Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
|
|
|
- Lilian Gibbs
- 5 years ago
- Views:
Transcription
1 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for the insertion of 5.0 French or larger central venous catheters (single and multilumen) through the use of a 20 gage extra-thin wall access needle and a specially designed.025 inch diameter Guide Wire using percutaneous entry (Seldinger) technique. All Minipuncture central venous catheters have a removable inner cannula, which must be removed after introduction. Micropuncture Access Sets And Trays: Used for the insertion of central venous catheters (single and multi-lumen) through the use of a 22gage access needle and a.018 inch diameter wire guide using percutaneous entry (Seldinger) technique. Most catheters for adult applications have a removable inner cannula, which must be removed after introduction. Intended Use The central venous catheter is designed for treatment of critically ill patients and is suggested for: 1. Continuous or intermittent drug infusions 2. Central venous blood pressure monitoring (CVP) 3. Acute hyperalimentation 4. Blood sampling 5. Delivery of whole blood or blood products 6. Simultaneous, separate infusion of drugs Contradictions Do not use heparin-coated central venous catheter in patient with a history of heparin-induced thrombocytopenia. Warnings Complications arising from the use of this device can result in serious injury or death; catheter tip can erode or perforate vascular walls. If heparin-induced thrombocytopenia is suspected, the heparin-coated catheter should be immediately removed. Every effort must be made to ascertain proper tip position in order to prevent erosion or perforation of central venous system. Tip position should be verified by xray and monitored on a routine basis. Periodic lateral view x-ray is suggested to assess tip location in relation to vessel wall. Tip position should appear to be parallel to vessel wall. To avoid vascular injury, do not use excessive force when advancing dilators. Use the smallest size dilator catheter placement will allow. Wire guide must always lead dilator by several centimeters. Do not advance dilator more than a few centimeters into the vessel. Do not power inject contrast medium through catheter. Catheter rupture may result. Use of 5 ml or Unauthorized Copying Prohibited Page 1 of 5
2 larger syringe will reduce the risk of catheter rupture. To distend great vessels and to prevent inadvertent air aspiration during catheter insertion, patient should be placed in trendelenburg position. Development of a hypersensitivity reaction should be followed by removal of the catheter and appropriate treatment at the discretion of the attending physician Sterile unless package opened or damaged Single use only. Do not reuse.reuse of the product might lead to infection.the catheter tip might kink. Disposable discard after use Sterilized by ethylene oxide Standard Interventional Procedure should be followed while inserting the catheter Precautions The catheter is intended for use by physicians trained and experienced in the placement of central Venous catheters using percutaneous entry (Seldinger) technique. Standard Seldinger technique for placement of percutaneous vascular access sheaths, catheters and wire guides should be employed during the placement of a central venous catheter. Do not re-sterilize catheter. Do not cut, trim or modify catheter or components prior to placement or intraoperatively. Patient movement can cause catheter tip displacement. Use should be limited to controlled hospital situations. Catheters placed via an antecubital vein have shown forward tip movement of up to 10 cm with motion of the extremity. Catheters placed from either a jugular or subclavian vein have demonstrated forward tip movement of 1-3 cm with neck and shoulder motion. Catheter should not be used for long-term indwelling Applications. If lumen flow is impeded, do not force injection or withdrawal of fluids. Notify attending physician immediately. Catheter should not be used for chronic hyperalimentation. Select puncture site and length of catheter needed by assessing patient anatomy and condition. Left subclavian and left jugular veins should be used only when other sites are not available Use of ECG, ultrasound and/or fluoroscopy is suggested for accurate catheter placement. PRODUCT RECOMMENDATIONS Catheter Size and Puncture Site: Preliminary reports indicate that catheter size can influence clotting; larger diameter catheters have more tendencies to promote clots. As reported by Amplatz and others, clot formation has less relation to type of catheter material than to size of catheter. The angle of the catheter tip to the vessel wall should be checked carefully. Blackshear reviewed the medical literature of catheter perforations, which have confirming x-rays, and found that an incident angle of the catheter to the vessel wall greater than 40 degrees was more likely to perforate. Another critical factor that can cause a catastrophic event is the choice of puncture site. Findings by Tocino and Watanabe indicate that the left subclavin and left jugular veins should be avoided when practical. Eighty percent of the perforations or erosions were found when these vessels were used. In addition, they have observed that the tip curve of a wedged Unauthorized Copying Prohibited Page 2 of 5
3 catheter can be detected with lateral view x-ray. The above discussion is meant to be a guide for catheter size and puncture site. As more data become available, other causal factors may become evident, but present information suggests that: The catheter size should be as small as the use will allow. Left subclavin and left jugular veins should be used only when other sites are not available. The following variables should be considered when selecting appropriate catheter and length: Patient history Size and age of patient Access site available Unusual anatomical variables Proposed use and duration of treatment plan Suggested Lumen Utilization: Double-Lumen: Distal exit port (end hole) whole blood or blood product delivery and sampling; any situation requiring more flow rate; CVP monitoring; medication delivery. It is strongly recommended that this lumen be used for all blood sampling. Proximal exit port medication delivery; acute hyperalimentation. Suggested Lumen Utilization: Triple-Lumen: Distal exit port (endhole) whole blood or blood product delivery and sampling; any situation requiring more flow rate; CVP monitoring; medication delivery. It is strongly recommended that this lumen be used for all blood sampling. Middle exit port medication delivery; acute hyperalimentation. proximal exit port medication delivery. NOTE: This catheter is not recommended for right atrial air aspiration because of inadequate side port surface area. Suggested Catheter Maintenance: Catheter entry site must be prepared and maintained in a manner consistent with standard procedure for central venous catheterization. To prevent clotting or possibility of air embolus, the double-lumen s #2 lumen, and the triple-lumen s #2 and #3 lumens should be filled with heparinized saline solution (100 units of heparin per cc of saline is usually adequate) prior to catheter introduction. After catheter is placed and prior to use, tip position and lumen patency should be confirmed by free aspiration of venous blood. If blood is not freely aspirated, physician should immediately reevaluate catheter tip position. Any unused lumens should be maintained with continuous saline or heparinized saline drip or locked with heparinized saline solution. Heparin-locked lumens should be reestablished at least every 8 hours. Before using any lumen already locked with heparin, lumen should be flushed with twice the indicated lumen volume using normal saline. Lumens should be flushed with normal saline between administrations of different infusates. After use, lumen should again be flushed with twice the indicated lumen volume using normal saline before reestablishing heparin lock. Strict aseptic technique must be adhered to while using and maintaining catheter. Unauthorized Copying Prohibited Page 3 of 5
4 Access Sites of Choice 1. High Internal Jugular 2. External Jugular 3. Low Internal Jugular 4. Supraclavicular 5. Infraclavicular 6. Femoral Directions for Use 1. Prepare the catheter for insertion by flushing each of the lumens and clamping or attaching the injection caps to the appropriate extensions. Leave the distal extension uncapped for wire guide passage. 2. Introduce thin wall percutaneous entry needle into vessel. Venous blood should beeasily aspirated to confirm position of needle tip within vessel. 3.Slide wire guide straightener (positioned on distal tip of wire guide) over J portion of wire guide. Pass straightened wire guide through needle; advance wire guide 5-10 cm into vessel. If straight wire is used, always advance soft, flexible end through needle hub and into vessel. If resistance is encountered during wire guide insertion, do not force wire guide. Withdrawal of wire guide through needle should be avoided; breakage may result. 4. While maintaining wire guide position, withdraw needle and wire guide straightener 5. Enlarge puncture site with number 11 scalpel blade, if required. If dilation is required, dilator can be advanced over wire guide and removed prior to insertion of central venous catheter. CAUTION: To avoid vascular injury, do not use excessive force when advancing dilators. Use the smallest size dilator catheter placement will allow. Wire guide must always lead dilator by several centimeters. Do not advance dilator more than a few centimeters into the vessel. 6. Measure catheter to be used against patient to determine approximate length of catheter needed from puncture site to central venous tip position. 7. Introduce the central venous catheter over wire guide. While maintaining wire guide position, advance catheter into vessel with a gentle twisting motion. NOTE: Do not advance catheter tip beyond distal tip of wire guide. Always have wire guide leading during catheter placement. Verify catheter tip position using radiography or appropriate technology. In order to guarantee extra pericardial location, the catheter tip should be located above the SVC-RA junction, within the lower 1/3 of the SVC.Every effort must be made to ascertain proper tip position in order to prevent erosion or perforation of the central venous system and to ensure proper delivery of infusates. 8. After catheter is in position, remove wire guide. Venous blood should be easily aspirated. Winged hub can now be sutured into place. If catheter is not introduced to its full length, additional suture should be carefully placed around catheter and affixed to the skin at entry site if movable suture wing is not included. This will help prevent backward or forward catheter movement. lumens should now be flushed with 5-10 cc normal saline prior to use or establishment of heparin lock. Unauthorized Copying Prohibited Page 4 of 5
5 NOTE: A wire guide that is at least twice as long as the catheter is recommended for catheter exchange procedure The technique for CVC insertion is the same for single, double, and triple lumen catheters as well as dialysis lines. BIORAD MEDISYS PVT. LTD. "Biorad House", No. 660, Eshwari Industrial Estate, Hulimavu Bannerghata Road, Bangalore , India. Customer Care: , Telefax: /4 biorad@bioradmedisys.com, Website: Labeling Symbols The following is an explanation of symbols that may be used on your product packaging Consult instructions for use Keep Away from Sunlight Lot Number Date of Manufacture Use By Keep dry Do not Resterilize Temperature Limit Do not re-use Sterilized using Ethylene Oxide Do not use if Package is Damaged Manufacturer Unauthorized Copying Prohibited Page 5 of 5
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Groshong* PICC and Catheters
 Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Mary Lou Garey MSN EMT-P MedFlight of Ohio
 Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
ompanionport Speciality Medical Devices For The Veterinary Community Surgical Suggestions
 Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
Polyurethane Radiology PICC
 Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Central Venous Line Insertion
 Central Venous Line Insertion Understand the indications and risks of CVC insertion Understand and troubleshoot the seldinger technique Understand available sites and select the appropriate site for clinical
Central Venous Line Insertion Understand the indications and risks of CVC insertion Understand and troubleshoot the seldinger technique Understand available sites and select the appropriate site for clinical
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Sterile Technique & IJ/Femoral Return Demonstration
 Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
PRODUCTS FOR THE DIFFICULT AIRWAY. Courtesy of Cook Critical Care
 PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
ICY Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC-3893A/
 Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
Radiology Polyurethane Catheter
 Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
Transjugular liver access and biopsy
 Transjugular liver access and biopsy An illustrated guide Illustrations by Lisa Clark LABS LIVER ACCESS AND BIOPSY SET MEDICAL Support your procedures by using products that are specifically designed for
Transjugular liver access and biopsy An illustrated guide Illustrations by Lisa Clark LABS LIVER ACCESS AND BIOPSY SET MEDICAL Support your procedures by using products that are specifically designed for
Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer
 Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
Quattro Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC4593/
 Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
i INTERACTIVE CATALOG FIND YOUR IDEAL CANNULAE
 i INTERACTIVE CATALOG FIND YOUR IDEAL CANNULAE Catalog 2017 i USER TIPS Easily navigate through the pages in our 2017 Cannula Catalog by using the mini-menu shown on upper right of each page. Navigate
i INTERACTIVE CATALOG FIND YOUR IDEAL CANNULAE Catalog 2017 i USER TIPS Easily navigate through the pages in our 2017 Cannula Catalog by using the mini-menu shown on upper right of each page. Navigate
Advancing Lives and the Delivery of Health Care. The High-Flow Port Designed for Apheresis
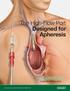 Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE
 Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Advocate Christ Medical Center CVC Placement Certification Course
 Advocate Christ Medical Center CVC Placement Certification Course July 12th, 2012 Hannah Watts, MD Medical Simulation Director Modified August 10, 2017 Taajwar Khan, MD Chief Resident of Internal Medicine
Advocate Christ Medical Center CVC Placement Certification Course July 12th, 2012 Hannah Watts, MD Medical Simulation Director Modified August 10, 2017 Taajwar Khan, MD Chief Resident of Internal Medicine
Xcela Hybrid PICC with PASV Valve Technology
 Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Port Design. Page 1. Port Placement, Removal, and Management. Selecting a Vascular Access Device. Thomas M. Vesely, MD
 Non-Dialysis Procedures Port Placement, Removal, and Management Thomas M. Vesely, MD Saint Louis, Missouri Selecting a Vascular Access Device Duration of use Number of lumens Frequency used Blood flow
Non-Dialysis Procedures Port Placement, Removal, and Management Thomas M. Vesely, MD Saint Louis, Missouri Selecting a Vascular Access Device Duration of use Number of lumens Frequency used Blood flow
Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore
 CENTRAL VENOUS CATHETERIZATION Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore OBJECTIVES Introduction Indications and Contraindications Complications Technique Basic principles Specifics by Site
CENTRAL VENOUS CATHETERIZATION Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore OBJECTIVES Introduction Indications and Contraindications Complications Technique Basic principles Specifics by Site
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
Optimize your hemodialysis procedural bundle.
 Optimize your hemodialysis procedural bundle. Cook Turbo-Flo HD ACUTE HEMODIALYSIS CATHETER SETS AND TRAYS Featuring Sof-Flex catheter material for balanced performance and feel. MEDICAL Choose from two
Optimize your hemodialysis procedural bundle. Cook Turbo-Flo HD ACUTE HEMODIALYSIS CATHETER SETS AND TRAYS Featuring Sof-Flex catheter material for balanced performance and feel. MEDICAL Choose from two
ARROW ENDURANCE. Extended Dwell Peripheral Catheter System. Rx only.
 ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS)
 Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Peripherally Inserted Central Catheter (PICC) Booklet
 Aintree University Hospital FT PICC Booklet: a real world example This local booklet is an example used in the NICE medical technology guidance adoption support resource for SecurAcath for securing percutaneous
Aintree University Hospital FT PICC Booklet: a real world example This local booklet is an example used in the NICE medical technology guidance adoption support resource for SecurAcath for securing percutaneous
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Instructions For Use. Implantable Ports. with Open-Ended and Groshong Catheters
 Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
Central Venous Access Devices. Stephanie Cunningham Amy Waters
 Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
PTA Balloon Dilatation Catheter 1/6(3211-0) February 2016 TIS-488-06272017 Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions
A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS
 A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS MAHURKAR * Elite acute dialysis catheter family EFFICIENCY OF DIALYSIS IN REVERSE FLOW Less than one percent recirculation Symmetrical tip design minimizes
A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS MAHURKAR * Elite acute dialysis catheter family EFFICIENCY OF DIALYSIS IN REVERSE FLOW Less than one percent recirculation Symmetrical tip design minimizes
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE
 LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement
 Title/Description: Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement Department: Patient Care Services Personnel: Nursing Services Effective Date: April
Title/Description: Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement Department: Patient Care Services Personnel: Nursing Services Effective Date: April
Hospital of the University of Pennsylvania NURSING. Insertion of Peripherally Inserted Central Catheter (PICC) and Midline Catheter (MLC)
 Page 1 of 11 KEYWORDS: Central Catheter Sterility REFER TO: 4B-02-09 Nursing Management of the Patient with a Central Venous Access Device HUP 1-12-24 Consent to Health Care Services SCOPE Registered Nurses
Page 1 of 11 KEYWORDS: Central Catheter Sterility REFER TO: 4B-02-09 Nursing Management of the Patient with a Central Venous Access Device HUP 1-12-24 Consent to Health Care Services SCOPE Registered Nurses
The High-Flow Port Designed & Indicated for Apheresis
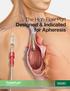 The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Exceptional Performance and Ease of Placement
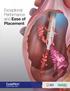 Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE
 DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
Central Line Care and Management
 Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
BARD. Instructions For Use
 BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use. Directions for Use. Contents
 NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Fundamentals of Flushing and Locking
 Fundamentals of Flushing and Locking Vascular Access Devices Fundamentals of Flushing and Locking Purpose, Product, and Process 2016 BD. BD and the BD Logo are trademarks of Becton, Dickinson and Company.
Fundamentals of Flushing and Locking Vascular Access Devices Fundamentals of Flushing and Locking Purpose, Product, and Process 2016 BD. BD and the BD Logo are trademarks of Becton, Dickinson and Company.
The University of Toledo Medical Center and its Medical Staff
 Name of Policy: Policy Number: Department: 3364-109-GEN-705 Infection Control Medical Staff Hospital Administration Approving Officer: Responsible Agent: Scope: Chair, Infection Control Committee Chief
Name of Policy: Policy Number: Department: 3364-109-GEN-705 Infection Control Medical Staff Hospital Administration Approving Officer: Responsible Agent: Scope: Chair, Infection Control Committee Chief
Pressure Injectable PICC Product. Venous Access Critical Care
 Pressure Injectable PICC Product Venous Access Critical Care Symbol Legend: Symbols and definitions of symbols are provided for reference. Some symbols may not apply to this product. Refer to product labeling
Pressure Injectable PICC Product Venous Access Critical Care Symbol Legend: Symbols and definitions of symbols are provided for reference. Some symbols may not apply to this product. Refer to product labeling
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Polyurethane Catheter
 References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
Lifecath Twin & Dualyse/Trilyse Permanent and Temporary Renal Catheters
 Lifecath Twin & Dualyse/Trilyse Permanent and Temporary Renal Catheters Accurate locking dose Excellent flow rates Safe connections vygon@vygon.co.uk www.vygon.co.uk Product Information Product Information
Lifecath Twin & Dualyse/Trilyse Permanent and Temporary Renal Catheters Accurate locking dose Excellent flow rates Safe connections vygon@vygon.co.uk www.vygon.co.uk Product Information Product Information
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Central Venous Catheter Insertion: Assisting
 Approved by: Central Venous Catheter Insertion: Assisting Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Santiago Ensenat Medical Director, Neonatology Neonatal
Approved by: Central Venous Catheter Insertion: Assisting Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Santiago Ensenat Medical Director, Neonatology Neonatal
Groshong Central Venous Catheters
 Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Catheters and Wires. Dr. Vaibhav Jain MD,DNB,MNAMS Senior Consultant, IR Medanta, the Medicity, Gurgaon
 Catheters and Wires Dr. Vaibhav Jain MD,DNB,MNAMS Senior Consultant, IR Medanta, the Medicity, Gurgaon Guidewires: Guidewires (solid wires navigated within the vascular system / extravascular tract) act
Catheters and Wires Dr. Vaibhav Jain MD,DNB,MNAMS Senior Consultant, IR Medanta, the Medicity, Gurgaon Guidewires: Guidewires (solid wires navigated within the vascular system / extravascular tract) act
FISH device S P. morris INNOVATIVE. SHEATH PROTECTED Femoral Introducer Sheath & Hemostasis Device
 SYSTEM COMPONENTS / DESCRIPTION The Femoral Introducer Sheath and Hemo stasis Device (FISH DEVICE) facilitates percutaneous entry of an intravascular device and aids in reducing time to hemostasis and
SYSTEM COMPONENTS / DESCRIPTION The Femoral Introducer Sheath and Hemo stasis Device (FISH DEVICE) facilitates percutaneous entry of an intravascular device and aids in reducing time to hemostasis and
Zenith Alpha T HORACIC ENDOVASCULAR GRAFT
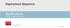 Deployment Sequence Zenith Alpha T HORACIC ENDOVASCULAR GRAFT www.cookmedical.com AI-D21183-EN-F Preparation and flush Proximal and distal components Remove the yellow hubbed inner stylet from the dilator
Deployment Sequence Zenith Alpha T HORACIC ENDOVASCULAR GRAFT www.cookmedical.com AI-D21183-EN-F Preparation and flush Proximal and distal components Remove the yellow hubbed inner stylet from the dilator
Phoenix Atherectomy System Product Overview /LB
 Phoenix Atherectomy System Product Overview Phoenix Atherectomy System- The next generation of atherectomy The first hybrid atherectomy system available Combines the benefits of existing atherectomy systems
Phoenix Atherectomy System Product Overview Phoenix Atherectomy System- The next generation of atherectomy The first hybrid atherectomy system available Combines the benefits of existing atherectomy systems
Kristin Wise, MD, FHM Division of General Internal Medicine and Geriatrics Hospital Medicine 2013
 Kristin Wise, MD, FHM Division of General Internal Medicine and Geriatrics Hospital Medicine 2013 Objectives for CVC Placement Understand the indications and contraindications Determine appropriate CVC
Kristin Wise, MD, FHM Division of General Internal Medicine and Geriatrics Hospital Medicine 2013 Objectives for CVC Placement Understand the indications and contraindications Determine appropriate CVC
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT
 PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
Designed to Reduce the Risk of Occlusion (1)
 Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
The Ceterix NovoStitch Disposable Suture Passer. Do Not Reuse
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
Research Article Can we predict the Position of Central Venous Catheter Tip Following Cannulation of Internal Jugular Vein?
 Cronicon OPEN ACCESS ANAESTHESIA Research Article Can we predict the Position of Central Venous Catheter Tip Following Cannulation of Internal Jugular Vein? Pradeep Marur Venkategowda 1, Surath Manimala
Cronicon OPEN ACCESS ANAESTHESIA Research Article Can we predict the Position of Central Venous Catheter Tip Following Cannulation of Internal Jugular Vein? Pradeep Marur Venkategowda 1, Surath Manimala
Appendix E: Overview of Vascular
 Appendix E: Overview of Vascular 56 Peripheral Short Catheter, less than 3 inches (7.5 cm) in length; over-the-needle catheter is most common. Inserted by percutaneous venipuncture, generally into a hand
Appendix E: Overview of Vascular 56 Peripheral Short Catheter, less than 3 inches (7.5 cm) in length; over-the-needle catheter is most common. Inserted by percutaneous venipuncture, generally into a hand
Overview of CVADs. Type of device commonly used. Dwell time Flushing requirement Associated complications. lumens
 Source: Clinical Skills Management of Vascular Access Devices Pre-course handbook. Adapted with permission from NHS Lothian Employee and Education Development Team. Overview of CVADs Type of device Veins
Source: Clinical Skills Management of Vascular Access Devices Pre-course handbook. Adapted with permission from NHS Lothian Employee and Education Development Team. Overview of CVADs Type of device Veins
Navigating Vascular Access Issues
 Navigating Vascular Access Issues The Oley Foundation 27 th Annual Consumer/Clinician Conference Redondo Beach, CA June, 27 2012 Anita Piano, BS, RN, VA-BC Administrative Nurse, PICC Service UCLA Health
Navigating Vascular Access Issues The Oley Foundation 27 th Annual Consumer/Clinician Conference Redondo Beach, CA June, 27 2012 Anita Piano, BS, RN, VA-BC Administrative Nurse, PICC Service UCLA Health
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports
 Disclosures A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports No conflicts of interest relevant to this presentation Jason W. Pinchot,
Disclosures A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports No conflicts of interest relevant to this presentation Jason W. Pinchot,
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
ENGLISH SHORT TERM HAEMODIALYSIS CATHETER
 SHORT TERM HAEMODIALYSIS CATHETER Guides & Instruction for use Intended use: Sterile single use device indicated for use in attaining short term access for Hemodialysis or aphaeresis. How supplied: The
SHORT TERM HAEMODIALYSIS CATHETER Guides & Instruction for use Intended use: Sterile single use device indicated for use in attaining short term access for Hemodialysis or aphaeresis. How supplied: The
Over the Wire Technique vs. Modified Seldinger Technique in Insertion of PICC
 Over the Wire Technique vs. Modified Seldinger Technique in Insertion of PICC Deniz Kasikci Department of Radiology, Jena University Hospital Friedrich-Schiller-University, Jena, Germany Disclosure Speaker
Over the Wire Technique vs. Modified Seldinger Technique in Insertion of PICC Deniz Kasikci Department of Radiology, Jena University Hospital Friedrich-Schiller-University, Jena, Germany Disclosure Speaker
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Protecting Patients. Preserving Access.
 MAHURKAR * Family of Acute Care Catheters Protecting Patients. Preserving Access. A full spectrum of acute care catheters for use in hemodialysis, apheresis, infusion and renal replacement therapy The
MAHURKAR * Family of Acute Care Catheters Protecting Patients. Preserving Access. A full spectrum of acute care catheters for use in hemodialysis, apheresis, infusion and renal replacement therapy The
Vascular access device selection & placement. Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University
 Vascular access device selection & placement Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University How to make the right choice of vascular access device.. Peripheral
Vascular access device selection & placement Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University How to make the right choice of vascular access device.. Peripheral
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices
 Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER. Instructions for Use
 Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Z-MED II BALLOON AORTIC AND PULMONIC VALVULOPLASTY CATHETER Instructions for Use CAUTION: Federal (USA) Law restricts this device to sale by or on the order of a physician. Manufactured for: B. Braun Interventional
Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully
 Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
Physician Training Bifurcated system Proximal suprarenal stent Modular (aortic main body and two iliac legs) Full thickness woven polyester graft material Fully supported by self-expanding z-stents H&L-B
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
