Fluocinolone Implant for Idiopathic Non-Infectious Posterior Uveitis
|
|
|
- Berenice Edwards
- 5 years ago
- Views:
Transcription
1 RP440_Berger-16.qxd:Poly 4/13/12 11:46 AM Page 1 May 2012 Fluocinolone Implant for Idiopathic Non-Infectious Posterior Uveitis PRESENTED BY
2 Fluocinolone Implant for Idiopathic Non-Infectious Posterior Uveitis For decades, corticosteroids have been the standardof-care, first-line therapy for non-infectious posterior uveitis. In most cases, they are administered by periocular or intravitreal injection or orally. Only one injectable corticosteroid is FDA approved for the treatment of uveitis (triamcinolone acetonide injectable suspension, Triesence, Alcon), but triamcinolone and other corticosteroids prepared extemporaneously for injection are routinely used for this purpose.* Numerous small studies and reviews of the literature have shown that steroid injections reduce ocular inflammation in patients with uveitis. 1-5 However, physicians must be cognizant of their usually short-term duration of action 11, 12 and the potential for drug- and injection-related side effects such as increased IOP, cataract formation, retinal 1, 3-5 detachment, endophthalmitis and vitreous hemorrhage. Steroids are the only oral therapy FDA approved for the treatment of noninfectious uveitis. They can reduce ocular inflammation and are used for patients in whom ocular steroid injections do not provide adequate control of posterior segment inflammation. Long-term use of oral corticosteroids, especially when high doses are required, may not be ideal 6 because of the potential for the development of cataract and/or increased IOP and serious systemic side effects, including high blood pressure, cardiovascular disease, excessive weight gain, psychiatric disorders, adrenal insufficiency, osteoporosis, osteonecrosis, increased risk of infection, myopathy, hyperglycemia and gastrointestinal problems When steroid treatment does not adequately control ocular inflammation or cannot be tolerated by the patient, other immunomodulatory medications are sometimes used. Antimetabolites, T-cell inhibitors, alkylating agents and some drugs in the newer biologics class, e.g., TNFalpha inhibitors, can provide effective ocular inflammation control but have also been shown to have the potential for severe systemic side effects None of these agents has been evaluated for treating ocular inflammation in randomized, controlled trials or approved by the FDA for the treatment of uveitis; most are intended for neurologic, Copyright 2012, Springer Science + Business Media. All Rights Reserved. rheumatologic or dermatologic conditions. Sustained-release ocular drug-delivery devices have been developed to control ocular inflammation and decrease the need for systemic therapy and its subsequent side effects. Two have been approved by the FDA for the treatment of non-infectious posterior uveitis. The first to gain approval, Retisert (fluocinolone acetonide intravitreal implant 0.59 mg, Bausch + Lomb), is surgically implanted into the vitreous and designed to release fluocinolone acetonide for approximately 2.5 years. Retisert is a corticosteroid indicated for the treatment of chronic noninfectious uveitis affecting the posterior segment of the eye. (Please see Important Risk Information for Retisert on page 3.) FDA approval of Retisert was based on 34-week results from two multicenter, double-masked, randomized, controlled clinical trials evaluating the safety and efficacy of the implant. 23 Subsequently, 3-year follow-up data were published. 24 The primary efficacy endpoint in the trials was the rate of recurrence of uveitis in the study eye in the 34 weeks prior to implantation compared with the rate of recurrence 34 weeks, 1 year, 2 years and 3 years after implantation. Retisert significantly reduced recurrence rates. 25 The second sustained-delivery device to be FDA approved for treating non-infectious posterior uveitis, Ozurdex (dexamethasone 0.7 mg intravitreal implant, Allergan), is injected into the vitreous using a specially designed applicator. Once in the vitreous, the implant slowly releases dexamethasone and biodegrades into lactic acid and glycolic acid. Here, I describe a case from my practice that illustrates how Retisert can fit into the treatment armamentarium for patients with non-infectious posterior uveitis and help to achieve treatment goals. RETISERT CASE STUDY A 60-year-old male, first presented to my office in September He had been seeing a retinal specialist in another city, who had diagnosed him with non-infectious Please see Important Risk Information for Retisert on page 3. *This product is not FDA-approved for this purpose. 2 RETINAL PHYSICIAN MAY 2012
3 BY BRIAN B. BERGER, MD posterior uveitis in the right eye. The uveitis could not be attributed to any specific ocular or systemic cause. Many 26, 27 cases of noninfectious uveitis are idiopathic. The patient had recently retired to Austin, Texas, where I practice, and was referred to me for continuing care. While under the care of the first retinal specialist, the patient had been given two periocular injections of triamcinolone, one in November 2001 and one in February The resulting improvement in ocular inflammation was short-lived following each injection. My first examination of the patient confirmed the previous physician s diagnosis. I did not see any sign of an infectious etiology, and the uveitis did not fit any welldefined type of the disease. It most closely resembled a syndrome that J. Donald Gass, MD, described as idiopathic vitritis. 28 The patient s cornea and anterior chamber were healthy, but he had a 3-year history of floaters, hazy vision and distortion OD. His visual acuity in the right eye was 20/80, and a posterior subcapsular cataract was present. Fluorescein angiography showed leakage from the disc and macula, suggesting low-grade inflammation. I noted 1+ haze in the vitreous, an epiretinal membrane in the macula and CME. At that time, OCT was not clinically available, so the macular thickening was not quantified. Based on the findings, four factors were contributing to the patient s decreased vision: uveitis, cataract, epiretinal membrane and CME. The patient was offered combined surgery as an option to treat both uveitis and the epiretinal membrane by both his previous retinal specialist and me. The patient agreed to proceed with the procedure and surgery was scheduled for December The patient was pre-treated with a posterior sub-tenon s injection of triamcinolone in an attempt to quiet the ocular inflammation prior to surgery. At the time of surgery, an anterior segment surgeon removed the cataract and implanted an IOL. Immediately after, I performed a vitrectomy, removed the epiretinal membrane and injected intravitreal triamcinolone, a commonly used strategy for controlling postoperative inflammation following cataract extraction in patients with uveitis. 29 Findings and next steps from key follow-up visits were: Four weeks after surgery, no inflammation was present, and visual acuity had improved to 20/40. The patient was somewhat pleased, but also disappointed that his vision did not improve to 20/20 in the operated eye. March 18, 2003: OCT, which by this time was in clinical use, showed that CME returned with a macular thickness of 377 µm. A fourth posterior sub-tenon s triamcinolone injection was given. June 5, 2003: Macular edema increased to 420 µm, and vision decreased from the last visit to 20/60. An intravitreal injection of triamcinolone was given. At a subsequent visit, visual acuity returned to 20/40. In an attempt to maintain visual acuity at that level, topical steroid and topical non-steroidal anti-inflammatory drops were prescribed. At this point, the clinical course of the Important Risk Information for Retisert Surgical placement of RETISERT (fluocinolone acetonide intravitreal implant 0.59 mg, Bausch + Lomb) is contraindicated in active viral, bacterial, mycobacterial or fungal infections of the eye. Based on clinical trials with RETISERT, during the 3-year post-implantation period, nearly all phakic eyes are expected to develop cataracts and require cataract surgery. As with any surgical procedure, there is risk involved. Potential complications accompanying intraocular surgery to place RETISERT into the vitreous cavity may include, but are not limited to, the following: cataract formation, choroidal detachment, temporary decreased visual acuity, endophthalmitis, hypotony, increased intraocular pressure, exacerbation of intraocular inflammation, retinal detachment, vitreous hemorrhage, vitreous loss, wound complication, wound site erythema and wound dehiscence. Following implantation of RETISERT, nearly all patients will experience an immediate and temporary decrease in visual acuity in the implanted eye which lasts for approximately one to four weeks post-operatively. Use of corticosteroids may result in elevated IOP and/or glaucoma. Based on clinical trials with RETISERT, within 3 years postimplantation, approximately 77% of patients will require IOP lowering medications to control intraocular pressure and 37% of patients will require filtering procedures to control intraocular pressure. Patients should be advised to have ophthalmologic follow-up examinations of both eyes at appropriate intervals following implantation of RETISERT. Physicians should periodically monitor the integrity of the implant by visual inspection. The most frequently reported ocular adverse events in clinical trials with RETISERT occurring in 50-90% of patients included: cataract, increased intraocular pressure, procedural complications and eye pain. Thirty five to forty percent (35-40%) of patients reported ocular/conjunctival hyperemia, reduced visual acuity and conjunctival hemorrhage. The most common non-ocular event reported was headache (>33%). Please see complete information about RETISERT in the accompanying full prescribing information on pages 7 and 8. RETINAL PHYSICIAN MAY
4 RP440_Berger-16.qxd:Poly 4/13/12 11:46 AM Page 4 Figure 1. Despite treatment with posterior sub-tenon s and intravitreal injections of triamcinolone, the right eye developed subretinal fluid and cystic macular thickening. (Oct. 26, 2004) patient s condition was similar to what is seen in IrvineGass syndrome, i.e., CME following cataract surgery, which is typically treated with these topical agents. Many of the available topical steroid and NSAID drops are specifically indicated for the reduction of ocular inflammation following cataract surgery, and it is generally accepted that their effectiveness against CME can be synergistic. With use of the drops, the patient s visual acuity was maintained at 20/40 for more than a year. I Oct. 26, 2004: By the time of this visit, the patient s ocular inflammation had recurred. OCT showed macular thickening to 428 µm as well as the development of subretinal fluid (Figure 1). Vision declined to 20/60. A fifth triamcinolone injection was given (sub-tenon s), and oral acetazolamide was prescribed. Systemic carbonic anhydrase inhibitors, used off-label, have been shown to be of some benefit for reducing subretinal fluid.30 I Dec. 23, 2004: Fluorescein angiography showed early and diffuse cystic leakage in the macula and disc (Figures 2a and 2b). A sixth sub-tenon s triamcinolone injection was given. For the next 9 months, vision was stable at 20/40. I Sept. 20, Visual acuity was 20/40, but OCT showed recurrent serous detachment of the macula and macular thickness of 324 µm. By this point in the patient s treatment, it was clear his condition was chronic. The Standardization of Uveitis Nomenclature (SUN) Working Group defined chronic uveitis as persistent and characterized by prompt relapse (less than 3 months) after discontinuation of therapy.31 None of the treatments that we tried were providing the desired continuous control of the ocular inflammation. We had not been able to improve his visual acuity to better than 20/40 and sometimes it would drop below that level. I preferred not to initiate systemic steroid or other immunomodulatory therapy, and risk the potential side effects, because the inflammation in this particular case was only in one eye, and the patient did not have an underlying condition that required either of those therapies. Also, approximately 5 months prior to his September 2005 visit, the FDA had approved the Retisert implant, giving us an alternative treatment to consider. I participated in the Retisert clinical trials and had seen some good results in the patients I treated. The patient was a good candidate for Retisert because his non-infectious posterior uveitis was chronic, and: I ocular disease, rather than a systemic condition, was driving treatment decisions Figures 2a and 2b. Prior to implantation of Retisert, fluorescein angiogram of the right eye showing early leakage at 53 seconds and diffuse cystic leakage in the macula and disc at 7 minutes 26 seconds. (Dec. 23, 2004) Please see Important Risk Information for Retisert on page 3. Retisert is a registered trademark of Bausch & Lomb Incorporated. All other brand/product names are trademarks of their respective owners. 4 R E T I N A L P H YS I C I A N M AY
5 Figures 3a and 3b. In 2009, following Retisert implantation in 2005, fluorescein angiography showed no leakage at 1 minute, 38 seconds or at 7 minutes, 26 seconds. periocular and intravitreal steroid injections had improved his condition for short periods, but continuous control of ocular inflammation had not yet been achieved periocular and intravitreal steroid injections had not increased his IOP he had no glaucomatous nerve damage. Weighing these factors against the potential risks of the Retisert implantation procedure and the known side effects of sustained-release ocular steroids, including cataract (the patient had already undergone cataract removal OD) and increased IOP, (See Important Risk Information for Retisert, on page 3), I recommended Retisert. 32 After discussing the potential benefits and risks with the patient, he elected to proceed. I implanted a Retisert on Nov. 2, Given that the eye had previously undergone a vitrectomy, I placed a transconjunctival 25-gauge infusion cannula during surgery to help maintain a safe pressure. Per my usual Uveitis Recurrence Rates Time Point Trial 1 Trial 2 (N=108) (N=116) 34 wks before implant 54% 40% 34 wks after implant 2% 13% 1 yr after implant 4% 13% 2 yrs after implant 10% 14% 3 yrs after implant 20% 17% 3 yrs after implant* 31% 24% (*imputed recurrences included) * Recurrences were imputed when a patient was not seen within 10 weeks of final scheduled visit. p-value<0.01 from McNemar s Chi squared test protocol, the patient returned for follow-up every 2-3 months for the first year post-implantation. Since the eye remained quiet, I extended the time between followup visits to every 3-4 months during the second year. After implantation, the patient experienced gradual and steady improvement in visual acuity. He did not experience elevated IOP and did not require any treatment other than Retisert for the uveitis. Visual acuity was 20/20 and macular thickness was normal at 203 through 2009 (Figures 3a and 3b). In the clinical trials which eventually led to FDA approval, two versions of the implant, 0.59-mg and 2.1-mg, were evaluated. Only efficacy data on the 0.59-mg implant were considered, and approval was based on 34-week results. 4 The primary efficacy endpoint in the studies was the rate of recurrence of uveitis in the study eye in the 34 weeks prior to implantation compared with the rate of recurrence 34 weeks, 1 year, 2 years and 3 years after implantation. In two randomized, double-masked, multicenter, controlled clinical trials, 224 patients received a 0.59-mg Retisert implant, which significantly reduced recurrence rates. (See Uveitis Recurrence Rates chart for specific results.) Additional secondary endpoints of the 3-year clinical trials showed that 23% of eyes that received the 0.59-mg implant had improved visual acuity (gain of >_ 3 lines) compared with 6% of fellow non-implanted eyes. 24 In addition, at 1 year after implantation in the 0.59-mg group, reduction in the area of CME was seen in 86% of eyes. At 3 years after implantation, the area of CME was decreased in 73% of eyes. Mean area of CME decreased from 33 mm 2 to 7 mm 2 from screening to 34 weeks after Please see Important Risk Information for Retisert on page 3. RETINAL PHYSICIAN MAY
6 implantation. The area of CME remained statistically significantly lower than baseline at the 1-, 2- and 3-year post-implantation visits. I typically do not remove an initial Retisert implant when the drug is depleted; I do however see these patients every 6 or 12 months to ensure the implant is not causing any problems. The patient described here is now 69 years old and seeing an eye doctor every 6 to 12 months is recommended. Although this patient did not experience any problems related to wound closure after Retisert implantation, I have since changed my Retisert suture strategy to bolster prevention of such problems over the long-term. I still use the recommended 8-0 propylene suture to secure the implant to the sclera, but before placing the recommended single 9-0 propylene suture on each side, I now often use 7-0 Vicryl absorbable sutures to tightly close the incision. This creates tighter wound closure without the risks of extra permanent sutures, such as conjunctival erosion, exposure or infection. The more recently approved sustained-release steroid implant Ozurdex may also alter my treatment protocol in certain cases. For example, Ozurdex, which is designed to release steroid for less time than Retisert, may sufficiently treat non-infectious posterior uveitis characterized by mild inflammation (but resistant to steroid injections) without exposing the eye to several years of drug. FORWARD PROGRESS In my opinion, the case I have presented here is an excellent illustration of how Retisert can be a useful addition to our treatment options for non-infectious posterior uveitis. The implant allowed us to achieve long-term remission of ocular inflammation for this patient without the use of any systemic therapy or ongoing need for local therapy. Dr. Berger is founder of the Retina Research Center, LLPC. He is active in ophthalmic research and has written several textbook chapters and published more than two dozen articles. He also teaches ophthalmology students at several universities in Texas. He can be reached at bberger@e-retina.net. REFERENCES 1. Yoshikawa K, Kotake S, Ichiishi A, et al. Posterior sub-tenon injections of repository corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol. 1995;39(1): Ozkiris A. Intravitreal triamcinolone acetonide injection for the treatment of posterior uveitis. Ocul Immunol Inflamm. 2006;14(4): Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: the past, the present, and the future. Surv Ophthalmol. 2008;53(2): Hogewind BF, Zijlstra C, Klevering BJ, Hoyng CB. Intravitreal triamcinolone for the treatment of refractory macular edema in idiopathic intermediate or posterior uveitis. Eur J Ophthalmol. 2008;18(3): Jonas JB, Kreissig I, Kamppeter B, Degenring RF. Intravitreal triamcinolone acetonide for the treatment of intraocular edematous and neovascular diseases. Ophthalmologe. 2004;101(2): Lee FF, Foster CS. Pharmacotherapy of uveitis. Expert Opin Pharmacother. 2010;11(7): Stanbury RM, Graham EM. Systemic corticosteroid therapy side effects and their management. Br J Ophthalmol. 1998;82(6): Treadwell BL, Salvage O, Sever ED, Copeman WS. Pituitary-adrenal function during corticosteroid therapy. Lancet. 1963;1(7277): Curtiss PH Jr, Clark WS, Herndon CH. Vertebral fractures resulting from prolonged cortisone and corticotropin therapy. J Am Med Assoc. 1954;156(5): Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med. 2010;123(10): McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20(2): Humes DJ, Fleming KM, Spiller RC, West J. Concurrent drug use and the risk of perforated colonic diverticular disease: a population-based case-control study. Gut. 2011;60(2): Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141(10): de Leeuw PW. Drug-induced hypertension. Recognition and management in older patients. Drugs Aging. 1997;11(3): El Afrit MA, Mazlout H, Trojet S, et al. Cortisone glaucoma: epidemiological, clinical, and therapeutic study. J Fr Ophtalmol. 2007;30(1): Garbe E, LeLorier J, Boivin JF, Suissa S. Risk of ocular hypertension or open-angle glaucoma in elderly patients on oral glucocorticoids. Lancet. 1997;350(9083): Cervantes-Castaneda RA, Bhat P, Fortuna E, Acevedo S, Foster CS. Induction of durable remission in ocular inflammatory diseases. Eur J Ophthalmol. 2009;19(1): Yeh S, Nussenblatt RB, Levy-Clarke GA. Emerging biologics in the treatment of uveitis. Expert Rev Clin Immunol. 2007;3(5): Plskova J, Greiner K, Forrester JV. Interferon-alpha as an effective treatment for noninfectious posterior uveitis and panuveitis. Am J Ophthalmol. 2007;144(1): Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4): Kempen JH, Gangaputra S, Daniel E, Levy-Clarke GA, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol. 2008;146(6): Goldstein DA. Uncovering the risks of immunosuppressive therapy in patients with uveitis. Arch Ophthalmol. 2009;127: Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T; Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirtyfour-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113(6): Callanan DG, Jaffe GJ, Martin DF, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9): Retisert full prescribing information; March Touch Briefings [Internet]. London: Business Briefings, Ltd.; c2011. U. S. Ophthalmic Review 2007/Uveitis-current standard of care: a report by Stephen Foster, MD, FACS, FACR, FAAO; [cited 2011 Mar 22]. Available at: touchbriefings.com/cdps/cditem.cfm?nid=2946&cid=5#uveitis. 27. The Merck Manuals Online Medical Library for Healthcare Professionals; c Eye disorders: uveitis. Available at: merckmanuals.com/professional/sec09/ch105/ch105a.html. 28. Brinton GS, Osher RH, Gass JD. Idiopathic vitritis. Retina. 1983;3(2): Alkawas AA, Hamdy AM, Shahien EA. Intraoperative intravitreal injection of triamcinolone acetonide for cataract extraction in patients with uveitis. Ocul Immunol Inflamm. 2010;18(5): Lashay AR, RahimiA, Chams H, et al. Evaluation of effect of acetazolamide on cystoid macular oedema in patients with Behcet's disease. Eye. 2003;17: Jabs DA, Nussenblatt RB, Rosenbaum JT and the Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3): Goldstein DA, Godfrey DG, Hall A, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol. 2007;125(11): Please see Important Risk Information for Retisert on page 3. Retisert is a registered trademark of Bausch & Lomb Incorporated. All other brand/product names are trademarks of their respective owners. 6 RETINAL PHYSICIAN MAY 2012
7
8 PH3810 Rev 5/11
Abbreviated Drug Evaluation: Fluocinolone acetonide intravitreal implant (Retisert )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
What you can expect with OZURDEX
 Important Information About Noninfectious Uveitis Affecting the Back Segment of the Eye and Treatment What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone intravitreal implant) is a prescription
Important Information About Noninfectious Uveitis Affecting the Back Segment of the Eye and Treatment What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone intravitreal implant) is a prescription
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Intravitreal Corticosteroid Implants
 Intravitreal Corticosteroid Implants Policy Number: 9.03.23 Last Review: 4/2018 Origination: 07/2015 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Intravitreal Corticosteroid Implants Policy Number: 9.03.23 Last Review: 4/2018 Origination: 07/2015 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Moncef Khairallah, MD
 Moncef Khairallah, MD Department of Ophthalmology, Fattouma Bourguiba University Hospital Faculty of Medicine, University of Monastir Monastir, Tunisia INTRODUCTION IU: anatomic form of uveitis involving
Moncef Khairallah, MD Department of Ophthalmology, Fattouma Bourguiba University Hospital Faculty of Medicine, University of Monastir Monastir, Tunisia INTRODUCTION IU: anatomic form of uveitis involving
Intravitreal Corticosteroid Implants. Description
 Subject: Intravitreal Corticosteroid Implants Page: 1 of 20 Last Review Status/Date: June 2015 Intravitreal Corticosteroid Implants Description An intravitreal implant is a drug delivery system, injected
Subject: Intravitreal Corticosteroid Implants Page: 1 of 20 Last Review Status/Date: June 2015 Intravitreal Corticosteroid Implants Description An intravitreal implant is a drug delivery system, injected
Medical Coverage Policy Intravitreal Corticosteroid Implants)
 Medical Coverage Policy Intravitreal Corticosteroid Implants) EFFECTIVE DATE:10 01 2015 POLICY LAST UPDATED: 02 07 2017 OVERVIEW An intravitreal implant is a drug delivery system, injected or surgically
Medical Coverage Policy Intravitreal Corticosteroid Implants) EFFECTIVE DATE:10 01 2015 POLICY LAST UPDATED: 02 07 2017 OVERVIEW An intravitreal implant is a drug delivery system, injected or surgically
Corporate Medical Policy
 Corporate Medical Policy Intravitreal Implant File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intravitreal_implant 11/2010 6/2017 6/2018 6/2017 Description of Procedure or Service
Corporate Medical Policy Intravitreal Implant File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intravitreal_implant 11/2010 6/2017 6/2018 6/2017 Description of Procedure or Service
Optometric Postoperative Cataract Surgery Management
 Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Successful Management of Uveitis in a Patient With Unilateral Multifocal Choroiditis
 Successful Management of Uveitis in a Patient With Unilateral Multifocal Choroiditis SPONSORED BY RETISERT PATIENT CASE STUDY SERIES Multifocal choroiditis is a panuveitis that predominantly affects women
Successful Management of Uveitis in a Patient With Unilateral Multifocal Choroiditis SPONSORED BY RETISERT PATIENT CASE STUDY SERIES Multifocal choroiditis is a panuveitis that predominantly affects women
Original Effective Date: 12/13/2017. Subject: Intravitreal corticosteroid implants: Retisert (fluocinolone acetonide intravitreal implant)
 Subject: Intravitreal corticosteroid implants: Retisert (fluocinolone acetonide intravitreal implant) Policy Number: MCP-302 Original Effective Date: 12/13/2017 Revision Date(s): Review Date: DISCLAIMER
Subject: Intravitreal corticosteroid implants: Retisert (fluocinolone acetonide intravitreal implant) Policy Number: MCP-302 Original Effective Date: 12/13/2017 Revision Date(s): Review Date: DISCLAIMER
INTRAVITREAL IMPLANTS
 INTRAVITREAL IMPLANTS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
INTRAVITREAL IMPLANTS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 20 Next Review: September 2017 Related Policies 9.03.21 Aqueous Shunts for Glaucoma Intravitreal Corticosteroid Implants Summary An intravitreal implant
FEP Medical Policy Manual Last Review: September 20 Next Review: September 2017 Related Policies 9.03.21 Aqueous Shunts for Glaucoma Intravitreal Corticosteroid Implants Summary An intravitreal implant
Update on management of Anterior Uveitis
 Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Intravitreal versus Posterior Subtenon Injection of Triamcinolone Acetonide for Diabetic Macular Edema
 Intravitreal versus Posterior Subtenon Injection of Triamcinolone Acetonide for Diabetic Macular Edema Young Jae Choi, MD, In Kyung Oh, MD, Jae Ryung Oh, MD, PhD, Kuhl Huh, MD, PhD Department of Ophthalmology,
Intravitreal versus Posterior Subtenon Injection of Triamcinolone Acetonide for Diabetic Macular Edema Young Jae Choi, MD, In Kyung Oh, MD, Jae Ryung Oh, MD, PhD, Kuhl Huh, MD, PhD Department of Ophthalmology,
What you can expect with OZURDEX
 Important Information About Macular Edema Following Branch or Central Retinal Vein Occlusion (RVO) and Treatment For patients with RVO What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone
Important Information About Macular Edema Following Branch or Central Retinal Vein Occlusion (RVO) and Treatment For patients with RVO What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone
Development of a dexamethasone intravitreal implant for the treatment of noninfectious posterior segment uveitis
 Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Pharmaceutical Science to Improve the Human Condition: Prix Galien 2014 Development of a dexamethasone intravitreal
Ann. N.Y. Acad. Sci. ISSN 0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Issue: Pharmaceutical Science to Improve the Human Condition: Prix Galien 2014 Development of a dexamethasone intravitreal
THE MULTICENTER UVEITIS STEROID TREATMENT (MUST) TRIAL: A Closer Look at the 7-Year Findings
 THE MULTICENTER UVEITIS STEROID TREATMENT (MUST) TRIAL: A Closer Look at the 7-Year Findings This supplement captures content from a roundtable discussion held in August 2017 at the American Society of
THE MULTICENTER UVEITIS STEROID TREATMENT (MUST) TRIAL: A Closer Look at the 7-Year Findings This supplement captures content from a roundtable discussion held in August 2017 at the American Society of
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company AAO October 25, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg
 An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg This guide is intended to provide injectors with information on the recommended injection technique and the important risks related
An Injector s Guide to OZURDEX (dexamethasone intravitreal implant) 0.7 mg This guide is intended to provide injectors with information on the recommended injection technique and the important risks related
Surgery in patients with uveitis. Lyndell Lim and Anthony Hall
 Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
For the PSV-FAI-001 Study Investigators
 An Injectable Fluocinolone Acetonide Insert Decreases the Incidence of Recurrence in Patients with Chronic Non-infectious Uveitis Affecting the Posterior Segment of the Eye: 12 Month Results Glenn J. Jaffe,
An Injectable Fluocinolone Acetonide Insert Decreases the Incidence of Recurrence in Patients with Chronic Non-infectious Uveitis Affecting the Posterior Segment of the Eye: 12 Month Results Glenn J. Jaffe,
Intravitreal Triamcinolone Acetonide for Macular Edema in HLA-B27 Negative Ankylosing Spondylitis
 105 This is an Open Access article licensed under the terms of the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the
105 This is an Open Access article licensed under the terms of the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the
A Guide to Administering
 A Guide to Administering INDICATIONS AND USAGE YUTIQ (fluocinolone acetonide intravitreal implant) 0.18 mg is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment
A Guide to Administering INDICATIONS AND USAGE YUTIQ (fluocinolone acetonide intravitreal implant) 0.18 mg is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment
84 Year Old with Rosacea
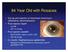 84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
Design: Retrospective, multi-center, interventional case study.
 Abstract (MUST be submitted as a separate file) Purpose: To evaluate outcomes in birdshot chorioretinopathy following intravitreal implantation of a fluocinolone acetonide containing drug delivery device.
Abstract (MUST be submitted as a separate file) Purpose: To evaluate outcomes in birdshot chorioretinopathy following intravitreal implantation of a fluocinolone acetonide containing drug delivery device.
ROLE OF LASER PHOTOCOAGULATION VERSUS INTRAVITREAL TRIAMCINOLONE ACETONIDE IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETES MELLITUS
 ORIGINAL ARTICLE ROLE OF LASER PHOTOCOAGULATION VERSUS INTRAVITREAL TRIAMCINOLONE ACETONIDE IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETES MELLITUS Aggarwal Somesh VP 1, Shah Sonali N 2, Bharwada Rekha M 3,
ORIGINAL ARTICLE ROLE OF LASER PHOTOCOAGULATION VERSUS INTRAVITREAL TRIAMCINOLONE ACETONIDE IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETES MELLITUS Aggarwal Somesh VP 1, Shah Sonali N 2, Bharwada Rekha M 3,
Misdiagnosed Vogt-Koyanagi-Harada (VKH) disease and atypical central serous chorioretinopathy (CSC)
 HPTER 12 Misdiagnosed Vogt-Koyanagi-Harada (VKH) disease and atypical central serous chorioretinopathy (S) linical Features VKH disease is a bilateral granulomatous panuveitis often associated with exudative
HPTER 12 Misdiagnosed Vogt-Koyanagi-Harada (VKH) disease and atypical central serous chorioretinopathy (S) linical Features VKH disease is a bilateral granulomatous panuveitis often associated with exudative
COMPARISON OF INTRAVITREAL TRIAMCINOLONE INJECTION VS LASER PHOTOCOAGULATION IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETIC RETINOPATHY
 Original Article COMPARISON OF INTRAVITREAL TRIAMCINOLONE INJECTION VS LASER PHOTOCOAGULATION IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETIC RETINOPATHY Aggarwal Somesh V 1, Shah Sonali N 2, Bharwada Rekha
Original Article COMPARISON OF INTRAVITREAL TRIAMCINOLONE INJECTION VS LASER PHOTOCOAGULATION IN ANGIOGRAPHIC MACULAR EDEMA IN DIABETIC RETINOPATHY Aggarwal Somesh V 1, Shah Sonali N 2, Bharwada Rekha
Cataract Surgery in Patients with Uveitis
 Cataract Surgery in Patients with Uveitis Chris Kalogeropoulos MD, PhD, FEBO Professor of Ophthalmology Faculty of Medicine, University of Ioannina President of Hellenic Society for the Study of Ocular
Cataract Surgery in Patients with Uveitis Chris Kalogeropoulos MD, PhD, FEBO Professor of Ophthalmology Faculty of Medicine, University of Ioannina President of Hellenic Society for the Study of Ocular
A retrospective nonrandomized study was conducted at 3
 Department of Ophthalmology, Kangbuk Samsung Hospital, Sungkyunkwan University College of Medicine 1, Seoul, Korea Hangil Eye Hospital 2, Incheon, Korea Seoul National University Bundang Hospital 3, Seongnam,
Department of Ophthalmology, Kangbuk Samsung Hospital, Sungkyunkwan University College of Medicine 1, Seoul, Korea Hangil Eye Hospital 2, Incheon, Korea Seoul National University Bundang Hospital 3, Seongnam,
PDF of Trial CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Sat, 09 Mar 2019 11:07:43 GMT) CTRI Number Last Modified On 08/08/2018 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Sat, 09 Mar 2019 11:07:43 GMT) CTRI Number Last Modified On 08/08/2018 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Regional vs. Systemic Therapy. Corticosteroids. Regional vs. Systemic Therapy for Uveitis. Considerations
 Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Recalcitrant Diabetic Macular Oedema: Therapeutic Options
 December 2007 A. Giridhar et al. - Recalcitrant DME 451 CONSULTATION S E C T I O N Recalcitrant Diabetic Macular Oedema: Therapeutic Options Dr. Cyrus M Shroff 1, Dr. N S Muralidhar 2, Dr. R Narayanan
December 2007 A. Giridhar et al. - Recalcitrant DME 451 CONSULTATION S E C T I O N Recalcitrant Diabetic Macular Oedema: Therapeutic Options Dr. Cyrus M Shroff 1, Dr. N S Muralidhar 2, Dr. R Narayanan
Why Is Imaging Critical in My Uveitis Practice?
 Why Is Imaging Critical in My Uveitis Practice? Dilraj S. Grewal, MD Developed in collaboration Imaging Is the Backbone of Uveitis Workup and Monitoring Treatment Response FP FAF B- scan Multimodal Imaging
Why Is Imaging Critical in My Uveitis Practice? Dilraj S. Grewal, MD Developed in collaboration Imaging Is the Backbone of Uveitis Workup and Monitoring Treatment Response FP FAF B- scan Multimodal Imaging
The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report
 European Review for Medical and Pharmacological Sciences The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report J. CISZEWSKA,
European Review for Medical and Pharmacological Sciences The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report J. CISZEWSKA,
POSTERIOR SUBTENON INJECTION OF TRIAMCINALONE ACETONIDE FOR CYSTOID DIABETIC MACULAR EDEMA
 POSTERIOR SUBTENON INJECTION OF TRIAMCINALONE ACETONIDE FOR CYSTOID DIABETIC MACULAR EDEMA Basel T. Ba arah MD, PhD*, Farid H. Al-Zawaideh MD*, Issam M. Al-Bataineh MD* ABSTRACT Objectives: To assess the
POSTERIOR SUBTENON INJECTION OF TRIAMCINALONE ACETONIDE FOR CYSTOID DIABETIC MACULAR EDEMA Basel T. Ba arah MD, PhD*, Farid H. Al-Zawaideh MD*, Issam M. Al-Bataineh MD* ABSTRACT Objectives: To assess the
Serge Bourgault 1,2*, Maryam Aroichane 3, Leah A Wittenberg 1, Andréane Lavallée 1,2 and Patrick E Ma 1
 Bourgault et al. Journal of Ophthalmic Inflammation and Infection 2013, 3:61 BRIEF REPORT Open Access Treatment of refractory uveitic macular edema with dexamethasone intravitreal implants in a pediatric
Bourgault et al. Journal of Ophthalmic Inflammation and Infection 2013, 3:61 BRIEF REPORT Open Access Treatment of refractory uveitic macular edema with dexamethasone intravitreal implants in a pediatric
Macular Hole Associated with Vogt-Koyanagi-Harada Disease at the Acute Uveitic Stage
 Published online: September 15, 2015 2015 The Author(s) Published by S. Karger AG, Basel 1663 2699/15/0063 0328$39.50/0 This article is licensed under the Creative Commons Attribution-NonCommercial 4.0
Published online: September 15, 2015 2015 The Author(s) Published by S. Karger AG, Basel 1663 2699/15/0063 0328$39.50/0 This article is licensed under the Creative Commons Attribution-NonCommercial 4.0
DIABETIC MACULAR edema
 CLINICAL SCIENCES Intravitreal Injection of Triamcinolone for Diffuse Diabetic Macular Edema Jost B. Jonas, MD; Ingrid Kreissig, MD; Antje Söfker, MD; Robert F. Degenring, MD Objective: To evaluate the
CLINICAL SCIENCES Intravitreal Injection of Triamcinolone for Diffuse Diabetic Macular Edema Jost B. Jonas, MD; Ingrid Kreissig, MD; Antje Söfker, MD; Robert F. Degenring, MD Objective: To evaluate the
OZURDEX. (dexamethasone intravitreal implant) 0.7 mg
 OZURDEX (dexamethasone intravitreal implant).7 mg HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OZURDEX safely and effectively. See full prescribing
OZURDEX (dexamethasone intravitreal implant).7 mg HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OZURDEX safely and effectively. See full prescribing
Optical coherence tomography findings in a child with posterior scleritis
 European Journal of Ophthalmology / Vol. 18 no. 6, 2008 / pp. 1007-1010 SHORT OMMUNITIONS & SE REPORTS Optical coherence tomography findings in a child with posterior scleritis H. ERDÖL, M. KOL,. TÜRK
European Journal of Ophthalmology / Vol. 18 no. 6, 2008 / pp. 1007-1010 SHORT OMMUNITIONS & SE REPORTS Optical coherence tomography findings in a child with posterior scleritis H. ERDÖL, M. KOL,. TÜRK
WGA. The Global Glaucoma Network
 The Global Glaucoma Network Fort Lauderdale April 30, 2005 Indications for Surgery 1. The decision for surgery should consider the risk/benefit ratio. Note: Although a lower IOP is generally considered
The Global Glaucoma Network Fort Lauderdale April 30, 2005 Indications for Surgery 1. The decision for surgery should consider the risk/benefit ratio. Note: Although a lower IOP is generally considered
Management of uveitis
 Management of uveitis DR. ANUPAMA KARANTH Anti-inflammatory agents -itis = inflammation Treatment : stop inflammation Use anti-inflammatory drugs Most potent of such agents : Corticosteroids Corticosteroids
Management of uveitis DR. ANUPAMA KARANTH Anti-inflammatory agents -itis = inflammation Treatment : stop inflammation Use anti-inflammatory drugs Most potent of such agents : Corticosteroids Corticosteroids
Sustained-Release Corticosteroid Options
 Sustained-Release Corticosteroid Options Mariana Cabrera, University of Miami Miller School of Medicine Steven Yeh, Emory University Thomas A Albini, University of Miami Miller School of Medicine Journal
Sustained-Release Corticosteroid Options Mariana Cabrera, University of Miami Miller School of Medicine Steven Yeh, Emory University Thomas A Albini, University of Miami Miller School of Medicine Journal
Uveitis unplugged: systemic therapy
 Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Facts About Diabetic Eye Disease
 Facts About Diabetic Eye Disease Points to Remember 1. Diabetic eye disease comprises a group of eye conditions that affect people with diabetes. These conditions include diabetic retinopathy, diabetic
Facts About Diabetic Eye Disease Points to Remember 1. Diabetic eye disease comprises a group of eye conditions that affect people with diabetes. These conditions include diabetic retinopathy, diabetic
Choroidal detachment following retinal detachment surgery: An analysis and a new hypothesis to minimize its occurrence in high-risk cases
 European Journal of Ophthalmology / Vol. 14 no. 4, 2004 / pp. 325-329 Choroidal detachment following retinal detachment surgery: An analysis and a new hypothesis to minimize its occurrence in high-risk
European Journal of Ophthalmology / Vol. 14 no. 4, 2004 / pp. 325-329 Choroidal detachment following retinal detachment surgery: An analysis and a new hypothesis to minimize its occurrence in high-risk
Evolving therapies for posterior uveitis. Infliximab (Remicade) Infliximab: pharmacology. FDA-approved monoclonal antibody therapy Target
 Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 19 September 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 September 2012 OZURDEX 700 micrograms, intravitreal implant in applicator Box of 1 sachet with applicator (CIP:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 September 2012 OZURDEX 700 micrograms, intravitreal implant in applicator Box of 1 sachet with applicator (CIP:
Original Effective Date: 10/24/2016. Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant)
 Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant) Original Effective Date: 10/24/2016 Policy Number: MCP-282 Revision Date(s): 12/13/2017 DISCLAIMER This Molina
Subject: Intravitreal corticosteroid implants: Ozurdex (dexamethasone intravitreal implant) Original Effective Date: 10/24/2016 Policy Number: MCP-282 Revision Date(s): 12/13/2017 DISCLAIMER This Molina
Choroidal Neovascularization in Sympathetic Ophthalmia
 Choroidal Neovascularization in Sympathetic Ophthalmia Lucia Sobrin, Miguel Cordero Coma, C. Stephen Foster Case Report A 49-year-old man presented after a ruptured globe repair of his left eye status
Choroidal Neovascularization in Sympathetic Ophthalmia Lucia Sobrin, Miguel Cordero Coma, C. Stephen Foster Case Report A 49-year-old man presented after a ruptured globe repair of his left eye status
COVER FOCUS AT A GLANCE. BY ANDREA ARRIOLA LÓPEZ, MD, MSc, and THOMAS ALBINI, MD
 THERAPEUTIC OPTIONS FOR UVEITIS Thanks to a plentiful number of choices available and in the pipeline, the future looks promising for patients with this condition. BY ANDREA ARRIOLA LÓPEZ, MD, MSc, and
THERAPEUTIC OPTIONS FOR UVEITIS Thanks to a plentiful number of choices available and in the pipeline, the future looks promising for patients with this condition. BY ANDREA ARRIOLA LÓPEZ, MD, MSc, and
You can C-ME after Uveitis
 You can C-ME after Uveitis Abstract: Approximately 50% of uveitis patients will present with vision loss secondary to cystoid macular edema[1]. Two patients with uveitis present with a constant decrease
You can C-ME after Uveitis Abstract: Approximately 50% of uveitis patients will present with vision loss secondary to cystoid macular edema[1]. Two patients with uveitis present with a constant decrease
EXP11677SK. Financial Disclosure. None to be Declared EXP11677SK
 Financial Disclosure None to be Declared Presentation overview Glaucoma Surgical History Complications of trabeculectomy Express Device Specifications Surgical Steps Clinical advantages, indications and
Financial Disclosure None to be Declared Presentation overview Glaucoma Surgical History Complications of trabeculectomy Express Device Specifications Surgical Steps Clinical advantages, indications and
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Health Technology Appraisal. Aflibercept for treating diabetic macular oedema.
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
Comparison Between 20- Gauge And 23-Gauge Vitrectomy In Diabetic Patients
 Asok Nataraj MS Abstract Aim: - Comparison Between 20- Gauge And 23-Gauge Vitrectomy In Diabetic Patients The purpose of this study was to directly compare the outcome, safety and efficacy of the 20G and
Asok Nataraj MS Abstract Aim: - Comparison Between 20- Gauge And 23-Gauge Vitrectomy In Diabetic Patients The purpose of this study was to directly compare the outcome, safety and efficacy of the 20G and
Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
 European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
Diabetic Retinopathy Clinical Research Network
 Diabetic Retinopathy Clinical Research Network Short-term Evaluation of Combination Corticosteroid+Anti- VEGF Treatment for Persistent Central-Involved Diabetic Macular Edema Following Anti-VEGF Therapy
Diabetic Retinopathy Clinical Research Network Short-term Evaluation of Combination Corticosteroid+Anti- VEGF Treatment for Persistent Central-Involved Diabetic Macular Edema Following Anti-VEGF Therapy
INFORMED CONSENT FOR AVASTIN TM (BEVACIZUMAB) INTRAVITREAL INJECTION
 INFORMED CONSENT FOR AVASTIN TM (BEVACIZUMAB) INTRAVITREAL INJECTION INDICATIONS: Age-related macular degeneration (AMD) is the leading cause of blindness in people over 50 years of age. It is caused by
INFORMED CONSENT FOR AVASTIN TM (BEVACIZUMAB) INTRAVITREAL INJECTION INDICATIONS: Age-related macular degeneration (AMD) is the leading cause of blindness in people over 50 years of age. It is caused by
Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema
 Original Research Article Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema Neha Kantilal Desai 1,*, Somesh Vedprakash Aggarwal 2, Sonali
Original Research Article Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema Neha Kantilal Desai 1,*, Somesh Vedprakash Aggarwal 2, Sonali
Noninfectious Posterior Uveitis: The Rationale for Steroid Use
 Insert to October 2016 CME Activity Noninfectious Posterior Uveitis: The Rationale for Steroid Use Thomas Albini, MD, moderator Nisha Acharya, MD Phoebe Lin, MD, PhD Quan Dong Nguyen, MD, MSc Steven Yeh,
Insert to October 2016 CME Activity Noninfectious Posterior Uveitis: The Rationale for Steroid Use Thomas Albini, MD, moderator Nisha Acharya, MD Phoebe Lin, MD, PhD Quan Dong Nguyen, MD, MSc Steven Yeh,
SCHEDULING STATUS Schedule 4. PROPRIETARY NAME AND DOSAGE FORM OZURDEX intravitreal implant
 Page 1 of 10 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM intravitreal implant COMPOSITION One intravitreal implant contains: Dexamethasone 700 μg Excipients: Ester terminated 50:50 poly
Page 1 of 10 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM intravitreal implant COMPOSITION One intravitreal implant contains: Dexamethasone 700 μg Excipients: Ester terminated 50:50 poly
Objectives. Tubes, Ties and Videotape: Financial Disclosure. Five Year TVT Results IOP Similar
 Tubes, Ties and Videotape: Surgical Video of Glaucoma Implants and Financial Disclosure I have no financial interests or relationships to disclose. Herbert P. Fechter MD, PE Eye Physicians and Surgeons
Tubes, Ties and Videotape: Surgical Video of Glaucoma Implants and Financial Disclosure I have no financial interests or relationships to disclose. Herbert P. Fechter MD, PE Eye Physicians and Surgeons
Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection
 Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection Kenalog/SS/ST/04.2012/v1.1 review 05.2013 Page 1 Introduction Your doctor has found that you have leakage of
Information for Patients undergoing Intravitreal Triamcinolone Acetonide (Kenalog) Injection Kenalog/SS/ST/04.2012/v1.1 review 05.2013 Page 1 Introduction Your doctor has found that you have leakage of
DATA SHEET. Presentation. Uses NAME OF MEDICINE. Actions. Pharmacokinetics. OZURDEX (dexamethasone) 700 µg implant
 DATA SHEET NAME OF MEDICINE OZURDEX (dexamethasone) 700 µg implant Presentation Dexamethasone is a white to cream-coloured crystalline powder with not more than a slight odour, and is practically insoluble
DATA SHEET NAME OF MEDICINE OZURDEX (dexamethasone) 700 µg implant Presentation Dexamethasone is a white to cream-coloured crystalline powder with not more than a slight odour, and is practically insoluble
Intraocular Radiation Therapy for Age-Related Macular Degeneration
 Medical Policy Manual Medicine, Policy No. 134 Intraocular Radiation Therapy for Age-Related Macular Degeneration Next Review: April 2019 Last Review: June 2018 Effective: August 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Medicine, Policy No. 134 Intraocular Radiation Therapy for Age-Related Macular Degeneration Next Review: April 2019 Last Review: June 2018 Effective: August 1, 2018 IMPORTANT REMINDER
Vanderbilt Eye Institute Clinical Trials
 April, 2010 Vanderbilt Eye Institute Clinical Trials Ophthalmology Actively Recruiting Studies For information on our clinical trials and other studies, please contact: Sandy Owings, COA, CCRP Clinic Director
April, 2010 Vanderbilt Eye Institute Clinical Trials Ophthalmology Actively Recruiting Studies For information on our clinical trials and other studies, please contact: Sandy Owings, COA, CCRP Clinic Director
Seong Joon Ahn *, Jooyoung Joung, Sang Hyup Lee and Byung Ro Lee *
 Ahn et al. BMC Ophthalmology (2018) 18:310 https://doi.org/10.1186/s12886-018-0985-x CASE REPORT Open Access Intravitreal dexamethasone implant therapy for the treatment of cystoid macular Oedema due to
Ahn et al. BMC Ophthalmology (2018) 18:310 https://doi.org/10.1186/s12886-018-0985-x CASE REPORT Open Access Intravitreal dexamethasone implant therapy for the treatment of cystoid macular Oedema due to
o White dot syndromes pattern recognition o Activity and damage o Quality of life o Key points o Idiopathic o Sarcoidosis o Multiple sclerosis
 Introduction Clinical Assessment of Posterior Uveitis Philip I. Murray Centre for Translational Inflammation Research University of Birmingham Birmingham and Midland Eye Centre o Classification of uveitis
Introduction Clinical Assessment of Posterior Uveitis Philip I. Murray Centre for Translational Inflammation Research University of Birmingham Birmingham and Midland Eye Centre o Classification of uveitis
ILUVIEN IN DIABETIC MACULAR ODEMA
 1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
When optical coherence tomography (OCT)
 Macular Imaging: SD-OCT in nterior Segment Surgical Practice Many pathologic processes of the macula can be visualized or quantified only with this modality. y Steven G. Safran, MD When optical coherence
Macular Imaging: SD-OCT in nterior Segment Surgical Practice Many pathologic processes of the macula can be visualized or quantified only with this modality. y Steven G. Safran, MD When optical coherence
Corporate Presentation August 2018
 Corporate Presentation August 218 Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words
Corporate Presentation August 218 Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words
Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington
 Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington I Jay M. Haynie, OD, FAAO have received honoraria from the following companies: Reichert Technologies Notal Vision Carl Zeiss Meditec
Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington I Jay M. Haynie, OD, FAAO have received honoraria from the following companies: Reichert Technologies Notal Vision Carl Zeiss Meditec
Intravitreal Corticosteroids in the Management of Diabetic Macular Edema
 Curr Ophthalmol Rep (2013) 1:144 149 DOI 10.1007/s40135-013-0015-3 DIABETIC RETINOPATHY: MEDICAL AND SURGICAL THERAPIES (PK KAISER, SECTION EDITOR) Intravitreal Corticosteroids in the Management of Diabetic
Curr Ophthalmol Rep (2013) 1:144 149 DOI 10.1007/s40135-013-0015-3 DIABETIC RETINOPATHY: MEDICAL AND SURGICAL THERAPIES (PK KAISER, SECTION EDITOR) Intravitreal Corticosteroids in the Management of Diabetic
Intravitreal Injection
 for patients Eye Clinic Ipswich Hospital Tel: 01473 703230 Intravitreal Injection What is an intravitreal injection? An intravitreal injection is the injection of a drug into the vitreous body (the jelly
for patients Eye Clinic Ipswich Hospital Tel: 01473 703230 Intravitreal Injection What is an intravitreal injection? An intravitreal injection is the injection of a drug into the vitreous body (the jelly
Diabetic Retinopathy: Managing the Extremes. J. Michael Jumper, MD West Coast Retina
 Diabetic Retinopathy: Managing the Extremes J. Michael Jumper, MD West Coast Retina Case 1: EC 65 y.o. HM No vision complaints Meds: Glyburide Metformin Pioglitazone Va: 20/20 OU 20/20 Case 2: HS 68 y.o.
Diabetic Retinopathy: Managing the Extremes J. Michael Jumper, MD West Coast Retina Case 1: EC 65 y.o. HM No vision complaints Meds: Glyburide Metformin Pioglitazone Va: 20/20 OU 20/20 Case 2: HS 68 y.o.
Controversies in the management of Irvine-Gass syndrome
 Incorporating current trials and technology into clinical practice Controversies in the management of Irvine-Gass syndrome by Daniel F. Kiernan, MD Seenu M. Hariprasad Practical Retina Editor Cystoid macular
Incorporating current trials and technology into clinical practice Controversies in the management of Irvine-Gass syndrome by Daniel F. Kiernan, MD Seenu M. Hariprasad Practical Retina Editor Cystoid macular
Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma
 Original Article Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma Irfan Qayyum Malik, M. Moin, A. Rehman, Mumtaz Hussain
Original Article Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma Irfan Qayyum Malik, M. Moin, A. Rehman, Mumtaz Hussain
Treatment chronic macular edema in Vogt-Koyanagi Harada syndrome with dexamethasone intravitreal implant: Description of Three Case
 Pacella F, Smaldone P, Albanese G, et al./senses Sci 2015; 2 (1):57-63 doi: 10.14616/sands-2015-1-57 63 OPE AC Treatment chronic macular edema in Vogt-Koyanagi Harada syndrome with dexamethasone intravitreal
Pacella F, Smaldone P, Albanese G, et al./senses Sci 2015; 2 (1):57-63 doi: 10.14616/sands-2015-1-57 63 OPE AC Treatment chronic macular edema in Vogt-Koyanagi Harada syndrome with dexamethasone intravitreal
ISPUB.COM. An Atypical Presentation of Posterior Scleritis. A Ramanathan, A Gaur CASE REPORT
 ISPUB.COM The Internet Journal of Ophthalmology and Visual Science Volume 8 Number 2 A Ramanathan, A Gaur Citation A Ramanathan, A Gaur.. The Internet Journal of Ophthalmology and Visual Science. 2009
ISPUB.COM The Internet Journal of Ophthalmology and Visual Science Volume 8 Number 2 A Ramanathan, A Gaur Citation A Ramanathan, A Gaur.. The Internet Journal of Ophthalmology and Visual Science. 2009
Comparison of Intravitreal Injection of Bevacizumab and Triamcinolone Acetonide in the Treatment of Uveitic Macular Edema
 ISSN 1735-1383 Iran. J. Immunol. June 2012, 9 (2), 136-144 Mansour Rahimi, Seyed Sahabaldin Shahrzad, Mohammad Banifatemi Comparison of Intravitreal Injection of Bevacizumab and Triamcinolone Acetonide
ISSN 1735-1383 Iran. J. Immunol. June 2012, 9 (2), 136-144 Mansour Rahimi, Seyed Sahabaldin Shahrzad, Mohammad Banifatemi Comparison of Intravitreal Injection of Bevacizumab and Triamcinolone Acetonide
Diabetic Retinopatathy
 Diabetic Retinopatathy Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl Zeiss Meditec Arctic DX Macula Risk Advanced
Diabetic Retinopatathy Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl Zeiss Meditec Arctic DX Macula Risk Advanced
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]
![Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]](/thumbs/82/86218480.jpg) Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Uveitis. What is Uveitis?
 Uveitis What is Uveitis? Uveitis [u-vee-i-tis] is a term for inflammation of the eye. It can occur in one eye or both eyes and affects the layer of the eye called the uvea [u-vee-uh]. It also can be associated
Uveitis What is Uveitis? Uveitis [u-vee-i-tis] is a term for inflammation of the eye. It can occur in one eye or both eyes and affects the layer of the eye called the uvea [u-vee-uh]. It also can be associated
Uveitis is a common cause of preventable blindness
 Treatment of Uveitic Cystoid Macular Edema Three case reports discuss the management of inflammatory CME. BY PRANJAL THAKURIA, MD; AND C. STEPHEN FOSTER, MD Uveitis is a common cause of preventable blindness
Treatment of Uveitic Cystoid Macular Edema Three case reports discuss the management of inflammatory CME. BY PRANJAL THAKURIA, MD; AND C. STEPHEN FOSTER, MD Uveitis is a common cause of preventable blindness
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
OCCLUSIVE VASCULAR DISORDERS OF THE RETINA
 OCCLUSIVE VASCULAR DISORDERS OF THE RETINA Learning outcomes By the end of this lecture the students would be able to Classify occlusive vascular disorders (OVD) of the retina. Correlate the clinical features
OCCLUSIVE VASCULAR DISORDERS OF THE RETINA Learning outcomes By the end of this lecture the students would be able to Classify occlusive vascular disorders (OVD) of the retina. Correlate the clinical features
DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 DATA SHEET 1. PRODUCT NAME OZURDEX 700 µg implant 2. QUALITATIVE AND QUANTITATIVE COMPOSITION dexamethasone 700 µg For full list of excipients, see section 6.1 List of Excipients. 3. PHARMACEUTICAL FORM
DATA SHEET 1. PRODUCT NAME OZURDEX 700 µg implant 2. QUALITATIVE AND QUANTITATIVE COMPOSITION dexamethasone 700 µg For full list of excipients, see section 6.1 List of Excipients. 3. PHARMACEUTICAL FORM
Emerging therapies for the treatment of uveitis: clinical trial observations
 Review: Clinical Trial Outcomes Emerging therapies for the treatment of uveitis: clinical trial observations Clin. Invest. (2013) 3(10), 951 966 Uveitis, a leading cause of blindness in the USA and western
Review: Clinical Trial Outcomes Emerging therapies for the treatment of uveitis: clinical trial observations Clin. Invest. (2013) 3(10), 951 966 Uveitis, a leading cause of blindness in the USA and western
Although photocoagulation and photodynamic PROCEEDINGS PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION *
 PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
PRODUCT MONOGRAPH. (dexamethasone) Intravitreal Implant 0.7 mg. Corticosteroid. Allergan Inc. Markham, ON L6G 0B5. Date of Revision: April 14, 2015
 PRODUCT MONOGRAPH Pr OZURDEX (dexamethasone) Intravitreal Implant 0.7 mg Corticosteroid Allergan Inc. Markham, ON L6G 0B5 Date of Revision: April 14, 2015 Submission Control No: 169671 Page 1 of 36 Table
PRODUCT MONOGRAPH Pr OZURDEX (dexamethasone) Intravitreal Implant 0.7 mg Corticosteroid Allergan Inc. Markham, ON L6G 0B5 Date of Revision: April 14, 2015 Submission Control No: 169671 Page 1 of 36 Table
Cataract and Diabetic macular edema: What should I do?
 Cataract and Diabetic macular edema: What should I do? Irini Chatziralli Ophthalmic Surgeon, University Scholar 2 nd Department of Ophthalmology, University of Athens (Director: Prof P. Theodossiadis)
Cataract and Diabetic macular edema: What should I do? Irini Chatziralli Ophthalmic Surgeon, University Scholar 2 nd Department of Ophthalmology, University of Athens (Director: Prof P. Theodossiadis)
Nausheen Khuddus, MD Melissa Elder, MD, PhD
 Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Trabeculectomy combined with cataract extraction: a follow-up study
 British Journal of Ophthalmology, 1980, 64, 720-724 Trabeculectomy combined with cataract extraction: a follow-up study R. S. EDWARDS From the Birmingham and Midland Eye Hospital, Church Street, Birmingham
British Journal of Ophthalmology, 1980, 64, 720-724 Trabeculectomy combined with cataract extraction: a follow-up study R. S. EDWARDS From the Birmingham and Midland Eye Hospital, Church Street, Birmingham
The US Food and Drug Administration
 Insert to July/August 2011 Injection Techniques for the Dexamethasone Intravitreal Implant Covered topics include: Profiles of the Experienced Injector Preparing the Patient for the Injection Injection
Insert to July/August 2011 Injection Techniques for the Dexamethasone Intravitreal Implant Covered topics include: Profiles of the Experienced Injector Preparing the Patient for the Injection Injection
Alastair Porteous * and Laura Crawley
 Porteous and Crawley BMC Ophthalmology 2018, 18(Suppl 1):219 https://doi.org/10.1186/s12886-018-0858-3 CASE REPORT Case report of secondary pigment dispersion glaucoma, recurrent uveitis and cystoid macular
Porteous and Crawley BMC Ophthalmology 2018, 18(Suppl 1):219 https://doi.org/10.1186/s12886-018-0858-3 CASE REPORT Case report of secondary pigment dispersion glaucoma, recurrent uveitis and cystoid macular
Adalimumab in Noninfectious Uveitis of Idiopathic Etiology Efficacy Across Different Anatomical Locations the VISUAL I and VISUAL II Trials
 9:00 AM Adalimumab in Noninfectious Uveitis of Idiopathic Etiology Efficacy Across Different Anatomical Locations the VISUAL I and VISUAL II Trials Pauline T. Merrill, MD Albert T. Vitale, MD Eric Fortin,
9:00 AM Adalimumab in Noninfectious Uveitis of Idiopathic Etiology Efficacy Across Different Anatomical Locations the VISUAL I and VISUAL II Trials Pauline T. Merrill, MD Albert T. Vitale, MD Eric Fortin,
