Transfusion-transmitted babesiosis caused by the
|
|
|
- Clare Smith
- 6 years ago
- Views:
Transcription
1 DONOR INFECTIOUS DISEASE TESTING Serologic screening of United States blood donors for Babesia microti using an investigational enzyme immunoassay Andrew E. Levin, 1 Phillip C. Williamson, 2 Evan M. Bloch, 3,4 Joan Clifford, 1 Sherri Cyrus, 2 Beth H. Shaz, 5 Debra Kessler, 5 Jed Gorlin, 6 James L. Erwin, 1 Neil X. Krueger, 1 Greg V. Williams, 1 Oksana Penezina, 1 Sam R. Telford IV, 7 John A. Branda, 8 Peter J. Krause, 9 Gary P. Wormser, 10 Anna M. Schotthoefer, 11 Thomas R. Fritsche, 11 and Michael P. Busch 3 BACKGROUND: The tick-borne pathogen Babesia microti has become recognized as the leading infectious risk associated with blood transfusion in the United States, yet no Food and Drug Administration licensed screening tests are currently available to mitigate this risk. The aim of this study was to evaluate the performance of an investigational enzyme immunoassay (EIA) for B. microti as a screening test applied to endemic and nonendemic blood donor populations. STUDY DESIGN AND METHODS: The study aimed to test 20,000 blood donors from areas of the United States considered endemic for B. microti and 10,000 donors from a nonendemic area with the investigational B. microti EIA. Repeat-reactive samples were retested by polymerase chain reaction (PCR), blood smear, immunofluorescent assay (IFA), and immunoblot assay. In parallel, serum samples from symptomatic patients with confirmed babesiosis were tested by EIA, IFA, and immunoblot assays. RESULTS: A total of 38 of 13,757 (0.28%) of the donors from New York, 7 of 4583 (0.15%) from Minnesota, and 11 of 8363 (0.13%) from New Mexico were found repeat reactive by EIA. Nine of the 56 EIA repeat-reactive donors (eight from New York and one from Minnesota) were positive by PCR. The specificity of the assay in a nonendemic population was 99.93%. Among IFA-positive clinical babesiosis patients, the sensitivity of the assay was 91.1%. CONCLUSION: The B. microti EIA detected PCRpositive, potentially infectious blood donors in an endemic population and exhibited high specificity among uninfected and unexposed individuals. The EIA promises to provide an effective tool for blood donor screening for B. microti in a format amenable to high-throughput and cost-effective screening. Transfusion-transmitted babesiosis caused by the tick-borne parasite Babesia microti has emerged in recent years as the foremost infectious risk of blood transfusion in the United States for which ABBREVIATIONS: CTS 5 Creative Testing Solutions; IFA 5 immunofluorescent assay; S/CO 5 signal/cutoff. From 1 Immunetics, Inc., Boston, Massachusetts; 2 Creative Testing Solutions, Tempe, Arizona; 3 Blood Systems Research Institute, San Francisco, California; 4 Johns Hopkins University School of Medicine, Baltimore, Maryland; 5 New York Blood Center, New York, New York; 6 Innovative Blood Resources/ Memorial Blood Centers, St Paul, Minnesota; 7 Tufts University Cummings School of Veterinary Medicine, North Grafton, Massachusetts; 8 Massachusetts General Hospital, Boston, Massachusetts; 9 Yale School of Public Health and Yale School of Medicine, New Haven, Connecticut; 10 New York Medical College, Valhalla, New York; and 11 Marshfield Clinic, Marshfield, Wisconsin. Address reprint requests to: Andrew E. Levin, PhD, Immunetics, Inc., 27 Drydock Avenue, Boston, MA 02210; alevin@immunetics.com. This project was supported by the National Heart Lung and Blood Institute at the National Institutes of Health through SBIR Phase I and Phase II Contract HHSN C, and by Blood Systems Research Institute Contract Received for publication January 12, 2016; revision received March 7, 2016; and accepted March 9, doi: /trf This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made. VC 2016 The Authors Transfusion published by Wiley Periodicals, Inc. on behalf of AABB TRANSFUSION 2016;56; TRANSFUSION Volume 56, July 2016
2 BLOOD DONOR SCREENING FOR BABESIA BY EIA no test licensed by the US Food and Drug Administration (FDA) is available. 1-3 Of the Babesia species that have been shown to cause human disease, B. microti is the overwhelmingly predominant species in the United States. 4 As B. microti is transmitted by the same Ixodes ticks that transmit Borrelia burgdorferi (the agent of Lyme disease), the two pathogens have similar endemic foci in the Northeast and Upper Midwest. Acquisition of B. microti through a tick bite most often results in transient, viral-like symptoms in healthy individuals, but those with compromised immune systems, including the very young, those of advanced age, and those without a spleen or with cancer or human immunodeficiency virus (HIV), are at risk for severe illness. 4,5 The ability of the parasite to establish asymptomatic infection in immunocompetent hosts, coupled with its survival for lengthy periods of time in blood donations puts transfusion recipients at risk. 1,5 More than 150 cases of transfusion-transmitted babesiosis have been described since 1979, approximately one-fifth of which were fatal. 6 At present there is no effective means to identify and remove infected blood donors from the donor pool, as risk factors for Babesia infection are insufficiently specific to be incorporated into donor health questionnaires and no blood screening tests for B. microti have been licensed by the FDA. Acute babesiosis has traditionally been diagnosed through examination of Giemsa-stained blood smears as for malaria. 4 More recently, polymerase chain reaction (PCR) tests have been developed which detect B. microti DNA Individuals who are infected with B. microti also develop an immune response which is detectable by serologic assays such as immunofluorescent assay (IFA), enzyme-linked immunosorbent assay, and immunoblot Antibody titers and direct markers of infectivity in asymptomatic blood donors, however, have only recently been evaluated Previously, we described the development of an enzyme immunoassay (EIA) for human IgG and IgM antibodies to B. microti based on a combination of immunodominant peptide antigens. 20 A study of approximately 27,000 US blood donors was carried out under an FDAapproved investigational new device exemption (IND) to determine the seroprevalence of B. microti in both endemic and nonendemic donor populations using the investigational B. microti EIA to support licensure of the assay. This report describes the results of the blood donor study and performance of the EIA on sera from clinical cases of babesiosis. MATERIALS AND METHODS Study design and donor populations The donor study was designed to measure B. microti seroprevalence with the investigational EIA in endemic and nonendemic regions of the United States. Donors at least 18 years of age in Suffolk and Nassau Counties, New York highly and moderately endemic areas, respectively were enrolled by New York Blood Center. Donors in Minneapolis, Minnesota a moderately endemic area were enrolled by Memorial Blood Centers division of Innovative Blood Resources. Donors in New Mexico, a nonendemic area, were enrolled by United Blood Services division of Blood Systems, Inc. All donor protocols were approved by the respective institution s institutional review board. Donors were provided factual information on this research study and enrollment was contingent upon signing a general informed consent permitting use of the donor s blood samples for Babesia research. Samples collected for the study represented routine donor collections at the study sites. Other than excluding some D and platelet donors, autologous donors, and all donors under 18 years of age, no other selective criteria were applied. A serum sample was obtained from each donor for testing by EIA, IFA, and immunoblot, and a whole blood sample, for PCR and blood smear. EIA, IFA, and blood smear testing were carried out by Creative Testing Solutions (CTS) in Tempe, Arizona,Chicago,Illinois,andBedford,Texas,whilePCRand immunoblot were carried out at Blood Systems Research Institute (San Francisco, CA) and Immunetics (Boston, MA), respectively. Per study design (Fig. 1), donor serum samples underwent initial testing by B. microti EIA at CTS. Initially reactive (defined as signal/cutoff[s/co] 1) and gray zone (0.934 S/CO < 1.0) samples were retested by EIA in duplicate at CTS. The purpose of the gray zone was to identify any donor samples that were reactive below the provisional cutoff (see below) and were positive by either PCR or blood smear, to determine a final cutoff based on study data. Repeat-reactive samples were defined as those with at least two of three reactive (S/CO 1.0) results. Repeat gray zone samples were defined as those with an initially reactive or gray zone result and at least one subsequent gray zone result. Samples that were initially nonreactive (S/CO < 0.934) as well as those with an initially reactive or gray zone result that were nonreactive in both duplicate retests were considered nonreactive. Serum aliquots from donors with repeat reactive or repeat gray zone EIA results were tested by IFA and immunoblot, while processed whole blood derived aliquots from the same donors were tested in parallel by PCR and blood smear. Donors that were found repeat reactive by EIA received notice within 1 week of donation and were deferred indefinitely from further blood donations. The associated blood products were not released for transfusion, but aliquots were retained by CTS for further research use. Clinical babesiosis study Serum samples were obtained from 129 symptomatic patients residing in endemic areas of the Northeast and Volume 56, July 2016 TRANSFUSION 1867
3 LEVIN ET AL. Fig. 1. Flow chart showing the study populations tested and the scheme for initial testing by EIA followed by retesting of repeatreactive samples by PCR, blood smear, IFA, and immunoblot. Cross-reactivity, interfering substances, and reproducibility studies The EIA was evaluated for cross-reactivity with other disease conditions by testing serum samples from patients with Trypanosoma cruzi, Leishmania, influenza, cytomegalovirus, Epstein-Barr virus, hepatitis C, HIV, Plasmodium falciparum, Plasmodium vivax, Toxoplasma gondii, B. burgdorferi, and Anaplasma phagocytophilum infections. Performance of the assay in the presence of rheumatoid factor and 18 other potentially interfering substances was also evaluated. Reproducibility was measured by testing replicates of a set of moderately reactive, weakly reactive, and negative samples across different EIA kit lots, operators, days, and sites. Fig. 2. Schematic of EIA principle. Four biotinylated peptides representing immunodominant sequences from B. microti antigens BMN1-9 and BMN1-17 were combined and immobilized to the microplate well. The assay detected both IgG and IgM antibodies in a single well. Midwest that had been diagnosed with babesiosis based on clinical presentation, history, and a positive PCR or blood smear result, irrespective of serologic status. Samples were tested with the B. microti EIA at CTS, by EIA and immunoblot at Immunetics, and by IFA at Tufts University. EIA The B. microti EIA used in the study has been described previously. 20 In brief, the EIA uses four peptide antigens derived from two members of the BMN1 gene family, BMN1-9 (also termed BmSA1) and BMN1-17, which have been shown to be immunodominant in prior studies The four peptide antigens were identified by screening candidate peptides against a library of sera from patients with clinically diagnosed babesiosis as well as healthy controls. The biotinylated peptides were immobilized on streptavidin-coated microplates (Fig. 2). Serum samples diluted 100-fold were incubated for 30 minutes on the microplate, after which unbound antibodies were removed by washing with buffer. Bound antibodies were detected by incubation with an anti-human IgG/IgM horseradish peroxidase (HRP) conjugate for 30 minutes, followed by further buffer washes. Microplates were incubated with a soluble peroxidase substrate (tetramethylbenzidine) for 10 minutes, after which a stop reagent was applied, and absorbance was read at 450 nm. A provisional cutoff based on prior studies 20 was calculated by adding a fixed value (0.355) to the mean absorbance in three negative control wells. IFA Blood donor samples were tested by IFA as described by Krause and colleagues 11 using B. microti substrate slides obtained from Fuller Laboratories. IgG and IgM antibodies were detected separately using corresponding fluorescein isothiocyanate labeled goat anti-human antibody conjugates (Jackson ImmunoResearch). Slides were analyzed with a microscope (Model BX43, Olympus) equipped with 1868 TRANSFUSION Volume 56, July 2016
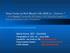
4 BLOOD DONOR SCREENING FOR BABESIA BY EIA Fig. 3. Representative B. microti immunoblots. Molecular weights (kda) of the six bands on which interpretive criteria are based are shown at top. Samples tested were (A) positive control; (B) PCR-positive blood donor from New York; (C) PCR-positive blood donor from Minnesota; (D) PCR-positive clinical babesiosis patient from Massachusetts; (E) blood smear-positive clinical babesiosis patient from Connecticut; (F) EIA-nonreactive blood donor from Arizona, a nonendemic region. rhodamine and fluorescein filters at 4003 magnification. IFA results were interpreted as positive or negative based on a cutoff of 1:128, which was selected based on studies with previously characterized clinical and blood donor samples (data not shown). Immunoblot Parasites harvested from infected hamster blood and enriched by differential centrifugation were lysed and extracted in sodium dodecyl sulfate buffer. The lysate was separated by polyacrylamide gel electrophoresis, and the proteins were transferred to nitrocellulose membranes. After incubation of membrane strips with human serum samples, bound IgG antibodies were detected using an anti-human IgG HRP conjugate. Immunoblot interpretive criteria were developed using panels of well-characterized sera from clinical babesiosis patients and controls. A positive result was defined as antibody reactivity with a 37- kda antigen band previously identified as BMN1-9 plus reactivity with at least one of a set of bands migrating at 34, 36, 69, 73, and 78 kda. All other band patterns were interpreted as negative (Fig. 3). With these scoring criteria, 87.3% (96/110) of PCR-positive or blood smear-positive clinical case sera and 97.6% (82/84) of IFA-positive sera, some of which were used in assay development, were immunoblot positive. Conversely, 100% (106/106) of a set of EIA nonreactive sera from low-risk blood donors in Arizona were immunoblot negative. Peripheral blood smear Peripheral blood smears were prepared from EDTApreserved whole blood samples using the method of Houwen 24 and allowed to air dry completely before staining. A minimum of two microscope slides were prepared as thin smears from each donor sample collected from B. microti-endemic areas. Smears were stained using Wright s-giemsa stain (Easy I, Azer Scientific). Microscopic examination to detect the presence of B. microti or other blood parasites, and quantification of the organisms detected, was performed according to the method of Blevins and coworkers 25 utilizing NCCLS-recommended standards (300 fields at per slide). Any positive or inconclusive results were confirmed by independent review before the final interpretation. PCR B. microti PCR was carried out according to an algorithm which employed two separate PCR assays, a screening assay followed by a confirmatory assay (Figure 4). The screening assay, based on primers Bab2 and Bab3, has been described previously. 9 The 95% limit of detection for this assay was approximately 13 parasites per 2 ml of whole blood. The confirmatory assay was adapted from a second previously described assay 7 and used a different primer pair, Bab8F/Bab8R. This assay was found to be similar in limit of detection to the screening assay (data not shown). EIA repeat-reactive samples were initially tested by the screening assay in triplicate; if any one of the triplicate results was positive (meeting both quantification cycle and melting temperature criteria), the sample was retested by the confirmatory assay in duplicate. Testing by both screening and confirmatory assays was repeated on those samples found positive by the initial screening assay run. A PCR result was considered as a confirmed positive when both of the confirmatory duplicates were positive (both duplicates meeting quantification cycle and melting temperature criteria) in either the initial or the repeat assay runs. RESULTS Donor enrollment began July 29 and ended November 15, 2013, yielding a total of 28,615 samples. After exclusion of samples that did not meet inclusion criteria, the total numbers of donor samples tested were 13,757 (New York), 4583 (Minnesota), and 8363 (New Mexico). The exact distribution of donors between Suffolk and Nassau Counties, New York, was not determined, but Nassau County was expected to account for the majority based on blood center collection practices. EIA and PCR reactivity at the provisional cutoff Of the 26,703 donor samples tested by EIA, 122 were found repeat reactive and 12 were in the gray zone at the provisional EIA cutoff. Further testing of repeat-reactive samples yielded 37 that were positive by IFA and 40 that were positive by immunoblot (Table 1). Among the 12 EIA Volume 56, July 2016 TRANSFUSION 1869
5 LEVIN ET AL. TABLE 1. Seroprevalence measured by B. microti EIA in endemic and nonendemic blood donor populations and clinical babesiosis patients at the provisional cutoff and revised cutoff, compared with detection by PCR, blood smear, IFA, and immunoblot* B. microti EIA repeat reactive Immunoblot positive Study population (A) Provisional C/O (B) Revised C/O (31.6) IFA positive, Out of (A) Out of (A) Out of (B) PCR positive Blood smear positive High-risk 0.54 (74) 0.28 (38) 0.22 (30) 0.24 (33) 0.15 (21) 0.06 (8) 0 (0) endemic (New York), n 5 13,757 Moderate-risk 0.35 (16) 0.15 (7) 0.04 (2) 0.02 (1) 0.02 (1) 0.02 (1) 0 (0) endemic (Minnesota), n Nonendemic 0.38 (32) 0.13 (11) 0.06 (5) 0.07 (6) 0.06 (5) 0 (0) 0 (0) (New Mexico), n Clinical babesiosis PCR/BS positive, 84.5 (109/129) 77.5 (100/129) 78.3 (101/129) 87.3 (96/110) 87.3 (96/110) 98.1 (102/104) 89.2 (74/83) n IFA positive, n (99/101) 91.1 (92/101) 100 (101/101) 97.6 (82/84) 97.6 (82/84) / (59/68) * Figures indicate percent and total number of reactive or positive samples in each category. Denominators indicate total number of samples tested; in some cases in the clinical babesiosis group, sample volume did not permit the test to be carried out. C/O 5 cutoff gray zone samples, three were IFA positive and two were immunoblot positive. PCR testing of the 134 repeat-reactive and gray zone donor samples yielded six donors who met both screening and confirmatory PCR criteria for positivity, while three donors met screening criteria but were positive by only one of the confirmatory replicates and were therefore identified as probable PCR positives (Table 2). All PCRpositive and probable-positive samples were from endemic regions. IFA titers for eight of the nine samples were at least 1024 and one was 512. All nine samples were positive by immunoblot (Fig. 3). At the provisional cutoff, the EIA S/CO values for the nine samples ranged from 1.62 to 5.75; none were in the gray zone. The frequencies of EIA repeat-reactive and PCR-positive samples were distributed randomly over the 16-week study period, with no obvious pattern. The distribution of EIA absorbance values for donors in the endemic versus nonendemic populations compared with EIA absorbance values for clinical babesiosis patients is shown in Fig. 5. None of the EIA repeat-reactive or gray zone samples were positive by blood smear examination. EIA reactivity at a revised cutoff An analysis of seroreactivity in blood donors versus EIA cutoff indicated that the cutoff could be increased up to 1.6-fold (the revised cutoff ) without sacrificing detection of any of the nine PCR-positive or probable positive donor samples. Concurrently, the rate of seroreactivity in the subset of donor samples that were PCR negative declined by 56%, to 0.20% in endemic areas, and by 66%, to 0.13% in the nonendemic area. Results calculated with the revised cutoff are shown in Table 2. Based on the revised cutoff, the repeat-reactive rate in the endemic population in New York was 0.28%, which was approximately twice the repeat-reactive rate in the endemic Minnesota population (0.15%) or the nonendemic New Mexico population (0.13%). At the revised cutoff, the difference between the frequency of EIA repeat-reactive samples in the endemic New York population and that in the nonendemic New Mexico population was significant (p ), while at the provisional cutoff, the difference was not significant (p 5 0.1). In the nonendemic (New Mexico) population, 32 of 8363 (0.38%) donors were repeat reactive at the provisional cutoff, decreasing to 11 of 8363 (0.13%) at the revised cutoff; of these 11, four were positive by IFA at titers of at least 256 and five were positive by immunoblot, leaving 6 of 8363 (0.07%) as the rate of EIA repeat reactivity among immunoblot-negative donors, presumed to be the EIA false-positive rate. EIA reactivity in clinical babesiosis patients Testing of serum samples from symptomatic patients who were diagnosed with babesiosis, with positive PCR or blood smear results, yielded 109 of 129 samples (84.5%, 95% confidence interval [CI], 77.1%-90.3%) with repeat-reactive results at the provisional EIA cutoff. By comparison, 101 of 129 (78.3%) were positive by IFA. Of the 20 EIA nonreactive clinical samples, 18 were available for further testing. Eleven of these 18 samples were negative by both IFA and immunoblot, while five samples yielded positive EIA results upon retesting. Includingthesefivesamples,98%of IFA-positive samples were reactive by EIA and the correlation between EIA results and IFA or immunoblot was TRANSFUSION Volume 56, July 2016
6 BLOOD DONOR SCREENING FOR BABESIA BY EIA Sample # TABLE 2. Characteristics of the PCR-positive blood donor samples identified in the donor study Origin Date Tested Screening PCR Confirmatory PCR EIA S/CO* IFA IgG IFA IgM Immunoblot 1 Nassau County, NY 8/2/2013 Pos Equiv Pos 2 Suffolk County, NY 8/1/2013 Pos Pos Pos 3 Suffolk County, NY 9/12/2013 Pos Pos Pos 4 Suffolk County, NY 9/20/2013 Pos Pos Pos 5 Suffolk County, NY 10/24/2013 Pos Pos Pos 6 Suffolk County, NY 11/9/2013 Pos Pos Pos 7 Suffolk County, NY 11/12/2013 Pos Equiv Pos 8 Suffolk County, NY 11/14/2013 Pos Equiv Pos 9 Hennepin County, MN 8/30/2013 Pos Pos Pos * At revised EIA cut-off. Fig. 4. Scheme utilized for PCR testing of EIA repeat-reactive blood donors. and 96%, respectively. Among the 101 IFA-positive patient sera, increasing the cutoff value 1.6-fold resulted in reclassification of nine samples (7%) as nonreactive,yielding 92 of 101 (91.1%) EIA repeat-reactive samples (Table 1 and Fig. 5). Reproducibility, interfering substances, and crossreactivity studies Reproducibility of the EIA as measured by replicate testing yielded coefficients of variation (CV) averaging less than 10% across kit lots, operators, days, and sites with no significant variance due to these factors (not shown). Likewise, none of the interfering substances tested produced any effect on EIA reactivity. Among potentially crossreactive conditions, only anaplasmosis and Lyme disease yielded one of 20 and four of 20 samples that were reactive in the EIA, respectively; all others were nonreactive. DISCUSSION A principal aim of this study was to determine the seroprevalence of B. microti in blood donors residing in endemic Fig. 5. Distribution of absorbance values in the B. microti EIA for blood donors from nonendemic (n ) and endemic areas (n 5 18,340) and for clinical babesiosis patients (n 5 129). The provisional and revised EIA cutoffs are shown as horizontal dashed lines. Volume 56, July 2016 TRANSFUSION 1871
7 LEVIN ET AL. and nonendemic regions of the United States using an investigational EIA. While serologic assays based on IFA have been used for detection of antibodies in clinical patients and blood donors, few EIAs have been developed for this purpose, 13,17,26 and none are currently licensed for blood screening. As the investigational EIA makes use of synthetic peptide antigens rather than whole B. microti cells obtained from hamster inoculation as in IFA methods, it offers the benefit of reproducible manufacture and performance, substantiated by the CV values in the reproducibility component of this study. The peptide sequences selected for use in the EIA were derived from known immunodominant antigens BMN1-9 and BMN1-17. EIA testing of well-characterized clinical babesiosis cases from endemic regions in the US Northeast and Midwest yielded high sensitivity of detection with these peptides, suggesting that the sequences are relatively conserved among B. microti strains that infect humans. Approximately 80% of PCR- or blood smear positive clinical case sera were reactive in the peptide EIA, similar to the 81.6% sensitivity reported for an IFA evaluated in blood donor studies by Moritz and colleagues. 19 Clinical case sera that were EIA negative were divided principally between those that showed no detectable antibody by IFA or immunoblot, potentially representing early-stage infections, and a subset in which spurious results were overturned by subsequent retesting. The strong overlap between clinical samples detected by EIA and those detected by IFA further suggests that the peptide antigens present in the EIA recapitulate the dominant antigenic components of the organism. How diverse B. microti sequences are in nature, and whether sequence diversity affects antigenicity, infectivity, or pathogenicity, are still unknown. One of the aims of the study was to optimize the EIA cutoff based on study results. Unlike blood-borne viruses for which antibodies are markers of chronic infection, babesiosis in healthy individuals is typically a transient infection, in which serum antibodies persist long after the infection is resolved. 27 Furthermore, exposure to B. microti through tick bites appears to be relatively frequent in endemic regions of the United States as evidenced by seroprevalence rates ranging from 1% to 2.5%. 14,28 This is consistent with our study results that showed that 90% of EIA repeat-reactive donors in endemic areas and 100% in nonendemic areas were PCR and blood smear negative, indicating that the majority of EIA-reactive donors are not likely to be infectious. Such EIA repeat-reactive donors, many of whom have antibodies to B. microti that can be verified by IFA or immunoblot, likely represent individuals with prior, resolved infections and with lingering but waning antibody as demonstrated in other studies. 15,29 However, exceptions may occur among seropositive donors with low levels of parasitemia that are detected inconsistently by currently used PCR assays, as shown in a recent study by Leiby and colleagues. 15 As high titers of antibody have been shown to correlate with active infection, 27,29 an optimal EIA cutoff would desirably discriminate active from prior, resolved infections. Study results indicated that a 1.6-fold increase in EIA cutoff above the provisional value substantially decreased the number of donors that were classified as EIA reactive and would hence be deferred, with no loss in detection of those EIA-reactive donors who were PCR positive and a very modest impact on detection of clinical babesiosis cases. This revised cutoff has been adopted for analysis of the study data for licensure by FDA and for ongoing use of the EIA in prospective screening under IND. With the revised cutoff, the rate of seroreactivity in endemic areas of New York was 0.28%, which was more than twice the rate of 0.13% measured in the nonendemic area (New Mexico). By comparison, the seroprevalence rate measured in a previous study carried out in New York using the same EIA during the previous year (2012) was approximately 0.91%, 20 and a rate of 0.75% was reported in a study of donors sampled in another endemic region in 2011 and tested by an IFA method. 19 The lower seroreactivity rate in endemic areas in this study reflects in part the application of a higher EIA cutoff, but more significantly, it likely reflects the New York blood donor population that was tested. This population comprised a large fraction of donors from Nassau County, where the prevalence of babesiosis reported in 2013 was approximately 24-fold lower than in Suffolk, a much higher risk area. 30 The causes of EIA reactivity in the 11 donors residing in the nonendemic region are unknown. A total of five of these 11 EIA-reactive donors had positive immunoblots, which may be due to exposure to either B. microti or a similar organism or may represent cross-reactivity from unrelated sources. Enzootic B. microti has been reported in Colorado, 31 and pathogenic Babesia species have been described in Mexico; 32 thus, exposure in or near New Mexico may be a possibility, in addition to the possibility of exposure during travel to other states. Sera from patients with Lyme disease and anaplasmosis were found to yield low levels of reactivity in the B. microti EIA, an expected result based on the known occurrence of coinfections by ticks carrying B. microti along with B. burgdorferi or A. phagocytophilum, 33,34 or alternatively of serial infections by these organisms leaving overlapping antibody titers in the human host. One question that pertains to donor screening policy is seasonality. Given that clinical babesiosis cases peak in midsummer and decline rapidly in autumn, 35 one option would be to limit screening for B. microti to those months when the risk is highest. Based on the apparently random distribution of PCR-positive and serologically positive donor samples detected each week between August and November, however, our study results are not consistent with seasonal screening limited to the summer months. This finding suggests that subclinical infection persists in 1872 TRANSFUSION Volume 56, July 2016
8 BLOOD DONOR SCREENING FOR BABESIA BY EIA at least some donors for months if not longer, consistent with evidence presented in prior studies. 14,29 On the other hand, culling of EIA-reactive donors has been shown to decrease their prevalence in the donor population, 36 which may conceivably leave a preponderance of donors with new, seasonal infections. A shortcoming of all serologic assays including the present EIA is the inability to detect window period infections, during which parasitemia precedes the appearance of circulating antibodies. Given the study design, which relied on EIA as the first-step screening test, the number of window period infections that were not detected is not known. Other studies have reported window phase infections in clinical patients 37 and, in one instance, in a blood donor. 28 Statistically, window period infections should account for a minor fraction of all donor infections given their presumed short duration and temporal limitation to seasonal periods of active transmission. Detection of window period infections would require methods other than serology, such as PCR or other direct parasite detection techniques, with the attendant relatively significant incremental cost. 38,39 However, even PCR is subject to limitations in sensitivity dependent on the target selected and on sample volume, which may vary between assays, and is capable of yielding false-negative results, in particular at low levels of parasitemia. 15 Volumes of whole blood utilized in published B. microti PCR protocols range from 200 ml to 2.0 ml, with corresponding analytical sensitivities ranging between 10 and 100 gene copies per PCR procedure Using immunoblot as a second step assay to define serological true and false positives in the nonendemic donor population, as was done in a previous study by Moritz and colleagues 19 with IFA as the screening assay, yielded a net specificity of 99.93% for the EIA (95% CI, 99.84% %). The ratio of PCR-positive, presumed infectious donors to EIA repeat-reactive donors in the combined endemic areas was 9:45 or 20% (21% in New York alone). This value is similar to, although slightly higher than, the 13% ratio of PCR-positive to IFA-positive donors in an endemic area reported by Moritz and coworkers. 19 Based on its efficiency of detection of potentially infectious donors in endemic areas, the EIA promises to be useful as a blood screening assay for B. microti. AsanEIAinstandard microplate format, it should be amenable to highthroughput processing and promises to meet costeffectiveness criteria as set forth in recent analyses. 40 The deferral rate of donors that are seropositive but uninfected, and by what means such donors might be reinstated, are issues that remain to be considered. Studies are ongoing to elucidate the kinetics of the antibody response in seroreactive donors over time, measured by the same B. microti EIA. Eventually, given the typically transient course of infection with B. microti, itmaybe important to determine whether a decrease in EIA reactivity could be one criterion in an algorithm for reentry of seroreactive, deferred donors. Given the recent recommendation by the Blood Products Advisory Committee for universal serologic screening of blood donors for B. microti, the seroprevalence data resulting from this study with an EIA intended for this application may be useful in informing further decisions on screening policy including timing and geography. ACKNOWLEDGMENT Technical assistance was provided by Janna Brancato, RN (Yale School of Public Health). CONFLICT OF INTEREST REFERENCES AEL, JC, JLE, GVW, OP, and NXK are employees of Immunetics, Inc. GPW has received research grants from Immunetics, Inc., Institute for Systems Biology, Rarecyte, Inc., and biomerieux SA. He owns equity in Abbott, has been an expert in malpractice cases involving babesiosis, and is an unpaid board member of the American Lyme Disease Foundation. JAB has received research grants from Immunetics, Inc., Diasorin, Inc., Alere, Inc., and bio- Merieux, Inc. The other authors have disclosed no conflicts of interest. 1. Leiby DA, Transfusion-transmitted Babesia spp.: bull s-eye on Babesia microti. Clin Microbiol Rev 2011;24: Blood Products Advisory Committee meeting issue summary strategies for implementation of antibody and nucleic acid-based testing for B. microti in blood donors [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2015 [cited 2016 Jan 8]. Available from: downloads/advisorycommittees/committeesmeetingmaterials/bloodvaccinesandotherbiologics/bloodproductsadvisorycommittee/ucm pdf. 3. Gubernot DM, Lucey CT, Lee KC, et al. Babesia infection through blood transfusions: reports received by the US Food and Drug Administration, Clin Infect Dis 2009;48: Vannier E, Krause P. Human babesiosis. N Engl J Med 2012; 366: Krause PJ, McKay K, Gadbaw J, et al. Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg 2003;68: Herwaldt BL, Linden JV, Bosserman E, et al. Transfusionassociated babesiosis in the United States: a description of cases. Ann Intern Med 2011;155: Teal AE, Habura A, Ennis J, et al. A new real-time PCR assay for improved detection of the parasite Babesia microti. JClin Microbiol 2012;50: Rollend L, Bent SJ, Krause PJ, et al. Quantitative PCR for detection of Babesia microti in Ixodes scapularis ticks and in human blood. Vector Borne Zoonotic Dis 2013; Volume 56, July 2016 TRANSFUSION 1873
9 LEVIN ET AL. 9. Bloch EM, Lee TH, Krause PJ, et al. Development of a realtime polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion 2013;53: Wang G, Wormser GP, Zhuge J, et al. Utilization of a realtime PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick Borne Dis 2015;6: Krause PJ, Telford SR, Ryan R, et al. Diagnosis of babesiosis: evaluation of a serologic test for the detection of Babesia microti antibody. J Infect Dis 1994;169: Ryan R, Krause PJ, Radolf J, et al. Diagnosis of Babesiosis using an immunoblot serologic test. Clin Diagn Lab Immunol 2001;8: Loa CC, Adelson ME, Mordechai E, et al. Serological diagnosis of human babesiosis by IgG enzyme-linked immunosorbent assay. Curr Microbiol 2004;49: Johnson ST, Cable RG, Tonnetti L, et al. Seroprevalence of Babesia microti in blood donors from Babesia-endemic areas of the northeastern United States: 2000 through Transfusion 2009;49: Leiby DA, Johnson ST, Won KY, et al. A longitudinal study of Babesia microti infection in seropositive blood donors. Transfusion 2014;54: Tonnetti L, Thorp AM, Deisting B, et al. Babesia microti seroprevalence in Minnesota blood donors. Transfusion 2013;53: Leiby DA, Chung AP, Gill JE, et al. Demonstrable parasitemia among Connecticut blood donors with antibodies to Babesia microti. Transfusion 2005;45: Popovsky MA, Lindberg LE, Syrek AL, et al. Prevalence of Babesia antibody in a selected blood donor population. Transfusion;28: Moritz ED, Winton CS, Johnson ST, et al. Investigational screening for Babesia microti in a large repository of blood donor samples from nonendemic and endemic areas of the United States. Transfusion 2014;54: Levin AE, Williamson PC, Erwin JL, et al. Determination of Babesia microti seroprevalence in blood donor populations using an investigational enzyme immunoassay. Transfusion 2014;54: Priest JW, Moss DM, Won K, et al. Multiplex assay detection of immunoglobulin G antibodies that recognize Babesia microti antigens. Clin Vaccine Immunol 2012;19: Lodes MJ, Houghton RL, Bruinsma ES, et al. Serological expression cloning of novel immunoreactive antigens of Babesia microti. Infect Immun 2000;68: Luo Y, Jia H, Terkawi MA, et al. Identification and characterization of a novel secreted antigen 1 of Babesia microti and evaluation of its potential use in enzyme-linked immunosorbent assay and immunochromatographic test. Parasitol Int 2011;60: Houwen B. Blood film preparation and staining procedures. Clin Lab Med 2002;22:1-14, v. 25. Blevins SM, Greenfield RA, Bronze MS. Blood smear analysis in babesiosis, ehrlichiosis, relapsing fever, malaria, and Chagas disease. Cleve Clin J Med 2008;75: Houghton RL, Homer MJ, Reynolds LD, et al. Identification of Babesia microti-specific immunodominant epitopes and development of a peptide EIA for detection of antibodies in serum. Transfusion 2002;42: Ruebush TK, Chisholm ES, Sulzer AJ, et al. Development and persistence of antibody in persons infected with Babesia microti. Am J Trop Med Hyg 1981;30: Johnson ST, Van Tassell ER, Tonnetti L, et al. Babesia microti real-time polymerase chain reaction testing of Connecticut blood donors: potential implications for screening algorithms. Transfusion 2013;53: Krause PJ, Spielman A, Telford SR, et al. Persistent parasitemia after acute babesiosis. N Engl J Med 1998;339: Reported cases by disease and county: AIDS cryptosporidiosis [Internet]. Albany: New York State Department of Health; 2013 Oct [cited 2016 Jan 8]. Available from: cases/1.htm. 31. Burkot TR, Schneider BS, Pieniazek NJ, et al. Babesia microti and Borrelia bissettii transmission by Ixodes spinipalpis ticks among prairie voles, Microtus ochrogaster, in Colorado. Parasitology 2000;121: Esteve-Gassent MD, Perez de Leon AA, Romero-Salas D, et al. Pathogenic landscape of transboundary zoonotic diseases in the Mexico-US border along the Rio Grande. Front Public Health 2014;2: Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 1996;275: Krause PJ, McKay K, Thompson CA, et al. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis 2002;34: Centers for Disease Control and Prevention (CDC). Babesiosis surveillance 18 States, MMWR Morb Mortal Wkly Rep 2012;61: Johnson ST, Cable RG, Leiby DA. Lookback investigations of Babesia microti-seropositive blood donors: seven-year experience in a Babesia-endemic area. Transfusion 2012;52: Devine P, Berardi V, Molloy P, et al. Babesia microti tests for blood donor screening. Transfusion 2011;51:204a. 38. Goodell AJ, Bloch EM, Krause PJ, et al. Costs, consequences, and cost-effectiveness of strategies for Babesia microti donor screening of the US blood supply. Transfusion 2014;54: Bish EK, Moritz ED, El-Amine H, et al. Cost-effectiveness of Babesia microti antibody and nucleic acid blood donation screening using results from prospective investigational studies. Transfusion 2015;55: Simon MS, Leff JA, Pandya A, et al. Cost-effectiveness of blood donor screening for Babesia microti in endemic regions of the United States. Transfusion 2014;54: TRANSFUSION Volume 56, July 2016
Babesiosis is a malaria-like illness caused by
 DONOR INFECTIOUS DISEASE TESTING Determination of Babesia microti seroprevalence in blood donor populations using an investigational enzyme immunoassay Andrew E. Levin, 1 Phillip C. Williamson, 2 James
DONOR INFECTIOUS DISEASE TESTING Determination of Babesia microti seroprevalence in blood donor populations using an investigational enzyme immunoassay Andrew E. Levin, 1 Phillip C. Williamson, 2 James
Babesia from a donor perspective
 Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Babesia from a donor perspective American Red Cross, Massachusetts Region Bryan Spencer, MPH Research Scientist American Society for Apheresis Annual Meeting May 8, 2015 San Antonio, TX The need is constant.
Babesia and Blood Safety
 Babesia and Blood Safety American Red Cross, New England Region Bryan Spencer, MPH Manager of Blood Research / REDS-III Coordinator ASM 46 th Annual Region I Meeting 27 October, 2011 Randolph, MA The need
Babesia and Blood Safety American Red Cross, New England Region Bryan Spencer, MPH Manager of Blood Research / REDS-III Coordinator ASM 46 th Annual Region I Meeting 27 October, 2011 Randolph, MA The need
Seroprevalence of Babesia microti in Individuals with Lyme Disease. Sabino R. Curcio, M.S, MLS(ASCP)
 Seroprevalence of Babesia microti in Individuals with Lyme Disease Sabino R. Curcio, M.S, MLS(ASCP) Lyme Disease Most common vectorborne illness in the United States Caused by the tick-transmitted spirochete
Seroprevalence of Babesia microti in Individuals with Lyme Disease Sabino R. Curcio, M.S, MLS(ASCP) Lyme Disease Most common vectorborne illness in the United States Caused by the tick-transmitted spirochete
Title: Public Health Reporting and National Surveillance for Babesiosis
 10-ID-27 Committee: Infectious Disease Title: Public Health Reporting and National Surveillance for Babesiosis I. Statement of the Problem: The increasing incidence of reported cases of babesiosis, the
10-ID-27 Committee: Infectious Disease Title: Public Health Reporting and National Surveillance for Babesiosis I. Statement of the Problem: The increasing incidence of reported cases of babesiosis, the
Interventions for Babesia (and Plasmodium)
 Interventions for Babesia (and Plasmodium) Susan L. Stramer, Ph.D. May 16 2017 www.aabb.org Babesiosis Malaria-like illness caused by Babesia spp. Asymptomatic fatal Non-specific symptoms (malaise, fever,
Interventions for Babesia (and Plasmodium) Susan L. Stramer, Ph.D. May 16 2017 www.aabb.org Babesiosis Malaria-like illness caused by Babesia spp. Asymptomatic fatal Non-specific symptoms (malaise, fever,
Traditional laboratory-based screening methods
 DONOR INFECTIOUS DISEASE TESTING Description of 15 DNA-positive and antibody-negative window-period blood donations identified during prospective screening for Babesia microti Erin D. Moritz, 1 Laura Tonnetti,
DONOR INFECTIOUS DISEASE TESTING Description of 15 DNA-positive and antibody-negative window-period blood donations identified during prospective screening for Babesia microti Erin D. Moritz, 1 Laura Tonnetti,
Guidance for Industry
 Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Reducing Babesia and Malaria Risks: Testing, Donor Selection, Inactivation. Susan L. Stramer PhD IPFA 23 rd Meeting May
 Reducing Babesia and Malaria Risks: Testing, Donor Selection, Inactivation Susan L. Stramer PhD IPFA 23 rd Meeting May 26 2016 Babesiosis Malaria-like illness caused by babesia spp. Asymptomatic fatal
Reducing Babesia and Malaria Risks: Testing, Donor Selection, Inactivation Susan L. Stramer PhD IPFA 23 rd Meeting May 26 2016 Babesiosis Malaria-like illness caused by babesia spp. Asymptomatic fatal
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS
 EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
Blood Smears Only 19 May Sample Preparation and Quality Control
 NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 9 May 205 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 9 May 205 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
Babesia spp. Emerging Transfusion Dilemmas
 Babesia spp. Emerging Transfusion Dilemmas David A. Leiby, PhD Transmissible Diseases Department American Red Cross Holland Laboratory Department of Microbiology and Tropical Medicine George Washington
Babesia spp. Emerging Transfusion Dilemmas David A. Leiby, PhD Transmissible Diseases Department American Red Cross Holland Laboratory Department of Microbiology and Tropical Medicine George Washington
Technical Bulletin No. 121
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 121 January 31, 2014 Lyme Blot, IgG and IgM - Now Performed at CPAL Contact: J. Matthew Groeller, 717.851.1416 Operations Manager, Clinical
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 121 January 31, 2014 Lyme Blot, IgG and IgM - Now Performed at CPAL Contact: J. Matthew Groeller, 717.851.1416 Operations Manager, Clinical
Screening for Babesia microti in the U.S. Blood Supply
 The new england journal of medicine Original Article Screening for Babesia microti in the U.S. Blood Supply Erin D. Moritz, Ph.D., Colleen S. Winton, S.B.B. (A.S.C.P.), Laura Tonnetti, Ph.D., Rebecca L.
The new england journal of medicine Original Article Screening for Babesia microti in the U.S. Blood Supply Erin D. Moritz, Ph.D., Colleen S. Winton, S.B.B. (A.S.C.P.), Laura Tonnetti, Ph.D., Rebecca L.
Update on Transfusion- Transmitted Infectious Diseases
 Update on Transfusion- Transmitted Infectious Diseases Scott Koepsell MD PhD Associate Professor University of Nebraska Medical Center I have no conflicts of interest to disclose. Global changes and the
Update on Transfusion- Transmitted Infectious Diseases Scott Koepsell MD PhD Associate Professor University of Nebraska Medical Center I have no conflicts of interest to disclose. Global changes and the
Therapeutic Parasite Reduction or Removal of Harmful Materials. Yanyun Wu, MD, PhD Chief Medical Officer
 Therapeutic Parasite Reduction or Removal of Harmful Materials Yanyun Wu, MD, PhD Chief Medical Officer None Conflict of Interest Puget Sound Blood Center is now Bloodworks Northwest After 70 years Puget
Therapeutic Parasite Reduction or Removal of Harmful Materials Yanyun Wu, MD, PhD Chief Medical Officer None Conflict of Interest Puget Sound Blood Center is now Bloodworks Northwest After 70 years Puget
KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING
 KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING Permission to Use: Please note that the presenter has agreed to make their presentation available. However, should you want to use some of the data
KNOWLEDGE INFUSION: FOCUS ON RISK-BASED DECISION-MAKING Permission to Use: Please note that the presenter has agreed to make their presentation available. However, should you want to use some of the data
SP.718 Special Topics at Edgerton Center: D-Lab Health: Medical Technologies for the Developing World
 MIT OpenCourseWare http://ocw.mit.edu SP.718 Special Topics at Edgerton Center: D-Lab Health: Medical Technologies for the Developing World Spring 2009 For information about citing these materials or our
MIT OpenCourseWare http://ocw.mit.edu SP.718 Special Topics at Edgerton Center: D-Lab Health: Medical Technologies for the Developing World Spring 2009 For information about citing these materials or our
Tickborne Disease Case Investigations
 Massachusetts Department of Public Health Bureau of Infectious Disease and Laboratory Sciences Tickborne Disease Case Investigations Anthony Osinski, MPH May 31, 2018 Factors Associated with Increasing
Massachusetts Department of Public Health Bureau of Infectious Disease and Laboratory Sciences Tickborne Disease Case Investigations Anthony Osinski, MPH May 31, 2018 Factors Associated with Increasing
Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis!
 Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis! Marie Kroun, MD Denmark Presentation in York, UK June
Ring Forms in Red Blood Cells (RBCs) Babesia? from Danish Chronically Ill Patients, All Clinically Suspect of Having Persistent Active Borreliosis! Marie Kroun, MD Denmark Presentation in York, UK June
Detection of Multiple Reactive Protein Species by Immunoblotting after Recombinant Outer Surface Protein A Lyme Disease Vaccination
 42 Detection of Multiple Reactive Protein Species by Immunoblotting after Recombinant Outer Surface Protein A Lyme Disease Vaccination Philip J. Molloy, 1,2 Victor P. Berardi, 2 David H. Persing, 2,3,a
42 Detection of Multiple Reactive Protein Species by Immunoblotting after Recombinant Outer Surface Protein A Lyme Disease Vaccination Philip J. Molloy, 1,2 Victor P. Berardi, 2 David H. Persing, 2,3,a
Screening donors and donations for transfusion transmissible infectious agents. Alan Kitchen
 Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Holarctic distribution of Lyme disease
 Babesia and Ehrlichia epidemiology: key points Holarctic distribution of Lyme disease 1. Always test for babesia and ehrlichia if Lyme disease is suspected 2. Endemic areas perhaps too broad a term; vector-borne
Babesia and Ehrlichia epidemiology: key points Holarctic distribution of Lyme disease 1. Always test for babesia and ehrlichia if Lyme disease is suspected 2. Endemic areas perhaps too broad a term; vector-borne
Blood Smears Only 20 May Sample Preparation and Quality Control
 NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 20 May 2014 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 20 May 2014 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
CEPHIA Consortium for the Evaluation and Performance of HIV Incidence Assays STANDARD OPERATING PROCEDURE
 CEPHIA Consortium for the Evaluation and Performance of HIV Incidence Assays STANDARD OPERATING PROCEDURE TITLE : SOP for (off board dilution) Less Sensitive Modified VITROS Enzyme Immunoassay CEPHIA DOCUMENT
CEPHIA Consortium for the Evaluation and Performance of HIV Incidence Assays STANDARD OPERATING PROCEDURE TITLE : SOP for (off board dilution) Less Sensitive Modified VITROS Enzyme Immunoassay CEPHIA DOCUMENT
Surveillance Report 2014
 Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Toxoplasma gondii IgM (Toxo IgM)
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Hira L. Nakhasi, Ph.D. CBER/USFDA July 3 rd 2015
 Strategies for Implementation of Antibody and Nucleic Acid-based Testing for Babesia microti in Blood Donations: Summary of May 13 th 2015 Blood Product Advisory Committee Meeting Hira L. Nakhasi, Ph.D.
Strategies for Implementation of Antibody and Nucleic Acid-based Testing for Babesia microti in Blood Donations: Summary of May 13 th 2015 Blood Product Advisory Committee Meeting Hira L. Nakhasi, Ph.D.
PRICE LIST Effective Date: October 16, 2017
 Effective October 16, 2017 we are offering our new tests for Lyme IGXSpot, Lyme Borreliosis, and Tick-borne Relapsing Fever Borreliosis The new ImmunoBlot tests have replaced the original Western Blot
Effective October 16, 2017 we are offering our new tests for Lyme IGXSpot, Lyme Borreliosis, and Tick-borne Relapsing Fever Borreliosis The new ImmunoBlot tests have replaced the original Western Blot
Transfusion-Transmitted Babesia spp.: Bull s-eye on Babesia microti
 CLINICAL MICROBIOLOGY REVIEWS, Jan. 2011, p. 14 28 Vol. 24, No. 1 0893-8512/11/$12.00 doi:10.1128/cmr.00022-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Transfusion-Transmitted
CLINICAL MICROBIOLOGY REVIEWS, Jan. 2011, p. 14 28 Vol. 24, No. 1 0893-8512/11/$12.00 doi:10.1128/cmr.00022-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Transfusion-Transmitted
Outbreak Investigation Guidance for Vectorborne Diseases
 COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
COMMUNICABLE DISEASE OUTBREAK MANUAL New Jersey s Public Health Response APPENDIX T3: EXTENDED GUIDANCE Outbreak Investigation Guidance for Vectorborne Diseases As per N.J.A.C. 8:57, viruses that are transmitted
The Alphabet Soup of Viral Hepatitis Testing
 The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
The New England Journal of Medicine ATOVAQUONE AND AZITHROMYCIN FOR THE TREATMENT OF BABESIOSIS. Study Population
 ATOVAQUONE AND AZITHROMYCIN FOR THE TREATMENT OF BABESIOSIS PETER J. KRAUSE, M.D., TIMOTHY LEPORE, M.D., VIJAY K. SIKAND, M.D., JOSEPH GADBAW, JR., M.D., GEORGINE BURKE, PH.D., SAM R. TELFORD III, SC.D.,
ATOVAQUONE AND AZITHROMYCIN FOR THE TREATMENT OF BABESIOSIS PETER J. KRAUSE, M.D., TIMOTHY LEPORE, M.D., VIJAY K. SIKAND, M.D., JOSEPH GADBAW, JR., M.D., GEORGINE BURKE, PH.D., SAM R. TELFORD III, SC.D.,
WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT. Product: Murex HIV Ag/Ab Combination Number: PQDx
 WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT Product: Murex HIV Ag/Ab Combination Number: PQDx 0144-043-00 Abstract Murex HIV Ag/Ab Combination with product codes 7G79-09 (GE41,
WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT Product: Murex HIV Ag/Ab Combination Number: PQDx 0144-043-00 Abstract Murex HIV Ag/Ab Combination with product codes 7G79-09 (GE41,
Toxoplasma gondii IgM ELISA Kit
 Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
DIAGNOSTIC AUTOMATION, INC.
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Identification of Microbes Lecture: 12
 Diagnostic Microbiology Identification of Microbes Lecture: 12 Electron Microscopy 106 virus particles per ml required for visualization, 50,000-60,000 magnification normally used. Viruses may be detected
Diagnostic Microbiology Identification of Microbes Lecture: 12 Electron Microscopy 106 virus particles per ml required for visualization, 50,000-60,000 magnification normally used. Viruses may be detected
Panel & Test Price List Effective Date: October 16, 2017
 -New tests now available: Bartonella IGXSpot, Bartonella Western Blot IgG & IgM -Tests Now available for New York residents: Lyme ImmunoBlots IgG & IgM *IGXSP IGXSpot Panel $442.50 Lyme IGXSpot 86352 Bartonella
-New tests now available: Bartonella IGXSpot, Bartonella Western Blot IgG & IgM -Tests Now available for New York residents: Lyme ImmunoBlots IgG & IgM *IGXSP IGXSpot Panel $442.50 Lyme IGXSpot 86352 Bartonella
MAJOR ARTICLE. John A. Branda, 1 Katy Linskey, 1 Yeowon A. Kim, 1 Allen C. Steere, 2 and Mary Jane Ferraro 1,2
 MAJOR ARTICLE Two-Tiered Antibody Testing for Lyme Disease With Use of 2 Enzyme Immunoassays, a Whole-Cell Sonicate Enzyme Immunoassay Followed by a VlsE C6 Peptide Enzyme Immunoassay John A. Branda, 1
MAJOR ARTICLE Two-Tiered Antibody Testing for Lyme Disease With Use of 2 Enzyme Immunoassays, a Whole-Cell Sonicate Enzyme Immunoassay Followed by a VlsE C6 Peptide Enzyme Immunoassay John A. Branda, 1
Learning Objectives. New HIV Testing Algorithm from CDC. Overview of HIV infection and disease 3/15/2016
 New HIV Testing Algorithm from CDC ASCLS-Michigan March 31, 2016 Dr. Kathleen Hoag Learning Objectives Following attendance and review of material provided, attendees will be able to: 1. Describe the new
New HIV Testing Algorithm from CDC ASCLS-Michigan March 31, 2016 Dr. Kathleen Hoag Learning Objectives Following attendance and review of material provided, attendees will be able to: 1. Describe the new
Laboratory diagnosis of congenital infections
 Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
SELECTED INFECTIONS ACQUIRED DURING TRAVELLING IN NORTH AMERICA. Lin Li, MD August, 2012
 SELECTED INFECTIONS ACQUIRED DURING TRAVELLING IN NORTH AMERICA Lin Li, MD August, 2012 Case 1 32 year old male working in Arizona; on leave back in Singapore Presented to hospital A for fever x (7-10)
SELECTED INFECTIONS ACQUIRED DURING TRAVELLING IN NORTH AMERICA Lin Li, MD August, 2012 Case 1 32 year old male working in Arizona; on leave back in Singapore Presented to hospital A for fever x (7-10)
LU:research Institutional Repository of Lund University
 LU:research Institutional Repository of Lund University This is an author produced version of a paper published in European journal of clinical microbiology & infectious diseases: official publication
LU:research Institutional Repository of Lund University This is an author produced version of a paper published in European journal of clinical microbiology & infectious diseases: official publication
HIV Update in Laboratory Testing. Patricia Slev, PhD, D(ABCC)
 HIV Update in Laboratory Testing Patricia Slev, PhD, D(ABCC) Objectives Explain the advances in HIV diagnostics, including fourth generation Ag/Ab combination HIV screening assays Describe the new CDC
HIV Update in Laboratory Testing Patricia Slev, PhD, D(ABCC) Objectives Explain the advances in HIV diagnostics, including fourth generation Ag/Ab combination HIV screening assays Describe the new CDC
HIV Diagnostic Testing
 In The name of God HIV Diagnostic Testing By : Dr. Shahzamani PhD of Medical virology Purpose of HIV Testing To identify asymptomatic individuals To diagnose HIV infection in those who practice high risk
In The name of God HIV Diagnostic Testing By : Dr. Shahzamani PhD of Medical virology Purpose of HIV Testing To identify asymptomatic individuals To diagnose HIV infection in those who practice high risk
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Documentation, Codebook, and Frequencies
 2 Documentation, Codebook, and Frequencies Laboratory Component: Hepatitis B: core antibody, surface antibody and surface antigen; Hepatitis C: confirmed antibody; Hepatitis D antibody Survey Years: 2003
2 Documentation, Codebook, and Frequencies Laboratory Component: Hepatitis B: core antibody, surface antibody and surface antigen; Hepatitis C: confirmed antibody; Hepatitis D antibody Survey Years: 2003
Toxoplasma gondii IgM ELISA Kit
 Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Module 3: Overview of HIV Testing Technologies
 Module 3: Overview of HIV Testing Technologies Purpose Pre-requisite Modules Module Time Learning Objectives To provide the participants with a basic knowledge of HIV testing and how HIV rapid test results
Module 3: Overview of HIV Testing Technologies Purpose Pre-requisite Modules Module Time Learning Objectives To provide the participants with a basic knowledge of HIV testing and how HIV rapid test results
See external label 2 C-8 C 96 tests Chemiluminescence. CMV IgM. Cat # Diluted samples, controls & calibrator 100 µl 30 minutes
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Screening the Blood Supply for West Nile Virus RNA by Nucleic Acid Amplification Testing
 The new england journal of medicine original article Screening the Blood Supply for West Nile Virus RNA by Nucleic Acid Amplification Testing Michael P. Busch, M.D., Ph.D., Sally Caglioti, M.T.(A.S.C.P.),
The new england journal of medicine original article Screening the Blood Supply for West Nile Virus RNA by Nucleic Acid Amplification Testing Michael P. Busch, M.D., Ph.D., Sally Caglioti, M.T.(A.S.C.P.),
Human Cytomegalovirus IgM ELISA Kit
 Human Cytomegalovirus IgM Catalog No: IRAPKT2012 ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgM ELISA is intended for use in the detection of IgM antibodies to Cytomegalovirus (CMV) infection in human
Human Cytomegalovirus IgM Catalog No: IRAPKT2012 ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgM ELISA is intended for use in the detection of IgM antibodies to Cytomegalovirus (CMV) infection in human
See external label 96 tests HSV 2 IgA. Cat #
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
WHO Prequalification of Diagnostics Programme PUBLIC REPORT
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: Murex HBsAg Version 3 with Murex HBsAg Confirmatory Version 3 Number: PQDx 0121-043-00 Abstract Murex HBsAg Version 3 with Murex HBsAg
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: Murex HBsAg Version 3 with Murex HBsAg Confirmatory Version 3 Number: PQDx 0121-043-00 Abstract Murex HBsAg Version 3 with Murex HBsAg
Human Cytomegalovirus Virus (CMV) IgG ELISA Kit
 Human Cytomegalovirus Virus Catalog No: IRAPKT1410 (CMV) IgG ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgG ELISA is intended for use in evaluating a patient s serologic status to cytomegalovirus (CMV)
Human Cytomegalovirus Virus Catalog No: IRAPKT1410 (CMV) IgG ELISA Kit Lot No: SAMPLE INTENDED USE The CMV IgG ELISA is intended for use in evaluating a patient s serologic status to cytomegalovirus (CMV)
Rubella virus IgG ELISA Kit
 Rubella virus IgG ELISA Kit Catalog Number KA0223 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
Rubella virus IgG ELISA Kit Catalog Number KA0223 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
Herpes Simplex Virus 2 IgM HSV 2 IgM
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
July Billing and Compliance. Chemistry. Help Us Help You. Referral Testing
 July 2018 Billing and Compliance New Medicare card mailings have begun for Minnesota and Wisconsin residents Chemistry Beta hydroxybutyrate, whole blood specimen requirement change Help Us Help You Preventing
July 2018 Billing and Compliance New Medicare card mailings have begun for Minnesota and Wisconsin residents Chemistry Beta hydroxybutyrate, whole blood specimen requirement change Help Us Help You Preventing
STATEMENT FOR MANAGING LYME DISEASE IN NOVA SCOTIA
 INFECTIOUS DISEASES EXPERT GROUP (IDEG) DEPARTMENT OF HEALTH AND WELLNESS STATEMENT FOR MANAGING LYME DISEASE IN NOVA SCOTIA Executive Summary: In 2016, the Public Health Agency of Canada (PHAC) modified
INFECTIOUS DISEASES EXPERT GROUP (IDEG) DEPARTMENT OF HEALTH AND WELLNESS STATEMENT FOR MANAGING LYME DISEASE IN NOVA SCOTIA Executive Summary: In 2016, the Public Health Agency of Canada (PHAC) modified
Pelagia Research Library. European Journal of Experimental Biology, 2015, 5(10):1-5
 Available online at www.pelagiaresearchlibrary.com European Journal of Experimental Biology, 2015, 5(10):1-5 ISSN: 2248 9215 CODEN (USA): EJEBAU Molecular diagnosis of human immuno deficiency virus (HIV)
Available online at www.pelagiaresearchlibrary.com European Journal of Experimental Biology, 2015, 5(10):1-5 ISSN: 2248 9215 CODEN (USA): EJEBAU Molecular diagnosis of human immuno deficiency virus (HIV)
Update on Lyme Disease Surveillance in Wisconsin for Providers and Laboratories
 Update on Lyme Disease Surveillance in Wisconsin for Providers and Laboratories Christopher Steward Division of Public Health Wisconsin Department of Health Services 04/10/14 Protecting and promoting the
Update on Lyme Disease Surveillance in Wisconsin for Providers and Laboratories Christopher Steward Division of Public Health Wisconsin Department of Health Services 04/10/14 Protecting and promoting the
Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran
 Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran Aliehsan Heidari, Manizheh Nourian, Hossein Keshavarz Associate Prof. Dept.
Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran Aliehsan Heidari, Manizheh Nourian, Hossein Keshavarz Associate Prof. Dept.
Emerging Pathogens that Impact the Canadian Blood Supply Alberta Vein to Vein Conference March 18-19, 2016
 Emerging Pathogens that Impact the Canadian Blood Supply Alberta Vein to Vein Conference March 18-19, 2016 Dr. Margaret Fearon Medical Director, Medical Microbiology, Canadian Blood Services March 18,
Emerging Pathogens that Impact the Canadian Blood Supply Alberta Vein to Vein Conference March 18-19, 2016 Dr. Margaret Fearon Medical Director, Medical Microbiology, Canadian Blood Services March 18,
Viral Hepatitis Diagnosis and Management
 Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
HIV-1 p24 Antigen ELISA Catalog Number:
 INTENDED USE The RETRO-TEK HIV-1 p24 Antigen ELISA is supplied for research purposes only. It is not intended for use in the diagnosis or prognosis of disease, or for screening and may not be used as a
INTENDED USE The RETRO-TEK HIV-1 p24 Antigen ELISA is supplied for research purposes only. It is not intended for use in the diagnosis or prognosis of disease, or for screening and may not be used as a
With the aim of continuous improvement of our products, Biokit increases the shelf life of bioelisa HIV 1+2 Ag/Ab from 10 to 12 months.
 1 NEWS BK NEWS # 377 /MKT DATE : 18-01-2016 TITLE : SHELF-LIFE EXTENSION OF bioelisa HIV 1+2 Ag/Ab With the aim of continuous improvement of our products, Biokit increases the shelf life of bioelisa HIV
1 NEWS BK NEWS # 377 /MKT DATE : 18-01-2016 TITLE : SHELF-LIFE EXTENSION OF bioelisa HIV 1+2 Ag/Ab With the aim of continuous improvement of our products, Biokit increases the shelf life of bioelisa HIV
Infectious Diseases Expert Group (IDEG) Department of Health and Wellness. Statement for Managing Lyme Disease in Nova Scotia
 Infectious Diseases Expert Group (IDEG) Department of Health and Wellness Statement for Managing Lyme Disease in Nova Scotia 2018 Executive Summary: In 2016, the Public Health Agency of Canada (PHAC) modified
Infectious Diseases Expert Group (IDEG) Department of Health and Wellness Statement for Managing Lyme Disease in Nova Scotia 2018 Executive Summary: In 2016, the Public Health Agency of Canada (PHAC) modified
Blood Smears Only 6 October Sample Preparation and Quality Control 15B-K
 NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 6 October 5 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
NEW YORK STATE Parasitology Proficiency Testing Program Blood Smears Only 6 October 5 The purpose of the New York State Proficiency Testing Program in the category of Parasitology - Blood Smears Only is
Guidance for Industry
 Guidance for Industry Adequate and Appropriate Donor Screening Tests for Hepatitis B; Hepatitis B Surface Antigen (HBsAg) Assays Used to Test Donors of Whole Blood and Blood Components, Including Source
Guidance for Industry Adequate and Appropriate Donor Screening Tests for Hepatitis B; Hepatitis B Surface Antigen (HBsAg) Assays Used to Test Donors of Whole Blood and Blood Components, Including Source
Center for Biologics Evaluation and Research
 Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
Guidance for Industry
 Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Research Article Decision on conducting HCV Immunoblot and HCV Viral Load Tests Dependent upon the Result of the Screening Tests
 IBIMA Publishing Journal of Virology & Microbiology http://www.ibimapublishing.com/journals/jvm/jvm.html Vol. 2013 (2013), Article ID 332501, 7 pages DOI: 10.5171/2013.332501 Research Article Decision
IBIMA Publishing Journal of Virology & Microbiology http://www.ibimapublishing.com/journals/jvm/jvm.html Vol. 2013 (2013), Article ID 332501, 7 pages DOI: 10.5171/2013.332501 Research Article Decision
Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone
 DOI 10.1186/s12941-017-0198-9 Annals of Clinical Microbiology and Antimicrobials RESEARCH Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone Ekaterina
DOI 10.1186/s12941-017-0198-9 Annals of Clinical Microbiology and Antimicrobials RESEARCH Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone Ekaterina
STARHS/RITA and Misclassification
 Optimization and Calibration of Less Sensitive and Avidity Modified Protocols for the Vitros Immunodiagnostic Products Anti-HIV- 1+2 Assay for Detection of Early HIV Infections Sheila M. Keating, Debra
Optimization and Calibration of Less Sensitive and Avidity Modified Protocols for the Vitros Immunodiagnostic Products Anti-HIV- 1+2 Assay for Detection of Early HIV Infections Sheila M. Keating, Debra
Lyme Disease and Tick Surveillance in British Columbia
 Lyme Disease and Tick Surveillance in British Columbia Muhammad Morshed, PhD, SCCM Program Head, Zoonotic Diseases & Emerging Pathogens BCCDC Public Health Microbiology and Reference Laboratory Provincial
Lyme Disease and Tick Surveillance in British Columbia Muhammad Morshed, PhD, SCCM Program Head, Zoonotic Diseases & Emerging Pathogens BCCDC Public Health Microbiology and Reference Laboratory Provincial
CYTOMEGALOVIRUS (CMV) IgM ELISA Kit Protocol
 CYTOMEGALOVIRUS (CMV) IgM ELISA Kit Protocol (Cat. No.:EK-310-91) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED USE The
CYTOMEGALOVIRUS (CMV) IgM ELISA Kit Protocol (Cat. No.:EK-310-91) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED USE The
3. Rapidly recognize influenza seasons in which the impact of influenza appears to be unusually severe among children.
 07-ID-14 Committee: Title: Infectious Disease Influenza-Associated Pediatric Mortality Statement of the Problem: In 2004, CSTE adopted influenza-associated pediatric mortality reporting with a provision
07-ID-14 Committee: Title: Infectious Disease Influenza-Associated Pediatric Mortality Statement of the Problem: In 2004, CSTE adopted influenza-associated pediatric mortality reporting with a provision
FDA Reentry Guidance
 FDA Reentry Guidance T. cruzi - Chagas, Hepatitis C and HIV Wednesday 6/6/18 Doug Denyer O Dina Hurlburt, SBB(ASCP)CM Outline Impact to Clients Review of FDA Guidance Documents Reentry Request Process
FDA Reentry Guidance T. cruzi - Chagas, Hepatitis C and HIV Wednesday 6/6/18 Doug Denyer O Dina Hurlburt, SBB(ASCP)CM Outline Impact to Clients Review of FDA Guidance Documents Reentry Request Process
MALARIA P. FALCIPARUM / P. VIVAX
 MALARIA P. FALCIPARUM / P. VIVAX 1. EXPLANATION OF THE TEST: Malaria is a serious, sometimes fatal, parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted
MALARIA P. FALCIPARUM / P. VIVAX 1. EXPLANATION OF THE TEST: Malaria is a serious, sometimes fatal, parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted
HIV Testing Technology and the Latest Algorithm
 HIV Testing Technology and the Latest Algorithm David Warshauer, PhD, D(ABMM) Deputy Director, Communicable Diseases Wisconsin State Laboratory of Hygiene HIV Testing has changed over time Patients with
HIV Testing Technology and the Latest Algorithm David Warshauer, PhD, D(ABMM) Deputy Director, Communicable Diseases Wisconsin State Laboratory of Hygiene HIV Testing has changed over time Patients with
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: Alere HIV Combo WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: Alere HIV Combo WHO reference number: PQDx 0243-013-00 Alere HIV Combo with product codes 7D2842, 7D2843, 7D2843SET manufactured by Alere
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: Alere HIV Combo WHO reference number: PQDx 0243-013-00 Alere HIV Combo with product codes 7D2842, 7D2843, 7D2843SET manufactured by Alere
Identifying false-positive syphilis antibody results using a semi-quantitative
 CVI Accepts, published online ahead of print on 20 April 2011 Clin. Vaccine Immunol. doi:10.1128/cvi.05066-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All
CVI Accepts, published online ahead of print on 20 April 2011 Clin. Vaccine Immunol. doi:10.1128/cvi.05066-11 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions. All
Measles IgM ELISA Kit
 Measles IgM ELISA Kit Catalog Number KA2257 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Measles IgM ELISA Kit Catalog Number KA2257 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Innovation in Diagnostics. ToRCH. A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES
 Innovation in Diagnostics ToRCH A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES EN TOXOPLASMOSIS Toxoplasmosis is a parasitic disease caused by with the obligate intracellular
Innovation in Diagnostics ToRCH A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES EN TOXOPLASMOSIS Toxoplasmosis is a parasitic disease caused by with the obligate intracellular
DCO Registration. If you have any questions contact the DCO help desk at:
 DCO Registration Please register using the two simple steps below: 1. Log-in or create a CME account: https://tiny.army.mil/r/zb8a/cme **Tip: If your facility is not listed as an option on the registration
DCO Registration Please register using the two simple steps below: 1. Log-in or create a CME account: https://tiny.army.mil/r/zb8a/cme **Tip: If your facility is not listed as an option on the registration
The Federal Tick-borne Disease Working Group and CDC's current activities on IPM for Lyme disease prevention and control
 The Federal Tick-borne Disease Working Group and CDC's current activities on IPM for Lyme disease prevention and control C. Ben Beard, Ph.D. Chief, Bacterial Diseases Branch CDC Division of Vector-Borne
The Federal Tick-borne Disease Working Group and CDC's current activities on IPM for Lyme disease prevention and control C. Ben Beard, Ph.D. Chief, Bacterial Diseases Branch CDC Division of Vector-Borne
SIV p27 Antigen ELISA Catalog Number:
 INTENDED USE The RETRO-TEK SIV p27 Antigen ELISA is for research use only and is not intended for in vitro diagnostic use. The RETRO-TEK SIV p27 Antigen ELISA is an enzyme linked immunoassay used to detect
INTENDED USE The RETRO-TEK SIV p27 Antigen ELISA is for research use only and is not intended for in vitro diagnostic use. The RETRO-TEK SIV p27 Antigen ELISA is an enzyme linked immunoassay used to detect
Diagnostic Methods of HBV and HDV infections
 Diagnostic Methods of HBV and HDV infections Zohreh Sharifi,ph.D Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine Hepatitis B-laboratory diagnosis Detection
Diagnostic Methods of HBV and HDV infections Zohreh Sharifi,ph.D Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine Hepatitis B-laboratory diagnosis Detection
Surveillance Report 2016
 Surveillance Report 2016 Executive summary We are pleased to present the fifth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2016 Executive summary We are pleased to present the fifth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
The Challenges in Developing and Commercializing HIV Tests that are Useful in Differentiating VISP/R VISP/R Workshop Bethesda, MD March 2013
 The Challenges in Developing and Commercializing HIV Tests that are Useful in Differentiating VISP/R VISP/R Workshop Bethesda, MD March 2013 Christopher Bentsen, M.S.,RAC, FRAPS Bio-Rad Laboratories, Redmond,
The Challenges in Developing and Commercializing HIV Tests that are Useful in Differentiating VISP/R VISP/R Workshop Bethesda, MD March 2013 Christopher Bentsen, M.S.,RAC, FRAPS Bio-Rad Laboratories, Redmond,
Situation of XMRV and Blood Transfusion. Celso Bianco, MD ISBT Working Party on TTID Lisbon, June 19, 2011
 Situation of XMRV and Blood Transfusion Celso Bianco, MD ISBT Working Party on TTID Lisbon, June 19, 2011 Lombardi et al. Science 326, 585 (2009) Conclusions: CFS and XMRV XMRV found in 67% of CFS patients
Situation of XMRV and Blood Transfusion Celso Bianco, MD ISBT Working Party on TTID Lisbon, June 19, 2011 Lombardi et al. Science 326, 585 (2009) Conclusions: CFS and XMRV XMRV found in 67% of CFS patients
Using administrative medical claims data to estimate underreporting of infectious zoonotic diseases
 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 40% Percentage of Yearly Cases 30% 25% 20% 15% 10% 5% 0% January Februar March April May June July August Septem October Novem Decem January Februar March
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 40% Percentage of Yearly Cases 30% 25% 20% 15% 10% 5% 0% January Februar March April May June July August Septem October Novem Decem January Februar March
ZIKA VIRUS TESTING GUIDANCE, UPDATED 7/20/2016
 Section 1: Which patients should be offered testing? SUMMARY OF CATEGORIES OF PATIENTS WHO SHOULD BE TESTED FOR ZIKA 1) Pregnant women who traveled to a Zika-affected area (see link to current list in
Section 1: Which patients should be offered testing? SUMMARY OF CATEGORIES OF PATIENTS WHO SHOULD BE TESTED FOR ZIKA 1) Pregnant women who traveled to a Zika-affected area (see link to current list in
A Summary of Clinical Evidence
 A Summary of Clinical Evidence Supporting the use of the Alere Determine HIV-1/2 Ag/Ab Combo Rapid Test to assist in the diagnosis of Human Immunodeficiency Virus (HIV) TAP HERE TO SEE THE PRODUCTS Table
A Summary of Clinical Evidence Supporting the use of the Alere Determine HIV-1/2 Ag/Ab Combo Rapid Test to assist in the diagnosis of Human Immunodeficiency Virus (HIV) TAP HERE TO SEE THE PRODUCTS Table
Serodiagnosis of Canine Babesiosis
 Serodiagnosis of Canine Babesiosis Xuenan XUAN, DVM, PhD National Research Center for Protozoan Diseases Obihiro University of Agriculture and Veterinary Medicine Obihiro, Hokkaido 080-8555, Japan Tel.:+81-155-495648;
Serodiagnosis of Canine Babesiosis Xuenan XUAN, DVM, PhD National Research Center for Protozoan Diseases Obihiro University of Agriculture and Veterinary Medicine Obihiro, Hokkaido 080-8555, Japan Tel.:+81-155-495648;
HIV Guideline Sakchai Dettrairat
 HIV Guideline 2016 Sakchai Dettrairat Division of Clinical Immunology Department of Medical Technology Faculty of Associated Medical Sciences Chiang Mai University Appearance of HIV markers in early HIV
HIV Guideline 2016 Sakchai Dettrairat Division of Clinical Immunology Department of Medical Technology Faculty of Associated Medical Sciences Chiang Mai University Appearance of HIV markers in early HIV
Received 24 November 1997/Returned for modification 11 February 1998/Accepted 6 April 1998
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, July 1998, p. 486 490 Vol. 5, No. 4 1071-412X/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. Seroprevalence of Antibodies
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, July 1998, p. 486 490 Vol. 5, No. 4 1071-412X/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. Seroprevalence of Antibodies
Malaria screening of donors and donations - issues and outcomes
 Malaria screening of donors and donations - issues and outcomes lan Kitchen National Transfusion Microbiology NHS Blood & Transplant London IPF/PEI, Rome, May 2014 Blood and Transplant Key elements Nature
Malaria screening of donors and donations - issues and outcomes lan Kitchen National Transfusion Microbiology NHS Blood & Transplant London IPF/PEI, Rome, May 2014 Blood and Transplant Key elements Nature
10/19/2012. Serologic Testing for Syphilis. Disclosures. Comparison of the Traditional and Reverse Screening Algorithms. Outline.
 Serologic Testing for Syphilis Comparison of the Traditional and Reverse Screening Algorithms Disclosures Elli S. Theel, Ph.D. Director, Infectious Diseases Serology Laboratory Assistant Professor of Laboratory
Serologic Testing for Syphilis Comparison of the Traditional and Reverse Screening Algorithms Disclosures Elli S. Theel, Ph.D. Director, Infectious Diseases Serology Laboratory Assistant Professor of Laboratory
