Protocol for an integrated data request of test results from the laboratories of pathological anatomy
|
|
|
- Evelyn Maxwell
- 6 years ago
- Views:
Transcription
1 Protocol for an integrated data request of test results from the laboratories of pathological anatomy Cancer diagnoses & early detection of cancer (breast, colorectal and cervix) CODAP page 1
2 Table of contents Introduction... 3 Contact information Data transfer: general principles Which data should be transferred? Data format Missing information Submission of protocols ( written reports ) Means of delivery Data transfer: detailed overview Cancer diagnoses A. Inclusion criteria B. Dataset for cancer diagnoses C. Detailed information about the variables Test results of breast and colorectal specimens A. Inclusion criteria B. Dataset for breast and colorectal specimens C. Detailed information about the variables Test results of cervical smears and biopsies-including HPV tests A. Inclusion criteria B. Dataset for cervical smears and biopsies-including HPV tests C. Detailed information about the variables CODAP page 2
3 Introduction This document will provide a set of instructions on how to transfer data on cancer diagnosis and test results of early cancer detection to the Belgian Cancer Registry. The aim is to provide a clear description of a standardized and integrated protocol describing how data should be transferred, allowing us to process this information and enabling the use of your data e.g. for national and international descriptive statistics on cancer incidence. Please transfer this document to your IT service and/or to the people responsible for assembling the dataset(s). The legal basis for the work of the Belgian Cancer Registry can be found in: The law of 13 December 2006 called: Wet houdende diverse bepalingen betreffende gezondheid (1), hoofdstuk VI, Artikel 39 Loi portant dispositions diverses en matière de santé (1), chapitre VI, Article 39 Recent changes in this law relevant to this data request were published in the Belgisch Staatsblad / Moniteur Belge on the 2nd of June 2010 (art. 29). Contact information For additional information or support, please contact the Belgian Cancer Registry at: 02/ info@kankerregister.org info@registreducancer.org CODAP page 3
4 1. Data transfer: general principles 1.1 Which data should be transferred? The Belgian Cancer Registry kindly asks you to transfer all data concerning these four topics: 1. One file containing all encoded cancer diagnoses and a separate file with the written reports (i.e. protocols ); 2. One file containing the encoded test results of all breast specimens and a separate file with the written reports (i.e. protocols ); 3. One file containing the encoded test results of all colorectal specimens and a separate file with the written reports (i.e. protocols ); 4. One file containing the encoded test results of all cervical smears and biopsies and a separate file with the written reports (i.e. protocols ). A detailed description of each data set is stated below. Remark: When following these procedures, it is normal that if a cancer is diagnosed in an early detection program, it will result in double registration: once in the cancer diagnosis file and once in the breast specimens, colorectal specimens or cervical smears and biopsies file. 1.2 Data format The file format is a tab separated text. 1.3 Missing information If certain information is not present in your database you can fill out the column with: #NS #NK #NA not stated or not present unknown not applicable CODAP page 4
5 1.4 Submission of protocols ( written reports ) The anonymous protocols should preferably be submitted in one file as a tab separated text format with a clear delimiter between the different protocols. The specimen number should be mentioned in the protocol. 1.5 Means of delivery Data are delivered preferably on CD-ROM secured by a password and sent to the Belgian Cancer Registry by a recorded delivery (aangetekende verzending / envoi recommandé) attended to the medical supervisor Dr. Liesbet Van Eycken at the following address. Stichting Kankerregister/Fondation Registre du Cancer For the attention of Dr. Liesbet Van Eycken, Medical Supervisor Koningsstraat Brussel Alternatively, the data can be sent by by means of a password secured zip file to the medical supervisor (elizabeth.vaneycken@kankerregister.org). In both cases, the password must be communicated to the Cancer Registry by telephone. CODAP page 5
6 2. Data transfer: detailed overview 2.1 Cancer diagnoses 2.1.A. Inclusion criteria Range to select: CODAP: all lesion codes equal or greater than 60+ codes, CIN3, GIN3, VIN3 and PIN3. (equivalent for ICD-O-3: 8000/0-9999/9.) All malignant tumours, invasive or in situ. All hematological tumours including the myelodysplastic syndromes and myeloproliferative diseases. All tumours of the central nervous system whatever the behavior of the tumour (benign, low malignant potential, malignant). All urothelial cell tumours (low malignant potential, in situ, invasive). Ovary: malignant and borderline malignant epithelial tumours. CODAP page 6
7 2.1.B. Dataset for cancer diagnoses Variable Compulsory Format Comment or optional 1 National number C 11 characters, Leading zero's should be conserved! (INSZ / NISS) text format without space 2 Last name O free text field Compulsory if INSZ/NISS unknown 3 First Name O free text field Compulsory if INSZ/NISS unknown 4 Sex C MALE or FEMALE 5 Date of birth C yyyymmdd 6 Date of death O yyyymmdd Only if applicable 7 Zip code C free text field 8 Country code C 2 characters, text format Code of the legal residence of the person: names_and_code_elements 9 Specimen number C free text field Should match the specimen number in the protocol. 10 Date specimen was taken C yyyymmdd 11 Requesting hospital O free text field 12 Organ C free text field All organ codes Name of the hospital that requests the pathological examination 13 Lesion C free text field Range to select: all lesion codes >= 60 and CIN3, GIN3, VIN3, PIN3. 14 pt O free text field Please use TNM 7th edition for all diagnoses from 2010 onwards 15 pn O free text field Please use TNM 7th edition for all diagnoses from 2010 onwards 16 pm O free text field Please use TNM 7th edition for all diagnoses from 2010 onwards 17 Degree of certainty O 1=uncertain 2=differential diagnosis 3=certain In grey the administrative block which is similar for all types of registration C = compulsory - O= optional : to be added CODAP page 7
8 2.1.C. Detailed information about the variables Date specimen was taken (Variable 10) Take care to fill in the date the specimen was taken and not the date of analysis. Requesting hospital (Variable 11) Fill in the hospital (campus/laboratory) that sent the specimen, requested the pathological examination and to which the result is reported. Lesion (Variable 13) If possible please encode lateralization (left or right). CODAP page 8
9 2.2 Test results of breast and colorectal specimens 2.2.A. Inclusion criteria The datasets for the breast and colorectal specimens are identical; however the test results should be delivered in separate files. Range to select: 1. For the file containing the encoded test results of all breast specimens: CODAP: organ codes 69XX (equivalent for ICD-O-3: C50.X) All encoded test results of breast specimens, including negative, benign and premalignant lesions. 2. For the file containing the encoded test results of all colorectal specimens: CODAP: organ codes 41XX, 42XX, 43XX (equivalent for ICD-O-3: C18.X, C19.X, C20.X, C21.X) All encoded test results of colorectal specimens, including negative, benign and premalignant lesions. CODAP page 9
10 2.2.B. Dataset for breast and colorectal specimens Variable Compulsory Format Comment or optional 1 National number C 11 characters, Leading zero's should be conserved! (INSZ / NISS) text format without space 2 Last name O free text field Compulsory if INSZ/NISS unknown 3 First Name O free text field Compulsory if INSZ/NISS unknown 4 Sex C MALE or FEMALE 5 Date of birth C yyyymmdd 6 Date of death O yyyymmdd Only if applicable 7 Zip code C free text field 8 Country code C 2 characters, text format Code of the legal residence of the person: names_and_code_elements 9 Specimen number C free text field Should match the specimen number in the protocol 10 Date specimen was taken C yyyymmdd 11 Requesting hospital O free text field 12 RIZIV/INAMI number of the demander of the test 13 Organ C free text field 14 Lesion 15 Degree of certainty O 1=uncertain 2=differential diagnosis 3=certain 16 Nomenclature number(s) C text format without space C = compulsory - O= optional : to be added C C 11 numbers text format without space free text field In grey the administrative block which is similar for all types of registration Name of the hospital that requests the pathological examination Range to select: File colorectal: 41XX,42XX 43XX File breast: 69XX All test results including negative tests Different numbers to be entered separated by commas "," CODAP page 10
11 2.2.C. Detailed information about the variables Date specimen was taken (Variable 10) Take care to fill in the date the specimen was taken and not the date of analysis. Requesting hospital (Variable 11) Please fill in the hospital (campus/laboratory) that sent the specimen, requested the pathological examination and to which the result is reported. RIZIV/INAMI number of the demander (Variable 12) Data bases for registration of screening results will be set up. One of the goals is to integrate all information from different sources to set up complete individual patient histories. Another tool that will be included in screening data bases is the fail-safe mechanism. This is a back-up mechanism which ensures that patients with an abnormal screening result will have the appropriate follow up. If this fails, we can identify what went wrong, by using the variable requesting hospital (variable 11) and the RIZIV/INAMI number of the demander (variable 12). Lesion (Variable 14) Please fill in the diagnostic codes. If possible encode lateralization (left or right). Provide a table with codes used for negative, benign and premalignant lesions if you have one. Nomenclature number(s) (Variable 16) Please fill in the nomenclature number(s) associated with the corresponding specimens if known. In case of more than one nomenclature number, they should be entered separated by a comma (, ). CODAP page 11
12 2.3 Test results of cervical smears and biopsies 2.3.A. Inclusion criteria Range to select: CODAP: organ codes 64XX, 65XX. (equivalent for ICD-O-3: C52.X, C53.X ) All cervical and vaginal cytology test results including negative tests (no abnormalities) and light or moderate cell abnormalities. All cervical histology test results including negative tests (no abnormalities) and light or moderate cell abnormalities. Results of high risk HPV tests. CODAP page 12
13 2.3.B. Dataset for test results of cervical smears and biopsies- including HPV tests Variable Compulsory Format Comment or optional 1 National number C 11 characters, Leading zero's should be conserved! (INSZ / NISS) text format without space 2 Last name O free text field Compulsory if INSZ/NISS unknown 3 First Name O free text field Compulsory if INSZ/NISS unknown 4 Sex C MALE or FEMALE 5 Date of birth C yyyymmdd 6 Date of death O yyyymmdd Only if applicable 7 Zip code C free text field 8 Country code C 2 characters, text format Code of the legal residence of the person: mes_and_code_elements 9 Specimen number C free text field Should match the specimen number in the protocol. 10 Date specimen was taken C yyyymmdd 11 Requesting hospital O free text field Name of the hospital that requests the pathological examination 12 RIZIV/INAMI number of the demander of the test 13 Quality of the specimen C C 11 numbers text format without space SUF+, SUF-, INSU Only for pap smears 14 Organ C free text field Range to select: 64XX, 65XX 15 Lesion C free text field All test results including negative tests, benign and premalignant lesions 16 Degree of certainty 17 HPV high risk test results 18 HPV high risk types detected 19 Nomenclature number(s) C C = compulsory - O= optional : to be added O C if HPV test performed 1=uncertain 2=differential diagnosis 3=certain HPV-, HPV+, HPVi HP16,HP18,,,, free text field text field without space In grey the administrative block which is similar for all types of registration O Applies to lesion Several answers possible Different HPV types to be entered separated by commas "," Several answers possible Different numbers to be entered separated by commas "," CODAP page 13
14 2.3.C. Detailed information about the variables Date specimen was taken (Variable 10) Take care to fill in the date the specimen was taken and not the date of analysis. Requesting hospital (Variable 11) Please fill in the hospital (campus/laboratory) that sent the specimen, requested the pathological examination and to which the result is reported. RIZIV/INAMI number of the demander (Variable 12) See also section 2.2.C. (detailed information about the variables of the dataset colorectal and breast specimens). Quality of the specimen (Variable 13) This variable is obligatory to be used, only for cervical smears. Please add this variable and corresponding codes. Possible codes SUF+ SUF- INSU Meaning of the codes Sufficient, with endocervical cells Sufficient but without endocervical cells Insufficient (no reliable test result could be determined or the specimen could not be evaluated at all because it was broken or incorrectly labeled) Organ (Variable 14) Select the CODAP organ codes 64xx and 65xx. Please use organ codes with four characters. Make a clear distinction between cytology and histology. For cytology use organ codes 64CY of 65CY. CODAP page 14
15 Lesion (Variable 15): o BEFORE 2011: The test results of the cervical smears and biopsies of the year s 2008, 2009 and 2010 can be delivered as now available in your data base. o FROM 01/01/2011 ON: introduction of new lesion codes (CERVIBASE 2011) Several new lesion codes will be introduced. These new codes and their significance are mentioned in the table below. PLEASE ADD THESE NEW LESION CODES TO THE EXISTING CODAP 2007 CODES FROM 01/01/2011 ON. CERVIBASE 2011 NEW LESION CODES New codes Meaning of the code ONLY FOR CYTOLOGY NILM ASCU Negative for epithelial cell abnormalities or any malignancy Atypical squamous cells of undetermined significance ASCH Atypical squamous cells, cannot exclude HSIL LSIL Low-grade squamous intraepithelial lesion HSIL High-grade squamous intraepithelial lesion, incl. in situ AGLC Atypical glandular cells (no distinction between any favor or origin) ONLY FOR HISTOLOGY ABST CIN1 Absent: no squamous, glandular or any dysplasias nor tumour Mild dysplasia CIN2 Moderate dysplasia CGIN Endocervical glandular dysplasia CODAP page 15
16 o Systematic overview of CERVIBASE 2011 coding By coding the test results, take care to make a clear distinction between cytology and histology. For correct coding of the cervical smears (cytology) and biopsies (histology), please follow the systematic overview of diagnostic codes as mentioned below. THIS SYSTEMATIC OVERVIEW OF CERVIBASE 2011 CODES SHOULD BE APPLIED TO ALL SPECIMENS TAKEN FROM 01/01/2011 ON. MAKE A CLEAR DISTINCTION BETWEEN CYTOLOGY AND HISTOLOGY. POSSIBLE CODES FOR CYTOLOGICAL AND HISTOLOGICAL LESIONS CYTOLOGY Lesion code Meaning of the code No cell abnormalities NILM Negative for epithelial cell abnormalities or any malignancy Squamous cell abnormalities ASCU Atypical squamous cells of undetermined significance ASCH Atypical squamous cells, cannot exclude HSIL LSIL Low-grade squamous intraepithelial lesion HSIL High-grade squamous intraepithelial lesion (incl. in situ) CODAP 2007 Squamous cell carcinoma Glandular cell abnormalities AGLC Atypical glandular cells (no distinction between any favor or origin) CODAP 2007 Adenocarcinoma in situ CODAP 2007 Adenocarcinoma Other malignancies CODAP 2007 Any other malignancy HISTOLOGY Code Meaning of the code No cell abnormalities ABST No dysplasias nor tumour (absent) Squamous cervical lesions CIN1 Mild dysplasia (CIN1) CIN2 Moderate dysplasia (CIN2) CODAP 2007 Severe dysplasia (CIN3) CODAP 2007 Carcinoma in situ CODAP 2007 Squamous carcinoma Glandular cervical lesions CGIN Endocervical glandular dysplasia CODAP 2007 Adenocarcinoma in situ CODAP 2007 Adenocarcinoma Other malignancies CODAP 2007 Any other malignancy new lesion codes, to be used from 2011 in addition to the CODAP 2007 codes currently used CODAP 2007 lesion codes CODAP page 16
17 Degree of certainty (Variable 16): This applies to lesion and not HPV test. HPV high risk test results (Variable 17): select only one code HPV high risk test results: select only one code Possible codes Meaning of the codes HPV- Negative HPV+ Positive HPVi Inconclusive HPV high risk types detected (Variable 18) Possible codes Meaning of the codes HP16 HPV Type 16 HP18 HPV Type 18 HPxx HPV Type xx Multiple HPV types to be entered in a text field separated by com mas ",": Nomenclature number(s) (Variable 19) Please fill in the nomenclature number(s) associated with the corresponding specimens if known. In case of more than one nomenclature number, they should be entered separated by a comma (, ). Additional information about cervical smears For the cervical smears we want to know the following information: o o o Which method do you use to analyze the samples (conventional or liquid based)? What laboratory performs the HPV tests? Which test is used for HPV detection? This information should be communicated to the Belgian Cancer Registry by mail to following address: info@kankerregister.org or info@registreducancer.org CODAP page 17
Protocol for an integrated data request of test results from the laboratories of pathological anatomy. CODAP - users version December 2016
 Protocol for an integrated data request of test results from the laboratories of pathological anatomy CODAP - users version December 2016 Cancer diagnoses & early detection of cancer (breast, colorectal
Protocol for an integrated data request of test results from the laboratories of pathological anatomy CODAP - users version December 2016 Cancer diagnoses & early detection of cancer (breast, colorectal
Protocol for an integrated data request of test results from the laboratories of pathological anatomy. SNOMED 3.5VF - users version December 2016
 Protocol for an integrated data request of test results from the laboratories of pathological anatomy SNOMED 3.5VF - users version December 2016 Cancer diagnoses & early detection of cancer (breast, colorectal
Protocol for an integrated data request of test results from the laboratories of pathological anatomy SNOMED 3.5VF - users version December 2016 Cancer diagnoses & early detection of cancer (breast, colorectal
BC Cancer Cervix Screening 2015 Program Results. February 2018
 BC Cancer Cervix Screening 2015 Program Results BC Cancer Cervix Screening 2015 Program Results 2 Table of Contents BC Cancer Cervix Screening 2015 Program Results... 1 Table of Contents... 2 Program Overview...
BC Cancer Cervix Screening 2015 Program Results BC Cancer Cervix Screening 2015 Program Results 2 Table of Contents BC Cancer Cervix Screening 2015 Program Results... 1 Table of Contents... 2 Program Overview...
Understanding Your Pap Test Results
 Understanding Your Pap Test Results Most laboratories in the United States use a standard set of terms called the Bethesda System to report pap test results. Normal: Pap samples that have no cell abnormalities
Understanding Your Pap Test Results Most laboratories in the United States use a standard set of terms called the Bethesda System to report pap test results. Normal: Pap samples that have no cell abnormalities
Appropriate Use of Cytology and HPV Testing in the New Cervical Cancer Screening Guidelines
 Appropriate Use of Cytology and HPV Testing in the New Cervical Cancer Screening Guidelines Tim Kremer, MD Ralph Anderson, MD 1 Objectives Describe the natural history of HPV particularly as it relates
Appropriate Use of Cytology and HPV Testing in the New Cervical Cancer Screening Guidelines Tim Kremer, MD Ralph Anderson, MD 1 Objectives Describe the natural history of HPV particularly as it relates
Colposcopy. Attila L Major, MD, PhD
 Colposcopy Attila L Major, MD, PhD Histology Colposcopy Cytology It has been estimated that annual Pap smear testing reduces a woman s chance of dying of cervical cancer from 4 in 1000 to about 5 in 10,000
Colposcopy Attila L Major, MD, PhD Histology Colposcopy Cytology It has been estimated that annual Pap smear testing reduces a woman s chance of dying of cervical cancer from 4 in 1000 to about 5 in 10,000
Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two
 Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two Seshu P. Sarma, MD, FAAP Emory University Regional Training Center Atlanta, Georgia Produced by the Alabama Department of Public Health
Faculty Pap Smear Guidelines: Family Planning Update 2008 Part Two Seshu P. Sarma, MD, FAAP Emory University Regional Training Center Atlanta, Georgia Produced by the Alabama Department of Public Health
Making Sense of Cervical Cancer Screening
 Making Sense of Cervical Cancer Screening New Guidelines published November 2012 Tammie Koehler DO, FACOG The incidence of cervical cancer in the US has decreased more than 50% in the past 30 years because
Making Sense of Cervical Cancer Screening New Guidelines published November 2012 Tammie Koehler DO, FACOG The incidence of cervical cancer in the US has decreased more than 50% in the past 30 years because
Chapter 10: Pap Test Results
 Chapter 10: Pap Test Results On completion of this section, the learner will be able to: 1. Identify how Pap test results are interpreted and the reasons for normal and abnormal results. 2. Describe the
Chapter 10: Pap Test Results On completion of this section, the learner will be able to: 1. Identify how Pap test results are interpreted and the reasons for normal and abnormal results. 2. Describe the
Clinical Practice Guidelines June 2013
 Clinical Practice Guidelines June 2013 General Principles: The Papanicolaou (Pap) smear is widely credited with reducing mortality from cervical cancer, and remains the single best method for the early
Clinical Practice Guidelines June 2013 General Principles: The Papanicolaou (Pap) smear is widely credited with reducing mortality from cervical cancer, and remains the single best method for the early
Cervical Screening for Dysplasia and Cancer in Patients with HIV
 Cervical Screening for Dysplasia and Cancer in Patients with HIV Adult Clinical Guideline from the New York State Department of Health AIDS Institute w w w.hivg uidelines.org Purpose of the Guideline Increase
Cervical Screening for Dysplasia and Cancer in Patients with HIV Adult Clinical Guideline from the New York State Department of Health AIDS Institute w w w.hivg uidelines.org Purpose of the Guideline Increase
Workshop for O& G trainees and paramedics 17 Dec 2011 Cytological Interpretation
 Workshop for O& G trainees and paramedics 17 Dec 2011 Cytological Interpretation May Yu Director of Cytology Laboratory Service Department of Anatomical & Cellular Pathology Prince of Wales Hospital Cervical
Workshop for O& G trainees and paramedics 17 Dec 2011 Cytological Interpretation May Yu Director of Cytology Laboratory Service Department of Anatomical & Cellular Pathology Prince of Wales Hospital Cervical
Cervical Cancer : Pap smear
 Taking a PAP SMEAR Cervical Cancer : Pap smear George N Papanicolaou introduced cervical cytology in clinical practice in 1940 In 1945, PAP smear was endorsed by American cancer society as an effective
Taking a PAP SMEAR Cervical Cancer : Pap smear George N Papanicolaou introduced cervical cytology in clinical practice in 1940 In 1945, PAP smear was endorsed by American cancer society as an effective
European Union survey on organization and quality control of cervical cancer screening and HPV vaccination programs
 European Union survey on organization and quality control of cervical cancer screening and HPV vaccination programs Introduction to the Survey The purpose of this project is to collect information regarding
European Union survey on organization and quality control of cervical cancer screening and HPV vaccination programs Introduction to the Survey The purpose of this project is to collect information regarding
EU guidelines for reporting gynaecological cytology
 EU guidelines for reporting gynaecological cytology Amanda Herbert Guy s & St Thomas Foundation NHS Trust 5th EFCS Annual Tutorial, Trondheim, Norway 28 th May 1 st June 2012 EU guidelines aim to harmonize
EU guidelines for reporting gynaecological cytology Amanda Herbert Guy s & St Thomas Foundation NHS Trust 5th EFCS Annual Tutorial, Trondheim, Norway 28 th May 1 st June 2012 EU guidelines aim to harmonize
Histopathology: Cervical HPV and neoplasia
 Histopathology: Cervical HPV and neoplasia These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need to learn about
Histopathology: Cervical HPV and neoplasia These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need to learn about
Screening for the Precursors of Cervical Cancer in the Era of HPV Vaccination. Dr Stella Heley Senior Liaison Physician Victorian Cytology Service
 Screening for the Precursors of Cervical Cancer in the Era of HPV Vaccination Dr Stella Heley Senior Liaison Physician Victorian Cytology Service Victorian Cytology Service Dr Stella Heley Dr Siobhan Bourke
Screening for the Precursors of Cervical Cancer in the Era of HPV Vaccination Dr Stella Heley Senior Liaison Physician Victorian Cytology Service Victorian Cytology Service Dr Stella Heley Dr Siobhan Bourke
Eradicating Mortality from Cervical Cancer
 Eradicating Mortality from Cervical Cancer Michelle Berlin, MD, MPH Vice Chair, Obstetrics & Gynecology Associate Director, Center for Women s Health June 2, 2009 Overview Prevention Human Papilloma Virus
Eradicating Mortality from Cervical Cancer Michelle Berlin, MD, MPH Vice Chair, Obstetrics & Gynecology Associate Director, Center for Women s Health June 2, 2009 Overview Prevention Human Papilloma Virus
Cervical Testing and Results Management. An Evidenced-Based Approach April 22nd, Debora Bear, MSN, MPH
 Cervical Testing and Results Management An Evidenced-Based Approach April 22nd, 2010 Debora Bear, MSN, MPH Assistant Medical Director for Planned Parenthood of New Mexico, Inc. Burden of cervical cancer
Cervical Testing and Results Management An Evidenced-Based Approach April 22nd, 2010 Debora Bear, MSN, MPH Assistant Medical Director for Planned Parenthood of New Mexico, Inc. Burden of cervical cancer
The society for lower genital tract disorders since 1964.
 The society for lower genital tract disorders since 1964. Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
The society for lower genital tract disorders since 1964. Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
1.Acute and Chronic Cervicitis - At the onset of menarche, the production of estrogens by the ovary stimulates maturation of the cervical and vaginal
 Diseases of cervix I. Inflammations 1.Acute and Chronic Cervicitis - At the onset of menarche, the production of estrogens by the ovary stimulates maturation of the cervical and vaginal squamous mucosa
Diseases of cervix I. Inflammations 1.Acute and Chronic Cervicitis - At the onset of menarche, the production of estrogens by the ovary stimulates maturation of the cervical and vaginal squamous mucosa
Cervical Cancer Screening for the Primary Care Physician for Average Risk Individuals Clinical Practice Guidelines. June 2013
 Cervical Cancer Screening for the Primary Care Physician for Average Risk Individuals Clinical Practice Guidelines General Principles: Since its introduction in 1943, Papanicolaou (Pap) smear is widely
Cervical Cancer Screening for the Primary Care Physician for Average Risk Individuals Clinical Practice Guidelines General Principles: Since its introduction in 1943, Papanicolaou (Pap) smear is widely
Management Algorithms for Abnormal Cervical Cytology and Colposcopy
 Management Algorithms for Abnormal Cervical Cytology and Colposcopy Table of Contents Standard Colposcopic Definitions... 1 Guidelines for the Assessment of Abnormal Cervical Cytology... 2 Ia: Persistent
Management Algorithms for Abnormal Cervical Cytology and Colposcopy Table of Contents Standard Colposcopic Definitions... 1 Guidelines for the Assessment of Abnormal Cervical Cytology... 2 Ia: Persistent
Manitoba Cervical Cancer Screening Program. Operations & Statistical Report and 2006
 anitoba Cervical Cancer Screening Program Operations & Statistical Report 2005 and 2006 1 MCCSP 2005-2006 Report ANITOBA CERVICAL CANCER SCREENING PROGRAM 2005 and 2006 Operations & Statistical Report
anitoba Cervical Cancer Screening Program Operations & Statistical Report 2005 and 2006 1 MCCSP 2005-2006 Report ANITOBA CERVICAL CANCER SCREENING PROGRAM 2005 and 2006 Operations & Statistical Report
Study Number: Title: Rationale: Phase: Study Period Study Design: Centres: Indication Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
!"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$
 !"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$ Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
!"#$%&'(#)*$+&,$-&.#,$/#0()1-$ ),1')$2(%&,2#,%$%(0'#$34567$ Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
Case Based Problems. Recommended Guidelines. Workshop: Case Management of Abnormal Pap Smears and Colposcopies. Disclosure
 Disclosure Workshop: Case Management of Abnormal Pap Smears and Colposcopies Rebecca Jackson, MD Associate Professor Obstetrics, Gynecology & Reproductive Sciences and Epidemiology & Biostatistics This
Disclosure Workshop: Case Management of Abnormal Pap Smears and Colposcopies Rebecca Jackson, MD Associate Professor Obstetrics, Gynecology & Reproductive Sciences and Epidemiology & Biostatistics This
Scottish Cervical Screening Programme. Colposcopy and Programme Management
 Scottish Cervical Screening Programme Colposcopy and Programme Management Addendum to NHSCSP Publication No 20 Second Edition Exceptions Applicable in NHS Scotland April 2013 (Final Version 2.8 to incorporate
Scottish Cervical Screening Programme Colposcopy and Programme Management Addendum to NHSCSP Publication No 20 Second Edition Exceptions Applicable in NHS Scotland April 2013 (Final Version 2.8 to incorporate
Lessons From Cases of Screened Women Who Developed Cervical Carcinoma
 Lessons From Cases of Screened Women Who Developed Cervical Carcinoma R. Marshall Austin MD,PhD Magee-Womens Hospital of University of Pittsburgh Medical Center raustin@magee.edu Why Focus Study On Cases
Lessons From Cases of Screened Women Who Developed Cervical Carcinoma R. Marshall Austin MD,PhD Magee-Womens Hospital of University of Pittsburgh Medical Center raustin@magee.edu Why Focus Study On Cases
Objectives. I have no financial interests in any product I will discuss today. Cervical Cancer Screening Guidelines: Updates and Controversies
 Cervical Cancer Screening Guidelines: Updates and Controversies I have no financial interests in any product I will discuss today. Jody Steinauer, MD, MAS University of California, San Francisco Objectives
Cervical Cancer Screening Guidelines: Updates and Controversies I have no financial interests in any product I will discuss today. Jody Steinauer, MD, MAS University of California, San Francisco Objectives
ICD-9-CM to ICD-10-CM DIAGNOSIS CODE MAPPING. Central Valley Public Health: Women's Way
 ICD-9-CM to ICD-10-CM DIAGNOSIS CODE MAPPING Central Valley Public Health: Women's Way ICD-9-CM (Top 35) ICD-10-CM ICD-10-CM Description COMMENTS V10.3: Personal history of malignant neoplasm of Z85.3
ICD-9-CM to ICD-10-CM DIAGNOSIS CODE MAPPING Central Valley Public Health: Women's Way ICD-9-CM (Top 35) ICD-10-CM ICD-10-CM Description COMMENTS V10.3: Personal history of malignant neoplasm of Z85.3
Cervical Cancer Screening Update. Melissa Hartman, DO Women s Health
 Cervical Cancer Screening Update Melissa Hartman, DO Women s Health Previous Cervical Cancer Screening Organization Recommendation ACS (2011) ACP (2008) NCI (2003) Age 21 or 3 years after first intercourse
Cervical Cancer Screening Update Melissa Hartman, DO Women s Health Previous Cervical Cancer Screening Organization Recommendation ACS (2011) ACP (2008) NCI (2003) Age 21 or 3 years after first intercourse
Cytology and Surgical Pathology of Gynecologic Neoplasms
 Cytology and Surgical Pathology of Gynecologic Neoplasms Current Clinical Pathology ANTONIO GIORDANO, MD, PHD SERIES EDITOR For further titles published in this series, go to http://www.springer.com/springer/series/7632
Cytology and Surgical Pathology of Gynecologic Neoplasms Current Clinical Pathology ANTONIO GIORDANO, MD, PHD SERIES EDITOR For further titles published in this series, go to http://www.springer.com/springer/series/7632
BCCCP Approved ICD-9 Code List Fiscal Year 2010
 BCCCP Approved ICD-9 List Fiscal Year 2010 Diagnosis Description 174.0 Malignant neoplasm of female breast; Nipple and areola 174.1 Malignant neoplasm of female breast; Central portion 174.2 Malignant
BCCCP Approved ICD-9 List Fiscal Year 2010 Diagnosis Description 174.0 Malignant neoplasm of female breast; Nipple and areola 174.1 Malignant neoplasm of female breast; Central portion 174.2 Malignant
REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2015 and after
 REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2015 and after The following lists are intended to assist you, as the reporter, in identifying the reportable neoplasms for your facility. Any reportable
REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2015 and after The following lists are intended to assist you, as the reporter, in identifying the reportable neoplasms for your facility. Any reportable
Your Colposcopy Visit
 Introduction Welcome to the colposcopy clinic. This booklet tells you about. The colposcopy examination.. Tests that are done in the colposcopy clinic.. What these tests look for Take a few minutes to
Introduction Welcome to the colposcopy clinic. This booklet tells you about. The colposcopy examination.. Tests that are done in the colposcopy clinic.. What these tests look for Take a few minutes to
ANALYSES OF CERVICAL CANCER IN RAJKOT POPULATION
 Electronic Journal of Pharmacology and Therapy Vol 4, 15-20 (2011) ISSN: 0973-9890 (Available online at wwwtcrjournalscom) Clinical Article ndexed in: ProQuest database Abstract, USA (ProQuest Science
Electronic Journal of Pharmacology and Therapy Vol 4, 15-20 (2011) ISSN: 0973-9890 (Available online at wwwtcrjournalscom) Clinical Article ndexed in: ProQuest database Abstract, USA (ProQuest Science
Management of Abnormal Cervical Cytology and Histology
 Management of Abnormal Cervical Cytology and Histology Assoc. Prof. Gökhan Tulunay Etlik Zübeyde Hanım Women s Diseases Teaching & Research Hospital Gynecologic Oncology Clinic Universally accepted guideline
Management of Abnormal Cervical Cytology and Histology Assoc. Prof. Gökhan Tulunay Etlik Zübeyde Hanım Women s Diseases Teaching & Research Hospital Gynecologic Oncology Clinic Universally accepted guideline
I have no financial interests in any product I will discuss today.
 Cervical Cancer Prevention: 2012 and Beyond George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics University of California,
Cervical Cancer Prevention: 2012 and Beyond George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics University of California,
Comprehensive ICD-10-CM Casefinding Code List for Reportable Tumors for 2018 (Effective Dates: 10/1/2017-9/30/2018)
 Comprehensive ICD-10-CM Casefinding Code List for Reportable Tumors for 2018 (Effective Dates: 10/1/2017-9/30/2018) Please refer to your standard setter(s) for specific reporting requirements before using
Comprehensive ICD-10-CM Casefinding Code List for Reportable Tumors for 2018 (Effective Dates: 10/1/2017-9/30/2018) Please refer to your standard setter(s) for specific reporting requirements before using
Cervical Cancer Screening. David Quinlan December 2013
 Cervical Cancer Screening David Quinlan December 2013 Cervix Cervical Cancer Screening Modest variation provincially WHO and UK begin at 25 stop at 60 Finland begin at 30 stop at 60 Rationale for
Cervical Cancer Screening David Quinlan December 2013 Cervix Cervical Cancer Screening Modest variation provincially WHO and UK begin at 25 stop at 60 Finland begin at 30 stop at 60 Rationale for
CASEFINDING. KCR Abstractor s Training
 CASEFINDING KCR Abstractor s Training 1 Introduction Casefinding Definition Purpose Methods Sources vs Resources Reportable Cancer Conditions Non-Reportable Conditions Ambiguous Terminology 2 https://www.google.com/search?q=puzzle+pieces&client=firefox-a&hs=mdc&rls=org.mozilla
CASEFINDING KCR Abstractor s Training 1 Introduction Casefinding Definition Purpose Methods Sources vs Resources Reportable Cancer Conditions Non-Reportable Conditions Ambiguous Terminology 2 https://www.google.com/search?q=puzzle+pieces&client=firefox-a&hs=mdc&rls=org.mozilla
Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary
 Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary Glossary of Terms Colposcopy is the examination of the cervix, vagina and, in
Clinical Guidance: Recommended Best Practices for Delivery of Colposcopy Services in Ontario Best Practice Pathway Summary Glossary of Terms Colposcopy is the examination of the cervix, vagina and, in
Screening for Cervical Cancer: Demystifying the Guidelines DR. NEERJA SHARMA
 Screening for Cervical Cancer: Demystifying the Guidelines DR. NEERJA SHARMA Cancer Care Ontario Cervical Cancer Screening Goals Increase patient participation in cervical screening Increase primary care
Screening for Cervical Cancer: Demystifying the Guidelines DR. NEERJA SHARMA Cancer Care Ontario Cervical Cancer Screening Goals Increase patient participation in cervical screening Increase primary care
I have no financial interests to disclose.
 Workshop: Case Management of Abnormal Pap Smears and Colposcopies Rebecca Jackson, MD Professor Obstetrics, Gynecology & Reproductive Sciences and Epidemiology & Biostatistics I have no financial interests
Workshop: Case Management of Abnormal Pap Smears and Colposcopies Rebecca Jackson, MD Professor Obstetrics, Gynecology & Reproductive Sciences and Epidemiology & Biostatistics I have no financial interests
Should Anal Pap Smears Be a Standard of Care in HIV Management?
 Should Anal Pap Smears Be a Standard of Care in HIV Management? Gordon Dickinson, M.D., FACP Professor of Medicine and Chief Infectious Diseases, Miller School of Medicine Short Answer: NO But 15-20 HPV
Should Anal Pap Smears Be a Standard of Care in HIV Management? Gordon Dickinson, M.D., FACP Professor of Medicine and Chief Infectious Diseases, Miller School of Medicine Short Answer: NO But 15-20 HPV
Cervical Dysplasia and HPV
 Cervical Dysplasia and HPV J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse HPV Double stranded DNA virus The HPV infect epithelial cells of the skin and mucous membranes Highest risk
Cervical Dysplasia and HPV J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse HPV Double stranded DNA virus The HPV infect epithelial cells of the skin and mucous membranes Highest risk
Dysplasia: layer of the cervical CIN. Intraepithelial Neoplasia. p16 immunostaining. 1, Cervical. Higher-risk, requires CIN.
 CLINICAL PRACTICE GUIDELINE Guideline Number: DHMP_DHMC_PG1015 Guideline Subject: Routine Cervical Cancer Screening Effective Date: 9/2018 Revision Date: 9/2019 Pages: 2 of 2 Quality Management Committee
CLINICAL PRACTICE GUIDELINE Guideline Number: DHMP_DHMC_PG1015 Guideline Subject: Routine Cervical Cancer Screening Effective Date: 9/2018 Revision Date: 9/2019 Pages: 2 of 2 Quality Management Committee
I have no financial interests in any product I will discuss today.
 Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Standards for Pathology Informatics in Australia (SPIA)
 Standards for Pathology Informatics in Australia (SPIA) Reporting Terminology and Codes Cytopathology (v3.0) Superseding and incorporating the Australian Pathology Units and Terminology Standards and Guidelines
Standards for Pathology Informatics in Australia (SPIA) Reporting Terminology and Codes Cytopathology (v3.0) Superseding and incorporating the Australian Pathology Units and Terminology Standards and Guidelines
What is a Pap smear?
 Pap smear What is a Pap smear? A Pap smear is a test that checks for changes in the cells of your cervix. The cervix is the lower part of the uterus that opens into the vagina. Developed over forty years
Pap smear What is a Pap smear? A Pap smear is a test that checks for changes in the cells of your cervix. The cervix is the lower part of the uterus that opens into the vagina. Developed over forty years
Cytyc Corporation - Case Presentation Archive - July 2002
 ThinPrep Pap Test History: 34 Year Old Female LMP: Day 20 Specimen Type: Cervical/Vaginal Case provided by Mark Tulecke, M.D. and Gabrielle Trawinski CT (ASCP), Mount Auburn Hospital, Cambridge, Massachusetts.
ThinPrep Pap Test History: 34 Year Old Female LMP: Day 20 Specimen Type: Cervical/Vaginal Case provided by Mark Tulecke, M.D. and Gabrielle Trawinski CT (ASCP), Mount Auburn Hospital, Cambridge, Massachusetts.
Cytology Report Format
 Squamous Precursor Lesions and Malignancies In Pap Test Dina R. Mody, MD, FCAP Director of Cytology The Methodist Hospital, Houston, TX Professor of Pathology and Laboratory Medicine Weill Medical College
Squamous Precursor Lesions and Malignancies In Pap Test Dina R. Mody, MD, FCAP Director of Cytology The Methodist Hospital, Houston, TX Professor of Pathology and Laboratory Medicine Weill Medical College
14. Cancer of the Cervix Uteri
 KEY FACTS 14. Cancer of the Cervix Uteri ICD-9 180 On average 78 cases of invasive cervical cancer were registered per year. Half of cases occurred under 49 years of age. 2% of female cancers. Higher than
KEY FACTS 14. Cancer of the Cervix Uteri ICD-9 180 On average 78 cases of invasive cervical cancer were registered per year. Half of cases occurred under 49 years of age. 2% of female cancers. Higher than
SESSION J4. What's Next? Managing Abnormal PAPs in 2014
 37th Annual Advanced Practice in Primary and Acute Care Conference: October 9-11, 2014 2:45 SESSION J4 What's Next? Managing Abnormal PAPs in 2014 Session Description: Linda Eckert, MD Review current guidelines
37th Annual Advanced Practice in Primary and Acute Care Conference: October 9-11, 2014 2:45 SESSION J4 What's Next? Managing Abnormal PAPs in 2014 Session Description: Linda Eckert, MD Review current guidelines
Cervical Conization. 1
 Cervical Conization www.zohrehyousefi.com 1 Cone Biopsy is a surgical procedure with removal of a cone shaped portion of the cervix The extent of involvement of epithelium on the ectocervix has been clearly
Cervical Conization www.zohrehyousefi.com 1 Cone Biopsy is a surgical procedure with removal of a cone shaped portion of the cervix The extent of involvement of epithelium on the ectocervix has been clearly
NATIONAL CERVICAL CANCER SCREENING PROGRAMME Monitor 2017
 a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a NATIONAL CERVICAL CANCER SCREENING PROGRAMME Monitor
a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a NATIONAL CERVICAL CANCER SCREENING PROGRAMME Monitor
HPV and Cervical Cancer, Screening and Prevention. John Ragsdale, MD July 12, 2018 CME Lecture Series
 HPV and Cervical Cancer, Screening and Prevention John Ragsdale, MD July 12, 2018 CME Lecture Series We have come a long Way Prevalence HPV in Young Adults in U.S HPV genotypes 55-60% of All cancers 20%
HPV and Cervical Cancer, Screening and Prevention John Ragsdale, MD July 12, 2018 CME Lecture Series We have come a long Way Prevalence HPV in Young Adults in U.S HPV genotypes 55-60% of All cancers 20%
Cervical Precancer: Evaluation and Management
 TAJ June 2002; Volume 15 Number 1 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Review fam Cervical Precancer: Evaluation and Management SM Khodeza Nahar Begum 1 Abstract Carcinoma of
TAJ June 2002; Volume 15 Number 1 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Review fam Cervical Precancer: Evaluation and Management SM Khodeza Nahar Begum 1 Abstract Carcinoma of
CASE 4 21/07/2017. Ectopic Prostatic Tissue in Cervix. Female 31. LLETZ for borderline nuclear abnormalities
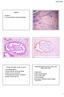 Female 31 CASE 4 LLETZ for borderline nuclear abnormalities PSA Ectopic Prostatic Tissue in Cervix AJSP 2006;30;209-215 usually incidental microscopic finding usually in ectocervical stroma? developmental
Female 31 CASE 4 LLETZ for borderline nuclear abnormalities PSA Ectopic Prostatic Tissue in Cervix AJSP 2006;30;209-215 usually incidental microscopic finding usually in ectocervical stroma? developmental
Done by khozama jehad. Neoplasia of the cervix
 Done by khozama jehad Neoplasia of the cervix An overview of cervical neoplasia very import. Most tumors of the cervix are of epithelial origin and are caused by oncogenic strains of human papillomavirus
Done by khozama jehad Neoplasia of the cervix An overview of cervical neoplasia very import. Most tumors of the cervix are of epithelial origin and are caused by oncogenic strains of human papillomavirus
California Cancer Registry Production Automation and Quality Control Unit Data Alert - Registrar
 Production Automation and Quality Control Unit Data Alert - Registrar 2015-061 REVISED Casefinding List FY2016 Please see additions and revisions noted below in red text. ICD-10-CM Codes have been adopted
Production Automation and Quality Control Unit Data Alert - Registrar 2015-061 REVISED Casefinding List FY2016 Please see additions and revisions noted below in red text. ICD-10-CM Codes have been adopted
Cervical Skills. Dr Margaret Laing Queen Elizabeth University Hospital
 Cervical Skills Dr Margaret Laing Queen Elizabeth University Hospital What is screening? Screening is a test offered to an apparently well person with the possibility of detecting a serious disease before
Cervical Skills Dr Margaret Laing Queen Elizabeth University Hospital What is screening? Screening is a test offered to an apparently well person with the possibility of detecting a serious disease before
We re on the Web! Visit us at VOLUME 19 ISSUE 1. January 2015
 VOLUME 19 ISSUE 1 January 2015 We re on the Web! Visit us at www.kumc.edu/kcr January is National Cervical Cancer Screening Month. Cervical cancer begins in the lining of the cervix (organ connecting the
VOLUME 19 ISSUE 1 January 2015 We re on the Web! Visit us at www.kumc.edu/kcr January is National Cervical Cancer Screening Month. Cervical cancer begins in the lining of the cervix (organ connecting the
King s Research Portal
 King s Research Portal DOI: 10.1111/cyt.12259 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA):
King s Research Portal DOI: 10.1111/cyt.12259 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA):
WELL WOMAN CLINIC-SCREENING PROGRAM FOR CERVICAL CARCINOMAS G. J. Vani Padmaja 1
 WELL WOMAN CLINIC-SCREENING PROGRAM FOR CERVICAL CARCINOMAS G. J. Vani Padmaja 1 HOW TO CITE THIS ARTICLE: G. J. Vani Padmaja. Well Woman Clinic-Screening Program for Cervical Carcinomas. Journal of Evolution
WELL WOMAN CLINIC-SCREENING PROGRAM FOR CERVICAL CARCINOMAS G. J. Vani Padmaja 1 HOW TO CITE THIS ARTICLE: G. J. Vani Padmaja. Well Woman Clinic-Screening Program for Cervical Carcinomas. Journal of Evolution
Can LBC Completely Replace Conventional Pap Smear in Developing Countries
 The Journal of Obstetrics and Gynecology of India (January February 2019) 69(1):69 76 https://doi.org/10.1007/s13224-018-1-7 ORIGINAL ARTICLE Can LBC Completely Replace Conventional Pap Smear in Developing
The Journal of Obstetrics and Gynecology of India (January February 2019) 69(1):69 76 https://doi.org/10.1007/s13224-018-1-7 ORIGINAL ARTICLE Can LBC Completely Replace Conventional Pap Smear in Developing
I have no financial interests in any product I will discuss today.
 Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
Cervical Cancer Screening Update and Implications for Annual Exams George F. Sawaya, MD Professor Department of Obstetrics, Gynecology and Reproductive Sciences Department of Epidemiology and Biostatistics
National Cervical Screening Program MBS Item Descriptors
 National Cervical Screening Program MBS Item Descriptors Item Item descriptor 73070 A test, including partial genotyping, for oncogenic human papillomavirus that may be associated with cervical pre-cancer
National Cervical Screening Program MBS Item Descriptors Item Item descriptor 73070 A test, including partial genotyping, for oncogenic human papillomavirus that may be associated with cervical pre-cancer
What is a Pap Smear and What do the results mean? Maria E Daheri RN Cervical Nurse Case Harris Health System
 What is a Pap Smear and What do the results mean? Maria E Daheri RN Cervical Nurse Case Manager @ Harris Health System What is a Pap Smear and when is it recommended? Pap smear The Pap smear is a screening
What is a Pap Smear and What do the results mean? Maria E Daheri RN Cervical Nurse Case Manager @ Harris Health System What is a Pap Smear and when is it recommended? Pap smear The Pap smear is a screening
Comparative Study of Pap Smear Quality by using Ayre s Spatula versus Ayre s Spatula and Cytobrush Combination
 ORIGINAL ARTICLE Comparative Study of Pap Smear Quality by using Ayre s Spatula versus Ayre s Spatula and Cytobrush Combination Numi Anjum 1, B Sindhoora 2 1. Tutor, Department of Obstetrics and Gynecology
ORIGINAL ARTICLE Comparative Study of Pap Smear Quality by using Ayre s Spatula versus Ayre s Spatula and Cytobrush Combination Numi Anjum 1, B Sindhoora 2 1. Tutor, Department of Obstetrics and Gynecology
Cervical Cancer Screening
 Cervical Cancer Screening MEGAN CHENEY, MD MPH MEDICAL DIRECTOR, THE WOMEN S CENTER ASSISTANT DIRECTOR, BGSMC OBGYN RESIDENCY PROGRAM SECTION CHIEF, THE WOMEN S HEALTH INSTITUTE Objectives Understand proper
Cervical Cancer Screening MEGAN CHENEY, MD MPH MEDICAL DIRECTOR, THE WOMEN S CENTER ASSISTANT DIRECTOR, BGSMC OBGYN RESIDENCY PROGRAM SECTION CHIEF, THE WOMEN S HEALTH INSTITUTE Objectives Understand proper
BRITISH COLUMBIA S CERVICAL CANCER SCREENING PROGRAM
 BRITISH COLUMBIA S CERVICAL CANCER SCREENING PROGRAM DATE: NOVEMBER 19, 2016 PRESENTER: DR. DIRK VAN NIEKERK 1 Conflict of Interest Disclosure Nothing to disclose 2 ..in the beginning of the malady it
BRITISH COLUMBIA S CERVICAL CANCER SCREENING PROGRAM DATE: NOVEMBER 19, 2016 PRESENTER: DR. DIRK VAN NIEKERK 1 Conflict of Interest Disclosure Nothing to disclose 2 ..in the beginning of the malady it
Utility of Pap Smear in Cervical Screening in a Tertiary Care Hospital
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 1 (2017) pp. 319-323 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2017.601.039
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 1 (2017) pp. 319-323 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2017.601.039
PAP SMEAR by Dr.Shantha Krishnamurthy MD Senior Consultant Pathology Fortis Hospitals
 PAP SMEAR by Dr.Shantha Krishnamurthy MD Senior Consultant Pathology Fortis Hospitals Historical Named after George Papanicolaou, a Greek American Studied cervical epithelium in menstrual cycle of guinea
PAP SMEAR by Dr.Shantha Krishnamurthy MD Senior Consultant Pathology Fortis Hospitals Historical Named after George Papanicolaou, a Greek American Studied cervical epithelium in menstrual cycle of guinea
Becoming a colposcopist: Colposcope case studies
 Becoming a colposcopist: Colposcope case studies Seon-Kyung Lee, M.D. Department of Obstetrics and Gynecology College of Medicine, Kyung Hee University Value of Colposcopy Cytology is an effective screening
Becoming a colposcopist: Colposcope case studies Seon-Kyung Lee, M.D. Department of Obstetrics and Gynecology College of Medicine, Kyung Hee University Value of Colposcopy Cytology is an effective screening
Estimated New Cancers Cases 2003
 Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz Estimated New Cancers Cases 2003 Images removed due to copyright reasons.
Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz Estimated New Cancers Cases 2003 Images removed due to copyright reasons.
SQUAMOUS CELLS: Atypical squamous cells (ASC) - of undetermined significance (ASC-US) - cannot exclude HSIL (ASC-H)
 SQUAMOUS CELLS: Atypical squamous cells (ASC) - of undetermined significance (ASC-US) - cannot exclude HSIL (ASC-H) ASC refers to cytologic changes suggestive of SIL, which are qualitativley or quantitatively
SQUAMOUS CELLS: Atypical squamous cells (ASC) - of undetermined significance (ASC-US) - cannot exclude HSIL (ASC-H) ASC refers to cytologic changes suggestive of SIL, which are qualitativley or quantitatively
GYN (Glandulars) Still Difficult After All These Years! Dina R Mody, MD Director of Cytology Laboratories and fellowship Program Methodist Hospital
 GYN (Glandulars) Still Difficult After All These Years! Dina R Mody, MD Director of Cytology Laboratories and fellowship Program Methodist Hospital and Bioreference Labs (Houston) Department of Pathology
GYN (Glandulars) Still Difficult After All These Years! Dina R Mody, MD Director of Cytology Laboratories and fellowship Program Methodist Hospital and Bioreference Labs (Houston) Department of Pathology
PRE TEST CERVICAL SCREENING MANAGEMENT COLPOSCOPY PATHOLOGIC DIAGNOSIS AND TREATMENT
 PRE TEST CERVICAL SCREENING MANAGEMENT COLPOSCOPY PATHOLOGIC DIAGNOSIS AND TREATMENT QUESTION #1 WHICH OF THE FOLLOWING IS NOT A RISK FACTOR FOR CERVICAL CANCER? A. HIGH RISK HPV B. CIGARETTE SMOKING C.
PRE TEST CERVICAL SCREENING MANAGEMENT COLPOSCOPY PATHOLOGIC DIAGNOSIS AND TREATMENT QUESTION #1 WHICH OF THE FOLLOWING IS NOT A RISK FACTOR FOR CERVICAL CANCER? A. HIGH RISK HPV B. CIGARETTE SMOKING C.
TISSUE TUMOR MARKER EXPRESSION IN
 TISSUE TUMOR MARKER EXPRESSION IN NORMAL CERVICAL TISSUE AND IN CERVICAL INTRAEPITHELIAL NEOPLASIA, FOR WOMEN WHO ARE AT HIGH RISK OF HPV (HUMAN PAPILLOMA VIRUS INFECTION). Raghad Samir MD PhD Verksamhet
TISSUE TUMOR MARKER EXPRESSION IN NORMAL CERVICAL TISSUE AND IN CERVICAL INTRAEPITHELIAL NEOPLASIA, FOR WOMEN WHO ARE AT HIGH RISK OF HPV (HUMAN PAPILLOMA VIRUS INFECTION). Raghad Samir MD PhD Verksamhet
Understanding My Pap Test Results
 Form: D-8766 Understanding My Pap Test Results For patients of the Gynecology Oncology Clinic Read this pamphlet to learn more about: why you are having a Pap test how to understand your test results what
Form: D-8766 Understanding My Pap Test Results For patients of the Gynecology Oncology Clinic Read this pamphlet to learn more about: why you are having a Pap test how to understand your test results what
Index. Cytoplasm, nonepithelial malignant tumor features 70
 Accurette device 23 Adenosarcoma, differential diagnosis 80, 81 Arias-Stella reaction 65 Atypical endocervical cells 8 Atypical endometrial cells 8 Atypical glandular cells (AGC) 8, 9 Atypical glandular
Accurette device 23 Adenosarcoma, differential diagnosis 80, 81 Arias-Stella reaction 65 Atypical endocervical cells 8 Atypical endometrial cells 8 Atypical glandular cells (AGC) 8, 9 Atypical glandular
National Surveillance System for Human Papillomavirus Infection and Related Disease in Scotland
 National Surveillance System for Human Papillomavirus Infection and Related Disease in Scotland Version Date: 10th October 2008 Proposal prepared by: M O Leary (EPIET), C. Robertson (Statistics), K. Sinka
National Surveillance System for Human Papillomavirus Infection and Related Disease in Scotland Version Date: 10th October 2008 Proposal prepared by: M O Leary (EPIET), C. Robertson (Statistics), K. Sinka
Hyperchromatic Crowded Groups: What is Your Diagnosis? Session 3000
 Hyperchromatic Crowded Groups: What is Your Diagnosis? Session 3000 Thomas A. Bonfiglio, M.D. Professor Emeritus, Pathology and Laboratory Medicine University of Rochester Disclosures In the past 12 months,
Hyperchromatic Crowded Groups: What is Your Diagnosis? Session 3000 Thomas A. Bonfiglio, M.D. Professor Emeritus, Pathology and Laboratory Medicine University of Rochester Disclosures In the past 12 months,
CERVICAL INTRAEPITHELIAL NEOPLASIA (CIN)
 CERVICAL INTRAEPITHELIAL NEOPLASIA (CIN) The cervix constitutes the lower third of the uterus. It is in two parts, the endocervix and the ectocervix. Ectocervix is covered with squamous epithelium. Endocervix
CERVICAL INTRAEPITHELIAL NEOPLASIA (CIN) The cervix constitutes the lower third of the uterus. It is in two parts, the endocervix and the ectocervix. Ectocervix is covered with squamous epithelium. Endocervix
January 15, 2009 (202) PROPOSALS TO IMPROVE CYTOLOGY PROFICIENCY TESTING REQUIRED BY THE CLINICAL LABORATORY IMPROVEMENT AMENDMENTS OF 1988
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Media Affairs CMS FACT SHEET FOR IMMEDIATE RELEASE Contact:
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Media Affairs CMS FACT SHEET FOR IMMEDIATE RELEASE Contact:
Cervicovaginal Cytology: Normal and Abnormal Cells and Adequacy of Specimens
 Cervicovaginal Cytology: Normal and Abnormal Cells and Adequacy of Specimens 3 Christine Bergeron, MD, PhD Introduction Carcinoma of the cervix is a slow growing cancer, which is preceded by precancerous
Cervicovaginal Cytology: Normal and Abnormal Cells and Adequacy of Specimens 3 Christine Bergeron, MD, PhD Introduction Carcinoma of the cervix is a slow growing cancer, which is preceded by precancerous
ASCCP 2013 Guidelines for Managing Abnormal Cervical Cancer Screening Tests
 ASCCP 2013 Guidelines for Managing Abnormal Cervical Cancer Screening Tests www.treatmentok.com Barbara S. Apgar, MD, MS Professor of Family Medicine University of Michigan Ann Arbor, Michigan Disclosures
ASCCP 2013 Guidelines for Managing Abnormal Cervical Cancer Screening Tests www.treatmentok.com Barbara S. Apgar, MD, MS Professor of Family Medicine University of Michigan Ann Arbor, Michigan Disclosures
These comments are an attempt to summarise the discussions at the manuscript meeting. They are not an exact transcript.
 Dear dr. Weber, We would like to thank you for the review of our manuscript entitled Cervical screening with an interval beyond five years requires different rescreen times for HPV-negative and HPVpositive,
Dear dr. Weber, We would like to thank you for the review of our manuscript entitled Cervical screening with an interval beyond five years requires different rescreen times for HPV-negative and HPVpositive,
The routine use of ZedScan within one colposcopy service in England. MC Macdonald, R Lyon, JE Palmer, JA Tidy
 The routine use of ZedScan within one colposcopy service in England MC Macdonald, R Lyon, JE Palmer, JA Tidy Introduction Colposcopic impression alone has been shown to be subjective with variable rates
The routine use of ZedScan within one colposcopy service in England MC Macdonald, R Lyon, JE Palmer, JA Tidy Introduction Colposcopic impression alone has been shown to be subjective with variable rates
A Study on Diagnostic Accuracy of Cervical Pap Smear by Correlating with Histopathology in a Tertiary Care Centre
 Original Article DOI: 10.21276/APALM.1878 A Study on Diagnostic Accuracy of Cervical Pap Smear by Correlating with Histopathology in a Tertiary Care Centre Rachana L Y, S.S. Hiremath*, Prabhu M H, S.S
Original Article DOI: 10.21276/APALM.1878 A Study on Diagnostic Accuracy of Cervical Pap Smear by Correlating with Histopathology in a Tertiary Care Centre Rachana L Y, S.S. Hiremath*, Prabhu M H, S.S
Taking Laboratory Coding for a Spin. Corrie Alvarez, CPC, CPMA, CPC-I, CEDC
 Taking Laboratory Coding for a Spin Corrie Alvarez, CPC, CPMA, CPC-I, CEDC Agenda Overview of Laboratory Discuss Common Laboratory Terms Coding Guidelines Review Drug Testing, Anatomical Pathology Discuss
Taking Laboratory Coding for a Spin Corrie Alvarez, CPC, CPMA, CPC-I, CEDC Agenda Overview of Laboratory Discuss Common Laboratory Terms Coding Guidelines Review Drug Testing, Anatomical Pathology Discuss
Pushing the Boundaries of the Lab Diagnosis in Asia
 Pushing the Boundaries of the Lab Diagnosis in Asia Diana Lim MBBS, FRCPA, FRCPath (UK) Senior Consultant National University Health System and National University of Singapore Department of Pathology
Pushing the Boundaries of the Lab Diagnosis in Asia Diana Lim MBBS, FRCPA, FRCPath (UK) Senior Consultant National University Health System and National University of Singapore Department of Pathology
An audit of liquid-based cervical cytology screening samples (ThinPrep and SurePath) reported as glandular neoplasia
 DOI:10.1111/j.1365-2303.2009.00695.x An audit of liquid-based cervical cytology screening samples (ThinPrep and SurePath) reported as glandular neoplasia S. A. Thiryayi, J. Marshall and D. N. Rana Manchester
DOI:10.1111/j.1365-2303.2009.00695.x An audit of liquid-based cervical cytology screening samples (ThinPrep and SurePath) reported as glandular neoplasia S. A. Thiryayi, J. Marshall and D. N. Rana Manchester
Cuid d Fheidhmeannacht na Seirbhíse Sláinte. Part of the Health Service Executive. CS/PR/PM-20 Rev 2 ISBN Programme Report 2014/2015
 Programme Report 2014/2015 Contents Summary points 2 Introduction to the statistics 2014/2015 3 Part 1 Cervical screening activity 3 Programme coverage 4 Laboratory turnaround time 7 Notification of results
Programme Report 2014/2015 Contents Summary points 2 Introduction to the statistics 2014/2015 3 Part 1 Cervical screening activity 3 Programme coverage 4 Laboratory turnaround time 7 Notification of results
CYTOLOGY: What every general practitioner should know ( and every future specialist)
 CYTOLOGY: What every general practitioner should know ( and every future specialist) 16 April 2014 Dr C Crause Pathologist and Senior Lecturer Department of Anatomical Pathology University of Pretoria/NHLS
CYTOLOGY: What every general practitioner should know ( and every future specialist) 16 April 2014 Dr C Crause Pathologist and Senior Lecturer Department of Anatomical Pathology University of Pretoria/NHLS
Mapping of Pap smear quality and results using routinely collected South African health facility data
 Mapping of Pap smear quality and results using routinely collected South African health facility data Caroline Makura 14 April 2016 Kathryn Schnippel, Pamela Michelow, Carla Chibwesha, Bridgette Goieman,
Mapping of Pap smear quality and results using routinely collected South African health facility data Caroline Makura 14 April 2016 Kathryn Schnippel, Pamela Michelow, Carla Chibwesha, Bridgette Goieman,
Effect of human papillomavirus vaccination on cervical cancer screening in Alberta
 Effect of human papillomavirus vaccination on cervical cancer screening in Alberta Presenter: Jong Kim 1 Team: Christopher Bell 2, Maggie Sun 3, Gordon Kliewer 3, Linan Xu 3, Maria McInerney 4, Lawrence
Effect of human papillomavirus vaccination on cervical cancer screening in Alberta Presenter: Jong Kim 1 Team: Christopher Bell 2, Maggie Sun 3, Gordon Kliewer 3, Linan Xu 3, Maria McInerney 4, Lawrence
APPENDIX I. Free-Standing Radiation Therapy Centers Cancer Case Identification Program
 APPENDIX I Free-Standing Radiation Therapy Centers Cancer Case Identification Program I-1 Sending Radiation Therapy data to FCDS Beginning January 1, 2003, all Florida Radiation Therapy Centers must send
APPENDIX I Free-Standing Radiation Therapy Centers Cancer Case Identification Program I-1 Sending Radiation Therapy data to FCDS Beginning January 1, 2003, all Florida Radiation Therapy Centers must send
