Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional Plasticity of Human Cortical and Spinal Motor Pathways
|
|
|
- Donald Arnold
- 5 years ago
- Views:
Transcription
1 City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional Plasticity of Human Cortical and Spinal Motor Pathways Luke Dixon Graduate Center, City University of New York Mohamed Ibrahim Graduate Center, City University of New York Danielle Santora Graduate Center, City University of New York How does access to this work benefit you? Let us know! Follow this and additional works at: Part of the Medical Neurobiology Commons, Neurology Commons, and the Physical Therapy Commons Recommended Citation Dixon, Luke; Ibrahim, Mohamed; and Santora, Danielle, "Paired Associative Transspinal and Transcortical Stimulation Produces Bidirectional Plasticity of Human Cortical and Spinal Motor Pathways" (2016). CUNY Academic Works. This Capstone Project is brought to you by CUNY Academic Works. It has been accepted for inclusion in All Dissertations, Theses, and Capstone Projects by an authorized administrator of CUNY Academic Works. For more information, please contact
2 PAIRED ASSOCIATIVE TRANSSPINAL AND TRANSCORTICAL STIMULATION PRODUCES BIDIRECTIONAL PLASTICITY OF HUMAN CORTICAL AND SPINAL MOTOR PATHWAYS by LUKE DIXON MOHAMED IBRAHIM DANIELLE SANTORA A capstone project submitted to the Graduate Faculty in Physical Therapy in partial fulfillment of the requirements for the degree of Doctor of Physical Therapy, The City University of New York 2016
3 2016 LUKE DIXON MOHAMED IBRAHIM DANIELLE SANTORA All Rights Reserved ii
4 This manuscript has been read and accepted for the Graduate Faculty in Physical Therapy in satisfaction of the capstone project requirement for the degree of DPT Maria Knikou, PT, PhD. Date Chair of Examining Committee Jeffrey Rothman, PT, Ed.D. Date Executive Officer THE CITY UNIVERSITY OF NEW YORK iii
5 Abstract PAIRED ASSOCIATIVE TRANSSPINAL AND TRANSCORTICAL STIMULATION PRODUCES BIDIRECTIONAL PLASTICITY OF HUMAN CORTICAL AND SPINAL MOTOR PATHWAYS by Luke Dixon Mohamed Ibrahim Danielle Santora Advisor: Maria Knikou, PT, PhD Anatomical, physiological, and functional connectivity exists between primary motor cortex (M1) and spinal cord neurons. Paired associative stimulation (PAS) produces enduring changes in M1 based on the Hebbian principle of associative plasticity. The present study aims to discover immediate neurophysiological changes on human corticomotor pathways by pairing noninvasive transspinal and transcortical stimulation via transcranial magnetic stimulation (TMS). We delivered paired transspinal and transcortical stimulation for 40-min at precise interstimulus intervals with TMS being delivered after (transspinal-transcortical PAS) or before (transcortical-transspinal PAS) transspinal stimulation. Transspinal-transcortical PAS markedly decreased intracortical inhibition, increased intracortical facilitation and M1 excitability with iv
6 concomitant decreases of motor threshold. Conversely, transcortical-transspinal PAS did not affect intracortical circuits and decreased M1 excitability. Both protocols affected the recruitment gain of spinal motoneurons. Transcortical-transspinal PAS reduced the soleus H- reflex postactivation depression, and transspinal-transcortical PAS reduced the low-frequency soleus H-reflex depression. These findings clearly indicate that pairing transspinal with transcortical stimulation produces both cortical and spinal plasticity, but excitability changes (inhibition or facilitation) in the human brain and spinal cord are reversed based on the timing interval and functional network interactions between the two associated inputs. Transspinaltranscortical PAS can be used as a therapeutic intervention to strengthen cortical networks and corticospinal connections in neurological disorders and thus reduce impairment associated with dysfunction of these neuronal networks. v
7 ACKNOWLEDGEMENTS We would like to thank the volunteers who participated in our study, especially the College of Staten Island Doctorate of Physical Therapy students, without whom this project would not have been possible. We are grateful for the support of the College of Staten Island Doctorate of Physical Therapy full-time faculty members, especially the Department Chair Dr. Jeffrey Rothman, for their continuous support. We are indebted to our advisor Dr. Maria Knikou, PT, PhD, for her irreplaceable guidance and assistance in all facets of this study from conception to completion. This project was funded by The New York State Department of Health, Spinal Cord Injury Research Board, Wadsworth Center and The Craig H. Neilsen Foundation, awarded to Dr. Maria Knikou, PT, PhD. vi
8 TABLE OF CONTENTS Section 1: Title Page... i Approval Page... iii Abstract... iv Acknowledgements... vi List of Figures... viii Section 2: Introduction...1 Materials and Methods...3 Results...10 Discussion...15 Limitations...20 Conclusion and Future Directions...21 References...32 vii
9 LIST OF FIGURES Figure 1: Paired Associative Stimulation Protocol...22 Figure 2: Intacortical Measures Before and After Transspinal-Transcortical PAS...24 Figure 3: Intracortical Measures Before and After Transcortical-Transspinal PAS...25 Figure 4: Corticospinal Excitability Before and After PAS...26 Figure 5: Spinal Excitability Before and After PAS...27 Figure 6: Spinal Inhibitory Measures Before and After PAS...28 Table 1: Tibialis Anterior Motor Evoked Potential Sigmoid Function Parameters...29 Table 2: Soleus Sigmoid Function Parameters: Transspinal-Transcortical PAS...30 Table 3: Soleus Sigmoid Function Parameters: Transcortical-Transspinal PAS...31 Sigmoid Function Equations...31 viii
10 Introduction The primary motor cortex (M1) engages in numerous physiological motor behaviors and sensorimotor learning (Lemon, 2008), and contributes to the flexible control of spinal circuits (Rothwell, 2012). Functional connectivity exists through anatomical and physiological connections between M1 neurons and spinal cord neurons (Porter and Lemon, 1993; Lemon, 2008). We have recently reported that long-lasting transspinal stimulation produces changes in cortical and corticospinal excitability (Knikou et al., 2015). However, the effects of pairing transspinal and transcortical stimulation on human motor pathways are not known. Paired associative stimulation (PAS) of presynaptic and postsynaptic cells produces spiketiming-dependent plasticity via synaptic mechanisms (Song et al., 2000). This form of plasticity has been studied extensively by monitoring single-cell in-vitro preparations, is associated with modifications in synaptic efficacy, and can modify synaptic efficacy in a bidirectional manner based on the timing of the two inputs (for review see, Dan and Poo, 2006; Markram et al., 2011). Potentiation occurs when the presynaptic input precedes the postsynaptic input, whereas depression occurs upon reverse order of the two inputs (Buonomano and Merzenich, 1998; Sjöström et al., 2008; Müller-Dahlhaus et al., 2010; Markram et al., 2011). Procedures for spiketiming-dependent and PAS-induced plasticity in humans differ, but both are based on the Hebbian principle of associative plasticity (Hebb, 1949) and thus may be mediated by similar mechanisms (Stefan et al., 2000, 2002). PAS-induced plasticity of M1 in humans is evident when transcranial magnetic stimulation (TMS)-M1 is paired with contralateral TMS-M1 (Rizzo et al., 2009), supplementary motor area (Arai et al., 2011), premotor cortex (Buch et al., 2011), posterior parietal cortex (Koch et al., 2013; Chao et al., 2015), somatosensory cortex (Wolters et 1
11 al., 2005), and peripheral nerve (Stefan et al., 2000; Mrachacz-Kersting et al., 2007) stimulation. In humans, PAS-induced plasticity and practice-induced plasticity likely share the same synapses or involve interactions of similar neuronal circuits (Ziemann et al., 2001; Stefan et al., 2006; Rosenkratz et al., 2007), supporting for a strong functional role of PAS-induced plasticity. In the current experiments, we applied associative stimulation to the spinal cord and M1 in healthy humans. We delivered paired transspinal and transcortical stimulation for 40-min at precise interstimulus intervals in which TMS was delivered after (transspinal-transcortical PAS) or before (transcortical-transspinal PAS) transspinal stimulation. In the transspinal-transcortical PAS, the transspinal stimulation served as the presynaptic input to cortical neurons. In the transcortical-transspinal PAS, transcortical stimulation served as the presynaptic input to spinal neurons. Our recent findings demonstrate that transspinal stimulation alone decreases spinal and increases corticospinal outputs (Knikou 2013, 2014; Knikou et al., 2015). We therefore hypothesized that transspinal-transcortical PAS will increase cortical output, whereas transcortical-transspinal PAS will increase spinal output. We found that transspinal-transcortical PAS at specific interstimulus intervals decreases intracortical inhibition, increases intracortical facilitation and M1 excitability with concomitant decreases of motor threshold. In contrast, transcortical-transspinal PAS did not affect intracortical circuits, and decreased corticospinal excitability. Both protocols affected the recruitment gain of spinal motoneurons. These findings support for a timing-dependent bilateral PAS-mediated plasticity of human motor pathways when pairing transspinal and transcortical stimulation. 2
12 Materials and Methods Subjects Nineteen healthy subjects participated in the study (mean age yrs SEM, ; 8 female). None of the subjects had a history of neurological or musculoskeletal disorder or was under medication that affects the central or peripheral nervous systems. All subjects were right leg dominant. Six experimental sessions in total were conducted on different days, and some subjects participated in more than one experiment. In this case, the experiment was conducted at a 3-week interval. In each experiment, 12 subjects participated unless stated otherwise. For all subjects, eligibility to the study was first established based on a TMS safety screening. Written informed consent was obtained before enrollment in the study. The blood pressure of all participants was monitored periodically during the experiment and no significant changes were noted. All experimental procedures were conducted in compliance with the Declaration of Helsinki after full Institutional Review Board (IRB) approval by the City University of New York IRB committee. EMG recordings Surface electromyography (EMG) was recorded by single bipolar differential electrodes (MA300-28, Motion Lab Systems Inc., Baton Rouge, LA) from the right tibialis anterior (TA), medial gastrocnemius (MG), and soleus muscles. EMG signals were amplified, filtered ( Hz), sampled at 2000 Hz via a 1401 plus (Cambridge Electronics Design Ltd., England), and stored for offline analysis. 3
13 Transcranial magnetic stimulation TMS focused over the left M1 was delivered via a Magstim stimulator (Magstim, UK) with a double-cone coil (diameter 110 mm) placed so the current of the coil flowed from a posterior to an anterior direction. The point where the lines between the inion and glabellum, and the left and right ear tragus met was marked on an EEG cap. The center of the double-cone coil was placed 1 cm posterior and 1 cm to the left from this intersection point (Knikou, 2014, Knikou et al., 2015). With the double cone coil held at this position, the stimulation intensity was gradually increased from zero and motor evoked potentials (MEPs) recorded from the right TA and soleus muscles were observed on a digital oscilloscope. When MEPs only in the TA muscle could not be evoked at low stimulation intensities, we moved the magnetic coil by a few mm to the left and the procedure was repeated. When the optimal position was determined, the TA MEP resting threshold was established and corresponded to the lowest stimulation intensity that induced repeatable MEPs with peak-to-peak amplitude of at least 100 μv in 3 out of 5 consecutive single TMS pulses (Rossini et al., 1994). Subjects answered a post-tms questionnaire the day after each experiment. One subject from the group reported mild remotely-related sleepiness and trouble concentrating. Transspinal and transcortical PAS protocol Paired stimulation included 240 pairs of TMS pulses delivered over the area of the motor cortex corresponding to the right TA muscle and cathodal transcutaneous stimulation to the spine. Both stimulations were delivered at 0.1 Hz for 40 min with subjects positioned in supine with kneeships flexed at 30 and ankles in a neutral position. For transspinal stimulation, the Thoracic 11 4
14 spinous process was identified via palpation, and a single cathode electrode (Uni-Patch TM EP84169, 10.2 cm 5.1 cm, Wabasha, MA) was placed along the vertebrae equally between the left and right paravertebral sides. Due to its size, the electrode covered from T11 to L1-2 vertebral levels. The cathode electrode was held under constant pressure and maintained in place via pre-wrap. Two reusable self-adhered electrodes (anode; same type as the cathode), connected to function as a single electrode, were placed bilaterally on the abdominal muscles. The cathode and anode electrodes were connected to a constant current stimulator (DS7A, Digitimer, UK) that was triggered by Spike 2 scripts (CED Ltd., UK). The interstimulus interval between transspinal and transcortical stimulation was customized for each subject. The transspinal-transcortical PAS interval allowed transspinal stimulation to evoke depolarization of spinal motoneurons and affect cortical circuits before descending motor volleys elicited by TMS arrived at the presynaptic terminals of corticospinal neurons (Fig. 1A). The transcortical-transspinal PAS interval allowed descending motor volleys elicited by TMS to arrive at the presynaptic terminals of corticospinal neurons before transspinal stimulation transynaptically evoked depolarization in spinal motoneurons and affected cortical circuits (Fig. 1B). The interstimulus interval for each subject was estimated using the relative onset latencies of EMG responses to TMS (TA MEP latency) and thoracolumbar transpinal stimulation (TA transspinal evoked potential-tep latency). The conduction time from M1 to corticospinal presynaptic terminals was estimated by adding 1.5 ms to the TA TEP latency, and the resultant value was subtracted from the TA MEP latency. The added 1.5 ms is the time required for synaptic transmission plus the conduction time needed to the lumbar nerve root at the vertebral foramina (Taylor and Martin, 2009; Bunday and Perez, 2012). This calculation resulted in 5
15 interstimulus intervals that ranged from 8 to 13.5 ms ( ms) across subjects for the transcortical-transspinal PAS. These intervals are consistent with the ms central conduction time of TA MEP (Rossini et al., 1994). In the transspinal-transcortical PAS, the interstimulus intervals ranged from 10 to 15 ms ( ms) across subjects, providing sufficient time for the transspinal stimulus to affect cortical networks. TA EMG recordings during transspinal-transcortical PAS indicate that TA TEPs can be easily separated from TA MEPs based on differential onset latencies and durations, whereas summation of MEPs and TEPs in the EMG occurs during transcortical-transspinal PAS (Fig. 1C). In the transspinal-transcortical PAS protocol, transspinal stimulation was delivered at ma ( TA TEP threshold), and TMS at MSO ( TA MEP resting threshold). In the transcortical-transspinal PAS protocol, transspinal stimulation was delivered at ma ( TA TEP threshold), and TMS at MSO ( TA MEP resting threshold). Experiments Experiment 1. In this experiment we assessed the short-latency intracortical inhibition and medium-latency intracortical facilitation before and after transspinal-transcortical and transcortical-transspinal PAS on different days. Intracortical inhibition and facilitation do not depend on changes in spinal excitability (Kujirai et al., 1993; Chen et al., 1998), and thus can accurately measure plasticity in the motor cortex. Paired TMS pulses over the left M1 were delivered via a Magstim BiStim 2 module (Magstim, UK) at interstimulus intervals of 2, 3, 4, 10, 15, and 30 ms. In the transspinal-transcortical PAS protocol, the conditioning TMS (first 6
16 stimulus) and the test TMS (second stimulus) were set at and at MEP resting threshold across subjects, respectively. In the transcortical-transspinal PAS protocol, the conditioning TMS and the test TMS were set at and MEP resting threshold across subjects, respectively. At the interstimulus interval of 30 ms, the test TMS was also delivered at 1.4 TA MEP resting threshold to establish whether intracortical facilitation depends on the MEP test size. Under control conditions and at all interstimulus intervals tested, at least 12 MEPs at 0.1 Hz at exactly the same stimulation intensities before and after PAS were recorded. Experiment 2. In this experiment we examined corticospinal neuroplasticity after PAS. For each subject, the TA MEP recruitment curve was assembled from stimulation intensities corresponding to 0.5 TA MEP resting threshold until maximum amplitudes were obtained. The MEP recruitment curves were assembled at the exact same intensities before and after transspinal-transcortical and transcortical-transspinal PAS on different days. Experiment 3. In this experiment we examined the immediate changes in spinal reflex excitability via soleus H-reflex recruitment curves, low-frequency (or homosynaptic) depression, and postactivation depression after PAS. The soleus M-wave and H-reflex recruitment curves were assembled before and after PAS. In assembling each soleus H-reflex recruitment curve, at least 120 H-reflexes were recorded at different stimulation intensities. The soleus maximal M- wave was evoked and its amplitude was used to determine the intensity required to elicit soleus H-reflexes on the ascending part of the recruitment curve ranging from % of the maximal M-wave. At this intensity, before and after PAS, 20 H-reflexes were recorded at 0.2, 0.33 and 1 7
17 Hz stimulation frequencies as well as at 0.2 Hz upon paired stimulation pulses at interstimulus intervals of 50 ms and 100 ms. Data analysis and statistics TA MEPs, soleus M-waves and soleus H-reflexes were measured as the area of the fullwave rectified EMG signal (Spike 2, CED Ltd., UK), while the soleus M-waves and H-reflexes from the recruitment curves were measured as peak-to-peak amplitude by customized Labview software. The latency of the TA MEP was measured based on the cumulative sum technique on the rectified waveform average (Ellaway, 1978). The TA MEPs evoked upon paired TMS pulses at different interstimulus intervals before and after PAS were measured and normalized to the mean control MEP recorded before PAS for each subject. The mean amplitude of the MEPs from each subject was grouped based on time of testing and PAS protocol. The Shapiro-Wilk test was performed to test data for normal distribution. A repeated-measures analysis of variance (rmanova) was performed to determine the effect of Time (before vs. after), Interstimulus Interval of paired TMS pulses, and PAS protocol on the TA MEPs recorded upon paired TMS pulses. Holm-Sidak t-tests for multiple comparisons were used to test for significant interactions between these factors. A Boltzmann sigmoid function (Eq. 1) was fitted to TA MEPs recorded at different stimulation intensities (recruitment curve) plotted against the actual stimulation intensities for each subject. The MEP slope and stimuli corresponding to MEP threshold, 50 % MEPmax, and maximal MEP were estimated based on equations 2, 3, and 4, respectively. These parameters were grouped from each subject based on Time of testing and PAS protocol. rmanova was 8
18 performed to determine the effect of Time and PAS protocol on each predicted parameter. Subsequently, the predicted stimulation intensity corresponding to 50 % MEPmax before PAS was used to normalize the TMS intensities. This was done for each subject separately so that MEP amplitudes at different stimulation intensities could be grouped across subjects. The average normalized MEP size was calculated in steps of 0.05 multiples of 50 % MEPmax stimulation intensities for each and across subjects. The soleus peak-to-peak M-wave amplitudes from all points of the recruitment curve were normalized to the homonymous maximal M-wave for each subject. A Boltzmann sigmoid function (Eq. 1) was fitted to the normalized M-waves plotted against the actual stimulation intensities (Devanne et al., 1997; Carroll et al., 2001). The soleus M-wave slope and the stimuli corresponding to M-wave threshold, 50 % Mmax, and maximal M-wave were estimated based on equations 2, 3, and 4, respectively. These parameters were grouped from each subject based on time of testing and PAS protocol. Paired t-tests were performed to determine changes in each parameter after PAS. rmanova was performed to determine the effect of Time and PAS protocol on each predicted parameter for the M-wave. The same analysis was also conducted for the soleus H-reflexes normalized to the homonymous maximal M-wave and plotted against the stimulation intensities ranging from below H-reflex threshold until maximal reflex amplitudes. In order to group the soleus M-wave and H-reflex recruitment curves across subjects, the predicted stimulation intensity at 50 % maximal M-wave before and after PAS was used to normalize the stimulation intensities and present them in multiples of motor threshold (MT). The average normalized soleus M-wave and H-reflex amplitudes were calculated in steps of 0.05 multiples of 50 % Mmax stimulation intensities first for each subject and then across subjects. 9
19 The soleus H-reflexes recorded at 0.2, 0.33, and 1 Hz were normalized to the homonymous maximal M-wave. H-reflex postactivation depression upon paired pulses at 100 and 50 ms were measured as the 2 nd soleus H-reflex amplitude normalized to the mean amplitude of the 1 st H- reflex. Paired t-tests were performed to determine changes in soleus H-reflex amplitudes after PAS for each protocol. rmanova was performed to determine the effect of Time of testing (before vs. after) and PAS protocol on the soleus H-reflex amplitude. T-tests, two-way and threeway rmanovas were performed as needed. In all tests, statistical significance was assumed when p < Data are presented as mean SEM. Results Experiment 1: Transspinal and transcortical PAS-induced immediate neurophysiological changes in cortical networks Short-latency intracortical inhibition and medium-latency intracortical facilitation, established via paired TMS pulses to the left M1, were assessed before and after each PAS protocol to characterize associative induced immediate changes in cortical networks. Non-rectified waveform averages of conditioned TA MEPs shown in Fig. 2A depict removal of short-latency intracortical inhibition and potentiation of intracortical facilitation after transspinal-transcortical PAS in both subjects. There was a significant effect of Time (F (1,6) = 9.68, p = 0.002) and Interstimulus Interval (F (1,6) = 7.69, p < 0.001) on conditioned TA MEP amplitudes, but the effect of Time and its interactions with Interstimulus Intervals was not significant (F (6) = 0.33, p = 0.92) (Fig. 2B). The conditioned TA MEPs were significantly different before and after PAS at interstimulus intervals of 2, 3, 4, 10, and 15 ms (t = 3.11, p = 0.002) (Fig. 2B). 10
20 Non-rectified waveform averages of conditioned TA MEPs shown in Fig. 3A depict depressant effects of transcortical-transspinal PAS on cortical inhibition in Subject 18A and marginal effects on cortical facilitation for both subjects. There was an overall significant effect of Time (F (1,6) = 7.27, p = 0.008) and Interstimulus Interval (F (1,6) = 8.18, p < 0.001), while the effect of Time and its interactions with Interstimulus Intervals was not significant (F (6) = 0.37, p = 0.89) for transcortical-transspinal PAS (N = 14, Fig. 3B). Two-way rmanova along with Holm-Sidak pairwise multiple comparisons for Time at each interstimulus interval showed no significant effects (2 ms: t = 0.55, p = 0.5; 3 ms: t = 0.39, p = 0.69; 4 ms: t = 0.28, p = 0.77; 10 ms: t = 1.23, p = 0.21; 15 ms: t = 1.85, p = 0.06; 30 ms at 1.2 MEPth: t = 1.39, p = 0.16; 30 ms at 1.4 MEPth: t = 1.39, p = 0.16). Three-way rmanova along with Holm-Sidak pairwise multiple comparisons showed a significant effect of PAS protocol for all interstimulus intervals (2 ms: t = 3.03, p = 0.003; 3 ms: t = 0.38, p < 0.001; 4 ms: t = 4.2, p < 0.001; 10 ms: t = 4.1, p < 0.001; 15 ms: t = 2.89, p = 0.004; 30 ms at 1.2 MEPth: t = 3.35, p < 0.001; and 30 ms at 1.4 MEPth: t = 1.65, p = 0.099). These findings indicate that intracortical inhibition and intracortical facilitation changed in a bidirectional manner after transspinal-transcortical and transcortical-transspinal PAS. Experiment 2: Transspinal and transcortical PAS-induced immediate neurophysiological changes in corticospinal networks The latency of the TA MEPs recorded at rest did not change in the transspinal-transcortical PAS protocol (before: ± 0.53 ms, after: ms, t = -0.08, p = 0.93) or in the transcortical-transspinal PAS protocol (before: ± 0.36 ms, after: ms, t = 0.46, p 11
21 = 0.64). The normalized TA MEP amplitudes plotted against multiples of stimulation intensities corresponding to 50 % MEPmax before PAS from all subjects and both protocols are shown in Figure 4. Two-way rmanova showed an overall significant effect of Time (F (1,15) = 7.53, p = 0.007) and normalized stimulation intensities (F (1,15) = 14.61, p < 0.001) on the actual MEP sizes in the transspinal-transcortical PAS protocol (Fig. 4A). The MEP size was increased from 0.9 to 1.2 MEP threshold (all p < 0.05) (Fig. 4A). The predicted stimulus corresponding to MEP threshold was decreased after transspinal-transcortical PAS (p = 0.01), but there were not statistically significant effects of Time on the predicted maximal MEP amplitude, MEP slope, or stimuli corresponding to 50 % MEPmax and maximal MEP (Table 1). Two-way rmanova showed that the actual normalized MEP sizes were decreased after transcortical-transspinal PAS at 1.3 MEP threshold (F (1,19) = 4.51, p = 0.035) (Fig. 4B). However, non-significant effects of Time were found on the predicted maximal MEP and MEP slope, as well as on stimuli corresponding to MEP-threshold, 50 % MEPmax, and maximal MEP (for all p > 0.05, Table 1). Two-way rmanova showed a significant effect of PAS protocol for m function (F (1,1) = 5.28, p = 0.02), and non-significant effects of PAS protocol for predicted MEP max amplitude (F (1,1) = , p = 0.97), MEP slope (F (1,1) = 1.42, p = 0.24), stimuli at MEP threshold (F (1,1) = 0.07, p = 0.78), stimuli at 50% MEPmax (F (1,1) = 0.14, p = 0.7), and stimuli at MEP max (F (1,1) = 0.87, p = 0.35). These findings indicate that transspinal-transcortical PAS increased MEP sizes that coincided with decreased membrane excitability of corticospinal neurons, whereas transcorticaltransspinal PAS decreased MEP amplitude without changing the MEP threshold. 12
22 Experiment 3: Transspinal and transcortical PAS-induced immediate neurophysiological changes in spinal networks The soleus M-wave and H-reflex recruitment input-output curves from all subjects and both PAS protocols are shown in Figs. 5A-B, and Figs. 5C-D, respectively. The associated sigmoid fits for H-reflexes from the ascending part of the recruitment input-output curve are shown in Fig. 5E and Fig. 5F, respectively, while the soleus H-reflexes recorded after PAS are normalized to stimulation intensities corresponding to 50 % Mmax before and after PAS. For the transspinal-transcortical PAS protocol, two-way rmanova showed a nonsignificant effect of Time on the actual H-reflex sizes (from 0.35 to 0.9 multiples of MT) when H-reflexes after PAS were grouped based on stimulation intensities normalized to the MT before (F (1,11) = 0.02, p = 0.86) or after (F (1,11) = 0.38, p = 0.53) PAS (Fig. 5C). The same analysis for the M-waves showed a significant effect of Time only when the M-waves after PAS were grouped based on stimulation intensities normalized to the MT before (F (1,11) = 15.63, p < 0.001) and not to the MT after (F (1,11) = 0.45, p = 0.5) PAS. Based on the sigmoid function results (Hreflexes or M-waves normalized to the homonymous maximal M-wave plotted against the actual stimulation intensities), the maximal H-reflex amplitude (t = 2.08, p = 0.03), and stimulus corresponding to 50 % Hmax (z = -2.1, p = 0.02) were decreased, while the H-reflex slope, m function of the sigmoid fit, and stimuli at threshold and at maximal intensities remained unaltered (for all p > 0.05; Table 2). Last, the m function of the sigmoid fit, M-wave slope, and stimuli corresponding to M-wave threshold, 50 % Mmax, and maximal M-wave were decreased (Wilcoxon Signed Ranked Test, Table 2). 13
23 For the transcortical-transspinal PAS protocol, two-way rmanova showed a nonsignificant effect of Time on the actual H-reflex sizes (from 0.35 to 0.9 multiples of MT) when H-reflexes recorded after PAS were grouped based on stimulation intensities normalized to the MT before PAS (F (1,11) = 0.02, p = 0.99) or to the MT after PAS (F (1,11) = 1.35, p = 0.24) (Fig. 5D). The same analysis for the M-waves showed a significant effect of Time only when the M- waves after PAS were grouped based on stimulation intensities normalized to the MT before (F (1,11) = 39.08, p < 0.001) and not to the MT after (F (1,11) = 0.06, p = 0.79) PAS, although for both cases a significant interaction between stimulation intensities and Time was found (F (11) = 3, p < 0.001). Based on the sigmoid function results (H-reflexes or M-waves normalized to the homonymous maximal M-wave plotted against the actual stimulation intensities), the predicted stimuli corresponding to 50 % Hmax (z = -2.9, p = 0.001) and H-reflex threshold (t = 4.11, p = 0.002) were decreased after PAS (Table 3). The H-reflex slope (t = -1.72, p = 0.11), maximal H- reflex amplitude (t = 2.08, p = 0.06) and m function of the sigmoid fit (t = 1, p = 0.33) remained unaltered (Table 3). The stimuli corresponding to M-wave threshold, 50 % Mmax, and maximal M-wave were decreased, while the M-wave slope and m function of the sigmoid fit remained unaltered (Wilcoxon Signed Ranked Test, Table 3). These findings indicate that both transspinaltranscortical and transcortical-transspinal PAS decreased the excitation thresholds of soleus motoneurons and soleus H-reflex amplitude, and transcortical-transspinal PAS decreased the excitation thresholds of Ia afferents. Transcortical-transspinal and transspinal-transcortical PAS substantially altered the soleus M-wave and H-reflex excitability thresholds (see inlets 1-3 in Fig. 6). Specifically, at the same stimulation intensity as that utilized before PAS, the soleus M-wave and H-reflex after PAS were 14
24 elicited at different points, i.e., at the derecruitment curve, (see inlet 3 in Fig. 6). After PAS, in order to evoke a soleus H-reflex on the ascending portion of the recruitment curve and an M- wave with an amplitude comparable to that before PAS, a substantial reduction in the stimulation intensity was required, consistent with our findings from the M-wave and H-reflex recruitment curves. Transcortical-transspinal PAS did not affect the soleus H-reflex homosynaptic depression (evoked at different stimulation frequencies; for all p > 0.05) but reduced the soleus H-reflex postactivation depression following paired pulses at interstimulus intervals of 100 ms (t = -2.88, p = 0.045) and 50 ms (t=-2.5, p = 0.02) at a constant stimulation frequency of 0.2 Hz (Fig. 6A). Transspinal-transcortical PAS reduced the soleus H-reflex homosynaptic depression at 0.2 Hz (t = -2.66, p = 0.032), 0.33 Hz (t = -2.82, p = 0.037) and 0.1 Hz (t = -2.25, p = 0.049). Further, the soleus H-reflex postactivation depression following paired pulses at interstimulus intervals of 100 ms (t = -1.17, p = 0.32) and 50 ms (t=-1.4, p = 0.18) at a constant stimulation frequency of 0.2 Hz remained unaltered (Fig. 6B). Three-way rmanova showed a significant effect of stimulation frequencies and interstimulus interval of paired pulses (F (4) = 26.36, p < 0.001), and time (F (1) = 13.62, p < 0.001), but not between protocols (F (1) = 1.76, p = 0.18). These findings indicate that both protocols affected the recruitment of soleus motoneurons, while transcorticaltransspinal PAS reduced the soleus H-reflex postactivation depression, and transspinaltranscortical PAS reduced the soleus H-reflex homosynaptic depression. Discussion This study demonstrates that PAS-induced plasticity can be achieved with precisely-timed pairing of transspinal and transcortical stimulation. This pairing paradigm produces both cortical 15
25 and spinal plasticity with opposite excitability changes (inhibition or facilitation) in the human brain and spinal cord based on timing interval and functional network interactions between the two associated inputs at a systems level. Before PAS was delivered, we successfully probed and recorded short-latency MEP inhibition and medium-latency MEP facilitation upon paired TMS pulses (Figs. 2B, 3B), in accordance with previous studies (Kujirai et al., 1993; Di Lazzaro et al., 1998). The use of a subthreshold (~0.7 MEP resting threshold) conditioning TMS pulse minimizes direct activation of cortical motoneurons (Ziemann et al., 1996). Consequently, the MEP short-latency inhibition and medium-latency facilitation are mediated by interneuronal circuits in the motor cortex (Ziemann et al., 1996). Short-latency intracortical inhibition is mediated by a low-threshold gamma-aminobutyric acid type A (GABA A ) receptor-dependent inhibitory pathway (Ilic et al., 2002). The cortical networks mediating intracortical facilitation, however, are more complex (Di Lazzaro et al., 2002). Evidence suggests that cortical facilitation is non-synaptic, occurs at the initial axon segment of cortical interneurons, involves a high threshold excitatory pathway, and is mediated by a separate population of interneurons (Kujirai et al., 1993; Ridding et al., 1995; Ziemann et al., 1996; Di Lazzaro et al., 1998, 2007; Sanger et al., 2001; Ilic et al., 2002). Transspinal-transcortical PAS decreases the amount of intracortical inhibition and increases the amount of intracortical facilitation (Fig. 2B). These neurophysiological changes are consistent with the decrease in intracortical inhibition and increase in intracortical facilitation following median or radial nerve stimulation (Aimonetti and Nielsen, 2001), and the MEP modulation when conditioned by transspinal stimulation at similar intervals (Knikou, 2014). After transspinal-transcortical PAS, the increase in MEP size at intensities ranging from 0.8 to 16
26 1.2 MEP resting threshold coincided with decreases in stimulus intensity corresponding to MEP threshold (Fig. 4A, Table 1). Increases in MEP amplitude likely reflect long-term potentiation (LTP)-like synaptic plasticity in neurons involved in modulation of motor cortex output, whereas changes of motor threshold are considered correlates of intrinsic plasticity (Delvendahl et al., 2012). Possible sources of changes in intracortical interneuronal circuits include modulation of interhemispheric connections by ipsilateral and contralateral thalamocortical circuits as a result of transspinal stimulation, and changes in interactions between I-waves (Ferbert et al., 1992; Di Lazzaro et al., 1999; Hanajima et al., 2002). Transspinal stimulation excites fibers at the spinal cord entry or at their exit from the spinal canal (Ladenbauer et al., 2010), generating action potentials that travel anti- and orthodromically along the posterior and anterior root fibers bilateral. Transspinal stimulation is associated with orthodromic excitation of motor axons and antidromic activation of muscle spindle afferents and all of their terminal branches leading to transynaptic excitation of motoneurons and interneurons close and far away from the stimulation site (Coburn, 1985; Maertens de Noordhout et al., 1988; Hunter and Ashby, 1994; Knikou, 2013; Gaunt et al., 2006; Ladenbauer et al., 2010). Stimulation of muscle afferents which terminate in the dorsal horn activates second order neurons in the ipsilateral dorsal spinocerebellar tract with bilateral projections to the brain hemispheres (Spanne and Jörntell, 2013). Hence, transspinal stimulation delivered before TMS at interstimulus intervals ranging from 10 to 15 ms ( ms) has the ability to affect cortical networks through the dorsal spinocerebellar tract. Cortical interneurons producing slow inhibitory or excitatory postsynaptic potentials are mainly distributed in layer II where the main sensory input to the motor cortex arrives through thalamocortical projections (Constantinople and Bruno, 17
27 2013). Therefore, somatosensory potentials induced by transspinal stimulation arrived at the cortex shortly before cortical neurons were depolarized by TMS. These potentials reinforced the TMS-induced descending volleys to corticospinal neurons so that a single TMS pulse induced a larger MEP, as well as directly affecting the cortical interneurons mediating cortical inhibition and facilitation. Transcortical-transspinal PAS did not affect the amount of intracortical inhibition or intracortical facilitation (Fig. 3B), and decreased the MEP size at an intensity of 1.3 MEP resting threshold without affecting motor threshold (Fig. 4B, Table 1). The decrease MEP size may be related to long-term depression-like synaptic plasticity of corticomotor neurons, and may be the result of changes between excitatory cortical neurons (Weise et al., 2013). Transcortical-transspinal and transspinal-transcortical PAS affect M-wave amplitudes when the M-waves recorded after PAS are normalized to MT before and not to that estimated after PAS. Additionally, both PAS protocols decrease the stimulation intensities needed to evoke M- waves at threshold, intermediate, and maximal amplitudes (Tables 2, 3). These changes verify that both PAS protocols affect the recruitment order of motor axons (Knikou, 2008). Decreases in the slope of the M-wave after transspinal-transcortical PAS suggest changes in the recruitment gain of the soleus motoneuron pool. In all subjects, a clear separation between Ia afferents and motor axons was possible, in that Ia afferent volleys from low to maximum intensities were evoked with a very small or completely absent M-wave. This implies that the soleus H-reflex reached its maximum amplitude before the antidromic motor volley collides with the orthodromic afferent volley (Knikou, 2008). Decreases in stimulation intensities needed to evoke H-reflexes at threshold, intermediate, and maximum amplitudes (Tables 2, 3) support the following notions: selective 18
28 changes in the ability of Ia afferents to depolarize the soleus motoneurons, altered excitability of Ia afferents, altered number of active motoneurons, or altered aggregate of spinal motoneurons on the subliminal fringe (Capaday and Stein, 1987; Knikou, 2008; Pierrot-Deseilligny and Burke, 2005). One may contend that the soleus H-reflex excitability changes after PAS were largely due to the altered recruitment gain of soleus motoneurons. However, this cannot be substantiated because sigmoid function results were obtained from each subject s H-reflex recruitment curves with actual stimulation intensities, and not normalized to the MT before or after PAS (Tables 2, 3). Potential sources of H-reflex excitability changes after PAS include modifications in the excitability profile of the motoneuron pool and properties of group Ia afferents. It is well known that the size of the monosynaptic reflex decreases during repetitive stimulation, and a frequency-dependent effect has been described both in the cat and human (Eccles and Rall, 1951; Crone and Nielsen, 1989) (Fig. 6). This type of reflex depression has been attributed to changes in the probability or amount of transmitter release at the synapse by the previously activated Ia afferents (Crone and Nielsen, 1989; Hultborn et al., 1996), and thus is presynaptic in origin. The soleus H-reflex size is also decreased upon paired stimulation pulses delivered at a constant stimulation frequency (Fig. 6). However, this depression does not characterize only the soleus H-reflex, since the M-wave size also decreases upon paired pulses (see inlet 4 in Fig. 6). Thus, this postactivation depression cannot be homosynaptic, and may involve interneurons such as Ib and Renshaw cells exerting postsynaptic inhibition. Transcortical-transspinal PAS decreases the soleus H-reflex postactivation depression but not the low-frequency depression (Fig. 6A), whereas transspinal-transcortical PAS has the exact 19
29 opposite effect (Fig. 6B). The same stimulation intensity after PAS evokes H-reflexes whose amplitude correspond to the derecruitment curve (see inlets 1-3 in Fig. 6), supporting the concept of altered recruitment of motor axons and Ia afferents after PAS, consistent with our findings of changes in the soleus M-wave and H-reflex recruitment curves. TMS at 0.9 MEP threshold intensities has been shown to decrease presynaptic inhibition of Ia afferents (Meunier and Pierrot-Deseilligny, 1998) and may therefore be involved in the decreased homosynaptic or postsynaptic depression in response to the PAS protocols. Alternatively, despite the lack of the direct evidence of transspinal stimulation on actions of presynaptic interneurons in humans, transspinal stimulation activates NMDA receptors which play an important role in the induction and maintenance of LTP-like plasticity (Stefan et al., 2002; Hunanyan et al., 2012). Limitations We did not use medium or high frequencies or pulse trains in our experiments to deliver PAS between M1 and spinal cord, although PAS-induced plasticity depends on the frequency and number of spike trains (Froemke and Dan, 2002; Froemke et al., 2006), because it is not feasible to pair TMS at medium or high frequencies. We chose a single 1-ms pulse duration at 0.1 Hz for both PAS protocols based on previous human and animal studies (Stefan et al., 2000; Hunanyan et al., 2012), and while this may have reduced the strength of plasticity, it cannot account for the neurophysiological differences we observed between the two PAS protocols. PAS was delivered for a prolonged time (40 min), but we did not perform the neurophysiological tests at different times after PAS termination, thus limiting our knowledge on the sustainability of neuroplasticity. Therefore further studies that will concentrate on examining the time course of the effects are 20
30 needed. Last, the extent to which similar associative plasticity can be observed in neurological disorders remains to be shown. These limitations are currently under investigation in our laboratory. Conclusion and future directions We show that pairing transspinal and transcortical stimulation produces both cortical and spinal PAS-mediated plasticity, but excitability changes (inhibition or facilitation) in the human brain and spinal cord are reversed based on the timing interval and functional network interactions between the two associated inputs. In opposition to the more traditional PAS technique (paired TMS and peripheral nerve stimulation) utilized in humans to produce PAS-induced plasticity, this new PAS paradigm has great translational impact because it can be used to alter intracortical circuits, corticospinal excitability, and properties of spinal motoneurons in people with cortical or spinal lesions. The latter warrants further tests for the development of targeted neural plasticity in this category of patients. 21
31 Figures Figure 1. Paired associative stimulation protocol. (A) Simplified illustration of the PAS protocol during which transspinal stimulation mediated volleys arrived at the cortex before the transcranial magnetic stimulation (TMS volley) delivered over the motor hot spot for the tibialis anterior (TA) muscle arrived at the corticospinal neurons. 22
32 (B) Simplified illustration of the PAS protocol during which corticospinal neurons activated via TMS arrived to the corticospinal neuron before spinal motoneurons were activated transynaptically by the transspinal stimulation. The interstimulus interval between paired pulses was customized for each subject. The interval allowed transspinal stimulation to affect cortical circuits before TMS-induced motor volleys arrived at the presynaptic terminals of corticospinal neurons or TMS-induced motor volleys to arrive at the presynaptic terminals of corticospinal neurons before transspinal stimulation evoked transynaptically depolarization in spinal motoneurons. (C) EMG recordings from the right TA muscle during the course of paired pulses show a clear separation of motor and transspinal evoked potentials (MEP, TEP) during transspinal-transcortical PAS (left sweeps), and summation of MEPs and TEPs during transcortical-transspinal PAS (right sweeps). 23
33 Figure 2. Intracortical measures before and after transspinal-transcortical PAS. Non-rectified waveform averages of conditioned tibialis anterior motor evoked potentials (TA MEPs) by subthreshold transcranial magnetic stimulation (TMS) from 2 subjects before (grey lines) and after (red lines) transspinal-transcortical PAS (A). The pool data plotted are normalized to the mean control MEP size before PAS (B). Error bars represent SEM. Analysis showed a significant effect of time on both intracortical inhibition and intracortical facilitation. *p < 0.05 compared with baseline (before PAS). 24
34 Figure 3. Intracortical measures before and after transcortical-transspinal PAS. Non-rectified waveform averages of conditioned tibialis anterior motor evoked potentials (TA MEPs) by subthreshold transcranial magnetic stimulation (TMS) from 2 subjects before (grey lines) and after (red lines) transcortical-transspinal PAS (A). The pool data plotted are normalized to the mean control MEP size before PAS (B). Error bars represent SEM. Analysis showed no significant effects of time on intracortical inhibition and intracortical facilitation. 25
35 Figure 4. Corticospinal excitability before and after PAS. Tibialis anterior motor evoked potentials (TA MEPs) recruitment curves from all subjects before ( ) and after ( ) transspinal-transcortical PAS (A) and transcorticaltransspinal PAS (B). The pool data are normalized to the maximal MEP size, are plotted in multiples of stimulation intensities corresponding to 50 % of the maximal MEP (MEPmax) both observed before PAS, and a sigmoid fit to the data is also shown. Analysis showed a significant effect of time for the transspinal-transcortical PAS with increased MEP sizes from 0.9 to 1.2 threshold, and decreased MEP sizes at 1.3 threshold for the transcorticaltransspinal PAS. 26
36 Figure 5. Spinal excitability before and after PAS. Soleus M-wave and H-reflex recruitment curves from all subjects before ( ) and after ( ) transspinal-transcortical PAS (A, C) and transcortical-transspinal PAS (B, D). The pool data illustrated in A-D are normalized to the homonymous maximal M-wave (Mmax), and are plotted in multiples of stimulation intensities corresponding to 50 % of the Mmax. The soleus H-reflexes recruitment curves assembled for stimulation intensities that H-reflexes were absent until they reached maximal amplitudes along with the sigmoid function fitted before ( ) and after ( )transspinal-transcortical PAS (E) and transcortical-transspinal PAS (F) are indicated with H-reflexes plotted against stimulation intensities that were grouped based on MT observed both before and after PAS. 27
37 Figure 6. Spinal inhibitory measures before and after PAS. Soleus H-reflexes sizes from all subjects before ( ) and after ( )transspinal-transcortical PAS (A) and transcortical-transspinal PAS (B) recorded at different stimulation frequencies and upon paired stimulation pulses at interstimulus intervals of 100 and 50 ms. The pool data are normalized to the maximal M-wave (Mmax). Error bars represent SEM. Analysis showed a significant effect of time for the soleus H-reflex postactivation depression (paired pulses) in the transcortical-transspinal PAS, and H-reflex low frequency dependent depression for the transspinal-transcortical PAS. *p < 0.05 compared with baseline (before PAS). Soleus H-reflexes recorded at 0.2 Hz before and after PAS at exactly the same stimulation intensities (Inlets 1 and 2) illustrate clearly that a shift in the recruitment order of motor axons and Ia afferents (Inlet 3) occurred after both PAS protocols. Soleus H-reflexes recorded upon paired pulses at 100 ms interstimulus interval (Inlet 4) illustrate clearly that postactivation depression occurs both in the H-reflex and M-wave upon paired pulses. 28
38 Tables Table 1. TA MEP sigmoid function parameters MEPmax (mvs) m slope 50 % MEPmax stimulus MEPth stimulus MEPmax stimulus Transspinaltranscortical PAS Before After p-value Transcorticaltransspinal PAS Before After p-value Results of predicted parameters from the sigmoid input output relation of non-normalized TA MEPs and stimulation intensities conducted for each subject separately and grouped based on time and paired-associative stimulation (PAS) protocol. Mean SEM. P-values shown are for before and after PAS comparisons. 29
Spinal Excitability Changes after Transspinal and Transcortical Paired Associative Stimulation in Humans
 City University of New York (CUNY) CUNY Academic Works Publications and Research College of Staten Island 1-16-217 Spinal Excitability Changes after Transspinal and Transcortical Paired Associative Stimulation
City University of New York (CUNY) CUNY Academic Works Publications and Research College of Staten Island 1-16-217 Spinal Excitability Changes after Transspinal and Transcortical Paired Associative Stimulation
Interactions Between Descending and Somatosensory Inputs in Humans
 City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 5-2015 Interactions Between Descending and Somatosensory Inputs in Humans Lisa Krivis
City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 5-2015 Interactions Between Descending and Somatosensory Inputs in Humans Lisa Krivis
Corticospinal Integration in Healthy Humans
 City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 6-2014 Corticospinal Integration in Healthy Humans Amanda Asmar Graduate Center, City
City University of New York (CUNY) CUNY Academic Works Dissertations, Theses, and Capstone Projects Graduate Center 6-2014 Corticospinal Integration in Healthy Humans Amanda Asmar Graduate Center, City
The Journal of Physiology Neuroscience
 J Physiol 591.19 (2013) pp 4903 4920 4903 The Journal of Physiology Neuroscience Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability David
J Physiol 591.19 (2013) pp 4903 4920 4903 The Journal of Physiology Neuroscience Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability David
Changes in intracortical excitability induced by stimulation of wrist afferents in man
 12359 Journal of Physiology (2001), 534.3, pp.891 902 891 Changes in intracortical excitability induced by stimulation of wrist afferents in man Jean-Marc Aimonetti and Jens Bo Nielsen * Laboratoire Développement
12359 Journal of Physiology (2001), 534.3, pp.891 902 891 Changes in intracortical excitability induced by stimulation of wrist afferents in man Jean-Marc Aimonetti and Jens Bo Nielsen * Laboratoire Développement
Water immersion modulates sensory and motor cortical excitability
 Water immersion modulates sensory and motor cortical excitability Daisuke Sato, PhD Department of Health and Sports Niigata University of Health and Welfare Topics Neurophysiological changes during water
Water immersion modulates sensory and motor cortical excitability Daisuke Sato, PhD Department of Health and Sports Niigata University of Health and Welfare Topics Neurophysiological changes during water
MOTOR EVOKED POTENTIALS AND TRANSCUTANEOUS MAGNETO-ELECTRICAL NERVE STIMULATION
 MOTOR EVOKED POTENTIAS AND TRANSCUTANEOUS MAGNETO-EECTRICA NERVE STIMUATION Hongguang iu, in Zhou 1 and Dazong Jiang Xian Jiaotong University, Xian, People s Republic of China 1 Shanxi Normal University,
MOTOR EVOKED POTENTIAS AND TRANSCUTANEOUS MAGNETO-EECTRICA NERVE STIMUATION Hongguang iu, in Zhou 1 and Dazong Jiang Xian Jiaotong University, Xian, People s Republic of China 1 Shanxi Normal University,
Spinal and Supraspinal Control of Reflexes: In health, under general anesthesia, and in Parkinson s disease. Jennifer C. Andrews
 Spinal and Supraspinal Control of Reflexes: In health, under general anesthesia, and in Parkinson s disease by Jennifer C. Andrews A thesis submitted in partial fulfillment of the requirements for the
Spinal and Supraspinal Control of Reflexes: In health, under general anesthesia, and in Parkinson s disease by Jennifer C. Andrews A thesis submitted in partial fulfillment of the requirements for the
Modulation of single motor unit discharges using magnetic stimulation of the motor cortex in incomplete spinal cord injury
 1 SHORT REPORT Division of Neuroscience and Psychological Medicine, Imperial College School of Medicine, Charing Cross Hospital, London W 8RF, UK H C Smith NJDavey D W Maskill P H Ellaway National Spinal
1 SHORT REPORT Division of Neuroscience and Psychological Medicine, Imperial College School of Medicine, Charing Cross Hospital, London W 8RF, UK H C Smith NJDavey D W Maskill P H Ellaway National Spinal
Paired-Pulse TMS to one Brain Region. Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation LEASE DO NOT COPY
 Paired-Pulse TMS to one Brain Region Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation Paired-Pulse Paradigms Sequential pulses applied to the same cortical region Variable
Paired-Pulse TMS to one Brain Region Joyce Gomes-Osman Research Fellow Berenson-Allen Center for Non-Invasive Stimulation Paired-Pulse Paradigms Sequential pulses applied to the same cortical region Variable
Doctoral Thesis. Modulation of Spinal Neural Circuits Induced by Corticospinal. Descending and Peripheral Afferent Inputs
 Doctoral Thesis Modulation of Spinal Neural Circuits Induced by Corticospinal Descending and Peripheral Afferent Inputs Shinji Kubota Division of Integrated Arts and Sciences Graduate School of Integrated
Doctoral Thesis Modulation of Spinal Neural Circuits Induced by Corticospinal Descending and Peripheral Afferent Inputs Shinji Kubota Division of Integrated Arts and Sciences Graduate School of Integrated
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Review Article A Review on Locomotor Training after Spinal Cord Injury: Reorganization of Spinal Neuronal Circuits and Recovery of Motor Function
 Hindawi Publishing Corporation Neural Plasticity Volume 2016, Article ID 1216258, 20 pages http://dx.doi.org/10.1155/2016/1216258 Review Article A Review on Locomotor Training after Spinal Cord Injury:
Hindawi Publishing Corporation Neural Plasticity Volume 2016, Article ID 1216258, 20 pages http://dx.doi.org/10.1155/2016/1216258 Review Article A Review on Locomotor Training after Spinal Cord Injury:
Nervous system. The main regulation mechanism of organism's functions
 Nervous system The main regulation mechanism of organism's functions Questions Neuron The reflex arc The nervous centers Properties of the nervous centers The general principles of coordination Inhibition
Nervous system The main regulation mechanism of organism's functions Questions Neuron The reflex arc The nervous centers Properties of the nervous centers The general principles of coordination Inhibition
Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle
 J Physiol (2003), 550.3, pp. 933 946 DOI: 10.1113/jphysiol.2003.042606 The Physiological Society 2003 www.jphysiol.org Differential modulation of intracortical inhibition in human motor cortex during selective
J Physiol (2003), 550.3, pp. 933 946 DOI: 10.1113/jphysiol.2003.042606 The Physiological Society 2003 www.jphysiol.org Differential modulation of intracortical inhibition in human motor cortex during selective
Using Transcranial magnetic stimulation to improve our understanding of Transverse Myelitis
 Using Transcranial magnetic stimulation to improve our understanding of Transverse Myelitis Kathy Zackowski, PhD, OTR Kennedy Krieger Institute Johns Hopkins University School of Medicine TMS (transcranial
Using Transcranial magnetic stimulation to improve our understanding of Transverse Myelitis Kathy Zackowski, PhD, OTR Kennedy Krieger Institute Johns Hopkins University School of Medicine TMS (transcranial
Locomotor training improves reciprocal and nonreciprocal inhibitory control of soleus motoneurons in human spinal cord injury
 J Neurophysiol 113: 27 26, 21. First published January 21, 21; doi:1.112/jn.872.21. Locomotor improves reciprocal and nonreciprocal inhibitory control of soleus motoneurons in human spinal cord injury
J Neurophysiol 113: 27 26, 21. First published January 21, 21; doi:1.112/jn.872.21. Locomotor improves reciprocal and nonreciprocal inhibitory control of soleus motoneurons in human spinal cord injury
Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley
 (2002), 542.3, pp. 963 976 DOI: 10.1113/jphysiol.2002.021683 The Physiological Society 2002 www.jphysiol.org Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated
(2002), 542.3, pp. 963 976 DOI: 10.1113/jphysiol.2002.021683 The Physiological Society 2002 www.jphysiol.org Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated
Cutaneomuscular reflexes recorded from the lower limb
 Journal of Physiology (1995), 487.1, pp.237-242 376 237 Cutaneomuscular reflexes recorded from the lower limb in man during different tasks J. Gibbs, Linda M. Harrison * and J. A. Stephens Department of
Journal of Physiology (1995), 487.1, pp.237-242 376 237 Cutaneomuscular reflexes recorded from the lower limb in man during different tasks J. Gibbs, Linda M. Harrison * and J. A. Stephens Department of
Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans
 11911 Journal of Physiology (2001), 533.3, pp.903 919 903 Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans Guillaume Nicolas, Véronique Marchand-Pauvert,
11911 Journal of Physiology (2001), 533.3, pp.903 919 903 Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans Guillaume Nicolas, Véronique Marchand-Pauvert,
Compound Action Potential, CAP
 Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Physiology. D. Gordon E. Robertson, PhD, FCSB. Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada
 Electromyography: Physiology D. Gordon E. Robertson, PhD, FCSB Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada Nervous System Central Nervous System (cerebellum,
Electromyography: Physiology D. Gordon E. Robertson, PhD, FCSB Biomechanics Laboratory, School of Human Kinetics, University of Ottawa, Ottawa, Canada Nervous System Central Nervous System (cerebellum,
Daina S. E. Dickins, 1 Marc R. Kamke, 1 and Martin V. Sale 1,2. 1. Introduction
 Hindawi Volume 217, Article ID 831949, 14 pages https://doi.org/1.1155/217/831949 Research Article Corticospinal Plasticity in Bilateral Primary Motor Cortices Induced by Paired Associative Stimulation
Hindawi Volume 217, Article ID 831949, 14 pages https://doi.org/1.1155/217/831949 Research Article Corticospinal Plasticity in Bilateral Primary Motor Cortices Induced by Paired Associative Stimulation
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
NEURAL CONTROL OF ECCENTRIC AND POST- ECCENTRIC MUSCLE ACTIONS
 NEURAL CONTROL OF ECCENTRIC AND POST- ECCENTRIC MUSCLE ACTIONS 1, 2 Daniel Hahn, 1 Ben W. Hoffman, 1 Timothy J. Carroll and 1 Andrew G. Cresswell 1 School of Human Movement Studies, University of Queensland,
NEURAL CONTROL OF ECCENTRIC AND POST- ECCENTRIC MUSCLE ACTIONS 1, 2 Daniel Hahn, 1 Ben W. Hoffman, 1 Timothy J. Carroll and 1 Andrew G. Cresswell 1 School of Human Movement Studies, University of Queensland,
Primary motor cortical metaplasticity induced by priming over the supplementary motor area
 J Physiol 587.20 (2009) pp 4845 4862 4845 Primary motor cortical metaplasticity induced by priming over the supplementary motor area Masashi Hamada 1, Ritsuko Hanajima 1, Yasuo Terao 1,ShingoOkabe 1, Setsu
J Physiol 587.20 (2009) pp 4845 4862 4845 Primary motor cortical metaplasticity induced by priming over the supplementary motor area Masashi Hamada 1, Ritsuko Hanajima 1, Yasuo Terao 1,ShingoOkabe 1, Setsu
Induction of plasticity in the human motor cortex by paired associative stimulation
 Brain (2000), 123, 572 584 Induction of plasticity in the human motor cortex by paired associative stimulation Katja Stefan, 1 Erwin Kunesch, 1 Leonardo G. Cohen, 2 Reiner Benecke 1 and Joseph Classen
Brain (2000), 123, 572 584 Induction of plasticity in the human motor cortex by paired associative stimulation Katja Stefan, 1 Erwin Kunesch, 1 Leonardo G. Cohen, 2 Reiner Benecke 1 and Joseph Classen
HEAD AND NECK PART 2
 HEAD AND NECK PART 2 INTEGRATED CURRICULUM = Integrate Basic Science and Clinical Training 1- ENT PATIENT EXAM IN ICS COURSE - Today and next week - Review/Preview Anatomy underlying ENT exam 2- NEUROANATOMY/NEUROLOGY
HEAD AND NECK PART 2 INTEGRATED CURRICULUM = Integrate Basic Science and Clinical Training 1- ENT PATIENT EXAM IN ICS COURSE - Today and next week - Review/Preview Anatomy underlying ENT exam 2- NEUROANATOMY/NEUROLOGY
Beyond Vanilla LTP. Spike-timing-dependent-plasticity or STDP
 Beyond Vanilla LTP Spike-timing-dependent-plasticity or STDP Hebbian learning rule asn W MN,aSN MN Δw ij = μ x j (v i - φ) learning threshold under which LTD can occur Stimulation electrode Recording electrode
Beyond Vanilla LTP Spike-timing-dependent-plasticity or STDP Hebbian learning rule asn W MN,aSN MN Δw ij = μ x j (v i - φ) learning threshold under which LTD can occur Stimulation electrode Recording electrode
Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes. Prof Richard Ribchester
 Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes Prof Richard Ribchester René Descartes Cogito, ergo sum The 21st century still holds many challenges to Neuroscience and Pharmacology
Neuroscience with Pharmacology 2 Functions and Mechanisms of Reflexes Prof Richard Ribchester René Descartes Cogito, ergo sum The 21st century still holds many challenges to Neuroscience and Pharmacology
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
CENTRAL CONTROL OF AN INSECT SENSORY INTERNEURONE
 J. Exp. Biol. (1970), S3, 137-145 With 4 text-figures Printed in Great Britain CENTRAL CONTROL OF AN INSECT SENSORY INTERNEURONE BY J. M. MCKAY* Department of Zoology, Makerere University College, Kampala,
J. Exp. Biol. (1970), S3, 137-145 With 4 text-figures Printed in Great Britain CENTRAL CONTROL OF AN INSECT SENSORY INTERNEURONE BY J. M. MCKAY* Department of Zoology, Makerere University College, Kampala,
Short latency inhibition of human hand motor cortex by somatosensory input from the hand
 Keywords: 9995 Journal of Physiology (2000), 523.2, pp. 503 513 503 Short latency inhibition of human hand motor cortex by somatosensory input from the hand H. Tokimura *, V. Di Lazzaro, Y. Tokimura *,
Keywords: 9995 Journal of Physiology (2000), 523.2, pp. 503 513 503 Short latency inhibition of human hand motor cortex by somatosensory input from the hand H. Tokimura *, V. Di Lazzaro, Y. Tokimura *,
συν together απτειν to clasp 2h Neuroscience with Pharmacology Functions and Mechanisms of Reflexes Cogito, ergo sum ( I think therefore I am ) Down
 2h Neuroscience with Pharmacology Functions and Mechanisms of Reflexes Neuroscience is studied at many different levels: from brain, to system, network, neurone, synapse, and molecule... Top Up Down René
2h Neuroscience with Pharmacology Functions and Mechanisms of Reflexes Neuroscience is studied at many different levels: from brain, to system, network, neurone, synapse, and molecule... Top Up Down René
Trans-spinal direct current stimulation: a novel tool to promote plasticity in humans
 Trans-spinal direct current stimulation: a novel tool to promote plasticity in humans Jean-Charles Lamy, PhD Brain and Spine Institute, Paris 1 Background Grecco et al., J Neuroresto, 2015 2 Background:
Trans-spinal direct current stimulation: a novel tool to promote plasticity in humans Jean-Charles Lamy, PhD Brain and Spine Institute, Paris 1 Background Grecco et al., J Neuroresto, 2015 2 Background:
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit. Supplementary Information. Adam W. Hantman and Thomas M.
 Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Chapter 17 Nervous System
 Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Introduction to TMS Transcranial Magnetic Stimulation
 Introduction to TMS Transcranial Magnetic Stimulation Lisa Koski, PhD, Clin Psy TMS Neurorehabilitation Lab Royal Victoria Hospital 2009-12-14 BIC Seminar, MNI Overview History, basic principles, instrumentation
Introduction to TMS Transcranial Magnetic Stimulation Lisa Koski, PhD, Clin Psy TMS Neurorehabilitation Lab Royal Victoria Hospital 2009-12-14 BIC Seminar, MNI Overview History, basic principles, instrumentation
(Received 10 April 1956)
 446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC
 1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
1 2 1 3 Supplementary figure 1: LII/III GIN-cells show morphological characteristics of MC 4 5 6 7 (a) Reconstructions of LII/III GIN-cells with somato-dendritic compartments in orange and axonal arborizations
Lecture VIII. The Spinal Cord, Reflexes and Brain Pathways!
 Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Physiology of synapses and receptors
 Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Physiology of synapses and receptors Dr Syed Shahid Habib Professor & Consultant Clinical Neurophysiology Dept. of Physiology College of Medicine & KKUH King Saud University REMEMBER These handouts will
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Cellular Bioelectricity
 ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture 24 Thursday, March 6, 2014 8. NEURAL ELECTROPHYSIOLOGY We will look at: Structure of the nervous system Sensory transducers and neurons Neural coding
ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture 24 Thursday, March 6, 2014 8. NEURAL ELECTROPHYSIOLOGY We will look at: Structure of the nervous system Sensory transducers and neurons Neural coding
The effects of cryotherapy on quadriceps corticospinal excitability in patients with patellofemoral pain
 The University of Toledo The University of Toledo Digital Repository Theses and Dissertations 2013 The effects of cryotherapy on quadriceps corticospinal excitability in patients with patellofemoral pain
The University of Toledo The University of Toledo Digital Repository Theses and Dissertations 2013 The effects of cryotherapy on quadriceps corticospinal excitability in patients with patellofemoral pain
purely monosynaptic e.p.s.p. is a prerequisite for the validity of the method. Experimental
 J. Physiol. (1987), 389, pp. 729-756 729 With 8 text-figures Printed in Great Britain ASSESSING CHANGES IN PRESYNAPTIC INHIBITION OF I a FIBRES: A STUDY IN MAN AND THE CAT BY H. HULTBORN*, S. MEUNIER,
J. Physiol. (1987), 389, pp. 729-756 729 With 8 text-figures Printed in Great Britain ASSESSING CHANGES IN PRESYNAPTIC INHIBITION OF I a FIBRES: A STUDY IN MAN AND THE CAT BY H. HULTBORN*, S. MEUNIER,
Interaction of paired cortical and peripheral nerve stimulation on human motor neurons
 Exp Brain Res (28) 188:13 21 DOI 1.17/s221-8-1334-8 RESEARCH ARTICLE Interaction of paired cortical and peripheral nerve stimulation on human motor neurons David E. Poon Francois D. Roy Monica A. Gorassini
Exp Brain Res (28) 188:13 21 DOI 1.17/s221-8-1334-8 RESEARCH ARTICLE Interaction of paired cortical and peripheral nerve stimulation on human motor neurons David E. Poon Francois D. Roy Monica A. Gorassini
Practical. Paired-pulse on two brain regions
 Practical Paired-pulse on two brain regions Paula Davila Pérez, MD Berenson-Allen Center for Noninvasive Brain Stimulation Beth Israel Deaconess Medical Center Harvard Medical School Plans for the afternoon
Practical Paired-pulse on two brain regions Paula Davila Pérez, MD Berenson-Allen Center for Noninvasive Brain Stimulation Beth Israel Deaconess Medical Center Harvard Medical School Plans for the afternoon
Neurophysiological Basis of TMS Workshop
 Neurophysiological Basis of TMS Workshop Programme 31st March - 3rd April 2017 Sobell Department Institute of Neurology University College London 33 Queen Square London WC1N 3BG Brought to you by 31 March
Neurophysiological Basis of TMS Workshop Programme 31st March - 3rd April 2017 Sobell Department Institute of Neurology University College London 33 Queen Square London WC1N 3BG Brought to you by 31 March
The Nervous System S P I N A L R E F L E X E S
 The Nervous System S P I N A L R E F L E X E S Reflexes Rapid, involuntary, predictable motor response to a stimulus Spinal Reflexes Spinal somatic reflexes Integration center is in the spinal cord Effectors
The Nervous System S P I N A L R E F L E X E S Reflexes Rapid, involuntary, predictable motor response to a stimulus Spinal Reflexes Spinal somatic reflexes Integration center is in the spinal cord Effectors
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Lecture 22: A little Neurobiology
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 22: A little Neurobiology http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Nervous system development Part of the ectoderm
Motor systems.... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington
 Motor systems... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington 1 Descending pathways: CS corticospinal; TS tectospinal; RS reticulospinal; VS
Motor systems... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington 1 Descending pathways: CS corticospinal; TS tectospinal; RS reticulospinal; VS
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal:
 Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Suppression of voluntary motor activity revealed using
 MS 2226, pp. 223-235 Journal of Physiology (1994), 477.2 223 Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man Nick J. Davey, Patricia
MS 2226, pp. 223-235 Journal of Physiology (1994), 477.2 223 Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man Nick J. Davey, Patricia
The sites of neural adaptation induced by resistance training in humans
 (2002), 544.2, pp. 641 652 DOI: 10.1113/jphysiol.2002.024463 The Physiological Society 2002 www.jphysiol.org The sites of neural adaptation induced by resistance training in humans Timothy J. Carroll,
(2002), 544.2, pp. 641 652 DOI: 10.1113/jphysiol.2002.024463 The Physiological Society 2002 www.jphysiol.org The sites of neural adaptation induced by resistance training in humans Timothy J. Carroll,
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Nervous System. The Peripheral Nervous System Agenda Review of CNS v. PNS PNS Basics Cranial Nerves Spinal Nerves Reflexes Pathways
 Nervous System Agenda Review of CNS v. PNS PNS Basics Cranial Nerves Spinal Nerves Sensory Motor Review of CNS v. PNS Central nervous system (CNS) Brain Spinal cord Peripheral nervous system (PNS) All
Nervous System Agenda Review of CNS v. PNS PNS Basics Cranial Nerves Spinal Nerves Sensory Motor Review of CNS v. PNS Central nervous system (CNS) Brain Spinal cord Peripheral nervous system (PNS) All
Fig Cervical spinal nerves. Cervical enlargement C7. Dural sheath. Subarachnoid space. Thoracic. Spinal cord Vertebra (cut) spinal nerves
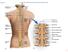 Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Nerve Conduction Studies NCS
 Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE
 TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
Nerve Conduction Studies NCS
 Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nerve Conduction Studies NCS Nerve conduction studies are an essential part of an EMG examination. The clinical usefulness of NCS in the diagnosis of diffuse and local neuropathies has been thoroughly
Nervous System: Spinal Cord and Spinal Nerves (Chapter 13)
 Nervous System: Spinal Cord and Spinal Nerves (Chapter 13) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Sources for figures and content: Marieb,
Nervous System: Spinal Cord and Spinal Nerves (Chapter 13) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Eastern Campus Primary Sources for figures and content: Marieb,
Trans spinal direct current stimulation modifies spinal cord excitability through synaptic and axonal mechanisms
 City University of New York (CUNY) CUNY Academic Works Publications and Research College of Staten Island September 4 Trans spinal direct current stimulation modifies spinal cord excitability through synaptic
City University of New York (CUNY) CUNY Academic Works Publications and Research College of Staten Island September 4 Trans spinal direct current stimulation modifies spinal cord excitability through synaptic
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM
 STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
Audit and Compliance Department 1
 Introduction to Intraoperative Neuromonitoring An intro to those squiggly lines Kunal Patel MS, CNIM None Disclosures Learning Objectives History of Intraoperative Monitoring What is Intraoperative Monitoring
Introduction to Intraoperative Neuromonitoring An intro to those squiggly lines Kunal Patel MS, CNIM None Disclosures Learning Objectives History of Intraoperative Monitoring What is Intraoperative Monitoring
FORSKNING INDENFOR NEUROREHABILITERING PÅ INSTITUT FOR MEDICIN OG SUNDHEDSTEKNOLOGI, AAU KIM DREMSTRUP AND NATALIE MRACHACZ-KERSTING
 FORSKNING INDENFOR NEUROREHABILITERING PÅ INSTITUT FOR MEDICIN OG SUNDHEDSTEKNOLOGI, AAU KIM DREMSTRUP AND NATALIE MRACHACZ-KERSTING Brain activity to control external devices and activities Brain-Computer-Interface
FORSKNING INDENFOR NEUROREHABILITERING PÅ INSTITUT FOR MEDICIN OG SUNDHEDSTEKNOLOGI, AAU KIM DREMSTRUP AND NATALIE MRACHACZ-KERSTING Brain activity to control external devices and activities Brain-Computer-Interface
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon
 Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
The Journal of Physiology
 J Physiol. (217) pp 1 16 1 Paired motor cortex and cervical epidural electrical stimulation timed to converge in the spinal cord promotes lasting increases in motor responses Asht M. Mishra 1,AjayPal 1,DishaGupta
J Physiol. (217) pp 1 16 1 Paired motor cortex and cervical epidural electrical stimulation timed to converge in the spinal cord promotes lasting increases in motor responses Asht M. Mishra 1,AjayPal 1,DishaGupta
Muscle fatigue changes cutaneous suppression of propriospinal drive to human upper limb muscles
 J Physiol 580.1 (2007) pp 211 223 211 Muscle fatigue changes cutaneous suppression of propriospinal drive to human upper limb muscles P. G. Martin, S. C. Gandevia and J. L. Taylor Prince of Wales Medical
J Physiol 580.1 (2007) pp 211 223 211 Muscle fatigue changes cutaneous suppression of propriospinal drive to human upper limb muscles P. G. Martin, S. C. Gandevia and J. L. Taylor Prince of Wales Medical
NEURO-MS TMS. Diagnostic Monophasic Magnetic Stimulator
 NEURO-MS Diagnostic Monophasic Magnetic Stimulator Diagnostics of neurological disorders Powerful monophasic stimulus Ergonomic and lightweight coils of different shapes and sizes Configurations for single
NEURO-MS Diagnostic Monophasic Magnetic Stimulator Diagnostics of neurological disorders Powerful monophasic stimulus Ergonomic and lightweight coils of different shapes and sizes Configurations for single
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
INFLUENCE OF SI ON INTERHEMISPHERIC INHIBITION
 INFLUENCE OF SI ON INTERHEMISPHERIC INHIBITION INFLUENCE OF PRIMARY SOMATOSENSORY CORTEX ON INTERHEMISPHERIC INHIBITION BY CHRISTOPHER M. ZAPALLOW, B.SC. A Thesis Submitted to the School of Graduate Studies
INFLUENCE OF SI ON INTERHEMISPHERIC INHIBITION INFLUENCE OF PRIMARY SOMATOSENSORY CORTEX ON INTERHEMISPHERIC INHIBITION BY CHRISTOPHER M. ZAPALLOW, B.SC. A Thesis Submitted to the School of Graduate Studies
Spike-Timing-Dependent Plasticity in Lower-Limb Motoneurons after Human Spinal Cord Injury
 Articles in PresS. J Neurophysiol (May 3, 2017). doi:10.1152/jn.00111.2017 1 2 Spike-Timing-Dependent Plasticity in Lower-Limb Motoneurons after Human Spinal Cord Injury 3 4 5 M.A. Urbin, Recep A. Ozdemir,
Articles in PresS. J Neurophysiol (May 3, 2017). doi:10.1152/jn.00111.2017 1 2 Spike-Timing-Dependent Plasticity in Lower-Limb Motoneurons after Human Spinal Cord Injury 3 4 5 M.A. Urbin, Recep A. Ozdemir,
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Non-therapeutic and investigational uses of non-invasive brain stimulation
 Non-therapeutic and investigational uses of non-invasive brain stimulation Robert Chen, MA, MBBChir, MSc, FRCPC Catherine Manson Chair in Movement Disorders Professor of Medicine (Neurology), University
Non-therapeutic and investigational uses of non-invasive brain stimulation Robert Chen, MA, MBBChir, MSc, FRCPC Catherine Manson Chair in Movement Disorders Professor of Medicine (Neurology), University
Memory Systems II How Stored: Engram and LTP. Reading: BCP Chapter 25
 Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
Memory Systems II How Stored: Engram and LTP Reading: BCP Chapter 25 Memory Systems Learning is the acquisition of new knowledge or skills. Memory is the retention of learned information. Many different
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Reflexes. Handout on The Basic Reflex Arc and Stretch and Tendon Reflexes. -55 mv -70 mv EPSP. By Noel Ways
 Reflexes Handout on The Basic Reflex Arc and Stretch and Tendon Reflexes By Noel Ways Basic Reflex Arch 2. : s are always unipolar and will conduct and impulse to a control center. In this case the control
Reflexes Handout on The Basic Reflex Arc and Stretch and Tendon Reflexes By Noel Ways Basic Reflex Arch 2. : s are always unipolar and will conduct and impulse to a control center. In this case the control
Long lasting effects of rtms and associated peripheral sensory input on MEPs, SEPs and transcortical reflex excitability in humans
 Journal of Physiology (2002), 540.1, pp. 367 376 DOI: 10.1113/jphysiol.2001.013504 The Physiological Society 2002 www.jphysiol.org Long lasting effects of rtms and associated peripheral sensory input on
Journal of Physiology (2002), 540.1, pp. 367 376 DOI: 10.1113/jphysiol.2001.013504 The Physiological Society 2002 www.jphysiol.org Long lasting effects of rtms and associated peripheral sensory input on
Resistant Against De-depression: LTD-Like Plasticity in the Human Motor Cortex Induced by Spaced ctbs
 Cerebral Cortex July 2015;25:1724 1734 doi:10.1093/cercor/bht353 Advance Access publication January 31, 2014 Resistant Against De-depression: LTD-Like Plasticity in the Human Motor Cortex Induced by Spaced
Cerebral Cortex July 2015;25:1724 1734 doi:10.1093/cercor/bht353 Advance Access publication January 31, 2014 Resistant Against De-depression: LTD-Like Plasticity in the Human Motor Cortex Induced by Spaced
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Paired stimulation to enhance spared circuit function after SCI
 Paired stimulation to enhance spared circuit function after SCI TMS Noam Y. Harel, MD, PhD James J. Peters VA Medical Center Icahn School of Medicine at Mt. Sinai CES Disclosures No financial or other
Paired stimulation to enhance spared circuit function after SCI TMS Noam Y. Harel, MD, PhD James J. Peters VA Medical Center Icahn School of Medicine at Mt. Sinai CES Disclosures No financial or other
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Neuroscience Letters
 Neuroscience Letters 529 (2012) 80 85 Contents lists available at SciVerse ScienceDirect Neuroscience Letters j ourna l ho me p ag e: www.elsevier.com/locate/neulet Loss of short-latency afferent inhibition
Neuroscience Letters 529 (2012) 80 85 Contents lists available at SciVerse ScienceDirect Neuroscience Letters j ourna l ho me p ag e: www.elsevier.com/locate/neulet Loss of short-latency afferent inhibition
Neural Basis of Motor Control. Chapter 4
 Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Neural Basis of Motor Control Chapter 4 Neurological Perspective A basic understanding of the physiology underlying the control of voluntary movement establishes a more comprehensive appreciation and awareness
Nerve. (2) Duration of the stimulus A certain period can give response. The Strength - Duration Curve
 Nerve Neuron (nerve cell) is the structural unit of nervous system. Nerve is formed of large numbers of nerve fibers. Types of nerve fibers Myelinated nerve fibers Covered by myelin sheath interrupted
Nerve Neuron (nerve cell) is the structural unit of nervous system. Nerve is formed of large numbers of nerve fibers. Types of nerve fibers Myelinated nerve fibers Covered by myelin sheath interrupted
Cranial Nerves and Spinal Cord Flashcards
 1. Name the cranial nerves and their Roman numeral. 2. What is Cranial Nerve I called, and what does it 3. Scientists who are trying to find a way to make neurons divide to heal nerve injuries often study
1. Name the cranial nerves and their Roman numeral. 2. What is Cranial Nerve I called, and what does it 3. Scientists who are trying to find a way to make neurons divide to heal nerve injuries often study
Ameen Alsaras. Ameen Alsaras. Mohd.Khatatbeh
 9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Timing and the cerebellum (and the VOR) Neurophysiology of systems 2010
 Timing and the cerebellum (and the VOR) Neurophysiology of systems 2010 Asymmetry in learning in the reverse direction Full recovery from UP using DOWN: initial return to naïve values within 10 minutes,
Timing and the cerebellum (and the VOR) Neurophysiology of systems 2010 Asymmetry in learning in the reverse direction Full recovery from UP using DOWN: initial return to naïve values within 10 minutes,
POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS
 J. exp. Biol. (1980), 85, 343-347 343 With a figures Printed in Great Britain POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS BY J. Y. KUWADA, G. HAGIWARA AND J. J. WINE
J. exp. Biol. (1980), 85, 343-347 343 With a figures Printed in Great Britain POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS BY J. Y. KUWADA, G. HAGIWARA AND J. J. WINE
Riluzole does not have an acute effect on motor thresholds and the intracortical excitability in amyotrophic lateral sclerosis
 J Neurol (1999) 246 [Suppl 3]: III/22 III/26 Steinkopff Verlag 1999 Martin Sommer Frithjof Tergau Stephan Wischer Carl-D. Reimers Wolfgang Beuche Walter Paulus Riluzole does not have an acute effect on
J Neurol (1999) 246 [Suppl 3]: III/22 III/26 Steinkopff Verlag 1999 Martin Sommer Frithjof Tergau Stephan Wischer Carl-D. Reimers Wolfgang Beuche Walter Paulus Riluzole does not have an acute effect on
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Gross Anatomy of Lower Spinal Cord
 Chapter 13 Spinal Cord, Spinal Nerves and Somatic Reflexes Spinal cord Spinal nerves Somatic reflexes Gross Anatomy of Lower Spinal Cord Meninges of Vertebra & Spinal Cord Spina Bifida Congenital defect
Chapter 13 Spinal Cord, Spinal Nerves and Somatic Reflexes Spinal cord Spinal nerves Somatic reflexes Gross Anatomy of Lower Spinal Cord Meninges of Vertebra & Spinal Cord Spina Bifida Congenital defect
CONTENTS. Foreword George H. Kraft. Henry L. Lew
 EVOKED POTENTIALS Foreword George H. Kraft xi Preface Henry L. Lew xiii Overview of Artifact Reduction and Removal in Evoked Potential and Event-Related Potential Recordings 1 Martin R. Ford, Stephen Sands,
EVOKED POTENTIALS Foreword George H. Kraft xi Preface Henry L. Lew xiii Overview of Artifact Reduction and Removal in Evoked Potential and Event-Related Potential Recordings 1 Martin R. Ford, Stephen Sands,
LESSON 3.3 WORKBOOK. Why does applying pressure relieve pain?
 Postsynaptic potentials small changes in voltage (membrane potential) due to the binding of neurotransmitter. Receptor-gated ion channels ion channels that open or close in response to the binding of a
Postsynaptic potentials small changes in voltage (membrane potential) due to the binding of neurotransmitter. Receptor-gated ion channels ion channels that open or close in response to the binding of a
