Spike-Timing-Dependent Plasticity in Lower-Limb Motoneurons after Human Spinal Cord Injury
|
|
|
- Lorraine Spencer
- 5 years ago
- Views:
Transcription
1 Articles in PresS. J Neurophysiol (May 3, 2017). doi: /jn Spike-Timing-Dependent Plasticity in Lower-Limb Motoneurons after Human Spinal Cord Injury M.A. Urbin, Recep A. Ozdemir, Toshiki Tazoe, and Monica A. Perez Department of Neurological Surgery, The Miami Project to Cure Paralysis, University of Miami, Miami, FL; Bruce W. Carter Department of Veterans Affairs Medical Center 1201 NW 16th Street, Miami, FL 33125, USA Section and Senior Editor: Abbreviated title: Leg STDP after SCI Number of figures: 7 Number of tables: 1 Number of pages: 37 Key words: Neuroplasticity, spinal cord injury, cortico-motoneuronal, spike timing-dependent plasticity, neurophysiology, corticospinal, foot drop Correspondence to: Monica A. Perez, PhD. Department of Neurological Surgery The Miami Project to Cure Paralysis University of Miami Bruce W. Carter Department of Veterans Affairs Medical Center Phone: (305) perezmo@miami.edu 1 Copyright 2017 by the American Physiological Society.
2 Abstract Recovery of lower-limb function after spinal cord injury (SCI) likely depends on transmission in the corticospinal pathway. Here, we examined whether paired corticospinalmotoneuronal stimulation (PCMS) changes transmission at spinal synapses of lower-limb motoneurons in humans with chronic incomplete SCI and aged-matched controls. We used 200 pairs of stimuli where corticospinal volleys evoked by transcranial magnetic stimulation (TMS) over the leg representation of the motor cortex were timed to arrive at corticospinalmotoneuronal synapses of the tibialis anterior (TA) muscle 2 ms before antidromic potentials evoked in motoneurons by electrical stimulation of the common peroneal nerve (PCMS+) or when antidromic potentials arrived 15 or 28 ms before corticospinal volleys (PCMS-) on separate days. Motor evoked potentials (MEPs) elicited by TMS and electrical stimulation were measured in the TA muscle before and after each stimulation protocol. After PCMS+, the size of MEPs elicited by TMS and electrical stimulation increased for up to 30 min in control and SCI participants. Notably, this was accompanied by increases in TA electromyographic (EMG) activity and ankle dorsiflexion force in both groups, suggesting that this plasticity has functional implications. After PCMS-, MEPs elicited by TMS and electrical stimulation were suppressed if afferent input from the common peroneal nerve reduced TA MEP size during paired stimulation in both groups. In conclusion, PCMS elicits spike-timing-dependent changes at spinal synapses of lower-limb motoneurons in humans and has potential to improve lower-limb motor output following SCI
3 59 New and Noteworthy Approaches that aim to enhance corticospinal transmission to lower-limb muscles following spinal cord injury (SCI) are needed. We demonstrate that paired cortico-motoneuronal stimulation (PCMS) can enhance plasticity at spinal synapses of lower-limb motoneurons in humans with and without SCI. We propose that PCMS has potential for improving motor output in leg muscles in individuals with damage to the corticospinal tract Introduction Transmission in the corticospinal tract projecting to lower-limb muscles can be impaired following spinal cord injury (SCI) (for review see Raineteau and Schwab 2001; Curt and Ellaway, 2012; Oudega and Perez 2012). Lesions of the corticospinal tract projecting to lowerlimb muscles often result in inadequate lift of the hindlimb during locomotion in the rat (Metz and Whishaw 2002; Muir et al. 2007), cat (Jiang and Drew 1996), and monkey (Courtine et al. 2005; Capogrosso et al. 2016). In agreement, studies in humans showed that damage to corticospinal projections to lower-limb muscles is often accompanied by foot drop or an inability to lift the foot during locomotion (Hansen et al. 2005; Thomas and Gorassini 2005; Burridge et al. 2011). Indeed, a relationship was found between foot drop and impairments in corticospinal transmission in humans with SCI (Barthelemy et al. 2010). Several neuromodulatory strategies have been used to improve the control of lower-limb muscles following SCI. For example, epidural electrical stimulation of the lumbar spinal cord, combined with motor training, improves adaptive locomotor outcomes and other related functions in people with SCI (Gerasimenko et al. 2007; Harkema et al. 2011; Angeli et al. 2014). 81 Operant conditioning of spinal reflexes results in faster and more symmetrical locomotion 3
4 (Thompson et al. 2013) while high frequency repetitive transcranial magnetic stimulation (TMS) over the leg motor cortex have some effects on decreasing spasticity in lower-limb muscles (Kumru et al. 2010, 2013). Paired-associative stimulation have been used to target corticospinal projections to leg muscles at a cortical level in humans with SCI (Roy et al. 2010). Although it is likely that interactions between corticospinal drive and spinal motoneurons contribute to the aftereffects of these forms of plasticity, strategies that aim to target cortico-motoneuronal synapses in lower-limb motoneurons in humans with SCI remain untested. In human upper-limb muscles, spinal synapses have been targeted noninvasively by using paired cortico-motoneuronal stimulation (PCMS), where corticospinal volleys evoked by TMS over the motor cortex are timed to arrive at cortico-motoneuronal synapses before or after antidromic potentials evoked in motoneurons by electrical stimulation of a peripheral nerve. PCMS increases corticospinal transmission and upper-limb motor output in controls (Taylor and Martin 2009; Fitzpatrick et al. 2016) and in humans with SCI (Bunday and Perez 2012). Evidence suggests that there is potential for inducing cortico-motoneuronal synaptic plasticity in lower-limb motoneurons in humans. Direct monosynaptic connections from the leg motor cortex to lower-limb motoneurons are likely present in both animals (Agnew et al. 1963; Jankowska et al. 1975) and humans (Brouwer and Ashby 1992; Nielsen and Petersen 1995), providing an opportunity for pairing presynaptic and postsynaptic inputs. Plasticity in spinal circuits involving lower-limb motoneurons is thought to contribute to improvements in voluntary motor output of leg muscles following motor training (Perez et al. 2005, 2007). Thus, we hypothesize that PCMS elicits spike-timing-dependent like-changes at spinal synapses of lower-limb motoneurons in humans and has potential to improve lower-limb motor output in humans with chronic anatomically incomplete SCI. 4
5 Materials and Methods Subjects. Eighteen individuals with SCI (39.7±15.3 years, 3 female) and 23 control subjects (30.5±8.6 years, 6 female) participated in the study. All subjects gave informed written consent to experimental procedures, which were approved by the local ethics committee at the University of Miami. SCI subjects had chronic ( 1 year) injuries with a neurological level between C2 and T11, an intact or impaired, but not absent innervation in dermatome L4-L5 during light touch and pin prick stimulus using the International Standards for Neurological Classification of Spinal Cord Injury sensory scores (Table 1). Two of the 18 were categorized as ASIA A (complete injury) due to the lack of sacral sparing, despite residual voluntary ankle movement. The remaining 16 subjects were classified as incomplete ASIA C or D. All SCI subjects were able to exert maximal isometric voluntary contractions (MVC) into ankle dorsiflexion but to a lesser extent than controls (controls=0.72±0.41 mv, SCI=0.28±0.16 mv; p<0.001). Subjects participated in 2 main experiments in which we examined the effect of PCMS+ and PCMS- on MEPs elicited by TMS on different days in a randomized order (PCMS+: controls, n=18 and SCI, n=15; PCMS-: controls, n=13, and SCI, n=7). Some participants completed 3 additional experiments, where we examined the effect of PCMS+ and PCMS- on MEPs elicited by 5
6 electrical stimulation on different days in a randomized order (PCMS+: controls, n=6 and SCI, n=7; PCMS-: controls, n=5 and SCI, n=4) and the effect of peripheral nerve stimulation (PNS) over the common peroneal nerve on TA MEP size at ISIs of 20, 30, and 40 ms (controls, n=13). Electromyographic (EMG) recordings. EMG was recorded from the right tibialis anterior (TA) muscle in control subjects and from the less affected leg in individuals with SCI through surface electrodes secured to the skin over the belly of each muscle (Ag AgCl, 10 mm diameter). EMG signals were amplified, filtered ( Hz), and sampled at 2 khz for off-line analysis (CED 1401 with Signal software, Cambridge Electronic Design, Cambridge, UK). PCMS. During testing subjects were seated in an armchair with the tested foot placed on a custom platform with the ankle connected to a force transducer and restrained by straps (Fig. 1A). At the start of the experiment, subjects were instructed to perform 2 brief MVCs for 3-5 s into dorsiflexion, separated by ~30 s of rest. Subjects participated in two PMCS protocols in a randomized order in different sessions separated by at least 2-3 days. In each protocol, tested at rest, 200 pairs of stimuli were delivered every 10 s (~34 min, 0.1 Hz) where corticospinal volleys evoked by TMS over the leg motor cortex were timed to arrive at cortico-motoneuronal synapses of the TA muscle 2 ms before antidromic potentials evoked in motoneurons by PNS of the common peroneal nerve (PCMS+) or when antidromic potentials arrived 15 ms or 30 ms before corticospinal volleys (PCMS-) on separate days (Figs. 1B and C). The PCMS+ protocol was intended to strengthen corticospinal transmission and the PCMS- protocol was intended to weaken corticospinal transmission (Taylor and Martin 2009; Bunday and Perez 2012). Previous studies using PCMS in upper-limb muscles administered 50 or 100 pairs of stimuli (Bunday and Perez 2012; Fitzpatrick et al. 2016). Our preliminary data in SCI subjects showed that 200 pairs 6
7 of stimuli were more effective in inducing consistent changes in TA MEP size in SCI participants consistent with the view that more pairs of stimuli exert more reliable changes in corticospinal excitability (Fitzpatrick et al. 2016). TMS. A double-cone coil (type number ) with a monophasic current waveform was used to deliver TMS (Magstim 200, Whitland, UK). We determined the optimal position for eliciting a motor evoked potential (MEP) in the TA muscle (hot spot) by moving the coil in small steps along the leg representation of the motor cortex. The hot spot was defined as the region where the largest MEP in the TA could be evoked with minimum intensity. The TMS coil was held with a custom coil holder, while the head was firmly secured to a headrest by straps. We used an intensity of 100% of the maximum stimulator output (MSO) for the TMS pulse in both groups during both protocols. PNS. A constant current stimulator (200 μs pulse duration, model DS7AH, Digitimer, Welwyn Garden City, UK) was used to deliver PNS to the common peroneal nerve with the stimulating electrode positioned under the head of the fibula (anode and cathode 3 cm apart) at an intensity of 1.5 x above the threshold to elicit maximal compound action potentials (M-max) in the TA muscle in both groups during both protocols. During F-wave testing signals were filtered using a high passed filter of 100 Hz to identify the earliest F-wave latency for calculating conduction times while the low pass filter was maintained at 1000 Hz (Khan et al. 2012). The F-wave onset latency was defined as the time at which a signal was ~20 µv above the mean baseline measured 100 ms before the stimulus artifact and it was estimated in individual trials to identify the response with the shortest latency (Perez and Rothwell 2015)
8 TMS and PNS interstimulus interval (ISI). TMS and PNS targeted the right TA muscle in control subjects and the less affected leg in SCI participants. ISIs for each protocol were tailored to individual subjects based on conduction times calculated from latencies of MEPs, F- wave, and M-max (Fig. 2). MEP and F-wave latencies were recorded during isometric ~10% of MVC into dorsiflexion to determine the shortest and clearest response for our estimations. The onset latency was defined as the time when each response exceeded 2 SD of the mean rectified pre-stimulus activity (100 ms) in the averaged waveform. Peripheral conduction time (PCT) from the stimulating electrode overlying the CPN to the TA motor pool was calculated using the following equation: (F-wave latency M-max latency) * 0.5 Central conduction time (CCT) from the motor cortex to the TA motor pool was calculated using the following equation: MEP latency - (PCT + M-max latency) We quantified the size of the F-wave and/or MEP elicited during paired stimulation throughout each protocol in both groups. Note that for analysis the 200 pairs of pulses were combined in 10 blocks of 20 pairs each. Repeated measure ANOVA showed no significant effect of TIME (F (9, 315) =1.31, p=0.14), GROUP (F (1, 35) =1.21, p=0.28), and in their interaction (F (9, 315) = 0.89, p=0.53) on responses measured during PCMS+ (Figs. 3A and C). Similarly, repeated measure ANOVA showed no effect of TIME (F (9, 144) =1.18, p=0.31), but GROUP (F (2, 16) =4.76, p=0.02) nor in their interaction (F (9, 144) =1.10, p=0.35) on responses measured during PCMS- (Figs. 3B and D). These results together indicate that the stimulation conditions were maintained constant across the 200 pairs of pulses. During PCMS+, due to the similar onset latency of the TA MEP and F-wave it is possible that both contributed to the response that was analyzed during 8
9 paired stimulation. During PCMS-, due to the interval between PNS and TMS, it is likely that the response present during paired stimulation was a MEP. During PCMS-, we observed in some control subjects that the MEP size was close to the MEP-max (79.5±7% of MEP-max, n=6) and in these individuals MEPs were not suppressed after PCMS-, therefore, we refer to them as nonresponders (Non-responders: control NR ). In the rest of the control subjects, MEPs evoked during PCMS- were smaller than the MEP-max (32.2±3.8% of MEP-max, n=7; Figs. 3B and D) and in these individuals MEPs were suppressed after PCMS-, therefore, we refer to these subjects as responders (Responders: control R ). Evidence showed that PNS of the common peroneal nerve applied 20 to 40 ms before a TMS pulse suppressed the size of the TA MEP (Kasai et al. 1992; Roy and Gorassini 2008; Roy et al. 2010; Zewdie et al. 2014). During PCMS-, for volleys to arrive at the spinal motoneurons 15 ms before descending volleys, we used an ISI between PNS and TMS of ~20 ms. To determine the best interval for suppressing the TA MEP in control NR during PCMS-, we completed an additional control experiment in which we examined the effect of PNS of the common peroneal nerve 20, 30, and 40 ms before the TMS pulse MEPs elicited by TMS. The active motor threshold (AMT) was defined as the minimal stimulus intensity required to induce MEPs >200 μv peak-to-peak amplitude above the background EMG in 5/10 consecutive trials in the contracting muscle (controls=42±11% of MSO, SCI=59±9% of MSO, p<0.001). The MEP-max was defined at rest by increasing stimulus intensities in 5% steps of maximal device output until the MEP amplitude did not show additional increases (controls=2.5±2.5 mv, SCI=1.1±0.8 mv, p=0.04). Twenty MEPs evoked by TMS over the leg motor cortex were acquired using an intensity needed to produce an MEP of ~5-10% of M-max (controls=4.7±2.7 % of M-max, SCI=7.0±6.4 % of M-max, p=0.2) at rest and MEP peak-to-peak 9
10 amplitude was measured before (baseline), immediately after (0), and 10, 20, and 30 min after each protocol. MEPs elicited by electrical stimulation. The corticospinal tract was stimulated using the D180 voltage stimulator (200 μs duration, expressing the intensity as a % of the stimulator output) passed between adhesive Ag-AgCl electrodes fixed to the scalp with the anode 2 cm lateral to the vertex and the cathode 2 cm anterior to the vertex (controls, n=5; SCI, n=4) to evoke motor responses in the TA. This stimulation likely activate axons of pyramidal tract neurons in the subcortical white matter (Nielsen et al. 1995). In addition, MEPs were elicited by passing high voltage electrical current (100 μs duration, Digitimer D , 750 V) between two surface electrodes fixed over the thoracic spine (thoracic MEP; controls, n=5; SCI, n=4) with the cathode positioned between the spinal processes of T9 and T10 vertebrae and the anode 10 cm above. For both types of stimulation, the intensity was set to elicit an MEP in the TA muscle of ~5-10% of the M-max (controls=8.2±2.1 % of M-max and SCI=10.1±5.9 % of M-max, p=0.3). The latency of MEPs elicited by spinal cord electrical stimulation (controls=18.55±2.35ms, SCI=20.56±1.26ms) were shorter than the latency of MEPs evoked by cortical stimulation (controls=29.06±2.34 ms, SCI=37.10±4.08 ms). Five to 10 MEPs elicited by cortical and thoracic electrical stimulation were tested at rest before (baseline), immediately after (0), and 30 min after each protocol. After PCMS+, we found a similar increased in the size of MEPs elicited by electrical stimulation of the leg motor cortex (controls=154.9±57.7%, SCI=192.2±10.6.1%) and the thoracic spinal cord (controls=142.2±34.8%, SCI=159.9±37.4%). Moreover, after PCMS-, MEPs elicited by electrical stimulation of the leg motor cortex (controls=80.8±10.3%, SCI=89.7±13.9%) and the thoracic spinal cord (controls=71.9±14.7%; SCI=66.2±19.0%) were 10
11 decreased below the baseline. Therefore, we grouped data together under MEPs elicited by electrical stimulation for statistical analysis for each muscle. If a subject completed both tests we used the results for MEPs elicited by cortical electrical stimulation for analysis Voluntary Motor Output. Ankle dorsiflexion force and TA EMG activity were measured during short, ballistic, isometric contractions into dorsiflexion using a custom LabView program (controls, n=7; SCI, n=8). One cursor showed the target force (10% MVC) and another cursor showed the force exerted by the subject. Subjects were instructed to dorsiflex the ankle to move the actual force to the target force as fast as possible without making corrections for errors in force production. After familiarization, four sets of 20 contractions were performed with partially stimulated visual feedback of the cursor. Here, subjects controlled the movement of the cursor up to 8% of MVC and the remaining 2% of MVC was simulated by LabView. During the simulation period the speed of the cursor was maintained constant (1 video frame: 15.6ms duration, range=0.61 to 0.67% MVC/ms), while the end point of the cursor was randomly varied within ±0.5% standard deviation of the target (10% of MVC). This strategy was used to avoid online real feedback of the force exerted to help to maintain subjects unaware of the possible effects of the stimulation (Bunday and Perez 2012). Force and EMG during each contraction were measured during a 250 ms window (125 ms before and 125 ms after the peak force and EMG values). Twenty contractions were performed before, immediately after (0), and 10, 20 and 30 min after PCMS Data analysis. Normal distribution was examined using the Shapiro-Wilk s test and Mauchly s test was examine to test sphericity. Data were log transformed when data was not normally 11
12 distributed. Greenhouse-Geisser correction statistics were used to reveal significant F values when sphericity could not be assumed. Repeated-measures analysis of variance (ANOVA) was performed to examine the effect of TIME (Baseline, 0, 10, 20, 30 min) and GROUP (control, SCI) on MEP size for PCMS+. Repeated-measures ANOVA was also performed to determine the effect of TIME and GROUP [control R, control NR, SCI] on MEP size for PCMS-. Bonferonni post-hoc tests were used to test for significant comparisons. The same test was performed to examine the effect of GROUP and TIME (Baseline, 0, 30 min) on MEPs elicited by electrical stimulation for PCMS+ and PCMS- and GROUP and BLOCK (1 to 20 blocks) on responses elicited during paired stimulation. Independent samples t-tests were used to examine differences in MEP and F-wave latencies, conduction times, MEP-max, AMT and MVC between groups. The significance level was set at P<0.05 and group data are presented as mean±sd in the text Results MEPs elicited by TMS Raw TA MEPs traces from a representative control and SCI participant are shown in Fig. 4A. Note that MEP size increased compared with baseline in both participants for 30 min after PCMS+. Repeated-measures ANOVA showed an effect of TIME (F (2.2, 61.42) =8.9, p<0.001) but not GROUP (F (1, 61.42) =0.5, p=0.50) nor their interaction (F (2.2, 61.42) =1.4, p=0.25) on TA MEP amplitude. Post-hoc tests showed that MEP amplitude increased immediately after (controls=169±82.7%, SCI=163.3±18.3%; p=0.03), 10 (controls=157.2±57.2%, SCI=176.1±95.3%; p=0.01), 20 (controls=144±59 %, SCI=131.7±152%; p<0.01), and 30 12
13 (controls=149.7±57.7%, SCI=214.27±118.4%; p<0.01) min after PCMS+ compared with baseline in both groups (Fig. 4B). Note that MEPs were facilitated in the majority of control (17/18) and SCI (14/15) subjects. Figure 5A illustrates raw MEPs traces from two representative control subjects [control R, control NR, see details in methods) and from one SCI participant before and after PCMS-. Here, note that MEP size decreased compared to baseline in the control R and SCI subject for 30 min after PCMS-, whereas, MEPs were facilitated in the control NR participant for 30 min after PCMS-. Repeated measures ANOVA showed no effect of TIME (F (2.1, 35.64) =1.48, p=0.24) but GROUP (F (2, 17) =32.7, p=0.001) and in their interaction (F (4.19, 41.05) =7.1, p=0.001) was found on TA MEP amplitude. Post-hoc tests indicated that MEPs were suppressed immediately after (control R =70.1±27.1%, p=0.003; SCI=73±20.2%, p=0.002), 10 (control R =62.3±15.5%, p<0.001; SCI=55.9±23.7%, p<0.001), 20 (control R =74.5±19.8%, p=0.007; SCI=60.5±26.6%, p<0.001), and 30 (control R =80.7±39.9%, p=0.01; SCI=75.4±21.2%, p=0.01) min after PCMS- compared with baseline. In the control NR group, MEPs were facilitated immediately after (p=0.03), 10 (p=0.01), 20 (p<0.01), and 30 (p<0.01) min after PCMS- (Fig. 5B). We completed additional experiments to examine the time at which PNS of the common peroneal nerve suppressed the size of the TA MEP (see details in methods). Repeated measured ANOVA showed an effect of ISI (F 3,33 =68.8, p<0.001), not GROUP (F 1, 11 =2.64, p=0.13) but in their interaction (F 3,33 =6.3, p=0.002) on TA MEP size. TA MEPs were suppressed at all ISIs in control R (p<0.001) but only at the 30 and 40 ms in control NR (p<0.001). Therefore, in two of the control NR we completed PCMS- using an interval between PNS and TMS of 30 ms. Based on their central and peripheral conduction times, we estimated that in these two subjects antidromic potentials arrived in spinal motoneurons 28 ms before corticospinal volleys. Using this adjusted 13
14 interval, MEPs were suppressed immediately after (81.3±27.1%), 10 (72.1±13.4%), 20 (73.8±12.2%), and 30 (67.5±3.9%) min after PCMS- compared to baseline. MEPs elicited by electrical stimulation Raw MEP traces recorded from the TA muscle after electrical stimulation of the motor cortex from a control and SCI participant are shown in Figs. 6A and C. Note that MEPs were facilitated after PCMS+ and suppressed after PCMS- in both participants. Repeated-measures ANOVA showed an effect of TIME (F (2, 18) =11.27, p<0.001) but not GROUP (F (1, 9) =0.2, p=0.67) nor their interaction (F (2, 18) =1.4, p=0.27) on TA MEPs in PCMS+. Post-hoc tests showed that MEP size increased immediately after (controls=167.08±17.39%, SCI=138.86±13.15%; p=0.001) and 30 min (controls=146.40±25.38%, ¾; SCI=169.35±19.18%, 7/7; p=0.005; Fig. 5B) after PCMS+ compared with baseline in most participants. Repeated measures ANOVA also showed an effect of TIME (F (2, 14) =12.71, p=0.001), but not GROUP (F (1, 7) =0.1, p=0.97) nor their interaction (F (2, 14) =1.5, p=0.26) on TA MEPs in PCMS-. Post-hoc tests indicated that MEPs were suppressed immediately after (controls=88.14±6.88%, SCI=79.15±4.15%; p=0.001) and 30 min (controls=84.56±7.69%, SCI=84.16±4.65%; p=0.001; Fig. 6D) after PCMS- in most control (4/5) and SCI (4/4) subjects Voluntary Motor Output Raw EMG and force recordings are shown in Fig. 7A for an SCI participant. Repeatedmeasures ANOVA showed an effect of TIME (F 4, 52 =14.0, p<0.001; F 2.51, 30.1 =10.8, p<0.001, respectively) but not GROUP (F (1,13) =1.8, p=0.20, F (1,12) =2.2, p=0.16, respectively) nor their interaction (F (4,52) =1.6, p=0.20, F (2.51,30.1) =0.6, p=0.6, respectively) on dorsiflexion force. Post- 14
15 hoc tests showed that force increased above baseline immediately after (control=120.8±11%, SCI=117.1±18.7%, p=0.003), 10 (control=138.7±28.2%, SCI=116±13.2%; p=0.001), 20 (controls=132.3±19.3%, SCI=128.6±34.1%, p=0.002), and 30 (controls=142.4±31.3%, SCI=122.5±20.5%; p=0.001) min after PCMS+ in both groups (Fig. 7B). Similarly, EMG increased above baseline immediately after (control=129.9±25.6%, SCI=130.9±27.6%; p=0.004), 10 (control=145.9±43.3%, SCI=133.1±41.4%; p=0.01), 20 (controls=132.6±28.1%, SCI=148.8±53.6%, p=0.01), and 30 (controls=144.2±44.3%, SCI=148.7±50.3%, p=0.01) min after PCMS+ in both groups (Fig. 7C). No correlation was found between changes in TA MEP size and EMG (controls: r=-0.47, p=0.06; SCI: r=0.44, p=0.11) and force (controls: r=0.20, p=0.62; SCI: r=0.52, p=0.18) after the PCM+ protocol in controls and SCI participants
16 Discussion Our novel findings demonstrate that 200 pairs of stimuli designed to produce spiketiming-dependent plasticity at cortico-motoneuronal synapses of the TA motoneuronal pool increases corticospinal transmission and voluntary motor output in lower-limb muscles in humans with and without SCI. We found that the size of MEPs elicited by TMS and by electrical stimulation increased when TMS-induced presynaptic volleys arrived 2 ms before antidromic volleys induced by PNS at cortico-motoneuronal synapses of the TA muscle (PCMS+). In contrast, the size of MEPs evoked by TMS and electrical stimulation was reduced when antidromic volleys arrived at the spinal cord 15 or 28 ms before presynaptic volleys (PCMS-). Dorsiflexion force and TA EMG activity increased after PCMS+ in both groups and SCI participants showed enhanced dorsiflexion angle and TA EMG activity during the early swing phase of locomotion. We propose that PCMS has potential to improve leg motor output in individuals with deficits in corticospinal transmission. Spike-timing-dependent changes in lower-limb motoneurons 16
17 PCMS elicits spike-timing-dependent changes at spinal synapses of upper-limb motoneurons in control (Taylor and Martin 2009; Fitzpatrick et al. 2016) and SCI (Bunday and Perez 2012) subjects. In the lower limb, paired-associative stimulation have been used to target corticospinal projections to leg muscles at a cortical level in humans with SCI (Roy et al. 2010) and controls (Mrachacz-Kersting et al. 2007; Cortes et al. 2011). Other have used repeated trains of PNS combined with TMS timing the volleys to arrive simultaneously at lower limb motoneurons to induce LTP-like plasticity in control subjects (Shulga et al. 2015). Here, for the first time, we demonstrate that spike-timing-dependent changes can be extended to at spinal synapses of lower-limb muscles. We targeted the TA muscle because it plays an important role in the control of the foot trajectory during the swing phase of the gait cycle (Winter and Bishop, 1992) and because foot drop is seen in individuals with cortical and spinal cord damage (Burridge et al. 2001; Westhout et al. 2007). We found that the size of TA MEPs elicited by TMS over the leg motor cortex increased after PCMS+ for 30 min in control and SCI participants. We followed up the effects of PCMS+ in a subgroup of individuals and found that MEP size returned to baseline ~60 min after the paired stimulation. PCMS relies on the ability of pairing presynaptic and postsynaptic inputs, therefore, muscles with direct monosynaptic connections to spinal motoneurons are good candidates for inducing these effects. In agreement, evidence showed that direct monosynaptic connections from the leg motor cortex to TA motoneurons are likely present in both animals (Agnew et al. 1963; Jankowska et al. 1975) and humans (Brouwer and Ashby 1992; Nielsen and Petersen 1995). Our result also showed that TA MEPs elicited by electrical stimulation of the leg motor cortex increased after PCMS+ for 30 min in both groups. MEPs elicited by electrical stimulation of the motor cortex with the anode 2 cm lateral to the vertex and the cathode 2 cm anterior to the vertex have shorter latencies than 17
18 MEPs evoked by TMS and vertex anodal stimulation (Nielsen et al. 1995). Recordings from the epidural space also indicate that a single pulse of electrical stimulation, in the same location as described above, provides a way to ensure D wave activation of corticospinal axons from the leg area (Di Lazzaro et al. 2001), supporting the view that lateral anodal stimulation penetrated deeper into the brain or that it activated corticospinal cells directly. Thus, increases in the size of TA MEPs elicited by TMS and electrical stimulation of the leg motor cortex and the thoracic spine suggests that changes after PCMS+ had a spinal origin. Although it is possible that this plasticity is not limited to direct cortico-motoneuronal synapses but also involves spinal interneurons this possibility needs to be tested in future studies. One of the important features of spike-timing-dependent plasticity is that these changes need to be reversible (Bi and Poo 1998; Dan and Poo 2004). We propose that our effects are related to spike-timing-dependent plasticity since when we reversed the order to arrival of volleys at the spinal cord, then antidromic volleys arrived at the spinal cord before presynaptic volleys, we found that TA MEPs elicited by TMS were suppressed after PCMS- for 30 min in both groups. Note that when antidromic volleys arrived at the spinal cord 15 ms before TMSinduced presynaptic volleys we observed two distinct responses in control subjects. In one group, the size of MEPs elicited during paired stimulation was reduced and in these subjects PCMSreduced corticospinal excitability. Whereas, in a second group, the size of MEPs elicited during paired stimulation when applying PCMS- was similar as MEP-max and in these subjects paired stimulation was ineffective at reducing corticospinal excitability. Evidence showed that electrical stimulation of homonymous and heteronymous nerves at the knee and/or at the ankle as well as stimulation of the skin innervated by the deep peroneal nerve, which contains the cutaneous branch of the common peroneal nerve, suppresses the TA MEP size 20 to 40 ms before a TMS 18
19 pulse over the leg motor cortex (Kasai et al. 1992; Roy and Gorassini 2008; Roy et al. 2010; Zewdie et al. 2014). This is consistent with the results from our control experiment showing that a conditioning pulse to the common peroneal nerve, given 20 to 40 ms before TMS, decreased TA MEP size. Importantly, in individuals in whom a conditioning pulse to the common peroneal nerve reduced the size of TA MEPs at 20 ms, PCMS- was effective in suppressing corticospinal excitability. However, in those individuals in whom a conditioning pulse to the common peroneal nerve reduced the size of TA MEPs at 30 and 40 ms, PCMS- was only effective when the ISI was adjusted to match this time. Thus, it is clear from our results that the effectiveness of PCMS- was linked to the suppressive effects of the conditioning pulse to the common peroneal nerve on TA MEP size. The fact that in some subjects common peroneal nerve stimulation suppressed the TA MEP at an early or later ISI is consistent with the range of effects previously reported from stimulation of homonymous and heteronymous nerves in the leg (Roy and Gorassini 2008; Roy et al. 2010). To examine possible contributions from homonymous vs. heteronymous connections to TA motoneurons (Simonetta-Moreau et al. 1999) future experiments could determine the effect of this plasticity by stimulating the common peroneal nerve and the deep peroneal nerve. The mechanisms involved in the TA MEP facilitation observed in control subjects not responding to PCMS- also need to be determined. A next important question is which neural mechanisms might have contributed to the MEP suppression after PCMS-. A possibility is that subcortical pathways contributed to this effect. This is supported by our finding showing that TA MEPs elicited by cortical electrical stimulation of the leg area of the motor cortex were suppressed after PCMS- for up to 30 min in both groups. Since we used suprathreshold stimulus intensity for PNS it is possible that circuits not only involving the homonymous common peroneal nerve but also heteromonymous nerves 19
20 and/or distant skin segments contributed to this effect. The short latency for this effect suggests reflex contributions through spinal pathways, such as high threshold cutaneous afferents (Kasai et al. 1992). In animals, Ia and Ib inhibitory interneurons projecting to lower-limb motoneurons are excited by high threshold cutaneous afferent fibers (Hultborn 1972; Lundberg et al. 1975) and these interneurons receive corticospinal inputs (Jankowska et al. 1975). In humans, stimulation of cutaneous afferents at similar conditioning latencies also suppresses a monosynaptic reflex response in the TA muscle (Delwaide et al. 1981; Delwaide and Crenna 1984). Pairs of TMS and PNS inputs inhibit the H-reflex in a calf muscle to the same degree as PNS pulses delivered at an ISI between 20 and 40 ms, suggesting that spinal postsynaptic mechanisms can also contribute to these effects (Poon et al. 2008). Another possibility is that Renshaw cells contributed to this effect. In humans, at the ISIs tested in the study there is ample time to activate Renshaw cells mediating recurrent inhibition to spinal motoneurons (Katz and Pierrot-Deseilligny 1999). Future studies are needed to examine the contribution from group Ia and skin cutaneous afferents, Renshaw cells and other sensory pathways to this plasticity. Sensory information from a peripheral nerve close to the knee can reach the motor cortex ~40 ms after the stimulation and a conditioning pulse to a heteronymous nerve facilitates TA MEP size at a cortical level at longer ISIs than those used in this study (Roy and Gorassini 2008), therefore, that it is less likely that cortical mechanisms contributed to our effects. Although most evidence suggest a spinal origin for our findings the precise spinal mechanisms remain to be tested. Functional considerations 20
21 Several neuromodulatory strategies have been used to improve the control of lower-limb muscles following SCI. For example, epidural electrical stimulation of the lumbar spinal cord has been combined with motor training to improve adaptive locomotor and other related functions in people with SCI (Gerasimenko et al. 2007; Harkema et al. 2011; Angeli et al. 2014). Operant conditioning of spinal reflexes results in faster and more symmetrical locomotion (Thompson et al. 2013) while high frequency repetitive TMS over the leg motor cortex have some effects on decreasing spasticity in lower-limb muscles (Kumru et al. 2010, 2013). In addition, it has been proposed that repeated noninvasive stimulation targeting more direct and indirect corticospinal volleys to spinal motoneurons might influence spinal plasticity in lowerlimb muscles (Cortes et al. 2011; Leukel et al. 2012). It is likely that interactions between corticospinal drive and motoneurons contribute to the aftereffects of all these forms of plasticity. Although we did not record directly from synaptic connections between corticospinal and spinal motoneurons, our protocol which is based on response latencies, targeted for the first time cortico-motoneuronal synapses in lower-limb motoneurons in humans with SCI. We found that paired stimuli precisely timed to arrive at the presynaptic terminal before postsynaptic depolarization results in improvements in voluntary motor output in dorsiflexor muscles in humans with chronic incomplete SCI. These observations support the results by Barthelemy and collaborators (2010) by showing that strengthening transmission in the corticospinal pathways improves TA EMG activity and ankle dorsiflexion during the early swing phase of the locomotor cycle in humans with SCI. Our results also support the view that targeted neuroplasticity might represent an avenue to enhance motor output in lower-limb muscles following SCI (Thompson and Wolpaw 2015). Our results, therefore, might be relevant for a number of motor disorders characterized by impaired corticospinal transmission. 21
22 References Agnew RF, Preston JB, Whitlock DG. Patterns of motor cortex effects on ankle flexor and extensor motoneurons in pyramidal cat preparation. Exp Neurol 8: , Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137: , Barthelemy DM, Willerslev-Olsen M, Lundell H, Conway BA, Knudsen H, Biering- Sorensen F, Nielsen JB. Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J Neurophysiol 104: , Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18: , Błażkiewicz M, Wit A. Comparison of sensitivity coefficients for joint angle trajectory between normal and pathological gait. Acta Bioeng Biomech 14: 83 91, Brouwer B, Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr Clin Neurophysiol 76: , Bunday KL, Perez MA. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol 22: , Burridge JH, Wood DE, Taylor PN, McLellan DL. Indices to describe different muscle activation patterns, identified during treadmill walking, in people with spastic drop-foot. Med Eng Phys 23: ,
23 Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WK, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, Courtine G. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539: , Cortes M, Thickbroom GW, Valls-Sole J, Pascual-Leone A, Edwards DJ. Spinal associative stimulation: a non-invasive stimulation paradigm to modulate spinal excitability. Clin Neurophysiol 122: , Courtine G, Roy RR, Raven J, Hodgson J, McKay H, Yang H, Zhong H, Tuszynski MH, Edgerton VR. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 128: , Curt A, Ellaway PH. Clinical neurophysiology in the prognosis and monitoring of traumatic spinal cord injury. Handb Clin Neurol 109: 63 75, Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron 44: 23 30, Di Lazzaro V, Oliviero A, Profice P, Meglio M, Cioni B, Tonali P, Rothwell JC. Descending spinal cord volleys evoked by transcranial magnetic and electrical stimulation of the motor cortex leg area in conscious humans. J Physiol 537: , Delwaide PJ, Crenna P. Cutaneous nerve stimulation and motoneuronal excitability. II. Evidence for non-segmental influences. J Neurol Neurosurg Psychiat 47: , Delwaide PJ, Crenna P, Fleron MH. Cutaneous nerve stimulation and motoneuronal excitability. I. Soleus and tibialis anterior excitability after ipsilateral and contralateral sural nerve stimulation. J Neurol Neurosurg Psychiat 44: , Fitzpatrick SC, Luu BL, Butler JE, Taylor JL. More conditioning stimuli enhance synaptic plasticity in the human spinal cord. Clin Neurophysiol 127: , Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol 98: , Hansen NL, Conway BA, Halliday DM, Hansen S, Pyndt HS, Biering-Sørensen F, Nielsen JB. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol 94: , Harkema S, Gerasimenko YP, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: ,
24 Hultborn H. Convergence on interneurones in reciprocal Ia inhibitory pathway to motoneurones. Acta Physiol Scand 85: 1 42, Jiang W, Drew T. Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J Neurophysiol 76: , Kasai T, Hayes KC, Wolfe DL, Allatt RD. Afferent conditioning of motor evoked potentials following transcranial magnetic stimulation of motor cortex in normal subjects. Electroencephalogr Clin Neurophysiol 85: , Kasai T, Komiyama T. Antagonist inhibition during rest and precontraction. Electroencephalogr Clin Neurophysiol 81: , Katz R, Pierrot-Deseilligny E. Recurrent inhibition in humans. Prog Neurobiol 57: , Khan SI, Giesebrecht S, Gandevia SC, Taylor JL. Activity-dependent depression of the recurrent discharge of human motoneurones after maximal voluntary contractions. J Physiol 590: , Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, Valero-Cabre A, Tormos JM, Pascual-Leone A. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair 24: , Kumru H, Benito J, Murillo N, Valls-Sole J, Valles M, Lopez-Blazquez R, Costa U, Tormos JM, Pascual-Leone A, Vidal J. Effects of high-frequency repetitive transcranial magnetic stimulation on motor and gait improvement in incomplete spinal cord injury patients. Neurorehabil Neural Repair 27: , Leukel C, Taube W, Beck S, Schubert M. Pathway- specific plasticity in the human spinal cord.eur J Neurosci 35: , Lundberg A, Malmgren K, Schomburg ED. Convergence from Ib cutaneous and joint afferents in reflex pathways to motoneurones. Brain Res 86: 81 84, Mrachacz-Kersting N, Fong M, Murphy BA, Sinkjaer T. Changes in excitability of the cortical projections to the human tibialis anterior after paired associative stimulation. J Neurophysiol 97: , Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods 115: , Muir GD, Webb AA, Kanagal S, Taylor L. (2007). Dorsolateral cervical spinal injury differentially affects forelimb and hindlimb action in rats. Eur J Neurosci 25: ,
25 Nielsen J, Petersen N. Evidence favouring different descending pathways to soleus motoneurones activated by magnetic brain stimulation in man. J Physiol 486: , Nielsen J, Petersen N, Ballegaard M. Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurones in man. J Physiol 484: , Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol 471: , Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol 590: , Perez MA, Lungholt BK and Nielsen JB. Presynaptic control of Ia afferents in relation to acquisition of a novel visuo-motor skill in healthy humans. J Physiology 568: , Perez MA, Lundbye-Jensen J and Nielsen JB. Task-specific depression of the soleus H-reflex size after co-contraction training of antagonistic ankle muscles. J Neurophysiol 98: , Poon DE, Roy FD, Gorassini MA, Stein RB. Interaction of paired cortical and peripheral nerve stimulation on human motor neurons. Exp Brain Res 188: 13 21, Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2: , Roy FD, Gorassini MA. Peripheral sensory activation of cortical circuits in the leg motor cortex of man. J Physiol 586: , Roy FD, Yang JF, Gorassini MA. Afferent regulation of leg motor cortex excitability after incomplete spinal cord injury. J Neurophysiol 103: , Shulga A, Lioumis P, Kirveskari E, Savolainen S, Mäkelä JP, Ylinen A. The use of F- response in defining interstimulus intervals appropriate for LTP-like plasticity induction in lower limb spinal paired associative stimulation. J Neurosci Methods 242: , 2015 Simonetta-Moreau M, Marque P, Marchand-Pauvert V, Pierrot-Deseilligny E. The pattern of excitation of human lower limb motoneurones by probable group II muscle afferents. J Physiol 517: , Taylor JL, Martin PG. Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. J. Neurosci 29: , Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 94: ,
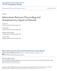
26 Thompson AK, Pomerantz FR, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33: , Thompson AK, Wolpaw JR. Targeted neuroplasticity for rehabilitation. Prog Brain Res 218: , Westhout FD, Paré LS, Linskey ME. Central causes of foot drop: rare and underappreciated differential diagnoses. J Spinal Cord Med 30: 62 66, Winter DA, Bishop PJ. Lower extremity injury. Biomechanical factors associated with chronic injury to the lower extremity. Sports Med 14: , Zewdie ET, Roy FD, Okuma Y, Yang JF, Gorassini MA. Long-latency, inhibitory spinal pathway to ankle flexors activated by homonymous group 1 afferents. J Neurophysiol 111: , Figure legends Figure 1. Experimental setup. A, Diagram showing the position of subjects during testing as well as the transcranial magnetic stimulation (TMS) coil, electromyographic (EMG) recording in the tibialis anterior (TA) muscle, and electrical stimulation of the common peroneal nerve. B, Diagram showing paired cortico-motoneuronal stimulation (PCMS) where corticospinal volleys evoked by TMS over the leg representation of the motor cortex were timed to arrive at corticomotoneuronal synapses of the tibialis anterior (TA) muscle 2 ms before antidromic potentials evoked in motoneurons by electrical stimulation of the common peroneal nerve (PCMS+) or when antidromic potentials arrived 15 or 28 ms before corticospinal volleys (PCMS-). We combined the data from subjects in whom antidromic potentials arrived at spinal motoneurons 15 and 28 ms before corticospinal volleys because they showed similar suppression of MEP size after PCMS-. C, Timeline of the experimental protocol. Following baseline testing, 200 pairs of stimuli were applied over the leg representation of the motor cortex for ~34 minutes. Recordings were taken immediately after (0), and 10, 20, and 30 min after the stimulation as shown by the open blocks. 26
27 Figure 2. Response latencies. A, Raw traces showing a motor evoked potential (MEP), an F- wave, and the maximal motor response (M-max) for representative subjects recorded from the TA muscle. B, MEP, F-wave and M-max latencies were used to calculate central and peripheral conduction time used to estimate time the arrival of pre- and post-synaptic volleys at the corticomotoneuronal synapse in control and SCI subjects (Mean±SD) Figure 3. Responses during paired stimulation. Raw traces from the TA muscle during paired stimulation in the PCMS+ (A) and PCMS- (B) protocol for representative subjects. Note that in the PCMS+ protocol, the M-max is followed by an F-wave likely combined with a MEP from cortical stimulation. Note that during paired stimulation a large MEP was present in a control subject that was a non-responder (control NR ) and a smaller MEP is present in a control subject that was a responder (control R ) to the PCMS- protocol. In responders to PCMS-, we found the MEPs were suppressed after paired stimulation. Data is also shown in a SCI participant. Note that in B, the M-max has multiple peaks because it included a TMS stimulus artifact. During PCMS+ and PCMS-, TMS was used over the leg motor cortex and PNS was given to the common peroneal nerve. Graphs show group data (C-D). The abscissa shows the number of pairs of stimuli during each protocol (a total of 200 pairs of stimuli). At each point, the average of 20 responses is shown. The ordinate shows the size of the conditioned response expressed as % of the M-max in control (black squares, n=18) and SCI (green circles, n=15) participants during PCMS+ (C) and in control R (black squares, n=7), control NR (black triangles, n=6) and SCI (blue circles, n=7) participants during PCMS- (D). Note that due to the similarities between the onset latency of the TA MEP and the TA F-wave it is likely that during the PCMS+ protocol both 726 responses were combined and this is what is reported in the analysis. Whereas, in the PCMS- 27
28 protocol the response reported it is likely to reflect the MEP size. Error bars indicate SEs. *P<0.05. Figure 4. MEPs elicited by TMS over the leg motor cortex before and after PCMS+. A, Raw MEP traces from the TA muscle in representative participants at rest before and after PCMS-. Waveforms represent the average of 20 trials. B, Graph shows box-plots group data (controls, n=18 and SCI=, n=15). The abscissa shows the time at which measurements were taken (baseline, immediately after (0), and 10, 20, and 30 min after the stimulation). The ordinate shows the size of the MEP expressed as % of the MEP at baseline in control (black circles) and SCI (green circles) participants. The horizontal broken line shows the MEP size at baseline. Error bars indicate SEs. *P<0.05. Figure 5. MEPs elicited by TMS over the leg motor cortex before and after PCMS-. A, Raw MEP traces from the TA muscle in a representative control subject that was a responder (control R, black traces), a control subject that was a non-responder (control NR, gray traces) to PCMS-, and in a SCI (blue traces) participant at rest before and after PCMS-. Waveforms represent the average of 20 trials. B, Graph shows group data (control R, n=7, control NR, n=6, and SCI, n=7). The abscissa shows the time at which measurements were taken (baseline, immediately after (0), and 10, 20, and 30 min after the stimulation). The ordinate shows the size of the MEP expressed as % of the MEP at baseline in control R (black circles), control NR (black triangles) and SCI (blue circles) participants. The horizontal broken line shows the MEP size at baseline. Error bars indicate SEs. *P<
29 Figure 6. MEPs elicited by cortical electrical stimulation before and after PCMS+ and PCMS-. Raw MEP traces from the TA muscle elicited by cortical electrical stimulation of the leg motor cortex in a representative control (A) and SCI (C) participant at rest before and after PCMS+ (upper traces) and PCMS- (lower traces). Waveforms represent the average of 5-10 trials. B, Graph shows group data (PCMS+: controls, n=6 and SCI, n=7; PCMS-: controls, n=5 and SCI, n=4). The abscissa shows the time at which measurements were taken (baseline, immediately after (0), and 30 min after the stimulation). The ordinate shows the size of the MEP expressed as % of the MEP at baseline in control (black bars) and SCI (green bars for PCMS+ and blue bars for PCMS-) participants. Data from individual subjects is shown in controls and SCI subjects (open circles). The horizontal broken line shows the MEP size at baseline. Error bars indicate SEs. *P<0.05. Figure 7. Voluntary motor output. A, Force (upper) and EMG (lower) raw traces from the TA muscle measured during brief, fast, ankle isometric voluntary contractions in the dorsiflexion direction before (baseline) and after (0, 10, 20, and 30 min) PCMS+ from a representative SCI subject. Waveforms represent the average of 20 force and EMG traces. Before PCMS+, 20 ankle isometric voluntary contractions were measured on 4 different times with periods of rest in between to establish the baseline. The average of these 4 measurements was used to estimate the baseline. Force and EMG during were measured 125 ms before and 125 ms after the peak force and EMG values. Group data (controls, n=7; SCI, n=8) shows force (B) and mean rectified EMG activity (C) in both groups. The abscissa shows the time at which measurements were taken [baseline (dotted line), immediately after (0), and 30 min after PCMS+]. The ordinate shows the mean force and EMG expressed as % of the baseline in control (black bars) and SCI (white bars 29
30 for PCMS+ and PCMS-) participants. The horizontal broken line shows the mean force and EMG activity at baseline. Bonferonni post-hoc tests were used to test for significant comparisons. Error bars indicate the SE. *P<
31 Figure 1 A B PCMS+2ms Depolarize Presynaptic Before Postsynaptic PCMS-15ms Depolarize Presynaptic After Postsynaptic C L pairs of stimuli Baseline 30 min Time (min)
Report. Motor Recovery after Spinal Cord Injury Enhanced by Strengthening Corticospinal Synaptic Transmission
 Current Biology 22, 2355 2361, December 18, 2012 ª2012 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2012.10.046 Motor Recovery after Spinal Cord Injury Enhanced by Strengthening Corticospinal
Current Biology 22, 2355 2361, December 18, 2012 ª2012 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2012.10.046 Motor Recovery after Spinal Cord Injury Enhanced by Strengthening Corticospinal
A novel cortical target to enhance hand motor output in humans with spinal cord injury
 doi:./brain/awx BRAIN 0: 0; A novel cortical target to enhance hand motor output in humans with spinal cord injury Jinyi Long, Paolo Federico and Monica A. Perez These authors contributed equally to this
doi:./brain/awx BRAIN 0: 0; A novel cortical target to enhance hand motor output in humans with spinal cord injury Jinyi Long, Paolo Federico and Monica A. Perez These authors contributed equally to this
Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional Plasticity of Human Cortical and Spinal Motor Pathways
 City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 6-2016 Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional
City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 6-2016 Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional
Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley
 (2002), 542.3, pp. 963 976 DOI: 10.1113/jphysiol.2002.021683 The Physiological Society 2002 www.jphysiol.org Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated
(2002), 542.3, pp. 963 976 DOI: 10.1113/jphysiol.2002.021683 The Physiological Society 2002 www.jphysiol.org Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated
Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans
 11911 Journal of Physiology (2001), 533.3, pp.903 919 903 Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans Guillaume Nicolas, Véronique Marchand-Pauvert,
11911 Journal of Physiology (2001), 533.3, pp.903 919 903 Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans Guillaume Nicolas, Véronique Marchand-Pauvert,
Spinal Excitability Changes after Transspinal and Transcortical Paired Associative Stimulation in Humans
 City University of New York (CUNY) CUNY Academic Works Publications and Research College of Staten Island 1-16-217 Spinal Excitability Changes after Transspinal and Transcortical Paired Associative Stimulation
City University of New York (CUNY) CUNY Academic Works Publications and Research College of Staten Island 1-16-217 Spinal Excitability Changes after Transspinal and Transcortical Paired Associative Stimulation
Motor and Gait Improvement in Patients With Incomplete Spinal Cord Injury Induced by High-Frequency Repetitive Transcranial Magnetic Stimulation
 Motor and Gait Improvement in Patients With Incomplete Spinal Cord Injury Induced by High-Frequency Repetitive Transcranial Magnetic Stimulation J. Benito, MD, 1 H. Kumru, PhD, 1 N. Murillo, PhD, 1 U.
Motor and Gait Improvement in Patients With Incomplete Spinal Cord Injury Induced by High-Frequency Repetitive Transcranial Magnetic Stimulation J. Benito, MD, 1 H. Kumru, PhD, 1 N. Murillo, PhD, 1 U.
Modulation of single motor unit discharges using magnetic stimulation of the motor cortex in incomplete spinal cord injury
 1 SHORT REPORT Division of Neuroscience and Psychological Medicine, Imperial College School of Medicine, Charing Cross Hospital, London W 8RF, UK H C Smith NJDavey D W Maskill P H Ellaway National Spinal
1 SHORT REPORT Division of Neuroscience and Psychological Medicine, Imperial College School of Medicine, Charing Cross Hospital, London W 8RF, UK H C Smith NJDavey D W Maskill P H Ellaway National Spinal
Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle
 J Physiol (2003), 550.3, pp. 933 946 DOI: 10.1113/jphysiol.2003.042606 The Physiological Society 2003 www.jphysiol.org Differential modulation of intracortical inhibition in human motor cortex during selective
J Physiol (2003), 550.3, pp. 933 946 DOI: 10.1113/jphysiol.2003.042606 The Physiological Society 2003 www.jphysiol.org Differential modulation of intracortical inhibition in human motor cortex during selective
Variety of muscle responses to tactile stimuli
 Variety of muscle responses to tactile stimuli Julita Czarkowska-Bauch Department of Neurophysiology, Nencki Institute of Experimental Biology, 3 Pasteur St., 02-093 Warsaw, Poland Abstract. Influences
Variety of muscle responses to tactile stimuli Julita Czarkowska-Bauch Department of Neurophysiology, Nencki Institute of Experimental Biology, 3 Pasteur St., 02-093 Warsaw, Poland Abstract. Influences
Doctoral Thesis. Modulation of Spinal Neural Circuits Induced by Corticospinal. Descending and Peripheral Afferent Inputs
 Doctoral Thesis Modulation of Spinal Neural Circuits Induced by Corticospinal Descending and Peripheral Afferent Inputs Shinji Kubota Division of Integrated Arts and Sciences Graduate School of Integrated
Doctoral Thesis Modulation of Spinal Neural Circuits Induced by Corticospinal Descending and Peripheral Afferent Inputs Shinji Kubota Division of Integrated Arts and Sciences Graduate School of Integrated
Changes in intracortical excitability induced by stimulation of wrist afferents in man
 12359 Journal of Physiology (2001), 534.3, pp.891 902 891 Changes in intracortical excitability induced by stimulation of wrist afferents in man Jean-Marc Aimonetti and Jens Bo Nielsen * Laboratoire Développement
12359 Journal of Physiology (2001), 534.3, pp.891 902 891 Changes in intracortical excitability induced by stimulation of wrist afferents in man Jean-Marc Aimonetti and Jens Bo Nielsen * Laboratoire Développement
FORSKNING INDENFOR NEUROREHABILITERING PÅ INSTITUT FOR MEDICIN OG SUNDHEDSTEKNOLOGI, AAU KIM DREMSTRUP AND NATALIE MRACHACZ-KERSTING
 FORSKNING INDENFOR NEUROREHABILITERING PÅ INSTITUT FOR MEDICIN OG SUNDHEDSTEKNOLOGI, AAU KIM DREMSTRUP AND NATALIE MRACHACZ-KERSTING Brain activity to control external devices and activities Brain-Computer-Interface
FORSKNING INDENFOR NEUROREHABILITERING PÅ INSTITUT FOR MEDICIN OG SUNDHEDSTEKNOLOGI, AAU KIM DREMSTRUP AND NATALIE MRACHACZ-KERSTING Brain activity to control external devices and activities Brain-Computer-Interface
Interactions Between Descending and Somatosensory Inputs in Humans
 City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 5-2015 Interactions Between Descending and Somatosensory Inputs in Humans Lisa Krivis
City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 5-2015 Interactions Between Descending and Somatosensory Inputs in Humans Lisa Krivis
REFLEX EFFECTS OF INDUCED MUSCLE CONTRACTION IN NORMAL AND SPINAL CORD INJURED SUBJECTS
 ABSTRACT: The modulation of the soleus H reflex in response to functional electrical stimulation (FES) of the rectus femoris (RF) muscle and its overlying skin was examined in 11 normal adults and 6 patients
ABSTRACT: The modulation of the soleus H reflex in response to functional electrical stimulation (FES) of the rectus femoris (RF) muscle and its overlying skin was examined in 11 normal adults and 6 patients
Locomotor training improves reciprocal and nonreciprocal inhibitory control of soleus motoneurons in human spinal cord injury
 J Neurophysiol 113: 27 26, 21. First published January 21, 21; doi:1.112/jn.872.21. Locomotor improves reciprocal and nonreciprocal inhibitory control of soleus motoneurons in human spinal cord injury
J Neurophysiol 113: 27 26, 21. First published January 21, 21; doi:1.112/jn.872.21. Locomotor improves reciprocal and nonreciprocal inhibitory control of soleus motoneurons in human spinal cord injury
Review Article A Review on Locomotor Training after Spinal Cord Injury: Reorganization of Spinal Neuronal Circuits and Recovery of Motor Function
 Hindawi Publishing Corporation Neural Plasticity Volume 2016, Article ID 1216258, 20 pages http://dx.doi.org/10.1155/2016/1216258 Review Article A Review on Locomotor Training after Spinal Cord Injury:
Hindawi Publishing Corporation Neural Plasticity Volume 2016, Article ID 1216258, 20 pages http://dx.doi.org/10.1155/2016/1216258 Review Article A Review on Locomotor Training after Spinal Cord Injury:
Muscle fatigue changes cutaneous suppression of propriospinal drive to human upper limb muscles
 J Physiol 580.1 (2007) pp 211 223 211 Muscle fatigue changes cutaneous suppression of propriospinal drive to human upper limb muscles P. G. Martin, S. C. Gandevia and J. L. Taylor Prince of Wales Medical
J Physiol 580.1 (2007) pp 211 223 211 Muscle fatigue changes cutaneous suppression of propriospinal drive to human upper limb muscles P. G. Martin, S. C. Gandevia and J. L. Taylor Prince of Wales Medical
NEURAL CONTROL OF ECCENTRIC AND POST- ECCENTRIC MUSCLE ACTIONS
 NEURAL CONTROL OF ECCENTRIC AND POST- ECCENTRIC MUSCLE ACTIONS 1, 2 Daniel Hahn, 1 Ben W. Hoffman, 1 Timothy J. Carroll and 1 Andrew G. Cresswell 1 School of Human Movement Studies, University of Queensland,
NEURAL CONTROL OF ECCENTRIC AND POST- ECCENTRIC MUSCLE ACTIONS 1, 2 Daniel Hahn, 1 Ben W. Hoffman, 1 Timothy J. Carroll and 1 Andrew G. Cresswell 1 School of Human Movement Studies, University of Queensland,
Rewiring of hindlimb corticospinal neurons after spinal cord injury
 Rewiring of hindlimb corticospinal neurons after spinal cord injury Arko Ghosh, Florent Haiss, Esther Sydekum, Regula Schneider, Miriam Gullo, Matthias T. Wyss, Thomas Mueggler, Christof Baltes, Markus
Rewiring of hindlimb corticospinal neurons after spinal cord injury Arko Ghosh, Florent Haiss, Esther Sydekum, Regula Schneider, Miriam Gullo, Matthias T. Wyss, Thomas Mueggler, Christof Baltes, Markus
Motor systems.... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington
 Motor systems... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington 1 Descending pathways: CS corticospinal; TS tectospinal; RS reticulospinal; VS
Motor systems... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington 1 Descending pathways: CS corticospinal; TS tectospinal; RS reticulospinal; VS
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit. Supplementary Information. Adam W. Hantman and Thomas M.
 Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Water immersion modulates sensory and motor cortical excitability
 Water immersion modulates sensory and motor cortical excitability Daisuke Sato, PhD Department of Health and Sports Niigata University of Health and Welfare Topics Neurophysiological changes during water
Water immersion modulates sensory and motor cortical excitability Daisuke Sato, PhD Department of Health and Sports Niigata University of Health and Welfare Topics Neurophysiological changes during water
thought to reflect complex interactions evoked by changes in afferent activity Southampton General Hospital, Southampton, S09 4XY
 J. Phy8iol. (1983), 337, pp. 497-58 497 With 6 text-ftgures Printed in Great Britain SPINAL INHIBITION IN MAN: DEPRESSION OF THE SOLEUS H REFLEX BY STIMULATION OF THE NERVE TO THE ANTAGONIST MUSCLE BY
J. Phy8iol. (1983), 337, pp. 497-58 497 With 6 text-ftgures Printed in Great Britain SPINAL INHIBITION IN MAN: DEPRESSION OF THE SOLEUS H REFLEX BY STIMULATION OF THE NERVE TO THE ANTAGONIST MUSCLE BY
(Received 10 April 1956)
 446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
Cutaneomuscular reflexes recorded from the lower limb
 Journal of Physiology (1995), 487.1, pp.237-242 376 237 Cutaneomuscular reflexes recorded from the lower limb in man during different tasks J. Gibbs, Linda M. Harrison * and J. A. Stephens Department of
Journal of Physiology (1995), 487.1, pp.237-242 376 237 Cutaneomuscular reflexes recorded from the lower limb in man during different tasks J. Gibbs, Linda M. Harrison * and J. A. Stephens Department of
Effect of Surface Spinal Stimulation (SSS) on H-reflex in Normal Individuals Narkeesh 1, A., Navroop kaur 2, N. & Sharma 3, S.
 Effect of Surface Spinal (SSS) on H-reflex in Normal Individuals Narkeesh 1, A., Navroop kaur 2, N. & Sharma 3, S. 1 Associate Professor, Email: narkeesh@gmail.com, 2 & 3 Post Graduate Students, Department
Effect of Surface Spinal (SSS) on H-reflex in Normal Individuals Narkeesh 1, A., Navroop kaur 2, N. & Sharma 3, S. 1 Associate Professor, Email: narkeesh@gmail.com, 2 & 3 Post Graduate Students, Department
Introduction to TMS Transcranial Magnetic Stimulation
 Introduction to TMS Transcranial Magnetic Stimulation Lisa Koski, PhD, Clin Psy TMS Neurorehabilitation Lab Royal Victoria Hospital 2009-12-14 BIC Seminar, MNI Overview History, basic principles, instrumentation
Introduction to TMS Transcranial Magnetic Stimulation Lisa Koski, PhD, Clin Psy TMS Neurorehabilitation Lab Royal Victoria Hospital 2009-12-14 BIC Seminar, MNI Overview History, basic principles, instrumentation
Crossed flexor reflex responses and their reversal in freely walking cats
 Brain Research, 197 (1980) 538-542 0 Elsevier/North-Holland Biomedical Press Crossed flexor reflex responses and their reversal in freely walking cats J. DUYSENS*, G. E. LOEB and B. J. WESTON Laboratory
Brain Research, 197 (1980) 538-542 0 Elsevier/North-Holland Biomedical Press Crossed flexor reflex responses and their reversal in freely walking cats J. DUYSENS*, G. E. LOEB and B. J. WESTON Laboratory
The Journal of Physiology Neuroscience
 J Physiol 591.19 (2013) pp 4903 4920 4903 The Journal of Physiology Neuroscience Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability David
J Physiol 591.19 (2013) pp 4903 4920 4903 The Journal of Physiology Neuroscience Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability David
MOTOR EVOKED POTENTIALS AND TRANSCUTANEOUS MAGNETO-ELECTRICAL NERVE STIMULATION
 MOTOR EVOKED POTENTIAS AND TRANSCUTANEOUS MAGNETO-EECTRICA NERVE STIMUATION Hongguang iu, in Zhou 1 and Dazong Jiang Xian Jiaotong University, Xian, People s Republic of China 1 Shanxi Normal University,
MOTOR EVOKED POTENTIAS AND TRANSCUTANEOUS MAGNETO-EECTRICA NERVE STIMUATION Hongguang iu, in Zhou 1 and Dazong Jiang Xian Jiaotong University, Xian, People s Republic of China 1 Shanxi Normal University,
NIH Public Access Author Manuscript J Neurosci. Author manuscript; available in PMC 2013 August 06.
 NIH Public Access Author Manuscript Published in final edited form as: J Neurosci. 2013 February 6; 33(6): 2365 2375. doi:10.1523/jneurosci.3968-12.2013. OPERANT CONDITIONING OF A SPINAL REFLEX CAN IMPROVE
NIH Public Access Author Manuscript Published in final edited form as: J Neurosci. 2013 February 6; 33(6): 2365 2375. doi:10.1523/jneurosci.3968-12.2013. OPERANT CONDITIONING OF A SPINAL REFLEX CAN IMPROVE
Localizing and Characterizing Neural Plasticity in Spinal Cord Injury
 PVA Summer 2012 Expo The Effect of Rehabilitation on the Neurobiology of MS and SCI Las Vegas, NV, August 30, 2012 Localizing and Characterizing Neural Plasticity in Spinal Cord Injury Keith Tansey, MD,
PVA Summer 2012 Expo The Effect of Rehabilitation on the Neurobiology of MS and SCI Las Vegas, NV, August 30, 2012 Localizing and Characterizing Neural Plasticity in Spinal Cord Injury Keith Tansey, MD,
Physiology. D. Gordon E. Robertson, PhD, FCSB. Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada
 Electromyography: Physiology D. Gordon E. Robertson, PhD, FCSB Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada Nervous System Central Nervous System (cerebellum,
Electromyography: Physiology D. Gordon E. Robertson, PhD, FCSB Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada Nervous System Central Nervous System (cerebellum,
Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex
 Archives of Disease in Childhood, 1988, 63, 1347-1352 Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex T H H G KOH AND J A EYRE Department of Child Health,
Archives of Disease in Childhood, 1988, 63, 1347-1352 Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex T H H G KOH AND J A EYRE Department of Child Health,
Considerable progress has been made in the last few years
 JOURNAL OF NEUROTRAUMA 34:1841 1857 (May 15, 2017) ª Mary Ann Liebert, Inc. DOI: 10.1089/neu.2016.4577 Rehabilitation Strategies after Spinal Cord Injury: Inquiry into the Mechanisms of Success and Failure
JOURNAL OF NEUROTRAUMA 34:1841 1857 (May 15, 2017) ª Mary Ann Liebert, Inc. DOI: 10.1089/neu.2016.4577 Rehabilitation Strategies after Spinal Cord Injury: Inquiry into the Mechanisms of Success and Failure
HUMAN MOTOR CONTROL. Emmanuel Guigon
 HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
The Physiology of the Senses Chapter 8 - Muscle Sense
 The Physiology of the Senses Chapter 8 - Muscle Sense www.tutis.ca/senses/ Contents Objectives... 1 Introduction... 2 Muscle Spindles and Golgi Tendon Organs... 3 Gamma Drive... 5 Three Spinal Reflexes...
The Physiology of the Senses Chapter 8 - Muscle Sense www.tutis.ca/senses/ Contents Objectives... 1 Introduction... 2 Muscle Spindles and Golgi Tendon Organs... 3 Gamma Drive... 5 Three Spinal Reflexes...
purely monosynaptic e.p.s.p. is a prerequisite for the validity of the method. Experimental
 J. Physiol. (1987), 389, pp. 729-756 729 With 8 text-figures Printed in Great Britain ASSESSING CHANGES IN PRESYNAPTIC INHIBITION OF I a FIBRES: A STUDY IN MAN AND THE CAT BY H. HULTBORN*, S. MEUNIER,
J. Physiol. (1987), 389, pp. 729-756 729 With 8 text-figures Printed in Great Britain ASSESSING CHANGES IN PRESYNAPTIC INHIBITION OF I a FIBRES: A STUDY IN MAN AND THE CAT BY H. HULTBORN*, S. MEUNIER,
Spinal and Supraspinal Control of Reflexes: In health, under general anesthesia, and in Parkinson s disease. Jennifer C. Andrews
 Spinal and Supraspinal Control of Reflexes: In health, under general anesthesia, and in Parkinson s disease by Jennifer C. Andrews A thesis submitted in partial fulfillment of the requirements for the
Spinal and Supraspinal Control of Reflexes: In health, under general anesthesia, and in Parkinson s disease by Jennifer C. Andrews A thesis submitted in partial fulfillment of the requirements for the
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE
 TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
Reflexes. Dr. Baizer
 Reflexes Dr. Baizer 1 Learning objectives: reflexes Students will be able to describe: 1. The clinical importance of testing reflexes. 2. The essential components of spinal reflexes. 3.The stretch reflex.
Reflexes Dr. Baizer 1 Learning objectives: reflexes Students will be able to describe: 1. The clinical importance of testing reflexes. 2. The essential components of spinal reflexes. 3.The stretch reflex.
Timing of cortical excitability changes during the reaction time of movements superimposed on tonic motor activity
 J Appl Physiol 97: 2220 2227, 2004; doi:10.1152/japplphysiol.00542.2004. Timing of cortical excitability changes during the reaction time of movements superimposed on tonic motor activity Cyril Schneider,
J Appl Physiol 97: 2220 2227, 2004; doi:10.1152/japplphysiol.00542.2004. Timing of cortical excitability changes during the reaction time of movements superimposed on tonic motor activity Cyril Schneider,
Corticospinal Integration in Healthy Humans
 City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 6-2014 Corticospinal Integration in Healthy Humans Amanda Asmar Graduate Center, City
City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 6-2014 Corticospinal Integration in Healthy Humans Amanda Asmar Graduate Center, City
Paired-Pulse TMS to one Brain Region. Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation LEASE DO NOT COPY
 Paired-Pulse TMS to one Brain Region Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation Paired-Pulse Paradigms Sequential pulses applied to the same cortical region Variable
Paired-Pulse TMS to one Brain Region Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation Paired-Pulse Paradigms Sequential pulses applied to the same cortical region Variable
The sites of neural adaptation induced by resistance training in humans
 (2002), 544.2, pp. 641 652 DOI: 10.1113/jphysiol.2002.024463 The Physiological Society 2002 www.jphysiol.org The sites of neural adaptation induced by resistance training in humans Timothy J. Carroll,
(2002), 544.2, pp. 641 652 DOI: 10.1113/jphysiol.2002.024463 The Physiological Society 2002 www.jphysiol.org The sites of neural adaptation induced by resistance training in humans Timothy J. Carroll,
Københavns Universitet
 university of copenhagen Københavns Universitet Interference in ballistic motor learning: specificity and role of sensory error signals Lundbye-Jensen, Jesper; Petersen, Tue Hvass; Rothwell, John C; Nielsen,
university of copenhagen Københavns Universitet Interference in ballistic motor learning: specificity and role of sensory error signals Lundbye-Jensen, Jesper; Petersen, Tue Hvass; Rothwell, John C; Nielsen,
Lecture VIII. The Spinal Cord, Reflexes and Brain Pathways!
 Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Recurrent inhibition between motor nuclei innervating opposing wrist muscles in the human upper limb
 5571 Journal of Physiology (1997), 499.1, pp.267-282 267 Recurrent inhibition between motor nuclei innervating opposing wrist muscles in the human upper limb C. Aymard*t, B. Decchit, R. Katzt, C. Lafittet,
5571 Journal of Physiology (1997), 499.1, pp.267-282 267 Recurrent inhibition between motor nuclei innervating opposing wrist muscles in the human upper limb C. Aymard*t, B. Decchit, R. Katzt, C. Lafittet,
The Journal of Physiology
 J Physiol. (217) pp 1 16 1 Paired motor cortex and cervical epidural electrical stimulation timed to converge in the spinal cord promotes lasting increases in motor responses Asht M. Mishra 1,AjayPal 1,DishaGupta
J Physiol. (217) pp 1 16 1 Paired motor cortex and cervical epidural electrical stimulation timed to converge in the spinal cord promotes lasting increases in motor responses Asht M. Mishra 1,AjayPal 1,DishaGupta
Interaction of paired cortical and peripheral nerve stimulation on human motor neurons
 Exp Brain Res (28) 188:13 21 DOI 1.17/s221-8-1334-8 RESEARCH ARTICLE Interaction of paired cortical and peripheral nerve stimulation on human motor neurons David E. Poon Francois D. Roy Monica A. Gorassini
Exp Brain Res (28) 188:13 21 DOI 1.17/s221-8-1334-8 RESEARCH ARTICLE Interaction of paired cortical and peripheral nerve stimulation on human motor neurons David E. Poon Francois D. Roy Monica A. Gorassini
Vestibular-induced Modulation Of Leg Motoneuron Pool Excitability In Standing And Prone Positions
 Article ID: WMC001436 2046-1690 Vestibular-induced Modulation Of Leg Motoneuron Pool Excitability In Standing And Prone Positions Corresponding Author: Mr. Akiyoshi Matsugi, Graduate Student, Physical
Article ID: WMC001436 2046-1690 Vestibular-induced Modulation Of Leg Motoneuron Pool Excitability In Standing And Prone Positions Corresponding Author: Mr. Akiyoshi Matsugi, Graduate Student, Physical
The Three Pearls DOSE FUNCTION MOTIVATION
 The Three Pearls DOSE FUNCTION MOTIVATION Barriers to Evidence-Based Neurorehabilitation No placebo pill for training therapy Blinded studies often impossible Outcome measures for movement, language, and
The Three Pearls DOSE FUNCTION MOTIVATION Barriers to Evidence-Based Neurorehabilitation No placebo pill for training therapy Blinded studies often impossible Outcome measures for movement, language, and
The effects of cryotherapy on quadriceps corticospinal excitability in patients with patellofemoral pain
 The University of Toledo The University of Toledo Digital Repository Theses and Dissertations 2013 The effects of cryotherapy on quadriceps corticospinal excitability in patients with patellofemoral pain
The University of Toledo The University of Toledo Digital Repository Theses and Dissertations 2013 The effects of cryotherapy on quadriceps corticospinal excitability in patients with patellofemoral pain
Brain-Computer Interfaces to Replace or Repair the Injured Central Nervous System
 Three approaches to restore movement Brain-Computer Interfaces to Replace or Repair the Injured Central Nervous System 1. Replace: Brain control of 2. Replace & Repair: Intra-Spinal Stimulation 3. Repair:
Three approaches to restore movement Brain-Computer Interfaces to Replace or Repair the Injured Central Nervous System 1. Replace: Brain control of 2. Replace & Repair: Intra-Spinal Stimulation 3. Repair:
Short latency inhibition of human hand motor cortex by somatosensory input from the hand
 Keywords: 9995 Journal of Physiology (2000), 523.2, pp. 503 513 503 Short latency inhibition of human hand motor cortex by somatosensory input from the hand H. Tokimura *, V. Di Lazzaro, Y. Tokimura *,
Keywords: 9995 Journal of Physiology (2000), 523.2, pp. 503 513 503 Short latency inhibition of human hand motor cortex by somatosensory input from the hand H. Tokimura *, V. Di Lazzaro, Y. Tokimura *,
Non-therapeutic and investigational uses of non-invasive brain stimulation
 Non-therapeutic and investigational uses of non-invasive brain stimulation Robert Chen, MA, MBBChir, MSc, FRCPC Catherine Manson Chair in Movement Disorders Professor of Medicine (Neurology), University
Non-therapeutic and investigational uses of non-invasive brain stimulation Robert Chen, MA, MBBChir, MSc, FRCPC Catherine Manson Chair in Movement Disorders Professor of Medicine (Neurology), University
XXVIII. Recording of Achilles tendon reflex
 XXVII. Examination of reflexes in man XXVIII. Recording of Achilles tendon reflex Physiology II - practice Dep. of Physiology, Fac. of Medicine, MU, 2016 Mohamed Al-Kubati Reflexes Reflex: is an involuntary
XXVII. Examination of reflexes in man XXVIII. Recording of Achilles tendon reflex Physiology II - practice Dep. of Physiology, Fac. of Medicine, MU, 2016 Mohamed Al-Kubati Reflexes Reflex: is an involuntary
Tapping the Neural Circuitry: Surface Spinal Stimulation in Spinal Cord Injury: A Case Report
 ISSN 0973-2020 (P) ISSN 2454-6089(E) Tapping the Neural Circuitry: Surface Spinal Stimulation in Spinal Cord Injury: A Case Report Bedi 1, Parneet Kaur; & Narkeesh 2, Arumugam Article Authorship & Affiliation
ISSN 0973-2020 (P) ISSN 2454-6089(E) Tapping the Neural Circuitry: Surface Spinal Stimulation in Spinal Cord Injury: A Case Report Bedi 1, Parneet Kaur; & Narkeesh 2, Arumugam Article Authorship & Affiliation
NIH Public Access Author Manuscript Exerc Sport Sci Rev. Author manuscript; available in PMC 2015 April 01.
 NIH Public Access Author Manuscript Published in final edited form as: Exerc Sport Sci Rev. 2014 April ; 42(2): 82 90. doi:10.1249/jes.0000000000000010. The Simplest Motor Skill: Mechanisms and Applications
NIH Public Access Author Manuscript Published in final edited form as: Exerc Sport Sci Rev. 2014 April ; 42(2): 82 90. doi:10.1249/jes.0000000000000010. The Simplest Motor Skill: Mechanisms and Applications
Trans-spinal direct current stimulation: a novel tool to promote plasticity in humans
 Trans-spinal direct current stimulation: a novel tool to promote plasticity in humans Jean-Charles Lamy, PhD Brain and Spine Institute, Paris 1 Background Grecco et al., J Neuroresto, 2015 2 Background:
Trans-spinal direct current stimulation: a novel tool to promote plasticity in humans Jean-Charles Lamy, PhD Brain and Spine Institute, Paris 1 Background Grecco et al., J Neuroresto, 2015 2 Background:
Increase in reciprocal I a inhibition during antagonist contraction in the human leg: a study of motor units and the H reflex
 433 Journal of Physiology (1995), 489.1, pp. 275-286 275 Increase in reciprocal I a inhibition during antagonist contraction in the human leg: a study of motor units and the H reflex Masaomi Shindo, Sohei
433 Journal of Physiology (1995), 489.1, pp. 275-286 275 Increase in reciprocal I a inhibition during antagonist contraction in the human leg: a study of motor units and the H reflex Masaomi Shindo, Sohei
The Journal of Physiology
 J Physiol 590.4 (2012) pp 919 935 919 The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex Laila Alibiglou 1,2 and Colum D.
J Physiol 590.4 (2012) pp 919 935 919 The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex Laila Alibiglou 1,2 and Colum D.
Spinal Interneurons. Control of Movement
 Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Neuronal mechanisms underlying retraining of walking after incomplete spinal cord injury
 Neuronal mechanisms underlying retraining of walking after incomplete spinal cord injury by Ephrem Takele Zewdie A thesis submitted in partial fulfilment of the requirement for the degree of Doctor of
Neuronal mechanisms underlying retraining of walking after incomplete spinal cord injury by Ephrem Takele Zewdie A thesis submitted in partial fulfilment of the requirement for the degree of Doctor of
Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans
 J Physiol 568.1 (2005) pp 343 354 343 Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans Monica A. Perez 2,Bjarke K. S. Lungholt 2 and Jens B.
J Physiol 568.1 (2005) pp 343 354 343 Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans Monica A. Perez 2,Bjarke K. S. Lungholt 2 and Jens B.
SEP Monitoring. Outline. Outline 1/22/2015. Development of SEPs Stimulation and recording techniques Predictive value of SEP Uses of SEP monitoring
 SEP Monitoring Andres A Gonzalez, MD Director, Surgical Neurophysiology Keck Medical Center of USC University of Southern California Outline Development of SEPs Stimulation and recording techniques Predictive
SEP Monitoring Andres A Gonzalez, MD Director, Surgical Neurophysiology Keck Medical Center of USC University of Southern California Outline Development of SEPs Stimulation and recording techniques Predictive
Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways
 J Physiol 580.1 (2007) pp 133 147 133 Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways E. Jankowska and K. Stecina Department
J Physiol 580.1 (2007) pp 133 147 133 Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways E. Jankowska and K. Stecina Department
Tibialis anterior stretch reflex in early stance is suppressed by repetitive transcranial magnetic stimulation
 J Physiol 587.8 (2009) pp 1669 1676 1669 RAPID REPORT Tibialis anterior stretch reflex in early stance is suppressed by repetitive transcranial magnetic stimulation Abraham T. Zuur 1,2,3,MarkS.Christensen
J Physiol 587.8 (2009) pp 1669 1676 1669 RAPID REPORT Tibialis anterior stretch reflex in early stance is suppressed by repetitive transcranial magnetic stimulation Abraham T. Zuur 1,2,3,MarkS.Christensen
Startle Stimuli Exert Opposite Effects on Human Cortical and Spinal Motor System Excitability in Leg Muscles
 Physiol. Res. 60 (Suppl. 1): S101-S106, 2011 Startle Stimuli Exert Opposite Effects on Human Cortical and Spinal Motor System Excitability in Leg Muscles T. V. ILIC 1,2, M. PÖTTER-NERGER 1, I. HOLLER 1,
Physiol. Res. 60 (Suppl. 1): S101-S106, 2011 Startle Stimuli Exert Opposite Effects on Human Cortical and Spinal Motor System Excitability in Leg Muscles T. V. ILIC 1,2, M. PÖTTER-NERGER 1, I. HOLLER 1,
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Percutaneous electrical stimulation of lumbosacral
 Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:174-181 Percutaneous electrical stimulation of lumbosacral roots in man A MAERTENS DE NOORDHOUT, J C ROTHWELL, P D THOMPSON, B L DAY, C D MARSDEN
Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:174-181 Percutaneous electrical stimulation of lumbosacral roots in man A MAERTENS DE NOORDHOUT, J C ROTHWELL, P D THOMPSON, B L DAY, C D MARSDEN
Functional electrical stimulation. FES applications
 Functional electrical stimulation FES applications For all therapeutic areas The functional electrical stimulation (FES) is highly important for the therapy and rehabilitation of paralytic illnesses. If
Functional electrical stimulation FES applications For all therapeutic areas The functional electrical stimulation (FES) is highly important for the therapy and rehabilitation of paralytic illnesses. If
Spinal Sensorimotor System Part IV: The PPSL Network Rick Wells July 7, 2003
 Spinal Sensorimotor System Part IV: The PPSL Network Rick Wells July 7, 2003 The PPSL Network The motoneuron level (MNL) network is the network of networks that directly converges on the spinal cord system
Spinal Sensorimotor System Part IV: The PPSL Network Rick Wells July 7, 2003 The PPSL Network The motoneuron level (MNL) network is the network of networks that directly converges on the spinal cord system
Nervous system. The main regulation mechanism of organism's functions
 Nervous system The main regulation mechanism of organism's functions Questions Neuron The reflex arc The nervous centers Properties of the nervous centers The general principles of coordination Inhibition
Nervous system The main regulation mechanism of organism's functions Questions Neuron The reflex arc The nervous centers Properties of the nervous centers The general principles of coordination Inhibition
Neurosoft TMS. Transcranial Magnetic Stimulator DIAGNOSTICS REHABILITATION TREATMENT STIMULATION. of motor disorders after the stroke
 Neurosoft TMS Transcranial Magnetic Stimulator DIAGNOSTICS REHABILITATION TREATMENT of corticospinal pathways pathology of motor disorders after the stroke of depression and Parkinson s disease STIMULATION
Neurosoft TMS Transcranial Magnetic Stimulator DIAGNOSTICS REHABILITATION TREATMENT of corticospinal pathways pathology of motor disorders after the stroke of depression and Parkinson s disease STIMULATION
Lateral view of human brain! Cortical processing of touch!
 Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Locomotor training improves premotoneuronal control after chronic spinal cord injury
 J Neurophysiol 111: 2264 2275, 2014. First published March 5, 2014; doi:10.1152/jn.00871.2013. Locomotor training improves premotoneuronal control after chronic spinal cord injury Maria Knikou 1,2,3,4
J Neurophysiol 111: 2264 2275, 2014. First published March 5, 2014; doi:10.1152/jn.00871.2013. Locomotor training improves premotoneuronal control after chronic spinal cord injury Maria Knikou 1,2,3,4
Reflex and Non-Reflex Torque Responses to Stretch of the Human Knee Extensors
 1 of 4 Reflex and Non-Reflex Torque Responses to Stretch of the Human Knee Extensors N. Mrachacz-Kersting, T. Sinkjaer Center for Sensory-Motor Interaction (SMI), Aalborg University, Aalborg, Denmark Abstract
1 of 4 Reflex and Non-Reflex Torque Responses to Stretch of the Human Knee Extensors N. Mrachacz-Kersting, T. Sinkjaer Center for Sensory-Motor Interaction (SMI), Aalborg University, Aalborg, Denmark Abstract
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
Nervous System. The Peripheral Nervous System Agenda Review of CNS v. PNS PNS Basics Cranial Nerves Spinal Nerves Reflexes Pathways
 Nervous System Agenda Review of CNS v. PNS PNS Basics Cranial Nerves Spinal Nerves Sensory Motor Review of CNS v. PNS Central nervous system (CNS) Brain Spinal cord Peripheral nervous system (PNS) All
Nervous System Agenda Review of CNS v. PNS PNS Basics Cranial Nerves Spinal Nerves Sensory Motor Review of CNS v. PNS Central nervous system (CNS) Brain Spinal cord Peripheral nervous system (PNS) All
Physiology of synapses and receptors
 Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Active Control of Spike-Timing Dependent Synaptic Plasticity in an Electrosensory System
 Active Control of Spike-Timing Dependent Synaptic Plasticity in an Electrosensory System Patrick D. Roberts and Curtis C. Bell Neurological Sciences Institute, OHSU 505 N.W. 185 th Avenue, Beaverton, OR
Active Control of Spike-Timing Dependent Synaptic Plasticity in an Electrosensory System Patrick D. Roberts and Curtis C. Bell Neurological Sciences Institute, OHSU 505 N.W. 185 th Avenue, Beaverton, OR
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Beyond Vanilla LTP. Spike-timing-dependent-plasticity or STDP
 Beyond Vanilla LTP Spike-timing-dependent-plasticity or STDP Hebbian learning rule asn W MN,aSN MN Δw ij = μ x j (v i - φ) learning threshold under which LTD can occur Stimulation electrode Recording electrode
Beyond Vanilla LTP Spike-timing-dependent-plasticity or STDP Hebbian learning rule asn W MN,aSN MN Δw ij = μ x j (v i - φ) learning threshold under which LTD can occur Stimulation electrode Recording electrode
Riluzole does not have an acute effect on motor thresholds and the intracortical excitability in amyotrophic lateral sclerosis
 J Neurol (1999) 246 [Suppl 3]: III/22 III/26 Steinkopff Verlag 1999 Martin Sommer Frithjof Tergau Stephan Wischer Carl-D. Reimers Wolfgang Beuche Walter Paulus Riluzole does not have an acute effect on
J Neurol (1999) 246 [Suppl 3]: III/22 III/26 Steinkopff Verlag 1999 Martin Sommer Frithjof Tergau Stephan Wischer Carl-D. Reimers Wolfgang Beuche Walter Paulus Riluzole does not have an acute effect on
Phase-Dependent Modulation of Percutaneously Elicited Multisegmental Muscle Responses After Spinal Cord Injury
 Phase-Dependent Modulation of Percutaneously Elicited Multisegmental Muscle Responses After Spinal Cord Injury Christine J. Dy, Yury P. Gerasimenko, V. Reggie Edgerton, Poul Dyhre-Poulsen, Grégoire Courtine
Phase-Dependent Modulation of Percutaneously Elicited Multisegmental Muscle Responses After Spinal Cord Injury Christine J. Dy, Yury P. Gerasimenko, V. Reggie Edgerton, Poul Dyhre-Poulsen, Grégoire Courtine
Compound Action Potential, CAP
 Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Abstract. Task Specific Effects of Repeated Unilateral Eccentric and Concentric Exercise on
 Abstract Task Specific Effects of Repeated Unilateral Eccentric and Concentric Exercise on Spinal Excitability of the Contralateral Homologous Plantar Flexors. By Ryan D. Hill April, 2011 Director of Thesis:
Abstract Task Specific Effects of Repeated Unilateral Eccentric and Concentric Exercise on Spinal Excitability of the Contralateral Homologous Plantar Flexors. By Ryan D. Hill April, 2011 Director of Thesis:
NEURO-MS TMS. Diagnostic Monophasic Magnetic Stimulator
 NEURO-MS Diagnostic Monophasic Magnetic Stimulator Diagnostics of neurological disorders Powerful monophasic stimulus Ergonomic and lightweight coils of different shapes and sizes Configurations for single
NEURO-MS Diagnostic Monophasic Magnetic Stimulator Diagnostics of neurological disorders Powerful monophasic stimulus Ergonomic and lightweight coils of different shapes and sizes Configurations for single
Suppression of voluntary motor activity revealed using
 MS 2226, pp. 223-235 Journal of Physiology (1994), 477.2 223 Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man Nick J. Davey, Patricia
MS 2226, pp. 223-235 Journal of Physiology (1994), 477.2 223 Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man Nick J. Davey, Patricia
Nervous System: Spinal Cord and Spinal Nerves (Chapter 13)
 Nervous System: Spinal Cord and Spinal Nerves (Chapter 13) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Sources for figures and content: Marieb,
Nervous System: Spinal Cord and Spinal Nerves (Chapter 13) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Sources for figures and content: Marieb,
Nerve Conduction Studies NCS
 Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Short interval intracortical inhibition and facilitation during the silent period in human
 J Physiol 583.3 (27) pp 971 982 971 Short interval intracortical inhibition and facilitation during the silent period in human Zhen Ni, Carolyn Gunraj and Robert Chen Division of Neurology, Krembil Neuroscience
J Physiol 583.3 (27) pp 971 982 971 Short interval intracortical inhibition and facilitation during the silent period in human Zhen Ni, Carolyn Gunraj and Robert Chen Division of Neurology, Krembil Neuroscience
Nerve Conduction Studies NCS
 Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
F acilitation of transmission in the lumbar interneuronal
 130 PAPER Modulation of the transmission in group II heteronymous pathways by tizanidine in spastic hemiplegic patients E Maupas, P Marque, C F Roques, M Simonetta-Moreau... See end of article for authors
130 PAPER Modulation of the transmission in group II heteronymous pathways by tizanidine in spastic hemiplegic patients E Maupas, P Marque, C F Roques, M Simonetta-Moreau... See end of article for authors
Neuro-MS/D Transcranial Magnetic Stimulator
 Neuro-MS/D Transcranial Magnetic Stimulator 20 Hz stimulation with 100% intensity Peak magnetic field - up to 4 T High-performance cooling: up to 10 000 pulses during one session Neuro-MS.NET software
Neuro-MS/D Transcranial Magnetic Stimulator 20 Hz stimulation with 100% intensity Peak magnetic field - up to 4 T High-performance cooling: up to 10 000 pulses during one session Neuro-MS.NET software
Neuro-MS/D DIAGNOSTICS REHABILITATION TREATMENT STIMULATION. Transcranial Magnetic Stimulator. of motor disorders after the stroke
 Neuro-MS/D Transcranial Magnetic Stimulator DIAGNOSTICS of corticospinal pathway pathology REHABILITATION of motor disorders after the stroke TREATMENT of depression and Parkinson s disease STIMULATION
Neuro-MS/D Transcranial Magnetic Stimulator DIAGNOSTICS of corticospinal pathway pathology REHABILITATION of motor disorders after the stroke TREATMENT of depression and Parkinson s disease STIMULATION
Journal of Electromyography and Kinesiology
 Journal of Electromyography and Kinesiology 20 (2010) 354 358 Contents lists available at ScienceDirect Journal of Electromyography and Kinesiology journal homepage: www.elsevier.com/locate/jelekin Role
Journal of Electromyography and Kinesiology 20 (2010) 354 358 Contents lists available at ScienceDirect Journal of Electromyography and Kinesiology journal homepage: www.elsevier.com/locate/jelekin Role
