EXPERT OPINION: REVIEWING CURRENT THINKING ON THE IN VIVO BEHAVIOUR OF PARTICLES IN THE EXTRA-FINE REGION
|
|
|
- Magdalen Chase
- 5 years ago
- Views:
Transcription
1 EXPERT OPINION: REVIEWING CURRENT THINKING ON THE IN VIVO BEHAVIOUR OF PARTICLES IN THE EXTRA-FINE REGION The inhalation route is a fast and effective way of delivering medication, both locally to the lungs and systemically to the body. Conventional wisdom for the development of inhaled products is that the preferred particle size for drug delivery is one to five microns and it is typically suggested that particles less than one micron are exhaled, but is this true? In this review, David Lewis, PhD, Head of Laboratory, Chippenham Research Centre, Chiesi UK Ltd, reports the latest research on the changing view of the behaviour of particles in the extra-fine region and their potential for enhancing inhaled drug therapies. Pulmonary drug delivery has generated a significant amount of interest in pharmaceutical research because of the lung s capacity to absorb pharmaceuticals for both local treatment and systemic delivery. 1 The large surface area and highly permeable air-toblood barrier provided by the respiratory system make it a highly receptive site for drug delivery, most especially for the local, rapid and effective treatment of, for example, asthma and chronic obstructive pulmonary disease. However, the development of inhaled drugs is complex, as to achieve targeted deposition three conditions must be met: 1) The inhaled aerosol formulation must be sized for drug deposition along the respiratory tract including in the deep lung 2) The drug delivery device and formulation must generate an aerosol cloud containing a high proportion of suitably sized particles 3) The deposition of the drug should translate into functional and clinical benefits. Better understanding of these parameters and their interrelationship helps to maximise the benefits and efficiency of pulmonary drug delivery systems but the majority of such research has focused on the behaviour of particles within the 1-5 µm range, which are known to deposit successfully in the pulmonary region. Particles larger than 5 µm will typically impact on the oropharynx and be swallowed. Assuming a continuum of behaviour, with finer and finer particles taking longer to deposit, the majority of particles less than 1 µm in size are expected to flow back out from the body on the exiting breath. Currently particles less than 1 µm in diameter are therefore not considered to be of consequence for drug delivery. TIME TO RE-EVALUATE? Recent technological developments have seen a wide range of industries look in more detail at particles in the 1-2 µm and nano range, and new evidence is emerging to suggest that assumptions about inhaled behaviour may not be correct. Indeed, studies investigating the deposition sites of extrafine and sub-micron particles have found that it is possible that these particles could in fact be effectively delivered to the small airways in the deep lung. 2 Clinically this might not only aid the efficiency of drug delivery, but could also result in a more uniform localised therapeutic response, if the drug was deposited in both the central and peripheral airways. 3,4 A recognised trait in certain asthma sufferers is persistent small airway dysfunction and Dr David Lewis Head of Laboratory Chippenham Research Centre Chiesi UK Address for Correspondence: Chiesi Ltd 333 Styal Road Manchester M22 5LG United Kingdom T: F: Copyright 2015 Frederick Furness Publishing Ltd
2 this seems to be associated with poor disease control. By targeting deep lung deposition extra-fine formulations of inhaled asthma therapies may therefore also have the potential to unlock the small airways compartment and improve treatment efficacy. 4,5 Furthermore there is evidence that when an aerosol is mainly composed of very fine particles the difference between in vitro aerosolisation and in vivo behaviour is less pronounced. 6 This suggests that aerosols with a finer particle-size distribution could exhibit behaviour that is relatively independent of flow rate, a feature that would enable the delivery of more controlled deposition across patient groups with different inspiratory profiles. Closer scrutiny of the behaviour of extrafine aerosols in terms of pulmonary delivery calls for data generated both in vitro and in vivo. Interpretation of such data can be used to answer the following questions and to provide greater insight: How do aerosols of extra-fine particles behave? How do extra-fine particles behave in the respiratory system? Are current in vitro and computer based particle behavioural models sufficient for describing this behaviour? Answering such questions will help resolve the potential pharmacological impacts and consequences of delivery of extra-fine aerosols for treatments for a wide range of applications. If particles around one micron and smaller are not simply exhaled then there are possible implications in terms of the sites of deposition with the lung, the dose received by the patient and the therapeutic activity of the drug. Further, improved insights may also provide a firm basis for future advancement in inhaled nano-medicine strategies. DEFINING EXTRA-FINE & SUB- MICRON AEROSOLS The development and quality control of orally inhaled and nasal drug products (OINDPs) relies on making in vitro measurements that reflect the likely success of drug delivery and targeted deposition. These include assessing the total quantity of drug emitted from the device and therefore available to the patient, and the aerodynamic size of the particles that make up the emitted aerosol. This impacts the percentage of the total dose that reaches the lungs during inhalation, as well as its regional intrapulmonary deposition, and is therefore therapeutically active. Inertial impaction, sedimentation and Brownian diffusion are all factors that are affected by particle characteristics and that influence the site of deposition in the airways. Influential particle characteristics include shape, density and, most especially, size. For inhaled product delivery, aerodynamic particle size measured by the technique of inertial cascade impaction, is the metric used as a primary indicator of deposition behaviour because of its relevance, which stems from a shared dependence on shape and density. The mass median aerodynamic diameter (MMAD), one of the most commonly used inhaled product metrics, defines the size of aerosol particles, taking into account their geometric diameter, shape and density. In terms of aerodynamic particle size, which is equal to the geometric particle diameter for spherical particles or unit density, a sub-micron particle can be identified simply as a particle with a diameter of less than one micron. 1 There is an increasing amount of research into extra-fine and sub-micron aerosols. However, as yet a definition has not been agreed. Aerosols can be either polydisperse, consisting of multiple particle sizes, or monodisperse, containing particles of uniform size. For a monodisperse aerosol to be sub-micron, the particles need to have an aerodynamic particle size of less than one micron. However, there are, in reality, no truly monodisperse aerosols most contain a distribution of particle sizes. For polydisperse aerosols, it has not been formalised as to what percentage of the particles need to be extra-fine or sub-micron to merit the respective classifications. Typically, when designing an inhaled delivery device, limits are set that define an optimal range for the MMAD of the particles and the fraction (based on mass or volume) of particles that can acceptably fall outside of this range. An evaluation of dry-powder inhalers (DPIs), conducted by Krishnaprasad, found that the majority of devices investigated, contained approximately 50% of particles within the fine particle fraction. 7 This suggests that many DPIs could already be delivering a proportion of the dose in the extra-fine range, even if the aerosol as a whole cannot be classified as extra-fine. This proportion can be quantified through analysis of the residual dose captured in the final filter of a cascade impactor. Recent technological developments have seen a wide range of industries look in more detail at particles in the 1-2 µm and nano range, and new evidence is emerging to suggest that assumptions about inhaled behaviour may not be correct The term extra-fine is now used routinely to describe inhaled particles and has been featured in several studies, 8 but there remain discrepancies in the term s definition that cause some confusion and make studies harder to compare. General consensus sees particles less than 1-2 µm referred to as extra-fine but some papers use this same term to refer to particles less than 0.1 µm in diameter. 2 For the purposes of this article, extra-fine particles are defined as those that have an aerodynamic particle size of less than 2 µm, to differentiate them clearly from particles that are traditionally defined as lying in the fine particle fraction. This figure has been selected on the basis of clinical relevance, it being the upper size limit of particles that are able to penetrate to the small airways of the lung. 9 PARTICLE BEHAVIOUR IN THE LUNG In pulmonary delivery, there are three factors that influence particle deposition behaviour: 1) The characteristics of the aerosol 2) The anatomy of the respiratory tract 3) The airflow patterns in the lung airways. 10 Deposition is quantified in terms of the ratio of the mass of particles deposited in the respiratory tract to the mass of particles inhaled 11 and governed, as mentioned earlier, by the mechanisms of impaction, sedimentation and diffusion. Copyright 2015 Frederick Furness Publishing Ltd 5
3 The mass of a particle affects its travelling velocity, which is also determined by the velocity of the respiratory airflow. Larger particles will tend to travel more slowly but also have greater inertia making them more prone to impaction. Gravitational deposition is dependent on residence time and particle settling velocity and is therefore promoted by larger particle size and the longer residence times that more easily occur in the small conducting airways and the alveolated lung region. 12 Diffusional displacement, on the other hand, becomes more pronounced as particle size decreases and is the factor with the highest probability of promoting the deposition of extra-fine particles in the lung and small airways. Settling velocity increases with the square of the particle diameter. This is why particles greater than 5 µm in size can quickly deposit after inhalation onto the oropharynx. Impaction is also an important mechanism for deposition in this area. The relationship between particle size and settling velocity means that extra-fine and sub-micron particles will take significantly longer to deposit, a primary reason why it is often thought that such particles are simply exhaled. A typical recommended breath-hold period after dose by an inhaler is ten seconds, though this may be an overly optimistic figure when compared with the reality of clinical practice. Clearly the extent of breath hold is likely to have a marked effect on particle deposition behaviour, most especially for extra-fine particles. 10 Indeed a suggestion for increasing the deposition of extra-fine and sub-micron particles, where desirable, is to introduce longer breath hold periods, but this would require the effective training of patients and may prove problematic for some groups including the elderly and young children. If we explore the premise that extra-fine and sub-micron particles are not simply exhaled then these particles have the potential to significantly contribute to product performance. The reduced net effect of oropharyngeal deposition (by impaction) and high pulmonary deposition in the upper respiratory region (as a result of slow settling times) could potentially counterbalance the impact of losing a certain fraction of particles in the extra-fine range on exhalation. Therefore such particles may be not only pharmacologically relevant but could even enhance dose effectiveness as a result Aerosols with a finer particle-size distribution could exhibit behaviour that is relatively independent of flow rate, a feature that would enable the delivery of more controlled deposition across patient groups with different inspiratory profiles of precise delivery to a targeted and therapeutically-relevant site. 2 Although extrafine particles only make up a very small contribution to particle mass, they may lead to significant dosing in terms of the number of particles deposited on a receptive surface. Research from Bodzenta-Lukaszyk and Kokot provides some support for the suggestion that the mechanisms at play during inhalation do not result in the complete exhalation of sub-micron particles. 13 This study concluded that, although almost half the mass of the inhaled aerosol from an MDI was composed of particles less than 1 µm, only approximately 10% of the emitted dose was exhaled, as measured by scintigraphy. Particles that are not exhaled and avoid deposition in the extrathoracic and tracheobronchial airways reach the alveolar region. Generally, it is thought that the principals of deposition within this region are sedimentation and via diffusion, which is of particular importance for those in the sub-micron range. 11 This reliance on diffusion and Brownian movement make the deposition of the extra-fine particles more pronounced in the alveolar region. Assessing the Impact of Agglomeration The preceding discussion assumes that inhaled extra-fine and sub-micron particles will remain discrete. However, at this size particles have a sharply increased tendency to agglomerate due to inter-particle forces which increase exponentially with decreasing particle size. That said, agglomerates are not fixed units, a primary factor in inhaled drug delivery, and can change their size and shape depending on the surrounding conditions. Larger agglomerates may break down into smaller particles, or smaller moieties may agglomerate further to form even larger particles. 14 In the design of a DPI, particle deagglomeration is actively promoted by inducing an inspiratory airflow that creates turbulence and aerosolisation within the device. Low-resistance devices result in high inspiratory flow rates while higher resistances induce lower air velocities. Due to the settling velocity and agglomeration tendencies of sub-micron particles, the development of inhalers with highly effective deagglomeration mechanisms, typically those with high internal resistance, are therefore more likely to provide greater lung deposition than those with a lower internal resistance. 15,16,17 At the same time as promoting successful de-agglomeration these devices deliver the low velocity needed to offset the slow settling rates and ensure the successful administration of extra-fine particles. 18 Accounting for the Effect of Humidity One further factor to consider when investigating the behaviour of particles in the respiratory system is the geometric expansion of particles that may occur as a result the high humidity levels (of 99.5%). This effect means that hygroscopic particles will have different deposition patterns from analogously sized non-hygroscopic alternatives. The size increase experienced by hygroscopic particles will directly affect settling velocity and therefore the location of deposition. In vitro it is very difficult to mimic the extreme humidity of the respiratory system due to the impact these conditions have on instrumentation. Interpretations and extrapolations therefore have to be made as to how 99.5% humidity will affect particle behaviour, with only in vivo investigations being able to demonstrate the impacts accurately. INVESTIGATING & MODELLING EXTRA-FINE PARTICLE BEHAVIOUR Experimental studies have demonstrated that factors including airway wall motion, inhalation waveform and geometric complexity all influence the deposition of aerosols in the respiratory system by affecting particle impaction and sedimentation. 19 As such, much work has been undertaken to investigate the best physical models to develop correlations of aerosol depositions that can be used to predict the doses deposited at target locations within the lung, including the alveolar dose. 6 Copyright 2015 Frederick Furness Publishing Ltd
4 In routine OINDP testing, multistage cascade impactors are the instrument of choice for measuring data related to deposition behaviour as they enable generation of an aerodynamic particle size distribution specifically for the active pharmaceutical ingredient, across an appropriate size range. These instruments are precision engineered and separate a sample via particle inertia. However, they are designed primarily to determine the consistency and quality of inhaled products and do not accurately represent the anatomical complexity of the human airways. The metric MMAD, generated from cascade impaction testing, excludes the particles depositing in the throat area of the apparatus. A number of studies have shown that extra-fine drug formulations with a low MMAD give greater lung deposition compared with larger particle formulations. 20 Physical models of the pulmonary acinus regions have been developed to investigate the deposition of pharmaceutical products further in order to predict therapeutic effect. Depending on the drug being delivered and its desired clinical effect, it can be necessary to target certain regions of the pulmonary system. For instance, for some pharmaceuticals, the alveolar region may be the target for deposition due to the need to address impaired performance in this region which is often an issue for asthma sufferers. 5,9 Conversely, for other drugs the tracheobronchial region may be the target and deposition in the alveolar region could potentially cause unwanted systemic exposure and increased side effects. 19 Individual alveolus models consisting of a single hemispherical shell or single alveolus attached to a tube have been used to characterise general transport within the alveolar region. The complexity of such models has since been increased to include channels with multiple hemispheres attached. Other models include rectangular alveoli compartments and bifurcating networks using a honeycomb structure of attached alveoli. 19 It has been found that the behaviour of particles varies greatly depending on the characteristics of the model used, which makes it difficult to compare the particle behaviours observed in different studies. There is an increasing amount of research into extra-fine and sub-micron aerosols. However, as yet a definition has not been agreed Models with multiple alveoli have been found to affect the flow field that pertains and particle trajectories, while bifurcations and complex airway geometry strongly 21, 22 influence aerosol deposition. Further, Navvab Khajeh-Hosseini- Dalasm and P Worth Longest found that alveolar deposition is dependent on deposition in the upper airways because of the impact this has on the remaining fraction of particles that enters the deep lung region. 19 Models mimicking wall motion, Copyright 2015 Frederick Furness Publishing Ltd 7
5 which drives alveolar airflow, have shown that such movement is also an important component with unsteady state flow having a large effect on particle transport and deposition. 23 It has also been suggested that truncated acinar models can be implemented to capture total deposition and, when combined with factors such as the impact of gravity, angles and sedimentation, these enable the development of new correlations to predict aerosol deposition from an inhaler device, from the tracheobronchial airways to the alveoli. 24 The measurement of extra-fine particle behaviour is technically challenging, regardless of the physical model. However for studies investigating the deposition of inhaled drugs at the extra-fine level, techniques that combine scintigraphy with computed tomography imaging do allow 3D assessment of the particles regional deposition with reasonable accuracy. 13 Since research suggests that targeting the peripheral airways with smaller drug particle aerosols certainly achieves comparable and in some cases superior drug efficacy, 4,5 such studies are important for the ongoing development of inhaled products. THE COMPLEMENTARY ROLE OF MODELLING When in vitro models are used to simulate discrete aspects of pulmonary particle behaviour, the data generated from such studies is often extrapolated using some form of computer model to provide a prediction as to how the results can be applied to the whole lung. Such models are used as a means to correlate in vitro and in vivo studies better by bridging the gap between in vivo behaviour and the data generated by cascade impactors and other physical set-ups that fail to capture particle behaviour precisely. As alveoli are extremely small, many studies use computational fluid dynamics (CFD) alongside scaled-up in vitro models for the analysis of aerosol transport and deposition. 8 This type of work is exemplified by work conducted by Khajeh-Hosseini- Dalasam and Longest assessing models used for the study of particle behaviour in the pulmonary system. 19 These researchers found that airway wall motion was important to match in vivo alveolar deposition data with one-dimensional (1D) models accurately. These models are used to implement analytical approximations of the The preceding discussion assumes that inhaled extra-fine and sub-micron particles will remain discrete. However, at this size particles have a sharply increased tendency to agglomerate various particle transport mechanisms to predict deposition at the level of individual bifurcations throughout the airways. 25 1D models, however, only consider distance travelled in a tubular network so omit the importance of considering oscillating flow and wall motion, which are important for matching particle behaviour and alveolar deposition in vivo. CFD uses mathematical algorithms to simulate the motion of particles, fluids and gases and their interactions with surfaces and is increasingly being used in medical studies. CFD approaches have recently been developed to simulate the delivery of pharmaceutical aerosols throughout the conducting airways. 11 The spray and jet effects of the inhaler are captured in addition to the patient s inhalation profile. By evaluating a sufficient number of stochastic individual path (SIP) models, the regional deposition fractions within the lung emerge. CFD models can successfully account for bifurcation asymmetry alveolar branches have a random orientation in the airways and physical effects on pharmaceutical aerosols, and enable the accurate prediction of highly localised deposition factors, which is crucial when studying the behaviour of extra-fine and sub-micron particles for pharmaceutical purposes. 26,27 These models can potentially also be used to help determine the aerosol penetration fraction that exits the bronchioles and enters the alveolar region over time. However, CFDs have not extensively been used for investigating alveolar deposition and are still in the developmental stages with respect to predicting deposition in this region. Physically relevant factors such as inhalation profiles consistent with pharmaceutical delivery still need to be taken into account. 19 However CFD models can relatively accurately model the two inhalation manoeuvers most commonly applied when actuating inhalers slow-and-deep and quick-and-deep, both of which are usually followed by a period of breath-hold. This period is often also included in CFD models to simulate particle deposition accurately. In terms of the specific results obtained with CFD studies, one study found a significant difference in the prediction of particle deposition with both inhalation manoeuvers using a CFD alveolated model with moving walls, compared with a 1D solution. 19 The conclusion from this work was that 1D models are not ideal for accurately predicting deposition in the alveolated airway. The CFD model however demonstrated the formation of accumulations of particles deposition hotspots and so could be used to predict regional drug delivery within the airways. The analysis of data from CFD models suggests that excellent approximations of in vitro extra-fine particle behaviour can be made in terms of velocity and deposition. Further comparisons of data with in vivo study findings see patients undergo CT scans after the administration of radiolabelled medication to establish deposition masks of the left and right lung regions, the oropharyngeal region and the gut. 28 Studies of this nature are crucial since it is widely recognised that in vivo investigations are the most pharmacologically relevant. The current limitations of computer simulations and in vitro methods means that well designed in vivo studies remain key to understanding the full potential and opportunity for extra-fine drug delivery to the lungs. LOOKING AHEAD Extra-fine aerosols potentially offer pharmacological benefit for pulmonary delivery. Not only is there evidence to suggest that extra-fine particles may reach the deep lung, rather than be exhaled, but also that this can result in a more uniform dosing 3,4. This can be a benefit for improved local therapeutic effect, and potentially for systemic drug delivery too. To progress this field of study further, large scale in vivo studies are needed to clinically determine the path of extra-fine and sub-micron drug particles through 8 Copyright 2015 Frederick Furness Publishing Ltd
6 the pulmonary region and to validate the in vitro and computational investigations being conducted. This is important to progress our understanding of the extrafine fraction already produced by existing inhaled products. The exploitation of extra-fine particle behaviour through the development of a new generation of inhalers designed to deliver nanosized particles is a separate challenge that lies well beyond current capabilities. REFERENCES 1. Demoly P, Hagedoorn P, de Boer A, Frijlink H, The clinical relevance of dry powder inhaler performance for drug delivery. Resp Med, 2014, Vol 108, pp Haughney J, Price D, Barnes N, Virchow C, Roche N, Chrystyn H, Choosing inhaler devices for people with asthma: Current knowledge and outstanding research needs. Resp Med, 2009, Vol 104, pp Scichilone N, Benfante A, Morandi L, Bellini F, Papi A, Impact of extrafine formulations of inhaled corticosteroids/long-acting beta-2 agonist combinations on patient-related outcomes in asthma and COPD. Patient Related Outcome Measures, 2014, Vol 5, pp , Academic Search Complete, EBSCOhost, viewed 16 April Patil J, Sarasija S, Pulmonary drug delivery strategies: A concise, systematic review. Lug India, 2012, Vol 29(1), pp Academic Search Complete, EBSCOhost (accessed April 28, 2015). 5. Usmani OS, Small airway disease in asthma: pharmacological considerations. Curr Opin Pulm Med, 2015, Vol 21, pp Usmani OS, Biddiscombe MF, Nightingale JA, Underwood SR, Barnes PJ, Effects of bronchodilator particle size in asthmatic patients using monodisperse aerosols. J Applied Physiol, 2003, Vol 95, pp Krishnaprasad K, Sobti V, Bhargava A, Evaluation of dry powder inhaleremitted aerosol of budesonide and formoterol demonstrated by Andersen Cascade Impactor using respirable fraction: An In vitro study. Int J Sci Study, 2014, Vol 2(9). 8. Worth Longest P, Tian G, Li X, Son Y-J, Hindle M, Performance of combination drug and hygroscopic excipient submicrometer particles from a softmist inhaler in a characteristic model of the airways. Ann Biomed Eng, 2013, Vol 40 (12), pp Lipworth B, Mangharan A, Anderson W, Unlocking the quiet zone: the small airway asthma phenotype. Lancet Respir Med, 2014, Vol 2, pp Lee M, Gibaldi s Drug Delivery Systems in Pharmaceutical Care, Yeh HC, Phalen RF, Raabe OG, Factors influencing the deposition of inhaled particles. Environmental Health Perspectives, 1976, Vol 15, pp Schulz H, Mechanisms and factors affecting intrapulmonary particle deposition: Implications for efficient inhalation therapies. GSF-National Research Centre for Environment and Health Institute for Inhalation Biology, Neuherberg/Munich Germany, Bodezenta-Lukaszyk A, Kokot M, Pharmacological consequences of inhaled drug delivery to small airways in the treatment of asthma. Adv Ther, 2014, /s Walter D, Primary particles-agglomerates-aggregates. Laboratories for Chemistry and Physics, Institute for Occupational and Social Medicine, Justus-Liebig-University, Giessen, Germany. 15. Chrystyn H, Price D, Not all asthma inhalers are the same: Factors to consider when prescribing an inhaler. Prim Care Respir J: J Gen Pract Airw Group, 2009, Vol 18, pp Ehtezazi T, Horsfield MA, Barry PW, O Callaghan C, Dynamic change of the upper airway during inhalation via aerosol delivery devices. J Aerosol Med Off J Int Soc Aerosols Med, 2004, Vol 17, pp de Koning JP, Dry powder inhalation: technical and physiological aspects, prescribing and use: Chapter 2: Effect of resistance to airflow on the inspiratory flow curve. University of Groningen, The Netherlands, Gjaltema D, Hagedoorn P, Frijlink HW, de Boer AH, Are extra-fine particles from dry powder inhalers likely to improve lung deposition? Eur Resp Soc, Thematic Poster: Asthma and COPD devices and treatments. 19. Khajeh-Hosseini-Dalasm N, Worth Longest P, Deposition of particles in the alveolar airways: Inhalation and Breath-hold with pharmaceutical aerosols. J of Aerosol Sci, Viewed April 10, Newman SP, Weisz AW, Talee N, Clarke SW, Improvement of drug delivery with a breath actuated pressurized aerosol for patients with poor inhaler techniques Thorax, 1991, Vol 46, pp Snitzman J, Heimshch T, Wildhaber JH, Tsuda A, Rosgen T, Respiratory flow phenomena and gravitational deposition in a three-dimensional space-filling model of the pulmonary acinar tree. J Biomed Eng, 2009, Vol 131, Berg E, Robinson RJ, Stereoscopic particle image velocimetry analysis of healthy and emphysemic alveolar sac models. J Biomech Eng, 2011, Vol 133, Balashazy I, Hofmann W, Farkas A, Madas BG, Three-dimensional model for aerosol transport and deposition in expanding and contracting alveoli. Inhalat Toxicol, 2008, Vol 20, pp Longest PW, Hindle M, Das Chaudhri S, Xi J, Comparison of ambient and spray aerosol deposition in a standard reduction part and more realistic mouth-throat geometry. J Aerosol Sci, 2008, Vol 39, pp Isaacs KK, Rosati JA, Martoren TB, Mechanisms of particle deposition. In Aerosols Handbook, 2005(Edited, Ruzer LS, Harley NH, Aerosols Handbook), pp 75-99, CRC Press City. 26. Worth Longest P, Holbrook LT, In silico models of aerosol delivery to the respiratory tract Development and applications. Adv Drug Del Rev, 2012, Vol 64, pp Fung YC, A model of the lung structure and its validation. J Appl Physiol, 1988, Vol 64, pp Leach CL, Huehl PJ, Chang R, Ketai L, Norenberg JP, McDonald JD, Characterization of respiratory deposition of fluticasone-salmeterol hydrofluoroalkane-134a beclomethasone in asthmatic patients Ann Allergy Asthma Immunol, 2012, Vol 108, pp Copyright 2015 Frederick Furness Publishing Ltd 9
PULMONARY DRUG DELIVERY
 P04 P14 P28 RETHINKING EXTRA-FINE PARTICLE BEHAVIOUR SHAKING UP AUTOMATED INHALER TESTING PATIENT PREFERENCE IN RESPIRATORY DEVICE TRAINING PULMONARY DRUG DELIVERY DECEMBER 1 ST 2015 ISSUE N O 62 Contents
P04 P14 P28 RETHINKING EXTRA-FINE PARTICLE BEHAVIOUR SHAKING UP AUTOMATED INHALER TESTING PATIENT PREFERENCE IN RESPIRATORY DEVICE TRAINING PULMONARY DRUG DELIVERY DECEMBER 1 ST 2015 ISSUE N O 62 Contents
Pulmonary deposition of inhaled drugs
 Pulmonary deposition of inhaled drugs Federico Lavorini Dept. Experimental and Clinical Medicine Careggi University Hospital Florence - Italy Presenter Disclosures F.L. has received in the last 5 years
Pulmonary deposition of inhaled drugs Federico Lavorini Dept. Experimental and Clinical Medicine Careggi University Hospital Florence - Italy Presenter Disclosures F.L. has received in the last 5 years
Deposition of Inhaled Particle in the Human Lung for Different Age Groups
 Deposition of Inhaled Particle in the Human Lung for Different Age Groups Xilong Guo 1, Qihong Deng 1* 1 Central South University (CSU), Changsha, China * Corresponding email: qhdeng@csu.edu.cn, qhdeng@gmail.com.
Deposition of Inhaled Particle in the Human Lung for Different Age Groups Xilong Guo 1, Qihong Deng 1* 1 Central South University (CSU), Changsha, China * Corresponding email: qhdeng@csu.edu.cn, qhdeng@gmail.com.
21/03/2011 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY. Fundamentals of aerosols
 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
Understanding cascade impaction and its importance for inhaler testing
 Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
FRI aerosol. deposition. A unique way to look at regional inhaled drug deposition.
 FRI aerosol deposition A unique way to look at regional inhaled drug deposition www.fluidda.com EXECUTIVE SUMMARY FRI - AN UPDATE OF SCINTIGRAPHY FRI deposition technology is based on CT imaging, and uses
FRI aerosol deposition A unique way to look at regional inhaled drug deposition www.fluidda.com EXECUTIVE SUMMARY FRI - AN UPDATE OF SCINTIGRAPHY FRI deposition technology is based on CT imaging, and uses
Advanced Drug Delivery Reviews
 ADR-12134; No of Pages 16 Advanced Drug Delivery Reviews xxx (2011) xxx xxx Contents lists available at ScienceDirect Advanced Drug Delivery Reviews journal homepage: www.elsevier.com/locate/addr In silico
ADR-12134; No of Pages 16 Advanced Drug Delivery Reviews xxx (2011) xxx xxx Contents lists available at ScienceDirect Advanced Drug Delivery Reviews journal homepage: www.elsevier.com/locate/addr In silico
Patient. Device Clinician. Safety & efficacy
 Patient Device Clinician Formulation Safety & efficacy 1. Modified from Daley-Yates et al., Expert Opin. Drug Deliv. 2011: 8(10):1297-1308 2. Modified from Laube et al., Eur Respir J 2011; 37: 1308 1331
Patient Device Clinician Formulation Safety & efficacy 1. Modified from Daley-Yates et al., Expert Opin. Drug Deliv. 2011: 8(10):1297-1308 2. Modified from Laube et al., Eur Respir J 2011; 37: 1308 1331
FRI aerosol. deposition. A unique way to look at regional inhaled drug deposition.
 FRI aerosol deposition A unique way to look at regional inhaled drug deposition www.fluidda.com EXECUTIVE SUMMARY Since 2005, Functional Respiratory Imaging (FRI) has been used many times to evaluate the
FRI aerosol deposition A unique way to look at regional inhaled drug deposition www.fluidda.com EXECUTIVE SUMMARY Since 2005, Functional Respiratory Imaging (FRI) has been used many times to evaluate the
Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung
 Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung Nia Stevens 9 th November 2016 Thanks to Richard Kaye, James Mitchell, Dave Prime at GSK Bahman Asgharian and Owen
Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung Nia Stevens 9 th November 2016 Thanks to Richard Kaye, James Mitchell, Dave Prime at GSK Bahman Asgharian and Owen
OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER
 OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER US FDA guidance for the in vitro demonstration of bioequivalence in a generic nebuliser directly references
OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER US FDA guidance for the in vitro demonstration of bioequivalence in a generic nebuliser directly references
Recommendations for Aerosol Applications of Silicone-Based Materials
 Recommendations for Aerosol Applications of Silicone-Based Materials September 2001 Revised March 2018 This document provides information and recommendations relevant to formulating aerosol products containing
Recommendations for Aerosol Applications of Silicone-Based Materials September 2001 Revised March 2018 This document provides information and recommendations relevant to formulating aerosol products containing
Spray Nebulizer Deposition Efficiency as a Function of Age. University of Denver Denver, CO Denver, CO
 ILASS Americas, 23 rd Annual Conference on Liquid Atomization and Spray Systems, Ventura, CA, May 2011 Spray Nebulizer Deposition Efficiency as a Function of Age L. Weber * and C.S. Lengsfeld 1 Department
ILASS Americas, 23 rd Annual Conference on Liquid Atomization and Spray Systems, Ventura, CA, May 2011 Spray Nebulizer Deposition Efficiency as a Function of Age L. Weber * and C.S. Lengsfeld 1 Department
Inhalation von Radionukliden physikalische und biologische Mechanismen
 Inhalation von Radionukliden physikalische und biologische Mechanismen Werner Hofmann Abteilung für Physik und Biophysik, Fachbereich Materialforschung und Physik, Universität Salzburg 1 LUNG DOSIMETRY
Inhalation von Radionukliden physikalische und biologische Mechanismen Werner Hofmann Abteilung für Physik und Biophysik, Fachbereich Materialforschung und Physik, Universität Salzburg 1 LUNG DOSIMETRY
The Use of Physics-Based Modeling to Better Design Drug- Device Interface. Yoen-Ju Son, PhD Merck Research Laboratory, Summit, NJ
 The Use of Physics-Based Modeling to Better Design Drug- Device Interface Yoen-Ju Son, PhD Merck Research Laboratory, Summit, NJ Presentation Outline Physics-based modeling in pharmaceutical industry Pulmonary
The Use of Physics-Based Modeling to Better Design Drug- Device Interface Yoen-Ju Son, PhD Merck Research Laboratory, Summit, NJ Presentation Outline Physics-based modeling in pharmaceutical industry Pulmonary
University of Groningen. Technology in practice Lexmond, Anne
 University of Groningen Technology in practice Lexmond, Anne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Technology in practice Lexmond, Anne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Transactions on Biomedicine and Health vol 2, 1995 WIT Press, ISSN
 Biomedical application of the supercomputer: targeted delivery of inhaled Pharmaceuticals in diseased lungs T.B. Martonen,* I. Katz,* D. Hwang,' Y.Yang* "Health Effects Research Laboratory, U.S.Environmental
Biomedical application of the supercomputer: targeted delivery of inhaled Pharmaceuticals in diseased lungs T.B. Martonen,* I. Katz,* D. Hwang,' Y.Yang* "Health Effects Research Laboratory, U.S.Environmental
Go With the Flow REGULATORY LANDSCAPE. Mark Copley at Copley Scientific
 Go With the Flow Image: Guzel Studio shutterstock.com Increasing global requirements for efficacious, inexpensive products to treat respiratory illnesses are driving the development of inhaled generics.
Go With the Flow Image: Guzel Studio shutterstock.com Increasing global requirements for efficacious, inexpensive products to treat respiratory illnesses are driving the development of inhaled generics.
CAPSULE-BASED DRY POWDER INHALERS, AN OPTIMAL SOLUTION FOR DIFFERENT INSPIRATIONAL RATES
 xxx Qualicaps CAPSULE-BASED DRY POWDER INHALERS, AN OPTIMAL SOLUTION FOR DIFFERENT INSPIRATIONAL RATES There is a wide range of devices available to deliver inhalation therapies, but there is increasing
xxx Qualicaps CAPSULE-BASED DRY POWDER INHALERS, AN OPTIMAL SOLUTION FOR DIFFERENT INSPIRATIONAL RATES There is a wide range of devices available to deliver inhalation therapies, but there is increasing
Particle Clearance in Human Bronchial Airways: Comparison of Stochastic Model Predictions with Experimental Data
 Ann. occup. Hyg., Vol. 46, Supplement 1, pp. 329 333, 2002 2002 British Occupational Hygiene Society Published by Oxford University Press DOI: 10.1093/annhyg/mef659 Particle Clearance in Human Bronchial
Ann. occup. Hyg., Vol. 46, Supplement 1, pp. 329 333, 2002 2002 British Occupational Hygiene Society Published by Oxford University Press DOI: 10.1093/annhyg/mef659 Particle Clearance in Human Bronchial
CLINICAL RELEVANCE OF IN VITRO PARTICLE SIZING DATA. Steve Newman, PhD Nottingham, UK November 2006
 IPAC-RS Conference 6-8 November 2006 CLINICAL RELEVANCE OF IN VITRO PARTICLE SIZING DATA Steve Newman, PhD Nottingham, UK November 2006 IPAC-RS Conference 6-8 November 2006 1 EFFECT OF PARTICLE SIZE ON
IPAC-RS Conference 6-8 November 2006 CLINICAL RELEVANCE OF IN VITRO PARTICLE SIZING DATA Steve Newman, PhD Nottingham, UK November 2006 IPAC-RS Conference 6-8 November 2006 1 EFFECT OF PARTICLE SIZE ON
Advanced Inhaler Technique. Learning Outcomes. Disclosure 1.1. Belgian Chocolate, French Champagne and Inhaled Medication: Too Good To Waste?
 Advanced Inhaler Technique Learning Outcomes Describe the mechanism of different inhalers Explain how inspiratory flow can effect drug delivery for different inhalers Counsel a patient on the correct use
Advanced Inhaler Technique Learning Outcomes Describe the mechanism of different inhalers Explain how inspiratory flow can effect drug delivery for different inhalers Counsel a patient on the correct use
Caption: The equipment required for testing Fluticasone Propionate (FP) Inhalation Powder in line with a new product-specific monograph (USP36-NF31).
 Product-specific FDA guidance, and product-specific pharmacopeial monographs, point to the use of test equipment, some of which isn t included in the general USP/Ph. Eur. chapters for orally inhaled products
Product-specific FDA guidance, and product-specific pharmacopeial monographs, point to the use of test equipment, some of which isn t included in the general USP/Ph. Eur. chapters for orally inhaled products
DISSOLUTION TESTING - Exploring the Link Between Particle Size & Dissolution Behavior for OINDPs
 Issue: March 2016, Posted Date: 3/1/2016 DISSOLUTION TESTING - Exploring the Link Between Particle Size & Dissolution Behavior for OINDPs INTRODUCTION In the development of orally inhaled and nasal products
Issue: March 2016, Posted Date: 3/1/2016 DISSOLUTION TESTING - Exploring the Link Between Particle Size & Dissolution Behavior for OINDPs INTRODUCTION In the development of orally inhaled and nasal products
Misty Max 10 nebulizer
 AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
Current Challenges and Opportunities in Demonstrating Bioequivalence
 Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
The Pressure Losses in the Model of Human Lungs Michaela Chovancova, Pavel Niedoba
 The Pressure Losses in the Model of Human Lungs Michaela Chovancova, Pavel Niedoba Abstract For the treatment of acute and chronic lung diseases it is preferred to deliver medicaments by inhalation. The
The Pressure Losses in the Model of Human Lungs Michaela Chovancova, Pavel Niedoba Abstract For the treatment of acute and chronic lung diseases it is preferred to deliver medicaments by inhalation. The
Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population
 Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population Contents: Motivation: Why modelling the paediatric dose to lung via in-vitro and in-silico
Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population Contents: Motivation: Why modelling the paediatric dose to lung via in-vitro and in-silico
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES
 COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients
 Thorax 1987;42:457-461 Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma D M MITCHELL, M A SOLOMON, S E J TOLFREE, M SHORT,
Thorax 1987;42:457-461 Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma D M MITCHELL, M A SOLOMON, S E J TOLFREE, M SHORT,
IMPROVING THE REALISM AND RELEVANCE OF MOUTH-THROAT MODELS FOR INHALED PRODUCT TESTING
 IMPROVING THE REALISM AND RELEVANCE OF MOUTH-THROAT MODELS FOR INHALED PRODUCT TESTING In this piece, Mark Copley, Sales Director of Copley Scientific, provides some background on mouth-throat models for
IMPROVING THE REALISM AND RELEVANCE OF MOUTH-THROAT MODELS FOR INHALED PRODUCT TESTING In this piece, Mark Copley, Sales Director of Copley Scientific, provides some background on mouth-throat models for
The FDA Critical Path Initiative
 The FDA Critical Path Initiative Clinical Considerations for Demonstration of Dose-response for Inhaled Corticosteroids - Exhaled Nitric Oxide Model Badrul A. Chowdhury, MD, PhD Director Division of Pulmonary
The FDA Critical Path Initiative Clinical Considerations for Demonstration of Dose-response for Inhaled Corticosteroids - Exhaled Nitric Oxide Model Badrul A. Chowdhury, MD, PhD Director Division of Pulmonary
Challenges in Nonclinical Development of Inhalation Drug Products
 Challenges in Nonclinical Development of Inhalation Drug Products Luqi Pei, Ph.D. Senior Pharmacologist DPARP, CDER August 6, 2015 Rockville, MD Disclaimer This speech reflects the views of the speaker
Challenges in Nonclinical Development of Inhalation Drug Products Luqi Pei, Ph.D. Senior Pharmacologist DPARP, CDER August 6, 2015 Rockville, MD Disclaimer This speech reflects the views of the speaker
What Do Cascade Impaction Measurements Tell Us: In Vitro Aspects. Context of Presentation
 What Do Cascade Impaction Measurements Tell Us: In Vitro Aspects Jolyon P. Mitchell, Ph.D. Trudell Medical International London, Canada IPAC-RS Conference November 2006 1 Context of Presentation This talk
What Do Cascade Impaction Measurements Tell Us: In Vitro Aspects Jolyon P. Mitchell, Ph.D. Trudell Medical International London, Canada IPAC-RS Conference November 2006 1 Context of Presentation This talk
Assessing the role of breathing simulators in OIP testing
 As first AppeAred in Inhalation April 2014 www.inhalationmag.com Assessing the role of breathing simulators in OIP testing Exploring how the application of patient-representative inhalation profiles can
As first AppeAred in Inhalation April 2014 www.inhalationmag.com Assessing the role of breathing simulators in OIP testing Exploring how the application of patient-representative inhalation profiles can
Lung Physiology and How Aerosol Deposits in the Lungs. 1. Physiological and Anatomical Background
 XA0100097 43 Lung Physiology and How Aerosol Deposits in the Lungs Toyoharu Isawa, M.D. 1. Physiological and Anatomical Background Weibel's morphologic data has been referred to not only for predicting
XA0100097 43 Lung Physiology and How Aerosol Deposits in the Lungs Toyoharu Isawa, M.D. 1. Physiological and Anatomical Background Weibel's morphologic data has been referred to not only for predicting
Inhalation Product Research at FDA
 Inhalation Product Research at FDA Changning Guo Ph. D., Chemist Division of Pharmaceutical Analysis FDA/CDER/OPS/OTR 2016 GPhA CMC workshop, May 17, 2016 Disclaimer: This presentation reflects the views
Inhalation Product Research at FDA Changning Guo Ph. D., Chemist Division of Pharmaceutical Analysis FDA/CDER/OPS/OTR 2016 GPhA CMC workshop, May 17, 2016 Disclaimer: This presentation reflects the views
Respiratory Therapy. Medical/Scientific/General Background
 Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
The problem with critical and non-critical inhaler errors
 The problem with critical and non-critical inhaler errors Federico Lavorini MD, PhD Dept. Experimental and Clinical Medicine Careggi University Hospital Florence, Italy Presenter disclosures Federico Lavorini
The problem with critical and non-critical inhaler errors Federico Lavorini MD, PhD Dept. Experimental and Clinical Medicine Careggi University Hospital Florence, Italy Presenter disclosures Federico Lavorini
The optimal particle size for beta-adrenergic aerosols in mild asthmatics*
 The optimal particle size for beta-adrenergic aerosols in mild asthmatics* Introduction The treatment of asthma and chronic obstructive pulmonary diseases (COPD) has improved considerably with the introduction
The optimal particle size for beta-adrenergic aerosols in mild asthmatics* Introduction The treatment of asthma and chronic obstructive pulmonary diseases (COPD) has improved considerably with the introduction
L. Borgström*, E. Bondesson*, F. Morén**, E. Trofast*, S.P. Newman +
 Eur Respir J, 1994, 7, 69 73 DOI: 10.1183/09031936.94.07010069 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1994 European Respiratory Journal ISSN 0903-1936 Lung deposition of budesonide
Eur Respir J, 1994, 7, 69 73 DOI: 10.1183/09031936.94.07010069 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1994 European Respiratory Journal ISSN 0903-1936 Lung deposition of budesonide
What you need to know about inhalers and how to use them Henry Chrystyn PhD, FRPharmS and David Price MA, MRCGP, DRCOG
 What you need to know about inhalers and how to use them Henry Chrystyn PhD, FRPharmS and David Price MA, MRCGP, DRCOG VM The authors describe the problems that arise with metered-dose and dry-powder inhalers,
What you need to know about inhalers and how to use them Henry Chrystyn PhD, FRPharmS and David Price MA, MRCGP, DRCOG VM The authors describe the problems that arise with metered-dose and dry-powder inhalers,
The effect of active and passive smoking on inhaled drugs in respiratory patients
 CHAPTER 12 The effect of active and passive smoking on inhaled drugs in respiratory patients G. Invernizzi*, A. Ruprecht*, P. Paredi #, R. Mazza*, C. De Marco*, R. Boffi* *Tobacco Control Unit, National
CHAPTER 12 The effect of active and passive smoking on inhaled drugs in respiratory patients G. Invernizzi*, A. Ruprecht*, P. Paredi #, R. Mazza*, C. De Marco*, R. Boffi* *Tobacco Control Unit, National
Using an Inhaler and Nebulizer
 Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Patricia KP Burnell Inhalation Product Development
 Patricia KP Burnell Inhalation Product Development Inhaled products: types, development The critical parameters In-vitro testing Ex-vivo testing What dose? Product Development: drug medicine Safety and
Patricia KP Burnell Inhalation Product Development Inhaled products: types, development The critical parameters In-vitro testing Ex-vivo testing What dose? Product Development: drug medicine Safety and
Device Design Similarity
 Device Design Similarity Dave Parkins Director DPI Product Development PQRI Workshop on Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products. Bethesda March 9-10, 2009 Device Similarity
Device Design Similarity Dave Parkins Director DPI Product Development PQRI Workshop on Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products. Bethesda March 9-10, 2009 Device Similarity
Revision 2. Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components
 Revision 2 Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components P. Worth Longest, Ph.D., 1,2* Mandana Azimi, Pharm.D., 2 Laleh Golshahi, Ph.D., 2 and Michael
Revision 2 Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components P. Worth Longest, Ph.D., 1,2* Mandana Azimi, Pharm.D., 2 Laleh Golshahi, Ph.D., 2 and Michael
IVIVC in Pediatric OIPs
 IPAC-RS/UF Orlando Conference 2014 March 20, 2014 IVIVC in Pediatric OIPs Herbert Wachtel Declaration of Conflicts of Interest H. Wachtel is employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Germany.
IPAC-RS/UF Orlando Conference 2014 March 20, 2014 IVIVC in Pediatric OIPs Herbert Wachtel Declaration of Conflicts of Interest H. Wachtel is employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Germany.
The Effects of Simulated Airway Diseases and Affected Flow Distributions on Aerosol Deposition
 The Effects of Simulated Airway Diseases and Affected Flow Distributions on Aerosol Deposition Gabriela Apiou-Sbirlea PhD, Ira M Katz PhD, and Ted B Martonen PhD BACKGROUND: Experimental and theoretical
The Effects of Simulated Airway Diseases and Affected Flow Distributions on Aerosol Deposition Gabriela Apiou-Sbirlea PhD, Ira M Katz PhD, and Ted B Martonen PhD BACKGROUND: Experimental and theoretical
Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi)
 Respiratory Drug Delivery Europe 2017 Nagel and Suggett Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi) Mark W. Nagel and Jason A. Suggett
Respiratory Drug Delivery Europe 2017 Nagel and Suggett Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi) Mark W. Nagel and Jason A. Suggett
Bioequivalence of Inhaled Corticosteroids. -with emphasis on Pharmacokinetic Tools.
 Bioequivalence of Inhaled Corticosteroids -with emphasis on Pharmacokinetic Tools? hochhaus@ufl.edu Topics related to Bioequivalence 10-60 % Deposited in lung Complete absorption from the lung Cl muc Mouth
Bioequivalence of Inhaled Corticosteroids -with emphasis on Pharmacokinetic Tools? hochhaus@ufl.edu Topics related to Bioequivalence 10-60 % Deposited in lung Complete absorption from the lung Cl muc Mouth
Achieving Optimal Particle Size Distribution in Inhalation Therapy
 Achieving Optimal Particle Size Distribution in Inhalation Therapy By Bob Bruno Inhalation therapy has proven to be an effective method of administering a number of pharmaceuticals for more than a century.
Achieving Optimal Particle Size Distribution in Inhalation Therapy By Bob Bruno Inhalation therapy has proven to be an effective method of administering a number of pharmaceuticals for more than a century.
Online supplementary material
 Online supplementary material Add-on long-acting β2-agonist (LABA) in a separate inhaler as asthma step-up therapy versus increased dose of inhaled corticosteroid (ICS) or ICS/LABA combination inhaler
Online supplementary material Add-on long-acting β2-agonist (LABA) in a separate inhaler as asthma step-up therapy versus increased dose of inhaled corticosteroid (ICS) or ICS/LABA combination inhaler
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI)
 I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
Assessing Quality of Inhaled Products And Links to Efficacy and Safety
 Assessing Quality of Inhaled Products And Links to Efficacy and Safety Prasad Peri, PhD ONDQA 2011 IPAC-RS Conference Bringing Value To The Patient In A Changing World March 30, 2011 1 Outline of the Presentation
Assessing Quality of Inhaled Products And Links to Efficacy and Safety Prasad Peri, PhD ONDQA 2011 IPAC-RS Conference Bringing Value To The Patient In A Changing World March 30, 2011 1 Outline of the Presentation
Asthma in Day to Day Practice
 Asthma in Day to Day Practice VIJAY.K.VANAM Financial relationships: Disclosures Employed at Mercy Medical Center, Mason City. Nonfinancial relationships: I receive no financial gain from any pharmaceutical
Asthma in Day to Day Practice VIJAY.K.VANAM Financial relationships: Disclosures Employed at Mercy Medical Center, Mason City. Nonfinancial relationships: I receive no financial gain from any pharmaceutical
Moving to More Realistic In Vitro Testing of OIDPs
 Moving to More Realistic In Vitro Testing of OIDPs IPAC-RS/UF 2014 Conference: Orlando Inhalation Conference - Approaches in International Regulation Renishkumar Delvadia, PhD. Food and Drug Administration
Moving to More Realistic In Vitro Testing of OIDPs IPAC-RS/UF 2014 Conference: Orlando Inhalation Conference - Approaches in International Regulation Renishkumar Delvadia, PhD. Food and Drug Administration
Computational toxicology: an in silico dosimetry model for risk assessment of air pollutants
 Computational toxicology: an in silico dosimetry model for risk assessment of air pollutants T. Martonen 1,2 & K. K. Isaacs 1,3 1 Experimental Toxicology Division, National Health and Environmental Effects
Computational toxicology: an in silico dosimetry model for risk assessment of air pollutants T. Martonen 1,2 & K. K. Isaacs 1,3 1 Experimental Toxicology Division, National Health and Environmental Effects
Everything for Inhalation
 Everything for Inhalation Everything for Inhalation Inhalation drug product development at Hovione has a strong focus on formulation for Dry Powder Inhalers (DPI), particularly for capsule-based and reservoir-based
Everything for Inhalation Everything for Inhalation Inhalation drug product development at Hovione has a strong focus on formulation for Dry Powder Inhalers (DPI), particularly for capsule-based and reservoir-based
Year in review. Vit Perlik Director of Regulatory Science and Clinical Development
 Year in review Vit Perlik Director of Regulatory Science and Clinical Development Content Year in review Covering September 2013 to September 2014 Where the regulation goes selection of events for illustration
Year in review Vit Perlik Director of Regulatory Science and Clinical Development Content Year in review Covering September 2013 to September 2014 Where the regulation goes selection of events for illustration
Higher lung deposition with Respimat Soft Mist Inhaler than HFA-MDI in COPD patients with poor technique
 ORIGINAL RESEARCH Higher lung deposition with Respimat Soft Mist Inhaler than in COPD patients with poor technique Peter Brand 1 Bettina Hederer 2 George Austen 3 Helen Dewberry 3 Thomas Meyer 4 1 RWTH,
ORIGINAL RESEARCH Higher lung deposition with Respimat Soft Mist Inhaler than in COPD patients with poor technique Peter Brand 1 Bettina Hederer 2 George Austen 3 Helen Dewberry 3 Thomas Meyer 4 1 RWTH,
TEPZZ _7584 A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (51) Int Cl.: A61K 9/00 ( ) A61K 31/439 (2006.
 (19) TEPZZ _784 A_T (11) EP 3 17 842 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 07.06.17 Bulletin 17/23 (1) Int Cl.: A61K 9/00 (06.01) A61K 31/439 (06.01) (21) Application number: 1197874.9
(19) TEPZZ _784 A_T (11) EP 3 17 842 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 07.06.17 Bulletin 17/23 (1) Int Cl.: A61K 9/00 (06.01) A61K 31/439 (06.01) (21) Application number: 1197874.9
Optimising the application of in vitro test methods for the demonstration of bioequivalence in orally inhaled products
 Optimising the application of in vitro test methods for the demonstration of bioequivalence in orally inhaled products Mark Copley, Director and Anna Sipitanou, Business Development Manager, Copley Scientific
Optimising the application of in vitro test methods for the demonstration of bioequivalence in orally inhaled products Mark Copley, Director and Anna Sipitanou, Business Development Manager, Copley Scientific
Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing
 INSTRUMENTATION» Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing Mark Copley Director Copley Scientific The recent introduction
INSTRUMENTATION» Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing Mark Copley Director Copley Scientific The recent introduction
Identification of crystalline forms suitable for inhalation in drug discovery
 Identification of crystalline forms suitable for inhalation in drug discovery Valentina Diana Di Lallo Chiesi Farmaceutici - Corporate R&D Preclinical Analytics & Early Formulations Department Crystallization:
Identification of crystalline forms suitable for inhalation in drug discovery Valentina Diana Di Lallo Chiesi Farmaceutici - Corporate R&D Preclinical Analytics & Early Formulations Department Crystallization:
Draft Guidance on Fluticasone Propionate; Salmeterol Xinafoate. Fluticasone Propionate; Salmeterol Xinafoate. Powder/Inhalation
 Reprinted from FDA s website by EAS Consulting Group, LLC Contains Nonbinding Recommendations Draft Guidance on Fluticasone Propionate; Salmeterol Xinafoate This draft guidance, once finalized, will represent
Reprinted from FDA s website by EAS Consulting Group, LLC Contains Nonbinding Recommendations Draft Guidance on Fluticasone Propionate; Salmeterol Xinafoate This draft guidance, once finalized, will represent
Understanding Regulatory Global Requirements for Nasal Drug Products. Julie D. Suman, Ph.D. April 8, 2016
 Understanding Regulatory Global Requirements for Nasal Drug Products Julie D. Suman, Ph.D. April 8, 2016 AGENDA NDA vs ANDA Regulatory Approaches for Bioequivalence (BE) FDA Drug Specific Guidances FDA,
Understanding Regulatory Global Requirements for Nasal Drug Products Julie D. Suman, Ph.D. April 8, 2016 AGENDA NDA vs ANDA Regulatory Approaches for Bioequivalence (BE) FDA Drug Specific Guidances FDA,
An update on inhalation devices
 OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
Controlled Particle Dispersion : Applying Vortical Flow to Optimize Nasal Drug Deposition By: Marc Giroux, Peter Hwang, MD; and Ajay Prasad, PhD
 Controlled Particle Dispersion : Applying Vortical Flow to Optimize Nasal Drug Deposition By: Marc Giroux, Peter Hwang, MD; and Ajay Prasad, PhD Nasal delivery of local and systemic medical therapies is
Controlled Particle Dispersion : Applying Vortical Flow to Optimize Nasal Drug Deposition By: Marc Giroux, Peter Hwang, MD; and Ajay Prasad, PhD Nasal delivery of local and systemic medical therapies is
INTEGRATED DESIGN SPACE TO DEVELOP BETTER DPI FORMULATIONS
 INTEGRATED DESIGN SPACE TO DEVELOP BETTER DPI FORMULATIONS Here, Filipa Maia, PhD, and Maria Palha, MSc, both Scientists at Hovione, report a study whose objective was to establish relationships between
INTEGRATED DESIGN SPACE TO DEVELOP BETTER DPI FORMULATIONS Here, Filipa Maia, PhD, and Maria Palha, MSc, both Scientists at Hovione, report a study whose objective was to establish relationships between
Inh l a t a i tional D i ev ces for the Ou O t u pat p ie i nts Dr Sunil Sharma Senior Resident
 Inhalational ldevices for the Outpatients Dr Sunil Sharma Senior Resident Introduction Inhalational ltherapy allows selective treatment t tof lungs achieving high concentrations in airway minimizing systemic
Inhalational ldevices for the Outpatients Dr Sunil Sharma Senior Resident Introduction Inhalational ltherapy allows selective treatment t tof lungs achieving high concentrations in airway minimizing systemic
Pulmonary Drug Delivery: A Promising Approach
 ISSN: 2231-3354 Received on: 10-04-2012 Revised on: 27-04-2012 Accepted on: 23-05-2012 DOI: 10.7324/JAPS.2012.2632 Pulmonary Drug Delivery: A Promising Approach ND Shah, VV Shah and ND Chivate ABSTRACT
ISSN: 2231-3354 Received on: 10-04-2012 Revised on: 27-04-2012 Accepted on: 23-05-2012 DOI: 10.7324/JAPS.2012.2632 Pulmonary Drug Delivery: A Promising Approach ND Shah, VV Shah and ND Chivate ABSTRACT
IPAC-RS/UF Orlando Inhalation Conference March 20, S.T. Horhota 1, C.B. Verkleij 2, P.J.G. Cornelissen 2, L. Bour 3, A. Sharma 3, M.
 IPAC-RS/UF Orlando Inhalation Conference March 20, 2014 Case Study: Pharmacokinetics and Pharmacodynamics of Tiotropium and Salmeterol Following Parallel Administration in COPD Patients Using Different
IPAC-RS/UF Orlando Inhalation Conference March 20, 2014 Case Study: Pharmacokinetics and Pharmacodynamics of Tiotropium and Salmeterol Following Parallel Administration in COPD Patients Using Different
Modelling Jet Nebulizers to Estimate Pulmonary Drug Deposition
 Modelling Jet Nebulizers to Estimate Pulmonary Drug Deposition by Wallace Bo-Neng Wee A thesis submitted in conformity with the requirements for the degree of Masters of Health Science Clinical Engineering,
Modelling Jet Nebulizers to Estimate Pulmonary Drug Deposition by Wallace Bo-Neng Wee A thesis submitted in conformity with the requirements for the degree of Masters of Health Science Clinical Engineering,
Respiratory System. Student Learning Objectives:
 Respiratory System Student Learning Objectives: Identify the primary structures of the respiratory system. Identify the major air volumes associated with ventilation. Structures to be studied: Respiratory
Respiratory System Student Learning Objectives: Identify the primary structures of the respiratory system. Identify the major air volumes associated with ventilation. Structures to be studied: Respiratory
Latex Free. An affordable, easy to use, high density, small volume nebulizer with a breath enhanced design! Breath Enhanced High Density Jet Nebulizer
 Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Anatomy & Physiology 2 Canale. Respiratory System: Exchange of Gases
 Anatomy & Physiology 2 Canale Respiratory System: Exchange of Gases Why is it so hard to hold your breath for Discuss! : ) a long time? Every year carbon monoxide poisoning kills 500 people and sends another
Anatomy & Physiology 2 Canale Respiratory System: Exchange of Gases Why is it so hard to hold your breath for Discuss! : ) a long time? Every year carbon monoxide poisoning kills 500 people and sends another
Effects of viscosity, pump mechanism and nozzle geometry on nasal spray droplet size
 ILASS Europe 21, 23rd Annual Conference on Liquid Atomization and Spray Systems, Brno, Czech Republic, September 21 P. Kippax 1, J. Suman 2, A. Virden 1* and G. Williams 3 1 Malvern Instruments, Grovewood
ILASS Europe 21, 23rd Annual Conference on Liquid Atomization and Spray Systems, Brno, Czech Republic, September 21 P. Kippax 1, J. Suman 2, A. Virden 1* and G. Williams 3 1 Malvern Instruments, Grovewood
Respiratory System. Organization of the Respiratory System
 Respiratory System In addition to the provision of oxygen and elimination of carbon dioxide, the respiratory system serves other functions, as listed in (Table 15 1). Respiration has two quite different
Respiratory System In addition to the provision of oxygen and elimination of carbon dioxide, the respiratory system serves other functions, as listed in (Table 15 1). Respiration has two quite different
INTRODUCTION. size and total nozzle area decrease with stage number. Volumetric air flow rate through
 CASCADE I M P A C T I O N Optimizing Cascade Impactor Testing for Characterizing Orally Inhaled & Nasal Drug Products By: Mark Copley INTRODUCTION Cascade impaction is a core analytical technique for characterizing
CASCADE I M P A C T I O N Optimizing Cascade Impactor Testing for Characterizing Orally Inhaled & Nasal Drug Products By: Mark Copley INTRODUCTION Cascade impaction is a core analytical technique for characterizing
OF COATING MATERIAL ON THE AERODYNAMIC PARTICLE SIZE DISTRIBUTION (PSD) OF OXIS TURBOHALER USING MIXING INLET WITH AN ANDERSEN CASCADE IMPACTOR (ACI)
 165 J App Pharm 2(3): 165-178 (2011) Khan et al., 2011 EFFECT OF COATING MATERIAL ON THE AERODYNAMIC PARTICLE SIZE DISTRIBUTION (PSD) OF OXIS TURBOHALER USING MIXING INLET WITH AN ANDERSEN CASCADE IMPACTOR
165 J App Pharm 2(3): 165-178 (2011) Khan et al., 2011 EFFECT OF COATING MATERIAL ON THE AERODYNAMIC PARTICLE SIZE DISTRIBUTION (PSD) OF OXIS TURBOHALER USING MIXING INLET WITH AN ANDERSEN CASCADE IMPACTOR
University of Groningen. Optimisation of dry powder inhalation Boer, Anne Haaije de
 University of Groningen Optimisation of dry powder inhalation Boer, Anne Haaije de IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Optimisation of dry powder inhalation Boer, Anne Haaije de IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask
 Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask Abstract This study s objective was to model the Amsino OneMask Oxygen Mask using Computational Fluid Dynamics (CFD). A three-dimensional
Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask Abstract This study s objective was to model the Amsino OneMask Oxygen Mask using Computational Fluid Dynamics (CFD). A three-dimensional
INTRODUCTION. Asthma Drug Delivery - 1
 Asthma Drug Delivery Laboratory Experiment Developed by: Alex Jannini, David Krause, Heather Malino and Kevin Sweeney, Rowan University, Department of Chemical Engineering Edited by: C. Stewart Slater
Asthma Drug Delivery Laboratory Experiment Developed by: Alex Jannini, David Krause, Heather Malino and Kevin Sweeney, Rowan University, Department of Chemical Engineering Edited by: C. Stewart Slater
EPAG Sponsored Workshop on Abbreviated Impactor Measurement (AIM) and Efficient Data Analysis (EDA) Concepts in Inhaler Testing. Overview of AIM EDA
 EPAG Sponsored Workshop on Abbreviated Impactor Measurement (AIM) and Efficient Data Analysis (EDA) Concepts in Inhaler Testing Overview of AIM EDA Jolyon Mitchell 1 and Mark Copley 2 1 Trudell Medical
EPAG Sponsored Workshop on Abbreviated Impactor Measurement (AIM) and Efficient Data Analysis (EDA) Concepts in Inhaler Testing Overview of AIM EDA Jolyon Mitchell 1 and Mark Copley 2 1 Trudell Medical
Luchtvervuiling en gezondheid: de mechanistische inzichten
 SYMPOSIUM Luchtvervuiling en gezondheid: de mechanistische inzichten Dr. Ir. Frans de Jongh Longfysioloog Medisch Spectrum Twente, Enschede, the Netherlands Academic Medical Centre, Amsterdam, the Netherlands
SYMPOSIUM Luchtvervuiling en gezondheid: de mechanistische inzichten Dr. Ir. Frans de Jongh Longfysioloog Medisch Spectrum Twente, Enschede, the Netherlands Academic Medical Centre, Amsterdam, the Netherlands
Important Principles of Aerosol Therapy for Your Patients
 Important Principles of Aerosol Therapy for Your Patients Disclaimer Appendix 3 Declaration of Vested Interest Form Name of presenter: David Henry RRT Name of employer: DeVilbiss Healthcare Inc. Definition:
Important Principles of Aerosol Therapy for Your Patients Disclaimer Appendix 3 Declaration of Vested Interest Form Name of presenter: David Henry RRT Name of employer: DeVilbiss Healthcare Inc. Definition:
PARTICLE DEPOSITION IN THE LUNG
 300 PARTICLE DEPOSITION IN THE LUNG See also: Bronchiectasis. Bronchiolitis. Chemokines, CXC: IL-8. Defensins. Interstitial Lung Disease: Cryptogenic Organizing Pneumonia. Further Reading Epler GR and
300 PARTICLE DEPOSITION IN THE LUNG See also: Bronchiectasis. Bronchiolitis. Chemokines, CXC: IL-8. Defensins. Interstitial Lung Disease: Cryptogenic Organizing Pneumonia. Further Reading Epler GR and
In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers
 In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers Ariel Berlinski MD BACKGROUND: Patients with cystic fibrosis perform airway clearance techniques and receive nebulized
In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers Ariel Berlinski MD BACKGROUND: Patients with cystic fibrosis perform airway clearance techniques and receive nebulized
? Pulmonary Respiratory System
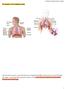 The Structure of The Respiratory System The respiratory system is composed of groups of organelles that filters and transports air into the lungs. The organs comprising of the respiratory system include
The Structure of The Respiratory System The respiratory system is composed of groups of organelles that filters and transports air into the lungs. The organs comprising of the respiratory system include
Tracking X-ray microscopy for alveolar dynamics in live intact mice
 SUPPLEMENTARY INFORMATION Tracking X-ray microscopy for alveolar dynamics in live intact mice Soeun Chang, Namseop Kwon, Byung Mook Weon, Jinkyung Kim, Chin Kook Rhee, Han Sung Choi, Yoshiki Kohmura, Masaki
SUPPLEMENTARY INFORMATION Tracking X-ray microscopy for alveolar dynamics in live intact mice Soeun Chang, Namseop Kwon, Byung Mook Weon, Jinkyung Kim, Chin Kook Rhee, Han Sung Choi, Yoshiki Kohmura, Masaki
The pulmonary route is gaining increasing
 ELECTRONICALLY REPRINTED FROM FEBRUARY 2014 Pulmonary Drug Delivery Particle Engineering for Inhaled Therapeutics Moderated by Adeline Siew, PhD Industry experts discuss the various factors affecting drug
ELECTRONICALLY REPRINTED FROM FEBRUARY 2014 Pulmonary Drug Delivery Particle Engineering for Inhaled Therapeutics Moderated by Adeline Siew, PhD Industry experts discuss the various factors affecting drug
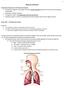
Chapter 10 Respiration
 1 Chapter 10 Respiration Introduction/Importance of the Respiratory System All eukaryotic organisms need oxygen to perform cellular respiration (production of ATP), either aerobically or anaerobically.
1 Chapter 10 Respiration Introduction/Importance of the Respiratory System All eukaryotic organisms need oxygen to perform cellular respiration (production of ATP), either aerobically or anaerobically.
Q. What are metered-dose inhalers? A. These are devices that dispense medicines directly into the lungs, in the form of a mist or aerosol in a
 1 2 Q. What are metered-dose inhalers? A. These are devices that dispense medicines directly into the lungs, in the form of a mist or aerosol in a specific dosage. In an MDI, the medicine is suspended
1 2 Q. What are metered-dose inhalers? A. These are devices that dispense medicines directly into the lungs, in the form of a mist or aerosol in a specific dosage. In an MDI, the medicine is suspended
Computer Modelling as a Tool in Characterization and Optimization of Aerosol Drug Delivery
 Aerosol and Air Quality Research, 15: 2466 2474, 2015 Copyright Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2015.03.0144 Computer Modelling as a Tool
Aerosol and Air Quality Research, 15: 2466 2474, 2015 Copyright Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2015.03.0144 Computer Modelling as a Tool
Respiratory System. Chapter 9
 Respiratory System Chapter 9 Air Intake Air in the atmosphere is mostly Nitrogen (78%) Only ~21% oxygen Carbon dioxide is less than 0.04% Air Intake Oxygen is required for Aerobic Cellular Respiration
Respiratory System Chapter 9 Air Intake Air in the atmosphere is mostly Nitrogen (78%) Only ~21% oxygen Carbon dioxide is less than 0.04% Air Intake Oxygen is required for Aerobic Cellular Respiration
Particle Size and Dust Inhalation
 Pneumoconiosis A disease of the lungs characterized by fibrosis and caused by the chronic inhalation of mineral dusts, especially silica and asbestos. Helen Lang Dept. Geology & Geography West Virginia
Pneumoconiosis A disease of the lungs characterized by fibrosis and caused by the chronic inhalation of mineral dusts, especially silica and asbestos. Helen Lang Dept. Geology & Geography West Virginia
Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults
 CLENIL MODULITE Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults Corticosteroids for the treatment of chronic asthma in adults and children aged 12
CLENIL MODULITE Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults Corticosteroids for the treatment of chronic asthma in adults and children aged 12
