Fecal immunochemical testing results and characteristics of colonic lesions
|
|
|
- Francis Green
- 5 years ago
- Views:
Transcription
1 Original article 111 Fecal immunochemical testing results and characteristics of colonic lesions Authors Sascha C. van Doorn 1, Inge Stegeman 2, An K. Stroobants 3, Marco W. Mundt 4, Thomas R. de Wijkerslooth 1, Paul Fockens 1, Ernst J. Kuipers 5, Patrick M. Bossuyt 2, Evelien Dekker 1 Institutions Institutions are listed at the end of article. submitted 2. August 214 accepted after revision 1. April 215 Bibliography DOI /s Published online: Endoscopy 215; 47: Georg Thieme Verlag KG Stuttgart New York ISSN X Corresponding author Evelien Dekker, MD, PhD Department of Gastroenterology and Hepatology Academic Medical Center Meibergdreef AZ, Amsterdam The Netherlands Fax: e.dekker@amc.uva.nl Background and study aims: Fecal immunochemical tests (FIT) are used to detect blood in feces, which might indicate the presence of colorectal neoplasia. The aim of this study was to investigate whether FIT results vary depending on the characteristics of colonic lesions. Patients and methods: This was a retrospective a- nalysis of lesions detected in a cohort of asymptomatic individuals (aged 5 75 years) who were invited to participate in a FIT-based screening pilot in The Netherlands. The mean FIT result was compared across subgroups of individuals defined by histopathology of the most advanced lesion detected. In addition, the results were compared with data from a primary colonoscopy screening trial, in which participants also completed a FIT. Results: In three rounds of FIT-based screening, a total of 877 FIT-positive individuals underwent colonoscopy. Higher mean FIT results (hemoglobin [Hb]/g feces) were observed in individuals with carcinomas (199 μg Hb/g) and advanced adenomas (87 μg Hb/g) compared with participants with nonadvanced adenomas (5 μg Hb/g) or those with serrated lesions (46 μg Hb/g) (P <.1). In the primary colonoscopy trial, 1256 participants completed a FIT test and underwent colonoscopy. The number of participants with nonadvanced adenomas as the most advanced lesion was comparable between this group and the FIT-based screening group (2 % vs. 22 %). Conclusion: In FIT-based screening, the mean FIT results varied depending on the characteristics of the most advanced colonic lesion. The proportion of participants with a nonadvanced adenoma as the most advanced lesion was similar in the FITbased screening group and in the primary colonoscopy screening group, suggesting that these lesions are coincidental findings rather than FITdetected findings. Introduction Colorectal cancer (CRC) is the second most common type of cancer in Western countries, and almost half of those diagnosed die from the disease [1]. The clinical and pathological stage at the time of diagnosis largely determines prognosis. Population screening for CRC aims to detect cancer and precancerous lesions at an early stage, and to reduce morbidity and mortality of the disease [2]. The fecal immunochemical test (FIT) is a quantitative fecal occult blood test that is specific for human blood and is used as a screening tool [3 7]. CRCs with a higher T stage are associated with higher FIT results [8]. Several trials have also reported significantly higher fecal hemoglobin levels in patients with advanced neoplasia than in patients with a normal colonoscopy [4, 5, 9, 1]. Further evaluation of the association between lesion characteristics and absolute FIT results (measured in μg Hb/g feces) in a screening population would improve the understanding of the performance of FIT. It would be useful to know which lesions are detected during FIT-based screening and which lesions are coincidental findings on colonoscopy, as this would help to develop other, more sensitive, screening tests or screening strategies. One recent study reported more positive FIT test results in individuals with more advanced lesions [11], and Digby et al. demonstrated an association between fecal hemoglobin and the severity of neoplastic disease in a large FIT-positive cohort undergoing colonoscopy [12]. The current study aimed to confirm these findings by comparing fecal hemoglobin concentrations obtained in FIT-based screening across subgroups defined by the most advanced lesion detected on subsequent colonoscopy. In addition, the findings in FIT-positive individuals were compared with results from a primary colonoscopy screening trial, performed in The Netherlands, in
2 112 Original article which participants also completed a FIT. In the latter study group, all participants underwent colonoscopy, regardless of their FIT result. This allowed the number and histopathology of lesions in FIT-positive individuals from FIT-based screening to be compared with findings from an asymptomatic primary colonoscopy screening group. The incidence of nonadvanced lesions was of interest, as the FIT results of these lesions are unknown. Patients and methods Population and design Data from pilot programs of FIT-based screening and from primary colonoscopy screening, collected from comparable regions in The Netherlands, were used in the study. FIT-based screening Data from FIT-based screening were collected in three rounds of a biennial FIT-based screening pilot in the Amsterdam region of The Netherlands. The study designs of the three consecutive screening rounds have been reported in detail elsewhere [4,13 15]. In brief, ethical approval was obtained from the Dutch Health Council (25 /3WBO, The Hague, The Netherlands). In each of three screening rounds, asymptomatic, average-risk, individuals aged between 5 and 75 years were invited to participate (see " Fig. e1, available online). This FIT-based pilot screening program was designed as a dynamic cohort study; individuals included in the first round were re-invited in subsequent rounds, as were individuals who had moved into the target area, and those who had reached the target age between screening rounds. Individuals who had moved out of the area or exceeded the age limit, or who had had a previous positive FIT result were not reinvited. Institutionalized people were excluded from participation. Eligible persons were identified using the electronic database of the regional municipal administration registration. Preannouncements and invitations were sent out by the regional Foundation of Population Screening Mid-West using a centralized invitation program (ICOLON). Only those who underwent a colonoscopy after a positive FIT test result were included in the present analyses. FIT-positive screening individuals were not invited for further FIT-based screening rounds and were therefore included only once in the analyses. Colonoscopy-based screening In the primary colonoscopy screening trial, asymptomatic, average-risk participants, who were in the same age group as individuals in the FIT-based pilot screening program, were randomly allocated to an invitation for primary CRC screening by colonoscopy or screening by computed tomography colonography (see " Fig. e2, available online). Ethical approval was obtained from the Dutch Health Council (29 /3WBO, The Hague, Netherlands). All participants gave written informed consent. The design and results of this randomized controlled trial (COCOS Colonoscopy Or CT colonography Screening) have been described in detail elsewhere [14, 16 18]. Only participants from the colonoscopy arm were eligible for the analyses in the current study. Between June 29 and July 21, a total of 66 individuals from the Amsterdam and Rotterdam regions of The Netherlands were randomly selected from the municipal administration registrations and invited for colonoscopy screening. Screening participants who were willing to undergo colonoscopy were additionally invited to complete one sample FIT before their screening colonoscopy. The FIT results of those who were allocated to and underwent primary colonoscopy and who provided written informed consent were included in the current analyses. Stool tests The same FIT test (OC Sensor; Eiken Chemical Co., Tokyo, Japan) was used for all three FIT-based screening rounds and for the primary colonoscopy screening trial. This test provides a quantitative measurement of fecal human hemoglobin. The FIT consists of a probe attached to a cap, which fits a collection tube containing hemoglobin-stabilizing buffer. In the Laboratory for General Clinical Chemistry for special techniques at the Academic Medical Center, Amsterdam, which is certified according to CCKL (ISO 91), the returned samples from both studies were analyzed using the OC Sensor automated instrument. The cut-off value for FIT positivity in the current study was 1 μg Hb/g feces, which corresponds to 5 ng Hb/mL buffer [7]. FIT-based screening In FIT-based screening, an invitation kit containing the FIT was sent by postal mail. In addition to the invitation letter, the kit also contained a detailed information brochure, a test instruction leaflet, and a return envelope. A signed informed consent form had to be enclosed in the return envelope. Participants could perform the test at home and return it by postal mail. Colonoscopy-based screening In the primary colonoscopy screening trial, screening participants received extensive, verbal instructions on performing the FIT during a consultation prior to the colonoscopy. They were asked to perform the FIT at home, within 48 hours prior to colonoscopy, but before starting the bowel preparation. Participants were asked to bring the FIT to the screening center, or to call the screening center immediately after performing the FIT, so that research staff could come and collect the FIT sample. Colonoscopy In FIT-based screening, colonoscopy was recommended only for individuals with a positive FIT test result. In the primary colonoscopy screening trial, all screenees underwent colonoscopy, regardless of their FIT result. The endoscopists who performed the colonoscopies were blinded to the FIT result. Participants eligible for colonoscopy were invited for a consultation at one of the screening centers. Individuals from the FITbased screening rounds who had undergone a colonoscopy in the 2 years prior to the positive FIT result were advised not to undergo a repeat colonoscopy. In the first two rounds, all colonoscopies were performed at the Academic Medical Center in Amsterdam by approximately 3 endoscopists. In the third round, colonoscopies were also performed at the Flevoziekenhuis in Almere by two endoscopists. Colonoscopies within the COCOS trial were performed at the Academic Medical Center in Amsterdam and in the Erasmus Medical Center in Rotterdam by five endoscopists. Colonoscopy quality indicators were recorded on a case record form. When polyps were detected, endoscopic removal of the lesions was attempted during the same procedure. If cancer was suspected or if endoscopic removal was not possible, biopsies were obtained and histopathological assessment provided a definitive diagnosis to inform further treatment decisions.
3 Original article 113 Histopathology All lesions were evaluated by an experienced gastrointestinal pathologist according to the Vienna criteria [19]. Lesions were classified as follows: adenocarcinoma, adenoma (tubular, tubulovillous, villous), hyperplastic, sessile serrated adenoma/polyp, traditional serrated adenoma, or miscellaneous. Hyperplastic polyps, sessile serrated adenomas/polyps, and traditional serrated adenomas were grouped as serrated lesions, as their endoscopic appearance is similar (mostly sessile or flat morphology, and all considered as nonbleeding lesions) [2]. Dysplasia was defined as either low grade or high grade. Advanced adenoma was defined as any adenoma 1 mm or an adenoma with > 25 % villous component or with high grade dysplasia. Cancers were staged according to the 7th edition of the American Joint Committee on Cancer classification [21, 22]. Data on location, size, macroscopic aspect, morphology, method of polypectomy, and endoscopic assessment of completeness of polypectomy were recorded for all lesions detected during colonoscopy. Analyses All screening participants who underwent colonoscopy were classified into subgroups, based on characteristics of the most advanced lesion detected during colonoscopy. Lesions were classified in the following order: carcinomas, advanced adenomas, nonadvanced adenomas, serrated lesions, and no colonic lesions. Subgroups were defined based on histopathology, morphology, size, and location. Histopathology subgroups were defined as: carcinoma, advanced adenomas, nonadvanced adenomas, serrated lesion, and no colonic lesions. Morphology subgroups were defined as flat, sessile, or pedunculated, as described by the Paris Classification for Colonic Lesions [23]. Size categories were: 1 5mm, 6 9mm, 1 mm. Location of colonic lesions was defined as proximal or distal to the splenic flexure. Location subgroups were alternatively defined by colonic segment (rectum, sigmoid, descending colon, splenic flexure, transverse colon, hepatic flexure, ascending colon, and cecum). In each of these subgroups, participants were classified only once (only the most advanced lesion detected per participant was analyzed); synchronous or metachronous lesions were not evaluated. Carcinomas were excluded from the morphology subgroups, as these lesions often present with a different morphology from regular polyps (e.g. depressed or ulcerated lesions). The mean and median FIT result was calculated in μg Hb/g feces for each of these subgroups. As FIT results are non-negative, a logarithmic transformation was first applied to better approach normality. Statistically significant differences in the mean results were tested between each set of subgroups (histopathology, morphology, size, and location) using one-way analysis of variance. Post hoc analyses were performed, applying a Bonferroni correction, to evaluate pairwise differences between subgroups. A multiple linear regression model was used to evaluate the conditional effects of polyp size, polyp morphology, and polyp location on the FIT result, adjusted for the other polyp features. The FIT result was the dependent variable, and the polyp characteristics were the independent variables. In addition, proportions of the respective colonic lesions identified in the FIT-positive individuals from FIT-based screening and in the primary colonoscopy screening participants of the primary colonoscopy screening trial were calculated. Those proportions were compared using the chi-squared test. Results Polyp characteristics in FIT-based screening In three consecutive rounds of FIT-based screening, 877 FIT-positive individuals underwent colonoscopy. FIT results ranged from 1 to 812 μg Hb/g feces; the mean amount of hemoglobin detected was 74 μg Hb/g feces. " Table 1 shows the mean and median FIT result in subgroups defined by polyp histopathology, morphology, size, and location (log-transformed FIT values were used for the analyses). Mean FIT results differed significantly between the subgroups defined by histopathology findings (P <.1). Post hoc analyses showed that screenees with an advanced adenoma or carcinoma had a significantly higher mean FIT result than screenees with no colorectal lesions (P =.6 and P <.1, respectively), nonadvanced adenomas (both P<.1) or serrated lesions only (P =.1 and P <.1, respectively). The mean FIT result of individuals with carcinomas was significantly higher than the FIT results of those with advanced adenomas (P <.1). Compared with individuals without polyps at colonoscopy, no differences in hemoglobin levels were found for those with serrated lesions (P =1.) or nonadvanced adenomas (P =.63). An additional a- nalysis was performed among the 85 individuals with a serrated lesion. No significant differences in FIT results were found between serrated lesions of different subtypes or size (P =.13; see Table e2, available online). The morphology of most carcinomas was characterized as different or stenotic. Excluding carcinomas, 592 lesions were included in the analysis of polyp morphology. Mean FIT results differed significantly between subgroups (P<.1) ( " Table 1, " Fig. 3). Pairwise differences were significant between pedunculated polyps and polyps with sessile or flat morphology (P <.1 and P=.1). Size was known for 636 of the 651 lesions. Lesions of 1 mm or larger had significantly higher FIT results than small polyps and diminutive polyps (P <.1): 26 lesions (41 %) were 1 mm, and these had a significantly higher FIT result than smaller polyps (6 9mm) and diminutive polyps (1 5mm) (P <.1). Hemoglobin concentrations were higher for polyps located in the distal colon than in the proximal colon. Analysis of polyp location specified for different colon segments also showed a significant difference (P =.1) (see Table e3, available online). This could be explained by higher FIT results in participants with lesions located in the sigmoid. Multiple linear regression Multiple linear regression was performed to evaluate whether polyp size, polyp morphology, and polyp location, respectively, were associated with differences in mean FIT results, when taking the other characteristics into account. The analysis showed that polyp location was no longer significantly associated with the amount of fecal hemoglobin when adjusting for the polyp size and morphology (P =.32). Polyp size and polyp morphology, however, remained significantly associated with the FIT result when adjusted for other polyp characteristics (P <.1 and P =.5, respectively). Polyp characteristics in colonoscopy-based screening In the primary colonoscopy trial, 1426 participants underwent a colonoscopy, of whom 1256 (88 %) also completed a FIT test [16]. FIT results ranged from to 67 μg Hb/g feces, with a mean of 9μg Hb/g feces.
4 114 Original article Table1 Fecal immunochemical test results and characteristics of the most advanced lesion detected at colonoscopy. FIT-based screening group Colonoscopy screening group n (%) Absolute FIT result, μg Hb/g feces P 1 n (%) Absolute FIT result, μg Hb/g feces P 1 Mean (SD) Median (IQR) Mean (SD) Median (IQR) Total (119) 26 (15 69) (47) ( 1) Histopathology 877 < <.1 No colorectal lesions 226 (26) 66 (111) 23 (14 53) 685 (55) 3 (2) ( ) Serrated lesion 2 85 (1) 46 (77) 2 (15 4) 191 (15) 5 (45) ( 1) Nonadvanced adenoma 195 (22) 5 (95) 19 (14 37) 261 (21) 5 (2) ( 2) Advanced adenoma 332 (38) 87 (127) 34 (17 93) 111 (9) 49 (118) 2 ( 27) Carcinoma 39 (4) 199 (185) 136 (57 37) 8 (< 1) 155 (15) 128 (25 241) Polyp morphology 592 < <.1 Flat 66 (11) 48 (66) 21 (14 53) 4 (7) 2 (3) ( 2) Sessile 318 (54) 54 (99) 2 (14 47) 48 (77) 1 (51) ( 2) Pedunculated 28 (35) 1 (139) 41 (19 19) 83 (16) 42 (112) 2 ( 2) Polyp size 636 < mm 26 (41) 19 (146) 47 (2 125) 1 (18) 52 (121) 2 ( 34) <.1 6 9mm 11 (17) 58 (14) 21 (14 43) 75 (13) 14 (5) ( 5) 1 5mm 266 (42) 54 (96) 21 (15 45) 394 (69) 7 (43) ( 1) Polyp location 647 < Proximal (to splenic flexure) 227 (35) 56 (91) 22 (15 53) 255 (45) 11 (54) ( 2) Distal (including splenic flexure) 42 (65) 88 (135) 31 (16 92) 316 (55) 2 (75) ( 4) FIT, fecal immunochemical test; IQR, interquartile range. 1 One-way analysis of variance. 2 Hyperplastic polyp, sessile serrated adenoma/polyp or traditional serrated adenoma Mean FIT results differed significantly depending on the histopathology results (P<.1) ( " Table 1). A post hoc analysis showed differences between subgroups that were comparable to those in the FIT-positive individuals in FIT-based screening, with significant differences between screenees with advanced adenomas and carcinomas compared with those without colorectal lesions (both P<.1). Participants with nonadvanced adenomas had significantly higher FIT results than those without colorectal lesions (P =.1). Of the 571 lesions detected in the colonoscopy screenees, morphology could be assessed in 538, and 531 remained after exclusion of the 7 carcinomas with known morphology. Mean FIT results differed significantly between groups (P <.1). Size was known for 569 of the 571 lesions. FIT results were significantly different among differently sized polyps. Of the 571 colorectal lesions, 255 were located proximal to the splenic flexure. Distally located lesions had a significantly higher hemoglobin concentration than proximally located lesions (P =.1), reflecting results in the FIT-based screening. FIT-positive screening vs. colonoscopy screening The distribution of colonic lesions was compared between the FIT-positive individuals in the FIT-based screening rounds and the participants undergoing primary colonoscopy screening. As expected, there were more advanced adenomas in the FIT-positive individuals than in the primary colonoscopy screenees (38 % vs. 8%), as well as more carcinomas (4 % vs. < 1%; P <.1) ( " Table 4). There was no difference in the number of screening participants with nonadvanced adenomas (22 % vs. 2 %, respectively; P =.32). Discussion This study showed that the mean FIT result for FIT-positive screening individuals with advanced adenomas and carcinomas is significantly higher than the mean in those with nonadvanced adenomas, serrated lesions, and those with no colorectal lesions. Screening individuals with pedunculated polyps had a higher mean FIT result than those with sessile and flat lesions, and screenees with polyps 1 mm had a higher FIT result than those with smaller lesions. As expected, significantly more participants had advanced adenomas and carcinomas in the FIT-positive group than in the screening colonoscopy group. The number of nonadvanced adenomas, however, was comparable in the two groups. Some limitations should be acknowledged. The analyses were based on the most advanced lesion detected during colonoscopy, and no adjustment was made for possible additional relevant pathology. Previous trials have evaluated the association between the occurrence of multiple adenomas and the amount of fecal hemoglobin [9, 1]; this could be relevant because multiple nonadvanced adenomas, as weak sources of bleeding, may together produce a positive FIT result. Such analyses were not performed in the current study, as it was believed that the absolute hemoglobin concentration is largely the result of the most advanced lesion, and the hypothesis was that more advanced lesions would show a higher FIT result. A previous cross-sectional study in symptomatic patients who were referred for colonoscopy reported that a higher T stage in CRC is associated with higher FIT values [8]. In the current study, only 47 carcinomas were detected in total, which prohibits precise analysis of the effect of stage on FIT values. The first round of the FIT-based screening program started in 26, and this allowed analysis of 877 FIT-positive individuals.
5 Original article 115 Histopathology subgroups Polyp morphology subgroups 6 FIT screening Colonoscopy screening 3 FIT screening Colonoscopy screening no lesions no lesions serrated lesions serrated lesions nonadvanced adenoma nonadvanced adenoma advanced adenoma advanced adenoma carcinoma carcinoma Pedunculated Pedunculated Sessile Sessile Flat Flat Polyp size subgroups FIT screening Colonoscopy screening FIT screening Colonoscopy screening mm 1 5 mm 6 9 mm 6 9 mm > 1 mm > 1 mm 2 1 Polyp location subgroups Proximal colon Proximal colon Distal colon Distal colon Fig. 3 Boxplots showing the differences between histopathology, morphology, size, and location subgroups and 25th percentiles fecal immunochemical test (FIT) in ng Hb/mL buffer. Experienced research staff registered all detected and removed polyps during the colonoscopies in both screening programs. In both screening pilots, most colonoscopies were performed by the same endoscopists. All polyps were sent to pathology for assessment, which allowed the analyses in the current study. Although both screening populations underwent a different screening route, they were all asymptomatic, comparable in age, lived largely in the same region, and all had a similar socioeconomic status. Previous trials have also reported higher fecal hemoglobin levels in patients with advanced adenomas and carcinomas compared with patients with nonadvanced adenomas or no neoplasia [9, 1, 24]. Two studies showed that individuals with nonadvanced adenomas > 6mm had a higher FIT result than those with a normal examination [9, 1]. The current findings did not confirm this, as the mean FIT result in the group of FIT-positive individuals with nonadvanced adenomas of any size was not higher than in individuals without lesions. Digby et al. demonstrated an association between fecal hemoglobin and the severity of neoplastic disease in a large FIT-positive cohort undergoing colonoscopy [12]. A limitation of this study was that the distributions of fecal hemoglobin could not be fully assessed, as the upper analytical limit was 1 ng Hb/mL buffer [12]. The current study demonstrated quantitative results of all FIT-positive individuals, with a cutoff level of 5 ng Hb/mL instead of 4 ng Hb/mL buffer. The difference in cutoff level and analytical limit may explain the difference in findings between the Digby study and the current study, as the latter describes a higher incidence of nonadvanced adenomas and hyperplastic polyps. Whereas Digby showed that lesion size was the only characteristic significantly related to fecal hemoglobin, the current study demonstrated that polyp morphology was also significantly related to the FIT result. Another limitation of the Digby study is that only FIT-positive participants underwent colonoscopy and could therefore be assessed [12]. To overcome this limitation, the current study also assessed FIT results from participants from an asymptomatic colonoscopy
6 116 Original article FIT-based screening group N=877 Primary colonoscopy screening group 1 N=1426 Histopathology, n (%) No colorectal lesions 226 (26) 778 (55) Serrated lesions 2 85 (1) 226 (16) <.1 Nonadvanced adenomas 195 (22) 292 (2).32 Advanced adenomas 332 (38) 121 (8) <.1 Carcinomas 39 (4) 9 (1) <.1 FIT, fecal immunochemical test. 1 Including both patients who performed colonoscopy and FIT and those who only performed colonoscopy. 2 Hyperplastic polyp or sessile serrated adenoma/polyp P Table4 Most advanced lesions detected at colonoscopy. screening trial. Results demonstrated that the same associations between polyp characteristics and FIT results could be evaluated, when FIT results below the cutoff level of 5 ng Hb/mL ( negative FIT results ) were also taken into account. FIT results in pedunculated polyps were higher than in sessile lesions. A previous study evaluating FIT levels and polyp features suggested that pedunculated lesions are often larger than sessile polyps, but the authors of that study did not detect a significant difference in FIT results between polyps of different sizes [9]. Haug et al. demonstrated a higher sensitivity of FIT for the detection of advanced neoplasia in the left vs. right colon, and they also found a significantly higher incidence of pedunculated shaped lesions in the left colon [25]. Although the authors only evaluated the association between FIT results and advanced neoplasia, they also found a higher median fecal hemoglobin level in pedunculated lesions. In their logistic regression analysis, pedunculated shape was statistically significantly associated with test sensitivity even when adjusted for size [25]. This is in line with the current results; in the multiple variable analyses, polyp size (higher FIT results for lesion of 1 mm) and polyp morphology (higher FIT results for pedunculated lesions) remained significantly associated with the FIT result when adjusted for other polyp characteristics. Carcinomas were excluded from the morphology analyses, as these lesions often present with different morphology from the regular polyp morphology according to the Paris classification [23]. It would be interesting to evaluate whether fecal hemoglobin levels differ with the shape of carcinomas, and to examine whether, for example, depressed carcinomas are more easily missed by FIT. Unfortunately, the number of carcinomas in the current study (n = 39) was too small for this type of analysis; larger additional studies are needed to evaluate this further. Whereas individuals in the FIT-based screening group who had the most advanced lesion in the proximal colon had a significantly lower FIT result compared with that in individuals with distally located adenomas, this was not observed in two other studies [9, 1]. However, polyp location was no longer significantly associated with fecal hemoglobin level when adjustment was made for the size and morphology, which is in line with previous studies [9, 1]. Haug et al. reported a higher sensitivity of FIT for distally located lesions [25]. The authors hypothesized that this might be caused by less hemoglobin degradation from left-sided lesions, and to difference in stool consistency in the left- vs. the rightsided colon and more homogeneously distributed blood in the stools from the right side. As described above, the authors detected more pedunculated lesions in the distal colon. Although in the current study the significant association between polyp location and FIT result disappeared after adjustment for polyp size and morphology, the morphology of polyps might be of larger influence of the fecal hemoglobin level than location. To our knowledge, this is the first study to analyze differences in FIT results for the different subtypes of serrated lesions. In two other trials, serrated adenomas were regarded as adenomatous, whereas all serrated lesions in the current study were regarded as separate findings [9, 1]. As the mean FIT result in individuals with serrated lesions was not higher than in those without lesions, one might conclude that serrated lesions are coincidental, nonbleeding findings in FIT-positive individuals, and that FIT does not seem to detect or differentiate between serrated lesions. Regrettably no analyses could be performed on dysplasia in serrated lesions, because the first screening round was performed in 26 and, at that time, dysplasia could not be assessed as reliably as it is today. As the mean FIT result in screenees with an advanced adenoma was approximately 87 μg Hb/g feces in the FITbased screening group and 49 μg Hb/g feces in the colonoscopy group, one could argue that the FIT positivity cutoff level of 1 μg Hb/g feces should be increased. In countries where colonoscopy capacity is limited, increasing the cutoff level within the screening trial will decrease the number of unnecessary colonoscopies in participants with a false-positive FIT and only nonadvanced lesions. However, as previous studies have shown, this would inevitably lead to missed carcinomas and advanced adenomas [5, 16]. As an alternative, the absolute FIT result could be incorporated into a risk model for advanced neoplasia. A recent publication demonstrated that individual FIT concentrations are predictive for the presence of colorectal neoplasia [24]. Our research group previously described several risk factors that were related to a false-negative or false-positive FIT result [26, 27]. Using a combination of the absolute FIT result and other risk factors to determine whether individuals are invited for colonoscopy following FIT-based screening, rather than using FIT positivity alone, could increase sensitivity without a loss in specificity, potentially improving the effectiveness of CRC programs [16, 27]. Although screening participants with advanced adenomas and carcinomas had elevated FIT results, FIT-positive individuals with nonadvanced adenomas did not have a higher FIT result than individuals without colorectal lesions. In addition, the number of screenees with nonadvanced adenomas was comparable in FIT-positive individuals and among primary colonoscopy screening participants. This combination of findings suggests that nonadvanced adenomas are coincidental findings in FIT-positive individuals, reflecting simply the background prevalence of these lesions in the age group targeted for screening. The fact that FIT does not seem to detect these lesions accurately can be regarded as an advantage. FIT-based screening aims to select participants with an increased risk of advanced lesions; only these individuals are invited for colonoscopy. As screening with FIT is typically repeated at fixed intervals, and given the slow progression rate of adenomas, participants with nonadvanced adenomas are likely
7 Original article 117 to be invited for colonoscopy in the future, but only when their adenomas have advanced to larger, bleeding lesions. Competing interests: None Institutions 1 Department of Gastroenterology and Hepatology, Academic Medical Center, Amsterdam, The Netherlands 2 Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Academic Medical Center, Amsterdam, The Netherlands 3 Department of Clinical Chemistry, Academic Medical Center, Amsterdam, The Netherlands 4 Department of Gastroenterology and Hepatology, Flevoziekenhuis Almere, The Netherlands 5 Department of Gastroenterology and Hepatology, Erasmus Medical Center, Rotterdam, The Netherlands Acknowledgment The study was supported by a grant from The Netherlands Organization for Health Research and Development of the Dutch Ministry of Health (ZonMW ). FIT tests and reagents needed for laboratory assessment for the 3rd pilot round of FIT screening were provided by Eiken Chemical Co., LTD., Japan. The sponsor was not involved in the study. References 1 Zavoral M. Colorectal cancer screening in Europe. World J Gastroenterol 29; 15: Libby G, Brewster DH, McClements PL et al. The impact of populationbased faecal occult blood test screening on colorectal cancer mortality: a matched cohort study. Br J Cancer 212; 17: Van Rossum LG, Van Rijn AF, Laheij RJ et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 28; 135: 82 4 Hol L, Wilschut JA, van Ballegooijen M et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer 29; 1: van Rossum LG, van Rijn AF, Laheij RJ et al. Cutoff value determines the performance of a semi-quantitative immunochemical faecal occult blood test in a colorectal cancer screening programme. Br J Cancer 29; 11: Van Roon AHC, Hol L, Van Vuuren AJ et al. Are fecal immunochemical test characteristics influenced by sample return time? A populationbased colorectal cancer screening trial. Am J Gastroenterol 212; 17: Fraser CG, Allison JE, Halloran SP et al. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst 212; 14: van Turenhout ST, van Rossum LG, Oort FA et al. Similar fecal immunochemical test results in screening and referral colorectal cancer. World J Gastroenterol 212; 18: Rozen P, Levi Z, Hazazi R et al. Identification of colorectal adenomas by a quantitative immunochemical faecal occult blood screening test depends on adenoma characteristics, development threshold used and number of tests performed. Aliment Pharmacol Ther 29; 29: Levi Z, Rozen P, Hazazi R et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med 27; 146: Cubiella J, Castro I, Hernandez V et al. Characteristics of adenomas detected by fecal immunochemical test in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev 214; 23: Digby J, Fraser CG, Carey FA et al. Faecal haemoglobin concentration is related to severity of colorectal neoplasia. J Clin Pathol 213; 66: Denters MJ, Deutekom M, Bossuyt PM et al. Lower risk of advanced neoplasia among patients with a previous negative result from a fecal test for colorectal cancer. Gastroenterology 212; 142: Stegeman I, de Wijkerslooth TR, Mallant-Hent RC et al. Implementation of population screening for colorectal cancer by repeated fecal immunochemical test (FIT): third round. BMC Gastroenterol 212; 12: Denters MJ, Deutekom M, Fockens P et al. Implementation of population screening for colorectal cancer by repeated fecal occult blood test in The Netherlands. BMC Gastroenterol 29; 9: De Wijkerslooth TR, Stoop EM, Bossuyt PM et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol 212; 17: Stoop EM, de Haan MC, de Wijkerslooth TR et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in populationbased screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 212; 13: De Wijkerslooth TR, de Haan MC, Stoop EM et al. Burden of colonoscopy compared to non-cathartic CT-colonography in a colorectal cancer screening programme: randomised controlled trial. Gut 212; 61: Schlemper RJ, Riddell RH, Kato Y et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2; 47: Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH et al. (eds.) WHO classification of tumours of the digestive system. France: Lyon: IARC; 21: Kim KH, Yang SS, Yoon YS et al. Validation of the seventh edition of the American Joint Committee on Cancer tumor-node-metastasis (AJCC TNM) staging in patients with stage II and stage III colorectal carcinoma: analysis of 2511 cases from a medical centre in Korea. Colorectal Dis 211; 13: e Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 21; 17: Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 25; 37: Liao C-S, Lin Y-M, Chang H-C et al. Application of quantitative estimates of fecal hemoglobin concentration for risk prediction of colorectal neoplasia. World J Gastroenterol 213; 19: Haug U, Kuntz KM, Knudsen AB et al. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br J Cancer 211; 14: Stegeman I, de Wijkerslooth TR, Stoop EM et al. Risk factors for false positive and for false negative test results in screening with fecal occult blood testing. Int J Cancer 213; 133: Stegeman I, de Wijkerslooth TR, Stoop EM et al. Combining risk factors with faecal immunochemical test outcome for selecting CRC screenees for colonoscopy. Gut 214; 63: Fig. e1, e2 and Table e2, e3 online content viewable at:
Citation for published version (APA): Wijkerslooth de Weerdesteyn, T. R. (2013). Population screening for colorectal cancer by colonoscopy
 UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
Earlier stages of colorectal cancer detected with immunochemical faecal occult blood tests
 O R I G I N A L A R T I C L E Earlier stages of colorectal cancer detected with immunochemical faecal occult blood tests L.G.M. van Rossum 1*, A.F. van Rijn 2, I.P. van Munster 3, J.B.M.J. Jansen 1, P.
O R I G I N A L A R T I C L E Earlier stages of colorectal cancer detected with immunochemical faecal occult blood tests L.G.M. van Rossum 1*, A.F. van Rijn 2, I.P. van Munster 3, J.B.M.J. Jansen 1, P.
Natural history of adenomas by CT colonography Evelien Dekker Charlotte Tutein Nolthenius, Jaap Stoker
 Natural history of adenomas by CT colonography Charlotte Tutein Nolthenius, Jaap Stoker Academic Medical Center Amsterdam, the Netherlands Possible conflicts of interest None Colonoscopy.. plus polypectomy
Natural history of adenomas by CT colonography Charlotte Tutein Nolthenius, Jaap Stoker Academic Medical Center Amsterdam, the Netherlands Possible conflicts of interest None Colonoscopy.. plus polypectomy
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines
 Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators
The Dutch bowel cancer screening program Relevant lessions for Ontario
 The Dutch bowel cancer screening program Relevant lessions for Ontario Ernst J Kuipers Erasmus MC University Medical Center Rotterdam - The Netherlands 1 Ismar Boas (1858 1938) Colorectal cancer screening
The Dutch bowel cancer screening program Relevant lessions for Ontario Ernst J Kuipers Erasmus MC University Medical Center Rotterdam - The Netherlands 1 Ismar Boas (1858 1938) Colorectal cancer screening
Population-based colorectal cancer screening by fecal immunochemical testing over multiple rounds van der Vlugt, M.
 UvA-DARE (Digital Academic Repository) Population-based colorectal cancer screening by fecal immunochemical testing over multiple rounds van der Vlugt, M. Link to publication Citation for published version
UvA-DARE (Digital Academic Repository) Population-based colorectal cancer screening by fecal immunochemical testing over multiple rounds van der Vlugt, M. Link to publication Citation for published version
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication
 UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
Sarvenaz Moosavi, 1 Robert Enns, 1 Laura Gentile, 2 Lovedeep Gondara, 2 Colleen McGahan, 2 and Jennifer Telford Introduction
 Canadian Gastroenterology and Hepatology Volume 2016, Article ID 5914048, 5 pages http://dx.doi.org/10.1155/2016/5914048 Research Article Comparison of One versus Two Fecal Immunochemical Tests in the
Canadian Gastroenterology and Hepatology Volume 2016, Article ID 5914048, 5 pages http://dx.doi.org/10.1155/2016/5914048 Research Article Comparison of One versus Two Fecal Immunochemical Tests in the
removal of adenomatous polyps detects important effectively as follow-up colonoscopy after both constitute a low-risk Patients with 1 or 2
 Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
The choice of methods for Colorectal Cancer Screening; The Dutch experience
 The choice of methods for Colorectal Cancer Screening; The Dutch experience Monique van Leerdam, Gastroenterologist, NKI-AVL, Amsterdam The Netherlands Colorectal cancer CRC 2 nd cause of cancer related
The choice of methods for Colorectal Cancer Screening; The Dutch experience Monique van Leerdam, Gastroenterologist, NKI-AVL, Amsterdam The Netherlands Colorectal cancer CRC 2 nd cause of cancer related
The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy
 ORIGINAL RESEARCH GASTROENTEROLOGY // INTERNAL MEDICINE The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy Răzvan Opaschi 1, Simona Băţagă 1, Ioan Macarie 2, Imola Török
ORIGINAL RESEARCH GASTROENTEROLOGY // INTERNAL MEDICINE The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy Răzvan Opaschi 1, Simona Băţagă 1, Ioan Macarie 2, Imola Török
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication
 UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION.
 The West London Medical Journal 2009 Vol No 1 pp 23-31 COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION. Competing interests: None declared ABSTRACT Sarah
The West London Medical Journal 2009 Vol No 1 pp 23-31 COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION. Competing interests: None declared ABSTRACT Sarah
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication
 UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
CHAPTER 7 Higher FIT cut-off levels: lower positivity rates but still acceptable detection rates for early stage colorectal cancers
 CHAPTER 7 Higher FIT cut-off levels: lower positivity rates but still acceptable detection rates for early stage colorectal cancers J.S. Terhaar sive Droste [1], F.A. Oort [1], R.W.M. van der Hulst [2],
CHAPTER 7 Higher FIT cut-off levels: lower positivity rates but still acceptable detection rates for early stage colorectal cancers J.S. Terhaar sive Droste [1], F.A. Oort [1], R.W.M. van der Hulst [2],
Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients
 Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients Caroline Addison NE BCSP Hub Director and Consultant Clinical Scientist What is FIT Type of Faecal Occult Blood test Designed
Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients Caroline Addison NE BCSP Hub Director and Consultant Clinical Scientist What is FIT Type of Faecal Occult Blood test Designed
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE
 SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
Objectives. Definitions. Colorectal Cancer Screening 5/8/2018. Payam Afshar, MS, MD Kaiser Permanente, San Diego. Colorectal cancer background
 Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
The Natural History of Right-Sided Lesions
 The Natural History of Right-Sided Lesions Jasper L.A. Vleugels Dept of Gastroenterology and Hepatology, Academic Medical Center, Amsterdam, the Netherlands. None Disclosures Agenda Is there evidence that
The Natural History of Right-Sided Lesions Jasper L.A. Vleugels Dept of Gastroenterology and Hepatology, Academic Medical Center, Amsterdam, the Netherlands. None Disclosures Agenda Is there evidence that
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer
 Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
This is the portion of the intestine which lies between the small intestine and the outlet (Anus).
 THE COLON This is the portion of the intestine which lies between the small intestine and the outlet (Anus). 3 4 5 This part is responsible for formation of stool. The large intestine (colon- coloured
THE COLON This is the portion of the intestine which lies between the small intestine and the outlet (Anus). 3 4 5 This part is responsible for formation of stool. The large intestine (colon- coloured
LIPPINCOTT WILLIAMS AND WILKINS
 AUTHOR QUERY FORM LIPPINCOTT WILLIAMS AND WILKINS JOURNAL NAME: MCG ARTICLE NO: JCG66 QUERIES AND / OR REMARKS QUERY NO. Details Required Author s Response GQ Q Q2 Q Please confirm that givennames (coloured
AUTHOR QUERY FORM LIPPINCOTT WILLIAMS AND WILKINS JOURNAL NAME: MCG ARTICLE NO: JCG66 QUERIES AND / OR REMARKS QUERY NO. Details Required Author s Response GQ Q Q2 Q Please confirm that givennames (coloured
Colon Cancer Screening. Layth Al-Jashaami, MD GI Fellow, PGY 4
 Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
WEO CRC SC Meeting. Barcelona, Spain October 23, 2015
 WEO CRC SC Meeting Barcelona, Spain October 23, 2015 Identification of serrated polyposis syndrome in the context of population-based CRC screening programs Evelien Dekker Academic Medical Center Amsterdam,
WEO CRC SC Meeting Barcelona, Spain October 23, 2015 Identification of serrated polyposis syndrome in the context of population-based CRC screening programs Evelien Dekker Academic Medical Center Amsterdam,
Colon Cancer Screening & Surveillance. Amit Patel, MD PGY-4 GI Fellow
 Colon Cancer Screening & Surveillance Amit Patel, MD PGY-4 GI Fellow Epidemiology CRC incidence and mortality rates vary markedly around the world. Globally, CRC is the third most commonly diagnosed cancer
Colon Cancer Screening & Surveillance Amit Patel, MD PGY-4 GI Fellow Epidemiology CRC incidence and mortality rates vary markedly around the world. Globally, CRC is the third most commonly diagnosed cancer
Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies Modeling Study for the US Preventive Services Task Force
 Clinical Review & Education US Preventive Services Task Force MODELING STUDY Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies Modeling Study for the US Preventive Services
Clinical Review & Education US Preventive Services Task Force MODELING STUDY Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies Modeling Study for the US Preventive Services
The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy
 ORIGINAL RESEARCH GASTROENTEROLOGY // INTERNAL MEDICINE The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy Răzvan Opaschi 1, Simona Bățagă 1, Ioan Macarie 2, Imola Török
ORIGINAL RESEARCH GASTROENTEROLOGY // INTERNAL MEDICINE The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy Răzvan Opaschi 1, Simona Bățagă 1, Ioan Macarie 2, Imola Török
Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2009;7:1341 1346 Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia JUN KATO,* TAMIYA MORIKAWA,* MOTOAKI KURIYAMA,*
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2009;7:1341 1346 Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia JUN KATO,* TAMIYA MORIKAWA,* MOTOAKI KURIYAMA,*
There is evidence that screening with the guaiac fecal
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2013;11:832 838 Association Between Early Stage Colon Neoplasms and False-negative Results From the Fecal Immunochemical Test HAN MO CHIU,*, YI CHIA LEE,*, CHIA
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2013;11:832 838 Association Between Early Stage Colon Neoplasms and False-negative Results From the Fecal Immunochemical Test HAN MO CHIU,*, YI CHIA LEE,*, CHIA
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Citation for published version (APA): Wijkerslooth de Weerdesteyn, T. R. (2013). Population screening for colorectal cancer by colonoscopy
 UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
Pathology in Slovenian CRC screening programme:
 Pathology in Slovenian CRC screening programme: Findings, organisation and quality assurance Snježana Frković Grazio University Medical Center Ljubljana, Slovenia Slovenia s population: 2 million Incidence
Pathology in Slovenian CRC screening programme: Findings, organisation and quality assurance Snježana Frković Grazio University Medical Center Ljubljana, Slovenia Slovenia s population: 2 million Incidence
Colorectal cancer screening
 26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
Challenges for Colorectal Cancer Screening
 Challenges for Colorectal Cancer Screening a Biomarker with No Standards! Prof. Emeritus Stephen P. Halloran University of Surrey W. Europe Top 20 Cancers Men Incidence & Mortality (2012) Women World -
Challenges for Colorectal Cancer Screening a Biomarker with No Standards! Prof. Emeritus Stephen P. Halloran University of Surrey W. Europe Top 20 Cancers Men Incidence & Mortality (2012) Women World -
Clinicopathological features of colorectal polyps in 2002 and 2012
 ORIGINAL ARTICLE Korean J Intern Med 2019;34:65-71 Clinicopathological features of colorectal polyps in 2002 and 2012 Yoon Jeong Nam, Kyeong Ok Kim, Chan Seo Park, Si Hyung Lee, and Byung Ik Jang Division
ORIGINAL ARTICLE Korean J Intern Med 2019;34:65-71 Clinicopathological features of colorectal polyps in 2002 and 2012 Yoon Jeong Nam, Kyeong Ok Kim, Chan Seo Park, Si Hyung Lee, and Byung Ik Jang Division
A TEST FOR COLORECTAL CANCER THAT IS 92% SENSITIVE AND NON-INVASIVE. Stool DNA test
 A TEST FOR COLORECTAL CANCER THAT IS 92% SENSITIVE AND NON-INVASIVE Stool DNA test THE NEW NON-INVASIVE SCREENING TEST FOR COLORECTAL CANCER Sensitive Clinically proven 1 Easy to use FDA approved COLOGUARD
A TEST FOR COLORECTAL CANCER THAT IS 92% SENSITIVE AND NON-INVASIVE Stool DNA test THE NEW NON-INVASIVE SCREENING TEST FOR COLORECTAL CANCER Sensitive Clinically proven 1 Easy to use FDA approved COLOGUARD
Colorectal Cancer Screening and Surveillance
 1 Colorectal Cancer Screening and Surveillance Jeffrey Lee MD, MAS Assistant Clinical Professor of Medicine University of California, San Francisco jeff.lee@ucsf.edu Objectives Review the various colorectal
1 Colorectal Cancer Screening and Surveillance Jeffrey Lee MD, MAS Assistant Clinical Professor of Medicine University of California, San Francisco jeff.lee@ucsf.edu Objectives Review the various colorectal
R. J. L. F. Loffeld, 1 P. E. P. Dekkers, 2 and M. Flens Introduction
 ISRN Gastroenterology Volume 213, Article ID 87138, 5 pages http://dx.doi.org/1.1155/213/87138 Research Article The Incidence of Colorectal Cancer Is Decreasing in the Older Age Cohorts in the Zaanstreek
ISRN Gastroenterology Volume 213, Article ID 87138, 5 pages http://dx.doi.org/1.1155/213/87138 Research Article The Incidence of Colorectal Cancer Is Decreasing in the Older Age Cohorts in the Zaanstreek
EVALUATION OF QUANTITATIVE DETECTION OF FECAL HUMAN HAEMOGLOBIN FOR COLORECTAL CANCER SCREENING
 EVALUATION OF QUANTITATIVE DETECTION OF FECAL HUMAN HAEMOGLOBIN FOR COLORECTAL CANCER SCREENING Kocna P., Vaníčková Z., Kovářová J., Krechler T., Kohout P., Beneš Z., Granátová J. Institute of Clinical
EVALUATION OF QUANTITATIVE DETECTION OF FECAL HUMAN HAEMOGLOBIN FOR COLORECTAL CANCER SCREENING Kocna P., Vaníčková Z., Kovářová J., Krechler T., Kohout P., Beneš Z., Granátová J. Institute of Clinical
The Importance of Complete Colonoscopy and Exploration of the Cecal Region
 The Importance of Complete Colonoscopy and Exploration of the Cecal Region Kuangi Fu, Takahiro Fujii, Takahisa Matsuda, and Yutaka Saito 2 2.1 The Importance of a Complete Colonoscopy Ever since case-control
The Importance of Complete Colonoscopy and Exploration of the Cecal Region Kuangi Fu, Takahiro Fujii, Takahisa Matsuda, and Yutaka Saito 2 2.1 The Importance of a Complete Colonoscopy Ever since case-control
Citation for published version (APA): Denters, M. J. (2013). Fecal immunochemical test based colorectal cancer screening
 UvA-DARE (Digital Academic Repository) Fecal immunochemical test based colorectal cancer screening Denters, M.J. Link to publication Citation for published version (APA): Denters, M. J. (013). Fecal immunochemical
UvA-DARE (Digital Academic Repository) Fecal immunochemical test based colorectal cancer screening Denters, M.J. Link to publication Citation for published version (APA): Denters, M. J. (013). Fecal immunochemical
Colorectal Cancer Screening: A Clinical Update
 11:05 11:45am Colorectal Cancer Screening: A Clinical Update SPEAKER Kevin A. Ghassemi, MD Presenter Disclosure Information The following relationships exist related to this presentation: Kevin A. Ghassemi,
11:05 11:45am Colorectal Cancer Screening: A Clinical Update SPEAKER Kevin A. Ghassemi, MD Presenter Disclosure Information The following relationships exist related to this presentation: Kevin A. Ghassemi,
Comparison of Immunochemical and Guaiac-Based Occult Fecal Tests with Colonoscopy Findings in Symptomatic Patients
 16 The Open Colorectal Cancer Journal, 2009, 2, 16-20 Open Access Comparison of Immunochemical and Guaiac-Based Occult Fecal Tests with Colonoscopy Findings in Symptomatic Patients Jean Louis Frossard
16 The Open Colorectal Cancer Journal, 2009, 2, 16-20 Open Access Comparison of Immunochemical and Guaiac-Based Occult Fecal Tests with Colonoscopy Findings in Symptomatic Patients Jean Louis Frossard
Incidence and Multiplicities of Adenomatous Polyps in TNM Stage I Colorectal Cancer in Korea
 Original Article Journal of the Korean Society of J Korean Soc Coloproctol 2012;28(4):213-218 http://dx.doi.org/10.3393/jksc.2012.28.4.213 pissn 2093-7822 eissn 2093-7830 Incidence and Multiplicities of
Original Article Journal of the Korean Society of J Korean Soc Coloproctol 2012;28(4):213-218 http://dx.doi.org/10.3393/jksc.2012.28.4.213 pissn 2093-7822 eissn 2093-7830 Incidence and Multiplicities of
General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer
 General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer Complexities of Pathological Assessment: Serrated Polyps/Adenomas Carolyn Compton, MD, PhD Professor of Life Sciences,
General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer Complexities of Pathological Assessment: Serrated Polyps/Adenomas Carolyn Compton, MD, PhD Professor of Life Sciences,
Management of pt1 polyps. Maria Pellise
 Management of pt1 polyps Maria Pellise Early colorectal cancer Malignant polyp Screening programmes SM Invasive adenocar cinoma Advances in diagnostic & therapeutic endoscopy pt1 polyps 0.75 5.6% of large-bowel
Management of pt1 polyps Maria Pellise Early colorectal cancer Malignant polyp Screening programmes SM Invasive adenocar cinoma Advances in diagnostic & therapeutic endoscopy pt1 polyps 0.75 5.6% of large-bowel
FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test
 FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test LIMBACH-LABORATORY H E I D E L B E R G H J Roth H Schmidt-Gayk Estimated incidence of cancer in Europe and European Union, 2006 Limbach
FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test LIMBACH-LABORATORY H E I D E L B E R G H J Roth H Schmidt-Gayk Estimated incidence of cancer in Europe and European Union, 2006 Limbach
A Proposal to Standardize Reporting Units for Fecal Immunochemical Tests for Hemoglobin
 doi: 10.1093/jnci/djs190 Advance Access publication on April 2, 2012. The Author 2012. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
doi: 10.1093/jnci/djs190 Advance Access publication on April 2, 2012. The Author 2012. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report.
 NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report. Sue Moss, Christopher Mathews Centre for Cancer Prevention, Wolfson Institute, Queen Mary University
NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report. Sue Moss, Christopher Mathews Centre for Cancer Prevention, Wolfson Institute, Queen Mary University
DIGESTIVE SYSTEM SURGICAL PROCEDURES May 1, 2015 INTESTINES (EXCEPT RECTUM) Asst Surg Anae
 ENDOSCOPY Z50 Duodenoscopy (not to be claimed if Z399 and/or Z00 performed on same patient within 3 months)... 92.10 Z9 Subsequent procedure (within three months following previous endoscopic procedure)...
ENDOSCOPY Z50 Duodenoscopy (not to be claimed if Z399 and/or Z00 performed on same patient within 3 months)... 92.10 Z9 Subsequent procedure (within three months following previous endoscopic procedure)...
Citation for published version (APA): Denters, M. J. (2013). Fecal immunochemical test based colorectal cancer screening
 UvA-DARE (Digital Academic Repository) Fecal immunochemical test based colorectal cancer screening Denters, M.J. Link to publication Citation for published version (APA): Denters, M. J. (2013). Fecal immunochemical
UvA-DARE (Digital Academic Repository) Fecal immunochemical test based colorectal cancer screening Denters, M.J. Link to publication Citation for published version (APA): Denters, M. J. (2013). Fecal immunochemical
Hyperplastische Polyps Innocent bystanders?
 Hyperplastische Polyps Innocent bystanders?? K. Geboes P th l i h O tl dk d Pathologische Ontleedkunde, KULeuven Content Historical Classification Relation Hyperplastic polyps carcinoma The concept cept
Hyperplastische Polyps Innocent bystanders?? K. Geboes P th l i h O tl dk d Pathologische Ontleedkunde, KULeuven Content Historical Classification Relation Hyperplastic polyps carcinoma The concept cept
Incidence and Management of Hemorrhage after Endoscopic Removal of Colorectal Lesions
 Showa Univ J Med Sci 12(3), 253-258, September 2000 Original Incidence and Management of Hemorrhage after Endoscopic Removal of Colorectal Lesions Masaaki MATSUKAWA, Mototsugu FUJIMORI, Takahiko KOUDA,
Showa Univ J Med Sci 12(3), 253-258, September 2000 Original Incidence and Management of Hemorrhage after Endoscopic Removal of Colorectal Lesions Masaaki MATSUKAWA, Mototsugu FUJIMORI, Takahiko KOUDA,
Page 1. Is the Risk This High? Dysplasia in the IBD Patient. Dysplasia in the Non IBD Patient. Increased Risk of CRC in Ulcerative Colitis
 Screening for Colorectal Neoplasia in Inflammatory Bowel Disease Francis A. Farraye MD, MSc Clinical Director, Section of Gastroenterology Co-Director, Center for Digestive Disorders Boston Medical Center
Screening for Colorectal Neoplasia in Inflammatory Bowel Disease Francis A. Farraye MD, MSc Clinical Director, Section of Gastroenterology Co-Director, Center for Digestive Disorders Boston Medical Center
Cost-effectiveness of adenoma surveillance - the Dutch guidelines -
 Cost-effectiveness of adenoma surveillance - the Dutch guidelines - WEO working group adenoma surveillance 20 May, 2016 Iris Lansdorp-Vogelaar, PhD On behalf of the SAP study-group Introduction Adenoma
Cost-effectiveness of adenoma surveillance - the Dutch guidelines - WEO working group adenoma surveillance 20 May, 2016 Iris Lansdorp-Vogelaar, PhD On behalf of the SAP study-group Introduction Adenoma
CT-colonography in population-based colorectal cancer screening de Haan, M.C.
 UvA-DARE (Digital Academic Repository) CT-colonography in population-based colorectal cancer screening de Haan, M.C. Link to publication Citation for published version (APA): de Haan, M. C. (2012). CT-colonography
UvA-DARE (Digital Academic Repository) CT-colonography in population-based colorectal cancer screening de Haan, M.C. Link to publication Citation for published version (APA): de Haan, M. C. (2012). CT-colonography
The addition of high magnifying endoscopy improves rates of high confidence optical diagnosis of colorectal polyps
 E140 The addition of high magnifying endoscopy improves rates of high confidence optical diagnosis of colorectal polyps Authors Mineo Iwatate 1, Yasushi Sano 1, Santa Hattori 1, Wataru Sano 1, Noriaki
E140 The addition of high magnifying endoscopy improves rates of high confidence optical diagnosis of colorectal polyps Authors Mineo Iwatate 1, Yasushi Sano 1, Santa Hattori 1, Wataru Sano 1, Noriaki
Bowel Cancer Screening Exploiting science brings better medicine
 Camberley & District Bowel Cancer Screening Exploiting science brings better medicine Prof Stephen P. Halloran World - All Cancers Men Incidence & Mortality (2012) Women Incidence Mortality GLOBOCAN 2012
Camberley & District Bowel Cancer Screening Exploiting science brings better medicine Prof Stephen P. Halloran World - All Cancers Men Incidence & Mortality (2012) Women Incidence Mortality GLOBOCAN 2012
How to characterize dysplastic lesions in IBD?
 How to characterize dysplastic lesions in IBD? Name: Institution: Helmut Neumann, MD, PhD, FASGE University Medical Center Mainz What do we know? Patients with IBD carry an increased risk of developing
How to characterize dysplastic lesions in IBD? Name: Institution: Helmut Neumann, MD, PhD, FASGE University Medical Center Mainz What do we know? Patients with IBD carry an increased risk of developing
Colorectal Neoplasia. Dr. Smita Devani MBChB, MRCP. Consultant Physician and Gastroenterologist Aga Khan University Hospital, Nairobi
 Colorectal Neoplasia Dr. Smita Devani MBChB, MRCP Consultant Physician and Gastroenterologist Aga Khan University Hospital, Nairobi Case History BT, 69yr male Caucasian History of rectal bleeding No change
Colorectal Neoplasia Dr. Smita Devani MBChB, MRCP Consultant Physician and Gastroenterologist Aga Khan University Hospital, Nairobi Case History BT, 69yr male Caucasian History of rectal bleeding No change
Missed Lesions at Endoscopy. Dr Russell Walmsley, MD, FRCP, FRACP Gastroenterologist WDHB Chair Endoscopy Guidance Group for New Zealand
 Missed Lesions at Endoscopy Dr Russell Walmsley, MD, FRCP, FRACP Gastroenterologist WDHB Chair Endoscopy Guidance Group for New Zealand Missed Lesions at Endoscopy Is there a problem? With Gastroscopy
Missed Lesions at Endoscopy Dr Russell Walmsley, MD, FRCP, FRACP Gastroenterologist WDHB Chair Endoscopy Guidance Group for New Zealand Missed Lesions at Endoscopy Is there a problem? With Gastroscopy
Friday, 15 May 2015: 10:00 12:00 * * * * *
 Washington 2015 7 th Meeting of the Expert Working Group (EWG) FIT for Screening Friday, 15 May 2015: 10:00 12:00 MEETING REPORT * * * * * Expert Working Group (EWG) founding members: Jim Allison, University
Washington 2015 7 th Meeting of the Expert Working Group (EWG) FIT for Screening Friday, 15 May 2015: 10:00 12:00 MEETING REPORT * * * * * Expert Working Group (EWG) founding members: Jim Allison, University
Endoscopic Corner CASE 1. Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R
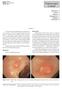 170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
Romanian Journal of Morphology and Embryology 2006, 47(3):
 Romanian Journal of Morphology and Embryology 26, 7(3):239 23 ORIGINAL PAPER Predictive parameters for advanced neoplastic adenomas and colorectal cancer in patients with colonic polyps a study in a tertiary
Romanian Journal of Morphology and Embryology 26, 7(3):239 23 ORIGINAL PAPER Predictive parameters for advanced neoplastic adenomas and colorectal cancer in patients with colonic polyps a study in a tertiary
Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it
 Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it Douglas K Rex 1 MD, MACG 1. Indiana University School of Medicine Division of Gastroenterology/Hepatology
Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it Douglas K Rex 1 MD, MACG 1. Indiana University School of Medicine Division of Gastroenterology/Hepatology
Screening for GI Cancer Past Present and Future. Prof. Bob Steele University of Dundee
 Screening for GI Cancer Past Present and Future Prof. Bob Steele University of Dundee Worldwide Cancer Incidence Rates UK Cancer Incidence Rates Screening The detection of disease in asymptomatic subjects
Screening for GI Cancer Past Present and Future Prof. Bob Steele University of Dundee Worldwide Cancer Incidence Rates UK Cancer Incidence Rates Screening The detection of disease in asymptomatic subjects
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Outcome High Priority
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care
 Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: The percentage
Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: The percentage
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Virtual Colonoscopy / CT Colonography Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Virtual Colonoscopy / CT Colonography Professional Institutional
Virtual Colonoscopy / CT Colonography Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Virtual Colonoscopy / CT Colonography Professional Institutional
Colorectal Cancer Screening
 Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
Characteristics of Adenomas Detected by Fecal Immunochemical Test in Colorectal Cancer Screening
 Research Article Cancer Epidemiology, Biomarkers & Prevention Characteristics of Adenomas Detected by Fecal Immunochemical Test in Colorectal Cancer Screening Joaquín Cubiella 1,Ines Castro 1, Vicent Hernandez
Research Article Cancer Epidemiology, Biomarkers & Prevention Characteristics of Adenomas Detected by Fecal Immunochemical Test in Colorectal Cancer Screening Joaquín Cubiella 1,Ines Castro 1, Vicent Hernandez
Synchronous and Subsequent Lesions of Serrated Adenomas and Tubular Adenomas of the Colorectum
 Tsumura T, et al 1 Synchronous and Subsequent Lesions of Serrated Adenomas and Tubular Adenomas of the Colorectum T. Tsumura a T. Hiyama d S. Tanaka b M. Yoshihara d K. Arihiro c K. Chayama a Departments
Tsumura T, et al 1 Synchronous and Subsequent Lesions of Serrated Adenomas and Tubular Adenomas of the Colorectum T. Tsumura a T. Hiyama d S. Tanaka b M. Yoshihara d K. Arihiro c K. Chayama a Departments
Neoplastic Colon Polyps. Joyce Au SUNY Downstate Grand Rounds, October 18, 2012
 Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
Surveying the Colon; Polyps and Advances in Polypectomy
 Surveying the Colon; Polyps and Advances in Polypectomy Educational Objectives Identify classifications of polyps Describe several types of polyps Verbalize rationale for polypectomy Identify risk factors
Surveying the Colon; Polyps and Advances in Polypectomy Educational Objectives Identify classifications of polyps Describe several types of polyps Verbalize rationale for polypectomy Identify risk factors
Risk scoring incorporating FIT in triage of symptomatic patients
 Risk scoring incorporating FIT in triage of symptomatic patients Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible conflicts of interest None Background Symptoms
Risk scoring incorporating FIT in triage of symptomatic patients Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible conflicts of interest None Background Symptoms
North West London Pathology. Faecal Occult Blood testing. Mrs Sophie Barnes FRCPath Consultant Clinical Scientist
 Faecal Occult Blood testing Mrs Sophie Barnes FRCPath Consultant Clinical Scientist Learning objectives Background Guidelines for colorectal cancer detection Tests available to detect occult blood in faeces
Faecal Occult Blood testing Mrs Sophie Barnes FRCPath Consultant Clinical Scientist Learning objectives Background Guidelines for colorectal cancer detection Tests available to detect occult blood in faeces
Polyp detection rates as quality indicator in clinical versus screening colonoscopy
 Polyp detection rates as quality indicator in clinical versus screening colonoscopy Authors G. Hoff 1, 2, 3,E.Botteri 2,O.Høie 4,K.Garborg 3, 5,H.Wiig 6,G.Huppertz-Hauss 7,V.Moritz 7, M. Bretthauer 3,
Polyp detection rates as quality indicator in clinical versus screening colonoscopy Authors G. Hoff 1, 2, 3,E.Botteri 2,O.Høie 4,K.Garborg 3, 5,H.Wiig 6,G.Huppertz-Hauss 7,V.Moritz 7, M. Bretthauer 3,
The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an open-label, randomized controlled trial
 Page1 of 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an
Page1 of 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 The effectiveness of telephone reminders and SMS messages on compliance with colorectal cancer screening: an
C olorectal cancer (CRC) is the second most common
 CANCER Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial J H Scholefield, S Moss, F Sufi, C M Mangham, J D Hardcastle... See end of
CANCER Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial J H Scholefield, S Moss, F Sufi, C M Mangham, J D Hardcastle... See end of
Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci. Colon polyps. Colorectal cancer
 Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci Colon polyps Colorectal cancer Harrison s Principles of Internal Medicine 18 Ed. 2012 Colorectal cancer 70% Colorectal cancer CRC and colon
Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci Colon polyps Colorectal cancer Harrison s Principles of Internal Medicine 18 Ed. 2012 Colorectal cancer 70% Colorectal cancer CRC and colon
05/07/2018. Organisation. The English screening programme what is happening? Organisation. Bowel cancer screening in the UK is:
 Organisation The English screening programme what is happening? Phil Quirke Lead Pathologist Bowel Cancer Screening PHE England Bowel Cancer Screening Pathology Committee Started 2006 with roll out 4 devolved
Organisation The English screening programme what is happening? Phil Quirke Lead Pathologist Bowel Cancer Screening PHE England Bowel Cancer Screening Pathology Committee Started 2006 with roll out 4 devolved
Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc.
 Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc. European Digestive Cancer Days, Prague - 26. September 2017 QUANTITATIVE FIT
Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc. European Digestive Cancer Days, Prague - 26. September 2017 QUANTITATIVE FIT
EARLY DETECTION OF COLORECTAL CANCER. Epidemiology of CRC
 Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
Optimizing implementation of fecal immunochemical testing in Ontario: A randomized controlled trial
 Optimizing implementation of fecal immunochemical testing in Ontario: A randomized controlled trial J. Tinmouth, N.N. Baxter, L.F. Paszat, E. Randell, M. Serenity, R. Sutradhar, L. Rabeneck Conflicts of
Optimizing implementation of fecal immunochemical testing in Ontario: A randomized controlled trial J. Tinmouth, N.N. Baxter, L.F. Paszat, E. Randell, M. Serenity, R. Sutradhar, L. Rabeneck Conflicts of
Friday, 23 October 2015: 10:15 12:00 * * * * *
 Barcelona 2015 8 th Meeting of the Expert Working Group (EWG) FIT for Screening Expert Working Group (EWG) founding members: Friday, 23 October 2015: 10:15 12:00 MEETING REPORT * * * * * Jim Allison, University
Barcelona 2015 8 th Meeting of the Expert Working Group (EWG) FIT for Screening Expert Working Group (EWG) founding members: Friday, 23 October 2015: 10:15 12:00 MEETING REPORT * * * * * Jim Allison, University
Measuring performance and quality indicators of CRC screening
 Measuring performance and quality indicators of CRC screening Ondřej MÁJEK Institute of Biostatistics and Analyses, Masaryk University Institute of Biostatistics and Analyses, Masaryk University, Brno
Measuring performance and quality indicators of CRC screening Ondřej MÁJEK Institute of Biostatistics and Analyses, Masaryk University Institute of Biostatistics and Analyses, Masaryk University, Brno
COLORECTAL CANCER SCREENING &THE FECAL IMMUNOCHEMICAL TEST (FIT) MATHEW ESTEY, PHD, FCACB CLINICAL CHEMIST
 COLORECTAL CANCER SCREENING &THE FECAL IMMUNOCHEMICAL TEST (FIT) MATHEW ESTEY, PHD, FCACB CLINICAL CHEMIST MATHEW.ESTEY@DYNALIFEDX.COM FACULTY /PRESENTER DISCLOSURE FACULTY: MATHEW ESTEY RELATIONSHIPS
COLORECTAL CANCER SCREENING &THE FECAL IMMUNOCHEMICAL TEST (FIT) MATHEW ESTEY, PHD, FCACB CLINICAL CHEMIST MATHEW.ESTEY@DYNALIFEDX.COM FACULTY /PRESENTER DISCLOSURE FACULTY: MATHEW ESTEY RELATIONSHIPS
EXPERT WORKING GROUP Surveillance after neoplasia removal. Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum
 EXPERT WORKING GROUP Surveillance after neoplasia removal Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum AIM To improve the quality of the evidences we have regarding post- polypectomy
EXPERT WORKING GROUP Surveillance after neoplasia removal Meeting Chicago, May 5th 2017 Chair: Rodrigo Jover Uri Ladabaum AIM To improve the quality of the evidences we have regarding post- polypectomy
Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests
 Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests Results of a systematic review, Kaiser experience, and implications for the Canton of Vaud Kevin Selby, M.D. Kevin.Selby@hospvd.ch
Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests Results of a systematic review, Kaiser experience, and implications for the Canton of Vaud Kevin Selby, M.D. Kevin.Selby@hospvd.ch
Faecal immunochemical test accuracy in patients referred for surveillance colonoscopy: a multicentre
 Terhaar sive Droste et al. BMC Gastroenterology 2012, 12:94 RESEARCH ARTICLE Open Access Faecal immunochemical test accuracy in patients referred for surveillance colonoscopy: a multicentre cohort study
Terhaar sive Droste et al. BMC Gastroenterology 2012, 12:94 RESEARCH ARTICLE Open Access Faecal immunochemical test accuracy in patients referred for surveillance colonoscopy: a multicentre cohort study
Why FIT (Faecal Immunochemical Test) is the best biomarker for CRC screening
 Why FIT (Faecal Immunochemical Test) is the best biomarker for CRC screening Prof. Stephen Halloran Royal Surrey County Hospital NHS Cancer Screening Programme University of Surrey gfobt Guaiacum Officinale
Why FIT (Faecal Immunochemical Test) is the best biomarker for CRC screening Prof. Stephen Halloran Royal Surrey County Hospital NHS Cancer Screening Programme University of Surrey gfobt Guaiacum Officinale
Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients
 Alimentary Pharmacology and Therapeutics Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients M. M. Widlak*,, C. L. Thomas, M. G. Thomas, C. Tomkins,
Alimentary Pharmacology and Therapeutics Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients M. M. Widlak*,, C. L. Thomas, M. G. Thomas, C. Tomkins,
Is the level of cleanliness using segmental Boston bowel preparation scale associated with a higher adenoma detection rate?
 ORIGINAL ARTICLE Annals of Gastroenterology (208) 3, 27-223 Is the level of cleanliness using segmental Boston bowel preparation scale associated with a higher adenoma detection rate? Abimbola Adike a,
ORIGINAL ARTICLE Annals of Gastroenterology (208) 3, 27-223 Is the level of cleanliness using segmental Boston bowel preparation scale associated with a higher adenoma detection rate? Abimbola Adike a,
Emerging Interventions in Endoscopy. Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital
 Emerging Interventions in Endoscopy Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital Colon Cancer Colon cancer is common. 1 in 20 people in the UK will develop the disease 19 000
Emerging Interventions in Endoscopy Margaret Vance Nurse Consultant in Gastroenterology St Mark s Hospital Colon Cancer Colon cancer is common. 1 in 20 people in the UK will develop the disease 19 000
Accepted Manuscript. En bloc resection for mm polyps to reduce post-colonoscopy cancer and surveillance. C. Hassan, M. Rutter, A.
 Accepted Manuscript En bloc resection for 10-20 mm polyps to reduce post-colonoscopy cancer and surveillance C. Hassan, M. Rutter, A. Repici PII: S1542-3565(19)30412-4 DOI: https://doi.org/10.1016/j.cgh.2019.04.022
Accepted Manuscript En bloc resection for 10-20 mm polyps to reduce post-colonoscopy cancer and surveillance C. Hassan, M. Rutter, A. Repici PII: S1542-3565(19)30412-4 DOI: https://doi.org/10.1016/j.cgh.2019.04.022
ColonCancerCheck Recommendations for Post-Polypectomy Surveillance
 ColonCancerCheck Recommendations for Post-Polypectomy Surveillance C. Dubé, B.R. McCurdy, T. Bronstein, A. Pollett, N.N. Baxter, D. Morgan, J. Tinmouth Table of Contents Background... 5 Methodology...
ColonCancerCheck Recommendations for Post-Polypectomy Surveillance C. Dubé, B.R. McCurdy, T. Bronstein, A. Pollett, N.N. Baxter, D. Morgan, J. Tinmouth Table of Contents Background... 5 Methodology...
