Cherokee Trail High School Blood Drive. Please help us to save lives! February 14th. 8:00 to 9:40 am & 11:00 am to 1:30 pm Upper Lecture Center
|
|
|
- Abner Mills
- 5 years ago
- Views:
Transcription
1 Cherokee Trail High School Blood Drive Please help us to save lives! February 14th 8:00 to 9:40 am & 11:00 am to 1:30 pm Upper Lecture Center To be eligible to donate you need to: weigh at least 110 pounds be at least 16-years-old & have signed Parental Consent form if you are 16 or 17 have not traveled to a malaria risk country in the past year eat a good meal and drink plenty of water before your donation receive teacher permission to donate during a class (cannot have an F in the class you will miss) bring your ID with you to the drive allow 1 hour to potentially save 3 lives! Questions? Contact Pam Graham ( ) or at pgraham3@cherrycreekschools.org It only takes about an hour to save up to 3 lives
2 Parental Acknowledgement Form CDO-013-PRD Acknowledgement from parent/guardian for minor to donate blood REV 11rev 18 ATTENTION MINOR AND PARENT/GUARDIAN: Please Read All Information on Front AND Back then Complete and Sign Form IMPORTANT FACTS FOR BLOOD DONORS REQUIRED ITEMS FOR MINOR TO BRING ON DAY OF DONATION If you are 16 or 17 years of age you must have the Parental Acknowledgment Form completely filled out and signed by your parent or guardian to be able to donate on the day of your drive. No matter your age, you must have some form of identification with you when you present to donate on the day of your drive. Examples of identification include but are not limited to: driver s license, work badge, school ID, credit card, mail, etc. LIFESAVING IMPACT OF BLOOD DONORS Giving blood and time makes you part of a local nonprofit mission and lifesaving cause. Nearly 8 percent of Bonfils blood collection comes from high school blood drives. Donating blood is an easy way to volunteer and save lives throughout the year. EATING AND DRINKING BEFORE AND AFTER DONATING BLOOD It is imperative that prior to your donation you eat within 2 hours and hydrate for hours so your donation process is smooth and you feel better during the process. After your donation it is also important to rest in our canteen area, have a snack and rehydrate so your body will have additional energy to compensate for your donation. PHYSICAL ACTIVITY AND SPORTS AFTER DONATING BLOOD Donating blood leads to a temporary decrease in the volume of oxygen-carrying red blood cells so strenuous activity after donation can lead to side effects like light-headedness or dizziness. Do not engage in strenuous activities such as lifting, running, pushing or picking up heavy objects for at least four to five hours after donating blood. If you have sports practice or a game on the day of the blood drive, we recommend you do not practice or play or if you are required to practice or play that you do not donate blood. Parental Acknowledgement Form In order to donate, this form MUST be filled out completely and printed clearly with a BLACK or BLUE ballpoint pen. Donor s Name: Donor's Last 4 SSN (if known): Donor's Date of Birth: Donor's Age: Donor's Address: CDO-013-PRD REV 11 City/State/Zip: Donor's Phone #: *Donor MUST present with a form of identification at time of donation. Donor is emancipated and has been verified by staff. I acknowledge that I have read and understand the information provided in the acknowledgement statements on the back side of this document and the blood donor requirements and I authorize the minor listed below, who is my son, daughter or someone for whom I am legally authorized to provide medical authorization, to provide a blood donation to Bonfils Blood Center. I also authorize a photo release if pictures are taken at the donation site. I understand that sensitive and personal information will be obtained from the donor prior to any donation as part of the routine donor screening process. This includes information required by the FDA pertaining to the donor s sexual history and Bonfils is required to define sexual content with explicit language that will be available on materials given to my son/daughter. Based upon this information, Bonfils will determine the suitability of the donor to donate blood. Information obtained from the donation is confidential and will not be released to third parties without the donor s consent or as otherwise required by law. I understand that I may be informed of my child's test results should Bonfils be unable to reach my child. Our blood center participates in research to improve blood safety. We may use your son/daughter s donor history information and a sample of your son/daughter s blood, in a confidential manner, for blood safety research, as described in the accompanying research information document(s). We are required to get parental consent for both 16- and 17- year old donors for this research. For more information about this research or blood donation, go to bonfils.org/reading. IN SIGNING THIS FORM, I ACKNOWLEDGE THAT MY SON/DAUGHTER IS 16 OR 17 YEARS OLD. Minor s Full Legal Name: Parent/Guardian s Name: Parent/Guardian s Signature: Phone # to call during minor s donation: Relationship to Donor: Date: PG 1 OF 2 Please call Bonfils Donor Relations department at or , opt. 1, if you have questions about your minor s donation.
3 ATTENTION MINOR AND PARENT/GUARDIAN: Please Read All Information on Front AND Back then Complete and Sign Form Below are the consent statements your son/daughter will be asked to read and sign before they donate. By signing the form on the front, you are consenting for your 16 or 17-year-old to make a voluntary blood donation. This signed form is the only verification that will be accepted - we cannot accept phone verifications. WHOLE BLOOD/PHLEBOTOMY ACKNOWLEDGEMENT Phlebotomy is the process of withdrawing blood from a vein. I am voluntarily consenting to the phlebotomy procedure for the donation of blood and have had the chance to refuse the phlebotomy procedure. Furthermore, I certify that I have answered truthfully all of the questions addressed to me regarding my present and prior illnesses, symptoms and physical conditions. I have read and truthfully answered the questions set forth in the donor questionnaire. I understand that by not disclosing all of the information set forth in the donor questionnaire, I could put myself at risk for complications or place others at risk of a transfusion transmitted disease as a result of this donation. In giving consent to Bonfils Blood Center to perform phlebotomy, I acknowledge that the procedure of phlebotomy has been explained to me and that I have had the opportunity to discuss the risks associated with phlebotomy and ask any and all questions. I understand that my blood will be TESTED for HIV, AIDS, hepatitis and other diseases. If these tests indicate that I should no longer donate blood or plasma because of a risk of transmitting disease, my name will be entered in a list of permanently deferred donors. I understand that I will be notified of an abnormal test result, which will be reported to authorities as required by law. Some tests may be unlicensed or used for research purposes. I understand that I SHOULD NOT DONATE blood if I am at risk for HIV/AIDS or hepatitis (refer to Making Your Blood Donation Safe ). If I consider myself to be a person at risk for spreading the virus known to cause AIDS, or other infectious diseases, I agree not to donate blood or other blood products for transfusion to another person or for further manufacture. I further understand that there are known COMPLICATIONS associated with donating blood. Complications can occur at the site where the needle was inserted and may extend beyond my arm and cause systemic complications throughout my body. Localized complications include: Pain Soreness Redness Bruising Swelling Bleeding vascular injury Tissue scarring Localized infection Systemic complications may include: Systemic infections Lightheadedness Fainting or passing out which may result in additional injuries if I fall or drive. I also understand that on rare occasions severe reactions to a phlebotomy procedure can have long-term or permanent effects including, but not limited to damage to nerve or muscle at or around the phlebotomy site which may result in numbness, pain or localized paralysis and the need for extended medical treatment. I understand that the blood I donate today may be used for transfusion to a patient or any other medical need Bonfils Blood Center has for its use. I also understand that my blood may be used for further manufacturing, research or investigational studies (no DNA analysis will be conducted). I give my consent to have a phlebotomist draw blood from me today. APHERESIS ACKNOWLEDGEMENT I hereby voluntarily give my permission to Bonfils Blood Center to remove blood from my body on this day by an automated procedure called apheresis. Apheresis is a procedure whereby needle(s) are placed into the vein(s) of one or both arms, whole blood is withdrawn from the donor and mixed with an anticoagulant (an agent which prevents or delays blood coagulation). The blood is then mechanically separated into various parts and a portion of that blood (red blood cells, platelets and/or plasma) is transferred to separate bags and saved. The remainder of the blood is returned to the donor (myself). This procedure may take between one and two hours to perform, during which time I will be connected to the machine and unable to get up and move around freely. Risks of apheresis range from mild discomfort to severe reactions and include but are not limited to: Anxiety, headache, pale skin tone, excessive tiredness or general weakness Hives or other allergic reaction Numbness and/or tingling of face, lips and/or fingers or muscle tension from the citrate during return of blood Low blood pressure or convulsions due to changes in blood volume Chills, fever or feelings of warmth Shortness of breath or hyperventilation Unpleasant taste sensation, nausea or vomiting PHOTO RELEASE Additional side effects for plateletpheresis (the removal of platelets during apheresis) may include a transient decrease in platelets and loss of a small amount of lymphocytes (a type of white blood cell) along with platelets. I have been informed that the effect of this loss is unclear. Loss of red blood cells due to leakage or breakage of the plastic tubing or containers may occur and thus prevent the return of red blood cells. Since the removal of blood and the return of blood are accomplished through the use of needles and tubing, it is possible that clotting could occur in the needles or tubing and this may lead to the termination of the individual procedure. There is a possibility that the red blood cells removed during the procedure could be broken down (hemolysis) due to a malfunction of the machine; however, this is rare. Although the machine is equipped with an air detector to prevent air bubbles, there is a remote possibility of an air bubble entering the donor. The consequence of this unlikely event could be severe. These, along with technical difficulties and side effects, could cause discomfort or serious problems. I understand that I will receive no medical benefit from this procedure, that my donation is voluntary and I could alternatively not undergo apheresis. I understand that I should avoid strenuous use of my arm(s) for about four hours after the donation. I have read the above statements. The procedure and risks have been explained to me. I have been given ample opportunity to ask questions about the procedure, the risks and anything I did not understand. I have had an opportunity to refuse permission and realize I can withdraw permission at any time. I give Bonfils Blood Center permission to use my son/daughters photos or likeness in future promotional materials. CDO-013-PRD REV 11rev 18 Please call Bonfils Donor Relations department at or , opt. 1, if you have questions about your minor s donation. PG 2 OF 2
4 Donor Prescreening Questions CDO-384-PRD REV 21rev Please read the following in its entirety to help guarantee your safety and the safety of the community blood supply and to help ensure you have a positive donation experience. Bonfils recommends that prior to your donation you eat within 2 hours and hydrate for hours. If you would like something to eat or drink before your donation please help yourself to the items in the canteen area. Donors are commonly deferred for the following questions. Bonfils asks these prescreening questions so donors do not spend time answering our full questionnaire only to find they are ineligible for a common reason. Even if you answer no to all of these prescreening questions, you may be ineligible to donate based on the answers given during the full questionnaire and interview process completed just prior to the actual donation. If you answer yes to any of these questions on the day you present to donate, you will NOT be eligible to donate at that time. If you have any questions related to travel or your eligibility to donate as a whole contact Bonfils Donor Relations department at or , opt. 1 or ask a Bonfils staff member at the time of your donation. HEALTH STATUS AND AGE 1. Do you weigh less than 110 pounds or more than 350 pounds? 2. Are you 15 years or younger? 3. Are you ill or not feeling well today? 4. Have you ever been diagnosed with lymphoma and/or leukemia? MEDICATIONS 5. Are you taking antibiotics today for an active infection? 6. Have you taken Accutane, Proscar or Propecia in the last month? 7. Have you taken Coumadin/Warfarin in the last 7 days? TRAVEL AND RESIDENCE ABROAD 8. From 1980 through 1990 were you in Belgium, Germany, the Netherlands or Holland as a member of the U.S. military, a civilian military employee or a dependent of a member of the U.S. military for 6 months or more?* 9. From 1980 through 1996 were you in Spain, Portugal, Turkey, Italy, Sicily or Greece as a member of the U.S. military, a civilian military employee or a dependent of a member of the U.S. military for 6 months or more?* 10. From 1980 to present have you spent time that adds up to 5 years or more in Europe?* 11. In the past 12 months have you spent more than 24 hours in (traveled to) any individual location outside of the U.S. or Canada?* a. If yes, did you travel to any of the countries or cities listed as a malarial risk area on the table on the back of this page? There are many ways to save lives with Bonfils. You can also help through Bonfils marrow program and volunteer opportunities. *Additionally, if you are deferred for these categories, you may still be eligible for research donations. Visit bonfils.org to learn more. PG 1 OF 2 Please call Bonfils Donor Relations department at or , opt. 1 for complete information on donor eligibility.
5 The table below is referenced in prescreening question 11a. Like the prescreening questions on this document that reference common deferrals but not all deferrals, this table includes common malarial risk areas but it is not an all-inclusive list. AFGHANISTAN AFRICA Angola Botswana Burundi Cameroon Central African Republic Chad Congo Ethiopia Gabon Gambia Ghana Ivory Coast Kenya Liberia Mozambique Niger Nigeria Rwanda Senegal Sierra Leone Somalia Sudan Tanzania Uganda Zaire Zambia Zimbabwe CAMBODIA CHINA Dali Banna Chuxiong City Dian Lake Jade Dragon Snow Mountain Jinghong Kunming Lijiang Nansha New Yuanyang Old Yunanyang Panzhihua Shangri-la Shilin Stone Forest Tiger Leaping Gorge Xinje Yunnan DOMINICAN REPUBLIC Any area outside Santiago or Santo Domingo HAITI HONDURAS Any area outside San Pedro Sula or Tegucigalpa (including the Bay Islands) INDIA INDONESIA IRAN MALAYSIA MEXICO Chihuahua City Copper Canyon El Fuerte Hidalgo del Parral Los Mochis Mazatlan Nuevo Mazatlan Nuevo Vallarta Punta Mita Sayulita PANAMA Any area east of the Panama Canal including the region of Colon SOUTH KOREA Any area north of Seoul, including the Demilitarized Zone (DMZ) VENEZUELA CDO-384-PRD REV 21ev Please call Bonfils Donor Relations department at or , opt. 1 for complete information on donor eligibility. PG 2 OF 2
6 Medical Guidelines BASIC REQUIREMENTS FOR BLOOD DONATION BD_4020 BD_001 rev 10 Must be at least 18 years of age or 16 or 17 with written parental or guardian acknowledgement. Weigh at least 110 pounds; if greater than 350 pounds, please call Bonfils Appointment Center at or , opt. 2. For your safety and to ensure a positive donation experience Bonfils recommends that prior to your donation you eat within 2 hours and hydrate for hours. If you would like something to eat or drink before your donation please help yourself to the items in the canteen area. Be in good general health. Proof of identification is required at time of donation. Examples include but are not limited to: driver s license, work badge, school ID, credit card, mail, etc. The donation interval after a whole blood donation is 56 days. Please contact Bonfils Blood Center for the specific interval for other blood product donations. You should not be under the influence of alcohol or recreational drugs at the time of donation. MEDICATIONS Donors should not discontinue taking medications prescribed or recommended by their physicians in order to be eligible to donate. Are you being treated with the following types of medication... Have you taken... which is also called... in the last Feldene piroxicam 2 days Anti-platelet agents Effient prasugrel (usually taken to prevent Brilinta ticagrelor 7 days stroke or heart Plavix clopidogrel attack) Ticlid ticlopidine 14 days Zontivity vorapaxar Xeralto rivaroxaban Fragmin dalteparin Lovenox enoxaparin 2 days Pradaxa dabigatran Anticoagulants or Eliquis abixaban blood thinners (usually to prevent blood Savaysa edoxaban clots in the leg and Coumadin lungs and to prevent Warfilone warfarin stokes) Jantoven Heparin, low 7 days molecular heparin weight heparin Arixta fondaparinux Acne treatment Accutane Amnesteem Absorica Claravis isotretinoin Myorisan 1 month Sotret Zenatane Hair loss remedy Propecia finasteride Proscar finasteride Prostate Symptoms Avodart Jalyn dutasteride 6 months Basal cell skin cancer Erivedge vismodegib 7 months Relapsing multiple sclerosis Aubagio Teriflunomide 2 years Psoriasis Soriatane acitretin 3 years Tegison etretinate Ever Hepatitis exposure Hepatitis B Immune Globulin HBIG 12 months HEALTH CONDITIONS Ambulatory Aid: For their own safety, donors using any type of temporary ambulatory aids including crutches, canes or other walking aids for a recent injury cannot donate until no longer requiring this assistance. Breastfeeding: Female donors that are breastfeeding are eligible to donate. Cancer: Donor is eligible with a history of basal cell or squamous cell skin cancer or carcinoma in situ. Lymphoma and leukemia are permanent deferrals. All other types of cancer will be assessed at time of donation, but 12 months must have passed since the last treatment and donor must be considered cancer free at time of donation. Colds: Donors are not eligible if they are not feeling well and healthy the day of donation. Diabetes: Donors with diabetes (type I or II) are eligible to donate. Diabetics who ever used bovine insulin manufactured in the United Kingdom are deferred. Heart Disease: Donors with a history of heart disease or heart attacks may be eligible to donate provided six months have elapsed from the incident and approval has been obtained from donor s physician. Donors with Wolff- Parkinson-White syndrome, hypertrophic cardiomyopathy and implanted/internal defibrillators are permanently ineligible. Hemochromatosis/Polycythemia: Donors diagnosed with hereditary hemochromatosis or polycythemia are asked to contact Bonfils Donor Relations. Hepatitis: Donors with a history of hepatitis B or C should refrain from donating. High Blood Pressure: Donors with high blood pressure are eligible to donate if their blood pressure is within an acceptable range when taken on the day of donation, regardless of medication. Malaria: Donor is eligible if symptom free for three years. Pregnant: Donors who are pregnant or have been pregnant in the last 6 weeks are not eligible to donate. Transfusion/Transplants: Donor is deferred for 12 months following a transplant or transfusion of blood or of blood products. PG 1 OF 2 Please call Bonfils Donor Relations department at or , opt. 1 for complete information on donor eligibility.
7 VACCINATIONS/IMMUNIZATION Vaccination/Immunization Antitoxins/animal serum injections Measles (rubeola) Mumps Polio Salk (oral vaccine) Typhoid (oral) Yellow fever German measles (rubella) BCG (Bacille Calmette-Guerin) for tuberculosis (TB) disease Hepatitis B (Recombivax, Energix-B, Heptavax) MMR (measles/mumps/rubella) Varicella zoster (chicken pox, Varivax) Shingles (Zostavax) Do Not Donate for 2 weeks 1 month (30 days) HIV/AIDS Individuals who have had a positive test for HIV/ AIDS or who are at risk for contracting HIV/AIDS are permanently deferred from donating blood. Risk factors include: Use of needles to take drugs, steroids or anything not prescribed by your doctor Taking money or drugs for sex The following risk factors represent a 12-month deferral for the donor from the date of occurrence: Male to male sexual contact in the past 12 months Intimate/sexual contact with any person in the above categories Accidental contact with another person s blood/ body fluid or an accidental needle stick Incarceration for more than 72 consecutive hours EUROPEAN TRAVEL/RESIDENCY CRITERIA CJD Variant Creutzfeldt-Jakob Disease (human form of mad cow disease). Total cumulative time spent in the United Kingdom (see list below) adding up to 3 months or more from indefinitely defers a donor from community donation. However, these donors may be eligible for research donations. Total cumulative time spent in the European Countries listed below of 5 years or more is cause for indefinite deferral from community donation, but does not defer the donor from research donations. COUNTRIES IN UNITED KINGDOM: Channel Islands England Falkland Islands EUROPEAN COUNTRIES: Albania Austria Belgium Bosnia-Herzegovina Bulgaria Channel Islands Croatia Czech Republic Denmark England Falkland Islands Finland France* Germany Gibraltar Isle of Man Northern Ireland Gibraltar Greece Hungary Isle of Man Kosovo Northern Ireland Republic of Ireland Italy (Sicily) Liechtenstein Luxembourg Macedonia Montenegro Netherlands (Holland) Norway * including its overseas departments (e.g., Martinique and others) ** including the Canary Islands and Spanish North African territories *** including the Azores ****formerly Federal Republic of Yugoslavia Scotland Wales Poland Portugal** Romania Scotland Serbia Slovak Republic Slovenia Spain*** Sweden Switzerland United Kingdom Yugoslavia**** Wales ACUPUNCTURE Acupuncture/Dry-needling: Donor is eligible if procedure was performed with single-use equipment. Otherwise, donor is deferred for 12 months. OTHER WAYS TO HELP You can save lives through Bonfils marrow program and volunteer opportunities. Additionally, if you are deferred for the reasons on this document, you may still be eligible for research donations. Visit bonfils.org to learn more. Please call Bonfils Donor Relations department at or , opt. 1 for complete information on donor eligibility. PG 2 OF 2
8 Zika Virus Research Information Sponsor / Study Title: Hologic, Inc. / Pre-pivotal Procleix Zika Virus Assay Testing of Donations From Donors of Whole Blood and Blood Components Protocol Number: B10383-ZIKVPS-CSP-01 Principal Investigator: Phillip Willaimson, PhD Telephone: Donor Relations: Additional Contacts: Kadi Schroeder: Please read this form carefully. Take time to ask the donor center staff as many questions about the use of your blood for research studies as you would like. The donor center staff can explain words or information that you do not understand. Reading this form and talking to the donor center staff may help you decide whether to donate or not. You are being asked to participate in a research study to evaluate a new test for detection of a mosquito-borne agent known as Zika virus. Zika is a virus that rarely causes paralytic nervous system damage, but in pregnancy, can cause loss of the baby or serious birth defects. Most people do not get sick after infection. Only one in five people will have fever, rash, joint pain, and conjunctivitis (red eyes) lasting a few days to a week. Zika is usually transmitted by the bite of an infected mosquito. It can also be transmitted by sex with an infected man, from a pregnant mother to her baby and by blood transfusion. This donor center is doing a research study to understand the effectiveness of a new test to detect the Zika virus in donated blood and prevent patient exposure. Some of this research is conducted with other institutions, such as blood bank organizations, academic centers and biomedical companies. Any remainder of your donation may be stored up to 3 years after the completion of the study and used for further research related to the Zika virus. Samples linked to your identifying information will be tested for ZIKA virus. If your test results suggest that you may be infected, this donation center will attempt to contact you to notify you and explain the significance of the results. The donation center will discuss the potential risk for sexual transmission of Zika Virus, and potential harm to the fetus during pregnancy. You will be notified in person, by phone, or by letter. If your test results suggest that you may be infected, you should discuss these results with your primary care physician. You may also visit the Centers for Disease Control and Prevention (CDC) website at for additional information regarding Zika virus. If the results suggest that you may have a Zika virus infection, you will be invited to participate in a voluntary follow-up study involving additional blood samples. Should you choose to participate, an additional informed consent process will be required. Your participation in this research study is entirely voluntary. You will not be paid for your participation in this study. Your participation will not require any additional procedures or time beyond the normal donation process. The risk of having your donation tested with the study test is not any greater than having your donation tested for other infectious diseases, although a positive result may alarm you. There is a very low chance that your blood sample may give a false positive result. If the test is positive, the blood that you donate will not be used for transfusion. There will be no costs or payments to you for your participation in this study. Although you may not receive a direct benefit from this study, the results may allow for better test systems to become available to protect the blood supply. CDO-VC-43 rev. 1 Page 1 of 2
9 Zika Virus Research Information The results of all testing on your donation during this study are confidential, except when reportable by law to public health authorities, and to authorized blood center personnel, the U.S. Food and Drug Administration (FDA), and Hologic, Inc. Your age, gender, general geographic location, and test results may be used to evaluate important information about Zika virus, but this information is combined with information about other donors and not identified with you. You may refuse to participate by notifying the blood collection staff that you will not be donating blood or blood components today. If you decline testing we will be unable to use your whole blood or red blood cells, however, we will inform you whether you may donate plasma or platelets. If you decide not to participate at this time, your decision will not change your future relationship with the blood center and there is no penalty to you. If you decide not to participate after your donation is taken, call the Principal Investigator at the number(s) above. An Independent Review Board (IRB) is a group of people who review research studies to protect the rights and welfare of research participants. If you have questions or complaints about your rights as a study participant contact the Chesapeake IRB: By mail: Study Subject Adviser Chesapeake IRB 6940 Columbia Gateway Drive, Suite 110 Columbia, MD or call toll free: or by adviser@chesapeakeirb.com Please reference the following number when contacting the Study Subject Adviser: Pro If you have scientific questions or questions about your participation in these studies, you may contact our Donor Counseling Service at , Monday Friday 8am-5pm. By signing your Blood Donation Record, you are giving consent to allow us to use a portion of your blood donation and associated information for research purposes related to Zika virus. Although you feel well and healthy at the time of your donation, please call us immediately at the phone number beside our donor center s name below if you develop any of the following symptoms within 14 days of your blood donation: Fever Rash Muscle or joint pain Eye pain or redness Headache By notifying us as soon as possible after developing any of these symptoms you may prevent the blood you donated today from being transfused and possibly infecting a patient. NOTE: If you recall being in an area where Zika is actively transmitted in the past 4 weeks, please call us. Although you are feeling well, you could be infected with Zika. Bonfils Blood Center ( CDO-VC-43 rev. 1 Page 2 of 2
Donor Prescreening Questions
 Donor Prescreening Questions CDO-384-PRD REV 22r18 Please read the following in its entirety to help guarantee your safety and the safety of the community blood supply and to help ensure you have a positive
Donor Prescreening Questions CDO-384-PRD REV 22r18 Please read the following in its entirety to help guarantee your safety and the safety of the community blood supply and to help ensure you have a positive
For Parents and Students: Minor Donor Permit and Information About Donating Blood
 DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
SLV Health Blood Drive
 SLV Health Blood Drive Friday, October 21 st From 10:30 a.m. until 2:30 p.m. Held at the SLV health Conference Center at 1921 Main To schedule your appointment, please contact Jho Cura at (719) 587-1226
SLV Health Blood Drive Friday, October 21 st From 10:30 a.m. until 2:30 p.m. Held at the SLV health Conference Center at 1921 Main To schedule your appointment, please contact Jho Cura at (719) 587-1226
DONOR ELIGIBILITY SPECIFIC INFORMATION
 To determine if you are eligible to donate we will: Ask about your health and travel Ask about medicines you are taking or have taken Ask about your risk for infections that can be transmitted by blood
To determine if you are eligible to donate we will: Ask about your health and travel Ask about medicines you are taking or have taken Ask about your risk for infections that can be transmitted by blood
SOP Revision 2
 SOP 02010 Revision 2 To determine if you are eligible to donate we will: Ask about your health and travel Ask about medicines you are taking or have taken Ask about your risk for infections that can be
SOP 02010 Revision 2 To determine if you are eligible to donate we will: Ask about your health and travel Ask about medicines you are taking or have taken Ask about your risk for infections that can be
Not Under Document Control If Printed. Donor Educational Materials. If you are deferred
 Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
BLOOD DONOR INTERVIEW AND PHYSICAL
 EXERCISE : BLOOD DONOR INTERVIEW AND PHYSICAL Skills: 20 points Objectives:. Determine if an individual is eligible to donate blood by proper performance of the donor interview. 2. Identify the conditions
EXERCISE : BLOOD DONOR INTERVIEW AND PHYSICAL Skills: 20 points Objectives:. Determine if an individual is eligible to donate blood by proper performance of the donor interview. 2. Identify the conditions
Cord Blood Medical History/ Health Assessment Questionnaire
 OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM
 UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM. A Pilot Study for Collection of Anti-Zika Immune Plasma
 A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
HEALTH SCREENING QUESTIONNAIRE CT Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE. Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
STUDENT MEDICAL FORM
 STUDENT MEDICAL FORM Welcome to MARYMOUNT UNIVERSITY! All students are required to complete demographic, insurance information, and immunization history. All Marymount students born after December 31,
STUDENT MEDICAL FORM Welcome to MARYMOUNT UNIVERSITY! All students are required to complete demographic, insurance information, and immunization history. All Marymount students born after December 31,
INFORMATION AND CONSENT FORM For Adults Aged 18 and Older Additional Facility Sites
 INFORMATION AND CONSENT FORM Program Title: Expanded Access IND Program to Provide Stamaril Vaccine to Persons in the United States for Vaccination Against Yellow Fever Program #: Sponsor: Sanofi Pasteur
INFORMATION AND CONSENT FORM Program Title: Expanded Access IND Program to Provide Stamaril Vaccine to Persons in the United States for Vaccination Against Yellow Fever Program #: Sponsor: Sanofi Pasteur
Donor Registration and Consent for HLA Typing
 Place NMDP Bar Code label here Jackie (left), donated to save the life of Paizley (right) Randy (left), donated to save the life of Luke (right) Tobias (left), donated to save the life of Betsy (right)
Place NMDP Bar Code label here Jackie (left), donated to save the life of Paizley (right) Randy (left), donated to save the life of Luke (right) Tobias (left), donated to save the life of Betsy (right)
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
First, Last Name DOB: Maternal Risk Questionnaire (MRQ)
 First, Last Name DOB: Cord Blood Bank Use Only: Maternal Risk Questionnaire (MRQ) NMDP CBU ID: Local CBU ID: NMDP Maternal ID: Local Maternal ID: Today's Date: Baby's Mother's Initials: Please read questions
First, Last Name DOB: Cord Blood Bank Use Only: Maternal Risk Questionnaire (MRQ) NMDP CBU ID: Local CBU ID: NMDP Maternal ID: Local Maternal ID: Today's Date: Baby's Mother's Initials: Please read questions
THE CARE WE PROMISE FACTS AND FIGURES 2017
 THE CARE WE PROMISE FACTS AND FIGURES 2017 2 SOS CHILDREN S VILLAGES INTERNATIONAL WHERE WE WORK Facts and Figures 2017 205 58 79 families and transit 31 Foster homes 162 8 3 173 214 2 115 159 136 148
THE CARE WE PROMISE FACTS AND FIGURES 2017 2 SOS CHILDREN S VILLAGES INTERNATIONAL WHERE WE WORK Facts and Figures 2017 205 58 79 families and transit 31 Foster homes 162 8 3 173 214 2 115 159 136 148
Current State of Global HIV Care Continua. Reuben Granich 1, Somya Gupta 1, Irene Hall 2, John Aberle-Grasse 2, Shannon Hader 2, Jonathan Mermin 2
 Current State of Global HIV Care Continua Reuben Granich 1, Somya Gupta 1, Irene Hall 2, John Aberle-Grasse 2, Shannon Hader 2, Jonathan Mermin 2 1) International Association of Providers of AIDS Care
Current State of Global HIV Care Continua Reuben Granich 1, Somya Gupta 1, Irene Hall 2, John Aberle-Grasse 2, Shannon Hader 2, Jonathan Mermin 2 1) International Association of Providers of AIDS Care
Question: 1. Are you currently taking an antibiotic?
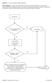 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Guidance for Travelers on Temporary Work Assignment Abroad
 infected person to immediately seek medical care but, prior to arrival, notify their healthcare provider that they may have been exposed to AI. For more information about avian influenza, see www.cdc.gov/flu/avian/facts.htm,
infected person to immediately seek medical care but, prior to arrival, notify their healthcare provider that they may have been exposed to AI. For more information about avian influenza, see www.cdc.gov/flu/avian/facts.htm,
Undetectable = Untransmittable. Mariah Wilberg Communications Specialist
 Undetectable = Untransmittable Mariah Wilberg Communications Specialist Undetectable=Untransmittable PLWH who get and stay undetectable have effectively no risk of transmitting HIV to their sex partners
Undetectable = Untransmittable Mariah Wilberg Communications Specialist Undetectable=Untransmittable PLWH who get and stay undetectable have effectively no risk of transmitting HIV to their sex partners
Report of Medical History
 Report of Medical History Students are required to have a current Report of Medical History if they plan to live in university housing. These records can be obtained from the high school, college or university
Report of Medical History Students are required to have a current Report of Medical History if they plan to live in university housing. These records can be obtained from the high school, college or university
FORM TITLE: Risk Behavior Assessment. 1. Are you a male who has had sex with another male in the preceding five years?
 RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
UNOS # NA. Person(s) Interviewed (First and last name) Relationship(s) to him/her. Print Name Title Signature
 UNOS # Form-0005 - Medical History and Behavioral Donor Name (First and last name) Date/Time of Interview Person(s) Interviewed (First and last name) Relationship(s) to him/her Person Conducting Interview
UNOS # Form-0005 - Medical History and Behavioral Donor Name (First and last name) Date/Time of Interview Person(s) Interviewed (First and last name) Relationship(s) to him/her Person Conducting Interview
Question: 1. Are you currently taking an antibiotic?
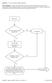 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
First, Last Name: DOB: F91148 Maternal Risk Questionnaire
 Maternal Risk Questionnaire (MRQ) NMDP CBU ID Local CBU ID NMDP Maternal ID Maternal Hospital ID Please read questions carefully and answer to the best of your knowledge: Check the box if you understand
Maternal Risk Questionnaire (MRQ) NMDP CBU ID Local CBU ID NMDP Maternal ID Maternal Hospital ID Please read questions carefully and answer to the best of your knowledge: Check the box if you understand
Making Your Blood Donation Safe
 BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
* Health Insurance Verification Form, submitted on line. Click on link. Mandatory Health Insurance Verification Form
 Residential Student: The Health Office welcomes you to residential living. It is our goal in collaboration with Residential Life, Safety, and Security, and the Dean of Students to promote health and wellness
Residential Student: The Health Office welcomes you to residential living. It is our goal in collaboration with Residential Life, Safety, and Security, and the Dean of Students to promote health and wellness
Make a difference! Your guide to saving lives through blood donation
 Make a difference! Your guide to saving lives through blood donation You have the power to change lives Every two seconds someone needs blood. Accidents happen 24/7, putting family, friends, and neighbors
Make a difference! Your guide to saving lives through blood donation You have the power to change lives Every two seconds someone needs blood. Accidents happen 24/7, putting family, friends, and neighbors
IRB Approval From: 3/8/2010 To: 10/28/2010
 UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Phase II Study to Assess the Safety and Immunogenicity of an Inactivated Swine-Origin H1N1 Influenza Vaccine in HIV-1 (Version 3.0, 16 FEB 2010) IRB Approval From:
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Phase II Study to Assess the Safety and Immunogenicity of an Inactivated Swine-Origin H1N1 Influenza Vaccine in HIV-1 (Version 3.0, 16 FEB 2010) IRB Approval From:
Annex 2 A. Regional profile: West Africa
 Annex 2 A. Regional profile: West Africa 355 million people at risk for malaria in 215 297 million at high risk A. Parasite prevalence, 215 Funding for malaria increased from US$ 233 million to US$ 262
Annex 2 A. Regional profile: West Africa 355 million people at risk for malaria in 215 297 million at high risk A. Parasite prevalence, 215 Funding for malaria increased from US$ 233 million to US$ 262
This also applies to all travellers transiting through countries with risk of transmission of yellow fever.
 JAMAICA YELLOW FEVER ENTRY REQUIREMENTS 29 MAY 2016 Vaccination against yellow fever is required to prevent the importation of yellow fever virus into Jamaica where the disease does not occur but where
JAMAICA YELLOW FEVER ENTRY REQUIREMENTS 29 MAY 2016 Vaccination against yellow fever is required to prevent the importation of yellow fever virus into Jamaica where the disease does not occur but where
HIBA ABDALRAHIM Capsca Focal Point Public Health Authority
 HIBA ABDALRAHIM Capsca Focal Point Public Health Authority Introduction Definition Symptom Transmission Global situation Local situation Control Content Introduction Yellow fever (YF) is a mosquito-borne
HIBA ABDALRAHIM Capsca Focal Point Public Health Authority Introduction Definition Symptom Transmission Global situation Local situation Control Content Introduction Yellow fever (YF) is a mosquito-borne
Eligibility List 2018
 The Global Fund s 2017-2022 strategy and allocation-based approach enables strategic investment to accelerate the end of HIV/AIDS, tuberculosis and malaria and build resilient and sustainable systems for
The Global Fund s 2017-2022 strategy and allocation-based approach enables strategic investment to accelerate the end of HIV/AIDS, tuberculosis and malaria and build resilient and sustainable systems for
Main developments in past 24 hours
 ECDC DAILY UPDATE Pandemic (H1N1) 2009 Update 02 October 2009, 09:00 hours CEST Main developments in past 24 hours Weekly Influenza Surveillance Overview to be published today; Media highlights and Eurosurveillance
ECDC DAILY UPDATE Pandemic (H1N1) 2009 Update 02 October 2009, 09:00 hours CEST Main developments in past 24 hours Weekly Influenza Surveillance Overview to be published today; Media highlights and Eurosurveillance
Terms and Conditions. VISA Global Customer Assistance Services
 Terms and Conditions VISA Global Customer Assistance Services Visa Global Customer Assistance Services (VGCAS) 1 The Visa Global Customer Assistance Services are co-ordinated by the Global Assistance Centre
Terms and Conditions VISA Global Customer Assistance Services Visa Global Customer Assistance Services (VGCAS) 1 The Visa Global Customer Assistance Services are co-ordinated by the Global Assistance Centre
Required Certificate of Immunization
 Required Certificate of Immunization Student Information Signature: Date: Required Immunization Information VACCINE HISTORY OF POSITIVE LAB/SEROLOGIC EVIDENCE MMR 1 Measles 1 Mumps 1 Rubella 1 Varicella
Required Certificate of Immunization Student Information Signature: Date: Required Immunization Information VACCINE HISTORY OF POSITIVE LAB/SEROLOGIC EVIDENCE MMR 1 Measles 1 Mumps 1 Rubella 1 Varicella
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
HEALTH OFFICE, Poughkeepsie, NY Residential Student:
 Residential Student: The Health Office welcomes you to residential living. It is our goal in collaboration with Residential Life, Safety, and Security, and the Dean of Students to promote health and wellness
Residential Student: The Health Office welcomes you to residential living. It is our goal in collaboration with Residential Life, Safety, and Security, and the Dean of Students to promote health and wellness
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT FOR OPTIONAL PROCEDURES AND SAMPLES COLLECTION AFTER WEEK 48
 CONSENT FOR OPTIONAL PROCEDURES AND SAMPLES COLLECTION AFTER WEEK 48 Protocol Title: A5354 Version 1.0, dated 4/19/16, Letter of Amendment #1, 12/13/16, Letter of Amendment #2, 11/7/17 Effect of Antiretroviral
CONSENT FOR OPTIONAL PROCEDURES AND SAMPLES COLLECTION AFTER WEEK 48 Protocol Title: A5354 Version 1.0, dated 4/19/16, Letter of Amendment #1, 12/13/16, Letter of Amendment #2, 11/7/17 Effect of Antiretroviral
Date of Birth Soc. Sec. or UD ID # Month Day Year. Country of Birth If not USA, indicate when you entered this country M/Y
 University of Delaware-Student Health Service, Laurel Hall, Newark, Delaware 19716-8101 Telephone: 302/831-2226 Fax: 302/831-6407 IMMUNIZATION DOCUMENTATION ALL OF THE FOLLOWING INFORMATION MUST BE COMPLETED
University of Delaware-Student Health Service, Laurel Hall, Newark, Delaware 19716-8101 Telephone: 302/831-2226 Fax: 302/831-6407 IMMUNIZATION DOCUMENTATION ALL OF THE FOLLOWING INFORMATION MUST BE COMPLETED
National Institute on Alcohol Abuse and Alcoholism. Environmental Approaches
 Environmental Approaches Consumption of 10+ and 21+ Drinks on an Occasion At Least Once in the Past Year, 2013 30 25 20 15 10+ drinks 15 25 10+ drinks 16 25 10 5 0 21+ drinks 3 2 21+ drinks 18-20 21-24
Environmental Approaches Consumption of 10+ and 21+ Drinks on an Occasion At Least Once in the Past Year, 2013 30 25 20 15 10+ drinks 15 25 10+ drinks 16 25 10 5 0 21+ drinks 3 2 21+ drinks 18-20 21-24
Donor Risk Assessment Interview (Donor >12 yrs old)
 Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Drug Prices Report Opioids Retail and wholesale prices * and purity levels,by drug, region and country or territory (prices expressed in US$ )
 1 / 11 Region/Subregion/ Country Africa Eastern Africa Kenya Madagascar Mauritius Uganda United Republic of Tanzania Northern Africa Algeria Egypt Libya Morocco Sudan Southern Africa Botswana Burkina Faso
1 / 11 Region/Subregion/ Country Africa Eastern Africa Kenya Madagascar Mauritius Uganda United Republic of Tanzania Northern Africa Algeria Egypt Libya Morocco Sudan Southern Africa Botswana Burkina Faso
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol No. VRX496-USA-11-001-LTFU: Long-term Follow-up
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM CONSENT TO PARTICIPATE IN A LONG TERM FOLLOW UP TO A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol No. VRX496-USA-11-001-LTFU: Long-term Follow-up
Q1 What age are you?
 Q1 What age are you? Answered: 504 Skipped: 0 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 4.56% 23 3.77% 19 4.56% 23 6.15% 31 3.97% 20 6.55% 33 5.95% 30 6.75% 34 6.35% 32 4.37% 22 6.75% 34 5.56%
Q1 What age are you? Answered: 504 Skipped: 0 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 4.56% 23 3.77% 19 4.56% 23 6.15% 31 3.97% 20 6.55% 33 5.95% 30 6.75% 34 6.35% 32 4.37% 22 6.75% 34 5.56%
MEDICAL HISTORY FORM ADMINISTRATION INSTRUCTIONS
 ADMINISTRATION INSTRUCTIONS CONFIRM THE STUDY BAR CODE ON PAGES 1 THROUGH 6 OF THIS FORM DO NOT PLACE A STUDY BAR CODE LABEL ON THIS INSTRUCTION PAGE. Interview Instructions The interview should be conducted
ADMINISTRATION INSTRUCTIONS CONFIRM THE STUDY BAR CODE ON PAGES 1 THROUGH 6 OF THIS FORM DO NOT PLACE A STUDY BAR CODE LABEL ON THIS INSTRUCTION PAGE. Interview Instructions The interview should be conducted
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 Gilead Sciences, Inc. GS-US-337-0115, 25-NOV-2013 A Phase 3, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination for 12 Weeks in Subjects
Gilead Sciences, Inc. GS-US-337-0115, 25-NOV-2013 A Phase 3, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Sofosbuvir/Ledipasvir Fixed-Dose Combination for 12 Weeks in Subjects
Douglas V. Faller, Ph.D., M.D., Susan P. Perrine M.D.
 ARGININE BUTYRATE + GANCICLOVIR IN EBV (+) MALIGNANCY A PHASE ONE TRIAL OF BUTYRATE IN COMBINATION WITH GANCICLOVIR IN EBV-INDUCED MALIGNANCIES AND LYMPHOPROLIFERATIVE DISEASE Principal Investigators:
ARGININE BUTYRATE + GANCICLOVIR IN EBV (+) MALIGNANCY A PHASE ONE TRIAL OF BUTYRATE IN COMBINATION WITH GANCICLOVIR IN EBV-INDUCED MALIGNANCIES AND LYMPHOPROLIFERATIVE DISEASE Principal Investigators:
Biology Report. Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis?
 Biology Report Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis? Introduction Tuberculosis is a serious disease caused by the bacterium Mycobacterium
Biology Report Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis? Introduction Tuberculosis is a serious disease caused by the bacterium Mycobacterium
Subject Name: Sponsor Consent Tmplt Date: Version 2 (17-Jul-2014) Unit Number:
 SCREENING CONSENT TO DETERMINE STUDY ELIGIBILITY AND OPTIONAL RESEARCH AUTHORIZATION TO DONATE ARCHIVED TISSUE SAMPLES FOR MOLECULAR CHARACTERIZATION OF TUMOR YALE UNIVERSITY SCHOOL OF MEDICINE YALE-NEW
SCREENING CONSENT TO DETERMINE STUDY ELIGIBILITY AND OPTIONAL RESEARCH AUTHORIZATION TO DONATE ARCHIVED TISSUE SAMPLES FOR MOLECULAR CHARACTERIZATION OF TUMOR YALE UNIVERSITY SCHOOL OF MEDICINE YALE-NEW
A Phase I Study to Assess the Safety of HIV and Hep C Vaccines Candidates When Given Separately or in Combination
 A Phase I Study to Assess the Safety of HIV and Hep C Vaccines Candidates When Given Separately or in Combination Sponsor: University of Oxford EudraCT No.: 2014-000730-30 REC: South Central Oxford A REC
A Phase I Study to Assess the Safety of HIV and Hep C Vaccines Candidates When Given Separately or in Combination Sponsor: University of Oxford EudraCT No.: 2014-000730-30 REC: South Central Oxford A REC
Title: Medication Deferral List Vault: DONOR CENTER - RELEASED; InfoCard#: DC-2185 Effective Date: 31 Jul 2017
 Medication Deferral List SOME MEDICATIONS MAY AFFECT YOUR ELIGIBILITY TO DONATE BLOOD. PLEASE TELL US IF YOU Have been treated with the following types of medications or for the following conditions...
Medication Deferral List SOME MEDICATIONS MAY AFFECT YOUR ELIGIBILITY TO DONATE BLOOD. PLEASE TELL US IF YOU Have been treated with the following types of medications or for the following conditions...
WCPT COUNTRY PROFILE December 2017 SWEDEN
 WCPT COUNTRY PROFILE December 2017 SWEDEN SWEDEN NUMBERS WCPT Members Practising physical therapists 11,043 Total number of physical therapist members in your member organisation 17,906 Total number of
WCPT COUNTRY PROFILE December 2017 SWEDEN SWEDEN NUMBERS WCPT Members Practising physical therapists 11,043 Total number of physical therapist members in your member organisation 17,906 Total number of
WCPT COUNTRY PROFILE December 2017 HUNGARY
 WCPT COUNTRY PROFILE December 2017 HUNGARY HUNGARY NUMBERS WCPT Members Practising physical therapists 727 Total number of physical therapist members in your member organisation 4,000 Total number of practising
WCPT COUNTRY PROFILE December 2017 HUNGARY HUNGARY NUMBERS WCPT Members Practising physical therapists 727 Total number of physical therapist members in your member organisation 4,000 Total number of practising
Iowa Methodist Medical Center Transplant Center. Informed Consent for Kidney Transplant Recipient
 Iowa Methodist Transplant Center Iowa Methodist Medical Center Transplant Center 1215 Pleasant Street, Suite 506 Des Moines, IA 50309 515-241-4044 Phone 515-241-4100 Fax Iowa Methodist Medical Center Transplant
Iowa Methodist Transplant Center Iowa Methodist Medical Center Transplant Center 1215 Pleasant Street, Suite 506 Des Moines, IA 50309 515-241-4044 Phone 515-241-4100 Fax Iowa Methodist Medical Center Transplant
WCPT COUNTRY PROFILE December 2017 SERBIA
 WCPT COUNTRY PROFILE December 2017 SERBIA SERBIA NUMBERS WCPT Members Practising physical therapists 622 Total number of physical therapist members in your member organisation 3,323 Total number of practising
WCPT COUNTRY PROFILE December 2017 SERBIA SERBIA NUMBERS WCPT Members Practising physical therapists 622 Total number of physical therapist members in your member organisation 3,323 Total number of practising
Hepatitis B and C Basics
 Hepatitis B and C Basics What is the liver? The liver is the largest internal organ that performs many important functions. Stores nutrients and vitamins Fights infection Stores energy Removes harmful
Hepatitis B and C Basics What is the liver? The liver is the largest internal organ that performs many important functions. Stores nutrients and vitamins Fights infection Stores energy Removes harmful
RECOMMENDATIONS ON INFLUENZA VACCINATION DURING THE WINTER SEASON
 RECOMMENDATIONS ON INFLUENZA VACCINATION DURING THE 2018 2019 WINTER SEASON October 2018 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for
RECOMMENDATIONS ON INFLUENZA VACCINATION DURING THE 2018 2019 WINTER SEASON October 2018 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for
2017 Bereavement Program Information
 A retreat for children with life-threatening illnesses and their families 2017 Bereavement Program Information Eligibility Guidelines Camp Sunshine offers bereavement programs for families who have had
A retreat for children with life-threatening illnesses and their families 2017 Bereavement Program Information Eligibility Guidelines Camp Sunshine offers bereavement programs for families who have had
JUST FOR KIDS SELECTED IMPORTANT SAFETY INFORMATION
 JUST FOR KIDS For children ages 6-17 with moderately to severely active Crohn s disease or ulcerative colitis (UC) who haven t responded well to other therapies SELECTED IMPORTANT SAFETY INFORMATION REMICADE
JUST FOR KIDS For children ages 6-17 with moderately to severely active Crohn s disease or ulcerative colitis (UC) who haven t responded well to other therapies SELECTED IMPORTANT SAFETY INFORMATION REMICADE
MEDICAL AND PERSONAL HISTORY
 MEDICAL AND PERSONAL HISTORY Last First MI Today s Date Name Age Mr. Mrs Ms Dr Address Home Phone City, State, Zip Work Phone Sex: M F Patient SS# Cell Phone Date of Birth / / Responsible Party Referring
MEDICAL AND PERSONAL HISTORY Last First MI Today s Date Name Age Mr. Mrs Ms Dr Address Home Phone City, State, Zip Work Phone Sex: M F Patient SS# Cell Phone Date of Birth / / Responsible Party Referring
Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis?
 Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis? Introduction Tuberculosis is a serious disease caused by the bacterium Mycobacterium tuberculosis.
Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis? Introduction Tuberculosis is a serious disease caused by the bacterium Mycobacterium tuberculosis.
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
WHO REGIONAL OFFICE FOR EUROPE RECOMMENDATIONS ON INFLUENZA
 WHO REGIONAL OFFICE FOR EUROPE RECOMMENDATIONS ON INFLUENZA September 2017 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe Marmorvej
WHO REGIONAL OFFICE FOR EUROPE RECOMMENDATIONS ON INFLUENZA September 2017 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe Marmorvej
Treatment with Rivaroxaban Xarelto
 Treatment with Rivaroxaban Xarelto Anticoagulation Clinic This handout explains the medicine rivaroxaban, a drug that helps prevent blood clots. What is rivaroxaban? Rivaroxaban (brand name Xarelto) is
Treatment with Rivaroxaban Xarelto Anticoagulation Clinic This handout explains the medicine rivaroxaban, a drug that helps prevent blood clots. What is rivaroxaban? Rivaroxaban (brand name Xarelto) is
ZIKA VIRUS. Causes, Symptoms, Treatment and Prevention
 ZIKA VIRUS Causes, Symptoms, Treatment and Prevention Introduction Zika virus is spread to people through mosquito bites. The most common symptoms of Zika virus disease are fever, rash, joint pain, and
ZIKA VIRUS Causes, Symptoms, Treatment and Prevention Introduction Zika virus is spread to people through mosquito bites. The most common symptoms of Zika virus disease are fever, rash, joint pain, and
European status report on alcohol and health Leadership, awareness and commitment
 European status report on alcohol and health 2014 Leadership, awareness and commitment Leadership, awareness and commitment Background Strong leadership from national and local governments is essential
European status report on alcohol and health 2014 Leadership, awareness and commitment Leadership, awareness and commitment Background Strong leadership from national and local governments is essential
BCG. and your baby. Immunisation. Protecting babies against TB. the safest way to protect your child
 BCG and your baby Protecting babies against TB Immunisation the safest way to protect your child This leaflet is about the BCG (Bacillus Calmette-Guerin) vaccination that is being offered to protect your
BCG and your baby Protecting babies against TB Immunisation the safest way to protect your child This leaflet is about the BCG (Bacillus Calmette-Guerin) vaccination that is being offered to protect your
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 IRC 002, Version 5.0, 20 AUG 2012: A Randomized, Open-Label, Phase 2, Multicenter Safety and Exploratory Efficacy Study of Investigational anti-influenza A Immune Plasma for the Treatment of Influenza
IRC 002, Version 5.0, 20 AUG 2012: A Randomized, Open-Label, Phase 2, Multicenter Safety and Exploratory Efficacy Study of Investigational anti-influenza A Immune Plasma for the Treatment of Influenza
Language for Consent Forms
 New York University University Committee on Activities Involving Human Subjects 665 Broadway, Suite 804, New York, NY 10012 VOICE: 212-998-4808 FAX: 212-995-4304 www.nyu.edu/ucaihs/ Language for Consent
New York University University Committee on Activities Involving Human Subjects 665 Broadway, Suite 804, New York, NY 10012 VOICE: 212-998-4808 FAX: 212-995-4304 www.nyu.edu/ucaihs/ Language for Consent
EUDY JUNIOR CAMP 2018 FIRST ANNOUNCEMENT
 EUDY JUNIOR CP 2018 FIRST ANNOUNCEMENT 21. 29. July 2018 Tesáre, SLOVAKIA IDENTITY OF THE DEAF EUROPEAN UNION OF THE DEAF YOUTH (EUDY) The European Union of the Deaf Youth is a European non-profit organisation
EUDY JUNIOR CP 2018 FIRST ANNOUNCEMENT 21. 29. July 2018 Tesáre, SLOVAKIA IDENTITY OF THE DEAF EUROPEAN UNION OF THE DEAF YOUTH (EUDY) The European Union of the Deaf Youth is a European non-profit organisation
SOUTHSIDE COMMUNITY ACUPUNCTURE, LLC. Financial Policies
 Disclosure of Information - Please Read the Following Carefully How to Prepare for Your First Visit : Plan on showing up a 15 minutes early to your first appointment and please wear, or bring with you
Disclosure of Information - Please Read the Following Carefully How to Prepare for Your First Visit : Plan on showing up a 15 minutes early to your first appointment and please wear, or bring with you
We will review the need for continuing treatment with you after 3 months and again after one year.
 Patient Information Drugs for Inflammatory Bowel Disease Adalimumab (Humira) Clinical Nurse Specialist - Allyson Lewis Clinical Nurse Specialist Gareth Lloyd-Ford Clinical Nurse Specialist Lynsey Hook
Patient Information Drugs for Inflammatory Bowel Disease Adalimumab (Humira) Clinical Nurse Specialist - Allyson Lewis Clinical Nurse Specialist Gareth Lloyd-Ford Clinical Nurse Specialist Lynsey Hook
Pro Active Physical Therapy & Sports Medicine
 Pro Active Physical Therapy & Sports Medicine Consent and Statement of Financial Responsibility 1. CONSENT FOR TREATMENT: I consent to and authorize my physical therapist, occupational therapist and other
Pro Active Physical Therapy & Sports Medicine Consent and Statement of Financial Responsibility 1. CONSENT FOR TREATMENT: I consent to and authorize my physical therapist, occupational therapist and other
SPECIALIZED FAMILY CARE Provider Training
 SPECIALIZED FAMILY CARE Provider Training Category: Health Issue Title: Zika Virus Materials: Centers for Disease Control Fact Sheet on Zika Virus Goal: Specialized Family Care Provider to learn the risks,
SPECIALIZED FAMILY CARE Provider Training Category: Health Issue Title: Zika Virus Materials: Centers for Disease Control Fact Sheet on Zika Virus Goal: Specialized Family Care Provider to learn the risks,
46825 (260) $UPONT
 Be wise. Immunize. Keeping track of the shots your children receive can be confusing. This is an important responsibility that is shared by you and your immunization providers. This booklet contains the
Be wise. Immunize. Keeping track of the shots your children receive can be confusing. This is an important responsibility that is shared by you and your immunization providers. This booklet contains the
Where we stand in EFORT
 Where we stand in EFORT Engaging with the new EU regulatory landscape for medical devices. Challenges & opportunities Brussel, Belgium April 6, 2018 Per Kjaersgaard-Andersen Associate Professor Section
Where we stand in EFORT Engaging with the new EU regulatory landscape for medical devices. Challenges & opportunities Brussel, Belgium April 6, 2018 Per Kjaersgaard-Andersen Associate Professor Section
CONSENT TO BE A RESEARCH PARTICIPANT Version of the Informed Consent DAIDS-ES Impact of CCR5 Blockade in HIV+ Kidney Transplant Recipients
 CONSENT TO BE A RESEARCH PARTICIPANT Version of the Informed Consent DAIDS-ES 20730 Impact of CCR5 Blockade in CONSENT VERSION 3.0 /October 2, 2017 PROTOCOL VERSION 3.0 /October 2, 2017 [NOTE: Site specific
CONSENT TO BE A RESEARCH PARTICIPANT Version of the Informed Consent DAIDS-ES 20730 Impact of CCR5 Blockade in CONSENT VERSION 3.0 /October 2, 2017 PROTOCOL VERSION 3.0 /October 2, 2017 [NOTE: Site specific
Your lung biopsy is scheduled for: Date: Time: Questions about your biopsy? Need to reschedule or cancel your appointment?
 Lung Biopsy: CT Guided Information for patients and families Your lung biopsy is scheduled for: Date: Time: Important: You must arrive 1 hour before your appointment Questions about your biopsy? Need to
Lung Biopsy: CT Guided Information for patients and families Your lung biopsy is scheduled for: Date: Time: Important: You must arrive 1 hour before your appointment Questions about your biopsy? Need to
EURO POLIO PAGE Data as of 04 October 2005 (Week 38)
 World Health Organization Regional Office for Europe EURO POLIO PAGE Data as of 04 October 2005 (Week 38) Vaccine-preventable Diseases and Immunization programme, Division of Technical Support website:
World Health Organization Regional Office for Europe EURO POLIO PAGE Data as of 04 October 2005 (Week 38) Vaccine-preventable Diseases and Immunization programme, Division of Technical Support website:
How does HBV affect the liver?
 Hepatitis B Why is the liver important? Your liver is a vital organ that performs many essential functions. It s the largest solid organ in the body and is located under your rib cage on the upper right
Hepatitis B Why is the liver important? Your liver is a vital organ that performs many essential functions. It s the largest solid organ in the body and is located under your rib cage on the upper right
Date of Birth: Age: Sex: Male Female Marital. Driver's Lic S M D. Status: Address:
 Houston Weight Loss and Lipo Centers Patient Name: Address: City, State : Apt: Zip: Email*: *By providing your email address you are agreeing to communication via email. Home Phone Primary contact Work
Houston Weight Loss and Lipo Centers Patient Name: Address: City, State : Apt: Zip: Email*: *By providing your email address you are agreeing to communication via email. Home Phone Primary contact Work
New Zealand Bone Marrow Donor Registry
 Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines-004-13 Date
Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines-004-13 Date
International Travel Medical Questionnaire
 International Travel Medical Questionnaire Name: of Birth: Gender: M/F Last First Month/Day/Year Circle One Address: Street City, State Zip Daytime Phone: Evening Phone: Primary care physician: Emergency
International Travel Medical Questionnaire Name: of Birth: Gender: M/F Last First Month/Day/Year Circle One Address: Street City, State Zip Daytime Phone: Evening Phone: Primary care physician: Emergency
Louisville '19 Attachment #69
 Telephone Meeting Approved and why I propose Using zoom to fulfill both Phone and Virtual video meeting Formats. The first established phone meeting Sanctioned by Gamblers Anonymous (listed on Trustee
Telephone Meeting Approved and why I propose Using zoom to fulfill both Phone and Virtual video meeting Formats. The first established phone meeting Sanctioned by Gamblers Anonymous (listed on Trustee
Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus
 Page 1 of 8 Informed Consent for Participation in a Research Study Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus Investigator Contact Information: Principal Investigator:
Page 1 of 8 Informed Consent for Participation in a Research Study Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus Investigator Contact Information: Principal Investigator:
1. What Panzyga is and what it is used for
 Package leaflet: Information for the user Panzyga, 100 mg/ml solution for infusion Human Normal Immunoglobulin (IVIg) This medicine is subject to additional monitoring. This will allow quick identification
Package leaflet: Information for the user Panzyga, 100 mg/ml solution for infusion Human Normal Immunoglobulin (IVIg) This medicine is subject to additional monitoring. This will allow quick identification
Extended Health Care Company Do you need any help retaining information about your health insurance coverage? Yes No
 PATIENT ENTRANCE FORM Date Circle: Male Female Name Birth Date (dd/mm/yy) Age Address Apt # City Province Postal Code Home # Cell # Work # E-MAIL Occupation Employer Name of Emergency Contact Contact #
PATIENT ENTRANCE FORM Date Circle: Male Female Name Birth Date (dd/mm/yy) Age Address Apt # City Province Postal Code Home # Cell # Work # E-MAIL Occupation Employer Name of Emergency Contact Contact #
