Isolation and Purification of Endotoxin by Hydrolytic Enzymes
|
|
|
- Britney Parks
- 5 years ago
- Views:
Transcription
1 INFECTION AND IMMUNITY, Dec. 1972, p Copyright 1972 American Society for Microbiology Vol. 6, No. 6 Printed in U.S.A. Isolation and Purification of Endotoxin by Hydrolytic Enzymes SAMUEL LEHRER1 AND ALOIS NOWOTNY Department of Microbiology and Immunology, Temple University School of Medicine, Philadelphia, Pennsylvania Received for publication 7 September 1972 Various commercial hydrolases were used in an attempt to degrade the endotoxic lipopolysaccharide macromolecule. Some inert components, such as and nucleic acids, could be removed from endotoxin preparations. As a result, endotoxic activity, measured by pyrogenicity, Shwartzman reaction, and mouse lethality, was increased. The remarkable resistance of endotoxin to hydrolases led to the use of such enzymes for the liberation and purification of endotoxin from whole bacterial cells. An increasing amount of evidence shows that the active site of bacterial endotoxic lipopolysaccharides lies in the lipid moiety of the molecule. No procedures are available presently for the isolation of the lipid moiety in intact form. Acid hydrolysis degrades the polysaccharides and will precipitate a lipid-rich fraction, which was called fraction A by Boivin and associates (3) or lipid A by Westphal and Luderitz (24). This precipitate, which consists of over 40 different fractions, shows 1 to 10% of the original activity of the parent endotoxin, as determined by various laboratories. These breakdown products derived from the lipid moiety of the molecule may resemble it, but are obviously not identical to it. Other attempts to isolate the lipid-rich moiety of endotoxin without degrading it employed hydrolytic enzymes. In our laboratories, over thirty commercially available hydrolytic enzymes were used, and, after digestion with these enzymes, their effect on endotoxin preparations was followed by (i) determination of changes in the most characteristic endotoxicity parameters and (ii) analyses of breakdown products liberated by the enzymes from the endotoxin preparation. The enzymes selected were those which are capable of cleaving certain linkages known or assumed to be present in the endotoxic lipopolysaccharide macromolecule. As will be shown, a remarkable resistance of endotoxin to these hydrolytic enzymes was observed. Although some contamination of the endotoxin preparations could be digested away and removed by enzymatic treatment, an enzyme-resistant core re- ' Present address: Scripps Clinic and Research Foundation, La Jolla, Calif mained, which showed no loss of biological activity. In several instances, with the right enzyme combination, an increase in biological potency could be observed. Based on these observations, a procedure was worked out for the direct extraction of endotoxin from bacterial cells by using enzymes, and the further purification of the crude preparation obtained by enzymatic procedures was developed. MATERIALS AND METHODS Hydrolytic enzymes. All enzyme preparations used in these studies were obtained from commercial sources. Determination of the activity of the enzyme preparations using recommended substrates under optimal conditions for the given enzyme-substrate system was carried out as described in the literature or as instructed by the vendor. Only sufficiently active enzyme preparations were used. Extraction of endotoxin by the Boivin procedure. Serratia marcescens 08 cells were grown by Merck, Sharp & Dohme, Rahway, N.J., and kindly provided for this entire project. The lyophilized cells were extracted with chloroform-methanol (1:1) in a Soxhlet to remove noncovalently bound lipids. The trichloroacetic acid (TA) extraction procedure of the lipid-extracted and dried bacterial cells was basically that described by Boivin et al. (2), as later modified by Nowotny et al. (17). This product was further purified by precipitation with alcohol and by sedimentation in an ultracentrifuge (TA endotoxin). Purification of trichloroacetic acid-extracted endotoxin by phenol-water was carried out as described (16) at 70 C by using freshly distilled phenol (TA-PW). The trichloroacetic acid endotoxin preparation was purified also by Pronase, in 0.1 M ammonium acetate buffer (ph 8.5). Enzyme (1 mg) was incubated with 100 mg of endotoxin dissolved in 25 ml of buffer at 37 C for 10 hr (TA- 928
2 VOL. 6, 1972 ISOLATION AND PURIFICATION OF ENDOTOXIN 929 ENZ). The purified endotoxins, TA-PW and TA- ENZ, were precipitated with methanol and centrifuged in ultracentrifuge. Extraction of endotoxin by hydrolytic enzymes. A 1-g amount of lyophilized, lipid-extracted, and waterwashed bacteria was suspended in 100 ml of 0.1 M ammonium acetate buffer, ph 8.5, and homogenized. The ammonium acetate buffer was prepared by adjusting the ph of 1 N ammonium hydroxide to 8.5 with 1 N acetic acid, and the molarity was adjusted to 0.1 M with ion-depleted water. A 10-mg amount of Pronase (Calbiochem, Los Angeles, Calif.) and 10 mg of threetimes crystallized lysozyme (General Biochemicals, Chagrin Falls, Ohio) were added to the suspension. The suspension, to which 1 ml of toluene had been added to prevent extensive bacterial growth, was shaken at 37 C for 10 hr. The digested material was centrifuged at 8,000 X g for 30 min. The pellet was discarded. Two further enzymes were added to the supernatant fluid, venom phosphodiesterase and alkaline phosphatase (from Escherichia coli). The ratios of these two enzymes to the original dried bacterial substrate in the case of phosphodiesterase was 1:500, and in the alkaline phosphatase was 1:200. The flasks were shaken for 2 hr at 37 C. The material was dialyzed against ion-depleted water for 3 days at 5 C temperature, the water being changed twice daily. The contents of the dialysis bags were filtered through Whatman no. 4 paper, adjusted to ph 7.5 with 0.1 M NaOH, and concentrated by vacuum distillation to approximately two-fifths of the original volume. The purification of this concentrated extract was achieved by adding two volumes of cold methanol. The precipitate was dissolved in water and centrifuged at 10,000 X g at 4 C for 30 min. The supernatant fluid was recentrifuged for 3 hr at 4 C at 105,000 X g. This lyophilized pellet obtained from the supernatant fraction was our enzyme-extracted and enzymepurified endotoxin (ENZ-ENZ). Chemical analysis. Analysis of the preparations obtained included determination of phosphorus by the method of Chen, Toribara, and Warner (4). The quantitative determination of the amino acid content was by the method of Moore and Stein (11). The amino sugar content was measured by the method of Rondle and Morgan (19). Carbohydrate content was measured after hydrolysis with 1 N sulfuric acid at 100 C for 8 hr. The hydrolysates were analyzed by paper chromatography as well as by the quantitative reducing carbohydrate method of Park and Johnson (18). Heptose was measured by the procedure of Dische (6) and the 2-keto-3-deoxy-octonate was measured by the thiobarbituric acid procedure of Weissbach and Hurwitz (23). Long-chain carboxylic acids were identified by gas-liquid chromatography and were quantitatively measured after transesterification with boron trifluoride in methanol (7). Ion-exchange column chromatographic analysis of the endotoxic preparations obtained. Amberlite XE220, kindly provided by Rohm and Haas Company, Philadelphia, Pa., was used to fractionate the endotoxin preparations, as described earlier (14-16). Biological activity of endotoxin. Biological activity was determined by using three different parameters. Mouse lethality in ICR male mice, the local Shwartzman skin assay, and the pyrogenicity assay in rabbits were carried out by routine procedures (13), and the febrile response was expressed as fever index (FI20; 1, 5) or minimal pyrogenic dose (MPD; 10). RESULTS An attempt has been made to combine all the findings related to enzymatic treatment of trichloroacetic acid endotoxin (TA-endotoxin) in Table 1. The only remarkable hydrolysis was obtained by proteolytic enzymes, especially with Pronase. Other enzymes, such as pepsin or ficin, were also active in liberating amino acids and from the endotoxin. A cellulase preparation (of microbiological origin), obtained through the courtesy of Rohm and Haas Company, Philadelphia, Pa., as well as a fungal hydrolase liberated some amino acids and, most probably due to contamination with proteolytic enzymes. The treatment with phosphatases set free hydrolytic products. Venom phosphodiesterase and spleen phosphodiesterase pretreatment rendered the endotoxin preparations sensitive to phosphomonoesterases, which liberated inorganic phosphorus. No significant cleavage of the polysaccharide or the lipid moiety could be detected by the analytical procedures applied here. The change in mouse lethality of the endotoxin preparations after treatment with hydrolytic enzymes is not shown in the table. Elevation of the lethality could be observed after proteolytic enzyme and phosphatase treatments. of the other enzymatic hydrolyses changed the toxicity of the preparations measurably. Similarly, as was done by using phosphatases, some other enzymes were also used in combination or in sequence. Pretreatment with proteolytic enzymes was followed by lipases or lipase-pretreated preparations were further subjected to proteolytic treatments. of these experiments resulted either in enhanced liberation of cleavage products or in changes in the biological activities tested. In these experiments, the activity of the second enzyme applied was measured in the presence of the previously used enzyme to determine its activity under these conditions. Those combinations in which no interference could be observed were used. Since such remarkable resistance of endotoxicity to the applied hydrolytic enzymes could be observed, and since mostly inert components (such as proteins or nucleic acids) of the trichloroacetic acid-extracted endotoxin prepara-
3 930 LEHRER AND NOWOTNY INFECT. IMMUNITY TABLE 1. Enzymatic hydrolysis of trichloroacetic acid endotoxin Enzyme activity Reduction of Enzyme (units/mg) turbidity Qualitative analysis Cleavage products detected Quantitative analysis Aminopeptidase (hog kidney) Carboxypeptidase (bovine pancreas)... Pronase (Streptomyces griseus)... Pepsin (hog stomach)... Trypsin... Alkaline protease (Bacillus).. Papain (papaya latex)... Ficin (fig latex)... Acid phosphatase (wheat germ)... Venom phosphodiesterase (venom)... Spleen phosphodiesterase (spleen)... Phospholipase C (Clostridium perfrinigens)... Phospholipase D (cabbage).. Lipase (wheat germ)... Lipase (steapsin)... Lipase (calf gland)... Lipase (pork pancreas)... Acylase (hog kidney)... a-amylase (bacterial)...,-amylase (sweet potato)... Cellulase 35 (microbiological... Chitinase (fungal)... Lysozyme (egg white)...,3-glucosidase (almond)... 1, 575 5, ,500 2, Amino acids Peptides Peptides Amino acids Phosphate, phosphate Phosphate a Mg of leucineb/ mg of endotoxin Mg of leucine/, mg of endotoxin 6.42 ug of leucine/mg of endotoxin 8.65 Mg of leucine/mg of endotoxin Ag of leucine/mg of endotoxin 0.18 Mg of P/mg of endotoxin 1.37c Ag P/mg endotoxin Mug P/mg endotoxin 0.70 ug of P/mg of endotoxin, 14.28,ug of leucine/mg of endotoxin, 1.67 gg of glucosed/mg of endotoxin 0.44 Mg of P/mg of endotoxin a, Not tested. I Total amino acid remaining in the supernatant fluid after enzyme digestion and sedimentation expressed as micrograms of leucine per milligrams of endotoxin. c Total phosphorus released by alkaline phosphatase from phosphodiesterase pretreated endotoxin. d Increase in reducing activity after enzyme digestion expressed as micrograms of glucose per milligram of endotoxin.
4 VOL. 6, 1972 ISOLATION AND PURIFICATION OF ENDOTOXIN 931 TABLE 1. Conitinued Enzyme Enzyme activity Reduction of (units/mg) turbidity Qualitative analysis Cleavage products detected Quantitative analysis Alkaline phosphatase (intestinal) Phosphate 0.75,sg of P/,mg of endotoxin Lipase (pancreas) Pectin methyl esterase (tomato) Carboxylesterase (liver) Lysozomal enzymes (liver) Hydrolase (fungal)... 3, mg of glucose/mg of endotoxin, 26.85,ug of leucine/mg of endotoxin tion (TA-endotoxin) seemed to undergo hydrolysis, efforts were concentrated on purification of endotoxins by enzymatic removal of those components which do not seem to belong to the active core. Chemical purification, such as phenol-water extraction of the trichloroacetic acid-extracted endotoxin (TA-PW), resulted in enhanced biological activity and reduced amino acid content. Similar effects could be obtained by exposing trichloroacetic acid-extracted endotoxin to those enzymes which liberated amino acids and, such as Pronase, as well as phosphatases, such as venom phosphodiesterase and alkaline phosphatase. The endotoxic activity (Table 2), as well as the data of chemical analyses (Table 3) of these endotoxin preparations have been compared with the enzyme-extracted and purified ENZ- ENZ preparations. As these tables show, considerable enhancement of mouse lethality could be obtained by exposing trichloroacetic acid-extracted endotoxin to enzymes (TA-ENZ) or by obtaining the endotoxin from whole bacterial cells by enzymatic extraction and purification. The yield in toxic material which expresses the number of median lethal doses isolated from 1 g of lyophilized bacterial cells was also highest with enzymatic liberation. The differences in activities are shown especially well in the case of pyrogenicity. Expressing the MPD in micrograms, by far the most active prepa, ration is the one which was liberated and purified from bacterial cells by enzymes. The pyrogenicity determined by the FT20 method did not agree with the MPD results. The FT20 of the ENZ-ENZ preparation was lower than that of the TA-PW. The local Shwartzman skin reactivity, which was expressed as the maximal dilution that still showed a positive skin reaction, did not reveal a significant difference between the different preparations. The ENZ-ENZ endotoxin did not show a greater activity here than other preparations. The chemical composition of the different endotoxin preparations is compared in Table 3. The trichloroacetic acid-extracted endotoxin, which was the lowest in biological activity, had the highest amount of phosphorus and a considerably high amount, almost 20%, of amino acids. Purification of this perparation by phenolwater extraction greatly reduced the amino acid content, but it was still close to 6%. Enzymatic treatment of the trichloroacetic acid-extracted endotoxin, which greatly enhanced the biological activity, did not reduce the amino acid content to a great degree. The highest amino acid content, over 27%, was detected in the enzyme-extracted and enzyme-purified endotoxin. Carbohydrate analysis revealed that the highest content is present in the TA-PW preparation. The lowest carbohydrate content was in the ENZ-ENZ preparation, which was also the most active endotoxin. The fatty acid content was highest in the TA-PW and in the ENZ-ENZ preparations. Column chromatographic analysis of the four preparations was carried out by using Amberlite XE220 anionic ion-exchange resin. Figure 1 shows the carbohydrate content of the chromatographic effluent, monitored by a Technicon AutoAnalyzer, as reported earlier (41). The carbohydrate content of the effluent reveals the presence of several carbohydrate-containing peaks in the effluent, and, as was observed earlier, the component which leaves the column shortly after 4 hr retention time showed endotoxic activity. The pattern obtained correlates with the
5 932 LEHRER AND NOWOTNY INFECT. IMMUNITY TABLE 2. Comparisonis of enidotoxic activities of various preparations Miouse lethal ToLocal Shwartz- Endotoxin toxicity (LDso Toxic material man reactivity FI20 (za) Pyrogenicity in mg) yield (a) (maximal MNPD (/Ag) I ~~~~~~~~~~dilutionin jag) TA-endotoxin TA-PW TA-ENZ ENZ-ENZ a The number of median lethal doses (LD5o) isolated from 1 g of bacterial cells. TABLE 3. Chemical analyses of the various endotoxini preparationsa Total Endotoxin ND; XAmino reducing Hexosamines Ht -ets3-deoxy Fatty Iacids carbohy- epsscarbohydrates acids drates TA-endotoxin TA-PW TA-ENZ ENZ-ENZ OD. a All values expressed as percentages RETEION TIME in HOURS FIG. 1. Columnz chromatographic fractionationz of various endotoxin preparationis. Endotoxins were fractionzatedby usingan Amberlite XE 220 resin, with a conlinluous gradient as described. Carbohydrate in the effluentt was detected by the orcinol reaction, anid the resulting chromogen was monitored at 420 nm by using thle Technicon autoanalysis system. 1, 3.75 mg of TA-endotoxin. 2, 3.75 mg of TA-P W endotoxin. 3, 3.75 mg of TA-ENZ endotoxini. 4, 3.75 mg of ENZ-ENZ enidotoxin. biological activities observed. The greatest amount of this component is present in the fourth preparation, which is the ENZ-ENZ endotoxin. In view of the fact that only minor peaks are observed in this preparation in zones which are inert as endotoxins, this preparation shows considerably greater homogeneity in comparison to all other materials. From the shape of the curve as well as from additional analysis, it could be seen that the band leaving after 4 hr of retention time is still not a chromatographically homogeneous substance. DISCUSSION A few reports in the literature describe inactivation or degradation of endotoxin by serum fractions (9, 21) or by organs (8, 20). Some of these are attributed to enzymatic processes, but isolation of the enzymes or characterization of their specificities has not yet been achieved. A microbial enzyme preparation was reported by Vincent and Cameron (22) to be able to degrade endotoxin, but reproducibility of the findings was not satisfactory. Dictyostelium discoideunm, a slime mold which utilizes bacterial cells as nutrients, appears to contain, among others, an endotoxin-inactivating enzyme, since no active endotoxin could be extracted from these bacteria plus slime mold cocultures by the phenol-water procedure. The substance obtained is nontoxic, and has a low percentage of fatty acid-containing, serologically fully active bacterial polysaccharide (A. M. Abdelnoor, Ph.D. thesis, University of Michigan, Ann Arbor, 1969; 12). Attempts to isolate the inactivating enzyme were not successful. Experiments were performed in our laboratories to find a microorganism which would provide the desired endotoxin-degrading enzyme. Several strains were isolated from soil samples, which could utilize crude trichloroacetic acid-
6 VOL. 6, 1972 ISOLATION AND PURIFICATION OF ENDOTOXIN 933 endotoxin preparation as the sole organic nutrient. These bacteria and fungi were able to utilize endotoxin as a source of nutrient; however, these culture filtrates could not reduce endotoxin lethality. Details of these experiments were described elsewhere (C. B. Lehrer, Ph.D. thesis, Temple University, Philadelphia, Pa., 1971). The fact that the macromolecule of endotoxin cannot be degraded or inactivated by known hydrolytic enzymes suggests that it requires more specific enzymes, possibly an endotoxinase. Biological inactivation and degradation of endotoxin, which probably takes place in the reticuloendothelial system, may require either a specific enzyme or a specific sequential effect of a number of hydrolases present in the cells of the reticuloendothelial system. The benefit of the high resistance of endotoxin to common hydrolases is that endotoxin can be liberated and purified from bacterial cells by exclusively enzymatic processes, avoiding harsh chemical treatments. The endotoxin obtained by such a procedure is more active in mouse lethality and in MPD determination than any other preparations tested in our laboratory earlier. The results reported here were obtained by using Serratia marcescens 08 cells. Whether the described enzymatic procedure can be applied to other endotoxin-containing gram-negative bacterial strains remains to be investigated. The high amino acid content of the ENZ- ENZ is probably due to the fact that native proteins, which are known to be more resistant, were subjected to proteolysis. If one assumes that these amino acids left in the ENZ-ENZ do not participate in endotoxin activity, their removal should further enhance the activity. Experiments with this aim are being conducted in our laboratory at the present time. ACKNOWLEDGMES This work was supported by Public Health Service AI , from the National Institute of Allergy and Infectious Diseases. LITERATURE CITED 1. Bennett, I. L., Jr Observations on the fever caused by bacterial pyrogens. I. A study of the relationship between fever caused by bacterial pyrogens and the fever accompanying acute infections. J. Exp. Med. 88: Boivin, A., J. Mesrobeanu, and L. Mesrobeanu, Extraction d'un complexe toxique et antigenique a partir du bacille d'aerotrycke. C. R. Soc. Biol. 114: Boivin, A., J. Mesrobeanu, and L. Mesrobeanu Technique pour la preparation des polysaccharides microbiens specifiques. C. R. Soc. Biol. 113: Chen, P. S., Jr., T. Y. Toribara, and H. Warner Microdetermination of phosphorus. Anal. Chem. 28: Cundy, K. R., and A. Nowotny Quantitative comr parison of toxicity parameters of bacterial endotoxins. Proc. Soc. Exp. Biol. Med. 127: Dische, Z Qualitative and quantitative colorimetric determination of heptoses. J. Biol. Chem. 204: Duron, 0. S., and A. Nowotny. Microdetermination of longchain carboxylic acids by transesterification with boron trifluoride. Anal. Chem. 35: Farrar, W. E., Jr., and L. M. Corwin The essential role of the liver in detoxification of endotoxin. Ann. N.Y. Acad. Sci. 133: Hegemann, F., and H. Lessmann Studies of the nature of the pyrogen-neutralizing factor in human blood. V. Occurrence of the endotoxin neutralizing serum factor in various animal species. Z. Immunitiitsforsch. 115: McClosky, W. T., C. W. Price, W. Van Winkle, Jr., H. Welch, and H. 0. Calvery Results of the first U.S.P. collaborative study on pyrogens. J. Amer. Pharm. Ass. 32: Moore, S., and W. H. Stein A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 211: Nigam, N. V., D. Malchow, E. T. Rietschel, 0. LUderitz, and 0. Westphal Die enzymatische Abspaltung langkettiger Fettsauren aus bakteriellen Lipopolysacchariden mittels Extrakten aus der Amobe von Dictyostelium discoideum. Hoppe-Seyler's Z. Physiol. Chem. 351: Nowotny, A Basic exercises in immunochemistry. Springer-Verlag, New York. 14. Nowotny, A Chemical and biological heterogeneity of endotoxins p In Weinbaum et al. (ed.), Microbial toxins, vol. 4. Academic Press Inc., New York. 15. Nowotny, A Heterogeneity of endotoxic bacterial lipopolysaccharides revealed by ion-exchange column chromatography. Nature (London) 210: Nowotny, A., K. R. Cundy, N. L. Neale, A. M. Nowotny, R. Radvany, S. P. Thomas, and D. J. Tripodi. Relation of structure to function in bacterial O-antigens. IV. Fractionation of the components. Ann. N.Y. Acad. Sci. 133: , Nowotny, A. M., S. Thomas, 0. S. Duron, and A. Nowotny Relation of structure to function in bacterial 0- antigens. I. Isolation methods. J. Bacteriol. 85: Park, J. T., and M. J. Johnson A submicrodetermination of glucose. J. Biol. Chem. 181: Rondle, C. J. M., and W. T. J. Morgan The determination of glucosamine and galactosamine. Biochem. J. 61: Rutenburg, S., A. Rutenburg, E. Smith, and J. Fine Detoxification of endotoxin by spleen. Ann. N.Y. Acad. Sci. 133: Skarnes, R. C The inactivation of endotoxin after interaction with certain proteins of normal serum. Ann. N.Y. Acad. Sci. 133: Vincent, W. F., and J. A. Cameron Kinetics of the enzymic dissociation of bacterial endotoxin. Biochim. Biophys. Acta 101: Weissbach, A., and J. Hurwitz The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coil B. J. Biol. Chem. 234: Westphal, O., and 0. Luderitz Chemische Erforschung von Lipopolysacchariden gram negativer Bakterien. Angew. Chem. 66:
Studies on the Glucanase of Sclerotinia libertiana. EBATA and Yukio SATOMURA
 Studies on the Glucanase of Sclerotinia libertiana By Junko EBATA and Yukio SATOMURA Faculty of Science, Osaka City University, Osaka Received December 13, 1962 The digestion of yeast cells with the glucanase
Studies on the Glucanase of Sclerotinia libertiana By Junko EBATA and Yukio SATOMURA Faculty of Science, Osaka City University, Osaka Received December 13, 1962 The digestion of yeast cells with the glucanase
STREPTOCOCCAL L FORMS
 STREPTOCOCCAL L FORMS II. CHEMICAL COMPOSITION' CHARLES PANOS, S. S. BARKULIS, AND J. A. HAYASHI Department of Biological Chemistry, University of Illinois College of Medicine, Chicago, Illinois Received
STREPTOCOCCAL L FORMS II. CHEMICAL COMPOSITION' CHARLES PANOS, S. S. BARKULIS, AND J. A. HAYASHI Department of Biological Chemistry, University of Illinois College of Medicine, Chicago, Illinois Received
INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY
 [Ravish, 2(2): Feb., 2013] ISSN: 2277-9655 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY Isolation And Characterization Of Proteolytic Bacteria And Its Protease Himani Ravish
[Ravish, 2(2): Feb., 2013] ISSN: 2277-9655 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY Isolation And Characterization Of Proteolytic Bacteria And Its Protease Himani Ravish
ELECTROPHORETIC STUDIES OF SONIC EXTRACTS OF PROTEUS VULGARIS
 ELECTROPHORETIC STUDIES OF SONIC EXTRACTS OF PROTEUS VULGARIS I. EFFECT OF GROWTH ENVIRONMENT ON ELECTROPHORETIC PATTERNS' SIDNEY D. RODENBERG Laboratory of Microbiology, Division of Biology, University
ELECTROPHORETIC STUDIES OF SONIC EXTRACTS OF PROTEUS VULGARIS I. EFFECT OF GROWTH ENVIRONMENT ON ELECTROPHORETIC PATTERNS' SIDNEY D. RODENBERG Laboratory of Microbiology, Division of Biology, University
ACTIVATION PHENOMENON OF CLOSTRIDIUM BOTULINUM TYPE E TOXIN
 ACTIVATION PHENOMENON OF CLOSTRIDIUM BOTULINUM TYPE E TOXIN JULIA GERWING, CLAUDE E. DOLMAN, AND DAVID A. ARNOTT Department of Bacteriology and Immunology, The University of British Columbia, Vancouver,
ACTIVATION PHENOMENON OF CLOSTRIDIUM BOTULINUM TYPE E TOXIN JULIA GERWING, CLAUDE E. DOLMAN, AND DAVID A. ARNOTT Department of Bacteriology and Immunology, The University of British Columbia, Vancouver,
TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
 Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
N-Glycosidase F Deglycosylation Kit
 For life science research only. Not for use in diagnostic procedures. FOR IN VITRO USE ONLY. N-Glycosidase F Deglycosylation Kit Kit for the deglycosylation of asparagine-linked glycan chains on glycoproteins.
For life science research only. Not for use in diagnostic procedures. FOR IN VITRO USE ONLY. N-Glycosidase F Deglycosylation Kit Kit for the deglycosylation of asparagine-linked glycan chains on glycoproteins.
Our data indicate that it is the lipid rather than. the polysaccharide moiety of LPS that is mitogenic. were used at 8 to 10 weeks of age.
 INFECTION AND IMMUNITY, Sept. 1973, p. 406-411 Copyright 0 1973 American Society for Microbiology Vol. 8, No. 3 Printed in U.S.A. In Vitro Transformation of Mouse Bone-Marrow-Derived (B) Lymphocytes Induced
INFECTION AND IMMUNITY, Sept. 1973, p. 406-411 Copyright 0 1973 American Society for Microbiology Vol. 8, No. 3 Printed in U.S.A. In Vitro Transformation of Mouse Bone-Marrow-Derived (B) Lymphocytes Induced
Solubilization and Activation of Membrane-bound Acid Protease of Aspergillus oryzae
 Agric. Biol. Chem., 41 (11), 2125 `2130, 1977 Solubilization and Activation of Membrane-bound Acid Protease of Aspergillus oryzae Yoshio TSUJITA and Akira ENDO Fermentation Research Laboratories Sankyo
Agric. Biol. Chem., 41 (11), 2125 `2130, 1977 Solubilization and Activation of Membrane-bound Acid Protease of Aspergillus oryzae Yoshio TSUJITA and Akira ENDO Fermentation Research Laboratories Sankyo
Experiment 1. Isolation of Glycogen from rat Liver
 Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
STUDIES OF STREPTOCOCCAL CELL WALLS
 STUDIES OF STREPTOCOCCAL CELL WALLS V. AMINO ACID COMPOSITION OF CELL WALLS OF VIRULENT AND AVIRULENT GROUP A HEMOLYTIC STREPTOCOCCI' B. S. TEPPER, J. A. HAYASHI, AND S. S. BARKULIS Department of Biological
STUDIES OF STREPTOCOCCAL CELL WALLS V. AMINO ACID COMPOSITION OF CELL WALLS OF VIRULENT AND AVIRULENT GROUP A HEMOLYTIC STREPTOCOCCI' B. S. TEPPER, J. A. HAYASHI, AND S. S. BARKULIS Department of Biological
Broad Spectrum Protease Inhibitor Cocktail
 Broad Spectrum Protease Inhibitor Cocktail Catalog number: AR1182 Boster s Broad Spectrum Protease Inhibitor Cocktail is a complex of various protease inhibitors, which has been tested for inhibiting proteases
Broad Spectrum Protease Inhibitor Cocktail Catalog number: AR1182 Boster s Broad Spectrum Protease Inhibitor Cocktail is a complex of various protease inhibitors, which has been tested for inhibiting proteases
Pepsin Microplate Assay Kit User Manual
 Pepsin Microplate Assay Kit User Manual Catalog # CAK1081 Detection and Quantification of Pepsin Activity in Urine, Serum, Plasma, Tissue extracts, Cell lysate, Cell culture media and Other biological
Pepsin Microplate Assay Kit User Manual Catalog # CAK1081 Detection and Quantification of Pepsin Activity in Urine, Serum, Plasma, Tissue extracts, Cell lysate, Cell culture media and Other biological
lactose-fermenting variants (reds). Appreciable lactose utilization variants. Hershey and Bronfenbrenner (1936) found the non-lactosefermenting
 THE LACTASE ACTIVITY OF ESCHERICHIA COLI- MUTABILE' CHARLES J. DEERE, ANNA DEAN DULANEY AND I. D. MICHELSON Department of Chemistry and Department of Bacteriology, University of Tennessee School of Biological
THE LACTASE ACTIVITY OF ESCHERICHIA COLI- MUTABILE' CHARLES J. DEERE, ANNA DEAN DULANEY AND I. D. MICHELSON Department of Chemistry and Department of Bacteriology, University of Tennessee School of Biological
LAB 3: Biomolecules and Digestion
 Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Volatile Fatty Acids and the Inhibition of Escherichia
 APPuan MICROBIOLOGY, Jan. 1969, p. 83-87 Copyright 1969 American Society for Microbiology Vol. 17, No. 1 Printed in U.S.A Volatile Fatty Acids and the of Escherichia coli Growth by Rumen Fluid1 MEYER J.
APPuan MICROBIOLOGY, Jan. 1969, p. 83-87 Copyright 1969 American Society for Microbiology Vol. 17, No. 1 Printed in U.S.A Volatile Fatty Acids and the of Escherichia coli Growth by Rumen Fluid1 MEYER J.
Chlorphenesin: an Antigen-Associated Immunosuppressant
 INFECTION AND IMMUNITY, JUlY 197, p. 6-64 Vol. 2, No. 1 Copyright 197 American Society for Microbiology Printed in U.S.A. Chlorphenesin: an Antigen-Associated Immunosuppressant H. Y. WHANG AND E. NETER
INFECTION AND IMMUNITY, JUlY 197, p. 6-64 Vol. 2, No. 1 Copyright 197 American Society for Microbiology Printed in U.S.A. Chlorphenesin: an Antigen-Associated Immunosuppressant H. Y. WHANG AND E. NETER
Chemical and Physical Processes of Digestion
 M57_MARI0000_00_SE_EX08.qxd 8/22/11 3:08 PM Page 394 8 E X E R C I S E Chemical and Physical Processes of Digestion Advance Preparation/Comments 1. Suggest to the students that they become familiar with
M57_MARI0000_00_SE_EX08.qxd 8/22/11 3:08 PM Page 394 8 E X E R C I S E Chemical and Physical Processes of Digestion Advance Preparation/Comments 1. Suggest to the students that they become familiar with
Hydrolysis of Irradiated Ovalbumin by Pepsin
 Hydrolysis of Irradiated Ovalbumin by Pepsin HECTOR A. DIEU and V. DESREUX From the Department of Physical Chemistry, University of Liege, Liege, Belgium ABSTRACT Solid ovalbumin has been irradiated at
Hydrolysis of Irradiated Ovalbumin by Pepsin HECTOR A. DIEU and V. DESREUX From the Department of Physical Chemistry, University of Liege, Liege, Belgium ABSTRACT Solid ovalbumin has been irradiated at
Chapter 9: Digestion Review Assignment
 _ Date: Mark: /45 Chapter 9: Digestion Review Assignment 45 Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following roles do
_ Date: Mark: /45 Chapter 9: Digestion Review Assignment 45 Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following roles do
STUDIES OF AN ENZYME PRODUCED BY BACILLUS FULMINANS
 STUDIES OF AN ENZYME PRODUCED BY BACILLUS FULMINANS THAT INACTIVATES BLOOD GROUP 0 SUBSTANCE1 INGEBORG NAYLOR AND HAROLD BAER Department of Microbiology, Tulane University School of Medicine, New Orleans,
STUDIES OF AN ENZYME PRODUCED BY BACILLUS FULMINANS THAT INACTIVATES BLOOD GROUP 0 SUBSTANCE1 INGEBORG NAYLOR AND HAROLD BAER Department of Microbiology, Tulane University School of Medicine, New Orleans,
OF TRANSAMINASE IN RAT TISUES
 OF TRANSAMINASE IN RAT TISUES KOZO YAMADA, SHUNJI SAWAKI, AKIRA FUKUMURA AND MASARU HAYASHID epartment of Internal Mcdicine, Faculty of Medicine, Nagoya University, agoya Showa-ku, N (Received July 30,
OF TRANSAMINASE IN RAT TISUES KOZO YAMADA, SHUNJI SAWAKI, AKIRA FUKUMURA AND MASARU HAYASHID epartment of Internal Mcdicine, Faculty of Medicine, Nagoya University, agoya Showa-ku, N (Received July 30,
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP
 CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
THE ISOLATION OF A MUCOPOLYSACCHARIDE FROM SYNOVIAL FLUID*
 THE ISOLATION OF A MUCOPOLYSACCHARIDE FROM SYNOVIAL FLUID* BY KARL MEYER, ELIZABETH M. SMYTH, AND MARTIN H. DAWSON (From the Department of Ophthalmology, College of Physicians and Surgeons, Columbia University,
THE ISOLATION OF A MUCOPOLYSACCHARIDE FROM SYNOVIAL FLUID* BY KARL MEYER, ELIZABETH M. SMYTH, AND MARTIN H. DAWSON (From the Department of Ophthalmology, College of Physicians and Surgeons, Columbia University,
ENZYMES QUESTIONSHEET 1
 QUESTIONSHEET 1 The apparatus illustrated below can be used to investigate the activity of the enzyme catalase, which is found in liver. The liver tissue has been ground up and mixed with a buffer solution.
QUESTIONSHEET 1 The apparatus illustrated below can be used to investigate the activity of the enzyme catalase, which is found in liver. The liver tissue has been ground up and mixed with a buffer solution.
Role of a Protease in Natural Activation of Clostridium botulinum Neurotoxin
 INFECTION AND IMMUNITY, OCt. 1972, p. 587-590 Copyright 1972 American Society for Microbiology Vol. 6, No. 4 Printed in U.S.A. Role of a Protease in Natural Activation of Clostridium botulinum Neurotoxin
INFECTION AND IMMUNITY, OCt. 1972, p. 587-590 Copyright 1972 American Society for Microbiology Vol. 6, No. 4 Printed in U.S.A. Role of a Protease in Natural Activation of Clostridium botulinum Neurotoxin
ON THE NATURE OF THE TRANSALDOLASE-DIHYDROXYACETONE
 VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
Screening of Rice Straw Degrading Microorganisms and Their Cellulase Activities
 Research 83 KKU Sci. J.37 (Supplement) 83-88 (2009) Screening of Rice Straw Degrading Microorganisms and Their Cellulase Activities Abstract Atcha Boonmee 1,2* Rice straw is one of the most abundant agricultural
Research 83 KKU Sci. J.37 (Supplement) 83-88 (2009) Screening of Rice Straw Degrading Microorganisms and Their Cellulase Activities Abstract Atcha Boonmee 1,2* Rice straw is one of the most abundant agricultural
Consequently, lipoprotein fractions have been analyzed
 THE PHOSPHOLIPID COMPOSITION OF HUMAN SERUM LIPOPROTEIN FRACTIONS SEPARATED BY ULTRACENTRIFUGATION * BY GERALD B. PHILLIPS (From the Departments of Biochemistry and Medicine, College of Physicians and
THE PHOSPHOLIPID COMPOSITION OF HUMAN SERUM LIPOPROTEIN FRACTIONS SEPARATED BY ULTRACENTRIFUGATION * BY GERALD B. PHILLIPS (From the Departments of Biochemistry and Medicine, College of Physicians and
ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA
 J. Gen. App!. Microbiol., 34, 213-219 (1988) ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA TOSHIRO HAYASHI, RYO IOROI,*
J. Gen. App!. Microbiol., 34, 213-219 (1988) ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA TOSHIRO HAYASHI, RYO IOROI,*
Inositol Phosphate Phosphatases of Microbiological Origin: the Inositol Pentaphosphate Products of Aspergillus ficuum
 JOURNAL OF BACTERIOLOGY, OCt. 1972, p. 434-438 Copyright 1972 American Society for Microbiology Vol. 112, No. 1 Printed in U.S.A. Inositol Phosphate Phosphatases of Microbiological Origin: the Inositol
JOURNAL OF BACTERIOLOGY, OCt. 1972, p. 434-438 Copyright 1972 American Society for Microbiology Vol. 112, No. 1 Printed in U.S.A. Inositol Phosphate Phosphatases of Microbiological Origin: the Inositol
Pelagia Research Library
 Available online at www.pelagiaresearchlibrary.com European Journal of Experimental Biology, 211, 1 (3):124-129 ISSN: 2248 9215 Production of Alkaline Protease by Bacillus subtilis (MTCC7312) using Submerged
Available online at www.pelagiaresearchlibrary.com European Journal of Experimental Biology, 211, 1 (3):124-129 ISSN: 2248 9215 Production of Alkaline Protease by Bacillus subtilis (MTCC7312) using Submerged
protein (Eaton 1936 a, 1937; Pappenheimer 1937). If other
 COMPARATIVE STUDIES ON THE PURIFICATION OF TETANUS AND DIPHTHERIA TOXINS MONROE D. EATON AND AXEL GRONAU Department of Bacteriology, Washington University School of Medicine, St. Louis, Missouri Received
COMPARATIVE STUDIES ON THE PURIFICATION OF TETANUS AND DIPHTHERIA TOXINS MONROE D. EATON AND AXEL GRONAU Department of Bacteriology, Washington University School of Medicine, St. Louis, Missouri Received
Sequential Extraction of Plant Metabolites
 ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 33-38 http://www.ijcmas.com Original Research Article Sequential Extraction of Plant Metabolites Shankar L. Laware* PG. Department of Botany, Fergusson College
ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 33-38 http://www.ijcmas.com Original Research Article Sequential Extraction of Plant Metabolites Shankar L. Laware* PG. Department of Botany, Fergusson College
Pyrogenicity and Immunogenicity of Lipid A Complexed with Bovine Serum Albumin or
 INFECTION AND IMMUNITY, Aug. 1973, p. 173--177 Copyright i 1973 American Society for Microbiology Vol. 8, No. 2 Printed in U.S.A. Pyrogenicity and Immunogenicity of Lipid A Complexed with Bovine Serum
INFECTION AND IMMUNITY, Aug. 1973, p. 173--177 Copyright i 1973 American Society for Microbiology Vol. 8, No. 2 Printed in U.S.A. Pyrogenicity and Immunogenicity of Lipid A Complexed with Bovine Serum
Phospholipase D Activity of Gram-Negative Bacteria
 JOURNAL OF BACTERIOLOGY, Dec. 1975, p. 1148-1152 Copyright 1975 American Society for Microbiology Vol. 124, No. 3 Printed in U.S.A. Phospholipase D Activity of Gram-Negative Bacteria R. COLE AND P. PROULX*
JOURNAL OF BACTERIOLOGY, Dec. 1975, p. 1148-1152 Copyright 1975 American Society for Microbiology Vol. 124, No. 3 Printed in U.S.A. Phospholipase D Activity of Gram-Negative Bacteria R. COLE AND P. PROULX*
(From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New Jersey)
 CRYSTALLIZATION OF SALT-FREE CHYMOTRYPSINOGEN AND CHYMOTRYPSIN FROM SOLUTION IN DILUTE ETHYL ALCOHOL BY M. KUNITZ (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New
CRYSTALLIZATION OF SALT-FREE CHYMOTRYPSINOGEN AND CHYMOTRYPSIN FROM SOLUTION IN DILUTE ETHYL ALCOHOL BY M. KUNITZ (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New
SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES
 1 SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES Proteins are important in food processing and food product development, as they are
1 SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES Proteins are important in food processing and food product development, as they are
Supporting Information
 Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS
 22 IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS Michael P. Lerner*, J. H. Anglin, Peggy L. Munson, Peggy J. Riggs, Nancy E. Manning, and Robert E. Nordquist Departments
22 IMMUNOLOGIC REACTIVITY IN HUMAN BREAST CANCER AGAINST CULTURED HUMAN BREAST TUMOR CELLS Michael P. Lerner*, J. H. Anglin, Peggy L. Munson, Peggy J. Riggs, Nancy E. Manning, and Robert E. Nordquist Departments
Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products)
 Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products) The target compound to be determined is 2, 4, 5-T. 1. Instrument Liquid Chromatograph-tandem mass spectrometer (LC-MS/MS)
Analytical Method for 2, 4, 5-T (Targeted to Agricultural, Animal and Fishery Products) The target compound to be determined is 2, 4, 5-T. 1. Instrument Liquid Chromatograph-tandem mass spectrometer (LC-MS/MS)
New Proteolytic Enzymes from Clostridium histolyticum Filtrates
 I No. 3, Volume 17 of the Journal of General Microbiology was issued on 13 December 1957 M4CLENNAN, J. D., MANDL, I. & HOWES, E. L. (1958). J. gen. Microbid. 18, 1-8 New Proteolytic Enzymes from Clostridium
I No. 3, Volume 17 of the Journal of General Microbiology was issued on 13 December 1957 M4CLENNAN, J. D., MANDL, I. & HOWES, E. L. (1958). J. gen. Microbid. 18, 1-8 New Proteolytic Enzymes from Clostridium
Journal of Chemical and Pharmaceutical Research, 2012, 4(8): Research Article
 Available online wwwjocprcom Journal of Chemical and Pharmaceutical Research, 212, 4(8):388-3812 Research Article ISSN : 975-7384 CODEN(USA) : JCPRC5 Isolation and partial purification of Protease from
Available online wwwjocprcom Journal of Chemical and Pharmaceutical Research, 212, 4(8):388-3812 Research Article ISSN : 975-7384 CODEN(USA) : JCPRC5 Isolation and partial purification of Protease from
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN
 THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
Scholars Research Library. Purification and characterization of neutral protease enzyme from Bacillus Subtilis
 Journal of Microbiology and Biotechnology Research Scholars Research Library J. Microbiol. Biotech. Res., 2012, 2 (4):612-618 (http://scholarsresearchlibrary.com/archive.html) Purification and characterization
Journal of Microbiology and Biotechnology Research Scholars Research Library J. Microbiol. Biotech. Res., 2012, 2 (4):612-618 (http://scholarsresearchlibrary.com/archive.html) Purification and characterization
Acid Protease Activity During Germination of Microcysts of the
 JOURNAL OF BACTERIOLOGY, Jan. 1976, p. 8-13 Copyright i 1976 American Society for Microbiology Vol. 125, No. 1 Printed in U.S.A. Acid Protease Activity During Germination of Microcysts of the Cellular
JOURNAL OF BACTERIOLOGY, Jan. 1976, p. 8-13 Copyright i 1976 American Society for Microbiology Vol. 125, No. 1 Printed in U.S.A. Acid Protease Activity During Germination of Microcysts of the Cellular
OPTIMISATION OF XYLOSE PRODUCTION USING XYLANASE
 Int. J. Chem. Sci.: 8(2), 2010, 909-913 OPTIMISATION OF XYLOSE PRODUCTION USING XYLANASE T. SATHISH a and N. Y. S. MURTHY * Department of Biotechnology, Malla Reddy Engineering College, HYDERABAD (A.P.)
Int. J. Chem. Sci.: 8(2), 2010, 909-913 OPTIMISATION OF XYLOSE PRODUCTION USING XYLANASE T. SATHISH a and N. Y. S. MURTHY * Department of Biotechnology, Malla Reddy Engineering College, HYDERABAD (A.P.)
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Molecule Energy Released Glucose 4 kcal/gram Sucrose 4 kcal/gram Lipid 9 kcal/gram Protein 4 kcal/gram
 Biology Keystone (PA Core) Quiz The Chemical Basis for Life - (BIO.A.2.2.3 ) Compare Carbohydrates, (BIO.A.2.3.1 ) Enzyme Role, (BIO.A.2.3.2) Enzyme Function Student Name: Teacher Name: Jared George Date:
Biology Keystone (PA Core) Quiz The Chemical Basis for Life - (BIO.A.2.2.3 ) Compare Carbohydrates, (BIO.A.2.3.1 ) Enzyme Role, (BIO.A.2.3.2) Enzyme Function Student Name: Teacher Name: Jared George Date:
 The Presence of Pyruvate Residues i TitleSimilar Polysaccharide (Commemorati Professor Sango Kunichika On the Oc Author(s) Hirase, Susumu; Watanabe, Kyoko Citation Bulletin of the Institute for Chemi University
The Presence of Pyruvate Residues i TitleSimilar Polysaccharide (Commemorati Professor Sango Kunichika On the Oc Author(s) Hirase, Susumu; Watanabe, Kyoko Citation Bulletin of the Institute for Chemi University
Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds
 Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds By Edward J. Leonard, M.D., and Stephen Hajdu, M.D. A plasma protein system of mammalian origin which increases the contractile
Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds By Edward J. Leonard, M.D., and Stephen Hajdu, M.D. A plasma protein system of mammalian origin which increases the contractile
Qualitative test of protein-lab2
 1- Qualitative chemical reactions of amino acid protein functional groups: Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product
1- Qualitative chemical reactions of amino acid protein functional groups: Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product
STUDIES ON CHOLINESTERASE*
 STUDIES ON CHOLINESTERASE* III. PURIFICATION OF THE ENZYME FROM ELECTRIC TISSUE BY FRACTIONAL AMMONIUM SULFATE PRECIPITATION BY MORTIMER A. ROTHENBERG AND DAVID NACHMANSOHN (From the Departments of Neurology
STUDIES ON CHOLINESTERASE* III. PURIFICATION OF THE ENZYME FROM ELECTRIC TISSUE BY FRACTIONAL AMMONIUM SULFATE PRECIPITATION BY MORTIMER A. ROTHENBERG AND DAVID NACHMANSOHN (From the Departments of Neurology
ENZYMATIC ACTIVITIES ASSOCIATED WITH CLOTTING OF FIBRINOGEN
 ENZYMATIC ACTIVITIES ASSOCIATED WITH CLOTTING OF FIBRINOGEN BY STAPHYLOCOAGULASE AND COAGULASE-REACTING FACTOR AND THEIR INHIBITION BY DIISOPROPYLFLUOROPHOSPHATE MARGARET C. DRUMMOND AND MORRIS TAGER Department
ENZYMATIC ACTIVITIES ASSOCIATED WITH CLOTTING OF FIBRINOGEN BY STAPHYLOCOAGULASE AND COAGULASE-REACTING FACTOR AND THEIR INHIBITION BY DIISOPROPYLFLUOROPHOSPHATE MARGARET C. DRUMMOND AND MORRIS TAGER Department
Digestive Lecture Test Questions Set 4
 Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
LIPOPOLYSACCHARIDE OF PSEUDOMONAS ARRUGINOSA WITH SPECIAL REFERENCE TO PYOCIN R RECEPTOR ACTIVITY' KAYOKO IKEDA2 AND FUJIO EGAMI'
 J. Gen. Appl. Microbiol., 19, 115-128 (1973) LIPOPOLYSACCHARIDE OF PSEUDOMONAS ARRUGINOSA WITH SPECIAL REFERENCE TO PYOCIN R RECEPTOR ACTIVITY' KAYOKO IKEDA2 AND FUJIO EGAMI' Department of Biophysics and
J. Gen. Appl. Microbiol., 19, 115-128 (1973) LIPOPOLYSACCHARIDE OF PSEUDOMONAS ARRUGINOSA WITH SPECIAL REFERENCE TO PYOCIN R RECEPTOR ACTIVITY' KAYOKO IKEDA2 AND FUJIO EGAMI' Department of Biophysics and
Collagenase Assay Kit
 Collagenase Assay Kit Catalog # 31 and 32 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION The collagenases are members of the matrix metalloproteinase (MMP) family and degrade collagen
Collagenase Assay Kit Catalog # 31 and 32 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION The collagenases are members of the matrix metalloproteinase (MMP) family and degrade collagen
THE ESTIMATION OF PEPSIN, TRYPSIN, PAPAIN, AND CATHEPSIN WITH HEMOGLOBIN
 Published Online: 20 September, 1938 Supp Info: http://doi.org/10.1085/jgp.22.1.79 Downloaded from jgp.rupress.org on July 1, 2018 THE ESTIMATION OF PEPSIN, TRYPSIN, PAPAIN, AND CATHEPSIN WITH HEMOGLOBIN
Published Online: 20 September, 1938 Supp Info: http://doi.org/10.1085/jgp.22.1.79 Downloaded from jgp.rupress.org on July 1, 2018 THE ESTIMATION OF PEPSIN, TRYPSIN, PAPAIN, AND CATHEPSIN WITH HEMOGLOBIN
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI
 METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI I. L- RHAMNOSE ISOMERASE DOROTHY M. WILSON1 AND SAM AJL Department of Bacteriology, Walter Reed Army Institute of Research, Washington, D. C. The methyl pentose,
METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI I. L- RHAMNOSE ISOMERASE DOROTHY M. WILSON1 AND SAM AJL Department of Bacteriology, Walter Reed Army Institute of Research, Washington, D. C. The methyl pentose,
INDUCTION OF β-glucanase FROM WHEY YEAST
 Page263 Research Article Pharmaceutical Sciences INDUCTION OF β-glucanase FROM WHEY YEAST M S.Dake 1*, A N Puntambekar 2, S V Amarapurkar 3 1*, 2, 3 Dr.D.Y.Patil Biotechnology & Bioinformatics Institute,
Page263 Research Article Pharmaceutical Sciences INDUCTION OF β-glucanase FROM WHEY YEAST M S.Dake 1*, A N Puntambekar 2, S V Amarapurkar 3 1*, 2, 3 Dr.D.Y.Patil Biotechnology & Bioinformatics Institute,
LOCALIZATION OF ACID AND ALKALINE PHOSPHATASES IN Myxococcus coralloides D
 LOCALIZATION OF ACID AND ALKALINE PHOSPHATASES IN Myxococcus coralloides D Francisco González, M.Magdalena Martínez-Cañamero, M.Esther Fárez-Vidal & José M.Arias Departamento de Microbiología, Facultad
LOCALIZATION OF ACID AND ALKALINE PHOSPHATASES IN Myxococcus coralloides D Francisco González, M.Magdalena Martínez-Cañamero, M.Esther Fárez-Vidal & José M.Arias Departamento de Microbiología, Facultad
Biology 2180 Laboratory #3. Enzyme Kinetics and Quantitative Analysis
 Biology 2180 Laboratory #3 Name Introduction Enzyme Kinetics and Quantitative Analysis Catalysts are agents that speed up chemical processes and the catalysts produced by living cells are called enzymes.
Biology 2180 Laboratory #3 Name Introduction Enzyme Kinetics and Quantitative Analysis Catalysts are agents that speed up chemical processes and the catalysts produced by living cells are called enzymes.
National Standard of the People s Republic of China. National food safety standard. Determination of pantothenic acid in foods for infants and
 National Standard of the People s Republic of China GB 5413.17 2010 National food safety standard Determination of pantothenic acid in foods for infants and young children, milk and milk products Issued
National Standard of the People s Republic of China GB 5413.17 2010 National food safety standard Determination of pantothenic acid in foods for infants and young children, milk and milk products Issued
Enzymatic Assay of PROTEASE (EC )
 Enzymatic Assay of PROTEASE PRINCIPLE: Hemoglobin + H 2 O Protease > Amino Acids CONDITIONS: T = 37 C, ph = 2.8, A 660nm, Light path = 1 cm METHOD: Colorimetric REAGENTS: A. 50 mm Potassium Phthalate Buffer,
Enzymatic Assay of PROTEASE PRINCIPLE: Hemoglobin + H 2 O Protease > Amino Acids CONDITIONS: T = 37 C, ph = 2.8, A 660nm, Light path = 1 cm METHOD: Colorimetric REAGENTS: A. 50 mm Potassium Phthalate Buffer,
Formation of Methylated and Phosphorylated Metabolites
 ANTMCROBAL AGENTS AND CHEMOTHERAPY, Aug. 1976, p. 363-369 Copyright 1976 American Society for Microbiology Vol. 10, No. 2 Printed in U.S.A. Formation of Methylated and Phosphorylated Metabolites During
ANTMCROBAL AGENTS AND CHEMOTHERAPY, Aug. 1976, p. 363-369 Copyright 1976 American Society for Microbiology Vol. 10, No. 2 Printed in U.S.A. Formation of Methylated and Phosphorylated Metabolites During
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Salmonella minnesota
 JOURNAL of BACTERIOLOGY, Dec. 1967, p. 1824-1836 Copyright 1967 American Society for Microbiology Vol. 94, No. 6 Printed in U.S.A. Endotoxic Glycolipid from a Heptoseless Mutant of Salmonella minnesota
JOURNAL of BACTERIOLOGY, Dec. 1967, p. 1824-1836 Copyright 1967 American Society for Microbiology Vol. 94, No. 6 Printed in U.S.A. Endotoxic Glycolipid from a Heptoseless Mutant of Salmonella minnesota
Degradation of a Pneumococcal Type-Specific Polysaccharide with Exposure of Group-Specificity*
 Proceedings of the National Academy of Sciences Vol. 67, No. 1, pp. 138-142, September 1970 Degradation of a Pneumococcal Type-Specific Polysaccharide with Exposure of Group-Specificity* John D. Higginbotham,
Proceedings of the National Academy of Sciences Vol. 67, No. 1, pp. 138-142, September 1970 Degradation of a Pneumococcal Type-Specific Polysaccharide with Exposure of Group-Specificity* John D. Higginbotham,
belonging to the pseudoglobulins, forming a heat-stable, dialysable vasoconstrictor (Received 2 April 1942)
 284 J. Physiol. (I942) IOI, 284-288 6I2.462.1:6I2.I46 PREPARATION AND SOME PROPERTIES OF HYPERTENSIN (ANGIOTONIN) BY P. EDMAN, U. S. VON EULER, E. JORPES AND 0. T. SJOSTRAND From the Physiology Department
284 J. Physiol. (I942) IOI, 284-288 6I2.462.1:6I2.I46 PREPARATION AND SOME PROPERTIES OF HYPERTENSIN (ANGIOTONIN) BY P. EDMAN, U. S. VON EULER, E. JORPES AND 0. T. SJOSTRAND From the Physiology Department
preparation which is available contains an oligo-1,4-0 1,4-glucantransferase
 THE PROPERTIES OF AN OLIGO-1,4 -- 1,4-GLUCANTRANSFERASE FROM ANIMAL. TISSUES* BY DAVID H. BROWN AND BARBARA ILLINGWORTH DEPARTMENT OF BIOLOGICAL CHEMISTRY, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE, SAINT
THE PROPERTIES OF AN OLIGO-1,4 -- 1,4-GLUCANTRANSFERASE FROM ANIMAL. TISSUES* BY DAVID H. BROWN AND BARBARA ILLINGWORTH DEPARTMENT OF BIOLOGICAL CHEMISTRY, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE, SAINT
Iodide transport in isolated cells of mouse submaxillary gland
 J. Biosci., Vol. 10, Number 3, September 1986, pp. 303 309. Printed in India. Iodide transport in isolated cells of mouse submaxillary gland R. K. BANERJEE*, A. K. BOSE, T. K. CHAKRABORTY, P. K. DE and
J. Biosci., Vol. 10, Number 3, September 1986, pp. 303 309. Printed in India. Iodide transport in isolated cells of mouse submaxillary gland R. K. BANERJEE*, A. K. BOSE, T. K. CHAKRABORTY, P. K. DE and
Fatty Acid and Aliphatic Hydrocarbon Composition of Sarcina lutea Grown in Three Different Media
 JOURNAL OF BACTERIOLOGY, Aug. 1967, p. 344-348 Copyright @ 1967 American Society for Microbiology Vol. 94, No. 2 Printed in U.S.A. Fatty Acid and Aliphatic Hydrocarbon Composition of Sarcina lutea Grown
JOURNAL OF BACTERIOLOGY, Aug. 1967, p. 344-348 Copyright @ 1967 American Society for Microbiology Vol. 94, No. 2 Printed in U.S.A. Fatty Acid and Aliphatic Hydrocarbon Composition of Sarcina lutea Grown
22 Bicozamycin (Bicyclomycin)
 22 Bicozamycin (Bicyclomycin) OH O H N O O OH HO [Summary of bicozamycin] C 12 H 18 N 2 O 7 MW: 302.3 CAS No.: 38129-37-2 Bicozamycin (BZM) is an antibiotic obtained from a fermented culture of Streptomyces
22 Bicozamycin (Bicyclomycin) OH O H N O O OH HO [Summary of bicozamycin] C 12 H 18 N 2 O 7 MW: 302.3 CAS No.: 38129-37-2 Bicozamycin (BZM) is an antibiotic obtained from a fermented culture of Streptomyces
THE MILK-CLOTTING ACTION OF PAPAIN*
 THE MILK-CLOTTING ACTION OF PAPAIN* BY A. K. BALLS.4ND SAM R. HOOVER (From the Food Research Division, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington) (Received for
THE MILK-CLOTTING ACTION OF PAPAIN* BY A. K. BALLS.4ND SAM R. HOOVER (From the Food Research Division, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington) (Received for
JOURNAL OF INTERNATIONAL ACADEMIC RESEARCH FOR MULTIDISCIPLINARY Impact Factor 1.625, ISSN: , Volume 2, Issue 11, December 2014
 COMPARISON OF ACTIVITY OF PECTINASE USING AGRICULTURAL WASTE SUBSTRATES A STUDY M.P.KUSUMA* DR M.V.V.CHANDANA LAKSHMI** *Assistant Professor, RBVRR College of Pharmacy, Osmania University, India **Associate
COMPARISON OF ACTIVITY OF PECTINASE USING AGRICULTURAL WASTE SUBSTRATES A STUDY M.P.KUSUMA* DR M.V.V.CHANDANA LAKSHMI** *Assistant Professor, RBVRR College of Pharmacy, Osmania University, India **Associate
SUPPLEMENTARY INFORMATION. Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was
 SUPPLEMENTARY INFORMATION Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was grown in a casein-based semisynthetic medium (C+Y) supplemented with yeast extract (1 mg/ml of
SUPPLEMENTARY INFORMATION Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was grown in a casein-based semisynthetic medium (C+Y) supplemented with yeast extract (1 mg/ml of
Substrate Specificity and Salt Inhibition of Five Proteinases Isolated from the Pyloric Caeca and Stomach of Sardine
 Agric. Biol. Chem., 46 (6), 1565~1569, 1982 1565 Substrate Specificity and Salt Inhibition of Five Proteinases Isolated from the Pyloric Caeca and Stomach of Sardine Minoru Noda, Thanh Vo Van, Isao Kusakabe
Agric. Biol. Chem., 46 (6), 1565~1569, 1982 1565 Substrate Specificity and Salt Inhibition of Five Proteinases Isolated from the Pyloric Caeca and Stomach of Sardine Minoru Noda, Thanh Vo Van, Isao Kusakabe
The effect of phosphatidyl choline on the degradation of phosphatidyl ethanolamine by the phospholipase of post-heparin plasma or snake venom
 The effect of phosphatidyl choline on the degradation of phosphatidyl ethanolamine by the phospholipase of post-heparin plasma or snake venom WILLIAM C. VOGEL, J. L. KOPPEL, and J. H. OLWIN Coagulation
The effect of phosphatidyl choline on the degradation of phosphatidyl ethanolamine by the phospholipase of post-heparin plasma or snake venom WILLIAM C. VOGEL, J. L. KOPPEL, and J. H. OLWIN Coagulation
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB5009.
 Translated English of Chinese Standard: GB5009.259-2016 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD GB OF THE PEOPLE S REPUBLIC OF CHINA National food safety standard Determination
Translated English of Chinese Standard: GB5009.259-2016 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD GB OF THE PEOPLE S REPUBLIC OF CHINA National food safety standard Determination
Effect of Fatty Acids on Staphylococcus aureus Delta-Toxin
 INFECTION AND IMMUNITY, Jan. 1976, p. 114-119 Copyright 0 1976 American Society for Microbiology Vol. 13, No. 1 Printed in U.S.A. Effect of Fatty Acids on Staphylococcus aureus Delta-Toxin Hemolytic Activity
INFECTION AND IMMUNITY, Jan. 1976, p. 114-119 Copyright 0 1976 American Society for Microbiology Vol. 13, No. 1 Printed in U.S.A. Effect of Fatty Acids on Staphylococcus aureus Delta-Toxin Hemolytic Activity
Interaction of an Endotoxin with Cationic Macromolecules
 J. gen. Mimobiol. (96), 26, 8-95 Printed in Great Britain 8 Interaction of an Endotoxin with Cationic Macromolecules BY P. T. MORA AND B. G. YOUNG National Cancer Institute, National Institutes of Health,
J. gen. Mimobiol. (96), 26, 8-95 Printed in Great Britain 8 Interaction of an Endotoxin with Cationic Macromolecules BY P. T. MORA AND B. G. YOUNG National Cancer Institute, National Institutes of Health,
Collagenase Assay Kit
 Collagenase Assay Kit Catalog # 31 and 32 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION Collagenases are members of the matrix metalloproteinase (MMP) family and degrade collagen types
Collagenase Assay Kit Catalog # 31 and 32 For Research Use Only - Not Human or Therapeutic Use INTRODUCTION Collagenases are members of the matrix metalloproteinase (MMP) family and degrade collagen types
PURIFICATION OF THE TOXIN IN A ZOAN PALYTHOA TUBERCULOSA.
 Title PURIFICATION OF THE TOXIN IN A ZOAN PALYTHOA TUBERCULOSA Author(s) Kimura, Shoji; Hashimoto, Yoshiro Citation PUBLICATIONS OF THE SETO MARINE BIO LABORATORY (1973), 20: 713-718 Issue Date 1973-12-19
Title PURIFICATION OF THE TOXIN IN A ZOAN PALYTHOA TUBERCULOSA Author(s) Kimura, Shoji; Hashimoto, Yoshiro Citation PUBLICATIONS OF THE SETO MARINE BIO LABORATORY (1973), 20: 713-718 Issue Date 1973-12-19
STUDIES OF THE HEMAGGLUTININ OF HAEMOPHILUS PERTUSSIS HIDEO FUKUMI, HISASHI SHIMAZAKI, SADAO KOBAYASHI AND TATSUJI UCHIDA
 STUDIES OF THE HEMAGGLUTININ OF HAEMOPHILUS PERTUSSIS HIDEO FUKUMI, HISASHI SHIMAZAKI, SADAO KOBAYASHI AND TATSUJI UCHIDA The National Institute of Health, Tokyo, Japan (Received: August 3rd, 1953) INTRODUCTION
STUDIES OF THE HEMAGGLUTININ OF HAEMOPHILUS PERTUSSIS HIDEO FUKUMI, HISASHI SHIMAZAKI, SADAO KOBAYASHI AND TATSUJI UCHIDA The National Institute of Health, Tokyo, Japan (Received: August 3rd, 1953) INTRODUCTION
Properties of Water. 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin.
 Name: ate: 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin. 1. Which of these enzymes function in the most similar ph range?.
Name: ate: 1. The graph shows the relationship between the rate of enzyme action and ph for three enzymes: pepsin, urease, and trypsin. 1. Which of these enzymes function in the most similar ph range?.
Phospholipid Fatty Acid (PLFA) Science, Inovation, Networks
 Phospholipid Fatty Acid (PLFA) Specific components of cell membranes that are only found in intact (viable) cells and rapidly degraded after cell death (White et.al, 1997 in Yao et.al, 2000) Cell membrane
Phospholipid Fatty Acid (PLFA) Specific components of cell membranes that are only found in intact (viable) cells and rapidly degraded after cell death (White et.al, 1997 in Yao et.al, 2000) Cell membrane
GLYCOGEN BEFORE THE LAB YOU HAVE TO READ ABOUT:
 GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
MIXED XYLANASE, β-glucanase ENZYME PREPARATION, produced by a strain of HUMICOLA INSOLENS
 MIXED XYLANASE, β-glucanase ENZYME PREPARATION, produced by a strain of HUMICOLA INSOLENS New specifications prepared at the 61st JECFA (2003) and published in FNP 52 Add 11 (2003). An ADI not specified
MIXED XYLANASE, β-glucanase ENZYME PREPARATION, produced by a strain of HUMICOLA INSOLENS New specifications prepared at the 61st JECFA (2003) and published in FNP 52 Add 11 (2003). An ADI not specified
Reading Comprehension of the digestive tract
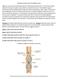 Reading Comprehension of the digestive tract Digestion is a process that break-down food into small molecule called nutrient. These small molecule called nutrients pass through the cell membrane or absorb
Reading Comprehension of the digestive tract Digestion is a process that break-down food into small molecule called nutrient. These small molecule called nutrients pass through the cell membrane or absorb
psittaci by Silver-Methenamine Staining and
 JOURNAL OF BACTERIOLOGY, July 1972, p. 267-271 Copyright 1972 American Society for Microbiology Vol. 111, No. 1 Printed in U.S.A. Location of Polysaccharide on Chlamydia psittaci by Silver-Methenamine
JOURNAL OF BACTERIOLOGY, July 1972, p. 267-271 Copyright 1972 American Society for Microbiology Vol. 111, No. 1 Printed in U.S.A. Location of Polysaccharide on Chlamydia psittaci by Silver-Methenamine
The incorporation of labeled amino acids into lens protein. Abraham Speclor and Jin H. Kinoshita
 The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19
 TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
THE RELATIONSHIP BETWEEN TWO METHODS FOR EVALUATING FIVE-CARBON SUGARS IN EUCALYPTUS EXTRACTION LIQUOR
 THE RELATIONSHIP BETWEEN TWO METHODS FOR EVALUATING FIVE-CARBON SUGARS IN EUCALYPTUS EXTRACTION LIQUOR Congcong Chi, a,b* Zeng Zhang, a Weiwei Ge, a and Hasan Jameel b Alkaline pre-extraction and hydrothermal
THE RELATIONSHIP BETWEEN TWO METHODS FOR EVALUATING FIVE-CARBON SUGARS IN EUCALYPTUS EXTRACTION LIQUOR Congcong Chi, a,b* Zeng Zhang, a Weiwei Ge, a and Hasan Jameel b Alkaline pre-extraction and hydrothermal
Proteases in germinating finger millet (Eleusine coracana) seeds
 Biosci., Vol. 5, Number 3, September 1983, pp. 219 224. Printed in India. Proteases in germinating finger millet (Eleusine coracana) seeds Introduction U. VIDYAVATHI, B. SHIVARAJ and T. N. PATTABIRAMAN
Biosci., Vol. 5, Number 3, September 1983, pp. 219 224. Printed in India. Proteases in germinating finger millet (Eleusine coracana) seeds Introduction U. VIDYAVATHI, B. SHIVARAJ and T. N. PATTABIRAMAN
A Revised Method for Determining Phosphate-Phosphorus Levels in Sugar Beet Leaf Petioles 1
 A Revised Method for Determining Phosphate-Phosphorus Levels in Sugar Beet Leaf Petioles 1 G. E. VARVEL, G. A. PETERSON and F. N. ANDERSON 2 &ceived/orpublicalionj..ne 1,1976 Knowledge of plant nutrient
A Revised Method for Determining Phosphate-Phosphorus Levels in Sugar Beet Leaf Petioles 1 G. E. VARVEL, G. A. PETERSON and F. N. ANDERSON 2 &ceived/orpublicalionj..ne 1,1976 Knowledge of plant nutrient
PRODUCT: RNAzol BD for Blood May 2014 Catalog No: RB 192 Storage: Store at room temperature
 PRODUCT: RNAzol BD for Blood May 2014 Catalog No: RB 192 Storage: Store at room temperature PRODUCT DESCRIPTION. RNAzol BD is a reagent for isolation of total RNA from whole blood, plasma or serum of human
PRODUCT: RNAzol BD for Blood May 2014 Catalog No: RB 192 Storage: Store at room temperature PRODUCT DESCRIPTION. RNAzol BD is a reagent for isolation of total RNA from whole blood, plasma or serum of human
Recombinant Trypsin, Animal Origin Free
 Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
