Table 1 Model of STRIDESMOOTH (Stride) and STRIDESMOOTH+ Microcatheter
|
|
|
- Owen Patterson
- 5 years ago
- Views:
Transcription
1
2 AMM-Q269 Ver.7 Page 2 of 5 Table 1 Model of STRIDESMOOTH (Stride) and STRIDESMOOTH+ Microcatheter Product name STRIDESMOOTH (Stride) Model No. STD105-22S STD125-22S STD125-22A STD130-22S STD150-22S STD105-26S STD125-26S STD150-26S Description STRIDESMOOTH (Stride) Microcatheter, 2.2 Fr, Straight, 105cm STRIDESMOOTH (Stride) Microcatheter, 2.2 Fr, Straight, 125cm STRIDESMOOTH (Stride) Microcatheter, 2.2 Fr, Angled, 125cm STRIDESMOOTH (Stride) Microcatheter, 2.2 Fr, Straight, 130cm STRIDESMOOTH (Stride) Microcatheter, 2.2 Fr, Straight, 150cm STRIDESMOOTH (Stride) Microcatheter, 2.6 Fr, Straight, 105cm STRIDESMOOTH (Stride) Microcatheter, 2.6 Fr, Straight, 125cm STRIDESMOOTH (Stride) Microcatheter, 2.6 Fr, Straight, 150cm Product name STRIDESMOOTH+ Model No. STP105-20S STP115-20S STP125-20S STP125-20A STP135-20S STP150-20S Description STRIDESMOOTH+ Microcatheter, 2.0 Fr, Straight, 105cm STRIDESMOOTH+ Microcatheter, 2.0 Fr, Straight, 115cm STRIDESMOOTH+ Microcatheter, 2.0 Fr, Straight, 125cm STRIDESMOOTH+ Microcatheter, 2.0 Fr, Angled, 125cm STRIDESMOOTH+ Microcatheter, 2.0 Fr, Straight, 135cm STRIDESMOOTH+ Microcatheter, 2.0 Fr, Straight, 150cm
3 AMM-Q269 Ver.7 Page 3 of 5 1. QA- RELATED STANDARDS EN ISO 13485:2012 AC:2012 ISO 13485:2003 Cor1:2009 EC Directive 93/42/EEC L Amd 1: 1998 Amd 2: 2000 Amd 3: 2002 Amd 4: 2003 Amd 5: PRODUCT- RELATED STANDARDS Table 2 Applied harmonized standards Medical devices Quality management systems Requirements for regulatory purposes Medical Devices Directive (2007) EN 556-1:2001 AC:2006 EN 556-2: 2003 ISO 594-2:1998 EN ISO : 2012 ISO : 2012 EN 1041:2008 EN 1707:1996 EN ISO :2009 ISO :1995 Amd 1:1999 Amd 2:2004 EN ISO :1997 C1:2002 ISO :1996 Cor 1:2002 EN ISO :2009 AC:2010 ISO :2009 Cor1:2010 EN ISO : 2006 ISO : 2006 EN ISO :2009 ISO :2002 Amd 1:2006 EN ISO :2009 ISO :2009 Sterilization of medical devices Requirements for medical devices to be designated "STERILE" Part 1: Requirements for terminally sterilized medical devices Sterilization of medical devices Requirements for medical devices to be designated "STERILE" - Part 2: Requirements for aseptically processed medical devices Conical fittings with a 6 % (Luer) taper for syringes, needles and certain other Part 2: Lock fittings Medical devices-symbols to be used with medical device labels, labeling and information to be supplied Part1: General requirements Information supplied by the manufacturer with medical devices Conical fittings with a 6 % (Luer) taper for syringes, needles and certain other Lock fittings Sterile, single-use intravascular catheters Part1: General requirement Sterile, single-use intravascular catheters Part2: Angiographic catheter Part 1: Evaluation and testing Biological evaluation of medical devices- Part 2: Animal welfare requirements Part 4: Selection of tests for interactions with blood Part 5: Tests for in vitro cytotoxicity
4 AMM-Q269 Ver.7 Page 4 of 5 EN ISO :2008 AC:2009 ISO :2008 Cor1:2009 EN ISO :2010 ISO :2010 EN ISO :2009 ISO :2006 EN ISO :2012 ISO :2012 EN ISO :2007 ISO :2007 EN ISO : 2006 ISO : 2006 EN ISO : 2009 ISO : 2006 EN ISO :2009 ISO :2006 EN ISO :2006: ISO :2006 EN ISO :2006 AC:2009 ISO :2006 Cor1:2007 EN ISO :2009 ISO :2009 EN ISO 14155: 2011 AC:2011 ISO 14155: 2011 Cor 1:2011 EN ISO 14161: 2009 ISO 14161: 2009 EN ISO :1999 ISO :1999 EN ISO : 2000 ISO : 2000 EN ISO :2005 ISO :2005 EN ISO : 2003 ISO : 2003 EN ISO : 2003 AC:2006 ISO : 2003 Cor 1: 2004 EN ISO 14971:2012 ISO 14971:2007 EN :1993 AC:1996 A1:1997 ISO 594-1:1986 Part 7: Ethylene oxide sterilization residuals Part 10: Tests for irritation and delayed-type hypersensitivity Part 11: Tests for systemic toxicity Part 12: Sample preparation and reference materials Sterilization of health care products - Ethylene oxide - Part 1:Requirements for development, validation and routine control of a sterilization process for medical devices Part 1: General requirements Part 2: Biological indicators for ethylene oxide sterilization processes Packaging for terminally sterilized medical devices Part 1: Requirements for materials, sterile barrier systems and packaging systems First edition Packaging for terminally sterilized medical devices Part 2: Validation requirements for forming, sealing and assembly processes First edition Sterilization of medical devices Microbiological methods Part 1: Determination of a population of microorganisms on products Sterilization of medical devices Microbiological methods Part 2: Determination of a population of microorganisms on products Clinical investigation of medical devices for human subjects Good clinical practice Guidance for the selection, use and interpretation of results First edition Part 1: Classifications of air cleanliness Part 2: Specifications for testing and monitoring to prove continued compliance with ISO First edition Part 3: Test menthods Cleanrooms and associated controlled environments - Biocontamination control - Part 1: General principles and methods Cleanrooms and associated controlled environments -- Biocontamination control - Part 2: Evaluation and interpretation of biocontamination data Medical device Application of risk management to medical devices Conical fittings with a 6% (Luer) taper for syringes, needles and certain other Part 1: General requirements
5 AMM-Q269 Ver.7 Page 5 of 5 3. PRODUCT- RELATED GUIDANCE MEDDEV : 2009 MEDDEV :2013 MEDDEV : 2012 EVALUATION OF CLINICAL DATA: A GUIDE FOR MANUFACTURERS AND NOTIFIED BODIES GUIDELINES ON A MEDICAL DEVICES VIGILANCE SYSTEM POST MARKET CLINICAL FOLLOW-UP STUDIES
EC Declaration of Conformity
 EC Declaration of Conformity MegaGen Implant Co., Ltd. 377-2, Gyochon-ri, Jain-myeon, Gyeongsan-si, Gyeongbuk, Korea declare that the medical devices described hereafter The Dental Implant Superstructure
EC Declaration of Conformity MegaGen Implant Co., Ltd. 377-2, Gyochon-ri, Jain-myeon, Gyeongsan-si, Gyeongbuk, Korea declare that the medical devices described hereafter The Dental Implant Superstructure
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 10555-5 Second edition 2013-06-15 Intravascular catheters Sterile and single-use catheters Part 5: Over-needle peripheral catheters Cathéters intravasculaires Cathéters stériles
INTERNATIONAL STANDARD ISO 10555-5 Second edition 2013-06-15 Intravascular catheters Sterile and single-use catheters Part 5: Over-needle peripheral catheters Cathéters intravasculaires Cathéters stériles
Intravascular catheters Sterile and single-use catheters. Part 5: Over-needle peripheral catheters
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-5 Second edition 2013-06-15 Intravascular catheters Sterile and single-use catheters Part 5: Over-needle peripheral catheters Cathéters intravasculaires
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-5 Second edition 2013-06-15 Intravascular catheters Sterile and single-use catheters Part 5: Over-needle peripheral catheters Cathéters intravasculaires
A high-level overview of the requirements of medical packaging standards
 Medical Packaging A high-level overview of the requirements of medical packaging standards Dec 2012 Thierry Wagner Regulatory Affairs Director Europe, Middle East and Africa DuPont Medical & Pharmaceutical
Medical Packaging A high-level overview of the requirements of medical packaging standards Dec 2012 Thierry Wagner Regulatory Affairs Director Europe, Middle East and Africa DuPont Medical & Pharmaceutical
Healthcare Sterilisation: Introduction and Standard Practices, Volume 1
 Contents 1. Overview of Sterilisation and Background... 1 1.1 Magic Wand... 1 1.1.1 Definition of Sterilisation... 2 1.1.2 Detectives or Forensic Scientists... 3 1.1.3 Idealised Process... 6 1.1.4 Lesser
Contents 1. Overview of Sterilisation and Background... 1 1.1 Magic Wand... 1 1.1.1 Definition of Sterilisation... 2 1.1.2 Detectives or Forensic Scientists... 3 1.1.3 Idealised Process... 6 1.1.4 Lesser
Healthcare Sterilisation: Challenging Practices Volume 2
 Contents 1 Traditional Chemical Sterilisation Methods... 1 1.1 Ethylene Oxide Sterilisation... 3 1.1.1 Green sterilisation (Benefits versus Risks)... 4 1.1.2 Ethylene Oxide Residuals... 8 1.1.3 Advantages
Contents 1 Traditional Chemical Sterilisation Methods... 1 1.1 Ethylene Oxide Sterilisation... 3 1.1.1 Green sterilisation (Benefits versus Risks)... 4 1.1.2 Ethylene Oxide Residuals... 8 1.1.3 Advantages
STERILITY TESTING OF PHARMACEUTICAL PRODUCTS
 STERILITY TESTING OF PHARMACEUTICAL PRODUCTS Tim Sandle CONTENTS Introduction xiii 1 STERILITY 1 Introduction 1 Sterility 3 Microorganisms and Microbial Growth 5 Types of microorganisms 7 Sterilization
STERILITY TESTING OF PHARMACEUTICAL PRODUCTS Tim Sandle CONTENTS Introduction xiii 1 STERILITY 1 Introduction 1 Sterility 3 Microorganisms and Microbial Growth 5 Types of microorganisms 7 Sterilization
Part 1: General requirements
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 10555-1 Second edition 2013-06-15 Corrected version 2014-01-15 Intravascular catheters Sterile and single-use catheters Part 1: General requirements
YY / ISO 11070:1998
 Translated English of Chinese Standard: YY0450.1-2003 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.120.30 C 48 YY 0450.1-2003
Translated English of Chinese Standard: YY0450.1-2003 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.120.30 C 48 YY 0450.1-2003
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 8537 Third edition 2016-03-15 Sterile single-use syringes, with or without needle, for insulin Seringues à insuline, stériles, non réutilisables, avec ou sans aiguille Reference
INTERNATIONAL STANDARD ISO 8537 Third edition 2016-03-15 Sterile single-use syringes, with or without needle, for insulin Seringues à insuline, stériles, non réutilisables, avec ou sans aiguille Reference
CATALOGUE OF MEDICAL DEVICES AND MEDICINAL PRODUCTS
 20 17 CATALOGUE OF MEDICAL DEVICES AND MEDICINAL PRODUCTS PARACÉLSIA - Indústria Farmacêutica, S.A. is a pharmaceutical company founded in 1930, exclusively owned by private capitals. We have been manufacturing
20 17 CATALOGUE OF MEDICAL DEVICES AND MEDICINAL PRODUCTS PARACÉLSIA - Indústria Farmacêutica, S.A. is a pharmaceutical company founded in 1930, exclusively owned by private capitals. We have been manufacturing
Sterile hypodermic needles for single use Requirements and test methods
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 7864 Fourth edition 2016-08-01 Sterile hypodermic needles for single use Requirements and test methods Aiguilles hypodermiques stériles, non réutilisables
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 7864 Fourth edition 2016-08-01 Sterile hypodermic needles for single use Requirements and test methods Aiguilles hypodermiques stériles, non réutilisables
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 22794 First edition 2007-07-15 Corrected version 2009-01-15 Dentistry Implantable materials for bone filling and augmentation in oral and maxillofacial surgery Contents of a
INTERNATIONAL STANDARD ISO 22794 First edition 2007-07-15 Corrected version 2009-01-15 Dentistry Implantable materials for bone filling and augmentation in oral and maxillofacial surgery Contents of a
Brazilian Official Gazette (Diário Oficial da União DOU) of 1 February 2010
 Brazilian Health Surveillance Agency www.anvisa.gov.br Draft Resolution No 10 of 27 January 2010. Brazilian Official Gazette (Diário Oficial da União DOU) of 1 February 2010 The Collegiate Directorate
Brazilian Health Surveillance Agency www.anvisa.gov.br Draft Resolution No 10 of 27 January 2010. Brazilian Official Gazette (Diário Oficial da União DOU) of 1 February 2010 The Collegiate Directorate
YY/T Translated English of Chinese Standard: YYT PHARMACEUTICAL INDUSTRY STANDARD
 Translated English of Chinese Standard: YYT0325-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA
Translated English of Chinese Standard: YYT0325-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA
YY Translated English of Chinese Standard: YY
 Translated English of Chinese Standard: YY0497-2005 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.040.20 C 31 Pharmaceutical National Standard of the People s Republic of China YY 0497-2005
Translated English of Chinese Standard: YY0497-2005 www.chinesestandard.net Sales@ChineseStandard.net YY ICS 11.040.20 C 31 Pharmaceutical National Standard of the People s Republic of China YY 0497-2005
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
ISO 8537 INTERNATIONAL STANDARD. Sterile single-use syringes, with or without needle, for insulin
 INTERNATIONAL STANDARD ISO 8537 Second edition 2007-10-01 Sterile single-use syringes, with or without needle, for insulin Seringues à insuline, stériles, non réutilisables, avec ou sans aiguille Reference
INTERNATIONAL STANDARD ISO 8537 Second edition 2007-10-01 Sterile single-use syringes, with or without needle, for insulin Seringues à insuline, stériles, non réutilisables, avec ou sans aiguille Reference
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Sterile hypodermic syringes for single use. Part 1: Syringes for manual use
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 7886-1 Second edition 2017-05 Sterile hypodermic syringes for single use Part 1: Syringes for manual use Seringues hypodermiques stériles, non
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 7886-1 Second edition 2017-05 Sterile hypodermic syringes for single use Part 1: Syringes for manual use Seringues hypodermiques stériles, non
Y s A P. n n. C o. e c. t o r s E R. e s. e l. e e d l I V. e d o s C l. vadsite The Transparent, Split Septum, Needleless Connector
 I V T H N E R e e d l A P e l e s Y s C o n n e c t o r s e d o s C l vadsite The Transparent, Split Septum, Needleless Connector What is vadsite? vadsite is a Neutral Displacement Needleless Connector
I V T H N E R e e d l A P e l e s Y s C o n n e c t o r s e d o s C l vadsite The Transparent, Split Septum, Needleless Connector What is vadsite? vadsite is a Neutral Displacement Needleless Connector
ISO INTERNATIONAL STANDARD. Sterile single-use intravascular catheter introducers
 INTERNATIONAL STANDARD ISO 11070 First edition 1998-05-01 Sterile single-use intravascular catheter introducers Introducteurs de cathéters intravasculaires stériles, non réutilisables A Reference number
INTERNATIONAL STANDARD ISO 11070 First edition 1998-05-01 Sterile single-use intravascular catheter introducers Introducteurs de cathéters intravasculaires stériles, non réutilisables A Reference number
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
Medidée Services SA. Nano-Tera.ch. 05 February 2015 part 4. Intro ISO GMP - GLP Pierre-Alain Sommer
 Nano-Tera.ch 05 February 2015 part 4 Intro ISO GMP - GLP Pierre-Alain Sommer Pierre-alain.sommer@medidee.com www.medidee.com Nano-Tera 2015 05.02.2015 Introduction to ISO 13485, cgmp s and GLP s Context
Nano-Tera.ch 05 February 2015 part 4 Intro ISO GMP - GLP Pierre-Alain Sommer Pierre-alain.sommer@medidee.com www.medidee.com Nano-Tera 2015 05.02.2015 Introduction to ISO 13485, cgmp s and GLP s Context
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD 7886- First edition 996-05-5 Sterile hypodermic syringes for Single use - Part : Syringes for use with power-driven Syringe Pumps Seringues hypodermiques st&iles, non r&mkables -
INTERNATIONAL STANDARD 7886- First edition 996-05-5 Sterile hypodermic syringes for Single use - Part : Syringes for use with power-driven Syringe Pumps Seringues hypodermiques st&iles, non r&mkables -
THE DOSING SPECIALIST
 04 THE DOSING SPECIALIST THE COMPANY WHO ARE WE? Over the past 30 years, ROVIPHARM has become the specialist manufacturer of medical and dosing devices for the pharmaceutical and healthcare industries.
04 THE DOSING SPECIALIST THE COMPANY WHO ARE WE? Over the past 30 years, ROVIPHARM has become the specialist manufacturer of medical and dosing devices for the pharmaceutical and healthcare industries.
Compounding Medication Regulations
 Association of Professors of Dermatology 2016 Annual Meeting Swissôtel, Chicago, IL Compounding Medication Regulations Christopher Bichakjian, MD Professor of Dermatology Comprehensive Cancer Center and
Association of Professors of Dermatology 2016 Annual Meeting Swissôtel, Chicago, IL Compounding Medication Regulations Christopher Bichakjian, MD Professor of Dermatology Comprehensive Cancer Center and
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family
 Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family MICRO-INTRODUCERS Micro-Introducer Kits Galt s Micro-Introducer Kits with smooth transition,
Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family MICRO-INTRODUCERS Micro-Introducer Kits Galt s Micro-Introducer Kits with smooth transition,
KEY DIFFERENCES BETWEEN THE NEW MEDICAL DEVICE AND THE OTC REGULATIONS IN EUROPE
 RNI Conseil 2017 Tous droits réservés Toute reproduction interdite sans l'autorisation de l'auteur. KEY DIFFERENCES BETWEEN THE NEW MEDICAL DEVICE AND THE OTC REGULATIONS IN EUROPE Anne Laure TARDY, PhD
RNI Conseil 2017 Tous droits réservés Toute reproduction interdite sans l'autorisation de l'auteur. KEY DIFFERENCES BETWEEN THE NEW MEDICAL DEVICE AND THE OTC REGULATIONS IN EUROPE Anne Laure TARDY, PhD
ISO INTERNATIONAL STANDARD. Anaesthetic and respiratory equipment Nebulizing systems and components
 INTERNATIONAL STANDARD ISO 27427 First edition 2009-05-01 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d'anesthésie et de réanimation respiratoire Systèmes de nébulisation
INTERNATIONAL STANDARD ISO 27427 First edition 2009-05-01 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d'anesthésie et de réanimation respiratoire Systèmes de nébulisation
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
ISO INTERNATIONAL STANDARD. Sterile hypodermic syringes for single use Part 4: Syringes with re-use prevention feature
 INTERNATIONAL STANDARD ISO 7886-4 First edition 2006-10-01 Sterile hypodermic syringes for single use Part 4: Syringes with re-use prevention feature Seringues hypodermiques stériles, non réutilisables
INTERNATIONAL STANDARD ISO 7886-4 First edition 2006-10-01 Sterile hypodermic syringes for single use Part 4: Syringes with re-use prevention feature Seringues hypodermiques stériles, non réutilisables
product catalogue Quality Results for life
 product catalogue ET CATHETERS OPU NEEDLES Quality Results for life IUI CATHETERS OOCYTE RETRIEVAL NEEDLES NEEDLES oocyte retrieval needles Manufactured from steel of excellent quality, its triple cut
product catalogue ET CATHETERS OPU NEEDLES Quality Results for life IUI CATHETERS OOCYTE RETRIEVAL NEEDLES NEEDLES oocyte retrieval needles Manufactured from steel of excellent quality, its triple cut
Bureau of Laboratory Quality Standards
 1. Condom -Lubricant ISO 4074:2002/Cor.1:2003 Annex C -Thickness ISO 4074:2002/Cor.1:2003 Annex F - Length ISO 4074:2002 / Cor1 : 2003 - Width ISO 4074 : 2002/Cor 1 : 2003 - Bursting pressure and Volume
1. Condom -Lubricant ISO 4074:2002/Cor.1:2003 Annex C -Thickness ISO 4074:2002/Cor.1:2003 Annex F - Length ISO 4074:2002 / Cor1 : 2003 - Width ISO 4074 : 2002/Cor 1 : 2003 - Bursting pressure and Volume
Product Sterility Testing... To Test or Not to Test? That Is the Question
 Product Sterility Testing... To Test or Not to Test? That Is the Question Elaine Daniell, Trabue Bryans, Kimbrell Darnell, Joyce Hansen, Victoria M. Hitchins, and Manuel Saavedra Abstract The applications
Product Sterility Testing... To Test or Not to Test? That Is the Question Elaine Daniell, Trabue Bryans, Kimbrell Darnell, Joyce Hansen, Victoria M. Hitchins, and Manuel Saavedra Abstract The applications
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS
 DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Environmental Monitoring in the Cell Processing Laboratory What to do with the data?
 Environmental Monitoring in the Cell Processing Laboratory What to do with the data? Shelly Heimfeld, Ph.D. Fred Hutchinson Cancer Research Center Seattle Cancer Care Alliance ISCT President Somatic Cell
Environmental Monitoring in the Cell Processing Laboratory What to do with the data? Shelly Heimfeld, Ph.D. Fred Hutchinson Cancer Research Center Seattle Cancer Care Alliance ISCT President Somatic Cell
Product: Spinal Needle. Zhejiang Runqiang Medical Instruments Co., Ltd
 Product: Spinal Needle Zhejiang Runqiang Medical Instruments Co., Ltd Parameter Method Specification Product Description - The product spinal needle is made up of three parts: - Spinal needle (needle tube
Product: Spinal Needle Zhejiang Runqiang Medical Instruments Co., Ltd Parameter Method Specification Product Description - The product spinal needle is made up of three parts: - Spinal needle (needle tube
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) Heinrich-Böll-Ring 10 53119
Notification of a Body in the framework of a technical harmonization directive From : Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) Heinrich-Böll-Ring 10 53119
DRAFT GUIDANCE DOCUMENT List of Recognized Standards
 DRAFT GUIDANCE DOCUMENT This guidance document is being distributed for comment purposes only. Published by authority of the Minister of Health Draft Date 2016/04/20 Health Products and Food Branch TABLE
DRAFT GUIDANCE DOCUMENT This guidance document is being distributed for comment purposes only. Published by authority of the Minister of Health Draft Date 2016/04/20 Health Products and Food Branch TABLE
Notification of a Body in the framework of a technical harmonization directive
 Notification of a Body in the framework of a technical harmonization directive From : Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) Heinrich-Böll-Ring 10 53119
Notification of a Body in the framework of a technical harmonization directive From : Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) Heinrich-Böll-Ring 10 53119
Retractable Technologies, Inc.
 Retractable Technologies, Inc. Product Retractable Needles Units per Boxes per 25G x 5/8 (16mm) 400 8 82091 25G x 1 (25mm) 400 8 82011 23G x 1 (25mm) 400 8 82031 Insulin Syringes Tuberculin Syringes Allergy
Retractable Technologies, Inc. Product Retractable Needles Units per Boxes per 25G x 5/8 (16mm) 400 8 82091 25G x 1 (25mm) 400 8 82011 23G x 1 (25mm) 400 8 82031 Insulin Syringes Tuberculin Syringes Allergy
BILATERAL SCREENING MEETING
 Negotiating Group for the Accession of the Republic of Serbia to the European Union - Chapter 28, Consumer and Health Protection BILATERAL SCREENING MEETING Chapter 28 Consumer and Health Protection Brussels,
Negotiating Group for the Accession of the Republic of Serbia to the European Union - Chapter 28, Consumer and Health Protection BILATERAL SCREENING MEETING Chapter 28 Consumer and Health Protection Brussels,
ASTM Workshop on Medical Device Cleanliness
 ASTM Workshop on Medical Device Cleanliness Steve Goldstine Consultants SteveGoldstine@Yahoo.com 11/16/2010 ASTM: Cleanliness of Medical Devices ASTM Workshop on Medical Device Cleanliness Standard Practices
ASTM Workshop on Medical Device Cleanliness Steve Goldstine Consultants SteveGoldstine@Yahoo.com 11/16/2010 ASTM: Cleanliness of Medical Devices ASTM Workshop on Medical Device Cleanliness Standard Practices
ISO/DIS Part 3: Sterile hypodermic syringes for single use. Auto-disabled syringes for fixed-dose immunization ISO/CEN PARALLEL PROCESSING
 DRAFT INTERNATIONAL STANDARD ISO/DIS 7886-3 ISO/TC 84 Sterile hypodermic syringes for single use Part 3: Auto-disabled syringes for fixed-dose immunization Seringues hypodermiques stériles, non réutilisables
DRAFT INTERNATIONAL STANDARD ISO/DIS 7886-3 ISO/TC 84 Sterile hypodermic syringes for single use Part 3: Auto-disabled syringes for fixed-dose immunization Seringues hypodermiques stériles, non réutilisables
01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation.
 Intraosseous Infusion System Directions for Use 01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation. EC REP Emerge
Intraosseous Infusion System Directions for Use 01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation. EC REP Emerge
Catheters and Wires. Dr. Vaibhav Jain MD,DNB,MNAMS Senior Consultant, IR Medanta, the Medicity, Gurgaon
 Catheters and Wires Dr. Vaibhav Jain MD,DNB,MNAMS Senior Consultant, IR Medanta, the Medicity, Gurgaon Guidewires: Guidewires (solid wires navigated within the vascular system / extravascular tract) act
Catheters and Wires Dr. Vaibhav Jain MD,DNB,MNAMS Senior Consultant, IR Medanta, the Medicity, Gurgaon Guidewires: Guidewires (solid wires navigated within the vascular system / extravascular tract) act
BACKFLUSH INSTRUMENTS
 BACKFLUSH INSTRUMENTS Versatility designed to handle all your surgical backflush needs. About VitreQ VitreQ creates instruments for the ophthalmic society with a special focus on vitreoretinal surgery.
BACKFLUSH INSTRUMENTS Versatility designed to handle all your surgical backflush needs. About VitreQ VitreQ creates instruments for the ophthalmic society with a special focus on vitreoretinal surgery.
Micro Production Part.
 Micro Production Part www.mipro-inc.com Worldwide activity 3 Who we are 4 Materials 5 Surface Treatment 6 Packaging 7 Letter from CEO 8 Cover 10 SOI Implant 11-12 TOI Implant 13-14 NSI Implant 15-16 SHI
Micro Production Part www.mipro-inc.com Worldwide activity 3 Who we are 4 Materials 5 Surface Treatment 6 Packaging 7 Letter from CEO 8 Cover 10 SOI Implant 11-12 TOI Implant 13-14 NSI Implant 15-16 SHI
INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION
 MIC * & MIC-KEY * Enterostomy Tubes INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE HEALTH PRODUCT CATALOGUE INNOVATIVE PRODUCTS - G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE
MIC * & MIC-KEY * Enterostomy Tubes INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE HEALTH PRODUCT CATALOGUE INNOVATIVE PRODUCTS - G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Medication errors. Impact of medication error guidance and regulation on drug-device combination products. June 2017 Dan Wozinski
 Medication errors Impact of medication error guidance and regulation on drug-device combination products June 2017 Dan Wozinski Agenda Understand the management of medication errors within global requirements
Medication errors Impact of medication error guidance and regulation on drug-device combination products June 2017 Dan Wozinski Agenda Understand the management of medication errors within global requirements
ADMINISTRATION SETS CATALOGUE
 ADMINISTRATION SETS CATALOGUE S1 2016 ADMINISTRATION SETS FOR VIRTUAL COLONOSCOPY (CT COLONOGRAPHY) AND INTUSSUSCEPTION REDUCTION... 2 Ref AS-3W-R35A... 2 Ref AS-3W-Y-R35A... 2 Ref AS-3W-H-R35A... 3 ADMINISTRATION
ADMINISTRATION SETS CATALOGUE S1 2016 ADMINISTRATION SETS FOR VIRTUAL COLONOSCOPY (CT COLONOGRAPHY) AND INTUSSUSCEPTION REDUCTION... 2 Ref AS-3W-R35A... 2 Ref AS-3W-Y-R35A... 2 Ref AS-3W-H-R35A... 3 ADMINISTRATION
STRENGTH FOR LIFE BONE CEMENTS FOR VERTEBRAL CONSOLIDATION
 STRENGTH FOR LIFE BONE CEMENTS FOR VERTEBRAL CONSOLIDATION who we are G-21 was set up in 2009 by expert entrepreneurs originating from the medical and pharmaceutical sector. G-21 is situated in proximity
STRENGTH FOR LIFE BONE CEMENTS FOR VERTEBRAL CONSOLIDATION who we are G-21 was set up in 2009 by expert entrepreneurs originating from the medical and pharmaceutical sector. G-21 is situated in proximity
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
TruGuard Custom Tongue and Jaw Positioner Instructions for Use
 TruGuard Custom Tongue and Jaw Positioner Instructions for Use Caution: Federal Law restricts this device to sale by or on the order of a licensed Physician or Radiation Therapist. DEVICE DESCRIPTION Bionix
TruGuard Custom Tongue and Jaw Positioner Instructions for Use Caution: Federal Law restricts this device to sale by or on the order of a licensed Physician or Radiation Therapist. DEVICE DESCRIPTION Bionix
Acute Therapy Systems. provencare Catheter Sets
 Acute Therapy Systems provencare Catheter Sets Catheter Sets Contents provencare Catheter Sets 4 provencare Product Features and Accessories 6 provencare Single Lumen Catheter Sets 8 provencare Paediatric
Acute Therapy Systems provencare Catheter Sets Catheter Sets Contents provencare Catheter Sets 4 provencare Product Features and Accessories 6 provencare Single Lumen Catheter Sets 8 provencare Paediatric
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 18746 First edition 2016-08-15 Traditional Chinese medicine Sterile intradermal acupuncture needles for single use Médecine traditionnelle chinoise Aiguilles d acupuncture intradermiques
INTERNATIONAL STANDARD ISO 18746 First edition 2016-08-15 Traditional Chinese medicine Sterile intradermal acupuncture needles for single use Médecine traditionnelle chinoise Aiguilles d acupuncture intradermiques
BD Eclipse Needle for Luer Lock Syringes - How to Use the BD Eclipse Needle for Luer Lock Syringes 3. Injection 1. Attachment
 BD Eclipse Needle How to Use the BD Eclipse Needle for Luer Lock Syringes 1. Attachment 1a. Firmly attach needle onto a Luer Lock syringe with a push and clockwise twist. 1b. Pull back on the safety cover
BD Eclipse Needle How to Use the BD Eclipse Needle for Luer Lock Syringes 1. Attachment 1a. Firmly attach needle onto a Luer Lock syringe with a push and clockwise twist. 1b. Pull back on the safety cover
High Flow Family. Short Term Dialysis Catheters. Dialysis
 High Flow Family Short Term Dialysis Catheters Dialysis High Flow Triple Lumen 13.5 Fr Unique tip design, superior to the classic sidehole design: Clotting risk is considerably reduced Adhesion risk to
High Flow Family Short Term Dialysis Catheters Dialysis High Flow Triple Lumen 13.5 Fr Unique tip design, superior to the classic sidehole design: Clotting risk is considerably reduced Adhesion risk to
Thora-Para catheter drainage systems
 Thora-Para catheter drainage systems Our thoracentesis-paracentesis catheter drainage devices are uniquely designed for safe and efficient diagnostic and therapeutic procedures. Thora-Para catheter drainage
Thora-Para catheter drainage systems Our thoracentesis-paracentesis catheter drainage devices are uniquely designed for safe and efficient diagnostic and therapeutic procedures. Thora-Para catheter drainage
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
EESTI STANDARD EVS-EN ISO :1999
 EESTI STANDARD EVS-EN ISO 10555-5:1999 Steriilsed ühekordselt kasutatavad intravaskulaarsed (soonesisesed) kateetrid. Osa 5: Üle nõela paigaldatavad perifeersed kateetrid Sterile, single-use intravascular
EESTI STANDARD EVS-EN ISO 10555-5:1999 Steriilsed ühekordselt kasutatavad intravaskulaarsed (soonesisesed) kateetrid. Osa 5: Üle nõela paigaldatavad perifeersed kateetrid Sterile, single-use intravascular
Endoscopic Ultrasound Endoskopischer Ultraschall
 Endoscopic Ultrasound Endoskopischer Ultraschall SonoTip Pro Control Endoscopic Ultrasound-Guided FNA System Single Use Twist-Lock Technology for Sheath & Needle Length Adjustments Offering Physician Controlled
Endoscopic Ultrasound Endoskopischer Ultraschall SonoTip Pro Control Endoscopic Ultrasound-Guided FNA System Single Use Twist-Lock Technology for Sheath & Needle Length Adjustments Offering Physician Controlled
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
The Interpretation of the relative Cytotoxic testing for gloves for the safety of the operator
 The Interpretation of the relative Cytotoxic testing for gloves for the safety of the operator Glove Selection factors Standards and guidelines for Gloves Cytotoxic testing BioClean ISO Class 4 100kg Washer
The Interpretation of the relative Cytotoxic testing for gloves for the safety of the operator Glove Selection factors Standards and guidelines for Gloves Cytotoxic testing BioClean ISO Class 4 100kg Washer
Provläsningsexemplar / Preview INTERNATIONAL STANDARD. Rubber condoms - Part 1: Requirements
 INTERNATIONAL STANDARD IS0 4074-I Second edition 1996-l 2-01 Rubber condoms - Part 1: Requirements Prbservatifs masculins en caoutchouc - Parfie 1: Exigences Reference number IS0 4074-I :1996(E) IS0 4074-I
INTERNATIONAL STANDARD IS0 4074-I Second edition 1996-l 2-01 Rubber condoms - Part 1: Requirements Prbservatifs masculins en caoutchouc - Parfie 1: Exigences Reference number IS0 4074-I :1996(E) IS0 4074-I
SVENSK STANDARD SS-EN 1615
 SVENSK STANDARD SS-EN 1615 Fastställd Utgåva Sida 2001-05-04 2 1 (1+14) Copyright SIS. Reproduction in any form without permission is prohibited. Enteral feeding catheters and enteral giving sets for single
SVENSK STANDARD SS-EN 1615 Fastställd Utgåva Sida 2001-05-04 2 1 (1+14) Copyright SIS. Reproduction in any form without permission is prohibited. Enteral feeding catheters and enteral giving sets for single
ISO INTERNATIONAL STANDARD. Sterile hypodermic syringes for single use Part 4: Syringes with re-use prevention feature
 INTERNATIONAL STANDARD ISO 7886-4 First edition 2006-10-01 Sterile hypodermic syringes for single use Part 4: Syringes with re-use prevention feature Seringues hypodermiques stériles, non réutilisables
INTERNATIONAL STANDARD ISO 7886-4 First edition 2006-10-01 Sterile hypodermic syringes for single use Part 4: Syringes with re-use prevention feature Seringues hypodermiques stériles, non réutilisables
QA Regulatory forum: Classification and Risk assessment according to MDR Date : T h e r e s e. A l b i n s s o m e d q t e c h.
 QA Regulatory forum: Classification and Risk assessment according to MDR Date : 05.04.2018 THERESE ALBINSSON T h e r e s e. A l b i n s s o n @ m e d q t e c h. s e MedQtech AB MedQtech AB is a consultant
QA Regulatory forum: Classification and Risk assessment according to MDR Date : 05.04.2018 THERESE ALBINSSON T h e r e s e. A l b i n s s o n @ m e d q t e c h. s e MedQtech AB MedQtech AB is a consultant
Webinar. Risk Assessment Reusable Polyester and Single Use Tyvek IsoClean Cleanroom Garments Dupont
 Webinar Risk Assessment Reusable Polyester and Single Use Tyvek IsoClean Cleanroom Garments Dupont 15 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical
Webinar Risk Assessment Reusable Polyester and Single Use Tyvek IsoClean Cleanroom Garments Dupont 15 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical
Needle free connectors, port protectors and infection risk today. Giancarlo Scoppettuolo A. Gemelli University Hospital Foundation, Rome
 Needle free connectors, port protectors and infection risk today Giancarlo Scoppettuolo A. Gemelli University Hospital Foundation, Rome 1 Pathogenesis of CRBSI and Prevention of extra and intraluminal
Needle free connectors, port protectors and infection risk today Giancarlo Scoppettuolo A. Gemelli University Hospital Foundation, Rome 1 Pathogenesis of CRBSI and Prevention of extra and intraluminal
PRODUCTS FOR THE DIFFICULT AIRWAY. Courtesy of Cook Critical Care
 PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
Environmental Monitoring, risk assessment and responsibilities
 Environmental Monitoring, risk assessment and responsibilities Medical Devices Directive -93/42/EEC ANNEX I ESSENTIAL REQUIREMENTS 8. Infection and microbial contamination 8.1. The devices and manufacturing
Environmental Monitoring, risk assessment and responsibilities Medical Devices Directive -93/42/EEC ANNEX I ESSENTIAL REQUIREMENTS 8. Infection and microbial contamination 8.1. The devices and manufacturing
Dentistry - Medical devices for dentistry Dental implants. Tandvård - Medicintekniska produkter för tandvård - Dentala implantat
 SIS FASTSTÄLLER OCH UTGER SVENSK STANDARD SAMT SÄLJER NATIONELLA, EUROPEISKA OCH INTERNATIONELLA STANDARDPUBLIKATIONER Dentistry - Medical devices for dentistry Dental implants Tandvård - Medicintekniska
SIS FASTSTÄLLER OCH UTGER SVENSK STANDARD SAMT SÄLJER NATIONELLA, EUROPEISKA OCH INTERNATIONELLA STANDARDPUBLIKATIONER Dentistry - Medical devices for dentistry Dental implants Tandvård - Medicintekniska
Y C o A P. n e. c t. o r s. E R e l. e s. e e d l I V. e d o s C l. bionector The Neutral Displacement Split Septum, Needleless Connector
 I V T N H e e d l E R e l A P e s s Y C o n n e c t o r s e d o s C l bionector The Neutral Displacement Split Septum, Needleless Connector Umbilical catheters What is bionector? bionector is a Neutral
I V T N H e e d l E R e l A P e s s Y C o n n e c t o r s e d o s C l bionector The Neutral Displacement Split Septum, Needleless Connector Umbilical catheters What is bionector? bionector is a Neutral
Anaesthetic Procedure Packs Ensuring maximum barrier precautions
 Anaesthetic Procedure Packs Ensuring maximum barrier precautions vygon@vygon.co.uk www.vygon.co.uk Anaesthetic Procedure Packs Ensuring maximum barrier precautions It has been estimated that infections
Anaesthetic Procedure Packs Ensuring maximum barrier precautions vygon@vygon.co.uk www.vygon.co.uk Anaesthetic Procedure Packs Ensuring maximum barrier precautions It has been estimated that infections
Meeting of the EU GLP Working Group, February GLP requirements in EU legislation and guidance medical devices
 Meeting of the EU GLP Working Group, 22-23 February 2017 Agenda item: Document title: Action required: Session 2, item 2a GLP requirements in EU legislation and guidance medical devices Requirements of
Meeting of the EU GLP Working Group, 22-23 February 2017 Agenda item: Document title: Action required: Session 2, item 2a GLP requirements in EU legislation and guidance medical devices Requirements of
IV Link Staff. Infection Prevention & Control A Learning Package for IV Link Staff
 IV Link Staff Infection Prevention & Control A Learning Package for IV Link Staff Purpose This learning package provides key infection prevention messages and direction for knowledge that you require as
IV Link Staff Infection Prevention & Control A Learning Package for IV Link Staff Purpose This learning package provides key infection prevention messages and direction for knowledge that you require as
FOR A HIGHER DEGREE OF CONFIDENCE
 SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS. > Introduction Manufacturing specialized products in a pharmaceutical manufacturing environment has a specific set of requirements
SELECTING THE RIGHT HAND PROTECTION WHEN WORKING WITH CHEMOTHERAPY DRUGS. > Introduction Manufacturing specialized products in a pharmaceutical manufacturing environment has a specific set of requirements
On the use of Amalgam for dental fillings in Sweden
 On the use of Amalgam for dental fillings in Sweden Director Medical Devices Medical Products Agency Uppsala, Sweden Content of this presentation Short regulatory introduction Current use of Amalgams in
On the use of Amalgam for dental fillings in Sweden Director Medical Devices Medical Products Agency Uppsala, Sweden Content of this presentation Short regulatory introduction Current use of Amalgams in
Safety Products Catalogue
 Safety Products Catalogue A Wide Array of Safety-Engineered Devices From One Company Safety matters to you... Needlestick and sharp injuries account for almost 1/5 of accidents to NHS staff. 2 95% of nurses
Safety Products Catalogue A Wide Array of Safety-Engineered Devices From One Company Safety matters to you... Needlestick and sharp injuries account for almost 1/5 of accidents to NHS staff. 2 95% of nurses
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 4074 First edition 2002-02-15 Natural latex rubber condoms Requirements and test methods Préservatifs masculins en latex de caoutchouc naturel Exigences et méthodes d'essai Reference
INTERNATIONAL STANDARD ISO 4074 First edition 2002-02-15 Natural latex rubber condoms Requirements and test methods Préservatifs masculins en latex de caoutchouc naturel Exigences et méthodes d'essai Reference
MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES
 MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES PUSHING BOUNDARIES Terumo Interventional Systems is committed to your success with innovative procedural solutions and ongoing support for your most challenging
MAXIMIZE RADIAL SOLUTIONS TO PERIPHERAL CHALLENGES PUSHING BOUNDARIES Terumo Interventional Systems is committed to your success with innovative procedural solutions and ongoing support for your most challenging
inspection body type A
 inspection body type A legal entity HygCen Austria GmbH Werksgelände 28, 5500 Bischofshofen web www.hygcen.at ident no. 0196 site HygCen Austria GmbH Werksgelände 28, 5500 Bischofshofen initial date of
inspection body type A legal entity HygCen Austria GmbH Werksgelände 28, 5500 Bischofshofen web www.hygcen.at ident no. 0196 site HygCen Austria GmbH Werksgelände 28, 5500 Bischofshofen initial date of
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml
 EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
Dental Instruments. Edition 2. Single Use Instruments. Absorbents. Wipes. Woundcare. Disposable Gloves. Patient Identification. First Aid.
 Single Use Instruments Absorbents Wipes Woundcare Disposable Gloves Patient Identification First Aid Dental Instruments Cotton Wool Personal Care Continence Care Edition 2 Instrapac Single Use Dental Instruments
Single Use Instruments Absorbents Wipes Woundcare Disposable Gloves Patient Identification First Aid Dental Instruments Cotton Wool Personal Care Continence Care Edition 2 Instrapac Single Use Dental Instruments
Why Choose OriGen? Mission Statement: We create medical products which improve peoples lives.
 Accessory Catalog S-FFP12 Mission Statement: We create medical products which improve peoples lives. Why Choose OriGen? Quality Commitment quality is the foundation of all product design at OriGen, and
Accessory Catalog S-FFP12 Mission Statement: We create medical products which improve peoples lives. Why Choose OriGen? Quality Commitment quality is the foundation of all product design at OriGen, and
Instructions for Use 1
 Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
Provläsningsexemplar / Preview. First edition
 Provläsningsexemplar / Preview TECHNICAL SPECIFICATION ISO/TS 19930 First edition 2017-12 Guidance on aspects of a risk-based approach to assuring sterility of terminally sterilized, single-use health
Provläsningsexemplar / Preview TECHNICAL SPECIFICATION ISO/TS 19930 First edition 2017-12 Guidance on aspects of a risk-based approach to assuring sterility of terminally sterilized, single-use health
Needle Valves. We deliver confidence through quality products
 We deliver confidence through quality products INTRODUCTION NEEDLE VALVES SD range of Needle valves come in various sizes, sealing styles, end connections and stem types. They are available in bar stock
We deliver confidence through quality products INTRODUCTION NEEDLE VALVES SD range of Needle valves come in various sizes, sealing styles, end connections and stem types. They are available in bar stock
Fundamentals of Flushing and Locking
 Fundamentals of Flushing and Locking Vascular Access Devices Fundamentals of Flushing and Locking Purpose, Product, and Process 2016 BD. BD and the BD Logo are trademarks of Becton, Dickinson and Company.
Fundamentals of Flushing and Locking Vascular Access Devices Fundamentals of Flushing and Locking Purpose, Product, and Process 2016 BD. BD and the BD Logo are trademarks of Becton, Dickinson and Company.
CLICK SEAL SYSTEM. Thermo Scientific Samco Clicktainer Vial. Unique click seal system providing sample protection and user safety
 LEAKPROOF 95KPA TESTED CE MARK CLICK SEAL SYSTEM Thermo Scientific Samco Clicktainer Vial Unique click seal system providing sample protection and user safety Thermo Scientific Samco Clicktainer Vial Providing
LEAKPROOF 95KPA TESTED CE MARK CLICK SEAL SYSTEM Thermo Scientific Samco Clicktainer Vial Unique click seal system providing sample protection and user safety Thermo Scientific Samco Clicktainer Vial Providing
ASEPT System ASEPT Pleural/Peritoneal. ASEPT Drainage Kits (600 ml ml) Accessories. Quality and Experience
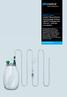 Quality and Experience ASEPT System ASEPT Pleural/Peritoneal Drainage System ASEPT Drainage Kits (600 ml + 000 ml) Accessories Recurring pleural effusions or malignant ascites can be treated on an outpatient
Quality and Experience ASEPT System ASEPT Pleural/Peritoneal Drainage System ASEPT Drainage Kits (600 ml + 000 ml) Accessories Recurring pleural effusions or malignant ascites can be treated on an outpatient
Product Catalog 2016 EMEA
 Product Catalog EMEA 2016 PORTFOLIO SOL-M Standard medical devices SOL-GUARD Basic safety medical devices SOL-CARE Innovative and advanced safety-engineered medical devices Sol-Millennium Europe Sp. z
Product Catalog EMEA 2016 PORTFOLIO SOL-M Standard medical devices SOL-GUARD Basic safety medical devices SOL-CARE Innovative and advanced safety-engineered medical devices Sol-Millennium Europe Sp. z
