Clinical equivalence of a novel nonchlorofluorocarbon-containing
|
|
|
- Lionel Paul
- 6 years ago
- Views:
Transcription
1 Clinical equivalence of a novel nonchlorofluorocarbon-containing salbutamol sulfate metered-dose inhaler and a conventional chlorofluorocarbon inhaler in patients with asthma Robert Dockhorn, MD," Jennifer A. Vanden Burgt, BS, b Bruce P. Ekholm, MS, b David Donnell, MRPharmS, ~ and Michael T. Cullen, MD b Lenexa, Kan., St. Paul, Minn., and Loughborough, England Background: New formulations of non-chlorofluorocarbon-containing propellants for pressurized metered-dose inhaler delivery systems must be developed in response to the forthcoming ban on chlorofluorocarbon (CFC) production. Objective: This study compared the bronchodilator effects of 100, 200, and 300 lag (base equivalent) of salbutamol in a novel CFC-free propellant system (Airomir in the 3M CFC-Free System; 3M Pharmaceuticals, St. Paul, Minn.; 108 IN of salbutamol sulfate or 90 IN of salbutamol base equivalent per inhalation) with that of 100 and 200 ixg of salbutamol base in a conventional CFC propellant system (Ventolin, CFC-11/12; Allen and Hanburys, Division of Glaxo Inc., Research Triangle Park, N.C.; 90 tn of salbutamol base per inhalation) and placebo. Methods: Twenty-six patients with chronic, stable asthma, who had a forced expiratory volume in 1 second (FEV1) between 50.0% and 75.0% of predicted normal value, entered this randomized, double-blind, double-dummy, 6-period, crossover study. FEV 1 was measured before and at multiple time points (ranging from 10 to 480 minutes) after administration of one, two, and three inhalations of salbutamol/cfc-free (100, 200, and 300 IN); one and two inhalations of salbutamol/cfc (100 and 200 IN); and placebo. Safety parameters included adverse events, heart rate, blood pressure, physical examinations, electrocardiograms, and clinical laboratory tests. Parametric analysis of variance models appropriate for a 6-period crossover design were used, along with multiple comparisons according to Tukey's method. Results: All active treatments produced significantly (p < O. 0001) greater bronchodilation than placebo. The bronchodilator effect, as measured by FEV z (peak percent change, peak as a percent of predicted value, duration, and area under the curve) after two inhalations of salbutumol/cfc-free was clinically comparable to two inhalations of salbutamol/cfc, with no clinically meaningful differences in safety parameters between the two delivery systems or between different dose levels. Conclusion: These results suggest that salbutamol/cfc-free may offer a suitable alternative for salbutamol/cfc when the need arises to change from CFC-containing salbutamol products. (J ALLERGY CLIN IMMUNOL 1995;96.'50-6.) Key words: Salbutamol, asthma, hydrofluoroalkane, HFA-134a, bronchodilator Chlorofluorocarbons (CFCs) are an essential component of current pressurized metered-dose inhalers (MDIs), which deliver drugs for the treat- From ainternational Medical Technical Consultants, Inc., Lenexa; b3m Pharmaceuticals, St. Paul; and C3M Health Care, Loughborough. Received for publication July 27, 1994; revised Jan. 10, 1995; accepted for publication Jan. 12, Supported by 3M Pharmaceuticals. ment of reversible obstructive airway disease (asthma). During the 1970s, scientists suggested that the growing nonmedicinal use of CFCs was reducing the concentration of the stratospheric ozone layer Reprint requests: J. A. Vanden Burgt, 3M Pharmaceuticals, 3M Center, 270-3A-01, St. Paul, MN Copyright 1995 by Mosby-Year Book, Inc /95 $ /1/
2 J ALLERGY CLIN IMMUNOL Dockhorn et al.!51 VOLUME 96, NUMBER 1 Abbreviations used ANOVA: Analysis of variance CFC: Chlorofluorocarbon FEVI: Forced expiratory volume in 1 second HFA: Hydrofluoroalkane MDI: Metered-dose inhaler with potentially harmful results. Under the auspices of the United Nations Environmental Program, a committee designated to study the effects of CFCs on the ozone layer organized the preparation of a CFC protocol. Ratified by over 100 nations, the Montreal Protocol will lead to the reduction in and eventual cessation of CFC production by January 1, Although other delivery systems (e.g., nebulizers and dry powder inhalers) do not use CFCs, MDIs are widely used and accepted and are the preferred method of treatment for many patients. The reformulation of MDI propellants from CFC-containing to CFC-free systems could have an impact on an estimated 250 million patients worldwide. One aim of reformulation would be to ensure continued availability of MDIs as a method of treatment while using a safe and effective system that addresses environmental concerns. A hydrofluoroalkane (HFA), designated propellant 134a (CF3CH2F), is considered to be one of the two present alternatives to CFC propellants. Like the CFCs, HFA-134a is chemically inert. 1,2 Unlike the CFCs, HFA-134a (tetrafluoroethane) has no chlorine and therefore no ozone-depleting potential. The preclinical toxicologic testing program of HFA-134a has been reviewed by the Committee for Proprietary Medicinal Products (CPMP, the European Regulatory Agency). The Committee stated that there were no significant pharmacologic, toxicologic, or safety issues found during their reviews. 3 For further information about HFA-134a, the reader is referred to Leach (1995) and Boulden (1994). 1, 2 In this study salbutamol sulfate (albuterol), a selective [32-adrenergic agonist currently available for inhalation asthma therapy, has been reformulated with the new HFA-134a propellant system. Because this formulation change incorporates HFA-134a, which is not yet licensed as an excipient for use in medicinal products, clinical trials to provide safety and efficacy data are required by relevant regulatory agencies worldwide. The objective of this study was to determine the dose of salbutamol sulfate in an HFA-134a propellant system (Alromir in the 3M CFC-Free System, 3M Pharmaceuticals, St. Paul, Minn.), referred to as salbutamol/cfc-free 108 txg of salbutamol sulfate or 90 p~g of salbutamol base equivalent per inhalation that produced clinical efficacy comparable with two inhalations of salbutamol base in a conventional CFC-11/12 propellant system (Ventolin, CFC-11/12, Allen and Hanburys, Division of Glaxo, Inc., Research Triangle Park, N.C.) referred to as salbutamol/cfc 90 txg of salbutamol base per inhalation, in patients with asthma. A placebo treatment was included as a reference. The primary objective measure was efficacy as assessed by forced expiratory volume in 1 second (FEV1). The safety parameters of the study were sitting heart rate and blood pressure, electrocardiograms, adverse events, physical examinations, and clinical laboratory tests. METHODS Study design This study was designed as a randomized, single-dose, double-blind, double-dummy, 6-period crossover trial of salbutamol/cfc-free, salbutamol/cfc, and placebo (a combination treatment of CFC and CFC/free systems) in 26 nonsmoking patients with stable bronchial asthma. A sample size of at least 24 subjects was necessary to provide at least 80% power to detect at alpha = 0.05 the differences between each of the active treatments and placebo. Patients had to demonstrate prestudy screening baseline FEV 1 values between 50.0% and 75.0% of the predicted normal value after washout of bronchodilator drugs and an increase in FEV1 of at least 20% within 30 minutes after an inhalation of 200 p.g of salbutamol/ CFC. Patients were randomized to groups of six so that a total of four patients was randomly assigned to each of the six possible sequences. Patients received a total of four inhalations at each of the six dosing periods with a 1-day washout between each period. To blind the study treatment and the dose level, patients received a companion placebo with each dose of study medication; they received a corresponding number of inhalations of the placebo to equal a four-inhalation dose (Table I). Clinical methods Before study initiation, the protocol was reviewed and approved by an institutional review board. Each patient voluntarily signed a written informed consent form. The study was conducted according to good clinical practice standards. Patients entered the clinical unit on the evening before study period 1 and remained there for the duration of the study. Use of asthma medications, with the exception of inhaled [3-agonists, was prohibited during the study. Inhaled [3-agonists were withheld for 8 hours before pulmonary function tests were performed
3 52 Dockhorn et al. J ALLERGY CLIN IMMUNOL JULY 1995 TABLE I. Treatment combinations 1 puff Salbutamol/CFC-free + 2 puffs Salbutamol/CFC-free + 3 puffs Salbutamol/CFC-free + 1 puff Salbutamol/CFC + 2 puffs Salbutamol/CFC + 3 puffs CFC-free placebo + at screening and each of the six study periods. Allergy medications were permitted. Patients continued to take their prescribed inhaled [3-agonists for the duration of the study, except for an 8-hour washout period before and during the testing period in each of the six study periods. None of the patients received inhaled steroids or cromolyn during the trial. Caffeine-containing beverages were withheld. Within 30 minutes before dosing in each of the six study periods, the baseline FEV1 was required to be within _+15.0% of the screening FEV1 and could not exceed 85% of predicted normal FEV 1. Patients who did not meet these criteria were allowed additional attempts, provided that they were able to complete the study within 16 days. Baseline measurements of blood pressure, heart rate, and electrocardiogram were taken, and a physical examination and laboratory tests were completed. Doses were administered between 7:00 and 9:00 AM and within 1 hour of the time of dosing in study period 1 for each of the consecutive study periods. Patients dosed themselves under the supervision of a study nurse. Patients inhaled a total of 4 puffs from two inhalers according to the randomization sequence. All inhalations per dose level were administered approximately 45 seconds apart (from the start of one inhalation to the start of the next). Time 0 was the time when the MDI was first actuated (i.e., the time of the first puff of the dose). Pulmonary function tests were performed at 10, 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, and 480 minutes from time 0 of the dose administration. All pulmonary function tests were performed at least three times in a standing position, in compliance with the American Thoracic Society statement on standardization of spirometry, 4 with a Puritan Bennett Processing (PS600) spirometer (Puritan-Bennett Corp., Overland Park, Kan.), which recorded and printed time volume curves. Patients were instructed to produce a maximal forced expiration over at least 6 seconds. Triplicate measurements of FEV1 were made with a 1-minute interval between each determination. The values recorded for FEV1 were from the best individual test. If TABLE II. Prestudy screening data for 26 3 puffs CFC patients entering the study placebo Response Value 2 puffs CFC placebo Predicted FEV 1 (L) 1 puff CFC Mean (SD) 4.07 (0.59) placebo Range puffs CFC-free Screening FEV 1 (L) placebo Mean (SD) 2.64 (0.44) 2 puffs CFC-free Range placebo Screening FEV 1 as a percent 1 puff CFC of predicted value placebo Mean (SD) 65.2 (6.9) Range Postreversibility FEV 1 (L) Mean (SD) 3.47 (0.57) Range Percent FEV 1 reversibility Mean (SD) 32.0 (13.3) Range these tests did not meet acceptability criteria, more tests were performed until acceptability criteria were met. Pulmonary function testing was stopped and rescue medication was given if a patient had a fall in FEV1 of 15% or greater from baseline measured in that study period and an accompanying increase in symptoms, a fall in FEV1 below 40.0% of predicted FEVt, or at the investigator's discretion if the patient was in distress. Statistical methods For the FEV 1 data, the following parameters were calculated for each patient and treatment. 1. Responders. A patient was considered a responder at a visit if his or her FEVz increased at least 15.0% over baseline within 30 minutes after study treatment administration. 2. Peak response as a percentage increase over baseline and as a percentage of the predicted value. Also, the peak change as a percentage of the maximum possible change was determined: Peak FEV1 - Predicted FEV1 - Baseline FEV1 Baseline FEV1 100% 3. Time of onset of effect. The onset of effect was determined by linear interpolation of the time that FEV 1 first exceeded 15.0% over baseline within 30 minutes after study treatment administration. 4. Duration of effect. The therapeutic response was considered to have ended when the FEV 1 fell below 15.0% over baseline for two consecutive measurements. The time of termination of effect was estimated by linear interpolation between the last measurement greater than 15.0% over baseline and the first of the two consecutive measurements less than
4 J ALLERGY CL]N IMMUNOL Dockhorn et al. 53 VOLUME 96, NUMBER 1 TABLE III. Results for responses based on FEV1 for 25 patients in each treatment group compared with placebo Response Placebo Salbutamol/CFC-free Salbutamol/CFC 1 Puff 2 Puffs 3 Puffs 1 Puff 2 Puffs p Value* AUC (% hr) Mean t 125.0t 98.1t 115.6t SD Peak % change Mean t 31.1t 31.6# 29.1t 31.2t SD Peak change (L) Mean t 0.81t 0.85t 0.77t 0.80t SD Peak as % of predicted value Mean t 89.4t 89.6t 88.0t 88.2t SD Peak change as % of max change Mean t 67.2t 70.4t 63.0t 65.3t SD Duration of effect (hr) Mean t 3.8t 4.0t 3.2t 3.6t SD responders No. 5 16t 17I" 20t 17t 17t % 20% 64% 68% 80% 68% 68% Time of onset of effect (min)$ Number Mean 11 6t 5# 6t 6t 5"~ SD Results are based on data from the 25 patients who completed all six periods. AUC, Area under the curve. *The p value tests the effect of treatment from an ANOVA for a 6-period crossover. tthe mean was significantly different from that of placebo as determined by ANOVA with Tukey's method of multiple comparisons. :~The time of onset was only defined for patients who responded to the assigned treatment. 15.0% over baseline. The duration of effect was defined as time of termination of effect minus time of onset of effect. For patients who did not respond at a visit, the duration of effect was defined to be zero for that visit. 5. Area under the curve. The area-under-the-curve measurements were calculated with the percentage change from baseline FEV 1 data, from onset of effect to termination of effect. Responses based on FEV1 were tested by an analysis of variance (ANOVA) with sequence, patient within sequence, treatment group, and period as factors in the model. The test of sequence effect was determined with the patient within sequence mean square as the error term in the denominator. Ap value of less than 0.05 was considered significant. If the treatment effect was considered significant, multiple comparisons were done with Tukey's method. The statistical software used to analyze the data was PROC GLM from SAS Version 6.07 (SAS Institute, Cary, N.C.). 5 RESULTS Study population Twenty-six patients (20 men and 6 women) with asthma history of at least 1 year participated in at least one treatment period. Of these, 25 completed all six treatment periods of the study. One patient was withdrawn from the study after one treatment period because of inability to meet the baseline variability criterion. Mean age was 28 _+ 9 years (range, 18 to 50 years). Mean height was 178 +_ 4 cm, and mean weight was kg. Eighteen patients (69%) also had allergies. FEV 1 Table II lists mean prestudy spirometry screening data. Before dosing in each of the study periods, the mean baseline FEV 1 was 2.80 L or 68.7% of predicted value. There were no significant differences in FEV~ before subjects entered
5 54 Dockhorn et al. J ALLERGY CLIN IMMUNOL JULY ~_ 80 "6 Ii Placebo 1 inhalation salbutamol/cfc-free 2 inhalations salbutamol/cfc-free 3 inhalations salbutamol/cfc-free 1 inhalation salbutamol/cfc 2 inhalations salbutamol(cfc normal FEV1 (with the normal prediction equation of Morris et al.). 6 Mean FEV1 values as a percentage of predicted FEV1 over time are presented in Fig. 1. The effects of all five active treatments were significantly greater than the effect of placebo throughout the first 90 minutes. For two and three inhalations of salbutamol/cfc-free, the results were significant throughout the first 240 minutes. Results of one and two inhalations of salbutamol/cfc were significant throughout the first 120 and 180 minutes, respectively. For this comparison, two inhalations of salbutamol/cfc-free most closely matched two inhalations of salbutamol/cfc ~ 0 6*0 1:;0 1;0 2z~0 3;0 3; ;0 Time (Minutes) FIG. 1. Mean FEV1 as a percentage of predicted value over time in 25 patients. the first period. As determined by ANOVA, there were no significant differences in baseline FEV1 values between the six treatment groups. A comparison of the mean FEV1 data for each of the six treatment groups indicated that the effect of two inhalations of salbutamol/cfc-free most closely matched the effect of two inhalations of salbutamol/cfc (Table III). There were no significant differences between any of the active formulations for any of the FEV~ responses. On the basis of peak percentage change, peak change, peak change as percentage of predicted value, peak change as percentage of maximum possible change, duration of effect, and time of onset, the results for all five active treatment groups (1, 2, and 3 puffs of salbutamol/cfc-free and 1 and 2 puffs of salbutamol/cfc) were significantly greater than that of placebo. For area under the curve, the results of all active treatment groups, with the exception of one inhalation of salbutamol sulfate, were significantly greater than placebo. Although a dose-response trend was seen for both salbutamol/cfc-free and salbutamol/cfc, there were no statistically significant differences between any of the five active treatments for any of the responses. The data were also analyzed after each patient's change in FEV1 was converted into a percentage change in FEV 1 relative to the patient's predicted Rescue bronchodilator use Pulmonary function testing was discontinued before completion of the 8-hour testing period for four patients on eight separate occasions (Table IV). No patient experienced any acute respiratory problems requiring rescue treatment within 60 minutes after administration of any of the six treatments, and no patient receiving salbutamol/ CFC-free required rescue treatment sooner than 300 minutes after dosing. All patients returned to baseline pulmonary function after receiving standard bronchodilator treatment. Safety Before dosing on each of the study days, the mean baseline pulse was 76 beats/min, and the mean baseline blood pressure was 115/70 mm Hg. As determined by ANOVA, there were no significant differences in baseline vital signs among the six treatment groups. For change from baseline pulse, according to ANOVA, the only significant treatment effect occurred at 300 minutes (p = 0.024). Because 13 tests of significance were done with these data, this was believed to be due to chance alone. There were no significant sequence or period effects. For change from baseline systolic and diastolic blood pressure, according to ANOVA, there were no significant differences among the six treatment groups, and there were no significant sequence or period effects. Comparison of prestudy and poststudy 12-lead electrocardiograms, results of physical examinations, and laboratory values revealed no clinically significant findings. A total of 19 adverse events were reported by 12 patients during the course of the study. All of these were mild or moderate in intensity, none of them resulted in patient withdrawal, and all were resolved by the end of the study. The most commonly reported adverse event was headache. A full listing
6 J ALLERGY CLIN IMMUNOL Dockhorn et al. 55 VOLUME 96, NUMBER 1 TABLE IV. Rescue bronchodilator administration Treatment Patient Dose Last PFT group No. (puffs) time (min) Reason Placebo FEV 1 dropped below 40% of predicted value FEV1 dropped ->15% from baseline/asthma symptoms Investigator's discretion Salbutamol/CFC FEV 1 dropped ->15% from baseline/asthma symptoms FEV~ dropped ->15% from baseline/asthma symptoms FEV~ dropped below 40% of predicted Salbutamol/CFC-free FEV 1 dropped below 40% of predicted Investigator's discretion TABLE V. Adverse events by treatment group Salbutamol/CFC-free Salbutamol/CFC Adverse Placebo 1 puff 2 puffs 3 puffs 1 puff 2 puffs event (n = 25) (n = 25} (n = 25) (n = 26) (n = 25) (n = 25) Headache " 1" 3" Rhinitis? Chest pain Dyspepsia 1~: 1 1:~ Dizziness 1 1 Nausea 1 Coughing 1 Abdominal pain 1 No. of subjects reporting adverse events *Subject 24 experienced a headache, which remained for three study periods. 1"Subject 6 experienced rhinitis, which was present before the study and remained unchanged throughout all periods. :~Subject 10 experienced dyspepsia, which was present for two study periods. of the number of subjects reporting an adverse event by treatment group is presented in Table V. It should be noted that because of the crossover design of the study, the total tally of adverse events does not match the data in Table V. This is because subjects 6, 10, and 24 each had one event that lasted for more than one treatment period. DISCUSSION This study determined that the reformulation of salbutamol in this CFC-free propellant system produced comparable bronchodilator effects at a dose equivalent to the leading currently marketed CFC-containing salbutamol product. The mean responses, based on FEV 1 data for all six treatment groups, indicated that the effect of two inhalations of salbutamol/cfc-free most closely matched the effect of two inhalations of salbutamol/cfc. This study examined FEVa response over time, up to 8 hours after dosing, and demonstrated comparable efficacy of two inhalations of the two products. All five treatments were significantly more effective than placebo, and although not statistically significant, a dose-response trend was evident for both the CFC-free and CFCcontaining products. These patients were taking only inhaled ~-agonists for treatment of their symptoms; therefore, no concurrent asthma therapy confounded the results. This is the first reported study of this salbutamol/cfc-free formulation in patients with asthma. Two previous studies (manuscripts in preparation) of healthy volunteer subjects demonstrated no significant differences in tolerability or safety between the CFC-free and CFC-containing propellant systems. In one study, patients were administered cumulative increasing doses of up to 16 inhalations of the CFC-free formulation compared with the CFC-containing system. In the second study, a long-term safety evaluation, subjects received 16 inhalations per day for 2 weeks, followed by 32 inhalations per day for 2 weeks.
7 56 Dockhorn et al. J ALLERGY CLIN IMMUNOL JULY 1995 The safety data presented here (i.e., adverse events, vital signs, electrocardiograms, clinical laboratory tests, and physical examinations) indicate that there were no clinically meaningful differences between salbutamol/cfc-free and salbutamol/ CFC and also no differences between the studied dose levels of each of the two products. On the basis of the safety data collected, the active and placebo treatments were well tolerated, and there were no safety concerns with the administration of the six study treatments. Further, there was no evidence of bronchoconstrictive effects immediately after placebo administration. In this study the new product has been assessed for its ability to produce bronchodilation equivalent to the presently marketed product. As pointed out by Wong and Hargreave, 7 [32-adrenergic agonists also manifest a bronchoprotective effect against nonsensitizing agents, such as methacholine or histamine, and against exercise. They suggest clinical equivalence with both responses should be sought until evidence supports the use of one or the other response or until the responses are equivalent. Comparative challenge studies evaluating the bronchoprotective effect of salbutamol/cfc-free are in progress. The results to date suggest that salbutamol/ CFC-free will offer a suitable alternative for salbutamol/cfc when the need arises to change from CFC-containing salbutamol products. We thank Altha Roberts Edgren for assistance in preparing the manuscript. REFERENCES 1. Leach CL. Approaches and challenges to use freon propellant replacements. Aerosol Sci Technol 1995 (in press). 2. Boulden ME. HFA 134a: the latest in propellant alternatives. Spray Technol Market 1994;4: CPMP on Possible Alternative to CFCs. Scrip 1994;1943: American Thoracic Society. Standardization of spirometry update. Am Rev Respir Dis 1987;136: SAS Institute Inc. SAS/STAT User's guide, version 6.4th ed. Vol. 1. Cary, North Carolina: SAS Institute Inc., Morris JF, Koski A, Johnson LC. Spirometric standards for healthy non-smoking adults. Am Rev Resp Dis 1971;103: Wong BJO, Hargreave FE. Bioequivalence of metered-dose inhaled medications. J ALLERGY CLIN IMMUNOL 1993;92:
Budesonide treatment of moderate and severe asthma in children: A doseresponse
 Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
Budesonide treatment of moderate and severe asthma in children: A doseresponse study Soren Pedersen, MD, PhD, and Ove Ramsgaard Hansen, MD Kolding, Denmark Objective: The purpose of the study was to evaluate
Cumulative Dose-Response Study of Non-CFC Propellant HFA 134a Salbutamol Sulfate Metered-Dose Inhaler in Patients With Asthma*
 Cumulative Dose-Response Study of Non-CFC Propellant HFA 134a Salbutamol Sulfate Metered-Dose Inhaler in Patients With Asthma* Eric C. Kleerup, MD; Donald P. Tashkin, MD; Ann C. Cline; and Bruce P. Ekholm,
Cumulative Dose-Response Study of Non-CFC Propellant HFA 134a Salbutamol Sulfate Metered-Dose Inhaler in Patients With Asthma* Eric C. Kleerup, MD; Donald P. Tashkin, MD; Ann C. Cline; and Bruce P. Ekholm,
Individual Study Table Referring to Part of the Dossier. Volume:
 Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Final Report M/100977/21Final Version () 2. SYNOPSIS A Title of Study: A PHASE IIa, RANDOMISED, DOUBLE-BLIND, MULTIPLE DOSE, PLACEBO CONTROLLED, 3 PERIOD CROSS-OVER, ASCENDING DOSE CLINICAL TRIAL TO ASSESS
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence
 Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
METERED DOSE INHALERS AND CFCs
 MD I?! Questions & Answers about... METERED DOSE INHALERS AND CFCs Environment Research centre Ministry of Environment, Energy and Water Written by: Fathimath Reema (ERC) Layout & Design: Aminath Nileysha
MD I?! Questions & Answers about... METERED DOSE INHALERS AND CFCs Environment Research centre Ministry of Environment, Energy and Water Written by: Fathimath Reema (ERC) Layout & Design: Aminath Nileysha
CFCs in inhalers for asthma and COPD
 CFCs in inhalers for asthma and COPD What s happening? 2009 United Nations Environment Programme Developed in association with the National Asthma Council Australia Supported by the Multilateral Fund for
CFCs in inhalers for asthma and COPD What s happening? 2009 United Nations Environment Programme Developed in association with the National Asthma Council Australia Supported by the Multilateral Fund for
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
DATE: 09 December 2009 CONTEXT AND POLICY ISSUES:
 TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
THE NEW ZEALAND MEDICAL JOURNAL
 THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1267 ISSN 1175 8716 Is Salamol less effective than Ventolin? A randomised, blinded, crossover study in New Zealand Catherina L Chang, Manisha Cooray, Graham Mills,
THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1267 ISSN 1175 8716 Is Salamol less effective than Ventolin? A randomised, blinded, crossover study in New Zealand Catherina L Chang, Manisha Cooray, Graham Mills,
SUMMARY THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 i SUMMARY ZENECA PHARMACEUTICALS FINISHED PRODUCT: ACTIVE INGREDIENT: ACCOLATE zafirlukast (ZD9188) Trial title (number): A Dose-ranging, Safety and Efficacy Trial with Zafirlukast (ACCOLATE ) in the Treatment
i SUMMARY ZENECA PHARMACEUTICALS FINISHED PRODUCT: ACTIVE INGREDIENT: ACCOLATE zafirlukast (ZD9188) Trial title (number): A Dose-ranging, Safety and Efficacy Trial with Zafirlukast (ACCOLATE ) in the Treatment
Effect of Study Design on Sample Size in Studies Intended to Evaluate Bioequivalence of Inhaled Short-Acting β-agonist Formulations
 Drug Development Effect of Study Design on Sample Size in Studies Intended to Evaluate Bioequivalence of Inhaled Short-Acting β-agonist Formulations The Journal of Clinical Pharmacology 28, 58(4) 457 465
Drug Development Effect of Study Design on Sample Size in Studies Intended to Evaluate Bioequivalence of Inhaled Short-Acting β-agonist Formulations The Journal of Clinical Pharmacology 28, 58(4) 457 465
Advance in inhaler technique: changes in delivery devices, Authorized Generics, and Advance in technology for monitoring inhaler adherence
 Advance in inhaler technique: changes in delivery devices, Authorized Generics, and Advance in technology for monitoring inhaler adherence Bruce Brown, MS, RRT, AE-C Nemours Healthcare System Disclosures
Advance in inhaler technique: changes in delivery devices, Authorized Generics, and Advance in technology for monitoring inhaler adherence Bruce Brown, MS, RRT, AE-C Nemours Healthcare System Disclosures
Correspondence: C-G. Löfdahl Dept of Respiratory Medicine and Allergology University Hospital S Lund Sweden.
 Eur Respir J 1997; 10: 2474 2478 DOI: 10.1183/09031936.97.10112474 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Differences in bronchodilating
Eur Respir J 1997; 10: 2474 2478 DOI: 10.1183/09031936.97.10112474 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Differences in bronchodilating
Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults
 CLENIL MODULITE Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults Corticosteroids for the treatment of chronic asthma in adults and children aged 12
CLENIL MODULITE Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults Corticosteroids for the treatment of chronic asthma in adults and children aged 12
Current Challenges and Opportunities in Demonstrating Bioequivalence
 Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
SYNOPSIS. First subject enrolled 15 August 2003 Therapeutic confirmatory (III) Last subject completed 03 February 2005
 Drug product: SYMBICORT pmdi 160/4.5 μg Drug substance(s): Budesonide/formoterol Study code: SD-039-0728 Edition No.: FINAL Date: 27 February 2006 SYNOPSIS A 52-week, randomized, double-blind, single-dummy,
Drug product: SYMBICORT pmdi 160/4.5 μg Drug substance(s): Budesonide/formoterol Study code: SD-039-0728 Edition No.: FINAL Date: 27 February 2006 SYNOPSIS A 52-week, randomized, double-blind, single-dummy,
Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products
 Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products Extended Breakout Session 2: Biomarker Strategies Richard C. Ahrens, M.D. Partha Roy, Ph.D. Dale P. Conner, Pharm.D. Regulatory
Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products Extended Breakout Session 2: Biomarker Strategies Richard C. Ahrens, M.D. Partha Roy, Ph.D. Dale P. Conner, Pharm.D. Regulatory
Performing a Methacholine Challenge Test
 powder for solution, for inhalation Performing a Methacholine Challenge Test Provocholine is a registered trademark of Methapharm Inc. Copyright Methapharm Inc. 2016. All rights reserved. Healthcare professionals
powder for solution, for inhalation Performing a Methacholine Challenge Test Provocholine is a registered trademark of Methapharm Inc. Copyright Methapharm Inc. 2016. All rights reserved. Healthcare professionals
Salmeterol, a new long acting inhaled,f2 adrenoceptor agonist: comparison with salbutamol in adult asthmatic patients
 Thorax 1988;43:674-678 Salmeterol, a new long acting inhaled,f2 adrenoceptor agonist: comparison with salbutamol in adult asthmatic patients ANDERS ULLMAN, NILS SVEDMYR From the Department of Clinical
Thorax 1988;43:674-678 Salmeterol, a new long acting inhaled,f2 adrenoceptor agonist: comparison with salbutamol in adult asthmatic patients ANDERS ULLMAN, NILS SVEDMYR From the Department of Clinical
Response to inhaled albuterol during nocturnal asthma
 Response to inhaled albuterol during nocturnal asthma Leslie Hendeles, PharmD, a Russell Beaty, MD, d Richard Ahrens, MD, e Gary Stevens, PhD, b and Eloise M. Harman, MD c Gainesville, Fla, Birmingham,
Response to inhaled albuterol during nocturnal asthma Leslie Hendeles, PharmD, a Russell Beaty, MD, d Richard Ahrens, MD, e Gary Stevens, PhD, b and Eloise M. Harman, MD c Gainesville, Fla, Birmingham,
The Impact of CFC to HFA Inhalers: What Pharmacists Need to Know
 The Impact of CFC to HFA Inhalers: What Pharmacists Need to Know by Marsha K. Millonig, MBA, RPh President,Catalyst Enterprises, LLC E.L.F. Publications, Inc. is accredited by the Accreditation Council
The Impact of CFC to HFA Inhalers: What Pharmacists Need to Know by Marsha K. Millonig, MBA, RPh President,Catalyst Enterprises, LLC E.L.F. Publications, Inc. is accredited by the Accreditation Council
COPD. Breathing Made Easier
 COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
Tolerance to bronchodilating effects of salmeterol in COPD
 Respiratory Medicine (2003) 97, 1014 1020 Tolerance to bronchodilating effects of salmeterol in COPD J.F. Donohue a, *, S. Menjoge b, S. Kesten b a University of North Carolina School of Medicine, 130
Respiratory Medicine (2003) 97, 1014 1020 Tolerance to bronchodilating effects of salmeterol in COPD J.F. Donohue a, *, S. Menjoge b, S. Kesten b a University of North Carolina School of Medicine, 130
Principal Investigator. Study center(s) This was a single-center study. Publications None at the time of writing this report.
 (For national authority SYNOPSIS use only) Date 3 March 2005 An open, randomized, two-way crossover study evaluating the pharmacokinetics of budesonide and formoterol from Symbicort pmdi versus Oxis Turbuhaler
(For national authority SYNOPSIS use only) Date 3 March 2005 An open, randomized, two-way crossover study evaluating the pharmacokinetics of budesonide and formoterol from Symbicort pmdi versus Oxis Turbuhaler
International co-ordinating investigator Dr Dencho Osmanliev, St Sofia, Pulmonary Dept, 19 D Nestorov Str, Sofia, 1431, Bulgaria.
 Drug product: SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0681 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0681 Date: 24 Oktober, 2003 (For national
Drug product: SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0681 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0681 Date: 24 Oktober, 2003 (For national
1 Bungoono City Hospital, Oita, Japan 2 Department of Respiratory Medicine and Infectious Diseases, Oita
 Original Manuscript Evaluation of Bioequivalence Between the New Procaterol Hydrochloride Hydrate Dry Powder Inhaler and the Approved Dry Powder Inhaler in Patients With Asthma in a Randomized, Double-Blind,
Original Manuscript Evaluation of Bioequivalence Between the New Procaterol Hydrochloride Hydrate Dry Powder Inhaler and the Approved Dry Powder Inhaler in Patients With Asthma in a Randomized, Double-Blind,
Challenges in Meeting International Requirements for Clinical Bioequivalence of Inhaled Drug Products
 Challenges in Meeting International Requirements for Clinical Bioequivalence of Inhaled Drug Products Tushar Shah, M.D. Sr. VP, Global Respiratory Research and Development TEVA Pharmaceuticals 1 Presentation
Challenges in Meeting International Requirements for Clinical Bioequivalence of Inhaled Drug Products Tushar Shah, M.D. Sr. VP, Global Respiratory Research and Development TEVA Pharmaceuticals 1 Presentation
ADASUVE (LOXAPINE) INHALATION POWDER. EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS
 ADASUVE (LOXAPINE) INHALATION POWDER EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS August2017 December 2012 PMR-JUN-2017-0017 ADASUVE Risk Evaluation and Mitigation Strategy (REMS) Education Program Content
ADASUVE (LOXAPINE) INHALATION POWDER EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS August2017 December 2012 PMR-JUN-2017-0017 ADASUVE Risk Evaluation and Mitigation Strategy (REMS) Education Program Content
Protection against methacholine-induced bronchospasm: salbutamol pmdi versus Clickhaler1 DPI
 Eur Respir J 2003; 21: 816 820 DOI: 10.1183/09031936.03.00045902 Printed in UK all rights reserved Copyright #ERS Journals Ltd 2003 European Respiratory Journal ISSN 0903-1936 Protection against methacholine-induced
Eur Respir J 2003; 21: 816 820 DOI: 10.1183/09031936.03.00045902 Printed in UK all rights reserved Copyright #ERS Journals Ltd 2003 European Respiratory Journal ISSN 0903-1936 Protection against methacholine-induced
Meenu Singh, Joseph L. Mathew, Prabhjot Malhi, B.R. Srinivas and Lata Kumar
 Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Strategic plan for harmonization phaseout of Metered Dose Inhaler (MDI) using chlorofluorocarbon (CFC) as propellant in Thailand.
 Strategic plan for harmonization phaseout of Metered Dose Inhaler (MDI) using chlorofluorocarbon (CFC) as propellant in Thailand. Presented by Por Punyaratabandhu PMU hired The Allergy and Immunology Society
Strategic plan for harmonization phaseout of Metered Dose Inhaler (MDI) using chlorofluorocarbon (CFC) as propellant in Thailand. Presented by Por Punyaratabandhu PMU hired The Allergy and Immunology Society
The Montreal Protocol and the Phase-out of CFC-based Metered Dose Inhalers. Paul Krajnik UNIDO, Montreal Protocol Branch
 The Montreal Protocol and the Phase-out of CFC-based Metered Dose Inhalers Paul Krajnik UNIDO, Montreal Protocol Branch 1 The Montreal Protocol Binding international agreement for the preservation and
The Montreal Protocol and the Phase-out of CFC-based Metered Dose Inhalers Paul Krajnik UNIDO, Montreal Protocol Branch 1 The Montreal Protocol Binding international agreement for the preservation and
The FDA Critical Path Initiative
 The FDA Critical Path Initiative Clinical Considerations for Demonstration of Dose-response for Inhaled Corticosteroids - Exhaled Nitric Oxide Model Badrul A. Chowdhury, MD, PhD Director Division of Pulmonary
The FDA Critical Path Initiative Clinical Considerations for Demonstration of Dose-response for Inhaled Corticosteroids - Exhaled Nitric Oxide Model Badrul A. Chowdhury, MD, PhD Director Division of Pulmonary
Content Indica c tion Lung v olumes e & Lung Indica c tions i n c paci c ties
 Spirometry Content Indication Indications in occupational medicine Contraindications Confounding factors Complications Type of spirometer Lung volumes & Lung capacities Spirometric values Hygiene &
Spirometry Content Indication Indications in occupational medicine Contraindications Confounding factors Complications Type of spirometer Lung volumes & Lung capacities Spirometric values Hygiene &
GINA. At-A-Glance Asthma Management Reference. for adults, adolescents and children 6 11 years. Updated 2017
 GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
National Transition Strategy to Replace CFC-based. MDIs with in the Commonwealth of Independent States. Paul Krajnik UNIDO, Montreal Protocol Branch
 National Transition Strategy to Replace CFC-based MDIs with HFA-MDIs in the Commonwealth of Independent States Paul Krajnik UNIDO, Montreal Protocol Branch 1 Starting point: total CFC phase-out CFC phase-out
National Transition Strategy to Replace CFC-based MDIs with HFA-MDIs in the Commonwealth of Independent States Paul Krajnik UNIDO, Montreal Protocol Branch 1 Starting point: total CFC phase-out CFC phase-out
SYNOPSIS. Date 15 June 2004
 Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
AllergyANDClinical Immunology
 THE JOURNAL OF AllergyANDClinical Immunology VOLUME 106 NUMBER 6 OFFICIAL JOURNAL OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA AND IMMUNOLOGY New products Series editors: Donald Y. M. Leung, MD, PhD, Harold
THE JOURNAL OF AllergyANDClinical Immunology VOLUME 106 NUMBER 6 OFFICIAL JOURNAL OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA AND IMMUNOLOGY New products Series editors: Donald Y. M. Leung, MD, PhD, Harold
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Lecture Notes. Chapter 3: Asthma
 Lecture Notes Chapter 3: Asthma Objectives Define asthma and status asthmaticus List the potential causes of asthma attacks Describe the effect of asthma attacks on lung function List the clinical features
Lecture Notes Chapter 3: Asthma Objectives Define asthma and status asthmaticus List the potential causes of asthma attacks Describe the effect of asthma attacks on lung function List the clinical features
Response to Albuterol MDI Delivered Through an Anti-Static Chamber During Nocturnal Bronchospasm
 Response to Albuterol MDI Delivered Through an Anti-Static Chamber During Nocturnal Bronchospasm Sreekala Prabhakaran MD, Jonathan Shuster PhD, Sarah Chesrown MD PhD, and Leslie Hendeles PharmD BACKGROUND:
Response to Albuterol MDI Delivered Through an Anti-Static Chamber During Nocturnal Bronchospasm Sreekala Prabhakaran MD, Jonathan Shuster PhD, Sarah Chesrown MD PhD, and Leslie Hendeles PharmD BACKGROUND:
D DAVID PUBLISHING. 1. Introduction
 Journal of Health Science 2 (2014) 591-598 doi: 10.17265/2328-7136/2014.12.004 D DAVID PUBLISHING Comparative Efficacy and Safety of Two Formulations of Ipratropium Bromide (IB) HFA pmdi in Patients with
Journal of Health Science 2 (2014) 591-598 doi: 10.17265/2328-7136/2014.12.004 D DAVID PUBLISHING Comparative Efficacy and Safety of Two Formulations of Ipratropium Bromide (IB) HFA pmdi in Patients with
Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views
 IPAC-RS/University of Florida Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views 20 th March 2014 Dr. Alfredo García - Arieta Head of the Service of Generic
IPAC-RS/University of Florida Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views 20 th March 2014 Dr. Alfredo García - Arieta Head of the Service of Generic
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline
 umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Asthma in the Athlete
 Asthma in the Athlete Jorge E. Gomez, MD Associate Professor Texas Children s Hospital Baylor College of Medicine Assist Team Physician UH Understand how we diagnose asthma Objectives Be familiar with
Asthma in the Athlete Jorge E. Gomez, MD Associate Professor Texas Children s Hospital Baylor College of Medicine Assist Team Physician UH Understand how we diagnose asthma Objectives Be familiar with
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE December 2014 Review Date: December 2017 Bulletin 206 : DuoResp Spiromax 160 / 4.5 and 320 / 9 budesonide & formoterol dry powder inhaler JPC Recommendations
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE December 2014 Review Date: December 2017 Bulletin 206 : DuoResp Spiromax 160 / 4.5 and 320 / 9 budesonide & formoterol dry powder inhaler JPC Recommendations
An update on inhalation devices
 OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
On completion of this chapter you should be able to: discuss the stepwise approach to the pharmacological management of asthma in children
 7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
Breakout Session # A 12:45 1:25. New Methods of Medication Delivery to the Lungs: Say Goodbye to the MDI as we know it
 Breakout Session # A 12:45 1:25 New Methods of Medication Delivery to the Lungs: Say Goodbye to the MDI as we know it Michael J. Welch MD, FAAP, FAAAAI, CPI Co-director, Allergy & Asthma Medical Group
Breakout Session # A 12:45 1:25 New Methods of Medication Delivery to the Lungs: Say Goodbye to the MDI as we know it Michael J. Welch MD, FAAP, FAAAAI, CPI Co-director, Allergy & Asthma Medical Group
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma and the competitive swimmer
 Asthma and the competitive swimmer Introduction: One in seven children and one in 25 adults in Great Britain have asthma and the number is growing. Thus every swim squad or club will have a number of asthmatics
Asthma and the competitive swimmer Introduction: One in seven children and one in 25 adults in Great Britain have asthma and the number is growing. Thus every swim squad or club will have a number of asthmatics
Spirometry: Introduction
 Spirometry: Introduction Dr. Badri Paudel 1 2 GMC Spirometry Spirometry is a method of assessing lung function by measuring the volume of air the patient can expel from the lungs after a maximal expiration.
Spirometry: Introduction Dr. Badri Paudel 1 2 GMC Spirometry Spirometry is a method of assessing lung function by measuring the volume of air the patient can expel from the lungs after a maximal expiration.
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C
 MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C Explain the importance of objective measures in the management of asthma Explain the different types of objective measures used in the management
MSRC AIR Course Karla Stoermer Grossman, MSA, BSN, RN, AE-C Explain the importance of objective measures in the management of asthma Explain the different types of objective measures used in the management
SYNOPSIS. Drug substance(s) Budesonide/formoterol Document No. Edition No. Study code SD Date 16 December 2004
 Drug product SYMBICORT pmdi 160/4.5 µg SYNOPSIS Drug substance(s) Budesonide/formoterol Document No. Edition No. Date 16 December 2004 A Twelve-Week, Randomized, Double-Blind, Double-Dummy, Placebo-Controlled
Drug product SYMBICORT pmdi 160/4.5 µg SYNOPSIS Drug substance(s) Budesonide/formoterol Document No. Edition No. Date 16 December 2004 A Twelve-Week, Randomized, Double-Blind, Double-Dummy, Placebo-Controlled
Anyone who smokes and/or has shortness of breath and sputum production could have COPD
 COPD DIAGNOSIS AND MANAGEMENT CHECKLIST Anyone who smokes and/or has shortness of breath and sputum production could have COPD Confirm Diagnosis Presence and history of symptoms: Shortness of breath Cough
COPD DIAGNOSIS AND MANAGEMENT CHECKLIST Anyone who smokes and/or has shortness of breath and sputum production could have COPD Confirm Diagnosis Presence and history of symptoms: Shortness of breath Cough
COMMITTEE ON MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 18 October 2007 Doc. Ref. CPMP/EWP/4151/00 Rev. 1 COMMITTEE ON MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 18 October 2007 Doc. Ref. CPMP/EWP/4151/00 Rev. 1 COMMITTEE ON MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE
Formoterol (OXIS s ) Turbuhaler s as a rescue therapy compared with salbutamol pmdi plus spacer in patients with acute severe asthma
 Respiratory Medicine (2003) 97, 1067 1074 Formoterol (OXIS s ) Turbuhaler s as a rescue therapy compared with salbutamol pmdi plus spacer in patients with acute severe asthma W. Boonsawat*, S. Charoenratanakul,
Respiratory Medicine (2003) 97, 1067 1074 Formoterol (OXIS s ) Turbuhaler s as a rescue therapy compared with salbutamol pmdi plus spacer in patients with acute severe asthma W. Boonsawat*, S. Charoenratanakul,
James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren, MD
 Therapeutic Effect of Zafirlukast as Monotherapy in Steroid-Naive Patients With Severe Persistent Asthma* James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren,
Therapeutic Effect of Zafirlukast as Monotherapy in Steroid-Naive Patients With Severe Persistent Asthma* James P. Kemp, MD; Margaret C. Minkwitz, PhD; Catherine M. Bonuccelli, MD; and Marshelle S. Warren,
GETTING STARTED WITH MANNITOL CHALLENGE TESTING. Rick Ballard, RRT, RPFT Applications/Education Specialist MGC Diagnostics
 GETTING STARTED WITH MANNITOL CHALLENGE TESTING Rick Ballard, RRT, RPFT Applications/Education Specialist MGC Diagnostics OBJECTIVES Indications for provocation testing Identify candidates for testing
GETTING STARTED WITH MANNITOL CHALLENGE TESTING Rick Ballard, RRT, RPFT Applications/Education Specialist MGC Diagnostics OBJECTIVES Indications for provocation testing Identify candidates for testing
Decramer 2014 a &b [21]
![Decramer 2014 a &b [21] Decramer 2014 a &b [21]](/thumbs/90/101390504.jpg) Buhl 2015 [19] Celli 2014 [20] Decramer 2014 a &b [21] D Urzo 2014 [22] Maleki-Yazdi 2014 [23] Inclusion criteria: Diagnosis of chronic obstructive pulmonary disease; 40 years of age or older; Relatively
Buhl 2015 [19] Celli 2014 [20] Decramer 2014 a &b [21] D Urzo 2014 [22] Maleki-Yazdi 2014 [23] Inclusion criteria: Diagnosis of chronic obstructive pulmonary disease; 40 years of age or older; Relatively
Pharmacodynamic steady state of tiotropium in patients with chronic obstructive pulmonary disease
 Eur Respir J 2002; 19: 639 644 DOI: 10.1183/09031936.02.00238002 Printed in UK all rights reserved Copyright #ERS Journals Ltd 2002 European Respiratory Journal ISSN 0903-1936 Pharmacodynamic steady state
Eur Respir J 2002; 19: 639 644 DOI: 10.1183/09031936.02.00238002 Printed in UK all rights reserved Copyright #ERS Journals Ltd 2002 European Respiratory Journal ISSN 0903-1936 Pharmacodynamic steady state
Study No Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Study Endpoints: Pharmacokinetics:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Asthma in Day to Day Practice
 Asthma in Day to Day Practice VIJAY.K.VANAM Financial relationships: Disclosures Employed at Mercy Medical Center, Mason City. Nonfinancial relationships: I receive no financial gain from any pharmaceutical
Asthma in Day to Day Practice VIJAY.K.VANAM Financial relationships: Disclosures Employed at Mercy Medical Center, Mason City. Nonfinancial relationships: I receive no financial gain from any pharmaceutical
SCREENING AND PREVENTION
 These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
Ventamol CFC-Free 100 micrograms, Pressurised Inhalation, Suspension (Salbutamol)
 Ventamol CFC-Free 100 micrograms, Pressurised Inhalation, Suspension (Salbutamol) For inhalation use Important: Read instructions carefully Wash your inhaler once a week and allow to dry What you need
Ventamol CFC-Free 100 micrograms, Pressurised Inhalation, Suspension (Salbutamol) For inhalation use Important: Read instructions carefully Wash your inhaler once a week and allow to dry What you need
History & Development
 RSPT 2317 Anticholinergic Bronchodilators () History & Development Prototypical parasympatholytic agent is atropine an alkaloid found naturally in the plants Atropa belladona (nightshade) and Datura species
RSPT 2317 Anticholinergic Bronchodilators () History & Development Prototypical parasympatholytic agent is atropine an alkaloid found naturally in the plants Atropa belladona (nightshade) and Datura species
SPIROMETRY. Marijke Currie (CRFS) Care Medical Ltd Phone: Copyright CARE Medical ltd
 SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual can
SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual can
Bronchodilator and Cardiac Effects of Isoprenaline, Orciprenaline, and Salbutamol Aerosols in Asthma
 Archives of Disease in Childhood, 1971, 46, 52. Bronchodilator and Cardiac Effects of Isoprenaline, Orciprenaline, and Salbutamol Aerosols in Asthma A. D. MILNER and D. INGRAM From The Hospital for Sick
Archives of Disease in Childhood, 1971, 46, 52. Bronchodilator and Cardiac Effects of Isoprenaline, Orciprenaline, and Salbutamol Aerosols in Asthma A. D. MILNER and D. INGRAM From The Hospital for Sick
JANUARY Guide to the WADA Prohibited List and Therapeutic Use Exemptions
 JANUARY 2018 Guide to the WADA Prohibited List and Therapeutic Use Exemptions Contents The WADA Prohibited List 3 Therapeutic Use Exemptions (TUEs) 5 Requirements for asthma TUEs 9 2 The WADA Prohibited
JANUARY 2018 Guide to the WADA Prohibited List and Therapeutic Use Exemptions Contents The WADA Prohibited List 3 Therapeutic Use Exemptions (TUEs) 5 Requirements for asthma TUEs 9 2 The WADA Prohibited
PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER
 PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER NAME OF MEDICINE Sodium Cromoglycate for Oral Inhalation. C 23 H 14 Na 2 O 11 Mol Wt. 512.3 CAS No. [15826-37-6] Sodium cromoglycate
PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER NAME OF MEDICINE Sodium Cromoglycate for Oral Inhalation. C 23 H 14 Na 2 O 11 Mol Wt. 512.3 CAS No. [15826-37-6] Sodium cromoglycate
Basic approach to PFT interpretation. Dr. Giulio Dominelli BSc, MD, FRCPC Kelowna Respiratory and Allergy Clinic
 Basic approach to PFT interpretation Dr. Giulio Dominelli BSc, MD, FRCPC Kelowna Respiratory and Allergy Clinic Disclosures Received honorarium from Astra Zeneca for education presentations Tasked Asked
Basic approach to PFT interpretation Dr. Giulio Dominelli BSc, MD, FRCPC Kelowna Respiratory and Allergy Clinic Disclosures Received honorarium from Astra Zeneca for education presentations Tasked Asked
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
G. Boyd on behalf of a UK Study group
 Eur Respir J, 1995, 8, 1494 1498 DOI: 10.1183/09031936.95.08091494 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1995 European Respiratory Journal ISSN 0903-1936 Salmeterol xinafoate in
Eur Respir J, 1995, 8, 1494 1498 DOI: 10.1183/09031936.95.08091494 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1995 European Respiratory Journal ISSN 0903-1936 Salmeterol xinafoate in
UMEC/VI vs. UMEC in subjects who responded to UMEC UMEC/VI vs. VI in subjects who responded to VI
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
behaviour are out of scope of the present review.
 explained about the test, a trial may be done before recording the results. The maneuver consists initially of normal tidal breathing. The subject then inhales to maximally fill the lungs. This is followed
explained about the test, a trial may be done before recording the results. The maneuver consists initially of normal tidal breathing. The subject then inhales to maximally fill the lungs. This is followed
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Pharmacokinetics, pharmacodynamics, and the delivery of pediatric bronchodilator therapy
 Pharmacokinetics, pharmacodynamics, and the delivery of pediatric bronchodilator therapy David P. Skoner, MD Pittsburgh, Pa β 2 -adrenergic receptor agonists have long been used for the amelioration of
Pharmacokinetics, pharmacodynamics, and the delivery of pediatric bronchodilator therapy David P. Skoner, MD Pittsburgh, Pa β 2 -adrenergic receptor agonists have long been used for the amelioration of
#8 - Respiratory System
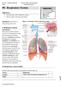 Page1 #8 - Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Equipment: Remember to bring photographic atlas. Figure 1. Structures of the respiratory system.
Page1 #8 - Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Equipment: Remember to bring photographic atlas. Figure 1. Structures of the respiratory system.
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION (FLUTICASONE FUROATE/VILANTEROL) (Breo Ellipta GlaxoSmithKline) Indication: Chronic Obstructive Pulmonary Disease Recommendation: The Canadian Drug Expert Committee (CDEC) recommends
CDEC FINAL RECOMMENDATION (FLUTICASONE FUROATE/VILANTEROL) (Breo Ellipta GlaxoSmithKline) Indication: Chronic Obstructive Pulmonary Disease Recommendation: The Canadian Drug Expert Committee (CDEC) recommends
Sponsor. Generic drug name. Trial indication(s) Protocol number. Protocol title. Clinical trial phase. Study Start/End Dates.
 Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
Asthma. Don Hayes, Jr., MD, FAAP, FACP
 Asthma Don Hayes, Jr., MD, FAAP, FACP Director, University of Kentucky Asthma Center Kentucky Children s Hospital & Assistant Professor of Pediatrics & Internal Medicine University of Kentucky College
Asthma Don Hayes, Jr., MD, FAAP, FACP Director, University of Kentucky Asthma Center Kentucky Children s Hospital & Assistant Professor of Pediatrics & Internal Medicine University of Kentucky College
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action
 Eur Respir J, 1996, 9, 1684 1688 DOI:.1183/09031936.96.09081684 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1996 European Respiratory Journal ISSN 0903-1936 Salmeterol versus formoterol
Eur Respir J, 1996, 9, 1684 1688 DOI:.1183/09031936.96.09081684 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1996 European Respiratory Journal ISSN 0903-1936 Salmeterol versus formoterol
Clinical effect of Diskus dry-powder inhaler at low and high inspiratory flow-rates in asthmatic children
 Eur Respir J 1998; 11: 35 354 DOI: 1.1183/931936.98.11235 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1998 European Respiratory Journal ISSN 93-1936 Clinical effect of Diskus dry-powder
Eur Respir J 1998; 11: 35 354 DOI: 1.1183/931936.98.11235 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1998 European Respiratory Journal ISSN 93-1936 Clinical effect of Diskus dry-powder
Philippine Strategy for the. Free Alternatives. under the National CFC Phase-out Plan (NCPP)
 Philippine Strategy for the Transition from CFCcontaining MDIs to CFC- Free Alternatives under the National CFC Phase-out Plan (NCPP) 1 Developing the Transition Strategy Findings of the study of the MDIs
Philippine Strategy for the Transition from CFCcontaining MDIs to CFC- Free Alternatives under the National CFC Phase-out Plan (NCPP) 1 Developing the Transition Strategy Findings of the study of the MDIs
Full Length Original Research Paper
 Copyright 2013 By IYPF All rights reserved Open Access Contents Int. J. Drug Dev. & Res. October - December 2013 Vol. 5 Issue 4 ISSN 0975-9344 www.ijddr.in Effect of Budesonide by metered dose inhaler
Copyright 2013 By IYPF All rights reserved Open Access Contents Int. J. Drug Dev. & Res. October - December 2013 Vol. 5 Issue 4 ISSN 0975-9344 www.ijddr.in Effect of Budesonide by metered dose inhaler
Chapter 7. Anticholinergic (Parasympatholytic) Bronchodilators. Mosby items and derived items 2008, 2002 by Mosby, Inc., an affiliate of Elsevier Inc.
 Chapter 7 Anticholinergic (Parasympatholytic) Bronchodilators Clinical Indications for Use Indication for anticholinergic bronchodilator COPD maintenance Indication for combined anticholinergic and β-agonist
Chapter 7 Anticholinergic (Parasympatholytic) Bronchodilators Clinical Indications for Use Indication for anticholinergic bronchodilator COPD maintenance Indication for combined anticholinergic and β-agonist
Pulmonary Function Testing: Concepts and Clinical Applications. Potential Conflict Of Interest. Objectives. Rationale: Why Test?
 Pulmonary Function Testing: Concepts and Clinical Applications David M Systrom, MD Potential Conflict Of Interest Nothing to disclose pertinent to this presentation BRIGHAM AND WOMEN S HOSPITAL Harvard
Pulmonary Function Testing: Concepts and Clinical Applications David M Systrom, MD Potential Conflict Of Interest Nothing to disclose pertinent to this presentation BRIGHAM AND WOMEN S HOSPITAL Harvard
Online supplementary material
 Online supplementary material Add-on long-acting β2-agonist (LABA) in a separate inhaler as asthma step-up therapy versus increased dose of inhaled corticosteroid (ICS) or ICS/LABA combination inhaler
Online supplementary material Add-on long-acting β2-agonist (LABA) in a separate inhaler as asthma step-up therapy versus increased dose of inhaled corticosteroid (ICS) or ICS/LABA combination inhaler
Air Flow Limitation. In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation.
 Asthma Air Flow Limitation In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation. True whether reversible, asthma and exercise-induced bronchospasm,
Asthma Air Flow Limitation In most serious respiratory disease, a key feature causing morbidity and functional disruption is air flow imitation. True whether reversible, asthma and exercise-induced bronchospasm,
Time factor in the measurement of response
 Thorax (1967), 22, 538. Time factor in the measurement of response to bronchodilators G. J. MUSHIN From Pritnce Heairv's Hospital, Melbournie, A ustralia Trhe measurement of response to bronchodilators
Thorax (1967), 22, 538. Time factor in the measurement of response to bronchodilators G. J. MUSHIN From Pritnce Heairv's Hospital, Melbournie, A ustralia Trhe measurement of response to bronchodilators
