AJNT. Review Article. Management of Pediatric Tumor Lysis Syndrome. Illias tazi *¹, Hatim Nafil¹, Jamila Elhoudzi², Lahoucine Mahmal¹, Mhamed Harif²
|
|
|
- Georgia Lynch
- 5 years ago
- Views:
Transcription
1 Sep;4(3): Review Article AJNT Management of Pediatric Tumor Lysis Syndrome Illias tazi *¹, Hatim Nafil¹, Jamila Elhoudzi², Lahoucine Mahmal¹, Mhamed Harif² 1. Hematology department, Chu Mohamed VI, Cadi Ayyad University, Marrakech, Morocco 2. Hematology and Pediatric Oncology department, Chu Mohamed VI, Cadi Ayyad University, Marrakech, Morocco Abstract Introduction: Tumor lysis syndrome (TLS) is a serious complication of malignancies and can result in renal failure or death. Review: In tumors with a high proliferative rate with a relatively large mass and a high sensitivity to cytotoxic agents, the initiation of therapy often results in the rapid release of intracellular anions, cations and the metabolic products of proteins and nucleic acids into the bloodstream. The increased concentrations of uric acid, phosphates, potassium and urea can overwhelm the body s homeostatic mechanisms to process and excrete these materials and result in the clinical spectrum associated with TLS. Typical clinical sequelae include gastrointestinal disturbances, neuromuscular effects, cardiovascular complications, acute renal failure and death. Tumor lysis syndrome can also compromise the efficacy or administration of curative therapies. Available evidence suggests that the incidence of clinical TLS is approximately 3 7% for acute leukemias and 4 11% for lymphomas. Pediatric cancers are the leading cause of death by disease in children. The most common pediatric cancers include the leukemias, lymphomas, central nervous system tumors and neuroblastoma. Thus, TLS is a major concern to practitioners caring for pediatric oncology patients. Given the complexity of TLS prevention and treatment, a multidisciplinary approach involving the collaboration of medical oncologists/ hematologists and nephrologists has the greatest potential of ensuring optimal patient outcomes. Rehydration is fundamental in the management of TLS in addition to the current standard therapy for hyperuricemia which include rasburicase and allopurinol. Conclusion: The early recognition and treatment of metabolic abnormalities often prevents the severe and life-threatening complications associated with tumor lysis syndrome. * Corresponding Author; Dr. Illias Tazi; Hematology Department, Chu Mohamed Vi, Cadi Ayyad University, Marrakech, Morocco; E mail: tazi_illias@hotmail.com Keyswords: Acute Renal Failure; Burkitt s Lymphoma; Hematologic Malignancies; Hydration; Tumor Lysis Syndrome The authors declared no conflict of interest Introduction Infection remains the leading cause of organ failure in critically ill cancer patients, but several reports point out the increasing proportion of patients admitted into intensive care units with organ failure related to the malignancy itself [1]. Some of these complications may be directly related to the extent of the malignancy. This may include acute renal failure, acute respiratory failure and coma. Most of these specific organ failures will require initiation of cancer therapy along with the initiation of organ support. Tumor lysis syndrome (TLS) is characterized by a number of metabolic abnormalities that may arise from rapid and massive lysis of malignant cells and the concomitant release of intracellular contents into the blood stream. Metabolic abnormalities characteristic of TLS include abnormally high serum uric acid levels (hyperuricemia) resulting from the breakdown of purine-containing nucleic acids and major electrolyte imbalances such as hyperkalemia, hyperphosphatemia and hypocalcemia [2, 3]. TLS typically occurs after the initiation of anti-cancer therapies, including cytotoxic drugs, biological agents, corticosteroids, hormones and radiation therapy, in patients with hematologic malignancies or solid tumors that are highly sensitive to treatment. Rarely, TLS arises spontaneously as a consequence of increased tumor cell lysis before any definitive anti-tumor therapy has been initiated [2]. Diagnosis of TLS may distinguish between laboratory TLS, defined by specific abnormalities in the serum concentrations of uric acid and/or electrolytes, and clinical TLS, defined as laboratory TLS accompanied by symptomatic complications of the underlying metabolic imbalances [4]. Clinical manifestations of TLS typically 147
2 Tazi et al occur hours after treatment initiation and may include renal failure, seizures, and cardiac arrhythmias [3, 5]. Because of the rapidity with which TLS progresses and the seriousness of common clinical consequences such as acute renal failure, TLS is associated with significant morbidity and potential mortality. Prevention of TLS relies on the identification of at-risk patients who would benefit from close monitoring and early implementation of prophylactic measures [4]. Given the complexity of TLS prevention and treatment, a multidisciplinary approach involving the collaboration of medical oncologists/hematologists and nephrologists has the greatest potential of ensuring optimal patient outcomes. The key to the management of TLS includes awareness of its causes, identification of high-risk patients, implementation of appropriate prophylactic measures, vigilant monitoring of electrolyte levels in patients undergoing chemotherapy, and initiation of more active treatment measures when necessary. Pediatric Considerations TLS which occurs in both pediatric and adult cancer patients is considered as a potential oncologic emergency. TLS is a constellation of clinical signs and symptoms resulting from severe electrolyte and metabolic abnormalities that usually occur in response to cytotoxic chemotherapy. Pediatric cancers are the leading cause of death by disease in children. The most commonly occurring pediatric cancers include leukemias (25%), lymphomas (23%), central nervous system tumors (16.6%) and neuroblastoma (8% to 10%) [6]. Lymphomas seen in the pediatric population include both Hodgkin s disease and non-hodgkin s lymphoma, such as Burkitt s lymphoma. Because TLS is most prevalent in patients with malignancies that are highly proliferative and that have a high tumor burden (most notably the lymphomas and leukemias), it is no surprise that pediatric patients with cancer are at significant risk for TLS during treatment. The precise incidence of TLS is not known, however, like in the adult population, it appears to occur with the greatest frequency in patients with Burkitt s lymphoma and lymphoblastic lymphoma [7]. Although TLS does occur in pediatric patients with solid tumors, it is relatively uncommon. However, it has been documented in patients with stage IV neuroblastoma [8]. Thus, while it does not exclusively affect pediatric patients, TLS is a major concern to practitioners caring for pediatric oncology patients. Pathophysiologic Characteristics In tumors with a high proliferative rate, a relatively large mass and a high sensitivity to cytotoxic agents, the initiation of therapy often results in the rapid release of intracellular anions, cations, and the metabolic products of proteins and nucleic acids into the blood stream [9]. The increased concentrations of uric acid, phosphates, potassium and urea can overwhelm the body s homeostatic mechanisms to process and excrete these materials and result in the clinical spectrum associated with TLS [10]. Hyperuricemia and its associated complications are the most frequently recognized manifestations of tumor lysis syndrome, and predispose to many of the other clinical derangements. Hyperuricemia results from rapid release and catabolism of intracellular nucleic acids. Purine nucleic acids are catabolized to hypoxanthine, then xanthine, and finally to uric acid by xanthine oxidase [11]. Hyperphosphatemia results from the rapid release of intracellular phosphates from malignant cells, which may contain as much as four times the amount of organic and inorganic phosphates as normal cells [5, 12]. Hyperphosphatemia can lead to the development of acute renal failure after precipitation with calcium in the renal tubules during tumor lysis syndrome. The serum concentration of calcium rapidly decreases as precipitation with phosphate occurs. Hypocalcemia is one of the most serious clinical manifestations of TLS and has been associated with the developmentof severe muscle cramping, tetany, and cardiac arrhythmias. Hyperkalemia may also be a life-threatening consequence of tumor lysis syndrome. Hyperkalemia results from the kidneys inability to clear the massive load of intracellular potassium released by lysed tumor cells. Neuromuscular signs and symptoms may include muscle weakness, cramps, paresthesias and possible paralysis. Cardiac manifestations may include asystole, ventricular tachycardia or fibrillation, syncope and possible sudden death [5, 8]. Increase in blood urea nitrogen and creatinine levels occur as a result of renal impairment associated with acute uric acid crystal nephropathy, calcium-phosphate crystals and nephrocalcinosis or a combination of both, leading to an acute obstructive uropathy syndrome. Acute clinical manifestations may include uremia, edema, hypertension, congestive heart failure and exacerbations of metabolic disturbances. Incidence and Risk Factors Most reports describing TLS portray patients with malignancies of hematopoietic origin, including leukemias and lymphomas. Determination of the incidence of TLS for specific leukemia and lymphoma subtypes is difficult because of differences among studies regarding patient populations analyzed and inconsistently applied definitions of TLS. Available evidence suggests that the incidence of clinical TLS is approximately 3 7% 148
3 Tumor lysis syndrome for acute leukemias and 4 11% for lymphomas [13, 14]. However, certain subgroups of leukemia patients, such as those with mature B acute lymphoblastic leukemia (ALL) and Burkitt s lymphoma/leukemia, have been reported to have incidences of TLS as high as approximately 25% [15, 16]. Rare cases of TLS have been reported for patients with solid tumors characterized by high cell proliferation rate, large tumor burden or high chemo-sensitivity, with hepatic metastases being an additional risk factor [17, 18]. However, TLS derived from solid tumors generally is far more unpredictable than that caused by hematologic malignancies in terms of tumor characteristics and timing of occurrence. For instance, solid tumor-related TLS may arise despite low chemosensitivity of the neoplastic disease and may occur a few weeks after initiation of anticancer treatment [17, 18]. Because available data for TLS in patients with solid tumors are limited to individual case reports and small case series, the overall incidence of solid tumor-related TLS is difficult to quantify but appears to be low. However, mortality is high in cases of fully manifested clinical TLS related to solid tumors [17, 18]. The risk of TLS is influenced by a number of characteristics including the type of malignancy, the type and intensity of anti-cancer treatment, and the presence of pre-existing conditions including renal insufficiency. In particular, high-risk patients are those who have malignancies with high rates of cell turnover and are highly sensitive to chemotherapy. The neoplastic cells associated with such malignancies are characterized by high nucleic acid and phosphorus content and have active purine metabolism[19]. These characteristics are typical of certain hematologic malignancies, especially acute lymphoid leukemia and high-grade Non Hodgkin lymphoma (NHL) [20]. Burkitt s lymphoma has one of the highest rates of cell division of any human tumor, putting patients with this particular type of NHL among those with the highest risk of TLS [21]. In patients with high tumor burden or with highly proliferative solid tumors, there is also a risk of spontaneous or therapyinduced TLS [9]. A number of pre-existing conditions, in particular those linked with renal insufficiency and reduced clearance of uric acid, also increase the risk of TLS. These include dehydration, oliguria/anuria, preexisting hyperuricemia and acidic urine which reduces the solubility and clearance of uric acid [22]. Clinical Consequences of Tumor Lysis Syndrome The clinical consequences of TLS-related hyperuricemia, hyperkalemia, and hyperphosphatemia are serious. Typical clinical sequelae include gastrointestinal disturbances, neuromuscular effects, cardiovascular complications, acute renal failure and death. Tumor lysis syndrome can also compromise the efficacy or administration of curative therapies [20]. Consequences of hyperkalemia Hyperkalemia may appear from 6 to 72 hours after the initiation of chemotherapy [7] and is the most serious manifestation of tumor lysis syndrome. Potassium is generally concentrated intracellularly. Cell lysis results in the liberation of large amounts of intracellular potassium into the extracellular fluid. Chronic kidney disease, acute renal failure or concurrent acidosis may exacerbate hyperkalemia as the excretory capacity of the kidney can be overwhelmed by transcellular shifts due to potassium release from lysing cells as well as acidosis. Symptoms of hyperkalemia include weakness, paresthesias, muscle cramps, nausea, vomiting, diarrhea, and anorexia. Severe hyperkalemia (>7mEq/liter) can adversely affect skeletal and cardiac muscle function. Electrocardiographic changes include widening of the QRS complex and peaked T waves. Hyperkalemia must be corrected rapidly before potentially fatal ventricular arrhythmias occur. Consequences of Hyperphosphatemia and Hypocalcemia Hyperphosphatemia develops from 24 to 48 hours following initiation of chemotherapy [23]. Release of intracellular phosphate can exceed the renal threshold for phosphate excretion, leading to hyperphosphatemia. Malignant hematologic cells may contain up to four times more intracellular phosphate compared with normal mature lymphoid cells [13]. Additionally, acute destruction of tumor cells during chemotherapy prevents the rapid reuse of phosphate for newly synthesized tumor cells. Precipitation of calcium phosphate occurs when the solubility product of calcium and phosphate is exceeded, possibly leading to hypocalcemia. Muscle cramps, tetany, cardiac arrhythmia and seizures can result. Acute nephrocalcinosis or precipitation of calcium phosphate in the renal tubules with an inflammatory response, may lead to acute renal failure. Calcium and phosphate abnormalities should be identified and treated early [24]. Consequences of Hyperuricemia Hyperuricemia develops from 48 to 72 hours following initiation of treatment [7]. Tumor cell lysis releases purine nucleic acids, which are metabolized into uric acid. Malignant cells carry a large burden of nucleic acid products due to their high cellular activity and turnover. The high turnover rate of neoplastic cells leads to ongoing DNA catabolism. The breakdown of purine nucleotides yields high amounts of hypoxanthine. Xanthine oxidase 149
4 Tazi et al catalyzes the conversion of hypoxanthine into uric acid. After spontaneous or cytotoxic therapy induced lysis, large amounts of uric acid are produced, leading to rapid increases in plasma and renal tubular concentrations of uric acid [13]. Hyperuricemia may lead to nausea and vomiting, oliguria or anuria, diarrhea, hematuria, and anorexia. Cardiovascular disease has also been linked with hyperuricemia [25]. However, acute renal failure represents one of the most frequent, serious clinical consequences of TLS and hyperuricemia [13]. Prevention and Treatment of Tumor Lysis Syndrome Epidemiologic considerations The potential severity of complications resulting from the development of TLS necessitates measures for prevention in high risk patients and prompts treatment if symptoms arise. Recognition of risk factors, close monitoring of at-risk patients and appropriate interventions are the key to preventing or managing TLS. General principles for the management of patients at risk of or presenting with TLS, after eliminating other potential causes for the metabolic abnormalities classically encountered in TLS, are aggressive volume repletion and/or expansion; therapy for hyperkalemia, hyperphosphatemia and secondary hypocalcemia; and prevention and therapy of hyperuricemia. These measures will have an impact on patient outcomes in general and kidney function in particular. Comorbidities predisposing to higher risk of developing TLS are elevated pre-treatment serum uric acid level, preexisting renal damage, tumor infiltration of the kidney, obstructive uropathy and advanced age [2, 26, 27]. Cytotoxic therapies more frequently associated withtls are those employing highly active, cycle specific drugs (cytosine arabinoside, etoposide and cisplatin). Corticosteroids have often been implicated in the pathogenesis of TLS probably because they are used as primary therapy for highly proliferating lymphoid disorders. Less frequently, TLS has been reported after administration of intrathecal methotrexate, monoclonal antibodies (rituximab), radiotherapy, interferon, hydroxyurea and imatinib. It has rarely been described also as a spontaneous event [28]. TLS occurs most commonly in Burkitt s lymphoma, lymphoblastic lymphoma, B-cell acute lymphoblastic leukemia (ALL) and T-cell ALL with hyperleukocytosis and extensive extramedullary disease because of a high proliferative fraction, large tumor burden or wide dissemination and high chemo-sensitivity [15, 29]. Incidence and complications of TLS has been analyzed by Wossmannet al in 1,791 children with NHL [14]. Patients with Burkitt s lymphoma or B-ALL had a higher incidence of TLS (8.4%) and anuria (4.4%). In particular, patients with B-ALL had the highest risk of developing a TLS (26.4%) and anuria (14.1%). The incidence of TLS was 19.1% for patients with LDH levels >1,000 u/l. TLS has also been documented in childhood solid tumor such as neuroblastoma, medulloblastoma and germ cell tumors [30]. Fluids and Hydration Supportive care should be implemented immediately to prevent delay in the initiation of cytotoxic therapy, which should be started as soon as it is safely possible. Volume depletion can further increase the risk of uric acid and calcium-phosphate precipitation. Volume repletion/ expansion is one of the most important interventions in patients at risk of or with TLS because it maintains renal blood flow and urine flow, promoting urinary excretion of potassium, uric acid and phosphate [31]. Aggressive hydration and diuresis are fundamental to the prevention and management of TLS. The combination of hydration and enhanced urine flow promotes the excretion of uric acid and phosphate by improving intravascular volume, renal blood flow, and glomerular filtration [29, 32]. The use of diuretics may also be necessary to maintain adequate urine output, but use of diuretics is contraindicated in patients with hypovolemia or obstructive uropathy. Pediatric patients should receive 2 to 3 liters/m2/day (or 200 ml/kg/day if weight 10 kg; volume adapted to patient age, cardiac function, and urine output) intravenous (IV) solution consisting of half of normal saline / 5% dextrose. Urine output should be monitored closely and be maintained within a range of 80 to 100 ml/m2/hour (4 to 6 ml/kg/hour if weight 10 kg) [2]. If there is no evidence of acute obstructive uropathy and/or hypovolemia, diuretics may be used to maintain output within this range if necessary. Because of the concurrent risks of hyperkalemia, hyperphosphatemia, and/or calcium phosphate precipitation, potassium, calcium and phosphate should be withheld initially from hydration fluids. Patients with oliguria that is not believed to be caused by volume depletion or is unresponsive to volume expansion should have an immediate evaluation by a nephrologist. This will permit a full evaluation for the potential presence of acute kidney injury (AKI) and identify the best therapeutic options based on volume status and other clinical and laboratory parameters. It will help also in deciding the role of diuretics and potential benefit of converting an established oliguric AKI to non-oliguric 150
5 Tumor lysis syndrome AKI as this may permit temporary management while renal replacement therapy is being considered. Urinary Alkalinization Solubility of the purine catabolism metabolites xanthine, hypoxanthine, and uric acid in urine differs and is dependent on urinary ph [33]. Uric acid solubility is low and increases as urinary ph becomes more alkaline. Although hypoxanthine is soluble, xanthine solubility increases to a much lesser degree in alkaline urine given its higher pka (log of dissociation constant for anacid) compared with uric acid [34]. The goal of urinary alkalinization is to maintain urinary ph at 7.0 to enhance uric acid solubility, therefore promoting its excretion. This can typically be achieved by adding meq/liter of IV sodium bicarbonate or a bolus with meq/kg for urine ph <7.0. Historically, urinary alkalinization has been recommended during treatment with allopurinol. Urinary alkalinization is not required with the use of rasburicase because the action of urate oxidase converts uric acid to allantoin, which is highly soluble and not dependent on urinary ph. Routine urinary alkalinization has recently become more controversial and possibly contraindicated [4, 33]. Levels of hypoxanthine and xanthine may be increased in patients treated with allopurinol and, as mentioned, urinary alkalinization does not significantly improve xanthine solubility. In addition, calcium phosphate is more soluble at an acidic ph; therefore, urinary alkalinization may lead to increased calciumphosphate crystallization and precipitation. There is also the risk of developing metabolic alkalosis, especially if the glomerular filtration rate (GFR) is compromised. These limitations and associated risks have led experts in the field not to recommend urinary alkalinization [4, 33]. Alkalinization may be considered in patients treated with allopurinol who have moderately increased uric acid levels, but no associated hyperphosphatemia. Allopurinol One approach to preventing or managing TLS associated hyperuricemia is to block the conversion of xanthine and hypoxanthine to uric acid. Allopurinol is a xanthine analog which, when converted in vivo to oxypurinol acts as a competitive inhibitor of xanthine oxidase, thereby blocking the conversion of the purine metabolites to uric acid [35]. Use of allopurinol has been shown to decrease the formation of uric acid and to reduce the incidence of obstructive uropathy caused by uric acid precipitation in patients at risk for developing TLS [35]. In pediatric patients, allopurinol is administered at a dose of 50 to100 mg/m 2 every 8 hours orally (maximum dose, 300 mg/m 2 / day) or 10 mg/kg/day divided every 8 hours (maximum dose, 800 mg/day). For patients unable to take allopurinol orally, IV administration may be considered, at a dose of 200 to 400 mg/m 2 /day in one to three divided doses (maximum dose, 600 mg/d) [36]. Non-recombinant Urate Oxidase (uricozyme) A second approach to the management of hyperuricemia is to promote the catabolism of uric acid. In most mammals, there exists an enzyme, urate oxidase, that converts uric acid into allantoin, which is five to 10 times more soluble in urine than uric acid [37]. However, this enzyme is not present in humans because of a nonsense mutation in the coding region. A non-recombinant form of urate oxidase with a high specific activity was initially isolated from Aspergillus flavus [38]. Kissel et al [39] reported results of a phase I/II study in 61 adults demonstrating that non- recombinant urate oxidase at a dose of 800 U/day reduced uric acid levels in the blood and increased allantoin excretion. A second study by Masera et al [40] demonstrated the efficacy of non-recombinant urate oxidase in 30 pediatric patients with leukemia. Toxicities included allergic reactions and methemoglobinemia or hemolytic anemia in patients with a deficiency of the glucose-6-phosphate dehydrogenase (G6PD) enzyme [41]. In a study of 134 pediatric patients treated with nonrecombinant urate oxidase from 1994 to 1996, Pui et al [42] reported that when compared with historical control patients treated with hyperhydration and allopurinol, nonrecombinant urate oxidase led to more rapid decreases in uric acid levels. Nearly 50% of patients in the nonrecombinant urate oxidase treatment cohort had urate concentrations less than 1 mg/dl after 2 days of therapy versus 2% of those treated with allopurinol (P<0.001). Recombinant Urate Oxidase (rasburicase). A second strategy for the treatment of hyperuricemiais to use rasburicase. Rasburicase is a recombinant urate oxidase that converts uric acid to the more water-soluble product allantoin. It is approved by the FDA for the prevention and management of hyperuricemia in pediatric and adult oncology patients at a dose of 0.20 mg/kg IV in a 30-minute infusion as a single dose for up to 5 days [43]. Because the enzyme can maintain its activity in blood samples ex vivo, it is imperative that blood samples obtained from patients who received rasburicase therapy be placed immediately on ice before transferring them to a diagnostic laboratory for measurement of serum uric acid. Failure to do so may result in false readings of low uric acid values. The safety, efficacy and pharmacokinetics of rasburicase for the prevention and treatment of hyperuricemia were evaluated in a phase 2 study of 131 patients aged 20 years with newly diagnosed 151
6 Tazi et al leukemia or lymphoma. Patients received rasburicase at a dose of 0.15 or 0.20 mg/kg for 5-7 consecutive days. Rasburicase was well tolerated and effective, leading to a rapid decrease in plasma uric acid levels in patients with hyperuricemia (median decrease from mg/dl after 4 hours) witha mean plasma half-life of 16.0 ± 6.3 and 21.1 ± 12.0 hours at doses of 0.15 and 0.20 mg/kg, respectively. Plasma uric acid levels remained low throughout the treatment [44]. The safety and efficacy of rasburicase were shown further in a compassionate-use study conducted in adults (n = 387) and pediatric patients (n = 682) in the United States and Canada [45]. Although the current FDA-approved rasburicase dose and schedule in pediatric and adult patients are 0.20 mg/kg/day for up to 5 days, several studies suggested that a shorter schedule may be sufficient to treat most patients. In an open-label multicenter randomized trial, Goldman et al [11] compared treatment with rasburicase to oral allopurinol in 52 pediatric patients with lymphoma (39 patients) or leukemia (13 patients) that were at high risk of developing TLS. All patients received approximately 3 liter/m 2 of hydration daily. Average uric acid levels at study initiation were 7.1 mg/dl in the rasburicase group and 7.8 mg/dl in the allopurinol group. Potential serious adverse reactions were rare and included anaphylaxis, rash, hemolysis, methemoglobulinemia, fever, neutropenia (with or without fever), respiratory distress, sepsis and mucositis. Other adverse reactions included vomiting, fever, nausea, headache and diarrhea [46]. Management of Acute Kidney Injury The role of the nephrologist becomes crucial in the care of patients at risk of or presenting with TLS to help prevent AKI and optimize the patient s condition as quickly as possible to avoid delay in initiation of cytoreductive therapy and dosage reduction. Supportive care must focus on managing volume status and addressing any metabolic abnormalities unless there is an acute indication for dialysis. Nephrotoxic medications should be avoided, and it is best to not use angiotensin converting enzyme inhibitors and angiotensin receptor blockers given their hyperkalemic and potentially GFR reducing effects, especially if in situations of hypovolemia or hypoperfusion. Iodinated contrast and gadolinium-based contrast agents also are best avoided in patients with established AKI or unstable kidney function [47]. Medication dosage should be based on GFR oradjusted for AKI. Dialysis therapy should be initiated when indicated using standard criteria for AKI. There are no specific benefits for initiating dialysis therapy for hyperuricemia per se. High-flux hemodialysis can be performed daily if needed, but excess ultrafiltration and hypotension should be avoided to prevent additional kidney injury. Peritoneal dialysis may not be effective in the setting of TLS given the need for high clearance rates and is not recommended as a modality of first choice [48]. Continuous venovenous hemodialysis is a renal replacement therapy option in patients with hypotension, large metabolic burden or fluid requirements has also been used in a prophylactic manner [49]. However, prophylactic dialysis or continuous renal replacement therapy in patients at risk of TLS has not been well studied and cannot be routinely recommended. It may need to be considered in patients with advanced Chronic kidney disease (CKD), end-stage renal disease or AKI at presentation. Patients should bemonitored carefully and assessed for renal recovery, which may be delayed in some cases. Conclusion Successful management and treatment of tumor lysis syndrome is highly dependent on the prompt identification of clinical and laboratory characteristics, signs and symptoms of patients at risk. Establishment of vascular access and the initiation of prophylactic measures, especially hydration and administration of allopurinol or rasburicase, are vital. The early recognition and treatment of metabolic abnormalities usually prevents the severe and life-threatening complications associated with tumor lysis syndrome. References 1. Azoulay E, Afessa B. The intensive care support of patients with malignancy: do everything that can be done. Intensive Care Med Jan;32(1): Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Brit J Haematol oct;127(1): Hochberg J, Cairo MS. Tumor lysis syndrome: current perspective. Haematologica Jan;93(1): Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26: Locatelli F, Rossi F. Incidence and pathogenesis of tumor lysis syndrome. Contrib Nephrol. 2005;147: Kobayashi D, Wofford MM, McLean TW, Lin JJ. Spontaneous tumor lysis syndrome in a child with T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer May;54(5): Flombaum CD. Metabolic emergencies in the cancer patient. Semin Oncol Jun;27(3):
7 Tumor lysis syndrome 8. Milano GM, De Sio L, Cozza R, Donfrancesco A. Tumor lysis syndrome and neuroblastoma. Med Pediatr Oncol Dec;41(6): Jeha S. Tumor lysis syndrome. Semin Hematol. 2001;38[4 Suppl 10]: Howard SC, Pui CH. Pitfalls in predicting tumor lysis syndrome. Leuk Lymphoma. 2006;47: Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, Tou C, Harvey E, Morris E, Cairo MS. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood May 15;97(10): Yarpuzlu AA. A review of clinical and laboratory findings and treatment of tumor lysis syndrome. Clin Chim Acta. 2003;333: Annemans L, Moeremans K, Lamotte M, Garcia Conde J, van den Berg H, Myint H, Pieters R, Uyttebroeck A. Incidence, medical resource utilisation and costs of hyperuricemia and tumour lysis syndrome in patients with acute leukaemia and non-hodgkin s lymphoma in four European countries. Leuk Lymphoma Jan;44(1): Wossmann W, Schrappe M, Meyer U, Zimmermann M, Reiter A. Incidence of tumor lysis syndrome in children with advanced stage Burkitt s lymphoma/ leukemia before and after introduction of prophylactic use of urateoxidase. Ann Hematol. 2003;82: Stapleton FB, Strother DR, Roy S 3rd, Wyatt RJ, McKay CP, Murphy SB. Acute renal failure at onset of therapy for advanced stage Burkitt lymphoma and B cell acute lymphoblastic lymphoma. Pediatrics Dec;82(6): Montesinos P, Lorenzo I, Martín G, Sanz J, Pérez- Sirvent ML, Martínez D, Ortí G, Algarra L, Martínez J, Moscardó F, de la Rubia J, Jarque I, Sanz G, Sanz MA. Tumor lysis syndrome in patients with acute myeloid leukemia: identification of risk factors and development of a predictive model. Haematologica Jan;93(1): Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51: Gemici C. Tumour lysis syndrome in solid tumours. Clin Oncol (R Coll Radiol). 2006;18: Ribeiro RC, Pui CH. Hyperuricemia in patients with cancer. Am J Cancer. 2002; 1: Levine AM. Challenges in the management of Burkitt s lymphoma. Clin Lymphoma Dec;3 Suppl 1:S Hecht JL, Aster JC. Molecular biology of Burkitt s lymphoma. J Clin Oncol Nov 1;18(21): Navolanic PM, Pui CH, Larson RA, Bishop MR, Pearce TE, Cairo MS, Goldman SC, Jeha SC, Shanholtz CB, Leonard JP, McCubrey JA. Elitek-rasburicase: an effective means to prevent and treat hyperuricemia associated with tumor lysis syndrome, a Meeting Report, Dallas, Texas, January Leukemia Mar;17(3): Ribeiro RC, Pui CH. Recombinant urate oxidase for prevention of hyperuricemia and tumor lysis syndrome in lymphoid malignancies. Clin Lymphoma Mar;3(4): Nicolin, G. Emergencies and their management. Eur J Cancer. 2002;38: Watanabe S, Kanellis J, Nakagawa T, et al. Reducing uric acid as a means to prevent cardiovascular and renal disease. Expert OpinTherPatents. 2002;12: Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-hodgkin s lymphoma. Am J Med Feb;94(2): Tsokos GC, Balow JE, Spiegel RJ, Magrath IT. Renal and metabolic complications of undifferentiated and lymphoblastic lymphomas. Medicine (Baltimore) May;60(3): Jasek AM, Day HJ. Acute spontaneous tumor lysis syndrome. Am J Hematol Oct;47(2): Jones DP, Mahmoud H, Chesney RW. Tumor lysis syndrome: pathogenesis and management. Pediatr Nephrol Apr;9(2): Pession A, Melchionda F, Castellini C. Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase). Biologics Mar;2(1): Conger JD, Falk SA. Intrarenal dynamics in the pathogenesis and prevention of acute urate nephropathy. J Clin Invest May;59(5): Silverman P, Distelhorst CW. Metabolic emergencies in clinical oncology. Semin Oncol Dec;16(6): Howard SC, Ribeiro RC, Pui C-H. Acute complications. In: Pui C-H, editor. Childhood Leukemias. 2nd ed. New York: Cambridge University Press. 2006:
8 Tazi et al 34. Seegmiller JE. Xanthine stone formation. Am J Med. 1968;45(5): Krakoff IH, Meyer RL. Prevention of hyperuricemia in leukemia and lymphoma: Use of alopurinol, a xanthineoxidase inhibitor. JAMA. 1965;193: Feusner J, Farber MS. Role of intravenous allopurinol in the management of acute tumor lysis syndrome. SeminOncol. 2001;28 (suppl 5): Yeldandi AV, Yeldandi V, Kumar S, Murthy CV, Wang XD, Alvares K, Rao MS, Reddy JK. Molecular evolution of the urate oxidase-encoding gene in hominoid primates: nonsense mutations. Gene Dec 30;109(2): Laboureur P, Langlois C. [Urate oxidase of Aspergillus flavus. I. Isolation, purification, properties]. Bull Soc ChimBiol (Paris). 1968;50: Kissel P, Lamarche M, Royer R. Modification of uricaemia and the excretion of uric acid nitrogen by an enzyme of fungal origin. Nature Jan 6;217(5123): Masera G, Jankovic M, Zurlo MG, Locasciulli A, Rossi MR, Uderzo C, Recchia M. Urate-oxidase prophylaxis of uric acid-induced renal damage in childhood leukemia. J Pediatr Jan;100(1): Ducros J, Saingra S, Rampal M, Coulange C, Barbe MC, Verzetti G. Hemolytic anemia due to G6PD deficiency and urate oxidase in a kidney-transplant patient. Clin Nephrol Feb;35(2): Pui CH, Relling MV, Lascombes F, Harrison PL, Struxiano A, Mondesir JM, Ribeiro RC, Sandlund JT, Rivera GK, Evans WE, Mahmoud HH. Urate oxidase in prevention and treatment of hyperuricemia associated with lymphoid malignancies. Leukemia Nov;11(11): Elitek [package insert]. Bridgewater, NJ: Sanofi- Aventis US LLC; Pui CH, Mahmoud HH, Wiley JM, Woods GM, Leverger G, Camitta B, Hastings C, Blaney SM, Relling MV, Reaman GH. Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients With leukemia or lymphoma. J Clin Oncol Feb 1;19(3): Jeha S, Kantarjian H, Irwin D, Shen V, Shenoy S, Blaney S, Camitta B, Pui CH. Efficacy and safety of rasburicase, a recombinant urate oxidase (Elitek), in the management of malignancy-associated hyperuricemia in pediatric and adult patients: final results of a multicenter compassionate use trial. Leukemia Jan;19(1): Ueng S. Rasburicase (Elitek): A novel agent for tumor lysis syndrome. Proc (BaylUniv Med Cent). 2005;18: Prince MR, Zhang H, Morris M, MacGregor JL, Grossman ME, Silberzweig J, DeLapaz RL, Lee HJ, Magro CM, Valeri AM. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology Sep;248(3): Kjellstrand CM, Cambell DC 2nd, von Hartitzsch B, Buselmeier TJ. Hyperuricemic acute renal failure. Arch Intern Med Mar;133(3): Choi KA, Lee JE, Kim YG, Kim DJ, Kim K, Ko YH, Oh HY, Kim WS, Huh W. Efficacy of continuous venovenous hemofiltration with chemotherapy in patients with Burkitt lymphoma and leukemia at high risk of tumor lysis syndrome. Ann Hematol Jul;88(7):
Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo
 Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo Outline Background/Definition Epidemiology/Risk Stratification Pathophysiology Treatment Renal Replacement Therapy Background/Definition
Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo Outline Background/Definition Epidemiology/Risk Stratification Pathophysiology Treatment Renal Replacement Therapy Background/Definition
Oncology Emergency Essentials: Addressing Tumor Lysis Syndrome in Your Practice
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Announcements. Learning Objectives. Introduction. Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome
 Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome Kamakshi V. Rao, Pharm.D., BCOP Oncology Clinical Pharmacy Specialist University of North Carolina Hospitals and Clinics
Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome Kamakshi V. Rao, Pharm.D., BCOP Oncology Clinical Pharmacy Specialist University of North Carolina Hospitals and Clinics
Iranian Journal of Blood & Cancer. The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
 IJBC 2016; 8(2): 33-37 Iranian Journal of Blood & Cancer Journal Home Page: www.ijbc.ir Original Article The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
IJBC 2016; 8(2): 33-37 Iranian Journal of Blood & Cancer Journal Home Page: www.ijbc.ir Original Article The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
2.1 mmol/l or 25% increase from baseline mmol/l or 25% decrease from baseline
 22.1 Hyperleukocytosis and tumour lysis syndrome The guideline is addressed for ALL patients with hyperleukocytosis (WBC 100 x109/l) only and should not be used without modifications in case of other diseases
22.1 Hyperleukocytosis and tumour lysis syndrome The guideline is addressed for ALL patients with hyperleukocytosis (WBC 100 x109/l) only and should not be used without modifications in case of other diseases
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES
 GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia
 Bangladesh Journal of Medical Science Vol. 11 No. 04 Oct 12 Original article Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia Sultana A 1,
Bangladesh Journal of Medical Science Vol. 11 No. 04 Oct 12 Original article Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia Sultana A 1,
Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase
 MOLECULAR AND CLINICAL ONCOLOGY 6: 955-959, 2017 Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase EISEKI USAMI 1, MICHIO KIMURA 1,
MOLECULAR AND CLINICAL ONCOLOGY 6: 955-959, 2017 Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase EISEKI USAMI 1, MICHIO KIMURA 1,
Guidelines for the Prevention of Tumour Lysis Syndrome
 1.0 Introduction For the purposes of this guideline, tumour lysis syndrome (TLS) is defined as metabolic derangement resulting from abrupt and massive breakdown of malignant cells and the release of intracellular
1.0 Introduction For the purposes of this guideline, tumour lysis syndrome (TLS) is defined as metabolic derangement resulting from abrupt and massive breakdown of malignant cells and the release of intracellular
SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature
 Case Report SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature Ashraf Ahmed Hussein Hashem 1, Tarek Abdel Hameed Mostafa Dowod 2, Mohsen Mahmooud Abdelmajeed 3 ABSTRACT We report
Case Report SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature Ashraf Ahmed Hussein Hashem 1, Tarek Abdel Hameed Mostafa Dowod 2, Mohsen Mahmooud Abdelmajeed 3 ABSTRACT We report
Acute tumor lysis syndrome (ATLS), although
 ACUTE TUMOR LYSIS SYNDROME Michele Cohen, DVM, DACVIM (Oncology) College of Veterinary Medicine Auburn University Auburn, Alabama Acute tumor lysis syndrome (ATLS), although uncommon, can be a serious
ACUTE TUMOR LYSIS SYNDROME Michele Cohen, DVM, DACVIM (Oncology) College of Veterinary Medicine Auburn University Auburn, Alabama Acute tumor lysis syndrome (ATLS), although uncommon, can be a serious
Tumour Lysis Syndrome (TLS)
 (TLS) Overview: Tumour lysis syndrome refers to a number of metabolic disturbances (hyperuricaemia, hyperphosphataemia, hyperkalaemia and hypocalcaemia) that occur as the result of rapid cell lysis. This
(TLS) Overview: Tumour lysis syndrome refers to a number of metabolic disturbances (hyperuricaemia, hyperphosphataemia, hyperkalaemia and hypocalcaemia) that occur as the result of rapid cell lysis. This
Managing patients with bulky cancers
 SIOP PODC Supportive Care Education (ICON 2016) Presentation Date: 23 rd January 2016 Recording Link at www.cure4kids.org: https://www.cure4kids.org/ums/home/conference_rooms/enter.php?room=p2pjfjp8nha
SIOP PODC Supportive Care Education (ICON 2016) Presentation Date: 23 rd January 2016 Recording Link at www.cure4kids.org: https://www.cure4kids.org/ums/home/conference_rooms/enter.php?room=p2pjfjp8nha
Contents Page No. 1. Outline of Procedure 2. Area of Application 3. Objective 4. Stages of the Process 5. Responsibilities
 Contents Page No. 1. Outline of Procedure 3 2. Area of Application 3 3. Objective 3 4. Stages of the Process 3 5. Responsibilities 8 6. Other useful information 8 7. References 8 Page 2 of 10 Clinical
Contents Page No. 1. Outline of Procedure 3 2. Area of Application 3 3. Objective 3 4. Stages of the Process 3 5. Responsibilities 8 6. Other useful information 8 7. References 8 Page 2 of 10 Clinical
Tumor lysis syndrome (TLS) is a group of PROCEEDINGS CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD
 CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD ABSTRACT Tumor lysis syndrome (TLS) is a life-threatening complication of cancer chemotherapy in which hyperuricemia
CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD ABSTRACT Tumor lysis syndrome (TLS) is a life-threatening complication of cancer chemotherapy in which hyperuricemia
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University
 RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL
 Original Article Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL Downloaded from ijpho.ssu.ac.ir at 1:59 IRDT on Friday July 1th 2018 Hashemi A 1,
Original Article Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL Downloaded from ijpho.ssu.ac.ir at 1:59 IRDT on Friday July 1th 2018 Hashemi A 1,
Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature
 Case Report 679 Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature Wan-Yee Teo, 1 MBBS, MRCPCH (Lond), Tsee-Foong Loh, 2 MBBS, MMed (Paeds),
Case Report 679 Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature Wan-Yee Teo, 1 MBBS, MRCPCH (Lond), Tsee-Foong Loh, 2 MBBS, MMed (Paeds),
Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults
 Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults Anne M. McDonnell, Pharm.D., Kristi L. Lenz, Pharm.D., Debra A. Frei-Lahr, M.D., John Hayslip, M.D., and Philip D. Hall,
Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults Anne M. McDonnell, Pharm.D., Kristi L. Lenz, Pharm.D., Debra A. Frei-Lahr, M.D., John Hayslip, M.D., and Philip D. Hall,
The pattern of metabolic derangements known as tumor. Tumor Lysis Syndrome. Resident Short Reviews
 Resident Short Reviews Tumor Lysis Syndrome Shelly M. Williams, MD; Anthony A. Killeen, MB, BCh, PhD Tumor lysis syndrome (TLS) is an acute, life-threatening disease among adults and children that is associated
Resident Short Reviews Tumor Lysis Syndrome Shelly M. Williams, MD; Anthony A. Killeen, MB, BCh, PhD Tumor lysis syndrome (TLS) is an acute, life-threatening disease among adults and children that is associated
Guidelines for use of RASBURICASE in adult Haematology and Oncology patients
 Network Guidance Document Guidelines for use of RASBURICASE in adult Haematology and Oncology patients Status: Expiry Date: Version Number: Publication Date: Final September 2013 6 September 2011 Page
Network Guidance Document Guidelines for use of RASBURICASE in adult Haematology and Oncology patients Status: Expiry Date: Version Number: Publication Date: Final September 2013 6 September 2011 Page
Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer
 Open Access Case Report DOI: 10.7759/cureus.1017 Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer Venkatkiran Kanchustambham 1, Swetha Saladi 2, Setu Patolia 2, David Stoeckel 2 1. Pulmonary
Open Access Case Report DOI: 10.7759/cureus.1017 Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer Venkatkiran Kanchustambham 1, Swetha Saladi 2, Setu Patolia 2, David Stoeckel 2 1. Pulmonary
Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy
 www.bioinformation.net Hypothesis Volume 10(11) Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy Mahmood Rasool 1 *, Arif Malik 2, Muhammad
www.bioinformation.net Hypothesis Volume 10(11) Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy Mahmood Rasool 1 *, Arif Malik 2, Muhammad
Electrolyte Imbalance and Resuscitation. Dr. Mehmet Okumuş Sütçü Imam University Faculty of Medicine Department of Emergency Medicine
 Electrolyte Imbalance and Resuscitation Dr. Mehmet Okumuş Sütçü Imam University Faculty of Medicine Department of Emergency Medicine Presentation plan Definition of the electrolyte disturbances Conditions
Electrolyte Imbalance and Resuscitation Dr. Mehmet Okumuş Sütçü Imam University Faculty of Medicine Department of Emergency Medicine Presentation plan Definition of the electrolyte disturbances Conditions
Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase)
 REVIEW Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase) Andrea Pession Fraia Melchionda Claudia Castellini Oncologia
REVIEW Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase) Andrea Pession Fraia Melchionda Claudia Castellini Oncologia
Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital
 Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital Case Number 1 43 year old male presenting with fevers, abdominal distension and weight gain over 2 weeks.
Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital Case Number 1 43 year old male presenting with fevers, abdominal distension and weight gain over 2 weeks.
Sponsor/Company: sanofi-aventis Drug substance: Elitek/Fasturtec (rasburicase, SR29142)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor/Company: sanofi-aventis Drug
The clinical management of tumour lysis syndrome in
 state of the art review The clinical management of tumour lysis syndrome in haematological malignancies Andrew Will 1 and Eleni Tholouli 2 1 Department of Paediatric Haematology, Royal Manchester Children
state of the art review The clinical management of tumour lysis syndrome in haematological malignancies Andrew Will 1 and Eleni Tholouli 2 1 Department of Paediatric Haematology, Royal Manchester Children
Tumour Lysis Syndrome: Implications for Cancer Therapy
 DOI:http://dx.doi.org/10.7314/APJCP.2012.13.8.3555 MINI-REVIEW Denish Mika, Sabrina Ahmad, C Guruvayoorappan* Abstract The tumour lysis syndrome (TLS) is a group of metabolic abnormalities caused by rapid
DOI:http://dx.doi.org/10.7314/APJCP.2012.13.8.3555 MINI-REVIEW Denish Mika, Sabrina Ahmad, C Guruvayoorappan* Abstract The tumour lysis syndrome (TLS) is a group of metabolic abnormalities caused by rapid
Nephrology / Urology. Hyperkalemia Causes and Definition Lecturio Online Medical Library. Definition. Epidemiology of Hyperkalemia.
 Nephrology / Urology Hyperkalemia Causes and Definition Lecturio Online Medical Library See online here Hyperkalemia is defined by the serum potassium level when it is higher than 5.5mEq/L. It is usually
Nephrology / Urology Hyperkalemia Causes and Definition Lecturio Online Medical Library See online here Hyperkalemia is defined by the serum potassium level when it is higher than 5.5mEq/L. It is usually
DERBY-BURTON LOCAL CANCER NETWORK FILENAME R-CODOX-M.DOC CONTROLLED DOC NO: HCCPG B115 CSIS Regimen Name: R-CODOXM. Rituximab + CODOX-M
 Rituximab + CODOX-M Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Burkitt
Rituximab + CODOX-M Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Burkitt
ACUTE PROMYELOCYTIC LEUKEMIA Rome (Italy), September 29-October 1, 2005
 4 th International Symposium on ACUTE PROMYELOCYTIC LEUKEMIA Rome (Italy), September 29-October 1, 2005 CORPORATE SYMPOSIUM TUMOR LYSIS SYNDROME: PATHOGENESIS AND CLINICAL ASPECTS Tosi P Seràgnoli Institute
4 th International Symposium on ACUTE PROMYELOCYTIC LEUKEMIA Rome (Italy), September 29-October 1, 2005 CORPORATE SYMPOSIUM TUMOR LYSIS SYNDROME: PATHOGENESIS AND CLINICAL ASPECTS Tosi P Seràgnoli Institute
tumor lysis syndrome
 2.0 ANCC CONTACT HOURS Oncology Emergency series Recognizing and preventing tumor lysis syndrome By Roberta Kaplow, PhD, APRN-CCNS, AOCNS, CCRN, and Karen Iyere, MSN, RN TUMOR LYSIS SYNDROME (TLS) is one
2.0 ANCC CONTACT HOURS Oncology Emergency series Recognizing and preventing tumor lysis syndrome By Roberta Kaplow, PhD, APRN-CCNS, AOCNS, CCRN, and Karen Iyere, MSN, RN TUMOR LYSIS SYNDROME (TLS) is one
Non-protein nitrogenous substances (NPN)
 Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
PRODUCT MONOGRAPH. (rasburicase) Powder for Injection Professed Standard
 PRODUCT MONOGRAPH Pr FASTURTEC (rasburicase) Powder for Injection Professed Standard 1.5 mg/vial (1.5 mg/ml/vial) Uricolytic Agent sanofi-aventis Canada Inc. Date of Revision: 2150 St. Elzear Blvd. West
PRODUCT MONOGRAPH Pr FASTURTEC (rasburicase) Powder for Injection Professed Standard 1.5 mg/vial (1.5 mg/ml/vial) Uricolytic Agent sanofi-aventis Canada Inc. Date of Revision: 2150 St. Elzear Blvd. West
Tumour lysis syndrome: new therapeutic strategies and classification
 review Tumour lysis syndrome: new therapeutic strategies and classification Mitchell S. Cairo 1 and Michael Bishop 2 1 Department of Pediatrics, Children s Hospital of New York Presbyterian, Columbia University,
review Tumour lysis syndrome: new therapeutic strategies and classification Mitchell S. Cairo 1 and Michael Bishop 2 1 Department of Pediatrics, Children s Hospital of New York Presbyterian, Columbia University,
Krystexxa. Krystexxa (pegloticase) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.14 Subject: Krystexxa Page: 1 of 5 Last Review Date: March 16, 2018 Krystexxa Description Krystexxa
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.14 Subject: Krystexxa Page: 1 of 5 Last Review Date: March 16, 2018 Krystexxa Description Krystexxa
Salicylate (Aspirin) Ingestion California Poison Control Background 1. The prevalence of aspirin-containing analgesic products makes
 Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
SERUM PHOSPHORUS TESTING
 MEDICAL POLICY For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS SERUM PHOSPHORUS TESTING Policy Number: CMP - 035 Effective Date: January 21, 2017 Table
MEDICAL POLICY For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS SERUM PHOSPHORUS TESTING Policy Number: CMP - 035 Effective Date: January 21, 2017 Table
Dr.Nahid Osman Ahmed 1
 1 ILOS By the end of the lecture you should be able to Identify : Functions of the kidney and nephrons Signs and symptoms of AKI Risk factors to AKI Treatment alternatives 2 Acute kidney injury (AKI),
1 ILOS By the end of the lecture you should be able to Identify : Functions of the kidney and nephrons Signs and symptoms of AKI Risk factors to AKI Treatment alternatives 2 Acute kidney injury (AKI),
CCRN Review - Renal. CCRN Review - Renal 10/16/2014. CCRN Review Renal. Sodium Critical Value < 120 meq/l > 160 meq/l
 CCRN Review Renal Leanna R. Miller, RN, MN, CCRN-CMC, PCCN-CSC, CEN, CNRN, CMSRN, NP Education Specialist LRM Consulting Nashville, TN Sodium 136-145 Critical Value < 120 meq/l > 160 meq/l Sodium Etiology
CCRN Review Renal Leanna R. Miller, RN, MN, CCRN-CMC, PCCN-CSC, CEN, CNRN, CMSRN, NP Education Specialist LRM Consulting Nashville, TN Sodium 136-145 Critical Value < 120 meq/l > 160 meq/l Sodium Etiology
Hospital, Chengdu , China. Hospital, Chengdu , China
 Hyperuricemia and acute renal failure secondary to spontaneous tumor lysis syndrome in low risk myelodysplastic syndrome. Yunlin Feng, M.D. 1,, Tao Jiang 2 Correspondence: Yunlin Feng fengyunlin@tsinghua.org.cn
Hyperuricemia and acute renal failure secondary to spontaneous tumor lysis syndrome in low risk myelodysplastic syndrome. Yunlin Feng, M.D. 1,, Tao Jiang 2 Correspondence: Yunlin Feng fengyunlin@tsinghua.org.cn
Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009
 www.ivis.org Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009 São Paulo, Brazil - 2009 Next WSAVA Congress : Reprinted in IVIS with the permission of the Congress Organizers HOW
www.ivis.org Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009 São Paulo, Brazil - 2009 Next WSAVA Congress : Reprinted in IVIS with the permission of the Congress Organizers HOW
Contrast Induced Nephropathy
 Contrast Induced Nephropathy O CIAKI refers to an abrupt deterioration in renal function associated with the administration of iodinated contrast media O CIAKI is characterized by an acute (within 48 hours)
Contrast Induced Nephropathy O CIAKI refers to an abrupt deterioration in renal function associated with the administration of iodinated contrast media O CIAKI is characterized by an acute (within 48 hours)
Subject: Rasburicase (Elitek )
 09-J2000-43 Original Effective Date: 10/15/15 Reviewed: 08/01/18 Revised: 09/15/18 Subject: Rasburicase (Elitek ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF
09-J2000-43 Original Effective Date: 10/15/15 Reviewed: 08/01/18 Revised: 09/15/18 Subject: Rasburicase (Elitek ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF
BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab
 BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab Protocol Code Tumour Group Contact Physicians LYCLLFLUDR Lymphoma Dr.
BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab Protocol Code Tumour Group Contact Physicians LYCLLFLUDR Lymphoma Dr.
4/20/2015. Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations. Learning Objectives. Ambulatory Oncology Infusion Center
 Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Explore the benefits
Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Explore the benefits
Basic Fluid and Electrolytes
 Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
Basic Fluid and Electrolytes Chapter 22 Basic Fluid and Electrolytes Introduction Infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte
CKD FOR INTERNISTS. Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College
 CKD FOR INTERNISTS Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College INTRODUCTION In 2002, the National Kidney Foundation s Kidney Disease Outcomes Quality Initiative(KDOQI)
CKD FOR INTERNISTS Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College INTRODUCTION In 2002, the National Kidney Foundation s Kidney Disease Outcomes Quality Initiative(KDOQI)
Composition: Each Tablet contains. Pharmacokinetic properties:
 Composition: Each Tablet contains Torsemide 5/10/20/40/100mg Pharmacokinetic properties: Torsemide is well absorbed from the gastrointestinal tract. Peak serum concentrations are achieved within 1 hour
Composition: Each Tablet contains Torsemide 5/10/20/40/100mg Pharmacokinetic properties: Torsemide is well absorbed from the gastrointestinal tract. Peak serum concentrations are achieved within 1 hour
Monitoring and Treatment of Acute Kidney Injury in Children with Acute Lymphoblastic Leukemia After High Dose Methotrexate Chemotherapy
 Iranian Journal of Pharmaceutical Research (2016), 15 (4): 957-961 Received: Feb 2015 Accepted: Jun 2016 Copyright 2016 by School of Pharmacy Shaheed Beheshti University of Medical Sciences and Health
Iranian Journal of Pharmaceutical Research (2016), 15 (4): 957-961 Received: Feb 2015 Accepted: Jun 2016 Copyright 2016 by School of Pharmacy Shaheed Beheshti University of Medical Sciences and Health
Non-Hodgkin s Lymphomas Version
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Hodgkin s Lymphomas Version 2.2015 NCCN.org Continue Supportive Care for NHL Tumor Lysis Syndrome (TLS) Laboratory hallmarks of TLS:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Hodgkin s Lymphomas Version 2.2015 NCCN.org Continue Supportive Care for NHL Tumor Lysis Syndrome (TLS) Laboratory hallmarks of TLS:
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Abdominal tumors, in children, 530 531 Alkalinization, in tumor lysis syndrome, 516 Allopurinol, in tumor lysis syndrome, 515 Anaphylaxis, drug
Note: Page numbers of article titles are in boldface type. A Abdominal tumors, in children, 530 531 Alkalinization, in tumor lysis syndrome, 516 Allopurinol, in tumor lysis syndrome, 515 Anaphylaxis, drug
IV Fluids. I.V. Fluid Osmolarity Composition 0.9% NaCL (Normal Saline Solution, NSS) Uses/Clinical Considerations
 IV Fluids When administering IV fluids, the type and amount of fluid may influence patient outcomes. Make sure to understand the differences between fluid products and their effects. Crystalloids Crystalloid
IV Fluids When administering IV fluids, the type and amount of fluid may influence patient outcomes. Make sure to understand the differences between fluid products and their effects. Crystalloids Crystalloid
Drugs Used to Treat Gout. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Drugs Used to Treat Gout Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Gout is a metabolic disease characterized by recurrent episodes of acute arthritis
Drugs Used to Treat Gout Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Gout is a metabolic disease characterized by recurrent episodes of acute arthritis
Major intra and extracellular ions Lec: 1
 Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Normal range of serum potassium is meq/l true hyperkalemia manifests clinically as : Clinical presentation : muscle and cardiac dysfunction
 Potassium Disorders hyperkalemia Potassium is mainly an cation? What is the major physiological role of potassium in the body? What is the major regulatory system of serum potassium level? Which part of
Potassium Disorders hyperkalemia Potassium is mainly an cation? What is the major physiological role of potassium in the body? What is the major regulatory system of serum potassium level? Which part of
Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome
 Maie et al. SpringerPlus 2014, 3:501 a SpringerOpen Journal RESEARCH Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome Open Access Koichiro Maie 1, Yasuhisa Yokoyama
Maie et al. SpringerPlus 2014, 3:501 a SpringerOpen Journal RESEARCH Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome Open Access Koichiro Maie 1, Yasuhisa Yokoyama
Heart Failure (HF) Treatment
 Heart Failure (HF) Treatment Heart Failure (HF) Complex, progressive disorder. The heart is unable to pump sufficient blood to meet the needs of the body. Its cardinal symptoms are dyspnea, fatigue, and
Heart Failure (HF) Treatment Heart Failure (HF) Complex, progressive disorder. The heart is unable to pump sufficient blood to meet the needs of the body. Its cardinal symptoms are dyspnea, fatigue, and
Objectives. Pre-dialysis CKD: The Problem. Pre-dialysis CKD: The Problem. Objectives
 The Role of the Primary Physician and the Nephrologist in the Management of Chronic Kidney Disease () By Brian Young, M.D. Assistant Clinical Professor of Medicine David Geffen School of Medicine at UCLA
The Role of the Primary Physician and the Nephrologist in the Management of Chronic Kidney Disease () By Brian Young, M.D. Assistant Clinical Professor of Medicine David Geffen School of Medicine at UCLA
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
Hematologic Emergencies. Udomsak Bunworasate Chulalongkorn University
 Hematologic Emergencies Udomsak Bunworasate Chulalongkorn University Hematologic Emergencies Hyperleukocytosis Tumor lysis syndrome SVC syndrome Spinal cord compression Hypercalcemia 1. Hyperleukocytosis
Hematologic Emergencies Udomsak Bunworasate Chulalongkorn University Hematologic Emergencies Hyperleukocytosis Tumor lysis syndrome SVC syndrome Spinal cord compression Hypercalcemia 1. Hyperleukocytosis
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
ULYRICE. Protocol Code. Lymphoma. Tumour Group. Dr. Laurie Sehn. Contact Physician
 BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
Pediatric GU Dysfunction
 Pediatric GU Dysfunction Assessment of pediatric renal function Signs and symptoms Laboratory tests Radiological tests Nursing considerations Psychosocial and developmental considerations GU Disorders
Pediatric GU Dysfunction Assessment of pediatric renal function Signs and symptoms Laboratory tests Radiological tests Nursing considerations Psychosocial and developmental considerations GU Disorders
Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer. Title. Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY
 Title Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer Author(s) Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY Citation Cochrane Database of Systematic Reviews,
Title Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer Author(s) Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY Citation Cochrane Database of Systematic Reviews,
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
Paul R. Bowlin, M.D. University of Colorado Denver. May 12 th, 2008
 Paul R. Bowlin, M.D. University of Colorado Denver May 12 th, 2008 Presentation Overview Background / Definitions History Indications for initiation of therapy Outcomes Studies Conclusions Questions Background
Paul R. Bowlin, M.D. University of Colorado Denver May 12 th, 2008 Presentation Overview Background / Definitions History Indications for initiation of therapy Outcomes Studies Conclusions Questions Background
Burkitt s Lymphoma or DLBCL with adverse features PATIENTS WITH GOOD PERFORMANCE STATUS
 Regimen R-CODOX M Indication Burkitt s Lymphoma or DLBCL with adverse features Therapeutic Intent Radical/Curative PATIENTS WITH GOOD PERFORMANCE STATUS Day Medication Dose Route Administration Details
Regimen R-CODOX M Indication Burkitt s Lymphoma or DLBCL with adverse features Therapeutic Intent Radical/Curative PATIENTS WITH GOOD PERFORMANCE STATUS Day Medication Dose Route Administration Details
Rasburicase 1.5 mg/ml powder and solvent for concentrate for solution for infusion. FASTURTEC
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
Jo Abraham MD Division of Nephrology University of Utah
 Jo Abraham MD Division of Nephrology University of Utah 68 year old male presented 3 weeks ago with a 3 month history of increasing fatigue He reported a 1 week history of increasing dyspnea with a productive
Jo Abraham MD Division of Nephrology University of Utah 68 year old male presented 3 weeks ago with a 3 month history of increasing fatigue He reported a 1 week history of increasing dyspnea with a productive
Case presentation. serum uric acid = 11.5 mg/dl 24-hour uric acid excretion = 300 mg
 GOUT 55 y/o male 12 hours pain in my big toe & ankle went to bed last night feeling fine felt as if had broken toe this morning similar problems in right ankle & left wrist Case presentation lab studies
GOUT 55 y/o male 12 hours pain in my big toe & ankle went to bed last night feeling fine felt as if had broken toe this morning similar problems in right ankle & left wrist Case presentation lab studies
GOOD MORNING! July 3, 2014
 GOOD MORNING! July 3, 2014 OUR PATIENT 4yo Female with: 2 days of fever, sore throat, swollen nodes in neck and abdominal pain PMH: Tonsillectomy age 2 Immunizations: UTD NKDA DIFFERENTIAL: OUR PATIENT
GOOD MORNING! July 3, 2014 OUR PATIENT 4yo Female with: 2 days of fever, sore throat, swollen nodes in neck and abdominal pain PMH: Tonsillectomy age 2 Immunizations: UTD NKDA DIFFERENTIAL: OUR PATIENT
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP
 Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
HIHIM 409 7/26/2009. Kidney and Nephron. Fermamdo Vega, M.D. 1
 Function of the Kidneys Nephrology Fernando Vega, M.D. Seattle Healing Arts Center Remove Wastes Regulate Blood Pressure Regulate Blood Volume Regulates Electrolytes Converts Vitamin D to active form Produces
Function of the Kidneys Nephrology Fernando Vega, M.D. Seattle Healing Arts Center Remove Wastes Regulate Blood Pressure Regulate Blood Volume Regulates Electrolytes Converts Vitamin D to active form Produces
The tumor lysis syndrome is the most common disease-related
 T h e n e w e ngl a nd j o u r na l o f m e dic i n e review article current concepts The Tumor Lysis Syndrome Scott C. Howard, M.D., Deborah P. Jones, M.D., and Ching-Hon Pui, M.D. From the Department
T h e n e w e ngl a nd j o u r na l o f m e dic i n e review article current concepts The Tumor Lysis Syndrome Scott C. Howard, M.D., Deborah P. Jones, M.D., and Ching-Hon Pui, M.D. From the Department
Spironolactone has not been demonstrated to elevate serum uric acid, to precipitate gout or to alter carbohydrate metabolism.
 SPIRONE Composition Each tablet contains Spironolactone 100 mg. Tablets Action Spironolactone is a specific pharmacologic antagonist of aldosterone, acting primarily through competitive binding of receptors
SPIRONE Composition Each tablet contains Spironolactone 100 mg. Tablets Action Spironolactone is a specific pharmacologic antagonist of aldosterone, acting primarily through competitive binding of receptors
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION. (rasburicase)
 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION Pr FASTURTEC (rasburicase) Powder for Injection Professed Standard 1.5 mg/vial (1.5 mg/ml/vial) Uricolytic Agent sanofi-aventis Canada Inc. 2905
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION Pr FASTURTEC (rasburicase) Powder for Injection Professed Standard 1.5 mg/vial (1.5 mg/ml/vial) Uricolytic Agent sanofi-aventis Canada Inc. 2905
Renal Function and Associated Laboratory Tests
 Renal Function and Associated Laboratory Tests Contents Glomerular Filtration Rate (GFR)... 2 Cockroft-Gault Calculation of Creatinine Clearance... 3 Blood Urea Nitrogen (BUN) to Serum Creatinine (SCr)
Renal Function and Associated Laboratory Tests Contents Glomerular Filtration Rate (GFR)... 2 Cockroft-Gault Calculation of Creatinine Clearance... 3 Blood Urea Nitrogen (BUN) to Serum Creatinine (SCr)
Management of Acute Kidney Injury in the Neonate. Carolyn Abitbol, M.D. University of Miami Miller School of Medicine / Holtz Children s Hospital
 Management of Acute Kidney Injury in the Neonate Carolyn Abitbol, M.D. University of Miami Miller School of Medicine / Holtz Children s Hospital Objectives Summarize the dilemmas in diagnosing & recognizing
Management of Acute Kidney Injury in the Neonate Carolyn Abitbol, M.D. University of Miami Miller School of Medicine / Holtz Children s Hospital Objectives Summarize the dilemmas in diagnosing & recognizing
PRODUCT CIRCULAR. Tablets COZAAR (losartan potassium) I. THERAPEUTIC CLASS II. INDICATIONS III. DOSAGE AND ADMINISTRATION PAK-CZR-T
 PRODUCT CIRCULAR Tablets I. THERAPEUTIC CLASS, the first of a new class of agents for the treatment of hypertension, is an angiotensin II receptor (type AT 1 ) antagonist. also provides a reduction in
PRODUCT CIRCULAR Tablets I. THERAPEUTIC CLASS, the first of a new class of agents for the treatment of hypertension, is an angiotensin II receptor (type AT 1 ) antagonist. also provides a reduction in
Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion.
 1. NAME OF THE MEDICINAL PRODUCT Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, 1 ml of Fasturtec concentrate
1. NAME OF THE MEDICINAL PRODUCT Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, 1 ml of Fasturtec concentrate
WATER, SODIUM AND POTASSIUM
 WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
5/18/2017. Specific Electrolytes. Sodium. Sodium. Sodium. Sodium. Sodium
 Specific Electrolytes Hyponatremia Hypervolemic Replacing water (not electrolytes) after perspiration Freshwater near-drowning Syndrome of Inappropriate ADH Secretion (SIADH) Hypovolemic GI disease (decreased
Specific Electrolytes Hyponatremia Hypervolemic Replacing water (not electrolytes) after perspiration Freshwater near-drowning Syndrome of Inappropriate ADH Secretion (SIADH) Hypovolemic GI disease (decreased
Gazyva. Gazyva (obinutuzumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.29 Subject: Gazyva Page: 1 of 7 Last Review Date: September 15, 2016 Gazyva Description Gazyva (obinutuzumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.29 Subject: Gazyva Page: 1 of 7 Last Review Date: September 15, 2016 Gazyva Description Gazyva (obinutuzumab)
Study of Clinical Profile and Prognostic Factors of Acute Kidney Injury (AKI) In Tertiary Referral Centre in Marathwada
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-853, p-issn: 2279-861.Volume 13, Issue 12 Ver. V (Dec. 214), PP 66-77 Study of Clinical Profile and Prognostic Factors of Acute Kidney
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-853, p-issn: 2279-861.Volume 13, Issue 12 Ver. V (Dec. 214), PP 66-77 Study of Clinical Profile and Prognostic Factors of Acute Kidney
PRODUCT INFORMATION UROCIT -K. Wax matrix tablets
 PRODUCT INFORMATION UROCIT -K Wax matrix tablets NAME OF DRUG Potassium Citrate CAS number- 6100-05-6 The empirical formula of potassium citrate is K 3 C 6 H 5 0 7.H 2 O, and its structural formula is:
PRODUCT INFORMATION UROCIT -K Wax matrix tablets NAME OF DRUG Potassium Citrate CAS number- 6100-05-6 The empirical formula of potassium citrate is K 3 C 6 H 5 0 7.H 2 O, and its structural formula is:
This is a controlled document and therefore must not be changed or photocopied L.80 - R-CHOP-21 / CHOP-21
 R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
Professor Dr. Saiyeedur Rahman Professor and Head Department of Medicine SBMCH, Barisal
 Professor Dr. Saiyeedur Rahman Professor and Head Department of Medicine SBMCH, Barisal What is Oncologic Emergency? An oncologic emergency is an acute, potentially life-threatening event resulting from
Professor Dr. Saiyeedur Rahman Professor and Head Department of Medicine SBMCH, Barisal What is Oncologic Emergency? An oncologic emergency is an acute, potentially life-threatening event resulting from
uric acid Non electrolytes of the plasma
 73 uric acid Non electrolytes of the plasma 1 Purines and uric acid Fig 2 JFI Uric acid is the major product of catabolism of the purine nucleosides adenosine and guanosine, Uric acid is sparingly soluble
73 uric acid Non electrolytes of the plasma 1 Purines and uric acid Fig 2 JFI Uric acid is the major product of catabolism of the purine nucleosides adenosine and guanosine, Uric acid is sparingly soluble
SAALT3W Sarcoma Dr. Meg Knowling. Protocol Code Tumour Group Contact Physician
 BCCA Protocol Summary for Etoposide, Ifosfamide-Mesna (SAIME) Alternating with vincristine, DOXOrubicin and Cyclophosphamide (with or without Mesna)(SAVAC or SAVACM) with Filgrastim Support at a THREE
BCCA Protocol Summary for Etoposide, Ifosfamide-Mesna (SAIME) Alternating with vincristine, DOXOrubicin and Cyclophosphamide (with or without Mesna)(SAVAC or SAVACM) with Filgrastim Support at a THREE
Acute Kidney Injury. Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1
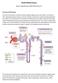 Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
Oncologic Emergencies
 Oncologic Emergencies Luca Delatore, MD James Emergency Department Medical Director Associate Professor Clinical Department of Emergency Medicine The Ohio State University Wexner Medical Center Prevalence
Oncologic Emergencies Luca Delatore, MD James Emergency Department Medical Director Associate Professor Clinical Department of Emergency Medicine The Ohio State University Wexner Medical Center Prevalence
Oncologic Emergencies
 Oncologic Emergencies Luca Delatore, MD James Emergency Department Medical Director Associate Professor Clinical Department of Emergency Medicine The Ohio State University Wexner Medical Center Prevalence
Oncologic Emergencies Luca Delatore, MD James Emergency Department Medical Director Associate Professor Clinical Department of Emergency Medicine The Ohio State University Wexner Medical Center Prevalence
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
HTN, retenopathy, edema, encephalopathy
 ARF Uremic syndrom Uremic syndrome (uremia) is a serious complication of CRF & ARF. It occurs when urea and other waste products build up in the body because the kidneys are unable to eliminate them. These
ARF Uremic syndrom Uremic syndrome (uremia) is a serious complication of CRF & ARF. It occurs when urea and other waste products build up in the body because the kidneys are unable to eliminate them. These
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck
 Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
