Transmembrane phospholipid distribution revealed by freeze-fracture replica labeling
|
|
|
- Anissa Carter
- 5 years ago
- Views:
Transcription
1 Journal of Cell Science 109, (1996) Printed in Great Britain The Company of Biologists Limited 1996 JCS Transmembrane phospholipid distribution revealed by freeze-fracture replica labeling Kazushi Fujimoto 1, *, Masato Umeda 2 and Toyoshi Fujimoto 1, 1 Department of Anatomy, Faculty of Medicine, Kyoto University, Yoshida-Konoecho, Sakyo-ku, Kyoto , Japan 2 Department of Inflammation Research, The Tokyo Metropolitan Institute of Medical Science, Honkomagome, Bunkyo-ku, Tokyo 113, Japan *Author for correspondence Present address: Department of Anatomy and Cell Biology, Gunma University School of Medicine, Showa-machi, Maebashi, Gunma 371, Japan Dedicated to Dr Pedro Pinto da Silva, on the occasion of his retirement SUMMARY We propose the use of membrane splitting by freezefracture for differential phospholipid analysis of protoplasmic and exoplasmic membrane leaflets (halves). Unfixed cells or tissues are quick-frozen, freeze-fractured, and platinum-carbon (Pt/C) shadowed. The Pt/C replicas are then treated with 2.5% sodium dodecyl sulfate (SDS) to solubilize unfractured membranes and to release cytoplasm or contents. While the detergent dissolves unfractured membranes, it would not extract lipids from split membranes, as their apolar domains are stabilized by their Pt/C replicas. After washing, the Pt/C replicas, along with attached protoplasmic and exoplasmic membrane halves, are processed for immunocytochemical labeling of phospholipids with antibody, followed by electron microscopic observation. Here, we present the application of the SDS-digested freeze-fracture replica labeling (SDS-FRL) technique to the transmembrane distribution of a major membrane phospholipid, phosphatidylcholine (PC), in various cell and intracellular membranes. Immunogold labeling revealed that PC is exclusively localized on the exoplasmic membrane halves of the plasma membranes, and the intracellular membranes of various organelles, e.g. nuclei, mitochondria, endoplasmic reticulum, secretory granules, and disc membranes of photoreceptor cells. One exception to this general scheme was the plasma membrane forming the myelin sheath of neurons and the Ca 2+ -treated erythrocyte membranes. In these cell membranes, roughly equal amounts of immunogold particles for PC were seen on each outer and inner membrane half, implying a symmetrical transmembrane distribution of PC. Initial screening suggests that the SDS-FRL technique allows in situ analysis of the transmembrane distribution of membrane lipids, and at the same time opens up the possibility of labeling membranes such as intracellular membranes not normally accessible to cytochemical labels without the distortion potentially associated with membrane isolation procedures. Key words: Membrane, Phospholipid, Freeze-fracture replica labeling, Immunogold electron microscopy INTRODUCTION The phospholipid distribution in membranes has been studied mainly by biochemical and physicochemical analyses conducted on artificial membranes, isolated cell homogenates, or non-complex blood elements, i.e. erythocytes and platelets. However, these approaches have significant limitations and pitfalls. For instance, evaluation of lipid distribution in an intracellular membrane requires cell lysis and isolation of the organelles. In some cases, ATP plays a pivotal role in membrane asymmetry (Zachowski, 1993). During the isolation procedure, the membrane is separated from cytoplasmic ATP, and a new lipid repartition may occur, different from that in situ (Schrier et al., 1992). A particularly interesting property of membrane lipids is that many eukaryotic cells exhibit an asymmetric distribution of lipids across the bilayer membrane (Zachowski, 1993): in the erythrocyte membrane, 75% of the phosphatidylcholine and >85% of the sphingomyelin are in the exoplasmic membrane half, while the protoplasmic membrane half contains 80-85% of the phosphatidylethanolamine and >96% of the phosphatidylserine. However, at present, this transmembrane asymmetrical phospholipid distribution cannot be independently verified visually by more direct methods. Our objective here is to add a new electron microscopic approach to study phospholipid transmembrane distribution in various cell and intracellular membranes: the sodium dodecyl sulfate-digested freeze-fracture replica labeling (SDS-FRL) technique is a method enabling us to look directly at the two-dimensional distribution of phospholipids within biological membranes. Fig. 1 shows schematically the rationale of our approach: after quick-freezing (Fig. 1a), freeze-fracture, and Pt/C shadowing (Fig. 1b), unfixed cells or intracellular organelles are treated with 2.5% SDS to dissolve unfractured membranes and contents (Fig. 1c). The cytoplasmic and exoplasmic halves of the membrane remain attached to the Pt/C cast. Although SDS dissolves unfractured portions of the membrane, it would not reach, micellize, and extract split membrane halves, as their
2 2454 a b c d K. Fujimoto, M. Umeda and T. Fujimoto PF Fig. 1. SDS-digested freeze-fracture replica labeling. (a) Cells are frozen. Only phospholipids are depicted. (b) Freeze-fracture splits the plasma membrane into inner protoplasmic (left side, PF) and outer exoplasmic (right side, EF) halves, and the fractured faces are stabilized by deposition of platinum/carbon (Pt/C). (c) Pt/C-casts are thawed and treated with SDS. The detergent dissolves unfractured areas of the plasma membrane, with release of the cytoplasm. (d) The replicas are then processed for immunogold labeling with antibody against a phospholipid. Note that in intracellular organelles, e.g. secretory granules and endoplasmic reticulum, the outer membrane half closest to the cytoplasm is designated the protoplasmic half, and the inner membrane half closest to the interior is designated the exoplasmic half. Thus, the fractured and replicated hydrophobic surface of the outer membrane half (labeled EF in Fig. 1b) is the P-face, and that of the inner membrane half (labeled PF in Fig. 1b) is the E-face. EF Pt/C apolar domains are positioned against, and stabilized by, their Pt/C casts. We reasoned that it should therefore be possible to label the cytoplasmic and exoplasmic leaflets of membranes in both isolated cells and tissue samples (Fig. 1d). In this paper, we first tested the feasibility of SDS-FRL for phospholipid using artificial membranes prepared from various phospholipids with an antibody, and chemically analyzed the replicas to estimate how much of the total phospholipids bound to Pt/C replicas remains after SDS-treatment. Then, we applied SDS-FRL to the immunolabeling of various cell and intracellular membranes. MATERIALS AND METHODS Artificial membranes Artificial membranes (multilamellar vesicles, MLVs) were prepared from mixtures of L-α-phosphatidylcholine (PC; isolated from bovine brain; Avanti Polar Lipids, Inc., Albaster, AL) and L-α-phosphatidyl- L-serine (PS; isolated from bovine brain; Sigma Chemical Co., St Louis, MO) in a 1:1 molar ratio; from mixtures of PS and sphingomyelin (SM; isolated from bovine brain; Avanti Polar Lipids, Inc., Albaster, AL) in a 1:1 molar ratio; from pure PC; and from pure PS according to the method of Kinsky et al. (1969). Small unilamellar vesicles (SUVs) ( nm in diameter) were prepared by using ultrasonic irradiation to break up suspensions of the MLV preparations. Aqueous dispersions of phospholipid vesicles were sandwiched between two well polished gold double replica carriers (Balzers Union, Liechtenstein) at 37 C, and then quickly frozen by plunging into a slush of partially solidified liquid nitrogen. Cells and tissues Erythrocytes were obtained from freshly drawn, heparinized human blood (blood type O or AB, Rh + ). The cells were washed four times, and suspended in cold Hepes buffer (145 mm NaCl, 5 mm KCl, 1 mm MgSO 4, 10 mm glucose, 0.1 mm EGTA, and Na-Hepes, ph 7.5). Some erythrocytes were incubated with Hepes buffer containing 1 mm CaCl 2 and 20 µm A23187 at 37 C for various times. Tissues (liver, pancreas, retina, and optic nerve from rats or mice) were cut into sections less than 100 µm thick by a Microslicer (Dosaka EM Co., Kyoto, Japan) or minced in small pieces, and collected in 0.01 M phosphate buffered saline (PBS). Erythrocytes and tissue slices were quick-frozen by contact with a copper block cooled with liquid helium (Heuser et al., 1979). SDS-digested freeze-fracture replica labeling The frozen samples were fractured in a Balzers BAF 400T freeze-etch unit (Balzers Union, Liechtenstein) at 110 C, replicated by deposition of Pt/C from an electron beam gun positioned at a 45 angle followed by carbon applied from overhead. To release the replicas from the specimen carrier, the carrier was immersed gently in 0.1% bovine serum albumin (fraction V, Miles Inc., Kankakee, IL) in PBS. After floating off, the pieces of replica were transferred to 5 ml of 2.5% SDS (Sigma Chemical Co., St Louis, MO) containing 10 mm Tris and 30 mm sucrose, ph 8.3. SDS-digestion was carried out for minutes at room temperature, with vigorous stirring. The replicas obtained from collagen-rich tissues such as pancreas and retinas were first treated with 0.1% collagenase (type I or type II; Sigma) containing 130 mm NaCl, 12 mm NaHCO 3, 3 mm NaH 2PO 4, 3 mm Na 2HPO 4, 2 mm MgSO 4 and 1 mm CaCl 2, for 1 hour at 37 C with stirring, and then treated with 2.5% SDS for 3 hours at room temperature, with vigorous stirring. After treatment with SDS, replicas (actually carbon-stabilized membrane halves) were rinsed for at least 1 hour, with four or more changes of PBS, and labeled with anti-pc monoclonal antibody (JE-1; Nam et al., 1990) diluted 1:40 in PBS for 1 hour at 37 C. After labeling, the replicas were washed three
3 Membrane phospholipid labeling 2455 times with PBS and incubated for 1 hour at 37 C with secondary goat anti-mouse IgM antibody-conjugated colloidal gold (Janssen Pharmaceuticals, Piscataway, NJ) diluted 1:40 in PBS. After immunogold labeling, the replicas were rinsed several times in PBS, fixed with 0.5% glutaraldehyde in 0.1 M phosphate buffer, ph 7.4, for 10 minutes at room temperature, washed twice with distilled water, and picked onto Formvar-coated grids. Electron microscopy was done using a JEOL 1200EX. Chemical measurement of phospholipids bound to Pt/C replicas The amount of phospholipids bound to SDS-treated Pt/C replicas was measured by the method of Zhou and Arthur (1992). Aqueous suspensions (2 µl) of small unilamellar vesicles (SUVs) of PC (10 mg/ml in Hepes buffer, ph 7.4) were sandwiched between two well polished gold double replica specimen carriers, quickly frozen by plunging into partially solidified liquid nitrogen, freeze-fractured, and Pt/C shadowed. Freeze-fracture with double replica specimen carriers gives two sets of replicas. Thus, one obtained from SUVs of PC was washed in distilled water, and the other was treated with 2.5% SDS for 30 minutes and then washed with distilled water. These two sets of replicas were picked on pre-cleaned coverglasses separately, and dried with nitrogen. To release inorganic phosphorus from the Pt/C shadowed membrane halves, the glass-bound replicas were treated with 70% perchloric acid at 200 C for 2 hours. The released phosphorus was measured by using malachite green. Some replicas of SUVs of PC were examined with a JEOL 1200EX electron microscope. RESULTS AND DISCUSSION Our approach is based on a hypothesis that the split membrane halves are physically stabilized (fixed) by carbon-shadowing onto the apolar fracture face, thus the carbon-fixed membrane halves would not be extracted with organic solvents or detergents (Andersson-Forsman and Pinto da Silva, 1988; Fujimoto, 1995; Fujimoto and Ogawa, 1991; Fujimoto and Pinto da Silva, 1988, 1989, 1992). We applied the SDS-FRL technique to the immunogold labeling of the intercellular junction proteins, e.g. gap junction protein (connexins), tight junction protein (occludin), and the findings suggested the reliability and the potential significance of SDS-FRL in the immunocytochemical labeling of integral membrane proteins (Fujimoto, 1995). Even if the carbon film acts to fix or retain the integral membrane proteins, it seems surprising that the membrane lipids really are retained after the SDS-treatment. To confirm this hypothesis, we first tested the feasibility of SDS-FRL for phospholipid using artificial membranes (MLVs) prepared from mixtures of phosphatidylcholine and phosphatidylserine (PC/PS) (molar ratio, 1:1), mixtures of phosphatidylserine and sphingomyelin (PS/SM) (molar ratio, 1:1), and from pure PS and pure SM with an anti-pc monoclonal antibody, JE-1. Fig. 2a and b show the labeling of PC/PS and pure PS membranes with JE-1, respectively. Although PC/PS membranes were intensely labeled by JE-1, the labeling of pure PS, PS/SM and pure SM membranes (data not shown) resulted in an absence of labeling. This suggests the reliability of phospholipid labeling by the SDS-FRL and the specificity of the antibody. An important question has arisen as to how much of the total phospholipid bound to Pt/C replica remains after SDS treatment. Our quantitative chemical analysis using SUVs of PC showed that SDS-untreated and SDS-treated replicas (7 mm 2 ) contain and moles of PC (average of two experiments), respectively. Although the ratio of PC present on the SDS-treated replica to that on the SDS-untreated is about 0.25, we should notice that in the control the Pt/C replica with carbon-stabilized membrane halves was only washed with distilled water. Therefore, a considerable amount of fractured or cross-fractured PC vesicles remain attached to the carbon cast, either because they are attached to the carbon stabilized inner monolayer of their fractured membrane (Fig. 3, Fracture plane B), or because they were cross-fractured and attached to the replica (Fig. 3, Fracture plane C). The ratio can be evaluated using the following assumptions: outer monolayer a and inner monolayer b are stabilized (fixed) by carbonshadowing onto the apolar fracture face, and bilayer c+d is attached to the carbon stabilized inner monolayer b, or bilayer e+f was cross-fractured and remains attached to the SDSuntreated replica (Fig. 3-1). Although SDS dissolves unfractured portions of the PC bilayers (Fig. 3-2, c +d and e +f ), it would not extract the Pt-C shadowed monolayers (Fig. 3-2, outer monolayer a and inner monolayer b ). Quantitative Fig. 2. SDS-FRL of artificial membranes (multilamellar vesicles) prepared from mixtures of L-α-phosphatidylcholine (PC) and L-α-phosphatidyl-L-serine (PS) (PC/PS molar ratio, 1:1) (a) and from PS (b) with anti-pc monoclonal antibody, JE-1. Mixtures composed of PC and PS are intensely labeled by JE-1 (a), while labeling of pure PS membranes results in an absence of labeling (b). Bars, 100 nm.
4 2456 K. Fujimoto, M. Umeda and T. Fujimoto Fracture plane A Fracture plane B Fracture plane C b 1 a c d b e f 2 3 a c d e f Fig. 3. Diagram for chemical analysis of total phospholipid bound to Pt/C replica after SDS-digestion. The fracture plane passes through the hydrophobic middle of the lipid bilayers (Fracture plane A and Fracture plane B), or crosses the interior of the vesicle (Fracture plane C). Thus, bilayers (c+d) and (e+f) are attached to the SDS-untreated replica (1). Although SDS dissolves unfractured bilayers (2, c +d and e +f ), it does not extract Pt/C-shadowed monolayers (2, a +b ). (3) Conventional freeze-fracture electron micrographs of the hydrophobic portion of outer (left) and inner monolayer (center) of the PC unilamellar vesicles, and the cross-fractured PC unilamellar vesicle (right). Bar, 200 nm. electron microscopy with random photography of ~200 freezefractured PC SUVs demonstrated that about 35% of the SUVs were fractured in Fracture plane A, 35% in Fracture plane B, and about 30% in Fracture plane C. With these assumptions, the ratio (X) of PC present on the SDS-treated replica to that on the SDS-untreated replica can be calculated by using the following equations: X=[(35 a )+(35 b )]/[(35 a)+35 (b+c+d)+30 (e+f)]; rearranging: X=[35 (a +b )]/[(35 (a+b)+35 (c+d)+30 (e+f)], a +b =a+b=c+d=e+f; thus: X=35/100=0.35. Comparing the calculated value (0.35) with the experimental data (0.25), we conclude that at least 70% of PC bound to the Pt/C replica remains after SDS treatment. We next applied the SDS-FRL technique to the immunolabeling of the human erythrocytes with JE-1. SDS (2.5%; 10 minutes at room temperature) dissolved most unfractured plasma membranes and released haemoglobin. The ultrastructure of the exoplasmic fracture face (E-face) and protoplasmic fracture face (P-face) of the erythrocyte membrane as revealed by SDS-FRL was equivalent to that of conventional freezefracture replicas cleaned by treatment with household bleach. A homogeneous distribution of intramembrane particles (IMPs) on the P- and E-faces was observed. More IMPs were visible on the P-face than on the E-face. The replicas of erythrocytes, which were labeled with JE-1, showed that the E- faces were densely labeled (Fig. 4a, EF), while the P-faces were virtually unlabeled (Fig. 4a, PF). The immunogold particles bound to the exoplasmic membrane half, which remains associated with the replica after the SDS digestion and labeling procedures, could be readily recognized through the replica (see Fig. 1d). Thus, the cytochemical labeling was seen superimposed on the image of an E-face. We conclude that the absence of PC immunolabeling on the P-faces implies a nearly complete asymmetrical transmembrane distribution of PC in the erythrocyte membranes. However, there is an alternative explanation for the absence of PC immunolabeling on P-faces; a peripheral membrane protein and/or a cytosolic protein matrix, e.g. membrane cytoskeleton, remain attached to the protoplasmic membrane half after SDStreatment, masks PC and inhibits antibody binding. To exclude this possibility we incubated the SDS-treated replicas of erythrocytes with protease (0.1 mg/ml trypsin or 1 µg/ml proteinase K) for 10 minutes at 37 C prior to cytochemical labeling. No difference in PC immunolabeling was observed (data not shown). Although this may support our conclusion, the influence of integral membrane proteins on PC immunolabeling is still debatable. The elevation of the cytoplasmic Ca 2+ level is currently assumed to activate a pathway for transbilayer diffusion of phospholipids, and to cause the disruption of lipid asymmetry of the plasma membrane of erythrocytes (Williamson et al., 1992). To re-evaluate the effect of Ca 2+ on the transmembrane phospholipid distribution by the SDS-FRL with JE-1, erythrocytes were incubated with Hepes buffer containing 1 mm
5 Membrane phospholipid labeling 2457 Fig. 4. SDS-FRL of human erythrocytes with JE-1. (a) Labeling of intact erythrocytes. The replicas of intact erythrocytes show that the E- faces (EF) are densely labeled with immunogold particles, while the P- faces (PF) are virtually unlabeled. (b) Labeling of 1 mm calcium/20 µm ionophore treated erythrocytes (10 minutes, 37 C). In contrast to intact erythrocytes, the labeling is observed on both the P-face (PF) and E-face (EF) revealed as smooth areas denuded of intramembrane particles. Cell attachment is frequently observed (arrowheads). (c) SDS-FRL of intact erythrocytes treated with phospholipase A 2. The immunogold labeling is completely diminished. Bars, 100 nm. CaCl 2 and 20 µm A23187 (Ca 2+ /ionophore) at 37 C for various times, frozen, and subjected to SDS-FRL for PC. Incubation with Ca 2+ /ionophore altered the fracture faces markedly. In contrast to intact erythrocytes, the IMPs of the P- and E-faces of Ca 2+ /ionophore-treated erythrocytes were not distributed randomly but were grouped in clusters. In addition to this morphological alteration, roughly equal amounts of immunogold particles for PC were seen on each membrane half in less than 10 minutes (Fig. 4b). These gold particles were confined to the intervening smooth areas between the clusters of IMPs. Cell attachment was frequently observed, and occurred only between the smooth areas denuded of IMP (Fig. 4b, hollow arrows). Incubation with either Ca 2+ or ionophore alone did not affect the asymmetrical distribution of PC and did not change the freeze-fracture morphology (data not shown). The specificity of the PC labeling was confirmed through several control experiments. In replicas treated with phospholipase A2 for 1 hour at 37 C prior to cytochemical labeling, the labeling of the E-face was completely diminished (Fig. 4c). A similar result was obtained when the primary antibody was adsorbed with excess amounts of pure PC multilammellar liposomes prior to immunocytochemical labeling, or the primary antibody was omitted in the staining procedure (data not shown). These initial experiments conducted on erythrocyte membranes show the asymmetrical transmembrane distribution of PC and its redistribution under a certain condition, and provide compelling evidence for our working hypothesis, that membrane phospholipids of split membrane halves remain attached to the Pt/C replicas even after SDStreatment. Next, we examined the distribution of PC in various cells and intracellular organelles. The fine structure of the fracture face observed in the Pt/C replicas of various cells and tissues subjected to SDS-FRL was the same as that of the conventional freeze-fractured faces. Therefore, although the immunogold particles were actually localized on the surface of carbonstabilized membrane halves attached to the replicas, we used here the conventional nomenclature of freeze-fractured faces (Branton et al., 1975). In this nomenclature, the membrane leaflet (half) closer to the cytoplasm, nucleoplasm, or mitochondrial plasm (mitochondrial matrix), is designated as the protoplasmic membrane leaflet or P leaflet, and the membrane leaflet closer to the exoplasmic, endoplasmic, or perinuclear space (cisterna) is designated as the exoplasmic leaflet or E leaflet. Thus the fractured and replicated membrane surfaces of P leaflet and E leaflet are P- and E-faces, respectively. SDS-FRL using JE-1 revealed that the E-faces of most intracellular membranes of various cell organelles, e.g. nuclei (Fig. 5b), mitochondria (Fig. 5c), secretory granules (Fig. 5d), endoplasmic reticulum (Fig. 5e), and discs of the outer segment of photoreceptor cells (Fig. 5f), and the E-faces of plasma membranes (Figs 5a,g and 6a) except for apical plasma membranes were densely immunolabeled, while the P-faces of these membranes generally displayed weaker labeling (Fig. 5a and h). All the results on the transmembrane distribution of PC obtained from the SDS-FRL clearly showed the preferential
6 2458 K. Fujimoto, M. Umeda and T. Fujimoto Fig. 5. Electron micrographs of rat hepatocytes (a-c), rat pancreatic acinar cells (d,e), and mouse retinal photoreceptor cells (f-h) processed for SDS-FRL with anti-pc monoclonal antibody, JE-1. The E-faces (EF) of most cellular membranes of intracellular organelles, e.g. nuclei (b), mitochondria (c), secretory granules (zymogen granules) (d), endoplasmic reticulum (e), and discs of the outer segment of photoreceptor cells (light-adapted; f) are densely labeled. The E-faces (EF) of most plasma membranes (a and g) are exclusively labeled. (a) This micrograph depicts the plasma membranes of adjacent hepatocytes. The fracture plane has jumped from one membrane to an adjacent membrane, thus both the E- (EF) and P-faces (PF) are present side by side. (b) The nuclear envelope consists of inner and outer nuclear membranes that are continuous at the margin of nuclear pores (arrowheads). The cavity between the inner and outer nuclear membranes is called the perinuclear space or cisterna (arrows). The fracture plane jumps from the less particulate E-face (EF) of the outer nuclear membrane to the more particulate P-face (PF) of the inner nuclear membrane. (c) Mitochondria are composed of two membranes, and in this freeze-fracture both the E-face (EF) of the outer mitochondrial membrane and the P-face (PF) of the inner mitochondrial membrane are present. More IMPs remain associated with the P-face than with the E-face. (d) The gently curving, convex E-face (EF) of the secretory granule (zymogen granule) membrane is easily distinguished from the concave P-face of the secretory granule membrane. (e) Oblique freeze-fracture through long narrow cisternae (arrowheads) of rough endoplasmic reticulum of rat pancreatic acinar cell. The cytoplasmic leaflet (PF) retains the majority of the IMPs, whereas the exoplasmic leaflet (EF) is largely devoid of particles. Cytoplasm (cyt) is present around the cisternae. (f) This micrograph illustrates the flattened membranous discs that are stacked in the rod outer segment of the photoreceptor cell (light-adapted). The E-faces (EF) of the disc membranes are densely labeled. (g and h) These micrographs show the labeling of JE-1 on the plasma membranes of mouse photoreceptor cells. There are two types of fracture faces associated with the outer segment plasma membrane in the retina, one concave E-face (g) and one convex P-face (h). As previously reported (Sjöstrand and Kreman, 1979), the P-faces show a particulate structure with dense arrangement of the particles in the range 8-10 nm in diameter. The particulate surface is interrupted by a smooth surface structure (h, arrows). The concave E-faces show some structure with particularly smooth areas (g, square brackets) surrounded by areas exposing a very fine particulate structure. The labeling is revealed as aggregates of immunogold particles on the particularly smooth areas (g, square brackets), while no labeling is observed on the P-face (h). There is no difference in the labeling of PC on the disc membranes and the plasma membranes between light- and dark-adapted photoreceptor cells (data not shown). Arrowheads indicate the cross-fractured disc membranes. Bars, 100 nm.
7 Membrane phospholipid labeling 2459 Fig. 6. SDS-FRL of rat pancreatic acinar cells (a,b) and myelin sheath (c) with JE-1. The labeling is not seen on the E-face of apical plasma membranes of the intact pancreatic acinar cells (a, ap), while the labeling can be observed on the E-face of that of unfixed, glycerol-impregnated (final concentration 30%; for 1 hour at 4 C) cells (b, ap). Arrow indicates desmosomes. (c) Labeling of myelin from rat optic nerve with JE-1. Although most of the fractured face of myelin exposed by freezefracturing are labeled, the P-face of the plasma membrane of the axon (AX) is not labeled with JE-1 (arrowheads). Bars, 100 nm. localization of PC to the exoplasmic membrane half (leaflet) of the cellular membrane. One exception to this general scheme was the apical (luminal) plasma membrane of glandular epithelial cells such as pancreatic acinar cells and the plasma membrane forming the myelin of neurons. In SDS-FRL of pancreatic acinar cells with JE-1, both the P- and E-faces of apical plasma membranes were virtually unlabeled (Fig. 6a). These findings are consistent with previous biochemical studies (Simons and van Meer, 1988) on the purified apical membrane fractions, suggesting that the apical plasma membrane is poor in PC, when compared to the basolateral plasma membrane. The plasma membrane of epithelial cells is divided into an apical and a basolateral domain by tight junctions (Fig. 6, TJ) that encircle the apex of each cell. Because it seemed likely that the tight junctions block membrane lipid diffusion, we next studied glycerol-impregnated material. Radical, qualitative changes in the ultrastructure of the tight junctions are observed as a result of differences in fixation and glycerol-impregnation (Pinto da Silva and Kachar, 1982). Thus, we expected that a barrier formed by the tight junctions to the diffusion of lipids in the exoplasmic (outer) membrane half might be affected by glycerol-impregnation. Interestingly, in chemically unfixed, glycerol-impregnated pancreatic acinar cells, the PC-immunogold complexes were detected on the E-faces of the apical plasma membranes as well as on the E-faces of the lateral plasma membranes (Fig. 6b). This implies that the tight-junctional barrier is broken by glycerol-impregnation, thus PC can freely diffuse between the lateral and apical plasma membranes. The myelin sheath of myelinated nerves is a morphologically and biochemically specialized membranous structure, as it contains exceptionally high levels of lipid, and very low levels of protein (Guidotti, 1972). Thus, this could be a useful model for our study. Interestingly, the immunogold labeling of PC was observed on both membrane halves (Fig. 6c), suggesting that the myelin sheath, with the symmetrical distribution of phospholipids, at least PC, is a unique example. Although Ca 2+ is involved in many biological membrane fusion phenomena (Hoekstra et al., 1985), the mechanism of its action is not well understood. When erythrocytes were incubated with Ca 2+ and ionophore, many cells came into sufficiently close contact, but the adjacent plasma membranes were not fused. In addition to the morphological event, at this stage, PC is distributed evenly in both membrane halves. Another example is the myelin sheath of the myelinated nerve with its indefinitely stable, extensive and extremely close contact of adjacent cell membranes. The SDS-FRL with JE-1 showed that in the myelin, the phospholipid distribution is almost symmetrical. These findings suggest, that the symmetrical distribution of phospholipids, at least PC, may be adapted
8 2460 K. Fujimoto, M. Umeda and T. Fujimoto to cell membrane contact. Further analysis on the distribution and movement of phospholipids using SDS-FRL, which occurs during membrane contact and fusion induced by various fusogens, will help elucidate the chemical property of membranes fusing to adjacent membranes. Conventional freeze-fracture replica electron microscopy reveals the precise macromolecular architecture of the membranes, but offers little information on the biochemical composition of cell membrane components. The SDS-FRL technique overcomes this obstacle, and may be useful both for ultrastructural observation and for the membrane lipid and protein cytochemistry of the biological membranes. In this study, SDS-FRL revealed that PC is exclusively localized on the exoplasmic membrane halves of the plasma membranes, and the intracellular membranes of various cell organelles. These findings seem to imply a nearly complete asymmetrical transmembrane distribution of PC in the membranes. However, there is an alternative explanation for the absence of labeling on the protoplasmic membrane halves; a peripheral membrane protein, a cytosolic protein matrix (e.g. membrane cytoskeleton), or an integral membrane protein, remains attached to the protoplasmic membrane half after SDS-treatment, masks PC and inhibits antibody binding. We cannot exclude this possibility at the present time. We gratefully acknowledge the helpful discussions with Drs Toru Noda and Masayuki Murata. We thank Drs Chizuka Ide and Kazuo Ogawa for their encouragement throughout this study. We thank Miss Sawako Nakamura and Kasumi Nomura for their secretarial assistance, and Mr Akira Uesugi for his help with the photographic work. We, and Dr K. Fujimoto in particular, want to express appreciation to Dr Masako Fujimoto for her excellent technical assistance. This work was supported by a research grant from the Ministry of Education, Science and Culture of Japan (to K. Fujimoto). REFERENCES Andersson-Forsman, C. and Pinto da Silva, P. (1988). Fracture-flip: new high-resolution images of cell surfaces after carbon stabilization of freezefractured membranes. J. Cell Sci. 90, Branton, D., Bullivant, S., Gilula, N. B., Karnovsky, M. J., Moor, H., Muhlethaler, K., Northcote, D. H., Packer, L., Satir, P., Speth, V., Staehelin, L. A., Steere, R. L. and Weinstein, R. S. (1975). Freeze-etching nomenclature. Science 190, Fujimoto, K. and Pinto da Silva, P. (1988). Macromolecular dynamics of the cell surface during the formation of coated pits is revealed by fracture-flip. J. Cell Sci. 91, Fujimoto, K. and Pinto da Silva, P. (1989). Surface views of nuclear pores in isolated rat liver nuclei as revealed by fracture-flip/triton-x. Eur. J. Cell Biol. 50, Fujimoto, K. and Ogawa, K. (1991). Fracture-flip and fracture-flip cytochemistry to study macromolecular architecture of membrane surfaces: practical procedures, interpretation and application. Acta Histochem. Cytochem. 24, Fujimoto, K. and Pinto da Silva, P. (1992). Fracture-flip/Triton X-100 reveals the cytoplasmic surface of human erythrocyte membranes. Acta Histochem. Cytochem. 25, Fujimoto, K. (1995). Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J. Cell Sci. 108, Guidotti, G. (1972). Membrane proteins. Annu. Rev. Biochem. 41, Heuser, J. E., Reese, T. S., Jan, L. Y., Dennis, M. J. and Evans, L. (1979). Synaptic vesicle exocytosis captured by quick-freezing and correlated with quantal transmitter release. J. Cell Biol. 81, Hoekstra, D., Wilschut, J. and Scherphof, G. (1985). Fusion of erythrocyte ghosts induced by calcium phosphate. Kinetic characteristics and the role of Ca 2+, phosphate and calcium-phosphate complexes. Eur. J. Biochem. 146, Kinsky, S. C., Haxby, J. A., Zopf, D. A., Alving, C. R. and Kinsky, C. B. (1969). Complement-dependent damage to liposomes prepared from pure lipids and Forssman hapten. Biochemistry 8, Nam, K. S., Igarashi, K., Umeda, M. and Inoue, K. (1990). Production and characterization of monoclonal antibodies that specifically bind to phosphatidylcholine. Biochim. Biophys. Acta 1046, Pinto da Silva, P. and Kachar, B. (1982). On tight-junction structure. Cell 28, Schrier, S. L., Zachowski, A., Herve, P., Kader, J.-P. and Devaux, P. F. (1992). Transmembrane redistribution of phospholipids of the human red cell membrane during hypotonic hemolysis. Biochim. Biophys. Acta 1105, Simons, K. and van Meer, G. (1988). Lipid sorting in epithelial cells. Biochemistry 27, Sjöstrand, F. S. and Kreman, M. (1979). Freeze-fracture analysis of structure of plasma membrane of photoreceptor cell outer segments. J. Ultrastruct. Res. 66, Williamson, P., Kulick, A., Zachowski, A., Schlegel, R. A. and Devaux, P. F. (1992). Ca 2+ induces transbilayer redistribution of all major phospholipids in human erythrocytes. Biochemistry 31, Zachowski, A. (1993). Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem. J. 294, Zhou, X. and Arthur, G. (1992). Improved procedures for the determination of lipid phosphorus by malachite green. J. Lipid Res. 33, (Received 16 August Accepted, in revised form, 27 June 1996)
PORE-LIKE STRUCTURES IN BIOLOGICAL MEMBRANES
 J. Cell Sci. 25, 157-161 (1977) 157 Printed in Great Britain PORE-LIKE STRUCTURES IN BIOLOGICAL MEMBRANES L. ORCI, A. PERRELET, FRANCINE MALAISSE-LAGAE AND P. VASSALLI* Institute of Histology and Embryology,
J. Cell Sci. 25, 157-161 (1977) 157 Printed in Great Britain PORE-LIKE STRUCTURES IN BIOLOGICAL MEMBRANES L. ORCI, A. PERRELET, FRANCINE MALAISSE-LAGAE AND P. VASSALLI* Institute of Histology and Embryology,
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
A COMPARISON OF MEMBRANE FRACTURE FACES OF FIXED AND UNFIXED GLYCERINATED TISSUE
 J. Cell Set. 21, 437-448 (1976) 43-7 Printed in Great Britain A COMPARISON OF MEMBRANE FRACTURE FACES OF FIXED AND UNFIXED GLYCERINATED TISSUE A. S. BREATHNACH, M. GROSS, B. MARTIN AND C. STOLINSKI Department
J. Cell Set. 21, 437-448 (1976) 43-7 Printed in Great Britain A COMPARISON OF MEMBRANE FRACTURE FACES OF FIXED AND UNFIXED GLYCERINATED TISSUE A. S. BREATHNACH, M. GROSS, B. MARTIN AND C. STOLINSKI Department
Cell Overview. Hanan Jafar BDS.MSc.PhD
 Cell Overview Hanan Jafar BDS.MSc.PhD THE CELL is made of: 1- Nucleus 2- Cell Membrane 3- Cytoplasm THE CELL Formed of: 1. Nuclear envelope 2. Chromatin 3. Nucleolus 4. Nucleoplasm (nuclear matrix) NUCLEUS
Cell Overview Hanan Jafar BDS.MSc.PhD THE CELL is made of: 1- Nucleus 2- Cell Membrane 3- Cytoplasm THE CELL Formed of: 1. Nuclear envelope 2. Chromatin 3. Nucleolus 4. Nucleoplasm (nuclear matrix) NUCLEUS
Basophilic. Basophilic structures are stained by basic dyes: Mnemonic: Basophilic = Blue
 Cell Overview Basophilic Basophilic structures are stained by basic dyes: Basic dyes are positive Basophilic structures are negative (ex. DNA, RNA, ribosomes, RER) Mnemonic: Basophilic = Blue Acidophilic
Cell Overview Basophilic Basophilic structures are stained by basic dyes: Basic dyes are positive Basophilic structures are negative (ex. DNA, RNA, ribosomes, RER) Mnemonic: Basophilic = Blue Acidophilic
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
This week s topic will be: Evidence for the Fluid Mosaic Model. Developing theories, testing hypotheses and techniques for visualizing cells
 Tutorials, while not mandatory, will allow you to improve your final grade in this course. Thank you for your attendance to date. These notes are not a substitute for the discussions that we will have
Tutorials, while not mandatory, will allow you to improve your final grade in this course. Thank you for your attendance to date. These notes are not a substitute for the discussions that we will have
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
The Annexin V Apoptosis Assay
 The Annexin V Apoptosis Assay Development of the Annexin V Apoptosis Assay: 1990 Andree at al. found that a protein, Vascular Anticoagulant α, bound to phospholipid bilayers in a calcium dependent manner.
The Annexin V Apoptosis Assay Development of the Annexin V Apoptosis Assay: 1990 Andree at al. found that a protein, Vascular Anticoagulant α, bound to phospholipid bilayers in a calcium dependent manner.
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Cell Structure. Present in animal cell. Present in plant cell. Organelle. Function. strength, resist pressure created when water enters
 Cell Structure Though eukaryotic cells contain many organelles, it is important to know which are in plant cells, which are in animal cells and what their functions are. Organelle Present in plant cell
Cell Structure Though eukaryotic cells contain many organelles, it is important to know which are in plant cells, which are in animal cells and what their functions are. Organelle Present in plant cell
COMPLEMENTARY PLASMA MEMBRANE FRACTURE FACES IN FREEZE-ETCH REPLICAS
 J. Cell Set. 12, 445-452 (1973) 445 Printed in Great Britain COMPLEMENTARY PLASMA MEMBRANE FRACTURE FACES IN FREEZE-ETCH REPLICAS N. E. FLOWER Physics and Engineering Laboratory, Department of Scientific
J. Cell Set. 12, 445-452 (1973) 445 Printed in Great Britain COMPLEMENTARY PLASMA MEMBRANE FRACTURE FACES IN FREEZE-ETCH REPLICAS N. E. FLOWER Physics and Engineering Laboratory, Department of Scientific
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Methods of studying membrane structure
 King Saud University College of Science Department of Biochemistry Biomembranes and Cell Signaling (BCH 452) Chapter 2 Methods of studying membrane structure Prepared by Dr. Farid Ataya http://fac.ksu.edu.sa/fataya
King Saud University College of Science Department of Biochemistry Biomembranes and Cell Signaling (BCH 452) Chapter 2 Methods of studying membrane structure Prepared by Dr. Farid Ataya http://fac.ksu.edu.sa/fataya
Cell Structure and Function. Biology 12 Unit 1 Cell Structure and Function Inquiry into Life pages and 68-69
 Cell Structure and Function Biology 12 Unit 1 Cell Structure and Function Inquiry into Life pages 45 59 and 68-69 Assignments for this Unit Pick up the notes/worksheet for this unit and the project There
Cell Structure and Function Biology 12 Unit 1 Cell Structure and Function Inquiry into Life pages 45 59 and 68-69 Assignments for this Unit Pick up the notes/worksheet for this unit and the project There
20X Buffer (Tube1) 96-well microplate (12 strips) 1
 PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
PROTOCOL MitoProfile Rapid Microplate Assay Kit for PDH Activity and Quantity (Combines Kit MSP18 & MSP19) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP20 Rev.1 DESCRIPTION MitoProfile Rapid Microplate
CELLS and TRANSPORT Student Packet SUMMARY CELL MEMBRANES ARE SELECTIVELY PERMEABLE DUE TO THEIR STRUCTURE Hydrophilic head
 CELLS and TRANSPORT Student Packet SUMMARY CELL MEMBRANES ARE SELECTIVELY PERMEABLE DUE TO THEIR STRUCTURE Hydrophilic head Hydrophobic tail Hydrophobic regions of protein Hydrophilic regions of protein
CELLS and TRANSPORT Student Packet SUMMARY CELL MEMBRANES ARE SELECTIVELY PERMEABLE DUE TO THEIR STRUCTURE Hydrophilic head Hydrophobic tail Hydrophobic regions of protein Hydrophilic regions of protein
PLASMA MEMBRANE. Submitted by:- DR.Madhurima Sharma PGGCG-II,Chandigarh
 PLASMA MEMBRANE Submitted by:- DR.Madhurima Sharma PGGCG-II,Chandigarh LIPID COMPONENTS OF THE PLASMA MEMBRANE The outer leaflet consists predominantly of phosphatidylcholine, sphingomyelin, and glycolipids,
PLASMA MEMBRANE Submitted by:- DR.Madhurima Sharma PGGCG-II,Chandigarh LIPID COMPONENTS OF THE PLASMA MEMBRANE The outer leaflet consists predominantly of phosphatidylcholine, sphingomyelin, and glycolipids,
COR 011 Lecture 9: ell membrane structure ept 19, 2005
 COR 011 Lecture 9: ell membrane structure ept 19, 2005 Cell membranes 1. What are the functions of cell membranes? 2. What is the current model of membrane structure? 3. Evidence supporting the fluid mosaic
COR 011 Lecture 9: ell membrane structure ept 19, 2005 Cell membranes 1. What are the functions of cell membranes? 2. What is the current model of membrane structure? 3. Evidence supporting the fluid mosaic
Human Epithelial Cells
 The Cell Human Epithelial Cells Plant Cells Cells have an internal structure Eukaryotic cells are organized Protective membrane around them that communicates with other cells Organelles have specific jobs
The Cell Human Epithelial Cells Plant Cells Cells have an internal structure Eukaryotic cells are organized Protective membrane around them that communicates with other cells Organelles have specific jobs
Cell Physiology
 Cell Physiology 21-10-2018 1 The two major parts of a typical cell are the nucleus and the cytoplasm. The nucleus is separated from the cytoplasm by a nuclear membrane, and the cytoplasm is separated from
Cell Physiology 21-10-2018 1 The two major parts of a typical cell are the nucleus and the cytoplasm. The nucleus is separated from the cytoplasm by a nuclear membrane, and the cytoplasm is separated from
AET-treated normal red cells (PNH-like cells)
 J. clin. Path., 1971, 24, 677-684 Electron microscope study of PNH red cells and AET-treated normal red cells (PNH-like cells) S. M. LEWIS, G. LAMBERTENGHI, S. FERRONE, AND G. SIRCHIA From the Department
J. clin. Path., 1971, 24, 677-684 Electron microscope study of PNH red cells and AET-treated normal red cells (PNH-like cells) S. M. LEWIS, G. LAMBERTENGHI, S. FERRONE, AND G. SIRCHIA From the Department
Part I => CARBS and LIPIDS. 1.5 Biological Membranes 1.5a Artificial Membranes 1.5b Lipid Bilayers
 Part I => CARBS and LIPIDS 1.5 Biological Membranes 1.5a Artificial Membranes 1.5b Lipid Bilayers Section 1.5a: Artificial Membranes Synopsis 1.5a - In water, apolar molecules such as fats and oils (eg
Part I => CARBS and LIPIDS 1.5 Biological Membranes 1.5a Artificial Membranes 1.5b Lipid Bilayers Section 1.5a: Artificial Membranes Synopsis 1.5a - In water, apolar molecules such as fats and oils (eg
Electronic Supporting Information
 Modulation of raft domains in a lipid bilayer by boundary-active curcumin Manami Tsukamoto a, Kenichi Kuroda* b, Ayyalusamy Ramamoorthy* c, Kazuma Yasuhara* a Electronic Supporting Information Contents
Modulation of raft domains in a lipid bilayer by boundary-active curcumin Manami Tsukamoto a, Kenichi Kuroda* b, Ayyalusamy Ramamoorthy* c, Kazuma Yasuhara* a Electronic Supporting Information Contents
Chapter 12: Membranes. Voet & Voet: Pages
 Chapter 12: Membranes Voet & Voet: Pages 390-415 Slide 1 Membranes Essential components of all living cells (define boundry of cells) exclude toxic ions and compounds; accumulation of nutrients energy
Chapter 12: Membranes Voet & Voet: Pages 390-415 Slide 1 Membranes Essential components of all living cells (define boundry of cells) exclude toxic ions and compounds; accumulation of nutrients energy
Membranes. Chapter 5
 Membranes Chapter 5 Membrane Structure The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membranes Chapter 5 Membrane Structure The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membrane Structure. Membrane Structure. Membrane Structure. Membranes
 Membrane Structure Membranes Chapter 5 The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membrane Structure Membranes Chapter 5 The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Chapter 7 Notes. Section 1
 Chapter 7 Notes Section 1 Cells Cells remained out of sight during most of human history until the invention of the first microscopes. It was not until the mid 1600s that scientists began to use microscopes
Chapter 7 Notes Section 1 Cells Cells remained out of sight during most of human history until the invention of the first microscopes. It was not until the mid 1600s that scientists began to use microscopes
Starch grains - excess sugars
 (a) Membrane system - site of light reactions (photosynthesis) - chlorpophyll pigments - enzymes - electron carriers - flattened, fluid-filled sacs (called thylakoids which are stacked to form grana) -
(a) Membrane system - site of light reactions (photosynthesis) - chlorpophyll pigments - enzymes - electron carriers - flattened, fluid-filled sacs (called thylakoids which are stacked to form grana) -
3UNIT. Photosynthesis and. Cellular Respiration. Unit PreQuiz? General Outcomes. Unit 3 Contents. Focussing Questions
 3UNIT Photosynthesis and Cellular Respiration General Outcomes In this unit, you will relate photosynthesis to the storage of energy in organic compounds explain the role of cellular respiration in releasing
3UNIT Photosynthesis and Cellular Respiration General Outcomes In this unit, you will relate photosynthesis to the storage of energy in organic compounds explain the role of cellular respiration in releasing
Membranes. Chapter 5. Membrane Structure
 Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Yara shwabkeh. Osama Alkhader. Heba Kalbouneh
 2 Yara shwabkeh Osama Alkhader Heba Kalbouneh CELL OVERVIEW -Note ; the important thing is to know how the organelles appear under the microscope - the stains we usually use in Histology are composed of
2 Yara shwabkeh Osama Alkhader Heba Kalbouneh CELL OVERVIEW -Note ; the important thing is to know how the organelles appear under the microscope - the stains we usually use in Histology are composed of
MEMBRANE STRUCTURE. Lecture 8. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
1 MEMBRANE STRUCTURE Lecture 8 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University Plasma Membrane 2 Plasma membrane: The outer boundary of the cell that separates it from the world
Cell Membranes Valencia college
 6 Cell Membranes Valencia college 6 Cell Membranes Chapter objectives: The Structure of a Biological Membrane The Plasma Membrane Involved in Cell Adhesion and Recognition Passive Processes of Membrane
6 Cell Membranes Valencia college 6 Cell Membranes Chapter objectives: The Structure of a Biological Membrane The Plasma Membrane Involved in Cell Adhesion and Recognition Passive Processes of Membrane
BIOL 158: BIOLOGICAL CHEMISTRY II
 BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
Cell Structure & Function. Source:
 Cell Structure & Function Source: http://koning.ecsu.ctstateu.edu/cell/cell.html Definition of Cell A cell is the smallest unit that is capable of performing life functions. http://web.jjay.cuny.edu/~acarpi/nsc/images/cell.gif
Cell Structure & Function Source: http://koning.ecsu.ctstateu.edu/cell/cell.html Definition of Cell A cell is the smallest unit that is capable of performing life functions. http://web.jjay.cuny.edu/~acarpi/nsc/images/cell.gif
CELL PARTS TYPICAL ANIMAL CELL
 AP BIOLOGY CText Reference, Campbell v.8, Chapter 6 ACTIVITY1.12 NAME DATE HOUR CELL PARTS TYPICAL ANIMAL CELL ENDOMEMBRANE SYSTEM TYPICAL PLANT CELL QUESTIONS: 1. Write the name of the cell part in the
AP BIOLOGY CText Reference, Campbell v.8, Chapter 6 ACTIVITY1.12 NAME DATE HOUR CELL PARTS TYPICAL ANIMAL CELL ENDOMEMBRANE SYSTEM TYPICAL PLANT CELL QUESTIONS: 1. Write the name of the cell part in the
Eukaryotic Cell Structure
 Eukaryotic Cell Structure Vocabulary listed for Chapter 7.3: cell wall, chromatin, nucleolus, ribosome, cytoplasm, endoplasmic reticulum, Golgi apparatus, vacuole, lysosome, chloroplast, plastid, chlorophyll,
Eukaryotic Cell Structure Vocabulary listed for Chapter 7.3: cell wall, chromatin, nucleolus, ribosome, cytoplasm, endoplasmic reticulum, Golgi apparatus, vacuole, lysosome, chloroplast, plastid, chlorophyll,
NOTES. Miami, Florida pores. from William Rawls, Baylor College of Medicine, of penicillin per ml, and 100,g of streptomycin
 JOURNAL OF VIROLOGY. Mar. 1976. p. 1038-1042 Copyright i 1976 American Society for Microbiology NOTES Vol. 17, No. 3 Printed in U.S.A. Nuclear Membrane Changes in Herpes Simplex Virus-Infected BHK-21 Cells
JOURNAL OF VIROLOGY. Mar. 1976. p. 1038-1042 Copyright i 1976 American Society for Microbiology NOTES Vol. 17, No. 3 Printed in U.S.A. Nuclear Membrane Changes in Herpes Simplex Virus-Infected BHK-21 Cells
1.4 Page 1 Cell Membranes S. Preston 1
 AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
RICHARD L. WOOD. From the Department of Biological Structure, University of Miami School of Medicine, Miami, Florida 33152
 A CLOSELY PACKED ARRAY OF MEMBRANE INTERCALATED PARTICLES AT THE FREE SURFACE OF HYDRA RICHARD L. WOOD. From the Department of Biological Structure, University of Miami School of Medicine, Miami, Florida
A CLOSELY PACKED ARRAY OF MEMBRANE INTERCALATED PARTICLES AT THE FREE SURFACE OF HYDRA RICHARD L. WOOD. From the Department of Biological Structure, University of Miami School of Medicine, Miami, Florida
I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins
 Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Lecture 6: Membranes and Cell Transport Biological Membranes I. Fluid Mosaic Model A. Biological membranes are lipid bilayers with associated proteins 1. Characteristics a. Phospholipids form bilayers
Biomembranes structure and function. B. Balen
 Biomembranes structure and function B. Balen All cells are surrounded by membranes Selective barrier But also important for: 1. Compartmentalization 2. Biochemical activities 3. Transport of dissolved
Biomembranes structure and function B. Balen All cells are surrounded by membranes Selective barrier But also important for: 1. Compartmentalization 2. Biochemical activities 3. Transport of dissolved
FOCUS SubCell. For the Enrichment of Subcellular Fractions. (Cat. # ) think proteins! think G-Biosciences
 169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
Renáta Schipp Gergely Berta Department of Medical Biology
 The cell III. Renáta Schipp Gergely Berta Department of Medical Biology Size and Biology Biology is a visually rich subject many of the biological events and structures are smaller than the unaided human
The cell III. Renáta Schipp Gergely Berta Department of Medical Biology Size and Biology Biology is a visually rich subject many of the biological events and structures are smaller than the unaided human
Structure & Function of Cells
 Anatomy & Physiology 101-805 Unit 4 Structure & Function of Cells Paul Anderson 2011 Anatomy of a Generalised Cell Attached or bound ribosomes Cilia Cytosol Centriole Mitochondrion Rough endoplasmic reticulum
Anatomy & Physiology 101-805 Unit 4 Structure & Function of Cells Paul Anderson 2011 Anatomy of a Generalised Cell Attached or bound ribosomes Cilia Cytosol Centriole Mitochondrion Rough endoplasmic reticulum
Thursday, October 16 th
 Thursday, October 16 th Good morning. Those of you needing to take the Enzymes and Energy Quiz will start very soon. Students who took the quiz Wednesday: Please QUIETLY work on the chapter 6 reading guide.
Thursday, October 16 th Good morning. Those of you needing to take the Enzymes and Energy Quiz will start very soon. Students who took the quiz Wednesday: Please QUIETLY work on the chapter 6 reading guide.
Notes Chapter 7 Cell Structure and Function Hooke looked at cork under a simple microscope and found tiny chambers he named cells.
 Notes Chapter 7 Cell Structure and Function 7.1 Cell discovery and Theory 1665 Hooke looked at cork under a simple microscope and found tiny chambers he named cells. Cells are the basic structural and
Notes Chapter 7 Cell Structure and Function 7.1 Cell discovery and Theory 1665 Hooke looked at cork under a simple microscope and found tiny chambers he named cells. Cells are the basic structural and
Biology 4410 First Examination Version B
 Biology 4410 Spring 2006 Name First Examination Version B This examination consists of two parts, a multiple-choice section and an essay section. Be sure to put your name on both the mark-sense sheet and
Biology 4410 Spring 2006 Name First Examination Version B This examination consists of two parts, a multiple-choice section and an essay section. Be sure to put your name on both the mark-sense sheet and
Cells: The Living Units
 Cells: The Living Units Introduction Life in general occurs in an aqueous environment All chemical processes essential to life occur within the aqueous environment of the cell and surrounding fluids contained
Cells: The Living Units Introduction Life in general occurs in an aqueous environment All chemical processes essential to life occur within the aqueous environment of the cell and surrounding fluids contained
Explain the reason for this difference in resolving power.
 1. (a) An electron microscope has a much greater resolving power than an optical microscope. (i) Explain the meaning of the term resolving power. Explain the reason for this difference in resolving power.
1. (a) An electron microscope has a much greater resolving power than an optical microscope. (i) Explain the meaning of the term resolving power. Explain the reason for this difference in resolving power.
The Cytoplasm Li Shulei Department of Histology & Embryology
 The Cytoplasm Li Shulei lishulei@tom.com Department of Histology & Embryology Cell components Cytoplasm Plasma membrane Organelles Cytoplasmic deposits Cytoskeleton Cytosol ( Matrix ) Nucleus Plasma membrane
The Cytoplasm Li Shulei lishulei@tom.com Department of Histology & Embryology Cell components Cytoplasm Plasma membrane Organelles Cytoplasmic deposits Cytoskeleton Cytosol ( Matrix ) Nucleus Plasma membrane
Cells. Unit 3 Cell Structure and Function. Cells. Plasma Membrane
 Unit 3 Cell Structure and Function Cells Cell theory The cell is the basic unit of life The cells of all living things exhibit the seven characteristics of life All living things are made of cells Cells
Unit 3 Cell Structure and Function Cells Cell theory The cell is the basic unit of life The cells of all living things exhibit the seven characteristics of life All living things are made of cells Cells
LIFE IS CELLULAR. Cell Theory. Cells Are Small. Prokaryotic Cell 10/4/15. Chapter 7 Cell Structure and Function
 Chapter 7 Cell Structure and Function The cell basic unit of life, all living things are made of a cell (unicellular) or more than one cell (multicellular). LIFE IS CELLULAR The invention of the microscope
Chapter 7 Cell Structure and Function The cell basic unit of life, all living things are made of a cell (unicellular) or more than one cell (multicellular). LIFE IS CELLULAR The invention of the microscope
Cellular Structure and Function. Chapter 7
 Cellular Structure and Function. Chapter 7 Cell Discovery and Theory. A cell is the basic structural and functional unit of all living organisms. The human body is made of trillions of cells that are too
Cellular Structure and Function. Chapter 7 Cell Discovery and Theory. A cell is the basic structural and functional unit of all living organisms. The human body is made of trillions of cells that are too
BIOLOGY 111. CHAPTER 3: The Cell: The Fundamental Unit of Life
 BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
BIOLOGY 111 CHAPTER 3: The Cell: The Fundamental Unit of Life The Cell: The Fundamental Unit of Life Learning Outcomes 3.1 Explain the similarities and differences between prokaryotic and eukaryotic cells
The Cell. Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62)
 The Cell Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62) Outline I. Prokaryotic vs. Eukaryotic II. Eukaryotic A. Plasma membrane transport across B. Main features of animal cells and their functions
The Cell Biology 105 Lecture 4 Reading: Chapter 3 (pages 47 62) Outline I. Prokaryotic vs. Eukaryotic II. Eukaryotic A. Plasma membrane transport across B. Main features of animal cells and their functions
10/13/11. Cell Theory. Cell Structure
 Cell Structure Grade 12 Biology Cell Theory All organisms are composed of one or more cells. Cells are the smallest living units of all living organisms. Cells arise only by division of a previously existing
Cell Structure Grade 12 Biology Cell Theory All organisms are composed of one or more cells. Cells are the smallest living units of all living organisms. Cells arise only by division of a previously existing
PMT. Contains ribosomes attached to the endoplasmic reticulum. Genetic material consists of linear chromosomes. Diameter of the cell is 1 µm
 1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice
 CELL STRUCTURE AND FUNCTION 8, 339-346 (1984) C by Japan Society for Cell Biology Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice Ikuo Yamaoka, Sumie Katsuta and Yoshimi
CELL STRUCTURE AND FUNCTION 8, 339-346 (1984) C by Japan Society for Cell Biology Silver-Impregnation of the Golgi Complex in Epididymal Epithelial Cells of Mice Ikuo Yamaoka, Sumie Katsuta and Yoshimi
Cytoskeleton. Provide shape and support for the cell. Other functions of the cytoskeleton. Nucleolus. Nucleus
 Chapter 4: Cell Structure and Function Cytoskeleton The cytoskeleton is a network of fibers that organizes structures and activities in the cell. Microtubules (the largest) Intermediate fibers Microfilaments
Chapter 4: Cell Structure and Function Cytoskeleton The cytoskeleton is a network of fibers that organizes structures and activities in the cell. Microtubules (the largest) Intermediate fibers Microfilaments
Chapter 7: Membrane Structure & Function
 Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Chapter 7: Membrane Structure & Function. 1. Membrane Structure. What are Biological Membranes? 10/21/2015. Why phospholipids? 1. Membrane Structure
 Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Cells & Tissues. Chapter 3
 Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Eukaryotic cells are essentially two envelope systems. Nuclear materials are separated from cytoplasm by nuclear membrane. Complex structure Also
 Dr. Gugale Pritesh Ramanlal M.Sc., Ph.D., B.Ed., D.M.L.T. Email id - pritesh.gugale09@gmail.com Contact numbernumber- 8446475310 Eukaryotic cells are essentially two envelope systems. Nuclear materials
Dr. Gugale Pritesh Ramanlal M.Sc., Ph.D., B.Ed., D.M.L.T. Email id - pritesh.gugale09@gmail.com Contact numbernumber- 8446475310 Eukaryotic cells are essentially two envelope systems. Nuclear materials
The Study of Cells The diversity of the cells of the body The following figure shows the proportion of cell size of the variety of cells in the body
 Adapted from Martini Human Anatomy 7th ed. Chapter 2 Foundations: The Cell Introduction There are trillions of cells in the body Cells are the structural building blocks of all plants and animals Cells
Adapted from Martini Human Anatomy 7th ed. Chapter 2 Foundations: The Cell Introduction There are trillions of cells in the body Cells are the structural building blocks of all plants and animals Cells
Peroxisomes. Endomembrane System. Vacuoles 9/25/15
 Contains enzymes in a membranous sac that produce H 2 O 2 Help survive environmental toxins including alcohol Help the cell use oxygen to break down fatty acids Peroxisomes Endo System Components of the
Contains enzymes in a membranous sac that produce H 2 O 2 Help survive environmental toxins including alcohol Help the cell use oxygen to break down fatty acids Peroxisomes Endo System Components of the
(d) are made mainly of lipids and of proteins that lie like thin sheets on the membrane surface
 Which of the following statements is no true? Biological membranes (a) are composed partly of amphipathic lipids (b) have hydrophobic and hydrophilic regions (c) are typically in a fluid state (d) are
Which of the following statements is no true? Biological membranes (a) are composed partly of amphipathic lipids (b) have hydrophobic and hydrophilic regions (c) are typically in a fluid state (d) are
Phospholipids. Extracellular fluid. Polar hydrophilic heads. Nonpolar hydrophobic tails. Polar hydrophilic heads. Intracellular fluid (cytosol)
 Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
The cell is the basic structural and functional unit of all organisms. The structure of cells is more or less the same in the simplest to the most
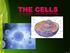 The cell is the basic structural and functional unit of all organisms. The structure of cells is more or less the same in the simplest to the most complicated plants and animals. Hand out pictures ANIMAL
The cell is the basic structural and functional unit of all organisms. The structure of cells is more or less the same in the simplest to the most complicated plants and animals. Hand out pictures ANIMAL
What Are Cell Membranes?
 What Are Cell Membranes? Chapter 5, Lesson 1 24 Directions Match each term in Column A with its meaning in Column B. Write the letter on the line. Column A 1. cytoplasm 2. cytosol 3. extracellular matrix
What Are Cell Membranes? Chapter 5, Lesson 1 24 Directions Match each term in Column A with its meaning in Column B. Write the letter on the line. Column A 1. cytoplasm 2. cytosol 3. extracellular matrix
FREEZE-ETCHED SURFACES OF MEMBRANES AND ORGANELLES IN THE CELLS OF PEA ROOT TIPS
 J. Cell Sci. 3, 199-206 (1968) I0.0. Printed in Great Britain FREEZE-ETCHED SURFACES OF MEMBRANES AND ORGANELLES IN THE CELLS OF PEA ROOT TIPS D. H. NORTHCOTE AND D. R. LEWIS Department of Biochemistry,
J. Cell Sci. 3, 199-206 (1968) I0.0. Printed in Great Britain FREEZE-ETCHED SURFACES OF MEMBRANES AND ORGANELLES IN THE CELLS OF PEA ROOT TIPS D. H. NORTHCOTE AND D. R. LEWIS Department of Biochemistry,
Cell Theory Vocabulary Flashcards
 Mr. Powner Biology Cell Theory Vocabulary Flashcards Instructions: Cut out the flashcards from the following pages. Use the following words to label the backside of the flashcards. The words are not listed
Mr. Powner Biology Cell Theory Vocabulary Flashcards Instructions: Cut out the flashcards from the following pages. Use the following words to label the backside of the flashcards. The words are not listed
Rama Abbady. Odai Bani-Monia. Diala Abu-Hassan
 5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
5 Rama Abbady Odai Bani-Monia Diala Abu-Hassan Lipid Rafts Lipid rafts are aggregates (accumulations) of sphingolipids. They re semisolid clusters (10-200 nm) of cholesterol and sphingolipids (sphingomyelin
PRE-LAB QUESTIONS: 1. What molecule makes up the bilayer of a cell s plasma membrane?
 LAB Name: Date Block A Bubblicious Membrane Model BACKGROUND INFORMATION: The cell s plasma membrane is a phospholipid bilayer with protein molecules imbedded in it. The protein molecules transport other
LAB Name: Date Block A Bubblicious Membrane Model BACKGROUND INFORMATION: The cell s plasma membrane is a phospholipid bilayer with protein molecules imbedded in it. The protein molecules transport other
AP Biology Cells: Chapters 4 & 5
 AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
AP Biology Cells: Chapters 4 & 5 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The was the first unifying principle of biology. a. spontaneous generation
Title: Sep 10 7:59 PM (1 of 36) Ch 3 Cell Organelles and Transport
 Title: Sep 10 7:59 PM (1 of 36) Ch 3 Cell Organelles and Transport Title: Sep 10 8:02 PM (2 of 36) Cell organelles Nucleus: contains DNA Title: Sep 10 8:03 PM (3 of 36) Nuclear envelope double membrane
Title: Sep 10 7:59 PM (1 of 36) Ch 3 Cell Organelles and Transport Title: Sep 10 8:02 PM (2 of 36) Cell organelles Nucleus: contains DNA Title: Sep 10 8:03 PM (3 of 36) Nuclear envelope double membrane
Chapter 4 Organization of the Cell
 Chapter 4 Organization of the Cell Cell basic unit of life o Small o Self-sufficient o Self-replicating Cell Theory organisms are composed of cells and all cells come from the division of other cells Cells
Chapter 4 Organization of the Cell Cell basic unit of life o Small o Self-sufficient o Self-replicating Cell Theory organisms are composed of cells and all cells come from the division of other cells Cells
Human height. Length of some nerve and muscle cells. Chicken egg. Frog egg. Most plant and animal cells Nucleus Most bacteria Mitochondrion
 10 m 1 m 0.1 m 1 cm Human height Length of some nerve and muscle cells Chicken egg Unaided eye 1 mm Frog egg 100 µm 10 µm 1 µm 100 nm 10 nm Most plant and animal cells Nucleus Most bacteria Mitochondrion
10 m 1 m 0.1 m 1 cm Human height Length of some nerve and muscle cells Chicken egg Unaided eye 1 mm Frog egg 100 µm 10 µm 1 µm 100 nm 10 nm Most plant and animal cells Nucleus Most bacteria Mitochondrion
Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE
 Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE The main aim of this module is to introduce the students to the types
Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE The main aim of this module is to introduce the students to the types
Cell Membranes and Signaling
 5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
Biology 12 Cell Structure and Function. Typical Animal Cell
 Biology 12 Cell Structure and Function Typical Animal Cell Vacuoles: storage of materials and water Golgi body: a series of stacked disk shaped sacs. Repackaging centre stores, modifies, and packages proteins
Biology 12 Cell Structure and Function Typical Animal Cell Vacuoles: storage of materials and water Golgi body: a series of stacked disk shaped sacs. Repackaging centre stores, modifies, and packages proteins
Renata Schipp Medical Biology Department
 Renata Schipp Medical Biology Department Deffinition of cell The cell is the smallest structural and functional unit of all known living organisms The cell was discovered by Robert Hooke in 1665 and also
Renata Schipp Medical Biology Department Deffinition of cell The cell is the smallest structural and functional unit of all known living organisms The cell was discovered by Robert Hooke in 1665 and also
Chapter 1 Plasma membranes
 1 of 5 TEXTBOOK ANSWERS Chapter 1 Plasma membranes Recap 1.1 1 The plasma membrane: keeps internal contents of the cell confined to one area keeps out foreign molecules that damage or destroy the cell
1 of 5 TEXTBOOK ANSWERS Chapter 1 Plasma membranes Recap 1.1 1 The plasma membrane: keeps internal contents of the cell confined to one area keeps out foreign molecules that damage or destroy the cell
Chapter 3: Cells. I. Overview
 Chapter 3: Cells I. Overview A. Characteristics 1. Basic structural/functional unit 2. Diameter is too small to see by the naked eye 3. Can be over 3 feet long 4. Trillions of cells in over 200 basic types
Chapter 3: Cells I. Overview A. Characteristics 1. Basic structural/functional unit 2. Diameter is too small to see by the naked eye 3. Can be over 3 feet long 4. Trillions of cells in over 200 basic types
Diabetologia 9 by Springer-Verlag 1978
 Diabetologia 15, 65-72 (1978) Diabetologia 9 by Springer-Verlag 1978 An Alteration in Internodal Myelin Membrane Structure in Large Sciatic Nerve Fibres in Rats with Acute Streptozotocin Diabetes and Impaired
Diabetologia 15, 65-72 (1978) Diabetologia 9 by Springer-Verlag 1978 An Alteration in Internodal Myelin Membrane Structure in Large Sciatic Nerve Fibres in Rats with Acute Streptozotocin Diabetes and Impaired
A ph-dependent Charge Reversal Peptide for Cancer Targeting
 Supporting Information A ph-dependent Charge Reversal Peptide for Cancer Targeting Naoko Wakabayashi 1, Yoshiaki Yano 1, Kenichi Kawano 1, and Katsumi Matsuzaki 1 1 Graduate School of Pharmaceutical Sciences,
Supporting Information A ph-dependent Charge Reversal Peptide for Cancer Targeting Naoko Wakabayashi 1, Yoshiaki Yano 1, Kenichi Kawano 1, and Katsumi Matsuzaki 1 1 Graduate School of Pharmaceutical Sciences,
1. (a) (i) Ability to distinguish points (close together); 1 (ii) Electrons have a shorter wavelength; 1
 1. (a) (i) Ability to distinguish points (close together); 1 Electrons have a shorter wavelength; 1 (b) (i) Golgi / nucleus / mitochondrion / endoplasmic reticulum / chromosome / larger ribosomes; R Membrane
1. (a) (i) Ability to distinguish points (close together); 1 Electrons have a shorter wavelength; 1 (b) (i) Golgi / nucleus / mitochondrion / endoplasmic reticulum / chromosome / larger ribosomes; R Membrane
CELL STRUCTURE AND FUNCTION. Chapter 7
 CELL STRUCTURE AND FUNCTION Chapter 7 WARM UP EXERCISE Please complete the pretest that you picked up as you came in. LIFE IS CELLULAR Robert Hooke- coined the term cells The Cell Theory All living things
CELL STRUCTURE AND FUNCTION Chapter 7 WARM UP EXERCISE Please complete the pretest that you picked up as you came in. LIFE IS CELLULAR Robert Hooke- coined the term cells The Cell Theory All living things
CELL MEMBRANES. CELL MEMBRANE- Structure and Function
 BIOLOGY 12 CELL MEMBRANES NAME: INTRODUCTION 1. The cell membrane the passage of molecules into and out of the cell. 2. Some types of molecules, particularly molecules, pass freely across the cell membrane
BIOLOGY 12 CELL MEMBRANES NAME: INTRODUCTION 1. The cell membrane the passage of molecules into and out of the cell. 2. Some types of molecules, particularly molecules, pass freely across the cell membrane
Unit 2: More on Matter & Energy in Ecosystems. Macromolecules to Organelles to Cells
 IN: Unit 2: More on Matter & Energy in Ecosystems Macromolecules to Organelles to Cells Where are cells on the biological scale? Sub-Atomic Particles Atoms Molecules Macromolecules (proteins, lipids, nucleic
IN: Unit 2: More on Matter & Energy in Ecosystems Macromolecules to Organelles to Cells Where are cells on the biological scale? Sub-Atomic Particles Atoms Molecules Macromolecules (proteins, lipids, nucleic
Cell Structure Animal/Human
 Cell Structure Animal/Human cell is basic unit of all life; structural and functional if its alive, must contain at least a single cell the function of an organism is the summation of functions of its
Cell Structure Animal/Human cell is basic unit of all life; structural and functional if its alive, must contain at least a single cell the function of an organism is the summation of functions of its
Cell Membranes. Dr. Diala Abu-Hassan School of Medicine Cell and Molecular Biology
 Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
Cell Membranes Dr. Diala Abu-Hassan School of Medicine Dr.abuhassand@gmail.com Cell and Molecular Biology Organelles 2Dr. Diala Abu-Hassan Membrane proteins Major components of cells Nucleic acids DNA
A. Major parts 1. Nucleus 2. Cytoplasm a. Contain organelles (see below) 3. Plasma membrane (To be discussed in Cellular Transport Lecture)
 Lecture 5: Cellular Biology I. Cell Theory Concepts: 1. Cells are the functional and structural units of living organisms 2. The activity of an organism is dependent on both the individual and collective
Lecture 5: Cellular Biology I. Cell Theory Concepts: 1. Cells are the functional and structural units of living organisms 2. The activity of an organism is dependent on both the individual and collective
10 The Golgi Apparatus: The First 100 Years
 2 Structure With no cell compartment or organelle has morphology served such a pivotal role in its discovery and investigation as with the apparatus of Golgi. The original description of the apparato reticulo
2 Structure With no cell compartment or organelle has morphology served such a pivotal role in its discovery and investigation as with the apparatus of Golgi. The original description of the apparato reticulo
Questions in Cell Biology
 Name: Questions in Cell Biology Directions: The following questions are taken from previous IB Final Papers on the subject of cell biology. Answer all questions. This will serve as a study guide for the
Name: Questions in Cell Biology Directions: The following questions are taken from previous IB Final Papers on the subject of cell biology. Answer all questions. This will serve as a study guide for the
Chapter 7. (7-1 and 7-2) A Tour of the Cell
 Chapter 7 (7-1 and 7-2) A Tour of the Cell Microscopes as Windows to the World of Cells Cells were first described in 1665 by Robert Hooke. By the mid-1800s, the accumulation of scientific evidence led
Chapter 7 (7-1 and 7-2) A Tour of the Cell Microscopes as Windows to the World of Cells Cells were first described in 1665 by Robert Hooke. By the mid-1800s, the accumulation of scientific evidence led
