COVER FOCUS AT A GLANCE. BY ANDREA ARRIOLA LÓPEZ, MD, MSc, and THOMAS ALBINI, MD
|
|
|
- Priscilla George
- 5 years ago
- Views:
Transcription
1 THERAPEUTIC OPTIONS FOR UVEITIS Thanks to a plentiful number of choices available and in the pipeline, the future looks promising for patients with this condition. BY ANDREA ARRIOLA LÓPEZ, MD, MSc, and THOMAS ALBINI, MD Uveitis is an inflammatory condition associated with visual impairment, blindness, and reduced quality of life. The condition often affects patients in their most active and economically productive years. 1 Uveitis has been demonstrated to cause between 5% and 20% of cases of legal blindness in the United States and the European Union, and up to 25% of cases of legal blindness in developing countries. 2 Immunomodulatory therapy is essential in controlling chronic noninfectious inflammation, with decreased risks compared with chronic corticosteroid use. Steroid-sparing immunomodulatory therapy, such as antimetabolites (methotrexate and mycophenolate), T-cell inhibitors, alkylating agents, and, more recently, biologics, are commonly used to manage uveitis. This article is an overview of newer available treatments for uveitis and therapies under clinical investigation (see Table, next page). LOCAL STEROIDS Treatment guidelines discourage use of systemic corticosteroids at doses higher than 10 mg daily due to complications associated with prolonged use. However, local steroids, especially sustained-delivery options, have had increasing importance in the management of uveitis. (87% stabilized or improved), reduced cystoid macular edema (CME), and decreased the need for adjunctive therapy. 5 The implant reduced uveitis recurrence (no more than one for any patient in the study) and the need for adjunctive therapy. 3,5 The MUST trial demonstrated statistically significantly better control of uveitis in the implant group (88% vs. 71%). 7,8 Associated adverse events included increased intraocular pressure (IOP), cataract, hypotony, and suture exposure. 3,4 The implant is contraindicated in active viral, bacterial, mycobacterial, and fungal eye infection. 7 A follow-up study of the patients in the MUST trial concluded that patients treated with systemic therapy had better visual acuity outcomes than those in the implant group. However, in the second half of the 7-year study period, less than 30% of patients in the implant arm had had an implant placed, dampening the strength of this conclusion. The implant appears to work as well as systemic therapy while it releases drug (ie, the first 3 years after implantation). Patients with chronic inflammation (Continued on page 56) AT A GLANCE Fluocinolone Acetonide Fluocinolone Acetonide Intravitreal Implant 0.59 mg The fluocinolone acetonide intravitreal implant 0.59 mg (Retisert, Bausch + Lomb) was approved by the US Food and Drug Administration (FDA) in It is surgically implanted to treat noninfectious intermediate uveitis, posterior uveitis, or panuveitis and releases drug for approximately 3 years. 3-7 Preclinical studies demonstrated that the implant is well tolerated, with no measurable systemic drug absorption. In a multicenter trial, the implant effectively controlled inflammation for 34 weeks, preserved vision Uveitis is a debilitating condition causing between 5% and 20% of legal blindness in the United States and the European Union. Immunomodulatory therapy is essential in controlling chronic inflammation, with decreased risks compared with chronic corticosteroid use. Numerous agents are in the pipeline for treatment of uveitis, including long-acting steroid delivery systems, local nonsteroidal agents, systemic therapies, gene therapies, and more. 54 RETINA TODAY OCTOBER 2017
2 TABLE. DRUGS FOR THE TREATMENT OF UVEITIS, AVAILABLE AND IN THE PIPELINE Therapeutic Agent (Manufacturer) Sutured fluocinolone implant (Retisert, Bausch + Lomb) Injectable fluocinolone implant (Iluvien, Alimera Sciences) Drug Class Delivery Mechanism Pivotal Clinical Trial (phase) Clinical Trial Number Corticosteroid Corticosteroid Sustained-release implant Sustained-release implant FDA approved Re-implantation of a fluocinolone acetonide implant for non-infectious uveitis affecting the posterior segment (phase 4) A Controlled, Multi-center Study of the Utilization and Safety of the MK II Inserter and the Safety of the FAI Insert in Subjects With Noninfectious Uveitis Affecting the Posterior Segment of the Eye (phase 3) NCT NCT Dexamethasone implant (Ozurdex, Allergan) Suprachoroidal space TA (CLS-TA, Clearside Biomedical) Sirolimus (Opsiria, Santen) Adalimumab (Humira, Abbvie) Corticosteroid Sustained-release implant Study of the Effectiveness of Ozurdex for the Control of Uveitis (phase 4) Corticosteroid Suprachoroidal space Suprachoroidal Injection of CLS-TA in Subjects With Non-infectious Uveitis (AZALEA) (phase 3) Suprachoroidal Injection of CLS-TA in Subjects With Macular Edema Associated With Noninfectious Uveitis (PEACHTREE) (phase 3) mtor inhibitor Intravitreal Intravitreal Sirolimus as Therapeutic Approach to Uveitis (SAVE-2) (phase 2) Study Assessing Double-masked Uveitis Treatment (SAKURA) (phase 3) A Phase 3b, Multinational, Multicenter, Open-Label Extension Study Assessing the Long-Term Safety of PRN Intravitreal Injections of DE-109 in Subjects With Non-Infectious Uveitis of the Posterior Segment of the Eye Who Have Participated in the SAKURA Development Program (32-009) Anti-TNF-alpha Subcutaneous Efficacy and Safety of Adalimumab in Patients With Active Uveitis (VISUAL l) (phase 3) Intravitreal Adalimumab Versus Subcutaneous Adalimumab in Noninfectious Uveitis (IVAS) (phase 2) A Study of the Long-term Safety and Efficacy of Adalimumab in Subjects With Intermediate-, Posterior-, or Pan-uveitis (VISUAL III) (phase 3) NCT NCT NCT NCT NCT NCT NCT NCT NCT OCTOBER 2017 RETINA TODAY 55
3 TABLE (Cont d.). DRUGS FOR THE TREATMENT OF UVEITIS, AVAILABLE AND IN THE PIPELINE Therapeutic Agent (Manufacturer) Tocilizumab (Actemra, Genentech) Sarilumab (Kevzara, Sanofi Genzyme) Anti IL-6 Intravenous Study of the Safety, Tolerability, and Bioactivity of Tocilizumab On Patients With Non-infectious UVEITIS: The STOP-Uveitis Study (STOP-Uveitis) (phase 1/2) NCT Anti IL-6 Subcutaneous SATURN study (phase 2) NCT EYS606 (Eyevensys) Anti TNF-alpha Electrotransfection injection system EGP-437 (iontophoretic dexamethasone phosphate, Eyegate Pharmaceuticals) Drug Class Delivery Mechanism Pivotal Clinical Trial (phase) Clinical Trial Number Phase 1b study Corticosteroid Iontophoresis Safety and Efficacy of Iontophoretic Dexamethasone Phosphate Ophthalmic Solution in Non-Infectious Anterior Uveitis (EGP )(phase 3) NCT NS2 (Aldeyra Therapeutics) Corticotropin (Acthar, Mallinckrodt Pharmaceuticals) Aldehyde trap Topical A Safety and Efficacy Study of NS2 in Patients With Anterior Uveitis (phase 2) Adrenocorticotropic hormone analogue Subcutaneous or intramuscular Phase 4 study NCT Abbreviations: anti IL-6, anti interleukin-6; anti TNF-alpha, anti tumor necrosis factor alpha; FDA, Food and Drug Administration; mtor, mammalian target of rapamycin; PRN, as needed; TA, triamcinolone acetonide Source: clinicaltrials.gov (Continued from page 54) may need to have the implant replaced every 30 months for optimum results. Other Fluocinolone Acetonide Intravitreal Implants The fluocinolone acetonide intravitreal implant 0.19 mg (Iluvien, Alimera Sciences) was FDA-approved in 2014 to treat eyes with diabetic macular edema. 3,6 The implant is inserted intravitreally with a 25-gauge needle. 3,6,7 In July, Alimera secured the rights to pursue a secondary indication for posterior uveitis in the European Union, the Middle East, and Africa. 9 Jaffe et al 3 found that the visual acuity of eyes with noninfectious intermediate uveitis, posterior uveitis, or panuveitis treated with the injectable fluocinolone acetonide implant stabilized or improved after 1 year. All 11 eyes in the study remained quiet and required less adjunctive therapy during 24 months of follow-up. 3 There was evidence of resolution of anterior chamber cells; reduced vitreous haze; decreased macular thickness and volume and decreased retinal nerve fiber layer thickness on optical coherence tomography (OCT); and absence of recurrences. Adverse effects included increased IOP and cataract progression. 3,6 The fluocinolone acetonide intravitreal implant 0.18 mg (Durasert, psivida) is currently under investigation for use in posterior segment noninfectious uveitis. Phase 3 pivotal trials of 3-year treatment with this implant have met their primary endpoint with a favorable safety profile, but results have not been published. 10,11 Dexamethasone The dexamethasone intravitreal implant 0.7 mg (Ozurdex, Allergan) is a sustained-release, injectable, biodegradable steroid implant approved in the United States and Europe for the treatment of noninfectious uveitis affecting the posterior segment. 6,7 Implanted with a 22-gauge injector, the device releases dexamethasone in a biphasic manner over a 6-month period. After the first 2 months, the steroid concentration declines until month 4, when it maintains a lower concentration until month 6. The HURON study compared the safety and efficacy of two doses (0.7 mg and 0.35 mg) of the dexamethasone intravitreal implant for the treatment of noninfectious intermediate or 56 RETINA TODAY OCTOBER 2017
4 posterior segment uveitis over 6 months. 12 Both doses were effective in controlling vitreous inflammation and improving visual acuity with reduction in CME, but the higher dose had a longer duration of action without a significant increase in side effects. A total of 47% of patients treated with the 0.7 mg implant achieved the primary outcome measure a vitreous haze score of zero at 8 weeks. 7,8 Observed side effects included raised IOP and cataract progression. The implant has also been shown to be effective in reducing macular thickness and increasing visual acuity in uveitic macular edema in vitrectomized eyes and in pediatric uveitis. 6 The dexamethasone intravitreal implant 0.7 mg is contraindicated in patients with periocular infections and advanced glaucoma and in patients whose posterior lens capsule is not intact, due to a risk of implant migration into the anterior chamber, seen especially in vitrectomized eyes. 7 [The dexamethasone intravitreal implant 0.7 mg] has also been shown to be effective in reducing macular thickness and increasing visual acuity in uveitic macular edema in vitrectomized eyes and in pediatric uveitis. Suprachoroidal Triamcinolone Acetonide Triamcinolone acetonide (CLS-TA, Clearside Biomedical) is in development as a single suprachoroidal 4.0 mg or 0.8 mg injection for treatment of macular edema associated with noninfectious uveitis. In the Dogwood trial, the primary endpoint of reducing retinal thickness was successfully achieved at 8 weeks after single treatment. The secondary endpoint of improving best corrected visual acuity (BCVA) was also achieved. 13 Although data are limited, the hope is that suprachoroidal injection will maximize triamcinolone s effect on the retina while minimizing its effect on the lens and trabecular meshwork. LOCAL NONSTEROIDAL AGENTS Sirolimus Sirolimus (Opsiria, Santen) is a macrolide compound derived from Streptomyces hygroscopicus. It has potent immunosuppressive and antineoplastic activity that depends on its binding to specific cytosolic proteins (immunophilins) to generate an immunosuppressive complex. 14 Sirolimus inhibits the activity of the serine/threonine protein kinase of mammalian target of rapamycin (mtor). Oral administration of sirolimus demonstrated complete inhibition of autoimmune uveitis in preclinical models. 2,15 However, this delivery route requires laboratory and clinical monitoring for systemic toxicity (anemia and hyperlipidemia). Bimonthly intravitreal sirolimus was effective at a dose of 440 µg without systemic side effects in a phase 1 study. 14 At the 6-month and 12-month primary endpoints of the SAVE study, sirolimus reduced vitreous haze and the need for systemic corticosteroids in patients with uveitis. 15 The phase 3 SAKURA 1 and 2 studies reported significant improvement in reducing ocular inflammation in patients with active noninfectious uveitis and successful tapering of corticosteroid dose (<5 mg/d). The results suggest that intravitreal injection of sirolimus can effectively resolve ocular inflammation, often without concomitant use of other local or systemic immunoregulators. 2,14-16 The most often encountered adverse event was worsening of inflammation, with minimal ocular hypertension or cataract progression over 2 years. 15 Systemic sirolimus (Rapamune, Pfizer) is approved for several indications. The ophthalmic formulation of sirolimus has been accepted for review by the FDA. SYSTEMIC THERAPY Adalimumab Adalimumab (Humira, Abbvie) is a recombinant human immunoglobulin G1 (IgG1) monoclonal antibody specific for tumor necrosis factor-alpha (TNF-alpha). Adalimumab binds TNF-alpha and blocks its interaction with its two known receptors. It is FDA-approved for the treatment of noninfectious intermediate uveitis, posterior uveitis, and panuveitis. Adalimumab is administered subcutaneously at a typical dose of 40 mg every other week. 6,17-20 Suhler et al showed that adalimumab was safe and effective in 68% of refractory uveitis patients 10 weeks after initial dose, and the effect was maintained in 39% after 1 year. 18 Jaffe et al reported that patients who received adalimumab had a significantly lower risk of treatment failure, defined as increased vitreous haze, new active inflammatory lesions, anterior chamber cell grade, or worsening of BCVA. 17 After discontinuation of immunomodulatory therapy, adalimumab achieved early and sustained disease control, reducing inflammation and visual impairment. 58 RETINA TODAY OCTOBER 2017
5 Its use is associated with significant reduction of macular thickness relative to baseline and with resolution of macular edema. 17,18,20 Adverse events reported include injection site infection, allergic reactions, active tuberculosis, lupus or lupus-like reaction, and demyelinating disorder. 17,20 Before initiation of treatment, baseline laboratory screening should be completed, consisting of complete blood count; comprehensive metabolic panel including liver function, renal function, and electrolytes; and screening for tuberculosis by tuberculin skin test and/or interferon gamma release assay. 17,19 Contraindications include any evidence of active infection, lymphoproliferative disorder diagnosed or treated within the previous 5 years, moderate or severe heart failure, chronic hepatitis B or C, presence of any demyelinating disorders, pneumonitis, or use in the perioperative period (1 week before and 1 week after surgery). 19 [Sarilumab] is indicated for the treatment of rheumatoid arthritis and is undergoing clinical trials for use in the management of posterior segment noninfectious uveitis. arthritis (JIA)-associated uveitis has begun. 6 The multicenter, randomized STOP-UVEITIS study assessing the safety, tolerability, and bioactivity of two doses of tocilizumab in intermediate, posterior, and panuveitis is under way in the United States. 6,23 Tocilizumab appears to be effective in reducing macular edema, controlling inflammation, and maintaining or improving visual acuity as long as patients are receiving infusions. 22,24 The adverse events reported are infections (including tuberculosis), infusion reactions, increased risk of gastrointestinal perforation, laboratory abnormalities (hyperlipidemia, elevated transaminases, neutropenia), augmented risk of neurologic disorders, and malignancies. 21,22 Sarilumab Sarilumab (Kevzara, Sanofi Genzyme) is a subcutaneously administered fully human monoclonal antibody directed against the alpha subunit of the IL-6 receptor complex. It is indicated for the treatment of rheumatoid arthritis and is undergoing clinical trials for use in the management of posterior segment noninfectious uveitis In the SATURN study, in patients with noninfectious uveitis, reductions in vitreous haze and steroid dosing were seen in treated patients. 25 Visual acuity and central macular thickness also improved with a 200-mg dose administered subcutaneously every 2 weeks. This drug targets a specific inflammatory mediator, and it has been associated with fewer side effects than other available therapies. 25,27 Neutropenia and elevated alanine aminotransferase levels were reported as adverse events. 26 Tocilizumab Tocilizumab (Actemra, Genentech) is a humanized monoclonal IgG1 antibody interleukin-6 receptor (IL-6R) synthesized by recombinant DNA technology. It is approved in the United States and other countries for treatment of several autoimmune disorders and is being studied for treatment of refractory uveitis-related macular edema. 6,14,21,22 Tocilizumab blocks IL-6 mediated signaling by binding both soluble and transmembrane IL-6 receptors. The drug consequently reduces T-cell activation, Th17 differentiation, antibody secretion, and hepatic acute phase protein production. Mesquida et al 21 reported a statistically significant reduction in central foveal thickness beginning the first month after initiation of therapy and continued and sustained through 24 months, associated with visual improvement. 6 A phase 1/2 clinical trial of tocilizumab in juvenile idiopathic Corticotropin Repository corticotropin injection (Acthar, Mallinckrodt Pharmaceuticals) is delivered by subcutaneous or intramuscular injection and targets melanocortin receptors. It is FDA-approved for a range of severe acute and chronic allergic and inflammatory ophthalmic conditions, including diffuse posterior uveitis. Little has been published on corticotropin s clinical efficacy in the treatment of patients with uveitis, 28,29 but the drug may be able to reduce inflammation with less systemic effects than steroids. GENE THERAPY EYS606 EYS606 (Eyevensys) is a nonviral gene therapy that uses a proprietary electrotransfection injection system to deliver plasmids encoding for the production of anti TNF-alpha into the ciliary muscle of the eye. TNF-alpha is a cytokine that has been shown to play a pivotal role in mediating intraocular inflammation in noninfectious uveitis. EYS606 is being investigated in an open-label phase 1b study in the United Kingdom that is expected to be completed toward the end of this year. 30 OCTOBER 2017 RETINA TODAY 59
6 OTHER TREATMENT OPTIONS EGP-437 EGP-437 (Eyegate Pharmaceuticals) is a dexamethasone phosphate solution that is delivered to targeted ocular tissues using transscleral iontophoresis. Iontophoresis is a noninvasive method in which low electrical current is applied to an ionizable substance to increase its mobility across a surface through electrochemical repulsion. 1,14,31,32 EGP-437 has been shown to have a prolonged duration of action and to be more effective compared with other delivery routes. In 2012, Cohen et al reported that EGP-437 was well tolerated and extremely effective, achieving anterior cell chamber scores of zero within 28 days after one treatment in 60% of participants with noninfectious anterior uveitis. 31 The most commonly reported adverse effects were conjunctival hyperemia, punctate keratitis, conjunctival edema, eye pain, and erythema and edema of the eyelid. 32 NS2 NS2 (Aldeyra Therapeutics) is an aldehyde-binding small molecule that traps aldehydes, neutralizing toxic aldehyde species that can lead to inflammation via activation of the nuclear factor kappa B pathway. Once the aldehyde is trapped, the aldehyde complex is degraded and aldehyde levels are diminished, which potentially reduces toxicity and inflammation. In a randomized, multicenter, investigator-masked, comparator-controlled, parallel-group phase 2 clinical trial, topical NS2 reduced anterior chamber cell count in patients with noninfectious uveitis. 33 Anterior chamber cell count was reduced 53% in those receiving NS2 after 8 weeks of treatment. The drug showed effectiveness equal to that of topical corticosteroids without IOP elevation or cataract production. No serious adverse events were reported. CONCLUSION Uveitis can be a debilitating condition, and it affects a significant percentage of the population. As the research outlined above demonstrates, much work is being done in many centers to find more and better ways to safely and effectively treat and manage uveitis. n 1. Babu K, Mahendradas P. Medical management of uveitis current trends. Indian J Ophthalmol. 2013;61(6): Nguyen QD, Merrill PT, Clark L, et al; Sirolimus study assessing double-masked uveitis treatment (SAKURA) study group. Intravitreal sirolimus for noninfectious uveitis: a phase III sirolimus study assessing double-masked uveitis treatment (SAKURA). Ophthalmology. 2016;123(11): Jaffe GJ, Lin P, Keenan RT, Ashton P, Skalak C, Stinnett SS. Injectable fluocinolone acetonide long-acting implant for noninfectious intermediate uveitis, posterior uveitis, and panuveitis. Two years results. Ophthalmology. 2016;123(9): Jaffe GJ, Ben-Nun J, Guo H, et al. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000;107(11): Jaffe GJ, Martin D, Callanan D, Pearson A, Levy B, Comstock T; for the Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis thirty-four week results of a multicenter randomized clinical study. Ophthalmology. 2006;113(6): Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol. 2014;8: Cabrera M, Yeh S, Albini TA. Sustained-release corticosteroid options. J Ophthalmol. 2014; Relhan N, Yeh S, Albini TA. Intraocular sustained-release steroids for uveitis. Int Ophthalmol Clin. 2015;55(3): Alimera Sciences to expand Iluvien indication in Europe for posterior uveitis [press release]. Alimera Sciences. July 10, Accessed September 18, psivida s Durasert three-year treatment for posterior segment uveitis successfully achieves primary efficacy endpoint in second phase 3 study [press release]. psivida. June 13, Accessed September 18, Safety and efficacy of an injectable fluocinolone acetonide intravitreal insert. Clinicaltrials.gov. NCT Updated December 22, Accessed September 18, Lowder C, Belfort R Jr, Lightman S, et al; for the Ozurdex HURON Study Group. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5): Clearside Biomedical, Inc. announces positive topline data from phase 2 clinical trial for the treatment of macular edema associated with noninfectious uveitis [press release]. Clearside Biomedical. Clearside-Biomedical-Announces-Positive-Topline-Data-Phase. Accessed September 18, Maya JR, Sadiq MA, Zapata LJ, et al. Emerging therapies for noninfectious uveitis: what may be coming to the clinics. J Ophthalmol. 2014;2014: Ibrahim MA, Sepah YJ, Watters A, et al. One-year outcomes of the SAVE study: sirolimus as a therapeutic approach for uveitis. Transl Vis Sci Technol. 2015;4(2): Nguyen QD, Ibrahim MA, Watters A, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE study. J Ophthalmic Inflamm Infect. 2013;3: Jaffe GJ, Dick AD, Brézin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016; 375(10): Suhler EB, Lowder CY, Goldstein DA, et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol. 2013;97(4): Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3): Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8): Mesquida M, Molins B, Llorenç V, et al. Twenty-four month follow-up of tocilizumab therapy for refractory uveitis-related macular edema [published online ahead of print May 16, 2017]. Retina. 22. Mesquida M, Molins B, Llorenç V, et al. Long term effects of tocilizumab therapy for refractory uveitis related macular edema. Ophthalmology. 2014;121(12): Study of the safety, tolerability, and bioactivity of tocilizumab on patients with non-infectious uveitis: the Stop-Uveitis Study (STOP-Uveitis). Clinicaltrails.gov. Updated April 26, Accessed September 18, Adán A, Mesquida M, Llorenç V, et al. Tocilizumab treatment for refractory uveitis-related cystoid macular edema. Graefes Arch Clin Exp Ophthalmol. 2013;251(11): Nguyen QD, Sundaram PA, Erickson K, et al; for the SATURN Study Group. The SATURN study (SARIL-NIU): sarilumab for the treatment of posterior segment non-infectious uveitis (NIU). Paper presented at: Association for Research in Vision and Ophthalmology Annual Meeting; May 3-7, 2015; Denver, CO. 26. Heissigerova J, Sundaram PA, Erickson K, et al. Sarilumab for the treatment of posterior segment non-infectious uveitis (NIU): the SATURN (SARIL-NIU) study. Acta Ophthalmol. 2015;93(S255): Nguyen QD, Sundaram PA, Erickson K, Varona R, Vinet L, Valerie Corp-dit-Genti, Robert Vitti, Marc D de Smet; Interim results from the SATURN Study: Sarilumab in Non-infectious Uveitis (SARIL-NIU). Invest Ophthalmol Vis Sci. 2016;57(12): Agarwal A, Hassan M, Sepah YJ, Do DV, Nguyen QD. Subcutaneous repository corticotropin gel for non-infectious panuveitis: reappraisal of an old pharmacologic agent. Am J Ophthalmol Case Rep. 2016;4: Ahmad KT, Sukumaran S, Brown KL, Ali TK. Use of corticotropin hormone for treatment of refractory uveitis secondary to juvenile idiopathic arthritis. Poster presented at: 9th International Symposium on Uveitis; August 20, 2016; Dublin, Ireland. 30. Eyevensys announces series A extension bringing total capital raised to 9 million [press release]. Eyevensys. September 15, html. Accessed September 18, Cohen AE, Assang C, Patane MA, et al; Avion Study Investigators. Evaluation of dexamethasone phosphate delivered by ocular iontophoresis for treating noninfectious anterior uveitis. Ophthalmology. 2012;119(1): Iontophoresis delivery of dexamethasone phosphate for noninfectious, non-necrotizing anterior scleritis, phase 1 dose-varying study. Clinicaltrials.gov. Updated April 20, Accessed September 18, Aldeyra Therapeutics announces positive results from phase II clinical trial in subjects with noninfectious anterior uveitis [press release]. Aldeyra Therapeutics. May 9, Accessed September 18, Thomas Albini, MD n associate professor of clinical ophthalmology at Bascom Palmer Eye Institute, University of Miami, Fla. n member of the Retina Today editorial advisory board n financial disclosures: consultant for Abbvie, Allergan, Bausch + Lomb, Mallinkrodt, and Santen n talbini@med.miami.edu Andrea Arriola López, MD, MSc n uveitis fellow at Bascom Palmer Eye Institute, University of Miami, Fla. n financial interest: none acknowledged n aearriola7@gmail.com Dr. Arriola would like to thank the Pan-American Ophthalmological Foundation and the Retina Research Foundation for funding her fellowship at Bascom Palmer Eye Institute. 60 RETINA TODAY OCTOBER 2017
Abbreviated Drug Evaluation: Fluocinolone acetonide intravitreal implant (Retisert )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
For the PSV-FAI-001 Study Investigators
 An Injectable Fluocinolone Acetonide Insert Decreases the Incidence of Recurrence in Patients with Chronic Non-infectious Uveitis Affecting the Posterior Segment of the Eye: 12 Month Results Glenn J. Jaffe,
An Injectable Fluocinolone Acetonide Insert Decreases the Incidence of Recurrence in Patients with Chronic Non-infectious Uveitis Affecting the Posterior Segment of the Eye: 12 Month Results Glenn J. Jaffe,
Noninfectious Posterior Uveitis: The Rationale for Steroid Use
 Insert to October 2016 CME Activity Noninfectious Posterior Uveitis: The Rationale for Steroid Use Thomas Albini, MD, moderator Nisha Acharya, MD Phoebe Lin, MD, PhD Quan Dong Nguyen, MD, MSc Steven Yeh,
Insert to October 2016 CME Activity Noninfectious Posterior Uveitis: The Rationale for Steroid Use Thomas Albini, MD, moderator Nisha Acharya, MD Phoebe Lin, MD, PhD Quan Dong Nguyen, MD, MSc Steven Yeh,
What you can expect with OZURDEX
 Important Information About Noninfectious Uveitis Affecting the Back Segment of the Eye and Treatment What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone intravitreal implant) is a prescription
Important Information About Noninfectious Uveitis Affecting the Back Segment of the Eye and Treatment What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone intravitreal implant) is a prescription
Sustained-Release Corticosteroid Options
 Sustained-Release Corticosteroid Options Mariana Cabrera, University of Miami Miller School of Medicine Steven Yeh, Emory University Thomas A Albini, University of Miami Miller School of Medicine Journal
Sustained-Release Corticosteroid Options Mariana Cabrera, University of Miami Miller School of Medicine Steven Yeh, Emory University Thomas A Albini, University of Miami Miller School of Medicine Journal
Uveitis unplugged: systemic therapy
 Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Regional vs. Systemic Therapy. Corticosteroids. Regional vs. Systemic Therapy for Uveitis. Considerations
 Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company AAO October 25, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
Update on management of Anterior Uveitis
 Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Adalimumab in Noninfectious Uveitis of Idiopathic Etiology Efficacy Across Different Anatomical Locations the VISUAL I and VISUAL II Trials
 9:00 AM Adalimumab in Noninfectious Uveitis of Idiopathic Etiology Efficacy Across Different Anatomical Locations the VISUAL I and VISUAL II Trials Pauline T. Merrill, MD Albert T. Vitale, MD Eric Fortin,
9:00 AM Adalimumab in Noninfectious Uveitis of Idiopathic Etiology Efficacy Across Different Anatomical Locations the VISUAL I and VISUAL II Trials Pauline T. Merrill, MD Albert T. Vitale, MD Eric Fortin,
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]
![Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]](/thumbs/82/86218480.jpg) Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 20 Next Review: September 2017 Related Policies 9.03.21 Aqueous Shunts for Glaucoma Intravitreal Corticosteroid Implants Summary An intravitreal implant
FEP Medical Policy Manual Last Review: September 20 Next Review: September 2017 Related Policies 9.03.21 Aqueous Shunts for Glaucoma Intravitreal Corticosteroid Implants Summary An intravitreal implant
Corporate Presentation August 2018
 Corporate Presentation August 218 Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words
Corporate Presentation August 218 Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words
Intravitreal Corticosteroid Implants. Description
 Subject: Intravitreal Corticosteroid Implants Page: 1 of 20 Last Review Status/Date: June 2015 Intravitreal Corticosteroid Implants Description An intravitreal implant is a drug delivery system, injected
Subject: Intravitreal Corticosteroid Implants Page: 1 of 20 Last Review Status/Date: June 2015 Intravitreal Corticosteroid Implants Description An intravitreal implant is a drug delivery system, injected
Medical Coverage Policy Intravitreal Corticosteroid Implants)
 Medical Coverage Policy Intravitreal Corticosteroid Implants) EFFECTIVE DATE:10 01 2015 POLICY LAST UPDATED: 02 07 2017 OVERVIEW An intravitreal implant is a drug delivery system, injected or surgically
Medical Coverage Policy Intravitreal Corticosteroid Implants) EFFECTIVE DATE:10 01 2015 POLICY LAST UPDATED: 02 07 2017 OVERVIEW An intravitreal implant is a drug delivery system, injected or surgically
Intravitreal Corticosteroid Implants
 Intravitreal Corticosteroid Implants Policy Number: 9.03.23 Last Review: 4/2018 Origination: 07/2015 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Intravitreal Corticosteroid Implants Policy Number: 9.03.23 Last Review: 4/2018 Origination: 07/2015 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Corporate Medical Policy
 Corporate Medical Policy Intravitreal Implant File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intravitreal_implant 11/2010 6/2017 6/2018 6/2017 Description of Procedure or Service
Corporate Medical Policy Intravitreal Implant File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intravitreal_implant 11/2010 6/2017 6/2018 6/2017 Description of Procedure or Service
Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis. Reference: NHS England: /PS
 Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis Reference: NHS England: 170010/PS Publications Gateway Reference: Document Purpose Policy 05527s Document Name
Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis Reference: NHS England: 170010/PS Publications Gateway Reference: Document Purpose Policy 05527s Document Name
Emerging therapies for the treatment of uveitis: clinical trial observations
 Review: Clinical Trial Outcomes Emerging therapies for the treatment of uveitis: clinical trial observations Clin. Invest. (2013) 3(10), 951 966 Uveitis, a leading cause of blindness in the USA and western
Review: Clinical Trial Outcomes Emerging therapies for the treatment of uveitis: clinical trial observations Clin. Invest. (2013) 3(10), 951 966 Uveitis, a leading cause of blindness in the USA and western
Multiple Technology Appraisal (MTA) Adalimumab, dexamethasone and sirolimus for treating non-infectious uveitis
 Multiple Technology Appraisal (MTA) Adalimumab, dexamethasone and sirolimus for treating non-infectious Response to consultee and commentator comments on the draft remit and draft scope (post-referral)
Multiple Technology Appraisal (MTA) Adalimumab, dexamethasone and sirolimus for treating non-infectious Response to consultee and commentator comments on the draft remit and draft scope (post-referral)
Evolving therapies for posterior uveitis. Infliximab (Remicade) Infliximab: pharmacology. FDA-approved monoclonal antibody therapy Target
 Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Nausheen Khuddus, MD Melissa Elder, MD, PhD
 Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Fluocinolone Implant for Idiopathic Non-Infectious Posterior Uveitis
 RP440_Berger-16.qxd:Poly 4/13/12 11:46 AM Page 1 May 2012 Fluocinolone Implant for Idiopathic Non-Infectious Posterior Uveitis PRESENTED BY Fluocinolone Implant for Idiopathic Non-Infectious Posterior
RP440_Berger-16.qxd:Poly 4/13/12 11:46 AM Page 1 May 2012 Fluocinolone Implant for Idiopathic Non-Infectious Posterior Uveitis PRESENTED BY Fluocinolone Implant for Idiopathic Non-Infectious Posterior
Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery.
 Poster #33516 Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery. JEFFREY H. LEVENSON, MD 1 (PRESENTING AUTHOR);
Poster #33516 Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery. JEFFREY H. LEVENSON, MD 1 (PRESENTING AUTHOR);
Charles C. Wykoff MD PhD Rahul N. Khurana MD
 HDWallpapers Suprachoroidal Triamcinolone Acetonide with & without Intravitreal Aflibercept for DME: Results of the 6 Month Prospective Phase 1/2 Hulk trial Blanton Eye Institute Charles C. Wykoff MD PhD
HDWallpapers Suprachoroidal Triamcinolone Acetonide with & without Intravitreal Aflibercept for DME: Results of the 6 Month Prospective Phase 1/2 Hulk trial Blanton Eye Institute Charles C. Wykoff MD PhD
NOVEL TREATMENT STRATEGIES
 Supplement to October 2018 NOVEL TREATMENT STRATEGIES for Macular Edema Associated With Noninfectious Uveitis Thomas Albini, MD, Moderator Diana V. Do, MD Charles C. Wykoff, MD, PhD Steven Yeh, MD A continuing
Supplement to October 2018 NOVEL TREATMENT STRATEGIES for Macular Edema Associated With Noninfectious Uveitis Thomas Albini, MD, Moderator Diana V. Do, MD Charles C. Wykoff, MD, PhD Steven Yeh, MD A continuing
Nguyen et al. Journal of Ophthalmic Inflammation and Infection 2013, 3:32
 Nguyen et al. Journal of Ophthalmic Inflammation and Infection 2013, 3:32 ORIGINAL RESEARCH Open Access Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients
Nguyen et al. Journal of Ophthalmic Inflammation and Infection 2013, 3:32 ORIGINAL RESEARCH Open Access Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients
Humira. (adalimumab) Drug Update Slideshow NEW INDICATION
 Humira (adalimumab) NEW INDICATION Drug Update Slideshow Introduction Brand name: Humira Generic name: Adalimumab Pharmacological class: Tumor necrosis factor (TNF) blocker Strength and Formulation: 10mg/0.2mL,
Humira (adalimumab) NEW INDICATION Drug Update Slideshow Introduction Brand name: Humira Generic name: Adalimumab Pharmacological class: Tumor necrosis factor (TNF) blocker Strength and Formulation: 10mg/0.2mL,
A Guide to Administering
 A Guide to Administering INDICATIONS AND USAGE YUTIQ (fluocinolone acetonide intravitreal implant) 0.18 mg is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment
A Guide to Administering INDICATIONS AND USAGE YUTIQ (fluocinolone acetonide intravitreal implant) 0.18 mg is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment
The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report
 European Review for Medical and Pharmacological Sciences The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report J. CISZEWSKA,
European Review for Medical and Pharmacological Sciences The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report J. CISZEWSKA,
Original Effective Date: 12/13/2017. Subject: Intravitreal corticosteroid implants: Retisert (fluocinolone acetonide intravitreal implant)
 Subject: Intravitreal corticosteroid implants: Retisert (fluocinolone acetonide intravitreal implant) Policy Number: MCP-302 Original Effective Date: 12/13/2017 Revision Date(s): Review Date: DISCLAIMER
Subject: Intravitreal corticosteroid implants: Retisert (fluocinolone acetonide intravitreal implant) Policy Number: MCP-302 Original Effective Date: 12/13/2017 Revision Date(s): Review Date: DISCLAIMER
ILUVIEN IN DIABETIC MACULAR ODEMA
 1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
INTRAVITREAL IMPLANTS
 INTRAVITREAL IMPLANTS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
INTRAVITREAL IMPLANTS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta460
 Adalimumab and dexamethasone for treating non-infectious uveitis Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta460 NICE 2017. All rights reserved. Subject to Notice of rights
Adalimumab and dexamethasone for treating non-infectious uveitis Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta460 NICE 2017. All rights reserved. Subject to Notice of rights
Development of a dexamethasone intravitreal implant for the treatment of noninfectious posterior segment uveitis
 Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Pharmaceutical Science to Improve the Human Condition: Prix Galien 2014 Development of a dexamethasone intravitreal
Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Pharmaceutical Science to Improve the Human Condition: Prix Galien 2014 Development of a dexamethasone intravitreal
Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant
 Ophthalmol Ther (2015) 4:51 58 DOI 10.1007/s40123-015-0028-0 CASE REPORT Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant Thomas Bertelmann
Ophthalmol Ther (2015) 4:51 58 DOI 10.1007/s40123-015-0028-0 CASE REPORT Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant Thomas Bertelmann
New Drugs for Uveitis. Medical Eye Unit St Thomas Hospital
 New Drugs for Uveitis Miles Stanford Medical Eye Unit St Thomas Hospital x Epithelium x x Antigen Y Y Y Y IgG m cd4 IL-2 Y m + IL-12 Cytotoxic T B pmn Ig s PG s. LTB4 O - IL-6 TNFα IFNγγ IL-2 Th1 IL-10
New Drugs for Uveitis Miles Stanford Medical Eye Unit St Thomas Hospital x Epithelium x x Antigen Y Y Y Y IgG m cd4 IL-2 Y m + IL-12 Cytotoxic T B pmn Ig s PG s. LTB4 O - IL-6 TNFα IFNγγ IL-2 Th1 IL-10
Postsurgical Inflammation in MIGS
 A CONTINUING MEDICAL EDUCATION PUBLICATION CME CONTINUING MEDICAL EDUCATION Postsurgical Inflammation in MIGS ISSUE 21 THOMAS W. SAMUELSON, MD Minimally invasive glaucoma surgery (MIGS) provides a relatively
A CONTINUING MEDICAL EDUCATION PUBLICATION CME CONTINUING MEDICAL EDUCATION Postsurgical Inflammation in MIGS ISSUE 21 THOMAS W. SAMUELSON, MD Minimally invasive glaucoma surgery (MIGS) provides a relatively
iologic Agents in the Treatment of Non-Infectious Uveitis
 What is New B iologic Agents in the Treatment of Non-Infectious Uveitis Amala George DNB The treatment of non-infectious uveitis is a challenge to clinicians. The treatment involves suppressing the deleterious
What is New B iologic Agents in the Treatment of Non-Infectious Uveitis Amala George DNB The treatment of non-infectious uveitis is a challenge to clinicians. The treatment involves suppressing the deleterious
An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg
 An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg This guide is intended to provide injectors with information on the recommended injection technique and the important risks related
An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg This guide is intended to provide injectors with information on the recommended injection technique and the important risks related
Serge Bourgault 1,2*, Maryam Aroichane 3, Leah A Wittenberg 1, Andréane Lavallée 1,2 and Patrick E Ma 1
 Bourgault et al. Journal of Ophthalmic Inflammation and Infection 2013, 3:61 BRIEF REPORT Open Access Treatment of refractory uveitic macular edema with dexamethasone intravitreal implants in a pediatric
Bourgault et al. Journal of Ophthalmic Inflammation and Infection 2013, 3:61 BRIEF REPORT Open Access Treatment of refractory uveitic macular edema with dexamethasone intravitreal implants in a pediatric
THE BURDEN OF NONINFECTIOUS UVEITIS OF THE POSTERIOR
 THE BURDEN OF NONINFECTIOUS UVEITIS OF THE POSTERIOR SEGMENT: A REVIEW New pharmacologic treatment options are urgently needed. BY STEVEN YEH, MD, and JESSICA G. SHANTHA, MD Noninfectious uveitis (NIU)
THE BURDEN OF NONINFECTIOUS UVEITIS OF THE POSTERIOR SEGMENT: A REVIEW New pharmacologic treatment options are urgently needed. BY STEVEN YEH, MD, and JESSICA G. SHANTHA, MD Noninfectious uveitis (NIU)
Actemra (tocilizumab) CG-DRUG-81
 Market DC Actemra (tocilizumab) CG-DRUG-81 Override(s) Prior Authorization Approval Duration 1 year Medications Line of Business Quantity Limit Actemra (tocilizumab) vials VA MCD and All L-AGP May be subject
Market DC Actemra (tocilizumab) CG-DRUG-81 Override(s) Prior Authorization Approval Duration 1 year Medications Line of Business Quantity Limit Actemra (tocilizumab) vials VA MCD and All L-AGP May be subject
Optometric Postoperative Cataract Surgery Management
 Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
COVER FOCUS AT A GLANCE. BY LISA J. FAIA, MD, and KIMBERLY A. DRENSER, MD, PhD
 PEDIATRIC UVEITIS: CHALLENGING FOR OPHTHALMOLOGISTS, PATIENTS, AND PARENTS Management of these complicated diseases differs between pediatric and adult patient populations. BY LISA J. FAIA, MD, and KIMBERLY
PEDIATRIC UVEITIS: CHALLENGING FOR OPHTHALMOLOGISTS, PATIENTS, AND PARENTS Management of these complicated diseases differs between pediatric and adult patient populations. BY LISA J. FAIA, MD, and KIMBERLY
Original Effective Date: 10/24/2016. Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant)
 Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant) Original Effective Date: 10/24/2016 Policy Number: MCP-282 Revision Date(s): 12/13/2017 DISCLAIMER This Molina
Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant) Original Effective Date: 10/24/2016 Policy Number: MCP-282 Revision Date(s): 12/13/2017 DISCLAIMER This Molina
Intravitreal Triamcinolone Acetonide for Macular Edema in HLA-B27 Negative Ankylosing Spondylitis
 105 This is an Open Access article licensed under the terms of the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the
105 This is an Open Access article licensed under the terms of the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the
Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study
 Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study Peter A. Campochiaro, MD, 1 Charles C. Wykoff, MD, 2 David M. Brown, MD, 2 David S. Boyer, MD, 3 Mark Barakat,
Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study Peter A. Campochiaro, MD, 1 Charles C. Wykoff, MD, 2 David M. Brown, MD, 2 David S. Boyer, MD, 3 Mark Barakat,
Cataract Surgery in Patients with Uveitis
 Cataract Surgery in Patients with Uveitis Chris Kalogeropoulos MD, PhD, FEBO Professor of Ophthalmology Faculty of Medicine, University of Ioannina President of Hellenic Society for the Study of Ocular
Cataract Surgery in Patients with Uveitis Chris Kalogeropoulos MD, PhD, FEBO Professor of Ophthalmology Faculty of Medicine, University of Ioannina President of Hellenic Society for the Study of Ocular
Surgery in patients with uveitis. Lyndell Lim and Anthony Hall
 Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 19 September 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 September 2012 OZURDEX 700 micrograms, intravitreal implant in applicator Box of 1 sachet with applicator (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 September 2012 OZURDEX 700 micrograms, intravitreal implant in applicator Box of 1 sachet with applicator (CIP:
Uveitis literature 2014: the year in review. Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA
 Uveitis literature 2014: the year in review Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA Disclosures RVG serves as Associate Editor of IOVS Editorial
Uveitis literature 2014: the year in review Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA Disclosures RVG serves as Associate Editor of IOVS Editorial
Uveitis. What is Uveitis?
 Uveitis What is Uveitis? Uveitis [u-vee-i-tis] is a term for inflammation of the eye. It can occur in one eye or both eyes and affects the layer of the eye called the uvea [u-vee-uh]. It also can be associated
Uveitis What is Uveitis? Uveitis [u-vee-i-tis] is a term for inflammation of the eye. It can occur in one eye or both eyes and affects the layer of the eye called the uvea [u-vee-uh]. It also can be associated
A comprehensive review of mtor-inhibiting pharmacotherapy for the treatment of noninfectious
 A comprehensive review of mtor-inhibiting pharmacotherapy for the treatment of noninfectious uveitis Blair, J; Barry, Robert; Moore, David; Denniston, Alastair DOI: 10.2174/1381612823666170111125550 Document
A comprehensive review of mtor-inhibiting pharmacotherapy for the treatment of noninfectious uveitis Blair, J; Barry, Robert; Moore, David; Denniston, Alastair DOI: 10.2174/1381612823666170111125550 Document
Learn Connect Succeed. JCAHPO Regional Meetings 2017
 Learn Connect Succeed JCAHPO Regional Meetings 2017 Intravitreal Injection Technique Updated Recommendations from an Expert Panel Sophie J. Bakri, M.D. Professor of Ophthalmology Vitreoretinal Diseases
Learn Connect Succeed JCAHPO Regional Meetings 2017 Intravitreal Injection Technique Updated Recommendations from an Expert Panel Sophie J. Bakri, M.D. Professor of Ophthalmology Vitreoretinal Diseases
Applications of Sustained Release Delivery Systems in Ocular Disease
 Applications of Sustained Release Delivery Systems in Ocular Disease Formulation and Delivery Systems For Peptide and Protein 2012 December 5, 2012 Christopher A. Rhodes, Ph.D. Principal, Christopher A
Applications of Sustained Release Delivery Systems in Ocular Disease Formulation and Delivery Systems For Peptide and Protein 2012 December 5, 2012 Christopher A. Rhodes, Ph.D. Principal, Christopher A
What prescribers need to know
 HUMIRA Citrate-free presentations in an Electronic Medical Record (EMR) What prescribers need to know 2 / This is your guide to identifying HUMIRA Citrate-free presentations in your Electronic Medical
HUMIRA Citrate-free presentations in an Electronic Medical Record (EMR) What prescribers need to know 2 / This is your guide to identifying HUMIRA Citrate-free presentations in your Electronic Medical
Advances in drug delivery to the posterior segment
 Advances in drug delivery to the posterior segment William Pearce, Emory University Jason Hsu, Retina Service of Wills Eye Hospital Steven Yeh, Emory University Journal Title: Current Opinion in Ophthalmology
Advances in drug delivery to the posterior segment William Pearce, Emory University Jason Hsu, Retina Service of Wills Eye Hospital Steven Yeh, Emory University Journal Title: Current Opinion in Ophthalmology
Why Is Imaging Critical in My Uveitis Practice?
 Why Is Imaging Critical in My Uveitis Practice? Dilraj S. Grewal, MD Developed in collaboration Imaging Is the Backbone of Uveitis Workup and Monitoring Treatment Response FP FAF B- scan Multimodal Imaging
Why Is Imaging Critical in My Uveitis Practice? Dilraj S. Grewal, MD Developed in collaboration Imaging Is the Backbone of Uveitis Workup and Monitoring Treatment Response FP FAF B- scan Multimodal Imaging
Design: Retrospective, multi-center, interventional case study.
 Abstract (MUST be submitted as a separate file) Purpose: To evaluate outcomes in birdshot chorioretinopathy following intravitreal implantation of a fluocinolone acetonide containing drug delivery device.
Abstract (MUST be submitted as a separate file) Purpose: To evaluate outcomes in birdshot chorioretinopathy following intravitreal implantation of a fluocinolone acetonide containing drug delivery device.
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO
 EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
FLUOCINOLONE ACETONIDE: STEROID LONG ACTING
 FLUOCINOLONE ACETONIDE: STEROID LONG ACTING Giuseppe Querques, MD PhD Department of Ophthalmology, IRCCS Ospedale San Raffaele, University Vita Salute San Raffaele, Milan, Italy Financial Disclosure ADVISORY
FLUOCINOLONE ACETONIDE: STEROID LONG ACTING Giuseppe Querques, MD PhD Department of Ophthalmology, IRCCS Ospedale San Raffaele, University Vita Salute San Raffaele, Milan, Italy Financial Disclosure ADVISORY
VMA at the macula resulting in VMT
 Ocriplasmina for pharmacologic treatment in VMT Teresio Avitabile 1 Introduction PVD is a normal, physiologic process that occurs with aging; however, in some cases, PVD is incomplete Incomplete PVD localized
Ocriplasmina for pharmacologic treatment in VMT Teresio Avitabile 1 Introduction PVD is a normal, physiologic process that occurs with aging; however, in some cases, PVD is incomplete Incomplete PVD localized
Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
 European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
Study Number CAIN457C2302 (core study) and CAIN457C2302E1 (extension study)
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient
 CM&R Rapid Release. Published online ahead of print September 20, 2012 as Aperture Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient Elizabeth
CM&R Rapid Release. Published online ahead of print September 20, 2012 as Aperture Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient Elizabeth
Investor Presentation October 2018
 Delivering Innovative Ophthalmic Products to Patients with Serious Eye Disorders Investor Presentation October 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION
Delivering Innovative Ophthalmic Products to Patients with Serious Eye Disorders Investor Presentation October 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION
ORIGINAL RESEARCH ARTICLE
 EJO ISSN 1120-6721 Eur J Ophthalmol 2017; 27 (3): 357-362 DOI: 10.5301/ejo.5000929 ORIGINAL RESEARCH ARTICLE Diabetic macular edema outcomes in eyes treated with fluocinolone acetonide 0.2 µg/d intravitreal
EJO ISSN 1120-6721 Eur J Ophthalmol 2017; 27 (3): 357-362 DOI: 10.5301/ejo.5000929 ORIGINAL RESEARCH ARTICLE Diabetic macular edema outcomes in eyes treated with fluocinolone acetonide 0.2 µg/d intravitreal
84 Year Old with Rosacea
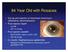 84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT OZURDEX 700 micrograms intravitreal implant in applicator 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One implant contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT OZURDEX 700 micrograms intravitreal implant in applicator 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One implant contains
ABSTRACT INTRODUCTION
 DOI 10.1007/s40123-017-0114-6 ORIGINAL RESEARCH Real-World Cost Savings Demonstrated by Switching Patients with Refractory Diabetic Macular Edema to Intravitreal Fluocinolone Acetonide (Iluvien): A Retrospective
DOI 10.1007/s40123-017-0114-6 ORIGINAL RESEARCH Real-World Cost Savings Demonstrated by Switching Patients with Refractory Diabetic Macular Edema to Intravitreal Fluocinolone Acetonide (Iluvien): A Retrospective
H.P. Acthar Gel (repository corticotropin) Prior Authorization Criteria Program Summary
 OBJECTIVE The intent of the H.P. Acthar Gel (repository corticotropin) Prior Authorization (PA) Criteria is to appropriately select patients for therapy according to product labeling and/or clinical studies
OBJECTIVE The intent of the H.P. Acthar Gel (repository corticotropin) Prior Authorization (PA) Criteria is to appropriately select patients for therapy according to product labeling and/or clinical studies
PDF of Trial CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Sat, 09 Mar 2019 11:07:43 GMT) CTRI Number Last Modified On 08/08/2018 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Sat, 09 Mar 2019 11:07:43 GMT) CTRI Number Last Modified On 08/08/2018 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
THE MULTICENTER UVEITIS STEROID TREATMENT (MUST) TRIAL: A Closer Look at the 7-Year Findings
 THE MULTICENTER UVEITIS STEROID TREATMENT (MUST) TRIAL: A Closer Look at the 7-Year Findings This supplement captures content from a roundtable discussion held in August 2017 at the American Society of
THE MULTICENTER UVEITIS STEROID TREATMENT (MUST) TRIAL: A Closer Look at the 7-Year Findings This supplement captures content from a roundtable discussion held in August 2017 at the American Society of
Intravitreal Corticosteroids in the Management of Diabetic Macular Edema
 Curr Ophthalmol Rep (2013) 1:144 149 DOI 10.1007/s40135-013-0015-3 DIABETIC RETINOPATHY: MEDICAL AND SURGICAL THERAPIES (PK KAISER, SECTION EDITOR) Intravitreal Corticosteroids in the Management of Diabetic
Curr Ophthalmol Rep (2013) 1:144 149 DOI 10.1007/s40135-013-0015-3 DIABETIC RETINOPATHY: MEDICAL AND SURGICAL THERAPIES (PK KAISER, SECTION EDITOR) Intravitreal Corticosteroids in the Management of Diabetic
Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema
 Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema V Swetha E Jeganathan 1,2 * and Karen Madill 3 1 Department of Ophthalmology,
Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema V Swetha E Jeganathan 1,2 * and Karen Madill 3 1 Department of Ophthalmology,
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd
 nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
WAEPS Medical Personnel Conference March 20, Deanne M. Nakamoto, MD Achieve Eye and Laser Specialists Silverdale, WA
 WAEPS Medical Personnel Conference March 20, 2015 Deanne M. Nakamoto, MD Achieve Eye and Laser Specialists Silverdale, WA Up to half of UVEITIS pa/ents have GLAUCOMA. What is uvei/s? Why should we care?
WAEPS Medical Personnel Conference March 20, 2015 Deanne M. Nakamoto, MD Achieve Eye and Laser Specialists Silverdale, WA Up to half of UVEITIS pa/ents have GLAUCOMA. What is uvei/s? Why should we care?
Local and systemic corticosteroids constitute first-line
 Future Therapies for Chronic Noninfectious Uveitis New drugs and innovative drug delivery systems will pave the way for future treatments in uveitis. By Emmett T. Cunningham, Jr, MD, PhD, MPH Local and
Future Therapies for Chronic Noninfectious Uveitis New drugs and innovative drug delivery systems will pave the way for future treatments in uveitis. By Emmett T. Cunningham, Jr, MD, PhD, MPH Local and
EFFICACY OF INTRAVITREAL TRIAMCINOLONE ACETONIDE FOR THE TREATMENT OF DIABETIC MACULAR EDEMA
 Basrah Journal Of Surgery EFFICACY OF INTRAVITREAL TRIAMCINOLONE ACETONIDE FOR THE TREATMENT OF DIABETIC MACULAR EDEMA Salah Zuhair Abed Al-Asadi MB,ChB, FICMS, Lecturer, Department of Surgery, College
Basrah Journal Of Surgery EFFICACY OF INTRAVITREAL TRIAMCINOLONE ACETONIDE FOR THE TREATMENT OF DIABETIC MACULAR EDEMA Salah Zuhair Abed Al-Asadi MB,ChB, FICMS, Lecturer, Department of Surgery, College
Management of uveitis
 Management of uveitis DR. ANUPAMA KARANTH Anti-inflammatory agents -itis = inflammation Treatment : stop inflammation Use anti-inflammatory drugs Most potent of such agents : Corticosteroids Corticosteroids
Management of uveitis DR. ANUPAMA KARANTH Anti-inflammatory agents -itis = inflammation Treatment : stop inflammation Use anti-inflammatory drugs Most potent of such agents : Corticosteroids Corticosteroids
You can C-ME after Uveitis
 You can C-ME after Uveitis Abstract: Approximately 50% of uveitis patients will present with vision loss secondary to cystoid macular edema[1]. Two patients with uveitis present with a constant decrease
You can C-ME after Uveitis Abstract: Approximately 50% of uveitis patients will present with vision loss secondary to cystoid macular edema[1]. Two patients with uveitis present with a constant decrease
Moncef Khairallah, MD
 Moncef Khairallah, MD Department of Ophthalmology, Fattouma Bourguiba University Hospital Faculty of Medicine, University of Monastir Monastir, Tunisia INTRODUCTION IU: anatomic form of uveitis involving
Moncef Khairallah, MD Department of Ophthalmology, Fattouma Bourguiba University Hospital Faculty of Medicine, University of Monastir Monastir, Tunisia INTRODUCTION IU: anatomic form of uveitis involving
New Treatments Following Cataract Surgery October 23, 2018
 Delivering Innovative Ophthalmic Products to Patients with Serious Eye Disorders New Treatments Following Cataract Surgery October 23, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE
Delivering Innovative Ophthalmic Products to Patients with Serious Eye Disorders New Treatments Following Cataract Surgery October 23, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE
Applying New Data to Improve the Standard of Care in Retinal Disease. With articles by Gaurav K. Shah, MD Carl D. Regillo, MD.
 Supplement to July/August 2012 CME Activity Applying New Data to Improve the Standard of Care in Retinal Disease With articles by Gaurav K. Shah, MD Carl D. Regillo, MD Release date: August 2012. Expiration
Supplement to July/August 2012 CME Activity Applying New Data to Improve the Standard of Care in Retinal Disease With articles by Gaurav K. Shah, MD Carl D. Regillo, MD Release date: August 2012. Expiration
Darren J. Bell, M.D. Texas A&M University College of Medicine College Station, Texas Doctor of Medicine
 Darren J. Bell, M.D. Education Texas A&M University College of Medicine College Station, Texas Doctor of Medicine 1997-2001 Texas A&M University College Station, Texas Bachelor of Science, Biomedical Engineering
Darren J. Bell, M.D. Education Texas A&M University College of Medicine College Station, Texas Doctor of Medicine 1997-2001 Texas A&M University College Station, Texas Bachelor of Science, Biomedical Engineering
ACTEMRA (tocilizumab)
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Actemra is an agent in the class of drugs known as biologic disease modifiers. It is used to treat adult onset rheumatoid (RA) arthritis, polyarticular
RATIONALE FOR INCLUSION IN PA PROGRAM Background Actemra is an agent in the class of drugs known as biologic disease modifiers. It is used to treat adult onset rheumatoid (RA) arthritis, polyarticular
Successful Management of Uveitis in a Patient With Unilateral Multifocal Choroiditis
 Successful Management of Uveitis in a Patient With Unilateral Multifocal Choroiditis SPONSORED BY RETISERT PATIENT CASE STUDY SERIES Multifocal choroiditis is a panuveitis that predominantly affects women
Successful Management of Uveitis in a Patient With Unilateral Multifocal Choroiditis SPONSORED BY RETISERT PATIENT CASE STUDY SERIES Multifocal choroiditis is a panuveitis that predominantly affects women
Package leaflet: information for the user. OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone
 Package leaflet: information for the user OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: information for the user OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone Read all of this leaflet carefully before you are given this medicine because it contains
Sclerokeratoplasty David S. Chu, M.D. Cases
 Sclerokeratoplasty David S. Chu, M.D. Cases Case 1 40 year-old female from Peru presented to our Service with inflamed OS for 2 months duration. Her symptoms began as red painful OS, which progressively
Sclerokeratoplasty David S. Chu, M.D. Cases Case 1 40 year-old female from Peru presented to our Service with inflamed OS for 2 months duration. Her symptoms began as red painful OS, which progressively
ACTEMRA (tocilizumab)
 ACTEMRA (tocilizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
ACTEMRA (tocilizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Seong Joon Ahn *, Jooyoung Joung, Sang Hyup Lee and Byung Ro Lee *
 Ahn et al. BMC Ophthalmology (2018) 18:310 https://doi.org/10.1186/s12886-018-0985-x CASE REPORT Open Access Intravitreal dexamethasone implant therapy for the treatment of cystoid macular Oedema due to
Ahn et al. BMC Ophthalmology (2018) 18:310 https://doi.org/10.1186/s12886-018-0985-x CASE REPORT Open Access Intravitreal dexamethasone implant therapy for the treatment of cystoid macular Oedema due to
Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema
 Original Research Article Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema Neha Kantilal Desai 1,*, Somesh Vedprakash Aggarwal 2, Sonali
Original Research Article Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema Neha Kantilal Desai 1,*, Somesh Vedprakash Aggarwal 2, Sonali
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project
 The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
of Systemic Immunosuppression for Autoimmune Uveitis.
 Review of Systemic Immunosuppression for Autoimmune Uveitis The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Castiblanco,
Review of Systemic Immunosuppression for Autoimmune Uveitis The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Castiblanco,
Combination Treatment of Diabetic Macular Edema with Anti-Vascular Endothelial Growth Factor and Steroids: Analysis of DRCR.
 REVIEW ARTICLE Combination Treatment of Diabetic Macular Edema with Anti-Vascular Endothelial Growth Factor and Steroids: Analysis of DRCR.net Protocol U Cindy Ung 1, Kareem Moussa 1, Yoshihiro Yonekawa
REVIEW ARTICLE Combination Treatment of Diabetic Macular Edema with Anti-Vascular Endothelial Growth Factor and Steroids: Analysis of DRCR.net Protocol U Cindy Ung 1, Kareem Moussa 1, Yoshihiro Yonekawa
