Foreword. FIT for Purpose
|
|
|
- Rose Kennedy
- 5 years ago
- Views:
Transcription
1 Focus on FIT 2017 Alpha Laboratories Ltd. Diagnostics 2017 Faecal Immunochemical Testing in Colorectal Cancer Pathways Introduction and Foreword 2 Getting FIT for the Future! 3 Evaluating a Faecal Immunochemical Test System 4-5 Introducing the FIT Service at NHS Tayside 6-7 A Tale of Two Settings - Screening or Symptomatic 8-9 A FIT Sample - Improve Testing Integrity 10 Which FIT's Best? - GMEC Study 11 A FIT System for Any Laboratory - HM-JACKarc 12
2 Foreword FIT for Purpose Faecal Immunochemical Tests (FIT) for Diagnosis of Significant Bowel Disease FOCUS ON FIT INTRODUCTION Alpha Laboratories has been at the forefront of faecal testing in the UK for nearly 20 years. This was initially as the market leader for guaiac-based faecal occult blood testing in hospital laboratories. Tender wins for bowel screening in all four UK countries followed this, as each launched its own screening programme, assessing the average risk asymptomatic populations. Continuing to provide leading edge products, Alpha Laboratories has been awarded the first contract for quantitative FIT as the front line test in the Scottish Bowel Screening Programme. This will employ the Kyowa Medex HM-JACKarc system. England will also be moving to a quantitative FIT method in the NHS Bowel Cancer Screening Programme in the near future. The use of FIT in the assessment of the symptomatic is changing too, as more publications have demonstrated that the use of quantitative FIT as a rule-out test has benefits to clinicians, laboratories and patients. In June 2015, NICE published the NG12 guidelines and advocated the introduction of tests for occult blood in faeces for certain patients presenting in primary care with lower abdominal symptoms but with a low risk of colorectal cancer. This sparked much controversy regarding the different types of tests available for occult blood detection, since many hospital laboratories had discontinued the provision of guaiac-based tests and did not want to go back to this old technology with its very poor analytical and clinical sensitivity and specificity. Whilst newer quantitative Faecal Immunochemical Test (FIT) methods have been widely introduced for bowel screening, publications on the use of this methodology in assessment of patients with lower abdominal symptoms have been increasing since Unfortunately, these had not been part of the data review for the June 2015 NG12 publication. In consequence, NICE have now reviewed the evidence for FIT in this clinical setting and are due to publish new guidelines entitled: Quantitative faecal immunochemical tests to assess symptomatic people who are at low risk of colorectal cancer in primary care. This assessment is focused on peer-reviewed publications on quantitative FIT methods in this particular clinical setting. These publications are concerned with studies on symptomatic patients being planned or considered for colonoscopy on whom a FIT had been performed. The quantitative FIT results were compared against the clinical findings. These publications identified that the FIT result could be used as a rule out test for significant colorectal disease (SCD), that is, colorectal cancer (CRC) plus higher risk adenoma (HRA: sometimes precursors of cancer) and inflammatory bowel disease (IBD: Crohn s and ulcerative colitis), since they have a very high negative predictive value (NPV) for SCD. In fact, using a cut-off of <10 µg/g Hb/ faeces, the NPV was almost 100% for cancer and >90% for SCD. The NICE Diagnostics Assessment Committee has published a number of documents online, including the draft diagnostic guidance: gid-dg10005/documents Differentiating patients with serious bowel disease from those with benign functional disorders, such as Irritable Bowel Syndrome (IBS), and minor colorectal disease such as haemorrhoids, hyperplastic polyps and simple diverticular disease, can be very challenging since the symptoms are very common and overlap in these conditions. The ability to use a simple, easy to use, inexpensive diagnostic test will provide additional assistance in determining the appropriate patient pathway for further investigation. Ahead of this publication, several hospitals and CCGs have already committed to the provision of FIT as a means of triaging all patients presenting in primary (and secondary) care with lower abdominal symptoms. This Focus on FIT publication summarises some recent progress and current thinking on the application of FIT in both symptomatic and screening populations. Toilet Normal Abnormal Blood Mucus 2
3 Getting FIT for the Future! FIT for Clinicians - Symptomatic Patients and Screening Programmes Why use FIT? Evidence Base NHS England spent approximately million during 2014 on performing colonoscopies (based on NHS tariff price), yet with approximately 40% of those no pathologies were found. Identifying and prioritising those patients more likely to require urgent intervention could save significant costs, reduce waiting times and improve care. Normal Low risk adenoma High risk adenoma Cancer Faecal Haemoglobin Publications support the Faecal Immunochemical Test (FIT) for Haemoglobin (Hb) as a rule out test (NPV of FIT at 10µg Hb/g faeces is 100% for Cancer, 94.6% High-Risk Adenoma (HRA), 93.5% Low-Risk Adenoma (LRA) and Inflammatory Bowel Disease 94% 1 ), and demonstrate that with an increased severity of disease a higher faecal Haemaglobin (F-Hb) concentration is detected. Thus FIT enables management of the patient pathway and most effective use of resources based on appropriate evidence. Personalised Medicine All patients are different and present with a range of symptoms and risk factors. The additional information provided by FIT testing can help determine the optimum management of each individual. Resource Management Waiting times for endoscopy resources are increasing. Performing an initial FIT test to categorise the patient could, with confidence, predict those for whom colonoscopy is not appropriate. This would remove 40% of patients from waiting lists, significantly improving the turn-around time for those remaining, and ensuring their treatment is optimised and actioned sooner. FIT for Patients - Informed choice Concerned about their condition, patients want quick answers, with minimal intervention. With FIT testing they can have access to more information about the symptoms they exhibit and the possible causes for them. Unfortunately IBS and other benign bowel disorders can exhibit similar symptoms to more serious conditions, such as colorectal cancers. As a consequence the longer it takes to resolve these concerns the more anxious patients become. Easier, More Convenient Sample Collection Compared to the traditional card format triple guaiac-based faecal occult blood test, sampling for the HM-JACKarc FIT test is quick and hygienic. Only one faecal sample is required and is collected using a small picker device that is then re-sealed in it s plastic vial ready for testing. Rapid Response For most, having a rapid non-invasive faecal test to get a faster diagnosis would be the preferred choice. Using a FIT result, about 40% of patients would be informed that no further follow up is necessary and hence relieved straight away. The remaining 60% would have the option of a prioritised process for colonoscopy and get their treatment solutions started sooner. Risk Management Invasive procedures are not without risk, and this is true of colonoscopy. 1 in 1,000 patients may suffer a perforated bowel during this procedure, with additional risk of morbidity. So, with a non-invasive alternative now available shouldn t that be the first choice? Additionally, delay in identifying any abnormal bowel pathology, also carries a higher risk of mortality. Hence, the ability to identify those at greater risk and then fast track these patients for appropriate colonoscopy and treatment is highly desirable. Treated early before it becomes invasive, bowel cancer has a 93% 5 year survival rate. Screening in the Asymptomatic Population Using FIT technology, such as the HM-JACKarc automated system, within a screening programme, enables the adjustment of positive cut off concentration. This helps to control the number of referrals for colonoscopy within the limits of available resources. In addition, the specificity of FIT eliminates false positives caused by dietary factors, ensuring positive results are a true indicator of pathology. Reference 1. Low faecal haemoglobin concentration potentially rules out significant colorectal disease PJ McDonald, et al. Accepted Article doi: / codi Find out more at 3
4 Focus on FIT Evaluating a Faecal Immunochemical Test System for Symptomatic Patient Assessments by Dr Ian Godber, Consultant Clinical Scientist, Clinical Lead (Biochemistry), Monklands Hospital, NHS Lanarkshire Matthew: How did you engage with all the necessary departments to progress the pilot project? Alan Hankinson, Senior Biomedical Scientist with the HM-JACKarc at Monklands Hospital Dr Ian Godber s team at NHS Lanarkshire has evaluated the HM-JACKarc automated FIT analytical system for assessment of patients referred from primary care to endoscopy because of lower GI symptoms. As a result of this successful study, the process of rolling out a FIT service to their local General Practitioners (GP) is now well under way. Matthew Davis, Alpha Laboratories Senior Product Manager, met recently with Ian to find out more from one of the first hospitals in the UK to offer a quantitative Faecal Immunochemical Test for haemoglobin (FIT) service. Matthew: What prompted your laboratory to consider providing the FIT service? Ian: We initiated investigations on Faecal Immunochemical Tests for haemoglobin (FIT) due to the withdrawal of traditional guaiac-based gfobt, following Professor Callum Fraser s audit (ACB Scotland 2005). This highlighted that gfobt should not be used to investigate symptomatic patients because of false positives and false negatives inherent when using this test. Our Gastroenterologists and GI surgeons agreed with the cessation of gfobt but the knockon effect has been an increased demand for lower GI tract endoscopy. In consequence, we needed to find a way to relieve this additional pressure on the endoscopy service. The directorate structure of NHS Lanarkshire aided discussion. The divisional management to which I report is the same for both Diagnostics and Cancer Services, with similar structure and goals. This helped to simplify communication and coordination on this project. Matthew: Where did the original enquiry for the FIT service come from? Ian: In 2013, we were approached by Alpha Labs, who asked if we would be interested in undertaking a pilot project. The current evidence in the literature at the time was limited but positive and showed favourable results, so we decided this project was worth progressing. Ian: There was sufficient data in publications on FIT to suggest that the ability to rule in patients who potentially had cancer and fast track them through endoscopy was good. In addition, the potential to rule out patients with no significant pathology was interesting from a cost saving and improved efficiency perspective. The proposal was taken to the Chief of Medicine for NHS Lanarkshire, at the Wishaw General Hospital site, Mr Hakim Ben Younes, who is also a GI surgeon, and he was keen to proceed. There was sufficient evidence to put the concept forward as a proposal to the Gastroenterologists, in order to relieve the pressure on the endoscopy service. This was a key factor in gaining approval to proceed with the project. Matthew: What were the outcomes from the pilot project? Ian: With support from Gastroenterologists and GI Surgeons, the project was driven forward to a successful conclusion, which resulted in a publication: Clin Chem Lab Med Apr;54(4): doi: / cclm The conclusions in the paper were essentially that the high negative predictive value of this test, at a cut off of 10µg Hb/g faeces, allows you to triage patients without missing any cancers, where the clinical sensitivity for colorectal cancer was 100%. In this study, all cancers had a f-hb concentration greater than 150 µg Hb/g faeces. Mucosal health Quantitative Calprotectin monitoring SUSPECTED CALPROTECT There still remain a number of questions, mainly around other bowel NEGATIVE pathologies that also generate a positive FIT result, and how these patients are managed. It may be that patients with very high f-hb concentrations, say 150µg Hb/g faeces are sent straight to endoscopy, FAECAL IMMUNOCHEMICAL TEST for Hb whereas those who are below that, but above 10 µg Hb/g faeces, are followed up clinically. We think that there is potential to develop a clinical pathway that involves an initial screen with FIT, with patients then being seen in a clinic if the result is positive. The patient characteristics such as age, as well as the symptoms, may lead the clinician down a different track, favouring referral to Gastroenterology, with the possibility of a calprotectin test being more appropriate. However, the higher the f-hb concentration initially the more important it is to initiate an urgent colonoscopy. 4
5 But, what about the negative FIT results? They may still need to be considered further and, depending on the clinical symptoms, there is possibly a case for following up these patients in 6 to12 months time with another FIT test. Matthew: How did you manage to secure funding for a new test? Ian: At present, the funding still isn t guaranteed and there is an on-going discussion as to where resources will come from. The NHS Board has indicated that the funding required could be moved from the endoscopy budget through into the laboratory budget. However, this becomes a bit of a grey area in that the test is actually being offered to patients seen in primary care, but the results are going back to secondary care, so it becomes part of the referral process for lower GI endoscopy. colonoscopy. Sometimes the patients' symptoms have resolved by the time they receive an appointment. Others are put on a long waiting list. Both the patient and the GP can now feel that something is being done at an earlier stage. Patient feedback will be surveyed once this approach is rolled out to a higher number of GPs. However, there is the potential for this not to work! There are over 100 general practices in NHS Lanarkshire and, when FIT kits were sent out during the pilot project, there was only a 50-60% return rate, so we really want to ensure we have full engagement with the GPs and patients before rolling it out any further. We would like to engage more with general practices first, to ensure they are fully briefed to gain maximum participation from the patients, before rolling it out to all of NHS Lanarkshire. Matthew: What were the outcomes from the new test implementation? Ian: The lessons to be learnt are to engage with all parties concerned, maybe forming a group to discuss the patient pathway and implementation involving the laboratories, GPs, GI surgeons and Gastroenterologists, as well as the endoscopy services. IBS/IBD IN TEST Matthew: How did you work with the community GPs to implement the new test service? Ian: We ve engaged with a limited number of GP practices to start with and, over the next few months, we are starting to review how we actually provide this service and then finetune it. We ve gone out and met with these practices and educated them, discussing how we are going to accept referrals for patients that present with lower GI symptoms. We explain that they would normally have been referred for a lower GI endoscopy but now they will first be provided with patient FIT collection kits, pre-paid envelopes and patient instructions for collection of faecal samples. So, the idea is that all referrals are done through an electronic mechanism for referral. At the time of referral in primary care, the patient is given an easy to use, hygienic, HM-JACKarc specimen collection device and asked to collect their sample and return it, with the laboratory request form, which identifies them, in the prepaid envelope provided. They re told to do that immediately, with their next bowel motion, and send it off as soon as possible. All the devices POSITIVE Refer Hospital for in Airdrie. colonoscopy go back to a central hub, here in Monklands We can t be seen as a delaying factor in triage. The endoscopy team receives the electronic referral, and they know to expect the f-hb concentration result, therefore there s a time window which is currently being fine-tuned for us to return a FIT result. Based on the FIT result, they may choose to fast track that patient straight to colonoscopy. Confirmed IBD: Monitoring Therapeutic effectiveness There is then the potential to avoid expensive, unpleasant and potentially risky colonoscopies where they are not necessary. Pretty much Anti-TNFα: serum drug level and anti-drug antibody monitoring every GP we ve spoken with, to date, thinks this is a useful addition to the health service. Remember, at present, they don t have gfobt, and currently the only option for patients with lower GI symptoms is to offer a referral for Impacting Abdominal Pain Patient Pathways Through Diagnostics Patient presents at GP with abdominal symptoms Patients triaged based on risk factors NEGATIVE Calprotectin test SUSPECTED COLORECTAL CANCER FAECAL IMMUNOCHEMICAL TEST for Hb POSITIVE Refer for urgent colonoscopy Find out more at 5
6 Focus on FIT Introducing the FIT Service at NHS Tayside Judith Strachan, Consultant Clinical Scientist, Ninewells Hospital & Medical School, NHS Tayside Gastroenterologists are increasingly challenged to provide an appropriate colonoscopy service to the population with more referrals and longer waiting times. In Tayside we d seen the number of colonoscopies going up year by year. However, the number of cancers being identified wasn t changing, despite an increasing number of people being subjected to an invasive and potentially risky procedure. NHS Tayside has a long standing history of bowel screening and, more recently of also analysing faecal haemoglobin in samples from symptomatic patients. Based on concerns about increasing colonoscopy lists, we became involved in discussions with our gastroenterology team exploring whether we could introduce quantitative faecal immunochemical testing (FIT) into our existing colorectal referral pathway. Could we triage people using a FIT result to refine whether they really needed a colonoscopy? Data from our initial investigation was published in GUT in GPs asked patients that they were already referring to gastroenterology to submit a faecal sample, so that we could analyse their faecal haemoglobin with FIT. This didn t affect the patients subsequent management and progression along the standard gastro pathway. We then, in retrospect, assessed clinical outcomes against the FIT result for each patient. This analysis showed that a negative FIT result correlated with a very low chance of having any significant bowel disease such as cancer or IBD. Using this data we were able to demonstrate that provision of FIT was viable, since we believed the number of unnecessary colonoscopies performed could be cut quite significantly. This was obviously very attractive, not only financially, but also in the potential for reducing the waiting times and pressures for the increasingly challenged personnel. Engaging the GPs Once we had obtained funding, the next step was to engage with the GPs. We went to them with a package early on to make it as easy as possible. It s important that the service fits local referral practice. At first there were some concerns from the GPs, because they were being asked to request another test and didn t want to delay an urgent referral. The GPs had input into the patient instruction leaflets and guidance on how to get samples back. The sampling aspect was new because the FIT test uses a faecal sampling device that s placed in a buffer tube. Faecal material in pots is not acceptable for FIT since there s so much evidence that faecal haemoglobin degrades. We send out FIT kits with patient instruction leaflets (Figure 1), directly to GP practices and the samples are returned using the normal laboratory transport arrangements. Patients are remarkably happy to do the test as advised and are absolutely fine using the FIT sampling device. We ve had no negative feedback or issues at all. Figure 1. Faecal Immunochemical Test (FIT) Sampling Device and Patient Instruction Leaflet. We promoted the FIT service through a series of joint key newsletters and s from gastroenterology and the laboratories to the GPs. This supported GP education and engagement so that they became more receptive to the idea. Involvement from gastro is essential and at Tayside the process was virtually led by them. This is really important as the service can t just be set up from a lab perspective and the joint approach is well received by the GPs. Triaging with FIT Tayside has been offering the FIT service for about 16 months now. It took about six months before we got full engagement from the GPs, because this was such a new process. Adding FIT onto our existing colorectal referral bundle through the electronic requesting system helps make it more straightforward. The FIT result is going back to the GP and to further support the process, we signpost them to the Tayside gastroenterology page which details what to do next and what the options are, dependent on the FIT result. About 75 to 80 percent of referred patients now have a FIT result. The tests are being analysed daily to make sure that we don t delay any referrals. So, by the time the gastroenterologists are seeing an electronic referral, either the FIT result is with that referral or they can look it up. The FIT result is being used as part of the decision making process as to what to do next. Do they go straight for colonoscopy or are they seen in a clinic? Sometimes if a referral is sent without a FIT, the gastroenterologist will request one, using the two way interactive electronic referral system. A crucial part of this process is to have the clinical input from the GPs as the FIT result can t be used in isolation. It has to be taken in conjunction with clinical signs and symptoms. If there is high clinical suspicion of a colorectal cancer or other serious condition, but the FIT result is negative, then of course that should not stop a referral. It s really important that this message is clear and FIT is used in combination to get the bigger picture. With the increased awareness and roll out of FIT, there is anecdotal feedback that the GPs really like the ability to offer this test. Some patients are reluctant to be referred to gastro because they are worried that they will end up having a colonoscopy. Now, the GP can offer this test first, before that decision is made. If the FIT result is positive then they know there are good grounds for proceeding to colonoscopy. If it is negative, then the chances are there is nothing seriously wrong and the patient is given that reassurance. We also have evidence that the referral status of people is changing. Previously where someone may have had a routine referral, now, if the FIT result is very high, the gastroenterologists may change that referral to urgent. So, individual patients are getting a colonoscopy and diagnosis of a cancer much faster. We re also starting to see a reduction in actual referrals into gastroenterology for the first time that anyone can remember. So, that shows that the GPs are gaining confidence that if there is a negative FIT the chances are that their patient doesn t have anything seriously wrong. The benefit is that by the reducing the number of people being referred unnecessarily, those that really do need it, are being seen much faster. 6
7 Reducing Colonoscopy Lists We are now getting real reductions in waiting times for colonoscopy in Tayside, with patients advancing through the system much faster. Without the FIT implementation I m sure referrals would have continued to grow, but they have now plateaued and fallen for the first time in years. FIT Service Success Factors Referral pathways differ across the UK, so it s important for any labs planning to set up a FIT service to fully understand their local procedures. It s essential to engage with the gastro service. There may not always be a seamless communication system. I think you ve got to adapt to whatever exists or take the opportunity to influence it. Labs have to understand the role of the GPs and you really need to know what your gastroenterologists want out of the testing. How are they going to use the test result, what turnaround time is appropriate and how many samples will there be? It s important to look at how many referrals there are to gastro and how many colonoscopies are being done. Without that information you can t really start to plan how the lab is going to offer the service. I think the key success factor for the Tayside implementation has been the team work between gastro and the labs. Flexibility and good communication on both sides has worked really well. We ve also learned a lot from the GPs over time and adapted to providing more kits, as we initially underestimated that. Feedback to the GPs has been really crucial and we liaise every two or three months through a newsletter. We feedback regularly about what we re actually funding, how many tests we ve performed, how many referrals there have been etc. Judith Strachan (left) with Becky McCann, using the HM-JACKarc FIT System to analyse samples for faecal haemoglobin We d also like to investigate sequential samples. If a patient has a negative FIT but still has symptoms, is it worth doing further tests? So there are lots of possibilities that we ve not really explored yet. The team is hoping to publish the work to date by mid There s an inevitable lag time in obtaining details of the clinical outcomes of these patients a lot of resource required to check on referral and clinical outcomes and this should not be underestimated. Reference 1. Mowat C, Digby J, Strachan JA, Wilson R, Carey FA, Fraser CG, Steele R. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut Sep; 65(9): Support from the supplier of the test is also crucial because you need their offering to work with the local system, provision of instruction leaflets and help with the whole package. You need a logistics solution to get the tubes from the lab to the GP and back again in a time frame that suits the service. Labs shouldn t underestimate the time taken to set this up. The next step for Tayside is really in refining the service, particularly regarding the detailing of the reports. We want to provide further guidance on what results mean to give GPs more confidence to make decisions. We are happy that our process is robust and just need to focus on further education of GP practices that haven t yet engaged. We re really interested to look at the population we have results from, to see if they are the demographic that is slow to engage with the GP. Find out more at 7
8 Focus on FIT A Tale of Two Settings by Professor Callum G. Fraser, Centre for Research into Cancer Prevention and Screening, University of Dundee, Ninewells Hospital and Medical School, Dundee Faecal Immunochemical Tests (FIT) are now used for asymptomatic population-based bowel (cancer) screening and also for the assessment of patients presenting with lower abdominal symptoms, particularly in primary care. Characteristic Target Population Aim FIT provide one investigation that is of significant value in these two very different clinical settings. These applications have different target populations, aims, faecal haemoglobin cut-offs, interpretation of results, potential harms, additional benefits, potential improvements and possible strategies for the future. Purpose Faecal Haemoglobin Cut-off Concentration Used It is important that these different aspects of FIT are appreciated as this test rolls out across the UK and the major advantages of increasing screening uptake and helping to decide which patients presenting in primary care would benefit most from colonoscopy are gained. Interpretation of Results The adjacent table clarifies the attributes. Potential Harms 8 Additional Benefits Potential Improvements
9 Distinguishing FIT in Screening from FIT in Assessment of the Symptomatic FIT in Screening FIT in the Symptomatic Asymptomatic individuals eligible to participate in structured screening programmes, which differ from nation to nation in the UK, being years in England, Wales and Northern Ireland, and years in Scotland along with those older individuals who choose to opt-in. To select those participants in screening programmes who have no symptoms, but are at highest risk of colorectal neoplasia cancer and higher-risk (advanced) adenoma. Patients of any age who present in primary care with lower abdominal symptoms such as rectal bleeding, a change in bowel habit to constipation or diarrhoea, unexplained weight loss, anaemia, abdominal pain, and abdominal or rectal mass. In addition, some patients seen at certain secondary care clinics such as gastroenterology, will benefit. To identify those patients who are most unlikely to have significant colorectal disease and would not benefit from referral for colonoscopy, saving resources and shortening waiting times, as well as identifying those who have significant colorectal disease and would benefit. Rule in neoplasia. Rule out significant colorectal disease (cancer + higher-risk adenoma + Inflammatory Bowel Disease). Rule in significant colorectal disease. High especially in colonoscopy constrained countries. Selected to give the screening programme performance characteristics desired, such as the positivity rate with which the available colonoscopy resource could cope. A positive result means that a risk of significant colorectal disease is present and further investigation is warranted. A negative result means the participant should be invited again at the set screening interval, currently two years in the UK. Not all colorectal neoplasia is detected interval cancer proportions are high when high faecal haemoglobin cutoffs are applied. Thus, a negative result does not mean that colorectal neoplasia is absent and participants receive information on lifestyle and symptoms. There is a reassurance effect of a negative result. Moreover, a positive result does not mean that colorectal neoplasia is present, but the participant will undergo an invasive and potentially harmful investigation. Not only cancer detected but also some higher-risk adenoma, sometimes precursors of cancer, and Inflammatory Bowel Disease. As FIT screening progresses and as the available colonoscopy resource expands, implementation of lower cut-offs over time would increase detection of cancer and even more higher-risk adenoma. Low 10 µg Hb/g faeces is widely recommended. Selected to ensure that patients with negative results, most unlikely to have significant colorectal disease, do not necessarily get early referral for colonoscopy. And, if positive, stimulates early referral to secondary care for further investigation. If the result is negative, there is considerable reassurance that significant colorectal disease is not present. A detectable faecal haemoglobin means that the patient warrants further investigation. FIT in assessment of the symptomatic is not perfect and some colorectal disease will be missed if a negative result is used as guidance for no referral. Most cancers are detected, but a slightly greater proportion of higher-risk adenoma and inflammatory bowel diseases are not detected. Thus, patients with negative results could be given reassurance, but possible alternatives such as watching and waiting, referral to secondary care clinics, or a repeat FIT might be warranted, particularly if symptoms persist. Not only possibility of significant colorectal disease being excluded, but cancer, higher-risk adenoma, sometimes precursors of cancer, and Inflammatory Bowel Disease detected. Investigation of more analytically sensitive methods for detection of faecal haemoglobin, since many patients have undetectable faecal haemoglobin with current methodology. Use of FIT result in combination with other variables such as blood haemoglobin. Find out more at 9
10 Focus on FIT A FIT Sample New Faecal Immunochemical Test Systems could Improve Sample Integrity for Faecal Haemoglobin One of the challenges in clinical diagnostics is the logistics of getting a quality sample from the patient to the laboratory for analysis. When considering the detection of faecal haemoglobin (f-hb), this is partly dependent on the technology to be employed. In the days of guaiacbased faecal testing, samples were sent in traditional blue-capped stool pots. This was clearly wrong, since haemoglobin in native faeces is very unstable (Brown and Fraser 1 ). It degrades rapidly at physiological and ambient temperatures. In passed faeces, which contains digestive enzymes, bacteria and fungi, it will degrade even more quickly. The moiety being examined in gfobt is the haem component of the haemoglobin molecule. Young et al. 2 demonstrated that, with gfobt, the degradation was more pronounced in the samples that were not dried, as when collected into a traditional faecal pot, versus a thin dried smeared sample (taken directly onto the gfobt card). The conclusion was that sampling directly onto the card should be made as soon as possible following defecation. In addition, analysis of gfobt should be delayed for a few days so that potential interference from plant peroxidases, leading to false positive results, can be minimised. 3 With the move to a more sensitive technology based on an immunoassay specific for human haemoglobin, it is vital to stabilise the haemoglobin present in the specimen collection device, prior to analysis, to protect it from degradation and maintain integrity. Brown and Fraser 1 performed a similar study to Young et al 2. However, they used both qualitative and quantitative FIT methods to analyse five haemoglobin spiked faecal samples, with daily sampling for up to 14 days. The conclusion was that false negative results for faecal haemoglobin could occur if sampling fresh into the tubes or onto the cards of FIT collection devices is delayed. With NICE, through a Diagnostics Assessment Committee, now focussing on the benefits of the application of FIT as a means to triage patients with lower gastrointestinal (GI) symptoms, it is important that any loss of f-hb is protected. Using a low level cut-off of 10µg of Hb/ g faeces, the negative predictive value (NPV) for cancer is very high (100 % in the hallmark study by McDonald et al. 4 ). Similarly high NPVs of 94%, are seen for higher risk adenomas (HRA) and Inflammatory Bowel Disease (IBD). Further studies in the UK have also shown a high degree of NPV when using FIT with cutoffs at this f-hb concentration. 5,6,7 The introduction of new quantitative FIT methods has vastly improved the method of faecal sample collection. This is an important aspect of the process since the clinical outcomes are dependent on the ability of the method to detect faecal haemoglobin at very low versus undetectable concentrations. The HM-JACKarc specimen collection device contains a proprietary buffer which can stabilise f-hb in samples for up to 14 days at ambient temperature (up to 25 C) or up to 120 days in the fridge (4 C). This was confirmed in a study by Carroll et al. 8 investigating the performance characteristics of four FIT methods. 7 With any method, sample integrity is key to the quality of result. Typically laboratories would be concerned that a layperson would be unable to provide a consistent sample. However, the collection device of the HM-JACKarc is unique, in that it has two hexagonal dimples on one side of the collection probe. This ensures that as the probe is pushed back into the collection device after sampling of the faeces, any excess faecal matter is removed and a consistent amount of sample is passed into the constant volume of buffer. This is irrespective of the consistency of the faecal sample which can vary from liquid to hard pellets. Use of the HM-JACKarc specimen collection devices ensures stability of f-hb and low variation in the ratio of faecal mass collected to volume of buffer. Such hygienic devices are simple for patients to use and encourage taking up the test in those who have concerns about handling faeces. For more information on the HM-JACKarc quantitative FIT method please visit References 1. Brown LF, Fraser CG. Effect of delay in sampling on haemoglobin determined by faecal immunochemical tests. Ann Clin Biochem. 2008;45: Young GP, Sinatra MA, St John DJ. Influence of delay in stool sampling on fecal occult blood test sensitivity. Clin Chem. 1996;42: Sinatra MA, St John DJ, Young GP. Interference of plant peroxidases with guaiac-based fecal occult blood tests is avoidable. Clin Chem. 1999;45: McDonald PJ, Digby J, Innes C, et al. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis. 2013;15:e Mowat C, et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut. 2016;65: Godber IM, et al. Use of a faecal immunochemical test for haemoglobin can aid in the investigation of patients with lower abdominal symptoms. Clin Chem Lab Med. 2016;54: Thomas CL, et al. Can immunochemical tests for faecal haemoglobin and faecal calprotectin be used to risk stratify patients for referral to colonoscopy for suspected colorectal cancer? Ann Clin Biochem. Biochemistry 2016;53 Suppl 1: Carroll MR, Seaman HE, Halloran SP Tests and investigations for colorectal cancer screening. Clin Biochem. 2014;47:
11 Which FIT's Best? Guildford Medical Device Evaluation Centre (GMEC) Evaluation of Quantitative Faecal Immunochemical Tests for Haemoglobin The excellent clinical outcome data demonstrated in many publications, require faecal haemoglobin cut-offs for referral for further investigation, at the low end of the analytical range of the available FIT systems. The choice of laboratory method is therefore important to the objective evaluation of patients, not only for haemoglobin stability in the specimen collection device, but also for its low bias and small imprecision at the lower limit of the analytical working range. Four FIT analytical systems were evaluated by the Guildford Medical Device Evaluation Centre (GMEC) in The resulting report is available online: activities/fit_reports/gmec_fit_evaluation_ report.pdf Extracts of data from that report are represented here. In this study, the HM-JACKarc system, supplied by Alpha Laboratories, was described as one of the more precise methods (Table 1). Its analytical working range correlated well to the expected values of spiked faecal samples. The ability to detect haemoglobin at both the lower and higher limits of the analytical range was confirmed (Figure 1). HM-JACKarc demonstrated a high sensitivity with a lower limit of detection of just 0.6 µg Hb/g faeces, making it ideal for symptomatic testing (Table 2). In addition, sample stability was proven at 20C throughout the 30 day period of the study (Table 3). Confirmed by GMEC: Stability of sample in the collection tube claims of the pack insert 120 days at 4-8 C 14 days at 25 C The Hook capacity greater than 200,000 µg of Hb / g faeces Linearity across the measurement range (7-400 µg of Hb / g faeces) Described as very sensitive at the low end with LOD as 0.6 µg of Hb / g faeces Table 1. HM-JACKarc Precision Imprecision measured against manufacturers mean concentrations Manufacturer data Buffer GMEC sr Samples data mean Verification σ mean σr value HM-JACKarc (µg Hb/ g faeces) Table 2. HM-JACKarc Sensitivity Measured lower limit of detection for each analyser. Quoted lower limits of detection were provided by each manufacturer in their data sheets. Mean concentration of 20 un-spiked collection tubes (µg Hb/g faeces) Standard deviation Lower limit of detection (µg Hb/g faeces) Consistent/ not consistent with claim NSD Consistent Consistent KEY: sr GMEC measured estimate of repeatability σr manufacturers claimed repeatability NSD Not statistically different from manufacturers claim Figure 1. HM-JACKarc Linearity Quoted lower limit of detection (µg Hb/g faeces) HM-JACKarc NS-PLUS C OC-SENSOR DIANA FOB Gold/BioMajesty Table 3. HM-JACKarc Sample Stability HM-JACKarc Measured Stability of Diluted Hb and Faecal Samples Spiked with Hb Temperature - 20 C 4 C 20 C Concentration (µg Hb/g faeces) All concs All concs All concs Hb in buffer STS STS STS Hb in faeces STS STS STS KEY: STS Stable throughout study (30 days) i.e. the concentration of Hb did not fall below 50% of the initial concentration during the study. Concs haemoglobin concentrations. Four concentrations of Hb were tested ranging from the detection limit to a strong positive FIT result. Find out more at 11
12 Focus on FIT A FIT System for Any Laboratory The HM-JACKarc is a high throughput, fully automated, compact bench top analyser that provides quantitative faecal haemoglobin (f-hb) concentration results on a simple to use platform. It has a wide dynamic range from 7ng/ml to 400 ng/ml (ng/ml = µg Hb/g faeces), making it ideal for use in clinical settings for both asymptomatic screening and assessment of the symptomatic. The latex reagent is specific for human haemoglobin and is measured by spherical turbidimetry. Quantitative Faecal Immunochemical Tests (FIT) offer significant advantage over traditional guaiac based tests and qualitative FIT methods, both of which have subjective end points with varying cut off levels. Advanced Sample Collection System Easy to use sample device collects a consistent sample size across different faecal matter Internal septum removes excess sample <2mg of sample in 2ml of buffer (ng/ml = µg Hb / g faeces) Tamper seal and window to confirm sample applied Unique bar code number for the tube (lot no./expiry date/tube no.) Collection buffer stabilises the faecal sample haemoglobin days at 4 C (refrigerated) - 14 days at 25 C (ambient temp) Fully Automated Instrument Compact and light bench top unit with touchscreen interface Uses Integrated Sphere Latex Turbidimetry to measure faecal haemoglobin concentration Sensitivity: 7 ng/ml Cut-off concentrations can be selected depending on requirements Screening or Symptomatic Testing No prozone effect up to 200,000 ng/ml High speed performance: 200 samples/hour - Time to first result 5.6 minutes - Additional results every 18 seconds Scattered Light Sensor LED Light Source Factory set Master curve with local 2 point recalibration - Calibrates system to local conditions and reagent combinations - Calibration weekly - System can store 2 calibration curves - Calibration of different lots of latex - Same lots with different calibrators Latex Reagent Provides a large concentration of capture antibodies Wide dynamic range 7ng/ml to 400 ng/ml (ng/ml = µg Hb/g faeces) High Hook capacity > 200,000ng/ml Ensures no samples give a falsely lower result Drives reaction kinetics to end stage equilibrium quickly To find out more visit Watch the HM-JACKarc in action at or contact us for support in setting up your FIT Service Direct Light Sensor The HM-JACKarc system is easy to set up and can be loaded with up to 80 samples at any one time. It can stand alone or be linked to the LIS, allowing flexible options for connectivity and full audit trail of results, QC and reagent lot numbers. Reaction Vessel Integrated Sphere Turbidimetry (IST) Scattered Light Direct Light Automated FIT Testing: Easy as 1-2-3! 40 Parham Drive, Eastleigh, Hampshire, SO50 4NU, UK Tel: marketing@alphalabs.co.uk Web: Registered in England
Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients
 Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients Caroline Addison NE BCSP Hub Director and Consultant Clinical Scientist What is FIT Type of Faecal Occult Blood test Designed
Faecal Immunochemical Testing (FIT) for Screening and Symptomatic Patients Caroline Addison NE BCSP Hub Director and Consultant Clinical Scientist What is FIT Type of Faecal Occult Blood test Designed
Information Pack for GP s The implementation of the Faecal Immunochemical Test (FIT) across the South West
 Information Pack for GP s The implementation of the Faecal Immunochemical Test (FIT) across the South West The South West Cancer Alliances have been awarded transformation funding to provide access to
Information Pack for GP s The implementation of the Faecal Immunochemical Test (FIT) across the South West The South West Cancer Alliances have been awarded transformation funding to provide access to
University of Dundee. Published in: Annals of Clinical Biochemistry DOI: / Publication date: 2017
 University of Dundee Application of NICE guideline NG12 to the initial assessment of patients with lower gastrointestinal symptoms Quyn, Aaron J.; Steele, Robert; Digby, Jayne; Strachan, Judith A.; Mowat,
University of Dundee Application of NICE guideline NG12 to the initial assessment of patients with lower gastrointestinal symptoms Quyn, Aaron J.; Steele, Robert; Digby, Jayne; Strachan, Judith A.; Mowat,
FIT - A Tale of Two Settings. Callum G Fraser Centre for Research into Cancer Prevention and Screening University of Dundee Scotland
 FIT - A Tale of Two Settings Callum G Fraser Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible Conflicts of Interest Consultant: Kyowa, Tokyo, Japan Consultant:
FIT - A Tale of Two Settings Callum G Fraser Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible Conflicts of Interest Consultant: Kyowa, Tokyo, Japan Consultant:
North West London Pathology. Faecal Occult Blood testing. Mrs Sophie Barnes FRCPath Consultant Clinical Scientist
 Faecal Occult Blood testing Mrs Sophie Barnes FRCPath Consultant Clinical Scientist Learning objectives Background Guidelines for colorectal cancer detection Tests available to detect occult blood in faeces
Faecal Occult Blood testing Mrs Sophie Barnes FRCPath Consultant Clinical Scientist Learning objectives Background Guidelines for colorectal cancer detection Tests available to detect occult blood in faeces
ENGAGING PRIMARY CARE IN BOWEL SCREENING
 ENGAGING PRIMARY CARE IN BOWEL SCREENING GP GOOD PRACTICE GUIDE SCOTLAND VERSION ENGAGING PRIMARY CARE IN BOWEL SCREENING GP GOOD PRACTICE GUIDE SCOTLAND VERSION CONTENT 2 Background & information on the
ENGAGING PRIMARY CARE IN BOWEL SCREENING GP GOOD PRACTICE GUIDE SCOTLAND VERSION ENGAGING PRIMARY CARE IN BOWEL SCREENING GP GOOD PRACTICE GUIDE SCOTLAND VERSION CONTENT 2 Background & information on the
FIT for symptomatic patients. Facilitator name
 FIT for symptomatic patients Facilitator name Context colorectal cancer Colorectal cancer in the UK 41,804 new cases in 2015 15,903 deaths in 2014 Fourth most common cancer Second most common cause of
FIT for symptomatic patients Facilitator name Context colorectal cancer Colorectal cancer in the UK 41,804 new cases in 2015 15,903 deaths in 2014 Fourth most common cancer Second most common cause of
The York Faecal Calprotectin Care Pathway for use in primary care. James Turvill
 The York Faecal Calprotectin Care Pathway for use in primary care James Turvill NICE guidance: dg11 Faecal calprotectin (FC) testing as an option in adults with recent onset of lower gastrointestinal symptoms
The York Faecal Calprotectin Care Pathway for use in primary care James Turvill NICE guidance: dg11 Faecal calprotectin (FC) testing as an option in adults with recent onset of lower gastrointestinal symptoms
Challenges for Colorectal Cancer Screening
 Challenges for Colorectal Cancer Screening a Biomarker with No Standards! Prof. Emeritus Stephen P. Halloran University of Surrey W. Europe Top 20 Cancers Men Incidence & Mortality (2012) Women World -
Challenges for Colorectal Cancer Screening a Biomarker with No Standards! Prof. Emeritus Stephen P. Halloran University of Surrey W. Europe Top 20 Cancers Men Incidence & Mortality (2012) Women World -
Dr Katie Elliott CRUK strategic GP Macmillan GP with NE &C Learning disability Network Assistant Clinical Lead Northern Cancer Alliance
 FIT for symptomatic patients Dr Katie Elliott CRUK strategic GP Macmillan GP with NE &C Learning disability Network Assistant Clinical Lead Northern Cancer Alliance AIMS Review National advice Consider
FIT for symptomatic patients Dr Katie Elliott CRUK strategic GP Macmillan GP with NE &C Learning disability Network Assistant Clinical Lead Northern Cancer Alliance AIMS Review National advice Consider
Bowel cancer risk in the under 50s. Greg Rubin Professor of General Practice and Primary Care
 Bowel cancer risk in the under 50s Greg Rubin Professor of General Practice and Primary Care Prevalence of GI problems in the consulting population Thompson et al, Gut 2000 Number of patients % of patients
Bowel cancer risk in the under 50s Greg Rubin Professor of General Practice and Primary Care Prevalence of GI problems in the consulting population Thompson et al, Gut 2000 Number of patients % of patients
Risk scoring incorporating FIT in triage of symptomatic patients
 Risk scoring incorporating FIT in triage of symptomatic patients Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible conflicts of interest None Background Symptoms
Risk scoring incorporating FIT in triage of symptomatic patients Centre for Research into Cancer Prevention and Screening University of Dundee Scotland Possible conflicts of interest None Background Symptoms
Cancer Screening Programmes BOWEL CANCER SCREENING. The Facts
 Cancer Screening Programmes BOWEL CANCER SCREENING The Facts What is the aim of this leaflet? This leaflet gives you information about bowel cancer, and the benefits and risks of bowel cancer screening.
Cancer Screening Programmes BOWEL CANCER SCREENING The Facts What is the aim of this leaflet? This leaflet gives you information about bowel cancer, and the benefits and risks of bowel cancer screening.
An Update on the Bowel Cancer Screening Programme. Natasha Djedovic, London Hub Director 17 th September 2018
 An Update on the Bowel Cancer Screening Programme Natasha Djedovic, London Hub Director 17 th September 2018 NHS Bowel Cancer Screening Programme 2006: 60-69 yr old men & women offered guaiac Faecal Occult
An Update on the Bowel Cancer Screening Programme Natasha Djedovic, London Hub Director 17 th September 2018 NHS Bowel Cancer Screening Programme 2006: 60-69 yr old men & women offered guaiac Faecal Occult
Diagnostics guidance Published: 26 July 2017 nice.org.uk/guidance/dg30
 Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care Diagnostics guidance Published: 26 July 2017 nice.org.uk/guidance/dg30 NICE 2018. All rights reserved. Subject
Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care Diagnostics guidance Published: 26 July 2017 nice.org.uk/guidance/dg30 NICE 2018. All rights reserved. Subject
Journal of Clinical Laboratory Instruments and Reagents, Vol. 34, No. 3 (June, 2011) Supplement
 Journal of Clinical Laboratory Instruments and Reagents, Vol. 34, No. 3 (June, 2011) Supplement Laboratory Instruments and Reagents 34(3): 387-392, 2011 Evaluation of the Extel Hemo Auto HS and the Hemo
Journal of Clinical Laboratory Instruments and Reagents, Vol. 34, No. 3 (June, 2011) Supplement Laboratory Instruments and Reagents 34(3): 387-392, 2011 Evaluation of the Extel Hemo Auto HS and the Hemo
Engaging Primary Care in bowel screening
 Engaging Primary Care in bowel screening GP good practice guide for Wales December 2018 Together we will beat cancer Contents Background 3 The FIT screening pathway in Wales 4 The role of GP practices
Engaging Primary Care in bowel screening GP good practice guide for Wales December 2018 Together we will beat cancer Contents Background 3 The FIT screening pathway in Wales 4 The role of GP practices
EVALUATION OF QUANTITATIVE FAECAL IMMUNOCHEMICAL TESTS FOR HAEMOGLOBIN
 EVALUATION OF QUANTITATIVE FAECAL IMMUNOCHEMICAL TESTS FOR HAEMOGLOBIN Date of original publication: 20 November 2013 Date of revision: 8 December 2014 Cover image: The Leggett Building, University of
EVALUATION OF QUANTITATIVE FAECAL IMMUNOCHEMICAL TESTS FOR HAEMOGLOBIN Date of original publication: 20 November 2013 Date of revision: 8 December 2014 Cover image: The Leggett Building, University of
Risk assessment tools for the symptomatic population Graham Radford-Smith Department of Gastroenterology and Hepatology Royal Brisbane and Women s
 Risk assessment tools for the symptomatic population Graham Radford-Smith Department of Gastroenterology and Hepatology Royal Brisbane and Women s Hospital GI inflammation IBD Group QIMR Berghofer Medical
Risk assessment tools for the symptomatic population Graham Radford-Smith Department of Gastroenterology and Hepatology Royal Brisbane and Women s Hospital GI inflammation IBD Group QIMR Berghofer Medical
Bowel health and screening: carers guide. A booklet for carers of people who use easy read materials
 Bowel health and screening: carers guide A booklet for carers of people who use easy read materials Contents About this booklet Page 3: Page 4: Page 5: Page 6: Page 6: Page 7: Page 8: Page 10: Page 10:
Bowel health and screening: carers guide A booklet for carers of people who use easy read materials Contents About this booklet Page 3: Page 4: Page 5: Page 6: Page 6: Page 7: Page 8: Page 10: Page 10:
Bowel cancer screening the facts. Bowel cancer screening: the facts
 Bowel cancer screening the facts Bowel cancer screening: the facts What is this leaflet about? This leaflet is about the benefits and risks of the Northern Ireland bowel cancer screening programme and
Bowel cancer screening the facts Bowel cancer screening: the facts What is this leaflet about? This leaflet is about the benefits and risks of the Northern Ireland bowel cancer screening programme and
Transforming Cancer Services for London
 Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Bowel cancer screening and prevention
 Bowel cancer screening and prevention Cancer Incidence and Mortality Victoria 2012 Number 6000 5000 4000 3000 2000 Incidences = 29,387 Mortality = 10,780 Incidence Mortality 1000 0 Prostate Breast Bowel
Bowel cancer screening and prevention Cancer Incidence and Mortality Victoria 2012 Number 6000 5000 4000 3000 2000 Incidences = 29,387 Mortality = 10,780 Incidence Mortality 1000 0 Prostate Breast Bowel
Why FIT (Faecal Immunochemical Test) is the best biomarker for CRC screening
 Why FIT (Faecal Immunochemical Test) is the best biomarker for CRC screening Prof. Stephen Halloran Royal Surrey County Hospital NHS Cancer Screening Programme University of Surrey gfobt Guaiacum Officinale
Why FIT (Faecal Immunochemical Test) is the best biomarker for CRC screening Prof. Stephen Halloran Royal Surrey County Hospital NHS Cancer Screening Programme University of Surrey gfobt Guaiacum Officinale
Bowel health and screening: carers guide. A booklet for carers of people who use easy read materials
 Bowel health and screening: carers guide A booklet for carers of people who use easy read materials Contents Page 3: About this booklet Page 4: What is the bowel? Page 5: Helping someone to have good bowel
Bowel health and screening: carers guide A booklet for carers of people who use easy read materials Contents Page 3: About this booklet Page 4: What is the bowel? Page 5: Helping someone to have good bowel
Friday, 17 October 2014: 08:30 11:30 * * * * *
 Vienna 2014 6 th Meeting of the Expert Working Group (EWG) FIT for Screening Expert Working Group (EWG) founding members: Friday, 17 October 2014: 08:30 11:30 MEETING REPORT * * * * * Jim Allison, University
Vienna 2014 6 th Meeting of the Expert Working Group (EWG) FIT for Screening Expert Working Group (EWG) founding members: Friday, 17 October 2014: 08:30 11:30 MEETING REPORT * * * * * Jim Allison, University
The Facts CANCER RESEARCH UK:::. Plain English Campaign NHS
 Published by the Department of Health in association with NHS Cancer Screening Programmes, with advice and support from the Cancer Research UK Primary Care Education Group. Clarity approved by,\vt Plain
Published by the Department of Health in association with NHS Cancer Screening Programmes, with advice and support from the Cancer Research UK Primary Care Education Group. Clarity approved by,\vt Plain
Colon cancer screening : age 50 or over I'll talk to my doctor about it
 Vaud Colon Cancer Screening Programme Colon cancer screening : age 50 or over I'll talk to my doctor about it Dépistage du cancer du colon Canton de Vaud Contents Help deciding and family doctor 3 Colon
Vaud Colon Cancer Screening Programme Colon cancer screening : age 50 or over I'll talk to my doctor about it Dépistage du cancer du colon Canton de Vaud Contents Help deciding and family doctor 3 Colon
02 Bowel Cancer UK - carer guide
 carers guide 02 Bowel Cancer UK - carer guide Introduction As a carer you can provide a key role in helping the person you support to make choices about their health on a daily basis. The contents of this
carers guide 02 Bowel Cancer UK - carer guide Introduction As a carer you can provide a key role in helping the person you support to make choices about their health on a daily basis. The contents of this
Implementation of Faecal Immunochemical Testing as the screening test for Bowel Screening. Programme in Wales
 Immunochemical Testing as the screening test for Bowel Screening. Programme in Wales Information to Public Health Wales Board prior to introduction Author: Dr Sharon Hillier, Acting Director Screening
Immunochemical Testing as the screening test for Bowel Screening. Programme in Wales Information to Public Health Wales Board prior to introduction Author: Dr Sharon Hillier, Acting Director Screening
Prevention of Bowel Cancer: which patients do I send for colonoscopy?
 Prevention of Bowel Cancer: which patients do I send for colonoscopy? Dr Chris Groves Consultant Gastroenterologist and Honorary Senior Lecturer St George s Hospital and Medical School Director, SW London
Prevention of Bowel Cancer: which patients do I send for colonoscopy? Dr Chris Groves Consultant Gastroenterologist and Honorary Senior Lecturer St George s Hospital and Medical School Director, SW London
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE
 SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
Friday, 23 October 2015: 10:15 12:00 * * * * *
 Barcelona 2015 8 th Meeting of the Expert Working Group (EWG) FIT for Screening Expert Working Group (EWG) founding members: Friday, 23 October 2015: 10:15 12:00 MEETING REPORT * * * * * Jim Allison, University
Barcelona 2015 8 th Meeting of the Expert Working Group (EWG) FIT for Screening Expert Working Group (EWG) founding members: Friday, 23 October 2015: 10:15 12:00 MEETING REPORT * * * * * Jim Allison, University
Implementing of Population-based FOBT Screening
 Implementing of Population-based FOBT Screening gfobt to FIT Experience from England Prof Stephen P. Halloran Guaiac FOBt Haem 2H 2 O 2 = 2H 2 0 + O 2 Oxidised guaiaconic acid is blue Biennial Bowel Cancer
Implementing of Population-based FOBT Screening gfobt to FIT Experience from England Prof Stephen P. Halloran Guaiac FOBt Haem 2H 2 O 2 = 2H 2 0 + O 2 Oxidised guaiaconic acid is blue Biennial Bowel Cancer
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS What is CRC? CRC (CRC) is cancer of the large intestine (colon), the lower part of the digestive system. Rectal cancer is cancer of the last several inches of the colon. Together,
FREQUENTLY ASKED QUESTIONS What is CRC? CRC (CRC) is cancer of the large intestine (colon), the lower part of the digestive system. Rectal cancer is cancer of the last several inches of the colon. Together,
NHS Bowel Cancer Screening Programme
 NHS Bowel Cancer Screening Programme Wolverhampton Bowel Cancer Screening Centre Annual Report April 2015 to March 2016 Introduction Bowel cancer is the fourth most common cancer in the UK (2012) accounting
NHS Bowel Cancer Screening Programme Wolverhampton Bowel Cancer Screening Centre Annual Report April 2015 to March 2016 Introduction Bowel cancer is the fourth most common cancer in the UK (2012) accounting
PATIENT BROCHURE. 441 Charmany Dr 1 Madison WI, RX Only
 PATIENT BROCHURE 441 Charmany Dr 1 Madison WI, 53719 844-870- 8870 Cologuard colorectal cancer screening test is a registered trademark of Exact Sciences Corporation. Indications for Use Cologuard is intended
PATIENT BROCHURE 441 Charmany Dr 1 Madison WI, 53719 844-870- 8870 Cologuard colorectal cancer screening test is a registered trademark of Exact Sciences Corporation. Indications for Use Cologuard is intended
Colorectal cancer screening
 26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography
 Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography 2019 Released 2019 health.govt.nz Citation: Ministry of Health. 2019. Referral Criteria for Direct Access
Referral Criteria for Direct Access Outpatient Colonoscopy or Computed Tomography Colonography 2019 Released 2019 health.govt.nz Citation: Ministry of Health. 2019. Referral Criteria for Direct Access
Get tested for. Colorectal cancer. Doctors know how to prevent colon or rectal cancer- and you can, too. Take a look inside.
 Get tested for Colorectal cancer Doctors know how to prevent colon or rectal cancer- and you can, too. Take a look inside. 1 If you re 50 or older, you need to get tested for colorectal cancer. It s one
Get tested for Colorectal cancer Doctors know how to prevent colon or rectal cancer- and you can, too. Take a look inside. 1 If you re 50 or older, you need to get tested for colorectal cancer. It s one
Rapid-VIDITEST. FOB+Tf. One step Fecal Occult Blood Card Test. Instruction manual
 Rapid-VIDITEST FOB+Tf One step Fecal Occult Blood Card Test Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Rapid-VIDITEST FOB+Tf One step Fecal Occult Blood Card Test Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Whilst IBS. should be. managed. IBS and IBD. and follow
 Introduction Faecal calprotectin testing is recommendr ded by NICE to help doctors distinguish between inflammatory bowel diseases (IBD),( suchh as Crohn s diseasee and ulcerative colitis, and non-inflammatory
Introduction Faecal calprotectin testing is recommendr ded by NICE to help doctors distinguish between inflammatory bowel diseases (IBD),( suchh as Crohn s diseasee and ulcerative colitis, and non-inflammatory
CerTest Turbilatex. A quantitative immunological latex method. FOB Calprotectin Transferrin H. pylori
 CerTest Turbilatex A quantitative immunological latex method FOB Calprotectin Transferrin H. pylori Turbidimetric technique. A latex turbidimetric assay The turbidimetric assay is based on the agglutination
CerTest Turbilatex A quantitative immunological latex method FOB Calprotectin Transferrin H. pylori Turbidimetric technique. A latex turbidimetric assay The turbidimetric assay is based on the agglutination
Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University WCC, Melbourne
 Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University. 2014 WCC, Melbourne Outline WHO criteria to justify screening Appropriateness: Global variation in incidence
Global colorectal cancer screening appropriate or practical? Graeme P Young, Flinders University. 2014 WCC, Melbourne Outline WHO criteria to justify screening Appropriateness: Global variation in incidence
Dr Alasdair Patrick. Dr Nagham Al-Mozany. 9:45-10:10 Where Are We Up To With Bowel Cancer Screening?
 Dr Alasdair Patrick Gastroenterologist and General Physician Middlemore Hospital Auckland Dr Nagham Al-Mozany Colorectal Surgeon Auckland City Hospital Clinical Senior Lecturer University of Auckland 9:45-10:10
Dr Alasdair Patrick Gastroenterologist and General Physician Middlemore Hospital Auckland Dr Nagham Al-Mozany Colorectal Surgeon Auckland City Hospital Clinical Senior Lecturer University of Auckland 9:45-10:10
NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report.
 NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report. Sue Moss, Christopher Mathews Centre for Cancer Prevention, Wolfson Institute, Queen Mary University
NHS Bowel Cancer Screening Programmes: Evaluation of pilot of Faecal Immunochemical Test : Final report. Sue Moss, Christopher Mathews Centre for Cancer Prevention, Wolfson Institute, Queen Mary University
Colorectal cancer screening in England
 Colorectal cancer screening in England critical analysis Prof Stephen P. Halloran Participation Rate 57% All Screens (1.9% +ve) 52% Prevalent 1 st Screen (age 60 years) 36% Prevalent Screen (2.2% +ve)
Colorectal cancer screening in England critical analysis Prof Stephen P. Halloran Participation Rate 57% All Screens (1.9% +ve) 52% Prevalent 1 st Screen (age 60 years) 36% Prevalent Screen (2.2% +ve)
ADHD clinic for adults Feedback on services for attention deficit hyperactivity disorder
 ADHD clinic for adults Feedback on services for attention deficit hyperactivity disorder Healthwatch Islington Healthwatch Islington is an independent organisation led by volunteers from the local community.
ADHD clinic for adults Feedback on services for attention deficit hyperactivity disorder Healthwatch Islington Healthwatch Islington is an independent organisation led by volunteers from the local community.
Guideline scope Diverticular disease: diagnosis and management
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Diverticular disease: diagnosis and management The Department of Health in England has asked NICE to develop a clinical guideline on diverticular
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Diverticular disease: diagnosis and management The Department of Health in England has asked NICE to develop a clinical guideline on diverticular
COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION.
 The West London Medical Journal 2009 Vol No 1 pp 23-31 COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION. Competing interests: None declared ABSTRACT Sarah
The West London Medical Journal 2009 Vol No 1 pp 23-31 COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION. Competing interests: None declared ABSTRACT Sarah
You re listening to an audio module from BMJ Learning. Hallo. I'm Anna Sayburn, Senior Editor with the BMJ Group s Consumer Health Team.
 Transcript of learning module Shared decision making (Dur: 26' 13") Contributors: Anna Sayburn and Alf Collins Available online at: http://learning.bmj.com/ V/O: You re listening to an audio module from
Transcript of learning module Shared decision making (Dur: 26' 13") Contributors: Anna Sayburn and Alf Collins Available online at: http://learning.bmj.com/ V/O: You re listening to an audio module from
Bowel scope screening
 NHS Bowel Cancer Screening Programme Bowel scope screening I ve been invited for NHS bowel scope screening. This leaflet gives you information to help you choose whether to have screening. NHS bowel scope
NHS Bowel Cancer Screening Programme Bowel scope screening I ve been invited for NHS bowel scope screening. This leaflet gives you information to help you choose whether to have screening. NHS bowel scope
Get FIT for the new year: a review of the role of faecal immunochemical test for haemoglobin in patients with symptoms of colorectal disease
 Review Article Page 1 of 9 Get FIT for the new year: a review of the role of faecal immunochemical test for haemoglobin in patients with symptoms of colorectal disease Neville Spiteri, Paul Skaife Department
Review Article Page 1 of 9 Get FIT for the new year: a review of the role of faecal immunochemical test for haemoglobin in patients with symptoms of colorectal disease Neville Spiteri, Paul Skaife Department
Calprest NG. The most accurate test for calprotectin in stool. Extended range ELISA assay for calprotectin in stool. Lead by perfection
 Calprest NG The most accurate test for calprotectin in stool. Human Recombinant Calprotectin Extended range ELISA assay for calprotectin in stool. Extended range Improved patient management NG Calprest
Calprest NG The most accurate test for calprotectin in stool. Human Recombinant Calprotectin Extended range ELISA assay for calprotectin in stool. Extended range Improved patient management NG Calprest
GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY
 Position Statement produced by BSG, AUGIS and ACPGBI GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY Introduction In 2011 the Independent Practice
Position Statement produced by BSG, AUGIS and ACPGBI GUIDANCE ON THE INDICATIONS FOR DIAGNOSTIC UPPER GI ENDOSCOPY, FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY Introduction In 2011 the Independent Practice
Information Booklet. Test Kit Helpline: Program Info Line:
 Information Booklet Test Kit Helpline: 1800 930 998 Program Info Line: 1800 118 868 Contents About the National Bowel Cancer Screening Program... 3 Bowel Cancer... 5 Your Test Kit... 9 Colonoscopy... 13
Information Booklet Test Kit Helpline: 1800 930 998 Program Info Line: 1800 118 868 Contents About the National Bowel Cancer Screening Program... 3 Bowel Cancer... 5 Your Test Kit... 9 Colonoscopy... 13
Information for families with a slightly increased risk of bowel cancer. Family History of Bowel Cancer
 Information for families with a slightly increased risk of bowel cancer Family History of Bowel Cancer How common is bowel cancer? Bowel cancer is the 3rd most common cancer in the UK. 1 in 20 people develop
Information for families with a slightly increased risk of bowel cancer Family History of Bowel Cancer How common is bowel cancer? Bowel cancer is the 3rd most common cancer in the UK. 1 in 20 people develop
Rapid-VIDITEST FOB Blister
 Rapid-VIDITEST FOB Blister One Step Fecal Occult Blood Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565,
Rapid-VIDITEST FOB Blister One Step Fecal Occult Blood Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565,
NHS KINGSTON. Contents
 NHS KINGSTON Contents 1. Background... 2 2. Targets and quality standards... 2 3. Service provision and performance... 3 Uptake... 3 Investigations... 6 Cancer detection... 7 Age extension... 7 4. Quality
NHS KINGSTON Contents 1. Background... 2 2. Targets and quality standards... 2 3. Service provision and performance... 3 Uptake... 3 Investigations... 6 Cancer detection... 7 Age extension... 7 4. Quality
Be it Resolved that FIT is the Best Way to Screen for Colorectal Cancer DEBATE
 Be it Resolved that FIT is the Best Way to Screen for Colorectal Cancer DEBATE DEBATE Presenters PRESENTATION MODERATOR Dr. Praveen Bansal -MD, CCFP FCFP Regional Primary Care Lead, Integrated Cancer Screening,
Be it Resolved that FIT is the Best Way to Screen for Colorectal Cancer DEBATE DEBATE Presenters PRESENTATION MODERATOR Dr. Praveen Bansal -MD, CCFP FCFP Regional Primary Care Lead, Integrated Cancer Screening,
Screening for GI Cancer Past Present and Future. Prof. Bob Steele University of Dundee
 Screening for GI Cancer Past Present and Future Prof. Bob Steele University of Dundee Worldwide Cancer Incidence Rates UK Cancer Incidence Rates Screening The detection of disease in asymptomatic subjects
Screening for GI Cancer Past Present and Future Prof. Bob Steele University of Dundee Worldwide Cancer Incidence Rates UK Cancer Incidence Rates Screening The detection of disease in asymptomatic subjects
BÜHLMANN fcal turbo. Calprotectin turbidimetric assay for professional use. Reagent Kit B-KCAL-RSET. Revision date:
 BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
Colorectal Cancer Screening
 Colorectal Cancer Screening An Integrated Care Pathway of the Collaborative Care Network Subject Matter Expert: Kevin Wolov, DO Pathway Custodian: Pat Czapp, MD First, a Friendly Reminder... This Integrated
Colorectal Cancer Screening An Integrated Care Pathway of the Collaborative Care Network Subject Matter Expert: Kevin Wolov, DO Pathway Custodian: Pat Czapp, MD First, a Friendly Reminder... This Integrated
Richard Watson, Chief Transformation Officer. Dr P Holloway, GP Clinical Lead for Cancer Lisa Parrish, Senior Transformation Lead
 GOVERNING BODY Agenda Item No. 08 Reference No. IESCCG 18-02 Date. 23 January 2018 Title Lead Chief Officer Author(s) Purpose Cancer Services Update Richard Watson, Chief Transformation Officer Dr P Holloway,
GOVERNING BODY Agenda Item No. 08 Reference No. IESCCG 18-02 Date. 23 January 2018 Title Lead Chief Officer Author(s) Purpose Cancer Services Update Richard Watson, Chief Transformation Officer Dr P Holloway,
Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests
 Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests Results of a systematic review, Kaiser experience, and implications for the Canton of Vaud Kevin Selby, M.D. Kevin.Selby@hospvd.ch
Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests Results of a systematic review, Kaiser experience, and implications for the Canton of Vaud Kevin Selby, M.D. Kevin.Selby@hospvd.ch
Centre for Specialist Psychological Treatments of Anxiety and Related Problems
 Centre for Specialist Psychological Treatments of Anxiety and Related Problems Information for people interested in accessing treatment at the Centre and those who already have a referral Welcome Welcome
Centre for Specialist Psychological Treatments of Anxiety and Related Problems Information for people interested in accessing treatment at the Centre and those who already have a referral Welcome Welcome
Dr. Coakley, so virtual colonoscopy, what is it? Is it a CT exam exactly?
 Virtual Colonoscopy Webcast January 26, 2009 Fergus Coakley, M.D. Please remember the opinions expressed on Patient Power are not necessarily the views of UCSF Medical Center, its medical staff or Patient
Virtual Colonoscopy Webcast January 26, 2009 Fergus Coakley, M.D. Please remember the opinions expressed on Patient Power are not necessarily the views of UCSF Medical Center, its medical staff or Patient
Suspected CANcer (SCAN) Pathway Information for patients
 Suspected CANcer (SCAN) Pathway Information for patients page 2 Your GP has advised you may benefit from investigation via the SCAN pathway. The SCAN pathway is part of a national programme called ACE
Suspected CANcer (SCAN) Pathway Information for patients page 2 Your GP has advised you may benefit from investigation via the SCAN pathway. The SCAN pathway is part of a national programme called ACE
Supporting people at higher risk of bowel cancer
 #never2young Never too young: Supporting people at higher risk of bowel cancer Campaign briefing Supporting people at higher risk of bowel cancer Bowel cancer is the second most common cause of cancer
#never2young Never too young: Supporting people at higher risk of bowel cancer Campaign briefing Supporting people at higher risk of bowel cancer Bowel cancer is the second most common cause of cancer
Cancer Control in the Workplace: A Corporate Standard
 Healthy Outcomes Conference, Whistler, BC March 31 April 2, 2009 Cancer Control in the Workplace: A Corporate Standard Dr. Alain Sotto, Hon.BSc, MD, CCFP(EM), FCBOM Chief Physician, Ontario Power Generation
Healthy Outcomes Conference, Whistler, BC March 31 April 2, 2009 Cancer Control in the Workplace: A Corporate Standard Dr. Alain Sotto, Hon.BSc, MD, CCFP(EM), FCBOM Chief Physician, Ontario Power Generation
Irritable Bowel Syndrome vs Inflammatory Bowel Disease
 Irritable Bowel Syndrome vs Inflammatory Bowel Disease Lana Bistritz MD FRCPC Royal Alexandra Hospital Faculty/Presenter Disclosure Faculty: Lana Bistritz Relationships with financial sponsors: Grants/Research
Irritable Bowel Syndrome vs Inflammatory Bowel Disease Lana Bistritz MD FRCPC Royal Alexandra Hospital Faculty/Presenter Disclosure Faculty: Lana Bistritz Relationships with financial sponsors: Grants/Research
Wellness Along the Cancer Journey: Cancer Types Revised October 2015 Chapter 4: Colorectal Cancer Overview
 Wellness Along the Cancer Journey: Cancer Types Revised October 2015 Chapter 4: Colorectal Cancer Overview Cancer Types Rev. 10.20.15 Page 35 Colorectal Cancer Overview Group Discussion True False Not
Wellness Along the Cancer Journey: Cancer Types Revised October 2015 Chapter 4: Colorectal Cancer Overview Cancer Types Rev. 10.20.15 Page 35 Colorectal Cancer Overview Group Discussion True False Not
Cancer Decision Support Tool (CDS) - FAQ's
 Cancer Decision Support Tool (CDS) - FAQ's What is the Cancer Decision Support (CDS) Tool Project? The Cancer Decision Support tool project is part of the wider Prevention and Diagnosis programme of work
Cancer Decision Support Tool (CDS) - FAQ's What is the Cancer Decision Support (CDS) Tool Project? The Cancer Decision Support tool project is part of the wider Prevention and Diagnosis programme of work
The Activity Students will read extracts from the Making sense of testing document, and answer questions about what they have read.
 Teacher Notes Introduction Students are asked to consider the use of health testing kits and the significance of results. They have to discuss how false positives and negatives arise and the effects they
Teacher Notes Introduction Students are asked to consider the use of health testing kits and the significance of results. They have to discuss how false positives and negatives arise and the effects they
Evaluation of the Bowel Cancer Awareness Pilot in SW and east of England, 31 st Jan to 18 th March 2011
 Evaluation of the Bowel Cancer Awareness Pilot in SW and east of England, 31 st Jan to 18 th March 2011 Dr Gina Radford Consultant in Public Health Anglia Cancer Network Why do a campaign Survival rates
Evaluation of the Bowel Cancer Awareness Pilot in SW and east of England, 31 st Jan to 18 th March 2011 Dr Gina Radford Consultant in Public Health Anglia Cancer Network Why do a campaign Survival rates
[Type here] CG [Type here]
![[Type here] CG [Type here] [Type here] CG [Type here]](/thumbs/78/77374363.jpg) TRTs: - Physicians: 5:20 - Patients & Advocacy: 11:52 - Company: 4:32 Exact Sciences FDA Broll SUGGESTED B- ROLL & GRAPHICS Opening (white background, black lettering) SOUNDBITES FDA Approves Cologuard
TRTs: - Physicians: 5:20 - Patients & Advocacy: 11:52 - Company: 4:32 Exact Sciences FDA Broll SUGGESTED B- ROLL & GRAPHICS Opening (white background, black lettering) SOUNDBITES FDA Approves Cologuard
Defeating Colon Cancer with Surgery
 Defeating Colon Cancer with Surgery Recorded on: September 19, 2012 Alessandro Fichera, M.D. Director, Colorectal Surgical Oncology Program, Seattle Cancer Care Alliance Please remember the opinions expressed
Defeating Colon Cancer with Surgery Recorded on: September 19, 2012 Alessandro Fichera, M.D. Director, Colorectal Surgical Oncology Program, Seattle Cancer Care Alliance Please remember the opinions expressed
FIT Laboratory update
 FIT Laboratory update Sally C Benton Consultant Clinical Biochemist, Berkshire and Surrey Pathology Services (BSPS) Director, Bowel Cancer Screening Southern Hub Date: 26 th February 2019 February 2017.
FIT Laboratory update Sally C Benton Consultant Clinical Biochemist, Berkshire and Surrey Pathology Services (BSPS) Director, Bowel Cancer Screening Southern Hub Date: 26 th February 2019 February 2017.
Cancer Research UK Tesco Charity of the Year Spotting the signs of cancer For men
 Cancer Research UK Tesco Charity of the Year 2012 Spotting the signs of cancer For men Thousands of people beat cancer every year. When cancer is diagnosed at an early stage, treatment is often simpler
Cancer Research UK Tesco Charity of the Year 2012 Spotting the signs of cancer For men Thousands of people beat cancer every year. When cancer is diagnosed at an early stage, treatment is often simpler
GLUTEN-FREE FOOD SCHEME. Information Pack
 GLUTEN-FREE FOOD SCHEME Information Pack The Tayside Gluten-Free Food Scheme is part of the Scottish Gluten-Free Food Service. There are variations from the Scottish Service and more information can be
GLUTEN-FREE FOOD SCHEME Information Pack The Tayside Gluten-Free Food Scheme is part of the Scottish Gluten-Free Food Service. There are variations from the Scottish Service and more information can be
The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean?
 The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean? Robert A. Smith, PhD Cancer Control, Department of Prevention and Early Detection American Cancer Society
The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean? Robert A. Smith, PhD Cancer Control, Department of Prevention and Early Detection American Cancer Society
SPOTTING THE SIGNS OF CANCER FOR MEN
 SPOTTING THE SIGNS OF CANCER FOR MEN Thousands of people survive cancer every year. When cancer is diagnosed at an early stage, treatment is more likely to be successful. So finding cancer early can make
SPOTTING THE SIGNS OF CANCER FOR MEN Thousands of people survive cancer every year. When cancer is diagnosed at an early stage, treatment is more likely to be successful. So finding cancer early can make
National Bowel Screening Programme. Quick Guide
 National Bowel Screening Programme Quick Guide What is the National Bowel Screening Programme? This is a free programme to help detect bowel cancer. The National Bowel Screening Programme is being rolled
National Bowel Screening Programme Quick Guide What is the National Bowel Screening Programme? This is a free programme to help detect bowel cancer. The National Bowel Screening Programme is being rolled
What is Colorectal Cancer?
 COLORECTAL CANCER (CRC) What is Colorectal Cancer? Colorectal cancer (also known as colon cancer) is cancer of the colon and/or rectum and occurs when a growth in the lining of the colon or rectum becomes
COLORECTAL CANCER (CRC) What is Colorectal Cancer? Colorectal cancer (also known as colon cancer) is cancer of the colon and/or rectum and occurs when a growth in the lining of the colon or rectum becomes
Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc.
 Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc. European Digestive Cancer Days, Prague - 26. September 2017 QUANTITATIVE FIT
Quantitative immunochemical tests: evidence on accuracy and implementation considerations in the Czech MUDr.. Petr Kocna, CSc. European Digestive Cancer Days, Prague - 26. September 2017 QUANTITATIVE FIT
Somerset, Wiltshire, Avon and Gloucestershire Cancer Alliance Peninsula Cancer Alliance. Transformation Funding Application
 Somerset, Wiltshire, Avon and Gloucestershire Cancer Alliance Peninsula Cancer Alliance Transformation Funding Application Achieving early diagnosis through the introduction of Quantitative Faecal Immunohistochemical
Somerset, Wiltshire, Avon and Gloucestershire Cancer Alliance Peninsula Cancer Alliance Transformation Funding Application Achieving early diagnosis through the introduction of Quantitative Faecal Immunohistochemical
Higher Risk, Lowered Age: New Colorectal Cancer Screening Guidelines
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/clinicians-roundtable/higher-risk-lowered-age-new-colorectal-cancerscreening-guidelines/10309/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/clinicians-roundtable/higher-risk-lowered-age-new-colorectal-cancerscreening-guidelines/10309/
Patient Survey Report Spring 2013
 Patient Survey Report Spring 2013 We invited the original group of Patients from last year s PPG to become part of a Virtual Forum once again this year and also sent text messages to all out patients inviting
Patient Survey Report Spring 2013 We invited the original group of Patients from last year s PPG to become part of a Virtual Forum once again this year and also sent text messages to all out patients inviting
With CRC Screening Rates on the Rise, Quality Control Becomes Center of Attention
 print this article In the News ISSUE: OCTOBER 2009 VOLUME: 60:10 With CRC Screening Rates on the Rise, Quality Control Becomes Center of Attention by Monica J. Smith New York In just the past year, New
print this article In the News ISSUE: OCTOBER 2009 VOLUME: 60:10 With CRC Screening Rates on the Rise, Quality Control Becomes Center of Attention by Monica J. Smith New York In just the past year, New
Standard of care Inflammatory Bowel Disease (IBD)
 Standard of care Inflammatory Bowel Disease (IBD) Inflammatory bowel disease (IBD) Tens of thousands of Australians live with the debilitating symptoms of Crohn s disease or ulcerative colitis the two
Standard of care Inflammatory Bowel Disease (IBD) Inflammatory bowel disease (IBD) Tens of thousands of Australians live with the debilitating symptoms of Crohn s disease or ulcerative colitis the two
Colon Cancer Screening and Surveillance. Louis V. Antignano, M.D. Wilson Gastroenterology October 11, 2011
 Colon Cancer Screening and Surveillance Louis V. Antignano, M.D. Wilson Gastroenterology October 11, 2011 Colorectal Cancer Preventable cancer Number 2 cancer killer in the USA Often curable if detected
Colon Cancer Screening and Surveillance Louis V. Antignano, M.D. Wilson Gastroenterology October 11, 2011 Colorectal Cancer Preventable cancer Number 2 cancer killer in the USA Often curable if detected
Case Study: Biomedical Scientist - Caroline
 Case Study: Biomedical Scientist - Caroline What do you do? I'm a biomedical scientist, in haematology. I work in an NHS hospital. We study the morphology of the cells - what they actually look like, such
Case Study: Biomedical Scientist - Caroline What do you do? I'm a biomedical scientist, in haematology. I work in an NHS hospital. We study the morphology of the cells - what they actually look like, such
Colorectal Cancer: Preventable, Beatable, Treatable. American Cancer Society
 Colorectal Cancer: Preventable, Beatable, Treatable American Cancer Society Reviewed/Revised May 2018 What we ll be talking about How common is colorectal cancer? What is colorectal cancer? What causes
Colorectal Cancer: Preventable, Beatable, Treatable American Cancer Society Reviewed/Revised May 2018 What we ll be talking about How common is colorectal cancer? What is colorectal cancer? What causes
Flu season. Making the most of online appointments. August 2018
 Flu season Making the most of online appointments August 2018 Dear colleagues, Over the last four years GP Online Services have been implemented by practices across England. Over 14 million patients -
Flu season Making the most of online appointments August 2018 Dear colleagues, Over the last four years GP Online Services have been implemented by practices across England. Over 14 million patients -
They know how to prevent colon cancer
 They know how to prevent colon cancer and you can, too. Take a look inside. If you re 50 or older, you need to get tested for colon cancer. It s one cancer that can actually be prevented! Colon cancer:
They know how to prevent colon cancer and you can, too. Take a look inside. If you re 50 or older, you need to get tested for colon cancer. It s one cancer that can actually be prevented! Colon cancer:
Let s look a minute at the evidence supporting current cancer screening recommendations.
 I m Dr. Therese Bevers, Medical Director of the Cancer Prevention Center and Professor of Clinical Cancer Prevention at The University of Texas MD Anderson Cancer Center. Today s lecture is on screening
I m Dr. Therese Bevers, Medical Director of the Cancer Prevention Center and Professor of Clinical Cancer Prevention at The University of Texas MD Anderson Cancer Center. Today s lecture is on screening
Blue Star Sunday. Increasing Awareness About Colon Cancer. Dear Faith Community,
 Blue Star Sunday Increasing Awareness About Colon Cancer Dear Faith Community, West Virginia s Cancer Coalition, Mountains of Hope, invites your faith community to participate in Colorectal Cancer Awareness
Blue Star Sunday Increasing Awareness About Colon Cancer Dear Faith Community, West Virginia s Cancer Coalition, Mountains of Hope, invites your faith community to participate in Colorectal Cancer Awareness
Constipation Information Leaflet THE DIGESTIVE SYSTEM. gutscharity.org.uk
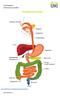 THE DIGESTIVE SYSTEM http://healthfavo.com/digestive-system-for-kids.html This factsheet is about Constipation Constipation is a symptom that can mean different things to different people but the usual
THE DIGESTIVE SYSTEM http://healthfavo.com/digestive-system-for-kids.html This factsheet is about Constipation Constipation is a symptom that can mean different things to different people but the usual
Bowel Cancer Information Leaflet THE DIGESTIVE SYSTEM
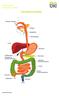 THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
