In Vitro Determination of the Main Effects in the Design of High-Flow Nasal Therapy Systems with Respect to Aerosol Performance
|
|
|
- Loraine Nash
- 5 years ago
- Views:
Transcription
1 Pulm Ther (2018) 4: ORIGINAL RESEARCH In Vitro Determination of the Main Effects in the Design of High-Flow Nasal Therapy Systems with Respect to Aerosol Performance Gavin Bennett. Mary Joyce. Louise Sweeney. Ronan MacLoughlin Received: March 1, 2018 / Published online: April 18, 2018 Ó The Author(s) 2018 ABSTRACT Introduction: The use of concurrent aerosol delivery during high-flow nasal therapy (HFNT) may be exploited to facilitate the delivery of a variety of prescribed medications for inhalation. Until now, a systematic approach to determine the conditions required to yield an optimal emitted dose has not been reported. The aim of this study was to establish the effects of gas flow rate, input droplet size, and nebulizer position on the amount of aerosol exiting the nasal cannula during HFNT and thus becoming available for inhalation. Methods: Testing was completed according to a factorial statistical design of experiments (DOE) approach. Emitted dose was characterized with a vibrating mesh nebulizer (Aerogen Solo, Aerogen Ltd) for an adult model of HFNT at three clinically relevant gas flow rates, using three nebulizers producing varying input droplet sizes and placed at two different nebulizer positions. Enhanced content To view enhanced content for this article go to G. Bennett (&) M. Joyce L. Sweeney R. MacLoughlin Aerogen Limited, IDA Business Park, Dangan, Galway, Ireland GBennett@aerogen.com Results: Increasing the gas flow rate significantly lowered the emitted dose, with a dose of 7.10% obtained at 10 LPM, 2.67% at 25 LPM, and 1.30% at 40 LPM (p \ ). There was a significant difference in emitted dose between nebulizers with different input droplet sizes, with increasing input droplet size associated with a reduced emitted dose (6.11% with an input droplet size of 3.22 lm, 2.76% with 4.05 lm, and 2.38% with 4.88 lm, p = , Pearson s r = ). In addition, the droplet size exiting the nasal cannula interface was lower than that produced by the aerosol generator for all devices under test. Positioning the nebulizer immediately after the humidification chamber yielded a marginally greater emitted dose (3.79%) than when the nebulizer was placed immediately upstream of the nasal cannula (3.39%). Flow rate, input droplet size, and nebulizer position were at the 0.10 level of significance, indicating that all three factors had significant effects on emitted dose. According to the DOE model, flow rate had the greatest influence on emitted dose, followed by input droplet size and then nebulizer position. Conclusion: Our findings indicate that in order to optimize the amount of aerosol exiting the nasal cannula interface during HFNT, it is necessary for gas flow rate to be low and the input droplet size to be small, while the nebulizer should be positioned immediately after the humidification chamber. Funding: Aerogen Limited.
2 74 Pulm Ther (2018) 4:73 86 Keywords: Aerosol; Design of experiments; Droplet size; Emitted dose; Flow rate; Highflow nasal therapy; Nebulizer INTRODUCTION High-flow nasal therapy (HFNT) is a means of delivering heated humidified air/oxygen mixes to patient airways that allows for higher flows than conventional low-flow therapy [1]. HFNT provides sufficient flow rates to equal or exceed patient inspiratory flow and reduces the inspiratory resistance associated with the nasopharynx, thus reducing the work of breathing [2]. Humidification during HFNT is essential for proper function of the epithelial lining and is an accepted standard of care. Without humidification, unidirectional inspiratory nasal airflow may lead to the drying of mucosa and the release of inflammation mediators [3]. Delivering inhaled medications to the lungs from a nasal cannula interface is particularly advantageous for medications with extended delivery times or a frequent dosing regimen. The use of concurrent aerosol delivery during HFNT may be exploited to facilitate the delivery of a variety of prescribed medications for inhalation. These include but are not limited to b-agonists and hypertonic saline [4, 5]. The mounting observations of response and clinical evidence in combination with its ease of use and patient tolerability have resulted in increasing adoption of HFNT, with particular interest in concurrent aerosol delivery during HFNT [6 10]. Morgan et al. showed that infants with acute bronchiolitis tolerated aerosolized b-agonist therapy better during HFNT than with a facemask, possibly preventing them from escalating to more invasive respiratory support [11]. In vitro studies indicate that aerosols can be efficiently delivered during HFNT [12, 13]. However, several factors influence both the amount of aerosol exiting the cannula and the amount available to the patient. These factors include the rate of gas delivered, the size of the nasal prongs, humidification, the size of the aerosol droplets, and the aerosol generator type and position [12 15]. Bhashyam et al. showed that aerosols can be delivered from a mesh nebulizer (Aerogen Solo, Aerogen, Ireland) through infant, pediatric, and adult nasal cannula at an oxygen flow rate of 3 LPM. However, gas flow rates of LPM are more commonly used for HFNT in adults [12]. Ari et al. reported aerosol delivery from a mesh nebulizer (Aerogen Solo) through an Optiflow (Fisher & Paykel, Auckland, New Zealand) pediatric nasal cannula at flow rates of 3 6 LPM with oxygen or heliox. The highest aerosol efficiency of * 10% was achieved at a flow rate of 3 LPM, and this decreased significantly to * 2% (with oxygen) as the flow rate was increased to 6 LPM [13]. Both of the aforementioned studies reported depositional losses in the delivery system in the range of 75 98% [12, 13]. Perry et al. reported the in vitro inspired dose of albuterol from a mesh nebulizer (Aerogen Solo) through a humidified HFNT system (Vapotherm, Stevensville, MD, USA). The inhaled doses (percent of the nominal dose) were 2.5%, 0.8%, 0.4%, and 0.2% for the adult cannula at 5, 10, 20, and 40 LPM, respectively. The authors concluded that high flow rates, small nasal prongs, humidification, and sharp changes in the direction of aerosol flow contributed to a low efficiency of in vitro aerosol delivery [15]. Several approaches to improve aerosol delivery during HFNT have been explored. During HFNT, much aerosol is lost due to impaction in the circuit due to the high flows used [14]. Longest et al. have attempted to generate submicron particles, which may reduce the chances of impaction in the circuit but it also reduces the drug mass available to the lungs [16, 17]. These studies notwithstanding, clinicians and users would benefit from a comprehensive review of the relevant factors that affect the amount of aerosol exiting the cannula. A systematic approach to establish the conditions required to yield an optimal emitted dose has not yet been reported. The objective of this study was to determine, using the wellestablished design of experiments approach, the main effects those of the gas flow rate, input droplet size, and nebulizer position on the amount of aerosol made available to the patient for inhalation during HFNT.
3 Pulm Ther (2018) 4: METHODS HFNT Circuit and Interfaces The Hudson RCI Neptune system (Teleflex Medical, High Wycombe, UK) was used with adult (P/N: KIT, ID 22 mm, length 1524 mm), pediatric (P/N: , ID 15 mm, length 1524 mm), and infant (P/N: , ID 10 mm, length 1524 mm) heated wire circuits. Adult (P/N: , ID 4 mm, length 500 mm), pediatric (P/N: , ID 3 mm, length 400 mm), and infant (P/N: , ID 2 mm, length 300 mm) nasal cannulas (Comfort Flo, Teleflex Medical) were used. Nebulizers were placed in the circuit using a standard T-piece connector positioned either proximally (immediately after the humidification chamber) or distally (immediately upstream of the cannula), as illustrated in Fig. 1. Nebulizers Experiments were performed with three vibrating mesh nebulizers (Aerogen Solo). The nebulizer performance characteristics, measured using laser diffraction (Spraytec, Malvern Instruments, Malvern, UK) as previously described [18], are outlined in terms of average input droplet size (lm volumetric median diameter, VMD) and aerosol output rate (ml/ min) (Table 1). Table 1 Input droplet size and output rate data with 0.9% saline (N = 3), direct from the nebulizer Input droplet size (lm VMD) 3.22 ± ± ± ± ± ± 0.02 Design of Experiments Aerosol output rate (ml/ min) Testing was completed according to a factorial statistical design of experiments (DOE) approach (Minitab 17 statistical software, 2010). Experimental runs (n = 60 in total) were created to fit a quadratic model, in order to establish the conditions necessary to maximize the emitted dose with an adult nasal cannula. The DOE approach automatically randomized the order in which experimental runs were completed. Randomization balances the effects of uncontrollable conditions and reduces the chance that these conditions will bias the results. Thus, the sample number for each experimental run is dictated by the DOE. A summary of the DOE study design is shown in Table 2. Inputs used included gas flow rate, droplet size, and nebulizer position within the circuit. Fig. 1 Illustration of test setup employed, indicating the two nebulizer placement positions within the HFNT circuit
4 76 Pulm Ther (2018) 4:73 86 Table 2 Study design obtained using a design of experiments approach Base design Replicates 4 Center points 20 Axial points 8 Total runs 60 Blocks 5 Emitted Dose 3 Factors, 8 runs Testing was performed in accordance with the DOE design presented in Table 3. Emitted dose was characterized during HFNT at three gas flow rates, using three nebulizers of varying input droplet size at two different nebulizer placement positions. Positions were chosen to reflect previous nebulizer placements in the literature. The nasal prongs from the cannula were placed distal to a filter (RespirGard II 303, Baxter, Ireland). To eliminate the potential for rained-out aerosol or humidity reaching the filter, a curved elbow was placed between the nasal prongs and the filter. This ensured that only aerosol droplets entrained in the gas flow were captured on the filter. The humidifier was powered on and allowed to come to temperature (37 C) and a 2 ml dose of albuterol sulphate (2 mg/ml) was nebulized. Albuterol was used as it is a commonly nebulized formulation used in the characterization of aerosol drug delivery systems, and is specified for use as a tracer aerosol in the international standard ISO 27427:2013 [19]. At the end of each dose, the drug captured on a filter was eluted using 10 ml of deionized water. The mass of drug was quantified by means of UV spectrophotometry at a wavelength of 276 nm and interpolation on a standard curve of albuterol sulphate concentrations ( lg/ml). Results for emitted dose were expressed as the percentage of the nominal dose placed in the nebulizer s medication cup. Table 3 Summary of testing completed to determine the effects of input droplet size, delivery gas flow rate, and nebulizer position on emitted dose using an adult cannula Input droplet size (lm VMD) Delivery gas flow rate Nebulizer position EMITTED DOSES AT PEDIATRIC AND INFANT CANNULAS Sample number (n) Proximal n =4 Distal n =4 25 Proximal n =0 Distal n =1 40 Proximal n =4 Distal n = Proximal n =1 Distal n =1 25 Proximal n =10 Distal n =10 40 Proximal n =1 Distal n = Proximal n =4 Distal n =4 25 Proximal n =1 Distal n =1 40 Proximal n =4 Distal n =4 In order to further investigate the effects of gas flow rate, input droplet size, and nebulizer position on emitted dose, testing was conducted using pediatric and infant nasal cannulas. The entire DOE was not completed for these cannulas only the extremes in terms of flow rate, droplet size, and nebulizer position. Flow rates were adjusted in accordance with the manufacturer s instructions. Testing was performed as outlined in Tables 4 and 5.
5 Pulm Ther (2018) 4: Table 4 Summary of testing completed to determine the effects of input droplet size, delivery gas flow rate, and nebulizer position on emitted dose using a pediatric cannula Input droplet size (lm VMD) Delivery gas flow rate Nebulizer position Sample number (n) Proximal n =3 20 Proximal n = Proximal n = Proximal n =3 Table 5 Summary of testing completed to determine the effects of input droplet size, delivery gas flow rate, and nebulizer position on emitted dose using an infant cannula Input droplet size (lm VMD) Delivery gas flow rate Nebulizer position Sample number (n) Proximal n =3 8 Proximal n = Proximal n = Proximal n =3 INFLUENCE OF NASAL CANNULA DESIGN The emitted dose was compared between two adult nasal cannula interfaces (Comfort Flo; P/N: and Comfort Flo Plus; P/N: , Hudson RCI Neptune, Teleflex Medical) when used in combination with the Hudson RCI Neptune circuit. The Comfort Flo cannula has an ID of 4 mm and length of 500 mm, while the Comfort Flo Plus cannula has an ID of 10 mm and length of 270 mm. A nebulizer of midpoint input droplet size (4.05 lm) was used. Testing was completed at three gas flow rates (10, 25, and 40 LPM). For the purposes of this test, the nebulizer was placed proximally within the HFNT circuit (wet side of the humidifier). AEROSOL DROPLET SIZE MEASUREMENT In order to characterize the potential aerosol distribution available to the patient, the mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were characterized for selected test combinations (Next Generation Impactor, Copley Scientific, Nottingham, UK), as per Pharmacopeia 34 \601[ [20]. The Aerogen Solo nebulizer and standard T-piece were presented to the throat of the impactor while operating at a vacuum of 15 LPM. A 2 ml dose of albuterol sulphate (2 mg/ml) was nebulized and drug was eluted from the collection stages of the impactor using 10 ml of deionized water. The MMAD at the nasal cannula outlet, across two delivery gas flow rates, was characterized as previously reported [21] by presenting the cannula to the impactor throat while operating at a vacuum of 15 LPM. The cannula was held in place against the opening of the impactor throat in a repeatable manner using a custom fixture. The Aerogen Solo nebulizer was positioned proximally in the HFNT circuit. MMAD was characterized at delivery gas flow rates of 10 and 40 LPM (n = 3). MMAD and GSD were calculated using the validated CITDAS software (Copley Scientific, UK). STATISTICAL DATA ANALYSIS A one-way analysis of variance (ANOVA) was conducted to establish if the emitted dose varied significantly depending on the gas flow rate
6 78 Pulm Ther (2018) 4:73 86 and when nebulizers with different input droplet sizes were used. A multiple comparison test (Tukey test) was performed to compare the mean of each column with the mean of every other column when comparing the doses emitted with different gas flow rates and by nebulizers of different input droplet sizes. A Student s t test was performed to determine if there was a significant difference in emitted dose between nebulizer positions in the circuit. p values of \ 0.05 were considered statistically significant. When testing was completed according to the statistical DOE approach, a bar graph of standardized effects was generated. If factors in the quadratic model reached the 0.10 level of significance, it was concluded that there was a significant relationship between those factors and emitted dose. Factors with longer bars in the graph had a greater influence on emitted dose. The prediction and optimization report showed factor settings that optimized emitted dose as well as alternative solutions that were nearly optimal. COMPLIANCE WITH ETHICS GUIDELINES This article does not contain any studies with human participants or animals performed by any of the authors. RESULTS Emitted Dose The mean ± standard deviation values of emitted dose (%) from the adult nasal cannula are outlined in Table 6. The emitted dose was assessed during HFNT at three different flow rates (10, 25, and 40 LPM), using three nebulizers with different input droplet sizes (3.22, 4.05, and 4.88 lm) and at two different nebulizer placement positions (proximal and distal). A bar graph of standardized effects was generated by the DOE model (Fig. 2). Gas flow rate, input droplet size, and nebulizer position were at the 0.10 level of significance, indicating that Table 6 Mean ± standard deviation values of emitted dose (%) for testing completed according to the DOE using an adult nasal cannula (Comfort Flo P/N: , Teleflex Medical) Input droplet size (lm VMD) Flow rate Nebulizer position Sample number (n) Emitted dose (%) Proximal n = ± 0.39 Distal n = ± Proximal n = 0 n/a Distal n = Proximal n = ± 0.81 Distal n = ± Proximal n = Distal n = Proximal n = ± 0.77 Distal n = ± Proximal n = Distal n = Proximal n = ± 0.52 Distal n = ± Proximal n = Distal n = Proximal n = ± 0.40 Distal n = ± 0.19 all three factors had a significant effect on emitted dose. Based on the results presented, gas flow rate had the greatest effect on emitted dose, followed by input droplet size and then nebulizer position. The optimal settings to maximize the emitted dose were as follows: input droplet size of 3.22 lm, gas flow rate of 10 LPM, and a proximal nebulizer position at the humidifier. The graph also highlights the effects when the settings of two factors were changed together. Altering the settings of input droplet size and gas flow rate (AB) or of input
7 Pulm Ther (2018) 4: Influence of Gas Flow Rate on Emitted Dose Fig. 2 Effects of flow rate, input droplet size, and nebulizer position on emitted dose. Blue bars in the graph show the factors that had significant effects on the emitted dose (at the 0.1 level of significance). Gray bars represent nonsignificant terms that were removed from the model. Flow rate had the greatest influence on emitted dose, followed by input droplet size and then nebulizer position Increasingthegasflowratereducedtheemitted dose, with a dose of 7.10% ± 0.86 obtained at 10 LPM, 2.67% ± 0.14 at 25 LPM, and 1.30% ± 0.21 at 40 LPM (Fig. 3). A one-way ANOVA was conducted to establish if the emitted dose varied statistically significantly with gas flow rate. The one-way ANOVA resulted in a p value of \ , indicating that different gas flow rates yielded significantly different emitted doses. A multiple comparison test (Tukey s test) showed thatgasflowratesof10and25lpmgavesignificantly different emitted doses (p\ ), as did 10 and 40 LPM (p \ ). The difference between the emitted doses obtained with gas flow rates of 25 and 40 LPM was not significant (p value = ). droplet size and nebulizer position (AC) simultaneously significantly affected the amount of drug exiting the cannula outlet. The main aim of this study was to establish how changes to a single factor affected the mean emitted dose. The prediction and optimization report showed factor settings that optimized the emitted dose as well as alternative solutions that were nearly optimal, as outlined in Table 7. The model explained 92.80% of the variation in emitted dose. Table 7 Top five alternative solutions to maximize emitted dose with an adult nasal cannula (Comfort Flo P/N: , Teleflex Medical) Input droplet size (lm VMD) Flow rate Nebulizer position Distal Proximal Distal Proximal Distal 4.37 Predicted emitted dose (%) Influence of Input Droplet Size on Emitted Dose Increasing the input droplet size reduced the emitted dose, with a dose of 6.11% ± 1.06 obtained with a droplet size of 3.22 lm, 2.76% ± 0.25 with 4.05 lm, and 2.38% ± 0.25 with 4.88 lm (Fig.4). A one-way ANOVA was conducted to establish if different droplet sizes yielded significantly different emitted dose between input droplet sizes (3.22, 4.05, and 4.88 lm). The one-way ANOVA resulted in a p value of , indicating that nebulizers with different input droplet sizes yield significantly different emitted doses. This relationship generated a Fig. 3 Effect of gas flow rate on emitted dose
8 80 Pulm Ther (2018) 4:73 86 Fig. 4 Effect of input droplet size on emitted dose Pearson correlation coefficient (r) of A multiple comparison test (Tukey s test) between nebulizers with input droplet sizes of 3.22 and 4.05 lm provided significantly different emitted doses (p = ), as did the nebulizers with input droplet sizes of 3.22 and 4.88 lm(p = ). The difference in the emitted doses of nebulizers with input droplet sizes of 4.05 and 4.88 lm wasnot significant (p = ). Influence of Nebulizer Position on Emitted Dose Mean ± standard deviation values of the emitted dose were 3.79 ± 0.68% for a proximal nebulizer placement position (immediately after the humidification chamber) and 3.39 ± 0.49% for a distal nebulizer placement position (immediately upstream of the nasal cannula) (Fig. 5). A Student s t test was performed to determine if the emitted dose varied significantly depending on the nebulizer position in the humidification circuit. The Student s t test resulted in a p value of , indicating that switching the nebulizer position resulted in a small but not statistically significant change in emitted dose. Influence of Nasal Cannula Design on Emitted Dose The doses emitted when using two adult nasal cannula interfaces (Comfort Flo and Comfort Fig. 5 Effect of nebulizer position on emitted dose Flo Plus, Hudson RCI Neptune, Teleflex Medical) with the same HFNT circuit were compared. A nebulizer of midpoint droplet size (4.05 lm) was used. Testing was also completed at three gas flow rates (10, 25, and 40 LPM). The mean ± standard deviation values of emitted dose are presented in Table 8. Emitted Dose Using Pediatric and Infant Cannulas The mean ± standard deviation values of emitted dose (%) from the pediatric and infant nasal cannulas are outlined in Tables 9 and 10, respectively. Just as seen when testing the adult cannula, a greater dose was emitted when a low gas flow rate was used with a small input droplet size and a proximal nebulizer position. Aerosol Droplet Size Measurement The MMAD and GSD for each nebulizer under test was first characterized. Results from droplet size characterization at gas flow rates of 10 and 40 LPM are shown in Table 11. The droplet size exiting the nasal cannula interface was consistently lower than that produced by the nebulizer (input droplet size). In addition, the droplet size at the adult nasal cannula outlet decreased with increasing gas flow rate: 1.82 lm at 10 LPM and 1.72 lm at 40 LPM with an input droplet size of 3.22 lm VMD, 1.90 lm at 10 LPM and 1.72 lm at 40 LPM with an input droplet
9 Pulm Ther (2018) 4: Table 8 Effect of nasal cannula design (delivery gas flow rate and cannula type) on emitted dose Delivery gas flow size of 4.05 lm VMD, and 1.68 lm at 10 LPM and 1.31 lm at 40 LPM with an input droplet size of 4.88 lm VMD. DISCUSSION Comfort Flo Comfort Flo Plus ± ± ± ± 0.98 The use of concurrent aerosol delivery during HFNT may be employed to facilitate the delivery of a variety of prescribed medications for inhalation. This study details a systematic approach to establish the conditions required to optimize the emitted dose during HFNT. The well-established design of experiments approach provided a robust model, thus generating usable and representative data without committing to exhaustive testing. For the majority of the testing completed in the study, nasal cannula interfaces with small inner bore diameters were utilized. Consequently, emitted dose (%) results were relatively low. Nevertheless, the purpose of this study was not to yield high amounts of aerosol, but to determine the settings necessary to optimize the emitted dose, regardless of the circuit and nasal cannula interface used. Increasing the gas flow rate was found to significantly reduce the emitted dose, from 7.10% at 10 LPM to 2.67% at 25 LPM and 1.30% at 40 LPM (p \ ). We found that a small input droplet size resulted in a significantly greater amount of aerosol exiting the cannula outlet, with a dose of 6.11% obtained with an input droplet size of 3.22 lm, 2.76% with 4.05 lm droplets, and 2.38% with 4.88 lm droplets (p = ). In addition, the droplet size exiting the nasal cannula interface was smaller than that produced by the aerosol generator for all devices under test. Positioning the nebulizer immediately after the humidification chamber yielded a marginally greater emitted dose (3.79%) than when the nebulizer was placed immediately upstream of the nasal cannula (3.39%). Flow rate, input droplet size, and nebulizer position were at the 0.10 level of significance, indicating that all three factors had a significant effect on emitted dose. Overall, flow rate had the greatest effect on emitted dose, followed by input droplet size and then nebulizer position (Fig. 2). Table 9 Mean ± standard deviation values of emitted dose (%) for testing completed using a pediatric cannula (Comfort Flo P/N: , Teleflex Medical) Input droplet size (lm VMD) Delivery gas flow rate Nebulizer position Sample number (n) Emitted dose (%) Proximal n = ± 1.50 Distal n = ± Proximal n = ± 0.35 Distal n = ± Proximal n = ± 0.79 Distal n = ± Proximal n = ± 0.26 Distal n = ± 0.07
10 82 Pulm Ther (2018) 4:73 86 Table 10 Mean ± standard deviation values of emitted dose (%) for testing completed using an infant cannula (Comfort Flo P/N: , Teleflex Medical) Input droplet size (lm VMD) Delivery gas flow rate Nebulizer position Sample number (n) Emitted dose (%) Proximal n = ± 1.05 Distal n = ± Proximal n = ± 0.61 Distal n = ± Proximal n = ± 1.12 Distal n = ± Proximal n = ± 0.38 Distal n = ± 0.15 Table 11 Mean ± standard deviation values of MMAD and GSD for each input droplet size at the nasal cannula with two different gas flow rates Input droplet size Adult cannula at 10 LPM Adult cannula at 40 LPM VMD (lm) MMAD (lm) GSD (lm) MMAD (lm) GSD (lm) MMAD (lm) GSD (lm) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± 0.43 Influence of Flow Rate on Emitted Dose According to the statistical DOE model, flow rate was the factor that had the greatest effect on emitted dose. Increasing the gas flow rate significantly reduced the dose emitted with an adult nasal cannula, from 7.10% ± 0.86 at 10 LPM to 2.67% ± 0.14 at 25 LPM and 1.30% ± 0.21 at 40 LPM (p \ ) (Fig. 3). Similarly, the emitted dose was flow-rate dependent when utilizing pediatric and infant nasal cannulas (Tables 9, 10). This is consistent with the findings of existing studies in the literature. Higher gas flow rates most likely caused greater depositional losses within the circuit tubing in comparison to when testing with lower flow rates. Ari et al. reported an aerosol delivery efficiency of * 10% at a flow rate of 3 LPM, and this decreased significantly to * 2% as the flow rate was increased to 6 LPM [13]. Similarly, Perry et al. reported that the in vitro inspired dose during HFNT decreased from 2.5% to 0.2% upon increasing the flow rate from 5 to 40 LPM [15]. Reminiac et al. also showed reductions in inhaled dose with increasing gas flow rate [22]. Influence of Input Droplet Size on Emitted Dose Our findings indicate that there was a significant difference in the doses emitted by nebulizers with different input droplet sizes, with an increasing input droplet size associated with a reduced emitted dose (6.11% ± 1.06 at 3.22 lm, 2.76% ± 0.25 at 4.05 lm, and 2.38% ± 0.25 at
11 Pulm Ther (2018) 4: lm) (Fig. 4), with a Pearson correlation coefficient (r) of One-way ANOVA resulted in a p value of , indicating that there was a significant difference in emitted dose between nebulizers with different input droplet sizes. A similar trend was observed when testing with pediatric and infant nasal cannulas (Tables 9, 10). Furthermore, the droplet size was subsequently characterized at the cannula outlet at gas flow rates of 10 and 40 LPM. MMAD is the diameter that is larger than 50% of the droplets and smaller than the other 50%, based on mass, and it is considered the best predictor of aerosol distribution throughout the lungs. Droplets less than 5 lm in size are predicted to deposit in the lungs of adults, whilst larger droplets are more likely to deposit in the upper airways [23, 24]. The droplet size exiting the nasal cannula interface was consistently lower than that produced by the nebulizer (input droplet size) for all three devices under test (Table 11). This finding is consistent with that of Bhashyam et al., who noted a similar decrease in aerosol size measurements at various points in a HFNT system [12]. This reduction is likely caused by inertial impaction within the delivery system, enabling aerosol droplets of a smaller droplet size to successfully transit through [12]. Those results suggest that losses throughout the circuit may be reduced by applying aerosol generators that produce a low MMAD initially [21, 25, 26]. We found that droplet size at the adult nasal cannula outlet decreased with increasing gas flow rate (10 40 LPM), in contrast with some studies in the literature. Reminiac et al. reported that larger particle size measurements were associated with high flow rates [21]. These contrasting results may be explained by the use of different test setups. Reminiac and colleagues placed the nasal cannula at the entry to the throat of the cascade impactor, whereas the nasal prongs were presented to the throat of the NGI via a fixture in this study. This fixture was employed to minimize rained-out humidity that may artificially increase droplet size measurements. Influence of Nebulizer Position on Emitted Dose The position of the nebulizer during simulated mechanical ventilation has previously been shown to exert a critical influence on the dose delivered, due to the aerosol being exposed to bias flow [27, 28]. The effect of nebulizer position during HFNT is not, however, well described. In this study, positioning the nebulizer immediately after the humidification chamber led to a larger emitted dose (3.79 ± 0.68%) than when the nebulizer was placed immediately upstream of the nasal cannula (3.39 ± 0.49%), although the difference in emitted dose was not statistically significant (Fig. 5). Placing the nebulizer close to the humidification chamber yielded a marginally higher emitted dose than the doses emitted when the nebulizer was placed in distal positions, such as immediately upstream of the nasal cannula, due to the point of entry of the aerosol into humidified/nonhumidified air [13, 15]. Reminiac et al. studied the effect of nebulizer position on aerosol delivery during simulated HFNT for adults in vitro. Nebulizers were attached before the humidification chamber, after the humidification chamber, or at the distal position immediately upstream from the nasal cannula. Reminiac and colleagues showed that when using the Aerogen-mesh nebulizer, the inhalable mass was significantly lower at the distal position immediately upstream of the cannula [22]. Influence of Nasal Cannula Design on Emitted Dose For the majority of the testing, nasal cannula interfaces with small dimensions were utilized. Consequently, emitted dose results were quite low. Nevertheless, the purpose of this study was not to yield high amounts of aerosol from nasal cannula, but to identify the conditions required to optimize the emitted dose, regardless of the circuit and interface used. A greater emitted dose was observed when the Comfort Flo Plus cannula was used rather than the Comfort Flo cannula. This is most likely explained by the larger inner bore diameter of the Comfort Flo
12 84 Pulm Ther (2018) 4:73 86 Plus nasal cannula interface. Small dimensions in the tubing and cannula components hinder the exit of aerosol from the cannula during HFNT [29]. Perry et al. reported a decrease in inspired dose during HFNT when smaller cannulas were utilized (pediatric and infant) rather than adult cannulas [15]. Pacocha et al. showed that the brand of nasal cannula interface also affects aerosol delivery in HFNT during simulated normal adult breathing [30]. Clinical Implications Several factors influence both the amount of aerosol exiting the cannula and the amount available to the patient during HFNT. The factors affecting the emitted dose have been systemically reviewed in this study and our findings may be clinically relevant as they could help to optimize the delivery of a variety of prescribed medications for inhalation. These include, but are not limited to, b-agonists and hypertonic saline [4, 5]. However, efficient aerosol delivery to the lungs during HFNT is challenging due to the high gas flow rates used clinically and the aforementioned small dimensions of the delivery tubing and nasal cannula. Furthermore, the nasal passages present a challenge for the aerosols to navigate before reaching the lungs [29]. The first in vivo study on aerosol delivery during HFNT reported low pulmonary aerosol deposition in healthy male subjects (1 4% of the dose placed in the nebulizer), regardless of the type of nebulizer used [31]. Therefore, the refinement of aerosol delivery systems may be necessary. A number of studies have assessed the effect of breathing pattern on aerosol delivery during HFNT and have obtained contrasting results. Sweeney et al. showed that the mean tracheal dose during simulated HFNT in vitro is significantly lower for a cystic fibrosis breathing profile than for normal adult breathing [32]. Conversely, Reminiac et al. reported that the respirable mass of drug was significantly higher during simulated respiratory distress than simulated quiet breathing [22]. Dailey et al. showed that with a distressed breathing pattern, aerosol delivery was greater at 30 and 50 LPM than with a quiet breathing pattern [33]. Study Limitations The intention of this study was to identify the main effects contributed by a high-flow therapy system design. This study did not assess how aerosol efficiency during HFNT is affected by simulated breathing, using an anatomically correct model. Future studies are required to investigate how patient factors affect aerosol delivery during HFNT, including breathing patterns and patient size (adult, pediatric, and infant). CONCLUSION In conclusion, our findings indicate that to optimize the amount of aerosol exiting the nasal cannula during HFNT, the gas flow rate should be low and the input aerosol droplet size should be small, while the nebulizer should be positioned close to the humidifier. Gas flow rate was the factor that had the greatest influence on emitted dose, followed by input droplet size and then nebulizer position. Whilst only a single commercially available high-flow therapy circuit was used in this assessment, clinicians, users, and system designers may nevertheless benefit from this comprehensive analysis of relevant factors when they attempt to optimize the delivery of medications for inhalation. Future studies may further investigate the factors that affect the amount of aerosol available to the patient during HFNT, including breathing patterns and patient size. ACKNOWLEDGEMENTS Funding. Funding for the study and article processing charges were provided by Aerogen Limited. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
13 Pulm Ther (2018) 4: Authorship. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Disclosures. Gavin Bennett is an employee of Aerogen Limited. Mary Joyce is an employee of Aerogen Limited. Louise Sweeney is an employee of Aerogen Limited. Ronan MacLoughlin is an employee of Aerogen Limited. Compliance with Ethics Guidelines. This article does not contain any studies with human participants or animals performed by any of the authors. Open Access. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License ( by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. REFERENCES 1. Golshahi L, Longest PW, Azimi M, Syed A, Hindle M. Intermittent aerosol delivery to the lungs during high-flow nasal cannula therapy. Respir Care. 2014;59(10): Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10): Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54(1): Corcoran TE, Godovchik JE, Donn KH, Busick DR, Goralski J, Locke LW, et al. Overnight delivery of hypertonic saline by nasal cannula aerosol for cystic fibrosis. Pediatr Pulmonol. 2017;52(9): MacLoughlin R, Power P, Wolny M, Duffy C. Evaluation of vibrating mesh nebulizer performance during nasal high flow therapy. J Aerosol Med Pulm Drug Deliv. 2013;26(2). 6. de Jongh BE, Locke R, Mackley A, Emberger J, Bostick D, Stefano J, et al. Work of breathing indices in infants with respiratory insufficiency receiving high-flow nasal cannula and nasal continuous positive airway pressure. J Perinatol Off J Calif Perinat Assoc. 2014;34(1): Kubicka ZJ, Limauro J, Darnall RA. Heated, humidified high-flow nasal cannula therapy: yet another way to deliver continuous positive airway pressure? Pediatrics. 2008;121(1): Spentzas T, Minarik M, Patters AB, Vinson B, Stidham G. Children with respiratory distress treated with high-flow nasal cannula. J Intensive Care Med. 2009;24(5): McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156(4): Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. 2007;20(4): Morgan SE, Mosakowski S, Solano P, Hall JB, Tung A. High-flow nasal cannula and aerosolized beta agonists for rescue therapy in children with bronchiolitis: a case series. Respir Care. 2015;60(9):e Bhashyam AR, Wolf MT, Marcinkowski AL, Saville A, Thomas K, Carcillo JA, et al. Aerosol delivery through nasal cannulas: an in vitro study. J Aerosol Med Pulm Drug Deliv. 2008;21(2): Ari A, Harwood R, Sheard M, Dailey P, Fink JB. In vitro comparison of heliox and oxygen in aerosol delivery using pediatric high flow nasal cannula. Pediatr Pulmonol. 2011;46(8): Hess DR. Aerosol therapy during noninvasive ventilation or high-flow nasal cannula. Respir Care. 2015;60(6): Perry SA, Kesser KC, Geller DE, Selhorst DM, Rendle JK, Hertzog JH. Influences of cannula size and flow rate on aerosol drug delivery through the vapotherm humidified high-flow nasal cannula system. Pediatr Crit Care Med. 2013;14(5):e Longest PW, Hindle M. Condensational growth of combination drug-excipient submicrometer particles for targeted high efficiency pulmonary delivery: comparison of CFD predictions with experimental results. Pharm Res. 2012;29(3):
14 86 Pulm Ther (2018) 4: Longest PW, Spence BM, Holbrook LT, Mossi KM, Son YJ, Hindle M. Production of inhalable submicrometer aerosols from conventional mesh nebulizers for improved respiratory drug delivery. J Aerosol Sci. 2012;51: MacLoughlin R, Higgins B, Laffey J, O Brien T. Optimized aerosol delivery to a mechanically ventilated rodent. J Aerosol Med Pulm Drug Deliv. 2009;22: ISO. Anaesthetic and respiratory equipment nebulizing systems and components. Geneva: International Organization for Standardization; US Pharmacopeia. 34 \ 601 [ Aerosols, nasal sprays. Metered-dose inhalers and dry powder inhalers. Rockville, MD: US Pharmacopeia; Reminiac F, Vecellio L, Loughlin RM, Le Pennec D, Cabrera M, Vourc h NH, et al. Nasal high flow nebulization in infants and toddlers: an in vitro and in vivo scintigraphic study. Pediatr Pulmonol. 2017;52(3): Reminiac F, Vecellio L, Heuze-Vourc h N, Petitcollin A, Respaud R, Cabrera M, et al. Aerosol therapy in adults receiving high flow nasal cannula oxygen therapy. J Aerosol Med Pulm Drug Deliv. 2016;29(2): Newman SP, Clarke SW. Therapeutic aerosols. 1: Physical and practical considerations. Thorax. 1983;38(12): Delvadia RR, Longest PW, Byron PR. In vitro tests for aerosol deposition. I: Scaling a physical model of the upper airways to predict drug deposition variation in normal humans. J Aerosol Med Pulm Drug Deliv. 2012;25(1): MacLoughlin R, Telfer C, Clark A, Fink J. Aerosol: a novel vehicle in pharmacotherapy in neonates. Curr Pharm Des. 2017;23(38): Holbrook L, Hindle M, Longest PW. Generating charged pharmaceutical aerosols intended to improve targeted drug delivery in ventilated infants. J Aerosol Sci. 2015;88: Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, Fink JB. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010;55(7): Berlinski A, Willis JR. Albuterol delivery by 4 different nebulizers placed in 4 different positions in a pediatric ventilator in vitro model. Respir Care. 2013;58(7): Golshahi L, Walenga RL, Longest PW, Hindle M. Development of a transient flow aerosol mixerheater system for lung delivery of nasally administered aerosols using a nasal cannula. Aerosol Sci Technol. 2014;48(10): Pacocha D, Dailey P, Gagnon G. Comparison of aerosol delivery with three high flow nasal cannula types and sizes. Poster presented at: AARC Congress; 2016 Oct 15 18; San Antonio, TX, USA. 31. Dugernier J, Hesse M, Jumetz T, Bialais E, Roeseler J, Depoortere V, et al. Aerosol delivery with two nebulizers through high-flow nasal cannula: a randomized cross-over single-photon emission computed tomography computed tomography study. J Aerosol Med Pulm Drug Deliv. 2017;30(5): Sweeney L, O Sullivan A, Mac Loughlin R. The effect of breathing profile on deposition using cystic fibrosis and healthy adult breathing profiles during nasal high flow oxygen therapy at three different gas flow rates. Poster presented at: Drug Delivery to the Lungs 26; 2015 Dec 9 11; Edinburgh, UK. 33. Dailey PA, Harwood R, Walsh K, Fink JB, Thayer T, Gagnon G, et al. Aerosol delivery through adult high flow nasal cannula with heliox and oxygen. Respir Care. 2017;62(9):
600 RESPIRATORY CARE MAY 2016 VOL 61 NO 5
 Quantifying Aerosol Delivery in Simulated Spontaneously Breathing Patients With Tracheostomy Using Different Humidification Systems With or Without Exhaled Humidity Arzu Ari PhD RRT PT CPFT FAARC, Robert
Quantifying Aerosol Delivery in Simulated Spontaneously Breathing Patients With Tracheostomy Using Different Humidification Systems With or Without Exhaled Humidity Arzu Ari PhD RRT PT CPFT FAARC, Robert
Aerosol Delivery Through Adult High Flow Nasal Cannula With Heliox and Oxygen
 Aerosol Delivery Through Adult High Flow Nasal Cannula With Heliox and Oxygen Patricia A Dailey RRT, Robert Harwood MSA RRT-NPS FAARC, Kyle Walsh, James B Fink PhD RRT FAARC, Tina Thayer RRT, Greg Gagnon,
Aerosol Delivery Through Adult High Flow Nasal Cannula With Heliox and Oxygen Patricia A Dailey RRT, Robert Harwood MSA RRT-NPS FAARC, Kyle Walsh, James B Fink PhD RRT FAARC, Tina Thayer RRT, Greg Gagnon,
In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System
 In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System Hui-Ling Lin MSc RRT RN FAARC, Robert J Harwood MSA RRT FAARC, James B Fink PhD RRT FAARC,
In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System Hui-Ling Lin MSc RRT RN FAARC, Robert J Harwood MSA RRT FAARC, James B Fink PhD RRT FAARC,
Revision 2. Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components
 Revision 2 Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components P. Worth Longest, Ph.D., 1,2* Mandana Azimi, Pharm.D., 2 Laleh Golshahi, Ph.D., 2 and Michael
Revision 2 Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components P. Worth Longest, Ph.D., 1,2* Mandana Azimi, Pharm.D., 2 Laleh Golshahi, Ph.D., 2 and Michael
High-Flow Nasal Cannula and Aerosolized Agonists for Rescue Therapy in Children With Bronchiolitis: A Case Series
 High-Flow Nasal Cannula and Aerosolized Agonists for Rescue Therapy in Children With Bronchiolitis: A Case Series Sherwin E Morgan RRT, Steve Mosakowski RRT, Patti Solano RRT, Jesse B Hall MD, and Avery
High-Flow Nasal Cannula and Aerosolized Agonists for Rescue Therapy in Children With Bronchiolitis: A Case Series Sherwin E Morgan RRT, Steve Mosakowski RRT, Patti Solano RRT, Jesse B Hall MD, and Avery
Pediatric Aerosol Therapy
 Pediatric Aerosol Therapy Ariel Berlinski MD Introduction Delivery Device Selection for the Treatment of Acute Pediatric Asthma Transnasal Aerosol Delivery in Pediatric Patients Delivery Device Selection
Pediatric Aerosol Therapy Ariel Berlinski MD Introduction Delivery Device Selection for the Treatment of Acute Pediatric Asthma Transnasal Aerosol Delivery in Pediatric Patients Delivery Device Selection
Misty Max 10 nebulizer
 AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
Frequently Asked Questions.
 Frequently Asked Questions www.aerogen.com 1 FAQs Frequently asked questions are answered below and specifically for each of our products. If you have a question that is not listed, please feel free to
Frequently Asked Questions www.aerogen.com 1 FAQs Frequently asked questions are answered below and specifically for each of our products. If you have a question that is not listed, please feel free to
Pediatrics in mechanical ventilation
 Pediatrics Optimization Intitulé du cours of aerosol therapy in mechanical ventilation Thèmes donnés Ermindo Di Paolo, PhD Departments of Pharmacy and Pediatrics Lausanne University Hospital Switzerland
Pediatrics Optimization Intitulé du cours of aerosol therapy in mechanical ventilation Thèmes donnés Ermindo Di Paolo, PhD Departments of Pharmacy and Pediatrics Lausanne University Hospital Switzerland
Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model
 Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model Arzu Ari PhD RRT PT CPFT FAARC, Khalid S Alwadeai MSc RRT-NPS, and
Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model Arzu Ari PhD RRT PT CPFT FAARC, Khalid S Alwadeai MSc RRT-NPS, and
Clinical White Paper
 Clinical White Paper Table of Contents 1 Abstract 1 2 High Efficiency Aerosol Drug Delivery During Ventilation 2 3 A Lung Recruitment Strategy for Patients with Respiratory Failure utilising Aerogen 5
Clinical White Paper Table of Contents 1 Abstract 1 2 High Efficiency Aerosol Drug Delivery During Ventilation 2 3 A Lung Recruitment Strategy for Patients with Respiratory Failure utilising Aerogen 5
ROLE OF PRESSURE IN HIGH FLOW THERAPY
 ROLE OF PRESSURE IN HIGH FLOW THERAPY Thomas L. Miller, PhD, MEd Director, Clinical Research and Education Vapotherm, Inc. Research Assistant Professor of Pediatrics Jefferson Medical College This information
ROLE OF PRESSURE IN HIGH FLOW THERAPY Thomas L. Miller, PhD, MEd Director, Clinical Research and Education Vapotherm, Inc. Research Assistant Professor of Pediatrics Jefferson Medical College This information
Rush University, College of Health Sciences
 Rush University, College of Health Sciences An Evaluation of the Prototype OxyMulti Mask Prototype Compared to the Oxymask Aerosol, OxyMulti Mask and Airlife Aerosol Mask for Aerosol Delivery in Adults
Rush University, College of Health Sciences An Evaluation of the Prototype OxyMulti Mask Prototype Compared to the Oxymask Aerosol, OxyMulti Mask and Airlife Aerosol Mask for Aerosol Delivery in Adults
In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System
 In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System Kimberly D Farney RRT, Brandon T Kuehne RRT MBA, Laurie A Gibson RT (N), Leif D Nelin MD, and Edward G Shepherd
In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System Kimberly D Farney RRT, Brandon T Kuehne RRT MBA, Laurie A Gibson RT (N), Leif D Nelin MD, and Edward G Shepherd
NIV and Aerosoltherapy
 NIV and Aerosoltherapy Workshop Jean-Bernard Michotte (Lausanne-CH) Simone Gambazza (Milano-IT) Jean-Bernard Michotte Haute Ecole de Santé Vaud, 1011 Lausanne - Suisse Cliniques Universitaires Saint-Luc,
NIV and Aerosoltherapy Workshop Jean-Bernard Michotte (Lausanne-CH) Simone Gambazza (Milano-IT) Jean-Bernard Michotte Haute Ecole de Santé Vaud, 1011 Lausanne - Suisse Cliniques Universitaires Saint-Luc,
Heated Humidified High Flow Nasal Cannula Treatment (HHHFNC)
 Heated Humidified High Flow Nasal Cannula Treatment (HHHFNC) 1. Introduction and Who Guideline applies to Heated humidified high flow nasal cannula treatment (HHHFNC), otherwise called as High flow oxygen
Heated Humidified High Flow Nasal Cannula Treatment (HHHFNC) 1. Introduction and Who Guideline applies to Heated humidified high flow nasal cannula treatment (HHHFNC), otherwise called as High flow oxygen
Nebulizers. Small Volume Nebulizers
 Small Volume MICRO MIST Nebulizer The MICRO MIST small volume nebulizer is designed for performance and economy and includes the design features needed by today s respiratory care practitioners. It can
Small Volume MICRO MIST Nebulizer The MICRO MIST small volume nebulizer is designed for performance and economy and includes the design features needed by today s respiratory care practitioners. It can
Respiratory Care in PICU Aerosol Therapy ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า
 Respiratory Care in PICU Aerosol Therapy น.อ.หญ ง ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า Aerosol liquid droplets or solid particles suspended in a gas(air) visible like fog Aerosol vs Humidity Humidity
Respiratory Care in PICU Aerosol Therapy น.อ.หญ ง ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า Aerosol liquid droplets or solid particles suspended in a gas(air) visible like fog Aerosol vs Humidity Humidity
Guidelines and Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide
 Guidelines Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide Patient Selection Diagnoses Patient presents with one or more of the following signs or symptoms of respiratory distress:
Guidelines Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide Patient Selection Diagnoses Patient presents with one or more of the following signs or symptoms of respiratory distress:
Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting J 2
 SALBUTAMOL DELIVERY IN AN IN VITRO PAEDIATRIC VENTILATOR-LUNG MODEL: COMPARISON OF JET, ULTRASONIC AND MESH NEBULISERS Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting
SALBUTAMOL DELIVERY IN AN IN VITRO PAEDIATRIC VENTILATOR-LUNG MODEL: COMPARISON OF JET, ULTRASONIC AND MESH NEBULISERS Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting
In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers
 In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers Ariel Berlinski MD BACKGROUND: Patients with cystic fibrosis perform airway clearance techniques and receive nebulized
In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers Ariel Berlinski MD BACKGROUND: Patients with cystic fibrosis perform airway clearance techniques and receive nebulized
Anaesthetic and respiratory equipment Nebulizing systems and components
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 27427 Third edition 2013-12-15 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d anesthésie et de réanimation
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 27427 Third edition 2013-12-15 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d anesthésie et de réanimation
Redefining Continuous Aerosol Drug Delivery 2015 PM223
 Redefining Continuous Aerosol Drug Delivery 2015 PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize
Redefining Continuous Aerosol Drug Delivery 2015 PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize
Redefining Continuous Aerosol Drug Delivery PM223
 Redefining Continuous Aerosol Drug Delivery PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize medication
Redefining Continuous Aerosol Drug Delivery PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize medication
Archives of Pulmonology and Respiratory Care
 v Clinical Group Archives of Pulmonology and Respiratory Care DOI CC By Marwa Mohsen 1, Ahmed A Elberry 2, Abeer Salah Eldin 3, Raghda RS Hussein 1 and Mohamed EA Abdelrahim 1,4 * Research Article Effects
v Clinical Group Archives of Pulmonology and Respiratory Care DOI CC By Marwa Mohsen 1, Ahmed A Elberry 2, Abeer Salah Eldin 3, Raghda RS Hussein 1 and Mohamed EA Abdelrahim 1,4 * Research Article Effects
Albuterol Delivery via Facial and Tracheostomy Route in a Model of a Spontaneously Breathing Child
 Albuterol Delivery via Facial and Tracheostomy Route in a Model of a Spontaneously Breathing Child Brandy Cooper and Ariel Berlinski MD BACKGROUND: Some pediatric patients receiving therapeutic aerosols
Albuterol Delivery via Facial and Tracheostomy Route in a Model of a Spontaneously Breathing Child Brandy Cooper and Ariel Berlinski MD BACKGROUND: Some pediatric patients receiving therapeutic aerosols
Effective Aerosol Delivery in Acute Care
 Effective Aerosol Delivery in Acute Care Jim Fink, RRT, PhD, FAARC, FCCP Chief Science Officer, Aerogen Pharma Corp., San Mateo, CA USA Visiting Professor, Dept of Physical therapy, Universidade Federal
Effective Aerosol Delivery in Acute Care Jim Fink, RRT, PhD, FAARC, FCCP Chief Science Officer, Aerogen Pharma Corp., San Mateo, CA USA Visiting Professor, Dept of Physical therapy, Universidade Federal
Guidelines and Best Practices for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) NICU POCKET GUIDE
 Guidelines and Best Practices for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) TM NICU POCKET GUIDE Patient Selection Diagnoses Patient presents with one or more of the following symptoms: These
Guidelines and Best Practices for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) TM NICU POCKET GUIDE Patient Selection Diagnoses Patient presents with one or more of the following symptoms: These
University of Groningen. Technology in practice Lexmond, Anne
 University of Groningen Technology in practice Lexmond, Anne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Technology in practice Lexmond, Anne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
High-flow nasal cannula use in a paediatric intensive care unit over 3 years
 High-flow nasal cannula use in a paediatric intensive care unit over 3 years Tracey I Wraight and Subodh S Ganu Respiratory illness and/or distress is the commonest reason for non-elective paediatric intensive
High-flow nasal cannula use in a paediatric intensive care unit over 3 years Tracey I Wraight and Subodh S Ganu Respiratory illness and/or distress is the commonest reason for non-elective paediatric intensive
Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask
 Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask Abstract This study s objective was to model the Amsino OneMask Oxygen Mask using Computational Fluid Dynamics (CFD). A three-dimensional
Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask Abstract This study s objective was to model the Amsino OneMask Oxygen Mask using Computational Fluid Dynamics (CFD). A three-dimensional
Continuous Aerosol Therapy
 PROCEDURE - : Page 1 of 5 Purpose Policy Physician's Order To standardize the administration of continuous aerosol therapy. Respiratory Care Services provides equipment and therapy according to physician
PROCEDURE - : Page 1 of 5 Purpose Policy Physician's Order To standardize the administration of continuous aerosol therapy. Respiratory Care Services provides equipment and therapy according to physician
Aerosolized Antibiotics in Mechanically Ventilated Patients
 Aerosolized Antibiotics in Mechanically Ventilated Patients Gerald C Smaldone MD PhD Introduction Topical Delivery of Antibiotics to the Lung Tracheobronchitis Aerosolized Antibiotic Delivery in the Medical
Aerosolized Antibiotics in Mechanically Ventilated Patients Gerald C Smaldone MD PhD Introduction Topical Delivery of Antibiotics to the Lung Tracheobronchitis Aerosolized Antibiotic Delivery in the Medical
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE
 Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study
 Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study Robert E Van Dyke MSc and Kurt Nikander BACKGROUND: Iloprost (Ventavis)
Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study Robert E Van Dyke MSc and Kurt Nikander BACKGROUND: Iloprost (Ventavis)
WILAflow Elite Neonatal Ventilator. Non-invasive treatment for the most delicate patients.
 EN WILAflow Elite Neonatal Ventilator Non-invasive treatment for the most delicate patients. 0197 Infant Ventilation redefined A new generation in Infant Ventilation WILAflow Elite is a microprocessor
EN WILAflow Elite Neonatal Ventilator Non-invasive treatment for the most delicate patients. 0197 Infant Ventilation redefined A new generation in Infant Ventilation WILAflow Elite is a microprocessor
Effect of Interval Between Actuations of Albuterol Hydrofluoroalkane Pressurized Metered-Dose Inhalers on Their Aerosol Characteristics
 Effect of Interval Between Actuations of Albuterol Hydrofluoroalkane Pressurized Metered-Dose Inhalers on Their Aerosol Characteristics Ariel Berlinski MD and David Pennington BACKGROUND: Albuterol hydrofluoroalkane
Effect of Interval Between Actuations of Albuterol Hydrofluoroalkane Pressurized Metered-Dose Inhalers on Their Aerosol Characteristics Ariel Berlinski MD and David Pennington BACKGROUND: Albuterol hydrofluoroalkane
JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI, PATRICK J. FAHEY, and MARTIN J. TOBIN
 Reconciling In Vitro and In Vivo Measurements of Aerosol Delivery from a Metered-Dose Inhaler during Mechanical Ventilation and Defining Efficiency-enhancing Factors JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI,
Reconciling In Vitro and In Vivo Measurements of Aerosol Delivery from a Metered-Dose Inhaler during Mechanical Ventilation and Defining Efficiency-enhancing Factors JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI,
Acute Paediatric Respiratory Pathway
 Guideline for the use of high Flow Nasal Cannula Oxygen Therapy (Optiflow or Airvo) in Children with Bronchiolitis or an acute respiratory illness Introduction: High flow nasal cannula (HFNC) oxygen enables
Guideline for the use of high Flow Nasal Cannula Oxygen Therapy (Optiflow or Airvo) in Children with Bronchiolitis or an acute respiratory illness Introduction: High flow nasal cannula (HFNC) oxygen enables
Oxygen administration and inhaled bronchodilators. Aerosol Therapy in Adults Receiving High Flow Nasal Cannula Oxygen Therapy
 JOURNAL OF AEROSOL MEDICINE AND PULMONARY DRUG DELIVERY Volume 28, Number 0, 2015 ª Mary Ann Liebert, Inc. Pp. 1 8 DOI: 10.1089/jamp.2015.1219 Aerosol Therapy in Adults Receiving High Flow Nasal Cannula
JOURNAL OF AEROSOL MEDICINE AND PULMONARY DRUG DELIVERY Volume 28, Number 0, 2015 ª Mary Ann Liebert, Inc. Pp. 1 8 DOI: 10.1089/jamp.2015.1219 Aerosol Therapy in Adults Receiving High Flow Nasal Cannula
Ultrasonic Humidifiers and Nebulisers
 Ultrasonic Humidifiers and Nebulisers for Medical Institutions DP100 NF EN ISO 13485 0459 SYST AM DP100, a n Container Ê Stands upright : convenient for preparing the session. Ê With simple and quick connector.
Ultrasonic Humidifiers and Nebulisers for Medical Institutions DP100 NF EN ISO 13485 0459 SYST AM DP100, a n Container Ê Stands upright : convenient for preparing the session. Ê With simple and quick connector.
Intracheal antibiotics administration
 Intracheal antibiotics administration Jean Chastre, M.D. www.reamedpitie.com Disclosure Conflicts of interest: Consulting or Lecture fees: Bayer, Pfizer, Cubist/Merck, Basilea, Kenta/Aridis, Roche, AstraZeneca/Medimmune
Intracheal antibiotics administration Jean Chastre, M.D. www.reamedpitie.com Disclosure Conflicts of interest: Consulting or Lecture fees: Bayer, Pfizer, Cubist/Merck, Basilea, Kenta/Aridis, Roche, AstraZeneca/Medimmune
Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects
 Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects Meagan N Dubosky MSc RRT, Yi-Fan Chen PhD, Mary E Henriksen MSc RRT, and David L Vines MHS RRT FAARC BACKGROUND:
Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects Meagan N Dubosky MSc RRT, Yi-Fan Chen PhD, Mary E Henriksen MSc RRT, and David L Vines MHS RRT FAARC BACKGROUND:
LRI Children s Hospital
 LRI Children s Hospital Humidified High Flow Nasal Cannula (HHFNC) Oxygen Therapy Staff relevant to: Medical and Nursing staff Team approval date: 5.01.18 Version: 1 Revision due: January 2020 Written
LRI Children s Hospital Humidified High Flow Nasal Cannula (HHFNC) Oxygen Therapy Staff relevant to: Medical and Nursing staff Team approval date: 5.01.18 Version: 1 Revision due: January 2020 Written
Understanding cascade impaction and its importance for inhaler testing
 Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
I. Subject: Continuous Aerosolization of Bronchodilators
 I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
Small Volume Nebulizer Treatment (Hand-Held)
 Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
Citation for published version (APA): Westerman, E. M. (2009). Studies on antibiotic aerosols for inhalation in cystic fibrosis s.n.
 University of Groningen Studies on antibiotic aerosols for inhalation in cystic fibrosis Westerman, Elisabeth Mechteld IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
University of Groningen Studies on antibiotic aerosols for inhalation in cystic fibrosis Westerman, Elisabeth Mechteld IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF)
Saline (0.9%) Nebuliser Guideline
 Saline (0.9%) Nebuliser Guideline Full Title of Guideline: Author (include email and role): Division & Speciality: Version: 3 Ratified by: Scope (Target audience, state if Trust wide): Review date (when
Saline (0.9%) Nebuliser Guideline Full Title of Guideline: Author (include email and role): Division & Speciality: Version: 3 Ratified by: Scope (Target audience, state if Trust wide): Review date (when
WILAflow Elite Neonatal Ventilator. Non-invasive treatment for the most delicate patients.
 EN WILAflow Elite Neonatal Ventilator Non-invasive treatment for the most delicate patients. 0197 Infant Ventilation redefined A new generation in Infant Ventilation WILAflow Elite is a microprocessor
EN WILAflow Elite Neonatal Ventilator Non-invasive treatment for the most delicate patients. 0197 Infant Ventilation redefined A new generation in Infant Ventilation WILAflow Elite is a microprocessor
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION
 CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS
 NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
Systems differ in their ability to deliver optimal humidification
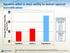 Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
1 2 3 Based on the contents detailed below, assess the substantial equivalence to a legally marketed predicate device. In that case, the standards, et
 Attachment 8 Application for Treatment Positive Airway Pressure Unit, etc. (1) Scope of application Continuous positive airway pressure units and Continuous-automated positive airway pressure units in
Attachment 8 Application for Treatment Positive Airway Pressure Unit, etc. (1) Scope of application Continuous positive airway pressure units and Continuous-automated positive airway pressure units in
Airway Clearance Devices
 Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
Nebulized Albuterol Delivery in a Model of Spontaneously Breathing Children With Tracheostomy
 Nebulized Albuterol Delivery in a Model of Spontaneously Breathing Children With Ariel Berlinski MD BACKGROUND: Nebulized therapy is commonly used in spontaneously breathing tracheostomized patients, despite
Nebulized Albuterol Delivery in a Model of Spontaneously Breathing Children With Ariel Berlinski MD BACKGROUND: Nebulized therapy is commonly used in spontaneously breathing tracheostomized patients, despite
ISO INTERNATIONAL STANDARD. Anaesthetic and respiratory equipment Nebulizing systems and components
 INTERNATIONAL STANDARD ISO 27427 First edition 2009-05-01 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d'anesthésie et de réanimation respiratoire Systèmes de nébulisation
INTERNATIONAL STANDARD ISO 27427 First edition 2009-05-01 Anaesthetic and respiratory equipment Nebulizing systems and components Matériel d'anesthésie et de réanimation respiratoire Systèmes de nébulisation
OG TAL ODUCT CA PR e e HEALTH
 PRODUCT CATALOG e e HEALTH MODEL: OC 5 HEALTH OXYGEN CONCENTRATOR ACCESSORIES FILTER AIR FILTER NASAL CANNULA BUBBLE HUMIDIFIER Oxygen concentrator is a device that generates oxygen with a purity of %90-92
PRODUCT CATALOG e e HEALTH MODEL: OC 5 HEALTH OXYGEN CONCENTRATOR ACCESSORIES FILTER AIR FILTER NASAL CANNULA BUBBLE HUMIDIFIER Oxygen concentrator is a device that generates oxygen with a purity of %90-92
OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER
 OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER US FDA guidance for the in vitro demonstration of bioequivalence in a generic nebuliser directly references
OPTIMISING ANALYTICAL STRATEGIES FOR THE DEMONSTRATION OF BIOEQUIVALENCE IN A GENERIC NEBULISER US FDA guidance for the in vitro demonstration of bioequivalence in a generic nebuliser directly references
Characterization of Ribavirin Aerosol With Small Particle Aerosol Generator and Vibrating Mesh Micropump Aerosol Technologies
 Characterization of Ribavirin Aerosol With Small Particle Aerosol Generator and Vibrating Mesh Micropump Aerosol Technologies Brian K Walsh RRT-NPS FAARC, Peter Betit RRT-NPS FAARC, James B Fink PhD RRT
Characterization of Ribavirin Aerosol With Small Particle Aerosol Generator and Vibrating Mesh Micropump Aerosol Technologies Brian K Walsh RRT-NPS FAARC, Peter Betit RRT-NPS FAARC, James B Fink PhD RRT
Inhalation Product Research at FDA
 Inhalation Product Research at FDA Changning Guo Ph. D., Chemist Division of Pharmaceutical Analysis FDA/CDER/OPS/OTR 2016 GPhA CMC workshop, May 17, 2016 Disclaimer: This presentation reflects the views
Inhalation Product Research at FDA Changning Guo Ph. D., Chemist Division of Pharmaceutical Analysis FDA/CDER/OPS/OTR 2016 GPhA CMC workshop, May 17, 2016 Disclaimer: This presentation reflects the views
Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing
 INSTRUMENTATION» Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing Mark Copley Director Copley Scientific The recent introduction
INSTRUMENTATION» Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing Mark Copley Director Copley Scientific The recent introduction
Assessing the role of breathing simulators in OIP testing
 As first AppeAred in Inhalation April 2014 www.inhalationmag.com Assessing the role of breathing simulators in OIP testing Exploring how the application of patient-representative inhalation profiles can
As first AppeAred in Inhalation April 2014 www.inhalationmag.com Assessing the role of breathing simulators in OIP testing Exploring how the application of patient-representative inhalation profiles can
Instruction Manual Aerogen, Inc. Part No. AG-AL1010 Rev. C
 Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Unit 5 Humidity/Aerosol Generators
 5-1 Unit 5 Humidity/Aerosol Generators GOAL On completion of this unit, the student should have an understanding of the principles of operation and proper use of different humidity/aerosol generators.
5-1 Unit 5 Humidity/Aerosol Generators GOAL On completion of this unit, the student should have an understanding of the principles of operation and proper use of different humidity/aerosol generators.
8/13/11. RSPT 1410 Humidity & Aerosol Therapy Part 3. Humidification Equipment. Aerosol Therapy
 1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
Respiratory Therapy. Medical/Scientific/General Background
 Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
21/03/2011 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY. Fundamentals of aerosols
 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
Understanding Regulatory Global Requirements for Nasal Drug Products. Julie D. Suman, Ph.D. April 8, 2016
 Understanding Regulatory Global Requirements for Nasal Drug Products Julie D. Suman, Ph.D. April 8, 2016 AGENDA NDA vs ANDA Regulatory Approaches for Bioequivalence (BE) FDA Drug Specific Guidances FDA,
Understanding Regulatory Global Requirements for Nasal Drug Products Julie D. Suman, Ph.D. April 8, 2016 AGENDA NDA vs ANDA Regulatory Approaches for Bioequivalence (BE) FDA Drug Specific Guidances FDA,
10/17/2016 OXYGEN DELIVERY: INDICATIONS AND USE OF EQUIPMENT COURSE OBJECTIVES COMMON CAUSES OF RESPIRATORY FAILURE
 OXYGEN DELIVERY: INDICATIONS AND USE OF EQUIPMENT J U L I E Z I M M E R M A N, R N, M S N C L I N I C A L N U R S E S P E C I A L I S T E L O I S A C U T L E R, R R T, B S R C C L I N I C A L / E D U C
OXYGEN DELIVERY: INDICATIONS AND USE OF EQUIPMENT J U L I E Z I M M E R M A N, R N, M S N C L I N I C A L N U R S E S P E C I A L I S T E L O I S A C U T L E R, R R T, B S R C C L I N I C A L / E D U C
Guidelines and Best Practices for High Velocity Nasal Insufflation (Hi-VNI Technology) Emergency Medicine Pocket Guide
 Guidelines and Best Practices for High Velocity Nasal Insufflation (Hi-VNI Technology) Emergency Medicine Pocket Guide Patient Selection Diagnoses When considering Vapotherm Hi-VNI Technology, the patient
Guidelines and Best Practices for High Velocity Nasal Insufflation (Hi-VNI Technology) Emergency Medicine Pocket Guide Patient Selection Diagnoses When considering Vapotherm Hi-VNI Technology, the patient
Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler
 Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler Ariel Berlinski MD, Dirk von Hollen, John N Pritchard PhD, and Ross HM Hatley PhD BACKGROUND: Inhaled
Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler Ariel Berlinski MD, Dirk von Hollen, John N Pritchard PhD, and Ross HM Hatley PhD BACKGROUND: Inhaled
KOALA. Adult Bacterial Viral Filters. Medical Pty Ltd
 Specialising in Anaesthetic Devices & Airway Management Laproscopic Surgery Maternity & Infant Care Adult Bacterial Viral Filters High efficiency up to 99.9 % @ 30L/Min Low resistance < 2.5 cmh2o @ 50
Specialising in Anaesthetic Devices & Airway Management Laproscopic Surgery Maternity & Infant Care Adult Bacterial Viral Filters High efficiency up to 99.9 % @ 30L/Min Low resistance < 2.5 cmh2o @ 50
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES
 COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
Latex Free. An affordable, easy to use, high density, small volume nebulizer with a breath enhanced design! Breath Enhanced High Density Jet Nebulizer
 Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Factors affecting bronchodilator delivery in mechanically ventilated adults
 LITERATURE REVIEW Factors affecting bronchodilator delivery in mechanically ventilated adults Arzu Ari and James B Fink ABSTRACT Background: Bronchodilators are increasingly being used in patients undergoing
LITERATURE REVIEW Factors affecting bronchodilator delivery in mechanically ventilated adults Arzu Ari and James B Fink ABSTRACT Background: Bronchodilators are increasingly being used in patients undergoing
VENTILATOR PRODUCT ACCESSORIES CATALOG
 VENTILATOR PRODUCT ACCESSORIES CATALOG Puritan Bennett TM 980 Ventilator Puritan Bennett TM 840 Ventilator PROMOTIONAL MATERIALS FOR USE IN THE U.S. TABLE OF CONTENTS Puritan Bennett 980 Ventilator Accessories...
VENTILATOR PRODUCT ACCESSORIES CATALOG Puritan Bennett TM 980 Ventilator Puritan Bennett TM 840 Ventilator PROMOTIONAL MATERIALS FOR USE IN THE U.S. TABLE OF CONTENTS Puritan Bennett 980 Ventilator Accessories...
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device
 I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
In-vitro Comparison of 4 Large-Volume Nebulizers in 8 Hours of Continuous Nebulization
 In-vitro Comparison of 4 Large-Volume Nebulizers in 8 Hours of Continuous Nebulization Ariel Berlinski MD, J Randy Willis RRT-NPS, and Tom Leisenring CRT BACKGROUND: Continuous albuterol nebulization has
In-vitro Comparison of 4 Large-Volume Nebulizers in 8 Hours of Continuous Nebulization Ariel Berlinski MD, J Randy Willis RRT-NPS, and Tom Leisenring CRT BACKGROUND: Continuous albuterol nebulization has
High Flow Nasal Cannula Oxygen HFNC. Dr I S Kalla Department of Pulmonology University of the Witwatersrand
 786 High Flow Nasal Cannula Oxygen HFNC Dr I S Kalla Department of Pulmonology University of the Witwatersrand Disclaimer I was a scep@c un@l I used it Now I am a firm believer HFNC The Fisher and Paykel
786 High Flow Nasal Cannula Oxygen HFNC Dr I S Kalla Department of Pulmonology University of the Witwatersrand Disclaimer I was a scep@c un@l I used it Now I am a firm believer HFNC The Fisher and Paykel
Delivering Aerosol Medication in ICU
 Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
INTRODUCTION. size and total nozzle area decrease with stage number. Volumetric air flow rate through
 CASCADE I M P A C T I O N Optimizing Cascade Impactor Testing for Characterizing Orally Inhaled & Nasal Drug Products By: Mark Copley INTRODUCTION Cascade impaction is a core analytical technique for characterizing
CASCADE I M P A C T I O N Optimizing Cascade Impactor Testing for Characterizing Orally Inhaled & Nasal Drug Products By: Mark Copley INTRODUCTION Cascade impaction is a core analytical technique for characterizing
Oxygen and aerosolized drug delivery: Matching the device to the patient
 REVIEW JOHN E. BURKHART, JR, BS. RRT Supervisor, Section of Respiratory Therapy, Department of Pulmonary and Critical Care Medicine, Cleveland Clinic. JAMES K. STOILER, MD Head, Section of Respiratory
REVIEW JOHN E. BURKHART, JR, BS. RRT Supervisor, Section of Respiratory Therapy, Department of Pulmonary and Critical Care Medicine, Cleveland Clinic. JAMES K. STOILER, MD Head, Section of Respiratory
5/3/2012. Goals and Objectives HFNC. High-Flow Oxygen Therapy: Real Benefit or Just a Fad?
 High-Flow Oxygen Therapy: Real Benefit or Just a Fad? Timothy R. Myers MBA, RRT-NPS Director, Women s & Children s Respiratory Care & Procedural Services and Pediatric Heart Center Rainbow Babies & Children
High-Flow Oxygen Therapy: Real Benefit or Just a Fad? Timothy R. Myers MBA, RRT-NPS Director, Women s & Children s Respiratory Care & Procedural Services and Pediatric Heart Center Rainbow Babies & Children
Portable Equine Nebuliser System. User Manual
 Portable Equine Nebuliser System User Manual Table of Contents INTENDED USE... 3 SAFETY INFORMATION... 3 TECHNICAL SPECIFICATION... 4 INSTRUCTIONS FOR USE... 6 MAINTENANCE... 12 TROUBLESHOOTING... 13 WARRANTY...
Portable Equine Nebuliser System User Manual Table of Contents INTENDED USE... 3 SAFETY INFORMATION... 3 TECHNICAL SPECIFICATION... 4 INSTRUCTIONS FOR USE... 6 MAINTENANCE... 12 TROUBLESHOOTING... 13 WARRANTY...
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF INHALATION AND NASAL PRODUCTS
 European Medicines Agency Inspections London, 16 February 2005 Doc Ref.: EMEA/CHMP/QWP/49313/2005 corr. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF
European Medicines Agency Inspections London, 16 February 2005 Doc Ref.: EMEA/CHMP/QWP/49313/2005 corr. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF
Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population
 Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population Contents: Motivation: Why modelling the paediatric dose to lung via in-vitro and in-silico
Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population Contents: Motivation: Why modelling the paediatric dose to lung via in-vitro and in-silico
2014 Full Year Results Presentation. Year ended 31 March 2014
 2014 Full Year Results Presentation Year ended 31 March 2014 26-30 May 2014 1 Full year result highlights 12 months to 31 March 2014 NZ$M PCP CC 1 Record net profit after tax 97.1 +26% +46% Record operating
2014 Full Year Results Presentation Year ended 31 March 2014 26-30 May 2014 1 Full year result highlights 12 months to 31 March 2014 NZ$M PCP CC 1 Record net profit after tax 97.1 +26% +46% Record operating
Comparison of patient spirometry and ventilator spirometry
 GE Healthcare Comparison of patient spirometry and ventilator spirometry Test results are based on the Master s thesis, Comparison between patient spirometry and ventilator spirometry by Saana Jenu, 2011
GE Healthcare Comparison of patient spirometry and ventilator spirometry Test results are based on the Master s thesis, Comparison between patient spirometry and ventilator spirometry by Saana Jenu, 2011
Aeroneb Solo System Instruction Manual
 www.aerogen.com Aeroneb Solo System Instruction Manual Aeroneb Solo System Instruction Manual Introduction 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 10 Installation
www.aerogen.com Aeroneb Solo System Instruction Manual Aeroneb Solo System Instruction Manual Introduction 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 10 Installation
Improving Care & Outcomes
 Improving Care & Outcomes Macquarie Technology Day, 20 October 2011 1 Improving Care & Outcomes The Care Continuum - Matthew Payton F&P Optiflow - Matthew Payton F&P Info Technologies - Lewis Gradon ICON
Improving Care & Outcomes Macquarie Technology Day, 20 October 2011 1 Improving Care & Outcomes The Care Continuum - Matthew Payton F&P Optiflow - Matthew Payton F&P Info Technologies - Lewis Gradon ICON
Nottingham Children s Hospital
 High Flow Nasal Cannula Therapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guide line for the use of HFNCT (High Flow Nasal Cannula Therapy) Contact Name
High Flow Nasal Cannula Therapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guide line for the use of HFNCT (High Flow Nasal Cannula Therapy) Contact Name
Special Problems in Aerosol Delivery: Artificial Airways
 Special Problems in Aerosol Delivery: Artificial Airways Rajiv Dhand MD Introduction Factors Influencing Aerosol Delivery through Endotracheal Tubes Endotracheal Tube Size Endotracheal Tube Material/Design
Special Problems in Aerosol Delivery: Artificial Airways Rajiv Dhand MD Introduction Factors Influencing Aerosol Delivery through Endotracheal Tubes Endotracheal Tube Size Endotracheal Tube Material/Design
Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation
 Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation Dorisanne D. Miller, Mohammad M. Amin, Lucy B. Palmer, Akbar R. Shah, and Gerald C. Smaldone Department of Respiratory Care,
Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation Dorisanne D. Miller, Mohammad M. Amin, Lucy B. Palmer, Akbar R. Shah, and Gerald C. Smaldone Department of Respiratory Care,
Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung
 Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung Nia Stevens 9 th November 2016 Thanks to Richard Kaye, James Mitchell, Dave Prime at GSK Bahman Asgharian and Owen
Use of Math Modelling to Understand Delivery of Biopharmaceutical Molecules to the Lung Nia Stevens 9 th November 2016 Thanks to Richard Kaye, James Mitchell, Dave Prime at GSK Bahman Asgharian and Owen
Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities
 Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities Goals 1. To meet respiratory care needs in patients who are on airborne
Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities Goals 1. To meet respiratory care needs in patients who are on airborne
Tissue Hypoxia and Oxygen Therapy
 Tissue Hypoxia and Oxygen Therapy ก ก ก ก ก ก 1. ก ก 2. ก ก 3. tissue hypoxia 4. ก ก ก 5. ก ก ก 6. ก กก ก 7. ก ก tissue hypoxia ก ก ก ก 1. Pathway of oxygen transport 2. Causes of tissue hypoxia 3. Effect
Tissue Hypoxia and Oxygen Therapy ก ก ก ก ก ก 1. ก ก 2. ก ก 3. tissue hypoxia 4. ก ก ก 5. ก ก ก 6. ก กก ก 7. ก ก tissue hypoxia ก ก ก ก 1. Pathway of oxygen transport 2. Causes of tissue hypoxia 3. Effect
IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room
 IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room 1) Decreasing Telemetry Alarms Janice Marlett, BSN, RN, Nursing Staff Educator To decrease tele alarms: Properly prep the skin Shave
IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room 1) Decreasing Telemetry Alarms Janice Marlett, BSN, RN, Nursing Staff Educator To decrease tele alarms: Properly prep the skin Shave
