SOP Revision 2
|
|
|
- Darrell Mervin Page
- 5 years ago
- Views:
Transcription
1 SOP Revision 2
2 To determine if you are eligible to donate we will: Ask about your health and travel Ask about medicines you are taking or have taken Ask about your risk for infections that can be transmitted by blood especially AIDS and viral hepatitis Take your blood pressure, temperature and pulse Take a blood sample to be sure your blood count is acceptable If you are eligible to donate we will: Clean your arm with an antiseptic. Tell us if you have any skin allergies Use a new, sterile, disposable needle to collect your blood Do not donate if you: Have AIDS or have ever had a positive HIV test Have EVER used needles to take any drugs not prescribed by your doctor Are a male who has had sexual contact with another male, IN THE PAST 12 MONTHS Have EVER taken money, drugs or other payment for sex Have had sexual contact in IN THE PAST 12 MONTHS with anyone described above Have had syphilis or gonorrhea IN THE PAST 12 MONTHS Have been in juvenile detention, lockup, jail or prison for more than 72 consecutive hours IN THE PAST 12 MONTHS DONOR ELIGIBILITY SPECIFIC INFORMATION Certain diseases, such as AIDS and hepatitis, can be spread through sexual contact and enter your bloodstream. We will ask specific questions about sexual contact. What do we mean by sexual contact? The words have sexual contact with and sex are used in some of the questions we will ask you, and apply to any of the activities below, whether-or-not a condom or other protection was used: Vaginal sex (contact between penis and vagina) Oral sex (mouth or tongue on someone s vagina, penis, or anus) Anal sex (contact between penis and anus) HIV/AIDS risk behaviors HIV is the virus that causes AIDS. It is spread mainly by sexual contact with an infected person OR by sharing needles or syringes used by an infected person for injecting drugs. Your blood can transmit infections, including HIV/AIDS, even if you feel well and all your tests are normal. This is because even the best tests cannot detect the virus for a period-of-time after you are infected. DO NOT donate to get a test! If you think you may be at risk for HIV/AIDS or any other infection, do not donate simply to get a test. Ask us where you can be tested outside the blood center. The following symptoms can be present before an HIV test turns positive: Fever Enlarged lymph glands Sore throat Rash DO NOT donate if you have these symptoms! Travel to or birth in other countries Blood donor tests may not be available for some infections that are found only in certain countries. If you were born in, have lived in, or visited certain countries, you may not be eligible to donate.
3 BLOOD DONOR EDUCATIONAL MATERIAL FOR EBOLA VIRUS DISEASE OR INFECTION This information applies following the CDC s classification of one or more countries as having widespread transmission or cases in urban areas with uncertain control measures at this link: west-africa/distribution-map.html. Blood collection facilities must reduce the risk of collecting blood and blood components from a donor who may be infected with Ebola virus. It is possible that an Ebola virus infected person may not have symptoms of infection during the incubation period. In addition, anyone who has EVER had Ebola virus infection or disease may be at risk for transmitting the virus through blood donation, regardless of the length of time since recovery. Ebola virus is transmitted from human to human by direct exposure to body fluids (such as blood, urine, stool, saliva, semen, vaginal fluids or vomit) from infected individuals. Healthcare workers, and family and friends providing care may have direct exposure to body fluids of infected patients. If direct exposure occurs, a person is at high risk of developing Ebola virus infection and must not donate blood or blood components. DO NOT DONATE BLOOD if: You have EVER had Ebola virus disease or infection In the PAST 8 WEEKS, you have lived in, or travelled to, a country with widespread Ebola virus disease or infection. In the PAST 8 WEEKS, you have had sexual contact with a person has EVER had Ebola virus disease or infection, regardless of the length of time since recovery. In the PAST 8 WEEKS, you have had direct exposure to the body fluids (such as blood, urine, stool, saliva, semen, vaginal fluids or vomit) of a person with Ebola virus disease or infection, including a person under investigation. In the PAST 8 WEEKS, you have you been notified by a public health authority that you may have been exposed to a person with Ebola virus disease or infection. PLEASE CONTACT THE DONOR CENTER, if you develop the following symptoms within the 8-week period following donation: Fever Diarrhea Severe Headache followed by: Vomiting Muscle Pain and Weakness Abdominal Pain Fatigue hemorrhage (bleeding or bruising) WHAT HAPPENS AFTER YOUR DONATION To protect patients, your blood is tested for several types of hepatitis, HIV, syphilis, and other infections. If your blood tests positive, it will not be given to a patient. There are times when your blood is not tested. If this occurs, you may not receive any notification. You will be notified about any positive test result which may disqualify you from donating in the future. The blood center will not release your test results without your written permission unless required by law (e.g. to the Health Department).
4
5 SOME MEDICATIONS MAY AFFECT YOUR ELIGIBILITY TO DONATE BLOOD. PLEASE TELL US IF YOU... Are being treated with the following types of medications... Anti-platelet agents (usually taken to prevent stroke or heart attack) or have taken... which is also called... anytime in the last... Feldene piroxicam 2 days Effient prasugrel Brilinta ticagrelor 7 days Plavix clopidogrel Ticlid ticlopidine 14 days Zontivity vorapaxar Xarelto rivaroxaban Fragmin dalteparin Lovenox enoxaparin Pradaxa dabigatran 2 days Anticoagulants or blood thinners (usually to prevent blood clots in the legs and lungs and to prevent strokes) Eliquis Savaysa Coumadin Warfilone Jantoven Heparin, low apixaban edoxaban warfarin molecular 7 days weight heparin heparin (unless listed separately) Arixtra fondaparinux Accutane Amnesteem Absorica Acne treatment Claravis isotretinoin Myorisan 1 Month Sotret Zenatane Hair loss remedy Propecia finasteride Proscar finasteride Prostate symptoms Avodart Jalyn dutasteride 6 Months Basal cell skin cancer Erivedge vismodegib Odomzo sonidegib 24 months Relapsing multiple sclerosis Aubagio teriflunomide 24 months Psoriasis Soriatane acitretin 36 months Tegison etretinate Ever Hepatitis B Hepatitis exposure Immune HBIG 12 months Globulin
6 Experimental Medication or Unlicensed (Experimental) Vaccine Growth hormone from human pituitary glands* Insulin from Cows (Bovine or Beef Insulin) manufactured in the United Kingdom* 12 months, or as indicated by Medical Director Ever Ever * No longer available in US DO NOT discontinue medications prescribed or recommended by your physicians in order to donate blood. Some medications affect your eligibility as a blood donor, for the following reasons: Anti-platelet agents affect platelet function, so people taking these drugs should not donate platelets for the indicated time; however, you may still be able to donate whole blood or red blood cells by apheresis. Anticoagulants or "blood thinners" are used to treat or prevent blood clots in the legs, lungs, or other parts of the body, and to prevent strokes. These medications affect the blood s ability to clot, which might cause excessive bruising or bleeding when you donate; however, you may still be able to donate whole blood or red blood cells by apheresis. Isotretinoin, finasteride, dutasteride acitretin and etretinate can cause birth defects. Your donated blood could contain high enough levels to damage the unborn baby if transfused to a pregnant woman. Erivedge (vismodegib), Odomzo (sonidegib), Aubagio (teriflunomide) can cause birth defects or the death of an unborn baby if transfused to a pregnant woman. Growth hormone from human pituitary glands was prescribed for children with delayed or impaired growth. The hormone was obtained from human pituitary glands, which are in the brain. Some people who took this hormone developed a rare nervous system condition called Creutzfeldt-Jakob Disease (CJD, for short). Insulin from cows (bovine, or beef, insulin) is an injected medicine used to treat diabetes. If this insulin came to the United States from the United Kingdom (where mad cow disease has occurred) it could contain material from cattle that have mad cow disease. Although no cases of the human type of mad cow disease have been reported in people treated with bovine (beef) insulin, there is concern that someone exposed to mad cow disease through beef insulin could transmit it to someone who receives their blood. Hepatitis B Immune Globulin (HBIG) is an injected material used to prevent hepatitis B infection following a possible or known exposure to hepatitis B. HBIG does not hepatitis B infection in every case, therefore, persons who have received HBIG must wait to donate blood. Experimental Medication or Unlicensed (Experimental) Vaccine is usually associated with a research study, and the effect on the safety of transfused blood is unknown. prevent Donors SHOULD NOT discontinue medications prescribed or recommended by their physician in order to donate blood.
7 These lists were prepared from the FDA Guidance for Industry Revised Preventive Measures to Reduce the Possible Risk of Transmission of Creutzfeldt-Jakob Disease and Variant Creutzfeldt-Jakob Disease by Blood and Blood Products, updated January 2016 vcjd countries of risk - United Kingdom Area Country Type of Travel or Residence Time Period Deferral Period Reason for Deferral UK Includes: 3 months or more Between Channel Islands England Falkland Islands Gibraltar Isle of Man Northern Ireland Scotland Wales Received a blood transfusion 1980 Present Indefinite Possible risk of Variant Creutzfeldt-Jakob Disease (vcjd). (there is no test for vcjd)
8 vcjd countries of risk -Europe Area Country Belgium Germany Netherlands Type of Travel or Residence 6 months or more associated with US military base. Time Period Between Deferral Period Reason for Deferral Greece Italy Portugal, including the Azores Spain, including the Canary Islands and Spanish North African territories Turkey 6 months or more associated with US military base. Between Indefinite Possible risk of Variant Creutzfeldt- Jakob Disease (vcjd). (there is no test for vcjd) France, including its overseas departments (e.g., Martinique and others) Received a blood transfusion 1980 Present Continue
9 vcjd countries of risk -Europe Area Country All Countries on this list Albania Austria Belgium Bosnia-Herzegovina Bulgaria Croatia Czech Republic Denmark Finland France, including its overseas departments (e.g., Martinique and others) Germany Greece Hungary Republic of Ireland Italy Kosovo Liechtenstein Luxembourg Macedonia Montenegro Netherlands Norway Poland Portugal, including the Azores Romania Serbia Slovak Republic Slovenia Spain, including the Canary Islands and Spanish North African territories Sweden Switzerland Turkey Type of Travel or Residence 5 years or more Time Period 1980 Present Deferral Period Indefinite Reason for Deferral Possible risk of Variant Creutzfeldt- Jakob Disease (vcjd). (there is no test for vcjd)
10 In the past 3 years, have you been outside the United States or Canada? Malaria is a serious and sometimes fatal disease that can caused by any of the following four Plasmodium species: P. falciparum; P. malariae; P. ovale; or P. vivax. About 1,500 cases of malaria are diagnosed in the United States each year, the vast majority of which are found among travelers and immigrants returning from countries where malaria transmission occurs, such as sub-saharan Africa and South Asia. People who contract malaria typically become very sick with high fevers, shaking chills and flu-like illness. Although malaria can be fatal, illness and death from this disease usually can be prevented. Check the CDC Yellow Book to see if you traveled to a malaria endemic area.
11 INFORMED CONSENT FOR PLASMAPHERESIS AND HIV TESTING 1. I understand that plasmapheresis is a process in which the liquid portion of the blood plasma is separated from the red cells. I understand an automated apheresis machine will be used, this process involves: a) A sterile, one-time use kit will be used for this procedure. b) During plasmapheresis donors rest on a donation bed. A needle is placed into a vein in the crux of whichever arm has the most robust vein. c) Removal of up to 15% of my total blood volume at one time. Whole blood is mixed with anticoagulant and separated from the red cells. The red cells are then returned to me. This process continues for several cycles. d) I may receive up to 250ml of anticoagulant (blood thinner). 2. I understand I can donate plasma one time in seven days, 3. I understand that more frequent donations could cause me complications. I will not participate in more than one plasmapheresis program at a time, and that the donation of Whole Blood or Red Blood Cells while participating in the plasmapheresis program may serve as a basis for an 8-week deferral or a 16- week deferral for a Red Blood Cell donation. 4. The pre-qualification process can take up to 1 hour. The process includes New Donor registration, donor education, health history questions and hands-on physical exam and blood tests. I will be notified within 2-3 days, if I qualify for the plasmapheresis program. 5. Routine donation sessions usually take less than 60 minutes total. You are encouraged to stay in the center after your donation and enjoy a healthy snack and juice. 6. I understand that prior to donation, I must provide written informed consent for the plasmapheresis procedure. 7. I understand my informed consent will be maintained electronically in my NuPlasma donor record file. Further, I understand that blood establishment records, including donor records, are subject to inspection by FDA and, if applicable, other regulatory agencies. 8. I acknowledge that before I signed the first copy of this informed consent, a qualified licensed nurse or physician explained the hazards of the procedure. Prior to signing consecutive consent forms I read about the potential hazards. The hazards of the procedure include the following: a) Light-headedness b) Fainting c) Vomiting d) Hyperventilation e) Hematoma formation at the site of venipuncture f) Syncopal reactions due to hypovolemia g) Post-Donation iron deficiency Reactions that are unique to plasmapheresis collection procedures include: a) Allergic symptoms such as skin redness, itching, and hives b) Chills (induced by infusion of room temperature saline or donor blood) c) Moderate hypocalcemia due to chelation of calcium by metabolized citrate (caused by infusion of anticoagulants containing citrate). This reaction is usually manifested by: Unusual taste or smell Tingling around the mouth or fingers
12 Muscle discomfort, muscle twitching, or spasms. d) Symptoms of severe hypocalcemia although rare include: Tetany Convulsions Cardiac arrhythmias e) Occasionally mild, temporary facial redness or flushing of donors has been reported, most often during return blood cycles. f) Hemolysis, air embolism and blood clotting g) Blood loss from the inability to return red blood cells during automated plasmapheresis, which may result in: The procedure being terminated, and Deferral from donation for 8 weeks. h) Complications such as a hematoma or localized infection at the venipuncture site. i) Nausea, vomiting, light-headedness, fainting, or seizures; j) Bleeding, infection, pain, and the potential need for additional procedures in the event of complications. 9. I understand that frequent plasmapheresis donors might experience changes in blood protein levels, hemoglobin, IgG levels which may necessitate deferment or removal from the program. I understand that NuPlasma will collect samples to test mean corpuscular volume (MCV), which is a measure of the average size of the red blood cells and is performed to diagnose anemia. Additionally, a total serum protein (SPE), including immunoglobulin levels, these samples will be collected and tested at least every 4 months, and if the results are not within required limits, I will be removed from the program until the values return to normal. Further, donor screening will be completed prior to each donation to check my total protein levels, blood pressure, temperature, pulse, weight, height and hematocrit and hemoglobin. If any of these levels are outside the required range, I understand I will be temporarily deferred from donating until they return to normal. 10. Donor Responsibilities: a) To reduce the risk of infection, I should leave my bandage in place for 2 hours after each donation. b) I understand I must actively participate in the accurate identification of myself. I will be asked for my social security number and my photograph will be taken to aid in my identification. I understand I will be asked for my full name and date of birth during each step of the process. I have provided NuPlasma a valid form of identification and my Social Security number, to ensure I am registered accurately. c) I understand I should have a nutritious meal before donating. I will drink plenty of noncaffeinated, non-alcoholic beverages before and after donation. d) If at any time in the donation process, I am not feeling well, I will notify a member of the staff to assist me. e) If I experience a reaction after leaving NuPlasma I agree to call the center or seek medical evaluation or treatment. If I refuse or decline to seek medical treatment or transportation to the emergency room or medical facility, I am assuming the risk that my condition may become worse or cause additional medical complications to develop, up to, and including death. 11. I consent to have my blood tested for the presence of transmissible disease agents and other antibodies. If the results of tests for communicable disease agents are reactive, positive, or outside of normal limits the consequences could include: a. Detection of infectious agents such as Human Immunodeficiency Virus (HIV), or hepatitis, West Nile Virus, Zika Virus. If I test positive for a transmissible disease I agree to be contacted by mail. b. I understand I may be temporarily or permanently deferred from donation, as required by FDA and State regulations. c. I understand my results will be reported to public health officials per state law, and a subsequent public health investigation may occur. d. I understand that I should not donate in order to receive infectious disease testing. 12. I understand there is a "window period" for infection (the time interval early in infection during which tests for diseases such as HIV or hepatitis may be negative although infection may still be transmitted). I
13 promise I am not participating in donation as a means of being tested, and that I am not at risk for transmitting HIV infection to others through this donation. 13. Female donors: I understand I cannot donate if I am pregnant. I will report any pregnancies I have ever had in the past to NuPlasma medical staff, and if I become pregnant I will stop donating until 6 weeks post-partum. Further, I consent to testing for Human Leukocyte Antigen (HLA), an antigen that can sometimes be developed during pregnancy, and is known to cause a rare lung disease in patients that receive a transfusion. 14. I have read and understand The NuPlasma Blood Bank pre-donation information as it applies to the plasmapheresis donation I am about to make. To my knowledge, I have answered all questions truthfully and accurately. I understand the information about the spread of the AIDS virus by blood and blood products. I agree not to donate blood/blood components for transfusion to another person if I think I am at risk for spreading the AIDS virus. 15. I voluntarily donate my blood/blood components to The NuPlasma Blood Bank to use in any way it deems advisable. For that purpose, I consent to related tests, examinations, and procedures determined appropriate by The NuPlasma Blood Bank. 16. I understand that trained personnel will insert a needle into my arm to collect my plasma. The donation of plasma is not completely risk free. I have read and understand these risks as presented in the predonation information. I have been given opportunity to ask questions and all the questions I have asked have been answered to my satisfaction. 17. I understand that I am free to withdraw my consent and to discontinue participation in the plasmapheresis program at any time. THANK YOU FOR DONATING TODAY
DONOR ELIGIBILITY SPECIFIC INFORMATION
 To determine if you are eligible to donate we will: Ask about your health and travel Ask about medicines you are taking or have taken Ask about your risk for infections that can be transmitted by blood
To determine if you are eligible to donate we will: Ask about your health and travel Ask about medicines you are taking or have taken Ask about your risk for infections that can be transmitted by blood
Not Under Document Control If Printed. Donor Educational Materials. If you are deferred
 Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Making Your Blood Donation Safe
 BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Cord Blood Medical History/ Health Assessment Questionnaire
 OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
BLOOD DONOR INTERVIEW AND PHYSICAL
 EXERCISE : BLOOD DONOR INTERVIEW AND PHYSICAL Skills: 20 points Objectives:. Determine if an individual is eligible to donate blood by proper performance of the donor interview. 2. Identify the conditions
EXERCISE : BLOOD DONOR INTERVIEW AND PHYSICAL Skills: 20 points Objectives:. Determine if an individual is eligible to donate blood by proper performance of the donor interview. 2. Identify the conditions
Donor Prescreening Questions
 Donor Prescreening Questions CDO-384-PRD REV 22r18 Please read the following in its entirety to help guarantee your safety and the safety of the community blood supply and to help ensure you have a positive
Donor Prescreening Questions CDO-384-PRD REV 22r18 Please read the following in its entirety to help guarantee your safety and the safety of the community blood supply and to help ensure you have a positive
Guidance for Industry
 Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
For Parents and Students: Minor Donor Permit and Information About Donating Blood
 DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
DIN NUMBER 102 Chestnut Ridge Road, Montvale NJ 07645 DID For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family,
Question: 1. Are you currently taking an antibiotic?
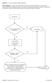 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
FORM TITLE: Risk Behavior Assessment. 1. Are you a male who has had sex with another male in the preceding five years?
 RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
Question: 1. Are you currently taking an antibiotic?
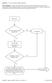 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Title: Medication Deferral List Vault: DONOR CENTER - RELEASED; InfoCard#: DC-2185 Effective Date: 31 Jul 2017
 Medication Deferral List SOME MEDICATIONS MAY AFFECT YOUR ELIGIBILITY TO DONATE BLOOD. PLEASE TELL US IF YOU Have been treated with the following types of medications or for the following conditions...
Medication Deferral List SOME MEDICATIONS MAY AFFECT YOUR ELIGIBILITY TO DONATE BLOOD. PLEASE TELL US IF YOU Have been treated with the following types of medications or for the following conditions...
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM. A Pilot Study for Collection of Anti-Zika Immune Plasma
 A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
HEALTH SCREENING QUESTIONNAIRE. Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
Cherokee Trail High School Blood Drive. Please help us to save lives! February 14th. 8:00 to 9:40 am & 11:00 am to 1:30 pm Upper Lecture Center
 Cherokee Trail High School Blood Drive Please help us to save lives! February 14th 8:00 to 9:40 am & 11:00 am to 1:30 pm Upper Lecture Center To be eligible to donate you need to: weigh at least 110 pounds
Cherokee Trail High School Blood Drive Please help us to save lives! February 14th 8:00 to 9:40 am & 11:00 am to 1:30 pm Upper Lecture Center To be eligible to donate you need to: weigh at least 110 pounds
Donor Risk Assessment Interview (Donor >12 yrs old)
 Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
WCPT COUNTRY PROFILE December 2017 SWEDEN
 WCPT COUNTRY PROFILE December 2017 SWEDEN SWEDEN NUMBERS WCPT Members Practising physical therapists 11,043 Total number of physical therapist members in your member organisation 17,906 Total number of
WCPT COUNTRY PROFILE December 2017 SWEDEN SWEDEN NUMBERS WCPT Members Practising physical therapists 11,043 Total number of physical therapist members in your member organisation 17,906 Total number of
WCPT COUNTRY PROFILE December 2017 HUNGARY
 WCPT COUNTRY PROFILE December 2017 HUNGARY HUNGARY NUMBERS WCPT Members Practising physical therapists 727 Total number of physical therapist members in your member organisation 4,000 Total number of practising
WCPT COUNTRY PROFILE December 2017 HUNGARY HUNGARY NUMBERS WCPT Members Practising physical therapists 727 Total number of physical therapist members in your member organisation 4,000 Total number of practising
WCPT COUNTRY PROFILE December 2017 SERBIA
 WCPT COUNTRY PROFILE December 2017 SERBIA SERBIA NUMBERS WCPT Members Practising physical therapists 622 Total number of physical therapist members in your member organisation 3,323 Total number of practising
WCPT COUNTRY PROFILE December 2017 SERBIA SERBIA NUMBERS WCPT Members Practising physical therapists 622 Total number of physical therapist members in your member organisation 3,323 Total number of practising
HEALTH SCREENING QUESTIONNAIRE CT Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
UNOS # NA. Person(s) Interviewed (First and last name) Relationship(s) to him/her. Print Name Title Signature
 UNOS # Form-0005 - Medical History and Behavioral Donor Name (First and last name) Date/Time of Interview Person(s) Interviewed (First and last name) Relationship(s) to him/her Person Conducting Interview
UNOS # Form-0005 - Medical History and Behavioral Donor Name (First and last name) Date/Time of Interview Person(s) Interviewed (First and last name) Relationship(s) to him/her Person Conducting Interview
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
For the Patient: Rituximab injection Other names: RITUXAN
 For the Patient: Rituximab injection Other names: RITUXAN Rituximab (ri tux' i mab) is a drug that is used to treat some types of cancer. It is a monoclonal antibody, a type of protein designed to target
For the Patient: Rituximab injection Other names: RITUXAN Rituximab (ri tux' i mab) is a drug that is used to treat some types of cancer. It is a monoclonal antibody, a type of protein designed to target
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM
 UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
Blood Transfusion Orientation & Information 2010
 Blood Transfusion Orientation & Information 2010 *if you are able to get online for this training: http://www.nhlbi.nih.gov/health/dci/diseases/bt/bt_whatis.html and you can read all information included
Blood Transfusion Orientation & Information 2010 *if you are able to get online for this training: http://www.nhlbi.nih.gov/health/dci/diseases/bt/bt_whatis.html and you can read all information included
UW MEDICINE PATIENT EDUCATION. Treating Blood Clots. What is a blood clot? DRAFT
 UW MEDICINE PATIENT EDUCATION Treating Blood Clots About deep vein thrombosis (DVT) and pulmonary embolism (PE) and how they are treated This handout explains blood clots, their symptoms, and how they
UW MEDICINE PATIENT EDUCATION Treating Blood Clots About deep vein thrombosis (DVT) and pulmonary embolism (PE) and how they are treated This handout explains blood clots, their symptoms, and how they
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
For the Patient: Bendamustine Other names: TREANDA
 For the Patient: Bendamustine Other names: TREANDA Bendamustine (ben'' da mus' teen) is a drug that is used to treat some types of cancer (lymphoma). It is a clear liquid that is injected into a vein.
For the Patient: Bendamustine Other names: TREANDA Bendamustine (ben'' da mus' teen) is a drug that is used to treat some types of cancer (lymphoma). It is a clear liquid that is injected into a vein.
IMPORTANT HEALTH INFORMATION
 IMPORTANT HEALTH INFORMATION SU-6523MI Page 1 of 8 Table of Contents Page What is an HIV test?..........................................1 Will the HIV test tell me if I have AIDS?............................1
IMPORTANT HEALTH INFORMATION SU-6523MI Page 1 of 8 Table of Contents Page What is an HIV test?..........................................1 Will the HIV test tell me if I have AIDS?............................1
Directed Semen Donor Eligibility Requirements
 Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
For the Patient: Fludarabine injection Other names: FLUDARA
 For the Patient: Fludarabine injection Other names: FLUDARA Fludarabine (floo-dare-a-been) is a drug that is used to treat many types of cancer. It is a clear liquid that is injected into a vein. Tell
For the Patient: Fludarabine injection Other names: FLUDARA Fludarabine (floo-dare-a-been) is a drug that is used to treat many types of cancer. It is a clear liquid that is injected into a vein. Tell
What is the most important information I should know about goserelin? What should I discuss with my healthcare provider before receiving goserelin?
 1 of 5 6/10/2016 4:04 PM Generic Name: goserelin (implant) (GOE se REL in) Brand Name: Zoladex What is goserelin? Goserelin is a man-made form of a hormone that regulates many processes in the body. Goserelin
1 of 5 6/10/2016 4:04 PM Generic Name: goserelin (implant) (GOE se REL in) Brand Name: Zoladex What is goserelin? Goserelin is a man-made form of a hormone that regulates many processes in the body. Goserelin
Package leaflet: Information for the patient. Avodart 0.5 mg soft capsules dutasteride
 Package leaflet: Information for the patient Avodart 0.5 mg soft capsules dutasteride Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient Avodart 0.5 mg soft capsules dutasteride Read all of this leaflet carefully before you start taking this medicine because it contains important information
Uniform Donor Risk Assessment Interview (Donor >12 yrs old)
 Your logo Uniform Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State
Your logo Uniform Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State
Pentostatin (Nipent )
 Pentostatin (Nipent ) Pentostatin (Nipent ) This leaflet is offered as a guide to you and your family. Your treatment will be fully explained by your doctor or nurse, who will be happy to answer any questions.
Pentostatin (Nipent ) Pentostatin (Nipent ) This leaflet is offered as a guide to you and your family. Your treatment will be fully explained by your doctor or nurse, who will be happy to answer any questions.
How does HBV affect the liver?
 Hepatitis B Why is the liver important? Your liver is a vital organ that performs many essential functions. It s the largest solid organ in the body and is located under your rib cage on the upper right
Hepatitis B Why is the liver important? Your liver is a vital organ that performs many essential functions. It s the largest solid organ in the body and is located under your rib cage on the upper right
ABO: Tissue#:
 Donor Name: Place of Interview: - Date-Time Interviewed: --/--/ --:-- Person Conducting Interview and Completing Form: - Person Interviewed: - Relationship to Potential Donor: - Address: - Phone: - Phone
Donor Name: Place of Interview: - Date-Time Interviewed: --/--/ --:-- Person Conducting Interview and Completing Form: - Person Interviewed: - Relationship to Potential Donor: - Address: - Phone: - Phone
In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise.
 Adapted From: Sexually Transmitted Infections Pamphlet. Public Health Agency of Canada, 2007 In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise.
Adapted From: Sexually Transmitted Infections Pamphlet. Public Health Agency of Canada, 2007 In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise.
First, Last Name DOB: Maternal Risk Questionnaire (MRQ)
 First, Last Name DOB: Cord Blood Bank Use Only: Maternal Risk Questionnaire (MRQ) NMDP CBU ID: Local CBU ID: NMDP Maternal ID: Local Maternal ID: Today's Date: Baby's Mother's Initials: Please read questions
First, Last Name DOB: Cord Blood Bank Use Only: Maternal Risk Questionnaire (MRQ) NMDP CBU ID: Local CBU ID: NMDP Maternal ID: Local Maternal ID: Today's Date: Baby's Mother's Initials: Please read questions
Welcome to Your Reading Assignment
 Welcome to Your Reading Assignment This workbook contains four reading assignments. It is filled with easy-to-read articles you can use to help keep yourself and those you care about safe. After each reading
Welcome to Your Reading Assignment This workbook contains four reading assignments. It is filled with easy-to-read articles you can use to help keep yourself and those you care about safe. After each reading
How is it transferred?
 STI s What is a STI? It is a contagious infection that is transferred from one person to another through sexual intercourse or other sexually- related behaviors. How is it transferred? The organisms live
STI s What is a STI? It is a contagious infection that is transferred from one person to another through sexual intercourse or other sexually- related behaviors. How is it transferred? The organisms live
STI s. (Sexually Transmitted Infections)
 STI s (Sexually Transmitted Infections) Build Awareness In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise. One of the primary defenses in the fight
STI s (Sexually Transmitted Infections) Build Awareness In Canada and around the world, the trend is clear: sexually transmitted infections (STIs) are on the rise. One of the primary defenses in the fight
Q1 What age are you?
 Q1 What age are you? Answered: 504 Skipped: 0 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 4.56% 23 3.77% 19 4.56% 23 6.15% 31 3.97% 20 6.55% 33 5.95% 30 6.75% 34 6.35% 32 4.37% 22 6.75% 34 5.56%
Q1 What age are you? Answered: 504 Skipped: 0 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 4.56% 23 3.77% 19 4.56% 23 6.15% 31 3.97% 20 6.55% 33 5.95% 30 6.75% 34 6.35% 32 4.37% 22 6.75% 34 5.56%
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT. Technescan MIBI 1 mg, kit for radiopharmaceutical preparation
 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Technescan MIBI 1 mg, kit for radiopharmaceutical preparation [Tetrakis(2-methoxy-2-methylpropyl-1 isocyanide)copper(i)] tetrafluoroborate Read all of this
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Technescan MIBI 1 mg, kit for radiopharmaceutical preparation [Tetrakis(2-methoxy-2-methylpropyl-1 isocyanide)copper(i)] tetrafluoroborate Read all of this
SPECIALIZED FAMILY CARE Provider Training
 SPECIALIZED FAMILY CARE Provider Training Category: Health Issue Title: Zika Virus Materials: Centers for Disease Control Fact Sheet on Zika Virus Goal: Specialized Family Care Provider to learn the risks,
SPECIALIZED FAMILY CARE Provider Training Category: Health Issue Title: Zika Virus Materials: Centers for Disease Control Fact Sheet on Zika Virus Goal: Specialized Family Care Provider to learn the risks,
For the Patient: Trastuzumab emtansine Other names: KADCYLA
 For the Patient: Trastuzumab emtansine Other names: KADCYLA Trastuzumab emtansine (tras tooz' ue mab em tan' seen) is a drug that is used to treat some types of cancer. Trastuzumab emtansine is a clear
For the Patient: Trastuzumab emtansine Other names: KADCYLA Trastuzumab emtansine (tras tooz' ue mab em tan' seen) is a drug that is used to treat some types of cancer. Trastuzumab emtansine is a clear
Sexually Transmitted Infections. Kim Dawson October 2010
 Sexually Transmitted Infections Kim Dawson October 2010 Objectives: You will learn about: Sexually Transmitted Infections (STI s). How they are transferred. High risk behavior. The most common STI s. How
Sexually Transmitted Infections Kim Dawson October 2010 Objectives: You will learn about: Sexually Transmitted Infections (STI s). How they are transferred. High risk behavior. The most common STI s. How
STI Review. CALM: STI/HIV - Lesson One (Handout 3) Bacteria/ Transmission. Symptoms. Disease. Virus
 STI Review Bacteria/ Virus? Transmission Chlamydia Bacteria Unprotected vaginal or anal sex with a person who has Chlamydia Genital Herpes Virus By direct contact with the sores or blisters of an infected
STI Review Bacteria/ Virus? Transmission Chlamydia Bacteria Unprotected vaginal or anal sex with a person who has Chlamydia Genital Herpes Virus By direct contact with the sores or blisters of an infected
For the Patient: Paclitaxel Other names: TAXOL
 For the Patient: Paclitaxel Other names: TAXOL Paclitaxel (pak'' li tax' el) is a drug that is used to treat many types of cancer. It is a clear liquid that is injected into a vein. Tell your doctor if
For the Patient: Paclitaxel Other names: TAXOL Paclitaxel (pak'' li tax' el) is a drug that is used to treat many types of cancer. It is a clear liquid that is injected into a vein. Tell your doctor if
Zika Virus. Frequently Asked Questions: Zika Virus and Pregnancy Version
 Zika Virus Frequently Asked Questions: Zika Virus and Pregnancy Version What is Zika? Zika is a viral infection that usually causes a mild illness that typically lasts between 2 and 7 days. 80% of people
Zika Virus Frequently Asked Questions: Zika Virus and Pregnancy Version What is Zika? Zika is a viral infection that usually causes a mild illness that typically lasts between 2 and 7 days. 80% of people
WHO REGIONAL OFFICE FOR EUROPE RECOMMENDATIONS ON INFLUENZA
 WHO REGIONAL OFFICE FOR EUROPE RECOMMENDATIONS ON INFLUENZA September 2017 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe Marmorvej
WHO REGIONAL OFFICE FOR EUROPE RECOMMENDATIONS ON INFLUENZA September 2017 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for Europe Marmorvej
Your lung biopsy is scheduled for: Date: Time: Questions about your biopsy? Need to reschedule or cancel your appointment?
 Lung Biopsy: CT Guided Information for patients and families Your lung biopsy is scheduled for: Date: Time: Important: You must arrive 1 hour before your appointment Questions about your biopsy? Need to
Lung Biopsy: CT Guided Information for patients and families Your lung biopsy is scheduled for: Date: Time: Important: You must arrive 1 hour before your appointment Questions about your biopsy? Need to
Nivolumab. Other Names: Opdivo. About this Drug. Possible Side Effects (More Common) Warnings and Precautions
 Nivolumab Other Names: Opdivo About this Drug Nivolumab is used to treat cancer. It is given in the vein (IV). Possible Side Effects (More Common) Bone marrow depression. This is a decrease in the number
Nivolumab Other Names: Opdivo About this Drug Nivolumab is used to treat cancer. It is given in the vein (IV). Possible Side Effects (More Common) Bone marrow depression. This is a decrease in the number
For the Patient: Cyclosporine injection Other names: SANDIMMUNE I.V.
 For the Patient: Cyclosporine injection Other names: SANDIMMUNE I.V. Cyclosporine (sye kloe spor een) is a drug that may be used to treat certain types of cancer. It may also be used to suppress your immune
For the Patient: Cyclosporine injection Other names: SANDIMMUNE I.V. Cyclosporine (sye kloe spor een) is a drug that may be used to treat certain types of cancer. It may also be used to suppress your immune
IRB Approval From: 3/8/2010 To: 10/28/2010
 UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Phase II Study to Assess the Safety and Immunogenicity of an Inactivated Swine-Origin H1N1 Influenza Vaccine in HIV-1 (Version 3.0, 16 FEB 2010) IRB Approval From:
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM Phase II Study to Assess the Safety and Immunogenicity of an Inactivated Swine-Origin H1N1 Influenza Vaccine in HIV-1 (Version 3.0, 16 FEB 2010) IRB Approval From:
Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another?
 Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another? HIV transmission can occur when blood, semen (including pre-seminal fluid or "pre-cum"),
Frequently Asked Questions about HIV/AIDS: Transmission and Prevention How is HIV passed from one person to another? HIV transmission can occur when blood, semen (including pre-seminal fluid or "pre-cum"),
The Johns Hopkins Bloomberg School of Public Health
 The Johns Hopkins Bloomberg School of Public Health CONSENT FORM A / NEW RESEARCH PROJECT Title of Research Project: A Randomized Trial of HAART in Acute/Early HIV Infection Version 3.0 Principal Investigator:
The Johns Hopkins Bloomberg School of Public Health CONSENT FORM A / NEW RESEARCH PROJECT Title of Research Project: A Randomized Trial of HAART in Acute/Early HIV Infection Version 3.0 Principal Investigator:
USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire. Table of Contents
 Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Paclitaxel (Taxol) and carboplatin
 Paclitaxel (Taxol) and carboplatin Paclitaxel (Taxol) and carboplatin This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may
Paclitaxel (Taxol) and carboplatin Paclitaxel (Taxol) and carboplatin This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may
1. What Panzyga is and what it is used for
 Package leaflet: Information for the user Panzyga, 100 mg/ml solution for infusion Human Normal Immunoglobulin (IVIg) This medicine is subject to additional monitoring. This will allow quick identification
Package leaflet: Information for the user Panzyga, 100 mg/ml solution for infusion Human Normal Immunoglobulin (IVIg) This medicine is subject to additional monitoring. This will allow quick identification
For the Patient: Lenalidomide Other names: REVLIMID
 For the Patient: Lenalidomide Other names: REVLIMID Lenalidomide (len a lid' oh mide) is a drug that is used to treat several types of cancer. It is a capsule that you take by mouth. Tell your doctor if
For the Patient: Lenalidomide Other names: REVLIMID Lenalidomide (len a lid' oh mide) is a drug that is used to treat several types of cancer. It is a capsule that you take by mouth. Tell your doctor if
INFORMATION AND CONSENT FORM For Adults Aged 18 and Older Additional Facility Sites
 INFORMATION AND CONSENT FORM Program Title: Expanded Access IND Program to Provide Stamaril Vaccine to Persons in the United States for Vaccination Against Yellow Fever Program #: Sponsor: Sanofi Pasteur
INFORMATION AND CONSENT FORM Program Title: Expanded Access IND Program to Provide Stamaril Vaccine to Persons in the United States for Vaccination Against Yellow Fever Program #: Sponsor: Sanofi Pasteur
RECOMMENDATIONS ON INFLUENZA VACCINATION DURING THE WINTER SEASON
 RECOMMENDATIONS ON INFLUENZA VACCINATION DURING THE 2018 2019 WINTER SEASON October 2018 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for
RECOMMENDATIONS ON INFLUENZA VACCINATION DURING THE 2018 2019 WINTER SEASON October 2018 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO Regional Office for
Nilotinib (nil ot' i nib) is a drug that is used to treat some types of cancer. It is a capsule that you take by mouth.
 For the Patient: Other names: TASIGNA (nil ot' i nib) is a drug that is used to treat some types of cancer. It is a capsule that you take by mouth. Blood tests may be taken regularly during treatment.
For the Patient: Other names: TASIGNA (nil ot' i nib) is a drug that is used to treat some types of cancer. It is a capsule that you take by mouth. Blood tests may be taken regularly during treatment.
LEBANON. WCPT COUNTRY PROFILE December 2018
 LEBANON WCPT COUNTRY PROFILE December 2018 LEBANON NUMBERS 1600 1400 1200 1000 800 600 400 200 0 Physical therapists in the country Members in MO 1,480 1,480 Total PTs in country 800000 700000 600000 500000
LEBANON WCPT COUNTRY PROFILE December 2018 LEBANON NUMBERS 1600 1400 1200 1000 800 600 400 200 0 Physical therapists in the country Members in MO 1,480 1,480 Total PTs in country 800000 700000 600000 500000
JUST FOR KIDS SELECTED IMPORTANT SAFETY INFORMATION
 JUST FOR KIDS For children ages 6-17 with moderately to severely active Crohn s disease or ulcerative colitis (UC) who haven t responded well to other therapies SELECTED IMPORTANT SAFETY INFORMATION REMICADE
JUST FOR KIDS For children ages 6-17 with moderately to severely active Crohn s disease or ulcerative colitis (UC) who haven t responded well to other therapies SELECTED IMPORTANT SAFETY INFORMATION REMICADE
Treatments for stroke prevention in Atrial Fibrillation as recommended by the Canadian Cardiovascular Society
 Treatments for stroke prevention in Atrial Fibrillation as recommended by the Canadian Cardiovascular Society Coumadin (Warfarin) Does this medication need ongoing monitoring of blood clotting times? Yes.
Treatments for stroke prevention in Atrial Fibrillation as recommended by the Canadian Cardiovascular Society Coumadin (Warfarin) Does this medication need ongoing monitoring of blood clotting times? Yes.
Quick Study: Sexually Transmitted Infections
 Quick Study: Sexually Transmitted Infections Gonorrhea What is it: A bacterial infection of the genitals, anus, or throat. How common: The CDC estimates 820,000 people in the United States get Gonorrhea
Quick Study: Sexually Transmitted Infections Gonorrhea What is it: A bacterial infection of the genitals, anus, or throat. How common: The CDC estimates 820,000 people in the United States get Gonorrhea
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
STD Notes. Myths about STDs
 STD Notes Sexually transmitted diseases (STD's) or sexually transmitted infections (STI's) are infectious diseases that spread from person to person through intimate contact. STD's can affect males and
STD Notes Sexually transmitted diseases (STD's) or sexually transmitted infections (STI's) are infectious diseases that spread from person to person through intimate contact. STD's can affect males and
For the Patient: Amsacrine Other names: AMSA PD
 For the Patient: Amsacrine Other names: AMSA PD Amsacrine (AM-sa-krin) is a drug that is used to treat some types of cancer. It is a clear orange-red liquid that is injected into a vein. Tell your doctor
For the Patient: Amsacrine Other names: AMSA PD Amsacrine (AM-sa-krin) is a drug that is used to treat some types of cancer. It is a clear orange-red liquid that is injected into a vein. Tell your doctor
SAFE BLOOD SAVE LIVES
 DIN BLOOD DONATION REGISTRATION FORM PART I: PRE-DONATION INFORMATION Thank you for coming to give blood today. Your donation could save and change the lives of the recipients. We sincerely request you
DIN BLOOD DONATION REGISTRATION FORM PART I: PRE-DONATION INFORMATION Thank you for coming to give blood today. Your donation could save and change the lives of the recipients. We sincerely request you
Preparation Instructions For Your Colonoscopy
 Page 1 of 16 PATIENT EDUCATION Preparation Instructions For Your Colonoscopy BARBARA WOODWARD LIPS PATIENT EDUCATION CENTER Page 2 of 16 Page 3 of 16 How to Prepare For Your Colonoscopy Please read these
Page 1 of 16 PATIENT EDUCATION Preparation Instructions For Your Colonoscopy BARBARA WOODWARD LIPS PATIENT EDUCATION CENTER Page 2 of 16 Page 3 of 16 How to Prepare For Your Colonoscopy Please read these
For the Patient: Eribulin Other names: HALAVEN
 For the Patient: Eribulin Other names: HALAVEN Eribulin (er'' i bue' lin) is a drug that is used to treat some types of cancer. It is a clear liquid that is injected into a vein. Tell your doctor if you
For the Patient: Eribulin Other names: HALAVEN Eribulin (er'' i bue' lin) is a drug that is used to treat some types of cancer. It is a clear liquid that is injected into a vein. Tell your doctor if you
Thyroid or Parathyroid Surgery
 PATIENT EDUCATION patienteducation.osumc.edu This handout gives you information about what to expect before, during and after your surgery. If you have questions, ask your nurse or doctor for more information.
PATIENT EDUCATION patienteducation.osumc.edu This handout gives you information about what to expect before, during and after your surgery. If you have questions, ask your nurse or doctor for more information.
A Patient s Guide to Blood Components and Products
 2014 A Patient s Guide to Blood Components and Products Contents What is a blood transfusion?... 1 Informed consent... 1 Frequently asked questions about blood transfusions... 2 What can I expect during
2014 A Patient s Guide to Blood Components and Products Contents What is a blood transfusion?... 1 Informed consent... 1 Frequently asked questions about blood transfusions... 2 What can I expect during
Where we stand in EFORT
 Where we stand in EFORT Engaging with the new EU regulatory landscape for medical devices. Challenges & opportunities Brussel, Belgium April 6, 2018 Per Kjaersgaard-Andersen Associate Professor Section
Where we stand in EFORT Engaging with the new EU regulatory landscape for medical devices. Challenges & opportunities Brussel, Belgium April 6, 2018 Per Kjaersgaard-Andersen Associate Professor Section
DENMARK. WCPT COUNTRY PROFILE December 2018
 DENMARK WCPT COUNTRY PROFILE December 2018 DENMARK NUMBERS 14000 12000 10000 8000 6000 4000 2000 0 Physical therapists in the country Members in MO 11,720 12,975 Total PTs in country 800000 700000 600000
DENMARK WCPT COUNTRY PROFILE December 2018 DENMARK NUMBERS 14000 12000 10000 8000 6000 4000 2000 0 Physical therapists in the country Members in MO 11,720 12,975 Total PTs in country 800000 700000 600000
Blood Transfusion. There are three types of blood cells: Red blood cells. White blood cells. Platelets.
 Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Sorafenib (so-ra-fe-nib) is a drug that is used to treat many types of cancer. It is a tablet that you take by mouth.
 For the Patient: Other names: Sorafenib NEXAVAR Sorafenib (so-ra-fe-nib) is a drug that is used to treat many types of cancer. It is a tablet that you take by mouth. A blood test may be taken before each
For the Patient: Other names: Sorafenib NEXAVAR Sorafenib (so-ra-fe-nib) is a drug that is used to treat many types of cancer. It is a tablet that you take by mouth. A blood test may be taken before each
Miralax (Polyethyene Glycol) Bowel Prep
 Miralax (Polyethyene Glycol) Bowel Prep The following instructions are your physician s specific instructions. Please follow the instructions carefully to ensure a successful prep. You can reach your physician
Miralax (Polyethyene Glycol) Bowel Prep The following instructions are your physician s specific instructions. Please follow the instructions carefully to ensure a successful prep. You can reach your physician
Donor Registration and Consent for HLA Typing
 Place NMDP Bar Code label here Jackie (left), donated to save the life of Paizley (right) Randy (left), donated to save the life of Luke (right) Tobias (left), donated to save the life of Betsy (right)
Place NMDP Bar Code label here Jackie (left), donated to save the life of Paizley (right) Randy (left), donated to save the life of Luke (right) Tobias (left), donated to save the life of Betsy (right)
PHILLY HEPATITIS ANSWERS ABOUT HEPATITIS
 PHILLY HEPATITIS ANSWERS ABOUT HEPATITIS TABLE OF CONTENTS WHAT IS HEPATITIS B? 2 HOW DO PEOPLE GET INFECTED WITH HEPATITIS B? 4 HOW DOES HEPATITIS B AFFECT MY BODY? 6 DOES HEPATITIS B AFFECT PREGNANCY?
PHILLY HEPATITIS ANSWERS ABOUT HEPATITIS TABLE OF CONTENTS WHAT IS HEPATITIS B? 2 HOW DO PEOPLE GET INFECTED WITH HEPATITIS B? 4 HOW DOES HEPATITIS B AFFECT MY BODY? 6 DOES HEPATITIS B AFFECT PREGNANCY?
What You Need to Know. Sexually Transmitted Infections (STIs)
 What You Need to Know Sexually Transmitted Infections (STIs) What You Need to Know About STIs What are STIs? Sexually transmitted infections (STIs) are diseases that spread through sexual contact. If you
What You Need to Know Sexually Transmitted Infections (STIs) What You Need to Know About STIs What are STIs? Sexually transmitted infections (STIs) are diseases that spread through sexual contact. If you
X-Plain Hepatitis B Reference Summary
 X-Plain Hepatitis B Reference Summary Introduction Hepatitis B is the most common serious liver infection. It is caused by the hepatitis B virus that attacks the liver. The virus is transmitted through
X-Plain Hepatitis B Reference Summary Introduction Hepatitis B is the most common serious liver infection. It is caused by the hepatitis B virus that attacks the liver. The virus is transmitted through
Treatment with Rivaroxaban Xarelto
 Treatment with Rivaroxaban Xarelto Anticoagulation Clinic This handout explains the medicine rivaroxaban, a drug that helps prevent blood clots. What is rivaroxaban? Rivaroxaban (brand name Xarelto) is
Treatment with Rivaroxaban Xarelto Anticoagulation Clinic This handout explains the medicine rivaroxaban, a drug that helps prevent blood clots. What is rivaroxaban? Rivaroxaban (brand name Xarelto) is
Sexually Transmitted Infections (STIs)
 Sexually Transmitted Infections (STIs) Overview Definition of STIs: What are they? Transmission: How are they spread? Types of infection: Bacterial (Chlamydia, Gonorrhea, Syphilis) Viral (Hepatitis B,
Sexually Transmitted Infections (STIs) Overview Definition of STIs: What are they? Transmission: How are they spread? Types of infection: Bacterial (Chlamydia, Gonorrhea, Syphilis) Viral (Hepatitis B,
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
