Complementary and Alternative Medicine in Treating Autism Spectrum Disorders. Request for Applications
|
|
|
- Harry Townsend
- 5 years ago
- Views:
Transcription
1 Complementary and Alternative Medicine in Treating Autism Spectrum Disorders Request for Applications Introduction Although medical care in the United States is increasingly driven by evidence-based practice, societal pressure for tolerance and incorporation of complementary and alternative forms of health care is significant. Many children (estimates vary from 50% to 75%) with autism spectrum disorders are treated with some form of complementary and alternative intervention, and approximately 1/3 of these are being so treated at the time of diagnostic evaluation. Parents of children with autism are often dissatisfied with their pediatricians lack of knowledge about complementary and alternative treatments. Families desire evidence with which they can judge intervention choices, providers want to determine what interventions are safe and effective, and funding sources of care want to know the potential public health effects of such interventions. All treatments should be held to the same scrutiny. The goal of this initiative is to identify and evaluate evidence-based integrative therapies and novel, untested treatment applications for autism spectrum disorders that include complementary and alternative forms of health care. Therapies and treatments should be clearly defined so as to enable replications or expansions of initial studies. Areas of special interest include but are not limited to biomedical interventions such as dietary treatments, nutritional supplements, among others and non-biomedical interventions such as sensory integration therapies and others. Awards Autism Speaks will make a limited number of research awards based on merit. An amount not to exceed 10% (inclusive) of the total award shall be used for Sponsoring Institution's indirect (overhead) costs. All awards are one-time, non-renewable research grants. Pilot Study Awards for proposals to establish proof of concept and develop preliminary data necessary to prepare and submit a competitive research grant application to a major federal funding agency (e.g., NIH, NSF, CDC, etc.) or its equivalent, will be for a maximum of $100,000 per year for one or two years. Research Awards for mature proposals including clinical trials will be for a maximum of $150,000 per year for three years. Eligibility Investigators holding full or part-time faculty appointments, professional affiliations or equivalent at accredited academic, medical, osteopathic, chiropractic, research or educational institutions are eligible to apply. Applications will NOT be accepted from individuals or proprietary organizations to support the research and development of products or treatments for profit. Each application is restricted to one type of project only pilot study or research award.
2 Letter of Intent The applicant must first submit a Letter of Intent (LOI) through the Autism Speaks Grants Administration web site that includes the following information: a. A concise description of the proposed project including: the type of project (pilot or research), specific aims, methods and expected results. b. A clear justification for the relevance and potential significance of the project to understanding the treatment, prevention or cure of autism spectrum disorders. c. Names, titles and institutional affiliations of active collaborators/coinvestigators in addition to the PI (excludes consultants, postdoctoral fellows, students and technicians). The Letter of Intent cannot exceed 2 pages including references. In addition, the LOI field requires that the applicant provide the following information at the time of submission: key word selections, proposal title, number of years requested, and RFA to which the PI is responding. Please refer to the Autism Speaks Grants Administration System for more detailed instructions. Only applicants whose Letters of Intent are approved by Autism Speaks will be invited to submit full proposals. Applicants whose Letter of Intent are approved will be invited to participate in a webinar to discuss the application and review process as well as topics relevant to the preparation and submission of a research application in response to this solicitation, such as common challenges in study design. These information sessions will be held in late October or early November. An invited proposal cannot deviate significantly from the project description in its approved Letter of Intent. Proposals received without first obtaining an approved LOI will be returned without review. Autism Speaks also reserves the right to return without review any proposal that in its judgment is not in compliance with its rules and procedures for proposal preparation and submission, is not responsive to its research goals, or exceeds its funding limits or available resources. Proposal Preparation Proposals should be written in 11 point Arial font. Use of smaller or difficult to read font may result in the proposal being returned without review. Please refer to the Autism Speaks Grants Administration System for more detailed instructions. A typical research proposal will include the following sections: a. Scientific/Technical Abstract (maximum 1 page) and Key Words: must be intelligible to a knowledgeable professional peer, and describe the project goal(s) and/or hypothesis, specific aims, research methods, expected results and significance/relevance. b. Lay Abstract (maximum 1 page): must be intelligible to a knowledgeable lay person and describe the project goal(s), general means, expected results and their significance/relevance to the treatment, prevention or cure of autism spectrum disorders. c. Research Plan (10 pages maximum for Pilot Study and 15 pages maximum for Research Award): 1. Background: Describe the historical development and existing empirical support for the integrative therapy or novel treatment application including: a. A detailed description of the integrative therapy or novel treatment application (supporting materials should be submitted in the appendix and referenced in the research plan). b. Explanation and justification of the case ascertainment or diagnostic parameters.
3 c. Justification for the appropriateness of the integrative therapy or novel treatment application for the target population. d. A description of how the integrative therapy or novel treatment application will address the needs of participating children and families, including specific functional outcomes targeted. 2. Study Design: Study participants should be from carefully selected populations and the selection criteria described and justified. Describe: the sampling strategy; implementation and evaluation methods; assessment of the therapeutic benefit; monitoring and reporting of side effects and toxicity; criteria and decision making process for suspending or ending the study prior to completion; the validation of the selected outcomes measures; assessment of child and family outcomes (including treatment acceptability by families); ecological validity of the treatment outcomes; the nature and relevance of control groups (including the use of historical or off-site control groups evaluated using the same methods but without the treatment component; the use of an historical control group should be justified) and precautions against bias in the study design and interpretation of results. Describe the feasibility of achieving study objectives within funding period (including sample availability and readiness to implement the proposed intervention). Describe the development and role of a Trial Steering Committee. Address any ethical issues implicit in study design. 3. Statistical Analysis: Describe proposed statistical analyses including how sample size will be determined and a power analysis to show that the sample size will produce statistically significant results. 4. Other Treatments: Describe any potential confounding factors or combined interventions (diet, medication, etc.) that parents are using in addition to and outside of the control of the treatment protocol, and their potential or known impact on the treatment protocol. d. Bibliography with complete literature citations including titles and all authors. e. Budget for each year of the project and a cumulative budget; the budget may include: 1. Personnel Costs (not to exceed the percent effort committed to the proposed project) a. Principal Investigator and/or Co-Investigator salaries and benefits (allowed for research proposals only; NOT ALLOWED for pilot studies). b. Technical support salaries and benefits. c. Postdoctoral fellow stipend and benefits. d. Graduate student stipend benefits (NOTE: Tuition reimbursement is not allowed). 2. Project Costs a. Research supplies, services and related expenses. b. Essential equipment not to exceed $15,000 per year or $30,000 total (allowed for research proposals only; NOT ALLOWED for pilot studies). A vendor estimate is required for a single item of equipment costing more than $5,000. c. Travel to professional meetings. d. Data analysis and publication costs. Budget justification: Justify personnel costs, project-dedicated resources, services and equipment and any unusually costly expenses or travel. Description of relevant facilities and necessary on-site and off-site resources. Biographical Sketches of the Principal Investigator and named Co-Investigators in NIH format, not to exceed 3 pages each; indicate education, professional
4 appointments and honors, total number of peer-reviewed publications, and complete citations (including title) of publications relevant to the proposed research. Current and Pending Support: indicate funding source, total award amount, award duration (inclusive dates) and project title; clearly explain any overlap with the proposed research including the extent to which the projects are redundant or complementary. Human Subjects Certifications must be documented with a copy of an official letter of approval (or equivalent for non-us applicants), which identifies the Principal Investigator, project title and date of approval, and is signed by the Review Committee Chair or equivalent responsible institutional/government official. Prior certification for another project cannot be substituted, but can be officially amended to include the proposed project (identified by project title). IMPORTANT: IRB (or equivalent ethical) certification is NOT required to submit an application; however, IRB (or equivalent ethical) certification must be submitted as soon as possible following official notification of an award. Autism Speaks will NOT issue the first funding increment of a grant until this certification is received. Rules governing human subjects (IRB) certifications for both US and non-us institutions can be found at: Letters of Collaboration or Agreement Appendices: Critical photographs or instrument descriptions requiring special formatting and other essential background material can be submitted as pdf files and referenced in the Research Plan. A maximum of 3 peer reviewed research publications (including manuscripts under review or accepted for publication) will be accepted; however, manuscripts not yet accepted for review, review articles, book chapters, popular press articles and meeting abstracts will NOT be accepted. WARNING! Appendix publications are provided as a courtesy to the reviewers who are under no obligation to read or consult them in evaluating a proposal. Submission of Letters of Intent and Proposals Only Letters of Intent and proposals submitted through the Autism Speaks Grants Administration System at will be accepted for review. Applicants should refer to the Grants Administration web site for specific requirements and instructions about electronic LOI and proposal submission. A successful electronic LOI or proposal submission will be confirmed by a system generated notification to the applicant. Letters of Intent or proposals submitted by regular mail or by will be returned without review. Letters of Intent are due on September 28, Proposals are due on February 15, IMPORTANT! Letters of Intent and proposals will NOT BE ACCEPTED after their due dates unless prior permission is obtained, and only for exceptional circumstances (e.g., child birth, serious illness or death in the family, natural disaster). It is the applicant s responsibility to insure that both the Letter of Intent and Proposal are in compliance with the policies and procedures prescribed in the RFA. Autism Speaks reserves the right to return without review any Letter of Intent or Proposal found to be not in compliance with the policies and procedures prescribed on the RFA at any time during the application and review process. Review and Selection The peer review panel will meet in March Funding selections and notification of awardees will be made in April 2008, with an expected award start date on or about May 2008.
5 Payment of Awards Awards will be paid annually and will be contingent upon registration in the Autism Speaks Grants Administration System of an official authorized to act for the institution receiving the award, and upon acceptance of the Autism Speaks award terms and conditions as described at including receipt of all required certifications. Funding for award years 02 and 03 will be contingent upon submission by the principal investigator of a satisfactory annual project progress report, and a financial statement showing expended and unexpended funds. Unexpended funds in excess of 25% of the annual award must be satisfactorily explained before those funds can be rolled over into the next year of the grant. In addition, an investigator may request a one-time, six-month non-cost extension from the termination date of the award in order to complete necessary work (including data analysis and preparation/submission of manuscripts for publication). Please address questions about proposal submission using the web-based Grants Administration System to Ms. Susan Kravitz, Grants Administrator by telephone at or by at: skravitz@autismspeaks.org. All other questions (including eligibility to apply) should be addressed to Dr. Charles D. Liarakos, Director of Scientific Review at: cliarakos@autismspeaks.org.
is to improve the future
 Autism Speaks Suzanne and Bob Wright Trailblazer Award Program 2013 Request for Applications Autism Speaks places a high priority on innovation and has designed its new Trailblazer Award to respond quickly
Autism Speaks Suzanne and Bob Wright Trailblazer Award Program 2013 Request for Applications Autism Speaks places a high priority on innovation and has designed its new Trailblazer Award to respond quickly
Environmen. Project. Background. etiology of II. to identify. approach. are strongly. Released. Page 1
 Environmen ntal Epidemiology of Autism Research Network (EEARN) RFA Environmental Epidemiological Approaches to Understanding Risk Factors for ASD: Collaborative Projects Submission, Review, & Notification
Environmen ntal Epidemiology of Autism Research Network (EEARN) RFA Environmental Epidemiological Approaches to Understanding Risk Factors for ASD: Collaborative Projects Submission, Review, & Notification
Request for Applications: 2019 Pre- and Postdoctoral Training Awards & Medical Student Gap Year Research Training Awards
 106 W 32ND STREET, SUITE 182 NEW YORK, NY 10001 P: 914-810-9100 AUTISMSCIENCEFOUNDATION.ORG Request for Applications: 2019 Pre- and Postdoctoral Training Awards & Medical Student Gap Year Research Training
106 W 32ND STREET, SUITE 182 NEW YORK, NY 10001 P: 914-810-9100 AUTISMSCIENCEFOUNDATION.ORG Request for Applications: 2019 Pre- and Postdoctoral Training Awards & Medical Student Gap Year Research Training
Autism Speaks Translational Postdoctoral Fellowship Program 2011 Request for Applications
 Autism Speaks Translational Postdoctoral Fellowship Program 2011 Request for Applications Deadlines: Letter of Intent: March 31, 2011, 8:00 p.m. (U.S. Eastern Time) Letters of recommendation and Application:
Autism Speaks Translational Postdoctoral Fellowship Program 2011 Request for Applications Deadlines: Letter of Intent: March 31, 2011, 8:00 p.m. (U.S. Eastern Time) Letters of recommendation and Application:
LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH 2017 REQUEST FOR APPLICATION (RFA)
 LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH FUNDING OPPORTUNITY DESCRIPTION 2017 REQUEST FOR APPLICATION (RFA) Lung Cancer Foundation of America (LCFA) and the International
LCFA/IASLC LORI MONROE SCHOLARSHIP IN TRANSLATIONAL LUNG CANCER RESEARCH FUNDING OPPORTUNITY DESCRIPTION 2017 REQUEST FOR APPLICATION (RFA) Lung Cancer Foundation of America (LCFA) and the International
UC Brain Tumor Center Molecular Therapeutics Pilot Grant Program 2013/2014
 Introduction An estimated 25,000 new cases of primary brain tumors will be diagnosed in the United States in 2013, and an estimated 170,000 patients will be diagnosed with brain metastases, most often
Introduction An estimated 25,000 new cases of primary brain tumors will be diagnosed in the United States in 2013, and an estimated 170,000 patients will be diagnosed with brain metastases, most often
Deadlines: Letters. change): Peer review panels: Winter disorders. physiology / risk factors prevention and. 3. Improve quality technologies
 Autism Speakss Dennis Weatherstone Predoctoral Fellowship Program 2013 Request for Applications Deadlines: Letter of Intent, System will open: September 10, 2012 Letter of Intent due on or before: October
Autism Speakss Dennis Weatherstone Predoctoral Fellowship Program 2013 Request for Applications Deadlines: Letter of Intent, System will open: September 10, 2012 Letter of Intent due on or before: October
RESEARCH GRANT APPLICATION
 2018-2019 RESEARCH GRANT APPLICATION Deadline: Friday, December 8, 2017 Osseointegration Foundation Research Grants The object of these grants is to provide funding for basic and applied scientific research
2018-2019 RESEARCH GRANT APPLICATION Deadline: Friday, December 8, 2017 Osseointegration Foundation Research Grants The object of these grants is to provide funding for basic and applied scientific research
2017 CURE - Sleep and Epilepsy Award Request for Applications (RFA) with LOI & Full Proposal Guidelines
 2017 CURE - Sleep and Epilepsy Award Request for Applications (RFA) with LOI & Full Proposal Guidelines About CURE CURE s mission is to cure epilepsy, transforming and saving millions of lives. We identify
2017 CURE - Sleep and Epilepsy Award Request for Applications (RFA) with LOI & Full Proposal Guidelines About CURE CURE s mission is to cure epilepsy, transforming and saving millions of lives. We identify
Research Grant Application
 2019-2020 Research Grant Application Application Deadline: December 14, 2018 Who We Are The Academy of Osseointegration and the Osseointegration Foundation foster the highest standards of research and
2019-2020 Research Grant Application Application Deadline: December 14, 2018 Who We Are The Academy of Osseointegration and the Osseointegration Foundation foster the highest standards of research and
Research commission: GUIDANCE FOR APPLICANTS
 SHAPe, the Sowerby Health intervention for Autistic People (SHAPe) study: Annual GP health checks for autistic adults to improve primary health access and address mortality and co-morbidities of autistic
SHAPe, the Sowerby Health intervention for Autistic People (SHAPe) study: Annual GP health checks for autistic adults to improve primary health access and address mortality and co-morbidities of autistic
Melanoma Research Foundation Request for Proposals (RFP) 2019
 Melanoma Research Foundation Request for Proposals (RFP) 2019 RESEARCH OVERVIEW: The Melanoma Research Foundation (MRF) is committed to advancing research across the spectrum of melanoma from prevention
Melanoma Research Foundation Request for Proposals (RFP) 2019 RESEARCH OVERVIEW: The Melanoma Research Foundation (MRF) is committed to advancing research across the spectrum of melanoma from prevention
Non-Pharmacological Strategies to Ameliorate Symptoms of Alzheimer s Disease and Related Dementia (NPSASA)
 Non-Pharmacological Strategies to Ameliorate Symptoms of Alzheimer s Disease and Related Dementia (NPSASA) Competition objectives: The Alzheimer s Association is launching a new initiative to stimulate
Non-Pharmacological Strategies to Ameliorate Symptoms of Alzheimer s Disease and Related Dementia (NPSASA) Competition objectives: The Alzheimer s Association is launching a new initiative to stimulate
ALS ACT (Accelerated Therapeutics)
 ALS ACT (Accelerated Therapeutics) Request for Proposals: Phase II Clinical development of novel, high-potential treatments for people with ALS Release Date 23 October 2015 Letter of intent due: November
ALS ACT (Accelerated Therapeutics) Request for Proposals: Phase II Clinical development of novel, high-potential treatments for people with ALS Release Date 23 October 2015 Letter of intent due: November
Award for a Physical Therapist
 Award for a Physical Therapist POLICIES AND GUIDELINES Published: October 4, 2018 Application Deadline: December 3, 2018 Cystic Fibrosis Foundation 4550 Montgomery Avenue, Suite 1100 N Bethesda, MD 20814
Award for a Physical Therapist POLICIES AND GUIDELINES Published: October 4, 2018 Application Deadline: December 3, 2018 Cystic Fibrosis Foundation 4550 Montgomery Avenue, Suite 1100 N Bethesda, MD 20814
MOVEMBER FOUNDATION DANISH PROSTATE CANCER PROJECT REQUEST FOR APPLICATIONS
 MOVEMBER FOUNDATION CANCER PROJECT REQUEST FOR APPLICATIONS CONTENTS ABOUT THE MOVEMBER FOUNDATION... 3 DESCRIPTION... 4 ELIGIBILITY... 5 ALLOWABLE COSTS... 6 INELIGIBLE COSTS... 6 COMMUNICATION REQUIREMENTS...
MOVEMBER FOUNDATION CANCER PROJECT REQUEST FOR APPLICATIONS CONTENTS ABOUT THE MOVEMBER FOUNDATION... 3 DESCRIPTION... 4 ELIGIBILITY... 5 ALLOWABLE COSTS... 6 INELIGIBLE COSTS... 6 COMMUNICATION REQUIREMENTS...
STTR TRANSFORMATIVE TEAM GRANTS APPLICATION
 2015 STTR TRANSFORMATIVE TEAM GRANTS APPLICATION Application Deadline: May 20, 2015 BLADDER BRAIN BREAST COLORECTAL HEAD & NECK LUNG OVARIAN PANCREAS PROSTATE APPLICATION REQUIREMENTS Solid Tumor Translational
2015 STTR TRANSFORMATIVE TEAM GRANTS APPLICATION Application Deadline: May 20, 2015 BLADDER BRAIN BREAST COLORECTAL HEAD & NECK LUNG OVARIAN PANCREAS PROSTATE APPLICATION REQUIREMENTS Solid Tumor Translational
Request for Applications Basic, Clinical and Population Science Pilot Awards
 Request for Applications Basic, Clinical and Population Science Pilot Awards Spring, 2013 SUMMARY The University of Wisconsin Carbone Cancer Center (UWCCC) is an NCI-designated Comprehensive Cancer Center
Request for Applications Basic, Clinical and Population Science Pilot Awards Spring, 2013 SUMMARY The University of Wisconsin Carbone Cancer Center (UWCCC) is an NCI-designated Comprehensive Cancer Center
Financial Administration and Control of Research and Special Funds
 1 2 3 Financial Administration and Control of Research and Special Funds 4 Financial Administration and Control of Research and Special Funds Presenter Name Colin Nicolson Presenter Title Assistant Manager
1 2 3 Financial Administration and Control of Research and Special Funds 4 Financial Administration and Control of Research and Special Funds Presenter Name Colin Nicolson Presenter Title Assistant Manager
Geriatrics / Gerontology Education
 2018 Geriatrics / Gerontology Education Call for Proposals Dr. Paula Rochon, RTO/ERO Chair in Geriatric Medicine, with trainees. RTO/ERO Foundation 2018 Grant Criteria & Call for Proposals February 20,
2018 Geriatrics / Gerontology Education Call for Proposals Dr. Paula Rochon, RTO/ERO Chair in Geriatric Medicine, with trainees. RTO/ERO Foundation 2018 Grant Criteria & Call for Proposals February 20,
Request for Proposals for a Clean Syringe Exchange Program
 ANNA M. ROTH, RN, MS, MPH HEALTH SERVICES DIRECTOR DAN PEDDYCORD, RN, MPA/HA DIRECTOR OF PUBLIC HEALTH C O N T R A C O S T A P U B L I C H E A L T H 597 CENTER AVENUE, SUITE 200 MARTINEZ, CALIFORNIA 94553
ANNA M. ROTH, RN, MS, MPH HEALTH SERVICES DIRECTOR DAN PEDDYCORD, RN, MPA/HA DIRECTOR OF PUBLIC HEALTH C O N T R A C O S T A P U B L I C H E A L T H 597 CENTER AVENUE, SUITE 200 MARTINEZ, CALIFORNIA 94553
REQUEST FOR PROPOSALS
 REQUEST FOR PROPOSALS My Seizure Gauge Initiative Bringing Big Data to Personalized Seizure Risk Assessment The Epilepsy Innovation Institute (Ei 2 ) is pleased to announce a Request for Proposals for
REQUEST FOR PROPOSALS My Seizure Gauge Initiative Bringing Big Data to Personalized Seizure Risk Assessment The Epilepsy Innovation Institute (Ei 2 ) is pleased to announce a Request for Proposals for
Paediatric and Adolescent HIV Research Grant Programme
 Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Paediatric and Adolescent HIV Research Grant Programme Call for Letter of Intent Table of Contents I. GRANT INFORMATION... 2
Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Paediatric and Adolescent HIV Research Grant Programme Call for Letter of Intent Table of Contents I. GRANT INFORMATION... 2
Information Technology Solutions
 Information Technology Solutions World Institute of Pain (WIP) Excellence in Pain Practice Award Award Applicant Site Inspection Handbook June 28, 2010 WIP EPP Award Site Inspection Handbook Page 2 Table
Information Technology Solutions World Institute of Pain (WIP) Excellence in Pain Practice Award Award Applicant Site Inspection Handbook June 28, 2010 WIP EPP Award Site Inspection Handbook Page 2 Table
Melanoma Research Foundation Request for Proposals (RFP) 2017
 Melanoma Research Foundation Request for Proposals (RFP) 2017 RESEARCH OVERVIEW: The Melanoma Research Foundation (MRF) is committed to advancing research across the spectrum of melanoma from prevention
Melanoma Research Foundation Request for Proposals (RFP) 2017 RESEARCH OVERVIEW: The Melanoma Research Foundation (MRF) is committed to advancing research across the spectrum of melanoma from prevention
Call for Research Proposals on the Interplay between Brain Health, Violence and Compassion
 Call for Research Proposals on the Interplay between Brain Health, Violence and Compassion Purpose The Avielle Foundation (TAF) is committed to supporting traditional neuroscience, public health, and clinical
Call for Research Proposals on the Interplay between Brain Health, Violence and Compassion Purpose The Avielle Foundation (TAF) is committed to supporting traditional neuroscience, public health, and clinical
REQUEST FOR PROPOSALS PROSTATE CANCER CLINICAL TRIAL AWARD SWITZERLAND AND FRANCE
 REQUEST FOR PROPOSALS PROSTATE CANCER CLINICAL TRIAL AWARD SWITZERLAND AND FRANCE 16 OCTOBER 2014 TABLE OF CONTENTS TABLE OF CONTENTS 2 RESEARCH PROGRAM OBJECTIVES 3 ABOUT THE MOVEMBER FOUNDATION 3 AIMS
REQUEST FOR PROPOSALS PROSTATE CANCER CLINICAL TRIAL AWARD SWITZERLAND AND FRANCE 16 OCTOBER 2014 TABLE OF CONTENTS TABLE OF CONTENTS 2 RESEARCH PROGRAM OBJECTIVES 3 ABOUT THE MOVEMBER FOUNDATION 3 AIMS
INOVIO PHARMACEUTICALS, INC. INVESTIGATOR CONFLICT OF INTEREST POLICY
 INOVIO PHARMACEUTICALS, INC. INVESTIGATOR CONFLICT OF INTEREST POLICY August 24, 2012 1. Purpose Public confidence and the reputation of the company are valuable business assets that Inovio strives to
INOVIO PHARMACEUTICALS, INC. INVESTIGATOR CONFLICT OF INTEREST POLICY August 24, 2012 1. Purpose Public confidence and the reputation of the company are valuable business assets that Inovio strives to
Diabetes Australia Research Program
 Diabetes Australia Research Program GUIDELINES 2019 General Grants 2019-2020 Millennium Awards Please read these Guidelines before completing your application. Closing date for applications: Friday, 4
Diabetes Australia Research Program GUIDELINES 2019 General Grants 2019-2020 Millennium Awards Please read these Guidelines before completing your application. Closing date for applications: Friday, 4
TRAUMA RECOVERY/HAP OPERATING GUIDELINES
 TRAUMA RECOVERY/HAP OPERATING GUIDELINES FOR THE NATIONAL TRAUMA RECOVERY NETWORK, THE TRAUMA RECOVERY NETWORK ASSOCIATIONS, AND THE TRAUMA RECOVERY NETWORK CHAPTERS Operating Guidelines These Operating
TRAUMA RECOVERY/HAP OPERATING GUIDELINES FOR THE NATIONAL TRAUMA RECOVERY NETWORK, THE TRAUMA RECOVERY NETWORK ASSOCIATIONS, AND THE TRAUMA RECOVERY NETWORK CHAPTERS Operating Guidelines These Operating
Student Case Study Contest
 Student Case Study Contest 2017 2018 guidelines application form appendix STUDENT CASE STUDY CONTEST 2017-2018 Introduction Massage & Myotherapy Australia, with AON, is pleased to invite students of massage,
Student Case Study Contest 2017 2018 guidelines application form appendix STUDENT CASE STUDY CONTEST 2017-2018 Introduction Massage & Myotherapy Australia, with AON, is pleased to invite students of massage,
Kansas Department of Health and Environment (KDHE) Kansas Data-Driven Prevention Initiative Request for Proposal (RFP) Fiscal Year 2019
 Kansas Department of Health and Environment (KDHE) Kansas Data-Driven Prevention Initiative Request for Proposal (RFP) Fiscal Year 2019 Kansas Department of Health and Environment Bureau of Health Promotion
Kansas Department of Health and Environment (KDHE) Kansas Data-Driven Prevention Initiative Request for Proposal (RFP) Fiscal Year 2019 Kansas Department of Health and Environment Bureau of Health Promotion
CENTER FOR COGNITIVE AND BEHAVIORAL BRAIN IMAGING. MISSION, ADMINISTRATIVE STRUCTURE and REGULATIONS
 CENTER FOR COGNITIVE AND BEHAVIORAL BRAIN IMAGING MISSION, ADMINISTRATIVE STRUCTURE and REGULATIONS MISSION The Center for Cognitive and Behavioral Brain Imaging is dedicated to pursuing structural and
CENTER FOR COGNITIVE AND BEHAVIORAL BRAIN IMAGING MISSION, ADMINISTRATIVE STRUCTURE and REGULATIONS MISSION The Center for Cognitive and Behavioral Brain Imaging is dedicated to pursuing structural and
Please pay attention to Question 15 in the questionnaire regarding contact information related to the payment process.
 2018 Program Changes/Additions International: We are continuing the program for students from the veterinary schools of France and the Netherlands. In addition, for the first time in 2018, two students
2018 Program Changes/Additions International: We are continuing the program for students from the veterinary schools of France and the Netherlands. In addition, for the first time in 2018, two students
CHAPTER 40 PROFESSIONAL LICENSING AND FACILITY REGULATION
 216-RICR-40-05-33 TITLE 216 - DEPARTMENT OF HEALTH CHAPTER 40 PROFESSIONAL LICENSING AND FACILITY REGULATION SUBCHAPTER 05 PROFESSIONAL LICENSING PART 33 - Speech Pathologists and Audiologists 33.1 Authority
216-RICR-40-05-33 TITLE 216 - DEPARTMENT OF HEALTH CHAPTER 40 PROFESSIONAL LICENSING AND FACILITY REGULATION SUBCHAPTER 05 PROFESSIONAL LICENSING PART 33 - Speech Pathologists and Audiologists 33.1 Authority
POLICY. Institutional Research Projects/Data Requests #7220
 POLICY 1. This policy is intended to ensure that data requests and research projects conducted by any college office, employee, student, or affiliate are sound and that they do not violate board policy,
POLICY 1. This policy is intended to ensure that data requests and research projects conducted by any college office, employee, student, or affiliate are sound and that they do not violate board policy,
REQUEST FOR PROPOSALS FOR CY 2019 FUNDING. Issue Date: Monday, July 30, Submission Deadline: 5:00 p.m., Friday, August 24, 2018
 REQUEST FOR PROPOSALS FOR CY 2019 FUNDING Issue Date: Monday, July 30, 2018 Submission Deadline: 5:00 p.m., Friday, August 24, 2018 NOTE: RFP proposals received after the deadline will not be considered.
REQUEST FOR PROPOSALS FOR CY 2019 FUNDING Issue Date: Monday, July 30, 2018 Submission Deadline: 5:00 p.m., Friday, August 24, 2018 NOTE: RFP proposals received after the deadline will not be considered.
VA Funding Opportunities & Research Resources
 VA Funding Opportunities & Research Resources Frederick G. Hamel, Ph.D. Acting Associate Chief of Staff/Research Professor Internal Medicine and Pharmacology/ UNMC October 19, 2015 NWI Way Contacts If
VA Funding Opportunities & Research Resources Frederick G. Hamel, Ph.D. Acting Associate Chief of Staff/Research Professor Internal Medicine and Pharmacology/ UNMC October 19, 2015 NWI Way Contacts If
The Financial Value of STTR for Non-Profit Research
 The Financial Value of STTR for Non-Profit Research Introduction Minimizing the Direct Labor Overhead Rate as a Metric for Success Greg Evans, Durestec For non-profit research institutions, managing PhD
The Financial Value of STTR for Non-Profit Research Introduction Minimizing the Direct Labor Overhead Rate as a Metric for Success Greg Evans, Durestec For non-profit research institutions, managing PhD
CFAR Supplement Announcement in HIV/AIDS FY2016
 CFAR Supplement Announcement in HIV/AIDS FY2016 The CFAR Program at the National Institutes of Health (NIH) invites applications from currently funded CFARs that are eligible for administrative supplements.
CFAR Supplement Announcement in HIV/AIDS FY2016 The CFAR Program at the National Institutes of Health (NIH) invites applications from currently funded CFARs that are eligible for administrative supplements.
Please find enclosed Dosimetry Service Licence No which replaces Dosimetry Service Licence No
 Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
NIH NEW CLINICAL TRIAL REQUIREMENTS AND FORMS-E
 NIH NEW CLINICAL TRIAL REQUIREMENTS AND FORMS-E HOW DOES THE NIH DEFINE CLINICAL TRIAL AND HOW DO I KNOW IF MY STUDY IS ONE? 01-18-18 A clinical trial is defined by the NIH as a research study in which
NIH NEW CLINICAL TRIAL REQUIREMENTS AND FORMS-E HOW DOES THE NIH DEFINE CLINICAL TRIAL AND HOW DO I KNOW IF MY STUDY IS ONE? 01-18-18 A clinical trial is defined by the NIH as a research study in which
TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE
 TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE RESEARCH PROPOSAL FORM This proposal is: (check where applicable) Dissertation Research: Grant Proposal: Funding Agency: Master's Thesis Research: Faculty
TENNESSEE STATE UNIVERSITY HUMAN SUBJECTS COMMITTEE RESEARCH PROPOSAL FORM This proposal is: (check where applicable) Dissertation Research: Grant Proposal: Funding Agency: Master's Thesis Research: Faculty
IC Chapter 1. Regulation of Chiropractors Creation of Board
 IC 25-10 ARTICLE 10. CHIROPRACTORS IC 25-10-1 Chapter 1. Regulation of Chiropractors Creation of Board IC 25-10-1-1 Definitions Sec. 1. As used in this article: (1) "Chiropractic" means the diagnosis and
IC 25-10 ARTICLE 10. CHIROPRACTORS IC 25-10-1 Chapter 1. Regulation of Chiropractors Creation of Board IC 25-10-1-1 Definitions Sec. 1. As used in this article: (1) "Chiropractic" means the diagnosis and
HIV and Drug Use Research Fellowship. Programme and Application Webinar
 HIV and Drug Use Research Fellowship Programme and Application Webinar Programme and Application Webinar HIV and Drug Use Research Fellowship Programme Intersection of HIV and Drug Use Objectives and Expected
HIV and Drug Use Research Fellowship Programme and Application Webinar Programme and Application Webinar HIV and Drug Use Research Fellowship Programme Intersection of HIV and Drug Use Objectives and Expected
Pfizer Independent Grants for Learning & Change Request for Proposals (RFP) Rheumatoid Arthritis Mono- and Combination DMARD Therapy in RA Patients
 Pfizer Independent Grants for Learning & Change Request for Proposals (RFP) Rheumatoid Arthritis Mono- and Combination DMARD Therapy in RA Patients I. Background The mission of Pfizer Independent Grants
Pfizer Independent Grants for Learning & Change Request for Proposals (RFP) Rheumatoid Arthritis Mono- and Combination DMARD Therapy in RA Patients I. Background The mission of Pfizer Independent Grants
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-15-1-0154 TITLE: Efficacy of the Direct Instruction Language for Learning (DI-LL) Program to Promote Expressive and Receptive Language in Children with Autism Spectrum Disorder PRINCIPAL
AWARD NUMBER: W81XWH-15-1-0154 TITLE: Efficacy of the Direct Instruction Language for Learning (DI-LL) Program to Promote Expressive and Receptive Language in Children with Autism Spectrum Disorder PRINCIPAL
Community Engagement Grants Informational Webinar. Carole Specktor, Senior Cessation Manager January, 2015
 Community Engagement Grants Informational Webinar Carole Specktor, Senior Cessation Manager January, 2015 Overview Background Funding initiative goals Overview of initiative: Size and duration of grants
Community Engagement Grants Informational Webinar Carole Specktor, Senior Cessation Manager January, 2015 Overview Background Funding initiative goals Overview of initiative: Size and duration of grants
SALISBURY UNIVERSITY COMMITTEE ON HUMAN RESEARCH APPLICATION FOR RESEARCH INVOLVING HUMAN SUBJECTS
 SALISBURY UNIVERSITY COMMITTEE ON HUMAN RESEARCH APPLICATION FOR RESEARCH INVOLVING HUMAN SUBJECTS If you have a full committee review: 1. Your proposal must be submitted at minimum 14 days before the
SALISBURY UNIVERSITY COMMITTEE ON HUMAN RESEARCH APPLICATION FOR RESEARCH INVOLVING HUMAN SUBJECTS If you have a full committee review: 1. Your proposal must be submitted at minimum 14 days before the
New NCCIH Funding Opportunities for Mind and Body Clinical Trials. April 24, 2017
 New NCCIH Funding Opportunities for Mind and Body Clinical Trials April 24, 2017 Introductions Wendy Weber, N.D., Ph.D., M.P.H. Branch Chief, Clinical Research in Complementary and Integrative Health Branch,
New NCCIH Funding Opportunities for Mind and Body Clinical Trials April 24, 2017 Introductions Wendy Weber, N.D., Ph.D., M.P.H. Branch Chief, Clinical Research in Complementary and Integrative Health Branch,
APPLICATION TO INVOLVE HUMAN SUBJECTS IN RESEARCH (Form IAUPRIRB-1)
 INTER AMERICAN UNIVERSITY OF PUERTO RICO INSTITUTIONAL REVIEW BOARD APPLICATION TO INVOLVE HUMAN SUBJECTS IN RESEARCH (Form IAUPRIRB-1) TITLE*: PRINCIPAL INVESTIGATOR S NAME, TELEPHONE AND POSTAL ADDRESS*:
INTER AMERICAN UNIVERSITY OF PUERTO RICO INSTITUTIONAL REVIEW BOARD APPLICATION TO INVOLVE HUMAN SUBJECTS IN RESEARCH (Form IAUPRIRB-1) TITLE*: PRINCIPAL INVESTIGATOR S NAME, TELEPHONE AND POSTAL ADDRESS*:
MICHIGAN OFFICE OF SERVICES TO THE AGING. Operating Standards For Service Programs
 Community SERVICE NAME Congregate Meals SERVICE NUMBER C-3 SERVICE CATEGORY SERVICE DEFINITION UNIT OF SERVICE Community The provision of nutritious meals to older individuals in congregate settings. Each
Community SERVICE NAME Congregate Meals SERVICE NUMBER C-3 SERVICE CATEGORY SERVICE DEFINITION UNIT OF SERVICE Community The provision of nutritious meals to older individuals in congregate settings. Each
Charles R. Mathews Summer Scholarship for Geriatrics Education and Research Student Policy Manual
 Charles R. Mathews Summer Scholarship for Geriatrics Education and Research Student Policy Manual The Charles R. Mathews Scholarship for Geriatrics Education and Research (Mathews Scholarship) was made
Charles R. Mathews Summer Scholarship for Geriatrics Education and Research Student Policy Manual The Charles R. Mathews Scholarship for Geriatrics Education and Research (Mathews Scholarship) was made
SAl N T UNIVERSITY. LO U I S TO: Applicants to the Graduate Program in Endodontics ADVANCED DENTAL EDUCATION
 SAl N T UNIVERSITY EST. 7818 LO U I S TO: Applicants to the Graduate Program in Endodontics FROM: Center for Advanced Dental Education CENTER FOR ADVANCED DENTAL EDUCATION Graduate Program in Endodontics
SAl N T UNIVERSITY EST. 7818 LO U I S TO: Applicants to the Graduate Program in Endodontics FROM: Center for Advanced Dental Education CENTER FOR ADVANCED DENTAL EDUCATION Graduate Program in Endodontics
SAINT LOUIS UNIVERSITY. Applicants to the Graduate Program in Endodontics. Program Requirements
 SAINT LOUIS UNIVERSITY TO: FROM: SUBJECT: Applicants to the Graduate Program in Endodontics Center for Advanced Dental Education Program Requirements 3320 Rutger Street Dreiling-Marshall Hall St. Louis,
SAINT LOUIS UNIVERSITY TO: FROM: SUBJECT: Applicants to the Graduate Program in Endodontics Center for Advanced Dental Education Program Requirements 3320 Rutger Street Dreiling-Marshall Hall St. Louis,
NA LEVEL 3 TECHNICAL QUALIFICATION RECOGNITION
 NA-019 - LEVEL 3 TECHNICAL QUALIFICATION RECOGNITION Introduction This procedure reflects the adoption of the EN4179-2014 wording as Australian Standard AS3669, and describes the NANDTB processes when
NA-019 - LEVEL 3 TECHNICAL QUALIFICATION RECOGNITION Introduction This procedure reflects the adoption of the EN4179-2014 wording as Australian Standard AS3669, and describes the NANDTB processes when
The NIH Biosketch. February 2016
 The NIH Biosketch February 2016 Outline NIH Biosketch Instructions NCBI My Bibliography Tools for creating a biosketch Word Template NCBI SciENcv Rules NIH Biosketch Questions are Welcome... Unanswerable
The NIH Biosketch February 2016 Outline NIH Biosketch Instructions NCBI My Bibliography Tools for creating a biosketch Word Template NCBI SciENcv Rules NIH Biosketch Questions are Welcome... Unanswerable
IRB EXPEDITED REVIEW
 IRB EXPEDITED REVIEW Research activities that (1) present no more than minimal risk* to human research participants, and (2) involve only procedures listed in one or more of the following categories may
IRB EXPEDITED REVIEW Research activities that (1) present no more than minimal risk* to human research participants, and (2) involve only procedures listed in one or more of the following categories may
How Being Trauma-Informed Improves Criminal Justice System Responses Train-the-Trainer (TTT) Event
 SOLICITATION FOR APP LICATIONS Training Opportunity: How Being Trauma-Informed Improves Criminal Justice System Responses Train-the-Trainer (TTT) Event PLEASE COMPLETE THIS APPLICATION IN ITS ENTIRETY
SOLICITATION FOR APP LICATIONS Training Opportunity: How Being Trauma-Informed Improves Criminal Justice System Responses Train-the-Trainer (TTT) Event PLEASE COMPLETE THIS APPLICATION IN ITS ENTIRETY
PROMOTION OF DOCTORAL STUDIES (PODS) SCHOLARSHIPS I & II - Guidelines
 PREFACE The mission of the Foundation for Physical Therapy is to fund physical therapy research supporting evidence-based practice, that enhances the quality of patient and client services and to develop
PREFACE The mission of the Foundation for Physical Therapy is to fund physical therapy research supporting evidence-based practice, that enhances the quality of patient and client services and to develop
Z E N I T H M E D I C A L P R O V I D E R N E T W O R K P O L I C Y Title: Provider Appeal of Network Exclusion Policy
 TheZenith's Z E N I T H M E D I C A L P R O V I D E R N E T W O R K P O L I C Y Title: Provider Appeal of Network Exclusion Policy Application: Zenith Insurance Company and Wholly Owned Subsidiaries Policy
TheZenith's Z E N I T H M E D I C A L P R O V I D E R N E T W O R K P O L I C Y Title: Provider Appeal of Network Exclusion Policy Application: Zenith Insurance Company and Wholly Owned Subsidiaries Policy
Request for Proposals. Enhancing and Updating the Canadian Evaluation Society Sanctioned. Logic Model Workshop. Canadian Evaluation Society
 Request for Proposals Enhancing and Updating the Canadian Evaluation Society Sanctioned Logic Model Workshop Canadian Evaluation Society March 2011 Contents 1.0 Proposal Content and Requirements... 1 2.0
Request for Proposals Enhancing and Updating the Canadian Evaluation Society Sanctioned Logic Model Workshop Canadian Evaluation Society March 2011 Contents 1.0 Proposal Content and Requirements... 1 2.0
Date January 20, Contact Information
 Curriculum Vitae Deborah Golant Badawi, MD Medical Director, Office of Genetics and People with Special Health Care Needs Maryland Department of Health and Mental Hygiene Date January 20, 2016 Contact
Curriculum Vitae Deborah Golant Badawi, MD Medical Director, Office of Genetics and People with Special Health Care Needs Maryland Department of Health and Mental Hygiene Date January 20, 2016 Contact
Consortium on Law and Values in Health, Environment & the Life Sciences
 Student Proposal Cover Page Consortium on Law and Values in Health, Environment & the Life Sciences Applicant Information Applicant Name: Geeta Naidu Date: 03/23/09 Project Title: Department: Increasing
Student Proposal Cover Page Consortium on Law and Values in Health, Environment & the Life Sciences Applicant Information Applicant Name: Geeta Naidu Date: 03/23/09 Project Title: Department: Increasing
MC IRB Protocol No.:
 APPLICATION FORM - INITIAL REVIEW INSTITUTIONAL REVIEW BOARD Room 117 Main Building 555 Broadway Dobbs Ferry NY 10522 Phone: 914-674-7814 / Fax: 914-674-7840 / mcirb@mercy.edu MC IRB Protocol No.: Date
APPLICATION FORM - INITIAL REVIEW INSTITUTIONAL REVIEW BOARD Room 117 Main Building 555 Broadway Dobbs Ferry NY 10522 Phone: 914-674-7814 / Fax: 914-674-7840 / mcirb@mercy.edu MC IRB Protocol No.: Date
RECERTIFICATION PROGRAMME FOR CONTINUING PROFESSIONAL DEVELOPMENT OF OPTOMETRISTS
 RECERTIFICATION PROGRAMME FOR CONTINUING PROFESSIONAL DEVELOPMENT OF OPTOMETRISTS Background The principal purpose of the Health Practitioners Competence Assurance Act 2003 (Act) is to protect public health
RECERTIFICATION PROGRAMME FOR CONTINUING PROFESSIONAL DEVELOPMENT OF OPTOMETRISTS Background The principal purpose of the Health Practitioners Competence Assurance Act 2003 (Act) is to protect public health
MDCH IRB REVIEW APPLICATION Authority: Code of Federal Regulations Title 45 Part 46
 The Michigan Department of Community Health Institutional Review Board for the Protection of Human Research Subjects Capitol View Building, 7 th Floor, 201 Townsend Street, Lansing, MI 48913 Phone: 517/241-1928
The Michigan Department of Community Health Institutional Review Board for the Protection of Human Research Subjects Capitol View Building, 7 th Floor, 201 Townsend Street, Lansing, MI 48913 Phone: 517/241-1928
NA-019 LEVEL 3 QUALIFICATION RECOGNITION FOR AS3669 AND EN 4179 / NAS 410
 NA-019 LEVEL 3 QUALIFICATION RECOGNITION FOR AS3669 AND EN 4179 / NAS 410 Initial Qualification Recognition This procedure describes how the NANDTB recognises NDT Level 3 personnel qualifications as meeting
NA-019 LEVEL 3 QUALIFICATION RECOGNITION FOR AS3669 AND EN 4179 / NAS 410 Initial Qualification Recognition This procedure describes how the NANDTB recognises NDT Level 3 personnel qualifications as meeting
DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT
 c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
Revised August 28, 2018
 Florida State University Human Subjects Committee Standard Operational Procedure (SOP) 7-IRB-26 Title: Responsible Executive: Approving Official: Prisoners as Research Subjects Gary K. Ostrander Gary K.
Florida State University Human Subjects Committee Standard Operational Procedure (SOP) 7-IRB-26 Title: Responsible Executive: Approving Official: Prisoners as Research Subjects Gary K. Ostrander Gary K.
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE BOARD OF PHYSICAL THERAPY GENERAL RULES
 DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE BOARD OF PHYSICAL THERAPY GENERAL RULES (By authority conferred on the director of the department of licensing and regulatory affairs by
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE BOARD OF PHYSICAL THERAPY GENERAL RULES (By authority conferred on the director of the department of licensing and regulatory affairs by
THE NEW YORK CITY AIDS FUND
 Request for Proposals Date Issued: Thursday, August 23, 2012 Proposal Deadline: Wednesday, October 10, 2012 BACKGROUND Founded in 1989, the New York City AIDS Fund (the AIDS Fund) is a group of grantmaking
Request for Proposals Date Issued: Thursday, August 23, 2012 Proposal Deadline: Wednesday, October 10, 2012 BACKGROUND Founded in 1989, the New York City AIDS Fund (the AIDS Fund) is a group of grantmaking
2. To provide trained coaches/ volunteers and specialized equipment at accessible facilities for sports clinics.
 Medstar NRH Adapted Sports Policy 1. Programs are open to anyone in the Washington Metropolitan area with a physical disability. Interested participants are pre-screened by coaches to determine eligibility
Medstar NRH Adapted Sports Policy 1. Programs are open to anyone in the Washington Metropolitan area with a physical disability. Interested participants are pre-screened by coaches to determine eligibility
Do not edit or delete any questions from this application. Doing so will result in immediate disqualification of your request.
 AIDS Walk of Oklahoma City, Inc. Request for Proposal (RFP) 2018 Proposal Application (for funding year 2019) Deadline: October 31, 2018 NOT VALID AFTER OCTOBER 31, 2018 General instructions can be downloaded
AIDS Walk of Oklahoma City, Inc. Request for Proposal (RFP) 2018 Proposal Application (for funding year 2019) Deadline: October 31, 2018 NOT VALID AFTER OCTOBER 31, 2018 General instructions can be downloaded
Scientific Misconduct September 15, Presented by May Al Kassar
 Research Non-Compliance & Scientific Misconduct September 15, 2010 Presented by May Al Kassar Responsible Research Conduct The ethical conduct of research is a shared responsibility among: The institution
Research Non-Compliance & Scientific Misconduct September 15, 2010 Presented by May Al Kassar Responsible Research Conduct The ethical conduct of research is a shared responsibility among: The institution
VENTURA COUNTY, HEALTH CARE AGENCY INVITES APPLICATIONS FOR: Occupational Therapist - Inpatient Psychiatric Unit 2007HCA-17AA (MI)
 VENTURA COUNTY, HEALTH CARE AGENCY INVITES APPLICATIONS FOR: Occupational Therapist - Inpatient Psychiatric Unit 2007HCA-17AA (MI) An Equal Opportunity Employer SALARY RANGE (approximate) $29.30 - $49.96
VENTURA COUNTY, HEALTH CARE AGENCY INVITES APPLICATIONS FOR: Occupational Therapist - Inpatient Psychiatric Unit 2007HCA-17AA (MI) An Equal Opportunity Employer SALARY RANGE (approximate) $29.30 - $49.96
Drug Testing Policy and Procedures Revised July2009
 Drug Testing Policy and Procedures Revised July2009 PLEASE NOTE: COACHES IN EACH SPORT MAY HAVE ADDITIONAL POLICIES THAT ARE STRICTER THAN DEPARTMENTAL POLICIES CITED HEREIN. Drug Policy Drug use (excluding
Drug Testing Policy and Procedures Revised July2009 PLEASE NOTE: COACHES IN EACH SPORT MAY HAVE ADDITIONAL POLICIES THAT ARE STRICTER THAN DEPARTMENTAL POLICIES CITED HEREIN. Drug Policy Drug use (excluding
The Congressionally Directed Medical Research Programs Funding Opportunities: Peer Reviewed Cancer Research Program
 The Congressionally Directed Medical Research Programs Funding Opportunities: Peer Reviewed Cancer Research Program Donna M. Kimbark, Ph.D. Program Manager, Peer Reviewed Cancer Research Program The views
The Congressionally Directed Medical Research Programs Funding Opportunities: Peer Reviewed Cancer Research Program Donna M. Kimbark, Ph.D. Program Manager, Peer Reviewed Cancer Research Program The views
St. Mary s Hospital Foundation Scholarship Program. Deadline: Must be postmarked by March 15, 2016
 St. Mary s Hospital Foundation Scholarship Program Deadline: Must be postmarked by March 15, 2016 MedStar St. Mary s Hospital Human Resources Department 25500 Point Lookout Road Leonardtown, MD 20650 For
St. Mary s Hospital Foundation Scholarship Program Deadline: Must be postmarked by March 15, 2016 MedStar St. Mary s Hospital Human Resources Department 25500 Point Lookout Road Leonardtown, MD 20650 For
REHABILITATION PSYCHOLOGY th Annual Conference Dallas, TX February 22-25, 2018 The Westin Galleria Dallas Pkwy Dallas, TX 75240
 REHABILITATION PSYCHOLOGY 2018 20 th Annual Conference Dallas, TX February 22-25, 2018 The Westin Galleria 13340 Dallas Pkwy Dallas, TX 75240 Promoting Access and Inclusion Celebrating Rehabilitation Psychology's
REHABILITATION PSYCHOLOGY 2018 20 th Annual Conference Dallas, TX February 22-25, 2018 The Westin Galleria 13340 Dallas Pkwy Dallas, TX 75240 Promoting Access and Inclusion Celebrating Rehabilitation Psychology's
SEA GRANT BUDGET FORM 90-4 Project Year: Year Duration: 12 Months Revised / Printed: 05/31/11 INSTITUTION: Stony Brook University
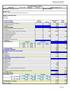 Project Year: Year 1-27 Duration: 12 Months Revised / Printed: 5/31/11 INSTITUTION: Stony Brook University a. Principal and Co-Principal Investigator(s): 1 2. 5,5 5,5 b. Associate Investigator(s): 1 2.
Project Year: Year 1-27 Duration: 12 Months Revised / Printed: 5/31/11 INSTITUTION: Stony Brook University a. Principal and Co-Principal Investigator(s): 1 2. 5,5 5,5 b. Associate Investigator(s): 1 2.
Request for Proposals: Junior Investigators in Genetics and Human Agency Letters of Intent Due March 1, 2016
 Request for Proposals: Junior Investigators in Genetics and Human Agency Letters of Intent Due March 1, 2016 The Department of Psychology at the University of Virginia is pleased to announce a $3.5 million
Request for Proposals: Junior Investigators in Genetics and Human Agency Letters of Intent Due March 1, 2016 The Department of Psychology at the University of Virginia is pleased to announce a $3.5 million
Section 32: BIMM Institute Student Disciplinary Procedure
 Section 32: BIMM Institute Student Disciplinary Procedure Introduction Academic Development & Quality Assurance Manual This Student Disciplinary Procedure provides a framework for the regulation of BIMM
Section 32: BIMM Institute Student Disciplinary Procedure Introduction Academic Development & Quality Assurance Manual This Student Disciplinary Procedure provides a framework for the regulation of BIMM
Request for Proposals
 Request for Proposals Innovative Model for Bringing Autism Expertise and Services to Rural Areas of Pennsylvania The Pennsylvania Department of Public Welfare (DPW) through the Tuscarora Intermediate Unit
Request for Proposals Innovative Model for Bringing Autism Expertise and Services to Rural Areas of Pennsylvania The Pennsylvania Department of Public Welfare (DPW) through the Tuscarora Intermediate Unit
We are inviting you to participate in a research study/project that has two components.
 Dear TEACCH Client: One of the missions of the TEACCH Autism Program is to support research on the treatment and cause of autism and related disorders. Therefore, we are enclosing information on research
Dear TEACCH Client: One of the missions of the TEACCH Autism Program is to support research on the treatment and cause of autism and related disorders. Therefore, we are enclosing information on research
IRB policy and procedures 1. Institutional Review Board: Revised Policy and Procedures Elmhurst College
 IRB policy and procedures 1 Institutional Review Board: Revised Policy and Procedures Elmhurst College IRB policy and procedures 2 Table of Contents A. Purpose and objectives... p. 3 B. Membership of the
IRB policy and procedures 1 Institutional Review Board: Revised Policy and Procedures Elmhurst College IRB policy and procedures 2 Table of Contents A. Purpose and objectives... p. 3 B. Membership of the
RULE-MAKING ORDER PERMANENT RULE ONLY. CR-103P (December 2017) (Implements RCW )
 RULE-MAKING ORDER PERMANENT RULE ONLY CODE REVISER USE ONLY CR-103P (December 2017) (Implements RCW 34.05.360) Agency: Department of Health- Dental Quality Assurance Commission Effective date of rule:
RULE-MAKING ORDER PERMANENT RULE ONLY CODE REVISER USE ONLY CR-103P (December 2017) (Implements RCW 34.05.360) Agency: Department of Health- Dental Quality Assurance Commission Effective date of rule:
RECERTIFICATION PROGRAMME FOR CONTINUING PROFESSIONAL DEVELOPMENT OF OPTOMETRISTS
 RECERTIFICATION PROGRAMME FOR CONTINUING PROFESSIONAL DEVELOPMENT OF OPTOMETRISTS Background The principal purpose of the Health Practitioners Competence Assurance Act 2003 (Act) is to protect public health
RECERTIFICATION PROGRAMME FOR CONTINUING PROFESSIONAL DEVELOPMENT OF OPTOMETRISTS Background The principal purpose of the Health Practitioners Competence Assurance Act 2003 (Act) is to protect public health
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE BOARD OF PHYSICAL THERAPY GENERAL RULES. Filed with the Secretary of State on
 DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE BOARD OF PHYSICAL THERAPY GENERAL RULES Filed with the Secretary of State on These rules take effect immediately upon filing with the Secretary
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE BOARD OF PHYSICAL THERAPY GENERAL RULES Filed with the Secretary of State on These rules take effect immediately upon filing with the Secretary
No An act relating to health insurance coverage for early childhood developmental disorders, including autism spectrum disorders. (S.
 No. 158. An act relating to health insurance coverage for early childhood developmental disorders, including autism spectrum disorders. (S.223) It is hereby enacted by the General Assembly of the State
No. 158. An act relating to health insurance coverage for early childhood developmental disorders, including autism spectrum disorders. (S.223) It is hereby enacted by the General Assembly of the State
AN ACT IN THE COUNCIL OF THE DISTRICT OF COLUMBIA
 AN ACT IN THE COUNCIL OF THE DISTRICT OF COLUMBIA Codification District of Columbia Official Code 2001 Edition 2004 Fall Supp. West Group Publisher To amend the District of Columbia Health Occupations
AN ACT IN THE COUNCIL OF THE DISTRICT OF COLUMBIA Codification District of Columbia Official Code 2001 Edition 2004 Fall Supp. West Group Publisher To amend the District of Columbia Health Occupations
Accreditation Requirements for the Geriatric Orthopaedic (GO) Fellowship
 Accreditation Requirements for the Geriatric Orthopaedic (GO) Fellowship IGFS desires to recognize geriatric orthopaedic fellowships that promote high quality post residency-training experience in geriatric
Accreditation Requirements for the Geriatric Orthopaedic (GO) Fellowship IGFS desires to recognize geriatric orthopaedic fellowships that promote high quality post residency-training experience in geriatric
The American Society of Echocardiography. Professional Benefits - Your Performance Stands Out, So Should You!
 The American Society of Echocardiography As the largest global organization for cardiovascular ultrasound imaging, the American Society of Echocardiography (ASE) is the leader and advocate, setting practice
The American Society of Echocardiography As the largest global organization for cardiovascular ultrasound imaging, the American Society of Echocardiography (ASE) is the leader and advocate, setting practice
System-level Workforce Capacity Building for Integrating HIV Primary Care in Community Health Care Settings Evaluation and Technical Assistance Center
 Pre-Application Technical Assistance Webinar System-level Workforce Capacity Building for Integrating HIV Primary Care in Community Health Care Settings Evaluation and Technical Assistance Center Competitive
Pre-Application Technical Assistance Webinar System-level Workforce Capacity Building for Integrating HIV Primary Care in Community Health Care Settings Evaluation and Technical Assistance Center Competitive
Neuroscience Seminar
 Neuroscience Seminar Series @CNY-149 *** Neuroscience Center Massachusetts General Hospital 149, 13th Street Charlestown, MA 02129 http://www.massgeneral.org/neuroscience/ *** Administrative Coordinator
Neuroscience Seminar Series @CNY-149 *** Neuroscience Center Massachusetts General Hospital 149, 13th Street Charlestown, MA 02129 http://www.massgeneral.org/neuroscience/ *** Administrative Coordinator
COOPERATIVE AGREEMENTS FOR STATE-BASED COMPREHENSIVE BREAST AND CERVICAL CANCER EARLY DETECTION PROGRAMS
 APRIL 2011 93.919 COOPERATIVE AGREEMENTS FOR STATE-BASED COMPREHENSIVE BREAST AND CERVICAL CANCER EARLY DETECTION PROGRAMS State Project/Program: NC BREAST AND CERVICAL CANCER CONTROL PROGRAM U. S. Department
APRIL 2011 93.919 COOPERATIVE AGREEMENTS FOR STATE-BASED COMPREHENSIVE BREAST AND CERVICAL CANCER EARLY DETECTION PROGRAMS State Project/Program: NC BREAST AND CERVICAL CANCER CONTROL PROGRAM U. S. Department
How Being Trauma-Informed Improves Criminal Justice System Responses Train-the-Trainer (TTT) Event for Individual Trainers
 SOLICITATION FOR APP LICATIONS Training Opportunity for Individual Trainers: How Being Trauma-Informed Improves Criminal Justice System Responses Train-the-Trainer (TTT) Event for Individual Trainers PLEASE
SOLICITATION FOR APP LICATIONS Training Opportunity for Individual Trainers: How Being Trauma-Informed Improves Criminal Justice System Responses Train-the-Trainer (TTT) Event for Individual Trainers PLEASE
New NCCIH Funding Opportunities for Natural Product Clinical Trials. May 9, 2017
 New NCCIH Funding Opportunities for Natural Product Clinical Trials May 9, 2017 The Webinar will start at 2:00 p.m. All participants have been placed on mute. We will take questions at the end of the presentation
New NCCIH Funding Opportunities for Natural Product Clinical Trials May 9, 2017 The Webinar will start at 2:00 p.m. All participants have been placed on mute. We will take questions at the end of the presentation
BARNARD COLLEGE Application for the Approval of the Use of Human Subjects in Research
 BARNARD COLLEGE Application for the Approval of the Use of Human Subjects in Research Project Title Principal Investigator Name and highest earned degree: Office Phone: Facsimile Phone Number: Department:
BARNARD COLLEGE Application for the Approval of the Use of Human Subjects in Research Project Title Principal Investigator Name and highest earned degree: Office Phone: Facsimile Phone Number: Department:
Advanced Education in Periodontics and Restorative Dentistry
 Office of Continuing Dental Education Rutgers, The State University of New Jersey 110 Bergen Street, B701 Newark, NJ 07103 cde.sdm.rutgers.edu cde@sdm.rutgers.edu p.973-972-6561 f. 973-972-7741 Advanced
Office of Continuing Dental Education Rutgers, The State University of New Jersey 110 Bergen Street, B701 Newark, NJ 07103 cde.sdm.rutgers.edu cde@sdm.rutgers.edu p.973-972-6561 f. 973-972-7741 Advanced
