|
|
|
- Suzanna Morris
- 5 years ago
- Views:
Transcription
1 1) Study title: Long-term Incidence of Venous Thromboembolism among Survivors of Childhood Cancer 2) Working group and investigators: The study of the long-term incidence of venous thromboembolism will be performed with the assistance of the Childhood Cancer Survivor Study (CCSS) Chronic Disease Working Group. Secondary oversight will be provided by the CCSS Epidemiology/Biostatistics Working Group. Roster: Name Contact information Arin Madenci Greg Armstrong Lisa Diller Todd Gibson Rebecca Howell Wendy Leisenring Larissa Nekhlyudov Kevin Oeffinger Christopher Tinkle Brent Weil Yutaka Yasui Christopher Weldon 3) Background and rationale: In the first five years after diagnosis, children with cancer may have up to 30-fold increased incidence of venous thromboembolism (VTE), compared to the general pediatric population. 1 The incidence of VTE ranges from events per 100,000 person-years in the general pediatric population and 152 events per 100,000 person-years among children with cancer. 1 4 Furthermore, the overall incidence of VTE has increased by 70-88% among all pediatric inpatients over the past decade. 5,6 This finding may reflect improvements in diagnostic tests or increasing exposure to risk factors for VTE, such as central venous catheters, 5 chemotherapy treatments, 7 and surgery. 4 Survivors of childhood cancer are often exposed to multiple risk factors, as a result of the underlying disease and manifold treatment modalities. Furthermore, the risk of VTE is elevated in the presence of an increasing number of medical risk factors, compared to a single medical risk factor. 8 However, long-term risk of VTE among childhood cancer survivors (CCS) has never been defined. Several aspects of multimodal cancer treatment may contribute to elevated VTE risk. From the adult literature, there is evidence that patients with breast cancer who are treated with chemotherapy possess an elevated risk of VTE, compared to those treated with placebo or hormonal therapy alone. 9 The mechanism by which chemotherapy contributes to a hypercoagulable state is posited to be related to endothelial cell dysfunction 10 or inflammatory mediators. 11 Among children with hematologic malignancies, treatments such as L-asparaginase and corticosteroids may contribute to thrombosis. 2 Inflammation and immobility from lower extremity lymphedema contribute to the development of deep venous thrombosis (DVT). 12 A recent series by Palmer and colleagues reported a 32% occurrence rate of lymphedema after inguinal dissection for children with melanoma. 13 An association between radiotherapy and lymphedema has also been reported in the breast and gynecologic cancer literature, with a synergistic effect on lymphedema among patients who undergo both surgery and 1
2 radiotherapy. 14,15 Similarly, functional limitations from surgery or chemotherapy-induced neuropathy may contribute to immobility and thereby predispose to VTE. Among a general population of children diagnosed with VTE, many underwent a prior surgical procedure. 3 The long-term effects on VTE of surgery and other treatments are unknown. Demographic and disease-related factors also may affect VTE risk. Older age was found to be associated with higher risk of VTE in several large database studies of children who develop VTE. 5 7 The prevalence of VTE varies with underlying malignancy type, although the relationship may be confounded by differential use of cancer treatments. In a 2011 study of the Pediatric Hospital Information System Database, O Brien and colleagues demonstrated an increased risk of VTE among adolescents and young adults with leukemia and sarcoma, compared with those with brain tumors. 7 The most common sites for VTE are the veins of the lower extremities (e.g. iliofemoral, popliteal), veins of the upper extremities (e.g. subclavian, axillary, brachial), and intra-abdominal central veins (e.g. inferior vena cava, portal vein, splenic vein). 6,16 In examining the long-term risk of VTE among childhood cancer survivors, several factors may pre-dispose to VTE years after cancer diagnosis. For example, oral contraceptive use (especially in the presence of ongoing tobacco use) confers an increased risk of VTE in the general population. 17 A recent meta-analysis by Meng and colleagues reported a 1.4% rate of VTE during pregnancy and the puerperium. 18 Stein and colleagues in analysis from the National Hospital Discharge Survey note that pregnancy among girls age was associated with double the incidence rate of VTE. 19 It is unknown if the rates of VTE with hormonal alterations are higher among CCS, compared to the already elevated risk of VTE with hormonal alterations in the general population. Finally, the development of second malignant neoplasms may be associated with increased VTE risk, although a recent trial reports a low overall occult cancer rate among the general adult population with newly diagnosed DVT. 20 These factors may be associated with a particularly elevated risk of VTE among CCS compared to the general adult population, which would be useful knowledge to guide counseling, screening, and prevention. Finally, the clinical significance of VTE in CCS is unknown. Among adults with cancer, VTE is the second leading cause of death. 21 The in-hospital mortality rate for children treated for VTE is approximately 9% and significantly higher than that of children admitted for reasons other than VTE, although a causal relationship between VTE and mortality has not been convincingly demonstrated. 6,22 Children with VTE may be at increased risk of mortality from sequelae such as pulmonary embolism or, alternatively, VTE may be a harbinger of severe disease rather than a mechanism of mortality. The proposed study will characterize the incidence, risk factors, and consequences of self-reported, late ( 5 years from diagnosis) VTE among survivors of childhood cancer. 4) Specific aims: a) Specific aim 1 To describe the cumulative incidence and incidence rate of late ( 5 years post-diagnosis) venous thromboembolism (VTE; i.e. deep vein thrombosis or pulmonary embolism) among childhood cancer survivors compared to siblings. Hypothesis: There is a higher cumulative incidence and incidence rate of long-term VTE among survivors compared to sibling controls. b) Specific aim 2 2
3 To identify factors associated with late ( 5 years post-diagnosis) VTE (i.e. deep vein thrombosis or pulmonary embolism) among childhood cancer survivors. Hypothesis: There is a higher incidence rate of late VTE among survivors who have specific demographic risk factors, cancer treatments (i.e. chemotherapy, radiotherapy, surgery including extremity surgery), and other long-term health measures (i.e. severe/disabling chronic health conditions defined as Common Terminology Criteria for Adverse Events [CTCAE] grade 3-4, joint replacement surgery, hospitalizations, second malignant neoplasm [SMN], lower physical activity status, history of pregnancy, oral contraceptive/estrogen replacement) compared to survivors without such factors. c) Specific aim 3 To define the incidence of late ( 5 years post-diagnosis) mortality among survivors who developed VTE, compared to those who did not develop VTE. Note: will separately assess: 1) VTE-specific mortality [CTCAE grade 5] and 2) mortality not related to SMN, progression, or external mortality. Hypothesis: There is a higher incidence rate of late mortality among survivors who developed VTE, compared to those who did not. 5) Analysis framework: a) Outcomes of interest The primary outcome is occurrence of late VTE ( 5 years post-diagnosis), defined as pulmonary embolism or deep vein thrombosis. This will be ascertained using the CTCAE grading system, including affirmative response to #F10 on the expansion baseline survey ( Blood clot in head, lung, arm, leg, or pelvis ), #G10 on 2007 follow-up survey ( Blood clot in head, lung, arm, leg, or pelvis ), #11M on FU2000, or F16 on the original baseline survey or as hospitalization for VTE based on #21 on 2000 follow-up survey ( Have you been admitted to a hospital? ). Survivors who documented an affirmative response to stroke (baseline #F9, baseline expansion #J14, FU2000 #10g, FU2007 #K14) during the same year as the VTE event will not be considered to have had VTE. The secondary outcome will be late mortality, defined in three ways: 1) as VTE-specific mortality [CTCAE grade 5], 2) mortality not related to SMN, progression/recurrence, or external mortality, and 3) all-cause mortality. Time of occurrence (based on age at occurrence) will be incorporated into the analysis for each outcome. b) Subject population We will include all childhood cancer survivors in the CCSS cohort (diagnosed ) who participated in the baseline survey. As comparators, we will include all siblings in the CCSS cohort. We will also compare long-term mortality (see above) among participants who developed VTE, compared to those who did not develop VTE. c) Exploratory variables Demographic and social variables o Age (continuous and categorical; Baseline #A1; ExpBaseline #A1) o Sex (categorical; Baseline #A2; ExpBaseline #A2) o Race and ethnicity (categorical: non-hispanic white, non-hispanic black, Hispanic, other; Baseline #A4, ExpBaseline #A5) 3
4 Chronic conditions o Common Terminology Criteria for Adverse Events (CTCAE) 23 No condition Grade 1 condition (mild) Grade 2 condition (moderate) Grade 3 condition (severe) Grade 4 condition (life-threatening or disabling) Grade 5 condition (fatal; secondary outcome: fatal VTE) o Tobacco use (categorical: never smoker, former smoker, current smoker; Baseline #N1-2, ExpBaseline #O1-8, LTFU 2003 #L1-8, LTFU 2007 #N7-14) o Physical activity (binary: active vs. inactive; Baseline #N9, ExpBaseline #O15, LTFU 2003 #D1-7, LTFU 2007 #N15-21) Active defined per Centers for Disease Control and Prevention guidelines as: 150 minutes/week of moderate intensity physical activity or 75 minutes/week of vigorous activity per week 24 o Body mass index (BMI; continuous and categorical: <18.5, , , , , 40; Baseline #A10-11, ExpBaseline #A3-4, LTFU 2003 #7-8, LTFU 2007 #A1-2) Calculated as BMI = (weight [kg]) / (height [m]) 2 o Hospitalizations (binary and ordinal: 0, 1, 2, >2 times; LTFU 2000 #21A- C, LTFU 2005 #A1-2) o Reproductive or endocrine variables Pregnancy (binary; Baseline #M9-11, ExpBaseline #N6, LTFU 2000 #19C, LTFU 2003 #N1, LTFU 2007 #F15, Q1) Parity (categorical; Baseline #M10, ExpBaseline #N7, LTFU 2003 #N1-3, LTFU 2007 #Q1-5) Use of oral contraceptive pills or hormone therapy including fertility treatment (binary; Baseline #2-3, ExpBaseline #B8, LTFU 2000 #6C, 19C, LTFU 2007 #C8, F15) o Cancer variables Second malignant neoplasm (binary; ExpBaseline #L1, LTFU 2003 #R1, LTFU 2005 #B1, LTFU 2007 #P1) Treatment variables (within 5 years of cancer diagnosis) o Any surgery (binary; MRAF for any surgery and LTFU 2007 #J1-37 for the below surgeries) Amputation (categorical; Baseline #I1, ExpBaseline #I1, LTFU 2007 #J1) Major joint replacement (categorical; Baseline #I5, ExpBaseline #I5, LTFU 2007 #J5) Limb-sparing procedure (categorical; Baseline #I4, #I6, ExpBaseline #I4, I6, LTFU 2007 #J4, J6) Will review Baseline/ExpBaseline #I6 and LTFU 2007 #J6 procedures to determine if applicable Splenectomy (categorical; ExpBaseline #I18, LTFU 2007 #J18) Nephrectomy (categorical; ExpBaseline #I37, LTFU 2007 #J37) 4
5 Thrombectomy (categorical; Baseline #I14) o Any chemotherapy (binary) Alkylating agent (binary) Cyclophosphamide equivalent dose (CED) score (categorical: 0, , , 8000mg/m 2 ) 25 Anthracycline (binary) Anthracycline score (categorical: 0, <250, 250 mg/m 2 ) 26 Platinum agent (binary) Platinum agent score (categorical: 0, 1, 2, 3) 27 o Any radiotherapy Total dose (categorical: 0,,, 20-29, 30-39, 40-49, >49 Gy) Body region dosimetry (continuous): Brain (categorical: 0,,, 20-29, 30-39, 40-49, >49 Gycontinous, average dose: 0, >0-1, 2-5, 6-10 Gy) Chest (categorical: 0,,, 20-29, 30-39, 40-49, >49 Gy continous, average dose: 0, >0-1, 2-5, 6-10 Gy) Abdomen/pelvis (categorical: 0,,, 20-29, 30-39, 40-49, >49 Gycontinous, average dose: 0, >0-1, 2-5, 6-10 Gy) Arms (categorical: 0,,, 20-29, 30-39, 40-49, >49 Gy continous, average dose: 0, >0-1, 2-5, 6-10 Gy) Legs (categorical: 0,,, 20-29, 30-39, 40-49, >49 Gycontinous, average dose: 0, >0-1, 2-5, 6-10 Gy) Medication variables o Hormone replacement therapy (see above) o Estrogens / Progesterones (see above) o Anticoagulants (categorical; including vitamin K antagonists, heparin medications, thrombin inhibitors; Baseline #B8, ExpBaseline #B8, LTFU 2000 #6, LTFU 2007 #C8) d) Statistical methods We will compare demographic and clinical characteristics between survivors who develop and do not develop VTE. Similarly, we will compare categorical variables and continuous variables between siblings who develop and do not develop VTE. For these initial univariable analyses, categorical variables will be compared between groups (survivors with VTE vs. survivors with no VTE; siblings with VTE vs. siblings with no VTE) using the Chisquare test or Fisher s exact test, when appropriate. Continuous variables will be compared between groups using the two-sample t-test (ANOVA for >2 groups) or Mann-Whitney U test (Wilcoxon rank-sum test for >2 groups), for non-normally distributed variables. (Table 1) In the overall cohort (survivors and siblings), cumulative incidence of VTE will be calculated for survivors and siblings. (Figure 1) Among survivors (excluding siblings), we will use piecewise exponential models to evaluate the association between late VTE and prior multimodal therapy (including surgery, chemotherapy, and radiotherapy) as well as relevant demographic and clinical factors. (Table 2) Analysis will be stratified as needed by 5
6 diagnosis, tumor location, and treatment variables. For example, cumulative incidence curves will be calculated for: 1) survivors who underwent surgery as prior treatment, compared to those who did not undergo surgery, 2) survivors with different dose categories of radiotherapy, 3) survivors who received chemotherapy as prior treatment, compared to those who did not receive chemotherapy, 4) survivors with different underlying diseases, and 5) female survivors who have been pregnant or used estrogen/progesterone-containing medications, compared to female survivors who have not been pregnant or used estrogen/progesterone-containing medications. (Figures 2A-C, 3) We will calculate rate ratios for VTE according to risk factors, conditioned on prior treatment with chemotherapy, surgery, or radiotherapy. (Table 3) Because the reporting of medications is restricted to the two years prior to each questionnaire, we will carry out a separate analysis evaluate the association of estrogen/progesterone-containing medications with the primary outcome of VTE using Cox regression models. In these models, relevant medication exposures will be coded as timedependent covariates and will be set to missing during time periods outside the 2 year windows in which a given subject has data. Effectively, this will mean subjects enter and leave the analysis corresponding to the two years prior to the age at which they responded to each survey. As such, only VTE events during the two years prior to a survey will be utilized in these analyses. Because of the reduced data available in these analyses, this model will be fit separately from those without the medication data. Finally, among survivors (and siblings, if not precluded by low event occurrence) we will use piecewise exponential models to compare mortality prior to (vs. following) the development of VTE. Mortality will be assessed in three ways: 1) VTE-related mortality, defined as CTCAE grade 5 VTE, 2) mortality not related to cancer progression/recurrence or external cause of mortality, and 3) all-cause mortality. Causes of mortality in the CCSS cohort have been ascertained by the National Death Index. 28 The models used for this analysis will again be adjusted for relevant demographic and clinical variables. Standardized mortality ratios for survivors and siblings before and after VTE will be calculated based on the expected number of deaths for age-, sex-, race/ethnicity-, and year-specific United States mortality rates. 28 (Table 4) All time-dependent multivariable analyses will be performed by incorporating age as a time-dependent variable because time since diagnosis will be the time scale for the model, however we plan to also use age as a second time variable. Multiple imputation will be employed when a participant experiences VTE, but age at time of VTE is missing. Mortality will be considered a competing risk to VTE. As such, cumulative incidence curves of death will be plotted alongside those of VTE. Person-years at risk for VTE will be calculated and will start at cohort entry (at 5 years since diagnosis of childhood cancer) and end at the earliest occurrence of censoring, VTE, or death. e) Examples of tables and figures Table 1. Comparison of demographic and treatment characteristics of childhood cancer survivors and siblings who did and did not develop venous thromboembolism (VTE) Survivors Siblings Variable Overall VTE No VTE P Overall VTE No VTE P Female Age at diagnosis, y 6
7 Race/ethnicity Non-Hispanic white Non-Hispanic black Hispanic Other Cancer diagnosis CNS Leukemia Lymphoma Wilms tumor Neuroblastoma Bone/soft tissue sarcoma Other Surgery No Yes 1 surgery 2 surgeries >2 surgeries Extremity surgery Major joint replacement Amputation Splenectomy Nephrectomy Childhood cardiac surgery Any chemotherapy Alkylating agent CED, mg/m >7999 Platinum agent score Anthracycline dose, mg/m 2 None < Radiotherapy, Gy (total dose) 7
8 >49 Brain radiotherapy, Gy Chest radiotherapy, Gy Abdomen/Pelvis radiotherapy, Gy Arms radiotherapy, Gy Legs radiotherapy, Gy 8
9 Hormonal medications Prior pregnancy Second malignant neoplasm Low physical activity Tobacco use Current Former Never BMI < >40 Year of diagnosis Follow-up, years (median, IQR) No. of severe/disabling/lifethreatening chronic conditions (CTCAE grade 3-4) CED, cyclophosphamide equivalent dose; CNS, central nervous system; IQR, interquartile range Formatted Table Table 2. Multivariable analysis of factors associated with venous thromboembolism (VTE) among childhood cancer survivors Variable Hazard ratio 95% Confidence interval P Cancer diagnosis CNS Leukemia Lymphoma Wilms tumor Neuroblastoma 9
10 Bone/soft tissue sarcoma Other Surgery No Yes 1 surgery 2 surgeries >2 surgeries Extremity surgery Major joint replacement Amputation Splenectomy Nephrectomy Childhood cardiac surgery Chemotherapy Alkylating agent CED, mg/m >7999 Platinum agent score Radiotherapy, Gy (total dose) >49 Brain radiotherapy, Gy Chest radiotherapy, Gy 10
11 Abdomen/Pelvis radiotherapy, Gy Arms radiotherapy, Gy Legs radiotherapy, Gy Hormonal medications Prior pregnancy Second malignant neoplasm Low physical activity Tobacco use Current Former Never BMI < >40 Female Race/ethnicity Non-Hispanic white Non-Hispanic black Formatted Table 11
12 Hispanic Other Year of diagnosis Age at diagnosis, y No. of severe/disabling/lifethreatening chronic conditions (CTCAE grade 3-4) BMI, body mass index; CED, cyclophosphamide equivalent dose; CNS, central nervous system Table 3. Treatment-specific rate ratios for venous thromboembolism (VTE) among childhood cancer survivors Radiotherapy Extremity surgery Chemotherapy Variable Rate ratio 95% CI P Rate ratio 12 95% CI P Rate ratio Number of risk factors 6 Any 5 Any 4 Any 3 Any 2 Any 1 None Individual and combinations of risk factors Obesity Sedentary Pregnancy/ Hormones CTCAE 3-4 SMN Tobacco 95% CI P
13 SMN + obesity SMN + tobacco SMN + sedentary No risk factors CI, confidence interval; CTCAE, Common Terminology Criteria for Adverse Events; SMN, second malignant neoplasm Table 4. Standardized mortality ratios (SMR) of childhood cancer survivors and siblings by VTE status Survivors Siblings Variable N Deaths SMR 95% Confidence interval N Deaths SMR 95% Confidence interval VTE No VTE SMR, standardized mortality ratio; VTE, venous thromboembolism Table 5. Association between estrogen- or progesterone-containing medication and VTE among childhood cancer survivors and siblings Survivors Siblings Variable Hazard Ratio (95% CI) P Hazard Ratio (95% CI) P Estrogen/progesteronecontaining medication No Yes CI, confidence interval Formatted: Font: Italic Figure 1. Cumulative incidence of venous thromboembolism among childhood cancer survivors vs. siblings, with mortality as a competing risk. Figure 2A. Cumulative incidence of venous thromboembolism among childhood cancer survivors with surgery as prior treatment vs. no surgery as prior treatment, with mortality as a competing risk. Figure 2B. Cumulative incidence of venous thromboembolism among childhood cancer survivors by radiotherapy dose categories, with mortality as a competing risk. Figure 2C. Cumulative incidence of venous thromboembolism among childhood cancer survivors with chemotherapy as prior treatment vs. no chemotherapy as prior treatment, with mortality as a competing risk. 13
14 Figure 3. Cumulative incidence of venous thromboembolism among childhood cancer survivors by type of first cancer diagnosis, with mortality as a competing risk. Figure 4. Cumulative incidence of mortality among childhood cancer survivors who did vs. did not develop venous thromboembolism. 6) Special consideration: N/A 14
15 References 1. Van Ommen, C. H. et al. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. J. Pediatr. 139, (2001). 2. Walker, A. J. et al. Venous thromboembolism in children with cancer - a population-based cohort study. Thromb. Res. 133, (2014). 3. Andrew, M. et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood 83, (1994). 4. Tuckuviene, R., Christensen, A. L., Helgestad, J., Johnsen, S. P. & Kristensen, S. R. Pediatric venous and arterial noncerebral thromboembolism in Denmark: a nationwide population-based study. J. Pediatr. 159, (2011). 5. Boulet, S. L. et al. Trends in venous thromboembolism-related hospitalizations, Pediatrics 130, e (2012). 6. Raffini, L., Huang, Y.-S., Witmer, C. & Feudtner, C. Dramatic increase in venous thromboembolism in children s hospitals in the United States from 2001 to Pediatrics 124, (2009). 7. O Brien, S. H., Klima, J., Termuhlen, A. M. & Kelleher, K. J. Venous thromboembolism and adolescent and young adult oncology inpatients in US children s hospitals, 2001 to J. Pediatr. 159, (2011). 8. Takemoto, C. M. et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J. Pediatr. 164, (2014). 9. Lee, A. Y. Y. & Levine, M. N. Venous thromboembolism and cancer: risks and outcomes. Circulation 107, I17 21 (2003). 10. Kirwan, C. C., McCollum, C. N., McDowell, G. & Byrne, G. J. Investigation of proposed mechanisms of chemotherapy-induced venous thromboembolism: endothelial cell activation 15
16 and procoagulant release due to apoptosis. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 21, (2015). 11. Sousou, T. & Khorana, A. A. New insights into cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 29, (2009). 12. Davies, Alun. Vascular Surgery. (Springer, 2006). 13. Palmer, P. E. et al. Complications in the surgical treatment of pediatric melanoma. J. Pediatr. Surg. 48, (2013). 14. Beesley, V. L. et al. Incidence, risk factors and estimates of a woman s risk of developing secondary lower limb lymphedema and lymphedema-specific supportive care needs in women treated for endometrial cancer. Gynecol. Oncol. 136, (2015). 15. Schünemann, H. & Willich, N. Lymphedema after breast carcinoma. A study of 5868 cases. Dtsch. Med. Wochenschr , (1997). 16. Sandoval, J. A. et al. Incidence, risk factors, and treatment patterns for deep venous thrombosis in hospitalized children: an increasing population at risk. J. Vasc. Surg. 47, (2008). 17. Pomp, E. R., Rosendaal, F. R. & Doggen, C. J. M. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am. J. Hematol. 83, (2008). 18. Meng, K., Hu, X., Peng, X. & Zhang, Z. Incidence of venous thromboembolism during pregnancy and the puerperium: a systematic review and meta-analysis. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 28, (2015). 16
17 19. Stein, P. D., Kayali, F. & Olson, R. E. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J. Pediatr. 145, (2004). 20. Carrier, M. et al. Screening for Occult Cancer in Unprovoked Venous Thromboembolism. N. Engl. J. Med. 373, (2015). 21. Khorana, A. A. Cancer and thrombosis: implications of published guidelines for clinical practice. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 20, (2009). 22. Setty, B. A., O Brien, S. H. & Kerlin, B. A. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr. Blood Cancer 59, (2012). 23. Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, (2006). 24. Physical Activity Guidelines Advisory Committee. Physical activity guidelines advisory committee report. (2008). 25. Green, D. M. et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 61, (2014). 26. Mulrooney, D. A. et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339, b4606 (2009). 27. Henderson, T. O. et al. Secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 99, (2007). 17
18 28. Mertens, A. C. et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 100, (2008). 18
1) Study Title: Long-term Gallbladder and Biliary Disease in Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study
 1) Study Title: Long-term Gallbladder and Biliary Disease in Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study 2) Working group and investigators: The study will be performed
1) Study Title: Long-term Gallbladder and Biliary Disease in Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study 2) Working group and investigators: The study will be performed
1. Study Title. Exercise and Late Mortality in 5-Year Survivors of Childhood Cancer: a Report from the Childhood Cancer Survivor Study.
 CCSS Analysis Concept Proposal Exercise, Mortality, & Childhood Cancer 1 1. Study Title. Exercise and Late Mortality in 5-Year Survivors of Childhood Cancer: a Report from the Childhood Cancer Survivor
CCSS Analysis Concept Proposal Exercise, Mortality, & Childhood Cancer 1 1. Study Title. Exercise and Late Mortality in 5-Year Survivors of Childhood Cancer: a Report from the Childhood Cancer Survivor
CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL
 CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL 1. Study title: Subsequent neoplasms among survivors of childhood cancer not previously treated with radiation 2. Working group and investigators:
CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL 1. Study title: Subsequent neoplasms among survivors of childhood cancer not previously treated with radiation 2. Working group and investigators:
Title: Incidence of chronic disease among childhood cancer survivors by treatment era and temporal trends in treatment exposure
 Childhood Cancer Survivor Study Analysis Concept Proposal Title: Incidence of chronic disease among childhood cancer survivors by treatment era and temporal trends in treatment exposure Working Group &
Childhood Cancer Survivor Study Analysis Concept Proposal Title: Incidence of chronic disease among childhood cancer survivors by treatment era and temporal trends in treatment exposure Working Group &
Exercise Behavior & Major Cardiac Events: CCSS 1 STUDY TITLE
 STUDY TITLE Exercise Behavior & Major Cardiac Events: CCSS 1 Association Between Exercise Behavior and Incidence of Major Cardiac Events in Adult Survivors of Childhood Cancer: A Report from the Childhood
STUDY TITLE Exercise Behavior & Major Cardiac Events: CCSS 1 Association Between Exercise Behavior and Incidence of Major Cardiac Events in Adult Survivors of Childhood Cancer: A Report from the Childhood
1. Study Title Long-term incidence of anorectal complications among childhood cancer survivors
 1. Study Title Long-term incidence of anorectal complications among childhood cancer survivors 2. Working group and investigators This proposed project will be undertaken with the assistance of the Childhood
1. Study Title Long-term incidence of anorectal complications among childhood cancer survivors 2. Working group and investigators This proposed project will be undertaken with the assistance of the Childhood
Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity
 Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity WORKING GROUP: This report will be written within the Cancer Control Working Group with oversight from the Second Malignant
Breast Cancer in Childhood Cancer Survivors: The Impact of Screening on Morbidity WORKING GROUP: This report will be written within the Cancer Control Working Group with oversight from the Second Malignant
a) Study Title: The impact of chronic disease on health care utilization in the CCSS cohort
 Analysis Concept Proposal a) Study Title: The impact of chronic disease on health care utilization in the CCSS cohort b) Working Group Investigators This proposed project will be developed through the
Analysis Concept Proposal a) Study Title: The impact of chronic disease on health care utilization in the CCSS cohort b) Working Group Investigators This proposed project will be developed through the
Childhood Cancer Survivor Study Analysis Concept Proposal
 Childhood Cancer Survivor Study Analysis Concept Proposal Title: Neurologic and Neurosensory Adverse Sequelae in Long-term Survivors of Childhood Brain Tumors: An Update and Expanded Risk Factor Analysis
Childhood Cancer Survivor Study Analysis Concept Proposal Title: Neurologic and Neurosensory Adverse Sequelae in Long-term Survivors of Childhood Brain Tumors: An Update and Expanded Risk Factor Analysis
Childhood Cancer Survivor Study Study Proposal: Male Health Questionnaire (MHQ) and CED February 2015
 Childhood Cancer Survivor Study Study Proposal: Male Health Questionnaire (MHQ) and CED February 2015 1. STUDY TITLE: Cyclophosphamide Equivalent Dosing and Male Health Late Effects Infertility, Erectile
Childhood Cancer Survivor Study Study Proposal: Male Health Questionnaire (MHQ) and CED February 2015 1. STUDY TITLE: Cyclophosphamide Equivalent Dosing and Male Health Late Effects Infertility, Erectile
Childhood Cancer Survivor Study Analysis Concept Proposal
 Title: Multiple Subsequent Neoplasms Working Group and Investigators: Childhood Cancer Survivor Study Analysis Concept Proposal This proposed publication will be within the Second Malignancy Working Group
Title: Multiple Subsequent Neoplasms Working Group and Investigators: Childhood Cancer Survivor Study Analysis Concept Proposal This proposed publication will be within the Second Malignancy Working Group
Second Neoplasms Working Group. CCSS Investigators Meeting June 2017
 Second Neoplasms Working Group CCSS Investigators Meeting June 2017 Second Neoplasms Working Group Overview Ongoing review, adjudication and entry of reported neoplasms into data set Initial review of
Second Neoplasms Working Group CCSS Investigators Meeting June 2017 Second Neoplasms Working Group Overview Ongoing review, adjudication and entry of reported neoplasms into data set Initial review of
CHILDHOOD CANCER SURVIVOR STUDY Long-Term Morbidity in Survivors of Childhood Leukemia with Down Syndrome Analysis Concept Proposal
 CHILDHOOD CANCER SURVIVOR STUDY Long-Term Morbidity in of Childhood Leukemia with Down Syndrome Analysis Concept Proposal Working Group and Investigators Genetics Working Group & Chronic Disease Working
CHILDHOOD CANCER SURVIVOR STUDY Long-Term Morbidity in of Childhood Leukemia with Down Syndrome Analysis Concept Proposal Working Group and Investigators Genetics Working Group & Chronic Disease Working
Childhood Cancer Survivor Study Analysis Concept Proposal
 Childhood Cancer Survivor Study Analysis Concept Proposal Title: Long-Term Outcomes of Childhood Central Nervous System Tumor Survivors: A Report from the Childhood Cancer Survivor Study Working Group
Childhood Cancer Survivor Study Analysis Concept Proposal Title: Long-Term Outcomes of Childhood Central Nervous System Tumor Survivors: A Report from the Childhood Cancer Survivor Study Working Group
CCSS Concept Proposal Working Group: Biostatistics and Epidemiology
 Draft date: June 26, 2010 CCSS Concept Proposal Working Group: Biostatistics and Epidemiology Title: Conditional Survival in Pediatric Malignancies: A Comparison of CCSS and SEER Data Proposed Investigators:
Draft date: June 26, 2010 CCSS Concept Proposal Working Group: Biostatistics and Epidemiology Title: Conditional Survival in Pediatric Malignancies: A Comparison of CCSS and SEER Data Proposed Investigators:
Childhood Cancer Survivor Study Analysis Concept Proposal
 Childhood Cancer Survivor Study Analysis Concept Proposal Title: Health outcomes among survivors of adolescent and young adult cancer in the Childhood Cancer Survivor Study. Working Group and Investigators:
Childhood Cancer Survivor Study Analysis Concept Proposal Title: Health outcomes among survivors of adolescent and young adult cancer in the Childhood Cancer Survivor Study. Working Group and Investigators:
3. BACKGROUND AND RATIONALE
 CHILDHOOD CANCER SURVIVOR STUDY Revised Analysis Concept Proposal 10-17 October 12, 2011 1. STUDY TITLE: Growth Hormone Exposure as a risk factor for the development of Subsequent Central Nervous System
CHILDHOOD CANCER SURVIVOR STUDY Revised Analysis Concept Proposal 10-17 October 12, 2011 1. STUDY TITLE: Growth Hormone Exposure as a risk factor for the development of Subsequent Central Nervous System
Evidence tables from the systematic literature search for premature ovarian insufficiency surveillance in female CAYA cancer survivors.
 Evidence tables from the systematic literature search for premature ovarian insufficiency surveillance in female CAYA cancer survivors. Who needs surveillance? Chiarelli et al. Early menopause and Infertility
Evidence tables from the systematic literature search for premature ovarian insufficiency surveillance in female CAYA cancer survivors. Who needs surveillance? Chiarelli et al. Early menopause and Infertility
Childhood Cancer Survivor Study Analysis Concept Proposal. Title: Analysis of Late Mortality by Treatment Era.
 Childhood Cancer Survivor Study Analysis Concept Proposal Title: Analysis of Late Mortality by Treatment Era Working Group & Investigators: Greg Armstrong Todd Gibson Ann Mertens Wendy Leisenring YutakaYasui
Childhood Cancer Survivor Study Analysis Concept Proposal Title: Analysis of Late Mortality by Treatment Era Working Group & Investigators: Greg Armstrong Todd Gibson Ann Mertens Wendy Leisenring YutakaYasui
Matthew J. Ehrhardt John T. Sandlund
 CCSS Analysis Concept Proposal Study Title: Risk for late effects of treatment in children newly diagnosed with mature B-cell non-hodgkin lymphoma: a report from the Childhood Cancer Survivor Study cohort
CCSS Analysis Concept Proposal Study Title: Risk for late effects of treatment in children newly diagnosed with mature B-cell non-hodgkin lymphoma: a report from the Childhood Cancer Survivor Study cohort
PEDIATRIC & ADOLESCENT CANCER SURVIVORSHIP. Denise Rokitka, MD, MPH
 PEDIATRIC & ADOLESCENT CANCER SURVIVORSHIP Denise Rokitka, MD, MPH Objectives Describe incidence of childhood cancer and survival rates and causes of early mortality. Understand the late effects of cancer
PEDIATRIC & ADOLESCENT CANCER SURVIVORSHIP Denise Rokitka, MD, MPH Objectives Describe incidence of childhood cancer and survival rates and causes of early mortality. Understand the late effects of cancer
Updated Analysis of Non-Surgical Premature Menopause in the Childhood Cancer Survivor Study
 Analysis Concept Proposal 1. Study Title Updated Analysis of Non-Surgical Premature Menopause in the Childhood Cancer Survivor Study 2. Working Group and Investigators CCSS Working Group: Chronic Disease
Analysis Concept Proposal 1. Study Title Updated Analysis of Non-Surgical Premature Menopause in the Childhood Cancer Survivor Study 2. Working Group and Investigators CCSS Working Group: Chronic Disease
Solid organ transplant after treatment for childhood cancer: A report from the Childhood Cancer Survivor Study.
 Your abstract submission has been received You have submitted the following abstract to the 2017 ASCO Annual Meeting (June 2-6, 2017). Receipt of this notice does not guarantee that your submission was
Your abstract submission has been received You have submitted the following abstract to the 2017 ASCO Annual Meeting (June 2-6, 2017). Receipt of this notice does not guarantee that your submission was
Childhood Cancer Survivor Study (U24 CA55727)
 (U24 CA55727) Report of the Epidemiology and Biostatistics Working Group Ann Mertens, PhD CCSS Investigator Meeting Williamsburg, VA June 9-10, 2010 Publications in past two years Dinu et al. Prediction
(U24 CA55727) Report of the Epidemiology and Biostatistics Working Group Ann Mertens, PhD CCSS Investigator Meeting Williamsburg, VA June 9-10, 2010 Publications in past two years Dinu et al. Prediction
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/188/20915 holds various files of this Leiden University dissertation. Author: Flinterman, Linda Elisabeth Title: Risk factors for a first and recurrent venous
Cover Page The handle http://hdl.handle.net/188/20915 holds various files of this Leiden University dissertation. Author: Flinterman, Linda Elisabeth Title: Risk factors for a first and recurrent venous
Childhood Cancer Survivor Study Analysis Concept Proposal
 Childhood Cancer Survivor Study Analysis Concept Proposal Date: August 6, 2012 Title: Longitudinal Evaluation of Chronic Health Conditions in Ewing Sarcoma Survivors: A Report of the Childhood Cancer Survivor
Childhood Cancer Survivor Study Analysis Concept Proposal Date: August 6, 2012 Title: Longitudinal Evaluation of Chronic Health Conditions in Ewing Sarcoma Survivors: A Report of the Childhood Cancer Survivor
Medical Late Effects of Childhood Cancer
 Medical Late Effects of Childhood Cancer Robert Goldsby Associate Professor of Clinical Pediatric UCSF Late Mortality in 5+ Year Survivors 1.00 US Female US Male Survival function estimate 0.70 0.75 0.80
Medical Late Effects of Childhood Cancer Robert Goldsby Associate Professor of Clinical Pediatric UCSF Late Mortality in 5+ Year Survivors 1.00 US Female US Male Survival function estimate 0.70 0.75 0.80
CONCEPT PROPOSAL. WORKING GROUP: This report will be written within the Chronic Disease Working Group. Proposed investigators include:
 CONCEPT PROPOSAL STUDY TITLE: Impact of Radiation Dose and Volume to the Pancreas on Subsequent Risk of Diabetes Mellitus in Long-term Survivors of Childhood Cancer Treated with Abdominal Radiation: A
CONCEPT PROPOSAL STUDY TITLE: Impact of Radiation Dose and Volume to the Pancreas on Subsequent Risk of Diabetes Mellitus in Long-term Survivors of Childhood Cancer Treated with Abdominal Radiation: A
Epidemiologia e clinica del tromboembolismo venoso. Maria Ciccone Sezione di Ematologia e Fisiopatologia della Coagulazione
 Epidemiologia e clinica del tromboembolismo venoso Maria Ciccone Sezione di Ematologia e Fisiopatologia della Coagulazione Thrombophilia may present clinically as one or more of several thrombotic manifestations
Epidemiologia e clinica del tromboembolismo venoso Maria Ciccone Sezione di Ematologia e Fisiopatologia della Coagulazione Thrombophilia may present clinically as one or more of several thrombotic manifestations
CHILDHOOD CANER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL
 CHILDHOOD CANER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL Project Title: Comparison of risks of mortality (all-cause, cause-specific) and invasive second or subsequent cancers: a Childhood Cancer Survivor
CHILDHOOD CANER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL Project Title: Comparison of risks of mortality (all-cause, cause-specific) and invasive second or subsequent cancers: a Childhood Cancer Survivor
Chronic Disease Working Group CCSS Investigator Meeting 2015
 Chronic Disease Working Group CCSS Investigator Meeting 2015 Kevin Oeffinger Charles (Chuck) Sklar Chronic Disease Working Group Next 10 yrs of CCSS may be more significant than the previous 10 yrs CCSS
Chronic Disease Working Group CCSS Investigator Meeting 2015 Kevin Oeffinger Charles (Chuck) Sklar Chronic Disease Working Group Next 10 yrs of CCSS may be more significant than the previous 10 yrs CCSS
Childhood Cancer Survivor Study Analysis Concept Proposal May 14, 2012
 Childhood Cancer Survivor Study Analysis Concept Proposal May 14, 2012 A. Study Title Estimating longterm outcomes in children newly diagnosed with standard risk acute lymphoblastic leukemia based on similarly
Childhood Cancer Survivor Study Analysis Concept Proposal May 14, 2012 A. Study Title Estimating longterm outcomes in children newly diagnosed with standard risk acute lymphoblastic leukemia based on similarly
Late Effects in Pediatric Cancer Survivors
 Late Effects in Pediatric Cancer Survivors LISA K OPP, DO ASSOCIATE PROFESSOR THE UNIVERSITY OF ARIZONA DEPARTMENT OF PEDIATRICS DIVISION OF HEMATOLOGY/ONCOLOGY/BMT Objectives Review Childhood Cancer and
Late Effects in Pediatric Cancer Survivors LISA K OPP, DO ASSOCIATE PROFESSOR THE UNIVERSITY OF ARIZONA DEPARTMENT OF PEDIATRICS DIVISION OF HEMATOLOGY/ONCOLOGY/BMT Objectives Review Childhood Cancer and
Analysis Concept Proposal
 Analysis Concept Proposal Title: Long-term outcomes among survivors of childhood acute myeloid leukemia Working Groups: Chronic Disease, Second Malignancy, Biostatistics Investigators: Lucie Turcotte,
Analysis Concept Proposal Title: Long-term outcomes among survivors of childhood acute myeloid leukemia Working Groups: Chronic Disease, Second Malignancy, Biostatistics Investigators: Lucie Turcotte,
CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL
 CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL STUDY TITLE: Infertility and the use of fertility treatments in female survivors of childhood cancer. WORKING GROUP Chronic Disease INVESTIGATORS:
CHILDHOOD CANCER SURVIVOR STUDY ANALYSIS CONCEPT PROPOSAL STUDY TITLE: Infertility and the use of fertility treatments in female survivors of childhood cancer. WORKING GROUP Chronic Disease INVESTIGATORS:
CCSS Investigator Meeting Plenary Session June 12, 2013 Memphis, TN. The Childhood Cancer Survivor Study
 CCSS Investigator Meeting Plenary Session June 12, 2013 Memphis, TN Schedule Wednesday 2:00 3:40pm Update Reports 4:00 7:00pm Career Development Awardee and Research Presentations 7:00 8:00pm Reception
CCSS Investigator Meeting Plenary Session June 12, 2013 Memphis, TN Schedule Wednesday 2:00 3:40pm Update Reports 4:00 7:00pm Career Development Awardee and Research Presentations 7:00 8:00pm Reception
CCSS Neurology Committee Report: 6/08
 Neurology Committee Report: 6/08 Roger J. Packer, MD Executive Director Neuroscience and Behavioral Medicine Chairman, Neurology Director, Brain Tumor Institute Children s s National Medical Center Washington,
Neurology Committee Report: 6/08 Roger J. Packer, MD Executive Director Neuroscience and Behavioral Medicine Chairman, Neurology Director, Brain Tumor Institute Children s s National Medical Center Washington,
Mabel Labrada, MD Miami VA Medical Center
 Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal. 1. TITLE: Longitudinal smoking patterns in adult survivors of childhood cancer
 CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal 1. TITLE: Longitudinal smoking patterns in adult survivors of childhood cancer 2. WORKING GROUP AND INVESTIGATORS Working group: This proposed
CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal 1. TITLE: Longitudinal smoking patterns in adult survivors of childhood cancer 2. WORKING GROUP AND INVESTIGATORS Working group: This proposed
VTE Risk Assessment. Challenges of Hemostasis in Cancer Patients. Cihan Ay, MD Associate Professor
 Challenges of Hemostasis in Cancer Patients VTE Risk Assessment Cihan Ay, MD Associate Professor Clinical Division of Haematology and Haemostaseology Department of Medicine I, Comprehensive Cancer Center
Challenges of Hemostasis in Cancer Patients VTE Risk Assessment Cihan Ay, MD Associate Professor Clinical Division of Haematology and Haemostaseology Department of Medicine I, Comprehensive Cancer Center
This proposal will be set within the Chronic Disease and Cancer Control Working Groups.
 Childhood Cancer Survivor Study Analysis Concept Proposal Date: 2/11/13 Title: Longitudinal Evaluation of Chronic Disease and Health Status in Soft Tissue Sarcoma Survivors: A Report from the Childhood
Childhood Cancer Survivor Study Analysis Concept Proposal Date: 2/11/13 Title: Longitudinal Evaluation of Chronic Disease and Health Status in Soft Tissue Sarcoma Survivors: A Report from the Childhood
Potpourri of Hematology Oncology. Jasmine Nabi, M.D. Oncology Associates Hall-Perrine Cancer Center at Mercy
 Potpourri of Hematology Oncology Jasmine Nabi, M.D. Oncology Associates Hall-Perrine Cancer Center at Mercy Lifestyle Modifications to Decrease the Risk of Colorectal Cancer Estimates for 2018 American
Potpourri of Hematology Oncology Jasmine Nabi, M.D. Oncology Associates Hall-Perrine Cancer Center at Mercy Lifestyle Modifications to Decrease the Risk of Colorectal Cancer Estimates for 2018 American
VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT
 VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT OBJECTIVE: To provide guidance on the recommended duration of anticoagulant therapy for venous thromboembolism (VTE). BACKGROUND: Recurrent episodes of VTE
VENOUS THROMBOEMBOLISM: DURATION OF TREATMENT OBJECTIVE: To provide guidance on the recommended duration of anticoagulant therapy for venous thromboembolism (VTE). BACKGROUND: Recurrent episodes of VTE
VTE in Children: Practical Issues
 VTE in Children: Practical Issues Wasil Jastaniah MBBS,FAAP,FRCPC Consultant Pediatric Hem/Onc/BMT May 2012 Top 10 Reasons Why Pediatric VTE is Different 1. Social, ethical, and legal implications. 2.
VTE in Children: Practical Issues Wasil Jastaniah MBBS,FAAP,FRCPC Consultant Pediatric Hem/Onc/BMT May 2012 Top 10 Reasons Why Pediatric VTE is Different 1. Social, ethical, and legal implications. 2.
1. STUDY TITLE: Impact of Chronic Disease on Neurocognitive and Psychosocial Functions.
 1. STUDY TITLE: Impact of Chronic Disease on Neurocognitive and Psychosocial Functions 2. WORKING GROUP AND INVESTIGATORS: 2.1. Working Groups: Psychology; Chronic Disease 2.2. Investigators: Neelam Jain
1. STUDY TITLE: Impact of Chronic Disease on Neurocognitive and Psychosocial Functions 2. WORKING GROUP AND INVESTIGATORS: 2.1. Working Groups: Psychology; Chronic Disease 2.2. Investigators: Neelam Jain
Deep Vein Thrombosis
 Deep Vein Thrombosis from NHS (UK) guidelines Introduction Deep vein thrombosis (DVT) is a blood clot in one of the deep veins in the body. Blood clots that develop in a vein are also known as venous thrombosis.
Deep Vein Thrombosis from NHS (UK) guidelines Introduction Deep vein thrombosis (DVT) is a blood clot in one of the deep veins in the body. Blood clots that develop in a vein are also known as venous thrombosis.
VENOUS THROMBOEMBOLISM AND CORONARY ARTERY DISEASE: IS THERE A LINK?
 VENOUS THROMBOEMBOLISM AND CORONARY ARTERY DISEASE: IS THERE A LINK? Ayman El-Menyar (1), MD, Hassan Al-Thani (2),MD (1)Clinical Research Consultant, (2) Head of Vascular Surgery, Hamad General Hospital
VENOUS THROMBOEMBOLISM AND CORONARY ARTERY DISEASE: IS THERE A LINK? Ayman El-Menyar (1), MD, Hassan Al-Thani (2),MD (1)Clinical Research Consultant, (2) Head of Vascular Surgery, Hamad General Hospital
Duration of Anticoagulant Therapy. Linda R. Kelly PharmD, PhC, CACP September 17, 2016
 Duration of Anticoagulant Therapy Linda R. Kelly PharmD, PhC, CACP September 17, 2016 Conflicts of Interest No conflicts of interest to report Objectives At the end of the program participants will be
Duration of Anticoagulant Therapy Linda R. Kelly PharmD, PhC, CACP September 17, 2016 Conflicts of Interest No conflicts of interest to report Objectives At the end of the program participants will be
What You Should Know
 1 New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know The American Society
1 New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know New 2018 ASH Clinical Practice Guidelines on Venous Thromboembolism: What You Should Know The American Society
Childhood Cancer Survivor Study (U24 CA55727)
 CCSS An NCI-funded Resource Childhood Cancer Survivor Study (U24 CA55727) Report of the Chronic Disease Working Group Charles Sklar, M.D. CCSS Investigator Meeting Williamsburg, VA June 9-10, 2010 Chronic
CCSS An NCI-funded Resource Childhood Cancer Survivor Study (U24 CA55727) Report of the Chronic Disease Working Group Charles Sklar, M.D. CCSS Investigator Meeting Williamsburg, VA June 9-10, 2010 Chronic
Working Group: This report will be written within the Second Malignancy Working Group.
 Title: Human papillomavirus (HPV)-associated malignancies as second cancers in childhood cancer survivors: a report from the Childhood Cancer Survivor Study Working Group: This report will be written within
Title: Human papillomavirus (HPV)-associated malignancies as second cancers in childhood cancer survivors: a report from the Childhood Cancer Survivor Study Working Group: This report will be written within
Childhood Cancer Survivor Study Study Proposal: Male Health Questionnaire (MHQ) November 6, 2012
 Childhood Cancer Survivor Study Study Proposal: Male Health Questionnaire (MHQ) November 6, 2012 1. STUDY TITLE: Perceptions of risk for Male Health Problems in childhood and adolescent cancer survivors:
Childhood Cancer Survivor Study Study Proposal: Male Health Questionnaire (MHQ) November 6, 2012 1. STUDY TITLE: Perceptions of risk for Male Health Problems in childhood and adolescent cancer survivors:
A Methodological Issue in the Analysis of Second-Primary Cancer Incidence in Long-Term Survivors of Childhood Cancers
 American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 158, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwg278 PRACTICE OF EPIDEMIOLOGY
American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 158, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwg278 PRACTICE OF EPIDEMIOLOGY
Dave Duddleston, MD VP and Medical Director Southern Farm Bureau Life
 Dave Duddleston, MD VP and Medical Director Southern Farm Bureau Life Sources of Risk for Venous Diseases Pulmonary embolism (thrombus) Bleeding from anticoagulation Mortality from underlying disease Chronic
Dave Duddleston, MD VP and Medical Director Southern Farm Bureau Life Sources of Risk for Venous Diseases Pulmonary embolism (thrombus) Bleeding from anticoagulation Mortality from underlying disease Chronic
Childhood Cancer Survivor Study Analysis Concept Proposal November 12, 2010
 Childhood Cancer Survivor Study Analysis Concept Proposal vember 12, 2010 1. Title: Radiation dose and benign thyroid conditions in the Childhood Cancer Survivor Study: analysis of radiation dose-response
Childhood Cancer Survivor Study Analysis Concept Proposal vember 12, 2010 1. Title: Radiation dose and benign thyroid conditions in the Childhood Cancer Survivor Study: analysis of radiation dose-response
THROMBOPHILIA TESTING: PROS AND CONS SHANNON CARPENTER, MD MS CHILDREN S MERCY HOSPITAL KANSAS CITY, MO
 THROMBOPHILIA TESTING: PROS AND CONS SHANNON CARPENTER, MD MS CHILDREN S MERCY HOSPITAL KANSAS CITY, MO DISCLAIMER I m a pediatrician I will be discussing this issue primarily from a pediatric perspective
THROMBOPHILIA TESTING: PROS AND CONS SHANNON CARPENTER, MD MS CHILDREN S MERCY HOSPITAL KANSAS CITY, MO DISCLAIMER I m a pediatrician I will be discussing this issue primarily from a pediatric perspective
Original Title: Long-term Follow up of Survivors of Childhood Osteosarcoma: A Report from the Childhood Cancer Survivor Study (CCSS)
 Childhood Cancer Survivor Study Analysis Concept Proposal Original Title: Long-term Follow up of Survivors of Childhood Osteosarcoma: A Report from the Childhood Cancer Survivor Study (CCSS) Working Groups:
Childhood Cancer Survivor Study Analysis Concept Proposal Original Title: Long-term Follow up of Survivors of Childhood Osteosarcoma: A Report from the Childhood Cancer Survivor Study (CCSS) Working Groups:
Endocrine Abnormalities in Aging Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study
 Endocrine Abnormalities in Aging Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study Sogol Mostoufi-Moab, University of Pennsylvania Kristy Seidel, Fred Hutchinson Cancer Research
Endocrine Abnormalities in Aging Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study Sogol Mostoufi-Moab, University of Pennsylvania Kristy Seidel, Fred Hutchinson Cancer Research
Risk and Impact of Pulmonary Complications in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study
 Risk and Impact of Pulmonary Complications in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study Andrew C. Dietz, Childrens Hosp Los Angeles Yan Chen, University of Alberta
Risk and Impact of Pulmonary Complications in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study Andrew C. Dietz, Childrens Hosp Los Angeles Yan Chen, University of Alberta
Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer
 Original Article Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer Gregory T. Armstrong, M.D., M.S.C.E., Yan Chen, M.M., Yutaka Yasui, Ph.D., Wendy Leisenring, Sc.D., Todd M. Gibson,
Original Article Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer Gregory T. Armstrong, M.D., M.S.C.E., Yan Chen, M.M., Yutaka Yasui, Ph.D., Wendy Leisenring, Sc.D., Todd M. Gibson,
*Corresponding Author:
 Audit of venous thromboembolism prophylaxis administered to general surgical patients undergoing elective and emergency operations at National Hospital, Sri Lanka *Migara Seneviratne 1, Asanka Hemachandra
Audit of venous thromboembolism prophylaxis administered to general surgical patients undergoing elective and emergency operations at National Hospital, Sri Lanka *Migara Seneviratne 1, Asanka Hemachandra
Early Ambulation Reduces the Risk of Venous Thromboembolism After Total Knee Replacement. Marilyn Szekendi, PhD, RN
 Early Ambulation Reduces the Risk of Venous Thromboembolism After Total Knee Replacement Marilyn Szekendi, PhD, RN ANA 7 th Annual Nursing Quality Conference, February 2013 Research Team Banafsheh Sadeghi,
Early Ambulation Reduces the Risk of Venous Thromboembolism After Total Knee Replacement Marilyn Szekendi, PhD, RN ANA 7 th Annual Nursing Quality Conference, February 2013 Research Team Banafsheh Sadeghi,
BLOOD RESEARCH. Venous thromboembolism in pediatric patients: a single institution experience in Korea
 BLOOD RESEARCH VOLUME 5 ㆍ NUMBER September 6 ORIGINAL ARTICLE Venous thromboembolism in pediatric patients: a single institution experience in Korea Hyoung Soo Choi, Chang Won Choi, Heon Min Kim, Hye Won
BLOOD RESEARCH VOLUME 5 ㆍ NUMBER September 6 ORIGINAL ARTICLE Venous thromboembolism in pediatric patients: a single institution experience in Korea Hyoung Soo Choi, Chang Won Choi, Heon Min Kim, Hye Won
Epidemiology/Biostatistics Working Group Report
 Epidemiology/Biostatistics Working Group Report CCSS Investigators Meeting June, 2015 1995 CCSS had 0 publication Dial-up access to Internet Amazon.com sold its 1 st book online I was a new PhD who got
Epidemiology/Biostatistics Working Group Report CCSS Investigators Meeting June, 2015 1995 CCSS had 0 publication Dial-up access to Internet Amazon.com sold its 1 st book online I was a new PhD who got
Member, St. Jude Children s Research Hospital (senior author)
 Title: Physical Functioning and Chronic Health Conditions in Pediatric Acute Lymphoblastic Leukemia and Non-Hodgkin Lymphoma Survivors Treated with Contemporary Therapy Working Group of Investigators:
Title: Physical Functioning and Chronic Health Conditions in Pediatric Acute Lymphoblastic Leukemia and Non-Hodgkin Lymphoma Survivors Treated with Contemporary Therapy Working Group of Investigators:
Combined oral contraceptives and risk of venous thromboembolism: nested case control studies using the QResearch and the CPRD databases
 Combined oral contraceptives and risk of venous thromboembolism: nested case control studies using the QResearch and the CPRD databases Yana Vinogradova, Carol Coupland, Julia Hippisley-Cox Web appendix:
Combined oral contraceptives and risk of venous thromboembolism: nested case control studies using the QResearch and the CPRD databases Yana Vinogradova, Carol Coupland, Julia Hippisley-Cox Web appendix:
DVT - initial management NSCCG
 Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge
 Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge What is a venous thromboembolism (VTE)? This is a medical term that describes a blood clot that develops in a deep vein
Reducing the risk of venous thrombo-embolism (VTE) in hospital and after discharge What is a venous thromboembolism (VTE)? This is a medical term that describes a blood clot that develops in a deep vein
4/3/2015. Obesity in Childhood Cancer Survivors: Opportunities for Early Intervention. Cancer in Children. Cancer is the #1 cause of diseaserelated
 Obesity in Childhood Cancer Survivors: Opportunities for Early Intervention Fang Fang Zhang, MD, PhD Friedman School of Nutrition Science and Policy, Tufts University UNTHSC Grant Rounds April 8, 2015
Obesity in Childhood Cancer Survivors: Opportunities for Early Intervention Fang Fang Zhang, MD, PhD Friedman School of Nutrition Science and Policy, Tufts University UNTHSC Grant Rounds April 8, 2015
Disclosures. This presentation is the intellectual property of the author. Contact them for permission to reprint and/or distribute.
 ALL in AYAs: Health Outcomes as a Criterion for Selecting Optimal Therapy David R. Freyer, DO, MS Director, Survivorship and Supportive Care Program, Children s Center for Cancer and Blood Diseases, Children
ALL in AYAs: Health Outcomes as a Criterion for Selecting Optimal Therapy David R. Freyer, DO, MS Director, Survivorship and Supportive Care Program, Children s Center for Cancer and Blood Diseases, Children
DEEP VEIN THROMBOSIS (DVT): TREATMENT
 DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients
 Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients What is a deep vein thrombosis (DVT)? A DVT is a blood clot that forms within a vein deep in the leg but can
Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients What is a deep vein thrombosis (DVT)? A DVT is a blood clot that forms within a vein deep in the leg but can
IRB protocol Yair Lev, MD 11/25/08
 IRB protocol Yair Lev, MD 11/25/08 Abdominal and Pelvic CT as a screening modality for occult malignant disease in unprovoked Venous Thromboembolism: A randomized, controlled prospective study. A. Study
IRB protocol Yair Lev, MD 11/25/08 Abdominal and Pelvic CT as a screening modality for occult malignant disease in unprovoked Venous Thromboembolism: A randomized, controlled prospective study. A. Study
Risk factors for the initiation and aggravation of lymphoedema after axillary lymph node dissection for breast cancer
 HEALTH SERVICES RESEARCH FUND Risk factors for the initiation and aggravation of lymphoedema after axillary lymph node dissection for breast cancer Key Messages 1. Previous inflammation or infection of
HEALTH SERVICES RESEARCH FUND Risk factors for the initiation and aggravation of lymphoedema after axillary lymph node dissection for breast cancer Key Messages 1. Previous inflammation or infection of
In the Clinic: Annals Sweta Kakaraparthi 1/23/15
 In the Clinic: Annals Sweta Kakaraparthi 1/23/15 Case Scenerio 56 year old female with breast cancer presents to the clinic for her 3 month followup! She is concerned about blood clots and asks you about
In the Clinic: Annals Sweta Kakaraparthi 1/23/15 Case Scenerio 56 year old female with breast cancer presents to the clinic for her 3 month followup! She is concerned about blood clots and asks you about
BSO, HRT, and ERT. No relevant financial disclosures
 BSO, HRT, and ERT Jubilee Brown, MD Professor & Associate Director, Gynecologic Oncology Levine Cancer Institute at the Carolinas HealthCare System Charlotte, North Carolina No relevant financial disclosures
BSO, HRT, and ERT Jubilee Brown, MD Professor & Associate Director, Gynecologic Oncology Levine Cancer Institute at the Carolinas HealthCare System Charlotte, North Carolina No relevant financial disclosures
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC
 Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC 1 st workshop: update to VTE guidelines in 2016 2 nd workshop: VTE controversies + new horizons André Roussin MD, FRCP, CSPQ CHUM
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC 1 st workshop: update to VTE guidelines in 2016 2 nd workshop: VTE controversies + new horizons André Roussin MD, FRCP, CSPQ CHUM
1. STUDY TITLE: Prevalence and Treatment-Related Predictors of Psychoactive Medication Use in Adult Survivors of Childhood Cancer
 1 1. STUDY TITLE: Prevalence and Treatment-Related Predictors of Psychoactive Medication Use in Adult Survivors of Childhood Cancer 2. WORKING GROUP AND INVESTIGATORS: 2.1. Working Groups: Psychology/Neuropsychology;
1 1. STUDY TITLE: Prevalence and Treatment-Related Predictors of Psychoactive Medication Use in Adult Survivors of Childhood Cancer 2. WORKING GROUP AND INVESTIGATORS: 2.1. Working Groups: Psychology/Neuropsychology;
Cancer and Thrombosis
 Cancer and Thrombosis The close relationship between venous thromboembolism and cancer has been known since at least the 19th century by Armand Trousseau. Thrombosis is a major cause of morbidity and mortality
Cancer and Thrombosis The close relationship between venous thromboembolism and cancer has been known since at least the 19th century by Armand Trousseau. Thrombosis is a major cause of morbidity and mortality
Locoregional treatment Session Oral Abstract Presentation Saulo Brito Silva
 Locoregional treatment Session Oral Abstract Presentation Saulo Brito Silva Background Post-operative radiotherapy (PORT) improves disease free and overall suvivallin selected patients with breast cancer
Locoregional treatment Session Oral Abstract Presentation Saulo Brito Silva Background Post-operative radiotherapy (PORT) improves disease free and overall suvivallin selected patients with breast cancer
Menopausal Hormone Therapy & Haemostasis
 Menopausal Hormone Therapy & Haemostasis The Haematologist Perspective Dr. Batia Roth-Yelinek Coagulation unit Hadassah MC Menopausal Hormone Therapy & Hemostasis Hemostatic mechanism Mechanism of estrogen
Menopausal Hormone Therapy & Haemostasis The Haematologist Perspective Dr. Batia Roth-Yelinek Coagulation unit Hadassah MC Menopausal Hormone Therapy & Hemostasis Hemostatic mechanism Mechanism of estrogen
CHILDHOOD CANCER SURVIVOR STUDY- Analysis Concept Proposal. 1. TITLE: Tobacco Use Among Adult Siblings of Childhood Cancer Survivors
 CHILDHOOD CANCER SURVIVOR STUDY- Analysis Concept Proposal 1. TITLE: Tobacco Use Among Adult Siblings of Childhood Cancer Survivors 2. WORKING GROUP INVESTIGATORS: This proposed study will be within the
CHILDHOOD CANCER SURVIVOR STUDY- Analysis Concept Proposal 1. TITLE: Tobacco Use Among Adult Siblings of Childhood Cancer Survivors 2. WORKING GROUP INVESTIGATORS: This proposed study will be within the
Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal
 CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal 1. TITLE: Follow-Up of Long Term Survivors of Hodgkin s Disease Diagnosed in Childhood and Adolescence A Report from the Childhood Cancer Survivors
CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal 1. TITLE: Follow-Up of Long Term Survivors of Hodgkin s Disease Diagnosed in Childhood and Adolescence A Report from the Childhood Cancer Survivors
Analysis Concept Proposal
 Analysis Concept Proposal Study Title: Long-term outcomes of survivors of childhood Acute Lymphoblastic Leukemia (ALL) across 30-years of treatment: a report from the Childhood Cancer Survivor Study Investigators:
Analysis Concept Proposal Study Title: Long-term outcomes of survivors of childhood Acute Lymphoblastic Leukemia (ALL) across 30-years of treatment: a report from the Childhood Cancer Survivor Study Investigators:
Cancer Control & Intervention Working Group Report 2017 CCSS Investigators Meeting
 Cancer Control & Intervention Working Group Report 2017 CCSS Investigators Meeting Paul Nathan, Jacqueline Casillas, Jennifer Ford, Kevin Oeffinger, Kiri Ness, Melissa Hudson, Tara Henderson, Wendy Leisenring
Cancer Control & Intervention Working Group Report 2017 CCSS Investigators Meeting Paul Nathan, Jacqueline Casillas, Jennifer Ford, Kevin Oeffinger, Kiri Ness, Melissa Hudson, Tara Henderson, Wendy Leisenring
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT)
 ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
Venous Thrombo-Embolism. John de Vos Consultant Haematologist RSCH
 Venous Thrombo-Embolism John de Vos Consultant Haematologist RSCH overview The statistics Pathogenesis Prophylaxis Treatment Agent Duration Incidental VTE Recurrence of VTE IVC filters CVC related thrombosis
Venous Thrombo-Embolism John de Vos Consultant Haematologist RSCH overview The statistics Pathogenesis Prophylaxis Treatment Agent Duration Incidental VTE Recurrence of VTE IVC filters CVC related thrombosis
The Current Pediatric Oncology Landscape
 The Current Pediatric Oncology Landscape An Imperative for Change Smita Bhatia, MD, MPH UAB Department of Pediatrics at Children s Hospital of Alabama Five-year Observed Survival Rates for Two Time Periods,
The Current Pediatric Oncology Landscape An Imperative for Change Smita Bhatia, MD, MPH UAB Department of Pediatrics at Children s Hospital of Alabama Five-year Observed Survival Rates for Two Time Periods,
ASH 2011: Clinically Relevant Highlights Regarding Venous Thromboembolism and Anticoagulation
 ASH 2011: Clinically Relevant Highlights Regarding Venous Thromboembolism and Anticoagulation Stephan Moll Department of Medicine, Division of Hematology-Oncology, University of North Carolina School of
ASH 2011: Clinically Relevant Highlights Regarding Venous Thromboembolism and Anticoagulation Stephan Moll Department of Medicine, Division of Hematology-Oncology, University of North Carolina School of
1. SCOPE of GUIDELINE:
 Page 1 of 35 CLINICAL PRACTICE GUIDELINE: Venous Thromboembolism (VTE) Prevention Guideline: Thromboprophylaxis AUTHORIZATION: VP, Medicine Date Approved: May 17, 2012 Date Revised: Vancouver Coastal Health
Page 1 of 35 CLINICAL PRACTICE GUIDELINE: Venous Thromboembolism (VTE) Prevention Guideline: Thromboprophylaxis AUTHORIZATION: VP, Medicine Date Approved: May 17, 2012 Date Revised: Vancouver Coastal Health
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second cancer
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second cancer
Obesity, renal failure, HIT: which anticoagulant to use?
 Obesity, renal failure, HIT: which anticoagulant to use? Mark Crowther with thanks to Dr David Garcia and others. This Photo by Unknown Author is licensed under CC BY-SA 1 2 Drug choices The DOACs have
Obesity, renal failure, HIT: which anticoagulant to use? Mark Crowther with thanks to Dr David Garcia and others. This Photo by Unknown Author is licensed under CC BY-SA 1 2 Drug choices The DOACs have
Inferior Venacaval Filters Valuable vs. Dangerous Valuable Annie Kulungowski. Department of Surgery Grand Rounds March 24, 2008
 Inferior Venacaval Filters Valuable vs. Dangerous Valuable Annie Kulungowski Department of Surgery Grand Rounds March 24, 2008 History of Vena Cava Filters Virchow-1846-Proposes PE originate from veins
Inferior Venacaval Filters Valuable vs. Dangerous Valuable Annie Kulungowski Department of Surgery Grand Rounds March 24, 2008 History of Vena Cava Filters Virchow-1846-Proposes PE originate from veins
PROGNOSIS AND SURVIVAL
 CANCER ASSOCIATED THROMBOSIS PROGNOSIS AND SURVIVAL Since French internist Armand Trousseau reported the occurrence of mysterious thrombotic disorders in cancer patients in the mid-19th century, the link
CANCER ASSOCIATED THROMBOSIS PROGNOSIS AND SURVIVAL Since French internist Armand Trousseau reported the occurrence of mysterious thrombotic disorders in cancer patients in the mid-19th century, the link
Oral contraceptives Epidemiological aspects Øjvind Lidegaard
 Oral contraceptives Epidemiological aspects Øjvind Lidegaard Professor Gynaecological Clinic 4232 Rigshospitalet Copenhagen University OC: Epidemiological aspects OC use OC and thrombosis - venous thromboembolism
Oral contraceptives Epidemiological aspects Øjvind Lidegaard Professor Gynaecological Clinic 4232 Rigshospitalet Copenhagen University OC: Epidemiological aspects OC use OC and thrombosis - venous thromboembolism
Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008
 Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Childhood Cancer Survivor study Study Proposal: Chronic Endocrine Disorders in Cancer Survivors February 2014
 Childhood Cancer Survivor study Study Proposal: Chronic Endocrine Disorders in Cancer Survivors February 2014 1. STUDY TITLE: Chronic endocrine disorders in adult survivors of childhood cancer: A Report
Childhood Cancer Survivor study Study Proposal: Chronic Endocrine Disorders in Cancer Survivors February 2014 1. STUDY TITLE: Chronic endocrine disorders in adult survivors of childhood cancer: A Report
NOTE: Deep Vein Thrombosis (DVT) Risk Factors
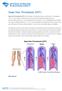 Deep Vein Thrombosis (DVT) Deep Vein Thrombosis (DVT) is the formation of a blood clot, known as a thrombus, in the deep leg vein. It is a very serious condition that can cause permanent damage to the
Deep Vein Thrombosis (DVT) Deep Vein Thrombosis (DVT) is the formation of a blood clot, known as a thrombus, in the deep leg vein. It is a very serious condition that can cause permanent damage to the
Ischemic Stroke in Critically Ill Patients with Malignancy
 Ischemic Stroke in Critically Ill Patients with Malignancy Jeong-Am Ryu 1, Oh Young Bang 2, Daesang Lee 1, Jinkyeong Park 1, Jeong Hoon Yang 1, Gee Young Suh 1, Joongbum Cho 1, Chi Ryang Chung 1, Chi-Min
Ischemic Stroke in Critically Ill Patients with Malignancy Jeong-Am Ryu 1, Oh Young Bang 2, Daesang Lee 1, Jinkyeong Park 1, Jeong Hoon Yang 1, Gee Young Suh 1, Joongbum Cho 1, Chi Ryang Chung 1, Chi-Min
