Directed Semen Donor Eligibility Requirements
|
|
|
- Randell Rich
- 6 years ago
- Views:
Transcription
1 Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant communicable disease agents. The UIC Andrology Lab is responsible for the processing, storage and distribution of semen from directed donors. Testing and screening are the responsibility of the donor/recipient. The donor/recipient must consult with a physician to determine donor eligibility by reviewing the donor s relevant medical records for risk factors for, and clinical evidence of, relevant communicable disease agents and diseases. Testing must be done using ViroMed Laboratories (Donor Screening Panel #139063). LabCorp testing centers exclusively offer this service. TESTING MUST BE COMPLETED WITHIN 7 DAYS OF EACH SEMEN COLLECTION 1) HIV, type 1 and HIV-1 NAT (nucleic acid amplification test method) 2) HIV, type2 3) Hepatitis B surface antigen (HBsAg) and Hepatitis B core antigen (anti-hbc) 4) Hepatitis C (anti-hcv) and HCV NAT (nucleic acid amplification test) 5) HTLV types I and II 6) Treponema pallidum (syphilis) [ FDA-licensed, approved or cleared test]. 7) CMV (cytomegalovirus) total IgG and IgM. ** See note at bottom of page. 8) Chlamydia trachomatis 9) Neisseria gonorrhea Copies of test results must be submitted to the requesting physician as well as the Andrology lab. Donor eligibility form will be completed and sent to physician. The physician will be notified of any reactive (positive) results. It will be up to the discretion of the physician whether or not to use the specimen. Semen specimens will be held in the quarantine tank until all testing is completed. Specimens with a reactive test result will be released with the following notification: Warning: Advise patient of communicable disease risks and Warning: Reactive test results for (name of the disease). Biohazard label included. ** For positive CMV results, include sheet explaining CMV Antibody Testing. Department of Urology (MC955) T University Andrology Laboratory F South Wood Street Chicago, Illinois Rev: 04/25/2017 Page 1 of 2
2 Zika Virus Screening for Directed Semen Donors FDA guidance recommends physician review of relevant medical records for directed semen donors including a review of travel history. The review must indicate that a potential donor is free from risk factors or clinical evidence of Zika virus infection for the purpose of determining donor eligibility. The directed donor will be considered ineligible if the following apply: 1. He has a medical diagnosis of Zika infection in the past 6 months. 2. Residence in, or travel to, an area with active Zika virus transmission within the past 6 months. 3. Sex within the past 6 months with a male who is known to have either of the risk factors listed above. The directed semen donor s physician must complete and sign the screening questionnaire below. Physician s Name Directed Semen Donor s Name Zika Virus Screening Questionnaire 1. Has the above named Directed Semen Donor had a medical diagnosis of Zika infection in the past six months? Circle or 2. Has the above named Directed Semen Donor resided in or traveled to an area with active Zika virus transmission within the past 6 months? 3. Has the above named Directed Semen Donor had sex within the past 6 months with a male who is known to have either of the risk factors listed above? I, the undersigned physician certify that I have screened the above named Directed Semen Donor for the Zika virus risk factors and have determined that he is eligible. Physician s Signature Date Department of Urology (MC955) T University Andrology Laboratory F South Wood Street Chicago, Illinois Rev: 04/25/2017 Page 2 of 2
3 DIRECTED DONOR MEDICAL HISTORY INTERVIEW FORM Directed Donor Name: Date: Photo Identification: ID Checked by: Cells / Tissues Donated: Sperm Date of Last Interview: Recovery Method: Donation Type: Masturbation Directed Donor yes no 1. (Men only) Have you had sex with another man in the preceding five years? yes no 2. Have you injected drugs for a non-medical reason in the preceding five years, including intravenous, intramuscular, or subcutaneous injections? yes no 3. Do you have hemophilia or another related clotting disorder? If yes, have you received human-derived clotting factor concentrates in the preceding five years? yes no 4. Have you engaged in sex in exchange for money or drugs in the preceding five years? yes no 5. Have you had sex in the preceding 12 months with any person described in the previous 4 items of this section or with any person known or suspected to have HIV infection, including a positive or reactive test for HIV virus, hepatitis B infection, or clinically active (symptomatic) hepatitis C infection? yes no 6. Have you been exposed in the preceding 12 months to known or suspected HIV, HBV, and /or HCV infected blood through percutaneous inoculation (e.g., needle-stick) or through contact with an open wound, non-intact skin, or mucous membrane? yes no 7. Have you been incarcerated for more than 72 consecutive hours during the previous 12 months? yes no 8 yes no 9. Have you lived with (resided in the same dwelling) another person who has hepatitis B or clinically active (symptomatic) hepatitis C infection in the preceding 12 months? Have you had a tattoo, ear piercing, or body piercing in the last 12 months in which sterile procedures were not used, e.g., contaminated instruments and/or ink were used, or shared instruments that had not been sterilized between uses were used? yes no 10. Have you been diagnosed with clinical, symptomatic viral hepatitis after your 11 th birthday? Unless evidence from the time of illness documents that the hepatitis was identified as being caused by hepatitis A virus(e.g., a reactive IgM anti-hav test), Epstein-Barr Virus (EBV), or cytomegalovirus (CMV)? yes no 11. Have you had a recent smallpox vaccination (vaccinia virus) in the last 60 days? Donors with no vaccinia complications should be deferred until the vaccination scab has separated spontaneously, or for 21 days post-vaccination, whichever is the later date, and until the physical examination or physical assessment includes confirmation that there is no scab at the vaccination site. In cases where the scab was removed before separating spontaneously, the donor should be deferred for two months after vaccination. For persons who experienced vaccinia complications, the donor should be deferred until 14 days after all vaccinia complications have been completely resolved. Directed Donor Medical History Interview Form-v Page 1 of 3
4 Directed Donor Name yes no 12. Do you have a clinically recognizable vaccinia virus infection contracted by close contact with someone who received the smallpox vaccine? If the answer is yes to this question, how and when was the scab lost? The donor s skin should be examined. Defer donation from living donors until the scab has spontaneously separated. If the scab was otherwise removed, defer donor for 3 months from the date of vaccination of the vaccine recipient. Defer persons who develop other complications of vaccinia infection acquired through contact with a vaccine recipient until 14 days after all vaccinia complications have completely resolved. yes no 13. Have you had a medical diagnosis or suspicion of WNV infection (based on symptoms and / or laboratory results, or confirmed West Nile Virus NV viremia)? If the answer is yes to this question defer donation for 120 days from diagnosis or onset of symptoms, whichever is the later date or 28 days after condition has resolved. yes no 14. Have you tested positive or reactive for WNV infection using and FDA-licensed or investigational WNV NAT donor screening test in the preceding 120 days? If the answer is yes to this question defer donation for 120 days from diagnosis or onset of symptoms, whichever is the later date or 28 days after condition has resolved. yes no 15. Have you had both a fever and a headache (simultaneously) during the 7 days prior to donation? If yes, defer donation for 120 days from the onset of illness. yes no 16. Have you been diagnosed with Zika virus infection, been in an area with active Zika virus transmission, or had sex with a male with either of those risk factors, within the past six months? yes no 17. Have you been treated for syphilis within the preceding 12 months? If yes, defer donation until evidence is presented that the treatment occurred more than 12 months ago and was successful. yes no 18. Have you been diagnosed with or treated for Chlamydia in the preceding 12 months? If yes, defer donation until evidence is presented that the treatment occurred more than 12 months ago and was successful. yes no 19. Have you been diagnosed with or treated for Gonorrhea in the preceding 12 months? If yes, defer donation until evidence is presented that the treatment occurred more than 12 months ago and was successful.. yes no 20. Have you ever been diagnosis with vcjd or any other form of CJD? yes no 21. Have you ever had a diagnosis of dementia or any degenerative or demyelinating disease of the central nervous system (CNS) or other neurological disease of unknown etiology? yes no 22. Have you ever received a non-synthetic dura mater transplant? yes no 23. Have you ever received human pituitary-derived growth hormone? yes no 24. Have you ever had a blood relative diagnosed with CJD? yes no 25. Have you spent three months or more cumulatively in the United Kingdom (U.K.) from the beginning of 1980 through the end of 1996? yes no 26. Are you a current or former U.S. military member, civilian military employee, or dependent of a military member or civilian employee who resided at U.S. military bases in rthern Europe (Germany, Belgium and the Netherlands) for 6 months or more cumulatively from 1980 through 1990, or elsewhere in Europe (Greece, Turkey, Spain, Portugal, and Italy) for 6 months or more cumulatively from 1980 through 1996? Directed Donor Medical History Interview Form-v Page 2 of 3
5 Directed Donor Name yes no 27. Have you lived 5 years or more cumulatively in Europe from 1980 until the present (note this criterion includes time spent in the U.K. from 1980 through 1996)? yes no 28. Have you received any transfusion of blood or blood components in the U.K. or France between 1980 and the present? yes no 29. Have you injected bovine insulin since 1980? Can you confirm that the product was not manufactured after 1980 from cattle in the U.K.? yes no 30. Are you or any of your close contacts (persons with whom you have engaged in activities that could result in intimate exchange of body fluids, including blood or saliva) a xenotransplantation product recipient? Have you, your sexual partner, or any member of his/her household ever had a transplant or other medical procedure that involved being exposed to live cells, tissues, or organs from a nonhuman animal source, or human body fluids, cells, tissues, or organs that have had ex vivo contact with live nonhuman animal cells, tissues, or organs? yes no 31. Have you had a transfusion or received blood or blood products in the last 48hrs? The following questions need only be asked if there is a SARS outbreak in the world. Contact the CDC website ( or call CDC ( ) to obtain the up-to-date information concerning areas affected by SARS. If there is cases of SARS ask the following questions, otherwise note N/A. yes no 32. Have you traveled to or resided (the areas affected) in the last 14 days? yes no 33. Have you had close contact with someone who has traveled to or resided (the areas affected) in the last 14 days? yes no 34. Have you been treated for SARS or suspected you had SARS in the last 28 days? yes no 35. Have you had close contact within the previous 14 days with persons with SARS or suspected SARS. Authorized person completing initial Medical History Interview form: Print Signature Date Directed Donor Medical History Interview Form-v Page 3 of 3
6 DIRECTED DONOR PHYSICAL ASSESSMENT FORM Directed Donor Name Date: Photo Identification ID Checked by Cells / Tissues Donated Sperm Date of Assessment Recovery Method: Donation Type: Masturbation Directed Donor PHYSICAL ASSESSMENT (is there evidence of the following - attach additional page for comments if needed) Poor Basic Hygiene yes no comments Genital lesions yes no comments Insertion trauma yes no comments Genital / Perianal Warts yes no comments Other Physical evidence yes no comments STD (Herpes/chancroid/ulcers) yes no comments n-medical injection sites yes no comments Home produced tattoo yes no comments Recent tattoo yes no comments Recent body piercing yes no comments Enlarged lymph nodes yes no comments Oral thrush yes no comments Blue Purple Spots / Lesions yes no comments Trauma / Infection yes no comments Fever / Rash yes no comments Jaundice / Icterus yes no comments Enlarged liver (hepatomegaly) yes no comments Scabs / Smallpox yes no comments Eczema Vaccinatum yes no comments Vaccinia necrosum yes no comments Corneal scarring yes no comments Swollen Eyelids yes no comments Directed Donor Physical Assessment Form-v Page 1 of 2
7 DONOR PHYSICAL ASSESSMENT FORM Directed Donor Name Key to schematics: (A) Abrasion (B) Blood draw site (C) Body Piercing -requires description and date of application. (D) Bruise / Contusion (E) Dressing / Bandage (F) Fracture / Dislocation (G) Hematoma (H) Laceration / Wound (I) Needle entry site (J) Organ recovery site (K) Rash (L) Scar (surgical / trauma) (M) Skin Lesion (N) Tattoo requires description and date of application Authorized person completing the initial physical examination: Print Signature Date Directed Donor Physical Assessment Form-v Page 2 of 2
FORM TITLE: Risk Behavior Assessment. 1. Are you a male who has had sex with another male in the preceding five years?
 RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
Question: 1. Are you currently taking an antibiotic?
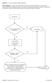 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic?
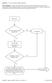 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Hot Topics in Tissue Safety
 Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
HEALTH SCREENING QUESTIONNAIRE. Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
The DRAI (Donors >12 yrs old) versus Requirements
 Q# or Pg # Pg 1 Question or section as it appears on the DRAI questionnaire (Donors >12 yrs old) 5-7-12 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Q# or Pg # Pg 1 Question or section as it appears on the DRAI questionnaire (Donors >12 yrs old) 5-7-12 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
The 2008 Guidelines for Gamete and Embryo Donation
 2008 Guidelines for gamete and embryo donation: a Practice Committee report Practice Committee of the American Society for Reproductive Medicine, and the Practice Committee of the Society for Assisted
2008 Guidelines for gamete and embryo donation: a Practice Committee report Practice Committee of the American Society for Reproductive Medicine, and the Practice Committee of the Society for Assisted
HEALTH SCREENING QUESTIONNAIRE CT Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
Current Infectious Disease Screening for the Live Organ Donor
 2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
Uniform DRAI - Donor >12 yrs old Requirements Crosswalk
 Q# or Pg # Pg 1 Question or section as it appears on the Uniform DRAI - Donor >12 yrs old 9-10-14 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Q# or Pg # Pg 1 Question or section as it appears on the Uniform DRAI - Donor >12 yrs old 9-10-14 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Case 1. FDA, Legal, and EBAA Medical Standards Panel. Case 1. Case 1 6/25/2012. Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies
 Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Hepatitis Case Investigation
 * indicates required fields Does patient also have: Hepatitis Case Investigation West Virginia Electronic Disease Surveillance System Division of Surveillance and Disease Control Infectious Disease Epidemiology
* indicates required fields Does patient also have: Hepatitis Case Investigation West Virginia Electronic Disease Surveillance System Division of Surveillance and Disease Control Infectious Disease Epidemiology
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legally binding More stringent - recommended Not legally binding and not recommended Non-reproductive tissues and cells
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legally binding More stringent - recommended Not legally binding and not recommended Non-reproductive tissues and cells
CORD BLOOD TRANSPLANTATION STUDY CBU EXCLUSION AND QUARANTINE RELEASE FORM
 (Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
(Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019
![[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019 [Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019](/thumbs/95/123651537.jpg) SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
A. Anti-HIV-1/2, HIV NAT Lookback: for those identified blood and blood components collected:
 PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
Cord Blood Medical History/ Health Assessment Questionnaire
 OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
2006 Guidelines for gamete and embryo donation
 2006 Guidelines for gamete and embryo donation The Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology Birmingham,
2006 Guidelines for gamete and embryo donation The Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology Birmingham,
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Increased Risk Donors for Organ Transplantation
 A Tool Kit to Assist Transplant Programs in the Use of Increased Risk Donors for Organ Transplantation February 2016 Table of Contents Table of Contents... 2 1.0 Increased Risk Donor Tool Kit... 3 1.1
A Tool Kit to Assist Transplant Programs in the Use of Increased Risk Donors for Organ Transplantation February 2016 Table of Contents Table of Contents... 2 1.0 Increased Risk Donor Tool Kit... 3 1.1
Non-reproductive tissues and cells
 Ministry of Health: Institute for Transplantation and Biomedicine / Colour key VIRAL HIV 1 and HIV 2 Hepatitis B Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More
Ministry of Health: Institute for Transplantation and Biomedicine / Colour key VIRAL HIV 1 and HIV 2 Hepatitis B Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis C Best Practice Guidelines For Local Health Departments
 Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding on national level More stringent - recommended on national level Not legy binding and not recommended on
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding on national level More stringent - recommended on national level Not legy binding and not recommended on
Donor Risk Assessment Interview (Donor >12 yrs old)
 Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Non-reproductive tissues and cells
 Colour key Tested pathogen VIRAL Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recommended on national level Not
Colour key Tested pathogen VIRAL Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recommended on national level Not
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recomd on national level Not legy binding and not recomd
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recomd on national level Not legy binding and not recomd
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Viral hepatitis. Supervised by: Dr.Gaith. presented by: Shaima a & Anas & Ala a
 Viral hepatitis Supervised by: Dr.Gaith presented by: Shaima a & Anas & Ala a Etiology Common: Hepatitis A Hepatitis B Hepatitis C Hepatitis D Hepatitis E Less common: Cytomegalovirus EBV Rare: Herpes
Viral hepatitis Supervised by: Dr.Gaith presented by: Shaima a & Anas & Ala a Etiology Common: Hepatitis A Hepatitis B Hepatitis C Hepatitis D Hepatitis E Less common: Cytomegalovirus EBV Rare: Herpes
Breastfeeding Center Milk Connection Questionnaire and Release for Donor
 Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
Uniform Donor Risk Assessment Interview (Donor >12 yrs old)
 Your logo Uniform Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State
Your logo Uniform Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State
ABO: Tissue#:
 Donor Name: Place of Interview: - Date-Time Interviewed: --/--/ --:-- Person Conducting Interview and Completing Form: - Person Interviewed: - Relationship to Potential Donor: - Address: - Phone: - Phone
Donor Name: Place of Interview: - Date-Time Interviewed: --/--/ --:-- Person Conducting Interview and Completing Form: - Person Interviewed: - Relationship to Potential Donor: - Address: - Phone: - Phone
UNOS # NA. Person(s) Interviewed (First and last name) Relationship(s) to him/her. Print Name Title Signature
 UNOS # Form-0005 - Medical History and Behavioral Donor Name (First and last name) Date/Time of Interview Person(s) Interviewed (First and last name) Relationship(s) to him/her Person Conducting Interview
UNOS # Form-0005 - Medical History and Behavioral Donor Name (First and last name) Date/Time of Interview Person(s) Interviewed (First and last name) Relationship(s) to him/her Person Conducting Interview
Name of Recipient: Recipient s DOB (if known) Relationship to Recipient: (Example: mother, father, sister, brother, friend, etc)
 The Christ Hospital Health Network DONOR REGISTRATION INFORMATION Phone: 513-585-2493 Fax: 513-585-0433 (Please be advised donor information is needed ONLY to register donor in the Christ Hospital system.
The Christ Hospital Health Network DONOR REGISTRATION INFORMATION Phone: 513-585-2493 Fax: 513-585-0433 (Please be advised donor information is needed ONLY to register donor in the Christ Hospital system.
Outbreak Investigation Guidance for Vectorborne Diseases
 COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
Patient Name: MRN: DOB: Treatment Location:
 Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC and its technical Directives (particularly 2006/17/EC) More stringent -legally binding, applies for all donations and all donor profiles
Colour key Minimum requirements as set out in Directive 2004/23/EC and its technical Directives (particularly 2006/17/EC) More stringent -legally binding, applies for all donations and all donor profiles
Hepatitis B and C Overview, Outbreaks, and Recommendations. Viral Hepatitis Language. Types of Viral Hepatitis 7/1/2013
 Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
Hepatitis B and C Overview, Outbreaks, and Recommendations Elizabeth Lawlor, MS Healthy Kansans living in safe and sustainable environments. Viral Hepatitis Language Acute infection is when the infection
Guidance for Industry
 Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
DONOR ELIGIBILITY EYE & TISSUE DREW TIMMONS, RN, CTBS TRANSPLANT SERVICES CENTER UT SOUTHWESTERN MEDICAL CENTER
 DONOR ELIGIBILITY EYE & TISSUE DREW TIMMONS, RN, CTBS TRANSPLANT SERVICES CENTER UT SOUTHWESTERN MEDICAL CENTER FACTORS IMPACTING EYE & TISSUE DONOR EVALUATION Institution Policy Clinical Experience Training
DONOR ELIGIBILITY EYE & TISSUE DREW TIMMONS, RN, CTBS TRANSPLANT SERVICES CENTER UT SOUTHWESTERN MEDICAL CENTER FACTORS IMPACTING EYE & TISSUE DONOR EVALUATION Institution Policy Clinical Experience Training
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
Screening donors and donations for transfusion transmissible infectious agents. Alan Kitchen
 Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B. Acute HBV Questionnaire
 BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B Informed Consent: Please read to individual and answer any questions There are about 60,000 people with hepatitis B in BC. We want to find
BC Enhanced Hepatitis Strain Surveillance Project Hepatitis B Informed Consent: Please read to individual and answer any questions There are about 60,000 people with hepatitis B in BC. We want to find
MEDICAL ADVISORY. July 18, 2017
 MEDICAL ADVISORY July 18, 2017 The following changes were made at the June 16, 2017 meeting of the Medical Advisory Board, and will become effective immediately. B1.100 Eye Bank Inspection The Accreditation
MEDICAL ADVISORY July 18, 2017 The following changes were made at the June 16, 2017 meeting of the Medical Advisory Board, and will become effective immediately. B1.100 Eye Bank Inspection The Accreditation
keyword: hepatitis Hepatitis
 www.bpac.org.nz keyword: hepatitis Hepatitis Key reviewers: Dr Susan Taylor, Microbiologist, Diagnostic Medlab, Auckland Dr Tim Blackmore, Infectious Diseases Physician and Microbiologist, Wellington Hospital,
www.bpac.org.nz keyword: hepatitis Hepatitis Key reviewers: Dr Susan Taylor, Microbiologist, Diagnostic Medlab, Auckland Dr Tim Blackmore, Infectious Diseases Physician and Microbiologist, Wellington Hospital,
Viral Hepatitis Diagnosis and Management
 Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Not Under Document Control If Printed. Donor Educational Materials. If you are deferred
 Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
BLOOD DONOR INTERVIEW AND PHYSICAL
 EXERCISE : BLOOD DONOR INTERVIEW AND PHYSICAL Skills: 20 points Objectives:. Determine if an individual is eligible to donate blood by proper performance of the donor interview. 2. Identify the conditions
EXERCISE : BLOOD DONOR INTERVIEW AND PHYSICAL Skills: 20 points Objectives:. Determine if an individual is eligible to donate blood by proper performance of the donor interview. 2. Identify the conditions
USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire. Table of Contents
 Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
Hepatitis STARS Program. Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003
 Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Non-reproductive tissues and cells VIRAL
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Non-reproductive tissues and cells VIRAL
Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests
 Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests Introduction As a potential donor, it is important you are aware of the screening tests
Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests Introduction As a potential donor, it is important you are aware of the screening tests
Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another?
 Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another? HIV transmission can occur when blood, semen (including pre-seminal fluid or "pre-cum"),
Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another? HIV transmission can occur when blood, semen (including pre-seminal fluid or "pre-cum"),
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 PAGE 1 of 5 PURPOSE To provide guidelines on the treatment and care of patients with Hepatitis. POLICY Hepatitis is an injury to hepatic cells and an inflammatory process in the liver. The major causes
PAGE 1 of 5 PURPOSE To provide guidelines on the treatment and care of patients with Hepatitis. POLICY Hepatitis is an injury to hepatic cells and an inflammatory process in the liver. The major causes
2017 CST-Astellas Canadian Transplant Fellows Symposium. Optimizing use of organs from Increased Risk Donors
 2017 CST-Astellas Canadian Transplant Fellows Symposium Optimizing use of organs from Increased Risk Donors Atual Humar, MD Atul Humar is a Professor in the Department of Medicine, University of Toronto.
2017 CST-Astellas Canadian Transplant Fellows Symposium Optimizing use of organs from Increased Risk Donors Atual Humar, MD Atul Humar is a Professor in the Department of Medicine, University of Toronto.
HIV/AIDS. Kuna High School Mr. Stanley
 HIV/AIDS Kuna High School Mr. Stanley Questions 1. Write an example of how your immune system helps prevent you from getting diseases. Terms to know Epidemic - a widespread occurrence of an infectious
HIV/AIDS Kuna High School Mr. Stanley Questions 1. Write an example of how your immune system helps prevent you from getting diseases. Terms to know Epidemic - a widespread occurrence of an infectious
Hepatitis C Virus (HCV)
 Clinical Practice Guidelines Hepatitis C Virus (HCV) OBJECTIVE The purpose is to guide the appropriate diagnosis and management of Hepatitis C Virus (HCV). GUIDELINE These are only guidelines, and are
Clinical Practice Guidelines Hepatitis C Virus (HCV) OBJECTIVE The purpose is to guide the appropriate diagnosis and management of Hepatitis C Virus (HCV). GUIDELINE These are only guidelines, and are
Increased Risk Donors. November 1, 2018 BC Kidney Days. Jagbir Gill MD MPH Associate Professor of Medicine UBC
 Increased Risk Donors November 1, 2018 BC Kidney Days Jagbir Gill MD MPH Associate Professor of Medicine UBC Median dialysis exposure prior to DDKT Case scenarios Which donors should we accept kidneys
Increased Risk Donors November 1, 2018 BC Kidney Days Jagbir Gill MD MPH Associate Professor of Medicine UBC Median dialysis exposure prior to DDKT Case scenarios Which donors should we accept kidneys
El Paso - Ambulatory Clinic Policy and Procedure
 Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
 Test Name *Minimum Collection Tubes Sample Stability ABO/Rh Blood Typing 50 µl cells EDTA whole blood (lavender-top) Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
Test Name *Minimum Collection Tubes Sample Stability ABO/Rh Blood Typing 50 µl cells EDTA whole blood (lavender-top) Within 14 days at 2-8⁰C. DO NOT FREEZE WHOLE BLOOD Micro Typing Systems 50 µl plasma
COMMUNICABLE DISEASE REPORT
 June 2011 Department of Health and Community Services Government of Newfoundland and Labrador COMMUNICABLE DISEASE REPORT Reporting Sexually Transmitted and Bloodborne Infections All laboratory-confirmed
June 2011 Department of Health and Community Services Government of Newfoundland and Labrador COMMUNICABLE DISEASE REPORT Reporting Sexually Transmitted and Bloodborne Infections All laboratory-confirmed
Confronting Ebola. Keeping NY patients and healthcare workers safe and healthy
 Confronting Ebola Keeping NY patients and healthcare workers safe and healthy All materials provided by Centers for Disease Control and Prevention. October 16, 2014 What You Need to Know about Ebola The
Confronting Ebola Keeping NY patients and healthcare workers safe and healthy All materials provided by Centers for Disease Control and Prevention. October 16, 2014 What You Need to Know about Ebola The
HIV, STI AND OTHER BLOOD- BORNE DISEASES. Kolářová M., EPI Autumn 2015
 HIV, STI AND OTHER BLOOD- BORNE DISEASES Kolářová M., EPI Autumn 2015 Chlamydia infection Genital warts Gonorrhoea Hepatitis B Hepatitis C HIV infection and AIDS Human papillomavirus infection Sexually
HIV, STI AND OTHER BLOOD- BORNE DISEASES Kolářová M., EPI Autumn 2015 Chlamydia infection Genital warts Gonorrhoea Hepatitis B Hepatitis C HIV infection and AIDS Human papillomavirus infection Sexually
Lymphogranuloma Venereum (LGV) Surveillance Project
 Lymphogranuloma Venereum (LGV) Surveillance Project Lymphogranuloma venereum (LGV) is a systemic, sexually transmitted disease (STD) caused by a type of Chlamydia trachomatis (serovars L1, L2, L3) that
Lymphogranuloma Venereum (LGV) Surveillance Project Lymphogranuloma venereum (LGV) is a systemic, sexually transmitted disease (STD) caused by a type of Chlamydia trachomatis (serovars L1, L2, L3) that
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
SAFE BLOOD SAVE LIVES
 DIN BLOOD DONATION REGISTRATION FORM PART I: PRE-DONATION INFORMATION Thank you for coming to give blood today. Your donation could save and change the lives of the recipients. We sincerely request you
DIN BLOOD DONATION REGISTRATION FORM PART I: PRE-DONATION INFORMATION Thank you for coming to give blood today. Your donation could save and change the lives of the recipients. We sincerely request you
STI s. (Sexually Transmitted Infections)
 STI s (Sexually Transmitted Infections) Build Awareness In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise. One of the primary defenses in the fight
STI s (Sexually Transmitted Infections) Build Awareness In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise. One of the primary defenses in the fight
The Alphabet Soup of Viral Hepatitis Testing
 The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
MONTEFIORE MEDICAL CENTER TRANSPLANT PROGRAM LIVING DONOR EVALUATION FORM History Questionnaire
 MONTEFIORE MEDICAL CENTER TRANSPLANT PROGRAM LIVING DONOR EVALUATION FORM History Questionnaire Donor s Name: Today s Date: Social Security #: Date of Birth Age Sex Address: Telephone #: (home) (work)
MONTEFIORE MEDICAL CENTER TRANSPLANT PROGRAM LIVING DONOR EVALUATION FORM History Questionnaire Donor s Name: Today s Date: Social Security #: Date of Birth Age Sex Address: Telephone #: (home) (work)
MEDICAL HISTORY FORM ADMINISTRATION INSTRUCTIONS
 ADMINISTRATION INSTRUCTIONS CONFIRM THE STUDY BAR CODE ON PAGES 1 THROUGH 6 OF THIS FORM DO NOT PLACE A STUDY BAR CODE LABEL ON THIS INSTRUCTION PAGE. Interview Instructions The interview should be conducted
ADMINISTRATION INSTRUCTIONS CONFIRM THE STUDY BAR CODE ON PAGES 1 THROUGH 6 OF THIS FORM DO NOT PLACE A STUDY BAR CODE LABEL ON THIS INSTRUCTION PAGE. Interview Instructions The interview should be conducted
*IMPORTANT* PLEASE FOLLOW THESE INSTRUCTIONS TO COMPLY WITH FLORIDA INTERNATIONAL UNIVERSITY S IMMUNIZATION POLICY
 *IMPORTANT* PLEASE FOLLOW THESE INSTRUCTIONS TO COMPLY WITH FLORIDA INTERNATIONAL UNIVERSITY S IMMUNIZATION POLICY The FIU Immunization Documentation Form must be processed prior to registering for your
*IMPORTANT* PLEASE FOLLOW THESE INSTRUCTIONS TO COMPLY WITH FLORIDA INTERNATIONAL UNIVERSITY S IMMUNIZATION POLICY The FIU Immunization Documentation Form must be processed prior to registering for your
Structure and duties of Blood service
 NATIONAL CENTER FOR TRANSFUSION MEDICINE Prevalence of infectious diseases among Mongolian blood donors Namjil Erdenebayar General director of The National Center for Transfusion Medicine, Mongolia NCTM,
NATIONAL CENTER FOR TRANSFUSION MEDICINE Prevalence of infectious diseases among Mongolian blood donors Namjil Erdenebayar General director of The National Center for Transfusion Medicine, Mongolia NCTM,
How is it transferred?
 STI s What is a STI? It is a contagious infection that is transferred from one person to another through sexual intercourse or other sexually- related behaviors. How is it transferred? The organisms live
STI s What is a STI? It is a contagious infection that is transferred from one person to another through sexual intercourse or other sexually- related behaviors. How is it transferred? The organisms live
Infection/Disease Control HEPATITIS CONTROL PROGRAM
 OPERATING PROCEDURE NO. 153-29 Florida State Hospital Chattahoochee, Florida July 8, 2009 Infection/Disease Control HEPATITIS CONTROL PROGRAM 1. Purpose: To establish consistent surveillance, reporting,
OPERATING PROCEDURE NO. 153-29 Florida State Hospital Chattahoochee, Florida July 8, 2009 Infection/Disease Control HEPATITIS CONTROL PROGRAM 1. Purpose: To establish consistent surveillance, reporting,
Bruce A. Luxon, MD, PhD, FACG Consultant: Vertex Speakers Bureau: Merck
 Bruce A. Luxon, MD, PhD, FACG Consultant: Vertex Speakers Bureau: Merck Bruce A. Luxon, MD, Ph.D. Anton and Margaret Fuisz Chair in Medicine Professor and Chair Department of Medicine Georgetown University
Bruce A. Luxon, MD, PhD, FACG Consultant: Vertex Speakers Bureau: Merck Bruce A. Luxon, MD, Ph.D. Anton and Margaret Fuisz Chair in Medicine Professor and Chair Department of Medicine Georgetown University
Taken From VBA s Adjudication Procedure Manual Section on Hepatitis
 Taken From VBA s Adjudication Procedure Manual Section on Hepatitis M21-1, Part III, Subpart iv, 4.I.2 Updated January 11, 2017 2. Hepatitis and Other Liver Disabilities Introduction Change Date January
Taken From VBA s Adjudication Procedure Manual Section on Hepatitis M21-1, Part III, Subpart iv, 4.I.2 Updated January 11, 2017 2. Hepatitis and Other Liver Disabilities Introduction Change Date January
EC CERTIFICATE. National Institute for Biological Standards and Control (NIBSC) Blanche Lane South Mimms Potters Bar Hertfordshire EN6 3QG UK
 National Institute for Biological Standards and Control (NIBSC) EC Certificate - Full Quality Assurance System Approval Certificate Annex IV (excluding sections 4 and 6) of Council Directive 98/79/EC on
National Institute for Biological Standards and Control (NIBSC) EC Certificate - Full Quality Assurance System Approval Certificate Annex IV (excluding sections 4 and 6) of Council Directive 98/79/EC on
Name Age Birthday / / Sex Last First MI. Home Address Street Apt City State Zip Code Home phone: ( ) Cell phone: ( ) Name of parent(s) or guardian:
 I. HEALTH HISTY- To be completed by the STUDENT (Required of all full-time students) Please answer all questions. Information requested in this form is strictly for the use of the Health Center in providing
I. HEALTH HISTY- To be completed by the STUDENT (Required of all full-time students) Please answer all questions. Information requested in this form is strictly for the use of the Health Center in providing
ACS BLOOD BORNE PATHOGEN TRAINING
 ACS BLOOD BORNE PATHOGEN TRAINING OBJECTIVE Define Blood borne pathogens Instruct how to recognize exposure to BBP Prevent or reduce risk of BBP exposure Identify high risk groups Review ACS exposure protocol
ACS BLOOD BORNE PATHOGEN TRAINING OBJECTIVE Define Blood borne pathogens Instruct how to recognize exposure to BBP Prevent or reduce risk of BBP exposure Identify high risk groups Review ACS exposure protocol
Blood Borne Pathogens. Becky Walch, R.N. Micheel Valdez, L.V.N.
 Blood Borne Pathogens Becky Walch, R.N. Micheel Valdez, L.V.N. Examples of Blood Borne Pathogens Hepatitis B Hepatitis C Other Hepatitis HIV Hepatitis Hepatitis means inflammation of the liver. Hepatitis
Blood Borne Pathogens Becky Walch, R.N. Micheel Valdez, L.V.N. Examples of Blood Borne Pathogens Hepatitis B Hepatitis C Other Hepatitis HIV Hepatitis Hepatitis means inflammation of the liver. Hepatitis
Relevant Communicable Diseases in HCT/Ps
 Relevant Communicable Diseases in HCT/Ps Phyllis I. Warkentin, MD Professor of Pathology and Pediatrics University of Nebraska Medical Center FACT Chief Medical Officer Relevant Communicable Diseases in
Relevant Communicable Diseases in HCT/Ps Phyllis I. Warkentin, MD Professor of Pathology and Pediatrics University of Nebraska Medical Center FACT Chief Medical Officer Relevant Communicable Diseases in
Commonly Asked Questions About Chronic Hepatitis C
 Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
CJD (Creutzfeldt-Jakob disease)
 CJD - lyodura and the risk of exposure during healthcare - frequently asked questions We would like to reassure all our patients that tight regulations govern all infection control processes at Addenbrooke's,
CJD - lyodura and the risk of exposure during healthcare - frequently asked questions We would like to reassure all our patients that tight regulations govern all infection control processes at Addenbrooke's,
RISK OF DISEASE TRANSMISSION FROM ORGAN DONORS PATIENT INFORMATION GUIDE
 Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
Public Health Image Library. CDC/ Cynthia Goldsmith. Image #
 Zika Virus Fredrick M. Abrahamian, D.O., FACEP, FIDSA Clinical Professor of Medicine UCLA School of Medicine Director of Education Department of Emergency Medicine Olive View-UCLA Medical Center Sylmar,
Zika Virus Fredrick M. Abrahamian, D.O., FACEP, FIDSA Clinical Professor of Medicine UCLA School of Medicine Director of Education Department of Emergency Medicine Olive View-UCLA Medical Center Sylmar,
More Fun Than Giving Blood CLINIC RECRUITED
 More Fun Than Giving Blood CLINIC RECRUITED Are you thinking of becoming a sperm donor? Choosing to become a sperm donor is a generous act, and by entering our sperm donor program you can help make someone
More Fun Than Giving Blood CLINIC RECRUITED Are you thinking of becoming a sperm donor? Choosing to become a sperm donor is a generous act, and by entering our sperm donor program you can help make someone
HONG KONG RED CROSS BLOOD TRANSFUSION SERVICE BLOOD DONATION REGISTRATION FORM
 I Bleed no. HONG KONG RED CROSS BLOOD TRANSFUSION SERVICE
I Bleed no. HONG KONG RED CROSS BLOOD TRANSFUSION SERVICE
For Parents and Students: Minor Donor Permit and Information About Donating Blood
 DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
Hepatitis. Dr. Mohamed. A. Mahdi 5/2/2019. Mob:
 Hepatitis Dr. Mohamed. A. Mahdi Mob: 0123002800 5/2/2019 Hepatitis Hepatitis means the inflammation of the liver. May cause by viruses or bacteria, parasites, radiation, drugs, chemical and toxins (alcohol).
Hepatitis Dr. Mohamed. A. Mahdi Mob: 0123002800 5/2/2019 Hepatitis Hepatitis means the inflammation of the liver. May cause by viruses or bacteria, parasites, radiation, drugs, chemical and toxins (alcohol).
Authorization for vaccination
 FOR USE BY CLSC INFORMATION RELATING TO VACCINATION Free Vaccination programs File no FIRST dose in grade 4 elementary school CONTRAINDICATION TO VACCINATION (specify) You must fill out the vaccination
FOR USE BY CLSC INFORMATION RELATING TO VACCINATION Free Vaccination programs File no FIRST dose in grade 4 elementary school CONTRAINDICATION TO VACCINATION (specify) You must fill out the vaccination
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
BLOOD COMPONENTS are regulated as
 Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More
Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More
