Daclizumab improves asthma control in patients with moderate to. severe persistent asthma: A randomized, controlled trial
|
|
|
- Darrell Blankenship
- 5 years ago
- Views:
Transcription
1 Daclizumab improves asthma control in patients with moderate to severe persistent asthma: A randomized, controlled trial William W. Busse, MD, Elliot Israel, MD, Harold S. Nelson, MD, James W. Baker, MD, B. Lauren Charous, MD, Donald Y. Young, Vladimir Vexler, PhD, Richard S. Shames, MD, and the Daclizumab Asthma Study Group Online Data Supplement
2 Methods Study Procedures Eligible patients were enrolled from October 2001 to December As each patient qualified for the randomized phase of the study, a member of the study site called the Interactive Voice Recording System (IVRS) center (Synteract, Inc.) to receive a randomized number. The randomization schedule was generated by PDL and sent to Synteract, Inc. for uploading to IVRS. The IVRS center communicated the patient s randomization number to the study center. All persons directly involved in the study were blinded to treatment including the patient, investigator(s), study coordinator, pharmacist, and PDL s medical monitor and Clinical Research Associates (CRA). Sites were supplied with blinded kits that were assigned to each patient. Spirometry was performed prior to dosing according to American Thoracic Society criteria to determine baseline FEV 1, forced vital capacity (FVC), FEV 1 /FVC, and forced expiratory flow (FEF) 25-75%. E1 Asthma symptoms, medication use, and peak flow recording were assessed using each patient s daily diary recording (by interactive voice response system). The daytime mean symptom scale used a range of response categories for each question from 0 to 6, indicating the least to the most asthma symptomatology. The nocturnal diary scale used
3 response categories ranging from 0 (indicating no awakening with asthma symptoms) to 3 (indicating awake all night). Daily daytime scale scores were computed as the mean total of the 4 questions on the daytime symptom scale. An overall diary score for the week was computed as the mean of at least 4 of 7 daily daytime scale scores. Weekly mean scores for the nocturnal diary scale were computed in a similar manner. A decrease in the weekly score for the daytime and nocturnal scales indicated an improvement in asthma symptoms. The change from baseline in the asthma scale scores was computed as the difference between the mean score from the last week of the pre-treatment run-in period (Days 7 to 1) and the last week of Treatment Period 1 (Days 77 through 83) and the last week of Treatment Period 2 (Days 134 to 140). Asthma-free days were defined as those days in which both the diurnal and nocturnal diaries indicated no symptoms. The method by which this variable was analyzed is dependent on the distribution of missing diary entries. If there were few missing data, changes in mean asthma-free days were analyzed. If patients were inconsistent in reporting their asthma symptoms, then the proportion of patients who experienced asthma-free days was compared by treatment group. Rescue use of β2-agonist metered dose inhaler (MDI) was recorded in the daytime and nocturnal symptom diaries. Patients were instructed to record the number of actuations of β2-agonist from an MDI that was used during the day in the daytime diary recording and the number of actuations of β2-agonist MDI that were used after going to sleep for the night in the nocturnal diary recording. The mean daily use of β2-agonist MDI was
4 computed for daily and nocturnal use for each week of diary recording. The change from baseline in the use of β2-agonist rescue medication was computed as the difference between the mean daily score from the week of the pretreatment run-in period (Days 7 to 1) and the last weeks of the Treatment Period 1 (Days 77 to 83) and the last week of Treatment Period 2 (Days 134 to 140). Serial serum chemistry panel, hematology, and whole blood eosinophils were monitored. At each visit, serum daclizumab levels and circulating human anti-daclizumab antibody were measured. Daclizumab levels were determined using an enzyme-linked immunosorbent assay (ELISA)-based system. Serum samples were first screened for antidaclizumab antibodies using a bridging ELISA format. Positive samples from the antidaclizumab assay were further evaluated in confirmatory and neutralizing assays. Serum eosinophil cationic protein (ECP) levels were measured by Immulite 2000 Automatic Immunoassay Analyzer (Diagnostic Products Corporation, Los Angeles, CA). A central laboratory performed these analyses. Patients recorded asthma symptoms, nocturnal awakenings due to asthma, rescue medications, and peak expiratory flow twice daily using an IVRS diary through Day 140 (Treatment Periods 1 and 2). Patients were instructed to call the study center if they met criteria for exacerbation, and study sites recorded exacerbation history at each visit. Investigators assessed the need for systemic steroid rescue. Statistical Methods
5 Within-group changes were evaluated by paired t-tests, and between-group significance by t-tests. Kaplan-Meier and log rank methods were used to assess time-to-event variables. Last-observation-carried-forward methods were used for patients who prematurely withdrew due to efficacy-related reasons. Available data were analyzed for patients who were premature non-efficacy related terminations. Asthma-free days were defined as days in which both diurnal and nocturnal diaries indicated no symptoms. Asthma exacerbations that were recorded following contact with the study site were included in the analysis. Patients who withdrew prior to completing the treatment and follow-up interval were included in the analysis up to the time the patient withdrew from the protocol. Reference E1. American Thoracic Society. Standardization of spirometry-1987 update. Am Rev Respir Dis 1987;136:
SYNOPSIS A two-stage randomized, open-label, parallel group, phase III, multicenter, 7-month study to assess the efficacy and safety of SYMBICORT
 Drug product: Drug substance(s): Edition No.: Study code: SYMBICORT pmdi 160/4.5 g Budesonide/formoterol D5896C00005 Date: 8 May 2006 SYNOPSIS A two-stage randomized, open-label, parallel group, phase
Drug product: Drug substance(s): Edition No.: Study code: SYMBICORT pmdi 160/4.5 g Budesonide/formoterol D5896C00005 Date: 8 May 2006 SYNOPSIS A two-stage randomized, open-label, parallel group, phase
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
SYNOPSIS THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
International co-ordinating investigator Dr Dencho Osmanliev, St Sofia, Pulmonary Dept, 19 D Nestorov Str, Sofia, 1431, Bulgaria.
 Drug product: SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0681 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0681 Date: 24 Oktober, 2003 (For national
Drug product: SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0681 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0681 Date: 24 Oktober, 2003 (For national
SYNOPSIS. Drug substance(s) Budesonide/formoterol Document No. Edition No. Study code SD Date 16 December 2004
 Drug product SYMBICORT pmdi 160/4.5 µg SYNOPSIS Drug substance(s) Budesonide/formoterol Document No. Edition No. Date 16 December 2004 A Twelve-Week, Randomized, Double-Blind, Double-Dummy, Placebo-Controlled
Drug product SYMBICORT pmdi 160/4.5 µg SYNOPSIS Drug substance(s) Budesonide/formoterol Document No. Edition No. Date 16 December 2004 A Twelve-Week, Randomized, Double-Blind, Double-Dummy, Placebo-Controlled
Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016
 Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016 Diagnosis: There is no lower limit to the age at which
Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016 Diagnosis: There is no lower limit to the age at which
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone
SYNOPSIS. First subject enrolled 15 August 2003 Therapeutic confirmatory (III) Last subject completed 03 February 2005
 Drug product: SYMBICORT pmdi 160/4.5 μg Drug substance(s): Budesonide/formoterol Study code: SD-039-0728 Edition No.: FINAL Date: 27 February 2006 SYNOPSIS A 52-week, randomized, double-blind, single-dummy,
Drug product: SYMBICORT pmdi 160/4.5 μg Drug substance(s): Budesonide/formoterol Study code: SD-039-0728 Edition No.: FINAL Date: 27 February 2006 SYNOPSIS A 52-week, randomized, double-blind, single-dummy,
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence
 Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Montelukast vs. Inhaled Low-Dose Budesonide as Monotherapy in the Treatment of Mild Persistent Asthma: A Randomized Double Blind Controlled Trial
 Montelukast vs. Inhaled Low-Dose Budesonide as Monotherapy in the Treatment of Mild Persistent Asthma: A Randomized Double Blind Controlled Trial by Vikram Kumar, a P. Ramesh, a Rakesh Lodha, a R. M. Pandey,
Montelukast vs. Inhaled Low-Dose Budesonide as Monotherapy in the Treatment of Mild Persistent Asthma: A Randomized Double Blind Controlled Trial by Vikram Kumar, a P. Ramesh, a Rakesh Lodha, a R. M. Pandey,
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
II: Moderate Worsening airflow limitations Dyspnea on exertion, cough, and sputum production; patient usually seeks medical
 Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
International Co-ordinating investigator None appointed.
 Drug product: Budesonide/formoterol SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0673 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0673 Date: 13 June,
Drug product: Budesonide/formoterol SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0673 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0673 Date: 13 June,
Treatment of acute asthmatic exacerbations with an increased dose of inhaled steroid
 12 Paediatrics and Child Health, Dunedin School of Medicine, PO Box 913, University of Otago Medical School, Dunedin, New Zealand J Garrett Preventive and Social Medicine, Dunedin School of Medicine S
12 Paediatrics and Child Health, Dunedin School of Medicine, PO Box 913, University of Otago Medical School, Dunedin, New Zealand J Garrett Preventive and Social Medicine, Dunedin School of Medicine S
Dual-Controller Asthma Therapy: Rationale and Clinical Benefits
 B/1 Dual-Controller Asthma Therapy: Rationale and Clinical Benefits MODULE B The 1997 National Heart, Lung, and Blood Institute (NHLBI) Expert Panel guidelines on asthma management recommend a 4-step approach
B/1 Dual-Controller Asthma Therapy: Rationale and Clinical Benefits MODULE B The 1997 National Heart, Lung, and Blood Institute (NHLBI) Expert Panel guidelines on asthma management recommend a 4-step approach
Asthma: Chronic Management. Yung-Yang Liu, MD Attending physician, Chest Department Taipei Veterans General Hospital April 26, 2015
 Asthma: Chronic Management Yung-Yang Liu, MD Attending physician, Chest Department Taipei Veterans General Hospital April 26, 2015 Global Strategy for Asthma Management and Prevention Evidence-based Implementation
Asthma: Chronic Management Yung-Yang Liu, MD Attending physician, Chest Department Taipei Veterans General Hospital April 26, 2015 Global Strategy for Asthma Management and Prevention Evidence-based Implementation
Asthma Assessment & Review
 ASTHMA RESOURCE PACK Section 5B Asthma Assessment & Review In this section: 1. Primary Care initial assessment and review Asthma Resource Pack Section 5B: Asthma Assessment & Review Version 3.0 Last Updated:
ASTHMA RESOURCE PACK Section 5B Asthma Assessment & Review In this section: 1. Primary Care initial assessment and review Asthma Resource Pack Section 5B: Asthma Assessment & Review Version 3.0 Last Updated:
SYNOPSIS. Date 15 June 2004
 Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
G. Boyd on behalf of a UK Study group
 Eur Respir J, 1995, 8, 1494 1498 DOI: 10.1183/09031936.95.08091494 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1995 European Respiratory Journal ISSN 0903-1936 Salmeterol xinafoate in
Eur Respir J, 1995, 8, 1494 1498 DOI: 10.1183/09031936.95.08091494 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1995 European Respiratory Journal ISSN 0903-1936 Salmeterol xinafoate in
Long Term Care Formulary RS -29
 RESTRICTED USE Asthma/COPD Management 1 of 6 PROTOCOL: Asthma Glossary of Medication Acronyms: SABA: short-acting beta agonist (e.g. salbutamol) SABD: short-acting bronchodilator (e.g. ipratropium or SABA)
RESTRICTED USE Asthma/COPD Management 1 of 6 PROTOCOL: Asthma Glossary of Medication Acronyms: SABA: short-acting beta agonist (e.g. salbutamol) SABD: short-acting bronchodilator (e.g. ipratropium or SABA)
Meenu Singh, Joseph L. Mathew, Prabhjot Malhi, B.R. Srinivas and Lata Kumar
 Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren, MD
 Therapeutic Effect of Zafirlukast as Monotherapy in Steroid-Naive Patients With Severe Persistent Asthma* James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren,
Therapeutic Effect of Zafirlukast as Monotherapy in Steroid-Naive Patients With Severe Persistent Asthma* James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren,
SYNOPSIS. Co-ordinating investigator Not applicable. Study centre(s) This study was conducted in Japan (57 centres).
 Drug product: Symbicort Turbuhaler Drug substance(s): ST (Symbicort Turbuhaler ) Edition No.: 1.0 Study code: D5890C00010 Date: 15 March 2007 SYNOPSIS An 8-week, randomised, double blind, parallel-group,
Drug product: Symbicort Turbuhaler Drug substance(s): ST (Symbicort Turbuhaler ) Edition No.: 1.0 Study code: D5890C00010 Date: 15 March 2007 SYNOPSIS An 8-week, randomised, double blind, parallel-group,
Chronic obstructive pulmonary disease (COPD) is characterized
 DANIEL E. HILLEMAN, PharmD ABSTRACT OBJECTIVE: To review the role of long-acting bronchodilators in the treatment of chronic obstructive pulmonary disease (COPD), including the importance of treatment
DANIEL E. HILLEMAN, PharmD ABSTRACT OBJECTIVE: To review the role of long-acting bronchodilators in the treatment of chronic obstructive pulmonary disease (COPD), including the importance of treatment
Study No.: SAM40012 Title: A multicentre, randomised, double-blind, double-dummy, parallel group comparison of three treatments : 1)
 Study No.: SAM40012 Title: A multicentre, randomised, double-blind, double-dummy, parallel group comparison of three treatments : 1) salmeterol/fluticasone propionate () (mcg strength) bd via DISKUS/ACCUHALER
Study No.: SAM40012 Title: A multicentre, randomised, double-blind, double-dummy, parallel group comparison of three treatments : 1) salmeterol/fluticasone propionate () (mcg strength) bd via DISKUS/ACCUHALER
SUMMARY THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 i SUMMARY ZENECA PHARMACEUTICALS FINISHED PRODUCT: ACTIVE INGREDIENT: ACCOLATE zafirlukast (ZD9188) Trial title (number): A Dose-ranging, Safety and Efficacy Trial with Zafirlukast (ACCOLATE ) in the Treatment
i SUMMARY ZENECA PHARMACEUTICALS FINISHED PRODUCT: ACTIVE INGREDIENT: ACCOLATE zafirlukast (ZD9188) Trial title (number): A Dose-ranging, Safety and Efficacy Trial with Zafirlukast (ACCOLATE ) in the Treatment
Individual Study Table Referring to Part of the Dossier. Volume:
 Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Clinical trial efficacy: What does it really tell you?
 Clinical trial efficacy: What does it really tell you? Joseph Spahn, MD Denver, Colo The primary goal of most clinical trials is an evaluation of the efficacy of the drug being evaluated. Therefore, it
Clinical trial efficacy: What does it really tell you? Joseph Spahn, MD Denver, Colo The primary goal of most clinical trials is an evaluation of the efficacy of the drug being evaluated. Therefore, it
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Scottish Medicines Consortium
 Scottish Medicines Consortium budesonide/formoterol 100/6, 200/6 turbohaler (Symbicort SMART ) No. (362/07) Astra Zeneca UK Limited 9 March 2007 (Issued May 2007) The Scottish Medicines Consortium (SMC)
Scottish Medicines Consortium budesonide/formoterol 100/6, 200/6 turbohaler (Symbicort SMART ) No. (362/07) Astra Zeneca UK Limited 9 March 2007 (Issued May 2007) The Scottish Medicines Consortium (SMC)
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL
 DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
Air Flow Limitation. In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation.
 Asthma Air Flow Limitation In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation. True whether reversible, asthma and exercise-induced bronchospasm,
Asthma Air Flow Limitation In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation. True whether reversible, asthma and exercise-induced bronchospasm,
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
DATE: 09 December 2009 CONTEXT AND POLICY ISSUES:
 TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
BUDESONIDE AND FORMOTEROL (SYMBICORT ): Α A REVIEW
 Volume 23, Issue 3 December 2007 BUDESONIDE AND FORMOTEROL (SYMBICORT ): A REVIEW Donna L. Smith, Pharm. D. Candidate More than 22 million people in the United States have asthma according to the Centers
Volume 23, Issue 3 December 2007 BUDESONIDE AND FORMOTEROL (SYMBICORT ): A REVIEW Donna L. Smith, Pharm. D. Candidate More than 22 million people in the United States have asthma according to the Centers
Accuracy of Parental and Child s Reports of Changes in Symptoms of Childhood Asthma
 7. Cecka JM, Gjertson DW, Terasaki PI. Pediatric renal transplantation - a review of the UNOS data. Pediatr Transplant 1997; 1: 55-64. 8. Alexander SR. Pediatric end stage renal disease. Am J Kidney Dis
7. Cecka JM, Gjertson DW, Terasaki PI. Pediatric renal transplantation - a review of the UNOS data. Pediatr Transplant 1997; 1: 55-64. 8. Alexander SR. Pediatric end stage renal disease. Am J Kidney Dis
Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products
 Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products Extended Breakout Session 2: Biomarker Strategies Richard C. Ahrens, M.D. Partha Roy, Ph.D. Dale P. Conner, Pharm.D. Regulatory
Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products Extended Breakout Session 2: Biomarker Strategies Richard C. Ahrens, M.D. Partha Roy, Ph.D. Dale P. Conner, Pharm.D. Regulatory
SHORT COMMUNICATION. Abstract. Kevin R. Murphy, 1 Tom Uryniak, 2 Ubaldo J. Martin 2 and James Zangrilli 2
 SHORT COMMUNICATION Drugs R D 212; 12 (1): 9-14 1179-691/12/1-9 ª 212 Murphy et al., publisher and licensee Adis Data Information BV. This is an open access article published under the terms of the Creative
SHORT COMMUNICATION Drugs R D 212; 12 (1): 9-14 1179-691/12/1-9 ª 212 Murphy et al., publisher and licensee Adis Data Information BV. This is an open access article published under the terms of the Creative
Clinical Practice Guideline: Asthma
 Clinical Practice Guideline: Asthma INTRODUCTION A critical aspect of the diagnosis and management of asthma is the precise and periodic measurement of lung function both before and after bronchodilator
Clinical Practice Guideline: Asthma INTRODUCTION A critical aspect of the diagnosis and management of asthma is the precise and periodic measurement of lung function both before and after bronchodilator
T he use of inhaled corticosteroids to control the inflammatory
 791 ORIGINAL ARTICLE Early asthma control and maintenance with eformoterol following reduction of inhaled corticosteroid dose D Price, D Dutchman, A Mawson, B Bodalia, S Duggan, P Todd on behalf of the
791 ORIGINAL ARTICLE Early asthma control and maintenance with eformoterol following reduction of inhaled corticosteroid dose D Price, D Dutchman, A Mawson, B Bodalia, S Duggan, P Todd on behalf of the
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Presented by the California Academy of Family Physicians 2013/California Academy of Family Physicians
 Family Medicine and Patient-Centered Asthma Care Presented by the California Academy of Family Physicians Faculty: Hobart Lee, MD Disclosures: Jeffrey Luther, MD, Program Director, Memorial Family Medicine
Family Medicine and Patient-Centered Asthma Care Presented by the California Academy of Family Physicians Faculty: Hobart Lee, MD Disclosures: Jeffrey Luther, MD, Program Director, Memorial Family Medicine
Learning the Asthma Guidelines by Case Studies
 Learning the Asthma Guidelines by Case Studies Timothy Craig, DO Professor of Medicine and Pediatrics Distinguished Educator Penn State University Hershey Medical Center Objectives 1. Learn the Asthma
Learning the Asthma Guidelines by Case Studies Timothy Craig, DO Professor of Medicine and Pediatrics Distinguished Educator Penn State University Hershey Medical Center Objectives 1. Learn the Asthma
Budesonide treatment of moderate and severe asthma in children: A doseresponse
 Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS
 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
Dual-controller therapy, or combinations REVIEW DUAL-CONTROLLER REGIMENS I: DATA FROM RANDOMIZED, CONTROLLED CLINICAL TRIALS.
 DUAL-CONTROLLER REGIMENS I: DATA FROM RANDOMIZED, CONTROLLED CLINICAL TRIALS Samy Suissa, PhD ABSTRACT Dual-controller therapy, or combinations of 2 or more pharmacotherapies with complementary mechanisms
DUAL-CONTROLLER REGIMENS I: DATA FROM RANDOMIZED, CONTROLLED CLINICAL TRIALS Samy Suissa, PhD ABSTRACT Dual-controller therapy, or combinations of 2 or more pharmacotherapies with complementary mechanisms
Asthma and Adherence to Inhaled Corticosteroids: Current Status and Future Perspectives
 Asthma and Adherence to Inhaled Corticosteroids: Current Status and Future Perspectives Camilla Boslev Bårnes MD and Charlotte Suppli Ulrik MD DMSc Introduction Methods Review of the Literature Adherence
Asthma and Adherence to Inhaled Corticosteroids: Current Status and Future Perspectives Camilla Boslev Bårnes MD and Charlotte Suppli Ulrik MD DMSc Introduction Methods Review of the Literature Adherence
The Asthma Guidelines: Diagnosis and Assessment of Asthma
 The Asthma Guidelines: Diagnosis and Assessment of Asthma Christopher H. Fanta, M.D. Partners Asthma Center Brigham and Women s Hospital Harvard Medical School Objectives Know how the diagnosis of asthma
The Asthma Guidelines: Diagnosis and Assessment of Asthma Christopher H. Fanta, M.D. Partners Asthma Center Brigham and Women s Hospital Harvard Medical School Objectives Know how the diagnosis of asthma
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS
 R2 (REVISED MANUSCRIPT BLUE 200208-877OC) ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS Mario Castro, M.D., M.P.H. Nina A. Zimmermann R.N. Sue
R2 (REVISED MANUSCRIPT BLUE 200208-877OC) ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS Mario Castro, M.D., M.P.H. Nina A. Zimmermann R.N. Sue
Pharmacological Management of Obstructive Airways in Humans. Introduction to Scientific Research. Submitted: 12/4/08
 Pharmacological Management of Obstructive Airways in Humans Introduction to Scientific Research Submitted: 12/4/08 Introduction: Obstructive airways can be characterized as inflammation or structural changes
Pharmacological Management of Obstructive Airways in Humans Introduction to Scientific Research Submitted: 12/4/08 Introduction: Obstructive airways can be characterized as inflammation or structural changes
Full Length Original Research Paper
 Copyright 2013 By IYPF All rights reserved Open Access Contents Int. J. Drug Dev. & Res. October - December 2013 Vol. 5 Issue 4 ISSN 0975-9344 www.ijddr.in Effect of Budesonide by metered dose inhaler
Copyright 2013 By IYPF All rights reserved Open Access Contents Int. J. Drug Dev. & Res. October - December 2013 Vol. 5 Issue 4 ISSN 0975-9344 www.ijddr.in Effect of Budesonide by metered dose inhaler
Los Angeles PRISMS Center
 Los Angeles PRISMS Center An mhealth Platform for Predicting Risk of Pediatric Asthma Exacerbation Using Personal Sensor Monitoring Systems Sep 2018 ASIC Meeting Rima Habre, ScD habre@usc.edu Assistant
Los Angeles PRISMS Center An mhealth Platform for Predicting Risk of Pediatric Asthma Exacerbation Using Personal Sensor Monitoring Systems Sep 2018 ASIC Meeting Rima Habre, ScD habre@usc.edu Assistant
Low- and high-dose fluticasone propionate in asthma; effects during and after treatment
 Eur Respir J 2000; 15: 11±18 Printed in UK ± all rights reserved Copyright #ERS Journals Ltd 2000 European Respiratory Journal ISSN 0903-1936 Low- and high-dose fluticasone propionate in asthma; effects
Eur Respir J 2000; 15: 11±18 Printed in UK ± all rights reserved Copyright #ERS Journals Ltd 2000 European Respiratory Journal ISSN 0903-1936 Low- and high-dose fluticasone propionate in asthma; effects
SYNOPSIS. ER OROS Paliperidone: Clinical Study Report R SCH-301
 SYNOPSIS Protocol No.: R076477-SCH-301 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study With an Open-Label Extension Evaluating Extended Release OROS Paliperidone in
SYNOPSIS Protocol No.: R076477-SCH-301 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study With an Open-Label Extension Evaluating Extended Release OROS Paliperidone in
Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended.
 Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended Bricanyl () DK/W/0017/pdWS/001 Rapporteur: Denmark Finalisation procedure
Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended Bricanyl () DK/W/0017/pdWS/001 Rapporteur: Denmark Finalisation procedure
Childhood Asthma. The pathophysiology of asthma is an interplay. CME Case Study. Case Study. By Moyez B. Ladhani, MD, CCFP, FAAP, FRCPC
 CME Case Study Childhood Asthma By Moyez B. Ladhani, MD, CCFP, FAAP, FRCPC Case Study A two-year-old child presents to your office with a cough, which has been present for three weeks. It is worse at nighttime
CME Case Study Childhood Asthma By Moyez B. Ladhani, MD, CCFP, FAAP, FRCPC Case Study A two-year-old child presents to your office with a cough, which has been present for three weeks. It is worse at nighttime
Potency ratio fluticasone propionate (Flixotide Diskus)/budesonide (Pulmicort Turbuhaler)
 Respiratory Medicine (2007) 101, 610 615 Potency ratio fluticasone propionate (Flixotide Diskus)/budesonide (Pulmicort Turbuhaler) Björn Ställberg a, Eva Pilman b, Bengt-Eric Skoogh c,, Bengt Arne Hermansson
Respiratory Medicine (2007) 101, 610 615 Potency ratio fluticasone propionate (Flixotide Diskus)/budesonide (Pulmicort Turbuhaler) Björn Ställberg a, Eva Pilman b, Bengt-Eric Skoogh c,, Bengt Arne Hermansson
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE January 6, 2016 SUBJECT EFFECTIVE DATE January 20, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of COPD Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE January 6, 2016 SUBJECT EFFECTIVE DATE January 20, 2016 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of COPD Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
SCREENING AND PREVENTION
 These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone
 Eur Respir J 2001; 18: 262 268 Printed in UK all rights reserved Copyright #ERS Journals Ltd 2001 European Respiratory Journal ISSN 0903-1936 Improved asthma control with budesonide/formoterol in a single
Eur Respir J 2001; 18: 262 268 Printed in UK all rights reserved Copyright #ERS Journals Ltd 2001 European Respiratory Journal ISSN 0903-1936 Improved asthma control with budesonide/formoterol in a single
RESPIRATORY CARE IN GENERAL PRACTICE
 RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
This is a cross-sectional analysis of the National Health and Nutrition Examination
 SUPPLEMENTAL METHODS Study Design and Setting This is a cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES) data 2007-2008, 2009-2010, and 2011-2012. The NHANES is
SUPPLEMENTAL METHODS Study Design and Setting This is a cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES) data 2007-2008, 2009-2010, and 2011-2012. The NHANES is
International Journal of Medical Research & Health Sciences
 International Journal of Medical Research & Health Sciences www.ijmrhs.com Volume 2 Issue 3 July - Sep Coden: IJMRHS Copyright @2013 ISSN: 2319-5886 Received: 23 th May 2013 Revised: 24 th Jun 2013 Accepted:
International Journal of Medical Research & Health Sciences www.ijmrhs.com Volume 2 Issue 3 July - Sep Coden: IJMRHS Copyright @2013 ISSN: 2319-5886 Received: 23 th May 2013 Revised: 24 th Jun 2013 Accepted:
Optimal Assessment of Asthma Control in Clinical Practice: Is there a role for biomarkers?
 Disclosures: Optimal Assessment of Asthma Control in Clinical Practice: Is there a role for biomarkers? Stanley Fineman, MD Past-President, American College of Allergy, Asthma & Immunology Adjunct Associate
Disclosures: Optimal Assessment of Asthma Control in Clinical Practice: Is there a role for biomarkers? Stanley Fineman, MD Past-President, American College of Allergy, Asthma & Immunology Adjunct Associate
ClinialTrials.gov Identifier: Sponsor/company: sanofi-aventis
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Inhaler Confusion. Today s Speaker Dr. Randall Brown. Director of Asthma Programs 6/7/2016. Dr. Randall Brown March 31, 2016
 + Inhaler Confusion Dr. Randall Brown March 31, 2016 + Today s Speaker Dr. Randall Brown Director of Asthma Programs Center for Managing Chronic Disease University of Michigan 1 ASTHMA ESSENTIALS IN PRIMARY
+ Inhaler Confusion Dr. Randall Brown March 31, 2016 + Today s Speaker Dr. Randall Brown Director of Asthma Programs Center for Managing Chronic Disease University of Michigan 1 ASTHMA ESSENTIALS IN PRIMARY
Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide.
 Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide. By: DR MOHD SHAMSUL AMRI Supervisor: Associate Professor Dr
Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide. By: DR MOHD SHAMSUL AMRI Supervisor: Associate Professor Dr
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Bronchial asthma. E. Cserháti 1 st Department of Paediatrics. Lecture for english speaking students 5 February 2013
 Bronchial asthma E. Cserháti 1 st Department of Paediatrics Lecture for english speaking students 5 February 2013 Epidemiology of childhood bronchial asthma Worldwide prevalence of 7-8 and 13-14 years
Bronchial asthma E. Cserháti 1 st Department of Paediatrics Lecture for english speaking students 5 February 2013 Epidemiology of childhood bronchial asthma Worldwide prevalence of 7-8 and 13-14 years
measured Improvement in am PEFR FP vs BUD gave +11l/min FP vs BDP gave nonsignificant improvement in PEFR +3l/min FP: BUD ratio 1.
 1 of 8 09/05/2018, 11:29 Guideline topic: Pharmacological management of asthma Evidence table 4.25: Budesonide vs Beclomethasone Different inhaled corticosteroids (ICS) flixotide propionate (FP) vs budesonide
1 of 8 09/05/2018, 11:29 Guideline topic: Pharmacological management of asthma Evidence table 4.25: Budesonide vs Beclomethasone Different inhaled corticosteroids (ICS) flixotide propionate (FP) vs budesonide
Rapid Effects of Inhaled Corticosteroids in Acute Asthma Gustavo J. Rodrigo, MD.
 Rapid Effects of Inhaled Corticosteroids in Acute Asthma Gustavo J. Rodrigo, MD WWW.chestjournal.org Background: Current reviews on the use of inhaled corticosteroids (ICS) for acute asthma underestimated
Rapid Effects of Inhaled Corticosteroids in Acute Asthma Gustavo J. Rodrigo, MD WWW.chestjournal.org Background: Current reviews on the use of inhaled corticosteroids (ICS) for acute asthma underestimated
Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial. Online Data Supplement
 Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial Nicolas Roche, Kenneth R. Chapman, Claus F. Vogelmeier, Felix JF Herth, Chau Thach, Robert Fogel, Petter Olsson,
Blood Eosinophils and Response to Maintenance COPD Treatment: Data from the FLAME Trial Nicolas Roche, Kenneth R. Chapman, Claus F. Vogelmeier, Felix JF Herth, Chau Thach, Robert Fogel, Petter Olsson,
Asthma for Primary Care: Assessment, Control, and Long-Term Management
 Asthma for Primary Care: Assessment, Control, and Long-Term Management Learning Objectives After participating in this educational activity, participants should be better able to: 1. Choose the optimal
Asthma for Primary Care: Assessment, Control, and Long-Term Management Learning Objectives After participating in this educational activity, participants should be better able to: 1. Choose the optimal
Improving the Management of Asthma to Improve Patient Adherence and Outcomes
 Improving the Management of Asthma to Improve Patient Adherence and Outcomes Robert Sussman, MD Atlantic Health System Overlook Medical Center Asthma Remains a Serious Health Risk in the US Every day in
Improving the Management of Asthma to Improve Patient Adherence and Outcomes Robert Sussman, MD Atlantic Health System Overlook Medical Center Asthma Remains a Serious Health Risk in the US Every day in
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
THE NEW ZEALAND MEDICAL JOURNAL
 THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1267 ISSN 1175 8716 Is Salamol less effective than Ventolin? A randomised, blinded, crossover study in New Zealand Catherina L Chang, Manisha Cooray, Graham Mills,
THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1267 ISSN 1175 8716 Is Salamol less effective than Ventolin? A randomised, blinded, crossover study in New Zealand Catherina L Chang, Manisha Cooray, Graham Mills,
Secondary Outcome/Efficacy Variable(s):
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
How to treat COPD? What is the mechanism of dyspnea? Smoking cessation
 : The Increasing Role of the FP Alan Kaplan, MD, CCFP(EM) Presented at the Primary Care Today: Education Conference and Medical Exposition, Toronto, Ontario, May 2006. Chronic obstructive pulmonary disease
: The Increasing Role of the FP Alan Kaplan, MD, CCFP(EM) Presented at the Primary Care Today: Education Conference and Medical Exposition, Toronto, Ontario, May 2006. Chronic obstructive pulmonary disease
Abstract Background Theophylline is widely used in the treatment of asthma, and there is evidence that theophylline has antiinflammatory
 Thorax 2000;55:837 841 837 National Heart and Lung Institute, Imperial College School of Medicine and Royal Brompton Hospital, London SW3 6LY, UK S Lim A Jatakanon K F Chung P J Barnes Napp Laboratories
Thorax 2000;55:837 841 837 National Heart and Lung Institute, Imperial College School of Medicine and Royal Brompton Hospital, London SW3 6LY, UK S Lim A Jatakanon K F Chung P J Barnes Napp Laboratories
SYNOPSIS. The study results and synopsis are supplied for informational purposes only.
 SYNOPSIS INN : CROMOGLICIC ACID Study number : (AAR-DE-301) Study title : A multicenter, double-blind, randomized group comparison study with two parallel groups over 12 weeks in children and adolescents
SYNOPSIS INN : CROMOGLICIC ACID Study number : (AAR-DE-301) Study title : A multicenter, double-blind, randomized group comparison study with two parallel groups over 12 weeks in children and adolescents
World Journal of Pharmaceutical and Life Sciences WJPLS
 wjpls, 2018, Vol. 4, Issue 10, 01-08 Research Article ISSN 2454-2229 Firas et al. WJPLS www.wjpls.org SJIF Impact Factor: 5.088 ASTHMA CONTROL Dr. Yaarub Madhloom Abbas 1, Dr. Firas Raad Shihab* 2 and
wjpls, 2018, Vol. 4, Issue 10, 01-08 Research Article ISSN 2454-2229 Firas et al. WJPLS www.wjpls.org SJIF Impact Factor: 5.088 ASTHMA CONTROL Dr. Yaarub Madhloom Abbas 1, Dr. Firas Raad Shihab* 2 and
Treatment Responses. Ronald Dahl, Aarhus University Hospital, Denmark
 Asthma and COPD: Are They a Spectrum Treatment Responses Ronald Dahl, Aarhus University Hospital, Denmark Pharmacological Treatments Bronchodilators Inhaled short-acting β -Agonist (rescue) Inhaled short-acting
Asthma and COPD: Are They a Spectrum Treatment Responses Ronald Dahl, Aarhus University Hospital, Denmark Pharmacological Treatments Bronchodilators Inhaled short-acting β -Agonist (rescue) Inhaled short-acting
Differential diagnosis
 Differential diagnosis The onset of COPD is insidious. Pathological changes may begin years before symptoms appear. The major differential diagnosis is asthma, and in some cases, a clear distinction between
Differential diagnosis The onset of COPD is insidious. Pathological changes may begin years before symptoms appear. The major differential diagnosis is asthma, and in some cases, a clear distinction between
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Asthma. chapter 7. Overview
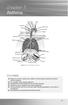 chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
Viral-Induced Asthma:
 Viral-Induced : Sorting through the Studies Malcolm R. Sears, MB, FRACP, FRCPC Presented at the Respirology Update Continuing Education Program, January 2005 Viral-associated wheezing is common and not
Viral-Induced : Sorting through the Studies Malcolm R. Sears, MB, FRACP, FRCPC Presented at the Respirology Update Continuing Education Program, January 2005 Viral-associated wheezing is common and not
Community COPD Service Protocol
 Community COPD Service Protocol Acknowledgements This protocol is based on the following documents: 1. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults
Community COPD Service Protocol Acknowledgements This protocol is based on the following documents: 1. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Not available 100/6mcg 2 BD formoterol (Fostair MDI) 100/6mcg 33
 COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP FLUTICASONE FUROATE/VILANTEROL COMBINATION INHALER - ASTHMA Policy agreed by Vale of York CCG (date) Drug, Treatment, Device name Fluticasone
COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP FLUTICASONE FUROATE/VILANTEROL COMBINATION INHALER - ASTHMA Policy agreed by Vale of York CCG (date) Drug, Treatment, Device name Fluticasone
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Co-Primary Outcomes/Efficacy Variables:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Lack of Subsensitivity to Albuterol After Treatment with Salmeterol in Patients with Asthma
 Lack of Subsensitivity to Albuterol After Treatment with Salmeterol in Patients with Asthma HAROLD S. NELSON, ROBERT B. BERKOWITZ, DAVID A. TINKELMAN, AMANDA H. EMMETT, KATHLEEN A. RICKARD, and STEVEN
Lack of Subsensitivity to Albuterol After Treatment with Salmeterol in Patients with Asthma HAROLD S. NELSON, ROBERT B. BERKOWITZ, DAVID A. TINKELMAN, AMANDA H. EMMETT, KATHLEEN A. RICKARD, and STEVEN
Presented by UIC College of Nursing
 Presented by UIC College of Nursing Describe COPD. Identify red flags for a COPD exacerbation. Identify COPD triggers or risk factors. Differentiate between long-acting inhalers and emergency use inhalers.
Presented by UIC College of Nursing Describe COPD. Identify red flags for a COPD exacerbation. Identify COPD triggers or risk factors. Differentiate between long-acting inhalers and emergency use inhalers.
Chronic Obstructive Pulmonary Disease 1/18/2018
 Presented by UIC College of Nursing Describe COPD. Identify red flags for a COPD exacerbation. Identify COPD triggers or risk factors. Differentiate between long acting inhalers and emergency use inhalers.
Presented by UIC College of Nursing Describe COPD. Identify red flags for a COPD exacerbation. Identify COPD triggers or risk factors. Differentiate between long acting inhalers and emergency use inhalers.
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
