Approval of a drug under this criteria document does not ensure full coverage of the drug.
|
|
|
- Kelly Palmer
- 5 years ago
- Views:
Transcription
1 Criteria Document: Reference #: PC/A011 Page 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan (PCHP) PreferredOne Insurance Company (PIC) Individual PreferredOne Insurance Company (PIC) Large Group PreferredOne Insurance Company (PIC) Small Group Coverage is subject to the terms of a member s benefit plan. To the extent there is any inconsistency between this criteria document or policy and the terms of a member s benefit plan, the member s benefit plan governs. Approval of a drug under this criteria document does not ensure full coverage of the drug. PURPOSE: The intent of the criteria document is to: Ensure the intended use is medically necessary; and Allow for both on-label use and National Comprehensive Cancer Network s (NCCN) Drug and Biologics Compendium recommended use; and Require a failed trial of self-administered drugs (unless contraindicated) before a provider administered drug, for prevention and/or treatment of osteoporosis/osteopenia and for continuation /non-emergent use of bisphosphonates for Paget s disease of bone; and To consider overall cost-effectiveness. Table 1: Provider Administered Medications FYI ONLY Drugs Route of Administration Generics Drug Class Generic Name available Boniva intravenous injection N ibandronate bisphosphonate pamidronate intravenous injection N/A pamidronate bisphosphonate subcutaneous injection given by a Prolia healthcare professional N denosumab RANKL inhibitor Reclast intravenous infusion Y zoledronic acid bisphosphonate geva subcutaneous injection given by a healthcare professional N denosumab RANKL inhibitor Zometa intravenous infusion Y zoledronic acid bisphosphonate
2 Criteria Document: Reference #: PC/A011 Page 2 of 8 GUIDELINES: Medical Necessity Criteria Must satisfy one of the following: I-III I. Initial request for denosumab (Prolia), ibandronate injection (Boniva), or zoledronic acid (Reclast or Zometa) for prevention or treatment of osteoporosis - must meet: A and either B or C A. The request is for one of the following medications must have one of: Denosumab (Prolia) - must have one of the following diagnoses: a or b a. On-label use one of the following: i-iii i. Treatment of postmenopausal women with osteoporosis at high-risk for fracture; or ii. Treatment to increase bone mass in men with osteoporosis at high-risk for fracture; or iii. Treatment of glucocorticoid induced osteoporosis (GIOP) in men and women at high-risk for fracture. b. NCCN recommended use in prostate cancer - prevention or treatment of osteoporosis during androgen-deprivation therapy for members with high fracture risk. 2. Ibandronate injectable (Boniva) - On-label use for treatment of osteoporosis in postmenopausal women. 3. Zoledronic acid (Reclast) On-label use - one of the following diagnoses: a-c a. Treatment and prevention of postmenopausal osteoporosis; or b. Treatment to increase bone mass in men with osteoporosis; or c. Treatment and prevention of glucocorticoid-induced osteoporosis. 4. Zoledronic acid (Zometa) - NCCN recommended use in prostate cancer for prevention or treatment of osteoporosis during androgen deprivation therapy for members with high fracture risk. B. The member has tried and failed one self-administered medication(s) (see Table 2) as evidenced by one of the following: 1 or 2 1. A decline in bone mineral density (BMD) of equal to or greater than 5%; or 2. Experienced a fragility (low-trauma) fracture. C. The member has severe renal impairment/chronic kidney disease (CKD) with a creatine clearance less than 35 ml/min.
3 Criteria Document: Reference #: PC/A011 Page 3 of 8 Table 2: Self-Administered Medications* Drugs Route of Administration Generics available FYI ONLY Generic Name Drug Class Actonel oral Y risedronate bisphosphonate Boniva oral Y ibandronate bisphosphonate Fortical, Miacalcin nasal, subcutaneous injection, intramuscular injection Y calcitonin polypeptide hormone selective estrogen Evista oral Y raloxifene receptor modulator recombinant human Forteo subcutaneous injection N teriparatide parathyroid hormone Fosamax oral Y alendronate bisphosphonate recombinant human Tymlos subcutaneous injection N abaloparatide parathyroid hormone * Listing of drugs in table above does not ensure coverage. Please check member s prescription benefit. Revised 11/30/17 II. Initial request for denosumab (Prolia or geva), ibandronate injection (Boniva), pamidronate, or zoledronic acid (Reclast or Zometa) for indications other than prevention or treatment of osteoporosis - must meet one of the following: A-E A. Denosumab (Prolia) - must meet: 1 or 2 1. On-label use - one of the following diagnoses: a or b a. Treatment to increase bone mass in men at high-risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer; or b. Treatment to increase bone mass in women at high-risk for fracture receiving adjuvant aromatase inhibitor therapy for invasive breast cancer. 2. NCCN recommended use in breast cancer, invasive - post-menopausal (natural or induced) members receiving adjuvant endocrine therapy along with calcium and vitamin D supplementation to maintain or improve bone mineral density and reduce risk of fractures. B. Denosumab (geva) must meet: 1 or 2 1. On-label use - must satisfy both of the following: a and b a. Treatment of hypercalcemia of malignancy; and b. Refractory to IV bisphosphonate therapy with pamidronate or Zometa (Table 1). 2. One of the following: a or b a. On-label use one of the following diagnoses: i or ii i. Prevention of skeletal-related events in members with bone metastases from solid tumor or multiple myeloma; or ii. Treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
4 Criteria Document: Reference #: PC/A011 Page 4 of 8 b. NCCN recommended use one of the following diagnoses: i-ix i. Bone cancer, giant cell tumor of the bone - as a single agent or combined with interferon alfa or radiation therapy for localized disease for giant cell tumor of the bone; or ii. Bone cancer, giant cell tumor of the bone - as a single agent for metastatic disease; or iii. Breast cancer, invasive - post-menopausal (natural or induced) members receiving adjuvant endocrine therapy along with calcium and vitamin D supplementation to maintain or improve bone mineral density and reduce risk of fractures; or iv. Breast cancer, invasive - with calcium and vitamin D supplementation in addition to chemotherapy or endocrine therapy, for bone metastases in members with expected survival of equal or greater than 3 months and adequate renal function; or v. Kidney cancer, clear cell and non-clear cell - as a component of best supportive care for bone metastases; or vi. Non-small cell lung cancer (NSCLC) - as a component of best supportive care for bone metastases; or vii. Prostate cancer - prevention of skeletal-related events in men with castration-recurrent prostate cancer who have documented bone metastases and creatinine clearance greater than 30mL/min; or viii. Thyroid carcinoma as palliative care for bone metastases (anaplastic); or ix. Thyroid carcinoma for bone metastases (follicular, Hurthle cell, medullary or papillary). C. Pamidronate - one of the following diagnoses: On-label use for moderate to severe Paget s disease of bone must meet: a or b a. Initial request allow 30mg daily on 3 consecutive days; or b. Continued use request contraindication to oral bisphosphonates - allow continued use for of the following: i or ii i. The member has a pre-existing condition that precludes the use of an oral bisphosphonate (Table 2); or ii. The member has gastroesophageal reflux disease (GERD) symptoms from a trial of an oral bisphosphonate (Table 2). [Note: Allow continued use of pamidronate only when there is a contraindication to oral bisphosphonates] 2. On-label use - one of the following diagnoses: a-c a. Moderate or severe hypercalcemia associated with malignancy, with or without bony metastases; or b. Osteolytic bone metastasis of breast cancer in conjunction with standard antineoplastic therapy; or c. Osteolytic lesions of multiple myeloma in conjunction with standard antineoplastic therapy. 3. NCCN recommended use - one of the following diagnoses: a g a. Breast cancer, invasive postmenopausal (natural or induced) members receiving adjuvant endocrine therapy along with calcium and vitamin D supplementation to maintain or improve bone mineral density and reduce risk of fractures; or b. Breast cancer, invasive with calcium and vitamin D supplementation in addition to chemotherapy or endocrine therapy for members with invasive breast cancer with bone metastases with expected survival of equal or greater than 3 months and adequate renal function; or
5 Criteria Document: Reference #: PC/A011 Page 5 of 8 c. Kidney cancer, clear cell and non-clear cell - as a component of best supportive care for bony metastases; or d. Multiple myeloma - in combination with primary therapy; or e. Non-small cell lung cancer (NSCLC) - supportive therapy in members with bone metastases; or f. Thyroid carcinoma, anaplastic - palliative care for bone metastases; or g. Thyroid carcinoma, follicular, Hurthle cell, medullary or papillary - for bone metastases. D. Zoledronic acid (Reclast) - on-label use - treatment of Paget s disease of bone in men and women: 1 or 2 1. Initial request allow a single 5mg infusion 2. Continued use request contraindication to oral bisphosphonates - allow continued use for of the following: a or b a. The member has a pre-existing condition that precludes the use of an oral bisphosphonate (Table 2); or b. The member has gastroesophageal reflux disease (GERD) symptoms from a trial of an oral bisphosphonate (Table 2). [Note: Allow continued use of zoledronic acid (Reclast) only when there is a contraindication to oral bisphosphonates] E. Zoledronic acid (Zometa) - must meet one of the following: 1 or 2 1. On-label use - must have one of the following diagnoses: a or b a. Hypercalcemia of malignancy; or b. Members with multiple myeloma and members with documented bone metastases from solid tumors, in conjunction with standard antineoplastic therapy. Prostate cancer should have progressed after treatment with at least one hormonal therapy. 2. NCCN recommended use - must have one of the following diagnoses: a-h a. Breast Cancer, invasive postmenopausal (natural or induced) members receiving adjuvant endocrine therapy along with calcium and vitamin D supplementation to maintain or improve bone mineral density and reduce risk of fractures; or b. Breast Cancer, invasive with calcium and vitamin D supplementation in addition to chemotherapy or endocrine therapy for bone metastasis in members with expected survival of equal or greater than 3 months and adequate renal function; or c. Kidney Cancer, clear cell and non-clear cell - as a component of best supportive care for bony metastases; or d. Multiple myeloma in combination with primary therapy; or e. Non-small cell lung cancer (NSCLC) supportive therapy in members with bone metastases; or
6 Criteria Document: Reference #: PC/A011 Page 6 of 8 f. Prostate cancer - prevention of skeletal-related events in men with castration-recurrent prostate cancer with documented bone metastases and creatinine clearance greater than 30mL/min; or g. Thyroid carcinoma, anaplastic as palliative care for bone metastases; or h. Thyroid carcinoma, follicular, Hurthle cell, medullary or papillary for bone metastases. III. Continuation request for denosumab (Prolia or geva), ibandronate injection (Boniva), pamidronate or zoledronic acid (Reclast or Zometa) - Allow an additional 24 months [Note: Continuation request for pamidronate and zoledronic acid (Reclast) for Paget s disease - see above for continued use criteria requirements.] DEFINITIONS: Contraindication/pre-existing condition that precludes the use of an oral bisphosphonate: The member cannot swallow; or The member has abnormalities that delay esophageal emptying (stricture, achalasia); or The member has esophageal lesions or esophageal ulcers; or The member cannot remain in an upright position post oral administration; or The member is status post restrictive bariatric procedure. High-risk for fracture: Low bone mineral density (BMD), advancing age, previous vertebral fracture/fragility fracture/low trauma fracture, glucocorticoid therapy, parental history of hip fracture, low body weight, current cigarette smoking, excessive alcohol consumption, rheumatoid arthritis, secondary osteoporosis (eg, hypogonadism or premature menopause, malabsorption, chronic liver disease, inflammatory bowel disease) Gastroesophageal reflux disease (GERD) symptoms from a trial of oral bisphosphonates: Documentation of patient education regarding taking medication with adequate amount of water while in an upright position, taking at an appropriate interval prior to or after the first food or drink of day, remaining in an upright position for 30-minutes after taking the medication; and Persistent GERD symptoms despite adequate treatment with use of H2-blockers or proton pump inhibitors. T-score: A measurement of bone density compared with what is normally expected in a healthy young adult of the same gender. It is expressed in number of units (standard deviations) that the bone density is above or below the average. T-score What the score means -1 and above Bone density is considered normal A sign of osteopenia, a condition in which bone density is below normal and may lead to Between -1 and -2.5 osteoporosis -2.5 and below Likely have osteoporosis
7 Criteria Document: Reference #: PC/A011 Page 7 of 8 FOR INTERNAL USE ONLY COVERAGE: Prior Authorization: Yes - initial authorize for 24 months (except where otherwise stated); continued use, authorize for 24 months Coverage is subject to the member s contract benefits. CODING: HCPCs J0897 injection, denosumab 1mg J1740 injection, ibandronate sodium, 1 mg J2430 injection, pamidronate disodium, per 30mg J3489 injection, zoledronic acid, 1mg RELATED CRITERIA/POLICIES: Integrated Healthcare Services Process Manual: UR015 Use of Medical Policy and Criteria REFERENCES: 1. Boniva injection [package insert]. South San Francisco, CA: Genentech USA, Inc; Pamidronate [package insert]. Bedford, OH: Bedford Laboratories; Prolia [package insert]. Thousand Oaks, CA: Amgen Inc; Reclast [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; geva [package insert]. Thousand Oaks, CA: Amgen Inc; Zometa [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; Zoledronic acid [package insert]. Schaumberg, IL: Sagent Pharmaceuticals; National Osteoporosis Foundation. Clinician s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation Retrieved from 9. Gnant M, Mlineritsch B, Stoeger, H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABSCG-12 randomised trial. Lancet Oncolo Jul;12(7): Valachis A, Polyzos NP, Coleman RE, Gnant M, et al. Adjuvant therapy with zoledronic acid in patients with breast cancer; a systematic review and meta-analysis. Oncologist 2013;18(4): He M, Fan W, Zhang. Adjuvant zoledronic acid therapy for patients with early stage breast cancer: an updated systematic review and meta-analysis. J Hematol Oncol Oct 23;6(1): NCCN Drugs & Biologics Compendium. Accessed 07/27/ Rosen, HN, Drezner MK. Overview of the management of osteoporosis in postmenopausal women. In UpToDate, Mulder, JE (Ed), UpToDate, Waltham, MA, Lewiecki EM. Osteoporotic fracture risk assessment. In UpToDate, Mulder, JE (Ed), UpToDate, Waltham, MA, DOCUMENT HISTORY: Created Date: 09/30/16 (previously part of PC/B009) Reviewed Date: 07/19/17, 01/18/18 Revised Date: 02/13/17, 03/20/17, 08/16/17, 10/10/17, 11/30/17, 01/18/18,, 06/06/18
8 Attachment A FDA APPROVED INDICATIONS DRUG Tx (*& prevent) PMO in women (^& are at high-risk for fracture) ( reduction in risk of invasive breast cancer in PMO w/osteoporosis; or at high-risk for invasive breast cancer) Tx to increase bone mass in men with (+primary or hypogonadal) osteoporosis (^& are at high risk for fracture) Tx (*& prevent) (# sustained systemic) glucocorticoid -induced osteoporosis (^& are at high risk for fracture) Hypercalcemia of malignancy Patients w/ multiple myeloma & patients with documented bone metastases from solid tumors, in conjunction with standard antineoplastic therapy Tx of Paget s disease of bone in men and women Tx to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer Tx to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer Tx to prevent skeletalrelated events in patients with bone metastases from solid tumors or multiple myeloma Tx of osteolytic bone metastases of breast cancer or osteolytic lesions of multiple myeloma Actonel (risedronate tablets) Boniva tablets (ibandronate) calcitonin * * * Evista (raloxifene tablets) Forteo (teriparatide subq injection) Fosamax (alendronate tablets & oral solution) Tymlos (abaloparatide subq injection) Boniva injection (ibandronate) pamidronate injection * ^ * ^ * +^ *#^ Prolia (denosumab injection) ^ ^ ^ Reclast (zoledronic acid injection) * * geva (denosumab injection) Zometa (zoledronic acid injection) Page 8 of 8 of PC/A011
9 PreferredOne Community Health Plan Nondiscrimination Notice PreferredOne Community Health Plan ( PCHP ) complies with applicable Federal civil rights laws and does not discriminate on the basis of race, color, national origin, age, disability, or sex. PCHP does not exclude people or treat them differently because of race, color, national origin, age, disability, or sex. PCHP: Provides free aids and services to people with disabilities to communicate effectively with us, such as: Qualified sign language interpreters Written information in other formats (large print, audio, accessible electronic formats, other formats) Provides free language services to people whose primary language is not English, such as: Qualified interpreters Information written in other languages If you need these services, contact a Grievance Specialist. If you believe that PCHP has failed to provide these services or discriminated in another way on the basis of race, color, national origin, age, disability, or sex, you can file a grievance with: Grievance Specialist PreferredOne Community Health Plan PO Box Minneapolis, MN Phone: (TTY: ) Fax: customerservice@preferredone.com You can file a grievance in person or by mail, fax, or . If you need help filing a grievance, a Grievance Specialist is available to help you. You can also file a civil rights complaint with the U.S. Department of Health and Human Services, Office for Civil Rights, electronically through the Office for Civil Rights Complaint Portal, available at or by mail or phone at: U.S. Department of Health and Human Services 200 Independence Avenue, SW Room 509F, HHH Building Washington, D.C , (TDD) Complaint forms are available at Language Assistance Services NDR PCHP LV (10/16)
10 PreferredOne Insurance Company Nondiscrimination Notice PreferredOne Insurance Company ( PIC ) complies with applicable Federal civil rights laws and does not discriminate on the basis of race, color, national origin, age, disability, or sex. PIC does not exclude people or treat them differently because of race, color, national origin, age, disability, or sex. PIC: Provides free aids and services to people with disabilities to communicate effectively with us, such as: Qualified sign language interpreters Written information in other formats (large print, audio, accessible electronic formats, other formats) Provides free language services to people whose primary language is not English, such as: Qualified interpreters Information written in other languages If you need these services, contact a Grievance Specialist. If you believe that PIC has failed to provide these services or discriminated in another way on the basis of race, color, national origin, age, disability, or sex, you can file a grievance with: Grievance Specialist PreferredOne Insurance Company PO Box Minneapolis, MN Phone: (TTY: ) Fax: customerservice@preferredone.com You can file a grievance in person or by mail, fax, or . If you need help filing a grievance, a Grievance Specialist is available to help you. You can also file a civil rights complaint with the U.S. Department of Health and Human Services, Office for Civil Rights, electronically through the Office for Civil Rights Complaint Portal, available at or by mail or phone at: U.S. Department of Health and Human Services 200 Independence Avenue, SW Room 509F, HHH Building Washington, D.C , (TDD) Complaint forms are available at Language Assistance Services NDR PIC LV (10/16)
FYI ONLY Generic Name. Generics available. zoledronic acid N/A
 Criteria Document: Reference #: PC/A011 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Criteria Document: Reference #: PC/A011 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents
 AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For
AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For
Department of Origin: Integrated Healthcare Services. Approved by: Chief Medical Officer Department(s) Affected: Date approved: 01/10/17
 Reference #: MP/D005 Page: 1 of 3 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MP/D005 Page: 1 of 3 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
TYMLOS (abaloparatide)
 TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other PROLIA, XGEVA 37012 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All requests
Generic Brand HICL GCN Exception/Other PROLIA, XGEVA 37012 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All requests
BONIVA (ibandronate sodium)
 BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
01/10/17. Replaces Effective Policy Dated: Amino Acid Based Elemental Formula (AABF) 09/28/15 Reference #: MP/A003 Page: 1 of 3
 Reference #: MP/A003 Page: 1 of 3 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MP/A003 Page: 1 of 3 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
PROLIA: Medical Coverage Policy Denosumab (Prolia and. Xgeva) EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Prior Authorization Required: Yes as shown below
 PROLIA, XGEVA (denosumab) MB9409 Covered Service: Prior Authorization Required: Additional Information Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes as shown below Must be
PROLIA, XGEVA (denosumab) MB9409 Covered Service: Prior Authorization Required: Additional Information Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes as shown below Must be
01/26/17. Replaces Effective Policy Dated: Autism Spectrum Disorders in Children: Assessment 01/19/16 and Evaluation Reference #: MP/A005 Page 1 of 4
 Reference #: MP/A005 Page 1 of 4 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MP/A005 Page 1 of 4 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
06/13/17. A. Completed a comprehensive diabetes education program within the past two years; and
 Reference #: MC/L011 Page 1 of 4 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA
Reference #: MC/L011 Page 1 of 4 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
03/13/18. A. Symptoms lasting for greater than or equal to 12 months that have resulted to significant impairment in activities of daily living; and
 Reference #: MC/I008 Page: 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/I008 Page: 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
PURPOSE: The intent of this policy is to provide guidelines for coverage of dental procedures under the medical benefit.
 Integrated Reference #: MP/D009 Page 1 of 4 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc.
Integrated Reference #: MP/D009 Page 1 of 4 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc.
Osteoporosis Treatment Overview. Colton Larson RFUMS October 26, 2018
 Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
09/12/17. I. Electrical Bone Growth Stimulator (invasive, semi-invasive, or non-invasive) any of the following: A-C
 Reference #: MC/F021 Page: 1 of 4 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/F021 Page: 1 of 4 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
Approved by: Integrated Health Quality Management Subcommittee Effective Date: Department of Origin: Integrated Healthcare Services.
 Reference #: MC/M020 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/M020 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Pharmacy Management Drug Policy
 Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Clinical Policy: Denosumab (Prolia, Xgeva) Reference Number: CP.PHAR.58
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only.
 This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: denosumab_prolia_xgeva 3/2011 9/2017 9/2018 9/2017 Description of Procedure or Service Receptor activator
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: denosumab_prolia_xgeva 3/2011 9/2017 9/2018 9/2017 Description of Procedure or Service Receptor activator
03/14/17. II. Initial early intensive-level behavioral and developmental therapy must have both of the following: A and B
 Reference #: MC/M024 Page 1 of 6 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/M024 Page 1 of 6 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
John J. Wolf, DO Family Medicine
 John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
DENOSUMAB (PROLIA & XGEVA )
 DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
Xgeva. Xgeva (denosumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
Parathyroid Hormone Analogs
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Reclast, Zometa) Reference Number: CP.PHAR.59 Effective Date: 03.11 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Reclast, Zometa) Reference Number: CP.PHAR.59 Effective Date: 03.11 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Xgeva. Xgeva (denosumab) Description. Section: Prescription Drugs Effective Date: January 1, 2016
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.18 Subject: Xgeva Page: 1 of 5 Last Review Date: December 3, 2015 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.18 Subject: Xgeva Page: 1 of 5 Last Review Date: December 3, 2015 Xgeva Description Xgeva (denosumab)
[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.
![[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4. [If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.](/thumbs/86/94943423.jpg) Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Date approved: 04/18/18. Approved by: Pharmacy and Therapeutics Quality Management Subcommittee Effective Date: Department of Origin: Pharmacy
 Integrated Healthcare Services and Criteria Document: Reference #: PC/V001 Page: 1 of 9 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services,
Integrated Healthcare Services and Criteria Document: Reference #: PC/V001 Page: 1 of 9 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services,
Tymlos (abaloparatide)
 Tymlos (abaloparatide) Policy Number: 5.01.638 Last Review: 11/2018 Origination: 10/2017 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Tymlos
Tymlos (abaloparatide) Policy Number: 5.01.638 Last Review: 11/2018 Origination: 10/2017 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Tymlos
3. Has bone specific alkaline phosphatase level increased OR does the member have symptoms related to active Paget s?
 Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
4.7 Studies of Quality Holy Cross Hospital Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017
 4.7 Studies of Quality Holy Cross Hospital 2017 Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017 Bone Health in Stage I ER/PR Positive Breast Cancer Patients To review
4.7 Studies of Quality Holy Cross Hospital 2017 Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017 Bone Health in Stage I ER/PR Positive Breast Cancer Patients To review
Medication Policy Manual. Topic: Prolia, denosumab Date of Origin: August 11, 2010
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
1
 www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
RADIOFREQUENCY ABLATION OF MISCELLANEOUS SOLID TUMORS EXCLUDING LIVER TUMORS
 EXCLUDING LIVER TUMORS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
EXCLUDING LIVER TUMORS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Clinical Policy: Abaloparatide (Tymlos) Reference Number: CP.CPA.306 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: (Tymlos) Reference Number: CP.CPA.306 Effective Date: 06.13 Last Review Date: 08.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Tymlos) Reference Number: CP.CPA.306 Effective Date: 06.13 Last Review Date: 08.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
Subject: Denosumab (Prolia ; Xgeva ) Injection
 09-J1000-25 Original Effective Date: 08/15/10 Reviewed: 03/14/18 Revised: 12/15/18 Next Review: 01/09/19 Subject: Denosumab (Prolia ; Xgeva ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-J1000-25 Original Effective Date: 08/15/10 Reviewed: 03/14/18 Revised: 12/15/18 Next Review: 01/09/19 Subject: Denosumab (Prolia ; Xgeva ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
The Bare Bones of Osteoporosis. Wendy Rosenthal, PharmD
 The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
The Bare Bones of Osteoporosis Wendy Rosenthal, PharmD Definition A systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection
 Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection Table of Contents Policy: Commercial Policy History References Coding Information Information Pertaining to All Policies
Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection Table of Contents Policy: Commercial Policy History References Coding Information Information Pertaining to All Policies
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 10/04/17 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 BAVENCIO (avelumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
BAVENCIO (avelumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Prolia /Xgeva (denosumab) Document Number: IC-0098
 / (denosumab) Document Number: IC-0098 Last Review Date: 5/30/2017 Date of Origin: 11/28/2011 Dates Reviewed: 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 03/2014, 06/2014, 09/2014, 12/2014,
/ (denosumab) Document Number: IC-0098 Last Review Date: 5/30/2017 Date of Origin: 11/28/2011 Dates Reviewed: 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 03/2014, 06/2014, 09/2014, 12/2014,
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital
 Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Osteoporosis Management
 Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Forteo (teriparatide) Prior Authorization Program Summary
 Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Name of Policy: Boniva (Ibandronate Sodium) Infusion
 Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab 120mg for Bone Metastases
 ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
The Osteoporosis Center at St. Luke s Hospital
 The Osteoporosis Center at St. Luke s Hospital Desloge Outpatient Center (on the west side of 141) 121 St. Luke s Center Drive, Suite 504 Chesterfield, MO 63017 Phone 314 205-6633 Fax 314 590-5909 NEW
The Osteoporosis Center at St. Luke s Hospital Desloge Outpatient Center (on the west side of 141) 121 St. Luke s Center Drive, Suite 504 Chesterfield, MO 63017 Phone 314 205-6633 Fax 314 590-5909 NEW
OSTEOPOROSIS MEDICINES
 Bone Basics 2010. NOF. All rights reserved. National Osteoporosis Foundation 1150 17th Street, NW, Suite 850 Washington, DC 20036 (800) 223-9994 www.nof.org OSTEOPOROSIS MEDICINES Although there is no
Bone Basics 2010. NOF. All rights reserved. National Osteoporosis Foundation 1150 17th Street, NW, Suite 850 Washington, DC 20036 (800) 223-9994 www.nof.org OSTEOPOROSIS MEDICINES Although there is no
Osteoporosis. Treatment of a Silently Developing Disease
 Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary
 Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Update on Osteoporosis 2016
 WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
denosumab (Prolia ) Policy # Original Effective Date: 07/21/2011 Current Effective Date: 04/19/2017
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Based on review of available data, the Company may consider the use of denosumab (Prolia) for the
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Breast Cancer and Bone Loss. One in seven women will develop breast cancer during a lifetime
 Breast Cancer and Bone Loss One in seven women will develop breast cancer during a lifetime Causes of Bone Loss in Breast Cancer Patients Aromatase inhibitors Bil Oophorectomy Hypogonadism Steroids Chemotherapy
Breast Cancer and Bone Loss One in seven women will develop breast cancer during a lifetime Causes of Bone Loss in Breast Cancer Patients Aromatase inhibitors Bil Oophorectomy Hypogonadism Steroids Chemotherapy
HOW I DO IT. Introduction. BARKIN J. How I Do It: Managing bone health in patients with prostate cancer. Can J Urol 2014;21(4):
 HOW I DO IT How I Do It: Managing bone health in patients with prostate cancer Jack Barkin, MD Department of Surgery, University of Toronto, Humber River Hospital, Toronto, Ontario, Canada BARKIN J. How
HOW I DO IT How I Do It: Managing bone health in patients with prostate cancer Jack Barkin, MD Department of Surgery, University of Toronto, Humber River Hospital, Toronto, Ontario, Canada BARKIN J. How
BREAST CANCER AND BONE HEALTH
 BREAST CANCER AND BONE HEALTH Rowena Ridout, MD, FRCPC Toronto Western Hospital Osteoporosis Program University Health Network / Mount Sinai Hospital rowena.ridout@uhn.ca None to declare Conflicts of Interest
BREAST CANCER AND BONE HEALTH Rowena Ridout, MD, FRCPC Toronto Western Hospital Osteoporosis Program University Health Network / Mount Sinai Hospital rowena.ridout@uhn.ca None to declare Conflicts of Interest
Dumfries and Galloway. Treatment Protocol for Osteoporosis
 Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Kristen M. Nebel, DO PENN/ LGHP Geriatrics. Temple Family Medicine Review
 Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
Medication Policy Manual. Topic: Prolia, Xgeva, denosumab Date of Origin: August 11, 2010
 Medication Policy Manual Policy No: dru223 Topic: Prolia, Xgeva, denosumab Date of Origin: August 11, 2010 Committee Approval Date: June 20, 2014 Next Review Date: June 2015 Effective Date: July 1, 2014
Medication Policy Manual Policy No: dru223 Topic: Prolia, Xgeva, denosumab Date of Origin: August 11, 2010 Committee Approval Date: June 20, 2014 Next Review Date: June 2015 Effective Date: July 1, 2014
PERCUTANEOUS BALLOON KYPHOPLASTY, RADIOFREQUENCY KYPHOPLASTY, AND MECHANICAL VERTEBRAL AUGMENTATION
 KYPHOPLASTY, AND MECHANICAL VERTEBRAL AUGMENTATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures,
KYPHOPLASTY, AND MECHANICAL VERTEBRAL AUGMENTATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures,
Calendar Year Deductible Annual Benefit Maximum. ADA Code Covered Services Member pays. n/a Office visit $5 per visit
 Blue Shield of California Dental HMO Plan Dental HMO Basic Benefit summary Effective January 1, 2018 THIS MATRIX IS INTENDED TO BE USED TO HELP YOU COMPARE COVERAGE BENEFITS AND IS A SUMMARY ONLY. THE
Blue Shield of California Dental HMO Plan Dental HMO Basic Benefit summary Effective January 1, 2018 THIS MATRIX IS INTENDED TO BE USED TO HELP YOU COMPARE COVERAGE BENEFITS AND IS A SUMMARY ONLY. THE
Calendar Year Deductible Annual Benefit Maximum. ADA Code Covered Services Member pays
 An independent member of the Blue Shield Association A50861-SG (1/19) Dental HMO Plan Dental HMO Standard Benefit summary Effective January 1, 2019 THIS MATRIX IS INTENDED TO BE USED TO HELP YOU COMPARE
An independent member of the Blue Shield Association A50861-SG (1/19) Dental HMO Plan Dental HMO Standard Benefit summary Effective January 1, 2019 THIS MATRIX IS INTENDED TO BE USED TO HELP YOU COMPARE
Osteoporosis and Lupus. Andrew Ruthberg, MD University Rheumatologists
 Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
GENETIC TESTING FOR TAMOXIFEN TREATMENT
 GENETIC TESTING FOR TAMOXIFEN TREATMENT Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical
GENETIC TESTING FOR TAMOXIFEN TREATMENT Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical
Clinical Practice. Presented by: Internist, Endocrinologist
 Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
PARSABIV (etelcalcetide)
 PARSABIV (etelcalcetide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
PARSABIV (etelcalcetide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
B. To assess an individual when clinical evaluation suggests use of non-prescribed medications or illegal substances; or
 Integrated Reference #: MP/D010 Page: 1 of 7 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services,
Integrated Reference #: MP/D010 Page: 1 of 7 PRODUCT APPLICATION: PreferredOne Community Health Plan (PCHP) PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services,
Bisphosphonates. Making intelligent drug choices
 Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Osteoporosis. Overview
 v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
Beyond the Break. After Breast Cancer: Osteoporosis in Survivorship. Dr Alexandra Ginty CCFP(EM) FCFP Regional Primary Care Lead CCO
 Beyond the Break After Breast Cancer: Osteoporosis in Survivorship Dr Alexandra Ginty CCFP(EM) FCFP Regional Primary Care Lead CCO Disclosures No disclosures Osteoporosis in Breast Cancer Survivorship
Beyond the Break After Breast Cancer: Osteoporosis in Survivorship Dr Alexandra Ginty CCFP(EM) FCFP Regional Primary Care Lead CCO Disclosures No disclosures Osteoporosis in Breast Cancer Survivorship
SOUTH TEXAS FRACTURE PREVENTION CLINIC PRE-DEXA PATIENT QUESTIONNAIRE
 OFFICE USE ONLY SOUTH TEXAS FRACTURE PREVENTION CLINIC PRE-DEXA PATIENT QUESTIONNAIRE PLACE STICKER HERE Name: Date of Birth: Male Female Primary Care Physician Name: Phone Number: Referring Physician
OFFICE USE ONLY SOUTH TEXAS FRACTURE PREVENTION CLINIC PRE-DEXA PATIENT QUESTIONNAIRE PLACE STICKER HERE Name: Date of Birth: Male Female Primary Care Physician Name: Phone Number: Referring Physician
12/12/17. I. Liver transplantation for children and adults (initial or retransplantation) - must satisfy the following: A and B
 Reference #: MC/T004 Page: 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/T004 Page: 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
MULTIMARKER SERUM TESTING RELATED TO OVARIAN CANCER
 MULTIMARKER SERUM TESTING RELATED TO OVARIAN CANCER Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures,
MULTIMARKER SERUM TESTING RELATED TO OVARIAN CANCER Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures,
STELARA (ustekinumab)
 STELARA (ustekinumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
STELARA (ustekinumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
INTRACAVITARY BALLOON BRACHYTHERAPY FOR MALIGNANT AND METASTATIC BRAIN TUMORS
 METASTATIC BRAIN TUMORS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
METASTATIC BRAIN TUMORS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
ENTYVIO (vedolizumab)
 ENTYVIO (vedolizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
ENTYVIO (vedolizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
PERJETA (pertuzumab) FOR TREATMENT OF MALIGNANCIES
 PERJETA (pertuzumab) FOR TREATMENT OF MALIGNANCIES Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures,
PERJETA (pertuzumab) FOR TREATMENT OF MALIGNANCIES Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures,
Medical Review. The following slides were medically reviewed by Dr. Nancy Dawson in June 2018.
 Bone Health Medical Review The following slides were medically reviewed by Dr. Nancy Dawson in June 2018. Presentation Overview 1. What is bone health? 2. How can cancer and cancer treatments affect your
Bone Health Medical Review The following slides were medically reviewed by Dr. Nancy Dawson in June 2018. Presentation Overview 1. What is bone health? 2. How can cancer and cancer treatments affect your
12/13/16. I. Liver transplantation for children and adults (initial or retransplantation) - must satisfy the following: A and B
 Reference #: MC/T004 Page: 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/T004 Page: 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss. A Publication of The Bone and Cancer Foundation
 Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
Medication Prior Authorization Form
 Reclast (Zoledronic Acid) Policy Number: 1050 Policy History Approve Date: 12/11/2015 Revise Dates: 8/22/2016 Next Review: 12/11/2016 Review Dates: Preauthorization All Plans Benefit plans vary in coverage
Reclast (Zoledronic Acid) Policy Number: 1050 Policy History Approve Date: 12/11/2015 Revise Dates: 8/22/2016 Next Review: 12/11/2016 Review Dates: Preauthorization All Plans Benefit plans vary in coverage
Osteoporosis. Definition
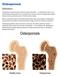 Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Bone Densitometry Pathway
 Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
Medical Policy. MP Specialty Drugs. Related Policies Guidelines for Prior Authorization of Pharmacologic Therapies
 Medical Policy Last Review: 04/30/2018 Effective Date: 07/01/2018 Section: Prescription Drug Related Policies 5.01.501 Guidelines for Prior Authorization of Pharmacologic Therapies DISCLAIMER Our medical
Medical Policy Last Review: 04/30/2018 Effective Date: 07/01/2018 Section: Prescription Drug Related Policies 5.01.501 Guidelines for Prior Authorization of Pharmacologic Therapies DISCLAIMER Our medical
RADIOFREQUENCY ABLATION OF PRIMARY OR METASTATIC LIVER TUMORS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
