CANADIAN HEMOPHILIA SOCIETY POSITION: CURRENT DONOR DEFERRAL CRITERIA ARE IN THE BEST INTERESTS OF BLOOD SAFETY
|
|
|
- Henry Horton
- 6 years ago
- Views:
Transcription
1 CANADIAN HEMOPHILIA SOCIETY POSITION: CURRENT DONOR DEFERRAL CRITERIA ARE IN THE BEST INTERESTS OF BLOOD SAFETY The Canadian Hemophilia Society (CHS) supports the current Canadian regulations regarding permanent deferrals of certain blood donors, including the restriction on donating blood by men who have had sex with a man (MSM) since These deferrals are mandated by Health Canada. The U.S. Food and Drug Administration (FDA) and the European Medicinal Evaluation Agency have similar regulations. (See annex 1 for the official CHS policy.) The CHS is making its position public in the context of a joint statement on March 9, 2006 by AABB, America s Blood Centers, of which Canadian Blood Services and Héma-Québec are members, and the American Red Cross which urged the FDA to modify the deferral period for MSM, 1 and the increasing number of protests against the current regulations on university campuses across Canada. The one and only goal of the CHS is to maintain the highest level of safety for all recipients of blood and blood products in Canada. Many people with bleeding disorders von Willebrand Disease, hemophilia B and other rare factor deficiencies receive plasma-derived blood products on a regular basis. Moreover, all people with bleeding disorders have a higher-than normal chance of needing fresh blood components such as red blood cells, platelets and fresh frozen plasma. These latter products cannot be virally inactivated during the manufacturing process. Our concerns are also for the hundreds of thousands of other Canadians who need fresh blood components for example, those with thalassemia, sickle cell disease, chronic anemias and cancer, and those who have had a serious accident or who require surgery or fractionated blood products to treat, for example, primary immune deficiencies. Examples of permanent deferrals in place to safeguard the blood system are: people who have taken illegal drugs or illegal steroids with a needle, even one time; people who have taken money or drugs for sex, even one time; men who have had sex with a man, even one time, since 1977; people who have ever taken clotting factor concentrates, such as hemophiliacs; people who have visited certain countries in Africa where a strain of HIV not detectable by current tests is prevalent. 2 Also permanently deferred are people who have spent more than three months in the United Kingdom or France between 1980 and This regulation is intended to reduce the risk from variant Creutzfeldt-Jakob disease (vcjd), caused by the ingestion of bovine products infected with bovine spongiform encephalopathy (BSE) or Mad Cow Disease. It was introduced years before the first case of transfusion-transmitted vcjd was identified. Since then the UK has reported four cases of vcjd from red blood cells, demonstrating the pertinence of this precautionary measure. While the other permanent deferrals are not challenged, the justification for the deferral of men who have had sex with a man has been questioned many times for more than a decade. Current epidemiological data shows that men who have had sex with a man are at greater risk for HIV/AIDS infection than other people. While HIV in Canada is not restricted to the MSM population, the infection rate is tragically high in this group. Assuming that 5% of the male Canadian population are MSM, current Canadian epidemiological data places the HIV/AIDS prevalence at approximately 4.2 per cent for MSM. This compares to per cent in the non- MSM, non IV-drug user male population. 3 In other words, using this analysis based on published Canadian data, a male truthfully answering YES to the MSM question is 263 times (4.2/0.016) more likely to be HIV infected than a male who truthfully answers NO. 1
2 The CHS position on this issue is the same as that adopted at the conclusion of the November 2001 Canadian Blood Services Héma-Québec consensus conference on donor selection. The consensus statement reads: Current laboratory tests used to test for HIV, HBV and HCV on collected blood are highly sensitive and can detect a unit as being potentially infectious for both prevalent infections and shortly after acquisition of infection. However, a small risk of undetected infectivity remains. Furthermore, there is a concern about unknown pathogens that may be transmitted in a similar way to that of known pathogens. It is prudent, therefore, to continue to select donors for donation through application of criteria that reduce the chance of infectious blood being collected. 4 The Canadian Hemophilia Society is unaware of any new scientific data which would put into question the conclusions of the conference. In November 2004, the Quebec Hemovigilance Committee, which advises the Quebec Minister of Health on blood safety, reviewed Héma-Québec s proposal to seek a change in the MSM deferral criteria from Health Canada. An excerpt from the summary of the meeting: Héma-Québec would like to know the position of the Hemovigilance Committee concerning the possibility of modifying the permanent exclusion to a temporary exclusion for men having had sex with another man. For example, men who had been abstinent for 12 months would no longer be deferred. Considering that: such a measure would only have a minor impact on the number of donors; the deferral would in any case continue to be seen as discriminatory; an increase in risk, even minor, is not acceptable; the incidence and prevalence of hepatitis B is higher in this group; the incidence of blood-borne sexually transmitted diseases is increasing in this population as in the general population; such a decision could cause patients to refuse a transfusion because of a perception of increased risk; no new element suggests a change to the conclusions of the consensus conference held in 2001 on this subject. The members of the Hemovigilance Committee voted unanimously in favour of maintaining the current deferral measure. 5 On June 21, 2007, Canadian Blood Services, following a literature review, analysis of surveillance data, assessment of international MSM policies, an independent risk assessment and consultations with the National Liaison Committee, the Canadian Federation of Students, Egale, healthcare professionals and associations representing recipients, came to this decision 6 : After careful consideration, the Board of Directors has determined that Canadian Blood Services will maintain the current policy while actively gathering knowledge to close the gaps in information that were identified through the risk assessment and the consultations. As such, we will take steps to better understand emerging pathogens, examine the risks and benefits of behavioural-based questions and monitor the experiences of blood agencies that have modified or changed their MSM deferral policies. 2
3 It is recognized that the MSM policy is discriminatory against men who had sex with another man; however, legal decisions have upheld the legality of such discrimination as it was judged to be justified in the interest of public health. One example of this is the decision of the Quebec Commission des droits de la personne et des droits de la jeunesse which in 1995 found that the Red Cross discriminatory deferral of men having had sex with another man was justified. 7 Much of the recent debate has centred around the risk of HIV transmission through the blood supply should the permanent deferral be relaxed to a 12-month deferral. Nucleic amplification testing (NAT) of each blood donation has reduced the risk of an HIV-positive donation escaping detection. The risk, however, is not zero. False negative tests and laboratory errors mean that testing is not foolproof. Recent epidemiological research has estimated that a change in regulation to a 12-month deferral would result in one infectious donation entering the blood system in Canada every 16 years. 8 This would result in three new HIV infections among recipients every 16 years as each donation is divided into three components. Secondary infection to a sexual partner increases the impact of relaxing the regulation. While this is a small increase in risk, it seems unreasonable to adopt a policy which would increase risk to recipients while at the same time regulators and blood system operators are exploring the introduction of new measures to further decrease risk, even marginally. Nucleic amplification testing for hepatitis B virus is one example. Viral reduction applied to fresh components through the use of filters and chemical processes will soon be another. Even more important from a safety perspective, in the opinion of the Canadian Hemophilia Society, is the increased risk from unknown or emerging blood-borne pathogens. The onset of symptoms could be several years. Evidence that the infection is blood-borne may take more time. Tests to detect the emerging pathogen may not yet be developed. HIV, for example, had an incubation period of between two and ten years between infection and first symptoms. Hepatitis C was suspected for many years before a test could detect its presence in blood donors, and it remained undetected in many victims for decades. In the intervals, thousands were infected. vcjd is thought to have an incubation period of 10 to 30 years. No test has yet been perfected. Sadly, blood-borne pathogens have had a history of propagating through the MSM, IV-drug user and sex trade populations, and recipients of blood and blood products. Hepatitis B and HIV are but two of the best-known examples. The Canadian Hemophilia Society believes it would be imprudent not to learn from history. Recent epidemiology gives credence to this. Human herpesvirus 8 (HHV-8) is a virus that has long been known to cause serious disease in those who are immunocompromised, and to be more prevalent in MSM than in the general population. In October 2006, an article in the New England Journal of Medicine showed HHV-8 was transmissible by blood transfusion. No test to detect HHV-8 in blood donations currently exists. 9 One can easily imagine the scenario in which a new blood-borne, sexually transmitted pathogen for which no screening test exists, and which has a lengthy period from infection to symptoms, enters the blood supply like hepatitis B or HIV. The donor selection questionnaire is the only tool to defend against this scenario. The tainted blood tragedy is a sad chapter in the history of public health in Canada. Over 1100 Canadians were infected with HIV through tainted blood. Three-quarters of them have passed away. Approximately 20,000 others were infected with hepatitis C after transfusions. The number of deaths from liver disease is not precisely known but could be in the thousands. Fortunately, lessons were learned. The Commission of Inquiry on the Blood System in Canada 10 (the Krever Commission) made recommendations which today are cited around the world. One of these was to take the precautionary approach when considering safety of the blood supply. Given the current evidence, the Canadian Hemophilia Society believes that the precautionary approach should apply to this particular issue. 3
4 By their very nature blood donor screening and deferral criteria are discriminatory; however, they are reasonably justifiable where they provide increased protection to public health. While the risk of HIV transmission through the transfusion of blood and blood products has been reduced significantly, the transmission of blood-borne pathogens including HIV remains significant among men who have sex with another man. In the absence of perfect testing, donor screening, including the existing MSM deferral, remains an essential component of blood safety. Created: March 31, 2006 Updated: February 12, 2008 The Canadian Hemophilia Society Blood Safety and Supply Committee: Tom Alloway, Ph.D.; Bill Featherstone; Michael King, M.D.; Martin Kukczyk; James Kreppner; Wilma McClure, R.N.; Bill Mindell, MPH; Tina Morgan; John Plater; Mohammad Qadura; David Page; Bruce Ritchie, M.D.; Craig Upshaw; Pam Wilton, R.N. 4
5 Annex 1 Canadian Hemophilia Society policy on donor deferral with regard to men having had sex with another man since 1977 Given that there are discussions underway within the Canadian blood system to change the donor deferral criteria regarding men having had sex with another man since 1977; Acknowledging that donor deferral criteria are by their very nature discriminatory, but that such discrimination can be justified for reasons of public health; Given that there is a continued high prevalence of HIV, hepatitis B and other blood-borne pathogens, and a higher risk of emergent blood-borne pathogens in this population; Given that screening tests are able to screen out known viruses, but unable to reduce risk from new or unidentified pathogens with long periods between infection and detection; Given the conclusions of the 2001 CBS - Héma-Québec consensus conference on donor selection; Given that any change to donor deferral criteria should be based on science; Given that no new science has emerged that would justify a change to the donor deferral criteria regarding men who have had sex with another man since 1977; Given the demonstrated value of the precautionary approach; Be it moved that the CHS support no change to the blood donor deferral criteria regarding men who have had sex with another man since Moved: Bruce Ritchie, M.D. Seconded: Tom Alloway, Ph.D. Carried unanimously by the CHS Board of Directors November
6 References 1 ABC Newsletter, March 24, Canadian Blood Services Donor Questionnaire 6ef3dabf8d ab9005caa3c?OpenDocument 3 Public Health Agency of Canada, HIV/AIDS Updates, May 2005 at 05/pdf/epi_05_e.pdf 4 S. M. King, J. AuBuchon, N. Barrowman, G. Follea, M. Giroux, W. Kim, J. Kreppner, P. Millson, B. Squires & R. Z. Shaul. Consensus statement from the consensus conference on blood-borne human immunodeficiency virus and hepatitis: optimizing the donor-selection process. Vox Sanguinis (2002) 83, Compte rendu de la 37 e réunion du Comité d hémovigilance, le 18 novembre 2004 (free translation) Commission des droits de la personne et des droits de la jeunesse Québec. L exclusion par la Croix-Rouge de dons de sang de la part de certaines personnes. Le 17 août M. Germain, R.S. Remis, G. Delage. The risks and benefits of accepting men who have had sex with men as blood donors. Transfusion, January Wolfgang Hladik, M.D., Sheila C. Dollard, Ph.D., Jonathan Mermin, M.D., Ashley L. Fowlkes, M.P.H., Robert Downing, Ph.D., Minal M. Amin, M.S., Flora Banage, M.B., Ch.B., Esau Nzaro, M.B., Ch.B., Peter Kataaha, M.B., Ch.B., Timothy J. Dondero, M.D., Philip E. Pellett, Ph.D., and Eve M. Lackritz, M.D. Transmission of Human Herpesvirus 8 by Blood Transfusion. N Engl J Med 2006;355: Commission of Inquiry on the Blood System in Canada disclaimer.html?orig=/100/200/301/hcan-scan/commission_blood_final_rep-e/vol1-e.pdf 6
MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE BACKGROUND
 MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE Countries around the world are reviewing the policy of deferring from blood donation men who have had sex with men (MSM). Both Canadian Blood Services
MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE Countries around the world are reviewing the policy of deferring from blood donation men who have had sex with men (MSM). Both Canadian Blood Services
Changing eligibility criteria for MSM: The Canadian Perspective
 Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
REPORT CARD on Canada s Blood System
 2005-2007 REPORT CRD on Canada s Blood System Ten Years after the Commission of Inquiry on the Blood System in Canada Prepared by the Blood Safety and Supply Committee of the Canadian Hemophilia Society
2005-2007 REPORT CRD on Canada s Blood System Ten Years after the Commission of Inquiry on the Blood System in Canada Prepared by the Blood Safety and Supply Committee of the Canadian Hemophilia Society
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services
 Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Pathogen Safety and BSE / variant CJD
 Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
July 14, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
Canada s Blood Donation Eligibility Criteria for Men Who Have Sex with Men
 Criteria for Men Who Have Sex with Men Dr. Graham D. Sher Chief Executive Officer, Canadian Blood Services Dr. Dana Devine Chief Medical and Scientific Officer, Canadian Blood Services Executive summary
Criteria for Men Who Have Sex with Men Dr. Graham D. Sher Chief Executive Officer, Canadian Blood Services Dr. Dana Devine Chief Medical and Scientific Officer, Canadian Blood Services Executive summary
Quick facts about mad cow disease
 Quick facts about mad cow disease Mad cow disease is the common name for a condition known technically as bovine spongiform encephalopathy, or BSE. Here are some quick facts about BSE, and its human offshoot,
Quick facts about mad cow disease Mad cow disease is the common name for a condition known technically as bovine spongiform encephalopathy, or BSE. Here are some quick facts about BSE, and its human offshoot,
REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH
 REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH (A-0) Revision of the Lifetime Deferral for Blood Donation of the Men Who Have Sex with Men (MSM) Population (Resolution, A-0) (Reference Committee E)
REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH (A-0) Revision of the Lifetime Deferral for Blood Donation of the Men Who Have Sex with Men (MSM) Population (Resolution, A-0) (Reference Committee E)
Zika Virus. Report to the Standing Committee on Health. Dr. Graham Sher Chief Executive Officer, Canadian Blood Services
 Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
Fleetbank House 1 st Floor, 2-6 Salisbury Square London EC4Y 8AE. Mr Simon Stevens Chief Executive of NHS England By .
 Fleetbank House 1 st Floor, 2-6 Salisbury Square London EC4Y 8AE Mr Simon Stevens Chief Executive of NHS England By email 05 July 2018 Dear Mr Stevens, Notice of retention/non-destruction of documents
Fleetbank House 1 st Floor, 2-6 Salisbury Square London EC4Y 8AE Mr Simon Stevens Chief Executive of NHS England By email 05 July 2018 Dear Mr Stevens, Notice of retention/non-destruction of documents
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
Government of Canada Federal AIDS Initiative Milestones
 HIV in Canada: Trends and Issues for Advancing Prevention, Care, Treatment and Support Through Knowledge Exchange Michael R Smith, Senior Policy Advisor, Programs and Coordination Division, Centre for
HIV in Canada: Trends and Issues for Advancing Prevention, Care, Treatment and Support Through Knowledge Exchange Michael R Smith, Senior Policy Advisor, Programs and Coordination Division, Centre for
HEALTH SCREENING QUESTIONNAIRE CT Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
INFECTED BLOOD INQUIRY TERMS OF REFERENCE
 INFECTED BLOOD INQUIRY TERMS OF REFERENCE What happened and why? 1. To examine the circumstances in which men, women and children 1 treated by national Health Services in the United Kingdom (collectively,
INFECTED BLOOD INQUIRY TERMS OF REFERENCE What happened and why? 1. To examine the circumstances in which men, women and children 1 treated by national Health Services in the United Kingdom (collectively,
HEALTH SCREENING QUESTIONNAIRE. Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
Change in deferrals for men who have sex with men: Assessing the risk of HIV transmission by transfusion
 Change in deferrals for men who have sex with men: Assessing the risk of HIV transmission by transfusion Josiane Pillonel, Rachid Djoudi, Florence Lot, Chistophe Martinaud, Claire Sauvage, Marie Jauffret-Roustide,
Change in deferrals for men who have sex with men: Assessing the risk of HIV transmission by transfusion Josiane Pillonel, Rachid Djoudi, Florence Lot, Chistophe Martinaud, Claire Sauvage, Marie Jauffret-Roustide,
DL (2018) 14. Dear Colleague UK INFECTED BLOOD INQUIRY RETENTION OF RECORDS
 The Scottish Government Population Health Directorate, Health Protection Division 17 July 2018 Dear Colleague UK INFECTED BLOOD INQUIRY RETENTION OF RECORDS Summary This Director s letter asks you to ensure
The Scottish Government Population Health Directorate, Health Protection Division 17 July 2018 Dear Colleague UK INFECTED BLOOD INQUIRY RETENTION OF RECORDS Summary This Director s letter asks you to ensure
patients with blood borne viruses Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled Document Lead:
 CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
[Submitted Electronically]
![[Submitted Electronically] [Submitted Electronically]](/thumbs/82/85246007.jpg) [Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
[Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
Permanent Donor Deferral Policy
 Permanent Donor Deferral Policy Background Rationale to have the questions relaxed/removed 1 Permanent donor deferral policy affects the supply of phenotypic blood extremely needed by individuals living
Permanent Donor Deferral Policy Background Rationale to have the questions relaxed/removed 1 Permanent donor deferral policy affects the supply of phenotypic blood extremely needed by individuals living
BLOOD TRANSFUSIONS. Answers. To Your. Questions
 BLOOD TRANSFUSIONS Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
BLOOD TRANSFUSIONS Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS
 EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
Current Infectious Disease Screening for the Live Organ Donor
 2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
Answers. To Your. Questions
 Blood Transfusions Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
Blood Transfusions Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
Chronic Wasting Disease (CWD)
 Blood Safety The American Red Cross (ARC) is denying blood donations from individuals who have spent six months or more in Europe since 1980, as well as that of any blood relative of a CJD victim. Sporadic
Blood Safety The American Red Cross (ARC) is denying blood donations from individuals who have spent six months or more in Europe since 1980, as well as that of any blood relative of a CJD victim. Sporadic
Source Plasma Safety
 Source Plasma Safety Plasma May 15-19, 2017 George Schreiber, ScD, PPTA Director, Epidemiology Three components: Plasma Product Safety Donor Quality Manufacturing Pool Quality Fractionation Process Safe
Source Plasma Safety Plasma May 15-19, 2017 George Schreiber, ScD, PPTA Director, Epidemiology Three components: Plasma Product Safety Donor Quality Manufacturing Pool Quality Fractionation Process Safe
ANNALS OF HEALTH LAW Advance Directive VOLUME 23 SPRING 2014 PAGES
 ANNALS OF HEALTH LAW Advance Directive VOLUME 23 SPRING 2014 PAGES 101-112 United States Blood Donor Policy on Gay Men: Adopting an Italian Individual Risk Assessment Policy Melissa Kong* I. INTRODUCTION
ANNALS OF HEALTH LAW Advance Directive VOLUME 23 SPRING 2014 PAGES 101-112 United States Blood Donor Policy on Gay Men: Adopting an Italian Individual Risk Assessment Policy Melissa Kong* I. INTRODUCTION
Not Under Document Control If Printed. Donor Educational Materials. If you are deferred
 Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Draft Agreed by Biologics Working Party December Draft Agreed by Blood Products Working party December 2010
 15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
Objectives. Methods. Results. Economic
 Octaplas Compared with Fresh Frozen Plasma to Reduce the Risk of Transmitting Lipid-Enveloped Viruses: An Economic Analysis and Budget Impact Analysis [Adapted from Membe SK, Coyle D, Husereau D, Cimon
Octaplas Compared with Fresh Frozen Plasma to Reduce the Risk of Transmitting Lipid-Enveloped Viruses: An Economic Analysis and Budget Impact Analysis [Adapted from Membe SK, Coyle D, Husereau D, Cimon
RISK OF DISEASE TRANSMISSION FROM ORGAN DONORS PATIENT INFORMATION GUIDE
 Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
BLOOD TRANSFUSIONS. Answers. To Your. Questions
 BLOOD TRANSFUSIONS Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
BLOOD TRANSFUSIONS Answers To Your Questions Answers to Your Questions This brochure is intended for people who may need a blood or blood product transfusion and for those who regularly receive transfusions.
Surveillance Report 2014
 Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Exploring the risks of liver cancer after successful treatment for hepatitis C virus
 CATIE-News CATIE s bite-sized HIV and hepatitis C news bulletins. Exploring the risks of liver cancer after successful treatment for hepatitis C virus 11 June 2013 In Canada and other high-income countries,
CATIE-News CATIE s bite-sized HIV and hepatitis C news bulletins. Exploring the risks of liver cancer after successful treatment for hepatitis C virus 11 June 2013 In Canada and other high-income countries,
Version for the Silent Procedure 29 April Agenda item January Hepatitis
 Version for the Silent Procedure 29 April 2014 134th session EB134.R18 Agenda item 10.5 25 January 2014 Hepatitis The Executive Board, Having considered the report on hepatitis, 1 RECOMMENDS to the Sixty-seventh
Version for the Silent Procedure 29 April 2014 134th session EB134.R18 Agenda item 10.5 25 January 2014 Hepatitis The Executive Board, Having considered the report on hepatitis, 1 RECOMMENDS to the Sixty-seventh
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
REPORT TO THE NZ BLOOD SERVICE: BEHAVIOURAL DONOR DEFERRAL CRITERIA REVIEW
 REPORT TO THE NZ BLOOD SERVICE: BEHAVIOURAL DONOR DEFERRAL CRITERIA REVIEW February 2014 Contents Preface... 5 Consultation: issues and responses... 5 Executive Summary... 9 Background... 9 Relevant issues...
REPORT TO THE NZ BLOOD SERVICE: BEHAVIOURAL DONOR DEFERRAL CRITERIA REVIEW February 2014 Contents Preface... 5 Consultation: issues and responses... 5 Executive Summary... 9 Background... 9 Relevant issues...
BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL?
 BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL? What if your patients want to donate blood? Are you aware that patients undergoing Acupuncture treatment, according to international guidelines,
BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL? What if your patients want to donate blood? Are you aware that patients undergoing Acupuncture treatment, according to international guidelines,
Policy Document. Blood Donation Deferral. Background
 Policy Document Blood Donation Deferral Background The Australian Medical Students Association (AMSA) is the peak representative body for medical students in Australia. Accordingly, AMSA advocates on issues
Policy Document Blood Donation Deferral Background The Australian Medical Students Association (AMSA) is the peak representative body for medical students in Australia. Accordingly, AMSA advocates on issues
Transfusion-transmitted Cytomegalovirus
 Transfusion-transmitted Cytomegalovirus Can you confidently abandon CMV seronegative products in the modern era of pre-storage leukoreduction? Jeannie Callum, BA, MD, FRCPC Really? Are we still talking
Transfusion-transmitted Cytomegalovirus Can you confidently abandon CMV seronegative products in the modern era of pre-storage leukoreduction? Jeannie Callum, BA, MD, FRCPC Really? Are we still talking
CJD (Creutzfeldt-Jakob disease)
 CJD - lyodura and the risk of exposure during healthcare - frequently asked questions We would like to reassure all our patients that tight regulations govern all infection control processes at Addenbrooke's,
CJD - lyodura and the risk of exposure during healthcare - frequently asked questions We would like to reassure all our patients that tight regulations govern all infection control processes at Addenbrooke's,
Report on Donor Selection Criteria Relating to Men Who Have Sex with Men
 Report on Donor Selection Criteria Relating to Men Who Have Sex with Men June 23, 2015 Table of Contents Executive Summary 3 Glossary 5 Abbreviations 6 1. Introduction 7 2. Known infectious risks 7 Table
Report on Donor Selection Criteria Relating to Men Who Have Sex with Men June 23, 2015 Table of Contents Executive Summary 3 Glossary 5 Abbreviations 6 1. Introduction 7 2. Known infectious risks 7 Table
Statement of Approach: Selection of witnesses to give oral evidence
 Statement of Approach: Selection of witnesses to give oral evidence Introduction 1. This Statement of Approach explains how the Inquiry will decide who to call to give oral evidence as a person who has
Statement of Approach: Selection of witnesses to give oral evidence Introduction 1. This Statement of Approach explains how the Inquiry will decide who to call to give oral evidence as a person who has
PUBLICATION OF THE SUPERIOR HEALTH COUNCIL No. 9291
 PUBLICATION OF THE SUPERIOR HEALTH COUNCIL No. 9291 Sexual risk behaviour and blood donation Part I: Blood donations by MSM This version was validated by the Board on October 2016 1 This advisory report
PUBLICATION OF THE SUPERIOR HEALTH COUNCIL No. 9291 Sexual risk behaviour and blood donation Part I: Blood donations by MSM This version was validated by the Board on October 2016 1 This advisory report
Hepatitis C Best Practice Guidelines For Local Health Departments
 Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Maximizing Cornea and Tissue Donation through Specimen Quality
 Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Maximizing Cornea and Tissue Donation through Specimen Quality Robert W. Bresler, Sydney D. Gastreich, Elias G. Koulouriotis, Linda S. Martin, Susan Diane Brockmeier, Chak-Sum Ho, PhD Abstract Purpose:
Babesia from a donor perspective
 Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Strategic partner for the Québec health system
 BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS Strategic partner for the Québec health system HUMAN TISSUES MOTHER S MILK CORPORATE PRESENTATION MISSION To efficiently meet the needs of the Québec population
BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS Strategic partner for the Québec health system HUMAN TISSUES MOTHER S MILK CORPORATE PRESENTATION MISSION To efficiently meet the needs of the Québec population
L 256/32 Official Journal of the European Union
 L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
In the United States and in Canada, as in most other
 BLOOD DONORS AND BLOOD COLLECTION The risks and benefits of accepting men who have had sex with men as blood donors M. Germain, R.S. Remis, and G. Delage BACKGROUND: It has been suggested that men who
BLOOD DONORS AND BLOOD COLLECTION The risks and benefits of accepting men who have had sex with men as blood donors M. Germain, R.S. Remis, and G. Delage BACKGROUND: It has been suggested that men who
KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING
 KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING Permission to Use: Please note that the presenter has agreed to make their presentation available. However, should you want to use some of the data
KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING Permission to Use: Please note that the presenter has agreed to make their presentation available. However, should you want to use some of the data
The Synthesis Report on the public consultation of the SCENIHR opinion on
 The Synthesis Report on the public consultation of the SCENIHR opinion on The Safety of Human-derived Products with regard to Variant Creutzfeldt-Jakob Disease 2 EXECUTIVE SUMMARY 1. Background... 5 2.
The Synthesis Report on the public consultation of the SCENIHR opinion on The Safety of Human-derived Products with regard to Variant Creutzfeldt-Jakob Disease 2 EXECUTIVE SUMMARY 1. Background... 5 2.
SUMMARY NOTES of the meeting of the Canadian Blood Services Community Liaison Committee (CLC) held in Winnipeg on Tuesday, 18 March 2003 at 2 p.m.
 SUMMARY NOTES of the meeting of the Canadian Blood Services Community Liaison Committee (CLC) held in Winnipeg on Tuesday, 18 March 2003 at 2 p.m. PRESENT: GUESTS: REGRETS: Myrtle Nichols, Centre Director,
SUMMARY NOTES of the meeting of the Canadian Blood Services Community Liaison Committee (CLC) held in Winnipeg on Tuesday, 18 March 2003 at 2 p.m. PRESENT: GUESTS: REGRETS: Myrtle Nichols, Centre Director,
Breastfeeding Center Milk Connection Questionnaire and Release for Donor
 Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
Provincial Infectious Diseases Advisory Committee Public Health Response to Hepatitis C
 Provincial Infectious Diseases Advisory Committee Public Health Response to Hepatitis C Dr. Doug Sider, Medical Director, Public Health Ontario Dr. Peggy Millson, Professor Emeritus, Dalla Lana School
Provincial Infectious Diseases Advisory Committee Public Health Response to Hepatitis C Dr. Doug Sider, Medical Director, Public Health Ontario Dr. Peggy Millson, Professor Emeritus, Dalla Lana School
How are testing technologies used to diagnose HIV infection?
 HIV testing technologies are used to determine if a person has HIV. Several types of HIV testing technologies are used in Canada. These tests differ in several ways, including where the test is conducted,
HIV testing technologies are used to determine if a person has HIV. Several types of HIV testing technologies are used in Canada. These tests differ in several ways, including where the test is conducted,
Question: 1. Are you currently taking an antibiotic?
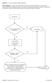 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Promoting hepatitis B vaccination
 Promoting hepatitis B vaccination Introduction Hepatitis B is a serious blood borne infection that can exacerbate hepatitis C infection, can cause serious liver damage and sometimes results in death. Hepatitis
Promoting hepatitis B vaccination Introduction Hepatitis B is a serious blood borne infection that can exacerbate hepatitis C infection, can cause serious liver damage and sometimes results in death. Hepatitis
The epidemiology of HIV in Canada
 FACTSHEET The epidemiology of HIV in Canada This fact sheet provides a snapshot of the HIV epidemic in Canada. It is one of a series of fact sheets on the epidemiology of HIV and hepatitis C in Canada.
FACTSHEET The epidemiology of HIV in Canada This fact sheet provides a snapshot of the HIV epidemic in Canada. It is one of a series of fact sheets on the epidemiology of HIV and hepatitis C in Canada.
Overview of Blood Transfusion System of Iran:
 Report Article Overview of Blood Transfusion System of Iran: 2002-2011 AM Cheraghali High Institute for Research and Education on Transfusion Medicine, Iran Blood Transfusion Organization and University
Report Article Overview of Blood Transfusion System of Iran: 2002-2011 AM Cheraghali High Institute for Research and Education on Transfusion Medicine, Iran Blood Transfusion Organization and University
THE BENCHMARK. UNAIDS and the polling company Zogby International surveyed the world on what people think about the AIDS epidemic and response.
 THE BENCHMARK UNAIDS and the polling company Zogby International surveyed the world on what people think about the AIDS epidemic and response. THE BENCHMARK UNAIDS and the polling company Zogby International
THE BENCHMARK UNAIDS and the polling company Zogby International surveyed the world on what people think about the AIDS epidemic and response. THE BENCHMARK UNAIDS and the polling company Zogby International
Blood Transfusion Orientation & Information 2010
 Blood Transfusion Orientation & Information 2010 *if you are able to get online for this training: http://www.nhlbi.nih.gov/health/dci/diseases/bt/bt_whatis.html and you can read all information included
Blood Transfusion Orientation & Information 2010 *if you are able to get online for this training: http://www.nhlbi.nih.gov/health/dci/diseases/bt/bt_whatis.html and you can read all information included
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
Patient Name: MRN: DOB: Treatment Location:
 Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Cord Blood Medical History/ Health Assessment Questionnaire
 OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P
 The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
Will I need a blood transfusion? Patient information
 Will I need a blood transfusion? Patient information Will I need a blood transfusion? Important information for all patients who may need a blood transfusion Like all medical treatments, a blood transfusion
Will I need a blood transfusion? Patient information Will I need a blood transfusion? Important information for all patients who may need a blood transfusion Like all medical treatments, a blood transfusion
FAMILY EDITION IN THIS ISSUE
 IN THIS ISSUE Introduction New Cerebrospinal Fluid Protein Tests Offered Genetics and Creutzfeldt-Jakob Disease Second Case of Variant CJD in Canada You Asked Us Consent Form for Donation of Biological
IN THIS ISSUE Introduction New Cerebrospinal Fluid Protein Tests Offered Genetics and Creutzfeldt-Jakob Disease Second Case of Variant CJD in Canada You Asked Us Consent Form for Donation of Biological
The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC
 The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC Ryan A. Maddox, PhD Epidemiologist CJD 2013 and the 11 th Annual CJD Foundation Family Conference July 14, 2013 National
The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC Ryan A. Maddox, PhD Epidemiologist CJD 2013 and the 11 th Annual CJD Foundation Family Conference July 14, 2013 National
Viral Hepatitis. WHO Regional Office for Europe July 2013
 Viral Hepatitis WHO Regional Office for Europe July 2013 What is Hepatitis? Hepatitis is a viral infection that causes inflammation of the liver There are five main types of viral hepatitis: A, B, C, D,
Viral Hepatitis WHO Regional Office for Europe July 2013 What is Hepatitis? Hepatitis is a viral infection that causes inflammation of the liver There are five main types of viral hepatitis: A, B, C, D,
Achieving Consensus on Increased Risk Donors to Improve Access to Organ Transplantation Executive Summary
 Achieving Consensus on Increased Risk Donors to Improve Access to Organ Transplantation Executive Summary Introduction To address the current shortage of organs for transplantation, (114,651 candidates
Achieving Consensus on Increased Risk Donors to Improve Access to Organ Transplantation Executive Summary Introduction To address the current shortage of organs for transplantation, (114,651 candidates
PHSKC HIV Testing Survey: Knowledge, Attitudes and Practices
 PHSKC HIV Testing Survey: Knowledge, Attitudes and Practices Page One This anonymous survey is intended to collect information about HIV testing attitudes and practices. Results will be used by Public
PHSKC HIV Testing Survey: Knowledge, Attitudes and Practices Page One This anonymous survey is intended to collect information about HIV testing attitudes and practices. Results will be used by Public
HIV AIDS and Other Infectious Diseases
 HIV AIDS and Other Infectious Diseases Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Chapter 1 - Introduction Despite the availability of a vaccine since
HIV AIDS and Other Infectious Diseases Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Chapter 1 - Introduction Despite the availability of a vaccine since
Hepatitis Trivia Game
 Hepatitis Trivia Game Materials: 30 cards with a multiple-choice, fill-in-the-blank, or true/false question written on them. Facilitator s answer sheet Description Trivia Game gives participants the opportunity
Hepatitis Trivia Game Materials: 30 cards with a multiple-choice, fill-in-the-blank, or true/false question written on them. Facilitator s answer sheet Description Trivia Game gives participants the opportunity
Question: 1. Are you currently taking an antibiotic?
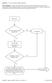 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Appendix A: Disease-Specific Chapters
 Ministry of Health and Long-Term Care Infectious Diseases Protocol Appendix A: Disease-Specific Chapters Chapter: Creutzfeldt-Jakob Disease, all types Revised March 2017 Creutzfeldt-Jakob Disease, all
Ministry of Health and Long-Term Care Infectious Diseases Protocol Appendix A: Disease-Specific Chapters Chapter: Creutzfeldt-Jakob Disease, all types Revised March 2017 Creutzfeldt-Jakob Disease, all
Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet
 JPAC 11-05 Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet 1. Paper for the JPAC meeting on: 10/3/11 2. Date submitted: 17 February 2011 3. Title (including version no.): Recommendations
JPAC 11-05 Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet 1. Paper for the JPAC meeting on: 10/3/11 2. Date submitted: 17 February 2011 3. Title (including version no.): Recommendations
donors and collecting blood and plasma in for-profit or a not for profit environment
 Remunerated versus non-remunerated donors and collecting blood and plasma in for-profit or a not for profit environment ISBT Amsterdam, June 4, 2013 Jan M. Bult President PPTA PATIENT CENTEREDNESS IT
Remunerated versus non-remunerated donors and collecting blood and plasma in for-profit or a not for profit environment ISBT Amsterdam, June 4, 2013 Jan M. Bult President PPTA PATIENT CENTEREDNESS IT
Guide to the preparation, use and quality assurance of blood components
 Contents Foreword...3 Members of the European Committee (Partial Agreement) on Blood Transfusion... 8 Members of the GTS working group... 22 Members of the TS066 working group... 30 Recommendation No.
Contents Foreword...3 Members of the European Committee (Partial Agreement) on Blood Transfusion... 8 Members of the GTS working group... 22 Members of the TS066 working group... 30 Recommendation No.
Khalid Alquthami (Correspondence) Regional Lab, Makkah. Saudi Arabia
 1 Regional Lab, Makkah. Saudi Arabia stract: Clinical specificity and genotype/subtype detection of viruses using the Cobas TaqScreen MPX system V 2.0, which is a nucleic acid test (NAT) that uses multiples
1 Regional Lab, Makkah. Saudi Arabia stract: Clinical specificity and genotype/subtype detection of viruses using the Cobas TaqScreen MPX system V 2.0, which is a nucleic acid test (NAT) that uses multiples
VIRAL HEPATITIS: SITUATION ANALYSIS AND PERSPECTIVES IN THE AFRICAN REGION. Report of the Secretariat. CONTENTS Paragraphs BACKGROUND...
 8 April 2014 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH PROGRAMME SUBCOMMITTEE Sixty-fourth session Brazzaville, Republic of Congo, 9 11 June 2014 Provisional agenda item 6 VIRAL HEPATITIS: SITUATION
8 April 2014 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH PROGRAMME SUBCOMMITTEE Sixty-fourth session Brazzaville, Republic of Congo, 9 11 June 2014 Provisional agenda item 6 VIRAL HEPATITIS: SITUATION
X-Plain Hepatitis B Reference Summary
 X-Plain Hepatitis B Reference Summary Introduction Hepatitis B is the most common serious liver infection. It is caused by the hepatitis B virus that attacks the liver. The virus is transmitted through
X-Plain Hepatitis B Reference Summary Introduction Hepatitis B is the most common serious liver infection. It is caused by the hepatitis B virus that attacks the liver. The virus is transmitted through
Lookback studies to assess viral risks The French experience
 Lookback studies to assess viral risks The French experience 2000-2012 S Laperche 1, MF Leconte des Floris 2, J Pillonel 3, L Hauser 2, C Lefort 2, JY Py 2, I Hervé 2, R Djoudi 2 For the French haemovigilance
Lookback studies to assess viral risks The French experience 2000-2012 S Laperche 1, MF Leconte des Floris 2, J Pillonel 3, L Hauser 2, C Lefort 2, JY Py 2, I Hervé 2, R Djoudi 2 For the French haemovigilance
VOLUME 34 NUMBER 1 WINTER 1999
 Hemophilia today VOLUME 34 NUMBER 1 WINTER 1999 SPECIAL ISSUE ON CJD AND BLOOD PRODUCTS Quarantine of Kogenate The Sequence of Events 1995 A doctor testifying at the Commission of Inquiry spoke of the
Hemophilia today VOLUME 34 NUMBER 1 WINTER 1999 SPECIAL ISSUE ON CJD AND BLOOD PRODUCTS Quarantine of Kogenate The Sequence of Events 1995 A doctor testifying at the Commission of Inquiry spoke of the
Infectious Disease Considerations in Organ Donation. Nikole A Neidlinger MD FACS Chief Medical Officer
 Infectious Disease Considerations in Organ Donation Nikole A Neidlinger MD FACS Chief Medical Officer Screening Donors and Infectious Disease considerations: Deceased Donor Screening Donors at increased
Infectious Disease Considerations in Organ Donation Nikole A Neidlinger MD FACS Chief Medical Officer Screening Donors and Infectious Disease considerations: Deceased Donor Screening Donors at increased
Fresh Frozen Plasma (FFP) and Cryoprecipitate Patient information
 Fresh Frozen Plasma (FFP) and Cryoprecipitate Patient information What are Fresh Frozen Plasma (FFP) and Cryoprecipitate? FFP and Cryoprecipitate (often just called cryo ) are both blood components made
Fresh Frozen Plasma (FFP) and Cryoprecipitate Patient information What are Fresh Frozen Plasma (FFP) and Cryoprecipitate? FFP and Cryoprecipitate (often just called cryo ) are both blood components made
VIRAL HEPATITIS: SITUATION ANALYSIS AND PERSPECTIVES IN THE AFRICAN REGION. Report of the Secretariat. CONTENTS Paragraphs BACKGROUND...
 5 November 2014 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-fourth session Cotonou, Republic of Benin, 3 7 November 2014 Provisional agenda item 11 VIRAL HEPATITIS: SITUATION ANALYSIS AND PERSPECTIVES
5 November 2014 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-fourth session Cotonou, Republic of Benin, 3 7 November 2014 Provisional agenda item 11 VIRAL HEPATITIS: SITUATION ANALYSIS AND PERSPECTIVES
Transmission of Human Herpesvirus 8 by Blood Transfusion
 The new england journal of medicine original article Transmission of Human Herpesvirus 8 by Blood Transfusion Wolfgang Hladik, M.D., Sheila C. Dollard, Ph.D., Jonathan Mermin, M.D., Ashley L. Fowlkes,
The new england journal of medicine original article Transmission of Human Herpesvirus 8 by Blood Transfusion Wolfgang Hladik, M.D., Sheila C. Dollard, Ph.D., Jonathan Mermin, M.D., Ashley L. Fowlkes,
The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC
 The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC Ryan A. Maddox, PhD Epidemiologist 2015 CJD Foundation Family Conference July 12, 2015 National Center for Emerging
The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC Ryan A. Maddox, PhD Epidemiologist 2015 CJD Foundation Family Conference July 12, 2015 National Center for Emerging
The Centers for Disease Control and Prevention Report: A CDC CJD Q&A
 The Centers for Disease Control and Prevention Report: A CDC CJD Q&A Ryan A. Maddox, PhD Epidemiologist 2016 CJD Foundation Family Conference July 10, 2016 National Center for Emerging and Zoonotic Infectious
The Centers for Disease Control and Prevention Report: A CDC CJD Q&A Ryan A. Maddox, PhD Epidemiologist 2016 CJD Foundation Family Conference July 10, 2016 National Center for Emerging and Zoonotic Infectious
Creutzfeldt-Jakob disease (CJD)
 Creutzfeldt-Jakob disease (CJD) A topic in the Alzheimer s Association series on understanding dementia. Dementia is a condition in which a person has significant difficulty with daily functioning because
Creutzfeldt-Jakob disease (CJD) A topic in the Alzheimer s Association series on understanding dementia. Dementia is a condition in which a person has significant difficulty with daily functioning because
Management Response. Canadian Blood Services Response to Questions from the Canadian Hemophilia Society
 Canadian Blood Services to Questions from the Canadian Hemophilia Society Introduction On Nov. 26, 1997, the final report of the Royal Commission of Inquiry on the in Canada was tabled in the House of
Canadian Blood Services to Questions from the Canadian Hemophilia Society Introduction On Nov. 26, 1997, the final report of the Royal Commission of Inquiry on the in Canada was tabled in the House of
Recent HIV testing behaviour among men having sex with men (MSM) in Montreal, results from the ARGUS 2005 survey
 Recent HIV testing behaviour among men having sex with men (MSM) in Montreal, results from the ARGUS 2005 survey G Lambert 1,2,3, J Cox 1,2,4, F Tremblay, M-A Gadoury, C Tremblay, M Alary, J Otis, R Lavoie,
Recent HIV testing behaviour among men having sex with men (MSM) in Montreal, results from the ARGUS 2005 survey G Lambert 1,2,3, J Cox 1,2,4, F Tremblay, M-A Gadoury, C Tremblay, M Alary, J Otis, R Lavoie,
Viruses. Objectives At the end of this sub section students should be able to:
 Name: 3.5 Responses to Stimuli Objectives At the end of this sub section students should be able to: 3.5.4 Viruses 1. Explain the problem of defining what a virus is - living or non-living? 2. show you
Name: 3.5 Responses to Stimuli Objectives At the end of this sub section students should be able to: 3.5.4 Viruses 1. Explain the problem of defining what a virus is - living or non-living? 2. show you
