ALKYLATING AGENTS (22)
|
|
|
- Vernon Allison
- 6 years ago
- Views:
Transcription
1 Serving the United States, Canada and North Central America MEMBER OF TEXAS HEALTH CARE ENTITIES A PRIVATE MEMBERSHIP MEDICAL ASSOCIATION 3105 MAIN STREET, ROWLETT, TX TEL: office@rgccusa.com WEBSITE: THESE LIST ARE UP TO DATE AS OF EVERY ITEM ON THESE 6 PAGES ARE TESTED ON EVERY CANCER PATIENT SHOWING EXACT % OF EFFECTIVENESS OF EACH PRODUCT. NO LAB IN THE WORLD EVEN COMES CLOSE!! (53) CHEMOTHERAPEUTIC AGENTS TESTED + (4) RESISTANCE FACTORS ALKYLATING AGENTS (22) ACNU NIMUSTINE ALTRETAMINE HEXALEN BCNU CARMUSTINE BENDAMUSTINE TREANDA BLEOMYCIN BLENOXANE CARBOPLATIN PARAPLATIN CCNU LOMUSTINE CHLORAMBUCIL LEUKERAN CISPLATIN PLATINOL CYCLOPHOSPHAMIDE CYTOXAN DTIC ESTRAMUSTINE EMCYT HYDROXYUREA DROXIA/HYDREA IFOSFAMIDE IFEX MELPHALAN ALKERAN MITOMYCIN MITOMYCIN C NEDAPLATIN AQUPLA OXALIPLATIN ELOXATIN PROCARBAZINE MATULANE TEMOZOLOMIDE TEMODAR TREOSULFAN TROFOSFAMIDE IXOTEN EPOTHILONES (1) IXABEPILONE IXEMPRA INHIBITORS OF TOPOISOMERASE I (3) CPT11 IRINOTECAN GIMATECAN TOPOTECAN HYCAMTIN ~ 1 ~ of 6
2 INHIBITORS OF TOPOISOMERASE II (9) AMRUBICIN-HYDROCHLORIDE CALSED DACTINOMYCIN COSMEGEN DAUNORUBICIN CERUBIDINE DOXORUBICIN ADRIAMYCIN EPIRUBICIN PHARMORUBICIN ETOPOSIDE VEPESID IDARUBICIN IDAMYCIN LIPOSOMAL DOXORUBICIN DOXIL MITOXANTRONE NOVANTRONE NUCLEUS SPINDLE STABILIZER I (5) ABRAXANE PACLITAXEL CABAZITAXEL JEVTANA DOCETAXEL TAXOTERE ERIBULIN HALAVEN PACLITAXEL TAXOL NUCLEUS SPINDLE STABILIZER II (3) VINBLASTINE VELBAN VINCRISTINE ONCOVIN VINORELBINE NAVELBINE NUCLEOSIDE ANALOGUES (10) 5FU 5-FLUOROURACIL CAPECITABINE XELODA CYTARABINE CYTOSINE ARABINOSIDE FLUDARABINE FLUDARA FUDR FLOXURIDINE GEMCITABINE GEMZAR MTX METHOTREXATE PEMETREXED ALIMTA RALTITREXED TOMUDEX UFT URACIL TEGAFUR RESISTANCE FACTORS (4) MDR1 MRP LRP GST CHEMOTHERAPEUTIC AGENTS TESTED CONTINUED ~ 2~ of 6
3 5-AZACYTIDINE VIDAZA // ABIRATERONE ZYTIGA // ALEMTUZUMAB CAMPATH // ANASTROZOLE ARIMIDEX // ANTIANDROGEN (GOSERELIN) // AXITINIB INLYTA // BEVACIZUMAB AVASTIN BORTEZOMIB VELCADE // BRENTUXIMAB VEDOTIN ADCETRIS // CATUMAXOMAB REMOVAB // CETUXIMAB (225) ERBITUX // CRIZOTINIB XALKORI // DASATINIB SPRYCEL // ERLOTINIB TARCEVA // EVEROLIMUS/TEMSIROLIMUS AFINITOR, ZORTRESS // EXEMESTANE AROMASIN // FULVESTRANT FASLODEX // GEFITINIB IRESSA // GEMTUZUMAB OZOGAMICIN MYLOTARG // GOSERELIN ZOLADEX // IBRITUMOMAB ZEVALIN // IMATINIB-MESYLATE GLEEVEC // IPILIMUMAB YERVOY // LAPATINIB TYKERB // LEUPROLIDE ELIGARD, LUPRON DEPOT // NILOTINIB TASIGNA // OCTREOTIDE SANDOSTATIN // OFATUMUMAB ARZERRA // OLAPARIB AZD-2281 // PANITUMUMAB VECTIBIX // PAZOPANIB VOTRIENT // PERTUZUMAB PERJETA // REGORAFENIB BAY // RITUXIMAB RITUXAN // RUXOLITINIB JAKAFI // SEMAXANIB SU5416 // SORAFENIB NEXAVAR // SUNITINIB SUTENT // TAMOXIFEN // TOSITUMOMAB BEXXAR // TRABECTEDIN YONDELIS // TRASTUZUMAB HERCEPTIN // VANDETANIB CAPRELSA // VEMURAFENIB ZELBORAF // VORINOSTAT ZOLINZA // BIOLOGICAL MODIFIERS TESTED (45) ~ 3 ~ of 6
4 GROWTH FACTORS & PROLIFERATION STIMULI (27) SS-r Progesterone Receptor Estrogen Receptor p180 COX2 5-LOX NFkB IkB(a,b,c) EGF Ras/Raf/MEK/Erk mtor c-erb-b1 c-erb-b2 Bcr-abl ALK EML-4-ALK NPM-ALK CD 117(c-kit) RET IGF-r 1 IGF-r-2 NR3C4-A Testosterone receptors NR3C4-B DHT receptors JAK ½ PTEN c-jun c-fos SELF REPAIR-RESISTANCE (14) HSP 27 HSP 72 HSP 90 Gamma GC (gene for drug resistance) DNA METHYLTRANSFERASE 1 DNA DEMETHYLASE 06-METHYL-DNA-TRAN TGF-b (transforming growth factor-beta) Histone deacylase dipeptide HDAC HAT CXCR4 CXCR12 CXCL12 (72) TUMOR RELATED GENES TESTED + (4) MARKERS ~ 4 ~ of 6
5 TUMOR RELATED GENES TESTED CONTINUED ANGIOGENESIS (5) VEGF (vascular endothelial growth factor FGF (fibroblast growth factor) PDGF (platelet derived growth factor) ANG 1 ANG 2 CELL CYCLE REGULATION & IMMORTALIZATION/APOPTOSIS (9) E2F1 CDC6 P27 (gene of the cycle-dependent kinase inhibit P53 (gene; DNA gene guardian) P16 (tumor suppressor gene; stops tumor cell death) BCL-2 H-TERT HUMAN TELOMERASE M2 Bax CD95 (fas-r) ANGIOGENESIS-METASTASIS (5) KISS-1-r Nm23 nonmetastatic gene 23 MMP (matrix metalloproteinase) c-met 67LR (Laminin receptor) DRUG METABOLISMS & TARGETS (13) CES1&2 carboxyesterase DPD dihydropyrimidine dehydrogenase UP Uridine phosphorylase NP Nucleoside phosphorylase TP (thymidine phosphorylase) TS DHFR (dihydrofolate reductase) SHMT serine Hydroxymethyl- transferase) GARFT RIBO-NUCLEOSIDE REDUCTASE CypB1 ERCC1 RRMI MARKERS (4) CD33 MYELOID CELL ORIGIN CD52 LEUKEMIA MARKER CD20 LYMPHOMA RELATED ANTIGEN EPCAM EPITHELIAL MARKER ~ 5 ~ of 6
6 NATURAL SUBSTANCE LIST TESTED (45) AHCC (Active Hexose Correlated Compound)// AMYGDALIN (B-17, LAETRILE)// ARABINOGALACTAN// AROMAT8-PN // ARTECIN // ASCORBIC ACID (INTRAVENOUS)// BIO Ae MULSION FORTE // BIO-D-MULSION OR NuMEDICA MICELLIZED D3 // CELLULAR VITALITY// CRUCIFEROUS COMPLETE// C-STATIN// CURCUMIN (TUMERIC) CURCUMA SORB (formally MERIVA )// CV247// DCA (dichloroacetate)// DEXTROL// EPIMUNE COMPLEX// FERMENTED SOY EXTRACT// FUCOIDAN// GENISTEIN// INDO 3 CARBINOL// INTENZYME FORTE // LYCOPENE// MAMMARY PMG // MELATONIN// METFORMIN // MISTLETOE// NALTREXONE// Nrf2 ACTIVATOR // OLEANDER EXTRACT ANVIRZEL // OPC SYNERGY // PAW-PAW// PME (poly mannose extract from aloe vera)// POLY-MVA // PROTEO-XYME // QUERCETIN// RESVERATROL// RETENZYME FORTE // SALICINIUM // SALVESTROL // SUPER ARTEMISININ// SUPEROXIDE DISMUTASE// THYMEX // UKRAIN// VIRXCAN // VITANOX// ~ 6 ~ of 6
Acute Lymphocytic Leukemia
 Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Chemotherapy 101 for Radiation Oncology Workers
 Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
Chemotherapy 101 for Radiation Oncology Workers James Sinclair, M.D. CCARE/Medical Director Scripps Cancer Center March 9, 2013 Category 1) Alkylating agents Alkylating agents directly damage DNA to prevent
R.G.C.C.-RESEARCH GENETIC CANCER CENTRE LTD
 ONCONOMICS 1 / 13 R.G.C.C.-RESEARCH GENETIC CANCER CENTRE LTD Dear colleague, Florina, / / We send you the results from the analysis on a patient suffering from carcinoma stage. The sample that was sent
ONCONOMICS 1 / 13 R.G.C.C.-RESEARCH GENETIC CANCER CENTRE LTD Dear colleague, Florina, / / We send you the results from the analysis on a patient suffering from carcinoma stage. The sample that was sent
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
Formulary Chemotherapy Agents: (Current as of 6/2018) Therapeutic Class
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
To help doctors give their patients the best possible care, the American
 Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Etiologies:
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
HCPCS Code/ generic (Brand) Name J7506. J8520 capecitabine (Xeloda) 1. J8521 capecitabine. J8530 cyclophosphamide. (Cytoxan) 1
 drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
Medication Review. Cancer Chemotherapy Drugs. Pharmacy Technician Training Systems Passassured, LLC
 Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Genetics in Cancer Therapy. Raju Kucherlapati, Ph.D. Harvard Medical School
 Genetics in Cancer Therapy Raju Kucherlapati, Ph.D. Harvard Medical School RK Financial interests I do not intend to discuss an off-label use of a product during this activity AVEO Pharmaceuticals KEW
Genetics in Cancer Therapy Raju Kucherlapati, Ph.D. Harvard Medical School RK Financial interests I do not intend to discuss an off-label use of a product during this activity AVEO Pharmaceuticals KEW
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
R.G.C.C.-RESEARCH GENETIC CANCER CENTRE EX-VIVO TEST
 R.G.C.C.-RESEARCH GENETIC CANCER CENTRE Dear Dr. Gilbard and Dr. Hammon, EX-VIVO TEST Florina, 16 / 9 / 8 We send you the results from the analysis made about a patient (??????????) suffering from lung
R.G.C.C.-RESEARCH GENETIC CANCER CENTRE Dear Dr. Gilbard and Dr. Hammon, EX-VIVO TEST Florina, 16 / 9 / 8 We send you the results from the analysis made about a patient (??????????) suffering from lung
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data
 Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
Hodgkin and Non-Hodgkin Lymphoma (LYM) Post-Infusion Data Registry Use Only Sequence Number: Date Received: CIBMTR Center Number: CIBMTR Research ID: Event date: / / Visit 100 day 6 months 1 year 2 years
R.G.C.C.-RESEARCH GENETIC CANCER CENTRE
 R.G.C.C.-RESEARCH GENETIC CANCER CENTRE Dear Dr. Gilbard and Dr. Hammon, EX-VIVO TEST Florina, 26 / 11 / 8 We send you the results from the analysis made about a patient (??????????????) suffering from
R.G.C.C.-RESEARCH GENETIC CANCER CENTRE Dear Dr. Gilbard and Dr. Hammon, EX-VIVO TEST Florina, 26 / 11 / 8 We send you the results from the analysis made about a patient (??????????????) suffering from
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Form 2023 R2.0: Ovarian Cancer Pre-HSCT Data
 Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Ovarian Cancer. compendia TREATMENT OF
 drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
drug & compendia TREATMENT OF Ovarian Cancer With each publication, ManagedCare Oncology s drug & Compendia highlights a single medication or a group of medications that could be utilized in the management
R.G.C.C.-RESEARCH GENETIC CANCER CENTRE Ltd
 R.G.C.C.-RESEARCH GENETIC CANCER CENTRE Ltd Dear Dr. Gilbard and Dr Hammon, Florina, 29 / 4 / 9 EX-VIVO TEST SCREEN We send you the results from the analysis made about a patient (??????????????) suffering
R.G.C.C.-RESEARCH GENETIC CANCER CENTRE Ltd Dear Dr. Gilbard and Dr Hammon, Florina, 29 / 4 / 9 EX-VIVO TEST SCREEN We send you the results from the analysis made about a patient (??????????????) suffering
Bladder Cancer (Urothelial) Pathways
 Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer (Urothelial) Pathways Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
DRUG PROPERTIES YOU NEED TO KNOW
 Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Introduction to Antineoplastic Prescribing
 Introduction to Antineoplastic Prescribing Robert Bradbury, R.Ph., BCPS Clinical Coordinator H. Lee Moffitt Cancer Center Objectives Meet the following goals concerning antineoplastic prescribing: Understand
Introduction to Antineoplastic Prescribing Robert Bradbury, R.Ph., BCPS Clinical Coordinator H. Lee Moffitt Cancer Center Objectives Meet the following goals concerning antineoplastic prescribing: Understand
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
Bladder Cancer Pathways (Urothelial)
 Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
Bladder Cancer Pathways (Urothelial) Patient Name: Date of Birth: Member Number: Treatment Start Date: ICD-10 Code: Pathology: Stage: 0a 0is I II III IV Recurrent Line of Treatment: Neoadjuvant/Pre-Op
MABCAMPATH mg 3 INJ GENZYME BIOPHARMACEUTICALS. MABCAMPBATH mg 3 INJ GENZYME BIOPHARMACEUTICALS
 ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA 250 720690001 250 mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL-2 18
ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA 250 720690001 250 mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL-2 18
Azacitidine Vidaza Non-transplant myelodysplastic syndrome Funded Funded Funded Funded Funded Funded Not Funded
 Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A
 Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Committee Approval Date: December 12, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
2. Date of most recent breast cancer diagnosis / / 3. Pathlogy: Padget s Phyllodes Tubular Medullary Mucinous
 Breast Cancer Patients: Begin on page 1, then skip to page 3 Lymphoma Patients: Begin on page 2 1. Newly diagnosed with Breast carcinoma Stage: (includes inflammatory breast and newly diagnosed recurrent
Breast Cancer Patients: Begin on page 1, then skip to page 3 Lymphoma Patients: Begin on page 2 1. Newly diagnosed with Breast carcinoma Stage: (includes inflammatory breast and newly diagnosed recurrent
Hazard Meds: Navigating the Warning Beacons
 Hazard Meds: Navigating the Warning Beacons Disclosures I have nothing to disclose at this time. Mark Twohey, Pharm.D., BCOP Palmetto Health Richland March 24, 2012 Objectives Understand the reasons for
Hazard Meds: Navigating the Warning Beacons Disclosures I have nothing to disclose at this time. Mark Twohey, Pharm.D., BCOP Palmetto Health Richland March 24, 2012 Objectives Understand the reasons for
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Overview of Cancer. Laura Bingell RN Transition Center Nurse for MFP (607)
 Overview of Cancer Laura Bingell RN Transition Center Nurse for MFP (607)962-8225 lbingell@ilny.org What is Cancer? A collection of related diseases in which some of the body s cells begin to divide abnormally
Overview of Cancer Laura Bingell RN Transition Center Nurse for MFP (607)962-8225 lbingell@ilny.org What is Cancer? A collection of related diseases in which some of the body s cells begin to divide abnormally
REGULATORY INFORMATION
 REGULATORY*INFORMATION/*CANCER*MEDICINES* Summary'of'regulatory'status'of'the'medicines' As#recommended#in#the#EML#Secretariat s#instructions#regarding#applications#for#the#addition#of#new# medicines#
REGULATORY*INFORMATION/*CANCER*MEDICINES* Summary'of'regulatory'status'of'the'medicines' As#recommended#in#the#EML#Secretariat s#instructions#regarding#applications#for#the#addition#of#new# medicines#
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
RGCC. Circulating Tumour Cells PRACTITIONER MANUAL
 RGCC Circulating Tumour Cells PRACTITIONER MANUAL NutriPATH Pty. Ltd. 18a Harker Street, Burwood, VIC, 3125, Australia Phone: 1300 688 522 (Australia) +61 3 9880 2900 (International) Fax: +61 3 9880 2999
RGCC Circulating Tumour Cells PRACTITIONER MANUAL NutriPATH Pty. Ltd. 18a Harker Street, Burwood, VIC, 3125, Australia Phone: 1300 688 522 (Australia) +61 3 9880 2900 (International) Fax: +61 3 9880 2999
FREEDOM OF INFORMATION SOUTH EAST SCOTLAND CANCER NETWORK
 Dear Date 06/02/09 Your Ref Our Ref RM/1220 Enquiries to Richard Mutch Extension 89441 Direct Line 0131-536-9441 Direct Fax 0131-536-9009 Email richard.mutch@lhnhslothian.scot.nhs.uk FREEDOM OF INFORMATION
Dear Date 06/02/09 Your Ref Our Ref RM/1220 Enquiries to Richard Mutch Extension 89441 Direct Line 0131-536-9441 Direct Fax 0131-536-9009 Email richard.mutch@lhnhslothian.scot.nhs.uk FREEDOM OF INFORMATION
Chapter 2 Clinical Anticancer Drugs for Cancer Treatment
 Chapter 2 Clinical Anticancer Drugs for Cancer Treatment Besides cytoreductive surgery and radiotherapy, chemotherapy is the most widely used therapeutic strategy in combating cancer. The high incidence
Chapter 2 Clinical Anticancer Drugs for Cancer Treatment Besides cytoreductive surgery and radiotherapy, chemotherapy is the most widely used therapeutic strategy in combating cancer. The high incidence
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
DRUGS YOU NEED TO KNOW
 jfitzake@d.umn.edu Page 1 of 7 DRUGS YOU NEED TO KNOW ALKYLATING AGENTS (blue cards) BUSULFAN CARMUSTINE (BCNU) CYCLOPHOSPHAMIDE DACARBAZINE LOMUSTINE (CCNU) MECHLORETHAMINE MELPHALAN THIOTEPA NATURAL
jfitzake@d.umn.edu Page 1 of 7 DRUGS YOU NEED TO KNOW ALKYLATING AGENTS (blue cards) BUSULFAN CARMUSTINE (BCNU) CYCLOPHOSPHAMIDE DACARBAZINE LOMUSTINE (CCNU) MECHLORETHAMINE MELPHALAN THIOTEPA NATURAL
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
CANCER CHEMOTHERAPY Michael Lea
 CANCER CHEMOTHERAPY 2010 Michael Lea Cancer Chemotherapy - Lecture Outline 1. Targets for cancer chemotherapy 2. Classification of anticancer drugs 3. Cell cycle specificity 4. Drug resistance 5. New approaches
CANCER CHEMOTHERAPY 2010 Michael Lea Cancer Chemotherapy - Lecture Outline 1. Targets for cancer chemotherapy 2. Classification of anticancer drugs 3. Cell cycle specificity 4. Drug resistance 5. New approaches
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Objectives. Principles of Cancer Treatment. Cancer Treatment Modalities. Cancer Treatment Modalities
 Objectives Principles of Cancer Treatment Juanita Madison, RN, MN, AOCN Oncology Clinical Nurse Specialist Seattle Cancer Care Alliance Describe the principles of cancer treatment Surgery Chemotherapy
Objectives Principles of Cancer Treatment Juanita Madison, RN, MN, AOCN Oncology Clinical Nurse Specialist Seattle Cancer Care Alliance Describe the principles of cancer treatment Surgery Chemotherapy
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
Cancer Drugs CANCER DRUGS NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS
 Cancer Drugs NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS by KONG KHOO, ROSEMARY COLUCCI, DANIEL GILLESPIE, JAMES D. GOWING, PIERRE MAJOR, JOSEPH RAGAZ, DAVID SALTMAN, COLLEEN SAVAGE Development of
Cancer Drugs NEW DRUGS AND INDICATIONS OVER THE PAST TEN YEARS by KONG KHOO, ROSEMARY COLUCCI, DANIEL GILLESPIE, JAMES D. GOWING, PIERRE MAJOR, JOSEPH RAGAZ, DAVID SALTMAN, COLLEEN SAVAGE Development of
DRUG PROPERTIES YOU NEED TO KNOW
 jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
Chemotherapy Agents 101
 Chemotherapy Agents 101 Jumana Ashy, Pharm D, BCOP Judith Misas, PharmD Candidate 2018 Jeremy Hutchinson, PharmD Sady Castance, PharmD September 12, 2017 1 Learning Objectives At the end of the presentation
Chemotherapy Agents 101 Jumana Ashy, Pharm D, BCOP Judith Misas, PharmD Candidate 2018 Jeremy Hutchinson, PharmD Sady Castance, PharmD September 12, 2017 1 Learning Objectives At the end of the presentation
Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist
 Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist Moderator: S. Terence Dunn, Ph.D. Associate Professor, Pathology Director, Molecular Pathology Laboratory University
Drug-targeted therapies and Predictive Prognosis: Changing Role for the Pathologist Moderator: S. Terence Dunn, Ph.D. Associate Professor, Pathology Director, Molecular Pathology Laboratory University
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Lynparza) Reference Number: CP.PHAR.360 Effective Date: 10.03.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Lynparza) Reference Number: CP.PHAR.360 Effective Date: 10.03.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Objectives
 Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
-2-
 -1- -2- -3- -4- -5- -6- -7- -8- blood sample CTC Isolation Molecular Back flush = Cell Characterisation Reflux Filtration Tumor cell collection Wash Cell Lysis Extraction immunomagnetic beads -9- stronger
-1- -2- -3- -4- -5- -6- -7- -8- blood sample CTC Isolation Molecular Back flush = Cell Characterisation Reflux Filtration Tumor cell collection Wash Cell Lysis Extraction immunomagnetic beads -9- stronger
AD regimen, 576 adrenal steroid inhibitor. aminoglutethimide, 23 25
 A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
BLOOD AND LYMPH CANCERS
 BLOOD AND LYMPH CANCERS 2 Blood and Lymph Cancers Highlights from the 2009 Annual Meeting of the American Society of Clinical Oncology Edited by Kenneth C. Anderson, MD Harvard Medical School and Dana-Farber
BLOOD AND LYMPH CANCERS 2 Blood and Lymph Cancers Highlights from the 2009 Annual Meeting of the American Society of Clinical Oncology Edited by Kenneth C. Anderson, MD Harvard Medical School and Dana-Farber
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
Anastrozole. Active Ingredient. Brand Name: Arimidex Strength: 1 mg Dosage Form: Tablets Pack Size: 28 Tabs Company: AstraZeneca
 Super Specialty Drugs by Anastrozole Arimidex 1 mg 28 Tabs AstraZeneca Armotraz 1 mg 10 Tabs Cipla Anastrozole Anabrez 1 mg 100 Tabs Sun Decitabine Dacogen 50 mg 1 Vial Eisai Inc. Erlotinib Erlocip-150
Super Specialty Drugs by Anastrozole Arimidex 1 mg 28 Tabs AstraZeneca Armotraz 1 mg 10 Tabs Cipla Anastrozole Anabrez 1 mg 100 Tabs Sun Decitabine Dacogen 50 mg 1 Vial Eisai Inc. Erlotinib Erlocip-150
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center
 Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Working Formulary January 2013 Oncology Chemotherapy Regimens
 Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
New Developments in Cancer Treatment. Dulcinea Quintana, MD
 New Developments in Cancer Treatment Dulcinea Quintana, MD Mortality Rates Goals of treatment 1 Cure Goal of treatment 2 Prolong life Goals of treatment 3 Improve Quality of Life Goals of treatment 4
New Developments in Cancer Treatment Dulcinea Quintana, MD Mortality Rates Goals of treatment 1 Cure Goal of treatment 2 Prolong life Goals of treatment 3 Improve Quality of Life Goals of treatment 4
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic Therapy
 Women s Empowerment Cancer Advocacy Network (WE CAN) Conference Bucharest, Romania October 2015 Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic
Women s Empowerment Cancer Advocacy Network (WE CAN) Conference Bucharest, Romania October 2015 Treatment Options for Breast Cancer in Low- and Middle-Income Countries: Adjuvant and Metastatic Systemic
Adjuvant Systemic Therapy in Early Stage Breast Cancer
 Adjuvant Systemic Therapy in Early Stage Breast Cancer Julie R. Gralow, M.D. Director, Breast Medical Oncology Jill Bennett Endowed Professor of Breast Cancer Professor, Global Health University of Washington
Adjuvant Systemic Therapy in Early Stage Breast Cancer Julie R. Gralow, M.D. Director, Breast Medical Oncology Jill Bennett Endowed Professor of Breast Cancer Professor, Global Health University of Washington
Supplementary Figures
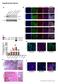 Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
1. Purpose Documentation Description.1 4. References 8 5. Cross-References.8 6. Development of the guideline.8
 CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer
 APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
The AngCN Antiemetic Guidelines
 The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Drug Name. J0129 Injection, abatacept (Orencia ), 10 mg Effective 01/01/2014. J0178 Injection, aflibercept (Eylea ), 1 mg Effective 04/01/2015
 J0129 Injection, abatacept (Orencia ), 10 J0178 Injection, aflibercept (Eylea ), 1 J0256 J0257 J0585 J0586 J0587 J0588 J0597 J0641 J0717 J0800 Injection, alpha 1-proteinase inhibitor, human (Aralast NP,
J0129 Injection, abatacept (Orencia ), 10 J0178 Injection, aflibercept (Eylea ), 1 J0256 J0257 J0585 J0586 J0587 J0588 J0597 J0641 J0717 J0800 Injection, alpha 1-proteinase inhibitor, human (Aralast NP,
Supplementary Table 1. Summary of FDA approval data for rare cancer indications from December 1987 to May 2011
 Rare Cancer : Lessons from FDA s by Gaddipati et al. Supplementary Table 1 Summary of FDA approval data for rare cancer indications from December 1987 to May 2011 1. mitoxantrone hydrochloride (NOVANTRONE
Rare Cancer : Lessons from FDA s by Gaddipati et al. Supplementary Table 1 Summary of FDA approval data for rare cancer indications from December 1987 to May 2011 1. mitoxantrone hydrochloride (NOVANTRONE
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Introduction to Targeted Therapy
 Introduction to Targeted Therapy Cancer remains the second leading cause of death in the United States, despite the significant advances in cancer therapy made over the past several decades. Many factors
Introduction to Targeted Therapy Cancer remains the second leading cause of death in the United States, despite the significant advances in cancer therapy made over the past several decades. Many factors
Cancer drug approvals for paediatric indications (n=43)
 Appendix: Supplementary material [posted as supplied by author] Figure A. Identification of cohort of drugs Total number of antineoplastic and immunomodulating products approved by the EMA up to 31 December
Appendix: Supplementary material [posted as supplied by author] Figure A. Identification of cohort of drugs Total number of antineoplastic and immunomodulating products approved by the EMA up to 31 December
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care
 Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and
 35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
StrandAdvantage Tissue-Specific Cancer Genomic Tests. Empowering Crucial First-Line Therapy Decisions for Your Patient
 StrandAdvantage Tissue-Specific Cancer Genomic Tests Empowering Crucial First-Line Therapy Decisions for Your Patient Harness the power of precision medicine with StrandAdvantage Precision medicine in
StrandAdvantage Tissue-Specific Cancer Genomic Tests Empowering Crucial First-Line Therapy Decisions for Your Patient Harness the power of precision medicine with StrandAdvantage Precision medicine in
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Verzenio) Reference Number: CP.PHAR.355 Effective Date: 10.24.17 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Verzenio) Reference Number: CP.PHAR.355 Effective Date: 10.24.17 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Verzenio) Reference Number: CP.PHAR.355 Effective Date: 10.24.17 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Verzenio) Reference Number: CP.PHAR.355 Effective Date: 10.24.17 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
3/8/2018. Treatment Modalities. Study Resources. Study Resources. New 2018 OCN Test Blueprint
 New 2018 OCN Test Blueprint Treatment Modalities Nancy Thompson, MSN, RN, AOCNS Director of Quality & Clinical Practice Swedish Cancer Institute March 17, 2018 Content Area Sub-Content Areas 2018 Test%
New 2018 OCN Test Blueprint Treatment Modalities Nancy Thompson, MSN, RN, AOCNS Director of Quality & Clinical Practice Swedish Cancer Institute March 17, 2018 Content Area Sub-Content Areas 2018 Test%
Hazardous Medication List
 Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Choice of Cancer Chemotherapy u Is it a science, art or voodoo medicine?
 PRINCIPLES OF CYTOTOXIC CHEMOTHERAPY Dr.Erdem Göker Ege Üniversitesi Tıp Fakültesi TÜLAY AKTAŞ ONKOLOJİ HASTANESİ Choice of Cancer Chemotherapy u Is it a science, art or voodoo medicine? BEST Tx OF CANCER
PRINCIPLES OF CYTOTOXIC CHEMOTHERAPY Dr.Erdem Göker Ege Üniversitesi Tıp Fakültesi TÜLAY AKTAŞ ONKOLOJİ HASTANESİ Choice of Cancer Chemotherapy u Is it a science, art or voodoo medicine? BEST Tx OF CANCER
Chemotherapy and biotherapy: guidelines and recommendations for practice.
 Complete Summary GUIDELINE TITLE Chemotherapy and biotherapy: guidelines and recommendations for practice. BIBLIOGRAPHIC SOURCE(S) Oncology Nursing Society (ONS). Chemotherapy and biotherapy: guidelines
Complete Summary GUIDELINE TITLE Chemotherapy and biotherapy: guidelines and recommendations for practice. BIBLIOGRAPHIC SOURCE(S) Oncology Nursing Society (ONS). Chemotherapy and biotherapy: guidelines
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Table of Contents. Introduction and Summary. KEI Research Note 2011:1 August 4, 2011 Paul Miano
 Cancer: Approval, ownership, market structure, and placement on WHO Model Essential Medicines List, for 100 new molecular entities (NMEs) on the NCI alpha list of cancer drugs and vaccines KEI Research
Cancer: Approval, ownership, market structure, and placement on WHO Model Essential Medicines List, for 100 new molecular entities (NMEs) on the NCI alpha list of cancer drugs and vaccines KEI Research
A themed review of patient safety incidents involving anti-cancer medicines 1 November June 2008
 A themed review of patient safety incidents involving anti-cancer medicines 1 November 2003 30 June 2008 October 2010 Full Report National Patient Safety Agency 2010. Copyright and other intellectual property
A themed review of patient safety incidents involving anti-cancer medicines 1 November 2003 30 June 2008 October 2010 Full Report National Patient Safety Agency 2010. Copyright and other intellectual property
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19
 Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Self Administered Oncology Agents Page 1 of 13 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Self Administered Oncology Agents Prime Therapeutics will review Prior
Self Administered Oncology Agents Page 1 of 13 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Self Administered Oncology Agents Prime Therapeutics will review Prior
4. Shown below is the structure of doxorubicin (Adriamycin). What is true about this agent?
 Midterm 2: 3 points each (except final question worth 1 point 1. A useful regimen for treating colorectal cancer is FOLFIRI. What is true about this regimen? A. The regimen includes folinic acid, 5-fluorouracil
Midterm 2: 3 points each (except final question worth 1 point 1. A useful regimen for treating colorectal cancer is FOLFIRI. What is true about this regimen? A. The regimen includes folinic acid, 5-fluorouracil
North of England Cancer Drugs Approval Group (NECDAG) Cumulative Table of Decisions August 2012
 Notes: This document is updated every 2 to 3 months; please check our website www.cancernorth.nhs.uk for latest version. This document does not all contain Interim Cancer Drugs Fund s, i.e. decisions made
Notes: This document is updated every 2 to 3 months; please check our website www.cancernorth.nhs.uk for latest version. This document does not all contain Interim Cancer Drugs Fund s, i.e. decisions made
Chemoradiation Interactions and Toxicity Management
 Chemoradiation Interactions and Toxicity Management Jonathan W. Thompson April 15, 2016 University of Alabama At Birmingham, Department of Radiation Oncology Radiation Oncology Associates of Acadiana,
Chemoradiation Interactions and Toxicity Management Jonathan W. Thompson April 15, 2016 University of Alabama At Birmingham, Department of Radiation Oncology Radiation Oncology Associates of Acadiana,
