Dr. Bernard Huber CEO. Bio-Europe
|
|
|
- Francis Parsons
- 5 years ago
- Views:
Transcription
1 Dr. Bernard Huber CEO Bio-Europe
2 ORYX Overview ORYX GmbH & Co. KG (ORYX) is a privately held company for translational oncology founded in 2007 and located in Baldham/Munich, Germany ORYX bridges the gap for new cancer therapies between leading academic research institutions and the pharmaceutical industry ORYX has been entrusted with three promising cancer immunotherapy substances by both the German Cancer Research Center (DKFZ) and the University of Heidelberg and has successfully developed these substances in clinical phase I/IIa trials ORYX has obtained valid safety results and already first promising efficacy data in all clinical phase I/IIa trials Bio-Europe
3 Three Cancer Immunotherapy Substances in Clinical Development Therapeutic Peptide Vaccines directed to constantly expressed tumor antigens MicOryx: VicOryx: First in class therapeutic vaccination against microsatellite instable (MSI-H) cancers Phase I/IIa: Colorectal Cancer First in class therapeutic vaccination against p16 INK4a expressing HPV-associated cancers Phase I/IIa: Cervical Cancer, Head and Neck Cancer, etc. Oncolytic Virus inducing a lasting bystander effect through oncolysis ParvOryx: First in class therapeutic use of Parvovirus H-1 (H-1PV) Phase I/IIa: Glioblastoma multiforme Bio-Europe
4 MicOryx: Rationale Several cancers arise from the lack of DNA mismatch repair (MMR) resulting in the accumulation of single deletions or insertions at coding microsatellites (MSI-H) MSI-H mutations lead to the expression of frameshift peptides (FSPs) FSPs are tumor specific antigens which are constantly expressed Cancers with MSI-H mutations include: 10-15% of colorectal cancers, 20-25% of endometrial cancers, 25-30% of upper urinary tract cancers, 15-20% of gastric cancers and 5-10% of pancreatic cancers Preclinical work shows a natural humoral and cellular immune response against FSPs in MSI-H colorectal cancer, which demonstrates that FSPs are recognized by the immune system and can trigger an immune response The therapeutic rationale is to induce a specific immune response against FSPs in MSI-H colorectal cancer patients Bio-Europe
5 MicOryx: Clinical Phase I/IIa Trial Single center, two part open label, prospective, 1 st part 6 patients, 2 nd part 16 patients (n = 22), UICC stage III/IV, MSI-H colorectal cancer Total of 12 s.c. applications with three FSPs one time/week for four consecutive weeks, followed by a four week rest period (one cycle) for a total of three cycles Subcutaneous injection of FSP mix and Montanide ISA 51 VG Monitoring of toxicity, immune response (including DTH) and tumor response Primary Objective: Safety 22/22 patients Secondary Objective: Efficacy (specific immune responses) 21/22 patients (95,5%) End of Study: Final Report H1/2015 Bio-Europe
6 VicOryx: Rationale About 5 % of all cancers are associated with human papilloma viruses (HPV) including: % of anal and vulvar cancers, % of vaginal and penile cancers, % of head and neck cancers In HPV-associated cancers, the human cyclindependent kinase inhibitor p16ink4a is expressed as an early consequence of HPVmediated cell transformation p16ink4a is a tumor antigen that is constantly expressed In normal cells, p16ink4a is rarely expressed and leads to immediate senescence Preclinical work shows a natural humoral and cellular immune response against p16 INK4a in HPV associated cancers, which demonstrates that p16 INK4a is recognized by the immune system and can trigger an immune response The therapeutic rationale is to induce a specific immune response against p16 INK4a in HPV associated cancers Bio-Europe
7 VicOryx: Clinical Phase I/IIa Trial Single center, two part open label, prospective, 1 st part 10 patients, 2 nd part 16 patients (n = 26) UICC stage III/IV, advanced HPV- and p16 INK4a - positive cervix, vulvar, vaginal, penile, anal or head and neck cancer Total of 12 s.c. applications with a specific p16 peptide one time/week for four consecutive weeks, followed by a four week rest period (one cycle) for a total of three cycles Subcutaneous injection of p16 and Montanide ISA 51 VG Monitoring of toxicity, immune response (including DTH) and tumor response Primary Objective: Safety 26/26 patients Secondary Objective: Efficacy (specific immune responses) 18/26 patients (69,23%) End of Study: Final Report H1/2015 Bio-Europe
8 ParvOryx: Rationale Oncolytic Parvovirus H-1 (H-1PV) is a wild type rat virus that infects and lyses tumor cells and tumor stem cells from a broad range of human tumors Human tumors sensitive to H-1PV include: glioblastoma multiforme pancreatic cancer breast cancer lung cancer melanoma, lymphoma pediatric tumors (e.g. neuroblastoma and medulloblastoma) prostate cancer renal cancer Parvovirus H-1: does not affect normal cells and is not pathogenic for humans acts at relatively low multiplicities of infection crosses the blood brain barrier Preclinical data showed that treatment with H-1PV causes tumor remissions in animal models bearing human tumors, e.g. glioblastoma multiforme and pancreatic cancer Bio-Europe
9 ParvOryx: Rationale H-1PV exerts a twofold therapeutic effect: selective lysis of tumor cells induction of a lasting bystander effect = stimulation of the adaptive immune system, preventing tumor progression, relapse and metastasis formation H-1PV oncolytic activity is predominantly mediated by non-structural protein (NS1) causing: dysregulation of cell transcription cell cycle arrest shut off of cell replication activation of cellular stress response induction of apoptotic and non-apoptotic cell death Bio-Europe
10 ParvOryx: Clinical Phase I/IIa Trial Single center, open label, prospective, dose escalating, two groups, 1st group (it) 12 patients, 2nd group (iv) 6 patients (n = 18) UICC Stage IV, progressive primary or recurrent glioblastoma multiforme It: half of the dose in the tumor, half of the dose in the wall of the resection cavity Iv: half of the dose in 5 consecutive injections, half of the dose in the wall of the resection cavity Primary Objective: Safety 18/18 patients Secondary Objective: OS 6 month, 76,47% (13 patients: 6,0 35,8 month) 3 patients still in 6 months follow up strong cellular immune response observed against glioma and viral proteins (bystander effect) End of Study: Final Report Q4/2015 Bio-Europe
11 ORYX - Partnering Opportunities Open for partnering discussions with global partners Contact ORYX GmbH & Co. KG Marktplatz Baldham, Germany Phone: Fax: info@oryx-medicine.com Bio-Europe
12 Disclaimer This Presentation includes and is based, inter alia, on forward-looking information and statements that are subject to risks and uncertainties that could cause actual results to differ. These statements and this Presentation are based on current expectations, estimates and projections, which generally are identifiable by statements containing words such as expects, believes, estimates or similar expressions. Important factors that could cause actual results to differ materially from those expectations include, among others, general economic and industry conditions in markets which are expected to be major markets for ORYX products, as well as risks and uncertainties related to product development, regulatory approvals, commercial partnerships, the outcome of intellectual property rights litigation and the competitive situation. Although ORYX believes that its expectations and the Presentation are based upon reasonable assumptions, it can give no assurance that those expectations will be achieved or that the actual results will be as set out in the Presentation. ORYX is making no representation or warranty, expressed or implied, as to the accuracy, reliability or completeness of the Presentation, and neither ORYX nor any of its directors, officers or employees will have any liability to you or any other persons resulting from your use of the information contained herein. This presentation was prepared for the 20th Annual International Partnering Conference Bio-Europe in Frankfurt, on November 3-5, 2014, and the information contained within will not be updated in this presentation. The slides should be read and considered in connection with other information provided by the company. Bio-Europe
ORYX Translational Medicine. Dr. Bernard Huber/ CEO
 ORYX Translational Medicine Dr. Bernard Huber/ CEO ORYX Overview ORYX GmbH & Co. KG (ORYX) is a privately held company for translational oncology founded in 2007 and located in Baldham/Munich, Germany
ORYX Translational Medicine Dr. Bernard Huber/ CEO ORYX Overview ORYX GmbH & Co. KG (ORYX) is a privately held company for translational oncology founded in 2007 and located in Baldham/Munich, Germany
ORYX Translational Medicine. Dr. Bernard Huber / CEO
 ORYX Translational Medicine Dr. Bernard Huber / CEO BIO International Convention June 2016 ORYX Overview ORYX GmbH & Co. KG (ORYX) is a privately held company for translational oncology founded in 2007
ORYX Translational Medicine Dr. Bernard Huber / CEO BIO International Convention June 2016 ORYX Overview ORYX GmbH & Co. KG (ORYX) is a privately held company for translational oncology founded in 2007
ORYX Translational Medicine. Dr. Dr. Sven Rohmann/ CBDO
 ORYX Translational Medicine Dr. Dr. Sven Rohmann/ CBDO ORYX Overview Ø ORYX GmbH & Co. KG (ORYX) is a privately held company for translational oncology founded in 2007 and located in Baldham/Munich, Germany
ORYX Translational Medicine Dr. Dr. Sven Rohmann/ CBDO ORYX Overview Ø ORYX GmbH & Co. KG (ORYX) is a privately held company for translational oncology founded in 2007 and located in Baldham/Munich, Germany
Arming the patient s immune system to fight cancer
 Arming the patient s immune system to fight cancer Redeye - Fight Cancer Seminar 10 th March 2017 Øystein Soug, CEO Important notice and disclaimer This report contains certain forward-looking statements
Arming the patient s immune system to fight cancer Redeye - Fight Cancer Seminar 10 th March 2017 Øystein Soug, CEO Important notice and disclaimer This report contains certain forward-looking statements
CANCER VACCINES THE NEXT WAVE IN IMMUNO-ONCOLOGY
 CANCER VACCINES THE NEXT WAVE IN IMMUNO-ONCOLOGY Peter Skorpil VP Business Development peter.skorpil@targovax.com Cutting Edge Oslo 2016-10-18 www.targovax.com 1 Important notice and disclaimer This report
CANCER VACCINES THE NEXT WAVE IN IMMUNO-ONCOLOGY Peter Skorpil VP Business Development peter.skorpil@targovax.com Cutting Edge Oslo 2016-10-18 www.targovax.com 1 Important notice and disclaimer This report
3Q 2016 presentation
 Arming the patient s immune system to fight cancer 3Q 2016 presentation 17 November 2016 Important notice and disclaimer This report contains certain forward-looking statements based on uncertainty, since
Arming the patient s immune system to fight cancer 3Q 2016 presentation 17 November 2016 Important notice and disclaimer This report contains certain forward-looking statements based on uncertainty, since
Company overview. Highlights for the 1 st quarter 2018 (January-March)
 Company overview Vaccibody is a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel immunotherapies. The company is a leader in the rapidly developing field of
Company overview Vaccibody is a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel immunotherapies. The company is a leader in the rapidly developing field of
Merck Pipeline. August 1, 2018
 Merck Pipeline August 1, 2018 Lead-in Language The chart below reflects the Company s research pipeline as of August 1, 2018. Candidates shown in Phase 3 include specific products and the date such candidate
Merck Pipeline August 1, 2018 Lead-in Language The chart below reflects the Company s research pipeline as of August 1, 2018. Candidates shown in Phase 3 include specific products and the date such candidate
Merck Pipeline. November 1, 2017
 Merck Pipeline November 1, 2017 Lead-in Language The chart below reflects the Company s research pipeline as of November 1, 2017. Candidates shown in Phase 3 include specific products and the date such
Merck Pipeline November 1, 2017 Lead-in Language The chart below reflects the Company s research pipeline as of November 1, 2017. Candidates shown in Phase 3 include specific products and the date such
MERCK ONCOLOGY OVERVIEW AACR 2018 APRIL 16, 2018
 MERCK ONCOLOGY OVERVIEW AACR 2018 APRIL 16, 2018 Forward-Looking Statement of Merck & Co., Inc., Kenilworth, NJ, USA This presentation of Merck & Co., Inc., Kenilworth, N.J., USA (the company ) includes
MERCK ONCOLOGY OVERVIEW AACR 2018 APRIL 16, 2018 Forward-Looking Statement of Merck & Co., Inc., Kenilworth, NJ, USA This presentation of Merck & Co., Inc., Kenilworth, N.J., USA (the company ) includes
Merck ASCO 2015 Investor Briefing
 Merck ASCO 2015 Investor Briefing Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation
Merck ASCO 2015 Investor Briefing Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation
Phase III ARISER trial data ASCO 2013 RENCAREX - Unique targeted antibody therapy for adjuvant treatment of non-metastatic clear cell renal cell
 Phase III ARISER trial data ASCO 2013 RENCAREX - Unique targeted antibody therapy for adjuvant treatment of non-metastatic clear cell renal cell carcinoma patients June 2013 Carbonic Anhydrase IX (CAIX):
Phase III ARISER trial data ASCO 2013 RENCAREX - Unique targeted antibody therapy for adjuvant treatment of non-metastatic clear cell renal cell carcinoma patients June 2013 Carbonic Anhydrase IX (CAIX):
Opdivo. Opdivo (nivolumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.53 Subsection: Antineoplastic Agents Original Policy Date: January 16, 2015 Subject: Opdivo Page:
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.53 Subsection: Antineoplastic Agents Original Policy Date: January 16, 2015 Subject: Opdivo Page:
Corporate Presentation May Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers
 Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY NFORMATON n format provided by Melero et al. (AUGUST 2015) Supplementary nformation S3 Combinations including two or more immunotherapy agents based on PD-1/PD-L1 blockade. (Source: https://clinicaltrials.gov/
SUPPLEMENTARY NFORMATON n format provided by Melero et al. (AUGUST 2015) Supplementary nformation S3 Combinations including two or more immunotherapy agents based on PD-1/PD-L1 blockade. (Source: https://clinicaltrials.gov/
Supplementary Online Content
 Supplementary Online Content Chacón MR, Enrico DH, Burton J, Waisberg FD, Videla VM. Incidence of placebo adverse events in randomized clinical trials of targeted and immunotherapy cancer drugs in the
Supplementary Online Content Chacón MR, Enrico DH, Burton J, Waisberg FD, Videla VM. Incidence of placebo adverse events in randomized clinical trials of targeted and immunotherapy cancer drugs in the
The immune biology of MSI cancers and its clinical implications
 The immune biology of MSI cancers and its clinical implications Magnus von Knebel Doeberitz, MD magnus.knebel-doeberitz@med.uni-heidelberg.de Department of Applied Tumor Biology Institute of Pathology
The immune biology of MSI cancers and its clinical implications Magnus von Knebel Doeberitz, MD magnus.knebel-doeberitz@med.uni-heidelberg.de Department of Applied Tumor Biology Institute of Pathology
Corporate Presentation September Nasdaq: ADXS
 Corporate Presentation September 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements regarding the ability and
Corporate Presentation September 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements regarding the ability and
CANCER 1.7 M 609,000 26% 15.5 M 73% JUST THE FACTS. More Than 1,100 Cancer Treatments in Clinical Testing Offer Hope to Patients
 CANCER MEDICINES IN DEVELOPMENT 2018 REPORT JUST THE FACTS MORE THAN 1.7 M ESTIMATED NEW CASES OF CANCER IN 2018 IN THE UNITED STATES MORE THAN 609,000 U.S. CANCER DEATHS ARE EXPECTED IN 2018 SINCE PEAKING
CANCER MEDICINES IN DEVELOPMENT 2018 REPORT JUST THE FACTS MORE THAN 1.7 M ESTIMATED NEW CASES OF CANCER IN 2018 IN THE UNITED STATES MORE THAN 609,000 U.S. CANCER DEATHS ARE EXPECTED IN 2018 SINCE PEAKING
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective
 PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center
 FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center 1430 Tulane Ave., Box SL-68, New Orleans, LA 70112-2699 J. Bennett Johnston Building, Mezzanine (Floor 1A), Suite A102 (504) 988-6060, fax (504) 988-6077,
FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center 1430 Tulane Ave., Box SL-68, New Orleans, LA 70112-2699 J. Bennett Johnston Building, Mezzanine (Floor 1A), Suite A102 (504) 988-6060, fax (504) 988-6077,
Personalized medecine Biomarker
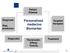 Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017
 SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
THE FUTURE OF IMMUNOTHERAPY IN COLORECTAL CANCER. Prof. Dr. Hans Prenen, MD, PhD Oncology Department University Hospital Antwerp, Belgium
 THE FUTURE OF IMMUNOTHERAPY IN COLORECTAL CANCER Prof. Dr. Hans Prenen, MD, PhD Oncology Department University Hospital Antwerp, Belgium DISCLAIMER Please note: The views expressed within this presentation
THE FUTURE OF IMMUNOTHERAPY IN COLORECTAL CANCER Prof. Dr. Hans Prenen, MD, PhD Oncology Department University Hospital Antwerp, Belgium DISCLAIMER Please note: The views expressed within this presentation
Merck Oncology Overview. The Development of MSI-H Cancer Therapy. Development of Anti-Cancer Drugs Forum Tokyo, Japan, 18, February 2017
 Merck Oncology Overview The Development of MSI-H Cancer Therapy Development of Anti-Cancer Drugs Forum Tokyo, Japan, 18, February 217 Andrew Joe, MD Executive Director, Late Stage Oncology Merck & Co.,
Merck Oncology Overview The Development of MSI-H Cancer Therapy Development of Anti-Cancer Drugs Forum Tokyo, Japan, 18, February 217 Andrew Joe, MD Executive Director, Late Stage Oncology Merck & Co.,
Opdivo. Opdivo (nivolumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.53 Subsection: Antineoplastic nts Original Policy Date: January 16, 2015 Subject: Opdivo Page: 1 of
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.53 Subsection: Antineoplastic nts Original Policy Date: January 16, 2015 Subject: Opdivo Page: 1 of
Promising Bifunctional Agents in Immuno- Oncology: A Roundtable Discussion with the Experts
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Change Log V1.3- v1.4
 Change Log V1.3- v1.4 This document shows the changes that were made to the SSDI manual and the Grade manual for the SEER*RSA version 1.4 release on (Date TBD). SSDI Manual Section: General Instructions
Change Log V1.3- v1.4 This document shows the changes that were made to the SSDI manual and the Grade manual for the SEER*RSA version 1.4 release on (Date TBD). SSDI Manual Section: General Instructions
MERCK ONCOLOGY OVERVIEW ASCO 2018 JUNE 4, 2018
 MERCK ONCOLOGY OVERVIEW ASCO 218 JUNE 4, 218 Forward-Looking Statement of Merck & Co., Inc., Kenilworth, NJ, USA This presentation of Merck & Co., Inc., Kenilworth, N.J., USA (the company ) includes forward
MERCK ONCOLOGY OVERVIEW ASCO 218 JUNE 4, 218 Forward-Looking Statement of Merck & Co., Inc., Kenilworth, NJ, USA This presentation of Merck & Co., Inc., Kenilworth, N.J., USA (the company ) includes forward
VeriStrat Poor Patients Show Encouraging Overall Survival and Progression Free Survival Signal; Confirmatory Phase 2 Study Planned by Year-End
 AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
Cloudbreak. January Cidara Therapeutics
 Cloudbreak January 2019 Cidara Therapeutics 2019 0 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities
Cloudbreak January 2019 Cidara Therapeutics 2019 0 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities
Pioneering vaccines that transform lives.
 Pioneering vaccines that transform lives. Immunomic Therapeutics, Inc. LAMP-Vax for General Oncology Presentation Outline 1. Immunomic Therapeutics: corporate overview 2. Unmet need in cancer immunotherapy
Pioneering vaccines that transform lives. Immunomic Therapeutics, Inc. LAMP-Vax for General Oncology Presentation Outline 1. Immunomic Therapeutics: corporate overview 2. Unmet need in cancer immunotherapy
Keytruda. Keytruda (pembrolizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 9 Last Review Date: September 20, 2018 Keytruda Description Keytruda
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 9 Last Review Date: September 20, 2018 Keytruda Description Keytruda
Approval of pembrolizumab (MSI- H/dMMR) and considerations for site-agnostic development of drugs in oncology
 Approval of pembrolizumab (MSI- H/dMMR) and considerations for site-agnostic development of drugs in oncology Steven Lemery, MD, MHS Associate Director, DOP2 Traditional development paradigm Based on tumor
Approval of pembrolizumab (MSI- H/dMMR) and considerations for site-agnostic development of drugs in oncology Steven Lemery, MD, MHS Associate Director, DOP2 Traditional development paradigm Based on tumor
Corporate Presentation October 2018 Nasdaq: ADXS
 Innovations in Immuno-Oncology Corporate Presentation October 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements
Innovations in Immuno-Oncology Corporate Presentation October 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements
CONSIDERATIONS IN DEVELOPMENT OF PEMBROLIZUMAB IN MSI-H CANCERS
 CONSIDERATIONS IN DEVELOPMENT OF PEMBROLIZUMAB IN MSI-H CANCERS December 2017 Christine K. Gause, Ph.D Executive Director, Biostatistics. 2 Microsatellite Instability-High Cancer - USPI KEYTRUDA is indicated
CONSIDERATIONS IN DEVELOPMENT OF PEMBROLIZUMAB IN MSI-H CANCERS December 2017 Christine K. Gause, Ph.D Executive Director, Biostatistics. 2 Microsatellite Instability-High Cancer - USPI KEYTRUDA is indicated
CANCER VACCINES: TECHNOLOGIES AND GLOBAL MARKETS
 CANCER VACCINES: TECHNOLOGIES AND GLOBAL MARKETS PHM173A January 2015 Shalini Shahani Dewan Project Analyst ISBN: 1-62296-008-4 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA 866-285-7215
CANCER VACCINES: TECHNOLOGIES AND GLOBAL MARKETS PHM173A January 2015 Shalini Shahani Dewan Project Analyst ISBN: 1-62296-008-4 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 02481 USA 866-285-7215
Investor Webcast: Initial Data from Phase 1a/1b Trial of Cabiralizumab/OPDIVO and Early Efficacy Signal in Pancreatic Cancer.
 Investor Webcast: Initial Data from Phase 1a/1b Trial of Cabiralizumab/OPDIVO and Early Efficacy Signal in Pancreatic Cancer November 8, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation
Investor Webcast: Initial Data from Phase 1a/1b Trial of Cabiralizumab/OPDIVO and Early Efficacy Signal in Pancreatic Cancer November 8, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation
Summary of Strategic Competitive Analysis and Publication Planning
 Summary of Strategic Competitive Analysis and Publication Planning Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Briefing
Summary of Strategic Competitive Analysis and Publication Planning Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Briefing
Keytruda. Keytruda (pembrolizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 9 Last Review Date: November 30, 2018 Keytruda Description Keytruda
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 9 Last Review Date: November 30, 2018 Keytruda Description Keytruda
Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies. Eric H. Rubin, MD Merck Research Laboratories
 Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies Eric H. Rubin, MD Merck Research Laboratories Outline Pembrolizumab P001 study - example of multiple expansion
Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies Eric H. Rubin, MD Merck Research Laboratories Outline Pembrolizumab P001 study - example of multiple expansion
Focus on Immunotherapy as a Targeted Therapy. Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct
 Focus on Immunotherapy as a Targeted Therapy Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct 18 2018 Disclosures I have nothing to disclose that is relevant to this presentation. Immunology @ Deeley
Focus on Immunotherapy as a Targeted Therapy Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct 18 2018 Disclosures I have nothing to disclose that is relevant to this presentation. Immunology @ Deeley
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015
 ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
World leader in navigated, non-invasive brain stimulation therapy and diagnosis
 World leader in navigated, non-invasive brain stimulation therapy and diagnosis Martin Jamieson CEO & Chairman of the Board Nexstim Plc Mikko Karvinen CFO Nexstim Plc Corporate Presentation, BioTrinity,
World leader in navigated, non-invasive brain stimulation therapy and diagnosis Martin Jamieson CEO & Chairman of the Board Nexstim Plc Mikko Karvinen CFO Nexstim Plc Corporate Presentation, BioTrinity,
New Developments in Cancer Treatment. Ian Rabinowitz MD
 New Developments in Cancer Treatment Ian Rabinowitz MD Treatment Outline Angiogenesis inhibition Targeted therapy Immunotherapy Personalization of therapy Genomics and cancer Stem cells and cancer Angiogenesis
New Developments in Cancer Treatment Ian Rabinowitz MD Treatment Outline Angiogenesis inhibition Targeted therapy Immunotherapy Personalization of therapy Genomics and cancer Stem cells and cancer Angiogenesis
8 of 21 (38.1%) Achieved RECIST v1.1 Durable Complete Response (CR) in Predicted Anti-PD-1 Non-Responder Melanoma Patients at 24 Weeks
 October 19, 2017 OncoSec Presents Positive Phase 2 Data for ImmunoPulse IL-12 in Combination with Pembrolizumab Demonstrating a Best Overall Response Rate (BORR) of 50% in Predicted Anti-PD-1 Non- Responder
October 19, 2017 OncoSec Presents Positive Phase 2 Data for ImmunoPulse IL-12 in Combination with Pembrolizumab Demonstrating a Best Overall Response Rate (BORR) of 50% in Predicted Anti-PD-1 Non- Responder
9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO
 9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO BERLIN, 13 NOVEMBER, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
9M RESULTS 2014 CONFERENCE CALL DR. MATTHIAS SCHROFF, CEO BERLIN, 13 NOVEMBER, 2014 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments,
Merck Pipeline. As of August 3, 2015
 Merck Pipeline As of August 3, 2015 Merck Pipeline as of August 3, 2015 Phase 2 Phase 2 Phase 3 Phase 3 Phase 3 Alzheimer s Disease MK-7622 Contraception, Next Generation Ring MK-8342B Allergy, House Dust
Merck Pipeline As of August 3, 2015 Merck Pipeline as of August 3, 2015 Phase 2 Phase 2 Phase 3 Phase 3 Phase 3 Alzheimer s Disease MK-7622 Contraception, Next Generation Ring MK-8342B Allergy, House Dust
Experimental Therapeutics I
 Experimental Therapeutics I Mary Hitt 5142 Katz Group Centre mhitt@ualberta.ca; or Mary.Hitt@albertahealthservices.ca 1 Specific Topics for Today Preclinical and clinical testing Gene therapy Nonviral
Experimental Therapeutics I Mary Hitt 5142 Katz Group Centre mhitt@ualberta.ca; or Mary.Hitt@albertahealthservices.ca 1 Specific Topics for Today Preclinical and clinical testing Gene therapy Nonviral
Immunotherapy on the Horizon
 Immunotherapy on the Horizon Andrew L. Coveler Assistant Professor of Medicine, Division of Oncology University of Washington Assistant Member Fred Hutchinson Cancer Research Center Image: NASA.gov 1 2
Immunotherapy on the Horizon Andrew L. Coveler Assistant Professor of Medicine, Division of Oncology University of Washington Assistant Member Fred Hutchinson Cancer Research Center Image: NASA.gov 1 2
The Current Status of Immune Checkpoint Inhibitors: Arvin Yang, MD PhD Oncology Global Clinical Research Bristol-Myers Squibb
 The Current Status of Immune Checkpoint Inhibitors: A Global Overview of the Field Arvin Yang, MD PhD Oncology Global Clinical Research Bristol-Myers Squibb Immune Checkpoint Inhibitors Conference, March
The Current Status of Immune Checkpoint Inhibitors: A Global Overview of the Field Arvin Yang, MD PhD Oncology Global Clinical Research Bristol-Myers Squibb Immune Checkpoint Inhibitors Conference, March
Cancer immunity and immunotherapy. General principles
 1 Cancer immunity and immunotherapy Abul K. Abbas UCSF General principles 2 The immune system recognizes and reacts against cancers The immune response against tumors is often dominated by regulation or
1 Cancer immunity and immunotherapy Abul K. Abbas UCSF General principles 2 The immune system recognizes and reacts against cancers The immune response against tumors is often dominated by regulation or
LAG-3: Validation Of Next Generation Checkpoint Pathways
 LAG-3: Validation Of Next Generation Checkpoint Pathways Frédéric Triebel, CO/CMO Immune Checkpoint Modulation & Combination Therapies April 13, 2016 London, UK. 1 AX:PRR; NADAQ:PBMD Notice: Forward Looking
LAG-3: Validation Of Next Generation Checkpoint Pathways Frédéric Triebel, CO/CMO Immune Checkpoint Modulation & Combination Therapies April 13, 2016 London, UK. 1 AX:PRR; NADAQ:PBMD Notice: Forward Looking
Photocure ASA Executing the Strategy
 Photocure ASA Executing the Strategy DECEMBER 6, 2012 KJETIL HESTDAL, CEO Disclaimer The information included in this Presentation contains certain forward-looking statements that address activities, events
Photocure ASA Executing the Strategy DECEMBER 6, 2012 KJETIL HESTDAL, CEO Disclaimer The information included in this Presentation contains certain forward-looking statements that address activities, events
Bihong Zhao, M.D, Ph.D Department of Pathology
 Bihong Zhao, M.D, Ph.D Department of Pathology 04-28-2009 Is tumor self or non-self? How are tumor antigens generated? What are they? How does immune system respond? Introduction Tumor Antigens/Categories
Bihong Zhao, M.D, Ph.D Department of Pathology 04-28-2009 Is tumor self or non-self? How are tumor antigens generated? What are they? How does immune system respond? Introduction Tumor Antigens/Categories
Immunotherapy in head and neck cancer and MSI in solid tumors
 Immunotherapy in head and neck cancer and MSI in solid tumors Brian Hunis, MD, MBA Associate Medical Director, Memorial Cancer Institute. Hollywood, FL »No disclosures Objectives»Discuss the role of immunology
Immunotherapy in head and neck cancer and MSI in solid tumors Brian Hunis, MD, MBA Associate Medical Director, Memorial Cancer Institute. Hollywood, FL »No disclosures Objectives»Discuss the role of immunology
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/22278 holds various files of this Leiden University dissertation. Author: Cunha Carvalho de Miranda, Noel Filipe da Title: Mismatch repair and MUTYH deficient
Cover Page The handle http://hdl.handle.net/1887/22278 holds various files of this Leiden University dissertation. Author: Cunha Carvalho de Miranda, Noel Filipe da Title: Mismatch repair and MUTYH deficient
Cloudbreak. March Cidara Therapeutics
 Cloudbreak March 2019 Cidara Therapeutics 2019 0 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities
Cloudbreak March 2019 Cidara Therapeutics 2019 0 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities
Bobby W. Sandage, Jr., PhD President & Chief Executive Officer. Lazard Capital Markets 8 th Annual Healthcare Conference
 Bobby W. Sandage, Jr., PhD President & Chief Executive Officer Lazard Capital Markets 8 th Annual Healthcare Conference Statements in this presentation that are not descriptions of historical facts are
Bobby W. Sandage, Jr., PhD President & Chief Executive Officer Lazard Capital Markets 8 th Annual Healthcare Conference Statements in this presentation that are not descriptions of historical facts are
Developing & Commercializing Targeted Small Molecule Drugs in Cancer
 Developing & Commercializing Targeted Small Molecule Drugs in Cancer SAFE HARBOR STATEMENT 2 Forward-looking statements made in the course of this presentation are made pursuant to the safe harbor provisions
Developing & Commercializing Targeted Small Molecule Drugs in Cancer SAFE HARBOR STATEMENT 2 Forward-looking statements made in the course of this presentation are made pursuant to the safe harbor provisions
RNAi in HBV, the next backbone therapy for use in combinations? Bruce D. Given, MD COO, Arrowhead Pharmaceuticals L.I.F.E.R.
 RNAi in HBV, the next backbone therapy for use in combinations? Bruce D. Given, MD COO, Arrowhead Pharmaceuticals L.I.F.E.R. October 20, 2017 Safe Harbor Statement This presentation contains forward-looking
RNAi in HBV, the next backbone therapy for use in combinations? Bruce D. Given, MD COO, Arrowhead Pharmaceuticals L.I.F.E.R. October 20, 2017 Safe Harbor Statement This presentation contains forward-looking
Leerink Immuno-Oncology Roundtable Conference
 Leerink Immuno-Oncology Roundtable Conference September 28, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private
Leerink Immuno-Oncology Roundtable Conference September 28, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private
Dicerna Pharmaceuticals Overview. Delivering RNAi-Based Breakthrough Therapies
 Pharmaceuticals Overview Delivering RNAi-Based Breakthrough Therapies Forward-Looking Statements This information may contain projections and other forward looking statements regarding future events, including
Pharmaceuticals Overview Delivering RNAi-Based Breakthrough Therapies Forward-Looking Statements This information may contain projections and other forward looking statements regarding future events, including
Wells Fargo Healthcare Conference September 6, 2018
 Wells Fargo Healthcare Conference September 6, 2018 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they are
Wells Fargo Healthcare Conference September 6, 2018 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they are
AVEO Oncology Announces Strategic Restructuring. AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET
 NEWS RELEASE FOR IMMEDIATE RELEASE AVEO Oncology Announces Strategic Restructuring AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET CAMBRIDGE, Mass., June 4, 2013 AVEO Oncology (NASDAQ: AVEO)
NEWS RELEASE FOR IMMEDIATE RELEASE AVEO Oncology Announces Strategic Restructuring AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET CAMBRIDGE, Mass., June 4, 2013 AVEO Oncology (NASDAQ: AVEO)
Cancer Immunotherapy Survey
 CHAPTER 8: Cancer Immunotherapy Survey All (N=100) Please classify your organization. Academic lab or center Small biopharmaceutical company Top 20 Pharma Mid-size pharma Diagnostics company Other (please
CHAPTER 8: Cancer Immunotherapy Survey All (N=100) Please classify your organization. Academic lab or center Small biopharmaceutical company Top 20 Pharma Mid-size pharma Diagnostics company Other (please
Keytruda. Keytruda (pembrolizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 7 Last Review Date: December 8, 2017 Keytruda Description Keytruda
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.50 Subject: Keytruda Page: 1 of 7 Last Review Date: December 8, 2017 Keytruda Description Keytruda
Limitations of Use: Imlygic has not been shown to improve overall survival or have an effect on visceral metastases (1).
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.66 Subject: Imlygic Page: 1 of 5 Last Review Date: June 22, 2017 Imlygic Description Imlygic (talimogene
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.66 Subject: Imlygic Page: 1 of 5 Last Review Date: June 22, 2017 Imlygic Description Imlygic (talimogene
Section D. Genes whose Mutation can lead to Initiation
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach
 Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach Oncolytic immunotherapy Oncolytic immunotherapy the use of a genetically modified virus to attack tumors and induce a systemic immune response
Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach Oncolytic immunotherapy Oncolytic immunotherapy the use of a genetically modified virus to attack tumors and induce a systemic immune response
DCVax Novel Personalized Immunotherapies for Solid Tumors
 DCVax Novel Personalized Immunotherapies for Solid Tumors Phaciliate Immunotherapy World Forum January 26, 2016 Disclaimer Certain statements made in this presentation are forward-looking statements of
DCVax Novel Personalized Immunotherapies for Solid Tumors Phaciliate Immunotherapy World Forum January 26, 2016 Disclaimer Certain statements made in this presentation are forward-looking statements of
AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC
 FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
Immuno-Oncology: Perspectives on Current Therapies & Future Developments
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/medical-industry-feature/immuno-oncology-perspectives-currenttherapies-future-developments/9502/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/medical-industry-feature/immuno-oncology-perspectives-currenttherapies-future-developments/9502/
BAVARIAN NORDIC BIO DEUTSCHLAND PRESENTATION OCTOBER 2014 CSE/OMX:BAVA, OTC:BVNRY
 BAVARIAN NORDIC BIO DEUTSCHLAND PRESENTATION OCTOBER 2014 CSE/OMX:BAVA, OTC:BVNRY This presentation includes "forward-looking statements" that involve risks, uncertainties and other factors, many of which
BAVARIAN NORDIC BIO DEUTSCHLAND PRESENTATION OCTOBER 2014 CSE/OMX:BAVA, OTC:BVNRY This presentation includes "forward-looking statements" that involve risks, uncertainties and other factors, many of which
Durable Response Rate in High Grade Glioma: an Emerging Endpoint for Immunotherapeutics. Timothy Cloughesy, MD University of California, Los Angeles
 Durable Response Rate in High Grade Glioma: an Emerging Endpoint for Immunotherapeutics Timothy Cloughesy, MD University of California, Los Angeles Disclosure 2 FDA Endpoints for the Approval of Cancer
Durable Response Rate in High Grade Glioma: an Emerging Endpoint for Immunotherapeutics Timothy Cloughesy, MD University of California, Los Angeles Disclosure 2 FDA Endpoints for the Approval of Cancer
Bayer to Present New Data on Advancing Oncology Portfolio. New Preclinical and Early Clinical Data Evaluating Innovative Compounds in Drug Development
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 105 th Annual Meeting American Association for Cancer Research Bayer to Present
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 105 th Annual Meeting American Association for Cancer Research Bayer to Present
Inovio Pharmaceuticals, Inc. (Exact name of registrant as specified in its charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES. Overview and Mechanism of Action Dr.
 BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
Clinical Policy: Nivolumab (Opdivo) Reference Number: CP.PHAR.121 Effective Date: Last Review Date: Line of Business: Medicaid
 Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07.15 Last Review Date: 01.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Opdivo) Reference Number: CP.PHAR.121 Effective Date: 07.15 Last Review Date: 01.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Summary of Research and Writing Activities in Oncology
 Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
Foreign antigens in human cancers
 Foreign antigens in human cancers Lorenzo Fanchi PhD student, Ton Schumacher Lab ESMO Preceptorship on Immuno-Oncology May 26th, 2017 IMMUNE CHECKPOINT INHIBITION SHOWS CLINICAL BENEFIT IN DIFFERENT TUMOR
Foreign antigens in human cancers Lorenzo Fanchi PhD student, Ton Schumacher Lab ESMO Preceptorship on Immuno-Oncology May 26th, 2017 IMMUNE CHECKPOINT INHIBITION SHOWS CLINICAL BENEFIT IN DIFFERENT TUMOR
Fighting Cancer with Data: Perspectives on MD Anderson Moon Shot Program
 Fighting Cancer with Data: Perspectives on MD Anderson Moon Shot Program Joxel Garcia, MD, MBA Executive Director, Cancer Prevention and Control Platform Member, Moon Shots Program Leadership Team The
Fighting Cancer with Data: Perspectives on MD Anderson Moon Shot Program Joxel Garcia, MD, MBA Executive Director, Cancer Prevention and Control Platform Member, Moon Shots Program Leadership Team The
THE MODERN GYNECOLOGIC EXAMINATION & SCREENING FOR GYNECOLOGIC MALIGNANCIES
 THE MODERN GYNECOLOGIC EXAMINATION & SCREENING FOR GYNECOLOGIC MALIGNANCIES Denise Uyar, MD Associate Professor OB/GYN Chief Gynecologic Oncology Medical College of Wisconsin April 12, 2019 NO DISCLOSURES
THE MODERN GYNECOLOGIC EXAMINATION & SCREENING FOR GYNECOLOGIC MALIGNANCIES Denise Uyar, MD Associate Professor OB/GYN Chief Gynecologic Oncology Medical College of Wisconsin April 12, 2019 NO DISCLOSURES
Immuno-Oncology Clinical Trials Update: Therapeutic Anti-Cancer Vaccines Issue 7 April 2017
 Delivering a Competitive Intelligence Advantage Immuno-Oncology Clinical Trials Update: Therapeutic Anti-Cancer Vaccines Issue 7 April 2017 Immuno-Oncology CLINICAL TRIALS UPDATE The goal of this MONTHLY
Delivering a Competitive Intelligence Advantage Immuno-Oncology Clinical Trials Update: Therapeutic Anti-Cancer Vaccines Issue 7 April 2017 Immuno-Oncology CLINICAL TRIALS UPDATE The goal of this MONTHLY
Clinical Policy: Nivolumab (Opdivo) Reference Number: ERX.SPA.302 Effective Date:
 Clinical Policy: (Opdivo) Reference Number: ERX.SPA.302 Effective Date: 03.01.19 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Opdivo) Reference Number: ERX.SPA.302 Effective Date: 03.01.19 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
MOLOGEN AG Jefferies 2014 London Healthcare Conference
 Jefferies 2014 London Healthcare Conference Dr. Matthias Schroff Chief Executive Officer London 20 November 2014 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring
Jefferies 2014 London Healthcare Conference Dr. Matthias Schroff Chief Executive Officer London 20 November 2014 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring
NeoTYPE Cancer Profiles
 NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
NeoTYPE Cancer Profiles Multimethod Analysis of 25+ Hematologic Diseases and Solid Tumors Anatomic Pathology FISH Molecular The next generation of diagnostic, prognostic, and therapeutic assessment NeoTYPE
C A N C É R O P Ô L E L Y O N A U V E R G N E R H Ô N E - A L P E S
 Cancer Research Cluster to Facilitate and Structure Oncology Research C A N C É R O P Ô L E L Y O N A U V E R G N E R H Ô N E - A L P E S s P E E D I N G U P P R O G R E S S CANCÉROPÔLE LYON AUVERGNE RHÔNE-ALPES
Cancer Research Cluster to Facilitate and Structure Oncology Research C A N C É R O P Ô L E L Y O N A U V E R G N E R H Ô N E - A L P E S s P E E D I N G U P P R O G R E S S CANCÉROPÔLE LYON AUVERGNE RHÔNE-ALPES
Radiation Oncology MOC Study Guide
 Radiation Oncology MOC Study Guide The following study guide is intended to give a general overview of the type of material that will be covered on the Radiation Oncology Maintenance of Certification (MOC)
Radiation Oncology MOC Study Guide The following study guide is intended to give a general overview of the type of material that will be covered on the Radiation Oncology Maintenance of Certification (MOC)
For personal use only
 Cancer killing viruses Oncolytic Virotherapeutics Bryan Dulhunty Managing Director Bell Potter Life Sciences Conference Building a Global Profile May 2102 bryan.dulhunty@viralytics.com www.viralytics.com
Cancer killing viruses Oncolytic Virotherapeutics Bryan Dulhunty Managing Director Bell Potter Life Sciences Conference Building a Global Profile May 2102 bryan.dulhunty@viralytics.com www.viralytics.com
Early Embryonic Development
 Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Merck Announces FDA Approval of KEYTRUDA. Provided to Investors as a Reference
 Merck Announces FDA Approval of KEYTRUDA Provided to Investors as a Reference Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions
Merck Announces FDA Approval of KEYTRUDA Provided to Investors as a Reference Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions
New Generation of T-cell Therapeutics
 New Generation of T-cell Therapeutics Corporate Overview January 2017 NASDAQ: TPIV 1 Safe Harbor Statement Certain statements contained herein are forward-looking statements within the meaning of the safe
New Generation of T-cell Therapeutics Corporate Overview January 2017 NASDAQ: TPIV 1 Safe Harbor Statement Certain statements contained herein are forward-looking statements within the meaning of the safe
BLA /S-048, S-049, S-050, S-051, S-052, S-061, S-062, S-064, S-065, and S-066 SUPPLEMENT APPROVAL
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 BLA 125554/S-048, S-049, S-050, S-051, S-052, S-061, S-062, S-064, S-065, and S-066 SUPPLEMENT APPROVAL Bristol-Myers
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 BLA 125554/S-048, S-049, S-050, S-051, S-052, S-061, S-062, S-064, S-065, and S-066 SUPPLEMENT APPROVAL Bristol-Myers
Determined to realize a future in which people with cancer live longer and better than ever before Q Conference Call
 Reimagining Cancer Treatment Determined to realize a future in which people with cancer live longer and better than ever before Q1 2016 Conference Call 1 Forward-Looking Statements Disclosure This presentation
Reimagining Cancer Treatment Determined to realize a future in which people with cancer live longer and better than ever before Q1 2016 Conference Call 1 Forward-Looking Statements Disclosure This presentation
37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE
 37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE January 2019 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE January 2019 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
REPROS THERAPEUTICS. Dedicated to Treating Male and Female Reproductive Disorders. Corporate Presentation. August 2017
 REPROS THERAPEUTICS Dedicated to Treating Male and Female Reproductive Disorders Corporate Presentation August 2017 Safe Harbor Any statements made by the Company that are not historical facts contained
REPROS THERAPEUTICS Dedicated to Treating Male and Female Reproductive Disorders Corporate Presentation August 2017 Safe Harbor Any statements made by the Company that are not historical facts contained
CELL BIOLOGY - CLUTCH CH CANCER.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF CANCER Cancer is a disease which is primarily caused from misregulated cell division, which form There are two types of tumors - Benign tumors remain confined
!! www.clutchprep.com CONCEPT: OVERVIEW OF CANCER Cancer is a disease which is primarily caused from misregulated cell division, which form There are two types of tumors - Benign tumors remain confined
