Colonoscopy MM /01/2010. PPO; HMO; QUEST Integration 10/01/2017 Section: Surgery Place(s) of Service: Outpatient
|
|
|
- Madlyn Reynolds
- 6 years ago
- Views:
Transcription
1 Colonoscopy Policy Number: Original Effective Date: MM /01/2010 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration 10/01/2017 Section: Surgery Place(s) of Service: Outpatient I. Description Colonoscopy is a visual examination of the lining of the colon with a fiberoptic endoscope. The endoscope is inserted through the anus and rectum and advanced through the large intestine under direct vision using the scope s optical system. Instrumentation can be passed through the scope for taking biopsies and removing polyps or suspicious lesions. Colonoscopy is one modality used for colorectal cancer screening. It allows for full structural examination of the colon and rectum in a single session and for the detection of both colorectal polyps and cancers with biopsy or polypectomy. The following criteria/guidelines for the use of colonoscopy for screening of individuals at increased risk for colorectal cancer (CRC) and for surveillance reflect the recommendations of the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer (USMSTF), which is comprised of representatives of the American College of Gastroenterology, American Gastroenterological Association and American Society of Gastrointestinal Endoscopy. II. Criteria/Guidelines A. Screening Colonoscopy 1. Screening colonoscopy is covered (subject to Limitations and Administrative Guidelines) to detect colorectal cancer (CRC) and adenomatous polyps (adenomas) in asymptomatic individuals at average risk, i.e., those without specific risk factors or family history of colorectal cancer or adenomas, beginning at age 50 years or 45 years for African Americans, and every 10 years thereafter until age Screening colonoscopy is covered (subject to Limitations and Administrative Guidelines) to detect colorectal cancer and adenomas in high risk individuals as follows: a. Family history of CRC or adenomas i. For individuals with one first degree relative (parent, sibling or child) with CRC or adenoma diagnosed before the age of 60 or two or more first-degree relatives with CRC or adenomas at any age, screening colonoscopy is covered beginning at age 40, or beginning at age 10 years younger than the age at diagnosis of the youngest affected relative, whichever comes first and every 5 years thereafter. ii. For individuals with one first degree relative with CRC or adenoma diagnosed at greater than or equal to age 60 years or two second degree relatives (grandparent,
2 Colonoscopy 2 aunt or uncle) with CRC or adenoma, screening colonoscopy is covered beginning at age 40 and every 10 years thereafter. NOTE: Individuals may choose to be screened with any recommended form of testing (see Colorectal Screening policy). b. For individuals affected by Lynch syndrome or individuals at risk (first-degree relatives of those affected), screening colonoscopy is covered every 1 to 2 years, beginning between ages 20 to 25 or 2 to 5 years before the youngest age of diagnosis of CRC in the family if diagnosed before age 25 years. c. For individuals with first degree relatives with serrated polyposis syndrome, also called hyperplastic polyposis syndrome, screening colonoscopy is covered at age 40, same age as the earliest diagnosis in the family of serrated polyposis if uncomplicated by CRC or 10 years earlier than earliest diagnosis in the family if complicated by CRC (whichever is earliest) and every five years if no polyps found. d. For individuals with inflammatory bowel disease, chronic ulcerative colitis, and Crohn s colitis, screening colonoscopy is covered beginning eight years after the onset of pancolitis or 12 to 15 years after the onset of left-sided colitis and every one to two years thereafter with biopsy to detect dysplasia. B. Surveillance Colonoscopy 1. Surveillance colonoscopy is covered (subject to Limitations and Administrative Guidelines) for asymptomatic patients after CRC resection as follows: a. Three to six months after curative cancer resection or upon completion of adjuvant therapy to rule out synchronous neoplasms, if not performed preoperatively.. b. One year after the curative resection (or one year following the colonoscopy that was performed to clear the colon of synchronous disease). c. Three years after the one year follow-up colonoscopy, if examination was normal and 5 years thereafter if the three year colonoscopy was normal. 2. Surveillance colonoscopy is covered (subject to Limitations and Administrative Guidelines) for asymptomatic patients with a personal history of adenoma or sessile serrated polyp (SSP) at prior colonoscopy as follows: a. For patients with one to two small (less than one centimeter) tubular adenomas or SSP without cytologic dysplasia, surveillance colonoscopy is covered five to ten years after the initial polypectomy (the precise time within this interval should be based on other clinical factors such as colonoscopy findings, family history, and the preferences of the patient and the judgment of the physician). If there are no adenomas or SSPs on the first surveillance colonoscopy, the second surveillance colonoscopy is covered in ten years. b. For patients with three to ten adenomas and/or SSPs, one adenoma or SSP greater than or equal to one centimeter, any adenoma with villous features or high-grade dysplasia, SSP with cytologic dysplasia, or traditional serrated adenoma that have been completely removed, surveillance colonoscopy is covered three years after the initial polypectomy. If the follow-up colonoscopy is normal or shows only one to two small tubular adenomas with low-grade dysplasia, then the interval for the subsequent colonoscopy is covered every five years. c. For patients with greater than 10 adenomas and/or SSPs on a single examination, surveillance colonoscopy is covered less than three years after the initial polypectomy.
3 Colonoscopy 3 d. For patients with incomplete or piecemeal polypectomy or polypectomy of large sessile polyps, repeat colonoscopy is covered two to six months following the initial polypectomy when necessary to verify complete removal. Once complete removal has been established based on endoscopic and pathologic assessments, subsequent surveillance needs to be individualized based on the physician s judgment.. e. For patients who meet the clinical criteria for serrated polyposis syndrome, colonoscopy is covered every year. Clinical criteria include the following: i. At least five serrated polyps proximal to the sigmoid colon, of which two or more are greater than or equal to ten millimeters ii. Any number of serrated polyps proximal to the sigmoid colon in an individual who has a first degree relative with serrated polyposis syndrome iii. Greater than 20 serrated polyps of any size, distributed throughout the colon f. For patients with hyperplastic polyps, surveillance colonoscopy is covered as follows: i. For patients with any number of hyperplastic polyps in the rectosigmoid that are each individually less than 10 millimeters, surveillance colonoscopy is covered 10 years after the initial polypectomy. ii. For patients with three or less hyperplastic polyps proximal to the sigmoid colon that are each 5 millimeters or less, surveillance colonoscopy is covered 10 years after the initial polypectomy. iii. For patients with four or more hyperplastic polyps proximal to the sigmoid colon that are of any size, surveillance colonoscopy is covered 5 years after the initial polypectomy. A longer subsequent follow-up interval may be appropriately applied when a follow-up exam shows improvement in findings, i.e., a reduction in the number of lesions. iv. For patients with any number of hyperplastic polyps proximal to the sigmoid colon that are each greater than 5 millimeters, surveillance colonoscopy is covered 5 years after the initial polypectomy. A longer subsequent follow-up interval may be appropriately applied when a follow-up exam shows improvement in findings, i.e., a reduction in the size of lesions. g. If colonoscopy is complete to the cecum and the preparation ultimately is deemed inadequate, colonoscopy (generally with a more aggressive preparation regimen) is covered within one year. When advanced neoplasia is detected, an interval shorter than one year is indicated. (NOTE: Preliminary assessment of preparation quality should be made in the rectosigmoid colon. If the preparation is clearly inadequate to allow polyp detection greater than 5 mm, the procedure should be terminated and rescheduled or alternatively, additional bowel cleansing can be attempted for colonoscopy to proceed that day). C. Diagnostic Colonoscopy Diagnostic colonoscopy is covered (subject to Limitations and Administrative Guidelines) for the evaluation of the following: 1. An abnormality discovered by barium enema that is likely to be clinically significant, such as a filling defect or stricture. 2. Unexplained gastrointestinal bleeding
4 Colonoscopy 4 3. Unexplained recent persistent change in bowel habit 4. Hematochezia that is not from the rectum or a perianal source 5. Melena after an upper gastrointestinal source has been excluded 6. Presence of fecal occult blood 7. Unexplained iron deficiency anemia 8. Suspected inflammatory bowel disease manifested by abdominal pain, fever, diarrhea, elevated sedimentation rate, etc. 9. Chronic inflammatory bowel disease of the colon when a more precise determination of the extent of disease will influence management 10. Clinically significant diarrhea of unexplained origin after appropriate work-up 11. Intraoperative identification of the site of a lesion that cannot be detected by palpation or gross inspection at surgery 12. Suspected disease of the terminal ileum 13. Metastatic adenocarcinoma of unknown primary when colon cancer is suspected 14. Acute colonic ischemia/ischemic bowel disease 15. Patients with streptococcus bovis endocarditis D. Therapeutic Colonoscopy Therapeutic colonoscopy is covered for the following: 1. Balloon dilation of stenotic lesions (e.g., anastomotic strictures) 2. Decompression of pseudo-obstruction of the colon 3. Palliative treatment of stenosing or bleeding neoplasms (e.g., laser, electrocoagulation, stenting) 4. Treatment of sigmoid volvulus 5. Treatment of bleeding from such lesions as vascular malformations/anomalies, ulceration, neoplasia or polypectomy site (e.g., electrocoagulation, heat probe, laser or injection therapy) 6. Preoperative marking" for localization of a lesion 7. Removal of a foreign body III. Limitations A. Repeat colonoscopy (or other screening procedures) for patients with small hyperplastic polyps performed at intervals less than that for average risk individuals is not covered. Individuals with small hyperplastic polyps are considered to have normal colonoscopy and should have colonoscopy or other screening options performed at intervals recommended for average-risk individuals. An exception is patients with a hyperplastic polyposis syndrome who are at increased risk for adenomas and CRC and need to be identified for intensive follow-up. B. Discontinuation of surveillance colonoscopy should be considered in patients with serious comorbidities who have life expectancies of less than 10 years according to the physician s judgment. C. Diagnostic colonoscopy is not covered for the following conditions: 1. Chronic, stable irritable bowel syndrome 2. Acute limited diarrhea 3. Hemorrhoids
5 Colonoscopy 5 4. Metastatic adenocarcinoma of unknown primary site in the absence of colonic symptoms and when a definitive site of origin will not influence management 5. Routine follow-up of inflammatory bowel disease 6. Upper gastrointestinal bleeding or melena with a demonstrated upper gastrointestinal source 7. Bright red rectal bleeding in patients with a convincing anorectal source via direct examination, anoscopy, or sigmoidoscopy AND no other symptoms suggestive of a more proximal bleeding source D. The routine use of anesthesia services for patients without high risk situations who are undergoing standard colonoscopy procedures is not covered. (See Anesthesia Services for Gastrointestinal Endoscopy Procedures policy.) IV. Administrative Guidelines A. Precertification is not required for colonoscopy when the above criteria are met. HMSA will from time to time perform retrospective reviews using the above criteria to validate if services rendered meet payment determination criteria. Supporting documentation including, but not limited to, gastroenterology notes, previous colonoscopy procedure reports and pathology reports with number and type of polyp (e.g., adenomatous, hyperplastic), features of polyp (e.g., tubular, villous), and degree of dysplasia (e.g., low grade, high grade) must be maintained in the patient s medical record and must be made available to HMSA on request. B. When both an upper and lower endoscopy are required for a patient, medical literature has established that it is both safe and efficient to do these concurrently. In addition, this is the professional standard of care and the most appropriate and cost effective delivery of the service. Therefore, if the two procedures are not going to be done on the same day, the medical record should clearly document the medical necessity for splitting the services and those records must be made available for review upon request. CPT Procedure Codes Code Description Colonoscopy through stoma; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) With biopsy, single or multiple With removal of foreign body With control of bleeding (eg, injection, bipolar cautery, unipolar cautery, laser, heater probe, stapler, plasma coagulator) With removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps or bipolar cautery With ablation of tumor(s), polyp(s), or other lesion(s) not amenable to removal by hot biopsy forceps, bipolar cautery or snare technique With removal of tumor(s), polyp(s), or other lesion(s) by snare technique
6 Colonoscopy With transendoscopic stent placement (includes predilation) Colonoscopy, rigid or flexible, transabdominal via colotomy, single or multiple Colonoscopy, flexible, proximal to splenic flexure; diagnostic, with or without collection of specimen(s) by brushing or washing, with or without colon decompression (separate procedure) With removal of a foreign body Colonoscopy, flexible, proximal to splenic flexure; with biopsy, single or multiple With directed submucosal injection(s), any substance Colonoscopy, flexible, proximal to splenic flexure; with control of bleeding (eg, injection, bipolar cautery, unipolar cautery, laser, heater probe, stapler, plasma coagulator) Colonoscopy, flexible, proximal to splenic flexure; with ablation of tumor(s), polyp(s), or other lesion(s) not amenable to removal by hot biopsy forceps, bipolar cautery or snare technique Colonoscopy, flexible, proximal to splenic flexure; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps or bipolar cautery Colonoscopy, flexible, proximal to splenic flexure; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique With dilation by balloon, 1 or more strictures (do not report in conjunction with 45387) With transendoscopic stent placement (includes predilation) With endoscopic ultrasound examination (do not report in conjunction with 45330, 45341, 45342, 45378, 76872) With transendoscopic ultrasound guided intramural or transmural fine needle aspiration (biopsy(s) HCPCS Procedure Codes G0105 G0121 ICD-10 Codes Code Description Colorectal cancer screening; colonoscopy on individual at high risk Colorectal cancer screening; colonoscopy on individual not meeting criteria for high risk Code Description Z80.0 Family history of malignant neoplasm of digestive organs Z83.71 Family history of colonic polyps Z84.81 Family history of carrier of genetic disease
7 Colonoscopy 7 Z12.12 Encounter for screening for malignant neoplasm of rectum Z12.11 Encounter for screening for malignant neoplasm of colon V. Important Reminder The purpose of this Medical Policy is to provide a guide to coverage. This Medical Policy is not intended to dictate to providers how to practice medicine. Nothing in this Medical Policy is intended to discourage or prohibit providing other medical advice or treatment deemed appropriate by the treating physician. Benefit determinations are subject to applicable member contract language. To the extent there are any conflicts between these guidelines and the contract language, the contract language will control. This Medical Policy has been developed through consideration of the medical necessity criteria under Hawaii s Patients Bill of Rights and Responsibilities Act (Hawaii Revised Statutes 432E-1.4), generally accepted standards of medical practice and review of medical literature and government approval status. HMSA has determined that services not covered under this Medical Policy will not be medically necessary under Hawaii law in most cases. If a treating physician disagrees with HMSA s determination as to medical necessity in a given case, the physician may request that HMSA reconsider the application of the medical necessity criteria to the case at issue in light of any supporting documentation. VI. References 1. Brooks DD, Winawer SJ, Rex DK, et al. Consensus guidelines from the U.S. multi-society task force on colorectal cancer and the American cancer society. Health care guideline: Colorectal cancer screening. June th Ed. 2. Levin B, Lieberman D, McFarland B, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008; 58: Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology. September :3; National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: Colorectal cancer screening. Version Qaseem A, Denberg TD, Hopkins RH Jr, Humphrey LL, Levine J, Sweet DE, Shekelle P. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Annals of Internal Medicine. March ; (5): U.S. Preventive Services Task Force. Screening for colorectal cancer. October Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin May-Jun; 56(3): Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology May; 130(6):
8 Colonoscopy 8 9. Rex, DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. September 2012; 107(9): Johnson D, Barkun A, Rex D, et al. Optimizing Adequacy of Bowel Cleansing for Colonoscopy: Recommendations From the US Multi-Society Task Force on Colorectal Cancer. American Journal Of Gastroenterology. October 2014;109(10): Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, Rafferty JF; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. Dis Colon Rectum Aug;58(8): Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Robertson DJ, Rex DK. Colonoscopy Surveillance After Colorectal Cancer Resection: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. American Journal Of Gastroenterology Mar;150(3): e Giardiello F, Allen J, Rex D, et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. American Journal Of Gastroenterology. August 2014;109(8): Rubenstein JH, Enns R, Heidelbaugh J, Barkun A; Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Lynch Syndrome. American Journal Of Gastroenterology Sep;149(3):
Historical. Note: The parenthetical numbers in the Clinical Indications section refer to the source documents cited in the References Section below.
 Clinical UM Guideline Subject: Colonoscopy Guideline #: CG-SURG-01 Current Effective Date: 01/21/2015 Status: Revised Last Review Date: 05/15/2014 Description Colonoscopy describes the direct visual inspection
Clinical UM Guideline Subject: Colonoscopy Guideline #: CG-SURG-01 Current Effective Date: 01/21/2015 Status: Revised Last Review Date: 05/15/2014 Description Colonoscopy describes the direct visual inspection
Clinical UM Guideline
 Subject: Guideline #: Current Effective Date: 06/28/2016 Status: Revised Last Review Date: 05/05/2016 Description This document addresses colonoscopy, an endoscopic procedure which allows direct visual
Subject: Guideline #: Current Effective Date: 06/28/2016 Status: Revised Last Review Date: 05/05/2016 Description This document addresses colonoscopy, an endoscopic procedure which allows direct visual
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines
 Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Colorectal Cancer Screening And Related Ancillary Services
 Manual: Policy Title: Reimbursement Policy Colorectal Cancer Screening And Related Ancillary Services Section: Preventive Services Subsection: None Date of Origin: 11/20/2015 Policy Number: RPM046 Last
Manual: Policy Title: Reimbursement Policy Colorectal Cancer Screening And Related Ancillary Services Section: Preventive Services Subsection: None Date of Origin: 11/20/2015 Policy Number: RPM046 Last
DIGESTIVE SYSTEM SURGICAL PROCEDURES May 1, 2015 INTESTINES (EXCEPT RECTUM) Asst Surg Anae
 ENDOSCOPY Z50 Duodenoscopy (not to be claimed if Z399 and/or Z00 performed on same patient within 3 months)... 92.10 Z9 Subsequent procedure (within three months following previous endoscopic procedure)...
ENDOSCOPY Z50 Duodenoscopy (not to be claimed if Z399 and/or Z00 performed on same patient within 3 months)... 92.10 Z9 Subsequent procedure (within three months following previous endoscopic procedure)...
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Colorectal Cancer Screening
 Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
The focus of Chapter 9 is on anoscopy, proctosigmoidoscopy, flexible sigmoidoscopy, and colonoscopy procedures and all
 9 Anoscopy, 45380 45380 45385 Proctosigmoidoscopy, Flexible Sigmoidoscopy, and Colonoscopy 45378 The focus of Chapter 9 is on anoscopy, proctosigmoidoscopy, flexible sigmoidoscopy, and colonoscopy procedures
9 Anoscopy, 45380 45380 45385 Proctosigmoidoscopy, Flexible Sigmoidoscopy, and Colonoscopy 45378 The focus of Chapter 9 is on anoscopy, proctosigmoidoscopy, flexible sigmoidoscopy, and colonoscopy procedures
Billing Guideline. Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/14 Last revision effective 4/16
 Billing Guideline Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/14 Last revision effective 4/16 Florida Hospital Care Advantage plans include full coverage of in-network
Billing Guideline Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/14 Last revision effective 4/16 Florida Hospital Care Advantage plans include full coverage of in-network
NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 Policy name: Preventive Health Guidelines - Men The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Policy name: Preventive Health Guidelines - Men The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
General and Colonoscopy Data Collection Form
 Identifier: Sociodemographic Information Type: Zip Code: Inpatient Outpatient Birth Date: m m d d y y y y Gender: Height: (inches) Male Female Ethnicity: Weight: (pounds) African American White, Non-Hispanic
Identifier: Sociodemographic Information Type: Zip Code: Inpatient Outpatient Birth Date: m m d d y y y y Gender: Height: (inches) Male Female Ethnicity: Weight: (pounds) African American White, Non-Hispanic
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer
 Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer Douglas K. Rex, MD, MACG 1, C. Richard Boland, MD 2, Jason A. Dominitz,
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer Douglas K. Rex, MD, MACG 1, C. Richard Boland, MD 2, Jason A. Dominitz,
NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 Policy name: Preventive Health Guidelines - Men The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Policy name: Preventive Health Guidelines - Men The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Title Description Type / Priority
 Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
Quality Measures In Colonoscopy: Why Should I Care?
 Quality Measures In Colonoscopy: Why Should I Care? David Greenwald, MD, FASGE Professor of Clinical Medicine Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York ACG/ASGE Best
Quality Measures In Colonoscopy: Why Should I Care? David Greenwald, MD, FASGE Professor of Clinical Medicine Albert Einstein College of Medicine Montefiore Medical Center Bronx, New York ACG/ASGE Best
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING
 CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 Policy name: Health Guidelines - Men This policy applies only to non-grandfathered plans as defined in the Affordable Care Act section 1251. The following chart contains procedure, diagnosis and modifier
Policy name: Health Guidelines - Men This policy applies only to non-grandfathered plans as defined in the Affordable Care Act section 1251. The following chart contains procedure, diagnosis and modifier
Colon Cancer Screening. Layth Al-Jashaami, MD GI Fellow, PGY 4
 Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Outcome High Priority
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer
 Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
REFERRAL GUIDELINES: ENDOSCOPY
 Outpatient Referral Guidelines Page 1 REFERRAL GUIDELINES: Referrals for endoscopy must be made using the Essential Referral Content Gastrointestinal Endoscopy Referral Form Please fax the completed referral
Outpatient Referral Guidelines Page 1 REFERRAL GUIDELINES: Referrals for endoscopy must be made using the Essential Referral Content Gastrointestinal Endoscopy Referral Form Please fax the completed referral
This is the portion of the intestine which lies between the small intestine and the outlet (Anus).
 THE COLON This is the portion of the intestine which lies between the small intestine and the outlet (Anus). 3 4 5 This part is responsible for formation of stool. The large intestine (colon- coloured
THE COLON This is the portion of the intestine which lies between the small intestine and the outlet (Anus). 3 4 5 This part is responsible for formation of stool. The large intestine (colon- coloured
FY 2017 MCRCEDP Procedure Code Reference Chart
 45378-53 45380 45381 45382 45384 45385 48388 45390 ; Colonoscopy, flexible, proximal to splenic flexure; diagnostic, with or without collection of specimen(s) by brushing or washing, with or without colon
45378-53 45380 45381 45382 45384 45385 48388 45390 ; Colonoscopy, flexible, proximal to splenic flexure; diagnostic, with or without collection of specimen(s) by brushing or washing, with or without colon
Preventive Services versus Diagnostic and/or Medical Services
 Manual: Policy Title: Reimbursement Policy Preventive Services versus Diagnostic and/or Medical Services Section: Administrative Subsection: None Date of Origin: 1/1/2000 Policy Number: RPM037 Last Updated:
Manual: Policy Title: Reimbursement Policy Preventive Services versus Diagnostic and/or Medical Services Section: Administrative Subsection: None Date of Origin: 1/1/2000 Policy Number: RPM037 Last Updated:
Neoplastic Colon Polyps. Joyce Au SUNY Downstate Grand Rounds, October 18, 2012
 Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
DATA COLLECTION GUIDE Direct Data Submission
 DATA COLLECTION GUIDE Colorectal Cancer Screening 2015 (07/01/2014 to 06/30/2015 Dates of Service) 1 Table of Contents Overview of the Process and Timeline 3 Measure Specifications 4 Summary of Changes
DATA COLLECTION GUIDE Colorectal Cancer Screening 2015 (07/01/2014 to 06/30/2015 Dates of Service) 1 Table of Contents Overview of the Process and Timeline 3 Measure Specifications 4 Summary of Changes
Quality ID #439: Age Appropriate Screening Colonoscopy National Quality Strategy Domain: Efficiency and Cost Reduction
 Quality ID #439: Age Appropriate Screening Colonoscopy National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Efficiency DESCRIPTION:
Quality ID #439: Age Appropriate Screening Colonoscopy National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Efficiency DESCRIPTION:
Joint Session with ACOFP and Cancer Treatment Centers of America (CTCA): Cancer Screening: Consensus & Controversies. Ashish Sangal, M.D.
 Joint Session with ACOFP and Cancer Treatment Centers of America (CTCA): Cancer Screening: Consensus & Controversies Ashish Sangal, M.D. Cancer Screening: Consensus & Controversies Ashish Sangal, MD Director,
Joint Session with ACOFP and Cancer Treatment Centers of America (CTCA): Cancer Screening: Consensus & Controversies Ashish Sangal, M.D. Cancer Screening: Consensus & Controversies Ashish Sangal, MD Director,
DATA COLLECTION GUIDE Direct Data Submission
 DATA COLLECTION GUIDE Updated 05/08/2014: Colorectal Cancer Screening 2014 (07/01/2013 to 06/30/2014 Dates of Service) Posted 05/08/2014 Final 1. Column V on page 31: Do not enter dates prior to 07/01/2013.
DATA COLLECTION GUIDE Updated 05/08/2014: Colorectal Cancer Screening 2014 (07/01/2013 to 06/30/2014 Dates of Service) Posted 05/08/2014 Final 1. Column V on page 31: Do not enter dates prior to 07/01/2013.
Objectives. Definitions. Colorectal Cancer Screening 5/8/2018. Payam Afshar, MS, MD Kaiser Permanente, San Diego. Colorectal cancer background
 Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
How to effectively code for Endoscopic procedures in Gastroenterology
 How to effectively code for Endoscopic procedures in Gastroenterology Ariwan Rakvit, MD Associate Professor Division of Gastroenterology Texas Tech University Health Science Center All rights reserved
How to effectively code for Endoscopic procedures in Gastroenterology Ariwan Rakvit, MD Associate Professor Division of Gastroenterology Texas Tech University Health Science Center All rights reserved
GI Coding Updates. Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC
 GI Coding Updates Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC Copyright/Disclaimer 2014 AAPC text CPT copyright 2016 American Medical Association. All rights reserved.
GI Coding Updates Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC Copyright/Disclaimer 2014 AAPC text CPT copyright 2016 American Medical Association. All rights reserved.
Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci. Colon polyps. Colorectal cancer
 Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci Colon polyps Colorectal cancer Harrison s Principles of Internal Medicine 18 Ed. 2012 Colorectal cancer 70% Colorectal cancer CRC and colon
Patologia sistematica V Gastroenterologia Prof. Stefano Fiorucci Colon polyps Colorectal cancer Harrison s Principles of Internal Medicine 18 Ed. 2012 Colorectal cancer 70% Colorectal cancer CRC and colon
Merit-based Incentive Payment system (MIPS) 2018 Qualified Clinical Data Registry (QCDR) Measure Specifications
 Merit-based Incentive Payment system (MIPS) 2018 Qualified Clinical Data Registry (QCDR) Measure Specifications This document contains a listing of the clinical quality measures which the New Hampshire
Merit-based Incentive Payment system (MIPS) 2018 Qualified Clinical Data Registry (QCDR) Measure Specifications This document contains a listing of the clinical quality measures which the New Hampshire
2012 FITWAY Allowable CPT Codes (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility)
 2012 FITWAY Allowable s (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility) Fecal Immunochemical Test (FIT) G0328/ 82274 Colorectal cancer screening
2012 FITWAY Allowable s (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility) Fecal Immunochemical Test (FIT) G0328/ 82274 Colorectal cancer screening
Supplementary Online Content
 Supplementary Online Content Tran AH, Ngor EWM, Wu BU. Surveillance colonoscopy in elderly patients: a retrospective cohort study. JAMA Intern Med. Published online August 11, 2014. doi:10.1001/jamainternmed.2014.3746
Supplementary Online Content Tran AH, Ngor EWM, Wu BU. Surveillance colonoscopy in elderly patients: a retrospective cohort study. JAMA Intern Med. Published online August 11, 2014. doi:10.1001/jamainternmed.2014.3746
2011 FITWAY Allowable CPT Codes (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility)
 2011 FITWAY Allowable s (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility) Fecal Immunochemical Test (FIT) G0328/ 82274 Colorectal cancer screening
2011 FITWAY Allowable s (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility) Fecal Immunochemical Test (FIT) G0328/ 82274 Colorectal cancer screening
Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER
 Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
2016 CPT coding changes and their effects
 18 2016 CPT coding changes and their effects by Linda Barney, MD, FACS, and Mark T. Savarise, MD, FACS Significant Current Procedural Terminology (CPT)* coding changes are being implemented in 2016. Notably,
18 2016 CPT coding changes and their effects by Linda Barney, MD, FACS, and Mark T. Savarise, MD, FACS Significant Current Procedural Terminology (CPT)* coding changes are being implemented in 2016. Notably,
Colon Cancer Screening & Surveillance. Amit Patel, MD PGY-4 GI Fellow
 Colon Cancer Screening & Surveillance Amit Patel, MD PGY-4 GI Fellow Epidemiology CRC incidence and mortality rates vary markedly around the world. Globally, CRC is the third most commonly diagnosed cancer
Colon Cancer Screening & Surveillance Amit Patel, MD PGY-4 GI Fellow Epidemiology CRC incidence and mortality rates vary markedly around the world. Globally, CRC is the third most commonly diagnosed cancer
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2018 Related Policies: None Virtual Colonoscopy/Computed Tomography Colonography Description Computed tomography colonography (CTC), also known as
FEP Medical Policy Manual Effective Date: January 15, 2018 Related Policies: None Virtual Colonoscopy/Computed Tomography Colonography Description Computed tomography colonography (CTC), also known as
EARLY DETECTION OF COLORECTAL CANCER. Epidemiology of CRC
 Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
GIQIC18 Appropriate follow-up interval of not less than 5 years for colonoscopies with findings of 1-2 tubular adenomas < 10 mm
 GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
Larry Hogan, Governor Boyd K. Rutherford, Lt. Governor Robert R. Neall, Secretary. CRF CPEST Program
 Larry Hogan, Governor Boyd K. Rutherford, Lt. Governor Robert R. Neall, Secretary May 18, 2018 Maryland Cigarette Restitution Fund Cancer Prevention, Education, Screening and Treatment (CRF CPEST) Program
Larry Hogan, Governor Boyd K. Rutherford, Lt. Governor Robert R. Neall, Secretary May 18, 2018 Maryland Cigarette Restitution Fund Cancer Prevention, Education, Screening and Treatment (CRF CPEST) Program
Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care
 Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: The percentage
Measure #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clincal Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: The percentage
Structured Follow-Up after Colorectal Cancer Resection: Overrated. R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007
 Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #185 (NQF 0659): Colonoscopy Interval for Patients with a History of Adenomatous Polyps Avoidance of Inappropriate Use National Quality Strategy Domain: Communication and Care Coordination 2018
Quality ID #185 (NQF 0659): Colonoscopy Interval for Patients with a History of Adenomatous Polyps Avoidance of Inappropriate Use National Quality Strategy Domain: Communication and Care Coordination 2018
2/14/2018 CODING PITFALLS IN THE ASC AVERAGE RISK VS HIGH RISK SCREENING TOP CODING PITFALLS
 CODING PITFALLS IN THE ASC Presented by: Kristin Vaughn, CPC, CGCS, CPMA, ICDCT-CM Healthcare Consultant and Lead Auditor AskMueller Consulting, LLC Kristin@askmuellerconsulting.com TOP CODING PITFALLS
CODING PITFALLS IN THE ASC Presented by: Kristin Vaughn, CPC, CGCS, CPMA, ICDCT-CM Healthcare Consultant and Lead Auditor AskMueller Consulting, LLC Kristin@askmuellerconsulting.com TOP CODING PITFALLS
2017 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY. MEASURE TYPE: Process
 Measure. #185 (NQF 0659): Colonoscopy Interval for Patients with a History of Adenomatous Polyps Avoidance of Inappropriate Use National Quality Strategy Domain: Communication and Care Coordination 2017
Measure. #185 (NQF 0659): Colonoscopy Interval for Patients with a History of Adenomatous Polyps Avoidance of Inappropriate Use National Quality Strategy Domain: Communication and Care Coordination 2017
OBSOLETE. NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 This document is obsolete. For current content, please see Preventive Health Guidelines - Women. Policy name: Preventive Health Guidelines - Women The following chart contains procedure and diagnosis code
This document is obsolete. For current content, please see Preventive Health Guidelines - Women. Policy name: Preventive Health Guidelines - Women The following chart contains procedure and diagnosis code
Appendix 1 (as supplied by the authors): Supplementary tables. Supplementary Table A1. Description of OHIP codes used in the current study.
 Appendix 1 (as supplied by the authors): Supplementary tables Supplementary Table A1. Description of OHIP codes used in the current study. OHIP Billing Code OHIP Billing Code Description Colonoscopy and
Appendix 1 (as supplied by the authors): Supplementary tables Supplementary Table A1. Description of OHIP codes used in the current study. OHIP Billing Code OHIP Billing Code Description Colonoscopy and
Colonic Polyp. Najmeh Aletaha. MD
 Colonic Polyp Najmeh Aletaha. MD 1 Polyps & classification 2 Colorectal cancer risk factors 3 Pathogenesis 4 Surveillance polyp of the colon refers to a protuberance into the lumen above the surrounding
Colonic Polyp Najmeh Aletaha. MD 1 Polyps & classification 2 Colorectal cancer risk factors 3 Pathogenesis 4 Surveillance polyp of the colon refers to a protuberance into the lumen above the surrounding
Colorectal Cancer Screening and Surveillance
 Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0148 Colorectal Cancer Screening and Surveillance Table of Contents Related Coverage Resources
Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0148 Colorectal Cancer Screening and Surveillance Table of Contents Related Coverage Resources
Information Technology Solutions
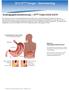 2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
CPT COD1NG UPDATES Gastroenterology CPT Advisors
 2014 CPT COD1NG UPDATES Gastroenterology CPT Advisors Joel V. Brill, MD, AGA CPT Advisor Daniel C. DeMarco, MD, ACG CPT Advisor Glenn D. Littenberg, MD, ASGE CPT Advisor The American College of Gastroenterology
2014 CPT COD1NG UPDATES Gastroenterology CPT Advisors Joel V. Brill, MD, AGA CPT Advisor Daniel C. DeMarco, MD, ACG CPT Advisor Glenn D. Littenberg, MD, ASGE CPT Advisor The American College of Gastroenterology
Colorectal Cancer Screening: A Clinical Update
 11:05 11:45am Colorectal Cancer Screening: A Clinical Update SPEAKER Kevin A. Ghassemi, MD Presenter Disclosure Information The following relationships exist related to this presentation: Kevin A. Ghassemi,
11:05 11:45am Colorectal Cancer Screening: A Clinical Update SPEAKER Kevin A. Ghassemi, MD Presenter Disclosure Information The following relationships exist related to this presentation: Kevin A. Ghassemi,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Virtual Colonoscopy / CT Colonography Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Virtual Colonoscopy / CT Colonography Professional Institutional
Virtual Colonoscopy / CT Colonography Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Virtual Colonoscopy / CT Colonography Professional Institutional
Ontario Association of Gastroenterology
 FEE GUIDE Ontario Association of Gastroenterology OHIP Gastroenterology Fee Guide October 2003* *The fee guide is derived from the Ontario Schedule of Benefits for Physician Services (OHIP fee guide) which
FEE GUIDE Ontario Association of Gastroenterology OHIP Gastroenterology Fee Guide October 2003* *The fee guide is derived from the Ontario Schedule of Benefits for Physician Services (OHIP fee guide) which
PO Box 2345, Beijing , China World J Gastroenterol 2005;11(44): World Journal of Gastroenterology ISSN
 PO Box 2345, Beijing 00023, China World J Gastroenterol 2005;(44):7007-703 www.wjgnet.com World Journal of Gastroenterology ISSN 007-9327 wjg@wjgnet.com ELSEVIER 2005 The WJG Press and Elsevier Inc. All
PO Box 2345, Beijing 00023, China World J Gastroenterol 2005;(44):7007-703 www.wjgnet.com World Journal of Gastroenterology ISSN 007-9327 wjg@wjgnet.com ELSEVIER 2005 The WJG Press and Elsevier Inc. All
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: colonoscopic surveillance for prevention of colorectal cancer in patients with ulcerative colitis, Crohn
2015 Gastroenterology Survival Guide
 2015 Gastroenterology Survival Guide Chapter 11: ICD-9 Coding Your first line of ICD-9 coding is to attach signs or symptoms to your claim, but even after your gastroenterologist performs a diagnostic
2015 Gastroenterology Survival Guide Chapter 11: ICD-9 Coding Your first line of ICD-9 coding is to attach signs or symptoms to your claim, but even after your gastroenterologist performs a diagnostic
ACG Clinical Guideline: Colorectal Cancer Screening
 ACG Clinical Guideline: Colorectal Cancer Screening Douglas K. Rex, MD, FACG 1, David A. Johnson, MD, FACG 2, Joseph C. Anderson, MD 3, Phillip S. Schoenfeld, MD, MSEd, MSc (Epi), FACG 4, Carol A. Burke,
ACG Clinical Guideline: Colorectal Cancer Screening Douglas K. Rex, MD, FACG 1, David A. Johnson, MD, FACG 2, Joseph C. Anderson, MD 3, Phillip S. Schoenfeld, MD, MSEd, MSc (Epi), FACG 4, Carol A. Burke,
Clinical Policy: Monitored Anesthesia Care for Gastrointestinal Endoscopy
 Clinical Policy: Monitored Anesthesia Care for Gastrointestinal Endoscopy Reference Number: CP.MP.161 Last Review Date: 05/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Monitored Anesthesia Care for Gastrointestinal Endoscopy Reference Number: CP.MP.161 Last Review Date: 05/18 See Important Reminder at the end of this policy for important regulatory and
Torisel (temsirolimus)
 Torisel (temsirolimus) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 11/1/2017 POLICY A. INDICATIONS The indications below
Torisel (temsirolimus) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 11/1/2017 POLICY A. INDICATIONS The indications below
Guidelines for Breast, Cervical and Colorectal Cancer Screening
 Guidelines for Breast, Cervical and Colorectal Cancer Screening Your recommendation counts. Talk to your patients about screening for cancer. CancerCare Manitoba provides organized, population-based screening
Guidelines for Breast, Cervical and Colorectal Cancer Screening Your recommendation counts. Talk to your patients about screening for cancer. CancerCare Manitoba provides organized, population-based screening
Tools of the Gastroenterologist: Introduction to GI Endoscopy
 Tools of the Gastroenterologist: Introduction to GI Endoscopy Objectives Endoscopy Upper endoscopy Colonoscopy Endoscopic retrograde cholangiopancreatography (ERCP) Endoscopic ultrasound (EUS) Endoscopic
Tools of the Gastroenterologist: Introduction to GI Endoscopy Objectives Endoscopy Upper endoscopy Colonoscopy Endoscopic retrograde cholangiopancreatography (ERCP) Endoscopic ultrasound (EUS) Endoscopic
Surveying the Colon; Polyps and Advances in Polypectomy
 Surveying the Colon; Polyps and Advances in Polypectomy Educational Objectives Identify classifications of polyps Describe several types of polyps Verbalize rationale for polypectomy Identify risk factors
Surveying the Colon; Polyps and Advances in Polypectomy Educational Objectives Identify classifications of polyps Describe several types of polyps Verbalize rationale for polypectomy Identify risk factors
Bariatric Surgery MM /11/2001. HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient; Inpatient
 Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient;
Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient;
FECAL OCCULT BLOOD TEST
 MEDICAL POLICY For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS FECAL OCCULT BLOOD TEST Policy Number: CMP - 023 Effective Date: January 1, 2018 Table
MEDICAL POLICY For use with the UnitedHealthcare Laboratory Benefit Management Program, administered by BeaconLBS FECAL OCCULT BLOOD TEST Policy Number: CMP - 023 Effective Date: January 1, 2018 Table
Carol A. Burke, MD, FACG
 Updated Guidelines for CRC C Screening and Surveillance Carol A. Burke MD, FACG, FASGE, FACP Cleveland Clinic, Cleveland, OH Gastroenterology t 2012;143:844 143 Gut 2010;59:666 1 Caveat for all Recommendations
Updated Guidelines for CRC C Screening and Surveillance Carol A. Burke MD, FACG, FASGE, FACP Cleveland Clinic, Cleveland, OH Gastroenterology t 2012;143:844 143 Gut 2010;59:666 1 Caveat for all Recommendations
1101 First Colonial Road, Suite 300, Virginia Beach, VA Phone (757) Fax (757)
 1101 First Colonial Road, Suite 300, Virginia Beach, VA 23454 www.vbgastro.com Phone (757) 481-4817 Fax (757) 481-7138 1150 Glen Mitchell Drive, Suite 208 Virginia Beach, VA 23456 www.vbgastro.com Phone
1101 First Colonial Road, Suite 300, Virginia Beach, VA 23454 www.vbgastro.com Phone (757) 481-4817 Fax (757) 481-7138 1150 Glen Mitchell Drive, Suite 208 Virginia Beach, VA 23456 www.vbgastro.com Phone
Colorectal Cancer Screening
 Colorectal Cancer Screening An Integrated Care Pathway of the Collaborative Care Network Subject Matter Expert: Kevin Wolov, DO Pathway Custodian: Pat Czapp, MD First, a Friendly Reminder... This Integrated
Colorectal Cancer Screening An Integrated Care Pathway of the Collaborative Care Network Subject Matter Expert: Kevin Wolov, DO Pathway Custodian: Pat Czapp, MD First, a Friendly Reminder... This Integrated
EGD Data Collection Form
 Sociodemographic Information Type Zip Code Gender Height (in inches) Race Ethnicity Inpatient Outpatient Male Female Birth Date Weight (in pounds) American Indian (Native American) or Alaska Native Asian
Sociodemographic Information Type Zip Code Gender Height (in inches) Race Ethnicity Inpatient Outpatient Male Female Birth Date Weight (in pounds) American Indian (Native American) or Alaska Native Asian
DIGESTIVE SYSTEM SURGICAL PROCEDURES December 22, 2015 (effective March 1, 2016) INTESTINES (EXCEPT RECTUM) Asst Surg Anae
 December 22, 2015 (effective March 1, 201) INTESTINES (EXCEPT RECTUM) Z513 Hydrostatic - Pneumatic dilatation of colon stricture(s) through colonoscope... 10.50 Z50 Fulguration of first polyp through colonoscope...
December 22, 2015 (effective March 1, 201) INTESTINES (EXCEPT RECTUM) Z513 Hydrostatic - Pneumatic dilatation of colon stricture(s) through colonoscope... 10.50 Z50 Fulguration of first polyp through colonoscope...
Detection of Colorectal Neoplasms in Asymptomatic Patients
 Detection of Colorectal Neoplasms in Asymptomatic Patients Scope This guideline provides recommendations for the detection of colorectal cancer and adenomas in asymptomatic patients. These recommendations
Detection of Colorectal Neoplasms in Asymptomatic Patients Scope This guideline provides recommendations for the detection of colorectal cancer and adenomas in asymptomatic patients. These recommendations
Sage Program Reimbursement Rates (Effective Jan 1, 2018 through Dec 31, 2018)
 Sage Program Reimbursement Rates Code Description of Service Allowable Rates New Patient 99201 History, exam, straight forward decision-making; 10 $44.47 99202 Expanded history; exam, straightforward decision-making;
Sage Program Reimbursement Rates Code Description of Service Allowable Rates New Patient 99201 History, exam, straight forward decision-making; 10 $44.47 99202 Expanded history; exam, straightforward decision-making;
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Analysis of Human DNA in Stool Samples as a Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Analysis of Human DNA in Stool Samples as a Technique for
Analysis of Human DNA in Stool Samples as a Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Analysis of Human DNA in Stool Samples as a Technique for
removal of adenomatous polyps detects important effectively as follow-up colonoscopy after both constitute a low-risk Patients with 1 or 2
 Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: capsule_endoscopy_wireless 5/2002 5/2016 5/2017 11/2016 Description of Procedure or Service Wireless capsule
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: capsule_endoscopy_wireless 5/2002 5/2016 5/2017 11/2016 Description of Procedure or Service Wireless capsule
2015 Procedural Reimbursement Guide for Endoscopy
 2015 Procedural Reimbursement Guide for Endoscopy THIS PROCEDURAL REIMBURSEMENT GUIDE, FOR SELECT ENDOSCOPY PROCEDURES, provides coding and reimbursement information for physicians and facilities. The
2015 Procedural Reimbursement Guide for Endoscopy THIS PROCEDURAL REIMBURSEMENT GUIDE, FOR SELECT ENDOSCOPY PROCEDURES, provides coding and reimbursement information for physicians and facilities. The
Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema
 Bahrain Medical Bulletin, Vol.24, No.3, September 2002 Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema Najeeb S Jamsheer, MD, FRCR* Neelam. Malik, MD, MNAMS** Objective: To
Bahrain Medical Bulletin, Vol.24, No.3, September 2002 Frequency of Diagnosis of Colorectal Cancer with Double Contrast Barium Enema Najeeb S Jamsheer, MD, FRCR* Neelam. Malik, MD, MNAMS** Objective: To
Cyramza (ramucirumab)
 Cyramza (ramucirumab) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 03/01/2017TBD03/01/2018 POLICY A. INDICATIONS The indications
Cyramza (ramucirumab) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 03/01/2017TBD03/01/2018 POLICY A. INDICATIONS The indications
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
Home Total Parenteral Nutrition for Adults
 Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
Policy Specific Section: March 1, 2005 January 30, 2015
 Medical Policy Fecal DNA Analysis for Colorectal Cancer Screening Type: Investigational / Experimental Policy Specific Section: Laboratory/Pathology Original Policy Date: Effective Date: March 1, 2005
Medical Policy Fecal DNA Analysis for Colorectal Cancer Screening Type: Investigational / Experimental Policy Specific Section: Laboratory/Pathology Original Policy Date: Effective Date: March 1, 2005
Colon Cancer Screening and Surveillance. Louis V. Antignano, M.D. Wilson Gastroenterology October 11, 2011
 Colon Cancer Screening and Surveillance Louis V. Antignano, M.D. Wilson Gastroenterology October 11, 2011 Colorectal Cancer Preventable cancer Number 2 cancer killer in the USA Often curable if detected
Colon Cancer Screening and Surveillance Louis V. Antignano, M.D. Wilson Gastroenterology October 11, 2011 Colorectal Cancer Preventable cancer Number 2 cancer killer in the USA Often curable if detected
GENERAL INFORMATION/RATIONALE
 MEASURE DESCRIPTION The percentage of adults age 50 through 75 who had a minimum of one colorectal cancer screening test during the one year measurement period. (Refer to Table CCS-3 for qualifying tests
MEASURE DESCRIPTION The percentage of adults age 50 through 75 who had a minimum of one colorectal cancer screening test during the one year measurement period. (Refer to Table CCS-3 for qualifying tests
Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care
 Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: The
Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: The
Genetic Testing of Inherited Cancer Predisposition Genetic Testing - Oncology
 Genetic Testing of Inherited Cancer Predisposition Genetic Testing - Oncology Policy Number: Original Effective Date: MM.02.010 05/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration
Genetic Testing of Inherited Cancer Predisposition Genetic Testing - Oncology Policy Number: Original Effective Date: MM.02.010 05/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration
Colorectal Cancer Screening. Dr Kishor Muniyappa 2626 Care Drive, Suite 101 Tallahassee, FL Ph:
 Colorectal Cancer Screening Dr Kishor Muniyappa 2626 Care Drive, Suite 101 Tallahassee, FL 32308 Ph: 850-297-0351 What we ll be talking about How common is colorectal cancer? What is colorectal cancer?
Colorectal Cancer Screening Dr Kishor Muniyappa 2626 Care Drive, Suite 101 Tallahassee, FL 32308 Ph: 850-297-0351 What we ll be talking about How common is colorectal cancer? What is colorectal cancer?
Somatuline Depot (lanreotide)
 Somatuline Depot (lanreotide) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 12/01/2017TBD POLICY A. INDICATIONS The indications
Somatuline Depot (lanreotide) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 12/01/2017TBD POLICY A. INDICATIONS The indications
Oncology, Colorectal Surgery, Gastroenterology, Internal Medicine, Family Practice, General Surgery, Geriatric Medicine, Multispecialty
 Measure : Follow-Up After Initial Diagnosis and Treatment Of Colorectal Cancer: Colonoscopy Owner: NQF (#0572) Measure Code: CLF Lab Data: N Steward: Health Benchmarks-IMS Health Rule Description: Percentage
Measure : Follow-Up After Initial Diagnosis and Treatment Of Colorectal Cancer: Colonoscopy Owner: NQF (#0572) Measure Code: CLF Lab Data: N Steward: Health Benchmarks-IMS Health Rule Description: Percentage
2017 Procedural Reimbursement Guide for Endoscopy
 2017 Procedural Reimbursement Guide for Endoscopy THIS PROCEDURAL REIMBURSEMENT GUIDE, FOR SELECT ENDOSCOPY PROCEDURES, provides coding and reimbursement information for physicians and facilities. The
2017 Procedural Reimbursement Guide for Endoscopy THIS PROCEDURAL REIMBURSEMENT GUIDE, FOR SELECT ENDOSCOPY PROCEDURES, provides coding and reimbursement information for physicians and facilities. The
Kyphoplasty and Vertebroplasty
 Kyphoplasty and Vertebroplasty Policy Number: Original Effective Date: MM.06.007 01/11/2005 Line(s) of Business: Current Effective Date: HMO; PPO 02/01/2012 Section: Surgery Place(s) of Service: Inpatient;
Kyphoplasty and Vertebroplasty Policy Number: Original Effective Date: MM.06.007 01/11/2005 Line(s) of Business: Current Effective Date: HMO; PPO 02/01/2012 Section: Surgery Place(s) of Service: Inpatient;
Hamideh Salimzadeh, PhD Assistant Professor, Digestive Diseases Research Center,Tehran University of Medical Sciences, Shariati Hospital, North
 Hamideh Salimzadeh, PhD Assistant Professor, Digestive Diseases Research Center,Tehran University of Medical Sciences, Shariati Hospital, North Kargar Avenue 14666 Tehran, Iran. Tel: +98-21-82415415 Fax:
Hamideh Salimzadeh, PhD Assistant Professor, Digestive Diseases Research Center,Tehran University of Medical Sciences, Shariati Hospital, North Kargar Avenue 14666 Tehran, Iran. Tel: +98-21-82415415 Fax:
Colorectal Cancer Prevention Quantity and Quality Count
 Colorectal Cancer Prevention Quantity and Quality Count Ernesto Drelichman, MD Gastrointestinal Surgery & Endoscopy Providence Hospital Key Messages Colorectal cancer can be prevented Screening reduces
Colorectal Cancer Prevention Quantity and Quality Count Ernesto Drelichman, MD Gastrointestinal Surgery & Endoscopy Providence Hospital Key Messages Colorectal cancer can be prevented Screening reduces
