Subtalar Arthroereisis
|
|
|
- Olivia Paul
- 5 years ago
- Views:
Transcription
1 Subtalar Arthroereisis Policy Number: Last Review: 5/2014 Origination: 5/2008 Next Review: 5/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for subtalar arthroereisis. This is considered investigational. When Policy Topic is covered Not Applicable When Policy Topic is not covered Subtalar arthroereisis is considered investigational. Description of Procedure or Service Arthroereisis (also referred to as arthrosis) is the limitation of excessive movement across a joint. Subtalar arthroereisis (STA) is performed by placing an implant in the sinus tarsi (a canal located between the talus and the calcaneus) and is designed to correct excessive talar displacement and calcaneal eversion. Background Flexible flatfoot is a common disorder, anatomically described as excessive pronation during weight bearing due to anterior and medial displacement of the talus. It may be congenital in nature or it may be acquired in adulthood due to posterior tibial tendon dysfunction, which in turn may be caused by trauma, overuse, and inflammatory disorders, among others. Symptoms include dull, aching and throbbing, cramping pain, which in children may be described as growing pains. Additional symptoms include refusal to participate in athletics or walking long distances. Conservative treatments include orthotics or shoe modifications. Surgical approaches for painful flatfoot deformities include tendon transfers, osteotomy, and arthrodesis. Arthroereisis with a variety of implant designs has also been investigated. Subtalar arthroereisis (STA) has been performed for more than 50 years, with a variety of implants designs, and compositions. Currently, the Maxwell-Brancheau Arthroereisis (MBA) implant is favored due to the simple and reversible implantation procedure, although other devices reported in the medical literature include the STA peg and a Kalix device. The MBA implant is described as reversible and easy to insert, with the additional advantage that it does not require bone cement. In children, insertion of the MBA implant may be offered as a stand-alone procedure, although children and adults often require adjunctive surgical procedures on bone and soft tissue to correct additional deformities. Regulatory Status A number of implants have received marketing clearance through the U.S. Food and Drug Administration s (FDA) 510(k) pathway. For example, the HyProCure Subtalar Implant System/Extra Osseos Fixation Device (GraMedica) received marketing clearance in 2004 (K042030), the SubFix arthroereisis implant (Memometal Technologies, Bruz, France) received FDA marketing clearance in 2010 (K093820) and the Arthrex ProStop Plus (Arthrex, Naples, FL) received marketing clearance in 2008 (K071456). The MBA implant (now owned by Integra LifeSciences Corp., Plainsboro, NJ)
2 received 510(k) marketing clearance in 1996 (K960692) because it was substantially equivalent to products on the market prior to device regulation. According to the FDA summary, the primary indication for the Subtalar MBA device is as a spacer for stabilization of the subtalar joint. It is designed to block the anterior and inferior displacement of the talus, thus allowing normal subtalar joint motion but blocking excessive pronation and the resulting sequela. (1) The MBA Resorb Implant received 510(k) marketing clearance in 2005 (K051611). This implant employs the same basic mechanical features as the predicate MBA implant but is composed of a material (poly l-lactic acid) that is resorbed by the body. Predicate devices include the Osteomed Talar-Fit (K031155), Nexa Orthopedics Subtalar Peg (K and K033046), Arthroereisis Implant Talus of Vilex (TOV, K041289), Instrateck (K080280), and Wright Medical Smith Sta-Peg (K792670). Rationale Periodic literature searches of the MEDLINE database on subtalar arthroereisis (STA) have identified minimal published studies, primarily consisting of single institution case series and individual case reports. The most recent literature update, using the MEDLINE database, was performed for the period of July 2012 through August 13, Following is a summary of the key literature to date. There are no controlled trials of subtalar arthroereisis compared to alternative treatments. The evidence base consists entirely of single-arm case series that report on success rates following this procedure. Interpretation of the current case series evidence is limited by the use of adjunctive procedures in addition to subtalar arthroereisis, creating difficulties in determining the extent to which each modality contributed to the outcomes. The evidence base is also limited by the lack of long-term follow-up, which may be particularly important for a procedure performed in children. In 2011, Metcalfe et al. published a systematic review of the literature on subtalar arthroereisis for pediatric flexible flatfoot. (2) Seventy-six case series or case reports (no controlled trials) were identified. Ten of the studies (756 feet) provided clinician-based assessment of the surgical result graded from excellent to poor with follow-up between 36 and 240 months. Six studies (212 feet) included estimates of overall patient satisfaction using non-validated outcome measures, while 1 study (16 feet) found significant improvement using a validated foot-specific patient outcome measure. Data from 15 studies that reported radiographic values were combined for analysis. Although 8 of 9 radiographic parameters showed statistically significant improvements following arthroereisis procedures, the relationship between radiographic and clinical outcomes is uncertain. The procedure was associated with a number of complications including sinus tarsi pain, device extrusion, and undercorrection. Complication rates ranged from 4.8% to 18.6%, with unplanned removal rates between 7.1% and 19.3% across all device types. The influence of adjunctive procedures on outcomes was not addressed in this review. One case series that was not confounded by adjunctive procedures and that had a relatively long follow-up was published by Graham et al. in This study reported mean 51-month follow-up of talotarsal stabilization in 117 feet using the HyProCure device. (3) Patients who received adjunctive procedures affecting the talotarsal joint were excluded from the analysis. Adult patients who met the inclusion/exclusion criteria were invited to participate in the study. Eighty-three patients gave consent to participate, and 78 completed the Maryland Foot Score Questionnaire; 5 patients who had 7 implants (6%) removed did not complete the questionnaire. There were 16 revision surgeries with HyProCure; 9 involved repositioning of a partially displaced device or a change in size of the device. Of the patients who retained the device, 52% reported complete alleviation of foot pain, 69% had no limitations in their foot functional abilities, and 80% reported complete satisfaction with the appearance of their feet. This case series is notable for its assessment of functional outcomes at medium-term follow-up in patients who did not have adjunct procedures. Other case series generally did not exclude the use of other adjunctive treatments. For example, in 1998 Vedantam and colleagues reported on a series of 78 children (140 feet) with neuromuscular disease who underwent STA with an STA-peg. (4) The stem of this implant is placed into the
3 calcaneous with the collar abutting the inferior surface of the lateral aspect of the talus, thus limiting motion. All but 5 of the children had additional procedures to balance the foot. Satisfactory results were reported in 96.4% of patients, although the contribution of the STA-peg cannot be isolated. In 2004, Nelson and colleagues reported on 37 patients (67 feet) who underwent Maxwell-Brancheau Arthroereisis (MBA) implant with an average of 18.4 months of follow-up. (5) While this study reported various improvements in anatomic measurements, there were no data on improvement in symptoms. Another series from 2006 reported significant improvements in pain and function in 78% of patients (23 patients, 28 feet) with use of a subtalar implant as a component of reconstructive foot and ankle surgery. (6) However, since results were not compared with controls receiving reconstructive surgery without STA, the contribution of the implants to these outcomes is unclear. In addition, the authors reported an overall complication rate of 46%, with surgical removal of 39% of the implants due to sinus tarsi pain. The authors also commented that postoperative sinus tarsi pain was unpredictable. Cicchinelli et al. reported on radiographic outcomes in a retrospective analysis of 28 feet in 20 pediatric patients treated with STA combined with gastrocnemius recession or with STA combined with gastrocnemius recession and medial column reconstruction. (7) Lucaccini et al. analyzed clinical and radiographic results of 14 patients (16 feet) with hallux valgus in abnormal pronation syndrome treated with distal osteotomy of the first metatarsal bone and STA performed in one stage. (8) In a 2010 study, Scharer and colleagues conducted a retrospective radiographic evaluation of 39 patients (68 feet) who had received the MBA implant for the treatment of painful pediatric flatfoot deformities. (9) The average age of the patients at the time of surgery was 12 years (range: 6-16 years). Additional procedures included 12 (18%) gastrocnemius recessions, 6 (9%) Achilles tendon lengthening, and 4 (6%) Kidner procedures. At an average 24-month follow-up (range: 6-61 months), there had been 10 (15%) complications requiring reoperation, including implant migration, undercorrection, overcorrection, and persistent pain. The implants were exchanged for either a larger or smaller implant. These case series do not allow comparison with nonsurgical interventions or with other surgical interventions. An example of a case series with longer follow-up is a 2012 retrospective study by Brancheau et al., which reported mean 36-month follow-up (range 18 to 48 months) in 35 patients (60 feet) after use of the Maxwell-Brancheau Arthroereisis (MBA) implant with adjunct procedures. (10) The mean age of the patients was 14.3 years (range, 5 to 46 years). Significant changes were observed in radiographic measures (talocalcaneal angle, calcaneocuboid angle, first to second intermetatarsal angle, calcaneal inclination angle, and talar declination angle). Seventeen percent of patients reported that 9 implants (15%) were removed after the initial surgery. Of the 24 patients (68.6%) who answered a subjective questionnaire (in person or by telephone at a mean of 33 months postoperatively), 95.8% reported resolution of the chief presenting complaint, and 79.2% said they were 100% satisfied with their surgical outcome. The contribution of the MBA implant to these results cannot be determined by this study design. Complications are frequently reported in the literature. Scher and colleagues reported 2 cases of extensive implant reaction in 2 children 2 years after a STA-peg procedure. (11) Due to the commonly seen complication of severe postoperative pain with failure to reconstitute the longitudinal arch on weight bearing and a residual flatfoot deformity, the authors do not recommend subtalar arthroereisis in the treatment of painful flexible flatfoot in children. A radiographic study on a bioabsorbable STA found poor outcomes in 3 of 6 patients who met the inclusion criteria and consented to additional imaging. (12) Two patients requested implant removal; a third patient had persistent pain but refused explantation. Radiographic measurement (magnetic resonance imaging or computed tomography) found that these 3 patients had smaller tarsal canal widths than the diameter of the inserted interference screw. The authors noted that the implant length also had to be reduced prior to implantation. They concluded that the current width and length of commercially available implants may need to be modified and that more research and long-term clinical study are needed. Cook et al. conducted a retrospective case-control study to identify factors that may contribute to failure (explantation) of titanium arthroereisis implants. (13) All patients who required removal of a self-locking wedge-type subtalar arthroereisis (n=22) were compared in a 1:2 ratio (n=44) to patients with
4 nonexplanted arthroereises who were treated during the same time period. Subjects were matched for preoperative radiographic measurements, age, gender, presenting diagnosis, and length of follow-up. Multivariate logistic regression showed no significant effect of age, gender, implant size, shape, length of follow-up, implant position, surgeon experience, or concomitant procedures. Patients who required explantation had slightly greater odds of radiographic undercorrection (odds ratio [OR]: 1.175) or residual transverse plane-dominant deformities (OR: 1.096). The percentage of explantations in this retrospective analysis was not described. Clinical Input Received through Physician Specialty Societies and Academic Medical Centers While the various physician specialty societies and academic medical centers may collaborate with and make recommendations during this process through the provision of appropriate reviewers, input received does not represent an endorsement or position statement by the physician specialty societies or academic medical centers, unless otherwise noted In response to requests, input was received through 1 physician specialty society (3 reviews) and 5 academic medical centers while this policy was under review in The input of reviewers was mixed regarding the medical necessity of arthroereisis In response to requests, input was received through 2 physician specialty societies and 2 academic medical centers while this policy was under review in Input was mixed, with a majority of reviewers considering this procedure to be investigational. Summary The evidence in the published medical literature on subalar athroereisis is inadequate to permit scientific conclusions. The main limitation is the lack of controlled studies comparing use of the implants with other surgical procedures, alone or in combination. Other limitations of the published data is the lack of long-term outcomes, particularly important since the procedure is often performed in growing children, and the difficulty in separating the effect of this procedure from that of other adjunctive treatments. In addition, some publications report high rates of complications and implant removal. Therefore, subtalar arthroereisis is considered investigational. Practice Guidelines and Position Statements 2009 Guidance from the United Kingdom s National Institute for Clinical Excellence (NICE) concluded that current evidence on the safety and efficacy of sinus tarsi implant insertion for mobile flatfoot is inadequate in quality and quantity. (14) Therefore this procedure should only be used with special arrangements for clinical governance, consent and audit, or research. The American College of Foot and Ankle Surgeons (ACFAS) published practice guidelines for the diagnosis and treatment of adult and pediatric flatfoot in 2004 and 2005 (these are not included in the ACFAS library of current clinical practice guidelines). (15, 16) The ACFAS guideline on adult flatfoot states: In the adult, arthroereisis is seldom implemented as an isolated procedure. Because of the long-term compensation and adaptation of the foot and adjunctive structures for flatfoot function, other ancillary procedures are usually used for appropriate stabilization. Long-term results of arthroereisis in the adult flexible flatfoot patient have not been established. Some surgeons advise against the subtalar arthroereisis procedure because of the risks associated with implantation of a foreign material, the potential need for further surgery to remove the implant, and the limited capacity of the implant to stabilize the medial column sag directly.
5 The ACFAS guideline on pediatric flatfoot states: proponents of this procedure (arthroereisis) argue that it is a minimally invasive technique that does not distort the normal anatomy of the foot. Others have expressed concern about placing a permanent foreign body into a mobile segment of a child s foot. The indication for this procedure remains controversial in the surgical community. Medicare National Coverage There is no national coverage determination. References 1. U.S. Food and Drug Administration. Summary for Kinetikos Medical Incorporated (KMI) Subtalar MBA System. Available online at: 2. Metcalfe SA, Bowling FL, Reeves ND. Subtalar joint arthroereisis in the management of pediatric flexible flatfoot: a critical review of the literature. Foot Ankle Int 2011; 32(12): Graham ME, Jawrani NT, Chikka A. Extraosseous talotarsal stabilization using HyProCure(R) in adults: a 5-year retrospective follow-up. J Foot Ankle Surg 2012; 51(1): Vedantam R, Capelli AM, Schoenecker PL. Subtalar arthroereisis for the correction of planovalgus foot in children with neuromuscular disorders. J Pediatr Orthop 1998; 18(3): Nelson SC, Haycock DM, Little ER. Flexible flatfoot treatment with arthroereisis: radiographic improvement and child health survey analysis. J Foot Ankle Surg 2004; 43(3): Needleman RL. A surgical approach for flexible flatfeet in adults including a subtalar arthroereisis with the MBA sinus tarsi implant. Foot Ankle Int 2006; 27(1): Cicchinelli LD, Pascual Huerta J, Garcia Carmona FJ et al. Analysis of gastrocnemius recession and medial column procedures as adjuncts in arthroereisis for the correction of pediatric pes planovalgus: a radiographic retrospective study. J Foot Ankle Surg 2008; 47(5): Lucaccini C, Zambianchi N, Zanotti G. Distal osteotomy of the first metatarsal bone in association with sub-talar arthroerisis, for hallux valgus correction in abnormal pronation syndrome. Chir Organi Mov 2008; 92(3): Scharer BM, Black BE, Sockrider N. Treatment of painful pediatric flatfoot with Maxwell-Brancheau subtalar arthroereisis implant a retrospective radiographic review. Foot Ankle Spec 2010; 3(2): Brancheau SP, Walker KM, Northcutt DR. An analysis of outcomes after use of the Maxwell- Brancheau Arthroereisis implant. J Foot Ankle Surg 2012; 51(1): Scher DM, Bansal M, Handler-Matasar S et al. Extensive implant reaction in failed subtalar joint arthroereisis: report of two cases. HSS J 2007; 3(2): Saxena A, Nguyen A. Preliminary radiographic findings and sizing implications on patients undergoing bioabsorbable subtalar arthroereisis. J Foot Ankle Surg 2007; 46(3): Cook EA, Cook JJ, Basile P. Identifying risk factors in subtalar arthroereisis explantation: a propensity-matched analysis. J Foot Ankle Surg 2011; 50(4): National Institute for Clinical Excellence (NICE). Sinus Tarsi Implant Insertion for Mobile Flatfoot: Interventional Procedure Guidance Available online at: Harris EJ, Vanore JV, Thomas JL et al. Clinical Practice Guideline Pediatric Flatfoot Panel: American College of Foot and Ankle Surgeons (ACFAS). Diagnosis and treatment of pediatric flatfoot. J Foot Ankle Surg 2004; 43(6): Lee MS, Vanore JV, Thomas JL et al. Clinical Practice Guideline Adult Flatfoot Panel: American College of Foot and Ankle Surgeons (ACFAS). Diagnosis and treatment of adult flatfoot. J Foot Ankle Surg 2005; 44(2): Billing Coding/Physician Documentation Information S2117 Arthroereisis, subtalar Unlisted procedure, foot or toes 0335T Extra-osseous subtalar joint implant for talotarsal stabilization
6 28725 Arthrodesis; subtalar, Arthrodesis, midtarsal or tarsometatarsal, multiple or transverse; with osteotomy (eg, flatfoot correction), Arthrodesis, midtarsal or tarsometatarsal, single joint and Arthroscopy, subtalar joint, surgical; with subtalar arthrodesis: may be used incorrectly. Additional Policy Key Words N/A Policy Implementation/Update Information 5/1/08 New policy; considered investigational. 5/1/09 No policy statement changes. 5/1/10 No policy statement changes. 5/1/11 No policy statement changes. 5/1/12 No policy statement changes. 5/1/13 No policy statement changes. 5/1/14 Updated regulatory status, added cpt codes. State and Federal mandates and health plan contract language, including specific provisions/exclusions, take precedence over Medical Policy and must be considered first in determining eligibility for coverage. The medical policies contained herein are for informational purposes. The medical policies do not constitute medical advice or medical care. Treating health care providers are independent contractors and are neither employees nor agents Blue KC and are solely responsible for diagnosis, treatment and medical advice. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, photocopying, or otherwise, without permission from Blue KC.
Topic: Subtalar Arthroereisis Date of Origin: January 3, Section: Surgery Last Reviewed Date: December 2013
 Medical Policy Manual Topic: Subtalar Arthroereisis Date of Origin: January 3, 2006 Section: Surgery Last Reviewed Date: December 2013 Policy No: 144 Effective Date: January 1, 2014 IMPORTANT REMINDER
Medical Policy Manual Topic: Subtalar Arthroereisis Date of Origin: January 3, 2006 Section: Surgery Last Reviewed Date: December 2013 Policy No: 144 Effective Date: January 1, 2014 IMPORTANT REMINDER
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: subtalar_arthroereisis 6/2009 2/2018 2/2019 2/2018 Description of Procedure or Service Arthroereisis (also
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: subtalar_arthroereisis 6/2009 2/2018 2/2019 2/2018 Description of Procedure or Service Arthroereisis (also
Subtalar Arthroereisis
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Subtalar Arthroereisis Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Subtalar Arthroereisis Professional Institutional Original Effective Date: September
Subtalar Arthroereisis Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Subtalar Arthroereisis Professional Institutional Original Effective Date: September
Subtalar Arthroereisis
 Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0486 Subtalar Arthroereisis Table of Contents Coverage Policy... 1 Overview... 1 General Background...
Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0486 Subtalar Arthroereisis Table of Contents Coverage Policy... 1 Overview... 1 General Background...
EVALUATION OF PATIENTS RECEIVING HYPROCURE EXTRAOSSEOUS TALOTARSAL STABILIZATION DEVICE
 C H A P T E R 1 9 EVALUATION OF PATIENTS RECEIVING HYPROCURE EXTRAOSSEOUS TALOTARSAL STABILIZATION DEVICE Jennifer N. Falk, DPM Gregory D. Clark, DPM INTRODUCTION Flatfoot deformity is one of the most
C H A P T E R 1 9 EVALUATION OF PATIENTS RECEIVING HYPROCURE EXTRAOSSEOUS TALOTARSAL STABILIZATION DEVICE Jennifer N. Falk, DPM Gregory D. Clark, DPM INTRODUCTION Flatfoot deformity is one of the most
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Clinical Policy Title: Subtalar arthroereisis (implant)
 Clinical Policy Title: Subtalar arthroereisis (implant) Clinical Policy Number: 14.03.05 Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Subtalar arthroereisis (implant) Clinical Policy Number: 14.03.05 Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next
SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT
 C H A P T E R 1 7 SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT William D. Fishco, DPM, MS INTRODUCTION Arthroereisis is a surgical procedure designed to limit the motion of a joint. Subtalar joint arthroereisis
C H A P T E R 1 7 SUBTALAR ARTHROEREISIS IN THE OLDER PATIENT William D. Fishco, DPM, MS INTRODUCTION Arthroereisis is a surgical procedure designed to limit the motion of a joint. Subtalar joint arthroereisis
Disclosure Subtalar Arthroereisis Survey: The Current Practice Patterns of Members of the AOFAS. Presenter: Neil S. Shah, M.D.
 Disclosure 2013 Subtalar Arthroereisis Survey: The Current Practice Patterns of Members of the AOFAS Presenter: Neil S. Shah, M.D. My disclosure is in the Final AOFAS Mobile App I have no potential conflicts
Disclosure 2013 Subtalar Arthroereisis Survey: The Current Practice Patterns of Members of the AOFAS Presenter: Neil S. Shah, M.D. My disclosure is in the Final AOFAS Mobile App I have no potential conflicts
Facet Arthroplasty. Policy Number: Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019
 Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Foot specialists globally,
 Extra-osseous Talotarsal Stabilization: An Overview This procedure is used when an arch support isn t sufficient and reconstructive surgery is too aggressive. BY MICHAEL E. GRAHAM, DPM Foot specialists
Extra-osseous Talotarsal Stabilization: An Overview This procedure is used when an arch support isn t sufficient and reconstructive surgery is too aggressive. BY MICHAEL E. GRAHAM, DPM Foot specialists
Horizon Subtalar. Surgical Technique
 Horizon Subtalar Surgical Technique Contents Product The BioPro Horizon Subtalar Implant is used for the treatment of flatfoot and posterior tibial tendon dysfunction in both children and adults. Implanted
Horizon Subtalar Surgical Technique Contents Product The BioPro Horizon Subtalar Implant is used for the treatment of flatfoot and posterior tibial tendon dysfunction in both children and adults. Implanted
Transtympanic Micropressure Applications as a Treatment of Meniere s Disease
 Transtympanic Micropressure Applications as a Treatment of Meniere s Disease Policy Number: 1.01.23 Last Review: 8/2014 Origination: 2/2006 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas
Transtympanic Micropressure Applications as a Treatment of Meniere s Disease Policy Number: 1.01.23 Last Review: 8/2014 Origination: 2/2006 Next Review: 8/2015 Policy Blue Cross and Blue Shield of Kansas
A Patient s Guide to Adult-Acquired Flatfoot Deformity
 A Patient s Guide to Adult-Acquired Flatfoot Deformity Glendale Adventist Medical Center 1509 Wilson Terrace Glendale, CA 91206 Phone: (818) 409-8000 DISCLAIMER: The information in this booklet is compiled
A Patient s Guide to Adult-Acquired Flatfoot Deformity Glendale Adventist Medical Center 1509 Wilson Terrace Glendale, CA 91206 Phone: (818) 409-8000 DISCLAIMER: The information in this booklet is compiled
Bioimpedance Devices for Detection and Management of Lymphedema
 Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2017 Origination: 1/2011 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2017 Origination: 1/2011 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Surgical Technique Horizon Subtalar Implant
 Surgical Technique Horizon Subtalar Implant Table of contents Introduction... 1 Features and Benefits... 1 Ordering Information...2-3 Indications... 4 Contraindications... 4 Surgical Technique... 5-6 Post
Surgical Technique Horizon Subtalar Implant Table of contents Introduction... 1 Features and Benefits... 1 Ordering Information...2-3 Indications... 4 Contraindications... 4 Surgical Technique... 5-6 Post
STS. Subtalar Spacer System SURGICAL TECHNIQUE
 STS Subtalar Spacer System SURGICAL TECHNIQUE Contents Chapter 1 4 Introduction 4 Device Description Chapter 2 5 Intended Use 5 Indications Chapter 3 6 Surgical Technique 9 Postoperative Protocol 9 Explant
STS Subtalar Spacer System SURGICAL TECHNIQUE Contents Chapter 1 4 Introduction 4 Device Description Chapter 2 5 Intended Use 5 Indications Chapter 3 6 Surgical Technique 9 Postoperative Protocol 9 Explant
Index. Clin Podiatr Med Surg 22 (2005) Note: Page numbers of article titles are in boldface type.
 Clin Podiatr Med Surg 22 (2005) 309 314 Index Note: Page numbers of article titles are in boldface type. A Abductor digiti minimi muscle, myectomy of, for tailor s bunionette, 243 Achilles tendon, lengthening
Clin Podiatr Med Surg 22 (2005) 309 314 Index Note: Page numbers of article titles are in boldface type. A Abductor digiti minimi muscle, myectomy of, for tailor s bunionette, 243 Achilles tendon, lengthening
Bioimpedance Devices for Detection and Management of Lymphedema
 Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2018 Origination: 1/2011 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Bioimpedance Devices for Detection and Management of Lymphedema Policy Number: 2.01.82 Last Review: 5/2018 Origination: 1/2011 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Outcomes after Lengthening Calcaneal Osteotomy for Flexible Flatfoot Deformity Evans- versus Hintermann-Osteotomy
 Outcomes after Lengthening Calcaneal Osteotomy for Flexible Flatfoot Deformity Evans- versus Hintermann-Osteotomy Ettinger, S. 1, Mattinger, T. 1, Yao, D. 1, Claassen, L. 1, Daniilidis, K. 2, Stukenborg-Colsman,
Outcomes after Lengthening Calcaneal Osteotomy for Flexible Flatfoot Deformity Evans- versus Hintermann-Osteotomy Ettinger, S. 1, Mattinger, T. 1, Yao, D. 1, Claassen, L. 1, Daniilidis, K. 2, Stukenborg-Colsman,
Increased pressures at
 Surgical Off-loading of Plantar Hallux Ulcerations These approaches can be used to treat DFUs. By Adam R. Johnson, DPM Increased pressures at the plantar aspect of the hallux leading to chronic hyperkeratosis
Surgical Off-loading of Plantar Hallux Ulcerations These approaches can be used to treat DFUs. By Adam R. Johnson, DPM Increased pressures at the plantar aspect of the hallux leading to chronic hyperkeratosis
Dynamic Spinal Visualization and Vertebral Motion Analysis
 Dynamic Spinal Visualization and Vertebral Motion Analysis Policy Number: 6.01.46 Last Review: 2/2019 Origination: 2/2006 Next Review: 2/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Dynamic Spinal Visualization and Vertebral Motion Analysis Policy Number: 6.01.46 Last Review: 2/2019 Origination: 2/2006 Next Review: 2/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Change of Alignment by Calcaneocuboid Distraction Arthrodesis for Acquired Flatfoot
 Change of Alignment by Calcaneocuboid Distraction Arthrodesis for Acquired Flatfoot Akira Taniguchi, Yasuhito Tanaka, Kunihiko Kadono, Kiyonori Tomiwa, So Kameda, Hiroaki Kurokawa, Takenori Matsuda, Tsukasa
Change of Alignment by Calcaneocuboid Distraction Arthrodesis for Acquired Flatfoot Akira Taniguchi, Yasuhito Tanaka, Kunihiko Kadono, Kiyonori Tomiwa, So Kameda, Hiroaki Kurokawa, Takenori Matsuda, Tsukasa
Case. 15 Y old boy presented with pain in the foot. No history of injury or any constitutional symptoms. Your diagnosis?
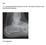 Case 15 Y old boy presented with pain in the foot. No history of injury or any constitutional symptoms Your diagnosis? Diagnosis: Calcaneo-navicular tarsal coalition. C sign Talar beaking Ant eaters nose
Case 15 Y old boy presented with pain in the foot. No history of injury or any constitutional symptoms Your diagnosis? Diagnosis: Calcaneo-navicular tarsal coalition. C sign Talar beaking Ant eaters nose
Electrical Stimulation for Scoliosis
 Electrical Stimulation for Scoliosis Policy Number: 1.01.509 Last Review: 8/2017 Origination: 8/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Electrical Stimulation for Scoliosis Policy Number: 1.01.509 Last Review: 8/2017 Origination: 8/2008 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Results of Calcaneal Osteotomy & Flexor Digitorum Longus transfer in Stage II Acquired Flatfoot Deformity
 Results of Calcaneal Osteotomy & Flexor Digitorum Longus transfer in Stage II Acquired Flatfoot Deformity Mr Amit Chauhan Mr Prasad Karpe Ms Maire-claire Killen Mr Rajiv Limaye University Hospital of North
Results of Calcaneal Osteotomy & Flexor Digitorum Longus transfer in Stage II Acquired Flatfoot Deformity Mr Amit Chauhan Mr Prasad Karpe Ms Maire-claire Killen Mr Rajiv Limaye University Hospital of North
DARCO. Bow 2 Plate SURGIC AL TECHNIQUE
 DARCO Bow 2 Plate SURGIC AL TECHNIQUE Contents 2 Preface 3 Chapter 1 4 Chapter 2 5 6 7 8 9 Appendix 10 10 11 Intended Use Indications/Contraindications Design Rationale Preoperative Planning Surgical Technique
DARCO Bow 2 Plate SURGIC AL TECHNIQUE Contents 2 Preface 3 Chapter 1 4 Chapter 2 5 6 7 8 9 Appendix 10 10 11 Intended Use Indications/Contraindications Design Rationale Preoperative Planning Surgical Technique
2017 SAFSA CONGRESS PROGRAMME
 2017 SAFSA CONGRESS PROGRAMME THURSDAY, MAY 25 07h45 07h55: WELCOME & INTRODUCTIONS Forefoot I: Hallux Valgus and Lesser Toes (08h00-10h00 Lectures) 08h00 08h30: Surgical Management of Hallux Valgus Rippstein,
2017 SAFSA CONGRESS PROGRAMME THURSDAY, MAY 25 07h45 07h55: WELCOME & INTRODUCTIONS Forefoot I: Hallux Valgus and Lesser Toes (08h00-10h00 Lectures) 08h00 08h30: Surgical Management of Hallux Valgus Rippstein,
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2014 Origination: 7/2013 Next Review: 1/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2014 Origination: 7/2013 Next Review: 1/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Double calcaneal osteotomy for severe adolescent flexible flatfoot reconstruction
 Xu et al. Journal of Orthopaedic Surgery and Research (2017) 12:153 DOI 10.1186/s13018-017-0655-3 RESEARCH ARTICLE Open Access Double calcaneal osteotomy for severe adolescent flexible flatfoot reconstruction
Xu et al. Journal of Orthopaedic Surgery and Research (2017) 12:153 DOI 10.1186/s13018-017-0655-3 RESEARCH ARTICLE Open Access Double calcaneal osteotomy for severe adolescent flexible flatfoot reconstruction
A Patient s Guide to Flatfoot Deformity (Pes Planus) in Children
 A Patient s Guide to Flatfoot Deformity (Pes Planus) in Children 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled
A Patient s Guide to Flatfoot Deformity (Pes Planus) in Children 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled
Clin Podiatr Med Surg 19 (2002) Index
 Clin Podiatr Med Surg 19 (2002) 335 344 Index Note: Page numbers of article titles are in bold face type. A Accessory soleus muscle, magnetic resonance imaging of, 300 Achilles tendon injury of, magnetic
Clin Podiatr Med Surg 19 (2002) 335 344 Index Note: Page numbers of article titles are in bold face type. A Accessory soleus muscle, magnetic resonance imaging of, 300 Achilles tendon injury of, magnetic
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
SURGICAL TECHNIQUE. Talex
 S U B TA L A R S TA B I L I Z AT I O N SY S T E M SURGICAL TECHNIQUE Talex S U B TA L A R S TA B I L I Z AT I O N SY S T E M Design Rationale The Talex Subtalar Stabilization System is the latest advancement
S U B TA L A R S TA B I L I Z AT I O N SY S T E M SURGICAL TECHNIQUE Talex S U B TA L A R S TA B I L I Z AT I O N SY S T E M Design Rationale The Talex Subtalar Stabilization System is the latest advancement
Medincenter GlavUpDK by the Ministry of Foreign Affairs of Russia, Moscow.
 Medincenter GlavUpDK by the Ministry of Foreign Affairs of Russia, Moscow. Berezhnoy Sergey. Percutaneous First Metatarsocuneiform Joint Arthrodesis in a Treatment of Metatarsus Primus Varus: a Prospective
Medincenter GlavUpDK by the Ministry of Foreign Affairs of Russia, Moscow. Berezhnoy Sergey. Percutaneous First Metatarsocuneiform Joint Arthrodesis in a Treatment of Metatarsus Primus Varus: a Prospective
Intracellular Micronutrient Analysis
 Intracellular Micronutrient Analysis Policy Number: 2.04.73 Last Review: 1/2019 Origination: 1/2013 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Intracellular Micronutrient Analysis Policy Number: 2.04.73 Last Review: 1/2019 Origination: 1/2013 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Index. Clin Podiatr Med Surg 23 (2006) Note: Page numbers of article titles are in boldface type.
 Clin Podiatr Med Surg 23 (2006) 233 239 Index Note: Page numbers of article titles are in boldface type. A Acclimatization, in sports preconditioning program, 197 Achilles tendon lengthening of, for equinus
Clin Podiatr Med Surg 23 (2006) 233 239 Index Note: Page numbers of article titles are in boldface type. A Acclimatization, in sports preconditioning program, 197 Achilles tendon lengthening of, for equinus
Vertebral Axial Decompression
 Vertebral Axial Decompression Policy Number: 8.03.09 Last Review: 11/2017 Origination: 11/2005 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Vertebral Axial Decompression Policy Number: 8.03.09 Last Review: 11/2017 Origination: 11/2005 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage
Extraarticular Lateral Ankle Impingement
 Extraarticular Lateral Ankle Impingement Poster No.: C-1282 Congress: ECR 2016 Type: Educational Exhibit Authors: C. Cevikol; Keywords: Trauma, Diagnostic procedure, MR, CT, Musculoskeletal system, Musculoskeletal
Extraarticular Lateral Ankle Impingement Poster No.: C-1282 Congress: ECR 2016 Type: Educational Exhibit Authors: C. Cevikol; Keywords: Trauma, Diagnostic procedure, MR, CT, Musculoskeletal system, Musculoskeletal
Managing Tibialis Posterior Tendon Injuries
 Managing Tibialis Posterior Tendon Injuries by Thomas C. Michaud, DC Published April 1, 2015 by Dynamic Chiropractic Magazine Tibialis posterior is the deepest, strongest, and most central muscle of the
Managing Tibialis Posterior Tendon Injuries by Thomas C. Michaud, DC Published April 1, 2015 by Dynamic Chiropractic Magazine Tibialis posterior is the deepest, strongest, and most central muscle of the
Foot and Ankle Systems Coding Reference Guide
 Foot and Ankle Systems Coding Reference Guide Physician Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis;
Foot and Ankle Systems Coding Reference Guide Physician Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis;
The Valgus Foot in Cerebral Palsy Equinovalgus not Plano-Valgus. Alfred D. Grant, M.D. David Feldman, M.D.
 The Valgus Foot in Cerebral Palsy Equinovalgus not Plano-Valgus Alfred D. Grant, M.D. David Feldman, M.D. Norman Otsuka, MD M.D. THE PURPOSE OF THIS PRESENTATION IS TO STATE CLEARLY THAT THE VALGUS FOOT
The Valgus Foot in Cerebral Palsy Equinovalgus not Plano-Valgus Alfred D. Grant, M.D. David Feldman, M.D. Norman Otsuka, MD M.D. THE PURPOSE OF THIS PRESENTATION IS TO STATE CLEARLY THAT THE VALGUS FOOT
There has been a struggle over the past few
 The Evidence Basis of Extra-osseous Talotarsal Stabilization (EOTTS) When compared to other surgical treatment options, the benefits far outweigh any potential risks. By Michael E. Graham, DPM There has
The Evidence Basis of Extra-osseous Talotarsal Stabilization (EOTTS) When compared to other surgical treatment options, the benefits far outweigh any potential risks. By Michael E. Graham, DPM There has
Assessment of percutaneous V osteotomy of the calcaneus with Ilizarov application for correction of complex foot deformities
 Acta Orthop. Belg., 2004, 70, 586-590 ORIGINAL STUDY Assessment of percutaneous V osteotomy of the calcaneus with Ilizarov application for correction of complex foot deformities Hani EL-MOWAFI From Mansoura
Acta Orthop. Belg., 2004, 70, 586-590 ORIGINAL STUDY Assessment of percutaneous V osteotomy of the calcaneus with Ilizarov application for correction of complex foot deformities Hani EL-MOWAFI From Mansoura
EXTRA-OSSEOUS TALOTARSAL STABILIZATION: NARROWING THE INDICATIONS
 EXTRA-OSSEOUS TALOTARSAL STABILIZATION: NARROWING THE INDICATIONS Michael E. Graham DPM, FACFAS, FAENS Macomb, Michigan, USA Introduction Sinus tarsi implants stabilize the talus on the tarsal mechanism
EXTRA-OSSEOUS TALOTARSAL STABILIZATION: NARROWING THE INDICATIONS Michael E. Graham DPM, FACFAS, FAENS Macomb, Michigan, USA Introduction Sinus tarsi implants stabilize the talus on the tarsal mechanism
Copyright 2004, Yoshiyuki Shiratori. All right reserved.
 Ankle and Leg Evaluation 1. History Chief Complaint: A. What happened? B. Is it a sharp or dull pain? C. How long have you had the pain? D. Can you pinpoint the pain? E. Do you have any numbness or tingling?
Ankle and Leg Evaluation 1. History Chief Complaint: A. What happened? B. Is it a sharp or dull pain? C. How long have you had the pain? D. Can you pinpoint the pain? E. Do you have any numbness or tingling?
Antigen Leukocyte Antibody Test
 Antigen Leukocyte Antibody Test Policy Number: 2.01.93 Last Review: 4/2014 Origination: 4/2014 Next Review: 4/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for
Antigen Leukocyte Antibody Test Policy Number: 2.01.93 Last Review: 4/2014 Origination: 4/2014 Next Review: 4/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for
.org. Posterior Tibial Tendon Dysfunction. Anatomy. Cause. Symptoms
 Posterior Tibial Tendon Dysfunction Page ( 1 ) Posterior tibial tendon dysfunction is one of the most common problems of the foot and ankle. It occurs when the posterior tibial tendon becomes inflamed
Posterior Tibial Tendon Dysfunction Page ( 1 ) Posterior tibial tendon dysfunction is one of the most common problems of the foot and ankle. It occurs when the posterior tibial tendon becomes inflamed
Timed Therapeutic Procedures
 Timed Therapeutic Procedures Policy Number: 10.01.526 Last Review: 4/2014 Origination: 4/2009 Next Review: 4/2015 Policy Documentation to support the reporting of timed procedure codes is required. The
Timed Therapeutic Procedures Policy Number: 10.01.526 Last Review: 4/2014 Origination: 4/2009 Next Review: 4/2015 Policy Documentation to support the reporting of timed procedure codes is required. The
Biofeedback as a Treatment of Headache
 Biofeedback as a Treatment of Headache Policy Number: 2.01.29 Last Review: 7/2018 Origination: 7/2008 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) may provide coverage
Biofeedback as a Treatment of Headache Policy Number: 2.01.29 Last Review: 7/2018 Origination: 7/2008 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) may provide coverage
March 19, Dear Ms. Strohkirch:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service March 19, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Treace
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service March 19, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Treace
*Rippstein, Trnka, Saragas, Hoffman
 THURS 25th MAY 07:00 07:10 Welcome and Introductions Paulo Ferrao Lecture 1: 07:10 09:45 Forefoot I: Hallux Valgus and Lesser Toes Mark Easley 40 mins 07:10 07:50 Surgical Management of Hallux Valgus 30
THURS 25th MAY 07:00 07:10 Welcome and Introductions Paulo Ferrao Lecture 1: 07:10 09:45 Forefoot I: Hallux Valgus and Lesser Toes Mark Easley 40 mins 07:10 07:50 Surgical Management of Hallux Valgus 30
Posterior Tibialis Tendon Dysfunction & Repair
 1 Posterior Tibialis Tendon Dysfunction & Repair Surgical Indications and Considerations Anatomical Considerations: The posterior tibialis muscle arises from the interosseous membrane and the adjacent
1 Posterior Tibialis Tendon Dysfunction & Repair Surgical Indications and Considerations Anatomical Considerations: The posterior tibialis muscle arises from the interosseous membrane and the adjacent
Physical Examination of the Foot & Ankle
 Inspection Standing, feet straight forward facing toward examiner Swelling Deformity Flatfoot (pes planus and hindfoot valgus) High arch (pes cavus and hindfoot varus) Peek-a-boo heel Varus Too many toes
Inspection Standing, feet straight forward facing toward examiner Swelling Deformity Flatfoot (pes planus and hindfoot valgus) High arch (pes cavus and hindfoot varus) Peek-a-boo heel Varus Too many toes
Vosevi (sofosbuvir/velpatasvir/voxilaprevir)
 Vosevi (sofosbuvir/velpatasvir/voxilaprevir) Policy Number: 5.01.646 Last Review: 10/2017 Origination: 10/2017 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Vosevi (sofosbuvir/velpatasvir/voxilaprevir) Policy Number: 5.01.646 Last Review: 10/2017 Origination: 10/2017 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Rippstein, Trnka, Saragas, Narramore
 THURS 25th MAY 07:45 07:55 Welcome and Introductions Paulo Ferrao Lecture 1: 08:00 10:20 Forefoot I: Hallux Valgus and Lesser Toes Mark Easley 30 mins 08:00 08:30 Surgical Management of Hallux Valgus Saragas,
THURS 25th MAY 07:45 07:55 Welcome and Introductions Paulo Ferrao Lecture 1: 08:00 10:20 Forefoot I: Hallux Valgus and Lesser Toes Mark Easley 30 mins 08:00 08:30 Surgical Management of Hallux Valgus Saragas,
Peritalar Dislocation After Tibio-Talar Arthrodesis: Fact or Fiction?
 AOFAS Annual Meeting, July 17-20th 2013 Hollywood, Florida Peritalar Dislocation After Tibio-Talar Arthrodesis: Fact or Fiction? Fabrice Colin, MD; Lukas Zwicky, MSc; Alexej Barg, MD; Beat Hintermann,
AOFAS Annual Meeting, July 17-20th 2013 Hollywood, Florida Peritalar Dislocation After Tibio-Talar Arthrodesis: Fact or Fiction? Fabrice Colin, MD; Lukas Zwicky, MSc; Alexej Barg, MD; Beat Hintermann,
Other Congenital and Developmental Diseases of the Foot. Department of Orthopedic Surgery St. Vincent s s Hospital, The Catholic University
 Other Congenital and Developmental Diseases of the Foot Department of Orthopedic Surgery St. Vincent s s Hospital, The Catholic University Contents Metatarsus Adductus Skewfoot Hallux Valgus Hallux Valgus
Other Congenital and Developmental Diseases of the Foot Department of Orthopedic Surgery St. Vincent s s Hospital, The Catholic University Contents Metatarsus Adductus Skewfoot Hallux Valgus Hallux Valgus
Foot & Ankle Disorders
 Foot & Ankle Disorders Hillingdon PGMC 6-7-2013 Htwe Zaw FRCS (Tr&Orth) Consultant Foot & Ankle and Trauma Surgeon Hillingdon Hospitals NHS Foundation Trust Overview Anatomy: hindfoot-midfoot coupling
Foot & Ankle Disorders Hillingdon PGMC 6-7-2013 Htwe Zaw FRCS (Tr&Orth) Consultant Foot & Ankle and Trauma Surgeon Hillingdon Hospitals NHS Foundation Trust Overview Anatomy: hindfoot-midfoot coupling
FACTS 1. Most need only Gastro aponeurotic release [in positive Silverskiold test]
![FACTS 1. Most need only Gastro aponeurotic release [in positive Silverskiold test] FACTS 1. Most need only Gastro aponeurotic release [in positive Silverskiold test]](/thumbs/83/88335212.jpg) FOOT IN CEREBRAL PALSY GAIT IN CEREBRAL PALSY I True Equinus II Jump gait III Apparent Equinus IV Crouch gait Group I True Equinus Extended hip and knee Equinus at ankle II Jump Gait [commonest] Equinus
FOOT IN CEREBRAL PALSY GAIT IN CEREBRAL PALSY I True Equinus II Jump gait III Apparent Equinus IV Crouch gait Group I True Equinus Extended hip and knee Equinus at ankle II Jump Gait [commonest] Equinus
Takhzyro (lanadelumab-flyo)
 Takhzyro (lanadelumab-flyo) Policy Number: 5.01.675 Last Review: 1/2019 Origination: 1/2019 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Takhzyro
Takhzyro (lanadelumab-flyo) Policy Number: 5.01.675 Last Review: 1/2019 Origination: 1/2019 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Takhzyro
Foot Injuries. Dr R B Kalia
 Foot Injuries Dr R B Kalia Overview Dramatic impact on the overall health, activity, and emotional status More attention and aggressive management Difficult appendage to study and diagnose. Aim- a stable
Foot Injuries Dr R B Kalia Overview Dramatic impact on the overall health, activity, and emotional status More attention and aggressive management Difficult appendage to study and diagnose. Aim- a stable
What Happens to the Paediatric Flat Foot? Peter J Briggs Freeman Hospital Newcastle upon Tyne
 What Happens to the Paediatric Flat Foot? Peter J Briggs Freeman Hospital Newcastle upon Tyne We don t know!! Population Studies 2300 children aged 4-13 years Shoe wearers Flat foot 8.6% Non-shoe wearers
What Happens to the Paediatric Flat Foot? Peter J Briggs Freeman Hospital Newcastle upon Tyne We don t know!! Population Studies 2300 children aged 4-13 years Shoe wearers Flat foot 8.6% Non-shoe wearers
Index. Clin Sports Med 23 (2004) Note: Page numbers of article titles are in boldface type.
 Clin Sports Med 23 (2004) 169 173 Index Note: Page numbers of article titles are in boldface type. A Achilles enthesopathy, calcaneal spur with, 133 clinical presentation of, 135 136 definition of, 131
Clin Sports Med 23 (2004) 169 173 Index Note: Page numbers of article titles are in boldface type. A Achilles enthesopathy, calcaneal spur with, 133 clinical presentation of, 135 136 definition of, 131
CURRICULUM VITAE. East Lansing, Michigan B.S. Biochemistry. Michigan State University
 NAME: EDUCATION: Michigan State University East Lansing, Michigan 1979-1983 B.S. Biochemistry Michigan State University College of Osteopathic Medicine East Lansing, Michigan Top 20% Class Standing 1983-1987
NAME: EDUCATION: Michigan State University East Lansing, Michigan 1979-1983 B.S. Biochemistry Michigan State University College of Osteopathic Medicine East Lansing, Michigan Top 20% Class Standing 1983-1987
Chamnanni Rungprai, M.D.
 Comparison pre- and post-operative alignment correction in patients with stage II flatfoot deformities treated with bony realignment procedure Co-authors 1,2 Chamnanni Rungprai, M.D. 1 Tyler G. Slayman,
Comparison pre- and post-operative alignment correction in patients with stage II flatfoot deformities treated with bony realignment procedure Co-authors 1,2 Chamnanni Rungprai, M.D. 1 Tyler G. Slayman,
Original Article. Annals of Rehabilitation Medicine INTRODUCTION
 Original Article Ann Rehabil Med 2014;38(3):369-375 pissn: 2234-0645 eissn: 2234-0653 http://dx.doi.org/10.5535/arm.2014.38.3.369 Annals of Rehabilitation Medicine Effects of Custom-Made Rigid Foot Orthosis
Original Article Ann Rehabil Med 2014;38(3):369-375 pissn: 2234-0645 eissn: 2234-0653 http://dx.doi.org/10.5535/arm.2014.38.3.369 Annals of Rehabilitation Medicine Effects of Custom-Made Rigid Foot Orthosis
Lower Limb Biomechanical Examination
 Lower Limb Biomechanical Examination Click here for completion instructions. Patient Name: Chief Complaint: History of problem: Nature of discomfort/pain Location (anatomic) Duration Onset Course Aggravating
Lower Limb Biomechanical Examination Click here for completion instructions. Patient Name: Chief Complaint: History of problem: Nature of discomfort/pain Location (anatomic) Duration Onset Course Aggravating
Addyi (flibanserin) When Policy Topic is covered Coverage of Addyi is recommended in those who meet the following criteria:
 Addyi (flibanserin) Policy Number: 5.01.605 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Addyi when
Addyi (flibanserin) Policy Number: 5.01.605 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Addyi when
ANKLE JOINT ANATOMY 3. TALRSALS = (FOOT BONES) Fibula. Frances Daly MSc 1 CALCANEUS 2. TALUS 3. NAVICULAR 4. CUBOID 5.
 ANKLE JOINT ANATOMY The ankle joint is a synovial joint of the hinge type. The joint is formed by the distal end of the tibia and medial malleolus, the fibula and lateral malleolus and talus bone. It is
ANKLE JOINT ANATOMY The ankle joint is a synovial joint of the hinge type. The joint is formed by the distal end of the tibia and medial malleolus, the fibula and lateral malleolus and talus bone. It is
Orthopaedic Surgery Foot
 Hyperpronation The Role of Subtalar Arthroereisis Michael E Graham Center for Foot and Ankle Disorders Abstract Excessive foot pronation is a common condition seen in children, adults and geriatric populations.
Hyperpronation The Role of Subtalar Arthroereisis Michael E Graham Center for Foot and Ankle Disorders Abstract Excessive foot pronation is a common condition seen in children, adults and geriatric populations.
Surgical Treatment of Bilateral Gynecomastia
 Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Surgical Interruption of Pelvic Nerve Pathways for Primary and Secondary Dysmenorrhea
 Surgical Interruption of Pelvic Nerve Pathways for Primary and Secondary Dysmenorrhea Policy Number: 4.01.17 Last Review: 11/2013 Origination: 11/2007 Next Review: 11/2014 Policy Blue Cross and Blue Shield
Surgical Interruption of Pelvic Nerve Pathways for Primary and Secondary Dysmenorrhea Policy Number: 4.01.17 Last Review: 11/2013 Origination: 11/2007 Next Review: 11/2014 Policy Blue Cross and Blue Shield
Minimally Invasive Bunion Surgery: Methods and Outcomes
 Minimally Invasive Bunion Surgery: Methods and Outcomes Hummira H. Abawi, DPM Diplomate ABPM, AACFAS Instructor of Orthopedics, University of Maryland Medical Center Director of Education, Maryland VA
Minimally Invasive Bunion Surgery: Methods and Outcomes Hummira H. Abawi, DPM Diplomate ABPM, AACFAS Instructor of Orthopedics, University of Maryland Medical Center Director of Education, Maryland VA
Foot Plating System Product rationale
 Product rationale CONTENTS Introduction 2 Foot plating system 2.7mm Universal Locking Plate 2.7mm 3 Metatarsophalangeal Plate 4 Opening Wedge Locking Plate 5 Opening Wedge Osteotomy Plate 5 Foot plating
Product rationale CONTENTS Introduction 2 Foot plating system 2.7mm Universal Locking Plate 2.7mm 3 Metatarsophalangeal Plate 4 Opening Wedge Locking Plate 5 Opening Wedge Osteotomy Plate 5 Foot plating
REPAIR OF THE DISPLACED AUSTIN OSTEOTOMY
 C H A P T E R 2 1 REPAIR OF THE DISPLACED AUSTIN OSTEOTOMY John V. Vanore, DPM INTRODUCTION Bunion surgery is frequently performed by foot and ankle surgeons. Generally, bunion surgery is quite predictable,
C H A P T E R 2 1 REPAIR OF THE DISPLACED AUSTIN OSTEOTOMY John V. Vanore, DPM INTRODUCTION Bunion surgery is frequently performed by foot and ankle surgeons. Generally, bunion surgery is quite predictable,
Conservative management of idiopathic clubfoot: Kite versus Ponseti method
 Journal of Orthopaedic Surgery 2009;17(1):67-71 Conservative management of idiopathic clubfoot: Kite versus Ponseti method AV Sanghvi, 1 VK Mittal 2 1 Department of Orthopaedics, Government Medical College
Journal of Orthopaedic Surgery 2009;17(1):67-71 Conservative management of idiopathic clubfoot: Kite versus Ponseti method AV Sanghvi, 1 VK Mittal 2 1 Department of Orthopaedics, Government Medical College
Intraoperative Fluorescence Imaging Systems
 Intraoperative Fluorescence Imaging Systems Policy Number: 10.01.530 Last Review: 01/2018 Origination: 01/2015 Next Review: 01/2019 Policy Intraoperative fluorescence imaging (SPY Imaging) to evaluate
Intraoperative Fluorescence Imaging Systems Policy Number: 10.01.530 Last Review: 01/2018 Origination: 01/2015 Next Review: 01/2019 Policy Intraoperative fluorescence imaging (SPY Imaging) to evaluate
How to approach the pediatric flatfoot
 Submit a Manuscript: http://www.wjgnet.com/esps/ Help Desk: http://www.wjgnet.com/esps/helpdesk.aspx DOI: 10.5312/wjo.v7.i1.1 World J Orthop 2016 January 18; 7(1): 1-7 ISSN 2218-5836 (online) 2016 Baishideng
Submit a Manuscript: http://www.wjgnet.com/esps/ Help Desk: http://www.wjgnet.com/esps/helpdesk.aspx DOI: 10.5312/wjo.v7.i1.1 World J Orthop 2016 January 18; 7(1): 1-7 ISSN 2218-5836 (online) 2016 Baishideng
TRIPLE ARTHRODESIS FOR ADULT ACQUIRED FLATFOOT
 TRIPLE ARTHRODESIS FOR ADULT ACQUIRED FLATFOOT Alan R. Catanzariti, DPM Brian T. Dix, DPM Phillip E. Richardson, DPM Robert W. Mendicino, DPM INTRODUCTION Triple arthrodesis for adult acquired flatfoot
TRIPLE ARTHRODESIS FOR ADULT ACQUIRED FLATFOOT Alan R. Catanzariti, DPM Brian T. Dix, DPM Phillip E. Richardson, DPM Robert W. Mendicino, DPM INTRODUCTION Triple arthrodesis for adult acquired flatfoot
PRELIMINARY RESULTS OF HALLUX VALGUS SURGERY USING MAGNESIUM SCREWS
 PRELIMINARY RESULTS OF HALLUX VALGUS SURGERY USING MAGNESIUM SCREWS AUTHOR: Timo Juutilainen, M.D., Ph.D. Head of Foot and Ankle Surgery Helsinki University Central Hospital (HUCH) Peijas Vantaa FINLAND
PRELIMINARY RESULTS OF HALLUX VALGUS SURGERY USING MAGNESIUM SCREWS AUTHOR: Timo Juutilainen, M.D., Ph.D. Head of Foot and Ankle Surgery Helsinki University Central Hospital (HUCH) Peijas Vantaa FINLAND
Type of Use (Select one or both, as applicable) Prescription Use (Part 21 CFR 801 Subpart D) Over-The-Counter Use (21 CFR 801 Subpart C)
 K153531 Page 1 of 2 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Indications for Use Form Approved: OMB No. 0910-0120 Expiration Date: January 31, 2017 See PRA Statement on last
K153531 Page 1 of 2 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Indications for Use Form Approved: OMB No. 0910-0120 Expiration Date: January 31, 2017 See PRA Statement on last
Technique Guide. 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot.
 Technique Guide 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot. Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
Technique Guide 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot. Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
The Lower Limb VII: The Ankle & Foot. Anatomy RHS 241 Lecture 7 Dr. Einas Al-Eisa
 The Lower Limb VII: The Ankle & Foot Anatomy RHS 241 Lecture 7 Dr. Einas Al-Eisa Ankle joint Synovial, hinge joint Allow movement of the foot in the sagittal plane only (1 degree of freedom): dorsiflexion:
The Lower Limb VII: The Ankle & Foot Anatomy RHS 241 Lecture 7 Dr. Einas Al-Eisa Ankle joint Synovial, hinge joint Allow movement of the foot in the sagittal plane only (1 degree of freedom): dorsiflexion:
Case 1 7 yo male Right elbow injury 3 months ago Medial elbow pain and tenderness over medial epicondyle Long arm cast given but off himself 1 month a
 Case presentations Case 1 7 yo male Right elbow injury 3 months ago Medial elbow pain and tenderness over medial epicondyle Long arm cast given but off himself 1 month after Progressive limited elbow flexion
Case presentations Case 1 7 yo male Right elbow injury 3 months ago Medial elbow pain and tenderness over medial epicondyle Long arm cast given but off himself 1 month after Progressive limited elbow flexion
THE FOOT S CONNECTED TOO... Evaluation Procedures for Orthotic Therapy Prescription 2005
 THE FOOT S CONNECTED TOO... Evaluation Procedures for Orthotic Therapy Prescription 2005 Unpublished Copyright Biomechanical Services, Inc. 2003 Biomechanical Services, Inc. 1050 Central Ave., Suite D
THE FOOT S CONNECTED TOO... Evaluation Procedures for Orthotic Therapy Prescription 2005 Unpublished Copyright Biomechanical Services, Inc. 2003 Biomechanical Services, Inc. 1050 Central Ave., Suite D
Ingrezza (valbenazine)
 Ingrezza (valbenazine) Policy Number: 5.01.635 Last Review: 7/2018 Origination: 07/2017 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Ingrezza
Ingrezza (valbenazine) Policy Number: 5.01.635 Last Review: 7/2018 Origination: 07/2017 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Ingrezza
AOFAS 2012 ANNUAL SUMMER MEETING. Subtalar Distraction Two Bone-Block Arthrodesis for Calcaneal Malunion
 AOFAS 2012 ANNUAL SUMMER MEETING Subtalar Distraction Two Bone-Block Arthrodesis for Calcaneal Malunion My disclosure is in the Final AOFAS Program Book. I have no potential conflicts with this presentation.
AOFAS 2012 ANNUAL SUMMER MEETING Subtalar Distraction Two Bone-Block Arthrodesis for Calcaneal Malunion My disclosure is in the Final AOFAS Program Book. I have no potential conflicts with this presentation.
The Vilex FUZETM. Dual Thread Screw & Intramedullary Nail in One Implant. The Ultimate TTC Arthrodesis Internal Fixator
 The Vilex FUZETM Dual Thread Screw & Intramedullary Nail in One Implant The Ultimate TTC Arthrodesis Internal Fixator Introduction The Vilex FUZE TM TTC Arthrodesis Compression Nail combines the attributes
The Vilex FUZETM Dual Thread Screw & Intramedullary Nail in One Implant The Ultimate TTC Arthrodesis Internal Fixator Introduction The Vilex FUZE TM TTC Arthrodesis Compression Nail combines the attributes
Cavus Foot: Subtle and Not-So-Subtle AOFAS Resident Review Course September 28, 2013
 Cavus Foot: Subtle and Not-So-Subtle Course September 28, 2013 Matthew M. Roberts, MD Associate Professor of Clinical Orthopaedic Surgery Co-Chief, Foot and Ankle Service Hospital for Special Surgery Disclosure
Cavus Foot: Subtle and Not-So-Subtle Course September 28, 2013 Matthew M. Roberts, MD Associate Professor of Clinical Orthopaedic Surgery Co-Chief, Foot and Ankle Service Hospital for Special Surgery Disclosure
Recognizing common injuries to the lower extremity
 Recognizing common injuries to the lower extremity Bones Femur Patella Tibia Tibial Tuberosity Medial Malleolus Fibula Lateral Malleolus Bones Tarsals Talus Calcaneus Metatarsals Phalanges Joints - Knee
Recognizing common injuries to the lower extremity Bones Femur Patella Tibia Tibial Tuberosity Medial Malleolus Fibula Lateral Malleolus Bones Tarsals Talus Calcaneus Metatarsals Phalanges Joints - Knee
Dorsal surface-the upper area or top of the foot. Terminology
 It is important to learn the terminology as it relates to feet to properly communicate with referring physicians when necessary and to identify the relationship between the anatomical structure of the
It is important to learn the terminology as it relates to feet to properly communicate with referring physicians when necessary and to identify the relationship between the anatomical structure of the
«Foot & Ankle Surgery» 04. Sept THE PAINFUL FLATFOOT. Norman Espinosa, MD
 THE PAINFUL FLATFOOT Norman Espinosa, MD Department of Orthopaedics University of Zurich Balgrist Switzerland www.balgrist.ch WHAT TO DO? INTRINSIC > EXTRINSIC ETIOLOGIES Repetitive microtrauma combined
THE PAINFUL FLATFOOT Norman Espinosa, MD Department of Orthopaedics University of Zurich Balgrist Switzerland www.balgrist.ch WHAT TO DO? INTRINSIC > EXTRINSIC ETIOLOGIES Repetitive microtrauma combined
Foot Anatomy. Midwest Bone & Joint Institute 2350 Royal Boulevard Suite 200 Elgin, IL Phone: Fax:
 A Patient s Guide to Foot Anatomy 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled from a variety of sources.
A Patient s Guide to Foot Anatomy 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled from a variety of sources.
CHRONIC FOOT PROBLEMS FOOT and ANKLE BASICS
 CHRONIC FOOT PROBLEMS FOOT and ANKLE BASICS ABC s of Comprehensive Musculoskeletal Care December 1 st, 2007 Stephen Pinney MD Chief, UCSF Foot and Ankle Service Chronic problems typically occur gradually
CHRONIC FOOT PROBLEMS FOOT and ANKLE BASICS ABC s of Comprehensive Musculoskeletal Care December 1 st, 2007 Stephen Pinney MD Chief, UCSF Foot and Ankle Service Chronic problems typically occur gradually
Complications following correction of the planovalgus foot in cerebral palsy by arthroereisis
 Acta Orthop. Belg., 2009, 75, 374379 ORIGIAL SUD Complications following correction of the planovalgus foot in cerebral palsy by arthroereisis Iakov OLAE, ietro ERSIAI, Lucian Lior ARCOVICI, Stefano ROSI,
Acta Orthop. Belg., 2009, 75, 374379 ORIGIAL SUD Complications following correction of the planovalgus foot in cerebral palsy by arthroereisis Iakov OLAE, ietro ERSIAI, Lucian Lior ARCOVICI, Stefano ROSI,
SAN ANTONIO, TEXAS DECEMBER 9-11, 2010 Hyatt Regency Hill Country Resort & Spa Resort, San Antonio, TX USA. Program Agenda
 6 th ANNUAL INTERNATIONAL EXTERNAL FIXATION SYMPOSIUM The Diabetic Charcot Foot SAN ANTONIO, TEXAS DECEMBER 9-11, 2010 Hyatt Regency Hill Country Resort & Spa Resort, San Antonio, TX USA Program Agenda
6 th ANNUAL INTERNATIONAL EXTERNAL FIXATION SYMPOSIUM The Diabetic Charcot Foot SAN ANTONIO, TEXAS DECEMBER 9-11, 2010 Hyatt Regency Hill Country Resort & Spa Resort, San Antonio, TX USA Program Agenda
