Full Length Original Research Paper
|
|
|
- Willis Weaver
- 5 years ago
- Views:
Transcription
1 Copyright 2013 By IYPF All rights reserved Open Access Contents Int. J. Drug Dev. & Res. October - December 2013 Vol. 5 Issue 4 ISSN Effect of Budesonide by metered dose inhaler with or without spacer & dry powder inhaler on Lung Function Page 233 Dilshad Ali Rizvi 1* Mohammad Tariq Salman 1 Joydeep Sircar 2 Ali Ahmad 1 1 Department of Pharmacology, Era s Lucknow Medical College, Sarfarazganj, Hardoi Road, Lucknow, India Department of Pulmonary Medicine, Era s Lucknow Medical College, Sarfarazganj, Hardoi Road, Lucknow, India Corresponding Authors: Dr. Dilshad Ali Rizvi Department of Pharmacology, Era s Lucknow Medical College, Sarfarazganj, Hardoi Road, Lucknow, India dr.dilshadali@yahoo.com. Introduction Inhaled corticosteroids are the most effective drugs for the treatment of asthma and they represent first-line therapy for all patients with persistent disease, irrespective of disease Severity. [1] The clinical benefit of inhaled corticosteroids therapy is determined by a Complex interplay between the nature and severity of the disease, the type of drug and its formulation, and characteristics of delivery device together with the patient s ability to use the device correctly. [2] Studies have demonstrated their efficacy in reducing symptom, frequency and severity of asthma exacerbations and asthma mortality. Inhaled corticosteroids are marketed with Abstract: Aims & objective-to compare the efficacy of Budesonide delivered by metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler on the lung function test parameters. Materials and Methods: This prospective study was undertaken to assess the effect of budesonide administered from fifty patients of chronic stable bronchial asthma were budesonide(400mcg) by metered dose inhaler, metered dose inhaler with spacer and by dry powder inhaler at day 14, 21 and 28 after enrolment respectively under direct supervision. Pulmonary function test was done before and one hour after administration of the drug on each visit. Results: There was no evidence of difference in peak expiratory flow rate (P=0.20), forced expiratory volume in one second (P=0.98), forced vital capacity (P=0.57) and forced expiratory volume in one second and forced vital capacity ratio (P=0.34) was seen after giving budesonide by different devices. Conclusion: Budesonide delivered by metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler have similar effect on lung function in patients of chronic stable bronchial asthma and may be used interchangeably. Keywords: Inhaled Corticosteroids, Drug Delivery Devices, Pulmonary Function Tests, Bronchial Asthma. different delivery devices, which have different lung deposition properties, in vivo dosage accuracy and dose variability. [3] The major advantage of inhaled therapy is that drugs are delivered directly into the airways producing higher local concentrations with significantly less risk of systemic side effects. Inhaled medications for asthma are available as pressurized metered dose inhaler, metered dose inhaler with spacer, breath-actuated metered dose inhaler, dry powder inhalers, soft mist inhalers and nebulized or wet aerosols. In most of studies the inhaled corticosteroids for the treatment of bronchial asthma have been administered by one or two of the devices as stated above. To the best of our knowledge there is no Indian study Full Length Original Research Paper
2 comparing clinical efficacy of budesonide The subjects fulfilling the following criteria were delivered via metered dose inhaler, metered dose considered to be suffering from chronic stable inhaler with spacer and dry powder inhaler in bronchial asthma as defined by American patients of chronic stable bronchial asthma. Thoracic society 1987.[6]. With inhaled corticosteroids being the 1. History suggestive of bronchial asthma Dr. Dilshad Ali Rizvi et al; Comparative Efficacy Of Budesonide By Different Devices mainstay of anti-inflammatory treatment in asthma, it is necessary to determine the comparative efficacy of different corticosteroids delivered through different inhaler devices. The present study was undertaken to assess the relative efficiency of budesonide administered from different delivery devices to adult patients of chronic stable bronchial asthma as measured by pulmonary function test parameters. Materials and Methods Effect of Budesonide by different delivery devices was studied in patients of chronic stable bronchial asthma attending out patient department of Tuberculosis and Chest Diseases. Individuals of either sex aged 18 years and above, who were residents of the local area and had a history of bronchial asthma for at least 6 months comprised the study unit. Approval for the study from the Institutional ethical committee was obtained and written and informed consent from all patients was taken. Sample size was calculated to be 36 on the basis of prior observations reported in a previous Study [4] using the formula: n-(s12+s2) (Z1-a/2+Z1-ß) 2/d2 where σ1=3.4, σ2=4.7, d=3.4, Z1a/2=1.96, Z1-ß=1.28, a=.05 and ß=0.1 (power 90%). But assuming loss to follow up cases to be 30% (10% for each step), the initial recruitment was calculated to be 46.8 which was further rounded off to 50 cases. 2. No acute exacerbation (episodes of progressive increase in shortness of breath, cough, wheezing, or chest tightness, or some combination of these symptoms) within the past one month 3. No history of receiving any corticosteroid therapy for past one month 4. Baseline forced expiratory volume in one second (FEV1) less than 80% of predicted value 5. Increase in FEV 1 equal or more than to 12% and peak expiratory flow rate (PEFR) equalor more than to 20% of baseline value 15 minutes after bronchodilator therapy. Exclusion criteria 1. Patients with past history of hypersensitivity to budesonide. 2. History of treatment of asthma within four weeks prior to study. 3. Pregnant and lactating females, 4. Subjects with hepatic, cardiac, renal and respiratory disorders and those with an upper respiratory tract or acute sinus infection within four weeks prior to enrollment. 5. Individuals with a smoking history of >10 packyears and those on immunotherapy who required a change in dosage regimen within 12 weeks prior to enrollment. Study design: Prospective, Open lebel Single dose of Budesonide 400mcg by metered dose inhaler was given on the second visit (day-14). On the third visit (day 21) single dose of Budesonide Page 234 Inclusion criteria 400mcg by metered dose inhaler with spacer was administered. Finally on the fourth visit (day 28) a
3 single dose of Budesonide 400mcg by dry powder salbutamol administration spirometry was inhaler was given. All study subjects underwent repeated and those patients who had an pulmonary function tests before and one hour increase of at least 12% absolute FEV1 and at after drug administration. least 20% PEFR were labeled as suffering from After a standardized initial evaluation, which bronchial asthma and enrolled in the study. Thus in included complete history taking, clinical all, patients had to visit the department for 4 times examination, investigations, asthma symptom including nomination, registration and 3 follow up score and spirometry, patients were requested to visits. follow up after two weeks and then weekly of two Drug was administered under direct supervision by Page 235 weeks. Each patient was given a card in which as needed salbutamol inhalation was to be mentioned by the patients themselves and they were requested to bring the card along with them when they came after two week. Each patient was given a diary to encircle asthma symptoms. The severity of Asthma was assessed by symptom score as mentioned by Coverley et al (2005) [7] that included major complains of asthma i.e. (i) shortness of breath, (ii) cough (iii) chest tightness (iv) night time awaken. The individual score of above four parameters were added up to get the cumulative asthma score. Graded scoring system was used to note patients complain and severity. Spirometry was done at the beginning of study (day-0). Before spirometry it was ascertained patient had not taken inhaled ß (salbutamol) therapy for at least 6 hours, theophylline therapy for at least 24 hours, and antihistamine therapy for at least 48 hours and coffee for at least 4 hours. Spirometry was performed with standard techniques and evaluated for validity according to American Thoracic Society criteria (1995) [5] using Medspiror (Medsystems Private Limited, Chandigarh). At least three spirometry maneuvers were done and highest FEV1 value was noted. Patients who had FEV1, less than 80% of predicted value were administered inhaled salbutamol 200mcg by nebulizer. Fifteen minutes after standard technique described by CMAJ(1999). Statistical analysis Data entry and statistical analysis was done using statistical package of social science (SPSS) software (version 17.0). pre-treatment and post treatment value were compared by student paired t test, pulmonary function test parameters on different days were compared by ANOVA followed by post hoc turkey s test. Asthma symptoms scores were compared by kruskalwallis test were used. P values less than 0.05 were considered significant. Results Initially 50 patients were enrolled in the study out of which, 3 did not turn up after second visit and 2 did not turn up after third visit. None of the patients experienced an acute exacerbation of asthma during the study period. Thus finally 5 patients were excluded due to loss to follow up and the data of the remaining 45 subjects (27 males and 18 females) was analyzed (Figure 1). Twenty four (53.3%) individuals were aged between years, 17 (37.7%) individuals were aged between years and 4 (8 individuals were aged between 61 mean age of the patients was found to be 42 years. Full Length Original Research Paper
4 asthma scores calculated from diary card FVC values at day-14, 21 and 28 before giving the entries varied between 1.97 to 2.09 on days of drug by different devices (P>0.05). [Table-3] visits. There was no significant difference in Pretreatment values of FEV1/FVC varied between patient s asthma symptom score per week at day %, % and % before 0, 14, 21 and 28 (P>0.05). Since there was no giving budesonide by metered dose inhaler (day- Dr. Dilshad Ali Rizvi et al; Comparative Efficacy Of Budesonide By Different Devices significant change in pulmonary function test Parameters (before the giving budesonide) at day-14, day-21, day-28, which shows that the patients were suffering from chronic stable bronchial asthma and there was no evidence of significant modification in the disease process during the course of the study. No significant Change in the asthma symptom scores and use of rescue medication during the study periods also shows that there was no acute exacerbation and the patients were stable. Pretreatment values of peak expiratory flow rate varied between %, % and % before giving budesonide by metered dose inhaler (day-14), metered dose inhaler with spacer (day-21) and dry powder inhaler (day-28) respectively. There was no significant difference in PEFR values at day-14, 21 and 28, before giving the drug by different devices (P>0.05). [Table-1] Pretreatment values of forced expiratory volume in 1 second varied between 60-77%, 62-75% and 58-77% before giving budesonide by inhaler (day-28) respectively. There was no significant difference in FEV1 values at day-14, 21 and 28 before giving the drug by different devices (P>0.05). [Table-2] Pretreatment values of forced vital capacity varied between %, % and % before giving budesonide by metered 14), metered dose inhaler with spacer (day-21) and dry powder inhaler (day-28) respectively. There was no significant difference in FEV1/FVC values at day-14, 21 and 28, before giving the drug by different devices (P>0.05). [Table-4] inhaler (day-28) there was highly significant increase in PEFR (P<0.001). The percentage change in PEFR was highest after giving budesonide dry powder inhaler (33-50%), followed by metered dose inhaler with spacer (36-53 %) and metered dose inhaler (36-50 %). However there was no significant difference in the PEFR after giving budesonide by any of the devices (P>0.05). the different devices at day-14, 21 and 28, there was highly significant increase in FEV1 (P<0.001). The post treatment values of FEV1 ranged between 63-81%, 64-79% and 66-82% by metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler respectively, the difference being statistically insignificant (P>0.05). different devices at day-14, 21 and 28, there was highly significant increase in FVC (P<0.001). The percentage change in FVC ranged between %, % and % by metered dose inhaler, metered dose inhaler with spacer and dry Page 236 dose inhaler (day-14), metered dose inhaler with powder inhaler respectively, the difference being spacer (day-21) and dry powder inhaler (day-28) statistically insignificant (P>0.05). respectively. There was no significant difference in
5 Page 237 different devices at day-14, 21 and 28 there was highly Significant increase in FEV1/FVC (P<0.001). The percentage change in FEV1/FVC ranged between , % and % by metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler respectively, the difference being statistically insignificant (P>0.05). The pulmonary function parameters showed a highly significant increase one hour after giving budesonide by any of the devices evaluated. There was no significant difference in post reatment values of peak expiratory flow rate (P=0.20), forced expiratory volume in one second (P=0.98), forced vital capacity (P=0.57) and forced expiratory volume in one second and forced vital capacity ratio (P=0.34) after giving budesonide by metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler respectively at day 14, 21 and 28. This shows a similar efficacy of budesonide delivered via the different devices studied. The pulmonary function parameters showed a highly significant increase one hour after giving budesonide by any of the devices evaluated. There was no significant difference in post treatment values of peak expiratory flow rate (P=0.20), forced expiratory volume in one second (P=0.98), forced vital capacity (P=0.57) and forced expiratory volume in one second and forced vital capacity ratio (P=0.34) after giving budesonide by metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler respectively at day 14, 21 and 28. This shows a similar efficacy of budesonide delivered via the different devices studied. Discussion Our study compared the effect budesonide delivered via metered dose inhaler, metered dose inhaler with spacer and by dry powder inhaler on lung functions and revealed that these devices have a similar effect on the lung function in patients of chronic stable bronchial asthma. inhaler (day-28) there was highly significant increase in PEFR in our study. Several studies have demonstrated an increase in peak expiratory flow rate after giving budesonide by nebulizer, metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler over a period of 1 to 12 weeks.[9-13] No significant difference in the PEFR was found after giving budesonide by any of the different devices used in our study which is in agreement with the study of Bisgaard et al (1998) [9] that compared the effect of budesonide gven as nebulized suspension verses metered dose inhaler in adult asthmatics. Spirometry at their clinic revealed no statistically significant difference between the treatments. Engel et al (1989) [10] also demonstrated that there was no significant difference in peak expiratory flow rate at clinic and evening peak exploratory flow rate after giving budesonide by metered dose inhaler or dry powder inhaler, however morning peak expiratory flow rate found from patient s diaries showed significantly higher values in the group receiving budesonide through dry powder inhaler. Reason of different effects of delivery devices on morning evening peak expiratory flow rate needs Full Length Original Research Paper to be further investigated.
6 second and forced vital capacity ratio (FEV1/FVC). There was no significant difference found in the FEV1/FVC after giving budesonide by inhaler (day-28) there was a highly significant any of the devices. Previous studies on inhaled increase in FEV1 in our study. Kerwin et al budesonide by different devices in patients of Dr. Dilshad Ali Rizvi et al; Comparative Efficacy Of Budesonide By Different Devices (2008)[11] observed a significant increase in FEV1 when budesonide was given by dry powder inhaler as compared to placebo. There was no significant difference found in the FEV1 after giving budesonide by any of the devices used in our study. Engel et al (1989) [10] compared inhaled budesonide delivered either via pressurized metered dose inhaler or turbuhaler and found that there was no significant difference in FEV1 between the two treatments. Bisgaard et al (1998) [9] compared the efficacy of budesonide as a nebulized suspension versus pressurized metered dose inhaler in adult asthmatics and revealed no statistically significant difference between the treatments. inhaler with spacer (day-21), dry powder inhaler (day-28) forced vital capacity also increased significantly. Although the percentage change in forced vital capacity was highest with metered dose inhaler followed by metered dose inhaler with spacer and dry powder inhaler but there was no significant difference in the FVC after giving budesonide by any of the devices. Engel et al (1989) [10] compared inhaled budesonide delivered via pressurized metered dose inhaler and turbuhaler and found no statistically significant differences in FVC. inhaler (day-28) there was highly significant chronic stable bronchial asthma have not reported the effect on FEV1/FVC. The present study found no significant differences on spirometric variables after giving budesonide via metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler. They may be used interchangeably depending on availability, cost and compliance of the patients. We conclude that budesonide delivered by different devices (metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler) have similar effect on lung function in patients of chronic stable bronchial asthma and may be used interchangeably. Acknowledgement: We are thankful to Dr Zeeshan Haider Zaidi Assistant Professor / biostatistician, Department of Community Medicine, Era s Lucknow MedicalCollege Lucknow for his help in statistical analysis. Table 1: Effect on Peak Expiratory Flow Rate (PEFR) (Predicted %) after giving Budesonide by Different Devices Drug Delivery Device Inhaler Inhaler with Spacer Dry Powder Inhaler ANOVA Pre-treatment ( ) ( ) ( ) Post-treatment ( ) ( ) ( ) F value P value Page 238 increase in forced expiratory volume in one
7 Page 239 Table 2: Effect on Forced Expiratory Volume in 1 second (FEV1) (Predicted %) after giving Budesonide by Different Devices Drug Delivery Device Inhaler Inhaler with Spacer Dry Powder Inhaler ANOVA Pre-treatment ( ) ( ) ( ) Post-treatment ( ) 73.4 ( ) ( ) F value P value Table 3: Effect on Forced Vital Capacity (FVC) (Predicted %) after giving Budesonide by different devices Drug Delivery Device Inhaler Inhaler with Spacer Dry Powder Inhaler ANOVA Pre-treatment ( ) ( ) ( ) Post-treatment ( ) ( ) ( ) F value P value Table 4: Effect on FEV1/FVC (Predicted %) after giving Budesonide by different devices Drug Delivery Device Inhaler Inhaler with Spacer Pre-treatment Post-treatment 0.73 ( ) 0.76 ( ) 0.74 ( ) 0.75 ( ) Dry Powder Inhaler 0.72 ( ) 0.73 ( ) Figure 1: Study Design and Execution After 14 days 50 Cases Enrolled (50 patients) Budesonide 400mcg delivered by MDI Three patients drop out after 21 days Budesonide 400mcg delivered by MDI with spacer (47 patients) Two patients drop out after 28 days References Budesonide 400mcg delivered by DPI (45 patients) 1) Global Initiative for Asthma. NHLBI/WHO workshop report. Global strategy for asthma management and prevention. National Institutes of Health, National Heart, Lung, and Blood Institute. Revised Publication no ) Barnes PJ, Seidenberg J, Magnan A, Pedersen S, Dal Negro R, Crompton GK, Virchow JC. Importance of inhaler devices in the management of airway disease. Respiratory Medicine 2008; ) Thorsson L, Geller D. Factors guiding the choice of delivery device for inhaled corticosteroids in the long-term management of stable asthma and COPD: focus on budesonide. Resp Med 2005; 99: ) Dane E, Funda C, Esra K U, Eser G Y, Mehmet K, Ercument E, Oktay G. Clinical effectiveness of nebulised budesonide in the treatment of acute asthma attacks. Tuberkulozve Torak Dergisi. 54(2): , ) Standardization of spirometry, 1994 update. American Thoracic Society. Am J Resspir Crit Full Length Original Research Paper ANOVA F value P value Care Med 1995; 152(3): ) American Thoracic Society: Standards for the diagnosis and care of patients with chronic 7) Obstructive pulmonary disease and asthma. AmRevRespir Dis1987: 136:
8 Dr. Dilshad Ali Rizvi et al; Comparative Efficacy Of Budesonide By Different Devices 8) Calverley P, Pauwels R, Lofdahl CG, SvenssonK, Higenbottam T, Carlsson IG, Stahl E. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. EurRespir J 2005; 26: ) Census of India: Provisional Population Total (Paper 2001). Registrar and Census Commissioner for India, New Delhi, ) Bisgaard H, Nikander K, Munch E. Comparative study of budesonide as a nebulized suspension vs pressurized metered dose inhaler in adult asthamatics. Respiratory Medicine 1998; 92: ) Engel T, Heinig JH, Malling HJ, Scharling B, Nikander K, Madsen F. Clinical comparison of inhaled budesonide delivered either via pressurized metered dose inhaler or Turbuhaler. Allergy 1989; 44: ) Kerwin EM, Pearlman DS, De Guia T, Carlsson LG, Gillen M, Uryniak T, Simonson SG. Evaluation of efficacy and safety of budesonide delivered via two dry powder inhalers. Curr Med Res Opin 2008; 24(5): ) Morice AH, Hochmuth L, Ekelund J, Thoren A, Puterman AS. Comparable long term safety and efficacy of a novel budesonide/formoterol pressurized metered dose inhaler versus budesonide/formoterolturbuhaler in adolescents and adults with asthma. PulmPharmacolTher 2008; 21(1): ) Volovitz B. inhaled budesonide in the management of acute worsening and exacerbations of asthma: a review of the evidence. Respir Med 2007 Apr; 101(4): Article History: Date of Submission: Date of Acceptance: Conflict of Interest: NIL Source of Support: NONE Page 240
Meenu Singh, Joseph L. Mathew, Prabhjot Malhi, B.R. Srinivas and Lata Kumar
 Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide.
 Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide. By: DR MOHD SHAMSUL AMRI Supervisor: Associate Professor Dr
Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide. By: DR MOHD SHAMSUL AMRI Supervisor: Associate Professor Dr
International Journal of Medical Research & Health Sciences
 International Journal of Medical Research & Health Sciences www.ijmrhs.com Volume 2 Issue 3 July - Sep Coden: IJMRHS Copyright @2013 ISSN: 2319-5886 Received: 23 th May 2013 Revised: 24 th Jun 2013 Accepted:
International Journal of Medical Research & Health Sciences www.ijmrhs.com Volume 2 Issue 3 July - Sep Coden: IJMRHS Copyright @2013 ISSN: 2319-5886 Received: 23 th May 2013 Revised: 24 th Jun 2013 Accepted:
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Correspondence: C-G. Löfdahl Dept of Respiratory Medicine and Allergology University Hospital S Lund Sweden.
 Eur Respir J 1997; 10: 2474 2478 DOI: 10.1183/09031936.97.10112474 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Differences in bronchodilating
Eur Respir J 1997; 10: 2474 2478 DOI: 10.1183/09031936.97.10112474 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Differences in bronchodilating
DATE: 09 December 2009 CONTEXT AND POLICY ISSUES:
 TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence
 Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
GINA. At-A-Glance Asthma Management Reference. for adults, adolescents and children 6 11 years. Updated 2017
 GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
On completion of this chapter you should be able to: discuss the stepwise approach to the pharmacological management of asthma in children
 7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Diagnosis, Treatment and Management of Asthma
 Diagnosis, Treatment and Management of Asthma Asthma is a complex disorder characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and an underlying inflammation.
Diagnosis, Treatment and Management of Asthma Asthma is a complex disorder characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and an underlying inflammation.
SHORT COMMUNICATION. Abstract. Kevin R. Murphy, 1 Tom Uryniak, 2 Ubaldo J. Martin 2 and James Zangrilli 2
 SHORT COMMUNICATION Drugs R D 212; 12 (1): 9-14 1179-691/12/1-9 ª 212 Murphy et al., publisher and licensee Adis Data Information BV. This is an open access article published under the terms of the Creative
SHORT COMMUNICATION Drugs R D 212; 12 (1): 9-14 1179-691/12/1-9 ª 212 Murphy et al., publisher and licensee Adis Data Information BV. This is an open access article published under the terms of the Creative
ADULT ASTHMA GUIDE SUMMARY. This summary provides busy health professionals with key guidance for assessing and treating adult asthma.
 ADULT ASTHMA GUIDE SUMMARY This summary provides busy health professionals with key guidance for assessing and treating adult asthma. Its source document Asthma and Respiratory Foundation NZ Adult Asthma
ADULT ASTHMA GUIDE SUMMARY This summary provides busy health professionals with key guidance for assessing and treating adult asthma. Its source document Asthma and Respiratory Foundation NZ Adult Asthma
Budesonide treatment of moderate and severe asthma in children: A doseresponse
 Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS
 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren, MD
 Therapeutic Effect of Zafirlukast as Monotherapy in Steroid-Naive Patients With Severe Persistent Asthma* James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren,
Therapeutic Effect of Zafirlukast as Monotherapy in Steroid-Naive Patients With Severe Persistent Asthma* James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren,
Montelukast vs. Inhaled Low-Dose Budesonide as Monotherapy in the Treatment of Mild Persistent Asthma: A Randomized Double Blind Controlled Trial
 Montelukast vs. Inhaled Low-Dose Budesonide as Monotherapy in the Treatment of Mild Persistent Asthma: A Randomized Double Blind Controlled Trial by Vikram Kumar, a P. Ramesh, a Rakesh Lodha, a R. M. Pandey,
Montelukast vs. Inhaled Low-Dose Budesonide as Monotherapy in the Treatment of Mild Persistent Asthma: A Randomized Double Blind Controlled Trial by Vikram Kumar, a P. Ramesh, a Rakesh Lodha, a R. M. Pandey,
COPD. Breathing Made Easier
 COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
SCREENING AND PREVENTION
 These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
This is a cross-sectional analysis of the National Health and Nutrition Examination
 SUPPLEMENTAL METHODS Study Design and Setting This is a cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES) data 2007-2008, 2009-2010, and 2011-2012. The NHANES is
SUPPLEMENTAL METHODS Study Design and Setting This is a cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES) data 2007-2008, 2009-2010, and 2011-2012. The NHANES is
A multitude of devices
 A multitude of devices Dr Andrew Scroop Respiratory Consultants 15 th September 2018 STEPWISE PHARMACOLOGICAL MANAGEMENT OF STABLE COPD COPD Inhalers MILD FEV 1 60 80% predicted few symptoms breathless
A multitude of devices Dr Andrew Scroop Respiratory Consultants 15 th September 2018 STEPWISE PHARMACOLOGICAL MANAGEMENT OF STABLE COPD COPD Inhalers MILD FEV 1 60 80% predicted few symptoms breathless
(Asthma) Diagnosis, monitoring and chronic asthma management
 Dubai Standards of Care 2018 (Asthma) Diagnosis, monitoring and chronic asthma management Preface Asthma is one of the most common problem dealt with in daily practice. In Dubai, the management of chronic
Dubai Standards of Care 2018 (Asthma) Diagnosis, monitoring and chronic asthma management Preface Asthma is one of the most common problem dealt with in daily practice. In Dubai, the management of chronic
Asthma. chapter 7. Overview
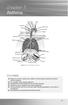 chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
Study of dynamic lung parameters in bronchial Asthma
 20; 4(1): 312-317 ISSN Print: 2394-7500 ISSN Online: 2394-5869 Impact Factor: 5.2 IJAR 20; 4(1): 312-317 www.allresearchjournal.com Received: 20-11-2017 Accepted: 21-12-2017 Dr. Madhuchhanda Pattnaik Associate
20; 4(1): 312-317 ISSN Print: 2394-7500 ISSN Online: 2394-5869 Impact Factor: 5.2 IJAR 20; 4(1): 312-317 www.allresearchjournal.com Received: 20-11-2017 Accepted: 21-12-2017 Dr. Madhuchhanda Pattnaik Associate
ADASUVE (LOXAPINE) INHALATION POWDER. EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS
 ADASUVE (LOXAPINE) INHALATION POWDER EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS August2017 December 2012 PMR-JUN-2017-0017 ADASUVE Risk Evaluation and Mitigation Strategy (REMS) Education Program Content
ADASUVE (LOXAPINE) INHALATION POWDER EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS August2017 December 2012 PMR-JUN-2017-0017 ADASUVE Risk Evaluation and Mitigation Strategy (REMS) Education Program Content
Performing a Methacholine Challenge Test
 powder for solution, for inhalation Performing a Methacholine Challenge Test Provocholine is a registered trademark of Methapharm Inc. Copyright Methapharm Inc. 2016. All rights reserved. Healthcare professionals
powder for solution, for inhalation Performing a Methacholine Challenge Test Provocholine is a registered trademark of Methapharm Inc. Copyright Methapharm Inc. 2016. All rights reserved. Healthcare professionals
Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma Full Report 2007
 Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma Full Report 2007 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Selecting Initial Therapy
Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma Full Report 2007 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Selecting Initial Therapy
BUDESONIDE AND FORMOTEROL (SYMBICORT ): Α A REVIEW
 Volume 23, Issue 3 December 2007 BUDESONIDE AND FORMOTEROL (SYMBICORT ): A REVIEW Donna L. Smith, Pharm. D. Candidate More than 22 million people in the United States have asthma according to the Centers
Volume 23, Issue 3 December 2007 BUDESONIDE AND FORMOTEROL (SYMBICORT ): A REVIEW Donna L. Smith, Pharm. D. Candidate More than 22 million people in the United States have asthma according to the Centers
Pharmacological Management of Obstructive Airways in Humans. Introduction to Scientific Research. Submitted: 12/4/08
 Pharmacological Management of Obstructive Airways in Humans Introduction to Scientific Research Submitted: 12/4/08 Introduction: Obstructive airways can be characterized as inflammation or structural changes
Pharmacological Management of Obstructive Airways in Humans Introduction to Scientific Research Submitted: 12/4/08 Introduction: Obstructive airways can be characterized as inflammation or structural changes
Asthma Management for the Athlete
 Asthma Management for the Athlete Khanh Lai, MD Assistant Professor Division of Pediatric Pulmonary and Sleep Medicine University of Utah School of Medicine 2 nd Annual Sports Medicine Symposium: The Pediatric
Asthma Management for the Athlete Khanh Lai, MD Assistant Professor Division of Pediatric Pulmonary and Sleep Medicine University of Utah School of Medicine 2 nd Annual Sports Medicine Symposium: The Pediatric
International co-ordinating investigator Dr Dencho Osmanliev, St Sofia, Pulmonary Dept, 19 D Nestorov Str, Sofia, 1431, Bulgaria.
 Drug product: SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0681 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0681 Date: 24 Oktober, 2003 (For national
Drug product: SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0681 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0681 Date: 24 Oktober, 2003 (For national
Treatment. Assessing the outcome of interventions Traditionally, the effects of interventions have been assessed by measuring changes in the FEV 1
 58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
Pathology of Asthma Epidemiology
 Asthma A Presentation on Asthma Management and Prevention What Is Asthma? A chronic disease of the airways that may cause Wheezing Breathlessness Chest tightness Nighttime or early morning coughing Pathology
Asthma A Presentation on Asthma Management and Prevention What Is Asthma? A chronic disease of the airways that may cause Wheezing Breathlessness Chest tightness Nighttime or early morning coughing Pathology
RESPIRATORY CARE IN GENERAL PRACTICE
 RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
Bronchial asthma. E. Cserháti 1 st Department of Paediatrics. Lecture for english speaking students 5 February 2013
 Bronchial asthma E. Cserháti 1 st Department of Paediatrics Lecture for english speaking students 5 February 2013 Epidemiology of childhood bronchial asthma Worldwide prevalence of 7-8 and 13-14 years
Bronchial asthma E. Cserháti 1 st Department of Paediatrics Lecture for english speaking students 5 February 2013 Epidemiology of childhood bronchial asthma Worldwide prevalence of 7-8 and 13-14 years
Supplementary Medications during asthma attack. Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus
 Supplementary Medications during asthma attack Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus Conflicts of Interest None Definition of Asthma Airway narrowing that is
Supplementary Medications during asthma attack Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus Conflicts of Interest None Definition of Asthma Airway narrowing that is
Asthma Assessment & Review
 ASTHMA RESOURCE PACK Section 5B Asthma Assessment & Review In this section: 1. Primary Care initial assessment and review Asthma Resource Pack Section 5B: Asthma Assessment & Review Version 3.0 Last Updated:
ASTHMA RESOURCE PACK Section 5B Asthma Assessment & Review In this section: 1. Primary Care initial assessment and review Asthma Resource Pack Section 5B: Asthma Assessment & Review Version 3.0 Last Updated:
MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C
 MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C Explain the importance of objective measures in the management of asthma Explain the different types of objective measures used in the management
MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C Explain the importance of objective measures in the management of asthma Explain the different types of objective measures used in the management
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A.
 aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
SPIROMETRY TECHNIQUE. Jim Reid New Zealand
 Jim Reid New Zealand The Basics Jim Reid Spirometry measures airflow and lung volumes, and is the preferred lung function test in COPD. By measuring reversibility of obstruction, it is also diagnostic
Jim Reid New Zealand The Basics Jim Reid Spirometry measures airflow and lung volumes, and is the preferred lung function test in COPD. By measuring reversibility of obstruction, it is also diagnostic
GUIDE LINE SUMMARY THE DIAGNOSIS AND TREATMENT OF ADULT ASTHMA BEST PRACTICE EV I DENCE-BASED KEY MESSAGES. Diagnosis.
 THE DIAGSIS AND TREATMENT OF ADULT ASTHMA The purpose of the guideline is to provide an evidence-based summary of the diagnostic management and treatment options available for asthma in the adult population
THE DIAGSIS AND TREATMENT OF ADULT ASTHMA The purpose of the guideline is to provide an evidence-based summary of the diagnostic management and treatment options available for asthma in the adult population
VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide
 VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide MODULE A: MAAGEMET OF COPD 1 2 Patient with suspected or confirmed COPD presents to primary care [ A ] See sidebar A Perform brief clinical
VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide MODULE A: MAAGEMET OF COPD 1 2 Patient with suspected or confirmed COPD presents to primary care [ A ] See sidebar A Perform brief clinical
Dual-Controller Asthma Therapy: Rationale and Clinical Benefits
 B/1 Dual-Controller Asthma Therapy: Rationale and Clinical Benefits MODULE B The 1997 National Heart, Lung, and Blood Institute (NHLBI) Expert Panel guidelines on asthma management recommend a 4-step approach
B/1 Dual-Controller Asthma Therapy: Rationale and Clinical Benefits MODULE B The 1997 National Heart, Lung, and Blood Institute (NHLBI) Expert Panel guidelines on asthma management recommend a 4-step approach
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL
 DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
National Asthma Educator Certification Board Detailed Content Outline
 I. THE ASTHMA CONDITION 9 20 1 30 A. Pathophysiology 4 6 0 10 1. Teach an individual with asthma and their family using simple language by illustrating the following with appropriate educational aids a.
I. THE ASTHMA CONDITION 9 20 1 30 A. Pathophysiology 4 6 0 10 1. Teach an individual with asthma and their family using simple language by illustrating the following with appropriate educational aids a.
Tips on managing asthma in children
 Tips on managing asthma in children Dr Ranjan Suri Consultant in Respiratory Paediatrics Bupa Cromwell Hospital Clinics: Friday (pm) Asthma in Children Making the diagnosis Patterns of childhood asthma
Tips on managing asthma in children Dr Ranjan Suri Consultant in Respiratory Paediatrics Bupa Cromwell Hospital Clinics: Friday (pm) Asthma in Children Making the diagnosis Patterns of childhood asthma
Comparison of the Effect of Short Course of Oral Prednisone in Patients with Acute Asthma
 ISPUB.COM The Internet Journal of Pulmonary Medicine Volume 7 Number 1 Comparison of the Effect of Short Course of Oral Prednisone in Patients with Acute Asthma E Razi, G Moosavi Citation E Razi, G Moosavi.
ISPUB.COM The Internet Journal of Pulmonary Medicine Volume 7 Number 1 Comparison of the Effect of Short Course of Oral Prednisone in Patients with Acute Asthma E Razi, G Moosavi Citation E Razi, G Moosavi.
Draft Guidance on Fluticasone Propionate; Salmeterol Xinafoate. Fluticasone Propionate; Salmeterol Xinafoate. Powder/Inhalation
 Reprinted from FDA s website by EAS Consulting Group, LLC Contains Nonbinding Recommendations Draft Guidance on Fluticasone Propionate; Salmeterol Xinafoate This draft guidance, once finalized, will represent
Reprinted from FDA s website by EAS Consulting Group, LLC Contains Nonbinding Recommendations Draft Guidance on Fluticasone Propionate; Salmeterol Xinafoate This draft guidance, once finalized, will represent
Respiratory Health. Asthma and COPD
 Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Bronchodilator Delivery and Nebuliser Trials in Adults
 Bronchodilator Delivery and Nebuliser Trials in Adults Acute Management Favour the use of MDI (+/- Spacer) If considering nebuliser Short term treatment Approx. < 3 weeks See optimisation of inhaled bronchodilators
Bronchodilator Delivery and Nebuliser Trials in Adults Acute Management Favour the use of MDI (+/- Spacer) If considering nebuliser Short term treatment Approx. < 3 weeks See optimisation of inhaled bronchodilators
Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016
 Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016 Diagnosis: There is no lower limit to the age at which
Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016 Diagnosis: There is no lower limit to the age at which
Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate)
 EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
SYNOPSIS. Drug substance(s) Budesonide/formoterol Document No. Edition No. Study code SD Date 16 December 2004
 Drug product SYMBICORT pmdi 160/4.5 µg SYNOPSIS Drug substance(s) Budesonide/formoterol Document No. Edition No. Date 16 December 2004 A Twelve-Week, Randomized, Double-Blind, Double-Dummy, Placebo-Controlled
Drug product SYMBICORT pmdi 160/4.5 µg SYNOPSIS Drug substance(s) Budesonide/formoterol Document No. Edition No. Date 16 December 2004 A Twelve-Week, Randomized, Double-Blind, Double-Dummy, Placebo-Controlled
CONTENTS. Ageing and Asthma Presentation of Asthma in the Elderly Diagnosis of Asthma in the Elderly... 2
 23 24 PREFACE Asthma in older people is common and is an increasingly serious health issue. It is estimated that the number of older people with asthma will rise in the next 20 years because of the worldwide
23 24 PREFACE Asthma in older people is common and is an increasingly serious health issue. It is estimated that the number of older people with asthma will rise in the next 20 years because of the worldwide
Daclizumab improves asthma control in patients with moderate to. severe persistent asthma: A randomized, controlled trial
 Daclizumab improves asthma control in patients with moderate to severe persistent asthma: A randomized, controlled trial William W. Busse, MD, Elliot Israel, MD, Harold S. Nelson, MD, James W. Baker, MD,
Daclizumab improves asthma control in patients with moderate to severe persistent asthma: A randomized, controlled trial William W. Busse, MD, Elliot Israel, MD, Harold S. Nelson, MD, James W. Baker, MD,
Allwin Mercer Dr Andrew Zurek
 Allwin Mercer Dr Andrew Zurek 1 in 11 people are currently receiving treatment for asthma (5.4 million people in the UK) Every 10 seconds, someone is having a potentially life-threatening asthma attack
Allwin Mercer Dr Andrew Zurek 1 in 11 people are currently receiving treatment for asthma (5.4 million people in the UK) Every 10 seconds, someone is having a potentially life-threatening asthma attack
Asthma: Chronic Management. Yung-Yang Liu, MD Attending physician, Chest Department Taipei Veterans General Hospital April 26, 2015
 Asthma: Chronic Management Yung-Yang Liu, MD Attending physician, Chest Department Taipei Veterans General Hospital April 26, 2015 Global Strategy for Asthma Management and Prevention Evidence-based Implementation
Asthma: Chronic Management Yung-Yang Liu, MD Attending physician, Chest Department Taipei Veterans General Hospital April 26, 2015 Global Strategy for Asthma Management and Prevention Evidence-based Implementation
ORIGINAL RESEARCH ARTICLE. A. Mirici, M. Meral and M. Akgun. Abstract
 ORIGINAL RESEARCH ARTICLE Clin Drug Invest 2003; 23 (1): 55-62 1173-2563/03/0001-0055/$230.00/0 Adis International Limited. All rights reserved. Comparison of the Efficacy of Nebulised Budesonide with
ORIGINAL RESEARCH ARTICLE Clin Drug Invest 2003; 23 (1): 55-62 1173-2563/03/0001-0055/$230.00/0 Adis International Limited. All rights reserved. Comparison of the Efficacy of Nebulised Budesonide with
Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended.
 Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended Bricanyl () DK/W/0017/pdWS/001 Rapporteur: Denmark Finalisation procedure
Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended Bricanyl () DK/W/0017/pdWS/001 Rapporteur: Denmark Finalisation procedure
Using an Inhaler and Nebulizer
 Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
TORCH: Salmeterol and Fluticasone Propionate and Survival in COPD
 TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
The clinical effectiveness and costeffectiveness. treatment of chronic asthma in children under the age of 12 years
 The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
PDF of Trial CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Wed, 31 Oct 2018 11:13:48 GMT) CTRI Number Last Modified On 17/01/2015 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Wed, 31 Oct 2018 11:13:48 GMT) CTRI Number Last Modified On 17/01/2015 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Principal Investigator. Study center(s) This was a single-center study. Publications None at the time of writing this report.
 (For national authority SYNOPSIS use only) Date 3 March 2005 An open, randomized, two-way crossover study evaluating the pharmacokinetics of budesonide and formoterol from Symbicort pmdi versus Oxis Turbuhaler
(For national authority SYNOPSIS use only) Date 3 March 2005 An open, randomized, two-way crossover study evaluating the pharmacokinetics of budesonide and formoterol from Symbicort pmdi versus Oxis Turbuhaler
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
beclometasone 100 MDI 2 puffs twice a day (recently changed to non CFC (Clenil Modulite))
 Case 1 Mr Thomson, a 32 year old asthmatic who is well known to you comes into your pharmacy. He is known to have a best peak flow of 640 L/min. He tells you that over the last few weeks he has been wakening
Case 1 Mr Thomson, a 32 year old asthmatic who is well known to you comes into your pharmacy. He is known to have a best peak flow of 640 L/min. He tells you that over the last few weeks he has been wakening
Presented by the California Academy of Family Physicians 2013/California Academy of Family Physicians
 Family Medicine and Patient-Centered Asthma Care Presented by the California Academy of Family Physicians Faculty: Hobart Lee, MD Disclosures: Jeffrey Luther, MD, Program Director, Memorial Family Medicine
Family Medicine and Patient-Centered Asthma Care Presented by the California Academy of Family Physicians Faculty: Hobart Lee, MD Disclosures: Jeffrey Luther, MD, Program Director, Memorial Family Medicine
Asthma in Day to Day Practice
 Asthma in Day to Day Practice VIJAY.K.VANAM Financial relationships: Disclosures Employed at Mercy Medical Center, Mason City. Nonfinancial relationships: I receive no financial gain from any pharmaceutical
Asthma in Day to Day Practice VIJAY.K.VANAM Financial relationships: Disclosures Employed at Mercy Medical Center, Mason City. Nonfinancial relationships: I receive no financial gain from any pharmaceutical
In 2002, it was reported that 72 of 1000
 REPORTS Aligning Patient Care and Asthma Treatment Guidelines Eric Cannon, PharmD Abstract This article describes how the National Asthma Education and Prevention Program Guidelines for the Diagnosis and
REPORTS Aligning Patient Care and Asthma Treatment Guidelines Eric Cannon, PharmD Abstract This article describes how the National Asthma Education and Prevention Program Guidelines for the Diagnosis and
Efficacy and Safety of Montelukast as Monotherapy in Children with Mild Persistent Asthma. Gautam Ghosh, Arun Kumar Manglik and Subhasis Roy
 Efficacy and Safety of Montelukast as Monotherapy in Children with Mild Persistent Asthma Gautam Ghosh, Arun Kumar Manglik and Subhasis Roy From the Shree Jain Hospital & Research Center, Howrah 711 102
Efficacy and Safety of Montelukast as Monotherapy in Children with Mild Persistent Asthma Gautam Ghosh, Arun Kumar Manglik and Subhasis Roy From the Shree Jain Hospital & Research Center, Howrah 711 102
Asthma Update A/Prof. John Abisheganaden. Senior Consultant, Dept Of Respiratory & Crit Care Medicine Tan Tock Seng Hospital
 Asthma Update - 2013 A/Prof. John Abisheganaden Senior Consultant, Dept Of Respiratory & Crit Care Medicine Tan Tock Seng Hospital Asthma A complex syndrome Multifaceted disease Heterogeneous Genetic and
Asthma Update - 2013 A/Prof. John Abisheganaden Senior Consultant, Dept Of Respiratory & Crit Care Medicine Tan Tock Seng Hospital Asthma A complex syndrome Multifaceted disease Heterogeneous Genetic and
Asthma training. Mike Levin Division of Asthma and Allergy Red Cross Hospital
 Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Clinical Practice Guideline: Asthma
 Clinical Practice Guideline: Asthma INTRODUCTION A critical aspect of the diagnosis and management of asthma is the precise and periodic measurement of lung function both before and after bronchodilator
Clinical Practice Guideline: Asthma INTRODUCTION A critical aspect of the diagnosis and management of asthma is the precise and periodic measurement of lung function both before and after bronchodilator
Do current treatment protocols adequately prevent airway remodeling in children with mild intermittent asthma?
 Respiratory Medicine (2006) 100, 458 462 Do current treatment protocols adequately prevent airway remodeling in children with mild intermittent asthma? Haim S. Bibi a,, David Feigenbaum a, Mariana Hessen
Respiratory Medicine (2006) 100, 458 462 Do current treatment protocols adequately prevent airway remodeling in children with mild intermittent asthma? Haim S. Bibi a,, David Feigenbaum a, Mariana Hessen
THE NEW ZEALAND MEDICAL JOURNAL
 THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1267 ISSN 1175 8716 Is Salamol less effective than Ventolin? A randomised, blinded, crossover study in New Zealand Catherina L Chang, Manisha Cooray, Graham Mills,
THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1267 ISSN 1175 8716 Is Salamol less effective than Ventolin? A randomised, blinded, crossover study in New Zealand Catherina L Chang, Manisha Cooray, Graham Mills,
Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol)
 EMA/126654/2014 Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for DuoResp Spiromax, which details the measures
EMA/126654/2014 Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for DuoResp Spiromax, which details the measures
Study No.: Title: Rationale: Phase: Study Period Study Design: Centres: Indication: Treatment: Objectives : Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Abnormal Spirometry Medical Risk in Aviation Conference Royal Aeronautical Society, Dec 2017
 Abnormal Spirometry Medical Risk in Aviation Conference Royal Aeronautical Society, Dec 2017 Professor Howard Branley MBChB MSc MD FCCP FRCP FRAeS Consultant in Respiratory Medicine What I want to cover
Abnormal Spirometry Medical Risk in Aviation Conference Royal Aeronautical Society, Dec 2017 Professor Howard Branley MBChB MSc MD FCCP FRCP FRAeS Consultant in Respiratory Medicine What I want to cover
Chitra C. Nair et al. Int. Res. J. Pharm. 2014, 5 (5) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article EVALUATION OF THE KNOWLEDGE OF PATIENTS, COMPLIANCE TO TREATMENT AND THE IMPACT OF PATIENT EDUCATION ON ASTHMA:
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article EVALUATION OF THE KNOWLEDGE OF PATIENTS, COMPLIANCE TO TREATMENT AND THE IMPACT OF PATIENT EDUCATION ON ASTHMA:
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Individual Study Table Referring to Part of the Dossier. Volume:
 Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Asthma in the Athlete
 Asthma in the Athlete Jorge E. Gomez, MD Associate Professor Texas Children s Hospital Baylor College of Medicine Assist Team Physician UH Understand how we diagnose asthma Objectives Be familiar with
Asthma in the Athlete Jorge E. Gomez, MD Associate Professor Texas Children s Hospital Baylor College of Medicine Assist Team Physician UH Understand how we diagnose asthma Objectives Be familiar with
Chronic obstructive pulmonary disease (COPD) is characterized
 DANIEL E. HILLEMAN, PharmD ABSTRACT OBJECTIVE: To review the role of long-acting bronchodilators in the treatment of chronic obstructive pulmonary disease (COPD), including the importance of treatment
DANIEL E. HILLEMAN, PharmD ABSTRACT OBJECTIVE: To review the role of long-acting bronchodilators in the treatment of chronic obstructive pulmonary disease (COPD), including the importance of treatment
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone
ASTHMA & RESPIRATORY FOUNDATION NZ ADULT ASTHMA GUIDELINES: A QUICK REFERENCE GUIDE 1
 ASTHMA & RESPIRATORY FOUNDATION NZ ADULT ASTHMA GUIDELINES: A QUICK REFERENCE GUIDE 1 1. Richard Beasley, Bob Hancox, Matire Harwood, Kyle Perrin, Betty Poot, Janine Pilcher, Jim Reid, Api Talemaitoga,
ASTHMA & RESPIRATORY FOUNDATION NZ ADULT ASTHMA GUIDELINES: A QUICK REFERENCE GUIDE 1 1. Richard Beasley, Bob Hancox, Matire Harwood, Kyle Perrin, Betty Poot, Janine Pilcher, Jim Reid, Api Talemaitoga,
Decramer 2014 a &b [21]
![Decramer 2014 a &b [21] Decramer 2014 a &b [21]](/thumbs/90/101390504.jpg) Buhl 2015 [19] Celli 2014 [20] Decramer 2014 a &b [21] D Urzo 2014 [22] Maleki-Yazdi 2014 [23] Inclusion criteria: Diagnosis of chronic obstructive pulmonary disease; 40 years of age or older; Relatively
Buhl 2015 [19] Celli 2014 [20] Decramer 2014 a &b [21] D Urzo 2014 [22] Maleki-Yazdi 2014 [23] Inclusion criteria: Diagnosis of chronic obstructive pulmonary disease; 40 years of age or older; Relatively
Study No.: SFCA3007 Title: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial Evaluating the Safety and Efficacy of the DISKUS
 Study No.: A3007 Title: A Randomized, Double-Blind, -Controlled, Parallel-Group Trial Evaluating the Safety and Efficacy of the DISKUS Formulations of Salmeterol (SAL) 50mcg BID and Fluticasone Propionate
Study No.: A3007 Title: A Randomized, Double-Blind, -Controlled, Parallel-Group Trial Evaluating the Safety and Efficacy of the DISKUS Formulations of Salmeterol (SAL) 50mcg BID and Fluticasone Propionate
Public Dissemination
 1. THE ASTHMA CONDITION 9 18 3 30 A. Pathophysiology 4 6 0 10 1. Teach an individual with asthma and their family using simple language by illustrating the following with appropriate educational aids a.
1. THE ASTHMA CONDITION 9 18 3 30 A. Pathophysiology 4 6 0 10 1. Teach an individual with asthma and their family using simple language by illustrating the following with appropriate educational aids a.
Asthma Medications: Information for Children and Families. What You Need to Know about Medicines for Asthma
 Page 1 of 8 PED-ALL-005-1992 Asthma Medications: Information for Children and Families What You Need to Know about Medicines for Asthma What Medicines Are used to Treat Asthma? There are two kinds of medicines:
Page 1 of 8 PED-ALL-005-1992 Asthma Medications: Information for Children and Families What You Need to Know about Medicines for Asthma What Medicines Are used to Treat Asthma? There are two kinds of medicines:
THE CHALLENGES OF COPD MANAGEMENT IN PRIMARY CARE An Expert Roundtable
 THE CHALLENGES OF COPD MANAGEMENT IN PRIMARY CARE An Expert Roundtable This activity is supported by an educational grant from Sunovion Pharmaceuticals Inc. COPD in the United States Third leading cause
THE CHALLENGES OF COPD MANAGEMENT IN PRIMARY CARE An Expert Roundtable This activity is supported by an educational grant from Sunovion Pharmaceuticals Inc. COPD in the United States Third leading cause
Relationship Between FEV1& PEF in Patients with Obstructive Airway Diseases
 OBSTRUCTIVE THE IRAQI POSTGRADUATE AIRWAY MEDICAL DISEASES JOURNAL Relationship Between FEV1& PEF in Patients with Obstructive Airway Diseases Muhammed.W.AL.Obaidy *, Kassim Mhamed Sultan*,Basil Fawzi
OBSTRUCTIVE THE IRAQI POSTGRADUATE AIRWAY MEDICAL DISEASES JOURNAL Relationship Between FEV1& PEF in Patients with Obstructive Airway Diseases Muhammed.W.AL.Obaidy *, Kassim Mhamed Sultan*,Basil Fawzi
Evaluation of Student Pharmacist and Pharmacist Impact on Disease State Management and Patient Satisfaction in Adult Patients with Asthma
 Evaluation of Student Pharmacist and Pharmacist Impact on Disease State Management and Patient Satisfaction in Adult Patients with Asthma Jamie L. McConaha, PharmD, CPG, TTS, BCACP; Brooke M. Jackson,
Evaluation of Student Pharmacist and Pharmacist Impact on Disease State Management and Patient Satisfaction in Adult Patients with Asthma Jamie L. McConaha, PharmD, CPG, TTS, BCACP; Brooke M. Jackson,
ASTHMA PROTOCOL CELLO
 ASTHMA PROTOCOL CELLO Leiden May 2011 1 Introduction This protocol includes an explanation of the clinical picture, diagnosis, objectives, non medical and medical therapy and a scheme for inhaler dosage.
ASTHMA PROTOCOL CELLO Leiden May 2011 1 Introduction This protocol includes an explanation of the clinical picture, diagnosis, objectives, non medical and medical therapy and a scheme for inhaler dosage.
Improving the Management of Asthma to Improve Patient Adherence and Outcomes
 Improving the Management of Asthma to Improve Patient Adherence and Outcomes Robert Sussman, MD Atlantic Health System Overlook Medical Center Asthma Remains a Serious Health Risk in the US Every day in
Improving the Management of Asthma to Improve Patient Adherence and Outcomes Robert Sussman, MD Atlantic Health System Overlook Medical Center Asthma Remains a Serious Health Risk in the US Every day in
Scottish Medicines Consortium
 Scottish Medicines Consortium budesonide/formoterol 100/6, 200/6 turbohaler (Symbicort SMART ) No. (362/07) Astra Zeneca UK Limited 9 March 2007 (Issued May 2007) The Scottish Medicines Consortium (SMC)
Scottish Medicines Consortium budesonide/formoterol 100/6, 200/6 turbohaler (Symbicort SMART ) No. (362/07) Astra Zeneca UK Limited 9 March 2007 (Issued May 2007) The Scottish Medicines Consortium (SMC)
