PEPTIDE-MHC-STABILITY DETERMINES THE SIZE OF THE CD8+ T CELL RESPONSE TOWARD AN IMMUNORECESSIVE TUMOR ANTIGEN DETERMINANT
|
|
|
- Valentine Barber
- 6 years ago
- Views:
Transcription
1 The Pennsylvania State University The Graduate School College of Medicine PEPTIDE-MHC-STABILITY DETERMINES THE SIZE OF THE CD8+ T CELL RESPONSE TOWARD AN IMMUNORECESSIVE TUMOR ANTIGEN DETERMINANT A Dissertation in Microbiology and Immunology by Alan Michael Watson 2011 Alan M. Watson Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2011
2 The dissertation of Alan M. Watson was reviewed and approved* by the following: Todd D. Schell Associate Professor of Microbiology and Immunology Dissertation Advisor Chair of Committee Neil D. Christensen Professor of Pathology, and Microbiology and Immunology Associate Chief, Division of Experimental Pathology David J. Spector Professor of Microbiology and Immunology Distinguished Educator Mary E. Truckenmiller Assistant Professor of Microbiology and Immunology Ronald P. Wilson Professor and Chair of the Department of Comparative Medicine Director, Penn State Hershey Animal Resource Program Richard J. Courtney Professor and Chair of the Department of Microbiology and Immunology *Signatures are on file in the Graduate School ii
3 ABSTRACT CD8+ T cells recognize peptide-determinants bound to major histocompatibility complex class-i (MHC-I) molecules on the surface of antigen presenting cells. Differences in the number of T cells responding to various peptide-determinants often results in the establishment of an immunodominance hierarchy in which T cells specific for one or more determinants predominate the overall response. T cells specific for determinants that mount a strong response are termed immunodominant whereas weaker responses are termed subdominant, cryptic or immunorecessive. A relationship between the affinity of peptide-determinants for MHC molecules and the size of both CD8+ and CD4+ T cell responses has been well established since the early 1990 s. Specifically, a few studies have established that the dissociation rate of peptides from their MHC complex (pmhc-stability) correlates strongly with immunodominance; those determinants that have a slow dissociation rate (high pmhc-stability) are generally dominant while rapid dissociation rates (low pmhc-stability) are often subdominant. However, the mechanism(s) that connect pmhc-stability and immunodominance is largely unknown. Using the virus-derived oncoprotein SV40 Large Tumor-Antigen (TAg) as a model we have addressed the relationship between pmhc-stability and immunodominance. TAg encodes four H-2 b -restricted CD8+ T cell determinants, sites I, II/III, IV and V. Upon immunization with TAg, a well characterized CD8+ T cell immunodominance hierarchy is established such that IV>I>II/III; responses to site V TAg ( ) are only detected following the deletion or inactivation of the other three determinants. The availability of T cell antigen receptor (TCR) transgenic mice that produce T cells specific for sites I and V make these determinants well suited for iii
4 characterizing the differences between immunodominant and immunorecessive determinants. The immunorecessive nature of site V is due in part to inefficient cross-priming of naïve site V-specific T cells such that only a fraction of naïve site V-specific T cells undergo priming. In contrast, nearly all site I-specific T cells undergo cross-priming in response to the same immunization. The cause of this discrepancy is unknown. However, site V forms low-stability pmhc whereas site I forms high-stability pmhc. Thus, it may be the low-stability of site V that results in inefficient cross-priming. The contribution of pmhc-stability to the regulation of naïve T cell cross-priming has never been directly addressed in the TAg system or others. Thus, in this study we have investigated whether the pmhc-stability of site V affects site V-specific T cell cross-priming. In order to address this question, we identified two point mutants, Q489A and G490A, which enhanced the pmhc-stability of site V and conserved epitope recognition by site V-specific T cells. We incorporated the mutations into TAg and produced immortalized cell lines. Immunization with Q489A or G490A cell lines resulted in a detectable endogenous site V-specific T cell response. This is the first time that an endogenous site V-specific T cell response has been observed following immunization with cell lines expressing the three dominant TAg determinants. Thus Q489A and G490A overcame the immunorecessive nature of site V. Our data suggest that this novel phenotype results from two distinct mechanisms: 1) As pmhc-stability increases, so does the efficiency of naïve T cell crosspriming such that a greater fraction of naïve site V-specific T cells undergo activation. iv
5 2) As pmhc-stability increases, so does the duration of site V-specific T cell cross-priming, driving enhanced accumulation of responding T cells. Our results indicate that following immunization with TAg immortalized cell lines approximately one-third of TCR transgenic T cells specific for site V (TCR-V cells) undergo cross-priming. In contrast, immunization with cell lines expressing Q489A or G490A TAg results in a two fold increase in the number of TCR-V cells that undergo cross-priming. Using a highly sensitive T cell magnetic-enrichment protocol, we demonstrate for the first time that TAg immortalized cells prime previously undetectable populations of endogenous site V-specific T cells. Although technical limitations hinder direct determination of the efficiency of endogenous site V-specific T cell priming, extrapolation of our data suggests that endogenous site V-specific T cells also experience inefficient cross-priming. Altogether, our results suggest that pmhc-stability determines the fraction of site V-specific T cells that is cross-primed. Our results also suggest that the size of the site V-specific T cell response increases as the duration of detectable cross-priming increases. Our results indicate that the window for cross-priming of TCR-V cells is limited to approximately hours. In contrast, cross-priming of TCR transgenic T cells specific for site I (TCR-I) is detected for at least seven days. We demonstrate that the Q489A and G490A substitutions resulted in an increase in the duration of site V-specific T cell cross-priming. Using two independent methods, our results suggest a direct relationship between the duration of site V-specific T cell cross-priming and the size of the site V-specific T cell response. We find that the duration of cross-priming determines the size of the T cell response via enhanced T cell accumulation of site V-specific T cells. Thus, our results suggest that pmhc-stability determines the size of the site V-specific T cell response by determining the duration of site V-specific T cell cross-priming. v
6 The results presented in this study suggest that pmhc-stability determines the size of the site V-specific T cell response by first determining the efficiency of naïve T cell cross-priming and subsequently facilitating the accumulation of activated T cells. Thus, the low peptide-mhc stability of site V contributes to its immunorecessive nature by reducing the fraction of T cells that are cross-primed and limiting their expansion. Our findings have implications for the optimization of T cell responses toward subdominant and immunorecessive determinants in the context of multivalent vaccines. Such optimization is critical for the successful development of tumor immunotherapy strategies and progress toward viral vaccines that limit immune escape. vi
7 Table of Contents List of Tables... ix List of Figures... x Abbreviations... xii Acknowledgements... xiv Chapter I: Introduction... 1 Chapter II: Literature Review... 7 Introduction... 7 Antigen Processing and Presentation... 8 Major Histocompatibility Complex Molecules... 8 Peptide binding and MHC-Stability Direct Presentation by MHC-I Presentation of Exogenous Antigen Cross-Presentation T Cell Immune Response...37 Antigen Recognition Antigen Presenting Cells T Cell Immune Response Antigen Stimulus Immunodominance pmhc-stability and the T Cell Response...60 SV40 Large T-Antigen and the CD8+ T Cell Immune Response...67 Chapter III: Materials and Methods...83 Chapter IV: Results...94 Increasing Peptide-MHC Stability Augments the Cross-Priming of Naïve CD8+ T Cells Specific for SV40 TAg Site V...94 The Duration of Cross-Presentation Determines the Accumulation but Not the Recruitment of Site V-Specific T Cells Chapter V: Discussion Proposed model explaining the findings from this study Our findings Implications and applications for CD8+ T cell immunity toward SV40 TAg vii
8 Implications and broad significance of our study References Appendix A: SV11 Mice Mount a Robust Site V-Specific T Cell Response Following Q489A Cell Immunization Introduction Results Discussion Appendix B: Comparison of Thy1.1+CD8+ Cells and B-Cells as a Static Population in Spike Experiments viii
9 List of Tables Table 1: Relative Affinity Values for the Interaction of H-2D b and Monosubstituted ASNENMETM Peptides...19 Table 2: Replacing Deleterious Residues in Low Relative Affinity Peptides Dramatically Increases Peptide Binding...21 Table 3: T Cell Surface Markers to Distinguish Antigen Experience...45 Table 4: Immunodominance Hierarchies...54 Table 5: Peptide Binding Affinity, pmhc-stability and Immunogenicity...63 Table 6: SV40 TAg H-2 b -Restricted T Cell Clones...73 Table 7: Cell Lines Used in this Study...86 Table 8: Rationale for Mutating TAg Site V-Residues at P1 and P ix
10 List of Figures Figure 1: Structure of MHC-I Molecule (H-2D b )...11 Figure 2: Structure of MHC-II Molecule (HLA-DR1)...13 Figure 3: The MHC class-i processing Figure 4: Proposed pathways for cross-presentation by MHC class I molecules...36 Figure 5: Cross-Priming of CD8+ T cells...43 Figure 6: CTL (H-2 b ) Determinants in SV40 TAg Figure 7: Peptide-MHC Stability of SV40 TAg H-2D b Determinants...78 Figure 8: Site V-specific T Cells are Poorly Cross-Primed...80 Figure 9: Characterization of Site V APLs...99 Figure 10: Q489A and G490A Cross-React with Clonal and Polyclonal Populations of Site V-specific T cells Figure 11: Visual Representation of the TAg Constructs Used for Cell Line Production Figure 12: Cell Lines Express Full-Length TAg and the Appropriate Site V-APL Figure 13: Q489A and G490A are Processed Figure 14: TAg Protein Stability Figure 15: TCR-V Cells Respond to Immunization with Q489A and G490A Cells Figure 16: Q489A and G490A Cells Overcome the Immunorecessive Nature of Site V Figure 17: Q489A and G490A Enhance the Fraction of Naïve Site V-Specific T cells that are Cross-Primed Figure 18: Bypass of Cross-Presentation Results in High Fraction of TCR-V Recruitment x
11 Figure 19: The Number of Naïve TCR-V Cells has No Effect on TCR-V Cell Recruitment Figure 20: Endogenous Site V-Specific T Cells are Primed Following WT-TAg Immunization Figure 21: TCR-V Cells have a Shortened Duration of Cross-Priming Compared to TCR-I Cells Figure 22: TCR-I and TCR-V Cells Have a Similar Avidity of Activation Figure 23: Q489A and G490A Cells Extend the Duration of TCR-V Cross-Priming Figure 24: TCR-V Cell Cross-Priming is Maintained Following Multiple Immunizations Figure 25: Multiple Immunizations Result in a Marked Expansion of TCR-V Cells Figure 26: Multiple Immunizations Enhance TCR-V Cell Accumulation but not the Fraction of Naive T Cell Recruitment Figure 27: Testing CD11c-DTR Mice Figure 28: The Duration of Cross-Priming Determines Accumulation but Not the Fraction of Naïve TCR-V Cell Recruitment Figure 29: Direct Presentation Does Not Affect Naïve T Cell Recruitment or Accumulation Figure 30: Proposed Model Describing the Relationship between SV40 TAg Site V- Specific T cell Cross-Priming and pmhc-stability Figure 31: SV11 Mice Mount a Robust Site V-Specific T Cell Response Following Q489A Cell Immunization Figure 32: Comparison of Thy1.1+ Cells and B-Cells as a Static Population in Spike Experiments xi
12 Abbreviations aa Amino Acid APC Antigen Presenting Cell APL Altered-Peptide Ligand B6 C57BL/6 Mouse CDR Complementarity Determining Region CLIP MHC class II associated invariant-chain peptide CTL Cytotoxic T Lymphocyte DC Dendtritic Cell DLN Draining Lymph Node DRiPs Defective Ribosomal Products ER Endoplasmic Reticulum ERAAP ER Resident Aminopeptidase associated with Antigen Processing HSP Heat Shock Protein IF Immunofluorescence IFN- Interferon-gamma Ii Invariant Chain LMP Low Molecular Weight Protein MHC Major Histocompatibility Complex xii
13 MHC-I Major Histocompatibility Complex Class-I MHC-II Major Histocompatibility Class-II Molecule papc Professional Antigen Presenting Cell PDL Programmed Cell Death Ligand PLC Peptide Loading Complex pmhc Peptide-bound Major Histocompatibility Complex pmhc-i Peptide-bound Major Histocompatibility Complex Class-II RA Relative Affinity SV40 Simian Virus 40 TAg SV40 Large Tumor-Antigen tag SV40 Small Tumor-Antigen TAP Transporter associated with Antigen Processing TCR T Cell Antigen Receptor TCR-I SV40 T Antigen Site I-Specific T Cell Receptor Transgenic T cells TCR-IV SV40 T Antigen Site IV-Specific T Cell Receptor Transgenic T cells TCR-V SV40 T Antigen Site V-Specific T Cell Receptor Transgenic T cells TLR Toll Like Receptor 2 m - 2 -Microglobulin xiii
14 Acknowledgements If I have seen a little further it is by standing on the shoulders of Giants. -Issac Newton Foremost, I must thank the faculty and staff of the Department of Microbiology and Immunology. I am fortunate to have completed my education with dedicated and talented researchers and teachers. I extend a special thanks to Dr. Richard Courtney whose direct involvement with graduate student recruitment was a significant factor when deciding to attend this graduate program. I would like to thank Billie Burns and Becky Yokey for always being available and having an answer when problems or questions arise. Finally, I thank the members of the kitchen, for their hard work and enjoyable company; research is much easier with their support. The most influential individual in my education has been my mentor, Dr. Todd Schell. Todd has practiced extraordinary support and patience during my tenure in his lab. He has provided me with freedom to pursue my project, and in doing so, he has granted me the opportunity to make mistakes and develop my own approach to science. Todd has always displayed the utmost integrity in his approach to research and has taught me to consider every result with skepticism and entertain every possibility before arriving at a conclusion. I am grateful for Todd s mentorship and to have had the opportunity to complete my thesis under his guidance. I have had the opportunity to work alongside many excellent people. Dr. Beth Vigliotti, Dr. Angela Tatum, Dr. Jodi Yorti, Dr. Christina Ryan, Gene Cozza, Lindsay xiv
15 Ward-Kavanagh and Mel Epler have been among those with whom I have shared the lab. Special thanks are given to Jeremy Haley who has been a constant and integral presence within the lab. Much of what I have accomplished would not have been completed without his skill and hard work. To all of you, I wish success. I would like to thank the members of my thesis committee Dr. David Spector, Dr. Mary E. Truckenmiller, Dr. Neil Christensen and Dr. Ronald Wilson. I am appreciative for your guidance, mentorship and hard work on my behalf. In addition, I owe thanks to individuals that have mentored me in the past. Dr. Satvir Tevethia, Dr. Larry Mylin, Dr. Leslie Parent, Dr. Phil Thuma, and Dr. Lawrence Samelson. Your influence has endowed me with a sense of who I wish to become as a scientist and the staggering realization of how far I must go. I am forever grateful. To those individuals who contributed to this document or the work described herein: Larry Mylin and Megan Thompson, thank you for providing mutants and cell lines. Sandip Savaliya, thank you for mouse colony management and cell line genomic DNA sequencing. Mel Epler, thank you for the production of reagents and helpful discussion. Jeremy Haley, thank you for the production of reagents, discussion and assistance with and contribution of experiments presented in this document. Credit is given where appropriate. Anuj Kalsy, thank you for helpful suggestions for this manuscript. I wish you success as you begin your graduate education, and I have no doubt that you will flourish. To my mother and father, Cathy Miller and Randy Watson: thank you for the investment that you made in me. Your unconditional love, support and encouragement have made it possible for me to pursue my Ph.D. and the wherewithal to be successful. I credit you most for my accomplishments. To my grandparents, Shirley and Richard xv
16 Watson: you have been a constant source of encouragement and powerful role models in my life. Gram, although you are no longer with us, you are always with me. To all of you, I love you. Finally, my wife: Suzzi has been a constant and steadfast source of support and encouragement throughout my graduate studies. She has been my closest companion, confidante and source of sanity throughout this process. The world may never know how much sanity I have siphoned from her simply to keep me afloat. Despite this, she has never complained about the long process of graduate school or the months that I neglected her as I wrote my thesis. Suzzi may be the hardest working person that I know. Her inspiring work ethic and devotion to her students should be the standard to which all teachers are held. As a result, she has motivated me to push myself and excel at my work. I cannot imagine a better person to have accompanied me through graduate school or to call my wife. Suzzi, I love you, and I am looking forward to beginning our family. xvi
17 I dedicate this dissertation to my grandparents Richard and Shirley Watson and to my daughter Abigail Watson. Together, you represent all that makes this worth perusing: unconditional love, support, and hope for the future. xvii
18 Chapter I: Introduction CD8+ cytotoxic T cells are important immune effectors against tumor cells and cells harboring viruses. CD8+ T cell responses are directed against major histocompatibility complex class-i (MHC-I) bound peptides that represent 8-10 amino acid segments of virus or self derived peptides. Although MHC-I molecules bind only one peptide at a time, they have a modular capability to associate with numerous peptide ligands as long as the peptide fits a general binding motif. The peptides that bind to MHC-I impart a range of peptide-mhc (pmhc) stabilities measured by the halflife of pmhc-retention at the plasma membrane. pmhc-stability must be sufficiently high in order to initiate a T cell response [1]. CD8+ T cell responses are often multivalent such that T cells are triggered against multiple determinants simultaneously. Commonly, CD8+ T cells specific for a particular determinant will expand to a higher frequency than those specific for other determinants. This phenomenon is known as immunodominance. Immunodominant determinants generate the highest frequency of T cells whereas subdominant and immunorecessive determinants generate low or undetectable responses, respectively. Among the factors that contribute to immunodominance are 1) antigen processing and presentation, 2) CD8+ T cell naïve precursor frequency, and 3) competition among T cells specific for different determinants. As a component of antigen processing and presentation, evidence suggests that pmhc-stability influences CD8+ T cell immunodominance hierarchies [2]; however, a mechanism has never been defined. Therefore, investigating these mechanisms has implications for the successful design of multivalent CD8+ T cell-based vaccines. 1
19 MHC-I molecules typically present peptides derived from de novo synthesized proteins; however, professional antigen presenting cells (papcs) can present MHC-I peptide determinants from exogenous antigens. This process is known as crosspresentation [3] and is required for the activation (cross-priming) of naïve T cells specific for antigens not directly presented by papcs such as tumor derived antigens (reviewed in [4]) and infectious agents that do not directly infect papcs. The importance of crosspresentation for initiating CD8+ T cell responses makes the process a likely candidate for the regulation of immunodominance in some systems. Previous studies suggest that peptides forming high-stability pmhc are presented more efficiently than peptides forming low-stability pmhc [5-7]; however, whether pmhc-stability affects cross-priming of CD8+ T cells remains unknown. Furthermore, determining whether pmhc-stability has effects on naïve T cell cross-priming may reveal the mechanism connecting pmhcstability and immunodominance. We are using SV40 Large T-Antigen (TAg) as a model to study mechanisms connecting pmhc-stability and immunodominance. TAg has four CD8+ T cell determinants; sites I, II/II, V (H-2D b -restricted) and IV (H-2K b -restricted). Site V from TAg is an immunorecessive determinant, indicating that it fails to induce a detectable CD8+ T cell response. Upon immunization with TAg constructs that lack the dominant TAg determinants; sites I, II/III, and IV, a detectable CD8+ T cell response to site V is obtained [8]. The mechanisms that contribute to this phenotype have not been fully defined; however, one study demonstrated that site V-specific T cells are inefficiently cross-primed such that only a fraction of site V-specific T cells are activated [9]. Furthermore, preliminary data indicate that the ability to cross-prime site V-specific T cells is quickly lost following immunization whereas the duration of site I-specific T cell cross-priming continues for at least 7 days [10]. One intrinsic difference between site V 2
20 and the other H-2D b -restricted TAg determinants is that site V forms relatively lowstability peptide-mhc complexes, making TAg an ideal system to evaluate how pmhcstability affects cross-priming and immunodominance. In this study, we address whether pmhc-stability determines the fraction of site V-specific T cells that undergo priming and the duration of site V-specific T cell crosspriming. Furthermore, we address the role that the fraction naïve T cells activated and the duration of site V-specific T cell cross-priming plays in the immunorecessive phenotype of site V. To address these points, we have developed the following hypothesis and specific aims: HYPOTHESIS: The low pmhc-stability of site V contributes to its immunorecessive nature by impacting the efficiency and the duration of naïve T cell cross-priming. SPECIFIC AIM 1: Determine whether pmhc-stability influences the immunorecessive nature of site V. 1. Identify site V point mutations that stabilize pmhc interactions. To determine whether the pmhc-stability influences the immunorecessive nature of site V, we first modified the pmhc-stability of site V. Therefore, our goal for this subaim was to identify point mutations within the site V determinant that result in enhanced pmhc-stability. 3
21 2. Determine whether the site V point mutations affect recognition by site V-specific T cells. To assess whether the site V point mutants influence the immunorecessive nature of site V, the mutants must be recognized by site V-specific T cells. Recognition of the site V point mutants by site V- specific T cells should allow us to evaluate the site V-specific T cell response using the same tools used for wild type site V including MHCtetramer reagents and TCR-V cells. Thus, in this subaim we determined whether our site V point mutants are recognized by endogenous site V- specific T cells and transgenic TCR-V cells. 3. Determine whether pmhc-stability alters the immunorecessive nature of site V. If pmhc-stability alters the immunorecessive nature of site V than an endogenous site V-specific T cell response should be detected in C57BL/6 mice. Thus, TAg immortalized cell lines were created that contain the site V point mutations. These cell lines were used for immunization, and endogenous T cell populations were monitored for the induction of a site V-specific T cell response. SPECIFIC AIM 2: Determine whether pmhc-stability affects cross-priming of site V-specific T cells. 1. Determine whether pmhc-stability affects the fraction of site V- specific T cells that undergo cross-priming. 4
22 The immunorecessive nature of site V is due in part to only a fraction of site V-specific T cells undergoing cross-priming following TAg immunization [9]. In this subaim, we determined whether pmhc-stability determines the fraction of site V-specific T cells that undergo crosspriming. We monitored the fraction of site V-specific T cells that undergo cross-priming through the use of TAg immortalized cell lines expressing site V point mutants that enhance pmhc-stability. 2. Determine whether pmhc-stability affects the duration of site V- specific T cell cross-priming. The stability of pmhc directly influences the length of time that pmhc molecules are retained on the plasma membrane. Thus, pmhc-stability may influence the duration that naïve T cells can encounter antigen and undergo cross-priming. In this subaim, we determined whether point mutants that enhance the pmhc-stability of site V affected the duration of naïve site V-specific T cell cross-priming. SPECIFIC AIM 3: Determine whether the duration of site V-specific T cell crosspriming affects the size of the site V-specific T cell response. 1. Determine whether the duration of site V-specific T cell crosspriming can be altered by administering multiple immunizations. Since site V-specific T cell cross-priming is limited to hours, studying site V cross-priming beyond hours following a single immunization remains a challenge. In this subaim we investigated 5
23 whether administering multiple immunizations of TAg immortalized cells at 24 hour intervals extended the duration of site V-specific T cell crosspriming beyond hours. Success of this method allowed us to study the effects that the duration of cross-priming has on the site V-specific T cell response. 2. Determine whether the duration of site V-specific T cell crosspriming alters the fraction of naïve site V-specific T cells that undergo activation. The duration of cross-priming may determine the fraction of naïve T cells that undergo cross-priming. In this subaim, we investigated whether the duration of site V-specific T cell cross-priming determines the fraction of naïve site V-specific T cells that undergo activation. 6
24 Chapter II: Literature Review Introduction A diverse array of cell types makes up the innate and adaptive arms of the immune response. Among the cells of the innate immune system are natural killer cells, macrophages and dendritic cells (DC) that respond to inflammatory environments and pathogen associated molecular patterns. Innate cell types are vital for the survival of an organism and constitute the primary defense against pathogens and disease (reviewed in [11]. However, the innate immune system is often inadequate following infection by aggressive pathogens or the development of tumors. The adaptive immune system augments innate immunity to specifically target these obstacles. Adaptive immunity hedges its arsenal against possible pathogens by generating a diverse array of receptors by random homologous recombination. B cells produce antibodies and CD4+ and CD8+ T cells recognize pathogen infected cells using specialized TCRs to prevent infection and eliminate infected cells, respectively. Innate immune cells work in concert with the adaptive arm of the immune response to control invading pathogens. CD8+ T cells are often referred to as cytotoxic T lymphocytes (CTL), because they induce targeted cell death upon ligation of their TCR. CTL are efficient weapons against intracellular pathogens and they can be effective in the control of established tumors. The general mechanisms that govern CD8+ T cell immune responses are well defined; however, a great deal of work remains before we can optimize responses towards individual antigens. For example, CD8+ T cell responses are often polarized toward particular targets within multivalent antigens while failing to respond to others, a phenomenon termed immunodominance. Understanding how to overcome immunodominance is imperative to the production of effective vaccines and tumor 7
25 immunotherapies eliciting a broad array of CD8+ T cell responses, which have been demonstrated to more effectively control viruses and tumors [12,13], rather than a limited subset. Below, a brief review of the literature discusses the factors that lead to the initiation of T cell responses and contribute to immunodominance. Antigen Processing and Presentation Understanding the role that CTL play in the immune response begins with understanding what makes CD8+ T cells an effective tool against viruses and tumors. CD8+ T cells simultaneously recognize an antigenic peptide-determinant derived from a protein antigen and an MHC-I molecule to which the determinant is bound and displayed on the plasma membrane of nucleated cells. Thus, the antigenic peptide-bound-mhc (pmhc) molecule, as it is recognized by a T cell, is very different from the antigen in its original protein context. The molecular manipulation leading from protein to pmhc is known as antigen processing and presentation. pmhc class-i molecules are necessary for T cell function from the initiation of the CD8+ T cell immune response to the targeting of virus infected or tumor cells for elimination. This section will introduce MHC molecules and discuss antigen processing and peptide presentation, focusing on MHC-I. Major Histocompatibility Complex Molecules MHC molecules are transmembrane heterodimers that constitute a variety of highly polymorphic alleles expressing proteins that bind and display peptides to the extracellular environment. Although each MHC allomorph binds only a restricted repertoire of peptides, the diversity of alleles within a given organism and the availability of peptide-containing antigens impart an immense diversity of determinants for T cell recognition. Due to the integral function of MHC in T cell immune responses and the 8
26 critical role of pmhc stability to this thesis, MHC molecules will be reviewed in detail below with an emphasis on peptide binding and complex stability. Antigen processing and MHC acquisition of peptides will be discussed in detail following the introduction to MHC. MHC molecules are categorized as class-i (MHC-I) and class-ii (MHC-II) subtypes. MHC-I molecules are expressed on all nucleated cells within the mammalian host, essentially every cell type with the exception of mature red blood cells. MHC-II molecules are found constitutively expressed on particular cell types known as professional antigen presenting cells (papc), as well as thymic epithelial cells. papcs are specialized for the presentation of antigen and the activation of T cells. The primary role of the MHC molecule is to bind and present peptides derived from pathogen and self proteins to CD8+ (recognize MHC-I) and CD4+ (recognize MHC-II) T cells. To accomplish this objective, MHC-I and MHC-II molecules have developed similar strategies of peptide association while evolving distinct structural differences. MHC Structure The distinctive structures of MHC-I and MHC-II molecules can be seen in Figure 1 and Figure 2, respectively. MHC class-i molecules consist of 1, 2, and 3 extracellular heavy chain domains [14] non-covalently bound to -microglobulin ( 2 m) [15] (Figure 1A). The 1 and 2 domains each contribute a network of -pleated sheets that line the floor of the peptide-binding groove and a -helical region that forms the sides of the groove [16] (Figure 1B). The peptide-binding groove for MHC-I molecules is 9
27 Figure 1 Structure of MHC-I Molecule (H-2D b ) MHC class I structure: (A) H-2D b (α chain in grey, β 2 m chain in blue) in complex with ASNENMETM (blue stick model, pdb 1HOC). (B) View from the top into the class I peptide binding groove. Conserved side chain residues that form hydrogen bonds with main chain atoms of the bound peptide are blue, polymorphic side chains are gray. Hydrogen bonds are indicated by dotted lines. The proposed tapasin binding region α is in red, and the α 2-1 helix is in cyan. (C) The main class I pockets A, B, and F are shown. This figure and legend were reproduced with from Yaneva et al. [17], with permission obtained from Elsevier Limited, The Boulevard, Langford Lane, Kidlington, Oxford, OX5 1GB, UK. License number:
28 Figure 1: Structure of MHC-I Molecule (H-2D b ) 11
29 Figure 2 Structure of MHC-II Molecule (HLA-DR1) MHC class II structure: (A) HLA DR1 (α chain in blue, β chain in gray) in complex with the peptide, PKYVKQNTLKLAT (blue stick model, pdb 1DLH). (B) View from the top into the class II peptide binding groove. Conserved side chain residues that form hydrogen bonds with main chain atoms of the bound peptide are blue, polymorphic side chains are gray. Hydrogen bonds are indicated by dotted lines. The proposed DM binding region α51 54 is in red, and the antibody epitope for empty class II, the β53-67 helix, is in cyan. (C) The main class II pockets P1, P4, P6, P7, and P9 are shown. This figure and legend were reproduced with from Yaneva et al. [17], with permission obtained from Elsevier Limited, The Boulevard, Langford Lane, Kidlington, Oxford, OX5 1GB, UK. License number:
30 Figure 2: Structure of MHC-II Molecule (HLA-DR1) 13
31 limited in size by bulky side chains on both ends, restricting the length of peptides that bind MHC-I to approximately 8-10 amino acids (aa) [18] (Figure 1C). The 1 and 2 domains are extremely polymorphic [19]; however, the overall superstructure of the binding-cleft contains only minor variability among MHC-I haplotypes. The 3 domain of the heavy chain serves two structural purposes: it anchors the MHC molecule to the plasma membrane by connecting to the transmembrane domain and it synergizes with 2 m in order to structurally support the peptide-binding cleft and stabilize the overall complex. The a3 domain also binds CD8 [20-24], a co-receptor important for the recognition of MHC molecules by CD8+ T cells. Many MHC structures have led to the overall consensus structure of MHC-I described here [25]. MHC-II molecules consist of a and chain heterodimer including 1 and 2, and 1 and 2 extracellular domains (Figure 2A). The 2 and 2 domains support the peptide-binding groove and anchor the heterodimer to the membrane whereas the 1 and 1 domains form the peptide-binding groove (Figure 2B). In a similar manner to MHC-I molecules, the groove consists of a floor of -sheets and two -helical regions. Unlike MHC-I molecules, MHC-II molecules are open at the ends of the peptide-binding groove, allowing relatively large peptides of differing lengths to bind (Figure 2C) [26]. MHC-II molecules bind peptides of 13-25aa [27], that may spill-out over the open ends of the binding groove. Although MHC-I and MHC-II molecules serve fundamentally similar functions, they have evolved unique structures allowing for the display of different peptide forms. 14
32 Peptide binding and MHC-Stability Although this thesis is not directly concerned with the mechanisms that lead to high-stability peptide-mhc (pmhc) interactions, understanding the factors that contribute to these interactions is crucial for our studies beginning in Chapter IV. pmhcstability may refer to both the affinity of interaction between peptide and MHC at equilibrium binding (a combination of the peptide s K on and K off ) or the rate / threshold at which the pmhc complexes dissociate (K off ). Traditionally, affinity is measured as equilibrium binding whereas stability measures the rate of pmhc decay from the plasma membrane in peptide-free media. Affinity is reported as relative affinity (RA) and stability is reported as a half-life. Often affinity and stability have a direct relationship (usually affinity is a predictor of stability, an example is shown in [28]); however, exceptions exist in which high-affinity MHC ligands form low-stability pmhc [1]. In this thesis, pmhc-stability strictly refers to the dissociation rate of a peptide from its cognate MHC. Thus, the term high-stability peptide, infers that a peptide, when bound to its cognate MHC, forms a pmhc interaction with a slow dissociation rate. Although affinity and stability are relative values, one study categorized low-stability peptides as having a half-life of <3 hours at 37 degrees Celsius and high-stability peptides as having a 3 hour half-life [1]. Generally a peptide that forms high-stability pmhc binds with high-affinity. Peptides that bind with high-affinity are often predictable based on aa sequence (see below); however, the specific attributes of a peptide resulting in high- or low-stability interactions are not as well understood and therefore are less predictable. The only way to determine stability is through empirical tests. Since pmhc-affinity is better understood, major peptide and MHC features that result in the formation of high-affinity interactions are discussed below. 15
33 A unique property of MHC molecules is the ability to bind a wide range of peptides with high affinity. MHC molecules have a general affinity for any peptide which fits the basic alignment of the binding groove due to sequence independent interactions. Such interactions include van der Waals forces and a conserved hydrogen bonding network with the -pleated sheets and the -helices of the binding cleft (reviewed in [25]), indicated by dotted lines in Figure 1B and Figure 2B. These sequence independent interactions are similar for both classes of MHC. High-affinity pmhc interactions are mediated by sequence-specific contacts that are determined by the polymorphic amino acids within the binding cleft. The polymorphic side chains within the H-2D b and HLA-DM1 molecules are represented as grey in Figure 1B and Figure 2B, respectively. The polymorphic side chains select for high-affinity peptides by creating pockets for peptide interactions in which preferred peptide residues interact with the MHC side chains (reviewed in [17,25]). Often two or three peptide residues, known as anchor residues, contribute a large share of the binding affinity [18,29] (reviewed in [30]). The importance of peptide anchor-residues and the mechanics of peptide binding are similar between MHC-I and MHC-II molecules, so for simplicity sake and direct relevance to this thesis, class-i MHC molecules, with an emphasis on H-2D b (Figure 1), will be the continued focus of discussion. The binding of a peptide to MHC-I initiates a conformational change in the complex [31,32] (reviewed in [33]). This change is not entirely understood because the flexibility of a peptide-free MHC-I molecule is such that crystal formation cannot be accomplished. However, it is known that peptide-induced changes in the structure are important for the binding of 2 m [34], a crucial component for MHC-I stability. One study demonstrated that the dissociation rate of 2 m decreases upon peptide association, especially peptides that form high-stability complexes [35]. Furthermore, antibody- 16
34 epitopes in MHC that are accessible in the open (peptide-free) conformation of MHC molecules are obscured following peptide association [36-42]. Such observations suggest that the formation of a tri-molecular complex of peptide/ -chain/ 2 m is critical for the overall stability of class-i molecules. As a result, the association of a high-stability peptide forms a complete pmhc which facilitates transport from the ER to the cell surface [34] and remains critical for the retention of pmhc complexes at the plasmamembrane. Generally, peptides bound to MHC-I interact strongly with the peptide-binding groove at the N-terminal (A and B) and C-terminal (F) pockets (Figure 1C). Often, peptide-residues are buried within the MHC structure (reviewed in [17,25]). The C- terminal region is particularly critical for peptide binding and MHC-stability; in one instance, crystallization of an MHC-I molecule was achieved with only a C-terminal fragment while N-terminal fragments were incapable of forming similarly stable interactions [43]. Thus, nearly all peptides bound to MHC-I contain C-terminal anchors [30]. H-2D b prefers to bind peptides of 9 aa in length with the anchors canonically found at peptide-residues 5 and 9 consisting of asparagine and methionine, respectively (leucine and isoleucine are common substitutes for methionine at position 9) [18]. The loss of any one of these anchors results in substantially lower binding affinities, generally eliminating MHC binding altogether. Although anchor residues are the primary contributor to peptide binding, nonanchor residues can contribute significantly. Altered-peptide ligands (APL) are valuable tools for assessing how each peptide residue contributes to peptide-mhc binding. In two papers that extensively assessed the role of non-anchor residues on H-2D b associated peptides, essentially every residue position was demonstrated to contribute 17
35 Table 1: Relative Affinity Values for the Interaction of H-2Db and Monosubstituted ASNENMETM Peptides The relative affinities for the interaction of H-2D b with the substituted peptides was determined, as explained in Materials and Methods of Sigal et al [45]. The position of the substituted AA in the peptide is indicated at the top of each column. The identity of the residue in the single letter amino acid code is indicated in the left column. Bold values are those obtained with ASNENMETM in each experiment. Values are those obtained in one representative experiment at that position. The data is from Sigal et al [28]. This figure and legend were reproduced from Sigal et al [45] with permission obtained from Elsevier Limited, The Boulevard, Langford Lane, Kidlington, Oxford, OX5 1GB, UK. License number:
36 Table 1: Relative Affinity Values for the Interaction of H-2D b and Monosubstituted ASNENMETM Peptides 19
37 Table 2: Replacing Deleterious Residues in Low Relative Affinity Peptides Dramatically Increases Peptide Binding The role of non-anchor residues in binding was tested by replacing deleterious amino acids in low binding peptides with amino acids that should improve binding. The relative affinities of the original and modified peptides were determined as indicated in Materials and Methods [45]. This figure and legend were reproduced from Sigal et al [45] with permission obtained from Elsevier Limited, The Boulevard, Langford Lane, Kidlington, Oxford, OX5 1GB, UK. License number:
38 Table 2: Replacing Deleterious Residues in Low Relative Affinity Peptides Dramatically Increases Peptide Binding 21
39 to binding affinity [28,44]. Table 1, reproduced from Sigal et al [45], demonstrates this by assessing the RA of peptide-point-mutants at each non-anchor residue of the H- 2D b binding peptide ASNENMETM from the influenza virus nucleoprotein. RA is the concentration of peptide necessary to obtain 50% of maximum MHC binding. Thus, lower RA values indicate greater peptide affinity. The authors results demonstrate that substitution of any individual non-anchor residue can result in substantial changes to RA, including significant improvements. To apply these results, Sigal et al. [45] took low RA peptides from influenza derived MHC-I ligands and substituted deleterious residues with residues that correlated with high RA (Table 2). The authors found that the substitution of two residues per peptide results in RA improvements upwards of 650 fold. For instance, when NP 17-25: GERQNATEI is substituted for GMPQNATEI, RA improved from > to 1.5 (>666 fold). Sigal et al. [45] assessed peptide and MHC binding through the addition of supra-physiological concentrations of exogenous peptide to MHC molecules. However, the natural loading of MHC-I molecules in the ER (see Direct Presentation for details) is from a heterogeneous pool of peptides containing as much as a 1000 fold excess (estimate) of low-affinity peptides [46]. For an MHC-I molecule that has a general affinity for any peptide conforming to the basic shape of the binding groove, how does it select for the high-affinity, and more specifically high-stability, binders? Numerous reports have suggested that tapasin, an ER resident chaperone, is important for high-stability peptide loading of MHC-I. Initial reports of tapasin deficient cells described a deficiency in peptide loading [47,48]. Later studies suggested that MHC-I molecules bind peptides in the absence of tapasin; however, the complexes rarely make it to the plasma membrane and those that do demonstrate low pmhc-stability [5]. A subsequent report demonstrated that tapasin prioritizes pmhc presentation according to the stability of the 22
40 complex [6]. These studies suggest that tapasin selects for peptides that form highstability pmhc interactions. Thus, tapasin prioritizes high-stability peptides for cell membrane presentation. HLA-DM provides a function similar to tapasin for loading highstability peptides onto MHC-II molecules [49]. The existence of independent mechanisms to ensure the loading of high-stability peptides underscores the importance of pmhc-stability for antigen presentation. Direct Presentation by MHC-I Antigen processing and presentation is the process by which protein is acquired, degraded into peptides, and trafficked to the cell membrane in complex with MHC-I. Peptides that are presented by MHC-I molecules are classically derived from proteins that are produced by the cell itself. Presentation provides a continuous representation of cell associated antigens to the immune system to alert CD8+ CTL to virus or pathogen invasion. Overall, MHC-I presentation provides representation of all proteins produced by the cell, albeit equal representation is neither possible nor achieved. Proteins destined for MHC-I presentation must undergo a series of processing events to repurpose the protein from its intended cellular function to that of immunosurveillance. Antigens must be fitted to MHC-I molecules through a number of trimming and transport steps that generally begins with the degradation of cell-associated proteins and terminates with the emergence of peptide bound MHC-I molecules on the plasma membrane. Peptides that escape complete degradation by proteases and peptidases, are selectively transported into the endoplasmic reticulum, bind to a compatible MHC-I molecule and are transported to the cell surface are among the relatively few peptides that represent the whole cell. A general illustration of this process is depicted in Figure 3. 23
41 Figure 3: The MHC class-i processing MHC class I heavy chains initially assemble with β2-microglobulin (β2m), followed by recruitment into the peptide-loading complex in the endoplasmic reticulum. Endogenous peptides, generated in the cytoplasm through the action of proteasomes and other peptidases, are transported into the endoplasmic reticulum via TAP. ERAAP mediates final amino-terminal trimming of peptides, before or after initial binding to MHC class I molecules. Components of the peptide loading complex promote peptide loading and exchange, providing a quality-control mechanism for the preferential export of kinetically stable peptide MHC class I complexes to the cell surface. This figure and ledged [50] were reproduced with permission from Nature Publishing Group. License Number:
42 Figure 3: The MHC class-i processing. 25
43 No known limitations exist on the types of proteins (eg. cellular location, structure, or function) that can enter the MHC-I presentation pathway. Proteins that donate peptides for class-i processing can be fully translated, functional products with a wide range of half-lives [51,52]. However, evidence suggests that incomplete or inaccurate translation products (Defective Ribosomal Products DRiPs) contribute the majority of MHC-I peptides [53] (reviewed in [54,55]). DRiPs is an all-encompassing term that describes any error / deviation from normal translation including misfolded proteins [56-58], alternate translation products [59,60], and mistranslation products [56]. As a result, MHC-I presentation is tightly linked to active protein synthesis [57,61-65], with a selective advantage given to newly acquired / actively synthesized proteins. Such a mechanism provides the cell with an early warning system for immunosurveillance of newly acquired foreign or altered-self antigens. The initial steps in peptide generation result from the degradation of protein substrates by the predominant cellular protease, the proteasome [66-68]. The proteasome is a constitutive, multi-subunit, ATP dependant protease that is responsible for the general maintenance recycling of cytosolic proteins. Proteasomes generally carve proteins into 2-25aa segments that better facilitates peptide delivery for MHC-I binding. Of specific importance is the immunoproteasome, an altered version of the constitutive proteasome that is always expressed in DC populations and is induced in other cell types by interferon-gamma (IFN-. The immunoproteasome generates alternative peptides not produced by the constitutive proteasome, and is more efficient at generating primary MHC-I binding peptides [69]. When 2 of the 3 subunits responsible for immunoproteasome formation, low molecular weight protein(lmp)-2 and LMP-7, are knocked-out in mice, altered CD8+ T cell repertoires and immune responses 26
44 are generated [70,71], suggesting that the immunoproteasome plays an important role in shaping CD8+ T cell responses. The quality of protein processing by the proteasome plays a critical role in determining which peptides will be available for MHC-I binding. The "constitutive" proteasome has a wide range of peptidase activities designed to shred proteins into small recyclable sizes (reviewed in [72]). However, the immunoproteasome demonstrates enhancements in the ability to cleave substrates at basic and hydrophobic residues [73-75] as well as reductions in postglutamyl activity [73] that cleaves after acidic or branched chain amino acids. These functions result in the production of peptides that have carboxyl ends containing hydrophobic or basic residues and a high association with MHC binding [30] and transport into the ER [76,77]. Changes in the specificity of peptide cleavage by the immunoproteasome suggest that the residues surrounding potential MHC-I peptides in the full length protein can affect peptide liberation and therefore shape the T cell response to potential peptides (discussed in more detail in immunodominance: antigen processing and presentation). Thus, understanding the mechanisms of peptide liberation is important for determining which peptides will be presented via MHC-I. Once peptides are liberated from their nascent proteins, they are transported into the ER before binding to MHC-I molecules. Transport is facilitated by the transporter associated with antigen processing (TAP). TAP describes the association of two distinct transmembrane subunits, TAP1 and TAP2 [78-80], which work in concert to deliver peptides of approximately 8-16 amino acids in length [81] from the cytosol through the ER membrane for MHC-I binding. TAP synergizes with MHC-I [47] and MHC-I loading machinery within the endoplasmic reticulum and forms an integral part of the peptide loading complex (PLC) [82]. Cell lines [83,84] or knock-out mice [85] that lack one of the 27
45 TAP subunits have severely reduced levels of membrane associated MHC-I, making them largely deficient in the presentation of MHC-I ligands. Such observations indicate that TAP is required for the loading of most MHC-I peptides and is a necessary component of the classical MHC-I pathway. Once peptides have entered the ER, they associate with additional members of the PLC [82,86] including the molecular chaperones tapasin and calreticulin, the ERresident disulfide isomerase ERp57, the MHC-I heavy chain and 2 m. Members of the PLC serve a critical function in the coordinated loading of peptides onto MHC-I molecules. No single member of the PLC can function alone to load MHC-I, rather all members of the PLC are required [50]. Calreticulin binds to empty MHC-I heavy chains [48] to ensure that the molecules are conformationaly suitable for peptide binding. Tapasin binds directly with TAP and facilitates recruitment of empty MHC-I and ERp57 [47]. Tapasin and ERp57 form a complex that binds to MHC-I molecules to load high affinity peptides [7] (see Peptide Binding and MHC Stability). Altogether, the PLC shuttles peptides directly to their nascent MHC-I molecules. Peptides that enter the ER are often longer than the 8-10 aa ligands that fit into MHC-I molecules. To accommodate large peptides, the endoplasmic reticulum aminopeptidase associated with antigen processing (ERAAP) trims the amino terminal ends [87]. ERRAP uses the MHC-I molecules as a template to determine the final size of the peptide [88]. An over-length peptide binds first to the MHC-I molecule and ERAAP trims the overhanging amino-terminal end of the peptide to the appropriate length. ERAAP therefore acts as an extension of the peptide liberation machinery and serves to increase the efficiency and the effectiveness of peptide production. 28
46 MHC-I molecules undergo a conformation change upon binding a peptide. The conformational change results in the release of the pmhc-i from TAP and the PLC [86,89]. The pmhc-i then undergoes export from the ER in vesicles bound for the plasma membrane where pmhc-i serves the purpose of immune surveillance. The average pmhc-i has a life-span on the cell surface of approximately 14 hours [90] resulting from a combination of constitutive recycling of MHC-I and the inherent instability of pmhc-i due to the dissociation of the bound peptide. MHC-I that undergo recycling are internalized by phagocytosis whereby the phagosomes fuse with early endosomes [91]. Estimates suggest that approximately 50% of the MHC-I molecules are degraded while the other 50% are returned to the plasma membrane for continued presentation [92]. Presentation of Exogenous Antigen Presentation of peptides is not limited to the cell in which the protein was produced. Acquisition of proteins by cell types specialized for the presentation of exogenous proteins can result in peptides being presented on MHC-I and/or MHC-II. The pathways for presentation by MHC-I and MHC-II are distinct. Below MHC-II presentation is briefly considered before MHC-I presentation is discussed in greater detail. MHC-II Expression of MHC-II molecules is restricted to papcs including dendritic cells, B-cells, and macrophages. Newly synthesized MHC-II molecules interact with the ER transmembrane chaperone referred to as the invariant chain (Ii). Ii acts as a surrogate peptide that stabilizes newly formed MHC-II molecules and serves as a guard against 29
47 binding of free peptides within the ER. The transmembrane domain of Ii targets the class-ii molecules for transport to the endosomes where proteasomal cleavage events result in the removal of Ii with the exception of the "surrogate peptide portion referred to as the MHC class II associated invariant-chain peptide (CLIP) [50]. Unlike MHC-I, MHC-II molecules await binding of an appropriate peptide within the endosomes before completing their journey to the plasma membrane. Peptides that are presented by MHC-II are restricted to those antigens that gain access to endosomal compartments via various endocytic mechanisms, generally from exogenously derived sources. The antigens undergo processing by endosomal peptidases which liberate peptides capable of binding to MHC-II molecules. With the assistance of HLA-DM, a MHC-II-like heterodimer, CLIP is displaced upon the binding of a peptide which confers sufficient pmhc-ii stability [50]. Peptides are thought to compete with CLIP for MHC-II binding, a process that is facilitated by HLA-DM. Reports have demonstrated that HLA-DM selects peptides according to their MHC-II pmhcstability [49,93]. Following the binding of a suitable peptide, MHC-II molecules are trafficked to the plasma membrane where they remain until peptides either dissociate or the pmhc-ii is recycled by normal cellular processes. Cross-Presentation MHC-I antigen presentation is critical for targeting activated CD8+ T cells towards the destruction of infected cells or cells harboring altered-self antigens. However, most cell types in which MHC-I presentation takes place are not located in the appropriate lymphoid compartments nor equipped with the co-stimulatory molecules necessary to activate naïve antigen specific T cells (see T cell priming). Naïve CD8+ T cell activation is reserved for professional antigen presenting cells (papc), primarily 30
48 dendritic cells (DC) (see APCs). Although activation of naïve CD8+ T cells can take place as the result of direct presentation from virus infected DCs [94,95], for example, it is likely that non-papcs may be the only reservoir of antigen. Proteins derived from viruses like influenza, which infects epithelial cells, or tumor derived proteins are generally confined to non-papc cell types. In this scenario, papcs must acquire proteins produced by another cell and cross-present the antigen to CD8+ T cells by providing peptides to the MHC-I pathway. Cross-presentation is a challenge on two fronts: 1) papcs must be specialized to take-up antigen from antigen producing cells and 2) papcs must shuttle exogenous antigen into the MHC-I processing and presentation pathway. The cross-presentation pathway is similar to classical or direct MHC-I presentation in terms of peptide loading onto empty MHC-I; however, there are distinct differences in the acquisition of antigen and where and how the proteins enter the MHC-I pathway. Cross-presentation takes place by a mechanism that is not yet well understood. Exceptions to the standard cross-presentation pathway, or perhaps alternative pathways, will be discussed below. A number of mechanisms have been proposed for the acquisition of antigen for cross-presentation. Such mechanisms include the transfer of: 1) Pre-processed peptides for immediate loading onto MHC-I 2) Fully formed pmhc-i 3) Exogenous non-processed protein that must then undergo processing and presentation by papc. The first two mechanisms will be considered briefly because less is known about them. The 3 rd point will be discussed in greater detail because this is the most accepted pathway for antigen acquisition and the best understood. 31
49 Pre-processed peptides Perhaps the simplest method of antigen transfer to papcs would be the direct transfer of cytosolic peptides from the donor cells to the papc. Conceivably, such peptides would be in abundant supply and capable of diffusing from donor cell to papc via physical contact or by phagocytosis. In fact, one study [96] has demonstrated that MHC-I ligands can diffuse through gap junctions to be presented by class-i molecules on an adjacent cell. The cells used in that study were not papcs; however, gap junctions are expressed in papcs such as follicular dendritic cells, B cells and macrophages [97] supporting the viability of this mechanism of cross-presentation. Another study demonstrated cross-priming of T cells specific for a cytosolic peptide expressed in the antigen donor cell [98]. The peptide was expressed at extremely high levels from a minigene encoded by a recombinant vaccinia virus. The ability of this particular peptide to be cross-presented was unique when compared with other peptides in the same study. The authors found that the peptide was cross-presented in a heat shock protein(hsp)-90 dependant manner suggesting that the molecular chaperone was protecting the peptide from degradation. Few examples exist of cross-presentation by way of cytosolic peptide transfer suggesting that it is a viable mechanism but probably the exception to the rule. Fully formed pmhc-i The transfer of fully formed pmhc-i from the donor cell to the papc usurps the need for MHC-I loading and presentation by the papc. This mechanism might represent the most efficient method of cross-presentation, saving papcs from independently processing the antigen. Numerous studies have demonstrated the in-vivo and in-vitro transfer of pmhc-i and pmhc-ii to papcs and the ability of those papcs to prime naïve T cells [99-104]. Studies have demonstrated the transfer of complexes from 32
50 allogenic cells [101,102], tumor cells [99], monocytes [103], and other DCs [102,104]. The acquisition of pmhc-i molecules, in some studies, appears to be dependent on the donor cells being apoptotic [99,100,103], which is consistent with studies demonstrating cross-presentation of antigen from apoptotic cells [105,106]. Both CD8+ and CD8- DC subsets are more efficient at this form of cross-presentation than non-dcs [104]. Such observations could explain findings that migratory CD8- DCs hand-off antigen to CD8+ DCs for priming of naïve T cells [ ]. To describe the process of pmhc transfer, Yewdell and Haeryfar [110] coined the term cross-dressing, suggesting that the donor cells are originally dressed in pmhc molecules before transferring their cargo onto another cell. Cross-dressing may be a primary mechanism of cross-presentation in some systems and an important mechanism for transferring antigen to CD8+ DCs for naïve T cell priming [ ]; however, the physiologic role of cross-dressing has yet to be determined. Exogenous non-processed protein The best understood mechanism for cross-presentation is the acquisition of exogenous proteins by papcs followed by the processing and presentation of those proteins. As with direct presentation, in cross-presentation there are particular types of proteins that undergo presentation more efficiently than others. For direct presentation, DRiPs are found to be the most sought-after antigens entering the MHC-I pathway. However, in the case of cross-presentation, highly stable proteins, with a long cellular half-life, appear to be the most efficiently cross-presented [111,112]. High stability may be necessary to ensure safe passage of non-processed protein into the papc. It is unclear whether proteins are transferred to papcs in a form that is fully mature and functional, denatured, or in pieces. Some studies suggest that protein is transferred in 33
51 the form of peptides (larger than the minimal 8-10aa antigenic determinants) in complex with heat shock proteins (HSPs) [ ]. However not all studies agree [116]. The source of exogenous antigen for papcs is not fully defined; however, phagocytosis and macropinocytosis in DCs provides an efficient route of antigen acquisition. The endocytic nature of immature DCs results in efficient capture and presentation of purified soluble proteins and proteins derived from various exogenous sources [117,118] (eg. latex beads, bacteria, opsonized particles, and apoptotic cells [105,106]). The presentation of antigen acquired by apoptotic cells is particularly efficient due in part to the expression of scavenger receptors on DCs such as CD36, which facilitates the uptake of apoptotic bodies [106], and the mannose receptor [119], which efficiently binds glycosylated antigens. Following the capture of exogenous antigen, papcs must make the protein available to the MHC-I processing machinery. In many systems, cross-presentation of antigen is proteasome and TAP dependant suggesting that the antigen must gain access to the cytosol before entering the classical MHC-I processing pathway. The steps that result in antigen gaining access to the cytosol are unknown (Figure 4). Processing may occur in relative proximity to the endocytic vesicle that houses the exogenous antigen. Some studies have demonstrated that ER components are present in the phagosomes of macrophages [120] consistent with studies demonstrating that the ER provides donor membranes for phagocytosis [121]. However, whether ER membranes are actually involved in phagocytosis is controversial [122,123]. Some authors have suggested that other ER components such as Sec61 are present in the phagosomes and assist in the translocation of phagosome-acquired antigens into the cytoplasm [124,125]. Sec61 is normally utilized in the ER for the removal of old and improperly folded proteins by retrotranslocation into the cytosol. After proteasomal 34
52 Figure 4: Proposed pathways for cross-presentation by MHC class I molecules (a) In the phagosome-to-cytoplasm pathway, particle- or cell-associated proteins or peptides are transported from the phagosome to the cytoplasm, where they enter the direct MHC class I pathway as substrates for proteasomes. The export mechanism is unknown. (b) The endoplasmic reticulum (ER) phagosome represents an essentially autonomous compartment for generating peptide MHC class I complexes from exogenous antigens. Components of the endoplasmic reticulum are incorporated into phagosomes that contain internalized antigens. Proteins are exported to the cytoplasm by the endoplasmic reticulum derived Sec61 translocation complex, where they become substrates for locally associated proteasomes. The resulting peptides are transported back into the endoplasmic reticulum phagosome by TAP, followed by binding to MHC class I molecules and transport to the cell surface. (c) Soluble proteins can be targeted to different processing pathways after internalization through receptor-mediated endocytosis. Proteins internalized by pinocytosis or scavenger receptors are targeted to lysosomes and are excluded from cross-presentation by MHC class I molecules. Proteins internalized through mannose receptors are targeted to a stable population of early endosomes, from which they are transported to the cytoplasm, entering the direct MHC class I pathway as proteasome substrates. Additional mechanisms for crosspresentation have been demonstrated. This figure and legend [50] were reproduced with permission from Nature Publishing Group. License Number:
53 Figure 4: Proposed pathways for cross-presentation by MHC class I molecules 36
54 degradation in the cytosol, normal class-i processing can take place by TAP-transport of peptides back into the phagosome or the ER before class-i loading and trafficking to the plasma-membrane (Figure 4a and b). Interestingly, one study suggested that antigen uptake by the mannose receptor but not scavenger receptors could supply antigen directly to the cross-presentation pathway [126] (Figure 4c). Cross-presentation is necessary for the activation of naive CD8+ T cells directed against tumors and viruses that do not infect papcs. In the case of tumor-derived antigens, cross-presentation is necessary for the induction of CD8+ T cells responses (reviewed in [4]). Therefore, mechanisms that regulate cross-presentation directly influence the outcome of CD8+ T cell responses to many pathogens and tumors. This thesis focuses on the CD8+ T cell response at early time points. Little is known concerning the role that pmhc-stability plays, if any, in the process of crosspresentation. Due to this knowledge gap, few studies have addressed whether pmhc-stability influences the CD8+ T cell responses to cross-presented antigen. However, numerous studies have demonstrated correlations between pmhc-stability and the T cell response (see pmhc Stability and the T Cell Response). Understanding the mechanisms that contribute to cross-presentation informs our experimental design and contributes to the interpretation of our data. T Cell Immune Response Antigen Recognition T cells bear a TCR for the specific recognition of pmhc complexes. The TCR is a transmembrane heterodimer composed of a and chain formed following the somatic recombination of variable (V), determining (D) and joining (J) elements similar to 37
55 those found in antibody heavy-chains [127]. VDJ recombination has the potential to result in extraordinary diversity, generating an estimated unique TCRs [128]. Diversity is mostly within the complementarity determining regions (CDR) CDR1, CDR2, and CDR3. The TCR uses the CDRs to simultaneously recognize the MHC molecule and the peptide. MHC molecules are recognized with the assistance of conserved residues in the CDR1 and CDR2 regions [129], and the peptide is recognized primarily using the CDR3 region (14 studies assessing different TCR:pMHC structures have been reviewed in [130]). Recognition of the pmhc by the TCR is assisted by co-receptor molecules CD4 or CD8. The CD4 and CD8 molecules determine TCR compatibility with MHC-I (CD8) or MHC-II (CD4) molecules, and they enhance the avidity of TCR:pMHC interactions [131]. T cells are generally considered to be exquisitely specific for their cognate pmhc. When considering the potential diversity of 8-10aa peptides that MHC-I molecules can bind (20 amino acids to the power of 8 or 10 = between potential peptide combinations), the discovery of natural peptide ligands that exhibit cross-reactivity is seemingly improbable. However, the requirement for the TCR to recognize both the MHC as well as the peptide has resulted in considerable flexibility within the TCR (reviewed in [130,132]) and cross-reactive TCRs have been discovered. An example of such flexibility was reported for a cross-reactive TCR recognizing both H- 2K b peptides pbm1; INFDFNTI, and VSV8, RGYVYQGL. The two peptides have different residues at every position but stimulate the same TCR. A structural shift in conformation within the CDR3 region of the TCR accommodates these peptide specific changes [133]. Similar observations have been noted by the same group concerning a TCR from H-2 k mice that cross-reacts with a H-2k b pmhc [134]. The implications of 38
56 TCR structural flexibility are that TCRs can often accommodate minor, and sometimes major [133], changes in MHC ligands, resulting in cross-reactivity. The potential for TCR promiscuity often results in the production of T cells that have low affinity towards self antigens. TCRs are selected in the thymus during T cell development. Following the expression of a functional TCR, T cells are tested for recognition of self antigens that are expressed and cross-presented in the thymus [135,136]. T cells that recognize self pmhc with sufficient affinity are deleted through a process known as central tolerance. However, autoreactive T cells of low affinity do escape into the periphery [ ]. The low affinity T cells may pose a risk for autoimmunity and/or may be beneficial for the control of tumors over expressing or uniquely expressing the self antigen. In this thesis, the activation of CD8+ T cells that escape central tolerance is explored by immunization with protein encoding high-stability CD8+ T cell determinants. The activation of these residual T cells may hold promise for the development of immunotherapy strategies targeting established brain tumors in a mouse model (Appendix A). Antigen Presenting Cells MHC-I molecules are expressed on all nucleated cell types, technically making all nucleated cells APCs. However, not all APCs are created equal. papcs are required for the activation of naïve T lymphocytes. papcs display the unique ability to crosspresent exogenous antigens via the MHC-I pathway. Cross-presentation is necessary for the activation of antigen inexperienced (naïve) T cells in circumstances where papcs do not otherwise directly present the antigen. 39
57 Cell types regarded as papc are bone marrow derived cells including dendritic cells (DCs), macrophages, and B cells. Each of these cell types expresses MHC-I molecules as well as MHC-II molecules, making them capable of activating both CD8+ and CD4+ T cells. Each papc expresses co-stimulatory molecules that interact with receptors expressed on naïve T cells providing a secondary / co-stimulatory signal required for T cell activation. Although all of these cell types can cross-present exogenous antigens on MHC-I molecules and prime naïve T cells under select conditions, DCs are the principle papcs that prime naïve CD8+ T cells in-vivo [110, ]. In experiments where CD11c+ cells were depleted (CD11c is a general marker for DCs), CD8+ T cell priming was abrogated [145,146], but priming was not affected when macrophages or B cells [145] were depleted. DCs were first described as a cell type that could assume a variety of branching forms [147]. Although in that seminal paper DCs were also described as non- phagocytic, we now know that DCs are highly phagocytic cells that are uniquely equipped to take up exogenous antigen and cross-present that antigen for the activation of naïve CD8+ T cells [144,148]. Antigens such as tumor antigen can drain directly into lymph nodes [149] where it is acquired by DCs and cross-presented; however, DCs typically acquire antigen in the peripheral tissues and migrate though the lymphatic system to the draining lymph nodes (DLNs). Shortly after migratory DCs arrive in the DLN they initiate the priming of naïve CD8+ T cells [150]. Studies suggest that priming does not result from direct interactions of T cells and migratory DCs; rather, priming results following what appears to be the passage of antigen from migratory DCs to lymph node resident CD8+ DCs [108]. CD8+ DCs are the primary DC subset responsible for naïve CD8+ T cell priming [145, ]. 40
58 After encountering foreign antigen in association with danger signals such as pathogen associated molecular patterns via toll like receptors (TLR) (e.g. LPS) or proinflammatory cytokines (e.g. IL-1, TNF-, and IL-6), DCs undergo maturation [154]. Maturation of DCs is a critical process for the activation of naïve T cells. Mature DCs upregulate expression of the co-stimulatory ligands B7-1 and B7-2 [155] (CD80 and CD86, reviewed in [156]) that subsequently interact with the T cell co-stimulatory receptor CD28 [157]. A lack of co-stimulation leads to suboptimal activation of T cells and results in reduced clonal expansion and anergy [158]. The upregulation of costimulatory molecules by papcs is assisted by ligation of the CD40 receptor by CD40L [159] expressed on activated CD4+ helper T cells. DCs that have not undergone maturation express negative regulatory factors such as programmed cell death ligands (PDLs) that interface with the inhibitory receptor PD1 on activated T cells and can induce T cell tolerance [160,161]. Thus, proper licensing of DCs is necessary to provide naïve CD8+ T cells with the signals necessary to undergo activation and division and protect activated T cells from death (Figure 5). T Cell Immune Response The CD8+ T cell response begins following the engagement of the TCR with a mature papc. Ligation of the TCR initiates intracellular signaling facilitated by the protein-tyrosine kinase Lck [162] and is enhanced by CD28 co-stimulation [163]. As T cells are activated, they modulate the expression of several cell surface proteins that are common markers used to identify the activation state of a T cell (Table 3). CD69, a c- 41
59 Figure 5: Cross-Priming of CD8+ T cells The molecular mechanisms involved in classical cross-priming are illustrated. Dendritic cells (DCs) take up antigen by distinct endocytosis mechanisms (not shown) and present it to CD4+ T helper (TH) cells through MHC class II molecules and crosspresent it to CD8+ cytotoxic T lymphocytes (CTLs) through MHC class I molecules. Activated CD4+ TH cells can stimulate CTLs through the production of interleukin-2 (IL- 2) and license DCs for cross-priming through CD40 ligand (CD40L) CD40 interactions. Licensed DCs upregulate expression of co-stimulatory molecules, such as CD70, CD80 and CD86, and downregulate inhibitory molecules, such as programmed cell death ligand (PDL1). Toll-like receptor (TLR) ligands further activate DCs and increase their cross-presentation activity. Cross-primed CTLs are programmed for survival and cease TNF-related apoptosis-inducing ligand (TRAIL) production. Helpless CTLs activated by unlicensed DCs die following secondary encounter with antigen in their effector phase (not shown). This figure and legend were partially reproduced from Kurts et al. [144]. Permission was received from Nature Publishing Group. License number:
60 Figure 5: Cross-Priming of CD8+ T cells 43
61 Table 3: T Cell Surface Markers to Distinguish Antigen Experience This table lists the common cell surface markers used to distinguish among the activation states of a T cell. 44
62 Table 3: T Cell Surface Markers to Distinguish Antigen Experience Antigen Experience Naïve Antigen Experienced Memory Cell Surface Markers CD44 lo, CD62L hi, CD69 lo, IL7R hi CD44 hi, CD62L lo, CD69 hi, IL7R lo CD44 hi, CD62L hi/lo, CD69 hi/lo, IL7R hi 45
63 type lectin and accessory signaling molecule that assists in T cell activation, is one of the earliest detectable cell-surface marker of T cell activation [164,165]. CD44 is a cell adhesion molecule that becomes upregulated following activation and may play a role in cell signaling/activation, tissue migration, and even actin-cytoskeleton dynamics (reviewed in [166]). CD62L is an adhesion molecule constitutively expressed on naïve T cells and downregulated on most T cells following activation [167]. CD62L is important for T cell homing to lymph nodes (reviewed in [168]); thus downregulation of CD62L following activation allows activated cells to traffic into the periphery. The number of naïve precursor T cells of each specificity has been estimated at between cells in mice [ ]. Following activation, naïve T cells undergo clonal expansion. Potentially millions of cells accumulate during clonal expansion, depending on intrinsic and extrinsic factors governing that particular T cell response. CD8+ T cells take on cytotoxic effector functions mediated by two predominant mechanisms, perforin and granzyme and fas:fas-ligand interactions [173]. Perforin and granzyme are contained in lytic granules that are released from the T cells following TCR recognition of a target cell. Perforin forms pores in the target cell membrane [174] and granzyme is a serine protease [175,176] that induces target cell apoptosis through direct cleavage of caspases as well as mitochondrial permeabilization (reviewed in [177]). Fas-ligand is expressed on activated T cells and upon interaction with fas on target cells can induce apoptosis by activation of caspases [178]. In addition to these two mechanisms, interferon- (IFN- ) is produced by activated T cells upon TCR stimulation and even plays a role in T cell activation [179]. IFN- acts as an effector molecule by binding to the IFN receptor expressed on nearly all tissues [180]. IFN- receptor stimulation results in the upregulation of MHC molecules and a host of genes important in antigen processing and presentation, including the immunoproteasome 46
64 (reviewed in [181]). Enhanced MHC expression and antigen processing assist the T cell in recognition of antigen and target cell elimination. Following clonal expansion, T cells enter a phase of contraction in which approximately 95% of effector cells undergo apoptosis. The remaining 5% of T cells transition into memory T cells [182]. Memory cell populations are retained throughout the life of the organism and provide rapid recall of the T cell response following reencounter with antigen. Memory cells are characterized by the expression of CD44 and IL-7 receptor [183] and can be broken into two distinct categories, central memory and effector memory, identified by hi or low expression of CD62L, respectively (Table 3). Effector memory populations are predominantly found in the peripheral tissues whereas central memory cells reside in the lymph nodes [184]. Central memory cells efficiently produce IL-2, a cytokine necessary for T cell proliferation, and therefore divide more rapidly in response to stimulus [184]. Central memory is thought to be the primary memory subset responsible for lifelong maintenance of immunological memory. Effector memory cells are quick to produce the effector cytokine IFN- [184] and are thought to provide peripheral surveillance for antigen and initiate rapid memory recall and immediate effector responses (reviewed in [185]). Antigen Stimulus The engagement of the TCR with its cognate pmhc complex is the primary factor leading to T cell activation. The exposure of naïve T cells to an inflammatory environment in the absence of specific TCR stimulation can result in the partial upregulation of activation markers but leads to apoptosis [186]. Although a single TCR:pMHC interaction can result in TCR signaling [187] (demonstrated using CD4+ T 47
65 cells), T cells have evolved mechanisms to promote high-avidity TCR:MHC interactions for the activation of naïve T cells. High-avidity TCR:pMHC binding is mediated in part by CD8 [188] which interfaces with the 3 domain of MHC-I molecules and is required for efficient activation of T cells [20]. When T cells interact with APCs they form an immunological synapse which results in the aggregation of TCR:pMHC complexes [189] and amplifies the signal transduction events [190] that lead to T cell activation. T cell activation requires more than a simple encounter of pmhc molecules on a mature papc. Signals are integrated in an additive fashion. Thus, small intermittent signals eventually lead to T cell activation [191]. Using intravital microscopy, a technique allowing the observation of cells within live organisms, studies have demonstrated that T cell activation results from sequential encounters with different papcs in three distinct phases [192]. The first eight hours consist of short encounters with papcs (lasting about 8 minutes) followed by approximately 12 hours of long-lived interactions (30minutes 3 hours in length) and ending with a second period of short encounters. These observations are consistent with other intravital microscopy studies and one ex-vivo study that demonstrated intermittent contacts between APCs and T cells [ ]. The abundance of pmhc displayed by APCs contributes to the strength of the T cell stimulus and accelerates the rate at which T cells progress though the three stages of priming [196], possibly due to the more rapid integration of TCR signals. Duration of antigen presentation Studies that have evaluated antigen stimulus beyond the point of T cell activation have found that antigen stimulation is not necessary for a T cell response. Eric Pamer s group first introduced the concept of T cell programming. The foundational study 48
66 demonstrated that the size and kinetics of the T cell responses to L. monocytogenes remain the same when infection is limited to 24 hours rather than the complete seven days required for bacterial clearance [197]. The study suggested that T cells only require an early encounter with antigen to initiate a normal immune response. Following this study, other groups demonstrated that as little as two hours of stimulation from a papc can result in T cell division and acquisition of effector functions [198]. The findings were later extended to show that stimulation for up to 20 hours is necessary to achieve maximum effector function and avoid abortive proliferation [199]. Together, these studies suggest that T cells initiate a program that results in the initiation of a T cell response following only a brief encounter with antigen. The implication of these findings is that T cells require no reencounter with antigen in order to complete their program. Although reencounter with antigen is not required for a productive T cell response, extending the duration of stimulus, in some systems out to 64 hours contributes to the size of the T cell response [ ]. In these studies, as those described above, the authors found that T cells are capable of undergoing a complete T cell response following only a short encounter with antigen. However, prolonged encounter with antigen results in an increase in the size of the T cell response. Recently, one study demonstrated that direct presentation at day five following immunization is necessary for maximal CD8+ T cell expansion toward a cell-associated tumor antigen [10]. The mechanisms that result in enhanced T cell responses following prolonged presentation remains unknown. However, one study demonstrated that longer antigen stimulation results in more efficient naïve T cell recruitment [200]. Not all studies agree that the duration of antigen stimulation determines the size of T cell 49
67 responses. For L. monocytogenes, the duration of infection has no effect on the size of the T cell response [203]. Antigen presentation can be detected for days following excision of virus infected tissue [204,205] or immunization with cell-associated antigen [10]. Assays to detect antigen presentation involve monitoring naive TCR-transgenic T cells for activation. Thus, in this context, persistence of antigen presentation is the persistence of naïve T cell cross-priming. Antigen may need to be presented in the context of a papc to promote T cell accumulation. One study suggested that accumulation is dependant on stimulation of T cells with B7-1 and IL-12, a co-stimulatory molecule and cytokine associated with papcs [200]. Thus, prolonged T cell cross-priming beyond the detection of virus infection may be an important mechanism for ensuring strong T cell responses. However, only one study has assessed the role of direct presentation on non-papcs and found that direct presentation is necessary for the accumulation of T cells specific for cell-associated antigen at day five and beyond following immunization [10]. All other studies have assessed the duration of antigen presentation in the context of papcs or by a method that makes no distinction between presentation on papcs and non-papcs. Whether enhanced T cell responses to persistent antigen presentation are due to presentation on papcs or non-papcs remains unresolved. Neither the mechanisms resulting in antigen persistence nor whether presentation by papcs is required for enhanced T cell accumulation has been determined. In many systems, the duration of antigen presentation plays an integral role in the accumulation of T cells. Thus, understanding the mechanisms that contribute to the duration of antigen presentation is paramount to the rational design of T cell-based vaccines. 50
68 In this thesis, we address whether pmhc-stability may contribute to the duration of antigen presentation and specifically investigate the role that the duration of cross-presentation by papcs plays in T cell accumulation. Naïve T Cell Recruitment The potency of naïve T cell priming can be judged by the fraction of total antigenspecific naïve T cells that undergo activation, referred to as naïve T cell recruitment. Little is known about what factors influence naïve T cell recruitment. The first T cells can be recruited within 24 hours following infection [203], and recruitment may continue through days three [206] and four [207]. These studies clearly demonstrated that persistence of antigen presentation over three to four days is necessary for efficient recruitment. No studies have specifically investigated other mechanisms that regulate T cell recruitment; however, antigen dose [196,208] and T cell avidity [209] have been implicated. Two studies have recently assessed the efficiency of naïve T cell recruitment in the context of virus infection [170,210]. The studies reached different conclusions, one finding that nearly all naïve T cells are recruited [210] and the other finding that the efficiency of recruitment varies among individual CD8+ T cell determinants [170]. Understanding factors that affect the efficiency of naïve T cell recruitment is pivotal for the rational design of vaccines. Enhancing the efficiency of naïve T cell recruitment may alter the overall size of T cell responses and change the control of infections or tumors. This thesis will investigate the role of pmhc-stability and the persistence of cross-presentation for determining naïve T cell recruitment. 51
69 Immunodominance Due to the abundance of T cell specificities and MHC ligands, T cell responses to a pathogen or tumor are often directed against multiple determinants. The size of the T cell response to each determinant may vary due to a phenomenon called immunodominance. Immunodominant determinants elicit the greatest T cell responses whereas determinants eliciting smaller responses are described as subdominant. Immunodominance hierarchies among determinants are apparent in numerous systems (for examples see Table 4) and are a subject of great interest (see reviews [211,212]). Understanding the mechanisms that contribute to establishing immunodominance hierarchies is crucial to the rational design of vaccines that elicit a broad spectrum T cell response, targeting both dominant and subdominant T cells. Some studies have suggested that subdominant determinants are important for the control of virus infections (e.g. HIV [12,213,214]) and tumors [137, ]. Thus, broad spectrum vaccines hold promise for the more efficient clearance of viral infection and the development of immunotherapy approaches. This thesis investigates whether pmhc-stability contributes to the subdominant nature of a tumor antigen determinant. A number of mechanisms have been proposed to contribute to immunodominance; however, no primary mechanism has been defined. Immunodominance in any given system may be unique and is probably the result of one or more contributing factors which fall into three categories: 1) Antigen processing and presentation 2) Naïve T cell precursor numbers 3) T cell competition 52
70 Table 4: Immunodominance Hierarchies This table lists commonly studied MHC-I restricted immunodominance hierarchies among various systems. Each peptide is listed with its MHC restriction and in the order of dominance from immunodominant (top) to subdominant (bottom). The pmhc half-life is presented along with a single reference where that data was presented. The reference provided for each determinant is not necessarily the only source for pmhc half-life; however, in order to provide a more accurate comparison among determinants, a deliberate effort was made to report only values obtained from a single study. In some cases (**), the half-life had to be estimated based on the published data, therefore the listed value is not the author s value. Values where the number is represented as >X is because the published data did not assay long enough to determine/estimate the true half-life. 53
71 Table 4: Immunodominance Hierarchies System Determinant Hierarchy * pmhc-stability Half-Life (hours) References SV40 TAg K b IV D b I D b II/III D b V L. monocytogenes K d LLO K d p K d p Influenza A Virus D b PA D b NP D b PB1-F LCMV D b /K b GP D b /K b GP D b NP D b GP K b GP Minor H Antigens K b H60 K b H4 K b H28 D b H13 Ovalbumin (OVA) K b K b >6 ~3.5 ** ~2 ** >6 >6 1 >9 ~4 ** ~3.5 ** >4 D b >4 K b ~1.5 ** D b >4 - K b >4 ~3 ** ~2 ** >6 >6 ~2 ~6 ~7.8 ~2.6 [219] [220] numbers reported in this review [221] [222] [223] [224] [225] * Listed in order of dominance: Top: dominant, Bottom: subdominant ** Estimated value 54
72 Antigen Processing and Presentation Ultimately pmhc presentation is the definitive factor governing the induction of a T cell immune response and therefore can play an important role in the establishment of immunodominance hierarchies. The presentation of appropriate numbers of pmhc is required for priming of naïve T cells. The number of pmhc molecules presented on a given cell is the result of numerous antigen processing steps and the quality of the peptide:mhc interaction. An excellent example of how antigen processing and presentation can affect immunodominance hierarchies is a study from Tenzer et al. [226] in which the authors demonstrated that pmhc presentation from overlapping dominant and subdominant determinants (including common escape variants) in HIV-1 is altered by effects on antigen processing. The authors demonstrated differential presentation of the determinants when constitutive proteasomal processing was compared to immunoproteasomal processing. The authors also demonstrated differential TAP transport affinities, peptide:mhc binding affinity and ERAAP-mediated trimming of peptides. The authors correlated the discrepancies in these antigen processing steps with the abundance of pmhc on the cell surface, and suggest that antigen processing and presentation influences CTL hierarchies by modulating pmhc abundance. The study discussed above is a broad view of how antigen processing may affect CTL hierarchies. However, in most cases, CTL hierarchies are not the result of overlapping determinants, and therefore differences in antigen processing may not have as profound an effect on the overall T cell response. Nonetheless, numerous examples exist where antigen processing results in changes in T cell immunodominance. Pang et al. [227] demonstrated that expression of the immunoproteasome is necessary for efficient presentation of the immunodominant FLU PA determinant. DCs deficient in immunoproteasomal subunits demonstrated decreased pmhc presentation 55
73 of PA and the immunodominance hierarchy is flipped in immunoproteasome subunit knockout mice making PA subdominant to NP These data were consistent with the author s previously published work demonstrating that altering the levels of pmhc presentation by relocation of peptide within the Flu neuraminidase protein can change the immunodominance hierarchy [228]. Other studies have also demonstrated that changes in protein context or residues flanking a determinant can alter processing [ ] and influence immunodominance [228,232]. None of these studies specifically addressed the role of cross-presentation in T cell immunodominance hierarchies. Tapasin along with ERp57 prioritizes the loading of high affinity peptides onto MHC-I molecules [7,233]. Specifically, tapasin preferentially loads peptides of highstability onto pmhc-i [5,6]. One study suggested that tapasin determines immunodominance by increasing the presentation of more stable complexes and in turn increasing the size of the T cell response toward high-stability peptides [234]. The authors demonstrated that in the absence of tapasin, the size of the T cell response to the dominant peptide decreases and a subdominant peptide becomes dominant. This study however did not assess changes in a traditional immunodominance hierarchy; rather they assessed the size of T cell responses to OVA-SIINFEKL APLs in isolation. A more complete discussion of the affects of pmhc-stability on the T cell response will be covered below (see pmhc Stability and the T Cell Response). Naïve T Cell Precursor Numbers If all factors leading to a T cell immune response were equal, including processing and presentation, then the number of naive T cells responding to antigen would dictate the hierarchy. Immunodominant T cell responses would be the result of large numbers of naïve T cells responding to antigen and subdominant T cell responses 56
74 would be due to small numbers of responding naïve T cells. Until recently, the number of naïve antigen specific T cells of a single specificity in a mouse was below the threshold of detection and indirect measurements were necessary to estimate precursor numbers [ ]. However, the Jenkins lab developed an assay to directly enumerate the number of naïve antigen-specific T cells in the lymphoid organs [171]. This method involves the isolation of multiple lymphoid organs and the magnetic labeling and positive selection of T cells with MHC-tetramer reagents. Isolation of naïve T cells by this method cannot assess the total number of naive T cells in a mouse since only a subset of the lymphoid organs is collected, cells are lost during tetramer enrichment, and variation in the avidity of T cell populations may affect the strength of tetramer binding. However, the method has spurred studies that have compared the number of naïve T cells specific for individual determinants. These studies have asked whether the ratios of naïve T cell precursors predict immunodominance [169,170,172]. A variation on Jenkins method is utilized in this thesis. The study initially published by Jenkins and colleagues [171] did not look at a traditional T cell immunodominance hierarchy; rather it assessed three CD4+ T cell determinants from different systems and evaluated the T cell response to those determinants following isolated peptide immunization. The authors found a direct relationship between the number of naïve T cells and the size of the T cell response following immunization. They concluded that the number of naïve T cells predicts the size of the T cell response and that the number of naïve T cells influences immunodominance hierarchies. A study published by the Lefrançois lab [172] showed similar results using an infectious model (recombinant VSV). In this study the authors inserted a peptide determinant into VSV and monitored the T cell response following infection. Like the Jenkin s study, the recombinant determinants had to be assessed in 57
75 isolation; although, the authors compared the immune response to both the recombinant determinant and a VSV determinant, and found that the precursor frequency is predictive of the immunodominance hierarchy between the two. The two studies that assessed traditional immunodominance hierarchies and the number of naïve T cells resulted in opposing conclusions. Sette s group assessed the relationship of precursor number on the hierarchy of LCMV determinants following LCMV infection [169]. They found that naïve T cell numbers are predictive of the immunodominance hierarchy for eight of the LCMV determinants. The authors also demonstrated a correlation with pmhc relative affinity (more will be discussed below). The study by the Turner lab monitored the dominance hierarchy of the Flu determinants following respiratory infection [170]. The authors found that the number of naïve precursors has no relationship to the Flu dominance hierarchy. In fact, the subdominant determinants have up to eight fold more naïve T cells than the dominant determinants. The authors determined that the naïve T cells specific for the subdominant determinants display incomplete recruitment of naïve T cells as well as a decreased rate of proliferation late in the immune response. This study and the others discussed in this section suggest that, if all else is equal, naïve T cell precursor numbers are probably predictive of the immunodominance hierarchy. However, in the case of Flu, major differences in naïve T cell recruitment and proliferation are more influential in the development of an immunodominance hierarchy. T Cell Competition One mechanism that can lead to differences in T cell responses among T cells of different specificities is competition. T cells of different specificities can compete with one another during priming [208, ]. This phenomenon was first demonstrated by Grufman et al. [238,241] using minor histocompatibility antigens and then observed 58
76 more directly by Kedl et al. [240] and Willis et al. [208] using OVA antigen peptide-pulsed DCs. These studies found that T cells specific for unique determinants compete for access to pmhc on antigen presenting cells. If a DC is presenting both a dominant and a subdominant peptide, T cells specific for the dominant determinant inhibits the response to the subdominant determinant in a dose dependant manner [240]. Thus, the presence of dominant T cells reduces the size of subdominant T cell responses [208,240], possibly influenced by poor naïve T cell recruitment [208]. When the dominant and subdominant peptides / antigens are presented on separate APCs or the APCs are in excess, competition is not observed or is limited. Competition manifests itself in other ways as well. One study demonstrated that competition during priming of CD4+ T cells with high-stability (previously shown to be dominant) and low-stability (subdominant) peptides resulted in the premature contraction of T cells responding to low-stability peptides. Premature contraction appeared to result in the establishment of the T cell hierarchy [239]. Another study demonstrated that preexisting T cell immunity to subdominant determinants could greatly impair the T cell response to the dominant determinants following rechallenge [242]. Conversely, Kotturi et al. [169] demonstrated that eliminating dominant determinants from LCMV resulted in an increase in the size of subdominant T cell responses; however, it did not change the overall dominance hierarchy. Thus, the size of T cell responses can be significantly altered by competition with T cells of an alternate specificity. In summary, immunodominance among T cells is a common phenomenon affecting immunity toward multivalent antigens. Although the cause of immunodominance is not fully defined, the factors that contribute to immunodominance 59
77 include differences in 1) antigen processing and presentation, 2) naïve T cell precursor numbers, and 3) T cell competition. The particular mechanism that contributes to immunodominance most likely varies among systems and may consist of a combination of these three factors. Understanding how immunodominance hierarchies are established and developing methods to enhance the size of subdominant and immunorecessive T cell responses is crucial to the development of effective multivalent T cell-based vaccines. Expansion of subdominant T cell responses is important for antiviral [12,213,214] and anti-tumor [137, ] immunity as a means of combating immune escape and effective targeting of both viruses and tumors. pmhc-stability and the T Cell Response Our basic understanding of CD8+ T cell responses is well defined. The dogmas that pervade T cell activation, expansion, effector function and the formation of immunological memory are textbook knowledge for any undergraduate. However, the mechanisms that contribute to the size of a T cell response following priming are somewhat ill-defined. Moreover, the influence of pmhc-stability on T cell priming and the size of the T cell response has progressed very little beyond phenomenological observations. In this section, I will describe what is known about the way pmhc-stability affects the size of the antigen specific T cell response. As noted previously, pmhcaffinity often directly determines pmhc-stability. Since many studies have assessed the role of pmhc-affinity in the T cell response, I will at times discuss pmhc-affinity, being sure to make the distinction between affinity and stability. I briefly discussed (in peptide binding and MHC stability) that tapasin and HLA- DM contribute to loading stable peptides onto MHC molecules. The presence of two 60
78 distinct molecules occupying a similar niche suggests that the selective loading of stable peptides onto MHC molecules has an evolutionary advantage. The idea that pmhcstability might correlate with the size or immunogenicity of the T cell response goes back to 1991 when pmhc-stability was first measured by Alain Townsend s group and suggested that the size of peptides bound to MHC molecules were optimized for highstability [243]. However, before pmhc-stability was empirically demonstrated to affect the size of the T cell response, others had determined first in the MHC-II system [244] then five years later with MHC-I [245] that peptides must bind MHC with relatively high affinity to initiate a detectable T cell response. The first substantial evidence that pmhcstability influenced the immunogenicity of a determinant was published in 1996 by van der Burg et al. [1]. The authors assessed pmhc-affinity of numerous peptides using a competitive binding assay to assess the concentration of peptide that resulted in 50% inhibition of binding of a reference peptide (IC 50 ). High-, moderate-, and low-affinity peptides were organized by IC 50 < 5 M, > 5mM / <15mM, and > 15mM, respectively. The authors affirmed that moderate to high pmhc-affinity is necessary to detect a T cell response. However, they observed that the immunogenicity of a determinant is more accurately predicted by the pmhc-stability (Table 5). In their system, pmhc-stability with a half-life of 3 hours was necessary to induce a detectable T cell response. Chen et al. [225] implicated pmhc-affinity as a regulator of immunodominance (e.g. higher affinity = greater likelihood of immunodominant phenotype) in However, not all studies agreed that pmhc-affinity correlates with immunodominance [246]. Chen et al. [225] also demonstrated that the two OVA determinants evaluated in the study demonstrate different pmhc-stabilities (Table 4), suggesting that pmhcstability might play a role in immunodominance. Lipford et al. [247] demonstrated that when APLs of OVA:SIINFEKL are created with different pmhc-stabilities, the 61
79 Table 5: Peptide Binding Affinity, pmhc-stability and Immunogenicity This table is reproduced from Table II in van der Burg et al. [1]. HLA-A*0201 determinants derived from HBV and HPV16 were tested for pmhc-affinity and pmhcstability. HLA-A*0201 transgenic mice were immunized with peptide emulsified in IFA with the HBV core derived helper determinant and judged to be immunogenic or nonimmunogenic based on cytotoxicity assays 6 days following immunization. The data are summarized in the table ranked first according to binding affinity, then by pmhcstability (dissociation time 50% - DT 50 ) and finally by immunogenicity. This table was reproduced with permission from The Journal of Immunology. Copyright The American Association of Immunologists, Inc. Annotation: All immunogenic peptides bind with intermediate or high affinity; however, of those peptides that bind with intermediate affinity, only high-stability peptides are immunogenic. Affirming previous studies, no peptides that bind with low affinity are immunogenic. 62
80 Table 5: Peptide Binding Affinity, pmhc-stability and Immunogenicity 63
81 immunogenicity of the APLs correlates with the pmhc-stability. Since then, numerous systems have demonstrated immunodominance hierarchies and have evaluated the pmhc-stabilities of the known determinants. Table 4 summarizes a number of CD8+ T cell hierarchies along with the pmhc-stabilities determined for each of the determinants. By and large, the immunodominance of a determinant correlates directly with the pmhcstability of that determinant; however, that is not to suggest that pmhc-stability is the primary property underlying the development of these hierarchies. Although a number of studies looking at CD8+ T cells have demonstrated a correlation between pmhc-stability and immunodominance, none have definitively shown a causal relationship. Perhaps the most work on the contribution of pmhc-stability and immunodominance has been done in the MHC-II system. Sant s group [248] carefully tested the premise that pmhc-stability determined immunodominance by engineering APLs of three determinants that naturally exhibit high, moderate and low pmhc-stability. The authors were able to decrease the pmhc-stability of the high-stability peptides, increase the stability of the low-stability peptides, and alter the stability of the intermediate peptides in both directions. The APLs were inserted into a protein vector that contained an established immunodominance hierarchy and the authors asked whether changes in the pmhc-stability of the determinants would alter the size of the resulting T cell response. In nearly every case, the size of the T cell response to the APLs directly mimics the pmhc-stability. Perhaps most convincing, when a dominant WT determinant is replaced with an APL that lowers the pmhc-stability in the context of the original protein, the determinant becomes cryptic. Sant s group later published a study that helps explain their observations [239]. The study demonstrated that competition during priming between T cells specific for high- and low-stability peptides 64
82 results in T cells specific for low-stability peptides undergoing abortive proliferation. When the high- and low-stability peptides were separated onto different papcs, competition was eliminated, and T cells specific for the low-stability peptides responded normally. A recent study by Baumgartner et al. [249] assessing pmhc-stability and the CD4+ T cell response concluded that as pmhc-stability increases, a more diverse array of CD4+ T cell clonotypes are recruited into the response. The authors found that lowstability peptides derived from pigeon and moth cytochrome C recruit high-avidity T cells. When high-stability variants of each peptide are used, both high- and low-avidity T cells are recruited and the overall diversity of TCRs utilized by antigen-specific T cells increases. This observation is consistent with a study with CD8+ T cells indicating that dominant T cell responses consist of more diverse TCR repertoires compared to subdominant responses [2]. The authors found that both high- and low-stability peptides elicit a similar T cell response size, suggesting that, for pigeon and moth cytochrome C, pmhc-stability may not determine immunodominance. However, the possibility that pmhc-stability influences the recruitment of antigen-specific T cells suggests an intriguing mechanism for the modulation of immunodominance in other systems. In this thesis, we explore a role for pmhc-stability in the recruitment of naïve T cells and immunodominance. Stability of pmhc directly correlates with the level of pmhc presentation on APCs [49,220,234,247, ]. Not only does the number of complexes presented increase as pmhc-stability increases, but in a scenario where antigen is limiting, presumably the persistence of pmhc presentation also increases with pmhc-stability (this has never been tested in vivo). As a result, the hypothesis is that an increase in the number and persistence of complexes on papcs results in an increase in T 65
83 cell priming and/or the size of the T cell response. A study by Henrickson et al. [196] simulated this scenario using synthetic-peptide labeled DCs. DCs were loaded with peptides of different stabilities and the authors demonstrated that the number of pmhc molecules displayed on DCs controls the rate at which naïve T cells undergo activation. The number / duration of pmhc presented on the DCs was determined by the pmhc half-life. The Henrickson study directly linked the number of pmhc molecules displayed on a papc with the efficiency of T cell priming. Their study serves as a proof of concept that pmhc-stability can affect T cell priming at near physiological levels of peptideloaded DCs. However it is necessary to point out that in other systems, the number of pmhc molecules displayed on papcs does not necessarily correlate with immunodominance [ ]. Although numerous studies have implicated pmhc-stability in immunodominance [2,219,225,252], only a few studies have specifically demonstrated that pmhc-stability correlates with the size of T cell responses [1,247,255,256]. A direct link to immunodominance has only been shown by one study examining CD4+ T cell responses [248]. Clues about the mechanisms that connect pmhc-stability and the size of T cell responses and/or immunodominance have implicated the recruitment of a greater diversity of T cells specific for high-stability peptides [2,249] and the more rapid activation of naïve T cells [196]. In this thesis, we elucidate mechanisms that connect pmhc-stability and the size of T cell responses by examining the role that pmhc-stability plays in the recruitment and the accumulation of T cells. 66
84 SV40 Large T-Antigen and the CD8+ T Cell Immune Response Simian Virus 40 (SV40) is a DNA tumor virus in the polyomavirus family that was discovered in 1960 as a contaminant of African green monkey kidney cells used to produce the polio virus vaccine [257]. Until that time, many individuals given the polio virus vaccination received the SV40 contaminant as well. Estimates of viral exposure were calculated at ~90% of children and 60% of adults before Although the natural host of SV40 is believed to be asian macaques, some evidence suggests that SV40 is circulating in the human population today and may be associated with or even the cause of some rare cancers [258]. As a result of high human exposure, SV40 became of great interest to the scientific community resulting in studies that would revolutionize the understanding of cancer development and spur entire fields of study including the discovery of p53 [259]. SV40 has an icosahedral capsid containing a double stranded DNA genome encoding early and late gene products required for viral replication and assembly, respectively. Among the early proteins expressed by the potent and promiscuous SV40 promoter are the large (T) and small (t) tumor antigens (TAg or tag). TAg and tag are capable of cell immortalization via a number of phenotypes collectively known as cellular transformation. Although tag provides functions that enhance cellular transformation, it is neither sufficient nor necessary for immortalization. TAg, on the other hand, is a 94 kda 708 amino acid nuclear-localized protein that when expressed alone results in immortalization of numerous cell types of different species. The transforming properties of TAg, although not fully understood, are largely attributed to its ability to bind active forms of the tumor suppressor p53 and the retinoblastoma tumor suppressor protein Rb as well as other cell cycle and metabolic regulators including CBP and p300. Mutants of TAg that fail to bind p53 or Rb lose the ability to immortalize cells. The sequestration of 67
85 Rb and p53 activate the cell cycle and block apoptosis [258,260,261]. Cultured cells undergoing trnsformation become less dependent on serum for growth, lose contactdependant growth inhibition, increase in growth rate, and will often form tumors in immunodeficient nude mice. In permissive monkey cells, TAg and tag play critical roles in the replication of SV40 and usually causes no adverse cellular side effects besides those resulting from the normal lifecycle of the virus. However, rarely the episomal genome of SV40 integrates into the host genome in a way that disrupts virus production but results in persistent TAg and tag expression and cellular transformation. Most human and rodent cells are not permissive for SV40 and therefore infection is aborted. However, integration of SV40 DNA will cause cellular transformation [258]. Transformation of many cell types may be achieved independent of virus infection by transfection of SV40 DNA. SV40 TAg Transgenic Mouse Lines Due to the unique transforming properties of SV40 TAg, TAg transgenic mouse lines have been engineered for the study of tumor development in-vivo. Generally the mouse lines were produced by expressing TAg, sometimes in combination with tag, driven by a tissue specific promoter [ ]. Such mouse models express tumor antigen at various stages of the mouse s development and/or in numerous tissues. Often, expression of TAg results in the induction of tumors, making such models valuable for tumor therapy and tumor prevention studies. The spontaneous nature of tumor development in SV40 TAg transgenic mice accurately mimics the natural development and biological environment of tumor development. Thus, TAg induced tumor models are often preferable to transplantation models in which tumor cells lines grown in culture are inoculated directly into host mice. Laboratories interested in tumor 68
86 vaccine or tumor immunotherapy research have utilized knowledge about the immune response to TAg to direct their studies. In appendix A, I apply the findings of this thesis to immunotherapy studies in a TAg induced tumor model of choroid plexus brain tumors. CD8+ T Cell Determinants in SV40 TAg C57BL/6 (B6) mice mount a specific immune response directed against SV40 TAg, resulting in the rejection of syngenic mouse cells expressing TAg [267]. Briefly, TAg-specific rejection is mediated by more than one unique H-2 b restricted CTL response [268]. SV40 TAg containd four CD8+ T cell determinants referred to as sites I, II/III, IV and V (Figure 6). The determinants were mapped following the isolation of cytotoxic T cell clones, and these clones were used to engineer transgenic mouse lines that produce TCR transgenic T cells specific for sites I, IV and V. The CTL clones, specificities and references associated with each are listed in Table 6. Following immunization with cell lines immortalized with wt-tag the immunodominance hierarchy among the TAg epitopes is IV > I > II/III [8,269]. A detectable response to site V is only observed upon immunization with TAg constructs in which sites I, II/III, and IV have been inactivated or deleted [8,269,270]. The mechanisms that lead to the immunodominance hierarchy among these determinants are not fully understood. However, using TAP1 -/- TAg-transformed cells that can only cross-present antigen, Chen et al. [271] demonstrated that the CD8+ T cell hierarchy is established during the cross-priming of naive T cells. The study indicated that the environment of the host prior to antigen exposure or events during SV40 TAg-specific T cell priming is sufficient to establish the dominance hierarchy. A recent study demonstrated that the hierarchy among T cells specific for sites I and IV can be reversed by providing additional naïve site I-specific T cells prior to immunization [10], suggesting 69
87 Figure 6: CTL (H-2 b ) Determinants in SV40 TAg. The 708 amino acid SV40 TAg encodes four H-2 b restricted CTL determinants (sites) which have been mapped to the indicated locations within the full-length protein. The minimal amino acid sequence of each determinant is listed including its MHC-I restriction. The immunodominance profile of the determinants following immunization of B6 mice with wt-tag immortalized syngenic cells is indicated. The immunorecessive designation indicates that a site V-specific T cell response has only been demonstrated in the absence of the other three TAg determinants. 70
88 Figure 6: CTL (H-2 b ) Determinants in SV40 TAg. 71
89 Table 6: SV40 TAg H-2 b -Restricted T Cell Clones This table lists the cytotoxic T cell clones isolated for each of the SV40 TAg H-2 b restricted determinants. References indicate the origin of the clone and/or studies where the clone was utilized for mapping the indicated determinant. Clones whose TCR and chain sequences were cloned for the production of TCR transgenic mice are indicated along with the references defining those mice. 72
90 Table 6: SV40 TAg H-2 b -Restricted T Cell Clones TAg Determinant Clone Name Reference Notes Site I Y-1 [272,273] TCR-I Transgenic Mice [274] K-11 [268] Site II/III Y-2 [273,275] Y-3 [273] K-19 [268] Site IV Y-4 [273,276] TCR-IV Transgenic Mice [277] Site V Y-5 [270,275] H-1 [8] B-3 [8] TCR-V Transgenic Mice [9] 73
91 that the number of naïve precursor cells at the time of priming may play a role in regulating the T cell response among the dominant determinants. The SV40 TAg CTL hierarchy is also characterized by differences in the pattern of tolerance in transgenic mouse lines. Two transgenic mouse lines that develop TAg induced choroid plexus tumors [262] or prostate tumors [263] demonstrate lower thresholds of tolerance to site V-specific T cells than the dominant epitopes [137,215,278]. Upon immunization with wt-tag immortalized cells, T cells specific for the dominant epitopes are not detected in these TAg transgenic mice whereas V-only immunization results in the recovery of site V-specific T cells. The reason that V-only immunization is required for the in-vivo expansion of site V-specific T cells is unknown; however, endogenous site V-specific T cell responses have never been observed under any circumstances following immunization with WT-TAg. The recovery of endogenous site V-specific T cells in these tumor models makes site V an opportune determinant for understanding how to target endogenous tumor-specific T cells for tumor immunotherapy. Uncovering the mechanisms that contribute to the immunorecessive nature of site V will aid in the development of multivalent vaccines targeting subdominant CD8+ T cell determinants and tumor immunotherapies. In this thesis, we examine the role that pmhc-stability plays in the immunorecessive nature of site V. SV40 TAg site V-specific T cell immune response The mechanisms that contribute to the immunorecessive nature of site V are not fully defined despite numerous studies. The following observations have been made 74
92 concerning the physical properties of site V and the CD8+ T cell responses directed toward site V: 1) The immunorecessive nature of site V is not due to a lack of direct presentation by the cells used for immunization, because cell lines immortalized with WT-TAg are efficiently lysed by site V-specific CTL clones [219,232,270]. 2) Differences in antigen processing and presentation of site V do not depend on protein context. In one study that exchanged site V and site I within TAg, neither the immunogenicity nor the placement of site V or site I in the immunodominance hierarchy was changed [232]. 3) Following liberation from TAg, the minimal site V determinant may undergo proteasomal degradation in the cytosol that can be overcome by addition of protease inhibitors [219]. Expression of the minimal site V determinant directly in the ER result in enhanced site V immunogenicity, suggesting that cytosolic degradation of the peptide has an effect on the site V-specific T cell response. Furthermore, cytosolic degradation can be overcome by the addition of alanine flanking residues to the peptide. The results suggest that the residues surrounding site V are important during peptide liberation from TAg and for protection from proteasomal degradation during normal antigen processing. This study used vaccinia virus encoded minigene-determinants rather than full-length TAg. Since the peptide sequence that is liberated from full-length TAg during protein processing is not known, the possibility remains that proteasomal degradation of the site V minimal peptide during direct- or cross-presentation results in the reduction of the number of peptides available for site V presentation. 4) Tolerance of endogenous site V-specific T cells in TAg transgenic mouse tumor models is less effective than tolerance towards the dominant determinants 75
93 [137,263]. These observations suggest that an intrinsic property of site V may affect the development of site V-specific T cells in transgenic animals. Targeting residual populations of site V-specific T cells in SV40 TAg-transgenic tumor models may prove useful for the study of CD8+ T cell-based immunotherapy studies. In this thesis, (Appendix A) we target these residual site V-specific T cells in a model of TAg induced brain tumors using a standard cell-based immunization approach. 5) The site V determinant forms lower stability pmhc than the other H-2D b determinants, sites I and II/III (Table 4 and Figure 7). Over the course of 6 hours pmhc- V dissociates from the cell surface at a much quicker rate than pmhc-i or pmhc-ii/iii [219]. Similarly, following the chemical inhibition of new pmhc complex formation in wt- TAg transformed target cells, site V-specific CTL no longer lyse targets after only four hours. In contrast, CTL specific for the dominant epitopes continued to lyse target cells for 10 hours [8]. These findings suggest that the low-stability of site V has functional consequences for T cell recognition and killing of target cells in-vivo. This thesis is the first study to directly explore the relationship between the pmhc-stability and the immunorecessive nature of the site V-specific T cell immune response. 6) Site V-specific TCR transgenic T cells are primed following immunization with wt-tag transformed cells. However, site V-specific T cells are cross-primed less efficiently than site I-specific T cells following the same immunization [9]. 95% of total site I-specific T cells divide following immunization with wt-tag transformed cell lines from either B6 or Tap1 -/- (Tap1 -/- cells only cross-present TAg). However, immunization with V-only TAg results in only a fraction of site V-specific T cell undergoing division, 82% following B6 cell immunization and 28% following Tap1 -/- cell immunization, leaving a significant population of undivided cells (Figure 8). Thus, nearly all site I-specific T cells are cross- 76
94 Figure 7: Peptide-MHC Stability of SV40 TAg H-2D b Determinants Relative decay of H-2D b complexes from the surface of RMA/s cells preincubated in the presence of synthetic peptides corresponding to H-2D b -restricted SV40 Tag CTL epitopes. RMA/s cells were incubated overnight at 28 C in RPMI medium supplemented with no peptide ( ) or 10 mm synthetic peptides corresponding to SV40 Tag CTL epitopes I (LT ) ( ), II/III (LT ) ( ), or V (LT ) ( ). The cells were washed free of peptide, and incubation was continued at 37 C. Samples were withdrawn at the times indicated and held on ice, and the relative abundance of cell surface H-2D b complexes was determined by flow cytometry using the conformation-sensitive, H-2D b - specific monoclonal antibody This figure and legend were reproduced with permission from the American Society for Microbiology. License number:
95 Figure 7: Peptide-MHC Stability of SV40 TAg H-2D b Determinants 78
96 Figure 8: Site V-specific T Cells are Poorly Cross- Primed Cells were analyzed for in-vivo proliferation of CFSE-labeled TCR transgenic T cells. Mice were adoptively transferred with CFSE-labeled TCR-I (top) or TCR-V (bottom) T cells and immunized the next day with the indicated B6 or TAP1 -/- cells or left unimmunized (HBSS). Three days after immunization, spleen cells were stained for CD8 and Db/I or Db/V tetramer, and the intensity of CFSE fluorescence on TCR-I and TCR-V T cells was determined by flow cytometry. This figure was reproduced with permission from The Journal of Immunology. Copyright The American Association of Immunologists, Inc. 79
97 Figure 8: Site V-specific T Cells are Poorly Cross- Primed 80
98 primed following immunization whereas only a small fraction of site V-specific T cells are primed. In this thesis, we explore a relationship between the pmhc-stability of site V and the efficiency of naïve T cell cross-priming. 7) The immunorecessive phenotype of site V is not due to a low number of site V precursors. When as many as 10 7 naïve site V-specific TCR transgenic T cells (TCR-V) were transferred into mice, WT-TAg immunized animals demonstrated less TCR-V cell expansion than mice immunized with V-only TAg [9]. These results suggest that the immunorecessive / subdominant nature of site V remains intact even for high numbers of naïve site V-precursor T cells. 8) Competition plays a role in the response to site V. Otahal et al. [9] demonstrated that the immunorecessive phenotype of site V is overcome when mice are co-immunized with separate cell lines transformed with wt-tag and V-only TAg. When wt-tag and V-only TAg are expressed in the same cell, site V remains immunorecessive. The results suggest that site V and the dominant determinants must be co-expressed in the same cell for site V-specific T cell responses to be suppressed. The author s results are reminiscent of a study in which competition during priming between naive CD8+ T cells specific for two determinants in chicken egg ovalbumin (OVA) resulted in suppression of T cell responses to the subdominant determinant [240]. In that study, OVA determinants had to be presented on the same DC in order for competition to take place. Whether a similar mechanism of competition is taking place among SV40 TAg determinants is not known. There are probably many reasons why site V is immunorecessive. However, an intrinsic property of the site V determinant is probably important since processing, presentation, and recognition of the site V determinant by site V-specific T cells are not 81
99 affected by expression of the dominant determinants [8]. Thus, instability of pmhc-v (discussed in point 5, above) is a likely candidate for consideration. Numerous studies have demonstrated a correlation [1,219,247] or a contributory relationship [1,196,248,255] between pmhc stability and the immunogenicity of CD8+ or CD4+ T cell determinants. However whether the stability of pmhc-v contributes directly to the immunorecessive nature of site V has never been addressed. Elucidating the mechanisms that contribute to the immunorecessive nature of CD8+ T cell determinants such as site V may allow for the design of more effective multivalent vaccines targeting similar determinants. Furthermore advancing our knowledge of CD8+ T cell responses toward tumor antigens like SV40 TAg is a step toward optimizing CD8+ T cell responses for the improved immunotherapy of cancer. The remainder of this document will explore the following hypothesis: The low pmhc-stability of site V contributes to its immunorecessive nature by impacting the efficiency and the duration of naïve T cell cross-priming. 82
100 Chapter III: Materials and Methods Mice C57BL/6 (B6; H-2 b ), B6.129S2-Tap1 tm1arp (TAP1 -/- ), and B6.PL-Thy1a/CyJ (Thy1.1) mice were purchased from The Jackson Laboratory, maintained in-house, and used between the ages of 8 and 20 weeks. TCR transgenic mice specific for the TAg site V determinant (TCR-V) [9] and TAg site I determinant (TCR-I) [274] are on the B6 background and were described previously (Table 7). TCR-V mice used in this study were bred onto the B6.SJL-Ptprc Pepc b /BoyJ (SJL; Taconic Farms) background so that donor cells express the CD45.1 congenic marker. All mice were bred and maintained under SPF conditions at the animal facility of Penn State Hershey Medical Center. All animal studies were preformed in accordance with guidelines established by the Penn State Hershey Institutional Animal Care and Use Committee under an approved protocol. Synthetic peptides, measurement of H-2D b complex stability, and intracellular cytokine staining Synthetic peptides were synthesized at the Penn State Hershey Macromolecular Core Facility using an automated peptide synthesizer (Milligen 9050 Fmoc peptide synthesizer; Millipore). Peptides were dissolved in DMSO and diluted to the appropriate concentration in RPMI 1640 medium with GlutaMAX (Invitrogen). Peptides used in this study correspond to the SV40 TAg site V (QGINNLDNL; Pep-V), site V variants Q489A (AGINNLDNL; Pep-Q489A), and G490A (QAINNLDNL; Pep-G490A), site I (SAINNYAQKL; Pep-I), and a control H-2D b -binding peptide corresponding to influenza virus nucleoprotein (ASNENMETM; Pep-Flu). The relative decay rate of 83
101 synthetic peptide-stabilized H-2D b complexes on TAP2-deficient RMA/s cells was carried out as previously described [219]. To detect the intracellular production of IFN- 1-2x10 6 spleen cells or cultured T cells were stimulated for 5-6 hours with synthetic peptides ranging in concentration from M in a solution of 1 g/ml of brefeldin A as previously described [279]. Intracellular IFN- was detected using the Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer s instructions. Cells were analyzed by flow cytometry as described below. Plasmid and mutagenesis Two independent clones, pamw 4-4 and pamw 4-8, expressing TAg-Q489A were produced via site directed mutagenesis of plm234 [8] (encoding wt-tag) using the Quikchange II XL Site-Directed Mutageniesis Kit (Stratagene) according to the manufacturer s instructions. All oligos used in this study were synthesized and Nensorb purified by the Penn State Hershey Macromolecular Core Facility. The oligos used for mutagenesis were 5 -TTGCCTTCAGGTGCTGGAATTAATAACCTGGAC-3 (sense) and 5 -GTCCAGGTTATTAATTCCAGCACCTGAAGGCAA-3 (anti-sense). The underlined nucleotides encode the alanine substitution. The G490A substitution was introduced into the SV40 TAg encoded by plasmid pselectesv [280] by the Altered Sites II mutagenesis procedure (Promega; [8]) alone (to produce plasmid pms02-7) or in combination with MHC anchor residue alanine substitutions that simultaneously inactivated sites I, II/III and IV (p413s06 C3-13). The G490A substitution (underlined) was introduced using the mutagenic oligonucleotide MYLI502 (5 -TTGCCTTCAGGTCAGGCTATTAATAACCTGGAC-3 ). SV40 TAg determinants I, II/III and IV were simultaneously inactivated by alanine substitutions 84
102 (underlined), respectively, by using the mutagenic oligonucleotides STEV 249 (N210A; 5 -GTGTCTGCTATTAATGCTTATGCTCAAAAATTG-3 ), STEV 250 (N227A; 5 - ATTTGTAAAGGGGTTGCTAAGGAATATTTGATG-3 ), and STEV294 (F408A; 5 - TCAGTGGTGTATGACGCTTTAAAATGCATGGTG-3 ). All mutagenic oligonucleotides were phosphorylated prior to use [8]. Generation of TAg Transformed cell lines and cell lines used in this study Cell lines generated and/or used for immunization in this study are summarized in Table 7. For simplicity, when referring to cell lines in this study the common name will be used. The formal name indicates the specific cell line information used in previous publications or for the specific identification of laboratory cell lines. The Q489A, TAP Q489A, and TAP G490A cell lines were produced by immortalization of primary mouse kidney cells from B6 or TAP -/- mice by the calcium phosphate precipitation method of transfection with QIAGEN Plasmid Maxi Kit (Qiagen) purified DNA [281] with the transforming agent listed in Table 7. G490A and G490A V-only cell lines were derived using purified plasmid DNA (Roche High Pure Plasmid Isolation Kit) and the Fugene 6 method of transfection (Roche) according to the manufacturer s instructions. Immortalized foci were harvested, expanded, and SV40 TAg expression was verified by indirect immunofluorescence [8] using the monoclonal antibody pab-419 [282,283]. K-3,1,4 cells [270] express a TAg variant harboring and Tyr-406 His mutations [284] rendering them resistant to lysis by T cells specific for TAg sites I, II/III and IV, while retaining presentation of wt-site V. Adherent cells were maintained in DMEM supplemented with 100U/ml penicillin, 100 g/ml streptomycin, 100 g/ml kanamycin, 2mM L -glutamine, 10 mm HEPES, 0.075% (w/v) NaHCO 3, and 5-10% FBS (Hyclone). 85
103 Table 7: Cell Lines Used in this Study Common Name Cell Background Transforming Agent Determinants Expressed Formal Name WT-TAg B6 SV40 I, II/III, IV, V T81-82 B6/WT- 19 V-Only B6 pslm V T03-35 B6/T 116A1 Cl-C Ref. [267] [269] Q489A B6 pamw 4-8 I, II/III, IV, Q489A G490A B6 pms02-7 I, II/III, IV, G490A T08-11 Q489A WT Bulk 4-8 T07-12 G490A WT C1.1 This Study This Study G490A B6 p413s06 C3- G490A T07-16 G490A V-Only 13 V-only D2.2 This Study Null B6 plmts364-1 None T B6/122B1 TAP WT TAP -/- ppvu0 I, II/III, IV, V T02-25 TAP WT-DNA [269] [9] TAP V-Only TAP -/- pslm V T02-32 TAP(361-11)c [9] TAP Q489A TAP -/- pamw 4-4 I, II/III, IV, Q489A T08-15 Tap-/- Q489A WT 4-4 B1 This Study TAP G490A TAP -/- pms02-7 I, II/III, IV, G490A T08-19 Tap-/- G490A WT A1 This Study K-3,1,4 B6 V T88-56 B6/K- 3,1,4 [282], - Produced by Lawrence M. Mylin and Megan M. Thompson. [270] 86
104 Immunizations Cell lines for immunization were grown under uniform conditions in a single batch for each experiment and were frozen at a concentration of 2 x 10 7 cells/ml at -80 C in DMEM culture medium (described above) supplemented with 10% FCS and 5% DMSO. On the day of immunization, cells were thawed at 37 C, washed twice with PBS, counted and resuspended at a concentration of 1 x 10 8 cells/ml in PBS. Mice were immunized i.p. with 5 x 10 7 cells (0.5 ml). Peptide immunizations were performed by subcutaneous injection at the base of the tail with 100 g of Pep-V, Pep-Q489A, or Pep-I and 160 g of the HBV core helper peptide emulsified in 100 l of incomplete Freund's adjuvant as previously described [285]. Immunization with recombinant vaccinia viruses rvv-es-v and rvv-es-i [219] were carried out by i.v. injection into the tail vein of 10 7 PFU in 0.2ml of PBS. Cytotoxic T cell lines and clones Primary site V-specific T cell cultures were initiated by incubation of 1x10 7 spleen cells from previously immunized B6 mice with 5x10 5 gamma-irradiated (10 Gy) K-3,1,4 cells per well of 12-well plates. Thereafter, T cell cultures were passed every 7 days into fresh wells containing stimulatory cells with 5U/ml of rh-il-2 (Amgen). TCR-V and TCR-I T cell lines were initiated in-vitro with RBC-depleted spleen cells from naïve TCR-V and TCR-I mice. At the time of primary culture, 100 nm synthetic site V or site I peptide was used for stimulation. TCR-V and TCR-I cultures were maintained as described above by restimulation with irradiated WT-TAg cells. The site II/III specific CTL clone K19 [268] and site IV-specific CTL line SV2168T [286] were maintained as described previously. T cells were used for assays on days 4-5 of the growth cycle. Lymphocytes and TAP2- deficient RMA/s cell lines [83,84] were maintained in complete RPMI 1640 medium with 87
105 GlutaMAX supplemented with 10% FBS, 100 U/ml penicillin, 100g/ml streptomycin, 25ng/ml sodium pyruvate, 35ul/L BME, and 10ml/L HEPES. Immunoprecipitation. and western blot and pulse-chase Monolayers of the indicated cell lines were harvested by incubation with trypsin (Gibco) in versene and lysed for 30 minutes on ice at a concentration of 1.5x10 7 cells/ml in lysis buffer (50mM Tris (ph 8.0), 5mM EDTA, 150mM NaCl, 0.5% NP-40) supplemented with TIU/ml aprotinin and 0.057mM PMSF. Debris was pelleted in a table top microcentrifuge at full speed for 2 minutes. 200 l of supernatant was immunoprecipitated with 40 l 50/50 protein A-sepharose beads (Sigma-Aldrich; P3391) and TAg-specific pab-901 [282,287] antibody supernatant for 2 hours at 4 C. Beads were washed 3x with SNNTE (50mM Tris, 5 mm EDTA, 0.5 M NaCl, 5% sucrose, and 1% NP-40), 1x with RIPA (50mM Tris (ph 7.4), 150mM NaCl, 0.1% SDS w/v, 1% Tritonx 100, 25.4mM deoxycholic acid) and eluted for 5 min at 95 C in 50 l of 2x sample buffer (100mM Tris-HCl, 4% SDS, 20% glycerol, 2% 2-ME, and 0.01% bromphenol blue). Proteins were separated on 12.5% SDS polyacrylamide gels under reducing conditions. Proteins were transferred to 0.45 m PVDF membrane (Millipore) and SV40 TAg was detected by blocking with 5% non-fat milk (Carnation) in TBST and blotting with 1 - pab-901 then with 2 -Goat- ouse-hpo (Source) in 1% non-fat milk. The blot was visualized using Pierce ECL Western Blotting Substrate (Thermo) and Kodak BioMax Light film. Pulse-chase experiments were preformed as previously described [9]. 88
106 Sequencing of cell line genomic DNA to confirm the incorporation of site V mutations Genomic DNA was extracted from the indicated cell lines using the Genomic DNA Purification Kit (Gentra Systems), and a C-terminal fragment of SV40 TAg, flanking the epitope V region, was amplified for 30 cycles using primers STEV398, 5 - CATAGAAGAATGGATGGCTGGA-3 and JTEV226, 5 -AGCAAACTCAGCCACAGGTC- 3. The amplified fragment (~600bp) was purified and then sequenced using STEV335, 5 -GAATTATGTGGGGGGAAAGCT-3 in the Penn State Hershey Molecular Genetics Core Facility using an ABI 3130XL Capillary sequencer. Cytotoxicity assay Cytotoxicity assays were performed as previously described [288]. Briefly, T-25 flasks of target fibroblasts were labeled overnight with 200 Ci of sodium 51 chromate. Target cells were treated with trypsin in versene and 10 4 target cells were seeded into 96-well V-bottom plates. Effector cells were plated with targets in triplicate at the indicated effector to target ratio (E:T) in a total volume of 200 l. The plates were incubated for 4 hours at 37 C and 5% CO 2 and then centrifuged in order to pellet cells and debris. 100 l of supernatant was removed from each well, and the radioactivity was counted on a Cobra gamma-counter (Packard Instruments). Percent specific lysis was determined as described previously [288]. Effector cell lines specific for sites I, II/III, IV, and V were as follows: TCR-I cells, clone K-19, line SV2168T, and TCR-V cells (Table 6). 89
107 Flow Cytometry Ex-vivo staining of lymphocytes was performed on single cell suspensions of spleens as previously described [269]. All staining was performed in FACS buffer (PBS supplemented with 2% FBS and 0.1% Sodium Azide) and completed at room temperature. Cells were incubated in a 1:100 dilution of FC block (rat anti-mouse CD16/CD32 (33 mg/ml [Pharmingen])) then washed 1x with FACS buffer. Fluorophore conjugated antibodies (1:150 dilution) and tetramer (1:200 dilution) were added to the cells and incubated for 15 minutes at room temperature. Cells were washed 3x with FACS buffer and fixed in 2% paraformaldehyde in PBS and analysed using a FACScan II, FACSCalibur, FACSCanto or LSR II flow cytometer (BD Biosciences). Unless otherwise noted, 100, ,000 live events were recorded based on FSC-A/SSC-A plotting, and data were analyzed using FlowJo software (Tree Star). MHC class I tetramer specific for H-2D b /TAg site V (Tet-V) or H-2D b /TAg site I (Tet-I) was produced and characterized as described previously [269]. The following antibody clones were purchased from ebiosciences: anti-mouse CD8a (clone ), anti-mouse CD45.1 (clone A20), and anti-mouse Thy1.1 (clone H1S51). anti-mouse IFN- (clone XMG1.2) was purchased from BD Pharmingen. In-vivo proliferation assays Single cell suspensions of RBC-depleted lymphocytes were derived from spleens and lymph nodes of TCR-V/CD45.1-transgenic mice and/or Thy1.1+ mice (described above). The frequency of site V-specific T cells was determined by staining with anti- CD8 and Tet-V. Cells were labeled with 5 M 5- and 6-CFSE (Molecular Probes) as described previously [9]. For "Thy1.1 spike" experiments (Figure 17A), lymphocytes from TCR-V and Thy1.1+ mice were resuspended in PBS and mixed. Donor mice 90
108 received 1x10 6 CD8+/Tet-V+ cells by i.v. injection. The day following T cell transfer, mice were immunized via the i.p. route with 5x10 7 TAg transformed cells. 3-7 days following immunization, spleens were harvested and TCR-V cells were visualized by staining for CD8 and CD45.1 in combination with Tet-V. Dilution of the CFSE label was used to determine whether naive T cells underwent division. For thy1.1 spike experiments, the number of undivided (UD) CFSE-Hi TCR-V and CD8+thy1.1+ cells was determined and a ratio was calculated using the thy1.1+cd8+ cell number as denominator (UD-TCR-V / UD-Thy1.1 = Ratio). Divided (D) TCR-V ratios were determined using the following equation (D-TCR-V / UD-Thy1.1 = Ratio). Data in each group were compared to the ratio derived from mice that received no immunization or immunization with a TAg immortalized cell line, B6/T 122B1, containing no known epitopes. Similar to the thy1.1 spike method, the B-cell spike method consists of transferring total spleen cells from CD45.1+ congenic TCR-V mice into experimental CD45.2+ mice. B-cells from donor mice were identified by staining with antibodies for CD45.1 and CD19 (B-cells). Ratios of UnDivided (UD) and Divided (D) TCR-V cells were determined by the following equation. (UD-TCR-V / UD-CD45.1+CD19+ = Ratio or D-TCR-V / UD-CD45.1+CD19+ = Ratio). Tetramer-based enrichment of site I- and site V-specific CD8+ T cells from naïve mice. At least 10 days prior to the experiment, positive control mice were immunized with WT-TAg or Q489A cells. At least 1 day prior to the experiment these same positive control mice received 10 5 naïve TCR-V cells i.v. Immunization plus adoptive transfer 91
109 yielded detectable populations of both site I- and site V-specific T cells to facilitate identification of positive cells in the gating strategy. A variation of previously published protocols [169,171,172] was utilized for tetramer enrichment. Briefly, on the day of enrichment, the spleen and the superficial cervical, brachial, inguinal, lumbar, and mesenteric lymph nodes were harvested. Organs were mechanically disrupted, and the resulting suspension was incubated in complete RPMI growth medium supplemented with 150u/ml collagenase A (Gibco, ) and 100u/ml recombinant DNAse I (Roche, ) at 37 C for 30 minutes with rocking. A single-cell suspension was created by pushing the particulate tissues through a stainless-steel 60 mesh screen (Sigma). RBCs were lysed using tris-amonium-chloride and the cells were washed once with FACS buffer. The cells were resuspended in 0.75ml of FACS buffer and 20ul of cells were saved for pre-sort staining. Cells were co-stained with tetramer-v-pe and tetramer-i-apc for 30 minutes at room temperature in FACS buffer containing Fc block. MHC tetramer stained cells were then washed 2x with 5ml of MACS buffer (PBS supplemented with 0.5% BSA and 2mM EDTA), and stained with 0.1ml each anti-pe and anti-apc MicroBeads (Miltenyi Biotech) in a total volume of 1ml for 15 minutes at 4 C. Cells were washed with 10ml MACS buffer and passed through a cell strainer (70 mesh, Becton Dickinson) before centrifugation. Cells were resuspended in 3ml MACS buffer and passed over an LS column (Miltenyi Biotech) at 4 C. Columns were washed 3x with 3ml MACS buffer and all flowthrough was collected for staining. Cells bound to the column were eluted 2x with 5ml of MACS buffer. Eluted cells were washed 1x with MACS buffer and 10ul was taken for counting. Pre-sort, flowthrough, and eluted cells were stained in a 96-well plate with the following antibodies: unlabeled Fc block (described above), FITC DUMP (antibodies to CD19, MHCII, F4/80, CD11b, and CD4), anti-cd8, anti-thy1.2, anti-cd44, Tetramer- 92
110 V-PE, and Tetramer-I-APC for 15 min at RT. Cells were washed 3x with FACS buffer and resuspended in 2% PFA. All samples were run on a BD FACSCanto or LSRII. The entire eluted cell fraction was acquired. As depicted in Figure 20A, cells were first gated on FITC-/CD8+ cells and then Thy1.2+ cells. Dot plots were displayed as Tet-I vs. Tet-V. In order to set the gates for each tetramer+ population, populations from positive control mice were used as a guide. 93
111 Chapter IV: Results Increasing Peptide-MHC Stability Augments the Cross-Priming of Naïve CD8+ T Cells Specific for SV40 TAg Site V The role that pmhc-stability plays in the initiation of T cell immune responses is not well understood. Evidence suggests that pmhc-stability determines the size of the T cell response [247,248] and more recent observations suggest that pmhc-stability may determine the rate at which naïve T cells undergo activation [196]. A mechanism describing why high pmhc-stability results in a T cell response of greater size has yet to be determined. The properties of the SV40 TAg site V determinant provide a unique model to elucidate the influence of pmhc-stability on the size of the T cell response and immunodominance. Site V is an immunorecessive CD8+ T cell determinant of SV40 TAg that forms low-stability pmhc compared to the dominant TAg determinants site I, II/II, and IV. Following immunization, only a subset of TCR transgenic T cells specific for site V undergo cross-priming whereas the majority of site I-specific T cells are crossprimed [9]. We can use high-stability APLs to determine the influence of pmhc-stability on the fraction of site V-specific T cells that are cross-primed. We can monitor changes in the fraction of naïve site V-specific T cell cross-priming as an indicator of changes to the efficiency of naïve T cell recruitment. Furthermore, the size of the site V-specific T cell response can be used as an indicator of changes to immunodominance. The properties of the CD8+ T cell response to site V makes SV40 TAg site V an ideal CD8+ T cell determinant for studying the role of pmhc-stability in immunodominance. 94
112 Identification of site V APLs that increase pmhc stability We first sought to identified point mutations within the site V minimal peptide that would result in an increased pmhc-half-life. By design, the APLs had to be recognized by site V-specific T cells specific for wt-site-v so that conclusions could be drawn regarding any changes to site V-specific T cell cross-priming or response size. APLs that cross-react with site V-specific T cells would allow the use of existing reagents such MHC-tetramers and TCR transgenic T cells (TCR-V). Site V (QGINNLDNL) already harbors the canonical H-2D b anchor residues at position (P) P5-N and P9-L (Table 8). Therefore, we chose to mutate non-anchor residues to stabilize pmhc binding but not affect TCR recognition. A number of studies have demonstrated the importance of nonanchor residues for high-affinity pmhc interactions [28,45,289]. However, not all nonanchor residues within site V were candidates for mutation since non-anchor residues also contribute to T cell recognition. P3-I and P8-N are important for recognition by site V-specific CTL [290] (Table 8), and studies focusing on H-2D b -binding peptides other than site V have indicated that P3 [291], P4, P6 [291,292], P7, and P8 [292] can protrude from the MHC binding cleft and/or serve as TCR contact residues. Thus, we altered residues at the remaining positions, P1-Q and P2-G. Since alanine residues at these positions are associated with high-affinity D b interactions [45] (Table 5), we chose to produce synthetic peptides with alanine substitutions at P1-Q and P2-G. The novel site V APLs are hereafter referred to as Q489A and G490A (Figure 9A). Synthetic peptides corresponding to Q489A and G490A were loaded onto TAP2- deficient RMA/s cells overnight. Peptide was washed off and aliquots of the cells were collected at the indicated time points and placed on ice. After all samples were collected, the cells were stained for surface H-2D b and evaluated by FACS. The results indicate that Q489A and G490A formed pmhc with greater stability than the site V 95
113 Table 8: Rationale for Mutating TAg Site V-Residues at P1 and P2 This table illustrates that rationale for mutating residues P1-Q and P2-G of TAgsite V in order to create APLs that form stable pmhc. Shaded residues were residues under consideration for mutation. Underlined residues were eliminated due to the rationale listed in the residue elimination column. Following elimination, shading was removed from the residues.. 96
114 Table 8: Rationale for Mutating TAg Site V-Residues at P1 and P2 Residue Elimination Mutation Candidates Position References WT-Site V Q G I N N L D N L [270,275] D b Anchors Q G I N N L D N L [18,30] Recognized by site V- Specific Clones H-1 or Y-5 Q G I N N L D N L [290] Often Exposed Residues or TCR- Contacts Residues Selected for Mutation Q G I N N L D N L [291,292] Q G 97
115 Figure 9: Characterization of Site V APLs Site V alanine substitutions increase pmhc stability. Synthetic peptides representing single alanine substitutions of site V were generated (A) and RMA/s cells were incubated with the peptides over night at 29 C. In the morning, peptides were washed free and the cells were allowed to incubate at 37 C. Samples were collected at the indicated times and held on ice until the last sample was collected. Cells were stained with a conformation dependant antibody specific for H-2D b. The data are plotted as a percent of maximum arbitrary fluorescence units. The dotted line represents 50% of maximum arbitrary fluorescence units. Results are from a single experiment and each point represents a single sample. 98
116 Figure 9: Characterization of Site V APLs 99
117 peptide (Figure 9B). Q489A formed the most stable pmhc with a half-life of >4 hours. The G490A mutation resulted in an intermediate stability between Q489A and site V, with a half-life of ~2.75 hr. Site V had a half-life of approximately 2 hours, similar to previous observations [219] (Table 4). Thus, both Q489A and G490A pmhc had enhanced stabilities. Characterization of Q489A and G490A Next we evaluated the effect of Q489A and G490A on recognition by site V-specific T cells. Site V-specific T cell lines derived from TCR transgenic mice [9] (Figure 10A & B) or polyclonal site V-specific T cells isolated from B6 mice (Figure 10C & D) were stimulated with synthetic peptide corresponding to site V, Q489A, G490A or control (Flu NP ). Five hours following incubation, T cells were evaluated for accumulation of intracellular IFN- by ICS. Both Q489A and G490A stimulated the production of IFN- (Figure 10A & C) over a wide range of peptide concentrations (Figure 10B & D), indicating that site V-specific T cells responded functionally toward both site V APLs. Clonal TCR-V (Figure 10B) and bulk (Figure 10D) T cell lines demonstrated a similar functional avidity towards Q489A, suggesting complete cross-reactivity. G490A stimulated the production of IFN- at all peptide concentrations; however, the functional avidity of TCR-V (Figure 10B) and polyclonal T cells (Figure 10D) was reduced by approximately 5 and 10 fold, respectively. 100
118 Figure 10: Q489A and G490A Cross-React with Clonal and Polyclonal Populations of Site V-specific T cells Cultures of TCR-V T cells (A & B) or bulk cultures of endogenous site V specific T cells (C & D) were stimulated with the indicated peptide at 10-7 M (A and C) or M (B and D) in the presence of BFA for 5 hours. Cells were evaluated for the production of intracellular IFN-. Results are representative of 2 independent experiments and each point represents a single sample. 101
119 Figure 10: Q489A and G490A Cross-React with Clonal and Polyclonal Populations of Site V-specific T cells 102
120 Taken together, these results indicate that Q489A did not compromise recognition by or functional avidity of TCR-V cells or polyclonal site V-specific T cells. G490A crossreacts with site V-specific T cells at all tested concentrations; however, it promoted lower functional avidity interactions than site V or Q489A from both TCR-V cells and polyclonal populations of site V-specific T cells. Production and characterization of cell lines expressing Q489A and G490A All previous work has characterized the site V-specific T cell response in the context of cell-based immunization. Therefore, to evaluate changes in the size of the site V-specific T cell response, immortalized cell lines were produced with mutants of TAg that incorporated Q489A and G490A. Full length SV40 TAg constructs containing Q489A or G490A were engineered in the context of both wild type and site V-only TAg (Figure 11), as described in the methods. Primary mouse B6 and Tap1 -/- kidney cells were immortalized with each of the unique constructs, and cell lines were derived for each of the constructs except Q489A V-only. Q489A V-only TAg was unable to immortalize primary kidney cells following multiple attempts. All cell lines are summarized in Table 7. Expression of TAg was verified by IP/western blot for each of the cell lines used for immunization in this study (Figure 12A), and the level of TAg protein expression was quantified. WT-TAg cells were set to 100% and a cell line expressing no TAg (No-TAg) was used as background. Thus, values represent differences in TAg protein expression relative to WT-TAg cells. Q489A and G490A cells expressed 29% and 23% more TAg than WT-TAg cells, respectively. TAP-WT cells expressed ~40% higher levels of TAg than WT-TAg cells; however, among the other TAP -/- cell lines, TAg expression only varied at most by 16%. The reason that TAg levels vary among cell lines is unknown. In future experiments, efforts to normalize the TAg proteins levels used for immunization may be undertaken by varying the number of cells 103
121 Figure 11: Visual Representation of the TAg Constructs Used for Cell Line Production 104
122 Figure 12: Cell Lines Express Full-Length TAg and the Appropriate Site V-APL Cell lines were derived from primary B6 or Tap -/- kidney cells immortalized with SV40 T Ag constructs containing Q489A or G490A. (A) Cell lines were evaluated for the level of TAg expression by immunoprecipitation and western blot of SV40 T Ag. (B) Cell lines were evaluated by immunofluorescence (IF) for nuclear-localization of TAg. G490A and G409A V-only cell lines were produced at Messiah College by Lawrence Mylin and Megan Thompson and IF data is not available. (C) Genomic DNA was isolated from each cell line and the epitope V region of TAg was sequenced. The double-underlined nucleotide sequence indicates the presence of the appropriate mutation. Thanks to Sandip Savaliya for the preparation of cell-line genomic DNA for sequencing. Results are representative of 2 independent experiments. Sequences were acquired once. 105
123 Figure 12: Cell Lines Express Full-Length TAg and the Appropriate Site V-APL 106
124 used from immunization based on levels of TAg expression. Expression of nuclear TAg in Q489A, TAP Q489A and TAP G490A cell lines was verified by immunofluorescence (IF) staining with TAg N-Terminal binding antibody pab- 419 [282,283] (Figure 12B). The results indicated that both Q489A- and G490A-TAg were targeted to the nucleus. IF data were not available for cell lines G490A and G490A V-only, produced by Lawrence Mylin and Megan Thompson from Messiah College. Additionally, genomic DNA was extracted from each cell line, and the region containing site V was sequenced in order to verify the expression of the appropriate site V point mutation (Figure 12C). These data indicate that full-length TAg was expressed in each of the cell lines and that the indicated site V APL was encoded. To determine whether Q489A and G490A were endogenously processed and presented from full-length TAg, cell lines expressing the Q489A- and G490A-TAg were tested by cytotoxicity assay (Figure 13). Each B6 cell line was tested for killing by CTL clones specific for each of the four TAg determinants. The site V-specific T cell line used in the assay was derived from bulk culture of TCR-V cells. The results indicate that Q489A and G490A were processed, presented, and recognized by site V-specific T cells. Furthermore, sites I, II/III, and IV were recognized in Q489A- and G490A-TAg immortalized cell lines. These data indicate that processing and presentation of the dominant TAg determinants as well as Q489A and G490A were maintained by Q489Aand G490A-TAg. Cell lines derived in the Tap1 -/- background were not tested by cytotoxicity assay due to their lack of cell surface MHC-I presentation [85]. Stable protein is required for cross-presentation. Although no studies have definitively determined the half-life necessary for cross-presentation, targeting otherwise stable proteins for rapid proteasomal degradation by genetically tagging them with an 107
125 Figure 13: Q489A and G490A are Processed and Presented from Full-Length TAg A 51 Cr cytotoxicity assay was performed on all cell lines from the B6 background. Effectors include T cells specific for each of the known TAg epitopes (see Materials and Methods). The results are representative of two independent experiments. 108
126 Figure 13: Q489A and G490A are Processed and Presented from Full-Length TAg 109
127 Figure 14: TAg Protein Stability Each cell line was tested for protein stability over 20 hours by pulse-chase as described in the methods. The results represent only one experiment. Plans include for this experiment to be repeated one or more times. 110
128 Figure 14: TAg Protein Stability 111
129 ubiquitin domain eliminates detectable cross-priming [112]. Since previous studies have determined that cross-priming of site V-specific T cells is necessary following cell-based immunization [9] and our study is focused on a the process of cross-priming, we assessed the stability of TAg in our cell lines using pulse-chase (Figure 14). TAg was detected in each cell line following a 20 hour chase. The detection of TAg at 20 hours suggests that the turnover of TAg did not vary substantially among cell lines and was significantly greater than proteins tagged with ubiquitin domains for immediate degradation. Thus, these data suggest that the stability of TAg in our cell lines was unlikely to affect the efficiency of T cell cross-priming. Immunization with Q489A and G490A cells induces detectable site V-specific T cell responses We wanted to determine whether Q489A and G490A TAg initiated a site V- specific T cell response. Otahal et al. [9] demonstrated that TCR-V cells respond at low levels to WT-TAg cell immunization, making TCR-V cells a sensitive indicator of a site V- specific T cell response. Mice received 10 6 naïve TCR-V T cells and were immunized with the cell lines: WT-TAg, V-only, Q489A, G490A, G490A V-only or no immunization. Seven days following immunization, spleens were harvested and site V-specific CD8+ T cells were visualized by MHC tetramer staining (Figure 15). Dot plots from representative mice are shown in Figure 15A and the mean frequency of the responding cells among groups is represented in Figure 15B. Similar to findings by Otahal et al. [9], immunization with WT-TAg resulted in a small increase in TCR-V cells compared to unimmunized mice. Immunization with V-only cells resulted in the accumulation of TCR- V cells to more than 10% of total CD8+ T cells per spleen. Q489A, G490A and G490A 112
130 Figure 15: TCR-V Cells Respond to Immunization with Q489A and G490A Cells Q489A and G490A cells prime TCR-V cells. B6 mice received 1x10 6 CD45.1 congenic TCR-V cells and were immunized with the indicated cell lines. 7 days following immunization, the peak of the TCR-V T cell response [217], spleens were harvested, and CD45.1+CD8+ TCR-V cells were quantified by staining with MHC tetramer. (A) Numbers represent the percentage of CD45.1 cells in the gate. (B) Bars represent the mean values of all mice (n=3) mice from panel A. Error bars are standard deviation. Results are representative of two independent experiments. 113
131 Figure 15: TCR-V Cells Respond to Immunization with Q489A and G490A Cells 114
132 V-only immunization resulted in the accumulation of TCR-V cells to levels similar to or more than immunization with WT-TAg, suggesting that the site V mutants were at least as immunogenic as wild type site V. Interestingly, when cell lines containing sites I, II/III, and IV (WT-TAg, Q489A, and G490A) were used for immunization, the size of the TCR- V response correlated with the pmhc-stability of the epitope V variants. Thus, increased pmhc-stability was associated with an increased T cell response. However, the mean frequency of the TCR-V cell responses to Q489A and G490A cells were not statistically different from the response to WT-TAg cells (95% confidence interval, unpaired T test). Therefore we can only conclude that both site V mutants were capable of initiating a TCR-V T cell response. In contrast to TCR-V transgenic T cells, endogenous site V-specific T cell responses are not detectable following immunization with WT-TAg, even following invitro restimulation [269]. To determine if endogenous site V-specific T cells responded to Q489A and G490A cells, we immunized B6 mice with the following cell lines: WT-TAg, V-only, Q489A, G490A and G490A V-only. 10 days following immunization, spleens were harvested and the lymphocytes were stained with site V MHC-tetramer (Tet-V) (Figure 16). The cell lines showed variable ability to produce a detectable ex-vivo site V tetramer-specific T cell response (Figure 16A, left panel). V-only immunization resulted in 0.38% and 1.02% of CD8+ T cells binding tetramer in mouse 1 and mouse 2 respectively, whereas mouse 3 did not have detectable tetramer positive T cells. Such mouse-to-mouse variability was typical in mice immunized with V-only TAg, a phenomena that has not been documented previously. Immunization with G490A V-only produced strong ex-vivo responses in all of the mice ranging from 1.00% to 2.75% of total CD8+ T cells, consistent with TCR-V cell responses (Figure 15). Surprisingly, 115
133 Figure 16: Q489A and G490A Cells Overcome the Immunorecessive Nature of Site V B6 mice were immunized with the indicated cell lines. 10 days following immunization spleens were harvested and CD8+ T cells specific for site V (A Left), I and IV (B & C) were quantified by staining with MHC tetramer. Endogenous T cells were restimulated twice in-vitro with K-3,1,4 cells, expressing site V-only TAg, and were evaluated for the expansion of site V-specific T cells by tetramer staining (A Right). The size of the ex-vivo site V response is compared with the site I (B) or site IV (C) response. Results are representative of at least 2 independent experiments. Horizontal bars represent the mean for each group of three mice. 116
134 Figure 16: Q489A and G490A Cells Overcome the Immunorecessive Nature of Site V 117
135 Figure 16: Q489A and G490A Cells Overcome the Immunorecessive Nature of Site V 118
136 Q489A immunization induced a weak but detectable population of Tet-V+ cells in mouse 1, and G490A immunization resulted in detectable populations in mice 1 and 3. All mice immunized with WT-TAg, Q489A, and G490A cells generated CD8+ T cell responses to sites I or IV, indicating that immunization resulted in the initiation of an immune response (Figure 16B and C). The overall hierarchy of the TAg determinants in each of the mice immunized with Q489A or G490A cells was the same as in mice immunized with WT- TAg cells; IV>I>V. To verify site V-specific T cell responses, splenocytes from each mouse were evaluated following in-vitro restimulation with K-3,1,4 cells, which express a TAg variant lacking sites I, II/III and IV [270] to expand residual populations of site V-specific T cells. With the exception of mouse 1 immunized with V-only TAg, mice that had detectable exvivo Tet-V+ cells showed a marked expansion of T cells in culture (Figure 16A, right panels). In addition, mouse 2 from the Q489A-immunized group showed accumulation of Tet-V+ cells despite the absence of a detectable ex-vivo population. Consistent with previous studies, no site V-specific T cells were detected after restimulation of splenocytes from WT-TAg-immunized mice. As expected, Tet-V+ cells did not expand from unimmunized mice following in-vitro culture, indicating that the expansion of site V- specific T cells was not due to in-vitro priming of naïve site V-specific T cells. The data revealed no discernable relationship between the size of the endogenous site V-specific T cell response and the response toward the dominant determinants (Figure 16B and C). These data suggest that, when expressed with the dominant TAg determinants in the context of full-length TAg, Q489A and G490A overcome the immunorecessive nature of site V. For the first time, we demonstrated an endogenous site V-specific T cell response following immunization with full-length TAg containing all three dominant 119
137 determinants. Thus, the Q489A and G490A mutations overcame the immunorecessive nature of site V. Q489A and G490A immunizations result in enhanced TCR-V cross-priming Otahal et al. [9] previously demonstrated that the site V determinant was inefficiently cross-presented from TAg in-vivo. The authors monitored CFSE labeled TCR-V cells for division and demonstrated that only a subset of naïve TCR-V cells proliferated following immunization with TAP-V-only cells [9] (Figure 8). Thus, increased immunogenicity of Q489A and G490A could be explained by an increase in the fraction of naïve TCR-V T cells triggered to proliferate. We developed an assay to accurately measure the fraction of naïve T cells that divide in response to immunization by monitoring the loss of cells from the naïve (CFSE-Hi) population. The assay normalizes the CFSE-Hi population to a marker population (Thy1.1+ cells) that does not divide. In addition, this novel approach corrects for variation in the number of TCR-V T cells transferred into different mice and allows for direct comparison among mice and treatment groups. In this approach, 10 6 CFSE-labeled TCR-V T cells were cotransferred ( spiked ) with CFSE-labeled naïve thy1.1+ spleen cells into recipient B6 mice (Figure 17A, left). When the mice were immunized, TCR-V cells divided (Figure 17A, top right panel) and Thy1.1+CD8+ T cells underwent no or negligible division (Figure 17A, bottom right panel). The initial ratio of TCR-V to CD8+Thy1.1+ cells was the same in all mice; therefore, the CFSE-Hi CD8+Thy1.1+ population served as a standard for normalizing the data. All data are represented as the ratio of # CFSE-hi TCR-V cells : # CFSE-hi Thy1.1+CD8+ cells. This ratio is directly proportional to the number of undivided TCR-V cells recovered from the spleen. The fraction of cells that divide following immunization can then be calculated by determining the difference in the 120
138 Figure 17: Q489A and G490A Enhance the Fraction of Naïve Site V-Specific T cells that are Cross-Primed Epitope V mutants induce a greater fraction of naïve TCR-V cells to undergo cross-priming. (A) Diagram of experimental protocol. (A-B) CFSE labeled spleen cells from a thy1.1 congenic mouse and a CD45.1 congenic TCR-V mouse were mixed together. 1x10 6 TCR-V T cells were transferred into B6 mice. The following day, the mice were immunized with the indicated cell lines. Three days following immunization, spleens were harvested and cells were stained with Tet-V, anti-cd8 and anti-thy1.1. (B) The bar graph displays the ratio of CFSE-Hi UnDivided TCR-V / CD8+Thy1.1+ cells from two combined independent experiments. A drop in the value of the ratio indicates an increase in the number of naïve TCR-V cells that underwent proliferation. (C) Demonstrates the CD44 and CD62L staining profile of UnDivided and Divided cell populations from representative mice. (D) The percent of naïve T cells that underwent division was calculated by the formula 100*(1-(I/U)), where I = the mean ratio from a group of immunized mice and U = the mean ratio from a group of unimmunized mice. P-values were determined by unpaired t-tests using normalized and combined data from both experiments. 121
139 Figure 17: Q489A and G490A Enhance the Fraction of Naïve Site V-Specific T cells that are Cross-Primed 122
140 Figure 17: Q489A and G490A Enhance the Fraction of Naïve Site V-Specific T cells that are Cross-Primed 123
141 Figure 17: Q489A and G490A Enhance the Fraction of Naïve Site V-Specific T cells that are Cross-Primed 124
142 ratios between an unimmunized / control immunized group and the mice receiving a specific immunization. Using this approach, we evaluated the effect of the Q489A and G490A mutations on the fraction of transgenic TCR-V T cells triggered by cross-priming. Since site V is presented in a TAP-dependant manner [9], any effect of direct presentation by TAg transformed cells was eliminated by immunizing B6 mice with TAP -/- cell lines. Following immunization with TAP-WT, a significant number (p=0.0013) of TCR-V cells underwent division as indicated by the reduction in undivided TVR-V cells compared to unimmunized animals (Figure 17B). Significantly more (p<0.0001) TCR-V cells underwent division following Q489A or G490A immunization. The distribution of CD44 and CD62L expression was similar among all groups of immunized mice for both UnDivided and Divided cell populations (Figure 17C), suggesting uniformity among the T cell populations regardless of the cell line used for immunization. Figure 17D indicates that 33.4% of TCR-V cells divided in response to immunization with TAP-WT whereas 69.9% and 69.4% of TCR-V cells divided in response to immunization with TAP-Q489A and TAP-G490A, respectively. These data indicate that Q489A and G490A increased the fraction of naïve site V-specific T cells that are cross-primed in-vivo. Bypassing cross-presentation leads to increased recruitment of TCR-V T cells Although a greater fraction of TCR-V cells divided following Q489A and G490A cell immunization, a third of the TCR-V cells did not divide. In contrast, Otahal et al. [9] demonstrated that nearly all TCR-I cells divided following immunization with WT-TAg. The possibility remains that TCR-V cells may be functionally heterogeneous, and therefore a subset of TCR-V cells may be incapable of dividing. To test this, mice received CFSE labeled TCR-V cells plus a Thy1.1 spike and were immunized with synthetic site V or Q489A peptide in incomplete Freund s adjuvant (IFA) [285] or a 125
143 Figure 18: Bypass of Cross-Presentation Results in High Fraction of TCR-V Recruitment B6 mice received CFSE labeled TCR-V cells plus thy1.1 spike and were immunized with peptide or recombinant vaccinia virus. Three days following immunization, spleens were harvested and TCR-V cells were evaluated for division. Representative CFSE plots of TCR-V cells 3 days following immunization are displayed in panel A. The ratio of UnDivided TCR-V / CD8+thy1.1+ cells (B) or the ratio of Divided TCR-V / CD8+Thy1.1+ cells (C) are displayed. Groups were comprised of 3 mice each. Data are representative of two independent experiments. P-values were determined by unpaired t-tests using normalized and combined data from two experiments. 126
144 Figure 18: Bypass of Cross-Presentation Results in High Fraction of TCR-V Recruitment 127
145 recombinant vaccinia virus expressing an ER targeted site V-minigene (rvv-es-v) [219] (Figure 18). Both immunization methods bypass cross-presentation for priming of naïve T cells. The results indicated that nearly all detectable TCR-V cells divided in response to peptide-v or peptide-q489a immunization, suggesting that nearly all TCR-V cells were capable of responding to antigen (Figure 18A and B). The majority of TCR-V T cells also divided following immunization with rvv-es-v. The lack of division after immunization with peptide-i or rvv-es-i demonstrates that division in response to peptide-v, peptide-q489a, and rvv-es-v was specific (Figure 18B). Interestingly, immunization with Q489A peptide resulted in significantly less proliferation than immunization with site V-peptide (Figure 18C). These data suggest that nearly all TCR- V cells are primed and divide following specific stimulus when cross-presentation is circumvented. The number of naive TCR-V cells does not affect the efficiency of TCR-V cell recruitment T cells of the same specificity can compete for access to their cognate pmhc on the dendritic cell surface [240]. Due to this type of competition, some naïve TCR-V cells may experience limited access to cross-presented antigen, resulting in sub-threshold levels of antigen stimulus needed to undergo priming. In an example of this phenomena, Cockburn et al. [293] recently demonstrated that more naïve T cells divide when fewer TCR-transgenic T cells specific for a malaria determinant are introduced into mice. Using the thy1.1 spike method, we transferred 10 6, 10 5 or 10 4 naive TCR-V cells into B6 mice prior to immunization with TAP-WT cells. Three days following immunization, spleens were harvested and the ratio of undivided TCR-V T cells was measured for each group (Figure 19A). The results demonstrated that a similar fraction of naïve TCR-V cells divided regardless of the number of TCR-V cells transferred. The 128
146 Figure 19: The Number of Naïve TCR-V Cells has No Effect on TCR-V Cell Recruitment Mice received 10 6, 10 5, or 10 4 CFSE-labeled TCR-V cells spiked with thy1.1+ cells and were immunized with TAP-WT cells the next day. Three days following immunization, spleens were harvested and the UnDivided TCR-V / Thy1.1+CD8+ ratio (A) and the Divided TCR-V / Thy1.1+CD8+ (B) ratios were determined. Representative histograms demonstrate the distribution and intensity of CFSE staining on the TCR-V cell populations in each group. The histogram scale is adjusted for each plot (C). The frequency of Thy1.1+ cells is displayed as a percent of total CD8+ cells / spleen. Each point is the mean frequency from all animals in the experiment. These data represent the only experiment conducted, n=3. (A-B) Values are not statistically significant as determined by unpaired t-tests. 129
147 Figure 19: The Number of Naïve TCR-V Cells has No Effect on TCR-V Cell Recruitment 130
148 similar efficiency of recruitment was not the result of altered TCR-V seeding efficiencies among groups because the percentage of CD8+ and Thy1.1+ cells across all groups formed a linear regression (Figure 19D). Interestingly, when 10 6 TCR-V cells were transferred into mice, the TCR-V cells proliferated more than when 10 5 or 10 4 TCR-V cells were transferred (Figure 19B), suggesting that TCR-V cells may proliferate more efficiently when more responding cells are present. These data suggest that as the naive site V-specific precursor numbers decrease, the fraction of naive T cells that are primed does not change. However, the number of site V-specific T cell precursors may influence the accumulation of responding cells, but priming of a limited fraction of naïve T cells results in limited to undetectable accumulation. WT-TAg cells prime endogenous site V-specific T cells Extrapolating our data from Figure 19A to include fewer naïve TCR-V cells, approaching values found in naïve animals, suggest that the fraction of naïve T cells that are cross-primed remains unchanged. Thus our findings suggest that endogenous site V-specific T cells are cross-primied following WT-TAg immunization. However, no direct evidence for priming of endogenous site V-specific T cells following WT-TAg cell immunization has been acquired, possibly due to low numbers of endogenous site V- specific T cells in naïve mice. Inefficient priming of a small population of endogenous cells could limit the size of the site V-specific T cell response making the T cells undetectable by conventional methods. Recent studies utilized a tetramer-based magnetic enrichment protocol to identify naïve precursors of both CD8+ and CD4+ T cells to track their expansion following specific immunization [169,171,172]. To determine whether endogenous site V-specific precursor T cells were being primed following WT-TAg cell immunization, we enriched endogenous site V-specific T cells 131
149 from individual mice. In parallel, we also enriched site I-specific T cells for direct comparison and as a positive control for T cell expansion following immunization. As detailed in the methods, spleen and lymph node cells from individual naïve or positive control mice (transferred with TCR-V cells and immunized with WT-TAg) were co-stained with APC-conjugated Tet-I and PE-conjugated Tet-V followed by magnetic enrichment with anti-pe and anti-apc magnetic beads. The positively sorted fractions were restained with Tet-I, Tet-V and antibodies specific for CD8, Thy1.2, and a panel of markers to eliminate non-t cells (DUMP gate). Negative controls consisted of cells obtained from naïve mice that were not stained with tetramer prior to magnetic sorting, but received the same post-sort staining. Samples were gated as described in the methods and depicted in Figure 20A. Our results demonstrate that on average 6.4 site V-specific T cells were detected per naïve B6 mouse, a significant difference from the 19.2 site I-specific T cells detected per mouse (p=0.0003, Mann-Whitney U-test) (Figure 20C). We directly compared the number of site I- and site V-specific T cells in individual mice to calculate the ratio of Tet-I : Tet-V-sorted cells directly for each mouse (Figure 20D). The results demonstrated that on average naive mice contained approximately 3.8 : 1 Tet-I- : Tet-V-specific precursor T cells. To test whether endogenous site V-specific cells were primed, we immunized B6 mice with WT-TAg. Mice were immunized with Q489A as a control for site V-specific T cell priming. Our results (Figure 20C) demonstrated that endogenous site V-specific T cells expanded 22 fold following WT-TAg immunization (6.4 cells cells). In contrast, immunization with Q489A resulted in a ~450 fold increase in the number of site V-specific T cells (6.4 cells 2868 cells), about a 20 fold enhancement over WT-TAg immunization. 132
150 Figure 20: Endogenous Site V-Specific T Cells are Primed Following WT-TAg Immunization Site V and site I tetramer positive cells were simultaneously enriched from total spleen and lymph node cells harvested from naïve mice (A) or mice that had been immunized 7 days prior with WT-TAg or Q489A cells (B). The gating scheme (A) as well as representative mice (A and B) from each experiment is shown. Each immunizations group was done at least two times. Data from all experiments are plotted below (C). Numbers indicate the total cells within a gate (A and B) or the mean number of tetramer+ cells acquired for each mouse (C). Lines indicate the mean number of Tetramer+ cells acquired for all mice (C). 133
151 Figure 20: Endogenous Site V-Specific T Cells are Primed Following WT-TAg Immunization 134
152 Figure 20: Endogenous Site V-Specific T Cells are Primed Following WT-TAg Immunization 135
153 These data represent the first time that an endogenous site V-specific T cell response has been demonstrated following WT-TAg cell immunization. These data also indicate a 6 fold increase in the site I-specific T cell response following Q489A immunization, suggesting that the Q489A cell line may be more immunogenic than WT-TAg. However, a 6 fold increase (3524 cells cells) in the general immunogenicity of Q489A would not account for the 20 fold increase in site V immunogenicity observed with the same cells (Figure 20C). Furthermore, immunization with Q489A cells resulted in a decrease in the ratio of site I-:site V-specific T cells 10 33:1 from the 70:1 ration obtained following immunization with WT-TAg (Figure 20D). If the increase in site V-specific T cells observed following Q489A immunization resulted from a general increase in immunogenicity of Q489A cells, then the ratio of site I-:site V-specific T cells should have remained the same. This result suggests that there was a greater accumulation of site V-specific T cells following Q489A cell immunization that cannot be explained by a general increase in immunogenicity of the cells. Overall, these data suggest that the difference between WT-TAg and Q489A cell-induced site V-specific T cell responses depended upon the Q489A determinant. 136
154 Summary of our findings Thus far, our results indicate that Q489A and G490A overcame the immunorecessive nature of site V. We have for the first time demonstrated endogenous site V-specific T cell responses following immunization with cells expressing the dominant TAg determinants. The increase in site V-specific T cell responses to Q489A and G490A cell immunization is in part due to an increase in the fraction of site V- specific T cells that are cross-primed. Thus pmhc-stability of site V determines the fraction of naïve T cells that are cross-primed. Furthermore, we demonstrated that WT- TAg immortalized cell lines induce endogenous site V-specific T cell proliferation. Previous work failed to detect the small populations of site V-specific T cells [269], and it was not known whether WT-TAg cells resulted in priming of endogenous site V-specific T cells. Therefore, we conclude that the low pmhc-stability of site V contributed to its immunorecessive nature by limiting the fraction of site V-specific T cells that are crossprimed. 137
155 The Duration of Cross-Presentation Determines the Accumulation but Not the Recruitment of Site V-Specific T Cells The use of the TCR-I and TCR-V cells led to the conclusion that site V-specific T cells are not cross-primed as well as site I-specific T cells [9]. Below, we look more closely at the differences between site I- and site V-specific T cell responses in an effort to understand the mechanisms that contribute to weak cross-priming and the size of the site V-specific T cell response. Specifically, we evaluated the role of pmhc-stability for determining the duration of naïve T cell cross-priming and subsequently the role that duration of cross-priming plays in the accumulation of T cells. The duration of site V-specific T cell cross-priming is limited Tatum et al. [10] demonstrated that the cross-priming of site I-specific T cells persists for at least seven days following immunization with WT-TAg cells. In that study, site I-specific T cell cross-priming appeared to decrease by day seven, similar to the weak cross-priming by site V-specific T cells observed following a standard immunization [9] (Figure 8). In cross-presentation of a particular determinant of TAg, the stability of pmhc molecules could determine the window for naïve T cells to encounter stimulus (eg. the duration of cross-priming). In this scenario, high-stability pmhc, like site I, may cross-prime T cells for a longer period of time than low-stability site V presented by the same papc. We asked whether high-stability peptides have a longer duration of cross-priming than low-stability peptides by comparing cross-priming of site I and site V. Unpublished preliminary observations by Pavel Otahal indicated that cross-priming of site I-specific T cells persists longer than cross-priming of site V-specific T cells following WT-TAg or V- only immunization, respectively. These experiments were repeated several times and 138
156 representative data are displayed in Figure 21. B6 mice were immunized with TAP-WT or TAP-V-only cells. Approximately 24, 48, or 72 hours following immunization, 10 6 CFSE-labeled TCR-V or TCR-I cells were transferred into the mice. Three days following transfer, spleens were harvested and the TCR-V and TCR-I cells were evaluated for proliferation. Proliferation was used to test (yes or no determination) for site I or site V cross-priming. The results indicated that following TAP-WT cell immunization, TCR-I cells could be cross-primed when transfer occurred 72 hours post immunization. Furthermore, consistent with previous observations [9], nearly all TCR-I cells proliferated. In contrast, TCR-V cross-priming was detected when the cells were transferred after 48 hours, and cross-priming decreased markedly after 24 hours. The duration of site V cross-priming was similar for TAP-WT and TAP-V-only cells suggesting that the persistence of site V-specific T cell cross-priming was not affected by the expression of the dominant TAg determinants. These data indicate that the duration of site V-specific T cell cross-priming is limited following TAP-WT cell immunization compared to the more dominant site I. The variation in the duration of TCR-I and TCR-V cell cross-priming could be explained by differences in the concentration of antigen required for activation (avidity of T cell activation) between naïve TCR-I and TCR-V cells. If all else was equal, but TCR- V cells had a lower avidity of activation than TCR-I cells, cross-priming of TCR-V cells might appear shorter than for TCR-I cells due to the threshold of pmhc required to trigger naïve T cell activation. To test this possibility, naïve TCR-I and TCR-V cells were incubated for 24 hours with various concentrations of peptides ranging from M. Activation of T cells was evaluated by FACS by expression of the early activation marker CD69. The results indicated that TCR-I and TCR-V cells had a similar avidity as assessed by CD69 expression (Figure 22). The 50% of maximum CD69 expression for 139
157 Figure 21: TCR-V Cells have a Shortened Duration of Cross-Priming Compared to TCR-I Cells Mice were immunized with TAP-WT or TAP-V-only cells 24, 48, or 72 hours prior to i.v. transfer of 10 6 CFSE labeled TCR-I or TCR-V transgenic T cells. Three days following transfer, spleens were harvested and the proliferation of the transgenic T cells was evaluated by tetramer staining and CFSE dilution. The numbers represent the percentage of CFSE-low cells in each plot. The results are representative of three experiments. The initial experiment was performed by Pavel Otahal. 140
158 Figure 21: TCR-V Cells have a Shortened Duration of Cross-Priming Compared to TCR-I Cells 141
159 Figure 22: TCR-I and TCR-V Cells Have a Similar Avidity of Activation Naïve TCR-I or TCR-V cells were incubated for 24 hours in culture media containing peptide at the indicated concentrations. The cells were harvested and stained with CD8 and CD69. Data is plotted as a percent of maximum CD8+CD69+ cells. These data represent one experiment. 142
160 Figure 22: TCR-I and TCR-V Cells Have a Similar Avidity of Activation 143
161 both cell types was between M. These data suggest that no significant differences in the avidity of activation exist between TCR-I and TCR-V cells. The similar activation avidity of TCR-I and TCR-V cells suggests that the duration of cross-priming is an intrinsic property of the site I and site V determinants. Thus, the duration of site V cross-priming might be influenced by pmhc-stability. To test this idea, we evaluated the influence of Q489A and G490A mutations on the duration of crosspriming using a similar method as that used in Figure 21. B6 mice were immunized with TAP-WT, TAP-Q489A or TAP-G490A cells. 24, 48, or 72 hours following immunization, CFSE-labeled TCR-V cells were transferred into the mice. Three days following transfer, spleens were harvested and the TCR-V cells were evaluated for proliferation. The results demonstrate that cross-priming of TCR-V cells occurred out to 72 hours following both TAP-Q489A and TAP-G490A immunization (Figure 23). These data indicate that the Q489A and G490A mutations enhanced the duration of TCR-V crosspriming; consistent with the idea that improvement in pmhc-stability can enhance the duration of cross-priming. Multiple immunizations result in extended site V-specific T cell cross-priming The increase in duration of TCR-V cross-priming might be the mechanism leading to enhanced recruitment of naïve TCR-V cells observed in Figure 17. We developed an assay to assess whether the duration of site V cross-priming influenced the fraction of recruited naïve T cells. We focused on extending the duration of site V cross-priming from TAP-WT cells in order to rule out any unknown variables that might arise from using the Q489A and G490A mutations such as altered efficiency of processing and presentation. Having demonstrated that site V cross-priming persists for approximately 24 hours following immunization (Figure 21), we determined whether cross-priming of site V could be continuously extended for 24 hour intervals (indicated as 144
162 Figure 23: Q489A and G490A Cells Extend the Duration of TCR-V Cross-Priming Mice were immunized with TAP-WT, TAP-Q489A or TAP-G490A cells 24, 48, or 72 hours prior to i.v. transfer of 1x10 6 CFSE labeled TCR-V cells. Three days following transfer, spleens harvested, and the proliferation of the transgenic T cells was measured by tetramer staining and CFSE dilution. The numbers represent the percentage of CFSE-hi or CFSE-low cell in each plot. Results are representative of two experiments. 145
163 Figure 23: Q489A and G490A Cells Extend the Duration of TCR-V Cross-Priming 146
164 days) by administering consecutive immunizations of TAP-WT cells. B6 mice were immunized (Figure 24A) once or for consecutive days prior to the transfer of CFSElabeled TCR-V cells. As a positive control for proliferation, TCR-I cells were transferred into mice that received a single immunization 4 days prior to transfer, because site I cross-priming has been demonstrated to persist beyond day 4 [10]. Three days following transfer of TCR-V and TCR-I cells, spleens were harvested and the percentage of divided cells was determined (Figure 24B and C). Each group that received multiple immunizations was compared to a background control group that received only a single immunization on the original day (e.g group 4,3,2,1 was compared to group 4). The results indicated that all groups receiving multiple immunizations out to three days prior to TCR-V cell transfer, groups: 3,2,1 and 2,1, had greater levels of divided cells than background groups receiving only a single immunization on day 3 or 2, respectively. Multiple immunizations beginning 4 days prior to transfer demonstrated no significant difference from the background group receiving a single immunization 4 days prior to transfer. These data suggest that cross-priming of TCR-V cells can be maintained for up to three days by immunizing mice with TAP-WT cells once every 24 hours. Multiple immunizations enhance the size of the TCR-V cell response Having demonstrated that multiple immunizations result in the extended crosspriming of site V-specific T cells, we asked whether multiple immunizations would have an effect on the size of the site V-specific T cell response. To ask this question, we immunized mice with WT-TAg cells that can both direct- and cross-present antigen to provide a more physiologically relevant stimulus for the site V-specific T cell response. Mice that received 10 6 TCR-V cells were immunized with WT-TAg cells 1 time or 4 times at 24 hour intervals (Figure 25A). 7 days following immunization, spleens were harvested and the percent of total CD8+tetramer-V+ cells was determined. The results 147
165 Figure 24: TCR-V Cell Cross-Priming is Maintained Following Multiple Immunizations B6 mice were immunized with TAP-WT cells on the indicated days prior to the transfer of 10 6 CFSE-labeled TCR-V or TCR-I cells into each mouse on day zero (A). Three days following transfer, spleens were harvested and the percent of divided (CFSE-low) TCR-V or TCR-I cells was calculated (B and C). Representative CFSE plots (B) and mean values (C) are plotted. The results represent the combined results of two independent experiments (C). N=6 mice per group. P-values were determined using an unpaired t-test. 148
166 Figure 24: TCR-V Cell Cross-Priming is Maintained Following Multiple Immunizations 149
167 indicated that multiple immunizations resulted in an increase in the percentage of TCR-V cells from ~5% of total CD8+ cell with only a single immunization to ~20% following multiple immunizations. Surprisingly, the TCR-V response to multiple immunizations was comparable to a single immunization with V-only cells. To determine whether the enhanced proliferation observed from TCR-V cells applied to endogenous site V-specific T cells, normal B6 mice were immunized 1 or 4 times with WT-TAg cells (Figure 25B). Ten days following immunization, spleens were harvested and the percent of CD8+tetramer+ cells in the spleen was evaluated. The results indicate no discernable difference between mice immunized one and multiple times. In fact, no mice immunized with WT-TAg resulted in a detectable T cell response above background. These data suggest that multiple immunizations are not capable of overcoming the immunorecessive nature of site V in normal B6 mice. Our experiments did not evaluate proliferation of site V-specific T cells by using the tetramer-based magnetic enrichment protocol, described previously. Therefore, the possibility remains that multiple immunizations did enhance the proliferation of endogenous site V-specific T cells; albeit, to levels below detection of traditional tetramer staining and FACS analysis. Multiple immunizations enhance TCR-V cell accumulation but not TCR-V cell recruitment Above, we demonstrated that Q489A and G490A cells resulted in an increase in the number of naïve TCR-V cells that were recruited following immunization (Figure 17). Enhanced TCR-V cell recruitment correlated with an increase in the site-v specific T cell response (Figure 16 and Figure 20). Thus, we hypothesize that the increased duration of site V-specific T cell cross-priming by Q489A and G490A cells contributing to the enhanced naïve TCR-V cell recruitment. To test whether the duration of crosspresentation determines the fraction of T cells that are cross-primed, we utilized a 150
168 Figure 25: Multiple Immunizations Result in a Marked Expansion of TCR-V Cells B6 mice did (A) or did not (B) receive 10 6 TCR-V cells and were immunized on the indicated days with WT-TAg cells or V-only cells where noted. At day 7 (A) or 10 (B) following the first immunization, spleens were harvested and the number of tetramer+ cells per spleen was determined as the percent of total CD8+ cells. 151
169 Figure 25: Multiple Immunizations Result in a Marked Expansion of TCR-V Cells 152
170 Figure 26: Multiple Immunizations Enhance TCR-V Cell Accumulation but not the Fraction of Naive T Cell Recruitment B6 mice received 10 6 CFSE-labeled TCR-V cells from CD45.1 congenic mice. The mice were immunized with TAP-WT cells on the indicated days following TCR-V transfer (A). On day five following transfer, spleens were harvested and the UnDivided / B-cell (B) and Divided / B-cell (C) ratios were calculated for each mouse. The data represent the normalized and combined results of two independent experiments. P- values were determined by an unpaired T test. 153
171 Figure 26: Multiple Immunizations Enhance TCR-V Cell Accumulation but not the Fraction of Naive T Cell Recruitment 154
172 method similar to the thy1.1 spike (Figure 17A); however, congenically marked B-cells were used as a static marker population rather than thy1.1+cd8+ cells (see appendix B for a comparison of the methods). Mice received CFSE-labeled splenocytes from TCR- V mice and were immunized 1, 2, or 3 days in a row with TAP-WT cells (Figure 26A). Two days following the final immunization (5 days following the initial immunization), spleens were harvested and the Undivided TCR-V / B-Cell (Figure 26B) and the Divided TCR-V / B-cell (Figure 26C) ratios were calculated. The results indicated that a statistically significant drop in the number of undivided TCR-V cells occurred following the second immunization and the number of undivided T cells did not decrease further following three immunizations. All other experiments have indicated a statistically significant drop following the first immunization with TAP-WT cells (Figure 17B and Figure 29), making this result unusual. This anomaly is due to unusual results obtained for the single immunization group (1) in one of the two experiments included in Figure 26A. Despite a fixed number of naïve T cells undergoing cross-priming following multiple immunizations; our results indicated an increase in the overall number of divided TCR-V cells with each subsequent immunization. These data suggest that multiple immunizations enhance the size of the TCR-V T cell response by promoting expansion of TCR-V cells. Future plans include a repeat of this experiment using the thy1.1 spike method. The duration of cross-priming enhances TCR-V cell accumulation but not TCR-V cell recruitment Thus far, we have demonstrated that multiple immunizations increase the duration of cross-priming and enhance the accumulation of TCR-V cells without affecting TCR-V cell recruitment. Our results in Figure 26 may be due to introducing large 155
173 amounts of antigen into the system that is not present following only a single immunization. Thus, we asked whether the duration of cross-priming was the factor leading to enhanced TCR-V cell recruitment following only a single Q489A or G490A immunization. To do this, we needed a system in which the duration of cross-priming could be controlled following a single immunization. CD11c-DTR mice [146] express a diphtheria toxin (DT) receptor-gfp fusion protein from the CD11c-promotor. Thus, administration of DT to CD11c-DTR mice results in the deletion of CD11c+ cells that can be monitored by the loss of GFP+ cells. Among the cells depleted are DCs required for the cross-priming of naïve T cells, including CD8+CD11c+ cells [153]. CD11c-DTR mice can be used to control the duration of TCR-V cell cross-priming by administering DT at specific times following immunization. We verified that 100ng of DT given to CD11c-DTR mice was sufficient for depletion of GFP+ cells (Figure 27). 100ng of DT in PBS was administered IP to CD11c- DTR mice and 24 hours following administration; spleens were harvested and stained for CD11c, CD11b, and CD8. A CD11c+CD11b+ population was depleted in CD11c-DTR mice when DT was administered but not in mice given PBS or in B6 mice (Figure 27, column 1). In CD11c-DTR mice, the CD11c+CD11b+ cells expressed GFP (Figure 27, column 2). A general depletion of GFP+ cells was observed in CD11c-DTR mice given DT (Figure 27, column 3). Furthermore, the GFP profile in depleted CD11c-DTR mice appeared similar to B6 mice, suggesting that CD11c+ cell depletion was efficient. Finally, CD11c+CD8+ cells were depleted in CD11c-DTR mice (Figure 27, column 4). These results suggest that DT administration to CD11c-DTR mice results in an efficient depletion of CD11c+CD8+ cells within 24 hours. Since CD11c+CD8+ DCs are the primary papc responsible for cross-priming of CD8+ T cells, these results suggest that 156
174 Figure 27: Testing CD11c-DTR Mice CD11c-DTR or B6 mice received 100ng of DT in 0.5ml PBS by the IP route or PBS alone. 24 hours following injection, spleens were harvested and stained for CD11c, CD11b, and CD8. The black line in the GFP column is the GFP staining profile of the B6 gated population referred to by the arrow. The red lines are the GFP staining profile for each mouse in the indicated row. Labels above each column indicate staining profile ( Y-axis X X-axis ). A single label indicates the X-axis parameter. The results are representative of one experiment. 157
175 Figure 27: Testing CD11c-DTR Mice 158
176 CD11c-DTR mice will be a useful system for controlling the duration of TCR-V crosspriming. Using the CD11c-DTR mice, we determined whether the duration of crosspriming affected the TCR-V response to TAP-Q489A cells. TAP-Q489A cells were tested because TCR-V cell cross-priming is detectable for at least 3 days following immunization (Figure 23). One-million CFSE-labeled TCR-V cells with Thy1.1+ spike were transferred into CD11c-DTR mice. Mice were immunized with TAP Q489A cells and DT was administered on day zero, one, two, or three following immunization (Figure 28A). Dot plots of representative mice indicating successful depletion of CD11c+CD11b+ cells are displayed in Figure 28B. Five days following the initial immunization, spleens were harvested and the ratios of UnDivided/Thy1.1 and Divided/Thy1.1 T cells were calculated. Administration of DT on day 1 (D1) resulted in a reduction in the number of T cells from the undivided CFSE-Hi population, and between days 2 and 3 (D2-D3) the number of undivided TCR-V cells remained unchanged (Figure 28C). In contrast, the number of divided cells significantly increased between days 1 and 2 (Figure 28D). The mean fraction of Divided TCR-V cells increased for D3 as well; although, the result was not significant. These data are consistent with our results using multiple immunizations and suggest that the duration of site V-specific T cell cross-priming determines TCR-V cell accumulation but not the fraction of naive T cells that are recruited. Direct presentation does not affect naïve T cell recruitment nor TCR-V division. We have addressed the role that cross-presentation plays in determining the size of the TCR-V response. Initial observations demonstrated that multiple immunizations with 159
177 Figure 28: The Duration of Cross-Priming Determines Accumulation but Not the Fraction of Naïve TCR-V Cell Recruitment CD11c-DTR mice received 10 6 CFSE-labeled TCR-V cells with thy1.1 spike. The following day, mice were immunized with TAP-Q489A cells. On the indicated days, mice received 100ng DT in PBS by the i.p. route (A). On day five, spleens were harvested and the successful depletion of CD11c+ cells was verified in each mouse by monitoring the loss of the CD11c+CD11b+ population indicated by the arrow (B, top row). A representative CFSE profile of TCR-V cells for mice in each group is also shown (B, bottom row). The histogram scale has been adjusted for each mouse. The UnDivided / thy1.1 (C) and Divided / thy1.1 (D) ratios were calculated. The data represent the combined results of two independent experiments. P-values were calculated by unpaired T test. 160
178 Figure 28: The Duration of Cross-Priming Determines Accumulation but Not the Fraction of Naïve TCR-V Cell Recruitment 161
179 Figure 28: The Duration of Cross-Priming Determines Accumulation but Not the Fraction of Naïve TCR-V Cell Recruitment 162
180 Figure 29: Direct Presentation Does Not Affect Naïve T Cell Recruitment or Accumulation B6 mice received 10 6 CFSE-labeled TCR-V cells with thy1.1 spike. Beginning the following day, the mice were immunized with WT-TAg cells once (WT-TAg) or three days in a row (WT-Multi) or TAP-WT cells once (TAP) or three days in a row (TAP-Multi). As a negative control, one group was immunized with null cells. Five days following the initial immunization, spleens were harvested and the UnDivided / thy1.1 (A) and the Divided / thy1.1 (B) ratios were calculated. P-values were calculated using an unpaired T test. The graphs represent the normalized and combined data from two independent experiments. 163
181 Figure 29: Direct Presentation Does Not Affect Naïve T Cell Recruitment or Accumulation 164
182 WT-TAg cells (Figure 25) or single immunizations with Q489A and G490A cells resulted in an enhanced endogenous T cell response (Figure 16 and Figure 20). These results were observed using B6 cells that could both direct- and cross-present antigen. Direct presentation enhances the size of the site I-specific T cell response at five days and beyond following immunization [10]. Thus, direct presentation may enhance the size of naïve TCR-V cell priming or expansion early in the immune response. The role that direct presentation plays in the response to site V was assessed by transferring 10 6 CFSE-labeled TCR-V cells plus thy1.1 spike into B6 mice. Mice were immunized with WT-TAg or TAP-WT cells one time or three times at 24 hour intervals. Five days following the initial immunization, spleens were harvested and the ratio of undivided and divided T cells to CD8+Thy1.1+ cells was calculated (Figure 29). The results indicate no significant difference between mice immunized with TAP -/- cell lines and mice immunized with B6 cell lines. Furthermore, no difference was observed between groups of mice immunized one or multiple times. These data suggest that direct presentation does not enhance the fraction of naïve T cells recruited or the accumulation of TCR-V cells at early time points following immunization. 165
183 Summary of our findings In this section, we have examined the role that the duration of cross-priming plays in determining that magnitude of site V-specific T cell expansion and the recruitment of naïve T cells. Our results indicate that the duration of site V-specific T cell cross-priming following TAP-WT cell immunization is limited to 1-2 days. In contrast, site I-specific T cell cross-priming persists for at least five days following immunization [10]. Q489A or G490A cells extend the duration of site V-specific T cell priming to at least three days. We found that as the duration of site V-specific T cell cross-priming increased, so did the size of site V-specific T cell accumulation. However, the duration of site V-specific T cell cross-priming had no effect on the fraction of naïve TCR-V cells that were recruited. Thus, from these data we have concluded that pmhc-stability affects the size of the site V-specific T cell response by enhancing site V-specific T cell accumulation but not naïve T cell recruitment. When these findings are considered with those from the previous section, a unifying conclusion regarding the role of pmhc-stability and the immunorecessive nature of SV40 TAg site V can be drawn. In the previous section, we concluded that pmhcstability determines the efficiency of naïve site V-specific T cell recruitment by a currently undefined mechanism. The current section concludes that pmhc-stability also determines the duration of site V-specific T cell cross-priming. Furthermore we demonstrated that the duration of site V-specific T cell cross-priming determines the size of the site V-specific T cell response. Thus, pmhc-stability determines the size of the site V-specific T cell response by first determining the fraction of naïve site V-specific T cells that are cross-primed and then by driving the accumulation of activated T cells. The combined effect of enhanced naïve T cell cross-priming and T cell accumulation results in Q489A and G490A cells overcoming the immunorecessive nature of site V. 166
184 Major Conclusions: First-half of study: 1) Q489A and G490A enhance the pmhc-stability of SV40 TAg site V and maintain cross-reactivity with site V-specific T cells. 2) Q489A and G490A cells overcome the immunorecessive nature of site V. 3) Q489A and G490A cells enhance the fraction of site V-specific T cells that are cross-primed. 4) Endogenous site V-specific T cells are primed following WT-TAg cell immunization but are undetectable using conventional methods. Second-half of study: 1) The duration of site V-specific T cell cross-priming is shorter then site I-specific T cell cross-priming. 2) Q489A and G490A cells increase the duration of site V-specific T cell crosspriming in-vivo. 3) The duration of cross-priming determines the magnitude of T cell accumulation but not the fraction of site V-specific T cells that are cross-primed. Broad conclusion: pmhc-stability determines the size of the site V-specific T cell response by determining the fraction of site V-specific T cell that are recruited and the duration of site V-specific T cell cross-priming. 167
185 Chapter V: Discussion In this study, we investigated the mechanisms by which pmhc-stability affects cross-priming of T cells specific for the immunorecessive site V determinant of SV40 TAg. A previous study demonstrated that only a fraction of site V-specific T cells are cross-primed following immunization whereas nearly all T cells specific for the dominant determinant site I undergo cross-priming [9]. Our results suggest that the low-pmhcstability of site V contributes to its immunorecessive nature, since increasing the pmhcstability of site V resulted in a detectable site V-specific T cell immune response. We demonstrate that increasing the pmhc-stability of site V augmented the efficiency of site V-specific T cell cross-priming such that a greater fraction of site V-specific T cells underwent cross-priming. Our data also demonstrate that pmhc-stability determines the duration of site V-specific T cell cross-priming. In our experiments, as the duration of cross-priming increased, so did the size of the site V-specific T cell response. Thus, we have concluded that pmhc-stability affects the site V-specific T cell response by two distinct mechanisms: 1) pmhc determines the fraction of naïve T cells that are crossprimed and 2) pmhc-stability influences the accumulation of site V-specific T cells by determining the duration of site V-specific T cell cross-priming. Proposed model explaining the findings from this study I propose a model (Figure 30) to explain the findings in this study. The model indicates that cross-presentation of SV40 TAg site V on CD11c+ papcs is necessary for priming of site V-specific T cells. However, the specific subset of CD11c+ papc that is required for priming and/or enhanced accumulation of site V-specific T cells in our 168
186 Figure 30: Proposed Model Describing the Relationship between SV40 TAg Site V-Specific T cell Cross-Priming and pmhc-stability The figure depicts the site V-specific T cell response to low-stability pmhc (left) and high-stability pmhc (right). Immunization with TAg immortalized cells results in the cross-presentation of pmhc molecules on CD11c+ papcs. Since, cross-presentation of high-stability pmhc may be more efficient (according to reports in the literature [5-7]), high-stability pmhc are depicted as presenting greater numbers of pmhc molecules (A). However, we have not tested the assumption of enhanced pmhc-presentation in our system, indicated by the grey box. Reflecting our results, the fraction of naïve site V- specific T cells primed against high-stability pmhc is greater than low-stability pmhc, depicted by 1/3 of T cells dividing in response to low-stability pmhc and 2/3 of T cells dividing in response to high-stability pmhc (B). As time progresses, pmhc half-life determines the level of cross-presentation. Enduring cross-presentation on CD11c+ papcs drives further accumulation of T cells, contributing to the overall size of the T cell response (C) 169
187 Figure 30: Proposed Model Describing the Relationship between SV40 TAg Site V-Specific T cell Cross-Priming and pmhc-stability 170
Chapter 6. Antigen Presentation to T lymphocytes
 Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
MHC class I MHC class II Structure of MHC antigens:
 MHC class I MHC class II Structure of MHC antigens: MHC class I antigens consist of a transmembrane heavy chain (α chain) that is non-covalently associated with β2- microglobulin. Membrane proximal domain
MHC class I MHC class II Structure of MHC antigens: MHC class I antigens consist of a transmembrane heavy chain (α chain) that is non-covalently associated with β2- microglobulin. Membrane proximal domain
General information. Cell mediated immunity. 455 LSA, Tuesday 11 to noon. Anytime after class.
 General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
Significance of the MHC
 CHAPTER 8 Major Histocompatibility Complex (MHC) What is is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) Significance of the MHC role in immune response role in organ
CHAPTER 8 Major Histocompatibility Complex (MHC) What is is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) Significance of the MHC role in immune response role in organ
Antigen presenting cells
 Antigen recognition by T and B cells - T and B cells exhibit fundamental differences in antigen recognition - B cells recognize antigen free in solution (native antigen). - T cells recognize antigen after
Antigen recognition by T and B cells - T and B cells exhibit fundamental differences in antigen recognition - B cells recognize antigen free in solution (native antigen). - T cells recognize antigen after
Significance of the MHC
 CHAPTER 8 Major Histocompatibility Complex (MHC) What is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) - MHC molecules were initially discovered during studies aimed
CHAPTER 8 Major Histocompatibility Complex (MHC) What is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) - MHC molecules were initially discovered during studies aimed
COURSE: Medical Microbiology, MBIM 650/720 - Fall TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12
 COURSE: Medical Microbiology, MBIM 650/720 - Fall 2008 TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12 FACULTY: Dr. Mayer Office: Bldg. #1, Rm B32 Phone: 733-3281 Email: MAYER@MED.SC.EDU
COURSE: Medical Microbiology, MBIM 650/720 - Fall 2008 TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12 FACULTY: Dr. Mayer Office: Bldg. #1, Rm B32 Phone: 733-3281 Email: MAYER@MED.SC.EDU
Antigen Presentation to T lymphocytes
 Antigen Presentation to T lymphocytes Immunology 441 Lectures 6 & 7 Chapter 6 October 10 & 12, 2016 Jessica Hamerman jhamerman@benaroyaresearch.org Office hours by arrangement Antibodies and T cell receptors
Antigen Presentation to T lymphocytes Immunology 441 Lectures 6 & 7 Chapter 6 October 10 & 12, 2016 Jessica Hamerman jhamerman@benaroyaresearch.org Office hours by arrangement Antibodies and T cell receptors
HLA and antigen presentation. Department of Immunology Charles University, 2nd Medical School University Hospital Motol
 HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
CELL BIOLOGY - CLUTCH CH THE IMMUNE SYSTEM.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF HOST DEFENSES The human body contains three lines of against infectious agents (pathogens) 1. Mechanical and chemical boundaries (part of the innate immune system)
!! www.clutchprep.com CONCEPT: OVERVIEW OF HOST DEFENSES The human body contains three lines of against infectious agents (pathogens) 1. Mechanical and chemical boundaries (part of the innate immune system)
HLA and antigen presentation. Department of Immunology Charles University, 2nd Medical School University Hospital Motol
 HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
B F. Location of MHC class I pockets termed B and F that bind P2 and P9 amino acid side chains of the peptide
 Different MHC alleles confer different functional properties on the adaptive immune system by specifying molecules that have different peptide binding abilities Location of MHC class I pockets termed B
Different MHC alleles confer different functional properties on the adaptive immune system by specifying molecules that have different peptide binding abilities Location of MHC class I pockets termed B
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
Key Concept B F. How do peptides get loaded onto the proper kind of MHC molecule?
 Location of MHC class I pockets termed B and F that bind P and P9 amino acid side chains of the peptide Different MHC alleles confer different functional properties on the adaptive immune system by specifying
Location of MHC class I pockets termed B and F that bind P and P9 amino acid side chains of the peptide Different MHC alleles confer different functional properties on the adaptive immune system by specifying
Basic Immunology. Lecture 5 th and 6 th Recognition by MHC. Antigen presentation and MHC restriction
 Basic Immunology Lecture 5 th and 6 th Recognition by MHC. Antigen presentation and MHC restriction Molecular structure of MHC, subclasses, genetics, functions. Antigen presentation and MHC restriction.
Basic Immunology Lecture 5 th and 6 th Recognition by MHC. Antigen presentation and MHC restriction Molecular structure of MHC, subclasses, genetics, functions. Antigen presentation and MHC restriction.
The Major Histocompatibility Complex (MHC)
 The Major Histocompatibility Complex (MHC) An introduction to adaptive immune system before we discuss MHC B cells The main cells of adaptive immune system are: -B cells -T cells B cells: Recognize antigens
The Major Histocompatibility Complex (MHC) An introduction to adaptive immune system before we discuss MHC B cells The main cells of adaptive immune system are: -B cells -T cells B cells: Recognize antigens
Major Histocompatibility Complex (MHC) and T Cell Receptors
 Major Histocompatibility Complex (MHC) and T Cell Receptors Historical Background Genes in the MHC were first identified as being important genes in rejection of transplanted tissues Genes within the MHC
Major Histocompatibility Complex (MHC) and T Cell Receptors Historical Background Genes in the MHC were first identified as being important genes in rejection of transplanted tissues Genes within the MHC
all of the above the ability to impart long term memory adaptive immunity all of the above bone marrow none of the above
 1. (3 points) Immediately after a pathogen enters the body, it faces the cells and soluble proteins of the innate immune system. Which of the following are characteristics of innate immunity? a. inflammation
1. (3 points) Immediately after a pathogen enters the body, it faces the cells and soluble proteins of the innate immune system. Which of the following are characteristics of innate immunity? a. inflammation
Significance of the MHC
 CHAPTER 7 Major Histocompatibility Complex (MHC) What is is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) Significance of the MHC role in immune response role in organ
CHAPTER 7 Major Histocompatibility Complex (MHC) What is is MHC? HLA H-2 Minor histocompatibility antigens Peter Gorer & George Sneell (1940) Significance of the MHC role in immune response role in organ
TCR, MHC and coreceptors
 Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Antigen processing and presentation. Monika Raulf
 Antigen processing and presentation Monika Raulf Lecture 25.04.2018 What is Antigen presentation? AP is the display of peptide antigens (created via antigen processing) on the cell surface together with
Antigen processing and presentation Monika Raulf Lecture 25.04.2018 What is Antigen presentation? AP is the display of peptide antigens (created via antigen processing) on the cell surface together with
Antigen Recognition by T cells
 Antigen Recognition by T cells TCR only recognize foreign Ags displayed on cell surface These Ags can derive from pathogens, which replicate within cells or from pathogens or their products that cells
Antigen Recognition by T cells TCR only recognize foreign Ags displayed on cell surface These Ags can derive from pathogens, which replicate within cells or from pathogens or their products that cells
The Adaptive Immune Response: T lymphocytes and Their Functional Types *
 OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Chapter 22: The Lymphatic System and Immunity
 Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
The major histocompatibility complex (MHC) is a group of genes that governs tumor and tissue transplantation between individuals of a species.
 Immunology Dr. John J. Haddad Chapter 7 Major Histocompatibility Complex The major histocompatibility complex (MHC) is a group of genes that governs tumor and tissue transplantation between individuals
Immunology Dr. John J. Haddad Chapter 7 Major Histocompatibility Complex The major histocompatibility complex (MHC) is a group of genes that governs tumor and tissue transplantation between individuals
Antigen Presentation and T Lymphocyte Activation. Abul K. Abbas UCSF. FOCiS
 1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
The Major Histocompatibility Complex
 The Major Histocompatibility Complex Today we will discuss the MHC The study of MHC is necessary to understand how an immune response is generated. And these are the extra notes with respect to slides
The Major Histocompatibility Complex Today we will discuss the MHC The study of MHC is necessary to understand how an immune response is generated. And these are the extra notes with respect to slides
LESSON 2: THE ADAPTIVE IMMUNITY
 Introduction to immunology. LESSON 2: THE ADAPTIVE IMMUNITY Today we will get to know: The adaptive immunity T- and B-cells Antigens and their recognition How T-cells work 1 The adaptive immunity Unlike
Introduction to immunology. LESSON 2: THE ADAPTIVE IMMUNITY Today we will get to know: The adaptive immunity T- and B-cells Antigens and their recognition How T-cells work 1 The adaptive immunity Unlike
Phase of immune response
 Antigen and antigen recognition by lymphocytes Antigen presentation to T lymphocytes Sanipa Suradhat Department of Veterinary Microbiology Faculty of Veterinary Science Phase of immune response 1 Phase
Antigen and antigen recognition by lymphocytes Antigen presentation to T lymphocytes Sanipa Suradhat Department of Veterinary Microbiology Faculty of Veterinary Science Phase of immune response 1 Phase
White Blood Cells (WBCs)
 YOUR ACTIVE IMMUNE DEFENSES 1 ADAPTIVE IMMUNE RESPONSE 2! Innate Immunity - invariant (generalized) - early, limited specificity - the first line of defense 1. Barriers - skin, tears 2. Phagocytes - neutrophils,
YOUR ACTIVE IMMUNE DEFENSES 1 ADAPTIVE IMMUNE RESPONSE 2! Innate Immunity - invariant (generalized) - early, limited specificity - the first line of defense 1. Barriers - skin, tears 2. Phagocytes - neutrophils,
The T cell receptor for MHC-associated peptide antigens
 1 The T cell receptor for MHC-associated peptide antigens T lymphocytes have a dual specificity: they recognize polymporphic residues of self MHC molecules, and they also recognize residues of peptide
1 The T cell receptor for MHC-associated peptide antigens T lymphocytes have a dual specificity: they recognize polymporphic residues of self MHC molecules, and they also recognize residues of peptide
Antigen Receptor Structures October 14, Ram Savan
 Antigen Receptor Structures October 14, 2016 Ram Savan savanram@uw.edu 441 Lecture #8 Slide 1 of 28 Three lectures on antigen receptors Part 1 (Today): Structural features of the BCR and TCR Janeway Chapter
Antigen Receptor Structures October 14, 2016 Ram Savan savanram@uw.edu 441 Lecture #8 Slide 1 of 28 Three lectures on antigen receptors Part 1 (Today): Structural features of the BCR and TCR Janeway Chapter
1. Overview of Adaptive Immunity
 Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Generation of the Immune Response
 Generation of the Immune Response Sheet 18 immunity I only added extra notes that were explained in the lecture, refer back to the slides. SLIDE 3: In the generation of Immune response whether by B or
Generation of the Immune Response Sheet 18 immunity I only added extra notes that were explained in the lecture, refer back to the slides. SLIDE 3: In the generation of Immune response whether by B or
the HLA complex Hanna Mustaniemi,
 the HLA complex Hanna Mustaniemi, 28.11.2007 The Major Histocompatibility Complex Major histocompatibility complex (MHC) is a gene region found in nearly all vertebrates encodes proteins with important
the HLA complex Hanna Mustaniemi, 28.11.2007 The Major Histocompatibility Complex Major histocompatibility complex (MHC) is a gene region found in nearly all vertebrates encodes proteins with important
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas
 Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas Chapter 04: Antigen Recognition in the Adaptive Immune System Test Bank MULTIPLE CHOICE 1. Most T lymphocytes
Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas Chapter 04: Antigen Recognition in the Adaptive Immune System Test Bank MULTIPLE CHOICE 1. Most T lymphocytes
Immunology - Lecture 2 Adaptive Immune System 1
 Immunology - Lecture 2 Adaptive Immune System 1 Book chapters: Molecules of the Adaptive Immunity 6 Adaptive Cells and Organs 7 Generation of Immune Diversity Lymphocyte Antigen Receptors - 8 CD markers
Immunology - Lecture 2 Adaptive Immune System 1 Book chapters: Molecules of the Adaptive Immunity 6 Adaptive Cells and Organs 7 Generation of Immune Diversity Lymphocyte Antigen Receptors - 8 CD markers
Antigen Presentation to T lymphocytes
 Antigen Presentation to T lymphocytes Immunology 441 Lectures 6 & 7 Chapter 6 October 10 & 12, 2016 Jessica Hamerman jhamerman@benaroyaresearch.org Office hours by arrangement Antigen processing: How are
Antigen Presentation to T lymphocytes Immunology 441 Lectures 6 & 7 Chapter 6 October 10 & 12, 2016 Jessica Hamerman jhamerman@benaroyaresearch.org Office hours by arrangement Antigen processing: How are
Principles of Adaptive Immunity
 Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
The Immune System. These are classified as the Innate and Adaptive Immune Responses. Innate Immunity
 The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
Third line of Defense. Topic 8 Specific Immunity (adaptive) (18) 3 rd Line = Prophylaxis via Immunization!
 Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Adaptive Immune System
 Short Course on Immunology Adaptive Immune System Bhargavi Duvvuri Ph.D IIIrd Year (Immunology) bhargavi@yorku.ca Supervisor Dr.Gillian E Wu Professor, School of Kinesiology and Health Sciences York University,
Short Course on Immunology Adaptive Immune System Bhargavi Duvvuri Ph.D IIIrd Year (Immunology) bhargavi@yorku.ca Supervisor Dr.Gillian E Wu Professor, School of Kinesiology and Health Sciences York University,
Alleles: the alternative forms of a gene found in different individuals. Allotypes or allomorphs: the different protein forms encoded by alleles
 Nomenclature Alleles: the alternative forms of a gene found in different individuals Allotypes or allomorphs: the different protein forms encoded by alleles Genotype: the collection of genes in an individual,
Nomenclature Alleles: the alternative forms of a gene found in different individuals Allotypes or allomorphs: the different protein forms encoded by alleles Genotype: the collection of genes in an individual,
The Adaptive Immune Response. T-cells
 The Adaptive Immune Response T-cells T Lymphocytes T lymphocytes develop from precursors in the thymus. Mature T cells are found in the blood, where they constitute 60% to 70% of lymphocytes, and in T-cell
The Adaptive Immune Response T-cells T Lymphocytes T lymphocytes develop from precursors in the thymus. Mature T cells are found in the blood, where they constitute 60% to 70% of lymphocytes, and in T-cell
Two categories of immune response. immune response. infection. (adaptive) Later immune response. immune response
 Ivana FELLNEROVÁ E-mail: fellneri@hotmail.com, mob. 732154801 Basic immunogenetic terminology innate and adaptive immunity specificity and polymorphism immunoglobuline gene superfamily immunogenetics MHC
Ivana FELLNEROVÁ E-mail: fellneri@hotmail.com, mob. 732154801 Basic immunogenetic terminology innate and adaptive immunity specificity and polymorphism immunoglobuline gene superfamily immunogenetics MHC
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 11 T-Cell Activation, Differentiation, and Memory Copyright 2013 by W. H. Freeman and
How T cells recognize antigen. How T cells recognize antigen -concepts
 Adaptive immunity How T cells recognize antigen Starting point: 2. Diversity in antigen recognition is accomplished, in part, by rearrangements in the TCR loci. This occurs in the thymus 3. The T cell
Adaptive immunity How T cells recognize antigen Starting point: 2. Diversity in antigen recognition is accomplished, in part, by rearrangements in the TCR loci. This occurs in the thymus 3. The T cell
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Nomenclature. HLA genetics in transplantation. HLA genetics in autoimmunity
 Nomenclature Alleles: the alternative forms of a gene found in different individuals Allotypes or allomorphs: the different protein forms encoded by alleles During pregnancy the mother tolerates the expression
Nomenclature Alleles: the alternative forms of a gene found in different individuals Allotypes or allomorphs: the different protein forms encoded by alleles During pregnancy the mother tolerates the expression
chapter 17: specific/adaptable defenses of the host: the immune response
 chapter 17: specific/adaptable defenses of the host: the immune response defense against infection & illness body defenses innate/ non-specific adaptable/ specific epithelium, fever, inflammation, complement,
chapter 17: specific/adaptable defenses of the host: the immune response defense against infection & illness body defenses innate/ non-specific adaptable/ specific epithelium, fever, inflammation, complement,
RAISON D ETRE OF THE IMMUNE SYSTEM:
 RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
General Overview of Immunology. Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center
 General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
Attribution: University of Michigan Medical School, Department of Microbiology and Immunology
 Attribution: University of Michigan Medical School, Department of Microbiology and Immunology License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution
Attribution: University of Michigan Medical School, Department of Microbiology and Immunology License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution
Determinants of Immunogenicity and Tolerance. Abul K. Abbas, MD Department of Pathology University of California San Francisco
 Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Profiling HLA motifs by large scale peptide sequencing Agilent Innovators Tour David K. Crockett ARUP Laboratories February 10, 2009
 Profiling HLA motifs by large scale peptide sequencing 2009 Agilent Innovators Tour David K. Crockett ARUP Laboratories February 10, 2009 HLA Background The human leukocyte antigen system (HLA) is the
Profiling HLA motifs by large scale peptide sequencing 2009 Agilent Innovators Tour David K. Crockett ARUP Laboratories February 10, 2009 HLA Background The human leukocyte antigen system (HLA) is the
Attribution: University of Michigan Medical School, Department of Microbiology and Immunology
 Attribution: University of Michigan Medical School, Department of Microbiology and Immunology License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution
Attribution: University of Michigan Medical School, Department of Microbiology and Immunology License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution
Andrea s SI Session PCB Practice Test Test 3
 Practice Test Test 3 READ BEFORE STARTING PRACTICE TEST: Remember to please use this practice test as a tool to measure your knowledge, and DO NOT use it as your only tool to study for the test, since
Practice Test Test 3 READ BEFORE STARTING PRACTICE TEST: Remember to please use this practice test as a tool to measure your knowledge, and DO NOT use it as your only tool to study for the test, since
Newsletter 2018 vol
 August 30, 2018 Newsletter 2018 vol.2 23-32 TARGETING TOOLS FOR A DNA VACCINE CARRIER, A DESIGN IN PREVENTION OF CANCER Sharif Mohammad Shaheen* and Mustafezur Rahman Faculty of Allied Health Sciences,
August 30, 2018 Newsletter 2018 vol.2 23-32 TARGETING TOOLS FOR A DNA VACCINE CARRIER, A DESIGN IN PREVENTION OF CANCER Sharif Mohammad Shaheen* and Mustafezur Rahman Faculty of Allied Health Sciences,
Immune surveillance: The immune system can recognize and destroy nascent malignant cells
 Immune surveillance: The immune system can recognize and destroy nascent malignant cells Control Escape APC T C T H B NKT NK Innate Tumor T cells are believed to play a major role in controlling tumor
Immune surveillance: The immune system can recognize and destroy nascent malignant cells Control Escape APC T C T H B NKT NK Innate Tumor T cells are believed to play a major role in controlling tumor
The Adaptive Immune Responses
 The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology
 Code : AS-2246 M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology A. Select one correct option for each of the following questions:- 2X10=10 1. (b)
Code : AS-2246 M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology A. Select one correct option for each of the following questions:- 2X10=10 1. (b)
Unit 6: Adaptive Immunity. Adaptive Immunity (Humoral Immunity; Cell-Mediated Immunity; Immunodeficiency; Hypersensitivity)
 Unit 6: Adaptive Immunity Adaptive Immunity (Humoral Immunity; Cell-Mediated Immunity; Immunodeficiency; Hypersensitivity) : ADAPTIVE IMMUNITY: AN OVERVIEW OF INNATE AND ADAPTIVE IMMUNITY Adaptive Immunity
Unit 6: Adaptive Immunity Adaptive Immunity (Humoral Immunity; Cell-Mediated Immunity; Immunodeficiency; Hypersensitivity) : ADAPTIVE IMMUNITY: AN OVERVIEW OF INNATE AND ADAPTIVE IMMUNITY Adaptive Immunity
Immunity and Cancer. Doriana Fruci. Lab di Immuno-Oncologia
 Immunity and Cancer Doriana Fruci Lab di Immuno-Oncologia Immune System is a network of cells, tissues and organs that work together to defend the body against attacks of foreign invaders (pathogens, cancer
Immunity and Cancer Doriana Fruci Lab di Immuno-Oncologia Immune System is a network of cells, tissues and organs that work together to defend the body against attacks of foreign invaders (pathogens, cancer
2014 Pearson Education, Inc. Exposure to pathogens naturally activates the immune system. Takes days to be effective Pearson Education, Inc.
 The innate immune interact with the adaptive immune system 1. Damage to skin causes bleeding = bradykinin activated, resulting in inflammation 2. Dendritic phagocytose pathogens Adaptive immunity 4. Dendritic
The innate immune interact with the adaptive immune system 1. Damage to skin causes bleeding = bradykinin activated, resulting in inflammation 2. Dendritic phagocytose pathogens Adaptive immunity 4. Dendritic
Cell Biology from an Immune Perspective
 Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Cell Biology from an Immune Perspective In this lecture we will very briefly
Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Cell Biology from an Immune Perspective In this lecture we will very briefly
IMMUNOINFORMATICS: Bioinformatics Challenges in Immunology
 Bioinformatics 1 -- Lecture 22 IMMUNOINFORMATICS: Bioinformatics Challenges in Immunology Most slides courtesy of Julia Ponomarenko, San Diego Supercomputer Center or Oliver Kohlbacher, WSI/ZBIT, Eberhard-Karls-
Bioinformatics 1 -- Lecture 22 IMMUNOINFORMATICS: Bioinformatics Challenges in Immunology Most slides courtesy of Julia Ponomarenko, San Diego Supercomputer Center or Oliver Kohlbacher, WSI/ZBIT, Eberhard-Karls-
Immunology Basics Relevant to Cancer Immunotherapy: T Cell Activation, Costimulation, and Effector T Cells
 Immunology Basics Relevant to Cancer Immunotherapy: T Cell Activation, Costimulation, and Effector T Cells Andrew H. Lichtman, M.D. Ph.D. Department of Pathology Brigham and Women s Hospital and Harvard
Immunology Basics Relevant to Cancer Immunotherapy: T Cell Activation, Costimulation, and Effector T Cells Andrew H. Lichtman, M.D. Ph.D. Department of Pathology Brigham and Women s Hospital and Harvard
Acquired Immunity 2. - Vaccines & Immunological Memory - Wataru Ise. WPI Immunology Frontier Research Center (IFReC) Osaka University.
 Acquired Immunity 2 - Vaccines & Immunological Memory - Wataru Ise WPI Immunology Frontier Research Center (IFReC) Osaka University Outline 1. What is vaccine (vaccination)? 2. What is immunological memory?
Acquired Immunity 2 - Vaccines & Immunological Memory - Wataru Ise WPI Immunology Frontier Research Center (IFReC) Osaka University Outline 1. What is vaccine (vaccination)? 2. What is immunological memory?
General Biology. A summary of innate and acquired immunity. 11. The Immune System. Repetition. The Lymphatic System. Course No: BNG2003 Credits: 3.
 A summary of innate and acquired immunity General iology INNATE IMMUNITY Rapid responses to a broad range of microbes Course No: NG00 Credits:.00 External defenses Invading microbes (pathogens). The Immune
A summary of innate and acquired immunity General iology INNATE IMMUNITY Rapid responses to a broad range of microbes Course No: NG00 Credits:.00 External defenses Invading microbes (pathogens). The Immune
I. Critical Vocabulary
 I. Critical Vocabulary A. Immune System: a set of glands, tissues, cells, and dissolved proteins that combine to defend against non-self entities B. Antigen: any non-self chemical that triggers an immune
I. Critical Vocabulary A. Immune System: a set of glands, tissues, cells, and dissolved proteins that combine to defend against non-self entities B. Antigen: any non-self chemical that triggers an immune
Immune System AP SBI4UP
 Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
There are 2 major lines of defense: Non-specific (Innate Immunity) and. Specific. (Adaptive Immunity) Photo of macrophage cell
 There are 2 major lines of defense: Non-specific (Innate Immunity) and Specific (Adaptive Immunity) Photo of macrophage cell Development of the Immune System ery pl neu mφ nk CD8 + CTL CD4 + thy TH1 mye
There are 2 major lines of defense: Non-specific (Innate Immunity) and Specific (Adaptive Immunity) Photo of macrophage cell Development of the Immune System ery pl neu mφ nk CD8 + CTL CD4 + thy TH1 mye
Adaptive Immunity: Humoral Immune Responses
 MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
Adaptive Immunity: Specific Defenses of the Host
 17 Adaptive Immunity: Specific Defenses of the Host SLOs Differentiate between innate and adaptive immunity, and humoral and cellular immunity. Define antigen, epitope, and hapten. Explain the function
17 Adaptive Immunity: Specific Defenses of the Host SLOs Differentiate between innate and adaptive immunity, and humoral and cellular immunity. Define antigen, epitope, and hapten. Explain the function
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
Overview: The immune responses of animals can be divided into innate immunity and acquired immunity.
 GUIDED READING - Ch. 43 - THE IMMUNE SYSTEM NAME: Please print out these pages and HANDWRITE the answers directly on the printouts. Typed work or answers on separate sheets of paper will not be accepted.
GUIDED READING - Ch. 43 - THE IMMUNE SYSTEM NAME: Please print out these pages and HANDWRITE the answers directly on the printouts. Typed work or answers on separate sheets of paper will not be accepted.
The Innate Immune Response
 The Innate Immune Response FUNCTIONS OF THE IMMUNE SYSTEM: Recognize, destroy and clear a diversity of pathogens. Initiate tissue and wound healing processes. Recognize and clear damaged self components.
The Innate Immune Response FUNCTIONS OF THE IMMUNE SYSTEM: Recognize, destroy and clear a diversity of pathogens. Initiate tissue and wound healing processes. Recognize and clear damaged self components.
T Cell Development. Xuefang Cao, MD, PhD. November 3, 2015
 T Cell Development Xuefang Cao, MD, PhD November 3, 2015 Thymocytes in the cortex of the thymus Early thymocytes development Positive and negative selection Lineage commitment Exit from the thymus and
T Cell Development Xuefang Cao, MD, PhD November 3, 2015 Thymocytes in the cortex of the thymus Early thymocytes development Positive and negative selection Lineage commitment Exit from the thymus and
Peptide Repertoire Changes Caused by Defects in Antigen Processing. Kristin Camfield Lind. A dissertation submitted in partial satisfaction of the
 Peptide Repertoire Changes Caused by Defects in Antigen Processing By Kristin Camfield Lind A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in
Peptide Repertoire Changes Caused by Defects in Antigen Processing By Kristin Camfield Lind A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in
Adaptive immune responses: T cell-mediated immunity
 MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
chapter 8 Antigen Processing and Presentation Self-MHC Restriction of T Cells
 8536d_ch08_185-199 8/22/02 11:49 AM Page 185 mac100 mac 100: 1268_tm:8536d:Goldsby et al. / Immunology 5e-: Antigen Processing and Presentation chapter 8 RECOGNITION OF FOREIGN PROTEIN ANTIGENS BY a T
8536d_ch08_185-199 8/22/02 11:49 AM Page 185 mac100 mac 100: 1268_tm:8536d:Goldsby et al. / Immunology 5e-: Antigen Processing and Presentation chapter 8 RECOGNITION OF FOREIGN PROTEIN ANTIGENS BY a T
Immunology for the Rheumatologist
 Immunology for the Rheumatologist Rheumatologists frequently deal with the immune system gone awry, rarely studying normal immunology. This program is an overview and discussion of the function of the
Immunology for the Rheumatologist Rheumatologists frequently deal with the immune system gone awry, rarely studying normal immunology. This program is an overview and discussion of the function of the
All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity
 1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
FOCiS. Lecture outline. The immunological equilibrium: balancing lymphocyte activation and control. Immunological tolerance and immune regulation -- 1
 1 Immunological tolerance and immune regulation -- 1 Abul K. Abbas UCSF FOCiS 2 Lecture outline Principles of immune regulation Self-tolerance; mechanisms of central and peripheral tolerance Inhibitory
1 Immunological tolerance and immune regulation -- 1 Abul K. Abbas UCSF FOCiS 2 Lecture outline Principles of immune regulation Self-tolerance; mechanisms of central and peripheral tolerance Inhibitory
AG MHC HLA APC Ii EPR TAP ABC CLIP TCR
 !! AG MHC HLA APC Ii EPR TAP ABC CLIP TCR Antigen Major Histocompartibility Complex Human Leukocyte Antigen Antigen Presenting Cell Invariant Chain Endoplasmatic Reticulum Transporters Associated with
!! AG MHC HLA APC Ii EPR TAP ABC CLIP TCR Antigen Major Histocompartibility Complex Human Leukocyte Antigen Antigen Presenting Cell Invariant Chain Endoplasmatic Reticulum Transporters Associated with
Lecture 11. Immunology and disease: parasite antigenic diversity
 Lecture 11 Immunology and disease: parasite antigenic diversity RNAi interference video and tutorial (you are responsible for this material, so check it out.) http://www.pbs.org/wgbh/nova/sciencenow/3210/02.html
Lecture 11 Immunology and disease: parasite antigenic diversity RNAi interference video and tutorial (you are responsible for this material, so check it out.) http://www.pbs.org/wgbh/nova/sciencenow/3210/02.html
a) The statement is true for X = 400, but false for X = 300; b) The statement is true for X = 300, but false for X = 200;
 1. Consider the following statement. To produce one molecule of each possible kind of polypeptide chain, X amino acids in length, would require more atoms than exist in the universe. Given the size of
1. Consider the following statement. To produce one molecule of each possible kind of polypeptide chain, X amino acids in length, would require more atoms than exist in the universe. Given the size of
Proteasomes. When Death Comes a Knock n. Warren Gallagher Chem412, Spring 2001
 Proteasomes When Death Comes a Knock n Warren Gallagher Chem412, Spring 2001 I. Introduction Introduction The central dogma Genetic information is used to make proteins. DNA RNA Proteins Proteins are the
Proteasomes When Death Comes a Knock n Warren Gallagher Chem412, Spring 2001 I. Introduction Introduction The central dogma Genetic information is used to make proteins. DNA RNA Proteins Proteins are the
C. Incorrect! MHC class I molecules are not involved in the process of bridging in ADCC.
 Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Lecture 6. Burr BIO 4353/6345 HIV/AIDS. Tetramer staining of T cells (CTL s) Andrew McMichael seminar: Background
 Lecture 6 Burr BIO 4353/6345 HIV/AIDS Andrew McMichael seminar: Background Tetramer staining of T cells (CTL s) 1. Vβ 19: There are 52 T cell receptor (TCR) Vβ gene segments in germ line DNA (See following
Lecture 6 Burr BIO 4353/6345 HIV/AIDS Andrew McMichael seminar: Background Tetramer staining of T cells (CTL s) 1. Vβ 19: There are 52 T cell receptor (TCR) Vβ gene segments in germ line DNA (See following
Antigen sampling and presentation
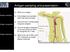 Antigen sampling and presentation ntigen sampling ntigen recognition ntigen clearance What is an antigen How antigens are sampled when they enter the body How do B and T lymphocytes recognize antigens
Antigen sampling and presentation ntigen sampling ntigen recognition ntigen clearance What is an antigen How antigens are sampled when they enter the body How do B and T lymphocytes recognize antigens
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
Summary and Discussion antigen presentation
 Summary and Discussion antigen presentation 247 248 Summary & Discussion Summary and discussion: antigen presentation For a cell to communicate information about its internal health and status to the immune
Summary and Discussion antigen presentation 247 248 Summary & Discussion Summary and discussion: antigen presentation For a cell to communicate information about its internal health and status to the immune
The Major Histocompatibility Complex of Genes
 The Major Histocompatibility Complex of Genes Topic 4 The Major Histocompatibility Complex Outline of Lectures The immunological reasons for transplant rejection How the MHC was discovered using inbred
The Major Histocompatibility Complex of Genes Topic 4 The Major Histocompatibility Complex Outline of Lectures The immunological reasons for transplant rejection How the MHC was discovered using inbred
Acquired Immunity Cells are initially and require before they can work Responds to individual microbes
 1 of 10 THE IMMUNE SYSTEM CHAPTER 43; PAGES 898 921 WHY DO WE NEED AN IMMUNE SYSTEM? It s a dirty, dirty world out there and we are vastly outnumbered Bacteria and parasites are everywhere The body has
1 of 10 THE IMMUNE SYSTEM CHAPTER 43; PAGES 898 921 WHY DO WE NEED AN IMMUNE SYSTEM? It s a dirty, dirty world out there and we are vastly outnumbered Bacteria and parasites are everywhere The body has
The Adaptive Immune Response. B-cells
 The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
