CIC Edizioni Internazionali. Clinical manifestations and management of Gaucher disease. Mini-review
|
|
|
- Aubrey Welch
- 5 years ago
- Views:
Transcription
1 Clinical manifestations and management of Gaucher disease Silvia Linari Giancarlo Castaman Center for Bleeding Disorders, Department of Heart and Vessels, Careggi University Hospital, Florence, Italy Address for correspondence: Silvia Linari, MD Center for Bleeding Disorders, Department of Heart and Vessels Careggi University Hospital Largo Brambilla Florence, Italy Phone: Fax: linaris@aou-careggi.toscana.it Summary Gaucher disease is a rare multi-systemic metabolic disorder caused by the inherited deficiency of the lysosomal enzyme β-glucocerebrosidase, which leads to the accumulation of its normal substrate, glucocerebroside, in tissue macrophages with damage to haematological, visceral and bone systems. Anaemia, thrombocytopenia, enlargement of liver and/or spleen, skeletal abnormalities (osteopenia, lytic lesions, pathological fractures, chronic bone pain, bone crisis, bone infarcts, osteonecrosis and skeletal deformities) are typical manifestations of the most prevalent form of the disease, the so-called non-neuronopathic type 1. However, severity and coexistence of different symptoms are highly variable. The determination of deficient β- glucocerebrosidase activity in leukocytes or fibroblasts by enzymatic assay is the gold standard for the diagnosis of Gaucher disease. Comprehensive and reproducible evaluation and monitoring of all clinically relevant aspects are fundamental for the effective management of Gaucher disease patients. Enzyme replacement therapy has been shown to be effective in reducing glucocerebroside storage burden and diminishing the deleterious effects caused by its accumulation. Tailored treatment plan for each patient should be directed to symptom relief, general improvement of quality of life, and prevention of irreversible damage. KEY WORDS: Gaucher disease; glucocerebroside; storage burden; activated macrophage; enzyme replacement therapy. Etiology and pathogenesis Gaucher disease (GD) is an inherited lysosomal storage disease caused by an autosomal recessive defect of the gene encoding β-glucocerebrosidase, enzyme responsible for the accumulation of glucosylceramide into reticuloendothelial cells, rendering GD a multi-organ chronic disorder (1, 2). These lipid-laden cells are called Gaucher cells and are predominantly found in the spleen, liver, bone marrow and rarely lung. As a consequence, hepatosplenomegaly, pancytopenia, bone complications and, in a small number of patients, lung involvement with interstitial lung disease and pulmonary hypertension can occur (2, 3). Massive infiltration by Gaucher cells alone cannot explain the multifaceted characteristics of the disease. The accumulation leads to a secondary activation of macrophages, inducing the release of various cytokines and lysosomal proteins (4). The most striking seems to be the increased expression of chitotriosidase, which can be raised 1000-fold in Gaucher patients and is produced in Gaucher cells. The plasma concentration strongly correlates with the accumulation of Gaucher cells in the body (5). In an animal model, an inflammatory infiltration of several organ systems, B-cell stimulation and expression of TNF-a and IL-1β were observed (6). These observations may explain the increased occurrence of auto-antibodies, B- cell lymphomas, gammopathies and multiple myeloma in Gaucher patients (7, 8). Similarly, most of the Gaucher changes in the long bones can be explained by the release of cytokines by storage cells in the bone marrow. To date, the pathogenic etiology of neurological involvement in GD is still incomplete (9). Diagnosis of GD Mini-review Residual levels of glucocerebrosidase in patients with GD have been variously estimated at 5-25% of normal activity. The measurement of β-glucocerebrosidase activity in leukocytes or in cultured fibroblasts obtained by skin biopsy is the gold standard for the diagnosis of GD (10). As for many other lysosomal enzymes, recently screening methods using dry blood spots have been developed also for β-glucocerebrosidase (11). The finding of a reduced β-glucocerebrosidase activity can be supplemented by detection of the genetic defect. The glucocerebrosidase gene (GBA) is located on chromosome 1q21; it contains 11 exons and 10 introns, covering 7.6 kilobases (kb) of sequence. There is a highly homologous pseudogene (psgba), spanning 5.7 kb with the same exon and intron number as the GBA, located 16 kb downstream, sharing approximately 96% of the sequence in coding regions. Almost 300 mutations and polymorphisms in GBA have been identified. Most are point mutations, but insertions or deletions, splice site alterations, and recombinating alleles, also with the nearby pseudogene, have also been described (12). Recently mutations in gene of saposin C, that is the β-glucocerebrosidase activator, have been also reported in association with GD (13). Four mutations account for over 90% of disease alleles in Ashkenazi Jewish patients: N370S, 84GG, L444P and IVS2+1G (14). Non-Jewish pa- Clinical Cases in Mineral and Bone Metabolism 2015; 12(2)
2 S. Linari et al. tients exhibit a much wider range of genotypes, although two mutations (N370S and L444P) are common in both populations. Of note, homozygosis for L444P normally results in neuronopathic disease whereas the presence of a single mutant N370S allele usually prevents neurological involvement. However, genotype-phenotype correlations are of limited clinical value and the clinical diversity of this single gene disorder suggests that modifier genes and environmental factors must play an important role (15). Genetic diagnosis can be performed during pregnancy using amniocentesis or chorionic villi sampling, but this test is only useful in populations with a high gene frequency or in families already known to be affected (16). The detection of Gaucher cells in bioptic samples is not required for diagnostic purpose, even if it often represents the step triggering the diagnostic suspicion in adult patients. In fact, approximately 60% of all new GD diagnoses are still made by a bone marrow biopsy or histology of a surgically removed spleen. Epidemiology GD is pan-ethnic, but it has a particular high prevalence among Ashkenazi Jews (17). Within this population, nonneuronopathic forms of GD occur with a frequency of approximately 1:850 and carrier frequency is 1:17. Based on epidemiological investigations in Italy a prevalence of 1:40,000-1:86,000 is assumed in the general population. Acute neurological forms occur even less frequently. A prevalence estimate of 1: may however be too low, as cases with fetal or neonatal manifestations (fetal hydrops, congenital ichthyosis) are not always properly diagnosed. Clinical classification Three major forms of GD have been clinically described. The most prevalent is the so-called non-neuronopathic form (type 1) characterized by anaemia, thrombocytopenia, enlargement of the spleen, skeletal abnormalities (18), and in a small number of patients, by lung involvement with interstitial lung disease (18) and pulmonary hypertension (19, 20). Type 1 is essentially a macrophage disorder, lacking primary central nervous system involvement. Patients with type 1 GD display a large variety of symptoms, ranging from asymptomatic subjects to those who display child-onset disease. Type 2 GD is an acute neuronopathic form with severe prognosis and survival limited to the first two or three years of life; it is characterized by neurological impairment in addition to visceral symptoms. The neurological symptoms start with oculomotor abnormalities followed by brainstem involvement. Type 3 GD is also characterized by neurological involvement but neurological symptoms generally appear later in life than in type 2 disease, and include abnormal eye movements, ataxia, seizures, and dementia, with patients surviving until their third or fourth decade (21). Recently, a clinical association has been reported between the presence of mutations in the β-glucocerebrosidase gene and Parkinsonism (22, 23). The identification of new phenotypes and appreciation that even patients with type 1 may develop some late-onset neurologic manifestations, may suggest is to consider GD as a continuum of disease states (Table 1) (24). Finally, a significant increased risk of haematological malignancies has been reported in GD. GD is frequently associated with immunologic abnormalities: polyclonal hypergammaglobulinemia may occur at diagnosis in 14-41% of adults (25-27); an increased prevalence of the pre-malignant condition mono- Table 1 - Clinical classification of the forms of GD. Recently the classic categories of types 1, 2 and 3 have blurry edges along a phenotypic continuum. Patients with GD can have a spectrum of symptoms, ranging from mild to severe neurological effects (24). Non-neuronopathic GD Acute neuronopathic GD Chronic neuronopathic GD Incidence 1: : :850 in Ashkenazi Jews <1: <1:50000 to <1: Ethnic group Panethnic, more common in Panethnic Panethnic Ashkenazi Jews Age at onset of disease Any age Infancy Childhood CNS symptoms (progressive) Hepatosplenomegaly Hematological symptoms Bone symptoms Clinical Cases in Mineral and Bone Metabolism 2015; 12(2)
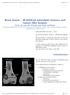
3 Clinical manifestations and management of Gaucher disease clonal gammopathy of undetermined significance (MGUS), up to 25%, has been described. In GD the increased risk of MM is reported as ranging from 5.9 (28) to 51.1 times that of normal population. An increased risk of other hematological malignancies, such as amyloidosis or B-cell non-hodgkin Lymphoma (NHL), has also been reported (29-31). Association between GD and solid organ malignancies is less clear but an increased incidence of hepatocellular carcinoma (HCC) has been described (29). Bone manifestations in GD Figure 1 - Coronal T1-weighted sequence of the femurs showing severe bone marrow infiltration with some focal areas. Figure 2 - A-P X-ray image of the distal femurs showing the Erlenmeyer flask deformity in the distal diametaphyseal transition zone. Skeletal involvement has a high prevalence in adult type 1 GD patients and represents actually the major morbidity for its frequent association with considerable pain, limitations in mobility and an extremely negative impact on the quality of life. The bone manifestations of GD are multifaceted and can include bone marrow infiltration, severe acute bone crises, chronic intermittent bone pain, bone infarction, lytic lesions, Erlenmeyer flask deformity of the distal femur, osteopenia, osteoporosis, osteonecrosis, subchondral joint collapse, pathologic fractures of long bones and vertebrae, and growth retardation in children (2, 9, 32, 33). Bone disease has a very high prevalence in type 1 GD, with a radiological evidence described in 93% of patients. Data from the International Collaborative Gaucher Group (ICGG) Registry show that at diagnosis bone pain is present in 50% of type 1 GD patients, bone marrow infiltration in 82%, Erlenmeyer flask deformity in 60%, osteonecrosis in 30%, localized or generalized decrease of bone mineral density (BMD) in 49% and in 36%, respectively (34). The underlying pathology of bone disease is related to the accumulation of Gaucher cells that infiltrate the bone marrow compartment and lead directly or indirectly to localized bone defects, including cortical thinning, osteonecrosis and lytic lesions. The pattern and the progression of the bone marrow infiltration vary from patient to patient, but in general the infiltration seems to begin in the lumbar spine, before entering the metaphysis and diaphysis of the femur, and then being seen in the epiphysis in the later stages. Magnetic resonance imaging (MRI) is the most sensitive diagnostic procedure for the detection of bone marrow changes. Affected bone areas have pathologically reduced signal using T1-weighted spin echo sequences. The reduced signal is caused by displacement of the signal-rich yellow bone marrow (Figure 1) (35). Bone marrow infiltration has also been connected with abnormal bone remodeling. The Erlenmeyer flask deformity is a common remodeling disorder, which occurs in over 60% of the patients. This manifests as a deformity of the distal long bones and generally affects the distal femur (Figure 2) or proximal tibia (36). The metaphyseal regions are expanded and show incorrect bone remodeling, which seems to be connected with a relative failure of osteoclast activity. Another pathological skeletal change seen in GD patients with progressive bone disease is osteonecrosis, also known as avascular necrosis. Osteonecrosis is ischaemic death of bone tissue due to chronic bone infarction. The patient has often suffered many bone crises prior to the onset of the necrotic process. However, vascular obstruction is not necessarily the primary pathological mechanism. Gaucher cells can also damage the vessel wall through the release of lysosomal contents, with localized osteonecrosis that may be extensive. Osteonecrosis usually occurs bilaterally in the head of the femur, but some patients have repeated episodes in the same hip. Involvement of the femoral neck can precede the pathological changes in the head of the femur (37). Osteonecrosis can affect medullary as well as cortical bone. Medullary osteonecrosis may also be entirely asymptomatic. These lesions may cause major disability from pathological fractures Clinical Cases in Mineral and Bone Metabolism 2015; 12(2)
4 S. Linari et al. and collapse of osseous endplates with joint disintegration and its replacement is often necessary to relieve pain and restore mobility. In children, the disorder is sometimes misdiagnosed as Legg-Calve-Perthes disease. One third of adult GD patients have a history of osteonecrosis (18); while specific genotypes, prior splenectomy, trauma, strenuous physical exertion, and pregnancies appear to be predisposing factors for the development of osteonecrosis, it is not possible to accurately predict in individual patients the future risk for its development. Bone crises can be a manifestation of the osteonecrotic process, when a sufficient proportion of the bone is affected. Extensive osteonecrosis can cause generalized systemic disease characterized by severe pain (due to edema in the bone cavity), high fever, shivering, complete disability, a high leukocyte count and an increased erythrocyte sedimentation rate. When a bone crisis occurs, it is the most incapacitating manifestation of GD, frequently requiring opiate analgesic. Differential diagnosis from septic osteomyelitis may be difficult. Patients with Gaucher crises have a negative blood culture. The best diagnostic option is a technetium- 99m bone scan within 2-3 days of onset of bone crises. It is cold in Gaucher bone crises and hot in septic osteomyelitis (38). Although bone pain usually occurs in GD, there are not always specific radiological findings. The nature of the bone pain differs, in fact this symptom may be dull, sharp, non-specific or severe and localized, like joint pain. Patients with GD are highly likely to suffer fractures, which relates to the increased prevalence of osteopenia, osteonecrosis, infarction and bone crises. Fractures can occur anywhere in the skeleton. Delayed skeletal growth is very common in children with GD, particularly in those with severe type 1. Gaucher cells can alternatively damage bone tissue since they are also activated macrophages which secrete several cytokines (TNFa, IL6, IL10, IL4 and monocyte chemo-attractant proteins) and/or interact with cells in their environment. The locally increased cytokines in bone stimulate production of osteoclast precursors, resulting in imbalances in bone remodeling to favour resorption over formation, leading to osteopenia or osteoporosis. A decreased bone mineralization can occur locally or diffusely and widespread, and can affect trabecular bone, as well as cortical bone (39). The lumbar spine represents an ideal location for the assessment of localized bone disease, as well as generalized osteoporosis. In fact, marrow infiltration is thought to begin in the lumbar vertebrae and then progress to the pelvis and the appendicular skeleton; moreover, the lumbar spine has a higher percentage of trabecular bone and may be more sensitive to changes in systemic bone mass than other sites where cortical bone predominates. Evaluations and monitoring for type 1 GD Challenges to patient care posed by clinical heterogeneity, variables progression rates, and potential permanent disability that can result from untreated or sub-optimally treated haematological, skeletal and visceral involvement dictate a need for comprehensive, serial monitoring. A consensus on minimum recommendations for effective monitoring of adult patients with type 1 GD had been developed by the ICGG Registry coordinators, with reproducible initial baseline and annual follow-up evaluations of all clinically relevant aspects of the disease. Assessment of disease severity includes blood tests, biochemical biomarkers, MRI or ultrasound for measurement of spleen and liver volume, a combination of modalities to evaluate trabecular and compact bone disease, including radiographs, bone densitometry by dual energy X- ray absorptiometry and MRI to assess the severity of bone marrow infiltration. Doppler echocardiogram, chest X-ray and electrocardiogram are useful to exclude pulmonary hypertension (Table 2) (40). Different biochemical biomarkers are evaluated to quantify dynamic changes in the clinical course over the years: ferritin, immunoglobulin, angiotensin-converting enzyme (ACE), tartrate resistant acid phosphatase (TRAP) (41). At the present time plasma levels of the hydrolase chitotriosidase and the CC-chemokine ligand 18/pulmonary activated-related chemokine (CCL18/PARC) are considered useful tools. Both parameters correlate well with the body burden of Gaucher cells. Chitotriosidase activity in serum can increase up to fold over the normal values in GD, and is reduced by treatment. However, 5-6% of the general population is homozygous for the chitotriosidase gene mutation causing complete deficiency of the enzyme activity (42). Chitotriosidase measurement is unreliable in these situations. Serum CCL18/PARC, which is not affected by any known genetic abnormality, is increased fold over the normal levels in GD and decreases during therapy with a pattern similar to chitotriosidase (43). Disease management GD is a multi-organ, chronic, heterogeneous disorder, requiring an individualized approach towards treatment. Many variables such as severity and rate of disease progression, concomitant pathological conditions, the impact of disease manifestations on quality of life and the phenotype/genotype relationship should be considered prior to the initiation of treatment in a patient with GD (10, 40, 44). At the present time, the therapeutic options for adult GD patients are enzyme replacement therapy (ERT) and substrate reduction therapy (SRT), although bone marrow transplantation and gene therapy have been applied in rare cases. It is generally accepted that a GD patient must be treated in the presence of complications such as anaemia, thrombocytopenia, bleeding tendency, skeletal disease, liver or lung involvement or organomegaly. Type 1 GD was the first lysosomal storage disorders for which an effective ERT was developed and it has become a prototype for treatments for related orphan diseases. After its introduction, in 1991, ERT has emerged as the standard of care for type 1 GD (45, 46). In order to establish the severity of disease and to tailor the initial and maintenance ERT dose, a classification in high- and low-risk type 1 GD patients has been suggested by a panel of experts (Tables 3, 4) (47). Over two decades since the introduction of therapy, it has become definitely clear that many of the symptoms and signs of visceral GD such as hepatosplenomegaly, as well as anaemia and thrombocytopenia, and often, skeletal or lung involvement, will respond adequately to ERT (48). Three different human recombinant enzymes have been approved; two of them are available in EU and USA: Imiglucerase (Cerezyme, Genzyme Corporation, Cambridge MA, USA) since 1994, and Velaglucerase alfa (VPRIV, Shire HGT, Cambridge MA, USA) since An additional preparation, 160 Clinical Cases in Mineral and Bone Metabolism 2015; 12(2)
5 Clinical manifestations and management of Gaucher disease Table 2 - Minimum recommendations for monitoring patients with non-neuronopathic GD (40). Table 3 - Type 1 GD Highest Risk Patients- One or More of the following symptoms (47). Symptomatic skeletal disease Moderate to severe osteopenia Avascular necrosis Chronic bone pain Pathological fractures Bone crises Joint replacement(s) Impaired quality of life due to GD Cardiopulmonary disease, including pulmonary hypertension Platelet count <60,000mm 3 or abnormal bleedings Symptomatic anaemia or haemoglobin < 8g/dl Transfusion dependency Significant liver disease Severe hepatomegaly (>2.5xnormal) Infarcts Varices Portal hypertension Hepatitis Significant spleen disease Severe splenomegaly (>15xnormal) Infarcts Significant renal disease Taliglucerase alfa (Elelyso, ProtalixBiotherapeutics, Carmiel, Israel) is available only in USA. The ICGG Registry has monitored the responses of a thousand patients world-wide to Imiglucerase (45). Decreased spleen and liver volumes and Table 4 - Type 1 GD Lower Risk Patients (47). Normal liver, cardiac, lung and renal function Minimal impairment of quality of life due to GD No obvious and recently rapid progression of disease manifestations Skeletal disease limited to mild osteopenia and Erlenmeyer flask deformity Haemoglobin>10.5 g/dl for females and >11.5 g/dl for males (or not more than 2 g/dl below lower limit of normal for age and sex) Platelet count >60,000mm 3 on three determinations Liver volume <2.5 x normal Spleen volume < 15 x normal increased haemoglobin levels and platelet counts usually occur within 6 months of advent of therapy with every-otherweek doses of units/kg body weight. Platelet count in patients with massively enlarged spleens may require longer periods to respond, but dramatic improvements usually continue within the first 2-4 years of therapy (46, 49). Normalization or near-normalization of haemoglobin and platelet count as well as liver and spleen volume, with reduction in bone pains and prevention of irreversible skeletal complications are the ultimate end-points. Improvement in bone marrow and in Clinical Cases in Mineral and Bone Metabolism 2015; 12(2)
6 S. Linari et al. Table 5 - Therapeutic goals for ERT in GD patients (49). the osseous skeleton in response to ERT has been observed to occur more slowly than the visceral and haematological responses. Increase of bone mineral density in response to ERT could take up to 8 years (50), and ERT is effective in ameliorating bone marrow infiltration only after years (Table 5) (51). Besides, pathological damage such as osteonecrosis, bone infarcts and fracture, once it has occurred, is irreversible, so the maximal benefit derived from ERT in terms of eliciting a response in the skeletal system may occur with early enzyme administration and preventive approaches. The use of biphosphonates can be an effective and safe mean to increase bone density and prevent complications (52). Supportive management for bone pains or bone crises is frequently required, and orthopedic surgery may be necessary in cases of pathologic fractures or osteonecrosis (53). Substrate synthesis inhibition therapy is an alternative oral approach, based on reduced synthesis of glucosylceramide by inhibiting the appropriate synthetic enzyme (i.e. glucosylceramide synthase), resultanting in decreased production of this dangerous lipid and the ability of the residual enzyme activity to restablish a new steady state. This approach was first tried with the iminosugar N-butyldeoxynojirimycin, Miglustat (Zavesca, Actelion Corp) (54), approved by EMA in 2002 for patients with mild-to-moderate GD who are unsuitable for ERT and by FDA in 2003 for patients in whom ERT is not a therapeutic option. Clinical trials demonstrated effects on the visceral organs in type 1 GD with some shrinking of hepatosplenomegaly and improvement of haematologic findings. However, the substantial adverse events related to the use of miglustat, in particular significant diarrhea, and controversial tremor and paresthesias, limited drug s acceptance. Ceramide analog of the substrate, Eliglustat (Genz ; Genzyme Corp) (55) is a novel agent with a better safety profile and higher potency than miglustat. Eliglustat was approved by FDA in August 2014 and approval procedures by EMA in January Therapeutic goals for anemia Increase hemoglobin levels within 12 to 24 months to 11g/dl for women and children 12g/dl for men Eliminate blood transfusion dependency and reduce fatigue, dyspnoea and angina Maintain improved hemoglobin values achieved after 12 to 24 months of therapy Therapeutic goals for thrombocytopenia Increase platelet count during the first year of therapy sufficiently, to prevent surgical, obstetrical and spontaneous bleeding Patients with splenectomy - normalization of platelet count by one year of treatment Moderate baseline thrombocytopenia - the platelet count should increase by 1.5- to 2-fold by year one and approach normal levels by year two Severe thrombocytopenia - the platelet count should increase by 1.5-fold by year one and continue to increase slightly during years two to five, but normalization is not expected Avoid splenectomy Maintain stable platelet counts to eliminate risk of bleeding Therapeutic goals for hepatomegaly and splenomegaly Reduce and maintain the liver volume to 1.0 to 1.5 times normal Reduce the liver volume by 20 to 30% within years one to two by 30 to 40% by years three to five Reduce and maintain spleen volume to less than two to eight times normal Reduce the spleen volume by reduce and maintain the 30 to 50% by year one and by 50 to 60% by years two to five Alleviate symptoms due to splenomegaly Eliminate hypersplenism Therapeutic goals for skeletal pathology Lessen or eliminate pain within one to two years Prevent bone crisis Prevent osteonecrosis and subchondral joint collapse Improve bone mineral density Increase trabecular bone mineral density by three to five years Pediatric patients: Attain normal or ideal peak skeletal mass Increase cortical and bone mineral density (BMD) by two years Therapeutic goals for growth in pediatric patients Normalise growth and achieve normal onset of puberty Therapeutic goals for pulmonary involvement Reverse hepatopulmonary syndrome and dependency on oxygen Ameliorate pulmonary hypertension (ERT+ adjuvant therapies) Improve functional status and quality of life Prevent sudden death Prevent pulmonary disease by timely initiation of ERT and avoidance of splenectomy 162 Clinical Cases in Mineral and Bone Metabolism 2015; 12(2)
7 Clinical manifestations and management of Gaucher disease Conclusion Gaucher is a rare disease with heterogeneous multisystem involvement. The non-neuronopathic GD may show different symptoms at any age. Related to the predominant manifestation, GD patient can refer to different specialists (pediatrician, internist, hematologist for hematological changes, gastroenterologist for hepatosplenomegaly, rheumatologist or orthopedic for bone disease), but the rarity of the disease and nonspecific and heterogeneous nature of GD symptoms may impede its consideration in the differential diagnosis. Since diagnosis of GD by enzyme testing is unequivocal, performing the test may be convenient in patients with dubious pathological signs. A delay in diagnosis can cause the occurrence of irreversible complications, such as avascular necrosis or MM, in a rare disease for which an effective treatment is available. Different aspects of pathophysiology and in particular determination of disease severity remains incompletely understood, and the precise relationship between GD and certain co-morbidities, especially cancer, remains unclear. Moreover, bone disease and its pathophysiology are yet to be fully understood to aid diagnosis, monitoring and treatment of GD. References 1. Brady RO, Kanfer JN, Bradley RM, et al. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher s disease. J Clin Invest. 1966;45(7): Grabowski GA, Petsko GA, Kolodny EH. Gaucher disease. In The Online Metabolic and Molecular Bases of Inherited Disease ( New York: McGraw-Hill, 2006 (revised July 2010). 3. Cox TM. Gaucher disease: understanding the molecular pathogenesis of sphingolipidoses. J Inherit Metab Dis. 2001:24(Suppl 2): Barak V, Acker M, Nisman B, et al. Cytokines in Gaucher s disease. Eur Cytokine Netw. 1999;10(2): Hollak CE, van Weely S, van Oers MH, et al. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93: Mizukami H, Mi Y, Wada R, et al. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002;109(9): Rosenbloom BE, Weinreb NJ, Zimran A, et al. Gaucher disease and cancer incidence: a study from the Gaucher Registry. Blood. 2005;105 (12): Arends M, van Dussen L, Biegstraaten M, et al. Malignancies and monoclonal gammopathy in Gaucher disease; a systematic review of the literature. Br J Haematol. 2013;161(6): Futerman AH, Zimran A. Gaucher Disease. Taylor & Francis Group, Boca Raton, FL, Charrow J, Esplin JA, Gribble TJ, et al. Gaucher disease: recommendations on diagnosis, evaluation, and monitoring. Arch Intern Med. 1998;158: Olivova P, Cullen E, Titlow M, et al. An improved high-throughpout dried blood spot screening method for Gaucher disease. Clin Chim Acta. 2008;398: Grabowsky GA. Gaucher Disease and other storage disorders. Hematology/the Education Program of American Society of Hematology. 2012: Tamargo RJ, Velayati A, Goldin E, et al. The role of saposin C in Gaucher disease. Mol Genet Metab. 2012;106: Koprivica V, Stone DL, Park JL, et al. Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet. 2000;66: Grabowsky GA. Gaucher Disease and other storage disorders. Hematology/the Education Program of American Society of Hematology. 2012: Zimran A, Elstein D, Abrahamov A, et al. Prenatal molecular diagnosis of Gaucher disease. Pren Diagn. 1995;15: Beutler E, Nguyen NJ, Henneberger MW, et al. Gaucher disease: gene frequencies in the Ashkenazi Jewish population. Am J Hum Genet. 1993;52(1): Charrow J, Andersson HC, Kaplan P, et al. The Gaucher Registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch Internal Med. 2000;160: Mistry PK, Sirrs S, Chan A, et al. Pulmonary hypertension in type 1 Gaucher s disease: genetic and epigenetic determinants of phenotype and response to therapy. Mol Genet Metab. 2002; 77: Beutler E, Grabowsky GA. Gaucher Disease. In: The Metabolic and Molecular Bases of Inherited Disease, 2001 Vol II (Ed. By Scriver CR, Sly WS, Childs B, Beaudet AL, Valle D, Kinzler KW, Vogelstein B): McGraw-Hill Inc., Columbus, USA. 21. Erikson A, Bembi B, Schiffmann R. Neuronopathic forms of Gaucher s disease. In: Gaucher s Disease, Vol. 10 (Ed. By A. Zimran), 1997, pp , BailliereTindall, London. 22. Aharon-Peretz J, Rosenbaum H, Geshoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson s disease in Ashkenazi Jews. N Engl J Med. 2004;351: Lwin A, Orvisky E, Goker-Alpan O. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81: Goker-Alpan O, Schiffmann R, Park JK. Phenotypic continuum in neuronopathic Gaucher disease: an intermediate phenotype between type 2 and type 3. Journal of Pediatrics. 2003;143: Brautbar A, Elstein D, Pines G, et al. Effect of enzyme replacement therapy on gammopathies in Gaucher Disease. Blood Cells Mol Dis. 2004;32: de Fost M, Out TA, de Wilde FA, et al. Immunoglobulin and free light chain abnormalities in Gaucher disease type 1: data from an adult cohort of 63 patients and review of the literature. Ann Hematol. 2008;87: Jurecka A, Gregorek H, Kleinotiene G, et al. Gaucher disease and dysgammaglobulinemia: a report of 61 patients, including 18 with GD type III. Blood Cells Mol Dis. 2011;46: Rosenbloom BE, Weinreb NJ, Zimran A, et al. Gaucher disease and cancer incidence: a study from the Gaucher Registry. Blood. 2005;105 (12): de Fost M, Von Dahl S, Weverling GJ, et al. Increased incidence of cancer in adult Gaucher disease in Western Europe. Blood Cells Mol Dis. 2006;36: Arends M, van Dussen L, Biegstraaten M, et al. Malignancies and monoclonal gammopathy in Gaucher disease; a systematic review of the literature. Br J Haematol. 2013;161(6): Taddei TH, Kacena KA, Yang M, et al. The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients. Am J Hematol. 2009;84: Zimran A. Gaucher s Disease. Ed. Balliere Tindall, Cambridge UK, Marcucci G, Zimran A, Bembi B, et al. Gaucher disease and bone manifestations. Calcif Tissue Int. 2014; 95: Gaucher Registry Annual Report 2012; gaucherregistry/. 35. Mass M, Poll LW, Terk MR. Imaging and quantifying skeletal involvement in Gaucher disease. Br J Radiol. 2002;75(suppl 1):A13-A Wenstrup RJ, Roce-Espiau M, Weinreb NJ. Skeletal aspects of Gaucher disease: a review. Br J Radiol. 2002;75(suppl 1):A2-A Katz K, Hover G, Grunebaum M. The natural history of osteonecrosis of the femoral head in children and adolescents who have Gaucher disease. J Bone Joint Surg Am. 1996;78: Bell RS, Mankin HJ, Doppelt SH. Osteomyelitis in Gaucher disease. J Bone Joint Surg Am. 1986;68: Pastores GM, Wallenstein S, Desnick RJ, et al. Bone density in type 1 Gaucher disease. J Bone Min Res. 1996;11: Weinreb NJ, Aggio MC, Andersson HC, et al. Gaucher disease type 1: revised recommendations on evaluations and monitoring for adult patients. Semin Hematol. 2004;41 (suppl 5): Aerts JM, Hollak CE, van Breemen M, et al. Identification and use of biomarkers in Gaucher disease and other lysosomal storage diseases. Acta Paediatr Suppl. 2005;94: Clinical Cases in Mineral and Bone Metabolism 2015; 12(2)
8 S. Linari et al. 42. Deegan PB, Cox TM. Clinical evaluation of biomarkers in Gaucher disease. Acta Paediatr Suppl. 2005;94: Boot RG, Verhoek M, de Fost M, et al. Marked elevation of the chemokine CCL18/PARC in Gaucher disease: a novel surrogate marker for assessing therapeutic intervention. Blood. 2004;103: Charrow J, Andersson HC, Kaplan P, et al. Enzyme replacement therapy and monitoring for children with type 1 Gaucher disease: consensus recommendations. J Pediatr. 2004;144: Weinreb NJ, Charrow J, Andersson HC, et al. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. Am J Med. 2002;113: Weinreb NJ, Goldblatt J, Villalobos J, et al. Long-term clinical outcomes in type 1 Gaucher disease following 10 years of imiglucerase treatment. J Inherit Metab Dis. 2013;36(3): Andersson HC, Charrow J, Kaplan P, et al. Individualization of long-time enzyme replacement therapy for Gaucher disease. Genet Med. 2005;7(2): Elstein D, Zimran A. Review of the safety and efficacy of imiglucerase treatment of Gaucher disease. Biologics. 2009;3: Pastores GM, Weinreb NJ, Aerts H, et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41: Wenstrup RJ, Kacena KA, Kaplan P, et al. Effect of enzyme replacement therapy with imiglucerase on BMD in type 1 Gaucher disease. J Bone Miner Res. 2007;22: Robertson PL, Maas M, Goldblatt J. Semiquantitative assessment of skeletal response to enzyme replacement therapy for Gaucher s disease using the bone marrow burden score. Am J Roentgenol. 2007;188: Wenstrup RJ, Baily L, Grabowsky GA, et al. Gaucher disease: alendronate disodium improves bone mineral density in adults receiving enzyme therapy. Blood. 2004;104: Giuffrida G, Cappellini MD, Carubbi F, et al. Management of bone disease in Gaucher disease type 1: clinical practice. Adv Ther. 2014;31(12): Cox TM, Aerts JM, Andria G, et al. The role of the iminosugar N- butyldeoxynojirimycin (miglustat) in the management of type I (nonneuronopathic) Gaucher disease. J Inherit Metab Dis. 2003;26: Mc Eachern KA, Fung J, Komarnitsky S, et al. A specific and potent inhibitor of glucosylceramide synthase for substrate reduction therapy of Gaucher disease. Mol Genet Metab. 2007;91: Clinical Cases in Mineral and Bone Metabolism 2015; 12(2)
Diagnosis, monitoring and treatment of adult Gaucher patients
 Diagnosis, monitoring and treatment of adult Gaucher patients Stephan vom Dahl, M.D., Professor of Medicine St. Franziskus Hospital, Köln, Germany Podčetrtek, Slovenia, April 22, 2006 Strokovni Sestanek
Diagnosis, monitoring and treatment of adult Gaucher patients Stephan vom Dahl, M.D., Professor of Medicine St. Franziskus Hospital, Köln, Germany Podčetrtek, Slovenia, April 22, 2006 Strokovni Sestanek
ARTICLE. The Clinical and Demographic Characteristics of Nonneuronopathic Gaucher Disease in 887 Children at Diagnosis
 ARTICLE The Clinical and Demographic Characteristics of Nonneuronopathic Gaucher Disease in 887 Children at Diagnosis Paige Kaplan, MBBCh; Hans C. Andersson, MD; Katherine A. Kacena, PhD; John D. Yee,
ARTICLE The Clinical and Demographic Characteristics of Nonneuronopathic Gaucher Disease in 887 Children at Diagnosis Paige Kaplan, MBBCh; Hans C. Andersson, MD; Katherine A. Kacena, PhD; John D. Yee,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Substrate Reduction Therapy Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Substrate Reduction Therapy! Prime Therapeutics will review Prior Authorization
Substrate Reduction Therapy Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Substrate Reduction Therapy! Prime Therapeutics will review Prior Authorization
Goal-oriented therapy with miglustat in Gaucher disease
 CURRENT MEDICAL RESEARCH AND OPINIONÕ 0300-7995 VOL. 25, NO. 1, 2009, 23 37 doi:10.1185/03007990802576518 ß 2009 Informa UK Ltd. All rights reserved: reproduction in whole or part not permitted REVIEW
CURRENT MEDICAL RESEARCH AND OPINIONÕ 0300-7995 VOL. 25, NO. 1, 2009, 23 37 doi:10.1185/03007990802576518 ß 2009 Informa UK Ltd. All rights reserved: reproduction in whole or part not permitted REVIEW
The following three cases of Gaucher Disease (GD) illustrate situations encountered in clinical practice; several common pitfalls are highlighted.
 SUPPLEMENT Management of Bone Disease in Gaucher Disease Type 1: Clinical Practice ABSTRACT Gaucher disease (GD) is a rare autosomal recessive disorder of glycosphingolipid metabolism resulting from deficient
SUPPLEMENT Management of Bone Disease in Gaucher Disease Type 1: Clinical Practice ABSTRACT Gaucher disease (GD) is a rare autosomal recessive disorder of glycosphingolipid metabolism resulting from deficient
Long-term treatment outcomes in Gaucher disease
 REVIEW Long-term treatment outcomes in Gaucher disease AJH Joel Charrow 1,2 * and C. Ronald Scott 3 Following the treatment of the first Gaucher disease patient with enzyme replacement therapy (ERT), it
REVIEW Long-term treatment outcomes in Gaucher disease AJH Joel Charrow 1,2 * and C. Ronald Scott 3 Following the treatment of the first Gaucher disease patient with enzyme replacement therapy (ERT), it
Gaucher Disease: a multiorgan rare disease in Internal Medicine. M.Domenica Cappellini Fondazione Policlinico IRCCS University of Milan
 Gaucher Disease: a multiorgan rare disease in Internal Medicine M.Domenica Cappellini Fondazione Policlinico IRCCS University of Milan XXXI Congreso Nacional de la Sociedad Espanola de Medicina Interna
Gaucher Disease: a multiorgan rare disease in Internal Medicine M.Domenica Cappellini Fondazione Policlinico IRCCS University of Milan XXXI Congreso Nacional de la Sociedad Espanola de Medicina Interna
The musculoskeletal manifestations of Gaucher's disease
 The musculoskeletal manifestations of Gaucher's disease Poster No.: C-2179 Congress: ECR 2010 Type: Educational Exhibit Topic: Musculoskeletal Authors: S. M. M. McDonald, M. A. Hopper, P. W. P. Bearcroft;
The musculoskeletal manifestations of Gaucher's disease Poster No.: C-2179 Congress: ECR 2010 Type: Educational Exhibit Topic: Musculoskeletal Authors: S. M. M. McDonald, M. A. Hopper, P. W. P. Bearcroft;
Report of Four Children with Gaucher Disease and Review of Literature
 http:// ijp.mums.ac.ir Case Report (Pages: 2287-2293) Report of Four Children with Gaucher Disease and Review of Literature Wajiha Maan 1, *Manoochehr Karjoo 1, Mirza Beg 112 1 Department of Pediatric
http:// ijp.mums.ac.ir Case Report (Pages: 2287-2293) Report of Four Children with Gaucher Disease and Review of Literature Wajiha Maan 1, *Manoochehr Karjoo 1, Mirza Beg 112 1 Department of Pediatric
TYPE 1 GAUCHER DISEASE PRESENTING AS PERSISTENT THROMBOCYTOPENIA, ASSOCIATED FACTOR XI DEFICIENCY & EMERGENT MYELOMA
 TYPE 1 GAUCHER DISEASE PRESENTING AS PERSISTENT THROMBOCYTOPENIA, ASSOCIATED FACTOR XI DEFICIENCY & EMERGENT MYELOMA Trish Hyland, Medical Scientist, Department of Haematology, Cork University Hospital
TYPE 1 GAUCHER DISEASE PRESENTING AS PERSISTENT THROMBOCYTOPENIA, ASSOCIATED FACTOR XI DEFICIENCY & EMERGENT MYELOMA Trish Hyland, Medical Scientist, Department of Haematology, Cork University Hospital
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (VPRIV) Reference Number: CP.PHAR.163 Effective Date: 02.01.16 Last Review Date: 05.18 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (VPRIV) Reference Number: CP.PHAR.163 Effective Date: 02.01.16 Last Review Date: 05.18 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important Reminder
Nadia Belmatoug Referral Centre for Lysosomal Diseases University Hospital Paris Nord-Val de Seine Assistante Publique Hôpitaux de Paris
 Nadia Belmatoug Referral Centre for Lysosomal Diseases University Hospital Paris Nord-Val de Seine Assistante Publique Hôpitaux de Paris Meeting initiated, organised and funded by Shire International GmbH
Nadia Belmatoug Referral Centre for Lysosomal Diseases University Hospital Paris Nord-Val de Seine Assistante Publique Hôpitaux de Paris Meeting initiated, organised and funded by Shire International GmbH
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Cerezyme) Reference Number: CP.PHAR.154 Effective Date: 02.01.16 Last Review Date: 05.18 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Cerezyme) Reference Number: CP.PHAR.154 Effective Date: 02.01.16 Last Review Date: 05.18 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important Reminder
Skeletal improvement in patients with Gaucher disease type 1: a phase 2 trial of oral eliglustat
 Skeletal Radiol (2014) 43:1353 1360 DOI 10.1007/s00256-014-1891-9 SCIENTIFIC ARTICLE Skeletal improvement in patients with Gaucher disease type 1: a phase 2 trial of oral eliglustat Ravi S. Kamath & Elena
Skeletal Radiol (2014) 43:1353 1360 DOI 10.1007/s00256-014-1891-9 SCIENTIFIC ARTICLE Skeletal improvement in patients with Gaucher disease type 1: a phase 2 trial of oral eliglustat Ravi S. Kamath & Elena
ULTRASTRUCTURAL FEATURES OF GAUCHER DISEASE TREATED WITH ENZYME REPLACEMENT THERAPY PRESENTING AS MESENTERIC MASS LESIONS
 Fetal and Pediatric Pathology, 25:241 248, 2006 Copyright # Informa Healthcare ISSN: 1551-3815 print/1551-3823 online DOI: 10.1080/15513810601123334 ULTRASTRUCTURAL FEATURES OF GAUCHER DISEASE TREATED
Fetal and Pediatric Pathology, 25:241 248, 2006 Copyright # Informa Healthcare ISSN: 1551-3815 print/1551-3823 online DOI: 10.1080/15513810601123334 ULTRASTRUCTURAL FEATURES OF GAUCHER DISEASE TREATED
2019 Update in Neuronopathic GD
 2019 Update in Neuronopathic GD Pramod K Mistry, MD, PhD, Professor of Medicine and Pediatrics Annual NYC Meeting, Museum of the City of New York October, 29, 2017 S L I D E 1 Disclosures Received research
2019 Update in Neuronopathic GD Pramod K Mistry, MD, PhD, Professor of Medicine and Pediatrics Annual NYC Meeting, Museum of the City of New York October, 29, 2017 S L I D E 1 Disclosures Received research
Substrate reduction therapy as a new treatment option for patients with Gaucher disease type 1: A review of literatures
 Review Article J Genet Med 2016;13(2):59-64 https://doi.org/10.5734/jgm.2016.13.2.59 ISSN 1226-1769 (Print) 2383-8442 (Online) Journal of JGM Genetic Medicine Substrate reduction therapy as a new treatment
Review Article J Genet Med 2016;13(2):59-64 https://doi.org/10.5734/jgm.2016.13.2.59 ISSN 1226-1769 (Print) 2383-8442 (Online) Journal of JGM Genetic Medicine Substrate reduction therapy as a new treatment
POTENTIAL LINK BETWEEN GAUCHER DISEASE PATHWAYS
 POTENTIAL LINK BETWEEN GAUCHER DISEASE PATHWAYS AND THOSE OF PARKINSON DISEASE Hanna Rosenbaum, MD Hematology and Bone Marrow Transplantation Rambam Medical Center and Bruce Rappaport Faculty of Medicine
POTENTIAL LINK BETWEEN GAUCHER DISEASE PATHWAYS AND THOSE OF PARKINSON DISEASE Hanna Rosenbaum, MD Hematology and Bone Marrow Transplantation Rambam Medical Center and Bruce Rappaport Faculty of Medicine
Pharmacy Medical Policy Alglucerase (Ceredase and Cerezyme ) for Gaucher Disease
 Pharmacy Medical Policy Alglucerase (Ceredase and Cerezyme ) for Gaucher Disease Table of Contents Policy: Commercial Policy History Endnotes Policy: Medicare Information Pertaining to All Policies Forms
Pharmacy Medical Policy Alglucerase (Ceredase and Cerezyme ) for Gaucher Disease Table of Contents Policy: Commercial Policy History Endnotes Policy: Medicare Information Pertaining to All Policies Forms
This policy addresses the coverage of Cerdelga (eliglustat) for the treatment of Gaucher disease Type 1 when appropriate criteria are met.
 Subject: Cerdelga (eliglustat) Original Effective Date: 12/5/2014 Policy Number: MCP-227 Revision Date(s): 12/15/2016; 6/22/2017 Review Dates: DISCLAIMER This Molina Clinical Policy (MCP) is intended to
Subject: Cerdelga (eliglustat) Original Effective Date: 12/5/2014 Policy Number: MCP-227 Revision Date(s): 12/15/2016; 6/22/2017 Review Dates: DISCLAIMER This Molina Clinical Policy (MCP) is intended to
Velaglucerase alfa in the treatment of Gaucher disease type 1: an update
 Velaglucerase alfa in the treatment of Gaucher disease type 1: an update Gaucher disease (GD) is an autosomal recessive lysosomal storage disorder caused by deficiency of the enzyme acid β-glucosidase.
Velaglucerase alfa in the treatment of Gaucher disease type 1: an update Gaucher disease (GD) is an autosomal recessive lysosomal storage disorder caused by deficiency of the enzyme acid β-glucosidase.
P.K. Tandon, PhD J. Alexander Cole, DSc. Use of Registries for Clinical Evaluation of Rare Diseases
 Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the Kakkis EveryLife Foundation, for educational purposes only. They are for use by drug development professionals and
Disclaimer: Presentation slides from the Rare Disease Workshop Series are posted by the Kakkis EveryLife Foundation, for educational purposes only. They are for use by drug development professionals and
Enzyme Replacement Therapy for Gaucher Disease: The Only Experience in Malaysia
 Enzyme Replacement Therapy for Gaucher Disease: The Only Experience in Malaysia L L Chan, FRCP, H P Lin, FRCP Department of Paediatrics, University of Malaya, 50603, Lembah Pantai, Kuala Lumpur Introduction
Enzyme Replacement Therapy for Gaucher Disease: The Only Experience in Malaysia L L Chan, FRCP, H P Lin, FRCP Department of Paediatrics, University of Malaya, 50603, Lembah Pantai, Kuala Lumpur Introduction
Gaucher disease type I : associated morbidities and long term efficacy of enzyme replacement therapy de Fost, M.
 UvA-DARE (Digital Academic Repository) Gaucher disease type I : associated morbidities and long term efficacy of enzyme replacement therapy de Fost, M. Link to publication Citation for published version
UvA-DARE (Digital Academic Repository) Gaucher disease type I : associated morbidities and long term efficacy of enzyme replacement therapy de Fost, M. Link to publication Citation for published version
A reappraisal of Gaucher disease Diagnosis and disease management algorithms
 AJH Educational Material Consensus Conference A reappraisal of Gaucher disease Diagnosis and disease management algorithms Pramod K. Mistry, 1 * Maria Domenica Cappellini, 2 Elena Lukina, 3 Hayri Özsan,
AJH Educational Material Consensus Conference A reappraisal of Gaucher disease Diagnosis and disease management algorithms Pramod K. Mistry, 1 * Maria Domenica Cappellini, 2 Elena Lukina, 3 Hayri Özsan,
Impact of Imiglucerase Supply Shortage on Clinical and Laboratory Parameters in Norrbottnian Patients with Gaucher Disease Type 3
 Arch. Immunol. Ther. Exp. (2015) 63:65 71 DOI 10.1007/s00005-014-0308-8 ORIGINAL ARTICLE Impact of Imiglucerase Supply Shortage on Clinical and Laboratory Parameters in Norrbottnian Patients with Gaucher
Arch. Immunol. Ther. Exp. (2015) 63:65 71 DOI 10.1007/s00005-014-0308-8 ORIGINAL ARTICLE Impact of Imiglucerase Supply Shortage on Clinical and Laboratory Parameters in Norrbottnian Patients with Gaucher
SCIENTIFIC DISCUSSION
 SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Cerezyme. This scientific discussion has been updated until 01 August 2003. For information on changes after
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Cerezyme. This scientific discussion has been updated until 01 August 2003. For information on changes after
Case series. Gaucher s disease: report of 11 cases with review of literature. Open Access
 Case series Open Access Gaucher s disease: report of 11 cases with review of literature Laila Essabar 1, Toufik Meskini 1,&, Najat Lamalmi 2, Said Ettair 1, Naima Erreimi 1, Nezha Mouane 1 1 FMPR, University
Case series Open Access Gaucher s disease: report of 11 cases with review of literature Laila Essabar 1, Toufik Meskini 1,&, Najat Lamalmi 2, Said Ettair 1, Naima Erreimi 1, Nezha Mouane 1 1 FMPR, University
A new severity score index for phenotypic classification and evaluation of responses to treatment in type I Gaucher disease
 A new severity score index for phenotypic classification and evaluation of responses to treatment in type I Gaucher disease Maja Di Rocco, 1 Fiorina Giona, 2 Francesca Carubbi, 3 Silvia Linari, 4 Fabrizio
A new severity score index for phenotypic classification and evaluation of responses to treatment in type I Gaucher disease Maja Di Rocco, 1 Fiorina Giona, 2 Francesca Carubbi, 3 Silvia Linari, 4 Fabrizio
ORIGINAL INVESTIGATION. Significant Disease Manifestations in Asymptomatic Homozygotes
 Type 1 Gaucher Disease ORIGINAL INVESTIGATION Significant Disease Manifestations in Asymptomatic Homozygotes Manisha Balwani, MD, MS; Laura Fuerstman, MA; Ruth Kornreich, PhD; Lisa Edelmann, PhD; Robert
Type 1 Gaucher Disease ORIGINAL INVESTIGATION Significant Disease Manifestations in Asymptomatic Homozygotes Manisha Balwani, MD, MS; Laura Fuerstman, MA; Ruth Kornreich, PhD; Lisa Edelmann, PhD; Robert
TREATMENTS FOR GAUCHER DISEASE
 TREATMENTS FOR GAUCHER DISEASE Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
TREATMENTS FOR GAUCHER DISEASE Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Review of miglustat for clinical management in Gaucher disease type 1
 REVIEW Review of miglustat for clinical management in Gaucher disease type 1 Can Ficicioglu The Children s Hospital of Philadelphia, Section of Biochemical Genetics Abstract: Gaucher disease is a progressive
REVIEW Review of miglustat for clinical management in Gaucher disease type 1 Can Ficicioglu The Children s Hospital of Philadelphia, Section of Biochemical Genetics Abstract: Gaucher disease is a progressive
IMAGING FINDINGS OF GAUCHER DISEASE IN ALL OVER THE BODY
 IMAGING FINDINGS OF GAUCHER DISEASE IN ALL OVER THE BODY V. Katsaros 1, P. Lampropoulou 2, M. Mitropoulou 1, A. Nikolaou 1, C. Drossos 2 Departments of CT and MRI 1 IKA Oncology Hospital and 2 Athens General
IMAGING FINDINGS OF GAUCHER DISEASE IN ALL OVER THE BODY V. Katsaros 1, P. Lampropoulou 2, M. Mitropoulou 1, A. Nikolaou 1, C. Drossos 2 Departments of CT and MRI 1 IKA Oncology Hospital and 2 Athens General
Selected Issues in Nonneoplastic. Orthopaedic Pathology. Diseases of Diarthrodial Joints and their Complications
 1 2 3 Selected Issues in Nonneoplastic Orthopaedic Pathology Diseases of Diarthrodial Joints and their Complications Biology of articular hyaline cartilage Arthritis Osteonecrosis Prosthetic Joints Metabolic
1 2 3 Selected Issues in Nonneoplastic Orthopaedic Pathology Diseases of Diarthrodial Joints and their Complications Biology of articular hyaline cartilage Arthritis Osteonecrosis Prosthetic Joints Metabolic
S2 Protein augmentation therapies for inherited disorders 1
 Disease category Disorder S2 Protein augmentation therapies for inherited 1 Augmented protein 2 Source of therapeutic protein / peptide Outcome References 3 Membrane transport Coagulation Cystic fibrosis
Disease category Disorder S2 Protein augmentation therapies for inherited 1 Augmented protein 2 Source of therapeutic protein / peptide Outcome References 3 Membrane transport Coagulation Cystic fibrosis
Taliglucerase alfa: safety and efficacy across 6 clinical studies in adults and children with Gaucher disease
 Zimran et al. Orphanet Journal of Rare Diseases (2018) 13:36 https://doi.org/10.1186/s13023-018-0776-8 REVIEW Taliglucerase alfa: safety and efficacy across 6 clinical studies in adults and children with
Zimran et al. Orphanet Journal of Rare Diseases (2018) 13:36 https://doi.org/10.1186/s13023-018-0776-8 REVIEW Taliglucerase alfa: safety and efficacy across 6 clinical studies in adults and children with
Evaluation of disease burden and response to treatment in adults with type 1 gaucher disease using a validated disease severity scoring system (DS3)
 Evaluation of disease burden and response to treatment in adults with type 1 gaucher disease using a validated disease severity scoring system (DS3) Neal J. Weinreb, University Research Foundation for
Evaluation of disease burden and response to treatment in adults with type 1 gaucher disease using a validated disease severity scoring system (DS3) Neal J. Weinreb, University Research Foundation for
A Lysosomal storage disease mimicking common paediatric symptoms?
 A Lysosomal storage disease mimicking common paediatric symptoms? Dominique Roland 1, François Eyskens 2 1 Institut de Pathologie et de Génétique, Génétique Humaine, Centre Agréé des maladies héréditaires
A Lysosomal storage disease mimicking common paediatric symptoms? Dominique Roland 1, François Eyskens 2 1 Institut de Pathologie et de Génétique, Génétique Humaine, Centre Agréé des maladies héréditaires
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Cerdelga) Reference Number: CP.PHAR.153 Effective Date: 07.01.18 Last Review Date: 05.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Cerdelga) Reference Number: CP.PHAR.153 Effective Date: 07.01.18 Last Review Date: 05.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end of this policy
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.241 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Reference Number: CP.CPA.241 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
MR Evaluation of Bone Marrow Disorders. Nisha Patel, MD
 MR Evaluation of Bone Marrow Disorders Nisha Patel, MD 1 Introduction Nearly all imaging modalities evaluate the marrow, which is a site of significant pathology Radiography Nuclear Medicine CT MR 2 Topics
MR Evaluation of Bone Marrow Disorders Nisha Patel, MD 1 Introduction Nearly all imaging modalities evaluate the marrow, which is a site of significant pathology Radiography Nuclear Medicine CT MR 2 Topics
Modelling Gaucher disease progression: long-term enzyme replacement therapy reduces the incidence of splenectomy and bone complications
 van Dussen et al. Orphanet Journal of Rare Diseases 24, 9:2 RESEARCH Open Access Modelling Gaucher disease progression: long-term enzyme replacement therapy reduces the incidence of splenectomy and bone
van Dussen et al. Orphanet Journal of Rare Diseases 24, 9:2 RESEARCH Open Access Modelling Gaucher disease progression: long-term enzyme replacement therapy reduces the incidence of splenectomy and bone
Inheritance of Gaucher Disease
 Sarah Mother of a child with Gaucher Working toward a healthy future Helping her son achieve his own dreams, too Straight Talk For Patients and Families Inheritance of Gaucher Disease Genzyme Corporation
Sarah Mother of a child with Gaucher Working toward a healthy future Helping her son achieve his own dreams, too Straight Talk For Patients and Families Inheritance of Gaucher Disease Genzyme Corporation
Screening for and Assessment of Osteonecrosis in Oncology Patients. Sue C. Kaste, DO SPR Postgraduate Course 2015
 Screening for and Assessment of Osteonecrosis in Oncology Patients Sue C. Kaste, DO SPR Postgraduate Course 2015 The author declares no potential conflicts of interest or financial disclosures Osteonecrosis
Screening for and Assessment of Osteonecrosis in Oncology Patients Sue C. Kaste, DO SPR Postgraduate Course 2015 The author declares no potential conflicts of interest or financial disclosures Osteonecrosis
How to Write a Life Care Plan for a Child with Hemoglobinopathy
 How to Write a Life Care Plan for a Child with Hemoglobinopathy Tamar Fleischer, BSN, MSN, CNLCP & Mona Yudkoff, RN, MPH, CRRN, CNLCP BalaCare Solutions March 2018 St. Peterburg, Florida What is Hemoglobinopathy?
How to Write a Life Care Plan for a Child with Hemoglobinopathy Tamar Fleischer, BSN, MSN, CNLCP & Mona Yudkoff, RN, MPH, CRRN, CNLCP BalaCare Solutions March 2018 St. Peterburg, Florida What is Hemoglobinopathy?
Enzyme replacement therapy in type 1 Gaucher disease and a review of the literature
 190 Case Report Enzyme replacement therapy in type 1 Gaucher disease and a review of the literature Tip 1 Gaucher hastalığında enzim replasman tedavisi ve literatürün gözden geçirilmesi Gökhan Kabaçam
190 Case Report Enzyme replacement therapy in type 1 Gaucher disease and a review of the literature Tip 1 Gaucher hastalığında enzim replasman tedavisi ve literatürün gözden geçirilmesi Gökhan Kabaçam
A Phase 2 Multi-center, Open-label, Switch-over Trial to Evaluate the Safety and Efficacy of Abcertin in Patients with Type 1 Gaucher Disease
 ORIGINAL ARTICLE Human Genetics & Genomics http://dx.doi.org/1.3346/jkms.215.3.4.378 J Korean Med Sci 215; 3: 378-384 A Phase 2 Multi-center, Open-label, Switch-over Trial to Evaluate the Safety and Efficacy
ORIGINAL ARTICLE Human Genetics & Genomics http://dx.doi.org/1.3346/jkms.215.3.4.378 J Korean Med Sci 215; 3: 378-384 A Phase 2 Multi-center, Open-label, Switch-over Trial to Evaluate the Safety and Efficacy
The role of the laboratory in diagnosing lysosomal disorders
 The role of the laboratory in diagnosing lysosomal disorders Dr Guy Besley, formerly Willink Biochemical Genetics Unit, Manchester Children s Hospital, Manchester M27 4HA, UK. Lysosomal disorders What
The role of the laboratory in diagnosing lysosomal disorders Dr Guy Besley, formerly Willink Biochemical Genetics Unit, Manchester Children s Hospital, Manchester M27 4HA, UK. Lysosomal disorders What
DATE: 22 December 2011 CONTEXT AND POLICY ISSUES
 TITLE: Eliglustat Tartrate, Miglustat, Imiglucerase, Velaglucerase or a Combination of These for the Treatment of Gaucher Disease: A Review of Clinical Effectiveness and Safety DATE: 22 December 2011 CONTEXT
TITLE: Eliglustat Tartrate, Miglustat, Imiglucerase, Velaglucerase or a Combination of These for the Treatment of Gaucher Disease: A Review of Clinical Effectiveness and Safety DATE: 22 December 2011 CONTEXT
Clinical and genetic characteristics of Gaucher disease according to phenotypic subgroups
 Original article http://dx.doi.org/.3345/kjp.22.55.2.4 Korean J Pediatr 22;55(2):4-53 Clinical and genetic characteristics of Gaucher disease according to phenotypic subgroups Ju-Young Lee, MD, Beom Hee
Original article http://dx.doi.org/.3345/kjp.22.55.2.4 Korean J Pediatr 22;55(2):4-53 Clinical and genetic characteristics of Gaucher disease according to phenotypic subgroups Ju-Young Lee, MD, Beom Hee
Non-commercial use only
 Hematology Reports 2012; volume 4:e21 Bone turnover markers in patients with type 1 Gaucher disease Gaetano Giuffrida, Maria Rocca Cingari, Nunziatina Parrinello, Alessandra Romano, Anna Triolo, Magda
Hematology Reports 2012; volume 4:e21 Bone turnover markers in patients with type 1 Gaucher disease Gaetano Giuffrida, Maria Rocca Cingari, Nunziatina Parrinello, Alessandra Romano, Anna Triolo, Magda
Combined miglustat and enzyme replacement therapy in two patients with type 1 Gaucher disease: two case reports
 Amato and Patterson Journal of Medical Case Reports (2018) 12:19 DOI 10.1186/s13256-017-1541-7 CASE REPORT Open Access Combined miglustat and enzyme replacement therapy in two patients with type 1 Gaucher
Amato and Patterson Journal of Medical Case Reports (2018) 12:19 DOI 10.1186/s13256-017-1541-7 CASE REPORT Open Access Combined miglustat and enzyme replacement therapy in two patients with type 1 Gaucher
DNA Polymorphism of Gaucher Disease in Iraqi Patients
 DNA Polymorphism of Gaucher Disease in Iraqi Patients *Shayma`a J. Ahmed (B.Sc., M.Sc., Ph.D.Sc. in Biotechnology) ** Mohammad F. Ibraheem (M.B.CH.B., D.C.H., F.I.C.M., C.A.B.P) *** Wissam Younis Al-temimi
DNA Polymorphism of Gaucher Disease in Iraqi Patients *Shayma`a J. Ahmed (B.Sc., M.Sc., Ph.D.Sc. in Biotechnology) ** Mohammad F. Ibraheem (M.B.CH.B., D.C.H., F.I.C.M., C.A.B.P) *** Wissam Younis Al-temimi
Evaluation of three biochemical markers in the monitoring of Gaucher disease
 585^592 # SSIEM and Springer. Printed in the Netherlands Evaluation of three biochemical markers in the monitoring of Gaucher disease A. Vellodi 1 *, Y. Foo 2 and T. J. Cole 3 1 Metabolic Unit, 2 Chemical
585^592 # SSIEM and Springer. Printed in the Netherlands Evaluation of three biochemical markers in the monitoring of Gaucher disease A. Vellodi 1 *, Y. Foo 2 and T. J. Cole 3 1 Metabolic Unit, 2 Chemical
Outcome of enzyme replacement therapy in Turkish patients with Gaucher disease: does late intervention affect the response?
 The Turkish Journal of Pediatrics 2011; 53: 499-507 Original Outcome of enzyme replacement therapy in Turkish patients with Gaucher disease: does late intervention affect the response? Zeynep Arıkan-Ayyıldız
The Turkish Journal of Pediatrics 2011; 53: 499-507 Original Outcome of enzyme replacement therapy in Turkish patients with Gaucher disease: does late intervention affect the response? Zeynep Arıkan-Ayyıldız
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cerezyme 200 U Powder for concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cerezyme 200 U Powder for concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
The glucocerebrosidase locus in Gaucher's disease: molecular analysis of a lysosomal
 J Med Genet 1993 30: 889-894 889 REVIEW ARTICLE Department of Medicine, University of Cambridge, Addenbrooke's Hospital, Hills Road, Cambridge CB2 2QQ, UK. P K Mistry T M Cox Correspondence to Dr Mistry.
J Med Genet 1993 30: 889-894 889 REVIEW ARTICLE Department of Medicine, University of Cambridge, Addenbrooke's Hospital, Hills Road, Cambridge CB2 2QQ, UK. P K Mistry T M Cox Correspondence to Dr Mistry.
Gaucher disease and other storage disorders
 3P SINAPOD Gaucher disease and other storage disorders Gregory A. Grabowski 1 1 Cincinnati Children s Hospital Medical Center, Cincinnati, OH In 1882, Philippe Gaucher described a 32-year-old woman with
3P SINAPOD Gaucher disease and other storage disorders Gregory A. Grabowski 1 1 Cincinnati Children s Hospital Medical Center, Cincinnati, OH In 1882, Philippe Gaucher described a 32-year-old woman with
Standardization of MRI and Scintigraphic Scores for Assessing the Severity of Bone Marrow Involvement in Adult Patients With Type 1 Gaucher Disease
 Musculoskeletal Imaging Original Research Mariani et al. Imaging Scoring Systems for Gaucher Disease Musculoskeletal Imaging Original Research Giuliano Mariani 1 Marzio Perri 1 Fabrizio Minichilli 2 Simona
Musculoskeletal Imaging Original Research Mariani et al. Imaging Scoring Systems for Gaucher Disease Musculoskeletal Imaging Original Research Giuliano Mariani 1 Marzio Perri 1 Fabrizio Minichilli 2 Simona
Hormone related problems (Endocrinopathies and osteoporosis) Vincenzo de Sanctis Ferrara.
 Hormone related problems (Endocrinopathies and osteoporosis) Vincenzo de Sanctis Ferrara vdesanctis@libero.it 6 th EUROPEAN SYMPOSIUM ON RARE ANAEMIAS 1 st Dutch-Belgian meeting for patients and health
Hormone related problems (Endocrinopathies and osteoporosis) Vincenzo de Sanctis Ferrara vdesanctis@libero.it 6 th EUROPEAN SYMPOSIUM ON RARE ANAEMIAS 1 st Dutch-Belgian meeting for patients and health
CEREZYME Genzyme. 70 mg (52 mg) (18 mg)
 CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
Sickle cell disease. Fareed Omar 10 March 2018
 Sickle cell disease Fareed Omar 10 March 2018 Physiology Haemoglobin structure HbA2: 2α and 2δ chains (2-3%) HbF: 2α and 2γ chains (
Sickle cell disease Fareed Omar 10 March 2018 Physiology Haemoglobin structure HbA2: 2α and 2δ chains (2-3%) HbF: 2α and 2γ chains (
How I treat Gaucher disease
 How I treat How I treat Gaucher disease Ari Zimran 1 1 Gaucher Clinic, Shaare Zedek Medical Center, Hebrew University Hadassah Medical School, Jerusalem, Israel This review presents a cohesive approach
How I treat How I treat Gaucher disease Ari Zimran 1 1 Gaucher Clinic, Shaare Zedek Medical Center, Hebrew University Hadassah Medical School, Jerusalem, Israel This review presents a cohesive approach
SICKLE CELL DISEASE. Dr. MUBARAK ABDELRAHMAN MD PEDIATRICS AND CHILD HEALTH. Assistant Professor FACULTY OF MEDICINE -JAZAN
 SICKLE CELL DISEASE Dr. MUBARAK ABDELRAHMAN MD PEDIATRICS AND CHILD HEALTH Assistant Professor FACULTY OF MEDICINE -JAZAN Objective: The student should be able: To identify the presentation, diagnosis,
SICKLE CELL DISEASE Dr. MUBARAK ABDELRAHMAN MD PEDIATRICS AND CHILD HEALTH Assistant Professor FACULTY OF MEDICINE -JAZAN Objective: The student should be able: To identify the presentation, diagnosis,
Hemolytic anemias (2 of 2)
 Hemolytic anemias (2 of 2) Sickle Cell Anemia The most common familial hemolytic anemia in the world Sickle cell anemia is the prototypical (and most prevalent) hemoglobinopathy Mutation in the β-globin
Hemolytic anemias (2 of 2) Sickle Cell Anemia The most common familial hemolytic anemia in the world Sickle cell anemia is the prototypical (and most prevalent) hemoglobinopathy Mutation in the β-globin
Randomized, Controlled Trial of Miglustat in Gaucher s Disease Type 3
 Randomized, Controlled Trial of Miglustat in Gaucher s Disease Type 3 Raphael Schiffmann, MD, 1 Edmond J. FitzGibbon, MD, 2 Chris Harris, PhD, 3 Catherine DeVile, MD, 4 Elin H. Davies, MSc, 4 Larry Abel,
Randomized, Controlled Trial of Miglustat in Gaucher s Disease Type 3 Raphael Schiffmann, MD, 1 Edmond J. FitzGibbon, MD, 2 Chris Harris, PhD, 3 Catherine DeVile, MD, 4 Elin H. Davies, MSc, 4 Larry Abel,
Chapter-6. Discussion
 Chapter-6 Discussion Discussion: LSD s are disorders which collectively constitute a significant burden in the community as collectively they constitute a prevalence of 1 in 5000. The present study here
Chapter-6 Discussion Discussion: LSD s are disorders which collectively constitute a significant burden in the community as collectively they constitute a prevalence of 1 in 5000. The present study here
An overview of Thalassaemias and Complications
 An overview of Thalassaemias and Complications Haemoglobin Haemoglobin is the most abundant protein in blood, and exists as three main types in normal adults: HbA ( ) - 97% HbA 2 ( ) - 2.5% HbF ( ) - 0.5%
An overview of Thalassaemias and Complications Haemoglobin Haemoglobin is the most abundant protein in blood, and exists as three main types in normal adults: HbA ( ) - 97% HbA 2 ( ) - 2.5% HbF ( ) - 0.5%
The Radiology Assistant : Bone tumor - ill defined osteolytic tumors and tumor-like lesions
 Bone tumor - ill defined osteolytic tumors and tumor-like lesions Henk Jan van der Woude and Robin Smithuis Radiology department of the Onze Lieve Vrouwe Gasthuis, Amsterdam and the Rijnland hospital,
Bone tumor - ill defined osteolytic tumors and tumor-like lesions Henk Jan van der Woude and Robin Smithuis Radiology department of the Onze Lieve Vrouwe Gasthuis, Amsterdam and the Rijnland hospital,
If unqualified, Complete remission is considered to be Haematological complete remission
 Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
RARE DISEASE TREATMENT RESOURCE GUIDE
 TABLE OF CONTENTS Cystinosis 2 Fabry Disease 3 Gaucher Disease 4 RARE DISEASE TREATMENT RESOURCE GUIDE Cystinosis Brand Name Procysbi TM Cystagon TM Cystaran TM Generic Name Cysteamine bitartrate delayed
TABLE OF CONTENTS Cystinosis 2 Fabry Disease 3 Gaucher Disease 4 RARE DISEASE TREATMENT RESOURCE GUIDE Cystinosis Brand Name Procysbi TM Cystagon TM Cystaran TM Generic Name Cysteamine bitartrate delayed
If unqualified, Complete remission is considered to be Haematological complete remission
 Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
Scroll right to see the database codes for Disease status and Response Diagnosis it refers to Disease status or response to treatment AML ALL CML CLL MDS or MD/MPN or acute leukaemia secondary to previous
Recombinant Macrophage Targeted Enzyme Replacement Therapy for Gaucher Disease in India
 R E S E A R C H P A P E R Recombinant Macrophage Targeted Enzyme Replacement Therapy for Gaucher Disease in India *A NAGRAL, *P MEWAWALLA, # S JAGADEESH, M KABRA, $ SR PHADKE, # IC VERMA, # RD PURI, #
R E S E A R C H P A P E R Recombinant Macrophage Targeted Enzyme Replacement Therapy for Gaucher Disease in India *A NAGRAL, *P MEWAWALLA, # S JAGADEESH, M KABRA, $ SR PHADKE, # IC VERMA, # RD PURI, #
Effect of Oral Eliglustat on Splenomegaly in Patients With Gaucher Disease Type 1 The ENGAGE Randomized Clinical Trial
 Research Original Investigation Effect of Oral on Splenomegaly in Patients With Gaucher Disease Type 1 The ENGAGE Randomized Clinical Trial Pramod K. Mistry, MD, PhD, FRCP; Elena Lukina, MD; Hadhami Ben
Research Original Investigation Effect of Oral on Splenomegaly in Patients With Gaucher Disease Type 1 The ENGAGE Randomized Clinical Trial Pramod K. Mistry, MD, PhD, FRCP; Elena Lukina, MD; Hadhami Ben
Eliglustat maintains long-term clinical stability in patients with Gaucher disease type 1 stabilized on enzyme therapy
 Regular Article From www.bloodjournal.org by guest on January 29, 2019. For personal use only. CLINICAL TRIALS AND OBSERVATIONS Eliglustat maintains long-term clinical stability in patients with Gaucher
Regular Article From www.bloodjournal.org by guest on January 29, 2019. For personal use only. CLINICAL TRIALS AND OBSERVATIONS Eliglustat maintains long-term clinical stability in patients with Gaucher
Pediatrics. Pyruvate Kinase Deficiency (PKD) Symptoms and Treatment. Definition. Epidemiology of Pyruvate Kinase Deficiency.
 Pediatrics Pyruvate Kinase Deficiency (PKD) Symptoms and Treatment See online here Pyruvate kinase deficiency is an inherited metabolic disorder characterized by a deficiency in the enzyme "pyruvate kinase"
Pediatrics Pyruvate Kinase Deficiency (PKD) Symptoms and Treatment See online here Pyruvate kinase deficiency is an inherited metabolic disorder characterized by a deficiency in the enzyme "pyruvate kinase"
The ZAGAL Study: Long- term Management and Follow- up of use of Miglustat in type 1 Gaucher disease in Spain.
 The ZAGAL Study: Long- term Management and Follow- up of use of Miglustat in type 1 Gaucher disease in Spain. Pilar Giraldo aematology Department. Miguel Servet University Hospital FEETEG Disclosures n
The ZAGAL Study: Long- term Management and Follow- up of use of Miglustat in type 1 Gaucher disease in Spain. Pilar Giraldo aematology Department. Miguel Servet University Hospital FEETEG Disclosures n
Eliglustat tartrate: an oral therapeutic option for Gaucher disease type 1
 For reprint orders, please contact: reprints@future-science.com Eliglustat tartrate: an oral therapeutic option for Gaucher disease type 1 Clin. Invest. (2014) 4(1), 45 53 Gaucher disease is an inborn
For reprint orders, please contact: reprints@future-science.com Eliglustat tartrate: an oral therapeutic option for Gaucher disease type 1 Clin. Invest. (2014) 4(1), 45 53 Gaucher disease is an inborn
Patients with type 1 Gaucher disease in South Florida, USA: demographics, genotypes, disease severity and treatment outcomes
 Orenstein et al. Orphanet Journal of Rare Diseases 2014, 9:45 RESEARCH Open Access Patients with type 1 Gaucher disease in South Florida, USA: demographics, genotypes, disease severity and treatment outcomes
Orenstein et al. Orphanet Journal of Rare Diseases 2014, 9:45 RESEARCH Open Access Patients with type 1 Gaucher disease in South Florida, USA: demographics, genotypes, disease severity and treatment outcomes
Speaker s Disclosure Statement. Starvation, Death and Destruction: The Battlefield of AVN. Objectives. Risk Factors
 Starvation, Death and Destruction: The Battlefield of AVN Speaker s Disclosure Statement I have no industry relationships to disclose I will discuss off-label use of medications Dana-Farber/Boston Children
Starvation, Death and Destruction: The Battlefield of AVN Speaker s Disclosure Statement I have no industry relationships to disclose I will discuss off-label use of medications Dana-Farber/Boston Children
DISEASES WITH ABNORMAL MATRIX
 DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
VPRIV EU-RMP Version 9.2. Elements for a Public Summary. Overview of Disease Epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of Disease Epidemiology Gaucher disease is a rare genetic illness due to the absence of a specific enzyme in the body. About 30,000 persons worldwide
VI.2 VI.2.1 Elements for a Public Summary Overview of Disease Epidemiology Gaucher disease is a rare genetic illness due to the absence of a specific enzyme in the body. About 30,000 persons worldwide
Highly specialised technologies guidance Published: 28 June 2017 nice.org.uk/guidance/hst5
 Eliglustat for treating type 1 Gaucher disease Highly specialised technologies guidance Published: 28 June 2017 nice.org.uk/guidance/hst5 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Eliglustat for treating type 1 Gaucher disease Highly specialised technologies guidance Published: 28 June 2017 nice.org.uk/guidance/hst5 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates in the Management of. Myeloma Bone Disease
 Bisphosphonates in the Management of Myeloma Bone Disease James R. Berenson, MD Medical & Scientific Director Institute for Myeloma & Bone Cancer Research Los Angeles, CA Myeloma Bone Disease Myeloma cells
Bisphosphonates in the Management of Myeloma Bone Disease James R. Berenson, MD Medical & Scientific Director Institute for Myeloma & Bone Cancer Research Los Angeles, CA Myeloma Bone Disease Myeloma cells
The lysosomal storage diseases currently number 45 and
 Recommendations on Diagnosis, Treatment, and Monitoring for Gaucher Disease Ana Maria Martins, MD, PhD, Eugenia Ribeiro Valadares, MD, PhD, Gilda Porta, MD, PhD, Janice Coelho, PhD, José Semionato Filho,
Recommendations on Diagnosis, Treatment, and Monitoring for Gaucher Disease Ana Maria Martins, MD, PhD, Eugenia Ribeiro Valadares, MD, PhD, Gilda Porta, MD, PhD, Janice Coelho, PhD, José Semionato Filho,
Medical and Surgical Complications of Sickle Cell Anemia
 Medical and Surgical Complications of Sickle Cell Anemia Ahmed Al-Salem Medical and Surgical Complications of Sickle Cell Anemia Ahmed Al-Salem Department of Surgery Dar A lalafia Medical Company Qatif
Medical and Surgical Complications of Sickle Cell Anemia Ahmed Al-Salem Medical and Surgical Complications of Sickle Cell Anemia Ahmed Al-Salem Department of Surgery Dar A lalafia Medical Company Qatif
Outcome of enzyme replacement therapy in children with Gaucher disease: The Egyptian experience
 The Egyptian Journal of Medical Human Genetics (2011) 12, 9 14 Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com ORIGINAL ARTICLE Outcome of enzyme
The Egyptian Journal of Medical Human Genetics (2011) 12, 9 14 Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com ORIGINAL ARTICLE Outcome of enzyme
Awaisheh. Mousa Al-Abbadi. Abdullah Alaraj. 1 Page
 f #3 Awaisheh Abdullah Alaraj Mousa Al-Abbadi 1 Page *This sheet was written from Section 1 s lecture, in the first 10 mins the Dr. repeated all the previous material relating to osteoporosis from the
f #3 Awaisheh Abdullah Alaraj Mousa Al-Abbadi 1 Page *This sheet was written from Section 1 s lecture, in the first 10 mins the Dr. repeated all the previous material relating to osteoporosis from the
Non-malignant hematologic disorders associated arthropathies: hemoglobinopathy-associated musculoskeletal manifestations, hemophilia
 Non-malignant hematologic disorders associated arthropathies: hemoglobinopathy-associated musculoskeletal manifestations, hemophilia HAEMOGLOBINOPATHIES = inherited disorders of globin divided into: Thalassaemia
Non-malignant hematologic disorders associated arthropathies: hemoglobinopathy-associated musculoskeletal manifestations, hemophilia HAEMOGLOBINOPATHIES = inherited disorders of globin divided into: Thalassaemia
Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD)
 McGovern et al. Orphanet Journal of Rare Diseases (2017) 12:41 DOI 10.1186/s13023-017-0572-x REVIEW Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD)
McGovern et al. Orphanet Journal of Rare Diseases (2017) 12:41 DOI 10.1186/s13023-017-0572-x REVIEW Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD)
Pharmacotherapy of Gaucher Disease: Current and Future Options
 Pharmacotherapy of Gaucher Disease: Current and Future Options Lunawati L. Bennett, PhD, PharmD, FACN; and Chris Fellner INTRODUCTION Gaucher disease (GD) is a rare lysosomal storage disease (LSD) affecting
Pharmacotherapy of Gaucher Disease: Current and Future Options Lunawati L. Bennett, PhD, PharmD, FACN; and Chris Fellner INTRODUCTION Gaucher disease (GD) is a rare lysosomal storage disease (LSD) affecting
Gaucher Disease: Clinical, Biological and Therapeutic Aspects
 Review Received: July 5, 2015 Accepted after revision: September 2, 2015 Published online: November 21, 2015 Gaucher Disease: Clinical, Biological and Therapeutic Aspects Azza Dandana Souhaira Ben Khelifa
Review Received: July 5, 2015 Accepted after revision: September 2, 2015 Published online: November 21, 2015 Gaucher Disease: Clinical, Biological and Therapeutic Aspects Azza Dandana Souhaira Ben Khelifa
MSK Tumors and Marrow Evaluation. Bone Marrow
 MSK Tumors and Marrow Evaluation Bone Marrow Thomas M. Link, MD Department of Radiology and Biomedical Imaging University of California, San Francisco (i) Introduction Bone marrow consists of trabecular
MSK Tumors and Marrow Evaluation Bone Marrow Thomas M. Link, MD Department of Radiology and Biomedical Imaging University of California, San Francisco (i) Introduction Bone marrow consists of trabecular
DONE BY : RaSHA RAKAN & Bushra Saleem
 DONE BY : RaSHA RAKAN & Bushra Saleem Hemolytic anemias (2 of 2) Sickle Cell Anemia The most common familial hemolytic anemia in the world Sickle cell anemia is the prototypical (and most prevalent) hemoglobinopathy
DONE BY : RaSHA RAKAN & Bushra Saleem Hemolytic anemias (2 of 2) Sickle Cell Anemia The most common familial hemolytic anemia in the world Sickle cell anemia is the prototypical (and most prevalent) hemoglobinopathy
Pediatric metabolic bone diseases
 Pediatric metabolic bone diseases Classification and overview of clinical and radiological findings M. Mearadji International Foundation for Pediatric Imaging Aid www.ifpia.com Introduction Metabolic bone
Pediatric metabolic bone diseases Classification and overview of clinical and radiological findings M. Mearadji International Foundation for Pediatric Imaging Aid www.ifpia.com Introduction Metabolic bone
Evangelia E. Vassalou MD,PhD Radiologist Department of Medical Imaging, Heraklion University Hospital Department of Medical Imaging, Sitia General
 Evangelia E. Vassalou MD,PhD Radiologist Department of Medical Imaging, Heraklion University Hospital Department of Medical Imaging, Sitia General Hospital Osteonecrosis pathophysiology epidemiology imaging
Evangelia E. Vassalou MD,PhD Radiologist Department of Medical Imaging, Heraklion University Hospital Department of Medical Imaging, Sitia General Hospital Osteonecrosis pathophysiology epidemiology imaging
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET VPRIV (velaglucerase alfa ghu) NAME OF THE MEDICINE VPRIV powder for solution for infusion. Active Ingredient: velaglucerase alfa ghu CAS number: 37228-64-1 DESCRIPTION Velaglucerase
NEW ZEALAND DATA SHEET VPRIV (velaglucerase alfa ghu) NAME OF THE MEDICINE VPRIV powder for solution for infusion. Active Ingredient: velaglucerase alfa ghu CAS number: 37228-64-1 DESCRIPTION Velaglucerase
In adults, the predominant Hb (HbA) molecule has four chains: two α and two β chains. In thalassemias, the synthesis of either the α or the β chains
 Thalassaemias Thalassemia Thalassemia is an inherited autosomal recessive blood disease. Associated with absence or reduction in a or b globin chains. Reduced synthesis of one of the globin chains can
Thalassaemias Thalassemia Thalassemia is an inherited autosomal recessive blood disease. Associated with absence or reduction in a or b globin chains. Reduced synthesis of one of the globin chains can
DXA When to order? How to interpret? Dr Nikhil Tandon Department of Endocrinology and Metabolism All India Institute of Medical Sciences New Delhi
 DXA When to order? How to interpret? Dr Nikhil Tandon Department of Endocrinology and Metabolism All India Institute of Medical Sciences New Delhi Clinical Utility of Bone Densitometry Diagnosis (DXA)
DXA When to order? How to interpret? Dr Nikhil Tandon Department of Endocrinology and Metabolism All India Institute of Medical Sciences New Delhi Clinical Utility of Bone Densitometry Diagnosis (DXA)
