Quality assessment service of the Institute for Quality Assurance Lübeck
|
|
|
- Hope Hancock
- 6 years ago
- Views:
Transcription
1 IfQ-Lübeck Institut für Qualitätssicherung Lübeck Quality assessment service of the Institute for Quality Assurance Lübeck IfQ-Lübeck Institut für Qualitätssicherung Lübeck Seekamp Lübeck (Germany) Tel Fax
2
3 Table of contents Institute for Quality Assurance Lübeck (IfQ-Lübeck)...5 IfQ staff...6 Quality assessment parameters...8 Characteristics of the IfQ-Lübeck quality assessment...8 Participation process...9 Example of an evaluation report...10 Quality assessment schemes for autoimmunity...12 Autoantibodies against thyroid gland...12 Autoantibodies against granulocytes...14 Antibodies in autoimmune kidney diseases...15 Antibodies in autoimmune liver diseases...16 Autoantibodies in pernicious anaemia...17 Autoantibodies against structural proteins of the skin...18 Antibodies against CCP...19 Autoantibodies against cell nuclei...20 Antibodies in autoimmune myopathies...21 Autoantibodies against phospholipids...22 Antibodies against tissue transglutaminase (ttg), endomysium and gliadin...23 Quality assessment schemes for infectious serology...24 Antibodies against Borrelia burgdorferi sensu lato...24 Antibodies against hepatitis E virus...25 Antibodies against herpes simplex virus...26 Antibodies against parvovirus B Antibodies against Epstein-Barr virus...28 Antibodies against arboviruses...29 Quality assessment schemes for allergology...30 Total IgE, specific IgE...30 Terms and conditions for participation and information on the QA schemes...31 Price list and timetable...35 Autoimmunity schemes...35 Infectious serology schemes...36 Allergology schemes
4 4
5 Institute for Quality Assurance Lübeck (IfQ-Lübeck) The Institute for Quality Assurance Lübeck was founded as an affiliated institution of EUROIMMUN Medizinische Labor diagnostika AG (hereinafter called EUROIMMUN AG) in It belongs 100 % to EURO- IMMUN AG, Lübeck (umbrella organisation). The Institute for Quality Assurance Lübeck is responsible for the organisation, management and evaluation of quality assessment schemes and the provision of professional support to participants. In December 2008 the Institute for Quality Assurance Lübeck received accreditation. The quality assessment service is designed to evaluate the capabilities of participating laboratories, based on performed analyses in comparison to reference values and results from other participating laboratories. It aims to provide an objective aid for assessing and determining the reliability of data obtained, for evaluating results and recognising problems. Great value is placed on the use of clinically and serologically well characterised samples. The reference values are determined by independent external reference laboratories, which use reagents from different manufacturers for analysis and share their test results. Our quality assessment schemes are not restricted to specific test systems. Based on the results, participating laboratories should introduce corrective measures, if necessary, to improve the quality of their services. The Institute for Quality Assurance Lübeck supports participants with competent scientific advice. Quality assessment schemes are held regularly in order to give the participating laboratories the chance to monitor their performance capabilities continuously. Further information can be found at IfQ staff Lübeck. Back row: Mareen Zimmermann-Ahmari, Dr. Monika Probst, Michaela Pfeiffer; front row: Jessica Nehlsen, Juliane Eggert, Anne Kahl 5
6 QA coordination Dr. Monika Probst Tel: Technical support and administration Michaela Pfeiffer Tel: Juliane Eggert Tel:
7 Mareen Zimmermann-Ahmari Tel: Jessica Nehlsen Tel.: Anne Kahl Tel.:
8 Quality assessment parameters Autoimmunity Thyroid gland Neuronal antigens ANCA Kidney Liver Structural proteins of the skin CCP Cell nuclei Autoimmune myopathies Phospholipids Coeliac disease Pernicious anaemia Registration possible until: 30 June for schemes during the second half-year Infectious serology Borrelia Hepatitis E virus Herpes simplex virus Parvovirus B19 Epstein-Barr virus Arboviruses (Zika virus, dengue virus, chikungunya virus) Allergology Total IgE, specific IgE Timetable on pages 35 and 36! 30 November for schemes during the first half-year Characteristics of the IfQ-Lübeck quality assessment Online registration, entry of results and evaluation No restriction to specific test systems Target values established by external reference laboratories Clinically characterised sera Two quality assessment schemes per year for each parameter Evaluation contains images (IFA, Westernblot) and results from reference labs Certificates for successful participation High number of participants from over 50 countries Numbers of participants I/2017 Number of participants (I/2017) Thyroid gland Neuronal ANCA Kidney Liver Skin CCP ANA Myopathies EBV Arboviruses Allergology Phospholipids Coeliac disease Borrelia HEV HSV Parvovirus B19 Participants other countries (light) Participants Germany (dark) Autoimmunity Infectious serology Allergology
9 Participation process General registration at Registration for the individual schemes Shipment of samples as indicated Data entry Evaluation available on the internet Certificates sent by postal mail 9
10 QA report Example of an evaluation report Dear Ms./Mr...., we thank you for your participation in the interlaboratory test. A total of 440 laboratories participated. The rate of correct results was 98 %. Characterisation of samples Clinical expression Target value (canca) Target value (anti-pr3) Target value (panca) Target value (anti-mpo) Sample 1 Wegener s granulomatosis pos pos neg neg Sample 2 Healthy blood donor neg neg neg neg Sample 3 Wegener s granulomatosis pos pos neg neg Rate of correct results per test method (IgG) Test methods Frequency of use Correct single results IIFT % ELISA (PR3 and MPO) % Lineblot (PR3 and MPO) % Westernblot (PR3 and MPO) % Other (PR3 and MPO) % The reference values from reference laboratories, immunofluorescence images of the interlaboratory samples and details about the performance and evaluation of EUROIMMUN interlaboratory test schemes are available through our quality assessment portal. The next interlaboratory test for the investigation of Autoantibodies against granulocytes will take place in October We hope you will participate again! Sincerely yours Dr. rer.nat. Monika Probst Quality Assessment Service Coordinator 10
11 Individual evaluation Participant: Test system used in your lab (class IgG): EUROIMMUN Anti-PR3-hn-hr ELISA Represented by: All users of this test system Parameter Intended result Your result Median [U/ml] Interval of 68 % of results n Correct [%] Sample 1 anti-pr3 positive positive U/ml Sample 2 anti-pr3 negative negative 2.0 U/ml 2,0 1,7-4, Sample 3 anti-pr3 positive positive U/ml Certificate: yes October 2016: yes May 2016: yes November 2015: yes May 2015: yes Rate of correct results of the different test systems 2. Anti-PR3 Correct results Test system Aeskulisa PR3 sensitive Anti-PR3-ELISA Anti-PR3-ELISA Anti-PR3-ELISA Anti-PR3-hn-hr ELISA Manufacturer Aesku.Diagnostics Biorad Diesse Eurodiagnostica EUROIMMUN Test method ELISA ELISA ELISA ELISA ELISA Number of participants Sample 1 target: anti-pr3 pos 100 % 100 % 100 % 100 % 99 % Sample 2 target: anti-pr3 neg 100 % 100 % 100 % 100 % 98 % Sample 3 target: anti-pr3 pos 100 % 100 % 100 % 100 % 98 % ANCA-Profile ELISA Quanta Lite PR3 EUROIMMUN Inova Diagnostics ELISA ELISA % 100 % 96 % 88 % 92 % 88 % Anti-proteinase 3 Antibodies against MPO and PR3 Other Other Other Other % 100 % 100 % 100 % 100 % 100 % All anti-pr3 test systems % 97 % 98 % Results of reference laboratories Anti-PR3 Units Cut-off Sample External ref. laboratory Test system (manufacturer) Laboratory 1 Anti-PR3-hn-hr ELISA (EUROIMMUN) U/ml < 2 1,302 Laboratory 2 Anti-PR3-hn-hr ELISA (EUROIMMUN) U/ml ,054 Laboratory 5 PR3 ANCA capture ELISA (Eurodiagnostica) IU/ml 7 pos neg pos Laboratory 5 PR3 ANCA direct ELISA (Phadia) IU/ml 3 63 < Laboratory 7 Anti-PR3-hn-hr ELISA (EUROIMMUN) U/ml ,100 Target value pos neg pos 11
12 Autoantibodies against thyroid gland April and October Number of samples: 3 Evaluated parameters: Sample volume: TG, TPO, TRAb 300 µl (double set available) Quality assessment no: QV 1010 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report Further available figures to complement the QA report (examples) positive: anti-tpo, anti-tg Test substrate: Thyroid gland (monkey) Test substrate: Thyroglobulin BIOCHIP Dilution 1:10 12
13 Autoantibodies against neuronal antigens April and October Number of samples: 3 Evaluated parameters: Sample volume: Amphiphysin, aquaporin 4, CASPR2, CV2, GAD, Hu, LGI1, Ma/Ta, NMDA receptor, Ri, Yo 200 µl (double set available) Quality assessment no: QV 1111 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report Further available figures to complement the QA report (examples) 50 within target range Number of participants outside target range no target range neg. bdl. pos. 1:10 1:32 1:100 1:320 1:1,000 Qualitative Titer 1 1 positive: anti-nmdar Cerebellum (monkey) Hippocampus (rat) Transfected cells Controltransfected cells Dilution 1:10 QV Sample 2 Test method: PNS Profile 12 Ag EUROLINE (EUROIMMUN) positive: anti-hu 13
14 Autoantibodies against granulocytes April and October Number of samples: 3 Evaluated parameters: Sample volume: ANCA, MPO, PR3 200 µl (double set available) Quality assessment no: QV 1200 Evaluation: QA report If immunofluorescence is used, only borderline and positive samples in the immunofluorescence must be confirmed by monospecific test systems to obtain a certificate. Further available figures to complement the QA report (examples) Number of participants c p neg. pos. Qualitative c p c p c p c p c p c p ,000 3,200 Quantitative dominant pattern (titer) -1 positive: canca inside target range outside target range p panca c canca Test substrate: Ethanol-fixed granulocytes Test substrate: HEp-2/ethanol-fixed granulocytes Test substrate: Formalin-fixed granulocytes Test substrate: PR3 BIOCHIP Dilution 1:10 QV Sample 1 Test method: ANCA Profile EUROLINE (EUROIMMUN) positive: anti-pr3 14
15 Antibodies in autoimmune kidney diseases April und October Number of samples: 3 Evaluated parameters: Sample volume: GBM, PLA2R, THSD7A 200 µl (double set available) Quality assessment no: QV 1250 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report Further available figures to complement the QA report (examples) Number of participants within target range outside target range no target range neg. pos. 1:10 1:32 1:100 1:320 1:1,000 1:3,200 Qualitative Titer positive: anti-gbm Kidney Antigen spots GBM PLA2R-transfected cells THSD7A-transfected cells Dilution 1:10 QV Sample 1 Test method: ANCA/-GBM Profile EUROLINE (EUROIMMUN) positive: anti-gbm 15
16 Antibodies in autoimmune liver diseases March and September Numbers of samples: 3 Evaluated parameters: Sample volume: AMA, ASMA, F-actin, gp210, LC-1, LKM-1, M2, nuclear dots, nuclear membrane, SLA/LP, Sp µl (double set available) Quality assessment no: QV 1300 Evaluation: QA report The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. Further available figures to complement the QA report (examples) Number of participants within target range outside target range no target range pos neg AMA ASMA cellular nuclear ,000 3,200 10,000 membrane dots Qualitative Pattern Quantitative dominant pattern (titer) positive: AMA HEp-2 cells Liver Kidney Antigen spots pos: Anti-M2 Dilution 1:100 QV Sample 1 Test method: Liver Profile EUROLINE (EUROIMMUN) positive: anti-gp210, anti-m2-3e, anti-m2 16
17 Autoantibodies in pernicious anaemia April and October Number of samples: 3 Evaluated parameters: Sample volume: Intrinsic factor, parietal cells (PCA) 200 µl (double set available) Quality assessment no: QV 1360 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report Further available figures to complement the QA report (examples) Number of participants within target range outside target range no target range neg. bdl. pos. 1:10 1:32 1:100 1:320 1:1, Qualitative Titer positive: anti-pca, anti-intrinsic faktor Test substrate: Stomach (monkey) Test substrate: Intrinsic faktor BIOCHIP Dilution 1:10 17
18 Autoantibodies against structural proteins of the skin April and October Numbers of samples: 3 Evaluated parameters: Sample volume: BP180, BP230, desmoglein 1, desmoglein 3, desmosomes, epidermal basement membrane, 200 µl (double set available) Quality assessment no: QV 1501 Evaluation: QA report The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. Further available figures to complement the QA report (examples) inside target range outside target range Number of particpants neg. 4 D EBM pos. 10 D desmosomes EBM epidermal basement membrane D EBM D EBM D EBM D EBM D EBM ,000 Qualitative Quantitative dominant pattern (titer) -1 positive: anti-desmosomes, anti-dsg 1, anti-dsg 3 Oesophagus Anti-Dsg 1 Anti-Dsg 3 Dilution 1:10 Dilution 1:100 Dilution 1:10 Dilution 1:10 18
19 Antibodies against CCP April and October Number of samples: 3 Evaluated parameter: Sample volume: CCP 200 µl (double set available) Quality assessment no: QV 1505 Evaluation: QA report The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. Only test systems that detect antibodies against CCP will be accepted. QA report without further figures 19
20 Autoantibodies against cell nuclei March and September Number of samples: 3 Evaluated parameters: Cell nuclei, centromeres, CENP A, CENP B, dsdna, nucleosomes, RNP, RNP / Sm, Scl-70, Sm, SS-A, SS-B Sample volume: 400 µl Quality assessment no: QV 1510 Evaluation: If immunofluorescence is used, only borderline and positive samples in the immunofluorescence must be confirmed by monospecific test systems to obtain a certificate. QA report Further available figures to complement the QA report (examples) within target range Number of participants outside target range no target range pos neg granular nucleolar homogenous granular dots cytoplasm nuclear ,000 3,200 10,000 Qualitative Pattern Quantitative dominant pattern (titer) positive: ANA (pattern: granular / nucleolar) HEp-2 cells Liver Antigen spots pos: SS-A; neg: nrnp/sm, Sm Antigen spots pos: SS-B; neg: Scl-70, Jo-1 Dilution 1:100 QV Sample 1 Test method: ANA Profile 5 EUROLINE (EUROIMMUN) positive: anti-ss-b, anti-ss-a, anti-ro-52 20
21 Antibodies in autoimmune myopathies March and September Number of samples: 3 Evaluated parameters: Sample volume: cn-1a, EJ, Jo-1, Ku, Mi-2, Mi-2α, Mi-2, OJ, PL-7, PL-12, PM-Scl, SRP 200 µl (double set available) Quality assessment no: QV 1530 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report Further available figures to complement the QA report (examples) QV Sample 1 Test method: Myositis Profile 3 EUROLINE (EUROIMMUN) positive: anti-jo-1, anti-ro-52 QV Sample 2 Test method: Myositis Profile 3 EUROLINE (EUROIMMUN) positive: anti-ku 21
22 Autoantibodies against phospholipids April and October Number of samples: 3 Evaluated parameters: Sample volume: Cardiolipin (IgG, IgM, IgAGM); 2-Glykoprotein (IgG, IgM, IgAGM) 150 µl (double set available) Quality assessment no: QV 1632 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report QA report without further figures 22
23 Antibodies against tissue transglutaminase (ttg), endomysium and gliadin April and October Number of samples: 3 Evaluated parameters: Tissue transglutaminase (ttg), endomysium, gliadin (IgA, IgG) Sample volume: 200 µl (double set available) Quality assessment no: QV 1913 Evaluation: Certificates will only be awarded to participants who use a test system that determines antibodies against ttg (IgA) or EMA (IgA) in combination with a coeliac-disease-specific IgG test system. The latter may be for the analysis of transglutaminase (IgG) or EMA (IgG), or an IgG test system based on deamidated gliadin. Test systems using native gliadin will not be certified. Further available figures to complement the QA report (examples) Number of participants QA rep ort within target range outside target range no target range neg 1 6 bdl pos :10 Qualitative : : : :1,000 1:3,200 Titer positive: anti-endomysium (IgA), anti-gliadin (IgA, IgG) Test substrate: Test substrate: Intestine Test substrate: Oesophagus Test substrate: Gliadin (deamidated) IgA Liver positive negative positive IgG positive Dilution 1:10 23
24 Antibodies against Borrelia burgdorferi sensu lato March and September Number of samples: 3 Evaluated parameters: Sample volume: Borrelia (IgG, IgM), serological complete diagnosis 250 µl (double set available) Quality assessment no: QV 2132 Evaluation: If a screening assay is used only borderline or positive samples have to be confirmed in a confirmation assay to obtain a certificate. QA report Further available figures to complement the QA report (examples) QV Sample 2 Test method: EUROLINE-WB (IgG) negative QV Sample 2 Test method: EUROLINE-WB (IgM) positive QV Sample 2 Test method: EUROLINE-RN-AT (IgG) negative QV Sample 2 Test method: EUROLINE-RN-AT (IgM) positive 24
25 Antibodies against hepatitis E virus Shipment March and September Number of samples: 3 Evaluated parameter: Sample volume: Hepatitis E virus (IgG, IgM, IgAGM) 200 µl (double set available) QA number: QV 2525 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report QA report without further figures 25
26 Antibodies against herpes simplex virus March and September Number of samples: 3 Evaluated parameters: Sample volume: HSV-1, HSV-2, HSV-1 / 2 (IgG, IgM) 200 µl (double set available) Quality assessment no: QV 2531 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report Further available figures to complement the QA report (examples) QV Sample 2 Test method: EUROLINE-WB (IgG) positive: anti-hsv-1 QV Sample 2 Test method: EUROLINE-WB (IgM) negative 26
27 Antibodies against parvovirus B19 March and September Number of samples: 3 Evaluated parameter: Sample volume: Parvovirus (IgG, IgM) 200 µl (double set available) QA number: QV 2580 Evaluation: The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. QA report Further available figures to complement the QA report (examples) QV Sample 2 Test method: EUROLINE (IgG) positive QV Sample 2 Test method: EUROLINE (IgM) positive 27
28 Antibodies against Epstein-Barr virus March and September Number of samples: 3 Evaluated parameters: Sample volume: EBV-CA (IgG, IgM), EBNA-1(IgG), avidity, serological complete diagnosis 200 µl (double set available) QA number: QV 2790 Evaluation: QA report The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. Further available figures to complement the QA report (examples) positive: anti-ebv-ca (IgG), anti-ebna (IgG); high avidity EBV-CA (IgG) EBV-CA (IgG) treated with urea EBV-CA (IgM) EBNA (IgG) Dilution 1:10 QV Sample 2 Test method: EUROLINE Profile 2 EBV (IgG) positive: anti-ebv-ca, anti-ebna-1 QV Sample 2 Test method: EUROLINE Profile 2 EBV (IgM) positive: anti-ebv-ea, anti-ebv-ca 28
29 Antibodies against arboviruses March and September Number of samples: 3 Evaluated parameters: Sample volume: Chikungunya virus, dengue virus, Zika virus (IgG, IgM) dengue virus NS1 antigen 300 µl (double set available) QA number: QV 2668 Evaluation: QA report The test systems are evaluated individually. A certificate is only awarded upon correct analysis of all three samples. Further available figures to complement the QA report (examples) positive: anti-zika virus (IgG, IgM) Zika virus Dengue virus Chikungunya virus IgM IgG Dilution 1:10 29
30 Allergology March and September Number of samples: 3 Evaluated parameters: Sample volume: Total IgE, specific IgE Total IgE 1 x 500 µl Total IgE / specific IgE 2 x 1000 µl QA number: QV 3000 Evaluation: QA report The test systems are evaluated individually. One certificate is be awarded for the correct analysis of total IgE and one for the correct analysis of specific IgE in each case. Further available figures to complement the QA report (examples) Participants: Quality Assessment Report QV , II/2015 "Allergology": Evaluation of your results Represented by: Test systems employed at your lab (class IgE): EUROIMMUN Allercoat 6 system (EAST class [0-6]) ELISA Intended result Parameter (EAST class [0-6]) Your Result Sample 2 w1 (common ragweed) ku/l: <0,35 Sample 3 w1 (common ragweed) ku/l: 6,11 Sample 3 (Number of participants) * * EAST class(0-6) Certificate: yes April Legend ** Your Result result accepted: assay All Your assay result not accepted: All assay Your assay Test method: EUROLINE Profile Inhalation Test method: EUROLINE Profile Food Test method: EUROLINE Profile Insect Venoms 30
31 Terms and conditions for participation and information on the QA schemes The Institute for Quality Assurance Lübeck (henceforth called IfQ Lübeck) organises quality assessment schemes for laboratory external quality control. Purpose of the quality assessment service The quality assessment service is designed to evaluate the performance capabilities of participating laboratories, based on performed laboratory tests in comparison to reference values and results from other participating laboratories. It provides an objective aid for assessing and determining the reliability of data obtained and for recognising problems. Based on the results, participating laboratories should introduce corrective measures, if necessary, to improve the quality of their services. Quality assessment schemes are held regularly in order to give the participating laboratories the chance to monitor their performance capabilities continuously. Participation in the quality assessment schemes should establish additional confidence for customers of participating laboratories. Quality assessment schemes are not aimed at evaluating the products (test systems) used and should not be drawn on to assess the performance of the products. The use of the quality assessment samples, certificates, participations certificates, reports and other information provided by the IfQ is only permitted within the framework of this intended use. Costs The participation fees for the quality assessment schemes are to be taken from the relevant valid price list (for Switzerland, please refer to the responsible EUROIMMUN subsidiary for information on the costs). Participants bear the costs for their reagents, time expenditure, etc. Quality assessment samples Quality assessment schemes take place twice a year, each time with three samples. Real clinically characterised samples are used preferably. These are obtained in collaboration with doctors or sample donors. The origin of each sample is known. The samples can be serum or plasma. Methods used by the participants must therefore be validated for both serum and plasma. Determination of expected results Expected results for quality assessment samples are determined before delivery of the samples in cooperation with competent external laboratories. A list of the reference laboratories is available on the quality assessment portal. For each quality assessment scheme at least one reference laboratory is commissioned which is accredited for performance of the respective analysis according to the corresponding laboratory norm (e.g. DIN EN ISO 15189, DIN EN ISO/ IEC 17025, DIN EN ISO 15195). The samples are measured and assessed by the appropriate reference laboratories. The qualitative result of the reference laboratories is taken as the expected result. Stability/preservation The quality assessment scheme samples are preserved, usually with sodium azide (< 1%). In individual cases, also other preservatives may be used. Avoid contact with the skin. Occasionally the used preservatives can interfere with certain test methods. To exclude such interference, consult the instructions in the test system used. Every quality assurance sample undergoes a stability check. In this check, transport and storage of the samples are simulated in stress tests. The stability check establishes that the sample is stable at the given storage temperature for the duration of the quality assessment round (generally 4 weeks). 31
32 Homogeneity Quality assurance samples are fluid and are mixed before and during filling to ensure homogeneity. The homogeneity of the samples is determined during process validation of the filling process. Safety warnings No antibodies against HCV, HIV-1 or HIV-2 are detected in quality assessment samples using CE-registered or FDA-approved test systems. Nevertheless, samples should be handled as carefully as infectious material. Registration of participants Any interested laboratory that routinely carries out the respective laboratory analyses can take part in the quality assessment. Participants are required to register on the internet at The quality assessment service portal allows participants to register for the different quality assessment schemes and later enter their results online. The address provided by the participant is used to provide effective communication between the IfQ Lübeck and the participants. Participants have to ensure availability via this address. Participants are required to notify the IfQ Lübeck via the portal of any changes in their personal details (e.g. or delivery address) and to keep their details up to date. If the provided address is incorrect, participants are not entitled to a new delivery or refund. Registration for quality assessment schemes, signing up for subscription, and procedure Registration, entering of results and issuing of reports take place online via the quality assessment portal. There, the dates for every quality assessment scheme are announced. Participants can apply to the quality assessment schemes during the registration period. The registered participants are informed on the start and end dates of the registration phase via . By registering to the quality assessment schemes, the participant agrees to the terms and conditions of the IfQ in its relevant valid version, to be found in the quality assessment portal. Registration for the quality assessment schemes can also be made via subscription. With registration via subscription, the participants are registered for all future quality assessment schemes offered by the IfQ until further notice. The participants can cancel the participation in the respective quality assessment scheme until the end of the registration period or cancel the subscription by this date. Revocation and cancellation can be done via . The conditions of participation apply in their relevant valid version. If the conditions of participation change, the participants are expressly informed about the change on the website ( Infobox: Changes in the conditions of participation ). Changed conditions of participation are published at the latest two weeks before the end of the registration period on the website and communicated via . An isolated revocation is not possible. The revocation always applies to the participation in the quality assessment scheme. Despite careful planning it may happen that the sample contingent is used up before the end of the registration phase. There is no entitlement to the number of samples being extended. Early registration ensures participation. After the end of the registration period, the quality assessment samples are sent by the IfQ Lübeck at the date announced in the portal. If samples are lost or damaged and the IfQ Lübeck is informed straightaway, replacements will be sent if possible. However, participants are not entitled to replacements. If a new delivery results in lateness or delayed service, there is no entitlement to the participant s results being taken into account in the evaluation of the quality assessment round. The participants commit to handle 32
33 and store the quality assessment samples in the same way as routine samples and measure them in their own laboratory. Participants must state their method(s) used (routine method) together with the results. Results obtained under routine conditions must be entered online in the portal by the respective deadline. No additional determinations or further methods that are not used for routine samples may be used to obtain results. It is not permitted to enter test results which are consolidated from different methods. If a test procedure consists of a screening test followed by a confirmatory test, both methods must be entered during registration on the portal, and results for the two methods must be given separately. If the confirmatory test is performed by an external laboratory this should be indicated. The certificate is only valid for tests performed by the participating laboratory; externally performed tests are therefore marked in the certificate. Falsification and secret communication between participating laboratories contradicts the goal of external quality control and is therefore not permitted. It is recommended that participants double-check that their results are entered correctly in the portal. Participants should also file a printout of their entry with their documentation. Errors in data entry can be corrected up until the deadline for entering the results. After the deadline no changes may be made to the entered results. In cases of necessity, for example faulty internet connection, results can be submitted to the IfQ Lübeck in writing (e.g. , fax). These will be entered into the portal by the quality assurance team, as long as the deadline is met. If results are not entered or transmitted within the time limit, no evaluation can be made. In this case, the costs are not refunded. Evaluation, quality assurance report and issuing of certificates The IfQ Lübeck evaluates the data promptly and places the evaluation online on the quality assessment portal. This includes the results of every participant (only available for the corresponding participant), a statistical total evaluation with anonymous and summarised results of all participating laboratories, and further helpful information (e.g. images from immunofluorescence tests, blot strips). Participants receive a message when the evaluation is available. Qualitative evaluation is crucial for the granting of a certificate. If the qualitative results of the participant for all samples is in agreement with the expected values, the quality assessment scheme has been passed and a certificate will be issued. Particularities of individual quality assessment schemes are to be found in detail in the respective information in the quality assessment portal. If a quality assessment scheme is not passed, the participant receives a participation confirmation instead of a certificate. In the result evaluation for each participant, the following statistical information is given alongside a comparison of the participant s results and the expected results. These values are based on all participants who participated in the scheme using the same test system. Median of results* 68 % result range* Number of participants Number of correct results (pass rate) *if at least 6 participants gave a quantitative result The median is the middle value of all measurement values when sorted by size, so that half of the values lie under the median and half over. If the number of measurement values is even, the arithmetic mean of the two middle values is taken as the median. The median rather than the arithmetic mean is used as a statistical parameter to reduce the influence of extreme values. Outlier tests are not performed.the 68 % result range gives the distribution range of quantitative results, within which the measurement values of 33
34 68 % of the participants lie. In the total evaluation the following information is given for each test that was used by participants in the quality assessment scheme: Number of participants Number of correct results (pass rate) The certificates and participation confirmations of the IfQ Lübeck are sent to the participants via mail to the address provided. The complete quality assessment scheme reports are provided in the quality assessment portal. Certificates, confirmations of participation and reports may be used within the framework of the intended used of the quality assessment schemes as described above. Misuse or tampering, or meaningful changes through manipulation of the documents, or by relating them with other documents is not allowed. Queries Complaints about the quality assessment carried out by the IfQ Lübeck should be sent in written form to the institute within 4 weeks of receiving the quality assessment report. After this deadline, complaints can no longer be considered. If it should happen that a quality assessment scheme becomes invalid through a mistake by the IfQ Lübeck, an additional scheme will be offered free of charge (with the same conditions for participation). Further claims on the IfQ Lübeck are excluded. Declaration of confidentiality The IfQ Lübeck treats all participant data as confidential. Merely the data required for invoicing are passed on to the responsible departments. In the quality assessment portal, the anonymised results of each quality assessment scheme are made accessible to the participants. Miscellaneous Further information about the quality assessment service can be found in the quality assessment portal. The IfQ Lübeck reserves the right to exclude a laboratory if it repeatedly does not return results or if a participant has falsified data or has made secret consultations or has tried to. This also applies to attempts to influence employees of the IfQ Lübeck. The IfQ reserves the right to introduce participant fees and to change individual quality assessment schemes with regard to their scope or to discontinue them completely. For further issues the general terms and conditions of EUROIMMUN AG apply. Please address any questions about the individual quality assessment schemes or the quality assessment service to the IfQ Lübeck at ifq@euroimmun.de Tel:
35 Time schedule 2018 Autoimmunity Scheme Format Dates QAS 2018/I Dates QAS 2018/II Autoantibodies against cell nuclei Cell nuclei, centromeres, CENP A, CENP B, dsdna, nucleosomes, RNP, RNP / Sm, Scl-70, Sm, SS-A, SS-B Antibodies in autoimmune myopathies cn-1a, EJ, Jo-1, Ku, Mi-2, Mi-2α, Mi-2, OJ, PL-7, PL-12, PM-Scl, SRP 1 set of specimen 3 x 400 µl Registration until: November 30, set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl March 20, 2018 Registration until: June 30, 2018 September 18, 2018 Antibodies in autoimmune liver diseases AMA, ASMA, F-actin, gp210, LC-1, LKM-1, M2, nuclear dots, nuclear membrane, SLA/LP, Sp100 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl Deadline: April 17, 2018 Deadline: October 16, 2018 Autoantibodies against thyroid gland TG, TPO, TRAb 1 set of specimen 2 sets of specimen 3 x 300 µl 3 x 600 µl Autoantibodies against neuronal antigens Amphiphysin, aquaporin 4, CASPR2, CV2, GAD, Hu, LGI1, Ma/Ta, NMDA receptor, Ri, Yo 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl Autoantibodies against granulocytes ANCA, MPO, PR3 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl Autoantibodies against structural proteins of the skin BP180, BP230, desmoglein 1 and 3, desmosomes, epidermal basement membrane Antibodies against CCP 1 set of specimen 2 sets of specimen 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl 3 x 200 µl 3 x 400 µl Registration until: November 30, 2017 April 24, 2018 Deadline: May 22, 2018 Registration until: June 30, 2018 October 16, 2018 Deadline: November 13, 2018 Autoantibodies against phospholipids Cardiolipin (IgG, IgM, IgAGM); 2-glycoprotein (IgG, IgM, IgAGM) 1 set of specimen 2 sets of specimen 3 x 150 µl 3 x 300 µl Antibodies against tissue transglutaminase, endomysium and gliadin (IgA, IgG) 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl Antibodies in autoimmune kidney diseases GBM, PLA2R, THSD7A 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl Autoantibodies in pernicious anaemia Intrinsic factor, parietal cells 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl 35
36 Time schedule 2018 Infectious serology Scheme Format Dates QAS 2018/I Dates QAS 2018/II Antibodies against Borrelia burgdorferi sensu lato (IgG, IgM), serological complete diagnosis 1 set of specimen 2 sets of specimen 3 x 250 µl 3 x 500 µl Antibodies against hepatitis E virus (IgG, IgM, IgAGM) 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl Antibodies against herpes simplex virus HSV-1, HSV-2, HSV-1/2 (IgG, IgM) 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl Registration until: November 30, 2017 Registration until: June 30, 2018 Antibodies against parvovirus B19 (IgG, IgM) 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl March 20, 2018 Deadline: April 17, 2018 September 18, 2018 Deadline: October 16, 2018 Antibodies against Epstein-Barr virus EBV-CA (IgG, IgM), EBNA-1 (IgG), avidity, serological complete diagnosis 1 set of specimen 2 sets of specimen 3 x 200 µl 3 x 400 µl Antibodies against arboviruses Chikungunya virus, dengue virus, Zika virus (IgG, IgM) 1 set of specimen 2 sets of specimen 3 x 300 µl 3 x 600 µl Allergology Scheme Format Dates QAS 2018/I Dates QAS 2018/II 1 set of specimen consisting of Registration until: November 30, 2017 Registration until: June 30, 2018 Allergology Total IgE, specific IgE Total IgE Total IgE/ specific IgE 1 x 500 µl 2 x 1000 µl March 20, 2018 September 18, 2018 Deadline: April 17, 2018 Deadline: October 16, 2018 Please ask your local distributor for prices. 36
37 Notes 37
38 Notes 38
39 Notes 39
40 IfQ-Lübeck Institut für Qualitätssicherung Lübeck IfQ-Lübeck Institut für Qualitätssicherung Lübeck Seekamp 31, Lübeck, Germany Tel: Fax: YV_0000_I_UK_B10, 09/2017
VASCULITIS PRODUCT HIGHLIGHTS
 VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
Assays. New. New. Combinations. Possibilities. Patents: EP , AU
 Assays Patents: EP 2362222, AU 2011217190 New Combinations New Possibilities Technology Classical Handling of Autoimmune Diagnostics 2-Step Diagnostics 1 st Screening 2 nd Confirmation Cell based IFA ELISA
Assays Patents: EP 2362222, AU 2011217190 New Combinations New Possibilities Technology Classical Handling of Autoimmune Diagnostics 2-Step Diagnostics 1 st Screening 2 nd Confirmation Cell based IFA ELISA
Differentiated ANCA diagnostics using BIOCHIP Mosaics
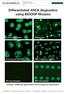 Differentiated ANCA diagnostics using BIOCHIP Mosaics Gran. MPO microdoplet Example: Antibodies against MPO and homogeneous ANA pattern Anti-MPO? ANA? panca positive, anti-mpo positive gran. Final result:
Differentiated ANCA diagnostics using BIOCHIP Mosaics Gran. MPO microdoplet Example: Antibodies against MPO and homogeneous ANA pattern Anti-MPO? ANA? panca positive, anti-mpo positive gran. Final result:
Autoimmune diagnostics. A comprehensive product line for the detection of autoantibodies
 Autoimmune diagnostics A comprehensive product line for the detection of autoantibodies Autoimmune diagnostics Autoimmune diseases are chronic inflammatory processes with an indeterminate etiology. They
Autoimmune diagnostics A comprehensive product line for the detection of autoantibodies Autoimmune diagnostics Autoimmune diseases are chronic inflammatory processes with an indeterminate etiology. They
Clinical Laboratory. 14:41:00 Complement Component 3 50 mg/dl Oct-18
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
IMTEC-ANA-LIA MAXX. Design Verification
 Design Verification IMTEC-ANA-LIA MAXX CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Specificity... 2 3 Interferences... 4 4 Imprecision... 6 4.1 Within-Run Imprecision... 6 4.2 Between-Run
Design Verification IMTEC-ANA-LIA MAXX CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Specificity... 2 3 Interferences... 4 4 Imprecision... 6 4.1 Within-Run Imprecision... 6 4.2 Between-Run
Product Guide. Valid from June 15 th, Simply innovative diagnostics
 Product Guide Valid from June 15 th, 2018 Simply innovative diagnostics ELISA Product group Page Thyroid TSH Receptor autoantibodies 6 Tg autoantibodies TPO autoantibodies Thyreoglobulin 7 TSH 8 Free
Product Guide Valid from June 15 th, 2018 Simply innovative diagnostics ELISA Product group Page Thyroid TSH Receptor autoantibodies 6 Tg autoantibodies TPO autoantibodies Thyreoglobulin 7 TSH 8 Free
Tel: Tel: Web: CODE DESCRIPTION UNITS
 New Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDIMOD2 Modulus2 2-4 Microplate Analyzer 1 Refurbished Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDINXGMI Refurbished Nexgen 4-Microplate
New Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDIMOD2 Modulus2 2-4 Microplate Analyzer 1 Refurbished Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDINXGMI Refurbished Nexgen 4-Microplate
ORDER INFORMATION AND PRODUCT DATA. Immunoglobulin Class A: IgA E: IgE G: IgG Product Code M: IgM O: IgGM Product Classification
 ORDER INFORMATION AND PRODUCT DATA FI 2531-1005 -1 G 1601: 16 single test strips 1208: 12 microplate strips with 8 wells each 9601: 96 individual break-apart wells (12 microplate strips with 8 wells each)
ORDER INFORMATION AND PRODUCT DATA FI 2531-1005 -1 G 1601: 16 single test strips 1208: 12 microplate strips with 8 wells each 9601: 96 individual break-apart wells (12 microplate strips with 8 wells each)
Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia
 Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia Foreign molecules = antigens Immune response Immune system non-specific specific cellular humoral
Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia Foreign molecules = antigens Immune response Immune system non-specific specific cellular humoral
Instructions for Use. Tg Antibody ELISA
 Instructions for Use Tg Antibody ELISA Enzyme Immunoassay for the Quantitative Determination of Autoantibodies to Thyroglobulin (Tg) in Serum and Plasma I V D REF EA618/96 12 x 8 2 8 C DLD Gesellschaft
Instructions for Use Tg Antibody ELISA Enzyme Immunoassay for the Quantitative Determination of Autoantibodies to Thyroglobulin (Tg) in Serum and Plasma I V D REF EA618/96 12 x 8 2 8 C DLD Gesellschaft
USA Product Listing. Serology Diagnostic Products
 USA Product Listing Serology Diagnostic Products Focus Diagnostics recognizes your testing needs because we identify and evaluate trends in diagnostic testing. The products listed in this brochure were
USA Product Listing Serology Diagnostic Products Focus Diagnostics recognizes your testing needs because we identify and evaluate trends in diagnostic testing. The products listed in this brochure were
EC Declaration of Conformity Authorised By: Version Released Amendments 3 04/03/2015
 Version Released Amendments 3 04/03/2015 Amend manufacturer s address to be the same format as printed on kit boxes. 4 09/06/2015 Removal of Pseudomonas aeruginosa products 5 07/01/2016 Reflect changes
Version Released Amendments 3 04/03/2015 Amend manufacturer s address to be the same format as printed on kit boxes. 4 09/06/2015 Removal of Pseudomonas aeruginosa products 5 07/01/2016 Reflect changes
Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18
![Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18 Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18](/thumbs/95/125595183.jpg) Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Rheumatoid Factor
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Rheumatoid Factor
Clinical Laboratory. [None
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
Anti-Nuclear Antibodies (ANA). (Incorporating Anti-double stranded DNA (dsdna) and Anti-Extractable Nuclear Antigen (ENA) Antibodies)
 Autoimmune Antibody Testing Points of Note: The interpretation of all autoantibody tests is highly dependent on the likelihood of disease in the patient. The results should always be interpreted with the
Autoimmune Antibody Testing Points of Note: The interpretation of all autoantibody tests is highly dependent on the likelihood of disease in the patient. The results should always be interpreted with the
AbBaltis. Product Catalogue. Contact us now for free samples!
 AbBaltis We are an award winning company supplying a wide variety of allergy and disease state human plasma and serum as well as clinical residual samples. Product Catalogue Additionally, we process blood
AbBaltis We are an award winning company supplying a wide variety of allergy and disease state human plasma and serum as well as clinical residual samples. Product Catalogue Additionally, we process blood
VGKC-Autoantibody Assay RIA
 Instructions for Use VGKC-Autoantibody Assay RIA 125I-Radio Immuno Assay for the Quantitative Determination of Antibodies to the Voltage-Gated Potassium Channel (VGKC) in Serum I V D REF RA115/25 25 2
Instructions for Use VGKC-Autoantibody Assay RIA 125I-Radio Immuno Assay for the Quantitative Determination of Antibodies to the Voltage-Gated Potassium Channel (VGKC) in Serum I V D REF RA115/25 25 2
PRODUCT CATALOG. All products are CE marked
 PRODUCT CATALOG 2017 All products are CE marked Content Company Profile 4 Allergy in-vitro Diagnostics Diagnostics for the IgE mediated Type I reaction (Immediate reaction) 6 Enzyme-Immuno-Assays on microtiterplates
PRODUCT CATALOG 2017 All products are CE marked Content Company Profile 4 Allergy in-vitro Diagnostics Diagnostics for the IgE mediated Type I reaction (Immediate reaction) 6 Enzyme-Immuno-Assays on microtiterplates
Immunoassay. Product Portfolio. Part of the IDS group
 Immunoassay Portfolio www.diametra.com info_diametra@idsplc.com Part of the IDS group Our Portfolio Cost-Effective Solutions for your Laboratory. All DiaMetra immunoassay kits in breakable well format.
Immunoassay Portfolio www.diametra.com info_diametra@idsplc.com Part of the IDS group Our Portfolio Cost-Effective Solutions for your Laboratory. All DiaMetra immunoassay kits in breakable well format.
University of Pretoria
 University of Pretoria Serodiagnostic Procedures Performed in the Department of Immunology Dr Pieter WA Meyer 1.Autoimmune Diseases Automated Anti-nuclear antibodies Anti-gliadin/ tissue transglutaminase
University of Pretoria Serodiagnostic Procedures Performed in the Department of Immunology Dr Pieter WA Meyer 1.Autoimmune Diseases Automated Anti-nuclear antibodies Anti-gliadin/ tissue transglutaminase
ELISA Range. VIROTECH Diagnostics GmbH. Lot independent. Reagent. System. IgG-Conjugate. Substrate. IgM-Conjugate. Washing Solution.
 Quelle: www.fotolia.com ELISA Range IgG-Conjugate IgM-Conjugate IgA-Conjugate Lot independent Reagent System Stop Solution Substrate Washing Solution Serum Dilution Dilution Buffer Incubation Time Cerebrospinal
Quelle: www.fotolia.com ELISA Range IgG-Conjugate IgM-Conjugate IgA-Conjugate Lot independent Reagent System Stop Solution Substrate Washing Solution Serum Dilution Dilution Buffer Incubation Time Cerebrospinal
Anti-TSH-Receptor ELISA
 Instructions for Use Anti-TSH-Receptor ELISA Enzyme Immuno Assay for the Quantitative Determination of TSH Receptor Autoantibodies (TRAb) in Serum I V D REF EA101/96 12 x 8 2 8 C DLD Gesellschaft für Diagnostika
Instructions for Use Anti-TSH-Receptor ELISA Enzyme Immuno Assay for the Quantitative Determination of TSH Receptor Autoantibodies (TRAb) in Serum I V D REF EA101/96 12 x 8 2 8 C DLD Gesellschaft für Diagnostika
ELISA. AccuDiag Autoimmune Diseases and Others. ANA Screen Qualitative ~135 min 90.4% 100%
 / AccuDiag Autoimmune Diseases and Others ANA Screen 5102-2 96 ~135 min 90.4% 100% ENA Profile-6 (Sm/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B) 2506-2 96 ~ 60 min 96% 100% ENA Combined Screen (6 antigens) Sm/RNP,
/ AccuDiag Autoimmune Diseases and Others ANA Screen 5102-2 96 ~135 min 90.4% 100% ENA Profile-6 (Sm/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B) 2506-2 96 ~ 60 min 96% 100% ENA Combined Screen (6 antigens) Sm/RNP,
DS-EIA-ANTI-HIV-UNIF (Ab) I DS-EIA-ANTI-HIV-UNIF (Ab) I DS-EIA-ANTI-HIV-UNIF (Ab) I
 * Sensitivity HIV DS-EIA-ANTI-HIV-UNIF (Ab) I-153 96 115 24 DS-EIA-ANTI-HIV-UNIF (Ab) I-150 192 115 24 DS-EIA-ANTI-HIV-UNIF (Ab) I-155 480 115 24 DS-EIA-HIV-AG-SCREEN I-1452 96 115 12 0.025 IU/ml DS-EIA-HIV-AGAB-SCREEN
* Sensitivity HIV DS-EIA-ANTI-HIV-UNIF (Ab) I-153 96 115 24 DS-EIA-ANTI-HIV-UNIF (Ab) I-150 192 115 24 DS-EIA-ANTI-HIV-UNIF (Ab) I-155 480 115 24 DS-EIA-HIV-AG-SCREEN I-1452 96 115 12 0.025 IU/ml DS-EIA-HIV-AGAB-SCREEN
Instructions for Use. IA2 Antibody ELISA. Enzyme Immuno Assay for the Quantitative Determination of Antibodies against IA-2 in Serum.
 Instructions for Use IA2 Antibody ELISA Enzyme Immuno Assay for the Quantitative Determination of Antibodies against IA-2 in Serum I V D REF EA114/96 12 x 8 2 8 C DLD Gesellschaft für Diagnostika und medizinische
Instructions for Use IA2 Antibody ELISA Enzyme Immuno Assay for the Quantitative Determination of Antibodies against IA-2 in Serum I V D REF EA114/96 12 x 8 2 8 C DLD Gesellschaft für Diagnostika und medizinische
Simultaneous comprehensive multiplex autoantibody analysis by CytoBead technology for Rapidly Progressive Glomerulonephritis.
 Simultaneous comprehensive multiplex autoantibody analysis by CytoBead technology for Rapidly Progressive Glomerulonephritis l Assays Indirect Immunofluorescence Goldstandard for Diagnosis of Autoimmune
Simultaneous comprehensive multiplex autoantibody analysis by CytoBead technology for Rapidly Progressive Glomerulonephritis l Assays Indirect Immunofluorescence Goldstandard for Diagnosis of Autoimmune
Autoantibodies panel ANA
 Autoantibodies panel ANA Anti-nuclear antibodies, ANA screening General: Anti-nuclear antibodies (ANA) contain all kinds of autoantibodies against nuclear antigens. Their targets are cell components in
Autoantibodies panel ANA Anti-nuclear antibodies, ANA screening General: Anti-nuclear antibodies (ANA) contain all kinds of autoantibodies against nuclear antigens. Their targets are cell components in
This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma tests/panels.
 February 20, 2017 Client Letter Test Update February 2017 Dear Colleague: This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma
February 20, 2017 Client Letter Test Update February 2017 Dear Colleague: This month, we are very pleased to introduce some new tests for Scleroderma as well as some test changes to our existing scleroderma
See external label 96 tests HSV 2 IgA. Cat #
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Controls & Calibrators. Disease Quality Controls
 Controls & Calibrators Infectious Disease Quality Controls Infectious Disease Quality Controls A broad selection of controls designed to monitor assay precision of hepatitis, retrovirus, sexually transmitted
Controls & Calibrators Infectious Disease Quality Controls Infectious Disease Quality Controls A broad selection of controls designed to monitor assay precision of hepatitis, retrovirus, sexually transmitted
Autoimmune bullous dermatoses
 Autoimmune bullous dermatoses Overview of serological diagnostics in autoimmune blister-forming diseases of the skin Pemphigoid diseases Pemphigus diseases Epidermolysis bullosa acquisita Dermatitis herpetiformis
Autoimmune bullous dermatoses Overview of serological diagnostics in autoimmune blister-forming diseases of the skin Pemphigoid diseases Pemphigus diseases Epidermolysis bullosa acquisita Dermatitis herpetiformis
Herpes Simplex Virus 2 IgM HSV 2 IgM
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
TSH Receptor Autoantibody ELISA
 Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it TSH Receptor Autoantibody ELISA Product Data Sheet Cat.
Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it TSH Receptor Autoantibody ELISA Product Data Sheet Cat.
AccuSet Autoimmune Performance Panel
 PACKAGE INSERT 0840-0001 INTENDED USE The 0840-0001 is intended for use by diagnostic manufacturers, researchers, and clinical laboratories to develop, evaluate, or troubleshoot autoimmune related test
PACKAGE INSERT 0840-0001 INTENDED USE The 0840-0001 is intended for use by diagnostic manufacturers, researchers, and clinical laboratories to develop, evaluate, or troubleshoot autoimmune related test
HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2017Apr19
 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2017Apr19
National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2017Apr19
June EQA schemes closed. Information on sample properties. Prof. Dr. Heinz Zeichhardt. Dr. Martin Kammel
 June 2018 EQA schemes closed Information on sample properties Prof. Dr. Heinz Zeichhardt Dr. Martin Kammel Issued by: INSTAND Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laborarien
June 2018 EQA schemes closed Information on sample properties Prof. Dr. Heinz Zeichhardt Dr. Martin Kammel Issued by: INSTAND Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laborarien
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital
 Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
EBV-EA IgG. Cat # 1415Z. EBV -EA IgG ELISA. ELISA: Enzyme Linked Immunosorbent Assay. ELISA - Indirect; Antigen Coated Plate
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
GAD 65 Antibody ELISA
 Instructions for Use GAD 65 Antibody ELISA Enzyme Immuno Assay for the Quantitative Determination of Antibodies against Glutamic Acid Decarboxylase in Serum I V D REF EA104/96 12 x 8 2 8 C DLD Gesellschaft
Instructions for Use GAD 65 Antibody ELISA Enzyme Immuno Assay for the Quantitative Determination of Antibodies against Glutamic Acid Decarboxylase in Serum I V D REF EA104/96 12 x 8 2 8 C DLD Gesellschaft
ELISA kits for infections (HIV; Hepatitis A, B, C, D, E; Syphilis, ToRCH and others), hormone pathologies and tumor markers.
 DSI S.r.l. DSI S.r.l. is one of the European leaders in the field of immunodiagnostic tests manufacturing. We are a research-focused company with the long-term microbiological scientific traditions. Our
DSI S.r.l. DSI S.r.l. is one of the European leaders in the field of immunodiagnostic tests manufacturing. We are a research-focused company with the long-term microbiological scientific traditions. Our
COMPLIANCE. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test. September 2018.
 September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
ANA Screen ELISA Kit. Cat. No.:DEIA3138 Pkg.Size:96T. Intended use. General Description. Principle Of The Test. Reagents And Materials Provided
 ANA Screen ELISA Kit Cat. No.:DEIA3138 Pkg.Size:96T Intended use The ANA Screen assay is a qualitative enzyme immunoassay (EIA) intended to screen for the presence of antinuclear antibodies (ANAs) in human
ANA Screen ELISA Kit Cat. No.:DEIA3138 Pkg.Size:96T Intended use The ANA Screen assay is a qualitative enzyme immunoassay (EIA) intended to screen for the presence of antinuclear antibodies (ANAs) in human
MP Biomedicals Asia Pacific Pte. Ltd. (formerly Genelabs Diagnostics Pte. Ltd.)
 Revision: 12 May 2005 1 WESTERN BLOT / IMMUNOBLOT HIV-1 BLOT Version 1.3* 11010-018 HIV-1 viral lysate Western Blot assay for the 11010-036 detection of antibodies to HIV-1 with serum 11010-108 108 strips
Revision: 12 May 2005 1 WESTERN BLOT / IMMUNOBLOT HIV-1 BLOT Version 1.3* 11010-018 HIV-1 viral lysate Western Blot assay for the 11010-036 detection of antibodies to HIV-1 with serum 11010-108 108 strips
June Pre-evaluation of the External Quality Assessment Schemes in Virus Diagnostics. Prof. Dr. Heinz Zeichhardt. Dr.
 June 2018 Pre-evaluation of the External Quality Assessment Schemes in Virus Diagnostics Prof. Dr. Heinz Zeichhardt Dr. Martin Kammel Issued by: INSTAND Gesellschaft zur Förderung der Qualitätssicherung
June 2018 Pre-evaluation of the External Quality Assessment Schemes in Virus Diagnostics Prof. Dr. Heinz Zeichhardt Dr. Martin Kammel Issued by: INSTAND Gesellschaft zur Förderung der Qualitätssicherung
Reflex Test Protocols
 Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive polyspecific DAT IgG DAT, C3 DAT Fetal Cell
H. pylori IgM CLIA kit
 H. pylori IgM CLIA kit Cat. No.:DEEL0251 Pkg.Size:96 tests Intended use Helicobacter pylori IgM Chemiluminescence ELISA is intended for use in evaluating the serologic status to H. pylori infection in
H. pylori IgM CLIA kit Cat. No.:DEEL0251 Pkg.Size:96 tests Intended use Helicobacter pylori IgM Chemiluminescence ELISA is intended for use in evaluating the serologic status to H. pylori infection in
IBD Tools to Aid in the Accurate Diagnosis of Inflammatory Bowel Disease
 IBD Tools to Aid in the Accurate Diagnosis of Inflammatory Bowel Disease Inflammatory Bowel Disease Experts in Autoimmunity Inova Diagnostics offers a complete array of methods to aid in the diagnosis
IBD Tools to Aid in the Accurate Diagnosis of Inflammatory Bowel Disease Inflammatory Bowel Disease Experts in Autoimmunity Inova Diagnostics offers a complete array of methods to aid in the diagnosis
Accredited entity according to ČSN EN ISO 15189:2013 Laboratoře Mikrochem a.s. Mikrochem Laboratories Nezvalova 984/2, Hodolany, Olomouc
 Medical laboratory locations: 1. Olomouc Nezvalova 984/2, 779 00 Olomouc 2. Přerov Čechova 8, 750 02 Přerov 3. Šumperk Nerudova 34, 787 01 Šumperk 4. Valašské Meziříčí Vsetínská 854, 757 01 Valašské Meziříčí
Medical laboratory locations: 1. Olomouc Nezvalova 984/2, 779 00 Olomouc 2. Přerov Čechova 8, 750 02 Přerov 3. Šumperk Nerudova 34, 787 01 Šumperk 4. Valašské Meziříčí Vsetínská 854, 757 01 Valašské Meziříčí
Primary Sample Manual Immunology Issue No Effective Date: 06/11/17 Page 1 of 19 EUROFINS BIOMNIS. Pr. Conleth Feighery, Immunology Consultant
 Issue No. 2.02 Effective Date: 06/11/17 Page 1 of 19 Written / Revised By: Barry O Dea, Medical Scientist Date: Reviewed By: Date: Pr. Conleth Feighery, Immunology Consultant Authorised By: Jean-Sébastien
Issue No. 2.02 Effective Date: 06/11/17 Page 1 of 19 Written / Revised By: Barry O Dea, Medical Scientist Date: Reviewed By: Date: Pr. Conleth Feighery, Immunology Consultant Authorised By: Jean-Sébastien
Panel # Panel CPT Code Price 2010 AUTOIMMUNE PROFILE - BASIC 99.00
 2010 AUTOIMMUNE PROFILE - BASIC 99.00 Anti-Nuclear Antibody (ANA) 86038 33.00 Rheumatoid Factor 86431 33.00 C1Q Immune Complex 86332 33.00 2011 AUTOIMMUNE PROFILE (COMPREHENSIVE) 210.00 Anti-Nuclear Antibody
2010 AUTOIMMUNE PROFILE - BASIC 99.00 Anti-Nuclear Antibody (ANA) 86038 33.00 Rheumatoid Factor 86431 33.00 C1Q Immune Complex 86332 33.00 2011 AUTOIMMUNE PROFILE (COMPREHENSIVE) 210.00 Anti-Nuclear Antibody
QUANTA Lite TM ANA ELISA
 Format to CLSI Standards GP2-A5 (Formerly NCCLS) Vol. 26 No. 12 Issue Date: 08/16/11 QUANTA Lite TM ANA ELISA 708750 For In Vitro Diagnostic Use CLIA Complexity: High Principles of the Procedure Highly
Format to CLSI Standards GP2-A5 (Formerly NCCLS) Vol. 26 No. 12 Issue Date: 08/16/11 QUANTA Lite TM ANA ELISA 708750 For In Vitro Diagnostic Use CLIA Complexity: High Principles of the Procedure Highly
H.Pylori IgG
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
H.Pylori IgG Cat # 1503Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC.
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
See external label 2 C-8 C Σ=96 tests Cat # EBV-VCA IgA. Cat # EBV -VCA IgA ELISA. ELISA: Enzyme Linked Immunosorbent Assay
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
H. pylori IgM. Cat # H. pylori IgM ELISA. ELISA: Enzyme Linked Immunosorbent Assay. ELISA - Indirect; Antigen Coated Plate
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com H. pylori
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com H. pylori
Toxoplasma gondii IgM (Toxo IgM)
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
INSTAND e.v. in cooperation with:
 Pre-evaluation of the EQA Schemes in Virus Diagnostics June 2015 Corrected version: 10 August 2015 (See Table 3; program 346) INSTAND e.v. in cooperation with: Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten
Pre-evaluation of the EQA Schemes in Virus Diagnostics June 2015 Corrected version: 10 August 2015 (See Table 3; program 346) INSTAND e.v. in cooperation with: Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten
.01_2018 A FIRENZE 2018_DEPL.1000 REV
 diagnostica senese CATALOGUE 2018 CATALOGUE 2018 DIESSE DIAGNOSTICA SENESE SPA Sales Office Via del Pozzo, 5 Loc. S. Martino 53035 Monteriggioni (SI) ITALY T. +39 0577 319560 / 61/ 50 F. +39 0577 318763
diagnostica senese CATALOGUE 2018 CATALOGUE 2018 DIESSE DIAGNOSTICA SENESE SPA Sales Office Via del Pozzo, 5 Loc. S. Martino 53035 Monteriggioni (SI) ITALY T. +39 0577 319560 / 61/ 50 F. +39 0577 318763
HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2016Oct28
 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2016Oct28
National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2016Oct28
See external label 2 C-8 C 96 tests Chemiluminescence. CMV IgM. Cat # Diluted samples, controls & calibrator 100 µl 30 minutes
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Schedule of Accreditation
 Schedule of Accreditation Organisation Name National Virus Reference Laboratory INAB Reg No 326MT Contact Name Eimear Malone Address University College Dublin, Belfield, Dublin Contact Phone No 01-7161319
Schedule of Accreditation Organisation Name National Virus Reference Laboratory INAB Reg No 326MT Contact Name Eimear Malone Address University College Dublin, Belfield, Dublin Contact Phone No 01-7161319
Instructions for Use. IA-2 Antibody RIA. for the Quantitative Determination of Antibodies against Protein-Tyrosine-Phosphatase (IA2) in Serum
 Instructions for Use IA-2 Antibody RIA 125I-Radioimmunoassay for the Quantitative Determination of Antibodies against Protein-Tyrosine-Phosphatase (IA2) in Serum REF RA101/50 RA104/100 50 100 2 8 C I V
Instructions for Use IA-2 Antibody RIA 125I-Radioimmunoassay for the Quantitative Determination of Antibodies against Protein-Tyrosine-Phosphatase (IA2) in Serum REF RA101/50 RA104/100 50 100 2 8 C I V
ENA screen (Extractable Nuclear Antigen) ELISA
 ENA screen (Extractable Nuclear Antigen) ELISA Catalog Number: ENA31-K01 Enzyme immunoassay for the determination of IgG antibodies to nuclear and cytoplasmic antigens in human serum and plasma. 20A Northwest
ENA screen (Extractable Nuclear Antigen) ELISA Catalog Number: ENA31-K01 Enzyme immunoassay for the determination of IgG antibodies to nuclear and cytoplasmic antigens in human serum and plasma. 20A Northwest
H.Pylori IgM Cat # 1504Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
FinTest IgG4 Screen 20 ELISA KIT
 FinTest IgG4 Screen 20 ELISA KIT Cat. No.:DEIA6196 Pkg.Size:96T Intended use Enzyme immunoassay (microtiter strips) for the detection and the quantitative determination of IgG4 antibodies against 20 Food
FinTest IgG4 Screen 20 ELISA KIT Cat. No.:DEIA6196 Pkg.Size:96T Intended use Enzyme immunoassay (microtiter strips) for the detection and the quantitative determination of IgG4 antibodies against 20 Food
Advances in Autoantibody Testing & Clinical Applications
 Advances in Autoantibody Testing & Clinical Applications Marvin J. Fritzler PhD MD Member: IUIS-WHO-AF-CDC Serology Committee Director: Advanced Diagnostics Laboratory University of Calgary Introduction
Advances in Autoantibody Testing & Clinical Applications Marvin J. Fritzler PhD MD Member: IUIS-WHO-AF-CDC Serology Committee Director: Advanced Diagnostics Laboratory University of Calgary Introduction
H.pylori IgA Cat #
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Detection of Anti-nuclear Antibodies in Women with Hyperprolactinaemia
 Detection of Anti-nuclear Antibodies in Women with Hyperprolactinaemia Salahdeen Ismail Mohammed 12, Alaaeldeen Balal Ahmed 1, Nuha Abdurhaman 2, Abdalla Hassan Sharief 2 1 Faculty of Medical Laboratory
Detection of Anti-nuclear Antibodies in Women with Hyperprolactinaemia Salahdeen Ismail Mohammed 12, Alaaeldeen Balal Ahmed 1, Nuha Abdurhaman 2, Abdalla Hassan Sharief 2 1 Faculty of Medical Laboratory
HSV-1 IgM ELISA. Catalog No (96 Tests) For Research Use Only. Not for use in Diagnostic Procedures.
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. HSV-1 IgM ELISA Kit is intended for the detection of IgM antibody to HSV-1 in human serum or plasma. SUMMARY AND
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. HSV-1 IgM ELISA Kit is intended for the detection of IgM antibody to HSV-1 in human serum or plasma. SUMMARY AND
Abnormal test results, interpretive comments and communication of results
 Abnormal test results, interpretive comments and communication of results Explain and justify an appropriate approach to the communication of abnormal test results to the ordering clinician 1.7.1.1. Define
Abnormal test results, interpretive comments and communication of results Explain and justify an appropriate approach to the communication of abnormal test results to the ordering clinician 1.7.1.1. Define
THROMBOSIS PRODUCT HIGHLIGHTS
 PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS has extensive experience and know-how in the Anti-Phospholipid Syndrome (APS) field and offers the most comprehensive panel of anti-phospholipid tests, employing highly
PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS has extensive experience and know-how in the Anti-Phospholipid Syndrome (APS) field and offers the most comprehensive panel of anti-phospholipid tests, employing highly
NOVA Lite ANCA (Ethanol) Kit with DAPI For In Vitro Diagnostic Use CLIA Complexity: High
 Format to CLSI Standards GP2-A5 (Formerly NCCLS) Vol. 26 No. 12 Issue Date: 09/11/13 NOVA Lite ANCA (Ethanol) 708301 Kit with DAPI For In Vitro Diagnostic Use CLIA Complexity: High Principles of the Procedure
Format to CLSI Standards GP2-A5 (Formerly NCCLS) Vol. 26 No. 12 Issue Date: 09/11/13 NOVA Lite ANCA (Ethanol) 708301 Kit with DAPI For In Vitro Diagnostic Use CLIA Complexity: High Principles of the Procedure
NEPHROCHECK Liquid Control Kit Package Insert
 NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
Autoantibodies in the Idiopathic Inflammatory Myopathies
 Autoantibodies in the Idiopathic Inflammatory Myopathies Steven R. Ytterberg, M.D. Division of Rheumatology Mayo Clinic Rochester, MN The Myositis Association Annual Conference St. Louis, MO Sept. 25,
Autoantibodies in the Idiopathic Inflammatory Myopathies Steven R. Ytterberg, M.D. Division of Rheumatology Mayo Clinic Rochester, MN The Myositis Association Annual Conference St. Louis, MO Sept. 25,
Zinc Transporter 8 (ZnT8) Antibody ELISA
 Instructions for Use Zinc Transporter 8 (ZnT8) Antibody ELISA Enzyme Immuno Assay for the Quantitative Determination of ZnT8 Autoantibodies in Serum I V D REF EA102/96 12 x 8 2 8 C DLD Gesellschaft für
Instructions for Use Zinc Transporter 8 (ZnT8) Antibody ELISA Enzyme Immuno Assay for the Quantitative Determination of ZnT8 Autoantibodies in Serum I V D REF EA102/96 12 x 8 2 8 C DLD Gesellschaft für
Herpes Simplex Virus 2 IgG HSV 2 IgG
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
See external label 2 C-8 C 96 tests CHEMILUMINESCENCE. CMV IgG. Cat # Step (20-25 C Room temp.) Volume
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
ANA Diagnostics Using Indirect Immunofluorescence
 ANA Diagnostics Using Indirect Immunofluorescence EUROIMMUN D-23560 Luebeck (Germany) Seekamp 31 Tel +49 45158550 Fax 5855591 E-mail euroimmun@euroimmun.de Table of Contents Autoantibodies against cell
ANA Diagnostics Using Indirect Immunofluorescence EUROIMMUN D-23560 Luebeck (Germany) Seekamp 31 Tel +49 45158550 Fax 5855591 E-mail euroimmun@euroimmun.de Table of Contents Autoantibodies against cell
CHEMILUMINESCENCE ENZYME IMMUNOASSAY (CLIA) Toxoplasma IgG. Cat # (20-25 C Room temp.) Volume
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 21 September 2006 EMEA/CHMP/BWP/298390/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 21 September 2006 EMEA/CHMP/BWP/298390/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON
Immunologicals Bulk Product Listing
 Immunologicals Bulk Product Listing The listing below is a guide to the range of products available from Immunologicals. Although our primary focus is on the supply of materials in quantities optimal to
Immunologicals Bulk Product Listing The listing below is a guide to the range of products available from Immunologicals. Although our primary focus is on the supply of materials in quantities optimal to
Reflex Test Protocols
 UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
UCH Clinical Laboratory Reflex Test Protocols Blood Bank Prepare RBCs for Transfusion (aka Crossmatch) N/A No Antibody Screen ordered Antibody Screen Direct Antiglobulin Test (DAT) Polyspecific DAT Positive
HIV Serology Quality Assessment Program Summary for Panel HIVSER 2017Apr19
 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory Public Health Agency of Canada HIV Serology Quality Assessment Program Summary
National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory Public Health Agency of Canada HIV Serology Quality Assessment Program Summary
Annual Report
 NATIONAL SYMPOSIUM on NON-INFECTIOUS SEROLOGY External Quality Evaluation (EQE) Non-Infectious Serology Annual Report 2009-2014 Aalst - June 6th 2015 M. Van Blerk & C. Van Campenhout Rue Juliette Wytsmanstraat
NATIONAL SYMPOSIUM on NON-INFECTIOUS SEROLOGY External Quality Evaluation (EQE) Non-Infectious Serology Annual Report 2009-2014 Aalst - June 6th 2015 M. Van Blerk & C. Van Campenhout Rue Juliette Wytsmanstraat
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx 0244-055-00 OraQuick HCV Rapid Antibody Test Kit with product codes 1001-0270
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx 0244-055-00 OraQuick HCV Rapid Antibody Test Kit with product codes 1001-0270
ELEGANCE Chlamydia pneumoniae IgG ELISA KIT
 INTENDED USE The ELEGANCE Chlamydia pneumoniae IgG ELISA has been designed for the in vitro diagnostic measurement of anti- Chlamydia pneumoniae IgG in the screening of human serum. PRINCIPLES OF THE ELEGANCE
INTENDED USE The ELEGANCE Chlamydia pneumoniae IgG ELISA has been designed for the in vitro diagnostic measurement of anti- Chlamydia pneumoniae IgG in the screening of human serum. PRINCIPLES OF THE ELEGANCE
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Anti-CCP2 testing. The Gold Standard in Diagnosis of Rheumatoid Arthritis. 1 of 11 P a g e
 Anti-CCP2 testing The Gold Standard in Diagnosis of Rheumatoid Arthritis 1 of 11 P a g e 2014-01- 07 Contents 1. Scope... 3 2. CCP2: Definition and Background... 3 3. The market for CCP2 assays... 3 4.
Anti-CCP2 testing The Gold Standard in Diagnosis of Rheumatoid Arthritis 1 of 11 P a g e 2014-01- 07 Contents 1. Scope... 3 2. CCP2: Definition and Background... 3 3. The market for CCP2 assays... 3 4.
Laboratory Diagnosis of Viral Infections. G. Jamjoom 2005
 Laboratory Diagnosis of Viral Infections G. Jamjoom 2005 Five Main Techniques: Virus Culture and Isolation Serology Rapid Detection of Viral Antigens Detection of Viral Nucleic Acid Electron Microscopy
Laboratory Diagnosis of Viral Infections G. Jamjoom 2005 Five Main Techniques: Virus Culture and Isolation Serology Rapid Detection of Viral Antigens Detection of Viral Nucleic Acid Electron Microscopy
Mycoplasma pneumoniae IgG ELISA Kit
 Mycoplasma pneumoniae IgG ELISA Kit Catalog Number KA2260 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Mycoplasma pneumoniae IgG ELISA Kit Catalog Number KA2260 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
RFQ:-HPCSA 03/2017 REQUEST FOR QUOTATION FOR OCCUPATIONAL HEALTH SERVICE ON BEHALF OF THE HEALTH PROFESSIONS COUNCIL OF SOUTH AFRICA
 RFQ:-HPCSA 03/2017 REQUEST FOR QUOTATION FOR OCCUPATIONAL HEALTH SERVICE ON BEHALF OF THE HEALTH PROFESSIONS COUNCIL OF SOUTH AFRICA Situated at: 553 Madiba Street, Arcadia, Pretoria Deadline for submission:
RFQ:-HPCSA 03/2017 REQUEST FOR QUOTATION FOR OCCUPATIONAL HEALTH SERVICE ON BEHALF OF THE HEALTH PROFESSIONS COUNCIL OF SOUTH AFRICA Situated at: 553 Madiba Street, Arcadia, Pretoria Deadline for submission:
HIV-1 ICx/CRx Kit Reagents for Immune Dissociation and Reactivity Confirmation
 INTENDED USE The RETRO-TEK HIV-1 IC x/cr x Kit is supplied for research purposes only. It is not intended for use in the diagnosis or prognosis of disease or for screening and may not be used as a confirmatory
INTENDED USE The RETRO-TEK HIV-1 IC x/cr x Kit is supplied for research purposes only. It is not intended for use in the diagnosis or prognosis of disease or for screening and may not be used as a confirmatory
HIV Viral Load Quality Assessment Program Summary for Panel HIVVL 2017Oct27
 1 The National Laboratory for HIV Reference Services is Accredited to ISO 15189 and ISO 17043 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology
1 The National Laboratory for HIV Reference Services is Accredited to ISO 15189 and ISO 17043 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology
Measurement of Antinuclear Antibodies: Assessment of Different Test Systems
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Jan. 2000, p. 72 78 Vol. 7, No. 1 1071-412X/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Measurement of Antinuclear
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Jan. 2000, p. 72 78 Vol. 7, No. 1 1071-412X/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Measurement of Antinuclear
See external label 2 C-8 C Σ=96 tests Cat # 1505Z. MICROWELL ELISA H.Pylori IgA Cat # 1505Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Name and Intended Use
 Name and Intended Use ENA screen is an indirect solid phase enzyme immunoassay (ELISA) for the qualitative screening of IgG class autoantibodies against extractable nuclear antigens (ENA) in human serum
Name and Intended Use ENA screen is an indirect solid phase enzyme immunoassay (ELISA) for the qualitative screening of IgG class autoantibodies against extractable nuclear antigens (ENA) in human serum
