Clinical Policy Title: Subtalar arthroereisis (implant)
|
|
|
- Katrina Reeves
- 5 years ago
- Views:
Transcription
1 Clinical Policy Title: Subtalar arthroereisis (implant) Clinical Policy Number: Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next Review Date: September 2018 Related policies: Policy contains: Subtalar arthroereisis. Sinus tarsi implant. Extra osseous talotarsal stabilization. Flatfoot (pes planus, pes planovalgus). CP# Total ankle replacement ABOUT THIS POLICY: AmeriHealth Caritas Northeast has developed clinical policies to assist with making coverage determinations. AmeriHealth Caritas Northeast s clinical policies are based on guidelines from established industry sources, such as the Centers for Medicare & Medicaid Services (CMS), state regulatory agencies, the American Medical Association (AMA), medical specialty professional societies, and peerreviewed professional literature. These clinical policies along with other sources, such as plan benefits and state and federal laws and regulatory requirements, including any state or plan specific definition of medically necessary, and the specific facts of the particular situation are considered by AmeriHealth Caritas Northeast when making coverage determinations. In the event of conflict between this clinical policy and plan benefits and/or state or federal laws and/or regulatory requirements, the plan benefits and/or state and federal laws and/or regulatory requirements shall control. AmeriHealth Caritas Northeast s clinical policies are for informational purposes only and not intended as medical advice or to direct treatment. Physicians and other health care providers are solely responsible for the treatment decisions for their patients. AmeriHealth Caritas Northeast s clinical policies are reflective of evidence based medicine at the time of review. As medical science evolves, AmeriHealth Caritas Northeast will update its clinical policies as necessary. AmeriHealth Caritas Northeast s clinical policies are not guarantees of payment. Coverage policy AmeriHealth Caritas Northeast considers the use of subtalar arthroereisis to be investigational and, therefore, not medically necessary. Limitations: All other uses of subtalar arthroereisis are not medically necessary. Alternative covered services: Orthotics. Physical therapy. Prescription drug therapy. Short leg walking cast or brace. Surgical procedures including, but not be limited to, the following: - Arthrodesis. 1
2 - Osteotomy. - Tendon transfer. Background Flatfoot (also called pes planus, pes planovalgus, or fallen arch ) occurs when the tendons that form the medial arch of the foot fail to pull together properly, allowing the entire sole of the foot to touch the floor while standing. Flatfoot is characterized as flexible (the medial arch flattens upon standing but normalizes during non weight bearing) and rigid (unaltered by weight bearing). It may result in alteration of foot biomechanics, subsequent foot misalignment, lower extremity pain, and limited physical activity. The etiology can be congenital or acquired through wear and tear stresses, injury to the tendon or bone, arthritis, and neuropathy. Most children are born with very little arch in the feet. As they grow and walk, the soft tissues along the bottom of the feet tighten, which gradually shapes the medial arches (American Academy of Orthopaedic Surgeons [AAOS], 2013). If flexible flatfoot continues into adolescence, a child may experience symptoms requiring treatment (AAOS, 2013; Dare, 2014). Other factors that can increase the risk for flatfoot include obesity, diabetes (e.g., Charcot foot), aging, and pregnancy (AAOS, 2013; Stolzman, 2015). Pain may be located in the medial, plantar, or lateral parts of the foot and extend into the lower extremities depending on etiology (Toullec, 2015). Most persons with flatfoot are asymptomatic and require no intervention. In general, treatment is indicated when a patient becomes symptomatic or withdraws from physical activity, but there is some debate regarding treatment necessity and best options. Treatment options typically begin with conservative medical management such as foot exercises, orthoses, and physical therapy. Acute painful flatfoot may require strict cast immobilization. Surgical options comprise arthrodesis, osteotomies, and numerous combinations of procedures to correct the underlying pathology (Toullec, 2014; Dare, 2014). Surgery is associated with operative morbidity and longer term sequelae, with eventual transfer of energy to non fused joints, nonunion, and growth plate disturbances. Subtalar arthroereisis: Subtalar arthroereisis (also called sinus tarsi implant or extra osseous talotarsal stabilization) involves extra articular placement of an implant that looks like a threaded cylinder within the sinus tarsi, with the goal of limiting excessive abnormal rotations of the tarsus. First described in congenital childhood flatfoot more than 60 years ago, subtalar arthroereisis is typically performed in conjunction with other tendon or bone procedures. As the least invasive surgical approach for flatfoot, it offers technical simplicity and rapid recovery but may not fully correct flatfoot. The main drawback is regular sinus tarsi pain, requiring removal of material while avoiding secondary correction loss (Toullec, 2015). 2
3 The U.S. Food and Drug Administration (FDA) does not regulate surgical procedures such as subtalar arthroereisis. However, FDA has cleared several implants used in the procedure for commercial use as Class II devices (FDA, 2016; product code HWC). Searches AmeriHealth Caritas Northeast searched PubMed and the databases of: UK National Health Services Centre for Reviews and Dissemination. Agency for Healthcare Research and Quality s National Guideline Clearinghouse and other evidence based practice centers. The Centers for Medicare & Medicaid Services (CMS). We conducted searches on August 1, Search terms were: "Subtalar Joint" (Mesh), "Joint Prosthesis"(Mesh), "Subtalar Joint/surgery"(Mesh), "Subtalar Joint/therapy" (Mesh), "Flatfoot" (Mesh) and free text terms arthroereisis and arthroerisis. We included: Systematic reviews, which pool results from multiple studies to achieve larger sample sizes and greater precision of effect estimation than in smaller primary studies. Systematic reviews use predetermined transparent methods to minimize bias, effectively treating the review as a scientific endeavor, and are thus rated highest in evidence grading hierarchies. Guidelines based on systematic reviews. Economic analyses, such as cost effectiveness, and benefit or utility studies (but not simple cost studies), reporting both costs and outcomes sometimes referred to as efficiency studies which also rank near the top of evidence hierarchies. Findings Two systematic reviews (Hayes, 2012 [updated 2015]; Metcalfe, 2011), one overview (National Institute for Health and Clinical Excellence [NICE], 2008) and three clinical practice guidelines (NICE, 2009; Lee, 2005; Harris, 2004) provided the basis for this policy. The reviews concur that evidence supporting the use of subtalar arthroereisis consists of small case series and case reports lacking long term follow up. The largest body of literature evaluated subtalar arthroereisis in pediatric populations. The most common indication for surgery was treatment of persistent, symptomatic flexible flatfoot, and most patients underwent concomitant procedures such as Achilles tendon lengthening. Studies varied in instrumentation, procedure, outcome measurement, and etiology, particularly between adult and pediatric patients. Two recent case series (116 total patients, 130 total feet) not included in the reviews confirm these findings (Ozan, 2015; Saxena, 2016). Subtalar arthroereisis for children has been used for decades and is a relatively simple intervention. The evidence suggests it has a lower risk of morbidity, shorter recovery time and favorable safety profile compared with other established surgical approaches. As no controlled studies have been published, it is not possible to determine definitive patient selection criteria or relative safety or efficacy of various implant devices. To note, other surgical options for symptomatic flatfoot, such as osteotomies and/or fusions, lack similar evidence from high quality comparative studies. 3
4 Evidence based guidelines reflect the uncertainty in the evidence base and the clinical controversy surrounding the utility of subtalar arthroereisis in pediatric and, to an even greater extent, adult populations. NICE (2009) recommended using the procedure in selected children with persistent mobile flatfoot due to neuromuscular disorder, skeletal dysplasia or systemic ligamentous laxity, whose treatment is supervised by a multidisciplinary team; the procedure may be indicated rarely in highly selected adult patients. Two guidelines by the American Orthopaedic Foot and Ankle Society (AOFAS) stated long term results of arthroereisis in adults have not been established, and the procedure in children remains controversial among surgeons (Lee, 2005; Harris, 2004). There appears to be greater acceptance of the procedure among podiatrists than orthopedists. Shah (2015) conducted a web based survey of AOFAS members for current practices regarding subtalar arthroereisis. A total of 572 respondents completed the survey (32 percent response rate), of whom 401 (70 percent) practice in the United States. Among U.S. practitioners, 40 percent have performed subtalar arthroereisis, and 60 percent of those still perform the procedure. Common indications were painful congenital flatfoot, posterior tibial tendon dysfunction, and flatfoot associated with accessory navicular. These results suggest while many doctors have performed subtalar arthroereisis, a significant number no longer do. Most doctors who still perform this procedure have removed the implants, commonly for pain. Policy updates: In 2017, we added an updated version of the Hayes systematic review (Hayes, 2012; updated 2016) with no change to its conclusions. No policy changes are warranted at this time. Summary of clinical evidence: Citation Hayes (2012, updated 2016) Subtalar arthroereisis for treatment of flatfoot Metcalfe (2011) Content, Methods, Recommendations Key points: Systematic review of six retrospective small case series of children and three case series of adults (10 37 patients per study). Update included one prospective comparison study, one prospective data analysis, two retrospective case series, one retrospective cohort study and one retrospective analysis. Most common indication = flexible flatfoot. Subtalar arthroereisis consistently improved pain, functionality, and radiographic findings not responsive to conservative treatment; effects observed for up to 12 years following the procedure. Favorable safety profile; pain and sinus tarsi tenderness being the most frequent complications. Infrequent explantation due to pain, implant dislocations or fractures. Large-scale randomized clinical trials (RCTs) comparing long-term outcomes of subtalar arthroereisis with other surgical interventions are needed to establish the efficacy and safety. Key points: 4
5 Citation Subtalar arthroereisis for pediatric flexible flatfoot NICE (2008) Content, Methods, Recommendations Systematic review of 76 case series and case reports. Eight of nine radiographic parameters showed significant improvement in increased static arch height and joint congruency; calcaneal inclination angle demonstrated the least change. Complications including sinus tarsi pain, device extrusion and under-correction: overall complication rates (4.8% to 18.6%); unplanned removal rate (7.1% to 19.3%) across all device types. Interplay between osseous alignment and dynamic stability within the foot may contribute to procedure effectiveness. Qualitative outcome data based on disease specific, validated outcome tools may improve current evidence and permit comparison of future study data. Key points: Sinus tarsi implant insertion for mobile flatfoot Review of eight case series and four case reports (643 total feet), including only one case series of 23 adults (28 implants). Overall quality: Low. Variation in procedures (design, size, instrumentation/insertion), unclear patient selection criteria. Many patients also had concomitant Achilles tendon procedures or other procedures. Can be done bilaterally or unilaterally but no evidence available about efficacy of bilateral implantation or laterality (left/right implantation). Indications for this procedure are different in pediatric and adult populations. Lack of consensus from experts on efficacy and safety, clinical utility, need for additional research, and the use of biodegradable and non-biodegradable implants. References Professional society guidelines/other: Harris EJ, Vanore JV, Thomas JL, et al. Diagnosis and treatment of pediatric flatfoot. J Foot Ankle Surg. 2004; 43(6): Lee MS, Vanore JV, Thomas JL, et al. Diagnosis and treatment of adult flatfoot. J Foot Ankle Surg. 2005; 44(2): OrthoInfo. Adult Acquired Flatfoot. Last reviewed December AAOS website. OrthoInfo. Flexible Flatfoot in Children. Last reviewed March AAOS website. Sinus tarsi implant insertion for mobile flatfoot. NICE interventional procedure guidance [IPG305]. Published date: July NICE website. Accessed August 10,
6 Peer reviewed references: Dare DM, Dodwell ER. Pediatric flatfoot: cause, epidemiology, assessment, and treatment. Curr Opin Pediatr. 2014; 26(1): FDA 510(k) Premarket Notification database searched using product code HWC. FDA website. Hayes Inc., Hayes Medical Technology Report. Subtalar Arthroereisis. Lansdale, Pa. Hayes Inc.; 2012 [Updated September 15, 2016]. Interventional procedure overview of sinus tarsi implant insertion for mobile flatfoot [IP 101]. National Institute for Health and Clinical Excellence (NICE) Interventional Procedures Programme. January NICE website Accessed August 10, Metcalfe SA, Bowling FL, Reeves ND. Subtalar joint arthroereisis in the management of pediatric flexible flatfoot: a critical review of the literature. Foot Ankle Int. 2011; 32(12): Ozan F, Dogar F, Gencer K, et al. Symptomatic flexible flatfoot in adults: subtalar arthroereisis. Ther Clin Risk Manag. 2015; 11: Saxena A, Via AG, Maffulli N, Chiu H. Subtalar arthroereisis implant removal in adults: a prospective study of 100 patients. J Foot Ankle Surg. 2016; 55(3): Shah NS, Needleman RL, Bokhari O, Buzas D Subtalar Arthroereisis Survey: the current practice patterns of members of the AOFAS. Foot Ankle Spec. 2015; 8(3): Stolzman S, Irby MB, Callahan AB, Skelton JA. Pes planus and paediatric obesity: a systematic review of the literature. Clin Obes. 2015; 5(2): Toullec E. Adult flatfoot. Orthop Traumatol Surg Res. 2015; 101(1 Suppl): S CMS National Coverage Determinations (NCDs): No NCDs identified as of the writing of this policy. Local Coverage Determinations (LCDs): L33392 Category III CPT Codes. CMS website. coveragedatabase/details/lcd details.aspx?lcdid=33392&ver=64. 6
7 L36219 Non Covered Services. CMS website. coveragedatabase/details/lcd details.aspx?lcdid=36219&ver=50. L34555 Non Covered Category III CPT Codes. CMS website. coveragedatabase/details/lcd details.aspx?lcdid=34555&ver=44. L35008 Non Covered Services. CMS website. coveragedatabase/details/lcd details.aspx?lcdid=35008&ver=63. L33777 Noncovered Services. CMS website. coveragedatabase/details/lcd details.aspx?lcdid=33777&ver=44. L35094 Services That Are Not Reasonable and Necessary. CMS website. database/details/lcd details.aspx?lcdid=35094&ver=136. Commonly submitted codes Below are the most commonly submitted codes for the service(s)/item(s) subject to this policy. This is not an exhaustive list of codes. Providers are expected to consult the appropriate coding manuals and bill accordingly. CPT Code Description Comments Unlisted procedure, foot or toes 0335T Extra-osseous subtalar joint implant for talotarsal stabilization ICD-10 Code Description Comments M21.40-M21.42 Flat foot [pes planus] (acquired) Q66.50-Q66.52 Congenital pes planus HCPCS Level II Code S2117 Description Arthroereisis, subtalar Comments 7
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: subtalar_arthroereisis 6/2009 2/2018 2/2019 2/2018 Description of Procedure or Service Arthroereisis (also
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: subtalar_arthroereisis 6/2009 2/2018 2/2019 2/2018 Description of Procedure or Service Arthroereisis (also
Clinical Policy Title: Computerized gait analysis
 Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: June 22, 2017 Next Review Date:
Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: June 22, 2017 Next Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Topic: Subtalar Arthroereisis Date of Origin: January 3, Section: Surgery Last Reviewed Date: December 2013
 Medical Policy Manual Topic: Subtalar Arthroereisis Date of Origin: January 3, 2006 Section: Surgery Last Reviewed Date: December 2013 Policy No: 144 Effective Date: January 1, 2014 IMPORTANT REMINDER
Medical Policy Manual Topic: Subtalar Arthroereisis Date of Origin: January 3, 2006 Section: Surgery Last Reviewed Date: December 2013 Policy No: 144 Effective Date: January 1, 2014 IMPORTANT REMINDER
Clinical Policy Title: Computerized gait analysis
 Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Strep testing
 Clinical Policy Title: Strep testing Clinical Policy Number: 07.01.09 Effective Date: December 1, 2017 Initial Review Date: October 19, 2017 Most Recent Review Date: November 16, 2017 Next Review Date:
Clinical Policy Title: Strep testing Clinical Policy Number: 07.01.09 Effective Date: December 1, 2017 Initial Review Date: October 19, 2017 Most Recent Review Date: November 16, 2017 Next Review Date:
Clinical Policy Title: Cardiac rehabilitation
 Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Clinical Policy Title: Bone growth stimulators for non-healing fractures
 Clinical Policy Title: Bone growth stimulators for non-healing fractures Clinical Policy Number: 14.02.03 Effective Date: January 1, 2015 Initial Review Date: July 16, 2014 Most Recent Review Date: March
Clinical Policy Title: Bone growth stimulators for non-healing fractures Clinical Policy Number: 14.02.03 Effective Date: January 1, 2015 Initial Review Date: July 16, 2014 Most Recent Review Date: March
Clinical Policy Title: Genicular nerve block
 Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering)
 Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering) Clinical Policy Number: 17.02.02 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent
Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering) Clinical Policy Number: 17.02.02 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent
SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT
 C H A P T E R 1 7 SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT William D. Fishco, DPM, MS INTRODUCTION Arthroereisis is a surgical procedure designed to limit the motion of a joint. Subtalar joint arthroereisis
C H A P T E R 1 7 SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT William D. Fishco, DPM, MS INTRODUCTION Arthroereisis is a surgical procedure designed to limit the motion of a joint. Subtalar joint arthroereisis
Clinical Policy Title: Zoster (shingles) vaccine
 Clinical Policy Title: Zoster (shingles) vaccine Clinical Policy Number: 18.02.10 Effective Date: June 1, 2018 Initial Review Date: April 10, 2018 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Zoster (shingles) vaccine Clinical Policy Number: 18.02.10 Effective Date: June 1, 2018 Initial Review Date: April 10, 2018 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next
Clinical Policy Title: Breast cancer index genetic testing
 Clinical Policy Title: Breast cancer index genetic testing Clinical Policy Number: 02.01.22 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2016
Clinical Policy Title: Breast cancer index genetic testing Clinical Policy Number: 02.01.22 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2016
Disclosure Subtalar Arthroereisis Survey: The Current Practice Patterns of Members of the AOFAS. Presenter: Neil S. Shah, M.D.
 Disclosure 2013 Subtalar Arthroereisis Survey: The Current Practice Patterns of Members of the AOFAS Presenter: Neil S. Shah, M.D. My disclosure is in the Final AOFAS Mobile App I have no potential conflicts
Disclosure 2013 Subtalar Arthroereisis Survey: The Current Practice Patterns of Members of the AOFAS Presenter: Neil S. Shah, M.D. My disclosure is in the Final AOFAS Mobile App I have no potential conflicts
Subtalar Arthroereisis
 Subtalar Arthroereisis Policy Number: 7.01.104 Last Review: 5/2014 Origination: 5/2008 Next Review: 5/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for subtalar
Subtalar Arthroereisis Policy Number: 7.01.104 Last Review: 5/2014 Origination: 5/2008 Next Review: 5/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for subtalar
Clinical Policy Title: Ketamine for treatment-resistant depression
 Clinical Policy Title: Ketamine for treatment-resistant depression Clinical Policy Number: 00.02.13 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: January
Clinical Policy Title: Ketamine for treatment-resistant depression Clinical Policy Number: 00.02.13 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: January
Clinical Policy Title: Tactile breast imaging
 Clinical Policy Title: Tactile breast imaging Clinical Policy Number: 05.01.07 Effective Date: February 1, 2018 Initial Review Date: November 16, 2017 Most Recent Review Date: January 11, 2018 Next Review
Clinical Policy Title: Tactile breast imaging Clinical Policy Number: 05.01.07 Effective Date: February 1, 2018 Initial Review Date: November 16, 2017 Most Recent Review Date: January 11, 2018 Next Review
Clinical Policy Title: Platelet rich plasma
 Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review
Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening
 Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab
 Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab Clinical Policy Number: 01.01.03 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent
Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab Clinical Policy Number: 01.01.03 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Hammer toe surgery
 Clinical Policy Title: Hammer toe surgery Clinical Policy Number: 18.03.04 Effective Date: July 1, 2016 Initial Review Date: July 15, 2016 Most Recent Review Date: June 22, 2017 Next Review Date: June
Clinical Policy Title: Hammer toe surgery Clinical Policy Number: 18.03.04 Effective Date: July 1, 2016 Initial Review Date: July 15, 2016 Most Recent Review Date: June 22, 2017 Next Review Date: June
Clinical Policy Title: Hammer toe surgery
 Clinical Policy Title: Hammer toe surgery Clinical Policy Number: 18.03.04 Effective Date: July 1, 2016 Initial Review Date: July 15, 2016 Most Recent Review Date: May 1, 2018 Next Review Date: May 2019
Clinical Policy Title: Hammer toe surgery Clinical Policy Number: 18.03.04 Effective Date: July 1, 2016 Initial Review Date: July 15, 2016 Most Recent Review Date: May 1, 2018 Next Review Date: May 2019
Clinical Policy Title: Ear tubes (tympanostomy)
 Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 11.03.05 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 11.03.05 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next
A Patient s Guide to Adult-Acquired Flatfoot Deformity
 A Patient s Guide to Adult-Acquired Flatfoot Deformity Glendale Adventist Medical Center 1509 Wilson Terrace Glendale, CA 91206 Phone: (818) 409-8000 DISCLAIMER: The information in this booklet is compiled
A Patient s Guide to Adult-Acquired Flatfoot Deformity Glendale Adventist Medical Center 1509 Wilson Terrace Glendale, CA 91206 Phone: (818) 409-8000 DISCLAIMER: The information in this booklet is compiled
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Subtalar Arthroereisis
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Clinical Policy Title: Platelet rich plasma
 Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2017 Next Review
Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2017 Next Review
Clinical Policy Title: Arthroscopic anterior cruciate ligament surgery skeletally immature
 Clinical Policy Title: Arthroscopic anterior cruciate ligament surgery skeletally immature Clinical Policy Number: 14.03.08 Effective Date: May 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review
Clinical Policy Title: Arthroscopic anterior cruciate ligament surgery skeletally immature Clinical Policy Number: 14.03.08 Effective Date: May 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Subtalar Arthroereisis Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Subtalar Arthroereisis Professional Institutional Original Effective Date: September
Subtalar Arthroereisis Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Subtalar Arthroereisis Professional Institutional Original Effective Date: September
Clinical Policy Title: Discography
 Clinical Policy Title: Discography Clinical Policy Number: 03.01.01 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2017 Next Review Date: October
Clinical Policy Title: Discography Clinical Policy Number: 03.01.01 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2017 Next Review Date: October
Subtalar Arthroereisis
 Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0486 Subtalar Arthroereisis Table of Contents Coverage Policy... 1 Overview... 1 General Background...
Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0486 Subtalar Arthroereisis Table of Contents Coverage Policy... 1 Overview... 1 General Background...
EVALUATION OF PATIENTS RECEIVING HYPROCURE EXTRAOSSEOUS TALOTARSAL STABILIZATION DEVICE
 C H A P T E R 1 9 EVALUATION OF PATIENTS RECEIVING HYPROCURE EXTRAOSSEOUS TALOTARSAL STABILIZATION DEVICE Jennifer N. Falk, DPM Gregory D. Clark, DPM INTRODUCTION Flatfoot deformity is one of the most
C H A P T E R 1 9 EVALUATION OF PATIENTS RECEIVING HYPROCURE EXTRAOSSEOUS TALOTARSAL STABILIZATION DEVICE Jennifer N. Falk, DPM Gregory D. Clark, DPM INTRODUCTION Flatfoot deformity is one of the most
A Patient s Guide to Flatfoot Deformity (Pes Planus) in Children
 A Patient s Guide to Flatfoot Deformity (Pes Planus) in Children 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled
A Patient s Guide to Flatfoot Deformity (Pes Planus) in Children 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled
Clinical Policy Title: Frenectomy for ankyloglossia
 Clinical Policy Title: Frenectomy for ankyloglossia Clinical Policy Number: 11.03.03 Effective Date: October 1, 2014 Initial Review Date: April 16, 2014 Most Recent Review Date: May 18, 2016 Next Review
Clinical Policy Title: Frenectomy for ankyloglossia Clinical Policy Number: 11.03.03 Effective Date: October 1, 2014 Initial Review Date: April 16, 2014 Most Recent Review Date: May 18, 2016 Next Review
.org. Posterior Tibial Tendon Dysfunction. Anatomy. Cause. Symptoms
 Posterior Tibial Tendon Dysfunction Page ( 1 ) Posterior tibial tendon dysfunction is one of the most common problems of the foot and ankle. It occurs when the posterior tibial tendon becomes inflamed
Posterior Tibial Tendon Dysfunction Page ( 1 ) Posterior tibial tendon dysfunction is one of the most common problems of the foot and ankle. It occurs when the posterior tibial tendon becomes inflamed
Clinical Policy Title: Arthroscopic anterior cruciate ligament surgery skeletally immature
 Clinical Policy Title: Arthroscopic anterior cruciate ligament surgery skeletally immature Clinical Policy Number: 14.03.08 Effective Date: May 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review
Clinical Policy Title: Arthroscopic anterior cruciate ligament surgery skeletally immature Clinical Policy Number: 14.03.08 Effective Date: May 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review
Foot and Ankle Systems Coding Reference Guide
 Foot and Ankle Systems Coding Reference Guide Physician Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis;
Foot and Ankle Systems Coding Reference Guide Physician Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis;
Clinical Policy Title: Interspinous dynamic stabilization devices
 Clinical Policy Title: Interspinous dynamic stabilization devices Clinical Policy Number: 14.02.07 Effective Date: April 1, 2016 Initial Review Date: November 18, 2015 Most Recent Review Date: January
Clinical Policy Title: Interspinous dynamic stabilization devices Clinical Policy Number: 14.02.07 Effective Date: April 1, 2016 Initial Review Date: November 18, 2015 Most Recent Review Date: January
Clinical Policy Title: Uvulopalatopharyngoplasty
 Clinical Policy Title: Uvulopalatopharyngoplasty Clinical Policy Number: 10.03.05 Effective Date: October 1, 2015 Initial Review Date: June 17, 2015 Most Recent Review Date: July 20, 2017 Next Review Date:
Clinical Policy Title: Uvulopalatopharyngoplasty Clinical Policy Number: 10.03.05 Effective Date: October 1, 2015 Initial Review Date: June 17, 2015 Most Recent Review Date: July 20, 2017 Next Review Date:
Clinical Policy: Growing Rods Spinal Surgery Reference Number: CP.MP.354
 Clinical Policy: Growing Rods Spinal Surgery Reference Number: CP.MP.354 Effective Date: 06/07 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy
Clinical Policy: Growing Rods Spinal Surgery Reference Number: CP.MP.354 Effective Date: 06/07 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy
Clinical Policy Title: Room humidifiers
 Clinical Policy Title: Room humidifiers Clinical Policy Number: 17.02.05 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review Date:
Clinical Policy Title: Room humidifiers Clinical Policy Number: 17.02.05 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review Date:
«Foot & Ankle Surgery» 04. Sept THE PAINFUL FLATFOOT. Norman Espinosa, MD
 THE PAINFUL FLATFOOT Norman Espinosa, MD Department of Orthopaedics University of Zurich Balgrist Switzerland www.balgrist.ch WHAT TO DO? INTRINSIC > EXTRINSIC ETIOLOGIES Repetitive microtrauma combined
THE PAINFUL FLATFOOT Norman Espinosa, MD Department of Orthopaedics University of Zurich Balgrist Switzerland www.balgrist.ch WHAT TO DO? INTRINSIC > EXTRINSIC ETIOLOGIES Repetitive microtrauma combined
Clinical Policy Title: Spinal cord stimulators for chronic pain
 Clinical Policy Title: Spinal cord stimulators for chronic pain Clinical Policy Number: 03.03.01 Effective Date: October 1, 2014 Initial Review Date: March 19, 2014 Most Recent Review Date: April 19, 2017
Clinical Policy Title: Spinal cord stimulators for chronic pain Clinical Policy Number: 03.03.01 Effective Date: October 1, 2014 Initial Review Date: March 19, 2014 Most Recent Review Date: April 19, 2017
Clinical Policy Title: Bunionectomy (hallux valgus surgery)
 Clinical Policy Title: Bunionectomy (hallux valgus surgery) Clinical Policy Number: 14.03.10 Effective Date: July 1, 2017 Initial Review Date: May 19, 2017 Most Recent Review Date: June 22, 2017 Next Review
Clinical Policy Title: Bunionectomy (hallux valgus surgery) Clinical Policy Number: 14.03.10 Effective Date: July 1, 2017 Initial Review Date: May 19, 2017 Most Recent Review Date: June 22, 2017 Next Review
Clinical Policy Title: Propel (drug eluting devices after sinus surgery)
 Clinical Policy Title: Propel (drug eluting devices after sinus surgery) Clinical Policy Number: 10.03.07 Effective Date: July 1, 2017 Initial Review Date: May 19, 2017 Most Recent Review Date: June 22,
Clinical Policy Title: Propel (drug eluting devices after sinus surgery) Clinical Policy Number: 10.03.07 Effective Date: July 1, 2017 Initial Review Date: May 19, 2017 Most Recent Review Date: June 22,
Clinical Policy Title: Pediatric rhinoplasty
 Clinical Policy Title: Pediatric rhinoplasty Clinical Policy Number: 11.03.06 Effective Date: October 1, 2017 Initial Review Date: August 17, 2017 Most Recent Review Date: September 21, 2017 Next Review
Clinical Policy Title: Pediatric rhinoplasty Clinical Policy Number: 11.03.06 Effective Date: October 1, 2017 Initial Review Date: August 17, 2017 Most Recent Review Date: September 21, 2017 Next Review
Clinical Policy Title: Epidermal nerve fiber density testing
 Clinical Policy Title: Epidermal nerve fiber density testing Clinical Policy Number: 09.01.12 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19,
Clinical Policy Title: Epidermal nerve fiber density testing Clinical Policy Number: 09.01.12 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19,
Calcaneus (Heel Bone) Fractures
 Page 1 of 8 Calcaneus (Heel Bone) Fractures A fracture of the calcaneus, or heel bone, can be a painful and disabling injury. This type of fracture commonly occurs during a high-energy event such as a
Page 1 of 8 Calcaneus (Heel Bone) Fractures A fracture of the calcaneus, or heel bone, can be a painful and disabling injury. This type of fracture commonly occurs during a high-energy event such as a
Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT)
 Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT) Clinical Policy Number: 09.01.02 Effective Date: September 1, 2013 Initial Review Date: February 18, 2013 Most
Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT) Clinical Policy Number: 09.01.02 Effective Date: September 1, 2013 Initial Review Date: February 18, 2013 Most
Managing Tibialis Posterior Tendon Injuries
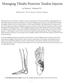 Managing Tibialis Posterior Tendon Injuries by Thomas C. Michaud, DC Published April 1, 2015 by Dynamic Chiropractic Magazine Tibialis posterior is the deepest, strongest, and most central muscle of the
Managing Tibialis Posterior Tendon Injuries by Thomas C. Michaud, DC Published April 1, 2015 by Dynamic Chiropractic Magazine Tibialis posterior is the deepest, strongest, and most central muscle of the
Clinical Policy Title: Autologous chondrocyte implantation
 Clinical Policy Title: Autologous chondrocyte implantation Clinical Policy Number: 14.03.07 Effective Date: March 1, 2017 Initial Review Date: February 15, 2017 Most Recent Review Date: February 6, 2018
Clinical Policy Title: Autologous chondrocyte implantation Clinical Policy Number: 14.03.07 Effective Date: March 1, 2017 Initial Review Date: February 15, 2017 Most Recent Review Date: February 6, 2018
Sympathetic Electrical Stimulation Therapy for Chronic Pain
 Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening
 Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Pharmocogenetic testing for warfarin (Coumadin ) sensitivity
 Clinical Policy Title: Pharmocogenetic testing for warfarin (Coumadin ) sensitivity Clinical Policy Number: 02.01.13 Effective Date: September 1, 2013 Initial Review Date: May 15, 2013 Most Recent Review
Clinical Policy Title: Pharmocogenetic testing for warfarin (Coumadin ) sensitivity Clinical Policy Number: 02.01.13 Effective Date: September 1, 2013 Initial Review Date: May 15, 2013 Most Recent Review
Clinical Policy Title: Noninvasive tests for rejection surveillance after heart transplantation
 Clinical Policy Title: Noninvasive tests for rejection surveillance after heart transplantation Clinical Policy Number: 04.01.04 Policy contains: Effective Date: January 1, 2016 Initial Review Date September
Clinical Policy Title: Noninvasive tests for rejection surveillance after heart transplantation Clinical Policy Number: 04.01.04 Policy contains: Effective Date: January 1, 2016 Initial Review Date September
Clinical Policy Title: Cryoneurolysis
 Clinical Policy Title: Cryoneurolysis Clinical Policy Number: 09.02.08 Effective Date: May 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review Date: April 19, 2017 Next Review Date: April 2018
Clinical Policy Title: Cryoneurolysis Clinical Policy Number: 09.02.08 Effective Date: May 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review Date: April 19, 2017 Next Review Date: April 2018
MP.090.MH Nerve Block, Paravertebral, Facet Joint, and SI Injections
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.090.MH Nerve Block, Paravertebral, Facet Joint, and SI This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.090.MH Nerve Block, Paravertebral, Facet Joint, and SI This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP
Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS)
 09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Clinical Policy Title: Epidermal nerve fiber density testing
 Clinical Policy Title: Epidermal nerve fiber density testing Clinical Policy Number: CCP.1263 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 2, 2018
Clinical Policy Title: Epidermal nerve fiber density testing Clinical Policy Number: CCP.1263 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 2, 2018
Clinical Policy Title: Tumor treatment fields for glioblastoma
 Clinical Policy Title: Tumor treatment fields for glioblastoma Clinical Policy Number: 05.02.05 Effective Date: July 1, 2015 Initial Review Date: March 18, 2015 Most Recent Review Date: April 19, 2017
Clinical Policy Title: Tumor treatment fields for glioblastoma Clinical Policy Number: 05.02.05 Effective Date: July 1, 2015 Initial Review Date: March 18, 2015 Most Recent Review Date: April 19, 2017
Clinical Policy Title: Outpatient diabetes self-management training (DSMT)
 Clinical Policy Title: Outpatient diabetes self-management training (DSMT) Clinical Policy Number: 06.02.02 Effective Date: July 1, 2013 Initial Review Date: April 23, 2013 Most Recent Review Date: March
Clinical Policy Title: Outpatient diabetes self-management training (DSMT) Clinical Policy Number: 06.02.02 Effective Date: July 1, 2013 Initial Review Date: April 23, 2013 Most Recent Review Date: March
Financial Disclosure. Turf Toe
 Seth O Brien, CP, LP Financial Disclosure Mr. Seth O'Brien has no relevant financial relationships with commercial interests to disclose. Turf Toe Common in athletes playing on firm, artificial turf Forceful
Seth O Brien, CP, LP Financial Disclosure Mr. Seth O'Brien has no relevant financial relationships with commercial interests to disclose. Turf Toe Common in athletes playing on firm, artificial turf Forceful
Clinical Policy: Radial Head Implant Reference Number: CP.MP.148
 Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
What Happens to the Paediatric Flat Foot? Peter J Briggs Freeman Hospital Newcastle upon Tyne
 What Happens to the Paediatric Flat Foot? Peter J Briggs Freeman Hospital Newcastle upon Tyne We don t know!! Population Studies 2300 children aged 4-13 years Shoe wearers Flat foot 8.6% Non-shoe wearers
What Happens to the Paediatric Flat Foot? Peter J Briggs Freeman Hospital Newcastle upon Tyne We don t know!! Population Studies 2300 children aged 4-13 years Shoe wearers Flat foot 8.6% Non-shoe wearers
CHRONIC FOOT PROBLEMS FOOT and ANKLE BASICS
 CHRONIC FOOT PROBLEMS FOOT and ANKLE BASICS ABC s of Comprehensive Musculoskeletal Care December 1 st, 2007 Stephen Pinney MD Chief, UCSF Foot and Ankle Service Chronic problems typically occur gradually
CHRONIC FOOT PROBLEMS FOOT and ANKLE BASICS ABC s of Comprehensive Musculoskeletal Care December 1 st, 2007 Stephen Pinney MD Chief, UCSF Foot and Ankle Service Chronic problems typically occur gradually
Case 1 7 yo male Right elbow injury 3 months ago Medial elbow pain and tenderness over medial epicondyle Long arm cast given but off himself 1 month a
 Case presentations Case 1 7 yo male Right elbow injury 3 months ago Medial elbow pain and tenderness over medial epicondyle Long arm cast given but off himself 1 month after Progressive limited elbow flexion
Case presentations Case 1 7 yo male Right elbow injury 3 months ago Medial elbow pain and tenderness over medial epicondyle Long arm cast given but off himself 1 month after Progressive limited elbow flexion
10/26/2017. Comprehensive & Coordinated Orthopaedic Management of Children with CP. Objectives. It s all about function. Robert Bruce, MD Sayan De, MD
 Comprehensive & Coordinated Orthopaedic Management of Children with CP Robert Bruce, MD Sayan De, MD Objectives Understand varying levels of intervention are available to optimize function of children
Comprehensive & Coordinated Orthopaedic Management of Children with CP Robert Bruce, MD Sayan De, MD Objectives Understand varying levels of intervention are available to optimize function of children
5 COMMON CONDITIONS IN THE FOOT & ANKLE
 5 COMMON CONDITIONS IN THE FOOT & ANKLE MICHAEL P. CLARE, MD FLORIDA ORTHOPAEDIC INSTITUTE TAMPA, FL USA IN A NUTSHELL ~ ALL ANATOMY & BIOMECHANICS >90% OF CONDITIONS IN FOOT & ANKLE DIAGNISED FROM GOOD
5 COMMON CONDITIONS IN THE FOOT & ANKLE MICHAEL P. CLARE, MD FLORIDA ORTHOPAEDIC INSTITUTE TAMPA, FL USA IN A NUTSHELL ~ ALL ANATOMY & BIOMECHANICS >90% OF CONDITIONS IN FOOT & ANKLE DIAGNISED FROM GOOD
Results of Calcaneal Osteotomy & Flexor Digitorum Longus transfer in Stage II Acquired Flatfoot Deformity
 Results of Calcaneal Osteotomy & Flexor Digitorum Longus transfer in Stage II Acquired Flatfoot Deformity Mr Amit Chauhan Mr Prasad Karpe Ms Maire-claire Killen Mr Rajiv Limaye University Hospital of North
Results of Calcaneal Osteotomy & Flexor Digitorum Longus transfer in Stage II Acquired Flatfoot Deformity Mr Amit Chauhan Mr Prasad Karpe Ms Maire-claire Killen Mr Rajiv Limaye University Hospital of North
Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT)
 Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT) Clinical Policy Number: 09.01.02 Effective Date: September 1, 2013 Initial Review Date: February 18, 2013 Most
Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT) Clinical Policy Number: 09.01.02 Effective Date: September 1, 2013 Initial Review Date: February 18, 2013 Most
Premier Health Plan considers Iontophoresis for Musculoskeletal Conditions medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL MP.036.PH - Iontophoresis for Musculoskeletal Conditions This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Premier Health Plan POLICY AND PROCEDURE MANUAL MP.036.PH - Iontophoresis for Musculoskeletal Conditions This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Clinical Policy Title: Bloodless heart transplant
 Clinical Policy Title: Bloodless heart transplant Clinical Policy Number: 05.03.05 Effective Date: July 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next Review Date:
Clinical Policy Title: Bloodless heart transplant Clinical Policy Number: 05.03.05 Effective Date: July 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next Review Date:
Clinical Policy Title: Home phototherapy for hyperbilirubinemia
 Clinical Policy Title: Home phototherapy for hyperbilirubinemia Clinical Policy Number: 11.02.04 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17,
Clinical Policy Title: Home phototherapy for hyperbilirubinemia Clinical Policy Number: 11.02.04 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17,
Clinical Policy Title: Seasonal influenza testing
 Clinical Policy Title: Seasonal influenza testing Clinical Policy Number: 07.01.08 Effective Date: October 1, 2017 Initial Review Date: August 17, 2017 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Seasonal influenza testing Clinical Policy Number: 07.01.08 Effective Date: October 1, 2017 Initial Review Date: August 17, 2017 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Genetic tests for Duchenne muscular dystrophy
 Clinical Policy Title: Genetic tests for Duchenne muscular dystrophy Clinical Policy Number: 02.01.23 Effective Date: February 1, 2017 Initial Review Date: January 18, 2017 Most Recent Review Date: January
Clinical Policy Title: Genetic tests for Duchenne muscular dystrophy Clinical Policy Number: 02.01.23 Effective Date: February 1, 2017 Initial Review Date: January 18, 2017 Most Recent Review Date: January
Clinical Policy Title: Inhaled nitric oxide
 Clinical Policy Title: Inhaled nitric oxide Clinical Policy Number: 11.02.02 Effective Date: June 1 2014 Initial Review Date: February 19, 2014 Most Recent Review Date: May 17, 2017 Next Review Date: March
Clinical Policy Title: Inhaled nitric oxide Clinical Policy Number: 11.02.02 Effective Date: June 1 2014 Initial Review Date: February 19, 2014 Most Recent Review Date: May 17, 2017 Next Review Date: March
Clinical Policy Title: Ear tubes (tympanostomy)
 Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 1135 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: August 1, 2018 Next Review
Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 1135 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: August 1, 2018 Next Review
Horizon Subtalar. Surgical Technique
 Horizon Subtalar Surgical Technique Contents Product The BioPro Horizon Subtalar Implant is used for the treatment of flatfoot and posterior tibial tendon dysfunction in both children and adults. Implanted
Horizon Subtalar Surgical Technique Contents Product The BioPro Horizon Subtalar Implant is used for the treatment of flatfoot and posterior tibial tendon dysfunction in both children and adults. Implanted
2017 SAFSA CONGRESS PROGRAMME
 2017 SAFSA CONGRESS PROGRAMME THURSDAY, MAY 25 07h45 07h55: WELCOME & INTRODUCTIONS Forefoot I: Hallux Valgus and Lesser Toes (08h00-10h00 Lectures) 08h00 08h30: Surgical Management of Hallux Valgus Rippstein,
2017 SAFSA CONGRESS PROGRAMME THURSDAY, MAY 25 07h45 07h55: WELCOME & INTRODUCTIONS Forefoot I: Hallux Valgus and Lesser Toes (08h00-10h00 Lectures) 08h00 08h30: Surgical Management of Hallux Valgus Rippstein,
Cavus Foot: Subtle and Not-So-Subtle AOFAS Resident Review Course September 28, 2013
 Cavus Foot: Subtle and Not-So-Subtle Course September 28, 2013 Matthew M. Roberts, MD Associate Professor of Clinical Orthopaedic Surgery Co-Chief, Foot and Ankle Service Hospital for Special Surgery Disclosure
Cavus Foot: Subtle and Not-So-Subtle Course September 28, 2013 Matthew M. Roberts, MD Associate Professor of Clinical Orthopaedic Surgery Co-Chief, Foot and Ankle Service Hospital for Special Surgery Disclosure
Clinical Policy Title: Pharmacogenomic tests for psychiatric medications
 Clinical Policy Title: Pharmacogenomic tests for psychiatric medications Clinical Policy Number: 02.02.01 Effective Date: October 1, 2015 Initial Review Date: April 15, 2015 Most Recent Review Date: May
Clinical Policy Title: Pharmacogenomic tests for psychiatric medications Clinical Policy Number: 02.02.01 Effective Date: October 1, 2015 Initial Review Date: April 15, 2015 Most Recent Review Date: May
Other Congenital and Developmental Diseases of the Foot. Department of Orthopedic Surgery St. Vincent s s Hospital, The Catholic University
 Other Congenital and Developmental Diseases of the Foot Department of Orthopedic Surgery St. Vincent s s Hospital, The Catholic University Contents Metatarsus Adductus Skewfoot Hallux Valgus Hallux Valgus
Other Congenital and Developmental Diseases of the Foot Department of Orthopedic Surgery St. Vincent s s Hospital, The Catholic University Contents Metatarsus Adductus Skewfoot Hallux Valgus Hallux Valgus
Subject: Ultrasound Osteogenesis Stimulators, Non Invasive
 Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Clinical Policy Title: Corneal implants
 Clinical Policy Title: Corneal implants Clinical Policy Number: 10.03.06 Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next Review Date:
Clinical Policy Title: Corneal implants Clinical Policy Number: 10.03.06 Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next Review Date:
Medincenter GlavUpDK by the Ministry of Foreign Affairs of Russia, Moscow.
 Medincenter GlavUpDK by the Ministry of Foreign Affairs of Russia, Moscow. Berezhnoy Sergey. Percutaneous First Metatarsocuneiform Joint Arthrodesis in a Treatment of Metatarsus Primus Varus: a Prospective
Medincenter GlavUpDK by the Ministry of Foreign Affairs of Russia, Moscow. Berezhnoy Sergey. Percutaneous First Metatarsocuneiform Joint Arthrodesis in a Treatment of Metatarsus Primus Varus: a Prospective
) Flexible Flatfoot Prevalence Correlating Factor Foot Pressure Physical Performance and Gait Analysis (Year III) NSC B
 ( ) Flexible FlatfootPrevalenceCorrelating FactorFoot Pressure Physical Performance and Gait Analysis (Year III) NSC91-2314-B-006-062 89 08 01 92 07 31 or severe FF noted among 377 preschool children after
( ) Flexible FlatfootPrevalenceCorrelating FactorFoot Pressure Physical Performance and Gait Analysis (Year III) NSC91-2314-B-006-062 89 08 01 92 07 31 or severe FF noted among 377 preschool children after
Ankle Pain After a Sprain.
 Ankle Pain After a Sprain www.fisiokinesiterapia.biz Anterior Drawer Stress Test Talar Tilt Talar Tilt (CFL) Difficult to isolate from subtalar ROM Slight plantar flexion (dorsi = relative subtalar isolation)
Ankle Pain After a Sprain www.fisiokinesiterapia.biz Anterior Drawer Stress Test Talar Tilt Talar Tilt (CFL) Difficult to isolate from subtalar ROM Slight plantar flexion (dorsi = relative subtalar isolation)
Clinical Policy Title: Renal denervation
 Clinical Policy Title: Renal denervation Clinical Policy Number: 09.03.04 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: January 11, 2018 Next Review Date:
Clinical Policy Title: Renal denervation Clinical Policy Number: 09.03.04 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: January 11, 2018 Next Review Date:
*Rippstein, Trnka, Saragas, Hoffman
 THURS 25th MAY 07:00 07:10 Welcome and Introductions Paulo Ferrao Lecture 1: 07:10 09:45 Forefoot I: Hallux Valgus and Lesser Toes Mark Easley 40 mins 07:10 07:50 Surgical Management of Hallux Valgus 30
THURS 25th MAY 07:00 07:10 Welcome and Introductions Paulo Ferrao Lecture 1: 07:10 09:45 Forefoot I: Hallux Valgus and Lesser Toes Mark Easley 40 mins 07:10 07:50 Surgical Management of Hallux Valgus 30
Clinical Policy Title: Propel (drug eluting devices after sinus surgery)
 Clinical Policy Title: Propel (drug eluting devices after sinus surgery) Clinical Policy Number: 10.03.07 Effective Date: July 1, 2017 Initial Review Date: May 19, 2017 Most Recent Review Date: May 1,
Clinical Policy Title: Propel (drug eluting devices after sinus surgery) Clinical Policy Number: 10.03.07 Effective Date: July 1, 2017 Initial Review Date: May 19, 2017 Most Recent Review Date: May 1,
