T-cells fighting B-cell lympho proliferative malignancies: the emerging field of CD19 CAR T-cell therapy
|
|
|
- Bartholomew Martin
- 5 years ago
- Views:
Transcription
1 REVIEW T-cells fighting B-cell lympho proliferative malignancies: the emerging field of CD19 CAR T-cell therapy D.M. Heijink 1, A.P. Kater 1, M.D. Hazenberg 1, A. Hagenbeek 1, M.J. Kersten 1 * 1 Department of Hematology, Academic Medical Center, Amsterdam, the Netherlands, *corresponding author: tel.: +31 (0) , fax: +31 (0) , m.j.kersten@amc.nl A B S T R A C T CAR T-cells are autologous T-cells transduced with a chimeric antigen receptor (CAR). The CAR contains an antigen recognition part (originating from an antibody), a T-cell receptor transmembrane and cytoplasmic signalling part, and one or more co-stimulatory domains. While CAR T-cells can be directed against any tumour target, most experience thus far has been obtained with targeting of the B-cell antigen CD19 that is expressed by B-cell acute lymphocytic leukaemia, chronic lymphocytic leukaemia and other B-cell lymphomas. The first clinical results are promising, although there are profound differences in response between patients with different haematological malignancies. Treatment-related side effects have been observed that require specific management. This review will explain the mechanism of action, summarise the experience to date and point out future directions for this hopeful new addition to the therapeutic armamentarium in the treatment of lymphoproliferative B-cell malignancies. KEYWORDS CAR T-cells, CD19, B-cell malignancies INTRODUCTION The cornerstone of the treatment of lymphoproliferative malignancies consists of chemotherapy, regularly followed by autologous or allogeneic stem cell transplantation. These treatments are not tumour cell specific and cause many side effects. In the last decade the focus has moved toward so-called targeted therapies using monoclonal antibodies and/or small molecules. Important examples in the current therapeutic arsenal are monoclonal antibodies against CD20, CD52 and CD30, cell surface antigens frequently, but not exclusively, expressed by lymphoproliferative malignancies such as lymphoma and lymphatic leukaemia. Other examples include small molecules targeting intracellular signalling pathways important for growth and survival of malignant cells, such as JAK inhibitors, BTK inhibitors, PI3K inhibitors and inhibitors of the proteasome. Most recently, an effective inhibitor of BCL-2, which induces apoptosis of malignant cells, has become available. 1 Another area that is attracting a lot of attention concerns autologous anti-tumour immune responses, in particular by T-lymphocytes. Most tumours are immunogenic but anti-tumour T-cell responses are efficiently suppressed by the tumour through membrane expression of immunosuppressive proteins such as PD-1 ligand and CTLA4 ligand. Interaction of these proteins with PD-1 and CTLA-4 on tumour specific T-cells renders these T-cells anergic. Antibodies that target PD-1 and CTLA-4 interactions (e.g. nivolumab, ipilimumab), release the breaks on the anti-tumour immune response and have been proven very effective. 2 Another example is blinatumomab, a bispecific T-cell engager (BiTe). This fusion protein consists of a CD3-specific part (recognising T-cells) and a CD19 specific part (recognising B-cells) and can thereby redirect T-cells directly towards malignant B-cells. Blinatumomab is approved for refractory or relapsed B-cell acute lymphocytic leukaemia (B-ALL). 3 Even more sophisticated is the use of engineered CAR T-cells, the topic of this review. ENGINEERED T-CELL THERAPY IN HAEMATOLOGY This completely new field involves the administration of autologous T-cells that have been engineered ex vivo to recognise tumour cells. Roughly, there are two ways Van Zuiden Communications B.V. All rights reserved. 147
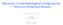
2 Figure 1. Schematic representation of CAR T-cell and TCR T-cell therapy = CD3ζ Third genera-on CAR Second genera-on CAR First genera-on CAR = co-s-mulatory domain = co-s-mulatory domain = hinge + scfv + linker = CD3 complex (δ ε γ ζ) An-gen (i.e. CD19) An-gen pep-de T-cell Tumor cell MHCI TCR α β T-cell CAR T-cell therapy TCR T-cell therapy to employ T-cells against haematological malignancies: T-cell receptor (TCR) engineered T-cells or chimeric antigen receptor (CAR) T-cells (figure 1). TCR T-cells are autologous T-cells transferred with a gene for the a and b chain of a specific T-cell receptor in order to direct the cells towards tumour-associated antigens (for example gp100 and MART-1), cancer germline antigens (for example NY-ESO-1 and MAGE) or cancer-specific mutations (for example in erbb2). TCR T-cells require a specific human leukocyte antigen (HLA) molecule for recognition of the target antigen. Therefore not all individuals are eligible for this treatment. Today, no results from clinical trials using TCR T-cells in haematological malignancies are known; however, several studies targeting the leukaemia antigen Wilms Tumor-1 (WT-1) are on-going. The great advantage of CAR T-cells is that tumour cell recognition can occur without MHC/HLA restriction. Evidence of clinical efficacy in lymphoproliferative malignancies is piling up quickly A hinge and transmembrane domain (often derived from CD8 α or CD28); 3. An intracellular signalling domain that is derived from the TCR (CD3 ζ chain) ensuring T-cell activation; 4. One or two co-stimulatory domains derived from CD28 and/or 4-1BB/CD137. These co-stimulatory domains have been included in the second- and third-generation CAR T-cells, respectively, in order to improve activation, replication and persistence of the CAR T-cells in vivo. Most of the research to date has focused on autologous CAR T-cells targeting CD19. CD19 is a transmembrane glycoprotein which is expressed on cells of the B-cell lineage, from early pro-b-cells in the bone marrow to mature B-cells in blood and tissues, but not on haematopoietic stem cells. Since CD19 is also expressed on the majority of malignant B-cells, the therapeutic potential of CD19-CAR T-cells is broad. 7,8 CAR T-CELLS CAR T-cells are characterised by four important elements (figure 1): 1. A single chain variable fragment (scfv) derived from a murine or humanised antibody which functions as the targeting domain directed against the target of choice. The scfv is attached to an extracellular spacer domain to ensure optimal binding freedom; GENERATION OF CAR T-CELLS The first step in the generation of autologous CAR T-cells is the collection of peripheral blood mononuclear cells through leukapheresis. The cells are cultured in the presence of a T-cell specific mitogenic stimulus, often using beads coated with anti-cd3/anti-cd28 monoclonal antibodies, resulting in the activation of T-cells with high replicative capacity. 7,8 The second step is transduction or transfection of the CAR construct, which is directed 148
3 against the target antigen of choice. Multiple methods have been tried including transposon-based systems introducing plasmid DNA into T-cells or electroporation of mrna into T-cells, but retroviral or lentiviral transduction is currently the transduction mode of choice. CAR T-cells are then cultured in the presence of cytokines (i.e. IL-2, IL-7, IL-12, IL-15). After expansion for several days, the cells are re-infused into the patient. In almost all CD19 CAR T-cell studies patients are lymphodepleted before infusing the engineered T-cells. In B-ALL patients the conditioning therapy mostly consists of fludarabine and cyclophosphamide, in lymphoma patients the choice depends on previous individual treatments. Pre-infusion lymphodepletion facilitates expansion and persistence of CAR T-cells in vivo CLINICAL USE OF CD19 CAR T-CELLS After preclinical evaluation, CD19 CAR T-cells were first tested in children and adults with relapsed/refractory ALL in adults with advanced relapsed and refractory chronic lymphatic leukaemia (CLL). Later patients with B-cell non-hodgkin lymphomas were included as well. Table 1 demonstrates the phase 1-2 trials which have been performed and published so far, each including ten or more patients Several smaller trials have been performed as well, which are reviewed in other papers. 24,25 Taken together, clear and deep responses are seen in a substantial percentage of B-ALL patients (complete responses in 70-94% of the cases). Durable complete responses correlate with persistence of the CAR T-cells and lasting B-cell depletion. It remains to be determined to what extent CD19 CAR-T cells may be curative for B-ALL and whether it has the potential to replace allogeneic stem cell transplantation in the future. More detailed studies will shed light on the causes of the disappearance and exhaustion of CAR T-cells that heralds B-ALL relapse ,18,21,23-25 Data on patients with diffuse large cell B-cell lymphomas are still scarce but so far complete response rates range from %. 19,20,22,25 Strikingly, patients with CLL appear to have lower response rates in the generally small trials performed (complete responses in 20-75% of the patients). 16,17,19,22,25 Although not fully elucidated yet, this might be due to disease-specific properties of CLL, in particular the interaction of CLL cells with the tumour microenvironment, which may cause exhaustion and dysfunction of (CAR) T-cells. 26 In other types of B-cell lymphomas (mantle cell lymphoma, follicular lymphoma) still only limited data are available. THE DOWNSIDES OF CD19 CAR T-CELLS There are several serious side effects of CAR T-cell therapy, which are mostly on-target toxicities. CD19 CAR T-cells specifically target CD19 positive B-cells, including healthy B-cells, resulting in long lasting B-cell aplasia. This aplasia a surrogate for the persistence of CAR T-cells puts successfully treated patients at risk for infections. Intravenous immunoglobulin therapy can prevent the majority of these infections. In addition, many patients develop cytokine release syndrome with symptoms ranging from mild and flu-like symptoms to severe reactions and multi-organ failure. The latter occurs in 30% of patients, typically 5-21 days after the infusion of CAR T-cells and is mediated by elevated levels of circulating cytokines, especially IL-6, IL-10 and interferon gamma. Treatment with the IL-6 inhibitor tocilizumab is highly effective; however, it is unclear whether this treatment affects the anti-tumour response. The same holds true for treatment with steroids. Finally, a number of patients have developed neurological symptoms ranging from seizures to aphasia and delirium. This type of toxicity seems transient and its pathophysiology is ill-understood. 7,8 FUTURE DIRECTIONS OF CAR T-CELL THERAPY Current research focuses on improving the safety and efficacy of CAR T-cell therapy. To be able to switch off the CAR T-cell response, for example in case of excessive toxicity, suicide genes have been incorporated into the T-cells. Examples are introduction of thymidine kinase, which can be inhibited by ganciclovir, expression of inducible caspase or Fas (pro-apoptotic genes) or expression of surface proteins rendering CAR T-cells susceptible to existing agents such as rituximab or cetuximab. Making CAR expression inducible instead of permanent could also increase safety. 4,27 To increase efficacy, multiple approaches are being explored. Firstly, it is investigated whether specific T-cell subsets provide superior antitumour efficacy. Currently, αβ T-cells (expressing a TCR consisting of an α and β chain) are used; however, therapy with γδ T-cells, specifically the Vγ9Vδ2 subset, may be more effective as these cells specifically recognise and efficiently kill cells with increased levels of phosphoantigens, which have been found in the majority of B-cell lymphomas. 28 Furthermore, it has become clear that selecting less differentiated T-cells such as naïve T-cells, T-memory stem cells and central memory T-cells for transduction results in more effective 149
4 Table 1. Overview of clinical trials using CD19 CAR T-cells in lymphoproliferative malignancies Author Year ALL CLL DLBCL Other Co-stimulation Construct Clinical response Maude et al BB Lentiviral 27/30 (90%) CR Grupp et al BB Lentiviral 50/53 (94%) CR (NB Extension of study reported by Maude) 13 Davila et al CD28 Retroviral 14/16 (88%) CR Porter et al BB Lentiviral 5/23 (22%) CR; 4/23 (17%) PR Porter et al BB Lentiviral 4/14 (29%) CR; 4/14 (29%) PR Lee et al CD28 Retroviral ALL: 14/20 (70%) CR Kochenderfer et al indolent CD28 Retroviral CLL: 3/4 (75%) CR; 1/4 (25%) PR DLBCL: 4/7 (57%) CR; 2/7 (29%) PR IL: 1/2 (50%) CR; 1/2 (50%) PR Schuster et al FL, 2 MCL 4-1BB Lentiviral DLBCL: 7/13 (54%) ORR FL: 7/7 (100%) ORR MCL: 1/2 (50%) ORR Turtle et al BB Lentiviral 15/18 (= 83%) CR Turtle et al FL, 4 MCL 4-1BB Lentiviral CLL: 3/6 (50%) CR; 1/6 (17%) PR DLBCL/FL/MCL: ORR 50 and 67% without and with fludarabine addition Park et al CD28 Retroviral 36/43 (84%) CR Kebriaei et al NHL CD28 Transposon ALL plus CLL: 10/21 (48%) CR (NB donor derived T-cells) Brudno et al MCL CD28 Retroviral ALL: 4/5 (80%) CR CLL: 1/5 (20%) CR, 1/5 (20%) PR DLBCL: 1/5 (20%) CR MCL: 1/5 (20%) PR (NB donor derived T-cells) CLL = chronic lymphocytic leukaemia; ALL = acute lymphoblastic leukaemia; DLBCL = diffuse large B cell lymphoma; FL = follicular lymphoma; MCL = mantle cell lymphoma; NHL = non-hodgkin lymphoma; PR = partial response; CR = complete response; ORR = overall response rate; IL = indolent lymphoma. in vivo expansion and survival. 4 To render CAR T-cells less susceptible to the suppressing effects of regulatory T-cells, T-cell signalling has been altered by increasing the expression of AKT or by modifying the CD28 signalling domain. 28 Interestingly, such an approach could also be of value in improving CAR T-cell therapy in CLL, as T-cells are globally dysfunctional in CLL with a few notable exceptions that could be enforced. 29 A second approach to improve efficacy is to develop CAR T-cells that express transgenes for IL-2, IL-12, IL-15 or IL-21. These cytokines are important for immune stimulation and may armour the infused CAR T-cells. In addition, chemokine receptors such as IL-7Rα have been built in to improve CAR T-cell survival. Thirdly, dual or polyspecific CAR T-cells might create higher tumour specificity. An example is the development of CAR T-cells targeting both CD19 and CD5 for CLL. 27 Furthermore, combination therapy with, for example, immune checkpoint inhibitors (inhibiting PD-1 and CTLA4) is being piloted which has the potential to overcome inhibition of the CAR T-cell response. 27,30 To make CAR T-cells more readily available ( off the shelf ) the use of allogeneic CAR T-cells is being explored. This obviously poses additional risks of graft-versus-hostdisease, which can be overcome by knockdown of the autologous T-cell receptor or introduction of a suicide gene
5 In the near future, several clinical trials with CAR T-cells will also become available in the Netherlands (for more information see CONCLUSION CAR T-cells provide a promising new type of treatment for lymphoproliferative malignancies. Clinical trials in patients with relapsed or refractory B-ALL and different types of B-cell lymphomas demonstrate impressive and often durable responses. The current clinical experience in haematology thus far mostly involves the use of CAR T-cells targeting the B-cell antigen CD19. Developments in novel and more specific target identification, dual target approaches, T-cell subset selection and CAR T-cell engineering will make this treatment even more safe, specific and effective. Hopefully, these developments will bring adoptive T-cell therapies further forward with the potential to replace stem cell transplantation in patients with lymphoproliferative malignancies. DISCLOSURES M.J. Kersten and A. Hagenbeek have received financial compensation for advisory boards for Novartis. REFERENCES 1. List of FDA approved drugs for hematology. 2. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nature Rev. 2015;14: Rogala R, Freyer CW, Ontiveros EP, et al. Blinatumomab: enlisting serial killer T-cells in the war against haematological malignancies. Expert Opin Biol Ther. 2015;15: Bonini C, Mondino A. Adoptive T-cell therapy for cancer: the era of engineered T cells. Eur J Immunol. 2015;45: Fujiwara H. Adoptive immunotherapy for haematological malignancies using T cells gene-modified to express tumor antigen-specific receptors. Pharmaceuticas. 2014;7: Uttenthal BJ, Chua I, Morris EC, Strauss HJ. Challenges in T cell receptor gene therapy. J Gene Med. 2012;14: Ghorashian S, Pule M, Amrolia P. CD19 chimeric antigen receptor T cell therapy for haematological malignancies. Br J Haematol. 2015;169: Maude S, Barrett DM. Current status of chimeric antigen receptor therapy for haematological malignancies. Br J Haematol. 2016;172: Mato A, Porter DL. A drive through cellular therapy for CLL in 2015: allogeneic cell transplantation and CARs. Blood. 2015;126: Kim JV, Latouche J-B, Rivière I, Sadelain M. The ABC of artificial antigen presentation. Nature Biotech. 2004;4: Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T-cells for sustained remissions in leukaemia. N Engl J Med. 2014;371: Grupp SA, Maude SL, Shaw PA, et al. Durable remissions in children with relapsed/refractory treated with T-cells engineered with a CD19 targeted chimeric antigen receptor (CTL019). Abstract 681, ASH Davila ML, Rivière I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukaemia. Sci Transl Med. 2014;19;6:224ra Porter DL, Freq NV, Melenhorst JJ, et al. Randomized, phase 2 dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Blood 2014, 124 (abstract). 15. Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukaemia. Sci Trans Med. 2015;7: Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: 1 phase 1 dose-escalation trial. Lancet. 2015:385: Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-cd19 chimeric antigen receptor. J Clin Oncol. 2015;33: Schuster SJ, Svoboda H, Nasta S, et al. Phase II1 trial of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. J Clin Oncol. 2015;33 (suppl; abstract 8516). 19. Turtle CJ, Berger C, Sommermeyer D, et al. Immunotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells of defined subset composition. J Clin Oncol. 2015;33 (suppl; abstract 3006). 20. Turtle CJ, Berger C, Sommermeyer D, et al. Anti-CD19 chimeric antigen receptor modified T cell therapy for B cell non-hodgkin lymphoma and chronic lymphocytic leukaemia: fludarabine and cyclophosphamide lymphodepletion improves in vivo expansion and persistence of CAR-T cells and clinical outcomes. Abstract, ASH Park JH, Rivière I, Wang X, et al. Implications of minimal residual disease negative complete remission (MRD-CR) and allogeneic stem cell transplant on safety and clinical outcome of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed, refractory B-cell ALL. Abstract 682, ASH Kebriaei P, Ciurea SO, Huls MH, et al. Pre-emptive donor lymphocyte infusion with CD19-directed, CAR-modified T cells infused after allogeneic hematopoietic cell transplantation for patients with advanced CD19+ malignancies. Abstract 862, ASH Brudno JN, Somerville R, Shi V, et al. Allogeneic T-cells expressing an anti-cd19 chimeric antigen receptor cause remissions of B-cell malignancies after allogenetic hematopoietic stem cell transplantation without causing graft-versus-host disease. Abstract 99, ASH Maude SL, Teachey DT, Porter DL, Grupp SA. CD-19 targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukaemia. Blood. 2015;125: Zhu Y, Tan Y, Ou R, et al. Anti-CD19 chimeric antigen receptor-modified T-cells for B-cell malignancies: a systemic review of efficacy and safety in clinical trials. Eur J Haematol Jun 25. doi: /ejh [Epub ahead of print] 26. Kater AP, Tonino SH, Egle A, Ramsay AG. How does lenalidomide target the chronic lymphocytic leukaemia microenvironment? Blood. 2014;124: Pegram HJ, Park JH, Brentjens RJ. CD28z CARs and armored CARs. Cancer J. 2014;20: Deniger DC, Switzer K, Mi T, et al. Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther. 2013;21: Te Raa GD, Pascutti MF, Garcia-Vallejo JJ, et al. CMV-specific CD8+ T-cell function is not impaired in chronic lymphocytic leukaemia. Blood. 2014;123: June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7:280ps7. 151
Conflict of Interest Natural Line of Defense: Update on Immunotherapy in Hematologic Malignancies. Immunogenicity of Cancer.
 Conflict of Interest Natural Line of Defense: Update on Immunotherapy in Hematologic Malignancies I have no professional/financial disclosures to report Katie Culos, PharmD BCOP Adult Stem Cell Transplant
Conflict of Interest Natural Line of Defense: Update on Immunotherapy in Hematologic Malignancies I have no professional/financial disclosures to report Katie Culos, PharmD BCOP Adult Stem Cell Transplant
Objectives. Emily Whitehead 10/11/2018. Chimeric Antigen Recepetor T-Cells (CAR-T) CAR-T Therapy: The Past, The Present, and The Future
 Objectives CAR-T Therapy: The Past, The Present, and The Future Nilay Shah, MD Michael Chargualaf, PharmD, BCOP WVU Medicine Mary Babb Randolph Cancer Center Review indications for FDA approved CAR-T therapy
Objectives CAR-T Therapy: The Past, The Present, and The Future Nilay Shah, MD Michael Chargualaf, PharmD, BCOP WVU Medicine Mary Babb Randolph Cancer Center Review indications for FDA approved CAR-T therapy
CAR-T Therapy: The Past, The Present, and The Future. Nilay Shah, MD Michael Chargualaf, PharmD, BCOP WVU Medicine Mary Babb Randolph Cancer Center
 CAR-T Therapy: The Past, The Present, and The Future Nilay Shah, MD Michael Chargualaf, PharmD, BCOP WVU Medicine Mary Babb Randolph Cancer Center Objectives Review indications for FDA approved CAR-T therapy
CAR-T Therapy: The Past, The Present, and The Future Nilay Shah, MD Michael Chargualaf, PharmD, BCOP WVU Medicine Mary Babb Randolph Cancer Center Objectives Review indications for FDA approved CAR-T therapy
CARs vs. BiTE in ALL. David L Porter, MD Jodi Fisher Horowitz Professor University of Pennsylvania Health System Abramson Cancer Center
 CARs vs. BiTE in ALL David L Porter, MD Jodi Fisher Horowitz Professor University of Pennsylvania Health System Abramson Cancer Center Disclosure Information David L Porter Speaker and members of study
CARs vs. BiTE in ALL David L Porter, MD Jodi Fisher Horowitz Professor University of Pennsylvania Health System Abramson Cancer Center Disclosure Information David L Porter Speaker and members of study
Abstract #163 Michael Kalos, PhD
 LONG TERM FUNCTIONAL PERSISTENCE, B CELL APLASIA AND ANTI LEUKEMIA EFFICACY IN REFRACTORY B CELL MALIGNANCIES FOLLOWING T CELL IMMUNOTHERAPY USING CAR REDIRECTED REDIRECTED T CELLS TARGETING CD19 Abstract
LONG TERM FUNCTIONAL PERSISTENCE, B CELL APLASIA AND ANTI LEUKEMIA EFFICACY IN REFRACTORY B CELL MALIGNANCIES FOLLOWING T CELL IMMUNOTHERAPY USING CAR REDIRECTED REDIRECTED T CELLS TARGETING CD19 Abstract
Immunotherapy on the Horizon: Adoptive Cell Therapy
 Immunotherapy on the Horizon: Adoptive Cell Therapy Joseph I. Clark, MD, FACP Professor of Medicine Loyola University Chicago Stritch School of Medicine Maywood, IL June 23, 2016 Conflicts of Interest
Immunotherapy on the Horizon: Adoptive Cell Therapy Joseph I. Clark, MD, FACP Professor of Medicine Loyola University Chicago Stritch School of Medicine Maywood, IL June 23, 2016 Conflicts of Interest
Exploiting the Immune System: Chimeric Antigen Receptor-T Cell Therapy for Hematologic Malignancies
 Exploiting the Immune System: Chimeric Antigen Receptor-T Cell Therapy for Hematologic Malignancies Maurice Alexander, PharmD, BCOP, CPP Clinical Specialist, Blood and Marrow Transplant UNC Bone Marrow
Exploiting the Immune System: Chimeric Antigen Receptor-T Cell Therapy for Hematologic Malignancies Maurice Alexander, PharmD, BCOP, CPP Clinical Specialist, Blood and Marrow Transplant UNC Bone Marrow
08/02/59. Tumor Immunotherapy. Development of Tumor Vaccines. Types of Tumor Vaccines. Immunotherapy w/ Cytokine Gene-Transfected Tumor Cells
 Tumor Immunotherapy Autologous virus Inactivation Inactivated virus Lymphopheresis Culture? Monocyte s Dendritic cells Immunization Autologous vaccine Development of Tumor Vaccines Types of Tumor Vaccines
Tumor Immunotherapy Autologous virus Inactivation Inactivated virus Lymphopheresis Culture? Monocyte s Dendritic cells Immunization Autologous vaccine Development of Tumor Vaccines Types of Tumor Vaccines
State of the art: CAR-T cell therapy in lymphoma
 State of the art: CAR-T cell therapy in lymphoma 14 th annual California Cancer Consortium conference Tanya Siddiqi, MD City of Hope Medical Center 8/11/18 Financial disclosures Consultant for Juno therapeutics
State of the art: CAR-T cell therapy in lymphoma 14 th annual California Cancer Consortium conference Tanya Siddiqi, MD City of Hope Medical Center 8/11/18 Financial disclosures Consultant for Juno therapeutics
2018 KSMO Immune Oncology Forum. Immune checkpoint inhibitors in hematologic. malignancies: evidences and perspectives 서울아산병원종양내과 홍정용
 2018 KSMO Immune Oncology Forum Immune checkpoint inhibitors in hematologic malignancies: evidences and perspectives 서울아산병원종양내과 홍정용 2018-07-18 Contents Introduction Immune checkpoint inhibtors in lymphomas
2018 KSMO Immune Oncology Forum Immune checkpoint inhibitors in hematologic malignancies: evidences and perspectives 서울아산병원종양내과 홍정용 2018-07-18 Contents Introduction Immune checkpoint inhibtors in lymphomas
ACTR (Antibody Coupled T-cell Receptor): A universal approach to T-cell therapy
 ACTR (Antibody Coupled T-cell Receptor): A universal approach to T-cell therapy European Medicines Agency Workshop on Scientific and Regulatory Challenges of Genetically Modified Cell-based Cancer Immunotherapy
ACTR (Antibody Coupled T-cell Receptor): A universal approach to T-cell therapy European Medicines Agency Workshop on Scientific and Regulatory Challenges of Genetically Modified Cell-based Cancer Immunotherapy
CAR-T CELLS: NEW HOPE FOR CANCER PATIENTS
 CAR-T CELLS: NEW HOPE FOR CANCER PATIENTS Natasha Kekre, MD, MPH, FRCPC Hematologist, Blood and Marrow Transplant Program, TOH Associate Scientist, Ottawa Hospital Research Institute Assistant Professor
CAR-T CELLS: NEW HOPE FOR CANCER PATIENTS Natasha Kekre, MD, MPH, FRCPC Hematologist, Blood and Marrow Transplant Program, TOH Associate Scientist, Ottawa Hospital Research Institute Assistant Professor
CAR T-Cell Therapy for Your Patients: What You Need To Know
 CAR T-Cell Therapy for Your Patients: What You Need To Know Marco L. Davila, MD, PhD Associate Member, Blood & Marrow Transplantation and Cellular Immunotherapy Medical Director Cell Therapy Facility H.
CAR T-Cell Therapy for Your Patients: What You Need To Know Marco L. Davila, MD, PhD Associate Member, Blood & Marrow Transplantation and Cellular Immunotherapy Medical Director Cell Therapy Facility H.
Immunocellular Therapies for Relapsed/ Refractory Heme Malignancies: A Focus on CAR T-Cell Therapy
 Immunocellular Therapies for Relapsed/ Refractory Heme Malignancies: A Focus on CAR T-Cell Therapy This transcript has been edited for style and clarity and includes all slides from the presentation. This
Immunocellular Therapies for Relapsed/ Refractory Heme Malignancies: A Focus on CAR T-Cell Therapy This transcript has been edited for style and clarity and includes all slides from the presentation. This
Chimeric An+gen Receptor (CAR) Modified T Cell Therapy: Mee#ng the Unmet Need in Follicular Lymphoma
 Chimeric An+gen Receptor (CAR) Modified T Cell Therapy: Mee#ng the Unmet Need in Follicular Lymphoma Stephen J. Schuster, M.D. Director, Lymphoma Program & Lymphoma Translational Research, Abramson Cancer
Chimeric An+gen Receptor (CAR) Modified T Cell Therapy: Mee#ng the Unmet Need in Follicular Lymphoma Stephen J. Schuster, M.D. Director, Lymphoma Program & Lymphoma Translational Research, Abramson Cancer
Sleeping Beauty: Current applications and future strategies. CAR-TCR Summit 2017 Partow Kebriaei, MD
 Sleeping Beauty: Current applications and future strategies CAR-TCR Summit 2017 Partow Kebriaei, MD Outline Chimeric antigen receptor (CAR) technology Viral versus nonviral vectors Results of current clinical
Sleeping Beauty: Current applications and future strategies CAR-TCR Summit 2017 Partow Kebriaei, MD Outline Chimeric antigen receptor (CAR) technology Viral versus nonviral vectors Results of current clinical
Immune checkpoint inhibitors in Hodgkin and non-hodgkin Lymphoma: How do they work? Where will we use them? Stephen M. Ansell, MD, PhD Mayo Clinic
 Immune checkpoint inhibitors in Hodgkin and non-hodgkin Lymphoma: How do they work? Where will we use them? Stephen M. Ansell, MD, PhD Mayo Clinic Conflicts of Interest Research Funding from Bristol Myers
Immune checkpoint inhibitors in Hodgkin and non-hodgkin Lymphoma: How do they work? Where will we use them? Stephen M. Ansell, MD, PhD Mayo Clinic Conflicts of Interest Research Funding from Bristol Myers
Background. Outcomes in refractory large B-cell lymphoma with traditional standard of care are extremely poor 1
 2-Year Follow-Up and High-Risk Subset Analysis of ZUMA-1, the Pivotal Study of Axicabtagene Ciloleucel (Axi-Cel) in Patients with Refractory Large B Cell Lymphoma Abstract 2967 Neelapu SS, Ghobadi A, Jacobson
2-Year Follow-Up and High-Risk Subset Analysis of ZUMA-1, the Pivotal Study of Axicabtagene Ciloleucel (Axi-Cel) in Patients with Refractory Large B Cell Lymphoma Abstract 2967 Neelapu SS, Ghobadi A, Jacobson
CAR-T cell therapy pros and cons
 CAR-T cell therapy pros and cons Stephen J. Schuster, MD Professor of Medicine Perelman School of Medicine of the University of Pennsylvania Director, Lymphoma Program & Lymphoma Translational Research
CAR-T cell therapy pros and cons Stephen J. Schuster, MD Professor of Medicine Perelman School of Medicine of the University of Pennsylvania Director, Lymphoma Program & Lymphoma Translational Research
Lymphoma and CLL EHA Madrid Professor John G Gribben Centre for Haemato-Oncology Barts Cancer Institute, London, UK
 Lymphoma and CLL EHA Madrid 2017 Professor John G Gribben Centre for Haemato-Oncology Barts Cancer Institute, London, UK Conflicts of Interest J Gribben I have the following financial relationships to
Lymphoma and CLL EHA Madrid 2017 Professor John G Gribben Centre for Haemato-Oncology Barts Cancer Institute, London, UK Conflicts of Interest J Gribben I have the following financial relationships to
Engineering an Immunity to Cancer: A New Era of Adoptive Cellular Therapy with Tisagenlecleucel (Kymriah) in Pediatric ALL
 Engineering an Immunity to Cancer: A New Era of Adoptive Cellular Therapy with Tisagenlecleucel (Kymriah) in Pediatric ALL Diana Schreier, Pharm.D., M.B.A. Pharmacy Grand Rounds October 17, 2017 2017 MFMER
Engineering an Immunity to Cancer: A New Era of Adoptive Cellular Therapy with Tisagenlecleucel (Kymriah) in Pediatric ALL Diana Schreier, Pharm.D., M.B.A. Pharmacy Grand Rounds October 17, 2017 2017 MFMER
Tumor Antigens in the Age of Engineered T cell Therapies
 Tumor Antigens in the Age of Engineered T cell Therapies September 30 th 2016 ESMO Preceptorship Course Amsterdam Carsten Linnemann, PhD Senior Scientist Kite Pharma EU B.V. Amsterdam Forward Looking Statements/Safe
Tumor Antigens in the Age of Engineered T cell Therapies September 30 th 2016 ESMO Preceptorship Course Amsterdam Carsten Linnemann, PhD Senior Scientist Kite Pharma EU B.V. Amsterdam Forward Looking Statements/Safe
Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant
 Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant Reporting Period January 1, 2012 June 30, 2012 Formula Grant Overview The Children s Hospital of Philadelphia received $3,521,179
Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant Reporting Period January 1, 2012 June 30, 2012 Formula Grant Overview The Children s Hospital of Philadelphia received $3,521,179
Engineered TCR and CAR Immunotherapeutics 2015:
 : A comparative analysis of the landscape of and business opportunities with TCR and CAR antibodies, T cells, NK cells, TILs, DLIs and CTLs released by La Merie Publishing on March 10, 2015 La Merie Publishing
: A comparative analysis of the landscape of and business opportunities with TCR and CAR antibodies, T cells, NK cells, TILs, DLIs and CTLs released by La Merie Publishing on March 10, 2015 La Merie Publishing
Advances in Immunotherapy for Lymphoma. Ashley Freeman, MD, FRCPC Immunotherapy Research Fellow
 Advances in Immunotherapy for Lymphoma Ashley Freeman, MD, FRCPC Immunotherapy Research Fellow The immune system and cancer Immunotherapies available for lymphoma Upcoming immunotherapy clinical trials
Advances in Immunotherapy for Lymphoma Ashley Freeman, MD, FRCPC Immunotherapy Research Fellow The immune system and cancer Immunotherapies available for lymphoma Upcoming immunotherapy clinical trials
CAR T-CELLS: ENGINEERING IMMUNE CELLS TO TREAT CANCER. Roman GALETTO, PhD 17 th Club Phase 1 Annual Meeting April 5 th Paris
 CAR T-CELLS: ENGINEERING IMMUNE CELLS TO TREAT CANCER Roman GALETTO, PhD 17 th Club Phase 1 Annual Meeting April 5 th 2018 - Paris Cellectis, 05-APR-2018 2 FORWARD-LOOKING STATEMENTS THIS PRESENTATION
CAR T-CELLS: ENGINEERING IMMUNE CELLS TO TREAT CANCER Roman GALETTO, PhD 17 th Club Phase 1 Annual Meeting April 5 th 2018 - Paris Cellectis, 05-APR-2018 2 FORWARD-LOOKING STATEMENTS THIS PRESENTATION
Treatments and Current Research in Leukemia. Richard A. Larson, MD University of Chicago
 Treatments and Current Research in Leukemia Richard A. Larson, MD University of Chicago 2 Acute (rapid progression) Myeloid Acute myeloid leukemia (AML) Acute promyelocytic leukemia (APL) Lymphoid Acute
Treatments and Current Research in Leukemia Richard A. Larson, MD University of Chicago 2 Acute (rapid progression) Myeloid Acute myeloid leukemia (AML) Acute promyelocytic leukemia (APL) Lymphoid Acute
Donor Lymphocyte Infusion for Malignancies Treated with an Allogeneic Hematopoietic Stem-Cell Transplant
 Last Review Status/Date: September 2014 Page: 1 of 8 Malignancies Treated with an Allogeneic Description Donor lymphocyte infusion (DLI), also called donor leukocyte or buffy-coat infusion is a type of
Last Review Status/Date: September 2014 Page: 1 of 8 Malignancies Treated with an Allogeneic Description Donor lymphocyte infusion (DLI), also called donor leukocyte or buffy-coat infusion is a type of
IMMUNOTHERAPY FOR CANCER A NEW HORIZON. Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust
 IMMUNOTHERAPY FOR CANCER A NEW HORIZON Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust ASCO Names Advance of the Year: Cancer Immunotherapy No recent
IMMUNOTHERAPY FOR CANCER A NEW HORIZON Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust ASCO Names Advance of the Year: Cancer Immunotherapy No recent
Stem Cell Sources 2/22/13. Cellular Therapy Today and Tomorrow. Cellular Therapy in HCT. Bone Marrow
 2/22/13 Cellular Therapy Today and Tomorrow Robert S. Negrin, MD Division Chief, Stanford Bone and Marrow Transplant Program Professor of Medicine Cellular Therapy in Clinical Medicine Established Hematopoietic
2/22/13 Cellular Therapy Today and Tomorrow Robert S. Negrin, MD Division Chief, Stanford Bone and Marrow Transplant Program Professor of Medicine Cellular Therapy in Clinical Medicine Established Hematopoietic
Update: New Treatment Modalities
 ASH 2008 Update: New Treatment Modalities ASH 2008: Update on new treatment modalities GA101 Improves tumour growth inhibition in mice and exhibits a promising safety profile in patients with CD20+ malignant
ASH 2008 Update: New Treatment Modalities ASH 2008: Update on new treatment modalities GA101 Improves tumour growth inhibition in mice and exhibits a promising safety profile in patients with CD20+ malignant
Adoptive Cellular Therapy SITC Primer October 2012
 Adoptive Cellular Therapy SITC Primer October 2012 Cassian Yee MD Member Fred Hutchinson Cancer Research Center Program in Immunology Clinical Research Division Professor University of Washington Division
Adoptive Cellular Therapy SITC Primer October 2012 Cassian Yee MD Member Fred Hutchinson Cancer Research Center Program in Immunology Clinical Research Division Professor University of Washington Division
Exploring Immunotherapies: Beyond Checkpoint Inhibitors
 Exploring Immunotherapies: Beyond Checkpoint Inhibitors Authored by: Jennifer Dolan Fox, PhD VirtualScopics (Now part of BioTelemetry Research) jennifer_fox@virtualscopics.com +1 585 249 6231 Introduction
Exploring Immunotherapies: Beyond Checkpoint Inhibitors Authored by: Jennifer Dolan Fox, PhD VirtualScopics (Now part of BioTelemetry Research) jennifer_fox@virtualscopics.com +1 585 249 6231 Introduction
Kymriah. Kymriah (tisagenlecleucel) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.101 Subject: Kymriah Page: 1 of 5 Last Review Date: September 20, 2018 Kymriah Description Kymriah
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.101 Subject: Kymriah Page: 1 of 5 Last Review Date: September 20, 2018 Kymriah Description Kymriah
CAR T-Cell Therapy for Lymphoma: Assessing Long-Term Durability. Julie M. Vose, MD, MBA
 CAR T-Cell Therapy for Lymphoma: Assessing Long-Term Durability Julie M. Vose, MD, MBA Relevant Disclosures Research Funding: Kite Pharma/Gilead, JUNO/Celgene, Novartis Honorarium/Ad Boards: Novartis,
CAR T-Cell Therapy for Lymphoma: Assessing Long-Term Durability Julie M. Vose, MD, MBA Relevant Disclosures Research Funding: Kite Pharma/Gilead, JUNO/Celgene, Novartis Honorarium/Ad Boards: Novartis,
New Evidence reports on presentations given at EHA/ICML Bendamustine in the Treatment of Lymphoproliferative Disorders
 New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
Adoptive cell therapy and modulation of the tumour microenvironment: new insights from ASCO 2016
 Adoptive cell therapy and modulation of the tumour microenvironment: new insights from ASCO 2016 Leila Khoja 1,2 and Bishal Gyawali 3 1 Royal Free Hospital, Pond Street, London NW3 2QG, UK. 2 Astrazeneca
Adoptive cell therapy and modulation of the tumour microenvironment: new insights from ASCO 2016 Leila Khoja 1,2 and Bishal Gyawali 3 1 Royal Free Hospital, Pond Street, London NW3 2QG, UK. 2 Astrazeneca
Applying Chimeric Antigen Receptor T cells (CAR-T) to hematologic malignancies
 Applying Chimeric Antigen Receptor T cells (CAR-T) to hematologic malignancies Anne W. Beaven, MD Associate Professor Duke University Medical Center Duke Debates April 21, 2017 Chimeric Antigen Receptor
Applying Chimeric Antigen Receptor T cells (CAR-T) to hematologic malignancies Anne W. Beaven, MD Associate Professor Duke University Medical Center Duke Debates April 21, 2017 Chimeric Antigen Receptor
Biogen Idec Oncology Pipeline. Greg Reyes, MD, PhD SVP, Oncology Research & Development
 Biogen Idec Oncology Pipeline Greg Reyes, MD, PhD SVP, Oncology Research & Development March 25, 2009 Biogen Idec Strategy in Lymphoma / Leukemia CLL RITUXAN NHL FC-RITUXAN GA101 RITUXAN-CVP RITUXAN-CHOP
Biogen Idec Oncology Pipeline Greg Reyes, MD, PhD SVP, Oncology Research & Development March 25, 2009 Biogen Idec Strategy in Lymphoma / Leukemia CLL RITUXAN NHL FC-RITUXAN GA101 RITUXAN-CVP RITUXAN-CHOP
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015
 ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
Engineered T cells: the promise and challenges of cancer immunotherapy
 Engineered T cells: the promise and challenges of cancer immunotherapy Andrew D. Fesnak, Carl H. June and Bruce L. Levine Abstract The immune system evolved to distinguish non-self from self to protect
Engineered T cells: the promise and challenges of cancer immunotherapy Andrew D. Fesnak, Carl H. June and Bruce L. Levine Abstract The immune system evolved to distinguish non-self from self to protect
Primer on Adoptive T cell Therapy. Saar Gill, MD, PhD University of Pennsylvania
 Primer on Adoptive T cell Therapy Saar Gill, MD, PhD University of Pennsylvania Presenter Disclosure Information Saar Gill The following relationships exist related to this presentation: Novartis, Research
Primer on Adoptive T cell Therapy Saar Gill, MD, PhD University of Pennsylvania Presenter Disclosure Information Saar Gill The following relationships exist related to this presentation: Novartis, Research
Chimeric Antigen Receptor - CAR T cell therapy. Frederick L. Locke, MD 2/17/2017
 Chimeric Antigen Receptor - CAR T cell therapy Frederick L. Locke, MD 2/17/2017 T cells are immune system cells that normally fight infection Each T cell recognizes a specific target T cells multiply and
Chimeric Antigen Receptor - CAR T cell therapy Frederick L. Locke, MD 2/17/2017 T cells are immune system cells that normally fight infection Each T cell recognizes a specific target T cells multiply and
Chimeric antigen receptor T cell therapies for lymphoma
 Chimeric antigen receptor T cell therapies for lymphoma Jennifer N. Brudno and James N. Kochenderfer Abstract New therapies are needed for patients with Hodgkin or non-hodgkin lymphomas that are resistant
Chimeric antigen receptor T cell therapies for lymphoma Jennifer N. Brudno and James N. Kochenderfer Abstract New therapies are needed for patients with Hodgkin or non-hodgkin lymphomas that are resistant
Newest Approaches in Immunotherapy for Non-Hodgkin s Lymphoma
 Newest Approaches in Immunotherapy for Non-Hodgkin s Lymphoma Immunotherapy in patients with relapsed/refractory non-hodgkin s lymphoma has shown promising progress. Research in the field of oncology has
Newest Approaches in Immunotherapy for Non-Hodgkin s Lymphoma Immunotherapy in patients with relapsed/refractory non-hodgkin s lymphoma has shown promising progress. Research in the field of oncology has
Priming the Immune System to Kill Cancer and Reverse Tolerance. Dr. Diwakar Davar Assistant Professor, Melanoma and Phase I Therapeutics
 Priming the Immune System to Kill Cancer and Reverse Tolerance Dr. Diwakar Davar Assistant Professor, Melanoma and Phase I Therapeutics Learning Objectives Describe the role of the immune system in cancer
Priming the Immune System to Kill Cancer and Reverse Tolerance Dr. Diwakar Davar Assistant Professor, Melanoma and Phase I Therapeutics Learning Objectives Describe the role of the immune system in cancer
CAR T-Cell Therapy for Acute Lymphoblastic Leukemia: Identifying Appropriate Patients and Maximizing Outcomes. Shannon L.
 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia: Identifying Appropriate Patients and Maximizing Outcomes Shannon L. Maude, MD, PhD CTL019 cell Lentiviral vector Anti-CD19 CAR construct CD19 Native
CAR T-Cell Therapy for Acute Lymphoblastic Leukemia: Identifying Appropriate Patients and Maximizing Outcomes Shannon L. Maude, MD, PhD CTL019 cell Lentiviral vector Anti-CD19 CAR construct CD19 Native
CAR-T. Subrena Powell RN, MSN, BMTCN
 CAR-T Subrena Powell RN, MSN, BMTCN Objectives Discuss the treatment timeline of a patient receiving CAR-T therapy Describe the side effects and management of symptoms of CAR-T therapy Treatment Schema
CAR-T Subrena Powell RN, MSN, BMTCN Objectives Discuss the treatment timeline of a patient receiving CAR-T therapy Describe the side effects and management of symptoms of CAR-T therapy Treatment Schema
National Institute for Health and Care Excellence. Single Technology Appraisal (STA)
 Single Technology Appraisal (STA) Tisagenlecleucel-T for previously treated B-cell acute lymphoblastic Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please
Single Technology Appraisal (STA) Tisagenlecleucel-T for previously treated B-cell acute lymphoblastic Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please
R/R DLBCL Treatment Landscape
 An Updated Analysis of JULIET, a Global Pivotal Phase 2 Trial of Tisagenlecleucel in Adult Patients With Relapsed or Refractory Diffuse Large B-Cell Lymphoma Abstract S799 Borchmann P, Tam CS, Jäger U,
An Updated Analysis of JULIET, a Global Pivotal Phase 2 Trial of Tisagenlecleucel in Adult Patients With Relapsed or Refractory Diffuse Large B-Cell Lymphoma Abstract S799 Borchmann P, Tam CS, Jäger U,
Precision CAR T Cell Therapeutics. Carl June October 15, 2018
 Precision CAR T Cell Therapeutics Carl June October 15, 2018 Disclosure Information Carl June I have the following financial relationships to disclose: Consultant for: Novartis, Immune Design, Viracta,
Precision CAR T Cell Therapeutics Carl June October 15, 2018 Disclosure Information Carl June I have the following financial relationships to disclose: Consultant for: Novartis, Immune Design, Viracta,
Emerging Targets in Immunotherapy
 Emerging Targets in Immunotherapy So Jin Shin, M.D. Department of Obstetrics and Gynecology, Keimyung University, School of Medicine, Daegu, Korea no-0ncology Todays is.. ancer Immunotherapy? nd immunotherapy
Emerging Targets in Immunotherapy So Jin Shin, M.D. Department of Obstetrics and Gynecology, Keimyung University, School of Medicine, Daegu, Korea no-0ncology Todays is.. ancer Immunotherapy? nd immunotherapy
AGRESSIVE LYMPHOMAS - FUTURE. Dr Stéphane Doucet CHUM
 AGRESSIVE LYMPHOMAS - FUTURE Dr Stéphane Doucet CHUM What are clinical trials? Clinical trials are carefully planned research studies where the most-promising discoveries and results from laboratory studies
AGRESSIVE LYMPHOMAS - FUTURE Dr Stéphane Doucet CHUM What are clinical trials? Clinical trials are carefully planned research studies where the most-promising discoveries and results from laboratory studies
Transforming patients lives through cellular immunotherapy. Next Generation Cellular Immunotherapy June 2017
 Transforming patients lives through cellular immunotherapy Next Generation Cellular Immunotherapy June 2017 1 Overview of Cell Medica Mission: Transform the treatment of cancer with cellular immunotherapy
Transforming patients lives through cellular immunotherapy Next Generation Cellular Immunotherapy June 2017 1 Overview of Cell Medica Mission: Transform the treatment of cancer with cellular immunotherapy
Yescarta (axicabtagene ciloleucel)
 Last Review Date: March 30, 2018 Effective Date: April 1, 2018 Number: MG.MM.PH.42 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Last Review Date: March 30, 2018 Effective Date: April 1, 2018 Number: MG.MM.PH.42 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Advances in haematological malignancies focus on lymphoid disease
 Advances in haematological malignancies focus on lymphoid disease Dr Kylie Mason MBBS PhD FRACP FRCPA Haematologist Bone marrow Transplant Physician Clinician Scientist The Royal Melbourne Hospital The
Advances in haematological malignancies focus on lymphoid disease Dr Kylie Mason MBBS PhD FRACP FRCPA Haematologist Bone marrow Transplant Physician Clinician Scientist The Royal Melbourne Hospital The
Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant
 Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant Reporting Period July 1, 2012 June 30, 2013 Formula Grant Overview The Children s Hospital of Philadelphia received $3,521,179
Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant Reporting Period July 1, 2012 June 30, 2013 Formula Grant Overview The Children s Hospital of Philadelphia received $3,521,179
Disclosures WOJCIECH JURCZAK
 Disclosures WOJCIECH JURCZAK ABBVIE (RESEARCH FUNDING), CELGENE (RESEARCH FUNDING); EISAI (RESEARCH FUNDING); GILEAD (RESEARCH FUNDING); JANSEN (RESEARCH FUNDING); MORPHOSYS (RESEARCH FUNDING), MUNDIPHARMA
Disclosures WOJCIECH JURCZAK ABBVIE (RESEARCH FUNDING), CELGENE (RESEARCH FUNDING); EISAI (RESEARCH FUNDING); GILEAD (RESEARCH FUNDING); JANSEN (RESEARCH FUNDING); MORPHOSYS (RESEARCH FUNDING), MUNDIPHARMA
Focus on Immunotherapy as a Targeted Therapy. Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct
 Focus on Immunotherapy as a Targeted Therapy Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct 18 2018 Disclosures I have nothing to disclose that is relevant to this presentation. Immunology @ Deeley
Focus on Immunotherapy as a Targeted Therapy Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct 18 2018 Disclosures I have nothing to disclose that is relevant to this presentation. Immunology @ Deeley
Adoptive Cell Therapy: Treating Cancer
 Adoptive Cell Therapy: Treating Cancer with Genetically Engineered T Cells Steven A. Feldman, Ph.D. Surgery Branch National Cancer Institute NCT Conference Heidelberg, Germany September 24, 2013 Three
Adoptive Cell Therapy: Treating Cancer with Genetically Engineered T Cells Steven A. Feldman, Ph.D. Surgery Branch National Cancer Institute NCT Conference Heidelberg, Germany September 24, 2013 Three
Checkpoint Blockade in Hematology and Stem Cell Transplantation
 Checkpoint Blockade in Hematology and Stem Cell Transplantation Saad S. Kenderian, MD Assistant Professor of Medicine and Oncology Mayo Clinic College of Medicine October 14, 2016 2015 MFMER slide-1 Disclosures
Checkpoint Blockade in Hematology and Stem Cell Transplantation Saad S. Kenderian, MD Assistant Professor of Medicine and Oncology Mayo Clinic College of Medicine October 14, 2016 2015 MFMER slide-1 Disclosures
Immuntherapie maligner Lymphome. Mathias Witzens-Harig Medizinische Klinik V Universität Heidelberg
 Immuntherapie maligner Lymphome Mathias Witzens-Harig Medizinische Klinik V Universität Heidelberg 20.02.2016 Immuntherapie maligner Lymphome Allogene Stammzelltransplantation Antikörper, z.b. Rituximab
Immuntherapie maligner Lymphome Mathias Witzens-Harig Medizinische Klinik V Universität Heidelberg 20.02.2016 Immuntherapie maligner Lymphome Allogene Stammzelltransplantation Antikörper, z.b. Rituximab
Current Applications and Future Directions of CAR-T cell therapies for B-cell Malignancies
 Current Applications and Future Directions of CAR-T cell therapies for B-cell Malignancies Nirav Shah, MD MSHP Assistant Professor of Medicine Medical College of Wisconsin 1 Financial Disclosure I currently
Current Applications and Future Directions of CAR-T cell therapies for B-cell Malignancies Nirav Shah, MD MSHP Assistant Professor of Medicine Medical College of Wisconsin 1 Financial Disclosure I currently
Bioassays for Quality Control of Cell & Gene Therapy Products
 Bioassays for Quality Control of Cell & Gene Therapy Products Erik Rutjens, Cell & Gene Therapy, Novartis Pharma AG CASSS Bioassays, Silver Spring, March2015 CTL019 Introduction CARTs = Chimeric Antigen
Bioassays for Quality Control of Cell & Gene Therapy Products Erik Rutjens, Cell & Gene Therapy, Novartis Pharma AG CASSS Bioassays, Silver Spring, March2015 CTL019 Introduction CARTs = Chimeric Antigen
OSCO/OU ASH-SABC Review. Lymphoma Update. Mohamad Cherry, MD
 OSCO/OU ASH-SABC Review Lymphoma Update Mohamad Cherry, MD Outline Diffuse Large B Cell Lymphoma Double Hit Lymphoma Follicular and Indolent B Cell Lymphomas Mantle Cell Lymphoma T Cell Lymphoma Hodgkin
OSCO/OU ASH-SABC Review Lymphoma Update Mohamad Cherry, MD Outline Diffuse Large B Cell Lymphoma Double Hit Lymphoma Follicular and Indolent B Cell Lymphomas Mantle Cell Lymphoma T Cell Lymphoma Hodgkin
CLL: future therapies. Dr. Nathalie Johnson
 CLL: future therapies Dr. Nathalie Johnson Disclosures Consultant and Advisory boards Roche, Abbvie, Gilead, Jansson, Lundbeck,Merck Research funding Roche, Abbvie, Lundbeck Outline Treatment of relapsed
CLL: future therapies Dr. Nathalie Johnson Disclosures Consultant and Advisory boards Roche, Abbvie, Gilead, Jansson, Lundbeck,Merck Research funding Roche, Abbvie, Lundbeck Outline Treatment of relapsed
Abstract 861. Stein AS, Topp MS, Kantarjian H, Gökbuget N, Bargou R, Litzow M, Rambaldi A, Ribera J-M, Zhang A, Zimmerman Z, Forman SJ
 Treatment with Anti-CD19 BiTE Blinatumomab in Adult Patients With Relapsed/Refractory B-Precursor Acute Lymphoblastic Leukemia (R/R ALL) Post-Allogeneic Hematopoietic Stem Cell Transplantation Abstract
Treatment with Anti-CD19 BiTE Blinatumomab in Adult Patients With Relapsed/Refractory B-Precursor Acute Lymphoblastic Leukemia (R/R ALL) Post-Allogeneic Hematopoietic Stem Cell Transplantation Abstract
Advances in Adoptive Cellular Therapy of Cancer. Melanoma Bridge Meeting December 5, 2014
 Advances in Adoptive Cellular Therapy of Cancer Melanoma Bridge Meeting December 5, 2014 David Stroncek, MD Chief, Cell Processing Section, DTM, CC, NIH Bethesda, Maryland, USA Disclosures None Focus
Advances in Adoptive Cellular Therapy of Cancer Melanoma Bridge Meeting December 5, 2014 David Stroncek, MD Chief, Cell Processing Section, DTM, CC, NIH Bethesda, Maryland, USA Disclosures None Focus
Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia
 OPTIMIZING TREATMENTS FOR HIGH-RISK ACUTE LYMPHOBLASTIC LEUKEMIA: WHAT IT TAKES TO MOVE THE NEEDLE Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia Noelle V. Frey and
OPTIMIZING TREATMENTS FOR HIGH-RISK ACUTE LYMPHOBLASTIC LEUKEMIA: WHAT IT TAKES TO MOVE THE NEEDLE Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia Noelle V. Frey and
Society for Immunotherapy of Cancer (SITC)
 Society for Immunotherapy of Cancer (SITC) Current Status of Chimeric and Adoptive T cell Therapy Lawrence G. Lum, MD, DSc Karmanos Cancer Institute and Wayne State University Advances in Cancer Immunotherapy
Society for Immunotherapy of Cancer (SITC) Current Status of Chimeric and Adoptive T cell Therapy Lawrence G. Lum, MD, DSc Karmanos Cancer Institute and Wayne State University Advances in Cancer Immunotherapy
CANCER IMMUNOTHERAPY. Cancer Research Center, Dpt. of Medicine & Service of Cytometry University of Salamanca. IBSAL
 FARMAFORUM 2015 FACULTAD FARMACIA CANCER IMMUNOTHERAPY Cancer Research Center, Dpt. of Medicine & Service of Cytometry University of Salamanca. IBSAL The immune response to tumors Oncogenic event The immune
FARMAFORUM 2015 FACULTAD FARMACIA CANCER IMMUNOTHERAPY Cancer Research Center, Dpt. of Medicine & Service of Cytometry University of Salamanca. IBSAL The immune response to tumors Oncogenic event The immune
Pharmacyclics Reports Updated Clinical Results from its Phase IA Trial of its First in Human BTK- Inhibitor PCI-32765
 Contact: Ramses Erdtmann Vice President of Finance Phone: 408-215-3325 Pharmacyclics Reports Updated Clinical Results from its Phase IA Trial of its First in Human BTK- Inhibitor PCI-32765 Company to Host
Contact: Ramses Erdtmann Vice President of Finance Phone: 408-215-3325 Pharmacyclics Reports Updated Clinical Results from its Phase IA Trial of its First in Human BTK- Inhibitor PCI-32765 Company to Host
Immunotherapy in haematological malignancies. Michele Ghielmini Oncology Institute of Southern Switzerland Bellinzona
 Immunotherapy in haematological malignancies Michele Ghielmini Oncology Institute of Southern Switzerland Bellinzona Conflicts of interest Roche Celgene Mundipharma Janssen Gilead Bayer Millenium What
Immunotherapy in haematological malignancies Michele Ghielmini Oncology Institute of Southern Switzerland Bellinzona Conflicts of interest Roche Celgene Mundipharma Janssen Gilead Bayer Millenium What
Yescarta. Yescarta (axicabtagene ciloleucel) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.105 Subject: Yescarta Page: 1 of 5 Last Review Date: September 20, 2018 Yescarta Description Yescarta
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.105 Subject: Yescarta Page: 1 of 5 Last Review Date: September 20, 2018 Yescarta Description Yescarta
Mariano Provencio Servicio de Oncología Médica Hospital Universitario Puerta de Hierro. Immune checkpoint inhibition in DLBCL
 Mariano Provencio Servicio de Oncología Médica Hospital Universitario Puerta de Hierro Immune checkpoint inhibition in DLBCL Immunotherapy: The Cure is Inside Us Our immune system prevents or limit infections
Mariano Provencio Servicio de Oncología Médica Hospital Universitario Puerta de Hierro Immune checkpoint inhibition in DLBCL Immunotherapy: The Cure is Inside Us Our immune system prevents or limit infections
Brave New World of Cancer Therapeutics (Back to the Future)
 Brave New World of Cancer Therapeutics (Back to the Future) Alexandra M. Levine, MD, MACP Chief Medical Officer Melinda & Norman Payson Professor of Medicine Professor of Hematology/HCT City of Hope National
Brave New World of Cancer Therapeutics (Back to the Future) Alexandra M. Levine, MD, MACP Chief Medical Officer Melinda & Norman Payson Professor of Medicine Professor of Hematology/HCT City of Hope National
Dr. C. Tom Kouroukis. New and upcoming treatments for Lymphoma
 Dr. C. Tom Kouroukis New and upcoming treatments for Lymphoma New and upcoming treatments for Lymphoma Dr. Tom Kouroukis Hamilton Convention Centre November 23, 2013 Overview Importance and design of clinical
Dr. C. Tom Kouroukis New and upcoming treatments for Lymphoma New and upcoming treatments for Lymphoma Dr. Tom Kouroukis Hamilton Convention Centre November 23, 2013 Overview Importance and design of clinical
Summary... 2 IMMUNOTHERAPY IN CANCER... 3
 ESMO 2016 Congress 7-11 October, 2016 Copenhagen, Denmark Table of Contents Summary... 2 IMMUNOTHERAPY IN CANCER... 3 Sequencing analysis reveals baseline tumour T cell receptor and neo antigen load associates
ESMO 2016 Congress 7-11 October, 2016 Copenhagen, Denmark Table of Contents Summary... 2 IMMUNOTHERAPY IN CANCER... 3 Sequencing analysis reveals baseline tumour T cell receptor and neo antigen load associates
Lymphoma 101. Nathalie Johnson, MDPhD. Division of Hematology Jewish General Hospital Associate Professor of Medicine, McGill University
 Lymphoma 101 Nathalie Johnson, MDPhD Division of Hematology Jewish General Hospital Associate Professor of Medicine, McGill University Disclosures Consultant and Advisory boards for multiple companies
Lymphoma 101 Nathalie Johnson, MDPhD Division of Hematology Jewish General Hospital Associate Professor of Medicine, McGill University Disclosures Consultant and Advisory boards for multiple companies
*Jagiellonian University, Kraków, Poland
 Single-Agent MOR208 in Relapsed or Refractory (R-R) Non-Hodgkin s Lymphoma (NHL): Results from Diffuse Large B-Cell Lymphoma (DLBCL) and Indolent NHL Subgroups of a Phase IIa Study Wojciech Jurczak, *
Single-Agent MOR208 in Relapsed or Refractory (R-R) Non-Hodgkin s Lymphoma (NHL): Results from Diffuse Large B-Cell Lymphoma (DLBCL) and Indolent NHL Subgroups of a Phase IIa Study Wojciech Jurczak, *
YESCARTA (axicabtagene ciloleucel)
 YESCARTA (axicabtagene ciloleucel) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
YESCARTA (axicabtagene ciloleucel) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
THERAPEUTIC IMPLICATIONS OF PREPARING AND ADMINISTERING INNATE IMMUNE CELLS. 9:40 am to 10:10 pm Laurence Cooper
 THERAPEUTIC IMPLICATIONS OF PREPARING AND ADMINISTERING INNATE IMMUNE CELLS 9:40 am to 10:10 pm Laurence Cooper ljncooper@ziopharm.com 05-16-2016 Forward-looking statements This presentation contains certain
THERAPEUTIC IMPLICATIONS OF PREPARING AND ADMINISTERING INNATE IMMUNE CELLS 9:40 am to 10:10 pm Laurence Cooper ljncooper@ziopharm.com 05-16-2016 Forward-looking statements This presentation contains certain
Product: Blinatumomab (AMG 103) Clinical Study Report: MT Date: 19 April 2013 Page 2 of 9510
 Date: 19 April 2013 Page 2 of 9510 2. SYNOPSIS Name of Sponsor: Amgen Research (Munich), formerly Micromet AG/Micromet GmbH, Staffelseestrasse 2, 81477 Munich, Germany Name of Finished Product: Blinatumomab
Date: 19 April 2013 Page 2 of 9510 2. SYNOPSIS Name of Sponsor: Amgen Research (Munich), formerly Micromet AG/Micromet GmbH, Staffelseestrasse 2, 81477 Munich, Germany Name of Finished Product: Blinatumomab
Haemato-Oncology ESMO PRECEPTORSHIP PROGRAMME IMMUNO-ONCOLOGY. Development and clinical experience Monique Minnema, hematologist
 Haemato-Oncology ESMO PRECEPTORSHIP PROGRAMME IMMUNO-ONCOLOGY Development and clinical experience Monique Minnema, hematologist Consultancy for disclosures Amgen, Celgene, Jansen Cilag, BMS, Takeda Immune
Haemato-Oncology ESMO PRECEPTORSHIP PROGRAMME IMMUNO-ONCOLOGY Development and clinical experience Monique Minnema, hematologist Consultancy for disclosures Amgen, Celgene, Jansen Cilag, BMS, Takeda Immune
Axicabtagene ciloleucel IV for relapsed/refractory acute lymphoblastic leukaemia in children and adolecents (aged 2-21 years) second line
 NIHR Innovation Observatory Evidence Briefing: August 2017 Axicabtagene ciloleucel IV for relapsed/refractory acute lymphoblastic leukaemia in children and adolecents (aged 2-21 years) second line NIHRIO
NIHR Innovation Observatory Evidence Briefing: August 2017 Axicabtagene ciloleucel IV for relapsed/refractory acute lymphoblastic leukaemia in children and adolecents (aged 2-21 years) second line NIHRIO
Bases for Immunotherapy in Multiple Myeloma
 Bases for Immunotherapy in Multiple Myeloma Paola Neri, MD, PhD Associate Professor of Medicine University of Calgary, Arnie Charbonneau Cancer Institute Disclosures Paola Neri MD, PhD Grants/research
Bases for Immunotherapy in Multiple Myeloma Paola Neri, MD, PhD Associate Professor of Medicine University of Calgary, Arnie Charbonneau Cancer Institute Disclosures Paola Neri MD, PhD Grants/research
Tumor responses (patients responding/ patients treated)
 Table 1. ACT clinical trial tumor responses and toxicities. a Target antigen Cancer(s) Receptor type Tumor responses (patients responding/ patients treated) Immune-mediated toxicities (patients experiencing
Table 1. ACT clinical trial tumor responses and toxicities. a Target antigen Cancer(s) Receptor type Tumor responses (patients responding/ patients treated) Immune-mediated toxicities (patients experiencing
Tumor Immunity and Immunotherapy. Andrew Lichtman M.D., Ph.D. Brigham and Women s Hospital Harvard Medical School
 Tumor Immunity and Immunotherapy Andrew Lichtman M.D., Ph.D. Brigham and Women s Hospital Harvard Medical School Lecture Outline Evidence for tumor immunity Types of tumor antigens Generation of anti-tumor
Tumor Immunity and Immunotherapy Andrew Lichtman M.D., Ph.D. Brigham and Women s Hospital Harvard Medical School Lecture Outline Evidence for tumor immunity Types of tumor antigens Generation of anti-tumor
Donor derived CD19 specific CAR + T cell therapy after haploidentical hematopoietic stem cell transplantation Laurence J.N. Cooper Hyatt Orlando
 Donor derived CD19 specific CAR + T cell therapy after haploidentical hematopoietic stem cell transplantation Laurence J.N. Cooper Hyatt Orlando International Airport Hotel December 3, 2015 (afternoon)
Donor derived CD19 specific CAR + T cell therapy after haploidentical hematopoietic stem cell transplantation Laurence J.N. Cooper Hyatt Orlando International Airport Hotel December 3, 2015 (afternoon)
BiTE in ALL and AML. Ibrahim Aldoss, MD Assistant Professor, City of Hope Hematology and Hematopoietic Cell Transplantation
 BiTE in ALL and AML Ibrahim Aldoss, MD Assistant Professor, City of Hope Hematology and Hematopoietic Cell Transplantation Disclosure I am consultant for Helocyte and Speaker Bureau for JAZZ Immune system
BiTE in ALL and AML Ibrahim Aldoss, MD Assistant Professor, City of Hope Hematology and Hematopoietic Cell Transplantation Disclosure I am consultant for Helocyte and Speaker Bureau for JAZZ Immune system
Important new concerns or changes to the current ones will be included in updates of YESCARTA s RMP.
 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for YESCARTA (axicabtagene ciloleucel) This is a summary of the risk management plan (RMP) for YESCARTA. The RMP details important
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for YESCARTA (axicabtagene ciloleucel) This is a summary of the risk management plan (RMP) for YESCARTA. The RMP details important
Rituximab, and autologous stem cell collection rate after induction therapy with Bortezomib-Rituximab. 4 Not yet recruiting
 1 Recruiting A Phase II Trial of Ofatumumab in Subjects With Waldenstrom's Conditions: Waldenstrom's ; Waldenstrom Macroglobulinaemia Intervention: Biological: Ofatumumab Sponsor: GlaxoSmithKline Number
1 Recruiting A Phase II Trial of Ofatumumab in Subjects With Waldenstrom's Conditions: Waldenstrom's ; Waldenstrom Macroglobulinaemia Intervention: Biological: Ofatumumab Sponsor: GlaxoSmithKline Number
Aggressive NHL and Hodgkin Lymphoma. Dr. Carolyn Faught November 10, 2017
 Aggressive NHL and Hodgkin Lymphoma Dr. Carolyn Faught November 10, 2017 What does aggressive mean? Shorter duration of symptoms Generally need treatment at time of diagnosis Immediate, few days, few weeks
Aggressive NHL and Hodgkin Lymphoma Dr. Carolyn Faught November 10, 2017 What does aggressive mean? Shorter duration of symptoms Generally need treatment at time of diagnosis Immediate, few days, few weeks
UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma
 UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Supported by a grant from Supported by a grant from UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Jonathan W.
UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Supported by a grant from Supported by a grant from UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Jonathan W.
DEVELOPMENT OF CELLULAR IMMUNOLOGY
 DEVELOPMENT OF CELLULAR IMMUNOLOGY 1880 s: Antibodies described (dominated studies of immunology until 1960 s) 1958: Journal of Immunology (137 papers) lymphocyte not listed in index Two papers on transfer
DEVELOPMENT OF CELLULAR IMMUNOLOGY 1880 s: Antibodies described (dominated studies of immunology until 1960 s) 1958: Journal of Immunology (137 papers) lymphocyte not listed in index Two papers on transfer
Tumor Immunology: A Primer
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Personalized medicine - cancer immunotherapy
 Personalized medicine - cancer immunotherapy Özcan Met, PhD Senior Staff Scientist, Cell Therapy Director Center for Cancer Immune Therapy Department of Hematology Department of Oncology University Hospital
Personalized medicine - cancer immunotherapy Özcan Met, PhD Senior Staff Scientist, Cell Therapy Director Center for Cancer Immune Therapy Department of Hematology Department of Oncology University Hospital
RXi Pharmaceuticals. Immuno-Oncology World Frontiers Conference. January 23, 2018 NASDAQ: RXII. Property of RXi Pharmaceuticals
 RXi Pharmaceuticals Immuno-Oncology World Frontiers Conference January 23, 2018 NASDAQ: RXII Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private
RXi Pharmaceuticals Immuno-Oncology World Frontiers Conference January 23, 2018 NASDAQ: RXII Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private
TRANSLATIONAL AND CLINICAL RESEARCH
 TRANSLATIONAL AND CLINICAL RESEARCH Concise Review: Emerging Principles from the Clinical Application of Chimeric Antigen Receptor T Cell Therapies for B Cell Malignancies MICHAEL D. JAIN a,b and MARCO
TRANSLATIONAL AND CLINICAL RESEARCH Concise Review: Emerging Principles from the Clinical Application of Chimeric Antigen Receptor T Cell Therapies for B Cell Malignancies MICHAEL D. JAIN a,b and MARCO
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES. Overview and Mechanism of Action Dr.
 BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
