Medical Laboratory Accredited to ISO15189:2012. Oncofocus. Precision Oncology ONCOFOCUS TEST REPORT
|
|
|
- Lorena Maxwell
- 5 years ago
- Views:
Transcription
1 Medical Laboratory Accredited to ISO15189:2012 Oncofocus Precision Oncology ONCOFOCUS TEST REPORT
2 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 1 of 15 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type - - Requester - - Contact details - - Date requested Tumour % 99% Cervix Tumour % - (macrodissected) Cervix Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus assay on which the sample was run met all assay specific quality metrics. Oncofocus currently targets 505 genes covering oncogenes, fusion genes, genes susceptible to copy number variation and tumour suppressors. Actionable genetic variants detected by Oncofocus are currently linked to 687 anti-cancer targeted therapies/therapy combinations. The following actionable variants were detected: Please note; The PIK3CA variant detected falls below our normal threshold for reporting, however repeat testing has confirmed its presence. Within the 'Current Clinical Trials Information' section of this report, starting on page 4, the NCT numbers are hyperlinks to the clinicaltrials.gov webpages which should be accessed to gain further trial specific information Sample Cancer Type: Cervical Cancer Clinically Significant Biomarkers Indicated Contraindicated Genomic Alteration Relevant Therapies (In this cancer type) Relevant Therapies (In other cancer type) Clinical Trials PIK3CA p.(e545k) c.1633g>a Clinical trials and/or off-label Clinical trials and/or off-label 12 CCND1 amplification Clinical trials and/or off-label Clinical trials and/or off-label 5 FGF19 amplification Clinical trials and/or off-label Clinical trials and/or off-label 4 FGF3 amplification Clinical trials and/or off-label Clinical trials and/or off-label 3 Sources included in relevant therapies: EMA1, FDA2, ESMO, NCCN Hotspot variants with >10% alternate allele reads are classified as detected with an assay sensitivity and positive predictive value(ppv) of 99%. Copy number variants; amplifications of CN> 6 with the 5% confidence value of 4 after normalization and deletions with 95% CI 1 are classified as present when the tumour% >50% with a sensitivity of 80% and PPV 100%. Gene Fusions are reported when occurring in >40 counts and meeting the thresholds of assay specific internal RNA quality control with a sensitivity of 92% and PPV of 99%. Supplementary technical information is available upon request.
3 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 2 of 15 Tier Criteria Met Genomic Alteration PIK3CA p.(e545k) c.1633g>a Tier: IIC CCND1 amplification Tier: IIC FGF19 amplification Tier: IIC FGF3 amplification Tier: IIC Tier Classification for Cervical Cancer IIC: Biomarker is an inclusion criteria for clinical trials IIC: Biomarker is an inclusion criteria for clinical trials IIC: Biomarker is an inclusion criteria for clinical trials IIC: Biomarker is an inclusion criteria for clinical trials Reference: Li et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn Jan;19(1):4-23. Relevant Therapy Summary In this cancer type In other cancer type In this cancer type and other cancer types Contraindicated Both for use and contraindicated No evidence PIK3CA p.(e545k) c.1633g>a Relevant Therapy EMA FDA ESMO NCCN Clinical Trials* MEK inhibitor + PIK3/mTOR inhibitor, PIK3/mTOR inhibitor (II/III) capivasertib + olaparib (II) copanlisib (II) everolimus (II) sirolimus (II) temsirolimus (II) ARQ-751 (I) capivasertib (I) GDC-0077 (I) gedatolisib + palbociclib (I) LY prexasertib (I) palbociclib + pictilisib, palbociclib + taselisib (I) * Most advanced phase (IV, III, II/III, II, I/II, I) is shown and multiple clinical trials may be available.
4 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 3 of 15 Relevant Therapy Summary (continued) In this cancer type In other cancer type In this cancer type and other cancer types Contraindicated Both for use and contraindicated No evidence CCND1 amplification Relevant Therapy EMA FDA ESMO NCCN Clinical Trials* abemaciclib (II) palbociclib (II) PNT chemotherapy (I/II) FGF19 amplification Relevant Therapy EMA FDA ESMO NCCN Clinical Trials* INCB (I/II) TAS-120 (I/II) E-7090 (I) INCB (I) FGF3 amplification Relevant Therapy EMA FDA ESMO NCCN Clinical Trials* TAS-120 (I/II) E-7090 (I) INCB (I) * Most advanced phase (IV, III, II/III, II, I/II, I) is shown and multiple clinical trials may be available.
5 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 4 of 15 Relevant Therapy Details Current Clinical Trials Information Clinical Trials information is current as of For the most up-to-date information regarding a particular trial, search by NCT ID or search local clinical trials authority website by local identifier listed in 'Other identifiers'. PIK3CA p.(e545k) c.1633g>a No NCT ID - see other identifier(s) Molecular selection of therapy in metastatic colorectal cancer: a molecularly stratified randomised controlled trial programme Cancer type: Cervical Cancer Variant class: PIK3CA mutation Other identifiers: 14893, CR13, CRUK/11/054, EudraCT Number: , FOCUS-4, FOCUS4, IRAS ID , ISRCTN , MREC N 13/SC/0111, UKCRN ID: Population segments: First line, Stage III, Stage IV Other inclusion criteria: PTEN underexpression Phase: II/III Therapies: MEK inhibitor + PIK3/mTOR inhibitor, PIK3/mTOR inhibitor Location: United Kingdom NCT A Phase I, Open-Label, Multicentre Study to Assess the Safety, Tolerability, Pharmacokinetics and Preliminary Antitumour Activity of Ascending Doses of AZD5363 Under Adaptable Dosing Schedules in Patients With Advanced Solid Malignancies Cancer type: Cervical Cancer Variant class: PIK3CA mutation Other identifiers: 0C-14-10, , , , , CR1322AZ, CSET 2365, D3610C00001, EudraCT Number: , IRAS ID: 62131, JapicCTI , M10AZD, NCI , NL , P1TGIVEN, PRO 09 Population segments: (N/A), Adenocarcinoma, Estrogen receptor positive, Fourth line or greater, HER2 positive, Hormone refractory, Second line, Stage III, Stage IV, Third line Phase: I Therapy: capivasertib Locations: Canada, Denmark, France, Italy, Japan, Singapore, Spain, United States US States: CA, CO, NY, OK, PA, TN, TX US Contact: AstraZeneca Clinical Study Information Center [ ; information.center@astrazeneca.com]
6 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 5 of 15 PIK3CA p.(e545k) c.1633g>a (continued) NCT Study to Evaluate the Safety and Efficacy of Sirolimus, in Subject With Refractory Solid Tumors Variant class: PIK3CA E545K mutation Other identifiers: , KCT , SMC Population segments: (N/A), Second line Phase: II Therapy: sirolimus Location: Republic of Korea NCT A Phase II Study of the PARP Inhibitor Olaparib (AZD2281) Alone and in Combination With AZD1775, AZD5363, or AZD6738 in Advanced Solid Tumors Variant class: PIK3CA activating mutation Other identifiers: , , NCI , OLAPCO, VICCMD1672 Population segments: First line, Second line, Stage IV Phase: II Therapy: capivasertib + olaparib Location: United States US States: CT, MA, OH, TN US Contact: Manuel Avedissian [ ; manuel.avedissian@yale.edu] NCT A Two-period, Multicenter, Randomized, Open-label, Phase II Study Evaluating the Clinical Benefit of a Maintenance Treatment Targeting Tumor Molecular Alterations in Patients With Progressive Locally-advanced or Metastatic Solid Tumors MOST: My own specific treatment Other identifiers: ET12-081, EudraCT number: , MOST, ProfiLER Population segments: Maintenance/Consolidation, Second line, Stage III, Stage IV Phase: II Therapy: everolimus Location: France Variant class: PIK3CA mutation
7 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 6 of 15 PIK3CA p.(e545k) c.1633g>a (continued) NCT Molecular Analysis for Therapy Choice (MATCH) Variant class: PIK3CA aberration Other identifiers: , CTSU/EAY131, EAY131, EAY131-A, EAY131-B, EAY131-C1, EAY131-C2, EAY131-E, EAY131-F, EAY131-G, EAY131-H, EAY131-I, EAY131-J, EAY131- L, EAY131-M, EAY131-MATCH, EAY131-N, EAY131-P, EAY131-Q, EAY131-R, EAY131-S1, EAY131-S2, EAY131-T, EAY131-U, EAY131-V, EAY131-W, EAY131-X, EAY131-Y, EAY131- Z1A, EAY131-Z1B, EAY131-Z1C, EAY131-Z1D, EAY131-Z1E, EAY131-Z1F, EAY131-Z1G, EAY131-Z1H, EAY131-Z1I, EAY131-Z1J, ECOGEAY131-M, MATCH, NCI , NCI- MATCH Population segments: (N/A), Aggressive, Classical, Fourth line or greater, HER2 positive, Indolent, Nodular lymphocyte-predominant, Second line, Stage III, Stage IV, Third line Phase: II Therapy: copanlisib Locations: Puerto Rico, United States US States: AK, AL, AR, AZ, CA, CO, CT, DC, DE, FL, GA, HI, IA, ID, IL, IN, KS, KY, LA, MA, MD, ME, MI, MN, MO, MS, MT, NC, ND, NE, NH, NJ, NM, NV, NY, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VA, VT, WA, WI, WV, WY US Contact: Multiple contacts: See for complete list of contacts. NCT Canadian Profiling and Targeted Agent Utilization Trial (CAPTUR): A Phase II Basket Trial Variant class: PIK3CA aberration Other identifiers: CA209-9DL, CAPTUR, ESR , ML39800, PM1, WI Population segments: Aggressive, Diffuse large B-cell lymphoma (DLBCL), Extranodal marginal zone B-cell lymphoma (MALT), First line, Follicular lymphoma (FL), Indolent, Lymphoblastic lymphoma (LBL), Mantle cell lymphoma (MCL), Other subtype, Second line, Stage III, Stage IV, Waldenstrom`s macroglobulinemia (WM) Phase: II Therapy: temsirolimus Location: Canada
8 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 7 of 15 PIK3CA p.(e545k) c.1633g>a (continued) NCT A Phase I, Open-Label, Dose-Escalation Study Evaluating the Safety, Tolerability, and Pharmacokinetics of GDC-0077 as a Single Agent in Patients With Locally Advanced or Metastatic PIK3CA-Mutant Solid Tumors and in Combination With Endocrine and Targeted Therapies in Patients With Locally Advanced or Metastatic PIK3CA-Mutant Hormone- Receptor Positive Breast Cancer Variant class: PIK3CA mutation Other identifiers: , EudraCT Number: , GO39374, NCI Population segments: Estrogen receptor positive, HER2 negative, Line of therapy N/A, Progesterone receptor positive, Stage III, Stage IV Phase: I Therapy: GDC-0077 Locations: Canada, France, Spain, United Kingdom, United States US States: MA, NY, TN US Contact: Reference Study ID Number: GO39374 [ ; global-rochegenentech-trials@gene.com] NCT Phase I Study of the CDK4/6 Inhibitor Palbociclib (PD ) in Combination With the PI3K/mTOR Inhibitor Gedatolisib (PF ) for Patients With Advanced Squamous Cell Lung, Pancreatic, Head & Neck and Other Solid Tumors Variant class: PIK3CA mutation Other identifiers: , NCI Population segments: Second line, Squamous Cell, Stage III, Stage IV Phase: I Therapy: gedatolisib + palbociclib Location: United States US State: MA US Contact: Dr. Nicole Chau [ ] NCT A Phase Ib Trial of LY in Combination With Chemotherapy or Targeted Agents in Advanced and/or Metastatic Tumors Cancer type: Unspecified Cancer Variant class: PIK3CA mutation Other identifiers: 15295, , I4D-MC-JTJF, NCI Population segments: Second line, Stage III, Stage IV Phase: I Therapy: LY prexasertib Location: United States US States: FL, OK, TN, TX US Contact: Eli Lilly and Company [ ]
9 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 8 of 15 PIK3CA p.(e545k) c.1633g>a (continued) NCT PIPA: A Phase Ib Study to Assess the Safety, Tolerability and Efficacy of the PI3K Inhibitors, Taselisib (GDC-0032) or Pictilisib (GDC-0941), in Combination With PAlbociclib, With the Subsequent Addition of Fulvestrant in PIK3CA-mutant Breast Cancers Other identifiers: CCR4191, EudraCT Number: , IRAS ID:159997, PIPA Population segments: Estrogen receptor positive, Fourth line or greater, HER2 negative, HER2 positive, KRAS, Stage III, Stage IV, Triple receptor negative Phase: I Therapies: palbociclib + pictilisib, palbociclib + taselisib Location: United Kingdom Variant class: PIK3CA mutation NCT A Phase I Dose Escalation Study of ARQ 751 in Adult Subjects With Advanced Solid Tumors With AKT1, 2, 3 Genetic Alterations, Activating PI3K Mutations, PTEN-null, or Other Known Actionable PTEN Mutations Variant class: PI3K activating mutation Other identifiers: , ARQ , PTEN-null Population segments: Second line, Stage III, Stage IV Phase: I Therapy: ARQ-751 Location: United States US State: TX US Contact: ArQule [ ; ClinicalTrials@arqule.com] CCND1 amplification NCT A Phase I/II Trial of Oral SRA737 (a Chk1 Inhibitor) Given in Combination With Gemcitabine Plus Cisplatin or Gemcitabine Alone in Subjects With Advanced Cancer Cancer type: Cervical Cancer Variant class: G1/S cell cycle pathway Other identifiers: , 30498, CRUKD/16/005, EudraCT Number: , PNT737-02, SRA Population segments: BRCA, Fourth line or greater, KRAS, Second line, Stage III, Stage IV, Third line Phase: I/II Therapy: PNT chemotherapy Locations: Spain, United Kingdom
10 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 9 of 15 CCND1 amplification (continued) NCT A Phase II Study of the CDK4/6 Inhibitor Abemaciclib in Patients With Solid Tumors Harboring Genetic Alterations in Genes Encoding D-type Cyclins or Amplification of CDK4 or CDK6 Variant class: CCND1 amplification Other identifiers: , NCI Population segments: First line, Stage III, Stage IV Phase: II Therapy: abemaciclib Location: United States US State: MA US Contact: Dr. Geoffrey Shapiro [ ; geoffrey_shapiro@dfci.harvard.edu] NCT Phase II Trial of the Cyclin-Dependent Kinase Inhibitor PD in Patients With Cancer Variant class: CCND1 amplification Other identifiers: NCI , Study 1006, UPCC 03909, UPCC03909 Population segments: Estrogen receptor positive, Fourth line or greater, HER2 negative, HER2 positive, Metastatic, Progesterone receptor positive, Second line, Stage III, Stage IV, Third line, Triple receptor negative Phase: II Therapy: palbociclib Location: United States US State: PA US Contact: Dr. Peter O. Dwyer [ ; PennCancerTrials@emergingmed.com] NCT A Phase II Study of Palbociclib in Progressive Brain Metastases Harboring Alterations in the CDK Pathway Variant class: CCND1 amplification Other identifiers: , NCI Population segments: CNS mets, Second line, Stage IV Phase: II Therapy: palbociclib Location: United States US State: MA US Contact: Dr. Priscilla Brastianos [ ; PBRASTIANOS@mgh.harvard.edu]
11 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 10 of 15 CCND1 amplification (continued) NCT Canadian Profiling and Targeted Agent Utilization Trial (CAPTUR): A Phase II Basket Trial Variant class: CCND1 aberration Other identifiers: CA209-9DL, CAPTUR, ESR , ML39800, PM1, WI Population segments: Aggressive, Diffuse large B-cell lymphoma (DLBCL), Extranodal marginal zone B-cell lymphoma (MALT), First line, Follicular lymphoma (FL), Indolent, Lymphoblastic lymphoma (LBL), Mantle cell lymphoma (MCL), Other subtype, Second line, Stage III, Stage IV, Waldenstrom`s macroglobulinemia (WM) Phase: II Therapy: palbociclib Location: Canada FGF19 amplification NCT Phase I/II Study of TAS-120 in Patients With Advanced Solid Tumors Harboring FGF/FGFR Aberrations Variant class: FGF19 amplification Other identifiers: , , 17-23, 17084, , CSET 2107, CT680, EudraCT Number: , IND Number , IRAS ID , IRAS ID , MC# 17-23, NCI , NL , NL , P 47317, REFMAL 340, TPU-TAS , UW18036 Population segments: FGFR, Second line, Stage III, Stage IV Phase: I/II Therapy: TAS-120 Locations: Australia, France, Hong Kong, Spain, United Kingdom, United States US States: AZ, CA, FL, MA, MN, NM, NY, PA, SC, TX, WA US Contact: Dr. Jerry Huang [jhuang@taihooncology.com] NCT A Phase I, Open-Label, Dose-Escalation and Expansion, Safety and Tolerability Study of INCB in Subjects With Advanced Hepatocellular Carcinoma and Other Malignancies Variant class: FGF19 aberration Other identifiers: , INCB , INCB , NCI , UMCC Population segments: Second line, Stage III, Stage IV Phase: I/II Therapy: INCB Locations: Belgium, United States US States: AL, AZ, IN, MI, NY US Contact: Incyte Corporation Call Center (US) [ ; medinfo@incyte.com]
12 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 11 of 15 FGF19 amplification (continued) NCT A Phase I, Open-Label, Dose-Escalation, Dose-Expansion, Safety and Tolerability Study of INCB in Japanese Subjects With Advanced Malignancies Variant class: FGF aberration Other identifier: INCB Population segments: Line of therapy N/A, Stage III, Stage IV Phase: I Therapy: INCB Location: Japan NCT A Phase I Study of E7090 in Subjects With Solid Tumor Variant class: FGF pathway Other identifiers: E7090-J , JapicCTI Population segments: Fourth line or greater, Stage III, Stage IV Phase: I Therapy: E-7090 Location: Japan FGF3 amplification NCT Phase I/II Study of TAS-120 in Patients With Advanced Solid Tumors Harboring FGF/FGFR Aberrations Variant class: FGF aberration Other identifiers: , , 17-23, 17084, , CSET 2107, CT680, EudraCT Number: , IND Number , IRAS ID , IRAS ID , MC# 17-23, NCI , NL , NL , P 47317, REFMAL 340, TPU-TAS , UW18036 Population segments: FGFR, Second line, Stage III, Stage IV Phase: I/II Therapy: TAS-120 Locations: Australia, France, Hong Kong, Spain, United Kingdom, United States US States: AZ, CA, FL, MA, MN, NM, NY, PA, SC, TX, WA US Contact: Dr. Jerry Huang [jhuang@taihooncology.com]
13 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 12 of 15 FGF3 amplification (continued) NCT A Phase I, Open-Label, Dose-Escalation, Dose-Expansion, Safety and Tolerability Study of INCB in Japanese Subjects With Advanced Malignancies Variant class: FGF aberration Other identifier: INCB Population segments: Line of therapy N/A, Stage III, Stage IV Phase: I Therapy: INCB Location: Japan NCT A Phase I Study of E7090 in Subjects With Solid Tumor Variant class: FGF pathway Other identifiers: E7090-J , JapicCTI Population segments: Fourth line or greater, Stage III, Stage IV Phase: I Therapy: E-7090 Location: Japan
14 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 13 of 15 Evidence Summary by Variant Class A variant class hierarchy was created to summarize gene variants with associated clinical evidence. Evidence items refers to citations across the different global data sources. PIK3CA p.(e545k) c.1633g>a Variant Class Evidence Items PI3K/AKT/MTOR pathway 0 PI3K activating mutation 1 PIK3CA activating mutation 1 PIK3CA E545 mutation 0 PIK3CA E545K mutation 1 PIK3CA aberration 2 PIK3CA mutation status 0 PIK3CA mutation 7 PIK3CA exon 9 mutation 0 PIK3CA E545 mutation 0 PIK3CA E545K mutation 1 PIK3CA activating mutation 1 PIK3CA E545 mutation 0 PIK3CA E545K mutation 1 CCND1 amplification Variant Class Evidence Items G1/S cell cycle pathway 1 CCND1 aberration 1 CCND1 amplification 3
15 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 14 of 15 Evidence Summary by Variant Class (continued) A variant class hierarchy was created to summarize gene variants with associated clinical evidence. Evidence items refers to citations across the different global data sources. FGF19 amplification Variant Class Evidence Items FGF pathway 2 FGF aberration 3 FGF19 aberration 1 FGF19 amplification 1 FGF3 amplification Variant Class Evidence Items FGF pathway 2 FGF aberration 3
16 Lead Clinical Scientist: - Pre-Reg Clinical Scientist: - Date: 15 of 15 Variant Details DNA Sequence Variants Gene Amino Acid Change Coding Variant ID Allele Frequency Transcript Variant Effect Gene Class Variant Class PIK3CA p.(e545k) c.1633g>a COSM % NM_ missense Gain of Function Hotspot Copy Number Variations Gene Locus Copy Number CCND1 chr11: FGF19 chr11: FGF3 chr11:
17
18 , Cambridge, CB10 1XL +44 (0) info@oncologica.com Ireland Italy Bymac Centre, Northwest Business Park, Blanchardstow, Dublin 15 Parco Tecnologico della Sardegna Pula, Località Piscinamanna
19 Medical Laboratory Accredited to ISO15189:2012 Immunofocus PD-1/PD-L1 TESTING FOR ANTI-PD-L1 DIRECTED IMMUNOTHERAPY IMMUNOFOCUS TEST REPORT
20 Lead Clinical Scientist: Pre-Reg Clinical Scientist: Date: 1 of 2 PD-L1 test Anti-PD-1/PD-L1 directed immunotherapies are an important group of agents used in the treatment of a range of different cancer types. These immunotherapies include pembrolizumab, atezolizumab, avelumab, nivolumab, and durvalumab. The immunofocus test is used to help identify those patients most likely to benefit from anti-pd-l1 directed immunotherapies. The immunofocus test assesses the proportion of tumour cells that express PD-L1 (Tumour Proportion Score) and the area occupied by tumour infiltrating PD-L1 positive immune cells. A range of cut-off/ threshold values for tumour proportion scores (>1%, >25%, >50%) and PD-L1 positive immune cells (>10%) have been identified as predictors of response to anti-pd-l1 directed therapies. These cut offs vary according to tumour type, whether the drug is being used in first or second line therapy and according to which immunotherapy is selected. The Oncologist will use the Immunofocus test results together with other clinicopathological parameters as a guide to determine whether a patient s tumour is likely to benefit from anti-pd-l1 directed therapies. PD-L1 Result The tumour shows a markedly heterogeneous pattern of PD-L1 expression. At the advancing margins the majority of tumour cells (80-90%) show strong or moderate intensity immunostaining for PD-L1 with complete patterns of surface membrane expression. In other areas of the tumour a smaller proportion of tumour cells show PD-L1 expression. Taken together the proportion of PD-L1 expressing tumour cells amounts to around 20-25% of the total tumour cell population. The tumour is associated with a focal sparse PD-L1 expressing immune cell (IC) infiltrate. PD-L1 expressing tumour infiltrating immune cells (ICs) cover around 4-5% of the tumour area occupied by tumour cells, intratumoural and contiguous peritumoural stroma. Summary; PD-L1 Tumour Proportion Score 20-25%; PD-L1 positive ICs 4-5% of tumour area
21 2 of 2 Report Authorised by Report reviewed by Signed Signed Printed Professor Gareth Williams Clinical Scientist Pathologist BMS!Senior] D 0 D Printed Keeda Hardisty Clinical Scientist Pathologist BMS!Senior] 0 D D
22 , Cambridge, CB10 1XL +44 (0) info@oncologica.com Ireland Italy Bymac Centre, Northwest Business Park, Blanchardstow, Dublin 15 Parco Tecnologico della Sardegna Pula, Località Piscinamanna
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 06 Jun 2017 1 of 13 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Female Histology
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 06 Jun 2017 1 of 13 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Female Histology
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 May 2017 1 of 13 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 May 2017 1 of 13 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 06 Apr 2017 1 of 16 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 06 Apr 2017 1 of 16 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 14 Dec 2016 1 of 18 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 14 Dec 2016 1 of 18 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Katherine Marquis Date: 05 Oct 2016 1 of 8 The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Katherine Marquis Date: 05 Oct 2016 1 of 8 The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 29 Mar 2017 1 of 8 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 29 Mar 2017 1 of 8 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 22 Dec 2016 1 of 4 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 22 Dec 2016 1 of 4 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 24 May 2017 1 of 14 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Histology
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 24 May 2017 1 of 14 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Histology
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 14 Dec 2016 1 of 18 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 14 Dec 2016 1 of 18 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 May 2017 1 of 13 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 May 2017 1 of 13 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 Jan 2018 1 of 25 ONC17 Surname Requester Forename Contact details DOB Date requested Gender Histology
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 Jan 2018 1 of 25 ONC17 Surname Requester Forename Contact details DOB Date requested Gender Histology
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 21 Apr 2017 1 of 25 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Histology
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 21 Apr 2017 1 of 25 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Histology
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 18 Jan 2017 1 of 25 Comment: The DNA and RNA extracted from this sample were of optimal quality. The
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 18 Jan 2017 1 of 25 Comment: The DNA and RNA extracted from this sample were of optimal quality. The
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 30 Nov 2016 1 of 11 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 30 Nov 2016 1 of 11 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 08 Feb 2017 1 of 6 Comment: The DNA and RNA extracted from this sample were of optimal quantity. The
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 08 Feb 2017 1 of 6 Comment: The DNA and RNA extracted from this sample were of optimal quantity. The
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 May 2017 1 of 28 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 May 2017 1 of 28 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 24 May 2017 1 of 14 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Histology
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 24 May 2017 1 of 14 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Histology
Workforce Data The American Board of Pediatrics
 Workforce Data 2009-2010 The American Board of Pediatrics Caution. Before using this report as a resource, please read the information below! Please use caution when comparing data in this version of the
Workforce Data 2009-2010 The American Board of Pediatrics Caution. Before using this report as a resource, please read the information below! Please use caution when comparing data in this version of the
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Retaking NPTE
 The table below lists the requirements for retaking the National Physical Therapy Exam (NPTE) for each jurisdiction. Summary Number of attempts on NPTE limited? 16 27 Number of attempts allowed before
The table below lists the requirements for retaking the National Physical Therapy Exam (NPTE) for each jurisdiction. Summary Number of attempts on NPTE limited? 16 27 Number of attempts allowed before
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Keeley Monsen Date: 26 May 2017 1 of 26 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Male Histology
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Keeley Monsen Date: 26 May 2017 1 of 26 ONC17 Surname Requesting Clinician Forename DOB Date requested Gender Male Histology
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 May 2017 1 of 28 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Tiffany Haddow Date: 04 May 2017 1 of 28 Surname Forename DOB Gender Histology # Primary site Tumour subtype Tissue Type
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Katherine Marquis Oncologica UK Ltd Date: 05 Aug 2016 1 of 18 Comment: The DNA and RNA extracted from this sample were of
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Katherine Marquis Oncologica UK Ltd Date: 05 Aug 2016 1 of 18 Comment: The DNA and RNA extracted from this sample were of
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Katherine Marquis Oncologica UK Ltd Date: 27 Jun 2016 1 of 36 Comment: The DNA and RNA extracted from this sample were of
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson Senior BMS: Katherine Marquis Oncologica UK Ltd Date: 27 Jun 2016 1 of 36 Comment: The DNA and RNA extracted from this sample were of
Different Types of Cancer
 Different Types of Cancer Cancer can originate almost anywhere in the body. Sarcomas (connective tissue) Ø arise from cells found in the supporting tissues of the body such as bone, cartilage, fat, connective
Different Types of Cancer Cancer can originate almost anywhere in the body. Sarcomas (connective tissue) Ø arise from cells found in the supporting tissues of the body such as bone, cartilage, fat, connective
Financial Impact of Lung Cancer in West Virginia
 Financial Impact of Lung Cancer in West Virginia John Deskins, Ph.D. Christiadi, Ph.D. Sara Harper November 2018 Bureau of Business & Economic Research College of Business & Economics West Virginia University
Financial Impact of Lung Cancer in West Virginia John Deskins, Ph.D. Christiadi, Ph.D. Sara Harper November 2018 Bureau of Business & Economic Research College of Business & Economics West Virginia University
Tecentriq (atezolizumab) (Intravenous)
 Tecentriq (atezolizumab) (Intravenous) Last Review Date: 06/01/2018 Date of Origin: 06/28/2016 Document Number: IC-0278 Dates Reviewed: 06/2016, 08/2016, 10/2016, 02/2017, 04/2017, 08/2017, 11/2017, 02/2018,
Tecentriq (atezolizumab) (Intravenous) Last Review Date: 06/01/2018 Date of Origin: 06/28/2016 Document Number: IC-0278 Dates Reviewed: 06/2016, 08/2016, 10/2016, 02/2017, 04/2017, 08/2017, 11/2017, 02/2018,
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Direct Access
 Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are two tables: Types and Limits Referrals TYPES AND
Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are two tables: Types and Limits Referrals TYPES AND
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Senior BMS: Katherine Marquis Date: 04 Apr 2016 17:01:50 PM 1 of 18 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus assay on which
Oncofocus Patient Test Report Senior BMS: Katherine Marquis Date: 04 Apr 2016 17:01:50 PM 1 of 18 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus assay on which
2016 COMMUNITY SURVEY
 1 Epilepsy Innovation Institute (Ei ) 016 COMMUNITY SURVEY INTRODUCTION From September 8th to November 9th, 016, epilepsy.com hosted a survey that asked the community the following: What are the aspects
1 Epilepsy Innovation Institute (Ei ) 016 COMMUNITY SURVEY INTRODUCTION From September 8th to November 9th, 016, epilepsy.com hosted a survey that asked the community the following: What are the aspects
RECOVERY SUPPORT SERVICES IN STATES
 RECOVERY SUPPORT SERVICES IN STATES An analysis of State recovery support services using the 16 17 Substance Abuse Block Grant (SABG) Behavioral Health Assessment and Plan THIS PROJECT IS BEING SUPPORTED
RECOVERY SUPPORT SERVICES IN STATES An analysis of State recovery support services using the 16 17 Substance Abuse Block Grant (SABG) Behavioral Health Assessment and Plan THIS PROJECT IS BEING SUPPORTED
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Direct Access
 Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are two tables: Types and Limits Referrals TYPES AND
Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are two tables: Types and Limits Referrals TYPES AND
Rituxan Hycela (rituximab and hyaluronidase human) (Subcutaneous)
 Rituxan Hycela (rituximab and hyaluronidase human) (Subcutaneous) Document Number: IC-0322 Last Review Date: 02/06/2018 Date of Origin: 7/20/2010 Dates Reviewed: 09/2010, 12/2010, 02/2011, 03/2011, 05/2011,
Rituxan Hycela (rituximab and hyaluronidase human) (Subcutaneous) Document Number: IC-0322 Last Review Date: 02/06/2018 Date of Origin: 7/20/2010 Dates Reviewed: 09/2010, 12/2010, 02/2011, 03/2011, 05/2011,
Personalized Gene Profile
 Sequence Variants: 6 4 ALTERATION MUTANT FRACTION FDA GUIDANCE (for indication) FDA GUIDANCE (for other indications) TRIALS (details below) c.38g>a; p.g13d 21.0% Cetuximab Contraindicated 22 Panitumumab
Sequence Variants: 6 4 ALTERATION MUTANT FRACTION FDA GUIDANCE (for indication) FDA GUIDANCE (for other indications) TRIALS (details below) c.38g>a; p.g13d 21.0% Cetuximab Contraindicated 22 Panitumumab
Velcade (bortezomib) Document Number: IC-0137
 Velcade (bortezomib) Document Number: IC-0137 Last Review Date: 11/21/2017 Date of Origin: 11/28/2011 Dates Reviewed: 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 09/2013, 12/2013, 03/2014,
Velcade (bortezomib) Document Number: IC-0137 Last Review Date: 11/21/2017 Date of Origin: 11/28/2011 Dates Reviewed: 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 09/2013, 12/2013, 03/2014,
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Foreign Educated Physical Therapists
 Requirements for Licensure Summary: Number of Jurisdictions that Require: Educational Credentials Review 50 Graduation from a program equivalent to CAPTE 37 Eligibility to practice in the country in which
Requirements for Licensure Summary: Number of Jurisdictions that Require: Educational Credentials Review 50 Graduation from a program equivalent to CAPTE 37 Eligibility to practice in the country in which
Overview of the HHS National Network of Quitlines Initiative
 Overview of the HHS National Network of Quitlines Initiative Prepared for the 2005 National Oral Health Conference 6th Joint Meeting of ASTDD and AAPHD Barbara Z. Park, RDH, MPH May 2, 2005 Background
Overview of the HHS National Network of Quitlines Initiative Prepared for the 2005 National Oral Health Conference 6th Joint Meeting of ASTDD and AAPHD Barbara Z. Park, RDH, MPH May 2, 2005 Background
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 18 Jan 2017 1 of 30 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Oncofocus Patient Test Report Lead Clinical Scientist: Keeda Snelson BMS: Tiffany Haddow Date: 18 Jan 2017 1 of 30 Comment: The DNA and RNA extracted from this sample were of optimal quality. The Oncofocus
Imfinzi (durvalumab) (Intravenous)
 Imfinzi (durvalumab) (Intravenous) Last Review Date: 09/05/2018 Date of Origin: 05/30/2017 Dates Reviewed: 05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 09/2018 Document Number: IC-0301 I. Length of Authorization
Imfinzi (durvalumab) (Intravenous) Last Review Date: 09/05/2018 Date of Origin: 05/30/2017 Dates Reviewed: 05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 09/2018 Document Number: IC-0301 I. Length of Authorization
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Direct Access
 Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are three tables: Types and Limits Specific Limits
Each licensing authority indicates the level of direct access allowed in the jurisdiction and the type of limitations that apply to this access. There are three tables: Types and Limits Specific Limits
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: Foreign Educated PTs and PTAs
 PT Requirements for Licensure Summary: Number of Jurisdictions that Require: Educational Credentials Review 50 from a program equivalent to CAPTE 37 Eligibility to practice in the country in which education
PT Requirements for Licensure Summary: Number of Jurisdictions that Require: Educational Credentials Review 50 from a program equivalent to CAPTE 37 Eligibility to practice in the country in which education
Oncofocus. Patient Test Report
 Oncofocus Patient Test Report Oncofocus Patient Test Report Oncologica UK Ltd, Suite 15-16, The Science Village, Chesterford Research Park, Cambridge, CB10 1XL www.oncologica.com - Tel: +44 (0)1223 785327
Oncofocus Patient Test Report Oncofocus Patient Test Report Oncologica UK Ltd, Suite 15-16, The Science Village, Chesterford Research Park, Cambridge, CB10 1XL www.oncologica.com - Tel: +44 (0)1223 785327
Nature Medicine: doi: /nm.3559
 Supplementary Note 1. A sample alteration report. Each alteration nominated by PHIAL is curated to answer specific fields that are intended to guide physician interpretation. Gene Alteration Patient ID
Supplementary Note 1. A sample alteration report. Each alteration nominated by PHIAL is curated to answer specific fields that are intended to guide physician interpretation. Gene Alteration Patient ID
Using Cancer Registry Data to Estimate the Percentage of Melanomas Attributable to UV Exposure
 Using Cancer Registry Data to Estimate the Percentage of Melanomas Attributable to UV Exposure Meg Watson, MPH Epidemiologist NAACCR Annual Conference June 16, 2016 National Center for Chronic Disease
Using Cancer Registry Data to Estimate the Percentage of Melanomas Attributable to UV Exposure Meg Watson, MPH Epidemiologist NAACCR Annual Conference June 16, 2016 National Center for Chronic Disease
USA National Mental Healthcare Nonprofit Exempt Organization Financial Analysis as of December 14, 2015 January 24, 2016 ANSA-H2
 USA National Mental Healthcare Nonprofit Exempt Organization Financial Analysis as of December 14, 2015 January 24, 2016 ANSA-H2 Prepared by David Yoo, HanaSoul Consulting, Omaha, Nebraska dcyoo@cox.net
USA National Mental Healthcare Nonprofit Exempt Organization Financial Analysis as of December 14, 2015 January 24, 2016 ANSA-H2 Prepared by David Yoo, HanaSoul Consulting, Omaha, Nebraska dcyoo@cox.net
Reducing Barriers to Risk Appropriate Cancer Genetics Services: Current Strategies
 Reducing Barriers to Risk Appropriate Cancer Genetics Services: Current Strategies Kara J. Milliron, MS, CGC Mark D. Pearlman, MD Disclosure I am a contract genetic counselor with Informed DNA, Inc. The
Reducing Barriers to Risk Appropriate Cancer Genetics Services: Current Strategies Kara J. Milliron, MS, CGC Mark D. Pearlman, MD Disclosure I am a contract genetic counselor with Informed DNA, Inc. The
Evidence-Based Policymaking: Investing in Programs that Work
 Evidence-Based Policymaking: Investing in Programs that Work August 4, 2015 The Policy Challenge Though policymakers want to make the best choices, the process often relies on inertia and anecdote Very
Evidence-Based Policymaking: Investing in Programs that Work August 4, 2015 The Policy Challenge Though policymakers want to make the best choices, the process often relies on inertia and anecdote Very
Improving Cancer Surveillance and Mortality Data for AI/AN Populations
 Improving Cancer Surveillance and Mortality Data for AI/AN Populations Melissa A. Jim, MPH (Diné) Epidemiologist, Cancer Surveillance Branch Assigned to IHS Division of Epidemiology and Disease Prevention
Improving Cancer Surveillance and Mortality Data for AI/AN Populations Melissa A. Jim, MPH (Diné) Epidemiologist, Cancer Surveillance Branch Assigned to IHS Division of Epidemiology and Disease Prevention
State of California Department of Justice. Bureau of Narcotic Enforcement
 State of California Department of Justice Bureau of Narcotic Enforcement Prescription Drugs in the U.S. At least half of all Americans take one prescription drug regularly, with one in six taking three
State of California Department of Justice Bureau of Narcotic Enforcement Prescription Drugs in the U.S. At least half of all Americans take one prescription drug regularly, with one in six taking three
Dates Reviewed: 12/2012, 3/2013, 6/2013, 9/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014,
 Perjeta (pertuzumab) Last Review Date: 5/30/2017 Date of Origin: 11/01/2012 Document Number: IC-0096 Dates Reviewed: 12/2012, 3/2013, 6/2013, 9/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014,
Perjeta (pertuzumab) Last Review Date: 5/30/2017 Date of Origin: 11/01/2012 Document Number: IC-0096 Dates Reviewed: 12/2012, 3/2013, 6/2013, 9/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014,
How to Get Paid for Doing EBD
 How to Get Paid for Doing EBD Robert D. Compton, DDS President Robert Compton, DDS Executive Director DentaQuest Institute Disclosure DentaQuest Institute President DentaQuest Benefits Senior VP & CDO
How to Get Paid for Doing EBD Robert D. Compton, DDS President Robert Compton, DDS Executive Director DentaQuest Institute Disclosure DentaQuest Institute President DentaQuest Benefits Senior VP & CDO
BY-STATE MENTAL HEALTH SERVICES AND EXPENDITURES IN MEDICAID, 1999
 STATE-BY BY-STATE MENTAL HEALTH SERVICES AND EXPENDITURES IN MEDICAID, 1999 James Verdier,, Ann Cherlow,, and Allison Barrett Mathematica Policy Research, Inc. Jeffrey Buck and Judith Teich Substance Abuse
STATE-BY BY-STATE MENTAL HEALTH SERVICES AND EXPENDITURES IN MEDICAID, 1999 James Verdier,, Ann Cherlow,, and Allison Barrett Mathematica Policy Research, Inc. Jeffrey Buck and Judith Teich Substance Abuse
How to faster integrate new technologies into clinical practice
 How to faster integrate new technologies into clinical practice 31JAN2017 Jens Bjørheim, CMO Ultimovacs Nordic Trial Alliance Stakeholders meeting 2 Oncology New drugs Landscape The current buzz Basket
How to faster integrate new technologies into clinical practice 31JAN2017 Jens Bjørheim, CMO Ultimovacs Nordic Trial Alliance Stakeholders meeting 2 Oncology New drugs Landscape The current buzz Basket
ANNUAL REPORT EXECUTIVE SUMMARY. The full report is available at DECEMBER 2017
 ANNUAL REPORT EXECUTIVE SUMMARY DECEMBER 2017 The full report is available at www.americashealthrankings.org EXECUTIVE SUMMARY OVERVIEW America s Health Rankings presents its 28th Annual Report, providing
ANNUAL REPORT EXECUTIVE SUMMARY DECEMBER 2017 The full report is available at www.americashealthrankings.org EXECUTIVE SUMMARY OVERVIEW America s Health Rankings presents its 28th Annual Report, providing
The Affordable Care Act and HIV: What are the Implications?
 The Affordable Care Act and HIV: What are the Implications? 2013 National Black AIDS Institute Webinar Series September 18, 2013 Jen Kates, Kaiser Family Foundation The Challenge Figure 1 30 years into
The Affordable Care Act and HIV: What are the Implications? 2013 National Black AIDS Institute Webinar Series September 18, 2013 Jen Kates, Kaiser Family Foundation The Challenge Figure 1 30 years into
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide Topic: License Renewal. License Renewal on Birthdays
 The table below lists information on the term and renewal date for each jurisdiction. Summary License Term 1 26 2 Renewal Date One date 31 Birthdays 6 Half in even years 4 4 License Term AL AK AZ AR CA
The table below lists information on the term and renewal date for each jurisdiction. Summary License Term 1 26 2 Renewal Date One date 31 Birthdays 6 Half in even years 4 4 License Term AL AK AZ AR CA
Expedited Partner Therapy (EPT)/Patient Deliver Partner Therapy (PDPT)
 Expedited Partner Therapy (EPT)/Patient Deliver Partner Therapy (PDPT) David Johnson STD Disparities Coordinator, Office of Health Equity Division of STD Prevention National Center for HIV, Viral Hepatitis,
Expedited Partner Therapy (EPT)/Patient Deliver Partner Therapy (PDPT) David Johnson STD Disparities Coordinator, Office of Health Equity Division of STD Prevention National Center for HIV, Viral Hepatitis,
Approach to Cancer Prevention through Policy, Systems, and Environmental Change in the U.S.
 Approach to Cancer Prevention through Policy, Systems, and Environmental Change in the U.S. Marcus Plescia, MD, MPH Director, Division of Cancer Prevention and Control Centers for Disease Control and Prevention
Approach to Cancer Prevention through Policy, Systems, and Environmental Change in the U.S. Marcus Plescia, MD, MPH Director, Division of Cancer Prevention and Control Centers for Disease Control and Prevention
How Often Do Americans Eat Vegetarian Meals? And How Many Adults in the U.S. Are Vegetarian? Posted on May 29, 2015 by The VRG Blog Editor
 How Often Do Americans Eat Vegetarian Meals? And How Many Adults in the U.S. Are Vegetarian? Posted on May 29, 2015 by The VRG Blog Editor The Vegetarian Resource Group asks in a 2015 National Survey Conducted
How Often Do Americans Eat Vegetarian Meals? And How Many Adults in the U.S. Are Vegetarian? Posted on May 29, 2015 by The VRG Blog Editor The Vegetarian Resource Group asks in a 2015 National Survey Conducted
LaTanya Runnells, Ph.D Program Manager December 6, 2016
 LaTanya Runnells, Ph.D Program Manager December 6, 2016 Mental Health First Aid USA is coordinated by the National Council for Behavioral Health, the Maryland Department of Health and Mental Hygiene, and
LaTanya Runnells, Ph.D Program Manager December 6, 2016 Mental Health First Aid USA is coordinated by the National Council for Behavioral Health, the Maryland Department of Health and Mental Hygiene, and
Consensus and Collaboration
 Consensus and Collaboration John Morton, MD, MPH, FACS, FASMBS Chief, Bariatric & Minimally Invasive Surgery Stanford School of Medicine Past-President, American Society of Metabolic and Bariatric Surgery,
Consensus and Collaboration John Morton, MD, MPH, FACS, FASMBS Chief, Bariatric & Minimally Invasive Surgery Stanford School of Medicine Past-President, American Society of Metabolic and Bariatric Surgery,
Multimedia Appendix 6 Educational Materials Table of Contents. Intervention Educational Materials Audio Script (version 1)
 Multimedia Appendix 6 Educational Materials Table of Contents Intervention Educational Materials... 1 Audio Script (version 1)... 1 Text (version 1)... 5 Slides (version 1)... 17 Audio Script (version
Multimedia Appendix 6 Educational Materials Table of Contents Intervention Educational Materials... 1 Audio Script (version 1)... 1 Text (version 1)... 5 Slides (version 1)... 17 Audio Script (version
Cyramza (ramucirumab) (Intravenous)
 Cyramza (ramucirumab) (Intravenous) Document Number: IC 0199 Last Review Date: 5/1/2018 Date of Origin: 06/24/2014 Dates Reviewed: 09/2014, 01/2015, 05/2015, 11/2015, 04/2016, 08/2016, 11/2016, 05/2017,
Cyramza (ramucirumab) (Intravenous) Document Number: IC 0199 Last Review Date: 5/1/2018 Date of Origin: 06/24/2014 Dates Reviewed: 09/2014, 01/2015, 05/2015, 11/2015, 04/2016, 08/2016, 11/2016, 05/2017,
THREE BIG IMPACT ISSUES
 THREE BIG IMPACT ISSUES Tim McAfee, MD, MPH Director CDC Office on Smoking and Health Presented at the National Cancer Policy Forum Workshop on Reducing Tobacco-Related Cancer Incidence and Mortality June
THREE BIG IMPACT ISSUES Tim McAfee, MD, MPH Director CDC Office on Smoking and Health Presented at the National Cancer Policy Forum Workshop on Reducing Tobacco-Related Cancer Incidence and Mortality June
EXAMPLE. ratio (%) Contraindication for treatment with panitumumab or cetuximab
 Dr Kate Goodhealth Goodhealth Medical Clinic 123 Address Road SUBURBTOWN NSW 2000 Referring Doctor Your ref Address Dr John Medico 123 Main Street, SUBURBTOWN NSW 2000 Phone 02 9999 9999 Requested 17 May
Dr Kate Goodhealth Goodhealth Medical Clinic 123 Address Road SUBURBTOWN NSW 2000 Referring Doctor Your ref Address Dr John Medico 123 Main Street, SUBURBTOWN NSW 2000 Phone 02 9999 9999 Requested 17 May
Christie Eheman, PhD NAACCR 6/2012
 Christie Eheman, PhD NAACCR 6/2012 Comparative Effectiveness Research CDC National Program of Cancer Registries Christie Eheman, PhD NAACCR 6/2012 CDC - Fran Michaud, Dave Butterworth, Linda Mulvihill,
Christie Eheman, PhD NAACCR 6/2012 Comparative Effectiveness Research CDC National Program of Cancer Registries Christie Eheman, PhD NAACCR 6/2012 CDC - Fran Michaud, Dave Butterworth, Linda Mulvihill,
Arkansas Prescription Monitoring Program
 Arkansas Prescription Monitoring Program FY 2016 Third Quarter Report January-March 2016 Arkansas Prescription Monitoring Program Quarterly Report January March, Fiscal year 2016 Act 304 of 2011 authorized
Arkansas Prescription Monitoring Program FY 2016 Third Quarter Report January-March 2016 Arkansas Prescription Monitoring Program Quarterly Report January March, Fiscal year 2016 Act 304 of 2011 authorized
Emerging Issues in Cancer Prevention and Control
 Emerging Issues in Cancer Prevention and Control Marcus Plescia, MD, MPH Director, Division of Cancer Prevention and Control Centers for Disease Control & Prevention National Center for Chronic Disease
Emerging Issues in Cancer Prevention and Control Marcus Plescia, MD, MPH Director, Division of Cancer Prevention and Control Centers for Disease Control & Prevention National Center for Chronic Disease
Response and resistance to BRAF inhibitors in melanoma
 Response and resistance to BRAF inhibitors in melanoma Keith T. Flaherty, M.D. Massachusetts General Hospital Cancer Center Disclosures Roche/Genentech: consultant GlaxoSmithKline: consultant BRAF mutations
Response and resistance to BRAF inhibitors in melanoma Keith T. Flaherty, M.D. Massachusetts General Hospital Cancer Center Disclosures Roche/Genentech: consultant GlaxoSmithKline: consultant BRAF mutations
Shu-Hong Zhu, PhD University of California, San Diego INTRODUCING THE ASIAN SMOKERS QUITLINE (ASQ)
 Shu-Hong Zhu, PhD University of California, San Diego INTRODUCING THE ASIAN SMOKERS QUITLINE (ASQ) THE REACH AND EFFICACY OF A MULTILINGUAL TELEPHONE QUITLINE FOR ASIAN SMOKERS English Quitlines in 1992
Shu-Hong Zhu, PhD University of California, San Diego INTRODUCING THE ASIAN SMOKERS QUITLINE (ASQ) THE REACH AND EFFICACY OF A MULTILINGUAL TELEPHONE QUITLINE FOR ASIAN SMOKERS English Quitlines in 1992
Improving Oral Health:
 Improving Oral Health: New Tools for State Policy Makers Cassie Yarbrough, MPP Lead Public Policy Analyst Health Policy Institute Lansing, MI June 1, 2017 The ADA Health Policy Institute 2017 American
Improving Oral Health: New Tools for State Policy Makers Cassie Yarbrough, MPP Lead Public Policy Analyst Health Policy Institute Lansing, MI June 1, 2017 The ADA Health Policy Institute 2017 American
6/22/2017 TARGETING THE TARGETS IN 2017 TARGETING THE TARGETS IN 2017
 TARGETING THE TARGETS IN 2017 Primary Care Focus Symposium July 1, 2017 Grace Wang MD I do not have any relevant financial relationships to disclose at this time TARGETING THE TARGETS IN 2017 What are
TARGETING THE TARGETS IN 2017 Primary Care Focus Symposium July 1, 2017 Grace Wang MD I do not have any relevant financial relationships to disclose at this time TARGETING THE TARGETS IN 2017 What are
Arkansas Prescription Monitoring Program
 Arkansas Prescription Monitoring Program FY 2017 Second Quarter Report October December 2016 Arkansas Prescription Monitoring Program Quarterly Report October December, Fiscal year 2017 Act 304 of 2011
Arkansas Prescription Monitoring Program FY 2017 Second Quarter Report October December 2016 Arkansas Prescription Monitoring Program Quarterly Report October December, Fiscal year 2017 Act 304 of 2011
Overview and Findings from ASTHO s IIS Interstate Data Sharing Meeting
 Overview and Findings from ASTHO s IIS Interstate Data Sharing Meeting Kim Martin Association of State and Territorial Health Officials (ASTHO) May 12, 2015 The Need for IIS Interstate Data Exchange States,
Overview and Findings from ASTHO s IIS Interstate Data Sharing Meeting Kim Martin Association of State and Territorial Health Officials (ASTHO) May 12, 2015 The Need for IIS Interstate Data Exchange States,
State Tobacco Control Programs
 State Tobacco Control Programs National Cancer Policy Forum Workshop Reducing Tobacco-Related Cancer Incidence and Mortality Karla S. Sneegas, MPH Chief Program Services Branch CDC Office on Smoking and
State Tobacco Control Programs National Cancer Policy Forum Workshop Reducing Tobacco-Related Cancer Incidence and Mortality Karla S. Sneegas, MPH Chief Program Services Branch CDC Office on Smoking and
Dental ER Visits: Evidence of a Failed System. Shelly Gehshan AACDP Conference April 29, 2012
 Dental ER Visits: Evidence of a Failed System Shelly Gehshan AACDP Conference April 29, 2012 Overview of Pew s s findings Preventable dental conditions were the primary diagnosis in 830,590 visits to hospital
Dental ER Visits: Evidence of a Failed System Shelly Gehshan AACDP Conference April 29, 2012 Overview of Pew s s findings Preventable dental conditions were the primary diagnosis in 830,590 visits to hospital
Squamous Cell NSCLC: Differentiating Between Progression and Pseudoprogression
 Squamous Cell NSCLC: Differentiating Between Progression and Pseudoprogression David R. Spigel, M.D. Program Director, Lung Cancer Research Sarah Cannon Research Institute Nashville, TN Case of NR: Initial
Squamous Cell NSCLC: Differentiating Between Progression and Pseudoprogression David R. Spigel, M.D. Program Director, Lung Cancer Research Sarah Cannon Research Institute Nashville, TN Case of NR: Initial
ADEA Survey of Dental School Seniors, 2015 Graduating Class Tables Report
 ADEA Survey of Dental School Seniors, 2015 Graduating Class Tables Report Published March 2016 Suggested Citation American Dental Education Association. (March 2016). ADEA Survey of Dental School Seniors,
ADEA Survey of Dental School Seniors, 2015 Graduating Class Tables Report Published March 2016 Suggested Citation American Dental Education Association. (March 2016). ADEA Survey of Dental School Seniors,
Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults
 Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults Astha Singhal BDS, MPH, PhD Assistant Professor, Health Policy & Health Services Research Boston University Henry
Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults Astha Singhal BDS, MPH, PhD Assistant Professor, Health Policy & Health Services Research Boston University Henry
Michael A. Preston, Ph.D., M.P.H. University of Arkansas for Medical
 Michael A. Preston, Ph.D., M.P.H. University of Arkansas for Medical Sciences mapreston@uams.edu @MDonP Community Health Centers of Arkansas Annual Conference Little Rock, AR 27 September 2018 No Financial
Michael A. Preston, Ph.D., M.P.H. University of Arkansas for Medical Sciences mapreston@uams.edu @MDonP Community Health Centers of Arkansas Annual Conference Little Rock, AR 27 September 2018 No Financial
Considerations for State Obesity Policy
 Considerations for State Obesity Policy Scott Kahan, MD, MPH Faculty, Johns Hopkins Bloomberg School of Public Health Director, National Center for Weight & Wellness Clinical Director, STOP Obesity Alliance,
Considerations for State Obesity Policy Scott Kahan, MD, MPH Faculty, Johns Hopkins Bloomberg School of Public Health Director, National Center for Weight & Wellness Clinical Director, STOP Obesity Alliance,
Director s Update Brief novel 2009-H1N1. Tuesday 21 JUL EDT Day 95. Week of: Explaining the burden of disease and aligning resources
 Director s Update Brief novel 9-H1N1 Tuesday 1 JUL 9 815 EDT Day 95 Week of: Explaining the burden of disease and aligning resources Key Events novel 9-H1N1 Declarations WHO: Pandemic Phase (11 JUN 9 1
Director s Update Brief novel 9-H1N1 Tuesday 1 JUL 9 815 EDT Day 95 Week of: Explaining the burden of disease and aligning resources Key Events novel 9-H1N1 Declarations WHO: Pandemic Phase (11 JUN 9 1
Black Women s Access to Health Insurance
 FACT SHEET Black Women s Access to Health Insurance APRIL 2018 Data released by the U.S. Census Bureau show that, despite significant health insurance gains since the Affordable Care Act (ACA) was implemented,
FACT SHEET Black Women s Access to Health Insurance APRIL 2018 Data released by the U.S. Census Bureau show that, despite significant health insurance gains since the Affordable Care Act (ACA) was implemented,
Voluntary Mental Health Treatment Laws for Minors & Length of Inpatient Stay. Tori Lallemont MPH Thesis: Maternal & Child Health June 6, 2007
 Voluntary Mental Health Treatment Laws for Minors & Length of Inpatient Stay Tori Lallemont MPH Thesis: Maternal & Child Health June 6, 2007 Introduction 1997: Nearly 300,000 children were admitted to
Voluntary Mental Health Treatment Laws for Minors & Length of Inpatient Stay Tori Lallemont MPH Thesis: Maternal & Child Health June 6, 2007 Introduction 1997: Nearly 300,000 children were admitted to
ARQ 087 Overview. FGFR Inhibitor. March 2017
 ARQ 087 Overview FGFR Inhibitor March 2017 Safe Harbor This presentation and other statements by ArQule contain forward-looking statements within the meaning of the Private Securities Litigation Reform
ARQ 087 Overview FGFR Inhibitor March 2017 Safe Harbor This presentation and other statements by ArQule contain forward-looking statements within the meaning of the Private Securities Litigation Reform
Oncology Drug Development
 Oncology Drug Development Advantages and challenges of using stratified clinical studies Simon J Hollingsworth The 18th Anti-Tumour Drug Development Forum 21 February Japan Stratified Medicine In The Clinic
Oncology Drug Development Advantages and challenges of using stratified clinical studies Simon J Hollingsworth The 18th Anti-Tumour Drug Development Forum 21 February Japan Stratified Medicine In The Clinic
A National and Statewide Perspective on the Opioid Crisis
 Insert CIO Name Here A National and Statewide Perspective on the Opioid Crisis Amy Peeples, MPA Deputy Director, National Center for Injury Prevention and Control Centers for Disease Control and Prevention
Insert CIO Name Here A National and Statewide Perspective on the Opioid Crisis Amy Peeples, MPA Deputy Director, National Center for Injury Prevention and Control Centers for Disease Control and Prevention
SNAP Outreach within Food Banks: A View From The Ground
 SNAP Outreach within Food Banks: A View From The Ground Shana Alford, Feeding America Colleen Heflin, University of Missouri Elaine Waxman, Feeding America FEEDING AMERICA + PARTNER NAME PARTNERSHIP DISCUSSION
SNAP Outreach within Food Banks: A View From The Ground Shana Alford, Feeding America Colleen Heflin, University of Missouri Elaine Waxman, Feeding America FEEDING AMERICA + PARTNER NAME PARTNERSHIP DISCUSSION
NCCN Non-Small Cell Lung Cancer V Meeting June 15, 2018
 Guideline Page and Request Illumina Inc. requesting to replace Testing should be conducted as part of broad molecular profiling with Consider NGS-based assays that include EGFR, ALK, ROS1, and BRAF as
Guideline Page and Request Illumina Inc. requesting to replace Testing should be conducted as part of broad molecular profiling with Consider NGS-based assays that include EGFR, ALK, ROS1, and BRAF as
MEDICAID FINANCING OF HPV VACCINE: Access for Low-Income Women
 MEDICAID FINANCING OF HPV VACCINE: Access for Low-Income Women For the American Public Health Association s 135 th Annual Meeting Alexandra Stewart, JD Department of Health Policy November 7, 2007 1 The
MEDICAID FINANCING OF HPV VACCINE: Access for Low-Income Women For the American Public Health Association s 135 th Annual Meeting Alexandra Stewart, JD Department of Health Policy November 7, 2007 1 The
Laws/Regulations Restricting Sale of Electronic Smoking Devices and Alternative Nicotine Products to Minors
 Laws/Regulations Restricting Sale of Electronic Smoking Devices and Alternative Nicotine to State E-Cigarettes Penalties f Penalties f Sale AL ALA. CODE 28-11-13 (2013) NO (under 19 prohibited) Civil citation
Laws/Regulations Restricting Sale of Electronic Smoking Devices and Alternative Nicotine to State E-Cigarettes Penalties f Penalties f Sale AL ALA. CODE 28-11-13 (2013) NO (under 19 prohibited) Civil citation
RAISE Network Webinar Series. Asian Smokers Quitline (ASQ): Promoting Cessation in Our Communities. March 17, :00 pm 2:00 pm PT
 RAISE Network Webinar Series Asian Smokers Quitline (ASQ): Promoting Cessation in Our Communities March 17, 2015 1:00 pm 2:00 pm PT National Council of Asian Pacific Americans Website: www.ncapaonline.org
RAISE Network Webinar Series Asian Smokers Quitline (ASQ): Promoting Cessation in Our Communities March 17, 2015 1:00 pm 2:00 pm PT National Council of Asian Pacific Americans Website: www.ncapaonline.org
Treatment Landscape in R/R DLBCL Novel Targets and Strategies. Wyndham H. Wilson, M.D., Ph.D. Senior Investigator
 Treatment Landscape in R/R DLBCL Novel Targets and Strategies Wyndham H. Wilson, M.D., Ph.D. Senior Investigator Gene-expression profiling of DLBCL subtypes Roschewski, M. et al. (2013) Nat. Rev. Clin.
Treatment Landscape in R/R DLBCL Novel Targets and Strategies Wyndham H. Wilson, M.D., Ph.D. Senior Investigator Gene-expression profiling of DLBCL subtypes Roschewski, M. et al. (2013) Nat. Rev. Clin.
Comprehensive Genomic Profiling, in record time. Accurate. Clinically Proven. Fast.
 Comprehensive Genomic Profiling, in record time Accurate. ly Proven. Fast. PCDx advantages Comprehensive genomic profiling, in record time PCDx Comprehensive Genomic Profiling (CGP) provides precise information
Comprehensive Genomic Profiling, in record time Accurate. ly Proven. Fast. PCDx advantages Comprehensive genomic profiling, in record time PCDx Comprehensive Genomic Profiling (CGP) provides precise information
Recent Update in Management of Breast Cancer: Medical Oncology. Jin Hee Ahn, M.D., PhD. 23-April-2015
 2015 GBCC & 4 th IBCS 1/37 Recent Update in Management of Breast Cancer: Medical Oncology Jin Hee Ahn, M.D., PhD. 23-April-2015 Department of Oncology, Asan Medical Center, UUCM, Seoul, Korea 2/37 3/37
2015 GBCC & 4 th IBCS 1/37 Recent Update in Management of Breast Cancer: Medical Oncology Jin Hee Ahn, M.D., PhD. 23-April-2015 Department of Oncology, Asan Medical Center, UUCM, Seoul, Korea 2/37 3/37
Eylea (aflibercept) Document Number: IC-0026
 Eylea (aflibercept) Document Number: IC-0026 Last Review Date: 3/1/2018 Date of Origin: 02/07/2013 Dates Reviewed: 03/07/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 04/2015,
Eylea (aflibercept) Document Number: IC-0026 Last Review Date: 3/1/2018 Date of Origin: 02/07/2013 Dates Reviewed: 03/07/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 04/2015,
Q1 What is your age?
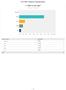 Q What is your age? Answ ered: 9 Skipped: Under 3 3-33 34-37 38-4 4+ Under 3 3-33 34-37 38-4 4+ 68.42% 3 5.79% 3.53% 2 5.26% Total 9 / 35 Q2 What is your gender? Answ ered: 9 Skipped: Male Female Male
Q What is your age? Answ ered: 9 Skipped: Under 3 3-33 34-37 38-4 4+ Under 3 3-33 34-37 38-4 4+ 68.42% 3 5.79% 3.53% 2 5.26% Total 9 / 35 Q2 What is your gender? Answ ered: 9 Skipped: Male Female Male
Report to Congressional Defense Committees
 Report to Congressional Defense Committees The Department of Defense Comprehensive Autism Care Demonstration Quarterly Report to Congress Second Quarter, Fiscal Year 2017 In Response to: Senate Report
Report to Congressional Defense Committees The Department of Defense Comprehensive Autism Care Demonstration Quarterly Report to Congress Second Quarter, Fiscal Year 2017 In Response to: Senate Report
Strategies to Increase Hepatitis C Treatment Within ADAPs
 Strategies to Increase Hepatitis C Treatment Within ADAPs Amanda Bowes National Alliance of State & Territorial AIDS Directors (NASTAD) Disclosures Presenter(s) has no financial interest to disclose. Learning
Strategies to Increase Hepatitis C Treatment Within ADAPs Amanda Bowes National Alliance of State & Territorial AIDS Directors (NASTAD) Disclosures Presenter(s) has no financial interest to disclose. Learning
Number of fatal work injuries,
 Number of fatal work injuries, 1992 2010 Number of fatal work injuries 7,000 6,217 6,331 6,632 6,275 6,202 6,238 6,055 6,054 5,920 5,915 6,000 5,534 5,575 5,764 5,734 5,840 5,657 5,214 5,000 4,551 4,690
Number of fatal work injuries, 1992 2010 Number of fatal work injuries 7,000 6,217 6,331 6,632 6,275 6,202 6,238 6,055 6,054 5,920 5,915 6,000 5,534 5,575 5,764 5,734 5,840 5,657 5,214 5,000 4,551 4,690
CDC s National Comprehensive Cancer Control Program (NCCCP): 2010 Priorities and New Program Opportunities
 CDC s National Comprehensive Cancer Control Program (NCCCP): 2010 Priorities and New Program Opportunities Laura Seeff MD Chief, Comprehensive Cancer Control Branch Division of Cancer Prevention and Control
CDC s National Comprehensive Cancer Control Program (NCCCP): 2010 Priorities and New Program Opportunities Laura Seeff MD Chief, Comprehensive Cancer Control Branch Division of Cancer Prevention and Control
