Epithelial Ovarian Cancer: Disease and Pipeline Analysis
|
|
|
- Patricia Hodge
- 6 years ago
- Views:
Transcription
1 Epithelial Ovarian Cancer: Disease and Pipeline Analysis January, 2015
2 Introduction Table of Contents Copyright and Usage Guidelines... 6 Chapter 1 Ovarian Cancer Disease Overview... 9 Ovarian Cancer10 Pathogenesis of Ovarian Cancer 11 Pathophysiology of Ovarian Cancer 12 Ovarian Cancer Risk Factors 13 Age 13 Reproductive History 13 Family History 13 Other factors 14 Infographic 15 Chapter 2 - Ovarian Cancer Epidemiology Ovarian Cancer Incidence 17 Ovarian Cancer Mortality 22 Chapter 3 Ovarian Cancer Diagnosis and Staging...24 Signs and Symptoms 25 Screening 25 Diagnosis 25 Serum CA Imaging 26 Malignancy Indices 27 Biopsy 27 Chapter 4 Ovarian Cancer Staging...28 Chapter 5 Treatment of Ovarian Cancer Surgery for Ovarian Cancer 33 Chemotherapy 33 Paclitaxel 33 Carboplatin Epithelial Ovarian Cancer: Disease and Pipeline Analysis 2
3 Introduction Cisplatin 34 Docetaxel 34 Pegylated Liposomal Doxorubicin (PLD) 34 Gemcitabine (Gemzar) 35 Topotecan 35 Pemetrexed 36 Yondelis (trabectedin) 36 Targeted Therapy 38 Bevacizumab (Avastin) 38 Treatment by Disease Stage and Type 40 Early Stage Ovarian Cancer 40 Late Stage Ovarian Cancer 40 Recurrent Ovarian Cancer 44 Chapter 6. Ovarian Cancer Prognosis...46 Chapter 7. Environmental Analysis...49 Areas of Unmet Need 50 Patient and Physician Awareness 50 Effective Screening and Early Diagnosis 50 Implementation of Clinical Best Practice 50 Clinical Trial Design Issues 51 Prevention of Tumour Recurrence 52 Targeted Approaches to Treatment 53 Major Market Analysis 54 Trial and Recruitment Environment 54 Regulatory Environment 55 Competitive Product Landscape 56 Pricing and Reimbursement Environment 57 Emerging Market Analysis 59 Trial and Recruitment Environment 59 Regulatory Environment 61 Competitive Product Landscape Epithelial Ovarian Cancer: Disease and Pipeline Analysis 3
4 Introduction Pricing and Reimbursement Environment 62 Patient Recruitment Environment 64 Drug Development Field 65 Chapter 8. Drug Development in Ovarian Cancer...66 Angiogenesis Inhibitors 70 Pazopanib (Votrient), GlaxoSmithKline 71 Cediranib, AstraZeneca 72 Nintedanib (Vargatef), Boehringer Ingelheim 74 Trebananib, Amgen 75 PARP (poly ADP ribose polymerase) inhibitors 76 Olaparib, AstraZeneca 79 Niraparib (MK-4827), Tesaro 80 Rucaparib, Clovis Oncology 81 Folate Receptor Overexpression 82 Vintafolide (Vynfinit), Merck and Endocyte 83 Farletuzumab, Eisai (Morphotek) 84 Immunotherapy 85 CVac, PrimaBiomed 86 Intracellular Signalling Cascades 88 Binimetinib (MEK162), Array Pharma/Novartis 88 Cytotoxics 91 Karenitecin, BioNumerik 92 Trabectedin (Yondelis), Janssen (J&J) 93 Opaxio (paclitaxel poliglumex), Cell Therapeutics 93 Paclical (encapsulated paclitaxel), Oasmia Pharmaceutical 94 Other Mechanisms 95 Citations...96 Ovarian Cancer Diagnosis and Treatment Guidelines...97 About Industry Standard Research Epithelial Ovarian Cancer: Disease and Pipeline Analysis 4
5 Introduction List of Tables Table 1. Incidence of Ovarian Cancer in the Major Markets 18 Table 2. Incidence of Ovarian Cancer in Emerging Markets 19 Table 3. Age at Diagnosis of Ovarian Cancer (US) 20 Table 4. Incidence Rate of Ovarian Cancer by Ethnicity (US) 21 Table 5. Ovarian Cancer Mortality in the Major Markets 22 Table 6. Age at Death from Ovarian Cancer (US) 22 Table 7. Ovarian Cancer Mortality in the Developing Markets23 Table 8. Classification of Ovarian Cancer 31 Table 9. 5-Year Relative Survival by Disease Stage (US) 47 Table 10. Ovarian Cancer - Major Markets Patient Population and Product Environment 54 Table 11. Ovarian Cancer - Emerging Markets Patient Population and Product Environment 59 Table 12. Number of Patients in Pharma Sponsored Ovarian Cancer Clinical Trials by Phase 65 Table 13. Late Stage Ovarian Cancer Pipeline 67 Table 14. Angiogenesis Inhibitors in Clinical Development for Ovarian Cancer 71 Table 15. PARP Inhibitors in Development for Ovarian Cancer 78 Table 16. Folate Receptor Antagonists in Development for Ovarian Cancer 83 Table 17. Immunotherapies in Development for Ovarian Cancer 83 Table 18. Intracellular Signalling Cascade Inhibitors for Ovarian Cancer 88 Table 19. Cytotoxics in Development for Ovarian Cancer 91 Table 20. Other Therapies in Development for Ovarian Cancer 95 List of Figures Figure 1. Female Reproductive System 10 Figure 2. Summary of Ovarian Cancer Subtypes 12 Figure 3. Global Incidence of Ovarian Cancer 17 Figure 4. Countries with the Highest Number of Ovarian Cancer Cases (2012) 20 Figure 5. Countries with the Highest ASR of Ovarian Cancer (2012) 20 Figure 6. Countries with the Highest Number of Annual Deaths from Ovarian Cancer 23 Figure 7. The Stages of Ovarian Cancer 30 Figure 8. Distribution of Active Industry Sponsored Ovarian Cancer Trials, by Phase 64 Figure 9. Major Intracellular Pathways Targeted for the Treatment of Ovarian Cancer Epithelial Ovarian Cancer: Disease and Pipeline Analysis 5
6 Introduction Thank you for your purchase of the 2015 Epithelial Ovarian Cancer: Disease and Pipeline Analysis report. Please read and observe the following articles associated with the use and distribution of the contents of this report. The purchase of a Single User License means that the report, either in whole or in part, cannot be distributed by any means to individuals or organizations beyond the original purchaser of the license. If at any point you wish to convert your Single User License to an Enterprise-wide License, Industry Standard Research will gladly do so. The purchase of an Enterprise-wide License means that the report may be circulated, either electronically or via hardcopy, within but not beyond the company holding the Enterprise-wide License. Any license holder should feel free to include elements of this report in his or her own work products (i.e., reports and presentations), providing that the elements carry with them a citation stating: Source: Industry Standard Research, No advertising or other promotional use can be made of the information in this report without the express prior written consent of Industry Standard Research. For such consent, please contact Andrew Schafer, President, at (919) or via at AndrewS@ISRreports.com Epithelial Ovarian Cancer: Disease and Pipeline Analysis 6
7 Introduction Table of Acronyms Acronym Description ACS American Cancer Society AJCC American Joint Committee on Cancer AMNOG Act on the Reform of the Market for Medicinal Products ASR Age Standardised Rate BLA Biologics Licence Application BRCA 1 and 2 Breast Cancer, early onset (gene) BRICK Brazil, Russia, India, China, South Korea CA 125 Cancer antigen 125 CHMP Committee for Medicinal Products for Human Use CT Computed Tomography CTD Clinical Trial Directive EGF(R) Epidermal Growth Factor (Receptor) EMA European Medicines Agency EU European Union FDA Food and Drugs Administration (United States) FGF(R) Fibroblast Growth Factor FIGO International Federation of Gynecology and Obstetrics FR Folate Receptor HR Hazard Ratio IBS Irritable Bowel Syndrome IP Intellectual Property LGSOC Low Grade Serous Ovarian Cancer MAA Marketing Authorization Application MRI Magnetic Resonance Imaging NCCN National Comprehensive Cancer Network NCI National Cancer Institute NHS National Health Service (UK) NICE National Institute for Health and Care Excellence PARP Poly ADP ribose polymerase PDGF(R) Platelet Derived Growth Factor (Receptor) PI3K Phosphatidylinositide 3-kinases PLD Pegylated liposomal doxorubicin SEER Surveillance, Epidemiology and End Results program TKI Tyrosine Kinase Inhibitor US United States VEGF(R) Vascular Endothelial Growth Factor (Receptor) WHO World Health Organization Industry Standard Research Epithelial Ovarian Cancer: Disease and Pipeline Analysis 7
8 Introduction Definitions Age Standardised Rate (ASR): A summary measure of the rate that a population would have if it had a standard age structure. Standardization is necessary when comparing several populations that differ with respect to age because age has a powerful influence on the risk of cancer. The ASR is a weighted mean of the age-specific rates; the weights are taken from population distribution of the standard population. The most frequently used standard population is the World Standard Population. It is expressed per 100,000 population Epithelial Ovarian Cancer: Disease and Pipeline Analysis 8
9 Chapter 1 Ovarian Cancer Disease Overview CHAPTER 1 Ovarian Cancer Disease Overview The ovaries are a pair of organs of the female reproductive system. They are located within the pelvic cavity on either side of the uterus and are attached to the uterus via the ovarian ligament and attached to the wall of the peritoneal cavity via a suspensory ligament. The ovary is comprised of a number of cell types including: Follicular cells epithelial cells which originate from surface epithelium covering the ovary Granulosa cells surround the follicular cells, are cuboidal, and proliferate to produce a stratified epithelium Gametes egg or haploid cell released into fallopian tubes and fertilized by sperm to form zygote Germinal epithelium the outermost layer of the ovary Ovarian cortex comprised of ovarian follicles and stroma Ovarian medulla the innermost layer of the ovary, usually without follicles Blood vessels and lymphatics Epithelial Ovarian Cancer: Disease and Pipeline Analysis 9
10 Chapter 1 Ovarian Cancer Disease Overview The ovaries are responsible for secretion of the hormones oestrogen, progesterone, and testosterone. Oestrogen is responsible for the appearance of secondary sex characteristics at puberty and the maturation and maintenance of the reproductive organs in their mature state. Progesterone readies the uterus for pregnancy and prepares the mammary glands for lactation. Together with oestrogen, progesterone promotes the changes which occur in the endometrium during the menstrual cycle. Testosterone has a number of functions including the development of secondary sex characteristics and growth of muscle mass and bone density. Figure 1. Female Reproductive System Ovarian Cancer Approximately 90% of cases of ovarian cancer are of epithelial origin. The remaining 10% are classified as non-epithelial ovarian cancer. Epithelial ovarian cancers are sub-classified by the World Health Organization as serous, endometrioid, clear cell, mucinous, Brenner (transitional cell), mixed epithelial, undifferentiated, and unclassified tumours. Although ovarian cancer is often considered clinically as one disease, histological subtype has prognostic significance, as does tumour grade. Tumour grade is determined using a number of grading systems, with no one system being universally accepted. It is becoming more widely recognised that different grading systems are appropriate for different histological subtypes. Characteristics of the tumour used in grading include architectural features, mitotic counts, and nuclear atypia. Up to 80% of advanced ovarian cancers are of the serous histological subtype. Those with mild to moderate cytologic atypia and low mitotic rates are classified as low-grade, whereas tumours with severe cytologic atypia and high mitotic rates are considered high-grade serous tumours. Many high grade serous tumours are considered to arise from the epithelium of the distal fallopian tube. Low grade serous Epithelial Ovarian Cancer: Disease and Pipeline Analysis 10
11 Chapter 1 Ovarian Cancer Disease Overview tumours account for only approximately 10% of all serous tumours. These tumours are more commonly diagnosed in younger women and are associated with longer survival. However, these tumours do not respond to traditional chemotherapy regimens. Mucinous tumours are the most heterogeneous group of epithelial tumours and tend to form large dominant masses. They comprise approximately 3% of all ovarian cancers. Endometrioid ovarian cancers account for approximately 10% of ovarian cancers and are typically early stage (stage 1) and low grade. Endometriosis and, in particular, endometriotic cysts have been implicated as precursors to endometrioid ovarian cancer. Clear cell cancers comprise approximately 5% of ovarian cancers; however, the incidence of this cancer type varies widely according to geography. It is more commonly diagnosed in Japanese women, compared with the US and Europe. Advanced stage clear cell cancer has a poorer prognosis than serous ovarian cancers due to resistance to standard ovarian cancer chemotherapies. Non-epithelial tumours include germ cell tumours, which are diagnosed principally in those under 20 years of age, and sex cord-stromal tumours. The treatment of these cancers is not covered by this report. Pathogenesis of Ovarian Cancer Ovarian cancer is a heterogeneous disease; a dualistic model for the pathogenesis of the disease is recognised which categorises epithelial tumours as type 1 or type 2 carcinomas. Type 1 carcinomas are frequently low-grade and indolent and include low-grade serous, endometrioid, mucinous, clear cell, and malignant Brenner tumours. They are relatively genetically stable and are characterised by mutations of the KRAS, BRAF, ERBb2, PTEN, PIK3CA, and ARID1A genes, which occur early in the evolution of the tumour. While a stepwise sequence of tumour development from benign precursor to malignant lesion is recognised for type 1 cancers, this is not clear for type 2 cancers. Type 2 cancers are high-grade, aggressive tumours comprising high-grade serous, high-grade endometrioid, malignant mixed mesodermal tumours, and undifferentiated tumours. These tumours are highly associated with TP53 gene mutations and these tumours also frequently carry BRCA 1 and 2 (Breast Cancer Early Onset) mutations Epithelial Ovarian Cancer: Disease and Pipeline Analysis 11
12 Chapter 1 Ovarian Cancer Disease Overview Figure 2. Summary of Ovarian Cancer Subtypes Ovarian Cancer (239,000 cases globally) Epithelial Ovarian Cancer 90% of cases Non-Epithelial Ovarian Cancer 10% of cases Tumour Sub- Type High Grade Serous 65% of cases Low Grade Serous 7% of cases Endometrioid 10% of cases Clear Cell 5% of cases Mucinous 3% 0f cases Sex Cord- Stromal Others e.g. Germ Cell Tumours Genetic Mutations and Pathway Alterations TP53, BRCA1&2, NF1, RB1, CDK12, HRRGs, Pathway alterations: PI3K/RAS/RAF /NOTCH BRAF, KRAS, NRAS, ERBB2 ARID1A, PIK3CA, PTEN ARID1A, PIK3CA, PTEN KRAS, HER2 amplification Clinical Characteristics Typically advanced stage at diagnosis Sensitive to chemotherapy High rate of recurrence. Tumours develop resistance to chemotherapy Resistant to chemotherapy Typically diagnosed in younger women Longer survival than for high grade serous Typically at advanced stage at diagnosis Typically low grade and early stage at diagnosis Higher incidence in Japan than in West Frequently low stage at diagnosis Poor prognosis Response rate of 15% to traditional chemotherapy Usually low stage at diagnosis Resistant to traditional chemotherapy regimens Pathophysiology of Ovarian Cancer Epithelial ovarian cancer is thought to arise from the de-differentiation of the epithelial cells overlying the ovary which may be incorporated into the ovary during ovulation. Ovarian cancer typically spreads to the surfaces of the peritoneal cavity and the omentum (fatty tissue attached to organs of the abdomen). Metastatic spread occurs in a number of ways including via local growth, implantation into the peritoneum, lymphatic or haematogenous dissemination, or invasion through the diaphragm. Intraperitoneal dissemination is the most common and recognized characteristic of ovarian cancer. Common sites of metastatic disease within the peritoneum include the under-surface of the diaphragm, the paracolic gutters (spaces between the colon and abdominal wall), the bladder, and the recto-urine pouch (cavity between the rectum and back wall of the uterus). Other common sites of metastatic spread are the surface of the liver, the mesentery and serosa of the large and small bowel, the omentum, the uterus, and paraaortic and pelvic lymph nodes. Outside the peritoneal cavity, epithelial ovarian cancer may spread to the pleural cavity, lungs, and lymph nodes of the groin Epithelial Ovarian Cancer: Disease and Pipeline Analysis 12
13 Chapter 1 Ovarian Cancer Disease Overview Ovarian Cancer Risk Factors The causes of ovarian cancer are unknown; however, a number of factors have been identified that affect the risk of development of ovarian cancer. Age Ovarian cancer is predominantly a disease of older, post-menopausal women. According to figures from the Surveillance, Epidemiology and Results Program (SEER) in the US, the median age at diagnosis of ovarian cancer is 63 years, with almost 70% of ovarian cancer diagnoses in women over the age of 55 years. Reproductive History Risk of development of ovarian cancer is highly associated with pregnancy and ovulation. Multiple pregnancies offer an increasingly protective effect against the development of ovarian cancer. Women who have been pregnant have a 50% decreased risk for developing ovarian cancer compared with nulliparous women. Women who have had multiple pregnancies are at lower risk of developing ovarian cancer than those with fewer pregnancies. Age at the time of first birth also affects risk. Women who give birth to their first child when they are under 25 years old have a 30-60% decreased risk. Conversely, women aged over 35 years at time of first birth have an increased risk of developing ovarian cancer. Early menarche and late menopause appear to contribute to a greater risk of ovarian cancer. The use of the oral contraceptive pill, tubal ligation, breastfeeding, and suppression of ovulation offer protection against ovarian cancer. These factors support the idea that risk for ovarian cancer is related to the number of ovulatory cycles. The incessant ovulation theory suggests that repeated ovarian epithelial trauma caused by follicular rupture and subsequent epithelial repair results in genetic alterations within the surface epithelium. The alternative gonadotropin theory proposes that persistent stimulation of the ovaries by gonadotropins, coupled with local effects of endogenous hormones, increases surface epithelial proliferation and subsequent mitotic activity. Both theories suggest that conditions that suppress the ovulatory cycle may play a protective role against ovarian cancer. Studies suggest that women taking hormone therapy, which extends the number of ovulatory cycles, are at increased risk of the development of ovarian cancer. Family History Family history plays an important role in the risk of development of ovarian cancer. The lifetime risk for developing ovarian cancer is 1.3% in the general population, but Epithelial Ovarian Cancer: Disease and Pipeline Analysis 13
14 Chapter 1 Ovarian Cancer Disease Overview increases to 4-5% when one first-degree family member is affected, and 7% when two relatives are affected. However, only 5-10% of ovarian cancer cases occur in women with a family history of the disease. Though women with hereditary ovarian cancer tend to develop the disease approximately 10 years earlier than women with non-hereditary ovarian cancer, the prognosis may be better for those with hereditary disease. In their non-mutated form, BRCA genes produce tumour suppressor proteins. Approximately one person in 4,000 in the general population carries a mutation of the BRCA 1 gene. An inherited BRCA 1 mutation confers a 15% 45% lifetime risk of developing ovarian cancer and 85% risk of developing breast cancer. A BRCA 2 mutation increases the lifetime risk of ovarian cancer to 10% 20% and breast cancer risk of 85%. In families with two first-degree relatives with premenopausal epithelial ovarian cancer, the likelihood of a female relative having an affected BRCA 1 or BRCA 2 gene is as high as 40%. Only approximately 10% of women with ovarian cancer have an identifiable genetic mutation such as the susceptibility genes BRCA 1 and BRCA 2. Due to the presence of mutated BRCA genes, women with a history of breast cancer have an increased risk of epithelial ovarian cancer. Other factors Lactose consumption and the use of talcum powder on the vulva and perineum may be associated with increased risk of epithelial ovarian cancer. Obesity has been postulated as a risk factor; however, it does not appear to be associated with the more aggressive types of ovarian cancer Epithelial Ovarian Cancer: Disease and Pipeline Analysis 14
15 Chapter 1 Ovarian Cancer Disease Overview OVARIAN CANCER SYMPTOMS Bloating Pelvic or abdominal pain Difficulty eating or feeling full quickly Urinary symptoms (urgency or frequency) TREATMENT surgery chemotherapy targeted therapy DEVELOPMENT PIPELINE Learn more in ISR s Epithelial Ovarian Cancer: Disease and Pipeline Analysis on ISRreports.com RISK FACTORS PROGNOSIS 63 GENETICS: BRCA1 and BRCA2 genes, Lynch Syndrome, and family history INCREASING AGE REPRODUCTIVE HISTORY &INFERTILITY HORMONE REPLACEMENT THERAPY INDIA, THE UNITED STATES, AND CHINA have the highest number of annual deaths from Ovarian cancer Median Age At Diagnosis WOMEN WILL BE DIAGNOSED WITH OVARIAN CANCER DURING THEIR LIFETIMES. PREVALENCE: In 2011, there were approximately 188,867 WOMEN living with ovary cancer in the United States. That s about the population of Tallahassee, FL. 1.3% of women are diagnosed with ovarian cancer in their lifetimes 44.6% AVG. 5 YEAR SURVIVAL RATE SOURCES: ISR Researach, seer.cancer.gov, CDC, Epithelial WHO Ovarian Cancer: Disease and ISRreports.com Pipeline Analysis PERCENT OF CASES BY STAGE Distant (metasticized) 61% Regional (spread to lymph nodes) 18% Localized 15% Unknown 6% 5 YEAR SURVIVAL Localized 92% Regional 72% Distant 27% Unknown 22%
16 Chapter 2 - Ovarian Cancer Epidemiology CHAPTER 2 Ovarian Cancer Epidemiology Epithelial Ovarian Cancer: Disease and Pipeline Analysis 16
17 Chapter 2 - Ovarian Cancer Epidemiology Ovarian Cancer Incidence The latest figures from the World Health Organization (WHO) [1] show that worldwide nearly 239,000 women are diagnosed with ovarian cancer each year. The global age standardized rate (ASR) of ovarian cancer is 6.1 cases per 100,000 women. Ovarian cancer is the 7 th most commonly diagnosed cancer in women, accounting for 3.6 % of female cancer diagnoses and 2% of all cancer diagnoses globally. Figure 3. Global Incidence of Ovarian Cancer World Female Estimated number of cancer cases, all ages (total 6,663,001) Other: 1,924, % Breast: 1,676, % Liver: 228, % Thyroid: 229, % Ovary: 238, % Corpus uteri: 319, % Stomach: 320, % Colorectum: 614, % Lung: 583, % Cervix uteri: 527, % Source: Globocan database [1]. Downloaded The incidence of ovarian cancer is highest in developed countries, with these countries having age standardized rates twice as high as those of developing countries. The highest rates of ovarian cancer are found in Northern, Central, and Eastern Europe, followed by Western Europe and the US. The lowest rates are found in Africa and parts of Asia. The estimated number of new ovarian cancer cases in Europe in 2012 was 65,538. In the US, WHO Globocan figures show that there were an estimated 20,874 newly diagnosed cases in The American Cancer Society (ACS) estimates that 21,980 new cases of ovarian cancer will be diagnosed in This represents 1.3% of all US cancer cases and approximately 3% of all cancers in US women. Ovarian cancer is the 17 th most common cancer in the US, but it is the 5 th most common cause of cancer deaths in women after lung cancer (26%), breast cancer (15%), colon cancer (9%), and pancreatic cancer (7%). In Europe, ovarian cancer is the 5 th most common cancer in females and the 13 th most common overall. In the US, the incidence of ovarian cancer has been decreasing slowly since the mid- 1980s [2] Epithelial Ovarian Cancer: Disease and Pipeline Analysis 17
18 Chapter 2 - Ovarian Cancer Epidemiology To 2010, the incidence decreased at a rate of 1.1% per year over the last ten years. In the UK, the incidence of ovarian cancer increased steadily until the early 2000s, after which it started to decrease. The biggest decreases in ovarian cancer incidence have been observed in the younger age groups and this is considered to be associated with the increase in use of the contraceptive pill, which decreases ovarian cancer risk. This trend has also been observed in Northern and Western Europe. In the major pharmaceutical markets, the UK has the highest ASR of ovarian cancer. This corresponds to the high ASRs observed in other countries in Northern Europe. The US has the highest ovarian cancer population of the major markets. The table below describes the ASRs and incidence of ovarian cancer in the major pharmaceutical markets. Table 1. Incidence of Ovarian Cancer in the Major Markets France Germany Italy Japan Spain UK US Total ASR Total Ovarian Cancer New 4,592 6,673 5,911 8,921 3,236 6,692 20,874 Cases (2012) Epithelial Ovarian Cancer 4,133 6,006 5,320 8,029 2,912 6,023 18,787 High Grade Serous Ovarian 2,985 4,337 3,842 5,174 2,103 4,350 13,568 Cancer Low Grade Serous Ovarian ,461 Cancer Endometrioid Ovarian Cancer ,087 Clear Cell Ovarian Cancer , ,044 Mucinous Ovarian Cancer Non-Epithelial Ovarian Cancer ,087 Source: Globocan 2012 [1]. ASR = Age Standardised Rate Epithelial Ovarian Cancer: Disease and Pipeline Analysis 18
19 Chapter 2 - Ovarian Cancer Epidemiology The leading emerging pharmaceutical markets of Brazil, India, and China have ASRs of half those of the major markets. However, due to the size of their total populations, both India and China have more ovarian cancer cases per year than the US. The annual incidence of ovarian cancer in Brazil is equivalent in size to that of the major European markets. Russia has an ASR of 11.3, reflecting the high incidence of ovarian cancer in Northern and Eastern Europe. This gives it a substantial patient population of over 12,000 new epithelial ovarian cancer cases per year. Table 2. Incidence of Ovarian Cancer in Emerging Markets Brazil India China South Korea Russia Total ASR Ovarian Cancer 5,804 26,834 34,575 2,349 13,373 Epithelial Ovarian Cancer 5,224 24,151 31,118 2,114 12,036 High Grade Serous Ovarian Cancer 3,773 17,442 22,474 1,527 8,692 Low Grade Serous Ovarian Cancer 406 1,878 2, Endometrioid Ovarian Cancer 580 2,683 3, ,337 Clear Cell Ovarian Cancer 290 1,342 1, Mucinous Ovarian Cancer , Non-Epithelial Ovarian Cancer 580 2,683 3, ,335 Source: Globocan 2012 [1]. ASR = Age Standardised Rate Epithelial Ovarian Cancer: Disease and Pipeline Analysis 19
20 Chapter 2 - Ovarian Cancer Epidemiology Figure 4 shows the countries with the highest numbers of newly diagnosed ovarian cancer patients each year. Figure 5 shows those countries with the highest ASRs. Figure 4. Countries with the Highest Number of Ovarian Cancer Cases (2012) left Figure 5. Countries with the Highest ASR of Ovarian Cancer (2012) right Based on figures from SEER, in the US [2], ovarian cancer has a median age at diagnosis of 63 years. Two-thirds of ovarian cancer diagnoses in the US are made in women 55 years of age or older; there are 12.3 new cases per 100,000 population per year and there is a 1.3% lifetime risk of suffering from ovarian cancer. Table 3. Age at Diagnosis of Ovarian Cancer (US) Age (years) % of Cases at Diagnosis < > Source: SEER (figures taken from ) Epithelial Ovarian Cancer: Disease and Pipeline Analysis 20
21 Chapter 2 - Ovarian Cancer Epidemiology The incidence rate of ovarian cancer is highest among White women (13.0) and lowest among Asian women (9.3). Table 4.Incidence Rate of Ovarian Cancer by Ethnicity (US) Ethnicity / Race New cases/100,000 All races 12.3 White 13.0 Black 9.8 Asian 9.3 Indigenous 9.8 Hispanic 10.9 Non-Hispanic 12.5 Source: SEER (figures taken from ) Epithelial Ovarian Cancer: Disease and Pipeline Analysis 21
22 Chapter 2 - Ovarian Cancer Epidemiology Ovarian Cancer Mortality The WHO estimates that globally almost 152,000 women lose their lives to ovarian cancer each year. This includes over 42,700 deaths in Europe and over 14,000 in the US. It causes 5% of cancer deaths and is responsible for more deaths annually than any other cancer of the female reproductive system. Improvements in surgical techniques, increased specialization of medical practitioners and treatment in specialist centres, together with the introduction of platinum-based chemotherapy regimens have significantly improved cancer survival rates since the 1970s. Despite these improvements, survival rates for ovarian cancer remain very poor. Figures from the US (SEER) show that between 2006 and 2010, the death rate from ovarian cancer in the US decreased by 2.8% per year among women aged under 65 years and 1.7% in those over 65 years. Relative 5 year survival has increased from 38% in the period to 44.6% in the period Table 5. Ovarian Cancer Mortality in the Major Markets Ovarian Cancer Mortality ASR Total Ovarian Cancer Deaths Source: WHO (Globocan) 2012 [1] France Germany Italy Japan Spain UK US ,389 5,379 3,617 4,986 1,878 4,040 15,377 The median age of death from ovarian cancer in the US is 71 years. The highest death rates occur in patients aged years (25.2%) and years (25.8%). Less than 14% of ovarian cancer deaths occur in patients aged under 55 years. Table 6. Age at Death from Ovarian Cancer (US) Age (years) % of cases at death < > Source: SEER (figures taken from ) Epithelial Ovarian Cancer: Disease and Pipeline Analysis 22
23 Chapter 2 - Ovarian Cancer Epidemiology Mortality rates in the developing markets are lower than those in the major markets, except for Russia. This reflects the lower incidence rates in Brazil, India, China, and South Korea and the higher incidence in Russia. Table 7. Ovarian Cancer Mortality in the Developing Markets Ovarian Cancer Mortality ASR Total Ovarian Cancer Deaths Brazil India China South Korea Russia ,846 19,549 14,676 1,054 7,971 Source: WHO (Globocan) 2012 India has the highest number of deaths from ovarian cancer and has a similar ratio of deaths to new cases annually as the US. China has a surprisingly low number of deaths from ovarian cancer compared to the number of new cases diagnosed annually. The mortality ASR for India is more than twice that of China. Figure 6. Countries with the Highest Number of Annual Deaths from Ovarian Cancer Source: Globocan Epithelial Ovarian Cancer: Disease and Pipeline Analysis 23
24 Chapter 3 Ovarian Cancer Diagnosis and Staging CHAPTER 3 Ovarian Cancer Diagnosis and Staging Epithelial Ovarian Cancer: Disease and Pipeline Analysis 24
25 Chapter 3 Ovarian Cancer Diagnosis and Staging Signs and Symptoms Diagnosis of early stage ovarian cancer is difficult due to the lack of symptoms experienced by the patient. Indeed, many patients with early stage disease have no symptoms and are diagnosed as a consequence of investigation for other conditions. Patients with advanced stage disease exhibit symptoms more commonly. Symptoms of ovarian cancer include abdominal or pelvic pain, constipation, diarrhea, urinary frequency, vaginal bleeding, abdominal distension or discomfort, indigestion and acid reflux, and fatigue. Abdominal distension, nausea, anorexia, dyspepsia, and early satiety are a consequence of an abdominal mass or ascites. Pleural effusions and development of respiratory symptoms such as shortness of breath may result from the extension of the disease across the diaphragm. Symptoms of irritable bowel syndrome (IBS) in women over the age of 50 years are considered significant, as IBS rarely presents for the first time in women of this age. Screening There is currently insufficient evidence for screening for ovarian cancer in the general female population using measurement of CA 125 (cancer antigen 125) and transvaginal ultrasound. Measurement of CA 125 is of limited value for the detection of early stage ovarian cancer as it is elevated in only approximately half of cases. The National Cancer Institute recommends that women considered to be at high-risk of ovarian cancer are recommended to seek advice from their physician and consider annual ultrasonographic examination and CA 125 testing. Women at high risk of development of ovarian cancer and no longer considering pregnancy may consider prophylactic oophorectomy. Diagnosis The presence of advanced ovarian cancer may be suspected from clinical presentation, but it can only be confirmed pathologically, either by removal of the ovaries or, when the disease is advanced, by sampling tissue or ascitic fluid. Serum CA 125 While of limited value in the detection of early stage ovarian cancer, measurement of serum CA 125 levels are routinely used to aid diagnosis. Elevated levels of CA 125 may be observed in only approximately half of patients diagnosed with Stage Epithelial Ovarian Cancer: Disease and Pipeline Analysis 25
26 Chapter 3 Ovarian Cancer Diagnosis and Staging I disease. However, elevated levels are observed in approximately 85% of patients diagnosed with advanced disease. Serum CA 125 levels may also be useful in the follow up of patients after treatment. When used as a diagnostic, CA 125 levels must be used in conjunction with histologic evaluation of the tumour, as CA 125 levels can also be elevated in malignancies such as breast, lung, colon, and pancreatic cancer, and benign gynaecologic problems such as endometriosis, ovarian cysts, and pelvic inflammatory disease. Imaging Ultrasound imaging of the abdomen and pelvis is the first imaging test recommended for women with suspected ovarian cancer. Transvaginal ultrasound offers improved visualisation of the ovaries and is superior to transabdominal ultrasound at differentiating between benign and malignant conditions. On ultrasound imaging, ovarian cancer is strongly associated with morphological findings such as the presence of a large lesion, multi-locular (many vesicles) cysts, solid papillary projections, irregular internal septations, and ascites. If the ultrasound, serum CA 125, and clinical status of the patient suggest ovarian cancer then computed tomography (CT) scans of the pelvis and abdomen are routinely used to determine the extent of disease and to aid in surgical planning. Chest CT or x-ray is used to determine the presence of pleural effusion or disease above the diaphragm. A pleural effusion must be cytologically evaluated in order to confirm the presence of malignancy. Magnetic resonance imaging (MRI) scans are not routinely used in the diagnosis of ovarian cancer. Mammography may be performed as part of the pre-operative assessment, usually in women older than 40 years who have not had a mammogram in the previous six months to one year. Ovarian tumours producing oestrogen may increase the risk of breast cancer and primary breast cancer may metastasize to the ovaries. Patients with diffuse carcinomatosis and gastrointestinal symptoms may require further imaging studies such as upper and/or lower endoscopy and barium enema Epithelial Ovarian Cancer: Disease and Pipeline Analysis 26
27 Chapter 3 Ovarian Cancer Diagnosis and Staging Malignancy Indices Following the initial ultrasound, a malignancy index (RMI I) (risk of malignancy) score is used to determine the referral of women to a specialist multidisciplinary team. The RMI I utilises the results from the serum CA 125 (CA125), menopausal status (M), and ultrasound score (U). RMI = U x M x CA125 The ultrasound result is scored 1 point for each of the following characteristics: multilocular cysts, solid areas, metastases, ascites, and bilateral lesions. The menopausal status is scored as 1 for pre-menopausal and 3 for post-menopausal. Serum CA 125 is measured in IU/ml and can vary between 0 and hundreds or thousands of units. All women with an RMI I score of 250 or greater should be referred for further evaluation. Biopsy In cases where patients do not undergo laparotomy/surgery, it is preferable to make a tissue diagnosis using histology rather than cytology. Histological samples are best obtained using percutaneous image-guided biopsy. If this is not feasible, a laparoscopic biopsy may performed. Fine-needle aspiration diagnostic paracentesis may be performed in patients with diffuse carcinomatosis or ascites without an obvious ovarian mass Epithelial Ovarian Cancer: Disease and Pipeline Analysis 27
28 Chapter 4 Ovarian Cancer Staging CHAPTER 4 Ovarian Cancer Staging Epithelial Ovarian Cancer: Disease and Pipeline Analysis 28
29 Chapter 4 Ovarian Cancer Staging Surgery is required to accurately stage ovarian cancer and obtain biopsies for tumour classification. Biopsies and cytological brushings are taken from the diaphragm, pelvic peritoneum, para-aortic and pelvic nodes, and infracolic omentum. Cytological washings are taken from the peritoneum. Ovarian epithelial cancer is staged according to the FIGO (The Féderation Internationale de Gynécologie et d Obstétrique) system, which was developed by The Féderation Internationale de Gynécologie et d Obstétrique and the American Joint Committee on Cancer (AJCC) [3]. According to this classification system, Stage I ovarian cancer is described as an encapsulated tumour within either one (Stage Ia) or both (Stage Ib) ovaries, or with tumour on the surface of the ovary (Stage Ic), and without the presence of malignant ascites. Stage II ovarian cancer is that in which the growth of the tumour(s) of the ovaries extends into the tissues of the pelvis. Stage III tumours are defined as those which have extended outside the pelvis, with or without presence of tumour cells within the regional lymph nodes. Stage IV describes disease with distant metastatic spread, such as pleural effusion or liver metastases Epithelial Ovarian Cancer: Disease and Pipeline Analysis 29
30 Chapter 4 Ovarian Cancer Staging Figure 7. The Stages of Ovarian Cancer Epithelial Ovarian Cancer: Disease and Pipeline Analysis 30
31 Chapter 4 Ovarian Cancer Staging Table 8. Classification of Ovarian Cancer Stage I Ia Ib Ic a II IIa IIb IIc a III IIIa IIIb IIIc IV Description Growth limited to the ovaries. Growth limited to one ovary; no ascites present containing malignant cells. No tumour on the external surface; capsule intact. Growth limited to both ovaries; no ascites present containing malignant cells. No tumour on the external surfaces; capsules intact. Tumour either stage Ia or Ib, but with tumour on surface of one or both ovaries, or with capsule ruptured, or with ascites present containing malignant cells, or with positive peritoneal washings. Growth involving one or both ovaries with pelvic extension. Extension and/or metastases to the uterus and/or fallopian tubes. Extension to other pelvic tissues. Tumour either stage IIa or IIb, but with tumour on surface of one or both ovaries, or with capsule(s) ruptured, or with ascites present containing malignant cells, or with positive peritoneal washings. Tumour involving one or both ovaries with histologically confirmed peritoneal implants outside the pelvis and/or positive regional lymph nodes. Superficial liver metastases. Tumour is limited to the true pelvis, but with histologically proven malignant extension to small bowel or omentum. Tumour grossly limited to the true pelvis, with negative nodes, but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces, or histologically proven extension to small bowel or mesentery. Tumour of one or both ovaries with histologically confirmed implants, peritoneal metastases of abdominal peritoneal surfaces, none exceeding 2 cm in diameter. Nodes are negative. Peritoneal metastases beyond the pelvis >2cm in diameter and / or positive regional lymph nodes. Growth involving one or both ovaries with distant metastases. If pleural effusion is present, positive cytology required to allocate case to Stage IV. Presence of parenchymal liver metastases. a In order to evaluate the impact on prognosis of the different criteria for allotting cases to stage Ic or IIc, it would be of value to know 1) if rupture of the capsule was spontaneous or caused by the surgeon and 2) if the source of malignant cells detected was peritoneal washings or ascites Epithelial Ovarian Cancer: Disease and Pipeline Analysis 31
32 Chapter 5 Treatment of Ovarian Cancer CHAPTER 5 Treatment of Ovarian Cancer Surgery and chemotherapy are the mainstays of treatment of ovarian cancer. Patient outcomes are highly associated with both the extent of surgical tumour removal and the duration of tumour sensitivity to chemotherapy with a platinum-based regimen Epithelial Ovarian Cancer: Disease and Pipeline Analysis 32
33 Chapter 5 Treatment of Ovarian Cancer Surgery for Ovarian Cancer Surgery is the initial treatment of choice for all stages of ovarian cancer. The aim of surgery is to confirm the diagnosis, determine the extent of the disease in order to accurately stage it, and resect all macroscopic disease. Optimal surgery for the treatment of late stage disease may include hysterectomy, bilateral salpingooophorectomy and removal of all involved omentum, suspicious or enlarged lymph nodes, and bilateral pelvic and para-aortic lymph node dissection, as well as radical pelvic dissection, bowel resection, surface stripping of the diaphragm and other peritoneal surfaces, splenectomy, and cholecystectomy. Repeat cytoreductive surgical intervention may be appropriate for patients who have disease recurrence more than six months after initial treatment. Chemotherapy Chemotherapy is a critical part of the treatment of the majority of women diagnosed with ovarian cancer. It is used in the adjuvant treatment setting as well as for the treatment of recurrent cancer. In a smaller number of patients, it is used in the neoadjuvant setting (prior to surgery), but its use in this manner remains controversial. The chemotherapies commonly used in the treatment of ovarian cancer include paclitaxel, docetaxel, carboplatin, cisplatin, doxorubicin, gemcitabine, topotecan, and pemetrexed. Paclitaxel Paclitaxel, in combination with carboplatin, forms the cornerstone of ovarian cancer chemotherapy. It is the treatment of choice for all ovarian cancer, unless the patient is deemed unsuitable. Paclitaxel was originally discovered by the National Cancer Institute (NCI) as part of a screening programme and is a compound extracted from the Pacific yew tree Taxus brevifolia. It was developed by Bristol-Myers Squibb and is sold under the brand name Taxol. Other formulations of paclitaxel include albumin-bound paclitaxel, which is sold by Abraxis Bioscience under the brand name Abraxane. Paclitaxel is on the World Health Organization s List of Essential Medicines, a list of the most important medication needed in a basic health system. Paclitaxel binds to tubulin and inhibits the disassembly of microtubules, thereby resulting in the inhibition of cell division and induction of apoptosis. It also induces apoptosis directly by binding to and blocking the function of the apoptosis inhibitor protein Bcl-2. Common side effects of treatment with paclitaxel include nausea and vomiting, loss of appetite, alopecia, arthralgia, myalgia, and neuropathy Epithelial Ovarian Cancer: Disease and Pipeline Analysis 33
34 Chapter 5 Treatment of Ovarian Cancer Carboplatin Carboplatin is a second-generation platinum agent with a broader spectrum of anticancer properties than first-generation cisplatin. It is activated intra-cellularly to form reactive platinum complexes which bind to GC-rich sites in DNA, inducing intra- and inter-strand cross-links and DNA-protein cross-links. This cross-linking leads to apoptosis and cell growth inhibition. Carboplatin has a significantly reduced side effect profile compared to cisplatin. Carboplatin was first approved in 1987 for the treatment of patients with ovarian cancer whose disease had recurred after treatment with cisplatin. It now forms a mainstay of treatment of ovarian cancer in combination with paclitaxel. However, as with cisplatin, initial tumour responsiveness is lost in most patients as the tumour becomes resistant to platinum therapy. Cisplatin Cisplatin was the first member of the platinum-containing class of anti-cancer drugs and was first approved for use by the FDA in Cisplatin is used as part of combination regimens to treat a wide range of cancers, including ovarian cancer, small cell lung cancer, testicular cancer, and lymphoma. The side effects of cisplatin therapy include nephrotoxicity and visual perception and hearing disorder. Docetaxel Docetaxel is a second-generation taxane, derived from a compound found in the European yew tree Taxus baccata. It binds to and stabilizes tubulin, thereby inhibiting microtubule disassembly, resulting in cell cycle arrest and cell death. It also inhibits pro-angiogenic factors such as VEGF and induces mediators of the inflammatory response. It was originally developed by Sanofi-aventis under the brand name Taxotere. Docetaxel is used in the treatment of recurrent ovarian cancer which has previously been treated with paclitaxel. Pegylated Liposomal Doxorubicin (PLD) PLD is a liposome-encapsulated form of the anthracycline antineoplastic antibiotic doxorubicin. It is marketed as Doxil and Caelyx. Doxorubicin intercalates between the base pairs of the DNA helix, preventing DNA replication and inhibiting protein Epithelial Ovarian Cancer: Disease and Pipeline Analysis 34
35 Chapter 5 Treatment of Ovarian Cancer synthesis. It also inhibits the enzyme topoisomerase II by stabilizing the topoisomerase II complex after it has broken the DNA chain for replication, preventing the DNA chain from being resealed and thereby preventing replication. Doxorubicin induces a number of common side effects including alopecia, myelosuppression, nausea and vomiting, oesophagitis, and skin reactions. Its most serious adverse effect is life-threatening heart toxicity. PLD was developed to improve the penetration of doxorubicin into tumours and decrease drug clearance, thereby increasing the duration of therapeutic effect. It also modulates toxicity, limiting the cardiac toxicity observed with doxorubicin. The most common side effects of treatment with PLD are hypersensitivity, stomatitis, and handfoot syndrome. PLD has shown response rates of up to 25% in the treatment of ovarian cancer patients with platinum-resistant disease recurrence. Gemcitabine (Gemzar) Gemcitabine was developed by Eli Lilly and marketed under the brand name Gemzar. However, it is now available in generic formulations. Gemcitabine is a nucleoside analogue which prevents DNA synthesis and also binds and inactivates the enzyme ribonucleotide reductase. This results in tumour cell apoptosis. Gemcitabine is used in the treatment of a wide range of cancer types including ovarian cancer, non-small cell lung cancer, pancreatic cancer, and breast cancer. Myelosuppression is the major dose-limiting factor associated with gemcitabine therapy. Other side effects of gemcitabine use include anaemia, neutropenia, and gastrointestinal side effects such as nausea and vomiting. In the treatment of platinum-resistant ovarian cancer, studies demonstrate gemcitabine response rates ranging from 13% to 19%. It has demonstrated noninferiority compared to PLD. Topotecan Topotecan is a semi-synthetic derivative of camptothecin. It stabilizes topoisomerase I-DNA covalent complexes during the S phase of the cell cycle, inhibiting the resealing of topoisomerase I-mediated single-strand DNA breaks and producing potentially lethal double-strand DNA breaks when complexes are encountered by the DNA replication machinery. It causes substantial myelosuppression. Other toxic effects Epithelial Ovarian Cancer: Disease and Pipeline Analysis 35
36 Chapter 5 Treatment of Ovarian Cancer include nausea and vomiting, alopecia, and asthenia. In ovarian cancer, topotecan is used in the treatment of platinum-resistant disease recurrence. In Phase II studies it has achieved objective response rates ranging from 13% to 17%. In combination with bevacizumab, an objective response rate of 25% was observed. Pemetrexed Pemetrexed is a folate antimetabolite which inhibits the enzymes thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase, used in purine and pyrimidine synthesis. By inhibiting the formation of purine and pyrimidine nucleotides, pemetrexed prevents the formation of DNA and RNA. The most common side effects of pemetrexed use include fatigue, and nausea and vomiting. Pemetrexed was developed and is marketed by Eli Lilly under the brand name Alimta. While not specifically approved for use in the treatment of ovarian cancer, pemetrexed has been evaluated for use in platinum-resistant recurrent ovarian cancer is several Phase II trials, demonstrating a response rate of between 9 and 21%. Yondelis (trabectedin) Trabectedin is a tetrahydroisoquinoline molecule, based on a compound originally isolated from the sea squirt Ecteinascidia turbinata. It was discovered and developed by PharmaMar, part of Zeltia, who markets the drug in Europe. It is licensed to Johnson & Johnson in the United States. Trabectedin binds to the minor groove of DNA causing DNA damage and affecting transcription regulation, leading to tumour cell cycle arrest and cell death. In cancer cells, the cell cycle arrest and cell death is not dependent on p53 status, and its effect is increased dramatically in cells deficient in homologous recombination tumour suppressor gene proteins such as BRCA 1 and 2. Trabectedin also has immunomodulatory effects, being selectively cytotoxic against monocytes and tumour-associated macrophages. It inhibits production of proinflammatory and angiogenic mediators, which induces changes in the tumour microenvironment and contributes to its antitumor activity. In September 2007, trabectedin was approved by the European Commission for the treatment of advanced or metastatic soft tissue sarcoma. In November 2009, it received approval in Europe for the treatment of relapsed platinum-sensitive ovarian cancer in Epithelial Ovarian Cancer: Disease and Pipeline Analysis 36
37 Chapter 5 Treatment of Ovarian Cancer combination with PLD. It has been granted orphan drug designation for both of these indications in the US and Europe. This approval was based on the results of a pivotal Phase III trial conducted in 672 women with recurrent ovarian cancer who had failed first-line platinum-based chemotherapy. The trial demonstrated that for patients who were sensitive to platinum therapy, there was a statistically significant improvement in progression-free survival, to 9.2 months, for patients treated with a combination of PLD plus trabectedin, compared with 7.5 months for patients treated with PLD alone (P =.017), and in the overall response rate. However, no improvement in progression-free survival or overall response rate was seen in platinum-resistant patients. While these results were sufficient to gain approval in Europe, Canada and other regions, for use in the treatment of platinum-sensitive recurrent ovarian cancer, a US Food and Drug Administration (FDA) advisory committee rejected the application for trabectedin by fourteen votes to one. The rejection was the result of a number of factors, including a lack of improvement in overall survival; results of an interim analysis showed overall survival times of 20.5 months for trabectedin plus PLD and 19.4 months for PLD alone. Toxicity was also raised as an issue by the committee; however, critics suggested that the concern was exaggerated as most of the reported events were not clinically important. A Phase III trial is currently ongoing in the US to evaluate the combination of trabectedin plus docetaxel versus docetaxel alone as a third-line treatment of platinum-sensitive, advanced relapsed ovarian cancer in patients who have received two previous lines of platinum-based chemotherapy. In order to satisfy regulators, the primary endpoint of the trial is overall survival, with progression-free survival as the secondary endpoint Epithelial Ovarian Cancer: Disease and Pipeline Analysis 37
38 Chapter 5 Treatment of Ovarian Cancer Targeted Therapy Bevacizumab (Avastin) Bevacizumab is monoclonal antibody which inhibits the action of the proangiogenesis growth factor vascular endothelial growth factor (VEGF). Ovarian cancer has been associated with high concentrations of VEGF. A correlation has been observed between high concentrations of VEGF and the development of ascites, and with poorer prognosis in ovarian cancer. Bevacizumab was developed by Genentech/Roche and is marketed as Avastin. It received its first approval in 2004 for the treatment of advanced colorectal cancer. Avastin is now approved in Europe, the US, and Japan for the treatment of colorectal cancer and non-small cell lung cancer. It is approved in Europe and the US for the treatment of kidney cancer and in Europe and Japan for the treatment of breast cancer and ovarian cancer. It is also approved in over 60 countries for use in the treatment of glioblastoma. In the treatment of ovarian cancer, bevacizumab is licensed in Europe for use in combination with carboplatin and paclitaxel for first-line treatment of advanced epithelial ovarian cancer, following approval in This approval was based on the results of the pivotal GOG 0128 and ICON7 studies. Bevacizumab is administered in addition to carboplatin and paclitaxel for up to 6 cycles of treatment (15 mg/kg of body weight given once every 3 weeks) followed by continued use of bevacizumab as a single agent until disease progression or for a maximum of 15 months or until unacceptable toxicity, whichever occurs earlier. Bevacizumab is also approved for use in combination with carboplatin and gemcitabine for the first recurrence of platinum-sensitive advanced ovarian cancer in patients who have not received prior therapy with bevacizumab or other VEGF inhibitors or VEGF receptor-targeted agents. This approval was based on the results of the Phase III OCEANS study (Ovarian Cancer Study Comparing Efficacy and Safety of Chemotherapy and Anti-Angiogenic Therapy in Platinum-Sensitive Recurrent Diseases). Bevacizumab is administered in combination with carboplatin and gemcitabine for 6 cycles (15 mg/kg of body weight given once every 3 weeks) and up to 10 cycles followed by continued use of bevacizmab as a single agent until disease progression. Bevacizumab is not licensed in the US for use in these indications. The UK s National Institute for Health and Care Excellence (NICE) does not recommend the use of bevacizumab by the National Health Service (NHS), either as a first-line treatment in combination with paclitaxel and carboplatin or for the treatment of recurrent platinum Epithelial Ovarian Cancer: Disease and Pipeline Analysis 38
39 Chapter 5 Treatment of Ovarian Cancer sensitive ovarian cancer, despite being approved by the European Medicines Agency (EMA) for use in both of these indications. Its guidance concludes that funding of the treatment by the NHS does not represent the best use of taxpayer money. In August 2014, Roche announced that the European Commission had approved the use of bevacizumab (10 mg/kg of body weight given once every 2 weeks as an intravenous infusion until disease progression or unacceptable toxicity) in combination with paclitaxel, topotecan, or PLD chemotherapy in women with recurrent ovarian cancer resistant to platinum-containing chemotherapy. The approval was based on results of the Phase III AURELIA study. In July 2014, the FDA announced that it had accepted Genentech s supplemental Biologics License Application (sbla) and granted bevacizumab priority review for the treatment of recurrent platinum-resistant ovarian cancer in combination with chemotherapy. In November 2014, the FDA approved the use of bevacizumab in this indication Epithelial Ovarian Cancer: Disease and Pipeline Analysis 39
40 Chapter 5 Treatment of Ovarian Cancer Treatment by Disease Stage and Type Early Stage Ovarian Cancer In patients with early stage disease (Stage Ia and Ib), surgery alone may be curative and should include hysterectomy, bilateral salpingo-oophorectomy, and omentectomy. It is important that patients with early stage cancer have biopsies of the underside of the diaphragm, including pelvic and abdominal peritoneal biopsies and pelvic and para-aortic lymph node biopsies to determine absence of spread to these areas. Bulky lymph nodes should be resected in order to remove all visible disease. In order to retain fertility, unilateral oophorectomy may be a possibility for young patients with grade I tumours, but there is an increased risk of disease recurrence associated with this surgery. Accurate staging of early stage tumours including the determination of histological grade and sub-type of the tumour and identifying the presence of microscopically positive lymph nodes is important in determining the need for adjuvant chemotherapy. Up to 30% of patients with apparently early epithelial ovarian cancer will be upstaged following initial surgery. Stage I patients who receive adjuvant chemotherapy using a platinum-based regimen have improved survival over those who do not receive adjuvant therapy. This is particularly true for patients at higher risk of tumour recurrence (stage 1B/C grade 2/3, any grade 3 or clear-cell histology). The optimal type and duration of treatment for these patients remains controversial. There is no evidence to suggest that six cycles of carboplatin and paclitaxel is more effective than three cycles, or that the combination of paclitaxel and carboplatin is superior to carboplatin alone. Treatment guidelines therefore state that it is reasonable to consider single-agent carboplatin for all women with intermediate- and high-risk stage I disease. Late Stage Ovarian Cancer Surgery Patients diagnosed with FIGO Stage II to IV ovarian cancers are treated with surgery and chemotherapy. Surgery should include total abdominal hysterectomy and bilateral salpingo-oophorectomy with omentectomy and debulking of as much gross tumour Epithelial Ovarian Cancer: Disease and Pipeline Analysis 40
41 Chapter 5 Treatment of Ovarian Cancer as can safely be performed. This may include intestinal resection, peritoneal stripping, diaphragmatic resection, removal of bulky para-aortic lymph nodes, and splenectomy. Complete cytoreduction of all macroscopically visible tumour tissue is associated with significantly increased overall survival and progression-free survival. The volume of residual disease remaining at the completion of the primary surgical procedure is a more powerful prognostic indicator than disease stage. The choice of additional treatments for late stage ovarian cancer is determined by whether the tumour was optimally or sub-optimally cytoreduced. Optimal cytoreduction is defined as total macroscopic tumour clearance with no residual visible disease. Evidence shows that patients who receive treatment in specialist centres with a high volume cases have significantly improved outcomes over those treated in generic treatment centres. Chemotherapy The risk of recurrence of ovarian cancer which has spread beyond the ovary (Stage II-IV) at diagnosis is significant. Therefore, adjuvant chemotherapy after surgery is recommended for all patients. Paclitaxel (175 mg/m2) plus carboplatin (AUC 6-5) administered intravenously every three weeks is the standard regimen, almost worldwide. Six cycles of treatment are administered and there is no evidence to suggest that further cycles are of benefit. The addition of a third chemotherapy drug to this regimen has not been shown to improve overall survival. Cisplatin (50-75 mg/ m2) plus paclitaxel has been demonstrated to be more toxic but non-inferior to carboplatin-paclitaxel and is a viable alternative to carboplatin-paclitaxel. When this combination is used, paclitaxel is administered as a 24-hour infusion to decrease the risk of neurotoxicity. Carboplatin plus docetaxel or PLD are used as alternative therapies in women who are allergic to, or intolerant of, paclitaxel. Docetaxel in combination with carboplatin is associated with less neurotoxicity but greater neutropenia than carboplatin-paclitaxel. Intraperitoneal Chemotherapy Studies have investigated the effect of intraperitoneal chemotherapy on the outcome of women diagnosed with ovarian cancer. The cytotoxic agent (usually the platinum agent) is introduced directly into the peritoneal cavity via a catheter. Introducing the agent intraperitoneally rather than intravenously has the theoretical advantage of enabling much higher concentrations of the agent to be obtained locally without the adverse systemic effects of this dose. The survival advantage demonstrated in these studies led to the National Cancer Institute (NCI) in the US to issue an alert in 1996 recommending that intraperitoneal therapy should be considered in patients with small volume (<1 cm) or no residual disease after surgery. The results of the GOG-0172 trial showed a median survival rate of 66 months for patients on the intraperitoneal treatment arm versus 50 months for patients who received IV administration of cisplatin and paclitaxel (P = 0.03). However, this treatment has not been widely adopted as a standard of care due to its greater toxicity and consequent Epithelial Ovarian Cancer: Disease and Pipeline Analysis 41
42 Chapter 5 Treatment of Ovarian Cancer difficulty in delivering all of the planned treatment. Toxicity issues and catheter-related problems such as infection, blockage, and discomfort contribute to a lower rate of completion of six cycles of treatment than with intravenous therapy. Modifications to the intraperitoneal regimen continue to be evaluated to improve the tolerability and practicality of this form of treatment. Treatment guidelines currently differ on the use of intraperitoneal chemotherapy. For example, the NCI suggests that all women with optimally cytoreduced disease should at least be offered intraperitoneal treatment. However, intraperitoneal chemotherapy is recommended by NICE in the UK as part of a clinical trial only. Dose-Dense Chemotherapy Dose-dense scheduling has been evaluated as a way to improve the effectiveness of paclitaxel chemotherapy, but is not considered standard of care. In studies using a paclitaxel days 1, 8, and 15 and carboplatin on day 1 schedule rather than the typical combination of paclitaxel and carboplatin given every 3 weeks (day 1 of a 21 day cycle), longer median progression-free survival and higher overall survival has been observed. However, early discontinuance due to increased toxicity has been reported. Targeted Therapy Two large randomised clinical trials (GOG-218 and ICON-7) were conducted to assess the addition of the anti-angiogenic agent bevacizumab to the combination of paclitaxel and carboplatin in front-line therapy. Patients in the experimental arm of these trials received bevacizumab intravenously every 3 weeks during the chemotherapy phase, followed by a period of maintenance treatment with the same schedule of bevacizumab. The studies differed in a number of ways. The ICON-7 trial used a lower dose of bevacizumab (7.5 mg/kg in the ICON-7 versus 15 mg/kg in the GOG-218). GOG-218 included only patients with stage III IV and macroscopic residual disease after surgery, but ICON-7 included patients also with high-risk early stage disease and patients in a more advanced stage but without macroscopic residual disease after surgery). The endpoint of both of the trials was progression-free survival. GOG-0218 included 1,873 women with stage III or IV disease, all of whom received chemotherapy carboplatin (AUC 6) and paclitaxel (175 mg/m2 for six cycles). Participants were randomly assigned to the following groups: 1) Control - chemotherapy plus placebo (cycles 2 through 22), 2) Bevacizumab Initiation - chemotherapy plus bevacizumab (15 mg/kg cycles 2 through 6) followed by placebo (cycles 7 through 22) or 3) Bevacizumab Throughout - chemotherapy plus bevacizumab (15mg/kg cycles 2 through 22). Results of GOG-0218 demonstrated no difference in progression-free survival between the control group and the Bevacizumab Initiation group. There was a statistically significant increase in progression-free survival in the Bevacizumab Epithelial Ovarian Cancer: Disease and Pipeline Analysis 42
43 Chapter 5 Treatment of Ovarian Cancer Throughout group when compared with the control group (14.1 vs months), with a Hazard Ratio (HR) of progression or death of in the Bevacizumab Throughout group (95% CI, ; P <.001). Median overall survival did not differ between the groups and was 39.3, 38.7, and 39.7 months for the control group, Bevacizumab Initiation group, and the Bevacizumab Throughout group, respectively. Quality of life did not differ between the three groups. Hypertension grade 2 or greater was more common with bevacizumab than with the placebo. There were more treatmentrelated deaths in the Bevacizumab Throughout arm (10 of 607) than in the control arm (6 of 601). ICON 7 randomly assigned 1,528 women after initial surgery to chemotherapy (carboplatin plus paclitaxel) for six cycles, or to chemotherapy plus bevacizumab (7.5 mg/kg for six cycles), followed by bevacizumab alone for an additional 12 cycles. Median progression-free survival was 17.3 months in the control group and 19 months in the bevacizumab group. HR for progression or death in the bevacizumab group was 0.81 (95% CI, ; P=.004). Bevacizumab has been licensed by the EMA at 15 mg/kg with carboplatin and paclitaxel and for 15 months or until progression. However, bevacizumab is not licensed for the initial treatment of ovarian cancer in the USA, and it is not consistently used in Europe. Neoadjuvant Chemotherapy Neoadjuvant chemotherapy is a consideration for patients whose disease is initially deemed to be inoperable or are unfit for surgery at the time of diagnosis. The aim of the treatment is to reduce the tumour burden sufficiently that the patient may become eligible for surgical cytoreduction followed by additional chemotherapy. It is likely that patients receiving this treatment have inferior outcomes to patients treated with initial maximal cytoreduction surgery followed by chemotherapy and therefore is only used when surgery is not suitable as the initial treatment. Maintenance Chemotherapy A number of studies have evaluated the use of maintenance chemotherapy to improve outcomes over fixed chemotherapy regimens. However, benefits must be balanced against additional toxicities and patient quality of life. A Phase III randomized trial exploring the impact of 12 monthly cycles of paclitaxel as maintenance chemotherapy was discontinued by the Data Safety and Monitoring Committee when an interim analysis revealed a highly statistically significant improvement in progression-free survival. Maintenance chemotherapy continues to be investigated as a way of aiming to extend the time to relapse or reduce the number of patients who suffer disease relapse following surgery and initial chemotherapy Epithelial Ovarian Cancer: Disease and Pipeline Analysis 43
44 Chapter 5 Treatment of Ovarian Cancer Recurrent Ovarian Cancer Approximately 70% of patients diagnosed with ovarian cancer will experience relapse of their disease within three years, despite optimal surgery and paclitaxel-carboplatin combination chemotherapy. For these patients, the benefits to overall survival of additional surgical cytoreduction are unclear and therefore not considered standard of care. There is some evidence that surgery at first relapse may confer survival benefit, but only when complete tumour resection is achieved. The role of radiation therapy in patients with recurrent ovarian cancer has not been defined. The probability of a patient responding to second and subsequent lines of chemotherapy is highly dependent on the progression-free interval from the last dose of the preceding line of chemotherapy. This factor has a significant impact on patient life expectancy. Platinum-refractory patients are defined as those progressing during therapy or within six months of receiving platinum-based therapy (GCIG 4th Ovarian Cancer Consensus Meeting criteria). By these criteria, patients are considered partially platinum-sensitive if progression is between 6 and 12 months following platinum therapy. Patients are considered platinum-sensitive when progression occurs more than twelve months following platinum treatment. Platinum-Refractory Ovarian Cancer Platinum-refractory ovarian cancer patients have a poor prognosis with overall survival of less than 12 months from diagnosis. Further treatment is used for the control of symptoms and to improve quality of life. Paclitaxel, topotecan, PLD, and gemcitabine have all shown some limited activity in these patients with progression-free survival of 3-4 months and response rates of approximately 15%. The choice of treatment is based on toxicity, clinical situation of the patient, and convenience of treatment administration. The Phase III AURELIA trial evaluated the use of bevacizumab in patients with platinum-resistant ovarian cancer. These patients had received no more than two previous lines of treatment. Patients received standard chemotherapy according to physician choice (weekly paclitaxel, PLD, or topotecan) and were randomised to receive either bevacizumab or no additional treatment, together with the physicianselected chemotherapy and then as maintenance therapy until progression. Results showed that the addition of bevacizumab to chemotherapy gave a clinically meaningful benefit, nearly doubling the median progression-free survival from 3.4 months to 6.7 months (HR=0.38, p<0.0001). The improvement in median overall survival of 16.6 months compared to 13.3 months for the control group was not significant (HR=0.87, p=0.27). Women who received bevacizumab in combination with chemotherapy had a significantly higher rate of objective response compared Epithelial Ovarian Cancer: Disease and Pipeline Analysis 44
45 Chapter 5 Treatment of Ovarian Cancer to women who received chemotherapy alone (28.2 percent versus 12.5 percent, p=0.0007). Platinum Sensitive Ovarian Cancer A carboplatin doublet chemotherapy regimen is the treatment of choice for patients considered platinum-sensitive or partially platinum-sensitive. Improvement in overall survival has been demonstrated with the use of carboplatin-paclitaxel and this is considered the optimal platinum doublet. Improvements in progression-free survival have been observed in studies using combinations of carboplatin with either gemcitabine or anthracycline. A carboplatin doublet is considered more effective than carboplatin alone. The Phase III OCEANS trial assessed the activity of bevacizumab in patients with measurable recurrent ovarian cancer following a platinum-free interval of over six months from first-line therapy (i.e. platinum-sensitive recurrence). In this doubleblind, placebo-controlled trial, all patients received a combination of carboplatin and gemcitabine at standard doses and were randomised to receive bevacizumab (15 mg/kg) or placebo administered every 3 weeks until disease progression. Median progression-free survival was significantly improved for patients receiving bevacizumab (12.4 months versus 8.4 months for those receiving a placebo). The effect of bevacizumab on HR to progression in patients assigned to the bevacizumab arm compared with placebo was (95% CI, ; P <.0001). Objective responses were increased for patients treated with chemotherapy in combination with bevacizumab versus chemotherapy alone (78.5% vs. 57.4%; P <.0001). The study allowed the crossover of patients from the control arm to the bevacizumab treatment arm on progression, thereby potentially confounding assessment of overall survival. No improvement in overall survival was demonstrated. Timing of Chemotherapy for Relapsed Disease Serial measurement of CA 125 following completion of first-line chemotherapy is widely used to detect disease which will eventually relapse clinically. However, there is no evidence to suggest that second-line treatment introduced at the detection of increased CA 125 is more effective than introducing second-line treatment at the onset of clinical symptoms in patients with complete clinical remission following platinum-based chemotherapy Epithelial Ovarian Cancer: Disease and Pipeline Analysis 45
46 Chapter 6. Ovarian Cancer Prognosis CHAPTER 6 Ovarian Cancer Prognosis Epithelial Ovarian Cancer: Disease and Pipeline Analysis 46
47 Chapter 6. Ovarian Cancer Prognosis The outcome for women diagnosed with ovarian cancer is poor, with an overall 5 year relative survival of approximately 44%. Disease stage at the time of diagnosis is a critical determinant of patient outcome, with a 92% relative 5-year survival for those diagnosed with localized disease and only a 27% relative 5 year survival rate for those diagnosed with late stage disease[2]. Unfortunately, due to the absence of a suitable screening test for the early detection of the disease in average risk women, the lack of specific symptoms caused by the disease, and poor patient and physician awareness, only 15% of patients with ovarian cancer are diagnosed with early stage disease. Table 9. 5-Year Relative Survival by Disease Stage (US) Disease Stage at Diagnosis Localised Disease Regional Metastasis Distant Metastasis % Patients Diagnosed 5-Year Relative Survival Unknown Source: SEER The general health of the patient at the time of presentation with ovarian cancer is a key parameter in determining outcome as this affects the choice of treatments available to the patient. It can determine suitability for surgery and the extent of surgery which can be performed as well as the tolerability of chemotherapy. Patient and disease parameters resulting in a more favourable prognosis include: Lower disease stage at diagnosis A younger age at diagnosis Good performance status, as this determines choice of treatment Cell type other than mucinous and clear cell Well-differentiated tumour (low grade) Smaller disease volume prior to any surgical debulking Absence of ascites Smaller residual tumour following primary debulking surgery The level of CA 125 at the time of diagnosis has no prognostic significance. However, it has a high correlation with survival when measured one month after the third course of chemotherapy for patients with stage III or stage IV disease Epithelial Ovarian Cancer: Disease and Pipeline Analysis 47
48 Chapter 6. Ovarian Cancer Prognosis Studies suggest that patients with tumours carrying BRCA 1 and BRCA 2 gene mutations have improved responses to chemotherapy when compared with patients with sporadic epithelial ovarian cancer. It is postulated that the deficient homologous DNA repair mechanism in these tumours may result in increased tumour sensitivity to chemotherapy Epithelial Ovarian Cancer: Disease and Pipeline Analysis 48
49 Chapter 7 Environmental Analysis CHAPTER 7 Environmental Analysis Epithelial Ovarian Cancer: Disease and Pipeline Analysis 49
50 Chapter 7 Environmental Analysis Areas of Unmet Need Patient and Physician Awareness Patient and physician awareness of ovarian cancer requires significant improvement, as the stage of the disease at diagnosis is a critical factor in determining patient outcomes. Patients frequently suffer from symptoms of potential ovarian cancer for a number of months prior to discussing these symptoms with their physician. Effective Screening and Early Diagnosis While measuring the patient s serum levels of CA 125 has some utility in monitoring tumour recurrence, levels are elevated in only approximately 50% of patients diagnosed with early stage ovarian cancer and 80% of late stage patients. More effective biomarkers are therefore required to aid in early detection of ovarian cancer. Screening of patients using currently available, practicable methods is effective only in patients identified as being at high risk of developing ovarian cancer. However, more effective identification and screening of high risk patients is still required to diagnose patients at an earlier stage of the disease or to determine patient risks in order to offer prophylactic intervention such as oophorectomy. Currently available techniques make screening in the general population ineffective and impractical. Implementation of Clinical Best Practice In addition to significant delays between presentation with symptoms and referral to a specialist, there are currently wide variations in clinical practice with respect to the detection, diagnosis, and management of women with ovarian cancer. These variations have been demonstrated to have a profound effect of patient outcomes. A study published in 2013 highlighted these discrepancies in the US and how they were costing patient lives. The study, which was based on the medical records of 13,321 women with ovarian cancer diagnosed from 1999 to 2006 in California, found that only 37% of patients received treatment that adhered to guidelines set by the National Comprehensive Cancer Network (NCCN). One of the principal reasons for sub-optimal care is that women are treated by doctors and hospitals that see few cases of ovarian cancer and lack expertise in the complex Epithelial Ovarian Cancer: Disease and Pipeline Analysis 50
51 Chapter 7 Environmental Analysis surgery and chemotherapy required for optimal outcomes. Debulking surgery should be performed by a gynaecologic oncologist rather than a gynaecologist or general surgeon. Surgeons who lack expertise in ovarian cancer should refer women to specialists but often do not. The study showed that surgeons who operated on ten or more women per year for ovarian cancer and hospitals that treated twenty or more per year were more likely to follow the NCCN guidelines. This adherence had a profound effect on the outcome for patients with advanced disease. For patients whose care met that of the guidelines, 35% survived at least five years, compared with 25% of those whose care fell short. However, more than 80% of patients were treated by surgeons with ten or fewer cases per year and at hospitals with twenty or fewer cases. There are a number of common reasons why patients fail to seek or receive specialist care. These include fear of offending their doctor or preferring to be treated by the obstetrician who delivered their babies. Many women feel an urgency to begin treatment immediately and do not feel that there is time to find a specialist. In a significant number of cases, a surgeon may find the tumour during a procedure for another condition and attempt to remove it. These factors combined with a smaller and less vocal advocacy community than exists for diseases such as breast cancer contribute to a general lack of awareness of the options available to the patient. A landmark study in 2006 showed that survival times of patients with ovarian cancer could be increased by over a year when chemotherapy is administered intraperitoneally rather than intravenously (median survival was 65.6 months, compared with 49.7 months). This led to a clinical announcement by the NCI and recommendation in professional guidelines for it to be offered to every patient considered suitable to receive it. However, this type of therapy is currently not in widespread use. There are a number of potential reasons for this. The regimen is highly toxic and not all patients will be able to tolerate it. The way doctors are paid in the US may be another issue. Patients receiving intraperitoneal therapy require long treatment sessions and a lot of intravenous fluids. Oncologists in private practice are paid per treatment and not for its duration; long treatment sessions block treatment chairs that could be used to treat other patients. Clinical Trial Design Issues Current FDA rules for the approval of new cancer therapies state that improvement in the median overall survival of patients is the required study endpoint and that improvement in progression-free survival alone is insufficient. This has been highlighted by the recent rejections for new ovarian cancer therapies that submitted data on significant progression-free survival alone such as bevacizumab, despite being approved for use in over 100 countries, including Europe Epithelial Ovarian Cancer: Disease and Pipeline Analysis 51
52 Chapter 7 Environmental Analysis Showing significant improvement in overall survival for a new agent over standard therapy is particularly problematic in ovarian cancer due to the many cycles of chemotherapy patients with ovarian cancer may receive. After patients have received therapy as part of a study, they are evaluated until disease progression and then followed for overall survival, but essentially go off study in this time. This means that, following early line treatment with a new agent, the patients may receive multiple, uncontrolled lines of therapy with multiple treatments at the discretion of their doctor. This makes it difficult to obtain a clear comparison of the overall survival impact of the novel therapy versus standard treatment. Critics of the FDA strategy claim that large trials confirm that progression-free survival is a suitable surrogate for overall survival, particularly in first-line therapy in advanced disease and in recurrent/persistent disease. Doctors fear that by not accepting progression-free survival as a primary outcome, new drug approvals for the treatment of ovarian cancer will stall in the US. This in turn may lead to the pharmaceutical industry failing to invest in the development of new therapies for the treatment of ovarian cancer. Supporters of the use of overall survival cite difficulties in the definition and accurate assessment of progression-free survival and claim that it may be susceptible to bias. Failure of the FDA to approve newer therapies based on lack of overall survival data, although considered appropriate in practice guidelines such as NCCN, drives use offlabel. This introduces further inconsistency of care within the treatment of ovarian cancer patients. Prevention of Tumour Recurrence Improvements in surgical techniques combined with adjuvant chemotherapy means that many women with ovarian cancer are clinically disease-free following initial therapy. However, tumour recurrence rates remain very high. Although platinum-based chemotherapy is currently the most effective treatment available, it eventually becomes ineffective due to resistance mechanisms within the tumour. New therapies which can prevent or overcome tumour drug resistance are required to maximize the efficacy of platinum agents while these agents remain standard of care. In addition, new therapies are required for patients with tumours resistant to conventional chemotherapies. A number of companies are studying the effect of the addition of a maintenance therapy continued beyond the initial 6 cycles of chemotherapy potentially until tumour recurrence. For all therapies administered over the long-term, a balance between the risk of tumour recurrence and quality of life and toxicity issues must be reached Epithelial Ovarian Cancer: Disease and Pipeline Analysis 52
53 Chapter 7 Environmental Analysis Biomarkers of tumour recurrence may be effective in providing early evidence of clinical tumour recurrence. However, the effects of early intervention would need to be firmly established. Currently, CA 125 levels are used to monitor patients for early signs of clinical disease recurrence. However, to date, no study has demonstrated that intervention with treatment at this stage, prior to evidence of gross tumour, has any impact on patient survival times. Targeted Approaches to Treatment While the concept of personalised medicine is becoming a reality in a number of cancer indications such as breast and lung cancer, the treatment of ovarian cancer continues to be dominated by chemotherapy. Some progress into identification and understanding the molecular processes involved in ovarian cancer has been made. However, this knowledge lags behind that of many other cancers types. Identification of the presence of BRCA mutations and other homologous recombination repair gene defects in ovarian cancers and the ongoing development of new therapies targeted to these processes show that research and development are moving in the right direction, albeit slowly. However, there remains a need to better understand the biological processes of ovarian cancer and to develop biomarkers which can effectively identify potential genetic susceptibilities in patients that can be targeted by new therapies. Targeted therapies may also offer the potential to effectively treat patients with poor performance status such as elderly patients. Up to half of the patients do not complete or are not suitable for paclitaxel-carboplatin first-line treatment due to toxicities Epithelial Ovarian Cancer: Disease and Pipeline Analysis 53
54 Chapter 7 Environmental Analysis Major Market Analysis Factors which impact the development and commercialization of new medicines in the major pharmaceutical markets include: (1) clinical trial and recruitment environment; (2) regulatory environment; (3) competitive product landscape; and (4) pricing and reimbursement environment. Table 10. Ovarian Cancer - Major Markets Patient Population and Product Environment Ovarian Cancer Incidence (2015) Epithelial Ovarian Cancer Incidence (2015) Trial and Recruitment Environment Regulatory Environment Competitive Product Landscape Reimbursement Environment US France Germany Italy Spain UK Japan 22,148 4,875 6,936 6,103 3,395 6,928 9,157 19,933 4,388 6,242 5,493 3,056 6,235 8,241 Source: Globocan 2012 and analyst calculations and assessment (E)=estimate Positive for the Pharmaceutical Industry Neutral for the Pharmaceutical Industry Negative for the Pharmaceutical Industry Trial and Recruitment Environment The clinical trial and recruitment environment is generally good for the major markets from the perspective of having high levels of medical and clinical trial expertise and substantial numbers of qualified oncology investigators. Several networks of ovarian cancer specialists are instrumental in conducting important clinical trials, such as the Gynecologic Oncology Group, which is a non-profit international organisation promoting excellence in the quality and integrity of gynecologic clinical trials and research Epithelial Ovarian Cancer: Disease and Pipeline Analysis 54
55 Chapter 7 Environmental Analysis According to data downloaded from clinicaltrials.gov in December 2014, of the 300 studies of known status currently open to patients with ovarian cancer, 194 are being conducted in North America (188 in the US) and 96 in Europe. Ovarian cancer trial activity in the rest of the world includes 5 studies in Japan, 5 in Russia, and 1 in South America. A number of recent late stage failures in the development of ovarian cancer therapies has led to criticism of the FDA s policy on the requirement for significant overall survival data from studies. Approvals such as for bevacizumab have been granted in Europe and elsewhere globally based on positive progression-free survival data alone. Critics suggest that an overhaul in clinical trial design is required particularly in respect to newer targeted therapies and point to the lack of control over the type and number of patient treatments after the end of the study phase, which could impact overall survival data. These difficulties could result in a lack of investment in development of new therapies for ovarian cancer. In Europe, the Clinical Trial Directive (CTD) was introduced to harmonize requirements and procedures across Europe. However, the inconsistent application and interpretation of the directive by the member states has resulted in an increased administrative burden and increased clinical trial costs. This increased complexity and cost has driven the conduct of clinical trials to other developed countries and to patient-rich developing countries. The number of clinical trials conducted in Europe decreased by 25% between 2007 and Data from the EMA showed that 2011 was the first year that the percentage of patients recruited to clinical trials used to support marketing applications to the EMA was higher for the rest of world than for either the US or European Economic Area plus Switzerland (37.3%, 31.5%, and 31.2% respectively). Revisions to the highly criticized CTD legislation are being implemented in order to make trials faster, easier, and less expensive. Key aspects of the revised regulations include (1) simplified approval processes and reporting and (2) better differentiation of sponsor obligations according to the risk the study poses to the participants. While recent guidelines have eased restrictions on accepting data from non-japanese clinical trials for drug approval in Japan, the reality is that most pivotal clinical trials as well as early pharmacokinetic/pharmacodynamics studies must be conducted in Japan. Slow recruitment and high costs are features of trials in the country. Regulatory Environment The regulatory environment is robust, transparent, and standardized across the major markets. A centralized approval process in Europe through the EMA allows marketing applications to be submitted and decisions made centrally, giving access to 27 countries. It is aimed at eliminating the potential for protectionist actions by individual Epithelial Ovarian Cancer: Disease and Pipeline Analysis 55
56 Chapter 7 Environmental Analysis countries that may result in the failure to approve medicines in competition with already approved domestically produced products. The EMA is the source of one-third of all new drugs released into the world market each year. A trial project is underway in Europe to speed up access to new medicines and address unmet medical needs. The project, known as Adaptive Licensing, aims to provide a mechanism by which drug makers can obtain restricted approval for new therapies much earlier in the development pathway. This is followed by provision of evolving data on benefits and risks gathered in a real world context which can be fed into the license in order to widen its scope. The findings from the pilot scheme will be evaluated at the end of In the US, the FDA is responsible for the approval of new medicines. The FDA could be considered to be increasingly risk averse and business leaders complain that regulation, fees, fines, and reporting requirements are stifling industry growth. However, the Orphan Drug status and the recent impetus from the FDA in promoting ongoing collaboration with developers through Breakthrough Therapy status mean that more concise drug development programme opportunities exist. The Pharmaceutical and Medical Devices Agency, part of the Ministry of Health, Labour and Welfare is responsible for pharmaceutical regulatory affairs in Japan. The review and approval process is slow and can take 2.5 years longer than in Europe and the US. Competitive Product Landscape The treatment of ovarian cancer is dominated by cytotoxic agents, many of which are no longer patent protected, making treatment of ovarian cancer relatively inexpensive compared with other cancer indications predominantly treated with targeted therapies. In recent years, a number of new formulations of cytotoxic agents such as pegylated doxorubicin have been approved and are aimed at producing less toxicity and higher tumour concentrations than the originator compounds. These agents command higher prices. Bevacizumab is the only targeted agent approved for use in ovarian cancer, however its approval is limited in the US and use as a firstline or maintenance therapy is considered off-label. To date, it has been used only in a limited capacity in ovarian cancer in the EU. Avastin (bevacizumab) sales for all approved indications in 2013 were $6.5bn. In the UK, NICE puts the cost of one course of bevacizumab therapy at 25,000 and while its use in a number of cancer indications including ovarian cancer has been rejected, it is the drug most frequently financed by the UK special cancer drugs fund. The ovarian cancer drugs market is estimated to be worth approximately $0.5bn (Decision Resources) in the seven major pharmaceutical markets. The approval of Epithelial Ovarian Cancer: Disease and Pipeline Analysis 56
57 Chapter 7 Environmental Analysis new targeted therapies and the increase in uptake of bevacizumab will see the market continue to grow. Pricing and Reimbursement Environment As healthcare budgets become increasingly constrained in the tough economic climate, so the pricing and reimbursement market becomes more challenging for pharmaceutical companies. The increasingly widespread use of comparative effectiveness assessments means that drug pricing is coming under increasing scrutiny and pressure from payers. Rebates and discounts are being increasingly forced on pharmaceutical companies. The US remains the most favourable healthcare environment for pharmaceutical companies, with high per capita healthcare spending and high usage of newly approved drugs. Under the Affordable Care Act, there is increasing coverage of the uninsured, increasing the potential patient population. However, payers are increasingly using comparative effectiveness strategies, rebates, and discounts. They are also less inclined to approve off-label use of available treatments. Higher copays are increasing medical expenses for patients. This higher co-pay situation is also becoming more evident in Japan which is under pressure to constrain its healthcare budget. Policy incentives aimed at growing the use of generics are a key component of Japan s healthcare strategy. Germany s recent reforms of its once favourable free market conditions have resulted in some of the most stringent pricing and reimbursement conditions across any of the major markets. The introduction of AMNOG (Act on the Reform of the Market for Medicinal Products) in 2011 with the intention of making cost savings to the health insurance system of over 2 billion resulted in the introduction of mandatory costbenefit evaluations for all new medicines. These evaluations have led to restricted prices and, in some cases, refusal of reimbursement. In addition, a review of marketed treatments has begun with the aim of cutting prices of some of the country s most popular medicines. The authorities can now seek to base the price of new medicines on those of much older generics. In some instances, this has led to a number of pharma companies including GSK, Eli Lilly, and Pfizer withholding introduction of new products onto the German market. The effect of these actions is also felt by pharma companies in countries which use Germany for reference pricing. On a more positive note, under the new regulations there has been a 30% increase in the number of submissions made for orphan drugs which are AMNOG-exempt, suggesting a refocus of pharmaceutical efforts away from so called me-too drug development in well covered disease indications and into areas of higher unmet need. In spite of widely publicized criticism of the reforms, Germany remains an attractive market in terms of high per capita pharmaceutical spending and a large ageing population Epithelial Ovarian Cancer: Disease and Pipeline Analysis 57
58 Chapter 7 Environmental Analysis A recent survey (PatientView) of patient groups in 12 countries showed that 41% of cancer patient groups said access to medicines was a problem. This is an increase of 13% on the 2012 survey. Of the countries surveyed, Germany ranked number one for access to medicines, followed by Italy and France in equal second position and the UK in fourth. While France has high usage of cancer drugs, particularly new agents, it has traditionally had low drug prices due to tight regulation and the use of price-volume agreements to control expenditure. This is set to continue as France aims to keep its healthcare budget down and rebates and discounts are increasingly applied by both public and private players. Spain, like Japan, has introduced measures to encourage higher levels of generic drug use. Its traditionally low prices are being maintained in light of the current economic crisis, and modifications to the Reference Pricing System and the price setting process are part of significant cut-backs recently introduced. Italy s vulnerable economy has led to the reduction of its already relatively low per capita spending on pharmaceuticals. This has been achieved by lowering drug prices and implementing cost-effectiveness analyses in its reimbursement decisions. It is also introducing higher co-pay tariffs to patients. The UK has included a cost-effectiveness analysis requirement in all of its reimbursement decisions for a number of years, implemented through NICE. Under this system, at least half of all initial reimbursement submissions are rejected due to lack of acceptable cost-effectiveness. This has driven an increasing use of riskshare agreements in order to get new drugs reimbursed. Under NICE direction, a low percentage of reimbursements are awarded for cancer indications compared to the number of cancer indications approved by EMA. This has resulted in the UK having some of the lowest usage of newer cancer drugs compared to other western markets. In addition, a recent report showed that there is up to a ten-fold difference in the use of new medicines between different areas of the country. The cancer drugs fund is used to give patients access to cancer medicines deemed too expensive for use generally throughout the NHS. Approximately 30 cancer drugs are available through the fund. The UK is introducing a new value-based pricing system with the aim of ensuring that the price the NHS pays for new medicines is more closely linked to their value to patients and society. The system was set to be in place in 2014 but has been repeatedly delayed. The pharmaceutical industry has raised concern that the new system puts too much emphasis on breakthrough treatments and not enough on incremental advances Epithelial Ovarian Cancer: Disease and Pipeline Analysis 58
59 Chapter 7 Environmental Analysis Emerging Market Analysis The BRICK countries (Brazil, Russia, India, China, South Korea) represent huge opportunities for the pharmaceutical industry in terms of their growing economies, improving healthcare priorities, and, in the case of India and China, very large populations. Table 11. Ovarian Cancer - Emerging Markets Patient Population and Product Environment Ovarian Cancer Incidence (2015) Epithelial Ovarian Cancer Incidence (2015) Trial and Recruitment Environment Regulatory Environment Competitive Product Landscape Reimbursement Environment Brazil Russia India China South Korea 6,282 13,496 28,970 37,324 2,516 5,654 12,146 26,073 33,592 2,264 Source: Globocan 2012 data and analyst calculations and assessment (E)=estimate Positive for the Pharmaceutical Industry Neutral for the Pharmaceutical Industry Negative for the Pharmaceutical Industry Trial and Recruitment Environment As clinical trial locations, the BRICK countries are destinations of increasing interest to pharmaceutical companies. They offer increasing investigator experience and motivated patient populations and are potentially low cost destinations. However, a review of studies registered on clinicaltrials.gov shows little ovarian cancer clinical trial activity ongoing in these countries. Of the 300 studies of known status open to patients with ovarian cancer in December 2014, five studies are being conducted in Russia, two in India, and one in Brazil Epithelial Ovarian Cancer: Disease and Pipeline Analysis 59
60 Chapter 7 Environmental Analysis Russia has built a reputation for fast recruitment, good patient retention, and high levels of investigator expertise, with patients keen to participate in order to gain access to the best medical care due to an inadequate national health system. Government policy to increase local research and development has seen most major pharmaceutical companies establish a presence in the country. This puts the pharmaceutical companies in a positive position to conduct clinical trials in the country. Average costs per patient of clinical trials in Russia are 60-70% less than in western countries. Standards are high, with the FDA conducting and passing two trial inspections in Russia in Medical institutions are accredited by the Ministry of Health. Constantly changing regulations, which result in decreased cost-effectiveness and increased timelines, are barriers to conducting clinical trials in the country. Russia requires the enrolment of local patients in clinical trials for the marketing approval of new drugs. China s healthcare system is characterized by very large hospitals situated in areas of high population density giving investigators access to large numbers of predominantly treatment naive patients. Fast recruitment introduces significant efficiencies which, in combination with lower wage costs, can decrease the price per patient of a clinical trial to only 30% of the price per patient in the US or EU. However, Clinical Trial Application Reviews can be slow and complicated, usually taking between 12 and 18 months to complete. China is cooperating with other Asian countries on the harmonization of clinical trials regulations. While declining clinical trial numbers in recent years are a feature of Brazil, India and Russia, the numbers are steadily increasing in China. Brazil has a large patient population with reduced access to care beyond the basiclevel care provided by the national healthcare programme. Urban hubs provide a concentrated patient population and a growing number of FDA-regulated investigators. The regulatory approval process for clinical trials is performed by ANVISA, the National Health Surveillance agency. Approvals in Brazil are among the slowest in Latin America and can take up to 12 months. However, the agency is working to simplify the process and accelerate timelines. Despite this, the number of clinical trials being performed in the country is decreasing as a number of large pharmaceutical companies reduce their presence. India has a large patient population with inadequate access to healthcare. Patients rely on private healthcare for treatment above basic care. Clinical trials offer the opportunity to receive treatments otherwise unavailable to them. While the majority of the population live in rural areas, urban areas offer high patient population hubs and boast facilities at near-western standards. The number of physicians is increasing annually, with many trained in the US and Europe. However, the clinical trial process is reported to be increasing in complexity, with an average approval time of approximately six months. Recent concerns over both the quality of trials conducted in India and their impact on the health of volunteers, as well as concerns over data Epithelial Ovarian Cancer: Disease and Pipeline Analysis 60
61 Chapter 7 Environmental Analysis authenticity have led to increased pressure on regulators to increase scrutiny of clinical trials. Tighter laws mean that trials must be conducted in GMP-compliant facilities, approved by ethics committees, registered with regulators, and subjected to random inspection. There has been a sharp decline in the number of trials conducted in India in recent years. It is reported that India started only 19 clinical trials in 2013, a decrease of 93% from the 262 started in 2012 and from a peak of 500 in South Korea is rapidly emerging as a global clinical trial hub due its centralized patient population, excellent infrastructure, and technology focus. It adopted ICH-GCP in 2000 and has a body (KONECT) focused on increasing the country s competitiveness as a clinical trial destination. Regulatory Environment One of the greatest concerns of the pharma industry regarding BRICK countries is the weakness of intellectual property (IP) protection. On the whole, the countries discussed in this report recognize the deficiencies within their systems and the requirement to strengthen their position if they wish to compete on the global stage. However, this must be balanced with local needs for access to innovative, affordable drugs. In this respect, Russia recently became party to the TRIPS agreement (Agreement on Trade Related Aspects of Intellectual Property Rights) through its accession to the World Trade Organization, which should drive improved IP regulation. However, manipulation of IP laws remains one of the ways in which poorer countries can be seen to provide their population with access to expensive treatments at affordable prices. As a result of implementing this methodology, India s IP situation is considered to be well below international standards. Its system does not recognize and thereby does not provide patent protection for what it considers to be incremental improvements to drugs. Therefore, few medicines receive exclusivity. In a recent challenge to the law by Novartis over its cancer medication Gleevec (Glivec), the Indian Supreme Court denied patent protection to the drug, resulting in the continued manufacture of cheaper generic copies in India. In 2012, an appeal by Bayer was overturned over India s granting of a compulsory license (its first) which enabled a local company to manufacture and sell Nexavar (sorafenib) for a 97% reduction in price over the original. A number of other drugs are being considered for compulsory licences. These actions form part of a political decision to encourage the wider use of generics, increase local industry, and expand access to free healthcare. In addition to these patent issues, India s regulatory system is considered to be overburdened with bureaucracy through its Central and State bodies which lack uniformity and skilled manpower. China has a stated commitment to biotechnology which is one of seven strategic Epithelial Ovarian Cancer: Disease and Pipeline Analysis 61
62 Chapter 7 Environmental Analysis emerging industries on which it is focused, with the aim of being an innovation-based economy by In order to achieve this goal, it has worked hard in recent years to improve its regulatory procedures and enhance IP protection through the China Food and Drug Administration. However, import of study drug requires full disclosure of manufacturing processes, raising doubt over data protection. However, in an effort to restrict the rapidly increasing healthcare budget, it has recently stated that it is considering the possibility of the introduction of compulsory licences in order to maintain the affordability of medicines. A fast-track approval process is being planned for new medicines which may exclude companies that do not conduct trials in the country. Some concerns remain over corruption within the regulatory system. Approval of new medicines can take up to three years over and above FDA and EMA timelines. Brazil is improving its regulatory system. New product registration takes two years or more, but a fast-track approval system is being investigated for life-saving drugs. The patent review timelines in Brazil are currently approximately eight years, but the aim is to reduce this to four years. Competitive Product Landscape As with the developed markets, the competitive product landscape for ovarian cancer is dominated by generic cytotoxic agents and limited by the lack of effective therapies for the treatment of recurrent disease. Pricing and Reimbursement Environment While Brazil has a growing pharma market, high per capita spend, and an increasingly affluent population, it also has strict price controls on medication and limited drug reimbursement to patients. Over 70% of the population is covered by a public health insurance programme, while the private healthcare system funds approximately 60% of the healthcare spend. Full patient reimbursement is available for a range of life-saving and chronic therapy drugs. Spending on expensive branded products is decreasing in favour of generics and the country has a strong domestic generic pharmaceuticals industry. In order to favour and boost domestic production, the government has increased import tariffs on foreign manufactured medicines and is increasing government investment, creating public-private partnerships and increasing access to medicines. International reference pricing and price referencing to local existing products is in operation. In addition, compulsory discounts of 24% are in force for government purchases Epithelial Ovarian Cancer: Disease and Pipeline Analysis 62
63 Chapter 7 Environmental Analysis In Russia, cost containment measures are being taken to limit what was previously a fairly liberal pricing environment. International reference pricing is in operation, utilising prices in 20 European countries and the prices of existing local products. Improvement in access to medications has increased the number of people eligible for reimbursement for the costs of their medication, but the government currently reimburses only a small percentage of the population and a high unmet demand for medications still exists. The Medicines Insurance System to be implemented will provide tiered reimbursement of medications to patients, but significant costs to patients will remain. Most oncology drugs in Russia are on the vital and essential drugs list which was implemented in 2010 to limit the number of drugs which had free pricing, resulting in the prices of drugs on this list now being controlled. Russia has recently suggested that it might penalize the import of foreign pharmaceuticals and limit the state purchasing of foreign medicines in order to encourage alliances between foreign pharma and domestic companies to build the country s domestic production. It is estimated that currently 85% of Russia s state drug spending is spent on foreign-made drugs. India spends very little of its budget on medical services, with less state contribution than Brazil, Russia, and China. Only approximately 5% of total spending on medicines in India is spent by the government with over 90% of medicine purchases not reimbursed to the patient. This high out of pocket expenditure limits the uptake of expensive new medicines. The recently introduced Price Control Order has brought over 600 non-patented drugs under government price control and is expected to reduce costs by up to 80%. While patented drugs are not currently included, there are increasing calls within India to include them. Government drug purchases are made through a process of sealed bids and usually awarded on price. China s expanding medical insurance protection has led to robust pharma market growth in the country. However, the use of expensive new medications is limited by patients ability to pay out of pocket expenses. In addition, cost-containment measures are to be introduced to contain the growing healthcare expenditure. Government mandated cuts of approximately 20% every three years are becoming commonplace. Though there are ongoing price cuts to the essential drugs list, the number of products on the list is continuously increasing with some basic cancer drugs now included. Korea s pharmaceutical market is dominated by spending on generics and cost containment policies. Between 2011 and 2012, 60% of drugs had their prices cut. The country operates a positive reimbursement list and negative decisions are made based on lack of cost effectiveness or lack of evidence. Price controls ensure that originator drug prices must drop 30% on generic entry to the market and generic prices must drop 59.5% versus the originator price. A raft of further price cuts are planned between 2013 and Epithelial Ovarian Cancer: Disease and Pipeline Analysis 63
64 Chapter 7 Environmental Analysis Patient Recruitment Environment The high recurrence rate of ovarian cancer combined with the development of resistance to the most effective paclitaxel-carboplatin chemotherapy regimens and lack of effective alternative treatment options drive patients into clinical trials to gain access to new medications. There are currently 300 studies open to ovarian cancer patients listed in clinicaltrials. gov (December 2014). That 119 of these trials are registered as being funded by industry shows healthy interest by the pharmaceutical industry in developing new drugs to treat ovarian cancer. Geographically, these industry-led trials are split approximately equally between the US and Europe (65 and 55 respectively). Within Europe, the UK is the most active location (27 trials), with the other major markets of Germany, France, Italy, and Spain hosting 16, 16, 10, and 17 trials respectively. Elsewhere globally, ovarian cancer trial activity is limited, with only 3 trials ongoing in Japan, 3 in China, 2 in India, 5 in Russia, and 2 in South Korea. Figure 8. Distribution of Active Industry Sponsored Ovarian Cancer Trials, by Phase Unknown: 1 Observational: 14 Phase III: 10 Phase I: 43 Phase II: 39 Phase I/II: 20 The 119 active, industry sponsored trials expect to recruit over 24,000 patients. Early phase (Phase I and I/II) trials comprise more than half of active trials (63 of119) and recruit an average of 82 patients for Phase I and 112 patients for Phase I/II. Phase II industry sponsored trials recruit an average of 99 patients and comprise 25% of trials. Ten active Phase III trials are registered (8% of ongoing ovarian cancer trials), recruiting an average of 634 patients Epithelial Ovarian Cancer: Disease and Pipeline Analysis 64
65 Chapter 7 Environmental Analysis Table 12. Number of Patients in Pharma Sponsored Ovarian Cancer Clinical Trials by Phase Trial Phase Number of Studies Total Number of Patients Mean Number of Patients Range of Patient Numbers I 43 3, I/II 20 2, II 30 2, III 10 6, ,500 IV Unclassified/ Observational 15 8, ,000 Total ,940 Source: clinicaltrials.gov December 2014 Drug Development Field A significant number of new therapies are currently in development for the treatment of ovarian cancer. While there have been several late stage failures very recently, the pipeline includes a number of late stage candidates with potential to reach clinical practise in the short term. A total of 80 new therapies that are in clinical trials for the treatment of ovarian cancer have been identified in this report. These therapies include a range of mechanisms of action including tyrosine kinase inhibitors, angiogenesis inhibitors, immunotherapies, chemotherapies, and apoptosis inducers. New therapies are discussed in more detail in the Chapter 8 of this report Epithelial Ovarian Cancer: Disease and Pipeline Analysis 65
66 Chapter 8 Drug Development in Ovarian Cancer CHAPTER 8 Drug Development in Ovarian Cancer Epithelial Ovarian Cancer: Disease and Pipeline Analysis 66
67 Chapter 8 Drug Development in Ovarian Cancer Women diagnosed with ovarian cancer continue to have poor outcomes despite the initial effectiveness of the carboplatin-paclitaxel chemotherapy doublet used in the first-line of treatment. This is due to the high rate of disease recurrence and the development of tumour resistance to therapy with platinum agents. The late-stage pipeline of new agents being investigated for use in various aspects of late stage ovarian cancer treatment is substantial and some of these therapies have the potential to reach clinical practice in the short term. Agents are being developed for use in first-line treatment in combination with standard of care paclitaxel-carboplatin doublet. There are also agents being developed as second or subsequent lines of therapy as individual agents or in combination with single agent chemotherapy, following treatment with platinum doublet. Work is also ongoing to assess the utility of agents as maintenance therapies following first-line treatment in order to both extend the disease free duration between lines of treatment and to extend survival. Table 13. Late Stage Ovarian Cancer Pipeline Drug Pazopanib (Votrient) Paclical Karenitecin (BNP1350) Farletuzumab (MORAb-003) Phase Submitted and Withdrawn III complete III failed Developing Company GSK/Oxigene Oasmia Pharma AB BioNumerik Pharma Mechanism kinase inhibitor (angiogenesis) Cytotoxic (paclitaxel) Cytotoxic (camptothecin analogue) III failed Eisai Ltd mab (folate receptor alpha) Cediranib III AstraZeneca Nintedanib (Vargatif) Trebananib (AMG386) Bevacizumab (Avastin) Binimetinib (MEK162) Niraparib (MK- 4827) Olaparib (AZD2281) III III III III Boehringer Ingelheim Amgen Roche Array Biopharma/ Novartis Kinase inhibitor (angiogenesis) Kinase inhibitor (FGFR, VEGFR, PDGFR) Anti-angiopoeitin peptibody mab (angiogenesis inhibitor) Kinase inhibitor (MEK) III Tesaro Inc PARP inhibitor III AstraZeneca PARP inhibitor Epithelial Ovarian Cancer: Disease and Pipeline Analysis 67
68 Chapter 8 Drug Development in Ovarian Cancer Drug Paclitaxel poliglumex (Opaxio) Phase III Developing Company Cell Therapeutics Mechanism Cytotoxic (cell cycle inhibitor) Rucaparib III Clovis Oncology PARP inhibitor Trebectedin (Yondelis) III PharmaMar Cytotoxic (DNA binder) Vintafolide (EC145) III (failed) MSD/Endocyte Peptide-drug conjugate Cvac II/III PrimaBiomed Ltd Immunotherapy As for other types of cancer, the understanding of the molecular processes involved in ovarian tumour growth, invasion, and metastasis is increasing and there is acceptance that a one treatment fits all approach is not appropriate, as epithelial ovarian cancer is a group of heterogeneous sub-types, each with their own molecular characteristics and clinical behaviour. This knowledge is leading to the investigation of the use of targeted therapies aimed at specific tumour characteristics and with the potential for lower toxicity and greater selectivity. Molecular pathways currently under investigation include angiogenesis inhibitors, PARP inhibitors, and inhibitors of the Ras/Raf/MEK/ERK and of the PI3K/AKT/mTOR intracellular signalling pathways. Figure 9 outlines the major intracellular pathways which impact tumour cell proliferation, migration, and survival as well as tumour angiogenesis Epithelial Ovarian Cancer: Disease and Pipeline Analysis 68
69 Chapter 8 Drug Development in Ovarian Cancer Figure 9. Major Intracellular Pathways Targeted for the Treatment of Ovarian Cancer In addition to the development of new targeted therapies, there is a requirement for associated development of biomarkers in order to appropriately identify patients most suitable for each therapy and also to assess patient response to therapy Epithelial Ovarian Cancer: Disease and Pipeline Analysis 69
See the latest estimates for new cases of ovarian cancer and deaths in the US and what research is currently being done.
 About Ovarian Cancer Overview and Types If you have been diagnosed with ovarian cancer or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. What Is
About Ovarian Cancer Overview and Types If you have been diagnosed with ovarian cancer or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. What Is
THERAPIES AND DIAGNOSTICS FOR OVARIAN CANCER. HLC143A June Usha Nagavarapu Project Analyst ISBN: X
 THERAPIES AND DIAGNOSTICS FOR OVARIAN CANCER HLC143A June 2013 Usha Nagavarapu Project Analyst ISBN: 1-56965-431-X BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 866-285-7215, 781-489-7301
THERAPIES AND DIAGNOSTICS FOR OVARIAN CANCER HLC143A June 2013 Usha Nagavarapu Project Analyst ISBN: 1-56965-431-X BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 866-285-7215, 781-489-7301
Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122
 Ovarian cancer: recognition and initial management Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Ovarian cancer: recognition and initial management Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
X-Plain Ovarian Cancer Reference Summary
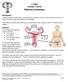 X-Plain Ovarian Cancer Reference Summary Introduction Ovarian cancer is fairly rare. Ovarian cancer usually occurs in women who are over 50 years old and it may sometimes be hereditary. This reference
X-Plain Ovarian Cancer Reference Summary Introduction Ovarian cancer is fairly rare. Ovarian cancer usually occurs in women who are over 50 years old and it may sometimes be hereditary. This reference
Staging and Treatment Update for Gynecologic Malignancies
 Staging and Treatment Update for Gynecologic Malignancies Bunja Rungruang, MD Medical College of Georgia No disclosures 4 th most common new cases of cancer in women 5 th and 6 th leading cancer deaths
Staging and Treatment Update for Gynecologic Malignancies Bunja Rungruang, MD Medical College of Georgia No disclosures 4 th most common new cases of cancer in women 5 th and 6 th leading cancer deaths
Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors
 Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors Overview Ovarian epithelial cancer, fallopian tube cancer, and primary peritoneal cancer are
Ovarian Cancer Includes Epithelial, Fallopian Tube, Primary Peritoneal Cancer, and Ovarian Germ Cell Tumors Overview Ovarian epithelial cancer, fallopian tube cancer, and primary peritoneal cancer are
GYNECOLOGIC MALIGNANCIES: Ovarian Cancer
 GYNECOLOGIC MALIGNANCIES: Ovarian Cancer KRISTEN STARBUCK, MD ROSWELL PARK CANCER INSTITUTE DEPARTMENT OF SURGERY DIVISION OF GYNECOLOGIC ONCOLOGY APRIL 19 TH, 2018 Objectives Basic Cancer Statistics Discuss
GYNECOLOGIC MALIGNANCIES: Ovarian Cancer KRISTEN STARBUCK, MD ROSWELL PARK CANCER INSTITUTE DEPARTMENT OF SURGERY DIVISION OF GYNECOLOGIC ONCOLOGY APRIL 19 TH, 2018 Objectives Basic Cancer Statistics Discuss
EDUCATIONAL COMMENTARY CA 125. Learning Outcomes
 EDUCATIONAL COMMENTARY CA 125 Learning Outcomes Upon completion of this exercise, participants will be able to: discuss the use of CA 125 levels in monitoring patients undergoing treatment for ovarian
EDUCATIONAL COMMENTARY CA 125 Learning Outcomes Upon completion of this exercise, participants will be able to: discuss the use of CA 125 levels in monitoring patients undergoing treatment for ovarian
Winship Cancer Institute of Emory University Optimizing First Line Treatment of Advanced Ovarian Cancer
 Winship Cancer Institute of Emory University Optimizing First Line Treatment of Advanced Ovarian Cancer Ira R. Horowitz, MD, SM, FACOG, FACS John D. Thompson Professor and Chairman Department of Gynecology
Winship Cancer Institute of Emory University Optimizing First Line Treatment of Advanced Ovarian Cancer Ira R. Horowitz, MD, SM, FACOG, FACS John D. Thompson Professor and Chairman Department of Gynecology
North of Scotland Cancer Network Clinical Management Guideline for Cancer of the Ovary
 North of Scotland Cancer Network Cancer of the Ovary Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by NOSCAN Gynaecology Cancer
North of Scotland Cancer Network Cancer of the Ovary Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by NOSCAN Gynaecology Cancer
Sarah Burton. Lead Gynae Oncology Nurse Specialist Cancer Care Cymru
 Sarah Burton Lead Gynae Oncology Nurse Specialist Cancer Care Cymru Gynaecological Cancers Cervical Cancers Risk factors Presentation Early sexual activity Multiple sexual partners Smoking Human Papiloma
Sarah Burton Lead Gynae Oncology Nurse Specialist Cancer Care Cymru Gynaecological Cancers Cervical Cancers Risk factors Presentation Early sexual activity Multiple sexual partners Smoking Human Papiloma
3 cell types in the normal ovary
 Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors (neoplasms) 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal
Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors (neoplasms) 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal
Hitting the High Points Gynecologic Oncology Review
 Hitting the High Points is designed to cover exam-based material, from preinvasive neoplasms of the female genital tract to the presentation, diagnosis and treatment, including surgery, chemotherapy, and
Hitting the High Points is designed to cover exam-based material, from preinvasive neoplasms of the female genital tract to the presentation, diagnosis and treatment, including surgery, chemotherapy, and
Ovarian Cancer: Implications for the Pharmacist
 Ovarian Cancer: Implications for the Pharmacist Megan May, Pharm.D., BCOP Objectives Describe the etiology and pathophysiology of ovarian cancer Outline the efficacy and safety of treatment options for
Ovarian Cancer: Implications for the Pharmacist Megan May, Pharm.D., BCOP Objectives Describe the etiology and pathophysiology of ovarian cancer Outline the efficacy and safety of treatment options for
Ovarian Cancer What you need to know
 Ovarian Cancer What you need to know www.ovarian.org.uk Contents Your body and your ovaries What is ovarian cancer? Your body and your ovaries 3 What is ovarian cancer? 3 Not one disease, but many 4 The
Ovarian Cancer What you need to know www.ovarian.org.uk Contents Your body and your ovaries What is ovarian cancer? Your body and your ovaries 3 What is ovarian cancer? 3 Not one disease, but many 4 The
Practice of Medicine-1 Ovarian Cancer Clinical Correlation
 Practice of Medicine-1 Ovarian Cancer Clinical Correlation Amir A. Jazaeri, M.D. Assistant Professor, Division of Gynecologic Oncology American Cancer Society Female Cancers 2000 Statistics Reprinted by
Practice of Medicine-1 Ovarian Cancer Clinical Correlation Amir A. Jazaeri, M.D. Assistant Professor, Division of Gynecologic Oncology American Cancer Society Female Cancers 2000 Statistics Reprinted by
CA125 in the diagnosis of ovarian cancer: the art in medicine
 CA125 in the diagnosis of ovarian cancer: the art in medicine Dr Marcia Hall Consultant Medical Oncology Mount Vernon Cancer Centre Hillingdon Hospital Wexham Park Hospital Epidemiology Ovarian cancer
CA125 in the diagnosis of ovarian cancer: the art in medicine Dr Marcia Hall Consultant Medical Oncology Mount Vernon Cancer Centre Hillingdon Hospital Wexham Park Hospital Epidemiology Ovarian cancer
New Cancer Cases By Site Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3%
 Uterine Malignancy New Cancer Cases By Site 2010 Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3% Cancer Deaths By Site 2010 Lung 26% Breast 15% Colo-Rectal 9% Pancreas 7%
Uterine Malignancy New Cancer Cases By Site 2010 Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3% Cancer Deaths By Site 2010 Lung 26% Breast 15% Colo-Rectal 9% Pancreas 7%
NAACCR Webinar Series 1 Q&A. Fabulous Prizes. Collecting Cancer Data: Ovary 11/3/2011. Collecting Cancer Data: Ovary
 NAACCR 2011 2012 Webinar Series Collecting Cancer Data: Ovary Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching this webinar
NAACCR 2011 2012 Webinar Series Collecting Cancer Data: Ovary Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching this webinar
Case Scenario 1. Pathology report Specimen from mediastinoscopy Final Diagnosis : Metastatic small cell carcinoma with residual lymphatic tissue
 Case Scenario 1 Oncology Consult: Patient is a 51-year-old male with history of T4N3 squamous cell carcinoma of tonsil status post concurrent chemoradiation finished in October two years ago. He was hospitalized
Case Scenario 1 Oncology Consult: Patient is a 51-year-old male with history of T4N3 squamous cell carcinoma of tonsil status post concurrent chemoradiation finished in October two years ago. He was hospitalized
Endometrial Cancer. Incidence. Types 3/25/2019
 Endometrial Cancer J. Anthony Rakowski DO, FACOOG MSU SCS Board Review Coarse Incidence 53,630 new cases yearly 8,590 deaths yearly 4 th most common malignancy in women worldwide Most common GYN malignancy
Endometrial Cancer J. Anthony Rakowski DO, FACOOG MSU SCS Board Review Coarse Incidence 53,630 new cases yearly 8,590 deaths yearly 4 th most common malignancy in women worldwide Most common GYN malignancy
What You Need to Know About Ovarian Cancer
 What You Need to Know About Ovarian Cancer About Us The Rhode Island Ovarian Cancer Alliance (RIOCA) was formed in honor and memory of Jessica Morris. Jessica was diagnosed with Stage IIIC Ovarian Cancer
What You Need to Know About Ovarian Cancer About Us The Rhode Island Ovarian Cancer Alliance (RIOCA) was formed in honor and memory of Jessica Morris. Jessica was diagnosed with Stage IIIC Ovarian Cancer
Carcinoma of the Fallopian Tube
 119 Carcinoma of the Fallopian Tube APM HEINTZ, F ODICINO, P MAISONNEUVE, U BELLER, JL BENEDET, WT CREASMAN, HYS NGAN and S PECORELLI STAGING Anatomy Primary site The Fallopian tube extends from the posterior
119 Carcinoma of the Fallopian Tube APM HEINTZ, F ODICINO, P MAISONNEUVE, U BELLER, JL BENEDET, WT CREASMAN, HYS NGAN and S PECORELLI STAGING Anatomy Primary site The Fallopian tube extends from the posterior
Gynecologic Cancers. What is Gynecologic Cancer. Who is at risk for GYN cancer? 3/1/2018 1
 What is Gynecologic Cancer Gynecologic Cancers Marge Ramsdell RN, MN, OCN Madigan Army Medical Center Any cancer that starts in a woman s reproductive organs Each GYN cancer is unique 5 main types Cervical
What is Gynecologic Cancer Gynecologic Cancers Marge Ramsdell RN, MN, OCN Madigan Army Medical Center Any cancer that starts in a woman s reproductive organs Each GYN cancer is unique 5 main types Cervical
Disease State Primer: Ovarian Cancer
 Q1 2012 Update Disease State Primer: Ovarian Cancer http://www.mayoclinic.com/images/image_popup/c7_ovarian_cancer.jpg Table of Contents I. Introduction Who is Lumleian and what is a disease state primer?
Q1 2012 Update Disease State Primer: Ovarian Cancer http://www.mayoclinic.com/images/image_popup/c7_ovarian_cancer.jpg Table of Contents I. Introduction Who is Lumleian and what is a disease state primer?
North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer
 THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
THIS DOCUMENT North of Scotland Cancer Network Clinical Management Guideline for Endometrial Cancer Based on WOSCAN CMG with further extensive consultation within NOSCAN UNCONTROLLED WHEN PRINTED DOCUMENT
GENERAL DATA. Sex : female Age : 40 years old Marriage status : married
 GENERAL DATA Sex : female Age : 40 years old Marriage status : married CHIEF COMPLAINT Bilateral ovarian tumors discovered by sonography accidentally PRESENT ILLNESS 2003-06-26 :bilateral ovarian tumors
GENERAL DATA Sex : female Age : 40 years old Marriage status : married CHIEF COMPLAINT Bilateral ovarian tumors discovered by sonography accidentally PRESENT ILLNESS 2003-06-26 :bilateral ovarian tumors
Cancer: recent advances and implications for underwriting
 Cancer: recent advances and implications for underwriting Robert Rubens Select 74 Bristol 25 February 2010 Agenda Epidemiology - changing mortality Evidence-base for underwriting breast cancer ovarian
Cancer: recent advances and implications for underwriting Robert Rubens Select 74 Bristol 25 February 2010 Agenda Epidemiology - changing mortality Evidence-base for underwriting breast cancer ovarian
Gynecologic Cancers are many diseases. Speaker Disclosure: Gynecologic Cancer Care in the Age of Precision Medicine. Controversies in Women s Health
 Gynecologic Cancer Care in the Age of Precision Medicine Gynecologic Cancers in the Age of Precision Medicine Controversies in Women s Health Lee-may Chen, MD Department of Obstetrics, Gynecology & Reproductive
Gynecologic Cancer Care in the Age of Precision Medicine Gynecologic Cancers in the Age of Precision Medicine Controversies in Women s Health Lee-may Chen, MD Department of Obstetrics, Gynecology & Reproductive
What is endometrial cancer?
 Uterine cancer What is endometrial cancer? Endometrial cancer is the growth of abnormal cells in the lining of the uterus. The lining is called the endometrium. Endometrial cancer usually occurs in women
Uterine cancer What is endometrial cancer? Endometrial cancer is the growth of abnormal cells in the lining of the uterus. The lining is called the endometrium. Endometrial cancer usually occurs in women
Maram Abdaljaleel, MD Dermatopathologist and Neuropathologist University of Jordan, School of Medicine
 Maram Abdaljaleel, MD Dermatopathologist and Neuropathologist University of Jordan, School of Medicine The most common non-skin malignancy of women 2 nd most common cause of cancer deaths in women, following
Maram Abdaljaleel, MD Dermatopathologist and Neuropathologist University of Jordan, School of Medicine The most common non-skin malignancy of women 2 nd most common cause of cancer deaths in women, following
Gynecologic Cancers are many diseases. Gynecologic Cancers in the Age of Precision Medicine Advances in Internal Medicine. Speaker Disclosure:
 Gynecologic Cancer Care in the Age of Precision Medicine Gynecologic Cancers in the Age of Precision Medicine Advances in Internal Medicine Lee-may Chen, MD Department of Obstetrics, Gynecology & Reproductive
Gynecologic Cancer Care in the Age of Precision Medicine Gynecologic Cancers in the Age of Precision Medicine Advances in Internal Medicine Lee-may Chen, MD Department of Obstetrics, Gynecology & Reproductive
Ovarian Cancer. What you should know. making cancer less frightening by enlightening
 Ovarian Cancer What you should know making cancer less frightening by enlightening ovarian cancer the facts Over 360 cases are diagnosed in Ireland annually It is the 6th most common cancer in women 4
Ovarian Cancer What you should know making cancer less frightening by enlightening ovarian cancer the facts Over 360 cases are diagnosed in Ireland annually It is the 6th most common cancer in women 4
L/O/G/O. Ovarian Tumor. Xiaoyu Niu Obstetrics and Gynecology Department Sichuan University West China Second Hospital
 L/O/G/O Ovarian Tumor Xiaoyu Niu Obstetrics and Gynecology Department Sichuan University West China Second Hospital Essentials classification of ovarian tumor clinical manifestation of ovarian tumor metastatic
L/O/G/O Ovarian Tumor Xiaoyu Niu Obstetrics and Gynecology Department Sichuan University West China Second Hospital Essentials classification of ovarian tumor clinical manifestation of ovarian tumor metastatic
3/25/2019. Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates
 J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates Signs
J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates Signs
Ovarian cancer. Quick reference guide. Issue date: April The recognition and initial management of ovarian cancer
 Issue date: April 2011 Ovarian cancer The recognition and initial management of ovarian cancer Developed by the National Collaborating Centre for Cancer About this booklet This is aquick reference guide
Issue date: April 2011 Ovarian cancer The recognition and initial management of ovarian cancer Developed by the National Collaborating Centre for Cancer About this booklet This is aquick reference guide
OVARIAN CARCINOMA Immune Therapy. Antibodies to CA-125 (Ovarex) Vaccine therapy
 OVARIAN CARCINOMA Immune Therapy Antibodies to CA-125 (Ovarex) Vaccine therapy OVARIAN CARCINOMA Targeted Therapy Bevacizumab (Avastin): GOG- 218 Anti-VEGF, angiogenesis inhibitor TLK 286 (Telcyta): Glutathione
OVARIAN CARCINOMA Immune Therapy Antibodies to CA-125 (Ovarex) Vaccine therapy OVARIAN CARCINOMA Targeted Therapy Bevacizumab (Avastin): GOG- 218 Anti-VEGF, angiogenesis inhibitor TLK 286 (Telcyta): Glutathione
2/21/2016. Cancer Precision Medicine: A Primer. Ovarian Cancer Statistics and Standard of Care in 2015 OUTLINE. Background
 Cancer Precision Medicine: A Primer Rebecca C. Arend, MD Division of Gyn Oncology OUTLINE Background Where we are Where we have been Where we are going Targeted Therapy in Ovarian Cancer How to Individualized
Cancer Precision Medicine: A Primer Rebecca C. Arend, MD Division of Gyn Oncology OUTLINE Background Where we are Where we have been Where we are going Targeted Therapy in Ovarian Cancer How to Individualized
Institute of Pathology First Faculty of Medicine Charles University. Ovary
 Ovary Barrett esophagus ph in vagina between 3.8 and 4.5 ph of stomach varies from 1-2 (hydrochloric acid) up to 4-5 BE probably results from upward migration of columnar cells from gastroesophageal junction
Ovary Barrett esophagus ph in vagina between 3.8 and 4.5 ph of stomach varies from 1-2 (hydrochloric acid) up to 4-5 BE probably results from upward migration of columnar cells from gastroesophageal junction
Ovarian Cancer Causes, Risk Factors, and Prevention
 Ovarian Cancer Causes, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors for ovarian cancer.
Ovarian Cancer Causes, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors for ovarian cancer.
Gynecologic Cancer Surveillance and Survivorship: Informing Practice and Policy
 Gynecologic Cancer Surveillance and Survivorship: Informing Practice and Policy Stephanie Yap, M.D. University Gynecologic Oncology Northside Cancer Institute Our Learning Objectives Review survival rates,
Gynecologic Cancer Surveillance and Survivorship: Informing Practice and Policy Stephanie Yap, M.D. University Gynecologic Oncology Northside Cancer Institute Our Learning Objectives Review survival rates,
Epithelial Ovarian Cancer
 Epithelial Ovarian Cancer GYNE/ONC Practice Guideline Dr. Alex Hammond Dr. Ian Kerr Dr. Akira Sugimoto Dr. Stephen Welch Kay Faroni Christine Gawlik Kerri Thornton Approval Date: This guideline is a statement
Epithelial Ovarian Cancer GYNE/ONC Practice Guideline Dr. Alex Hammond Dr. Ian Kerr Dr. Akira Sugimoto Dr. Stephen Welch Kay Faroni Christine Gawlik Kerri Thornton Approval Date: This guideline is a statement
GYN Malignancies. Anatomy of the Female Reproductive System. Overview of Major GYN Cancer Characteristics. Multiple Primary and Histology Coding Rules
 GYN Malignancies Anatomy of the Female Reproductive System Overview of Major GYN Cancer Characteristics Multiple Primary and Histology Coding Rules Collaborative Stage Data Collection System (CSv2) C.S.
GYN Malignancies Anatomy of the Female Reproductive System Overview of Major GYN Cancer Characteristics Multiple Primary and Histology Coding Rules Collaborative Stage Data Collection System (CSv2) C.S.
Anatomy of the Female Reproductive System. Overview of Major GYN Cancer Characteristics. Multiple Primary and Histology Coding Rules
 GYN Malignancies Anatomy of the Female Reproductive System Overview of Major GYN Cancer Characteristics Multiple Primary and Histology Coding Rules Collaborative Stage Data Collection System (CSv2) C.S.
GYN Malignancies Anatomy of the Female Reproductive System Overview of Major GYN Cancer Characteristics Multiple Primary and Histology Coding Rules Collaborative Stage Data Collection System (CSv2) C.S.
What is ovarian cancer?
 What is ovarian cancer? Ovarian cancer is a type of cancer that forms in tissues of the ovary. Most ovarian cancers are either ovarian epithelial cancers (cancer that begins in the cells on the surface
What is ovarian cancer? Ovarian cancer is a type of cancer that forms in tissues of the ovary. Most ovarian cancers are either ovarian epithelial cancers (cancer that begins in the cells on the surface
OVARIAN CANCER FACTSHEET. What is ovarian cancer?
 OVARIAN CANCER FACTSHEET What is ovarian cancer? ENGAGe is releasing a series of factsheets to raise awareness of gynaecological cancers and to support its network to work at grassroots level. Ovarian
OVARIAN CANCER FACTSHEET What is ovarian cancer? ENGAGe is releasing a series of factsheets to raise awareness of gynaecological cancers and to support its network to work at grassroots level. Ovarian
One of the commonest gynecological cancers,especially in white Americans.
 Gynaecology Dr. Rozhan Lecture 6 CARCINOMA OF THE ENDOMETRIUM One of the commonest gynecological cancers,especially in white Americans. It is a disease of postmenopausal women with a peak incidence in
Gynaecology Dr. Rozhan Lecture 6 CARCINOMA OF THE ENDOMETRIUM One of the commonest gynecological cancers,especially in white Americans. It is a disease of postmenopausal women with a peak incidence in
Index. B Bilateral salpingo-oophorectomy (BSO), 69
 A Advanced stage endometrial cancer diagnosis, 92 lymph node metastasis, 92 multivariate analysis, 92 myometrial invasion, 92 prognostic factors FIGO stage, 94 histological grade, 94, 95 histologic cell
A Advanced stage endometrial cancer diagnosis, 92 lymph node metastasis, 92 multivariate analysis, 92 myometrial invasion, 92 prognostic factors FIGO stage, 94 histological grade, 94, 95 histologic cell
Gynecologic Malignancies. Kristen D Starbuck 4/20/18
 Gynecologic Malignancies Kristen D Starbuck 4/20/18 Outline Female Cancer Statistics Uterine Cancer Adnexal Cancer Cervical Cancer Vulvar Cancer Uterine Cancer Endometrial Cancer Uterine Sarcoma Endometrial
Gynecologic Malignancies Kristen D Starbuck 4/20/18 Outline Female Cancer Statistics Uterine Cancer Adnexal Cancer Cervical Cancer Vulvar Cancer Uterine Cancer Endometrial Cancer Uterine Sarcoma Endometrial
Stage 3c breast cancer survival rate
 Stage 3c breast cancer survival rate There are five main subtypes of ovarian carcinoma, of which high-grade serous carcinoma is the most common. [3]. Testicular cancer is generally found in young men.
Stage 3c breast cancer survival rate There are five main subtypes of ovarian carcinoma, of which high-grade serous carcinoma is the most common. [3]. Testicular cancer is generally found in young men.
Chapter 2: Initial treatment for endometrial cancer (including histologic variant type)
 Chapter 2: Initial treatment for endometrial cancer (including histologic variant type) CQ01 Which surgical techniques for hysterectomy are recommended for patients considered to be stage I preoperatively?
Chapter 2: Initial treatment for endometrial cancer (including histologic variant type) CQ01 Which surgical techniques for hysterectomy are recommended for patients considered to be stage I preoperatively?
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
MPH Quiz. 1. How many primaries are present based on this pathology report? 2. What rule is this based on?
 MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
Fast Facts: Ovarian Cancer
 Fast Facts Fast Facts: Ovarian Cancer Christina Fotopoulou MD PhD Consultant Gynaecological Oncologist Queen Charlotte s and Chelsea Hospital London, UK Thomas J Herzog MD Professor of Obstetrics and Gynecology
Fast Facts Fast Facts: Ovarian Cancer Christina Fotopoulou MD PhD Consultant Gynaecological Oncologist Queen Charlotte s and Chelsea Hospital London, UK Thomas J Herzog MD Professor of Obstetrics and Gynecology
H&E, IHC anti- Cytokeratin
 Cat No: OVC2281 - Ovary cancer tissue array Lot# Cores Size Cut Format QA/QC OVC228101 228 1.1mm 4um 12X19 H&E, IHC anti- Cytokeratin Recommended applications: For Research use only. RNA or protein ovary
Cat No: OVC2281 - Ovary cancer tissue array Lot# Cores Size Cut Format QA/QC OVC228101 228 1.1mm 4um 12X19 H&E, IHC anti- Cytokeratin Recommended applications: For Research use only. RNA or protein ovary
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA91: Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for the treatment of advanced ovarian
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA91: Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for the treatment of advanced ovarian
of 20 to 80 and subsequently declines [2].
![of 20 to 80 and subsequently declines [2]. of 20 to 80 and subsequently declines [2].](/thumbs/80/81450506.jpg) - - According to the 2014 World Health Organization (WHO) classification and tumor morphology, primary ovarian tumors are subdivided into three categories: epithelial (60%), germ cell (30%), and sex-cord
- - According to the 2014 World Health Organization (WHO) classification and tumor morphology, primary ovarian tumors are subdivided into three categories: epithelial (60%), germ cell (30%), and sex-cord
ENDOMETRIAL CANCER: A GUIDE FOR PATIENTS
 ENDOMETRIAL CANCER: A GUIDE FOR PATIENTS PATIENT INFORMATION BASED ON ESMO CLINICAL PRACTICE GUIDELINES This guide for patients has been prepared by Reliable Cancer Therapies (RCT) as a service to patients,
ENDOMETRIAL CANCER: A GUIDE FOR PATIENTS PATIENT INFORMATION BASED ON ESMO CLINICAL PRACTICE GUIDELINES This guide for patients has been prepared by Reliable Cancer Therapies (RCT) as a service to patients,
LCA Gynaecological Cancer Clinical Guidelines
 LCA Gynaecological Cancer Clinical Guidelines July 2014 LCA GYNAECOLOGICAL CANCER CLINICAL GUIDELINES Contents Introduction... 5 Executive summary... 6 1 The Structure of Gynaecology Oncology Services...
LCA Gynaecological Cancer Clinical Guidelines July 2014 LCA GYNAECOLOGICAL CANCER CLINICAL GUIDELINES Contents Introduction... 5 Executive summary... 6 1 The Structure of Gynaecology Oncology Services...
Pathology of the female genital tract
 Pathology of the female genital tract Common illnesses of the female genital tract Before menarche Developmental anomalies Tumors (ovarial teratoma) Amenorrhea Fertile years PCOS, ovarian cysts Endometriosis
Pathology of the female genital tract Common illnesses of the female genital tract Before menarche Developmental anomalies Tumors (ovarial teratoma) Amenorrhea Fertile years PCOS, ovarian cysts Endometriosis
INTRODUCTION Ovarian cancer is the leading cause of mortality from gynecologic malignancies in the industrialized countries and is responsible for
 INTRODUCTION Ovarian cancer is the leading cause of mortality from gynecologic malignancies in the industrialized countries and is responsible for more deaths than both cervical and endometrial tumours.
INTRODUCTION Ovarian cancer is the leading cause of mortality from gynecologic malignancies in the industrialized countries and is responsible for more deaths than both cervical and endometrial tumours.
North of Scotland Cancer Network Clinical Management Guideline for Carcinoma of the Uterine Cervix
 THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
It is a malignancy originating from breast tissue
 59 Breast cancer 1 It is a malignancy originating from breast tissue including both early stages which are potentially curable, and metastatic breast cancer (MBC) which is usually incurable. Most breast
59 Breast cancer 1 It is a malignancy originating from breast tissue including both early stages which are potentially curable, and metastatic breast cancer (MBC) which is usually incurable. Most breast
Oncology 101. Cancer Basics
 Oncology 101 Cancer Basics What Will You Learn? What is Cancer and How Does It Develop? Cancer Diagnosis and Staging Cancer Treatment What is Cancer? Cancer is a group of more than 100 different diseases
Oncology 101 Cancer Basics What Will You Learn? What is Cancer and How Does It Develop? Cancer Diagnosis and Staging Cancer Treatment What is Cancer? Cancer is a group of more than 100 different diseases
6 Week Course Agenda. Today s Agenda. Ovarian Cancer: Risk Factors. Winning the War 11/30/2016 on Women s Cancer Gynecologic Cancer Prevention
 6 Week Course Agenda Winning the War 11/30/2016 on Women s Cancer Gynecologic Cancer Prevention Lee-may Chen, MD Director, Division of Gynecologic Oncology Professor Department of Obstetrics, Gynecology
6 Week Course Agenda Winning the War 11/30/2016 on Women s Cancer Gynecologic Cancer Prevention Lee-may Chen, MD Director, Division of Gynecologic Oncology Professor Department of Obstetrics, Gynecology
OVARIAN CANCER STATISTICS
 bioprognos OncoOVARIAN Dx Non-invasive blood test useful to suggest a possible diagnosis in patients with suspected malignancy in the ovarians, reduce inappropriate diagnostic tests, days of hospitalization,
bioprognos OncoOVARIAN Dx Non-invasive blood test useful to suggest a possible diagnosis in patients with suspected malignancy in the ovarians, reduce inappropriate diagnostic tests, days of hospitalization,
Stage 3 ovarian cancer survival rate
 Search Stage 3 ovarian cancer survival rate 19-5-2017 If you've been diagnosed with ovarian cancer, it's natural to wonder about your prognosis. Learn about survival rates, outlook, and more. Take the
Search Stage 3 ovarian cancer survival rate 19-5-2017 If you've been diagnosed with ovarian cancer, it's natural to wonder about your prognosis. Learn about survival rates, outlook, and more. Take the
Surgery to Reduce the Risk of Ovarian Cancer. Information for Women at Increased Risk
 Surgery to Reduce the Risk of Ovarian Cancer Information for Women at Increased Risk Centre for Genetics Education NSW Health 2017 The Centre for Genetics Education NSW Health Level 5 2C Herbert St St
Surgery to Reduce the Risk of Ovarian Cancer Information for Women at Increased Risk Centre for Genetics Education NSW Health 2017 The Centre for Genetics Education NSW Health Level 5 2C Herbert St St
Ovarian Cancer. Georgia McCann, MD. Division of Gynecologic Oncology
 Ovarian Cancer Georgia McCann, MD Division of Gynecologic Oncology Myth: Ovarian cancer is a silent killer Non-specific Abdominal pain, discomfort Bloating, back pain Urinary urgency Constipation Tiredness
Ovarian Cancer Georgia McCann, MD Division of Gynecologic Oncology Myth: Ovarian cancer is a silent killer Non-specific Abdominal pain, discomfort Bloating, back pain Urinary urgency Constipation Tiredness
Stage 3 ovarian cancer survival rate
 Stage 3 ovarian cancer survival rate Gogamz Menu The latest ovarian cancer survival statistics for the UK for Health Professionals. See data for age, trends over time, stage at diagnosis and more. 5-8-2014
Stage 3 ovarian cancer survival rate Gogamz Menu The latest ovarian cancer survival statistics for the UK for Health Professionals. See data for age, trends over time, stage at diagnosis and more. 5-8-2014
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER CERVIX
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER CERVIX Site Group: Gynecology Cervix Author: Dr. Stephane Laframboise 1. INTRODUCTION 3 2. PREVENTION 3 3. SCREENING AND
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER CERVIX Site Group: Gynecology Cervix Author: Dr. Stephane Laframboise 1. INTRODUCTION 3 2. PREVENTION 3 3. SCREENING AND
SAMPLE. Rencarex (Renal Cell Carcinoma)- Analysis and Forecasts to Reference Code: GDHC0004RCCDVR Publication Date: March 2012
 Rencarex (Renal Cell Carcinoma)- Analysis and Forecasts to Reference Code: GDHC0004RCCDVR Publication Date: March 2012 The Renal Cell Carcinoma (RCC) Disease Therapeutics Market GlobalData s analysis suggests
Rencarex (Renal Cell Carcinoma)- Analysis and Forecasts to Reference Code: GDHC0004RCCDVR Publication Date: March 2012 The Renal Cell Carcinoma (RCC) Disease Therapeutics Market GlobalData s analysis suggests
3 cell types in the normal ovary
 Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal cells
Ovarian tumors 3 cell types in the normal ovary Surface (coelomic epithelium) the origin of the great majority of ovarian tumors 90% of malignant ovarian tumors Totipotent germ cells Sex cord-stromal cells
Proposed All Wales Vulval Cancer Guidelines. Dr Amanda Tristram
 Proposed All Wales Vulval Cancer Guidelines Dr Amanda Tristram Previous FIGO staging FIGO Stage Features TNM Ia Lesion confined to vulva with
Proposed All Wales Vulval Cancer Guidelines Dr Amanda Tristram Previous FIGO staging FIGO Stage Features TNM Ia Lesion confined to vulva with
[EPUB] RARE LOW GRADE OVARIAN CANCER
![[EPUB] RARE LOW GRADE OVARIAN CANCER [EPUB] RARE LOW GRADE OVARIAN CANCER](/thumbs/82/85256417.jpg) 09 June, 2018 [EPUB] RARE LOW GRADE OVARIAN CANCER Document Filetype: PDF 540.53 KB 0 [EPUB] RARE LOW GRADE OVARIAN CANCER Although there are ongoing trials specifically for low-grade ovarian cancers.
09 June, 2018 [EPUB] RARE LOW GRADE OVARIAN CANCER Document Filetype: PDF 540.53 KB 0 [EPUB] RARE LOW GRADE OVARIAN CANCER Although there are ongoing trials specifically for low-grade ovarian cancers.
Springer Healthcare. Understanding and Diagnosing Ovarian Cancer. Concise Reference: Krishnansu S Tewari, Bradley J Monk
 Concise Reference: Understanding and Diagnosing Ovarian Cancer Krishnansu S Tewari, Bradley J Monk Extracted from: The 21 st Century Handbook of Clinical Ovarian Cancer Published by Springer Healthcare
Concise Reference: Understanding and Diagnosing Ovarian Cancer Krishnansu S Tewari, Bradley J Monk Extracted from: The 21 st Century Handbook of Clinical Ovarian Cancer Published by Springer Healthcare
receive adjuvant chemotherapy
 Women with high h risk early stage endometrial cancer should receive adjuvant chemotherapy Michael Friedlander The Prince of Wales Cancer Centre and Royal Hospital for Women The Prince of Wales Cancer
Women with high h risk early stage endometrial cancer should receive adjuvant chemotherapy Michael Friedlander The Prince of Wales Cancer Centre and Royal Hospital for Women The Prince of Wales Cancer
Chapter 8 Adenocarcinoma
 Page 80 Chapter 8 Adenocarcinoma Overview In Japan, the proportion of squamous cell carcinoma among all cervical cancers has been declining every year. In a recent survey, non-squamous cell carcinoma accounted
Page 80 Chapter 8 Adenocarcinoma Overview In Japan, the proportion of squamous cell carcinoma among all cervical cancers has been declining every year. In a recent survey, non-squamous cell carcinoma accounted
Work up of a Pelvic Mass
 Work up of a Pelvic Mass Considerations from the north where primary care and CON clinic / GPO work interface Dr. Shannon Douglas, GPO Vanderhoof with support by Dr Margaret Smith and Dr. Ursula Lee Nov
Work up of a Pelvic Mass Considerations from the north where primary care and CON clinic / GPO work interface Dr. Shannon Douglas, GPO Vanderhoof with support by Dr Margaret Smith and Dr. Ursula Lee Nov
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Locally advanced or metastatic bladder cancer Bladder cancer is the sixth most common cancer. It is more common in men than in
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Locally advanced or metastatic bladder cancer Bladder cancer is the sixth most common cancer. It is more common in men than in
BRCA mutation carrier patient: How to manage?
 BRCA mutation carrier patient: How to manage? Clinical Case Presentation Katarzyna Sosińska-Mielcarek Department of Oncology and Radiotherapy University Clinical Center Gdansk, Poland esmo.org DISCLOSURE
BRCA mutation carrier patient: How to manage? Clinical Case Presentation Katarzyna Sosińska-Mielcarek Department of Oncology and Radiotherapy University Clinical Center Gdansk, Poland esmo.org DISCLOSURE
Specialised Services Policy: CP02 Hyperthermic Intraperitoneal Chemotherapy (HIPEC) and Cytoreductive Surgery for treatment of Pseudomyxoma Peritonei
 Specialised Services Policy: CP02 Hyperthermic Intraperitoneal Chemotherapy (HIPEC) of Pseudomyxoma Peritonei Document Author: Assistant Medical Director Executive Lead: Medical Director Approved by: Management
Specialised Services Policy: CP02 Hyperthermic Intraperitoneal Chemotherapy (HIPEC) of Pseudomyxoma Peritonei Document Author: Assistant Medical Director Executive Lead: Medical Director Approved by: Management
Table Selected Clinical Trials of Anti-Angiogenesis Therapy in Gynecologic Malignancies
 Table Selected Clinical Trials of Anti-Angiogenesis Therapy in Gynecologic Malignancies Uterus Study N Eligibility Regimen RR (No. of Responses) Median OS Grade 3/4 Toxicities Nimeiri et al[42] Total:
Table Selected Clinical Trials of Anti-Angiogenesis Therapy in Gynecologic Malignancies Uterus Study N Eligibility Regimen RR (No. of Responses) Median OS Grade 3/4 Toxicities Nimeiri et al[42] Total:
Cancers of unknown primary : Knowing the unknown. Prof. Ahmed Hossain Professor of Medicine SSMC
 Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
OVAIRES PROTOCOLES PTES PHASE DESCRIPTION
 SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG II PRÉVENTION A Randomized Phase II Double-Blind Placebo-Controlled Trials of Acetylsalicylic
SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG II PRÉVENTION A Randomized Phase II Double-Blind Placebo-Controlled Trials of Acetylsalicylic
Bases biológicas del cáncer de ovario en el siglo XXI
 Bases biológicas del cáncer de ovario en el siglo XXI Iñigo Espinosa, M.D. Clínica Universidad de Navarra Epithelial Ovarian Tumors WHO 1973-2014 Serous Mucinous Endometrioid Clear cell Transitional Squamous
Bases biológicas del cáncer de ovario en el siglo XXI Iñigo Espinosa, M.D. Clínica Universidad de Navarra Epithelial Ovarian Tumors WHO 1973-2014 Serous Mucinous Endometrioid Clear cell Transitional Squamous
3 Summary of clinical applications and limitations of measurements
 CA125 (serum) 1 Name and description of analyte 1.1 Name of analyte Cancer Antigen 125 (CA125) 1.2 Alternative names Mucin-16 1.3 NLMC code To follow 1.4 Description of analyte CA125 is an antigenic determinant
CA125 (serum) 1 Name and description of analyte 1.1 Name of analyte Cancer Antigen 125 (CA125) 1.2 Alternative names Mucin-16 1.3 NLMC code To follow 1.4 Description of analyte CA125 is an antigenic determinant
Key Recommendations. Gynecologic management of women with inherited risk of gynecologic cancer
 Gynecologic management of women with inherited risk of gynecologic cancer C. Bethan Powell MD Kaiser Permanente Northern California Gynecologic Oncology Program Lead, Kaiser Permanente Northern California
Gynecologic management of women with inherited risk of gynecologic cancer C. Bethan Powell MD Kaiser Permanente Northern California Gynecologic Oncology Program Lead, Kaiser Permanente Northern California
Radiation Oncology MOC Study Guide
 Radiation Oncology MOC Study Guide The following study guide is intended to give a general overview of the type of material that will be covered on the Radiation Oncology Maintenance of Certification (MOC)
Radiation Oncology MOC Study Guide The following study guide is intended to give a general overview of the type of material that will be covered on the Radiation Oncology Maintenance of Certification (MOC)
BREAST PATHOLOGY. Fibrocystic Changes
 BREAST PATHOLOGY Lesions of the breast are very common, and they present as palpable, sometimes painful, nodules or masses. Most of these lesions are benign. Breast cancer is the 2 nd most common cause
BREAST PATHOLOGY Lesions of the breast are very common, and they present as palpable, sometimes painful, nodules or masses. Most of these lesions are benign. Breast cancer is the 2 nd most common cause
through the cell cycle. However, how we administer drugs also depends on the combinations that we give and the doses that we give.
 Hello and welcome to this lecture. My name is Hillary Prescott. I am a Clinical Pharmacy Specialist at The University of Texas MD Anderson Cancer Center. My colleague, Jeff Bryan and I have prepared this
Hello and welcome to this lecture. My name is Hillary Prescott. I am a Clinical Pharmacy Specialist at The University of Texas MD Anderson Cancer Center. My colleague, Jeff Bryan and I have prepared this
Cervical Cancer: 2018 FIGO Staging
 Cervical Cancer: 2018 FIGO Staging Jonathan S. Berek, MD, MMS Laurie Kraus Lacob Professor Stanford University School of Medicine Director, Stanford Women s Cancer Center Senior Scientific Advisor, Stanford
Cervical Cancer: 2018 FIGO Staging Jonathan S. Berek, MD, MMS Laurie Kraus Lacob Professor Stanford University School of Medicine Director, Stanford Women s Cancer Center Senior Scientific Advisor, Stanford
PORTEC-4. Patient seqnr. Age at inclusion (years) Hospital:
 May 2016 Randomisation Checklist Form 1, page 1 of 2 Patient seqnr. Age at inclusion (years) Hospital: Eligible patients should be registered and randomised via the Internet at : https://prod.tenalea.net/fs4/dm/delogin.aspx?refererpath=dehome.aspx
May 2016 Randomisation Checklist Form 1, page 1 of 2 Patient seqnr. Age at inclusion (years) Hospital: Eligible patients should be registered and randomised via the Internet at : https://prod.tenalea.net/fs4/dm/delogin.aspx?refererpath=dehome.aspx
General history. Basic Data : Age :62y/o Date of admitted: Married status : Married
 General history Basic Data : Age :62y/o Date of admitted:940510 Married status : Married General history Chief Complain : bilateral ovarian cyst incidentally being found out during pap smear. Present Illness
General history Basic Data : Age :62y/o Date of admitted:940510 Married status : Married General history Chief Complain : bilateral ovarian cyst incidentally being found out during pap smear. Present Illness
Gynecologic Oncologist. Surgery Chemotherapy Radiation Therapy Hormonal Therapy Immunotherapy. Cervical cancer
 Gynecologic Oncology Pre invasive vulvar, vaginal, & cervical disease Vulvar Cervical Endometrial Uterine Sarcoma Fallopian Tube Ovarian GTD Gynecologic Oncologist Surgery Chemotherapy Radiation Therapy
Gynecologic Oncology Pre invasive vulvar, vaginal, & cervical disease Vulvar Cervical Endometrial Uterine Sarcoma Fallopian Tube Ovarian GTD Gynecologic Oncologist Surgery Chemotherapy Radiation Therapy
Case 1. Gynaecology Case Presentation. Objectives. Disclosures 22/10/ year old female Clinical history: Assess right ovarian cyst
 Gynaecology Case Presentation Organ Imaging 2016 University of Toronto Sarah Johnson 39 year old female Clinical history: Assess right ovarian cyst Clinically diagnosed endometriosis Started fertility
Gynaecology Case Presentation Organ Imaging 2016 University of Toronto Sarah Johnson 39 year old female Clinical history: Assess right ovarian cyst Clinically diagnosed endometriosis Started fertility
Guidelines for Assigning Summary Stage 2000
 Guidelines for Assigning Summary Stage 2000 Mary Lewis, CTR National Program of Cancer Registries 2014 NCRA Annual Meeting May 17, 2014 National Center for Chronic Disease Prevention and Health Promotion
Guidelines for Assigning Summary Stage 2000 Mary Lewis, CTR National Program of Cancer Registries 2014 NCRA Annual Meeting May 17, 2014 National Center for Chronic Disease Prevention and Health Promotion
What is Ovarian Cancer?
 Ovarian Cancer What is Ovarian Cancer? Let us answer some of your questions. ESMO Patient Guide Series based on the ESMO Clinical Practice Guidelines esmo.org Ovarian cancer Ovarian cancer An ESMO guide
Ovarian Cancer What is Ovarian Cancer? Let us answer some of your questions. ESMO Patient Guide Series based on the ESMO Clinical Practice Guidelines esmo.org Ovarian cancer Ovarian cancer An ESMO guide
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
