REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH
|
|
|
- Lizbeth Gregory
- 5 years ago
- Views:
Transcription
1 REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH (A-0) Revision of the Lifetime Deferral for Blood Donation of the Men Who Have Sex with Men (MSM) Population (Resolution, A-0) (Reference Committee E) EXECUTIVE SUMMARY Objectives. To review the data on the prevalence of human immunodeficiency virus (HIV) in the men who have sex with men (MSM) population, examine the potential increase in the blood supply that would result from increased donations by the MSM population, and discuss any increased risk to the safety of the blood supply should the lifetime deferral of MSM from blood donation be removed. Data Sources. Literature searches were conducted in the PubMed database for English-language articles published between and 00 using the search term men who have sex with men blood donation or men who have sex with men blood deferral. The World Wide Web was searched, using the Google search engine, using the search term men who have sex with men blood donation deferral. Results. Current Food and Drug Administration (FDA) blood donor deferral criteria require that men who have had sex with men even once since be permanently deferred from blood donation. A policy change with respect to blood donation deferral is a risk management decision wherein the risks of introducing additional infected units for transfusion over the current residual risk must be balanced against the benefits of increasing the pool of blood donors. Also important are ethical and societal factors. Current prevalence rates of HIV in the MSM population and the residual risk with the current deferral policy suggest an unacceptable increase in risk should the MSM population no longer be deferred. Targeting blood donation deferral to a set of high risk behaviors is not practical. While, the increased risk with a -year abstinence from blood donation from the last MSM contact would be very small, it is not zero. This small but scientifically real increase in risk represents a clear violation of ethical principles and therefore is not tolerable. If a - or -year deferral policy is considered, risk management calculations would yield risks at a level that many might consider acceptable. Data suggest that men who have abstained from sex with other men for more than years essentially present no greater risk than the general population and that while it is a matter of judgment as to whether a -year deferral period would pass the risk hurdle, it may be reasonable to consider. However, many argue that the rights of blood transfusion recipients outweigh any asserted rights of blood donors and that the right to receive safe blood is the overriding responsibility of blood collection agencies. Conclusions. Men who have had sex with men since are currently permanently deferred from blood donation. This FDA policy recommendation has generated controversy due concerns that it may be discriminatory and that it stigmatizes the MSM population. Any policy decision on blood donation deferral of the MSM population must be governed by the best available scientific evidence but there are inherent weaknesses in mathematical models used in the risk assessments on this issue that continue to generate some uncertainty. With respect to the MSM population, it appears that a policy change from a permanent lifetime deferral to a -year deferral following the last MSM contact may be supportable, but societal and ethical consequences must be analyzed should this decision be advanced. Action of the AMA House of Delegates 00 Annual Meeting: Council on Science and Public Health Report Recommendation Adopted as Amended, and Remainder of Report Filed.
2 REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH CSAPH Report -A-0 Subject: Presented by: Revision of the Lifetime Deferral for Blood Donation of the Men Who Have Sex with Men (MSM) Population (Resolution, A-0) Mary Anne McCaffree, MD, Chair 0 0 Referred to: Reference Committee E (Shannon M. Kilgore, MD, Chair) Resolution (A-0), introduced by the New York Delegation and referred to the Board of Trustees, asked: That our American Medical Association (AMA) advocate to the Food and Drug Administration (FDA) that its guidance is discriminatory to large populations of potential blood donors and that this policy has not kept pace with screening technology and with the spread of specific diseases; and That our AMA advocate to the FDA that a uniform screening of donors be put in place for all populations and that the lifetime restriction for men who have had sex with men since be eliminated. This Council report reviews data on the prevalence of human immunodeficiency virus (HIV) in the men who have sex with men (MSM) population, examines the potential increase in the blood supply that would result from increased donations by the MSM population, and discusses any increased risk to the safety of the blood supply should the lifetime deferral from blood donation be removed. The report does not discuss social and ethical issues that surround the current FDA guidance on this issue. Data Sources Literature searches conducted in the PubMed database for English-language articles published between and 00 using the search terms men who have sex with men blood donation or men who have sex with men blood deferral yielded a total of references; articles/reviews directly relevant to the risk management of blood donations were selected for further review. An additional references were culled from the articles selected for further review. The World Wide Web was searched, using the Google search engine, using the search term men who have sex with men blood donation deferral. Relevant Web references were examined for accuracy and appropriateness. Electronic references cited in this report were revisited to verify availability as of March, 00. Introduction Over the past few years, interest has been expressed in changing the current FDA blood donor deferral criteria for the MSM population. Men who have had sex with men even once since are permanently deferred from blood donation. It has been proposed that permanent deferral be changed to a specific time of abstinence from MSM behavior, after which the individual should be allowed to Action of the AMA House of Delegates 00 Annual Meeting: Council on Science and Public Health Report Recommendation Adopted as Amended, and Remainder of Report Filed.
3 CSAPH Rep. - A-0 -- page donate blood. Several time lengths have been proposed for the deferral period; however, a -year deferral has received the most interest. The primary reasons why this policy change is being considered are the view that the current policy is discriminatory toward the gay population and that the volatility of the US blood supply would be eased by relaxation of the current policy. This matter is difficult to address based purely on scientific data. Clearly, this is a risk management decision where the best available scientific evidence must be balanced against the needs of society, both in terms of the blood supply itself (i.e., safety and quantity) and in terms of cultural and ethical norms. This report presents the current scientific data on blood donation deferral and the MSM population. It recommends that an analysis of the societal and ethical implications of revising the lifetime deferral policy for MSM populations be undertaken by the Council on Ethical and Judicial Affairs in order that the risk management equation be balanced. Indeed, the FDA suggests that this risk management decision is constantly changing based on new scientific data and has committed to convening expert panels to review the evidence regularly. It is also reasonable to expect that changing societal norms will play a major role in public acceptance of any such policy change. The FDA states that should future information support a change in the current policy for the MSM population, it will be seriously considered. Additionally, the FDA has stated that it is working with the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health to reach consensus on this issue. Of note, both Canada and the European Union have a similar lifetime blood donation deferral policy for the MSM population and following recent review have chosen to maintain the status quo. Blood Donation in the United States The current US blood supply is remarkably safe. However, the potential for new, as yet unidentified, bloodborne pathogens for which no tests exist, analogous to hepatitis C in the late 0s and West Nile virus in this decade, requires that stringent donor selection criteria remain firmly in place. While the ultimate responsibility for keeping the US blood supply safe lies with the individual establishments that collect the blood, the FDA is tasked with keeping blood donations as safe as possible. To accomplish this, the FDA has issued guidance that recommends multi-layered protections for donated blood to ensure its safety. There are five levels: Donor Screening: Donors are first informed about potential risks that may compromise the blood supply, and then through a detailed questionnaire are required to answer questions about factors that may bear on the safety of their blood. For example, donors with a history of intravenous (IV) drug abuse are permanently deferred. Studies indicate that donor screening is effective; for example, one study indicates that donors deferred via standard blood donor questions regarding risk of viral hepatitis as well as those with a history of IV drug use were more likely to have higher hepatitis marker rates than those who were not deferred. Of note, prior to the availability of tests for HIV and hepatitis C, the risks of post-transfusion hepatitis C and HIV infection were managed via donor selection criteria, such as the use of voluntary donors and deferral of those with known risk conditions. Blood Testing: After donation, every unit of donated blood undergoes a series of tests for hepatitis B and C viruses (HBV and HCV), HIV and, human T-lymphotropic virus (HTLV types I and II), West Nile virus, and syphilis. These tests have become more and more sophisticated, and the current use of nucleic acid testing (NAT) has dramatically reduced the risk of contracting HIV and HCV from the blood supply., However, despite the improved bloodborne pathogen testing, there still remains a window period for several of these pathogens during which the tests will not detect
4 CSAPH Rep. - A-0 -- page recent infection of the donor. For HIV, this window is now about days and for HCV days (although a range of to 0 days is possible). Donor Lists: All blood collection establishments are required to keep a current list of deferred donors and use it to ensure they do not collect blood from anyone on the list. Quarantine: Donated blood must be quarantined and not used for transfusion until it is tested and shown to be free of known infectious agents. Problems and Deficiencies: Should manufacturing problems occur, blood collection establishments are required to investigate immediately and correct all deficiencies. The FDA must be notified when product deviations occur in distributed products. Current Lifetime Blood Donation Deferral Criteria Current FDA guidance is followed in an FDA-approved AABB- (formerly known as the American Association of Blood Banks) developed Donor History Questionnaire, which is used by blood collection agencies, such as AABB members, America s Blood Centers, and the American Red Cross. This recommends that any person fitting the following conditions be permanently deferred for blood donation; Is repeatedly reactive to screening tests for HBV, HCV, HIV, HTLV-I/II (on two independent donations), and has antibodies to core antigen of HBV (on two independent donations); Has a history of hepatitis since age years; Has a history of hemophilia or other inherited bleeding disease; Has a history of IV drug use; Has a history of Chagas' disease; Has a blood relative with Creutzfeldt-Jakob disease; Has received growth hormone of human pituitary origin or dura mater graft; Has lived in the United Kingdom for months or more between 0 and, or in Europe for years overall or more since 0; Has received a transfusion in the United Kingdom or France since 0; Has a history of hematologic cancer; Is a male who had sex with a male even once since ; or Has received money or drugs for sex. In addition to these permanent deferrals, there are also deferrals for specific time periods, which are determined by the risk factor and may be implemented at the medical director s discretion. Thus, if someone self-identifies as having had acupuncture, electrolysis, or a body piercing, they are deferred from donating blood for year. Even ear piercings, if not performed in a physician s office, may necessitate a -year deferral. Dental work may require a -day deferral while a root canal procedure may call for a -day deferral. Residual Risk of Contracting a Bloodborne Pathogen from a Blood Transfusion Despite these efforts, certain challenges to efficacy of the donor screening process remain. First, the potential donor must be able to fully understand the screening questions in order to answer them accurately. For example, it has been shown that donors have a varying range of definitions of sex that may be due to different concepts of risk activities. Second, there will always be some underlying level of unreported deferrable risk, with younger donors more likely to not report
5 CSAPH Rep. - A-0 -- page deferrable risk., Third, there is the risk of quarantine release errors, in which a unit of blood waiting for testing is accidentally released. Finally, despite donor screening, rare testing errors will occur, although some experts believe these to be so low in frequency as to be inconsequential. When all factors are considered, the risk that an infectious unit of blood will be undetected and enter the blood supply is called the residual risk. In the United States, this residual risk is currently estimated to be about in,,000 donations for HCV and in,,000 donations for HIV, with the combined use of NAT and serologic screening of donations., Hepatitis B virus residual risk, using a combination of anti-hepatitis B core and hepatitis B surface antigen testing, is about unit in 00,000 to 00,00 donations. NAT is available for HBV but is not required as a routine screen due to the marginal added benefit of its use with pooled donor samples. However, it is being performed under an investigational new drug (IND) application in selected blood centers. It is important to note that incidence rates for all these pathogens and for HTLV are twice as high in first-time donors, which emphasizes the importance of the testing process, even after donor deferral. At this time, the residual risk of West Nile virus is estimated to in 0,000 donation. While six cases of transfusion-associated transmission of West Nile virus have been identified since 00 (when minipool NAT was introduced), there have been no cases since individual donation NAT in endemic regions was implemented., Prevalence of the MSM Population in the United States Although few definitive reports exist on the prevalence of MSM in the US population, one carefully performed and frequently cited survey from reported that.% of males aged years or older self-identified as being homosexual or gay. Data from the General Social Surveys conducted between and 000 indicate the rate of MSM to be in the range of.% to.%. Prevalence of HIV and Other Bloodborne Pathogens in the MSM Population Surveillance data from the CDC indicate that three decades into the HIV epidemic, the MSM population comprised more than two-thirds (%) of all men living with HIV in 00, even though only about % to % of US men have reported having sex with other men. Additionally, data from the National HIV Behavioral Surveillance system suggest that HIV prevalence ranges from about % to 0%, with a median of % in this population. This translates to about 00,000 to 00,000 MSM who are infected with HIV. Significantly, % of the MSM who were HIV-positive were unaware of their infection, and more than half of those who were unaware had not had an HIV test in the previous year. Other reports suggest a lower HIV prevalence (about %) in the MSM population, perhaps reflecting differences in the sample populations studied,,0 with % of HIVinfected MSM unaware of their infection. However, the incidence of HIV in the MSM population remains fairly stable, ranging between % to % per year for those with high risk behaviors and % for those with low risk behaviors., With respect to HBV and HCV prevalence, while levels have declined over the last 0 years, the primary risk factors for infection have not. Thus, about % to 0% of MSM have markers of previous HBV infection, while about % have markers of HCV infection. - Incidence of HBV infection in the MSM population averages about %. Notably, HCV prevalence in the MSM population is no more than twice that of the general population, and with the high sensitivity of anti- HCV enzyme immunoassay and the redundancy of HCV NAT, deferral of blood donations from the MSM population plays at best a marginal role in preventing HCV transmission.
6 CSAPH Rep. - A-0 -- page With respect to HBV, it is important to recognize that % of HBV infections resolve, with an average window period of about 0 days. Thus, with any deferral policy that is greater than year following the high risk activity, the primary risk to the blood supply lies in those who are chronically infected. The prevalence of chronic HBV infection in the MSM population is about %. Even then, these donors would test positive with the two HBV antibody tests and thus the primary risk defaults to donation during the window period following infection. Of more recent relevance to the MSM population is human herpesvirus (HHV-), the causative agent for Kaposi s sarcoma. At the May 00 meeting of the Department of Health and Human Services (HHS) Advisory Committee on Blood Safety and Availability, it was reported that the prevalence of HHV- in HIV-negative MSM was about % to %. However, it appears that while HHV- may be transmitted via blood transfusions, the rate is about % to % of seropositive units., Finally, the prevalence of HHV- among the general population of donors is quite high (at least.%) and there are no reports of increased Kaposi's sarcoma incidence, even when many of these units are transfused into immunosuppressed patients. 0 Risk Assessment of the Donor Deferral Criteria for the MSM Population Several factors must be considered in any decision to change the current lifetime deferral criteria for blood donation for the MSM population. The first is whether a deferral standard can be created that would result in no significant increase in risk over the current lifetime deferral criteria. This makes the assumption that the US public would not accept any situation that would result in a blood supply that is not as safe as reasonably possible. The second is whether any change in the deferral criteria would increase donor numbers sufficiently to make a significant impact on the current blood supply. In this regard, the 00 Nationwide Blood Collection and Utilization Report indicates that while blood shortages were less frequent, when they did occur they were more acute, and some studies indicate that any change in deferral standards may only marginally improve recruitment of MSM donors. Third, ethical and societal issues must be considered and these include the perception of discrimination against the MSM population should deferral criteria not be supported by scientific data. Indeed, lawsuits are beginning to be filed against blood collection agencies for refusing to accept blood donations from the MSM population. Several studies (some unpublished, but presented at the FDA s March 00 Behavior-Based Donor Deferrals in the NAT Era meeting) have examined these issues. Leiss and colleagues examined different deferral criteria that included: () No MSM deferral; () change to a -year deferral period; () change to a -year deferral period; and () change to a -year deferral period. Their analysis concluded that with the current prevalence rates of HIV in the MSM population and the residual risk with the current deferral policy, there would be an unacceptable increase in risk should the MSM population no longer be deferred, thereby making the safety of the blood supply rely solely on blood testing. This finding is supported by a 00 study in Australia, which reported that those potential donors most likely to become infected with HIV and donate blood during the testing window period were MSM. The Leiss study also concluded that targeting blood donation deferral to a set of highrisk behaviors is not practical. In particular, such a practice would require the screening process to ask questions that focus directly and in detail on very sensitive and intimate sexual behavior, questions that many donors would find awkward to answer truthfully. Furthermore, behaviors change over time and this strategy would create many challenges for administrators of blood collection agencies. The example used by the authors is an individual who has previously donated but now declares a sexual behavior risk. A -Year Deferral Policy: Germain and co-workers have examined the impact on the US blood supply of a -year deferral policy. They calculate an % increase in HIV risk or additional HIV-
7 CSAPH Rep. - A-0 -- page contaminated unit for every,000 additional donations and estimate that the number of donations would increase by.%. The authors conclude that while the increased risk with a -year abstinence from blood donation from the last MSM contact would be very small, it is not zero. A study by Soldan and Sinka estimated that in the United Kingdom the risk of an HIV-infected unit being released to the public would increase by 0% with a policy change from lifetime deferral to a -year deferral from last MSM contact, reflecting an increase from the current risk of 0. per year to 0. per year. These authors also state that the increase in non-infected donations with such a policy shift would be small (perhaps % of current donations), and they favor maintaining permanent de-selection of MSM, irrespective of the risk of HIV-infectious donations. Finally, in 00, Andrew Dayton summarized to the HHS Advisory Committee on Blood Safety and Availability the findings from a March 00 FDA workshop on deferral of the MSM population., His mathematical modeling using data from the FDA s Biological Product Deviation Report suggested that a -year deferral policy would increase HIV risk by.% of the current risk. However, the same model using older data from New York state yielded an increased HIV risk of 0% of the current risk, which translates to an increase of about infectious units transfused (in this model, Dr. Dayton assumed a background residual risk of infected units). Analysis of such a policy change in 000 indicates that the pool of blood donors could be increased by,000. Leiss and colleagues suggest that similar to changing to a no-deferral policy, this small but scientifically real increase in risk is a clear violation of ethical principles and therefore not acceptable. However, testimony from Celso Bianco representing the AABB at the May 00 Advisory Committee on Blood Safety and Availability meeting argued that assumptions made in these studies have been too conservative; the AABB s analysis suggests that moving to a -year deferral policy would increase the number of HIV-infected donations being transfused by in million donations, or case every. years. A -Year Deferral Policy: If a - or -year deferral policy is considered, risk management calculations would yield risks at a level that many might consider acceptable. A study by Sanchez and colleagues found that compared to blood donors who did not report MSM contact, the prevalence of reactive screening test results was fivefold higher among those who reported the behavior within the past years. However, in those who last practiced male-to-male sex more than years ago, no significant difference was found. The authors suggest that a -year deferral following MSM contact may be a good starting point for consideration in changing blood donation deferral policy. At the March 00 FDA workshop, Andrew Dayton presented data indicating that a -year deferral policy would increase HIV risk by.% of the current risk. This is using the newer data from the FDA s Biological Product Deviation Report. Using the older New York state data yielded an estimate of increased HIV risk of % over the current residual risk (allowing more infectious components to be transfused). With respect to HHV-, the FDA estimates that changing the MSM deferral to to years would increase blood recipient exposure to HHV- by % to %. Michael Busch presented information indicating that with abstinence of less than months or for to years, the presence of positive infectious disease markers was to times that of the general donor population. However, with abstinence for years or longer, the marker rate was similar to that of the general donor population. Thus, data suggest that men who have abstained from sex with other men for more than years essentially present no greater risk than the general population.,, Additionally, at the May 00 Advisory Committee on Blood Safety and Availability meeting, data were presented indicating that a
8 CSAPH Rep. - A-0 -- page year deferral period would provide a temporal safety net that would allow unidentified pathogens that may emerge to be recognized before they enter the blood supply. A policy change consistent with these data was examined by the FDA in 000 and it was estimated that about,00 new donors would be added to the donor pool. In their risk management analysis, Leiss and co-workers suggest that while it is a matter of judgment as to whether a -year deferral period would pass the risk hurdle, it may be within the ballpark for discussion. They also suggest potential societal and ethical benefits from considering this policy change. These include the utilitarian benefit of potentially increasing the pool of blood donors, and the social benefit of reducing the perceived stigma associated with the MSM population. However, it must be noted that this remains controversial. Many argue that the rights of blood transfusion recipients outweigh any asserted rights of blood donors, that the right to receive safe blood is the overriding responsibility of blood collection agencies, and that there is no direct discrimination in the current lifetime deferral policy since the purpose of selection is to prevent virus infections, including HIV, with which the MSM population are disproportionately affected. A Perspective on Risk Assessment Any mathematical model for risk management can only provide an estimate of the potential risk. To put this into perspective, the residual risk that an HIV-infected unit of blood will enter the blood supply is estimated at about infected donation for every. million donations, which translates to a residual risk of about infected units every year there are about. million blood donations annually. However, it is clear that HIV-infected units do not enter the US blood supply annually undetected. In fact, since the implementation of NAT in, there have been four incidences where HIV has been transmitted via a blood transfusion, with the last in 00 (C. Bianco, America s Blood Centers, personal communication, April 00). In all four of these transmissions, the donors denied any risk factors at screening, rendering the length of donor deferral moot (J. MacPherson, America s Blood Centers, personal communication, April 00). In the eight years since the implementation of NAT, more than million units of whole blood/red blood cells, and about million total blood components, have been transfused annually. A rudimentary analysis would suggest that out of more than million whole blood units transfused, only resulted in HIV transmission. Clearly, this is far lower than is predicted by the risk models. Whether this is due to the lifetime deferral or to the fact that there is short, finite -day window period during which the risk of an infected donor s blood cannot be adequately tested, cannot be determined. In the absence of actual data to supplement the risk assessments, these risk assessments will only be as good as the assumptions used in the modeling. Indeed, this is a position echoed by the blood collection agencies. The Position of Blood Collection Agencies Blood collection agencies do not support the current lifetime deferral recommendation for men who have had sex with men even once since. In a statement submitted to the March 00 FDA Workshop on Behavior-Based Donor Deferrals in the NAT Era, the American Red Cross, America s Blood Centers, and the AABB stated that they believe that the current lifetime deferral for men who have had sex with other men is medically and scientifically unwarranted and recommend that deferral criteria be modified and made comparable with criteria for other groups at increased risk for sexual transmission of transfusion-transmitted infection. 0 These three organizations, which represent virtually all the blood collection agencies in the United States, also specify that they acknowledge the concern that relaxation of deferral criteria may increase the number of presenting donors who are marker positive. They go on to state, [h]owever, this impact has not been measured directly; it has only been modeled using what may be incomplete assumptions. The blood collectors are willing to assist in collecting data regarding the actual impact of changes in the
9 CSAPH Rep. - A-0 -- page 0 0 deferral, in order to allow for informed decision-making, and/or for the development of additional, appropriate interventions to ameliorate the impact. Conclusions Men who have had sex with men since are currently permanently deferred from blood donation. This FDA policy recommendation has generated controversy due concerns that it may be discriminatory and that it stigmatizes the MSM population. It is clear that a policy change with respect to blood donation deferral is a risk management decision wherein the risks of introducing additional infected units for transfusion over the current residual risk must be balanced against the benefits of increasing the pool of blood donors. Also important are ethical and societal factors, which this report does not address. Any policy decision on blood donation deferral of the MSM population must be governed by the best available scientific evidence but there are inherent weaknesses in mathemathical models used in the risk assessments on this issue that continue to generate some uncertainty. With respect to the MSM population, it appears that a policy change from a permanent lifetime deferral to a -year deferral following the last MSM contact may be supportable, but societal and ethical consequences must be analyzed should this decision be advanced. Such an analysis should include discussion of what society would consider acceptable risk with respect to safety of the blood supply, as that will determine to what extent a precautionary principle must be factored into any policy decision. Finally, should such a policy change occur, blood collection agencies must be marshaled to collect data that will provide actual data for future risk assessments to improve decision-making on this issue. RECOMMENDATION The Council on Science and Public Health recommends that the following statement be adopted in lieu of Resolution (A-0), and that the remainder of this report be filed: That our American Medical Association (AMA) recognize that based on existing scientific evidence and risk assessment models, a shift to a -year deferral policy for blood donation from men who have sex with men (MSM) is supportable. (New HOD Policy) Fiscal Note: $,00
10 CSAPH Rep. - A-0 -- page REFERENCES. Food and Drug Administration. Keeping Blood Transfusions Safe: FDA's Multi-layered Protections for Donated Blood. Available at: Accessed: Zou S, Fujii K, Johnson S, et al. Prevalence of selected viral infections among blood donors deferred for potential risk to blood safety. Transfusion. 00;:-00.. Tan L, Khan MK, Hawk JC, III. Use of blood therapeutically drawn from hemochromatosis patients. Council on Scientific Affairs, American Medical Association. Transfusion. ;:-.. Stramer SL, Glynn SA, Kleinman SH, et al. Detection of HIV- and HCV infections among antibody-negative blood donors by nucleic acid-amplification testing. N Engl J Med. 00;:0-.. Goodman JL. The safety and availability of blood and tissues--progress and challenges. N Engl J Med. 00;:-.. American Association of Blood Banks. Blood Donor History Questionnaire. Available at: Donor_History_Questionnaire. Accessed: O'Brien SF, Ram SS, Yi QL, Goldman M. Donor's understanding of the definition of sex as applied to predonation screening questions. Vox Sang. 00;. Damesyn MA, Glynn SA, Schreiber GB, et al. Behavioral and infectious disease risks in young blood donors: implications for recruitment. Transfusion. 00;:-0.. Van der Bij AK, Coutinho RA, Van der Poel CL. Surveillance of risk profiles among new and repeat blood donors with transfusion-transmissible infections from through 00 in the Netherlands. Transfusion. 00;:-.. Leiss W, Tyshenko M, Krewski D. Men having sex with men donor deferral risk assessment: an analysis using risk management principles. Transfus Med Rev. 00;:-.. Dodd RY, Notari EP, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 00;:-.. Stramer SL. Current risks of transfusion-transmitted agents: a review. Arch Pathol Lab Med. 00;:0-0.. Bihl F, Castelli D, Marincola F, Dodd RY, Brander C. Transfusion-transmitted infections. J Transl Med. 00;:. West Nile virus transmission through blood transfusion--south Dakota, 00. MMWR Morb Mortal Wkly Rep. 00;:-.
11 CSAPH Rep. - A-0 -- page. Laumann EO, Gagnon JH, Michael RT, Michaels S. The Social Organization of Sexuality in the United States. Chicago: University of Chicago Press;.. Anderson JE, Stall R. Increased reporting of male-to-male sexual activity in a national survey. Sex Transm Dis. 00;:-.. Centers for Disease Control and Prevention. Cases of HIV infection and AIDS in the United States and Dependent Areas, 00. Available at: Accessed: Sifakis F, Flynn CP, Metsch LR, et al. HIV Prevalence, Unrecognized Infection, and HIV Testing Among Men Who Have Sex with Men --- Five U.S. Cities, June 00--April 00. MMWR. 00;:-0.. Pollack LM, Osmond DH, Paul JP, Catania JA. Evaluation of the Center for Disease Control and Prevention's HIV behavioral surveillance of men who have sex with men: sampling issues. Sex Transm Dis. 00;:-. 0. McQuillan GM, Kruszon-Moran D, Kottiri BJ, et al. Prevalence of HIV in the US household population: the National Health and Nutrition Examination Surveys, to 00. J Acquir Immune Defic Syndr. 00;:-.. Unrecognized HIV infection, risk behaviors, and perceptions of risk among young black men who have sex with men--six U.S. cities, -. MMWR Morb Mortal Wkly Rep. 00;:-.. McFarland W, Stoyanoff SR, Shehan D, et al. HIV Incidence Among Young Men Who Have Sex With Men -- Seven U.S. Cities, MMWR. Morb Mortal Wkly Rep. 00;0:0-.. Food and Drug Administration. Behavior-Based Donor Deferrals in the NAT Era. Available at: Accessed: Rhodes SD, DiClemente RJ, Yee LJ, Hergenrather KC. Hepatitis B vaccination in a high risk MSM population: the need for vaccine education. Sex Transm Infect. 000;:0-0.. Remis RS, Dufour A, Alary M, et al. Association of hepatitis B virus infection with other sexually transmitted infections in homosexual men. Omega Study Group. Am J Public Health. 000;0:0-.. Wasley A, Miller JT, Finelli L. Surveillance for acute viral hepatitis--united States, 00. MMWR Surveill Summ. 00;:-.. Dayton, A. I. Deferral of MSM: Policy Analysis. Available at: Accessed: Department of Health and Human Services. Blood Safety and Availability Advisory Committee May 00 meeting transcript. Available at: Accessed: --00.
12 CSAPH Rep. - A-0 -- page. Hladik W, Dollard SC, Mermin J, et al. Transmission of human herpesvirus by blood transfusion. N Engl J Med. 00;:-. 0. America's Blood Centers. Study Finds Evidence of Transmission of HHV- by Transfusion. ABC Newsletter. 00;:-.. Department of Health and Human Services. 00 Nationwide Blood Collection and Utilization Survey. Available at: Accessed: Spencer B, Rios J, Cable R. Marginal recruitment impact of potential alterations to FDA deferral criteria for men who have sex with men. Transfusion. 00;:-0.. Garmaise D. Gay man launches suit over refusal to accept blood donation. HIV AIDS Policy Law Rev. 00;:0-.. Musto JA, Seed CR, Law M, Keller AJ, Kaldor JM. Estimating the risk of blood donation associated with HIV risk behaviours. Transfus Med. 00;:-.. Germain M, Remis RS, Delage G. The risks and benefits of accepting men who have had sex with men as blood donors. Transfusion. 00;:-.. Soldan K, Sinka K. Evaluation of the de-selection of men who have had sex with men from blood donation in England. Vox Sang. 00;:-.. Sanchez AM, Schreiber GB, Nass CC, et al. The impact of male-to-male sexual experience on risk profiles of blood donors. Transfusion. 00;:0-.. Brooks JP. The rights of blood recipients should supersede any asserted rights of blood donors. Vox Sang. 00;:0-.. Franklin IM. Is there a right to donate blood? Patient rights; donor responsibilities. Transfus Med. 00;:-. 0. Kleinman S: Behavior-Based Blood Donors Deferrals in the Era of Nucleic Acid Testing (NAT): AABB Statement presented at the FDA Behavior-Based Blood Donors Deferrals in the Era of Nucleic Acid Testing (NAT) meeting, 00.
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P
 The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations Jackson B R, Busch M P, Stramer S L, AuBuchon J P Record Status This is a critical abstract of an economic evaluation that meets
MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE BACKGROUND
 MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE Countries around the world are reviewing the policy of deferring from blood donation men who have had sex with men (MSM). Both Canadian Blood Services
MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE Countries around the world are reviewing the policy of deferring from blood donation men who have had sex with men (MSM). Both Canadian Blood Services
[Submitted Electronically]
![[Submitted Electronically] [Submitted Electronically]](/thumbs/82/85246007.jpg) [Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
[Submitted Electronically] Dr. Matthew J. Kuehnert, Director Office of Blood, Organ, and Other Tissue Safety Division of Healthcare Quality Promotion National Center for Emerging and Zoonotic Infectious
Changing eligibility criteria for MSM: The Canadian Perspective
 Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
Changing eligibility criteria for MSM: The Canadian Perspective Mindy Goldman MD Canadian Blood Services ASFA & WAA Joint Meeting, San Francisco April 4, 2014 Outline - Historical background - International
CANADIAN HEMOPHILIA SOCIETY POSITION: CURRENT DONOR DEFERRAL CRITERIA ARE IN THE BEST INTERESTS OF BLOOD SAFETY
 CANADIAN HEMOPHILIA SOCIETY POSITION: CURRENT DONOR DEFERRAL CRITERIA ARE IN THE BEST INTERESTS OF BLOOD SAFETY The Canadian Hemophilia Society (CHS) supports the current Canadian regulations regarding
CANADIAN HEMOPHILIA SOCIETY POSITION: CURRENT DONOR DEFERRAL CRITERIA ARE IN THE BEST INTERESTS OF BLOOD SAFETY The Canadian Hemophilia Society (CHS) supports the current Canadian regulations regarding
Guidance for Industry
 Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
Current Infectious Disease Screening for the Live Organ Donor
 2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
Change in deferrals for men who have sex with men: Assessing the risk of HIV transmission by transfusion
 Change in deferrals for men who have sex with men: Assessing the risk of HIV transmission by transfusion Josiane Pillonel, Rachid Djoudi, Florence Lot, Chistophe Martinaud, Claire Sauvage, Marie Jauffret-Roustide,
Change in deferrals for men who have sex with men: Assessing the risk of HIV transmission by transfusion Josiane Pillonel, Rachid Djoudi, Florence Lot, Chistophe Martinaud, Claire Sauvage, Marie Jauffret-Roustide,
Surveillance Report 2014
 Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
Policy Document. Blood Donation Deferral. Background
 Policy Document Blood Donation Deferral Background The Australian Medical Students Association (AMSA) is the peak representative body for medical students in Australia. Accordingly, AMSA advocates on issues
Policy Document Blood Donation Deferral Background The Australian Medical Students Association (AMSA) is the peak representative body for medical students in Australia. Accordingly, AMSA advocates on issues
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
July 14, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
July 14, 2015 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2015-D-1211
Surveillance Report 2015
 Surveillance Report 2015 Executive summary We are pleased to present the fourth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2015 Executive summary We are pleased to present the fourth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
ANNALS OF HEALTH LAW Advance Directive VOLUME 23 SPRING 2014 PAGES
 ANNALS OF HEALTH LAW Advance Directive VOLUME 23 SPRING 2014 PAGES 101-112 United States Blood Donor Policy on Gay Men: Adopting an Italian Individual Risk Assessment Policy Melissa Kong* I. INTRODUCTION
ANNALS OF HEALTH LAW Advance Directive VOLUME 23 SPRING 2014 PAGES 101-112 United States Blood Donor Policy on Gay Men: Adopting an Italian Individual Risk Assessment Policy Melissa Kong* I. INTRODUCTION
In the United States and in Canada, as in most other
 BLOOD DONORS AND BLOOD COLLECTION The risks and benefits of accepting men who have had sex with men as blood donors M. Germain, R.S. Remis, and G. Delage BACKGROUND: It has been suggested that men who
BLOOD DONORS AND BLOOD COLLECTION The risks and benefits of accepting men who have had sex with men as blood donors M. Germain, R.S. Remis, and G. Delage BACKGROUND: It has been suggested that men who
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services
 Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
transfusion-transmissible HCV and HIV in Italy Haemovigilance and the residual risk of
 Haemovigilance and the residual risk of transfusion-transmissible Giuliano Grazzini, MD National Blood Centre, National Institute of Health, Rome, Italy & International Federation of Blood Donor Organizations
Haemovigilance and the residual risk of transfusion-transmissible Giuliano Grazzini, MD National Blood Centre, National Institute of Health, Rome, Italy & International Federation of Blood Donor Organizations
MSM DONOR POLICY: STUDIES FROM THE USA AND OTHER COUNTRIES. Brian Custer IPFA PEI Workshop Rome 22 May 2014
 MSM DONOR POLICY: STUDIES FROM THE USA AND OTHER COUNTRIES Brian Custer IPFA PEI Workshop Rome 22 May 2014 Financial Disclosure Nothing to disclose International Context for Blood Donation by MSM Country
MSM DONOR POLICY: STUDIES FROM THE USA AND OTHER COUNTRIES Brian Custer IPFA PEI Workshop Rome 22 May 2014 Financial Disclosure Nothing to disclose International Context for Blood Donation by MSM Country
HEALTH SCREENING QUESTIONNAIRE. Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors
 Journal of the Egyptian Society of Haematology & Research, Vol. 7, No. 2, September: 1-5, 2011 Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors HISHAM
Journal of the Egyptian Society of Haematology & Research, Vol. 7, No. 2, September: 1-5, 2011 Routine Use of Mini-Pool Nucleic Acid Testing (MP-NAT) Multiplex Assay for Sero-Negative Blood Donors HISHAM
Epidemiology of Acute Hepatitis C Infection in Canada Results from the Enhanced Hepatitis Strain Surveillance System (EHSSS)
 Epidemiology of Acute Hepatitis C Infection in Canada Results from the Enhanced Hepatitis Strain Surveillance System (EHSSS) At a Glance Reported rates of acute HCV declined from.5 per, population in to.
Epidemiology of Acute Hepatitis C Infection in Canada Results from the Enhanced Hepatitis Strain Surveillance System (EHSSS) At a Glance Reported rates of acute HCV declined from.5 per, population in to.
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS
 EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
R Offergeld, D Faensen, S Ritter, O Hamouda Department for Infectious Disease Epidemiology, Robert Koch Institute, Berlin, Germany
 Eurosurveillance, Volume 10, Issue 2, 01 February 2005 Surveillance report HUMAN IMMUNODEFICIENCY VIRUS, HEPATITIS C AND HEPATITIS B INFECTIONS AMONG BLOOD DONORS IN GERMANY 2000-2002: RISK OF VIRUS TRANSMISSION
Eurosurveillance, Volume 10, Issue 2, 01 February 2005 Surveillance report HUMAN IMMUNODEFICIENCY VIRUS, HEPATITIS C AND HEPATITIS B INFECTIONS AMONG BLOOD DONORS IN GERMANY 2000-2002: RISK OF VIRUS TRANSMISSION
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey
 Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Source Plasma Safety
 Source Plasma Safety Plasma May 15-19, 2017 George Schreiber, ScD, PPTA Director, Epidemiology Three components: Plasma Product Safety Donor Quality Manufacturing Pool Quality Fractionation Process Safe
Source Plasma Safety Plasma May 15-19, 2017 George Schreiber, ScD, PPTA Director, Epidemiology Three components: Plasma Product Safety Donor Quality Manufacturing Pool Quality Fractionation Process Safe
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
HEALTH SCREENING QUESTIONNAIRE CT Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
patients with blood borne viruses Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled Document Lead:
 CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
Directed Semen Donor Eligibility Requirements
 Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
EDMA HIV-AIDS TEAM Fact Sheet November 2007
 EDMA HIV-AIDS TEAM Fact Sheet November 2007 1. HIV Facts AIDS epidemic update UNAIDS Epidemic Update, November 2007 (1) 760,000 people to be living with HIV in Western and Central Europe in 2007. 31,000
EDMA HIV-AIDS TEAM Fact Sheet November 2007 1. HIV Facts AIDS epidemic update UNAIDS Epidemic Update, November 2007 (1) 760,000 people to be living with HIV in Western and Central Europe in 2007. 31,000
A. Anti-HIV-1/2, HIV NAT Lookback: for those identified blood and blood components collected:
 PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
PURPOSE: MATERIALS: To outline a procedure for removing, recalling and/or correcting blood products that do not meet the requirements of the FDA, AABB, or the blood center. Recall/Market Withdrawal Form
BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL?
 BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL? What if your patients want to donate blood? Are you aware that patients undergoing Acupuncture treatment, according to international guidelines,
BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL? What if your patients want to donate blood? Are you aware that patients undergoing Acupuncture treatment, according to international guidelines,
Surveillance Report 2016
 Surveillance Report 2016 Executive summary We are pleased to present the fifth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2016 Executive summary We are pleased to present the fifth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Canada s Blood Donation Eligibility Criteria for Men Who Have Sex with Men
 Criteria for Men Who Have Sex with Men Dr. Graham D. Sher Chief Executive Officer, Canadian Blood Services Dr. Dana Devine Chief Medical and Scientific Officer, Canadian Blood Services Executive summary
Criteria for Men Who Have Sex with Men Dr. Graham D. Sher Chief Executive Officer, Canadian Blood Services Dr. Dana Devine Chief Medical and Scientific Officer, Canadian Blood Services Executive summary
Increased Risk Donors for Organ Transplantation
 A Tool Kit to Assist Transplant Programs in the Use of Increased Risk Donors for Organ Transplantation February 2016 Table of Contents Table of Contents... 2 1.0 Increased Risk Donor Tool Kit... 3 1.1
A Tool Kit to Assist Transplant Programs in the Use of Increased Risk Donors for Organ Transplantation February 2016 Table of Contents Table of Contents... 2 1.0 Increased Risk Donor Tool Kit... 3 1.1
Source of effectiveness data Effectiveness data were derived from a single study combined with a review of previously completed studies.
 Declining value of alanine aminotransferase in screening of blood donors to prevent posttransfusion hepatitis B and C virus infection Busch M P, Korelitz J J, Kleinman S H, Lee S R, Aubuchon J P, Schreiber
Declining value of alanine aminotransferase in screening of blood donors to prevent posttransfusion hepatitis B and C virus infection Busch M P, Korelitz J J, Kleinman S H, Lee S R, Aubuchon J P, Schreiber
Public Health, Infections and Transplantation
 Public Health, Infections and Transplantation Dr Kerry Chant Chief Health Officer & Deputy Director General Population and Public Health NSW Ministry of Health May2014 Public Health Infection and Transplantation
Public Health, Infections and Transplantation Dr Kerry Chant Chief Health Officer & Deputy Director General Population and Public Health NSW Ministry of Health May2014 Public Health Infection and Transplantation
Guidance for Industry
 Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Transfusion-transmitted Cytomegalovirus
 Transfusion-transmitted Cytomegalovirus Can you confidently abandon CMV seronegative products in the modern era of pre-storage leukoreduction? Jeannie Callum, BA, MD, FRCPC Really? Are we still talking
Transfusion-transmitted Cytomegalovirus Can you confidently abandon CMV seronegative products in the modern era of pre-storage leukoreduction? Jeannie Callum, BA, MD, FRCPC Really? Are we still talking
Process or performance improvement is often discussed
 Process Improvement in Transfusion Medicine Ongoing efforts to decrease costs in the clinical laboratory make continuous process improvement especially important in difficult economic times. Process improvement
Process Improvement in Transfusion Medicine Ongoing efforts to decrease costs in the clinical laboratory make continuous process improvement especially important in difficult economic times. Process improvement
Virtual Mentor Ethics Journal of the American Medical Association September 2005, Volume 7, Number 9
 Virtual Mentor Ethics Journal of the American Medical Association September 2005, Volume 7, Number 9 The Living Code The Power of the Code by Andrew Nelson The American Medical Association s Code of Medical
Virtual Mentor Ethics Journal of the American Medical Association September 2005, Volume 7, Number 9 The Living Code The Power of the Code by Andrew Nelson The American Medical Association s Code of Medical
Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States
 545 CONCISE COMMUNICATION Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States Carolyn Gardella, 1,2 Anthony A. Marfin, 1,a Richard H. Kahn, 1 Emmett Swint,
545 CONCISE COMMUNICATION Persons with Early Syphilis Identified through Blood or Plasma Donation Screening in the United States Carolyn Gardella, 1,2 Anthony A. Marfin, 1,a Richard H. Kahn, 1 Emmett Swint,
REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH. AMA Policy Consolidation: Influenza and Influenza Vaccine
 REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH CSAPH Report 5-I-12 Subject: Presented by: Referred to: AMA Policy Consolidation: Influenza and Influenza Vaccine Sandra A. Fryhofer, MD, Chair Reference
REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH CSAPH Report 5-I-12 Subject: Presented by: Referred to: AMA Policy Consolidation: Influenza and Influenza Vaccine Sandra A. Fryhofer, MD, Chair Reference
Anitha M.*, Sreedhar Babu K. V., Praveen M. D., Sriranjitha T. V. N.
 International Journal of Research in Medical Sciences Anitha M et al. Int J Res Med Sci. 2017 Aug;5(8):3438-3442 www.msjonline.org pissn 2320-6071 eissn 2320-6012 Original Research Article DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20173536
International Journal of Research in Medical Sciences Anitha M et al. Int J Res Med Sci. 2017 Aug;5(8):3438-3442 www.msjonline.org pissn 2320-6071 eissn 2320-6012 Original Research Article DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20173536
Objectives. Methods. Results. Economic
 Octaplas Compared with Fresh Frozen Plasma to Reduce the Risk of Transmitting Lipid-Enveloped Viruses: An Economic Analysis and Budget Impact Analysis [Adapted from Membe SK, Coyle D, Husereau D, Cimon
Octaplas Compared with Fresh Frozen Plasma to Reduce the Risk of Transmitting Lipid-Enveloped Viruses: An Economic Analysis and Budget Impact Analysis [Adapted from Membe SK, Coyle D, Husereau D, Cimon
Question: 1. Are you currently taking an antibiotic?
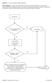 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Zika Virus. Report to the Standing Committee on Health. Dr. Graham Sher Chief Executive Officer, Canadian Blood Services
 Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Version for the Silent Procedure 29 April Agenda item January Hepatitis
 Version for the Silent Procedure 29 April 2014 134th session EB134.R18 Agenda item 10.5 25 January 2014 Hepatitis The Executive Board, Having considered the report on hepatitis, 1 RECOMMENDS to the Sixty-seventh
Version for the Silent Procedure 29 April 2014 134th session EB134.R18 Agenda item 10.5 25 January 2014 Hepatitis The Executive Board, Having considered the report on hepatitis, 1 RECOMMENDS to the Sixty-seventh
Question: 1. Are you currently taking an antibiotic?
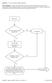 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Detection of HIV-1 and HCV Infections among Antibody-Negative Blood Donors by Nucleic Acid Amplification Testing
 The new england journal of medicine original article Detection of HIV-1 and HCV Infections among Antibody-Negative Blood Donors by Nucleic Acid Amplification Testing Susan L. Stramer, Ph.D., Simone A.
The new england journal of medicine original article Detection of HIV-1 and HCV Infections among Antibody-Negative Blood Donors by Nucleic Acid Amplification Testing Susan L. Stramer, Ph.D., Simone A.
RISK OF DISEASE TRANSMISSION FROM ORGAN DONORS PATIENT INFORMATION GUIDE
 Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
DHMH Activities toward Implementing Requirements of Md. Code Ann., Health-General , Hepatitis C Prevention and Control within Maryland
 DHMH Activities toward Implementing Requirements of Md. Code Ann., Health-General 18-1001, Hepatitis C Prevention and Control within Maryland Submitted by: Maryland Department of Health and Mental Hygiene
DHMH Activities toward Implementing Requirements of Md. Code Ann., Health-General 18-1001, Hepatitis C Prevention and Control within Maryland Submitted by: Maryland Department of Health and Mental Hygiene
SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification
 SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification Hepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States.
SUBJECT: Hepatitis C Virus (HCV) Counseling/Education, Testing, Referral, and Partner Notification Hepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States.
Original article J Bas Res Med Sci 2017; 4(2):4-8.
 Prevalence of HIV, hepatitis B and C infections among volunteer blood donors at the blood transfusion center of Ilam city, Iran Hasan Boustani 1, Enayat Anvari 2, Sedighe Saiadi Sartang 2, Mehdi Omidi
Prevalence of HIV, hepatitis B and C infections among volunteer blood donors at the blood transfusion center of Ilam city, Iran Hasan Boustani 1, Enayat Anvari 2, Sedighe Saiadi Sartang 2, Mehdi Omidi
Provincial Infectious Diseases Advisory Committee Public Health Response to Hepatitis C
 Provincial Infectious Diseases Advisory Committee Public Health Response to Hepatitis C Dr. Doug Sider, Medical Director, Public Health Ontario Dr. Peggy Millson, Professor Emeritus, Dalla Lana School
Provincial Infectious Diseases Advisory Committee Public Health Response to Hepatitis C Dr. Doug Sider, Medical Director, Public Health Ontario Dr. Peggy Millson, Professor Emeritus, Dalla Lana School
Felix Yao DOCTOR OF PHILOSOPHY
 Investigation on the Risk of Viral Infection in Musculoskeletal Grafts Felix Yao The collection of these publications is presented for the degree of DOCTOR OF PHILOSOPHY of The University of Western Australia
Investigation on the Risk of Viral Infection in Musculoskeletal Grafts Felix Yao The collection of these publications is presented for the degree of DOCTOR OF PHILOSOPHY of The University of Western Australia
APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION*
 11 APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION* Hisami IKEDA** Asian Med. J. 44(1): 11 21, 2000 Abstract: In the field of blood transfusion, risk management is defined
11 APPROACH TO RISK MANAGEMENT IN MEDICAL PRACTICE: STANDPOINT OF THE BLOOD TRASFUSION* Hisami IKEDA** Asian Med. J. 44(1): 11 21, 2000 Abstract: In the field of blood transfusion, risk management is defined
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
LL - LL-ROHINI (NATIONAL REFERENCE 135091650 Age 49 Years Gender Male 29/8/2017 120000AM 29/8/2017 100248AM 29/8/2017 105306AM Ref By Final HEATITIS, VIRAL, COMREHENSIVE ANEL HEATITIS A ANTIBODY (ANTI
Guidance for Industry
 Reprinted from FDA s website by EAS Consulting Group, LLC Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening
Reprinted from FDA s website by EAS Consulting Group, LLC Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening
CORD BLOOD TRANSPLANTATION STUDY CBU EXCLUSION AND QUARANTINE RELEASE FORM
 (Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
(Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil,
 236 BJID 2004; 8 (June) Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil, 1991-2001 Emil Kupek Department of Public Health, Federal University of Santa Catarina,
236 BJID 2004; 8 (June) Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil, 1991-2001 Emil Kupek Department of Public Health, Federal University of Santa Catarina,
Overview of Blood Transfusion System of Iran:
 Report Article Overview of Blood Transfusion System of Iran: 2002-2011 AM Cheraghali High Institute for Research and Education on Transfusion Medicine, Iran Blood Transfusion Organization and University
Report Article Overview of Blood Transfusion System of Iran: 2002-2011 AM Cheraghali High Institute for Research and Education on Transfusion Medicine, Iran Blood Transfusion Organization and University
Viral Hepatitis. WHO Regional Office for Europe July 2013
 Viral Hepatitis WHO Regional Office for Europe July 2013 What is Hepatitis? Hepatitis is a viral infection that causes inflammation of the liver There are five main types of viral hepatitis: A, B, C, D,
Viral Hepatitis WHO Regional Office for Europe July 2013 What is Hepatitis? Hepatitis is a viral infection that causes inflammation of the liver There are five main types of viral hepatitis: A, B, C, D,
The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood. Liz Caffrey, Patricia Hewitt and John Barbara
 CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
REPORT TO THE NZ BLOOD SERVICE: BEHAVIOURAL DONOR DEFERRAL CRITERIA REVIEW
 REPORT TO THE NZ BLOOD SERVICE: BEHAVIOURAL DONOR DEFERRAL CRITERIA REVIEW February 2014 Contents Preface... 5 Consultation: issues and responses... 5 Executive Summary... 9 Background... 9 Relevant issues...
REPORT TO THE NZ BLOOD SERVICE: BEHAVIOURAL DONOR DEFERRAL CRITERIA REVIEW February 2014 Contents Preface... 5 Consultation: issues and responses... 5 Executive Summary... 9 Background... 9 Relevant issues...
Draft Agreed by Biologics Working Party December Draft Agreed by Blood Products Working party December 2010
 15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
Breastfeeding Center Milk Connection Questionnaire and Release for Donor
 Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
The Alphabet Soup of Viral Hepatitis Testing
 The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
Government of Canada Federal AIDS Initiative Milestones
 HIV in Canada: Trends and Issues for Advancing Prevention, Care, Treatment and Support Through Knowledge Exchange Michael R Smith, Senior Policy Advisor, Programs and Coordination Division, Centre for
HIV in Canada: Trends and Issues for Advancing Prevention, Care, Treatment and Support Through Knowledge Exchange Michael R Smith, Senior Policy Advisor, Programs and Coordination Division, Centre for
Pathogen Safety and BSE / variant CJD
 Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Screening the Blood Supply for West Nile Virus RNA by Nucleic Acid Amplification Testing
 The new england journal of medicine original article Screening the Blood Supply for West Nile Virus RNA by Nucleic Acid Amplification Testing Michael P. Busch, M.D., Ph.D., Sally Caglioti, M.T.(A.S.C.P.),
The new england journal of medicine original article Screening the Blood Supply for West Nile Virus RNA by Nucleic Acid Amplification Testing Michael P. Busch, M.D., Ph.D., Sally Caglioti, M.T.(A.S.C.P.),
Outbreak Investigation Guidance for Vectorborne Diseases
 COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
Khalid Alquthami (Correspondence) Regional Lab, Makkah. Saudi Arabia
 1 Regional Lab, Makkah. Saudi Arabia stract: Clinical specificity and genotype/subtype detection of viruses using the Cobas TaqScreen MPX system V 2.0, which is a nucleic acid test (NAT) that uses multiples
1 Regional Lab, Makkah. Saudi Arabia stract: Clinical specificity and genotype/subtype detection of viruses using the Cobas TaqScreen MPX system V 2.0, which is a nucleic acid test (NAT) that uses multiples
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
Update on Transfusion- Transmitted Infectious Diseases
 Update on Transfusion- Transmitted Infectious Diseases Scott Koepsell MD PhD Associate Professor University of Nebraska Medical Center I have no conflicts of interest to disclose. Global changes and the
Update on Transfusion- Transmitted Infectious Diseases Scott Koepsell MD PhD Associate Professor University of Nebraska Medical Center I have no conflicts of interest to disclose. Global changes and the
New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 2016
 New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 6 Table of Contents. Introduction.... Methodology... 3. Data Limitations.... Definitions used... 3 5. Overview of STBBI epidemiology
New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 6 Table of Contents. Introduction.... Methodology... 3. Data Limitations.... Definitions used... 3 5. Overview of STBBI epidemiology
BLOOD COMPONENTS are regulated as
 Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More
Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More
The HIV Medicine Association (HIVMA) of the Infectious Diseases Society of America (IDSA)
 Written Testimony for the Record to the House Subcommittee on Labor, Health and Human Services, Education and Related Agencies regarding FY 2018 Appropriations for HIV/AIDS Programs Submitted by Dr. Wendy
Written Testimony for the Record to the House Subcommittee on Labor, Health and Human Services, Education and Related Agencies regarding FY 2018 Appropriations for HIV/AIDS Programs Submitted by Dr. Wendy
Maintaining a Safe and Adequate Blood Supply in the Event of Pandemic Influenza. Guidelines for National Blood Transfusion Services
 Maintaining a Safe and Adequate Blood Supply in the Event of Pandemic Influenza Guidelines for National Blood Transfusion Services 19 May 2006 1 Rationale Current global concern that an occurrence of pandemic
Maintaining a Safe and Adequate Blood Supply in the Event of Pandemic Influenza Guidelines for National Blood Transfusion Services 19 May 2006 1 Rationale Current global concern that an occurrence of pandemic
Case 1. FDA, Legal, and EBAA Medical Standards Panel. Case 1. Case 1 6/25/2012. Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies
 Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Hepatitis C Best Practice Guidelines For Local Health Departments
 Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Babesia from a donor perspective
 Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Exploring the risks of liver cancer after successful treatment for hepatitis C virus
 CATIE-News CATIE s bite-sized HIV and hepatitis C news bulletins. Exploring the risks of liver cancer after successful treatment for hepatitis C virus 11 June 2013 In Canada and other high-income countries,
CATIE-News CATIE s bite-sized HIV and hepatitis C news bulletins. Exploring the risks of liver cancer after successful treatment for hepatitis C virus 11 June 2013 In Canada and other high-income countries,
Research Article Decreasing Prevalence of Transfusion Transmitted Infection in Indian Scenario
 e Scientific World Journal, Article ID 173939, 4 pages http://dx.doi.org/10.1155/2014/173939 Research Article Decreasing Prevalence of Transfusion Transmitted Infection in Indian Scenario Tulika Chandra,
e Scientific World Journal, Article ID 173939, 4 pages http://dx.doi.org/10.1155/2014/173939 Research Article Decreasing Prevalence of Transfusion Transmitted Infection in Indian Scenario Tulika Chandra,
2017 CST-Astellas Canadian Transplant Fellows Symposium. Optimizing use of organs from Increased Risk Donors
 2017 CST-Astellas Canadian Transplant Fellows Symposium Optimizing use of organs from Increased Risk Donors Atual Humar, MD Atul Humar is a Professor in the Department of Medicine, University of Toronto.
2017 CST-Astellas Canadian Transplant Fellows Symposium Optimizing use of organs from Increased Risk Donors Atual Humar, MD Atul Humar is a Professor in the Department of Medicine, University of Toronto.
Title: Revision of the Surveillance Case Definition for HIV Infection and AIDS Among children age > 18 months but < 13 years
 06-ID-02 Committee: Infectious Disease Title: Revision of the Surveillance Case Definition for HIV Infection and AIDS Among children age > 18 months but < 13 years Statement of problem: Advances in HIV
06-ID-02 Committee: Infectious Disease Title: Revision of the Surveillance Case Definition for HIV Infection and AIDS Among children age > 18 months but < 13 years Statement of problem: Advances in HIV
Report on Donor Selection Criteria Relating to Men Who Have Sex with Men
 Report on Donor Selection Criteria Relating to Men Who Have Sex with Men June 23, 2015 Table of Contents Executive Summary 3 Glossary 5 Abbreviations 6 1. Introduction 7 2. Known infectious risks 7 Table
Report on Donor Selection Criteria Relating to Men Who Have Sex with Men June 23, 2015 Table of Contents Executive Summary 3 Glossary 5 Abbreviations 6 1. Introduction 7 2. Known infectious risks 7 Table
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091608 Age 26 Years Gender Female 30/8/2017 92900AM 30/8/2017 94717AM 31/8/2017 90216AM Ref By Final HEATITIS ACUTE VIRUS CONFIRMATION HEATITIS A ANTIBODY (ANTI HAV),
LL - LL-ROHINI (NATIONAL REFERENCE 135091608 Age 26 Years Gender Female 30/8/2017 92900AM 30/8/2017 94717AM 31/8/2017 90216AM Ref By Final HEATITIS ACUTE VIRUS CONFIRMATION HEATITIS A ANTIBODY (ANTI HAV),
PUBLICATION OF THE SUPERIOR HEALTH COUNCIL No. 9291
 PUBLICATION OF THE SUPERIOR HEALTH COUNCIL No. 9291 Sexual risk behaviour and blood donation Part I: Blood donations by MSM This version was validated by the Board on October 2016 1 This advisory report
PUBLICATION OF THE SUPERIOR HEALTH COUNCIL No. 9291 Sexual risk behaviour and blood donation Part I: Blood donations by MSM This version was validated by the Board on October 2016 1 This advisory report
seroprevalence of infectious markers & their trends in blood donors in a hospital based blood bank in north india
 Indian J Med Res 142, September 2015, pp 317-322 DOI:1103/0971-5916.166598 seroprevalence of infectious markers & their trends in blood donors in a hospital based blood bank in north india R.N. Makroo,
Indian J Med Res 142, September 2015, pp 317-322 DOI:1103/0971-5916.166598 seroprevalence of infectious markers & their trends in blood donors in a hospital based blood bank in north india R.N. Makroo,
Improving Utilization of Organs from Increased Risk Donors. Matthew J. Kuehnert, MD CDC
 Improving Utilization of Organs from Increased Risk Donors Matthew J. Kuehnert, MD CDC Conflict of Interest Disclosure I have no relevant financial relationships to disclose. 2016 AST Improving Utilization
Improving Utilization of Organs from Increased Risk Donors Matthew J. Kuehnert, MD CDC Conflict of Interest Disclosure I have no relevant financial relationships to disclose. 2016 AST Improving Utilization
risk of Transfusion-Transmitted Transmitted Viral Infections (HIV, HCV and HBV)
 Prevention and ResidualR risk of Transfusion-Transmitted Transmitted Viral Infections (HIV, HCV and HBV) Syria Laperche L (Centre National de référence r rence pour les hépatites h B et C en Transfusion)
Prevention and ResidualR risk of Transfusion-Transmitted Transmitted Viral Infections (HIV, HCV and HBV) Syria Laperche L (Centre National de référence r rence pour les hépatites h B et C en Transfusion)
Frequency of occult hepatitis B in HBsAg seronegative blood donors in a tertiary care hospital in kerala,south India.
 Frequency of occult hepatitis B in HBsAg seronegative blood donors in a tertiary care hospital in kerala,south India. Cinzia Keechilot, Veena Shenoy 1,V Anil kumar 2,Lalita Biswas 3. MBBS student * Transfusion
Frequency of occult hepatitis B in HBsAg seronegative blood donors in a tertiary care hospital in kerala,south India. Cinzia Keechilot, Veena Shenoy 1,V Anil kumar 2,Lalita Biswas 3. MBBS student * Transfusion
Taken From VBA s Adjudication Procedure Manual Section on Hepatitis
 Taken From VBA s Adjudication Procedure Manual Section on Hepatitis M21-1, Part III, Subpart iv, 4.I.2 Updated January 11, 2017 2. Hepatitis and Other Liver Disabilities Introduction Change Date January
Taken From VBA s Adjudication Procedure Manual Section on Hepatitis M21-1, Part III, Subpart iv, 4.I.2 Updated January 11, 2017 2. Hepatitis and Other Liver Disabilities Introduction Change Date January
