DIFFUSION- AND REACTION RATE-LIMITED REDOX PROCESSES MEDIATED BY QUINONES THROUGH BILAYER LIPID MEMBRANES
|
|
|
- Alexis Sharp
- 6 years ago
- Views:
Transcription
1 DIFFUSION- AND REACTION RATE-LIMITED REDOX PROCESSES MEDIATED BY QUINONES THROUGH BILAYER LIPID MEMBRANES ASHER ILANI AND TAMAR KRAKOVER Department ofphysiology, Hebrew University, Hadassah Medical School, Jerusalem, Israel ABSTRACT The mediation of redox reactions through bilayer lipid membranes was studied. With an appropriate choice of electron acceptors the redox process can be limited either by the chemical reaction rate between the mediator and the reactants or by the shuttle frequency of the mediator through the membrane. Both modes were demonstrated for redox reactions mediated by 2,6 dichlorobenzoquinone (DCBQ) and by a-tocopherol with ascorbate entrapped inside vesicles using ferricyanide (a mild oxidant) or hexachloroiridate (a strong oxidant) in the external solution. The redox processes were reaction rate-limited and diffusion-limited for ferricyanide and hexachloroiridate, respectively. The kinetics of the redox processes in the diffusion- and the reaction rate-limited modes allows the determination of the shuttle frequencies and of the interfacial reaction rates of the mediators, respectively. The shuttle frequencies of DCBQ and a-tocopherol were -8 and 0.08 s-', respectively, in L-a-dipalmitoyl phosphatidylcholine (DPPC) cholesterol vesicles at 250C. Interfacial reaction rates between the mediators and ferricyanide were about two- and tenfold lower compared with bulk reaction rates for DCBQ (water) and tocopherol (50% ethanol solution), respectively, i.e., tocopherol is relatively less accessible to aqueous oxidants at the membrane interface. Tocopherol and oxidized tocopherol are reversible hydrophobic redox couples that interact very rapidly with strong oxidants. In both modes of mediation DCBQ was more effective than ca-tocopherol. INTRODUCTION Redox reactions across bilayer lipid membranes mediated by various additives have been demonstrated by several investigators (see, for example, Hinkle, 1970; Hauska, 1977; Futami et al., 1979a, b; Runquist and Loach, 1981). A detailed analysis of a kinetic model has not been presented. However, in one of the above studies (Runquist and Loach, 1981), it was explicitly stated that the rate of the redox reaction was limited by the reaction at one side of the interface and not by the shuttle of the mediator across the membrane. This paper deals theoretically and empirically with the mediation of redox reactions across lipid vesicles by quinone-like substances. The distinction between diffusionlimited and reaction rate-limited redox processes is a central theme of this presentation. Such an analysis enables the determination of the shuttle frequency of the mediator molecules across the membrane and of the rate constants of the reactions at the membrane interfaces. By comparing the latter with the rate constant of the reaction in bulk phases, it is possible to arrive at some conclusions regarding the protrusion of the reactive part of the mediator at the water-membrane interface. THEORY In the following model the oxidized and reduced forms of the mediator will be denoted by Q and QH2, respectively. The total amount of the mediator, i.e., Q + QH2, will be denoted by Q. The nominal concentration of the mediator added to the preparation will be denoted by brackets, e.g., [QJ], the concentration in the aqueous phase as [Q]w and [QH2],, and its presence in the membrane will be expressed in terms of surface density (Q) and (QH2). It is assumed that and (Q) = [Q]W (QH2) = O[QH2]., (1) in which the constant d has the dimension of length transforming volume concentration to surface concentration. The mediation of redox reactions through lipid membranes will be divided into apparent three stages: (a) The chemical reaction between the reduced mediator, QH2, and the oxidant, Ox, at the oxidizing (outer) side of the vesicle. This stage can be further divided into both the reaction in the aqueous medium given by k aox + QH2(aqueous) -- Q(aqueous) + ared, (2a) BIOPHYS. J. Biophysical Society * /87/02/161/07 $1.00 Volume 51 February
2 and the reaction with the mediator confined to the membrane interface, aox + QH2(membranous) k' Q(membranous) + ared, (2b) in which a is a stoichiometric factor, usually 2, and Red is the reduced form of the oxidant. Although one molecule of reduced mediator eventually transfers two electrons, the rates of reactions 2a and 2b vary linearly with oxidant concentration rather than its square, since the intermediate semiquinone is highly reactive. The back-reactions of 2a and 2b are assumed to be negligible for the oxidants used in this study. (b) The chemical reaction at the reducing (inner) side of the vesicle, which can be written in analogy to Eqs. 2a and 2b as follows: and k Q(aqueous) + ared' QH2(aqueous) + aox' (3a) Q(membranous) + ared' QH2(membranous) + aox' (3b) in which Red' and Ox' are the redox species present at the reducing side of the membrane. In this presentation the situation will be analyzed for the case in which the interior of the vesicles contains a strong reductant, Red', at a relatively high concentration so that all the mediators in the inner side of the vesicles are in the reduced form, i.e., reactions 3a and 3b do not constitute a limit on the rate of the redox process. (c) The shuttle of the mediator Q and QH2, through the membrane, i.e., 2 (Q) out - (Q)in (Q2H2)OUt (Q2H2)"'s (4) where Q is the shuttle frequency. Superscripts "in" and "out" denote inner and outer sides of the vesicles, respectively. In Eqs. 1 and 4, it is assumed that there is no difference between Q and QH2 in terms of their interaction with the membrane, i.e., Q and fd are assumed to be the same for Q and QH2. At a particular vesicle concentration the amount of the mediator adsorbed to the vesicle membrane area, A, constitutes a constant fraction, a, of the mediator added to the vesicle preparation. Therefore [Q,]w = (1 - a)[q,] (5) Total Q in membrane = a[q,] V (6) and (Q,) = a[q,] V/2A, (7) in which [Q,] is the nominal mediator concentration in the vesicle preparation, V is the volume of the preparation, and (Q,) is the surface concentration of Q + QH2 at each interface. The factor 2 in Eq. 7 signifies that the total interfacial area is about twice the vesicle area. The factor a is a function of,b and the total vesicle area. By dividing Eq. 5 by Eq. 7 and using Eq. 1 it follows that = av/2a(1 - a), (8) from which an expression for a can be obtained, i.e., a = 2A#/(V + 2A13). (9) The redox process will be analyzed next for the two extreme situations: a diffusion (or permeability)-limited process and a reaction rate-limited process. Diffusion (or Permeability)-limited Process This applies to the case in which ak'[ox] + (1 - a)k[ox] >> 2. (10) This means that all the mediator in the outer side of the vesicle is in the oxidized form, Q. The rate of the redox process is determined, therefore, by the rate of arrival of the reduced mediator at the oxidizing interface, dqh2/dt. The rate that QH2 molecules leave the reducing side of the vesicles is determined by the shuttle frequency, Q, and the surface concentration of QH2 in the inner side of the membrane, (QH2)'n, i.e., dqh2/dt = QA(QH2)'n. (1 1) Therefore, the rate of reduction of Ox is - Vd[Ox]/dt = aqa(qh2)i'. In a steady state the rate of QH2 leaving the vesicles is equal to the rate of Q entering the vesicles. Therefore, (QH2)in= (Q)out = (Qt). Introducing Eq. 7 into Eq. 12, it follows that Therefore, - Vd[Ox]dt = 0.5a[Q,]QaV. d[ox]/dt = -0.5 ara[q,]. (12) (13) (14) (15) Eq. 15 predicts a linear decrease of [Ox] with time. As noted before the value of a is generally 2. If a can be determined independently the value of Q for a mediator can be calculated. Except for a direct measurement of a, which can be performed in principle by straightforward dialytic experiments, Eq. 15 allows an estimate of a by comparing the 162 BIOPHYSICAL JOURNAL VOLUME
3 rate of the redox reaction in a diluted vesicle preparation with the rate at the original vesicle preparation. If [QJ is kept constant the reaction rate should vary only through the change in a. From the dependence of a on the total vesicles area (see Eq. 9), it can be shown that = 1/f + (I -a)/a' (16) in which f is the factor of dilution and af is the value of a for the diluted preparation. Reaction Rate-limited Process This applies to the case in which»>> ak'[oxj + (1 - a)k[ox]. (17) This condition implies that most of the mediator in the outer side of the membrane is in its reduced form. In a steady state the formation rate of Q in the bulk and at the interface is balanced by the entry rate of Q into the vesicles, which in turn is equal to the exit rate of QH2 from the vesicles. The reduction rate of the oxidant is determined by Eqs. 2a and 2b. If k' in Eq. 2b has the same dimensions as k, it follows that k'. [Ox] * (QH2) represents the change d(qh2)/dt. To express the reaction 2b in terms of d[ox]/ dt, the above product should be multiplied not only by the factor a, but also by the ratio of total vesicles area to the volume of the vesicle preparation, i.e., d[ox]/dt = -k'[ox](qh2) * a * (A/V). (17a) Since (QH2) = (Qt), by introducing Eq. 7 into the above expression and adding the term due to reaction 2a, and taking into account Eq. 5, it follows that -d[ox]dt = ak[ox] [Qj(1 - a) ak'a[q,] [Ox]. (18) From Eq. 18 it follows that -d In[Ox]/dt = [ak(i - a) ak'a] [Qj. (19) Integration of Eq. 19 leads to in which [Ox] = [Ox]. exp - (t/t), (20) /Tr= [ak(l - a) + 0.5ak'al[Q,] (21) and [Ox]0 is the concentration of the oxidant at time zero. Since k can be determined by studying the reaction rate between the oxidant and the mediator in an aqueous solution, the value of k' can be calculated if a is known. For extremely hydrophobic mediators where a = 1 Eq. 19 allows a direct estimate of k'. Eq. 21 shows that 1/T varies linearly with the nominal mediator concentration [Qj]. The change of 11/r with a depends on the difference between k and 0.5k'. Thus, for k > 0.5k', 1 /r should increase with the decrease of a; this means that diluting the vesicles while keeping [QJ] constant should lead to a decrease in r. The model presented is not inconsistent with an equilibrium situation always prevailing between mediators in the bulk aqueous solution and those in the interface. Such an equilibrium is assumed to occur in the diffusion-limited processes. In the reaction rate-limited processes all that is required is that only a small fraction of the outside facing mediators are oxidized. This small fraction should give rise to an influx of oxidized mediators that is equal to the amount of oxidized mediators formed by the sum of reactions 2a and 2b. Although there is a realistic possibility that an equilibrium is established instantaneously between the interface and the bulk phase, there is nothing in the model or the results that excludes a nonequilibrium state. METHODS Liposomes made of DPPC (L-a-dipalmitoyl phosphatidylcholine) and cholesterol at a ratio of 10:1 were prepared using the injection method (Batzri and Korn, 1973); an ethanolic solution of the DPPC-cholesterol mixture was injected into a well stirred aqueous solution at a ratio of 1:12.5. Since DPPC has a transition temperature of 410C the aqueous solution was preheated to a temperature over 600C. The final DPPC concentration was 1.85 mm. The average liposome diameter was -200 A, as shown electron microscopically. The outer solution was then changed using Sephadex G columns following the method used by Radian and Kanner (1985). In this procedure l-ml minicolumns were filled with the Sephadex preswollen by the solution planned to be outside. Before use, the minicolumns were placed in glass tubes and centrifuged at low speed (- 1,500 g) for 1.5 min. Small portions of the liposome preparation (0.22 ml) were then carefully applied onto the precentrifuged columns which had been placed in clean glass tubes. Another centrifugation at the above speed and time yielded liposomes free of ethanol with the desired solutions inside and outside. In a sample of vesicle preparations the phospholipid concentration was determined before and after passage through the column and was found to be the same. In the experiments in which ferricyanide was used as oxidant the inner solution contained 0.5 M ascorbic acid and 20 mm potassium phosphate adjusted with KOH to ph 5.8. The outer solution contained 0.5 M KCI and 20 mm potassium phosphate adjusted to the same ph. When hexachloroiridate was used as an oxidant the inner solution contained 0.5 M ascorbic acid and 20 mm potassium acetate adjusted with KOH to ph 5.0. The outer solution contained 0.5 KCI and 20 mm potassium acetate adjusted to the same ph. a-tocopherol was dissolved in the ethanolic liposome-forming solution. Its final concentration in the liposome preparation varied between 5 x 10 and 2 x 10-4 M as indicated in the figures and tables. The spectrum of the a-tocopherol-containing vesicles showed a clear peak at 292 nm. From the size of this peak it was inferred that tocopherol concentration after passage through the column was at least 90% of the original concentration. The reductive capacity of the vesicle preparations with and without tocopherol were about the same. It is inferred, therefore, that tocopherol did not affect significantly the size of the vesicles. A 2,6 dichlorobenzoquinone (DCBQ) aqueous solution was prepared as follows: a portion of DCBQ ethanolic solution was dried in a glass tube with a stream of N2. The desired outer solution was then added and was well stirred. Small amounts of this solution were applied to the already formed liposome preparation. DCBQ concentration varied between 5 x I0-' and 5 x I0 - M as indicated in the figure legends and tables. DPPC, cholesterol, and a-tocopherol were purchased from Sigma ILANI AND KRAKOVER Diffusion- and Reaction Rate-limited Redox Processes 163
4 Chemical Co., (St. Louis, MO), potassium hexachloroiridate from Alfa Scientific Inc. (Hayward, CA), and DCBQ from Eastman Kodak Co. (Rochester, NY). The reduction of ferricyanide and hexachloroiridate were followed by a spectrophotometer (Uvikon 810, Kontron Analytical, Everett, MA) at 420 nm and 487 nm, respectively. The reactions were carried out at temperatures of C. RESULTS I. Hexachloroiridate-Ascorbate Redox Reactions Mediated by DCBQ and a-tocopherol To satisfy the conditions for a diffusion-limited rate process the strong oxidizer hexachloroiridate, Ir(Cl)6-, was used as an oxidant. Fig. 1 depicts the reduction of hexachloroiridate with time. It can be seen that with the addition of DCBQ the reduction proceeds linearly with time and that linearity is maintained for consecutive additions of the oxidant. It is clear from Fig. 1 that hexachloroiridate disappears exponentially even in the absence of any defined mediator. Although this reaction is significant compared with the stability of hexachloroiridate in an aqueous solution of the same composition, it is small compared with the mediated redox reaction. Fig. 2 shows the reduction of hexachloroiridate mediated by a-tocopherol. Here too, reduction proceeds linearly with time, and linearity is maintained until the internal ascorbate is exhausted. Table I summarizes the results for the rates of redox reactions between hexachloroiridate and ascorbate mediated by DCBQ or tocopherol for various mediator and lipid concentrations. Note that in diluting the vesicles by the factors of 5 and 20 and keeping A &C= M AA =0.010 A t 0.25 Q25 I Ir(CI)6-- lc= MI la= min I t1t mm FIGURE 2 Time course of reduction of hexachloroiridate mediated by a-tocopherol after consecutive additions (arrows) of the oxidant to a vesicle preparation containing 0.5 M ascorbate inside the vesicles. The amount of hexachloroiridate added is indicated in terms of its concentration in the preparation. The vesicle preparation contained DPPC at 1.85 mm, cholesterol at 0.18 mm, and a-tocopherol at 0.2 mm. the DCBQ concentration constant, the rate of the redox process decreased only by the factors of 0.62 and 0.22, respectively. According to Eq. 15 this means that a dilution by the factors of 5 and 20 led to a decrease of a by 0.62 and 0.22, respectively. By Eq. 16 it can be shown that this corresponds to a value for a of 0.83 at 1.85 mm DPPC. When the values of a and [Qt] are introduced into Eq. 15, the value of Q for DCBQ becomes -8/s. The value of a for tocopherol is very close to 1. The shuttle frequency, Q, for tocopherol according to the figures presented in Table I is, therefore, -0.08/s. II. Ferricyanide-Ascorbate Redox Reactions Mediated by DCBQ and a-tocopherol Fig. 3 shows typical reduction experiments of ferricyanide in the presence of DCBQ. Curve A shows that in the TABLE I REDUCTION RATES OF HEXACHLOROIRIDATE IN VESICLE PREPARATIONS CONTAINING 0.5 M POTASSIUM ASCORBATE Time (min) FIGURE 1 Concentration change in hexachloroiridate as monitored by the decrease in absorbance at 487 nm after the addition of potassium hexachloroiridate at -0.5 mm to a vesicle preparation containing 0.5 M ascorbate inside the vesicles. Curve A represents the results without any mediator. Curves B and C represent the absorbance change after consecutive additions of hexachloroiridate to the same preparation in the presence of 5 x 10-7 M DCBQ. The points on curve A correspond to an exponential decrease in concentration with a time constant of 20 min. At the end of the reaction there is a small constant increment in absorbance relative to the initial value. The vesicle preparations contained DPPC at 1.85 mm and cholesterol at 0.18 mm. 164 Mediator Concentration [DPPC] a d[ox]/dt M mm lo0 mci/cm3. s DCBQ 5 x 10' * 3.5 ± 0.66 (24) DCBQ 5 x 10-' * 2.17 ± 0.28 (3) DCBQ 5 x 10-' * 0.76 ± 0.08 (3) a-tocopherol 5 x 10-' ± 0.5 (5) a-tocopherol 10-s (2) The outer medium contained 0.5 M KCI. Both sides contained 20 mm acetate at ph 5.0. Numbers in parentheses denote number of determinations. ±, standard deviations. *Calculated values according to Eqs. 15 and 16. BIOPHYSICAL JOURNAL VOLUME
5 1.5 r * E c0.75 c E 1.0 * 'U U ** Time (min) FIGURE 3 Time course of reduction of ferricyanide mediated by DCBQ as monitored by the change in absorbance at 420 nm after its addition to a vesicle preparation containing 0.5 M ascorbate inside the vesicles. Note that in the absence of the mediator the reaction is negligible. Curves B and C represent results of experiments in which the amount of ferricyanide added was less or more, respectively, as compared with the equivalent amount of ascorbate inside the vesicles. The points on the curves represent a calculated exponential course with time constants of 4.8 and 4.0 min for curves B and C, respectively. Note that curve B is a single exponential throughout the entire range. absence of the quinone the decline of ferricyanide is extremely slow. Curves B and C represent experiments in which the amount of ferricyanide added to the liposome solution was less or more, respectively, compared with the equivalent amount of ascorbate present inside the vesicles. It can be seen that for the case where ascorbate is in excess the decline of ferricyanide in the solution follows an exponential course throughout the entire reaction. On the other hand when ferricyanide is in excess the reaction rate toward the end of the process decreases relative to the initial rate. This is of course expected since under these conditions, as the internal ascorbate is exhausted, the reaction at the reducing interface becomes rate limiting. These results validate the assumption that as long as the ascorbate concentration does not drop below -50 mm, the reduction of the quinone at the reducing interface is not rate limiting. The "pure" exponential nature of curve B implies also that the vesicles in the preparation were quite homogeneous in terms of an area/volume ratio, a fact that corresponds to the electron microscopic picture of the vesicles. Fig. 4 shows the results of several experiments in which the timie constant of the exponential decay of ferricyanide was measured. As expected from Eq. 21 the time constant, r, of the redox reaction process is inversely proportional to the nominal concentration of DCBQ in the solution. An example for a tocopherol-mediated redox process with ferricyanide is shown in Fig. 5. Table II summarizes the results of the time constant determinations of redox reactions between ascorbate and ferricyanide mediated by DCBQ and tocopherol. +4 * [DCBQ] 10-5 M FIGURE 4 The reciprocal of the time constant of ferricyanide reduction by ascorbate-containing vesicles as a function of DCBQ concentration. The results were obtained for a particular vesicle preparation containing 1.85 mm DPPC and 0.18 mm cholesterol. It is noteworthy that the time constant of DCBQmediated reactions decreases when the vesicles are diluted. This should happen according to Eq. 21 if k > 0.5'. The rate constant of the reaction between ferricyanide and DCBQ in water, k, was found to be.400 M-' s-'. Using the value of r from Table II and the value of a (0.83) from Table I, the value of k' according to Eq. 21 is -100 M' s. A factor of 2 between k and k' is to be expected since a mediator in the aqueous solution can be approached by a ferricyanide molecule from a spatial angle of 4ir steradians compared with a spatial angle of 27r steradians for an interfacially confined mediator. Thus it can be concluded that the interfacial reaction rate between DCBQ and ferricyanide is only slightly less than the reaction rate in the aqueous solution Fe(CN) E H ( Q0O I 30.0 Time (min) FIGURE 5 Time course of a-tocopherol-mediated ferricyanide reduction by ascorbate-containing vesicles. The mediator was included in the vesicle membranes at a final concentration of 0.2 mm. The other constituents of the vesicle membrane were DPPC at 1.85 mm and cholesterol at 0.18 mm. ILANI AND KRAKOVER Diffusion- and Reaction Rate-limited Redox Processes 165
6 TABLE II TIME CONSTANT, r, FOR THE EXPONENTIAL DECREASE OF FERRICYANIDE IN VESICLE SOLUTIONS CONTAINING 0.5 M POTASSIUM ASCORBATE INSIDE THE VESICLES Mediator Concentration DPPC T concentration M mm min DCBQ 2 x 10' ± 0.3 (7) DCBQ 2 x 10' ± 0.5 (5) a-tocopherol 2 x ± 3.0 (3) a-tocopherol 5 x 10' ± 6.0 (3) The outer medium contained 0.5 M KCl. Both solutions contained 20 mm phosphate at ph 5.7. Numbers in parentheses denote number of determinations. ±, standard deviations. The reaction rate constant between tocopherol and ferricyanide, k', is, according to the results presented in Table II, only -2 M-' s-1, i.e., almost two orders of magnitude smaller than the value of DCBQ. We have studied the reaction rate constant between reduced tocopherol and ferricyanide in 50% ethanol solution. The rate constant was found to be 40 M-' s-. Thus the ratio between the interfacial reaction rate for tocopherol vs. that for DCBQ reveals a fivefold discrepancy, indicating that the interfacial reactive part of tocopherol is less accessible to reaction with ferricyanide in comparison to DCBQ. DISCUSSION The results presented in this study demonstrate a clear distinction between reaction rate-limited and diffusionlimited mediation of redox processes through lipid membranes. This distinction is shown for two types of mediators, a small quinone and a-tocopherol. Comparing the mediation by these compounds we did not find an unusual phenomenon like that described by Hauska and his colleagues (Hauska, 1977; Futami et al., 1979a). They showed that tocopherol- and isoprenoid-containing quinones were much more effective in mediating redox processes than were small molecular weight quinones that do not contain isoprenoid tails. The opposite is found in our study. Thus in the diffusion-limited mode of reaction (ascorbate, in; hexachloroiridate, out) the calculated 2 is -100-fold higher for DCBQ than for a-tocopherol. In the reaction rate-limited mode (ascorbate vs. ferricyanide) the calculated interfacial reaction rate between ferricyanide and the reduced DCBQ is higher by a factor of.50, being -100 and 2 M' s-' for DCBQ and tocopherol, respectively. Therefore, in both modes, DCBQ was much more potent as a mediator than was tocopherol. A rigorous comparison between the results of the experiments reported in this study and those of Futami et al. (1 979a) is not possible because of the difference in the nature of lipids 166 used and the preparation method of vesicles. Yet, by comparing the data that appeared in Table I of Futami et al. (1979a) at a mediator concentration of -5 x 10-4 M and converting it according to Eq. 15 to levels of concentration used in our study for the diffusion limited-mode of mediation, it can be gathered that the difference between these two studies relates primarily to the small molecular weight quinones; their efficacy as mediators in the system of Futami et al. is at least three orders of magnitude lower than that of DCBQ in our system. It should be noted that in the study by Futami et al. the reductant was dithionite rather than ascorbate. It is possible that the interaction between the strong reductant dithionite and quinone mediators in the aqueous solution produces hyperreduced species that are either "destroyed" irreversibly in the water or extracted from the membrane because of their hydrophilic nature. A significant result of this study is related to the clear redox mediation effect of tocopherol. It should be stressed that ubiquinones (we tried ubiquinone-6 and ubiquinone- 10) were hardly effective in mediating redox processes in our system, most probably due to their low redox potential which precluded a reaction (or a fast enough reaction) with ascorbate. On the other hand tocopherol was readily reduced by ascorbate. It is implied, therefore, that tocopherol forms an effective hydrophobic reversible redox species with a redox potential higher than zero and lower than 0.4 V (vs. standard hydrogen electrode). Thermodynamically it can be reduced by cellular reductants such as glutathione. The fact that its interfacial rate of reaction with ferricyanide is significantly lower than the bulk rateconstant, implies that it is available in its reduced form in the interior of the membrane. Therefore reduced tocopherol can act as a membranal antioxidant agent. The implication from this study is that the antioxidant potency of tocopherol can be restored by occasional interfacial reactions with appropriate aqueous reductants. To assess the significance of interfacial redox reactions of tocopherol in biological membranes it is necessary to study its interaction with glutathione, reduced NAD and NADP, and ascorbate at physiological concentrations. These topics are being explored in our laboratory. The study of redox reaction mediation in lipid vesicles constitutes a new way of probing molecular dynamics in the bilayer structure. Thus, the study of diffusion-limited processes can yield the value of transmembrane shuttle frequencies. It is noteworthy that for DCBQ the figure is very small; only -8 s-'. For a membrane -30 A thick, this corresponds to a nominal diffusion coefficient lower than 10-2 cm2/s. The lateral diffusion coefficient of pyrene in the same type of membrane at the same temperature was found to be I0O- cm2/s (Daems et al., 1985). Thus we are led to the conclusion that DCBQ is concentrated mostly in the interfacial region of the bilayer and is excluded from the lipid bilayer interior by a significant barrier. This is consistent with the fact that k' for DCBQ is only slightly BIOPHYSICAL JOURNAL VOLUME
7 less than that expected from the bulk water reaction rate, k (see Results). This indicates that the quinones in the membrane are readily available for direct oxidative attack by an aqueous oxidant. If they were mostly dissolved in the bulk lipid phase the value of k' should have been much smaller than k. This is also in accord with the observation of the high a value for DCBQ (see Table I), which nominally corresponds to a bulk partition coefficient between hydrophobic solvent and water of more than 1,000, whereas the actual number for benzene-type molecules is only in the range of (Katz and Diamond, 1974). The notion that quinones are mostly adsorbed at the membrane-water interface was already suggested by a completely independent observation regarding photodriven electron transport from porphyrin to quinone in lipid bilayer membranes (Krakover et al., 1981). Another feature that can be explored by these studies is the relative availability of the active moiety of the mediator in the membrane to an aqueous reactant. This can be achieved by a careful comparison between the bimolecular reaction rate at the membrane interface with the reaction rate between the two species in bulk phases. As noted in this study it is inferred that the active reduced form of a-tocopherol is relatively less accessible to oxidation by an aqueous oxidant than is DCBQ. This work was supported by a grant from Dr. Fred S. Singer through The Authority for Research and Development of the Hebrew University. T. Krakover is a recepient of the postdoctoral award from the Jacob and Mini Bacaner foundation. Receivedfor publication 15 April 1986 and infinalform 31 July REFERENCES Batzri, S., and E. D. Korn Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta. 298: Daems, D., M. Van den Zegel, N. Boens, and F. C. De Schryver Fluorescence decay of pyrene in small and large unilamellar L a- dipalmitoylphosphatidyl choline vesicles above and below the phase transition temperature. Eur. Biophys. J. 12: Futami, A., E. Hurt, and G. Hauska. 1979a. Vectorial redox reactions of physiological quinones. I. Requirement of a minimal length of the isoprenoid side chain. Biochim. Biophys. Acta. 547: Futami, A., and G. Hauska. 1979b. Vectorial redox reactions of physiological quinones. II. A study of transient semiquinone formation. Biochim. Biophys. Acta. 547: Hauska, G Plasto and ubiquinone as translocators of electrons and protons through membranes. FEBS (Fed. Eur. Biochem. Soc.) Lett. 79: Hinkle, P A model system for mitochondrial ion transport and respiratory control. Biochem. Biophys. Res. Commun. 41: Katz, Y., and J. H. Diamond Termodynamic constants for nonelectrolyte partition between dimyristoil lecithin and water. J. Membr. Biol. 17: Krakover, T., A. Ilani, and D. Mauzerall Apparent inhibition of photoredox reactions of magnesium octaethyl porphyrin at the lipid bilayer-water interface by neutral quinones. Biophys. J. 35: Radian, R., and B. Kanner Reconstitution and purification of the sodium and chlorid-coupled y-aminobutyric acid transporter from rat brain. J. Biol. Chem. 260: Runquist, J. A., and P. A. Loach Catalysis of electron transfer across phospholipid bilayers by iron-porphyrin complexes. Biochim. Biophys. Acta. 637: ILANI AND KRAKOVER Diffusion- and Reaction Rate-limited Redox Processes 167
Chapter 2 Transport Systems
 Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Protection of DPPC phospholipid liposomal membrane against radiation oxidative damage by antioxidants
 Protection of DPPC phospholipid liposomal membrane against radiation oxidative damage by antioxidants D. L. Marathe, B. N. Pandey and K. P. Mishra Radiation Biology Division, Bhabha Atomic Research Centre,
Protection of DPPC phospholipid liposomal membrane against radiation oxidative damage by antioxidants D. L. Marathe, B. N. Pandey and K. P. Mishra Radiation Biology Division, Bhabha Atomic Research Centre,
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Chapter 7: Membranes
 Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Chapter 7: Membranes Roles of Biological Membranes The Lipid Bilayer and the Fluid Mosaic Model Transport and Transfer Across Cell Membranes Specialized contacts (junctions) between cells What are the
Membrane transport. Pharmacy Dr. Szilvia Barkó
 Membrane transport Pharmacy 04.10.2017 Dr. Szilvia Barkó Cell Membranes Cell Membrane Functions Protection Communication Import and and export of molecules Movement of the cell General Structure A lipid
Membrane transport Pharmacy 04.10.2017 Dr. Szilvia Barkó Cell Membranes Cell Membrane Functions Protection Communication Import and and export of molecules Movement of the cell General Structure A lipid
Singlet Oxygen Production Photosensitized by Fluorescein in Reversed Micellar Solutions. Norio Miyoshi and Giiti Tomita*
 Singlet Oxygen Production Photosensitized by Fluorescein in Reversed Micellar Solutions Norio Miyoshi and Giiti Tomita* Institute of Biophysics, Faculty of Agriculture, Kyushu University, Fukuoka 812,
Singlet Oxygen Production Photosensitized by Fluorescein in Reversed Micellar Solutions Norio Miyoshi and Giiti Tomita* Institute of Biophysics, Faculty of Agriculture, Kyushu University, Fukuoka 812,
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. (a) Uncropped version of Fig. 2a. RM indicates that the translation was done in the absence of rough mcirosomes. (b) LepB construct containing the GGPG-L6RL6-
Supplementary Figures Supplementary Figure 1. (a) Uncropped version of Fig. 2a. RM indicates that the translation was done in the absence of rough mcirosomes. (b) LepB construct containing the GGPG-L6RL6-
Cell Membranes and Signaling
 5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
5 Cell Membranes and Signaling Concept 5.1 Biological Membranes Have a Common Structure and Are Fluid A membrane s structure and functions are determined by its constituents: lipids, proteins, and carbohydrates.
RETINOID-PHOSPHOLIPID INTERACTIONS AS STUDIED BY MAGNETIC RESONANCE. Stephen R. Wassail* and William Stillwellt
 Vol.''% No. 3 85 RETINOID-PHOSPHOLIPID INTERACTIONS AS STUDIED BY MAGNETIC RESONANCE Stephen R. Wassail* and William Stillwellt Departments of Physics* and Biology+ Indiana University-Purdue University
Vol.''% No. 3 85 RETINOID-PHOSPHOLIPID INTERACTIONS AS STUDIED BY MAGNETIC RESONANCE Stephen R. Wassail* and William Stillwellt Departments of Physics* and Biology+ Indiana University-Purdue University
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم -Please refer to the slides from (4-20) -Slides (4, 5) -Oxidative phosphorylation consists of 2 parts: 1.electron transport chain (series of electron transport proteins much filled
بسم هللا الرحمن الرحيم -Please refer to the slides from (4-20) -Slides (4, 5) -Oxidative phosphorylation consists of 2 parts: 1.electron transport chain (series of electron transport proteins much filled
Fall Name Student ID
 Name Student ID PART 1: Matching. Match the organelle to its function (11 points) 1.Proton motive force 2. Fluid Mosiac 3. Oxidative Phosphorylation 4. Pyruvate dehydrogenase 5. Electrochemical Force 6.
Name Student ID PART 1: Matching. Match the organelle to its function (11 points) 1.Proton motive force 2. Fluid Mosiac 3. Oxidative Phosphorylation 4. Pyruvate dehydrogenase 5. Electrochemical Force 6.
BIOPHYSICS II. By Prof. Xiang Yang Liu Department of Physics,
 BIOPHYSICS II By Prof. Xiang Yang Liu Department of Physics, NUS 1 Hydrogen bond and the stability of macromolecular structure Membrane Model Amphiphilic molecule self-assembly at the surface and din the
BIOPHYSICS II By Prof. Xiang Yang Liu Department of Physics, NUS 1 Hydrogen bond and the stability of macromolecular structure Membrane Model Amphiphilic molecule self-assembly at the surface and din the
NANO 243/CENG 207 Course Use Only
 L9. Drug Permeation Through Biological Barriers May 3, 2018 Lipids Lipid Self-Assemblies 1. Lipid and Lipid Membrane Phospholipid: an amphiphilic molecule with a hydrophilic head and 1~2 hydrophobic tails.
L9. Drug Permeation Through Biological Barriers May 3, 2018 Lipids Lipid Self-Assemblies 1. Lipid and Lipid Membrane Phospholipid: an amphiphilic molecule with a hydrophilic head and 1~2 hydrophobic tails.
Molecular Cell Biology. Prof. D. Karunagaran. Department of Biotechnology. Indian Institute of Technology Madras
 Molecular Cell Biology Prof. D. Karunagaran Department of Biotechnology Indian Institute of Technology Madras Module 4 Membrane Organization and Transport Across Membranes Lecture 1 Cell Membrane and Transport
Molecular Cell Biology Prof. D. Karunagaran Department of Biotechnology Indian Institute of Technology Madras Module 4 Membrane Organization and Transport Across Membranes Lecture 1 Cell Membrane and Transport
Cell membrane & Transport. Dr. Ali Ebneshahidi Ebneshahidi
 Cell membrane & Transport Dr. Ali Ebneshahidi Cell Membrane To enclose organelles and other contents in cytoplasm. To protect the cell. To allow substances into and out of the cell. To have metabolic reactions
Cell membrane & Transport Dr. Ali Ebneshahidi Cell Membrane To enclose organelles and other contents in cytoplasm. To protect the cell. To allow substances into and out of the cell. To have metabolic reactions
EXPERIMENT 3 ENZYMATIC QUANTITATION OF GLUCOSE
 EXPERIMENT 3 ENZYMATIC QUANTITATION OF GLUCOSE This is a team experiment. Each team will prepare one set of reagents; each person will do an individual unknown and each team will submit a single report.
EXPERIMENT 3 ENZYMATIC QUANTITATION OF GLUCOSE This is a team experiment. Each team will prepare one set of reagents; each person will do an individual unknown and each team will submit a single report.
Transport through membranes
 Transport through membranes Membrane transport refers to solute and solvent transfer across both cell membranes, epithelial and capillary membranes. Biological membranes are composed of phospholipids stabilised
Transport through membranes Membrane transport refers to solute and solvent transfer across both cell membranes, epithelial and capillary membranes. Biological membranes are composed of phospholipids stabilised
Flip-Flop Induced Relaxation Of Bending Energy: Implications For Membrane Remodeling
 Biophysical Journal, Volume 97 Supporting Material Flip-Flop Induced Relaxation Of Bending Energy: Implications For Membrane Remodeling Raphael Jeremy Bruckner, Sheref S. Mansy, Alonso Ricardo, L. Mahadevan,
Biophysical Journal, Volume 97 Supporting Material Flip-Flop Induced Relaxation Of Bending Energy: Implications For Membrane Remodeling Raphael Jeremy Bruckner, Sheref S. Mansy, Alonso Ricardo, L. Mahadevan,
CELL MEMBRANES. A schematic of how the lipids are arranged in the membrane is shown enlarged below the electronmicrograph.
 CELL MEMBRANES We first thought that cell membranes were just lipid bilayers. Here we have a picture of a cell membrane taken with an electron microscope (called an electron-micrograph ). The thickness
CELL MEMBRANES We first thought that cell membranes were just lipid bilayers. Here we have a picture of a cell membrane taken with an electron microscope (called an electron-micrograph ). The thickness
μ i = chemical potential of species i C i = concentration of species I
 BIOE 459/559: Cell Engineering Membrane Permeability eferences: Water Movement Through ipid Bilayers, Pores and Plasma Membranes. Theory and eality, Alan Finkelstein, 1987 Membrane Permeability, 100 Years
BIOE 459/559: Cell Engineering Membrane Permeability eferences: Water Movement Through ipid Bilayers, Pores and Plasma Membranes. Theory and eality, Alan Finkelstein, 1987 Membrane Permeability, 100 Years
Cell membranes. Stef Elorriaga 4/11/2016 BIO102
 Cell membranes Stef Elorriaga 4/11/2016 BIO102 Announcements Lab report 2 is due now Quiz 2 is on Wednesday on cells, part of the cells, plasma membrane, and enzymes Outline of the day Activity on the
Cell membranes Stef Elorriaga 4/11/2016 BIO102 Announcements Lab report 2 is due now Quiz 2 is on Wednesday on cells, part of the cells, plasma membrane, and enzymes Outline of the day Activity on the
Synthesis of ATP, the energy currency in metabolism
 Synthesis of ATP, the energy currency in metabolism Note that these are simplified summaries to support lecture material Either Substrate-level phosphorylation (SLP) Or Electron transport phosphorylation
Synthesis of ATP, the energy currency in metabolism Note that these are simplified summaries to support lecture material Either Substrate-level phosphorylation (SLP) Or Electron transport phosphorylation
Cell Structure and Function Exam Study Guide Part I
 Cell Structure and Function Exam Study Guide Part I 1. Which image best depicts the hot water, which the cold? 2. What is the relationship between temperature and the speed of molecular motion? 3. If a
Cell Structure and Function Exam Study Guide Part I 1. Which image best depicts the hot water, which the cold? 2. What is the relationship between temperature and the speed of molecular motion? 3. If a
Cell Membranes. Q: What components of the cell membrane are in a mosaic pattern?
 Cell Membranes The cell / plasma membrane is. Selective in that it allows things in and some things out of the cell. Recall that phospholipids have hydrophobic and hydrophilic. The term to describe this
Cell Membranes The cell / plasma membrane is. Selective in that it allows things in and some things out of the cell. Recall that phospholipids have hydrophobic and hydrophilic. The term to describe this
Membrane Structure and Function
 Membrane Structure and Function Chapter 7 Objectives Define the following terms: amphipathic molecules, aquaporins, diffusion Distinguish between the following pairs or sets of terms: peripheral and integral
Membrane Structure and Function Chapter 7 Objectives Define the following terms: amphipathic molecules, aquaporins, diffusion Distinguish between the following pairs or sets of terms: peripheral and integral
TRANSPORT ACROSS MEMBRANES
 Unit 2: Cells, Membranes and Signaling TRANSPORT ACROSS MEMBRANES Chapter 5 Hillis Textbook TYPES OF TRANSPORT ACROSS THE CELL (PLASMA) MEMBRANE: What do you remember? Complete the chart with what you
Unit 2: Cells, Membranes and Signaling TRANSPORT ACROSS MEMBRANES Chapter 5 Hillis Textbook TYPES OF TRANSPORT ACROSS THE CELL (PLASMA) MEMBRANE: What do you remember? Complete the chart with what you
MitoCheck Complex I Activity Assay Kit
 MitoCheck Complex I Activity Assay Kit Item No. 700930 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
MitoCheck Complex I Activity Assay Kit Item No. 700930 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Oxidative Phosphorylation
 Oxidative Phosphorylation Energy from Reduced Fuels Is Used to Synthesize ATP in Animals Carbohydrates, lipids, and amino acids are the main reduced fuels for the cell. Electrons from reduced fuels are
Oxidative Phosphorylation Energy from Reduced Fuels Is Used to Synthesize ATP in Animals Carbohydrates, lipids, and amino acids are the main reduced fuels for the cell. Electrons from reduced fuels are
The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11
 1 February 26, The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11 Introduction I 2 Biological membranes are phospholipid bilayers with associated proteins. Current data support a fluid
1 February 26, The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11 Introduction I 2 Biological membranes are phospholipid bilayers with associated proteins. Current data support a fluid
Chemical Surface Transformation 1
 Chemical Surface Transformation 1 Chemical reactions at Si H surfaces (inorganic and organic) can generate very thin films (sub nm thickness up to µm): inorganic layer formation by: thermal conversion:
Chemical Surface Transformation 1 Chemical reactions at Si H surfaces (inorganic and organic) can generate very thin films (sub nm thickness up to µm): inorganic layer formation by: thermal conversion:
Methods and Materials
 a division of Halcyonics GmbH Anna-Vandenhoeck-Ring 5 37081 Göttingen, Germany Application Note Micostructured lipid bilayers ANDREAS JANSHOFF 1), MAJA GEDIG, AND SIMON FAISS Fig.1: Thickness map of microstructured
a division of Halcyonics GmbH Anna-Vandenhoeck-Ring 5 37081 Göttingen, Germany Application Note Micostructured lipid bilayers ANDREAS JANSHOFF 1), MAJA GEDIG, AND SIMON FAISS Fig.1: Thickness map of microstructured
X-ray diffraction study on interdigitated structure of phosphatidylcholines in glycerol
 X-ray diffraction study on interdigitated structure of phosphatidylcholines in glycerol Hiroshi Takahashi 1,*, Noboru Ohta 2 and Ichiro Hatta 2 1 Department of Physics, Gunma University, 4-2 Aramaki, Maebashi
X-ray diffraction study on interdigitated structure of phosphatidylcholines in glycerol Hiroshi Takahashi 1,*, Noboru Ohta 2 and Ichiro Hatta 2 1 Department of Physics, Gunma University, 4-2 Aramaki, Maebashi
Reading Assignments. A. Energy and Energy Conversions. Lecture Series 9 Cellular Pathways That Harvest Chemical Energy. gasoline) or elevated mass.
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11
 1 The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11 Introduction I 2 Biological membranes are phospholipid bilayers with associated proteins. Current data support a fluid mosaic model
1 The Cell Membrane & Movement of Materials In & Out of Cells PACKET #11 Introduction I 2 Biological membranes are phospholipid bilayers with associated proteins. Current data support a fluid mosaic model
Unit 1 Matter & Energy for Life
 Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different
Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different
Diffusion, Osmosis and Active Transport
 Diffusion, Osmosis and Active Transport Particles like atoms, molecules and ions are always moving Movement increases with temperature (affects phases of matter - solid, liquid, gas) Solids - atoms, molecules
Diffusion, Osmosis and Active Transport Particles like atoms, molecules and ions are always moving Movement increases with temperature (affects phases of matter - solid, liquid, gas) Solids - atoms, molecules
Phospholipids. Extracellular fluid. Polar hydrophilic heads. Nonpolar hydrophobic tails. Polar hydrophilic heads. Intracellular fluid (cytosol)
 Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Life Needs Energy. The Rules (Laws of Thermodynamics) 1) energy can not be created or destroyed, but it can be changed from one form to another
 Intro to Metabolism Learning Outcomes Explain laws governing energy and energy transfers. Describe enzymes and how they work. Explain what is meant by selectively permeable. Explain the differences between
Intro to Metabolism Learning Outcomes Explain laws governing energy and energy transfers. Describe enzymes and how they work. Explain what is meant by selectively permeable. Explain the differences between
Membrane Structure and Membrane Transport of Small Molecules. Assist. Prof. Pinar Tulay Faculty of Medicine
 Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
Membrane Structure and Membrane Transport of Small Molecules Assist. Prof. Pinar Tulay Faculty of Medicine Introduction Cell membranes define compartments of different compositions. Membranes are composed
OXIDATIVE STRESS STUDIES ON LIPID MODEL MEMBRANES
 OXIDATIVE STRESS STUDIES ON LIPID MODEL MEMBRANES MARCELA ELISABETA BARBINTA-PATRASCU *, LAURA TUGULEA * * Faculty of Physics, University of Bucharest, Romania Received December 21, 2004 The liposomes
OXIDATIVE STRESS STUDIES ON LIPID MODEL MEMBRANES MARCELA ELISABETA BARBINTA-PATRASCU *, LAURA TUGULEA * * Faculty of Physics, University of Bucharest, Romania Received December 21, 2004 The liposomes
Cholesterol determination using protein-templated fluorescent gold nanocluster probes
 Electronic Supplementary Information for Cholesterol determination using protein-templated fluorescent gold nanocluster probes Xi Chen and Gary A. Baker* Department of Chemistry, University of Missouri-Columbia,
Electronic Supplementary Information for Cholesterol determination using protein-templated fluorescent gold nanocluster probes Xi Chen and Gary A. Baker* Department of Chemistry, University of Missouri-Columbia,
Membranes. Chapter 5
 Membranes Chapter 5 Membrane Structure The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membranes Chapter 5 Membrane Structure The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Chapter 7: Membrane Structure & Function
 Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Cellular Neurophysiology I Membranes and Ion Channels
 Cellular Neurophysiology I Membranes and Ion Channels Reading: BCP Chapter 3 www.bioelectriclab All living cells maintain an electrical potential (voltage) across their membranes (V m ). Resting Potential
Cellular Neurophysiology I Membranes and Ion Channels Reading: BCP Chapter 3 www.bioelectriclab All living cells maintain an electrical potential (voltage) across their membranes (V m ). Resting Potential
Chapter 7: Membrane Structure & Function. 1. Membrane Structure. What are Biological Membranes? 10/21/2015. Why phospholipids? 1. Membrane Structure
 Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Chapter 7: Membrane Structure & Function 1. Membrane Structure 2. Transport Across Membranes 1. Membrane Structure Chapter Reading pp. 125-129 What are Biological Membranes? Hydrophilic head WATER They
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Membranes. Chapter 5. Membrane Structure
 Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
Membranes Chapter 5 Membrane Structure Lipid Bilayer model: - double phospholipid layer - Gorter & Grendel: 1925 Fluid Mosaic model: consist of -phospholipids arranged in a bilayer -globular proteins inserted
3.1 Background. Preformulation Studies
 Preformulation Studies 3.1 Background Delivery of any drug requires a suitable dosage form to get optimum therapeutic effects. The development of such dosage forms fundamental properties of the drug molecule
Preformulation Studies 3.1 Background Delivery of any drug requires a suitable dosage form to get optimum therapeutic effects. The development of such dosage forms fundamental properties of the drug molecule
Membrane Structure. Membrane Structure. Membrane Structure. Membranes
 Membrane Structure Membranes Chapter 5 The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Membrane Structure Membranes Chapter 5 The fluid mosaic model of membrane structure contends that membranes consist of: -phospholipids arranged in a bilayer -globular proteins inserted in the lipid bilayer
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Biological membranes are composed of lipid bilayers
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Biological membranes are composed of lipid bilayers
Investigating Lipids
 Investigating Lipids In today s culture, there is a stigma associated with the word fat. While it is true that too much fat can lead to health problems, fats (or lipids) play very important roles in biology.
Investigating Lipids In today s culture, there is a stigma associated with the word fat. While it is true that too much fat can lead to health problems, fats (or lipids) play very important roles in biology.
Interaction of Functionalized C 60 Nanoparticles with Lipid Membranes
 Interaction of Functionalized C 60 Nanoparticles with Lipid Membranes Kevin Gasperich Advisor: Dmitry Kopelevich ABSTRACT The recent rapid development of applications for fullerenes has led to an increase
Interaction of Functionalized C 60 Nanoparticles with Lipid Membranes Kevin Gasperich Advisor: Dmitry Kopelevich ABSTRACT The recent rapid development of applications for fullerenes has led to an increase
Physical Cell Biology Lecture 10: membranes elasticity and geometry. Hydrophobicity as an entropic effect
 Physical Cell Biology Lecture 10: membranes elasticity and geometry Phillips: Chapter 5, Chapter 11 and Pollard Chapter 13 Hydrophobicity as an entropic effect 1 Self-Assembly of Lipid Structures Lipid
Physical Cell Biology Lecture 10: membranes elasticity and geometry Phillips: Chapter 5, Chapter 11 and Pollard Chapter 13 Hydrophobicity as an entropic effect 1 Self-Assembly of Lipid Structures Lipid
The Discovery of the Cell
 The Discovery of the Cell 7-1 Life Is Cellular Review the cell in relation to: - Its definition - The origin of life - The characteristics of life - The hierarchy of biological organization - The science
The Discovery of the Cell 7-1 Life Is Cellular Review the cell in relation to: - Its definition - The origin of life - The characteristics of life - The hierarchy of biological organization - The science
Constant Motion of Molecules. Kinetic Theory of Matter Molecules move randomly and bump into each other and other barriers
 CELL TRANSPORT Constant Motion of Molecules Kinetic Theory of Matter Molecules move randomly and bump into each other and other barriers Solution homogenous liquid throughout which two or more substances
CELL TRANSPORT Constant Motion of Molecules Kinetic Theory of Matter Molecules move randomly and bump into each other and other barriers Solution homogenous liquid throughout which two or more substances
Coarse grained simulations of Lipid Bilayer Membranes
 Coarse grained simulations of Lipid Bilayer Membranes P. B. Sunil Kumar Department of Physics IIT Madras, Chennai 600036 sunil@iitm.ac.in Atomistic MD: time scales ~ 10 ns length scales ~100 nm 2 To study
Coarse grained simulations of Lipid Bilayer Membranes P. B. Sunil Kumar Department of Physics IIT Madras, Chennai 600036 sunil@iitm.ac.in Atomistic MD: time scales ~ 10 ns length scales ~100 nm 2 To study
1.4 Page 1 Cell Membranes S. Preston 1
 AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.3 Cell Membranes and Transport Page 1 1.3 Cell Membranes and Transport from your syllabus l. Cell Membrane Structure 1. Read and
Chapter 12: Membranes. Voet & Voet: Pages
 Chapter 12: Membranes Voet & Voet: Pages 390-415 Slide 1 Membranes Essential components of all living cells (define boundry of cells) exclude toxic ions and compounds; accumulation of nutrients energy
Chapter 12: Membranes Voet & Voet: Pages 390-415 Slide 1 Membranes Essential components of all living cells (define boundry of cells) exclude toxic ions and compounds; accumulation of nutrients energy
Classification of Lipids
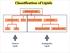 Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Classification of Lipids Neutral Lipids Amphipathic Lipids Amphipathic Lipids Most cell-membrane lipids are one of two main classes of amphipathic hydrolyzable lipids. Glycerophospholipids (phosphoglycerides):
Biology. Membranes.
 1 Biology Membranes 2015 10 28 www.njctl.org 2 Vocabulary active transport carrier protein channel protein concentration gradient diffusion enzymatic activity facilitated diffusion fluid mosaic hypertonic
1 Biology Membranes 2015 10 28 www.njctl.org 2 Vocabulary active transport carrier protein channel protein concentration gradient diffusion enzymatic activity facilitated diffusion fluid mosaic hypertonic
Reduction of p-benzoquinone on lipid-modified electrodes: effect of the alkyl chain length of lipids on the electron transfer reactions
 www.elsevier.nl/locate/jelechem Journal of Electroanalytical Chemistry 484 (2000) 131 136 Reduction of p-benzoquinone on lipid-modified electrodes: effect of the alkyl chain length of lipids on the electron
www.elsevier.nl/locate/jelechem Journal of Electroanalytical Chemistry 484 (2000) 131 136 Reduction of p-benzoquinone on lipid-modified electrodes: effect of the alkyl chain length of lipids on the electron
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/2/12/e1601838/dc1 Supplementary Materials for General and programmable synthesis of hybrid liposome/metal nanoparticles Jin-Ho Lee, Yonghee Shin, Wooju Lee, Keumrai
advances.sciencemag.org/cgi/content/full/2/12/e1601838/dc1 Supplementary Materials for General and programmable synthesis of hybrid liposome/metal nanoparticles Jin-Ho Lee, Yonghee Shin, Wooju Lee, Keumrai
The main biological functions of the many varied types of lipids include: energy storage protection insulation regulation of physiological processes
 Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Big Idea In the biological sciences, a dehydration synthesis (condensation reaction) is typically defined as a chemical reaction that involves the loss of water from the reacting molecules. This reaction
Total Phosphatidic Acid Assay Kit
 Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
Product Manual Total Phosphatidic Acid Assay Kit Catalog Number MET- 5019 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Phosphatidic Acid (PA) is a critical precursor
Interfacial Reactions (Part III)
 NPTEL Chemical Engineering Interfacial Engineering Module 7: Lecture 3 Interfacial Reactions (Part III) Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati 781039
NPTEL Chemical Engineering Interfacial Engineering Module 7: Lecture 3 Interfacial Reactions (Part III) Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati 781039
Movement of substances across the cell membrane
 Ch 4 Movement of substances across the cell membrane Think about (Ch 4, p.2) 1. The structure of the cell membrane can be explained by the fluid mosaic model. It describes that the cell membrane is mainly
Ch 4 Movement of substances across the cell membrane Think about (Ch 4, p.2) 1. The structure of the cell membrane can be explained by the fluid mosaic model. It describes that the cell membrane is mainly
IAM Chromatography. Introduction
 IAM Chromatography IAM Drug Discovery Columns Introduction References: 1. Regis 1998-99 Chromatography Catalog A simple, rapid method to predict drug transport across biological barriers has been a long
IAM Chromatography IAM Drug Discovery Columns Introduction References: 1. Regis 1998-99 Chromatography Catalog A simple, rapid method to predict drug transport across biological barriers has been a long
Homeostasis, Transport & The Cell Membrane. Chapter 4-2 (pg 73 75) Chapter 5
 Homeostasis, Transport & The Cell Membrane Chapter 4-2 (pg 73 75) Chapter 5 Unit 5: Lecture 1 Topic: The Cell Membrane Covers: Chapter 5, pages 95-96 Chapter 4, pages 73-75 The Cell Membrane The chemistry
Homeostasis, Transport & The Cell Membrane Chapter 4-2 (pg 73 75) Chapter 5 Unit 5: Lecture 1 Topic: The Cell Membrane Covers: Chapter 5, pages 95-96 Chapter 4, pages 73-75 The Cell Membrane The chemistry
Chapter 14 - Electron Transport and Oxidative Phosphorylation
 Chapter 14 - Electron Transport and Oxidative Phosphorylation The cheetah, whose capacity for aerobic metabolism makes it one of the fastest animals Prentice Hall c2002 Chapter 14 1 14.4 Oxidative Phosphorylation
Chapter 14 - Electron Transport and Oxidative Phosphorylation The cheetah, whose capacity for aerobic metabolism makes it one of the fastest animals Prentice Hall c2002 Chapter 14 1 14.4 Oxidative Phosphorylation
Membrane Structure, Resting membrane potential, Action potential. Biophysics seminar
 Membrane Structure, Resting membrane potential, Action potential Biophysics seminar 09.09.2013. Membrane structure Biological membranes consists of lipids and proteins to bind with non-covalent bond. Phospholipids
Membrane Structure, Resting membrane potential, Action potential Biophysics seminar 09.09.2013. Membrane structure Biological membranes consists of lipids and proteins to bind with non-covalent bond. Phospholipids
Biology 2201 Unit 1 Matter & Energy for Life
 Biology 2201 Unit 1 Matter & Energy for Life 2.2 Cell Membrane Structure Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different conditions
Biology 2201 Unit 1 Matter & Energy for Life 2.2 Cell Membrane Structure Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different conditions
Chapter 7: Membrane Structure and Function. Key Terms:
 Key Terms: Selectively permeable Fluid mosaic model Amphipathic Phospholipid Bilayer Hydrophilic Hydrophobic Phosphate head Fatty acid tail Davson-Danielli Singer-Nicolson Freeze-Fracture EM Unsaturated
Key Terms: Selectively permeable Fluid mosaic model Amphipathic Phospholipid Bilayer Hydrophilic Hydrophobic Phosphate head Fatty acid tail Davson-Danielli Singer-Nicolson Freeze-Fracture EM Unsaturated
Transport. Slide 1 of 47. Copyright Pearson Prentice Hall
 & Transport 1 of 47 Learning Targets TN Standard CLE 3216.1.3 Explain how materials move into and out of cells. CLE 3216.1.5 Investigate how proteins regulate the internal environment of a cell through
& Transport 1 of 47 Learning Targets TN Standard CLE 3216.1.3 Explain how materials move into and out of cells. CLE 3216.1.5 Investigate how proteins regulate the internal environment of a cell through
1. Membrane proteins have a variety of functions. State four membrane protein functions. A. B. C. D.
 Part I: Short answers 1. Membrane proteins have a variety of functions. State four membrane protein functions. A. B. C. D. Part II: Label the components 2. Label the components of a biological membrane
Part I: Short answers 1. Membrane proteins have a variety of functions. State four membrane protein functions. A. B. C. D. Part II: Label the components 2. Label the components of a biological membrane
MARTINI Coarse-Grained Model of Triton TX-100 in Pure DPPC. Monolayer and Bilayer Interfaces. Supporting Information
 MARTINI Coarse-Grained Model of Triton TX-100 in Pure DPPC Monolayer and Bilayer Interfaces. Antonio Pizzirusso a, Antonio De Nicola* a, Giuseppe Milano a a Dipartimento di Chimica e Biologia, Università
MARTINI Coarse-Grained Model of Triton TX-100 in Pure DPPC Monolayer and Bilayer Interfaces. Antonio Pizzirusso a, Antonio De Nicola* a, Giuseppe Milano a a Dipartimento di Chimica e Biologia, Università
Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE
 Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE The main aim of this module is to introduce the students to the types
Paper 4. Biomolecules and their interactions Module 22: Aggregates of lipids: micelles, liposomes and their applications OBJECTIVE The main aim of this module is to introduce the students to the types
Topic 3: Movement of substances across cell membrane
 Topic 3: Movement of substances across cell membrane 1. What is/are the role(s) of structure A? (1) For cell recognition. (2) For carrying water-soluble substances across cell membrane. (3) For supporting
Topic 3: Movement of substances across cell membrane 1. What is/are the role(s) of structure A? (1) For cell recognition. (2) For carrying water-soluble substances across cell membrane. (3) For supporting
Chapter 1 Membrane Structure and Function
 Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
Chapter 1 Membrane Structure and Function Architecture of Membranes Subcellular fractionation techniques can partially separate and purify several important biological membranes, including the plasma and
REPORT DOCUMENTATION PAGE (SF298) (Continuation Sheet)
 REPRT DCUMENTATIN PAGE (SF298) (Continuation Sheet) Key Features of the Report: (i) (ii) (iii) (iv) We have synthesized a polymer that could form both unimolecular micelles and inverse micelles, depending
REPRT DCUMENTATIN PAGE (SF298) (Continuation Sheet) Key Features of the Report: (i) (ii) (iii) (iv) We have synthesized a polymer that could form both unimolecular micelles and inverse micelles, depending
VOLTAGE-DEPENDENT LIPID FLIP-FLOP INDUCED
 VOLTAGE-DEPENDENT LIPID FLIP-FLOP INDUCED BY ALAMETHICIN JAMES E. HALL, Department ofphysiology, California College ofmedicine, University of California, Irvine, California 92717 U.S.A. ABSTRACT Alamethicin
VOLTAGE-DEPENDENT LIPID FLIP-FLOP INDUCED BY ALAMETHICIN JAMES E. HALL, Department ofphysiology, California College ofmedicine, University of California, Irvine, California 92717 U.S.A. ABSTRACT Alamethicin
Cell Structure and Function C H A P T E R 7
 Cell Structure and Function C H A P T E R 7 EQ: What Scientists and inventions helped aid in creating Cell Theory? 7.1 Cell Theory (Cells and Living Things) Cells are the basic building block of all life
Cell Structure and Function C H A P T E R 7 EQ: What Scientists and inventions helped aid in creating Cell Theory? 7.1 Cell Theory (Cells and Living Things) Cells are the basic building block of all life
Chapter 5 Ground Rules of Metabolism Sections 6-10
 Chapter 5 Ground Rules of Metabolism Sections 6-10 5.6 Cofactors in Metabolic Pathways Most enzymes require cofactors Energy in ATP drives many endergonic reactions Table 5-1 p86 Cofactors and Coenzymes
Chapter 5 Ground Rules of Metabolism Sections 6-10 5.6 Cofactors in Metabolic Pathways Most enzymes require cofactors Energy in ATP drives many endergonic reactions Table 5-1 p86 Cofactors and Coenzymes
A) Choose the correct answer: 1) Reduction of a substance can mostly occur in the living cells by:
 Code: 1 1) Reduction of a substance can mostly occur in the living cells by: (a) Addition of oxygen (b) Removal of electrons (c) Addition of electrons (d) Addition of hydrogen 2) Starting with succinate
Code: 1 1) Reduction of a substance can mostly occur in the living cells by: (a) Addition of oxygen (b) Removal of electrons (c) Addition of electrons (d) Addition of hydrogen 2) Starting with succinate
https://www.youtube.com/watch?v=-asfob8cmic Chapter 8
 https://www.youtube.com/watch?v=-asfob8cmic Chapter 8 Cell membrane Cytoplasm Ribosomes ALL cells have DNA cytoplasm In prokaryotes the DNA is in the. nucleus In eukaryotes the DNA is in the. Function
https://www.youtube.com/watch?v=-asfob8cmic Chapter 8 Cell membrane Cytoplasm Ribosomes ALL cells have DNA cytoplasm In prokaryotes the DNA is in the. nucleus In eukaryotes the DNA is in the. Function
Chromatography on Immobilized Artificial Membrane
 Chromatography on Immobilized Artificial Membrane Principles of measuring compounds affinity to phospholipids using immobilized artificial membrane chromatography Chromatography on Immobilized Artificial
Chromatography on Immobilized Artificial Membrane Principles of measuring compounds affinity to phospholipids using immobilized artificial membrane chromatography Chromatography on Immobilized Artificial
User s Manual and Instructions
 User s Manual and Instructions Mitochondria Activity Assay (Cytochrome C Oxidase Activity Assay) Kit Catalog Number: KC310100 Introduction Mitochondria are the eukaryotic subcellular organelles that contain
User s Manual and Instructions Mitochondria Activity Assay (Cytochrome C Oxidase Activity Assay) Kit Catalog Number: KC310100 Introduction Mitochondria are the eukaryotic subcellular organelles that contain
The Working Cell: G: Membrane Transport & H: Enzymes. Chapter 5
 The Working Cell: G: Membrane Transport & H: Enzymes Chapter 5 Standards Unit G: Membrane Transport I can recognize the fluid mosaic model and accurately identify and describe the function of the components.
The Working Cell: G: Membrane Transport & H: Enzymes Chapter 5 Standards Unit G: Membrane Transport I can recognize the fluid mosaic model and accurately identify and describe the function of the components.
The Plasma Membrane - Gateway to the Cell
 The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
The Plasma Membrane - Gateway to the Cell 1 Photograph of a Cell Membrane 2 Cell Membrane The cell membrane is flexible and allows a unicellular organism to move 3 Homeostasis Balanced internal condition
CHAPTER 28 LIPIDS SOLUTIONS TO REVIEW QUESTIONS
 28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
28 09/16/2013 17:44:40 Page 415 APTER 28 LIPIDS SLUTINS T REVIEW QUESTINS 1. The lipids, which are dissimilar substances, are arbitrarily classified as a group on the basis of their solubility in fat solvents
Summary and general discussion
 Summary and general discussion Ingestion of contaminated soil can be an important route of exposure to soil-borne contaminants, especially for children (1). To estimate the health risk associated to this
Summary and general discussion Ingestion of contaminated soil can be an important route of exposure to soil-borne contaminants, especially for children (1). To estimate the health risk associated to this
Biology 4410 First Examination Version B
 Biology 4410 Spring 2006 Name First Examination Version B This examination consists of two parts, a multiple-choice section and an essay section. Be sure to put your name on both the mark-sense sheet and
Biology 4410 Spring 2006 Name First Examination Version B This examination consists of two parts, a multiple-choice section and an essay section. Be sure to put your name on both the mark-sense sheet and
Controlled via the Cell Membrane
 CELL TRANSPORT 1 Controlled via the Cell Membrane Passive Transport Does NOT require energy, moves from HIGH concentrations to LOW concentrations Active Transport DOES require energy, moves from LOW concentrations
CELL TRANSPORT 1 Controlled via the Cell Membrane Passive Transport Does NOT require energy, moves from HIGH concentrations to LOW concentrations Active Transport DOES require energy, moves from LOW concentrations
Bio 103 Section A02 Summer 2003 Exam #2 Study Guide Dr. Largen
 Chapter 4 - Cell Structure Bio 103 Section A02 Summer 2003 Exam #2 Study Guide Dr. Largen Microscopes provide windows to the world of the cell compare light versus electron microscopes illumination type
Chapter 4 - Cell Structure Bio 103 Section A02 Summer 2003 Exam #2 Study Guide Dr. Largen Microscopes provide windows to the world of the cell compare light versus electron microscopes illumination type
I. Chemical Properties of Phospholipids. Figure 1: Phospholipid Molecule. Amphiphatic:
 I. Chemical Properties of Phospholipids Figure 1: Phospholipid Molecule Amphiphatic: a) The amphiphatic nature & cylindrical shape of phospholipids contributes to their ability to assume bilayers in an
I. Chemical Properties of Phospholipids Figure 1: Phospholipid Molecule Amphiphatic: a) The amphiphatic nature & cylindrical shape of phospholipids contributes to their ability to assume bilayers in an
Plasma Membrane Function
 Plasma Membrane Function Cells have to maintain homeostasis, they do this by controlling what moves across their membranes Structure Double Layer of phospholipids Head (polar) hydrophiliclikes water -
Plasma Membrane Function Cells have to maintain homeostasis, they do this by controlling what moves across their membranes Structure Double Layer of phospholipids Head (polar) hydrophiliclikes water -
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 21 pages 709-717 717 (Animal( Cell Adhesion) Review Chapter 12 Membrane Transport Review Chapter
Ion Diffusion Selectivity in Lecithin-Water Lamellar Phases
 Ion Diffusion Selectivity in Lecithin-Water Lamellar Phases Y. LANGE and C. M. GARY BOBO From the Laboratoire de Physiologie Cellulaire, College de France, Paris, France. Dr. Lange's present address is
Ion Diffusion Selectivity in Lecithin-Water Lamellar Phases Y. LANGE and C. M. GARY BOBO From the Laboratoire de Physiologie Cellulaire, College de France, Paris, France. Dr. Lange's present address is
Cell Membrane and Transport
 Cell Membrane and Transport 29/06/2015 11:08 AM Describe the Characteristics of the phospholipid Bilayer. The Phospholipid bilayer is made up of a double layer of membrane lipids that have a hydrophobic
Cell Membrane and Transport 29/06/2015 11:08 AM Describe the Characteristics of the phospholipid Bilayer. The Phospholipid bilayer is made up of a double layer of membrane lipids that have a hydrophobic
