Modelling Jet Nebulizers to Estimate Pulmonary Drug Deposition
|
|
|
- Marylou Holmes
- 5 years ago
- Views:
Transcription
1 Modelling Jet Nebulizers to Estimate Pulmonary Drug Deposition by Wallace Bo-Neng Wee A thesis submitted in conformity with the requirements for the degree of Masters of Health Science Clinical Engineering, Institute of Biomaterials and Biomedical Engineering University of Toronto Copyright by Wallace Wee 010
2 ii Modelling Jet Nebulizers to Estimate Pulmonary Drug Deposition Abstract Wallace Wee Masters of Health Science Clinical Engineering, Institute of Biomaterials and Biomedical Engineering University of Toronto 010 Administration of medication directly to diseased lungs reduces adverse systemic side effects. For cystic fibrosis, jet nebulizers are the standard aerosol delivery system since they can aerosolize drugs that require relatively large volumes of liquid. Selection of the appropriate nebulizer for a given drug is crucial to ensure delivery of the therapeutic dose. This selection, ideally, requires knowledge of the pulmonary drug deposition (PDD). The gold standard for accurately measuring PDD is nuclear medicine techniques, which exposes the subject to radiation and therefore cannot be used repeatedly to test multiple devices. An alternative is to characterize the nebulizer using in vitro experiments and estimate the device s in vivo performance. However these techniques are time-consuming and can only collect data for one breathing pattern and drug-device combination. Therefore this study is to formulate mathematical models for jet nebulizers that can estimate PDD based on the drug-device combination and patient s breathing patterns.
3 iii Acknowledgments I am deeply grateful to Dr. Allan Coates for his guidance, patience and encouragement throughout this project. His insightful advice and support has made my experience in aerosol science eye-opening and exciting, and has played a substantial part in my development as a medical researcher. I am very appreciative to Kitty Leung for teaching me the lab techniques necessary to becoming an aerosol scientist and having the serenity and fortitude to explain these complex concepts. I would like to thank my thesis committee Prof. Tom Chau, Dr. Joseph Fisher and Dr. James Duffin for offering their insight and advice during the project. I am also very thankful to my family for their inspiration, motivation and continual support.
4 iv Table of Contents Acknowledgments...iii Table of Contents... iv List of Tables... vi List of Figures...vii List of Appendices... x List of Abbreviations... xi Chapter 1 Overview Introduction... 1 Background Patient Respiration Particle Distributions Particle Distributions Inertial Impaction Gravitational Sedimentation Diffusion Jet Nebulizers Conventional Unvented Jet Nebulizer Breath-Enhanced Jet Nebulizer Breath-Actuated Jet Nebulizer Chapter Predicting Inhaled Mass Materials and Methods Mathematical Modelling Experimental Setup Experimental Procedure... 3
5 v Steady State Conditions Dynamic Conditions Experimental Results... 5 Chapter 3 Predicting Pulmonary Drug Deposition Materials and Methods Mathematical Modelling Inhaled Mass Model Pulmonary Drug Deposition Model Experimental Setup Experimental Procedure Steady State Conditions Experimental Results Chapter 4 Discussions In Vitro Results: Predicting Inhaled Mass In Vivo Results: Predicting Pulmonary Drug Deposition Chapter 5 Conclusions References Appendix A Inhaled Mass Model Derivation... 53
6 vi List of Tables Table 1: Coefficients of the Quadratic Equations (y = a + b x c x ) for the Rate of Output and Regression Coefficient (r), where x is the entrained flow through the devices and n is the number of devices characterized Table : Coefficient of Variation of breath-enhanced nebulizers... 8 Table 3: Breath-Actuated AeroEclipse II in vitro data Aerosol collected on the inspiratory filter compared to the model s predicted inhaled mass. The standard patient breathing pattern is V T =0.6 L, Ti/Te = 0.4/0.6 and 15 BPM Table 4: Subject breathing patterns and physical characteristics.... 4
7 vii List of Figures Figure1: Poisson distribution... 7 Figure : Lognormal distribution... 8 Figure 3: Deposition Processes... 9 Figure 4: Inertial Impaction Figure 5: Gravitational Sedimentation... 1 Figure 6: Unvented Jet Nebulizer Figure 7: Bernoulli Effect Figure 8: Breath-enhanced Jet Nebulizer (LC Star) Figure 9: Breath-actuated Jet Nebulizer (AeroEclipse) Figure 10: Previously reported characterization curves for various nebulizers Figure 11: Equating flows... 0 Figure 1: Scenarios... 1 Figure 13: Dynamic conditions setup... 5 Figure 14: Sample characterization curves... 7 Figure 15: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Stars with varying tidal volumes and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red... 9 Figure 16: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Stars with varying
8 viii duty cycles and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red... 9 Figure 17: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Stars with varying respiration rates and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red Figure 18: Bland and Altman limits of agreement plot of the difference between the drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Stars with varying parameters (tidal volume, duty cycle and respiration rates) and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red Figure 19: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Pluses with varying tidal volumes and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red Figure 0: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Pluses with varying duty cycles and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red Figure 1: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Pluses with varying respiration rates and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red... 3 Figure : Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Pluses with varying all parameters (tidal volume, duty cycle and respiration rates) and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red... 3
9 ix Figure 3: Nebulizer characterization curves with RF for the Breath-Enhanced (A) LC Star and (B) LC Plus Figure 4: Visual representation of drug delivered to the patient (white) after eliminating aerosol trapped in the dead space (black) Figure 5: Particle size distribution measurement setup using the Malvern Mastersizer X Figure 6: Bland and Altman limits of agreement plot of the difference between the in vivo inhaled mass data and the model s predicted inhaled mass for normal subjects. Bias represented in blue and 95% Confidence Interval is in red Figure 7: Bland and Altman limits of agreement plot of the difference between the in vivo PDD data and the model s predicted PDD for normal subjects. Bias represented in blue and 95% Confidence Interval is in red Figure 8: Bland and Altman limits of agreement plot of the difference between the in vivo inhaled mass data and the model s predicted inhaled mass for CF patients. Bias represented in blue and 95% Confidence Interval is in red Figure 9: Bland and Altman limits of agreement plot of the difference between the in vivo PDD data and the model s predicted PDD for CF patients. Bias represented in blue and 95% Confidence Interval is in red
10 x List of Appendices Appendix A: Inhaled Mass Model Derivation
11 xi List of Abbreviations BPM: Breathes Per Minute DPI: Dry Powder Inhalers LPM: Liters Per Minute MDI: Metered Dose Inhalers MMAD: Mass Median Aerodynamic Diameter MMD: Mass Median Diameter PDD: Pulmonary Drug Deposition RF: Respirable Fraction UV: Ultraviolet
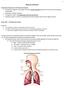
12 1 Chapter 1 Overview 1 Introduction Lung diseases, like asthma and cystic fibrosis, are conditions that can affect the patient s ability to breathe, exchange gases and/or become prone to lung infections. Therefore, where possible, it makes sense to deliver medications directly to the lungs in the treatment of respiratory diseases, as this will minimize systemic exposure and adverse side effects 3. The ideal situation would be to achieve successful delivery, which by definition, is noninvasively overcoming the protective mechanisms and barriers of the airway for distal lung deposition 4. The anatomical barriers that may need to be overcome include the nose, posterior pharynx and the various airway bifurcations. As a result, successful treatment can be accomplished by generating an aerosol that can provide sufficient drug deposition but also bypass anatomical barriers. While smaller aerosol particles are less likely to be trapped by the airway defence mechanisms, the tradeoff is that these small particles would carry little drug since the particle volume is proportional to radius cubed (volume α r 3 ) 3. For the clinician to ensure that the patient is receiving adequate medication they require information about the aerosol particle size and the drug administration route (orally or nasally) 5. The particle size of most medical aerosols follow a Poisson distribution and can be described by the mass median diameter. Aerosols inhaled orally encounter different anatomical structures as opposed to those administered nasally. For normal adults, aerosols inhaled through the nose
13 experience greater turbulent flow at the level of nasal turbinates which filters the particle size significantly in comparison to oral administration where particles greater than 5 µm are filtered, usually at the posterior pharynx and vocal cords. The respirable fraction (RF) is defined as the fraction deposited in the lungs in relation to the total amount that entered the airway opening. Aerosol devices like the metered dose inhalers (MDI) and dry powder inhalers (DPI) are devices widely used in asthma management. However these devices are highly dependent on the properties of the drug being aerosolized and are limited to high potency medications such as powders, suspensions and solutions. Specifically, high potency for asthma medication refers to drugs active in the microgram range. For inhaled drugs such as antibiotics which are only effective in the milligram range, these convenient and efficient delivery systems are less optimal. The jet nebulizer, on the other hand, is the standard aerosol delivery system for cystic fibrosis since it is able to aerosolize drugs like antibiotics and mucolytic agents 6;7. There are many classes of jet nebulizers that range from unvented to breath-enhanced to breath-actuated. Briefly, the unvented nebulizer generates aerosols based solely on the compressor, independent of the patient s breathing pattern. More efficient nebulizers, like the breath-enhanced and breath-actuated devices, are able to minimize the amount of drug generated and lost during exhalation 7. While many of these devices may offer similar outputs, selecting the appropriate nebulizer for a given drug is crucial to ensure that the patient receives the therapeutic dose. Unlike intravenous administration, where the clinician knows the exact dose administered to the
14 3 patient, aerosol medication delivery varies markedly. Although higher technology devices approach 30% efficiency, the old unvented ones have typical values hovering around 5% 8;9. In infant patients using a facemask the nebulizer efficacy drops even more to about 1% 10. This implies that without proper selection of the device and drug combination the delivery could be less than the therapeutic range or in the worst case cause toxicity in the patient. Another reason to select the appropriate nebulizer for a given drug is that many of the drugs are expensive, a factor that favours efficient delivery. The selection of a suitable nebulizer should, ideally, be based on measurements of pulmonary deposition. However, this is difficult for three reasons. First, there is a lack of characterization data for a given device and agent combination. Secondly, nebulizer performance data by most manufacturers are determined using a saline solution. For aqueous based drug solutions this does not pose a significant problem for the particle size distribution, however this still does not provide an accurate indication of the drug output because the output is often highly dependent on the physical properties (viscosity and surface tension) of the agent being nebulized 11. Lastly, the sheer number of nebulizers on the market from unvented to breath-enhanced to breath-actuated and the variations in efficiency within each class of nebulizers makes it hard to systematically compare devices 6. The current gold standard for accurately measuring pulmonary drug deposition is to use nuclear medicine techniques. However this technique is not only time consuming but also faces a number of challenges affecting its accuracy 1;13. These include the selection of an appropriate
15 4 radioactive label, accommodation of different tissue attenuations and D images to infer deposition in a 3D structure. On top of this, the nuclear medicine technique is only able to collect data on one patient with a given device and drug combination at a time and at the expense of exposing the patient to radiation. This also becomes more complicated when dealing with tests on pediatric patients. Therefore nuclear medicine techniques are not conducive for evaluating multiple nebulizers for use in patients groups who vary widely in size. An alternative method is to characterize jet nebulizers using in vitro experiments and then estimate the in vivo performance of the device 14. These in vitro experiments can be grouped into two phases. In the first phase the device is evaluated with a known drug to determine the output particle size distribution at a given entrained flow. The second phase incorporates the dynamic situation with a patient breath simulator, typically using a double half sinusoidal breathing pattern 15. Other equipment utilized for these experiments include the electronic balance, osmometer, ultraviolet (UV) spectrophotometer and laser diffraction particle sizer. Unfortunately, while the in vitro methodology provides a method of evaluating the device it is also time-consuming and again is only able to collect data for one breathing pattern, device and drug combination. A better solution for evaluating and comparing different nebulizers is to use a mathematical model that is built upon the patient s breathing pattern, nebulizer and drug combination. Although not entirely independent of in vitro experiments, the mathematical model will only require the steady state information collected in the first phase of the in vitro
16 5 experiments, which would reduce experimental time. Moreover the model is more flexible, providing the clinician with the ability to alter any parameter, allowing them to observe trends, by providing more than 1 data point. Lastly the model can incorporate additional concepts like the respirable fraction, which is lacking in the in vitro experiments, thus giving more accurate data for comparison. As a result the focus of this thesis project is to formulate a mathematical model to bridge the gap presented by the in vivo and in vitro experiments. This model will provide the means to effectively determine the amount of pulmonary drug deposition in a patient by taking into account the nebulizer and drug combination and the patient s breathing pattern. The validity of the model will be established from data gathered in both normal adults (Chapter ) and cystic fibrosis patients (Chapter 3), pediatric and adult, where both pattern of breathing through a nebulizer and drug deposition is known. Background The goal of aerosol treatment is to successfully delivery aerosolized medication directly to the lungs. To accomplish this requires an understanding of aerosol physics, pulmonary physiology and technologies. The main concepts that will be touched upon in this section include patient patterns of breathing, particle size distribution, deposition processes and nebulizer performance.
17 6.1 Patient Respiration When a normal individual breathes, air flows in and out of the lungs because of the pressure differential between the atmosphere and the lungs. The pressure gradient is achieved either by expanding the lungs during inspiration creating a drop in pressure in the lungs causing air to flow inwards or vice-versa by contracting the lungs during expiration causing air to flow outwards. When air travels to the alveoli, during inspiration, it experiences different resistances and obstructions as it travels through various cavities and airway tracts, which help to filter unwanted particulate matter. For example air inhaled through the nose experiences greater flow resistance and turbulence as opposed to air being inhaled through the mouth.. Particle Distributions Previously it has been indirectly mentioned that the successful delivery of medical aerosols is in part dependent on the particle size. This is because the size determines how much drug is carried and whether the particle will deposit in the lungs. Generally aerosols can be classified as either monodisperse (particles having all the same size) or polydisperse (aerosol particles with varying sizes). In practice most medical aerosols are polydisperse and follow the Poisson distribution, shown in figure 1, which relates particle size with the particle number of an aerosol. The geometric standard deviation (σ g ) measures the extent of how polydisperse the Poisson distribution is, where a larger σ g implies greater polydispersion.
18 7 Figure1: Poisson distribution The Poisson distribution, however, does not provide the full picture because small particles carry little drug. Recall that the particle s volume is proportional to the 3 rd power of the radius (r 3 ). Therefore the convention is to express the particle size distribution on a logarithmic scale of the diameter. Most medical aerosols fit this scale to generate a lognormal distribution, shown in figure.
19 8 Figure : Lognormal distribution Additionally, these distributions can be described using a number of different methods. Two useful statistical values include the mass median diameter (MMD) and mass median aerodynamic diameter (MMAD). The MMD by definition is the diameter at which one half of the total mass of spherical particles of unit density is attributed to particles larger than the MMD. The MMAD is similar to the MMD but is used to describe aerosol particles of non-spherical and/or different densities. By definition the aerodynamic diameter is the diameter of a unitdensity sphere having the same terminal settling velocity as the particle in question. From past research studies the ideal aerosol particle size ranges for pulmonary deposition in normal adults (inhaled orally) is from 1 to 5 µm, but if administered nasally this range decreases. In addition, there is evidence that the smaller the size of the patient (i.e. pediatric
20 9 patients) the particle size required for deposition drops even more. The respirable fraction accounts for the discrepancy between the nebulizer drug output and the amount that deposits in the lungs is the concept. By multiplying the respirable fraction by the inhaled mass, one can more accurately determine the amount of drug delivered. The inhaled mass, by definition, is the amount of aerosol delivered to the mouth of the patient. For in vitro experiments the inhaled mass corresponds to the mass of aerosol collected on the inspiratory filter, which is the mouth of a mechanical breath simulator..3 Particle Distributions Aerosolized medication delivered to the patient is deposited in the lungs by one of three processes; inertial impaction, sedimentation and diffusion (shown in figure 3). Figure 3: Deposition Processes 1
21 Inertial Impaction Large aerosol particles (greater than a few micrometers in diameter) have increased inertia and as a result are less able to follow a column of air as it turns 1. Another way to describe this process is that these particles cannot navigate around sharp corners (like the airway bifurcation) and will continue along their original path, illustrated in figure 4. Since inertia is defined as the resistance of an object to a change in its state of motion, inertial impaction occurs when the particle fails to make a turn and exits the column of air carrying it into the lungs. Inertial impaction against baffles within a nebulizer removes particles too big for lung deposition and determines the particle size distribution of the device output. Figure 4: Inertial Impaction [
22 11 Three factors that influence a particle s probability of undergoing inertial impaction are the particle size, flow speed and aerosol path. The first two factors can be thought of as follows, the larger or faster a particle is the more time it requires to negotiate around a turn, which increases the likelihood of inertial impaction. Since the particle is moving at the same speed as the column of air, in which it is suspended, speed and flow are closely linked. The last factor is the path that the aerosol takes to reach the lungs. The reason for this is that depending on where the aerosol travels it will encounter a number of different anatomical barriers. A prime example is the administration of an aerosol nasally or orally. The anatomical structures of the nose increase flow resistance and turbulence, which traps more drug in the nasal pharynx before it can enter the lungs. This is because one of the functional purposes of the nose is to remove particulate matter from inhaled air before it can deposit in the lungs and it does this job well, preventing many infections..3. Gravitational Sedimentation In gravitational sedimentation the aerosol particles begin to rain out, due to gravity, and deposit themselves in the lungs, illustrated in figure 5. This deposition process is time-dependent and therefore is proportional to the time the drug spends in the lungs. In other words the longer the drug has to dwell in the lungs the greater the amount of deposition. This follows that the amount of aerosol deposition increases with slow breathing and breath holding because the aerosolized medication has more time to travel along the respirable tract and sediment. The
23 1 typical range of particle size diameters that experience sedimentation are from 1 to 5µm. Particles 1µm have a high surface area to weight ratio and remain suspended for longer periods than their larger sibling. This time of suspension can be such that they are exhaled before deposition..3.3 Diffusion Figure 5: Gravitational Sedimentation [ Diffusion is the primary deposition mechanism for small aerosol particles (diameters less than 1 µm). For this deposition process, the particles can be modeled as small spheres that follow the kinetic theory of gases and the concept of Brownian motion. This is where the particles move about randomly, colliding with other gas molecules until they eventual impact with the airways of the lungs. The main intermolecular force that contributes to diffusion is electrostatic. As in the case with gravitational sedimentation, the rate of diffusion deposition is inversely proportional the patient s breathing rate, specifically the inspiratory time (T i ). The slower the patient breathes the more time these small particles have to randomly hit the walls of the lungs. However the amount of drug carried by these tiny particles is so small (since the
24 13 amount of drug carried is proportional to r 3 ) that diffusion plays a minor role in drug delivery. The exception is in the field of air pollution with very potent agents like diesel particulate matter..4 Jet Nebulizers The current nebulizers on the market utilize three aerosol generation techniques; ultrasound, jet nebulization and vibrating membrane. This section will focus on the jet nebulizer as it is the basis for the models. Within this nebulizer class there are significant differences in the aerosol particle size distribution, cost and design of the different jet nebulizer types (i.e. unvented vs. breath enhanced vs. breath actuated)..4.1 Conventional Unvented Jet Nebulizer Conventional unvented jet nebulizers are inexpensive and disposable. These devices generate aerosols by passing compressed gas through a small orifice above the drug solution, as shown in figure 6. Passing a gas through a small hole creates a high velocity jet, and based on the Bernoulli Effect (figure 7), will cause a drop in pressure. This pressure differential draws the drug solution up adjacent capillary tubes towards the jet stream. When the drug reaches the jet stream it is sheared into an aerosol and travels toward the outlet of the device. A baffle system located above the orifice filters the particulates to generate a specific particle size distribution. Larger aerosols that are unable to navigate through the baffles will impact these baffles and fall
25 14 back down into the original drug solution. In addition, since the only air entering the nebulizing chamber comes from the compressor, nothing the patient does influences output. Low Pressure Figure 6: Unvented Jet Nebulizer ρ = density υ = velocity z = change in height p = pressure g = gravity Figure 7: Bernoulli Effect The above equation of the Bernoulli Effect explains the process in which the drug solution is pulled towards the jet stream. Although the equation is comprised of multiple parameters, for simplicity, it can be assumed that ρgz is equal to 0 since the change in height of
26 15 the jet stream is insignificant in comparison to the gas velocity. As a result, the velocity and pressure are inversely proportional..4. Breath-Enhanced Jet Nebulizer More sophisticated jet nebulizers are the breath-enhanced and breath-actuated nebulizers. These nebulizers are more efficient because they are able to minimize the amount of drug expelled during exhalation. The breath-enhanced nebulizer generates aerosols in a manner similar to the conventional unvented nebulizer. However the output of the device can be broken down into two stages based on the patient s inspiration and expiration phases. A schematic of a breath-enhanced nebulizer in operation is shown in figure 8. Inspiration Expiration Figure 8: Breath-enhanced Jet Nebulizer (LC Star)
27 16 From the above figure, it can be seen that the aerosol is continually generated by a high velocity jet that shears the medication into particulates. During inspiration, the nebulizer s vent (entrained flow valve) opens, allowing entrained air to enter the nebulizer and carry aerosolized medication around the baffles to the patient. During expiration, the vent closes as the expiratory valve opens. This allows the patient s expired air to escape to the atmosphere instead of entering the nebulizer. This stops the expired air from carrying drug out of the vent thereby reducing drug loss since most of the drug generated during expiration either impacts on the baffles or rains out back into the nebulizer reservoir. Relating this back to modelling the devices, the amount of aerosol generated during the patient s expiratory phase is minute in comparison to the dose delivered during inspiration and therefore can be considered negligible..4.3 Breath-Actuated Jet Nebulizer While the breath-actuated nebulizer follows the same principles in generating the aerosol, this device performs slightly better than the breath-enhanced nebulizer by generating medication only during inspiration 6. A schematic of the AeroEclipse II breath-actuated jet nebulizer is shown in figure 9.
28 Figure 9: Breath-actuated Jet Nebulizer (AeroEclipse) 17
29 18 Chapter Predicting Inhaled Mass 1 Materials and Methods 1.1 Mathematical Modelling The output of breath-enhanced and breath-actuated devices is dependent on the patient s respiration cycle (breathing pattern) and drug-device combination. The breathing pattern can be described using the patient s flow, V pt(t), which is divided into two phases; inspiratory and expiratory. Inspiration is the phase of interest since the device is delivering aerosol to the patient. This phase can be modeled as a half sinusoidal function, shown below: VTωi V ' pt( t) = sin ( ω t),[l/min] i ω = i Π T i,[rad/min] Where V T is the tidal volume in liters, ω i is the frequency of inspiration in radians per second, t is time in seconds, T i is the total inspiration time, T e is the total expiration time and T tot
30 19 is the sum of inspiration and expiration. For patients breathing from nebulizers the typical breathing pattern has a T i /T tot around The characterization of the drug-device combination is based on the jet nebulizer s rate of output versus entrained flow. This relationship can be modeled by a quadratic formula shown below. Examples of characterization curves are shown in figure 10. O' tot ( V ' ent( t) ) = a+ bv ' ent( t) cv ' ent( t),[mg/min] Where O tot is the rate of output in milligram per second, V ent is the entrained flow in liters per minute and coefficients a [mg/min], b [mg/l], c [mg x min / L ] characterize the drug-device combination. Figure 10: Previously reported characterization curves for various nebulizers 6 The operation of the nebulizer (illustrated in figure 11) needs to be incorporated to relate the rate of output to the patient s breathing pattern. Since the device has negligible height and air is a Newtonian fluid, one can assume the summation of flows into and out of the device is approximately 0 liters per minutes (lpm). This is described in the following equation:
31 0 ( t) = V ' ( t) V ' ent( t),[l/min] V ' pt N + Where V N is the nebulizer flow (compressor flow) in lpm. Entrained Flow Patient Flow Compressor Flow Figure 11: Equating flows An added complexity accounted in the model is the time when the inspiratory valve opens. For the breath-enhanced nebulizers this occurs when the patient flow is equal to the compressor flow. For the breath-actuated nebulizers is when the inspiratory effort reaches the manufacturer s specified flow. In general, this creates three operation scenarios; beginning of inspiration, inspiration and end of inspiration (as illustrated in figure 1).
32 1 Beginning of Inspiration Inspiration End of Inspiration 0 t 1 t Figure 1: Scenarios The total output of the devices can be derived for one inspiration phase based on the above equations. The detailed derivation can be found in Appendix A. Breath-Enhanced Nebulizers Otot = V T a V ' N V V ω t t T T 1 ( 1 cos( ωt )) + A( t t ) B [ cos( ωt ) cos( ωt )] + C,[mg/breath] Breath-Actuated Nebulizers ( ωt ) sin( ωt ) BVT CVT ω t t1 sin 1 Otot= A( t t1) [ cos( ωt ) cos( ωt1) ] +,[mg/breath] 4 4 ω
33 1. Experimental Setup Three reusable jet-nebulizer types were chosen for this project and are as follows: Breath-enhanced Pari LC Star (Pari Respiratory Equipment) Breath-enhanced Pari LC Plus (Pari Respiratory Equipment) Breath-actuated AeroEclipse II (Trudell Medical International) The number of devices evaluated varied for each nebulizer type. Nebulizers were tested on their performance under steady-state and dynamic conditions. In each experiment the nebulizers were driven by compressors at the manufacturer s recommended flows. The breath-enhanced nebulizers used the Pari Proneb Ultra compressor (flow of 4.1 L/min) and the breath-actuated device was driven by the Invacare Mobilaire (flow of 7.1 L/min). Flows were measured and verified at the start of each experiment using a flowcalibration instrument (TSI) placed at the mouth piece of the device. Compressors were utilized over the hospital dry gas source in order to mimic the home environment, reduce evaporative losses 4 and generate enhanced output with correct particle sizes. The active ingredient Salbutamol (Ventolin Respirator Solution) was selected for the in vitro experiments. The reasoning for this is that Salbutamol is water soluble and undergoes almost identical nebulization characteristics to other water soluble drugs currently in use or being investigated for treatment of cystic fibrosis such as tobramycin, hypertonic saline, denufosol. In addition, this drug solution is not only less costly compared to tobramycin but also contains a chromophore which lends itself to ultraviolet (UV) spectroscopy, which is used for measuring concentration.
34 3 1.3 Experimental Procedure Steady State Conditions As mentioned above the jet nebulizer s rate of output with respect to entrained flow can be modeled using a quadratic formula. These characterization curves were obtained by in vitro testing of each device under steady state conditions with varying entrained flows (ranging from 0 to 35 L/min). For each experiment the nebulizers were vertically clamped. The device output was determined after 4 minutes (for the breath-enhanced nebulizers LC Star and LC Plus) and.5 minutes (for the breath-actuated device AeroEclipse II) of nebulization. The reason for the different runtimes is that the AeroEclipse II, when operated at high entrained flows, reached end-nebulization around 3 minutes. Therefore to keep the initial dose and fill volume the same, the runtimes were reduced. Furthermore a set duration was selected, as opposed to letting the device run till end nebulization, in order to calculate rate of output. The output of the device was determined by gavimetrically weighing the device dry, after filling, and after nebulization. The residual volume (V r ) was measured, which is defined as the change in device weight before and after nebulization. To account for evaporative effects and the resulting change in concentration of the drug solution, osmolality was obtained before prenebulization and after post-nebulization weighing. In each case a 0 microliter sample was assayed for osmolality by vapour pressure osmometry (Advanced Micro-Osmometer 330). With the device s change in weight and osmolality, the total drug output can be determined. The nebulizer output was calculated based on the initial dose (initial fill volume multiplied by the drug concentration) minus the mass of the drug left in the well of the nebulizer
35 4 at the end of nebulization (residual volume multiplied by the initial concentration) multiplied by the ratio of final-to-initial osmolality, to take into account evaporative effects. Subsequently the rate of output is calculated to be the total output divided by the runtime. Otot f ( V ' ent( t) ) = D V C,[mg] i r i osm osm i Otot O ' tot = ( V ' ent( t) ),[mg/min] t run Where D i is the initial dose in mg, V r is the residual volume in milliliters, C i is the initial drug concentration in milligrams per milliliter, osm is the osmolalilty in milliosmols and t run is the runtime. The initial dose of salbutamol was 4 mg at a concentration of 0.65 mg/ml Dynamic Conditions To determine how the nebulizers would perform during a patient s respiration cycle, each device was tested under dynamic conditions. These conditions tested the device s performance under an idealized patient respiration cycle with varying duty cycles, volumes and breathing frequencies. The nebulizer was connected to a T-connector attached to an inspiratory filter and an expiratory filter with a unidirectional valve. The mouth of the above setup was connected to the Harvard pump. This pump is a Harvard Model 613 Volume-controlled Large Animal Ventilator (Harvard Apparatus Canada, US) capable of generating half sine-wave patterns of breathing. The overall setup is shown in figure 13.
36 5 After each 4 minute run was performed, aerosol generated was trapped on the inspiratory filter, expiratory filter and connector. The total drug output was then determined as the amount of drug left in the nebulizer and trapped in the filters/connectors. The concentration of drug collected on the filters and connector was determined from UV spectroscopy at an absorbance wavelength of 8 nm. The data provided information about the total device output and inhaled mass (amount of drug on inspiratory filter). In addition the respirable mass was determined based on the drug collected on the inspiratory filter multiplied by the respirable fraction. 1-way Filters Nebulizer Compressor Breathing Simulator Connectors Figure 13: Dynamic conditions setup 1.4 Experimental Results The characterization of the jet nebulizers is crucial in obtaining the performance coefficients for the model. The quadratic fits used to model the output rate of the device show a high correlation (r > 0.95) with the in vitro steady state performance data, as shown in figure 14 and in table 1. In addition comparison of the steady state output rates for breath-enhanced devices of the same type using the coefficients of variation showed similar performance (table ). The next step is to evaluate the models prediction with dynamic in vitro data. The models for the LC Star and LC Plus were tested with breathing patterns that varied the tidal volume, duty cycle and respiration rate. The standard breathing pattern is a tidal volume of 0.6 L, duty cycle of 40/60 and respiration rate of 15 BPM. The above parameters were varied
37 6 independently to observe how the model accommodates varying breathing patterns. Tidal volume variations included 0. L, 0.4 L and 0.6 L. Duty cycle was tested at 40/60 and 50/50. Previous studies suggest that patient breathing patterns have a 40/60 duty cycle, whereas the European standard uses a 50/50 duty cycle. The respiration rates tested included 15 BPM and 30 BPM. This range was chosen to observe how the increased respiration rates affect the maximum flows into the device and the subsequent drug output to the patient. Figures 16 to 18 are the Bland and Altman limits of agreement between the dynamic in vitro inhaled mass of the LC Stars with the model s predicted inhaled mass using device specific coefficients. These experiments were tested on 4 devices, with each device experimented 3 times. All figures show biases around zero with tight 95% confidence intervals. Figure 18 (which is the consolidation of data points for all parameter variations) illustrates the strong agreement between the model and the in vitro data. Similarly for the LC Plus, figures 19 to are the Bland and Altman limits of agreement between the dynamic in vitro inhaled mass of the LC Pluses with the model s predicted inhaled mass using device specific coefficients. These experiments were tested on 3 devices, with each device experimented 3 times. All figures show biases around zero and tight 95% confidence intervals, with the exception of figure 0 due to the short range of x-axis values. Overall when all data points are consolidated onto the same Bland and Altman plot (figure ), it demonstrates the strong agreement between the model and in vitro data. Due to a lack of in vivo data for the AeroEclipse II, the model for this device was only tested on the standard breathing pattern (tidal volume of 0.6 L, duty cycle of 40/60 and respiration rate of 15 BPM).
38 7 A Output Rate [mg/min] LC Star A Characterization Curve y = x x R = Entrained Flow [L/min] B AeroEclipse II Characterization Curve Output Rate [mg/min] y = x x R = Entrained Flow [L/min] Figure 14: Sample characterization curves (A) breath-enhanced nebulizer (B) breath-actuated nebulizer
39 8 Table 1: Coefficients of the Quadratic Equations (y = a + b x c x ) for the Rate of Output and Regression Coefficient (r), where x is the entrained flow through the devices and n is the number of devices characterized. n a b c r Pari LC Star 4 9.e e -.3e Pari LC Plus e e e AeroEclipse II 1.69e e -.98e Table : Coefficient of Variation of breath-enhanced nebulizers Type n Coefficient of Variation [%] at Varying Entrained Flows [lpm] Pari LC Star Pari LC Plus
40 9 LC Star Inhaled Mass for Varying Tidal Volumes with 4 minute Runtime Inhaled Mass - Model [mg] Average Inhaled Mass [mg] Figure 15: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Stars with varying tidal volumes and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red LC Star Inhaled Mass for Varying Duty Cycle with 4 minute Runtime Inhaled Mass - Model [mg] Average Inhaled Mass [mg] Figure 16: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Stars with varying duty cycles and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red.
41 30 LC Star Inhaled Mass for Varying BPM with 4 minute Runtime 0.0 Inhaled Mass - Model [mg] Average Inhaled Mass [mg] Figure 17: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Stars with varying respiration rates and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red LC Star Inhaled Mass for Varying Parameters with 4 minute Runtime Inhaled Mass - Model [mg] Average Inhaled Mass [mg] Figure 18: Bland and Altman limits of agreement plot of the difference between the drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Stars with varying parameters (tidal volume, duty cycle and respiration rates) and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red.
42 31 LC Plus Inhaled Mass for Varying Tidal Volumes with 4 minute Runtime 0.10 Inhaled Mass - Model [mg] Average Inhaled Mass [mg] Figure 19: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Pluses with varying tidal volumes and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red. LC Plus Inhaled Mass with Varying Duty Cycle with 4 minute Runtime 0.06 Total Inhaled Mass - Model [mg] Average Inhaled Mass [mg] Figure 0: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Pluses with varying duty cycles and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red.
43 3 LC Plus Inhaled Mass for Varying BPM with 4 minute Runtime 0.04 Inhaled Mass - Model [mg] Average Inhaled Mass [mg] Figure 1: Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Pluses with varying respiration rates and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red. LC Plus Inhaled Mass for Varying Parameters with 4 minute Runtime Inhaled Mass - Model [mg] Average Inhaled Mass [mg] Figure : Bland and Altman limits of agreement plot of the difference between drug collected on the inspiratory filter from in vitro experiments of the Breath-Enhanced LC Pluses with varying all parameters (tidal volume, duty cycle and respiration rates) and the model s predicted output using the device-drug specific characterization coefficients. Bias represented in blue and 95% Confidence Interval is in red.
44 33 Table 3: Breath-Actuated AeroEclipse II in vitro data Aerosol collected on the inspiratory filter compared to the model s predicted inhaled mass. The standard patient breathing pattern is V T =0.6 L, Ti/Te = 0.4/0.6 and 15 BPM Device Breathing Pattern Inhaled Mass [mg] Error [%] In Vitro Model A Standard C Standard
45 34 Chapter 3 Predicting Pulmonary Drug Deposition 1 Materials and Methods 1.1 Mathematical Modelling The mathematical models derived in the previous chapter provided the foundation to predict the inhaled mass (the amount of aerosol delivered to the mouth of the patient) generated by jet nebulizers. This model was validated using the in vitro experiments that tested the jet nebulizer s output during dynamic conditions. The next step is to test the model against in vivo nuclear medicine studies on a wide range of patients, from normal to cystic fibrosis adults. The in vivo data set was conducted on breathenhanced jet nebulizers and therefore the following sections will focus on the derivation of breath-enhanced models Inhaled Mass Model Please refer to Chapter Section.1 for the derivation of this model Pulmonary Drug Deposition Model
46 35 The inhaled mass model provided the means to predict the amount of aerosol delivered to the mouth of the patient. Enhancing this model to estimate PDD requires integrating the RF, patient s dead space, nebulizer output cut-off and the plateau effect. For the model to predict the PDD, it is necessary to include the respirable fraction (RF), the fraction of aerosol particles 5 µm in diameter. This cutoff diameter was chosen as these particles are likely to deposit in the central region of the lungs for adult patients 4. The respirable fraction varies with respect to the entrained flow (V ent ) through the device. Therefore to integrate the RF, the fraction of particles 5 µm in diameter was multiplied against the nebulizer s output rate at each level of entrained flow. The resulting RF characterization curves are shown in the figure 31 below.
47 36 A LC Star W-1 Characterization y = x x R = Output Rate [mg/min] y = x x R = Entrained Flow [L/min] B LC Plus A Characterization y = x x R = Output Rate [mg/min] y = x x R = Entrained Flow [L/min] Figure 3: Nebulizer characterization curves with RF for the Breath-Enhanced (A) LC Star and (B) LC Plus. The model was further enhanced by incorporating the patient s dead space, the portion of the patient s airway where inhaled aerosol is immediately exhaled before impaction can occur. Previous studies have suggested that for normal patient s dead space can be approximated as. ml of volume per kg of weight 7. In addition, the amount of aerosol that is trapped in the dead space occurs during the end of inspiration.
48 37 Calculating the amount of aerosol caught in the patient s dead space, requires the time during inspiratory phase when the dead space volume is being filled. The mathematical derivation is shown below: V deadspace t = V ' pt( t) dt = t i t i t VTωi sin ( ω t) dt,[l/min] i cos -1 V t= deadspace VT + ω i cos( ωiti),[min] After solving for the time, the amount of aerosol in the dead space can be calculated and subtracted from the predicted amount. A visual representation of this is shown in figure 3, below. 0.5 Nebulizer Output During Inspiration Aerosol caught in dead space 0. Output [mg/min] Aerosol delivered to patient Time [min] Figure 4: Visual representation of drug delivered to the patient (white) after eliminating aerosol trapped in the dead space (black).
8/13/11. RSPT 1410 Humidity & Aerosol Therapy Part 3. Humidification Equipment. Aerosol Therapy
 1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
Misty Max 10 nebulizer
 AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask
 Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask Abstract This study s objective was to model the Amsino OneMask Oxygen Mask using Computational Fluid Dynamics (CFD). A three-dimensional
Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask Abstract This study s objective was to model the Amsino OneMask Oxygen Mask using Computational Fluid Dynamics (CFD). A three-dimensional
Latex Free. An affordable, easy to use, high density, small volume nebulizer with a breath enhanced design! Breath Enhanced High Density Jet Nebulizer
 Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Understanding cascade impaction and its importance for inhaler testing
 Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
Respiratory Therapy. Medical/Scientific/General Background
 Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
Deposition of Inhaled Particle in the Human Lung for Different Age Groups
 Deposition of Inhaled Particle in the Human Lung for Different Age Groups Xilong Guo 1, Qihong Deng 1* 1 Central South University (CSU), Changsha, China * Corresponding email: qhdeng@csu.edu.cn, qhdeng@gmail.com.
Deposition of Inhaled Particle in the Human Lung for Different Age Groups Xilong Guo 1, Qihong Deng 1* 1 Central South University (CSU), Changsha, China * Corresponding email: qhdeng@csu.edu.cn, qhdeng@gmail.com.
Citation for published version (APA): Westerman, E. M. (2009). Studies on antibiotic aerosols for inhalation in cystic fibrosis s.n.
 University of Groningen Studies on antibiotic aerosols for inhalation in cystic fibrosis Westerman, Elisabeth Mechteld IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
University of Groningen Studies on antibiotic aerosols for inhalation in cystic fibrosis Westerman, Elisabeth Mechteld IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
Pediatrics in mechanical ventilation
 Pediatrics Optimization Intitulé du cours of aerosol therapy in mechanical ventilation Thèmes donnés Ermindo Di Paolo, PhD Departments of Pharmacy and Pediatrics Lausanne University Hospital Switzerland
Pediatrics Optimization Intitulé du cours of aerosol therapy in mechanical ventilation Thèmes donnés Ermindo Di Paolo, PhD Departments of Pharmacy and Pediatrics Lausanne University Hospital Switzerland
A PRACTICAL GUIDE TO nebulization
 A PRACTICAL GUIDE TO nebulization therapy a initiative This book is based on sources believed to be reliable in providing information that is complete and generally in accordance with the standards accepted
A PRACTICAL GUIDE TO nebulization therapy a initiative This book is based on sources believed to be reliable in providing information that is complete and generally in accordance with the standards accepted
21/03/2011 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY. Fundamentals of aerosols
 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
Redefining Continuous Aerosol Drug Delivery 2015 PM223
 Redefining Continuous Aerosol Drug Delivery 2015 PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize
Redefining Continuous Aerosol Drug Delivery 2015 PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize
Redefining Continuous Aerosol Drug Delivery PM223
 Redefining Continuous Aerosol Drug Delivery PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize medication
Redefining Continuous Aerosol Drug Delivery PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize medication
Latex Free. An affordable, easy to use, high density, small volume nebulizer with a revolutionary breath enhanced design!
 Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a revolutionary breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients
Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a revolutionary breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients
University of Groningen. Technology in practice Lexmond, Anne
 University of Groningen Technology in practice Lexmond, Anne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Technology in practice Lexmond, Anne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Anatomy & Physiology 2 Canale. Respiratory System: Exchange of Gases
 Anatomy & Physiology 2 Canale Respiratory System: Exchange of Gases Why is it so hard to hold your breath for Discuss! : ) a long time? Every year carbon monoxide poisoning kills 500 people and sends another
Anatomy & Physiology 2 Canale Respiratory System: Exchange of Gases Why is it so hard to hold your breath for Discuss! : ) a long time? Every year carbon monoxide poisoning kills 500 people and sends another
Chapter 10. The Respiratory System Exchange of Gases. Copyright 2009 Pearson Education, Inc.
 Chapter 10 The Respiratory System Exchange of Gases http://www.encognitive.com/images/respiratory-system.jpg Human Respiratory System UPPER RESPIRATORY TRACT LOWER RESPIRATORY TRACT Nose Passageway for
Chapter 10 The Respiratory System Exchange of Gases http://www.encognitive.com/images/respiratory-system.jpg Human Respiratory System UPPER RESPIRATORY TRACT LOWER RESPIRATORY TRACT Nose Passageway for
Chapter 10 The Respiratory System
 Chapter 10 The Respiratory System Biology 2201 Why do we breathe? Cells carry out the reactions of cellular respiration in order to produce ATP. ATP is used by the cells for energy. All organisms need
Chapter 10 The Respiratory System Biology 2201 Why do we breathe? Cells carry out the reactions of cellular respiration in order to produce ATP. ATP is used by the cells for energy. All organisms need
Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing
 INSTRUMENTATION» Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing Mark Copley Director Copley Scientific The recent introduction
INSTRUMENTATION» Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing Mark Copley Director Copley Scientific The recent introduction
Recommendations for Aerosol Applications of Silicone-Based Materials
 Recommendations for Aerosol Applications of Silicone-Based Materials September 2001 Revised March 2018 This document provides information and recommendations relevant to formulating aerosol products containing
Recommendations for Aerosol Applications of Silicone-Based Materials September 2001 Revised March 2018 This document provides information and recommendations relevant to formulating aerosol products containing
Achieving Optimal Particle Size Distribution in Inhalation Therapy
 Achieving Optimal Particle Size Distribution in Inhalation Therapy By Bob Bruno Inhalation therapy has proven to be an effective method of administering a number of pharmaceuticals for more than a century.
Achieving Optimal Particle Size Distribution in Inhalation Therapy By Bob Bruno Inhalation therapy has proven to be an effective method of administering a number of pharmaceuticals for more than a century.
CHAPTER 7.1 STRUCTURES OF THE RESPIRATORY SYSTEM
 CHAPTER 7.1 STRUCTURES OF THE RESPIRATORY SYSTEM Pages 244-247 DO NOW What structures, do you think, are active participating in the breathing process? 2 WHAT ARE WE DOING IN TODAY S CLASS Finishing Digestion
CHAPTER 7.1 STRUCTURES OF THE RESPIRATORY SYSTEM Pages 244-247 DO NOW What structures, do you think, are active participating in the breathing process? 2 WHAT ARE WE DOING IN TODAY S CLASS Finishing Digestion
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
The Pressure Losses in the Model of Human Lungs Michaela Chovancova, Pavel Niedoba
 The Pressure Losses in the Model of Human Lungs Michaela Chovancova, Pavel Niedoba Abstract For the treatment of acute and chronic lung diseases it is preferred to deliver medicaments by inhalation. The
The Pressure Losses in the Model of Human Lungs Michaela Chovancova, Pavel Niedoba Abstract For the treatment of acute and chronic lung diseases it is preferred to deliver medicaments by inhalation. The
Effects of viscosity, pump mechanism and nozzle geometry on nasal spray droplet size
 ILASS Europe 21, 23rd Annual Conference on Liquid Atomization and Spray Systems, Brno, Czech Republic, September 21 P. Kippax 1, J. Suman 2, A. Virden 1* and G. Williams 3 1 Malvern Instruments, Grovewood
ILASS Europe 21, 23rd Annual Conference on Liquid Atomization and Spray Systems, Brno, Czech Republic, September 21 P. Kippax 1, J. Suman 2, A. Virden 1* and G. Williams 3 1 Malvern Instruments, Grovewood
Respiratory System. Student Learning Objectives:
 Respiratory System Student Learning Objectives: Identify the primary structures of the respiratory system. Identify the major air volumes associated with ventilation. Structures to be studied: Respiratory
Respiratory System Student Learning Objectives: Identify the primary structures of the respiratory system. Identify the major air volumes associated with ventilation. Structures to be studied: Respiratory
Appendix M: Device Technique
 Nursing Care of Dyspnea: The 6th Vital Sign in Individuals with Chronic Obstructive Pulmonary Disease (COPD) Appendix M: Device Technique Medications: Inhalation Devices Medications come in many forms.
Nursing Care of Dyspnea: The 6th Vital Sign in Individuals with Chronic Obstructive Pulmonary Disease (COPD) Appendix M: Device Technique Medications: Inhalation Devices Medications come in many forms.
Nebulizers. Small Volume Nebulizers
 Small Volume MICRO MIST Nebulizer The MICRO MIST small volume nebulizer is designed for performance and economy and includes the design features needed by today s respiratory care practitioners. It can
Small Volume MICRO MIST Nebulizer The MICRO MIST small volume nebulizer is designed for performance and economy and includes the design features needed by today s respiratory care practitioners. It can
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS
 NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
Invacare Aerosol Products. Stratos Aerosol Compressors Nebulizers Asthma Management
 Invacare Aerosol Products Stratos Aerosol Compressors Nebulizers Asthma Management Invacare Aerosol Products Invacare is proud to offer an updated aerosol product line that includes the Stratos Compact,
Invacare Aerosol Products Stratos Aerosol Compressors Nebulizers Asthma Management Invacare Aerosol Products Invacare is proud to offer an updated aerosol product line that includes the Stratos Compact,
Pulmonary deposition of inhaled drugs
 Pulmonary deposition of inhaled drugs Federico Lavorini Dept. Experimental and Clinical Medicine Careggi University Hospital Florence - Italy Presenter Disclosures F.L. has received in the last 5 years
Pulmonary deposition of inhaled drugs Federico Lavorini Dept. Experimental and Clinical Medicine Careggi University Hospital Florence - Italy Presenter Disclosures F.L. has received in the last 5 years
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES
 COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
Respiratory Physiology In-Lab Guide
 Respiratory Physiology In-Lab Guide Read Me Study Guide Check Your Knowledge, before the Practical: 1. Understand the relationship between volume and pressure. Understand the three respiratory pressures
Respiratory Physiology In-Lab Guide Read Me Study Guide Check Your Knowledge, before the Practical: 1. Understand the relationship between volume and pressure. Understand the three respiratory pressures
15 cm Reservoir Tubing. 210 cm Tubing
 12 Aerosol Therapy nebulisers AEROSOL THERAPY MICRO MIST SMALL VOLUME NEBULISER The MICRO MIST small volume nebuliser is designed for performance and economy and can be used for hand-held or in-line treatments.
12 Aerosol Therapy nebulisers AEROSOL THERAPY MICRO MIST SMALL VOLUME NEBULISER The MICRO MIST small volume nebuliser is designed for performance and economy and can be used for hand-held or in-line treatments.
Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study
 Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study Robert E Van Dyke MSc and Kurt Nikander BACKGROUND: Iloprost (Ventavis)
Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study Robert E Van Dyke MSc and Kurt Nikander BACKGROUND: Iloprost (Ventavis)
Chapter 10 Respiration
 1 Chapter 10 Respiration Introduction/Importance of the Respiratory System All eukaryotic organisms need oxygen to perform cellular respiration (production of ATP), either aerobically or anaerobically.
1 Chapter 10 Respiration Introduction/Importance of the Respiratory System All eukaryotic organisms need oxygen to perform cellular respiration (production of ATP), either aerobically or anaerobically.
OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER
 OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER US FDA guidance for the in vitro demonstration of bioequivalence in a generic nebuliser directly references
OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER US FDA guidance for the in vitro demonstration of bioequivalence in a generic nebuliser directly references
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI)
 I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
Inhalation von Radionukliden physikalische und biologische Mechanismen
 Inhalation von Radionukliden physikalische und biologische Mechanismen Werner Hofmann Abteilung für Physik und Biophysik, Fachbereich Materialforschung und Physik, Universität Salzburg 1 LUNG DOSIMETRY
Inhalation von Radionukliden physikalische und biologische Mechanismen Werner Hofmann Abteilung für Physik und Biophysik, Fachbereich Materialforschung und Physik, Universität Salzburg 1 LUNG DOSIMETRY
Anaesthetic and respiratory equipment Nebulizing systems and components
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 27427 Third edition 2013-12-15 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d anesthésie et de réanimation
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 27427 Third edition 2013-12-15 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d anesthésie et de réanimation
Analysis of Lung Function
 Computer 21 Spirometry is a valuable tool for analyzing the flow rate of air passing into and out of the lungs. Flow rates vary over the course of a respiratory cycle (a single inspiration followed by
Computer 21 Spirometry is a valuable tool for analyzing the flow rate of air passing into and out of the lungs. Flow rates vary over the course of a respiratory cycle (a single inspiration followed by
Unit 5 Humidity/Aerosol Generators
 5-1 Unit 5 Humidity/Aerosol Generators GOAL On completion of this unit, the student should have an understanding of the principles of operation and proper use of different humidity/aerosol generators.
5-1 Unit 5 Humidity/Aerosol Generators GOAL On completion of this unit, the student should have an understanding of the principles of operation and proper use of different humidity/aerosol generators.
MEDIUM-FLOW PNEUMOTACH TRANSDUCER
 MEDIUM-FLOW PNEUMOTACH TRANSDUCER SS11LA for MP3x and MP45 System TSD117 & TSD117-MRI for MP150/MP100 System RX117 Replacement Airflow Head See also: AFT series of accessories for airflow and gas analysis
MEDIUM-FLOW PNEUMOTACH TRANSDUCER SS11LA for MP3x and MP45 System TSD117 & TSD117-MRI for MP150/MP100 System RX117 Replacement Airflow Head See also: AFT series of accessories for airflow and gas analysis
NebuTech nebulizer from Salter. Striving to be #1 IN PATIENT SATISFACTION. To help you and your patients. easy. breathe
 nebulizer from Salter To help you and your patients breathe easy Striving to be #1 IN PATIENT SATISFACTION nebulizer from Salter Let go to work for you and your patients today. Performance Versatility
nebulizer from Salter To help you and your patients breathe easy Striving to be #1 IN PATIENT SATISFACTION nebulizer from Salter Let go to work for you and your patients today. Performance Versatility
B Unit III Notes 6, 7 and 8
 The Respiratory System Why do we breathe? B. 2201 Unit III Notes 6, 7 and 8 Respiratory System We know that our cells respire to produce ATP (energy). All organisms need energy to live, so that s why we
The Respiratory System Why do we breathe? B. 2201 Unit III Notes 6, 7 and 8 Respiratory System We know that our cells respire to produce ATP (energy). All organisms need energy to live, so that s why we
In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers
 In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers Ariel Berlinski MD BACKGROUND: Patients with cystic fibrosis perform airway clearance techniques and receive nebulized
In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers Ariel Berlinski MD BACKGROUND: Patients with cystic fibrosis perform airway clearance techniques and receive nebulized
Respiratory System. Organization of the Respiratory System
 Respiratory System In addition to the provision of oxygen and elimination of carbon dioxide, the respiratory system serves other functions, as listed in (Table 15 1). Respiration has two quite different
Respiratory System In addition to the provision of oxygen and elimination of carbon dioxide, the respiratory system serves other functions, as listed in (Table 15 1). Respiration has two quite different
Respiratory Care in PICU Aerosol Therapy ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า
 Respiratory Care in PICU Aerosol Therapy น.อ.หญ ง ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า Aerosol liquid droplets or solid particles suspended in a gas(air) visible like fog Aerosol vs Humidity Humidity
Respiratory Care in PICU Aerosol Therapy น.อ.หญ ง ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า Aerosol liquid droplets or solid particles suspended in a gas(air) visible like fog Aerosol vs Humidity Humidity
RESPIRATORY PHYSIOLOGY Pre-Lab Guide
 RESPIRATORY PHYSIOLOGY Pre-Lab Guide NOTE: A very useful Study Guide! This Pre-lab guide takes you through the important concepts that where discussed in the lab videos. There will be some conceptual questions
RESPIRATORY PHYSIOLOGY Pre-Lab Guide NOTE: A very useful Study Guide! This Pre-lab guide takes you through the important concepts that where discussed in the lab videos. There will be some conceptual questions
Comparison of patient spirometry and ventilator spirometry
 GE Healthcare Comparison of patient spirometry and ventilator spirometry Test results are based on the Master s thesis, Comparison between patient spirometry and ventilator spirometry by Saana Jenu, 2011
GE Healthcare Comparison of patient spirometry and ventilator spirometry Test results are based on the Master s thesis, Comparison between patient spirometry and ventilator spirometry by Saana Jenu, 2011
Rush University, College of Health Sciences
 Rush University, College of Health Sciences An Evaluation of the Prototype OxyMulti Mask Prototype Compared to the Oxymask Aerosol, OxyMulti Mask and Airlife Aerosol Mask for Aerosol Delivery in Adults
Rush University, College of Health Sciences An Evaluation of the Prototype OxyMulti Mask Prototype Compared to the Oxymask Aerosol, OxyMulti Mask and Airlife Aerosol Mask for Aerosol Delivery in Adults
Spirometry and Flow Volume Measurements
 Spirometry and Flow Volume Measurements Standards & Guidelines December 1998 To serve the public and guide the medical profession Revision Dates: December 1998 Approval Date: June 1998 Originating Committee:
Spirometry and Flow Volume Measurements Standards & Guidelines December 1998 To serve the public and guide the medical profession Revision Dates: December 1998 Approval Date: June 1998 Originating Committee:
Important Principles of Aerosol Therapy for Your Patients
 Important Principles of Aerosol Therapy for Your Patients Disclaimer Appendix 3 Declaration of Vested Interest Form Name of presenter: David Henry RRT Name of employer: DeVilbiss Healthcare Inc. Definition:
Important Principles of Aerosol Therapy for Your Patients Disclaimer Appendix 3 Declaration of Vested Interest Form Name of presenter: David Henry RRT Name of employer: DeVilbiss Healthcare Inc. Definition:
1. When a patient fails to ventilate or oxygenate adequately, the problem is caused by pathophysiological factors such as hyperventilation.
 Chapter 1: Principles of Mechanical Ventilation TRUE/FALSE 1. When a patient fails to ventilate or oxygenate adequately, the problem is caused by pathophysiological factors such as hyperventilation. F
Chapter 1: Principles of Mechanical Ventilation TRUE/FALSE 1. When a patient fails to ventilate or oxygenate adequately, the problem is caused by pathophysiological factors such as hyperventilation. F
Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience
 Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience 8th Annual North Regional Respiratory Care Conference Minnesota & Wisconsin Societies for Respiratory Care Mayo Civic
Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience 8th Annual North Regional Respiratory Care Conference Minnesota & Wisconsin Societies for Respiratory Care Mayo Civic
This letter is to notify you of recent changes to the PULMOZYME product monograph. Please note, additions/changes have been highlighted in yellow.
 April 9, 2015 Subject: Revised Product Monograph - PULMOZYME (dornase alfa) Dear Drug Information / Poison Control Centres, This letter is to notify you of recent changes to the PULMOZYME product monograph.
April 9, 2015 Subject: Revised Product Monograph - PULMOZYME (dornase alfa) Dear Drug Information / Poison Control Centres, This letter is to notify you of recent changes to the PULMOZYME product monograph.
Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung
 Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung Nia Stevens 9 th November 2016 Thanks to Richard Kaye, James Mitchell, Dave Prime at GSK Bahman Asgharian and Owen
Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung Nia Stevens 9 th November 2016 Thanks to Richard Kaye, James Mitchell, Dave Prime at GSK Bahman Asgharian and Owen
Respiratory Drug Delivery. Be inspired. Nebulizer and compressor catalog
 Respiratory Drug Delivery Be inspired Nebulizer and compressor catalog Introduction Inspiring innovation Philips is committed to enhancing patients lives by delivering cost-effective solutions that drive
Respiratory Drug Delivery Be inspired Nebulizer and compressor catalog Introduction Inspiring innovation Philips is committed to enhancing patients lives by delivering cost-effective solutions that drive
Revision 2. Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components
 Revision 2 Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components P. Worth Longest, Ph.D., 1,2* Mandana Azimi, Pharm.D., 2 Laleh Golshahi, Ph.D., 2 and Michael
Revision 2 Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components P. Worth Longest, Ph.D., 1,2* Mandana Azimi, Pharm.D., 2 Laleh Golshahi, Ph.D., 2 and Michael
Transactions on Biomedicine and Health vol 2, 1995 WIT Press, ISSN
 Biomedical application of the supercomputer: targeted delivery of inhaled Pharmaceuticals in diseased lungs T.B. Martonen,* I. Katz,* D. Hwang,' Y.Yang* "Health Effects Research Laboratory, U.S.Environmental
Biomedical application of the supercomputer: targeted delivery of inhaled Pharmaceuticals in diseased lungs T.B. Martonen,* I. Katz,* D. Hwang,' Y.Yang* "Health Effects Research Laboratory, U.S.Environmental
2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus
 2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus Patient Perspective Alpha-1 1 Foundation John W. Walsh Patient Focus Personal perspective Challenges and lessons Importance
2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus Patient Perspective Alpha-1 1 Foundation John W. Walsh Patient Focus Personal perspective Challenges and lessons Importance
Pharmacokinetics I. Dr. M.Mothilal Assistant professor
 Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
NIV and Aerosoltherapy
 NIV and Aerosoltherapy Workshop Jean-Bernard Michotte (Lausanne-CH) Simone Gambazza (Milano-IT) Jean-Bernard Michotte Haute Ecole de Santé Vaud, 1011 Lausanne - Suisse Cliniques Universitaires Saint-Luc,
NIV and Aerosoltherapy Workshop Jean-Bernard Michotte (Lausanne-CH) Simone Gambazza (Milano-IT) Jean-Bernard Michotte Haute Ecole de Santé Vaud, 1011 Lausanne - Suisse Cliniques Universitaires Saint-Luc,
Spray Nebulizer Deposition Efficiency as a Function of Age. University of Denver Denver, CO Denver, CO
 ILASS Americas, 23 rd Annual Conference on Liquid Atomization and Spray Systems, Ventura, CA, May 2011 Spray Nebulizer Deposition Efficiency as a Function of Age L. Weber * and C.S. Lengsfeld 1 Department
ILASS Americas, 23 rd Annual Conference on Liquid Atomization and Spray Systems, Ventura, CA, May 2011 Spray Nebulizer Deposition Efficiency as a Function of Age L. Weber * and C.S. Lengsfeld 1 Department
An update on inhalation devices
 OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
Name Class Date. Complete each of the following sentences by choosing the correct term from the word bank.
 Skills Worksheet Chapter Review USING KEY TERMS Complete each of the following sentences by choosing the correct term from the word bank. red blood cells veins white blood cells arteries lymphatic system
Skills Worksheet Chapter Review USING KEY TERMS Complete each of the following sentences by choosing the correct term from the word bank. red blood cells veins white blood cells arteries lymphatic system
Inhalation Therapy. Inhalation Therapy
 Matching Device and Patient Matching Patient to the Device Søren Pedersen University of Southern Denmark Kolding Hospital Anatomical factors (age) Training and education Delivered dose Psycomotor skills
Matching Device and Patient Matching Patient to the Device Søren Pedersen University of Southern Denmark Kolding Hospital Anatomical factors (age) Training and education Delivered dose Psycomotor skills
Physics of the Cardiovascular System
 Dentistry College Medical Physics Physics of the Cardiovascular System The cells of the body act like individual engines. In order for them to function they must have: - 1. Fuel from our food to supply
Dentistry College Medical Physics Physics of the Cardiovascular System The cells of the body act like individual engines. In order for them to function they must have: - 1. Fuel from our food to supply
Tuesday, December 13, 16. Respiratory System
 Respiratory System Trivia Time... What is the fastest sneeze speed? What is the surface area of the lungs? (hint... think of how large the small intestine was) How many breaths does the average person
Respiratory System Trivia Time... What is the fastest sneeze speed? What is the surface area of the lungs? (hint... think of how large the small intestine was) How many breaths does the average person
The Respiratory System
 13 PART A The Respiratory System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Organs of the Respiratory
13 PART A The Respiratory System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Organs of the Respiratory
ISO INTERNATIONAL STANDARD. Anaesthetic and respiratory equipment Nebulizing systems and components
 INTERNATIONAL STANDARD ISO 27427 First edition 2009-05-01 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d'anesthésie et de réanimation respiratoire Systèmes de nébulisation
INTERNATIONAL STANDARD ISO 27427 First edition 2009-05-01 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d'anesthésie et de réanimation respiratoire Systèmes de nébulisation
Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi)
 Respiratory Drug Delivery Europe 2017 Nagel and Suggett Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi) Mark W. Nagel and Jason A. Suggett
Respiratory Drug Delivery Europe 2017 Nagel and Suggett Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi) Mark W. Nagel and Jason A. Suggett
PULMONARY FUNCTION TEST(PFT)
 PULMONARY FUNCTION TEST(PFT) Objectives: By the end of the present lab, students should be able to: 1. Record lung volumes and capacities and compare them with those of a typical person of the same gender,
PULMONARY FUNCTION TEST(PFT) Objectives: By the end of the present lab, students should be able to: 1. Record lung volumes and capacities and compare them with those of a typical person of the same gender,
The Respiratory System Structures of the Respiratory System Structures of the Respiratory System Structures of the Respiratory System Nose Sinuses
 CH 14 D.E. Human Biology The Respiratory System The Respiratory System OUTLINE: Mechanism of Breathing Transport of Gases between the Lungs and the Cells Respiratory Centers in the Brain Function Provides
CH 14 D.E. Human Biology The Respiratory System The Respiratory System OUTLINE: Mechanism of Breathing Transport of Gases between the Lungs and the Cells Respiratory Centers in the Brain Function Provides
Choice of Nebulizer for Inhaled Tobramycin Treatment in Cystic Fibrosis
 Respiratory Drug Delivery 2010 Müllinger et al. Choice of Nebulizer for Inhaled Tobramycin Treatment in Cystic Fibrosis Bernhard Müllinger, 1 Thomas C. Topini, 2 Anna L. Valeri, 2 Juliane Gleske, 1 Philipp
Respiratory Drug Delivery 2010 Müllinger et al. Choice of Nebulizer for Inhaled Tobramycin Treatment in Cystic Fibrosis Bernhard Müllinger, 1 Thomas C. Topini, 2 Anna L. Valeri, 2 Juliane Gleske, 1 Philipp
Ventilator Waveforms: Interpretation
 Ventilator Waveforms: Interpretation Albert L. Rafanan, MD, FPCCP Pulmonary, Critical Care and Sleep Medicine Chong Hua Hospital, Cebu City Types of Waveforms Scalars are waveform representations of pressure,
Ventilator Waveforms: Interpretation Albert L. Rafanan, MD, FPCCP Pulmonary, Critical Care and Sleep Medicine Chong Hua Hospital, Cebu City Types of Waveforms Scalars are waveform representations of pressure,
Respiratory System. Introduction. Atmosphere. Some Properties of Gases. Human Respiratory System. Introduction
 Introduction Respiratory System Energy that we consume in our food is temporarily stored in the bonds of ATP (adenosine triphosphate) before being used by the cell. Cells use ATP for movement and to drive
Introduction Respiratory System Energy that we consume in our food is temporarily stored in the bonds of ATP (adenosine triphosphate) before being used by the cell. Cells use ATP for movement and to drive
Testing inhalers. One of the longstanding challenges facing the
 Testing inhalers This article investigates how the industry can test inhalers in a way that is most representative of typical use. One of the longstanding challenges facing the United States Pharmacopeia
Testing inhalers This article investigates how the industry can test inhalers in a way that is most representative of typical use. One of the longstanding challenges facing the United States Pharmacopeia
Pulsating Aerosols Prove Highly Effective in Delivering Medications to the Sinuses
 PARI Respiratory Equipment, Inc. White Paper 2943 Oak Lake Blvd. Midlothian, VA. 23112 800 327 8632 www.pari.com Pulsating Aerosols Prove Highly Effective in Delivering Medications to the Sinuses Bob Bruno,
PARI Respiratory Equipment, Inc. White Paper 2943 Oak Lake Blvd. Midlothian, VA. 23112 800 327 8632 www.pari.com Pulsating Aerosols Prove Highly Effective in Delivering Medications to the Sinuses Bob Bruno,
Using an Inhaler and Nebulizer
 Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Go With the Flow REGULATORY LANDSCAPE. Mark Copley at Copley Scientific
 Go With the Flow Image: Guzel Studio shutterstock.com Increasing global requirements for efficacious, inexpensive products to treat respiratory illnesses are driving the development of inhaled generics.
Go With the Flow Image: Guzel Studio shutterstock.com Increasing global requirements for efficacious, inexpensive products to treat respiratory illnesses are driving the development of inhaled generics.
Chapter 11 The Respiratory System
 Biology 12 Name: Respiratory System Per: Date: Chapter 11 The Respiratory System Complete using BC Biology 12, page 342-371 11.1 The Respiratory System pages 346-350 1. Distinguish between A. ventilation:
Biology 12 Name: Respiratory System Per: Date: Chapter 11 The Respiratory System Complete using BC Biology 12, page 342-371 11.1 The Respiratory System pages 346-350 1. Distinguish between A. ventilation:
PULMONARY FUNCTION TESTS
 Chapter 4 PULMONARY FUNCTION TESTS M.G.Rajanandh, Department of Pharmacy Practice, SRM College of Pharmacy, SRM University. OBJECTIVES Review basic pulmonary anatomy and physiology. Understand the reasons
Chapter 4 PULMONARY FUNCTION TESTS M.G.Rajanandh, Department of Pharmacy Practice, SRM College of Pharmacy, SRM University. OBJECTIVES Review basic pulmonary anatomy and physiology. Understand the reasons
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF INHALATION AND NASAL PRODUCTS
 European Medicines Agency Inspections London, 16 February 2005 Doc Ref.: EMEA/CHMP/QWP/49313/2005 corr. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF
European Medicines Agency Inspections London, 16 February 2005 Doc Ref.: EMEA/CHMP/QWP/49313/2005 corr. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF
Delivering Aerosol Medication in ICU
 Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
University of Groningen. Optimisation of dry powder inhalation Boer, Anne Haaije de
 University of Groningen Optimisation of dry powder inhalation Boer, Anne Haaije de IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Optimisation of dry powder inhalation Boer, Anne Haaije de IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
I. Subject: Continuous Aerosolization of Bronchodilators
 I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
Lung Physiology and How Aerosol Deposits in the Lungs. 1. Physiological and Anatomical Background
 XA0100097 43 Lung Physiology and How Aerosol Deposits in the Lungs Toyoharu Isawa, M.D. 1. Physiological and Anatomical Background Weibel's morphologic data has been referred to not only for predicting
XA0100097 43 Lung Physiology and How Aerosol Deposits in the Lungs Toyoharu Isawa, M.D. 1. Physiological and Anatomical Background Weibel's morphologic data has been referred to not only for predicting
The Human Respiration System
 The Human Respiration System Nasal Passage Overall function is to filter, warm and moisten air as it enters the body. The nasal passages are the primary site of air movement we tend to be nose breathers.
The Human Respiration System Nasal Passage Overall function is to filter, warm and moisten air as it enters the body. The nasal passages are the primary site of air movement we tend to be nose breathers.
It is recommended that a mask and protective eyewear be worn when providing care to a patient with a cough
 UNIVERSITY HEALTH NETWORK POLICY #: PAGE 1 OF 7 POLICY AND PROCEDURE MANUAL: RESPIRATORY THERAPY DEPT PATIENT CARE SECTION ORIGINAL DATE: 04/03 ISSUED BY: SITE LEADER APPROVED BY: Infection Prevention
UNIVERSITY HEALTH NETWORK POLICY #: PAGE 1 OF 7 POLICY AND PROCEDURE MANUAL: RESPIRATORY THERAPY DEPT PATIENT CARE SECTION ORIGINAL DATE: 04/03 ISSUED BY: SITE LEADER APPROVED BY: Infection Prevention
LUNGS. Requirements of a Respiratory System
 Respiratory System Requirements of a Respiratory System Gas exchange is the physical method that organisms use to obtain oxygen from their surroundings and remove carbon dioxide. Oxygen is needed for aerobic
Respiratory System Requirements of a Respiratory System Gas exchange is the physical method that organisms use to obtain oxygen from their surroundings and remove carbon dioxide. Oxygen is needed for aerobic
Chapter 10. Respiratory System and Gas Exchange. Copyright 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Chapter 10 Respiratory System and Gas Exchange Function of the Respiratory System To obtain oxygen (O 2 ) for all cells in the body. To rid the cells of waste gas (CO 2 ). Oxygen (O 2 ) is vital chemical
Chapter 10 Respiratory System and Gas Exchange Function of the Respiratory System To obtain oxygen (O 2 ) for all cells in the body. To rid the cells of waste gas (CO 2 ). Oxygen (O 2 ) is vital chemical
Energy is needed for cell activities: growth,reproduction, repair, movement, etc...
 Respiration Energy is needed for cell activities: growth,reproduction, repair, movement, etc... Metabolism refers to all of the chemical reactions in the body, where molecules are synthesized (anabolism)
Respiration Energy is needed for cell activities: growth,reproduction, repair, movement, etc... Metabolism refers to all of the chemical reactions in the body, where molecules are synthesized (anabolism)
Challenges in Nonclinical Development of Inhalation Drug Products
 Challenges in Nonclinical Development of Inhalation Drug Products Luqi Pei, Ph.D. Senior Pharmacologist DPARP, CDER August 6, 2015 Rockville, MD Disclaimer This speech reflects the views of the speaker
Challenges in Nonclinical Development of Inhalation Drug Products Luqi Pei, Ph.D. Senior Pharmacologist DPARP, CDER August 6, 2015 Rockville, MD Disclaimer This speech reflects the views of the speaker
Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE BREATHING DEVICE
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Treatments POLICY NUMBER: 421 Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Treatments POLICY NUMBER: 421 Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE
The respiratory system
 The respiratory system Practical 1 Objectives Respiration, ventilation Intrapleural and intrapulmonary pressure Mechanism of inspiration and expiration Composition of the atmosphere and the expired air
The respiratory system Practical 1 Objectives Respiration, ventilation Intrapleural and intrapulmonary pressure Mechanism of inspiration and expiration Composition of the atmosphere and the expired air
ADDITIONAL TECHNOLOGIES FOR PRESSURIZED METERED DOSE INHALERS. Steve Newman Scientific Consultant Nottingham, UK
 THE PRESS-AND-BREATHE pmdi ADDITIONAL TECHNOLOGIES FOR PRESSURIZED METERED DOSE INHALERS Steve Newman Scientific Consultant Nottingham, UK steve.newman@physics.org Compact, portable, convenient Asthma
THE PRESS-AND-BREATHE pmdi ADDITIONAL TECHNOLOGIES FOR PRESSURIZED METERED DOSE INHALERS Steve Newman Scientific Consultant Nottingham, UK steve.newman@physics.org Compact, portable, convenient Asthma
The science of nebulised drug delivery
 Thorax 1997;52(Suppl 2):S31 S44 S31 Department of Child Health, University of Leicester, Leicester Royal Infirmary, PO Box 65, Leicester LE2 7LX, UK C O Callaghan P W Barry Correspondence to: Dr C O Callaghan.
Thorax 1997;52(Suppl 2):S31 S44 S31 Department of Child Health, University of Leicester, Leicester Royal Infirmary, PO Box 65, Leicester LE2 7LX, UK C O Callaghan P W Barry Correspondence to: Dr C O Callaghan.
One-Compartment Open Model: Intravenous Bolus Administration:
 One-Compartment Open Model: Intravenous Bolus Administration: Introduction The most common and most desirable route of drug administration is orally by mouth using tablets, capsules, or oral solutions.
One-Compartment Open Model: Intravenous Bolus Administration: Introduction The most common and most desirable route of drug administration is orally by mouth using tablets, capsules, or oral solutions.
Development of a Dry Powder Multi-dose Inhaler using Computational Modeling
 ENGINEER - Vol. XXXXII, No. 03, pp. [57-65], 2009 The Institution of Engineers, Sri Lanka Development of a Dry Powder Multi-dose Inhaler using Computational Modeling M.A.D.A.Sudeera, V.P.C.Dassanayake,
ENGINEER - Vol. XXXXII, No. 03, pp. [57-65], 2009 The Institution of Engineers, Sri Lanka Development of a Dry Powder Multi-dose Inhaler using Computational Modeling M.A.D.A.Sudeera, V.P.C.Dassanayake,
