Introduction CORNEA. Jacek P. Szaflik 1,2 & Joanna Major 1,2 & Justyna Izdebska 1,2 & Mieczysław Lao 1,2 & Jerzy Szaflik 1,2
|
|
|
- Britton Young
- 5 years ago
- Views:
Transcription
1 Graefes Arch Clin Exp Ophthalmol (2016) 254: DOI /s CORNEA Systemic immunosuppression with mycophenolate mofetil to prevent corneal graft rejection after high-risk penetrating keratoplasty: a 2-year follow-up study Jacek P. Szaflik 1,2 & Joanna Major 1,2 & Justyna Izdebska 1,2 & Mieczysław Lao 1,2 & Jerzy Szaflik 1,2 Received: 2 February 2015 /Accepted: 20 October 2015 /Published online: 9 November 2015 # The Author(s) This article is published with open access at Springerlink.com Abstract Purpose In this study, we aimed to evaluate the efficacy and safety of systemic immunosuppression with mycophenolate mofetil (MMF) to prevent corneal graft rejection after highrisk penetrating keratoplasty. Methods One hundred and ninety-six consecutive patients who underwent high-risk penetrating keratoplasty defined as the presence of deep vascularization in more than two quadrants, keratouveitis, emergency keratoplasties, and retransplantations were enrolled in the study. Ninety-eight prospectively followed up patients were treated with MMF [with dose adjustment based on mycophenolic acid (MPA) serum concentration], and 98 patients were in the non MMF-treated retrospectively assessed control group. Results During a mean of 24 months of observation, immune reactions occurred in eight cases (8 %) and graft rejection with subsequent graft failure occurred in three cases (3 %) in the MMF group. In the control group, graft rejection occurred in 76 cases (78 %) and failure due to graft rejection occurred in 30 cases (31 %). Kaplan Meier analysis demonstrated that 93 % of the grafts in the MMF-treated group and 47 % in the control group showed no immune rejection (p<0.01, log-rank test) after a year. Cox regression analysis proved that Clinical Trial Registration number if required Not required. * Jacek P. Szaflik szaflik@ophthalmology.pl 1 2 Department of Ophthalmology, Medical University of Warsaw, Warszawa, Poland Independent Public University Eye Hospital, ul. J. Sierakowskiego 13, Warszawa, Poland MMF treatment decreased the risk of graft rejection 11 times (RR=11, 95.0 % CI , p<0.0001). Among 98 MMFtreated patients, 13 had gastric discomfort, three developed leucopenia, and two had anemia that resolved after MMF dose reduction. Conclusions MMF treatment after high risk penetrating keratoplasty is safe and reduces the incidence of immune graft rejection and graft failure. Side effects were rare and reversible in all but one case. Keywords High-risk penetrating keratoplasty. Corneal graft failure. Corneal graft rejection. Immunosuppression. Mycophenolate mofetil Introduction The cornea is the most frequently transplanted tissue, with the exception of blood transfusions. The overall survival rate of low-risk corneal grafts reaches 85 % 90 % in 10 years of follow-up [1 3]. However, despite the progress in medical treatment and microsurgical procedures, penetrating keratoplasty in high-risk patients (high-risk penetrating keratoplasty [HRPK]) remains a challenging procedure due to the risk of irreversible allograft rejection. Allograft rejection occurs in % of these patients during the first year after transplantation [4 6], and immune graft rejection is the main cause of corneal graft failure and loss of its transparency [7]. The use of cyclosporine A (CsA) is limited due to its toxicity and the risk of neocancerogenesis. Side effects such as nephrotoxicity, hepatotoxicity, hypertension, altered glucose metabolism, and gingival hyperplasia occur in up to 40 % of patients [8, 9], but probably the most threatening complication is the increased incidence of malignancy (cancers, lymphomas) [10, 11]. Reis et al. [12] and Birnbaum et al. [9]showed
2 308 Graefes Arch Clin Exp Ophthalmol (2016) 254: that mycophenolate mofetil (MMF) and CsA were equally effective and MMF treatment had a favorable safety profile, as the complications associated with MMF administration have mainly included gastrointestinal and hematologic problems (e.g., leucopenia, anemia), all of which are reversible after cessation of the drug s dosage[13 15]. The aim of our study was to evaluate the efficacy and safety of systemic immunosuppression with MMF in the prevention of corneal graft rejection after HRPK with 2 years of followup. Material and methods Patients, treatment schedule, and follow-up A total of 196 patients were included in the analysis. All patients underwent HRPK in the University Ophthalmic Hospital in Warsaw. Ninety-eight consecutive patients were treated with MMF and prospectively followed up. The control group included 98 consecutive patients assessed retrospectively. In 2009, results of a multicenter, prospective and randomized clinical study concerning the efficacy of mycophenolate mofetil after high-risk corneal transplantation were published [16]. Recruitment for the study s control group was prematurely stopped due to a statistically significant difference between treated patients and the non-mmf-treated control group (in favor of the group treated with MMF). That is why the control group was assessed retrospectively in our study; we found it unethical not to administer effective therapy [16]. Informed consents were obtained, and the local bioethical committee approved the study. We defined high-risk keratoplasty as retransplanted keratoplasties, emergency transplantations in case of corneal perforation due to bacterial infections, presence of deep vascularization in three or four quadrants, and transplantation in patients with keratouveitis (Table 1). Exclusion criteria included perforation due to unknown cause, viral or fungal corneal infections, and glaucoma diagnosed prior to the surgery. The indications for corneal transplantation are listed in Table 2. HLA and ABO blood group antigen matching had not been performed before the surgery. All donor corneas were stored in Eusol GS medium at 4 C (mean time of storage, 5 days). The mean endothelial cell density of donor corneal button was 2400 cells/mm 2 ( cells/mm 2 ). All patients underwent penetrating keratoplasty or penetrating keratoplasty with extracapsular cataract extraction (Table 3). The mean diameter of the donor corneal discs was 8.0±0.5 mm, 0.5 mm larger than the diameter of the recipient bed. In cases of emergency keratoplasties, interrupted 10-0 nylon sutures were used; in other cases, a combination of interrupted and continuous 10-0 nylon sutures was applied. All knots were buried in the peripheral host cornea. Directly after surgery, all patients received antibiotic eye drops (fluoroquinolone), and subconjunctival injection of dexamethasone was administered. Patient data are listed in Tables 3 and 4. Group 1 was prospectively followed up and received mycophenolate mofetil (CellCept, Roche Pharma AG, Grenzach-Wyhlen, Germany), according to a set protocol. All group 1 patients were examined by the transplantologist, and a thorough assessment was conducted comprising the patient s history of peptic ulcer, malignancy, and chronic infections; blood pressure measurement; and laboratory tests, including complete blood cell count, serum creatinine, and liver functions. Afterwards, systemic immunosuppression was introduced. A day before surgery, MMF was administered at an initial dose of mg. On the day of surgery and for 2 days after, the patients received methylprednisolone sodium succinate intravenously (1st day, 500 mg; 2nd day, 250 mg; 3rd day, 250 mg). On the fourth day, methylprednisolone was administered orally (0.4 mg/kg daily). MPA serum concentration was measured on the seventh day after surgery (a predose MPA serum concentration reached about 2 μg/ml and no more than 5 μg/ml). Methylprednisolone was tapered during the first month after surgery to reach the lowest possible dose of 5 10 mg daily, and was discontinued after 11 months. A month after the operation, MMF was also tapered to mg, after 6 months to mg, and a year after surgery MMF was discontinued. Since immunosuppressive therapy may activate viral infections (e.g., herpes simplex virus, cytomegalovirus, human papillomavirus) and induce atypical infections (Pneumocystis jirovecii), prophylaxis for 6 months with acyclovir (4 400 mg daily) and co-trimazole (480 mg twice daily for 3 months tapered to 480 mg daily) was introduced. Apart from systemic treatment, topical steroid drops were administered (loteprednol etabonate). Group 2 consisted of 98 consecutive patients assessed retrospectively with no systemic immunosuppressive therapy administered. On the day of the surgery and in the next 2 days after surgery, the patients received methylprednisolone sodium succinate intravenously (500 mg once daily). On the fourth day, the patients received methylprednisolone (1 mg/kg). The mean dose of methylprednisolone after a month was 0.5 mg/ kg daily; after 2 months, it was 0.3 mg/kg daily; and after 6 months, it was 5 10 mg daily. Patients in both groups received (from the first day after surgery) topical antibiotic drops or ointment (fluoroquinolone) until epithelial healing was complete, and afterwards they received topical steroid for a year or longer, depending on their response, until all sutures were removed (loteprednol was administered in the MMF group and dexamethasone in the control groups four to six times daily for 2 months, three times daily until the sixth month, and two times daily thereafter for 6 months or longer).
3 Graefes Arch Clin Exp Ophthalmol (2016) 254: Table 1 Indications for HRPK (Fisher s exact test: p=0.247; no statistical difference) Diagnosis Group 1 (MMF) Group 2 (control) Total 3 or 4 quadrants with deep vascularization (n) or 4 quadrants of retransplanted corneal button with deep vascularization Emergency transplantations (n) Retransplantations (n) Active recurrent or chronic uveitis (n) Postoperative examinations were performed each day during the first week after surgery, and then on the following schedule: 1, 3, 6, 12, and 24 months after surgery. The examination included visual acuity for distance and near vision, intraocular pressure measurement, and slit lamp examination. Apart from the ophthalmic examination, each patient was examined by the transplantologist. Side effects (gastrointestinal, hematologic) and MPA plasma concentration were closely monitored. Predose MPA plasma concentration was measured a week after surgery, a month after surgery, 3 months after surgery, and each time the dosage of MMF was changed (during the follow-up time, an average of three measurements were taken). The main outcomes of the study were occurrence of immune graft rejection and graft survival. Immune reactions were diagnosed on the basis of a patient s complaints (redness, discomfort, pain, worse visual acuity, photophobia, lacrimation) and slit lamp examination findings, such as endothelial precipitates, Khodadoust line, noninfectious subepithelial infiltrates, and stromal edema [17]. In case of immune graft rejection, patients in both groups were treated with topical dexamethasone drops every hour and with ointment at bedtime. In case of severe reactions (more than five keratic precipitates, inflammatory cells in stroma not due to infection, endothelial rejection line, stromal oedema, cells in aqueous humor) [18], the patients (group 1 and 2) were admitted to hospital and treated with intravenous methylprednisolone sodium succinate (500 mg for 3 days) and oral methylprednisolone acetate (1 mg/kg) afterwards, in addition to topical treatment as mentioned above. Patients in group 1 also had their MMF dosage increased to mg daily. Clear graft survival was determined when there was no opacity in the 4-mm central zone of the corneal button. Graft failure was diagnosed with the presence of irreversible corneal edema and graft opacity. Statistical analysis Statistical analysis was carried out using R statistical software. Groups 1 and 2 were compared using Student s t-test (patient age, visual acuity, graft diameter, follow-up time) and Mann Whitney U test (endothelial cell density, donor age, donor tissue storage time). Chi-squared test was performed to compare surgical procedures employed. The Kaplan Meier estimator was used to establish clear graft survival and rejection-free interval. Statistical significance was determined using the log-rank test. For all tests, a p value below 0.05 was considered statistically significant. Proportional Table 2 Indication for the corneal transplant Diagnosis Group 1 (MMF) n=98 Group 2 (control) n=98 Total Corneal opacity and neovascularization due to severe dry eye syndrome Alkali burn Trauma ulcer Graft failure due to graft rejection Graft failure due to nonhealing epithelial defect Graft failure due to corneal melt Graft failure due to bacterial corneal ulcer Acute perforation of non-infectious corneal ulceration Refractory bacterial keratitis Acute perforation of corneal ulcer Keratouveitis of idiopathic etiology 5 2 7
4 310 Graefes Arch Clin Exp Ophthalmol (2016) 254: Table 3 Type of surgery (χ 2 = 1.42, p=0,54; no statistical difference) Group 1 (MMF) Group 2 (control) Total Penetrating keratoplasty (n) Penetrating keratoplasty+extracapsular cataract extraction (n) Penetrating keratoplasty+extracapsular cataract extraction+intraocular lens implantation (n) hazards model (Cox regression) was used to establish risk factors of graft rejection. Results Demography One hundred and ninety-six consecutive patients (103 women and 93 men, aged years) were enrolled in the study. Of these, 98 were prospectively followed up and treated with systemic immunosuppression with MMF (group 1), and 98 were included in a retrospective control group with no systemic immunosuppression with MMF administered (group 2). None of the patients were lost for the follow-up. Mean follow-up time was 56±31 weeks in group 1 and 51±39 weeks in group 2. There was no statistically significant difference between the two groups regarding preoperative best correct visual acuity, recipient age, donor age, quality of the donor corneal disc, tissue storage time, preoperative graft endothelial cell density, graft diameter, or the type of surgery performed. Patient data are shown in Tables 1, 2, 3 and 4. Efficacy The mean time of observation was 95 weeks (94±21 weeks in group 1 and 97±18 weeks in group 2). At this time, 84 of all 196 patients (43 %) experienced immune graft rejection, and graft failure due to immune reactions occurred in 33 patients (almost 17 %). Graft failure due to other causes occurred in only 10 cases (5 %). In group 1, immune reactions occurred in eight cases (8 %) during and despite MMF treatment, and five of them were reversible; in four cases (50 %), the rejection was treated as severe reaction. Graft failure due to graft rejection occurred in three cases (3 % of patients). Another six cases (6 %) experienced graft failures due to other causes (nonhealing, persistent erosions, glaucoma, or infection). Regarding the underlying diagnosis: 50 % of the rejected emergency and repeated transplants failed (lost transparency). In case of patients with retransplanted vascularized corneas, none of the rejected grafts lost transparency and none of the patients with the keratouveitis rejected the graft (Tables 5 and 6). In group 2, graft rejection occurred in 76 cases (77 %); in 15 of these cases, there was more than one episode of immune reaction during the follow-up, and in 33 cases (43 %) the rejection was severe. In 45 of the 76 cases, the reaction was reversible. Failure due to graft rejection occurred in 30 cases, and other causes for graft failure (nonhealing, persistent erosions, glaucoma) occurred in four cases. Regarding the underlying diagnosis: 50 % of the rejected emergency grafts, 40 % oftherepeatedtransplantsand44%retransplantswith vascularized corneal bed failed (lost transparency). None of the grafts of patients with uveitis and immune reaction after the transplantation failed (Tables 5 and 6). Efficacy data are shown in Tables 5 and 6. According to Kaplan Meier curves (Fig. 1), after 12 months of therapy, grafts without immune rejections accounted for 93 % of group 1 (MMF treated) and 47 % of group 2 (control group) (p<0.01 in log-rank test). Cox regression analysis proved that MMF treatment decreased the risk of graft rejection 11 times (95.0 % CI , p<0.0001). Furthermore, the other group characteristics (recipient s age and sex, quality of the transplanted tissue, endothelial cell Table 4 Patient data Group 1 (MMF) Group 2 (control) Total p value Patients (n) Male/female (n) 51/47 42/56 93/ (ns) * Age (mean±sd) 58±16 56± (ns) ** Follow-up (days) 394.9± ± (ns) *** ns, no statistical difference. * Pearson s χ 2 test; ** Mann Whitney U test; *** Student s t test.
5 Graefes Arch Clin Exp Ophthalmol (2016) 254: Table 5 Efficacy Group 1 (MMF) Group 2 (control) p value * Graft rejection (n) 8 76 < 0.01 Graft failure due to graft rejection (n) 3 30 < 0.01 Graft failure due to other causes (n) 6 4 Nonhealing, persistent epithelial defects (n) 4 3 Glaucoma decompensation (n) 1 1 Infection (n) 1 0 * log rank test count of the donor tissue, diameter of the corneal button, donor age, time of storage, diagnosis, type of the surgery) included in the model had no effect on graft rejection. This indicates that the diminished risk of an immune response observed in group 1 (MMF treated) was a result of the therapy, and other factors that characterized the treatment group (recipient s age and sex, quality of the transplanted tissue, endothelial cell count of the donor tissue, diameter of the corneal button, donor age, time of storage, diagnosis, type of the surgery) did not influence the result. The Kaplan Meier plot illustrating clear graft survival (Fig. 2) shows that after a year of therapy, only 2 % of grafts failed in the MMF group compared to 19 % in the control group. Safety In the MMF group, 13 patients (13.5 %) complained of gastrointestinal disturbances, but immunosuppressive treatment only had to be stopped in one case with refractive diarrhea. In other cases, proton pump inhibitors or MMF enteric-coated mycophenolate sodium were administered, and the discomfort subsided. There were also two cases of anemia and three of leucopenia, which resolved after reduction of the MMF dosage. Discussion There are three main immunologic risk factors for graft rejection: vascularization, inflammation, and previous graft rejection [19, 20], which we accounted for in the inclusion criteria of our study. In the Collaborative Corneal Transplantation Studies (CCTS) high-risk keratoplasty was defined as having two or more quadrants with deep stromal vessels; if corneas had vascularization in four quadrants, the risk for immune graft rejection doubled [4]. These findings were confirmed in other studies. The depth and extent of vessels seem to be critical factors for corneal graft survival (the more vessels, the higher risk for graft rejection) [4, 20, 21]. Active inflammation at the time of surgery is also a risk factor for corneal graft rejection and graft failure [4, 5, 7] as well as regrafting, especially if the failure was the result of immune graft rejection [4, 6, 21]. Topical application of corticosteroids is the gold standard after low-risk keratoplasties because it provides effective immunosuppression and good anterior chamber penetration [22]. But the impact of topical steroids is often insufficient in preventing graft rejection in high-risk patients. These procedures require an increased use of the eye drops, which can cause side effects such as cataract, glaucoma, or delayed wound healing. These impediments explain why the search for optimal treatment schedules for such patients remains ongoing. To our knowledge, only two immunosuppressives are routinely used after high-risk keratoplasties: CsA and MMF. Table 6 Rejected and failed grafts based on the underlying diagnosis Diagnosis Group 1 (MMF) Group 2 (control) Rejection (n=8) Failure (n=3) Rejection (n=76) Failure (n = 30) 3 or 4 quadrants with deep vascularization 0 0 (0 %) 8 0 (0 %) (n=12) 3 or 4 quadrants of retransplanted corneal 2 0 (0 %) 18 8 (44 %) button with deep vascularization (n=20) Emergency transplantations (n=30) 4 2 (50 %) (50 %) Retransplantations (n=31) 2 1 (50 %) (41 %) Active recurrent or chronic uveitis (n=5) 0 0(0%) 1 0(0%)
6 312 Graefes Arch Clin Exp Ophthalmol (2016) 254: Fig. 1 Kaplan-Meier survival curve concerning immune rejection free graft survival (log-rank test: p<0.01) CsA was introduced in ophthalmology in the mid-1980s, and its use in ophthalmology is well established. Many publications report good effects from CsA use after HRPK, but some report no benefits. In a meta-analysis of randomized controlled trials with two studies on CsA versus placebo, pooled analysis of clear graft survival showed no differences between the two groups [23]. CsA may be the cause of significant side effects, including hypertension, nephrotoxicity, neurotoxicity, and malignancies [10, 11, 24, 25]. In a prospective, randomized, multicenter study conducted by Birnbaum et al. [9], MMF was administered for 6 months after high-risk keratoplasty. In this study, HLA loci were typed, but the HLA matching was not performed. In the MMF group (n=50), there were eight cases (16 %) of graft rejections, and two of them were irreversible; whereas, in the control group (n=37), there were 12 cases (32 %) of immune graft rejection and seven were irreversible. The difference Fig. 2 Kaplan-Meier survival curve concerning clear graft survival (logrank test: p<0.01) between the groups in graft rejection episodes was statistically significant (p=0.044). It is worth mentioning that all rejection episodes in the MMF group occurred after cessation of MMF. In our study, the patients were treated with MMF at full dosage (1000 mg twice daily) for about a month. Under optimal conditions, the dosage was tapered to 750 or 500 mg twice daily after a month and continued for the next 5 months. During the seventh month, the dosage was reduced to 2 x 250 mg daily, and MMF was discontinued after 12 months. In control group 2, the doses of methylprednisolone were higher than in the MMF group (1 mg/kg vs. 0.4 mg/kg at the beginning, 0.5 mg/kg vs mg daily after 2 months, 0.3 mg/kg vs mg daily after 3 months). The doses in both groups equaled after 6 months. It should also be stressed that most of the patients in group 2 (76 of 98, 77,6 %) did not follow the scheme, as when graft rejection occurred, the dosage of methylprednisolone was increased to 1 mg/kg daily, so mean doses of methylprednisolone were actually higher. We also observed statistically significant differences in immune graft rejection and graft failure between the MMF and control groups (p<0.01). There were eight episodes (8 %) of graft rejection in the MMF-treated group (n=98), with three irreversible cases; in the control group (n=98), there were 75 (almost 77 %) patients with graft rejection, and 30 of them eventually had graft failure. In the present study, about 40 % of grafts in both groups had irreversible immune rejection. Compared with the study of Birnbaum et al. [9], our groups were larger, which may explain why the effect of MMF treatment was even more pronounced. Taking into account the underlying diagnosis, the same percentage of rejected emergency grafts in both groups failed (50 % of rejected MMF-treated and MMF-non-treated grafts lost transparency). These results are comparable to other studies, as other researchers found % of emergency graft lost their transparency [26, 27]. As far as retransplantations are concerned, respectively, 50 % of grafts (one patient) in group 1 and 40 % of grafts (12 patients) in group 2 failed. The literature shows that about 33 to 46 % of repeated grafts fail in 2 years of follow-up [26, 28 30]. Forty-four percent of neovascularized regrafts failed in the control group, which is less than other researchers found (50-83 %) [26, 27]. The results are summarized in Tables 5 and 6. Emergency transplants and retransplantations seem to be high-risk factors for permanent loss of graft transparency. The main side effects in our MMF group (n=98) were gastrointestinal disorders in 13 cases (13 %) and hematologic changes (anemia, leucopenia) in five cases (5 %). Apart from one patient with refractory diarrhea, all side effects were well tolerated and reversible. In the study of Birnbaum et al. [16], out of 57 MMF-treated patients, 36 (63 %) suffered from side effects, but the authors noted that they were probably the effect of systemic
7 Graefes Arch Clin Exp Ophthalmol (2016) 254: immunosuppression with MMF in only 24 cases (42 %). In that study, 15 patients (26 %) suffered from gastrointestinal disturbances; in one case, the MMF therapy was stopped, eight patients (14 %) developed infections, and one had anemia. The authors also reported two cases of malignancies (3 and 5 months after surgery) [16]. In the Reis et al. [12]study, Hodgkin s lymphoma was diagnosed in the MMF group a month after surgery. Cancerogenesis due to MMF use cannot be excluded, but it seems unlikely. In 2005, a study based on data collected on almost 7000 patients in the Organ Procurement and Transplantation Network, United Network for Organ Sharing and Collaborative Transplant Study registry showed that there was no statistically significant difference between MMF-treated and non MMF-treated de novo renal transplant recipients in 3 years of follow-up study for the development of lymphomas (p=0.999) and other malignancies (p=0.088) [15]. MMF is considered less active in cancerogenesis than CsA or tacrolimus, and treatment of less than 6 months seems too short to induce neoplastic disease. It is more probable that the patients were asymptomatic prior to keratoplasty. Nevertheless, other studies reported more side effects than we observed. Because MMF treatment seems rather safe, most authors do not monitor MPA serum level. Taking into account each patient s variability and the dynamic relationship between the pharmacokinetics and pharmacodynamics of the drug, we believe that monitoring of MPA serum concentration should be mandatory, because it helps to avoid exceeding safe drug levels and permits individual dosage adjustment. In conclusion, our study provides further evidence to confirm that systemic MMF treatment after HRPK considerably reduces the number of patients with immune graft rejection and graft failure. Most of the side effects are transient or reversible after adjusting the therapy, and the treatment is relatively safe. Since it was previously reported that pharmacologically induced immune tolerance may decrease after a certain time and MMF treatment may be insufficient, long-term graft survival studies need to be conducted [31]. Compliance with ethical standards Funding No funding was received for this research. Conflict of interest All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent Informed consent was obtained from all MMFtreated individual participants included in the study (for the control group, formal consent was not required, as it was assessed retrospectively). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. References 1. Pramanik S, Musch DC, Sutphin JE, Farjo AA (2006) Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology 113: Thompson RW Jr, Price MO, Bowers PJ, Price FW Jr (2003) Longterm graft survival after penetrating keratoplasty. Ophthalmology 110: Muraine M, Sanchez C, Watt L, Retout A, Brasseur G (2003) Longterm results of penetrating keratoplasty. A 10-year-plus retrospective study. Graefes Arch Clin Exp Ophthalmol 241: Maguire MG, Stark WJ, Gottsch JD, Stulting RD, Sugar A, Fink NE, Schwartz A (1994) Risk factors for graft failure and rejection in collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology 101: Maier P, Böhringer D, Reinhard T (2007) Clear graft survival and immune reactions following emergency keratoplasty. Graefes Arch Clin Exp Ophthalmol 245: Sangwan VS, Ramamurthy B, Shah U, Garg P, Sridhar MS, Rao GN (2005) Outcome of corneal transplant rejection: a 10-year study. Clin Experiment Ophthalmol 33: Pletey U, Forrester JV, (eds) (2010) Uveitis and immunological disorders. Progress III. Essentials in ophthalmology. Springer, pp Reinhard T, Sundmacher R, Godehardt E, Heering P (1997) Preventive systemic cyclosporin A after keratoplasty at increased risk for immune reactions as the only elevated risk factor. Ophthalmologe 94: Birnbaum F, Böhringer D, Sokolovska Y, Sundmacher R, Reinhard T (2005) Immunosuppression with cyclosporine A and mycophenolate mofetil after penetrating high-risk keratoplasty: a retrospective study. Transplantation 79: Gallagher MP, Kelly PJ, Jardine M, Perkovic V, Cass A, Craig JC, Eris J, Webster AC (2010) Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol 21: Vogt P, Frei U, Repp H, Bunzendahl H, Oldhafer K, Pichlmayr R (1990) Malignant tumours in renal transplant recipients receiving cyclosporin: survey of 598 first-kidney transplantations. Nephrol Dial Transplant 5: Reis A, Reinhard T, Voiculescu A, Kutkuhn B, Godehardt E, Spelsberg H, Althaus C, Sundmacher R (1999) Mycophenolate mofetil versus cyclosporin A in high risk keratoplasty patients: a prospectively randomised clinical trial. Br J Ophthalmol 83: Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47: Shipkova M, Armstrong VW, Oellerich M, Wieland E (2005) Mycophenolate mofetil in organ transplantation: focus on
8 314 Graefes Arch Clin Exp Ophthalmol (2016) 254: metabolism, safety and tolerability. Expert Opin Drug Metab Toxicol 1: European Mycophenolate Mofetil Cooperative Study Group (1999) Mycophenolate mofetil in renal transplantation: 3-year results from the placebo-controlled trial. Transplantation 68: Birnbaum F, Mayweg S, Reis A, Böhringer D, Seitz B, Engelmann K, Messmer EM, Reinhard T (2009) Mycophenolate mofetil (MMF) following penetrating high-risk keratoplasty: long-term results of a prospective, randomised, multicentre study. Eye (Lond) 23: Alldredge OC, Krachmer JH (1981) Clinical types of corneal transplant rejection: Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol 99: The Collaborative CornealTransplantation Studies Research Group (1993) Design and methods of The Collaborative Corneal Transplantation Studies. Cornea 12: Brightbill FS, McDonnell PJ, McGhee NJ, Fajo AA, Serdarevic ON, (eds) (2009) Corneal surgery: theory, technique and tissue. Fourth edition. Mosby, pp Tham VM, Abbott RL (2002) Corneal graft rejection: recent updates. Int Ophthalmol Clin 42(1): Williams KA, Lowe MT, Bartlett CM, Kelly L, Coster DJ, (eds) (2007) The Australian corneal graft registry report. Flinders University Press, Adelaide, Shimazaki J, Iseda A, Satake Y, Shimazaki-Den S (2012) Efficacy and safety of long-term corticosteroid eye drops after penetrating keratoplasty: a prospective, randomized, clinical trial. Ophthalmology 119: Wei X, Chen XM, Wang L, Song JP, Deng YP (2011) Effects of immunosuppressants after penetrating keratoplasty: meta-analysis of randomized controlled trials. Int J Ophthalmol 4: Shimazaki J, Den S, Omoto M, Satake Y, Shimmura S, Tsubota K (2011) Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am J Ophthalmol 152: Nussenblatt RB, Palestine AG (1986) Cyclosporine: immunology, pharmacology and therapeutic uses. Surv Ophthalmol 31: Yu AL, Kaiser M, Schaumberger M, Messmer E, Kook D, Welge- Lussen U (2014) Perioperative and postoperative risk factors for corneal graft failure. Clin Ophthalmol 28;8: Rijneveld WJ, Wolff R, Völker-Dieben HJ, Pels E (2011) Validation of tissue quality for donor corneas, designated for emergency cases: corneal graft survival. Acta Ophthalmol 89(8): Weisbrod DJ, Sit M, Naor J, Slomovic AR (2003) Outcomes of repeat penetrating keratoplasty and risk factors for graft failure. Cornea 22(5): Al-Mezaine H, Wagoner MD, King Khaled Eye Specialist Hospital Cornea Transplant Study Group (2006) Repeat penetrating keratoplasty: indications, graft survival, and visual outcome. Br J Ophthalmol 90(3): Fasolo A, Capuzzo C, Fornea M, Franch A, Birattari F, Carito G, Cucco F, Prosdocimo G, Sala M, Delle Noci N, Primavera V, Frigo AC, Grigoletto F, Ponzin D, CORTES Study Group (2011) Risk factors for graft failure after penetrating keratoplasty: 5-year followup from the corneal transplantepidemiological study. Cornea 30(12): Reinhard T, Reis A, Böhringer D, Malinowski M, Voiculescu A, Heering P, Godehardt E, Sunmacher R (2001) Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in highrisk keratoplasty patients: 3 years' results of a randomized prospective clinical trial. Graefes Arch Clin Exp Ophthalmol 239(5):
Corneal graft rejection in African Americans at Howard University Hospital
 Saudi Journal of Ophthalmology (2011) 25, 285 289 King Saud University Saudi Journal of Ophthalmology www.saudiophthaljournal.com www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Corneal graft rejection
Saudi Journal of Ophthalmology (2011) 25, 285 289 King Saud University Saudi Journal of Ophthalmology www.saudiophthaljournal.com www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Corneal graft rejection
OCULAR HERPES simplex virus
 CLINICAL SCIENCES Oral Acyclovir After Penetrating Keratoplasty for Herpes Simplex Keratitis Fabiana P. Tambasco, MD; Elisabeth J. Cohen, MD; Lien H. Nguyen, MD; Christopher J. Rapuano, MD; Peter R. Laibson,
CLINICAL SCIENCES Oral Acyclovir After Penetrating Keratoplasty for Herpes Simplex Keratitis Fabiana P. Tambasco, MD; Elisabeth J. Cohen, MD; Lien H. Nguyen, MD; Christopher J. Rapuano, MD; Peter R. Laibson,
PENETRATING KERATOPLASTY
 CLINICAL SCIENCES Risk Factors for Various Causes of Failure in Initial Corneal Grafts Marianne O. Price, PhD; Robert W. Thompson, Jr, MD; Francis W. Price, Jr, MD Objective: To determine the risk factors
CLINICAL SCIENCES Risk Factors for Various Causes of Failure in Initial Corneal Grafts Marianne O. Price, PhD; Robert W. Thompson, Jr, MD; Francis W. Price, Jr, MD Objective: To determine the risk factors
Efficacy and Safety of Thymoglobulin and Basiliximab in Kidney Transplant Patients at High Risk for Acute Rejection and Delayed Graft Function
 ArtIcle Efficacy and Safety of Thymoglobulin and Basiliximab in Kidney Transplant Patients at High Risk for Acute Rejection and Delayed Graft Function Guodong Chen, 1 Jingli Gu, 2 Jiang Qiu, 1 Changxi
ArtIcle Efficacy and Safety of Thymoglobulin and Basiliximab in Kidney Transplant Patients at High Risk for Acute Rejection and Delayed Graft Function Guodong Chen, 1 Jingli Gu, 2 Jiang Qiu, 1 Changxi
Repeat penetrating keratoplasty: indications and prognosis,
 European Journal of Ophthalmology / Vol. 19 no. 3, 2009 / pp. 362-368 Repeat penetrating keratoplasty: indications and prognosis, 1995-2005 ZULEYHA YALNIZ-AKKAYA, AYSE BURCU NUROZLER, ELVIN HATICE YILDIZ,
European Journal of Ophthalmology / Vol. 19 no. 3, 2009 / pp. 362-368 Repeat penetrating keratoplasty: indications and prognosis, 1995-2005 ZULEYHA YALNIZ-AKKAYA, AYSE BURCU NUROZLER, ELVIN HATICE YILDIZ,
Outcome of Penetrating Keratoplasty from a Corneal Unit in Pakistan
 Original Article Outcome of Penetrating Keratoplasty from a Corneal Unit in Pakistan Muhammad Nasir Bhatti, Yawar Zaman, P.S. Mahar, Azizur Rahman, Muhammad Fazal Kamal, Mazhar-ul-Hassan, Partab Rai Pak
Original Article Outcome of Penetrating Keratoplasty from a Corneal Unit in Pakistan Muhammad Nasir Bhatti, Yawar Zaman, P.S. Mahar, Azizur Rahman, Muhammad Fazal Kamal, Mazhar-ul-Hassan, Partab Rai Pak
Condition: Herpes Simplex Keratitis
 Condition: Herpes Simplex Keratitis Description: Herpes simplex infection is very common but usually remains latent. When the virus is reactivated it travels along the trigeminal nerve to cause local infection
Condition: Herpes Simplex Keratitis Description: Herpes simplex infection is very common but usually remains latent. When the virus is reactivated it travels along the trigeminal nerve to cause local infection
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
Assistant Professor, Department of Ophthalmology, Rajindra Hospital, Patiala, Punjab, India 3
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 79-85, p-issn: 79-86.Volume 6, Issue 7 Ver. X (July. 7), PP 5-5 www.iosrjournals.org A Randomized Controlled Prospective Study To Assess
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 79-85, p-issn: 79-86.Volume 6, Issue 7 Ver. X (July. 7), PP 5-5 www.iosrjournals.org A Randomized Controlled Prospective Study To Assess
Immunosuppressants. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Immunosuppressants Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Immunosuppressive Agents Very useful in minimizing the occurrence of exaggerated or inappropriate
Immunosuppressants Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Immunosuppressive Agents Very useful in minimizing the occurrence of exaggerated or inappropriate
JMSCR Volume 03 Issue 01 Page January 2015
 www.jmscr.igmpublication.org Impact Factor 3.79 ISSN (e)-2347-176x Pterygium Excision and Conjunctival Autograft A Study Authors Dr. M. Premanandam 1, Dr. A. Geetha 2, Dr. Himabindu 3 1 MS, Associate Professor,
www.jmscr.igmpublication.org Impact Factor 3.79 ISSN (e)-2347-176x Pterygium Excision and Conjunctival Autograft A Study Authors Dr. M. Premanandam 1, Dr. A. Geetha 2, Dr. Himabindu 3 1 MS, Associate Professor,
Nursing care of the patient with cornea graft rejection
 Department of Ophthalmology and Visual Sciences Publications 12-1-1995 Nursing care of the patient with cornea graft rejection Jill Fishbaugh University of Iowa Copyright 1995 American Society of Ophthalmic
Department of Ophthalmology and Visual Sciences Publications 12-1-1995 Nursing care of the patient with cornea graft rejection Jill Fishbaugh University of Iowa Copyright 1995 American Society of Ophthalmic
Various Landmark. Clinical Clinical Trials: Trials: Cornea. Cornea is the first refractive media and also is the major. Sandeep Gupta MS,DNB
 Clinical Clinical Trials: Trials: Cornea Various Landmark Corneal Clinical Trials Sandeep Gupta MS,DNB Sandeep Gupta MS,DNB, Parth Patel MBBS, B.V. Rao MS,DNB, Gagandeep Kaur MBBS, V.S. Gurunadh MS, M.A.
Clinical Clinical Trials: Trials: Cornea Various Landmark Corneal Clinical Trials Sandeep Gupta MS,DNB Sandeep Gupta MS,DNB, Parth Patel MBBS, B.V. Rao MS,DNB, Gagandeep Kaur MBBS, V.S. Gurunadh MS, M.A.
INVELTYS (loteprednol etabonate ophthalmic suspension) 1%, for topical ophthalmic use Initial U.S. Approval: 1998
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
Effects of deep lamellar keratoplasty on severe necrotizing stromal keratitis.
 Biomedical Research 2018; 29 (4): 702-707 ISSN 0970-938X www.biomedres.info Effects of deep lamellar keratoplasty on severe necrotizing stromal keratitis. Xiaoru Shi, Yang Liu, Hui Jia, Lei Liu, Chunmei
Biomedical Research 2018; 29 (4): 702-707 ISSN 0970-938X www.biomedres.info Effects of deep lamellar keratoplasty on severe necrotizing stromal keratitis. Xiaoru Shi, Yang Liu, Hui Jia, Lei Liu, Chunmei
Human lamellar tendon graft in corneal surgery
 Human lamellar tendon graft in corneal surgery Armando Signorelli, Jr, MD, Carlos Roberto Signorelli, MD, Ernest Rifgatovich Muldashev, MD Refractive and Corneal surgery - 1993 - V.9(2) - P. 135-139 ABSTRACT
Human lamellar tendon graft in corneal surgery Armando Signorelli, Jr, MD, Carlos Roberto Signorelli, MD, Ernest Rifgatovich Muldashev, MD Refractive and Corneal surgery - 1993 - V.9(2) - P. 135-139 ABSTRACT
INDICATIONS For steroid responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye globe.
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
To Evaluate the Sociodemographic Factors And Etiology of Corneal Neovascularisation at out Patient Department of M.L.B Medical College, Jhansi.(U.
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 15, Issue 6 Ver. IV (June. 2016), PP 129-134 www.iosrjournals.org To Evaluate the Sociodemographic Factors
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 15, Issue 6 Ver. IV (June. 2016), PP 129-134 www.iosrjournals.org To Evaluate the Sociodemographic Factors
Dr Jo-Anne Pon. Dr Sean Every. 8:30-9:25 WS #70: Eye Essentials for GPs 9:35-10:30 WS #80: Eye Essentials for GPs (Repeated)
 Dr Sean Every Ophthalmologist Southern Eye Specialists Christchurch Dr Jo-Anne Pon Ophthalmologist Southern Eye Specialists, Christchurch Hospital, Christchurch 8:30-9:25 WS #70: Eye Essentials for GPs
Dr Sean Every Ophthalmologist Southern Eye Specialists Christchurch Dr Jo-Anne Pon Ophthalmologist Southern Eye Specialists, Christchurch Hospital, Christchurch 8:30-9:25 WS #70: Eye Essentials for GPs
Transplant Hepatology
 Transplant Hepatology Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the certified
Transplant Hepatology Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the certified
Subject Index. Atopic keratoconjunctivitis (AKC) management 16 overview 15
 Subject Index Acanthamoeba keratitis, see Infective keratitis Acute allergic conjunctivitis AKC, see Atopic keratoconjunctivitis Allergy acute allergic conjunctivitis 15 atopic keratoconjunctivitis 15
Subject Index Acanthamoeba keratitis, see Infective keratitis Acute allergic conjunctivitis AKC, see Atopic keratoconjunctivitis Allergy acute allergic conjunctivitis 15 atopic keratoconjunctivitis 15
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
Lamellar Keratoplasty for the Treatment of Fungal Keratitis
 Cornea 21(1): 33 37, 2002. 2002 Lippincott Williams & Wilkins, Inc., Philadelphia Lamellar Keratoplasty for the Treatment of Fungal Keratitis Lixin Xie, M.D., Weiyun Shi, M.D., Zhaosheng Liu, M.D., and
Cornea 21(1): 33 37, 2002. 2002 Lippincott Williams & Wilkins, Inc., Philadelphia Lamellar Keratoplasty for the Treatment of Fungal Keratitis Lixin Xie, M.D., Weiyun Shi, M.D., Zhaosheng Liu, M.D., and
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia
 Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Issued: September 2013 guidance.nice.org.uk/ipg466 NICE has accredited the process used
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Issued: September 2013 guidance.nice.org.uk/ipg466 NICE has accredited the process used
PATIENT INFORMATION ON CORNEAL GRAFT
 PATIENT INFORMATION ON CORNEAL GRAFT (TRANSPLANT) SURGERY M ANANDAN What is the cornea? The clear window of the eye approximately 0.5mm thick and 12mm across. It lies in front of the fluid filled anterior
PATIENT INFORMATION ON CORNEAL GRAFT (TRANSPLANT) SURGERY M ANANDAN What is the cornea? The clear window of the eye approximately 0.5mm thick and 12mm across. It lies in front of the fluid filled anterior
Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
 Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
European Risk Management Plan. Measures impairment. Retreatment after Discontinuation
 European Risk Management Plan Table 6.1.4-1: Safety Concern 55024.1 Summary of Risk Minimization Measures Routine Risk Minimization Measures Additional Risk Minimization Measures impairment. Retreatment
European Risk Management Plan Table 6.1.4-1: Safety Concern 55024.1 Summary of Risk Minimization Measures Routine Risk Minimization Measures Additional Risk Minimization Measures impairment. Retreatment
BK virus infection in renal transplant recipients: single centre experience. Dr Wong Lok Yan Ivy
 BK virus infection in renal transplant recipients: single centre experience Dr Wong Lok Yan Ivy Background BK virus nephropathy (BKVN) has emerged as an important cause of renal graft dysfunction in recent
BK virus infection in renal transplant recipients: single centre experience Dr Wong Lok Yan Ivy Background BK virus nephropathy (BKVN) has emerged as an important cause of renal graft dysfunction in recent
Donor Risk Factors for Graft Failure in the Cornea Donor Study
 CLINICAL SCIENCE Donor Risk Factors for Graft Failure in the Cornea Donor Study Joel Sugar, MD,* Monty Montoya, MBA, Mariya Dontchev, MPH, Jean Paul Tanner, MPH, Roy Beck, MD, PhD, Robin Gal, MSPH, Shawn
CLINICAL SCIENCE Donor Risk Factors for Graft Failure in the Cornea Donor Study Joel Sugar, MD,* Monty Montoya, MBA, Mariya Dontchev, MPH, Jean Paul Tanner, MPH, Roy Beck, MD, PhD, Robin Gal, MSPH, Shawn
Influence of Cyclosporin on Steroid-induced Cataracts After Renal Transplantation
 Influence of Cyclosporin on Steroid-induced Cataracts After Renal Transplantation Takako Nakamura*, Hiroshi Sasaki*, Kouta Nagai*, Kuruto Fujisawa*, Kazuyuki Sasaki*, Kouji Suzuki and Ryuzo Tsugawa Departments
Influence of Cyclosporin on Steroid-induced Cataracts After Renal Transplantation Takako Nakamura*, Hiroshi Sasaki*, Kouta Nagai*, Kuruto Fujisawa*, Kazuyuki Sasaki*, Kouji Suzuki and Ryuzo Tsugawa Departments
D90 (27/10/2005) Final SmPC NL/H/653/01
 1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
GENERAL INFORMATION CORNEAL TRANSPLANTATION
 GENERAL INFORMATION CORNEAL TRANSPLANTATION WHAT IS CORNEAL TRANSPLANTATION? A corneal transplant is an operation where a damaged or diseased cornea is replaced with donated, healthy tissue. Also called
GENERAL INFORMATION CORNEAL TRANSPLANTATION WHAT IS CORNEAL TRANSPLANTATION? A corneal transplant is an operation where a damaged or diseased cornea is replaced with donated, healthy tissue. Also called
Proton Pump Inhibitors do not Interact with the Immunosuppressant Enteric-Coated Mycophenolate Sodium
 Proton Pump Inhibitors do not Interact with the Immunosuppressant Enteric-Coated Mycophenolate Sodium S. Kofler, C. Wolf, Z. Sisic, J. Behr, M. Vogeser, M. Shipkova, B. Meiser, G. Steinbeck, B. Reichart,
Proton Pump Inhibitors do not Interact with the Immunosuppressant Enteric-Coated Mycophenolate Sodium S. Kofler, C. Wolf, Z. Sisic, J. Behr, M. Vogeser, M. Shipkova, B. Meiser, G. Steinbeck, B. Reichart,
Corneal transplantation: HLA and age
 L Aquila, 24 novembre 2012 Corneal transplantation: HLA and age Federico Genzano Besso*, Paola Magistroni, Piera Santoro*, Silvano Battaglio*, Raffaella Giacometti, Morteza Mansouri and Antonio Amoroso
L Aquila, 24 novembre 2012 Corneal transplantation: HLA and age Federico Genzano Besso*, Paola Magistroni, Piera Santoro*, Silvano Battaglio*, Raffaella Giacometti, Morteza Mansouri and Antonio Amoroso
Evolving therapies for posterior uveitis. Infliximab (Remicade) Infliximab: pharmacology. FDA-approved monoclonal antibody therapy Target
 Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Serum samples from recipients were obtained within 48 hours before transplantation. Pre-transplant
 SDC, Patients and Methods Complement-dependent lymphocytotoxic crossmatch test () Serum samples from recipients were obtained within 48 hours before transplantation. Pre-transplant donor-specific CXM was
SDC, Patients and Methods Complement-dependent lymphocytotoxic crossmatch test () Serum samples from recipients were obtained within 48 hours before transplantation. Pre-transplant donor-specific CXM was
CORNEAL CONDITIONS CORNEAL TRANSPLANTATION
 GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients
 Pediatr Transplantation 2013: 17: 436 440 2013 John Wiley & Sons A/S. Pediatric Transplantation DOI: 10.1111/petr.12095 Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients
Pediatr Transplantation 2013: 17: 436 440 2013 John Wiley & Sons A/S. Pediatric Transplantation DOI: 10.1111/petr.12095 Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients
JOURNAL OF OPHTHALMOLOGY AND RELATED SCIENCES
 JOURNAL OF OPHTHALMOLOGY AND RELATED SCIENCES BILATERAL ACUTE TRANSILLUMINATION OF THE IRIS Kavitha Avadhani 1, MD, MS, Jay Kalliath 1, MS, FRCS 1 Department of Ophthalmology, NMC Speciality Hospital,
JOURNAL OF OPHTHALMOLOGY AND RELATED SCIENCES BILATERAL ACUTE TRANSILLUMINATION OF THE IRIS Kavitha Avadhani 1, MD, MS, Jay Kalliath 1, MS, FRCS 1 Department of Ophthalmology, NMC Speciality Hospital,
Oral azithromycin combined with topical anti-inflammatory agents in the treatment of blepharokeratoconjunctivitis in children
 Oral azithromycin combined with topical anti-inflammatory agents in the treatment of blepharokeratoconjunctivitis in children Daniel S. Choi, BA, and Ali Djalilian, MD Author affiliations: Department of
Oral azithromycin combined with topical anti-inflammatory agents in the treatment of blepharokeratoconjunctivitis in children Daniel S. Choi, BA, and Ali Djalilian, MD Author affiliations: Department of
Original Article A retrospective clinical study of Xinjiang Uygur patients with corneal allograft rejection
 Int J Clin Exp Med 2015;8(3):4356-4362 www.ijcem.com /ISSN:1940-5901/IJCEM0005912 Original Article A retrospective clinical study of Xinjiang Uygur patients with corneal allograft Reziwan Maimaitiming
Int J Clin Exp Med 2015;8(3):4356-4362 www.ijcem.com /ISSN:1940-5901/IJCEM0005912 Original Article A retrospective clinical study of Xinjiang Uygur patients with corneal allograft Reziwan Maimaitiming
Steroid Minimization: Great Idea or Silly Move?
 Steroid Minimization: Great Idea or Silly Move? Disclosures I have financial relationship(s) within the last 12 months relevant to my presentation with: Astellas Grants ** Bristol Myers Squibb Grants,
Steroid Minimization: Great Idea or Silly Move? Disclosures I have financial relationship(s) within the last 12 months relevant to my presentation with: Astellas Grants ** Bristol Myers Squibb Grants,
2009 Eye Banking Statistical Report Eye Bank Association of America th Street, N.W. Suite 1010 Washington, DC Phone (202) Fax
 2009 Eye Banking Statistical Report Eye Bank Association of America 1015 18th Street, N.W. Suite 1010 Washington, DC 20036 Phone (202) 775-4999 Fax (202) 429-6036 www.restoresight.org Introduction 2009
2009 Eye Banking Statistical Report Eye Bank Association of America 1015 18th Street, N.W. Suite 1010 Washington, DC 20036 Phone (202) 775-4999 Fax (202) 429-6036 www.restoresight.org Introduction 2009
Systemic Immunomodulatory Strategies in High-risk Corneal Transplantation
 Systemic Immunomodulatory Strategies in High-risk Corneal Transplantation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Systemic Immunomodulatory Strategies in High-risk Corneal Transplantation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient
 CM&R Rapid Release. Published online ahead of print September 20, 2012 as Aperture Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient Elizabeth
CM&R Rapid Release. Published online ahead of print September 20, 2012 as Aperture Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient Elizabeth
SELECTED ABSTRACTS. All (n) % 3-year GS 88% 82% 86% 85% 88% 80% % 3-year DC-GS 95% 87% 94% 89% 96% 80%
 SELECTED ABSTRACTS The following are summaries of selected posters presented at the American Transplant Congress on May 5 9, 2007, in San Humar A, Gillingham KJ, Payne WD, et al. Review of >1000 kidney
SELECTED ABSTRACTS The following are summaries of selected posters presented at the American Transplant Congress on May 5 9, 2007, in San Humar A, Gillingham KJ, Payne WD, et al. Review of >1000 kidney
Increased Early Rejection Rate after Conversion from Tacrolimus in Kidney and Pancreas Transplantation
 Increased Early Rejection Rate after Conversion from Tacrolimus in Kidney and Pancreas Transplantation Gary W Barone 1, Beverley L Ketel 1, Sameh R Abul-Ezz 2, Meredith L Lightfoot 1 1 Department of Surgery
Increased Early Rejection Rate after Conversion from Tacrolimus in Kidney and Pancreas Transplantation Gary W Barone 1, Beverley L Ketel 1, Sameh R Abul-Ezz 2, Meredith L Lightfoot 1 1 Department of Surgery
Sclerokeratoplasty David S. Chu, M.D. Cases
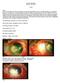 Sclerokeratoplasty David S. Chu, M.D. Cases Case 1 40 year-old female from Peru presented to our Service with inflamed OS for 2 months duration. Her symptoms began as red painful OS, which progressively
Sclerokeratoplasty David S. Chu, M.D. Cases Case 1 40 year-old female from Peru presented to our Service with inflamed OS for 2 months duration. Her symptoms began as red painful OS, which progressively
Emerging Drug List EVEROLIMUS
 Generic (Trade Name): Manufacturer: Everolimus (Certican ) Novartis Pharmaceuticals NO. 57 MAY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence: For use with cyclosporine
Generic (Trade Name): Manufacturer: Everolimus (Certican ) Novartis Pharmaceuticals NO. 57 MAY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence: For use with cyclosporine
Mooren s ulcer in China: a study of clinical characteristics and treatment
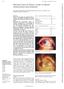 1244 Zhongshan Ophthalmic Center, Sun Yat-sen University of Medical Sciences, Guangzhou, PR China J Chen H Xie Z Wang B Yang Z Liu L Chen X Gong Y Lin Correspondence to: Dr Jiaqi Chen, Zhongshan Ophthalmic
1244 Zhongshan Ophthalmic Center, Sun Yat-sen University of Medical Sciences, Guangzhou, PR China J Chen H Xie Z Wang B Yang Z Liu L Chen X Gong Y Lin Correspondence to: Dr Jiaqi Chen, Zhongshan Ophthalmic
Original Article Retrospective analysis of corneal transplantation: a tertiary hospital database study over ten years
 Int J Clin Exp Med 2018;11(7):6824-6831 www.ijcem.com /ISSN:1940-5901/IJCEM0069798 Original Article : a tertiary hospital database study over ten years Yaping Jiang 1,2, Minjie Sheng 1, Yue Zhang 3, Xiaoyan
Int J Clin Exp Med 2018;11(7):6824-6831 www.ijcem.com /ISSN:1940-5901/IJCEM0069798 Original Article : a tertiary hospital database study over ten years Yaping Jiang 1,2, Minjie Sheng 1, Yue Zhang 3, Xiaoyan
Abbreviated Drug Evaluation: Fluocinolone acetonide intravitreal implant (Retisert )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Acute Eyes for ED. Enis Kocak. The Alfred Ophthalmology
 Acute Eyes for ED Enis Kocak The Alfred Ophthalmology The problem with eyes Things to cover Ocular anatomy Basic assessment Common presentations Eye first aid and procedures Ophthalmic emergencies What
Acute Eyes for ED Enis Kocak The Alfred Ophthalmology The problem with eyes Things to cover Ocular anatomy Basic assessment Common presentations Eye first aid and procedures Ophthalmic emergencies What
Overview of New Approaches to Immunosuppression in Renal Transplantation
 Overview of New Approaches to Immunosuppression in Renal Transplantation Ron Shapiro, M.D. Professor of Surgery Surgical Director, Kidney/Pancreas Transplant Program Recanati/Miller Transplantation Institute
Overview of New Approaches to Immunosuppression in Renal Transplantation Ron Shapiro, M.D. Professor of Surgery Surgical Director, Kidney/Pancreas Transplant Program Recanati/Miller Transplantation Institute
Photodynamic therapy for IMMK in horses
 Photodynamic therapy for IMMK in horses Overview Immune mediated keratitis in horses Traditional treatment options Sustained release implant Photodynamic therapy Use in veterinary medicine Treatment for
Photodynamic therapy for IMMK in horses Overview Immune mediated keratitis in horses Traditional treatment options Sustained release implant Photodynamic therapy Use in veterinary medicine Treatment for
UCSF UC San Francisco Previously Published Works
 UCSF UC San Francisco Previously Published Works Title Unilateral Posterior Interstitial Keratitis as a Clinical Presentation of Herpes Simplex Virus Disease. Permalink https://escholarship.org/uc/item/8970f2fq
UCSF UC San Francisco Previously Published Works Title Unilateral Posterior Interstitial Keratitis as a Clinical Presentation of Herpes Simplex Virus Disease. Permalink https://escholarship.org/uc/item/8970f2fq
Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes The cornea
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes The cornea
Long-term prognosis of BK virus-associated nephropathy in kidney transplant recipients
 Original Article Kidney Res Clin Pract 37:167-173, 2018(2) pissn: 2211-9132 eissn: 2211-9140 https://doi.org/10.23876/j.krcp.2018.37.2.167 KIDNEY RESEARCH AND CLINICAL PRACTICE Long-term prognosis of BK
Original Article Kidney Res Clin Pract 37:167-173, 2018(2) pissn: 2211-9132 eissn: 2211-9140 https://doi.org/10.23876/j.krcp.2018.37.2.167 KIDNEY RESEARCH AND CLINICAL PRACTICE Long-term prognosis of BK
History. Examination. Diagnosis/Course
 History A 51 year-old female with a history of chronic dry eyes and photosensitivity was referred for evaluation. She reported a five year history of symptoms of frequent irritation and photophobia in
History A 51 year-old female with a history of chronic dry eyes and photosensitivity was referred for evaluation. She reported a five year history of symptoms of frequent irritation and photophobia in
Deep Anterior Lamellar Keratoplasty - Techniques
 Deep Anterior Lamellar Keratoplasty - Techniques SHERAZ DAYA MD FACP FACS FRCS(Ed) FRCOphth Financial Disclosure Company Code 1. Abbott Medical Optics Inc. S 2. Bausch + Lomb C,L 3. Carl Zeiss Meditec
Deep Anterior Lamellar Keratoplasty - Techniques SHERAZ DAYA MD FACP FACS FRCS(Ed) FRCOphth Financial Disclosure Company Code 1. Abbott Medical Optics Inc. S 2. Bausch + Lomb C,L 3. Carl Zeiss Meditec
Herpetic Eye Disease Jason Duncan, OD, FAAO Diplomate, American Board of Optometry Associate Professor, Southern College of Optometry
 Herpetic Eye Disease Jason Duncan, OD, FAAO Diplomate, American Board of Optometry Associate Professor, Southern College of Optometry I have what?! How to break the news Meet the Herpes Quick virology
Herpetic Eye Disease Jason Duncan, OD, FAAO Diplomate, American Board of Optometry Associate Professor, Southern College of Optometry I have what?! How to break the news Meet the Herpes Quick virology
Post-LASIK infections
 Post-LASIK infections By Mohamed El-moddather Assiss. Prof. and head of department of ophthalmology AL-Azhar unizersity Assuit LASIK has become a common refractive procedure and is generally considered
Post-LASIK infections By Mohamed El-moddather Assiss. Prof. and head of department of ophthalmology AL-Azhar unizersity Assuit LASIK has become a common refractive procedure and is generally considered
For Immediate Release Contacts: Jenny Keeney Astellas US LLC (847)
 For Immediate Release Contacts: Jenny Keeney Astellas US LLC (847) 317-5405 Lauren McDonnell GolinHarris (312) 729-4233 ASTELLAS RECEIVES FDA APPROVAL FOR USE OF PROGRAF (TACROLIMUS) IN CONJUNCTION WITH
For Immediate Release Contacts: Jenny Keeney Astellas US LLC (847) 317-5405 Lauren McDonnell GolinHarris (312) 729-4233 ASTELLAS RECEIVES FDA APPROVAL FOR USE OF PROGRAF (TACROLIMUS) IN CONJUNCTION WITH
Innovation In Ophthalmology
 Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma
 Original Article Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma Irfan Qayyum Malik, M. Moin, A. Rehman, Mumtaz Hussain
Original Article Role of Initial Preoperative Medical Management in Controlling Post-Operative Anterior Uveitis in Patients of Phacomorphic Glaucoma Irfan Qayyum Malik, M. Moin, A. Rehman, Mumtaz Hussain
Treatment of fungal keratitis by penetrating keratoplasty
 1070 Shandong Eye Institute and Hospital, Qingdao 266071, PR China L Xie X Dong W Shi Correspondence to: Lixin Xie, MD, Shandong Eye Institute and Hospital, 5 Yanerdao Road, Qingdao 266071, PR China lixinxie@public.qd.sd.cn
1070 Shandong Eye Institute and Hospital, Qingdao 266071, PR China L Xie X Dong W Shi Correspondence to: Lixin Xie, MD, Shandong Eye Institute and Hospital, 5 Yanerdao Road, Qingdao 266071, PR China lixinxie@public.qd.sd.cn
Cataract and cornea. Miltos O. Balidis PhD, FEBOphth,ICOphth ATHENS
 Cataract and cornea Miltos O. Balidis PhD, FEBOphth,ICOphth CATARACT and Stromal opacities Keratoplasty Keratoconus Endothelial pathology Scars PTK Trypan blue 0.01%. Work at the transparent side of cornea
Cataract and cornea Miltos O. Balidis PhD, FEBOphth,ICOphth CATARACT and Stromal opacities Keratoplasty Keratoconus Endothelial pathology Scars PTK Trypan blue 0.01%. Work at the transparent side of cornea
Therapeutic keratoplasty in
 Brit. J. Ophthal. (I 97 I) 55, 326 Therapeutic keratoplasty in Pseudomonas pyocyaneus corneal ulcers S. R. K. MALIK AND GURBAX SINGH Maulana Azad Medical College and Associated Irwin and G.B. Pant Hospital,
Brit. J. Ophthal. (I 97 I) 55, 326 Therapeutic keratoplasty in Pseudomonas pyocyaneus corneal ulcers S. R. K. MALIK AND GURBAX SINGH Maulana Azad Medical College and Associated Irwin and G.B. Pant Hospital,
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endothelial_keratoplasty 9/2009 6/2018 6/2019 6/2018 Description of Procedure or Service Endothelial keratoplasty
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endothelial_keratoplasty 9/2009 6/2018 6/2019 6/2018 Description of Procedure or Service Endothelial keratoplasty
Acanthamoeba Keratitis in a Non-contact Lens Wearer: A Challenge in Diagnosis and Management
 JOURNAL OF CASE REPORTS 2014;4(2):419-423 Acanthamoeba Keratitis in a Non-contact Lens Wearer: A Challenge in Diagnosis and Management Dian Eka Putri, Lukman Edwar, Made Susiyanti Department of Ophthalmology,
JOURNAL OF CASE REPORTS 2014;4(2):419-423 Acanthamoeba Keratitis in a Non-contact Lens Wearer: A Challenge in Diagnosis and Management Dian Eka Putri, Lukman Edwar, Made Susiyanti Department of Ophthalmology,
Medical Science. Research Paper. 2nd yr post graduate, Dept. Of Ophthalmology, SVS Medical College
 Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Clinical Indications for Penetrating Keratoplasty in Maharaj Nakorn Chiang Mai Hospital,
 Thai J Ophthalmol Clinical Indications for Penetrating Keratoplasty in Maharaj Nakorn Chiang Mai Hospital, 1990-1 995 Somsanguan Ausayakhun, M.D.* Jinda Juntaramanee** ABSTRACT The preoperative clinical
Thai J Ophthalmol Clinical Indications for Penetrating Keratoplasty in Maharaj Nakorn Chiang Mai Hospital, 1990-1 995 Somsanguan Ausayakhun, M.D.* Jinda Juntaramanee** ABSTRACT The preoperative clinical
JMSCR Vol 06 Issue 11 Page November 2018
 www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 79.54 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i11.77 Short-Term Outcome of Penetrating
www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 79.54 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i11.77 Short-Term Outcome of Penetrating
Reduced graft function (with or without dialysis) vs immediate graft function a comparison of long-term renal allograft survival
 Nephrol Dial Transplant (2006) 21: 2270 2274 doi:10.1093/ndt/gfl103 Advance Access publication 22 May 2006 Original Article Reduced graft function (with or without dialysis) vs immediate graft function
Nephrol Dial Transplant (2006) 21: 2270 2274 doi:10.1093/ndt/gfl103 Advance Access publication 22 May 2006 Original Article Reduced graft function (with or without dialysis) vs immediate graft function
DALK IN DANGEROUS INFECTIONS
 32 INTERNATIONAL CONGRESS of the HELLENIC SOCIETY OF INTRAOCULAR IMPLANT AND REFRACTIVE SURGERY CORNEA ROUND TABLE: STROMAL REPAIR DALK IN DANGEROUS INFECTIONS, MD Clinica Degli Occhi Sarnicola, Grosseto
32 INTERNATIONAL CONGRESS of the HELLENIC SOCIETY OF INTRAOCULAR IMPLANT AND REFRACTIVE SURGERY CORNEA ROUND TABLE: STROMAL REPAIR DALK IN DANGEROUS INFECTIONS, MD Clinica Degli Occhi Sarnicola, Grosseto
HERPES SIMPLEX VIRUS (HSV)
 CLINICAL SCIENCES Modalities to Decrease Stromal Herpes Simplex Keratitis Reactivation Rates John D. Sheppard, MD; Michael L. Wertheimer, MD; Stephen V. Scoper, MD Objective: To evaluate the efficacy of
CLINICAL SCIENCES Modalities to Decrease Stromal Herpes Simplex Keratitis Reactivation Rates John D. Sheppard, MD; Michael L. Wertheimer, MD; Stephen V. Scoper, MD Objective: To evaluate the efficacy of
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Visual outcome in patients undergoing penetrating keratoplasty
 International Journal of Research in Medical Sciences Singh G et al. Int J Res Med Sci. 2015 Jan;3(1):244-249 www.msjonline.org pissn 2320-6071 eissn 2320-6012 Research Article DOI: 10.5455/2320-6012.ijrms20150144
International Journal of Research in Medical Sciences Singh G et al. Int J Res Med Sci. 2015 Jan;3(1):244-249 www.msjonline.org pissn 2320-6071 eissn 2320-6012 Research Article DOI: 10.5455/2320-6012.ijrms20150144
Trifluridine Ophthalmic Solution, 1% Sterile
 Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
Non-Descemet Stripping Automated Endothelial Keratoplasty for Bullous Keratopathy in Buphthalmic Eye
 DOI: 10.1159/000446103 Published online: June 2, 2016 2016 The Author(s) Published by S. Karger AG, Basel This article is licensed under the Creative Commons Attribution-NonCommercial 4.0 International
DOI: 10.1159/000446103 Published online: June 2, 2016 2016 The Author(s) Published by S. Karger AG, Basel This article is licensed under the Creative Commons Attribution-NonCommercial 4.0 International
Summary of Results for Laypersons
 What was the Study Called? Summary of Results for Laypersons A Multicenter, Four Arm, Randomized, Open Label Clinical Study Investigating Optimized Dosing in a Prograf -/Advagraf -Based Immunosuppressive
What was the Study Called? Summary of Results for Laypersons A Multicenter, Four Arm, Randomized, Open Label Clinical Study Investigating Optimized Dosing in a Prograf -/Advagraf -Based Immunosuppressive
Herpes Zoster Ophtalmicus in a HIV positive patient: A Case Report
 ISPUB.COM The Internet Journal of Neurology Volume 9 Number 2 Herpes Zoster Ophtalmicus in a HIV positive patient: A Case Report G Lopez Bejerano, Y Graza Fernandez Citation G Lopez Bejerano, Y Graza Fernandez..
ISPUB.COM The Internet Journal of Neurology Volume 9 Number 2 Herpes Zoster Ophtalmicus in a HIV positive patient: A Case Report G Lopez Bejerano, Y Graza Fernandez Citation G Lopez Bejerano, Y Graza Fernandez..
Influence of advanced recipient and donor age on the outcome of corneal transplantation
 British Journal of Ophthalmology 1997;81:835 839 835 Department of Ophthalmology, Flinders University of South Australia, Australia K A Williams S M Muehlberg R F Lewis D J Coster Correspondence to: Dr
British Journal of Ophthalmology 1997;81:835 839 835 Department of Ophthalmology, Flinders University of South Australia, Australia K A Williams S M Muehlberg R F Lewis D J Coster Correspondence to: Dr
Mustard Gas Induced Ocular Surface Disorders
 Challenging Case Mustard Gas Induced Ocular Surface Disorders Section Editor: Alireza Baradaran-Rafii, MD Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran Sulfur
Challenging Case Mustard Gas Induced Ocular Surface Disorders Section Editor: Alireza Baradaran-Rafii, MD Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran Sulfur
Clinical Practice Guide for the Diagnosis, Treatment and Management of Anterior Eye Conditions. April 2018
 Clinical Practice Guide for the Diagnosis, Treatment and Management of Anterior Eye Conditions This Clinical Practice Guide provides evidence-based information about current best practice in the management
Clinical Practice Guide for the Diagnosis, Treatment and Management of Anterior Eye Conditions This Clinical Practice Guide provides evidence-based information about current best practice in the management
Choroidal Neovascularization in Sympathetic Ophthalmia
 Choroidal Neovascularization in Sympathetic Ophthalmia Lucia Sobrin, Miguel Cordero Coma, C. Stephen Foster Case Report A 49-year-old man presented after a ruptured globe repair of his left eye status
Choroidal Neovascularization in Sympathetic Ophthalmia Lucia Sobrin, Miguel Cordero Coma, C. Stephen Foster Case Report A 49-year-old man presented after a ruptured globe repair of his left eye status
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
IMMUNOSUPPRESSION IN CORNE AL TR ANSPLANTATION
 IMMUNOSUPPRESSION IN CORNE AL TR ANSPLANTATION JOHN C. HILL Cape Town, South Africa SUMMARY This paper reviews the clinical post-operative management of keratoplasty and the management of corneal graft
IMMUNOSUPPRESSION IN CORNE AL TR ANSPLANTATION JOHN C. HILL Cape Town, South Africa SUMMARY This paper reviews the clinical post-operative management of keratoplasty and the management of corneal graft
TITLE: Proton Pump Inhibitors (PPIs) in Renal Transplant Patients: Evidence for PPI of Choice
 TITLE: Proton Pump Inhibitors (PPIs) in Renal Transplant Patients: Evidence for PPI of Choice DATE: 25 August 2008 CONTEXT AND POLICY ISSUES: Gastrointestinal (GI) complications including gastroduodenal
TITLE: Proton Pump Inhibitors (PPIs) in Renal Transplant Patients: Evidence for PPI of Choice DATE: 25 August 2008 CONTEXT AND POLICY ISSUES: Gastrointestinal (GI) complications including gastroduodenal
Misdiagnosed Vogt-Koyanagi-Harada (VKH) disease and atypical central serous chorioretinopathy (CSC)
 HPTER 12 Misdiagnosed Vogt-Koyanagi-Harada (VKH) disease and atypical central serous chorioretinopathy (S) linical Features VKH disease is a bilateral granulomatous panuveitis often associated with exudative
HPTER 12 Misdiagnosed Vogt-Koyanagi-Harada (VKH) disease and atypical central serous chorioretinopathy (S) linical Features VKH disease is a bilateral granulomatous panuveitis often associated with exudative
Eye (2009) 23, & 2009 Macmillan Publishers Limited All rights reserved X/09 $32.00
 (2009) 23, 543 548 & 2009 Macmillan Publishers Limited All rights reserved 0950-222X/09 $32.00 www.nature.com/eye Effects of the duration of initial oral corticosteroid treatment on the recurrence of inflammation
(2009) 23, 543 548 & 2009 Macmillan Publishers Limited All rights reserved 0950-222X/09 $32.00 www.nature.com/eye Effects of the duration of initial oral corticosteroid treatment on the recurrence of inflammation
Protocol. Endothelial Keratoplasty
 Protocol Endothelial Keratoplasty (90322) Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization No Review Dates: 01/14, 11/14, 11/15, 11/16, 11/17 Preauthorization is not required.
Protocol Endothelial Keratoplasty (90322) Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization No Review Dates: 01/14, 11/14, 11/15, 11/16, 11/17 Preauthorization is not required.
The Evolution of Corneal Transplantation
 e-issn 2329-0358 DOI: 10.12659/AOT.905498 Received: 2017.05.25 Accepted: 2017.07.19 Published: 2017.12.15 The Evolution of Corneal Transplantation Authors Contribution: Study Design A Data Collection B
e-issn 2329-0358 DOI: 10.12659/AOT.905498 Received: 2017.05.25 Accepted: 2017.07.19 Published: 2017.12.15 The Evolution of Corneal Transplantation Authors Contribution: Study Design A Data Collection B
Pediatric Kidney Transplantation
 Pediatric Kidney Transplantation Vikas Dharnidharka, MD, MPH Associate Professor Division of Pediatric Nephrology Conflict of Interest Disclosure Vikas Dharnidharka, MD, MPH Employer: University of Florida
Pediatric Kidney Transplantation Vikas Dharnidharka, MD, MPH Associate Professor Division of Pediatric Nephrology Conflict of Interest Disclosure Vikas Dharnidharka, MD, MPH Employer: University of Florida
UPDATE ON CORNEAL TRANSPLANTATION. Frank S. Hwang M.D. Assistant Professor Cornea, External Disease and Refractive Surgery
 UPDATE ON CORNEAL TRANSPLANTATION Frank S. Hwang M.D. Assistant Professor Cornea, External Disease and Refractive Surgery OBJECTIVES Types of corneal transplantation Donor Selection of corneal tissue Penetrating
UPDATE ON CORNEAL TRANSPLANTATION Frank S. Hwang M.D. Assistant Professor Cornea, External Disease and Refractive Surgery OBJECTIVES Types of corneal transplantation Donor Selection of corneal tissue Penetrating
Optometric Postoperative Cataract Surgery Management
 Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
How to improve long term outcome after liver transplantation?
 How to improve long term outcome after liver transplantation? François Durand Hepatology & Liver Intensive Care University Paris Diderot INSERM U1149 Hôpital Beaujon, Clichy PHC 2018 www.aphc.info Long
How to improve long term outcome after liver transplantation? François Durand Hepatology & Liver Intensive Care University Paris Diderot INSERM U1149 Hôpital Beaujon, Clichy PHC 2018 www.aphc.info Long
Introduction. Fuchs Endothelial Corneal Dystrophy (FECD) represents a non-inflammatory dystrophy of the corneal endothelial layer which
 Romanian Journal of Ophthalmology, Volume 59, Issue 3, July-September 2015. pp:159-163 GENERAL ARTICLE FUCHS ENDOTHELIAL CORNEAL DYSTROPHY: IS FEMTOSECOND LASER ASSISTED CATARACT SURGERY THE RIGHT APPROACH?
Romanian Journal of Ophthalmology, Volume 59, Issue 3, July-September 2015. pp:159-163 GENERAL ARTICLE FUCHS ENDOTHELIAL CORNEAL DYSTROPHY: IS FEMTOSECOND LASER ASSISTED CATARACT SURGERY THE RIGHT APPROACH?
Endothelial Keratoplasty
 Endothelial Keratoplasty Policy Number: 9.03.22 Last Review: 11/2017 Origination: 11/2015 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Endothelial
Endothelial Keratoplasty Policy Number: 9.03.22 Last Review: 11/2017 Origination: 11/2015 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Endothelial
Case Report Outcome of Two Corneal Collagen Crosslinking Methods in Bullous Keratopathy due to Fuchs Endothelial Dystrophy
 Case Reports in Medicine, Article ID 463905, 5 pages http://dx.doi.org/10.1155/2014/463905 Case Report Outcome of Two Corneal Collagen Crosslinking Methods in Bullous Keratopathy due to Fuchs Endothelial
Case Reports in Medicine, Article ID 463905, 5 pages http://dx.doi.org/10.1155/2014/463905 Case Report Outcome of Two Corneal Collagen Crosslinking Methods in Bullous Keratopathy due to Fuchs Endothelial
