Comparison of oral prednisolone and intramuscular depot triamcinolone in patients with severe chronic
|
|
|
- Cory Stevenson
- 5 years ago
- Views:
Transcription
1 Thorax 1984;39: Comparison of oral prednisolone and intramuscular depot triamcinolone in patients with severe chronic asthma RICHARD F WILLEY, RONALD J FERGUSSON, DAVID J GODDEN, GRAHAM K CROMPTON, IAN WB GRANT From the Respiratory Unit, Northern General Hospital, Edinburgh ABSTRACT In a double blind crossover study oral prednisolone was compared with intramuscular depot triamcinolone in the treatment of 20 patients with severe chronic asthma. A short term study comparing each treatment over four weeks showed only minor differences in therapeutic efficacy, but at the end of 24 week periods on each of the two treatments triamcinolone was significantly more effective than prednisolone in terms of forced expiratory volume in one second and forced vital capacity. Better control of asthma was accompanied by a significant fall in weight and some evidence of reduced adrenal suppression (improved cortisol response following a short tetracosactrin test). Side effects, including menstrual irregularities, muscle pain, and hirsuitism, were, however, more common during treatment with triamcinolone. Although treatment with corticosteroids by inhalation controls symptoms in many patients with chronic asthma, those with either severe asthma or a poor response to inhaled corticosteroid aerosols often require supplementary systemic treatment. Traditionally this is given in the form of oral prednisolone. A maintenance dose of 10 mg or more a day is required in only a small proportion of patients with chronic asthma, but such patients form a significant number of those attending asthma clinics and they are prone to develop side effects that may be seriously disabling and occasionally life threatening İt has been suggested in an open study' that better control is achieved, with no greater incidence of side effects, when corticosteroid treatment is given in the form of injections of depot triamcinolone at intervals of four weeks. This paper describes the results of studies designed to compare treatment with oral prednisolone and intramuscular triamcinolone in patients with poorly controlled asthma. A double blind crossover study of four weeks of each treatment was undertaken first, but as it showed only Address for reprint requests: Dr RF Willey, Royal Lancaster Infirmary, Lancaster LA1 4RP. Accepted 4 January 1984 minor differences in therapeutic efficacy it was followed by a study of the same design in which each treatment was continued for 24 weeks. Patients and methods Twenty outpatients (8 male and 12 female) with chronic bronchial asthma were included in the study. Their ages ranged from 15 to 76 years (mean 48.9). Informed consent for inclusion in the study was obtained in all cases. All the patients had previously shown an improvement in FEV, of at least 25% after treatment with an aqueous salbutamol aerosol (5 mg) delivered by intermittent positive pressure breathing (IPPB). They all had an FEV, of less than 75% of the predicted normal despite regular treatment with oral prednisolone, 10 mg daily, and inhaled beclomethasone, 400,ug daily. At the time of entry all had required at least two courses of prednisolone in increased dosage for exacerbations of asthma in the preceding 12 months but not in the last four weeks. Initially a short term double blind crossover study was performed on 20 patients. The treatment regimens were identical with those used in the long term study described below, except that each was continued for only four weeks. The following treatment regimens were then compared in a long term study, which was also of the 340
2 Comparison of oral prednisolone and intramuscular depot triamcinolone double blind crossover type: (1) prednisolone, 5 mg (one tablet) twice daily for 24 weeks, with an intramuscular injection of saline, 2 ml, on the first day of each four week period; (2) placebo "prednisolone" tablets, one twice daily for 24 weeks, with an intramuscular injection of triamcinolone acetonide suspension (Kenalog: ER Squibb and Sons Ltd), 80 mg on the first day of each four week period. Each treatment regimen was continued for 24 weeks, the order being decided by random allocation but with equal numbers of patients started on each treatment. No "washout" period was included because of the severity of asthma in the patients included in the study, but some observations were limited to the later parts of each treatment period to allow for possible carry over effects. Clinical assessments were made on entry and every four weeks thereafter. At each visit FEV1 and forced vital capacity (FVC) before and after administration of salbutamol by IPPB, body weight, blood pressure, and concentrations of urea, electrolytes, and plasma cortisol were measured. A short tetracosactrin test (250,ug given by intramuscular injection) was performed at entry and at 12, 24, 36, and 48 weeks. Patients' faces were photographed at 0, 24, and 48 weeks to assess the degree of Cushingoid appearance. If an exacerbation of asthma severe enough to warrant an increased dose of prednisolone occurred, patients were asked to stop the trial tablets and take 20 mg of prednisolone daily for seven days. The number of times this happened was recorded, together with any side effects attributed to treatment. Patients were withdrawn from the trial if their symptoms were uncontrolled or if they developed intolerable side effects. Patients continued on treatment with beclomethasone dipropionate, 100 jig four times daily, throughout the study. Inhaled bronchodilator treatment was also continued, but oral 18 agonists and theophylline preparations were not given. Results were analysed with Student's t test for paired comparisons. For body weight, systolic and diastolic blood pressure, potassium, urea, and cortisol, values obtained at the end of each period were compared with each other and with pretrial values. For FEVy, FVC, and increases in FEV, and FVC after the administration of salbutamol by IPPB, the mean results for the last eight and 16 weeks of each treatment period were compared. Single pretrial values are included in table 2 but these were not used in the analysis. Results were not compared over the whole 24 weeks of each treatment period since they might have been influenced by a carry over effect. For the same reason, the number of courses of prednisolone required for acute episodes of asthma was compared only over the last eight and 16 weeks of each treatment period. Results 341 The results of the short term study are shown in table 1. No significant differences between the treatment periods were found for FEV, or FVC (before and after salbutamol), but at all times the values were significantly better than pretrial values. The basal plasma cortisol concentrations did not change but the tetracosactrin response after prednisolone was lower than the pretrial response (p < 0.001) and lower than the response after triamcinolone (p < 0.01). In the long term study two patients failed to complete the full period of assessment. One was withdrawn 16 weeks after the start of the first treatment period (with triamcinolone) because of gradually increasing symptoms culminating in a severe attack of asthma that required treatment in hospital for three weeks. The other was withdrawn because of the development of severe asthmatic symptoms 22 weeks after the start of the second treatment period (with prednisolone). Results from all the 18 patients who completed 48 weeks of assessment were included in the analysis. The results are shown in tables 2-4. Mean values for FEV1 after the last 16 and the last eight weeks were significantly higher during treatment with triamcinolone than with prednisolone (p < 0-05 and p < 0-02 respectively). The mean values for FVC over the last 16 weeks were similarly higher during treatment with triamcinolone (p < 0.01), but there were no significant differences between the treatments for the mean increases in FEV, or FVC after the administration of salbutamol by IPPB. Patients required fewer courses of prednisolone during triamcinolone treatment periods, but this difference did not reach significance. Plasma urea concentrations were lower after treatment with triamcinolone (p < 0*001 when compared with the pretrial values, p < 0*01 when compared with the values after treatment with prednisolone); but the differences did not appear to be of clinical importance. The mean body weight at the end of the triamcinolone treatment period was significantly lower than that after treatment with prednisolone (p < 0-001). The mean morning basal plasma cortisol concentration was not significantly different after the two treatments, nor in either case did it differ from the very low pretrial value (0.08 (SD 0-1 1),umolll). Table 4 shows the mean increase in cortisol after a short tetracosactrin test. The increases after 12 and 24 weeks of triamcinolone
3 342 Table 1 Willey, Fergusson, Godden, Crompton, Grant Results ofshort term study on 20 patients (mean values with standard deviations in parentheses) Measurement Before trial Prednisolone treatment Tinamcinolone treatnent Prednisolone v triamcinolone: After 2 weeks After 4 weeks After 2 weeks After 4 weeks 2 and 4 week comparisons Weight (kg) 76-8 (16-5) 76-7 (16-6) 76-5 (16-2) 76*5 (16-5) 76-3 (16.2) NS Blood pressure (mm Hg) systolic ) 134 (12) 133 (13) 135 ( (14) NS diastolic 84 12) 84 (9) 83 (9) 84 (12 86 (9) NS FEV, (1) 1-44 (0.34) 1-81 (0-48) 1-78 (0-45) 1-80 (056) 1-81 (0.56) NS* FVC (1) 2-33 (0-64) 2-64 (0-74) 2-73 (0.65) 2-64 (0-71) 2-60 (0.64) NS* FEV after salbutamol (1) 1-95 (0-43) 2-31 (0-57) 2-24 (0-48) 2-33 (0-57) 2-26 (0-61) NS* FVC after salbutamol (I) 2-92 (0.66) 3-22 (0-61) 3-24 (0.56) 3-25 (0.69) 3-22 (0.69) NS* Plasma urea (mmolil) 56 (1.6) 5-5 (1-5) 5-8 (1-5) 5-7 (1.5) 5-5 (1-4) NS Plasma potassium (mmovl) 3*75 (0.32) 3-82 (0.42) 3-64 (0-51) 3-69 (0-34) 3 65 (0.38) NS Serum cortisol (JAmoVIl) 0-13 (0-15) 0.09 (0-17) 0-11 (0-21) 0-14 (0-17) 0-14 (0-16) NS Cortisol response to tetracosactrin (tmovl) 0-16 (0-14) 0.05 (0.07) 0.21 (0-19) p = 0-01** *No significant difference between treatment periods, but at all times values were significantly higher than before treatment (p < 0.05 or p < 0.005) **Pretrial v prednisolone period: p < 0-001; pretrial v triamcinolone period: NS. Conversion: SIto traditional units-urea: 1 mmovi = mg/100 ml; potassium: 1 mmovi = 1 meq/l; cortisol: 1 itmovli ig/100 ml. FVC-forced vital capacity; NS-not significant. Table 2 Results oflong term study on 18 patients (mean values for last 16 and last eight weeks ofa 24 week period of treatment with standard deviatons in parentheses) Measurement Before trial Last 16 weeks Last 8 weeks Prednisolone Triamcinolone p value Prednisolone Triamcinolone p value FEV, (l) 1-64 (0.26) 1-64 (0.40) 1-82 (0.43) < (0.37) 1-84 (0.45) < 0-02 FVC (1) 2-42 (0-62) 2-44 (0-60) 2-65 (0-61) < (0-58) 2-63 (0-66) NS Increase in FEV after salbutamol (l) 0-41 (0.29) 0-32 (0-17) 0-35 (0-17) NS 0-32 (0-20) 0-29 (0-16) NS Increase in FVC after salbutamol (1) 0.60 (0.49) 0-41 (0.36) 0.37 (0-25) NS 0-41 (0-36) 0-34 (0.25) NS Prednisolone courses (total per patient 1-56 (1-89) 0.61 (0.92) NS 0-89 (1-18) 0-33 (0-59) NS FVC-forced vital capacity; NS-not significant. Table 3 Results oflong term study on 18 patients (mean values with standard deviations in parentheses) Measurement Before trial (A) After 24 weeks Comparisons Prednisolone (B) Triamcinolone (C) A v B A v C B v C Weight (kg) 78-8 (17-5) 80-0 (18-7) 77-6 (18-1) NS NS p < Blood pressure (mm Hg) systolic (18-0) (16-9) (16-6) NS NS NS diastolic 87-4 (9-1) (10-7) 89 5 (12-2) NS NS NS Plasma potassium (mmol/1) 3-70 (0-45) 3.75 (0.35) 3-65 (0-32) NS NS NS Plasma urea (mmol/1) 5-99 (1-29) 6-08 (1-66) 4-94 (1-32) NS p < p < 0-01 Morning basal plasma cortisol (/mo l) 0-08 (0-11) 0.12 (0-13) 0.08 (0.07) NS NS NS Conversion: SI to traditional units-potassium: 1 mmol/l = 1 meq/l; urea: 1 mmovl = mg/100 ml; cortisol: 1.tmol/l 36-23,ug/100 ml. NS-not significant.
4 Comparison of oral prednisolone and intramuscular depot triamcinolone Table 4 Response to tetracosactrin 30 minutes after dose of250 pg in 18 patients (mean values with standard deviations in parentheses) Before trial (A) After 12 weeks After 24 weeks Prednisolone (B) Triamcinolone (C) p value Prednisolone (D) Triamcinolone (E) p value (B v C) (D v E) Increase in plasma cortisol after tetracosactrin (,umol/l) 0-17 (0-13) *0-10 (0-11) 0-18 (0-12) < (0-13) 0-22 (0-14) < 0-01 *Significantly less than pretrial value (p < 0.05). Conversion: SI to traditional values-cortisol: 1,umol/Il 36-23,ug/100 ml. were greater than those after equivalent periods of prednisolone (p < 0-02 and p < 0-01 respectively) and that after 12 weeks of prednisolone was significantly smaller than the pretrial increase (p < 0-05). A normal response to tetracosactrin was defined as an increase in plasma cortisol concentration of 0-2,umoIIl (7.25 mg/100 ml) or more, 30 minutes after the intramuscular injection of 250,ug, from a baseline level of 0-3-0O7,umol/l ( ,ug/100 ml) to at least 055 umol/l (19.2,ug/100 ml). A subnormal response was recorded in every patient throughout the study, although one patient had a normal response at the time of entry. SIDE EFFECTS Side effects (table 5) were reported by 18 of the 20 patients during treatment with triamcinolone and by 12 during treatment with prednisolone. The most troublesome were menstrual disturbances and muscle aching and weakness. Four premenopausal women were included in the study and of these three reported menstrual side effects. Two developed amenorrhoea lasting for two and five months and resolving within four weeks of stopping treatment with triamcinolone; the third developed menorrhagia during treatment with triamcinolone. One postmenopausal patient developed vaginal bleeding after two triamcinolone injections. Uterine curettings were normal and the vaginal bleeding stopped Table 5 Numbers ofpatients developing side effects during treatment with triamcinolone and prednisolone Side effect Triamcinolone Prednisolone Muscle aching and weakness 6 2 Rashes 5 2 Menstrual problems 4 Facial hirsuitism 4 1 Depression 3 Oedema 2 3 Hypokalaemia 2 2 Hypertension 1 Nausea 1 1 Bruising 3 during the first month after the change to prednisolone. Muscle aching and weakness occurred in six patients while they were receiving triamcinolone and in two during treatment with prednisolone. One patient developed considerable weakness and wasting of the shoulder muscles. His symptoms started during treatment with prednisolone and increased during the first three months of triamcinolone, but then subsided despite continued treatment. Rashes occurred in five patients during triamcinolone treatment (two acneiform, two non-specific, one typical of psoriasis) and in two patients while they were having prednisolone (one acneiform, one nonspecific). One patient with mild hypertension required treatment for a gradually increasing diastolic blood pressure during the triamcinolone period. Glycosuria was not detected in any of the 18 patients during the study, but glucose tolerance tests were not performed. No patient had to be withdrawn from the trial because of side effects. CUSHINGOID APPEARANCE The patient's photographs were shown in a double blind fashion to two experienced clinical endocrinologists, who were asked to assess the degree of Cushingoid appearance. Using a simple scoring system they were unable to recognise any consistent differences between the photographs taken after each treatment period either by direct comparison or when they were compared with photographs taken on entry to the study. Discussion 343 The patients included in this study were known to have had poorly controlled asthma, often for several years, during which time they had been maintained on regular oral prednisolone and yet had often required courses of high dose treatment and hospital admission because of deterioration in their symptoms. The short term study was undertaken to see
5 344 whether significant differences between the effects of oral prednisolone and intramuscular triamcinolone could be detected after periods of four weeks, but the results were inconclusive, It was, however, of interest that at the end of both treatment periods in the short term study FEV, and FVC before and after salbutamol were significantly higher than the pretrial values, even although one of the regimens was identical with the pretrial treatment. This suggests that some patients had not been taking the prescribed dose of prednisolone (10 mg daily) regularly during the pretrial period, and that an increase in "true" corticosteroid dosage during the close supervision of the study could also have accounted-at least partially-for the poorer tetracosactrin responses at the end of the treatment periods than before the trial. In the long term study triamcinolone was shown to have a significant advantage over prednisolone in terms of FEV, and FVC, but experimentally it is known to have at most 20% more glucocorticoid activity than prednisolone,2 and 80 mg every 28 days should be equivalent to a mean daily dose of only 3*43 mg of prednisolone. This would seem to suggest that triamcinolone is a more potent agent in terms of clinical activity than is prednisolone (perhaps because of superior bioavailability or intrinsic pharmacological properties); but if that is the case the observation made in this study that the response to tetracosactrin was reduced to a lesser degree by triamcinolone than by prednisolone is Willey, Fergusson, Godden, Crompton, Grant unexpected and unexplained. The significant reduction in body weight at the end of the triamcinolone period might suggest less corticosteroid effect in respect of fluid retention and obesity, but loss of muscle mass from subclinical myopathy could have at least partly accounted for this observation. This study confirms the view expressed by Peake et al' that intramuscular depot triamcinolone is a potentially useful alternative to prednisolone for the control of intractable chronic asthma. We would not normally recommend it in preference to prednisolone because some side effects, particularly menstrual irregularities, are often unacceptable and others, such as myopathy and osteoporosis, have not yet been fully assessed. It may, however, be of value in patients with severe chronic asthma in whom treatment with prednisolone has proved ineffective. We would like to thank Dr CM Feek and Dr RS Gray of the deparment of endocrine and metabolic diseases, Western General Hospital, Edinburgh, for assessing the patients' photographs. References 'Peake MD, Cayton RM, Howard P. Triamcinolone in corticosteroid-resistant asthma. Br J Dis Chest 1979; 73: Liddle GW. Clinical pharmacology of the antiinflammatory steroids. Clin Pharmacol Ther 1961; 2:615. Thorax: first published as /thx on 1 May Downloaded from on 15 September 2018 by guest. Protected by copyright.
Intramuscular triamcinolone acetonide in chronic severe asthma
 Thorax 1985;40: 840-845 Intramuscular triamcinolone acetonide in chronic severe asthma DT McLEOD, SJ CAPEWELL, JENNIFER LAW, W MAcLAREN, A SEATON From the Chest Unit, City Hospital, and Institute of Occupational
Thorax 1985;40: 840-845 Intramuscular triamcinolone acetonide in chronic severe asthma DT McLEOD, SJ CAPEWELL, JENNIFER LAW, W MAcLAREN, A SEATON From the Chest Unit, City Hospital, and Institute of Occupational
Salmeterol, a new long acting inhaled,f2 adrenoceptor agonist: comparison with salbutamol in adult asthmatic patients
 Thorax 1988;43:674-678 Salmeterol, a new long acting inhaled,f2 adrenoceptor agonist: comparison with salbutamol in adult asthmatic patients ANDERS ULLMAN, NILS SVEDMYR From the Department of Clinical
Thorax 1988;43:674-678 Salmeterol, a new long acting inhaled,f2 adrenoceptor agonist: comparison with salbutamol in adult asthmatic patients ANDERS ULLMAN, NILS SVEDMYR From the Department of Clinical
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Serum potassium responses to nebulized salbutamol administered during an
 Archives of Emergency Medicine, 1989, 6, 22-26 Serum potassium responses to nebulized salbutamol administered during an acute asthmatic attack D. DaCRUZ & C. HOLBURN Accident and Emergency Department,
Archives of Emergency Medicine, 1989, 6, 22-26 Serum potassium responses to nebulized salbutamol administered during an acute asthmatic attack D. DaCRUZ & C. HOLBURN Accident and Emergency Department,
Budesonide treatment of moderate and severe asthma in children: A doseresponse
 Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
SYNOPSIS THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
The clinical effectiveness and costeffectiveness. treatment of chronic asthma in children under the age of 12 years
 The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
On completion of this chapter you should be able to: discuss the stepwise approach to the pharmacological management of asthma in children
 7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Treatment of acute asthmatic exacerbations with an increased dose of inhaled steroid
 12 Paediatrics and Child Health, Dunedin School of Medicine, PO Box 913, University of Otago Medical School, Dunedin, New Zealand J Garrett Preventive and Social Medicine, Dunedin School of Medicine S
12 Paediatrics and Child Health, Dunedin School of Medicine, PO Box 913, University of Otago Medical School, Dunedin, New Zealand J Garrett Preventive and Social Medicine, Dunedin School of Medicine S
Dual-Controller Asthma Therapy: Rationale and Clinical Benefits
 B/1 Dual-Controller Asthma Therapy: Rationale and Clinical Benefits MODULE B The 1997 National Heart, Lung, and Blood Institute (NHLBI) Expert Panel guidelines on asthma management recommend a 4-step approach
B/1 Dual-Controller Asthma Therapy: Rationale and Clinical Benefits MODULE B The 1997 National Heart, Lung, and Blood Institute (NHLBI) Expert Panel guidelines on asthma management recommend a 4-step approach
GINA. At-A-Glance Asthma Management Reference. for adults, adolescents and children 6 11 years. Updated 2017
 GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
A comparison of global questions versus health status questionnaires as measures of the severity and impact of asthma
 Eur Respir J 1999; 1: 591±596 Printed in UK ± all rights reserved Copyright #ERS Journals Ltd 1999 European Respiratory Journal ISSN 93-1936 A comparison of global questions versus health status questionnaires
Eur Respir J 1999; 1: 591±596 Printed in UK ± all rights reserved Copyright #ERS Journals Ltd 1999 European Respiratory Journal ISSN 93-1936 A comparison of global questions versus health status questionnaires
Corticosteroids. Abdulmoein Al-Agha, FRCPCH Professor of Pediatric Endocrinology, King Abdulaziz University Hospital,
 Corticosteroids Abdulmoein Al-Agha, FRCPCH Professor of Pediatric Endocrinology, King Abdulaziz University Hospital, http://aagha.kau.edu.sa History 1855 Addison's disease 1856 Adrenal glands essential
Corticosteroids Abdulmoein Al-Agha, FRCPCH Professor of Pediatric Endocrinology, King Abdulaziz University Hospital, http://aagha.kau.edu.sa History 1855 Addison's disease 1856 Adrenal glands essential
ADULT ASTHMA GUIDE SUMMARY. This summary provides busy health professionals with key guidance for assessing and treating adult asthma.
 ADULT ASTHMA GUIDE SUMMARY This summary provides busy health professionals with key guidance for assessing and treating adult asthma. Its source document Asthma and Respiratory Foundation NZ Adult Asthma
ADULT ASTHMA GUIDE SUMMARY This summary provides busy health professionals with key guidance for assessing and treating adult asthma. Its source document Asthma and Respiratory Foundation NZ Adult Asthma
G. Boyd on behalf of a UK Study group
 Eur Respir J, 1995, 8, 1494 1498 DOI: 10.1183/09031936.95.08091494 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1995 European Respiratory Journal ISSN 0903-1936 Salmeterol xinafoate in
Eur Respir J, 1995, 8, 1494 1498 DOI: 10.1183/09031936.95.08091494 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1995 European Respiratory Journal ISSN 0903-1936 Salmeterol xinafoate in
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Treatment. Assessing the outcome of interventions Traditionally, the effects of interventions have been assessed by measuring changes in the FEV 1
 58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
Medicine Dr. Kawa Lecture 4 - Treatment of asthma :
 Medicine Dr. Kawa Lecture 4 - Treatment of asthma : Avoiding allergens. Hyposensitization :Subcutaneous injections of inially very small, but gradually increasing doses of allergens (desensitization or
Medicine Dr. Kawa Lecture 4 - Treatment of asthma : Avoiding allergens. Hyposensitization :Subcutaneous injections of inially very small, but gradually increasing doses of allergens (desensitization or
THE NEW ZEALAND MEDICAL JOURNAL
 THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1267 ISSN 1175 8716 Is Salamol less effective than Ventolin? A randomised, blinded, crossover study in New Zealand Catherina L Chang, Manisha Cooray, Graham Mills,
THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1267 ISSN 1175 8716 Is Salamol less effective than Ventolin? A randomised, blinded, crossover study in New Zealand Catherina L Chang, Manisha Cooray, Graham Mills,
Acute Wheezing Emergencies: From Young to Old! Little Wheezers in the ED: Managing Acute Pediatric Asthma
 Acute Wheezing Emergencies: From Young to Old! Little Wheezers in the ED: Managing Acute Pediatric Asthma Talk Outline Case Delivery of bronchodilators Meter-dose inhalers and spacers Continuous nebulization
Acute Wheezing Emergencies: From Young to Old! Little Wheezers in the ED: Managing Acute Pediatric Asthma Talk Outline Case Delivery of bronchodilators Meter-dose inhalers and spacers Continuous nebulization
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone
Individual Study Table Referring to Part of the Dossier. Volume:
 Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma Full Report 2007
 Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma Full Report 2007 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Selecting Initial Therapy
Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma Full Report 2007 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Selecting Initial Therapy
beclometasone 100 MDI 2 puffs twice a day (recently changed to non CFC (Clenil Modulite))
 Case 1 Mr Thomson, a 32 year old asthmatic who is well known to you comes into your pharmacy. He is known to have a best peak flow of 640 L/min. He tells you that over the last few weeks he has been wakening
Case 1 Mr Thomson, a 32 year old asthmatic who is well known to you comes into your pharmacy. He is known to have a best peak flow of 640 L/min. He tells you that over the last few weeks he has been wakening
Meenu Singh, Joseph L. Mathew, Prabhjot Malhi, B.R. Srinivas and Lata Kumar
 Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL
 DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
Drugs acting on the respiratory tract
 Drugs acting on the respiratory tract Antiasthmatic drugs Asthma Asthma is a chronic inflammatory disease characterized by episodes of reversible airways obstruction due to bronchial hyperresponsiveness;
Drugs acting on the respiratory tract Antiasthmatic drugs Asthma Asthma is a chronic inflammatory disease characterized by episodes of reversible airways obstruction due to bronchial hyperresponsiveness;
Secondary Outcome/Efficacy Variable(s):
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Sponsor. Generic drug name. Trial indication(s) Protocol number. Protocol title. Clinical trial phase. Study Start/End Dates.
 Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients
 Thorax 1987;42:457-461 Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma D M MITCHELL, M A SOLOMON, S E J TOLFREE, M SHORT,
Thorax 1987;42:457-461 Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma D M MITCHELL, M A SOLOMON, S E J TOLFREE, M SHORT,
Diagnosis, Treatment and Management of Asthma
 Diagnosis, Treatment and Management of Asthma Asthma is a complex disorder characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and an underlying inflammation.
Diagnosis, Treatment and Management of Asthma Asthma is a complex disorder characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and an underlying inflammation.
NEW ZEALAND DATA SHEET SEREVENT Accuhaler
 NEW ZEALAND DATA SHEET SEREVENT Accuhaler Salmeterol xinafoate (50 mcg per inhalation) Presentation SEREVENT Accuhaler is a moulded plastic device containing a foil strip with 60 regularly placed blisters
NEW ZEALAND DATA SHEET SEREVENT Accuhaler Salmeterol xinafoate (50 mcg per inhalation) Presentation SEREVENT Accuhaler is a moulded plastic device containing a foil strip with 60 regularly placed blisters
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Abstract Background Theophylline is widely used in the treatment of asthma, and there is evidence that theophylline has antiinflammatory
 Thorax 2000;55:837 841 837 National Heart and Lung Institute, Imperial College School of Medicine and Royal Brompton Hospital, London SW3 6LY, UK S Lim A Jatakanon K F Chung P J Barnes Napp Laboratories
Thorax 2000;55:837 841 837 National Heart and Lung Institute, Imperial College School of Medicine and Royal Brompton Hospital, London SW3 6LY, UK S Lim A Jatakanon K F Chung P J Barnes Napp Laboratories
SYNOPSIS. Co-ordinating investigator Not applicable. Study centre(s) This study was conducted in Japan (57 centres).
 Drug product: Symbicort Turbuhaler Drug substance(s): ST (Symbicort Turbuhaler ) Edition No.: 1.0 Study code: D5890C00010 Date: 15 March 2007 SYNOPSIS An 8-week, randomised, double blind, parallel-group,
Drug product: Symbicort Turbuhaler Drug substance(s): ST (Symbicort Turbuhaler ) Edition No.: 1.0 Study code: D5890C00010 Date: 15 March 2007 SYNOPSIS An 8-week, randomised, double blind, parallel-group,
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Roflumilast (Daxas) for chronic obstructive pulmonary disease
 Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Chronotherapy of asthma with inhaled steroids: The effect of dosage timing on drug efficacy
 Chronotherapy of asthma with inhaled steroids: The effect of dosage timing on drug efficacy Diane J. Pincus, MD, Stanley J. Szefler, MD, Lynn M. Ackerson, PhD, and Richard J. Martin, MD Denver, Colo. Background:
Chronotherapy of asthma with inhaled steroids: The effect of dosage timing on drug efficacy Diane J. Pincus, MD, Stanley J. Szefler, MD, Lynn M. Ackerson, PhD, and Richard J. Martin, MD Denver, Colo. Background:
Comparison of the Effect of Short Course of Oral Prednisone in Patients with Acute Asthma
 ISPUB.COM The Internet Journal of Pulmonary Medicine Volume 7 Number 1 Comparison of the Effect of Short Course of Oral Prednisone in Patients with Acute Asthma E Razi, G Moosavi Citation E Razi, G Moosavi.
ISPUB.COM The Internet Journal of Pulmonary Medicine Volume 7 Number 1 Comparison of the Effect of Short Course of Oral Prednisone in Patients with Acute Asthma E Razi, G Moosavi Citation E Razi, G Moosavi.
SYNOPSIS. First subject enrolled 15 August 2003 Therapeutic confirmatory (III) Last subject completed 03 February 2005
 Drug product: SYMBICORT pmdi 160/4.5 μg Drug substance(s): Budesonide/formoterol Study code: SD-039-0728 Edition No.: FINAL Date: 27 February 2006 SYNOPSIS A 52-week, randomized, double-blind, single-dummy,
Drug product: SYMBICORT pmdi 160/4.5 μg Drug substance(s): Budesonide/formoterol Study code: SD-039-0728 Edition No.: FINAL Date: 27 February 2006 SYNOPSIS A 52-week, randomized, double-blind, single-dummy,
Assessment of reversibility of airway obstruction. disease. in patients with chronic obstructive airways. FEVy after salbutamol occurred in the
 19 Regional Thoracic Unit, Fazakerley Hospital, Liverpool M Nisar M Walshaw J E Earis M G Pearson P M A Calverley Address for reprint requests: Dr P M A Calverley, Fazakerley Hospital, Liverpool, L9 7AL.
19 Regional Thoracic Unit, Fazakerley Hospital, Liverpool M Nisar M Walshaw J E Earis M G Pearson P M A Calverley Address for reprint requests: Dr P M A Calverley, Fazakerley Hospital, Liverpool, L9 7AL.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 1 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION mg per tablet Active: Prednisolone 1.0 Other ingredients:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 1 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION mg per tablet Active: Prednisolone 1.0 Other ingredients:
West of Scotland Difficult Asthma Group Statement of Practice
 West of Scotland Difficult Asthma Group Statement of Practice Member Health Boards: Ayrshire and Arran Dumfries and Galloway Forth Valley Lanarkshire Glasgow ADVICE NOTE FOR INTRAMUSCULAR TRIAMCINOLONE
West of Scotland Difficult Asthma Group Statement of Practice Member Health Boards: Ayrshire and Arran Dumfries and Galloway Forth Valley Lanarkshire Glasgow ADVICE NOTE FOR INTRAMUSCULAR TRIAMCINOLONE
RESPIRATORY CARE IN GENERAL PRACTICE
 RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
Study No.: Title: Rationale: Phase: Study Period Study Design: Centres: Indication: Treatment: Objectives : Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren, MD
 Therapeutic Effect of Zafirlukast as Monotherapy in Steroid-Naive Patients With Severe Persistent Asthma* James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren,
Therapeutic Effect of Zafirlukast as Monotherapy in Steroid-Naive Patients With Severe Persistent Asthma* James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren,
BUDESONIDE AND FORMOTEROL (SYMBICORT ): Α A REVIEW
 Volume 23, Issue 3 December 2007 BUDESONIDE AND FORMOTEROL (SYMBICORT ): A REVIEW Donna L. Smith, Pharm. D. Candidate More than 22 million people in the United States have asthma according to the Centers
Volume 23, Issue 3 December 2007 BUDESONIDE AND FORMOTEROL (SYMBICORT ): A REVIEW Donna L. Smith, Pharm. D. Candidate More than 22 million people in the United States have asthma according to the Centers
Tips on managing asthma in children
 Tips on managing asthma in children Dr Ranjan Suri Consultant in Respiratory Paediatrics Bupa Cromwell Hospital Clinics: Friday (pm) Asthma in Children Making the diagnosis Patterns of childhood asthma
Tips on managing asthma in children Dr Ranjan Suri Consultant in Respiratory Paediatrics Bupa Cromwell Hospital Clinics: Friday (pm) Asthma in Children Making the diagnosis Patterns of childhood asthma
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS
 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
SYNOPSIS. Date 15 June 2004
 Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
Original Article. Kishore Kumar K 1, Sai Samrat K 2, Sony Reddy S 3 INTRODUCTION
 Original Article A Comparative Study between Intramuscular and Oral Methylprednisolone Acetate in the Treatment of Asthma Exacerbation Following Discharge from the Hospital Kishore Kumar K 1, Sai Samrat
Original Article A Comparative Study between Intramuscular and Oral Methylprednisolone Acetate in the Treatment of Asthma Exacerbation Following Discharge from the Hospital Kishore Kumar K 1, Sai Samrat
(Asthma) Diagnosis, monitoring and chronic asthma management
 Dubai Standards of Care 2018 (Asthma) Diagnosis, monitoring and chronic asthma management Preface Asthma is one of the most common problem dealt with in daily practice. In Dubai, the management of chronic
Dubai Standards of Care 2018 (Asthma) Diagnosis, monitoring and chronic asthma management Preface Asthma is one of the most common problem dealt with in daily practice. In Dubai, the management of chronic
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
UNUSUAL CAUSE OF ADRENAL INSUFFICIENCY. Dr.Khushboo Dr.S.Balasubramanian s unit
 UNUSUAL CAUSE OF ADRENAL INSUFFICIENCY Dr.Khushboo Dr.S.Balasubramanian s unit BRIEF HISTORY 7 year old male child presented with Fever : 3 days Vomiting : 3 days h/0 Acute encephalopathy with fever O/E
UNUSUAL CAUSE OF ADRENAL INSUFFICIENCY Dr.Khushboo Dr.S.Balasubramanian s unit BRIEF HISTORY 7 year old male child presented with Fever : 3 days Vomiting : 3 days h/0 Acute encephalopathy with fever O/E
Asthma. chapter 7. Overview
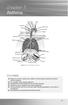 chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
Allwin Mercer Dr Andrew Zurek
 Allwin Mercer Dr Andrew Zurek 1 in 11 people are currently receiving treatment for asthma (5.4 million people in the UK) Every 10 seconds, someone is having a potentially life-threatening asthma attack
Allwin Mercer Dr Andrew Zurek 1 in 11 people are currently receiving treatment for asthma (5.4 million people in the UK) Every 10 seconds, someone is having a potentially life-threatening asthma attack
Chronic persistent asthma presenting to an accident and emergency department compliance with B.T.S. guidelines
 Archives of Emergency Medicine, 1993, 10, 347-353 Chronic persistent asthma presenting to an accident and emergency department compliance with B.T.S. guidelines J. R. THOMPSON & M. A. LAMBERT Accident
Archives of Emergency Medicine, 1993, 10, 347-353 Chronic persistent asthma presenting to an accident and emergency department compliance with B.T.S. guidelines J. R. THOMPSON & M. A. LAMBERT Accident
Sudden death in asthma
 Thorax, 1979, 34, 40-44 Sudden death in asthma J R M BATEMAN AND S W CLARKE From the Department of Thoracic Medicine, The Royal Free Hospital, London NW3 2QG, UK ABSTRACT Two deaths after sudden severe
Thorax, 1979, 34, 40-44 Sudden death in asthma J R M BATEMAN AND S W CLARKE From the Department of Thoracic Medicine, The Royal Free Hospital, London NW3 2QG, UK ABSTRACT Two deaths after sudden severe
Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet
 1 Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet 1 Name of Guideline / Policy/ Procedure MANAGEMENT OF ACUTE PAEDIATRIC ASTHMA Purpose of Procedure/
1 Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet 1 Name of Guideline / Policy/ Procedure MANAGEMENT OF ACUTE PAEDIATRIC ASTHMA Purpose of Procedure/
COPD. Breathing Made Easier
 COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
THE ACUTE AND CHRONIC BRONCHODILATOR
 Br. J. clin. Pharmac. (1975), 2, 533-537 THE ACUTE AND CHRONIC BRONCHODILATOR EFFECTS OF EPHEDRINE IN ASTHMATIC PATIENTS C.S. MAY, M.E. PICKUP & J.W. PATERSON Asthma Research Council Clinical Pharmacology
Br. J. clin. Pharmac. (1975), 2, 533-537 THE ACUTE AND CHRONIC BRONCHODILATOR EFFECTS OF EPHEDRINE IN ASTHMATIC PATIENTS C.S. MAY, M.E. PICKUP & J.W. PATERSON Asthma Research Council Clinical Pharmacology
II: Moderate Worsening airflow limitations Dyspnea on exertion, cough, and sputum production; patient usually seeks medical
 Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Asthma Medications: Information for Children and Families. What You Need to Know about Medicines for Asthma
 Page 1 of 8 PED-ALL-005-1992 Asthma Medications: Information for Children and Families What You Need to Know about Medicines for Asthma What Medicines Are used to Treat Asthma? There are two kinds of medicines:
Page 1 of 8 PED-ALL-005-1992 Asthma Medications: Information for Children and Families What You Need to Know about Medicines for Asthma What Medicines Are used to Treat Asthma? There are two kinds of medicines:
West of Scotland Difficult Asthma Group Statement of Practice
 West of Scotland Difficult Asthma Group Statement of Practice Member Health Boards: Ayrshire and Arran Dumfries and Galloway Forth Valley Lanarkshire Glasgow INFORMATION ON THE USE OF BRONCHIAL THERMOPLASTY
West of Scotland Difficult Asthma Group Statement of Practice Member Health Boards: Ayrshire and Arran Dumfries and Galloway Forth Valley Lanarkshire Glasgow INFORMATION ON THE USE OF BRONCHIAL THERMOPLASTY
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Recurrent wheezing illnesses 24.9% Similar to Australia Above global averages
 Prof Mike South Department of General Medicine Royal Children s Hospital Melbourne Australia www.mikesouth.org.au Asthma is very common in Australia Approx 25% children have recurrent wheezing illnesses
Prof Mike South Department of General Medicine Royal Children s Hospital Melbourne Australia www.mikesouth.org.au Asthma is very common in Australia Approx 25% children have recurrent wheezing illnesses
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence
 Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol)
 EMA/126654/2014 Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for DuoResp Spiromax, which details the measures
EMA/126654/2014 Summary of the risk management plan (RMP) for DuoResp Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for DuoResp Spiromax, which details the measures
Prof Neil Barnes. Respiratory and General Medicine London Chest Hospital and The Royal London Hospital
 Prof Neil Barnes Respiratory and General Medicine London Chest Hospital and The Royal London Hospital ASTHMA: WHEN EVERYTHING FAILS WHAT DO YOU DO? South GP CME 2013, Dunedin Saturday 17 th August 2013
Prof Neil Barnes Respiratory and General Medicine London Chest Hospital and The Royal London Hospital ASTHMA: WHEN EVERYTHING FAILS WHAT DO YOU DO? South GP CME 2013, Dunedin Saturday 17 th August 2013
Air Flow Limitation. In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation.
 Asthma Air Flow Limitation In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation. True whether reversible, asthma and exercise-induced bronchospasm,
Asthma Air Flow Limitation In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation. True whether reversible, asthma and exercise-induced bronchospasm,
3 RESPIRATORY SYSTEM
 3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
Viral-Induced Asthma:
 Viral-Induced : Sorting through the Studies Malcolm R. Sears, MB, FRACP, FRCPC Presented at the Respirology Update Continuing Education Program, January 2005 Viral-associated wheezing is common and not
Viral-Induced : Sorting through the Studies Malcolm R. Sears, MB, FRACP, FRCPC Presented at the Respirology Update Continuing Education Program, January 2005 Viral-associated wheezing is common and not
Sample. Affix patient label within this box.
 Instructions for completing orders Complete pages 1-3 for General Inpatient Orders. All pathway compatible orders (indicated by ) within the General Inpatient Orders will be followed automatically. Optional
Instructions for completing orders Complete pages 1-3 for General Inpatient Orders. All pathway compatible orders (indicated by ) within the General Inpatient Orders will be followed automatically. Optional
Omalizumab (Xolair ) ( Genentech, Inc., Novartis Pharmaceuticals Corp.) September Indication
 ( Genentech, Inc., Novartis Pharmaceuticals Corp.) September 2003 Indication The FDA recently approved Omalizumab on June 20, 2003 for adults and adolescents (12 years of age and above) with moderate to
( Genentech, Inc., Novartis Pharmaceuticals Corp.) September 2003 Indication The FDA recently approved Omalizumab on June 20, 2003 for adults and adolescents (12 years of age and above) with moderate to
Supplementary Medications during asthma attack. Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus
 Supplementary Medications during asthma attack Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus Conflicts of Interest None Definition of Asthma Airway narrowing that is
Supplementary Medications during asthma attack Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus Conflicts of Interest None Definition of Asthma Airway narrowing that is
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
Adult Summary flowchart for Asthma Switch and Step Down to preferred inhaler choices
 HVCCG Adult Asthma Switch and Step Down Algorithms - Approved by Hertfordshire Medicines Management Committee June 2016 Page 1 of 6 Adult Summary flowchart for Asthma Switch and Step Down to preferred
HVCCG Adult Asthma Switch and Step Down Algorithms - Approved by Hertfordshire Medicines Management Committee June 2016 Page 1 of 6 Adult Summary flowchart for Asthma Switch and Step Down to preferred
the use of inhaled corticosteroids for at least 3 months preceding the study;
 The cost effectiveness of chlorofluorocarbon-free beclomethasone dipropionate in the treatment of chronic asthma: a cost model based on a 1-year pragmatic, randomised clinical study Price D, Haughney J,
The cost effectiveness of chlorofluorocarbon-free beclomethasone dipropionate in the treatment of chronic asthma: a cost model based on a 1-year pragmatic, randomised clinical study Price D, Haughney J,
Aerospan (flunisolide)
 STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
Asthma in Pregnancy. Asthma. Chronic Airway Inflammation. Objective Measures of Airflow. Peak exp. flow rate (PEFR)
 Chronic Airway Inflammation Asthma in Pregnancy Robin Field, MD Maternal Fetal Medicine Kaiser Permanente San Francisco Asthma Chronic airway inflammation increased airway responsiveness to a variety of
Chronic Airway Inflammation Asthma in Pregnancy Robin Field, MD Maternal Fetal Medicine Kaiser Permanente San Francisco Asthma Chronic airway inflammation increased airway responsiveness to a variety of
Getting Asthma treatment right. Dr David Cremonesini Specialist Pediatrician American Hospital
 Getting Asthma treatment right Dr David Cremonesini Specialist Pediatrician American Hospital cdavid@ahdubai.com } Consultant Paediatrician from UK of 5.5 years } Speciality in Allergy / Asthma (PG Certificate)
Getting Asthma treatment right Dr David Cremonesini Specialist Pediatrician American Hospital cdavid@ahdubai.com } Consultant Paediatrician from UK of 5.5 years } Speciality in Allergy / Asthma (PG Certificate)
Antifungal treatment of severe asthma
 Antifungal treatment of severe asthma David W. Denning University Hospital of South Manchester [Wythenshawe Hospital] The University of Manchester Severe asthma Bel EH, Severe asthma. Breath magazine Dec
Antifungal treatment of severe asthma David W. Denning University Hospital of South Manchester [Wythenshawe Hospital] The University of Manchester Severe asthma Bel EH, Severe asthma. Breath magazine Dec
Asthma training. Mike Levin Division of Asthma and Allergy Red Cross Hospital
 Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Monocast Description Indications
 Monocast Tablet Description The active ingredient of Monocast tablet is Montelukast Sodium INN. Montelukast is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl
Monocast Tablet Description The active ingredient of Monocast tablet is Montelukast Sodium INN. Montelukast is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl
Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide.
 Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide. By: DR MOHD SHAMSUL AMRI Supervisor: Associate Professor Dr
Step-down approach in chronic stable asthma: A comparison of reducing dose Inhaled Formoterol/ Budesonide with maintaining Inhaled Budesonide. By: DR MOHD SHAMSUL AMRI Supervisor: Associate Professor Dr
Bronchodilator and Cardiac Effects of Isoprenaline, Orciprenaline, and Salbutamol Aerosols in Asthma
 Archives of Disease in Childhood, 1971, 46, 52. Bronchodilator and Cardiac Effects of Isoprenaline, Orciprenaline, and Salbutamol Aerosols in Asthma A. D. MILNER and D. INGRAM From The Hospital for Sick
Archives of Disease in Childhood, 1971, 46, 52. Bronchodilator and Cardiac Effects of Isoprenaline, Orciprenaline, and Salbutamol Aerosols in Asthma A. D. MILNER and D. INGRAM From The Hospital for Sick
measured Improvement in am PEFR FP vs BUD gave +11l/min FP vs BDP gave nonsignificant improvement in PEFR +3l/min FP: BUD ratio 1.
 1 of 8 09/05/2018, 11:29 Guideline topic: Pharmacological management of asthma Evidence table 4.25: Budesonide vs Beclomethasone Different inhaled corticosteroids (ICS) flixotide propionate (FP) vs budesonide
1 of 8 09/05/2018, 11:29 Guideline topic: Pharmacological management of asthma Evidence table 4.25: Budesonide vs Beclomethasone Different inhaled corticosteroids (ICS) flixotide propionate (FP) vs budesonide
Q. What are metered-dose inhalers? A. These are devices that dispense medicines directly into the lungs, in the form of a mist or aerosol in a
 1 2 Q. What are metered-dose inhalers? A. These are devices that dispense medicines directly into the lungs, in the form of a mist or aerosol in a specific dosage. In an MDI, the medicine is suspended
1 2 Q. What are metered-dose inhalers? A. These are devices that dispense medicines directly into the lungs, in the form of a mist or aerosol in a specific dosage. In an MDI, the medicine is suspended
ASTRAZENECA v GLAXOSMITHKLINE
 CASE AUTH/1833/5/06 ASTRAZENECA v GLAXOSMITHKLINE CONCEPT study leavepiece AstraZeneca complained that a leavepiece issued by Allen & Hanburys, part of GlaxoSmithKline, did not present a fair and balanced
CASE AUTH/1833/5/06 ASTRAZENECA v GLAXOSMITHKLINE CONCEPT study leavepiece AstraZeneca complained that a leavepiece issued by Allen & Hanburys, part of GlaxoSmithKline, did not present a fair and balanced
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Predicting, Preventing and Managing Asthma Exacerbations. Heather Zar Department of Paediatrics & Child Health University of Cape Town South Africa
 Predicting, Preventing and Managing Asthma Exacerbations Heather Zar Department of Paediatrics & Child Health University of Cape Town South Africa Asthma exacerbations Predicting exacerbation recognising
Predicting, Preventing and Managing Asthma Exacerbations Heather Zar Department of Paediatrics & Child Health University of Cape Town South Africa Asthma exacerbations Predicting exacerbation recognising
Adult Summary flowchart for Asthma Switch and Step Down to ENHCCG preferred inhaler choices
 ENHCCG Adult Asthma Switch and Step Down Algorithms - Approved by Hertfordshire Medicines Management Committee June 2016 Page 1 of 6 Adult Summary flowchart for Asthma Switch and Step Down to ENHCCG preferred
ENHCCG Adult Asthma Switch and Step Down Algorithms - Approved by Hertfordshire Medicines Management Committee June 2016 Page 1 of 6 Adult Summary flowchart for Asthma Switch and Step Down to ENHCCG preferred
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
I. Subject: Continuous Aerosolization of Bronchodilators
 I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
TREAMENT OF RECURRENT VIRUS-INDUCED WHEEZING IN YOUNG CHILDREN. Dr Lại Lê Hưng Respiratory Department
 TREAMENT OF RECURRENT VIRUS-INDUCED WHEEZING IN YOUNG CHILDREN Dr Lại Lê Hưng Respiratory Department Literature review current through: Feb 2013. This topic last updated: Aug 14, 2012 INTRODUCTION Wheezing
TREAMENT OF RECURRENT VIRUS-INDUCED WHEEZING IN YOUNG CHILDREN Dr Lại Lê Hưng Respiratory Department Literature review current through: Feb 2013. This topic last updated: Aug 14, 2012 INTRODUCTION Wheezing
