Diabetes Subcommittee of PTAC meeting held 3 March (minutes for web publishing)
|
|
|
- Brent Bridges
- 5 years ago
- Views:
Transcription
1 Diabetes Subcommittee of PTAC meeting held 3 March 2011 (minutes for web publishing) Diabetes Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and Therapeutics Advisory Committee (PTAC) and PTAC Subcommittees Note that this document is not necessarily a complete record of the Diabetes Subcommittee meeting; only the relevant portions of the minutes relating to Diabetes Subcommittee discussions about an Application or PHARMAC staff proposal that contain a recommendation are generally published. The Diabetes Subcommittee may: (a) recommend that a pharmaceutical be listed by PHARMAC on the Pharmaceutical Schedule and the priority it gives to such a listing; (b) defer a final recommendation, and give reasons for the deferral (such as the supply of further information) and what is required before further review; or (c) recommend that PHARMAC decline to list a pharmaceutical on the Pharmaceutical Schedule. These Subcommittee minutes were reviewed by PTAC at its meeting on 5 & 6 May 2011, the record of which will be available in June Contents 1 Diabetes test meters and test strip review Continuous Subcutaneous Insulin Infusion (Insulin Pumps)...3 1
2 1 Diabetes test meters and test strip review 1.1 The Subcommittee reviewed the information on diabetes test strip dispensing with other diabetes pharmaceuticals provided by PHARMAC extracted from pharmaceutical claims data. Members noted the average number of test strips collected by patients on metformin or diet alone appeared high when considered with international guidelines. Members noted that Best Practice Advocacy Centre (BPAC) had provided clinicians guidance on the use of test strips and that the usage figures also appeared high compared to the BPAC recommendations. Members noted that the number of test strips used by patients on insulin appeared low compared to international guideline recommendations. 1.2 The Subcommittee considered that type I diabetics may over or under use due to their personal preference with respect to their diabetes and level of care. Members considered that patients currently had ample opportunity to access test strips and it was up to the clinician and patient to agree to the appropriate level of testing. Members did not consider that PHARMAC should enter into any campaigns to target testing in this patient group. 1.3 The Subcommittee noted that patients on metformin or diet alone were not eligible for subsidised blood glucose diagnostic test meters as these were only subsidised for patients on insulin or sulphonylureas or who were pregnant. Members noted that clinicians received sample meters from pharmaceutical companies to initiate patients on, which allowed this patient group access to a meter. 1.4 Members noted that clinicians often initiated newly diagnosed type II diabetics on test strips to allow them to see what effect diet and exercise have on their blood glucose. Members considered that long term testing may be more damaging as increased testing may lead to increased anxiety and worry. Members considered that patients should only be testing if they are likely to change something, for example the insulin dose they are about to use. 1.5 The Subcommittee noted that for type II diabetics, HbA1c was the appropriate test to evaluate glycaemic control. Members noted that the HbA1c speedometer available to general practice and diabetes centres was an excellent tool to explain blood glucose control to patients. Members noted that prior to insulin initiation in type II patients it was appropriate to initiate regular blood glucose testing. Members noted that some clinicians were progressing directly to insulin from metformin in type II diabetics 1.6 The Subcommittee considered that there was likely to be an over use of blood glucose testing in rest homes. 1.7 The Subcommittee recommended that PHARMAC consider a campaign to promote optimal use of blood glucose testing strips aimed at the Primary Health Organisation (PHO) level. Members noted that this campaign should be targeted for practice nurses and general practitioners. Members noted that PHOs should have an education coordinator and that they would be the appropriate people to discuss this type of program with. 2
3 1.8 The Subcommittee reviewed the patient application for the funding of test strips for patients on home total parenteral nutrition (TPN). Members considered that patients with glucose intolerance may require test strips to monitor blood glucose; otherwise there was not any unmet clinical need. Members noted that patients receiving TPN in hospital were monitored and would not need to self monitor blood glucose. 1.9 The Subcommittee recommended that PHARMAC fund blood glucose test strips with a high priority for patients on home TPN with the following restriction: Patients on home TPN at risk of hypoglycaemia or hyperglycaemia 1.10 The Subcommittee considered that patients with a disorder of glucose homeostasis, either acquired or genetic, could not currently receive funded blood glucose test strips. Members considered there were approximately 40 to 50 patients nationally who may require blood glucose test strips for a disorder of glucose homeostasis. Members noted that currently clinicians would be prescribing these and endorsing the script inappropriately or applying for Hospital Exceptional Circumstances The Subcommittee recommended the PHARMAC fund blood glucose test strips with a high priority for patients with impaired glucose homeostasis with the following restriction Patients with a genetic or an acquired disorder of glucose homeostasis not including Type I or Type II diabetes or metabolic syndrome. 2 Continuous Subcutaneous Insulin Infusion (Insulin Pumps) 2.1 The Subcommittee reviewed a PHARMAC generated application for Insulin pumps. Members noted the significant number of responses to the PHARMAC request for information from clinicians, District Health Board, patients and suppliers. 2.2 The Subcommittee noted that insulin pumps were a method of delivering rapid acting insulin in both varying basal amounts and as a bolus when required. Members noted that insulin pumps were similar in action to multiple daily injections (MDI) of insulin, including long and short acting insulin, but allowed greater sophistication of dosage and control. 2.3 The Subcommittee considered the evidence for insulin pumps from randomised controlled trials (RCTs) was of moderate quality but weak strength for both improvement in control of HbA1c and also reduction of hypoglycaemic events. The Subcommittee considered the evidence for insulin pumps from observational studies was of moderate strength but weak quality for the same outcomes. 2.4 The Subcommittee noted that the RCTs excluded the population groups that it considered were most likely to benefit from insulin pumps, namely those with recurrent hypoglycaemic events. The Subcommittee considered that RCTs may target patients with already good glycaemic control, rather than those with less good control. The Subcommittee noted a confounder for insulin pump observational studies could be that the insulin pump acts as a talisman, in that the services surrounding the insulin pump may provide much of the benefit. 3
4 2.5 The Subcommittee considered that patients using an insulin pump used approximately 20% less insulin than those on MDI. Members noted that patients would maintain one or two vials or pens of long acting insulin at home in case of insulin pump failure. Members noted that patients would likely increase the number of blood glucose test strips prior to and post initiation of an insulin pump. Members considered that there would likely be a small increase in the usage of ketone test strips for patients on insulin pumps. 2.6 The Subcommittee considered that in its experience insulin pumps provided lifestyle benefits for patients and a definite improvement in clinical markers of diabetes including a reduction in HbA1c of between 0.5 and 0.7% and a reduction in the number of hypoglycaemic events. Members also considered it likely that insulin pumps provided greater glycaemic stability (lower peak and trough variation). Members considered that the Diabetes Control and Complications Trial was the seminal study for diabetes control with respect to outcomes. 2.7 The Subcommittee considered that patients on insulin pumps had an increased risk of ketoacidosis if the tube was occluded as there was less circulating insulin reserve, and that patients could rapidly develop ketoacidosis overnight if this occurred. Members also noted that patients had to be motivated to receive any clinical benefit and that if the patient (or caregiver) was not motivated then insulin pumps provided no benefit over MDI. 2.8 The Subcommittee considered that the benefit of insulin pumps in pregnancy is not well demonstrated. The Subcommittee considered there may be some merit where pumps facilitate control of HbA1c less than 6.5%, but it would expect much of this benefit to be lost if this level of control was not achieved in the first 8-10 weeks of gestation. Members noted that for patients wanting to become pregnant (and during early pregnancy) maintaining an HbA1c under 6.5 % may result in a reduction in foetal abnormalities. Members noted that currently pharmaceutical companies loaned insulin pumps to patients wishing to become pregnant and recovered them post partum, and that although this may be clinically defensible most patients wished to retain the pump. 2.9 The Subcommittee noted that there was currently a large inequity in New Zealand for the funding of insulin pumps and consumables from DHBs. In addition, funding from charities made it easier for some groups but not others to receive pumps. Members noted that Maori and Pacific Island Type I diabetics were less likely to access insulin pumps currently due to economic constraints. Members noted that if considered appropriate for funding that PHARMAC would likely fund both pumps and consumables. Members noted that the funding restrictions for consumables should be aligned with the requirements for the pumps to avoid patients that diabetic teams would not consider good candidates purchasing a pump, and gaining access to funded consumables [or vice versa] The Subcommittee noted that funding of insulin pumps and consumables would require intensive nurse and clinician time. Members noted that there would be capacity limitations at that level which would be a rate limiting step in access in some DHBs The Subcommittee considered that patients should only be initiated on an insulin pump by a diabetic team. Members noted that patients should undergo intensive MDI prior to application for an insulin pump. Members noted that applicants should undergo training in carbohydrate counting, insulin pump usage and if possible undergo a psychiatric test. 4
5 2.12 The Subcommittee recommended that insulin pumps should be restricted as there was unlikely to be a benefit for all patients. Members considered that the New Zealand Society for the Study of Diabetes (NZSSD) 2008 recommendations were appropriate restrictions. Members noted that for paediatric patients the guidelines should include highly motivated patients with poor control, recurrent hypoglycaemia or early signs of disease progression (e.g. retinopathy) (or, interested, highly motivated caregivers if the patient is very young). Members also considered that co-morbidities should be eligible e.g. coeliac or cystic fibrosis patients The Subcommittee noted that patients should initially be reviewed after three months and then annually. Members noted that if patients were not showing a benefit then ongoing subsidy should be withdrawn. Members considered that NZSSD could be approached to advise on exit criteria The Subcommittee recommended that if PHARMAC was to provide subsidy for insulin pumps then PHARMAC should consider price, quality, warranty, service and back-up and an appropriate interface for ease of use The Subcommittee recommended listing insulin pumps and consumables for patients who meet the entry criteria with a high priority. 5
Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus
 Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus Version No. Changes Made Version of July 2018 V0.5 Changes made to the policy following patient engagement including: - the
Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus Version No. Changes Made Version of July 2018 V0.5 Changes made to the policy following patient engagement including: - the
THE SHEFFIELD AREA PRESCRIBING GROUP. Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes. Date: March 2018.
 THE SHEFFIELD AREA PRESCRIBING GROUP Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes Date: March 2018 Overview Freestyle Libre is a flash glucose sensor device that measures *interstitial
THE SHEFFIELD AREA PRESCRIBING GROUP Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes Date: March 2018 Overview Freestyle Libre is a flash glucose sensor device that measures *interstitial
Mental Health Subcommittee of PTAC meeting held 21 June (minutes for web publishing)
 Mental Health Subcommittee of PTAC meeting held 21 June 2010 (minutes for web publishing) Mental Health Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Mental Health Subcommittee of PTAC meeting held 21 June 2010 (minutes for web publishing) Mental Health Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Audit support for continuous subcutaneous insulin infusion for the treatment of diabetes mellitus (review of technology appraisal guidance 57)
 Audit support for continuous subcutaneous insulin (review of technology appraisal guidance 57) Issue date: 2008 Audit support Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus
Audit support for continuous subcutaneous insulin (review of technology appraisal guidance 57) Issue date: 2008 Audit support Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus
Placename CCG. Policies for the Commissioning of Healthcare
 Placename CCG Policies for the Commissioning of Healthcare Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus. This document is part
Placename CCG Policies for the Commissioning of Healthcare Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus. This document is part
(minutes for web publishing)
 Gastrointestinal Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 28 March 2017 (minutes for web publishing) Gastrointestinal Subcommittee minutes are published
Gastrointestinal Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 28 March 2017 (minutes for web publishing) Gastrointestinal Subcommittee minutes are published
Monitoring in Type 2 Diabetes. Learning Outcomes. Type 2 Diabetes. Senga Hunter Community Diabetes Specialist Nurse
 Monitoring in Type 2 Diabetes Senga Hunter Community Diabetes Specialist Nurse Learning Outcomes Understand why blood monitoring is necessary Understand the blood tests for monitoring diabetes Understand
Monitoring in Type 2 Diabetes Senga Hunter Community Diabetes Specialist Nurse Learning Outcomes Understand why blood monitoring is necessary Understand the blood tests for monitoring diabetes Understand
Approval of Multiple Diabetes Management Products and Mesalazine proposals
 8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic
8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA151 Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus This guidance was issued in
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA151 Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus This guidance was issued in
NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system
 NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system The Freestyle Libre flash glucose monitoring system is a sensor based, factory-calibrated system that measures interstitial fluid
NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system The Freestyle Libre flash glucose monitoring system is a sensor based, factory-calibrated system that measures interstitial fluid
Walsall CCG Supported Blood Glucose Meters April 2018
 Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Diabetes in children and young people: diagnosis and management of type 1 and type 2 diabetes in children and young people
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Diabetes in children and young people: diagnosis and management of type 1 and type 2 diabetes in children and young people
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Continuous subcutaneous insulin infusion for the treatment of diabetes (review) Final scope Appraisal objective To review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Continuous subcutaneous insulin infusion for the treatment of diabetes (review) Final scope Appraisal objective To review
Respiratory Subcommittee of PTAC meeting held 5 February (minutes for web publishing)
 Respiratory Subcommittee of PTAC meeting held 5 February 2010 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Respiratory Subcommittee of PTAC meeting held 5 February 2010 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
APPLICATION FOR SUBSIDY BY SPECIAL AUTHORITY
 APPLICANT (stamp sticker acceptable) Page 1 Fm SA1603 Insulin Pumps INITIAL APPLICATION - permanent neonatal diabetes Applications only from a relevant specialist nurse practitioner. Approvals valid f
APPLICANT (stamp sticker acceptable) Page 1 Fm SA1603 Insulin Pumps INITIAL APPLICATION - permanent neonatal diabetes Applications only from a relevant specialist nurse practitioner. Approvals valid f
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Commissioning Statement
 Commissioning Statement Treatment/ device For the treatment of Commissioning position Flash Glucose Monitoring Systems (including Freestyle Libre ) Monitoring glucose levels in adults and children over
Commissioning Statement Treatment/ device For the treatment of Commissioning position Flash Glucose Monitoring Systems (including Freestyle Libre ) Monitoring glucose levels in adults and children over
SFHDiabIPT01. Assess the suitability of insulin pump therapy for an individual with Type 1 diabetes. Overview
 Assess the suitability of insulin pump therapy for an individual with Type Overview This standard covers the activities associated with assessing the suitability of insulin pump therapy for individuals
Assess the suitability of insulin pump therapy for an individual with Type Overview This standard covers the activities associated with assessing the suitability of insulin pump therapy for individuals
Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus
 Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus Version No. Changes Made Version of 05.10.2018 V1 Policy ratified by Healthier
Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus Version No. Changes Made Version of 05.10.2018 V1 Policy ratified by Healthier
Respiratory Subcommittee of PTAC Meeting held 4 March 2015
 Respiratory Subcommittee of PTAC Meeting held 4 March 2015 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and
Respiratory Subcommittee of PTAC Meeting held 4 March 2015 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and
PHARMAC responds on long-acting insulin analogues
 Vol 118 No 1224 ISSN 1175 8716 PHARMAC responds on long-acting insulin analogues A Special Series article in August by Dr Jeremy Krebs discussed the timing of funding of long-acting insulin analogues in
Vol 118 No 1224 ISSN 1175 8716 PHARMAC responds on long-acting insulin analogues A Special Series article in August by Dr Jeremy Krebs discussed the timing of funding of long-acting insulin analogues in
In keeping with the Scottish Diabetes Group criteria, use should be restricted to those who:
 Advice Statement 009-18 July 2018 Advice Statement What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin
Advice Statement 009-18 July 2018 Advice Statement What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin
Opinion 18 December 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 18 December 2013 LANTUS 100 units/ml, solution for injection in a vial B/1 vial of 10 ml (CIP: 34009 359 464 9 2)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 18 December 2013 LANTUS 100 units/ml, solution for injection in a vial B/1 vial of 10 ml (CIP: 34009 359 464 9 2)
Placename CCG. Policies for the Commissioning of Healthcare
 Placename CCG Policies for the Commissioning of Healthcare Policy for the funding of insulin pumps and continuous glucose monitoring devices for patients with diabetes 1 Introduction 1.1 This document
Placename CCG Policies for the Commissioning of Healthcare Policy for the funding of insulin pumps and continuous glucose monitoring devices for patients with diabetes 1 Introduction 1.1 This document
Insulin detemir (Levemir)... 2 Leuprorelin... 4 Levetiracetam (Keppra)... 5 Budesonide/eformoteral (Symbicort) widening access...
 August 2005 PTAC Meeting PTAC minutes are published in accordance with the following definitions from the PTAC Guidelines 2002: Minute means that part of the record of a PTAC or Sub-committee meeting (including
August 2005 PTAC Meeting PTAC minutes are published in accordance with the following definitions from the PTAC Guidelines 2002: Minute means that part of the record of a PTAC or Sub-committee meeting (including
Type 1 Diabetes: diagnosis and management. Stakeholder workshop
 Type 1 Diabetes: diagnosis and management Stakeholder workshop 28 th May 2012 Royal College of Paediatrics & Child Health: 5-11 Theobalds Road, London WC1X 8SH Summary notes The stakeholder scoping workshop
Type 1 Diabetes: diagnosis and management Stakeholder workshop 28 th May 2012 Royal College of Paediatrics & Child Health: 5-11 Theobalds Road, London WC1X 8SH Summary notes The stakeholder scoping workshop
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Gestational diabetes: risk assessment, testing, diagnosis and management bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
Gestational diabetes: risk assessment, testing, diagnosis and management bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
Changes to the National Diabetes Services Scheme (NDSS)
 Changes to the National Diabetes Services Scheme (NDSS) June 2016 FREQUENTLY ASKED QUESTIONS 1. What are the changes from 1 July 2016? 2. Why are the supply changes happening? 3. What is the role of Diabetes
Changes to the National Diabetes Services Scheme (NDSS) June 2016 FREQUENTLY ASKED QUESTIONS 1. What are the changes from 1 July 2016? 2. Why are the supply changes happening? 3. What is the role of Diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
NHS Leeds CCG. Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults
 NHS Leeds CCG Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults Produced by: Jo Alldred, Medicines Effectiveness Lead, NHS Leeds CCG Dr Bryan Power, Long Term Conditions
NHS Leeds CCG Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults Produced by: Jo Alldred, Medicines Effectiveness Lead, NHS Leeds CCG Dr Bryan Power, Long Term Conditions
COPYRIGHTED MATERIAL. Chapter 1 An Introduction to Insulin Pump Therapy WHAT IS INSULIN PUMP THERAPY?
 Chapter 1 An Introduction to Insulin Pump Therapy This chapter will provide information on what insulin pump therapy is, and how insulin pumps have developed from the early models introduced in the 1970s
Chapter 1 An Introduction to Insulin Pump Therapy This chapter will provide information on what insulin pump therapy is, and how insulin pumps have developed from the early models introduced in the 1970s
Diabetes and Pregnancy
 Diabetes and Pregnancy Dr Warren Gillibrand Deputy Director of Postgraduate Education Department of Nursing & Midwifery Department of AHP and Sports Science w.p.gillibrand@hud.ac.uk Aims of the session
Diabetes and Pregnancy Dr Warren Gillibrand Deputy Director of Postgraduate Education Department of Nursing & Midwifery Department of AHP and Sports Science w.p.gillibrand@hud.ac.uk Aims of the session
Endocrinology Subcommittee of PTAC Meeting held 17 June (minutes for web publishing)
 Endocrinology Subcommittee of PTAC Meeting held 17 June 2014 (minutes for web publishing) Endocrinology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Endocrinology Subcommittee of PTAC Meeting held 17 June 2014 (minutes for web publishing) Endocrinology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Analgesic Subcommittee of PTAC Meeting held 1 March 2016
 Analgesic Subcommittee of PTAC Meeting held 1 March 2016 (minutes for web publishing) The Analgesic Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and
Analgesic Subcommittee of PTAC Meeting held 1 March 2016 (minutes for web publishing) The Analgesic Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and
Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline
 Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline 6 November 2014 Gary Tonkin Today Process to develop the guideline What the guideline recommends
Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline 6 November 2014 Gary Tonkin Today Process to develop the guideline What the guideline recommends
DIABETES STRUCTURED EDUCATION IN WORCESTERSHIRE Information for Healthcare Professionals May 2011
 DIABETES STRUCTURED EDUCATION IN WORCESTERSHIRE Information for Healthcare Professionals May 2011 What is Structured Education? Diabetes Structured Education is referred to in the Diabetes NSF standards
DIABETES STRUCTURED EDUCATION IN WORCESTERSHIRE Information for Healthcare Professionals May 2011 What is Structured Education? Diabetes Structured Education is referred to in the Diabetes NSF standards
Commissioning Statement. Flash Glucose Monitoring system (FreeStyle Libre ) March 2018
 Technology Commissioning Statement Flash Glucose Monitoring system (FreeStyle Libre ) March 2018 FreeStyle Libre (Abbott) Flash Glucose Monitoring System for use in adults, young people and children. Recommendation
Technology Commissioning Statement Flash Glucose Monitoring system (FreeStyle Libre ) March 2018 FreeStyle Libre (Abbott) Flash Glucose Monitoring System for use in adults, young people and children. Recommendation
medicines_management/correspondence/pathway-for-the-managed- Access-of-FreeStyle-Libre.
 Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland www.hscboard.hscni.net/download/publications/pharmacy_and_
Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland www.hscboard.hscni.net/download/publications/pharmacy_and_
EFFECTIVE SHARE CARE AGREEMENT. FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY
 Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Insulin pumps (CSII): Using Modern technology to communicate with families of children with type 1 diabetes
 Insulin pumps (CSII): Using Modern technology to communicate with families of children with type 1 diabetes Grace Harris and Rita Sigley Paediatric Diabetes Nurse Specialists Starship children s Health
Insulin pumps (CSII): Using Modern technology to communicate with families of children with type 1 diabetes Grace Harris and Rita Sigley Paediatric Diabetes Nurse Specialists Starship children s Health
Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (<18 years of age)
 Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (
Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (
The new face of diabetes care in New Zealand
 UPFRONT The new face of diabetes care in New Zealand ON 1 JULY, 2012 the Get Checked programme, under which diabetes follow-up care in New Zealand is funded, will cease to exist. In its place will be the
UPFRONT The new face of diabetes care in New Zealand ON 1 JULY, 2012 the Get Checked programme, under which diabetes follow-up care in New Zealand is funded, will cease to exist. In its place will be the
SFHDiabPT03 Provide dietary education for an individual with Type 1 diabetes who is contemplating insulin pump therapy
 Provide dietary education for an individual with Type 1 diabetes who is contemplating insulin pump therapy Overview This standard concerns the activities of helping an individual with diabetes understand
Provide dietary education for an individual with Type 1 diabetes who is contemplating insulin pump therapy Overview This standard concerns the activities of helping an individual with diabetes understand
Description of the technology
 Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
The principles of insulin adjustment guidance
 The principles of insulin adjustment guidance Tips for insulin titration Blood glucose (BG) monitoring is needed to help identify the efficacy of treatment in diabetes. Monitor blood glucose according
The principles of insulin adjustment guidance Tips for insulin titration Blood glucose (BG) monitoring is needed to help identify the efficacy of treatment in diabetes. Monitor blood glucose according
(minutes for web publishing)
 Dermatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 20 October 2017 (minutes for web publishing) Dermatology Subcommittee minutes are published in accordance
Dermatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 20 October 2017 (minutes for web publishing) Dermatology Subcommittee minutes are published in accordance
Cardiovascular Subcommittee of PTAC Meeting held 17 February 2016
 Cardiovascular Subcommittee of PTAC Meeting held 17 February 2016 (minutes for web publishing) Cardiovascular Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Cardiovascular Subcommittee of PTAC Meeting held 17 February 2016 (minutes for web publishing) Cardiovascular Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Monitoring polypharmacy and reducing problematic prescribing
 CLINICAL AUDIT Monitoring polypharmacy and reducing problematic prescribing Valid to November 2020 bpac nz better medicin e Audit Focus Patients taking ten or more medicines are at an increased risk of
CLINICAL AUDIT Monitoring polypharmacy and reducing problematic prescribing Valid to November 2020 bpac nz better medicin e Audit Focus Patients taking ten or more medicines are at an increased risk of
Comments. Type 2 Diabetes
 Stakeholder organisation(s) (or your name if you are commenting as an individual): Name of commentator (leave blank if you are commenting as an individual): Document Comment (full version, short version
Stakeholder organisation(s) (or your name if you are commenting as an individual): Name of commentator (leave blank if you are commenting as an individual): Document Comment (full version, short version
Insulin Pump Therapy in children. Prof. Abdulmoein Al-Agha, FRCPCH(UK)
 Insulin Pump Therapy in children Prof. Abdulmoein Al-Agha, FRCPCH(UK) aagha@kau.edu.sa Highlights Evolution of insulin pump Pumps mimics Pancreas Goals of diabetes care What lowers HbA1c Criteria for selection
Insulin Pump Therapy in children Prof. Abdulmoein Al-Agha, FRCPCH(UK) aagha@kau.edu.sa Highlights Evolution of insulin pump Pumps mimics Pancreas Goals of diabetes care What lowers HbA1c Criteria for selection
PHARMACOLOGY AND PHARMACOKINETICS
 DRUG GUIDELINE Insulin, human neutral (Actrapid ) Intravenous Infusion for SCOPE (Area): FOR USE IN: Critical Care Unit, Emergency Department and Operating Suite EXCLUSIONS: Paediatrics (seek Paediatrician
DRUG GUIDELINE Insulin, human neutral (Actrapid ) Intravenous Infusion for SCOPE (Area): FOR USE IN: Critical Care Unit, Emergency Department and Operating Suite EXCLUSIONS: Paediatrics (seek Paediatrician
August 2004 PTAC meeting. PTAC minutes are published in accordance with the following definitions from the PTAC Guidelines 2002:
 August 2004 PTAC meeting PTAC minutes are published in accordance with the following definitions from the PTAC Guidelines 2002: Minute means that part of the record of a PTAC or Sub-committee meeting (including
August 2004 PTAC meeting PTAC minutes are published in accordance with the following definitions from the PTAC Guidelines 2002: Minute means that part of the record of a PTAC or Sub-committee meeting (including
DANII Foundation. Pre-Budget Submission Extending Lifesaving CGM Technology and
 DANII Foundation Pre-Budget Submission 2018-19 Extending Lifesaving CGM Technology and Addressing Unmet Need in Diabetes Education and Support in Australian Schools Executive Summary Diabetes is recognised
DANII Foundation Pre-Budget Submission 2018-19 Extending Lifesaving CGM Technology and Addressing Unmet Need in Diabetes Education and Support in Australian Schools Executive Summary Diabetes is recognised
The Vision. The Objectives
 The Vision Older people participate to their fullest ability in decisions about their health and wellbeing and in family, whānau and community life. They are supported in this by co-ordinated and responsive
The Vision Older people participate to their fullest ability in decisions about their health and wellbeing and in family, whānau and community life. They are supported in this by co-ordinated and responsive
(minutes for web publishing)
 Rheumatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 17 October 2017 (minutes for web publishing) Rheumatology Subcommittee minutes are published in
Rheumatology Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 17 October 2017 (minutes for web publishing) Rheumatology Subcommittee minutes are published in
Trust Guideline for the Management of: Insulin Pump Therapy in Children with Type 1 Diabetes
 A clinical guideline recommended for use In: By: For: Key words: Written by: Supported by: Approved by: Jenny Lind Children s Department Jenny Lind Diabetes Team Children on CSII therapy CSII, Insulin
A clinical guideline recommended for use In: By: For: Key words: Written by: Supported by: Approved by: Jenny Lind Children s Department Jenny Lind Diabetes Team Children on CSII therapy CSII, Insulin
Type 2 Diabetes Recommended SMBG
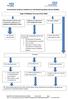 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Anneli, Martina s daughter In better control with her pump since 2011 MY CHILD HAS TYPE 1 DIABETES
 Anneli, Martina s daughter In better control with her pump since 2011 MY CHILD HAS TYPE 1 DIABETES Many parents whose child is diagnosed with Type 1 diabetes wonder: Why is this happening to my child?
Anneli, Martina s daughter In better control with her pump since 2011 MY CHILD HAS TYPE 1 DIABETES Many parents whose child is diagnosed with Type 1 diabetes wonder: Why is this happening to my child?
Guidelines for the care of Children with Diabetes Mellitus undergoing Surgery
 Guidelines for the care of Children with Diabetes Mellitus undergoing Surgery Background Surgery places physical and emotional stress on the body. This, alongside new surroundings, parental anxiety and
Guidelines for the care of Children with Diabetes Mellitus undergoing Surgery Background Surgery places physical and emotional stress on the body. This, alongside new surroundings, parental anxiety and
Cystic Fibrosis Panel Applications (Dornase Alfa) Contents
 Cystic Fibrosis Panel Applications (Dornase Alfa) Contents Page 2-4: Entry and Stopping Criteria for Treatment with Dornase Alfa Page 5-9: Application and consent forms for a one month trail and long term
Cystic Fibrosis Panel Applications (Dornase Alfa) Contents Page 2-4: Entry and Stopping Criteria for Treatment with Dornase Alfa Page 5-9: Application and consent forms for a one month trail and long term
Insulin Pump An information session to help you decide if you are ready to use an insulin pump.
 Insulin Pump An information session to help you decide if you are ready to use an insulin pump. Welcome Welcome to the insulin pump information session. We hope this session helps you to decide whether
Insulin Pump An information session to help you decide if you are ready to use an insulin pump. Welcome Welcome to the insulin pump information session. We hope this session helps you to decide whether
QOF Indicator DM013:
 QOF Indicator DM013: The percentage of patients with diabetes, on the register, who have a record of a dietary review by a suitably competent professional in the preceding 12 months Note: the bold signposts
QOF Indicator DM013: The percentage of patients with diabetes, on the register, who have a record of a dietary review by a suitably competent professional in the preceding 12 months Note: the bold signposts
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations
 Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
Benefit Criteria for Diabetic Equipment and Supplies Home Health Services Changing July 1, 2011
 Benefit Criteria for Diabetic Equipment and Supplies Home Health Services Changing July 1, 2011 Information posted May 13, 2011 Effective for dates of service on or after July 1, 2011, the benefit criteria
Benefit Criteria for Diabetic Equipment and Supplies Home Health Services Changing July 1, 2011 Information posted May 13, 2011 Effective for dates of service on or after July 1, 2011, the benefit criteria
Nutritional products (Special Foods) supplied by Nutricia
 2 April 2012 Nutritional products (Special Foods) supplied by Nutricia PHARMAC is pleased to announce the funding of a number of nutritional products (Special Foods) supplied by Nutricia. These products
2 April 2012 Nutritional products (Special Foods) supplied by Nutricia PHARMAC is pleased to announce the funding of a number of nutritional products (Special Foods) supplied by Nutricia. These products
Proposal on subsidy changes for some respiratory inhalation products and access restrictions to combination inhalers.
 30 August 2011 Proposal on changes for some respiratory products and access restrictions to combination inhalers. As notified on 16 June 2011, PHARMAC has been considering options for managing the funding
30 August 2011 Proposal on changes for some respiratory products and access restrictions to combination inhalers. As notified on 16 June 2011, PHARMAC has been considering options for managing the funding
Diabetes in England NHS Medical Directorate. Dr Rowan Hillson MBE National Clinical Director for Diabetes
 Diabetes in England 2010 Dr Rowan Hillson MBE National Clinical Director for Diabetes I strongly believe that Everyone with diabetes deserves the highest standards of personalised diabetes care, no matter
Diabetes in England 2010 Dr Rowan Hillson MBE National Clinical Director for Diabetes I strongly believe that Everyone with diabetes deserves the highest standards of personalised diabetes care, no matter
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Consultation Group: Dr Amalia Mayo, Paediatric Consultant. Review Date: March Uncontrolled when printed. Version 2. Executive Sign-Off
 Policy For The Adjustment Of Insulin Injections By Paediatric Diabetes Specialist Nurses/Community Paediatric Nurses Diabetes Working With Children Within NHS Grampian Co-ordinators: Lead Paediatric Diabetes
Policy For The Adjustment Of Insulin Injections By Paediatric Diabetes Specialist Nurses/Community Paediatric Nurses Diabetes Working With Children Within NHS Grampian Co-ordinators: Lead Paediatric Diabetes
Social, political and economic implications of self-blood glucose monitoring in type 2 diabetes management.
 Social, political and economic implications of self-blood glucose monitoring in type 2 diabetes management. A qualitative study of how Australian health care professionals form their convictions viewed
Social, political and economic implications of self-blood glucose monitoring in type 2 diabetes management. A qualitative study of how Australian health care professionals form their convictions viewed
Blood Glucose Test Strip Guidelines
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Immunisation Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 26 July (minutes for web publishing)
 Immunisation Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 26 July 2017 (minutes for web publishing) Immunisation Subcommittee minutes are published in accordance
Immunisation Subcommittee of the Pharmacology and Therapeutics Advisory Committee (PTAC) Meeting held on 26 July 2017 (minutes for web publishing) Immunisation Subcommittee minutes are published in accordance
Proposals regarding the Cholesterol Lowering Pharmaceuticals Atorvastatin and Ezetimibe
 26 April 2010 Proposals regarding the Cholesterol Lowering Pharmaceuticals Atorvastatin and Ezetimibe PHARMAC is seeking feedback on the following proposals relating to the cholesterol lowering pharmaceuticals
26 April 2010 Proposals regarding the Cholesterol Lowering Pharmaceuticals Atorvastatin and Ezetimibe PHARMAC is seeking feedback on the following proposals relating to the cholesterol lowering pharmaceuticals
15 th Annual DAFNE collaborative meeting Tuesday 28 th June 2016
 15 th Annual DAFNE collaborative meeting Tuesday 28 th June 2016 Sponsored by: Abbott Diabetes Care and Lilly Diabetes The REPOSE Trial (Relative Effectiveness of Pumps over Structured Education) Background
15 th Annual DAFNE collaborative meeting Tuesday 28 th June 2016 Sponsored by: Abbott Diabetes Care and Lilly Diabetes The REPOSE Trial (Relative Effectiveness of Pumps over Structured Education) Background
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes
 Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
Special Foods Subcommittee of PTAC Meeting held 5 December (minutes for web publishing)
 Special Foods Subcommittee of PTAC Meeting held 5 December 2013 (minutes for web publishing) Special Foods Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Special Foods Subcommittee of PTAC Meeting held 5 December 2013 (minutes for web publishing) Special Foods Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Personal statement on Continuous Subcutaneous Insulin Infusion Professor John Pickup
 1 Personal statement on Continuous Subcutaneous Insulin Infusion Professor John Pickup King s College London School of Medicine, Guy s Hospital, London SE1 9RT Experience of the technology I am the lead
1 Personal statement on Continuous Subcutaneous Insulin Infusion Professor John Pickup King s College London School of Medicine, Guy s Hospital, London SE1 9RT Experience of the technology I am the lead
Diabetes Medical Management Plan
 SCHOOL DISTRICT OF LEE COUNTY HEALTH SERVICES Print Form Date of Plan Diabetes Medical Management Plan This plan should be completed by the student's personal health care team and parents/guardian. It
SCHOOL DISTRICT OF LEE COUNTY HEALTH SERVICES Print Form Date of Plan Diabetes Medical Management Plan This plan should be completed by the student's personal health care team and parents/guardian. It
Proposal to fund a number of Nutritional products (Special Foods) supplied by Nutricia
 16 February 2012 Proposal to fund a number of Nutritional products (Special Foods) supplied by Nutricia PHARMAC is seeking feedback on a proposal to fund a number of nutritional products (Special Foods)
16 February 2012 Proposal to fund a number of Nutritional products (Special Foods) supplied by Nutricia PHARMAC is seeking feedback on a proposal to fund a number of nutritional products (Special Foods)
Anti-Infective Subcommittee of PTAC Meeting held 13 October (minutes for web publishing)
 Anti-Infective Subcommittee of PTAC Meeting held 13 October 2016 (minutes for web publishing) Anti-Infective Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Anti-Infective Subcommittee of PTAC Meeting held 13 October 2016 (minutes for web publishing) Anti-Infective Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Managing comorbid health problems in people with eating disorders bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated
Managing comorbid health problems in people with eating disorders bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated
Provincial Clinical Knowledge Topic Insulin Pump Therapy, Pediatric and Adult Acute Care V 1.0
 Provincial Clinical Knowledge Topic Insulin Pump Therapy, Pediatric and Adult Acute Care V 1.0 Copyright: 2018, Alberta Health Services. This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives
Provincial Clinical Knowledge Topic Insulin Pump Therapy, Pediatric and Adult Acute Care V 1.0 Copyright: 2018, Alberta Health Services. This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives
Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin)
 14 July 2015 Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) PHARMAC is seeking feedback on a proposal relating to the funding of the TNF-inhibitor medicines
14 July 2015 Proposal relating to the funding of TNF inhibitors (Humira and Enbrel) and gabapentin (Neurontin) PHARMAC is seeking feedback on a proposal relating to the funding of the TNF-inhibitor medicines
Executive Summary Management of Type 1 Diabetes Mellitus during illness in children and young people under 18 years (Sick Day Rules)
 Executive Summary Management of Type 1 Diabetes Mellitus during illness in children and young people under 18 years (Sick Day Rules) SETTING FOR STAFF PATIENTS Medical and nursing staff Children and young
Executive Summary Management of Type 1 Diabetes Mellitus during illness in children and young people under 18 years (Sick Day Rules) SETTING FOR STAFF PATIENTS Medical and nursing staff Children and young
Approach to the Young child & Parent with Child with DM Best Structure for Continued Care
 Approach to the Young child & Parent with Child with DM Best Structure for Continued Care M.S. Limbe MD Paediatric Endcocrinologist Aga Khan University Hospital, Nairobi Approach to the Young Child & Parent
Approach to the Young child & Parent with Child with DM Best Structure for Continued Care M.S. Limbe MD Paediatric Endcocrinologist Aga Khan University Hospital, Nairobi Approach to the Young Child & Parent
DISCOVER THE POWER OF CONNECTION MINIMED 640G
 DISCOVER THE POWER OF CONNECTION MINIMED 640G INSULIN PUMP THERAPY CHANGING LIVES TODAY Have you just been diagnosed with insulin dependent diabetes? Perhaps you ve been on multiple daily injection therapy
DISCOVER THE POWER OF CONNECTION MINIMED 640G INSULIN PUMP THERAPY CHANGING LIVES TODAY Have you just been diagnosed with insulin dependent diabetes? Perhaps you ve been on multiple daily injection therapy
Standard Operating Procedure 11. Completion of DAFNE Data Collection: Full Post Course Form F04.010, Version 8
 Standard Operating Procedure 11 Completion of DAFNE Data Collection: Full Post Course Form F04.010, Version 8 Date Version Issue Review Contact Approved Date Date Person October 2013 9 October 2013 March
Standard Operating Procedure 11 Completion of DAFNE Data Collection: Full Post Course Form F04.010, Version 8 Date Version Issue Review Contact Approved Date Date Person October 2013 9 October 2013 March
Paolo Di Bartolo U.O di Diabetologia Dip. Malattie Digestive & Metaboliche AULS Prov. di Ravenna. Ipoglicemie e Monitoraggio Glicemico
 Paolo Di Bartolo U.O di Diabetologia Dip. Malattie Digestive & Metaboliche AULS Prov. di Ravenna Ipoglicemie e Monitoraggio Glicemico Management of Hypoglycaemia.if hypoglycemia is a problem, the principles
Paolo Di Bartolo U.O di Diabetologia Dip. Malattie Digestive & Metaboliche AULS Prov. di Ravenna Ipoglicemie e Monitoraggio Glicemico Management of Hypoglycaemia.if hypoglycemia is a problem, the principles
CLINICAL AUDIT. Initiating Insulin. in Patients with Type 2 Diabetes
 CLINICAL AUDIT Initiating Insulin in Patients with Type 2 Diabetes bpac nz better medicin e Focus This focus of this audit is patients with type 2 diabetes on oral anti-diabetic medications who have poor
CLINICAL AUDIT Initiating Insulin in Patients with Type 2 Diabetes bpac nz better medicin e Focus This focus of this audit is patients with type 2 diabetes on oral anti-diabetic medications who have poor
New NICE guidance: Changes in practice for multidisciplinary teams. Part 1: Type 1 diabetes in children and young people
 Article New NICE guidance: Changes in practice for multidisciplinary teams. Part 1: Type 1 diabetes in children and young people Helen Thornton This is the first of two articles on the 2015 NICE NG18 guideline,
Article New NICE guidance: Changes in practice for multidisciplinary teams. Part 1: Type 1 diabetes in children and young people Helen Thornton This is the first of two articles on the 2015 NICE NG18 guideline,
DIABETES WITH PREGNANCY
 DIABETES WITH PREGNANCY Prof. Aasem Saif MD,MRCP(UK),FRCP (Edinburgh) Maternal and Fetal Risks Diabetes in pregnancy is associated with risks to the woman and to the developing fetus. Maternal and Fetal
DIABETES WITH PREGNANCY Prof. Aasem Saif MD,MRCP(UK),FRCP (Edinburgh) Maternal and Fetal Risks Diabetes in pregnancy is associated with risks to the woman and to the developing fetus. Maternal and Fetal
DIABETES MANAGEMENT PLAN 2019
 SCHOOL SETTING Insulin pump therapy Use in conjunction with Action Plan This plan has been adapted from the original work of Diabetes Victoria, Monash Children s Hospital and the Royal Children s Hospital,
SCHOOL SETTING Insulin pump therapy Use in conjunction with Action Plan This plan has been adapted from the original work of Diabetes Victoria, Monash Children s Hospital and the Royal Children s Hospital,
Pre admission & surgery Pre-admission Nurses Association SIG Catherine Prochilo Credentialled Diabetes Nurse Educator Sat 23 March 2013
 Pre admission & surgery Pre-admission Nurses Association SIG Catherine Prochilo Credentialled Diabetes Nurse Educator Sat 23 March 2013 www.diabetesvic.org.au Plan/ overview Issue/ presenting problems
Pre admission & surgery Pre-admission Nurses Association SIG Catherine Prochilo Credentialled Diabetes Nurse Educator Sat 23 March 2013 www.diabetesvic.org.au Plan/ overview Issue/ presenting problems
GESTATIONAL DIABETES (DIET/INSULIN/ METFORMIN) CARE OF WOMEN IN BIRTHING SUITE
 GESTATIONAL DIABETES (DIET/INSULIN/ METFORMIN) CARE OF WOMEN IN BIRTHING SUITE DEFINITION A disorder characterised by hyperglycaemia first recognised during pregnancy due to increased insulin resistance
GESTATIONAL DIABETES (DIET/INSULIN/ METFORMIN) CARE OF WOMEN IN BIRTHING SUITE DEFINITION A disorder characterised by hyperglycaemia first recognised during pregnancy due to increased insulin resistance
9 Diabetes care. Back to contents
 Back to contents Diabetes is a major risk factor for the development of peripheral vascular disease and 349/628 (55.6%) of the patients in this study had diabetes. Hospital inpatients with diabetes are
Back to contents Diabetes is a major risk factor for the development of peripheral vascular disease and 349/628 (55.6%) of the patients in this study had diabetes. Hospital inpatients with diabetes are
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens
 EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
Diabetes Technology Continuous Subcutaneous Insulin Infusion Therapy And Continuous Glucose Monitoring In Adults: An Endocrine Society Clinical
 Diabetes Technology Continuous Subcutaneous Insulin Infusion Therapy And Continuous Glucose Monitoring In Adults: An Endocrine Society Clinical Practice Guideline Task Force Members Anne Peters, MD (Chair)
Diabetes Technology Continuous Subcutaneous Insulin Infusion Therapy And Continuous Glucose Monitoring In Adults: An Endocrine Society Clinical Practice Guideline Task Force Members Anne Peters, MD (Chair)
