Hospital Pharmaceuticals Review PTAC, Hospital Pharmaceuticals Subcommittee & Respiratory Subcommittee minutes for web publishing
|
|
|
- Moses Stokes
- 5 years ago
- Views:
Transcription
1 Hospital Pharmaceuticals Review PTAC, Hospital Pharmaceuticals Subcommittee & Respiratory Subcommittee minutes for web publishing Respiratory System and Allergies therapeutic group PTAC and Subcommittee of PTAC minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics Advisory Committee (PTAC) and PTAC Subcommittees This document contains minutes relevant to the consultation document of 25 September 2012 relating to products in the Respiratory System and Allergies therapeutic group. Note that this document is not a complete record of the relevant PTAC and Subcommittee meetings; only the relevant portions of the minutes relating PTAC and its Subcommittees advice on the review of Hospital Pharmaceuticals are included. Contents Hospital Pharmaceuticals Subcommittee 5 July Respiratory Subcommittee 16 February Hospital Pharmaceuticals Subcommittee 1 May Pharmacology and Therapeutics Advisory Committee 10 & 11 May A
2 Hospital Pharmaceuticals Subcommittee 5 July Antiallergy Preparations 1.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Antiallergy Preparations heading. 1.2 The Subcommittee noted that the following pharmaceuticals are commonly used in Bee venom allergy treatments - Maintenance kit - 6 vials 120 µg freeze dried venom, 6 diluent 1.8 ml - Treatment kit - 1 vial 550 µg freeze dried venom, 1 diluent 9 ml, 3 diluent 1.8 ml Wasp venom allergy treatment - Treatment kit (paper wasp venom) - 1 vial 500 µg freeze dried polister venom, 1 diluent 9 ml, 1 diluent 1.8 ml - Treatment kit (yellow jacket venom) - 1 vial 550 µg freeze dried vespula venom, 1 diluent 9 ml, 1 diluent 1.8 ml Beclomethasone dipropionate - Aqueous nasal spray 50 µg per dose - Aqueous nasal spray 100 µg per dose Budesonide - Aqueous nasal spray 50 µg per dose - Aqueous nasal spray 100 µg per dose Fluticasone propionate - Aqueous nasal spray 50 µg per dose Ipratropium bromide - Aqueous nasal spray 0.03% Sodium cromoglycate - Aqueous nasal spray 4% Cetirizine hydrochloride - Oral liq 1 mg per ml - Tab 10 mg Chlorpheniramine maleate - Inj 10 mg per ml, 1 ml ampoule - Oral liq 2 mg per 5 ml Cyproheptadine hydrochloride - Tab 4 mg Fexofenadine hydrochloride - Tab 60 mg - Tab 120 mg - Tab 180 mg Loratadine - Oral liq 1 mg per ml - Tab 10 mg Promethazine hydrochloride - Inj 25 mg per ml, 2 ml ampoule A
3 - Oral liq 5 mg per 5 ml - Tab 10 mg - Tab 25 mg Trimeprazine tartrate - Oral liq 30 mg per 5 ml 1.3 The Subcommittee recommended that the listing of bee and wasp venom allergy kits in a national PML be subject to restrictions on their use that are in line with the Special Authority restrictions for them in the Pharmaceutical Schedule. 1.4 The Subcommittee noted that allergen desensitisation kits for substances other than bee or wasp venom were not widely used in DHB hospitals, and were not subsidised in the Pharmaceutical Schedule, and recommended that they not be included in a national PML. Members noted that separate to the issue of desensitisation kits are allergy testing kits, and considered that these should remain available in hospitals. 1.5 The Subcommittee noted that omalizumab is not widely used in DHB hospitals, and noted that the Respiratory Subcommittee had previously recommended against its listing in the Pharmaceutical Schedule. The Subcommittee recommended that it not be included in a national PML. 1.6 The Subcommittee noted that there is some use of adrenaline auto-injectors by DHB hospital staff, such as district nurses and carers in children s wards. The Subcommittee considered that as these are not funded in the Pharmaceutical Schedule, listing in a national PML could not be justified, as inclusion in a national PML would result in de facto community funding. The Subcommittee recommended that adrenaline auto-injectors not be included in a national PML, and considered that current use by hospital staff could be replaced by ampoules or prefilled syringes and adequate training. 2 Anticholinergic Agents 2.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Anticholinergic Agents heading. 2.2 The Subcommittee noted that the following pharmaceuticals are commonly used in Ipratropium bromide - Aerosol inhaler 20 µg per dose - Nebuliser soln 250 µg per ml, 1 ml - Nebuliser soln 250 µg per ml, 2 ml Tiotropium bromide - Powder for inhalation 18 µg per dose Salbutamol with ipratropium bromide - Aerosol inhaler 100 µg with ipratropium bromide 20 µg per dose - Nebuliser soln 2.5 mg with ipratropium bromide 0.5 mg per 2.5 ml vial 2.3 The Subcommittee recommended that the listing of tiotropium in a national PML be subject to restrictions on its use that are in line with the Special Authority restriction for it in the Pharmaceutical Schedule. A
4 3 Beta-Adrenoceptor Agonists 3.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Beta-Adrenoceptor Agonists heading. 3.2 The Subcommittee noted that the following pharmaceuticals are commonly used in Salbutamol - Aerosol inhaler, 100 µg per dose - Infusion 1 mg per ml, 5 ml ampoule - Inj 500 µg per ml, 1 ml ampoule - Nebuliser soln 1 mg per ml, 2.5 ml - Nebuliser soln 2 mg per ml, 2.5 ml - Oral liq 2 mg per 5 ml Terbutaline sulphate - Powder for inhalation 250 µg per dose - Inj 0.5 mg per ml, 1 ml amp 3.3 Members noted that one DHB had reported using terbutaline nebuliser solution for a patient with salbutamol allergy. The Subcommittee considered that this use should be managed through an exceptions mechanism, rather than by listing on a national PML. 4 Cough Suppressants 4.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Cough Suppressants heading. 4.2 The Subcommittee considered that there was limited evidence in support of cough suppressants, and noted that none are subsidised in the Pharmaceutical Schedule. Members noted that in the palliative care setting it was likely that strong opioids would be used for this purpose rather than using lower strength over-the-counter opioid containing cough suppressants. 4.3 The Subcommittee considered that excluding all cough suppressants from a national PML could be difficult, and recommended that the most commonly used product, pholcodine oral liq 1 mg per ml, be included in a national PML. 4.4 The Subcommittee recommended that dextromethorphan, diphenhydramine, opiate squill and 2 mg per ml and 3 mg per ml strengths of pholcodine be excluded from a national PML. 4.5 The Subcommittee noted that there may be some use of dextromethorphan in the surgical setting, and requested that the view of anaesthetists be sought on its exclusion. 5 Decongestants 5.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Decongestants heading. A
5 5.2 The Subcommittee noted that the following pharmaceuticals are commonly used in Oxymetazoline hydrochloride - Aqueous nasal spray 0.25 mg per ml - Aqueous nasal spray 0.5 mg per ml Pseudoephedrine hydrochloride - Tab 60 mg Sodium chloride - Aqueous nasal spray 6.5 mg per ml Sodium chloride with sodium bicarbonate - Soln for nasal irrigation Xylometazoline hydrochloride - Aqueous nasal spray 0.05% - Aqueous nasal spray 0.1% - Nasal drops 0.05% - Nasal drops 0.1% 5.3 Members noted that sodium chloride nasal spray is generally used for compounding with other products, not as a therapeutic product in its own right. The Subcommittee considered that it was not necessary for the other strength of this product (7.4 mg per ml) to be included in a national PML. 5.4 Members noted that different packs of sodium chloride with sodium bicarbonate nasal irrigation (NeilMed Sinus Rinse) are available, and that the starter kit is the one that is predominantly used and should be listed. 5.5 The Subcommittee noted that while xylometazoline is more widely used than oxymetazoline, and is also a less expensive agent, having both agents on a national PML would provide a backup in the event of long-term supply issues, which have occurred previously. 5.6 The Subcommittee considered that, as menthol capsules are not widely used in DHB hospitals, and are not subsidised in the Pharmaceutical Schedule, they did not need to be included in a national PML 5.7 The Subcommittee considered that, as dexchlorpheniramine maleate is not widely used in DHB hospitals, and is not fully subsidised in the Pharmaceutical Schedule, it did not need to be included in a national PML. 6 Inhaled Corticosteroids 6.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Inhaled Corticosteroids heading. 6.2 The Subcommittee noted that the following pharmaceuticals are commonly used in Beclomethasone dipropionate A
6 - Aerosol inhaler 50 µg per dose - Aerosol inhaler 100 µg per dose - Aerosol inhaler 250 µg per dose Budesonide - Powder for inhalation 100 µg per dose - Powder for inhalation 200 µg per dose - Powder for inhalation 400 µg per dose Fluticasone - Aerosol inhaler 50 µg per dose - Aerosol inhaler 125 µg per dose - Aerosol inhaler 250 µg per dose - Powder for inhalation 50 µg per dose - Powder for inhalation 100 µg per dose - Powder for inhalation 250 µg per dose 6.3 The Subcommittee noted that there is some use of nebulised budesonide in DHB hospitals, and recommended that the 500 µg in 2 ml nebuliser solution should be included in a national PML, but that there was no need for the other presentation (1 mg in 2 ml) to also be included. The Subcommittee requested that the view of the Respiratory Subcommittee be sought on the need for budesonide nebuliser solution in a national PML. 7 Inhaled Long-Acting Beta-Adrenoceptor Agonists 7.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Inhaled Long-Acting Beta-Adrenoceptor Agonists heading. 7.2 The Subcommittee noted that the following pharmaceuticals are commonly used in Eformoterol fumarate - Powder for inhalation 6 µg per dose - Powder for inhalation 12 µg per dose Salmeterol - Aerosol inhaler 25 µg per dose - Powder for inhalation 50 µg per dose Budesonide with eformoterol - Aerosol inhaler 100 µg with eformoterol fumarate 6 µg - Aerosol inhaler 200 µg with eformoterol fumarate 6 µg - Powder for inhalation 100 µg with eformoterol fumarate 6 µg - Powder for inhalation 200 µg with eformoterol fumarate 6 µg - Powder for inhalation 400 µg with eformoterol fumarate 12 µg Fluticasone with salmeterol - Aerosol inhaler 50 µg with salmeterol 25 µg - Aerosol inhaler 125 µg with salmeterol 25 µg - Powder for inhalation 100 µg with salmeterol 50 µg - Powder for inhalation 250 µg with salmeterol 50 µg A
7 7.3 The Subcommittee recommended that the listing of budesonide with eformoterol and fluticasone with salmeterol in a national PML be subject to restrictions on their use that are in line with the Special Authority restriction for them in the Pharmaceutical Schedule. 7.4 The Subcommittee considered that, because the higher dose products of fluticasone with salmeterol (aerosol inhaler 250 µg with salmeterol 25 µg, powder for inhalation 500 µg with salmeterol 50 µg) were not subsidised in the Pharmaceutical Schedule, they should not be included in a national PML. 8 Leukotriene Receptor Antagonists 8.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to montelukast. 8.2 The Subcommittee recommended that, as montelukast is not subsidised in the Pharmaceutical Schedule, and as it does not have a unique use within hospitals, that it not be included in a national PML. 9 Mast Cell Stabilisers 9.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Mast Cell Stabilisers heading. 9.2 The Subcommittee noted that the following pharmaceuticals are commonly used in Nedocromil - Aerosol inhaler 2 mg per dose Sodium cromoglicate - Aerosol inhaler 5 mg per dose - Powder for inhalation 20 µg per dose 10 Methylxanthines 10.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Methylxanthines heading The Subcommittee noted that the following pharmaceuticals are commonly used in Aminophylline - Inj 25 mg per ml, 10 ml Theophylline - Oral liq 80 mg per 15 ml - Tab long-acting 250 mg A
8 10.3 The Subcommittee noted that one DHB had reported using aminophylline tablets. The Subcommittee recommended that, as these are not widely used in DHB hospitals, and as they are not subsidised in the Pharmaceutical Schedule, that they not be included in a national PML. 11 Mucolytics and Expectorants 11.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Mucolytics and Expectorants heading The Subcommittee noted that the following pharmaceuticals are commonly used in Bromhexine hydrochloride - Tab 8 mg - Oral liq 8 mg per 5 ml Dornase alfa - Nebuliser soln 2.5 mg per 2.5 ml ampoule Sodium chloride - Nebuliser soln 7% 11.3 The Subcommittee requested that the view of the Respiratory Subcommittee be sought on the benefits of bromhexine hydrochloride, and on the need for its inclusion in a national PML The Subcommittee recommended that the listing of dornase alfa in a national PML be subject to restrictions on its use that are in line with the restrictions for it in the Pharmaceutical Schedule The Subcommittee noted that one DHB had reported using a lower strength of bromhexine oral liquid (12 mg per 15 ml), but considered that this did not need to be included in a national PML The Subcommittee recommended that as guaifenesin and guaifenesin with bromhexine hydrochloride were not widely used in DHB hospitals, and as they are not subsidised in the Pharmaceutical Schedule, they not be included in a national PML. 12 Pulmonary Surfactants 12.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Pulmonary Surfactants heading The Subcommittee noted that the following pharmaceuticals are commonly used in Beractant - Soln 200 mg per 8 ml vial A
9 Poractant alfa - Soln 120 mg per 1.5 ml vial - Soln 240 mg per 3 ml vial 12.3 The Subcommittee noted that having two agents included would be useful from a supply security perspective, and also that some patients may be unable to take one or the other for religious reasons. 13 Respiratory Stimulants 13.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to products under the Respiratory Stimulants heading The Subcommittee noted that the following pharmaceuticals are commonly used in Caffeine citrate - Inj 20 mg per ml, 2.5 ml ampoule (caffeine base 10 mg per ml) - Oral liq 20 mg per ml (caffeine base 10 mg per ml) Doxapram - Inj 20 mg per ml, 5 ml vial 13.3 The Subcommittee noted that doxapram was an older product that was not widely used, and requested the view of anaesthetists and of the Respiratory Subcommittee on the use of it and the need for its inclusion in a national PML. 14 Sclerosing Agents 14.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to talc The Subcommittee noted that most DHB hospitals used sterile talc as a sclerosing agent, either as a powder or as a slurry (soln 100 mg per ml, 50 ml), and recommended that both be included in a national PML. 15 Tumour Necrosis Factor (TNF) Inhibitors 15.1 The Subcommittee reviewed the information from DHB hospitals and PHARMAC in relation to the use of infliximab for sarcoidosis The Subcommittee noted that infliximab is funded in Auckland DHB for use in treatment-resistant sarcoidosis, but not a formulary item in any other DHBs for this indication The Subcommittee noted that treatment of sarcoidosis is not an approved indication for infliximab, and that the evidence for its use is mostly limited to case reports. However, the Subcommittee noted the information that had previously been considered by the Auckland DHB Hospital Medicines Committee, and considered that in the case of life-threatening refractory disease, treatment with infliximab appeared to be reasonable. A
10 15.4 The Subcommittee recommended that infliximab be included in a national PML for the treatment of life-threatening sarcoidosis that is refractory to other treatments. A
11 Respiratory Subcommittee 16 February Hospital Pharmaceuticals 16.1 The Subcommittee noted a paper and presentation by PHARMAC staff outlining the developments and future initiatives planned in regards to PHARMAC finalising a national Preferred Medicines List (PML) and eventually taking responsibility for funding and procurement of DHB hospital medicines. The Subcommittee did not raise any concerns or issues in regards to this project The Subcommittee reviewed a series of recommendations by the Hospital Pharmaceuticals Subcommittee in regards to which pharmaceuticals relevant to respiratory medicine should be included on a national PML. The Subcommittee noted that PHARMAC had invited feedback from relevant colleges and professional societies, and noted the responses that were received. Anticholinergic agents 16.3 The Subcommittee noted and agreed with the recommendations in relation to anticholinergic agents. The Subcommittee commented that combination products such as salbutamol with ipratropium bromide are rarely used in hospitals, with prescribing of separate inhalers being preferred Beta-adrenoceptor agonists 16.4 The Subcommittee noted and agreed with the recommendations in relation to betaadrenoceptor agonists. The Subcommittee noted that while there is little evidence to support the use of salbutamol oral liquid in asthma, there appears to be some benefit from its use in children with neuromuscular disease as a strengthening agent. Cough suppressants 16.5 The Subcommittee noted that the Hospital Pharmaceutical Subcommittee had recommended that pholcodine oral liquid 1 mg per ml should be included in the PML. The Subcommittee considered that more than one product would not be required. The Subcommittee noted that cough preparations had been delisted from the Section B of the Pharmaceutical Schedule in The Subcommittee noted that other agents, such as codeine and morphine, can also be used as cough suppressants in appropriate circumstances. Decongestants 16.6 The Subcommittee noted and agreed with the recommendations in relation to decongestants. Inhaled corticosteroids 16.7 The Subcommittee noted the recommendations in relation to inhaled corticosteroids The Subcommittee noted that the Hospital Pharmaceuticals Subcommittee had recommended that one form of budesonide nebuliser solution (250 mcg per ml, 2 ml) be included in a national PML, but that a higher strength (500 mcg per ml, 2 ml) not be included. The Subcommittee considered that the nebulising solution was used in A
12 the treatment of croup and in the intensive care setting post extubation as an alternative to parenteral steroids The Subcommittee noted that clinicians may need to administer doses of up to 2 mg of nebulised budesonide, which would require 8 ml of the lower dose solution to be used, and as such having the both strengths of this product available would be an advantage Members noted that nebulised steroids would likely only be used if the treating physician was unable to administer parental dexamethasone (or other steroids) in a patient unable to tolerate oral preparations, this may be a problem in smaller hospitals with les specialised skill sets. Leukotriene receptor agonists The Subcommittee noted and agreed with the recommendations in relation to leukotriene receptor antagonists. Long-acting beta-adrenoceptor agonists The Subcommittee noted and agreed with the recommendations in relation to longacting beta-adrenoceptor agonists. Mast cell stabilisers The Subcommittee noted and agreed with the recommendations in relation to mast cell stabilisers. Methylxanthines The Subcommittee noted and agreed with the recommendations in relation to methylxanthines. Mucolytics and expectorants The Subcommittee noted the recommendations in relation to mucolytics and expectorants The Subcommittee noted that the Hospital Pharmaceuticals Subcommittee had recommended that some formulations of bromhexine hydrochloride be included in a national PML. The Subcommittee considered that there was insufficient evidence to support the inclusion of bromhexine in a national PML and that a Cochrane review shows that it has little or no benefit, and recommended that it not be included at this time. The Subcommittee noted that the product could be re-assessed at a later date if there were requests for it The Subcommittee noted the recommendation that dornase alfa should be listed in a national PML subject to the same access criteria that apply in the community. The Subcommittee noted that dornase alfa is sometime used in hospital to treat children experiencing a semi-acute cystic fibrosis exacerbation and may be used for up to 2 to 4 weeks Members considered that it would be important to provide access to dornase alfa in this acute situation, but that there would need to be established national criteria for A
13 such use. The Subcommittee recommended this issue be referred to the Cystic Fibrosis Panel for discussion, including a review of the evidence, and comment. Pulmonary surfactants The Subcommittee noted and agreed with the recommendations in relation to pulmonary surfactants. Respiratory stimulants The Subcommittee noted and agreed with the recommendations in relation to respiratory stimulants The Subcommittee noted that doxapram is reserved for infants born at more extreme prematurity who have been extubated and continue to have central apnoeas, despite continuous positive airway pressure and maximal caffeine, in whom neonatologists believe reventilation would represent a major setback. The Subcommittee considered that doxapram should be listed in a national PML with a high priority as it is used in tertiary units around the country and perhaps also in the higher-level secondary units such as Hawke s Bay. Sclerosing agents The Subcommittee noted and agreed with the recommendations in relation to respiratory stimulants The Subcommittee noted that talc had been recommended for inclusion, but that other agents were also used as sclerosing agents, such as bleomycin, doxycycline and tetracycline. Tumour necrosis factor (TNF) inhibitors The Subcommittee noted that the Hospital Pharmaceuticals Subcommittee has recommended that infliximab be funded for the treatment of life-threatening pulmonary sarcoidosis that is refractory to other treatments. The Hospital Pharmaceuticals Subcommittee noted that infliximab is funded in Auckland DHB for use in treatment-resistant pulmonary sarcoidosis but is not used in any other DHB The Subcommittee noted a January 2005 application from [a clinician] to Auckland DHB s Hospital Medicines Committee for funding of infliximab in the treatment of pulmonary sarcoidosis. The outcome of the application was that infliximab be added to the Auckland DHB formulary for the treatment of resistant pulmonary sarcoidosis restricted to prescribing by [that clinician] and supply of a report on each patient treated. The Subcommittee noted that [the clinician] is an expert in the treatment of sarcoidosis The subcommittee noted five clinical trials supplied by PHARMAC: Brougham et al (Am J Respir Crit Care Med 2006;174: ) was a phase II double blind, multicentre, placebo-controlled randomised clinical trial. 138 patients with chronic pulmonary sarcoidosis were randomised to receive intravenous infusions infliximab 3 or 5 mg/kg or placebo at weeks 0, 2, 6, 12, 18 and 24 with follow up to week 52. The primary 1 Name of clinician withheld under section 9(2)(a) of the Official information Act 1982 A
14 endpoint was the change from baseline to week 24 in percent of predicted FVC. There was a mean increase of 2.5% FVC in the combined infliximab groups at week 24 (statistically significant but of uncertain clinical significance). There was no change in functional secondary outcome such as SGRQ or Borg s dyspnea score at week 24. Pneumonia was more common in the infliximab patients at 7% vs 2%. Post hoc exploratory analysis suggested that patients with more severe disease tended to benefit more from infliximab treatment. A major criticism of the study is that patients were stable for at least one month prior to the initiation of treatment, potentially biasing a possible treatment effect, and had only moderate disease at best making extrapolation to severe treatment resistant patients difficult. Brougham et al (Chest 2009;136: ). This paper assessed the reproducibility of reading the chest roentgenogram from the 138 patients in the Am J Respir Crit Care Med 2006 study using three different evaluation methods: the Scadding staging system, the Muers scoring, and a global assessment of the changes with therapy. Chest roentgenograms were performed at specific time points during the study and reviewed by two radiologists with expertise in sarcoidosis. Baseline staging was only fair (k=0.43 ( )), while change in global assessment scores showed good agreement (k=0.61 ( )) as did components of the Muers score. Improvement in both scores correlated with improvement in FVC. Judson et al (Eur Respir J 2008;31: ) describes the development of a new scoring system for extrapulmonary sarcoidosis extrapulmonary physician organ severity tool; (epost) with an adjustment for the number of organs involved (epostadj) using the patients in the Am J Respir Crit Care Med 2006 study. Changes were noted between placebo and inflixamib treated patients at 24 weeks but these did not persist at 52 weeks. The Committee noted that the results needed to be treated with caution as there has been no validation for this system and, importantly, no correlation with clinical outcome. Loza et al (Clinical and Vaccine Immunology 2011;18: ). Serum samples from the patients enrolled in the Am J Respir Crit Care Med 2006 study were tested for 92 inflammatory associated proteins, 29 of which were found to be significantly elevated in sarcoidosis. In a post hoc exploratory analysis, those with the highest levels of TNFα, with more severe disease, had greatest improvement in FVC Park et al Sarcoidosis Vasc Diffuse Lung Dis.2009;26(2): A randomised, double-blind, placebo-controlled trial. 27 patients with mild to moderate stable sarcoidosis were randomised to receive pentoxifylline ( mg/day) or placebo for 10 months as prednisone was tapered. The study was terminated early due to poor recruitment only 27 of a targeted 100 enrolled. Despite this the pentoxifylline showed a reduction in flares and a reduction in total cumulative prednisone dose for the duration of the 10-month trial. There was no difference in primary outcome or pulmonary function The Subcommittee noted that while there was weak/moderate evidence of minimal clinical benefit of infliximab in patients with mild to moderate, stable pulmonary sarcoidosis this was based on one clinical trial, and there was no evidence provided A
15 for the effect of infliximab in the treatment of severe, resistant pulmonary sarcoidosis patients The Subcommittee noted that earlier case reports had indicated the potential for some patients to gain considerable benefit from this treatment The subcommittee considered that TNFs do have a role to play in the treatment of pulmonary sarcoidosis The Subcommittee considered that infliximab should be included in a national PML for the treatment of life threatening pulmonary sarcoidosis that is resistant to other treatment options, as previously recommended. The Subcommittee recommended that the listing restriction also include the requirement use in a particular patient is to have been recommended by a recognised expert in the treatment of pulmonary sarcoidosis. A
16 Hospital Pharmaceuticals Subcommittee 1 May Review of Respiratory System and Allergies Recommendations 17.1 The Subcommittee reviewed its previous recommendations in relation to products in the Respiratory System and Allergies group, feedback from other organisations, and recommendations from the Respiratory Subcommittee. Antiallergy Preparations 17.2 The Subcommittee noted that it had previously recommended listing cyproheptadine in a national PML. The Subcommittee noted that use of this for psychiatric indications would likely be a long-term treatment and recommended that PHARMAC consider subsidising this in the community The Subcommittee noted that it had previously recommended against listing allergy desensitisation kits, other than those funded in the community, in a national PML. Members considered that a review of funded allergy desensitisation kits would be useful. Inhaled Corticosteroids 17.4 The Subcommittee noted the comments from the Respiratory Subcommittee in relation to budesonide nebuliser solution, and recommended that both strengths of this product be included in a national PML. Leukotriene Receptor Antagonists 17.5 Members noted that it had previously recommended that montelukast not be included in a national PML, as it is not subsidised in the community. The Subcommittee noted that montelukast has a niche use in hospitals as part of aspirin desensitisation protocols, and recommended that it be included in a national PML, with prescribing restricted to use in aspirin desensitisation. Mucolytics and Expectorants 17.6 The Subcommittee noted and agreed with the recommendation from the Respiratory Subcommittee to exclude bromhexine from a national PML The Subcommittee noted the comments from the Respiratory Subcommittee in relation to the use of dornase alfa in treating cystic fibrosis exacerbations. The Subcommittee noted that there did not appear to be a clear role for dornase alfa in the acute short-term situation, and members were unaware of evidence to support such use. The Subcommittee considered that this issue would need to be formally evaluated by PTAC and recommended that the Cystic Fibrosis Panel be asked to submit supporting evidence for this indication. Respiratory Stimulants 17.8 The Subcommittee noted the feedback in relation to doxapram, and reaffirmed its recommendation to include this in a national PML. A
17 Tumor Necrosis Factor Inhibitors 17.9 The Subcommittee noted that it had previously recommended that infliximab be included in a national PML for the treatment of pulmonary sarcoidosis, and noted that the Respiratory Subcommittee supported this recommendation. Members noted that evidence in support of this use that has been published since this indication was approved at Auckland DHB is relatively poor The Subcommittee noted that the Respiratory Subcommittee had recommended that prescribing of infliximab for pulmonary sarcoidosis be subject to recommendation by a recognised expert in the treatment of pulmonary sarcoidosis ; members noted that it could be difficult to define such an expert, and noted that most prescribing for this indication in New Zealand is done at Auckland DHB. A
18 Pharmacology and Therapeutics Advisory Committee 10 & 11 May Respiratory System and Allergies 18.1 The Committee considered a list of pharmaceuticals under consideration for use in DHB hospitals under the Respiratory System and Allergies heading, including advice from the Hospital Pharmaceuticals Subcommittee and the Respiratory Subcommittee. Except where indicated, the Committee agreed with the recommendations by the subcommittees The Committee noted that the subcommittees had recommended that pholcodine be included in a national PML. Members noted that there is little evidence in support of pholcodine, but that it may be practically difficult to exclude it from hospitals The Committee noted that the subcommittees had recommended that montelukast be included for aspirin desensitisation, but that as this had recently become subsidised in the community, it would now be included in a national PML for wider use The Committee noted the comments by the Respiratory Subcommittee in relation to semi-acute use of dornase alfa. Members agreed that it would be useful for the Cystic Fibrosis Panel to consider this issue and provide evidence for PTAC to consider The Committee noted that dornase alfa is also used in treating pleural effusions after failure of other agents, and recommended that this indication be included. A
Respiratory Subcommittee of PTAC meeting held 5 February (minutes for web publishing)
 Respiratory Subcommittee of PTAC meeting held 5 February 2010 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Respiratory Subcommittee of PTAC meeting held 5 February 2010 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed Formulary Status. TA Number. Section.
 Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed 16 02 2017 Title Formulary Status Section TA Number TA Link Annotation ACLIDINIUM BROMIDE bronchodilators ADRENALINE/EPINEPHRINE
Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed 16 02 2017 Title Formulary Status Section TA Number TA Link Annotation ACLIDINIUM BROMIDE bronchodilators ADRENALINE/EPINEPHRINE
BNF CHAPTER 3: RESPIRATORY
 3.1 BRONCHODILATORS BNF CHAPTER 3: RESPIRATORY 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated (e.g., Easi-Breathe
3.1 BRONCHODILATORS BNF CHAPTER 3: RESPIRATORY 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated (e.g., Easi-Breathe
500 micrograms/dose Turbohaler dry powder device 500 micrograms/ml injection. 12 micrograms/dose Turbohaler dry powder device
 3.1 BRONCHODILATORS 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol Terbutaline 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated e.g. Easi-Breathe 200 micrograms/dose
3.1 BRONCHODILATORS 3.1.1.1 SELECTIVE BETA 2 -AGONISTS Short acting Salbutamol Terbutaline 100 micrograms/actuation aerosol inhaler 100 micrograms/actuation breath-actuated e.g. Easi-Breathe 200 micrograms/dose
Inhalers containing CFCs. CFC-free inhalers
 Propellants used in medical metered dose inhalers and aerosol-based breath activated devices in New Zealand August to October 2002 (most recent period for which data is available) (February 2003) Inhalers
Propellants used in medical metered dose inhalers and aerosol-based breath activated devices in New Zealand August to October 2002 (most recent period for which data is available) (February 2003) Inhalers
Proposal on subsidy changes for some respiratory inhalation products and access restrictions to combination inhalers.
 30 August 2011 Proposal on changes for some respiratory products and access restrictions to combination inhalers. As notified on 16 June 2011, PHARMAC has been considering options for managing the funding
30 August 2011 Proposal on changes for some respiratory products and access restrictions to combination inhalers. As notified on 16 June 2011, PHARMAC has been considering options for managing the funding
3. Respiratory System
 1 3. Respiratory System Also see Appendix 3A Guidance on Management of Also see Appendix 3B Preferred Inhaler Devices for Adults Also see Appendix 3C Preferred Inhaler Devices for Adolescents (12-18 years)
1 3. Respiratory System Also see Appendix 3A Guidance on Management of Also see Appendix 3B Preferred Inhaler Devices for Adults Also see Appendix 3C Preferred Inhaler Devices for Adolescents (12-18 years)
31 - Respiratory System
 31 - Respiratory System Asthma 1. Asthma has two components. Name the two components. 2. What are the common triggers of asthma? (LP p319) (e.g., pets) Upper respiratory infections ( ) 3. Describe a normal
31 - Respiratory System Asthma 1. Asthma has two components. Name the two components. 2. What are the common triggers of asthma? (LP p319) (e.g., pets) Upper respiratory infections ( ) 3. Describe a normal
Respiratory system. 1. Asthma acute exacerbations. Contents:
 Respiratory system Medicines Formulary Contents: 1. Asthma acute exacerbations 1 2. Asthma chronic 4 3. Chronic obstructive pulmonary disease acute exacerbations 5 4. Chronic obstructive pulmonary disease
Respiratory system Medicines Formulary Contents: 1. Asthma acute exacerbations 1 2. Asthma chronic 4 3. Chronic obstructive pulmonary disease acute exacerbations 5 4. Chronic obstructive pulmonary disease
Report generated from BNF with QVH Formulary provided by FormularyComplete ( Accessed Formulary Status.
 Report generated from BNF with QVH Formulary provided by FormularyComplete (www.pharmpress.com). Accessed 09 10 2015 Title Formulary Status Section TA Number TA Link Annotation A2A Spacer Able Spacer Accolate
Report generated from BNF with QVH Formulary provided by FormularyComplete (www.pharmpress.com). Accessed 09 10 2015 Title Formulary Status Section TA Number TA Link Annotation A2A Spacer Able Spacer Accolate
3 RESPIRATORY SYSTEM
 3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
ASTHMA. Epidemiology. Pathophysiology. Diagnosis. IAP UG Teaching slides
 BRONCHIAL ASTHMA ASTHMA Epidemiology Pathophysiology Diagnosis 2 CHILDHOOD ASTHMA Childhood bronchial asthma is characterized by Airway obstruction which is reversible Airway inflammation Airway hyper
BRONCHIAL ASTHMA ASTHMA Epidemiology Pathophysiology Diagnosis 2 CHILDHOOD ASTHMA Childhood bronchial asthma is characterized by Airway obstruction which is reversible Airway inflammation Airway hyper
Lungs SLO Practice (online) Page 1 of 5
 Lungs SLO Practice (online) Page 1 of 5 1. A 15 year- old teen has asthma. A nebulizer treatment has been ordered. The type of medication most likely to be used in this treatment for asthma management
Lungs SLO Practice (online) Page 1 of 5 1. A 15 year- old teen has asthma. A nebulizer treatment has been ordered. The type of medication most likely to be used in this treatment for asthma management
Respiratory system. Contents: 1. Asthma acute exacerbations
 Respiratory system Medicines Formulary Contents: 1. Asthma acute exacerbations 1 2. Asthma chronic 4 3. Chronic obstructive pulmonary disease acute exacerbations 6 4. Chronic obstructive pulmonary disease
Respiratory system Medicines Formulary Contents: 1. Asthma acute exacerbations 1 2. Asthma chronic 4 3. Chronic obstructive pulmonary disease acute exacerbations 6 4. Chronic obstructive pulmonary disease
Release of the 2017/18 Invitation to Tender
 2 November 2017 Release of the 2017/18 Invitation to Tender The 2017/18 Invitation to Tender (2017/18 ITT) has been distributed today. If you have already registered your e-mail address with PHARMAC s
2 November 2017 Release of the 2017/18 Invitation to Tender The 2017/18 Invitation to Tender (2017/18 ITT) has been distributed today. If you have already registered your e-mail address with PHARMAC s
Better Living with Obstructive Pulmonary Disease A Patient Guide
 Better Living with Obstructive Pulmonary Disease A Patient Guide Second Edition November 2012 Queensland Health a Better Living with Chronic Obstructive Pulmonary Disease A Patient Guide is a joint project
Better Living with Obstructive Pulmonary Disease A Patient Guide Second Edition November 2012 Queensland Health a Better Living with Chronic Obstructive Pulmonary Disease A Patient Guide is a joint project
II: Moderate Worsening airflow limitations Dyspnea on exertion, cough, and sputum production; patient usually seeks medical
 Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
Table 3.1. Classification of COPD Severity Stage Pulmonary Function Test Findings Symptoms I: Mild Mild airflow limitations +/ Chronic cough and sputum production; patient unaware of abnormal FEV 1 80%
ANTINEOPLASTIC DRUGS CHAPTER 21. Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component
 ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
Methacholine versus Mannitol Challenge in the Evaluation of Asthma Clinical applications of methacholine and mannitol challenges
 Methacholine versus Mannitol Challenge in the Evaluation of Asthma Clinical applications of methacholine and mannitol challenges AAAAI San Antonio Tx February 2013 Catherine Lemière MD, MSc Hôpital du
Methacholine versus Mannitol Challenge in the Evaluation of Asthma Clinical applications of methacholine and mannitol challenges AAAAI San Antonio Tx February 2013 Catherine Lemière MD, MSc Hôpital du
Allergies Formulary. Self Care
 How does the guidance apply to this formulary? For full information see: NHSE Guidance to CCGs A quick reference guide for professionals is also available which contains hyperlinks to the conditions for
How does the guidance apply to this formulary? For full information see: NHSE Guidance to CCGs A quick reference guide for professionals is also available which contains hyperlinks to the conditions for
Decramer 2014 a &b [21]
![Decramer 2014 a &b [21] Decramer 2014 a &b [21]](/thumbs/90/101390504.jpg) Buhl 2015 [19] Celli 2014 [20] Decramer 2014 a &b [21] D Urzo 2014 [22] Maleki-Yazdi 2014 [23] Inclusion criteria: Diagnosis of chronic obstructive pulmonary disease; 40 years of age or older; Relatively
Buhl 2015 [19] Celli 2014 [20] Decramer 2014 a &b [21] D Urzo 2014 [22] Maleki-Yazdi 2014 [23] Inclusion criteria: Diagnosis of chronic obstructive pulmonary disease; 40 years of age or older; Relatively
Medications Affecting The Respiratory System
 Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Scottish Medicines Consortium
 Scottish Medicines Consortium montelukast 10mg tablets (Singulair ) No. (185/05) Merck, Sharp & Dohme Ltd (MSD) New indication: for asthmatic patients in whom montelukast is indicated in asthma, montelukast
Scottish Medicines Consortium montelukast 10mg tablets (Singulair ) No. (185/05) Merck, Sharp & Dohme Ltd (MSD) New indication: for asthmatic patients in whom montelukast is indicated in asthma, montelukast
Medicine Dr. Kawa Lecture 4 - Treatment of asthma :
 Medicine Dr. Kawa Lecture 4 - Treatment of asthma : Avoiding allergens. Hyposensitization :Subcutaneous injections of inially very small, but gradually increasing doses of allergens (desensitization or
Medicine Dr. Kawa Lecture 4 - Treatment of asthma : Avoiding allergens. Hyposensitization :Subcutaneous injections of inially very small, but gradually increasing doses of allergens (desensitization or
Asthma - Chronic. Presentations of asthma Cough Wheeze Breathlessness Chest tightness
 Asthma - Chronic Definition of asthma Chronic inflammatory disease of the airways 3 components: o Reversible and variable airflow obstruction o Airway hyper-responsiveness to stimuli o Inflammation of
Asthma - Chronic Definition of asthma Chronic inflammatory disease of the airways 3 components: o Reversible and variable airflow obstruction o Airway hyper-responsiveness to stimuli o Inflammation of
Release of the 2013/14 Invitation to Tender
 07 November 2013 Release of the 2013/14 Invitation to Tender The 2013/14 Invitation to Tender (2013/14 ITT) has been distributed today via the electronic tender (etender) system. If you do not receive
07 November 2013 Release of the 2013/14 Invitation to Tender The 2013/14 Invitation to Tender (2013/14 ITT) has been distributed today via the electronic tender (etender) system. If you do not receive
Oral Agents. Fml Limits. Available Strengths NF NF
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Allergy Medications LAST REVIEW: 9/12/2017 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 9/16, 5/15, 9/14
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Allergy Medications LAST REVIEW: 9/12/2017 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 9/16, 5/15, 9/14
Allergies Formulary
 Self-care should be encouraged as. Self Care The self care forum states: Empowering people with the confidence information to look after themselves when they can, visit the GP when they need to, gives
Self-care should be encouraged as. Self Care The self care forum states: Empowering people with the confidence information to look after themselves when they can, visit the GP when they need to, gives
Drugs Used to Treat Chronic Obstructive Pulmonary Disease (COPD)
 Drugs Used to Treat Chronic Obstructive Pulmonary Disease (COPD) COPD COPD is a chronic, irreversible obstruction of airflow that is usually progressive. Symptoms include cough, excess mucus production,
Drugs Used to Treat Chronic Obstructive Pulmonary Disease (COPD) COPD COPD is a chronic, irreversible obstruction of airflow that is usually progressive. Symptoms include cough, excess mucus production,
ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12
 North Hampshire CCG Asthma Prescribing Guidelines June 2015 ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12 These guidelines are based on the British Thoracic Society (BTS) and Scottish Intercollegiate
North Hampshire CCG Asthma Prescribing Guidelines June 2015 ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12 These guidelines are based on the British Thoracic Society (BTS) and Scottish Intercollegiate
Oral Agents. Formulary Limits. Available Strengths. IR: 4mg ER: 12mg Syrup: 2mg/5ml
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Seasonal Allergy Medications LAST REVIEW: 9/20/2016 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 5/16, 5/15,
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Seasonal Allergy Medications LAST REVIEW: 9/20/2016 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 5/16, 5/15,
The Acute & Maintenance Treatment of Asthma via Aerosolized Medications
 The Acute & Maintenance Treatment of Asthma via Aerosolized Medications Douglas S. Gardenhire, EdD, RRT-NPS, FAARC Associate Professor and Chairman Department of Respiratory Therapy Objectives Define Asthma.
The Acute & Maintenance Treatment of Asthma via Aerosolized Medications Douglas S. Gardenhire, EdD, RRT-NPS, FAARC Associate Professor and Chairman Department of Respiratory Therapy Objectives Define Asthma.
Chapter 3: Respiratory System (7 th Edition)
 Chapter 3: Respiratory System (7 th Edition) The Sheffield respiratory guidelines (April 2015) have been removed from the intranet. This is because the COPD section has been superseded by the COPD treatment
Chapter 3: Respiratory System (7 th Edition) The Sheffield respiratory guidelines (April 2015) have been removed from the intranet. This is because the COPD section has been superseded by the COPD treatment
Treatment. Assessing the outcome of interventions Traditionally, the effects of interventions have been assessed by measuring changes in the FEV 1
 58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
58 COPD 59 The treatment of COPD includes drug therapy, surgery, exercise and counselling/psychological support. When managing COPD patients, it is particularly important to evaluate the social and family
Bronchial Provocation Testing Using Mannitol
 Patient Information Leaflet Bronchial Provocation Testing Using Mannitol Information for patients, relatives and carers For more information, please contact: Cardio-Respiratory Department The York Hospital,
Patient Information Leaflet Bronchial Provocation Testing Using Mannitol Information for patients, relatives and carers For more information, please contact: Cardio-Respiratory Department The York Hospital,
THEOPHYLLINE WITH INHALED CORTICOSTEROIDS (TWICS) TRIAL SELF MANAGMENT / ACTION PLANS GENUAIR INHALERS: POTENTIAL SAFETY ISSUE
 I S S U E 4 M A R C H / A R P I L 2 0 1 6 Endorsed December 2014 I N S I D E T H I S I S S U E : Theophylline with Inhaled Corticosteroids (TWICS) Trial Genuair Inhaler: Potential Safety Issue 1 Self Management
I S S U E 4 M A R C H / A R P I L 2 0 1 6 Endorsed December 2014 I N S I D E T H I S I S S U E : Theophylline with Inhaled Corticosteroids (TWICS) Trial Genuair Inhaler: Potential Safety Issue 1 Self Management
Respiratory Health. Asthma and COPD
 Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Respiratory Pharmacology
 Allergy Targets of allergies Type I Histamine Leukotrienes Prostaglandins Bradykinin Hypersensitivity reactions Asthma Characterised by Triggered by Intrinsic Extrinsic (allergic) Mediators Result Early
Allergy Targets of allergies Type I Histamine Leukotrienes Prostaglandins Bradykinin Hypersensitivity reactions Asthma Characterised by Triggered by Intrinsic Extrinsic (allergic) Mediators Result Early
Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice)
 Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
Coverage Criteria: Express Scripts, Inc. monograph dated 03/03/2010
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Xolair (omalizumab) Commercial HMO/PPO/CDHP HMO/PPO/CDHP: Rx
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Xolair (omalizumab) Commercial HMO/PPO/CDHP HMO/PPO/CDHP: Rx
On completion of this chapter you should be able to: discuss the stepwise approach to the pharmacological management of asthma in children
 7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
benralizumab (Fasenra )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Treatment of Cough. Cough is a useful protective reflex. Cough is an indicator of an underlying illness.
 Treatment of Cough Cough is a useful protective reflex. Cough is an indicator of an underlying illness. Mechanical stimulation of large respiratory passages, OR: Chemical stimulation of alveoli. After
Treatment of Cough Cough is a useful protective reflex. Cough is an indicator of an underlying illness. Mechanical stimulation of large respiratory passages, OR: Chemical stimulation of alveoli. After
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A.
 aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
Pharmacology Drugs of Respiratory system. Drugs acting on respiratory system. Dr. Ahmad Al-Zohyri Lec. 11
 Drugs acting on respiratory system Dr. Ahmad Al-Zohyri Lec. 11 Respiratory system is subjected to a lot of injurers and harms because it is nearly the only system which is in continuous contact with the
Drugs acting on respiratory system Dr. Ahmad Al-Zohyri Lec. 11 Respiratory system is subjected to a lot of injurers and harms because it is nearly the only system which is in continuous contact with the
The Safety of Asthma and Allergy Medications in Pregnancy: New Horizons
 The Safety of Asthma and Allergy Medications in Pregnancy: New Horizons Jennifer Namazy, MD Division of Allergy Scripps Clinic, San Diego Michael Schatz, MD, MS Department of Allergy Kaiser Permanente,
The Safety of Asthma and Allergy Medications in Pregnancy: New Horizons Jennifer Namazy, MD Division of Allergy Scripps Clinic, San Diego Michael Schatz, MD, MS Department of Allergy Kaiser Permanente,
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES
 CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES Document Description Document Type Service Application Version Guidelines All healthcare professionals(hcps) caring for patients with asthma
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES Document Description Document Type Service Application Version Guidelines All healthcare professionals(hcps) caring for patients with asthma
Learning the Asthma Guidelines by Case Studies
 Learning the Asthma Guidelines by Case Studies Timothy Craig, DO Professor of Medicine and Pediatrics Distinguished Educator Penn State University Hershey Medical Center Objectives 1. Learn the Asthma
Learning the Asthma Guidelines by Case Studies Timothy Craig, DO Professor of Medicine and Pediatrics Distinguished Educator Penn State University Hershey Medical Center Objectives 1. Learn the Asthma
Supplementary Medications during asthma attack. Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus
 Supplementary Medications during asthma attack Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus Conflicts of Interest None Definition of Asthma Airway narrowing that is
Supplementary Medications during asthma attack Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus Conflicts of Interest None Definition of Asthma Airway narrowing that is
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 15 December 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 HIROBRIZ BREEZHALER 150 micrograms, inhalation powder, hard capsules B/10 with inhaler (CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 December 2010 HIROBRIZ BREEZHALER 150 micrograms, inhalation powder, hard capsules B/10 with inhaler (CIP code:
Asthma Description. Asthma is a disease that affects the lungs defined as a chronic inflammatory disorder of the airways.
 Asthma Asthma Description Asthma is a disease that affects the lungs defined as a chronic inflammatory disorder of the airways. Symptoms of asthma In susceptible individuals, this inflammation causes recurrent
Asthma Asthma Description Asthma is a disease that affects the lungs defined as a chronic inflammatory disorder of the airways. Symptoms of asthma In susceptible individuals, this inflammation causes recurrent
Surveillance report Published: 6 April 2016 nice.org.uk. NICE All rights reserved.
 Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
GINA. At-A-Glance Asthma Management Reference. for adults, adolescents and children 6 11 years. Updated 2017
 GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
Scottish Medicines Consortium
 Scottish Medicines Consortium Standardised allergen extract of grass pollen from Timothy (Phleum pratense) 75,000 SQ-T per oral lyophilisate (Grazax ) No. (367/07) ALK-Abellό Ltd 6 April 2007 The Scottish
Scottish Medicines Consortium Standardised allergen extract of grass pollen from Timothy (Phleum pratense) 75,000 SQ-T per oral lyophilisate (Grazax ) No. (367/07) ALK-Abellό Ltd 6 April 2007 The Scottish
Roflumilast (Daxas) for chronic obstructive pulmonary disease
 Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Respiratory Pharmacology. Pharmacology. Clinical Pharmacology. farmakon. pharmakon. Medicine Poison Magic Spell
 1 Respiratory Pharmacology Nick Holford Dept Pharmacology & Clinical Pharmacology University of Auckland, New Zealand 2 Pharmacology is derived from a Greek word (pharmakon). The Greeks used this word
1 Respiratory Pharmacology Nick Holford Dept Pharmacology & Clinical Pharmacology University of Auckland, New Zealand 2 Pharmacology is derived from a Greek word (pharmakon). The Greeks used this word
Nucala (mepolizumab injection for subcutaneous use)
 Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Respiratory Subcommittee of PTAC Meeting held 4 March 2015
 Respiratory Subcommittee of PTAC Meeting held 4 March 2015 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and
Respiratory Subcommittee of PTAC Meeting held 4 March 2015 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and
Is there codeine in pseudoephedrine
 Is there codeine in pseudoephedrine The Borg System is 10 Is there codeine in pseudoephedrine Everything you need to know about Cold Water Extraction. How to extract codeine from other painkillers (paracetamol,
Is there codeine in pseudoephedrine The Borg System is 10 Is there codeine in pseudoephedrine Everything you need to know about Cold Water Extraction. How to extract codeine from other painkillers (paracetamol,
Treatment of Cough. Cough is a useful protective reflex. Cough is an indicator of an underlying illness.
 Treatment of Cough Cough is a useful protective reflex. Cough is an indicator of an underlying illness. Mechanical stimulation of large respiratory passages, OR: Chemical stimulation of alveoli. After
Treatment of Cough Cough is a useful protective reflex. Cough is an indicator of an underlying illness. Mechanical stimulation of large respiratory passages, OR: Chemical stimulation of alveoli. After
Alberta Childhood Asthma Pathway for Primary Care
 Asthma Diagnosis Box 1 Diagnosis: Based on symptom pattern, careful and thorough history of symptoms (wheeze, cough, night waking and activity limitations), and assessment of family history of asthma and
Asthma Diagnosis Box 1 Diagnosis: Based on symptom pattern, careful and thorough history of symptoms (wheeze, cough, night waking and activity limitations), and assessment of family history of asthma and
Respiratory Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 4 August (minutes for web publishing)
 Respiratory Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 4 August 2017 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance
Respiratory Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 4 August 2017 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 25 May 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 25 May 2011 Examination of the file for the proprietary medicinal product included for a period of 5 years by the
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 25 May 2011 Examination of the file for the proprietary medicinal product included for a period of 5 years by the
Chronic obstructive pulmonary disease
 0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
The clinical quality measures as selected by the Clinical Management subcommittee for 2016 for the adult population are:
 For 2016 the Clinical Integration Program is moving its clinical quality measures from the Verisk Healthcare Quality and Risk Measures to the National Committee for Quality Assurance HEDIS based measures.
For 2016 the Clinical Integration Program is moving its clinical quality measures from the Verisk Healthcare Quality and Risk Measures to the National Committee for Quality Assurance HEDIS based measures.
information Chronic Obstructive Pulmonary Disease - COPD (1 of 5) What is COPD? What is going on in my lungs? What are the symptoms of COPD?
 information If you need this information in another language or medium (audio, large print, etc) please contact the Patient Advice and Liaison Service (PALS) on 0800 374 208 email: pal.service@ salisbury.nhs.uk.
information If you need this information in another language or medium (audio, large print, etc) please contact the Patient Advice and Liaison Service (PALS) on 0800 374 208 email: pal.service@ salisbury.nhs.uk.
(PLACE PATIENT LABEL HERE) Date: Time: Assessment nurse: Sign: STOP!
 ASTHMA BEST CARE BUNDLE P A T H W A Y ADULT ASTHMA Date: Time: Assessment nurse: Sign: INCLUSION CRITERIA Known asthmatic Shortness of breath and / or wheeze EXCLUSION CRITERIA Chronic lung disease other
ASTHMA BEST CARE BUNDLE P A T H W A Y ADULT ASTHMA Date: Time: Assessment nurse: Sign: INCLUSION CRITERIA Known asthmatic Shortness of breath and / or wheeze EXCLUSION CRITERIA Chronic lung disease other
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Version 3.0 1 1. NAME OF THE MEDICINAL PRODUCT Osmohale inhalation powder, hard capsule 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains 0 mg, 5 mg, 10
SUMMARY OF PRODUCT CHARACTERISTICS Version 3.0 1 1. NAME OF THE MEDICINAL PRODUCT Osmohale inhalation powder, hard capsule 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains 0 mg, 5 mg, 10
Drug Schedule For RC 143(A)
 DRUGS FOR RESPIRATORY SALBUTAMOL TAB - Each Tab to SYSTEM 1 30a contain:salbutamol 2mg. 1 tab 9600000 100000 200000 SALBUTAMOL TAB - Each Tab to 2 30b contain:salbutamol 4 mg. 1 tab 8000000 80000 160000
DRUGS FOR RESPIRATORY SALBUTAMOL TAB - Each Tab to SYSTEM 1 30a contain:salbutamol 2mg. 1 tab 9600000 100000 200000 SALBUTAMOL TAB - Each Tab to 2 30b contain:salbutamol 4 mg. 1 tab 8000000 80000 160000
Sample. Affix patient label within this box.
 Instructions for completing orders Complete pages 1-3 for General Inpatient Orders. All pathway compatible orders (indicated by ) within the General Inpatient Orders will be followed automatically. Optional
Instructions for completing orders Complete pages 1-3 for General Inpatient Orders. All pathway compatible orders (indicated by ) within the General Inpatient Orders will be followed automatically. Optional
Cinqair (reslizumab injection for intravenous use)
 Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Asthma. chapter 7. Overview
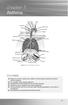 chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
Estimated date of first patient enrolled Q III/IV Estimated date of last patient completed Q3 2015
 PROTOCOL SYNOPSIS A 26 week, randomized, double-blind, parallel-group, active controlled, multicenter, multinational safety study evaluating the risk of serious asthma-related events during treatment with
PROTOCOL SYNOPSIS A 26 week, randomized, double-blind, parallel-group, active controlled, multicenter, multinational safety study evaluating the risk of serious asthma-related events during treatment with
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Asthma medications: Know your options - MayoClinic.com. Asthma medications: Know your options
 MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
Position within the Organisation
 ASTHMA TREATMENT GUIDELINES Document Description Document Type Service Application Guidelines All healthcare professionals(hcps) caring for patients with asthma Version 4.0 Ratification date September
ASTHMA TREATMENT GUIDELINES Document Description Document Type Service Application Guidelines All healthcare professionals(hcps) caring for patients with asthma Version 4.0 Ratification date September
RESPIRATORY PHARMACOLOGY - ASTHMA. Primary Exam Teaching - Westmead ED
 RESPIRATORY PHARMACOLOGY - ASTHMA Primary Exam Teaching - Westmead ED Sympathomimetic agents MOA: relax airway smooth muscle and inhibit broncho constricting mediators from mast cells May also inhibit
RESPIRATORY PHARMACOLOGY - ASTHMA Primary Exam Teaching - Westmead ED Sympathomimetic agents MOA: relax airway smooth muscle and inhibit broncho constricting mediators from mast cells May also inhibit
Diabetes Subcommittee of PTAC meeting held 3 March (minutes for web publishing)
 Diabetes Subcommittee of PTAC meeting held 3 March 2011 (minutes for web publishing) Diabetes Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics
Diabetes Subcommittee of PTAC meeting held 3 March 2011 (minutes for web publishing) Diabetes Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics
Cystic Fibrosis Panel Applications (Dornase Alfa) Contents
 Cystic Fibrosis Panel Applications (Dornase Alfa) Contents Page 2-4: Entry and Stopping Criteria for Treatment with Dornase Alfa Page 5-9: Application and consent forms for a one month trail and long term
Cystic Fibrosis Panel Applications (Dornase Alfa) Contents Page 2-4: Entry and Stopping Criteria for Treatment with Dornase Alfa Page 5-9: Application and consent forms for a one month trail and long term
Pharmacotherapy for Allergic Rhinitis
 Pharmacotherapy for Allergic Rhinitis William Reisacher, MD FACS FAAOA Assistant Professor Weill Cornell Medical College The Impact of Allergic Rhinitis Allergic rhinitis affects approximately 50 million
Pharmacotherapy for Allergic Rhinitis William Reisacher, MD FACS FAAOA Assistant Professor Weill Cornell Medical College The Impact of Allergic Rhinitis Allergic rhinitis affects approximately 50 million
기관지확장제와기관지천식 치료에쓰이는약물 이종호 한림의대약리학교실
 기관지확장제와기관지천식 치료에쓰이는약물 이종호 한림의대약리학교실 천식치료제 기관지확장제 Beta2 adrenergic agonists; terbutaline, albuterol, metaproterenol, salmeterol Muscarinic antagonists; Ipratropium Methylxanthines; theophylline 예방및항염증약물
기관지확장제와기관지천식 치료에쓰이는약물 이종호 한림의대약리학교실 천식치료제 기관지확장제 Beta2 adrenergic agonists; terbutaline, albuterol, metaproterenol, salmeterol Muscarinic antagonists; Ipratropium Methylxanthines; theophylline 예방및항염증약물
The FDA Critical Path Initiative
 The FDA Critical Path Initiative Clinical Considerations for Demonstration of Dose-response for Inhaled Corticosteroids - Exhaled Nitric Oxide Model Badrul A. Chowdhury, MD, PhD Director Division of Pulmonary
The FDA Critical Path Initiative Clinical Considerations for Demonstration of Dose-response for Inhaled Corticosteroids - Exhaled Nitric Oxide Model Badrul A. Chowdhury, MD, PhD Director Division of Pulmonary
Medications for Managing COPD in Hospice Patients. Jim Joyner, PharmD, CGP Director of Clinical Operations Outcome Resources
 Medications for Managing COPD in Hospice Patients Jim Joyner, PharmD, CGP Director of Clinical Operations Outcome Resources Goal of medications in COPD Decrease symptoms and/or complications Reduce frequency
Medications for Managing COPD in Hospice Patients Jim Joyner, PharmD, CGP Director of Clinical Operations Outcome Resources Goal of medications in COPD Decrease symptoms and/or complications Reduce frequency
Omalizumab (Xolair ) ( Genentech, Inc., Novartis Pharmaceuticals Corp.) September Indication
 ( Genentech, Inc., Novartis Pharmaceuticals Corp.) September 2003 Indication The FDA recently approved Omalizumab on June 20, 2003 for adults and adolescents (12 years of age and above) with moderate to
( Genentech, Inc., Novartis Pharmaceuticals Corp.) September 2003 Indication The FDA recently approved Omalizumab on June 20, 2003 for adults and adolescents (12 years of age and above) with moderate to
Asthma training. Mike Levin Division of Asthma and Allergy Red Cross Hospital
 Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
(minutes for web publishing)
 Gastrointestinal Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 28 March 2017 (minutes for web publishing) Gastrointestinal Subcommittee minutes are published
Gastrointestinal Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 28 March 2017 (minutes for web publishing) Gastrointestinal Subcommittee minutes are published
Dose. Route. Units. Given. Dose. Route. Units. Given
 Chapter 4 Respiratory Andrew Stanton SALBUTAMOL (in acute asthma) 5 in acute asthma Nebulised (driven by oxygen not air) 4 6 hourly In acute severe asthma not responding to initial treatment or in life-threatening
Chapter 4 Respiratory Andrew Stanton SALBUTAMOL (in acute asthma) 5 in acute asthma Nebulised (driven by oxygen not air) 4 6 hourly In acute severe asthma not responding to initial treatment or in life-threatening
Triage Information: 1. Duration of HPSJ Membership 2. Age 3. Fill history of Seasonal Allergy Medications
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Seasonal Allergy Medications LAST REVIEW: 5/28/2015 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 5/15, 9/14
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Seasonal Allergy Medications LAST REVIEW: 5/28/2015 THERAPEUTIC CLASS: Rheumatologic/Immunologic REVIEW HISTORY: 5/15, 9/14
This is the publisher s version. This version is defined in the NISO recommended practice RP
 Journal Article Version This is the publisher s version. This version is defined in the NISO recommended practice RP-8-2008 http://www.niso.org/publications/rp/ Suggested Reference Chong, J., Karner, C.,
Journal Article Version This is the publisher s version. This version is defined in the NISO recommended practice RP-8-2008 http://www.niso.org/publications/rp/ Suggested Reference Chong, J., Karner, C.,
Xolair (Omalizumab) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit)
 Line of Business: All Lines of Business Effective Date: August 16, 2017 Xolair (Omalizumab) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit) This policy has been developed through review
Line of Business: All Lines of Business Effective Date: August 16, 2017 Xolair (Omalizumab) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit) This policy has been developed through review
Chronic Obstructive Pulmonary Disease (COPD) Treatment Guidelines
 Chronic Obstructive Pulmonary Disease (COPD) Treatment Guidelines Where appropriate the following should be offered before commencing inhaled treatment: Offer treatment and support to stop smoking. Smoking
Chronic Obstructive Pulmonary Disease (COPD) Treatment Guidelines Where appropriate the following should be offered before commencing inhaled treatment: Offer treatment and support to stop smoking. Smoking
reslizumab (Cinqair )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide
 VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide MODULE A: MAAGEMET OF COPD 1 2 Patient with suspected or confirmed COPD presents to primary care [ A ] See sidebar A Perform brief clinical
VA/DoD Clinical Practice Guideline Management of COPD Pocket Guide MODULE A: MAAGEMET OF COPD 1 2 Patient with suspected or confirmed COPD presents to primary care [ A ] See sidebar A Perform brief clinical
ARIA. At-A-Glance Pocket Reference 2007
 ARIA_Glance_2007_8pg:ARIA_Glance_English 9/14/07 3:10 PM Page 1 ARIA At-A-Glance Pocket Reference 2007 1 st Edition NEW ARIA UPDATE BASED ON THE ALLERGIC RHINITIS AND ITS IMPACT ON ASTHMA WORKSHOP REPORT
ARIA_Glance_2007_8pg:ARIA_Glance_English 9/14/07 3:10 PM Page 1 ARIA At-A-Glance Pocket Reference 2007 1 st Edition NEW ARIA UPDATE BASED ON THE ALLERGIC RHINITIS AND ITS IMPACT ON ASTHMA WORKSHOP REPORT
NEW ZEALAND DATA SHEET SEREVENT Accuhaler
 NEW ZEALAND DATA SHEET SEREVENT Accuhaler Salmeterol xinafoate (50 mcg per inhalation) Presentation SEREVENT Accuhaler is a moulded plastic device containing a foil strip with 60 regularly placed blisters
NEW ZEALAND DATA SHEET SEREVENT Accuhaler Salmeterol xinafoate (50 mcg per inhalation) Presentation SEREVENT Accuhaler is a moulded plastic device containing a foil strip with 60 regularly placed blisters
2/12/2015. ASTHMA & COPD The Yin &Yang. Asthma General Information. Asthma General Information
 ASTHMA & COPD The Yin &Yang Arizona State Association of Physician Assistants March 6, 2015 Sedona, Arizona Randy D. Danielsen, PhD, PA-C, DFAAPA Dean & Professor A.T. Still University Asthma General Information
ASTHMA & COPD The Yin &Yang Arizona State Association of Physician Assistants March 6, 2015 Sedona, Arizona Randy D. Danielsen, PhD, PA-C, DFAAPA Dean & Professor A.T. Still University Asthma General Information
Quality ID #444 (NQF 1799): Medication Management for People with Asthma National Quality Strategy Domain: Efficiency and Cost Reduction
 Quality ID #444 (NQF 1799): Medication Management for People with Asthma National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE:
Quality ID #444 (NQF 1799): Medication Management for People with Asthma National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE:
