Journal of Chromatography A, 1157 (2007) 51 55
|
|
|
- Kristopher Baldwin
- 5 years ago
- Views:
Transcription
1 Journal of Chromatography A, 1157 (2007) Extraction and ultra-performance liquid chromatography of hydrophilic and lipophilic bioactive components in a Chinese herb Radix Salviae Miltiorrhizae Mei Liu a,b, Yongguo Li b,c,, Guixin Chou b,c, Xuemei Cheng c, Mian Zhang a, Zhengtao Wang a,b,c, a Department of Pharmacognosy, China Pharmaceutical University, Nanjing , China b Key Laboratory of Standardization of Chinese Medicines of Ministry of Education, Shanghai University of Traditional Chinese Medicine, Shanghai , China c Shanghai R&D Centre for Standardization of Chinese Medicines, Shanghai , China Received 18 October 2006; received in revised form 21 April 2007; accepted 7 May 2007 Available online 10 May 2007 Abstract An ultra-performance liquid chromatography (UPLC) method has been developed and validated for the quality evaluation of a Chinese herb Radix Salviae Miltiorrhizae (Danshen root) by chemical fingerprinting analysis. Meanwhile a novel sample preparation procedure has been designed which can simultaneously extract both the hydrophilic and lipophilic components for a single run LC system. Comparing to the conventional HPLC method, UPLC showed many advantages including reduced run time, less solvent consumption and increased peak capacities. Using the established method, 20 target components were detected based on the retention times and on-line UV spectra by referencing to the available standards and reported data. Eleven crude drug samples from different sources were evaluated using the UPLC fingerprints. The developed method proved practicable and reliable for quality control of herbal products with multivalent components in a complicated matrix Elsevier B.V. All rights reserved. Keywords: Radix Salviae Miltiorrhizae; Fingerprinting; Salvianolic acids; Tanshinone; UPLC; HPLC 1. Introduction The quality assessment of herbal medicines has always been a challenging task due to the diversity of the multi-components existing in a complicated matrix. The chromatographic fingerprinting (CFP) method has become one of the most frequently applied approaches [1,2], which can provide the whole profile of not only the marker compounds but also the unknown components. Nevertheless development and operation of CFP on HPLC is a rigorous operation as it generally needs about one hour for a single run and the resolution seems still poor due to Corresponding author at: Key Laboratory of Standardization of Chinese Medicines of Ministry of Education, Shanghai University of Traditional Chinese Medicine, No Cai Lun Road, Zhangjiang Hi-Tech Park, Shanghai , China. Tel.: ; fax: Co-corresponding author. addresses: yongguoli@yahoo.com (Y. Li), wangzht@shutcm.edu.cn (Z. Wang). the extreme complexity of the components contained in herbal products. Ultra-performance liquid chromatography (UPLC) is a newly developed Waters instrumentation with high power in separation and analysis speed over traditional HPLC [3]. There were several publications introducing the use of UPLC in pharmaceutical analysis [4 9], however, no application on quality assessment or chemical fingerprint of Chinese herbal medicines using this technique has been reported up to date. Danshen, root of Salvia miltiorrhiza, is one of the most popularly used Chinese herbs and is now experiencing a particularly strong and rapid demand for treatment and prevention of cardiovascular system disorders [10]. The major bioactive constituents of Danshen can be classified into the hydrophilic depsides derived from caffeic acid, e.g. salvianolic acids, and lipophilic components including diterpenoids tanshinones (see Supplementary data, Fig. S1). It is well known that the accumulations of the secondary metabolites in natural herb, like Danshen root are seriously affected by ecological environments and cultivation conditions, and therefore the quality /$ see front matter 2007 Elsevier B.V. All rights reserved. doi: /j.chroma
2 52 M. Liu et al. / J. Chromatogr. A 1157 (2007) Table 1 Data of normalized peak areas of UPLC fingerprints of Danshen roots from different sources Peak D01 D02 D03 D04 D05 D06 D07 D08 D09 D10 D11 RT RRT Average RSD (%) Note: Peak normalization was calculated based on the integrative peaks (the peaks area larger than 3000 V, or the peaks height larger than 30 V). RRT (relative retention time) was calculated by referring to peak 20 (tanshinone IIA). D01 D11: Danshen root collected from different sources. D01, from Shanghai, D02 from Bozhou, Anhui, D03 from Hefei, Anhui, D04 from Nanning, Guangxi, D05 from Zhengzhou,Henan, D06 from Anguo,Hebei, D07 from Jinan, Shandong, D08 from Shanghai, D09 from Guangzhou, Guangdong, D10 from Zhangshu, Jiangxi and D11 from Germany. evaluation of Danshen root has drawn extraordinary attention [11 15]. CFP analyses of Danshen using HPLC or high-speed countercurrent chromatography (HSCCC) have been reported [16,17], nevertheless, in those methods, the hydrophilic or lipophilic components were extracted and analysed separately, which were undoubted time-consuming and inconvenient. A specific designed pattern for fingerprinting analysis of Danshen to extract simultaneously both the hydrophilic and lipophilic components proved a challenging task due to their diverse chemical and physical properties of the two groups of compounds. The present research aimed to develop a new CFP method using UPLC to demonstrate both the hydrophilic and lipophilic components coexisting in Danshen in a single run. As a comparison, a routine HPLC chromatographic method was also developed and optimized. The Danshen roots from different sources were evaluated using the developed CFP method as well (See Supplementary data, Fig. S1). 2. Experimental 2.1. Chemicals, reagents and samples HPLC grade acetonitrile and phosphoric acid (Merck, Darmstadt, Germany) were used for HPLC and UPLC analyses. Deionised water was purified by a Milli-Q system (Millipore, Bedford, MA, USA). Other chemicals and solvents were of analytical grade. The reference standards of protocatechuicalhydryde, danshensu, caffeic acid, rosmarinic acid, lithospermic acid, salvianolic acid B, salvianolic acid A and tanshinone IIA were provided by Shanghai R&D Centre for Standardization of Chinese Medicines and their purities are of more than 98% by HPLC-Diode-Array-Detector analysis based on a peak area normalization method. The samples were collected from Germany and several sources within China (Table 1) and were authenticated by Professor ZhengtaoWang, and voucher samples were deposited in the laboratory of institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine Apparatus UPLC was performed using a commercial Acquity System from Waters and 100 mm columns with an I.D. of 2.1 mm packed with 1.7 m Acquity C 18 BEH particles. The UPLC system was equipped with a 500 nl flow cell and a Rheodyne injector, an ultraviolet detector and an auto sampler. HPLC analysis was performed on an Agilent 1100 series HPLC system with an auto sampler and a diode array detector. The analysis was conducted on an Agilent Zorbax SB-C 18 column 250 mm 4.6 mm and 5 m packing material Preparation of sample solutions One gram of the Danshen root powder, accurately weighed, was transferred to a 50 ml tampon centrifuge tube. The preparations in triplicate were extracted with 10 ml of 10% methanol for 30 min by ultra-sonication and subsequently centrifuged for 10 min at 2000 g; the supernatant was taken as solution 1. The residue was treated with 25 ml of 90% methanol for another 30 min by ultra-sonication, after centrifugation, the supernatant was taken as solution 2. Finally, 5 ml of solution 1 and 20 ml of
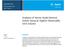
3 M. Liu et al. / J. Chromatogr. A 1157 (2007) solution 2 were mixed in a 25 ml volumetric flask, 75% methanol was added to make up to the mark if necessary. The final solution was allowed to stand for 30 min, and filtered through a 0.45 m membrane before injection Preparation of the reference solutions Accurately weighed quantities (5 mg for each) of the reference compounds were combined and made to a 10 ml volume using 75% methanol in a volumetric flask, This solution was then filtered through a 0.45 m membrane and used as the mixed reference solution for UPLC and HPLC analyses Chromatographic conditions The HPLC fingerprinting analysis was carried out on an Agilent Zorbax SB-C 18 column (250 mm 4.6 mm I.D., 5 m). Chromatographic separation was carried out using a gradient elution of (A) acetonitrile, (B) 0.1% phosphoric acid in water and (C) methanol as follows: 0 30 min, A 5 25%, B 95 75%, min, A 25 50%, B 75 50%, min, A 70%, B 10%, C 20%. The flow rate was kept at 1.0 ml/min, column temperature at 30 C, injection 10 l, the wavelength of PDA detector ranged from 200 to 400 nm and detected at 280 nm. The condition of UPLC fingerprinting was conducted on an Acquity C 18 BEH column ( mm I.D., 1.7 m), using a binary mobile phase of (A) acetonitrile and (B) 0.1% phosphoric acid: 0 5 min, A 10 25%; 5 10 min, A 25 50%; min, A 80%. The flow rate was kept at 0.5 ml/min whilst the column temperature kept at 30 C, injection volumes were 2 l, and the chromatogram was recorded at 280 nm. 3. Results and discussion 3.1. Sample procedure development Due to the diverse physicochemical properties, the hydrophilic and lipophilic components co-existing in Danshen root had been extracted individually and determined separately with time-consuming procedures [18]. A recent report [19] described a method to extract both the two types of components, but which seemed unsatisfactory as the lipophilic components gave lower recovery, and furthermore, the hydrophilic component salvianolic acid B displayed so prominent a peak that the profiling figure seemed extremely disproportionate. In our present research, a new sample preparation approach was developed by integrating two extraction steps into one procedure for simultaneous analysis of both the hydrophilic and the lipophilic components. Briefly, the first step was to extract the hydrophilic components with dilute aqueous methanol. Four concentrations of methanol (0, 5, 10 and 15%) were tested and the results showed that 0, 5 and 10% methanol gave the same high extraction efficiencies as indicated by the recovery of salvianolic acid B (97.4%), whilst there was no lipophilic component taken out at this strength of methanol. Consequently 10% methanol was used for extraction of the hydrophilic components (solution 1). For the extraction of the lipophilic components, three concentrations (90, 95 and 100%) of methanol were tried which gave the similar extraction efficiencies, as estimated by the recovery of tanshinone IIA (>97%) and at last, 90% methanol was applied to obtain lipophilic components (solution 2). Finally, 5 ml of solution 1 and 20 ml of solution 2 were mixed well in a 25 ml volumetric flask and make to the mark with 75% methanol if necessary. Using this integrated method both the two types of chemically diverse analytes were sufficiently extracted and more importantly a perfect CFP image was constructed UPLC fingerprint analysis of Danshen root To obtain good separation and ideal peak distribution of CFP, the system conditions including mobile phase, column temperature, detection wavelength and flow rate were investigated respectively. By referring to the established HPLC conditions, it was determined that acetonitrile was a better organic mobile phase than methanol for the separation of salvianolic acids. In order to improve the resolution and symmetry of the peaks, different strengths of buffers in the mobile phase was tested. It was found that 0.1% of phosphoric acid achieved better separation and suppressed any tailing peaks. It was also suggested that the optimised separation could be achieved when the column temperature was kept at 30 C rather than 25 or 40 C and the flow rate set at 0.5 ml min 1. For detection, both the tanshinones and salvianolic acids displayed the maximal UV absorption at 280 nm. CFP of Danshen root obtained by UPLC as shown in Fig. 1A using the given chromatographic parameters, in which the target peaks were well separated from each other as well as from other unidentified components with a minimum resolution (R s, min) of 1.8 being obtained using a binary solvent system. By referring to the retention times and UV spectra of the standards, eight peaks (2, 4, 5, 9,10,11,13 and 20) in the UPLC profile were identified. These were danshensu, protocatechuicalhydryde, caffeic acid, rosmarinic acid, lithospermic acid, salvianolic acid B, salvianolic acid A and tanshinone IIA, respectively Comparison of the fingerprints between UPLC and HPLC The CFP analysis of Danshen root by HPLC-PDA was also constructed using the given chromatographic parameters (Fig. 1B). In this CFP profile, the target peaks were also well separated from each other with a minimum resolution (R s, min ) of 1.1, but a ternary solvent system had to be used for the separation of peaks 18 and 19. Eight peaks (2, 4, 5, 9, 10, 11, 13 and 20) in the HPLC fingerprint were characterized as the same as in UPLC profile. By comparing the obtained data and chromatograms generated from the UPLC and HPLC, the advances in UPLC over HPLC could be summarized as follows. (1) the single running time of UPLC (17 min) was about five times shorter than that of HPLC (70 min) which may be most important for huge batch analysis; (2) a combination of the shortened running time with a smaller flow rate of 0.5 ml/min reduced solvent (mostly harmful to human body) consumption to only 7.5 ml, while solvent
4 54 M. Liu et al. / J. Chromatogr. A 1157 (2007) be caused by the different geographic or cultivating conditions. (See Supplementary data, Fig. S2). 4. Conclusion Fig. 1. Chromatographic fingerprint of Danshen root obtained by (A) UPLC and (B) HPLC were the coexisting peaks: 2, Danshensu; 5, Protocatechuic alhydryde; 6, caffeic acid; 9, rosmarinci acid; 10, lithospermic acid; 11, salvianolic acid B; 13, salvianolic acid A; 20, tanshinoneiia. The chromatograms were detected at 280 nm. usage for a single run in HPLC was up to 70 ml, (3) the peak capacities obtained by UPLC [20] were 87, more efficient than that obtained by HPLC (peak capacities of 65), (4) in the condition described above, the two critical pairs of peaks 9 and 10, 18 and 19 were well separated by UPLC. In summary, the UPLC method had its advantages over HPLC in terms of time-saving, solvent-saving, high performance and high efficiency, and has been successively applied to CFP analysis and quality evaluation of Danshen root Fingerprint analysis of representative Danshen samples using UPLC Eleven Danshen samples collected from different locations were analysed using the established methods and the UPLC fingerprint profiles were obtained. Twenty peaks were found common in all the analysed samples. The normalized peak areas of these 20 co-existing peaks in CFP of Danshen from different sources were summarized in Table 1 and the comparison of the chromatograms were shown in Supplementary data, Fig. S2. The data revealed that the main components and the peaks distribution were much similar among these samples, but the variation of normalization peak area clearly appeared, for instance, the predominant hydrophilic component (peak 11, salvianolic acid B), and the lipophilic component (peak 20, tanshinone IIA) showed their RSD of 20 and 34%, respectively, which might UPLC methods have been developed in recent years for pharmaceutical analysis but not yet utilized in quality control for Chinese herbal medicines until this research. In the present study a UPLC method has been established and validated for the first time in the chemical fingerprint analysis of Danshen root. The advantages of UPLC in the analysis of multi-components in a complicated matrix has been demonstrated, with shorter analysis times, reduced solvent consumption and improved peak resolution compared to conventional HPLC. Meanwhile, an integrated sample preparation method has been established to demonstrate both the hydrophilic and lipophilic components in a single run and obtain perfect chromatographic profiles. In addition, 20 common peaks were detected in all the analysed samples and eight of them were unambiguously identified by comparing their retention times and the on-line UV spectra with the available reference compounds, which will further contribute to the quality assessment of Danshen root from different populations and geographic conditions. Most importantly, the UPLC fingerprinting method established in the present report will serve as a valuable reference for quality evaluation and standardization of Chinese herbal drugs and final products. Acknowledgement The authors gratefully acknowledge the technical support of Mr. Jie Chen in Waters Corporation, Shanghai, China. Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi: /j.chroma References [1] P.S. Xie, S.B. Chen, Y.Z. Liang, X.H. Wang, R.T. Tian, R. Upton, J. Chromatogr. A 1112 (2006) 171. [2] Y.Z. Liang, P.S. Xie, K. Chan, J. Chromatogr. B 812 (2004) 53. [3] J.W. Thompson, J.S. Mellors, J.W. Eschelbach, J.W. Jorgenson, LC GC 24 (2006) 16. [4] L. Novakova, L. Matysova, P. Solich, Talanta 68 (2006) 908. [5] D.T.T. Nguyen, D. Guillarme, S. Rudaz, J.L. Veuthey, J. Chromatogr. A 1128 (2006) 105. [6] A.D. Villiers, F. Lestremau, R. Szucs, S. Gélébart, F. David, P. Sandra, J. Chromatogr. A 1127 (2006) 60. [7] E. Barceló-Barrachina, E. Moyano, M.T. Galceran, J.L. Lliberia, B. Bagó, M.A. Cortes, J. Chromatogr. A 1125 (2006) 195. [8] G. Desmet, J. Chromatogr. A 1116 (2006) 89. [9] C.C. Leandro, P. Hancock, R.J. Fussell, B.J. Keely, J. Chromatogr. A 1103 (2006) 94. [10] Z.H. Dong, J. She, H.Z. Zheng (Eds.), Modern Study of Traditional Chinese Medicine, vol. 2, Xue Yuan Press, Beijing, 2001, p [11] X.H. Fan, Y.Y. Cheng, Z.L. Ye, R.C. Lin, Z.Z. Qian, Anal. Chim. Acta 555 (2006) 217. [12] J.L. Zhang, M. Cui, Y. He, J. Pharm. Biomed. Anal. 36 (2005) 1029.
5 M. Liu et al. / J. Chromatogr. A 1157 (2007) [13] P. Hu, G. An Luo, Z.Z. Zhao, Z.H. Jiang, Chem. Pharm. Bull. 53 (2005) 705. [14] L.M. Zhou, M. Chow, Z. Zuo, J. Pharm. Biomed. Anal. 41 (2006) 744. [15] D. Yun, Y.N. Pan, W.W. Fu, Chem. Pharm. Bull. 53 (2005) 508. [16] P. Hu, Q.L. Liang, G. An Lou, Z.Z. Zhao, Z.H. Jiang, Chem. Pharm. Bull. 53 (2005) 677. [17] M. Gu, Z.G. Su, J. Chromatogr. A 1022 (2004) 139. [18] Z.H. Shi, J.T. He, T.T. Yao, W.H. Chang, M.P. Zhao, J. Pharm. Biomed. Anal. 37 (2005) 481. [19] P. Hu, G. An Luo, Z.Z. Zhao, Z.H. Jiang, Chem. Pharm. Bull. 53 (2005) 481. [20] S.A.C. Wren, J. Pharm. Biomed. Anal. 38 (2005) 337.
Simultaneous Determination of Seven Active Components in Mian anning Mixture by HPLC Under Multiple UV Wavelengths
 measurement for the determination of ribavirin by HPLC [J]. Chin J Pharm Anal(), 2007, 27(11): 1803-1805. [9] LIU Y L, LI D M, FEN L, et al. Uncertainty of measurement in the HPLC determination of Fufangdanshen
measurement for the determination of ribavirin by HPLC [J]. Chin J Pharm Anal(), 2007, 27(11): 1803-1805. [9] LIU Y L, LI D M, FEN L, et al. Uncertainty of measurement in the HPLC determination of Fufangdanshen
Effective use of Pharmacopeia guidelines to reduce cost of chromatographic analysis for Fluticasone propionate
 Effective use of Pharmacopeia guidelines to reduce cost of chromatographic analysis for Fluticasone propionate Application Note Pharmaceutical QA/QC Author Siji Joseph Agilent Technologies, Inc. Bangalore,
Effective use of Pharmacopeia guidelines to reduce cost of chromatographic analysis for Fluticasone propionate Application Note Pharmaceutical QA/QC Author Siji Joseph Agilent Technologies, Inc. Bangalore,
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Artepillin C, is it a good marker for quality control of Brazilian Green Propolis? Cui-ping Zhang 1, Xiao-ge Shen 1, Jia-wei Chen 1, Xia-sen Jiang 1, Kai Wang 2, Fu-liang Hu 1 *
SUPPLEMENTARY MATERIAL Artepillin C, is it a good marker for quality control of Brazilian Green Propolis? Cui-ping Zhang 1, Xiao-ge Shen 1, Jia-wei Chen 1, Xia-sen Jiang 1, Kai Wang 2, Fu-liang Hu 1 *
ISSN: ; CODEN ECJHAO E-Journal of Chemistry 2011, 8(3),
 ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.e-journals.net 2011, 8(3), 1275-1279 Simultaneous Determination of Paracetamol, Phenylephrine Hydrochloride, Oxolamine Citrate and Chlorpheniramine
ISSN: 0973-4945; CODEN ECJHAO E- Chemistry http://www.e-journals.net 2011, 8(3), 1275-1279 Simultaneous Determination of Paracetamol, Phenylephrine Hydrochloride, Oxolamine Citrate and Chlorpheniramine
Application Note. Agilent Application Solution Analysis of ascorbic acid, citric acid and benzoic acid in orange juice. Author. Abstract.
 Agilent Application Solution Analysis of ascorbic acid, citric acid and benzoic acid in orange juice Application Note Author Food Syed Salman Lateef Agilent Technologies, Inc. Bangalore, India 8 6 4 2
Agilent Application Solution Analysis of ascorbic acid, citric acid and benzoic acid in orange juice Application Note Author Food Syed Salman Lateef Agilent Technologies, Inc. Bangalore, India 8 6 4 2
CHAPTER INTRODUCTION OF DOSAGE FORM AND LITERATURE REVIEW
 132 CHAPTER 6 DEVELOPMENT AND VALIDATION OF A STABILITY-INDICATING RP-HPLC METHOD FOR SIMULTANEOUS DETERMINATION OF PARACETAMOL, TRAMADOL HYDROCHLORIDE AND DOMPERIDONE IN A COMBINED DOSAGE FORM 6.1 INTRODUCTION
132 CHAPTER 6 DEVELOPMENT AND VALIDATION OF A STABILITY-INDICATING RP-HPLC METHOD FOR SIMULTANEOUS DETERMINATION OF PARACETAMOL, TRAMADOL HYDROCHLORIDE AND DOMPERIDONE IN A COMBINED DOSAGE FORM 6.1 INTRODUCTION
CHAPTER INTRODUCTION OF DOSAGE FORM AND LITERATURE REVIEW
 51 CHAPTER 2 SIMULTANEOUS ESTIMATION OF PIOGLITAZONE, GLIMEPIRIDE AND GLIMEPIRIDE IMPURITIES IN COMBINATION DRUG PRODUCT BY A VALIDATED STABILITY-INDICATING RP-HPLC METHOD 2.1 INTRODUCTION OF DOSAGE FORM
51 CHAPTER 2 SIMULTANEOUS ESTIMATION OF PIOGLITAZONE, GLIMEPIRIDE AND GLIMEPIRIDE IMPURITIES IN COMBINATION DRUG PRODUCT BY A VALIDATED STABILITY-INDICATING RP-HPLC METHOD 2.1 INTRODUCTION OF DOSAGE FORM
Available online at Scholars Research Library
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (3):157-161 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (3):157-161 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Increasing resolution using longer columns while maintaining analysis time Advantages of the wide power range of the Agilent 1290 Infinity LC System
 Increasing resolution using longer columns while maintaining analysis time Advantages of the wide power range of the Agilent 129 Infinity LC System Application Note Pharmaceutical and Chemical Analysis
Increasing resolution using longer columns while maintaining analysis time Advantages of the wide power range of the Agilent 129 Infinity LC System Application Note Pharmaceutical and Chemical Analysis
Bangladesh J. Bot. 46(4): , 2017 (December)
 Bangladesh J. Bot. 46(4): 1333-1340, 2017 (December) COMPARATIVE ANALYSIS OF GINSENOSIDES IN DIFFERENT GROWTH AGES AND PARTS OF ASIAN GINSENG (PANAX GINSENG C.A. MEYER) AND AMERICAN GINSENG (PANAX QUINQUEFOLIUS
Bangladesh J. Bot. 46(4): 1333-1340, 2017 (December) COMPARATIVE ANALYSIS OF GINSENOSIDES IN DIFFERENT GROWTH AGES AND PARTS OF ASIAN GINSENG (PANAX GINSENG C.A. MEYER) AND AMERICAN GINSENG (PANAX QUINQUEFOLIUS
YAKUGAKU ZASSHI 126(11) (2006) 2006 The Pharmaceutical Society of Japan
 YAKUGAKU ZASSHI 126(11) 1185 1190 (2006) 2006 The Pharmaceutical Society of Japan 1185 Determination of Liquiritin, Naringin, Hesperidin, Thymol, Imperatorin, Honokiol, Isoimperatorin, and Magnolol in
YAKUGAKU ZASSHI 126(11) 1185 1190 (2006) 2006 The Pharmaceutical Society of Japan 1185 Determination of Liquiritin, Naringin, Hesperidin, Thymol, Imperatorin, Honokiol, Isoimperatorin, and Magnolol in
Pharmacokinetics and excretion of cryptotanshinone after intravenous injection in pigs
 Asian Journal of Drug Metabolism and Pharmacokinetics Paper ID 1608-2281-2005-0503-00231-05 Copyright by Hong Kong Medical Publisher Received April 5, 2005 ISSN 1608-2281 2005; 5(3):231-235 Accepted June
Asian Journal of Drug Metabolism and Pharmacokinetics Paper ID 1608-2281-2005-0503-00231-05 Copyright by Hong Kong Medical Publisher Received April 5, 2005 ISSN 1608-2281 2005; 5(3):231-235 Accepted June
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(2):770-775 Validation of Rapid Liquid Chromatographic
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(2):770-775 Validation of Rapid Liquid Chromatographic
Comparison of conventional HPLC with UPLC method for determination of albuterol sulfate and it s impurities in pharmaceutical formulation
 Oriental Journal of Chemistry Vol. 24(2), 537-544 (2008) Comparison of conventional HPLC with UPLC method for determination of albuterol sulfate and it s impurities in pharmaceutical formulation P.N. DALVI,
Oriental Journal of Chemistry Vol. 24(2), 537-544 (2008) Comparison of conventional HPLC with UPLC method for determination of albuterol sulfate and it s impurities in pharmaceutical formulation P.N. DALVI,
RP-HPLC Analysis of Temozolomide in Pharmaceutical Dosage Forms
 Asian Journal of Chemistry Vol. 22, No. 7 (2010), 5067-5071 RP-HPLC Analysis of Temozolomide in Pharmaceutical Dosage Forms A. LAKSHMANA RAO*, G. TARAKA RAMESH and J.V.L.N.S. RAO Department of Pharmaceutical
Asian Journal of Chemistry Vol. 22, No. 7 (2010), 5067-5071 RP-HPLC Analysis of Temozolomide in Pharmaceutical Dosage Forms A. LAKSHMANA RAO*, G. TARAKA RAMESH and J.V.L.N.S. RAO Department of Pharmaceutical
Transferring a Method for Analysis of DNPH-Derivatized Aldehydes and Ketones from HPLC to UHPLC
 Transferring a Method for Analysis of DNPH-Derivatized Aldehydes and Ketones from HPLC to UHPLC Application Note Environmental Author Melanie Metzlaff Agilent Technologies, Inc. Waldbronn, Germany Abstract
Transferring a Method for Analysis of DNPH-Derivatized Aldehydes and Ketones from HPLC to UHPLC Application Note Environmental Author Melanie Metzlaff Agilent Technologies, Inc. Waldbronn, Germany Abstract
Analytical Method Development for USP Related Compounds in Paclitaxel Using an Agilent Poroshell 120 PFP
 Analytical Method Development for USP Related Compounds in Paclitaxel Using an Agilent Poroshell 1 PFP Application Note Clinical Research Author Rongjie Fu Agilent Technologies (Shanghai) Co. Ltd Abstract
Analytical Method Development for USP Related Compounds in Paclitaxel Using an Agilent Poroshell 1 PFP Application Note Clinical Research Author Rongjie Fu Agilent Technologies (Shanghai) Co. Ltd Abstract
Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column
 Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Maximizing chromatographic peak capacity with the Agilent 1290 Infinity LC system
 Maximizing chromatographic peak capacity with the Agilent 1290 Infinity LC system A practical guide on how to use parameters to increase peak capacity Application Note Pharmaceutical and Chemical Author
Maximizing chromatographic peak capacity with the Agilent 1290 Infinity LC system A practical guide on how to use parameters to increase peak capacity Application Note Pharmaceutical and Chemical Author
ACQUITY UPLC WITH PDA DETECTION: DETERMINING THE SENSITIVITY LIMITS OF OXYBUTYNIN AND RELATED COMPOUNDS
 ACQUITY UPLC WITH PDA DETECTION: DETERMINING THE SENSITIVITY LIMITS OF OXYBUTYNIN AND RELATED COMPOUNDS Tanya Jenkins Waters Corporation, Milford, MA, USA INTRODUCTION Some of the most challenging methods
ACQUITY UPLC WITH PDA DETECTION: DETERMINING THE SENSITIVITY LIMITS OF OXYBUTYNIN AND RELATED COMPOUNDS Tanya Jenkins Waters Corporation, Milford, MA, USA INTRODUCTION Some of the most challenging methods
Development, Estimation and Validation of Lisinopril in Bulk and its Pharmaceutical Formulation by HPLC Method
 ISSN: 0973-4945; CODEN ECJAO E- Chemistry http://www.e-journals.net 2012, 9(1), 340-344 Development, Estimation and Validation of Lisinopril in Bulk and its Pharmaceutical Formulation by PLC Method V.
ISSN: 0973-4945; CODEN ECJAO E- Chemistry http://www.e-journals.net 2012, 9(1), 340-344 Development, Estimation and Validation of Lisinopril in Bulk and its Pharmaceutical Formulation by PLC Method V.
STANDARD OPERATING PROTOCOL (SOP)
 1 STANDARD PERATING PRTCL (SP) Subject: To determine the Contents of isoflavone in soybean products by HPLC Analysis Project/Core No.: Core B Total 9 Pages SP No.: CB0102 Modified Date: 05/15/01 BTANICAL
1 STANDARD PERATING PRTCL (SP) Subject: To determine the Contents of isoflavone in soybean products by HPLC Analysis Project/Core No.: Core B Total 9 Pages SP No.: CB0102 Modified Date: 05/15/01 BTANICAL
METHOD DEVELOPMENT AND VALIDATION BY RP-HPLC FOR ESTIMATION OF ZOLPIDEM TARTARATE
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ramalakshmi et al. SJIF Impact Factor 6.647 Volume 7, Issue 2, 1010-1018 Research Article ISSN 2278 4357 METHOD DEVELOPMENT AND VALIDATION BY RP-HPLC
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ramalakshmi et al. SJIF Impact Factor 6.647 Volume 7, Issue 2, 1010-1018 Research Article ISSN 2278 4357 METHOD DEVELOPMENT AND VALIDATION BY RP-HPLC
Title Revision n date
 A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
Comparison of a UPLC Method across Multiple UHPLC Systems
 Comparison of a UPLC Method across Multiple UHPLC Systems Tanya Jenkins Waters Corporation, Milford, MA, U.S. INTRODUCTION In 2004, Waters introduced the ACQUITY UPLC System. Since this launch, many liquid
Comparison of a UPLC Method across Multiple UHPLC Systems Tanya Jenkins Waters Corporation, Milford, MA, U.S. INTRODUCTION In 2004, Waters introduced the ACQUITY UPLC System. Since this launch, many liquid
Scholars Research Library. Der Pharmacia Lettre, 2015, 7 (5):44-49 (
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (5):44-49 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (5):44-49 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography
 Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography Application ote Food Safety Authors Chen-Hao Zhai and Yun Zou Agilent Technologies Co. Ltd.
Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography Application ote Food Safety Authors Chen-Hao Zhai and Yun Zou Agilent Technologies Co. Ltd.
Validation of Changes to the USP Assay Method for Ibuprofen Tablets
 Validation of Changes to the USP Assay Method for Ibuprofen Extraction and Filtration Techniques Lynn Massad, Pam Anderson, James Ward, Philip Burns, and Ranga Velagaleti* This article discusses changes
Validation of Changes to the USP Assay Method for Ibuprofen Extraction and Filtration Techniques Lynn Massad, Pam Anderson, James Ward, Philip Burns, and Ranga Velagaleti* This article discusses changes
Simultaneous determination of chlorogenic and isochlorogenic acid contents in Xiao'er Yanbian granule by HPLC.
 Biomedical Research 2014; 25 (4): 477-482 ISSN 0970-938X http://www.biomedres.info Simultaneous determination of chlorogenic and isochlorogenic acid contents in Xiao'er Yanbian granule by HPLC. Chunyan
Biomedical Research 2014; 25 (4): 477-482 ISSN 0970-938X http://www.biomedres.info Simultaneous determination of chlorogenic and isochlorogenic acid contents in Xiao'er Yanbian granule by HPLC. Chunyan
Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page:
 Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com ANALYTICAL METHOD DEVELOPMENT AND VALIDATION OF GEFITINIB
Research Article CODEN: AJPAD7 ISSN: 2321-0923 Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry Journal home page: www.ajpamc.com ANALYTICAL METHOD DEVELOPMENT AND VALIDATION OF GEFITINIB
Comparison of ferulic acid content in Radix Angelicae Sinensis, Danggui Buxue Tang and Danggui Sini Tang
 1364 Comparison of ferulic acid content in Radix Angelicae Sinensis, Danggui Buxue Tang and Danggui Sini Tang JIA MEI YAO 1, LI JIN YU 2, QIONG CHEN 1, ZE QI CHEN 2, DONG SHENG WANG 2, XIN JIAN QIU 2 and
1364 Comparison of ferulic acid content in Radix Angelicae Sinensis, Danggui Buxue Tang and Danggui Sini Tang JIA MEI YAO 1, LI JIN YU 2, QIONG CHEN 1, ZE QI CHEN 2, DONG SHENG WANG 2, XIN JIAN QIU 2 and
Antioxidant activity and components of a traditional chinese medicine formula consisting of Crataegus pinnatifida and Salvia miltiorrhiza
 Chen et al. BMC Complementary and Alternative Medicine 2013, 13:99 RESEARCH ARTICLE Open Access Antioxidant activity and components of a traditional chinese medicine formula consisting of Crataegus pinnatifida
Chen et al. BMC Complementary and Alternative Medicine 2013, 13:99 RESEARCH ARTICLE Open Access Antioxidant activity and components of a traditional chinese medicine formula consisting of Crataegus pinnatifida
Optimization of extraction method and profiling of plant phenolic compounds through RP-HPLC
 Chapter III Optimization of extraction method and profiling of plant phenolic compounds through RP-HPLC 1. INTRODUCTION Phenolics compounds are naturally present antioxidants, found in a variety of plant
Chapter III Optimization of extraction method and profiling of plant phenolic compounds through RP-HPLC 1. INTRODUCTION Phenolics compounds are naturally present antioxidants, found in a variety of plant
HPLC to UHPLC Transfer of USP Method for Amlodipine Besylate Using the Agilent 1290 Infinity II LC
 HPLC to UHPLC Transfer of USP Method for Amlodipine Besylate Using the Agilent 129 Infinity II LC Application Note Small Molecule Pharmaceuticals Authors Gerd Vanhoenacker, Mieke Steenbeke, Koen Sandra,
HPLC to UHPLC Transfer of USP Method for Amlodipine Besylate Using the Agilent 129 Infinity II LC Application Note Small Molecule Pharmaceuticals Authors Gerd Vanhoenacker, Mieke Steenbeke, Koen Sandra,
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(1):138-144 Simultaneous RP HPLC determination of Latanoprost
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(1):138-144 Simultaneous RP HPLC determination of Latanoprost
Available online at ScienceDirect. IERI Procedia 5 (2013 )
 Available online at www.sciencedirect.com ScienceDirect IERI Procedia 5 (213 ) 351 356 213 International Conference on Agricultural and Natural Resources Engineering Pre-column Derivatization RP-HPLC Determination
Available online at www.sciencedirect.com ScienceDirect IERI Procedia 5 (213 ) 351 356 213 International Conference on Agricultural and Natural Resources Engineering Pre-column Derivatization RP-HPLC Determination
Journal of Chromatography A, 1064 (2005) 53 57
 Journal of Chromatography A, 1064 (2005) 53 57 Preparative isolation and purification of three flavonoids from the Chinese medicinal plant Epimedium koreamum Nakai by high-speed counter-current chromatography
Journal of Chromatography A, 1064 (2005) 53 57 Preparative isolation and purification of three flavonoids from the Chinese medicinal plant Epimedium koreamum Nakai by high-speed counter-current chromatography
A high-performance liquid chromatography (HPLC) method for determination of chlorogenic acid and emodin in Yinhuang Jiangzhi Tea
 Journal of Hainan Medical University 2016; 22(12): 17-21 17 Journal of Hainan Medical University http://www.jhmuweb.net/ A high-performance liquid chromatography (HPLC) method for determination of chlorogenic
Journal of Hainan Medical University 2016; 22(12): 17-21 17 Journal of Hainan Medical University http://www.jhmuweb.net/ A high-performance liquid chromatography (HPLC) method for determination of chlorogenic
Rapid Gradient and Elevated Temperature UHPLC of Flavonoids in Citrus Fruit
 Rapid Gradient and Elevated Temperature UHPLC of Flavonoids in Citrus Fruit Application Note General Chromatography, Food Industry Authors John W. Henderson Jr., Judy Berry, Anne Mack, William Long Agilent
Rapid Gradient and Elevated Temperature UHPLC of Flavonoids in Citrus Fruit Application Note General Chromatography, Food Industry Authors John W. Henderson Jr., Judy Berry, Anne Mack, William Long Agilent
HPLC Determination of Eight Polyphenols in the Leaves of Crataegus pinnatifida Bge. var. major
 HPLC Determination of Eight Polyphenols in the Leaves of Crataegus pinnatifida Bge. var. major Xixiang Ying 1,2, Rongxiang Wang 1, Jing Xu 1, Wenjie Zhang 1, Haibo Li 3, Chaoshen Zhang 1, and Famei Li
HPLC Determination of Eight Polyphenols in the Leaves of Crataegus pinnatifida Bge. var. major Xixiang Ying 1,2, Rongxiang Wang 1, Jing Xu 1, Wenjie Zhang 1, Haibo Li 3, Chaoshen Zhang 1, and Famei Li
RP-HPLC METHOD DEVELOPMENT AND VALIDATION FOR THE ESTIMATION OF BACLOFEN IN BULK AND PHARMACEUTICAL DOSAGE FORMS
 Int. J. Chem. Sci.: 11(1), 2013, 390-398 ISSN 0972-768X www.sadgurupublications.com RP-HPLC METHOD DEVELOPMENT AND VALIDATION FOR THE ESTIMATION OF BACLOFEN IN BULK AND PHARMACEUTICAL DOSAGE FORMS SAROJ
Int. J. Chem. Sci.: 11(1), 2013, 390-398 ISSN 0972-768X www.sadgurupublications.com RP-HPLC METHOD DEVELOPMENT AND VALIDATION FOR THE ESTIMATION OF BACLOFEN IN BULK AND PHARMACEUTICAL DOSAGE FORMS SAROJ
SIMULTANEOUS DETERMINATION OF ATORVASTATIN AND EZETIMIBE BY RP-HPLC IN PURE AND PHARMACEUTICAL DOSAGE FORM
 Int. J. Chem. Sci.: 6(3), 2008, 1576-1582 SIMULTANEOUS DETERMINATION OF ATORVASTATIN AND EZETIMIBE BY RP-HPLC IN PURE AND PHARMACEUTICAL DOSAGE FORM B. NEELIMA, P. RAVI KUMAR, M. MURALI KRISHNA, V. HIMA
Int. J. Chem. Sci.: 6(3), 2008, 1576-1582 SIMULTANEOUS DETERMINATION OF ATORVASTATIN AND EZETIMIBE BY RP-HPLC IN PURE AND PHARMACEUTICAL DOSAGE FORM B. NEELIMA, P. RAVI KUMAR, M. MURALI KRISHNA, V. HIMA
Mass-Based Purification of Natural Product Impurities Using an Agilent 1260 Infinity II Preparative LC/MSD System
 Application Note Food Testing and Agriculture Mass-Based Purification of Natural Product Impurities Using an Agilent 126 Infinity II Preparative LC/MSD System Authors Florian Rieck and Jörg Hippler Agilent
Application Note Food Testing and Agriculture Mass-Based Purification of Natural Product Impurities Using an Agilent 126 Infinity II Preparative LC/MSD System Authors Florian Rieck and Jörg Hippler Agilent
ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC
 ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC METHOD APPROVALS Norvin Pharma Inc. Author Analytical Laboratory Approver Analytical Laboratory Group Leader Approver Manager Quality Control Chemistry
ASSAY AND IMPURITY METHOD FOR DURACOR TABLETS BY HPLC METHOD APPROVALS Norvin Pharma Inc. Author Analytical Laboratory Approver Analytical Laboratory Group Leader Approver Manager Quality Control Chemistry
Development and validation of stability indicating RP-LC method for estimation of calcium dobesilate in pharmaceutical formulations
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (11):236-242 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (11):236-242 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
CHAPTER 8 HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) ANALYSIS OF PHYTOCHEMICAL CONSTITUENTS OF M. ROXBURGHIANUS AND P. FRATERNUS PLANT EXTRACTS
 CHAPTER 8 HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) ANALYSIS OF PHYTOCHEMICAL CONSTITUENTS OF M. ROXBURGHIANUS AND P. FRATERNUS PLANT EXTRACTS CHAPTER 8: HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC)
CHAPTER 8 HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) ANALYSIS OF PHYTOCHEMICAL CONSTITUENTS OF M. ROXBURGHIANUS AND P. FRATERNUS PLANT EXTRACTS CHAPTER 8: HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC)
Development and Validation of Improved RP-HPLC method for Identification and Estimation of Ellagic and Gallic acid in Triphala churna
 International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.2, No.3, pp 1486-1493, July-Sept 2010 Development and Validation of Improved RP-HPLC method for Identification and Estimation
International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.2, No.3, pp 1486-1493, July-Sept 2010 Development and Validation of Improved RP-HPLC method for Identification and Estimation
2D-LC as an Automated Desalting Tool for MSD Analysis
 2D-LC as an Automated Desalting Tool for MSD Analysis Direct Mass Selective Detection of a Pharmaceutical Peptide from an MS-Incompatible USP Method Application Note Biologics and Biosimilars Author Sonja
2D-LC as an Automated Desalting Tool for MSD Analysis Direct Mass Selective Detection of a Pharmaceutical Peptide from an MS-Incompatible USP Method Application Note Biologics and Biosimilars Author Sonja
Journal of Chemical and Pharmaceutical Research, 2018, 10(3): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2018, 10(3):142-147 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Development of Reverse Phase HPLC Method and Validation
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2018, 10(3):142-147 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Development of Reverse Phase HPLC Method and Validation
Development and validation of related substances method for Varenicline and its impurities
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (1):304-309 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (1):304-309 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Pelagia Research Library
 Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2015, 6(1):6-10 ISSN: 0976-8688 CODEN (USA): PSHIBD Validated RP-HPLC method for simultaneous estimation of metformin hydrochloride
Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2015, 6(1):6-10 ISSN: 0976-8688 CODEN (USA): PSHIBD Validated RP-HPLC method for simultaneous estimation of metformin hydrochloride
Available online Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(1):171-176 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Reverse phase high performance liquid chromatography
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2016, 8(1):171-176 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Reverse phase high performance liquid chromatography
SIMULTANEOUS ESTIMATION OF VALSARTAN AND HYDROCHLOROTHIAZIDE IN TABLETS BY RP-HPLC METHOD
 170 Original Article SIMULTANEOUS ESTIMATION OF VALSARTAN AND HYDROCHLOROTHIAZIDE IN TABLETS BY RP-HPLC METHOD *Lakshmana Rao A, 1 Bhaskara Raju V *V.V. Institute of Pharmaceutical Sciences, Gudlavalleru,
170 Original Article SIMULTANEOUS ESTIMATION OF VALSARTAN AND HYDROCHLOROTHIAZIDE IN TABLETS BY RP-HPLC METHOD *Lakshmana Rao A, 1 Bhaskara Raju V *V.V. Institute of Pharmaceutical Sciences, Gudlavalleru,
Rb 1. Results of variance analysis REFERENCES Chin J Mod Appl Pharm, 2014 September, Vol.31 No
 5 Tab. 5 Results of variance analysis F A 0.589 2 0.295 22.9 B 0.065 0 2 0.032 5 2.5 C 0.286 2 0.143 11.14 D( ) 0.025 8 2 0.012 9 F [4] F 0.05(2,2) =19 F 0.01(2,2) =99 Note: Chek the table of F values
5 Tab. 5 Results of variance analysis F A 0.589 2 0.295 22.9 B 0.065 0 2 0.032 5 2.5 C 0.286 2 0.143 11.14 D( ) 0.025 8 2 0.012 9 F [4] F 0.05(2,2) =19 F 0.01(2,2) =99 Note: Chek the table of F values
Application Note. Small Molecule Pharmaceuticals. 2-Ethyl-2-phenylmalonamide Ethosuximide Primidone Carbamazepine. Carbamazepine-
 Analysis of Pharmaceutical Substances Using HPLC and UHPLC Methods Backward Compatibility for Validated Analytical Procedures and Methods Comparing the Infi nity LC and the Infi nity II LC Application
Analysis of Pharmaceutical Substances Using HPLC and UHPLC Methods Backward Compatibility for Validated Analytical Procedures and Methods Comparing the Infi nity LC and the Infi nity II LC Application
Application Note. Authors. Abstract. Food
 Determination of Hormones in Shrimp by Agilent 129 Infinity LC with Agilent Poroshell 12 LC Column and Agilent Bond Elut QuEChERS for Sample Preparation Application Note Food Authors Rongjie Fu and Andy
Determination of Hormones in Shrimp by Agilent 129 Infinity LC with Agilent Poroshell 12 LC Column and Agilent Bond Elut QuEChERS for Sample Preparation Application Note Food Authors Rongjie Fu and Andy
Journal of Chemical and Pharmaceutical Research, 2014, 6(3): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(3):1337-1341 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Preliminary study on serum pharmacochemistry of
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(3):1337-1341 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Preliminary study on serum pharmacochemistry of
Analysis of Counterfeit Antidiabetic Drugs by UHPLC with the Agilent 1220 Infinity Mobile LC
 Analysis of Counterfeit Antidiabetic Drugs by UPLC with the Agilent 1 Infinity Mobile LC Application Note Small Molecule Pharmaceuticals & Generics Author Sonja Schneider Agilent Technologies, Inc. Waldbronn,
Analysis of Counterfeit Antidiabetic Drugs by UPLC with the Agilent 1 Infinity Mobile LC Application Note Small Molecule Pharmaceuticals & Generics Author Sonja Schneider Agilent Technologies, Inc. Waldbronn,
DEVELOPMENT AND VALIDATION OF NEW HPLC METHOD FOR THE ESTIMATION OF PALIPERIDONE IN PHARMACEUTICAL DOSAGE FORMS
 ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in DEVELOPMENT AND VALIDATION OF NEW HPLC METHOD FOR THE ESTIMATION OF PALIPERIDONE IN PHARMACEUTICAL
ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in DEVELOPMENT AND VALIDATION OF NEW HPLC METHOD FOR THE ESTIMATION OF PALIPERIDONE IN PHARMACEUTICAL
Converting a CHP Method for Insulin to Agilent Poroshell 120 Columns
 Converting a CHP Method for Insulin to Agilent Poroshell Columns Application Note Biopharm Author Rongjie Fu Agilent Technologies (Shanghai) Co., Ltd. 4 Ying Lun Road Shanghai 3 China Abstract A regulatory
Converting a CHP Method for Insulin to Agilent Poroshell Columns Application Note Biopharm Author Rongjie Fu Agilent Technologies (Shanghai) Co., Ltd. 4 Ying Lun Road Shanghai 3 China Abstract A regulatory
Development and validation of an HPLC assay for fentanyl and related substances in fentanyl citrate injection, USP
 Journal of Pharmaceutical and Biomedical Analysis 20 (1999) 705 716 Development and validation of an HPLC assay for fentanyl and related substances in fentanyl citrate injection, USP John Lambropoulos
Journal of Pharmaceutical and Biomedical Analysis 20 (1999) 705 716 Development and validation of an HPLC assay for fentanyl and related substances in fentanyl citrate injection, USP John Lambropoulos
Validated RP- HPLC Method for the Quantitative Estimation of Valsartan in Bulk and Pharmaceutical Dosage Forms
 International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.2, No.2, pp 1194-1198, April-June 2010 Validated RP- HPLC Method for the Quantitative Estimation of Valsartan in Bulk
International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.2, No.2, pp 1194-1198, April-June 2010 Validated RP- HPLC Method for the Quantitative Estimation of Valsartan in Bulk
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR QUANTITATIVE ANALYSIS TOLBUTAMIDE IN PURE AND PHARMACEUTICAL FORMULATIONS
 Int. J. Chem. Sci.: 11(4), 2013, 1607-1614 ISSN 0972-768X www.sadgurupublications.com DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR QUANTITATIVE ANALYSIS TOLBUTAMIDE IN PURE AND PHARMACEUTICAL FORMULATIONS
Int. J. Chem. Sci.: 11(4), 2013, 1607-1614 ISSN 0972-768X www.sadgurupublications.com DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR QUANTITATIVE ANALYSIS TOLBUTAMIDE IN PURE AND PHARMACEUTICAL FORMULATIONS
36 J App Pharm Vol. 6; Issue 1: 36-42; January, 2014 Rao et al., 2014
 36 J App Pharm Vol. 6; Issue 1: 36-42; January, 2014 Rao et al., 2014 Original Research Article SIMPLE AND RAPID LIQUID CHROMATOGRAPHIC METHOD FOR REAL-TIME QUANTIFICATION OF NAPROXEN / ESOMEPRAZOLE MAGNESIUM
36 J App Pharm Vol. 6; Issue 1: 36-42; January, 2014 Rao et al., 2014 Original Research Article SIMPLE AND RAPID LIQUID CHROMATOGRAPHIC METHOD FOR REAL-TIME QUANTIFICATION OF NAPROXEN / ESOMEPRAZOLE MAGNESIUM
Ultrafast analysis of synthetic antioxidants in vegetable oils using the Agilent 1290 Infinity LC system
 Ultrafast analysis of synthetic antioxidants in vegetable oils using the Agilent 19 Infinity LC system Application Note Food Authors Standard solution Gerd Vanhoenacker, Frank David, Pat Sandra Research
Ultrafast analysis of synthetic antioxidants in vegetable oils using the Agilent 19 Infinity LC system Application Note Food Authors Standard solution Gerd Vanhoenacker, Frank David, Pat Sandra Research
Amudha S et al., Asian Journal of Pharmthiaceutical Technology & Innovation, 04 (21); 2016; Research Article
 Asian Journal of Pharmaceutical Technology & Innovation ISSN: 2347-8810 Research Article Received on: 09-11-2016 Accepted on: 20-11-2016 Published on: 15-12-2016 Corresponding Author: * Amudha S, Dept.
Asian Journal of Pharmaceutical Technology & Innovation ISSN: 2347-8810 Research Article Received on: 09-11-2016 Accepted on: 20-11-2016 Published on: 15-12-2016 Corresponding Author: * Amudha S, Dept.
Determination of Sedative Component in Chinese Medicines by High Resolution Chromatography-Electron Impact-Mass Spectrometry
 Journal of Analytical Sciences, Methods and Instrumentation, 2012, 2, 13-17 http://dx.doi.org/10.4236/jasmi.2012.21003 Published Online March 2012 (http://www.scirp.org/journal/jasmi) 13 Determination
Journal of Analytical Sciences, Methods and Instrumentation, 2012, 2, 13-17 http://dx.doi.org/10.4236/jasmi.2012.21003 Published Online March 2012 (http://www.scirp.org/journal/jasmi) 13 Determination
[ APPLICATION NOTE ] Profiling Mono and Disaccharides in Milk and Infant Formula Using the ACQUITY Arc System and ACQUITY QDa Detector
![[ APPLICATION NOTE ] Profiling Mono and Disaccharides in Milk and Infant Formula Using the ACQUITY Arc System and ACQUITY QDa Detector [ APPLICATION NOTE ] Profiling Mono and Disaccharides in Milk and Infant Formula Using the ACQUITY Arc System and ACQUITY QDa Detector](/thumbs/83/88757464.jpg) Profiling Mono and Disaccharides in Milk and Infant Formula Using the ACQUITY Arc System and ACQUITY QDa Detector Mark Benvenuti, Gareth Cleland, and Jennifer Burgess Waters Corporation, Milford, MA, USA
Profiling Mono and Disaccharides in Milk and Infant Formula Using the ACQUITY Arc System and ACQUITY QDa Detector Mark Benvenuti, Gareth Cleland, and Jennifer Burgess Waters Corporation, Milford, MA, USA
Hyderabad, India. Department of Pharmaceutical Chemistry, Glocal University, Saharanpur, India.
 International Journal On Engineering Technology and Sciences IJETS RP-HPLC Method development and validation for the Simultaneous Estimation of Metformin and Empagliflozine in Tablet Dosage Form Shaik
International Journal On Engineering Technology and Sciences IJETS RP-HPLC Method development and validation for the Simultaneous Estimation of Metformin and Empagliflozine in Tablet Dosage Form Shaik
Development and Validation of a Polysorbate 20 Assay in a Therapeutic Antibody Formulation by RP-HPLC and Charged Aerosol Detector (CAD)
 LIFE SCIENCES AR ENOUGH? HOW DO YOU KNOW RAW MATERIALS ARE PURE? HOW DO YOU EVALUATE PRODUCT PACKAGING? HOW DO YOU DO YOU KNOW WHAT ANALYTICAL TECHNIQUE TO USE? HOW DO YOU SIMULTANEOUSLY TEST FOR TWO BYPRODUCTS?
LIFE SCIENCES AR ENOUGH? HOW DO YOU KNOW RAW MATERIALS ARE PURE? HOW DO YOU EVALUATE PRODUCT PACKAGING? HOW DO YOU DO YOU KNOW WHAT ANALYTICAL TECHNIQUE TO USE? HOW DO YOU SIMULTANEOUSLY TEST FOR TWO BYPRODUCTS?
Flupyradifurone. HPLC Method
 HPLC Method CIPAC Collaboration Trial according to CIPAC Information Sheet No 308 by Alexandra Michel Crop Science Division Bayer Aktiengesellschaft Alfred-Nobel-Str. 50, Building 6820 40789 Monheim am
HPLC Method CIPAC Collaboration Trial according to CIPAC Information Sheet No 308 by Alexandra Michel Crop Science Division Bayer Aktiengesellschaft Alfred-Nobel-Str. 50, Building 6820 40789 Monheim am
NOVEL RP-HPLC METHOD. B.Lakshmi et a. concentration range KEY INTRODUCTION. Diltiazem is used to. . It works by of contractionn of the. (dilate).
 B.Lakshmi et a ISSN-2319-2119 al, The Experiment, January. 2013 Vol..6(4), 365-371 A NOVEL RP-HPLC METHOD FOR THE DETERMINATION OF DILTIAZEM IN PHARMACEUTICAL DRUG PRODUCTS ABSTRACT High resolution RP-HPLC
B.Lakshmi et a ISSN-2319-2119 al, The Experiment, January. 2013 Vol..6(4), 365-371 A NOVEL RP-HPLC METHOD FOR THE DETERMINATION OF DILTIAZEM IN PHARMACEUTICAL DRUG PRODUCTS ABSTRACT High resolution RP-HPLC
CHAPTER-5. Sumatriptan Succinate
 110 CHAPTER-5 Sumatriptan Succinate 111 CHAPTER-5 Chapter-5 : Sumatriptan succinate S. No. Name of the Sub- Title Page No. 5.1 Introduction 112-113 5.2 Experimental 114-121 5.3 Method validation Procedure
110 CHAPTER-5 Sumatriptan Succinate 111 CHAPTER-5 Chapter-5 : Sumatriptan succinate S. No. Name of the Sub- Title Page No. 5.1 Introduction 112-113 5.2 Experimental 114-121 5.3 Method validation Procedure
Research on Extraction Process of Gallic Acid from Penthorum chinense Pursh by Aqueous Ethanol
 Green and Sustainable Chemistry, 2015, 5, 63-69 Published Online May 2015 in SciRes. http://www.scirp.org/journal/gsc http://dx.doi.org/10.4236/gsc.2015.52009 Research on Extraction Process of Gallic Acid
Green and Sustainable Chemistry, 2015, 5, 63-69 Published Online May 2015 in SciRes. http://www.scirp.org/journal/gsc http://dx.doi.org/10.4236/gsc.2015.52009 Research on Extraction Process of Gallic Acid
USP Method Transfer of Ziprasidone HCl from HPLC to UPLC
 Mia Summers and Kenneth J. Fountain Waters Corporation, Milford, MA, USA APPLICATION BENEFITS Updating USP Methods from HPLC to UPLC using sub-2 µm columns 85% decrease in analysis time, faster throughput
Mia Summers and Kenneth J. Fountain Waters Corporation, Milford, MA, USA APPLICATION BENEFITS Updating USP Methods from HPLC to UPLC using sub-2 µm columns 85% decrease in analysis time, faster throughput
Study of the British Pharmacopeia method on methimazole (thiamazole) content in carbimazole tablets
 Journal of Pharmaceutical and Biomedical Analysis 16 (1998) 785 792 Study of the British Pharmacopeia method on methimazole (thiamazole) content in carbimazole tablets Maria Aletrari *, Popi Kanari, Dora
Journal of Pharmaceutical and Biomedical Analysis 16 (1998) 785 792 Study of the British Pharmacopeia method on methimazole (thiamazole) content in carbimazole tablets Maria Aletrari *, Popi Kanari, Dora
ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006)
 September 2006 RESTRICTED ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006) This document was provided by a contracted quality control laboratory.
September 2006 RESTRICTED ZIDOVUDINE, LAMIVUDINE AND ABACAVIR TABLETS Draft proposal for The International Pharmacopoeia (September 2006) This document was provided by a contracted quality control laboratory.
UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes
 UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes Catalin E. Doneanu, Weibin Chen, and Jeffrey R. Mazzeo Waters Corporation, Milford, MA, U.S. A P P L I C AT ION B E N E F
UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes Catalin E. Doneanu, Weibin Chen, and Jeffrey R. Mazzeo Waters Corporation, Milford, MA, U.S. A P P L I C AT ION B E N E F
THIN LAYER CHROMATOGRAPHY
 THIN LAYER CHROMATOGRAPHY Thin layer chromatography is the best known technique of plant biochemistry. TLC is used for preliminary separation and determination of plant constituents. It is helpful for
THIN LAYER CHROMATOGRAPHY Thin layer chromatography is the best known technique of plant biochemistry. TLC is used for preliminary separation and determination of plant constituents. It is helpful for
Qualitative and quantitative determination of phenolic antioxidant compounds in red wine and fruit juice with the Agilent 1290 Infinity 2D-LC Solution
 Qualitative and quantitative determination of phenolic antioxidant compounds in red wine and fruit juice with the Agilent 1290 Infinity 2D-LC Solution Application Note Food Testing Author Edgar Naegele
Qualitative and quantitative determination of phenolic antioxidant compounds in red wine and fruit juice with the Agilent 1290 Infinity 2D-LC Solution Application Note Food Testing Author Edgar Naegele
ISSN (Print)
 Scholars Academic Journal of Pharmacy (SAJP) Sch. Acad. J. Pharm., 2014; 3(3): 240-245 Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com
Scholars Academic Journal of Pharmacy (SAJP) Sch. Acad. J. Pharm., 2014; 3(3): 240-245 Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com
Ultra High Performance Liquid Chromatograph. Nexera X2 C196-E079
 Ultra High Performance Liquid Chromatograph Nexera X2 C196-E79 Maximizing the Potential of UHPLC/HPLC Analyses Intelligence Intelligent data solutions, including a new i -PDeA peak calculation method for
Ultra High Performance Liquid Chromatograph Nexera X2 C196-E79 Maximizing the Potential of UHPLC/HPLC Analyses Intelligence Intelligent data solutions, including a new i -PDeA peak calculation method for
Research Article Reverse Phase High Performance Liquid Chromatographic Determination of Flunarazine in Tablet Dosage Form
 Research Article Reverse Phase High Performance Liquid Chromatographic Determination of Flunarazine in Tablet Dosage Form MS. Palled*, Prachi Naik and AR. Bhat Department of Pharmaceutical Analysis, KLE
Research Article Reverse Phase High Performance Liquid Chromatographic Determination of Flunarazine in Tablet Dosage Form MS. Palled*, Prachi Naik and AR. Bhat Department of Pharmaceutical Analysis, KLE
Pelagia Research Library
 Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2014, 5(5):91-98 ISSN: 0976-8688 CODEN (USA): PSHIBD A novel RP-HPLC method development and validation of Perindopril Erbumine in
Available online at www.pelagiaresearchlibrary.com Der Pharmacia Sinica, 2014, 5(5):91-98 ISSN: 0976-8688 CODEN (USA): PSHIBD A novel RP-HPLC method development and validation of Perindopril Erbumine in
ANALYTICAL TECHNIQUES FOR THE QUALITY OF ANTI- AIDS DRUGS
 Research Article N. Bala Krishna, IJPRBS, 2013; Volume 2(1): 63-77 ISSN: 2277-8713 IJPRBS N.BALAA KRISHNA, Dr. M.V.V NAGESWARA REDDY IJPRBS-QR CODE PAPER-QR CODE DEVELOPMENT OF NEW ANALYTICAL TECHNIQUES
Research Article N. Bala Krishna, IJPRBS, 2013; Volume 2(1): 63-77 ISSN: 2277-8713 IJPRBS N.BALAA KRISHNA, Dr. M.V.V NAGESWARA REDDY IJPRBS-QR CODE PAPER-QR CODE DEVELOPMENT OF NEW ANALYTICAL TECHNIQUES
PAPRIKA EXTRACT SYNONYMS DEFINITION DESCRIPTION FUNCTIONAL USES CHARACTERISTICS
 PAPRIKA EXTRACT Prepared at the 77 th JECFA, published in FAO JECFA Monographs 14 (2013), superseding tentative specifications prepared at the 69 th JECFA (2008). An ADI of 0-1.5 mg/kg bw was allocated
PAPRIKA EXTRACT Prepared at the 77 th JECFA, published in FAO JECFA Monographs 14 (2013), superseding tentative specifications prepared at the 69 th JECFA (2008). An ADI of 0-1.5 mg/kg bw was allocated
Application Note. Agilent Application Solution Analysis of fat-soluble vitamins from food matrix for nutrition labeling. Abstract.
 Agilent Application Solution Analysis of fat-soluble vitamins from food matrix for nutrition labeling Application Note Food 1 Agilent 12 Infinity 1 Author Siji Joseph Agilent Technologies, Inc. Bangalore,
Agilent Application Solution Analysis of fat-soluble vitamins from food matrix for nutrition labeling Application Note Food 1 Agilent 12 Infinity 1 Author Siji Joseph Agilent Technologies, Inc. Bangalore,
[Application Note] TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY
![[Application Note] TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY [Application Note] TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY](/thumbs/74/70091865.jpg) TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY Camille Chatenay, 1,2 Fabien Bévalot, 1,2 Cyril Mounier, 1 Jean Michel Prévosto 1 1 Hôpital d Instruction des Armées Desgenettes,
TOXICOLOGY SCREENING BY UPLC/PDA IN COMBINATION WITH AN EXTENSIVE COMPOUND LIBRARY Camille Chatenay, 1,2 Fabien Bévalot, 1,2 Cyril Mounier, 1 Jean Michel Prévosto 1 1 Hôpital d Instruction des Armées Desgenettes,
Umapathi et al. Keywords: Fixed dose combination; Rifampin quinone; Tuberculosis; Sodium ascorbate; Quantitative determination; HPLC
 Tropical Journal of Pharmaceutical Research December 2010; 9 (6): 587-593 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online
Tropical Journal of Pharmaceutical Research December 2010; 9 (6): 587-593 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online
Method Development and Validation for Simultaneous Estimation of Atorvastatin and Ezetimibe in Pharmaceutical Dosage Form by HPLC
 World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
Isolation of five carotenoid compounds from tangerine tomatoes
 Isolation of five carotenoid compounds from tangerine tomatoes Thesis Thomas Haufe Advisor: Steven J. Schwartz, Ph.D Department of Food Science and Technology Presented in fulfillment of the requirements
Isolation of five carotenoid compounds from tangerine tomatoes Thesis Thomas Haufe Advisor: Steven J. Schwartz, Ph.D Department of Food Science and Technology Presented in fulfillment of the requirements
CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS
 129 CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS 130 6.1. Introduction Valsartan is an orally active specific angiotensin II blocker effective in lowering
129 CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS 130 6.1. Introduction Valsartan is an orally active specific angiotensin II blocker effective in lowering
Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin in Pharmaceutical Dosage Form using RP-HPLC Method
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.4, pp 1736-1744, Oct-Dec 2013 Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.4, pp 1736-1744, Oct-Dec 2013 Development and Validation for Simultaneous Estimation of Sitagliptin and Metformin
Isocratic Reversed Phase Liquid Chromatographic Method Validation for the Determination of Cilostazol in Pure and Formulations
 Human Journals Research Article October 2015 Vol.:4, Issue:3 All rights are reserved by Rambabu K et al. Isocratic Reversed Phase Liquid Chromatographic Method Validation for the Determination of Cilostazol
Human Journals Research Article October 2015 Vol.:4, Issue:3 All rights are reserved by Rambabu K et al. Isocratic Reversed Phase Liquid Chromatographic Method Validation for the Determination of Cilostazol
AN HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION OF RISPERIDONE AND TRIHEXYPHENIDYL HYDROCHLORIDE FROM BULK AND DOSAGE FORMS.
 Research article Hygeia.J.D.Med.vol.3 (1), 2011, 29-33. www.hygeiajournal.com HYGEIA JOURNAL FOR DRUGS AND MEDICINES ISSN 2229 3590 (online) ISSN 0975 6221 (print) AN HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION
Research article Hygeia.J.D.Med.vol.3 (1), 2011, 29-33. www.hygeiajournal.com HYGEIA JOURNAL FOR DRUGS AND MEDICINES ISSN 2229 3590 (online) ISSN 0975 6221 (print) AN HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION
Available Online through (or) IJPBS Volume 2 Issue 4 OCT-DEC Research Article Pharmaceutical Sciences
 Page247 Research Article Pharmaceutical Sciences ULTRA PERFORMANCE LIQUID CHROMATOGRAPHIC METHOD DEVELOPMENT AND VALIDATION FOR THE QUANTIFICATION OF IMPURITIES AND DEGRADATION PRODUCTS IN THE METOPROLOL
Page247 Research Article Pharmaceutical Sciences ULTRA PERFORMANCE LIQUID CHROMATOGRAPHIC METHOD DEVELOPMENT AND VALIDATION FOR THE QUANTIFICATION OF IMPURITIES AND DEGRADATION PRODUCTS IN THE METOPROLOL
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR ESTIMATION OF LACOSAMIDE IN BULK AND ITS PHARMACEUTICAL FORMULATION
 http://www.rasayanjournal.com Vol.4, No.3 (2011), 666-672 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR IN BULK AND ITS PHARMACEUTICAL FORMULATION V.Kalyan Chakravarthy*
http://www.rasayanjournal.com Vol.4, No.3 (2011), 666-672 ISSN: 0974-1496 CODEN: RJCABP DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR IN BULK AND ITS PHARMACEUTICAL FORMULATION V.Kalyan Chakravarthy*
Analysis of Isoflavones with the PerkinElmer Flexar FX-15 UHPLC System Equipped with a PDA Detector
 application Note UHPLC Author Njies Pedjie PerkinElmer, Inc. Shelton, CT 06484 USA Analysis of Isoflavones with the PerkinElmer Flexar FX-15 UHPLC System Equipped with a PDA Detector Introduction Foods
application Note UHPLC Author Njies Pedjie PerkinElmer, Inc. Shelton, CT 06484 USA Analysis of Isoflavones with the PerkinElmer Flexar FX-15 UHPLC System Equipped with a PDA Detector Introduction Foods
Estimation of Zanamivir Drug present in Tablets using RP-HPLC Method
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol. 3, No.1, pp 180-186, Jan-Mar 2011 Estimation of Zanamivir Drug present in Tablets using RP-HPLC Method Ravindra Reddy.Y*,
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol. 3, No.1, pp 180-186, Jan-Mar 2011 Estimation of Zanamivir Drug present in Tablets using RP-HPLC Method Ravindra Reddy.Y*,
Development and Validation of a Simultaneous HPLC Method for Quantification of Amlodipine Besylate and Metoprolol Tartrate in Tablets
 Journal of PharmaSciTech 0; ():- Research Article Development and Validation of a Simultaneous HPLC Method for Quantification of Amlodipine Besylate and Metoprolol Tartrate in Tablets * Sayyed Hussain,
Journal of PharmaSciTech 0; ():- Research Article Development and Validation of a Simultaneous HPLC Method for Quantification of Amlodipine Besylate and Metoprolol Tartrate in Tablets * Sayyed Hussain,
