Symptom or faecal immunochemical test based referral criteria for colorectal cancer detection in symptomatic patients: a diagnostic tests study
|
|
|
- Bryce Cole
- 5 years ago
- Views:
Transcription
1 Herrero et al. BMC Gastroenterology (2018) 18:155 RESEARCH ARTICLE Symptom or faecal immunochemical test based referral criteria for colorectal cancer detection in symptomatic patients: a diagnostic tests study Jesús-Miguel Herrero 1,2, Pablo Vega 1,2, María Salve 1,2, Luis Bujanda 3 and Joaquín Cubiella 1,2* Open Access Abstract Background: Symptom based referral criteria for colorectal cancer (CRC) detection are the cornerstone of the strategy to improve prognosis in CRC. In 2017, the National Institute for Health and Care Excellence (NICE) updated their referral criteria (2017 NG12). Recently, several studies have evaluated the faecal haemoglobin (f-hb) concentration in this setting. The aim of this study is to evaluate the diagnostic accuracy of the 2017 NG12 referral criteria and to compare them with the CG27 referral criteria, the f-hb concentration and two f-hb based prediction model: COLONPREDICT and FAST Score. Methods: This is a post-hoc diagnostic test study performed within the COLONPREDICT study database (1572 patients, CRC prevalence 13.6%). We assessed symptoms, the 2017 NG12 and CG27 referral criteria and determined the f-hb before performing a colonoscopy. We compared the discriminatory ability using the area under the curve (AUC) and the sensitivity and specificity at pre-stablished thresholds with the McNemar s test. Results: The 2017 NG12 referral criteria discriminatory ability (AUC 0.53; 95% confidence interval- CI ) was inferior to the CG27 version (AUC 0.59; 95% CI ; p = 0.01), the f-hb concentration (AUC 0.86; 95% CI ; p < 0.001), the COLONPREDICT Score (AUC 0.92; 95% CI ; p < 0.001) or the FAST Score (AUC 0.87; 95% CI ; p < 0.001). The number of patients meeting each criteria were as follows: 2017 NG12 and CG27 = 94.1% and 52. 2%; f-hb 20 and 10 μg/g faeces = 38.6 and 44.3%; COLONPREDICT Score 5.6 and 3.2 = 29.4 and 63.2% and FAST Score 4.50 and 2.12 = 37.1 and 87.0%. The 2017 NG12 criteria were more sensitive (100%) than the CG27 criteria (68. 2%), the f-hb ( 20 μg/g) (91.2%), the f-hb ( 10 μg/g) (93.5%), the COLONPREDICT Score ( 5.6) (90.1%) and the FAST Score ( 4.50) (89.8%) (p 0.001) and equivalent to the COLONPREDICT Score ( 3.5) (99.5%) or the FAST Score ( 2.12) (100.0%) (p = 1). However, their specificity (6.8%) was significantly lower than any of the evaluated criteria (50.3%, 69.6%, 63.4%, 78.7%, 45.8%, 71.3%, 13.9%; p <0.001). Conclusion: Referral criteria based on f-hb measurement, either as a single test or within prediction models, are more accurate than symptom-based referral criteria for CRC detection in symptomatic patients. Keywords: Colorectal cancer, Faecal immunochemical test, Diagnostic accuracy, Risk stratification * Correspondence: joaquin.cubiella.fernandez@sergas.es 1 Department of Gastroenterology, Complexo Hospitalario Universitario de Ourense, Ourense, Spain 2 Instituto de Investigación Biomédica Galicia Sur, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd),, Ourense, Spain Full list of author information is available at the end of the article The Author(s) Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( applies to the data made available in this article, unless otherwise stated.
2 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 2 of 10 Background Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancerrelated death [1]. Two strategies are widely used to detect the disease at an early stage and, thus, improve the prognosis: CRC screening and early diagnosis strategies in symptomatic patients [2, 3]. Although screening programmes have been progressively implemented, most CRC are still detected when symptoms become apparent [4]. In addition, although gastrointestinal symptoms are extremely common in the population, the probability of CRC detection associated with any one symptom is low [5 7]. Thus, risk classification scores have been developed based on symptoms to determine which patients are most at risk of CRC with the aim of reducing this interval between the initial consultation and diagnostic colonoscopy [8, 9]. In this regard, one of the best known referral criteria for CRC detection are the National Institute for Health and Care Excellence (NICE) referral guideline for suspected cancer (CG27) [3]. This referral system has been extensively evaluated showing a low specificity and a variable sensitivity for CRC detection [6, 10 12]. In order to improve these results, the updated version of 2015 (NG12) introduced two significant changes. First, they recommended referral for those symptoms with a positive predictive value of 3% instead of previous 5%. Second, for the first time, testing for occult blood in faeces was recommended in several symptom scenarios with a positive predictive value below 3% [13]. However, the guideline did not recommend any particular method to determine occult blood in faeces. Faecal immunochemical tests for haemoglobin (FIT) allow for quantitation of faecal haemoglobin concentration (f-hb). FIT has proven to be the best currently available non-invasive test for CRC screening in asymptomatic individuals and an excellent test for rule-in of CRC and rule-out of significant colonic lesions (SCL) in patients presenting with lower gastrointestinal symptoms [14 21]. On the basis of the available evidence [22], the NICE diagnostic guidance (DG30) recommends the use of FIT with a 10 μg Hb/g faeces to guide referral for colorectal cancer in primary care [23]. However, the effect of the NG12 is not well understood and only one study has evaluated the diagnostic accuracy of this guidance [24]. In July 2017, NG 12 was amended and testing for occult blood in faeces was recommended in patients without rectal bleeding but with unexplained symptoms that do not meet the criteria for a suspected cancer pathway [13]. We have recently developed and validated two f-hb based prediction models for CRC detection: COLON- PREDICT and FAST. The database of the COLONPRE- DICT Score derivation cohort [25, 26]. is an excellent platform to compare the most widely symptom based referral criteria with the f-hb concentration based strategies. In this database, an extensive collection of information regarding symptoms as well as several blood and faecal determinations are included. This information allowed us to perform a post hoc analysis in order to evaluate the diagnostic accuracy of the 2017 NG12, compare these criteria with the CG27, the f-hb concentration and two CRC prediction models based on the f-hb concentration: COLONPREDICT and FAST Scores [25, 26]. Methods Study design The current study is a post hoc analysis performed within the COLONPREDICT study: a multicentre, cross-sectional, blinded study of diagnostic tests. The study aimed to create and validate a CRC prediction index based on available biomarkers, clinical and demographical data. We performed this post hoc analysis in the 1572 patients included in the derivation previously described [25]. Brief description of the COLONPREDICT study The details of the study have been described extensively elsewhere and are summarized here [25, 26]. We used the Colonoscopy Research into Symptom Prediction questionnaire (CRISP) to record symptoms and demographic data [27]. Based on this questionnaire, they determined if patients met the CG27 referral criteria for CRC detection [3]. f-hb concentration was assessed using the automated OC-SENSOR MICRO analyser (Eiken Chemical Co., Ltd., Tokyo, Japan). The faeces for the f-hb determination were collected using the OC-Sensor probe. Moreover, we determined blood haemoglobin (b-hb) and mean corpuscular volume with a Beckman Coulter Autoanalyzer (Beckman Coulter Inc., CA, USA). Colonoscopy was performed blind for the questionnaire and analytical results NG12 referral criteria and the f-hb based prediction models calculation On the basis of the information obtained from the CRISP questionnaire and the analysis performed (f-hb, b-hb and mean corpuscular volume), we determined which of the 2017 NG12 criteria for CRC suspicion were met. Two researchers (JMH and JC) independently decided the equivalence between each NICE criteria and the information collected. Finally, they reached a consensus version. NG12 referral criteria are shown in Table 1 [13]. We considered a positive faecal occult blood test if the f-hb concentration was 10 μg Hb/g faeces. COLONPREDICT score is a CRC prediction model based on a multivariable logistic regression analysis [25]. The COLONPREDICT score is based in eleven variables and the mathematical formula is as follows: x
3 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 3 of 10 Table 1 Criteria to refer people using a suspected cancer pathway referral for CRC according to the NG12 referral criteria. The number of patients meeting each of the referral criteria is shown Criteria Number of patients (n = 1572) Patients 40 years with unexplained weight loss and 196 (12.5%) abdominal pain Patients 50 years with unexplained rectal bleeding 811 (51.6%) Patients 60 years with: iron deficiency anaemia or changes in their bowel habit 890 (56.7%) Patients with a rectal or abdominal mass 80 (5.1%) Adults < 50 years with rectal bleeding and any of the following unexplained symptoms or findings: abdominal pain, change in bowel habit, weight loss or iron-deficiency anaemia. Offer testing for occult blood in faeces to assess for colorectal cancer in adults without rectal bleeding who but with unexplained symptoms that do not meet the criteria for a suspected cancer pathway referral A positive test for occult blood in faeces was considered if the haemoglobin concentration was 10 μg Hb/g faeces. 124 (7.9%) 78 (4.9%) Any of the referral criteria 1479 (94.1%) rectal bleeding x change in bowel habit x rectal mass x benign anorectal lesions x f-hb 20 μg Hb/g faeces x b-hb (< 10 g/dl) x b-hb (10 12 g/dl) x CEA 3 ng/ml x age (years) x sex (male) x previous colonoscopy (last 10 years) x continuous treatment with aspirin. It shows a high diagnostic accuracy for CRC detection. Two thresholds have been defined with 90% and 99% sensitivity for CRC: 5.6 and 3.5. FAST Score is a CRC prediction model based on a multivariable logistic regression analysis [26]. The FAST score is based on three variables and the mathematical formula is as follows: 0 x f-hb (0) μg Hb/g faeces x f-hb (1, 19) x f-hb [20, 200) μg Hb/g faeces x f-hb 200 μg Hb/g faeces x age (years) x sex (male). Two thresholds have been defined with 90% and 99% sensitivity for CRC: 4.50 and Outcomes The main outcome was CRC detection. According to previous studies evaluating FIT in symptomatic patients, [14 21] we considered significant colonic lesion (SCL) detection as the secondary outcome. We defined SCL as CRC, advanced adenoma ( 10 mm, villous histology, high-grade dysplasia), polyposis (> 10 polyps of any histology, including serrated lesions), histologically confirmed colitis (any aetiology), polyps 10 mm, complicated diverticular disease (diverticulitis, bleeding), colonic ulcer and/ or bleeding angiodysplasia. Statistical analysis First, we performed a descriptive analysis of the population included in the study. In order to determine differences in diagnostic accuracy between the NG12 referral criteria and the rest of diagnostic criteria, the CG27 referral criteria, the f-hb concentration, the COLONPRE- DICT and the FAST score we performed two analysis. First, we determined the number of individuals with a positive result and the sensitivity and the specificity for CRC and SCL detection. We determined if the differences between the sensitivity and the specificity of the NG12 referral criteria and the rest of diagnostic criteria, CG27 referral criteria, the COLONPREDICT and the FAST scores at the pre-stablished thresholds and the f-hb at a 10 and 20 μg Hb/g faeces concentration threshold, were statistically significant using the McNemar s test. Finally, we also calculated the positive and negative predictive value (PPV, NPV), the positive and negative likelihood ratios (LR) and the diagnostic Odds Ratio (OR) of all the diagnostic tests. Diagnostic OR is defined as the odds of positivity in subjects with disease relative to the odds in subjects without disease. In a second step, we evaluated the discriminatory ability using receiver-operating characteristic (ROC) curves for CRC and SCL diagnosis, and we calculated the area under the curve (AUC). We determined whether there were statistically significant differences using the chi-square test of homogeneity of areas. Additionally, we determined if there were differences in the discriminatory ability of each of the diagnostic criteria according to the healthcare level referring the patient to colonoscopy. Primary healthcare referral was determined when a general practitioner was requesting the colonoscopy and secondary healthcare referral was determined when a specialist (gastroenterologist, surgeon..) was requesting the exploration. We report differences with 95% confidence intervals (CI) and their significance. We consider a p-value < 0.05 statistically significant. We carried out the analyses using the IBM SPSS Statistics for Windows version 21.0 (IBM Corp, Armonk, USA) and EPIDAT 3.1 (Dirección Xeral de Saúde Pública, Santiago de Compostela, Spain). Results Description of the cohort Among the 1572 patients included in the derivation cohort of the COLONPREDICT, a CRC was detected in 214 (13.6%) patients and a SCL in 463 (29.5%) patients: advanced adenomas in 251 (16.0%), a polyp 10 mm with non-adenoma histology in 6 (0.4%), colitis in 36 (2.3%) and other SCLs in 6 (0.4%) patients. Direct referrals from primary care to endoscopic evaluation accounted for 22.9% of the patients included.
4 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 4 of 10 As we show in the Table 1, 1,479 out of the 1572 (94.1%) met at least one of the 2017 NG12 referral criteria. In contrast, 52.2% of the patients met any of the CG27 referral criteria, 38.7% had a f-hb concentration 20 μg Hb/g faeces, 44.4% had a f-hb concentration 10 μg Hb/g faeces, 30.9% had a COLONPREDICT Score 5.6, 60.5% had a COLONPREDICT Score 3.5, 37.1% had a FAST Score 4.50 and 88.0% had a FAST Score Analysis of the diagnostic accuracy The sensitivity of the 2017 NG12 referral criteria for CRC detection reaches 100% at the expense of a low specificity (6.8%). As we show in the Table 2, the sensitivity of the 2017 NG12 referral criteria is superior to the sensitivity of the CG27 referral criteria, the f-hb ( 20 μg Hb/g and 10 μg Hb/g faeces), the COLON- PREDICT Score at a 5.6 threshold (p < 0.001) and the FAST Score at a 4.50 threshold. In contrast, the sensitivity is similar to the COLONPREDICT Score at a 3.2 threshold and the FAST Score at a 2.12 threshold (p =1) and the specificity is inferior to any of the other criteria (p < 0.001). The rest of the diagnostic accuracy analysis is displayed in Table 2. On the other hand, 2017 NG12 referral criteria allows the diagnosis of 98.9% of SCL. As in the diagnostic accuracy for CRC detection, the specificity is extremely low (7.9%). As we show in the Table 3, the sensitivity of the 2017 NG12 referral criteria is similar to the FAST Score at a 2.12 threshold (p = 1) and superior the rest of the evaluated criteria. In contrast, the specificity of the 2017 NG12 criteria is inferior to any of the additional criteria evaluated (p < 0.001). We show the PPV, NPV, positive and negative LR and the diagnostic OR in Table 2. Analysis of the discriminatory ability The analysis of the discriminatory ability for CRC detection of the NICE referral criteria, the f-hb concentration, the COLONPREDICT and the FAST Score is shown in Fig. 1. The discriminatory ability of the 2017 NG12 referral criteria is inferior to any of the evaluated criteria in the Chi-square homogeneity test comparison of AUC. Additionally, we found no differences in the performance of each diagnostic test in the evaluation of the discriminatory ability according to the healthcare referring the patient to colonoscopy: 2017 NG12 referral criteria (primary = 0.53, 95% CI ; secondary = 0.53, 95% CI ; p = 0.1), CG27 referral criteria (primary = 0.60, 95% CI ; secondary = 0.59, 95% CI ; p = 0.7), f-hb concentration (primary = 0.85, 95% CI ; secondary = 0.86, 95% CI ; p = 0.5), COLONPREDICT Score (primary = 0.90, 95% CI ; secondary = 0.93, 95% CI ; p = 0.1), FAST Score (primary = 0.84, 95% CI ; secondary = 0.88, 95% CI ; p = 0.1). Fig. 2 shows the discriminatory ability for SCL detection of the diagnostic tests evaluated. The discriminatory ability of the 2017 NG12 referral criteria is similar to the CG27 referral criteria and inferior to the rest of the evaluated criteria in the Chi-square homogeneity test comparison of AUC. Discussion Summary We have evaluated the diagnostic accuracy of the 2017 NG12 referral criteria for suspected CRC. As we clearly show, these updated criteria are more sensitive than CG27 version. However, they produce a marked increase in the number of patients meeting them and a reduction in the specificity. Furthermore, we had the opportunity to compare them with the f-hb concentration and two f-hb based prediction model. As we clearly show, both diagnostic tools have a higher discriminatory ability than the NICE referral criteria. Strengths and limitations We have used a wide cohort of consecutive patients referred to colonoscopy due to gastrointestinal symptoms. Patients were evaluated homogenously using a symptom questionnaire and several analytics, including a FIT, were performed before colonoscopy. This questionnaire allowed us to gather all the details regarding type of symptoms, duration and evolution. Thus, we could evaluate the 2017 NG12 referral criteria for suspected CRC for the first time. Furthermore, we have been able to evaluate the use of FIT, with the 10 μg Hb/g faeces, as recommended in the DG30 [23]. On the other hand, our study has several limitations that must be taken in consideration. We cannot exclude a risk of bias of selection, as long as the symptomatic patients included in the study were previously selected for colonoscopy evaluation. However, we have included all consecutive patients referred both from primary and secondary healthcare to colonoscopy. Comparison with existing literature The update of the NICE referral criteria is based on reducing the PPV threshold required to refer patients from primary care using a suspected cancer pathway referral. The guideline development group agreed to use a threshold value of 3% PPV to underpin their recommendations [13]. So, those patients with symptoms (i.e 40 years with unexplained weight loss) with a PPV > 3% for CRC should be referred for further testing. Our results clearly demonstrate that this strategy increases the sensitivity for CRC detection in comparison with previous criteria. However, these criteria certainly introduce a risk of over investigation. In fact, what our analysis
5 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 5 of 10 Table 2 Evaluation of the diagnostic accuracy of the evaluated strategies for colorectal cancer detection Sensititvity 1 P 2 Specificity 1 P 3 Positive PV 1 Negative PV 1 Positive LR 4 Negative LR 4 Diagnostic OR NG12 referral criteria (n = 1479) 100% ( ) 6.8% ( ) 14.5% ( ) 100% ( ) 1.07 ( ) NE NE CG27 referral criteria (n = 821) 68.2% ( ) p < % ( ) p < % ( ) 91.0% ( ) 1.4 ( ) 0.6 ( ) 2.2 ( ) f-hb 20 μg Hb/g faeces (n = 607) 91.2% ( ) p < % ( ) p < % ( ) 98.0% ( ) 3.0 ( ) 0.1 ( ) 23.6 ( ) f-hb 10 μg Hb/g faeces (n = 696) 93.5% ( ) p = % ( ) p < % ( ) 98.4 ( ) 2.6 ( ) 0.1 ( ) 24.8 ( ) COLONPREDICT Score 5.6 (n = 463) 90.1% ( ) p < % ( ) p < % ( ) 98.0% ( ) 4.2 ( ) 0.1 ( ) 33.8 ( ) COLONPREDICT Score 3.2 (n = 994) 99.5% ( ) % ( ) p < % ( ) 99.8% ( ) 1.8 ( ) 0.01 ( ) 179 ( ) FAST Score 4.50 (n = 583) 89.8% ( ) p < % ( ) p < % ( ) 97.8% ( ) 3.13 ( ) 0.14 ( ) 21.8 ( ) FAST Score 2.12 (n = 1383) 100.0% ( ) p = % ( ) p < % ( ) 100% ( ) 1.16 ( ) NE NE 1 Values are expressed as percentages and its 95% confidence interval 2 Significance of the sensitivity differences when compared with the NG12 referral criteria in McNemar s test. Differences with p < 0.05 are considered statistically significant 3 Significance of the specificity differences when compared with the NG12 referral criteria in McNemar s test. Differences with p < 0.05 are considered statistically significant 4 Values are expressed as absolute numbers and its 95% confidence interval PV, predictive value; LR, likelihood ratio; OR, odds ratio; f-hb, faecal haemoglobin; NE, non evaluable
6 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 6 of 10 Table 3 Evaluation of the diagnostic accuracy of the evaluated strategies for significant colonic lesion detection Sensititvity 1 P 2 Specificity 1 P 3 Positive PV 1 Negative PV 1 Positive LR 4 Negative LR 4 Diagnostic OR NG12 referral criteria (n = 1479) 98.9% ( ) 7.9% ( ) 30.9% ( ) 94.6% ( ) 1.07 ( ) 0.14 ( ) 7.9 ( ) CG27 referral criteria (n = 821) 58.3% ( ) p < % ( ) p < % ( ) 74.3% ( ) 1.17 ( ) 0.83 ( ) 1.4 ( ) f-hb 20 μg Hb/g faeces (n = 607) 74.2% ( ) p < % ( ) p < % ( ) 87.6% ( ) 3.11 ( ) 0.34 ( ) 9.2 ( ) f-hb 10 μg Hb/g faeces (n = 696) 79.4% ( ) p < % ( ) p < % ( ) 89.1% ( ) 2.67 ( ) 0.29 ( ) 9.1 ( ) COLONPREDICT Score 5.6 (n = 463) 64.2% ( ) p < % ( ) p < % ( ) 84.7% ( ) 3.79 ( ) 0.43 ( ) 8.8 ( ) COLONPREDICT Score 3.2 (n = 994) 88.7% ( ) p < % ( ) p < % ( ) 91.5% ( ) 1.82 ( ) 0.22 ( ) 8.3 ( ) FAST Score 4.50 (n = 583) 72.7% ( ) p < % ( ) p < % ( ) 87.2% ( ) 3.28 ( ) 0.35 ( ) 9.4 ( ) FAST Score 2.12 (n = 1383) 97.8% ( ) p = % ( ) p < % ( ) 94.7% ( ) 1.17 ( ) 0.13 ( ) 8.7 ( ) 1 Values are expressed as percentages and its 95% confidence interval 2 Significance of the sensitivity differences when compared with the NG12 referral criteria in McNemar s test. Differences with p < 0.05 are considered statistically significant 3 Significance of the specificity differences when compared with the NG12 referral criteria in McNemar s test. Differences with p < 0.05 are considered statistically significant 4 Values are expressed as absolute numbers and its 95% confidence interval PV, predictive value; LR, likelihood ratio; OR, odds ratio; f-hb, faecal haemoglobin; NE, non evaluable
7 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 7 of 10 Fig. 1 ROC curves of the NICE referral criteria, faecal haemoglobin concentration and the COLONPREDICT and FAST Scores for colorectal cancer detection. The area under the curve of the ROC curves are shown. 1 Significance of the discriminatory ability differences when compared with the NG12 referral criteria in Chi square homogeneity test. Differences with p < 0.05 are considered statistically significant. ROC, Receiver-operating characteristics; NICE, National Institute for Health and Care Excellence confirms is that the discriminatory ability of any group of symptoms for CRC detection is suboptimal [6]. An additional innovation of the 2017 NG12 referral criteria is the inclusion of the faecal occult blood test in the evaluation of symptomatic patients. However, its use is only limited to patients without rectal bleeding and with unexplained symptoms that do not meet the criteria for a suspected cancer pathway referral [13]. Our results confirm the data previously published: the f-hb concentration measured with a FIT shows a higher discriminatory ability for CRC detection than the NICE referral criteria [14 19]. So, probably, the strategy for the evaluation of the risk of CRC detection in symptomatic patients should be based on the f-hb concentration irrespective of symptoms. Actually, in the COLONPRE- DICT Score, patients with a f-hb concentration 20 μg Hb/g faeces have 17.0 times more risk of CRC detection. In contrast, patients with rectal bleeding or a change in bowel habit have 2.2 and 1.7 times more risk of CRC detection, respectively [25]. Recently, an article has evaluated the diagnostic accuracy of the 2017 NG12 referral criteria for CRC and SCL detection and compared these criteria with the f-hb concentration [24]. This study used the database of three diagnostic tests studies evaluating FIT in symptomatic patients [15, 17, 19]. and shows that the discriminatory ability of the 2017 NG12 referral criteria are inferior to the f-hb concentration. This cohort has significant differences with ours: the prevalence of symptoms related to CRC diagnosis, rectal bleeding, changes in bowel habit, iron-deficiency anaemia or rectal mass, is inferior as well as the prevalence of CRC or SCL. Probably, these differences are responsible for the differences in the number of patients that meet 2017 NG12 referral criteria and in the inferior discriminatory ability documented in our study. However, are results are consistent
8 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 8 of 10 Fig. 2 ROC curves of the NICE referral criteria, faecal haemoglobin concentration and the COLONPREDICT and FAST Scores for significant colonic lesion detection. The area under the curve of the ROC curves are shown. 1 Significance of the discriminatory ability differences when compared with the NG12 referral criteria in Chi square homogeneity test. Differences with p < 0.05 are considered statistically significant. ROC, Receiveroperating characteristics; NICE, National Institute for Health and Care Excellence in the comparison of the 2017 NG12 referral criteria with the f-hb concentration. Implications for research and/or practice One of the main lessons learned in these years from the CRC screening programs is that lack of symptoms or the presence of non-specific symptoms do not exclude a CRC in adult population. Up to 20% of the incident CRC are detected in asymptomatic patients within a CRC screening program based in a guaiac faecal occult blood test [4]. So, the strategies for CRC detection in symptomatic patients should determine which patients require urgent referral, which require a normal referral and, finally, in what situations no additional evaluation is required. The NICE referral criteria only determine the scenarios where an urgent referral is required. Due to the increased discriminatory ability of the FIT for CRC, either the f-hb concentration alone or a f-hb based prediction model can allow to establish these three risk groups with different diagnostic strategies. As we have recently proposed, at least 90% of CRC should be detected in a high-risk group, requiring a fast-track referral to colonoscopy. In contrast, in a low-risk group, where no additional explorations are required, the probability of a missing CRC should be well below 1%, so that the risk of CRC is balanced with the risk of colonoscopy complications, mainly perforation [28]. Conclusions To conclude, the discriminatory ability of any symptom based criteria is limited when compared with a f-hb concentration based strategy. An urgent evaluation of the diagnostic accuracy of FIT in symptomatic patients attending primary care is required.
9 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 9 of 10 Abbreviations AUC: Area under the curve; CEA: Carcinoembryonic antigen; CI: Confidence interval; CRC: Colorectal cancer; CRISP: Colonoscopy Research into Symptom Prediction questionnaire; f-hb: faecal haemoglobin; FIT: Faecal immunochemical test; LR: Likelihood ratio; NICE: National Institute for Health and Care Excellence; NPV: Negative predictive value; OR: Odds Ratio; PPV: Positive predictive value; ROC: Receiver-operating characteristic; SCL: Significant colonic lesion Meeting presentations This research was partially presented in the XIX Reunión Nacional de la Asociación Española de Gastroenterologia held in Madrid 1-4th March, Funding This study was supported by a grant from Instituto de Salud Carlos III, Madrid, Spain (PI11/00094). Instituto de Salud Carlos III had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. JC has received an intensification grant through the European Commission funded BIOCAPS project (FP-7-REGPOT , Grant agreement no. FP ). Availability of data and materials All data generated or analysed during this study are included in this published article and its supplementary information files. Authors contributions The authors contributions were as follows: JH and JC designed the analysis, PV, MS and LB collected the data, JH and JC performed the analysis and wrote the manuscript, and all the authors decided to submit the article for publication. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. JC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript. Ethics approval and consent to participate Galician Clinical Research Ethics Committee approved this study (Code 2011/ 038) under resolution dated 2nd March We accessed patients clinical histories for study purposes in accordance with the research protocols laid down by clinical documentation departments. Patients provided written informed consent. Consent for publication Not applicable. Competing interests JC and MS had financial support from Instituto de Salud Carlos III for the submitted work but they had no financial relationships with any organisations that might have an interest in the submitted work in the previous five years, and no other relationships or activities that could appear to have influenced the submitted work. The remaining authors had no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous five years and no other relationships or activities that could appear to have influenced the submitted work. Publisher s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Author details 1 Department of Gastroenterology, Complexo Hospitalario Universitario de Ourense, Ourense, Spain. 2 Instituto de Investigación Biomédica Galicia Sur, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd),, Ourense, Spain. 3 Donostia Hospital, Biodonostia Institute, University of the Basque Country UPV/EHU, CIBERehd, San Sebastian, Spain. Received: 18 March 2018 Accepted: 16 October 2018 References 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN Int J Cancer. 2010; 127: Zauber AG, Knudsen AB, Rutter CM, Lansdorp-Vogelaar I, Savarino JE, van Ballegooijen M, Kuntz KM. Cost-Effectiveness of CT Colonography to Screen for Colorectal Cancer. Technol. Assess. Rep. 2009: nlm.nih.gov/pubmed/ NICE Clinical Guideline 27- Referral guidelines for suspected cancer. April [Internet] [cited 2005 May 20]. Available from: 4. Mansouri D, McMillan DC, Crearie C, Morrison DS, Crighton EM, Horgan PG. Temporal trends in mode, site and stage of presentation with the introduction of colorectal cancer screening: a decade of experience from the west of Scotland. Br J Cancer. 2015;113: Ford AC. Veldhuyzen van Zanten SJO, Rodgers CC, Talley NJ, Vakil NB, Moayyedi P. diagnostic utility of alarm features for colorectal cancer: systematic review and meta-analysis. Gut. 2008;57: Jellema P, van der Windt DA, Bruinvels DJ, Mallen CD, van Weyenberg SJ, Mulder CJ, et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ. 2010;340:c Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011;61:e Cotterchio M, Manno M, Klar N, McLaughlin J, Gallinger S. Colorectal screening is associated with reduced colorectal cancer risk: a case-control study within the population-based Ontario familial colorectal cancer registry. Cancer Causes Control. 2005;16: Williams TGS, Cubiella J, Griffin SJ, Walter FM, Usher-Smith JA. Risk prediction models for colorectal cancer in people with symptoms: a systematic review. BMC Gastroenterol. 2016;16: Zafar A, Mak T, Whinnie S, Chapman MA. The 2-week wait referral system does not improve 5-year colorectal cancer survival. Colorectal Dis. 2012;14: e Rai S, Kelly MJ. Prioritization of colorectal referrals: a review of the 2-week wait referral system. Color Dis. 2007;9: Thorne K, Hutchings HA, Elwyn G. The effects of the Two-Week Rule on NHS colorectal cancer diagnostic services: a systematic literature review. BMC Health Serv Res. 2006;6: National Institute for Health and Clinical Excellence. Suspected cancer: recognitioin and referral [Internet]. NICE Guidel [cited 2018 Mar 18]. Available from: Oono Y, Iriguchi Y, Doi Y, Tomino Y, Kishi D, Oda J, et al. A retrospective study of immunochemical fecal occult blood testing for colorectal cancer detection. Clin. Chim. Acta. 2010;411: McDonald PJ, Digby J, Innes C, Strachan JA, Carey FA, Steele RJC, et al. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis. [Internet] [cited 2013 Jan 20];15:e151 e Cubiella J, Salve M, Díaz-Ondina M, Vega P, Alves MT, Iglesias F, et al. Diagnostic accuracy of the faecal immunochemical test for colorectal cancer in symptomatic patients: comparison with NICE and SIGN referral criteria. Color Dis. 2014;16:O Mowat C, Digby J, Strachan JA, Wilson R, Carey FA, Fraser CG, et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut. 2016;65(9): Rodríguez-Alonso L, Rodríguez-Moranta F, Ruiz-Cerulla A, Lobatón T, Arajol C, Binefa G, et al. An urgent referral strategy for symptomatic patients with suspected colorectal cancer based on a quantitative immunochemical faecal occult blood test. Dig Liver Dis. 2015;47: Godber IM, Todd LM, Fraser CG, MacDonald LR. Younes H ben. Use of a faecal immunochemical test for haemoglobin can aid in the investigation of patients with lower abdominal symptoms. Clin. Chem. Lab. Med. 2016;54: Auge JM, Fraser CG, Rodriguez C, Lopez-Ceron M, Castells A, Roset A, et al. Clinical utility of one versus two faecal immunochemical test samples in the detection of advanced colorectal neoplasia in symptomatic patients. Clin Chem Lab Med. 2015;54:
10 Herrero et al. BMC Gastroenterology (2018) 18:155 Page 10 of Widlak MM, Thomas CL, Thomas MG, Tomkins C, Smith S, O Connell N, et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment Pharmacol Ther. 2017;45: Westwood M, Lang S, Armstrong N, van Turenhout S, Cubiella J, Stirk L, et al. Faecal immunochemical tests (FIT) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: a systematic review conducted to inform new NICE DG30 diagnostic guidance. BMC Med. 2017;15: Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care [Internet]. [cited 2017 Mar 11]. Available from: Quyn AJ, Steele RJ, Digby J, Strachan JA, Mowat C, McDonald PJ, et al. Application of NICE guideline NG12 to the initial assessment of patients with lower gastrointestinal symptoms: not FIT for purpose? Ann Clin Biochem. 2017;0: Cubiella J, Vega P, Salve M, Díaz-Ondina M, Alves MT, Quintero E, et al. Development and external validation of a faecal immunochemical testbased prediction model for colorectal cancer detection in symptomatic patients. BMC Med. 2016;14: Cubiella J, Digby J, Rodríguez-Alonso L, Vega P, Salve M, Díaz-Ondina M, et al. The fecal hemoglobin concentration, age and sex test score: development and external validation of a simple prediction tool for colorectal cancer detection in symptomatic patients. Int. J. cancer [internet]. 2017;140: Adelstein B-A, Irwig L, Macaskill P, Katelaris PH, Jones DB, Bokey L. A self administered reliable questionnaire to assess lower bowel symptoms. BMC Gastroenterol. 2008;8: Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31 53.
Risk assessment tools for the symptomatic population Graham Radford-Smith Department of Gastroenterology and Hepatology Royal Brisbane and Women s
 Risk assessment tools for the symptomatic population Graham Radford-Smith Department of Gastroenterology and Hepatology Royal Brisbane and Women s Hospital GI inflammation IBD Group QIMR Berghofer Medical
Risk assessment tools for the symptomatic population Graham Radford-Smith Department of Gastroenterology and Hepatology Royal Brisbane and Women s Hospital GI inflammation IBD Group QIMR Berghofer Medical
University of Dundee. Published in: Annals of Clinical Biochemistry DOI: / Publication date: 2017
 University of Dundee Application of NICE guideline NG12 to the initial assessment of patients with lower gastrointestinal symptoms Quyn, Aaron J.; Steele, Robert; Digby, Jayne; Strachan, Judith A.; Mowat,
University of Dundee Application of NICE guideline NG12 to the initial assessment of patients with lower gastrointestinal symptoms Quyn, Aaron J.; Steele, Robert; Digby, Jayne; Strachan, Judith A.; Mowat,
Risk scoring incorporating FIT in triage of symptomatic patients
 Risk scoring incorporating FIT in triage of symptomatic patients Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible conflicts of interest None Background Symptoms
Risk scoring incorporating FIT in triage of symptomatic patients Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible conflicts of interest None Background Symptoms
Get FIT for the new year: a review of the role of faecal immunochemical test for haemoglobin in patients with symptoms of colorectal disease
 Review Article Page 1 of 9 Get FIT for the new year: a review of the role of faecal immunochemical test for haemoglobin in patients with symptoms of colorectal disease Neville Spiteri, Paul Skaife Department
Review Article Page 1 of 9 Get FIT for the new year: a review of the role of faecal immunochemical test for haemoglobin in patients with symptoms of colorectal disease Neville Spiteri, Paul Skaife Department
Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients
 Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients Caroline Addison NE BCSP Hub Director and Consultant Clinical Scientist What is FIT Type of Faecal Occult Blood test Designed
Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients Caroline Addison NE BCSP Hub Director and Consultant Clinical Scientist What is FIT Type of Faecal Occult Blood test Designed
Information Pack for GP s The implementation of the Faecal Immunochemical Test (FIT) across the South West
 Information Pack for GP s The implementation of the Faecal Immunochemical Test (FIT) across the South West The South West Cancer Alliances have been awarded transformation funding to provide access to
Information Pack for GP s The implementation of the Faecal Immunochemical Test (FIT) across the South West The South West Cancer Alliances have been awarded transformation funding to provide access to
Bowel cancer risk in the under 50s. Greg Rubin Professor of General Practice and Primary Care
 Bowel cancer risk in the under 50s Greg Rubin Professor of General Practice and Primary Care Prevalence of GI problems in the consulting population Thompson et al, Gut 2000 Number of patients % of patients
Bowel cancer risk in the under 50s Greg Rubin Professor of General Practice and Primary Care Prevalence of GI problems in the consulting population Thompson et al, Gut 2000 Number of patients % of patients
North West London Pathology. Faecal Occult Blood testing. Mrs Sophie Barnes FRCPath Consultant Clinical Scientist
 Faecal Occult Blood testing Mrs Sophie Barnes FRCPath Consultant Clinical Scientist Learning objectives Background Guidelines for colorectal cancer detection Tests available to detect occult blood in faeces
Faecal Occult Blood testing Mrs Sophie Barnes FRCPath Consultant Clinical Scientist Learning objectives Background Guidelines for colorectal cancer detection Tests available to detect occult blood in faeces
Dr Katie Elliott CRUK strategic GP Macmillan GP with NE &C Learning disability Network Assistant Clinical Lead Northern Cancer Alliance
 FIT for symptomatic patients Dr Katie Elliott CRUK strategic GP Macmillan GP with NE &C Learning disability Network Assistant Clinical Lead Northern Cancer Alliance AIMS Review National advice Consider
FIT for symptomatic patients Dr Katie Elliott CRUK strategic GP Macmillan GP with NE &C Learning disability Network Assistant Clinical Lead Northern Cancer Alliance AIMS Review National advice Consider
FIT for symptomatic patients. Facilitator name
 FIT for symptomatic patients Facilitator name Context colorectal cancer Colorectal cancer in the UK 41,804 new cases in 2015 15,903 deaths in 2014 Fourth most common cancer Second most common cause of
FIT for symptomatic patients Facilitator name Context colorectal cancer Colorectal cancer in the UK 41,804 new cases in 2015 15,903 deaths in 2014 Fourth most common cancer Second most common cause of
Challenges for Colorectal Cancer Screening
 Challenges for Colorectal Cancer Screening a Biomarker with No Standards! Prof. Emeritus Stephen P. Halloran University of Surrey W. Europe Top 20 Cancers Men Incidence & Mortality (2012) Women World -
Challenges for Colorectal Cancer Screening a Biomarker with No Standards! Prof. Emeritus Stephen P. Halloran University of Surrey W. Europe Top 20 Cancers Men Incidence & Mortality (2012) Women World -
Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients
 Alimentary Pharmacology and Therapeutics Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients M. M. Widlak*,, C. L. Thomas, M. G. Thomas, C. Tomkins,
Alimentary Pharmacology and Therapeutics Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients M. M. Widlak*,, C. L. Thomas, M. G. Thomas, C. Tomkins,
Diagnostics guidance Published: 26 July 2017 nice.org.uk/guidance/dg30
 Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care Diagnostics guidance Published: 26 July 2017 nice.org.uk/guidance/dg30 NICE 2018. All rights reserved. Subject
Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care Diagnostics guidance Published: 26 July 2017 nice.org.uk/guidance/dg30 NICE 2018. All rights reserved. Subject
Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography
 Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography 2019 Released 2019 health.govt.nz Citation: Ministry of Health. 2019. Referral Criteria for Direct Access
Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography 2019 Released 2019 health.govt.nz Citation: Ministry of Health. 2019. Referral Criteria for Direct Access
FIT - A Tale of Two Settings. Callum G Fraser Centre for Research into Cancer Prevention and Screening University of Dundee Scotland
 FIT - A Tale of Two Settings Callum G Fraser Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible Conflicts of Interest Consultant: Kyowa, Tokyo, Japan Consultant:
FIT - A Tale of Two Settings Callum G Fraser Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible Conflicts of Interest Consultant: Kyowa, Tokyo, Japan Consultant:
Development and evaluation of a risk assessment tool to improve clinical triage accuracy for colonoscopic investigations
 Lord et al. BMC Cancer (2018) 18:229 https://doi.org/10.1186/s12885-018-4140-0 RESEARCH ARTICLE Development and evaluation of a risk assessment tool to improve clinical triage accuracy for colonoscopic
Lord et al. BMC Cancer (2018) 18:229 https://doi.org/10.1186/s12885-018-4140-0 RESEARCH ARTICLE Development and evaluation of a risk assessment tool to improve clinical triage accuracy for colonoscopic
IJC International Journal of Cancer
 IJC International Journal of Cancer Fecal immunochemical test accuracy in familial risk colorectal cancer screening Ines Castro 1, Joaquın Cubiella 1, Concepcion Rivera 1, Carmen Gonzalez-Mao 2, Pablo
IJC International Journal of Cancer Fecal immunochemical test accuracy in familial risk colorectal cancer screening Ines Castro 1, Joaquın Cubiella 1, Concepcion Rivera 1, Carmen Gonzalez-Mao 2, Pablo
Citation for published version (APA): Wijkerslooth de Weerdesteyn, T. R. (2013). Population screening for colorectal cancer by colonoscopy
 UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
Effect of aspirin on the diagnostic accuracy of the faecal immunochemical test for colorectal advanced neoplasia
 Original Article Effect of aspirin on the diagnostic accuracy of the faecal immunochemical test for colorectal advanced neoplasia United European Gastroenterology Journal 2018, Vol. 6(1) 123 130! Author(s)
Original Article Effect of aspirin on the diagnostic accuracy of the faecal immunochemical test for colorectal advanced neoplasia United European Gastroenterology Journal 2018, Vol. 6(1) 123 130! Author(s)
Prevention of Bowel Cancer: which patients do I send for colonoscopy?
 Prevention of Bowel Cancer: which patients do I send for colonoscopy? Dr Chris Groves Consultant Gastroenterologist and Honorary Senior Lecturer St George s Hospital and Medical School Director, SW London
Prevention of Bowel Cancer: which patients do I send for colonoscopy? Dr Chris Groves Consultant Gastroenterologist and Honorary Senior Lecturer St George s Hospital and Medical School Director, SW London
Risk prediction models for colorectal cancer in people with symptoms: a systematic review
 Williams et al. BMC Gastroenterology (2016) 16:63 DOI 10.1186/s12876-016-0475-7 RESEARCH ARTICLE Risk prediction models for colorectal cancer in people with symptoms: a systematic review Tom G. S. Williams
Williams et al. BMC Gastroenterology (2016) 16:63 DOI 10.1186/s12876-016-0475-7 RESEARCH ARTICLE Risk prediction models for colorectal cancer in people with symptoms: a systematic review Tom G. S. Williams
The value of colonoscopy in isolated abdominal pain. Dr Aaditya Narendra, Dr Yi Ling Adeline Lim, Dr Casper Francois Pretorius
 The value of colonoscopy in isolated abdominal pain Dr Aaditya Narendra, Dr Yi Ling Adeline Lim, Dr Casper Francois Pretorius Background Abdominal pain is a commonly reported symptom in the general population
The value of colonoscopy in isolated abdominal pain Dr Aaditya Narendra, Dr Yi Ling Adeline Lim, Dr Casper Francois Pretorius Background Abdominal pain is a commonly reported symptom in the general population
NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report.
 NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report. Sue Moss, Christopher Mathews Centre for Cancer Prevention, Wolfson Institute, Queen Mary University
NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report. Sue Moss, Christopher Mathews Centre for Cancer Prevention, Wolfson Institute, Queen Mary University
Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc.
 Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc. European Digestive Cancer Days, Prague - 26. September 2017 QUANTITATIVE FIT
Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc. European Digestive Cancer Days, Prague - 26. September 2017 QUANTITATIVE FIT
Objectives. Definitions. Colorectal Cancer Screening 5/8/2018. Payam Afshar, MS, MD Kaiser Permanente, San Diego. Colorectal cancer background
 Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Colorectal Cancer Screening
 Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
The York Faecal Calprotectin Care Pathway for use in primary care. James Turvill
 The York Faecal Calprotectin Care Pathway for use in primary care James Turvill NICE guidance: dg11 Faecal calprotectin (FC) testing as an option in adults with recent onset of lower gastrointestinal symptoms
The York Faecal Calprotectin Care Pathway for use in primary care James Turvill NICE guidance: dg11 Faecal calprotectin (FC) testing as an option in adults with recent onset of lower gastrointestinal symptoms
WEO CRC SC Meeting. Barcelona, Spain October 23, 2015
 WEO CRC SC Meeting Barcelona, Spain October 23, 2015 THE HEMOGLOBIN CONCENTRATION IN A NEGATIVE RESULT AS A PREDICTOR FOR ADVANCED NEOPLASIA Isabel Portillo, Eunare Arana-Arri, Isabel Idigoras, Lorea Martínez-Indart.
WEO CRC SC Meeting Barcelona, Spain October 23, 2015 THE HEMOGLOBIN CONCENTRATION IN A NEGATIVE RESULT AS A PREDICTOR FOR ADVANCED NEOPLASIA Isabel Portillo, Eunare Arana-Arri, Isabel Idigoras, Lorea Martínez-Indart.
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE
 SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
The Dutch bowel cancer screening program Relevant lessions for Ontario
 The Dutch bowel cancer screening program Relevant lessions for Ontario Ernst J Kuipers Erasmus MC University Medical Center Rotterdam - The Netherlands 1 Ismar Boas (1858 1938) Colorectal cancer screening
The Dutch bowel cancer screening program Relevant lessions for Ontario Ernst J Kuipers Erasmus MC University Medical Center Rotterdam - The Netherlands 1 Ismar Boas (1858 1938) Colorectal cancer screening
Guideline scope Diverticular disease: diagnosis and management
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Diverticular disease: diagnosis and management The Department of Health in England has asked NICE to develop a clinical guideline on diverticular
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Diverticular disease: diagnosis and management The Department of Health in England has asked NICE to develop a clinical guideline on diverticular
Digestive Health Southwest Endoscopy 2016 Quality Report
 Digestive Health 2016 Quality Report Our 2016 our quality and value management program focused on one primary area of interest: Performing high quality colonoscopy High quality Colonoscopy We selected
Digestive Health 2016 Quality Report Our 2016 our quality and value management program focused on one primary area of interest: Performing high quality colonoscopy High quality Colonoscopy We selected
GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY
 Position Statement produced by BSG, AUGIS and ACPGBI GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY Introduction In 2011 the Independent Practice
Position Statement produced by BSG, AUGIS and ACPGBI GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY Introduction In 2011 the Independent Practice
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer
 Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Fecal immunochemical testing results and characteristics of colonic lesions
 Original article 111 Fecal immunochemical testing results and characteristics of colonic lesions Authors Sascha C. van Doorn 1, Inge Stegeman 2, An K. Stroobants 3, Marco W. Mundt 4, Thomas R. de Wijkerslooth
Original article 111 Fecal immunochemical testing results and characteristics of colonic lesions Authors Sascha C. van Doorn 1, Inge Stegeman 2, An K. Stroobants 3, Marco W. Mundt 4, Thomas R. de Wijkerslooth
Characteristics of Adenomas Detected by Fecal Immunochemical Test in Colorectal Cancer Screening
 Research Article Cancer Epidemiology, Biomarkers & Prevention Characteristics of Adenomas Detected by Fecal Immunochemical Test in Colorectal Cancer Screening Joaquín Cubiella 1,Ines Castro 1, Vicent Hernandez
Research Article Cancer Epidemiology, Biomarkers & Prevention Characteristics of Adenomas Detected by Fecal Immunochemical Test in Colorectal Cancer Screening Joaquín Cubiella 1,Ines Castro 1, Vicent Hernandez
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING
 CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an open-label, randomized controlled trial
 Page1 of 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an
Page1 of 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an
FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test
 FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test LIMBACH-LABORATORY H E I D E L B E R G H J Roth H Schmidt-Gayk Estimated incidence of cancer in Europe and European Union, 2006 Limbach
FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test LIMBACH-LABORATORY H E I D E L B E R G H J Roth H Schmidt-Gayk Estimated incidence of cancer in Europe and European Union, 2006 Limbach
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer
 Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer Douglas K. Rex, MD, MACG 1, C. Richard Boland, MD 2, Jason A. Dominitz,
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer Douglas K. Rex, MD, MACG 1, C. Richard Boland, MD 2, Jason A. Dominitz,
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines
 Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
American Journal of Clinical Cancer Research
 American Journal of Clinical Cancer Research Clinical Cancer Research http://www.ivyunion.org/index.php/ajcre Page 1 of 7 Vol. 2, Article ID 201400343, 7 pages Research Article American Journal of Diagnostic
American Journal of Clinical Cancer Research Clinical Cancer Research http://www.ivyunion.org/index.php/ajcre Page 1 of 7 Vol. 2, Article ID 201400343, 7 pages Research Article American Journal of Diagnostic
Sarvenaz Moosavi, 1 Robert Enns, 1 Laura Gentile, 2 Lovedeep Gondara, 2 Colleen McGahan, 2 and Jennifer Telford Introduction
 Canadian Gastroenterology and Hepatology Volume 2016, Article ID 5914048, 5 pages http://dx.doi.org/10.1155/2016/5914048 Research Article Comparison of One versus Two Fecal Immunochemical Tests in the
Canadian Gastroenterology and Hepatology Volume 2016, Article ID 5914048, 5 pages http://dx.doi.org/10.1155/2016/5914048 Research Article Comparison of One versus Two Fecal Immunochemical Tests in the
Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University WCC, Melbourne
 Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University. 2014 WCC, Melbourne Outline WHO criteria to justify screening Appropriateness: Global variation in incidence
Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University. 2014 WCC, Melbourne Outline WHO criteria to justify screening Appropriateness: Global variation in incidence
GIQIC18 Appropriate follow-up interval of not less than 5 years for colonoscopies with findings of 1-2 tubular adenomas < 10 mm
 GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
Faecal calprotectin in patients with suspected colorectal cancer:
 Research James Turvill, Assad Aghahoseini, Nala Sivarajasingham, Kazim Abbas, Murtaza Choudhry, Kostantinos Polyzois, Kostantinos Lasithiotakis, Dimitra Volanaki, Baek Kim, Fiona Langlands, Helen Andrew,
Research James Turvill, Assad Aghahoseini, Nala Sivarajasingham, Kazim Abbas, Murtaza Choudhry, Kostantinos Polyzois, Kostantinos Lasithiotakis, Dimitra Volanaki, Baek Kim, Fiona Langlands, Helen Andrew,
Suspected cancer: National Collaborating Centre for Cancer. recognition and management of suspected cancer in children, young people and adults
 National Collaborating Centre for Cancer : recognition and management of suspected cancer in children, young people and adults Clinical Guideline Appendices A - E June 2015 Final version Commissioned by
National Collaborating Centre for Cancer : recognition and management of suspected cancer in children, young people and adults Clinical Guideline Appendices A - E June 2015 Final version Commissioned by
Bowel cancer screening and prevention
 Bowel cancer screening and prevention Cancer Incidence and Mortality Victoria 2012 Number 6000 5000 4000 3000 2000 Incidences = 29,387 Mortality = 10,780 Incidence Mortality 1000 0 Prostate Breast Bowel
Bowel cancer screening and prevention Cancer Incidence and Mortality Victoria 2012 Number 6000 5000 4000 3000 2000 Incidences = 29,387 Mortality = 10,780 Incidence Mortality 1000 0 Prostate Breast Bowel
Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2009;7:1341 1346 Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia JUN KATO,* TAMIYA MORIKAWA,* MOTOAKI KURIYAMA,*
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2009;7:1341 1346 Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia JUN KATO,* TAMIYA MORIKAWA,* MOTOAKI KURIYAMA,*
Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema
 Bahrain Medical Bulletin, Vol.24, No.3, September 2002 Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema Najeeb S Jamsheer, MD, FRCR* Neelam. Malik, MD, MNAMS** Objective: To
Bahrain Medical Bulletin, Vol.24, No.3, September 2002 Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema Najeeb S Jamsheer, MD, FRCR* Neelam. Malik, MD, MNAMS** Objective: To
White Rose Research Online URL for this paper:
 This is an author produced version of Unrestricted faecal calprotectin testing performs poorly in the diagnosis of inflammatory bowel disease in patients in primary care. White Rose Research Online URL
This is an author produced version of Unrestricted faecal calprotectin testing performs poorly in the diagnosis of inflammatory bowel disease in patients in primary care. White Rose Research Online URL
Foreword. FIT for Purpose
 Focus on FIT 2017 Alpha Laboratories Ltd. Diagnostics 2017 Faecal Immunochemical Testing in Colorectal Cancer Pathways Introduction and Foreword 2 Getting FIT for the Future! 3 Evaluating a Faecal Immunochemical
Focus on FIT 2017 Alpha Laboratories Ltd. Diagnostics 2017 Faecal Immunochemical Testing in Colorectal Cancer Pathways Introduction and Foreword 2 Getting FIT for the Future! 3 Evaluating a Faecal Immunochemical
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2018 Related Policies: None Virtual Colonoscopy/Computed Tomography Colonography Description Computed tomography colonography (CTC), also known as
FEP Medical Policy Manual Effective Date: January 15, 2018 Related Policies: None Virtual Colonoscopy/Computed Tomography Colonography Description Computed tomography colonography (CTC), also known as
Diagnostics guidance Published: 10 May 2017 nice.org.uk/guidance/dg28
 Virtual chromoendoscopy to assess colorectal polyps during colonoscopy Diagnostics guidance Published: 10 May 2017 nice.org.uk/guidance/dg28 NICE 2017. All rights reserved. Subject to Notice of rights
Virtual chromoendoscopy to assess colorectal polyps during colonoscopy Diagnostics guidance Published: 10 May 2017 nice.org.uk/guidance/dg28 NICE 2017. All rights reserved. Subject to Notice of rights
The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean?
 The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean? Robert A. Smith, PhD Cancer Control, Department of Prevention and Early Detection American Cancer Society
The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean? Robert A. Smith, PhD Cancer Control, Department of Prevention and Early Detection American Cancer Society
This is the portion of the intestine which lies between the small intestine and the outlet (Anus).
 THE COLON This is the portion of the intestine which lies between the small intestine and the outlet (Anus). 3 4 5 This part is responsible for formation of stool. The large intestine (colon- coloured
THE COLON This is the portion of the intestine which lies between the small intestine and the outlet (Anus). 3 4 5 This part is responsible for formation of stool. The large intestine (colon- coloured
R. J. L. F. Loffeld, 1 P. E. P. Dekkers, 2 and M. Flens Introduction
 ISRN Gastroenterology Volume 213, Article ID 87138, 5 pages http://dx.doi.org/1.1155/213/87138 Research Article The Incidence of Colorectal Cancer Is Decreasing in the Older Age Cohorts in the Zaanstreek
ISRN Gastroenterology Volume 213, Article ID 87138, 5 pages http://dx.doi.org/1.1155/213/87138 Research Article The Incidence of Colorectal Cancer Is Decreasing in the Older Age Cohorts in the Zaanstreek
Appendix 1: Description of the 25 studies excluded from the meta-analysis of Sensitivity
 Supplementary Data Appendix 1: Description of the 25 studies excluded from the meta-analysis of Sensitivity and Specificity. Below we list each study excluded from our meta-analysis of the sensitivity
Supplementary Data Appendix 1: Description of the 25 studies excluded from the meta-analysis of Sensitivity and Specificity. Below we list each study excluded from our meta-analysis of the sensitivity
Understanding test accuracy research: a test consequence graphic
 Whiting and Davenport Diagnostic and Prognostic Research (2018) 2:2 DOI 10.1186/s41512-017-0023-0 Diagnostic and Prognostic Research METHODOLOGY Understanding test accuracy research: a test consequence
Whiting and Davenport Diagnostic and Prognostic Research (2018) 2:2 DOI 10.1186/s41512-017-0023-0 Diagnostic and Prognostic Research METHODOLOGY Understanding test accuracy research: a test consequence
ORE Open Research Exeter
 ORE Open Research Exeter TITLE The diagnostic performance of scoring systems to identify symptomatic colorectal cancer compared to current referral guidance AUTHORS Marshall, T; Lancashire, R; Sharp, D;
ORE Open Research Exeter TITLE The diagnostic performance of scoring systems to identify symptomatic colorectal cancer compared to current referral guidance AUTHORS Marshall, T; Lancashire, R; Sharp, D;
Title Description Type / Priority
 Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
PENETRANCE ACTIONABILITY SIGNIFICANCE/BURDEN OF DISEASE NEXT STEPS. YES ( 1 of above) YES (Proceed to Stage II)
 Stage I: Binning Dashboard GENE/GENE PANEL: APC ACTIONABILITY 1. Is there a qualifying resource, such as a practice guideline or systematic review, for the genetic condition? 2. Does the practice guideline
Stage I: Binning Dashboard GENE/GENE PANEL: APC ACTIONABILITY 1. Is there a qualifying resource, such as a practice guideline or systematic review, for the genetic condition? 2. Does the practice guideline
Background. Aim. Design. Method. Results. Conclusion. Keywords
 Research Margaret Astin, Tom Griffin, Richard D Neal, Peter Rose and William Hamilton The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review Abstract Background Over
Research Margaret Astin, Tom Griffin, Richard D Neal, Peter Rose and William Hamilton The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review Abstract Background Over
Running title: Adenomas with a positive FIT result in CRC screening. Address for correspondence: Dr. Joaquín Cubiella Fernández.
 CHARACTERISTICS OF ADENOMAS DETECTED BY FECAL IMMUNOCHEMICAL TEST IN COLORECTAL CANCER SCREENING Joaquín Cubiella 1, Inés Castro 1, Vicent Hernandez 2,3, Carmen González-Mao 3,4, Concepción Rivera 1, Felipe
CHARACTERISTICS OF ADENOMAS DETECTED BY FECAL IMMUNOCHEMICAL TEST IN COLORECTAL CANCER SCREENING Joaquín Cubiella 1, Inés Castro 1, Vicent Hernandez 2,3, Carmen González-Mao 3,4, Concepción Rivera 1, Felipe
Debate: General surveillance/screening for colon cancer in a resource constrained environment is imperative
 Debate: General surveillance/screening for colon cancer in a resource constrained environment is imperative Dr. Meryl Oyomno Department of surgery, University of Pretoria INTRODUCTION Screening is the
Debate: General surveillance/screening for colon cancer in a resource constrained environment is imperative Dr. Meryl Oyomno Department of surgery, University of Pretoria INTRODUCTION Screening is the
CHAPTER 7 Higher FIT cut-off levels: lower positivity rates but still acceptable detection rates for early stage colorectal cancers
 CHAPTER 7 Higher FIT cut-off levels: lower positivity rates but still acceptable detection rates for early stage colorectal cancers J.S. Terhaar sive Droste [1], F.A. Oort [1], R.W.M. van der Hulst [2],
CHAPTER 7 Higher FIT cut-off levels: lower positivity rates but still acceptable detection rates for early stage colorectal cancers J.S. Terhaar sive Droste [1], F.A. Oort [1], R.W.M. van der Hulst [2],
The 2012 SAGE Wait Time Program: Survey of Access to GastroEnterology in Canada Can J Gastroenterol 2013;27:83-9.
 The 2012 SAGE Wait Time Program: Survey of Access to GastroEnterology in Canada Can J Gastroenterol 2013;27:83-9. Desmond Leddin MB, David Armstrong MD, Mark Borgaonkar MD, Ronald J Bridges MD, Carlo A
The 2012 SAGE Wait Time Program: Survey of Access to GastroEnterology in Canada Can J Gastroenterol 2013;27:83-9. Desmond Leddin MB, David Armstrong MD, Mark Borgaonkar MD, Ronald J Bridges MD, Carlo A
EVALUATION OF QUANTITATIVE DETECTION OF FECAL HUMAN HAEMOGLOBIN FOR COLORECTAL CANCER SCREENING
 EVALUATION OF QUANTITATIVE DETECTION OF FECAL HUMAN HAEMOGLOBIN FOR COLORECTAL CANCER SCREENING Kocna P., Vaníčková Z., Kovářová J., Krechler T., Kohout P., Beneš Z., Granátová J. Institute of Clinical
EVALUATION OF QUANTITATIVE DETECTION OF FECAL HUMAN HAEMOGLOBIN FOR COLORECTAL CANCER SCREENING Kocna P., Vaníčková Z., Kovářová J., Krechler T., Kohout P., Beneš Z., Granátová J. Institute of Clinical
C olorectal adenomas are reputed to be precancerous
 568 COLORECTAL CANCER Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese Y Yamaji, T Mitsushima, H Ikuma, H Watabe, M Okamoto, T
568 COLORECTAL CANCER Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese Y Yamaji, T Mitsushima, H Ikuma, H Watabe, M Okamoto, T
Research Article Development of Polyps and Cancer in Patients with a Negative Colonoscopy: A Follow-Up Study of More Than 20 Years
 ISRN Gastroenterology, Article ID 261302, 4 pages http://dx.doi.org/10.1155/2014/261302 Research Article Development of Polyps and Cancer in Patients with a Negative Colonoscopy: A Follow-Up Study of More
ISRN Gastroenterology, Article ID 261302, 4 pages http://dx.doi.org/10.1155/2014/261302 Research Article Development of Polyps and Cancer in Patients with a Negative Colonoscopy: A Follow-Up Study of More
Colorectal cancer screening
 26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
COLORECTAL CANCER SCREENING &THE FECAL IMMUNOCHEMICAL TEST (FIT) MATHEW ESTEY, PHD, FCACB CLINICAL CHEMIST
 COLORECTAL CANCER SCREENING &THE FECAL IMMUNOCHEMICAL TEST (FIT) MATHEW ESTEY, PHD, FCACB CLINICAL CHEMIST MATHEW.ESTEY@DYNALIFEDX.COM FACULTY /PRESENTER DISCLOSURE FACULTY: MATHEW ESTEY RELATIONSHIPS
COLORECTAL CANCER SCREENING &THE FECAL IMMUNOCHEMICAL TEST (FIT) MATHEW ESTEY, PHD, FCACB CLINICAL CHEMIST MATHEW.ESTEY@DYNALIFEDX.COM FACULTY /PRESENTER DISCLOSURE FACULTY: MATHEW ESTEY RELATIONSHIPS
Rx Only. Detecting Cancer In Blood.
 Epi procolon is an FDA-approved blood test for colorectal cancer screening for patients who are unwilling or unable to be screened by recommended methods. Rx Only Intended Use, Contraindications, Warnings,
Epi procolon is an FDA-approved blood test for colorectal cancer screening for patients who are unwilling or unable to be screened by recommended methods. Rx Only Intended Use, Contraindications, Warnings,
FIT for purpose: enhanced applications for faecal immunochemical tests
 Review Article Page 1 of 11 FIT for purpose: enhanced applications for faecal immunochemical tests Erin L. Symonds 1,2, Robert J. L. Fraser 3, Graeme P. Young 2 1 Bowel Health Service, Flinders Medical
Review Article Page 1 of 11 FIT for purpose: enhanced applications for faecal immunochemical tests Erin L. Symonds 1,2, Robert J. L. Fraser 3, Graeme P. Young 2 1 Bowel Health Service, Flinders Medical
Clinical features of bowel disease in patients aged <50 years in primary care:
 Research Sally A Stapley, Greg P Rubin, Deborah Alsina, Elizabeth A Shephard, Matthew D Rutter and William T Hamilton Clinical features of bowel disease in patients aged
Research Sally A Stapley, Greg P Rubin, Deborah Alsina, Elizabeth A Shephard, Matthew D Rutter and William T Hamilton Clinical features of bowel disease in patients aged
WEO CRC SC Meeting. Vienna, Austria October 14, 2016
 WEO CRC SC Meeting Vienna, Austria October 14, 216 Possibleconflictsofinterest The Bowel Health Service receives material support from Eiken Chemical Company. Erin Symonds FIT positivity rate is highest
WEO CRC SC Meeting Vienna, Austria October 14, 216 Possibleconflictsofinterest The Bowel Health Service receives material support from Eiken Chemical Company. Erin Symonds FIT positivity rate is highest
Transforming Cancer Services for London
 Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Sequential screening in the early diagnosis of colorectal cancer in the community
 Journal of Public Health: From Theory to Practice https://doi.org/10.1007/s10389-019-01024-0 ORIGINAL ARTICLE Sequential screening in the early diagnosis of colorectal cancer in the community Ming-sheng
Journal of Public Health: From Theory to Practice https://doi.org/10.1007/s10389-019-01024-0 ORIGINAL ARTICLE Sequential screening in the early diagnosis of colorectal cancer in the community Ming-sheng
Screening for GI Cancer Past Present and Future. Prof. Bob Steele University of Dundee
 Screening for GI Cancer Past Present and Future Prof. Bob Steele University of Dundee Worldwide Cancer Incidence Rates UK Cancer Incidence Rates Screening The detection of disease in asymptomatic subjects
Screening for GI Cancer Past Present and Future Prof. Bob Steele University of Dundee Worldwide Cancer Incidence Rates UK Cancer Incidence Rates Screening The detection of disease in asymptomatic subjects
Haemoglobin level at previous negative FIT and risk of neoplasia at subsequent screening rounds. Carlo SENORE
 Haemoglobin level at previous negative FIT and risk of neoplasia at subsequent screening rounds Carlo SENORE Possible conflicts of interest None related to the presentation Carlo Senore AIMS To estimate
Haemoglobin level at previous negative FIT and risk of neoplasia at subsequent screening rounds Carlo SENORE Possible conflicts of interest None related to the presentation Carlo Senore AIMS To estimate
Endoscopic Corner CASE 1. Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R
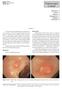 170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
Epi procolon 2.0 CE is a blood test for colorectal cancer screening.
 Epi procolon 2.0 CE is a blood test for colorectal cancer screening. About Epi procolon 2.0 CE... 2 Screening Recommendations... 3 What You Should Know About Epi procolon 2.0 CE and the Septin 9 DNA Biomarker...
Epi procolon 2.0 CE is a blood test for colorectal cancer screening. About Epi procolon 2.0 CE... 2 Screening Recommendations... 3 What You Should Know About Epi procolon 2.0 CE and the Septin 9 DNA Biomarker...
NICE guideline on Suspected cancer: recognition and referral (2015) Education package for GPs and Nurse Practitioners Case scenarios
 NICE guideline on Suspected cancer: recognition and referral (2015) Education package for GPs and Nurse Practitioners Case scenarios How to use the case scenarios The case scenarios can be used in a training
NICE guideline on Suspected cancer: recognition and referral (2015) Education package for GPs and Nurse Practitioners Case scenarios How to use the case scenarios The case scenarios can be used in a training
Transition to Fecal Immunochemical Testing (FIT)
 Transition to Fecal Immunochemical Testing (FIT) Frequently Asked Questions for Primary Care Providers October 2017 Version 1.1 Overview Ontario will be transitioning from the guaiac fecal occult blood
Transition to Fecal Immunochemical Testing (FIT) Frequently Asked Questions for Primary Care Providers October 2017 Version 1.1 Overview Ontario will be transitioning from the guaiac fecal occult blood
THE NEW ZEALAND MEDICAL JOURNAL
 THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1258 ISSN 1175 8716 A survey of colonoscopy capacity in New Zealand s public hospitals Andrew Yeoman, Susan Parry Abstract Aims Population screening for colorectal
THE NEW ZEALAND MEDICAL JOURNAL Vol 120 No 1258 ISSN 1175 8716 A survey of colonoscopy capacity in New Zealand s public hospitals Andrew Yeoman, Susan Parry Abstract Aims Population screening for colorectal
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
Friday, 23 October 2015: 10:15 12:00 * * * * *
 Barcelona 2015 8 th Meeting of the Expert Working Group (EWG) FIT for Screening Expert Working Group (EWG) founding members: Friday, 23 October 2015: 10:15 12:00 MEETING REPORT * * * * * Jim Allison, University
Barcelona 2015 8 th Meeting of the Expert Working Group (EWG) FIT for Screening Expert Working Group (EWG) founding members: Friday, 23 October 2015: 10:15 12:00 MEETING REPORT * * * * * Jim Allison, University
Colon Cancer Screening. Layth Al-Jashaami, MD GI Fellow, PGY 4
 Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Colorectal Cancer Screening. Paul Berg MD
 Colorectal Cancer Screening Paul Berg MD What is clinical integration? AMA Definition The means to facilitate the coordination of patient care across conditions, providers, settings, and time in order
Colorectal Cancer Screening Paul Berg MD What is clinical integration? AMA Definition The means to facilitate the coordination of patient care across conditions, providers, settings, and time in order
Be it Resolved that FIT is the Best Way to Screen for Colorectal Cancer DEBATE
 Be it Resolved that FIT is the Best Way to Screen for Colorectal Cancer DEBATE DEBATE Presenters PRESENTATION MODERATOR Dr. Praveen Bansal -MD, CCFP FCFP Regional Primary Care Lead, Integrated Cancer Screening,
Be it Resolved that FIT is the Best Way to Screen for Colorectal Cancer DEBATE DEBATE Presenters PRESENTATION MODERATOR Dr. Praveen Bansal -MD, CCFP FCFP Regional Primary Care Lead, Integrated Cancer Screening,
Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis
 Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis BMJ 2010;340:c1269 doi:10.1136/bmj.c1269 Mr DC 62 year old man Borderline anaemia
Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis BMJ 2010;340:c1269 doi:10.1136/bmj.c1269 Mr DC 62 year old man Borderline anaemia
COLORECTAL CARCINOMA
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
Measuring the quality of colonoscopy: Where are we now and where are we going?
 Measuring the quality of colonoscopy: Where are we now and where are we going? Timothy D. Imler, MD Division of Gastroenterology and Hepatology, Indiana University School of Medicine, Indianapolis, Indiana
Measuring the quality of colonoscopy: Where are we now and where are we going? Timothy D. Imler, MD Division of Gastroenterology and Hepatology, Indiana University School of Medicine, Indianapolis, Indiana
Friday, 15 May 2015: 10:00 12:00 * * * * *
 Washington 2015 7 th Meeting of the Expert Working Group (EWG) FIT for Screening Friday, 15 May 2015: 10:00 12:00 MEETING REPORT * * * * * Expert Working Group (EWG) founding members: Jim Allison, University
Washington 2015 7 th Meeting of the Expert Working Group (EWG) FIT for Screening Friday, 15 May 2015: 10:00 12:00 MEETING REPORT * * * * * Expert Working Group (EWG) founding members: Jim Allison, University
Title: Serrated polyposis syndrome associated with long-standing inflammatory bowel disease
 Title: Serrated polyposis syndrome associated with long-standing inflammatory bowel disease Authors: Jesús Castro, Miriam Cuatrecasas, Francesc Balaguer, Elena Ricart, María Pellisé DOI: 10.17235/reed.2017.5068/2017
Title: Serrated polyposis syndrome associated with long-standing inflammatory bowel disease Authors: Jesús Castro, Miriam Cuatrecasas, Francesc Balaguer, Elena Ricart, María Pellisé DOI: 10.17235/reed.2017.5068/2017
COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION.
 The West London Medical Journal 2009 Vol No 1 pp 23-31 COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION. Competing interests: None declared ABSTRACT Sarah
The West London Medical Journal 2009 Vol No 1 pp 23-31 COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION. Competing interests: None declared ABSTRACT Sarah
Polyp detection rates as quality indicator in clinical versus screening colonoscopy
 Polyp detection rates as quality indicator in clinical versus screening colonoscopy Authors G. Hoff 1, 2, 3,E.Botteri 2,O.Høie 4,K.Garborg 3, 5,H.Wiig 6,G.Huppertz-Hauss 7,V.Moritz 7, M. Bretthauer 3,
Polyp detection rates as quality indicator in clinical versus screening colonoscopy Authors G. Hoff 1, 2, 3,E.Botteri 2,O.Høie 4,K.Garborg 3, 5,H.Wiig 6,G.Huppertz-Hauss 7,V.Moritz 7, M. Bretthauer 3,
Dr Alasdair Patrick Gastroenterologist
 Dr Alasdair Patrick Gastroenterologist Bowel Cancer screening Dr Alasdair Patrick Gastroenterologist MacMurray Gastroenterology Case- Patient for Screening? 62 year old lady Father diagnosed with advanced
Dr Alasdair Patrick Gastroenterologist Bowel Cancer screening Dr Alasdair Patrick Gastroenterologist MacMurray Gastroenterology Case- Patient for Screening? 62 year old lady Father diagnosed with advanced
University of Dundee. Published in: Journal of Medical Screening DOI: / Publication date: 2016
 University of Dundee Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited Digby, Jayne; Fraser, Callum G.; Carey, Francis A.; Lang,
University of Dundee Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited Digby, Jayne; Fraser, Callum G.; Carey, Francis A.; Lang,
