ORDER INFORMATION AND PRODUCT DATA. Immunoglobulin Class A: IgA E: IgE G: IgG Product Code M: IgM O: IgGM Product Classification
|
|
|
- Blanche Doyle
- 6 years ago
- Views:
Transcription
1 ORDER INFORMATION AND PRODUCT DATA FI G 1601: 16 single test strips 1208: 12 microplate strips with 8 wells each 9601: 96 individual break-apart wells (12 microplate strips with 8 wells each) 5001: 50 individual tubes 0001: 100 individual tubes 1003: 10 slides with 3 fields each 1005: 10 slides with 5 fields each 2005: 20 slides with 5 fields each 1010: 10 slides with 10 fields each 1050: 10 slides with 50 fields each 1096: 10 slides with 96 fields each 1001: 10 slides to be incubated with 1 patient serum each 1002: 10 slides to be incubated with 2 patient sera each Immunoglobulin Class A: IgA E: IgE G: IgG Product Code M: IgM O: IgGM Product Classification P: Polyvalent (IgM) AF: Fluorescence-labelled antibodies for EUROIMMUN IIFT X: low-avide antibodies of class IgG CA: Control for EUROIMMUN IIFT (autoimmunity) H: high-avide antibodies of class IgG CI: Control for EUROIMMUN IIFT (infectious serology) CV: Control for EUROIMMUN IIFT (determination of further antibodies) CW: Control for EUROIMMUN Westernblot/EUROLINE-WB DA: EUROASSAY test system (autoimmunity) DL: EUROLINE test system (autoimmunity) DN: EUROLINE test system (infectious serology) DP: EUROLINE test system (allergology) DW: Westernblot/EUROLINE-WB test system (autoimmunity) DY: Westernblot/EUROLINE-WB test system (infectious serology) EA: Microplate ELISA test system (autoimmunity) EI: Microplate ELISA test system (infectious serology) EP: Microplate ELISA test system (allergology) EQ: Microplate ELISA test system (serum proteins and tumour markers) EV: Microplate ELISA test system (determination of further antibodies) FA: Test system for indirect immunofluorescence (autoimmunity) FB: Single slides for indirect immunofluorescence (autoimmunity) FI: Test system for indirect immunofluorescence (infectious serology) FK: Single slides for indirect immunofluorescence (infectious serology) FV: Test system for indirect immunofluorescence (determination of further antibodies) FX: Single slides for indirect immunofluorescence (determination of further antibodies) MN: EUROArray for Molecular Genetic Determinations RA: Radioimmunoassay (autoimmunity) RD: Radioimmunoassay (direct detection of autoantigens, hormone determination) ZF: Further reagents for EUROIMMUN IIFT ZZ: Other items for EUROIMMUN IIFT When placing an order, please indicate quantity, order number and designation of the required product. Examples: 2 x FA liver, monkey (2 test systems with 10 slides each, 10 fields per slide) 1 x FA kidney, rat / stomach, rat (1 test system with 20 slides, 5 fields per slide) 2 x FI G Measles virus (2 IgG class test systems with 10 slides each, 3 fields per slide) Test systems comprise all reagents needed to perform the serological investigation, for diagnostics in indirect immunofluorescence, e.g. slides, FITC-labelled antibodies human immunoglobulin, positive and negative control sera (not available for some products) as well as embedding medium, cover glasses, sachets of PBS and Tween 20. EUROSORB for the determination of IgM class antibodies is not included in the immunofluorescence test systems. Single slides are supplied with cover glasses only. Substrates consisting of cell cultures and tissues which do not appear in this catalogue can be made to specification. In addition, BIOCHIP Mosaics can be produced according to individual requirements. Apart from the customary package sizes and slide formats, special sizes are available as well. Quotations can be provided upon request. 62
2 Fluorescence-Labelled : Fluorescein (FITC) for EUROIMMUN IIFT Antiserum AF FITC-labelled anti-human IgA 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) AF FITC-labelled anti-human IgG 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) AF FITC-labelled anti-human IgM 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) AF FITC-labelled anti-human IgM 1.5 ml (ready for use) AF (IgA + IgG + IgM goat) 6.0 ml (ready for use) AF FITC-labelled anti-human Ig 1.5 ml (ready for use) AF (IgA + IgG goat) 6.0 ml (ready for use) AF FITC-labelled anti-human C3c 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) AF FITC-labelled anti-human IgG 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) primate absorbed AF FITC-labelled anti-human IgA 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) with Evans Blue AF FITC-labelled anti-human IgG 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) with Evans Blue AF FITC-labelled anti-human IgM 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) with Evans Blue AF FITC-labelled anti-human IgM 1.5 ml (ready for use) AF (IgA + IgG + IgM goat) 6.0 ml (ready for use) with Evans Blue AF FITC-labelled anti-human Ig 1.5 ml (ready for use) AF (IgA + IgG goat) 6.0 ml (ready for use) with Evans Blue Fluorescence- Labelled AF FITC-labelled anti-human C3c 1.5 ml (ready for use) AF (goat) 6.0 ml (ready for use) with Evans Blue 63
3 Controls for EUROIMMUN IIFT: Organ-Specific Autoantibodies Control (Ready for Use) CA autoantibody-free control IgG 0.1 ml (aab negative) CA antibodies thyroid microsomes IgG 0.1 ml and thyroglobulin (MAb + TAb control) CA antibodies thyroid microsomes IgG 0.1 ml (MAb control) CA antibodies thyroglobulin IgG 0.1 ml (TAb control) CA antibodies pancreas islets IgG 0.1 ml (islet cell ab control) CA antibodies pancreas islets IgG 0.1 ml control with indication of titer and JDF units CA antibodies glutamic acid decarboxylase IgG 0.1 ml (GAD ab control) CA antibodies parathyroid gland IgG 0.1 ml (parathyroid gland ab control) CA antibodies adrenal cortex IgG 0.1 ml (adrenal cortex ab control) CA antibodies ovary: theca cells IgG 0.1 ml (ovary ab control) CA antibodies ovary: zona pellucida IgG 0.1 ml (zona pellucida ab control) Controls for the Determination of Autoantibodies CA antibodies testis: Leydig cells IgG 0.1 ml (testis ab control) CA antibodies spermatozoa IgG 0.1 ml (spermatozoa ab control) CA antibodies pituitary gland antigens IgG 0.1 ml (pituitary gland ab control) CA antibodies brain: grey matter IgG 0.1 ml (stiff man syndrome; SMS control) CA antibodies Purkinje cell cytoplasm IgG 0.1 ml (Yo ab control) CA antibodies neurone nuclei: Ri antigen IgG 0.1 ml (Ri ab control) CA antibodies neurone nuclei: Hu antigen IgG 0.1 ml (Hu ab control) CA antibodies myelin IgG 0.1 ml (myelin ab control) CA antibodies myelin-associated IgG 0.1 ml glycoprotein (M ab control) CA antibodies neuroendothelium IgG 0.1 ml (neuroendothelium ab control) CA antibodies neurofilaments IgG 0.1 ml (neurofilament ab control) CA antibodies non-medullated nerves IgG 0.1 ml (non-medullated nerves ab control) CA antibodies granulocyte cytoplasm, IgG 0.1 ml cytoplasmic pattern (canca control, PR3-ab control) CA antibodies granulocyte cytoplasm, IgG 10 x 60 µl, ready for use CA aliquots from positive control for ANCA diagnostics 80 x 60 µl, ready for use (10 different fluorescence patterns) CA antibodies granulocyte cytoplasm, cytoplasmic pattern IgG 0.1 ml (canca control), control serum with indication of titer 64
4 Controls for EUROIMMUN IIFT: Organ-Specific Autoantibodies Control (Ready for Use) CA antibodies granulocyte cytoplasm, perinuclear pattern IgG 0.1 ml (panca control), control serum with indication of titer CA antibodies granulocyte cytoplasm, perinuclear pattern IgG 0.1 ml (panca control, MPO ab control) CA antibodies granulocyte cytoplasm, perinuclear pattern IgG 0.1 ml (panca control, MPO ab control) control serum with indication of titer CA antibodies granulocyte cytoplasm, IgG 0.1 ml perinuclear pattern (xanca control) CA antibodies thrombocytes IgG 0.1 ml (thrombocyte ab control) CA antibodies renal glomeruli IgG 0.1 ml (Goodpasture syndrome; GBM ab control) CA antibodies renal tubuli IgG 0.1 ml (TBM ab control) CA antibodies alveolar basement membrane IgG 0.1 ml (ABM ab control) CA antibodies liver-specific protein IgG 0.1 ml (LSP ab control) CA antibodies liver membrane IgG 0.1 ml (LMA control) CA M antibodies bile ducts IgM 0.1 ml (bile ducts IgM control) CA antibodies bile canaliculi IgG 0.1 ml (bile canaliculi ab control) CA antibodies liver-kidney microsomes IgG 0.1 ml (LKM ab control) CA antibodies parietal cells IgG 0.1 ml (PCA control) CA antibodies intrinsic factor IgG 0.1 ml (intrinsic factor ab control) CA antibodies parietal cells + intrinsic factor IgG 0.1 ml (PCA + intrinsic factor ab control) Controls for the Determination of Autoantibodies CA A antibodies intestinal goblet cells IgA 0.1 ml (ulcerative colitis; UC IgA control) CA G antibodies intestinal goblet cells IgG 0.1 ml (ulcerative colitis; UC IgG control) CA antibodies pancreas acini IgG 0.1 ml (Crohn s disease 1 control) CA antibodies pancreas secretion IgG 0.1 ml (Crohn s disease 2 control) CA antibodies striated muscles IgG 0.1 ml (myasthenia gravis control) CA antibodies striation IgG 0.1 ml (heart 1 ab control) CA antibodies intercalated discs IgG 0.1 ml (heart 2 ab control) CA antibodies epidermis: desmosomes IgG 0.1 ml (pemphigus vulgaris control) CA antibodies epidermis: basement membrane IgG 0.1 ml (bullous pemphigoid control) CA antibodies keratin IgG 0.1 ml (filaggrin ab control) 65
5 Controls for EUROIMMUN IIFT: Systemic Autoantibodies Control (Ready for Use) CA antibodies cell nuclei IgG 0.1 ml (ANA control), homogeneous pattern CA antibodies cell nuclei (ANA control), IgG 8 aliquots concentrate 50 µl each CA homogeneous pattern, control serum with 96 aliquots concentrate 50 µl each indication of titer, reference serum W 1064 (66/233) CA antibodies cell nuclei, IgG 20 x 60 µl, ready for use CA aliquots from positive control for ANA diagnostics 80 x 60 µl, ready for use (20 different fluorescence patterns) CA antibodies cell nuclei (ANA control), IgG 0.1 ml homogeneous pattern control serum with indication of titer CA antibodies dsdna IgG 0.1 ml (dsdna ab control) CA antibodies cell nuclei IgG 0.1 ml (ANA control), nucleolar pattern CA antibodies RNA IgG 0.1 ml (RNA ab control) CA antibodies cell nuclei IgG 0.1 ml (ANA control), granular pattern CA antibodies U1-nRNP IgG 0.1 ml CA antibodies Sm IgG 0.1 ml CA antibodies SS-A (Ro) IgG 0.1 ml Controls for the Determination of Autoantibodies CA antibodies SS-B (La) IgG 0.1 ml CA antibodies Scl-70 IgG 0.1 ml CA antibodies PCNA (cyclin I) IgG 0.1 ml CA antibodies mitosin (cyclin II) IgG 0.1 ml CA antibodies Sp100 (nuclear granula, IgG 0.1 ml nuclear dots; PBC-associated) CA antibodies Ku IgG 0.1 ml (Ku ab control) CA antibodies nuclear membrane IgG 0.1 ml CA antibodies cell nuclei IgG 0.1 ml (ANA control), centromere pattern CA antibodies spindle fibers (MSA-1) IgG 0.1 ml CA antibodies mitochondria IgG 0.1 ml (AMA control) CA antibodies mitochondria IgG 0.1 ml (AMA M2 control; PBC-associated) CA antibodies mitochondria IgG 0.1 ml (AMA M9 control) CA antibodies ribosomes IgG 0.1 ml CA antibodies Golgi apparatus IgG 0.1 ml CA antibodies F-actin IgG 0.1 ml (F-actin control) CA antibodies vimentin IgG 0.1 ml CA antibodies histidyl-trna synthetase IgG 0.1 ml (Jo-1 ab control) 66
6 Controls for EUROIMMUN IIFT: Systemic Autoantibodies Control (Ready for Use) CA antibodies smooth muscles IgG 0.1 ml (ASMA control) CA A antibodies endomysium IgA 0.1 ml (endomysium IgA control) CA A antibodies endomysium plus gliadin (GAF-3X) IgA 0.1 ml (endomysium plus gliadin GAF-3X IgA control) CA antibodies reticulin IgG 0.1 ml (reticulin ab control) CA G antibodies elastin IgG 0.1 ml (elastin ab control) CA G antibodies vascular endothelium IgG 0.1 ml (vascular endothelium ab control) Further control sera for the determination of autoantibodies available upon request. Controls for the Determination of Autoantibodies 67
7 Controls for EUROIMMUN IIFT: Infectious Serology Control (Ready for Use) CI A antibodies Bordetella pertussis IgA 0.1 ml IgA positive control CI G antibodies Bordetella pertussis IgG 0.1 ml CI M antibodies Bordetella pertussis IgM 0.1 ml IgM positive control CI Z Bordetella pertussis/parapertussis IgA, IgG, IgM 0.1 ml negative control CI A antibodies Bordetella parapertussis IgA 0.1 ml IgA positive control CI G antibodies Bordetella parapertussis IgG 0.1 ml CI M antibodies Bordetella parapertussis IgM 0.1 ml IgM positive control CI G * antibodies Haemophilus influenzae IgG 0.1 ml CI Z Haemophilus influenzae IgA, IgG, IgM 0.1 ml negative control CI A antibodies Helicobacter pylori IgA 0.1 ml IgA positive control CI G antibodies Helicobacter pylori IgG 0.1 ml CI M antibodies Helicobacter pylori IgM 0.1 ml IgM positive control CI Z Helicobacter pylori IgA, IgG, IgM 0.1 ml negative control Controls for Infectious Serology CI A antibodies Campylobacter jejuni IgA 0.1 ml IgA positive control CI G antibodies Campylobacter jejuni IgG 0.1 ml CI Z Campylobacter jejuni/coli IgA, IgG, IgM 0.1 ml negative control CI G * antibodies Campylobacter coli IgG 0.1 ml CI G * antibodies Klebsiella pneumoniae IgG 0.1 ml CI Z * Klebsiella pneumoniae IgA, IgG, IgM 0.1 ml negative control CI G antibodies Treponema pallidum IgG 0.1 ml FTA CI M antibodies Treponema pallidum IgM 0.1 ml FTA IgM positive control CI Z Treponema pallidum IgA, IgG, IgM 0.1 ml FTA negative control CI G antibodies Treponema pallidum IgG 50 µl serum concentrate for FTA absorption CI G antibodies Reiter spirochetes IgG 0.1 ml unspecific FTA CI G antibodies Borrelia afzelii/b. burgdorferi/b. garinii IgG 0.1 ml CI M antibodies Borrelia afzelii/b. burgdorferi/b. garinii IgM 0.1 ml IgM positive control *) Currently not available as IVD in the European Union. 68
8 Controls for EUROIMMUN IIFT: Infectious Serology Control (Ready for Use) CI Z Borrelia afzelii/b. burgdorferi/b. garinii IgA, IgG, IgM 0.1 ml negative control CI 213a-0101 M antibodies Borrelia (OspC antigen) IgM 0.1 ml IgM positive control CI 213b-0101 G antibodies Borrelia (VlsE antigen) IgG 0.1 ml CI Z * Listeria monocytogenes IgA, IgG, IgM 0.1 ml negative control CI G * antibodies Listeria monocytogenes 1/2a IgG 0.1 ml CI G * antibodies Listeria monocytogenes 4b IgG 0.1 ml CI P antibodies Legionella pneumophila serotypes 1 or 4 IgM 0.1 ml positive control CI G * antibodies Legionella pneumophila serotype 1 IgG 0.1 ml CI Z Legionella pneumophila/non-pneumophila IgA, IgG, IgM 0.1 ml negative control CI 215b-0101 P antibodies Legionella pneumophila mixture IgM 0.1 ml IgM positive control CI 215d-0101 P antibodies Legionella pneumophila mixture IgM 0.1 ml IgM positive control CI 215f-0101 P antibodies Legionella pneumophila mixture IgM 0.1 ml IgM positive control CI 215h-0101 P antibodies Legionella pneumophila mixture IgM 0.1 ml IgM positive control CI G * antibodies Yersinia enterocolitica IgG 0.1 ml (for diagnosing various forms of arthritis) CI Z * Yersinia enterocolitica IgA, IgG, IgM 0.1 ml negative control (for diagnosing various forms of arthritis) CI A antibodies Chlamydia trachomatis IgA 0.1 ml IgA positive control Controls for Infectious Serology CI G antibodies Chlamydia trachomatis IgG 0.1 ml CI M antibodies Chlamydia trachomatis IgM 0.1 ml IgM positive control CI Z Chlamydia IgA, IgG, IgM 0.1 ml negative control CI A antibodies Chlamydia pneumoniae IgA 0.1 ml IgA positive control CI G antibodies Chlamydia pneumoniae IgG 0.1 ml CI M antibodies Chlamydia pneumoniae IgM 0.1 ml IgM positive control CI A antibodies Chlamydia psittaci IgA 0.1 ml IgA positive control CI G antibodies Chlamydia psittaci IgG 0.1 ml CI 219a-0101 G * antibodies Afipia felis IgG 0.1 ml CI 219a-0101 Z * Afipia felis IgA, IgG, IgM 0.1 ml negative control *) Currently not available as IVD in the European Union. 69
9 Controls for EUROIMMUN IIFT: Infectious Serology Control (Ready for Use) CI 219b-0101 G antibodies Bartonella henselae IgG 0.1 ml CI 219b-0101 M antibodies Bartonella henselae IgM 0.1 ml IgM positive control CI 219b-0101 Z Bartonella henselae/quintana IgA, IgG, IgM 0.1 ml negative control CI 219d-0101 G antibodies Bartonella quintana IgG 0.1 ml CI 219d-0101 M antibodies Bartonella quintana IgM 0.1 ml IgM positive control CI G antibodies Mycoplasma hominis IgG 0.1 ml CI Z Mycoplasma hominis/pneumoniae and IgA, IgG, IgM 0.1 ml Ureaplasma urealyticum negative control CI G antibodies Mycoplasma pneumoniae IgG 0.1 ml CI M antibodies Mycoplasma pneumoniae IgM 0.1 ml IgM positive control CI G antibodies Ureaplasma urealyticum IgG 0.1 ml CI A antibodies Leishmania donovani IgA 0.1 ml IgA positive control CI G antibodies Leishmania donovani IgG 0.1 ml CI M antibodies Leishmania donovani IgM 0.1 ml IgM positive control Controls for Infectious Serology CI Z Leishmania donovani IgA, IgG, IgM 0.1 ml negative control CI G antibodies Plasmodia IgG 0.1 ml CI Z Plasmodia IgA, IgG, IgM 0.1 ml negative control CI A antibodies Echinococcus granulosus IgA 0.1 ml IgA positive control CI G antibodies Echinococcus granulosus IgG 0.1 ml CI Z Echinococcus granulosus IgA, IgG, IgM 0.1 ml negative control CI A * antibodies Toxoplasma gondii IgA 0.1 ml IgA positive control CI G antibodies Toxoplasma gondii IgG 0.1 ml CI H high-avid antibodies Toxoplasma gondii IgG 0.1 ml CI M antibodies Toxoplasma gondii IgM 0.1 ml IgM positive control CI X low-avid antibodies Toxoplasma gondii IgG 0.1 ml CI Z Toxoplasma gondii IgA, IgG, IgM 0.1 ml negative control CI G * antibodies HIV-1 IgG 0.1 ml CI Z * HIV-1/2 IgA, IgG, IgM 0.1 ml negative control *) Currently not available as IVD in the European Union. 70
10 Controls for EUROIMMUN IIFT: Infectious Serology Control (Ready for Use) CI G * antibodies HIV-2 IgG 0.1 ml CI G antibodies Herpes simplex virus 1 (HSV-1) IgG 0.1 ml CI M antibodies Herpes simplex virus 1 (HSV-1) IgM 0.1 ml IgM positive control CI Z HSV-1/HSV-2 IgA, IgG, IgM 0.1 ml negative control CI G antibodies Herpes simplex virus 2 (HSV-2) IgG 0.1 ml CI M antibodies Herpes simplex virus 2 (HSV-2) IgM 0.1 ml IgM positive control CI G antibodies human herpes virus 6 (HHV-6) IgG 0.1 ml CI M antibodies human herpes virus 6 (HHV-6) IgM 0.1 ml IgM positive control CI Z human herpes virus 6 (HHV-6) IgA, IgG, IgM 0.1 ml negative control CI A antibodies Cytomegalovirus (CMV) IgA 0.1 ml IgA positive control CI G antibodies Cytomegalovirus (CMV) IgG 0.1 ml CI H high-avid antibodies Cytomegalovirus (CMV) IgG 0.1 ml CI M antibodies Cytomegalovirus (CMV) IgM 0.1 ml IgM positive control CI X low-avid antibodies Cytomegalovirus (CMV) IgG 0.1 ml CI Z Cytomegalovirus (CMV) IgA, IgG, IgM 0.1 ml negative control CI G antibodies Rubella virus IgG 0.1 ml CI H high-avid antibodies Rubella virus IgG 0.1 ml Controls for Infectious Serology CI X low-avid antibodies Rubella virus IgG 0.1 ml CI Z Rubella virus IgA, IgG, IgM 0.1 ml negative control CI G antibodies SARS coronavirus IgG 0.1 ml CI Z SARS coronavirus IgA, IgG, IgM 0.1 ml negative control CI G antibodies Measles virus IgG 0.1 ml CI M antibodies Measles virus IgM 0.1 ml IgM positive control CI Z Measles virus IgA, IgG, IgM 0.1 ml negative control CI G antibodies Mumps virus IgG 0.1 ml CI M antibodies Mumps virus IgM 0.1 ml IgM positive control *) Currently not available as IVD in the European Union. 71
11 Controls for EUROIMMUN IIFT: Infectious Serology Control (Ready for Use) CI Z Mumps virus IgA, IgG, IgM 0.1 ml negative control CI A antibodies Varicella zoster virus (VZV) IgA 0.1 ml IgA positive control CI G antibodies Varicella zoster virus (VZV) IgG 0.1 ml CI H high-avid antibodies Varicella zoster virus (VZV) IgG 0.1 ml CI M antibodies Varicella zoster virus (VZV) IgM 0.1 ml IgM positive control CI X low-avid antibodies Varicella zoster virus (VZV) IgG 0.1 ml CI Z Varicella zoster virus (VZV) IgA, IgG, IgM 0.1 ml negative control CI G antibodies TBE virus IgG 0.1 ml CI M antibodies TBE virus IgM 0.1 ml IgM positive control CI Z TBE virus IgA, IgG, IgM 0.1 ml negative control CI G antibodies West Nile virus IgG 0.1 ml CI H high-avid antibodies West Nile virus IgG 0.1 ml CI M antibodies West Nile virus IgM 0.1 ml IgM positive control Controls for Infectious Serology CI X low-avid antibodies West Nile virus IgG 0.1 ml CI Z West Nile virus IgA, IgG, IgM 0.1 ml negative control CI G antibodies Japanese encephalitis virus IgG 0.1 ml CI M antibodies Japanese encephalitis virus IgM 0.1 ml IgM positive control CI Z antibodies Japanese encephalitis virus IgA, IgG, IgM 0.1 ml negative control CI G antibodies Yellow fever virus IgG 0.1 ml CI M antibodies Yellow fever virus IgM 0.1 ml IgM positive control CI Z Yellow fever virus IgA, IgG, IgM 0.1 ml negative control CI 266a-0101 G antibodies Dengue virus IgG 0.1 ml CI 266a-0101 M antibodies Dengue virus IgM 0.1 ml IgM positive control CI 266a-0101 Z Dengue virus IgA, IgG, IgM 0.1 ml negative control CI A antibodies Respiratory syncytial virus (RSV) IgA 0.1 ml IgA positive control CI G antibodies Respiratory syncytial virus (RSV) IgG 0.1 ml CI M antibodies Respiratory syncytial virus (RSV) IgM 0.1 ml IgM positive control 72
12 Controls for EUROIMMUN IIFT: Infectious Serology Control (Ready for Use) CI Z Respiratory syncytial virus (RSV) IgA, IgG, IgM 0.1 ml negative control CI A antibodies Adenovirus IgA 0.1 ml IgA positive control CI G antibodies Adenovirus IgG 0.1 ml CI M antibodies Adenovirus IgM 0.1 ml IgM positive control CI Z Adenovirus IgA, IgG, IgM 0.1 ml negative control CI A antibodies Influenza virus type A IgA 0.1 ml IgA positive control CI G antibodies Influenza virus type A IgG 0.1 ml CI M antibodies Influenza virus type A IgM 0.1 ml IgM positive control CI Z Influenza virus type A/B IgA, IgG, IgM 0.1 ml negative control CI A antibodies Influenza virus type B IgA 0.1 ml IgA positive control CI G antibodies Influenza virus type B IgG 0.1 ml CI M antibodies Influenza virus type B IgM 0.1 ml IgM positive control CI A antibodies Parainfluenza virus types 1-4 IgA 0.1 ml IgA positive control CI G antibodies Parainfluenza virus types 1-4 IgG 0.1 ml CI M antibodies Parainfluenza virus types 1-4 IgM 0.1 ml IgM positive control CI Z Parainfluenza virus IgA, IgG, IgM 0.1 ml negative control CI G antibodies Coxsackie virus IgG 0.1 ml Controls for Infectious Serology CI Z Coxsackie virus IgA, IgG, IgM 0.1 ml negative control CI 275a-0101 G antibodies Echo virus IgG 0.1 ml CI 275a-0101 Z Echo virus IgA, IgG, IgM 0.1 ml negative control CI 277a G * antibodies Sandfly fever virus IgG 0.1 ml CI 277a Z * Sandfly fever virus IgA, IgG, IgM 0.1 ml negative control CI 278h G antibodies Hantavirus IgG 0.1 ml CI 278h M antibodies Hantavirus IgM 0.1 ml IgM positive control CI 278h Z Hantavirus IgA, IgG, IgM 0.1 ml negative control CI A antibodies Epstein-Barr virus capsid antigen (EBV-CA) IgA 0.1 ml IgA positive control *) Currently not available as IVD in the European Union. 73
13 Controls for EUROIMMUN IIFT: Infectious Serology Control (Ready for Use) CI G antibodies Epstein-Barr virus capsid antigen (EBV-CA) IgG 0.1 ml CI H high-avid antibodies Epstein-Barr virus capsid antigen (EBV-CA) IgG 0.1 ml CI M antibodies Epstein-Barr virus capsid antigen (EBV-CA) IgM 0.1 ml IgM positive control CI X low-avid antibodies Epstein-Barr virus (EBV-CA) IgG 0.1 ml CI Z Epstein-Barr virus (EBV) IgA, IgG, IgM 0.1 ml negative control CI C antibodies Epstein-Barr virus nuclear antigen (EBNA) IgG 0.1 ml complement-binding, positive control CI A antibodies Epstein-Barr virus early antigen (EBV-EA) IgA 0.1 ml IgA positive control CI G antibodies Epstein-Barr virus early antigen (EBV-EA) IgG 0.1 ml CI H high-avid antibodies Epstein-Barr virus early antigen (EBV-EA) IgG 0.1 ml CI X low-avid antibodies Epstein-Barr virus early antigen (EBV-EA) IgG 0.1 ml CI 279a-0101 Z * Crimean Congo fever virus IgA, IgG, IgM 0.1 ml negative control CI A antibodies Candida albicans IgA 0.1 ml IgA positive control CI G antibodies Candida albicans IgG 0.1 ml Controls for Infectious Serology CI M antibodies Candida albicans IgM 0.1 ml IgM positive control CI Z Candida albicans IgA, IgG, IgM 0.1 ml negative control CI 293a-0101 G antibodies Chikungunya virus IgG 0.1 ml CI 293a-0101 M antibodies Chikungunya virus IgM 0.1 ml IgM positive control CI 293a-0101 Z Chikungunya virus IgA, IgG, IgM 0.1 ml negative control Further control sera for infectious serology available upon request. Controls for EUROIMMUN IIFT: Determination of Further Control (Ready for Use) CV A antibodies Saccharomyces cerevisiae IgA 0.1 ml IgA positive control CV G antibodies Saccharomyces cerevisiae IgG 0.1 ml CV M antibodies Saccharomyces cerevisiae IgM 0.1 ml IgM positive control CV Z Saccharomyces cerevisiae IgA, IgG, IgM 0.1 ml negative control CV A antibodies gliadin (GAF-3X) IgA 0.1 ml (gliadin-gaf-3x IgA control) CV G antibodies gliadin (GAF-3X) IgG 0.1 ml (gliadin-gaf-3x IgG control) 74
14 Controls for EUROIMMUN Westernblots/EUROLINE-WB Control (50-fold concentrate) CW A antibodies Helicobacter pylori IgA 0.1 ml IgA positive control CW G antibodies Helicobacter pylori IgG 0.1 ml CW ZA antibodies Helicobacter pylori IgA 0.1 ml IgA negative control CW ZG antibodies Helicobacter pylori IgG 0.1 ml IgG negative control CW YM antibodies Treponema pallidum IgM 0.1 ml IgM weakly reactive control CW G antibodies Borrelia afzelii IgG 0.1 ml (Westernblot/EUROLINE-WB) CW M antibodies Borrelia afzelii IgM 0.1 ml IgM positive control (Westernblot/EUROLINE-WB) CW YM antibodies Borrelia afzelii IgM 0.1 ml IgM weakly reactive control (Westernblot/EUROLINE-WB) CW ZG antibodies Borrelia afzelii IgG 0.1 ml IgG negative control (Westernblot/EUROLINE-WB) CW ZM antibodies Borrelia afzelii IgM 0.1 ml IgM negative control (Westernblot/EUROLINE-WB) CW G antibodies Borrelia afzelii IgG 0.1 ml 2 (Westernblot/EUROLINE-WB) CW G antibodies Borrelia burgdorferi IgG 0.1 ml CW M antibodies Borrelia burgdorferi IgM 0.1 ml IgM positive control CW YM antibodies Borrelia burgdorferi IgM 0.1 ml IgM weakly reactive control CW ZG antibodies Borrelia burgdorferi IgG 0.1 ml IgG negative control Controls for Infectious Serology CW ZM antibodies Borrelia burgdorferi IgM 0.1 ml IgM negative control CW G antibodies Borrelia garinii IgG 0.1 ml CW M antibodies Borrelia garinii IgM 0.1 ml IgM positive control CW YM antibodies Borrelia garinii IgM 0.1 ml IgM weakly reactive control CW ZG antibodies Borrelia garinii IgG 0.1 ml IgG negative control CW ZM antibodies Borrelia garinii IgM 0.1 ml IgM negative control CW A antibodies Yersinia enterocolitica IgA 0.1 ml IgA positive control (for diagnosing various forms of arthritis) CW G antibodies Yersinia enterocolitica IgG 0.1 ml (for diagnosing various forms of arthritis) 75
15 Controls for EUROIMMUN Westernblots/EUROLINE-WB Control (50-fold concentrate) CW YA antibodies Yersinia enterocolitica IgA 0.1 ml IgA weakly reactive control (for diagnosing various forms of arthritis) CW ZA antibodies Yersinia enterocolitica IgA 0.1 ml IgA negative control (for diagnosing various forms of arthritis) CW ZG antibodies Yersinia enterocolitica IgG 0.1 ml IgG negative control (for diagnosing various forms of arthritis) CW G antibodies Herpes simplex virus (HSV) IgG 0.1 ml CW ZG antibodies Herpes simplex virus (HSV) IgG 0.1 ml IgG negative control CW ZM antibodies Herpes simplex virus (HSV) IgM 0.1 ml IgM negative control CW G antibodies Epstein-Barr virus (EBV) IgG 0.1 ml Controls for Infectious Serology 76
16 EUROASSAY for the Determination of Autoantibodies (Test Systems) Substrate Slides x Fields DA G thyroid peroxidase (TPO) IgG EUROASSAY strip 10 x 03 DA G thyroglobulin (TG) with antigens 10 x 05 DA G myeloperoxidase (MPO) IgG EUROASSAY strip 10 x 03 DA G proteinase 3 (PR3) with antigens 10 x 05 DA G AMA M2, LKM-1, SLA/LP IgG EUROASSAY strip 10 x 03 with antigens DA G LKM-1, LC-1, SLA/LP IgG EUROASSAY strip 10 x 03 DA G with antigens 10 x 05 DA G Liver Profile IgG EUROASSAY strip 10 x 03 AMA M2, LKM-1, LC-1, SLA/LP with antigens DA G Anti-ENA ProfilePlus IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B, Scl-70, with antigens 10 x 05 Jo-1 separately) DA G Anti-ENA ProfilePlus with AMA M2 IgG EUROASSAY strip 10 x 03 (nrnp/sm, Sm, SS-A, SS-B, Scl-70, with antigens Jo-1, AMA M2 separately) DA G SLE Profile IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B, dsdna, with antigens 10 x 05 histones, ribosomal P-proteins separately) DA G Anti-ENA ProfilePlus with dsdna IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, dsdna, Jo-1, with antigens 10 x 05 SS-A, SS-B, Scl-70 separately) DA G Anti ENA Profile IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B separately) with antigens 10 x 05 DA G Anti-ENA ProfilePlus with centromere protein B IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B, Scl-70, with antigens 10 x 05 Jo-1, centromere protein B separately) DA G Anti-ENA ProfilePlus with histones IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B, Scl-70, with antigens 10 x 05 Jo-1, histones separately) DA G Anti-ENA ProfilePlus with PM-Scl IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B, Scl-70, with antigens 10 x 05 Jo-1, PM-Scl separately) DA G SLE Profile 2 IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B, dsdna, with antigens 10 x 05 histones, nucleosomes separately) DA G SLE Profile 3 IgG EUROASSAY strip 10 x 03 DA G (dsdna, histones, nucleosomes, with antigens 10 x 05 ribosomal P-proteins separately) EUROASSAY/EUROLINE Westernblot DA G Anti-ENA ProfilePlus with Ro-52 IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B, Scl-70, with antigens 10 x 05 Jo-1, Ro-52 separately) DA G Scl-70, Jo-1, PM-Scl IgG EUROASSAY strip 10 x 03 DA G with antigens 10 x 05 DA G Anti-ENA ProfilePlus with ribosomal P-proteins IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, SS-A, SS-B, Scl-70, with antigens 10 x 05 Jo-1, ribosomal P-proteins separately) DA G Anti-ENA ProfilePlus with nucleosomes IgG EUROASSAY strip 10 x 03 DA G (nrnp/sm, Sm, nucleosomes, Jo-1, with antigens 10 x 05 SS-A, SS-B, Scl-70 separately) DA O AMA Profile IgGM EUROASSAY strip 10 x 03 (AMA M2, M4, M9 separately) with antigens If requested the EUROASSAY can be manufactured individually, thus offering special antigen combinations, e.g. dsdna, histones, nucleosomes, PM-Scl, nrnp/sm, Sm, SS-A (60 kda), Ro-52, SS-B, Scl-70, Jo-1, ribosomal P-proteins, centromere protein B, AMA M2, M4, M9, SLA/LP, LC-1, LKM-1. Slides with only one field can be delivered as special format. 77
17 EUROLINE for the Determination of Autoantibodies (Test Systems) Substrate DL G Neuronal Antigens Profile IgG membrane strip with antigens 16 x 01 (amphiphysin, PNMA2 (Ma-2), Ri, Yo, Hu separately) DL G Gangliosides Profile 1 IgG membrane strip with antigens 16 x 01 (GM1, GD1b, GQ1b separately) DL M Gangliosides Profile 1 IgM membrane strip with antigens 16 x 01 (GM1, GD1b, GQ1b separately) DL G Gangliosides Profile 2 IgG membrane strip with antigens 16 x 01 (GM1, GM2, GM3, GD1a, GD1b, GT1b, GQ1b separately) DL M Gangliosides Profile 2 IgM membrane strip with antigens 16 x 01 (GM1, GM2, GM3, GD1a, GD1b, GT1b, GQ1b separately) DL G myeloperoxidase (MPO) IgG membrane strip with antigens 16 x 01 proteinase 3 (PR3) DL G myeloperoxidase (MPO) IgG membrane strip with antigens 16 x 01 proteinase 3 (PR3) glomerular basement membrane (GBM) DL G Liver Profile 2 IgG membrane strip with antigens 16 x 01 (AMA M2, M2-3E, LKM-1, LC-1, SLA/LP separately) DL G Liver Profile IgG membrane strip with antigens 16 x 01 (AMA M2, LKM-1, LC-1, SLA/LP separately) DL G Profile Autoimmune Liver Diseases IgG membrane strip with antigens 16 x 01 (AMA M2, M2-3E, Sp100, PML, gp210, LC-1, LKM-1, SLA/LP, Ro-52 separately) DL G Myositis Profile IgG membrane strip with antigens 16 x 01 (Mi-2, Ku, PM-Scl, Jo-1, PL-7, PL-12, Ro-52 separately) DL G Myositis Profile 2 IgG membrane strip with antigens 16 x 01 (Mi-2, Ku, PM-Scl, U1-nRNP 70K, U1-nRNP A, U1-nRNP C, SRP, Jo-1, PL-7, PL-12, OJ, EJ, Ro-52 separately) EUROASSAY/EUROLINE Westernblot DL G Anti-ENA ProfilePlus 1 IgG membrane strip with antigens 16 x 01 (nrnp/sm, Sm, SS-A, Ro-52, SS-B, Scl-70, Jo-1 separately) DL G ANA Profile 3 IgG membrane strip with antigens 16 x 01 (nrnp/sm, Sm, SS-A, Ro-52, SS-B, Scl-70, PM-Scl, Jo-1, centromere protein B, PCNA, dsdna, nucleosomes, histones, ribosomal P-proteins, AMA M2 separately) DL G ANA Profile 1 IgG membrane strip with antigens 16 x 01 (nrnp/sm, Sm, SS-A, Ro-52, SS-B, Scl-70, Jo-1, centromere protein B, dsdna, nucleosomes, histones, ribosomal P-proteins separately) 78
18 EUROLINE for Infectious Serology (Test Systems) Substrate DN G EUROLINE Borrelia-RN-AT IgG membrane strip with antigens 32 x 01 DN G (p18, p19, p20, p21, p58, 240 x 01 OspC (p25), p39, p83, LBb, LBa, VlsE Bg, VlsE Bb, VlsE Ba separately) DN M EUROLINE Borrelia-RN-AT IgM membrane strip with antigens 32 x 01 DN M (OspC Bg nativ, OspC Bb nativ, 240 x 01 OspC Ba nativ, p39, VlsE Bb separately) DN G Malaria Profile 1 IgG membrane strip with antigens 16 x 01 (Plasmodium falciparum HRP-2 and MSP-2, Plasmodium vivax MSP and CSP separately) DN G * TO.R.C.H. Profile IgG membrane strip with antigens 16 x 01 (Toxoplasma gondii, Rubella virus, CMV, HSV-1, HSV-2 separately) DN M * TO.R.C.H. Profile IgM membrane strip with antigens 16 x 01 (Toxoplasma gondii, Rubella virus, CMV, HSV-1, HSV-2 separately) DN 278h G Hanta virus Profile 1 IgG membrane strip with antigens 16 x 01 PUUV (Puumala virus) DOBV (Dobrava virus) HNTV (Hantaan virus) DN 278h M Hanta virus Profile 1 IgM membrane strip with antigens 16 x 01 PUUV (Puumala virus) DOBV (Dobrava virus) HNTV (Hantaan virus) DN G EBV Profile 2 IgG membrane strip with antigens 16 x 01 (VCA gp125, VCA p19, EBNA-1, p22, EA-D separately) DN M EBV Profile 2 IgM membrane strip with antigens 16 x 01 (VCA gp125, VCA p19, EBNA-1, p22, EA-D separately) EUROLINE for Allergology (Test Systems) From , all allergy profiles will contain cross-reactive carbohydrate determinants (CCD). Substrate DP E allergy profile inhalation IgE membrane strip with allergens 16 x 01 (g1, g3, g6, g12, t2, t3, t4, t7, w1, w6, w9, d1, d2, e1, e2, e3, m1, m2, m3, m6) DP E allergy profile inhalation 2 IgE membrane strip with allergens 16 x 01 (g6, g12, t2, t3, t4, w6, w9, d1, d2, e1, e2, e3, e6, e82, e84, es4, m1, m2, m3, m6) EUROASSAY/EUROLINE Westernblot DP E allergy profile pediatric inhalation IgE membrane strip with allergens 16 x 01 (g6, g12, t2, t3, t4, w6, w8, w9, d1, d2, e1, e2, e3, e6, e82, e84, m1, m2, m3, m6) DP E allergy profile Mediterranean inhalation IgE membrane strip with allergens 16 x 01 (g2, g6, t3, t4, t9, t11, t23, t210, w1, w6, w9, w19, d1, d2, d70, e1, e2, e3, m2, m6) DP E allergy profile inhalation South East Asia IgE membrane strip with allergens 16 x 01 (gs1, ts19, t104, t119, t223, ds1, i6, e1, e2, es172, e6, e71, e82, e84, ms1, ms4, m5, m12, m45, u134) DP E allergy profile inhalation Korea 1 IgE membrane strip with allergens 16 x 01 (g2, g3, g6, g12, ts3, ts11, ts14, ts15, t4, t7, t12, t19, t71, w100, f1, f2, f14, f23, f234, f95) DP E allergy profile inhalation Korea 2 IgE membrane strip with allergens 16 x 01 (w1, w6, w8, w11, w14, w12, m1, m2, m3, m6, e1, e2, i6, h1, d1, d2, g7, ts4, t1, t16) DP E allergy profile inhalation China IgE membrane strip with allergens 16 x 01 (gs23, ts21, t3, t8, t11, t12, t14, t70, ws18, w1, w6, w9, es1, d1, d2, i6, ms5, m1, u73, u80) *) Currently not available as IVD in the European Union. 79
19 EUROLINE for Allergology (Test Systems) From , all allergy profiles will contain cross-reactive carbohydrate determinants (CCD). Substrate DP E allergy profile inhalation Middle East IgE membrane strip with allergens 16 x 01 (g1, g6,g12, t2, t3, t7, t9, w1, w6, w8, d1, d2, i6, e1, e84, m1, m2, m3, m5, m6) DP E allergy profile inhalation Gulf IgE membrane strip with allergens 16 x 01 (g6, g12, t2, t3, t7, t9, w1, w6, d1, d2, i6, e1, e2, e3, e17, m1, m2, m3, m5, m6) DP E allergy profile inhalation Turkey IgE membrane strip with allergens 16 x 01 (gs12, gs15, gs21, g12, ts23, ts24, t9, t70, ws19, ws20, ws21, d1, d2, i6, es2, es172, e1, e2, e3, e4, e6, e80, e81, ms11, ms12, m1, m2, m3, m6) DP E * allergy profile inhalation India IgE membrane strip with allergens 16 x 01 (g6, g12, g20, t18, w4, w27, w29, ds1, d2, i6, e1, e2, e11, e85, m3, m37, u81, u126, u129, u140) DP E allergy profile food IgE membrane strip with allergens 16 x 01 (f1, f75, f2, f45, f4, f5, f9, f13, f14, f17, f20, f49, f84, f237, f25, f31, f35, f85, f3, f23) DP E allergy profile food 2 IgE membrane strip with allergens 16 x 01 (f1, f75, f2, f78, f4, f5, f14, f10, f13, f17, f20, f49, f84, f95, f25, f31, f35, f85, f3, f23) DP E allergy profile food South East Asia 1 IgE membrane strip with allergens 16 x 01 (f1, f75, f2, f4, f9, f10, f14, f13, f17, f63, f64, f83, fs10, fs14, f23, f24, f80, f179, f105, f336) DP E * allergy profile food South East Asia 2 IgE membrane strip with allergens 16 x 01 (f1, f75, f2, f4, f9, f10, f14, f13, f17, f63, f340, f83, fs10, fs14, f23, f24, f80, f179, f105, f336) DP E allergy profile food Korea 1 IgE membrane strip with allergens 16 x 01 (f1, f2, f14, f81, f3, f23, f40, f41, f234, f26, f27, f83, fs15, f95, f4, f6, f9, f292, f47, f48) DP E allergy profile food Korea 2 IgE membrane strip with allergens 16 x 01 (f1, f2, f14, f81, f3, f23, f40, f41, f234, f26, f27, f83, fs15, f95, f4, f6, f9, f292, f47, f48) EUROASSAY/EUROLINE Westernblot DP E allergy profile food China IgE membrane strip with allergens 16 x 01 (f1, f2, f4, f7, f27, f88, fs35, f13, f14, fs40, f25, f292, fs42, f23, f234, f3, f41, f56, fs41, fs77) DP E allergy profile food Middle East IgE membrane strip with allergens 16 x 01 (f1, f75, f2, f78, e204, f4, f14, f45, f13, f17, f20, f33, f49, f29, f25, f31, f85, f48, f89, f88) DP E allergy profile food Gulf IgE membrane strip with allergens 16 x 01 (f1, f75, f2, f105, f4, f14, f45, fs36, f13, f29, f33, f44, f93, f25, f31, f48, f83, f88, f3, f23) DP E allergy profile food Cyprus 1 IgE membrane strip with allergens 16 x 01 (f1, f2, f75, f76, f77, f78, f81, f4, f45, f5, f14, f7, f79, f9, f10, f13, f144, f17, f20, f49) DP E allergy profile food Cyprus 2 IgE membrane strip with allergens 16 x 01 (f177, f23, f234, f24, f258, f3, f37, f40, f41, f80, f48, f108, f132, f212, f25, f292, f31, f35, f47, f96) DP E allergy profile food Cyprus 3 IgE membrane strip with allergens 16 x 01 (f26, f63, f83, f88, f237, f29, f32, f328, f33, f44, f49, f72, f84, f95, f281, f86, f89, f90, f105, f93) DP E allergy profile food Turkey IgE membrane strip with allergens 16 x 01 (f1, f75, f2, f169, f78, f4, f79, f9, f14, f10, f13, f17, f144, U87, f222, f73, f33, f44, f49, f92, f84, f146, f328, f25, f31, f35, f48, f95, f97, f122, f132, fs14, fs10, fs43, f83) DP E allergy profile food India IgE membrane strip with allergens 16 x 01 (f2, f75, f168, f4, f9, f14, f13, f36, f49, f50, f35, f38, f48, f244, f83, f89, f74, f240, f23, f24) DP E allergy profile atopy IgE membrane strip with allergens 16 x 01 (g6, g12, t3, w6, d1, e1, e2, e3, m2, m6, f1, f2, f3, f4, f9, f14, f17, f31, f35, f49) *) Currently not available as IVD in the European Union. 80
20 EUROLINE for Allergology (Test Systems) From , all allergy profiles will contain cross-reactive carbohydrate determinants (CCD). Substrate DP E allergy profile atopy 3 IgE membrane strip with allergens 16 x 01 (g6, t3, t4, w6, d1, d2, e1, e2, e3, m2, m3, f1, f75, f2, f3, f4, f13, f14, f31, f49) DP E allergy profile pollen-food cross reactions IgE membrane strip with allergens 16 x 01 (g6, t3, w6, f4, f5, f13, f17, f20, f48, f89, f271, f275, f44, f49, f348, f237, f328, f31, f35, f85) DP E allergy profile pediatrics IgE membrane strip with allergens 16 x 01 (gx, t3, w6, d1, d2, e1, e2, e3, m2, m3, m6,f1, f2, f3, f4, f9, f13, f14, f17, f31, f35, f49, f75, f76, f77, f78) DP E allergy profile atopy China IgE membrane strip with allergens 16 x 01 (ts20, w1, w6, ds1, h1, e1, e2, i6, ms1, u80, f1, f2, f13, f14, f27, f88, fs33, fs34, f23, f234) DP E allergy profile atopy China 2 IgE membrane strip with allergens 16 x 01 (w18, w6, u80, ds1, es1, ms5, f245, fs56, fs43, fs42, fs34) DP E allergy profile atopy China 3 IgE membrane strip with allergens 16 x 01 (ts22, w6, us1, ds1, es1, h1, ms5, f245, fs9, fs43, fs56, fs34, fs44) DP E allergy profile insect venoms IgE membrane strip with allergens 16 x 01 (i1, i3) Westernblot/EUROLINE-WB for the Determination of Autoantibodies (Test Systems) Antigen and Antigen Source DW G EUROLINE-WB whole antigen, SDS extract IgG 16 x 01 DW G neuronal antigens (Hu, Yo, Ri) of primate cerebellum 24 x 01 plus recombinant Hu, Yo and Ri plus recombinant Hu, Yo and Ri DW G EUROLINE-WB whole antigen, SDS extract IgG 16 x 01 DW G HEp-2 cell antigens of HEp-2 cells plus native SS-A 30 x 01 (nucleolar, cytoplasmic, mitochondrial) and recombinant CENP B and Ro-52 plus CENP B, SS-A and Ro-52 Westernblot/EUROLINE-WB for Infectious Serology (Test Systems) Antigen and Antigen Source EUROASSAY/EUROLINE Westernblot DY A Helicobacter pylori whole antigen, IgA 16 x 01 DY A SDS extract 30 x 01 DY G Helicobacter pylori whole antigen, IgG 16 x 01 DY G SDS extract 30 x 01 DY G Treponema pallidum 15 kda, 17 kda, IgG 16 x 01 DY G 45 kda (tmpa), 47 kda 24 x 01 DY M Treponema pallidum 15 kda, 17 kda, IgM 16 x 01 DY M 45 kda (tmpa), 47 kda 24 x 01 DY G EUROLINE-WB 15 kda, 17 kda, 45 kda (tmpa) 47 kda, IgG 16 x 01 DY G Treponema pallidum plus purified cardiolipin 24 x 01 plus cardiolipin DY M EUROLINE-WB 15 kda, 17 kda, 45 kda (tmpa) 47 kda, IgM 16 x 01 DY M Treponema pallidum plus purified cardiolipin 24 x 01 plus cardiolipin DY G Borrelia afzelii whole antigen, SDS extract of IgG 16 x 01 DY G Borrelia afzelii 30 x 01 DY G 32 x 01 DY G 240 x 01 81
21 Westernblot/EUROLINE-WB for Infectious Serology (Test Systems) Antigen and Antigen Source DY M Borrelia afzelii whole antigen, SDS extract of IgM 16 x 01 DY M Borrelia afzelii 30 x 01 DY M 32 x 01 DY M 240 x 01 DY G EUROLINE-WB whole antigen, SDS extract of IgG 16 x 01 DY G Borrelia Borrelia afzelii plus VlsE 30 x 01 DY G 240 x 01 DY M EUROLINE-WB whole antigen, SDS extract of IgM 16 x 01 DY M Borrelia Borrelia afzelii plus VlsE 30 x 01 DY M 240 x 01 DY G Borrelia burgdorferi whole antigen, SDS extract of IgG 16 x 01 DY G Borrelia burgdorferi sensu stricto 30 x 01 DY G 32 x 01 DY G 240 x 01 DY M Borrelia burgdorferi whole antigen, SDS extract of IgM 16 x 01 DY M Borrelia burgdorferi sensu stricto 30 x 01 DY M 32 x 01 DY M 240 x 01 DY G Borrelia garinii whole antigen, SDS extract IgG 16 x 01 DY G of Borrelia garinii 30 x 01 DY G 32 x 01 DY G 240 x 01 DY M Borrelia garinii whole antigen, SDS extract of IgM 16 x 01 DY M Borrelia garinii 30 x 01 DY M 32 x 01 DY M 240 x 01 DY A Yersinia enterocolitica isolated virulence factors IgA 16 x 01 DY A Detection of antibodies virulence factors of the (release proteins, Yop) 30 x 01 Yersinia enterocolitica pathogen in human serum of Yersinia enterocolitica (for diagnosing various forms of arthritis). EUROASSAY/EUROLINE Westernblot DY G Yersinia enterocolitica isolated virulence factors IgG 16 x 01 DY G Detection of antibodies virulence factors of the (release proteins, Yop) 30 x 01 Yersinia enterocolitica pathogen in human serum of Yersinia enterocolitica (for diagnosing various forms of arthritis). DY G Echinococcus granulosus whole antigen, IgG 16 x 01 DY G SDS extract 24 x 01 DY G EUROLINE-WB whole antigen, SDS extract of the IgG 16 x 01 DY G Herpes simplex virus 1 (HSV-1) HSV-1 strain McIntire plus gg2 24 x 01 plus HSV-2 type-specific purified by affinity chromatography glycoprotein G2 DY M EUROLINE-WB whole antigen, SDS extract of the IgM 16 x 01 DY M Herpes simplex virus 1 (HSV-1) HSV-1 strain McIntire plus gg2 24 x 01 plus HSV-2 type-specific purified by affinity chromatography glycoprotein G2 DY G * Cytomegalovirus whole antigen, IgG 16 x 01 DY G * (CMV) SDS extract 24 x 01 DY M * Cytomegalovirus whole antigen, IgM 16 x 01 DY M * (CMV) SDS extract 24 x 01 DY G Rubella virus whole antigen IgG 24 x 01 DY G Epstein-Barr virus whole antigen, IgG 16 x 01 DY G (EBV) SDS extract 24 x 01 DY M Epstein-Barr virus whole antigen, IgM 16 x 01 DY M (EBV) SDS extract 24 x 01 *) Currently not available as IVD in the European Union. 82
22 Microplate ELISA for the Determination of Autoantibodies (Test Systems) Antigen and Antigen Source Calibration EA G thyroid peroxidase recombinant, expression with IgG 10/50/500 IU/ml 96 x 01 (TPO) Baculovirus vector in insect cells EA G thyroglobulin native, human IgG 20/100/1000 IU/ml 96 x 01 (TG) thyroid gland EA G TSH receptor native, IgG 0/1/2/8/40 IU/l 96 x 01 (thyrotropin receptor) porcine thyrocytes EA G TSH receptor native, IgG 0.1/1/2/8/40 IE/l 96 x 01 (thyrotropin receptor) porcine thyrocytes Fast ELISA EA G GAD recombinant IgG 5/15/35/120/ 96 x 01 glutamic acid decarboxylase 250/2000 IU/ml EA G GAD/IA2 Pool recombinant IgG 4/10/20/70/ 96 x 01 glutamic acid decarboxylase/ 145/450 IU/ml tyrosine phosphatase EA G IA2 recombinant IgG 10/20/75/250/400/ 96 x 01 tyrosine phosphatase 4000 IU/ml EA G ANCA Profile (proteinase 3, MPO, elastase, native, human IgG semi-quantitative 12 x 08 cathepsin G, BPI, lactoferrin separately) neutrophil granulocytes recombinant, human c-dna expressed in human cell line EA G canca: native, human IgG 2/20/200 RU/ml 96 x 01 proteinase 3 neutrophil granulocytes (capture test) EA G canca: native, human IgG 2/20/200 RU/ml 96 x 01 proteinase 3 neutrophil granulocytes (PR3-hn-hr) recombinant, human c-dna expressed in human cell line EA G panca: native, human IgG 2/20/200 RU/ml 96 x 01 myeloperoxidase (MPO) neutrophil granulocytes EA G glomerular basement membrane alpha-3-chain of type IV collagen, IgG 2/20/200 RU/ml 96 x 01 (GBM) incl. NC1 domain, from bovine kidney EA G soluble liver antigen/ recombinant SLA/LP IgG 2/20/200 RU/ml 96 x 01 liver-pancreas antigen (SLA/LP) expression in E. coli EA G cytosolic recombinant formiminotransferase IgG semi-quantitative 96 x 01 liver antigen type 1 cyclodeaminase, expression with (LC-1) baculovirus vector in insect cells EA G liver-kidney microsomes recombinant cytochrome P450 IID6, IgG 2/20/200 RU/ml 96 x 01 LKM-1 expression with baculovirus vector in insect cells EA G parietal cells native, H+/K+ ATPase, IgG 2/20/200 RU/ml 96 x 01 (PCA) porcine gastric mucosa EA G intrinsic factor native, porcine IgG 2/20/200 RU/ml 96 x 01 gastric mucosa Microplate ELISA for the Determination of Autoantibodies EA G BP180-4X recombinant, expression in E. coli, IgG 2/20/200 RU/ml 48 x 01 tetramer of the NC16A domain EA G cyclic citrullinated peptides highly purified synthetic IgG 1/5/20/100/200 RU/ml 96 x 01 (CCP) CCP (second generation) EA G histones native, mixture of individually isolated IgG 2/20/200 RU/ml 96 x 01 histone types H1/H2a/H2b/H3/H4, bovine EA G double-stranded DNA highly purified genomic IgG 10/100/800 IU/ml 96 x 01 (dsdna) double-stranded DNA EA G dsdna-ncx highly purified genomic IgG 10/100/800 IU/ml 96 x 01 double-stranded DNA, complexed with nucleosomes EA G C1q native, IgG 2/20/200 RU/ml 96 x 01 human C1q 83
Seite Infektionsserologie Infectious serology 142
 142 Infectious serology Infectious serology 143 Bacteria Bordetella Borrelia Treponema Chlamydia For more information on this subject scan the QR code or enter the Quick Link code q008 at www.euroimmun.com
142 Infectious serology Infectious serology 143 Bacteria Bordetella Borrelia Treponema Chlamydia For more information on this subject scan the QR code or enter the Quick Link code q008 at www.euroimmun.com
Immunologicals Bulk Product Listing
 Immunologicals Bulk Product Listing The listing below is a guide to the range of products available from Immunologicals. Although our primary focus is on the supply of materials in quantities optimal to
Immunologicals Bulk Product Listing The listing below is a guide to the range of products available from Immunologicals. Although our primary focus is on the supply of materials in quantities optimal to
VASCULITIS PRODUCT HIGHLIGHTS
 VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
AbBaltis. Product Catalogue. Contact us now for free samples!
 AbBaltis We are an award winning company supplying a wide variety of allergy and disease state human plasma and serum as well as clinical residual samples. Product Catalogue Additionally, we process blood
AbBaltis We are an award winning company supplying a wide variety of allergy and disease state human plasma and serum as well as clinical residual samples. Product Catalogue Additionally, we process blood
Quality assessment service of the Institute for Quality Assurance Lübeck
 IfQ-Lübeck Institut für Qualitätssicherung Lübeck Quality assessment service of the Institute for Quality Assurance Lübeck IfQ-Lübeck Institut für Qualitätssicherung Lübeck Seekamp 31 23560 Lübeck (Germany)
IfQ-Lübeck Institut für Qualitätssicherung Lübeck Quality assessment service of the Institute for Quality Assurance Lübeck IfQ-Lübeck Institut für Qualitätssicherung Lübeck Seekamp 31 23560 Lübeck (Germany)
ELISA. AccuDiag Autoimmune Diseases and Others. ANA Screen Qualitative ~135 min 90.4% 100%
 / AccuDiag Autoimmune Diseases and Others ANA Screen 5102-2 96 ~135 min 90.4% 100% ENA Profile-6 (Sm/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B) 2506-2 96 ~ 60 min 96% 100% ENA Combined Screen (6 antigens) Sm/RNP,
/ AccuDiag Autoimmune Diseases and Others ANA Screen 5102-2 96 ~135 min 90.4% 100% ENA Profile-6 (Sm/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B) 2506-2 96 ~ 60 min 96% 100% ENA Combined Screen (6 antigens) Sm/RNP,
Tel: Tel: Web: CODE DESCRIPTION UNITS
 New Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDIMOD2 Modulus2 2-4 Microplate Analyzer 1 Refurbished Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDINXGMI Refurbished Nexgen 4-Microplate
New Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDIMOD2 Modulus2 2-4 Microplate Analyzer 1 Refurbished Instruments MICROPLATE ANALYZERS DESCRIPTION UNITS MDINXGMI Refurbished Nexgen 4-Microplate
Autoimmune diagnostics. A comprehensive product line for the detection of autoantibodies
 Autoimmune diagnostics A comprehensive product line for the detection of autoantibodies Autoimmune diagnostics Autoimmune diseases are chronic inflammatory processes with an indeterminate etiology. They
Autoimmune diagnostics A comprehensive product line for the detection of autoantibodies Autoimmune diagnostics Autoimmune diseases are chronic inflammatory processes with an indeterminate etiology. They
.01_2018 A FIRENZE 2018_DEPL.1000 REV
 diagnostica senese CATALOGUE 2018 CATALOGUE 2018 DIESSE DIAGNOSTICA SENESE SPA Sales Office Via del Pozzo, 5 Loc. S. Martino 53035 Monteriggioni (SI) ITALY T. +39 0577 319560 / 61/ 50 F. +39 0577 318763
diagnostica senese CATALOGUE 2018 CATALOGUE 2018 DIESSE DIAGNOSTICA SENESE SPA Sales Office Via del Pozzo, 5 Loc. S. Martino 53035 Monteriggioni (SI) ITALY T. +39 0577 319560 / 61/ 50 F. +39 0577 318763
Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia
 Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia Foreign molecules = antigens Immune response Immune system non-specific specific cellular humoral
Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia Foreign molecules = antigens Immune response Immune system non-specific specific cellular humoral
Panel # Panel CPT Code Price 2010 AUTOIMMUNE PROFILE - BASIC 99.00
 2010 AUTOIMMUNE PROFILE - BASIC 99.00 Anti-Nuclear Antibody (ANA) 86038 33.00 Rheumatoid Factor 86431 33.00 C1Q Immune Complex 86332 33.00 2011 AUTOIMMUNE PROFILE (COMPREHENSIVE) 210.00 Anti-Nuclear Antibody
2010 AUTOIMMUNE PROFILE - BASIC 99.00 Anti-Nuclear Antibody (ANA) 86038 33.00 Rheumatoid Factor 86431 33.00 C1Q Immune Complex 86332 33.00 2011 AUTOIMMUNE PROFILE (COMPREHENSIVE) 210.00 Anti-Nuclear Antibody
Clinical Laboratory. 14:41:00 Complement Component 3 50 mg/dl Oct-18
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Thyroid Peroxidase (TPO) Antibody 5.0 IU/mL [0.0-9.0] 18-289-900139 16-Oct-18 Complement Component 3 50 mg/dl 18-289-900139
EC Declaration of Conformity Authorised By: Version Released Amendments 3 04/03/2015
 Version Released Amendments 3 04/03/2015 Amend manufacturer s address to be the same format as printed on kit boxes. 4 09/06/2015 Removal of Pseudomonas aeruginosa products 5 07/01/2016 Reflect changes
Version Released Amendments 3 04/03/2015 Amend manufacturer s address to be the same format as printed on kit boxes. 4 09/06/2015 Removal of Pseudomonas aeruginosa products 5 07/01/2016 Reflect changes
Product Guide. Valid from June 15 th, Simply innovative diagnostics
 Product Guide Valid from June 15 th, 2018 Simply innovative diagnostics ELISA Product group Page Thyroid TSH Receptor autoantibodies 6 Tg autoantibodies TPO autoantibodies Thyreoglobulin 7 TSH 8 Free
Product Guide Valid from June 15 th, 2018 Simply innovative diagnostics ELISA Product group Page Thyroid TSH Receptor autoantibodies 6 Tg autoantibodies TPO autoantibodies Thyreoglobulin 7 TSH 8 Free
Anti-Nuclear Antibodies (ANA). (Incorporating Anti-double stranded DNA (dsdna) and Anti-Extractable Nuclear Antigen (ENA) Antibodies)
 Autoimmune Antibody Testing Points of Note: The interpretation of all autoantibody tests is highly dependent on the likelihood of disease in the patient. The results should always be interpreted with the
Autoimmune Antibody Testing Points of Note: The interpretation of all autoantibody tests is highly dependent on the likelihood of disease in the patient. The results should always be interpreted with the
The Way Forward. Allergy Autoimmune Urinalysis Infectious Disease. International Diagnostic Products Catalogue
 The Way Forward Allergy Autoimmune Urinalysis Infectious Disease International Diagnostic Products Catalogue We are at your service. The Way Forward with a New Attitude and Increased Global Vision The
The Way Forward Allergy Autoimmune Urinalysis Infectious Disease International Diagnostic Products Catalogue We are at your service. The Way Forward with a New Attitude and Increased Global Vision The
Controls & Calibrators. Disease Quality Controls
 Controls & Calibrators Infectious Disease Quality Controls Infectious Disease Quality Controls A broad selection of controls designed to monitor assay precision of hepatitis, retrovirus, sexually transmitted
Controls & Calibrators Infectious Disease Quality Controls Infectious Disease Quality Controls A broad selection of controls designed to monitor assay precision of hepatitis, retrovirus, sexually transmitted
Infectious Disease Antibodies and Antigens. ...Viro Stat. Supplying Researchers and Manufacturers Since
 Infectious Disease Antibodies and Antigens...Viro Stat Supplying Researchers and Manufacturers Since 1985 Infectious Disease Antibodies and Antigens...Viro Stat Supplying Researchers and Manufacturers
Infectious Disease Antibodies and Antigens...Viro Stat Supplying Researchers and Manufacturers Since 1985 Infectious Disease Antibodies and Antigens...Viro Stat Supplying Researchers and Manufacturers
Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18
![Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18 Clinical Laboratory. 14:42:00 SSA-52 (Ro52) (ENA) Antibody, IgG 1 AU/mL [0-40] Oct-18](/thumbs/95/125595183.jpg) Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Rheumatoid Factor
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Rheumatoid Factor
Accredited entity according to ČSN EN ISO 15189:2013 Laboratoře Mikrochem a.s. Mikrochem Laboratories Nezvalova 984/2, Hodolany, Olomouc
 Medical laboratory locations: 1. Olomouc Nezvalova 984/2, 779 00 Olomouc 2. Přerov Čechova 8, 750 02 Přerov 3. Šumperk Nerudova 34, 787 01 Šumperk 4. Valašské Meziříčí Vsetínská 854, 757 01 Valašské Meziříčí
Medical laboratory locations: 1. Olomouc Nezvalova 984/2, 779 00 Olomouc 2. Přerov Čechova 8, 750 02 Přerov 3. Šumperk Nerudova 34, 787 01 Šumperk 4. Valašské Meziříčí Vsetínská 854, 757 01 Valašské Meziříčí
Freiburg Medical Laboratory ME LLC, P.O.Box 3068, Dubai Tel: Fax:
 Autoantibodies acetylcholine receptor alveolar basal membrane C3-nephritis factor cold agglutinin cytoplasmatic antigen (parotis) DNA, double stranded (ds DNA) DNA, single stranded (ss DNA) endomysium,
Autoantibodies acetylcholine receptor alveolar basal membrane C3-nephritis factor cold agglutinin cytoplasmatic antigen (parotis) DNA, double stranded (ds DNA) DNA, single stranded (ss DNA) endomysium,
ELISA Range. VIROTECH Diagnostics GmbH. Lot independent. Reagent. System. IgG-Conjugate. Substrate. IgM-Conjugate. Washing Solution.
 Quelle: www.fotolia.com ELISA Range IgG-Conjugate IgM-Conjugate IgA-Conjugate Lot independent Reagent System Stop Solution Substrate Washing Solution Serum Dilution Dilution Buffer Incubation Time Cerebrospinal
Quelle: www.fotolia.com ELISA Range IgG-Conjugate IgM-Conjugate IgA-Conjugate Lot independent Reagent System Stop Solution Substrate Washing Solution Serum Dilution Dilution Buffer Incubation Time Cerebrospinal
Differentiated ANCA diagnostics using BIOCHIP Mosaics
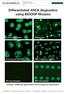 Differentiated ANCA diagnostics using BIOCHIP Mosaics Gran. MPO microdoplet Example: Antibodies against MPO and homogeneous ANA pattern Anti-MPO? ANA? panca positive, anti-mpo positive gran. Final result:
Differentiated ANCA diagnostics using BIOCHIP Mosaics Gran. MPO microdoplet Example: Antibodies against MPO and homogeneous ANA pattern Anti-MPO? ANA? panca positive, anti-mpo positive gran. Final result:
Autoantibodies panel ANA
 Autoantibodies panel ANA Anti-nuclear antibodies, ANA screening General: Anti-nuclear antibodies (ANA) contain all kinds of autoantibodies against nuclear antigens. Their targets are cell components in
Autoantibodies panel ANA Anti-nuclear antibodies, ANA screening General: Anti-nuclear antibodies (ANA) contain all kinds of autoantibodies against nuclear antigens. Their targets are cell components in
Assays. New. New. Combinations. Possibilities. Patents: EP , AU
 Assays Patents: EP 2362222, AU 2011217190 New Combinations New Possibilities Technology Classical Handling of Autoimmune Diagnostics 2-Step Diagnostics 1 st Screening 2 nd Confirmation Cell based IFA ELISA
Assays Patents: EP 2362222, AU 2011217190 New Combinations New Possibilities Technology Classical Handling of Autoimmune Diagnostics 2-Step Diagnostics 1 st Screening 2 nd Confirmation Cell based IFA ELISA
MOLECULAR DIAGNOSTIC PANELS and REAGENT KITS for NUCLEIC ACID EXTRACTION and RT-PCR of INFECTIOUS DISEASES and GENOMICS. Sep 2018
 MOLECULAR DIAGNOSTIC PANELS and REAGENT KITS for NUCLEIC ACID EXTRACTION and RT-PCR of INFECTIOUS DISEASES and GENOMICS Sep 2018 STANDARDISED & EASY APPROACH TO PCR DIAGNOSTICS The diagnostic kits of MOLgen
MOLECULAR DIAGNOSTIC PANELS and REAGENT KITS for NUCLEIC ACID EXTRACTION and RT-PCR of INFECTIOUS DISEASES and GENOMICS Sep 2018 STANDARDISED & EASY APPROACH TO PCR DIAGNOSTICS The diagnostic kits of MOLgen
Catalogue Product Name Tests Number ELISA IVD 96 Test kits AC-96 CHAGAS-96 DEN-G DEN-M DEN-G/M EH-96 EG-96 FC-96 FI-96 LEISH-96 LEPTO-G LEPTO-M
 Catalogue Number Product Name Tests ELISA IVD 96 Test kits AC-96 Ascaris Antibody Detection Test kit 96 Test CHAGAS-96 Chagas Antibody Detection Test kit 96 Test DEN-G Dengue IgG Antibody Detection Test
Catalogue Number Product Name Tests ELISA IVD 96 Test kits AC-96 Ascaris Antibody Detection Test kit 96 Test CHAGAS-96 Chagas Antibody Detection Test kit 96 Test DEN-G Dengue IgG Antibody Detection Test
Storage conditions. roomtemperature or incubator at 36 C. Other: 2-8 C Blood: roomtemperature or incubator at. A.s.a.p. max 24h. A.s.a.p.
 Extract Vademecum Department of Medical Microbiology Status: Code: Version: Approval date: Geldig MMB-PR-04/1 1 Juni 2017 Name request Specimen Material needed Transport Bacteriology Bacterial culture
Extract Vademecum Department of Medical Microbiology Status: Code: Version: Approval date: Geldig MMB-PR-04/1 1 Juni 2017 Name request Specimen Material needed Transport Bacteriology Bacterial culture
Clinical Laboratory. [None
 Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
Clinical Laboratory Procedure Result Units Ref Interval Accession Collected Received Double-Stranded DNA (dsdna) Ab IgG ELISA Detected * [None 18-289-900151 Detected] Double-Stranded DNA (dsdna) Ab IgG
Microbiology EQA Product Portfolio
 Labquality EQAS Microbiology EQA Product Portfolio Clinically relevant external quality assessment program for microbiology Bacterial serology Bacteriology Mycology Parasitology Preanalytics Virology Labquality
Labquality EQAS Microbiology EQA Product Portfolio Clinically relevant external quality assessment program for microbiology Bacterial serology Bacteriology Mycology Parasitology Preanalytics Virology Labquality
University of Pretoria
 University of Pretoria Serodiagnostic Procedures Performed in the Department of Immunology Dr Pieter WA Meyer 1.Autoimmune Diseases Automated Anti-nuclear antibodies Anti-gliadin/ tissue transglutaminase
University of Pretoria Serodiagnostic Procedures Performed in the Department of Immunology Dr Pieter WA Meyer 1.Autoimmune Diseases Automated Anti-nuclear antibodies Anti-gliadin/ tissue transglutaminase
ANA Diagnostics Using Indirect Immunofluorescence
 ANA Diagnostics Using Indirect Immunofluorescence EUROIMMUN D-23560 Luebeck (Germany) Seekamp 31 Tel +49 45158550 Fax 5855591 E-mail euroimmun@euroimmun.de Table of Contents Autoantibodies against cell
ANA Diagnostics Using Indirect Immunofluorescence EUROIMMUN D-23560 Luebeck (Germany) Seekamp 31 Tel +49 45158550 Fax 5855591 E-mail euroimmun@euroimmun.de Table of Contents Autoantibodies against cell
Code TEST SPECIMEN REQUIREMENT TAT Referral centre. 1 week BP GM (Specimen stability: 2-8 C for 48 hours) 3240 Anti-ds DNA 3 ml Plain Blood/Serum
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
A007 Allergy Basic Profile (28 parameters) 5 ml Plain Blood/Serum week BPGM
 B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
B.P. CLINICAL LAB SDN. BHD. ESOTERIC TEST LIST Note: For campaign profile, please notify processing lab 3 weeks in advanced for reagent and consumables preparation. Failure to pre-notify lab will cause
DS-EIA-ANTI-HIV-UNIF (Ab) I DS-EIA-ANTI-HIV-UNIF (Ab) I DS-EIA-ANTI-HIV-UNIF (Ab) I
 * Sensitivity HIV DS-EIA-ANTI-HIV-UNIF (Ab) I-153 96 115 24 DS-EIA-ANTI-HIV-UNIF (Ab) I-150 192 115 24 DS-EIA-ANTI-HIV-UNIF (Ab) I-155 480 115 24 DS-EIA-HIV-AG-SCREEN I-1452 96 115 12 0.025 IU/ml DS-EIA-HIV-AGAB-SCREEN
* Sensitivity HIV DS-EIA-ANTI-HIV-UNIF (Ab) I-153 96 115 24 DS-EIA-ANTI-HIV-UNIF (Ab) I-150 192 115 24 DS-EIA-ANTI-HIV-UNIF (Ab) I-155 480 115 24 DS-EIA-HIV-AG-SCREEN I-1452 96 115 12 0.025 IU/ml DS-EIA-HIV-AGAB-SCREEN
+++ neu +++ new +++ neu HIGHLIGHTS 2018
 ++ neu +++ new +++ n HIGHLIGHTS 2018 MIKROGEN BASED IN MUNICH, GERMANY Product Overview PRODUCT OVERVIEW Bacterial infections Anaplasma / Ehrlichia Adenovirus Babesia Astrovirus Bordetella Chikungunya
++ neu +++ new +++ n HIGHLIGHTS 2018 MIKROGEN BASED IN MUNICH, GERMANY Product Overview PRODUCT OVERVIEW Bacterial infections Anaplasma / Ehrlichia Adenovirus Babesia Astrovirus Bordetella Chikungunya
p. 111 p. 185 p. 197 p. 238
 Editorial Board Contributors Preface General Methods p. 1 General Methods: Antibody-Based and Molecular p. 3 Introduction p. 5 Antibody-Based Methods p. 6 p. xiii p. xv p. xxv Introduction to Molecular
Editorial Board Contributors Preface General Methods p. 1 General Methods: Antibody-Based and Molecular p. 3 Introduction p. 5 Antibody-Based Methods p. 6 p. xiii p. xv p. xxv Introduction to Molecular
AMPLIRUN TOTAL A RELIABLE QUALITY CONTROL SOURCE FOR NUCLEIC ACID TESTS
 AMPLIRUN TOTAL A RELIABLE QUALITY CONTROL SOURCE FOR NUCLEIC ACID TESTS Take Control, Molecular Control. 02 04 05 06 06 07 08 08 AMPLIRUN TOTAL RESPIRATORY INFECTIONS TUBERCULOSIS INFECTIONS GASTROINTESTINAL
AMPLIRUN TOTAL A RELIABLE QUALITY CONTROL SOURCE FOR NUCLEIC ACID TESTS Take Control, Molecular Control. 02 04 05 06 06 07 08 08 AMPLIRUN TOTAL RESPIRATORY INFECTIONS TUBERCULOSIS INFECTIONS GASTROINTESTINAL
PRODUCT CATALOG. All products are CE marked
 PRODUCT CATALOG 2017 All products are CE marked Content Company Profile 4 Allergy in-vitro Diagnostics Diagnostics for the IgE mediated Type I reaction (Immediate reaction) 6 Enzyme-Immuno-Assays on microtiterplates
PRODUCT CATALOG 2017 All products are CE marked Content Company Profile 4 Allergy in-vitro Diagnostics Diagnostics for the IgE mediated Type I reaction (Immediate reaction) 6 Enzyme-Immuno-Assays on microtiterplates
Specific Panels. Celiac disease panel. Pancreas Panel:
 Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
Specific Panels Celiac disease panel Anti Endomysium IgA Anti Endomysium IgG Anti Gliadin IgA Anti Gliadin IgG Anti Transglutaminase IgA Anti Transglutaminase IgG Total IgA Total IgG Stool analysis +Sudan
Immunoassay. Product Portfolio. Part of the IDS group
 Immunoassay Portfolio www.diametra.com info_diametra@idsplc.com Part of the IDS group Our Portfolio Cost-Effective Solutions for your Laboratory. All DiaMetra immunoassay kits in breakable well format.
Immunoassay Portfolio www.diametra.com info_diametra@idsplc.com Part of the IDS group Our Portfolio Cost-Effective Solutions for your Laboratory. All DiaMetra immunoassay kits in breakable well format.
ELISA kits for infections (HIV; Hepatitis A, B, C, D, E; Syphilis, ToRCH and others), hormone pathologies and tumor markers.
 DSI S.r.l. DSI S.r.l. is one of the European leaders in the field of immunodiagnostic tests manufacturing. We are a research-focused company with the long-term microbiological scientific traditions. Our
DSI S.r.l. DSI S.r.l. is one of the European leaders in the field of immunodiagnostic tests manufacturing. We are a research-focused company with the long-term microbiological scientific traditions. Our
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC and its technical Directives (particularly 2006/17/EC) More stringent -legally binding, applies for all donations and all donor profiles
Colour key Minimum requirements as set out in Directive 2004/23/EC and its technical Directives (particularly 2006/17/EC) More stringent -legally binding, applies for all donations and all donor profiles
WESTERN BLOTS & LINE IMMUNO ASSAYS
 1 WESTERN BLOTS & LINE IMMUNO ASSAYS FEBRUARY 2010 AUTOIMMUNE DISEASE NEW! DT-ANA8D-24 BlueDot ANA 8 Sm; Sm/RNP; SSA(Ro); SSB(La); Jo-1; Scl-70; PM-Scl; CENP-A/B NEW! DT-ANA10D-24 BlueDot ANA 10 Sm; Sm/RNP;
1 WESTERN BLOTS & LINE IMMUNO ASSAYS FEBRUARY 2010 AUTOIMMUNE DISEASE NEW! DT-ANA8D-24 BlueDot ANA 8 Sm; Sm/RNP; SSA(Ro); SSB(La); Jo-1; Scl-70; PM-Scl; CENP-A/B NEW! DT-ANA10D-24 BlueDot ANA 10 Sm; Sm/RNP;
IMTEC-ANA-LIA MAXX. Design Verification
 Design Verification IMTEC-ANA-LIA MAXX CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Specificity... 2 3 Interferences... 4 4 Imprecision... 6 4.1 Within-Run Imprecision... 6 4.2 Between-Run
Design Verification IMTEC-ANA-LIA MAXX CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Specificity... 2 3 Interferences... 4 4 Imprecision... 6 4.1 Within-Run Imprecision... 6 4.2 Between-Run
Simultaneous comprehensive multiplex autoantibody analysis by CytoBead technology for Rapidly Progressive Glomerulonephritis.
 Simultaneous comprehensive multiplex autoantibody analysis by CytoBead technology for Rapidly Progressive Glomerulonephritis l Assays Indirect Immunofluorescence Goldstandard for Diagnosis of Autoimmune
Simultaneous comprehensive multiplex autoantibody analysis by CytoBead technology for Rapidly Progressive Glomerulonephritis l Assays Indirect Immunofluorescence Goldstandard for Diagnosis of Autoimmune
Viral Antigens Recombinant Proteins. Life Science, Inc. Large Scale Manufacturing, R&D and Custom Services.
 Viral Antigens Recombinant Proteins Large Scale Manufacturing, R&D and Custom Services Life Science, Inc. www.meridianlifescience.com Life Science, Inc. Meridian Life Science, (MLS) is an industry leader
Viral Antigens Recombinant Proteins Large Scale Manufacturing, R&D and Custom Services Life Science, Inc. www.meridianlifescience.com Life Science, Inc. Meridian Life Science, (MLS) is an industry leader
JAJ International, Inc./LuSys Laboratories, Inc.
 JAJ International, Inc./LuSys Laboratories, Inc. 10054 Mesa Ridge Court, Suite #120 San Diego, California 92121 USA Tel: 1(858) 866-0788, Fax: 1(858) 866-1688 Email: info@jajinternational.com Website:
JAJ International, Inc./LuSys Laboratories, Inc. 10054 Mesa Ridge Court, Suite #120 San Diego, California 92121 USA Tel: 1(858) 866-0788, Fax: 1(858) 866-1688 Email: info@jajinternational.com Website:
Supplementary Figure Legends
 Supplementary Figure Legends Supplementary Figure 1. Comparison of RNP IC-mediated NET formation. Quantification of DNA release induced by ICs consisting of SmRNP combined with SLE IgG 961 (n = 10), 1032
Supplementary Figure Legends Supplementary Figure 1. Comparison of RNP IC-mediated NET formation. Quantification of DNA release induced by ICs consisting of SmRNP combined with SLE IgG 961 (n = 10), 1032
ANA Diagnostics Using Indirect Immunofluorescence
 ANA Diagnostics Using Indirect Immunofluorescence EUROIMMUN AG Seekamp 31 23560 Lübeck (Germany) Tel +49 451/ 58 55-0 Fax 58 55-591 info@euroimmun.de www.euroimmun.com Table of contents Autoantibodies
ANA Diagnostics Using Indirect Immunofluorescence EUROIMMUN AG Seekamp 31 23560 Lübeck (Germany) Tel +49 451/ 58 55-0 Fax 58 55-591 info@euroimmun.de www.euroimmun.com Table of contents Autoantibodies
Autoantibodies giving rise to cytoplasmic IIF staining using HEp-2 cell substrate
 Autoantibodies giving rise to cytoplasmic IIF staining using HEp-2 cell substrate Some associations of anticytoplasmic antibodies with clinical diagnoses and features HEp-2 IIF: the gold standard for ANA
Autoantibodies giving rise to cytoplasmic IIF staining using HEp-2 cell substrate Some associations of anticytoplasmic antibodies with clinical diagnoses and features HEp-2 IIF: the gold standard for ANA
Atlas of Antinuclear Antibodies
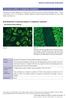 Fluorescence patterns of cytoplasmic autoantigens There are many important organelles in the cytoplasm which fulfill various functions of the cell as described in section 1. Various autoantibodies, known
Fluorescence patterns of cytoplasmic autoantigens There are many important organelles in the cytoplasm which fulfill various functions of the cell as described in section 1. Various autoantibodies, known
Mikrogen GmbH Product Catalogue
 roduct Catalogue Content Serology Bacterial infections...4 Bordetella pertussis... 4 Borrelia burgdorferi... 4 Brucella... 4 Campylobacter... 4 Chlamydia... 5 Coxiella burnetii (Q-Fever)... 5 Diphtheria
roduct Catalogue Content Serology Bacterial infections...4 Bordetella pertussis... 4 Borrelia burgdorferi... 4 Brucella... 4 Campylobacter... 4 Chlamydia... 5 Coxiella burnetii (Q-Fever)... 5 Diphtheria
M O L E C U L A R G E N E T I C S
 MOLECULAR GENETICS ADVANTAGES OF MOLECULAR GENETICS Molecular genetics is a dynamic and transformative area of diagnostics, leading to insights in research and treatment in many disease states that are
MOLECULAR GENETICS ADVANTAGES OF MOLECULAR GENETICS Molecular genetics is a dynamic and transformative area of diagnostics, leading to insights in research and treatment in many disease states that are
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital
 Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
الحترمونا من خري الدعاء
 الحترمونا من خري الدعاء Instructions for candidates The examination consists of 30 multiple choice questions, each divided into 5 different parts. Each part contains a statement which could be true or
الحترمونا من خري الدعاء Instructions for candidates The examination consists of 30 multiple choice questions, each divided into 5 different parts. Each part contains a statement which could be true or
USA Product Listing. Serology Diagnostic Products
 USA Product Listing Serology Diagnostic Products Focus Diagnostics recognizes your testing needs because we identify and evaluate trends in diagnostic testing. The products listed in this brochure were
USA Product Listing Serology Diagnostic Products Focus Diagnostics recognizes your testing needs because we identify and evaluate trends in diagnostic testing. The products listed in this brochure were
Advances in Autoantibody Testing & Clinical Applications
 Advances in Autoantibody Testing & Clinical Applications Marvin J. Fritzler PhD MD Member: IUIS-WHO-AF-CDC Serology Committee Director: Advanced Diagnostics Laboratory University of Calgary Introduction
Advances in Autoantibody Testing & Clinical Applications Marvin J. Fritzler PhD MD Member: IUIS-WHO-AF-CDC Serology Committee Director: Advanced Diagnostics Laboratory University of Calgary Introduction
NEWER TESTS IN NEUROLOGY DR RAJESH V BENDRE HOD, IMMUNOCHEMISTRY METROPOLIS, MUMBAI
 NEWER TESTS IN NEUROLOGY DR RAJESH V BENDRE HOD, IMMUNOCHEMISTRY METROPOLIS, MUMBAI The Central Nervous System was considered an Immunological Privileged Site Blood brain barrier (BBB) Proapoptotic molecules
NEWER TESTS IN NEUROLOGY DR RAJESH V BENDRE HOD, IMMUNOCHEMISTRY METROPOLIS, MUMBAI The Central Nervous System was considered an Immunological Privileged Site Blood brain barrier (BBB) Proapoptotic molecules
Product List Infectious Disease Diagnostics Serology CSF Stool
 VIROTECH Diagnostics GmbH Product List Infectious Disease Diagnostics Serology CSF Stool VIROTECH An Overview Since December 1st, 2016 VIROTECH Diagnostics GmbH Anniversary year 2016 2015 2014 Cooperation
VIROTECH Diagnostics GmbH Product List Infectious Disease Diagnostics Serology CSF Stool VIROTECH An Overview Since December 1st, 2016 VIROTECH Diagnostics GmbH Anniversary year 2016 2015 2014 Cooperation
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Microbiology Department Contact: Dr David Ashburn Zone 3 Raigmore Hospital Tel: +44 (0) 1463 704108 Old Perth Road Fax: +44 (0) 1463 705648
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Microbiology Department Contact: Dr David Ashburn Zone 3 Raigmore Hospital Tel: +44 (0) 1463 704108 Old Perth Road Fax: +44 (0) 1463 705648
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK West of Scotland Specialist Virology Centre New Lister Building Level 5 Glasgow Royal Infirmary 10-16 Alexandra Parade Glasgow G31 2ER Contact:
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK West of Scotland Specialist Virology Centre New Lister Building Level 5 Glasgow Royal Infirmary 10-16 Alexandra Parade Glasgow G31 2ER Contact:
Innovation in Diagnostics. ToRCH. A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES
 Innovation in Diagnostics ToRCH A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES EN TOXOPLASMOSIS Toxoplasmosis is a parasitic disease caused by with the obligate intracellular
Innovation in Diagnostics ToRCH A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES EN TOXOPLASMOSIS Toxoplasmosis is a parasitic disease caused by with the obligate intracellular
REFLEX TESTING LIST March 2012
 Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Aeromonas/Plesiomonas Positive findings Organism ID & Susceptibility April 2011 Culture *AFB Culture and Stain Positive findings Organism ID & Susceptibility Nov. 1999 *AFB Culture Only Positive findings
Product List Autobio Diagnostics. Autobio. autobio diagnostics an autobio group company
 Product List Autobio Diagnostics Autobio.. autobio diagnostics an autobio group company www.autobio-diagnostics.com Autobio Diagnostics Co., Ltd. was established in 1998 and has become one of the largest
Product List Autobio Diagnostics Autobio.. autobio diagnostics an autobio group company www.autobio-diagnostics.com Autobio Diagnostics Co., Ltd. was established in 1998 and has become one of the largest
HPSC - Weekly Infectious Disease Report
 HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 33, 2018 (Notification
HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 33, 2018 (Notification
HPSC - Weekly Infectious Disease Report
 HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 50, 2018 (Notification
HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 50, 2018 (Notification
HPSC - Weekly Infectious Disease Report
 HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 39, 2018 (Notification
HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 39, 2018 (Notification
HPSC - Weekly Infectious Disease Report
 HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 49, 2018 (Notification
HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 49, 2018 (Notification
HPSC - Weekly Infectious Disease Report
 HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 38, 2018 (Notification
HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 38, 2018 (Notification
HPSC - Weekly Infectious Disease Report
 HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 22, 2018 (Notification
HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 22, 2018 (Notification
HPSC - Weekly Infectious Disease Report
 HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 3, 2019 (Notification
HPSC - Weekly Infectious Disease Report Statutory Notifications of Infectious Diseases reported in Ireland via the Computerised Infectious Disease Reporting (CIDR) system for: Week 3, 2019 (Notification
DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations
 Dear User, ISSUE: M008 DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations In order to comply with national quality guidance and as part of our
Dear User, ISSUE: M008 DEPARTMENT OF MICROBIOLOGY IMPORTANT NOTICE TO USERS Turnaround Times (TATs) for Microbiology Investigations In order to comply with national quality guidance and as part of our
Supplemental Figure S1. Neutralization of HIV-1 strain HxB2 by MAbs 2F5, 4E10 and CL15. Recombinant HIV-1HxB2 virions, competent for a single round
 Supplemental Figure S1. Neutralization of HIV-1 strain HxB2 by MAbs 2F5, 4E10 and CL15. Recombinant HIV-1HxB2 virions, competent for a single round of infection, were generated using the luciferase reporter
Supplemental Figure S1. Neutralization of HIV-1 strain HxB2 by MAbs 2F5, 4E10 and CL15. Recombinant HIV-1HxB2 virions, competent for a single round of infection, were generated using the luciferase reporter
LU:research Institutional Repository of Lund University
 LU:research Institutional Repository of Lund University This is an author produced version of a paper published in European journal of clinical microbiology & infectious diseases: official publication
LU:research Institutional Repository of Lund University This is an author produced version of a paper published in European journal of clinical microbiology & infectious diseases: official publication
At the forefront of multiplex PCR
 Prodesse At the forefront of multiplex PCR 2005 PRODUCT CATALOG Distributed by Standard Format Assays Real Time Format Assays Hexaplex Influenza A Influenza B Parainfluenza 1 Parainfluenza 2 Parainfluenza
Prodesse At the forefront of multiplex PCR 2005 PRODUCT CATALOG Distributed by Standard Format Assays Real Time Format Assays Hexaplex Influenza A Influenza B Parainfluenza 1 Parainfluenza 2 Parainfluenza
Viral Structure Herpes virus. Pathology of viral disease. Viral Structure. Topics for the first lecture. Virus size
 Pathology of viral disease Viral Structure Herpes virus Ila Singh, MD, PhD Department of Pathology P & S 14-453 is132@columbia.edu Envelope Tegument Spikes Nucleocapsid Genome Principles of Virology: Molecular
Pathology of viral disease Viral Structure Herpes virus Ila Singh, MD, PhD Department of Pathology P & S 14-453 is132@columbia.edu Envelope Tegument Spikes Nucleocapsid Genome Principles of Virology: Molecular
LIAISON Measles IgG The fully automated solution for quantitative antibody detection
 LIAISON Measles IgG The fully automated solution for quantitative antibody detection FOR OUTSIDE THE US AND CANADA ONLY LIAISON Measles IgG Number of tests 100 Key assay features Method Assay range Solid
LIAISON Measles IgG The fully automated solution for quantitative antibody detection FOR OUTSIDE THE US AND CANADA ONLY LIAISON Measles IgG Number of tests 100 Key assay features Method Assay range Solid
Rheumatologic Testing in Primary Care
 Rheumatologic Testing in Primary Care Fernando Vega, MD October 4, 2008 To help establish a diagnosis in pt with clinical features suggestive of an autoimmune disorder To exclude such disorders in pt with
Rheumatologic Testing in Primary Care Fernando Vega, MD October 4, 2008 To help establish a diagnosis in pt with clinical features suggestive of an autoimmune disorder To exclude such disorders in pt with
BioPlex 2200 Infectious Disease Panels
 BioPlex 2200 System BioPlex 2200 Infectious Disease Panels An Expanding Multiplexed Assay Menu Lyme HIV Ag-Ab MMV IgM Syphilis Total & RPR MMRV EBV HSV-1 & HSV-2 EBV IgM ToRC IgM ToRC Leading the way with
BioPlex 2200 System BioPlex 2200 Infectious Disease Panels An Expanding Multiplexed Assay Menu Lyme HIV Ag-Ab MMV IgM Syphilis Total & RPR MMRV EBV HSV-1 & HSV-2 EBV IgM ToRC IgM ToRC Leading the way with
Potential Reimbursement CPT Codes
 BioFire FilmArray Blood Culture Identification (BCID) Panel Medicare All targets (n) 87150 n x * *BioFire BCID Panel is comprised of 27 total targets. The number of targets allowed for reimbursement may
BioFire FilmArray Blood Culture Identification (BCID) Panel Medicare All targets (n) 87150 n x * *BioFire BCID Panel is comprised of 27 total targets. The number of targets allowed for reimbursement may
MENU PRODUCT MOLECULAR. International Product Listing. Simplexa Molecular Kits Integrated Cycler Molecular Reagents and Primer Pairs
 International Product Listing MOLECULAR PRODUCT MENU Simplexa Molecular Kits Integrated Cycler Molecular Reagents and Primer Pairs Products are not intended for donor screening. Simplexa Molecular Kits
International Product Listing MOLECULAR PRODUCT MENU Simplexa Molecular Kits Integrated Cycler Molecular Reagents and Primer Pairs Products are not intended for donor screening. Simplexa Molecular Kits
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Royal Liverpool & Broadgreen University Hospitals NHS Trust Department of Virology Liverpool Clinical Laboratories Royal Liverpool and Broadgreen
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Royal Liverpool & Broadgreen University Hospitals NHS Trust Department of Virology Liverpool Clinical Laboratories Royal Liverpool and Broadgreen
PRODUCTS. Autoimmune Disease Antigens. Custom Design Antigens. Infectious Disease Antigens
 T H E E X P E R T S O N Y O U R S I D E Antigen Catalog PRODUCTS Autoimmune Disease Antigens Outstanding assay performance with the most complete panel of recombinant and native autoimmune antigens for
T H E E X P E R T S O N Y O U R S I D E Antigen Catalog PRODUCTS Autoimmune Disease Antigens Outstanding assay performance with the most complete panel of recombinant and native autoimmune antigens for
Basic Metabolic Panel
 Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
Basic Metabolic Panel Order Name: CHEM 8 Test Number: 2028100 REV DATE:2/5/2008 Glucose Urea Nitrogen, Blood (BUN) Creatinine Sodium Potassium Serum/Plasma Chloride Bicarbonate Calcium Anion Gap Calculated
ELISA System Line. Analyzers and Reagents. CoreLab DX ELISA. > Instruments for all kinds of laboratories > Broad range of parameters > Proven quality
 ELISA System Line Analyzers and Reagents > Instruments for all kinds of laboratories > Broad range of parameters > Proven quality ELISA CoreLab DX ELISA System Line Unique Solutions Your Advantages > Instruments
ELISA System Line Analyzers and Reagents > Instruments for all kinds of laboratories > Broad range of parameters > Proven quality ELISA CoreLab DX ELISA System Line Unique Solutions Your Advantages > Instruments
ELISA System Line. Analyzers and Reagents ELISA. > Instruments for all kinds of laboratories > Broad range of parameters > Proven quality
 ELISA System Line Analyzers and Reagents > Instruments for all kinds of laboratories > Broad range of parameters > Proven quality ELISA ELISA System Line Unique Solutions Your Advantages > Instruments
ELISA System Line Analyzers and Reagents > Instruments for all kinds of laboratories > Broad range of parameters > Proven quality ELISA ELISA System Line Unique Solutions Your Advantages > Instruments
4. KIDNEYS AND AUTOIMMUNE DISEASE
 How to Cite this article: Kidneys and Autoimmune Disease - ejifcc 20/01 2009 http://www.ifcc.org 4. KIDNEYS AND AUTOIMMUNE DISEASE Maksimiljan Gorenjak 4.1 Autoimmune diseases The human immune system limits
How to Cite this article: Kidneys and Autoimmune Disease - ejifcc 20/01 2009 http://www.ifcc.org 4. KIDNEYS AND AUTOIMMUNE DISEASE Maksimiljan Gorenjak 4.1 Autoimmune diseases The human immune system limits
Is it Autoimmune or NOT! Presented to AONP! October 2015!
 Is it Autoimmune or NOT! Presented to AONP! October 2015! Four main jobs of immune system Detects Contains and eliminates Self regulates Protects Innate Immune System! Epithelial cells, phagocytic cells
Is it Autoimmune or NOT! Presented to AONP! October 2015! Four main jobs of immune system Detects Contains and eliminates Self regulates Protects Innate Immune System! Epithelial cells, phagocytic cells
Chlamydia MIF IgM. Performance Characteristics. Product Code IF1250M Rev. I. Not for Distribution in the United States
 Product Code IF1250M Rev. I Performance Characteristics Not for Distribution in the United States EXPECTED VALUES Community Acquired Pneumonia Population Two outside investigators assessed the Focus Chlamydia
Product Code IF1250M Rev. I Performance Characteristics Not for Distribution in the United States EXPECTED VALUES Community Acquired Pneumonia Population Two outside investigators assessed the Focus Chlamydia
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Clinical Microbiology QMC Campus Derby Road Nottingham NG7 2UH Contact: Jil Bowskill Tel: +44 (0) 1905 760192 Ext 30659 E-Mail: jilean.bowskill@nuh.nhs.uk
2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK Clinical Microbiology QMC Campus Derby Road Nottingham NG7 2UH Contact: Jil Bowskill Tel: +44 (0) 1905 760192 Ext 30659 E-Mail: jilean.bowskill@nuh.nhs.uk
About the LIAISON MDX
 The quality of treatment starts with diagnosis International Molecular Product MENU Simplexa Molecular Kits LIAISON MDX Molecular Reagents Primer Pairs About the LIAISON MDX The LIAISON MDX is an innovative
The quality of treatment starts with diagnosis International Molecular Product MENU Simplexa Molecular Kits LIAISON MDX Molecular Reagents Primer Pairs About the LIAISON MDX The LIAISON MDX is an innovative
Version number CAT 2016/01
 Version number CAT 2016/01 Contents An Introduction to the QCMD EQA Schemes 4 Disease Groups Blood Borne Virus 5 Central Nervous System 5 Congenital Infections 6 Drug Resistance 6 Exotic/Emerging Diseases
Version number CAT 2016/01 Contents An Introduction to the QCMD EQA Schemes 4 Disease Groups Blood Borne Virus 5 Central Nervous System 5 Congenital Infections 6 Drug Resistance 6 Exotic/Emerging Diseases
Comparison of indirect immunofluorescence and line immunoassay for autoantibody detection
 Comparison of indirect immunofluorescence and line immunoassay for autoantibody detection Y.L. Jeon, M.H. Kim, W.I. Lee, S.Y. Kang Department of Laboratory Medicine, KyungHee University School of Medicine,
Comparison of indirect immunofluorescence and line immunoassay for autoantibody detection Y.L. Jeon, M.H. Kim, W.I. Lee, S.Y. Kang Department of Laboratory Medicine, KyungHee University School of Medicine,
INSTAND e.v. in cooperation with:
 Pre-evaluation of the EQA Schemes in Virus Diagnostics June 2015 Corrected version: 10 August 2015 (See Table 3; program 346) INSTAND e.v. in cooperation with: Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten
Pre-evaluation of the EQA Schemes in Virus Diagnostics June 2015 Corrected version: 10 August 2015 (See Table 3; program 346) INSTAND e.v. in cooperation with: Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten
INFECTIOUS DISEASE. Page 2
 Infectious disease Advantages OF TESTING INFECTIOUS DISEASE We are in the middle of a paradigm shift in infectious disease diagnostic testing. As we move from targeted infectious disease testing to a syndromic
Infectious disease Advantages OF TESTING INFECTIOUS DISEASE We are in the middle of a paradigm shift in infectious disease diagnostic testing. As we move from targeted infectious disease testing to a syndromic
ON O C N O C H O E H M E A M T A O T L O O L G O Y
 REAL TIME PCR KITS von AB Analitica www.bioproducts.at ONKOHÄMATOLOGIE INFEKTIOLOGIE HUMANGENTIK CE IVD gleiches PCR Protokoll REALQUALITY CATALOGUE REALQUALITY REAL TIME PCR KITS Characteristics: CE IVD
REAL TIME PCR KITS von AB Analitica www.bioproducts.at ONKOHÄMATOLOGIE INFEKTIOLOGIE HUMANGENTIK CE IVD gleiches PCR Protokoll REALQUALITY CATALOGUE REALQUALITY REAL TIME PCR KITS Characteristics: CE IVD
BactoReal Kits for detection of bacteria
 BactoReal Kits for detection of bacteria BactoReal Kit Bartonella spp. Detection of the glta gene of B. clarridgeiae, B. elizabethae, B. grahamii, B. henselae, B. koehlerae, B. quintana, B. volans and
BactoReal Kits for detection of bacteria BactoReal Kit Bartonella spp. Detection of the glta gene of B. clarridgeiae, B. elizabethae, B. grahamii, B. henselae, B. koehlerae, B. quintana, B. volans and
Test Requested Specimen Ordering Recommendations
 Microbiology Essentials Culture and Sensitivity (C&S) Urine C&S Catheter Surgical (excluding kidney aspirates) Voided Requisition requirements o Specific method of collection MUST be indicated o Indicate
Microbiology Essentials Culture and Sensitivity (C&S) Urine C&S Catheter Surgical (excluding kidney aspirates) Voided Requisition requirements o Specific method of collection MUST be indicated o Indicate
