Host Cellular Immune Response to Pneumococcal Lung Infection in Mice
|
|
|
- Bruno Ryan
- 5 years ago
- Views:
Transcription
1 INFECTION AND IMMUNITY, Feb. 2000, p Vol. 68, No /00/$ Copyright 2000, American Society for Microbiology. All Rights Reserved. Host Cellular Immune Response to Pneumococcal Lung Infection in Mice ARAS KADIOGLU, 1 NEILL A. GINGLES, 1 KATE GRATTAN, 1 ALISON KERR, 2 TIM J. MITCHELL, 2 AND PETER W. ANDREW 1 * Department of Microbiology & Immunology, University of Leicester, Leicester, 1 and Division of Infection & Immunity, University of Glasgow, Glasgow, 2 United Kingdom Received 17 June 1999/Returned for modification 9 September 1999/Accepted 5 November 1999 Although there is substantial evidence that pneumolysin is an important virulence factor in pneumococcal pneumonia, relatively little is known about how it influences cellular infiltration into the lungs. We investigated how the inability of mutant pneumococci to produce pneumolysin altered the pattern of inflammation and cellular infiltration into the lungs. The effect on bacterial growth in the lungs also was assessed. There were three phases of growth of wild-type bacteria in the lungs: a decline followed by a rapid increase and then stasis or decline. The absence of pneumolysin was associated with a more rapid early decline and then a much slower increase in numbers. The pattern of inflammatory-cell accumulation also had distinct stages, and the timing of these stages was influenced by the presence of pneumolysin. Neutrophils began to accumulate about 12 to 16 h after infection with wild-type pneumococci. This accumulation occurred after the early decline in pneumococcal numbers but coincided with the period of rapid growth. Following infection with pneumococci unable to make pneumolysin, neutrophil influx was slower and less intense. Coincident with the third stage of pneumococcal growth was an accumulation of T and B lymphocytes at the sites of inflammation, but the accumulation was not associated with an increase in the total number of lymphocytes in the lungs. Lymphocyte accumulation in the absence of pneumolysin occurred but was delayed. Streptococcus pneumoniae is an important respiratory pathogen of humans, causing pneumonia (lobar and bronchopneumonia), septicemia, otitis media, and meningitis. The pneumococcus produces several factors that may be important in the development of disease. One such factor is the pneumococcal toxin pneumolysin. We have shown that pneumolysin is a multifunctional toxin that exhibits cytolytic activity (hemolysis), and at sublytic concentrations it is known to alter the functioning of immune cells (1, 15). This modulation of cells and thus the activity of the immune system includes the inhibition of ciliary beat on human respiratory epithelium (8, 9), the stimulation of tumor necrosis factor alpha and interleukin-1 release from human monocytes (12), the activation of phospholipase A 2 in pulmonary cells (20), and the inhibition of the neutrophil respiratory burst (18). The toxin also activates the classical complement pathway in the absence of antipneumolysin antibody (16). Pneumolysin plays an important but as yet not completely defined role in the development of bronchopneumonia. It has been previously shown that pneumococci not expressing pneumolysin have reduced virulence in the mouse compared to the wild-type organism, with slower pneumococcal growth in the lungs and delayed development of associated septicemia, culminating in a general reduction in the severity of the inflammatory response (6). It has also been shown that immunization with a genetically engineered toxoid version of pneumolysin protects mice from bronchopneumonia (2). It is also worth noting that pneumolysin alone can reproduce the symptoms of pneumococcal disease in the lungs (9). Hence, either by direct damage to the host or by stimulation of host inflammatory mediators leading to bronchopneumonia, pneumolysin may play an important role in the induction and further maintenance of the inflammatory response. Despite a considerable amount of work illustrating the requirement for pneumolysin in bronchopneumonia, studies examining host tissue pathogenesis and the interactions between bacterial and host factors in bronchopneumonia are rare. In this paper, we discuss how bronchopneumonia develops in a murine model of bronchopneumonia and septicemia due to both wild-type Streptococcus pneumoniae and a pneumolysinnegative mutant. We monitored for the first time in this particular animal model the early events involved in the initial progression of bronchopneumonia and its subsequent development after intranasal infection. We have focused not only on bacterial growth kinetics but also on the host tissue response in terms of the onset of inflammation, the timing of inflammatory cell infiltrate into lungs, the nature and type of host immune cells involved in these processes, and the development of tissue histopathology. Our hypothesis is that in pneumococcal bronchopneumonia, pneumolysin is the major trigger of inflammation and toxemia and that it acts by activating a cascade of host factors responsible for the recruitment of inflammatory cells. We will be looking to see how the pattern of bacterial growth and dissemination, host morbidity and mortality, and the progress of inflammation and production of host factors develop by using our model of bronchopneumonia and septicemia due to parental wild-type and pneumolysin-negative mutant pneumococci. By combining these results with data on bacterial growth in the lungs and blood, we intend to develop a picture of certain aspects of the host inflammatory response to pneumococcal infection. Downloaded from on January 16, 2019 by guest * Corresponding author. Mailing address: Department of Microbiology & Immunology, Medical Sciences Building, University Rd., Leicester LE1 9HN, United Kingdom. Phone: (116) Fax: (116) pwa@le.ac.uk. MATERIALS AND METHODS Pneumococcal strains. The wild-type S. pneumoniae strain used was serotype 2 strain D39, NCTC 7466 (National Collection of Type Cultures, London, United Kingdom). The pneumolysin-negative mutant used, PLN-A, was made by 492
2 VOL. 68, 2000 ROLE OF PNEUMOLYSIN IN BRONCHOPNEUMONIA 493 insertion duplication mutagenesis (5). Pneumococci were cultured on blood agar base containing 5% (vol/vol) horse blood or in brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) containing 20% (vol/vol) fetal bovine serum (FBS; Gibco, Paisley, United Kingdom) supplemented with 1 mg of erythromycin (Sigma, Poole, United Kingdom) per ml for PLN-A. Preparation of the challenge dose. S. pneumoniae was passaged through mice as described previously (6), and aliquots were stored at 70 C. Pneumococci can be stored for at least 3 months at 70 C with no significant loss of viability. When required, the suspension was thawed at room temperature and bacteria were harvested by centrifugation before being resuspended in sterile phosphate-buffered saline (PBS). Intranasal challenge of mice. Female MF1 outbred mice weighing 30 to 35 g (Harlan Olac, Bicester, United Kingdom) were lightly anesthetized with 2.5% (vol/vol) fluothane (Zeneca, Macclesfield, United Kingdom) over oxygen (1.5 to 2 liters/min). A 50- l volume of PBS containing 10 6 CFU of S. pneumoniae wild type or PLN-A was administered to the nostrils of mice held vertical. Mice were monitored for symptoms of disease for 48 h (longer for those infected with PLN-A) or until they became moribund, at which point the experiment was ended. Experiments were done to determine the growth of bacteria in vivo. At preselected time intervals following infection, groups of mice were deeply anesthetized with 5% (vol/vol) fluothane and blood was collected by cardiac puncture. Following this procedure, the mice were killed immediately by cervical dislocation. The lung were removed into 10 ml of sterile distilled water, weighed, and then homogenized in a Stomacher-Lab blender (Seward Medical, London, United Kingdom). Viable counts in homogenates and in blood were determined as described previously (6). The presence of a type 2 polysaccharide capsule was confirmed by the Quellung reaction. Enumeration and differential analysis of lung leukocyte counts. At the same prechosen intervals following infections as above, lungs from preselected groups of mice were removed, as above, and leukocytes were prepared by a modification of a previously published method (7, 13). After removal of the lungs from sacrificed animals, the tissue was placed into 10 ml of Hanks balanced salt solution. The lungs were then cut into small pieces and homogenized in 5 ml of digestion buffer (5% [vol/vol] FBS in RPMI 1640 with collagenase [Sigma] at 0.5 mg/ml [207 collagen digestion units] and DNase I from bovine pancreas [Sigma] at 30 g/ml [87 units]) through a tea strainer a total of three times. After homogenization, lung samples were incubated at 37 C for 30 min. Subsequently, digested tissue samples were pipetted to break up tissue fragments and passed through a column containing approximately 1 cm of nonabsorbent cotton wool in a glass Pasteur pipette to remove large pieces of debris. Cells collected in Falcon 2052 tubes (Becton Dickinson) were centrifuged at 322 g for 5 min at 4 C. The supernatant was removed, and the cells resuspended in 1 ml of 1 lysis solution (Pharmingen, San Diego, Calif.). After 5 min at room temperature to lyse the erythrocytes, the remaining cells were brought to isotonicity by adding an excess volume of ice-cold 1 PBS. Following centrifugation at 322 g for 5 min at 4 C, the cells were washed with 1 ml of 1 PBS before a final resuspension in 1 ml of 5% FBS in RPMI The cells were then enumerated with a hemocytometer (Improved Neubauer; Weber Scientific International Ltd.) with the addition of trypan blue in 1 PBS at a 1:1 volume ratio of stain to cell suspension. The total number of cells used from each lung sample was diluted with 5% (vol/vol) FBS in RPMI 1640 to give a total of to cells per 50 l. For differential analysis, cytocentrifugation of these cells was performed with 50 l of cell suspension centrifuged onto cytospin slides (Shandon) in a cytocentrifuge (Cytospin 2; Shandon) at 108 g for 3 min. Following centrifugation, the slides were air dried briefly and then fixed in 100% methanol for 10 min. After fixation, differential staining was performed with Giemsa stain (BDH, Poole, United Kingdom). The slides were quantified independently by two observers at 500 magnification with a graticule-equipped eyepiece, and mononuclear leukocytes, lymphocytes, and polymorphonuclear leukocytes were identified. At least 200 cells were counted on each slide in total. By using the percentage of each type of leukocyte obtained from each slide, cell numbers of each leukocyte population were calculated from the total number of cells counted per milliliter. All slides were read by investigators blinded to their identity, and the coefficient of intraobserver variation was 3.4%. Histology. At prechosen intervals, whole-lung samples from infected mice were excised, embedded in Tissue Tek OCT, and frozen in liquid nitrogen with an isopentane heat buffer to prevent snap freezing and tissue damage. Once frozen, the samples were stored at 70 C until required. At 1 day before sectioning, the samples were moved to 20 C. On the day of sectioning, 15- m sections were taken at 18 to 25 C on a Bright microtome. The sections were allowed to dry at room temperature for 20 min, and the embedding compound surrounding the tissue was peeled away and discarded. Once dried, the sections were stained with hematoxylin and eosin. After being stained, the sections were fixed with DPX mountant (BDH) for permanent storage. Immunohistochemistry. Leukocyte recruitment into lung tissue was analyzed by an alkaline phosphatase anti-alkaline phosphatase (APAAP) staining method as described previously (14). Rat anti-mouse monoclonal antibodies to T cells (CD3), B cells (CD19), macrophages (F/480), and neutrophils (7/4) (Serotec, Oxford, United Kingdom) were used. Four sections from each lung collected at chosen time points were used for each antibody to be tested, along with three sections for negative controls: (i) an isotype-matched control antibody; (ii) exclusion of the primary antibody (or the secondary enzyme-conjugated antibody); and (iii) a sample not incubated with the substrate-chromogen solution. Once stained, the sections were analyzed by one observer (A.K.) and positively stained cells within the vicinity of inflamed bronchioles were enumerated. The distribution patterns of positively stained leukocytes within the lungs were also observed. Statistical analysis. Data were analyzed by a one-tailed Mann-Whitney U test, Student s t test, and one-way analysis of variance. Statistical significance was assumed at P RESULTS Symptoms following infection. All 75 mice challenged intranasally with 10 6 CFU of wild-type S. pneumoniae showed signs of illness (starry coat and hunched appearance) by 24 h postinfection. By 48 h postinfection, all the mice had become moribund. In contrast, the most extreme symptom in mice infected with PLN-A was starry coat, seen in all 30 mice at 48 h. Thereafter, none of the 20 mice observed showed symptoms within the remaining 9 days of the experiment. Growth of wild-type and PLN-A pneumococci in lung tissue and blood. The growth of the pneumolysin-negative strain, PLN-A, in lung tissue and blood was different from that of the parental wild-type strain. In the lungs, over the first 16 h for the wild type and 8 to 10 h for PLN-A, bacterial numbers declined sharply; they began to increase again 12 to 16 h postinfection (Fig. 1a). PLN-A growth was significantly slower (P 0.05) (maximum doubling time, 178 min) than wild-type growth (maximum doubling time, 120 min), although both showed identical growth patterns when grown in vitro in brain heart infusion medium (data not shown). Both wild-type and PLN-A growth showed no further increase after 24 h postinfection. There was, however, a statistically significant difference between wild-type and PLN-A levels at 2, 4, 6, 20, 24, and 48 h (P 0.05 for all time points). There was no significant difference at other time points. Levels of PLN-A in lung tissue decreased by 72 h and significantly so by 96 h compared to the 48-h levels (P 0.05), but the bacterium still persisted 264 h postinfection (Fig. 1b). Neither the wild type nor PLN-A was detected in the blood until 12 h postinfection, at which time bacteria were isolated from all mice (five mice for each infection) (Fig. 2a). The two organisms showed comparable rates of increase until 16 h, at which time wild-type numbers increased rapidly (maximum doubling time, 145 min), reaching a peak by 24 to 48 h, whereas the numbers of PLN-A organisms did not increase after 20 to 24 h postinfection (maximum doubling time, 307 min). These differences between the wild type and PLN-A at 24 and 48 h were statistically significant (P 0.01 for both). Levels of PLN-A organisms began to decrease after 48 h, but organisms still persisted in the blood for at least 11 days (264 h) postinfection (Fig. 2b), with no signs of illness in infected mice at times after 48 h. Production of a type 2 capsule was confirmed by Quellung reactions at 24 and 48 h postinfection for wildtype and PLN-A organisms recovered from both lungs and blood. Histological examination of lung tissue. Histological analysis of lung tissue sections from mice infected with wild-type pneumococci showed inflammation and cellular infiltration centered around bronchioles and perivascular areas. The foci of inflammation were restricted to certain bronchioles and perivascular areas close to these bronchioles at 24 h postinfection. Inflammation presented itself as hypertrophy of bronchiole walls, heavy cellular infiltration around such bronchioles, and some edema. Bacteria were detected within alveoli and around inflamed bronchioles. By 48 h postinfection, bronchiole wall thickening had increased, and solid fibrous tissue and exudate filling the bron- Downloaded from on January 16, 2019 by guest
3 494 KADIOGLU ET AL. INFECT. IMMUN. Downloaded from FIG. 1. (a) Time course of the change in numbers of S. pneumoniae wild type ( ) and PLN-A ({) in lungs of MF1 mice infected intranasally with 10 6 CFU (n 5 for each time point; error bars indicate standard error of the mean [SEM]). P 0.05 for wild-type values at 20, 24 and 48 h compared to PLN-A. (b) Time course of the change in numbers of S. pneumoniae PLN-A in lungs of MF1 mice infected intranasally with 10 6 CFU (n 5 for each time point; error bars indicate SEM). FIG. 2. (a) Time course of the change in numbers of S. pneumoniae wild type and PLN-A in blood of MF1 mice infected intranasally with 10 6 CFU (n 5 for each time point; error bars indicate SEM). P 0.01 for wild-type values at 24 and 48 h compared to PLN-A values. Time course of the change in numbers of S. pneumoniae PLN-A in blood of MF1 mice infected intranasally with 10 6 CFU (n 5 for each time point; error bars indicate SEM). chioles and alveolar spaces had appeared. Additionally, cellular infiltration had increased, with extension of inflammatory cells from bronchioles and perivascular areas into the surrounding lung parenchyma and with several focal areas of consolidation becoming larger and more diffuse. The presence of alveoli in lung sections at this time point was hardly distinguishable due to intensive tissue edema. Overall, during this period of infection, inflammation and tissue injury had encompassed nearly all of the lung surface (Fig. 3a and b). The histological changes seen in the lungs of mice infected with PLN-A were generally delayed compared to those in the lungs of mice infected with the wild type and were less severe, exhibiting considerably less tissue inflammation and cellular infiltration into perivascular areas between infected bronchioles at 24, 48, 72, and 96 h. However, despite this lower severity and the lower levels of pathologic tissue damage, some bronchioles did exhibit signs of inflammation by 48 h, with moderate levels of cellular infiltration and hyperplasia. Compared to wild-type-infected mice, however, the cellular infiltration around such bronchioles appeared to be less intense and did not extend into the perivascular areas. Loss of alveolar structure, parenchymal involvement, and interstitial edema were greatly reduced, and no focal areas of tissue consolidation were present. General tissue edema was mild, and tissue fibrosis was absent (Fig. 4). Total and differential leukocyte analysis of cytocentrifuged lung homogenates. Total leukocyte numbers and individual lymphocyte, macrophage, and polymorphonuclear cell numbers were enumerated over the time course of infection in wild-type- and PLN-A infected mice. Total leukocyte levels in wild-type-infected lung tissue homogenates increased by 12 h and reached significantly increased values (P 0.05) by 24 h postinfection (Fig. 5) compared to the time zero levels. In contrast, total leukocyte levels in PLN-A infected lung tissue homogenates showed no signif- on January 16, 2019 by guest
4 VOL. 68, 2000 ROLE OF PNEUMOLYSIN IN BRONCHOPNEUMONIA 495 Downloaded from FIG. 3. Light microscopy of lung tissue from mice infected with 10 6 CFU of wild-type S. pneumoniae, sacrificed at 24 h (a) (the large single arrow indicates an area of cellular infiltration, the small single arrow indicates edema, and the large double arrow indicates bronchiole wall thickening) and 48 h (b) (the large arrows indicate areas of fibrosis, the small arrows indicate edema, and the open arrowhead indicates heavy cellular infiltration) postinfection. Magnifications, 400. icant change until 48 h postinfection, when they were significantly greater than those at time zero (P 0.01). Interestingly, the total leukocyte levels in PLN-A infected mice were lower at 72 h postinfection than at 48 h. The total leukocyte levels also appeared to be lower in PLN-A infected than in wildtype-infected tissue at each equivalent time point; however, between 12 and 48 h the differences did not reach statistical significance. When individual cell types were analyzed in wild-type-infected total-lung homogenates, polymorphonuclear cell numbers in the lungs showed significant increases by 12, 24, and 48 h postinfection (Fig. 6a) compared to time zero (P 0.05, 0.01, and 0.05 respectively). Macrophage levels decreased by 24h(P 0.01), whereas lymphocyte levels showed no significant change throughout the 48-h time course (Fig. 6a). When the same analysis was carried out for PLN-A infected totallung homogenates, a different picture emerged. Although polymorphonuclear cell levels were again significantly higher at 24 and 48 h postinfection (P 0.01 and 0.05, respectively) than at time zero (Fig. 6b), there was no increase at 12 h and the number of cells was significantly smaller than the number observed for wild-type-infected tissue at each equivalent time point (P 0.01). Additionally, as was the case for total leukocyte levels, the levels of polymorphonuclear cells in PLN-A infected tissue also decreased substantially by 72 h compared to the 24- and 48-h levels. Macrophage levels at 24 h postinfection were significantly higher than in wild-type-infected tissue at the equivalent time point (P 0.05), but a significant decrease in macrophage levels in PLN-A infected tissue occurred 72 h postinfection compared to time zero (P 0.01). A similar decrease had occurred at 24 h in wild-type-infected tissue. As in wild-type-infected lungs, lymphocyte levels in PLN-A infected lungs showed no significant changes throughout the time course (Fig. 6b). The levels of each cell type were on January 16, 2019 by guest
5 496 KADIOGLU ET AL. INFECT. IMMUN. Downloaded from FIG. 4. Light microscopy of lung tissue from mice infected with 10 6 CFU of S. pneumoniae PLN-A, sacrificed at 24 h (a) (the large arrow indicates a bronchiole, and the small arrow indicates slight cellular infiltration) and 48 h (b) (the large arrow indicates a bronchiole, and the small arrow indicates cellular infiltration) postinfection. Magnifications, 300 (a) and 400 (b). the same (P 0.05) at time zero for both wild-type- and PLN-A infected mice, and when data were analyzed with each cell population as a percentage instead of total numbers of cells, the same patterns were obtained (data not shown). Immunohistochemical analysis of inflammatory cell infiltrates. To further analyze leukocyte infiltration into lung tissue, in situ analysis of leukocyte numbers, distribution patterns, and their anatomical localization in lung tissue over the time course of infection with the wild type and PLN-A was performed by immunohistochemistry. Positively stained cells were enumerated in inflamed areas of sectioned lung tissue only. In inflamed areas of wild-type-infected lung, the numbers of neutrophils showed the greatest increase, reaching a statistically significant peak (P 0.05) at 24 h compared to time zero (Fig. 7). This also reflected the equivalent increase seen in total-lung homogenate counts of neutrophils. Neutrophils were observed within inflamed bronchioles and in bronchiole walls but also to a much greater extent in the perivascular areas surrounding inflamed bronchioles and in alveolar spaces. These areas of lung tissue were heavily infiltrated with neutrophils at 24 h. However, the numbers of neutrophils in tissue, especially in and around inflamed bronchioles, decreased significantly by 48 h postinfection compared to 24 h (P 0.05). The numbers of neutrophils were still larger than those of macrophages or lymphocytes at equivalent time points, as was also the case for the numbers of total-lung homogenate. T lymphocytes showed interesting patterns of distribution, with the numbers of cells in inflamed areas increasing significantly (P 0.05) by 24 and 48 h postinfection compared to time zero. The numbers of T cells were large in tissue surrounding inflamed bronchioles and somewhat smaller in close proximity to the bronchiole walls themselves by 24 h (Fig. 8a). By 48 h, however, the numbers of T cells around the bronchiole on January 16, 2019 by guest
6 VOL. 68, 2000 ROLE OF PNEUMOLYSIN IN BRONCHOPNEUMONIA 497 FIG. 5. Total leukocyte counts from whole-lung homogenate cytospins from MF1 mice infected intranasally with 10 6 CFU of S. pneumoniae wild type and PLN-A (n 5 for each time point; error bars indicate SEM). P 0.05 for wild-type leukocyte levels at 24 h compared to time zero; P 0.01 for PLN-A at 48 h compared to time zero; P 0.05 for PLN-A at 72 h compared to 48 h. FIG. 6. (a) Differential leukocyte counts from whole-lung homogenate cytospins from MF1 mice infected intranasally with 10 6 CFU of wild-type S. pneumoniae (n 5 for each time point; error bars indicate SEM). P 0.05 for polymorphonuclear cell levels at 12 and 48 h and P 0.01 for levels at 24 h compared to time zero. P 0.01 for macrophage levels at 24 h compared to time zero. (b) Differential leukocyte counts from whole-lung homogenate cytospins from MF1 mice infected intranasally with 10 6 CFU of S. pneumoniae PLN-A (n 5 for each time point; error bars indicate SEM). P 0.01 for polymorphonuclear cell levels at 24 h and P 0.05 for cell levels at 48 h compared to time zero. P 0.01 for macrophage levels at 72 h compared to time zero. walls had decreased but the numbers in perivascular tissue and around inflamed bronchioles had increased, as had the total number of T cells dispersed throughout the tissue as a whole (Fig. 8b). Thus, there is a shift in distribution patterns from bronchioles to tissue spaces between the inflamed bronchioles. The total numbers of macrophages in inflamed areas remained constant at 0, 24, and 48 h postinfection. However, the numbers of macrophages localized inside inflamed bronchioles and in perivascular tissue areas surrounding such bronchioles increased by 24 h compared to time zero. Macrophages were also seen in alveolar spaces by this time point. The number of B lymphocytes in lung tissue increased steadily by 24 and 48 h postinfection. This increase was observed in tissue in close proximity to inflamed bronchioles and to a lesser extent within alveolar spaces. By 48 h, B lymphocytes were also observed within inflamed bronchioles. In inflamed areas of PLN-A infected lung tissue, neutrophil numbers increased by 24 h and continued to do so until reaching a peak significantly greater than the initial value (P 0.05 compared to time zero levels) by 48 h (Fig. 9), again in keeping with equivalent increases in neutrophil counts in total-lung homogenate. Neutrophil numbers in perivascular areas around inflamed bronchioles increased somewhat by 24 h and to a greater extent by 48 h, although the numbers within bronchioles did not increase. Neutrophil numbers in PLN-A infected tissue were significantly smaller at 24 h postinfection (P 0.05) and smaller again at 48 h postinfection compared to the numbers found at the equivalent time points in wild-typeinfected tissue. However, neutrophils exhibited a similar anatomical localization pattern in both types of infected lungs. No changes were seen in the numbers of T lymphocytes, B lymphocytes, and macrophages in PLN-A infected tissue from 0 to 24 h postinfection, unlike in wild-type-infected tissue (Fig. 9). By 48 h postinfection, small increases in the numbers of such cells were observed. Both macrophages and T lymphocytes showed increased numbers by 48 h, with macrophage numbers increasing within inflamed bronchioles and especially around inflamed bronchiole tissue. T-cell numbers, on the other hand, showed small increases in and around inflamed bronchioles (Fig. 8c) but considerably less than for wild-typeinfected tissue. A similar pattern was also observed for B lymphocytes. The total number of cells was much smaller than that observed in wild-type-infected tissue sections, however. The main differences between cell infiltrates into wild-typeand PLN-A infected lung tissue were the changing distribution patterns for T lymphocytes and neutrophils, the greater numbers of infiltrating cells in wild-type-infected lung sections, and a general decrease in numbers of infiltrating cells by 48 h postinfection in wild-type-infected tissue compared to 72 h postinfection in PLN-A infected tissue. DISCUSSION We have previously shown that pneumolysin is crucially involved in the pathogenesis of pneumococcal pneumonia (6). Downloaded from on January 16, 2019 by guest
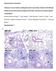
7 498 KADIOGLU ET AL. INFECT. IMMUN. FIG. 7. Numbers of leukocytes in tissue sections from lung samples of MF1 mice infected intranasally with 10 6 CFU of wild-type S. pneumoniae (n 4 for each time point; error bars indicate SEM). We found that a pneumolysin-negative mutant grew more slowly in the lungs and induced much less inflammation than did compared to the parent wild-type organism (6). In this paper, we have analyzed pneumococcal growth in the lungs and blood of mice in more detail than before and have characterized the pattern of inflammatory-cell influx. These data revealed that the pattern of survival and cell influx of wild-type and PLN-A pneumococci is more complex than we previously saw. When the growth kinetics of both wild-type and PLN-A organisms in lungs was examined, three phases were seen in the first 48 h postinfection: (i) an early and sharp decline in numbers of pneumococci, (ii) an increase in numbers, and (iii) a stage where pneumococcal numbers remained constant or declined. Although the two strains showed similar patterns, the most obvious difference between the wild type and PLN-A was the extent of the change in two of the phases. The early, sharp decline of pneumococcal numbers was much more evident for PLN-A and the increase in numbers after 16 h was much sharper with wild-type pneumococci. Thus, pneumolysin appears to be crucial to the survival of the pneumococcus at two distinct stages of the infection. The occurrence of these three phases of pneumococcal decline or growth at different times after infection implies that different antipneumococcal systems emerged over time or that their effectiveness changed with time. This view is reinforced by the second period of decline in numbers of bacteria in the lungs seen with PLN-A after 48 h postinfection. The pattern of influx of inflammatory cells was consistent with this idea. When pneumococcal growth in blood was examined, distinct stages were seen again: a rapid increase in the numbers of pneumococci and the subsequent stabilization of these numbers. A notable feature here was the much lower plateau of the PLN-A level reached in the blood compared with the level of wild-type organisms. Another feature was the asymptomatic persistence of PLN-A in the blood. In these experiments, pneumolysin did not influence the time of appearance of pneumococcal bacteremia or the early rate of increase in bacterial numbers. However, it did influence the sensitivity of pneumococci to the mechanism that eventually limits their numbers. Whatever the nature of this mechanism, it is one of stasis rather than cidal action. Previous investigation of bacteremia with the same strain of pneumococci also showed the persistence of PLN-A in blood up to 7 days postinfection, with 50% survival of infected animals (3). The authors suggested that the absence of pneumolysin during the early hours of infection prevents the development of sepsis and delays death by at least several days. Our previous finding that a delay in the appearance of pneumococci in the blood is associated with a delay in the time of death would also suggest that the onset of bacteremia is an important determinant of the time of death (6). Previous work with a model of endotracheal instillation (21) has shown that PLN-A has a reduced capacity to injure the alveolar-capillary barrier and hence a reduced capacity to multiply within lung tissue. It has also been shown that PLN-A failed to cause the separation of tight junctions between epithelial cells. It was suggested that as a consequence, adherence to separated epithelial cell edges and invasion of lung tissue were decreased (19). PLN-A is also known to be less successful in penetrating the interstitium of the lung from the alveoli and invading the bloodstream than is the wild type. When purified pneumolysin is coinstilled with PLN-A, however, the pattern of multiplication is similar to that of the wild type (21). Although using a different model, the authors showed that pneumolysin facilitated the intra-alveolar replication of pneumococci, as well as the penetration of these bacteria from alveoli into deeper lung tissue, eventually resulting in the presence of pneumococci in the bloodstream (21). This previous work, combined with the observations described in this paper, suggests that the cytotoxic properties of pneumolysin are essential for bacterial multiplication in the alveoli. Pneumolysin appears to play an important role especially during the first 6 to 8 h of infection, when bacterial colonization and growth within host tissue crucially occur to form the basis of future inflammatory reactions and systemic infection. To begin to explain the host mechanisms underlying these patterns of pneumococcal behavior, we analyzed the influx of inflammatory cells into infected lungs. We found a sequential infiltration pattern of inflammatory cells which failed to eliminate wild-type pneumococci from lungs and resulted in bacterial proliferation, bacteremia, and eventual host death. Recently published work with CD1 Swiss mice intranasally challenged with S. pneumoniae (4) also showed a sequential movement of different inflammatory cells into the lungs. As with our observations, it was reported that an early neutrophil influx was followed by an increase in the number of lymphocytes, but in contrast to our data, a large increase in macrophages was seen. However, the data of Bergeron et al. (4) were Downloaded from on January 16, 2019 by guest FIG. 8. (a) Light microscopy of APAAP-stained T lymphocytes (darkly stained cells) surrounding a bronchiole in lung tissue infected with 10 6 CFU of wild-type S. pneumoniae 24 h postinfection. Arrows indicate positively stained T cells. (b) Light microscopy of APAAP-stained T lymphocytes (darkly stained cells) surrounding a bronchiole in lung tissue infected with 10 6 CFU of wild-type S. pneumoniae 48 h postinfection. Large arrows indicate positively stained T cells, and the small arrow indicates the bronchiole. (c) Light microscopy of APAAP-stained T lymphocytes (darkly stained cells) surrounding a bronchiole in lung tissue infected with 10 6 CFU of S. pneumoniae PLN-A 24 h postinfection. Large arrows indicate the small number of T cells. The small arrow indicates the bronchiole. Magnifications, 400 (a) and 320 (b and c).
8 Downloaded from on January 16, 2019 by guest 499
9 500 KADIOGLU ET AL. INFECT. IMMUN. FIG. 9. Numbers of leukocytes in tissue sections from lung samples of MF1 mice infected intranasally with 10 6 CFU of S. pneumoniae PLN-A (n 4 for each time point; error bars indicate SEM). obtained by studying lavage fluid, not whole-lung homogenates as in our work. When total or individual leukocyte levels in the lungs are analyzed, it is clear that the early sharp decline in wild-type or PLN-A pneumococci was well under way before measurable cellular influx had begun. The nature of the antimicrobial system at this stage is unknown, but the absence of pneumolysin increases its effect on pneumococci. Candidates could include surfactants, complement, or resident macrophages. It has been shown previously that pneumolysin interacts with complement (16) and monocytes (17), but its interaction with surfactant is unknown. Eventually, the sensitivity of the pneumococci to this early killing system wanes and is not restored by the appearance of inflammatory cells, so that beginning around 16 h, wild-type pneumococci appear to enter a period of unrestrained growth. This occurs in spite of the concomitant influx of neutrophils at this time. Therefore, it would suggest that these infiltrating neutrophils are able to kill wild-type pneumococci. This appears not to be true for PLN-A pneumococci; here, the rate of increase in the number of bacteria in the second stage is much lower even though the influx of neutrophils is slower and less intense. Thus, it might be concluded that pneumolysin increases the timing and extent of the influx of neutrophils but significantly inhibits their activity on arrival. This conclusion is entirely consistent with the previously reported activity of pneumolysin in vitro, whereby it significantly depressed a variety of phagocytic functions, such as the respiratory burst and the release of lysosomal enzymes, and pneumolysin-treated phagocytes had a depressed ability to kill S. pneumoniae in vitro (17). An interesting observation was the early accumulation of T and B lymphocytes at the sites of inflammation. Three associated observations are worth noting. First, the peak of accumulation is coincident with the beginning of the phase when pneumococcal growth ceases (Fig. 1a and 7), which is seen as stasis of the wild-type strain or eventual decline of PLN-A numbers. Second, the time of maximum accumulation of lymphocytes is delayed in the absence of pneumolysin and also is less intense (Fig. 9). Resolution of cause and effect in these observations is clearly required before their significance can be assessed. Finally, the accumulation of lymphocytes is not reflected in a significant increase in the total number of lymphocytes in the lungs. Previous work has demonstrated the presence of a large T-cell population within the extravascular compartment of the rat lung, which is distributed randomly throughout the alveolar septal walls (11). This population is said to be at least five times larger than the peripheral blood T-cell pool in SPF rats (10). It is possible that the accumulation of T cells within certain areas of lung tissue, without significant increases in total numbers of infiltrating T cells, may reflect a shift in the pattern of accumulation of resident T cells rather than infiltrating cells. Another interesting feature is the localization of inflammatory cells within the lungs. It appears that these cells migrate toward specific areas of lung tissue; they first travel to tissue surrounding inflamed bronchioles and around bronchiole walls, but they then decrease in number in these areas and increase in number in perivascular areas away from the inflamed bronchioles and throughout the lung parenchyma. This shifting migration pattern suggests that immune system cells migrate within lung tissue toward areas of bacterial confluence. The chemotactic factors that mediate these events are not known, but the CXC and CC chemokines are obvious candidates. The role of these chemokines is the subject of our continuing investigations. In conclusion, the capability of the host to confine pneumococcal numbers and to survive infection by pneumococci unable to make pneumolysin indicates the vital role of this toxin in disease. Although much remains to be determined about the events described above, this paper highlights a number of new questions that must be addressed if we are to fully understand the interaction of pneumococci and the host during pneumonia. REFERENCES 1. Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24: Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62: Benton, K. A., M. P. Everson, and D. E. Briles A pneumolysinnegative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63: Bergeron, Y., N. Ouellet, A.-M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66: Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57: Canvin, J. R., A. P. Marvin, M. Sivakumaran, J. C. Paton, G. J. Boulnois, P. W. Andrew, and T. J. Mitchell The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 172: Curtis, J. L., G. B. Huffnagle, G. H. Chen, M. L. Warnock, M. R. Gyetko, R. A. McDonald, P. J. Scott, and G. B. Toews Experimental murine pulmonary cryptococcus-differences in pulmonary inflammation and lymphocyte recruitment induced by encapsulated strains of Cryptococcus neoformans. Lab. Investig. 71: Feldman, C., T. J. Mitchell, P. W. Andrew, G. J. Boulnois, S. C. Reed, H. C. Todd, P. J. Cole, and R. Wilson The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb. Pathog. 9: Feldman, C., R. Reed, A. Rutman, N. C. Munro, D. K. Jeffrey, A. Brain, V. Lund, T. J. Mitchell, P. W. Andrew, G. J. Boulnois, H. C. Todd, P. J. Cole, and R. Wilson The effect of Streptococcus pneumoniae on intact respiratory epithelium. Eur. Respir. J. 5: Downloaded from on January 16, 2019 by guest
10 VOL. 68, 2000 ROLE OF PNEUMOLYSIN IN BRONCHOPNEUMONIA Holt, P. G., A. Degebrodt, and T. Venaille Preparation of interstitial lung cells by digestion of tissue slices: preliminary characterisation by morphology and performance in functional assays. Immunity 54: Holt, P. G., and M. A. Schon-Hegard Localisation of T cells, macrophages and dendritic cells in rat respiratory tract tissue: implications for immune function studies. Immunity 62: Houldsworth, S., P. W. Andrew, and T. J. Mitchell Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 by human mononuclear phagocytes. Infect. Immun. 62: Huffnagle, G. B., R. M. Strieter, T. J. Standiford, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4 T-cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 155: Kadioglu, A., and P. Sheldon Steroid pulse therapy for rheumatoid arthritis: effect on lymphocyte subsets and mononuclear adhesion. Br. J. Rheumatol. 37: Mitchell, T. J., P. W. Andrew, G. J. Boulnois, C. J. Lee, R. A. Lock, and J. C. Paton Molecular studies of pneumolysin as an aid to vaccine design. Zentbl. Bakteriol. 23: Mitchell, T. J., P. W. Andrew, F. K. Saunders, A. N. Smith, and G. J. Editor: E. I. Tuomanen Boulnois Complement activation and antibody binding by pneumolysin via a region homologous to a human acute phase protein. Mol. Microbiol. 5: Nandoskar, M., A. Ferrante, E. J. Bates, N. Hurst, and J. C. Paton Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunity 59: Paton, J. C., and A. Ferrante Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41: Rayner, C. F. J., A. D. Jackson, A. Rutman, A. Dewar, T. J. Mitchell, P. W. Andrew, P. J. Cole, and R. Wilson Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect. Immun. 63: Rubins, J. B., P. W. Andrew, T. J. Mitchell, and D. E. Niewoehner Pneumolysin activates phospholipase A 2 in pulmonary artery endothelial cells. Infect. Immun. 62: Rubins, J. B., D. Charboneau, J. C. Paton, T. J. Mitchell, and P. W. Andrew Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Investig. 95: Downloaded from on January 16, 2019 by guest
11 ERRATUM Host Cellular Immune Response to Pneumococcal Lung Infection in Mice ARAS KADIOGLU, NEILL A. GINGLES, KATE GRATTAN, ALISON KERR, TIM J. MITCHELL, AND PETER W. ANDREW Department of Microbiology & Immunology, University of Leicester, Leicester, and Division of Infection & Immunity, University of Glasgow, Glasgow, United Kingdom Volume 68, no. 2, p , The symbols in Fig. 2, 5, 6, 7, and 9 should have been defined as follows. Page 494, Fig. 2:, PLN-A;, wild type. Page 497, Fig. 5:, PLN-A;, wild type. Figure 6:, lymphocytes;, macrophages; E, polymorph. Page 498, Fig. 7:, CD3;, macrophages; E, neutrophils;, CD19. Page 500, Fig. 9:, CD3;, macrophages; E, neutrophils;, CD
Upper and Lower Respiratory Tract Infection by Streptococcus pneumoniae Is Affected by Pneumolysin Deficiency and Differences in Capsule Type
 INFECTION AND IMMUNITY, June 2002, p. 2886 2890 Vol. 70, No. 6 0019-9567/02/$04.00 0 DOI: 10.1128/IAI.70.6.2886 2890.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Upper and
INFECTION AND IMMUNITY, June 2002, p. 2886 2890 Vol. 70, No. 6 0019-9567/02/$04.00 0 DOI: 10.1128/IAI.70.6.2886 2890.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Upper and
Streptococcus pneumonia
 Streptococcus pneumonia The pneumococci (S. pneumoniae) are gram-positive diplococci. Often lancet shaped or arranged in chains, possessing a capsule of polysaccharide that permits typing with specific
Streptococcus pneumonia The pneumococci (S. pneumoniae) are gram-positive diplococci. Often lancet shaped or arranged in chains, possessing a capsule of polysaccharide that permits typing with specific
Serotypes of Streptococcus pneumoniae
 INFECrION AND IMMUNITY, Dec. 1994, p. 5683-5688 0019-9567/94/$04.00+0 Copyright C 1994, American Society for Microbiology Vol. 62, No. 12 Immunization of Mice with Pneumolysin Toxoid Confers a Significant
INFECrION AND IMMUNITY, Dec. 1994, p. 5683-5688 0019-9567/94/$04.00+0 Copyright C 1994, American Society for Microbiology Vol. 62, No. 12 Immunization of Mice with Pneumolysin Toxoid Confers a Significant
Pneumococcal Neuraminidases A and B Both Have Essential Roles during Infection of the Respiratory Tract and Sepsis
 INFECTION AND IMMUNITY, July 2006, p. 4014 4020 Vol. 74, No. 7 0019-9567/06/$08.00 0 doi:10.1128/iai.01237-05 Copyright 2006, American Society for Microbiology. All Rights Reserved. Pneumococcal Neuraminidases
INFECTION AND IMMUNITY, July 2006, p. 4014 4020 Vol. 74, No. 7 0019-9567/06/$08.00 0 doi:10.1128/iai.01237-05 Copyright 2006, American Society for Microbiology. All Rights Reserved. Pneumococcal Neuraminidases
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Immune System AP SBI4UP
 Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Lymphoid System: cells of the immune system. Answer Sheet
 Lymphoid System: cells of the immune system Answer Sheet Q1 Which areas of the lymph node have most CD3 staining? A1 Most CD3 staining is present in the paracortex (T cell areas). This is towards the outside
Lymphoid System: cells of the immune system Answer Sheet Q1 Which areas of the lymph node have most CD3 staining? A1 Most CD3 staining is present in the paracortex (T cell areas). This is towards the outside
Biological Consulting Services
 Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Sensitivities of Human Monocytes and Epithelial Cells to Pneumolysin Are Different
 INFECTION AND IMMUNITY, Feb. 2002, p. 1017 1022 Vol. 70, No. 2 0019-9567/02/$04.00 0 DOI: 10.1128/IAI.70.2.1017 1022.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Sensitivities
INFECTION AND IMMUNITY, Feb. 2002, p. 1017 1022 Vol. 70, No. 2 0019-9567/02/$04.00 0 DOI: 10.1128/IAI.70.2.1017 1022.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Sensitivities
Immunity. Chapter 38 Part 1
 Immunity Chapter 38 Part 1 Impacts, Issues Frankie s Last Wish Infection with a common, sexually transmitted virus (HPV) causes most cervical cancers including the one that killed Frankie McCullogh 38.1
Immunity Chapter 38 Part 1 Impacts, Issues Frankie s Last Wish Infection with a common, sexually transmitted virus (HPV) causes most cervical cancers including the one that killed Frankie McCullogh 38.1
BACTERIAL PATHOGENESIS
 BACTERIAL PATHOGENESIS A pathogen is a microorganism that is able to cause disease. Pathogenicity is the ability to produce disease in a host organism. Virulence a term which refers to the degree of pathogenicity
BACTERIAL PATHOGENESIS A pathogen is a microorganism that is able to cause disease. Pathogenicity is the ability to produce disease in a host organism. Virulence a term which refers to the degree of pathogenicity
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
CELL MEDIATED IMMUNE RESPONSE
 CELL MEDIATED IMMUNE RESPONSE Chapter IV - CELL MEDIATED IMMUNE RESPONSE Sujatha, M. 2013. Evaluation of Immunological changes in Fish, Catla catla administered with bacterial pathogen, Aeromonas hydrophila,
CELL MEDIATED IMMUNE RESPONSE Chapter IV - CELL MEDIATED IMMUNE RESPONSE Sujatha, M. 2013. Evaluation of Immunological changes in Fish, Catla catla administered with bacterial pathogen, Aeromonas hydrophila,
Pathogenesis of Infectious Diseases. CLS 212: Medical Microbiology
 Pathogenesis of Infectious Diseases CLS 212: Medical Microbiology Definitions Path- means disease. Pathogenesis The steps or mechanisms involved in the development of a disease. Infection The presence
Pathogenesis of Infectious Diseases CLS 212: Medical Microbiology Definitions Path- means disease. Pathogenesis The steps or mechanisms involved in the development of a disease. Infection The presence
Blood consists of red and white blood cells suspended in plasma Blood is about 55% plasma and 45% cellular elements Plasma 90% water 10% dissolved
 Bio 100 Guide 21 Blood consists of red and white blood cells suspended in plasma Blood is about 55% plasma and 45% cellular elements Plasma 90% water 10% dissolved inorganic ions, proteins, nutrients,
Bio 100 Guide 21 Blood consists of red and white blood cells suspended in plasma Blood is about 55% plasma and 45% cellular elements Plasma 90% water 10% dissolved inorganic ions, proteins, nutrients,
The Immune System. These are classified as the Innate and Adaptive Immune Responses. Innate Immunity
 The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
The Immune System Biological mechanisms that defend an organism must be 1. triggered by a stimulus upon injury or pathogen attack 2. able to counteract the injury or invasion 3. able to recognise foreign
Histopathology: pulmonary pathology
 Histopathology: pulmonary pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need to learn about these
Histopathology: pulmonary pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need to learn about these
10.00 PBS OVA OVA+isotype antibody 8.00 OVA+anti-HMGB1. PBS Methatroline (mg/ml)
 RESEARCH ARTICLE Penh (100% of PBS) 1 PBS 8.00 +anti-hmgb1 6.00 4.00 p=0.054 Cellular & Molecular Immunology advance online publication, PBS 3.12 6.25 Methatroline (mg/ml) Neutrophil isolation and culture
RESEARCH ARTICLE Penh (100% of PBS) 1 PBS 8.00 +anti-hmgb1 6.00 4.00 p=0.054 Cellular & Molecular Immunology advance online publication, PBS 3.12 6.25 Methatroline (mg/ml) Neutrophil isolation and culture
EXPERIMENTAL SALMONELLOSIS
 EXPERIMENTAL SALMONELLOSIS INTRACELLULAR GROWTH OF Salmonella enteritidis INGESTED IN MONONUCLEAR PHAGOCYTES OF MICE, AND CELLULAR BASIS OF IMMUNITY SUSUMU MITSUHASHI, ICHIEI SATO, AND TOKUMITSU TANAKA
EXPERIMENTAL SALMONELLOSIS INTRACELLULAR GROWTH OF Salmonella enteritidis INGESTED IN MONONUCLEAR PHAGOCYTES OF MICE, AND CELLULAR BASIS OF IMMUNITY SUSUMU MITSUHASHI, ICHIEI SATO, AND TOKUMITSU TANAKA
PATHOGENICITY OF MICROORGANISMS
 PATHOGENICITY OF MICROORGANISMS Some microorganisms are : 1- Harmless microorganism, as normal flora 2- Harmfull microorganism, as pathogenic. A pathogenic microorganism is defined as one that causes or
PATHOGENICITY OF MICROORGANISMS Some microorganisms are : 1- Harmless microorganism, as normal flora 2- Harmfull microorganism, as pathogenic. A pathogenic microorganism is defined as one that causes or
Chapter 16 Innate Immunity: Nonspecific Defenses of the Host
 Module 10 Chapter 16 Innate Immunity: Nonspecific Defenses of the Host The concept of immunity Immunity: ability to protect against from microbes and their o Aka, Susceptibility: vulnerability or lack
Module 10 Chapter 16 Innate Immunity: Nonspecific Defenses of the Host The concept of immunity Immunity: ability to protect against from microbes and their o Aka, Susceptibility: vulnerability or lack
BIOH122 Session 8 Non-Specific Disease Resistance
 BIOH122 Session 8 Non-Specific Disease Resistance To complete this worksheet, select: Module: Disease Resistance Activity: Animations Title: Non-Specific Disease Resistance Introduction 1. Name five general
BIOH122 Session 8 Non-Specific Disease Resistance To complete this worksheet, select: Module: Disease Resistance Activity: Animations Title: Non-Specific Disease Resistance Introduction 1. Name five general
INNATE IMMUNITY Non-Specific Immune Response. Physiology Unit 3
 INNATE IMMUNITY Non-Specific Immune Response Physiology Unit 3 Protection Against Infection The body has several defenses to protect itself from getting an infection Skin Mucus membranes Serous membranes
INNATE IMMUNITY Non-Specific Immune Response Physiology Unit 3 Protection Against Infection The body has several defenses to protect itself from getting an infection Skin Mucus membranes Serous membranes
I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms. Table 2: Innate Immunity: First Lines of Defense
 I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms Table 2: Innate Immunity: First Lines of Defense Innate Immunity involves nonspecific physical & chemical barriers that are adapted for
I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms Table 2: Innate Immunity: First Lines of Defense Innate Immunity involves nonspecific physical & chemical barriers that are adapted for
Quantifying Bordetella-induced Neutrophil Infiltration Using a Myeloperoxidase Assay
 Quantifying Bordetella-induced Neutrophil Infiltration Using a Myeloperoxidase Assay Sandra M. Fuentes, Research Scholar Faculty Mentor: Dr. Eric Harvill The Pennsylvania State University Abstract Bacteria
Quantifying Bordetella-induced Neutrophil Infiltration Using a Myeloperoxidase Assay Sandra M. Fuentes, Research Scholar Faculty Mentor: Dr. Eric Harvill The Pennsylvania State University Abstract Bacteria
SUPPLEMENTARY INFORMATION. CXCR4 inhibitors could benefit to HER2 but not to Triple-Negative. breast cancer patients
 SUPPLEMENTARY INFORMATION CXCR4 inhibitors could benefit to HER2 but not to Triple-Negative breast cancer patients Lefort S. 1,2, Thuleau A. 3, Kieffer Y. 1,2, Sirven P. 1,2, Bieche I. 4, Marangoni E.
SUPPLEMENTARY INFORMATION CXCR4 inhibitors could benefit to HER2 but not to Triple-Negative breast cancer patients Lefort S. 1,2, Thuleau A. 3, Kieffer Y. 1,2, Sirven P. 1,2, Bieche I. 4, Marangoni E.
Module 10 Innate Immunity
 Module 10 Innate Immunity Chapter 16 Innate Immunity Lectures Lectures prepared prepared by by Christine HelmutL.Kae Case The Concept of Immunity Immunity: ability to protect against disease from microbes
Module 10 Innate Immunity Chapter 16 Innate Immunity Lectures Lectures prepared prepared by by Christine HelmutL.Kae Case The Concept of Immunity Immunity: ability to protect against disease from microbes
ISOLATION OF EXFOLIATED SOMATIC CELLS FROM BUFFALO MILK
 Original Article Buffalo Bulletin (March 2013) Vol.32 No.1 ISOLATION OF EXFOLIATED SOMATIC CELLS FROM BUFFALO MILK A.K. Dang*, Joydip Mukherjee, Manu Jamwal, Surender Singh, A.K. Mohanty, Shiv Prasad,
Original Article Buffalo Bulletin (March 2013) Vol.32 No.1 ISOLATION OF EXFOLIATED SOMATIC CELLS FROM BUFFALO MILK A.K. Dang*, Joydip Mukherjee, Manu Jamwal, Surender Singh, A.K. Mohanty, Shiv Prasad,
Chapter 14. In Vitro Measurement of Phagocytosis and Killing of Cryptococcus neoformans by Macrophages. André Moraes Nicola and Arturo Casadevall
 Chapter 14 In Vitro Measurement of Phagocytosis and Killing of Cryptococcus neoformans by Macrophages André Moraes Nicola and Arturo Casadevall Abstract Macrophages are pivotal cells in immunity against
Chapter 14 In Vitro Measurement of Phagocytosis and Killing of Cryptococcus neoformans by Macrophages André Moraes Nicola and Arturo Casadevall Abstract Macrophages are pivotal cells in immunity against
Explain the laboratory diagnosis of Rabies?
 Explain the laboratory diagnosis of Rabies? The standard test for rabies testing is dfa. This test has been thoroughly evaluated for more than 40 years, and is recognized as the most rapid and reliable
Explain the laboratory diagnosis of Rabies? The standard test for rabies testing is dfa. This test has been thoroughly evaluated for more than 40 years, and is recognized as the most rapid and reliable
Innate Immunity. Bởi: OpenStaxCollege
 Innate Immunity Bởi: OpenStaxCollege The vertebrate, including human, immune system is a complex multilayered system for defending against external and internal threats to the integrity of the body. The
Innate Immunity Bởi: OpenStaxCollege The vertebrate, including human, immune system is a complex multilayered system for defending against external and internal threats to the integrity of the body. The
Defense mechanism against pathogens
 Defense mechanism against pathogens Immune System What is immune system? Cells and organs within an animal s body that contribute to immune defenses against pathogens ( ) Bacteria -Major entry points ;open
Defense mechanism against pathogens Immune System What is immune system? Cells and organs within an animal s body that contribute to immune defenses against pathogens ( ) Bacteria -Major entry points ;open
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
4b. Innate (nonspecific) Immunity
 4b. Innate (nonspecific) Immunity Chapter 16: Innate (nonspecific) Immunity! Some terms:! Susceptibility: Lack of immunity to a disease.! Immunity: Ability to ward off disease.! Innate immunity: Defenses
4b. Innate (nonspecific) Immunity Chapter 16: Innate (nonspecific) Immunity! Some terms:! Susceptibility: Lack of immunity to a disease.! Immunity: Ability to ward off disease.! Innate immunity: Defenses
Disease causing organisms Resistance Immunity
 Part 1 Disease causing organisms Resistance Immunity Bacteria Most common pathogens Anthrax Cholera Staphylococcus epidermidis bacteria Bacterial diseases Tuberculosis Cholera Bubonic Plague Tetanus Effects
Part 1 Disease causing organisms Resistance Immunity Bacteria Most common pathogens Anthrax Cholera Staphylococcus epidermidis bacteria Bacterial diseases Tuberculosis Cholera Bubonic Plague Tetanus Effects
Immunology Lecture- 1
 Immunology Lecture- 1 Immunology and Immune System Immunology: Study of the components and function of the immune system Immune System a network collected from cells, tissues organs and soluble factors
Immunology Lecture- 1 Immunology and Immune System Immunology: Study of the components and function of the immune system Immune System a network collected from cells, tissues organs and soluble factors
1. Lymphatic vessels recover about of the fluid filtered by capillaries. A. ~1% C. ~25% E. ~85% B. ~10% D. ~50%
 BIOL2030 Huaman A&P II -- Exam 3 -- XXXX -- Form A Name: 1. Lymphatic vessels recover about of the fluid filtered by capillaries. A. ~1% C. ~25% E. ~85% B. ~10% D. ~50% 2. Special lymphatic vessels called
BIOL2030 Huaman A&P II -- Exam 3 -- XXXX -- Form A Name: 1. Lymphatic vessels recover about of the fluid filtered by capillaries. A. ~1% C. ~25% E. ~85% B. ~10% D. ~50% 2. Special lymphatic vessels called
Innate Immunity: Nonspecific Defenses of the Host
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 16 Innate Immunity: Nonspecific Defenses of the Host Host Response to Disease Resistance- ability
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 16 Innate Immunity: Nonspecific Defenses of the Host Host Response to Disease Resistance- ability
Chapter 13 Lymphatic and Immune Systems
 The Chapter 13 Lymphatic and Immune Systems 1 The Lymphatic Vessels Lymphoid Organs Three functions contribute to homeostasis 1. Return excess tissue fluid to the bloodstream 2. Help defend the body against
The Chapter 13 Lymphatic and Immune Systems 1 The Lymphatic Vessels Lymphoid Organs Three functions contribute to homeostasis 1. Return excess tissue fluid to the bloodstream 2. Help defend the body against
Influenza A virus infection predisposes hosts to secondary infection with different
 Supplementary information Influenza A virus infection predisposes hosts to secondary infection with different Streptococcus pneumoniae serotypes with similar outcome but serotype-specific manifestation.
Supplementary information Influenza A virus infection predisposes hosts to secondary infection with different Streptococcus pneumoniae serotypes with similar outcome but serotype-specific manifestation.
Prothrombin (Human) ELISA Kit
 Prothrombin (Human) ELISA Kit Catalog Number KA0496 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
Prothrombin (Human) ELISA Kit Catalog Number KA0496 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay... 3 General
THE BRITISH JOURN OF VOL. LII OCTOBER, 1971 NO. 5 EXPERIMENTAL PATHOLOGY DISAGGREGATED CELLS OF THE TRANSMISSIBLE VENEREAL TUMOUR OF THE DOG
 Vol. Lll, No. 4 (August, 1971) was issued 2.9.71. THE BRITISH JOURN OF EXPERIMENTAL PATHOLOGY VOL. LII OCTOBER, 1971 NO. 5 A PHENOMENON RESEMBLING OPSONIC ADHERENCE SHOWN BY DISAGGREGATED CELLS OF THE
Vol. Lll, No. 4 (August, 1971) was issued 2.9.71. THE BRITISH JOURN OF EXPERIMENTAL PATHOLOGY VOL. LII OCTOBER, 1971 NO. 5 A PHENOMENON RESEMBLING OPSONIC ADHERENCE SHOWN BY DISAGGREGATED CELLS OF THE
In vitro bactericidal assay Fig. S8 Gentamicin protection assay Phagocytosis assay
 In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
EXPERIMENTAL PNEUMONIA IN MICE FOLLOWING THE INHALATION OF STREPTOCOCCUS H2EMOLYTICUS AND OF FRIEDLANDER'S BACILLUS.
 EXPERIMENTAL PNEUMONIA IN MICE FOLLOWING THE INHALATION OF STREPTOCOCCUS H2EMOLYTICUS AND OF FRIEDLANDER'S BACILLUS. BY ERNEST G. STILLMAN, M.D., AND ARNOLD BRANCH, M.D. (From the Hospital of The Rockefeller
EXPERIMENTAL PNEUMONIA IN MICE FOLLOWING THE INHALATION OF STREPTOCOCCUS H2EMOLYTICUS AND OF FRIEDLANDER'S BACILLUS. BY ERNEST G. STILLMAN, M.D., AND ARNOLD BRANCH, M.D. (From the Hospital of The Rockefeller
Product Use HPSC-CC are for research use only. It is not approved for human or animal use, or for application in in vitro diagnostic procedures.
 HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
SUPPLEMENTARY INFORMATION. Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was
 SUPPLEMENTARY INFORMATION Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was grown in a casein-based semisynthetic medium (C+Y) supplemented with yeast extract (1 mg/ml of
SUPPLEMENTARY INFORMATION Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was grown in a casein-based semisynthetic medium (C+Y) supplemented with yeast extract (1 mg/ml of
HISTO-PHYSIOLOGY HISTO-PHYSIOLOGY HISTO-PHYSIOLOGY. 09-Mar-15. Dr. Muhammad Tariq Javed. RESPIRATORY SYSTEM Lec-1
 RESPIRATORY SYSTEM Lec-1 Dr. Muhammad Tariq Javed Professor Department of Pathology, University of Agriculture, Faisalabad. Email: mtjaved@uaf.edu.pk Web: http://www.geocities.ws/mtjaved 1 2 Conducting
RESPIRATORY SYSTEM Lec-1 Dr. Muhammad Tariq Javed Professor Department of Pathology, University of Agriculture, Faisalabad. Email: mtjaved@uaf.edu.pk Web: http://www.geocities.ws/mtjaved 1 2 Conducting
What is the immune system? Types of Immunity. Pasteur and rabies vaccine. Historical Role of smallpox. Recognition Response
 Recognition Response Effector memory What is the immune system? Types of Immunity Innate Adaptive Anergy: : no response Harmful response: Autoimmunity Historical Role of smallpox Pasteur and rabies vaccine
Recognition Response Effector memory What is the immune system? Types of Immunity Innate Adaptive Anergy: : no response Harmful response: Autoimmunity Historical Role of smallpox Pasteur and rabies vaccine
Supplementary Figure 1. Ent inhibits LPO activity in a dose- and time-dependent fashion.
 Supplementary Information Supplementary Figure 1. Ent inhibits LPO activity in a dose- and time-dependent fashion. Various concentrations of Ent, DHBA or ABAH were pre-incubated for 10 min with LPO (50
Supplementary Information Supplementary Figure 1. Ent inhibits LPO activity in a dose- and time-dependent fashion. Various concentrations of Ent, DHBA or ABAH were pre-incubated for 10 min with LPO (50
EXPERIMENTAL SHIGELLA INFECTIONS: CHARACTERISTICS OF A FATAL
 EXPERIMENTAL SHIGELLA INFECTIONS: CHARACTERISTICS OF A FATAL INFECTION PRODUCED IN GUINEA PIGS' SAMUEL B. FORMAL, GUSTAVE J. DAMMIN, E. H. LABREC, AND H. SCHNEIDER Walter Reed Army Institute of Research,
EXPERIMENTAL SHIGELLA INFECTIONS: CHARACTERISTICS OF A FATAL INFECTION PRODUCED IN GUINEA PIGS' SAMUEL B. FORMAL, GUSTAVE J. DAMMIN, E. H. LABREC, AND H. SCHNEIDER Walter Reed Army Institute of Research,
Human IL-2. Pre-Coated ELISA Kit
 Human IL-2 (Interleukin 2) Pre-Coated ELISA Kit Catalog No: 90-2083 1 96 well Format (96 tests) Detection Range: 31.2 2000 pg/ml Sensitivity: < 18.75 pg/ml This immunoassay kit allows for the in vitro
Human IL-2 (Interleukin 2) Pre-Coated ELISA Kit Catalog No: 90-2083 1 96 well Format (96 tests) Detection Range: 31.2 2000 pg/ml Sensitivity: < 18.75 pg/ml This immunoassay kit allows for the in vitro
Supplemental Experimental Procedures
 Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Streptococcus pneumoniae Causes Experimental Meningitis following Intranasal and Otitis Media Infections via a Nonhematogenous Route
 INFECTION AND IMMUNITY, Dec. 2001, p. 7318 7325 Vol. 69, No. 12 0019-9567/01/$04.00 0 DOI: 10.1128/IAI.69.12.7318 7325.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Streptococcus
INFECTION AND IMMUNITY, Dec. 2001, p. 7318 7325 Vol. 69, No. 12 0019-9567/01/$04.00 0 DOI: 10.1128/IAI.69.12.7318 7325.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Streptococcus
Pathology of Pneumonia
 Pathology of Pneumonia Dr. Atif Ali Bashir Assistant Professor of Pathology College of Medicine Majma ah University Introduction: 5000 sq meters of area.! (olympic track) Filters >10,000 L of air / day!
Pathology of Pneumonia Dr. Atif Ali Bashir Assistant Professor of Pathology College of Medicine Majma ah University Introduction: 5000 sq meters of area.! (olympic track) Filters >10,000 L of air / day!
Chapter 17. The Lymphatic System and Immunity. Copyright 2010, John Wiley & Sons, Inc.
 Chapter 17 The Lymphatic System and Immunity Immunity Innate Immunity Fast, non-specific and no memory Barriers, ph extremes, Phagocytes & NK cells, fever, inflammation, complement, interferon Adaptive
Chapter 17 The Lymphatic System and Immunity Immunity Innate Immunity Fast, non-specific and no memory Barriers, ph extremes, Phagocytes & NK cells, fever, inflammation, complement, interferon Adaptive
CHAPTER 4 IMMUNOLOGICAL TECHNIQUES
 CHAPTER 4 IMMUNOLOGICAL TECHNIQUES Nitroblue Tetrazolium Chloride (NBT) Reduction test NBT reduction test was evaluated by employing the method described by Hudson and Hay,1989 based upon principle that
CHAPTER 4 IMMUNOLOGICAL TECHNIQUES Nitroblue Tetrazolium Chloride (NBT) Reduction test NBT reduction test was evaluated by employing the method described by Hudson and Hay,1989 based upon principle that
Mouse Hepatic Progenitor Organoid Culture: Supplementary Protocols
 TECHNICAL BULLETIN Mouse Hepatic Progenitor Organoid Culture: The following are supplementary protocols for the culture of hepatic organoids with HepatiCult Organoid Growth Medium (Mouse) (Catalog #06030).
TECHNICAL BULLETIN Mouse Hepatic Progenitor Organoid Culture: The following are supplementary protocols for the culture of hepatic organoids with HepatiCult Organoid Growth Medium (Mouse) (Catalog #06030).
Assessment of Keratitis Damage in an Age Dependent Mouse Model Using Analytical Software
 Assessment of Keratitis Damage in an Age Dependent Mouse Model Using Analytical Software Quincy C. Moore III, PhD, 1 Emmanuel B. Olorunyomi, 1 Miles E. DuBose, 2 and Cleveland O. Lane Jr, PhD 1 1 Prairie
Assessment of Keratitis Damage in an Age Dependent Mouse Model Using Analytical Software Quincy C. Moore III, PhD, 1 Emmanuel B. Olorunyomi, 1 Miles E. DuBose, 2 and Cleveland O. Lane Jr, PhD 1 1 Prairie
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep
 The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep invaders out of the body (pp. 772 773; Fig. 21.1; Table
The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep invaders out of the body (pp. 772 773; Fig. 21.1; Table
Innate Immunity. Natural or native immunity
 Innate Immunity 1 Innate Immunity Natural or native immunity 2 When microbes enter in the body 3 Secondly, it also stimulates the adaptive immune system 4 Immunologic memory 5 Components of Innate Immunity
Innate Immunity 1 Innate Immunity Natural or native immunity 2 When microbes enter in the body 3 Secondly, it also stimulates the adaptive immune system 4 Immunologic memory 5 Components of Innate Immunity
bacteria review 1. Which of the following structures is not found in bacteria?
 Name: Date: 1. Which of the following structures is not found in bacteria? 5. How do human diseases caused by bacteria and diseases caused by viruses react to antibiotics? A. ribosome B. cytoplasm C. cell
Name: Date: 1. Which of the following structures is not found in bacteria? 5. How do human diseases caused by bacteria and diseases caused by viruses react to antibiotics? A. ribosome B. cytoplasm C. cell
L6 GLUT4myc Cell Growth Protocol
 L6 GLUT4myc Cell Growth Protocol Background: Parental L6 cells selected for high fusion (2, 3) were stably transfected with a rat GLUT4 cdna carrying a myc epitope (recognized by the commercially available
L6 GLUT4myc Cell Growth Protocol Background: Parental L6 cells selected for high fusion (2, 3) were stably transfected with a rat GLUT4 cdna carrying a myc epitope (recognized by the commercially available
Acute Inflammation. Dr. G Mahendra Department of Pathology
 Acute Inflammation Dr. G Mahendra Department of Pathology Inflammation Inflammation is a physiological response to tissue injury. Tissue injury Reaction/response of body Inflammation Inflammation is not
Acute Inflammation Dr. G Mahendra Department of Pathology Inflammation Inflammation is a physiological response to tissue injury. Tissue injury Reaction/response of body Inflammation Inflammation is not
Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice
 Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice Publication from the Dr. Rath Research Institute Experimental
Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice Publication from the Dr. Rath Research Institute Experimental
Mass Histology Service
 Mass Histology Service A complete anatomical pathology laboratory www.masshistology.com Telephone: (877) 286-6004 Report on Pathology A Time Course Study of the Local Effects of Intramuscular XXXXXXX Injection
Mass Histology Service A complete anatomical pathology laboratory www.masshistology.com Telephone: (877) 286-6004 Report on Pathology A Time Course Study of the Local Effects of Intramuscular XXXXXXX Injection
Secretory antibodies in the upper respiratory tract
 Secretory antibodies in the upper respiratory tract B lymphocytes IgM (pneumococcus) Dimeric IgA J chain Polymeric immunoglobulin receptor (PigR) Polysaccharide capsule Epithelial cell Basolateral Secretory
Secretory antibodies in the upper respiratory tract B lymphocytes IgM (pneumococcus) Dimeric IgA J chain Polymeric immunoglobulin receptor (PigR) Polysaccharide capsule Epithelial cell Basolateral Secretory
Follicle Dermal Papilla Cell
 Follicle Dermal Papilla Cell Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product Description
Follicle Dermal Papilla Cell Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product Description
Streptococcus pyogenes
 Streptococcus pyogenes From Wikipedia, the free encyclopedia Streptococcus pyogenes S. pyogenes bacteria at 900x magnification. Scientific classification Kingdom: Eubacteria Phylum: Firmicutes Class: Cocci
Streptococcus pyogenes From Wikipedia, the free encyclopedia Streptococcus pyogenes S. pyogenes bacteria at 900x magnification. Scientific classification Kingdom: Eubacteria Phylum: Firmicutes Class: Cocci
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System Multiple-Choice Questions
 Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 24 The Immune System 24.1 Multiple-Choice Questions 1) The body's innate defenses against infection include A) several nonspecific
CHRONIC INFLAMMATION
 CHRONIC INFLAMMATION Chronic inflammation is an inflammatory response of prolonged duration often for months, years or even indefinitely. Its prolonged course is proved by persistence of the causative
CHRONIC INFLAMMATION Chronic inflammation is an inflammatory response of prolonged duration often for months, years or even indefinitely. Its prolonged course is proved by persistence of the causative
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
محاضرة مناعت مدرس المادة :ا.م. هدى عبدالهادي علي النصراوي Immunity to Infectious Diseases
 محاضرة مناعت مدرس المادة :ا.م. هدى عبدالهادي علي النصراوي Immunity to Infectious Diseases Immunity to infection depends on a combination of innate mechanisms (phagocytosis, complement, etc.) and antigen
محاضرة مناعت مدرس المادة :ا.م. هدى عبدالهادي علي النصراوي Immunity to Infectious Diseases Immunity to infection depends on a combination of innate mechanisms (phagocytosis, complement, etc.) and antigen
Pre-Lec. + Questions
 Sheet 14 (part 2) made by : Majd abu-fares corrected by: Shatha khtoum date:8/11/2016 Pre-Lec. + Questions *Pus: secretion of {WBCs + product of WBCs + product of M.O} *WBCs can produce enzymes cytokines
Sheet 14 (part 2) made by : Majd abu-fares corrected by: Shatha khtoum date:8/11/2016 Pre-Lec. + Questions *Pus: secretion of {WBCs + product of WBCs + product of M.O} *WBCs can produce enzymes cytokines
Acquired Immunity Cells are initially and require before they can work Responds to individual microbes
 1 of 10 THE IMMUNE SYSTEM CHAPTER 43; PAGES 898 921 WHY DO WE NEED AN IMMUNE SYSTEM? It s a dirty, dirty world out there and we are vastly outnumbered Bacteria and parasites are everywhere The body has
1 of 10 THE IMMUNE SYSTEM CHAPTER 43; PAGES 898 921 WHY DO WE NEED AN IMMUNE SYSTEM? It s a dirty, dirty world out there and we are vastly outnumbered Bacteria and parasites are everywhere The body has
Cell Mediated Immunity CELL MEDIATED IMMUNITY. Basic Elements of Cell Mediated Immunity (CMI) Antibody-dependent cell-mediated cytotoxicity (ADCC)
 Chapter 16 CELL MEDIATED IMMUNITY Cell Mediated Immunity Also known as Cellular Immunity or CMI The effector phase T cells Specificity for immune recognition reactions TH provide cytokines CTLs do the
Chapter 16 CELL MEDIATED IMMUNITY Cell Mediated Immunity Also known as Cellular Immunity or CMI The effector phase T cells Specificity for immune recognition reactions TH provide cytokines CTLs do the
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Where are we heading?
 Unit 4: Where are we heading? Unit 4: Introduction Unit 1: Why should we care about infectious diseases? Unit 2: What does it mean to have an infectious disease? Unit 3: When does a microbe become a pathogen?
Unit 4: Where are we heading? Unit 4: Introduction Unit 1: Why should we care about infectious diseases? Unit 2: What does it mean to have an infectious disease? Unit 3: When does a microbe become a pathogen?
Key Difference - Pleural Effusion vs Pneumonia
 Difference Between Pleural Effusion and Pneumonia www.differencebetween.com Key Difference - Pleural Effusion vs Pneumonia Pleural effusion and pneumonia are two conditions that affect our respiratory
Difference Between Pleural Effusion and Pneumonia www.differencebetween.com Key Difference - Pleural Effusion vs Pneumonia Pleural effusion and pneumonia are two conditions that affect our respiratory
As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the
 3 RESULTS As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the DKFZ in Heidelberg (Dept. of Cellular and Molecular pathology) contributed to this work by performing
3 RESULTS As outlined under External contributions (see appendix 7.1), the group of Prof. Gröne at the DKFZ in Heidelberg (Dept. of Cellular and Molecular pathology) contributed to this work by performing
Lecture 2: Virology. I. Background
 Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Haemophilus influenzae
 Haemophilus influenzae type b Severe bacterial infection, particularly among infants During late 19th century believed to cause influenza Immunology and microbiology clarified in 1930s Haemophilus influenzae
Haemophilus influenzae type b Severe bacterial infection, particularly among infants During late 19th century believed to cause influenza Immunology and microbiology clarified in 1930s Haemophilus influenzae
ANATOMY OF THE IMMUNE SYSTEM
 Immunity Learning objectives Explain what triggers an immune response and where in the body the immune response occurs. Understand how the immune system handles exogenous and endogenous antigen differently.
Immunity Learning objectives Explain what triggers an immune response and where in the body the immune response occurs. Understand how the immune system handles exogenous and endogenous antigen differently.
Unit 5 The Human Immune Response to Infection
 Unit 5 The Human Immune Response to Infection Unit 5-page 1 FOM Chapter 21 Resistance and the Immune System: Innate Immunity Preview: In Chapter 21, we will learn about the branch of the immune system
Unit 5 The Human Immune Response to Infection Unit 5-page 1 FOM Chapter 21 Resistance and the Immune System: Innate Immunity Preview: In Chapter 21, we will learn about the branch of the immune system
Body Defense Mechanisms
 BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 13 Body Defense Mechanisms Lecture Presentation Anne Gasc Hawaii Pacific University and University of
BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 13 Body Defense Mechanisms Lecture Presentation Anne Gasc Hawaii Pacific University and University of
Faculty of Veterinary Medicine Universiti Malaysia Kelantan. Immunology and Serology (DVT2153)
 Faculty of Veterinary Medicine Universiti Malaysia Kelantan Immunology and Serology (DVT2153) By Dr. Erkihun Aklilu 1 Immune Response AgAinst Various infectious agents Immunology and Serology (DVT2153)
Faculty of Veterinary Medicine Universiti Malaysia Kelantan Immunology and Serology (DVT2153) By Dr. Erkihun Aklilu 1 Immune Response AgAinst Various infectious agents Immunology and Serology (DVT2153)
An Investigation into the Effects of the Addition of Synthetic Receptor on Chemokine Induced Jurkat T-Cell Migration
 An Investigation into the Effects of the Addition of Synthetic Receptor on Chemokine Induced Jurkat T-Cell Migration Jessica Jurado, Jianfang Hu, Avery August, PhD PSU Undergraduate Animal Bioscience July
An Investigation into the Effects of the Addition of Synthetic Receptor on Chemokine Induced Jurkat T-Cell Migration Jessica Jurado, Jianfang Hu, Avery August, PhD PSU Undergraduate Animal Bioscience July
1. The barriers of the innate immune system to infection
 Section 3.qxd 16/06/05 2:11 PM Page 12 12 SECTION THREE: Fleshed out 1. The barriers of the innate immune system to infection Questions What are the three characteristics of the innate immune system? What
Section 3.qxd 16/06/05 2:11 PM Page 12 12 SECTION THREE: Fleshed out 1. The barriers of the innate immune system to infection Questions What are the three characteristics of the innate immune system? What
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury
 Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Histopathology: Glomerulonephritis and other renal pathology
 Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
FIG S1 Replication rates of S. suis strain 10, 10Δsly, and 10cpsΔEF on mono- and virus pre-infected porcine PCLS.
 A strain 10 10cps EF B strain 10 H1N1 + strain 10 10cps EF H1N1 + 10cps EF 10 8 10 sly 10 7 H3N2 + strain 10 H3N2 + 10cps EF CFU/ml media 10 7 10 6 10 5 10 4 CFU/ml media 10 6 10 5 10 4 10 3 0 2 4 8 12
A strain 10 10cps EF B strain 10 H1N1 + strain 10 10cps EF H1N1 + 10cps EF 10 8 10 sly 10 7 H3N2 + strain 10 H3N2 + 10cps EF CFU/ml media 10 7 10 6 10 5 10 4 CFU/ml media 10 6 10 5 10 4 10 3 0 2 4 8 12
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit
 INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
INSTRUCTIONS Pierce Primary Cardiomyocyte Isolation Kit 88281 Number Description 88281 Pierce Primary Cardiomyocyte Isolation Kit, contains sufficient reagents to isolate cardiomyocytes from 50 neonatal
Where are we heading?
 Unit 5: Where are we heading? Unit 5: Introduction Unit 1: Why should we care about infectious diseases? Unit 2: What does it mean to have an infectious disease? Unit 3: When does a microbe become a pathogen?
Unit 5: Where are we heading? Unit 5: Introduction Unit 1: Why should we care about infectious diseases? Unit 2: What does it mean to have an infectious disease? Unit 3: When does a microbe become a pathogen?
Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit
 PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
RODENT Hepatocytes Care Manual
 RODENT Hepatocytes Care Manual INSTRUCTION MANUAL ZBM0054.02 SHIPPING CONDITIONS Rodent Hepatocytes cryopreserved Orders are delivered via Federal Express courier. All US and Canada orders are shipped
RODENT Hepatocytes Care Manual INSTRUCTION MANUAL ZBM0054.02 SHIPPING CONDITIONS Rodent Hepatocytes cryopreserved Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Natural Defense Mechanisms
 Color code: Important in red Extra in blue For team error adjustments, click here Natural Defense Mechanisms Objectives To know First (non-specific immunity) and second (adaptive immunity) lines of defense
Color code: Important in red Extra in blue For team error adjustments, click here Natural Defense Mechanisms Objectives To know First (non-specific immunity) and second (adaptive immunity) lines of defense
value as a medium for the in vivo cultivation of different
 THE BEHAVIOR OF THE VIRUS OF EQUINE ENCEPH- ALOMYELITIS ON THE CHORIOALLANTOIC MEMBRANE OF THE DEVELOPING CHICK' ELIZABETH HIGBIE AND BEATRICE HOWITT George Williams Hooper Foundation, University of California,
THE BEHAVIOR OF THE VIRUS OF EQUINE ENCEPH- ALOMYELITIS ON THE CHORIOALLANTOIC MEMBRANE OF THE DEVELOPING CHICK' ELIZABETH HIGBIE AND BEATRICE HOWITT George Williams Hooper Foundation, University of California,
Innate vs Adaptive Response
 General Immunology Innate vs Adaptive Response Innate- non-specific (4 types of barriers) anatomic- ato mechanical ca (skin), ph, mucous, normal flora Physiologic- temperature, ph, chemicals (lysozyme,
General Immunology Innate vs Adaptive Response Innate- non-specific (4 types of barriers) anatomic- ato mechanical ca (skin), ph, mucous, normal flora Physiologic- temperature, ph, chemicals (lysozyme,
INTRODUCTION TO UPPER RESPIRATORY TRACT DISEASES
 Upper Respiratory Tract Infections Return to Syllabus INTRODUCTION TO UPPER RESPIRATORY TRACT DISEASES General Goal: To know the major mechanisms of defense in the URT, the major mechanisms invaders use
Upper Respiratory Tract Infections Return to Syllabus INTRODUCTION TO UPPER RESPIRATORY TRACT DISEASES General Goal: To know the major mechanisms of defense in the URT, the major mechanisms invaders use
