GATTEX (Teduglutide) for Injection REMS Program: Prescriber Education
|
|
|
- Margaret Merritt
- 5 years ago
- Views:
Transcription
1 GATTEX (Teduglutide) for Injection REMS Program: Prescriber Education Shire-NPS Pharmaceuticals, Inc. 300 Shire Way, Lexington, MA A REMS (Risk Evaluation and Mitigation Strategy) is a program required by the FDA to manage known or potential serious risks associated with a drug product. The GATTEX Prescriber Education Slide Deck is required by the FDA as part of the GATTEX REMS Program. S /18
2 Table of Contents Topic Slide Indication 3 Overview: Important Adverse Reactions of Special Interest 4 Possible Acceleration of Neoplastic Growth 5 Possible Enhanced Growth of Colorectal Polyps 7 Gastrointestinal Obstruction 9 Gallbladder and Biliary Tract Disease 11 Pancreatic Disease 13 Fluid Overload 15 Increased Absorption of Concomitant Oral Medication 18 2
3 Indication GATTEX (teduglutide) for injection is indicated for the treatment of adult patients with Short Bowel Syndrome (SBS) who are dependent on parenteral support. Teduglutide is a recombinant analog of GLP-2 GLP-2, glucagon-like peptide-2 3
4 Overview Important Adverse Reactions of Special Interest Possible safety risks with GATTEX Possible acceleration of neoplastic growth and enhanced growth of colorectal polyps Gastrointestinal obstruction Gallbladder, biliary tract and pancreatic disease Increased absorption of fluids leading to fluid overload in patients with cardiovascular disease Increased absorption of oral medications with narrow therapeutic index 4
5 Possible Acceleration of Neoplastic Growth GLP-2 receptors are localized mainly in the GI tract 1 GATTEX promotes growth of intestinal epithelial cells in the GI tract It can not be excluded that GATTEX promotes growth of existing neoplasms in the GI tract 3 patients on GATTEX were reported with neoplasms*: 2 cases of lung cancer with smoking history 1 case of GI metastatic adenocarcinoma (unknown origin) following abdominal radiation for Hodgkin s disease 1. Munroe DG et al. Proc Natl Acad Sci. 1999; 96: * As of January 24,
6 Possible Acceleration of Neoplastic Growth GATTEX Label Warnings and Precautions Possible Acceleration of Neoplastic Growth Based on the pharmacologic activity and findings in animals, GATTEX has the potential to cause hyperplastic changes including neoplasia. Based on benign tumor findings in the rat carcinogenicity study, patients should be monitored clinically for small bowel neoplasia. If a benign neoplasm is found, it should be removed. In case of small bowel cancer, GATTEX therapy should be discontinued. In patients with active gastrointestinal malignancy (GI tract, hepatobiliary, pancreatic), GATTEX therapy should be discontinued. In patients with active non-gastrointestinal malignancy, the clinical decision to continue GATTEX should be made based on risk-benefit considerations. In patients at increased risk for malignancy, the clinical decision to use GATTEX should be considered only if the benefits outweigh the risks. 6
7 Possible Enhanced Growth of Colorectal Polyps 11/173 (6.4%) GATTEX-treated patients developed GI polyps in pooled Phase III SBS studies* 2 villous adenomas 3 hyperplastic 3 tubular adenomas 1 serrated adenomas 1 inflammatory 1 biopsy not done GATTEX mechanism of action and nonclinical data are consistent with a potential to enhance growth of polyps * As of January 24,
8 Possible Enhanced Growth of Colorectal Polyps GATTEX Label Warnings and Precautions Colorectal Polyps Colonoscopy of the entire colon with removal of polyps should be done within 6 months prior to starting treatment with GATTEX. A follow-up colonoscopy (or alternate imaging) is recommended at the end of 1 year of GATTEX. Subsequent colonoscopies should be done every 5 years or more often as needed. If a polyp is found, adherence to current polyp follow-up guidelines is recommended. In case of diagnosis of colorectal cancer, GATTEX therapy should be discontinued. 8
9 Gastrointestinal Obstruction 12 patients experienced one or more episodes of intestinal obstruction/stenosis* 6 in SBS placebo-controlled studies 3/77 (3.9%) on GATTEX, 0.05 mg/kg/day 3/32 (9.4%) on GATTEX, 0.10 mg/kg/day None in placebo-group Onset 1 day to 6 months 6 in the extension studies (all on GATTEX, 0.05 mg/kg/day) Onset 6 days to 19 months Of all of these patients, 2 patients required endoscopic dilatation; and one required surgical intervention * As of January 24,
10 Gastrointestinal Obstruction GATTEX Label Warnings and Precautions Intestinal Obstruction Intestinal obstruction has been reported in clinical trials. In patients who develop intestinal or stomal obstruction, GATTEX should be temporarily discontinued while the patient is clinically managed. GATTEX may be restarted when the obstructive presentation resolves, if clinically indicated. 10
11 Gallbladder and Biliary Tract Disease 13/173 (7.5%) of GATTEX-treated patients reported biliary events, including cholecystitis and gallstones/sludge in pooled Phase III SBS studies* 5 patients had a history of biliary disease None of these events resulted in study withdrawal * As of January 24,
12 Gallbladder and Biliary Tract Disease GATTEX Label Warnings and Precautions Gallbladder and Biliary Tract Disease Cholecystitis, cholangitis, and cholelithiasis have been reported in clinical studies. Patients must undergo initial (within 6 months prior) laboratory assessment of bilirubin and alkaline phosphatase. Subsequent laboratory assessments are recommended every 6 months; if a clinically meaningful elevation is seen imaging of the biliary tract is recommended to identify possible obstruction. 12
13 Pancreatic Disease 3/173 (1.7%) of GATTEX-treated patients developed pancreatitis in pooled Phase III SBS studies* All 3 patients had a history of pancreatitis None of these events resulted in study withdrawal * As of January 24,
14 Pancreatic Disease GATTEX Label Warnings and Precautions Pancreatic Disease Pancreatitis has been reported in clinical studies. Patients must undergo initial (within 6 months prior) laboratory assessment of lipase and amylase. Subsequent laboratory assessments are recommended every 6 months; if a clinically meaningful elevation is seen imaging of the pancreas is recommended to identify possible obstruction. 14
15 Fluid Overload 23/173 (13.3%) of patients treated with GATTEX reported fluid overload in pooled Phase III SBS studies* Fluid overload should be considered when administering GATTEX in patients with underlying heart disease * As of January 24,
16 Fluid Overload GATTEX Label Warnings and Precautions Cardiovascular Disease Due to increased intestinal fluid absorption, patients with cardiovascular disease, such as cardiac insufficiency and hypertension, should be monitored with regard to fluid overload, especially during initiation of therapy. Parenteral nutrition/intravenous (PN/IV) fluid volume should be reassessed relative to signs of fluid overload. In case of a significant deterioration of the cardiovascular disease, the need for continued GATTEX treatment should be reassessed. 16
17 PN/IV Volume Adjustment In order to reduce risk for fluid overload the following PN/IV volume adjustment algorithm is suggested Determine pre-treatment urine output (ideally 1 to 2 L/day) Determine urine output 2 to 4 weeks after starting treatment Reduce weekly PN/IV volume by 10% to 30% if urine output increased at least 10% compared with pre-treatment volume Evaluate if the patient tolerated the PN/IV reduction 1 to 2 weeks later Continue monitoring urine output on a regular basis and adjust PN/IV volume accordingly with the goal of reducing or achieving complete independence from PN/IV support and maintaining clinical nutrition status 17
18 Increased Absorption of Concomitant Oral Medication Based on the pharmacodynamic effect of GATTEX, there is a potential for increased absorption of concomitant oral medications Considerations should be given for dosage adjustment of concomitant oral medication requiring titration or that have a narrow therapeutic index 18
19 Increased Absorption of Concomitant Oral Medication GATTEX Label Warnings and Precautions Risks Resulting from Increased Absorption of Concomitant Oral Medication Altered mental status in association with GATTEX has been observed in patients on benzodiazepines in clinical trials. Patients on concomitant oral drugs (e.g., benzodiazepines, phenothiazines) requiring titration or with a narrow therapeutic index may require dose adjustment while on GATTEX. 19
Gattex. Gattex (teduglutide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.03 Subject: Gattex Page: 1 of 5 Last Review Date: March 16, 2018 Gattex Description Gattex (teduglutide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.03 Subject: Gattex Page: 1 of 5 Last Review Date: March 16, 2018 Gattex Description Gattex (teduglutide)
Gattex. Gattex (teduglutide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.09.03 Subject: Gattex Page: 1 of 5 Last Review Date: September 18, 2015 Gattex Description Gattex (teduglutide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.09.03 Subject: Gattex Page: 1 of 5 Last Review Date: September 18, 2015 Gattex Description Gattex (teduglutide)
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility 3 DOSAGE FORMS AND STRENGTHS
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GATTEX safely and effectively. See full prescribing information for GATTEX. GATTEX (teduglutide [rdna
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GATTEX safely and effectively. See full prescribing information for GATTEX. GATTEX (teduglutide [rdna
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION
 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION Pr REVESTIVE * Teduglutide for injection Powder for solution for injection 5 mg per vial Alimentary tract and metabolism products ATC Code: A16AX08
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION Pr REVESTIVE * Teduglutide for injection Powder for solution for injection 5 mg per vial Alimentary tract and metabolism products ATC Code: A16AX08
PRODUCT INFORMATION. REVESTIVE (teduglutide)
 PRODUCT INFORMATION REVESTIVE (teduglutide) NAME OF THE MEDICINE REVESTIVE Active ingredient: teduglutide The active ingredient teduglutide (rdna origin) is a 33 amino acid glucagon-like peptide-2 (GLP-2)
PRODUCT INFORMATION REVESTIVE (teduglutide) NAME OF THE MEDICINE REVESTIVE Active ingredient: teduglutide The active ingredient teduglutide (rdna origin) is a 33 amino acid glucagon-like peptide-2 (GLP-2)
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
teduglutide [rdna origin] (Gattex )
![teduglutide [rdna origin] (Gattex ) teduglutide [rdna origin] (Gattex )](/thumbs/87/95830651.jpg) Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
GATTEX (teduglutide [rdna origin])
![GATTEX (teduglutide [rdna origin]) GATTEX (teduglutide [rdna origin])](/thumbs/89/99443265.jpg) GATTEX (teduglutide [rdna origin]) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
GATTEX (teduglutide [rdna origin]) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Developing treatment options for patients with rare gastrointestinal and endocrine disorders and unmet medical need
 Developing treatment options for patients with rare gastrointestinal and endocrine disorders and unmet medical need Luke Beshar, senior vice president and chief financial officer BioNJ BioPartnering 2011
Developing treatment options for patients with rare gastrointestinal and endocrine disorders and unmet medical need Luke Beshar, senior vice president and chief financial officer BioNJ BioPartnering 2011
Understanding the Benefits and Risks
 LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The LOTRONEX REMS Program Prescriber Education Slide Deck LOTRONEX is a registered trademark of Prometheus
LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The LOTRONEX REMS Program Prescriber Education Slide Deck LOTRONEX is a registered trademark of Prometheus
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines
 Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Outcomes from a 12-Week, Open-Label, Multicenter Clinical Trial of Teduglutide in Pediatric Short Bowel Syndrome.
 Outcomes from a 12-Week, Open-Label, Multicenter Clinical Trial of Teduglutide in Pediatric Short Bowel Syndrome. Carter BA 1, Cohran VC 2, Cole CR 3, Corkins MR 4, Dimmitt RA 5, Duggan C 6, Hill S 7,
Outcomes from a 12-Week, Open-Label, Multicenter Clinical Trial of Teduglutide in Pediatric Short Bowel Syndrome. Carter BA 1, Cohran VC 2, Cole CR 3, Corkins MR 4, Dimmitt RA 5, Duggan C 6, Hill S 7,
Management of Short Bowel Syndrome in the Era of Teduglutide. Charlene Compher, PhD, RD University of Pennsylvania
 Management of Short Bowel Syndrome in the Era of Teduglutide Charlene Compher, PhD, RD University of Pennsylvania compherc@nursing.upenn.edu Disclosures Research funding for clinical trials by NPS Pharmaceuticals
Management of Short Bowel Syndrome in the Era of Teduglutide Charlene Compher, PhD, RD University of Pennsylvania compherc@nursing.upenn.edu Disclosures Research funding for clinical trials by NPS Pharmaceuticals
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Outcome High Priority
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Safety and Efficacy of Teduglutide After 52 Weeks of Treatment in Patients With Short Bowel Syndrome Intestinal Failure
 Safety and Efficacy of Teduglutide After 52 Weeks of Treatment in Patients With Short Bowel Syndrome Intestinal Failure O keefe, S.J.D et al, Clinical Gastroenterology and Hepatology Accepted date: 21
Safety and Efficacy of Teduglutide After 52 Weeks of Treatment in Patients With Short Bowel Syndrome Intestinal Failure O keefe, S.J.D et al, Clinical Gastroenterology and Hepatology Accepted date: 21
Surveying the Colon; Polyps and Advances in Polypectomy
 Surveying the Colon; Polyps and Advances in Polypectomy Educational Objectives Identify classifications of polyps Describe several types of polyps Verbalize rationale for polypectomy Identify risk factors
Surveying the Colon; Polyps and Advances in Polypectomy Educational Objectives Identify classifications of polyps Describe several types of polyps Verbalize rationale for polypectomy Identify risk factors
Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care
 Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: The percentage
Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: The percentage
LOTRONEX and its authorized generic alosetron hydrochloride:
 LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The Prescribing Program for LOTRONEX TM Prescriber Education Slide Deck PROMETHEUS and the Link Design
LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The Prescribing Program for LOTRONEX TM Prescriber Education Slide Deck PROMETHEUS and the Link Design
Dosing and Administration Guide for once-daily NATPARA (parathyroid hormone) for Injection
 Dosing and Administration Guide for once-daily NATPARA (parathyroid hormone) for Injection Indications and Usage NATPARA is a parathyroid hormone indicated as an adjunct to calcium and vitamin D to control
Dosing and Administration Guide for once-daily NATPARA (parathyroid hormone) for Injection Indications and Usage NATPARA is a parathyroid hormone indicated as an adjunct to calcium and vitamin D to control
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary
 Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Agent Indication Dosing and Administration Natpara (parathyroid hormone) subcutaneous
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Agent Indication Dosing and Administration Natpara (parathyroid hormone) subcutaneous
GIQIC18 Appropriate follow-up interval of not less than 5 years for colonoscopies with findings of 1-2 tubular adenomas < 10 mm
 GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
Pharmacotherapy IV: Liraglutide for Chronic Weight Management SARAH CAWSEY MD, FRCPC 2 ND ANNUAL OBESITY UPDATE SEPTEMBER 22, 2018
 Pharmacotherapy IV: Liraglutide for Chronic Weight Management SARAH CAWSEY MD, FRCPC 2 ND ANNUAL OBESITY UPDATE SEPTEMBER 22, 2018 Disclosures Faculty Assistant Clinical Professor, Department of Medicine,
Pharmacotherapy IV: Liraglutide for Chronic Weight Management SARAH CAWSEY MD, FRCPC 2 ND ANNUAL OBESITY UPDATE SEPTEMBER 22, 2018 Disclosures Faculty Assistant Clinical Professor, Department of Medicine,
AESOP Overview and Inclusion/Exclusion Criteria Richard Pencek, PhD
 747-207 AESOP Overview and Inclusion/ Criteria Richard Pencek, PhD Sr Director, Clinical Research, Intercept Pharmaceuticals, Inc. 2 PSC Forum 2 AESOP: A Phase 2 Randomized, Placebo-Controlled Trial, Dose-Finding
747-207 AESOP Overview and Inclusion/ Criteria Richard Pencek, PhD Sr Director, Clinical Research, Intercept Pharmaceuticals, Inc. 2 PSC Forum 2 AESOP: A Phase 2 Randomized, Placebo-Controlled Trial, Dose-Finding
Gastroenterology Fellowship Program
 Gastroenterology Fellowship Program Outpatient Clinical Rotations I. Overview A. Three Year Continuity Clinic Experience All gastroenterology fellows will be required to have a ½ day continuity clinic
Gastroenterology Fellowship Program Outpatient Clinical Rotations I. Overview A. Three Year Continuity Clinic Experience All gastroenterology fellows will be required to have a ½ day continuity clinic
Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci. Colon polyps. Colorectal cancer
 Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci Colon polyps Colorectal cancer Harrison s Principles of Internal Medicine 18 Ed. 2012 Colorectal cancer 70% Colorectal cancer CRC and colon
Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci Colon polyps Colorectal cancer Harrison s Principles of Internal Medicine 18 Ed. 2012 Colorectal cancer 70% Colorectal cancer CRC and colon
Appendix 4: WHO Classification of Tumours of the pancreas 17
 S3.01 The WHO histological tumour type must be recorded. CS3.01a The histological type of the tumour should be recorded based on the current WHO classification 17 (refer to Appendices 4-7). Appendix 4:
S3.01 The WHO histological tumour type must be recorded. CS3.01a The histological type of the tumour should be recorded based on the current WHO classification 17 (refer to Appendices 4-7). Appendix 4:
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer
 Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Case Scenario 1. Discharge Summary
 Case Scenario 1 Discharge Summary A 69-year-old woman was on vacation and noted that she was becoming jaundiced. Two months prior to leaving on that trip, she had had a workup that included an abdominal
Case Scenario 1 Discharge Summary A 69-year-old woman was on vacation and noted that she was becoming jaundiced. Two months prior to leaving on that trip, she had had a workup that included an abdominal
Pathology in Slovenian CRC screening programme:
 Pathology in Slovenian CRC screening programme: Findings, organisation and quality assurance Snježana Frković Grazio University Medical Center Ljubljana, Slovenia Slovenia s population: 2 million Incidence
Pathology in Slovenian CRC screening programme: Findings, organisation and quality assurance Snježana Frković Grazio University Medical Center Ljubljana, Slovenia Slovenia s population: 2 million Incidence
6 semanas de embarazo. Tubulovillous adenoma with dysplasia icd 10. Inicio / Embarazo / 6 semanas de embarazo
 Inicio / Embarazo / 6 semanas de embarazo 6 semanas de embarazo Tubulovillous adenoma with dysplasia icd 10 Free, official coding info for 2018 ICD-10-CM D13.2 - includes detailed rules, notes, synonyms,
Inicio / Embarazo / 6 semanas de embarazo 6 semanas de embarazo Tubulovillous adenoma with dysplasia icd 10 Free, official coding info for 2018 ICD-10-CM D13.2 - includes detailed rules, notes, synonyms,
PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG. (goserelin)
 ONC.000-092-861.10.0 PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG (goserelin) Name of the medicinal product Zoladex LA 10.8mg depot Qualitative and quantitative composition Goserelin acetate (equivalent to 10.8
ONC.000-092-861.10.0 PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG (goserelin) Name of the medicinal product Zoladex LA 10.8mg depot Qualitative and quantitative composition Goserelin acetate (equivalent to 10.8
A LEADER IN ADVANCED ENDOSCOPY AND HEPATOBILIARY SURGERY
 A LEADER IN ADVANCED ENDOSCOPY AND HEPATOBILIARY SURGERY St. Peter s Hospital Advanced Endoscopy & Hepatobiliary Center Welcome The St. Peter s Hospital Advanced Endoscopy & Hepatobiliary Center is a leader
A LEADER IN ADVANCED ENDOSCOPY AND HEPATOBILIARY SURGERY St. Peter s Hospital Advanced Endoscopy & Hepatobiliary Center Welcome The St. Peter s Hospital Advanced Endoscopy & Hepatobiliary Center is a leader
Gastroenterology. Certification Examination Blueprint. Purpose of the exam
 Gastroenterology Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the certified gastroenterologist
Gastroenterology Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the certified gastroenterologist
Colonic Polyp. Najmeh Aletaha. MD
 Colonic Polyp Najmeh Aletaha. MD 1 Polyps & classification 2 Colorectal cancer risk factors 3 Pathogenesis 4 Surveillance polyp of the colon refers to a protuberance into the lumen above the surrounding
Colonic Polyp Najmeh Aletaha. MD 1 Polyps & classification 2 Colorectal cancer risk factors 3 Pathogenesis 4 Surveillance polyp of the colon refers to a protuberance into the lumen above the surrounding
Lahey Clinic Internal Medicine Residency Program: Curriculum for Gastroenterology
 Lahey Clinic Internal Medicine Residency Program: Curriculum for Gastroenterology Faculty representative: David L. Burns, MD, CNSP Resident representative: Tom Castiglione, MD Revision date: March 6, 2006
Lahey Clinic Internal Medicine Residency Program: Curriculum for Gastroenterology Faculty representative: David L. Burns, MD, CNSP Resident representative: Tom Castiglione, MD Revision date: March 6, 2006
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
Case Study: #3: Gallbladder Carcinoma?
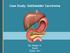 Case Study: #3: Gallbladder Carcinoma? By: Megan Wyatt K. SON Wyatt 225 2B1 RDMS, RVT Patient: Male 85 YOA Caucasian Indication: Elevated Alkaline Phosphatase History Annual physical showed elevated alkaline
Case Study: #3: Gallbladder Carcinoma? By: Megan Wyatt K. SON Wyatt 225 2B1 RDMS, RVT Patient: Male 85 YOA Caucasian Indication: Elevated Alkaline Phosphatase History Annual physical showed elevated alkaline
Pathology in Slovenian CRC screening programme: Organisation and quality assurance. Snježana Frković Grazio and Matej Bračko
 Pathology in Slovenian CRC screening programme: Organisation and quality assurance Snježana Frković Grazio and Matej Bračko June 2009 to December 2013 (first three rounds) 33 969 colonoscopies were performed
Pathology in Slovenian CRC screening programme: Organisation and quality assurance Snježana Frković Grazio and Matej Bračko June 2009 to December 2013 (first three rounds) 33 969 colonoscopies were performed
General and Colonoscopy Data Collection Form
 Identifier: Sociodemographic Information Type: Zip Code: Inpatient Outpatient Birth Date: m m d d y y y y Gender: Height: (inches) Male Female Ethnicity: Weight: (pounds) African American White, Non-Hispanic
Identifier: Sociodemographic Information Type: Zip Code: Inpatient Outpatient Birth Date: m m d d y y y y Gender: Height: (inches) Male Female Ethnicity: Weight: (pounds) African American White, Non-Hispanic
Enteral and parenteral nutrition in GI failure and short bowel syndrome
 Enteral and parenteral nutrition in GI failure and short bowel syndrome Alastair Forbes University College London Intestinal failure Inadequate functional intestine to allow health to be maintained by
Enteral and parenteral nutrition in GI failure and short bowel syndrome Alastair Forbes University College London Intestinal failure Inadequate functional intestine to allow health to be maintained by
Parenteral Nutrition. Outline. Potential Biomarkers for Use in Intestinal Adaptation. Jejunum is primary site of digestion and absorption
 Outline Potential Biomarkers for Use in Intestinal Adaptation Kelly A. Tappenden, Ph.D., R.D. Professor of Nutrition and GI Physiology 1. Intestinal Adaptation potential regulators 2. Intestinal mucosal
Outline Potential Biomarkers for Use in Intestinal Adaptation Kelly A. Tappenden, Ph.D., R.D. Professor of Nutrition and GI Physiology 1. Intestinal Adaptation potential regulators 2. Intestinal mucosal
Natpara. Natpara (parathyroid hormone) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.21 Subject: Natpara Page: 1 of 5 Last Review Date: December 3, 2015 Natpara Description Natpara (parathyroid
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.21 Subject: Natpara Page: 1 of 5 Last Review Date: December 3, 2015 Natpara Description Natpara (parathyroid
ACG Clinical Guideline: Primary Sclerosing Cholangitis
 ACG Clinical Guideline: Primary Sclerosing Cholangitis Keith D. Lindor, MD, FACG 1, Kris V. Kowdley, MD, FACG 2, and M. Edwyn Harrison, MD 3 1 College of Health Solutions, Arizona State University, Phoenix,
ACG Clinical Guideline: Primary Sclerosing Cholangitis Keith D. Lindor, MD, FACG 1, Kris V. Kowdley, MD, FACG 2, and M. Edwyn Harrison, MD 3 1 College of Health Solutions, Arizona State University, Phoenix,
WELCOME TO YOUR WORLD OF PBC TREATMENT Adding OCALIVA (obeticholic acid) to your treatment plan starts here.
 WELCOME TO YOUR WORLD OF PBC TREATMENT Adding OCALIVA (obeticholic acid) to your treatment plan starts here. Photograph of an actual patient What is OCALIVA? OCALIVA is a prescription medicine used to
WELCOME TO YOUR WORLD OF PBC TREATMENT Adding OCALIVA (obeticholic acid) to your treatment plan starts here. Photograph of an actual patient What is OCALIVA? OCALIVA is a prescription medicine used to
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary
 Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 2 Available Product Indication Dosing and Administration Natpara (parathyroid hormone)
Natpara (parathyroid hormone) Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 2 Available Product Indication Dosing and Administration Natpara (parathyroid hormone)
Gastro Intestinal Pathology
 Duration: 04 weeks (20 days) Gastro Intestinal 3/SBM-4/01 Alimentation in health Topic/ Concept Objectives Time Dept. 1. recall digestion, absorption and metabolism relating to, carbohydrates, proteins,
Duration: 04 weeks (20 days) Gastro Intestinal 3/SBM-4/01 Alimentation in health Topic/ Concept Objectives Time Dept. 1. recall digestion, absorption and metabolism relating to, carbohydrates, proteins,
GASTROINTESTINAL IMAGING STUDY GUIDE
 GASTROINTESTINAL IMAGING STUDY GUIDE Pharynx Diverticula Foreign bodies Trauma o Motility Disorders Esophagus Diverticula Trauma Esophagitis Barrett esophagus Rings, webs, and strictures Varices Benign
GASTROINTESTINAL IMAGING STUDY GUIDE Pharynx Diverticula Foreign bodies Trauma o Motility Disorders Esophagus Diverticula Trauma Esophagitis Barrett esophagus Rings, webs, and strictures Varices Benign
NATPARA (parathyroid hormone) for injection Risk Evaluation and Mitigation Strategy (REMS) Program
 NATPARA (parathyroid hormone) for injection Risk Evaluation and Mitigation Strategy (REMS) Program TRAINING MODULE FOR PHARMACY REPRESENTATIVES 1 Contents Introduction NATPARA (parathyroid hormone) for
NATPARA (parathyroid hormone) for injection Risk Evaluation and Mitigation Strategy (REMS) Program TRAINING MODULE FOR PHARMACY REPRESENTATIVES 1 Contents Introduction NATPARA (parathyroid hormone) for
What is the right calcium balance?
 For patients with hypoparathyroidism What is the right calcium balance? Indications and Usage1 NATPARA is a parathyroid hormone indicated as an adjunct to calcium and vitamin D to control hypocalcemia
For patients with hypoparathyroidism What is the right calcium balance? Indications and Usage1 NATPARA is a parathyroid hormone indicated as an adjunct to calcium and vitamin D to control hypocalcemia
Colon Cancer Screening. Layth Al-Jashaami, MD GI Fellow, PGY 4
 Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
EVALUATION FORM WSGNA 2018 Fall Conference Endoscopy for Malignant and Premalignant Lesions of the GI Tract 10/27/2018
 EVALUATION FORM WSGNA 2018 Fall Conference Endoscopy for Malignant and Premalignant Lesions of the GI Tract 10/27/2018 Please respond to the following items on a scale from 5 (highest) to 1 (lowest). The
EVALUATION FORM WSGNA 2018 Fall Conference Endoscopy for Malignant and Premalignant Lesions of the GI Tract 10/27/2018 Please respond to the following items on a scale from 5 (highest) to 1 (lowest). The
NEURO- GASTRO- ENTEROLOGY & MOTILITY OESO- PHAGUS LOGY. Room A Room B Room C Room E1 Room E2 Room M Room N1 Room N2 Room L8 Room 1.
 ONCO ENTERO RADIO & SATURDAY SUNDAY MONDAY TUESDAY WEDNESDAY Saturday, October 15, 2016 Room A Room B Room C Room E1 09:00-10:30 Peptic ulcer : Still important for gastroenterologists 11:00-13:00 Video
ONCO ENTERO RADIO & SATURDAY SUNDAY MONDAY TUESDAY WEDNESDAY Saturday, October 15, 2016 Room A Room B Room C Room E1 09:00-10:30 Peptic ulcer : Still important for gastroenterologists 11:00-13:00 Video
Title Description Type / Priority
 Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
GASTROENTEROLOGY Maintenance of Certification (MOC) Examination Blueprint
 GASTROENTEROLOGY Maintenance of Certification (MOC) Examination Blueprint ABIM invites diplomates to help develop the Gastroenterology MOC exam blueprint Based on feedback from physicians that MOC assessments
GASTROENTEROLOGY Maintenance of Certification (MOC) Examination Blueprint ABIM invites diplomates to help develop the Gastroenterology MOC exam blueprint Based on feedback from physicians that MOC assessments
Stage 4 gastric adenocarcinoma icd 10
 > Stage 4 gastric adenocarcinoma icd 10 stage iii; Carcinoma of colon, stage iv; Colon cancer metastatic to unspecified site; Hereditary nonpolyposis colon cancer; Malignant tumor of colon; Metastasis.
> Stage 4 gastric adenocarcinoma icd 10 stage iii; Carcinoma of colon, stage iv; Colon cancer metastatic to unspecified site; Hereditary nonpolyposis colon cancer; Malignant tumor of colon; Metastasis.
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION OZEMPIC. semaglutide injection. 2 mg/pen (1.34 mg/ml)
 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION OZEMPIC semaglutide injection 2 mg/pen (1.34 mg/ml) Pre-filled pen delivering doses of 0.25 mg or 0.5 mg and Pre-filled pen delivering doses of
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION OZEMPIC semaglutide injection 2 mg/pen (1.34 mg/ml) Pre-filled pen delivering doses of 0.25 mg or 0.5 mg and Pre-filled pen delivering doses of
Appendix 1 (as supplied by the authors): Supplementary tables. Supplementary Table A1. Description of OHIP codes used in the current study.
 Appendix 1 (as supplied by the authors): Supplementary tables Supplementary Table A1. Description of OHIP codes used in the current study. OHIP Billing Code OHIP Billing Code Description Colonoscopy and
Appendix 1 (as supplied by the authors): Supplementary tables Supplementary Table A1. Description of OHIP codes used in the current study. OHIP Billing Code OHIP Billing Code Description Colonoscopy and
NATPARA (parathyroid hormone) for injection Risk Evaluation and Mitigation Strategy (REMS) Program
 NATPARA (parathyroid hormone) for injection Risk Evaluation and Mitigation Strategy (REMS) Program PRESCRIBER TRAINING MODULE 1 Contents Introduction NATPARA (parathyroid hormone) for injection Indication
NATPARA (parathyroid hormone) for injection Risk Evaluation and Mitigation Strategy (REMS) Program PRESCRIBER TRAINING MODULE 1 Contents Introduction NATPARA (parathyroid hormone) for injection Indication
Objectives. Definitions. Colorectal Cancer Screening 5/8/2018. Payam Afshar, MS, MD Kaiser Permanente, San Diego. Colorectal cancer background
 Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Case 1- B.N. 66 yr old F with PMHx of breast cancer s/ p mastectomy, HTN, DM presented with dysphagia to solids and liquids.
 Case 1- B.N 66 yr old F with PMHx of breast cancer s/ p mastectomy, HTN, DM presented with dysphagia to solids and liquids. Reports retching to clear esophagus. Case 1- B.N EGD: Stricture in the distal
Case 1- B.N 66 yr old F with PMHx of breast cancer s/ p mastectomy, HTN, DM presented with dysphagia to solids and liquids. Reports retching to clear esophagus. Case 1- B.N EGD: Stricture in the distal
DOSING AND ADMINISTRATION GUIDE
 DOSING AND ADMINISTRATION GUIDE Indication TAVALISSE is a kinase inhibitor indicated for the treatment of thrombocytopenia in adult patients with chronic immune thrombocytopenia (ITP) who have had an insufficient
DOSING AND ADMINISTRATION GUIDE Indication TAVALISSE is a kinase inhibitor indicated for the treatment of thrombocytopenia in adult patients with chronic immune thrombocytopenia (ITP) who have had an insufficient
DATA SHEET. 1. CREON 10,000 Capsules CREON 25,000 Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 3. PHARMACEUTICAL FORM 4. CLINICAL PARTICULARS
 1. CREON 10,000 Capsules CREON 25,000 Capsules DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Pancreatin, produced from porcine pancreatic tissue For a full list of excipients, see Section 6.1
1. CREON 10,000 Capsules CREON 25,000 Capsules DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Pancreatin, produced from porcine pancreatic tissue For a full list of excipients, see Section 6.1
Management of Patients with Past or Present Hepatic Abnormalities
 S21 Management of Patients with Past or Present Hepatic Abnormalities Evidence Based Medicine Official recommendations Expert opinion Course of action before tocilizumab therapy in patients with a history
S21 Management of Patients with Past or Present Hepatic Abnormalities Evidence Based Medicine Official recommendations Expert opinion Course of action before tocilizumab therapy in patients with a history
Corporate Medical Policy
 Corporate Medical Policy Chromoendoscopy as an Adjunct to Colonoscopy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: chromoendoscopy_as_an_adjunct_to_colonoscopy 7/2012 11/2017
Corporate Medical Policy Chromoendoscopy as an Adjunct to Colonoscopy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: chromoendoscopy_as_an_adjunct_to_colonoscopy 7/2012 11/2017
Summary of the risk management plan (RMP) for Synjardy (empagliflozin / metformin)
 EMA/217413/2015 Summary of the risk management plan (RMP) for Synjardy (empagliflozin / metformin) This is a summary of the risk management plan (RMP) for Synjardy, which details the measures to be taken
EMA/217413/2015 Summary of the risk management plan (RMP) for Synjardy (empagliflozin / metformin) This is a summary of the risk management plan (RMP) for Synjardy, which details the measures to be taken
Summary. Cezary ŁozińskiABDF, Witold KyclerABCDEF. Rep Pract Oncol Radiother, 2007; 12(4):
 Rep Pract Oncol Radiother, 2007; 12(4): 201-206 Original Paper Received: 2006.12.19 Accepted: 2007.04.02 Published: 2007.08.31 Authors Contribution: A Study Design B Data Collection C Statistical Analysis
Rep Pract Oncol Radiother, 2007; 12(4): 201-206 Original Paper Received: 2006.12.19 Accepted: 2007.04.02 Published: 2007.08.31 Authors Contribution: A Study Design B Data Collection C Statistical Analysis
Hyperplastische Polyps Innocent bystanders?
 Hyperplastische Polyps Innocent bystanders?? K. Geboes P th l i h O tl dk d Pathologische Ontleedkunde, KULeuven Content Historical Classification Relation Hyperplastic polyps carcinoma The concept cept
Hyperplastische Polyps Innocent bystanders?? K. Geboes P th l i h O tl dk d Pathologische Ontleedkunde, KULeuven Content Historical Classification Relation Hyperplastic polyps carcinoma The concept cept
When is a programmed follow-up meaningful and how should it be done? Professor Alastair Watson University of Liverpool
 When is a programmed follow-up meaningful and how should it be done? Professor Alastair Watson University of Liverpool Adenomas/Carcinoma Sequence Providing Time for Screening Normal 5-20 yrs 5-15 yrs
When is a programmed follow-up meaningful and how should it be done? Professor Alastair Watson University of Liverpool Adenomas/Carcinoma Sequence Providing Time for Screening Normal 5-20 yrs 5-15 yrs
Navigating the Biliary Tract with CT & MR: An Imaging Approach to Bile Duct Obstruction
 Navigating the Biliary Tract with CT & MR: An Imaging Approach to Bile Duct Obstruction Ann S. Fulcher, MD Medical College of Virginia Virginia Commonwealth University Richmond, Virginia Objectives To
Navigating the Biliary Tract with CT & MR: An Imaging Approach to Bile Duct Obstruction Ann S. Fulcher, MD Medical College of Virginia Virginia Commonwealth University Richmond, Virginia Objectives To
Clinical Management of Obscure- Overt Gastrointestinal Bleeding. Presented by Dr. 張瀚文
 Clinical Management of Obscure- Overt Gastrointestinal Bleeding Presented by Dr. 張瀚文 Definition Obscure: : hard to understand; not clear. Overt: : public; not secret. Occult: : hidden from the knowledge
Clinical Management of Obscure- Overt Gastrointestinal Bleeding Presented by Dr. 張瀚文 Definition Obscure: : hard to understand; not clear. Overt: : public; not secret. Occult: : hidden from the knowledge
DIGESTIVE SYSTEM SURGICAL PROCEDURES May 1, 2015 INTESTINES (EXCEPT RECTUM) Asst Surg Anae
 ENDOSCOPY Z50 Duodenoscopy (not to be claimed if Z399 and/or Z00 performed on same patient within 3 months)... 92.10 Z9 Subsequent procedure (within three months following previous endoscopic procedure)...
ENDOSCOPY Z50 Duodenoscopy (not to be claimed if Z399 and/or Z00 performed on same patient within 3 months)... 92.10 Z9 Subsequent procedure (within three months following previous endoscopic procedure)...
GI Coding Updates. Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC
 GI Coding Updates Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC Copyright/Disclaimer 2014 AAPC text CPT copyright 2016 American Medical Association. All rights reserved.
GI Coding Updates Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC Copyright/Disclaimer 2014 AAPC text CPT copyright 2016 American Medical Association. All rights reserved.
An otherwise healthy 45 year-old man underwent
 Carol Rees Parrish, M.S., R.D., Series Editor Short Bowel Syndrome in Adults Part 5 Trophic Agents in the Treatment of Short Bowel Syndrome John K. DiBaise Carol Rees Parrish An important goal when treating
Carol Rees Parrish, M.S., R.D., Series Editor Short Bowel Syndrome in Adults Part 5 Trophic Agents in the Treatment of Short Bowel Syndrome John K. DiBaise Carol Rees Parrish An important goal when treating
Familial Adenomatous Polyposis
 Familial Adenomatous Polyposis 1 in 10,000 incidence 100 s to 1000 s of colonic adenomas by teens Cancer risk: colon, gastric, duodenum (periampulla), small bowel, pancreas, papillary thyroid, childhood
Familial Adenomatous Polyposis 1 in 10,000 incidence 100 s to 1000 s of colonic adenomas by teens Cancer risk: colon, gastric, duodenum (periampulla), small bowel, pancreas, papillary thyroid, childhood
Dsuvia (sufentanil) NEW PRODUCT SLIDESHOW
 Dsuvia (sufentanil) NEW PRODUCT SLIDESHOW Introduction Brand name: Dsuvia Generic name: Sufentanil Pharmacological class: Opioid agonist Strength and Formulation: 30mcg; sublingual tabs (housed in a disposable,
Dsuvia (sufentanil) NEW PRODUCT SLIDESHOW Introduction Brand name: Dsuvia Generic name: Sufentanil Pharmacological class: Opioid agonist Strength and Formulation: 30mcg; sublingual tabs (housed in a disposable,
University of Bristol - Explore Bristol Research
 Hunt, L., Ben-Shlomo, Y., Whitehouse, M., Porter, M., & Blom, A. (2017). The Main Cause of Death Following Primary Total Hip and Knee Replacement for Osteoarthritis: A Cohort Study of 26,766 Deaths Following
Hunt, L., Ben-Shlomo, Y., Whitehouse, M., Porter, M., & Blom, A. (2017). The Main Cause of Death Following Primary Total Hip and Knee Replacement for Osteoarthritis: A Cohort Study of 26,766 Deaths Following
Quality Measures In Colonoscopy: Why Should I Care?
 Quality Measures In Colonoscopy: Why Should I Care? David Greenwald, MD, FASGE Professor of Clinical Medicine Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York ACG/ASGE Best
Quality Measures In Colonoscopy: Why Should I Care? David Greenwald, MD, FASGE Professor of Clinical Medicine Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York ACG/ASGE Best
SOMATULINE DEPOT (lanreotide) injection, for subcutaneous use Initial U.S. Approval: 2007
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SOMATULINE safely and effectively. See full prescribing information for SOMATULINE. SOMATULINE (lanreotide)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SOMATULINE safely and effectively. See full prescribing information for SOMATULINE. SOMATULINE (lanreotide)
An Introduction to Serrated Polyposis Syndrome (SPS)
 An Introduction to Serrated Polyposis Syndrome (SPS) An Introduction to Serrated Polyposis Syndrome (SPS) 3 An Introduction to Serrated Polyposis Syndrome (SPS) Contents What is Serrated Polyposis Syndrome
An Introduction to Serrated Polyposis Syndrome (SPS) An Introduction to Serrated Polyposis Syndrome (SPS) 3 An Introduction to Serrated Polyposis Syndrome (SPS) Contents What is Serrated Polyposis Syndrome
removal of adenomatous polyps detects important effectively as follow-up colonoscopy after both constitute a low-risk Patients with 1 or 2
 Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Child-Pugh Class B or C or Patients with a Prior Decompensation Event a 5 mg once daily 5 mg once weekly. Compensated Child- Pugh Class A
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OCALIVA safely and effectively. See full prescribing information for OCALIVA. OCALIVA (obeticholic
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OCALIVA safely and effectively. See full prescribing information for OCALIVA. OCALIVA (obeticholic
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
REFERRAL GUIDELINES: GALLSTONES
 REFERRAL GUIDELINES: GALLSTONES Document Purpose To ensure patients with gallstones disease are managed appropriately in primary/ secondary care Oxford Radcliffe Hospital Surgical Department Surgical Registrar
REFERRAL GUIDELINES: GALLSTONES Document Purpose To ensure patients with gallstones disease are managed appropriately in primary/ secondary care Oxford Radcliffe Hospital Surgical Department Surgical Registrar
Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary
 Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,2 Lotronex (alosetron) a Indication For women with severe diarrheapredominant irritable
Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,2 Lotronex (alosetron) a Indication For women with severe diarrheapredominant irritable
Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema
 Bahrain Medical Bulletin, Vol.24, No.3, September 2002 Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema Najeeb S Jamsheer, MD, FRCR* Neelam. Malik, MD, MNAMS** Objective: To
Bahrain Medical Bulletin, Vol.24, No.3, September 2002 Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema Najeeb S Jamsheer, MD, FRCR* Neelam. Malik, MD, MNAMS** Objective: To
COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION.
 The West London Medical Journal 2009 Vol No 1 pp 23-31 COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION. Competing interests: None declared ABSTRACT Sarah
The West London Medical Journal 2009 Vol No 1 pp 23-31 COLORECTAL SCREENING PROGRAMME: IMPACT ON THE HOSPITAL S PATHOLOGY SERVICES SINCE ITS INTRODUCTION. Competing interests: None declared ABSTRACT Sarah
PATHOLOGY MCQs. The Pancreas
 PATHOLOGY MCQs The Pancreas A patient with cystic fibrosis is characteristically: A. more than 45 years of age B. subject to recurring pulmonary infections C. obese D. subject to spontaneous fractures
PATHOLOGY MCQs The Pancreas A patient with cystic fibrosis is characteristically: A. more than 45 years of age B. subject to recurring pulmonary infections C. obese D. subject to spontaneous fractures
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SAXENDA safely and effectively. See full prescribing information for SAXENDA. SAXENDA (liraglutide
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SAXENDA safely and effectively. See full prescribing information for SAXENDA. SAXENDA (liraglutide
Active ingredients: Metoclopramide Hydrochloride mg Equivalent to metoclopramide hydrochloride anhydrous mg
 Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
Journal of Interventional Gastroenterology A Combination of Snare Polypectomy and APC Therapy for Prolapsing Common Bile Duct Adenoma
 Journal of Interventional Gastroenterology A Combination of Snare Polypectomy and APC Therapy for Prolapsing Common Bile Duct Adenoma --Manuscript Draft-- Manuscript Number: Full Title: Article Type: Section/Category:
Journal of Interventional Gastroenterology A Combination of Snare Polypectomy and APC Therapy for Prolapsing Common Bile Duct Adenoma --Manuscript Draft-- Manuscript Number: Full Title: Article Type: Section/Category:
Amyloidosis & the GI Tract
 Amyloidosis & the GI Tract John O. Clarke, M.D. Director, Esophageal Program Clinical Associate Professor of Medicine Stanford University john.clarke@stanford.edu 2017 Topics to cover 1) Patterns of GI
Amyloidosis & the GI Tract John O. Clarke, M.D. Director, Esophageal Program Clinical Associate Professor of Medicine Stanford University john.clarke@stanford.edu 2017 Topics to cover 1) Patterns of GI
REFERRAL GUIDELINES: ENDOSCOPY
 Outpatient Referral Guidelines Page 1 REFERRAL GUIDELINES: Referrals for endoscopy must be made using the Essential Referral Content Gastrointestinal Endoscopy Referral Form Please fax the completed referral
Outpatient Referral Guidelines Page 1 REFERRAL GUIDELINES: Referrals for endoscopy must be made using the Essential Referral Content Gastrointestinal Endoscopy Referral Form Please fax the completed referral
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. Teduglutide for injection
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr REVESTIVE * Teduglutide for injection Read this carefully before you start taking REVESTIVE and each time you get
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr REVESTIVE * Teduglutide for injection Read this carefully before you start taking REVESTIVE and each time you get
Colonoscopy Quality Data
 Colonoscopy Quality Data www.dhsgi.com Introduction Colorectal cancer is the second leading cause of cancer related deaths in the United States, in men and women combined. In 2016, there are expected to
Colonoscopy Quality Data www.dhsgi.com Introduction Colorectal cancer is the second leading cause of cancer related deaths in the United States, in men and women combined. In 2016, there are expected to
CLINICAL PRESENTATION AND RADIOLOGY QUIZ QUESTION
 Donald L. Renfrew, MD Radiology Associates of the Fox Valley, 333 N. Commercial Street, Suite 100, Neenah, WI 54956 8/27/2011 Radiology Quiz of the Week # 35 Page 1 CLINICAL PRESENTATION AND RADIOLOGY
Donald L. Renfrew, MD Radiology Associates of the Fox Valley, 333 N. Commercial Street, Suite 100, Neenah, WI 54956 8/27/2011 Radiology Quiz of the Week # 35 Page 1 CLINICAL PRESENTATION AND RADIOLOGY
Why would fatty foods aggravate the patient s RUQ pain? What effect does cholecystokinin (CCK) have on gastric emptying?
 CASE 28 A 43-year-old woman presents to the emergency department with the acute onset of abdominal pain. Her pain is located to the right upper quadrant (RUQ) and radiates to the right shoulder. She reports
CASE 28 A 43-year-old woman presents to the emergency department with the acute onset of abdominal pain. Her pain is located to the right upper quadrant (RUQ) and radiates to the right shoulder. She reports
Barrett s Esophagus Burn, Resect, Freeze, or Just Watch Strategies for Evaluation and Surveillance of Barrett s Amitabh Chak, MD OBJECTIVES: NOTES:
 Speaker 1 Barrett s Esophagus Burn, Resect, Freeze, or Just Watch Strategies for Evaluation and Surveillance of Barrett s Amitabh Chak, MD 1. List the epidemiology of Barrett s esophagus. 2. Review the
Speaker 1 Barrett s Esophagus Burn, Resect, Freeze, or Just Watch Strategies for Evaluation and Surveillance of Barrett s Amitabh Chak, MD 1. List the epidemiology of Barrett s esophagus. 2. Review the
ENDOSCOPY IN COMPETITION DIAGNOSTICS. Dr. med. Dirk Hartmann Klinikum Ludwigshafen
 Falk Symposium 166 GI Endoscopy Standards and Innovations Mainz, 18. 19. September 2008 ENDOSCOPY IN COMPETITION DIAGNOSTICS Dr. med. Dirk Hartmann Klinikum Ludwigshafen ENDOSCOPY IN COMPETITION Competing
Falk Symposium 166 GI Endoscopy Standards and Innovations Mainz, 18. 19. September 2008 ENDOSCOPY IN COMPETITION DIAGNOSTICS Dr. med. Dirk Hartmann Klinikum Ludwigshafen ENDOSCOPY IN COMPETITION Competing
