UROLOGY NSSG (Lancs & South Cumbria)
|
|
|
- Peregrine Gibbs
- 5 years ago
- Views:
Transcription
1 UROLOGY NSSG (Lancs & South Cumbria) Constitution and Terms of Reference 2015 Agreements: Mr Mohan Pillai Chair, Urology NSSG (Lancs & South Cumbria) Date: 4 June 2015 Urology NSSG Date: 4 June 2015 Dr Gerry Skailes Clinical Lead for Cancer GML&SC SCN Date: 10 July 2015
2 INTRODUCTION The Urology NSSG is a multi-professional group made up of health professionals from organisations across the Lancashire & South Cumbria Cancer Network covering a population of 1.6 million. This document outlines the Urology NSSG Constitution and Terms of Reference and will be reviewed on an annual basis. NETWORK CONFIGURATION OF TEAMS Measure 14-1C-101 Diagnostic / Local MDTs and Referring catchment population Referrals and diagnostic assessment and basic surgical services will be performed within each of the four local cancer units. Network Specialist MDT Complex pelvic surgery is delivered at the Royal Blackburn Hospital and Royal Preston Hospital sites. Each of the four local cancer units also manage patients who are too frail or have serious co-existing disease or advanced metastatic disease making them unsuitable for referral to the Specialist Teams (although this decision will be made after a discussion at the Network Urology MDT). Patients are referred to the Network MDT when a kidney tumour has invaded the inferior vena cava or where there is thrombus extension to the heart. These patients may be referred to specialist cardiac centres for treatment eg University Hospital of South Manchester. Local and Specialist Teams Trust Local Diagnostic Teams/MDTs Lead Clinician Specialist MDT Hospital Site and Trust Complex pelvic surgery undertaken across two sites: CCGs Population Lancashire Teaching Hospitals Foundation Trust MDT location: Royal Preston Hospital Lead Clinician: Mr Pradip Javle Consultant Urological Surgeon MDT frequency: weekly Royal Preston Hospital part of Lancashire Teaching Hospitals NHS Trust (patients from Central Lancs, Blackpool, North Lancs (Fylde & Wyre) Video-conference MDT: weekly Population: 1.6m Greater Preston Chorley & South Ribble 212, ,686 Blackpool, Fylde & Wyre Hospitals Trust MDT location: Blackpool Victoria Hospital Lead Clinician: Mr Naeem Saghir Consultant Urological Surgeon Blackpool Fylde/Wyre 172, ,372 MDT frequency: weekly East Lancashire Hospitals Trust MDT location: Royal Blackburn Hospital Lead Clinician Mr Guy Wemyss-Holden Consultant Urological Surgeon MDT frequency: weekly Royal Blackburn Hospital part of East Lancashire Hospitals NHS Trust (patients from Cumbria (South Lakes), North Lancs (Lancaster), East Lancs and Blackburn with Darwen) Video Conference MDT: weekly Population: 1.6m Blackburn with Darwen East Lancashire 169, ,435 University of Morecambe Bay Hospitals Trust MDT location: Royal Lancaster Infirmary Lead Clinicians Mr Ashutosh Jain Consultant Urological Surgeon Cumbria (Furness and South Lakeland) North Lancashire (Lancaster) 176, ,537 MDT frequency: weekly 2
3 Supranetwork MDT for penile cancer: Referring Networks/Urology MDTs Catchment Population Penile Cancer Supranetwork MDT Greater Manchester & Cheshire Local urology MDTs 3.125m Total Catchment Population Merseyside & Cheshire Local urology MDTs 2.3m Lancashire & South Cumbria - Local MDTs BFWHT Blackpool Victoria Hospital ELHT Royal Blackburn Hospital LTHT Royal Preston Hospital UHMBT - Royal Lancaster Infirmary 1.6m Hosted by Christie Hospitals NHS Trust 7,725,000 North Wales Local Urology MDTs 0.6m Supranetwork MDT for testicular cancer Referring Networks/Urology MDTs Catchment Population Testicular Cancer Supranetwork MDT Total Catchment Population Greater Manchester & Cheshire Local Urology MDTs 3.125m Lancashire & South Cumbria Local urology MDTs BFWHT Blackpool Victoria Hospital ELHT Royal Blackburn Hospital LTHT Royal Preston Hospital UHMBT - Royal Lancaster Infirmary 1.6m Hosted by Christie Hospitals NHS Trust 5,425,000 North Wales Local Urology MDTs 0.6m The above arrangements were agreed as part of the LSCCN Urology IOG Action Plan (2004) which was agreed by all the constituent organisations of the Network and agreed by the Cancer Action Team in
4 TERMS OF REFERENCE Membership Measure 14-1C-102 Mr Mohan Pillai (Chair) Consultant Urological Surgeon ELHT Mr Guy Wemyss-Holden Consultant Urological Surgeon (MDT LC) ELHT Mr Mohammad Masaarane Consultant Urological Surgeon ELHT Dr Santhi Kumar Consultant Histopathologist ELHT Dr Tom Newton Consultant Radiologist ELHT Ms Deborah Dobson Urology Nurse Specialist ELHT Ms Deborah Hesketh Urology Nurse Specialist ELHT Mr Pradip Javle Consultant Urological Surgeon (MDT LC) LTHT Mr Shyam Matanhelia Consultant Urological Surgeon LTHT Miss Rosie Blades Consultant Urological Surgeon LTHT Dr Sadathulla Sharief Consultant Histopathologist LTHT Dr Susan Cox Consultant Radiologist LTHT Dr Alison Birtle Consultant Oncologist LTHT Dr Omi Parikh (Vice Chair) Consultant Oncologist LTHT Dr Marcus Wise Consultant Oncologist LTHT Mrs Stephanie Keenan Urology Nurse Specialist LTHT Ms Jennifer Herdman Urology Nurse Specialist LTHT Mr Naeem Saghir Consultant Urological Surgeon (MDT LC) BTHT Mr Nasir Khan Consultant Urological Surgeon BTHT Mr Robert Saul Urology Nurse Specialist BTHT Ms Melanie Fluss Urology Nurse Specialist BTHT Ms Helen Bright Urology Nurse Specialist BTHT Mr Ashutosh Jain Consultant Urological Surgeon (MDT LC) UHMBT Mr Colin Cutting Consultant Urological Surgeon UHMBT Mr Richard Turner Urology Nurse Specialist UHMBT Ms Lorraine Rigg Urology Nurse Specialist UHMBT Ms Kath Boit Urology Nurse Specialist UHMBT Mr Bill Ryder Mr George Niven Patient Rep Patient Rep Vicki Wagstaff Admin support Strategic Clinical Network BFWHT: Blackpool, Fylde & Wyre Hospitals Trust ELHT: East Lancashire Hospitals Trust LTHT: Lancashire Teaching Hospitals Trust UHMBT: University Hospitals of Morecambe Bay Trust MDT LC: MDT Lead Clinician The group will be deemed to be quorate if there is representation from three of the four localities. Network Group meetings Measure 14-1C-103 The group will meet twice a year as a minimum and educational meetings will be arranged when required. Notes of the meeting will be produced and attendance recorded. Annual Report and Work Programme Measure 14-1C-104 The NSSG will produce an annual report and work programme. 4
5 The Urology NSSG will have a Chair elected from within the membership of the NSSG. A Vice Chair will also be nominated for succession planning purposes and for leading the NSSG in the absence of the Chair. The current Chair is Mr Mohan Pillai, Consultant Urological Surgeon, ELHT. Dr Omi Parikh, Consultant Oncologist, LTHT, has been appointed Vice-Chair. One of the NHS employed members will be nominated as having specific responsibility for user issues and information for patients/carers. Stephanie Keenan is the nominated member with such responsibility. A member of the NSSG responsible for ensuring that recruitment into trials and other well designated studies is integrated into the function of the NSSG. Dr Alison Birtle, Consultant Oncologist is the nominated member. Extended membership - the group will identify and recommend membership of other appropriate professionals as required, to achieve the objectives of the group. Role and Function of Group The role and purpose of the Urology NSSG is to improve the experience and outcomes of cancer care for urology patients in the Lancashire & South Cumbria Network. This involves consideration of strategies and plans for service improvement and service development across the patient pathway, incorporating all aspects of care at appropriate stages of the patient s journey. The aim is to achieve the best possible outcomes and best quality of life for all patients who use urological cancer services within the Network. The Urology NSSG: Is the Network s primary source of clinical opinion on issues relating to urological cancer for the Network Is the Group with corporate responsibility, delegated by the Network Board, for co-ordination and consistency across the Network for policy, practice guidelines, audit, research and service improvement relating to urological cancer. Will consult with the relevant cross-cutting Network groups on issues involving chemotherapy, cancer imaging, histopathology and laboratory investigation and specialist palliative care; and with the Head of Service on issues involving radiotherapy. Members of the NSSG will be responsible for feeding back information/decisions from the NSSG to their clinical and managerial colleagues. The responsibilities and core business of the NSSG can be expressed under six broad headings, which are contained within the Manual of Cancer Service Standards: 1. Service Planning 2. Service Improvement/Redesign (Modernisation) 3. Service Monitoring and Evaluation 4. Workforce Development 5. Research and Development 6. Annual Report and Work Plan 5
6 NETWORK CLINICAL AND REFERRAL GUIDELINES Measures 14-1C-105 to 109 The NSSG will be responsible for developing Urology Network Clinical and Referral Guidelines and will ensure that the guidelines are reviewed every three years, unless new guidance becomes available earlier. The Network guidelines cover the treatment of: Bladder, Kidney, and Prostate cancers. NICE Prostate Guideline 176 was issued in There are a number of recommendations in the Guideline which cannot currently be met by the Acute Trusts. This detail is contained in the Work Programme NICE bladder guidelines have been incorporated Penile Cancer Supranetwork Guidelines have been developed in collaboration with the Supranetwork Penile MDT. Testicular Cancer Supranetwork Guidelines have been developed in collaboration with the Supranetwork Testicular MDT. The Guidelines have been circulated to all Urology MDTs in the Network. Chemotherapy Treatment Algorithms Measure 14-1C-110 The NSSG, in consultation with the Network Chemotherapy Group, will agree a list of acceptable chemotherapy treatment algorithms which will be updated bi-annually. Algorithms produced for the following: Chemoradiation for bladder cancer; Chemotherapy algorithm for non TCC bladder cancer Squamous cell carcinoma; Chemotherapy Algorithm for Non Transitional Cell Bladder Cancer Primary sarcoma or carcinosarcoma of the bladder; Chemotherapy Algorithm for Non-Transitional Cell Bladder Cancer small cell carcinoma of the bladder; Chemotherapy algorithm for non-transitional cell bladder cancer adenocarcinoma or urachal cancer of the bladder; Chemotherapy algorithm for TCC bladder cancer; Chemotherapy algorithm for upper urinary tract TCC; Systemic treatment algorithm for renal cell cancer; Treatment algorithm for testicular germ cell tumours. Treatment algorithm for prostate cancer A copy of the agreed algorithms can be found at Appendix 1. Copies of the chemotherapy protocols for all urological tumours are available on the Strategic Clinical Network Website: 6
7 Patient Pathways for Kidney Cancer Measure 14-1C-111 The NSSG has produced a patient pathway for kidney cancer which details the named services, hospitals and MDTs which a patient should be referred to according to named indications, during their investigation, treatment, psychological and social support, rehabilitation and follow-up. Patient Pathways for Bladder Cancer Measure 14-1C-112 The NSSG has produced a patient pathway for bladder cancer which details the named services, hospitals and MDTs which a patient should be referred to according to named indications, during their investigation, treatment, psychological and social support, rehabilitation and follow-up. Patient Pathways for Prostate Cancer Measure 14-1C-113 The NSSG has produced a patient pathway for prostate cancer which details the named services, hospitals and MDTs which a patient should be referred to according to named indications, during their investigation, treatment, psychological and social support, rehabilitation and follow-up. Patient Pathways for Testicular Cancer Measure 14-1C-114 The NSSG has produced a patient pathway for testicular cancer which details the named services, hospitals and MDTs which a patient should be referred to according to named indications, during their investigation, treatment, psychological and social support, rehabilitation and follow-up. Patient Pathways for Penile Cancer Measure 14-1C-115 The NSSG has produced a patient pathway for penile cancer which details the named services, hospitals and MDTs which a patient should be referred to according to named indications, during their investigation, treatment, psychological and social support, rehabilitation and follow-up. 7
8 Referral Blackpool, Fylde & Wyre Hospitals NHS Trust East Lancashire Hospitals NHS Trust Lancashire Teaching Hospitals NHS Foundation Trust University Hospitals of Morecambe Bay NHS Trust Diagnostics Blackpool, Fylde & Wyre Hospitals NHS Trust East Lancashire Hospitals NHS Trust Lancashire Teaching Hospitals NHS Foundation Trust University Hospitals of Morecambe Bay NHS Trust Local MDT Blackpool, Fylde & Wyre Hospitals NHS Trust East Lancashire Hospitals NHS Trust Lancashire Teaching Hospitals NHS Foundation Trust University Hospitals of Morecambe Bay NHS Trust Level 1 - Local Management o Surgery o Post-op care o Chemotherapy o Follow-up o Palliative Care Blackpool, Fylde & Wyre Hospitals NHS Trust East Lancashire Hospitals NHS Trust Lancashire Teaching Hospitals NHS Foundation Trust University Hospitals of Morecambe Bay NHS Trust Network MDT Complex cases Supra Network MDT REFERRAL PATHWAY Level 2 Patients from Blackpool, Fylde & Wyre to Preston Level 2 Patients from Cumbria and Lancaster to Blackburn Radiotherapy/Chemotherapy Preston Complex Renal surgery referred to Manchester Network Some cases referred back for local management and/or palliative care Level 3 - Treatments at Christie Hospital Retroperitoneal node dissection. Brachytherapy, until service provided within the Network. All Penile cancer Level 3 treatments at Royal Preston Hospital Testicular chemotherapy/radiotherapy Selected penile chemotherapy/radiotherapy University Hospital of South Manchester Renal tumours with IVC thrombus requiring cardiac bypass/caval excision Level 1 treatment Local care Level 2 treatment Specialist care Level 3 treatment Supranetwork care 8
9 Patient Experience Measure 14-1C-116 The NSSG will annually review patient feedback of the associated local MDTs and any actions implemented and will agree an improvement programme with them. This review and associated improvement programme will be captured in the Annual Report. Clinical Outcomes Indicators and Network Audit Measure 14-1C 117 North West Regional Urology Audit The NSSG supports the annual North West Regional Urology Audit programme and the NSSG will annually review progress and outcomes of the North West Urology Audits. The NSSG will also consider any other ad hoc audits put forward by any of the local urology MDTs. The North West Regional Urology Audit for 2014 topic was Penile and Testicular cancer. Information is contained within the Annual Report. Discussion of Clinical Trials Measure 14-1C-118 The NSSG will annually discuss the MDTs reports on clinical trials and agree an improvement programme with each MDT. The outcome of the discussion will be contained within the Annual Report. 9
10 Appendix 1 Chemotherapy Treatment Algorithms for Urology Cancer Chemoradiation for bladder cancer; Chemotherapy algorithm for non TCC bladder cancer Squamous cell carcinoma; Chemotherapy Algorithm for Non Transitional Cell Bladder Cancer Primary sarcoma or carcino-sarcoma of the bladder; Chemotherapy Algorithm for Non-Transitional Cell Bladder Cancer small cell carcinoma of the bladder; Chemotherapy algorithm for non-transitional cell bladder cancer adenocarcinoma or urachal cancer of the bladder; Chemotherapy algorithm for TCC bladder cancer; Chemotherapy algorithm for upper urinary tract TCC; Systemic treatment algorithm for renal cell cancer; Treatment algorithm for testicular germ cell tumours. Treatment algorithm for prostate cancer 10
11 Chemo Radiation Algorithm for Transitional Cell Carcinoma of the Urinary Bladder T2-T4No TCC Performance status 0-2, EGFR > 25mls per minute, fit for radical radiotherapy and for chemotherapy: Concurrent 5-FU and Mitomycin C fraction 1-4 of radiotherapy 5-FU fraction of radiotherapy as per BC2001 Phase III Data (ASCO 2010, ASTRO 2010, JCO 2010). 11
12 Chemotherapy Algorithm for Non-Transitional Cell Bladder Cancer Adenocarcinoma or urachal cancer of the urinary bladder T2-T4 potentially resectable tumours: Neoadjuvant chemotherapy is not evidence based and should not be given Resected T2-T4N+ or T4No: Minimal data in support of adjuvant treatment Metastatic disease, performance status 0-1, EGFR> 50 mls/min and LVEF > 45 mls: 3-6 cycles of Epirubicin-Cisplatin and Capecitabine if fit, performance status 0-1, EGFR> 50 mls/min and LVEF > 45 mls. Reassess after 3 cycles and if responding continue to 6 cycles Metastatic disease with performance status 2 or EGFR < 50 mls/min or LVEF < 45 mls/min, or other comorbidities making platinum ineligible but still fit for chemotherapy: 3 cycles of Gemcitabine-Carboplatin 21 day cycle, reassess after 3 cycles and if responding continue to 6 cycles 12
13 Chemotherapy Algorithm for Non-Transitional Cell Bladder Cancer Small cell carcinoma of the bladder NB transitional cell carcinoma with small cell differentiation should be treated according to TCC algorithm Limited stage disease post cystectomy or local excision, performance status 0-1, EGFR > 50mls/min, fit for cisplatin: 4-6 cycles of Etoposide and Cisplatin adjuvant chemotherapy Limited stage disease post cystectomy or local excision, performance status 2 or EGFR < 50mls/min, or unfit for Cisplatin due to comorbidities: 4-6 cycles of Etoposide and Carboplatin adjuvant chemotherapy Extensive or metastatic disease: Follow algorithm above for choice of regime dependent on comorbidities, performance status and EGFR. For 3 cycles, reassess and if response continue to 6 cycles Recurrent disease: If > 6 months from initial treatment follow algorithm above and re-treat If < 6 months from initial treatment and patient fit for further chemotherapy, consider second-line chemotherapy as per small cell lung protocol as there is no evidence based choice of second-line chemotherapy in metastatic small cell carcinoma of the bladder 13
14 Chemotherapy Algorithm for Non-Transitional Cell Bladder Cancer Squamous cell carcinoma of the bladder NB Transitional cell carcinoma with squamous differentiation should be treated according to TCC algorithm T2-T4 potentially resectable tumours: Neoadjuvant chemotherapy is not evidence based and should not be given Resected T2-T4N+ or T4No: Minimal data in support of adjuvant treatment Metastatic disease, performance status 0-1, EGFR> 50 mls/min: 3-6 cycles of Cisplatin and continuous infusional 5FU or Cisplatin and Capecitabine Reassess after 3 cycles and if responding continue to 6 cycles Metastatic disease, performance status 2, or EGFR < 50mls/min or other comorbidities rendering ineligible for Cisplatin: 3-6 cycles of Mitomycin C and continuous infusional 5FU or Mitomycin C and Capecitabine. Reassess after 3 cycles and if responding continue to 6 cycles Recurrent disease: If > 6 months from initial treatment follow algorithm above and re-treat If < 6 months from initial treatment and patient fit for further chemotherapy, there are no regimes with strong supportive data 14
15 Chemotherapy Algorithm for Non-Transitional Cell Bladder Cancer Primary sarcoma or carcino-sarcoma of the urinary bladder NB Transitional cell carcinoma with sarcomatoid features should be treated according to TCC algorithm For all stages of refer to algorithm for treatment of sarcoma 15
16 Chemotherapy Algorithm for Bladder Cancer Transitional cell carcinoma-first line chemotherapy T2-T3NoMo Fit for radical radiotherapy or radical cystectomy Performance status 0-1, EGFR > 50mls/min 3 cycles of neoadjuvant chemotherapy Gemcitabine-Cisplatin x 21 day cycle Post cystectomy adverse pathology e.g. pt3/4, or N+ Performance status 0-1, EGFR > 50 mls/min In selected patients ONLY, on case by case discussion 4 cycles of adjuvant chemotherapy Gemcitabine-Cisplatin x 21 day cycle T2-T4 N+ or M1 Performance status 0-1, EGFR > 50 mls/min 3-6 cycles of Gemcitabine- Cisplatin x 21 day cycle Interval CT after 3 cycles, if responding continue to 6 cycles Performance status 2 EGFR mls/min Other comorbidities rendering unsuitable for Cisplatin 3-6 cycles of Gemcitabine- Carboplatin x 21 day cycle Interval CT after 3 cycles, if responding continue to 6 cycles Second line chemotherapy for transitional cell carcinoma If > 6 months since last treatment, re-challenge with same regime and follow algorithm above for EGFR, performance status and number of cycles If < 6 months consider second-line chemotherapy with weekly Taxol if performance status 0-1 Reassess after 12 weeks of treatment Consider clinical trial 16
17 Chemotherapy Algorithm for Upper Urinary Tract Transitional Cell Carcinoma T2-T4 No or N+ Mo apparently resectable tumour pre radical nephroureterectomy: No evidence for neoadjuvant chemotherapy. Neoadjuvant chemotherapy should not be given Completely resected T2-T4NoMo, or T1-T4N+ Mo post radical nephroureterectomy: Consider adjuvant chemotherapy versus surveillance as part of clinical study e.g. the POUT trial Follow bladder TCC algorithm for choice of regimen For selected patients outside of a clinical study, adjuvant chemotherapy may be considered Follow bladder TCC algorithm for treatment of choice Non-resectable locally advanced and/or node positive or metastatic disease: Follow bladder TCC algorithm Recurrent disease: Follow bladder TCC second-line algorithm 17
18 Systemic Treatment Algorithm for Renal Cell Cancer ADJUVANT THERAPY No standard treatment, consider entry into clinical trial LOCALLY ADVANCED / RECURRENT DISEASE Treat as metastatic disease or consider metastatectomy METASTATIC DISEASE First Line: Sunitinib Entry into trial (Sunitinib +/- vaccine) Pazopanib If poor performance status or nonclear cell histology consider Temsirolimus If appropriate consider referral for high dose Interleukin 2 2 nd line options: Everolimus 3 rd line options: Consider entry into clinical trial (none currently open locally) No standard treatments Consider referral for high dose Interleukin 2 Consider role of Sorafenib 18
19 Treatment Algorithm Testicular Germ Cell Tumours Abdominal Orchiectomy Stage I Seminoma Stage 1 Non- Seminoma All others Low risk: Surveillance or SA Carboplatin All others: SA Carboplatin AUC7 Surveillance Prognostic stage Chemotherapy* 19
20 Chemotherapy Treatment Algorithm for Prostate Cancer Metastatic Hormone Refractory Prostate Carcinoma First Line Chemotherapy Docetaxel + Prednisolone 6 10 cycles Alternative Mitozantrone + Prednisolone 6 cycles Second Line Chemotherapy Cabazitaxel + Prednisolone up to 10 cycles 20
21 Appendix 2 Patient Pathway for Bladder Cancer Referral Urgent GP suspected renal cancer referral received by local Trust. Patient contacted and offered appointment within 14 days First definitive treatment: TURBT Local MDT First seen Patient attends an OPD appointment in dedicated haematuria clinic to facilitate rapid access to investigative procedures including imaging and cystoscopy Local MDT Meeting Patient discussed at MDT with histology/imaging results available. If age appropriate notify TYA MDT at Christie (see pathway on page 3). Local team will manage all patients diagnosed with low risk superficial bladder cancer. Metastatic Carcinoma of Unknown Primary Refer to CUP MDT for discussion First definitive surgical treatment: cases to be notified to Specialist MDT: All patients diagnosed with high risk superficial bladder cancer: o Grade 3 transitional cell carcinoma with no sub-mucosal invasion (G3pTa); o Grade 3 Transitional cell carcinoma with sub-mucosal invasion (G3pT1); o Extensive Grade 2; o Recurrent Grade 2 or multifocal Grade 2; o Carcinoma in situ All patients diagnosed with muscle invasive bladder cancer (T2-T4); All patients who present with metastatic bladder cancer prior to management by the local team. Following discussion at Specialist MDT: Patients who are suitable for radical cystectomy and ileal conduit/bladder reconstruction will be managed by the Specialist team; Patients who are not suitable for radical cystectomy and require urinary diversion can by managed by the local team; Patients who are suitable for radiotherapy +/- neoadjuvant or adjuvant chemotherapy will be referred to the oncologist from the local team. Follow-up As per EUA guidelines See Clinical Guidelines 21
22 Patient Pathway for Kidney Cancer Referral Urgent GP suspected renal cancer referral received by local Trust. Patient contacted and offered appointment within 14 days First seen Patient attends an OPD appointment and assessment carried out. Urgent investigations requested CT Scan/Ultrasound Local MDT Meeting Patient discussed at MDT with imaging results available. If age appropriate notify TYA MDT at Christie (see pathway on page 3) First definitive treatment: Radical excision Radiofrequency ablation/cryotherapy Systemic treatment Specialist palliative care Following discussion at MDT: Metastatic Carcinoma of Unknown Primary Refer to CUP MDT for discussion Patients who are suitable for radical nephrectomy will be referred back to the local team for management, but should be offered laparoscopic surgery where clinically appropriate. Patients who are suitable for partial nephrectomy should be referred back to the clinicians within the Network who have appropriate experience. Patients with metastatic disease who are suitable for angiogenesis inhibitors / immunotherapy will be referred to the oncologist from the local team. Treatment will be given by the specialist team at present, pending involvement of medical oncologist. Refer to Specialist MDT prior to local management for the following: T1 tumours for which nephron sparing surgery may be possible; Tumours which have or may have invaded major blood vessels; Presence of metastases; May benefit from resection of primary and metastases; Bi-lateral disease or patient may require dialysis; Have Von Hippel-Lindau disease or hereditary papillary tumours Follow-up See table overleaf 22
23 Agreed follow-up scheme: Proposed surveillance schedule following treatment for RCC, taking into account patient risk profile and treatment efficacy. Risk strategy as Mayo scheme. Local teams have responsibility for follow up Risk profile Treatment 6mo 1yr 2yr 3yr 4yr 5yr >5yr low RN /PNonly US CT US CT US CT discharge intermediate RN/PN/cryo/RFA CT CT CT US CT CT CT every 2 years high RN /PN/cryo/RFA CT CT CT CT CT CT CT every 2 years Cryo = cryotherapy; CT = computed tomography of chest and abdomen, or MRI = magnetic resonance imaging; PN = partial nephrectomy; RFA = radiofrequency ablation; RN = radical nephrectomy; US = ultrasound of abdomen, kidneys and renal bed 23
24 Patient Pathway for Penile Cancer Referral Urgent GP suspected penile cancer referral received by local Trust. Patient contacted and offered appointment within 14 days First seen Patient attends an OPD appointment and assessment carried out. Urgent investigations requested penile biopsy Local/Specialist Network MDT Meeting Review histology and imaging; ensure referral already sent to Supranetwork MDT Metastatic Carcinoma of Unknown Primary Refer to CUP MDT for discussion Refer to Supranetwork MDT: Patients to be notified to the next Supranetwork MDT following diagnosis and reviewed by Supranetwork MDT core member. Chemotherapy/Radiotherapy undertaken by Supranetwork MDT at Christie Hospital. After Supranetwork MDT discussion, patients wishing palliative chemotherapy or palliative radiation given locally are referred back to a named member of the extended Supranetwork MDT. Follow-up Follow up care under the guidance of the Supranetwork Team. Follow up may be delivered locally under pre-defined guidelines from the Supranetwork MDT if patient wishes 24
25 Patient Pathway for Prostate Cancer Referral Urgent GP suspected prostate cancer referral received by local Trust. Patient contacted and offered appointment within 14 days First seen Patient attends an OPD in dedicated prostate clinic for assessment and prostate biopsy Local MDT Patients with localised prostatic carcinoma will be notified to the Specialist MDT; Patients who may be suitable for chemotherapy should be referred to Specialist MDT for discussion; Patients with localised prostatic carcinoma, requiring active monitoring, will be managed by the local team; Patients with locally advanced prostatic carcinoma; Patients with metastatic disease requiring hormone manipulation; Patients not suitable for radiotherapy will be managed with hormone therapy alone; Palliative radiotherapy for symptom control refer to local oncologist. Local teams will counsel patients in order for them to select their treatment option. Metastatic Carcinoma of Unknown Primary Refer to CUP MDT for discussion Specialist MDT Patients with local prostatic carcinoma, requiring radical prostatectomy will be managed by the specialist surgical or oncology team; Patients who may be suitable for chemotherapy should be referred from the local MDT to Specialist MDT for discussion. All patients requiring brachytherapy will be referred to the Uro-Oncology team at Christie Hospital Patients with localised prostatic carcinoma, requiring radiotherapy, will be referred to the local oncologist and managed by the local team; All patients suitable for inclusion in a trial to be discussed at Specialist MDT Follow-up As per EUA guidelines See Clinical Guidelines 25
26 Patient Pathway for Testicular Cancer Referral Urgent GP suspected testicular cancer referral received by local Trust. Patient contacted and offered appointment within 14 days First seen Patient attends an OPD appointment and assessment carried out. Testes ultrasound and clinical examination and listed for orchidectomy TYA MDT Christie If age appropriate notify TYA MDT at Christie (see pathway on page 3). Carcinoma of Unknown Primary refer to CUP MDT Orchidectomy Local MDT Meeting MDT with histology results. Arrange CT chest, abdomen and pelvis Expedite referral to the snmdt prior to orchidectomy for: Obvious metastatic disease; Very high tumour markers; Non-gonadal germ cell tumour of (mediastinum, retroperitoneum, brain); Severe constitutional symptoms. Parameters for Good Prognosis and High Risk: This information is contained within the Clinical Guidelines Positive histology refer to Supranetwork MDT for discussion and review Delivery of Chemotherapy and Radiotherapy Patients requiring chemotherapy and radiotherapy as part of their treatment will be treated by core members of the Supranetwork MDT. This can be delivered locally. Supranetwork Testicular MDT All cases to be notified to Supranetwork MDT at time of surgery Follow-up Follow up undertaken by Supranetwork MDT Oncologists (includes extended MDT members) 26
27 MSCC Clinical Indicators for Referral to Urology Cancer Rehabilitation Pathway: Patients are at risk of developing or experiencing the following clinical indicators and should be assessed for referral to rehabilitation pathway interventions at all stages in the cancer care pathway as described below: Please ask the patient for their consent before referring to Rehabilitation services Diagnosis & Care Planning Treatment Post Treatment Monitoring/ Survivorship Palliative Care End of Life Care Consider level of intervention required: Information support General rehabilitation services Specialist Oncology/ palliative rehabilitation Ensure patient has contact details for timely future access to rehabilitation services (see local cancer services directory rehabilitation services) Physiotherapy Difficulties with function, movement and symptom control: Difficulty walking/ getting around Breathing difficulties/ cough/perioperative respiratory problems Fatigue/ tiredness Weakness/ loss of muscle strength (focal or generalized) Impaired balance Equipment/ Information needs Pain/sensory changes MSCC Urinary Continence Difficulties with functional activities of daily living, leisure and work resulting from: Difficulty with functional mobility Breathing difficulties/ cough Fatigue/ tiredness Impaired balance/ weakness Anxiety/ role and function change/ body image s Cognitive impairment Equipment/ information needs Pain/sensory changes MSCC Urinary Continence Occupational Therapy Dietetics Communication and eating/ drinking: Impaired or risk of impaired swallowing Impaired or risk of impaired speech/voice/communication Equipment/information needs Nutrition and Diet: Reduced appetite Malnutrition Weight loss/ weight management (cachexia) Fatigue/ tiredness Nausea and vomiting Difficulties swallowing Information needs Speech and Language Therapy 27
28 TEENAGE AND YOUNG ADULT PATHWAY YEARS Designated TYA Hospitals Suspected cancer GP Referral or other route referral Site Specific Team (SiSpMDT) Site-Specific Diagnostic Pathway SiSpMDT meeting - diagnosis and treatment decision TYA MDT Notified TYA team, advice & support to SSMDT +/- patient/family TYA Notification Pro-Forma to: chn-tr.mdt@nhs.net Fax: Post to: Kerrie Waterhouse, TYAMDT Coordinator, Young Oncology Unit, The Christie NHS Foundation Trust, M20 4BX Charlene Jones TYA Clinical Nurse Specialist (Lancs & South Cumbria) Jointly-agreed* MDT decision Treatment plan, clinical trial, informed patient choice re place of care. TYAMDT meeting diagnosis, treatment & care package INTEGRATED TREATMENT PLAN AND KEY WORKER AGREED Treatment in TYA designated hospital. Coordinated by Site Specific MDT in conjunction with PTC Treatment in Principal Treatment Centre. Coordinated by TYAMDT Follow-up by SiSpMDT to integrated plan agreed with the TYAMDT Follow-up by TYAMDT to integrated plan agreed with the SiSpMDT *NB Jointly agreed refers to the MDT discussion. The patient will remain under the clinical care of the site specialist clinician until a formal referral for transfer of care to the TYA Unit Lead Clinician has been accepted. 28
Chemotherapy Treatment Algorithms for Urology Cancer
 Chemotherapy Treatment Algorithms for Urology Cancer Chemoradiation for bladder cancer; Chemotherapy algorithm for non TCC bladder cancer Squamous cell carcinoma; Chemotherapy Algorithm for Non Transitional
Chemotherapy Treatment Algorithms for Urology Cancer Chemoradiation for bladder cancer; Chemotherapy algorithm for non TCC bladder cancer Squamous cell carcinoma; Chemotherapy Algorithm for Non Transitional
Appendix 4 Urology Care Pathways
 Appendix 4 Urology Care Pathways Cancer Care Pathways outline the steps and stages in the patient journey from referral through to diagnostics, staging, treatment, follow up, rehabilitation and if applicable
Appendix 4 Urology Care Pathways Cancer Care Pathways outline the steps and stages in the patient journey from referral through to diagnostics, staging, treatment, follow up, rehabilitation and if applicable
Bladder Cancer Guidelines
 Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
PROTOCOLS FOR THE MANAGEMENT OF PATIENTS WITH UROLOGICAL MALIGNANCY
 Lancashire & South Cumbria PROTOCOLS FOR THE MANAGEMENT OF PATIENTS WITH UROLOGICAL MALIGNANCY 2017 Guidelines developed by the Urology NSSG (Lancashire & South Cumbria) Reviewed: April 2017 Review date:
Lancashire & South Cumbria PROTOCOLS FOR THE MANAGEMENT OF PATIENTS WITH UROLOGICAL MALIGNANCY 2017 Guidelines developed by the Urology NSSG (Lancashire & South Cumbria) Reviewed: April 2017 Review date:
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Acute Oncology. Protocols & Competencies Acute Oncology Introduction
 Acute Oncology Protocols & Competencies Acute Oncology Introduction Acute Oncology elearning Module Throughout the Acute Oncology FACTS elearning courses you will be able to access Network Protocols by
Acute Oncology Protocols & Competencies Acute Oncology Introduction Acute Oncology elearning Module Throughout the Acute Oncology FACTS elearning courses you will be able to access Network Protocols by
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
3.1 Investigations for Patients Presenting with Haematuria Table 1
 3.1 Investigations for Patients Presenting with Haematuria Table 1 Patients at risk of bacterial endocarditis should be given antibiotic prophylaxis as per local guidelines. Patients with heart valve replacements
3.1 Investigations for Patients Presenting with Haematuria Table 1 Patients at risk of bacterial endocarditis should be given antibiotic prophylaxis as per local guidelines. Patients with heart valve replacements
Oncological Treatment of Urological Cancer
 Network Guidance Document Oncological Treatment of Urological Cancer Status: Expiry Date: Version Number: Publication Date: Final March 2014 8 March 2012 Page 1 of 13 Contents Contents... 2 Oncology Provision...
Network Guidance Document Oncological Treatment of Urological Cancer Status: Expiry Date: Version Number: Publication Date: Final March 2014 8 March 2012 Page 1 of 13 Contents Contents... 2 Oncology Provision...
Attachment #2 Overview of Follow-up
 Attachment #2 Overview of Follow-up Provided below is a general overview of follow-up and this may vary based on specific patient or cancer characteristics. Of note, Labs and imaging can be performed closer
Attachment #2 Overview of Follow-up Provided below is a general overview of follow-up and this may vary based on specific patient or cancer characteristics. Of note, Labs and imaging can be performed closer
BLADDER CANCER PATHWAY (E&W) Version 1
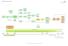 BLADDER CANCER PATHWAY (E&W) Version 1 Flexi cysto FU (via cystoscopy) ptag1/2 and OP instillations MMC ptag3/cis BCG BCG cancer One-stop Haematuria Clinic TURBT Histology presented Decision to refer to
BLADDER CANCER PATHWAY (E&W) Version 1 Flexi cysto FU (via cystoscopy) ptag1/2 and OP instillations MMC ptag3/cis BCG BCG cancer One-stop Haematuria Clinic TURBT Histology presented Decision to refer to
Attachment #2 Overview of Follow-up
 Attachment #2 Overview of Follow-up Provided below is a general overview of follow-up and this may vary based on specific patient or cancer characteristics. Of note, Labs and imaging can be performed closer
Attachment #2 Overview of Follow-up Provided below is a general overview of follow-up and this may vary based on specific patient or cancer characteristics. Of note, Labs and imaging can be performed closer
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER
 MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
MUSCLE-INVASIVE AND METASTATIC BLADDER CANCER (Text update March 2008) A. Stenzl (chairman), N.C. Cowan, M. De Santis, G. Jakse, M. Kuczyk, A.S. Merseburger, M.J. Ribal, A. Sherif, J.A. Witjes Introduction
COLORECTAL NSSG (Lancs & South Cumbria) Constitution and Terms of Reference 2015
 COLORECTAL NSSG (Lancs & South Cumbria) Constitution and Terms of Reference 2015 Agreements: Agreed by: Mr Tom Raymond, Consultant Colorectal Surgeon, University Hospitals of Morecambe Bay NHS Trust and
COLORECTAL NSSG (Lancs & South Cumbria) Constitution and Terms of Reference 2015 Agreements: Agreed by: Mr Tom Raymond, Consultant Colorectal Surgeon, University Hospitals of Morecambe Bay NHS Trust and
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
Guidelines for the Management of Bladder Cancer
 Guidelines for the Management of Bladder Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Version 3 and 4 Sections 5.2 and 8 updated Page 1 of 9 1. Scope of
Guidelines for the Management of Bladder Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Version 3 and 4 Sections 5.2 and 8 updated Page 1 of 9 1. Scope of
LCA Lung Clinical Forum. 21 st October 2014
 LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs. Gynaecological sarcomas Version 1
 Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Gynaecological sarcomas Version 1 Background This guidance is to provide direction for the management of patients with sarcomas
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Gynaecological sarcomas Version 1 Background This guidance is to provide direction for the management of patients with sarcomas
(Lancs & South Cumbria)
 HAEMATOLOGY NSSG (Lancs & South Cumbria) Constitution and Terms of Reference 2015 Page 1 of 22 The constitution has been agreed by: Position: Chair of Haematology NSSG Name: Dr Mark Grey Organisation:
HAEMATOLOGY NSSG (Lancs & South Cumbria) Constitution and Terms of Reference 2015 Page 1 of 22 The constitution has been agreed by: Position: Chair of Haematology NSSG Name: Dr Mark Grey Organisation:
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
Guideline for the Management of Vulval Cancer
 Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM)
 INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT NECN N Tees & Hartlepool North Tees And Hartlepool Date Self Assessment Completed 23rd July 2009 Date of IV Review 19th June 2009
INTERNAL VALIDATION REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT NECN N Tees & Hartlepool North Tees And Hartlepool Date Self Assessment Completed 23rd July 2009 Date of IV Review 19th June 2009
A06/S(HSS)b Ex-vivo partial nephrectomy service (Adult)
 A06/S(HSS)b 2013/14 NHS STANDARD CONTRACT FOR EX-VIVO PARTIAL NEPHRECTOMY SERVICE (ADULT) PARTICULARS, SCHEDULE 2 THE SERVICES, A - SERVICE SPECIFICATION Service Specification No. Service Commissioner
A06/S(HSS)b 2013/14 NHS STANDARD CONTRACT FOR EX-VIVO PARTIAL NEPHRECTOMY SERVICE (ADULT) PARTICULARS, SCHEDULE 2 THE SERVICES, A - SERVICE SPECIFICATION Service Specification No. Service Commissioner
DRAFT SARCOMA MEASURES
 Gateway 15612 Draft Sarcoma Measures Page 1 of 44 DH INFORMATION READER BOX Policy Estates HR / Workforce Commissioning Management IM & T Policy Planning / Finance Clinical Social Care / Partnership Working
Gateway 15612 Draft Sarcoma Measures Page 1 of 44 DH INFORMATION READER BOX Policy Estates HR / Workforce Commissioning Management IM & T Policy Planning / Finance Clinical Social Care / Partnership Working
Audit Report Report of the 2012 Clinical Audit Data
 Urological Cancer Managed Clinical Network Audit Report Report of the 2012 Clinical Audit Data Mr Seamus Teahan MCN Clinical Lead Tom Kane MCN Manager Sandie Ker Information Officer Urological Cancer Audit
Urological Cancer Managed Clinical Network Audit Report Report of the 2012 Clinical Audit Data Mr Seamus Teahan MCN Clinical Lead Tom Kane MCN Manager Sandie Ker Information Officer Urological Cancer Audit
Activity Report July 2012 June 2013
 Urological Cancers Managed Clinical Network Activity Report July 2012 June 2013 Mr Seamus Teahan Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager 1 CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
Urological Cancers Managed Clinical Network Activity Report July 2012 June 2013 Mr Seamus Teahan Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager 1 CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
Manchester Cancer. Guidelines for the management of renal cancer
 Guidelines for the management of renal cancer Approved by the urology pathway board September 2014 To be reviewed September 2016 Renal Cancer Guidelines 1. Introduction 1.1 Kidney cancer accounts for 3%
Guidelines for the management of renal cancer Approved by the urology pathway board September 2014 To be reviewed September 2016 Renal Cancer Guidelines 1. Introduction 1.1 Kidney cancer accounts for 3%
MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER
 10 MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER Recommendations from the EAU Working Party on Muscle Invasive and Metastatic Bladder Cancer G. Jakse (chairman), F. Algaba, S. Fossa, A. Stenzl, C. Sternberg
10 MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER Recommendations from the EAU Working Party on Muscle Invasive and Metastatic Bladder Cancer G. Jakse (chairman), F. Algaba, S. Fossa, A. Stenzl, C. Sternberg
Work Programme/Service Delivery Plan 2010/2013
 Essex and East Suffolk Gynaecological Cancer Network Site Specific Group Work Programme/Service Delivery Plan 2010/2013 Version Number 1.2 Author Members of the NSSG Date Written June 2010 Reviewed May
Essex and East Suffolk Gynaecological Cancer Network Site Specific Group Work Programme/Service Delivery Plan 2010/2013 Version Number 1.2 Author Members of the NSSG Date Written June 2010 Reviewed May
SKIN NSSG. (Lancs & South Cumbria) Constitution and Terms of Reference 2015
 SKIN NSSG (Lancs & South Cumbria) Constitution and Terms of Reference 2015 VERSION : May 2015 1 The constitution has been agreed by: Position: Chair of Skin NSSG Name: Chris Dobson Organisation: Lancashire
SKIN NSSG (Lancs & South Cumbria) Constitution and Terms of Reference 2015 VERSION : May 2015 1 The constitution has been agreed by: Position: Chair of Skin NSSG Name: Chris Dobson Organisation: Lancashire
RENAL CANCER GUIDELINES
 Greater Manchester and Cheshire Cancer Network RENAL CANCER GUIDELINES Agreed by Urology CSG: July 2010 Review Date: July 2012 Renal Cancer Guidelines 1. Introduction 1.1 Kidney cancer accounts for 3%
Greater Manchester and Cheshire Cancer Network RENAL CANCER GUIDELINES Agreed by Urology CSG: July 2010 Review Date: July 2012 Renal Cancer Guidelines 1. Introduction 1.1 Kidney cancer accounts for 3%
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2
 Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2 Background Sarcomas that arise in the lung de novo are
Shared Care Pathway for Soft Tissue Sarcomas Presenting to Site Specialised MDTs Lung /chest wall sarcomas incl. pulmonary metastatectomy Version 2 Background Sarcomas that arise in the lung de novo are
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO)
 North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
Cancer of Unknown Primary Service
 Cancer of Unknown Primary Service Dr Maurice Fernando Consultant In Specialist Palliative Care and CUP lead Doncaster and Bassetlaw Hospitals NHS FT Wakefield meeting -14-07-2016 CUP service CUP MDT
Cancer of Unknown Primary Service Dr Maurice Fernando Consultant In Specialist Palliative Care and CUP lead Doncaster and Bassetlaw Hospitals NHS FT Wakefield meeting -14-07-2016 CUP service CUP MDT
West Midlands Sarcoma Advisory Group
 West Midlands Sarcoma Advisory Group Guideline for the Initial Investigation and Referral to Sarcoma Specialist Multi Disciplinary Team for Suspected Sarcoma of Soft Tissue Extremities (limbs and trunk
West Midlands Sarcoma Advisory Group Guideline for the Initial Investigation and Referral to Sarcoma Specialist Multi Disciplinary Team for Suspected Sarcoma of Soft Tissue Extremities (limbs and trunk
Cancer of Unknown Primary (CUP) Protocol
 1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
NICE BULLETIN Diagnosis & treatment of prostate cancer
 Diagnosis & treatment of prostate cancer NICE provided the content for this booklet which is independent of any company or product advertised Diagnosis and treatment of prostate cancer Introduction In
Diagnosis & treatment of prostate cancer NICE provided the content for this booklet which is independent of any company or product advertised Diagnosis and treatment of prostate cancer Introduction In
UROLOGY CANCER 2009 COMPARATIVE AUDIT REPORT
 Urological Cancer Audit 2009 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT UROLOGY CANCER 2009 COMPARATIVE AUDIT REPORT Dr Prasad Bollina, NHS Lothian SCAN Lead Urology Cancer Clinician Dr
Urological Cancer Audit 2009 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT UROLOGY CANCER 2009 COMPARATIVE AUDIT REPORT Dr Prasad Bollina, NHS Lothian SCAN Lead Urology Cancer Clinician Dr
UROLOGICAL CANCER 2010 COMPARATIVE AUDIT REPORT
 SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT UROLOGICAL CANCER 2010 COMPARATIVE AUDIT REPORT Dr Prasad Bollina, NHS Lothian SCAN Lead Urology Cancer Clinician Dr Prasad Bollina, NHS Lothian
SOUTH EAST SCOTLAND CANCER NETWORK PROSPECTIVE CANCER AUDIT UROLOGICAL CANCER 2010 COMPARATIVE AUDIT REPORT Dr Prasad Bollina, NHS Lothian SCAN Lead Urology Cancer Clinician Dr Prasad Bollina, NHS Lothian
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services. Cancer of Unknown Primary Network Site Specific Group. Clinical Guidelines
 Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Supra Network Sarcoma Advisory Group (SAG) Annual Report
 London and South East Sarcoma Network Supra Network Sarcoma Advisory Group (SAG) Annual Report 2011-2012 Hosted by Date: December 2012 Version: 2 Review Date: September 2013 London Cancer Integrated Cancer
London and South East Sarcoma Network Supra Network Sarcoma Advisory Group (SAG) Annual Report 2011-2012 Hosted by Date: December 2012 Version: 2 Review Date: September 2013 London Cancer Integrated Cancer
B14/S/b 2013/14 NHS STANDARD CONTRACT FOR PENILE CANCER SECTION B PART 1 - SERVICE SPECIFICATIONS. Service Specification No.
 B14/S/b 2013/14 NHS STANDARD CONTRACT FOR PENILE CANCER SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider Lead Period Date of Review B14/S/b Penile
B14/S/b 2013/14 NHS STANDARD CONTRACT FOR PENILE CANCER SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider Lead Period Date of Review B14/S/b Penile
Cancer of Unknown Primary (CUP)
 Cancer of Unknown Primary (CUP) Pathways and Guidelines V1.0 London Cancer September 2013 The following pathways and guidelines document has been compiled by the London Cancer CUP technical subgroup and
Cancer of Unknown Primary (CUP) Pathways and Guidelines V1.0 London Cancer September 2013 The following pathways and guidelines document has been compiled by the London Cancer CUP technical subgroup and
PATHWAY MANAGEMENT OF METASTATIC SPINAL CORD COMPRESSION (MSCC) THE CHRISTIE, GREATER MANCHESTER & CHESHIRE
 PATHWAY MANAGEMENT OF METASTATIC SPINAL CORD COMPRESSION (MSCC) THE CHRISTIE, GREATER MANCHESTER & CHESHIRE Procedure Reference: Document Owner: Dr V. Misra Version: Accountable Committee: V3 MSCC Network
PATHWAY MANAGEMENT OF METASTATIC SPINAL CORD COMPRESSION (MSCC) THE CHRISTIE, GREATER MANCHESTER & CHESHIRE Procedure Reference: Document Owner: Dr V. Misra Version: Accountable Committee: V3 MSCC Network
Improving services for upper GI (OG) cancer Application template (Version 2)
 Trust Clinical lead Improving services for upper GI (OG) cancer Application template (Version 2) Managerial lead Date completed 14 June 2013 Barnet & Chase Farm Hospitals NHS Trust Dr Marta Carpani Upper
Trust Clinical lead Improving services for upper GI (OG) cancer Application template (Version 2) Managerial lead Date completed 14 June 2013 Barnet & Chase Farm Hospitals NHS Trust Dr Marta Carpani Upper
NICE Quality Standards and COF
 NICE Quality Standards and COF David Baldwin Consultant Respiratory Physician NUH Hon Senior Lecturer Nottingham University Clinical lead NICE lung cancer GL Chair NICE QS Topic Expert Group Quality Standards
NICE Quality Standards and COF David Baldwin Consultant Respiratory Physician NUH Hon Senior Lecturer Nottingham University Clinical lead NICE lung cancer GL Chair NICE QS Topic Expert Group Quality Standards
National Prostate Cancer Audit. Bill Cross June 2015
 National Prostate Cancer Audit Bill Cross June 2015 National Prostate Cancer Audit aim of assessing the process of care and its outcomes in men diagnosed with prostate cancer in England and Wales National
National Prostate Cancer Audit Bill Cross June 2015 National Prostate Cancer Audit aim of assessing the process of care and its outcomes in men diagnosed with prostate cancer in England and Wales National
Activity Report July 2014 June 2015
 Urological Cancers Managed Clinical Network Activity Report July 2014 June 2015 Mr Gren Oades Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION 5
Urological Cancers Managed Clinical Network Activity Report July 2014 June 2015 Mr Gren Oades Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION 5
National Standards for Sarcoma Services
 National Standards for Sarcoma Services 2009 TABLE OF CONTENTS 1. INTRODUCTION TO THE NATIONAL CANCER STANDARDS...2 2. METHODOLOGY...4 3. FORMAT...4 4. INTRODUCTION TO SARCOMA...5 STANDARDS TOPIC: ORGANISATION...10
National Standards for Sarcoma Services 2009 TABLE OF CONTENTS 1. INTRODUCTION TO THE NATIONAL CANCER STANDARDS...2 2. METHODOLOGY...4 3. FORMAT...4 4. INTRODUCTION TO SARCOMA...5 STANDARDS TOPIC: ORGANISATION...10
National Standards for Sarcoma Services 2009
 National Standards for Sarcoma Services 2009 Crown copyright July 2010 F1101011 Contents 1. Introduction to the National Cancer Standards 2. Methodology 3. Format 4. Introduction to Sarcoma Standards Topic:
National Standards for Sarcoma Services 2009 Crown copyright July 2010 F1101011 Contents 1. Introduction to the National Cancer Standards 2. Methodology 3. Format 4. Introduction to Sarcoma Standards Topic:
Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Prostate Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
GUIDELINES FOR THE MANAGEMENT OF BLADDER CANCER
 GUIDELINES FOR THE MANAGEMENT OF BLADDER CANCER For approvals and version control see Document Management Record on page 9 Ref: AngCN-SSG-U4 Page 1 of 11 Approved and Published: Aug 2012 TABLE OF CONTENTS
GUIDELINES FOR THE MANAGEMENT OF BLADDER CANCER For approvals and version control see Document Management Record on page 9 Ref: AngCN-SSG-U4 Page 1 of 11 Approved and Published: Aug 2012 TABLE OF CONTENTS
PROSTATE CANCER STRATIFIED FOLLOW UP. Hilary Baker Lead CNS for Uro-oncology MSc, BSc, RGN.
 PROSTATE CANCER STRATIFIED FOLLOW UP Hilary Baker Lead CNS for Uro-oncology MSc, BSc, RGN. LEARNING OBJECTIVES To refresh your knowledge about prostate cancer. To discuss the purpose and patient benefits
PROSTATE CANCER STRATIFIED FOLLOW UP Hilary Baker Lead CNS for Uro-oncology MSc, BSc, RGN. LEARNING OBJECTIVES To refresh your knowledge about prostate cancer. To discuss the purpose and patient benefits
Guideline for the Follow-up of Patients with Gynaecological Malignancies
 Guideline for the Follow-up of Patients with Gynaecological Malignancies Version History Version Date Summary of Change/Process 2.0 20.02.08 Endorsed by the Governance Committee 2.1 18.11.10 Circulated
Guideline for the Follow-up of Patients with Gynaecological Malignancies Version History Version Date Summary of Change/Process 2.0 20.02.08 Endorsed by the Governance Committee 2.1 18.11.10 Circulated
Treatment Testicular Cancer Guidelines
 Treatment Testicular Cancer Guidelines Thank you very much for reading. As you may know, people have look hundreds times for their chosen readings like this, but end up in infectious downloads. Rather
Treatment Testicular Cancer Guidelines Thank you very much for reading. As you may know, people have look hundreds times for their chosen readings like this, but end up in infectious downloads. Rather
Urological Tumours 1 Kidney tumours 2 Bladder tumours
 Urological Tumours 1 Kidney tumours 2 Bladder tumours Tim Bracey SpR Histopathology Derriford Hospital Kidney tumours What are we going to talk about?! Anatomy of urinary tract! Types of kidney tumours!
Urological Tumours 1 Kidney tumours 2 Bladder tumours Tim Bracey SpR Histopathology Derriford Hospital Kidney tumours What are we going to talk about?! Anatomy of urinary tract! Types of kidney tumours!
Improving Outcomes for People with Sarcoma
 NHS National Institute for Health and Clinical Excellence Guidance on Cancer Services Improving Outcomes for People with Sarcoma List of All Recommendations March 2006 Developed for NICE by the National
NHS National Institute for Health and Clinical Excellence Guidance on Cancer Services Improving Outcomes for People with Sarcoma List of All Recommendations March 2006 Developed for NICE by the National
GUIDELINES ON RENAL CELL CARCINOMA
 GUIDELINES ON RENAL CELL CARCINOMA B. Ljungberg (chairman), D.C. Hanbury, M.A. Kuczyk, A.S. Merseburger, P.F.A. Mulders, J-J. Patard, I.C. Sinescu Introduction This EAU guideline was prepared to help urologists
GUIDELINES ON RENAL CELL CARCINOMA B. Ljungberg (chairman), D.C. Hanbury, M.A. Kuczyk, A.S. Merseburger, P.F.A. Mulders, J-J. Patard, I.C. Sinescu Introduction This EAU guideline was prepared to help urologists
Commissioning Urology Services in New NHS
 Commissioning Urology Services in New NHS Oct 2013 Commissioning Urology Services NHS England s Role Role of the new Clinical Reference Groups Current Issues / Work Programme for Urology Clinical Reference
Commissioning Urology Services in New NHS Oct 2013 Commissioning Urology Services NHS England s Role Role of the new Clinical Reference Groups Current Issues / Work Programme for Urology Clinical Reference
Guidelines for Management of Penile Cancer
 Guidelines for Management of Penile Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Sections 3, 5, 6 and 16 updated. Page 1 of 10 1. Scope
Guidelines for Management of Penile Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Sections 3, 5, 6 and 16 updated. Page 1 of 10 1. Scope
Quality Standards for Diagnosis and Treatment in Breast Units Across Greater Manchester
 Quality Standards for Diagnosis and Treatment in Breast Units Across Greater Manchester Greater Manchester Cancer Clinical Director: Mr Mohammed Absar Pathway Manager: Rebecca Price Pathway approval: 24
Quality Standards for Diagnosis and Treatment in Breast Units Across Greater Manchester Greater Manchester Cancer Clinical Director: Mr Mohammed Absar Pathway Manager: Rebecca Price Pathway approval: 24
Testicular Germ Cell Cancer Explained
 The Beatson West of Scotland Cancer Centre Pan Glasgow Urology / Oncology Patient Information Testicular Germ Cell Cancer Explained The Beatson West of Scotland Cancer Centre 1053 Great Western Road, Glasgow
The Beatson West of Scotland Cancer Centre Pan Glasgow Urology / Oncology Patient Information Testicular Germ Cell Cancer Explained The Beatson West of Scotland Cancer Centre 1053 Great Western Road, Glasgow
Costing report: Bladder cancer
 Putting NICE guidance into practice Costing report: Bladder cancer Implementing the NICE guideline on bladder cancer (NG2) Published: February 2015 Updated September 2015 to update the unit cost of transurethral
Putting NICE guidance into practice Costing report: Bladder cancer Implementing the NICE guideline on bladder cancer (NG2) Published: February 2015 Updated September 2015 to update the unit cost of transurethral
Subject Index. Androgen antiandrogen therapy, see Hormone ablation therapy, prostate cancer synthesis and metabolism 49
 OOOOOOOOOOOOOOOOOOOOOOOOOOOOOO Subject Index Androgen antiandrogen therapy, see Hormone ablation therapy, synthesis and metabolism 49 Bacillus Calmette-Guérin adjunct therapy with transurethral resection
OOOOOOOOOOOOOOOOOOOOOOOOOOOOOO Subject Index Androgen antiandrogen therapy, see Hormone ablation therapy, synthesis and metabolism 49 Bacillus Calmette-Guérin adjunct therapy with transurethral resection
Brain and CNS Clinical Network Group (CNG)
 Brain and CNS Clinical Network Group (CNG) Annual Report 2013-2014 This Annual Report has been agreed by: Title Name Date Agreed Brain and CNS CNG Chair CMSCN Cancer Clinical Lead CWW Area Team Medical
Brain and CNS Clinical Network Group (CNG) Annual Report 2013-2014 This Annual Report has been agreed by: Title Name Date Agreed Brain and CNS CNG Chair CMSCN Cancer Clinical Lead CWW Area Team Medical
Urological cancers why we need to change. Stakeholder workshop 14 March 2013
 Urological cancers why we need to change Stakeholder workshop 14 March 2013 Introduction and welcome Today s event aims to cover: 1. Background, clinical evidence and London Cancer s recommendations Q&As
Urological cancers why we need to change Stakeholder workshop 14 March 2013 Introduction and welcome Today s event aims to cover: 1. Background, clinical evidence and London Cancer s recommendations Q&As
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases
 Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Trimodality Therapy for Muscle Invasive Bladder Cancer
 Trimodality Therapy for Muscle Invasive Bladder Cancer Brita Danielson, MD, FRCPC Radiation Oncologist, Cross Cancer Institute Assistant Professor, Department of Oncology University of Alberta Edmonton,
Trimodality Therapy for Muscle Invasive Bladder Cancer Brita Danielson, MD, FRCPC Radiation Oncologist, Cross Cancer Institute Assistant Professor, Department of Oncology University of Alberta Edmonton,
DEPARTMENT OF ONCOLOGY ELECTIVE
 DEPARTMENT OF ONCOLOGY ELECTIVE 2015-2016 www.uwo.ca/oncology Oncology Elective Program Administrator: Ms. Kimberly Trudgeon Room A4-901C (Admin) LHSC London Regional Cancer Centre (Victoria Campus) Phone:
DEPARTMENT OF ONCOLOGY ELECTIVE 2015-2016 www.uwo.ca/oncology Oncology Elective Program Administrator: Ms. Kimberly Trudgeon Room A4-901C (Admin) LHSC London Regional Cancer Centre (Victoria Campus) Phone:
West Midlands Sarcoma Advisory Group
 West Midlands Sarcoma Advisory Group Guideline for the Initial Investigation and Referral to Specialist Sarcoma Multi Disciplinary Team for Suspected Bone Sarcoma Version History Version Date Brief Summary
West Midlands Sarcoma Advisory Group Guideline for the Initial Investigation and Referral to Specialist Sarcoma Multi Disciplinary Team for Suspected Bone Sarcoma Version History Version Date Brief Summary
Open clinical uro-oncology trials in Canada
 Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS WITH STAGE T1
Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS WITH STAGE T1
MANAGEMENT OF PATIENTS WITH METASTATIC SPINAL CORD COMPRESSION
 CLINICAL POLICY MANAGEMENT OF PATIENTS WITH METASTATIC SPINAL CORD COMPRESSION DOCUMENT REF: PCLASCORD (Version No. 1.4) Name and designation of policy author(s) Approved by (committee, group, manager)
CLINICAL POLICY MANAGEMENT OF PATIENTS WITH METASTATIC SPINAL CORD COMPRESSION DOCUMENT REF: PCLASCORD (Version No. 1.4) Name and designation of policy author(s) Approved by (committee, group, manager)
Activity Report April June 2012
 Urological Cancers Managed Clinical Network Activity Report April 2011- June 2012 Mr Seamus Teahan Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
Urological Cancers Managed Clinical Network Activity Report April 2011- June 2012 Mr Seamus Teahan Consultant Urologist MCN Clinical Lead Tom Kane MCN Manager CONTENTS EXECUTIVE SUMMARY 3 1. INTRODUCTION
Index. Surg Oncol Clin N Am 14 (2005) Note: Page numbers of article titles are in boldface type.
 Surg Oncol Clin N Am 14 (2005) 433 439 Index Note: Page numbers of article titles are in boldface type. A Abdominosacral resection, of recurrent rectal cancer, 202 215 Ablative techniques, image-guided,
Surg Oncol Clin N Am 14 (2005) 433 439 Index Note: Page numbers of article titles are in boldface type. A Abdominosacral resection, of recurrent rectal cancer, 202 215 Ablative techniques, image-guided,
Pathway specification for urological cancers
 London Cancer: Pathway specification for urological cancers Originally published May 2012, updated December 2012 Version 2.0 Contents 1. Introduction... 2 1.1. London Cancer... 2 1.2. Pathway specifications...
London Cancer: Pathway specification for urological cancers Originally published May 2012, updated December 2012 Version 2.0 Contents 1. Introduction... 2 1.1. London Cancer... 2 1.2. Pathway specifications...
ADJUVANT CHEMOTHERAPY...
 Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Open clinical uro-oncology trials in Canada
 CLINICAL TRIALS Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada ADRENOCORTICAL MALIGNANCIES
CLINICAL TRIALS Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada ADRENOCORTICAL MALIGNANCIES
Testicular cancer and other germ cell tumours. London Cancer Jonathan Shamash
 Testicular cancer and other germ cell tumours London Cancer 2018 Jonathan Shamash Background Testicular germ cell tumours are the commonest cancers of young men Overall they are curable but long term side
Testicular cancer and other germ cell tumours London Cancer 2018 Jonathan Shamash Background Testicular germ cell tumours are the commonest cancers of young men Overall they are curable but long term side
Update on Management of Malignant Spinal Cord Compression. Heino Hugel Consultant in Palliative Medicine University Hospital Aintree
 Update on Management of Malignant Spinal Cord Compression Heino Hugel Consultant in Palliative Medicine University Hospital Aintree Current Guidelines The symptoms of MSCC may be subtle and therefore careful
Update on Management of Malignant Spinal Cord Compression Heino Hugel Consultant in Palliative Medicine University Hospital Aintree Current Guidelines The symptoms of MSCC may be subtle and therefore careful
A. Service Specifications
 SCHEDULE 2 THE SERVICES A. Service Specifications Service Specification No: Service B14/S/c Urological cancers Specialised Testicular Cancer services Commissioner Lead For local completion Provider Lead
SCHEDULE 2 THE SERVICES A. Service Specifications Service Specification No: Service B14/S/c Urological cancers Specialised Testicular Cancer services Commissioner Lead For local completion Provider Lead
Health Board/Region: All-Wales
 Peer Review: Cancer Sub-site: Gynaecology Health Board/Region: All-Wales Cycle: Second Date of review: February 2018 This report describes the findings and themes observed by clinical review panels during
Peer Review: Cancer Sub-site: Gynaecology Health Board/Region: All-Wales Cycle: Second Date of review: February 2018 This report describes the findings and themes observed by clinical review panels during
North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer
 THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD
 CLINICAL TRIALS Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS
CLINICAL TRIALS Open clinical uro-oncology trials in Canada Eric Winquist, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer A PHASE II PROTOCOL FOR PATIENTS
CLINICAL TRIALS Open clinical uro-oncology trials in Canada George Rodrigues, MD, Eric Winquist, MD
 Open clinical uro-oncology trials in Canada George Rodrigues, MD, Eric Winquist, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer AN OPEN-LABEL, MULTICENTER, RANDOMIZED PHASE II
Open clinical uro-oncology trials in Canada George Rodrigues, MD, Eric Winquist, MD London Health Sciences Centre, London, Ontario, Canada bladder cancer AN OPEN-LABEL, MULTICENTER, RANDOMIZED PHASE II
Update on Haematuria and Bladder Cancer
 Update on Haematuria and Bladder Cancer Hugh Mostafid FRCS(urol) FEBU Consultant Urologist, Royal Surrey County Hospital and Honorary Senior Lecturer, University of Surrey Guildford None Declarations Recent
Update on Haematuria and Bladder Cancer Hugh Mostafid FRCS(urol) FEBU Consultant Urologist, Royal Surrey County Hospital and Honorary Senior Lecturer, University of Surrey Guildford None Declarations Recent
Clinical/Surgical trials that will change my practice
 Clinical/Surgical trials that will change my practice Mr Jim M Adshead Herts and Beds Urological Cancer Centre, Lister Hospital What s changed and where do I feel we are clutching at straws? Regional Specialist
Clinical/Surgical trials that will change my practice Mr Jim M Adshead Herts and Beds Urological Cancer Centre, Lister Hospital What s changed and where do I feel we are clutching at straws? Regional Specialist
Open clinical uro-oncology trials in Canada George Rodrigues, MD, Mary J. Mackenzie, MD, Eric Winquist, MD
 Open clinical uro-oncology trials in Canada George Rodrigues, MD, Mary J. Mackenzie, MD, Eric Winquist, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A MULTICENTRE, RANDOMIZED
Open clinical uro-oncology trials in Canada George Rodrigues, MD, Mary J. Mackenzie, MD, Eric Winquist, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A MULTICENTRE, RANDOMIZED
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012
 Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
Upper GI Malignancies Imaging Guidelines for the Management of Gastric, Oesophageal & Pancreatic Cancers 2012 Version Control This is a controlled document please destroy all previous versions on receipt
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER CERVIX
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER CERVIX Site Group: Gynecology Cervix Author: Dr. Stephane Laframboise 1. INTRODUCTION 3 2. PREVENTION 3 3. SCREENING AND
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER CERVIX Site Group: Gynecology Cervix Author: Dr. Stephane Laframboise 1. INTRODUCTION 3 2. PREVENTION 3 3. SCREENING AND
NICaN Testicular Germ Cell Tumours SACT protocols
 Reference No: Title: Author(s) Ownership: Approval by: Systemic Anti-Cancer Therapy (SACT) Guidelines for Germ Cell Tumours Dr Audrey Fenton Consultant Medical Oncologist, Dr Vicky Coyle Consultant Medical
Reference No: Title: Author(s) Ownership: Approval by: Systemic Anti-Cancer Therapy (SACT) Guidelines for Germ Cell Tumours Dr Audrey Fenton Consultant Medical Oncologist, Dr Vicky Coyle Consultant Medical
Guidelines for the Management of Urological Cancers within North Trent
 North Trent Cancer Network Guidelines for the Management of Urological Cancers within North Trent Version 4.0 May 2013 Produced by the North Trent Urology Group For Review 1 st May 2014 CONTENTS 1.0 Network
North Trent Cancer Network Guidelines for the Management of Urological Cancers within North Trent Version 4.0 May 2013 Produced by the North Trent Urology Group For Review 1 st May 2014 CONTENTS 1.0 Network
Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction
 Version History Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction Version Summary of change Date Issued 2.0 Endorsed by the Governance Committee 20.02.08 2.1 Circulated
Version History Guideline for the Management of Patients Suitable for Immediate Breast Reconstruction Version Summary of change Date Issued 2.0 Endorsed by the Governance Committee 20.02.08 2.1 Circulated
Patient Selection for Ablative Therapies. Adrian D Joyce Leeds UK
 Patient Selection for Ablative Adrian D Joyce Leeds UK Therapy Renal Cell Ca USA: 30,000 new cases annually >12,000 deaths RCC accounts for 3% of all adult malignancy 40% of patients will die from their
Patient Selection for Ablative Adrian D Joyce Leeds UK Therapy Renal Cell Ca USA: 30,000 new cases annually >12,000 deaths RCC accounts for 3% of all adult malignancy 40% of patients will die from their
Guideline for the referral to the Late Effects Multi Disciplinary Team
 Guideline for the referral to the Late Effects Multi Disciplinary Team Version History Version Date Summary of Change\Process 0.1 July 2010 Reviewed and approved by West Midlands Children s Cancer Network
Guideline for the referral to the Late Effects Multi Disciplinary Team Version History Version Date Summary of Change\Process 0.1 July 2010 Reviewed and approved by West Midlands Children s Cancer Network
Patient Group Direction for Rotavirus vaccine Version: ROTAVIRUS (Rotarix ) Start Date: 1 st July 2013 Expiry Date:30 th June 2015
 Patient Group Direction for Rotavirus vaccine Version: ROTAVIRUS-2013. 1 (Rotarix ) Start 1 st July 2013 Expiry 30 th June 2015 THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS:
Patient Group Direction for Rotavirus vaccine Version: ROTAVIRUS-2013. 1 (Rotarix ) Start 1 st July 2013 Expiry 30 th June 2015 THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS:
Acute Oncology Induction Training Specification
 Acute Oncology Induction Training Specification Author: Acute Oncology/Cancer of Unknown Primary Clinical Network Group (AO/CUP CNG) V. 2.0 Agreed: 30.11.2012 Reviewed: May 2015 Review : May 2016 MCCN
Acute Oncology Induction Training Specification Author: Acute Oncology/Cancer of Unknown Primary Clinical Network Group (AO/CUP CNG) V. 2.0 Agreed: 30.11.2012 Reviewed: May 2015 Review : May 2016 MCCN
Open clinical uro-oncology trials in Canada
 Open clinical uro-oncology trials in Canada George Rodrigues, MD, Eric Winquist, MD, Mary J. Mackenzie, MD London Health Sciences Centre, London, Ontario, Canada ADRENOCORTICAL MALIGNANCIES CISPLATIN-BASED
Open clinical uro-oncology trials in Canada George Rodrigues, MD, Eric Winquist, MD, Mary J. Mackenzie, MD London Health Sciences Centre, London, Ontario, Canada ADRENOCORTICAL MALIGNANCIES CISPLATIN-BASED
Faster Cancer Treatment Indicators: Use cases
 Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
