STUDIENLISTE Februar 2018 Studien offen für Accrual DIM Onkologie/Hämatologie und Brustzentrum Kantonsspital St. Gallen. fett = neu Zuständigkeit
|
|
|
- Virgil Wilkerson
- 5 years ago
- Views:
Transcription
1 15/ L E U K Ä M I E SAKK GRAALL 2014 Multicenter trial for the treatment of Acute Lymphoblastic Leukemia (ALL) in younger adults (18-59 years) SAKK CLL-13 SAKK CLL-13: A phase 3 multicenter, randomized, prospective, open-label trial of standard chemoimmunotherapy (FCR/BR) versus rituximab plus venetoclax (RVe) versus obinutuzumab (GA101) plus venetoclax (GVe) versus obinutuzumab plus ibrutinib plus venetoclax (GIVe) in fit patients with previously untreated chronic lymphocytic leukemia (CLL) without del(17p) or TP53 mutation (GAIA Trial) MDS 14 Multicentre Observational Study of MDS Patients in Switzerland Swiss MDS Registry Blincyto An Observational Study of Blinatumomab Safety and Effectiveness, Utilisation, and Treatment Practices 13/082 L Y M P H O M IELSG 37 A randomized, open-label, multicentre, twoarm phase III comparative study assessing the role of mediastinal radiotherapiy after Rituximab containing chemotherapy regimens to patients with newly diagnosed Prinamry Mediastinal Large B-Cell Lymphoma (PMLBCL) 15/033 SAKK 36/13 Combination of ibrutinib and bortezomib followed by ibrutinib maintenance to treat patients with relapsed and refractory mantle cell lymphoma; a multicenter Phase I/II trial / Start Phase II am /104 SAKK 35/14 Rituximab with or without Ibrutinib for untreated patients with advanced follicular lymphoma in need of therapy. A randomized, double-blinded, SAKK and NLG collaborative Phase II trial SAKK 35/15 A phase I trial of obinutuzumab in combination with venetoclax in previously untreated follicular lymphoma patients SAKK HD 21 Treatment optimization trial in the first-line treatment of advanced stage Hodgkin lymphoma; comparison of 6 cycles of escalated BEACOPP with 6 cycles of BrECADD SAKK TRIANGLE TRIANGLE: autologous Transplantation after a Rituximab/Ibrutinib/Ara-c containing induction in Generalized mantle cell Lymphoma a randomized European MCL Network trial T. Silzle M.Baumann Michael.baumann@zlmsg.ch M.Fehr Martin.fehr@kssg.ch F. Hitz S. Mayr / P.Caminada M.Steiner M. Steiner K.Knöpel P.Caminada / R. Demmer Melanom Myelom /16 OptiPOM Protocol SAKK 39/16 OptiPOM Alternate day dosing of Pomalidomide in patients with refractory Multiple Myeloma. A multicenter, single arm phase II trial S. Mayr Studienliste Februar /kk/jn (ersetzt Liste Januar 2018) Seite 1 von 5
2 Titanium EDO-S Phase 1/2 Open-Label Trial of Tinostamustine Conditioning and Autologous Stem Cell Transplantation for Salvage Treatment in Relapsed / Refractory Multiple Myeloma (Titanium 1) HNO /061 Gehirn SAKK 67/15 BASILEA An open-label Phase 1/2a study of BAL administered as intravenous 48-hour infusion in adult patients with advanced solid tumors D. Hess dagmar.hess@kssg.ch M A M M A Adjuvant TAXIS 23/16 SAKK 23/16: Tailored AXIllary Surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically nodepositive breast cancer. A multicenter randomized phase III trial (TAXIS) 14/158 POSITIVE IBCSG IBCSG - Touch 14/057 Metastasierend SAKK 96/12* A study evaluating the pregnancy outcomes and safety of interrupting endocrine therapy for young women with endocrine responsive breast cancer IBCSG Touche Phase II open label, multicenter, randomized trial of neoadjuvant palbociclib in combination with hormonal therapy and HER2 blockade versus paclitaxel in combination with HER2 blockade for elderly patients with hormone receptor positive/her2 positive early breast cancer (To reduce the use of Chemotherapy in elderly patients with ER-positive and HER2-posoitive breast cancer: the TOUCH trial) Prevention of Symptomatic Skeletal Events with Denosumab Administered every 4 Weeks versus every 12 Weeks A Non-Inferiority Phase III Trial 15/026 SAKK 25/14 Eribulin as 1st line treatment in elderly patients ( 70 years) with advanced breast cancer: a multicenter phase II trial / SAKK 24/14 Anti-EGFR-immunoliposomes loaded with doxorubicin in patients with advanced triple negative EGFR positive breast cancer A multicenter single arm phase II trial BLADDER SAKK 06/17 Neoadjuvant and adjuvant durvalumab in combination with neoadjuvant chemotherapie in patients with operable urothelial cancer. A multicentre,single-arm phase II trial BAY17403 A randomized, open label, multicenter, seamless Phase 2/3 study to evaluate the efficacy and safety of rogaratinib (BAY ) compared to chemotherapy in patients with FGFRpositive locally advanced or metastatic urothelial carcinoma who have received prior platinum-containing chemotherapy M.Knauer Michael.Knauer@kssg.ch S.Riniker Salome.iniker@kssg.ch P.Weder Patrik.Weder@kssg.ch Sabine.schmid@kssg.ch U.Hasler-Strub Ursula.hasler-strub@kssg.ch U.Hasler Ursula.hasler-strub@kssg.ch S.Schmied T.Zeller R.Demmer/ R.Demmer T.Zeller/ K.Knö pfel Studienliste Februar /kk/jn (ersetzt Liste Januar 2018) Seite 2 von 5
3 PB_ L U N G E MK ; PEARLS A randomized, phase 3 trial with anti-pd-1 monoclonal antibody pembrolizumab (MK- 3475) versus placebo for patients with early stage NSCLC after resection and completion of standard adjuvant therapy SAKK19/16 Binimetinib, pemetrexed and cisplatin, followed by maintenance with binimetinib and pemetrexed, in patients with advanced nonsmall cell lung cancer with KRAS mutations: A multicenter phase IB trial BOOSTER ETOP BOOSTER: A randomised phase II trial of osimertinib and bevacizumab versus osimertinib alone as second-line treatment in stage IIIb-IVb NSCLC with confirmed EGFRm and T790M MERU A Randomized, Double-Blind, Placebo- Controlled Phase 3 Study of Rovalpituzumab Tesirine as Maintenance Therapy Following First-Line Platinum-Based Chemotherapy in Subjects with Extensive Stage Small Cell Lung Cancer M16-298(MERU) SAKK 19/17 First line durvalumab in patients with PD-L1 positive, advanced NSCLC with performance status 2 unsuitable for combination chemotherapy. A multicenter, single-arm phase II trial MK : KeylmPaCT A Phase 2 Precision Oncology Study of Biomarker-Directed, Pembrolizumab- (MK- 3475, SCH ) Based Combination Therapy for Advanced Non-Small Cell Lung Cancer (KEYNOTE-495; KeyImPaCT). C.Appenzeller Christina.appenzeller@kssg.ch K. Knöpfel K.Klag/ S. Mayr/ S.Mayr/ K O L O N / R E K T A L 15/127 SAKK 41/13 Adjuvant aspirin treatment in PIK3CA mutated colon cancer patients. A randomized, doubleblinded, placebo-controlled, phase III trial PhenoTox Hunting the missing heritability of early-onset fluoropyrimdine-related toxicity in cancer 15/156 SAKK 41/14 O E S O P H A G E A L BMS CA SAKK PRODIGE 32 patients: an observational multi-center study. Protocol SAKK 41/14 ACTIVE-2 Physical activity program in patients with metastatic colorectal cancer who receive palliative firstline chemotherapy. A multicenter open label randomized controlled phase III trial A Randomized, Multicenter, Double Blind, Phase III Study of Nivolumab or Placebo in Subjects with Resected Lower Esophageal, or Gastroesophageal Junction Cancer Systematic surgery vs monitoring and salvage surgery in operable oesophageal cancer in complete clinical response after chemoradiation Strategic multicenter randomized phase II- III trial U. Güller Ulrich.gueller@kssg.ch D.Horber Daniel.horber@kssg.ch Ch.Weisshaupt Christian.weisshaupt@kssg.ch Th.Ruhstaller Thomas.ruhstaller@kssg.ch J.Kehl/ R.Demmer/ K. Knöpfel N I E R E SAKK 07/17 Nivolumab in combination with Ipilimumab in patients with metastatic renal cell carcinoma: A multicentre single-arm phase li trial C.Rothermundt K.Klag/ Studienliste Februar /kk/jn (ersetzt Liste Januar 2018) Seite 3 von 5
4 MAGEN MK A Randomized, Multicenter, Double Blind, Phase III Study of Nivolumab or Placebo in Subjects with Resected Lower Esophageal, or Gastroesophageal Junction Cancer (CheckMate 577: CHECKpoint pathway and nivolumab clinical Trial Evaluation 577) CA /057 P R O S T A T A SAKK 96/12* SAKK 08/14 IMPROVE 14/155 PRO-PLAT MK Pembro Prostata Prevention of Symptomatic Skeletal Events with Denosumab Administered every 4 Weeks versus every 12 Weeks A Non-Inferiority Phase III Trial Investigation of Metformin in patients with castration resistant prostate cancer in combination with Enzalutamide vs. Enzalutamide alone A randomized, open label, phase II trial Single arm open lable phase II pilot study of Carboplatin in patients with metastatic castration-resistant prostate cancer (CRPC) and PTEN-LOSS Phase II Trial of Pembrolizumab (MK-3475) in Subjects with Metastatic Castration-Resistant Prostate Cancer (mcrpc) Previously Treated with Chemotherapy (KEYNOTE-199) SAKK 08/16 ODM-201 maintenance therapy in patients with metastatic castration resistant prostate cancer (mcrpc) previously treated with one novel hormonal agent first line and non-progressive disease after second line treatment with a taxane: A multicenter randomized double-blind placebo-controlled phase II trial 13/083 H O D E N K R E B S SAG TCCS Prospektive Datenerhebung bei Patienten mit Hodenkrebs U. Güller Ulrich.gueller@kssg.ch Sabine.schmid@kssg.ch Aurelius.Omlin@kssg.ch S.Mayr M.Steiner/ Urogenital /061 P H A S E I S T U D I E N SAKK 67/15 An open-label Phase 1/2a study of BAL BASILEA administered as intravenous 48-hour infusion in adult patients with advanced solid tumors MP0274 A phase 1, first-in-human, single-arm, multicenter, open-label, repeated-dose, dose escalation study to assess safety, tolerability, and pharmacokinetics of MP0274 in patients with advanced HER2-positive solid tumors NIR178 A Phase 2, multi-center, open label study of NIR178 in combination with PDR001 in patients with selected advanced solid tumors and non-hodgkin lymphoma /190 Bayer An open-label, first-in-human, dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and maximum tolerated dose and / or recommended Phase II dose of the ATR M.Joerger M.Joerger Studienliste Februar /kk/jn (ersetzt Liste Januar 2018) Seite 4 von 5
5 inhibitor BAY in patients with advanced solid tumors and lymphomas SAKK 65/16 TLD-1, a novel Liposomal Doxorubicin, in Patients with Advanced Solid Tumors; a multicenter open-label single-arm phase I trial. M. Jörger / D. Hess Sakrom EuroEwings SAKK57/16 NAPAGE Euro Ewing 2012: International Randomised Controlled Trial for the Treatment of Newly Diagnosed Ewing's Sarcoma Family of Tumours NAb-PAclitaxel and GEmcitabine in advanced soft tissue sarcoma. A multicenter open-label single arm phase Ib/IIa trial. Ch. Appenzeller Christina.Appenzeller@kssg.ch P.Caminada A. Harder J. Niedermann Die Studienliste ist abrufbar auf *Diese Studien können grösstenteils auswärts durchgeführt werden. Studienliste Februar /kk/jn (ersetzt Liste Januar 2018) Seite 5 von 5
STUDIENLISTE August2018 Studien offen für Accrual DIM Onkologie/Hämatologie und Brustzentrum Kantonsspital St. Gallen
 14-007 15/193 2016-00548 L E U K Ä M I E SAKK GRAALL 2014 2016-02559 SAKK 30/10 Hovon 103 Selinexor Multicenter trial for the treatment of Acute Lymphoblastic Leukemia (ALL) in younger adults (18-59 years)
14-007 15/193 2016-00548 L E U K Ä M I E SAKK GRAALL 2014 2016-02559 SAKK 30/10 Hovon 103 Selinexor Multicenter trial for the treatment of Acute Lymphoblastic Leukemia (ALL) in younger adults (18-59 years)
OPEN TRIALS Accruals counted until 30-April Current Accrual
 Disease group Breast OPEN TRIALS Trial Title Accrual Target IBCSG 48-14 POSITIVE IBCSG 50-14 OLYMPIA IBCSG 52-15 PALLAS SAKK 21/12 A study evaluating the pregnancy outcomes and safety of interrupting endocrine
Disease group Breast OPEN TRIALS Trial Title Accrual Target IBCSG 48-14 POSITIVE IBCSG 50-14 OLYMPIA IBCSG 52-15 PALLAS SAKK 21/12 A study evaluating the pregnancy outcomes and safety of interrupting endocrine
Studienverzeichnis Medizinische Onkologie
 Studienverzeichnis Medizinische Onkologie Mammakarzinom, Gynäkologie 21/12 24/14 25/14 96/12 PALLAS I I Transdermal CR1447 (4-OH-testosterone) in endocrine responsive-her2 negative and triple negative-androgen
Studienverzeichnis Medizinische Onkologie Mammakarzinom, Gynäkologie 21/12 24/14 25/14 96/12 PALLAS I I Transdermal CR1447 (4-OH-testosterone) in endocrine responsive-her2 negative and triple negative-androgen
Open Trials as of end of March 2016
 EORTC 10085 PRO EORTC 10085 prospective part, Clinical and biological characterization of Male Breast Cancer: an international EORTC, BIG and NABCG inter study 30 30-Jun-2016 estelle.cassoly@sakk.ch IBCSG
EORTC 10085 PRO EORTC 10085 prospective part, Clinical and biological characterization of Male Breast Cancer: an international EORTC, BIG and NABCG inter study 30 30-Jun-2016 estelle.cassoly@sakk.ch IBCSG
OPEN TRIALS Accruals counted until 31-August Current Accrual
 Disease group Breast Trial Title Accrual Target IBCSG 48-14 POSITIVE IBCSG 50-14 OLYMPIA IBCSG 52-15 PALLAS A study evaluating the pregnancy outcomes and safety of interrupting endocrine therapy for young
Disease group Breast Trial Title Accrual Target IBCSG 48-14 POSITIVE IBCSG 50-14 OLYMPIA IBCSG 52-15 PALLAS A study evaluating the pregnancy outcomes and safety of interrupting endocrine therapy for young
Trial Trial Name Opening of the first site. Enzalutamide in combination with metformin vs. enzalutamide in patients with CRPC
 TRIALS TO BE ACTIVATED 2016/2017 Trial Trial Name Opening of the first site SAKK 41/13 SAKK 08/14 (IMPROVE) SAKK 16/14 SAKK 21/12 (Phase II part) GRAALL-2014 HD21 HOVON 103 SEL SAKK 30/10 IELSG-42 IELSG-43
TRIALS TO BE ACTIVATED 2016/2017 Trial Trial Name Opening of the first site SAKK 41/13 SAKK 08/14 (IMPROVE) SAKK 16/14 SAKK 21/12 (Phase II part) GRAALL-2014 HD21 HOVON 103 SEL SAKK 30/10 IELSG-42 IELSG-43
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY NFORMATON n format provided by Melero et al. (AUGUST 2015) Supplementary nformation S3 Combinations including two or more immunotherapy agents based on PD-1/PD-L1 blockade. (Source: https://clinicaltrials.gov/
SUPPLEMENTARY NFORMATON n format provided by Melero et al. (AUGUST 2015) Supplementary nformation S3 Combinations including two or more immunotherapy agents based on PD-1/PD-L1 blockade. (Source: https://clinicaltrials.gov/
Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona
 Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona Solid Tumor: USON 15180 / A Multicenter, Open-Label Phase 1b/2 Trial of Lenvatinib (E7080) Plus Pembrolizumab
Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona Solid Tumor: USON 15180 / A Multicenter, Open-Label Phase 1b/2 Trial of Lenvatinib (E7080) Plus Pembrolizumab
CLINICAL TRIALS ACC. Jul 2016
 CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
Checkpoint Regulators Cancer Immunotherapy takes centre stage. Dr Oliver Klein Department of Medical Oncology 02 May 2015
 Checkpoint Regulators Cancer Immunotherapy takes centre stage Dr Oliver Klein Department of Medical Oncology 02 May 2015 Adjuvant chemotherapy improves outcome in early breast cancer FDA approval of Imatinib
Checkpoint Regulators Cancer Immunotherapy takes centre stage Dr Oliver Klein Department of Medical Oncology 02 May 2015 Adjuvant chemotherapy improves outcome in early breast cancer FDA approval of Imatinib
Etudes cliniques Service d Oncologie - Radiothérapie
 Etudes cliniques Service d Oncologie - Radiothérapie Décembre 2017 SEIN NEO ADJUVANT WO39392 (Impassion 031) : A phase III randomized study to investigate the efficacy and safety of Atezolizumab in combination
Etudes cliniques Service d Oncologie - Radiothérapie Décembre 2017 SEIN NEO ADJUVANT WO39392 (Impassion 031) : A phase III randomized study to investigate the efficacy and safety of Atezolizumab in combination
London Cancer New Drugs Group. February London Cancer New Drugs Group (LCNDG) Work Plan for the London Cancer Drugs Fund list.
 February 2013 London Cancer New s Group (LCNDG) Work Plan for the London Cancer s Fund London Cancer s Fund List This Cancer s Fund (CDF) list of medicines and s is in two parts. 1. The standard list of
February 2013 London Cancer New s Group (LCNDG) Work Plan for the London Cancer s Fund London Cancer s Fund List This Cancer s Fund (CDF) list of medicines and s is in two parts. 1. The standard list of
Studies proceeding under pre HRA-Approval system (NHS Permission)
 15/NW/0004 162679 Studies proceeding under pre HRA-roval system (NHS Permission) A MULTI-CENTRE RANDOMISED CLINICAL TRIAL OF BIOMARKER-DRIVEN MAINTENANCE TREATMENT FOR FIRST- LINE METASTATIC COLORECTAL
15/NW/0004 162679 Studies proceeding under pre HRA-roval system (NHS Permission) A MULTI-CENTRE RANDOMISED CLINICAL TRIAL OF BIOMARKER-DRIVEN MAINTENANCE TREATMENT FOR FIRST- LINE METASTATIC COLORECTAL
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT
 NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including accrual, and
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including accrual, and
General Information, efficacy and safety data
 Horizon Scanning in Oncology Horizon Scanning in Oncology 23 rd Prioritization 2 nd quarter 2015 General Information, efficacy and safety data Eleen Rothschedl Anna Nachtnebel Priorisierung XXIII HSS Onkologie
Horizon Scanning in Oncology Horizon Scanning in Oncology 23 rd Prioritization 2 nd quarter 2015 General Information, efficacy and safety data Eleen Rothschedl Anna Nachtnebel Priorisierung XXIII HSS Onkologie
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT
 NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Fall Conference Call 23 November 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Etudes cliniques Service d Oncologie - Radiothérapie
 Etudes cliniques Service d Oncologie - Radiothérapie Juillet 2017 SEIN NEO ADJUVANT Neo-RHEA : NEOadjuvant Biomarker ResearcH Study of Palbociclib in Estrogen Receptor Positive HER2 Negative Breast CAncer
Etudes cliniques Service d Oncologie - Radiothérapie Juillet 2017 SEIN NEO ADJUVANT Neo-RHEA : NEOadjuvant Biomarker ResearcH Study of Palbociclib in Estrogen Receptor Positive HER2 Negative Breast CAncer
MEDICAL PRIOR AUTHORIZATION
 MEDICAL PRIOR AUTHORIZATION TAXOTERE (docetaxel) DOCEFREZ(docetaxel) docetaxel (generic) POLICY I. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
MEDICAL PRIOR AUTHORIZATION TAXOTERE (docetaxel) DOCEFREZ(docetaxel) docetaxel (generic) POLICY I. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
National Cancer Drugs Fund List - Approved
 National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
Date of HRA. Date Site Invited. Date Site Selected. Approval. Recruited. Date
 14/LO/0259 134883 RE-AKT: A randomised Phase II study of enzalutamide (MDV3100) in combination with AZD5363 in s with Metastatic Castration - Resistant Prostate Cancer Yes 08/01/2018 01/07/2016 01/07/2017
14/LO/0259 134883 RE-AKT: A randomised Phase II study of enzalutamide (MDV3100) in combination with AZD5363 in s with Metastatic Castration - Resistant Prostate Cancer Yes 08/01/2018 01/07/2016 01/07/2017
Summary of Research and Writing Activities in Oncology
 Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
SCIENTIFIC PROGRAM. ASCO Direct 15CME HOURS. SCfHS ACCREDITED FOR. Current Update Sponsored by:
 ASCO Direct Current Update 2017 24-25 November 2017 Crowne Plaza Hotel, Jeddah, KSA SCIENTIFIC PROGRAM Organized by: SCfHS ACCREDITED FOR 15CME HOURS Licensed by: Sponsored by: (+966) 11 462 2515 Ext.
ASCO Direct Current Update 2017 24-25 November 2017 Crowne Plaza Hotel, Jeddah, KSA SCIENTIFIC PROGRAM Organized by: SCfHS ACCREDITED FOR 15CME HOURS Licensed by: Sponsored by: (+966) 11 462 2515 Ext.
Keytruda. Keytruda (pembrolizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 9 Last Review Date: November 30, 2018 Keytruda Description Keytruda
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 9 Last Review Date: November 30, 2018 Keytruda Description Keytruda
General Information, efficacy and safety data
 Horizon Scanning in Oncology Horizon Scanning in Oncology 28 th Prioritization 3 rd quarter 2016 General Information, efficacy and safety data Nicole Grössmann Claudia Wild Please note: Within this document
Horizon Scanning in Oncology Horizon Scanning in Oncology 28 th Prioritization 3 rd quarter 2016 General Information, efficacy and safety data Nicole Grössmann Claudia Wild Please note: Within this document
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center
 Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Immune checkpoint inhibitors in NSCLC
 1 Immune checkpoint inhibitors in NSCLC Rolf Stahel University Hospital of Zürich Zürich, November 3, 2017 2 What can we learn from the clinical experience of second line immunotherapy of advanced NSCLC?
1 Immune checkpoint inhibitors in NSCLC Rolf Stahel University Hospital of Zürich Zürich, November 3, 2017 2 What can we learn from the clinical experience of second line immunotherapy of advanced NSCLC?
Date Site Confirmed By Sponsor. Non- Confirmation Status. HRA Approval Date. Date Site Confirmed
 Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial First Patient Recruited? Date of First Patient Recruited Invited Selected Approval
Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial First Patient Recruited? Date of First Patient Recruited Invited Selected Approval
WASHINGTON. Spokane Cheney. Olympia. Tri-Cities. Walla Walla. Portland North Central Oregon City OREGON. Mt. Vernon. Port Angeles
 2 3 Mt. Vernon Port Angeles Bremerton Tacoma 5 5 Olympia Everett Edmonds Seattle 90 WASHINGTON 90 Spokane Cheney Pullman Longview 82 Tri-Cities Portland North Central Oregon City 5 84 OREGON Walla Walla
2 3 Mt. Vernon Port Angeles Bremerton Tacoma 5 5 Olympia Everett Edmonds Seattle 90 WASHINGTON 90 Spokane Cheney Pullman Longview 82 Tri-Cities Portland North Central Oregon City 5 84 OREGON Walla Walla
Target date to recruit patients agreed? Target range minimum. Target range maximum. Agreed 1 1 Agreed 01/01/ /01/2016 Recruitment Finished
 No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date No of patients recruited
No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date No of patients recruited
The role of immune checkpoint inhibitors in non-small cell lung cancer
 Review Article Page 1 of 9 The role of immune checkpoint inhibitors in non-small cell lung cancer Tiffany L. George 1, Erin M. Bertino 2 1 Divisions of Hematology and Medical Oncology, 2 Division of Medical
Review Article Page 1 of 9 The role of immune checkpoint inhibitors in non-small cell lung cancer Tiffany L. George 1, Erin M. Bertino 2 1 Divisions of Hematology and Medical Oncology, 2 Division of Medical
Docetaxel. Class: Antineoplastic agent, Antimicrotubular, Taxane derivative.
 Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
CENTER FOR CLINICAL TRIALS St. Luke s Medical Center. Ongoing Clinical Trials CANCER INSTITUTE (36)
 CENTER FOR CLINICAL TRIALS St. Luke s Medical Center Ongoing Clinical Trials CANCER INSTITUTE (36) BLOOD TITLE : A Randomized, Two By Two Arm, Multicenter, Open-Label Phase III Study Of BMS-354825 Administered
CENTER FOR CLINICAL TRIALS St. Luke s Medical Center Ongoing Clinical Trials CANCER INSTITUTE (36) BLOOD TITLE : A Randomized, Two By Two Arm, Multicenter, Open-Label Phase III Study Of BMS-354825 Administered
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN BY PRODUCT
 PAGE 175 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN BY PRODUCT Summary of risk management plan for pembrolizumab This is a summary of the risk management plan (RMP) for pembrolizumab. The RMP details
PAGE 175 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN BY PRODUCT Summary of risk management plan for pembrolizumab This is a summary of the risk management plan (RMP) for pembrolizumab. The RMP details
Roche Investor Relations ASCO Planner 2016
 Roche Investor Relations ASCO Planner 2016 Friday, June 3 Session Title: Pediatric Oncology I Date: Fri, June 3 Location: S504 Time: 3:00 PM - 6:00 PM Speaker Name: Veronique Minard-Colin 5:12 PM - 5:24
Roche Investor Relations ASCO Planner 2016 Friday, June 3 Session Title: Pediatric Oncology I Date: Fri, June 3 Location: S504 Time: 3:00 PM - 6:00 PM Speaker Name: Veronique Minard-Colin 5:12 PM - 5:24
Checkpoint regulators a new class of cancer immunotherapeutics. Dr Oliver Klein Medical Oncologist ONJCC Austin Health
 Checkpoint regulators a new class of cancer immunotherapeutics Dr Oliver Klein Medical Oncologist ONJCC Austin Health Cancer...Immunology matters Anti-tumour immune response The participants Dendritc cells
Checkpoint regulators a new class of cancer immunotherapeutics Dr Oliver Klein Medical Oncologist ONJCC Austin Health Cancer...Immunology matters Anti-tumour immune response The participants Dendritc cells
HRA Approval Date Site Date Site Sponsor. Non- Confirmation Status. Date Site Ready To Start
 Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial Date of Receipt of Date of Valid Research Application First Patient Recruited? Date
Research Ethics Committee Reference Number Integrated Research Application System Number Submission Type Name of Trial Date of Receipt of Date of Valid Research Application First Patient Recruited? Date
Keytruda (pembrolizumab)
 Keytruda (pembrolizumab) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 07/24/2017TBD03/01/2018 POLICY A. INDICATIONS The
Keytruda (pembrolizumab) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 07/24/2017TBD03/01/2018 POLICY A. INDICATIONS The
A JOHNS HOPKINS SINGAPORE ANNUAL RESEARCH REPORT 2015
 A JOHNS HOPKINS SINGAPORE ANNUAL RESEARCH REPORT 2015 15 years of excellence in patient care, research, and education The Promise of Medicine: The research team at Johns Hopkins Singapore provides unparalleled
A JOHNS HOPKINS SINGAPORE ANNUAL RESEARCH REPORT 2015 15 years of excellence in patient care, research, and education The Promise of Medicine: The research team at Johns Hopkins Singapore provides unparalleled
Metastatic NSCLC: Expanding Role of Immunotherapy. Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian
 Metastatic NSCLC: Expanding Role of Immunotherapy Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian Disclosures: No relevant disclosures Please note that some of the studies reported in
Metastatic NSCLC: Expanding Role of Immunotherapy Evan W. Alley, MD, PhD Abramson Cancer Center at Penn Presbyterian Disclosures: No relevant disclosures Please note that some of the studies reported in
6/22/2017 TARGETING THE TARGETS IN 2017 TARGETING THE TARGETS IN 2017
 TARGETING THE TARGETS IN 2017 Primary Care Focus Symposium July 1, 2017 Grace Wang MD I do not have any relevant financial relationships to disclose at this time TARGETING THE TARGETS IN 2017 What are
TARGETING THE TARGETS IN 2017 Primary Care Focus Symposium July 1, 2017 Grace Wang MD I do not have any relevant financial relationships to disclose at this time TARGETING THE TARGETS IN 2017 What are
Histology independent indications in Oncology
 CHMP Oncology Working Party Workshop Histology independent indications in Oncology What have we learnt from the anti PD1- PDL1 story? J Camarero (CHMP alternate ES, OncWP) Disclaimers the views presented
CHMP Oncology Working Party Workshop Histology independent indications in Oncology What have we learnt from the anti PD1- PDL1 story? J Camarero (CHMP alternate ES, OncWP) Disclaimers the views presented
The Royal Marsden NHS Foundation Trust Medicines Formulary. NICE register for medicines initiated at the trust. Type and code. Publication.
 NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
NICE register for medicines initiated at the trust. CG denotes Clinical Guideline TA denotes Technical Appraisal For further information about NICE and full guidelines please refer to www.nice.org.uk Title
Keytruda. Keytruda (pembrolizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 9 Last Review Date: September 20, 2018 Keytruda Description Keytruda
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 9 Last Review Date: September 20, 2018 Keytruda Description Keytruda
HSHS St. Vincent Hospital Regional Cancer Center Adult Active Study List
 NHLBI-MDS ECOG-A NCT02775383 Blood Disorders-Other The National Myelodysplastic Syndromes (MDS) Study PNH Registry M07-001 DRUG CO NCT01374360 Blood Disorders-Other PNH Registry A071102 ALLIANCE NCT02152982
NHLBI-MDS ECOG-A NCT02775383 Blood Disorders-Other The National Myelodysplastic Syndromes (MDS) Study PNH Registry M07-001 DRUG CO NCT01374360 Blood Disorders-Other PNH Registry A071102 ALLIANCE NCT02152982
EORTC LUNG CANCER GROUP GROUP MEETING
 Sunday 7 th October 2018 Time slot Topic / Title of presentation Chair 9.00 11.00 Board meeting 11.00 11.30 Arrivals & Welcome coffee 11.30 11.50 Welcome & Introduction New Board,, Membership status, Calendar
Sunday 7 th October 2018 Time slot Topic / Title of presentation Chair 9.00 11.00 Board meeting 11.00 11.30 Arrivals & Welcome coffee 11.30 11.50 Welcome & Introduction New Board,, Membership status, Calendar
Immunotherapy in non-small cell lung cancer
 Immunotherapy in non-small cell lung cancer Geoffrey Peters and Thomas John Olivia Newton-John Cancer Research Institute, Heidelberg, Victoria, Australia. Email: Geoffrey.peters@austin.org.au Abstract
Immunotherapy in non-small cell lung cancer Geoffrey Peters and Thomas John Olivia Newton-John Cancer Research Institute, Heidelberg, Victoria, Australia. Email: Geoffrey.peters@austin.org.au Abstract
New Developments in Cancer Treatment. Dulcinea Quintana, MD
 New Developments in Cancer Treatment Dulcinea Quintana, MD Mortality Rates Goals of treatment 1 Cure Goal of treatment 2 Prolong life Goals of treatment 3 Improve Quality of Life Goals of treatment 4
New Developments in Cancer Treatment Dulcinea Quintana, MD Mortality Rates Goals of treatment 1 Cure Goal of treatment 2 Prolong life Goals of treatment 3 Improve Quality of Life Goals of treatment 4
Etudes cliniques Service d Oncologie - Radiothérapie
 Etudes cliniques Service d Oncologie - Radiothérapie Mars 2017 SEIN ADJUVANT OLYMPIA : A Phase III study to assess the efficacy and safety of olaparib versus placebo as adjuvant therapy in BRCA mutated
Etudes cliniques Service d Oncologie - Radiothérapie Mars 2017 SEIN ADJUVANT OLYMPIA : A Phase III study to assess the efficacy and safety of olaparib versus placebo as adjuvant therapy in BRCA mutated
(generic name: ipilimumab) Injection 50 mg ( Yervoy ), a human anti-human CTLA-4 monoclonal. August 21, 2018
 August 21, 2018 Opdivo Approved for Supplemental Applications for Expanded Indications of Malignant Pleural Mesothelioma and Adjuvant Treatment of Melanoma, Change in Dosage and Administration (D&A) of
August 21, 2018 Opdivo Approved for Supplemental Applications for Expanded Indications of Malignant Pleural Mesothelioma and Adjuvant Treatment of Melanoma, Change in Dosage and Administration (D&A) of
Performance in Initiating Clinical Research Q2 2016/17
 Performance in Initiating Clinical Research Q2 2016/17 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Performance in Initiating Clinical Research Q2 2016/17 Research Ethics Committee Reference Number Integrated Research Application System Number Name of Trial Date of Receipt of Valid Research Application
Single Technology Appraisal (STA)
 Single Technology Appraisal (STA) Durvalumab for maintenance treatment of locally advanced unresectable non-small cell lung cancer that has not progressed after platinum-based chemoradiation therapy Response
Single Technology Appraisal (STA) Durvalumab for maintenance treatment of locally advanced unresectable non-small cell lung cancer that has not progressed after platinum-based chemoradiation therapy Response
KTE-C19 for relapsed or refractory mantle cell lymphoma
 NIHR Innovation Observatory Evidence Briefing: July 2017 KTE-C19 for relapsed or refractory mantle cell lymphoma NIHRIO (HSRIC) ID: 11846 NICE ID: 9122 LAY SUMMARY Mantle cell lymphoma is an uncommon type
NIHR Innovation Observatory Evidence Briefing: July 2017 KTE-C19 for relapsed or refractory mantle cell lymphoma NIHRIO (HSRIC) ID: 11846 NICE ID: 9122 LAY SUMMARY Mantle cell lymphoma is an uncommon type
Title Cancer Drug Phase Status
 Clinical Trial Identifier Title Cancer Drug Phase Status NCT01164995 Study With Wee-1 Inhibitor MK-1775 and Carboplatin to Treat p53 Mutated Refractory and Resistant Ovarian Cancer Epithelial Ovarian Cancer
Clinical Trial Identifier Title Cancer Drug Phase Status NCT01164995 Study With Wee-1 Inhibitor MK-1775 and Carboplatin to Treat p53 Mutated Refractory and Resistant Ovarian Cancer Epithelial Ovarian Cancer
Options for first-line cisplatin-eligible patients
 The Past Options for first-line cisplatin-eligible patients Metastatic urothelial cancer Cisplatin-eligible Gemcitabine/ cisplatin MVAC or high-dose intensity MVAC Paclitaxel/ cisplatin/ gemcitabine Bellmunt
The Past Options for first-line cisplatin-eligible patients Metastatic urothelial cancer Cisplatin-eligible Gemcitabine/ cisplatin MVAC or high-dose intensity MVAC Paclitaxel/ cisplatin/ gemcitabine Bellmunt
Policy. Medical Policy Manual Approved Revised: Do Not Implement until 6/30/2019. Nivolumab
 Medical Manual Approved Revised: Do Not Implement until 6/30/2019 Nivolumab NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB
Medical Manual Approved Revised: Do Not Implement until 6/30/2019 Nivolumab NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB
N. Ireland Cancer Trials Centre/ N. Ireland Cancer Trials Network Trials Open to Recruitment June 2013
 N. Ireland Cancer Trials Centre/ N. Ireland Cancer Trials Network Trials Open to Recruitment June 2013 Bladder HABIO Haematuria Biomarker Study Bowel Cancer NSCCG National Study Of Colorectal Cancer Genetics
N. Ireland Cancer Trials Centre/ N. Ireland Cancer Trials Network Trials Open to Recruitment June 2013 Bladder HABIO Haematuria Biomarker Study Bowel Cancer NSCCG National Study Of Colorectal Cancer Genetics
Policy. Medical Policy Manual Approved Revised: Do Not Implement Until 3/2/19. Nivolumab (Intravenous)
 Nivolumab (Intravenous) NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3734-XX Opdivo 240
Nivolumab (Intravenous) NDC CODE(S) 00003-3772-XX Opdivo 40 MG/4ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3774-XX Opdivo 100 MG/10ML SOLN (B-M SQUIBB U.S. (PRIMARY CARE)) 00003-3734-XX Opdivo 240
Updates in Immunotherapy for Urothelial Carcinoma
 Updates in Immunotherapy for Urothelial Carcinoma Andrew J Armstrong MD ScM FACP DUA 2018 Copyright 2006 SciMed. Talk Outline Immunotherapy progress in 2017: 5 new approved PD-1/PD-L1 inhibitory agents
Updates in Immunotherapy for Urothelial Carcinoma Andrew J Armstrong MD ScM FACP DUA 2018 Copyright 2006 SciMed. Talk Outline Immunotherapy progress in 2017: 5 new approved PD-1/PD-L1 inhibitory agents
Target date to recruit patients agreed? Target range minimum. Target range maximum
 No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date Total no of patients recruited
No REC Reference IRAS No Title Target number of patients agreed? Target range minimum Target range maximum Target date to recruit patients agreed? Planned Recruitment end date Total no of patients recruited
Challenging Genitourinary Tumors: What s New in 2017
 Challenging Genitourinary Tumors: What s New in 2017 David J. Vaughn, MD Genitourinary Medical Oncology Professor Please note that some of the studies reported in this presentation were presented as an
Challenging Genitourinary Tumors: What s New in 2017 David J. Vaughn, MD Genitourinary Medical Oncology Professor Please note that some of the studies reported in this presentation were presented as an
General Information, efficacy and safety data
 Horizon Scanning in Oncology Horizon Scanning in Oncology 27 th Prioritization 2 nd quarter 2016 General Information, efficacy and safety data Nicole Grössmann Claudia Wild Please note: Within this document
Horizon Scanning in Oncology Horizon Scanning in Oncology 27 th Prioritization 2 nd quarter 2016 General Information, efficacy and safety data Nicole Grössmann Claudia Wild Please note: Within this document
IMMUNE CHECKPOINT THERAPY FOR GENITOURINARY CANCERS: KIDNEY CANCER AND TRANSITIONAL CELL CARCINOMA
 IMMUNE CHECKPOINT THERAPY FOR GENITOURINARY CANCERS: KIDNEY CANCER AND TRANSITIONAL CELL CARCINOMA Kathleen Mahoney, M.D., Ph.D. Instructor of Medicine, Harvard Medical School Attending, Beth Israel Deaconess
IMMUNE CHECKPOINT THERAPY FOR GENITOURINARY CANCERS: KIDNEY CANCER AND TRANSITIONAL CELL CARCINOMA Kathleen Mahoney, M.D., Ph.D. Instructor of Medicine, Harvard Medical School Attending, Beth Israel Deaconess
Prostate cancer Management of metastatic castration sensitive cancer
 18 th Annual Advances in Oncology - 2017 Prostate cancer Management of metastatic castration sensitive cancer Urothelial carcinoma Non-muscle invasive urothelial carcinoma Updates in metastatic urothelial
18 th Annual Advances in Oncology - 2017 Prostate cancer Management of metastatic castration sensitive cancer Urothelial carcinoma Non-muscle invasive urothelial carcinoma Updates in metastatic urothelial
SUPPLEMENTARY INFORMATION In format provided by Sebti et al. (NOVEMBER 2011)
 Supplementary Information S4 Clinical trials with farnesyltransferase inhibitors Drug(s) Disease Phase Patients Median Age Tipifarnib CR Clinical response PR HI SD PD MD FT or Prenylation Response rate
Supplementary Information S4 Clinical trials with farnesyltransferase inhibitors Drug(s) Disease Phase Patients Median Age Tipifarnib CR Clinical response PR HI SD PD MD FT or Prenylation Response rate
We re Reaching Ludicrous Speed: New Immunotherapy Oncology Medications
 We re Reaching Ludicrous Speed: New Immunotherapy Oncology Medications Adam Peele, PharmD, BCPS, BCOP Oncology Pharmacy Manager Cone Health Disclosures Merck Pharmaceuticals Speaker s Bureau 1 Objectives
We re Reaching Ludicrous Speed: New Immunotherapy Oncology Medications Adam Peele, PharmD, BCPS, BCOP Oncology Pharmacy Manager Cone Health Disclosures Merck Pharmaceuticals Speaker s Bureau 1 Objectives
PERIOPERATIVE TREATMENT OF NON SMALL CELL LUNG CANCER. Virginie Westeel Chest Disease Department University Hospital Besançon, France
 PERIOPERATIVE TREATMENT OF NON SMALL CELL LUNG CANCER Virginie Westeel Chest Disease Department University Hospital Besançon, France LEARNING OBJECTIVES 1. To understand the potential of perioperative
PERIOPERATIVE TREATMENT OF NON SMALL CELL LUNG CANCER Virginie Westeel Chest Disease Department University Hospital Besançon, France LEARNING OBJECTIVES 1. To understand the potential of perioperative
Clinical Policy: Nivolumab (Opdivo) Reference Number: CP.PHAR.121
 Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07/15 Last Review Date: 04/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07/15 Last Review Date: 04/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Immunotherapy and Targeted Therapies: The new face of cancer treatment
 Immunotherapy and Targeted Therapies: The new face of cancer treatment Abdulazeez Salawu MBBS, MSc, PhD, MRCP Academic Clinical Lecturer Weston Park Hospital, Sheffield Novel Systemic Anti-cancer Therapies
Immunotherapy and Targeted Therapies: The new face of cancer treatment Abdulazeez Salawu MBBS, MSc, PhD, MRCP Academic Clinical Lecturer Weston Park Hospital, Sheffield Novel Systemic Anti-cancer Therapies
2018 KSMO Immune Oncology Forum. Immune checkpoint inhibitors in hematologic. malignancies: evidences and perspectives 서울아산병원종양내과 홍정용
 2018 KSMO Immune Oncology Forum Immune checkpoint inhibitors in hematologic malignancies: evidences and perspectives 서울아산병원종양내과 홍정용 2018-07-18 Contents Introduction Immune checkpoint inhibtors in lymphomas
2018 KSMO Immune Oncology Forum Immune checkpoint inhibitors in hematologic malignancies: evidences and perspectives 서울아산병원종양내과 홍정용 2018-07-18 Contents Introduction Immune checkpoint inhibtors in lymphomas
Principales presentaciones de AstraZeneca/MedImmune en el congreso de ESMO 2017
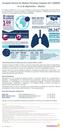 Principales presentaciones de AstraZeneca/MedImmune en el congreso de ESMO 2017 Autor principal Título del abstract Detalles de la presentación Cáncer de pulmón Paz-Ares L Ramalingam S Zhou C PACIFIC:
Principales presentaciones de AstraZeneca/MedImmune en el congreso de ESMO 2017 Autor principal Título del abstract Detalles de la presentación Cáncer de pulmón Paz-Ares L Ramalingam S Zhou C PACIFIC:
HSHS St. Vincent Hospital Regional Cancer Center Adult Active Study List
 NHLBI-MDS ECOG-A NCT02775383 Blood Disorders-Other The National Myelodysplastic Syndromes (MDS) Study A221101 ALLIANCE NCT01781468 Brain A Phase III Randomized, Double-Blind Placebo Controlled Study of
NHLBI-MDS ECOG-A NCT02775383 Blood Disorders-Other The National Myelodysplastic Syndromes (MDS) Study A221101 ALLIANCE NCT01781468 Brain A Phase III Randomized, Double-Blind Placebo Controlled Study of
Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations
 Gong et al. Journal for ImmunoTherapy of Cancer (2018) 6:8 DOI 10.1186/s40425-018-0316-z REVIEW Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration
Gong et al. Journal for ImmunoTherapy of Cancer (2018) 6:8 DOI 10.1186/s40425-018-0316-z REVIEW Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration
General Information, efficacy and safety data
 Horizon Scanning in Oncology Horizon Scanning in Oncology 33 r d Prioritization 4 t h quarter 2017 General Information, efficacy and safety data Nicole Grössmann Sarah Wolf Please note: Within this document
Horizon Scanning in Oncology Horizon Scanning in Oncology 33 r d Prioritization 4 t h quarter 2017 General Information, efficacy and safety data Nicole Grössmann Sarah Wolf Please note: Within this document
Cancer drug approvals for paediatric indications (n=43)
 Appendix: Supplementary material [posted as supplied by author] Figure A. Identification of cohort of drugs Total number of antineoplastic and immunomodulating products approved by the EMA up to 31 December
Appendix: Supplementary material [posted as supplied by author] Figure A. Identification of cohort of drugs Total number of antineoplastic and immunomodulating products approved by the EMA up to 31 December
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD
 Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
NEW DRUG FUNDING PROGRAM (NDFP) & EVIDENCE BUILDING PROGRAM (EBP) Approved Drugs and Eligibility Criteria
 NEW DRUG FUNDING PROGRAM (NDFP) & EVIDENCE BUILDING PROGRAM (EBP) Approved Drugs and Eligibility Criteria The information in this document is a summary of the funding criteria for the New Drug Funding
NEW DRUG FUNDING PROGRAM (NDFP) & EVIDENCE BUILDING PROGRAM (EBP) Approved Drugs and Eligibility Criteria The information in this document is a summary of the funding criteria for the New Drug Funding
Bone Marrow Transplant Gynecologic Immunotherapy Phase 1. Breast Head & Neck Melanoma/Skin Sarcoma
 Karmanos offers clinical trials for many different forms of cancer. Below are the most current clinical trials open to new participants as of this publication's issue date. If you have any questions regarding
Karmanos offers clinical trials for many different forms of cancer. Below are the most current clinical trials open to new participants as of this publication's issue date. If you have any questions regarding
Chemotherapy Treatment Algorithms for Urology Cancer
 Chemotherapy Treatment Algorithms for Urology Cancer Chemoradiation for bladder cancer; Chemotherapy algorithm for non TCC bladder cancer Squamous cell carcinoma; Chemotherapy Algorithm for Non Transitional
Chemotherapy Treatment Algorithms for Urology Cancer Chemoradiation for bladder cancer; Chemotherapy algorithm for non TCC bladder cancer Squamous cell carcinoma; Chemotherapy Algorithm for Non Transitional
Target date to recruit patients agreed? Target range. maximum. minimum. Date. 5 Agreed 26/01/ /11/2015 Recruitment Finished.
 No REC Reference IRAS No Title Target number of agreed? Target range minimum Target range maximum Target date to recruit agreed? Planned Recruitment end date Total no of recruited at the agreed target
No REC Reference IRAS No Title Target number of agreed? Target range minimum Target range maximum Target date to recruit agreed? Planned Recruitment end date Total no of recruited at the agreed target
First Phase 3 Results Presented for a PD-1 Immune Checkpoint Inhibitor
 September 30, 2014 Positive Phase 3 Data for Opdivo (nivolumab) in Advanced Melanoma Patients Previously Treated with Yervoy @ (ipilimumab) Presented at the ESMO 2014 Congress First Phase 3 Results Presented
September 30, 2014 Positive Phase 3 Data for Opdivo (nivolumab) in Advanced Melanoma Patients Previously Treated with Yervoy @ (ipilimumab) Presented at the ESMO 2014 Congress First Phase 3 Results Presented
For Health Professionals Who Care For Cancer Patients
 April 2018 Volume 21, No. 4 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Palbociclib for Metastatic Breast Cancer, Adjuvant Capecitabine for Breast
April 2018 Volume 21, No. 4 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Palbociclib for Metastatic Breast Cancer, Adjuvant Capecitabine for Breast
National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) Trial design:
 Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A PHASE III STUDY OF IRESSA
Open clinical uro-oncology trials in Canada Eric Winquist, MD, Mary J. Mackenzie, MD, George Rodrigues, MD London Health Sciences Centre, London, Ontario, Canada BLADDER CANCER A PHASE III STUDY OF IRESSA
Bone Marrow Transplant Gynecologic Immunotherapy Phase 1. Breast Head & Neck Melanoma/Skin Sarcoma
 Karmanos offers clinical trials for many different forms of cancer. Below are the most current clinical trials open to new participants as of this publication's issue date. If you have any questions regarding
Karmanos offers clinical trials for many different forms of cancer. Below are the most current clinical trials open to new participants as of this publication's issue date. If you have any questions regarding
Perfomance in Delivering (Commercial Trials)
 Perfomance in Delivering (Commercial Trials) REC Ref IRAS ID Name of Trial Target Of? Minimum Of Maximum Of Target Date To Recruit? Total Date Of to recruit target number Recruited At of patients The Target
Perfomance in Delivering (Commercial Trials) REC Ref IRAS ID Name of Trial Target Of? Minimum Of Maximum Of Target Date To Recruit? Total Date Of to recruit target number Recruited At of patients The Target
ASCO 2014 Highlights*
 ASCO 214 Highlights* Investor Meeting June 2, 214 *American Society of Clinical Oncology, May 3 June 3, 214 Forward-Looking Information During this meeting, we will make statements about the Company s
ASCO 214 Highlights* Investor Meeting June 2, 214 *American Society of Clinical Oncology, May 3 June 3, 214 Forward-Looking Information During this meeting, we will make statements about the Company s
Media Release. Roche highlights personalised medicines and cancer immunotherapies at 2016 American Society of Clinical Oncology (ASCO) Annual Meeting
 Media Release Basel, 3 May 2016 Roche highlights personalised medicines and cancer immunotherapies at 2016 American Society of Clinical Oncology (ASCO) Annual Meeting 19 Roche medicines are included in
Media Release Basel, 3 May 2016 Roche highlights personalised medicines and cancer immunotherapies at 2016 American Society of Clinical Oncology (ASCO) Annual Meeting 19 Roche medicines are included in
Keytruda. Keytruda (pembrolizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 7 Last Review Date: December 8, 2017 Keytruda Description Keytruda
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 7 Last Review Date: December 8, 2017 Keytruda Description Keytruda
7 Recruiting Carfilzomib, Rituximab and Dexamethasone in Waldenstrom's Macroglobulinemia Condition: Waldenstrom's Macroglobulinemia
 1 Recruiting Questionnaire and Tissue Banking For Multiple Myeloma, Waldenstrom and Related Disorders Conditions: Multiple Myeloma; Waldenstrom's ; Smoldering Multiple Myeloma; Lymphoblastic Lymphoma Intervention:
1 Recruiting Questionnaire and Tissue Banking For Multiple Myeloma, Waldenstrom and Related Disorders Conditions: Multiple Myeloma; Waldenstrom's ; Smoldering Multiple Myeloma; Lymphoblastic Lymphoma Intervention:
Benefit Risk Analysis Of Decision-Making: Oncology
 Benefit Risk Analysis Of Decision-Making: Oncology G.K. Raju, Ph.D. December 13 th 2016 Outline Background Approach Application to Oncology Non-Small Cell Lung Cancer Learnings & Ongoing Work Acknowledgements
Benefit Risk Analysis Of Decision-Making: Oncology G.K. Raju, Ph.D. December 13 th 2016 Outline Background Approach Application to Oncology Non-Small Cell Lung Cancer Learnings & Ongoing Work Acknowledgements
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Pembrolizumab (Keytruda) Reference Number: CP.PHAR.322 Effective Date: 07.01.18 Last Review Date: 11.17 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the
Clinical Policy: Pembrolizumab (Keytruda) Reference Number: CP.PHAR.322 Effective Date: 07.01.18 Last Review Date: 11.17 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the
Triple Negative Breast Cancer: Part 2 A Medical Update
 Triple Negative Breast Cancer: Part 2 A Medical Update April 29, 2015 Tiffany A. Traina, MD Breast Medicine Service Memorial Sloan Kettering Cancer Center Weill Cornell Medical College Overview What is
Triple Negative Breast Cancer: Part 2 A Medical Update April 29, 2015 Tiffany A. Traina, MD Breast Medicine Service Memorial Sloan Kettering Cancer Center Weill Cornell Medical College Overview What is
Azacitidine Vidaza Non-transplant myelodysplastic syndrome Funded Funded Funded Funded Funded Funded Not Funded
 Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
2016 Updates in Oncology & Malignant Hematology Brendan Curley, DO
 2016 Updates in Oncology & Malignant Hematology Brendan Curley, DO Disclosures I received final support from ASCO Conquer Cancer foundation in two Merit Awards and one travel award I am on the speakers
2016 Updates in Oncology & Malignant Hematology Brendan Curley, DO Disclosures I received final support from ASCO Conquer Cancer foundation in two Merit Awards and one travel award I am on the speakers
Cancer: Can we Afford the Cure? Current Trends in Oncology Treatment
 Cancer: Can we Afford the Cure? Current Trends in Oncology Treatment Julie Kennerly-Shah, PharmD, MS, MHA, BCPS May 30, 2018 The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer
Cancer: Can we Afford the Cure? Current Trends in Oncology Treatment Julie Kennerly-Shah, PharmD, MS, MHA, BCPS May 30, 2018 The Ohio State University Comprehensive Cancer Center Arthur G. James Cancer
My name is Dr. David Ilson, Professor of Medicine at Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center in New York, New York.
 Welcome to this CME/CE-certified activity entitled, Integrating the Latest Advances Into Clinical Experience: Data and Expert Insights From the 2016 Meeting on Gastrointestinal Cancers in San Francisco.
Welcome to this CME/CE-certified activity entitled, Integrating the Latest Advances Into Clinical Experience: Data and Expert Insights From the 2016 Meeting on Gastrointestinal Cancers in San Francisco.
Macmillan Publications
 S1 S2 S3 S3 S3 S4 S5 S6 S7 S8 S8 S9 S10 S11 S11 S12 S13 S14 S15 S17 S18 S19 Bladder Cancer: Non-Invasive, Invasive and Advanced Bone Cancer: Primary, Secondary Colon Cancer, Anal Cancer, Rectal Cancer
S1 S2 S3 S3 S3 S4 S5 S6 S7 S8 S8 S9 S10 S11 S11 S12 S13 S14 S15 S17 S18 S19 Bladder Cancer: Non-Invasive, Invasive and Advanced Bone Cancer: Primary, Secondary Colon Cancer, Anal Cancer, Rectal Cancer
National Horizon Scanning Centre. Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer. December 2007
 Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
Weitere Kombinationspartner der Immunotherapie
 1 Weitere Kombinationspartner der Immunotherapie Rolf Stahel University Hospital of Zürich Zürich, 9.12.216 2 Immunotherapy in a multimodality approach NSCLC Advanced disease Checkpoint inhibitors for
1 Weitere Kombinationspartner der Immunotherapie Rolf Stahel University Hospital of Zürich Zürich, 9.12.216 2 Immunotherapy in a multimodality approach NSCLC Advanced disease Checkpoint inhibitors for
